Abstract
Mitochondrial shaping proteins are categorized as fusion and fission factors based on their opposing actions on the architecture of mitochondria. Mammalian Fis1 fragments mitochondria into small units and was initially considered a major mitochondrial fission factor, with this major role being challenged. Yu et al (2019) define that Fis1 induces mitochondrial fragmentation by blocking mitochondrial fusion. This study highlights the limitation of categorizing mitochondrial dynamics proteins based on architecture measurements, given the general tendency to associate fragmentation with activation of fission.
Subject Categories: Membrane & Intracellular Transport
A common response to stress involves a change in mitochondrial architecture. Certain stresses cause a transition of the connected mitochondrial tubules into fragmented spheres, a transition we refer to as fragmentation. Thus, mitochondrial fragmentation is one of the earliest phenotypes observed during adaptation to stress. Beyond the fact that fragmentation is an approachable phenotype to test stress, it is functionally meaningful as well. Fragmentation enables each individual mitochondrion to function independently of their cell‐mates, allowing quality control mechanisms to assess the function of each individual mitochondrion. Fragmentation may play a role in changes in mitochondrial fuel preference as well by unclear mechanisms. Furthermore, when mitochondria are divided into individual units, they become isolated and no longer share content through fusion events. This isolation enables the development of different subtypes of mitochondria within the individual cell. Consequently, defining the molecular mechanisms behind mitochondrial fragmentation can provide answers on how the response to stress, mitochondrial quality control and specialization are occurring. Mitochondrial fragmentation is commonly induced by the inhibition of mitochondrial fusion with unopposed mitochondrial fission occurring at normal rates (Fig 1). Activation of mitochondrial fission can contribute to fragmentation. However, with some notable exceptions kept in mind, in most cases, the induction of fission alone is not sufficient to lead to fragmentation. This notion kept us all puzzled about the mechanisms responsible for fragmentation induced by Fis1.
Figure 1. The dual role of Fis1 in mitochondrial morphology.
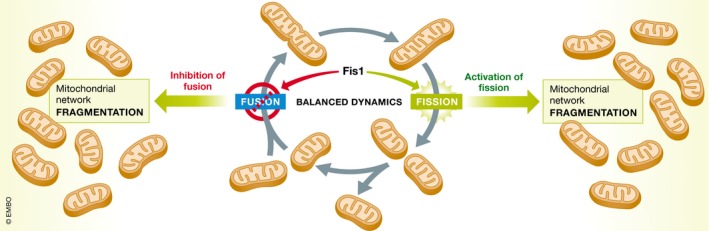
Fis1 can promote mitochondrial fragmentation by two mechanisms: activating fission and reducing mitochondrial fusion events. Mitochondrial fragmentation is commonly induced by the inhibition of fusion rather than the activation of fission. Similarly, elongated mitochondria are commonly found in conditions associated with the inhibition of the fission machinery rather than the overexpression of the fusion proteins.
The mitochondrial protein Fis1 has been around the block, so to speak. A bit of history is in order. This protein was originally discovered in yeast as Fis1p, where it is the sole receptor for the yeast fission dynamin, Dnm1 (Mozdy et al, 2000). Fis1 overexpression can cause mitochondrial fission in mammalian cells, and it can contribute to stress‐induced fission (i.e., during apoptosis; Iwasawa et al, 2011). Fis1 is, nevertheless, not essential for the recruitment of Drp1 to mitochondria. Over the years, other receptors for Drp1 were identified (Gandre‐Babbe & van der Bliek, 2008), relegating Fis1 to the role of second fiddle by acting as a modulating factor in the fission process. New life was breathed into Fis1 with the discovery that it participates in other downstream processes such as mitophagy, where it attenuates LC3 lipidation through binding interactions between Fis1 and the Rab7 GAP TBC1D15 (Shen et al, 2014; Yamano et al, 2014). A U‐turn in this pathway was suggested by the discovery that Fis1 and TBC1D15 interactions with Rab7 on lysosomes activate mitochondrial fission through Drp1 (Wong et al, 2018). If this circuitous route was not already enough, Fis1 also acts as a tether between mitochondria and ER through its binding interactions with the ER protein Bap31 (Iwasawa et al, 2011). This tethering might also be providing an additional mechanism by which Fis1 is promoting fragmentation, as recent evidence demonstrates that the ER marks future mitochondrial Drp1‐mediated fission sites (Friedman et al, 2011). In summary, while Fis1 has additional roles not directly related just to mitochondrial fission, it appears that when it is inducing fragmentation, it requires Drp1. Thus, one could conclude that: (i) Drp1 does not require Fis1 to induce fission, and (ii) mitochondrial fragmentation regulated by Fis1 is mostly executed by Drp1‐mediated fission.
In this context, Yu et al (2019) focused on answering the question: Why Fis1 overexpression can still induce fragmentation in the absence of Drp1? They first tested the domains within Fis1 required to induce Drp1‐independent fragmentation and found that the presence of both cytosolic and transmembrane domains of Fis1 is required for fragmentation. Of interest, deleting the cytosolic domain caused perinuclear clustering of mitochondria, suggesting that the cytosolic domain of Fis1 could be modulating mitochondrial motility. This finding is interesting in the light that the cytosolic domain of Fis1 is anchoring mitochondria to the ER via BAP31 (Iwasawa et al, 2011). Thus, if the Fis1 anchor is absent, this could cause an unrestricted transport to the perinuclear area. Moreover, the authors show that the effects of Fis1 overexpression on mitochondrial morphology are suppressed by latrunculin B. Actin assembly between ER and mitochondria is known to play an important role during Drp1‐dependent fission. The results of Yu et al (2019) now suggest that Fis1 also contributes to mitochondrial dynamics in an actin‐dependent, but Drp1‐independent manner. It remains to be seen whether these actions of Fis1 also occur at the MAM or elsewhere. These results do already suggest that Fis1 is a highly adaptable adaptor.
From there, Yu et al (2019) test whether Fis1 might be promoting fission by recruiting dynamin 2 (Dyn2), previously shown to regulate Drp1‐induced fission. The finding that Fis1 can still fragment mitochondria in Drp1−/− cells knocked down for Dyn2 supports that Fis1 overexpression fragments mitochondria by other non‐fission‐related mechanisms. This led to Yu et al (2019) to test whether Fis1 could be inhibiting mitochondrial fusion, which also leads to mitochondrial fragmentation. They find that endogenous Fis1 immunoprecipitates with Mfn1, Mfn2 and OPA1 and that Fis1 is reducing the GTPase activity of Mfn1, Mfn2 and OPA1 when measured using cell‐free approaches. Finally, they show, by using two independent methodologies, that Fis1 overexpression limits the exchange of matrix content between mitochondria, a process dependent both on mitochondrial fusion and on motility. Consequently, these data demonstrate that fragmentation induced by Fis1 overexpression is associated with reduced mitochondrial fusion events. An interesting follow‐up study would be to establish the relative contribution of altered motility, as well as changes in inter‐organellar contacts, versus Mfn/OPA1 GTPase activity inhibition by Fis1. In this regard, it is unknown whether the blockage of GTPase activity induced by Fis1 observed in the cell‐free assay will be conserved in vivo.
Fission and fusion appear to be two sides of the same coin. These processes are often coupled, as is evident when fission follows fusion at the same place and in rapid succession. Overlap between fission and fusion is suggested by the presence of Mfn2 in fission spots during apoptosis (Karbowski et al, 2004) and its role as a tether to the ER where it marks the sites of future mitochondrial fission (Friedman et al, 2011). Functional overlap between fission and fusion is further suggested by the ability of the inner membrane fusion protein Opa1 to induce fission as well, clearly diffusing the line between fission and fusion processes (Anand et al, 2014). Given these ambiguities, it is not surprising that Fis1 may wear several hats, playing diverse roles in different aspects of mitochondrial dynamics. The precise mechanics of Fis1 functions are as yet unclear, but the results of Yu et al (2019) have, nevertheless, uncovered an exciting new area of research. Remarkably, the findings by Yu et al (2019) illustrate that a fragmented mitochondrial network is not unequivocally associated with increased mitochondrial fission, even when the protein orthologue was demonstrated to do so.
The EMBO Journal (2019) 38: e101839
See also: R Yu et al
Contributor Information
Marc Liesa, Email: mliesa@mednet.ucla.edu.
Alexander Van der Bliek, Email: avan@mednet.ucla.edu.
Orian S Shirihai, Email: oshirihai@mednet.ucla.edu.
References
- Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, Langer T (2014) The i‐AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol 204: 919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK (2011) ER tubules mark sites of mitochondrial division. Science 334: 358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandre‐Babbe S, van der Bliek AM (2008) The novel tail‐anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell 19: 2402–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasawa R, Mahul‐Mellier AL, Datler C, Pazarentzos E, Grimm S (2011) Fis1 and Bap31 bridge the mitochondria‐ER interface to establish a platform for apoptosis induction. EMBO J 30: 556–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Arnoult D, Chen H, Chan DC, Smith CL, Youle RJ (2004) Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J Cell Biol 164: 493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozdy AD, McCaffery JM, Shaw JM (2000) Dnm1p GTPase‐mediated mitochondrial fission is a multi‐step process requiring the novel integral membrane component Fis1p. J Cell Biol 151: 367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Yamano K, Head BP, Kawajiri S, Cheung JT, Wang C, Cho JH, Hattori N, Youle RJ, van der Bliek AM (2014) Mutations in Fis1 disrupt orderly disposal of defective mitochondria. Mol Biol Cell 25: 145–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YC, Ysselstein D, Krainc D (2018) Mitochondria‐lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 554: 382–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano K, Fogel AI, Wang C, van der Bliek AM, Youle RJ (2014) Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy. Elife 3: e01612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Jin SB, Lendahl U, Nistér M, Zhao J (2019) Human Fis1 regulates mitochondrial dynamics through inhibition of the fusion machinery. EMBO J 38: e99748 [DOI] [PMC free article] [PubMed] [Google Scholar]


