Abstract
As concerns about the safety of systemic oral pharmacologic treatments for knee osteoarthritis (OA) mount, clinicians have increased the use of intra-articular hyaluronic acid (IA-HA) in managing mild-to-moderate knee OA. Supartz (sodium hyaluronate; Seikagaku Corporation, Tokyo, Japan) is the first IA-HA product to be approved in the world and has the longest history of global use. In this review, we summarize evidence supporting Supartz efficacy and safety, including data from pivotal clinical trials that resulted in approval of Supartz in the United States and Japan, the safety of single and repeated courses of Supartz, and Supartz efficacy using objective outcomes and in special populations. There is strong evidence that single 5-week courses of Supartz provide clinically meaningful reductions in pain and improved function for up to 6 months without risk of serious side effects or complications. Repeated courses of Supartz are as safe as single courses and have an extremely low risk of infection. Findings from promising initial studies, which suggest that Supartz may improve muscle strength, gait pattern, and balance, should be confirmed in randomized controlled trials.
Keywords: Knee osteoarthritis, hyaluronic acid, Supartz, intra-articular injections, viscosupplementation
Prevalence and Burden of Knee Osteoarthritis
Osteoarthritis (OA) is one of the most common joint diseases worldwide and a leading cause of chronic pain and disability in the United States1 and other developed countries.2,3 In 2007-2008, 14 million individuals in the United States had symptomatic knee OA including nearly 2 million people under the age of 45 years, 6 million aged 45 to 64 years, and 6 million aged 65 or older.4 The prevalence of radiographic knee OA appears to be higher in Japan than in the United States, although this may be due, in part, to varying definitions of knee OA across epidemiological studies.5 Knee OA accounts for more than 80% of the OA total burden2 and imposes a substantial clinical and economic burden on patients and society by causing pain, functional limitations, and physical disability resulting in reduced quality of life, lost earnings, and increased healthcare utilization and costs.6,7
Utilization of Intra-articular Hyaluronan Injections for Treatment of Knee OA
Intra-articular injections of hyaluronan (IA-HA) are used to reduce pain and improve function in patients with mild-to-moderate knee OA, or for patients with severe knee OA who wish to delay joint replacement surgery or for whom surgery is contraindicated.8 In clinical practice, first-line therapy for knee OA is often prescription oral nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen even though NSAIDs are associated with gastrointestinal and cardiovascular risks9 and acetaminophen is minimally effective for the treatment of OA.10 Patients who fail to respond to other oral analgesics may be prescribed opioids, which are no more effective than non-opioid analgesics for treating moderate-to-severe chronic pain associated with knee OA11 and are associated with side effects and a high risk of serious adverse events (AEs).12 Due to the limitations of systemic analgesics, clinicians may elect to use IA-HA as an alternative to analgesics and NSAIDs in patients of advanced age and those with a high risk for gastrointestinal and cardiovascular complications.8 A study of real-word use of pharmacologic treatments for knee OA found that utilization of IA-HA is associated with reductions in the use of NSAIDs, opioids, and IA corticosteroids in patients who have not undergone total knee arthroplasty.13 IA-HA may be preferable to IA corticosteroids for diabetic patients who may experience transient but potentially dangerous increases in blood glucoses levels following IA corticosteroid injections.14
Intra-articular Hyaluronan: Mechanisms of Action
Unmodified hyaluronan has a relatively short resident time when injected into the joint (<48 h), but its clinical effects can last 6 to 12 months, suggesting that exogenous hyaluronan triggers biological responses that contribute to long-term efficacy. Pre-clinical and clinical data indicate that hyaluronan may work through multiple mechanical and physiological mechanisms including shock absorption, joint lubrication, anti-inflammatory effects, pain reduction, chondroprotection, proteoglycan synthesis, and cartilage matrix alterations. Hyaluronan is a naturally occurring molecule present in high levels in cartilage and synovial fluid (SF) and prevents cartilage degradation by lubricating and cushioning the joint.15 Exogenous hyaluronan reduces nociceptive pain by restoring the shock-absorbing and lubricating abilities of depleted SF and suppressing the expression of mediators of nociceptive pain, such as prostaglandin E2, cyclooxygenase-2, and adenosine 5′-triphosphate.16-21 Hyaluronan blunts the inflammatory cascade implicated in the pathogenesis of OA by reducing the production of pro-inflammatory cytokines (eg, tumor necrosis factor alpha, interleukin [IL]-1β, IL-6),22,23 chemokines (eg, IL-8),22 proteases (eg, matrix metalloproteinases, a disintegrin, and metalloproteinase with thrombospondin motifs),18,22,24,25 and reactive oxygen species,26 and inhibiting activation of transcription factors (eg, NF-κB, Phospho-p38 MARK, Phospho-ERK).17,18,24 Hyaluronan stimulates synthesis and deposition of extracellular matrix (ECM) molecules that are suppressed and degraded in joints with OA.27,28 Clinical studies have shown that IA-HA reduces levels of biomarkers associated with cartilage degradation (eg, chondroitin sulfate-6, keratan sulfate) and stimulates production of biomarkers of ECM synthesis (eg, C-propeptide of collagen II).19,27,29
Supartz (Sodium Hyaluronan)
Supartz (also sold under the brand names Supartz FX, Artz, ArtzDispo, Artzal, and Visco-3) is the first worldwide approved IA-HA product and has been available in Japan and the United States since 1987 and 2001, respectively, and is CE marked in 15 countries. Because multiple commercially available IA-HA products list sodium hyaluronan as the active ingredient, but have different properties, including molecular weight, production method, and presence of molecular cross-linking,30 we will use the product’s commercial name (Supartz) throughout this article as this review is solely focused on outcomes related to Supartz and excludes studies of other commercially available IA-HA products with sodium hyaluronan as the active ingredient.
Supartz is a sterile, viscoelastic, non-pyogenic injectable solution containing highly purified, high molecular weight (620-1170 kDa) sodium hyaluronate extracted from chicken combs. Each 1 mL of Supartz contains 10 mg of sodium hyaluronate dissolved in physiological saline (1.0% solution). A treatment course or cycle of Supartz consists of 3 to 5 injections for a total dose of 75 to 125 mg of sodium hyaluronate.
This review summarizes data on the efficacy and safety of Supartz, which has the longest history of use among IA-HA products, for the treatment of knee OA. Data from randomized controlled trials (RCTs) that supported product approval in Japan and the United States, prospective and retrospective studies that evaluated the effects of Supartz on objective clinical outcomes, and studies documenting the safety and efficacy of single and repeated treatment courses were included. Efforts were made to include research that is not well known outside of Japan.
Article Selection
We conducted a search of electronic databases (MEDLINE/PubMed, EMBASE, BIOSIS, and DDFU, SELMIC, Ichusi-Web) to identify relevant articles written in English and Japanese published between 1983 and 2017. The search terms used were Supartz, Supartz FX, Artz, Artzal, ArtzDispo, Osteoartz, SPH, hyaluronic acid, hyaluronate, and hyaluranon. This review exclusively focuses on clinical research with the exception of survey studies; animal and pre-clinical studies were not included in the review. The abstracts of 84 articles were reviewed and 29 were selected for inclusion. Of these 29 studies, 17 were written in Japanese and 12 in English.
Efficacy Studies in Support of Supartz Approval in Japan
The approval of Supartz for the treatment of knee of OA in Japan was supported by evidence of efficacy derived from a multicenter, open-label, dose-finding study31 and 2 multicenter, randomized, double-blind, placebo-controlled studies (Table 1).32,33 Treatment in the dose-finding trial varied by dose, dosing interval, and number of administrations and was conducted over 4 to 8 weeks; in the 2 RCTs, treatment consisted of weekly IA Supartz injections over 5 weeks. Clinical outcomes were mostly consistent across trials. Patients rated subjective symptoms (pain while at rest, walking, and going up/down stairs; flexion/extension pain; oppressive pain (tenderness); swelling; and sense of fever [1 study]) at each weekly visit. Objective symptoms assessed during the clinical exam included patellar ballottement, estimated amount of synovial effusion (1 study), and range of motion (ROM). During clinic visits, patients rated their ability to perform 4 activities of daily living (ADLs), including a 10-min walk, going up/down stairs, squatting to pick up something on the floor, and sitting on the floor. Patients and clinicians provided a global assessment of change in symptoms at each visit (excellently improved, improved, fairly improved, unchanged, slightly worsened, worsening, markedly worsened).
Table 1.
Designs of pivotal Supartz clinical trials for Japan and United States regulatory approval.
| Study/country | N | Inclusion criteria | Treatment/study duration | Co-interventions permitted | Outcomes |
|---|---|---|---|---|---|
| Oshima et al31/Japan | 206 | Symptomatic and radiographic evidence of knee OA; age ⩾18 years | 4-8 wk/4-8 wk | Prior anti-inflammatory or analgesic medication or physical therapy | Pain while at rest, walking, going up/down stairs pain, during flexion/extension; oppressive pain (tenderness); swelling; patellar ballottement; synovial effusion; ROM; impairment in ADLs; patient- and clinician-rated global improvement |
| Shichikawa et al32/Japan | 107 | Radiographic knee OA with pain on movement; age ⩾18 years | 5 wk/5 wk | Prior physical therapy | Pain while at rest, walking, going up/down stairs pain, during flexion/extension; oppressive pain (tenderness); swelling; patellar ballottement; synovial effusion; ROM; impairment in ADLs; patient- and clinician-rated global improvement |
| Shichikawa et al33/Japan | 228 | Radiographic knee OA with pain on exercise; age ⩾18 years | 5 wk/5 wk | Prior anti-inflammatory medication or physical therapy | Pain while at rest, walking, going up/down stairs pain, duringflexion/extension; oppressive pain (tenderness); swelling; patellar ballottement; synovial effusion; ROM; impairment in ADLs; patient- and clinician-rated global improvement |
| Puhl et al34/Germany | 209 | Radiographic knee OA; age 40-75 years | 5 wk/14 wk | Paracetamol rescue | Primary: Lequesne, paracetamol use Secondary: VAS, investigator/patient global assessment |
| Lohmander et al35/Sweden | 240 | Radiographic OA; VAS ⩾10 mm; age 40-75 years; Lequesne score ⩾4 at baseline | 5 wk/20 wk | Paracetamol rescue | Primary: Lequesne, VAS ratings for knee function, pain, ROM, and activity level Secondary: paracetamol use, investigator/patient global assessment |
| Day et al36/Australia | 203 | Mild/moderate early OA; pain on walking; age 40-75 years | 5 wk/18 wk | Paracetamol rescue | Primary: WOMAC pain, stiffness, and disability Secondary: Lequesne, paracetamol use, investigator and patient global assessment |
| United Kingdom (unpublished)37 | 231 | Radiographic OA; moderate pain >3 months | 5 wk/25 wk | Co-proxomol as rescue | Primary: VAS pain Secondary: Lequesne, paracetamol use, investigator/patient global assessment |
| France (unpublished)37 | 254 | Radiographic OA; VAS global pain ⩾35 mm | 5 wk/13 wk 3 wk/13 wk |
Paracetamol as rescue | Primary: Lequesne Secondary: VAS pain, paracetamol use, investigator global assessment |
Abbreviations: ADL, activities of daily living; OA, osteoarthritis; ROM, range of motion; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
A preliminary dose-finding study evaluated the efficacy of Supartz in 206 patients with clinical and radiographic evidence of OA.31 Subjects received one of 2 doses (2.5 or 5.0 mL) at one of 2 dosing intervals (once weekly or every other week); those assigned to weekly treatment had 4 to 7 total injections and those assigned to biweekly treatment had 4 injections. Four subjects were excluded due to protocol violations and 12 dropped out of the study, resulting in 190 evaluable subjects of whom 134 received 2.5 mL Supartz weekly, 29 received 2.5 mL Supartz every other week, 23 received 5.0 mL Supartz weekly, and 4 received 5.0 mL Supartz every other week. Subjects were assessed at baseline, after each injection, and on completion of the study (which varied depending on the number of administrations received).
A larger proportion of patients receiving weekly vs biweekly administration had an overall effectiveness rating of “excellent” or “good” at the end of the study (2.5 mL: 67.9% vs 48.3%, P < .01; 5.0 mL: 65.2% vs 25.0%, P = NS). There was no significant difference in response between subjects who received weekly injections of 2.5 or 5.0 mL. For subjects who received weekly 2.5 mL injections, maximum efficacy was achieved after 5 injections. Results indicated that the optimal efficacy was obtained by once weekly administration of 2.5 mL for a total of 4 to 5 weeks.
In the first Supartz RCT, 107 patients received Supartz (n = 52) or placebo (n = 55) with 98 patients (Supartz, n = 48; placebo, n = 50) available for the final efficacy analysis.32 At each week during the 5-week treatment, a significantly greater proportion of patients treated with Supartz was rated by investigators as having excellent or good improvement compared with the placebo group. About twice as many Supartz-treated patients were rated as being significantly or moderately improved compared with the placebo group (60% vs 34%, P < .001) at the final assessment. At 5 weeks, patients in the Supartz group had significantly less pain when walking, going up/down stairs, and during flexion than those in the placebo group (P < .05 for all comparisons). No significant differences were noted in swelling, ROM, and patellar ballottement. At 5 weeks, patients who received Supartz reported better ability to squat to pick up an item from the floor (P < .05), but in a subsequent RCT there were no statistically significant between-group differences in the ability to perform ADLs.
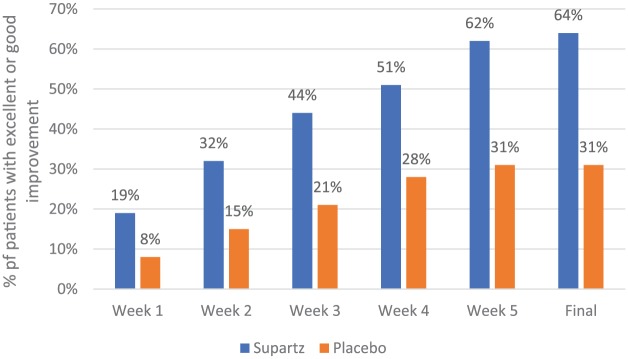
In a second RCT, 228 patients were randomized to Supartz (n = 114) or placebo (n = 114) with 208 patients available for the final efficacy analysis (Supartz, n = 103; placebo, n = 105).33 At each week during the trial, a significantly higher proportion of Supartz-treated patients received a global assessment rating (which considered improvement in subjective and objective symptoms during the entire trial) of “excellent” or “good” compared with placebo-treated patients (Figure 1). At the final assessment, twice as many Supartz-treated patients were rated having excellent or good improvement compared with the placebo group (64% vs 31%, P < .001).33 After 5 weeks of treatment, patients in the Supartz group reported significantly less pain when at rest (P < .01), walking (P < .01), going up/down stairs (P < .001), during flexion/extension (P < .01), and oppressive pain (P < .01) than those in the placebo group. Groups did not differ in swelling, patellar ballottement, synovial effusion, ROM, or the ability to perform ADLs after 5 weeks.
Figure 1.
Global effectiveness of Supartz vs placebo during a 5-week clinical trial.33 All between-group comparisons were statistically significant (P< .001) at each time point during the trial.
Efficacy Studies Supporting Approval of Supartz in the United States
Approval of Supartz in the United States was based on an integrated analysis of 5 similarly designed, multicenter, double-blind, randomized, placebo-controlled trials conducted in Australia,36 France, Germany,34 Sweden,35 and the United Kingdom summarized in Table 2.37,38 The placebo treatment consisted of IA saline injections except in the German study which used a diluted 1% Supartz formulation as the vehicle control.34 Each study followed a standard protocol of 5 weekly IA injections of 2.5 mL Supartz apart from the French study, which incorporated an additional arm of 3 weekly HA injections followed by 2 injections of control.37,38 The RCTs enrolled patients with symptomatic OA, ⩾40 years old (⩾50 in Sweden), with radiographically confirmed OA of the tibiofemoral compartment. Predominantly unilateral disease was specified in all but 1 RCT (Germany). All protocols excluded patients with inflammatory arthropathies, IA injections during the prior 3- to 6 -month period, and clinically relevant instability or malalignment and/or severe effusion.
Table 2.
Adverse events occurring in >4% of Supartz-treated patients who received a single treatment course.37
| Adverse event | Supartz (n = 619) | Placebo (n = 537) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Arthralgia | 110 | 17.8 | 95 | 17.7 |
| Arthropathy/arthosis/arthritis | 68 | 11.0 | 57 | 10.6 |
| Back pain | 40 | 6.5 | 26 | 4.8 |
| Pain (non-specific) | 37 | 6.0 | 26 | 4.8 |
| Injection site reactiona | 35 | 5.7 | 18 | 3.4 |
| Headache | 27 | 4.4 | 23 | 4.3 |
| Injection site pain | 26 | 4.2 | 22 | 4.1 |
no statistically significant differences were found between groups for any adverse events but does not provide p values.
A total of 1155 patients from all 5 trials were included in an intent-to-treat pooled analysis. Efficacy was assessed at weeks 5 and 13 in all trials and in week 9 in 4 trials; there were additional assessments of efficacy at weeks 17, 20, and/or 25 in 3 of the 5 RCTs. The Lequesne Index, an algofunctional, validated, composite index of pain and function, was a primary measure in 3 studies (France, Germany, and Sweden) and a secondary measure in 2 trials (Australia, United Kingdom).
The mean reduction in total Lequesne Index score from baseline over all visits was significantly larger in the Supartz vs placebo group, 0.68 vs 2.00; between-group difference of 0.68; 95% confidence interval = 0.56 to 0.79; P = .0026.37 In the Australian study, there were statistically significant differences in favor of the treatment group in WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) pain scores at weeks 14 and 18, WOMAC stiffness at weeks 10 and 14, and WOMAC disability at week 18 (all comparisons P < .05).36 Puhl et al34 reported a decrease in rescue paracetamol consumption over weeks 1 to 5 in Supartz patients compared with placebo although this difference versus placebo did not reach statistical significance (0.85 vs 0.89; P > .05).
In the Swedish study, among patients aged >60 years with more severe baseline disease (Lequesne Index Score > 10), Supartz showed a consistent pattern of improvement vs placebo.35 Visual analog scale (VAS) pain scores were significantly lower in Supartz-treated vs placebo-treated patients at week 1 (P = .008), week 13 (P = .014), and week 20 (P = .004). Mean reductions in pain VAS were reported to be more than 28 mm at weeks 4 and 20. Likewise, significant advantages for Supartz were seen with respect to other endpoints such as VAS scores for activity level, Lequesne Index, and global assessments by the patient and assessor. These differences were reproduced both at discrete time points during follow-up and when assessed as a mean treatment effect over the 20-week trial period by area under the curve measurements for both the intent-to-treat and per-protocol populations.
Objective Measures of Treatment Efficacy
Although the Japanese pivotal studies included several measures of objective efficacy (eg, amount of synovial effusion, ROM), these measures were only briefly described, and no statistically significant differences were seen between the treatment groups. More recently conducted studies have investigated the potential benefits of Supartz for improving essential aspects of objective function, such as muscle strength, gait pattern, and balance, which we summarize below.
To evaluate whether pain relief due to Supartz indirectly improves muscle strength in patients with knee OA (who typically have muscular atrophy due to disuse), Nishiura conducted a prospective, open-label study in 31 patients (38 knees) with knee OA. Most patients received 5 weekly IA-HA injections, but 10 knees received 10 injections (1 injection weekly for 5 weeks and then 5 injections every other week).39 Muscle strength of quadriceps femoris and hamstrings on both sides was measured using a KIN-COM 500 H before and within 1 week after the completion of each Supartz course. A qualitative analysis showed that quadriceps strength was improved in 50% of knees, unchanged in 29%, and worsened in 21%. Although quantitative analyses showed a trend toward improved quadriceps and hamstring strength following treatment, these changes were not statistically significant. A post hoc analysis of patients aged <70 years (27 knees in 23 subjects) showed that these patients experienced an increase in quadriceps femoris and hamstrings strength following treatment (both P < .05) but that the muscles in the treated knee were approximately 20% weaker than in patients’ normal untreated knees. The 19 patients who received Supartz in 1 knee experienced a significant increase in isokinetic concentric and eccentric quadriceps strength following treatment (both P < .05).
A prospective controlled study compared the efficacy of Supartz in improving muscle activation in 23 patients with knee OA and 14 age-matched subjects with normal knees.40 Electromyographic activity was recorded during walking for 5 consecutive trials to assess muscle activation patterns before and following completion of 5 weekly IA Supartz injections and at 3- and 6-month follow-up. Treatment with Supartz reduced co-contraction of the quadriceps, hamstring, tibialis anterior, and medial gastrocnemius muscles and improved motor activities of these muscles for at least 6 months, such that muscle activation in treated knees was recovered and similar to the control group.
The impact of Supartz on gait patterns and sagittal ground reactions (GRFs) was examined by comparing prospective outcomes in 15 treated patients with knee OA (30 knees) and 15 age-, body mass-, and sex-matched healthy controls (30 knees).41 Patients were assessed at baseline, after 5 weeks of IA Supartz, and at 3- and 6-month follow-up. Prior to treatment, patients with knee OA exhibited abnormal joint loading, as shown by a loss of the distinctive 2-peak GRF signal, and an altered gait pattern with slower walking velocity and cadence and longer stride time than the control group (P < .05 for all comparisons). Supartz treatment reversed and somewhat restored gait patterns and GRFs in patients with knee OA with an almost immediate clinical effect that lasted up to 6 months.
A prospective study examined frontal and sagittal joint kinetics in 25 patients with bilateral symptomatic knee OA and 15 age-, height-, and weight-matched healthy controls.42 In the treated OA group, gait analyses were performed at baseline and at 1 week, 3 months, and 6 months following completion of treatment. Pain VAS and Lequesne Index total scores were significantly improved at 1 week (P < .05) after treatment and were maintained over 6 months (P < .05). At baseline, the OA knee group had significantly slower walking speed (P < .001) and shorter step length (P = .01) than healthy subjects. Walking speed significantly increased at 1 week, 3 months, and 6 months after the completion of treatment. Step length significantly increased at 1 week after completion of treatment. Larger hip adduction moments at early stance and larger knee adduction moments at early and terminal stance were significantly improved at 1 week, 3 months, and 6 months following treatment (all P < .05 compared with baseline). VAS pain scores were negatively correlated with increased knee adduction moments following treatment (r = −0.656; P < .001). Authors cautioned that reduced pain following IA-HA may result in increased joint loading on the osteoarthritic knee joints, which could accelerate the rate of joint degeneration, noting that there is a 6.5 times increased risk of knee OA progression for each 1% increase in knee abduction moments.
Balance, an important component of performance for transfer, ambulatory tasks, and many ADLs, is often impaired in the elderly OA population. A 6-month, prospective, observer-blind, controlled study in 68 patients aged ⩾65 years with clinical symptoms and radiographic evidence of unilateral mild-to-moderate knee OA evaluated the effects of Supartz on pain, functional ability, and balance (single-leg stance test, functional reach test, timed “up and go” test, and Berg balance scale).43 Fifty-six of 68 participants completed the 6-month study and were compared with 50 age-, body mass-, and sex-matched healthy controls. The VAS and Lequesne Index scores were significantly reduced from pre-treatment to week 1 after the fifth injection with benefits lasting for 6 months (P < .001 for all comparisons relative to baseline). Prior to treatment, patients with knee OA had worse scores on the 4 balance tests than the control group (P < .001 for all comparisons). After receiving Supartz, patients with knee OA significantly improved in all 4 balance tests at 1 week, 1 month, 3 months, and 6 months after the fifth injection (P ⩽ 0.001 for all comparisons).
Large knee joint sounds are often produced when patients with end-stage OA bend and stretch their knees. A study evaluated the effect of IA Supartz in patients with end-stage knee OA and patellofemoral (PF) joint lesions using the joint sounds as a measure of lubrication.44 Subjects were 30 patients (5 men and 25 women) with end-stage OA whose average age was 69.5 years. Artificial joint replacement and high tibial osteotomy (HTO) were performed in all patients. Supartz 2.5 mL was injected once into the knees (n = 30) of these patients before the operation, and knee sounds while bending and stretching the knees were recorded before and immediately after the injection. A subset of these 30 subjects (20 knees in 20 patients) were injected with a drug mixture of lidocaine 2 mL and steroid after some unspecified interval following the Supartz injection, and knee joint sounds were measured in a similar way. Similarly, the changes in the joint sounds were examined in 35 patients with PF disorders who produced joint sounds when they bent and stretched their knees. The average frequency in the end-stage OA group significantly decreased from 422.1 ± 141.1 Hz before the Supartz injection to 280.2 ± 76.6 Hz immediately after the injection (P < .0001). In contrast, the average frequency before and after the IA injection of lidocaine plus steroid was unchanged (420 ± 10 Hz vs 417 ± 6.3 Hz). The average frequency in subjects with PF disorders significantly decreased immediately after the IA-HA injection (544.6 ± 154.9 Hz vs 368.4 ± 113.2 Hz; P < .01). Results of this study support the lubricating properties of Supartz; however, results should be replicated in an RCT with a longer duration of follow-up.
Safety of Supartz
Below, we summarize the evidence supporting the safety of single and repeated Supartz treatments including infection rates in large patient populations treated with Supartz.
Safety of single course treatment
An integrated analysis of 5 RCTs evaluating single treatments of Supartz found that the most common AEs were arthralgia (joint pain with no evidence of inflammation), arthropathy/arthrosis/arthritis (joint pain with evidence of inflammation), back pain, pain (non-specific), injection site reaction, headache, and injection site pain (Table 2).37,38 There were no statistically significant differences in the incidence rates of these AEs between groups. None of the AEs were considered serious or related to Supartz and all resolved when treatment ended without sequelae. The rates of discontinuation due to AEs were similar across active (Supartz) and control groups in both the individual trials and integrated analysis: 1.8% of patients receiving Supartz and 3.2% receiving control injections discontinued treatment early due to an AE.38
Safety of repeated treatment
There is no evidence of an increased safety risk associated with repeated Supartz treatment. A post-marketing surveillance study in Japan from 1987 to 1993 evaluated data of 7404 patients with knee OA from 675 medical institutions who received IA injections of Supartz.45 Of the 7404 patients, 3614 (49%) received more than 5 injections. Supartz was well tolerated with 37 of 7404 patients (0.5%) reporting 58 adverse reactions, of which 83% occurred at the injection site. AEs occurred most frequently (53 of 58) during the first course of treatment (ie, first 5 injections) and typically occurred with a few hours of injection.
Of the 58 AEs, the most frequent were 29 reports of pain at the injection site (50%), 16 reports of swelling (27.6%), and 3 cases of redness at the injection site (5.2%). No significant difference was observed in the incidence rate of AEs between patients aged <65 vs ⩾65 years (0.37% vs 0.47%). The frequency and severity of AEs occurring during repeat treatment cycles were similar to those reported for a single treatment cycle.37
Four prospective uncontrolled studies from Japan included 127 patients with knee OA who received repeated courses of Supartz injections over an average of 12.5 months (range: 4-35 months).46-49 No AEs or abnormal laboratory results related to Supartz were reported in any of the studies.
A retrospective study from the United States analyzed the safety of 2 or more courses of Supartz in 303 osteoarthritic knees from 220 patients.50 A total of 26 AEs were reported (20 mild and 6 moderate), yielding an incidence of 8.6%. Of the 26 AEs, 25 were determined as possibly related and 1 as definitely related to the injection. All AEs resolved spontaneously and without medical intervention.
Risk of infection associated with Supartz
IA-HA and IA corticosteroids are both associated with low infection incidence rates. A single physician in Japan administered approximately 225 870 IA injections (212 220 IA-HA and 13 650 IA corticosteroid injections) over a 14.5-year period and identified only 5 cases of infectious knee OA possibly related to the injection.51 The infection rate after IA corticosteroid injection (0.015% or 2/13 650) was nearly 10.4-fold higher (P = .0308) than the infection rate after IA-HA injections (0.0014% or 3/212 220). The overall infection rate was 0.0022% (5/225870).
When to permit taking a shower or bath after an IA injection has been a controversial subject.52 Data from a recent study suggest that patients can safely bathe on the first day after receiving an IA-HA or steroid injection.52 A total of 324 patients with knee (76%) or shoulder (24%) disease received 2018 IA-HA (25 mg Artz or Suvenyl) or corticosteroid (triamcinolone acetonide 10 mg) injections and were instructed to have a bath on the day after receiving the injection. Most patients (93%) received an IA-HA injection. Patients were clinically evaluated for infection at 1, 2, 4, 12, and 24 weeks after injection. Bacterial culture tests for SF and blood tests (white blood cell count, C-reactive protein, and erythrocyte sedimentation rate) were performed at the time of the first injection and at 2, 4, and 12 weeks after injection. The data showed that there were no infections or bacteria detected in the SF of any patients. The other laboratory values were not significantly changed during the period of evaluation.
Efficacy of Supartz in Special Populations
Patient undergoing HTO
The efficacy of Supartz in patients undergoing HTO has not been well studied. A prospective, non-randomized, controlled cohort study investigated the effects of Supartz on pain and ROM in patients who underwent HTO.53 Subjects were 9 knees in 9 patients (6 men and 3 women) who were diagnosed with knee OA and underwent HTO. Supartz was injected into the affected knees of these patients once a week for 5 weeks from the first week after the operation (HA group). The control group, who were matched on age, body weight, and baseline ROM and Japanese Orthopedic Association (JOA) scores, consisted of 9 knees in 9 patients (2 men and 7 women) with knee OA who underwent HTO but were not injected with Supartz (non-HA group). Preoperative ROM was similar in the HA and non-HA groups (134° vs 129°). At 5 weeks after HTO, ROM was significantly higher in the HA group compared with the non-HA group (118° vs 93°, P < .03) although there was no statistically significant between-groups difference in ROM (134° vs 132°) or JOA scores 1 year after surgery. The early improvement in ROM following IA-HA administration may help alleviate pain and shorten the rehabilitation period. The magnitude, clinical relevance, and duration of benefits of IA-HA following HTO should be evaluated in future studies.
Patients with subchondral bone lesions
Alleviation of pain caused by subchondral bone marrow lesions (BML), which are a risk factor for the development and progression of OA, may be a potential therapeutic mechanism of IA-HA. To evaluate this hypothesis, Naraoka et al studied the relationship between pain VAS and Japanese Knee Osteoarthritis Measure (JKOM) scores and magnetic resonance imaging (MRI)-detected BML scores in 34 patients with knee OA (37 knees) treated with or without Supartz.54 The JKOM comprises 25 items that assess pain and stiffness in the knee (8 items), state of daily living (10 items), everyday activities (5 items), and health conditions (2 items). Treatment was determined by patient preference with 17 knees treated with IA-HA once weekly for 5 weeks (HA group) and 20 knees that were not treated with IA-HA (non-HA group). Patients in the non-HA group were given a pamphlet of muscle training and instructed to perform self-exercise therapy at home and could take oral NSAIDs.
A follow-up examination at 3 months was completed for 9 of the 17 knees in the HA group and 13 of the 20 knees in the non-HA group. MRI was performed in 5 knees in each group at 3 months. There was a significant improvement in BML score at 3 months (P < .05) in the HA group but no statistically significant improvement in BML score in the non-HA group. The VAS score was improved in all cases with an 8 or lower baseline BML score, and so 8 was used as the cut-off for determining severity of baseline BML. In the HA group, there was a significant improvement in VAS (P < .05) and JKOM (P < .05) scores in cases with a BML score of ⩽8 compared with those with a BML score of >8. In contrast, in the non-HA group, there was no significant difference in VAS or JKOM improvement by baseline BML score. Results suggest that IA-HA may have a greater pain-relieving effect in patients with lower baseline BML scores. In addition, the reduction in BML scores seen 3 months after initiation of IA-HA could indicate that BML may be a useful biomarker for evaluation of the therapeutic efficacy of IA-HA.
Several limitations of this study should be noted. This was a small non-randomized controlled study and the control group may have differed from the treatment group in important but unknown ways. Another limitation was the failure to examine meniscus injury in detail as this is a key differential diagnosis of BML. Finally, only a limited number of cases had MRI follow-up data.
Efficacy of Supartz Maintenance Treatment
In the United States, payers typically allow eligible patients to receive a course of IA-HA every 6 months for treatment of knee OA.55 In contrast, it is accepted practice in Japan to administer IA-HA continuously in consideration of patient symptoms and this frequency of treatment may be reimbursed by insurers in Japan.56 A multicenter, randomized, clinical study was conducted in Japan to compare the effectiveness of a single course of Supartz (5 weekly injections; n = 120) vs Supartz maintenance (10 injections administered every other week after the initial 5 weekly injections; n = 119) in 239 patients with knee OA.56 Thirty-three patients with missing data at week 0 or 6 were excluded from the analyses leaving 106 in the maintenance group and 100 in the single-cycle group. Concomitant therapy with anti-inflammatory agents or exercise was permitted, but subjects could not switch or discontinue the concomitant treatment between 3 weeks prior to the initiation of the first treatment cycle and the final observation day. Pain VAS, JKOM scores, articular ROM, and hydrarthrosis were assessed at the initiation of the first cycle (week 0) and at weeks 6, 12, and 24. At week 24, a global evaluation by the patient and orthopedic doctor was assessed with a 7-point rating scale (considerably improved, moderately improved, slightly improved, unchanged, slightly deteriorated, moderately deteriorated, and considerably deteriorated).
At weeks 6, 12, and 24, VAS and JKOM scores and articular ROM were significantly improved in both groups compared with week 0 (P < .05 for all comparisons). With week 6 as the baseline assessment (after 1 treatment cycle), the group that received maintenance treatment had significantly greater improvement in VAS scores at week 12 and JKOM scores at week 12 and 24 compared with the single-cycle group (P < .05 for all comparisons); there were no between-group differences in articular ROM or hydrarthrosis at week 12 or 24. The patient global assessment indicated no worsening of symptoms, and the proportion of patients rating themselves as considerably or moderately improved were 70% and 61% in the maintenance and single-cycle groups, respectively (P = .344). Similarly, orthopedic doctors indicated no deteriorated cases, with the proportion of patients considerably or moderately improved at 75% and 68% in the maintenance and single-cycle groups, respectively (P = .825). Investigators noted that, although a single cycle of Supartz could be effective for up to 6 months, continuous maintenance treatment was more effective for improving knee OA symptoms and ADLs compared with single-cycle treatment. It should be noted that this study had important methodological weaknesses, including quasi-randomization, allowance of resumption of treatment in the single-cycle group due to recurrence of pain, and the lack of sham injections for patients randomized to the single-cycle group, which may have influenced results. The potential benefits of single-cycle vs maintenance treatment should be evaluated in additional studies that use a more rigorous methodology.
Conclusions
Supartz is an effective and safe treatment for the management of knee OA.57,58 IA-HA is a suitable alternative to systemic pharmacologic treatments (eg, NSAIDs, opioids) in patients who have an inadequate treatment response to these medications or who experience or are at risk for the significant side effects and complications associated with these agents. Patients with knee OA tend to be older and often have contraindications for NSAIDs and opioids, leaving IA-HA and IA corticosteroids as the sole non-surgical treatment options. Large RCTs conducted in multiple countries have demonstrated that a single treatment cycle (3-5 weekly injections) of Supartz significantly reduces pain and improves subjective function in patients with mild to moderate symptomatic knee OA. Demonstrating that Supartz improves objective measures of function has been more challenging, but some small studies have yielded promising findings with respect to the potential effects of Supartz on muscle strength and activation, gait patterns, and balance, which should be replicated in RCTs. Supartz has a long history of clinical use and post-marketing surveillance studies have shown that repeated courses of Supartz present no significant safety risks compared with single courses of treatment. The potential benefits of maintenance Supartz treatment are intriguing and should be evaluated in rigorously designed clinical trials to determine whether this regimen is superior to single-cycle treatment and which types of patients may benefit from more frequent administration.
Footnotes
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/ or publication of this article: The author(s) received financial support from Seikagaku Corporation for the preparation of this article.
Author Contributions: AB was primarily responsible for preparing the initial and final drafts of the paper. JTN and THL prepared the first draft of several sections of the paper. VD provided important critical commentary. All authors approved the final version of the manuscript.
References
- 1. Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 4. Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ. Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity. 2016;68:1743–1750. doi: 10.1002/acr.22897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muraki S, Tanaka S, Yoshimura N. Epidemiology of knee osteoarthritis. OA Sports Med. 2013;1:21. [Google Scholar]
- 6. Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10:437–441. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 7. Sharif B, Garner R, Sanmartin C, Flanagan WM, Hennessy D, Marshall DA. Risk of work loss due to illness or disability in patients with osteoarthritis: a population-based cohort study. Rheumatology. 2016;55:861–868. doi: 10.1093/rheumatology/kev428. [DOI] [PubMed] [Google Scholar]
- 8. Bruyere O, Cooper C, Pelletier JP, et al. A consensus statement on the European society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—from evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45:S3–S11. doi: 10.1016/j.semarthrit.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 9. Pelletier J-P, Martel-Pelletier J, Rannou F, Cooper C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45:S22–S27. doi: 10.1016/j.semarthrit.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 10. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. Jama. 2018;319:872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. da Costa BR, Nuesch E, Kasteler R, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2014;9:CD003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McIntyre LF, Beach W, Bhattacharyya S, Yadalam S, Bisson B, Kim K. Impact of hyaluronic acid injections on utilization of pain management medications. Am J Pharm Benefits. 2017;9:195–199. [Google Scholar]
- 14. Habib GS, Miari W. The effect of intra-articular triamcinolone preparations on blood glucose levels in diabetic patients: a controlled study. J Clin Rheumatol. 2011;17:302–305. doi: 10.1097/RHU.0b013e31822acd7c. [DOI] [PubMed] [Google Scholar]
- 15. Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi: 10.1186/s12891-015-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boettger MK, Kummel D, Harrison A, Schaible HG. Evaluation of long-term antinociceptive properties of stabilized hyaluronic acid preparation (NASHA) in an animal model of repetitive joint pain. Arthritis Res Ther. 2011;13:R110. doi: 10.1186/ar3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yasuda T. Hyaluronan inhibits prostaglandin E2 production via CD44 in U937 human macrophages. Tohoku J Exp Med. 2010;220:229–235. [DOI] [PubMed] [Google Scholar]
- 18. Hashizume M, Koike N, Yoshida H, Suzuki M, Mihara M. High molecular weight hyaluronic acid relieved joint pain and prevented the progression of cartilage degeneration in a rabbit osteoarthritis model after onset of arthritis. Mod Rheumatol. 2010;20:432–438. doi: 10.1007/s10165-010-0299-1. [DOI] [PubMed] [Google Scholar]
- 19. Ikeda K. Changes in synovial fluid markers and clinical effect after intra-articular injection of sodium hyaluronate with particular reference to the anti-inflammatory effect in terms of prostagladin E2 concentration. J Tokyo Women’s Med. 1998;68:22–36. [Google Scholar]
- 20. Kumahashi N, Naitou K, Nishi H, et al. Correlation of changes in pain intensity with synovial fluid adenosine triphosphate levels after treatment of patients with osteoarthritis of the knee with high-molecular-weight hyaluronic acid. Knee. 2011;18:160–164. doi: 10.1016/j.knee.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 21. Kusayama Y, Akamatsu Y, Kumagai K, Kobayashi H, Aratake M, Saito T. Changes in synovial fluid biomarkers and clinical efficacy of intra-articular injections of hyaluronic acid for patients with knee osteoarthritis. J Exp Orthop. 2014;1:16. doi: 10.1186/s40634-014-0016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang CT, Lin YT, Chiang BL, Lin YH, Hou SM. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis Cartilage. 2006;14:1237–1247. doi: 10.1016/j.joca.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 23. Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A. Molecular size hyaluronan differently modulates toll-like receptor-4 in LPS-induced inflammation in mouse chondrocytes. Biochimie. 2010;92:204–215. doi: 10.1016/j.biochi.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 24. Kataoka Y, Ariyoshi W, Okinaga T, et al. Mechanisms involved in suppression of ADAMTS4 expression in synoviocytes by high molecular weight hyaluronic acid. Biochem Biophys Res Commun. 2013;432:580–585. doi: 10.1016/j.bbrc.2013.02.043. [DOI] [PubMed] [Google Scholar]
- 25. Chang CC, Hsieh MS, Liao ST, et al. Hyaluronan regulates PPARγ and inflammatory responses in IL-1β-stimulated human chondrosarcoma cells, a model for osteoarthritis. Carbohydr Polym. 2012;90:1168–1175. doi: 10.1016/j.carbpol.2012.06.071. [DOI] [PubMed] [Google Scholar]
- 26. Mongkhon JM, Thach M, Shi Q, Fernandes JC, Fahmi H, Benderdour M. Sorbitol-modified hyaluronic acid reduces oxidative stress, apoptosis and mediators of inflammation and catabolism in human osteoarthritic chondrocytes. Inflamm Res. 2014;63:691–701. doi: 10.1007/s00011-014-0742-4. [DOI] [PubMed] [Google Scholar]
- 27. Shimizu M, Higuchi H, Takagishi K, Shinozaki T, Kobayashi T. Clinical and biochemical characteristics after intra-articular injection for the treatment of osteoarthritis of the knee: prospective randomized study of sodium hyaluronate and corticosteroid. J Orthop Sci. 2010;15:51–56. doi: 10.1007/s00776-009-1421-0. [DOI] [PubMed] [Google Scholar]
- 28. Bagga H, Burkhardt D, Sambrook P, March L. Long-term effects of intraarticular hyaluronan on synovial fluid in osteoarthritis of the knee. J Rheumatol. 2006;33:946–950. [PubMed] [Google Scholar]
- 29. Hasegawa M, Nakoshi Y, Tsujii M, et al. Changes in biochemical markers and prediction of effectiveness of intra-articular hyaluronan in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:526–529. doi: 10.1016/j.joca.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 30. Altman RD, Bedi A, Karlsson J, Sancheti P, Schemitsch E. Product differences in intra-articular hyaluronic acids for osteoarthritis of the knee. Am J Sports Med. 2016;44:2158–2165. doi: 10.1177/0363546515609599. [DOI] [PubMed] [Google Scholar]
- 31. Oshima Y, Azuma H, Namiki O, et al. Intra-articular injection therapy of high molecular weight sodium hyaluronate (SPH) on osteoarthritis of the knee joint. Jpn Phar Ther. 1983;11:2253–2267. [Google Scholar]
- 32. Shichikawa K. Clinical evaluation of high molecular weight sodium hyaluronate (SPH) on osteoarthritis of the knee: multicenter well controlled comparative study. Jpn J Clin Pharmacol Ther. 1983;14:545–558. [Google Scholar]
- 33. Shichikawa K, Maeda A, Ogawa N. [Clinical evaluation of sodium hyaluronate in the treatment of osteoarthritis of the knee]. Ryumachi. 1983;23:280–290. [PubMed] [Google Scholar]
- 34. Puhl W, Bernau A, Greiling H, et al. Intra-articular sodium hyaluronate in osteoarthritis of the knee: a multicenter, double-blind study. Osteoarthritis Cartilage. 1993;1:233–241. [DOI] [PubMed] [Google Scholar]
- 35. Lohmander LS, Dalen N, Englund G, et al. Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: a randomised, double blind, placebo controlled multicentre trial. Ann Rheum Dis. 1996;55:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Day R, Brooks P, Conaghan PG, Petersen M. A double blind, randomized, multicenter, parallel group study of the effectiveness and tolerance of intraarticular hyaluronan in osteoarthritis of the knee. J Rheumatol. 2004;31:775–782. [PubMed] [Google Scholar]
- 37. Seikagaku Corporation. Supartz (Sodium Hyaluronate): US Prescribing Information. Memphis, TN: Smith & Nephew, Inc; 2007. [Google Scholar]
- 38. Strand V, Conaghan PG, Lohmander LS, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14:859–866. [DOI] [PubMed] [Google Scholar]
- 39. Nishiura M. A study of the effect of high molecular weight sodium hyaluronate on osteoarthritis of the knee-quantitative evaluation with the measurement of the strength using KIN-COM 500H. Joint Surg. 1995;22:18–23. [Google Scholar]
- 40. Tang AC, Hong WH, Chen HC, Tang SF. Intra-articular intervention by hyaluronic acid for knee osteoarthritis can modify locomotor pattern of muscle activity. Clin Neurol Neurosurg. 2015;129:S16–S20. doi: 10.1016/S0303-8467(15)30006-8. [DOI] [PubMed] [Google Scholar]
- 41. Tang SF, Chen CP, Chen MJ, Pei YC, Lau YC, Leong CP. Changes in sagittal ground reaction forces after intra-articular hyaluronate injections for knee osteoarthritis. Arch Phys Med Rehabil. 2004;85:951–955. [DOI] [PubMed] [Google Scholar]
- 42. Tang AC, Tang SF, Hong WH, Chen HC. Kinetics features changes before and after intra-articular hyaluronic acid injections in patients with knee osteoarthritis. Clin Neurol Neurosurg. 2015;129:S21–S26. doi: 10.1016/S0303-8467(15)30007-X. [DOI] [PubMed] [Google Scholar]
- 43. Sun SF, Hsu CW, Hwang CW, et al. Hyaluronate improves pain, physical function and balance in the geriatric osteoarthritic knee: a 6-month follow-up study using clinical tests. Osteoarthritis Cartilage. 2006;14:696–701. doi: 10.1016/j.joca.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 44. Matsuura I. The influence that is given to knee joint sound by the injection of hyaluronic acid knee joint. Clin Rheumatol. 1999;11:113–116. [Google Scholar]
- 45. Ueno Y, Kuramoto K, Konno N, Koizumi T, Hoshiba T, Kamohara S. Investigation on result of use after launch of ARTZ and ARTZ Dispo: evaluation on the efficacy, safety, and utility in the medication for osteoarthritis of the knee and periarthritis of the shoulder. Jpn Pharmacol Ther. 1995;23:2151–2170. [Google Scholar]
- 46. Hashimoto Y. Multicenter clinical studies of ARTZ (high molecular weight sodium hyaluronate) in the long term treatment of osteoarthritis of the knee. Jpn Pharmacol Ther. 1992;20:347–360. [Google Scholar]
- 47. Igrashi M, Arai M, Morito H, et al. Multicentre clinical studies of high molecular weight sodium hyaluronate in the long-term treatment of osteoarthritis of the knee. Jpn Pharmacol Ther. 1983;11:4871–4888. [Google Scholar]
- 48. Suzu F, Hirasawa Y. Results of long term treatment with ARTZ for the knee osteoarthritis. Jpn Pharmacol Ther. 1990;18:4979–4984. [Google Scholar]
- 49. Yoh K, Tsuji H, Maruoka T, Tateishi H. Clinical results of intra-articular treatment with sodium hyaluronate in osteoarthritis of the knee. Clin Rheumatol. 1989;2:132–136. [Google Scholar]
- 50. Whitman CS, Allen D, Comadoll JL, Thomason HC, Oweida SJ. A retrospective study of SUPARTZ® and repeat treatment for osteoarthritis pain in the knee. J Manag Care Med. 2010;13:43–47. [Google Scholar]
- 51. Ijiri S. Infection rate associated with over 220,000 intra-articular injections and precautions taken to avoid infection. J Jpn Clin Orthop Assoc. 2015;40:1–11. [Google Scholar]
- 52. Suzuki T, Seito S, Hirozane T, et al. Safety of bathing after intra-articular injection on the first day. Clin Orthop Surg. 2016;57:665–668. [Google Scholar]
- 53. Miyanishi K, Miura H, Nagamine R, Urabe K, Iwamoto Y. Clinical effect of intra-articular injection of sodium hyaluronate on patients with high tibial osteotomy. Orthopedics Traumatol. 1998;47:390–393. [Google Scholar]
- 54. Naraoka T. Relationship between the knee injection of sodium hyaluronan and MRI-detected bone marrow lesions in knee osteoarthritis. J East Jpn Orthop Traumatol. 2013;25:7–13. [Google Scholar]
- 55. Watson S. A guide to knee injections for osteoarthritis. Medical News Today. 2018. https://www.medicalnewstoday.com/articles/310557.php.
- 56. Hirose J, Mizuta H, Kataoka M, Tsumura H, Watanabe S, Chosa E. Evaluation of long-term intra-articular injection of hyaluronan for knee osteoarthritis; a prospective controlled multicenter trial. Orthopedic Surg. 2012;63:1321–1326. [Google Scholar]
- 57. Curran MP. Hyaluronic acid (Supartz(R)): a review of its use in osteoarthritis of the knee. Drugs Aging. 2010;27:925–941. doi: 10.2165/11205920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 58. Altman RD, Dasa V, Takeuchi J. Review of the mechanism of action for Supartz FX in knee osteoarthritis. Cartilage. 2018;9:11–20. doi: 10.1177/1947603516684588. [DOI] [PMC free article] [PubMed] [Google Scholar]