Short abstract
Background
Many adults with post-traumatic stress disorder (PTSD) are unable to access healthcare services for treatment due to logistical, social, and attitudinal barriers. Interventions delivered via mobile applications (apps) may help overcome these barriers.
Objective
The aim of this study is to systematically evaluate the most recent evidence from trials investigating the efficacy of mobile apps for treating PTSD.
Methods
PubMed, Web of Science, Embase, PsycINFO, and Medline were searched in February 2018. Randomised controlled trials (RCTs) were included if they quantitatively evaluated the efficacy of a mobile app for treating PTSD as part of the primary aim. Findings were presented in a narrative synthesis.
Results
In the five identified RCTs, the use of app-based interventions appeared to be associated with reductions in PTSD symptoms. However, the strength of evidence for this association appeared to be inconsistent, and there was little evidence that those using the apps experienced greater reductions in PTSD symptoms than those in control conditions. Nonetheless, there was some evidence that app-based interventions are both a feasible and acceptable treatment pathway option.
Conclusions
Included studies were often limited by small sample sizes, brief intervention, and follow-up periods, and self-reported measures of PTSD. Evidence for the efficacy of mobile interventions for treating PTSD was inconclusive, but promising. Healthcare professionals should exercise caution in recommending app-based interventions until the potentially adverse effects of app use are better understood and larger-scale studies have taken place.
Keywords: PTSD, review, mental health, mobile phone, mobile health
Introduction
Post-traumatic stress disorder (PTSD) is an anxiety disorder that can arise following exposure to a traumatic event, and is estimated to effect around 4.4% of the UK adult population.1 Those with PTSD may require long-term treatment, but access to support can be hampered by a range of barriers including limited resources available to mental health services, perceived stigma discouraging help-seeking, preference for resolving the problem without help and difficulties accessing help.2,3
Emerging healthcare interventions supported by electronic information and communication processes, such as the use of mobile applications (apps), could provide a means to overcome these barriers and reach individuals who are unable or reluctant to access mental healthcare.4,5 Mobile phones are owned by an estimated 85% of the UK adult population, with ownership trends expected to rise even further, and, therefore, provide an opportunity for delivering interventions at a population-level using apps.6 In the last decade, computer and web-based health technologies (such as mobile phone-based alcohol app interventions5 and machine-learning approaches to detect PTSD3) have been harnessed to increase reach, provide real-time monitoring, and offer a more holistic treatment pathway via digital support platforms and tele-health.7–10 A recent survey of veterans receiving treatment for PTSD indicated that those who had access to a mobile device were interested in using a mobile health intervention via apps to manage and monitor symptoms.11
App-based interventions might be a far-reaching, ubiquitous, and desirable treatment option for people with PTSD. However, despite the increase in available app-based interventions, a 2015 review found that compared to other mental health disorders, the evidence base for mobile health technologies in PTSD was lacking (see Olff12 for review). The primary aim of this review was to systematically evaluate recent evidence for the efficacy of mobile apps for treating PTSD. We summarise the best available evidence by focusing on results from randomised controlled trials (RCTs), which are widely considered the gold standard in experimental research design. We also summarise secondary findings of the included studies relating to outcomes other than PTSD, and the feasibility and acceptability of the interventions under study.
Methods
Design
We conducted a systematic review of RCTs, following Cochrane methodology and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.13 The review was registered with PROSPERO (CRD42018107815).
Search strategy
PubMed, Web of Science, Embase, PsycINFO, and Medline electronic bibliographic databases were searched in February 2018 to identify relevant, peer-reviewed studies. As smartphone technology is rapidly evolving, the search was limited to papers published since 2007, the year the first iPhone was released.14 The following search strategy was applied to each electronic database search engine, restricted to abstracts only:
(smartphone* OR apps OR application OR mhealth OR m-health OR mobile* OR uhealth) AND (PTSD OR post traumatic stress disorder OR posttraumatic stress disorder OR post-traumatic stress disorder)
The asterisk denotes truncation. Reference lists of relevant studies and systematic reviews were searched in addition to the electronic databases.
Eligibility criteria
Studies were eligible to be included in the current review if they (i) were published in English, (ii) were published since 2007, (iii) were RCTs, (iv) as part of the primary study aim, quantitatively evaluated the efficacy of a mobile app (exposure) for PTSD treatment (outcome), and (v) included participants with subthreshold or full PTSD caused by any type of trauma or traumatic event.
Studies were excluded from the review if they (i) assessed the efficacy of mobile apps developed for the sole purpose of screening for PTSD, and (ii) assessed the use of passive interventions such as teletherapy.
Article selection process and data collection
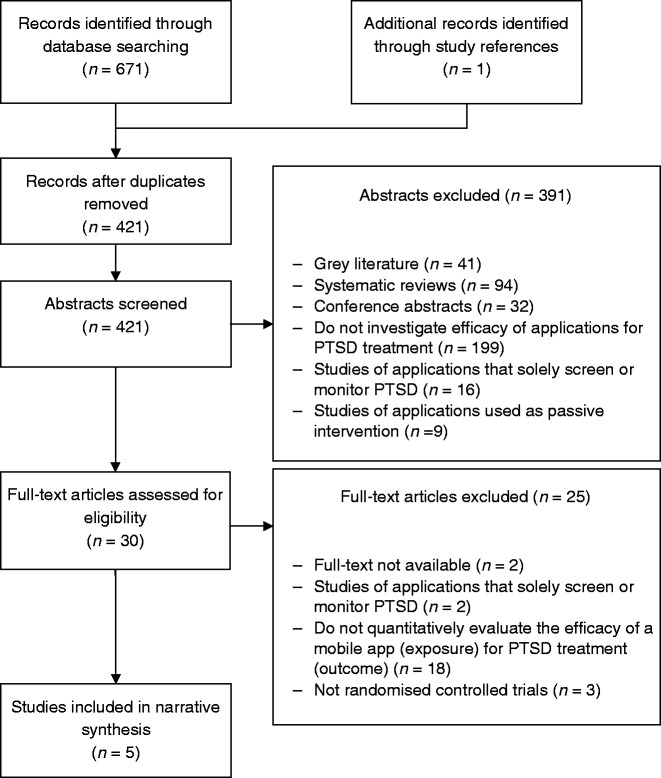
PRISMA flow diagram can be found in Figure 1. Following the removal of duplicates in Mendeley, titles and abstracts of articles were independently reviewed for eligibility by PMP, DL, and AW between May and September 2018. Full texts of studies deemed potentially eligible were independently evaluated further. If any discrepancies arose, these were dis cussed by the two reviewers until a consensus was reached. Data were extracted by PMP and AW on study design (intervention type, study participants, and PTSD measurement instruments used) and on study findings (with a specific focus on findings relating to PTSD outcomes). Findings were summarised in a narrative synthesis.
Figure 1.
Flow diagram of screened studies.
Assessment of quality
The methodological quality of the included studies was assessed using the Cochrane Handbook for Systematic Reviews of Interventions.15 The handbook recommends using the Cochrane risk of bias tool16 to allocate a risk of bias classification for each study. Grading with this approach was dependent on certain issues affecting bias such as reporting of randomisation, blinding and allocation concealment, and whether outcome measures were fully reported. Each study was reviewed by two authors (DL and VW), given an overall risk of bias grade of low, unclear or high, and then discussed for consensus if there was disagreement in grading.
Results
Study characteristics
The characteristics of the five included studies are summarised in Table 1. All studies were conducted in the USA. Mean sample ages ranged from 32 to 46 years. Two of the five included studies were pilot RCTs,19,20 both of which are from the same research group and had the lowest sample sizes (n = 49 and n = 20, respectively). All studies used variants of the PTSD Checklist to measure PTSD.22 Three of the included studies compared an app intervention to a waitlist control group and/or a standard-of-care comparator,17–19 while the remaining two compared different ways of delivering an app intervention.20,21 The majority of studies screened for PTSD symptoms for inclusion.18–21
Table 1.
Characteristics of included studies.
| Lead author, year, study location | Intervention | Study design | Study participants (sample size, gender, average age in years) | PTSD measurement instrument(s) used | Relevant findings |
|---|---|---|---|---|---|
| Kahn, 2016, USA17 | 16 weeks Mission Reconnect (MR) use | Four-arm randomised controlled trial:(1) MR only (n = 80) (2) Prevention and Relationship Enhancement Program (PREP) only (n = 80) (3) both programs together (n = 80) (4) waitlist control (n = 80) | Community-based veteran-partner dyads where the veteran had a history of deployment in a post-9/11 combat operationn = 320 (160 dyads) Veterans: 147 men, 34 womenAge M = 33.4 (SD = 6.6) Partners: 10 men, 129 womenAge M = 32.4 (SD = 7.0) | PCL-CAssessment at baseline, eight weeks and 16 weeks. | Within-group comparisonsVeterans: intent-to-treat paired t-tests indicated that in the MR-only arm, veterans’ PCL-C scores declined between baseline (M = 38.4, SD = 16.5) and eight weeks (M = 32.3, SD = 15.8, p < 0.05). These improvements were also sustained at 16 weeks (M = 31.3, SD = 15.7, p < 0.05). PCL-C scores also declined in the other study arms, but to a lesser extent, and only reaching statistical significance in the PREP arm at 16 weeks. Partners: in the MR-only arm, partners’ PCL-C scores declined between baseline (M = 33.7, SD = 12.6) and eight weeks (M = 29.1, SD = 12.7, p < 0.05). In the MR and PREP arm, PCL-C scores declined between baseline (M = 33.4, SD = 15.0) and 16 weeks (M = 28.7, SD = 14.4, p < 0.05). There were no other significant changes in PCL-C scores. Between-group comparisonsVeterans: after adjusting for multiple comparisons, two-sample t-tests showed no significant between-arm differences in PCL-C scores at eight or 16 weeks (p > 0.05). Partners: no significant between-arm differences. |
| Kuhn, 2017, USA18 | Three months of PTSD Coach use | Two-arm randomised controlled trial: (1) PTSD Coach (n = 62) (2) waitlist control (n = 58) | Adults exposed to a traumatic event more than one month previously, with PTSD symptoms (score ≥35 on PCL-C), but not receiving PTSD treatment. n = 120 37 men, 83 womenaAge M = 39.3a | PCL-CAssessment at baseline, three months and six months. | Within-group comparisonsIn the PTSD Coach arm, PCL-C scores declined between baseline (M = 63.19, SD = 11.78) and three months (M = 51.93, SD = 14.04) (no significance test reported). There were no further significant changes between three and six months (M = 49.15, SD = 13.94, t(61) = 1.61, p = 0.113). In the waitlist control arm, PCL-C scores also declined between baseline (M = 60.59, SD = 10.24) and three months (M = 53.90, SD = 13.78) (no significance test reported). Between-group comparisonsIn an intent-to-treat repeated measures ANOVA, PCL-C scores were more reduced between baseline and three months in the PTSD Coach arm than in the waitlist control arm (F(1,177) = 4.55, p = 0.035). The condition by time interaction effect size was statistically significant (Cohen’s d = 0.41, p < 0.05). However, at three months, the mean PCL-C scores did not differ between arms (t(118) = 0.73, p = 0.466). |
| Miner, 2016, USA19 | One month of PTSD Coach use | Two-arm pilot randomised controlled trial: (1) PTSD Coach (n = 25) (2) waitlist control (n = 24) | Community trauma survivors with PTSD symptoms (score ≥25 on PCL-C) but not receiving PTSD treatment. n = 49 9 men, 40 womenAge M = 45.7 (SD = 13.9) | PCL-C Assessment at baseline, post-condition, and one-month follow-up. | Within-group comparisonsIn intent-to-treat exploratory paired sample t-tests, PCL-C scores declined between baseline and post-condition in the PTSD Coach arm (t(24) = –2.06, p = 0.040, d = -0.59), and this was sustained at one-month follow-up (t(24) = –2.89, p = 0.004, d = –0.97). In the waitlist control arm, PCL-C scores did not significantly decline between baseline and post-condition (t(23) = –1.70, p = 0.093, d = –0.31), but did decline once assigned they were assigned to use PTSD Coach (t(23) = –2.80, p = 0.006, d = –0.61). Between-group comparisonsIn intent-to-treat between groups repeated measures ANOVAs, the condition by time interaction effect size from baseline to post-condition was non-significant (d = –0.25, p > 0.05). |
| Possemato, 2016, USA20 | Eight weeks of PTSD Coach use | Two-arm pilot randomised controlled trial: (1) Self-Managed (SM) PTSD Coach (n = 10) (2) Clinician-supported (CS) PTSD Coach (n = 10) | Veterans Affairs primary care patients with military-related trauma resulting in a PCL-S score ≥40, but not intending to enter specialty PTSD treatment before study completion. n = 20 19 men, 1 womanaAge M = 42 (SD = 12) | PCL-S Assessments at baseline, eight weeks, 12 weeks and 16 weeks. | Within-group comparisonsIntent-to-treat within-group analysis showed that PCL-S scores significantly declined between baseline and post-treatment in both the SM arm (t(9) = 2.8, p = 0.02, Cohen’s d = 0.41) and the CS arm (t(9) = 5.4, p < 0.01, Cohen’s d = 1.4). Results from 12- and 16-week follow-up were not reported as they were thought to be more reflective of care received following the intervention under study. Between-group comparisonsThose in the CS condition appeared to see a greater reduction in PCL-S scores than those in the SM condition, but the condition by time interaction effect was not significant (F(1,18) = 0.93, p = 0.30, d = 0.54). |
| Roy, 2017, USA21 | LifeArmor, PE Coach, Positive Activity Jackpot, Eventful, Tactical Breather, Virtual Hope Box, Daily Yoga, Simply Yoga. Six weeks of app use. | Two-arm randomised controlled trial: (1) resilience enhancement (RE; daily directed app use via text) (n = 72) (2) Control (CT, app access without daily direction) (n = 72) | Adults within the military healthcare system who had either experienced deployment or a stressful life event and had a PCL score between 28 and 49 (no PTSD diagnosis) n = 144 77 men, 67 womenAge M = 33.6a | PCL Assessment at screening, baseline, weekly for six weeks of intervention, and at three-, six-, and 12-month follow-up. | Within-group comparisonsCompared to screening and baseline, there were significant reductions in overall PCL scores at three-month follow-up in both study arms. There were also significant reductions in overall PCL scores over the six weeks of intervention for both arms. At 6- and 12-month follow-up, there was a partial rebound in overall PCL scores. Between-group comparisonsRE and CT did not significantly differ in their overall PCL scores. |
aInferred from information provided in the article.
M: mean; SD: standard deviation; PCL-C: PTSD Checklist – civilian version; PCL-S: PTSD Checklist – specific version; PCL: PTSD Checklist.
Intervention periods ranged from one month to four months. Three of the five included studies investigated the PTSD Coach app. PTSD Coach is intended to provide psycho-education, self-assessment, symptom-management tools, and links to resources for individuals with PTSD. A thorough overview of the app and its global application can be found in Kuhn et al.23 The remaining two studies investigated the efficacy of apps that were not specifically designed for PTSD. Kahn et al.17 reported results from a study examining the impact of Mission Reconnect, a dyadic intervention program for veterans and their partners, which supports psychological, social, and physical outcomes. Finally, Roy et al.21 outline evidence from a variety of healthcare apps delivered together to foster a range of positive activities including social engagement, psycho-education, and relaxation.
PTSD-related outcomes
Key findings relating to PTSD-related outcomes are described in Table 1. All included studies reported within-group comparisons, which were variously suggestive of improvements in PTSD symptoms immediately following app intervention. Particularly promising was the find by Kahn et al. that veterans who entered their study with high levels of PTSD saw clinically significant reductions in PTSD CheckList – Civilian Version (PCL-C) scores following app use.17 However, where reported, effect sizes for PTSD improvements following app use were typically weak to moderate.19,20 Only clinician-supported use of PTSD Coach produced a large effect size for immediate within-group changes in PTSD, but with a sample size of n = 10 in the relevant study arm, this test was not adequately powered.20 Included studies also reported evidence of sustained improvements in PTSD symptoms over the following weeks or months.17–19,21 However, the study with the longest follow-up period also reported a partial rebound in PCL-C scores at six and 12 months.21 Overall, there was little evidence for a strong, long-term improvement in PTSD symptoms following app use.
In addition, there was little to no evidence that those who used the apps experienced a greater improvement in PTSD symptoms compared to those who received a standard-of-care comparator or who did not receive any intervention at all.17–19 Kuhn et al.17 did report a greater reduction in PTSD scores following app use compared to waitlist control, but the effect size for this interaction was weak (d = 0.41), and a further analysis showed that the post-treatment PTSD scores for two groups did not significantly differ from each other.18 Similarly, two studies found that a higher proportion of participants achieved clinically significant improvements in their PTSD following app use than waitlist control, but only Kuhn et al. found this association to be statistically significant.18,19
Several studies also investigated how the extent and type of app use might be related to PTSD outcomes. There was no significant evidence to suggest that PTSD improvements were significantly enhanced by providing daily directions for app use,21 or by delivering the apps with clinician support.20 This was despite both features appearing to encourage more frequent app use. Consistent with this, two other included studies did not find extent of app use to significantly correlate with changes in outcomes.18,19 However, clinician-supported app use did appear to increase the likelihood of participants accepting referrals for mental health treatment than self-managed app use.20 Finally, there was no evidence to suggest that app use benefited particular groups, with Miner et al.19 reporting that among those who used the app, changes in PCL scores were not significantly related to gender, education, or smartphone ownership.
Other findings
Most of the included studies investigated a range of other outcome variables, including stress, anxiety, depression, self-compassion, sleep quality, pain, psychosocial functioning, and quality of life.17,18,20,21 Findings regarding these outcomes were inconsistent both within and between the included studies, such that no firm conclusions can be drawn. It is noteworthy that self-managed PTSD Coach use was significantly associated with worsening social quality of life.20
However, there was evidence to suggest that the apps under study were both feasible and acceptable interventions. Veterans and partners in Kahn et al.17 used Mission Reconnect for over one hour per week on average throughout the intervention period, and in the final survey, were highly likely to recommend it to a friend. In Miner et al.,19 participants opened PTSD Coach between two and three times per week on average, and only one of the 43 participants reported not using the app at all. The app was used at a range of times and places, and few barriers to use were endorsed. The majority of participants agreed that they had learned new tools to cope with their symptoms from the app, and only five out of the 43 participants reported that the app was of no use to them.19
Brief overview of study quality
The included studies shared some common strengths. In particular, they all had clear aims and objectives, randomised participants to study condition, and had low attrition rates. However, the included studies also had some key limitations. Two of the included studies were pilot studies and were, therefore, underpowered with particularly small sample sizes.19,20 Additionally, while the quality of reporting was typically good, there were some gaps. For instance, Kahn et al.17 did not report the results of their within-groups comparisons in full, and Roy et al.21 did not report any numerical results, instead presenting all results graphically, limiting proper scrutiny of their findings.
Kahn et al.17 was the only study to include a waitlist control arm and a standard-of-care comparison arm. Kuhn et al.18 and Miner et al.19 only included a waitlist comparator, which, alone, does not offer the most stringent comparison, and Possemato et al.20 and Roy et al.21 did not include any such control arms, such that the overall potential benefit of their different forms of app use cannot be fully ascertained. In addition, across the studies, intervention and follow-up periods were typically brief, thereby limiting the window to instigate and detect beneficial change in participants.
All included studies were vulnerable to response bias, using self-report measures of PTSD and sometimes of app usage (used as an adherence measure). The clinical relevance of self-reported PTSD symptoms are more doubtful than clinician-administered diagnostic measures, but several studies did attempt to isolate findings which were clinically relevant.17–20
Studies were also vulnerable to sampling bias, most often with concerns around participants self-selecting into the studies. Additionally, there was very limited discussion of participant blinding, perhaps because the concealment of group allocation is particularly challenging for this kind of intervention. In Kahn et al.,17 all participants were explicitly told the importance of their study arm, which may have particularly biased their activities and self-reporting throughout the trial. Finally, it should be noted that all included studies were conducted in the USA, and, therefore, might not be representative of other national samples.
Risk of bias
Of the five studies, two were classified as having an overall low risk of bias17,24 based on qualities such as clearly defined randomisation process reporting, appropriate blinding of group allocation where possible, and complete reporting for all outcome measures. The remaining studies18–20 did not report or clarify measures taken to avoid bias, and, therefore, were given ratings of either unclear risk or high risk. A summary of the risk of bias assessment can be found in Supplementary Table 1.
Discussion
The aim of this systematic review was to summarise evidence on the efficacy of apps for treating PTSD. We focused on RCTs as the gold standard in experimental research design. Findings on the efficacy of app-based interventions were, overall, promising but weak, with risk of bias present in several studies. There was little evidence that they had a greater effect than standard-of-care or receiving no intervention, and directing or supporting app use added little benefit. However, despite the limited clinical benefits of app use, explorations of app use and satisfaction indicated that the app-based interventions appeared both feasible and acceptable to participants.
The impact of the reviewed apps on PTSD outcomes is disappointingly inconclusive. It is difficult to identify the exact cause due to the small number of studies. It may be explained by differences in determining PTSD status (such as clinical diagnosis and self-report probable PTSD questionnaires) and the duration of follow-up. Other study designs have shown varying degrees of improvement in PTSD symptoms associated with apps that target PTSD,25–27 and even with apps that primarily target anger and pain management.28–30 But the promising feasibility and acceptability of the app-based interventions is more consistent with previous studies. PTSD Coach in particular has been downloaded several thousand times and has received predominantly positive reviews and user satisfaction ratings, with many describing the app as helpful for improving symptoms and knowledge of PTSD.26–31 These findings are also consistent with the known interest in mobile health interventions among veterans in PTSD treatment.11
One of the included studies found changes in PTSD symptoms to be unrelated to the gender, education, or smartphone ownership of users.19 However, existing literature suggests that app use may in the first instance be associated with user computer literacy and skills, expected usefulness of the app, and social influence,25,32 and that app satisfaction may be higher among smartphone owners than non-owners.31 Given that one of the key motivations for developing app-based interventions is to ensure that otherwise neglected groups can access treatment, predictors of app use, satisfaction, and efficacy warrant further research and attention.
Some caution is warranted around the use of app-based interventions in this population. PTSD has been linked to problematic smartphone use,33,34 and problematic smartphone use has, in turn, been linked to poorer mental health outcomes.35 These associations could conceivably be exacerbated by encouraging the use of app-based interventions. There is also limited but concerning evidence around the potentially adverse effects of app-based interventions. One of the included studies found self-directed app use to be associated with worsening social quality of life.20 Additionally, in a recent study of aggregate mobile analytics data from downloads of PTSD Coach, one user reported feelings of increased stress in response to technical difficulties in using the app,26 and focus groups in another study found some aspects of the app frustrating.31 These incidents highlight the need to consider that, like all treatments options, app-based interventions can induce both positive and negative outcomes. It should be noted that none of the included studies specifically sought to identify negative outcomes of the app-based interventions under study, and future trials should aim to capture possible adverse effects.
As yet, app-based interventions are not explicitly recommended in the National Institute for Health and Care Excellence PTSD treatment guidelines as a treatment pathway in the UK.36 Indeed, this review finds the efficacy of apps as a treatment for PTSD to be weak. However, the results remain promising: when delivered with clinician support, app-based interventions may prompt users to seek further care,20 and studies suggest that they may be a feasible and acceptable treatment option. Future RCTs should, therefore, continue to investigate the efficacy of app-based interventions for PTSD and should seek to address some of the methodological constraints of the included studies by ensuring adequately powered samples, using clinician-administered diagnostic tools to measure PTSD outcomes, and including suitable control groups to allow comparison with the existing standard of care. Moreover, considering the known drawbacks of increased mobile phone use, a greater effort should be made to ensure that there are no unintended adverse effects of app use in this population.
Limitations
This review provides a systematic, up-to-date overview of the current evidence around mobile health and PTSD. We summarise the highest-quality available evidence by focusing on results from published, peer-reviewed RCTs. However, there are some limitations to the review and of the studies included. First, by focusing on RCTs whose primary aim was to evaluate the efficacy of app-based interventions for treating PTSD, we do not capture evidence from other study designs or from studies that investigated this topic as their secondary aim. While this ensures the quality of the reported results, other important findings and considerations may have been missed. Second, owing to time constraints, we did not contact key researchers in the field to help identify relevant studies.
Third, as outlined in the results section, the included studies had some limitations, thereby impacting on the quality of the review. There were particular concerns around sample sizes (particularly in the case of both pilot studies), brief intervention and follow-up periods, the use of self-report measures of PTSD symptoms and app usage, and sometimes incomplete reporting of results. Future research should adopt larger samples, include longer intervention and follow-up periods, and consider using clinician-administered diagnostic measures of PTSD. Fourth, the review identified only five published peer-reviewed studies. Thus, the results should be interpreted taking this low number of studies into account.
Finally, to capture as much available evidence as possible, we did not screen according to various study features. For instance, the included studies vary in whether they screened participants for PTSD as part of their inclusion criteria, which trauma types they included, whether they instructed participants on app use, and what sort of control group condition was included, if any. The results should, therefore, be interpreted taking this variability into consideration.
Conclusions
Overall, evidence for the efficacy of mobile interventions for treating PTSD is weak but promising. While studies did show app-based treatments to be associated with reductions in PTSD symptoms, these improvements were not consistently significant, sustained, or more substantial than improvements seen in control conditions. But, despite this, the apparent feasibility and acceptability of these interventions is noteworthy, and it remains possible that they can overcome various barriers associated with access to mental health services. Additional usability and programme development is still needed to further optimise user experience, and there are gaps in knowledge with regards to whether apps may inadvertently worsen mental health symptoms for certain individuals. As such, healthcare professionals must ensure that such apps are recommended cautiously with appropriate safeguards in place to support patients. Future research should continue to investigate the viability of app-based interventions for PTSD using rigorous, large-scale studies, and app developers should continue to use this growing evidence base and user feedback to minimise potential frustrations and other adverse effects that can be associated with app use.
Supplemental Material
Supplemental Material for Efficacy of mobile application interventions for the treatment of post-traumatic stress disorder: A systematic review by Alice Wickersham, Petros Minas Petrides, Victoria Williamson and Daniel Leightley: the STRONG STAR Consortium in Digital Health
Authors’ Note
Alice Wickersham and Petros Minas Petrides have contributed equally to this article and both should be considered as joint first author.
Contributorship
PMP, AW, and DL conceived the study and contributed to protocol development. PMP wrote the first draft of the manuscript. AW and DL revised subsequent versions. DL and VW conducted risk of bias.
Declaration of conflicting interests
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Ethical approval
Ethical approval was not required for this study.
Funding
DL and AW were funded by the US Department of Defense (grant number W81XWH-14-1-0079). AW is in receipt of a PhD studentship funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. The funders had no influence over the work plan, data analysis, or data interpretation.
Guarantor
DL.
Peer review
This manuscript was reviewed by two individuals who have chosen to remain anonymous.
Supplemental material
Supplemental material is available for this article online.
References
- 1.Fear NT, Bridges S, Hatch S, et al. Posttraumatic stress disorder. Adult Psychiatr Morb Surv 2016; 2014: 1–25. [Google Scholar]
- 2.Salaheddin K, Mason B. Identifying barriers to mental health help-seeking among young adults in the UK: a cross-sectional survey. B. J Gen Pract 2016; 66(651): e686–e692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leightley D, Williamson V, John Darby J, et al. Identifying probable post-traumatic stress disorder: applying supervised machine learning to data from a UK military cohort. Journal of Mental Health 2018; 28: 1: 34–41. [DOI] [PubMed] [Google Scholar]
- 4.Martinez SG, Badillo-Urquiola KA, Leis RA, et al. Investigation of multimodal mobile applications for improving mental health. In: Schmorrow D., Fidopiastis C. (eds) foundations of augmented cognition: neuroergonomics and operational neuroscience 2016; 9744: 333–343. [Google Scholar]
- 5.Leightley D, et al. A smartphone app and personalized text messaging framework (InDEx) to monitor and reduce alcohol use in ex-serving personnel: development and feasibility study JMIR mHealth uHealth 2018; 6(9): e10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deloitte. State of the smart consumer and business usage patterns. Deloitte, 2017. December, pp.3–52.
- 7.Cikajlo I, Rudolf M, Goljar N, et al. Telerehabilitation using virtual reality task can improve balance in patients with stroke. Disabil Rehabil 2012; 34(1): 13–18. [DOI] [PubMed] [Google Scholar]
- 8.Li B, Maxwell M, Leightley D, et al. Development of Exergame-based virtual trainer for physical therapy using Kinect. Games Health 2014; 29(4): 230–232. [Google Scholar]
- 9.Yohannes A. Telehealthcare management for patients with chronic obstructive pulmonary disease. Expert Rev Respir Med 2012; 6(3): 239–242. [DOI] [PubMed] [Google Scholar]
- 10.Brennan DM, Mawson S, Brownsell S. Telerehabilitation: Enabling the Remote Delivery of Healthcare, Rehabilitation, and Self Management In: Gaggioli, Keshner, Weiss et al. (eds) Advanced technologies in rehabilitation (Vol. 145). Fairfax, VA: IOS Press, 2009; 231–248. [PubMed] [Google Scholar]
- 11.Erbes CR, et al. Access, utilization, and interest in mHealth applications among veterans receiving outpatient care for PTSD. Mil Med 2014; 179(11): 1218–1222. [DOI] [PubMed] [Google Scholar]
- 12.Olff M. Mobile mental health: a challenging research agenda. Eur J Psychotraumatol 2015; 6(1): 27882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritchie R. 11 years ago today, Steve Jobs introduced the iPhone. iMore, https://www.imore.com/history-iphone-original (2018, accessed October 2018). [Google Scholar]
- 15.Higgins JP, Green S. (eds) Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons, Ltd, 2008. [Google Scholar]
- 16.Higgins JPT, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343(2): d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahn JR, Collinge W, Soltysik R. Post-9/11 veterans and their partners improve mental health outcomes with a self-directed mobile and web-based wellness training program: a randomized controlled trial. J Med Internet Res 2016; 18(9): e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn E, Kanuri N, Hoffman JE, et al. A randomized controlled trial of a smartphone app for posttraumatic stress disorder symptoms. J Consult Clin Psychol 2017; 85(3): 267–273. [DOI] [PubMed] [Google Scholar]
- 19.Miner A, Kuhn E, Hoffman JE, et al. Feasibility, acceptability, and potential efficacy of the PTSD Coach app: a pilot randomized controlled trial with community trauma survivors. Psychol Trauma Theory, Res Pract Policy 2016; 8(3): 384–392. [DOI] [PubMed] [Google Scholar]
- 20.Possemato K, et al. Using PTSD Coach in primary care with and without clinician support: a pilot randomized controlled trial. Gen Hosp Psychiatry 2016; 38: 94–98. [DOI] [PubMed] [Google Scholar]
- 21.Roy MJ, Costanzo ME, Highland KB, et al. An app a day keeps the doctor away: guided education and training via smartphones in subthreshold post traumatic stress disorder. Cyberpsychology, Behav Soc Netw 2017; 20(8): 470–478. [DOI] [PubMed] [Google Scholar]
- 22.Weathers FW, Litz BT, Herman DS, et al. The PTSD Checklist: reliability, validity and diagnostic utility. In: Annual meeting of the International Society for Traumatic Stress Studies, San Antoni, 1994, Boston, USA: National Center for PTSD.
- 23.Kuhn E, et al. PTSD Coach around the world. mHealth 2018; 4(2): 15–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brief DJ, Rubin A, Enggasser JL, et al. Web-based intervention for returning veterans with symptoms of posttraumatic stress disorder and risky alcohol use. J Contemp Psychother 2011; 41(4): 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keen SM, Roberts N. Preliminary evidence for the use and efficacy of mobile health applications in managing posttraumatic stress disorder symptoms. Heal Syst 2017; 6(2): 122–129. [Google Scholar]
- 26.Owen JE, Jaworski BK, Kuhn E, et al. mHealth in the wild: using novel data to examine the reach, use, and impact of PTSD Coach. JMIR Ment Heal 2015; 2(1): e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith SK, et al. Cancer distress coach: pilot study of a mobile app for managing posttraumatic stress. Psychooncology 2018; 27(1): 350–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson SS, Levesque DA, Broderick LE, et al. Pain self-management for veterans: development and pilot test of a stage-based mobile-optimized intervention. JMIR Med Informatics 2017; 5(4): e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackintosh M-A, Niehaus J, Taft CT, et al. Using a mobile application in the treatment of dysregulated anger among veterans. Mil Med 2017; 182(11): e1941–e1949. [DOI] [PubMed] [Google Scholar]
- 30.Morland LA, Niehaus J, Taft C, et al. using a mobile application in the management of anger problems among veterans: a pilot study. Mil Med 2016; 181(9): 990–995. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn E, et al. Preliminary evaluation of PTSD Coach, a smartphone app for post-traumatic stress symptoms. Mil Med 2014; 179(1): 12–18. [DOI] [PubMed] [Google Scholar]
- 32.Frisbee KL. Variations in the use of mHealth tools: the VA mobile health study. JMIR mHealth uHealth 2016; 4(3): e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contractor AA, Frankfurt SB, Weiss NH, et al. Latent-level relations between DSM-5 PTSD symptom clusters and problematic smartphone use. Comput Human Behav 2017; 72: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Contractor AA, Weiss NH, Tull MT, et al. PTSD’s relation with problematic smartphone use: mediating role of impulsivity. Comput Human Behav 2017; 75: 177–183. [Google Scholar]
- 35.Thomée S, Härenstam A, Hagberg M. . Mobile phone use and stress, sleep disturbances, and symptoms of depression among young adults - a prospective cohort study. BMC Public Health 2011; 11(1): 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The National Institute for Health and Care Excellence (NICE). Post-traumatic stress disorder, NICE: London, 2005. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Efficacy of mobile application interventions for the treatment of post-traumatic stress disorder: A systematic review by Alice Wickersham, Petros Minas Petrides, Victoria Williamson and Daniel Leightley: the STRONG STAR Consortium in Digital Health