Abstract
Background:
With an expectation of excellent locoregional control, ongoing efforts to de-intensify therapy for patients with HPV associated squamous cell oropharyngeal cancer necessitate a better understanding of the metastatic risk for patients with this disease.
The objective was to determine what factors affect the risk of metastases in patients with squamous cell cancers of the oropharynx
Methods
Under a shared use agreement, 547 RTOG 0129 and 0522 patients with non. metastatic oropharyngeal squamous cell cancers who had known p16 status and smoking status were analyzed to assess the association of clinical features with the development of distant metastases. Factors analyzed included p16 status, gender, T stage, N Stage, age, and smoking history.
Results:
Multivariable analysis of 547 patients with a median follow-up of 4.6 years revealed that age ≥ 50 (HR=3.28, p=0.003), smoking > 0 pack-years (HR=3.09, p<0.001), N3 disease (HR=2.64, p<0.001), T4 disease (HR=1.63, p=0.030) and negative P16 status(HR=1.60, p=0.044) were all factors associated with an increased risk of distant disease.
Conclusions:
Age, smoking, N3 disease, T4 disease and negative p16 status were associated with the development of distant metastases in patients with squamous cell cancers of the oropharynx treated definitively with concurrent chemo-radiation.
Trial Registration:
See clinicaltrials.gov. RTOG 0129 was entitled Chemotherapy and Radiation Therapy With or Without Surgery in Treating Patients With Head and Neck Cancer, NCT00047008; RTOG 0522 was entitled Radiation Therapy and Cisplatin With or Without Cetuximab in Treating Patients With Stage III or Stage IV Head and Neck Cancer, NCT00265941;
Keywords: HPV, Distant Metastases, Oropharynx, Smoking, Age, Nodal Disease No Conflict of Interests
Precis:
After analyzing over 500 patients with squamous cell cancer of the oropharynx, smoking > 0 pack-years (p<0.001), N3 disease (p<0.001), age ≥ 50 (p=0.003), T4 disease (p=0.030) and negative P16 status (p=0.044) were associated with an increased risk of distant disease. Increased age and p16 were newly identified risk factors for the development of metastases in patients with squamous cell cancers of the oropharynx.
Introduction:
HPV-associated squamous cell cancer of the oropharynx(HPV+ORO) is associated with improved survival compared to classic squamous cell cancers of the oropharynx(HPV. ORO). The observed improvement in survival has been attributed to significantly higher rates of local and regional tumor control. In RTOG 0129, the 3.year rate of locoregional failure for patients with HPV-positive tumors was 13.6% vs. 35.1% for HPV-negative cancers(P<0.001)1. At 3 years, the distant metastatic rates for HPV+ORO were 8.7% versus 14.6% in HPV-negative patients (p=0.26). However, this does not tell the whole story. Subsequent studies have shown that both the pattern2, and pace3 of distant metastatic spread differ between the HPV-associated and HPV-negative cancers. The pattern may reflect more osseous and fewer pulmonary metastases in HPV-associated cancers2, while the time frame to the development of distant disease may extend to 5 years, instead of being more commonly confined to 2 years which is more typical for HPV-negative disease3. As health care providers seek to de-intensify therapy for patients with HPV+ORO, understanding the metastatic risk for these patients becomes more relevant. Our goal was utilize prospectively collected data to better understand the metastatic potential of HPV+ORO.
Methods:
Patient Selection
Under a shared use agreement, data from RTOG 0129 and RTOG 0522 were received from the NRG. Because the data were de-identified, our Institutional Review Board did not require a review of this effort. RTOG 0129 compared standard fractionation radiation versus accelerated fractionation with concurrent cisplatin (two cycles with accelerated fractionation and three cycles with standard fractionation). Inclusion criteria included AJCC 7th Edition4 Stage III or IV(except T1N+ or T2N1)patients who were undergoing definitive treatment for squamous cell cancers of the oral cavity, oropharynx, hypopharynx and larynx. RTOG 0522 tested whether adding cetuximab to the concurrent radiation-cisplatin platform improved progression-free survival (PFS)5. Inclusion stage criteria were identical and eligible primary sites were similar, but RTOG 0522 did not include patients with squamous cell cancers of the oral cavity.
For purposes of definition, if a patient had a positive p16 stain, the patient was considered to have HPV-associated disease.
Statistical Methods
Factors thought to possibly affect the rate of distant metastases and available in the dataset included p16 status, gender, T stage (AJCC 7th Edition), N Stage (AJCC 7th Edition), age, and smoking history3. Patients missing p16 status or smoking history were excluded from analysis. Gender, T stage, N stage, and age were available for all patients, and patient characteristics were compared across p16 status using chi. squared tests. Distant metastasis-free survival (DMFS) was defined as time from date of randomization to date of distant metastasis or death, where those who were alive without distant metastasis at last follow-up were censored at last follow-up date. For analysis, age was categorized as <45, 45–49, 50–54, 55–59, 60–64, 65–69, and ≥70 years. Those with 0 smoking pack-years were considered never smokers, those with >40 smoking pack-years were considered heavy smokers, and further cut points were explored at 10 and 20 smoking pack-years. Further groupings of age levels, smoking pack-year levels, as well as N-stage and T-stage levels were assessed using Kaplan. Meier plots and estimates of yearly DMFS. Survival analyses of DMFS were performed, where univariate Cox proportional hazards models were fit followed by a multivariable analysis using backward elimination with a p-value of 0.1 for removal criteria. As it is the primary predictor of interest, p16 status was fixed into the multivariable model. Univariate and multivariable Cox models were also generated for p16+ patients. Model assumptions were checked and verified. Statistical analyses were performed using SAS 9.4, and significance was assessed at the 0.05 level.
Results:
The studies identified 547 patients with non-metastatic oropharyngeal squamous cell cancers who had known p16 status and smoking status (See Figure 2 & Supplemental Table 1S). The mean follow-up was 4.8 years. The descriptive statistics are shown (Supplemental Table 2S). T stage, gender, and smoking history were significantly associated with p16 status (Supplemental Table 3S).
Figure 2:
Consort Diagram
Univariate analysis of DMFS found that age, smoking history, nodal burden, T stage and p16 status were significantly associated with DMFS (Table 1a). We then looked at each clinical factor by small bin to see if we could simplify the cut-points.
Table 1a -.
Distant metastasis-free survival – UV
| Distant metastasis-free survival (years) | ||||
|---|---|---|---|---|
| - | ||||
| Covariate | Level | N | Hazard Ratio (95% CI) | HR P-value |
| p16 status | Negative | 145 | 1.98 (1.27–3.09) | 0.002 |
| Positive | 402 | - | - | |
| Gender | Female | 68 | 0.81 (0.41–1.63) | 0.561 |
| Male | 479 | - | - | |
| T stage | T4 | 156 | 1.74 (1.06–2.87) | 0.029 |
| T3 | 173 | 0.95 (0.55–1.63) | 0.845 | |
| T2 | 218 | - | - | |
| N stage | N3 | 50 | 2.43 (0.88–6.69) | 0.086 |
| N2c | 139 | 1.12 (0.42–2.97) | 0.820 | |
| N2b | 199 | 1.14 (0.44–2.92) | 0.787 | |
| N2a | 63 | 0.74 (0.24–2.34) | 0.611 | |
| N1 | 61 | 0.54 (0.16–1.86) | 0.327 | |
| N0 | 35 | - | - | |
| Age | 70 and older | 24 | 2.38 (0.59–9.52) | 0.221 |
| 65–69 | 46 | 3.85 (1.25–11.83) | 0.018 | |
| 60–64 | 102 | 2.14 (0.72–6.32) | 0.169 | |
| 55–59 | 133 | 1.98 (0.68–5.74) | 0.210 | |
| 50–54 | 121 | 2.05 (0.70–5.96) | 0.189 | |
| 45–49 | 79 | 0.41 (0.09–1.85) | 0.248 | |
| 44 and younger | 42 | - | - | |
| Smoking history (pack-years) | >40 | 118 | 4.21 (2.07–8.56) | <.001 |
| >20, <=40 | 117 | 3.51 (1.70–7.24) | <.001 | |
| >10, <=20 | 59 | 3.88 (1.74–8.67) | <.001 | |
| >0, <=10 | 79 | 2.96 (1.34–6.51) | 0.007 | |
| 0 | 174 | - | - | |
There was a statistically significant association between increasing age in 5-year increments and DMFS from ≤ 44 y/o to ≥70 y/o (Supplemental Figure 1aS, log-rank p=0.0064). An age cut-point of ≥ 50 y/o was associated with significant separation between the DMFS curves ((log-rank p=0.0005), Figure 1a).
Figure 1a.
Distant Metastasis-Free Survival by Age, <50 y/o versus ≥ 50 y/oAgeNo. of Subject Event Censored Median Survival (95% CI) 1 Yr Survival 2 Yr Survival 3 Yr Survival 4 Yr Survival 5 Yr Survival<50 121 7 (6%) 114 (94%) NA (NA, NA) 98.3% (93.3%, 99.6%) 94.7% (88.5%, 97.6%) 93.7% (87.3%, 97.0%) 93.7% (87.3%, 97.0%) 93.7% (87.3%, 97.0%)>=50 426 78 (18%) 348 (82%) NA (NA, NA) 90.3% (86.9%, 92.8%) 83.6% (79.6%, 87.0%) 82.5% (78.4%, 86.0%) 80.5% (76.1%, 84.2%) 79.6% (75.0%, 83.4%)
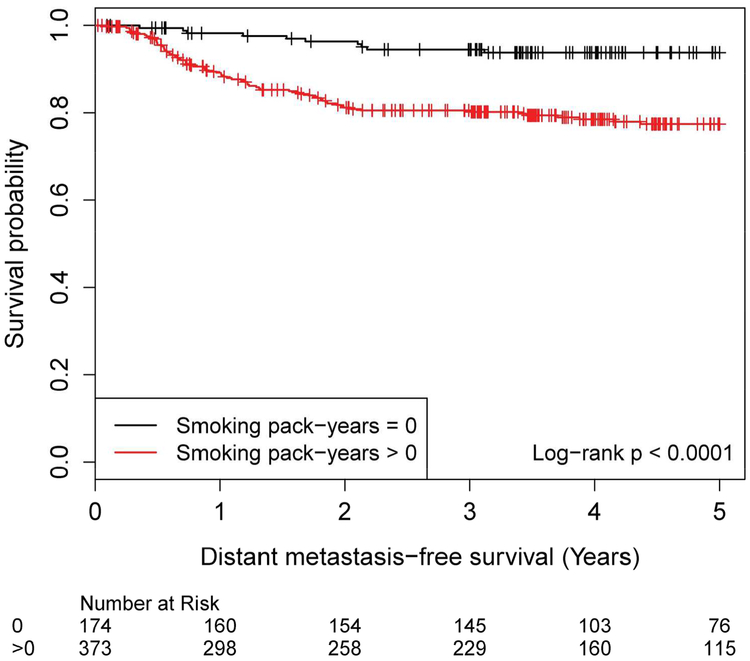
There was a statistically significant association between increasing smoking history and DMFS (Smoking history was evaluated using 5 bins with log-rank p=0.001 (Supplemental Figure 1bS). Patients with any smoking history (>0 pack years) had inferior DMFS compared to never smokers (0 pack years, log-rank p<0.001, Figure 1b).
Figure 1b.
Distant Metastasis-Free Survival by Smoking History, 0 versus >0 Pack Years Smoking history (pack-years) No. of Subject Event Censored Median Survival (95% CI) 1 Yr Survival 2 Yr Survival 3 Yr Survival 4 Yr Survival 5 Yr Survival 0 174 11 (6%) 163 (94%) NA (NA, NA) 98.2% (94.6%, 99.4%) 96.4% (92.1%, 98.3%) 94.5% (89.6%, 97.1%) 93.8% (88.7%, 96.6%) 93.8% (88.7%, 96.6%) >0 373 74 (20%) 299 (80%) NA (NA, NA) 89.1% (85.4%, 92.0%) 81.2% (76.6%, 85.0%) 80.5% (75.9%, 84.4%) 78.5% (73.6%, 82.6%) 77.4% (72.3%, 81.7%)
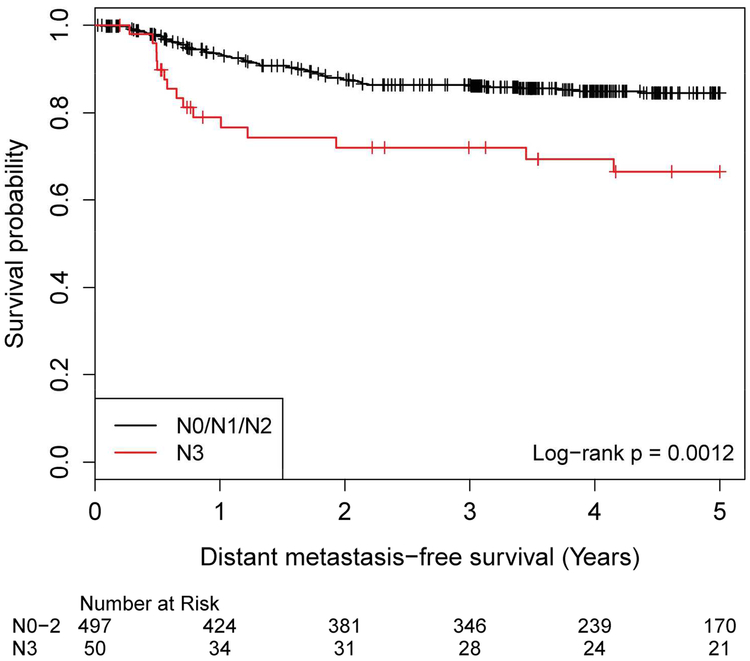
Nodal disease was investigated with 6 subgroups from the AJCC 7th edition. The Kaplan-Meier analysis showed N3 DMFS was clearly distinct from the other groups (Figure 1cS, log-rank p=0.018). Patients with N3 disease had worse DMFS compared to those with lesser nodal disease burden (Figure 1c, log-rank p=0.001).
Figure 1c.
Distant Metastasis-Free Survival by AJCC 7th Edition Nodal Staging, N0/N1/N2 versus N3 N stage No. of Subject Event Censored Median Survival (95% CI) 1 Yr Survival 2 Yr Survival 3 Yr Survival 4 Yr Survival 5 Yr Survival N0/N1/N2 497 70 (14%) 427 (86%) NA (NA, NA) 93.4% (90.8%, 95.3%) 87.6% (84.2%, 90.3%) 86.4% (82.9%, 89.2%) 84.9% (81.2%, 88.0%) 84.5% (80.8%, 87.6%) N3 50 15 (30%) 35 (70%) NA (NA, NA) 79.0% (64.4%, 88.1%) 72.0% (56.6%, 82.7%) 72.0% (56.6%, 82.7%) 69.4% (53.6%, 80.7%) 66.5% (50.3%, 78.4%)
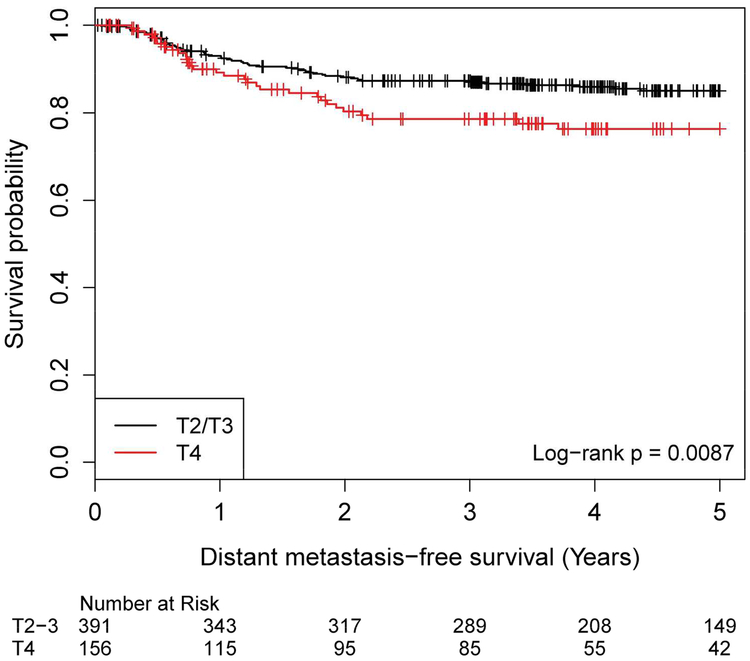
Tumor stage was evaluated from T2 to T4. Patients with T2 and T3 disease had virtually identical outcomes, superior to those with T4 disease (Figure 1dS, log-rank p=0.032). Those with T4 disease had significantly worse DMFS compared to those with T2/T3 disease (Figure 1d, log-rank p = 0.009).
Figure 1d.
Distant Metastasis-Free Survival by AJCC 7th Edition T Stage, T2/T2 versus T4 T stage No. of Subject Event Censored Median Survival (95% CI) 1 Yr Survival 2 Yr Survival 3 Yr Survival 4 Yr Survival 5 Yr Survival T2/T3 391 53 (14%) 338 (86%) NA (NA, NA) 93.1% (90.0%, 95.2%) 88.2% (84.4%, 91.1%) 87.3% (83.5%, 90.3%) 86.0% (81.9%, 89.2%) 85.1% (80.8%, 88.4%) T4 156 32 (21%) 124 (79%) NA (NA, NA) 89.3% (82.8%, 93.4%) 80.3% (72.4%, 86.2%) 78.6% (70.4%, 84.7%) 76.3% (67.7%, 82.9%) 76.3% (67.7%, 82.9%)
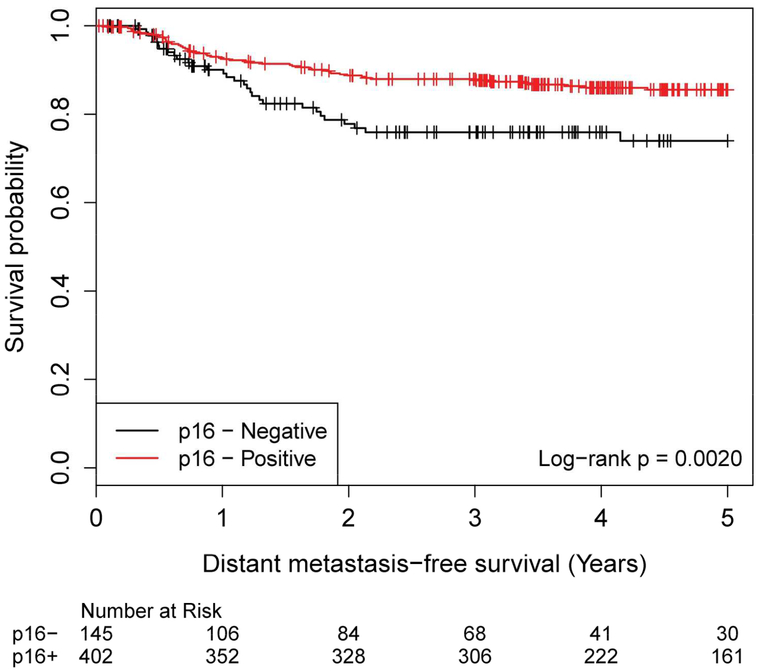
Patients with P16 negative disease had inferior DMFS compared to those with P16 positive disease, p=0.002 (Figure 1e).
Figure 1e.
Distant Metastasis-Free Survival by p16 Status, Negative versus Positive p16 status No. of Subject Event Censored Median Survival (95% CI) 1 Yr Survival 2 Yr Survival 3 Yr Survival 4 Yr Survival 5 Yr Survival Negative 145 31 (21%) 114 (79%) NA (NA, NA) 90.1% (83.5%, 94.1%) 77.8% (69.2%, 84.2%) 75.9% (67.2%, 82.7%) 75.9% (67.2%, 82.7%) 74.0% (64.5%, 81.3%) Positive 402 54 (13%) 348 (87%) NA (NA, NA) 92.8% (89.7%, 94.9%) 88.7% (85.1%, 91.5%) 87.9% (84.2%, 90.8%) 86.0% (82.0%, 89.1%) 85.5% (81.4%, 88.8%)
Using the groupings defined above, univariable analysis found that all variables except gender were significantly associated with DMFS (Table 1b). In multivariable analysis (Table 2), the following variables had a statistically significant association with worsening DMFS: age ≥50 vs. <50 (HR: 3.28, 95% CI: 1.51–7.11, p=0.003) smoking pack-years > 0 vs. 0 (HR: 3.09, 95% CI: 1.62–5.88, p<0.001), N3 vs. N0/N1/N2 (HR: 2.64, 95% CI: 1.50–4.65, p<0.001), T4 vs. T2/T3 disease (HR: 1.63, 95% CI: 1.05–2.54, p=0.030) and p16.negative status(HR: 1.60, 95% CI 1.01–2.53, p=0.044).
Table 1b -.
Distant metastasis-free survival – UV
| Distant metastasis-free survival (years) | ||||
|---|---|---|---|---|
| - | ||||
| Covariate | Level | N | Hazard Ratio (95% CI) | HR P-value |
| p16 status | Negative | 145 | 1.98 (1.27–3.09) | 0.002 |
| Positive | 402 | - | - | |
| Gender | Female | 68 | 0.81 (0.41–1.63) | 0.561 |
| Male | 479 | - | - | |
| T stage | T4 | 156 | 1.79 (1.15–2.77) | 0.010 |
| T2/T3 | 391 | - | - | |
| N stage | N3 | 50 | 2.45 (1.40–4.28) | 0.002 |
| N0/N1/N2 | 497 | - | - | |
| Age | >=50 | 426 | 3.59 (1.66–7.79) | 0.001 |
| <50 | 121 | - | - | |
| Smoking history (pack-years) | >0 | 373 | 3.65 (1.93–6.87) | <.001 |
| 0 | 174 | - | - | |
Table 2 -.
Distant metastasis-free survival – MV
| Distant metastasis-free survival (years) | |||
|---|---|---|---|
| - | |||
| Covariate | Level | Hazard Ratio | HR P-value |
| p16 status | Negative | 1.60 (1.01–2.53) | 0.044 |
| Positive | - | - | |
| T stage | T4 | 1.63 (1.05–2.54) | 0.030 |
| T2/T3 | - | - | |
| N stage | N3 | 2.64 (1.50–4.65) | <.001 |
| N0/N1/N2 | - | - | |
| Age | >=50 | 3.28 (1.51–7.11) | 0.003 |
| <50 | - | - | |
| Smoking history (pack-years) | >0 | 3.09 (1.62–5.88) | <.001 |
| 0 | - | - | |
Number of observations in the original data set = 547. Number of observations used = 547.
Backward selection with an alpha level of removal of 0.1 was used. The following variables were removed from the model: Gender.
In a subset analysis of patients with p16.positive disease, age, smoking and N3 disease were significantly associated with the development of distant metastases, but T4 status was not (Table 3).
Table 3 -.
Distant metastasis-free survival - MV - HPV-pos
| Distant metastasis-free survival (years) | |||
|---|---|---|---|
| - | |||
| Covariate | Level | Hazard Ratio | HR P-value |
| N stage | N3 | 2.18 (1.10–4.35) | 0.026 |
| N0/N1/N2 | - | - | |
| Age | >=50 | 2.75 (1.09–6.93) | 0.032 |
| <50 | - | - | |
| Smoking history (pack-years) | >0 | 3.73 (1.76–7.91) | <.001 |
| 0 | - | - | |
Number of observations in the original data set = 402. Number of observations used = 402.
Backward selection with an alpha level of removal of 0.1 was used. The following variables were removed from the model: Gender, and T stage.
Discussion:
In this study, increasing age was associated with a greater risk of distant metastases in oropharyngeal squamous cell carcinoma. The group from Princess Margaret, with limited numbers, had found age over 70 associated with inferior overall survival in +p16 patients with T4 or N3 disease6. Unlike our series, only 3 of the 27 patients in the risk group who were over 70 received chemotherapy, and the implication was that increased age was not as important to survival as the lack of chemotherapy for patients with T4 or N3 disease. Potential clinical explanations for the observation that increased age was associated with a greater risk of distant metastases include either a decreased tolerability or effectiveness of systemic therapy in eradicating micrometastatic disease in older patients. A meta-analysis has suggested a decreasing benefit of chemotherapy with increasing age for patients with non-metastatic head and neck squamous cell carcinoma7.
Long-term results of RTOG 91–11 found that deaths not attributed to larynx cancer or treatment were 30.8% in the concomitant cisplatin arm, 20.8% in the induction cisplatin flourouracil arm, and 16.9% in the radiation alone arm8. These “excess” deaths in the concomitant arm were observed beyond 4.5 years after treatment. Randomized data with follow-up beyond 5 years in patients with head and neck cancer is relatively scarce but Cooper et al have published long-term results of RTOG 9501 which tested the value of concurrent chemotherapy and radiation versus radiation alone in the post. operative setting9 Overall, for those patients whose deaths were not related to the study cancer, there were 22 more deaths in the concurrent cisplatin arm, suggesting that the excess non-study related cancer death rate was 11%(22 excess deaths divided by the 202 patients randomized to the concurrent arm). It is possible that in the +HPV population with relatively good long-term survival, the late mortality of concurrent cisplatin8–10 may outweigh its relatively modest gains in distant control.
Another potential explanation for an increasing risk of distant metastases is increasing immune senescence in the elderly. The mechanisms of the immune system in retarding the development of distant metastases have been described11. We note that because of the paucity of patients and events in our group of patients in the ≥ 70 y/o category, the data for this subgroup was not reliable.
Smoking history has previously been recognized as a risk factor for distant metastatic disease. Previous studies identified a cut-point at 10 pack-years3 or by dividing patients into never/former smokers versus current smokers12. Our own analysis found an association between increasing smoking history and DMFS, with a major distinction between never smokers and those with any smoking history. While one may hypothesize that temporally distant smoking may have less impact on DMFS than recent or continued smoking, we do not have data to evaluate this theory.
The association of N3 disease with worse DMFS in our analysis is consistent with the AJCC 8th edition of staging. In the clinical staging of +p16 associated oropharyngeal cancer, N3 remains lymph nodes > 6 cms, while the -p16 N3a clinical staging is > 6 cm without evidence of extranodal extension. The N3b category for clinically staging -p16 oropharyngeal cancers includes any nodal disease with extranodal extension and we regret that our data does not allow us to address the importance or non-importance of extranodal extension, particularly in the +p16 oropharyngeal cancer patients. T4 disease and N3 disease bring up the possible confounding variables of socioeconomic support. It is reasonable to suspect that patients presenting with a greater burden of disease are more likely to face socioeconomic challenges, such as limitations in access to care, health literacy, and comorbidities.
Examining a cohort of over 500 patients, we found that p16 negative disease was associated with a worse DMFS after accounting for age, smoking status, N stage, and T stage. In their prospectively collected dataset of 899 patients with oropharyngeal cancer treated from 2001 to 2009 at Princess Margaret Hospital3, 505 patients were analyzed with known p16 status, and +p16 patients were considered to have HPV associated disease. Patients with AJCC 7TH Edition Stage I and II patients were treated with radiation alone, while more advanced patients were treated with concurrent chemoradiation unless they were frail, >70 year of age, or declined chemotherapy. Forty-nine percent of HPV-associated oropharyngeal cancers received radiation therapy without systemic therapy. There were no significant differences in the distant failure rate between HPV-positive and HPV-negative patients which, at 3 years was 90% versus 86%, p=0.53. With a median follow-up of 3.9 years, there were not enough events for multivariate analysis of either local control, regional control or distant metastases. In a recursive partitioning analysis (RPA) stratified by HPV status, AJCC 7th edition T4 and N3 status were risk factors for development of distant metastases, similar to our own analysis. Potential explanations for the differences between our own findings and those observed in the Princess Margaret analysis include the differences in patient population (our own analysis includes only patients with more advanced disease) and our uniform use of chemotherapy. Since the Princess Margaret data suggested that distant failures continued to manifest through 5 years after treatment in the HPV-associated cancer group, the longer follow-up in our series would tend to make the risk of distant failure in the HPV-associated group higher.
A recent analysis of predictors of distant disease from Cleveland Clinic included 310 HPV+ORO patients with AJCC 7th Edition Stage III to IVb disease who were treated with either cisplatin-based chemoradiation or cetuximab with concurrent external radiation13. Determination of HPV status was by either in situ hybridization for HPV DNA or p16 staining. In a series containing only patients with HPV+ORO patients, it was not possible to examine the relevance of viral associated disease to the risk of distant metastases. Multivariate analysis suggested cetuximab as opposed to cisplatin-based therapy was associated with increased metastases, as were current smoker and T4 disease status. Could cisplatin be more effective in preventing distant disease among patients with HPV+ORO cancers than it is for other head and neck cancers? Univariate analysis had found that neither pack-years smoking on a continuous variable status or pack-years using 20 pack-years as a cut-off were not quite statistically significant (p=0.0751 and 0.0635 respectively) with regard to distant metastases. AJCC 7th edition nodal disease was not significantly associated with increased risk of distant metastases. The Cleveland Clinic data also showed that distant disease in the HPV-associated oropharyngeal cancer population continued to manifest through approximately 5 years from treatment. The median follow-up for this series was 44 months.
Because all of our patients received systemic therapy, our data may not reflect the true “natural” history of distant metastases in this population. Limitations of our own study include the fact that our data does not allow us to explore the importance of the interval since smoking, or the effects of current smoking or even the potential resumption of smoking after treatment. We also cannot ascertain the importance of radiologically detected or pathologic extranodal extension. Concurrent cisplatin-5FU may have some limited effect on distant control, at least in head and neck patients studied prior to the HPV era with a meta-analysis reporting absolute differences in metastatic rates at 5 years ± sd of −2.9 ± 2.7 %7. RTOG 0129 and RTOG 0522 did not require PET scanning and did not include AJCC 7th edition T1NanyM0 or T2N1M0 patients.
The importance of T4 disease seems consistent across our study as well as the studies from Princess Margaret and Cleveland clinic. It is unclear how to reconcile the disparate findings from our present study and the findings from Princess Margaret and Cleveland Clinic. Owing to the higher number of distant metastases in our study (85 versus 31 for Cleveland Clinic and 45 in the Princess Margaret series), the present study was better powered to detect an association between p16 status and the risk of distant disease. The differences in results between the studies may also be attributable to the somewhat longer follow-up in the present study. All three studies involved retrospective analysis of prospectively collected or registry data and analyzed a fairly large number of patients, but varied in the patient population, treatment delivered, and in the structure of the analysis. Despite these acknowledged limitations, it is imperative that we seek further insight into which patients are at greatest risk of distant metastases as treatment algorithms are redefined in the era of HPV-associated oropharyngeal cancers.
Conclusions:
Analysis of 547 patients with non-metastatic oropharyngeal squamous cell cancers treated in RTOG 0129 and RTOG 0522 identified a higher rate of distant metastatic disease in those patients with any smoking history, age ≥50 years, N3 disease, T4 disease, and p16 negative disease.
Supplementary Material
Research Support:
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller S, Khuri FR, Kono SA, Beitler JJ, Shin DM, Saba NF. HPV positive squamous cell carcinoma of the oropharynx. Are we observing an unusual pattern of metastases? Head Neck Pathol. 2012;6: 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31: 543–550. [DOI] [PubMed] [Google Scholar]
- 4.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Handbook: Springer, 2010. [Google Scholar]
- 5.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32: 2940–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang SH, Xu W, Waldron J, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM Stage and Prognostic Groups for Human Papillomavirus-Related Oropharyngeal Carcinomas. J Clin Oncol. 2015;33: 836–845. [DOI] [PubMed] [Google Scholar]
- 7.Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92: 4–14. [DOI] [PubMed] [Google Scholar]
- 8.Forastiere AA, Zhang Q, Weber RS, et al. Long-term results of RTOG 91–11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper JS, Zhang Q, Pajak TF, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84: 1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groot HJ, Lubberts S, de Wit R, et al. Risk of Solid Cancer After Treatment of Testicular Germ Cell Cancer in the Platinum Era. J Clin Oncol. 2018;36: 2504–2513. [DOI] [PubMed] [Google Scholar]
- 11.Janssen LME, Ramsay EE, Logsdon CD, Overwijk WW. The immune system in cancer metastasis: friend or foe? J Immunother Cancer. 2017;5: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liskamp CP, Janssens GO, Bussink J, Melchers WJ, Kaanders JH, Verhoef CG. Adverse effect of smoking on prognosis in human papillomavirus-associated oropharyngeal carcinoma. Head Neck. 2016;38: 1780–1787. [DOI] [PubMed] [Google Scholar]
- 13.Weller MA, Ward MC, Berriochoa C, et al. Predictors of distant metastasis in human papillomavirus-associated oropharyngeal cancer. Head Neck. 2017;39: 940–946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.