Abstract
Objective:
To determine whether antenatal depression is associated with oxidative stress and inflammation, and secondarily, whether the association between antenatal depression and spontaneous preterm birth (SPTB) is mediated by these biomarkers.
Study design:
The primary outcome included urine oxidative stress biomarkers 8-hydroxydeoxyguanosine(8-OHdG) and 8-isoprostane and plasma inflammatory biomarkers measured at 10, 18, and 26 weeks and averaged within individual. Linear and logistic regression models were used, adjusting for age, race, parity, and pre-pregnancy body mass index.
Results:
Among 462 women, 8-isoprostane was higher among depressed women (geometric mean: 299.96 pg/mL vs. 237.01 pg/mL;p=0.001). In multivariable analyses, antenatal depression was significantly associated with an increase in average 8-isoprostane (β: 0.25; 95%CI: 0.05-0.44;p=0.01). The association of antenatal depression with SPTB was partially mediated by 8-isoprostane. Antenatal depression was not associated with 8-OHdG or inflammatory biomarkers.
Conclusions:
Antenatal depression was associated with higher oxidative stress across pregnancy, namely 8-isoprostane, and may impact SPTB via oxidative stress.
Keywords: inflammation, oxidative stress, pregnancy, depression, preterm birth
INTRODUCTION
Preterm birth (PTB) is a significant public health problem, impacting 1 out of every 10 births in the US, and >70% of these PTBs follow spontaneous preterm labor or preterm premature rupture of membranes (PPROM), which are together designated as spontaneous PTB (SPTB) (1). The underlying mechanisms leading to SPTB remain poorly understood, and oxidative stress and inflammation as well as antenatal depression have been implicated as possible risk factors (2–5). Outside of pregnancy, raised inflammatory markers have been associated with maternal depressive symptoms (6, 7). In pregnancy, some studies have suggested an association between inflammatory markers and antenatal depression (8–14), but this finding has not been universally observed (15–17). Additionally, depressed pregnant women treated with an antidepressant may have lower level of inflammatory cytokines (12, 18). Importantly, the association between oxidative stress and antenatal depression remains to be characterized.
A number of biomarkers with known physiologic effects exist to measure both oxidative stress and inflammation (19, 20). Pro-inflammatory cytokines are some of the most well-studied markers associated with PTB, including interleukin-6 (IL-6) (21, 22), interleukin-10 (IL-10) (23), and C-reactive protein (CRP). Oxidative stress, defined as an imbalance between antioxidant capacity and reactive oxygen species (ROS) generation, has received less attention, but has been implicated as playing a role in PTB (24). 8-isoprostane is a useful biomarker of oxidative stress due to its stability, sensitivity to oxidant injury, and specificity (25). 8-hydroxydeoxyguanosine (8-OHdG), an oxidized nucleoside that is released in the repair of damaged DNA, is also a commonly used marker of oxidative stress (26).
In prior analyses, we found that oxidative stress, particularly 8-isoprotane, and inflammatory cytokine biomarkers were associated to SPTB across pregnancy (24, 27) and a shortened cervical length (28). Additionally, we have found that antenatal depression was associated with SPTB (29). Unanswered questions that remain include: whether oxidative stress is associated with antenatal depression; and whether the association between antenatal depression and SPTB is mediated by oxidative stress and inflammation. A better understanding of the associations between antenatal depression, oxidative stress and inflammation, and PTB, can help address clinical barriers to depression care in pregnancy, including a lack of biomarkers, difficulty in making a diagnosis, and reluctance to use psychotropic medications.
We hypothesized that 1) oxidative stress and inflammation are associated with antenatal depression, and 2) the mechanism by which antenatal depression affects SPTB could be via altering levels of oxidative stress and inflammation. The primary aim of the current study was to determine whether antenatal depression was associated with two biomarkers of oxidative stress, 8-OHdG and 8-Isoprostane, and five biomarkers of inflammation, including IL-6, IL-10, interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), and CRP, collected across pregnancy. The secondary aim was to assess whether the association between antenatal depression and SPTB was mediated by those biomarkers found to be significant in the primary aim.
MATERIAL AND METHODS
Study population:
The current study was a secondary analysis of a case-control study of PTB nested within a prospective cohort study (LIFECODES birth cohort) at Brigham and Women’s Hospital (Boston, MA) from 2006 to 2008 (30). Briefly, in the nested case-control study, we selected 130 cases of preterm birth from the birth cohort as well as 352 randomly selected singleton term controls (N=352) (30). Similar to prior analyses from this dataset investigating secondary research questions, our goal was to characterize the association between biomarkers of oxidative stress and inflammation and antenatal depression in a population that would be generalizable to the original cohort. Thus, we utilized inverse probability weightings for the primary aim when the study outcomes were oxidative stress and inflammatory biomarkers, which were created from the probability of selection from the parent study population for cases (90.1%) and controls (33.9%) (28, 31). This adjustment made these results more generalizable to pregnant women in the base cohort population (32). All enrolled women provided written informed consent. The study was approved by the Institutional Review Board at Brigham and Women’s Hospital and the University of Michigan.
During the first trimester (median 10 weeks gestation), enrolled women completed a demographic questionnaire, as well as supplied urine and plasma for biomarker analysis. Participants also provided urine and plasma samples at follow-up visits (median 18 and 26 weeks weeks gestation). Results of oxidative stress and inflammatory biomarkers at all time points in relation to PTB have been presented elsewhere (24, 27). Specimen collection at approximately 10, 18, and 26 weeks gestation were scheduled for each trimester coinciding with scheduled prenatal labs when possible, and were not related to the current secondary analysis.
Study variables:
The following demographic variables were assessed at enrollment, including: age, race/ethnicity, parity, education, insurance status, medical comorbidities, and pre-pregnancy body mass index (BMI) (calculated by dividing a subject’s weight by the square of her height, kg/m2). A history of prior PTB included any past delivery, regardless of clinical indication, between >20 and <37 weeks gestation. Gestational age was defined by the best obstetrical estimate, including last menstrual period (LMP) and early ultrasound dating.
The primary exposure was antenatal depression, defined as a clinical diagnosis of depression at anytime in pregnancy documented by the obstetric or the primary care provider in the electronic medical record (EMR) of the study institution. The following depression-related variables were also collected: exposure to an antidepressant medication during pregnancy, including selective serotonin reuptake inhibitor (SSRI), benzodiazepine, antipsychotic, mood stabilizer, or other psychotropic medication, and a history of depression before pregnancy. A study obstetrician (KKV) retrospectively reviewed the medical record of each participant, including both obstetrical visits as well as visits with other healthcare providers, including mental health and primary care, to identify a diagnosis of antenatal depression and use of a anti-depressive medication. The study obstetrician was blinded to outcomes of oxidative stress and inflammation when reviewing each medical record. With regards to antidepressant medication use in pregnancy, we compared women with antenatal depression exposed to an antidepressant and women with antenatal depression not exposed to an antidepressant to women without antenatal depression (i.e. 3-level variable).
For the primary aim, the outcome was the mean difference in the subject-specific geometric average (hereafter referred to as average) of oxidative stress biomarkers, including 8-OHdG and 8-Isoprostane, and inflammation biomarkers, including IL-6, IL-10, CRP, IL-1β, and TNF-α, from measurements available from three visits (median 10, 18, and 26 weeks gestation). Oxidative stress biomarkers were corrected for urinary specific gravity across the three study visits.
For the secondary aim, the outcome was SPTB compared to no PTB. All PTB cases, defined as <37 weeks of gestation, were independently reviewed and validated by two board certified Maternal Fetal Medicine physicians. When disagreement in pregnancy outcome or characteristic arose, the case was re-reviewed and a consensus conference held to determine the final characterization. SPTB, often characterized by intrauterine or decidual inflammation, was defined as birth <37 weeks due to PPROM or spontaneous labor (33). Two further classifications of PTB were also assessed: medically indicated PTB (MPTB) due to preeclampsia or fetal growth restriction and PTB due to another maternal of fetal indication, both of which were excluded when the outcome was SPTB in the secondary aim.
Oxidative stress and inflammatory biomarker analysis:
Details about how oxidative stress and inflammatory biomarkers were measured and analyzed are described in detail in prior analyses (24, 27). Briefly, plasma samples were analyzed for inflammatory biomarkers at the University of Michigan Cancer Center Immunological Monitoring Core (Ann Arbor, MI). Cytokines were analyzed using Milliplex MAP High Sensitivity Human Cytokine Magnetic Bead Panel (EMD Millipore Corp., St. Charles, MO), and for individual measures below the limit of detection (LOD) (0.128 pg/mL for all cytokines), values reported numerically were kept as is. CRP was measured using a DuoSet enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) and the lower LOD was 10 pg/mL and upper LOD was 100 pg/mL.
For oxidative stress markers, urine samples were stored at −80C after collection until measurement. Both 8-OHdG and 8-isoprostane were measured by Cayman Chemical (Ann Arbor, MI). For total 8-isoprostane, urine samples were hydrolyzed to deconjugate 8-isoprostane esterified to phospholipids and were passed through affinity columns for purification. Eluted samples were then dried and resuspended in a buffer before measurement with enzyme immunoassay (EIA)(24). The LOD was 3.9 pg/mL. For 8-OHdG, samples were diluted directly into buffer without purification, and concentrations were measured by EIA. The LOD was 10.3 pg/mL. Consistent with prior analyses, to account for urine dilution, specific gravity was measured in urine samples with a digital handheld refractometer (Atago Co, Ltd, Tokyo, Japan). Concentrations of oxidative stress markers were corrected for specific gravity with the following formula: OSC = OS([1.015 – 1]/[SG – 1]) (34). OSc represents the corrected biomarker concentration; OS is the uncorrected urinary concentration; 1.015 is the median specific gravity in all samples; and SG is the specific gravity of the sample.
Statistical analysis:
Distributions for both inflammation and oxidative stress biomarkers were ln-transformed (i.e. natural log transformed) for data analyses. For the primary aim, we used linear regression models to determine whether antenatal depression was associated with the average concentration of serum inflammatory and urine specific gravity adjusted oxidative stress biomarkers. Covariates were selected a priori based on a directed acyclic graph of variables related to depression, oxidative stress/inflammation, and PTB (Table 1). All multivariable models adjusted for the following covariates: age, race, parity, and pre-pregnancy BMI. As described above, we utilized inverse probability weightings for the primary aim, which were created from the probability of selection from the parent study population when presenting frequency distributions and conducting regression analyses.
Table 1.
Conceptual model for primary analysis (total effect) and secondary analysis (mediated effect)
| Analysis | Covariates (V) | Exposure (A) | Mediator (M) | Outcome (Y) |
|---|---|---|---|---|
| Primary analysis (total effect) | Age Race Parity Body mass index |
Antenatal depression | Oxidative stress biomarkers, specific gravity adjusted: 8-isoprostane 8-OHdG Inflammatory biomarkers: IL-6 IL-10 IL-1β TNF-α CRP |
|
| Secondary analysis (mediated effect) | Age Race Parity Body mass index |
Antenatal depression | 8-isoprostane | SPTB |
For the secondary aim assessing for mediation of 8-isoprostane (i.e. mediating variable) between antenatal depression (i.e. exposure) and SPTB (i.e. outcome), we utilized multivariable logistic regression models, adjusting for the same covariates as above. Given the outcome for the secondary aim was SPTB, women with PTB for another indication (including MPTB or PTB for another maternal or fetal indication) were excluded. We assessed mediation of 8-isoprostane because this was the biomarker that was identified to be significantly associated with antenatal depression in the primary aim, and with SPTB in our previous analysis (24). This multivariable analysis was conducted sequentially as follows: 1) association between antenatal depression and 8-isoprostane, 2) association between 8-isoprostane and SPTB (previously published as a conference abstract) (24), 3) association between antenatal depression and SPTB (previously published) (29), and 4) association of both antenatal depression and 8-isoprostance as covariates and SPTB (35, 36). Within the causal framework, we investigated the natural direct effect (primary aim) and the natural indirect or mediated effect (secondary aim), as illustrated in Table 1 (37). We performed 2 tests to assess the significance of the mediation or indirect effect of 8-isoprostane, namely 1) the Sobel test, which determined whether the reduction in the effect of antenatal depression on SPTB after including 8-isoprostane was significant (i.e. whether the mediation effect was significant); and 2) bootstrapping over 1,000 iterations to assess the mediation effect of 8-isoprostane in a logistic regression model between antenatal depression and SPTB (STATA command “ldecomp”) (38, 39). All analyses were performed using STATA (STATACORP, version 10.0, College Station, TX).
RESULTS
Among the 482 women included in the original case-control study of PTB, 462 (96%) women had an available EMR for abstraction of antenatal depression on oxidative stress and inflammatory biomarkers. This analysis is conducted among these 462 women. There were no statistically significant differences (p<0.05) between women in the current analysis (n=462) compared to excluded women (n=20) by maternal age, race, parity, or gestational age at delivery (data not shown).
Among the 462 pregnant women observed, the mean age was 32.1 years (SD 5.49), less than half were white (40.7%), and most were multiparous (55.4%). In table 2, we present weighted percentages for all frequencies. Seventy-three (15.8%; 73/462) women had antenatal depression of whom 48 (64.3%; 48/72) were exposed to an antidepressant medication during pregnancy. Sixty-three women (11.7%) reported a prior history of depression before pregnancy.
Table 2.
Maternal characteristics overall and by antenatal depression status by weighted percentages
| Overall N=462 N (%) |
Antenatal depression N=72 N (%) |
No antenatal depression N=390 N (%) |
p-value | |
|---|---|---|---|---|
| Maternal age <25 years | 52/462 (11.9) | 9/72 (15.7) | 43/390 (11.3) | 0.71 |
| Non-white race | 191/462 (40.7) | 23/72 (33.8) | 168/390 (41.8) | 0.07 |
| Parity ≥1 | 258/462 (55.4) | 44/72 (61.5) | 214/390 (54.4) | 0.32 |
| College graduate | 179/453 (39.5) | 16/71 (22.5) | 163/382 (42.7) | 0.001 |
| Public insurance | 90/452 (19.9) | 23/70 (32.9) | 67/382 (17.5) | 0.003 |
| Obese (Body mass index>30 kg/m2) | 87/454 (18.1) | 24/71 (34.8) | 63/383 (15.4) | 0.001 |
| History of PTB <37 weeks among parous women | 50/251 (19.9) | 13/43 (30.2) | 37/208 (17.8) | 0.06 |
| PTB<37 weeks in current pregnancy | 125/461 (12.2) | 30/72 (21.0) | 95/389 (10.8) | 0.002 |
| SPTB <37 weeks in current pregnancy | 69/461 (6.7) | 16/72 (11.2) | 53/389 (6.0) | 0.06 |
| History of depression before pregnancy | 63/462 (11.7) | 36/72 (47.6) | 27/390 (5.9) | <0.001 |
| Antidepressant medication use during current pregnancy | 48/462 (8.9) | 48/72 (64.3) | -- | -- |
We present weighted percentages for all frequencies based on inverse probability weighting (i.e. the probability of selection from the parent study) as outlined in the methods.
Student’s t-test was used to compare continuous variables and chi-squared test for categorical variables by depression status.
Consistent with the original case-control study, 125 women (weighted %=12.2; 125/461) had a PTB <37 weeks. Close to a tenth of women (9.1%; 55/450) reported a history of PTB in a prior pregnancy. Sixty-nine women (7.1%; 69/406) had a SPTB, which was 2 times more frequent among women with antenatal depression compared to those without (12.4 vs. 6.3%; odd ratio, OR: 2.11 [95% CI: 1.10 – 4.04]; p=0.02), which we have previously published (29).
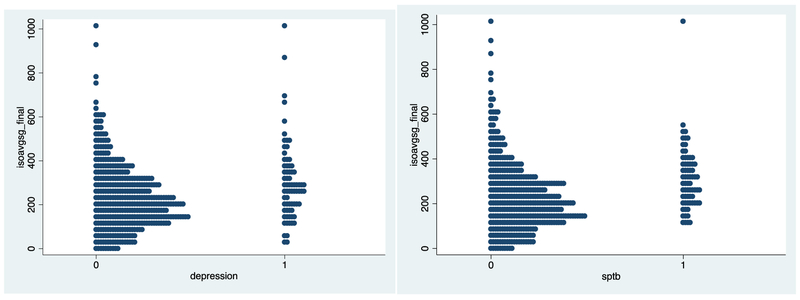
Women with antenatal depression had significantly higher levels of specific gravity corrected 8-isoprostane compared to those without antenatal depression (geometric mean: 299.96 pg/mL vs. 237.01 pg/mL; p=0.001) (Table 3). Figure 1 presents a scatter plot of average 8-isoprostane levels by antenatal depression and SPTB status. With regards to the impact of antidepressant exposure, women with antenatal depression exposed to antidepressants during pregnancy (geometric mean: 268.74 pg/mL) had lower levels of 8-isoprostane compared to women with antenatal depression not exposed to antidepressants (geometric mean: 362.40 pg/mL) (p=0.03), but both groups with antenatal depression had higher 8-isoprostane levels compared to women without antenatal depression (geometric mean: 237.01 pg/mL; ANOVA p=0.02). Although not statistically significant, 8-OHDG as well as the inflammatory biomarker CRP were higher among women with antenatal depression compared to those without.
Table 3.
Distribution of specific gravity corrected oxidative stress and inflammatory biomarkers overall and by antenatal depression status
| Biomarkers | Overall geometric mean (SD) |
Geometric mean by antenatal depression status |
p-value | |
|---|---|---|---|---|
| Antenatal depression (N=72) |
No antenatal depression (N=390) |
|||
| Oxidative stress markers | ||||
| 8-Isoprostane (pg/mL) (n=462) | 246.82 (156.72) | 299.96 | 237.01 | 0.001 |
| 8-OHDG (ng/mL) (n=462) | 127.50 (48.75) | 132.66 | 126.54 | 0.32 |
| Inflammatory markers | ||||
| IL-6 (pg/mL) (n=460) | 5.21 (27.80) | 5.20 (17.66) | 5.22 (29.29) | 0.99 |
| IL-10 (pg/mL) (n=460) | 30.23 (117.72) | 32.40 (114.96) | 29.83 (118.36) | 0.86 |
| CRP (ug/mL) (n=460) | 9.41 (10.10) | 11.13 (11.27) | 9.10 (9.85) | 0.11 |
| IL-1β (pg/mL) (n=460) | 1.07 (4.76) | 0.73 (1.64) | 1.13 (5.12) | 0.51 |
| TNF-α (pg/mL) (n=460) | 1.09 (0.57) | 1.11 (0.52) | 1.09 (0.58) | 0.76 |
Figure 1.
Scatter plot of average oxidative stress biomarker 8-isoprostane (1A) by antenatal depression and SPTB (1B)
We present unadjusted and adjusted analyses of the association between antenatal depression in association with the geometric average of oxidative stress and inflammatory biomarkers in Table 4. In adjusted analyses, antenatal depression was significantly associated with an increase in average 8-isoprostane levels (β: 0.25; 95% CI: 0.05–0.44, p=0.01). Antenatal depression was not associated with 8-OHdG nor biomarkers of inflammation. In table 5, we present the adjusted analyses of the association between antenatal depression and each of the biomarkers separately at 10, 18, and 26 weeks gestation, in which antenatal depression was significantly associated with the oxidative stress marker 8- OHdG at 18 weeks gestation only.
Table 4.
Unadjusted and adjusted analyses of the association between antenatal depression with average oxidative stress and inflammation biomarkers across pregnancy
| Unadjusted analysis β (95% CI); p-value |
Adjusted analysis* β (95% CI); p-value |
|
|---|---|---|
| Oxidative stress biomarkers | ||
| 8-Isoprostane | 0.32 (0.12, 0.52); 0.001 | 0.25 (0.05, 0.44); 0.01 |
| 8-OHdG | 0.04 (−0.06, 0.15); 0.42 | 0.015 (−0.09, 0.13); 0.79 |
| Inflammatory biomarkers | ||
| IL-6 | 0.13 (−0.17, 0.44); 0.39 | 0.08 (−0.24, 0.41); 0.61 |
| IL-10 | 0.03 (−0.21, 0.28); 0.78 | 0.06 (−0.20, 0.33); 0.64 |
| CRP | 0.21 (−0.02, 0.44); 0.07 | 0.06 (−0.15, 0.29); 0.55 |
| IL-1β | 0.07 (−0.24, 0.39); 0.64 | 0.08 (−0.24, 0.41); 0.63 |
| TNF-α | 0.01 (−0.12, 0.15); 0.81 | −0.01 (−0.16, 0.13); 0.82 |
Bolded results reflect statistically significant finding (p<0.05).
Models adjusted for: age, race, parity, and pre-pregnancy body mass index. Oxidative stress biomarkers were specific gravity adjusted values.
Models adjusted with inverse probability weightings.
Table 5.
Visit-specific adjusted analyses of the association between antenatal depression and oxidative stress and inflammation biomarkers at 10, 18, and 26 weeks gestation
| 10 weeks gestation Adjusted analysis* β (95% CI); p-value |
18 weeks gestation Adjusted analysis* β (95% CI); p-value |
26 weeks gestation Adjusted analysis* β (95% CI); p-value |
|
|---|---|---|---|
| Oxidative stress biomarkers | |||
| 8-Isoprostane | 0.26 (0.02, 0.50); 0.03 | 0.12 (0.17, 0.41); 0.42 | 0.26 (0.03, 0.49); 0.02 |
| 8-OHdG | −0.01 (−0.23, 0.21); 0.96 | 0.10 (0.00, 0.21); 0.05 | −0.04 (−0.26, 0.18); 0.70 |
| Inflammatory biomarkers | |||
| IL-6 | −0.07 (0.43, 0.27); 0.66 | 0.03 (−0.38, 0.44); 0.87 | 0.03 (−0.27, 0.35); 0.80 |
| IL-10 | −0.03 (−0.32, 0.26); 0.82 | 0.19 (−0.15, 0.53); 0.28 | −0.01 (−0.24, 0.22); 0.90 |
| CRP | 0.13 (−0.18, 0.46); 0.39 | 0.05 (−0.18, 0.29); 0.64 | −0.03 (−0.31, 0.24); 0.81 |
| IL-1β | −0.07 (−0.40, 0.25); 0.64 | 0.12 (−0.26, 0.51); 0.52 | 0.22 (−0.15, 0.60); 0.23 |
| TNF-α | −0.06 (−0.20, 0.08); 0.40 | 0.02 (−0.14, 0.19); 0.75 | −0.01 (−0.18, 0.17); 0.95 |
Bolded results reflect statistically significant finding (p<0.05).
Models adjusted for: age, race, parity, and pre-pregnancy body mass index. Oxidative stress biomarkers were specific gravity adjusted values.
Models adjusted with inverse probability weightings.
The association between antenatal depression and average 8-isoprostane levels persisted regardless of inverse probability weighting (β: 0.23; 95% CI: 0.02–0.44, p=0.02), as well as further adjustment for a history of prior PTB (β: 0.20; 95% CI: 0.01–0.41, p=0.04). To assess the response to anti-depressant exposure, we created a three-level exposure (no depression compared to depression without medication and depression with medication) in which the association between depression without medication and 8-isoprostane was no longer significant (p=0.23), but a trend was still observed for depression with medication and 8-isoprostane compared to no depression (p=0.06).
Given the significant association between antenatal depression and increasing 8-isoprostane, we sought to determine whether 8-isoprostane mediated the association between antenatal depression and SPTB after excluding 55 women with MPTB or PTB for other indications that we previously observed (29). To do so, we recreated models of the association between 8-isoprostane and SPTB and between antenatal depression and SPTB that were examined in our previous work (24, 29). Models adjusting for the same covariates as above were constructed so that they used a consistent sample (i.e., those with antenatal depression information as well as biomarker measurements). In these adjusted models, 8-isoprostane was significantly associated with SPTB (AOR: 3.85, 95% CI: 2.22–6.67, p<0.001). Antenatal depression was significantly associated with SPTB (AOR: 2.09, 95% CI: 1.09–4.03, p=0.02). The association between 8-isoprostane and antenatal depression with SPTB were both attenuated when both covariates were analyzed in the same regression model, suggesting partial mediation (AOR for 8-isoprostane: 3.72, 95% CI: 2.14–6.46, p<0.001; and AOR for antenatal depression: 1.68, 95% CI: 0.85–3.34, p=0.13). Stated differently, the effect estimate for the association between antenatal depression and SPTB with the inclusion of 8-isoprostane in the model changed from an AOR of 2.09 to 1.68, and was no longer statistically significant. The above steps suggested that 8-isoprostane partially mediated the relationship between antenatal depression and SPTB. Using bootstrapping over 1,000 iterations to separate out the mediating effect of 8-isoprostane in the logistic regression model above of antenatal depression and SPTB, 27% of the effect of antenatal depression on SPTB was explained by 8-isoprostane. A p-value of 0.07 was calculated based on assessing the statistical significance of the mediation effect by the Sobel test.
DISCUSSION
Antenatal depression was significantly associated with higher levels of the oxidative stress biomarker 8-isoprostane across pregnancy. Additionally, the association between antenatal depression and SPTB also showed statistical mediation by 8-isoprostane. These data suggest that antenatal depression may at least partially contribute to SPTB via oxidative stress.
To our knowledge, this is the first study to document an association between antenatal depression and oxidative stress. After adjusting for multiple covariates, we found a robust association between antenatal depression and the urinary oxidative stress biomarker 8-isoprostane, but notably we did not find an association with plasma inflammatory biomarkers, including IL10, IL6, CRP, IL-1β, and TNF-α, and the urinary oxidative stress biomarker 8-OHdG. Women with antenatal depression exposed to an antidepressant had lower levels of 8-isoprostane compared to women with antenatal depression not exposed to an antidepressant as well as women without antenatal depression. Prior data have suggested that pregnant women with depression on antidepressants have lower levels of inflammation, including IL10, IL6, IL-1β, and TNF-α (12, 17, 40). Prior studies have been inconsistent with regards to an association between plasma inflammatory markers and antenatal depression with some investigators noting an association (8–14), while others have not (15–17). Given the relatively high proportion of anti-depressant use among women with depression in the current study (>50%), it is possible that some women with depression were undiagnosed, their diagnosis was not documented in the medical record, or they were managed by psychotherapy. It is possible that inclusion of women with less severe depression may have biased these findings toward the null. Differences in results across studies may be due to the relatively small sample sizes (on average 50–100 women), heterogeneity in study design, serum assay techniques, collection of varying inflammatory markers, gestational age at collection, and patient populations studied, as well as timing and varying assessment of antenatal depression.
Given the association in the current study between antenatal depression and 8-isoprostane, and prior analyses from this dataset demonstrating an association between 8-isoprostane and SPTB (31) as well as antenatal depression and SPTB (29), we found that the relationship between antenatal depression and SPTB was at least partially mediated by 8-isoprostane. A prior study found that the relationship between antenatal depression and adverse pregnancy outcomes, including PTB, growth restriction, fetal demise, and hypertensive disorders of pregnancy, was not mediated by the inflammatory cytokine IL-6 despite an association between depression and IL-6 (14). The current study was conducted before the provision of progesterone to women at high-risk for SPTB, and it is possible that therapeutic progesterone exposure may alter the association between antenatal depression, oxidative stress, and SPTB, which will need to be investigated in future studies. Though the mechanism underlying the connection between antenatal depression and SPTB is likely multifactorial and remains to be defined, these results suggest one possible pathway may be via oxidative stress that warrants further study.
This study must be interpreted within the context of its design. This is a secondary analysis and diagnosis and treatment of antenatal depression was assessed by retrospective chart review, and before routine screening for depression using the Edinburgh Postnatal Depression Scale (EPDS) was implemented in Massachusetts, which currently is universally documented in the medical record (41). We only included clinic visits within our institution’s healthcare network, which included the patient’s primary obstetrical provider. However, it is possible that some women may have been evaluated by another out-of-network mental health provider. Hence, it is possible that the true prevalence of depression and its treatment was underestimated. We are unable to comment about when during pregnancy women were first diagnosed with depression and the duration of antidepressant medication exposure, which could impact the association with inflammatory and oxidative stress biomarkers. However, the association between depression and these biomarkers did not change when analyzed separately at different time points during pregnancy. Further studies are needed to further disentangle the temporal and possibly causal relationship between depression, oxidative stress/inflammation, and PTB. While in the current study we assessed whether oxidative stress mediated the relationship between depression and PTB, it is possible that depression mediates the relationship between oxidative stress and PTB. Additionally, this does not imply that inflammatory biomarkers and 8-OHdG do not mediate as well. Future studies are needed to investigate multiple mediators concurrently (42, 43). We did not have data with regards to depression severity, including measurement by a validated scale, and relied on documentation of a clinical diagnosis in the EMR. We are unable to comment on the impact of depression severity on inflammation and oxidative stress. The current study utilized plasma and urinary biomarkers, and it is possible that other markers, such as amniotic, umbilical cord, cerebrospinal, or cervicovaginal fluid, would be more strongly associated with antenatal depression (40). This is a secondary analysis of nested case-control study of PTB, and these results may not be generalizable to all women. We utilized inverse probability weighting to adjust for the effect of oversampling PTB so that these results would be more generalizable to pregnant women in the base cohort population.
In conclusion, we observed that antenatal depression was associated with higher levels of the oxidative stress biomarker 8-isoprostane, and that oxidative stress may partially mediate the association between antenatal depression and SPTB. A better understanding and characterization of the role of oxidative stress in antenatal depression could lead to future targeted prevention and treatment strategies for depression in pregnancy and SPTB.
Acknowledgments
FUNDING: This study was funded by the following grant from the National Institutes of Health (R01ES018872 NIH/NIEHS). Funding for Ferguson KK was provided by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST: Dr. Venkatesh has served on an advisory board for Sage Pharmaceuticals. The other authors report no conflict of interest.
REFERENCES
- 1.Iams J, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371(9607):164–75. [DOI] [PubMed] [Google Scholar]
- 2.Wei S, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstetrics and gynecology. 2010;116(2.1):393–401. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg R, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. New England Journal of Medicine. 2000;342(20):1500–7. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesh K, Riley L, Castro VM, Perlis RH, Kaimal AJ. Association of Antenatal Depression Symptoms and Antidepressant Treatment With Preterm Birth. Obstetrics and gynecology. 2016;127(5):926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straub H, Adams M, Kim JJ, Silver RK. Antenatal depressive symptoms increase the likelihood of preterm birth. American Journal of Obstetrics and Gynecology. 2012;207(4):329. [DOI] [PubMed] [Google Scholar]
- 6.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. Journal of Affective Disoders. 2013;150(3):736–44. [DOI] [PubMed] [Google Scholar]
- 7.Howren M, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic Medicine. 2009;71(2):171–86. [DOI] [PubMed] [Google Scholar]
- 8.Christian L, Franco A, Glaser R, Iams JD. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain, Behavior, and Immunity. 2009;23(6):750–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassidy-Bushrow A, Peters RM, Johnson DA, Templin TN. Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. Journal of Reproductive Immunology. 2012;94(2):202–9. [DOI] [PubMed] [Google Scholar]
- 10.Azar R, Mercer D. Mild depressive symptoms are associated with elevated C-reactive protein and proinflammatory cytokine levels during early to midgestation: a prospective pilot study. Journal of Womens Health. 2013;22(4):385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haeri S, Baker AM, Ruano R. Do pregnant women with depression have a pro-inflammatory profile? Journal of obstetrics and gynecology research. 2013;39(5):948–52. [DOI] [PubMed] [Google Scholar]
- 12.Latendresse G, Ruiz RJ, Wong B. Psychological distress and SSRI use predict variation in inflammatory cytokines during pregnancy. Open journal of obstetrics and gynecology. 2013;3(1A):184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maes M, Ombelet W, De Jongh R, Kenis G, Bosmans E. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. Journal of Affective Disoders. 2001;63(1–3):85–92. [DOI] [PubMed] [Google Scholar]
- 14.Miller E, Grobman WA, Culhane J, Adam E, Buss C, Entringer S, et al. Does maternal inflammation mediate the relationship between antenatal depression and adverse pregnancy outcomes? Society for Maternal Fetal Medicine 37th Annual Meeting; 2017 January 23–28, 2017; Las Vegas. [Google Scholar]
- 15.Blackmore E, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O’Connor TG. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosomatic Medicine. 2011;73(8):656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackmore E, Groth SW, Chen DG, Gilchrist MA, O’Connor TG, Moynihan JA. Depressive symptoms and proinflammatory cytokines across the perinatal period in African American women. Journal of psychosomatic obstetrics and gynecology. 2014;35(1):8–15. [DOI] [PubMed] [Google Scholar]
- 17.Miller E, Grobman WA, Culhane J, Adam E, Buss C, Entringer S, et al. Do psychotropic medications reduce inflammation in women with antenatal depression/anxiety? Society for Maternal Fetal Medicine 37th Annual Meeting; 2017 January 23–28, 2017; Las Vegas. [Google Scholar]
- 18.Edvinsson Å, Bränn E, Hellgren C, Freyhult E, White R, Kamali-Moghaddam M, et al. Lower inflammatory markers in women with antenatal depression brings the M1/M2 balance into focus from a new direction. Psychoneuroendocrinology. 2017;80:15–25. [DOI] [PubMed] [Google Scholar]
- 19.Bastek J, Elovitz MA. The role and challenges of biomarkers in spontaneous preterm birth and preeclampsia. Fertility and sterility. 2013;99(4):1117–23. [DOI] [PubMed] [Google Scholar]
- 20.Brou L, Almli LM, Pearce BD, Bhat G, Drobek CO, Fortunato S, et al. Dysregulated biomarkers induce distinct pathways in preterm birth. BJOG. 2012;119(4):458–73. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Seminars in reproductive medicine. 2007;25(1):21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Challis J, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F. Inflammation and pregnancy. Reproductive sciences. 2009;16(2):206–15. [DOI] [PubMed] [Google Scholar]
- 23.Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, et al. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. Journal of Immunology. 2000;164(11):5721–8. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson K, McElrath TF, Chen YH, Loch-Caruso R, Mukherjee B, Meeker JD. Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. American Journal of Obstetrics and Gynecology. 2015;212(2):e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts L, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free radical biology & medicine. 2000;28(4):505–13. [DOI] [PubMed] [Google Scholar]
- 26.Wu L, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clinica chimica acta. 2004;339(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson K, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Longitudinal profiling of inflammatory cytokines and C-reactive protein during uncomplicated and preterm pregnancy. American Journal of Reproductive Immunology. 2014;72(3):326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venkatesh K, Cantonwine DE, Ferguson K, Arjona M, Meeker JD, McElrath TF. Inflammatory and oxidative stress markers associated with decreased cervical length in pregnancy. American Journal of Reproductive Immunology. 2016;76(5):376–82. [DOI] [PubMed] [Google Scholar]
- 29.Venkatesh K, Ferguson KK, Smith NA, Cantonwine D, McElrath TF. Association of maternal depression in pregnancy with clinical pathways of preterm birth. Society for Maternal-Fetal Medicine 37th Annual Meeting 2018 February 2, 2018; Dallas, TX. [Google Scholar]
- 30.Ferguson K, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatrics. 2014;168(1):61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferguson K, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ Health Perspect. 2015;123(3):210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Y, Scott AJ, Wild CJ. Secondary analysis of case-control data. Statistics in Medicine. 2006;25:1323–39. [DOI] [PubMed] [Google Scholar]
- 33.McElrath T, Hecht JL, Dammann O, Boggess K, Onderdonk A, Markenson G, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. American Journal of Epidemiology. 2008;168(9):980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meeker J, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, et al. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ Health Perspect. 2009;117(10):1587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Judd C, Kenny DA, McClelland GH. Estimating and testing mediation and moderation in within-subject designs. Psychological methods. 2001;6(2):115–34. [DOI] [PubMed] [Google Scholar]
- 36.Kenny D. Mediation 2016 [January 25, 2018]. Available from: http://davidakenny.net/cm/mediate.htm.
- 37.Ferguson K, Chen YH, VanderWeele TJ, McElrath TF, Meeker JD, Mukherjee B. Mediation of the Relationship between Maternal Phthalate Exposure and Preterm Birth by Oxidative Stress with Repeated Measurements across Pregnancy. Environ Health Perspect. 2017;125(3):488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buis M. Direct and indirect effects in a logit model. Stata Journal. 2010;10(1):11–29. [PMC free article] [PubMed] [Google Scholar]
- 39.Preacher K, Leonardelli GJ. Calculation for the Sobel test: An interactive calculation tool for mediation tests 2018 [January 25, 2018]. Available from: http://quantpsy.org/sobel/sobel.htm.
- 40.Miller E, Sakawicz A, Roy A, Clayberger C, Sullivan JT, Grobman WA, et al. The association of SSRIs with inflammatory cytokines in maternal cerbrospinal fluid, peripheral blood and umblical cord blood. Society for Maternal Fetal Medicine 37th Annual Meeting; 2017 January 23–28, 2017; Las Vegas. [Google Scholar]
- 41.Venkatesh K, Nadel H, Blewett D, Freeman M, Kaimal A, Riley L.. Implementation of universal screening for depression during pregnancy: Feasibility and impact on obstetric care. American Journal of Obstetrics and Gynecology. 2016. [DOI] [PubMed] [Google Scholar]
- 42.Vanderweele T Mediation analysis with multiple versions of the mediator. Epidemiology. 2012;23(3):454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daniel R, De Stavola BL, Cousens SN, Vansteelandt S. Causal mediation analysis with multiple mediators. Biometrics. 2015;71(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]