Abstract
Malaria prevalence has declined in the past 10 years, especially outside of Sub-Saharan Africa. However, the proportion of cases due to Plasmodium vivax is increasing, accounting for up to 90–100% of the malaria burden in endemic regions. Nonetheless, investments in malaria research and control still prioritize Plasmodium falciparum while largely neglecting P. vivax. Specific biological features of P. vivax, particularly invasion of reticulocytes, occurrence of dormant liver forms of the parasite, and the potential for transmission of sexual stage parasites prior to onset of clinical illness, promote its persistence and hinder development of research tools and interventions. This review discusses recent advances in P. vivax research, current knowledge of its unique biology, and proposes priorities for P. vivax research and control efforts.
Keywords: Malaria, Plasmodium vivax, research
The Case for P. vivax-specific Research Innovations
Soon after the turn of this century, the fight against malaria entered a new era in which elimination, and ultimately eradication, is the goal [1]. A concerted international commitment to provide the scientific, political, and financial means to achieve this objective has since shown remarkable progress, achieving a 41% decrease in global malaria incidence and a 62% decline in mortality from 2000 to 2015 [2]. These gains reflect efforts mobilized predominantly against Plasmodium falciparum, the most prevalent and deadly of the five Plasmodium species that can infect and commonly cause malaria in humans, while largely neglecting malaria caused by Plasmodium vivax and other species. However, P. vivax contributes to greater morbidity and has a wider geographic distribution than P. falciparum (Figure 1), with approximately 35% of the world’s population (2.5 billion people) at risk of infection [3]. Furthermore, having shown an apparently slower decline in prevalence than P. falciparum, it has become the predominant cause of malaria in nearly all endemic regions outside of sub-Saharan Africa, where it accounts for 41% of all reported clinical cases [4]. Although the overall incidence of malaria is declining in these areas, the prevalence of cases due to P. vivax is increasing, particularly in countries in the pre-elimination or elimination phase [4]. P. vivax is therefore likely to persist as a barrier to malaria elimination and eradication without continued investments in the development of species-specific research tools and interventions.
Figure 1. The spatial distribution of Plasmodium falciparum and Plasmodium vivax malaria in 2010.

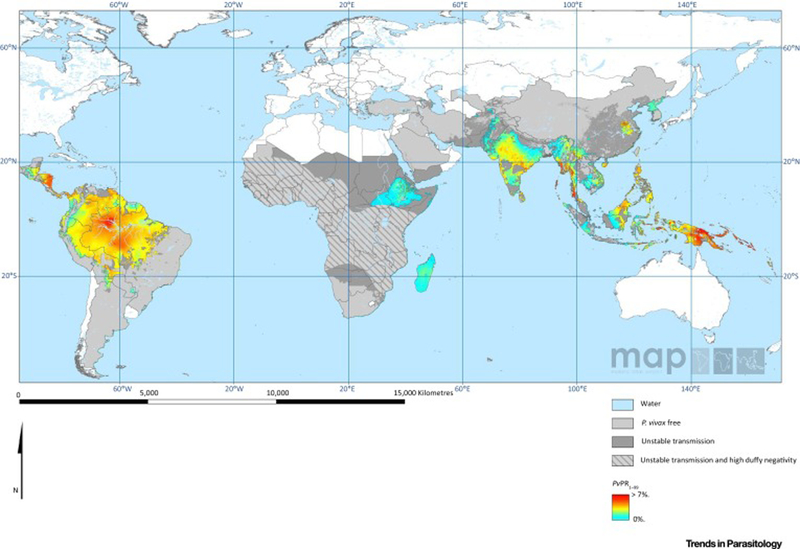
A) Estimates of the age-standardized annual mean P. falciparum parasite rate in two to ten year olds (PfPR2–10), within the spatial limits of stable transmission, stratified into four levels of risk. Areas of no risk and unstable risk (PfAPI < 0.1%) are also shown [139]. B) Mean point estimates of the age-standardized annual mean P. vivax parasite prevalence in two to ten year olds, within the spatial limits of stable transmission. Areas of no risk and unstable risk are also shown. Areas where Duffy negative prevalence was estimated as ≥ 90% are hatched to provide additional context for the impact of P. vivax on the local population within these areas [3]. Maps downloaded from the Malaria Atlas Project http://www.map.ox.ac.uk/and reproduced under the Creative Commons Attribution 3.0 Unported (CC BY 3.0) license (https://creativecommons.org/licenses/by/3.0/).
Advances concerning basic research tools have enabled breakthroughs in our understanding of Plasmodium biology and the subsequent discovery and development of diagnostics, anti-malarial drugs, and vaccines. Establishment of in vivo animal models, laboratory colonization of mosquito vectors, and perhaps most importantly, in vitro culture of erythrocytic asexual and sexual stages, opened new frontiers and enabled study of malaria outside of endemic areas. This progress has been facilitated largely by a significant increase in research and development (R&D) funding in recent decades, from $121 million in 1993 to $610 million in 2011 [5]. However, P. vivax remains largely neglected, accounting for no more than 5% of malaria R&D funding compared to nearly half of all research funds being dedicated to P. falciparum [5].
Unsurprisingly, this has resulted in the development of research tools and control strategies designed for the elimination of P. falciparum. While much of the research on P. falciparum has direct application to other Plasmodium species many control interventions have limited or no effectiveness against P. vivax. As a consequence, our understanding of the unique aspects of P. vivax biology and development of specific interventions to tackle them has lagged significantly behind.
Biological Barriers to P. vivax Research and Development
The life cycle of P. vivax is in general like that of P. falciparum in that it requires a competent female Anopheles mosquito for transmission and a human host for its survival and propagation. However, P. vivax exhibits a number of specific attributes to support its persistence in more diverse ecological habitats, and which hinder research and control efforts (Table 1). Following an infectious mosquito bite, sporozoites are delivered into the dermal tissues where they penetrate small blood vessels, circulate to the liver, and begin pre-erythrocytic or liver stage growth. Within hepatocytes, parasites replicate via asexual reproduction, termed schizogony (see Glossary), over a period of 7–9 days, after which thousands of merozoites are released into the blood circulation where they then invade red blood cells (RBCs) and begin the erythrocytic, or blood-stage cycle of infection. Alternatively, P. vivax sporozoites can pause in early liver stage development as small dormant forms, called hypnozoites (see Glossary), and then awaken weeks to years after the initial attack to cause relapsing infections. Hypnozoite activation is thought to cause the majority of blood stage infections, up to 90% in some areas [6].
Table 1.
Biological features and available research tools for P. vivax as compared to other human and simian Plasmodium species.
| Human | Simian | |||
|---|---|---|---|---|
| P. vivax | P. falciparum | P. cynomolgi | P. knowlesi | |
| Pre-erythrocytic cycle | ||||
| Hypnozoites | Yes | No | Yes | No |
| Erythrocytic cycle | ||||
| Duration (h) | 48 | 48 | 48 | 24 |
| Host cell | Reticulocytes | Normocytes, Reticulocytes | Reticulocytes | Normocytes, Reticulocytes |
| Gametocytes | ||||
| Time to maturation | 2–3d | 12–14d | 58h | 31h |
| Lifespan | ≤3d | 2.5d | ≤12h | 5h |
| In vitro cultivation | ||||
| Asexual blood stages | Short-term ex vivo | Continuous in vitro | Short-term ex vivo | Continuous in vitro |
| Gametocytes | No | Yes | No | No |
| Liver stages | Yes | Yes | Yes | No |
| In vivo models | ||||
| Old World monkeys | No development | Complete | Complete | Complete |
| New World monkeys | Partial (no hypnozoites) | Complete | Partial (no gametocytes) | Partial (no gametocytes) |
| Humanized mice | Liver stage | Complete | No | No |
| CHMI | Yes | Yes | No | No |
| Research tools | ||||
| Genetic modification | Episomes, Point mutations | Episomes, mutagenesis, homologous recombination | Episomal centromeres | Episomes, homologous recombination |
Within RBCs, parasites undergo asexual replication via schizogony in 48-hour cycles in established infections. Unlike P. falciparum, which can invade and develop in RBCs of all ages, P. vivax has evolved to preferentially invade reticulocytes (see Glossary). These cells occur more frequently in the bone marrow than in circulation, resulting in low-density or sub-microscopic parasitemia in the peripheral blood that are often undetectable. A small proportion of merozoites differentiate into male and female gametocytes, which following ingestion by a mosquito during bloodfeeding, undergo sexual reproduction and generation of infectious sporozoites via sporogony (see Glossary), thus completing the entire parasite life cycle. In contrast to P. falciparum gametocytes, which undergo prolonged development over 10–12 days, P. vivax gametocytes develop rapidly, possibly with the first generation of merozoites released from the liver, and appear in the blood nearly simultaneously with asexual blood stage parasites [7]. Therefore, transmission to mosquitoes can occur prior to the onset of clinical illness. This ability of P. vivax to form infectious gametocytes quickly and continuously, coupled with activation of hypnozoites, promotes highly effective and persistent transmission that renders ineffective the strategies designed to control falciparum malaria. Here, we review recent advances in models and technologies to enable P. vivax research, with a focus on these unique biological features., We furthermore identify gaps where investments are needed to better target research for development of interventions to intensify control of, and possibly eliminate, P. vivax malaria.
In Vitro Cultivation of Erythrocytic Stages
A reliable, long-term, continuous in vitro culture system for the P. vivax asexual cycle remains elusive, despite attempts to do so over more than 100 years. Whereas the ability of P. falciparum and P. knowlesi to invade and develop within RBCs of all ages enabled establishment of robust and reproducible protocols for their long-term cultivation [8–11], similar methods have proven inadequate to achieve reproducible, stable in vitro growth of P. vivax [12–15]. This is most likely related to the marked tropism of P. vivax for reticulocytes. Progress to characterize and obtain viable host cells and optimize culture conditions for P. vivax has been slow. These efforts continue to support ex vivo invasion assays and short-term growth, but fall short of a continuous long-term in vitro culture system needed for experimental laboratory-based research.
Obtaining and Characterizing Reticulocytes
Given the low concentration (0.5–1.5%) and short duration (~24 hours) of reticulocytes in circulating blood, maintaining sufficient abundance is a major challenge for P. vivax culture. The most successful attempt at P. vivax in vitro culture resulted in a doubling of the population of a non-human primate (NHP) adapted strain at each of eight cycles when cultured in McCoy’s 5A medium with regular supplementation of reticulocytes enriched from the blood of hemochromatosis patients [15]. Attempts to reproduce this protocol have been unsuccessful [16] More recently, fresh and cryopreserved reticulocytes enriched from leukocyte-depleted human cord blood have been used, with short-term success [17–19]; however, variability among reticulocytes and the need to have continuous access to hemochromatosis patients and newborns limit these approaches. While blood from these sources have relatively higher concentrations of reticulocytes compared to normal human blood, enrichment is a crucial step in all of these protocols, and the method employed is likely to affect the in vitro invasion and development of P. vivax. Data suggests that while differential centrifugation is more laborious, reticulocytes enriched using this technique better support P. vivax invasion than density gradients [18, 20] or immuno-magnetic purification [15, 16].
More recent advances in regard to hematopoietic stem cells (HSC) provided a reproducible in vitro source of fresh reticulocytes to support P. vivax culture [21–23]. Relatively large quantities of HSCs can be enriched from human cord blood by magnetic microbead selection on the basis of their expression of either CD34 or CD133, and then used immediately or cryopreserved as aliquots for initiating new cultures as needed. CD34/CD133 HSCs cultured in the presence of specific cytokines and growth factors (stem cell factor, IL-3, hydrocortisone, erythropoietin), with or without co-culture with mouse stromal cells, can produce erythroid cell populations with up to 20% reticulocytes. Unfortunately, the many variables inherent in optimizing production of HSC-derived reticulocytes, as well as the high cost, have limited its use to support P. vivax culture. In addition, the presence of fetal hemoglobin within these HSC-derived reticulocytes and the low expression level of some surface receptors (e.g., DARC) may negatively impact parasite growth [17, 24].
It should be noted that reticulocytes are a heterogeneous population of erythrocytes that, once released into the peripheral circulation, undergo significant morphological, biochemical, and metabolic changes during their rapid maturation to normocytes (see Glossary). A recent report has demonstrated that clinical isolates of P. vivax preferentially invade immature reticulocytes expressing high levels of the transferrin receptor, CD71, which are typically restricted to the bone marrow [25]. This is a significant step forward in characterizing the preferred host cell for P. vivax, but identification of additional reticulocyte surface markers, particularly the receptor(s) for P. vivax invasion, would further advance these efforts. Since small numbers of very immature stages of reticulocytes are present in peripheral blood, continued efforts to cultivate P. vivax may benefit by further improving enrichment methods for the young subpopulation of reticulocytes that better support invasion.
Culture Conditions
Given the limited success to date of efforts to cultivate P. vivax in vitro, it is evident that optimal culture conditions have yet to be determined and are unlikely to be due to limited availability of reticulocytes. Historically, McCoy’s 5A medium has better supported P. vivax multiplication than the RPMI 1640 medium that is used for P. falciparum and Plasmodium knowlesi culture, although the formulation of the two media differs only slightly in the concentrations of a few vitamins and amino acids. Addition of a more stable form of L-glutamine to McCoy’s 5A medium was recently found to promote longer persistence of parasites in vitro [16], suggesting that P. vivax is sensitive to the byproducts (ammonia and pyrrolidone carboxylic acid) resulting from the spontaneous degradation of this amino acid. Reports on the benefit of shaking P. vivax cultures are conflicting [14, 15, 17], but given the fragility of mature P. vivax schizonts, shifting from static to shaking conditions at the appropriate time may improve parasite maturation, egress, and/or re-invasion [16]. Finally, a higher starting hematocrit (12% vs. 6%) better supports parasite development, and may reflect the need for a particular parasite or total cell density to promote cell to cell communication [16].
In vivo Models
Non-human Primates
NHP models have been critical to P. vivax research for over 50 years, and remain highly relevant in the absence of an in vitro culture system. A number of P. vivax isolates from diverse endemic regions have been adapted to chimpanzees and New World monkeys of the genera Aotus (owl monkeys) and Saimiri (squirrel monkeys) [26, 27]. These models facilitate study of all P. vivax life stages, including hypnozoites [28], and provide a critical renewable source of parasite material for in-depth omics studies [29, 30], adaptation to in vitro culture (as described above), and perhaps most importantly, a reliable laboratory method for transmission to mosquitoes. NHP models are also useful for evaluating pre-erythrocytic [31–33], blood-stage [34–37], and transmission-blocking [38–40] candidate vaccines, as well as antimalarial drugs [41–44]. In recent years, numerous countries, including the United States, Australia, Japan, and New Zealand, and the European Union have banned or severely limited chimpanzee-based research. Large investments to further primate research, including the use of chimpanzees, in countries such as China and Thailand, present new opportunities for conducting P. vivax research [45]. However, in light of continuing ethical, financial, and availability issues surrounding the use of other primates, alternative in vivo models for P. vivax research should be pursued.
Humanized Mice
In recent years, several humanized mouse models, which are reliant on immunodeficient mouse strains to support xenotransplantation, have been developed to support studies on different stages of the Plasmodium life cycle. To date, only the NOD [46] and NSG [47] mouse models have been implemented for P. falciparum asexual and sexual stage [48] development. In terms of blood-stage development, these mice can support limited engraftment and differentiation of HSCs following treatment with, or transgenic expression of, human cytokines such as interleukin-3, erythropoietin, and thrombopoietin, which offers the potential that they may be suitable for the study of P. vivax [49]. In addition, clodronate-treated NSG mice are receptive to direct engraftment by intravenous injection of human RBCs to levels sufficient for support of P. falciparum infections [47, 50].
For pre-erythrocytic stage studies, the livers of multiple types of immunodeficient mice have been repopulated sufficiently with human hepatocytes to support Plasmodium liver stage development. The Alb-uPA liver-humanized mouse has been shown to be a suitable model for P. falciparum liver stage infection [51–53], while FRG KO huHep mice have also been successful in supporting P. vivax sporozoite infection, liver stage development, and hypnozoite formation [54, 55]. When backcrossed on the NOD background, FRG-NOD huHep mice further support the transition of P. falciparum pre-erythrocytic merozoites to blood stage infection [55]. The DRAG humanized mouse model also supports the complete P. falciparum life cycle, including transmission to mosquitoes [56], while TK-NOG humanized mice allow liver and blood stage development of both P. falciparum and Plasmodium ovale [57]. The presence of late-developing P. ovale schizonts in the livers of these mice suggests the eventual activation of quiescent parasites. Therefore, TK-NOG mice may also serve as a suitable model for P. vivax liver stage infection. Mouse models that support humanized liver tissue engraftment and/or human reticulocytes could be crucial to furthering our understanding of hypnozoite biology and relapse, and erythrocytic host cell requirements for parasite invasion and growth.
Controlled Human Malaria Infections
Deliberate infection of patients with P. vivax began more than a century ago, with the development of malariotherapy to treat neurosyphilis [58]. Infections of prisoners from the 1940s to the 1970s provided a wealth of information on P. vivax biology, antimalarial efficacy, and immunization with irradiated sporozoites [59–62]. In recent years, controlled human malaria infection (CHMI; See Glossary) models for P. vivax have been developed in Colombia, the United States, and Australia. CHMI initiated via mosquito bite closely resembles natural infection [63–69], but relies on access to and production of infected mosquitoes or sporozoites, and risks hypnozoite formation and potential relapse. Thus, these studies will require pre-screening of participants for Duffy blood group, as well as glucose-6-phosphate dehydrogenase (G6PD) deficiency and intermediate metabolizer CYP2D6 phenotypes in order to avoid hemolysis induced by treatment with primaquine. While CHMI initiated with blood-stage parasites does not mimic natural P. vivax infection [70, 71], it circumvents these issues, and allows for control over parasite strain, inoculum size, and study location. Assuming the risks of P. vivax CHMI are managed effectively, it has the potential to provide a platform for early proof-of-concept drug and vaccine testing, as well as small, inexpensive Phase II efficacy trials.
Sexual Stages and Experimental Transmission
Studies on P. vivax gametocytes, mosquito stages, and transmission rely on either experimentally infected NHPs or human subjects, although moving forward, humanized mouse models may also be suitable for this purpose. Protocols have been established to infect mosquitoes by direct skin feeding [72], and isolated blood can be used in membrane feeding assays (see Glossary) to infect mosquitoes [73–78] and generate mosquito life stages, including gametes and ookinetes, in vitro [79, 80]. Given the historical reliance on chimpanzees for robust production of NHP-adapted P. vivax sporozoites [81–84], and the recent phase-out of chimpanzee-based research in most countries, it will be crucial to improve existing New World monkey models to maximize P. vivax transmission and sporozoite production. Development of protocols to infect mosquitoes with P. vivax following short-term culture of cryopreserved clinical isolates [85], and to cryopreserve infectious sporozoites [86–88], will help to overcome limitations surrounding NHP and human research and expand capabilities to investigate P. vivax transmission and liver stage biology in both in vivo and ex vivo models. When coupled with G6PD and CYP2D6 screening for CHMI as described above, such advances may also further expand our capabilities to explore novel approaches to P. vivax vaccination, including chemoprophylaxis with sporozoites, as has recently been demonstrated for P. falciparum [89].
In Vitro Models for Liver Stage Development and Hypnozoites
HepG2 [90] and HC04 [91] immortalized human hepatoma cell lines have successfully been used to study P. vivax liver stage infection and evaluate antimalarial drugs in vitro. However, these hepatocytoma cells exhibit aberrant gene expression, signaling, metabolism, and abnormal cellular architecture that fail to adequately recapitulate that of normal primary human hepatocytes in vivo. Furthermore, to attain even low infection efficiencies (0.05–2%) requires substantial numbers of sporozoites, and excessive proliferation of both HepG2 and HC04 cells limits their use and prohibits long-term culture that is necessary to study hypnozoites. Use of primary human hepatocytes may solve these problems, but reports to date are that even in co-cultures optimized with supportive stromal cells, P. vivax infection efficiencies of hepatoma cell lines are poor [92, 93], and are highly variable among donors. Other promising modifications to in vitro cultures of primary human hepatocytes include the use of microfluidic devices with flow [94], or the use of hepatocyte-like cells derived from human induced pluripotent stem cells (iPSC) originating from human fibroblasts, which can be permissive to P. vivax [95] and may also support genetic modification of hepatocytes. Long-term in vitro cultivation of Macaca fascicularis primary hepatocytes with the closely related simian malaria parasite Plasmodium cynomologi has recently been reported to support hypnozoite persistence and reactivation for up to 40 days [96], and offers a viable alternative. These innovations further enable our ability to study P. vivax liver stage biology and reflect remarkable progress towards development of a high-throughput in vitro assay for discovery and evaluation of novel drugs that target pre-erythrocytic stages and hypnozoites.
Development of Genetic Tools
Completion of the first P. vivax reference genome sequence generated from the NHP-adapted Salvador I strain in 2008 [29] together with rapidly advancing genomics technologies, have led to a growing number of P. vivax whole genomes being sequenced. In-depth genomics approaches have provided new insights concerning P. vivax genetic diversity and population genetics, mechanisms of human RBC invasion [97–99], characterization of multi-gene families, parasite evolution [100], drug resistance [101], and relapse [102]. Recent advances in single-cell genomics [103, 104] and techniques for utilizing cryopreserved Plasmodium samples [105] provide a promising approach for detailed analyses of noncultivable parasites from complex multi-species or multi-clonal infections, which are common among P. vivax infections in humans and NHPs [105–107]. The known P. vivax genome has further facilitated development of platforms for immunoscreening [108–110] and protein-protein interactions [111] to identify novel vaccine candidate antigens, and provides a basis for transcriptomic [66, 112–115] and proteomic [30, 116, 117] studies.
Genetic Modification
Whereas P. falciparum research has greatly benefited from the use of genetic modification tools, development of P. vivax transgenic parasites has suffered from the lack of an in vitro culture system, low parasite densities observed in NHP and patient infections, and the existence of only a limited number of genetic markers and drugs for selection. Despite these challenges, significant steps have been taken towards rendering P. vivax more amenable to molecular investigation. Transient episomal expression of a luciferase reporter was achieved ex vivo using blood-stage parasites isolated from a Saimiri monkey [118]. More recently, zinc finger nucleases (ZFNs) have been used to stably introduce drug-resistance mutations into the dihydrofolate reductase (DHFR) gene of P. vivax parasites in vivo in Saimiri monkeys [119]. Development of a stable transgenic fluorescent or luciferase-expressing P. vivax line that could be used in the aforementioned in vivo and in vitro models would greatly advance efforts to understand the unique features of P. vivax biology and will be fundamental for high-throughput drug and vaccine research.
Heterologous Expression of P. vivax Genes
In the absence of robust genetic modification systems, heterologous expression of P. vivax genes in other Plasmodium species, and even other organisms, can provide important information about parasite biology and permit evaluation of antimalarial drugs and candidate vaccine antigens. Episomal overexpression of the P. vivax chloroquine resistance transporter (PvCRT-O) in P. falciparum and Dictyostelium discoideum allowed for analyses of drug sensitivity and PvCRT function in chloroquine transport and accumulation [120]. Similarly, double crossover-homologous recombination (see Glossary) and piggyBac transposition systems (see Glossary) have been used to stably integrate wild type and mutant P. vivax DHFR alleles in P. falciparum [121] and P. berghei [122] to confirm the involvement of these mutations in pyrimethamine resistance. The rise of CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats-CRISPR-associated protein 9 nuclease)-based genome editing tools, which have recently been successfully established for P. falciparum [123–126], should be used to improve targeting efficiency and reduce the time required to stably modify target genes, potentially without the need for drug-selectable marker genes [127, 128]. Development of chimeric P. berghei expressing P. vivax circumsporozoite protein (CSP) [129, 130] and cell traversal protein for ookinetes and sporozoites (CelTOS) [131] genes has provided a mechanism for early in vivo evaluation of the safety and efficacy of candidate vaccine antigens that target these proteins. P. falciparum and P. berghei are quite genetically and evolutionarily distinct from P. vivax, and differences in gene expression, regulation [132], or function may limit the utility of these models.
Simian Models for P. vivax
To improve upon genetic modification strategies for P. vivax, there is opportunity to harness recent innovations for two closely related simian malaria parasites, P. cynomolgi and P. knowlesi. Development of a transgenic fluorescent P. cynomolgi reporter line using an episomal centromere has enabled imaging of live parasites in infected rhesus monkey blood, infected mosquitoes, in vitro liver stage assays, and purification of hypnozoites for further study [133]. P. knowlesi grown in either rhesus monkey [11] or human erythrocytes [10] is also genetically tractable, and homologous recombination techniques have recently been utilized to investigate erythrocyte invasion and remodeling by this parasite [134, 135]. It has been demonstrated that P. knowlesi recognizes regulatory sequences for other Plasmodium species and can be used to express heterologous constructs [136], including the P. vivax PHISTb gene, which was shown to be associated with the host cell cytoskeleton [137]. Recent efforts to optimize direct electroporation of P. knowlesi schizonts may also have direct application to the genetic transformation of P. vivax [138]. The adaptation of P. knowesi to long term in vitro culture and the proven methods for its genetic modification provide new insights and should be applied to efforts for P. vivax.
Concluding Remarks and Future Perspectives
Overcoming decades of neglect, an international cadre of dedicated scientists has persisted, advancing research technologies and models for P. vivax research to uncover new facets of this parasite’s unique biology with the goal of developing P. vivax-specific interventions. New opportunities abound, with recent advances having been made in stem cell research and characterizing reticulocyte invasion, introducing human and animal models for the complete life cycle, and investigating hepatocyte biology, There are clear priorities on which we should focus to expand upon existing tools and maximize our capabilities (see Outstanding Questions Box). Namely, we must 1) identify and source the preferred host cell for invasion and blood stage development, 2) recapitulate in vitro the hepatocyte phenotypes required to support pre-erythrocytic development and hypnozoite formation, 3) improve access to a renewable source(s) of single clonal P. vivax populations, and 4) establish genetic modification methods for stable parasite transfection.
Outstanding Questions.
What are the primary receptors for P. vivax merozoite invasion of reticulocytes? Can subpopulations of cells expressing these receptors be identified and enriched from existing sources, or can stem cells be modified to express them in order to facilitate in vitro culture?
Considering its similar pathobiology, can P. cynomolgi be adapted for blood-stage in vitro growth in human erythrocytes to provide insights into P. vivax biology and evaluate new therapies?
Can P. vivax be further adapted to New World monkeys to produce robust, highly transmissible infections without the need for sporozoites derived from chimpanzee infections?
Can humanized mice be engrafted with both primary human hepatocytes and CD71+ reticulocytes to levels that support liver-to-blood-stage transition of P. vivax?
What hepatocyte phenotype(s) and growth conditions support improved ex vivo invasion efficiencies and long-term growth of P. vivax? Are these comparable to what is achieved in natural human infections?
Can one or more of the laboratory models validate early transmissibility to mosquitoes, especially whether first-generation merozoites emerge out of the liver develop into infectious gametocytes.
Can a repository of different P. vivax strains consisting of a single, genetically clonal population of parasites be established to allow for routine use in experimental models?
Can advances in genomics, particularly single-cell approaches, be utilized to decipher the basis of unique P. vivax biology, namely determinants of hypnozoite formation, early transmission, and reticulocyte invasion, for development of more vivax effective targeted therapies?
Can transfection methods developed using the closely related P. knowlesi and P. cynomolgi be adapted to genetically modify P. vivax? Can we develop alternative drug-selectable markers that will facilitate selection of genetically modified P. vivax in vivo?
The recent discovery that P. vivax merozoites have a predilection for a subset of immature, reticulocytes expressing high levels of CD71 that are largely restricted to the bone marrow has important implications for establishing an in vitro continuous culture system for this parasite. Further characterization of these cells, development of improved methods to obtain them, and clear definition of the culture conditions required to support P. vivax in vitro invasion, growth, and egress are clearly needed. However, while establishing a continuous culture system is correctly often described as the major challenge facing the P. vivax research community, development of CHMI, improved NHP and humanized mouse models, and especially in vitro hepatocyte culturing, provide a greatly expanded framework for conducting a plethora of ex vivo studies, including the evaluation of antimalarial drugs and vaccines. Renewable sources of well-characterized, single clonal populations of P. vivax, perhaps grown in NHPs, would allow us to better leverage these models. By further harnessing recent advances in genetics to develop transgenic fluorescent or luciferase-expressing P. vivax reporter lines, these models could support in vivo live imaging, particularly for the study of hypnozoites, and high-throughput assays for drug discovery and evaluation. The closely related P. cynomolgi and P. knowlesi provide suitable models to pursue investigations in the absence of P. vivax, including reticulocyte invasion and hypnozoite formation and reactivation. These endeavors will improve upon existing resources and provide essential new tools to advance P. vivax research and development efforts, and accelerate progress towards the elimination and eradication of this parasite.
Trends Box.
The predilection of P. vivax for immature red blood cells (RBCs), its ability to form hypnozoites, and early transmission of gametocytes are significant barriers to research and control
Improved understanding of the preferred host cell for P. vivax invasion (reticulocytes expressing high levels of CD71) has enabled development of ex vivo assays and short-term growth, but long-term continuous culture remains elusive.
Non-human primates (NHPs) remain the best model for P. vivax research, but controlled human malaria infections (CHMI), and pending further studies, humanized mice, are promising alternatives.
In-depth genomics and systems biology approaches have provided new insight into P. vivax genetics, RBC invasion, multi-gene families, evolution, drug resistance, and relapse.
Genetic modification of P. vivax remains challenging, but successes with P. cynomolgi and P. knowlesi may provide insight necessary to achieve this goal.
Acknowledgments
This work was supported in part by grants from the Bill and Melinda Gates Foundation (OPP#1023643) and NIH (R01AI064478) to J.H.A., as well as the Division of Intramural Research and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA. We thank T. E. Wellems, J. M. Sá, and R. R. Moraes Barros for useful discussion and comments on early versions of the manuscript.
Glossary
- Controlled human malaria infection (CHMI)
the intentional infection of human volunteers with Plasmodium by mosquito-bite or injection of sporozoites or blood-stage parasites
- Double-crossover homologous recombination
A form of genetic modification in which foreign DNA with sequences identical to those flanking the target gene is introduced into the cell, causing the target gene DNA to be replaced with the foreign DNA sequence in between those homologous regions during replication
- Hypnozoite
a persistent, non-growing and non-dividing uninucleate hepatic stage parasite observed in infections caused by relapsing species, such as P. vivax, P. ovale, and P. cynomolgi. Hypnozoites may remain dormant for weeks to years, and are apparently only sensitive to treatment with primaquine
- Membrane feeding assay
a functional assay to measure transmission of Plasmodium parasites to mosquitoes. Plasmodium-infected blood is delivered to mosquitoes via an artificial membrane attached to a water-jacketed glass feeder that is maintained at 37°C via circulating water bath
- Normocyte
amature erythrocyte that is normal in size, shape, and color
- piggyBac transposition system
A form of genetic modification in which the piggybac transposon can be mobilized between vectors and chromosomes via a cut and paste mechanism to target genes of interest located between specific inverted terminal repeat sequences (ITRs). The piggybac transposase recognizes the ITRs located on both ends of the transposon vector, removes the DNA sequence between them, and integrates it into TTAA chromosomal sites
- Relapse
blood-stage infection resulting from activation of a hypnozoite(s) in the liver
- Reticulocyte
an immature erythrocyte lacking a nucleus, and containing granular or reticular matter that can be stained with new methylene blue or Giemsa for identification
- Schizogony
haploid asexual replication by multiple fission of the nucleus of the parasite within a host cell, followed by segmentation of the cytoplasm to give rise to multiple identical daughter cells. When occurring within the liver, a Plasmodium sporozoite gives rise to merozoites that are released into the bloodstream. During blood-stage infection, a merozoite gives rise to additional merozoites that maintain the erythrocytic cycle
- Sporogony
formation of sporozoites within the mosquito vector during the sexual stage of the Plasmodium life cycle. Fusion of male and female gametocytes gives rise to a motile zygote, or ookinete, that invades and traverses the mosquito midgut where it undergoes division by multiple fission within an oocyst, resulting in production of sporozoites
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alonso PL et al. (2011) A research agenda to underpin malaria eradication. PLoS Med 8 (1), e1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO, World Malaria Report 2016, WHO, Geneva, 2016. [Google Scholar]
- 3.Gething PW et al. (2012) A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis 6 (9), e1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO, Control and Elimination of Plasmodium vivax Malaria, WHO, Geneva, 2015. [Google Scholar]
- 5.PATH, From Pipeline to Product: Malaria R&D Funding Needs into the Next Decade, PATH, Seattle, 2013. [Google Scholar]
- 6.Adekunle AI et al. (2015) Modeling the dynamics of Plasmodium vivax infection and hypnozoite reactivation in vivo. PLoS Negl Trop Dis 9 (3), e0003595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendis K et al. (2001) The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg 64 (1–2 Suppl), 97–106. [DOI] [PubMed] [Google Scholar]
- 8.Trager W and Jensen JB (1976) Human malaria parasites in continuous culture. Science 193 (4254), 673–5. [DOI] [PubMed] [Google Scholar]
- 9.Lim C et al. (2013) Expansion of host cellular niche can drive adaptation of a zoonotic malaria parasite to humans. Nat Commun 4, 1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon RW et al. (2013) Adaptation of the genetically tractable malaria pathogen Plasmodium knowlesi to continuous culture in human erythrocytes. Proc Natl Acad Sci U S A 110 (2), 531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kocken CHM (2002) Plasmodium knowlesi provides a rapid in vitro and in vivo transfection system that enables double-crossover gene knockout studies. Infect Immun 70 (2), 655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larrouy G et al. (1981) [Obtaining intraerythrocytic forms of Plasmodium vivax by in vitro culture]. C R Seances Acad Sci III 292 (16), 929–30. [PubMed] [Google Scholar]
- 13.Brockelman CR et al. (1985) Observation on complete schizogony of Plasmodium vivax in vitro. J Protozool 32 (1), 76–80. [DOI] [PubMed] [Google Scholar]
- 14.Lanners HN (1992) Prolonged in vitro cultivation of Plasmodium vivax using Trager’s continuous-flow method. Parasitol Res 78 (8), 699–701. [DOI] [PubMed] [Google Scholar]
- 15.Golenda CF et al. (1997) Continuous in vitro propagation of the malaria parasite Plasmodium vivax. Proc Natl Acad Sci U S A 94 (13), 6786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw-Saliba K et al. (2016) Insights into an optimization of Plasmodium vivax Sal-1 in vitro culture: The Aotus primate model. PLoS Negl Trop Dis 10 (7), e0004870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Udomsangpetch R et al. (2007) Short-term in vitro culture of field isolates of Plasmodium vivax using umbilical cord blood. Parasitol Int 56 (1), 65–9. [DOI] [PubMed] [Google Scholar]
- 18.Russell B et al. (2011) A reliable ex vivo invasion assay of human reticulocytes by Plasmodium vivax. Blood 118 (13), e74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borlon C et al. (2012) Cryopreserved Plasmodium vivax and cord blood reticulocytes can be used for invasion and short term culture. Int J Parasitol 42 (2), 155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sriprawat K et al. (2009) Effective and cheap removal of leukocytes and platelets from Plasmodium vivax infected blood. Malar J 8, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panichakul T et al. (2007) Production of erythropoietic cells in vitro for continuous culture of Plasmodium vivax. Int J Parasitol 37 (14), 1551–7. [DOI] [PubMed] [Google Scholar]
- 22.Noulin F et al. (2012) Cryopreserved reticulocytes derived from hematopoietic stem cells can be invaded by cryopreserved Plasmodium vivax isolates. PLoS One 7 (7), e40798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuya T et al. (2014) Reticulocytes from cryopreserved erythroblasts support Plasmodium vivax infection in vitro. Parasitol Int 63 (2), 278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasvol G et al. (1977) Effects of foetal haemoglobin on susceptibility of red cells to Plasmodium falciparum. Nature 270 (5633), 171–3. [DOI] [PubMed] [Google Scholar]
- 25.Malleret B et al. (2015) Plasmodium vivax: restricted tropism and rapid remodeling of CD71-positive reticulocytes. Blood 125 (8), 1314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt LH (1973) Infections with Plasmodium falciparum and Plasmodium vivax in the owl monkey--model systems for basic biological and chemotherapeutic studies. Trans R Soc Trop Med Hyg 67 (4), 446–74. [DOI] [PubMed] [Google Scholar]
- 27.Collins WE (2002) Nonhuman primate models. I. Nonhuman primate host-parasite combinations. Methods Mol Med 72, 77–84. [DOI] [PubMed] [Google Scholar]
- 28.Joyner C et al. (2015) No more monkeying around: primate malaria model systems are key to understanding Plasmodium vivax liver-stage biology, hypnozoites, and relapses. Front Microbiol 6, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlton JM et al. (2008) Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature 455 (7214), 757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson DC et al. (2015) Plasmodium vivax trophozoite-stage proteomes. J Proteomics 115, 157–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins WE (1992) South American monkeys in the development and testing of malarial vaccines--a review. Mem Inst Oswaldo Cruz 87 (Suppl 3), 401–406. [DOI] [PubMed] [Google Scholar]
- 32.Yang C et al. (1997) Induction of protective antibodies in Saimiri monkeys by immunization with a multiple antigen construct (MAC) containing the Plasmodium vivax circumsporozoite protein repeat region and a universal T helper epitope of tetanus toxin. Vaccine 15 (4), 377–86. [DOI] [PubMed] [Google Scholar]
- 33.Collins WE et al. (1997) Protective immunity induced in squirrel monkeys with a multiple antigen construct against the circumsporozoite protein of Plasmodium vivax. Am J Trop Med Hyg 56 (2), 200–10. [DOI] [PubMed] [Google Scholar]
- 34.Collins WE et al. (1999) Testing the efficacy of a recombinant merozoite surface protein (MSP-119) of Plasmodium vivax in Saimiri boliviensis monkeys. Am J Trop Med Hyg 60 (3), 350–356 [DOI] [PubMed] [Google Scholar]
- 35.Arevalo-Herrera M et al. (2005) Immunogenicity and protective efficacy of recombinant vaccine based on the receptor-binding domain of the Plasmodium vivax Duffy binding protein in Aotus monkeys. Am J Trop Med Hyg 73 (5 Suppl), 25–31. [DOI] [PubMed] [Google Scholar]
- 36.Castellanos A et al. (2007) Plasmodium vivax thrombospondin related adhesion protein: immunogenicity and protective efficacy in rodents and Aotus monkeys. Mem Inst Oswaldo Cruz 102 (3), 411–6. [DOI] [PubMed] [Google Scholar]
- 37.Rojas-Caraballo J et al. (2009) Immunogenicity and protection-inducing ability of recombinant Plasmodium vivax rhoptry-associated protein 2 in Aotus monkeys: a potential vaccine candidate. Vaccine 27 (21), 2870–6. [DOI] [PubMed] [Google Scholar]
- 38.Hisaeda H et al. (2000) Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect Immun 68 (12), 6618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arevalo-Herrera M et al. (2005) Induction of transmission-blocking immunity in Aotus monkeys by vaccination with a Plasmodium vivax clinical grade PVS25 recombinant protein. Am J Trop Med Hyg 73 (5 Suppl), 32–7. [DOI] [PubMed] [Google Scholar]
- 40.Collins WE et al. (2006) Assessment of transmission-blocking activity of candidate Pvs25 vaccine using gametocytes from chimpanzees. Am J Trop Med Hyg 74 (2), 215–221. [PubMed] [Google Scholar]
- 41.Collins WE et al. (1992) The susceptibility of the Indonesian I/CDC strain of Plasmodium vivax to chloroquine. J Parasitol 78 (2), 344–9. [PubMed] [Google Scholar]
- 42.Obaldia N et al. (1997) WR 238605, chloroquine, and their combinations as blood schizonticides against a chloroquine-resistant strain of Plasmodium vivax in Aotus monkeys. Am J Trop Med Hyg 56 (5), 508–510. [DOI] [PubMed] [Google Scholar]
- 43.Guan J et al. (2005) Antimalarial activities of new pyrrolo[3,2-f]quinazoline-1,3-diamine derivatives. Antimicrob Agents Chemother 49 (12), 4928–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birrell GW et al. (2015) JPC-2997, a new aminomethylphenol with high in vitro and in vivo antimalarial activities against blood stages of Plasmodium. Antimicrob Agents Chemother 59 (1), 170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang XL et al. (2014) Experimental primates and non-human primate (NHP) models of human diseases in China: current status and progress. Dongwuxue Yanjiu 35 (6), 447–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angulo-Barturen I et al. (2008) A murine model of falciparum-malaria by in vivo selection of competent strains in non-myelodepleted mice engrafted with human erythrocytes. PLoS One 3 (5), e2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnold L et al. (2011) Further improvements of the P. falciparum humanized mouse model. PLoS One 6 (3), e18045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duffier Y et al. (2016) A humanized mouse model for sequestration of Plasmodium falciparum sexual stages and in vivo evaluation of gametocytidal drugs. Sci Rep 6, 35025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amaladoss A et al. (2015) De novo generated human red blood cells in humanized mice support Plasmodium falciparum infection. PLoS One 10 (6), e0129825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnold L et al. (2010) Analysis of innate defences against Plasmodium falciparum in immunodeficient mice. Malar J 9, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morosan S et al. (2006) Liver-stage development of Plasmodium falciparum, in a humanized mouse model. J Infect Dis 193 (7), 996–1004. [DOI] [PubMed] [Google Scholar]
- 52.Sacci JB Jr. et al. (2006) Plasmodium falciparum infection and exoerythrocytic development in mice with chimeric human livers. Int J Parasitol 36 (3), 353–60. [DOI] [PubMed] [Google Scholar]
- 53.Yang AS et al. (2017) Cell traversal activity is important for Plasmodium falciparum liver infection in humanized mice. Cell Rep 18 (13), 3105–3116. [DOI] [PubMed] [Google Scholar]
- 54.Mikolajczak SA et al. (2015) Plasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric mice. Cell Host Microbe 17 (4), 526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaughan AM et al. (2015) Plasmodium falciparum genetic crosses in a humanized mouse model. Nat Methods 12 (7), 631–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wijayalath W et al. (2014) Humanized HLA-DR4.RagKO.IL2RgammacKO.NOD (DRAG) mice sustain the complex vertebrate life cycle of Plasmodium falciparum malaria. Malar J 13, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soulard V et al. (2015) Plasmodium falciparum full life cycle and Plasmodium ovale liver stages in humanized mice. Nat Commun 6, 7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitrow M (1990) Wagner-Jauregg and fever therapy. Med Hist 34 (3), 294–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coatney HR et al. (1949) Studies in human malaria; trials of quinacrine, colchicine (SN 12,080) and quinine against Chesson strain vivax malaria. Am J Hyg 50 (2), 194–9. [PubMed] [Google Scholar]
- 60.Clyde DF et al. (1973) Specificity of protection of man immunized against sporozoite-induced falciparum malaria. Am J Med Sci 266 (6), 398–403. [DOI] [PubMed] [Google Scholar]
- 61.Miller LH et al. (1976) The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med 295 (6), 302–4. [DOI] [PubMed] [Google Scholar]
- 62.McCarthy VC and Clyde DF (1977) Plasmodium vivax: correlation of circumsporozoite precipitation (CSP) reaction with sporozoite-induced protective immunity in man. Exp Parasitol 41 (1), 167–71. [DOI] [PubMed] [Google Scholar]
- 63.Herrera S et al. (2009) Successful sporozoite challenge model in human volunteers with Plasmodium vivax strain derived from human donors. Am J Trop Med Hyg 81 (5), 740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herrera S et al. (2011) Consistent safety and infectivity in sporozoite challenge model of Plasmodium vivax in malaria-naive human volunteers. Am J Trop Med Hyg 84 (2 Suppl), 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arevalo-Herrera M et al. (2014) Plasmodium vivax sporozoite challenge in malaria-naive and semi-immune Colombian volunteers. PLoS One 9 (6), e99754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rojas-Pena ML et al. (2015) Transcription profiling of malaria-naive and semi-immune Colombian volunteers in a Plasmodium vivax sporozoite challenge. PLoS Negl Trop Dis 9 (8), e0003978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arevalo-Herrera M et al. (2016) Antibody profiling in naive and semi-immune individuals experimentally challenged with Plasmodium vivax sporozoites. PLoS Negl Trop Dis 10 (3), e0004563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arevalo-Herrera M et al. (2016) Protective efficacy of Plasmodium vivax radiation-attenuated sporozoites in Colombian volunteers: a randomized controlled trial. PLoS Negl Trop Dis 10 (10), e0005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bennett JW et al. (2016) Phase ½a trial of Plasmodium vivax malaria vaccine candidate VMP001/AS01B in valaria-naive adults: safety, immunogenicity, and efficacy. PLoS Negl Trop Dis 10 (2), e0004423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCarthy JS et al. (2013) Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J Infect Dis 208 (10), 1688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Griffin P et al. (2016) Safety and reproducibility of a clinical trial system using induced blood stage Plasmodium vivax infection and its potential as a model to evaluate malaria transmission. PLoS Negl Trop Dis 10 (12), e0005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sattabongkot J et al. (2003) Comparison of artificial membrane feeding with direct skin feeding to estimate the infectiousness of Plasmodium vivax gametocyte carriers to mosquitoes. Am J Trop Med Hyg 69 (5), 529–35. [PubMed] [Google Scholar]
- 73.Rios-Velasquez CM et al. (2013) Experimental Plasmodium vivax infection of key Anopheles species from the Brazilian Amazon. Malar J 12, 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moreno M et al. (2014) Infection of laboratory-colonized Anopheles darlingi mosquitoes by Plasmodium vivax. Am J Trop Med Hyg 90 (4), 612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andolina C et al. (2015) The suitability of laboratory-bred Anopheles cracens for the production of Plasmodium vivax sporozoites. Malar J 14, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vallejo AF et al. (2016) Plasmodium vivax gametocyte infectivity in sub-microscopic infections. Malar J 15, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vallejo AF et al. (2016) Optimization of a membrane feeding assay for Plasmodium vivax infection in Anopheles albimanus. PLoS Negl Trop Dis 10 (6), e0004807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang C et al. (2016) A reassessment of the artificial infection of three predominant mosquito species with Plasmodium vivax in Shandong Province, China. J Vector Borne Dis 53 (3), 208–14. [PubMed] [Google Scholar]
- 79.McClean CM et al. (2010) Optimized in vitro production of Plasmodium vivax ookinetes. Am J Trop Med Hyg 83 (6), 1183–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bounkeua V et al. (2011) Lack of molecular correlates of Plasmodium vivax ookinete development. Am J Trop Med Hyg 85 (2), 207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Collins WE et al. (1986) Infection of mosquitoes with Plasmodium vivax from chimpanzees using membrane feeding. Am J Trop Med Hyg 35 (1), 56–60. [DOI] [PubMed] [Google Scholar]
- 82.Ponnudurai T et al. (1990) Large-scale production of Plasmodium vivax sporozoites. Parasitology 101 (03), 317–320. [DOI] [PubMed] [Google Scholar]
- 83.Sullivan JS et al. (1996) Plasmodium vivax infections in chimpanzees for sporozoite challenge studies in monkeys. Am J Trop Med Hyg 55 (3), 344–9. [DOI] [PubMed] [Google Scholar]
- 84.Collins WE et al. (2008) Observations on the sporozoite transmission of Plasmodium vivax to monkeys. J Parasitol 94 (1), 287–8. [DOI] [PubMed] [Google Scholar]
- 85.Shaw-Saliba K et al. (2016) Infection of laboratory colonies of Anopheles mosquitoes with Plasmodium vivax from cryopreserved clinical isolates. Int J Parasitol 46 (11), 679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lupton EJ et al. (2015) Enhancing longevity of Plasmodium vivax and P. falciparum sporozoites after dissection from mosquito salivary glands. Parasitol Int 64 (2), 211–8. [DOI] [PubMed] [Google Scholar]
- 87.Singh N et al. (2016) A simple and efficient method for cryopreservation and recovery of viable Plasmodium vivax and P. falciparum sporozoites. Parasitol Int 65 (5 Pt B), 552–557. [DOI] [PubMed] [Google Scholar]
- 88.Patrapuvich R et al. (2016) Viability and infectivity of cryopreserved Plasmodium vivax sporozoites. Southeast Asian J Trop Med Public Health 47 (2), 171–81. [PubMed] [Google Scholar]
- 89.Mordmüller B et al. (2017) Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 542 (7642), 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chattopadhyay R et al. (2010) Establishment of an in vitro assay for assessing the effects of drugs on the liver stages of Plasmodium vivax malaria. PLoS One 5 (12), e14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sattabongkot J et al. (2006) Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and Plasmodium vivax. Am J Trop Med Hyg 74 (5), 708–715. [PubMed] [Google Scholar]
- 92.March S et al. (2013) A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe 14 (1), 104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.March S et al. (2015) Micropatterned coculture of primary human hepatocytes and supportive cells for the study of hepatotropic pathogens. Nat Protoc 10 (12), 2027–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maher SP et al. (2014) Microphysical space of a liver sinusoid device enables simplified long-term maintenance of chimeric mouse-expanded human hepatocytes. Biomed Microdevices 16 (5), 727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ng S et al. (2015) Human iPSC-derived hepatocyte-like cells support Plasmodium liver-stage infection in vitro. Stem Cell Reports 4 (3), 348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dembele L et al. (2014) Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nat Med 20 (3), 307–12. [DOI] [PubMed] [Google Scholar]
- 97.Hester J et al. (2013) De novo assembly of a field isolate genome reveals novel Plasmodium vivax erythrocyte invasion genes. PLoS Negl Trop Dis 7 (12), e2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Menard D et al. (2013) Whole genome sequencing of field isolates reveals a common duplication of the Duffy binding protein gene in Malagasy Plasmodium vivax strains. PLoS Negl Trop Dis 7 (11), e2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hostetler JB et al. (2016) Independent origin and global distribution of distinct Plasmodium vivax Duffy binding protein gene duplications. PLoS Negl Trop Dis 10 (10), e0005091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Loy DE et al. (2017) Out of Africa: origins and evolution of the human malaria parasites Plasmodium falciparum and Plasmodium vivax. Int J Parasitol 47 (2–3), 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Flannery EL et al. (2015) Next-generation sequencing of Plasmodium vivax patient samples shows evidence of direct evolution in drug-resistance genes. ACS Infect Dis 1 (8), 367–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin JT et al. (2015) Using amplicon deep sequencing to detect genetic signatures of Plasmodium vivax relapse. J Infect Dis 212 (6), 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nair S et al. (2014) Single-cell genomics for dissection of complex malaria infections. Genome Res 24 (6), 1028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cubi R et al. (2017) Laser capture microdissection enables transcriptomic analysis of dividing and quiescent liver stages of Plasmodium relapsing species. Cell Microbiol 19 (8), e12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chan ER et al. (2015) Comparative analysis of field-isolate and monkey-adapted Plasmodium vivax genomes. PLoS Negl Trop Dis 9 (3), e0003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Henry-Halldin CN et al. (2011) High-throughput molecular diagnosis of circumsporozoite variants VK210 and VK247 detects complex Plasmodium vivax infections in malaria endemic populations in Papua New Guinea. Infect Genet Evol 11 (2), 391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chan ER et al. (2012) Whole genome sequencing of field isolates provides robust characterization of genetic diversity in Plasmodium vivax. PLoS Negl Trop Dis 6 (9), e1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen JH et al. (2010) Immunoproteomics profiling of blood stage Plasmodium vivax infection by high-throughput screening assays. J Proteome Res 9 (12), 6479–89. [DOI] [PubMed] [Google Scholar]
- 109.Lu F et al. (2014) Profiling the humoral immune responses to Plasmodium vivax infection and identification of candidate immunogenic rhoptry-associated membrane antigen (RAMA). J Proteomics 102, 66–82. [DOI] [PubMed] [Google Scholar]
- 110.Chuquiyauri R et al. (2015) Genome-scale protein microarray comparison of human antibody responses in Plasmodium vivax relapse and reinfection. Am J Trop Med Hyg 93 (4), 801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hostetler JB et al. (2015) A library of Plasmodium vivax recombinant merozoite proteins reveals new vaccine candidates and protein-protein interactions. PLoS Negl Trop Dis 9 (12), e0004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bozdech Z et al. (2008) The transcriptome of Plasmodium vivax reveals divergence and diversity of transcriptional regulation in malarai parasites. Proc Natl Acad Sci U S A 105 (42), 16290–16295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Westenberger SJ et al. (2010) A systems-based analysis of Plasmodium vivax lifecycle transcription from human to mosquito. PLoS Negl Trop Dis 4 (4), e653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boopathi PA et al. (2016) Design, construction and validation of a Plasmodium vivax microarray for the transcriptome profiling of clinical isolates. Acta Trop 164, 438–447. [DOI] [PubMed] [Google Scholar]
- 115.Zhu L et al. (2016) New insights into the Plasmodium vivax transcriptome using RNA-Seq. Sci Rep 6, 20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Acharya P et al. (2011) Clinical proteomics of the neglected human malarial parasite Plasmodium vivax. PLoS One 6 (10), e26623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moreno-Pérez DA et al. (2015) Determining the Plasmodium vivax VCG-1 strain blood stage proteome. J Proteomics 113, 268–280. [DOI] [PubMed] [Google Scholar]
- 118.Pfahler JM et al. (2006) Transient transfection of Plasmodium vivax blood stage parasites. Mol Biochem Parasitol 149 (1), 99–101. [DOI] [PubMed] [Google Scholar]
- 119.Moraes Barros RR et al. (2015) Editing the Plasmodium vivax genome, using zinc-finger nucleases. J Infect Dis 211 (1), 125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sá JM et al. (2006) Expression and function of pvcrt-o, a Plasmodium vivax ortholog of pfcrt, in Plasmodium falciparum and Dictyostelium discoideum. Mol Biochem Parasitol 150 (2), 219–28. [DOI] [PubMed] [Google Scholar]
- 121.Auliff AM et al. (2012) Functional analysis of Plasmodium vivax dihydrofolate reductase-thymidylate synthase genes through stable transformation of Plasmodium falciparum. PLoS One 7 (7), e40416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Somsak V et al. (2011) Transgenic Plasmodium parasites stably expressing Plasmodium vivax dihydrofolate reductase-thymidylate synthase as in vitro and in vivo models for antifolate screening. Malar J 10, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ghorbal M et al. (2014) Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat Biotechnol 32 (8), 819–21. [DOI] [PubMed] [Google Scholar]
- 124.Zhang C et al. (2014) Efficient editing of malaria parasite genome using the CRISPR/Cas9 system. MBio 5 (4), e01414–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wagner JC et al. (2014) Efficient CRISPR-Cas9-mediated genome editing in Plasmodium falciparum. Nat Methods 11 (9), 915–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee MC and Fidock DA (2014) CRISPR-mediated genome editing of Plasmodium falciparum malaria parasites. Genome Med 6 (8), 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lu J et al. (2016) A redesigned CRISPR/Cas9 system for marker-free genome editing in Plasmodium falciparum. Parasit Vectors 9, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mogollon CM et al. (2016) Rapid generation of marker-free P. falciparum fluorescent reporter lines using modified CRISPR/CAS9 constructions and selection protocol. PLoS One 11 (12), e0168362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Espinosa DA et al. (2013) Development of a chimeric Plasmodium berghei strain expressing the repeat region of the P. vivax circumsporozoite protein for in vivo evaluation of vaccine efficacy. Infect Immun 81 (8), 2882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mizutani M et al. (2016) Development of a Plasmodium berghei transgenic parasite expressing the full-length Plasmodium vivax circumsporozoite VK247 protein for testing vaccine efficacy in a murine model. Malar J 15 (1), 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alves E et al. (2017) Evaluation of Plasmodium vivax cell-traversal protein for ookinetes and sporozoites as a preerythrocytic P. vivax vaccine. Clin Vaccine Immunol 24 (4), e00501–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Azevedo MF and del Portillo HA (2007) Promoter regions of Plasmodium vivax are poorly or not recognized by Plasmodium falciparum. Malar J 6, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Voorberg-van der Wel A. et al. (2013) Transgenic fluorescent Plasmodium cynomolgi liver stages enable live imaging and purification of malaria hypnozoite-forms. PLoS One 8 (1), e54888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hill RJ et al. (2016) Regulation and essentiality of the StAR-related lipid transfer (START) domain-containing phospholipid transfer protein PFA0210c in malaria parasites. J Biol Chem 291 (46), 24280–24292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Moon RW et al. (2016) Normocyte-binding protein required for human erythrocyte invasion by the zoonotic malaria parasite Plasmodium knowlesi. Proc Natl Acad Sci U S A 113 (26), 7231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van der Wel AM et al. (1997) Transfection of the primate malaria parasite Plasmodium knowlesi using entirely heterologous constructs. J Exp Med 185 (8), 1499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tarr SJ et al. (2014) A conserved domain targets exported PHISTb family proteins to the periphery of Plasmodium infected erythrocytes. Mol Biochem Parasitol 196 (1), 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moraes Barros RR et al. (2017) Comparison of two methods for transformation of Plasmodium knowlesi: Direct schizont electroporation and spontaneous plasmid uptake from plasmid-loaded red blood cells. Mol Biochem Parasitol doi: 10.1016/j.molbiopara.2017.10.001 [DOI] [PMC free article] [PubMed]
- 139.Gething PW et al. (2011) A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J 10, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]


