Abstract
Background:
Dynamic MRI has been proposed as a non-invasive, child-friendly, reproducible, and repeatable imaging method providing a three-dimensional view of the velopharyngeal structures and function during speech. However, the value of dynamic MRI as compared to imaging methods such as nasopharyngoscopy is not well understood. The aim of this study was to compare the ability of nasopharyngoscopy and dynamic MRI to accurately identify velopharyngeal closure patterns among adults without cleft palate.
Methods:
Participants included 34 healthy adults with normal anatomy between 19 and 33 years of age (mean = 23 years; SD = 4.1 years). Participants underwent dynamic MRI and nasopharyngoscopy studies and comparisons were performed to determine the intra- and inter-rater reliability for accurately determining closure pattern. The MRI acquisition was a dynamic acquisition of a 2D plane.
Results:
Strong inter- (κ = .824; p = <0.001) and intra-rater (Rater 1: κ = 0.879, p = <0.001, 94% agreement between ratings; Rater 2 with 100% agreement) agreement was observed for the identification of closure pattern using nasopharyngoscopy. Inter-rater agreement for ratings using MRI demonstrated moderate agreement (κ = .489; p = <0.004). Examining point agreement, revealed only 27 of the 33 ratings of MRI showed agreement (80%).
Conclusion:
This demonstrates that inter-rater reliability for determining closure patterns from nasopharyngoscopy is good, however, ratings using MRI was less reliable at determining closure patterns. It is likely that future improvements in dynamic imaging with MRI to enable 3D visualizations are needed for improved diagnostic accuracy for assessing velopharyngeal closure patterns.
Keywords: nasopharyngoscopy, magnetic resonance imaging, velopharyngeal function
INTRODUCTION
It is estimated between 20–30% of children with a repaired cleft palate will continue to have hypernasal speech and will require additional surgeries to create normal resonance (McWilliams, 1990; Bicknell et al., 2002). The clinical paradigm for resonance evaluations prior to secondary surgery for velopharyngeal dysfunction includes a perceptual assessment by a trained speech-language pathologist followed by instrumental evaluation (Paal et al., 2005). Although listener judgment by a trained speech-language pathologist remains the clinical gold standard for assessing resonance, imaging plays an important role in the evaluation process.
Nasopharyngoscopy and multiview videofluoroscopy are the most commonly used clinical tools for direct assessments of velopharyngeal function (Skolnick and Cohn, 1989; Witzel and Stringer, 1990; Sader et al., 1994; Hess et al., 1996; Lam et al., 2006; Kummer et al., 2012). Survey results suggest nasopharyngoscopy is more commonly used in clinical care compared to videofluoroscopy (Kummer et al., 2012). Numerous studies (Henningsson and Isberg, 1991; Havstam et al., 2005; Lam et al., 2006) have compared nasopharyngoscopy and multiview videofluoroscopy. Studies have used different methods for comparisons between imaging techniques. Although findings vary, all studies agree that both imaging methods present advantages and limitations as imaging tools in velopharyngeal assessments. Given each imaging modality provides varied viewpoints, it is likely that multiple imaging methods may be beneficial in the evaluation of a child with velopharyngeal dysfunction.
The ideal imaging method should be non-invasive, child-friendly, reproducible, and repeatable without harm or risk to the child and should provide a comprehensive three-dimensional view of the velopharyngeal function during speech (Beer et al., 2004). There is presently no single imaging method that meets all of these criteria (Beer et al., 2004; Silver et al., 2011). Several research groups have proposed that dynamic magnetic resonance imaging (MRI) may be a tool that can meet many of these criteria for successful imaging (Beer et al., 2004; Silver et al., 2011; Perry et al., 2014; Sagar and Nimkin, 2015; Scott et al., 2014; Perry et al., 2017). However, currently MRI is not routine in clinical cleft care. Similar to studies on more traditional imaging methods, MRI also presents with advantages and disadvantages in velopharyngeal assessments. The greatest limitation of dynamic MRI has typically been attributed to high costs and limited views with either slow imaging or low spatial coverage. However, a typical whole head MRI scan is generally between $250 and $400 and more recently research groups have demonstrated higher imaging speeds of up to 100 frames per second (Fu et al., 2015; Fu et al., 2017). Current dynamic MRI scans of speech utilize a single 2D imaging plane to increase speed. Several imaging planes may be acquired in separate scans with the same speech sample, but temporal alignment of the different imaging planes is challenging due to timing differences in repetitions of speech samples. New engineering developments are showing promising results in obtaining full 3D images of the vocal tract, but such methods have not been applied in large studies yet (Fu et al., 2017).
A second reason for lack of clinical implementation of MRI in cleft care is due to the limited investigations comparing MRI to traditional imaging methods. As a result, it is not clear what role dynamic MRI plays in the clinical evaluation process. Ozgur and colleagues (2000) compared static MRI data during sustained phonation to that of nasopharyngoscopy. Results, although largely qualitative, demonstrate static MRI (during rest and sustained phonation) can provide similar information as nasopharyngoscopy. Specifically, both methods could display the velopharyngeal gap, lateral pharyngeal wall displacements, and velar position between rest and sustained phonation. Additionally, MRI allowed for direct in-plane measurements of the gap size that could not be obtained on the nasopharyngoscopy. The authors propose the combination of imaging methods may be useful in clinical care. Static MRI, however, is not an optimal clinical evaluation tool because sustained phonation tasks have been shown to present with exaggerated velopharyngeal function compared to natural speech (Sorenson, 1989). Research is needed to compare true dynamic MRI methods to traditional dynamic imaging methods, such as nasopharyngoscopy.
Sagar and Nimkin (2015) used 2D dynamic or cine MRI with simultaneous audio recordings among five children with velopharyngeal dysfunction to compare methods to nasopharyngoscopy and videofluoroscopy. Results indicated that velopharyngeal insufficiency was correctly identified with MRI across all five children. MRI was determined to be superior in assessing the anatomy of the velopharynx, quantifying adenoid and tonsillar tissue sizes and locations, and assessing discontinuity in the levator veli palatini musculature. MRI accurately identified closure pattern in 3 out of 5 children, showing a 60% accuracy rate compared to nasopharyngoscopy. However, given this is a small sample size, intra- and inter-rater reliability measures were not reported or examined. Additionally, the cine MRI used in Sagar and Nimkin (2015) only achieved 2 images per second, which limits the ability to visualize closure and contact. Further studies are needed to examine the reliability of dynamic MRI in assessing velopharyngeal closure patterns and using a larger sample size.
Technological advances are bringing MRI closer to clinical implementation (Sutton et al., 2009; Sutton et al., 2010; Scott et al., 2012; Perry et al., 2017; Fu et al., 2017) and improvements in imaging speed and spatial coverage provide a tool that is more similar to traditional imaging methods, such as nasopharyngoscopy. Perry et al. (2014) demonstrated a method of using dynamic and static MRI data for assessing velopharyngeal function among adult participants. A follow up study, using similar methods, was applied to young children using sentence-level speech stimuli (Perry et al., 2017). Dynamic MRI methods have also been proposed for use on clinical MRI scanners (Scott et al., 2012; Sagar and Nimkin, 2015). Fu et al. (2015, 2017) demonstrated the application of high speeds of up to 100 frames per second and fully 3D imaging acquisitions. Despite these advances, the value of 2D dynamic MRI as compared to imaging methods such as nasopharyngoscopy is not well understood. The aim of this study was to compare the ability of nasopharyngoscopy and dynamic MRI to accurately identify velopharyngeal closure patterns among noncleft adults.
METHODS
Participants
In accordance with the local Institutional Review Boards, 34 healthy adult participants, with normal anatomy between 19 and 33 years of age (mean = 23 years; SD = 4.1 years) participated in the study, 21 males and 13 females. All participants were native English speakers and indicated no history of craniofacial anomalies, permanent hearing loss, swallowing disorders, sleep apnea, or neurologic disorders that might affect measures of structures and their movements. All participants were judged by a speech-language pathologist to have normal oral-to-nasal resonance balance and presented with no observable oral-nasal abnormalities. Articulation skills were assessed to be normal. Participants all completed a flexible nasopharyngoscopy and dynamic MRI study using the same speech stimuli described below.
Flexible nasopharyngoscopy and dynamic MRI
An otolaryngologist with over 10 years of experience in imaging of the velopharyngeal system performed nasopharyngoscopy assessments using a JedMed Machida 3.2 mm diameter Flexible Nasopharyngoscope #49–5030, which were digitized and stored using a subject code. Movie files were clipped to remove any identifying information (e.g., image of the patient before the scope or patient number). Images were obtained with a resolution of 63.8 X 63.8 pixels. The participant’s nose was decongested bilaterally using Rhinall and then anesthetized with Lidocaine. A flexible nasopharyngoscope was passed through the middle meatus of the most patent nostril and positioned above the velopharyngeal port. The participants were instructed to repeat the production of “ansa,” “ampa,” and sustain an /s/ sound for 10 seconds. Participants’ repetition of stimuli “ansa” and “ampa” were paced using a metronome beat at a rate of 120 beats per minute (BPM) which resulted in one syllable per beat and three repetitions in 3 seconds. The speech stimuli were selected to represent velar movements and transitions between a lowered (nasals) and elevated (consonants) velar position. The repetitive nature of the stimuli was optimal for the parameters of the dynamic MRI, as outlined by Goud Lingala et al. (2016) in response to a consensus statement from an International Society for Magnetic Resonance in Medicine (ISMRM)-endorsed speech MRI summit.
Participants were imaged in the supine position using a Siemens 3 Tesla Trio (Erlangen, Germany) MRI scanner and a 12-channel Siemens Trio head coil. Participants wore an MR-compatible headset with an attached optical microphone (Dual Channel-FOMRI, Optoacoustics Ltd., Or Yehuda, Israel) for active cancellation of the loud MR gradient noise while preserving the speech samples. Simultaneous speech recordings were obtained and audio and video files were aligned using the acquisition simulation software provided by the vendor of the MRI scanner. This software allows for accurate simulations of sequence timing using the exact acquisition protocol, giving information about the actual time location of data acquisition events with 10 μs accuracy.
The imaging protocol used a non-Cartesian spiral sequence to obtain a fast-gradient echo Fast Low Angle Shot (FLASH) multi-shot spiral technique to acquire 15.8 frames per second (fps) as previously described by Sutton et al. (2010). The sequence uses a time-efficient acquisition of a six-shot spiral pulse sequence with an alternating echo time (TE) between 1.3 and 1.8 ms to allow for dynamic estimation and correction of the magnetic field map. Images were obtained at 1.87 X 1.875 X 8 mm3 spatial resolution. Multiple saturation bands were used to decrease the signal created from regions of higher fat concentrations (such as the cheeks) and to suppress the signal from regions outside of the area of interest. Fast frame rates are achieved through the use of an optimized acquisition strategy coupled with an image reconstruction method that corrects for effects caused by imperfections in the magnetic field in the oropharyngeal region (Sutton et al., 2010). The oblique coronal plane was sampled through the belly and along the length of the levator veli palatini muscle. The speech sample was carefully selected to represent movements of the velum between lowered (i.e., nasal consonants) and fully elevated (i.e., oral pressure consonants), as well as transitions between the lowered and elevated articulatory positions. A metronome was played over the headphones at a rate of 120 BPM to instruct the subject on the pace of one syllable per beat for the “ampa” and “ansa” repetitions. Figure 1 demonstrates a selection of images across the production of “ansa” to demonstrate the structures of interest including lateral pharyngeal walls, velum, and posterior pharyngeal wall in both dynamic MRI and nasopharyngoscopy. Figure 2 demonstrates the planes and cross-section utilized to obtain dynamic MR imaging and, although not a purpose of this study, the levator muscle can be visualized in this image as well as in the MR images in Figure 2b and 2c.
Figure 1.
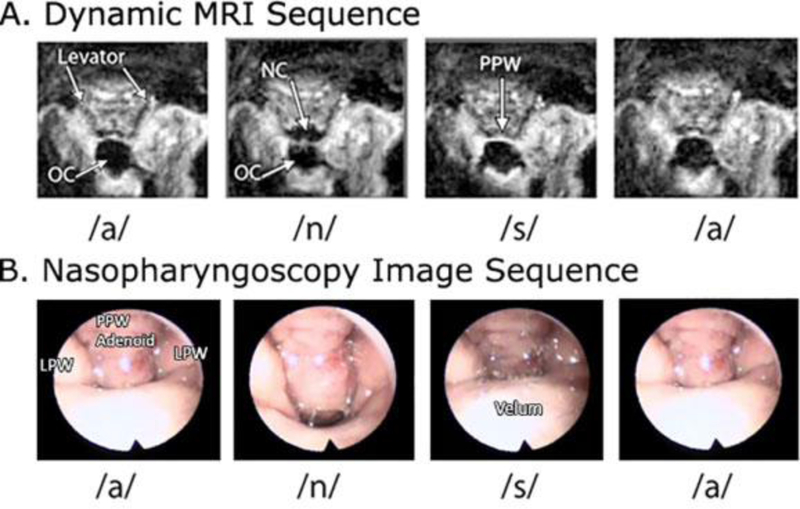
Still images captured from dynamic MRI and nasopharyngoscopy during the production of /ansa/. OC, oral cavity; NC, nasal cavity; PPW, posterior pharyngeal wall; LPW, lateral pharyngeal wall.
Figure 2.

MRI imaging planes and oblique coronal (in-plane) cross section obtained during dynamic MRI acquisition.
Images were reconstructed with an output time-driven sliding window process and images were exported at 30 fps, in which a single image is gathered from the data closest to the desired time point. This results in a minimal amount of interpolation across time. The sliding window reconstruction process minimizes redundant information in adjacent time points and minimizes temporal blurring (Sutton et al., 2009).
Measures obtained and statistical analyses
Two speech-language pathologists (senior author and study coauthor) served as raters for the investigation. Both raters have training and extensive experience in the clinical evaluation of individuals with cleft palate and routinely perform diagnostic evaluations using nasopharyngoscopy. Additionally, both raters have experience in measuring MRI data and have published methodology for such measures. Raters completed a training session which involved MRI and nasopharyngoscopy data from a selected group of six participants. The raters discussed the methods for determination of the four identified closure patterns including 1) coronal, 2) circular, 3) circular with Passavant’s ridge, and 4) sagittal. Definitions for determining categorical ratings were based on definitions created from nasopharyngoscopy studies by Armour et al. (2005), Skolnick et al. (1973), and Finklestein et al. (1995), with the exception of Finklestein’s “coronal with lateral wall movement” category. Following the training session, raters independently rated the sampled training dataset and compared ratings. The raters discussed areas of discrepancy until 100% agreement was found on all sampled training data. Following the training session, nasopharyngoscopy and MRI data were randomized and raters independently rated the samples. Both MRI and nasopharyngoscopy videos were able to be replayed, paused, and/or viewed frame-by-frame or with slower playback functions standard in computer movie viewer programs while making judgements on closure pattern. Two weeks later, the raters again rated another randomized dataset, rating 100% of the participant data to determine intra-rater reliability. A Cohen’s kappa coefficient was used because of its ability to assess the agreement between and within raters based on categorical variables (closure pattern). Specifically, we measured the inter- and intra-rater agreement for the categorical ratings of closure pattern.
RESULTS
Examination of nasopharyngoscopy data displayed strong agreement (κ = .81 – 1.00, Landis and Koch, 1977). Inter-rater reliability for the raters was found to be κ = .824 (p = < .001) between raters indicating that raters agreed 91% of the time. Inspection of the percent of 100% agreement displayed only three out of 34 ratings of nasopharyngoscopy data that showed disagreement (9%). Of the total 34 participants, all demonstrated either coronal or circular closure pattern, based on the nasopharyngoscopy ratings.
Inter-rater reliability of MRI ratings showed moderate agreement (κ =.41-.60, Landis and Koch, 1977) of κ = .489 (p = < .004) between raters indicating raters agreed 80% of the time. This demonstrates that inter-rater reliability for determining closure patterns from nasopharyngoscopy is good, however, ratings using MRI was less reliable for determining closure patterns.
Intra-rater reliability for nasopharyngoscopy also showed high agreement (Rater 1: κ = 0.879, p = < .001, 94% agreement between ratings; Rater 2 with 100% agreement). The category for Rater 1 rating of coronal and circular changed However, MRI ratings showed slight/fair agreement (κ = .0-.40, Landis and Koch, 1977). The intra-rater reliability for rater one was κ = .176 (p = .244; 60% agreement between ratings) and the intra-rater reliability for rater two was κ = .284 (p = < .092; 68% agreement between ratings). This intra-rater discrepancy demonstrates that ratings using MRI is less reliable in evaluating closure pattern compared to that of nasopharyngoscopy. Raters were three times more likely to change the rating of closure in MRI from coronal to circular than from circular to coronal.
All participants displayed velopharyngeal closure as evidenced by no gap. No participant was observed to display sagittal or circular with Passavant’s ridge pattern. This was expected in that all participants had normal velopharyngeal mechanisms. Both raters more frequently identified participants to have a coronal closure pattern when viewing from MRI compared to that of nasopharyngoscopy (Table 1).
Table 1.
Number of Closure Patterns Labeled Using MRI Versus Nasopharyngoscopy.a
| Reliability | ||
|---|---|---|
| Imaging Modality | Interrater k | Percentage Agreement |
| Nasopharyngoscopy | 0.824 | 91% |
| Magnetic resonance imaging | 0.489 | 80% |
No participant was observed to display sagittal or circular with passavant ridge closure pattern.
DISCUSSION
Nasopharyngoscopy is a commonly used instrumental tool for assessing velopharyngeal dysfunction in children with cleft palate and hypernasal speech. It is particularly beneficial because it is able to determine closure patterns, assess velopharyngeal motions, and detect a velopharyngeal gap size and location. Past research has suggested the combined benefit of nasopharyngoscopy and MRI for the evaluation of velopharyngeal closure (Ozgur et al., 2000; Sagar and Nimkin, 2015; Perry et al., 2017). However, small sample sizes and the limited ability to dynamically view the velopharyngeal structures during speech tasks were limiting factors of previous studies. To the best of our knowledge, no studies have examined the reliability of dynamic MRI in assessing closure patterns, as compared to that of nasopharyngoscopy. Results from this study demonstrate that the rate and image resolution of the current dynamic MRI data from this study was less reliable than nasopharyngoscopy at determining closure pattern. The low intra-rater reliability further indicates that the use of dynamic MRI for labeling closure pattern requires further investigation.
It is likely that the alternate viewpoints provided by nasopharyngoscopy and dynamic MRI contribute to the differences in assessing closure patterns. Nasopharyngoscopy gives a bird’s eye view of the mechanism while providing depth-perception cues including shadows, bubbling of mucous at the portal, and a view of structures above and below the plane of velopharyngeal closure. However, nasopharyngoscopy has been criticized for the distortion created by such depth cues (Pigott, 2002). Objects further from the lens of the scope appear disproportionately smaller than objects closure to the lens. These depth distortion cues limit the accuracy of measurements of the velopharyngeal portal (e.g., velopharyngeal depth or portal width) but provide accuracy in determining closure patterns (Pigott and Makepeace, 1982; Pigott, 2002; Sagar and Nimkin, 2015). Studies have demonstrated inter-rater reliability of nasopharyngoscopy ratings ranging from poor to good, indicating room for improvement (Yoon et al., 2006; Sie et al., 2008; Tieu et al., 2012). In contrast, the dynamic 2D MRI that is currently used provides a single slice or image along the oblique coronal plane, in line with velopharyngeal closure and offers no depth cues that are common in nasopharyngoscopy. Due to the lack of depth distortion and the in-plane nature of MRI, it is superior at making precise measures of the velopharyngeal portal at the plane of closure (Sagar and Nimkin, 2015; Perry et al., 2015). Specifically, the width and depth of the portal can be measured and the size of the velopharyngeal gap can be determined.
Figure 3 demonstrates the coronal and circular closure patterns in which both raters had 100% agreement in the classification. As evident in the image, the coronal closure pattern has primary closure via the posterior movement of the velum and the circular shows greater involvement of the lateral pharyngeal walls creating a sphincter-like closure. In the dynamic MRI, the images on the right demonstrate a moment of closure just before full closure. In the MR images during coronal closure patter, the velopharyngeal portal appears as a rectangular bar that gets smaller closing from front to back. In contrast, the circular closure pattern appears as a distinctive circular dark whole, getting progressively smaller with closure. This can only be appreciated during the movement from open to close and during fully closed, this observation is less distinct. However, the distinctive circle (as seen in Figure 3, bottom right image) was not consistently visible and thus was labeled coronal when the scope demonstrated lateral pharyngeal wall motion and sphincter-like closure receiving a label of circular.
Figure 3.

Demonstration of the coronal and circular closure patterns in which both raters had 100% agreement in the classification. In the dynamic MRI, the images on the far right demonstrate a moment of closure just before full closure to highlight the bar-shaped portal opening compared to the circular portal pattern seen in the circular closure pattern.
In the present study, participants were more likely to be labeled having a coronal closure pattern when viewing their MRI data. This suggests that dynamic MRI methods used in the present study may be better at assessing anterior-to-posterior movements but may be limited in assessing lateral pharyngeal wall motions. Using planar x-ray tomography, Iglesias et al. (1980) demonstrated that maximal mesial displacement of the lateral pharyngeal walls for various speech sounds among a group of normal adult participants occurs at a level of and just below the plane of the hard palate. In all of the participants in the current study, the MRI plane of view is somewhat above this level. Thus, the maximal level of mesial displacement of the lateral pharyngeal walls was below the visual plane of the MRI section. As a result, the MR images did not capture the major inward motion of the lateral pharyngeal walls thereby possibly under-representing their contribution that would lead to a judgment of circular closure of the velopharyngeal port. Because 2D dynamic MRI methods were used in the present study, the level of closure plane could not be adjusted. However, developments with 3D dynamic acquisition (Fu et al., 2017) will provide great flexibility is adjusting the image plane during speech. It is likely that different positioning of the image plane from 3D dynamic MRI data would be of clinical value, particularly in assessing velar and pharyngeal wall motions, and detection of a velopharyngeal gap.
Because all participants in the present study were non-cleft adults with normal velopharyngeal function, there were no instances of a velopharyngeal gap observed by the raters to determine velopharyngeal gap size. However, using MRI for measuring velopharyngeal gap estimates, velopharyngeal portal dimensions, and describing soft tissue motions (e.g., levator veli palatini muscle, velum, and lateral and posterior pharyngeal wall motions) have been previously demonstrated (Ozgur et al., 2000; Vadodaria et al., 2000; Scott et al., 2012; Scott et al., 2014; Perry et al., 2014; Sagar and Nimkin, 2015; Perry et al., 2017). Although quantitative data appears to be a strength of MRI, the present study demonstrates that qualitative assessments of velopharyngeal closure pattern, is currently an inherent weakness of the dynamic MRI methods used in the present study. In the present study, stimuli were chosen to display transitions in velopharyngeal structures between open and closed positions. However, for clinical population in determining the presence of velopharyngeal dysfunction, stimuli should not contain oral and nasal stimuli within the same samples (as in the present study). Developments in MRI using sentence-level stimuli have been described and are examples of more suitable speech stimuli for clinical populations (Perry et al., 2017).
It is possible that an axial MRI viewpoint, as used by Sagar and Nimkin (2015) may provide improved visualization of the closure patterns instead of the oblique coronal plane used in the present study. The oblique coronal image plane was used as the plane of closure in the present study because it follows the trajectory of the levator veli palatini muscular contraction thus moving the velum superiorly and posteriorly toward the posterior pharyngeal wall. By positioning the image plane along the levator veli palatini muscle, muscle contraction properties can be visualized (Ha et al., 2007; Tian et al., 2010; Sagar and Nimkin, 2015; Perry et al., 2015; Perry et al., 2017). However, a viewpoint along the axial plane may be more similar to that seen in nasopharyngoscopy. The current study utilized 2D methods for dynamic acquisition, thus the images cannot be viewed from multiple planes. Three dimensional imaging methods at high frame rates, such as that by Fu et al. (2015, 2017), would allow examination of closure from multiple planes. Future studies should compare velopharyngeal closure patterns along the oblique coronal image plane to that of the axial image plane to determine optimal image plane of acquisition. The results of such a study are also needed to establish guidelines for imaging studies to produce comparable data between dynamic MRI studies.
All imaging methods present with advantages and disadvantages. The primary disadvantage of nasopharyngoscopy is the invasive nature of the procedure, which also may not be well tolerated by young children. Additionally, the distorted depth cues and wide angle distortions can limit the interpretation of size of structure, extent of closure, and validity and reliability estimates of velopharyngeal orifice estimates (Pigott and Makepeace, 1982; Sinclair et al., 1982; Karnell et al., 1983; Henningsson and Isberg, 1991; Pigott, 2002; Lam et al., 2006). The involvement of a topical anesthetic, when used, is also seen as a limitation of nasopharyngoscopy. Advantages of nasopharyngoscopy include the ability to assess and directly visualize velar movement, posterior and lateral pharyngeal wall movements, and anatomical structures or defects. Additional features such as degree or type of closure, gap location, portal shape, and amount of velar movement can be assessed using nasopharyngoscopy.
MRI is advantageous because it is non-invasive, does not use radiation, and provides a view of the exact plane of closure without the depth-distortion effect commonly seen in nasopharyngoscopy. However, as shown in the present study, dynamic MRI with the parameters reported in the present study is less accurate than nasopharyngoscopy at determining closure patterns. Many authors have described the process of using accurate assessments of velopharyngeal motions and types of closure as a means to tailoring a surgical intervention during secondary surgery for velopharyngeal dysfunction (Shprintzen et al., 1979; Peat et al., 1994; Cable and Canady, 2003; Marsh, 2003). Interpretations based on nasopharyngoscopy alone are limited to inferences of muscle function based on gross positional changes of the velum. MRI offers a wider view of oropharyngeal area (Witt et al., 2000; Shinagawa et al., 2005) and provides valuable information about the internal velopharyngeal muscles. The primary advantage of MRI is that it is the only imaging modality that can demonstrate the muscle function, morphology, and positioning in vivo (Kuehn et al., 2001, 2004). Studies have already demonstrated how dynamic MRI can be used for assessing muscle function (Tian et al., 2010; Perry et al., 2012; Scott et al., 2012; Perry et al., 2014; Sagar and Nimkin, 2015). However, research is needed to understand how insights related to muscle function direct surgical planning and/or improve outcomes. Results from the present study also elucidate the need for technological advances in dynamic acquisition to increase imaging speed and spatial coverage in three dimensions prior to clinical implementation for the purpose of assessing closure patterns.
Methods are being development to address the issues of needed imaging speed to resolve the closure event and 3D visualization to visualize multiple, arbitrary imaging planes simultaneously. Fast, 3D MRI will combine the advantages of MRI with the depth viewing of nasopharyngoscopy. For example, in Fu et al. (2017), a novel approach to imaging spatial and temporal information in images was developed that enables full vocal tract imaging in 3D at 166 frames per second. These methods require specialized MRI pulse sequences and computational environments to perform the experiment and reconstruct the 3D movie of the speech sample and hence are not widely available yet. In addition, visualization and analysis tools must be developed to be able to utilize these methods in the clinic.
Limitations of the present study include the lack of participants with cleft palate. Additionally, only two of the four closure pattern types were observed among the participants in the present study. Therefore, results are limited to interpretations of dynamic MRI for the two closure patterns presented in this study (coronal and circular). It is possible that the apparent advantages associated with imaging methods may become more evident with a wider range of closure patterns across participants and among those with velopharyngeal dysfunction. These two closure patterns are the most frequent closure pattern types, which likely explains why only these two types were observed. The upright nature of nasopharyngoscopy studies compared to the supine position used during MRI may also have influenced closure pattern. Although closure pattern between supine and upright has not been directly assessed, studies have demonstrated differences in velar height during speech between upright and supine body positions particularly for adults (Perry, 2011; Kollara and Perry, 2014). Siegel-Sadewitz and Shprintzen (1982) demonstrated a degree of physiologic plasticity in the ability of an individual to change velopharyngeal valving patterns. However, it is unclear if a patient might change valving patterns between the two imaging studies and/or the influence of gravity (body position) on ones’ ability to change their closure pattern. The use of a nasal decongestant during nasopharyngoscopy but not during dynamic MRI may have also produced differences that were not examined in the present study.
Although ultra-fast dynamic imaging methods have been created by our collaborative research group (Fu et al., 2015, 2017), nasopharyngoscopy data were not obtained on those participants and thus were not able to be used in the comparison for the present study. It is likely that given the increased frame rate and increased spatial coverage, improvements in the diagnostic accuracy of MRI for assessing closure patterns may be possible.
CONCLUSION
Continued improvements in dynamic imaging quality for MRI will allow for improved diagnostic accuracy of MRI for assessing velopharyngeal closure patterns. Although rater reliability was high for traditional imaging modalities, interpretations of velopharyngeal function based on nasopharyngoscopy alone are limited to inferences of muscle function based on gross positional changes of the velum. MRI offers a wider view of oropharyngeal area (Witt et al., 2000; Shinagawa et al., 2005) and provides valuable information about the internal velopharyngeal musculature. However, in the preset study, ratings of velopharyngeal closure pattern using dynamic 2D MRI was not as reliable as nasopharyngoscopy. This likely points to the need for three dimensional visualization in the assessment of closure patterns and future MRI acquisition techniques will fill this technical gap.
Acknowledgments
Grant Credit: 1R03DC009676-01A1 from the National Institute of Deafness and Other Communicative Disorder
References
- Armour A, Fischbach S, Klaiman P, Fisher DM. Does velopharyngeal closure pattern affect the success of pharyngeal flap pharyngoplasty? Plast Reconstr Surg. 2005;115:45–52. [PubMed] [Google Scholar]
- Beer AJ, Hellerhoff P, Zimmermann A, Mady K, Sader R, Rummeny EJ, Hannig C. Dynamic near-real-time magnetic resonance imaging for analyzing the velopharyngeal closure in comparison with videofluoroscopy. J Magn Reson Imaging. 2004;20:791–797. [DOI] [PubMed] [Google Scholar]
- Bicknell S, McFadden LR, Curran JB. Frequency of pharyngoplasty after primary repair of cleft palate. J Can Dent Assoc. 2002;68(11):688–692. . [PubMed] [Google Scholar]
- Cable BB, Canady JW. The endoscopically assisted pharyngeal flap. Cleft Palate Craniofac J. 2003;40:114–115. [DOI] [PubMed] [Google Scholar]
- Ettema SL, Kuehn DP, Perlman AL, Alperin N. Magnetic resonance imaging of the levator veli palatini muscle during speech. Cleft Palate Craniofac J. 2002;39:130–144. [DOI] [PubMed] [Google Scholar]
- Finklestein Y, Shapiro-Feinberg M, Talmi YP, Nachmani A, DeRowe A, Ophir D. Axial configuration of the velopharyngeal valve and its valving mechanism. Cleft Palat Craniofac J. 1995;32:299–305. [DOI] [PubMed] [Google Scholar]
- Fu M, Barlaz MS, Holtrop JL, Perry JL, Kuehn DP, Shosted RK, Liang Z, Sutton BP. High-resolution full-vocal-tract 3D dynamic speech imaging. Magn Reson Med. 2017;77:1619–1629. Doi: 10.1002/mrm.26248. . [DOI] [PubMed] [Google Scholar]
- Fu M, Bo Z, Shosted RK, Perry JL, Kuehn DP, Liang Z, Sutton BP. High-resolution dynamic speech imaging with joint low-rank and sparsity constraints. Magn Reson Med. 2015;73:1820–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud Lingala S, Sutton BP, Miquel ME, Nayak KS. Recommendations for real-time speech MRI. J Magn Reson Imaging. 2016;43:28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S, Kuehn DP, Cohen M, Alperin N. Magnetic resonance imaging of the levator veli palatini muscle in speakers with a repaired cleft palate. Cleft Palate Craniofac J. 2007;44:494–505. [DOI] [PubMed] [Google Scholar]
- Henningsson GE, Isberg AM. Velopharyngeal movement patterns in patients alternating between oral and glottal articulation: a clinical and cineradiographical study. Cleft Palate J. 1986;23:1–9. [PubMed] [Google Scholar]
- Havstam C, Lohmander A, Persson C, Dotevall H, Lith A, Lilja J. Evaluation of VPI assessment with videofluoroscopy and naasoendoscopy. Brit J Plast Surg. 2005;58:922–931. [DOI] [PubMed] [Google Scholar]
- Hess U, Hannig C, Sader R, Cavallaro A, Wuttge-Hannig A, Zeilhofer H. Evaluation of velopharyngeal closure in preoperative planning of maxillary advancement. Rontgenpraxis. 1996;49:25–26. [PubMed] [Google Scholar]
- Iglesias A, Kuehn DP, Morris HL. Simultaneous assessment of pharyngeal wall and velar displacement for selected speech sounds. J Speech Hear Res. 1980;23:429–46. [DOI] [PubMed] [Google Scholar]
- Karnell MP, Ibuki K, Morris HL, Van Demark DR. Reliability of nasopharyngeal fiberscope (NPF) for assessing velopharyngeal function: analysis by judgment. Cleft Palate J. 1983;20:199–208. [PubMed] [Google Scholar]
- Kollara L, Perry JL. Effects of gravity on the velopharyngeal structures in children using upright magnetic resonance imaging. Cleft Palate Craniofac J. 2014;51:669–676. DOI: 10.1597/13-107. [DOI] [PubMed] [Google Scholar]
- Kuehn DP. A cineradiographic investigation of velar movement variables in two normals. Cleft Palate J. 1976;13:88–103. [PubMed] [Google Scholar]
- Kuehn DP, Ettema SL, Goldwasser MS, Barkmeier JC. Magnetic resonance imaging of the levator veli palatini muscle before and after primary palatoplasty. Cleft Palate Craniofac J. 2004;41:584–592. [DOI] [PubMed] [Google Scholar]
- Kuehn DP, Ettema SL, Goldwasser MS, Barkmeier JC, Wachtel JM. Magnetic resonance imaging in the evaluation of occult submucous cleft palate. Cleft Palate Craniofac J. 2001;38:421–431. [DOI] [PubMed] [Google Scholar]
- Kummer AW, Clark SL, Redle EE, Thomsen LL, Billmire DA. Current practice in assessing and reporting speech outcomes of cleft palate and velopharyngeal surgery: a survey of cleft palate/craniofacial professionals. Cleft Palate Craniofac J. 2012;49:146–152. [DOI] [PubMed] [Google Scholar]
- Lam DJ, Starr JR, Perkins JA, Lewis CW, Eblen LE, Dunlap J, Sie KC. A comparison of nasendoscopy and multiview videofluoroscopy in assessing velopharyngeal insufficiency. Otolaryngol Head Neck Surg. 2006;134:394–402. [DOI] [PubMed] [Google Scholar]
- Landis J & Koch G (1977) Measurement of observer agreement for categorical data, Biometrics. 33: 159–174. [PubMed] [Google Scholar]
- Marsh JL. Management of velopharyngeal dysfunction: differential diagnosis for differential management. J Craniofac Surg. 2003;14:621–628. [DOI] [PubMed] [Google Scholar]
- McWilliams BJ. The long-term speech results of primary and secondary surgical correction of palatal clefts In: Bardach J, Morris HL, eds. Multidisciplinary Management of Cleft Lip and Palate. Philadelphia: WB Saunders; 1990:815–819. [Google Scholar]
- Ozgur F, Tuncbilek G, Cila A. Evaluation for velopharyngeal insufficiency with magnetic resonance imaging and nasendoscopy. Ann Plast Surg. 2000;44:8–13. [DOI] [PubMed] [Google Scholar]
- Paal S, Reulbach U, Strobel-Schwarthoff K, Nkenke E, Schuster M. Evaluation of speech disorders in children with cleft lip and palate. J Orofac Orthop. 2005;66:270–278. [DOI] [PubMed] [Google Scholar]
- Peat BG, Albery EH, Jones K, Piggott RW. Tailoring velopharyngeal surgery: the influence of etiology and type of operation. Plast Reconstr Surg. 1994;93:948–953. [PubMed] [Google Scholar]
- Perry JL. Variations in velopharyngeal structures between supine and upright postures using open-type magnetic resonance imaging. Cleft Palate Craniofac J. 2011;48:123–133. [DOI] [PubMed] [Google Scholar]
- Perry JL, Kuehn DP, Sutton BP, Fang X. Velopharyngeal structural and functional assessments of speech in young children using dynamic magnetic resonance imaging. Cleft Palate Craniofac J. 2017; in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Sutton BP, Kuehn DP, Gamage JK. Using MRI for assessing velopharyngeal structures and function. Cleft Palate Craniofac J. 2014;51(4):476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott RW. An analysis of the strengths and weaknesses of endoscopic and radiological investigations of the velopharyngeal incompetence based on 20 year experience of simultaneous recording. Br J Plast Surg. 2002;55:32–34. [DOI] [PubMed] [Google Scholar]
- Pigott RW, Makepeace AP. Some characteristics of endoscopic and radiological systems used in elaboration of the diagnosis of velopharyngeal incompetence. Br J Plast Surg. 1982;35:19–32. [DOI] [PubMed] [Google Scholar]
- Sader R, Horch HH, Herzog M, Zeilhofer HF, Hannig C, Hess U, Bünte E, Böhme G. High-frequency videocinematography for the objective imaging of the velopharyngeal closure mechanism in cleft palate patients. Fortschr Kieferorthop. 1994;55:169–175. [DOI] [PubMed] [Google Scholar]
- Sagar P, Nimkin K. Feasibility study to assess clinical applications of 3-T cine MRI coupled with synchronous audio recordings during speech in evaluation of velopharyngeal insufficiency in children. Pediatr Radiol. 2015;45:217–227. [DOI] [PubMed] [Google Scholar]
- Scott AD, Boubertakh R, Birch MJ, Miquel ME. Towards clinical assessment of velopharyngeal closure using MRI: evaluation of real-time MRI sequences at 1.5 and 3T. Br J Radiol. 2012;85:e1083–e1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AD, Wylezinska M, Birch MJ, Miquel ME. Speech MRI: morphology and function. Phys Med. 2014;30:604–618. [DOI] [PubMed] [Google Scholar]
- Shinagawa H, Ono T, Honda E, Masaki S, Shimada Y, Fujimoto I, Sasaki T, Iriki A, Ohyama K. Dynamic analysis of articulatory movement using magnetic resonance imaging movies: methods and implications in cleft lip and palate. Cleft Palate Craniofac J. 2005;42:225–230. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ, Lewin ML, Croft CB, et al. A comprehensive study of pharyngeal flap surgery: tailor made flaps. Cleft Palate J. 1979;16:46–55. [PubMed] [Google Scholar]
- Sie KC, Starr JR, Bloom DC, Cunningham M, de Serres LM, et al. Multicenter interrater and intrarater reliability in the endoscopic evaluation of velopharyngeal insufficiency. Arch Otolaryngol Head Neck Surg. 2008;134(7):757–63. doi: 10.1001/archotol.134.7.757. [DOI] [PubMed] [Google Scholar]
- Siegel-Sadewitz VL, Shprintzen RJ. Nasopharyngoscopy of the normal velopharyngeal sphincter: An experiment of biofeedback. Cleft Palate J. 1982;19:194–200. [PubMed] [Google Scholar]
- Silver AL, Nimkin K, Ashland JE, Ghosh SS, van der Kouwe AJ, Brigger MT, Hartnick CJ. Cine magnetic resonance imaging with simultaneous audio to evaluate pediatric velopharyngeal insufficiency. Arch Otolaryngol Head Neck Surg. 2011;137:258–263. [DOI] [PubMed] [Google Scholar]
- Sinclair SW, Davies DM, Bracka A. Comparative reliability of nasal pharyngoscopy and videofluorography in the assessment of velopharyngeal incompetence. Br J Plast Surg. 1982;35:113–117. [DOI] [PubMed] [Google Scholar]
- Skolnick ML, Cohn ER. Videofluoroscopic Studies of Speech in Patients with Cleft Palate. New York: Springer-Verlag; 1989:1–48. [Google Scholar]
- Sorenson DN. A fundamental frequency investigation of children ages 6–10 years old. J Commun Disord. 1989;22(2):115–23. [DOI] [PubMed] [Google Scholar]
- Sutton BP, Conway C, Bae Y, Brinegar C, Liang ZP, Kuehn DP. Dynamic imaging of speech and swallowing with MRI. Conf Proc IEEE Eng Med Biol Soc. 2009;6651–6654. [DOI] [PubMed] [Google Scholar]
- Sutton BP, Conway CA, Bae Y, Seethamraju R, Kuehn DP. Faster dynamic imaging of speech with field inhomogeneity correlated spiral fast low angle shot (FLASH) at 3 T. J Magn Reson Imaging. 2010;32:1228–1237. [DOI] [PubMed] [Google Scholar]
- Tian W, Li Y, Yin H, Zhao SF, Li S, Wang Y, Shi B. Magnetic resonance imaging assessment of velopharyngeal motion in Chinese children after primary palatal repair. J Craniofac Surg. 2010a;21:578–587. [DOI] [PubMed] [Google Scholar]
- Tieu DD, Gerber ME, Milczuk HA, Parikh SR, Perkins JA, Yoon PJ, Sie KC. Generation consensus in the application of a rating scale to nasendoscopic assessment of velopharyngeal function. Arch Otolaryngol Head Neck Surg. 2012;138(10):923–8. doi: 10.1001/archotol.2013.203. [DOI] [PubMed] [Google Scholar]
- Vadodaria S, Goodacre TE, Anslow P. Does MRI contribute to the investigation of palatal function? Br J Plast Surg. 2000;53:191–199. [DOI] [PubMed] [Google Scholar]
- Witt PD, Marsh JL, McFarland EG, Riski JE. The evolution of velopharyngeal imaging. Ann Plast Surg. 2000;45:665–673 [DOI] [PubMed] [Google Scholar]
- Witzel MA, Stringer DA. Methods of assessing velopharyngeal function In: Bardach J, Morris HL, eds. Secondary Surgical Treatment of Cleft Palate. Philadelphia: WB Saunders; 1990:763–773. [Google Scholar]
- Yoon PJ, Starr JR, Perkins JA, Bloom D, Sie KC. Interrater and intrarater reliability in the evaluation of velopharyngeal insufficiency within a single institution. Arch Otolaryngol Head Neck Surg. 2006;132(9):947–51. [DOI] [PubMed] [Google Scholar]


