Abstract
Ultraviolet (UV) absorbance measurements provide a rapid and reliable method to determine protein concentrations. the National Institute of standards and technology (NIST) has developed Standard Reference Material (SRM) 2082 as a pathlength standard for UV absorbance measurements for use with the new generation of microvolume spectrophotometers and short-pathlength cuvettes. short pathlengths are used with high-concentration targets to ensure that absorbance values are within the optimal range. the short-pathlength instruments and cuvettes also reduce the required volumes to conserve valuable samples. the authors compared the results obtained with high-quality dual-beam spectrophotometers and short-pathlength cuvettes to the results obtained from a microvolume spectrophotometer and a microvolume plate reader. SRM 2082 can be used to accurately calculate pathlength values, thereby increasing the accuracy in subsequent measurements using the short-pathlength cuvettes and microvolume absorbance instruments. RM 8671 (reference material, the NISTmAb) can then be used to ensure the accuracy and reproducibility of protein concentration measurements by providing an industrially relevant reference material, a well-characterized monoclonal antibody.
Ultraviolet (UV) and visible spectrophotometers are found in ever y bioanalytical laboratory and are frequently used to measure concentrations of both proteins (1) and nucleic acids (2). Concentration of an analyte is related to absorbance at a specified wavelength by Beer’s Law. Absorbance of light at a specific wavelength is defined as the log10 (I0/I), where I0 is the intensity of the light before it enters the sample and I is the intensity of the light after it passes through the sample. This definition of absorbance assumes light is either absorbed by the solution or transmitted, and that scattered or reflected light is a minor component (3). Measurements of the light transmitted by solutions of large complex molecules that may contain particles that may scatter a significant amount of light are more properly termed decadic attenuance (DA). DA refers to the base 10 logarithm of the ratio of the incident to the reduced light intensity measured in the sample without correction due to scattering or reflection. Protein solutions such as monoclonal antibodies (mAbs), for example, may contain particles capable of light scatter in formulation conditions that induce colloidal instability.
The formulation of protein biopharmaceuticals, such as mAbs in many cases, requires high concentrations to reduce the volumes administered to patients. It is advantageous to do direct measurements on these protein solutions to determine the concentration for quality control programs. This has been made possible by the development of efficient analytical methods and instruments. DA measurements of proteins at high concentrations directly without dilutions requires short pathlengths to ensure that sufficient light is detected by the spectrophotometer. Short-pathlength cuvettes can be used in conventional double-beam spectrophotometers, or a new generation of spectrophotometers have been developed that can measure the full absorbance spectrum of samples using microliter volume (MV) samples using short pathlengths.
A variable pathlength spectroscopic instrument that utilizes a fiber optic probe to measure absorbances at multiple pathlengths (0.01 mm to 15 mm) and the resulting “slope spectroscopy” data can be used to measure highly concentrated protein samples (4–6).
NIST has developed Standard Reference Material (SRM) 2082, a pathlength standard for these MV spectrophotometers and short-pathlength cuvettes (7). SRM 2082 is composed of solutions of tryptophan and uracil in a buffer, and these solutions can be used to evaluate pathlengths in the UV range. The authors propose using SRM 2082 to calibrate short-pathlength cuvettes/instruments and/or determine if MV spectrophotometers are operating correctly within the specifications. Additionally, once pathlength and instrument performance have been verified using SRM 2082, a reference material such as the NIST mAb reference material (RM 8671) may be used to confirm spectrophotometer performance with a protein that has similar biophysical properties to many biopharmaceuticals. The reference value for the concentration of RM 8671 (NISTmAb) was determined using DA measurements at 280 nm, and this reference material has been well characterized by many additional measurement methods (8, 9).
This study, using two example applications, was designed to illustrate how the NIST standards can be used to achieve reproducible optical pathlength and protein concentration measurements. The authors have demonstrated how SRM 2082 can be used to calibrate short-pathlength cuvettes to increase the measurement accuracy and also to benchmark the absorbance measurements of MV spectrophotometers and MV plate readers. RM 8671 was then used as a relevant biopharmaceutical sample to confirm the pathlength calibrations done with SRM 2082 and benchmark the DA measurements using a MV spectrophotometer and a MV plate reader.
MATERIALS AND METHODS
Samples
The development and characterization of SRM 2082 have been detailed (7). SRM 2082 is composed of solutions of tryptophan and uracil in a buffer, 10 mmol/L 2-amino-2-hydroxymethyl-propane-1,3 diol, pH 8.0 buffer (TRIS buffer, included in the standard for a blank sample). SRM 2082 is stored at −20 °C, but can be kept for at least three months at 4 °C. It is important to fully mix the samples by bringing the solutions to ambient temperature and then invert the vials 20 times to ensure a uniform solution.
The NISTmAb (Reference Material 8671, lot 14HB-D-002) is a humanized IgG1κ mAb at a concentration of nominal 10 mg/ mL (9). The NISTmAb is in a buffer composed of 12.5 mmol/L L-histidine, 12.5 mmol/L L-histidine HCl, pH 6.0 (His buffer). Samples were kept at −80 °C for long-term storage. The samples were brought to ambient temperature for approximately 30 min and then inverted 10 times. Samples were aliquoted and stored at −80 °C. The thawed samples could be kept at 4 °C for up to a month.
Certified absorbance values of SRM 2082 and reference values of RM 8671
The certified values of the tryptophan and uracil solutions of NIST SRM 2082 were measured using NIST calibrated cuvettes (0.01 cm to 0.2 cm) and the NIST material measurement laboratory transfer spectrophotometer (traceable to the NIST high-accuracy spectrophotometer) (10). The measurements were done at a spectral bandwidth of 0.8 nm and a temperature of 22 °C. Additional details are available from the certificate of analysis for SRM 2082 (NIST SRM webpage, www.nist.gov/srm) and Lang et al. (7). The reference value measurement of the NISTmAb samples is described in the report of investigation for RM 8671 (also available at www.nist.gov/srm) and a recent publication (9). The NISTmAb RM 8761 (lot 14HB-D-002) reference mass concentration value is 10.003 mg/mL with a standard Type A uncertainty of 0.176 % (Reference Material 8671, www.nist.gov/srm). The protein concentration was calculated using the measured DA values and a theoretical extinction coefficient (1.42 mL·mg−1·cm−1) (9, 11–13). The DA value and uncertainty (Table I) were from the reference measurement of NIST RM 8671 (9), normalized to a 1-cm pathlength.
Table I.
Certified absorbance values for the components of SRM 2082 and the reference value decadic attenuance (DA) for RM 8671. SRM 2082 is Standard Reference Material (SRM) 2082.
| Test sample | Absorbance 260 nm | Absorbance/DA 280 nm |
|---|---|---|
| Tryptophan | 8.350 ± 0.003* | |
| Uracil | 7.990 ± 0.003* | |
| NISTmAb (DA) | 14.204 ± 0.026** | |
| NISTmAb (wavelenth dependent corrected scattering at 320 nm to 340nm) | 14.125 (S.D. 0.008)*** | |
| NISTmAb (simple correction at 320 nm) | 14.145 | |
| NISTmAb (simple correction at 340 nm) | 14.174 |
The uncertainties were calculated as described in the NIST certificate of analysis for SRM 2082 and
decadic attenuance (DA), standard type A uncertainty (9), report of investigation for RM 8671 (see certificates at www.nist.gov/srm).
The DA was corrected for protein scattering done using the DA values from 320 nm to 340 nm as described in the text (13).
Measurements on the MV spectrophotometer and MV plate reader
The SRM 2082 and RM 8671 samples were measured using a MV spectrophotometer (Nanodrop One C, Thermo Fisher Scientific). Samples of the His or TRIS buffer were run to blank the instrument. MV spectrophotometer measurements were performed by applying samples to the measurement surfaces with a pipette (2–3 μL). Between replicates, the sample stage was wiped off with a laboratory wipe, cleaned with water, wiped again, and a new sample applied for measurement. The instrument was operated at ambient temperature (22 °C) in the NIST laboratories. The measurements at 260 nm and 280 nm using the MV spectrophotometer were corrected by the instrument software using the absorbance at 340 nm (non-absorbing region). The MV spectrophotometer was checked to ensure the correct operation based on the recommendations of the manufacturer. The baseline correction from the absorbance at 340 nm is an adjustment to zero the baseline of the sample at that wavelength and is not a wave length-dependent correction specific for protein scattering.
Samples of SRM 2082 and RM 8671 were measured using a MV plate reader (BioTek Synergy MX microvolume plate reader) with a Take3 microvolume plate and operated according to the manufacturer’s instructions. A basic system test was run and evaluated to confirm full operation of the reader’s motors, lamp, the detector, and various subsystems. A calibrated absorbance test plate was used to confirm mechanical alignment, including optical density accuracy, linearity, and repeatability, and wavelength accuracy for the following wavelengths: 405 nm, 450 nm, 490 nm, 550 nm, 620 nm, 630 nm, 690 nm, and 750 nm. An absorbance liquid test was performed to confirm repeatability and alignment of the reader when a solution is used in a micro-plate. The Take3 microvolume plate was pathlength calibrated as described in the manufacturer’s instructions. Briefly, 2 μl of buffer were loaded onto the microspot slide for the blank sample. Both the microspot slide and top slide were cleaned using a dry laboratory wipe and deionized water. Samples of interest were loaded onto the microspot slide and absorbance measured at 260 nm for the uracil component of SRM 2082 and 280 nm for the tryptophan component of SRM 2082 and RM 8671 samples. All values were imported via the Gen5 software and exported into excel for analysis. The values at 260 nm and 280 nm were corrected by subtracting values at 320 nm (non-absorbing region) and normalized to a pathlength of 1 cm. The baseline correction from the absorbance at 320 nm is an adjustment to zero-the-baseline of the sample at that wavelength and is not a wavelength-dependent correction specific for protein scattering.
Results and discussion
Test samples
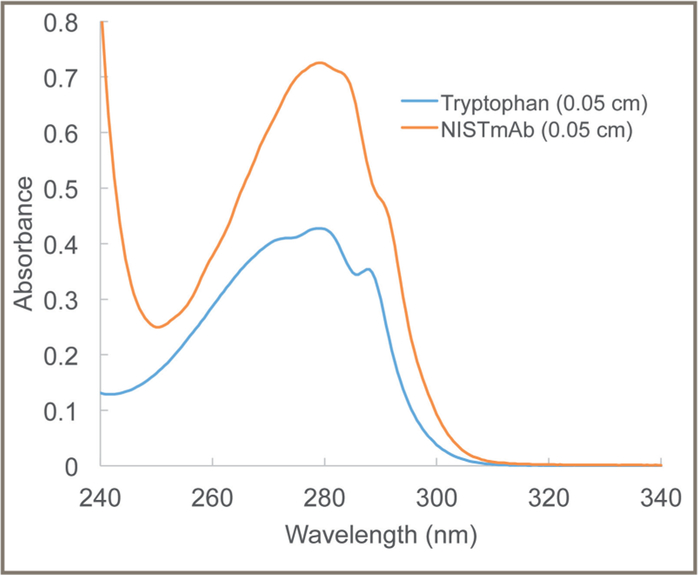
The respective UV spectra of the tryptophan component of SRM 2082 and RM 8671 (the NISTmAb) were measured with a double-beam spectrophotometer (Figure 1). They have a similar shape with a broad peak at 280 nm. To ensure accurate measurements of protein and nucleic acid concentrations, it is important to ensure that the absorbance and pathlength values follow Beer’s Law. Beer’s Law (shown in Equation 1) describes the fundamental relationship among absorbance, target analyte, concentration, and pathlength:
| Eq. 1 |
where A is absorbance, ε is the extinction coefficient, b is pathlength, and c is concentration. The units for the extinction coefficient (ε) are usually mol−1·L·cm−1, for the pathlength cm, and mol· L−1 for concentration (c). Alternatively, the extinction coefficient can have units of mg−1·L·cm−1 resulting in concentrations of mg/mL.
Figure 1:
Ultraviolet spectra of the tryptophan component of SRM 2082 and RM 8671 (the NISTmAb) at 0.05-cm pathlength. The spectral band width was 0.8 nm. SRM 2082 is Standard Reference Material 2082.
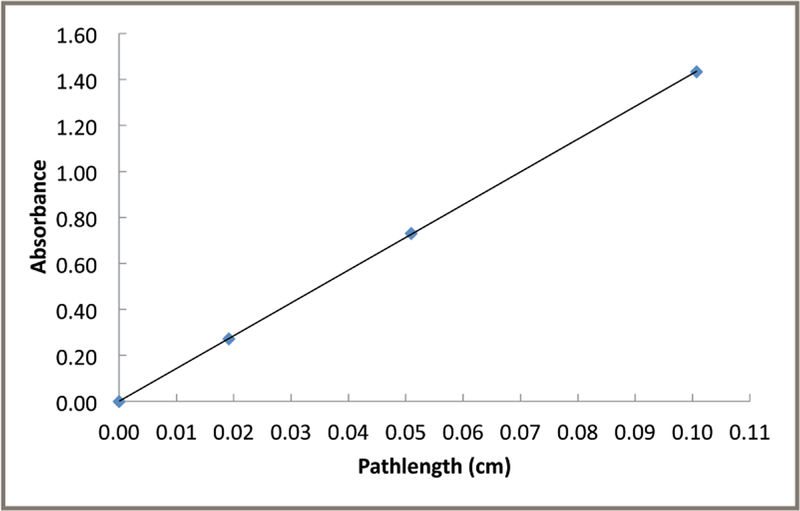
Absorbance is the -log10 of transmittance of light, and it has a proportional (linear) relationship with concentration when solution and instrument conditions follow Beer’s Law. Measurements of the absorbance of target analytes at high concentrations may have serious limitations. An instrumental limitation is that, at high absorbance, very little light is transmitted by the solution and reaches the detector. For instance, at an absorbance of 3, only 0.1 % of the incident light reaches the detector, potentially leading to serious signal-to-noise issues. Shorter pathlengths can help keep the absorbance values in the optimal range for measurements (0.6 absorbance unit [AU]). Figure 2 shows the relationship between the DA of RM 8671 (corrected for absorbance of the HIS buffer) and the pathlengths of the NIST calibrated cuvettes. These results show that the RM 8671 measurements have a linear correlation of DA and pathlength using these conditions. Lang et al. also showed that the tryptophan and uracil components of SRM 2082 also followed a proportional (linear) relationship of UV absorbance with path-length over this same range (7).
Figure 2:
Proportionality of the decadic attenuance (DA) measurements of RM 8671 with cuvette pathlength. Samples of RM 8671 were measured in the NIST calibrated cuvettes at 280 nm with the indicated pathlengths at 22 °C. Single point DA measurements with the NIST calibrated cuvettes had an R2 value for the linear regression of 1.0. The uncertainty in the measurments is approximately 1%.
Protein solutions can contain particulates that scatter light in spectroscopic measurements. Various methods have been used to correct the measurements for light scattering. These methods use measurements at higher wavelengths (above 320 nm), where proteins do not typically absorb light (6, 13, 14). Rayleigh light scattering predicts that if the protein aggregates are smaller than the wavelength of light, then the scattering is inversely proportional to the fourth power of the wavelength of light (λ); shorter wavelengths will scatter greater amounts of light than longer wavelengths (14). Using this theory the corrected absorbance at 280 nm (A280) can be calculated by subtracting the protein light due to scattering by measuring the absorbance at non-absorbing reference wavelengths (Aref) and using the wavelength dependence ((λref/λ280)4) of scattered light, as shown in Equation 2 below (adapted from Maity et al. [13]):
| Eq. 2 |
The authors did the correction for light scattering on the data from the NISTmAb samples (RM 8671, lot 14HB-D-002) using this equation for measurements at the reference wavelengths of 320 nm to 340 nm (average of the data at 0.5 nm resolution) (Table I). This correction is not large (approximately 0.6%) in this case, but it can be significant in some samples.
The reference absorbance values for the components of SRM 2082 and RM 8671, normalized to a 1-cm pathlength to facilitate comparisons, were derived from their reference values measured by NIST (Table I). These values are based on many previous measurements of the NIST reference materials from many measurements, including investigation of several sources of uncertainty in the measurements (www.nist.gov/srm). Simple baseline corrections for the NIST mAb reference values were done by subtracting the DA at 340 nm and 320 nm to facilitate the comparisons of the reference values for MV spectrophotometer and the MV plate reader, respectively (Table I), because the software for these instruments does this correction.
The authors have included two examples of applications using the N IST standards to ensure the qualitiy of the measurments of protein biopharmaceuticals. The first is the use of SRM 2082 to calibrate the pathlength of a short-pathlength cuvette, followed by using the calibrated pathlength of the cuvette to determine the DA of RM 8671 (NISTmAb) and calculating the resulting protein concentration using the theoretical extinction coeficient (13) as a control of the calibration using a typical protein pharmaceutical modeled by RM 8671.
Example #1: calibration of a short-pathlength cuvette
The components of SR M 2082 were used to illustrate the calibration of a short-pathlength cuvette. The cuvette was obtained from a vendor with an approximately (nominal) pathlength of 0.05 cm. This cuvette had been previously calibrated at NIST using interferometry and other dimensional metrology methods (7) as 0.05115 cm.
Although NIST has the higher-order metrology equipment to precisely measure the pathlength, these resources may not be readily available to many biopharmaceutical laboratories. The authors wanted to determine how well a typical laboratory could do a calibration on a short-pathlength cuvette using conventional commercial dual beam spectrophotometers. The absorbance values of the tryptophan (at 280 nm) and uracil (at 260nm) components were measured using two different dual beam spectrophotometers. Equations 3 and 4 were used to calculate the pathlengths (in cm) of the cuvette using three measurements at the respective maximal absorbance wavelength for tryptophan and uracil:
| Eq. 3 |
| Eq. 4 |
Table II shows the pathlengths calculated using measurements from tryptophan, uracil, and the average of both. These measurements were done at a spectral band width of 0.8 nm and a temperature of 22 °C. The coefficients of variation (CVs) for the dual-beam spectrophotometers are less the 0.25% for these measurements (Table II).
Table II.
SRM 2082 components used to calculate the pathlengths of a 0.05 cm cuvette and to calculate the concentration of RM 8671 on two dual-beam (DB) spectrophotometers. SRM 2082 is Standard Reference Material (SRM) 2082
| Method to calculate | Calculatted pathlength,cm | DA 280 nm, RM 8671 (normalized to 1 cm) | Resulting protein (mg/mL) |
Difference (%) |
|---|---|---|---|---|
| DB Spectrophotometer A | ||||
| SRM 2082 Trp. 280 nm | 0.05080 (0.00008, n=3) | 14.249 (0.035) | 10.035 (0.025) | 0.32 |
| SRM 2082 Uracil 260 nm | 0.05096 (0.00001, n=3) | 14.205 (0.035) | 10.003 (0.025) | 0.01 |
| SRM 2082 both | 0.05088 (0.00005, n=3) | 14.227 (0.035) | 10.019 (0.025) | 0.16 |
| Calibration by dimensional metrology and IF | 0.05115 (0.00004)* | 14.152 (0.035) | 9.966 (0.025) | −0.37 |
| Approximate value | 0.05 | 14.477 (0.036) | 10.195 (0.025) | 1.92 |
| DB Spectrophotometer B | ||||
| SRM 2082 Trp. 280 nm | 0.05094 (0.00005, n=3) | 14.291 (0.007) | 10.064 (0.005) | 0.61 |
| SRM 2082 Uracil 260 nm | 0.05129 (0.00004, n=3) | 14.195 (0.007) | 9.997 (0.005) | −0.06 |
| SRM 2082 both | 0.05111 (0.00005, n=3) | 14.243 (0.007) | 10.030 (0.005) | 0.28 |
| Calibration by dimensional metrology and IF | 0.05115 (0.00004)* | 14.233 (0.007) | 10.024 (0.005) | 0.2 |
| Approximate value | 0.05 | 14.560 (0.007) | 10.253 (0.005) | 2.51 |
NIST uncertainty values for the cuvette measurements from previous NIST measurements (Report of Analysis for SRM 2082).
TRP is tryptophan.
IF is infrared spectroscopy.
The next step was to measure the DA of RM 8671 (NISTmAb) using the same cuvette and conditions (spectral band width of 0.8 nm and a temperature of 22 °C) on two dual-beam commercial spectrophotometers. The NISTmAb was measured three times, and the DA values at 280 nm were normalized to 1 cm using the calculated pathlengths shown in Table II. The DA values were then used to calculate an apparent protein concentration by dividing by the theoretical extinction coefficient (1.42 mL·mg−1·cm−1 (14). The differences between the calculated protein concentration and the reference value (10.003 mg/mL) were calculated as a percentage of the reference value. In this manner, SRM 2082 components were used to calculate the pathlengths, and RM 8671 was used as a protein biopharmaceutical control with an established concentration to benchmark the concentration measurements.
The results show that using the approximate (nominal) pathlength for the 0.05 cm cuvette, as provided by the manufacturer, leads to calculated absorbances/DA (and resulting concentration values) 2% to 3% higher than the reference value (for this example). Using the pathlength values obtained using SRM 2082 reduces the differences to less than 0.3%, showing that the laboratories can perform pathlength calibrations of short-pathlength cuvettes to ensure accurate protein concentration measurements.
Example #2: benchmarking the performance of a MV spectrophotometer and a MV plate reader
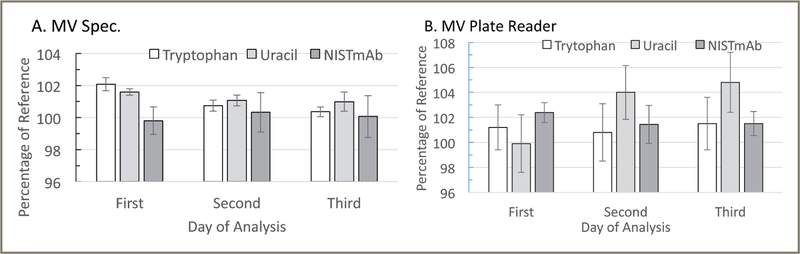
Figure 3 shows typical data for absorbance measurements taken over three days for the components of SRM 2082 obtained using a MV spectrophotometer and a MV microplate reader. The measurements over the three days were normalized as the percentage of the reference values (Table I) to facilitate comparison between the different samples.
Figure 3:
Absorbance and decadic attenuance (DA) measurements normalized to the percentage of reference values for a MV spectrophotometer (A) and a MV plate reader (B). The reference values for the NISTmAb were the values simple-corrected for absorbance at 340 nm and 320 nm (Table I) for the MV spectrophotometer and the MV plate reader measurements, respectively. Measurements were done on three separate days. The measurements were normalized to a 1-cm pathlength and then the values were calculated as the percentage of the reference values (Table I). For the NISTmAb measurements, the MV spectrophotometer and the reference values were corrected by subtracting the 340 nm values and the MV plate reader measurement, and reference values were corrected by subtracting the 320 nm values. The error bars are 1 coeficient of variation from n=3 measurements for the MV spectrophtometer and n=16 measurements for the MV plate reader.
These results are typical among those obtained by the authors over many measurements with these instruments. The path-length of the MV spectrophotometer can vary from 0.003 cm to 0.1 cm (adjusted automatically), and the MV plate reader is approximately 0.05 cm, according to the instrument manufacturers. The tryptophan and uracil, components at a 0.05-cm pathlength, would have absorbances of approximately 0.42 and 0.40, respectively. These values are close to the optimal range for absorbance measurements. Samples that are significantly lower in concentration will be a challenge to measure producing low absorbances that can increase variability among replicate values.
The data for the MV spectrophotometer and the MV plate reader for three days of measurements (shown in Figure 3) were analyzed to determine if there were evidence for biases in the measurements. If a statistical analysis provides evidence for bias, then a pathlength calibration might be justified to improve the quality of the measurements. The average of the measurements and a 95% confidence interval for the three days of data were calculated (Table III). The accuracy (difference from the measured value to the reference certified values) for the tryptophan and uracil samples for the MV spectrophotometer range from 1.1% to 1.2%, and for the MV plate reader the range was 1.2% to 2.9% (Table III).
Table III.
Average percentage of reference values and 95% confidence interval.
| Instrument | Tryptophan (95% C.I.) | Uracil (95% C.I.) | NISTmAb (95 C.I.%) |
|---|---|---|---|
| MV Spectrophotometer | 101.1 (103.3–98.8) | 101.2 (102.1–100.4) | 100.1 (101.3–99.4) |
| MV Plate Reader | 101.2 (102.0–100.3) | 102.9 (109.4–96.4) | 101.8 (103.1–100.5) |
The 95% confidence intervals for the tryptophan and uracil measurements on the MV spectrophotometer and the MV plate readers contains the values of 100% for both samples, indicating that these data do not provide statistically significant evidence that would indicate the benefit of a new calibration based on these data. The 95% confidence interval of the measurements from the NISTmAb (RM 8671) sample using the MV spectrophotometer and MV plate reader also contains the value of 100%, indicating that these data do not provide statistically significant evidence that would support the need for a new calibration.
CONCLUSION
In this example, the most appropriate application of SRM 2082 for MV instruments would be as a standard to benchmark the pathlength measurements to ensure that the instrument was working correctly within the 95% confidence intervals, based on the absorbance measurements for the particular instrument. Because the MV instruments have a higher variability (CVs) in the measurements compared to the dual beam instruments, measuring the 95% confidence interval using the SRM 2082 components is useful to ensure that the instruments are operating within their pathlength specifications.
A statistically significant change in the absorbance values of SRM 2082 components outside the 95% confidence interval would indicate a problem, and the instrument manufacturer should be contacted.
The development of spectrophotometers that can make sophisticated spectral measurements on microliter volume samples has been a major technological accomplishment. The straightforward sample preparation and speed of making multiple measurements, while conserving precious samples, make these instruments highly desirable for analysis of many biopharmaceutical samples.
This study shows the utility of NIST standards in a quality-control program for protein biopharmaceuticals. SR M 2082 can be used to calibrate short-path-lengths cuvettes and ensure the correct working of MV instrument using materials that have a spectrum similar to the major targets in bioprocessing research, proteins and nucleic acids (15–20). The components of SRM 2082 materials have significant advantages over existing dichromate-based absorbance standards including safety, stability, and absorbance peak shapes that match proteins and nucleic acids (7). NIST RM 8671 is a well-characterized mAb for many measurements with the chemical and physical properties similar to antibody-based biopharmaceuticals. These standards can be used to assist in the quality control of concentration measurements of biopharmaceuticals.
ACKNOWLEDGEMENT
The authors wish to thank Steve Lund (NIST, Statistical Engineering Division) for helpful comments regarding the manuscript.
REFERENCES
- 1.Grimsley GR and Pace CN, “Current Protocols in Protein Science,” Supplement. 33 (3.1) 1–9 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Cavaluzzi MJ and Borer PN, Nucleic Acids Res. 32 (e13) 11–19 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verhoven JW, Pure Appl. Chem 68, 2223–2286 (1996). [Google Scholar]
- 4.Huffman S and Soni K, Ferralolo J, Bioproc. Int 12, 66–73 (2009). [Google Scholar]
- 5.Anderle H and Weber H, Bioproc. Int 29, 42–50 (2016). [Google Scholar]
- 6.Thakkar SV et al. , J. Pharm. Sci 101, 3051–3061 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Lang B et al. , NIST J Research. 122, 1–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiel JE, Davis DL, and Borisov OV, State-of-the-Art and Emerging Technologies for Therapeutic Monoclonal Antibody Characterization Volume 1. Monoclonal Antibody Therapeutics: Structure, Function, and Regulatory Space (American Chemical Society, Washington, DC, 2014). [Google Scholar]
- 9.Schiel JE, et al. , Anal. Bioanal. Chem 410, 2127–2139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Travis JC, et al. , “Certified Transmittance Density Uncertainties for Standard Reference Materials Using a Transfer Spectrophotometer,” NIST Technical Note, number 1715, 2011. [Google Scholar]
- 11.Pace CN et al. , Protein Sci. 4, 2411–2423 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gokarn Y et al. , “Biophysical Techniques for Characterizing the Higher Order Structure and Interactions of Monoclonal Antibodies,” State-of-the-Art and Emerging Technologies for Therapeutic Monoclonal Antibody Characterization Volume 2. Biopharmaceutical Characterization: The NISTmAb Case Study, Schiel JE, Davis DL, Borisov OV, Eds. (American Chemical Society, Washington DC, 2015), pp. 285–327. [Google Scholar]
- 13.Maity H et al. , Int. J. Biol. Macromol 77, 260¬265 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Maity H, Karkaria C, and Davagnino J, Curr. Pharm. Biotech 10, 609–625 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Ploeser JM and Loring HS, J. Biol. Chem 178, 431–437 (1949). [PubMed] [Google Scholar]
- 16.Voet D et al. , Biopolymers. 1, 193–208 (1963). [Google Scholar]
- 17.Shugar D and Fox J, Biochem. Biophys. Acta 9, 199–218 (1952). [DOI] [PubMed] [Google Scholar]
- 18.Dunn DB and Hall RH, “Purines, Pyrimidines, Nucleosides, and Nucleotides: Phyical Constants and Spectral Properties,” Handbook of Biochemistry and Molecular Biology, Fasman G, Ed., (CRC Press, Cleveland, OH, 3rd edition, 1975), pp. 65–215. [Google Scholar]
- 19.Mihalyi E, J. Chem. Eng. Data 13, 179–182 (1968). [Google Scholar]
- 20.Edelhoch H, Biochem. 6, 1948–1954 (1967). [DOI] [PubMed] [Google Scholar]