Short abstract
High frequency spontaneous activity in injured primary afferents has been proposed as a pathological mechanism of neuropathic pain following nerve injury. Although spinal infusion of glial cell line-derived neurotrophic factor reduces the activity of injured myelinated A-fiber neurons after fifth lumbar (L5) spinal nerve ligation in rats, the implicated molecular mechanism remains undetermined. The fast-inactivating transient A-type potassium current (IA) is an important determinant of neuronal excitability, and five voltage-gated potassium channel (Kv) alpha-subunits, Kv1.4, Kv3.4, Kv4.1, Kv4.2, and Kv4.3, display IA in heterologous expression systems. Here, we examined the effect of spinal glial cell line-derived neurotrophic factor infusion on IA and the expression of these five Kv mRNAs in injured A-fiber neurons using the in vitro patch clamp technique and in situ hybridization histochemistry. Glial cell line-derived neurotrophic factor infusion reversed axotomy-induced reduction of the rheobase, elongation of first spike duration, and depolarization of the resting membrane potential. L5 spinal nerve ligation significantly reduced the current density of IA and glial cell line-derived neurotrophic factor treatment reversed the reduction. Among the examined Kv mRNAs, only the change in Kv4.1-expression was parallel with the change in IA after spinal nerve ligation and glial cell line-derived neurotrophic factor treatment. These findings suggest that glial cell line-derived neurotrophic factor should reduce the hyperexcitability of injured A-fiber primary afferents by IA recurrence. Among the five IA-related Kv channels, Kv4.1 should be a key channel, which account for this IA recurrence.
Keywords: Glial cell line-derived neurotrophic factor, neuropathic pain, primary afferent neurons, spontaneous activity, Kv channels, potassium currents
Introduction
Peripheral nerve injury causes high frequency spontaneous activity (SA) in primary afferent neurons.1 This hyperexcitability of primary afferent neurons has long been implicated as a pathological mechanism of neuropathic pain.2 After simple peripheral nerve lesion at a rather proximal portion, such as fifth lumbar (L5) spinal nerve ligation (SNL), SA occurs selectively in primary afferent A-fibers, but not C-fibers,3–7 while some other types of injury could also induce C-fiber SA.8–11 Intrathecal infusion of glial cell line-derived neurotrophic factor (GDNF) prevents/reverses L5 SNL-induced mechanical allodynia.3,12–14 Boucher et al.3 have suggested that GDNF exerts its analgesic effects by reducing SA through the prevention of de novo expression of a specific type of voltage-gated sodium channel (Nav) alpha-subunit, Nav1.3, in the injured dorsal root ganglion (DRG). However, a Nav1.3 knockout study failed to prevent the occurrence of SA.15 Therefore, changes in the expression of Nav alpha subunits cannot explain the SA mechanism.
Hyperexcitability of primary afferent neurons is a common observation in various painful neuropathy animal models and is often associated with K+ current reduction.16–19 K+ currents are major outward currents of all neurons leading to hyperpolarization of the cell membrane and a resultant reduction in cell excitability. Following peripheral nerve injury, primary afferent neurons show reduced K+ currents, and topical application of K+ channel blockers greatly increases SA in injured primary afferents.20–22 K+ currents are divided into roughly three distinct types based on their kinetics and pharmacological sensitivity: slow-inactivating sustained K-current (IK), fast-inactivating transient A-current (IA), and slow-inactivating transient D-current (ID). Among them, IA is an important determinant of action potential threshold, waveform, and firing frequency.23–25 In mammals, IA can be generated by voltage-gated potassium channel (Kv) alpha-subunits encoded by the Kv1.4, Kv3.4, Kv4.1, Kv4.2, and Kv4.3 genes.26,27 It has been demonstrated that DRG neurons express some of these Kv alpha-subunits and that axotomy downregulates them.28–33
While intrathecal GDNF infusion prevents several phenotypic changes in the injured DRG,3,13,14,34,35 its effect on Kv channel expression is not known. The present study was designed to test the hypothesis that GDNF would restore reduced K+ currents and would reverse hyperexcitability of injured A-fiber primary afferent neurons following L5 SNL. We further aimed to identify Kv channel genes related to these electrophysiological effects. We used the in vitro patch clamp technique and in situ hybridization (ISH) histochemistry for this purpose.
Materials and methods
Animals
In total, 39 adult male Sprague-Dawley rats weighing 210 to 240 g (Nippon Dobutsu Co., Nishinomiya Japan) were used for histochemical experiments, and 21 rats weighing 85 to 90 g were used for electrophysiological experiments. These rats were housed in plastic cages, three to four per cage, and food and water were available ad libitum. The room was maintained on a 12-h light/dark cycle in a constant 22°C to 24°C temperature. All experiments were reviewed and approved by the Animal Research Committee at Hyogo College of Medicine and were performed in accordance with the National Institutes of Health guidelines for animal care.
L5 SNL and delayed GDNF infusion
All surgical procedures were performed under adequate anesthesia with sodium pentobarbital (50 mg/kg, i.p.). For intrathecal infusion of GDNF, a laminectomy of the S1–S2 vertebrae was performed, and a polyethylene tube (O.D. = 0.64 mm, Silascon™, Kaneka Medix Co., Osaka, Japan) was inserted into the subarachnoid space, terminating near the L5 DRG level. After the muscle and fascia were sutured in layers with 3-0 silk thread, the remaining portion of the tube was implanted subcutaneously. It took 25 to 30 min to finish this surgery. Three days later, the rats received L5 SNL or sham surgery as previously described.36 Briefly, an approximately 2-cm length skin incision was made in the midline at the low back level, and the muscles were separated from the spinal process and vertebral arch on the left side at the L6 level. After removal of the L6 transverse spinal process, the L5 spinal nerve was identified and tightly ligated with 4-0 silk sutures. In sham-operated rats, the left L5 spinal nerve was isolated without ligation. These surgeries required 15 to 20 min. The animals were divided into three groups; L5 SNL+ GDNF treatment (SNL + GDNF), L5 SNL + saline treatment (SNL + saline), and sham + saline treatment (sham + saline). After an additional three days, the implanted tube was identified and cleared with 8 to 12 µl saline and then connected to a mini-osmotic pump (1 µl/h, 7d infusion; model #2001; Alzet, Cupertino, CA) filled with recombinant human GDNF (0.5 µg/µl in saline; Prospec, Rehovot, Israel) or saline (n = 7, each). The pump was implanted subcutaneously, and the skin was sutured with 3-0 silk thread. This operation was over within 30 min. This GDNF dose has also been used in previous studies,3,12,13 and our protocol successfully reversed established mechanical allodynia on day 10.14 Additionally, we used a smaller pump (model #1007D; 0.5 µl/h, 7 days; Alzet) containing GDNF or saline (n = 7, each) for the electrophysiological study using the smaller rats. We always use adult rats (210–240 g) for behavioral and histochemical studies. In such matured rats, however, it is difficult to acutely dissociate DRG neurons each other with minimum damage because of hard cell-cell and cell-matrix connections. So, we decided to use juvenile rats for electrophysiological study. In order to match GDNF dose to rat’s body weight at the end of GDNF infusion (10 days after L5 SNL) using the two capacities of mini-osmotic pumps (1 µl/h, 7 days and 0.5 µl/h, 7 days), we used rats weighing 85 to 90 g for electrophysiology experiments. The adult and juvenile rats grew up 320 to 350 g and 160 to 170 g, respectively, when they were sacrificed.
Electrophysiological study
The electrophysiological recording was conducted on the final day of 7-day-long GDNF infusion (10 days after L5 SNL). Under 2% isoflurane anesthesia, the ipsilateral (left) L5 DRG was rapidly harvested from the naïve, SNL + saline, and SNL + GDNF groups and incubated at 37°C in 5% CO2 for 40 min in serum free, advanced Dulbecco’s modified eagle medium (DMEM)/F12 containing 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA), collagenase (type IV; 2 mg/ml; Worthington Biomedical, Lakewood, NJ), and dispase (2 mg/ml, Worthington). After washing thrice with advanced DMEM/F12 containing 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MI; without enzyme), the DRG was dissociated by trituration in DMEM containing 10% fetal bovine serum, 1% penicillin/streptomycin, 625 µM L-glucose, 4.5 g/l D-glucose, and 110 mg/l sodium pyruvate. DRG neurons were plated on poly-L-lysine-coated glass coverslips (Becton Dickenson Labware, Bedford, MA) and incubated at 37°C in 5% CO2 for 2 to 8 h before recording. Then, the coverslips were transferred to the recording chamber (volume, 0.5 ml) continuously perfused (0.5 ml/min) with a standard external solution containing (in mM) 155 NaCl, 3 KCl, 1 CaCl2, 1 MgCl2, 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), and 20 glucose (pH 7.3) and mounted onto an inverted microscope (Nikon, Tokyo, Japan) equipped with phase contrast, a video camera, and two micromanipulators.
We performed whole-cell patch-clamp analysis on the large neurons (diameter ≥36 µm) to determine whether GDNF modulated the excitability of injured A-fiber neurons. We used this size criterion since 36 µm in diameter is equivalent to 1000 µm2 in cross-sectional area, and all neuronal profiles larger than 1000 µm2 can be immunostained with the monoclonal antibody N52, a marker of myelinated primary afferent neurons.37 Whole cell recordings were conducted as described.38 Fire-polished patch-pipettes (2–5 MΩ) were filled with (in mM) 120 potassium methanesulfonate, 20 KCl, 7.5 HEPES, and 2 ethylene glycol-bis-(b-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA, pH 7.3). In current-clamp mode, we elicited action potentials by depolarizing current pulses (50–1000 pA, 50–100-pA steps, 200 ms) and determined the rheobase (the minimum current required to elicit an action potential). Spike duration was determined as the duration of the first spike at the level of half amplitude. To study outward K+ currents, the solution was changed to one containing (in mM) 150 choline chloride, 3 KCl, 1 MgCl2, 10 HEPES, and 20 glucose (pH 7.35). We separated IA and K-type currents (IK) as previously described.38 Outward potassium currents were elicited by stepping to a conditioning voltage of either –40 mV or –120 mV from the holding potential of –60 mV for 300 ms; then the membrane was depolarized from –40 mV to +60 mV in increments of 10 mV, with +60 mV producing the largest peak current in each recording. Current- and voltage-clamp recordings were conducted with an Axopatch 200B amplifier (Molecular Devices, Foster city, CA). Signals were low-pass filtered at 1 or 5 kHz and digitized at 10 kHz. Access resistance was monitored throughout the experiments, and no significant changes were found. All recordings were performed at room temperature.
Tissue preparations
After the final behavioral measurement on day 10, the rats were deeply anesthetized with sodium pentobarbital (75 mg/kg body weight, i.p.) and fixed with paraformaldehyde (Merck, Darmstadt, Germany) as described.14 The ipsilateral L5 DRG was dissected, postfixed, frozen in powdered dry ice, cut into 12-µm-thick sections, and thaw-mounted onto MAS-coated glass slides (Matsunami, Osaka, Japan). We prepared slides containing the ipsilateral L5 DRG sections obtained from 21 animals (seven sections from each of the three groups) in order to examine the effect of GDNF under the same histochemical conditions. Other rats that underwent L5 SNL 1, 3, 7, and 28 days previously or sham 7 days previously (n = 3, each) were killed by decapitation under deep pentobarbital anesthesia. The ipsilateral L5 DRGs were freshly dissected and frozen. The DRGs were cut into 12-µm-thick sections for the time course study. Three other naïve rats were similarly processed for control.
ISH histochemistry and immunohistochemistry
Partial cDNAs of rat Kv1.4 (GenBank Accession No.X16002; base 2309-2742), Kv3.4 (X62841; 2345-2743), Kv4.1 (U89873; 1080-1400), Kv4.2 (S64320; 1850-2376), and Kv4.3 (U42975; 571-975) were synthesized by reverse transcription polymerase chain reaction from rat DRG-derived total RNA, cloned into p-GEM T-easy vector (Promega, Madison, WI), and sequenced as previously described.39 These vectors were digested by a restriction enzyme SpeI or NcoI (Takara Bio, Otsu, Japan) in adequate buffer, and alpha 35S-UTP-labeled antisense RNA probes were synthesized using T7 or SP6 RNA polymerase (Promega). ISH and emulsion autoradiography were performed as described.39
Image analysis
We captured the tissue images using a Nikon DXM-1200 digital CCD camera connected to a Nikon Diaphoto-300 microscope, saved them as TIFF images, and objectively evaluated the ISH signals using NIH image version 1.61 as described.39 The percentage of grain-occupied area of each neuronal profile was divided by the background grain density to obtain the signal intensity (×background). The signal intensities were plotted against the neuronal cross-sectional area. We defined signal intensities larger than 10× background as positively labeled for the mRNA and calculated the percentages of positively labeled profiles.
Statistical analysis
Data are expressed as mean ± SEM. For electrophysiological experiments, data were stored on a computer disk for off-line analysis (pClamp 8.0; Axon Instruments Inc., Foster City, CA). The differences were analyzed using one-way analysis of variance followed by Bonferroni’s multiple comparison tests. For ISH histological experiments, the differences in signal intensity were analyzed using the Kruskal–Wallis test followed by Dunn’s multiple comparisons tests. The differences in the percentages were analyzed using one-way analysis of variance followed by Bonferroni’s multiple comparison tests or unpaired t tests. All tests were performed using Prism4 (GraphPad Software Inc, San Diego, CA). Two-tailed P values lower than 0.05 were considered to be significant.
Results
Our surgical procedures did not affect health status of the rats, except for neuropathic pain behavior in the ipsilateral hind paw.
GDNF reverses hyperexcitability of injured large neurons
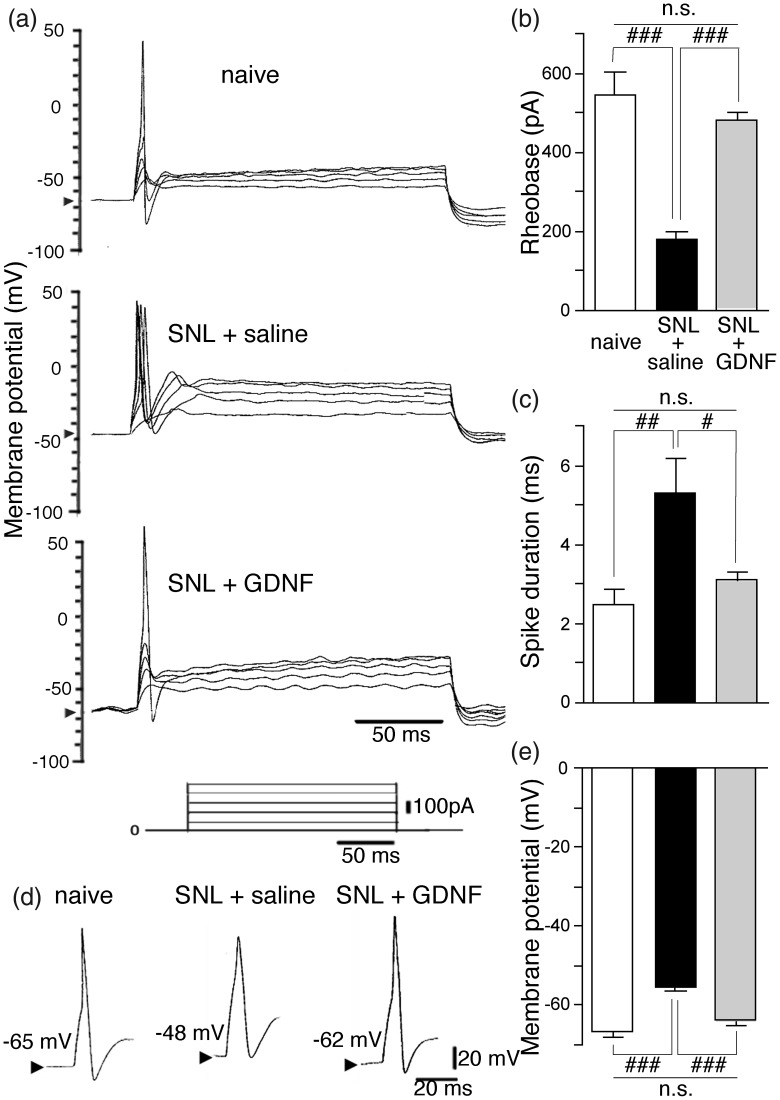
Large neurons (diameter ≥36 μm) from the SNL + saline group had a significantly lower rheobase (Figure 1(a) and (b); P < 0.001), significantly longer first spike duration (Figure 1(c) and (d); P < 0.01), and significantly more depolarized resting membrane potential (Figure 1(d) and (e); P < 0.001) compared to naïve neurons, indicating that injured A-fiber neurons become hyperexcitable. GDNF treatment completely reversed all these changes (Fig. 1).
Figure 1.
Delayed GDNF treatment reversed SNL-induced hyperexcitability of injured large (diameter > 36 μm; exclusively with A-fiber) DRG neurons. (a) Changes in the excitability during depolarizing current pulses (200 ms, 100-pA step pulses) of the ipsilateral L5 DRG large neurons obtained from the indicated groups. Arrowheads in the y-axis indicate the resting membrane potentials. (b) Reversal of the rheobase (the minimum current required to elicit an action potential) in the injured large neurons by GDNF. (c) Reversal of spike duration in the injured large neurons by GDNF. (d) Representative shape of the first action potential in each group. Arrowheads indicate the resting membrane potentials. (e) Reversal of resting membrane potential in the injured large neurons by GDNF. Values represent mean ± SEM (n = 7). n.s. P > 0.05, #P < 0.05, ##P < 0.01, ###P < 0.001 by one-way ANOVA followed by Bonferroni’s multiple comparison tests.
DRG: dorsal root ganglion; GDNF: glial cell line-derived neurotrophic factor; n.s.: not significant; SNL: spinal nerve ligation.
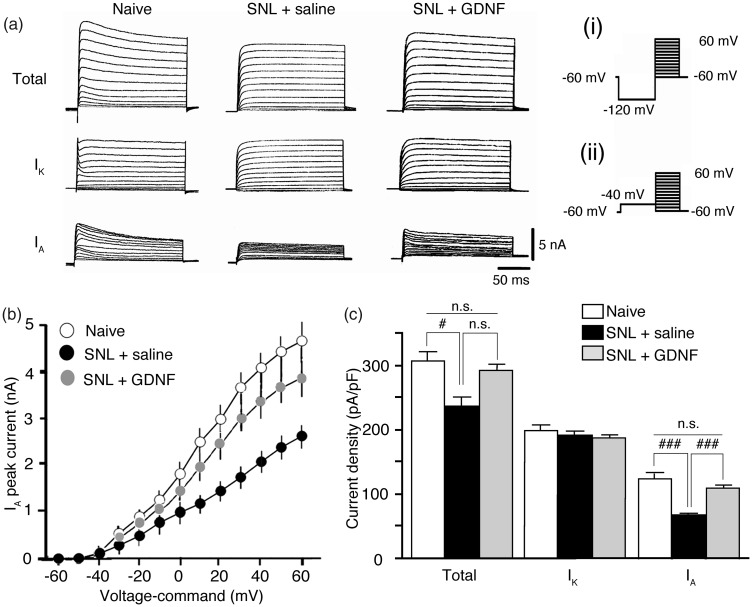
In order to explain the mechanisms of these changes in excitability of large neurons after nerve injury and GDNF treatment, we examined the changes in potassium currents in these neurons (Figure 2). While total potassium current density was significantly lower in the SNL + saline group compared to naïve rats (P < 0.05 vs. naïve), the effect of GDNF treatment on this reduction did not reach statistical significance (Figure 2(c); P > 0.05 vs. SNL + saline). Therefore, we separated total potassium currents into IA and IK (Figure 2(a)). IK density was not influenced by SNL surgery or GDNF treatment (Figure 2(a) and (c)). In contrast, IA density was significantly decreased in the SNL + saline group (P < 0.001 vs. naïve) and GDNF treatment completely counteracted the reduction (Figure 2(a) to (c); P < 0.001 vs. SNL + saline).
Figure 2.
Delayed GDNF treatment reversed SNL-induced reduction of IA of injured large DRG neurons. (a) Comparison of representative waveforms of the depolarization-activated potassium currents of the ipsilateral (left) L5 DRG neurons obtained from the indicated groups. Total potassium currents and IK were initiated via a prepulse of –120 mV (i) and –40 mV (ii) protocol, respectively, followed by a series test pulses rising from –60 mV to +60 mV in 10-mV steps. Subtraction total – IK reveals IA. (b) I–V relationships of IA obtained from each group. (c) Comparison of current densities obtained from each group. Values represent mean ± SEM (n = 7). n.s. P > 0.05, #P < 0.05, ###P < 0.001 by one-way ANOVA followed by Bonferroni’s multiple comparison tests. DRG: dorsal root ganglion; GDNF: glial cell line-derived neurotrophic factor; IA: fast-inactivating transient A-current; IK: slow-inactivating sustained K-current; n.s.: not significant; SNL: spinal nerve ligation.
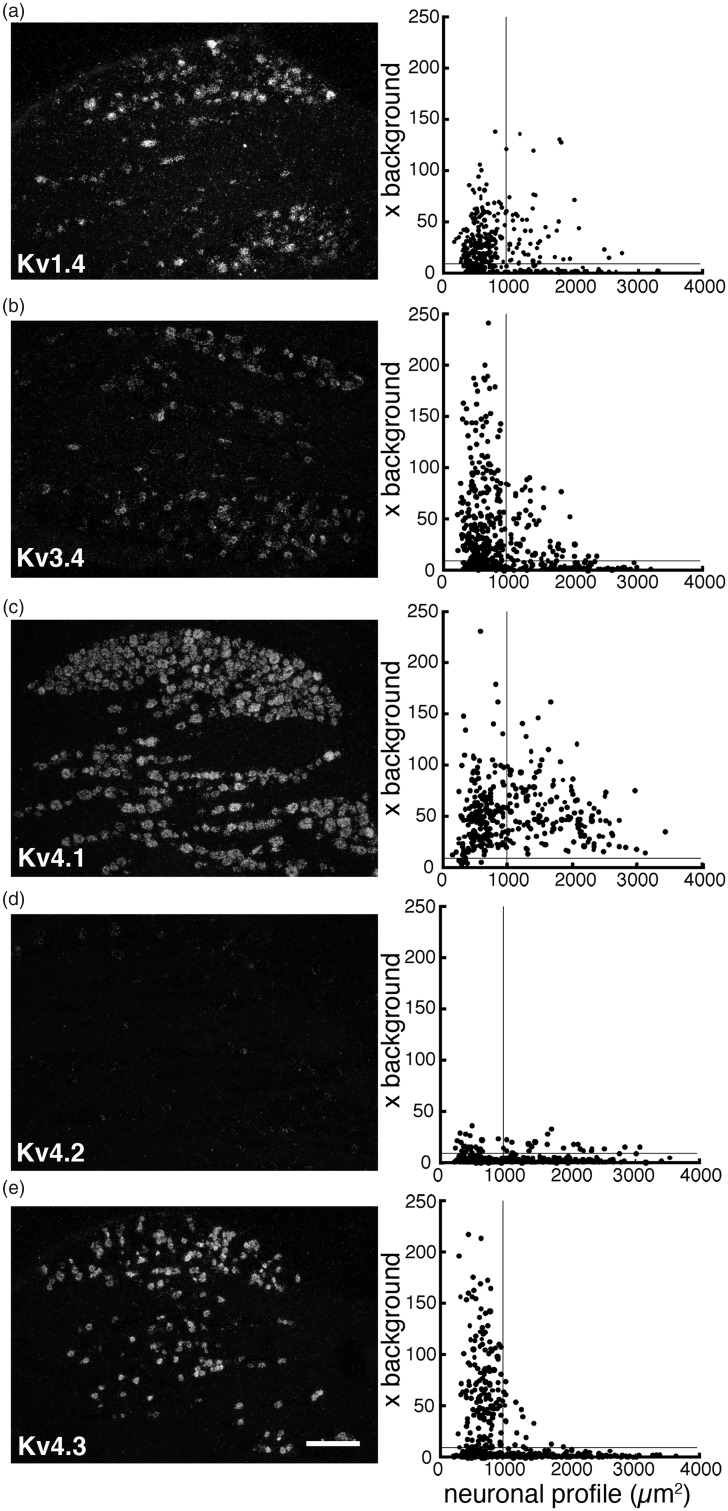
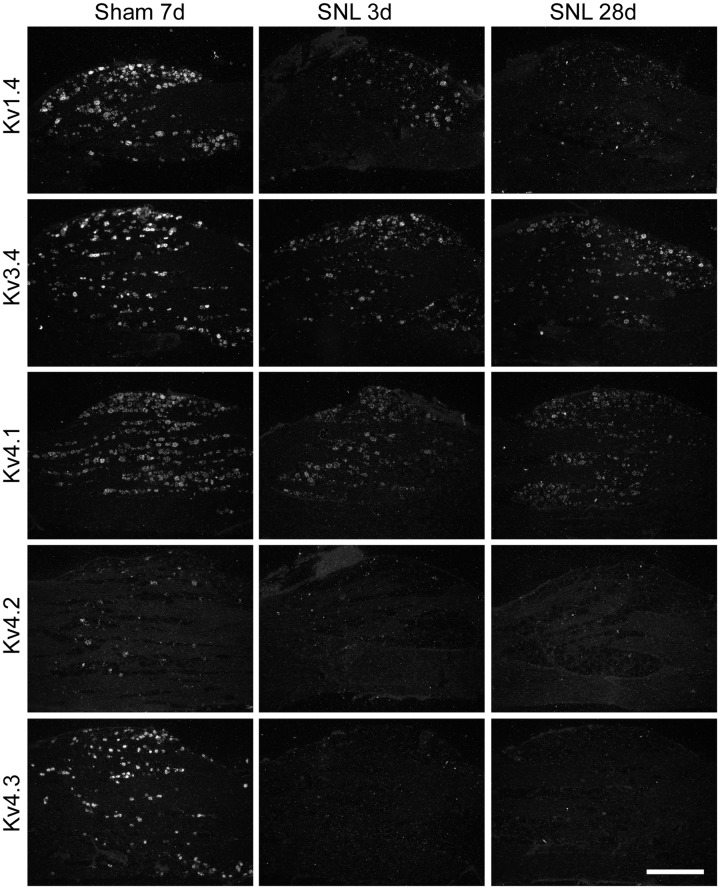
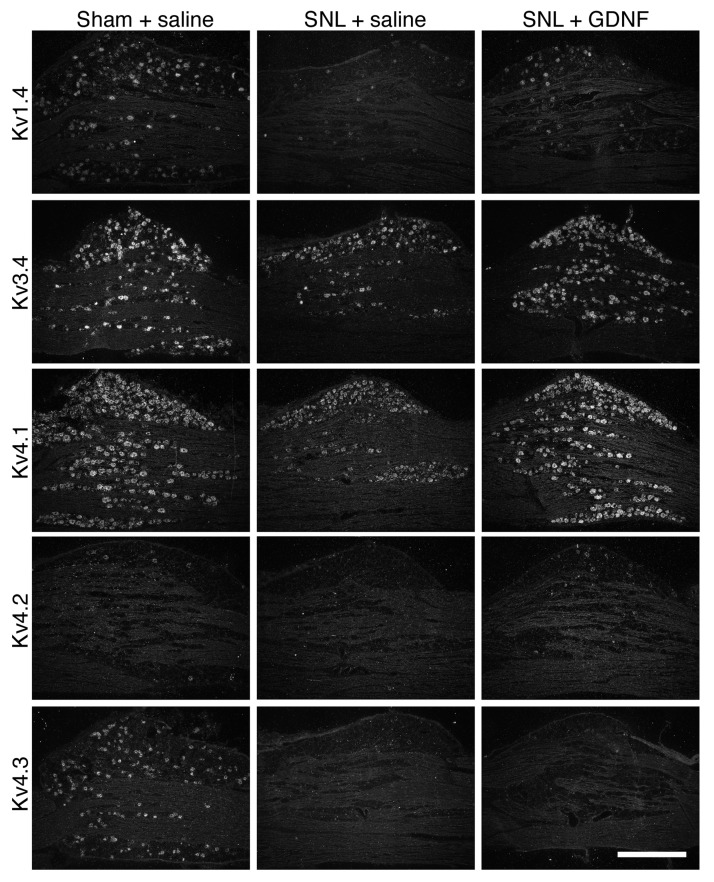
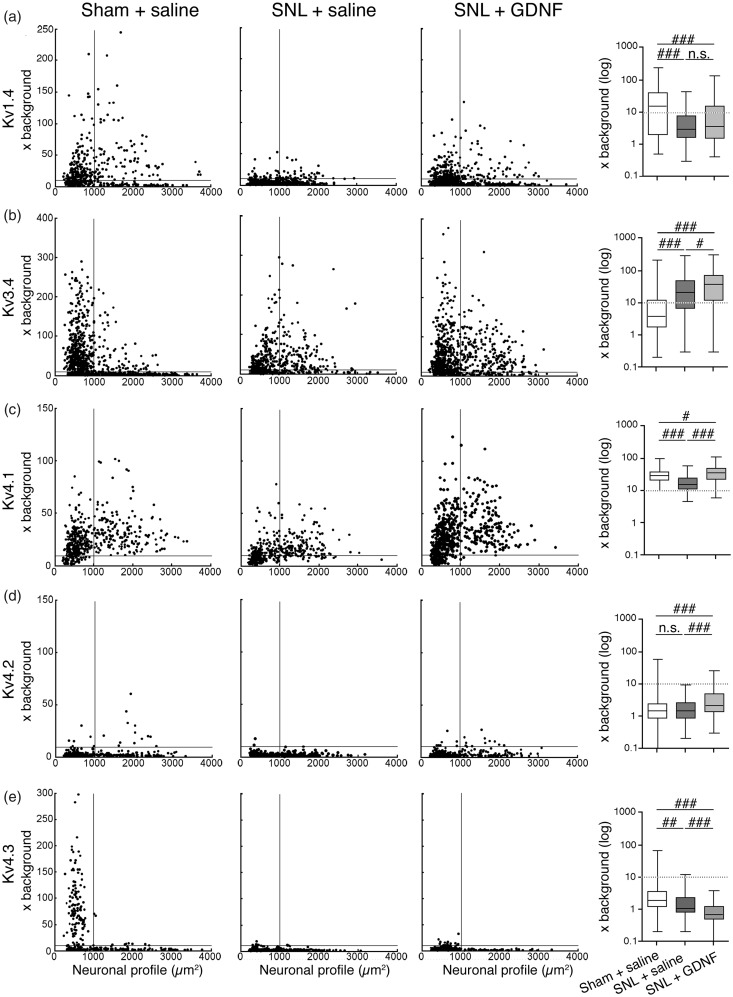
Next, we examined the change in expression of IA-encoding Kv alpha-subunit mRNAs by ISH histochemistry using 35S-labeled RNA probes and emulsion autoradiography. For histochemical studies, we classified the DRG neurons into small (profile < 1000 µm2; all C-fibers and small A-fibers) and large (≥1000 µm2; exclusively A-fibers).37 In naïve or sham L5 DRGs (Table 1 and Figure 3), Kv1.4 and Kv3.4 mRNAs were preferentially expressed in small neurons. Kv4.1 mRNA was expressed in almost all DRG neurons, while Kv4.3 mRNA was almost exclusively expressed in small neurons, and signals for Kv4.2 mRNA were rarely detected. After L5 SNL, overall expression of these mRNAs clearly decreased in the L5 DRG. These changes were obvious on day 3, when we started GDNF infusion, and continued for at least 28 days (Figure 4). Although Kv1.4 expression appeared partially reversed by GDNF treatment (Figures 5 and 6(a)), signal intensities in large neurons were not significantly reversed. Interestingly, Kv3.4 expression was increased in large neurons but decreased in small neurons after SNL (Figures 5 and 6(b)). GDNF treatment further increased Kv3.4 mRNA expression in large neurons (Figure 6(b)). Kv4.1 expression was significantly reduced by SNL and GDNF completely reversed the reduction (Figures 5 and 6(c)). Kv4.2 expression was quite low in DRGs in each group (Figure 5), while a significant change was detected after GDNF treatment (Figure 6(d)). Kv4.3 signals disappeared after SNL, and GDNF could not reverse the downregulation (Figures 5 and 6(e)).
Table 1.
Percentages of naïve DRG neurons expressing five IA-encoding Kv mRNAs.
| Subtype | Small | Large |
|---|---|---|
| Kv1.4 | 71.5% (196/274) | 42.6% (46/108) |
| Kv3.4 | 70.9% (258/364) | 35.5% (55/155) |
| Kv4.1 | 95.1% (193/206) | 100% (146/146) |
| Kv4.2 | 6.3% (25/392) | 10.0% (20/201) |
| Kv4.3 | 56.2% (159/283) | 8.4% (14/167) |
Note: DRG neurons were classified into small (cross-sectional area ≤1000 µm2; all C-fiber neurons and smaller A-fiber neurons) and large (>1000 µm2; exclusively A-fiber neurons) groups (See18). Expression of each mRNA was examined by in situ hybridization histochemistry using 35S-labeled RNA probe and visualized by emulsion autoradiography. Cell profiles with the silver grain densities ≥ 10× background were taken as positive cells. Numbers in parenthesis are positive cells/total counted cells.
Figure 3.
Expression of five IA-encoding Kv alpha-subunit mRNAs in a naïve rat DRG. DRG sections were processed for ISH histochemistry with 35S-labeled RNA probes and visualized by emulsion autoradiography. Left panels: Representative dark-field photomicrographs of the DRG showing ISH signals for Kv1.4 (a), Kv3.4 (b), Kv4.1 (c), Kv4.2 (d), and Kv4.3 (e) mRNAs, respectively. Scale bar = 500 μm. Right panels: Scatterplot diagrams of the left-hand panels. Individual neuronal profiles are plotted according to the cross-sectional area (μm2; along the x-axis) and signal intensity (x background; along the y-axis). The lines at S/N ratio = 10 indicate the borderline between the negatively and positively labeled profiles.DRG: dorsal root ganglion; ISH: in situ hybridization; Kv: voltage-gated potassium channel; S/N: signal to noise.
Figure 4.
Time course change in expression of five IA-encoding Kv alpha-subunit mRNAs in the ipsilateral L5 DRG after nerve injury. Rats underwent left L5 SNL or sham surgery (sham) at the indicated previous days. The ipsilateral L5 DRG sections were obtained, mounted on the same slide glass, processed for ISH histochemistry, and visualized by emulsion autoradiography. Representative dark-field photomicrographs of the indicated Kv mRNAs. Scale bar = 500 μm. IA: fast-inactivating transient A-current; Kv: voltage-gated potassium channel; DRG: dorsal root ganglion; SNL: spinal nerve ligation; ISH: in situ hybridization.
Figure 5.
Effect of delayed GDNF treatment on the expression of five IA-encoding Kv α-subunit mRNAs in the ipsilateral L5 DRG after L5 SNL. Representative dark-field photomicrographs of the DRG sections processed for ISH histochemistry with35 S-labeled RNA probes and visualized by emulsion autoradiography. These sections in the same lines were obtained from the indicated groups and mounted on the same slide glass. Scale bar = 500 μm. DRG: dorsal root ganglion; GDNF: glial cell line-derived neurotrophic factor; IA: fast-inactivating transient A-current; ISH: in situ hybridization; Kv: voltage-gated potassium channel; SNL: spinal nerve ligation.
Figure 6.
Objective quantification of the ISH signals for five IA-encoding Kv α-subunit mRNAs of individual DRG neurons in the ipsilateral L5 DRG after L5 SNL and GDNF treatment. Left scatterplot diagrams showing the ISH signal densities (× background) for these mRNAs and cell size of the ipsilateral L5 DRG neurons obtained from the indicated groups. At least 350 neuronal profiles pooled from seven rats were plotted. The horizontal lines at 10× background denote the borderline between positively and negatively labeled cells. All cells larger than 1000 μm2, which are designated by a vertical line, are A-fiber neurons.37 Right graphs are box-and-whisker plots showing the distribution of signal densities (× background in log scale) of large (>1000 μm2) neurons. The whiskers indicate the maximum and minimum values, and the box indicates the upper quartile, median, and lower quartile. The dotted lines at 10× background indicate the borderline between positively and negatively labeled cells. n.s. P > 0.05, #P < 0.05, ##P < 0.01, ###P < 0.001 by the Kruskal–Wallis test followed by Dunn’s multiple comparison tests. DRG: dorsal root ganglion; GDNF: glial cell line-derived neurotrophic factor; IA: fast-inactivating transient A-current; ISH: in situ hybridization; Kv: voltage-gated potassium channel; n.s.: not significant; SNL: spinal nerve ligation.
Discussion
We demonstrated that injured putative A-fiber neurons showed increased excitability with a significantly lower rheobase and more depolarized resting membrane potential compared to naïve neurons. Similar electrophysiological changes have been reported previously.17,18 Under such conditions, normally subthreshold depolarization by chemokines and cytokines derived from Schwann cells and macrophages around the injured axons could cause action potentials, leading to spontaneous firing.40,41 We found, for the first time, that GDNF reversed the electrophysiological hyperexcitability of these putative A-fiber neurons. In previous studies, while SA was suppressed by topical or systemic application of a COX-2 inhibitor,42 gabapentin,43 and low-dose local anesthetics,7 neuronal hyperexcitability per se was only reversed by perineural injection of clonidine, an alpha2 adrenergic receptor agonist, at the site of injury.18 The effectiveness of GDNF and clonidine may reflect the upregulation of receptors for these agents in injured DRG neurons.34,39,44,45
We detected a significant reduction of IA, but not IK, in injured A-fiber neurons. A previous study reported that IA and IK decreased, but ID was unaffected, after axotomy.46 Although the details remain obscure, this difference may be due to the age or weight of the rats used, differences in the injured site or nerve, and/or interval after injury: 10 days versus 2 to 3 weeks. Our GDNF treatment also reversed the reduction of IA in the injured putative A-fiber neurons. Given that IA is an important determinant of neuronal excitability, GDNF should suppress SA through IA recurrence.
We demonstrated that naïve DRG neurons expressed five IA-encoding Kv alpha-subunits at various percentages. Kv1.4 and Kv3.4 mRNAs were predominantly expressed by small neurons and some large neurons, and these observations were in line with the findings of previous immunohistochemical studies.28,31 While a previous study using ISH with digoxigenin-labeled RNA probes reported that Kv4.1 mRNA is expressed by approximately 60% of DRG neurons with all ranges of cell sizes,33 our result showed that almost all large neurons had this transcript, perhaps due to the higher sensitivity of our ISH method. Signals for Kv4.2 mRNA were observed only in a limited number of neurons. An immunohistochemical and quantitative reverse transcription polymerase chain reaction study also reported findings consistent with ours.32 Selective distribution of the Kv4.3 protein in some C-fiber neurons has been revealed by immunohistochemistry.31 Our ISH also showed high expression of Kv4.3 mRNA in small neurons with only just detectable signals in some medium-sized and large neurons.
Kv alpha-subunits are divided into four major gene families, Kv1, Kv2, Kv3, and Kv4, and each Kv alpha-subunit can assemble with the members of the same family, but not with those of other families, to constitute functional Kv channels.47 DRG neurons express many Kv alpha-subunit mRNAs.48 Although each Kv alpha-subunit examined in this study displays IA-type currents in heterologous expression systems, the precise composition of functional IA-type channels in DRG neurons was not determined. If large neurons express other Kv1 or Kv3 alpha-subunits, each of them could assemble with Kv1.4 or Kv3.4 to constitute heteromeric channels26 and the electrophysiological characteristics of these channels may differ from those of IA.49,50 Conversely, all three members of the Kv4 family (Kv4.1, Kv4.2, and Kv4.3) encode IA. All DRG large neurons expressed Kv4.1 mRNA, and less than 10% of these neurons expressed the other two Kv4 mRNAs (Figure 3 and Table 1). In addition, L5 SNL decreased IA as well as Kv4.1 expression and GDNF fully reversed both changes. Therefore, our data indicate that most A-fiber neurons should have IA mediated by the homotetrameric Kv4.1 channel, while some neurons may have heteromeric Kv4 channels and suggest that the change in IA in injured large neurons is mainly due to change in Kv4.1.
Contrary to other Kv mRNAs, Kv3.4 mRNA increased selectively in the injured large neurons after SNL. A previous study found overall reduction of immunoreactivity for Kv3.4 in injured DRG neurons after L5/6 SNL.31 Those authors, however, did not separate small neurons from large neurons and appeared not to have recognized the remaining Kv3.4 staining in large neurons. If this alpha-subunit forms homotetrameric channels, this upregulation could partially compensate for the reduction of Kv4.1-mediated IA.
Naturally, other mechanisms that could influence IA in the injured A-fiber neurons, such as change in the phosphorylation state of Kv alpha-subunits and assembly with beta-subunit, cannot be excluded.51–53 Whether these changes contribute to hyperexcitability of the injured A-fibers and the effect of GDNF treatment in this model remains to be elucidated.
As a matter of course, parallel changes in excitability and Kv4.1 expression in the injured primary afferent neurons do not demonstrate causality. However, we identified a powerful candidate for molecular mechanism of abnormal hyperexcitability in injured A-fibers among more than 30 Kv alpha subunits. Development of a Kv4.1-specific opener will be a useful strategy for future research and pain therapy. Peripheral nerve block of damaged nerve is often effective in human neuropathic pain, indicating the involvement of abnormal excitability of injured primary afferents. A Kv4.1-specific opener may be effective in human. Otherwise, quantitative/comparative analysis of Kv expression in the injured primary afferent neurons may reveal the key Kv alpha-subunit in human neuropathic pain mechanism.
In conclusion, the regulatory effects of GDNF on Kv4.1 expression could be a main molecular mechanism through which GDNF reverses the hyperexcitability and reduces the spontaneous firing of injured A-fibers in rats.
Acknowledgments
The authors would like to thank Editage (www.editage.jp) for English language editing.
Author Contributions
MS and TF: design of the experiments; MS and MT: electrophysiological studies and data analysis; TF: surgery, behavioral tests, in situ hybridization histochemistry, and data analysis; MS and TF: writing the manuscript; KI and KN: supervise the experiments and editing the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by JSPS KAKENHI grant numbers 19603019 and 21600016 for TF; grant numbers 24590737, 22792021, 24659832 and in part by Research grants from Sato and Uemura Funds from Nihon University School of Dentistry for MS (2012) and grant from the Dental Research Center, Nihon University School of Dentistry; Nihon University multidisciplinary research grant; and Individual Research Grant.
References
- 1.Wall PD, Gutnick M. Properties of afferent nerve impulses originating from a neuroma. Nature 1974; 248: 740–743. [DOI] [PubMed] [Google Scholar]
- 2.Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res 2009; 196: 115–128. [DOI] [PubMed] [Google Scholar]
- 3.Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science 2000; 290: 124–127. [DOI] [PubMed] [Google Scholar]
- 4.Liu CN, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain 2000; 85: 503–521. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Eschenfelder S, Blenk KH, Janig W, Habler H. Spontaneous activity of axotomized afferent neurons after L5 spinal nerve injury in rats. Pain 2000; 84: 309–318. [DOI] [PubMed] [Google Scholar]
- 6.Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol 2003; 89: 1588–1602. [DOI] [PubMed] [Google Scholar]
- 7.Sukhotinsky I, Ben-Dor E, Raber P, Devor M. Key role of the dorsal root ganglion in neuropathic tactile hypersensibility. Eur J Pain 2004; 8: 135–143. [DOI] [PubMed] [Google Scholar]
- 8.Michaelis M, Blenk KH, Janig W, Vogel C. Development of spontaneous activity and mechanosensitivity in axotomized afferent nerve fibers during the first hours after nerve transection in rats. J Neurophysiol 1995; 74: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 9.Banik RK, Brennan TJ. Spontaneous discharge and increased heat sensitivity of rat C-fiber nociceptors are present in vitro after plantar incision. Pain 2004; 112: 204–213. [DOI] [PubMed] [Google Scholar]
- 10.Grossmann L, Gorodetskaya N, Teliban A, Baron R, Janig W. Cutaneous afferent C-fibers regenerating along the distal nerve stump after crush lesion show two types of cold sensitivity. Eur J Pain 2009; 13: 682–690. [DOI] [PubMed] [Google Scholar]
- 11.Kirillova I, Teliban A, Gorodetskaya N, Grossmann L, Bartsch F, Rausch VH, Struck M, Tode J, Baron R, Janig W. Effect of local and intravenous lidocaine on ongoing activity in injured afferent nerve fibers. Pain 2011; 152: 1562–1571. [DOI] [PubMed] [Google Scholar]
- 12.Nagano M, Sakai A, Takahashi N, Umino M, Yoshioka K, Suzuki H. Decreased expression of glial cell line-derived neurotrophic factor signaling in rat models of neuropathic pain. Br J Pharmacol 2003; 140: 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R, Guo W, Ossipov MH, Vanderah TW, Porreca F, Lai J. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience 2003; 121: 815–824. [DOI] [PubMed] [Google Scholar]
- 14.Fukuoka T, Noguchi K. A potential anti-allodynic mechanism of GDNF following L5 spinal nerve ligation; Mitigation of NPY up-regulation in the touch sense pathway. Neuroscience 2015; 304: 240–249. [DOI] [PubMed] [Google Scholar]
- 15.Nassar MA, Baker MD, Levato A, Ingram R, Mallucci G, McMahon SB, Wood JN. Nerve injury induces robust allodynia and ectopic discharges in Nav1.3 null mutant mice. Mol Pain 2006; 2: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang JM, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol 1999; 82: 3359–3366. [DOI] [PubMed] [Google Scholar]
- 17.Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in the excitability of rat dorsal root ganglion neurons. J Neurophysiol 2001; 85: 630–643. [DOI] [PubMed] [Google Scholar]
- 18.Liu B, Eisenach JC. Hyperexcitability of axotomized and neighboring unaxotomized sensory neurons is reduced days after perineural clonidine at the site of injury. J Neurophysiol 2005; 94: 3159–3167. [DOI] [PubMed] [Google Scholar]
- 19.Takeda M, Tsuboi Y, Kitagawa J, Nakagawa K, Iwata K, Matsumoto S. Potassium channels as a potential therapeutic target for trigeminal neuropathic and inflammatory pain. Mol Pain 2011; 7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devor M. Potassium channels moderate ectopic excitability of nerve-end neuromas in rats. Neurosci Lett 1983; 40: 181–186. [DOI] [PubMed] [Google Scholar]
- 21.Burchiel KJ, Russell LC. Effects of potassium channel-blocking agents on spontaneous discharges from neuromas in rats. J Neurosurg 1985; 63: 246–249. [DOI] [PubMed] [Google Scholar]
- 22.Kajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett 1992; 138: 225–228. [DOI] [PubMed] [Google Scholar]
- 23.Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv4.1 subunits. J Neurosci 1998; 18: 3124–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tkatch T, Baranauskas G, Surmeier DJ. Kv4.2 mRNA abundance and A-type K(+) current amplitude are linearly related in basal ganglia and basal forebrain neurons. J Neurosci 2000; 20: 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrasquillo Y, Burkhalter A, Nerbonne JM. A-type K+ channels encoded by Kv4.2, Kv4.3 and Kv1.4 differentially regulate intrinsic excitability of cortical pyramidal neurons. J Physiol 2012; 590: 3877–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci 1999; 868: 233–285. [DOI] [PubMed] [Google Scholar]
- 27.Pongs O. Voltage-gated potassium channels: from hyperexcitability to excitement. FEBS Lett 1999; 452: 31–35. [DOI] [PubMed] [Google Scholar]
- 28.Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A 2001; 98: 13373–13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SY, Choi JY, Kim RU, Lee YS, Cho HJ, Kim DS. Downregulation of voltage-gated potassium channel alpha gene expression by axotomy and neurotrophins in rat dorsal root ganglia. Mol Cells 2003; 16: 256–259. [PubMed] [Google Scholar]
- 30.Yang EK, Takimoto K, Hayashi Y, de Groat WC, Yoshimura N. Altered expression of potassium channel subunit mRNA and alpha-dendrotoxin sensitivity of potassium currents in rat dorsal root ganglion neurons after axotomy. Neuroscience 2004; 123: 867–874. [DOI] [PubMed] [Google Scholar]
- 31.Chien LY, Cheng JK, Chu D, Cheng CF, Tsaur ML. Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J Neurosci 2007; 27: 9855–9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phuket TR, Covarrubias M. Kv4 channels underlie the subthreshold-operating A-type K-current in nociceptive dorsal root ganglion neurons. Front Mol Neurosci 2009; 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuyoshi H, Takimoto K, Yunoki T, Erickson VL, Tyagi P, Hirao Y, Wanaka A, Yoshimura N. Distinct cellular distributions of Kv4 pore-forming and auxiliary subunits in rat dorsal root ganglion neurons. Life Sci 2012; 91: 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci 1998; 18: 3059–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci 1998; 12: 256–268. [DOI] [PubMed] [Google Scholar]
- 36.Fukuoka T, Yamanaka H, Kobayashi K, Okubo M, Miyoshi K, Dai Y, Noguchi K. Re-evaluation of the phenotypic changes in L4 dorsal root ganglion neurons after L5 spinal nerve ligation. Pain 2012; 153: 68–79. [DOI] [PubMed] [Google Scholar]
- 37.Fukuoka T, Noguchi K. Comparative study of voltage-gated sodium channel alpha-subunits in non-overlapping four neuronal populations in the rat dorsal root ganglion. Neurosci Res 2011; 70: 164–171. [DOI] [PubMed] [Google Scholar]
- 38.Takeda M, Tanimoto T, Ikeda M, Nasu M, Kadoi J, Yoshida S, Matsumoto S. Enhanced excitability of rat trigeminal root ganglion neurons via decrease in A-type potassium currents following temporomandibular joint inflammation. Neuroscience 2006; 138: 621–630. [DOI] [PubMed] [Google Scholar]
- 39.Fukuoka T, Kobayashi K, Yamanaka H, Obata K, Dai Y, Noguchi K. Comparative study of the distribution of the alpha-subunits of voltage-gated sodium channels in normal and axotomized rat dorsal root ganglion neurons. J Comp Neurol 2008; 510: 188–206. [DOI] [PubMed] [Google Scholar]
- 40.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol 2009; 194: 417–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uceyler N, Schafers M, Sommer C. Mode of action of cytokines on nociceptive neurons. Exp Brain Res 2009; 196: 67–78. [DOI] [PubMed] [Google Scholar]
- 42.Zhao FY, Spanswick D, Martindale JC, Reeve AJ, Chessell IP. GW406381, a novel COX-2 inhibitor, attenuates spontaneous ectopic discharge in sural nerves of rats following chronic constriction injury. Pain 2007; 128: 78–87. [DOI] [PubMed] [Google Scholar]
- 43.Pan HL, Eisenach JC, Chen SR. Gabapentin suppresses ectopic nerve discharges and reverses allodynia in neuropathic rats. J Pharmacol Exp Ther 1999; 288: 1026–1030. [PubMed] [Google Scholar]
- 44.Cho HJ, Kim DS, Lee NH, Kim JK, Lee KM, Han KS, Kang YN, Kim KJ. Changes in the alpha 2-adrenergic receptor subtypes gene expression in rat dorsal root ganglion in an experimental model of neuropathic pain. Neuroreport 1997; 8: 3119–3122. [DOI] [PubMed] [Google Scholar]
- 45.Birder LA, Perl ER. Expression of alpha2-adrenergic receptors in rat primary afferent neurones after peripheral nerve injury or inflammation. J Physiol 1999; 515(Pt 2): 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J Neurophysiol 1999; 82: 700–708. [DOI] [PubMed] [Google Scholar]
- 47.Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev 2004; 84: 803–833. [DOI] [PubMed] [Google Scholar]
- 48.Bocksteins E, Van de Vijver G, Van Bogaert PP, Snyders DJ. Kv3 channels contribute to the delayed rectifier current in small cultured mouse dorsal root ganglion neurons. Am J Physiol Cell Physiol 2012; 303: C406–C415. [DOI] [PubMed] [Google Scholar]
- 49.Hernandez-Pineda R, Chow A, Amarillo Y, Moreno H, Saganich M, Vega-Saenz de Miera EC, Hernandez-Cruz A, Rudy B. Kv3.1-Kv3.2 channels underlie a high-voltage-activating component of the delayed rectifier K+ current in projecting neurons from the globus pallidus. J Neurophysiol 1999; 82: 1512–1528. [DOI] [PubMed] [Google Scholar]
- 50.Baranauskas G, Tkatch T, Nagata K, Yeh JZ, Surmeier DJ. Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons. Nat Neurosci 2003; 6: 258–266. [DOI] [PubMed] [Google Scholar]
- 51.Cerda O, Trimmer JS. Analysis and functional implications of phosphorylation of neuronal voltage-gated potassium channels. Neurosci Lett 2010; 486: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pongs O, Schwarz JR. Ancillary subunits associated with voltage-dependent K+ channels. Physiol Rev 2010; 90: 755–796. [DOI] [PubMed] [Google Scholar]
- 53.Vacher H, Trimmer JS. Diverse roles for auxiliary subunits in phosphorylation-dependent regulation of mammalian brain voltage-gated potassium channels. Pflugers Arch 2011; 462: 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]