Abstract
Introduction
Rotator cuff tear surgical repair techniques have significantly progressed. However, tendon retear following primary repair persistently occurs at high rates. Rehabilitation protocols, surgical fixation techniques, biologic therapy with scaffolds, platelet-rich plasma, and even stem cell applications are under study to promote adequate tendon healing.
Methods
A nonsystematic query of the PubMed database was conducted in July 2016 utilizing the search terms “rotator cuff repair,” “tear,” “rehabilitation,” “scaffold,” “platelet-rich plasma,” and “stem cell” to identify, analyze, and summarize relevant studies.
Conclusion
Individualized rehabilitation protocols may be the best approach for small to medium sized tears. Surgical fixation will continue to be debated as modifications to single-row technique and increases in suture number have improved tensile strength. Double-row repairs have been associated with higher costs. Transosseous equivalent technique exhibits comparable subjective and objective outcomes to single- and double-row repair at two-year follow-up. Biocompatible scaffold augmentation has showed inconsistent short-term results. Platelet-rich plasma has lacked uniformity in treatment preparation, administration, and outcome measurement with mixed results. Few human studies have suggested decreased retear rates and improved repair maintenance following bone marrow-derived mesenchymal stem cell augmentation. This review reiterated the necessity of additional high-quality, large-sample studies to develop any final verdict regarding efficacy.
Keywords: platelet-rich plasma, rehabilitation, rotator cuff repair, rotator cuff tear, scaffold, stem cell
Introduction
Rotator cuff tear surgical fixation and biologic healing strategies pose significant challenges. Rotator cuff tears are present in around 20% of the population and are responsible for more than 4.5 million office visits in the United States annually.1,2 Age-related degeneration particularly plays an integral role in tendon injury as full-thickness rotator cuff tears are found in roughly 50% of patients over the age of 70.3 Moreover, untreated rotator cuff tears may increase in size over time, resulting in additional tendon retraction and irreversible muscle atrophy.3 These injuries are similarly noticeable as half of asymptomatic patients become symptomatic within three years of initial diagnosis.3
Multiple strategies exist for rotator cuff tear treatment.4–11 Initial conservative management consists of activity modification, nonsteroidal anti-inflammatory drugs, and corticosteroid injections.4 In nonoperative cases, physical therapy is also employed to strengthen shoulder and scapular musculature and to address postural disturbances that may be responsible for patient symptoms.4 Postoperatively, physical therapy is similarly utilized to minimize joint stiffness and to strengthen the surrounding musculature.4
Although definitive indication for surgical intervention is still debated, surgical repair is commonly utilized in more than 200,000 procedures annually.5–7 Prognostic factors associated with successful recovery can be broken in to four categories: demographic factors, clinical factors, factors related to cuff integrity, and surgical procedure factors.12 Demographic factors include younger age and male gender, which have been associated with improved outcomes.12 Clinical factors leading to successful recovery include high bone mineral density, absence of diabetes mellitus, increased preoperative level of sports activity, greater preoperative range of motion (ROM), and absence of obesity.8,12 Moreover, cuff integrity plays an important part in successful repair with smaller sagittal lesion size, limited tendon retraction, decreased fatty infiltrate, and absence of multiple tendon involvement associated with improved tendon healing.12 Multiple prognostic factors are also associated with negative predictive values and generally impact cuff integrity or functional outcomes at follow-up.13–15 The most significant prognostic factors that lead to impaired cuff integrity include increasing age, larger tear sizes, smoking history, subscapularis pathology, and concomitant biceps or acromioclavicular joint procedures, whereas smoking and workers’ compensation cases are closely associated with worse functional outcomes after rotator cuff repair (RCR).8,12–15
Despite surgical intervention, retearing after primary repair occurs in 20–94% of patients, often resulting in persistent morbidity and loss of function.16–21 Similarly, roughly 1 in 25 patients undergo rotator cuff revision surgery in the following year.5 Retears can be classified by location in the tissue at two common sites: (1) the tendon at the bone–tendon repair interface or (2) the musculotendinous junction with preservation of the healed footprint.22–25 Contributing factors to retear susceptibility include larger rotator cuff tears (>3 cm), increased fatty infiltration of the muscle, and advanced age.5 Data suggest impaired fixation and biologic healing pose a significantly negative impact on clinical outcomes.16,26 Furthermore, revision procedures are associated with increased risk of complication and financial burden.16–18,26
Identifying effective methods of restoring the preinjured integrity of the native enthesis is a major research focus. Biomechanical surgical fixation techniques to improve maintenance of tendon fixation are a recent focus of effort.27–36 Moreover, a variety of postoperative rehabilitation protocols are studied, yet have not demonstrated significant improvements for rotator cuff healing.37–46 The emergence of platelet-rich plasma (PRP) as a biological adjunct to RCR has also been under investigation; however, routine use during RCR is still under question.9 As the search for the solution to biological augment rotator cuff healing continues stem cells treatments near the forefront of interest.47–50 Stem cell therapies represent a desirable treatment option due to their proliferative abilities and propensity to differentiate into various tissue types, including tendon.50–52 Yet, while initial results are promising, a comprehensive understanding of both past and present evidence is necessary to make well-informed conclusions concerning its current efficacy.
Therefore, the purpose of this manuscript is to review and summarize the literature on rehabilitation protocols, surgical techniques, PRP, biocompatible scaffolds, and stem cell therapy aimed at improving the integrity of rotator cuff repair. During the month of July 2016, we conducted a nonsystematic query of the PubMed database utilizing the search terms “rotator cuff repair,” “tear,” “rehabilitation,” “scaffold,” “platelet-rich plasma,” and “stem cell” with the Boolean operators “AND” and “OR.” Relevant studies were identified, and results were analyzed and summarized. We aim to conclude with comments on the current state of rotator cuff repair augmentation and propose further direction necessary to advance understanding of this topic.
Augmenting healing with tendon physiologic stimulation: Delayed therapy versus early rehabilitation?
As RCR failure typically occurs within the first 3–6 months postoperatively, the rehabilitation protocol is integral to reduce pain, promote a favorable environment for healing, and return preinjury function.53,54 Because tendons require adequate loading to facilitate healing, removal of this stimulus may be detrimental to tendon healing.55 Yet, the protection of the tendon insertion site and avoidance of its mechanical disruption during early rehabilitation also warrants consideration.56 Currently, two approaches for postoperative rehabilitation after RCR are popularized: (1) delayed therapy and (2) early rehabilitation.37,40–43,45,46,57,58
Delayed therapy protocols were popularized when open repairs were prominent.38,40,42,43 Recovery from open approaches favored postoperative periods of relative immobilization.46 This theory was largely based on prior animal and cadaveric studies suggesting low-level muscle contractions and stresses place tension on the site of repair during passive motion, creating vulnerability for tendon repair damage during early healing after open repair.57,58 More recently in 2012, Cuff and Pupello39 led a randomized control trial (RCT) of postoperative arthroscopic RCR patients comparing a cohort (n = 33) initiating passive ROM at two days post-op and another group (n = 35) who would begin therapy six weeks later. Similar improvement in preoperative to postoperative American Shoulder and Elbow Surgeon (ASES) and Simple Shoulder test scores were observed between groups.39 Moreover, no significant differences were found in patient satisfaction or healing rates between cohorts.39 Similarly, a comparable study conducted by Lee et al.43 in 2012 reported significant improvements in strength, ROM, and function in both aggressive and limited passive ROM groups (n = 64) in patients receiving single-row arthroscopic RCR at one-year follow-up.
In contrast, a number of studies suggest potential benefits of early return to function. In 2011, Duzgun et al.40 performed an RCT comparing slow (n = 16) and accelerated (n = 13) rehabilitation protocols with 24 weeks follow-up and found that patients in the accelerated cohort experienced less activity-related pain as early as week 5. Patients in the accelerated cohort also had superior patient-reported functional scores at weeks 8, 12, and 16 postoperatively.40 Two other studies conducted by Arndt et al.37 (n = 100) and Raab et al.45 (n = 26) found similar results, supporting the benefits of early, aggressive rehabilitation protocols in pain reduction and functional benefits.
Despite conflicting evidence supporting both rehabilitation strategies (Table 1), results are mixed regarding superiority of one intervention over the other.41,42,46 Results from a 2015 meta-analysis of delayed versus passive ROM conducted by Chang et al.38 strongly support the idea that individualization of postoperative protocols may be the best method of preserving tendon integrity during the healing process. The study included six RCTs and found significantly greater shoulder flexion improvement at six and 12 months with early ROM; interestingly, retear rates were found to significantly increase when two studies that only included small- and medium-sized tears were removed from the analysis.38 Hence, early rehabilitation protocols may be more appropriate for patients with small- to medium-sized tears versus a delayed approach in patients with large sized tears.38 Because prevention of rotator cuff retear relies on numerous variables, further insight into improving rehabilitation protocols may provide opportunity to preserve the integrity of the healing tendon.44
Table 1.
Overview of human studies evaluating therapy protocols of rotator cuff repair.
| Study | Study type | Sample size | Follow-up | Results and conclusions |
|---|---|---|---|---|
| Chang et al.38 | Systematic review and meta-analysis | n = 482 (six RCT) | Baseline, three, and six months | Early ROM exercise protocols accelerated recovery and postoperative stiffness, but likely result in improper tendon healing with large tears. |
| Sheps et al.46 | RCT | n = 189 | Six weeks and three, six, 12, and 24 months | The early motion group demonstrated increased abduction and scapular plane elevation (p = 0.002) at six weeks, but no difference afterward. No difference in clinical outcomes or ADLs for early mobilization or immobilized groups at 24 months. |
| Koh et al.42 | RCT | n = 100 | Six and 24 months | No difference in healing rate between four and eight months of immobilization after RCR for medium-sized rotator cuff tears. |
| Kim et al.16 | Case series | n = 221 | Three and 12 months | Retears are infrequent after three months if sufficient healing and integrity demonstrated by repair at three months. |
| Iannotti et al.53 | Cohort study | n = 113 | Two, six, 12, 16, 26, 39, and 52 weeks | Recurrent retear rate on MRI evaluation of RCR is 17% within one year. Retears primarily occur between six and 26 weeks. |
| Cuff and Pupello39 | RCT | n = 68 | Nine and 12 months | Slightly higher rotator cuff healing rate in delayed PROM versus early PROM, but similar outcomes at one year in both groups. |
| Lee et al.43 | RCT | n = 64 | Three, six, and 12 months | Increased risk of anatomic failure may occur with aggressive early passive rehabilitation versus limited early passive rehabilitation. |
| Kim et al.41 | RCT | n = 105 | Three, six, and 12 months | Early PROM exercise did not statistically improve early gains in ROM or alleviate pain, but did not negatively affect cuff healing. |
| Arndt et al.37 | Randomized prospective study | n = 100 | Three, six, 12, and final follow-up | Early passive motion demonstrated improved functional results with no significant difference in healing. |
| Duzgun et al.40 | RCT | n = 29 | One, three, five, eight, 12, 16, and 24 weeks | Accelerated protocols were superior to slow protocol in functional activity level and pain during activity and at night. |
| Raab et al.45 | RCT | n = 26 | Three months | CPM had a beneficial effect on ROM and pain relief for female patients and patients ≥60 years old, but no effect on overall shoulder score at three months. |
ADL: activities of daily living; CPM: continuous passive motion; MRI: magnetic resonance imaging; PROM: passive range of motion; RCR: rotator cuff repair; RCT: randomized control trial; ROM: range of motion.
Biomechanical surgical fixation techniques
As past and present surgical techniques yielded imperfect RCR preservation, surgical means of improving tendon fixation has been a focus of effort (Table 2). The major aims of RCR surgical fixation strategies are to provide high initial strength, reconstruct the anatomic footprint, maximize approximation of the fixation, and maintain stabilization throughout the healing period.62 The open transosseous technique was classically utilized as the gold standard, providing effective restoration of the anatomic footprint.62 However, this method was performed with the open technique and highly dependent on bone quality, often failing with sutures tearing through the soft, metaphyseal bone of the humerus.63 After an arthroscopic transosseous technique was developed and associated with similar failures, development of a distally located cortical bone tunnel was applied.63 Yet risk of axillary nerve injury associated with subaxillary portal placement has prevented wide acceptance of this approach.63
Table 2.
Overview of human studies evaluating surgical techniques of rotator cuff repair.
| Study | Study type | Sample size | Follow-up | Results and conclusions |
|---|---|---|---|---|
| McCormick et al.59 | Retrospective cohort | n = 63 | Minimum two years | Arthroscopic RCR demonstrated significant improvement in subjective and objective outcomes at minimum two-year follow-up with all three techniques (SR, DR, TOE). |
| Xu et al.34 | Meta-analysis | Nine studies (five RCTs) | N/A | DR RCR technique provides a significantly lower retear rate, higher ASES score, increased IR ROM compared to SR techniques. |
| Chen et al.35 | Systematic review | Six RCTs | N/A | DR RCR significantly higher rate of intact tendon healing compared to SR, especially in large to massive rotator cuff tears. No clinical difference in functional improvement between techniques. |
| Koh et al.60 | RCT | n = 71 | Two and six weeks, three, six, 12, and 24 months | Retear rates and clinical outcomes for DR RCR with one additional medial suture anchor was not significantly different than SR RCR with two lateral suture anchors in medium to large tears. |
| Duquin et al.36 | Systematic review | n = 1252 (23 Studies) | >1 year | DR RCR demonstrates significantly lower retear rates than SR technique in tears >1 cm. |
| Burks et al.61 | RCT | n = 40 | Six weeks, three months, one year | No clinical or MRI differences noted between SR or DR RCR techniques. |
ASES: American Shoulder and Elbow Surgeon; DR: double row; IR: internal rotation; MRI: magnetic resonance imaging; RCT: randomized control trial; RCR: rotator cuff repair; ROM: range of motion; SR: single row; TOE: transosseous equivalent.
Attention has shifted to suture-anchor fixation methods which have outperformed transosseous techniques in resilience to applied cyclic loads.64 The desire to achieve the maximum cuff fixation has led to the development of novel arthroscopic suture anchors.65 The success and improvements of new anchors have been matched with new complications.65–69 Early studies have evaluated anchors in vivo and in vitro, necessitating the need for further evaluation to truly understand the optimal anchor.65 Several trends do appear in laboratory testing of anchor failure. Braided polyester suture options are limited with nonmetallic PEEK and biodegradable polymers.70 Larger fully threaded screw designs require high failure strengths where suture failure is the most common reason for failure.70 Eyelet location is another common anchor variation where the distal crossbar eyelet, most commonly observed in biodegradable polymer anchor designs, fails prior to the screw threads.70 Further innovations including venting strategies and bone marrow stimulation provide additional options for surgeons to evaluate.
Of these repair methods, single- and double-row repairs are debated in the literature. In early arthroscopic repair techniques, it was believed that repairs utilizing a single row of anchors failed to recreate the contact area of the anatomic footprint.71 Thus, two rows of anchors were later found to better simulate the biological footprint by increasing the surface area of the cuff.72 The theory was that this would deliver a more favorable environment for tendon healing.72 Results of early biomechanical studies were encouraging with double-row repairs outperforming single-row repairs in initial fixation strength, ultimate tensile load, gap formation, and footprint area strain.27–29 However, upon later evaluation it was found that these studies compared double-row repairs with simple single-row suture techniques.27–29 Later increases in suture number as well as modification of the Mason–Allen technique significantly improved the tensile strength of single-row repairs.30,73 Hence, subsequent biomechanical testing showed similar performance between the two techniques with regards to cyclic displacement and ultimate failure load.31–33,74 Development of double-row repair has also evolved over the past decade with the transosseous equivalent technique, which exhibits comparable subjective and objective outcomes to single- and double-row repair at two-year follow-up.59
Improved performance of double-row repairs in lab studies has failed to consistently translate to clinical outcomes.34–36,60,61 A recent meta-analysis conducted by Xu et al.34 concluded that double-row repair was associated with significantly improved ASES scores, ROM, and reduced retear rates compared to single-row repairs. Similarly, a systematic review by Duquin et al.36 and meta-analysis conducted by Chen et al.35 both reported double-row repair as superior in management of large sized tears. Despite these results, numerous studies have equally reported absence of significant clinical difference between the two treatments.35,60,61 Increased costs associated with the double-row technique have also necessitated clinical outcome justification for its use over the cheaper single-row technique.74 Increases in surgical time, procedure complexity, and implant costs all contribute to the expenses associated with double-row technique.74 Correspondingly, an economic analysis conducted by Genuario et al.75 concluded that double-row repairs were not cost effective, regardless of the cuff tear size. Given these points, mixed clinical outcomes, lack of evidence to demonstrate restoration of the preinjury enthesis, and additional cost concerns of double-row repairs require further evaluation to establish a clear primary surgical option for RCR.
With numerous techniques and anchors available and a wide range of outcomes, cost–benefit analyses will continue to have a significant effect on the future of RCR. Black et al.76 evaluated 344 consecutive patients who received a transosseous equivalent or transosseous repair. Overall, there was no difference in surgical time between the techniques for medium and large repairs; however, the transosseous procedure produced substantial implant-related cost savings for small, medium, large, and massive RCRs.76
Biocompatible scaffolds: Targeting intrinsic tendon repair
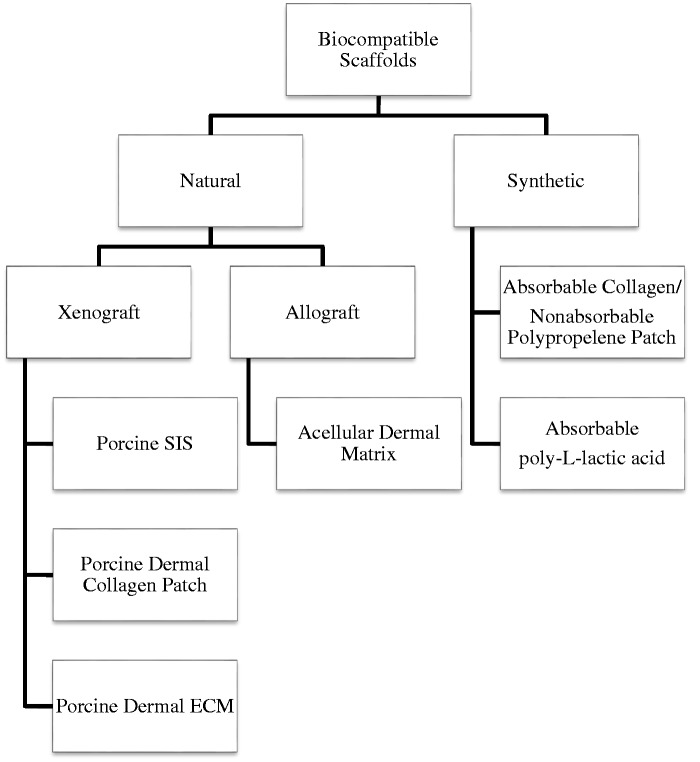
Despite advancement in repair techniques which have improved biomechanical properties of fixation, methods to improve rotator cuff healing quality have yet to be established. Biocompatible scaffolds (Figure 1) have been evaluated to stimulate intrinsic factors of tendon repair.18,77–81 Synthetic scaffolds are primarily believed to augment mechanical stabilization of the repair construct, while natural scaffolds composed of extracellular matrix and bioactive mediators additionally provide an inductive milieu for host cell repair.18
Figure 1.
Biocompatible scaffolds evaluated in clinical studies. ECM: extracellular matrix; SIS: small intestinal submucosa.
Clinical studies investigating natural xenograft augmentation of RCR yield mixed results concerning treatment efficacy. Three case series with a mean follow-up ranging from 2 to 4½ years found porcine xenografts to produce significant improvements in functional outcomes.77–79 However, clinical improvements have not been reflected in quality of tendon repair.80 A 2009 RCT conducted by Iannotti et al.80 found persistently high failure rates in large to massive rotator cuff tears treated with small intestinal submucosa (SIS) scaffolds. In particular, augmentation with SIS scaffolds has been poorly received as 20–40% of patients in clinical studies experienced severe postoperative inflammatory reactions.79–81
Consequently, acellular human dermal matrices have been studied as potential solutions to avoiding these adverse reactions.82 Therapeutic effects of these allograft patches have been overall positive with significant improvements in clinical outcomes; notably, Barber et al.82 published a RCT which found patients treated for dermal allografts in large (>3 cm) cuff tears involving two tendons to have 85% intact repairs versus 40% in controls at mean 24-month follow-up (p < 0.01). In addition, there were no adverse reactions observed in this treatment group (n = 22).82
Persistent concern for residual DNA in allografts causing inflammation has also led to investigation of a number of synthetic patches which have similarly produced varied results.47,83,84 A single cohort study addressing an absorbable collagen/nonabsorbable polypropylene patch and two case series evaluating adjunct repair with absorbable poly-l-lactic acid scaffolds in the treatment of large to massive tears showed improved functional outcomes yet conflicting data regarding retear rates.47,83,84 The Ciampi et al.84 cohort study showed significantly improved UCLA scores, scapular plane elevation, abduction strength (p < 0.01), and lower retear rates (17%, p = 0.001) for the polypropylene treatment group (n = 52) compared to the control (n = 51) and biological patch (n = 49). However, despite significant improvements in functional shoulder outcome scores PENN, ASES, Single Assessment Numeric Evaluation scores in both absorbable poly-l-lactic acid scaffolds studies, reported retear rates were 62% by Lenart et al.83 (n = 16) and 22% (n = 18) by Proctor.47 Currently, justification for scaffold use is limited for large to massive rotator cuff tears and cases with degenerative tendon tissue.10 Further high-quality, long-term RCTs including cost-effective analyses for future scaffolds are necessary before evidence-based recommendations can be made concerning biocompatible scaffold augmentation of RCR.48 Results are additionally summarized in Table 3.
Table 3.
Overview of human studies evaluating scaffold augmentation of rotator cuff repair.
| Study | Study type | Sample size | Follow-up | Results and conclusions |
|---|---|---|---|---|
| Lenart et al.83 | Case series | n = 16 | Mean 1.5 years | PLL patch augmentation reported significant improvements in functional outcomes but had a retear rate of 62% |
| Ciampi et al.84 | Cohort study | n = 152 | Two, 12, 36 months | Polypropylene patch augmentation for RCR significantly improved function, strength, and retear rates at 36 months. |
| Proctor47 | Case series | n = 18 | Six, 12, and 42 months | PLL bioabsorbable synthetic patch successfully reinforced surgical repair of large to massive RCR at 12 (83%) and 42 (78%) months. |
| Encalada-Diaz et al.18 | Case series | n = 10 | Two and four weeks Three, six, and 12 months | PCPU patch was well tolerated with a 10% retear rate at 12 months. |
| Gupta et al.77 | Case series | n = 27 | Minimum two years | Dermal tissue matrix xenograft group demonstrated improved pain, AROM, and subjective outcomes (ASES and SF-12 scores) and US revealed intact cuff reconstruction. |
| Barber et al.82 | RCT | n = 42 | Mean 14.5 months | Acellular dermal human allograft augmentation of large (>3 cm) tears involving two tendons showed better ASES and Constant scores. MRI revealed 85% intact repair in treatment versus 40% in controls (p < 0.01). |
| Phipatanakul and Petersen79 | Case series | n = 11 | Minimum two years | Porcine SIS xenograft did not reconstitute rotator cuff tissue or improve the quality of the RCR. |
| Badhe et al.78 | Case series | n = 10 | Six weeks, three and 12 months | PDC patch demonstrated excellent pain relief with moderate improvement in AROM and strength for chronic tears. |
| Walton et al.81 | Case control | n = 19 | Three, six, and 24 months | The ROI xenograft group had persisting cuff deficits, no clinical advantage compared to the control group, and a high proportion suffered a severe inflammatory reaction. |
| Iannotti et al.80 | RCT | n = 30 | One year | Porcine SIS augmentation did not improve tendon healing or clinical outcomes for large and massive RCR compared to controls. |
AROM: active range of motion; ASES: American Shoulder and Elbow Surgeon; MRI: magnetic resonance imaging; PCPU: polycarbonate polyurethane; PDC: porcine dermal collagen; PLL: poly-L-lactide; RCR: rotator cuff repair; RCT: randomized control trial; ROI: Restore orthobiologic implant; SIS: small intestinal submucosa; US: ultrasound.
PRP: Mixed results
In addition to biocompatible patches, orthobiologics researchers have aggressively pursued PRP as an RCR adjunct agent of interest (Table 4). PRP is a fraction of whole blood, typically isolated via centrifugation to yield a plasma product with supraphysiologic platelet concentrations.11,93,94 Upon platelet degranulation, growth factors and chemokines are released, honing migration of cells including fibroblasts, macrophages, and mesenchymal stem cells (MSCs) to the site of tendon injury.11 Through the healing cascade, growth factors including insulin-like growth factor, fibroblast growth factor, platelet-derived growth factor, transforming growth factor, and vascular endothelial growth factor are made available at the site of injury, providing a favorable environment for tendon healing.93 PRP has additionally been found to enhance MSC proliferation and differentiation.94 However, despite the proposed benefits of this therapy, research results have been mixed, hindering a consensus concerning its efficacy in promoting rotator cuff healing.85–90
Table 4.
Overview of studies evaluating PRP augmentation of rotator cuff repair.
| Study | Study type | Sample size | Follow-up | Results and conclusions |
|---|---|---|---|---|
| Saltzman et al.9 | Systematic review of meta-analyses | n = 3193 (seven meta-analyses) | 12–31 months | No universally accepted evidence that PRP use at time of arthroscopic RCR improves retear rates or clinical outcome scores. |
| Malavolta et al.85 | RCT | n = 54 | Three, six, 12, and 24 months | PRP prepared using apheresis and applied in a liquid state with thrombin did not produce better clinical results at 24 months compared to controls. |
| Weber et al.86 | RCT | n = 60 | Three, six, nine, and 12 weeks | PRFM did not significantly improve perioperative morbidity, clinical outcomes, or structural repair integrity. |
| Bergeson et al.87 | Cohort | n = 37 | 12 months | PRFM augmentation for “at-risk” RCRs did not improve retear rates or functional outcomes scores compared to controls. |
| Randelli et al.88 | RCT | n = 53 | Three, seven, 14, and 30 days and three, six, 12, and 24 months | Autologous PRP reduced pain in the first few months after RCR. PRP may have positively affected cuff healing for grade 1 and 2 tears. |
| Barber et al.89 | Case–control | n = 40 | Four, 12, 24, and 36 months | RCR augmented with two additional PRP fibrin matrix constructs resulted in lower retear rates on MRI evaluation and no postoperative clinical difference compared to controls. |
| Castricini et al.90 | RCT | n = 88 | 16 months | Autologous PRFM does not improve healing for augmentation of small to medium DR RCR. |
| Castillo et al.91 | Laboratory | n = 5 | N/A | Three different PRP concentration/separation systems produced different concentrations of GFs and WBCs. |
| Sundman et al.92 | Laboratory | n = 11 | N/A | Two commercial PRP preparation systems produced varying concentrations of GFs, cytokines, and WBCs. Preparation systems of PRP should be considered depending on the clinical application. |
DR: double row; GF: growth factor; MRI: magnetic resonance imaging; PRFM: platelet rich fibrin-matrix; PRP: platelet-rich plasma; RCR: rotator cuff repair; RCT: randomized control trial; WBC: white blood cell.
Few clinical studies have yielded positive results supporting PRP as an adjunct to RCR.85–90 Randelli et al.88 performed a double-blind RCT comparing patients receiving PRP with an autologous thrombin component during arthroscopic RCR versus surgical controls. Results showed significantly greater pain reduction in the treatment group (n = 26) within the first 30 days.88 Notably, in patients with grade 1 and 2 tears, the treatment group showed significant long-term superiority in tendon retraction on MRI evaluation and strength at two-year follow-up.88 Similarly, a comparative series by Barber et al.89 evaluated patients receiving a platelet-rich fibrin matrix construct sutured into a single-row repair (six of 20, 30%) against patients undergoing surgery alone (12 of 20, 60%) and found significantly decreased retear rates upon MRI evaluation at a mean 31 months (p = 0.03). Though regardless of improved structural integrity, the treatment group (n = 20) in this study failed to experience significant clinical outcomes evaluated with ASES (p = 0.35), Single Assessment Numeric Evaluation (p = 0.37), Simple Shoulder Test (p = 0.41), and Constant scores (p = 0.19).89
Despite the findings in the above studies, results have largely been unable to support PRP conferring benefits in tendon healing.85–87,90 Castricini et al.90 published results of an RCT evaluating double-row arthroscopic repair with autologous platelet-rich fibrin matrix (n = 43) against control subjects (n = 45) and found no difference in total Constant score or MRI tendon score between groups at 16 months. Congruently, a handful of other clinical trials have similarly been unable to show evidence of therapeutic benefit.85,86 Moreover, a prospective cohort study performed by Bergeson et al.87 found patients who received single-row arthroscopic repair augmentation with PRP fibrin matrix adjunct (n = 16) to have worsened repair outcomes versus historic controls (n = 21) with retear rates of 56.2 and 38.1% (p = 0.024), respectively.
The heterogeneous outcome results in the literature are likely reflective of the nonuniformity of PRP administered to study treatment groups. Treatment protocols in these studies are notably diverse with regards to delivery, activation, formulation, and associated fixation technique.85–90 Moreover, while little is known of the clinical implications of increased leukocyte concentrations on cuff repair, leukocytes are inherently present in PRP and have been associated with both catabolic and immunomodulatory activities.91,92 Hence, attempts at leukoreduction during PRP preparation must also be considered. Nevertheless, existing results on PRP efficacy are equivocal at best.9,85–92 Findings are in agreement with a recent meta-analyses of seven level II and III studies (Quality of Reporting of Meta-Analyses scores each > 15) conducted by Saltzman et al.9 of 3193 overlapping patients that concluded intraoperative use of PRP during RCR has not universally been shown to improve retear rates or clinical outcomes effectiveness.95 Vavken et al.95 found PRP to demonstrate no cost-effective benefit even in small- to medium-sized tears.95
Stem cells: The early evidence
Following the initial enthusiasm surrounding applications of PRP, biologics research has largely shifted focus to stem cell therapy.49 As the term stem cell generally encompasses both embryonic and adult subtypes, we focus our review on adult multipotent stem cells which possess tissue differentiation capabilities toward a single germ layer.49 MSCs are mesodermal in origin and have exhibited preference toward development into mesenchymal tissue including cartilage, bone, fat, and tendon.49 While literature has largely focused on clinical applications for cartilage and bone, therapeutic success in the treatment of recalcitrant lateral epicondylitis and patellar tendinopathy supports the notion that stem cell augmentation of RCR healing is a worthwhile endeavor.51,52,96 A summary of studies evaluating stem cell surgical enhancement is represented in Table 5.
Table 5.
Overview of human studies evaluating stem cell enhancement of rotator cuff repair.
| Study | Study type | Sample size | Follow-up | Results and conclusions |
|---|---|---|---|---|
| Gomes et al.97 | Cohort study | n = 14 | 12-month minimum | Rotator cuffs repaired with BMSC injections at the repair borders showed MRI preservation in 14/14 (100%) cases at 12 months. UCLA shoulder scores significantly improved at 12 months and remained stable in 13/14 (93%) patients at 24 months. |
| Hernigou et al.98 | Case–control study | n = 90 | 10 years | BMSC-treated repairs healed two months faster than controls via MRI and US evaluation. All treatment repairs fully healed at six months versus 30/45 (67%) in the control group. Long-term maintenance of tendon integrity of 39/45 (87%) in the treatment group versus 44% in the control group (p < 0.05). |
BMSC: bone marrow-derived mesenchymal stem cell; MRI: magnetic resonance imaging; UCLA: University of California at Los Angeles; US: ultrasound.
Early human studies on rotator cuff stem cell applications focused on identification and isolation of viable stem cell sources. Prior work demonstrates implanted tenocytes are able to produce collagen matrix and tendon regeneration.49,51,52,96 Thus, the pursuit of tenocyte-like progenitor cells poses an attractive solution to potentiate rotator cuff healing. In 2007, a study conducted by Bi et al.99 was the first to establish existence of a tendon progenitor cell population in human hamstring tendons and murine patellar tendons. These findings led to subsequent studies which further characterized progenitor cells at a number of sources within the shoulder including the subacromial bursa and the long head of the biceps tendon.50,100,101
Notably, the Mazzocca research group102 investigated intraoperative methods of stem cell harvest. A 2010 study (n = 23) demonstrated safety of bone marrow-derived mesenchymal stem cells (BMSCs) harvest and isolation from the proximal humerus during arthroscopy, negating necessity for cell digestion and expansion steps that are unable to be completed during a single surgery.102 The viability of this newly established source was confirmed later by Beitzel et al.,103 who found comparable levels of MSCs in the proximal humerus (n = 55) and distal femur (n = 29), proposing both sites as reliable, reproducible sources of bone marrow harvest. Mazzocca et al.104 also led a 2011 study (n = 11) which found that BMSC aspirated, purified, and exposed to physiologic insulin levels during arthroscopic RCR showed significantly increased gene expression of tendon-like markers, tendon-specific protein, and increased cell surface receptors in comparison to controls. Such results introduced the potential use of insulin to coax stem cell differentiation and enable repair augmentation during a single operative procedure.104
Despite paucity of clinical studies directly evaluating augmentation of RCR, two human studies have shown early evidence of stem cell efficacy. In 2012, Gomes et al.97 published a cohort study evaluating efficacy of conventional RCR with MSC adjunct at minimum 12-month follow-up.97 Fourteen patients underwent mini-open RCR with transosseous stitch.97 Iliac-derived MSCs were prepared and injected at tendon borders.97 At 12 months, MRI findings showed preserved tendon integrity in all cases (100%, 12/12).97 Likewise, mean UCLA scores significantly improved at 12 months and remained stable at 24 months in 13/14 patients (92.9%).97 Despite the small sample and absence of control group, these early results suggest improved maintenance of repair compared to prior reported rates of retear.16,17,19
To address stem cell treatment safety, Centeno et al.105 conducted a large multicenter investigation which monitored reported adverse events for adult patients (n = 2372) undergoing BMSC therapy for orthopedic conditions (n = 3012) at 18 centers. The total adverse event rate for all BMSC treatment groups was a reported 12.1% with a lower rate for patients undergoing a standard BMSC-only injection.105 The majority of the reported adverse effects were postprocedural pain or pain from the progression of degenerative joint disease.105 No clinical evidence suggested an increased risk or rate of neoplasm.105 However, despite current evidence supporting the safety of BMSC for orthopedic conditions, larger and longer quality studies are needed to confirm these findings.105
More recently, Hernigou et al.98 published a 10-year study further adding evidence to stem cell application for RCR augmentation. Forty-five patients were selected and designated to a treatment group undergoing arthroscopic single-row RCR with iliac crest BMSC injections delivered at tendon fixation and compared to 45 matched controls.98 Both groups underwent the same conservative postoperative surgery protocol.98 With equal lesion size, treatment patients healed two months faster than the controls upon ultrasound and MRI evaluation.98 All treatment repairs fully healed at six months compared to 67% in the control group.98 Long-term follow-up was also notable for 87% maintenance of tendon integrity in the treatment cohort compared to 44% in the control group (p < 0.05).98 These findings further indicate MSCs’ potential to improve healing rates and long-term repair integrity.98 An additional case–control study (n = 80) conducted by the same group showed maintenance of tendon integrity after rotator cuff revision at two-year follow-up.106 However, despite these impressive results, larger high-quality studies are needed before firm conclusions can be made concerning therapeutic efficacy.
Additional findings from the Hernigou et al.107 research group have advanced our understanding of chronic tendon disease and the potential role for stem cell therapy in improving the healing response. In a 2015 paper, they hypothesized that there was decreased MSC concentration at the bone–tendon interface in patients with rotator cuff injury requiring surgery.107 This was tested by analyzing bone marrow aspirate collected from the humeral tuberosities of patients (n = 125) with symptomatic full-thickness rotator cuff tears undergoing repair.107 The treatment group experienced significant decrease in MSCs with 56 (45%) shoulders showing a 30–50% moderate decrease in MSC content and 40 (32%) shoulders exhibiting a severe 50–70% decrease in comparison to controls (n = 75) undergoing arthroscopy without evidence of cuff tear.107 Patient age, amount of fatty degeneration observed, and chronicity of injury before surgical management were associated with poor outcomes.107 While this adds credence to the idea that supplementing stem cell deficiencies may improve RCR, it also introduces patient demographics that must be considered when identifying potential candidates for RCR. Such considerations are integral to tailoring patient treatments and promoting favorable outcomes. After development of stem cell therapies to assist with RCR and additional clinical results in a human population, a thorough cost-effective analysis is crucial to determine if there is a potential role in RCR.108
Conclusion
Understanding of rotator cuff repair augmentation has expanded considerably with investigation into the role of rehabilitation protocols, surgical fixation methods, biocompatible scaffolds, PRP, and stem cell therapy yielding fascinating results. Individualized rehabilitation protocols may be the best method of approach with early rehabilitation facilitating early return of ROM and return to daily activity for small to medium tears and delayed therapy providing adequate time for tendon healing for large tears in the postoperative period. With regard to surgical fixation, it will continue to be debated as modifications to single-row technique and increases in suture number have improved the tensile strength of single-row repairs. The transosseous equivalent technique was developed and exhibits comparable subjective and objective outcomes to single- and double-row repair at two-year follow-up. Continued studies that evaluate long-term clinical outcomes will be essential to optimize the surgical standard of care as currently double-row repairs have been associated with higher cost and a thorough cost analysis may not support clinical benefit. Moreover, biocompatible scaffold augmentation has showed inconsistent results with long-term RCTs needed before conclusions concerning efficacy may be determined. Current investigation into PRP has lacked uniformity in terms of treatment preparation, administration, and outcome measurement and has also displayed varied results concerning clinical efficacy. While a few small human studies have suggested decreased retear rates and improved maintenance of repair following BMSC augmentation, more high-quality and large-sample studies are needed before a final verdict is reached concerning their efficacy.
Despite efforts to provide a comprehensive review on this topic, this study is not without its limitations. The literature search was not conducted systematically, and other relevant studies may have been inadvertently omitted from this review. A literature search was conducted for each topic of the manuscript and the results were reviewed for any applicable titles for each section. Moreover, paucity of studies assessing functional outcomes, which drive clinical decision making for revision surgery, significantly limited conclusions that could be made about stem cell augmentation at this time. It is also possible that the lack of effect observed in studies assessing rehab protocols, surgical technique, and PRP was observed in trials of low quality. Nonetheless, care was taken to draw major conclusions from RCTs and meta-analyses when possible. We believe that the current literature has highlighted many of the complexities which deserve consideration when assessing treatment to improve rotator cuff repair. Further directions of study of treatment modalities, particularly biocompatible scaffolds, PRP, and stem cells, should involve treatment standardization in high-powered clinical trials to further delineate the relationship between treatment and patient outcomes.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Review and Patient Consent
There was no review board approval required for this literature review and there were no patient consents as this was a literature review without any patients.
References
- 1.Oh LS, Wolf BR, Hall MP, et al. Indications for rotator cuff repair: a systematic review. Clin Orthop 2007; 455: 52–63. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto A, Takagishi K, Osawa T, et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg 2010; 19: 116–120. [DOI] [PubMed] [Google Scholar]
- 3.Park JG, Cho NS, Song JH, et al. Rotator cuff repair in patients over 75 years of age: clinical outcome and repair integrity. Clin Orthop Surg 2016; 8: 420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osborne JD, Gowda AL, Wiater B, et al. Rotator cuff rehabilitation: current theories and practice. Phys Sportsmed 2016; 44: 85–92. [DOI] [PubMed] [Google Scholar]
- 5.McElvany MD, McGoldrick E, Gee AO, et al. Rotator cuff repair published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med 2015; 43: 491–500. [DOI] [PubMed] [Google Scholar]
- 6.Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears: a randomized controlled study in 103 cases with a five-year follow-up. J Bone Joint Surg Am 2014; 96: 1504–1514. [DOI] [PubMed] [Google Scholar]
- 7.Kukkonen J, Joukainen A, Itälä A, et al. Operatively treated traumatic versus non-traumatic rotator cuff ruptures: a registry study. Ups J Med Sci 2013; 118: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaudreuil J, Dhénain M, Coudane H, et al. Clinical practice guidelines for the surgical management of rotator cuff tears in adults. Orthop Traumatol Surg Res 2010; 96: 175–179. [DOI] [PubMed] [Google Scholar]
- 9.Saltzman BM, Jain A, Campbell KA, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy 2015; 32: 906–918. [DOI] [PubMed] [Google Scholar]
- 10.Thangarajah T, Pendegrass CJ, Shahbazi S, et al. Augmentation of rotator cuff repair with soft tissue scaffolds. Orthop J Sports Med 2015; 3: 2325967115587495–2325967115587495. (online only). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anz AW, Hackel JG, Nilssen EC, et al. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg 2014; 22: 68–79. [DOI] [PubMed] [Google Scholar]
- 12.Fermont AJM, Wolterbeek N, Wessel RN, et al. Prognostic factors for successful recovery after arthroscopic rotator cuff repair: a systematic literature review. J Orthop Sports Phys Ther 2014; 44: 153–163. [DOI] [PubMed] [Google Scholar]
- 13.Lambers Heerspink FO, Dorrestijn O, van Raay JJAM, et al. Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg 2014; 23: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 14.Woollard JD, Bost JE, Piva SR, et al. The relationship of preoperative factors to patient-reported outcome in rotator cuff repair: a systematic review. Phys Ther Rev 2016; 21: 138–150. [Google Scholar]
- 15.Santiago-Torres J, Flanigan DC, Butler RB, et al. The effect of smoking on rotator cuff and glenoid labrum surgery: a systematic review. Am J Sports Med 2015; 43: 745–751. [DOI] [PubMed] [Google Scholar]
- 16.Kim HM, Caldwell J-ME, Buza JA, et al. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am 2014; 96: 106–112. [DOI] [PubMed] [Google Scholar]
- 17.Lafosse L, Brzoska R, Toussaint B, et al. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am 2008; 90: 275–286. [DOI] [PubMed] [Google Scholar]
- 18.Encalada-Diaz I, Cole BJ, MacGillivray JD, et al. Rotator cuff repair augmentation using a novel polycarbonate polyurethane patch: preliminary results at 12 months’ follow-up. J Shoulder Elbow Surg 2011; 20: 788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am 2004; 86: 219–224. [DOI] [PubMed] [Google Scholar]
- 20.Galatz LM. Nicotine delays tendon-to-bone healing in a rat shoulder model. J Bone Joint Surg Am 2006; 88: 2027–2027. [DOI] [PubMed] [Google Scholar]
- 21.Harryman DT, Mack LA, Wang KY, et al. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am 1991; 73: 982–989. [PubMed] [Google Scholar]
- 22.Cho NS, Yi JW, Lee BG, et al. Retear patterns after arthroscopic rotator cuff repair: single-row versus suture bridge technique. Am J Sports Med 2010; 38: 664–671. [DOI] [PubMed] [Google Scholar]
- 23.Gusmer PB, Potter HG, Donovan WD, et al. MR imaging of the shoulder after rotator cuff repair. Am J Roentgenol 1997; 168: 559–563. [DOI] [PubMed] [Google Scholar]
- 24.Magee TH, Gaenslen ES, Seitz R, et al. MR imaging of the shoulder after surgery. Am J Roentgenol 1997; 168: 925–928. [DOI] [PubMed] [Google Scholar]
- 25.Owen RS, Iannotti JP, Kneeland JB, et al. Shoulder after surgery: MR imaging with surgical validation. Radiology 1993; 186: 443–447. [DOI] [PubMed] [Google Scholar]
- 26.Sugaya H, Maeda K, Matsuki K, et al. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. J Bone Joint Surg Am 2007; 89: 953–960. [DOI] [PubMed] [Google Scholar]
- 27.Baums MH, Spahn G, Steckel H, et al. Comparative evaluation of the tendon-bone interface contact pressure in different single- versus double-row suture anchor repair techniques. Knee Surg Sports Traumatol Arthrosc 2009; 17: 1466–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun S, Millett PJ, Yongpravat C, et al. Biomechanical evaluation of shear force vectors leading to injury of the biceps reflection pulley: a biplane fluoroscopy study on cadaveric shoulders. Am J Sports Med 2010; 38: 1015–1024. [DOI] [PubMed] [Google Scholar]
- 29.Kim DH, ElAttrache NS, Tibone JE, et al. Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. Am J Sports Med 2006; 34: 407–414. [DOI] [PubMed] [Google Scholar]
- 30.Gerber C, Schneeberger AG, Beck M, et al. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br 1994; 76-B: 371–380. [PubMed] [Google Scholar]
- 31.Mazzocca AD, Millett PJ, Guanche CA, et al. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med 2005; 33: 1861–1868. [DOI] [PubMed] [Google Scholar]
- 32.Nelson CO, Sileo MJ, Grossman MG, et al. Single-row modified Mason-Allen versus double-row arthroscopic rotator cuff repair: a biomechanical and surface area comparison. Arthroscopy 2008; 24: 941–948. [DOI] [PubMed] [Google Scholar]
- 33.Mahar A, Tamborlane J, Oka R, et al. Single-row suture anchor repair of the rotator cuff is biomechanically equivalent to double-row repair in a bovine model. Arthroscopy 2007; 23: 1265–1270. [DOI] [PubMed] [Google Scholar]
- 34.Xu C, Zhao J, Li D. Meta-analysis comparing single-row and double-row repair techniques in the arthroscopic treatment of rotator cuff tears. J Shoulder Elbow Surg 2014; 23: 182–188. [DOI] [PubMed] [Google Scholar]
- 35.Chen M, Xu W, Dong Q, et al. Outcomes of single-row versus double-row arthroscopic rotator cuff repair: a systematic review and meta-analysis of current evidence. Arthroscopy 2013; 29: 1437–1449. [DOI] [PubMed] [Google Scholar]
- 36.Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing?: a systematic review. Am J Sports Med 2010; 38: 835–841. [DOI] [PubMed] [Google Scholar]
- 37.Arndt J, Calvert P, Mielcarek P, et al. Immediate passive motion versus immobilization after endoscopic supraspinatus tendon repair: a prospective randomized study. Orthop Traumatol Surg Res 2012; 98: S131–S138. [DOI] [PubMed] [Google Scholar]
- 38.Chang K-V, Hung C-Y, Han D-S, et al. Early versus delayed passive range of motion exercise for arthroscopic rotator cuff repair a meta-analysis of randomized controlled trials. Am J Sports Med 2015; 43: 1265–1273. [DOI] [PubMed] [Google Scholar]
- 39.Cuff DJ, Pupello DR. Prospective randomized study of arthroscopic rotator cuff repair using an early versus delayed postoperative physical therapy protocol. J Shoulder Elbow Surg 2012; 21: 1450–1455. [DOI] [PubMed] [Google Scholar]
- 40.Duzgun I, Baltaci G, Atay AO. Comparison of slow and accelerated rehabilitation protocol after arthroscopic rotator cuff repair: pain and functional activity. Acta Orthop Traumatol Turc 2011; 45: 23–33. [DOI] [PubMed] [Google Scholar]
- 41.Kim Y-S, Chung SW, Kim JY, et al. Is early passive motion exercise necessary after arthroscopic rotator cuff repair? Am J Sports Med 2012; 40: 815–821. [DOI] [PubMed] [Google Scholar]
- 42.Koh KH, Lim TK, Shon MS, et al. Effect of immobilization without passive exercise after rotator cuff repair: randomized clinical trial comparing four and eight weeks of immobilization. J Bone Joint Surg Am 2014; 96: e44–e44. [DOI] [PubMed] [Google Scholar]
- 43.Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy 2012; 28: 34–42. [DOI] [PubMed] [Google Scholar]
- 44.McCormick F, Wilcox RB, Alqueza A. Postoperative rotator cuff repair rehabilitation and complication management. Oper Tech Orthop 2015; 25: 76–82. [Google Scholar]
- 45.Raab MG, Rzeszutko D, O’Connor W, et al. Early results of continuous passive motion after rotator cuff repair: a prospective, randomized, blinded, controlled study. Am J Orthop 1996; 25: 214–220. [PubMed] [Google Scholar]
- 46.Sheps DM, Bouliane M, Styles-Tripp F, et al. Early mobilisation following mini-open rotator cuff repair: a randomised control trial. Bone Joint J 2015; 97-B: 1257–1263. [DOI] [PubMed] [Google Scholar]
- 47.Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg 2014; 23: 1508–1513. [DOI] [PubMed] [Google Scholar]
- 48.Burkhart SS, Ricchetti ET, Levine WN, et al. Challenges and controversies in treating massive rotator cuff tears. Instr Course Lect 2016; 65: 93–108. [PubMed] [Google Scholar]
- 49.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147. [DOI] [PubMed] [Google Scholar]
- 50.Utsunomiya H, Uchida S, Sekiya I, et al. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med 2013; 41: 657–668. [DOI] [PubMed] [Google Scholar]
- 51.Wang A, Breidahl W, Mackie KE, et al. Autologous tenocyte injection for the treatment of severe, chronic resistant Lateral Epicondylitis a pilot study. Am J Sports Med 2013; 41: 2925–2932. [DOI] [PubMed] [Google Scholar]
- 52.Clarke AW, Alyas F, Morris T, et al. Skin-derived tenocyte-like cells for the treatment of patellar tendinopathy. Am J Sports Med 2011; 39: 614–623. [DOI] [PubMed] [Google Scholar]
- 53.Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair. J Bone Joint Surg Am 2013; 95: 965–971. [DOI] [PubMed] [Google Scholar]
- 54.Kim JH, Hong IT, Ryu KJ, et al. Retear rate in the late postoperative period after arthroscopic rotator cuff repair. Am J Sports Med 2014; 42: 2606–2613. [DOI] [PubMed] [Google Scholar]
- 55.Galatz LM, Charlton N, Das R, et al. Complete removal of load is detrimental to rotator cuff healing. J Shoulder Elbow Surg 2009; 18: 669–675. [DOI] [PubMed] [Google Scholar]
- 56.Peltz CD, Sarver JJ, Dourte LM, et al. Exercise following a short immobilization period is detrimental to tendon properties and joint mechanics in a rat rotator cuff injury model. J Orthop Res 2010; 28: 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCann PDMD, Wootten MEMS, Kadaba MP, et al. A kinematic and electromyographic study of shoulder rehabilitation exercises. Clin Orthop 1993; 288: 179–188. [PubMed] [Google Scholar]
- 58.Hatakeyama Y, Itoi E, Pradhan RL, et al. Effect of arm elevation and rotation on the strain in the repaired rotator cuff tendon a Cadaveric study. Am J Sports Med 2001; 29: 788–794. [DOI] [PubMed] [Google Scholar]
- 59.McCormick F, Wilson H, Gupta A, et al. Single-row, double-row, and transosseous equivalent techniques for isolated supraspinatus tendon tears with minimal atrophy: a retrospective comparative outcome and radiographic analysis at minimum 2-year followup. Int J Shoulder Surg 2014; 8: 15–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koh KH, Kang KC, Lim TK, et al. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy 2011; 27: 453–462. [DOI] [PubMed] [Google Scholar]
- 61.Burks RT, Crim J, Brown N, et al. A prospective randomized clinical trial comparing arthroscopic single- and double-row rotator cuff repair: magnetic resonance imaging and early clinical evaluation. Am J Sports Med 2009; 37: 674–682. [DOI] [PubMed] [Google Scholar]
- 62.Provencher MT, Kercher JS, Galatz LM, et al. Evolution of rotator cuff repair techniques: are our patients really benefiting? Instr Course Lect 2011; 60: 123–136. [PubMed] [Google Scholar]
- 63.Denard PJ, Burkhart SS. The evolution of suture anchors in arthroscopic rotator cuff repair. Arthroscopy 2013; 29: 1589–1595. [DOI] [PubMed] [Google Scholar]
- 64.Burkhart SS, Diaz Pagàn JL, Wirth MA, et al. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy 1997; 13: 720–724. [DOI] [PubMed] [Google Scholar]
- 65.Skaliczki G, Paladini P, Merolla G, et al. Early anchor displacement after arthroscopic rotator cuff repair. Int Orthop 2015; 39: 915–920. [DOI] [PubMed] [Google Scholar]
- 66.Benson EC, MacDermid JC, Drosdowech DS, et al. The incidence of early metallic suture anchor pullout after arthroscopic rotator cuff repair. Arthroscopy 2010; 26: 310–315. [DOI] [PubMed] [Google Scholar]
- 67.Galland A, Airaudi S, Gravier R, et al. Pullout strength of all suture anchors in the repair of rotator cuff tears: a biomechanical study. Int Orthop 2013; 37: 2017–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaar TK, Schenck RC, Wirth MA, et al. Complications of metallic suture anchors in shoulder surgery: a report of 8 cases. Arthroscopy 2001; 17: 31–37. [DOI] [PubMed] [Google Scholar]
- 69.Barber FA, Herbert MA, Beavis RC. Cyclic load and failure behavior of arthroscopic knots and high strength sutures. Arthroscopy 2009; 25: 192–199. [DOI] [PubMed] [Google Scholar]
- 70.Alan Barber F, Herbert MA, Hapa O, et al. Biomechanical analysis of pullout strengths of rotator cuff and glenoid anchors: 2011 update. Arthroscopy 2011; 27: 895–905. [DOI] [PubMed] [Google Scholar]
- 71.Apreleva M, Ozbaydar M, Fitzgibbons PG, et al. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair site area. Arthroscopy 2002; 18: 519–526. [DOI] [PubMed] [Google Scholar]
- 72.Meier SW, Meier JD. Rotator cuff repair: the effect of double-row fixation on three-dimensional repair site. J Shoulder Elbow Surg 2006; 15: 691–696. [DOI] [PubMed] [Google Scholar]
- 73.Gerber C, Schneeberger AG, Perren SM, et al. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg 1999; 81: 1281–1290. [DOI] [PubMed] [Google Scholar]
- 74.Lorbach O, Baums MH, Kostuj T, et al. Advances in biology and mechanics of rotator cuff repair. Knee Surg Sports Traumatol Arthrosc 2015; 23: 530–541. [DOI] [PubMed] [Google Scholar]
- 75.Genuario JW, Donegan RP, Hamman D, et al. The cost-effectiveness of single-row compared with double-row arthroscopic rotator cuff repair. J Bone Joint Surg 2012; 94: 1369–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Black EM, Austin LS, Narzikul A, et al. Arthroscopic anchorless vs. anchored rotator cuff repair: are the cost savings worth the hassle? J Shoulder Elbow Surg 2015; 24: e238–e239. [Google Scholar]
- 77.Gupta AK, Hug K, Boggess B, et al. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med 2013; 41: 872–879. [DOI] [PubMed] [Google Scholar]
- 78.Badhe SP, Lawrence TM, Smith FD, et al. An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elbow Surg 2008; 17: S35–S39. [DOI] [PubMed] [Google Scholar]
- 79.Phipatanakul WP, Petersen SA. Porcine small intestine submucosa xenograft augmentation in repair of massive rotator cuff tears. Am J Orthop 2009; 38: 572–575. [PubMed] [Google Scholar]
- 80.Iannotti JP, Codsi MJ, Kwon YW, et al. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am 2006; 88: 1238–1244. [DOI] [PubMed] [Google Scholar]
- 81.Walton JR, Bowman NK, Khatib Y, et al. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am 2007; 89: 786–791. [DOI] [PubMed] [Google Scholar]
- 82.Barber FA, Burns JP, Deutsch A, et al. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy 2012; 28: 8–15. [DOI] [PubMed] [Google Scholar]
- 83.Lenart BA, Martens KA, Kearns KA, et al. Treatment of massive and recurrent rotator cuff tears augmented with a poly-l-lactide graft, a preliminary study. J Shoulder Elbow Surg 2015; 24: 915–921. [DOI] [PubMed] [Google Scholar]
- 84.Ciampi P, Scotti C, Nonis A, et al. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears a 3-year follow-up study. Am J Sports Med 2014; 42: 1169–1175. [DOI] [PubMed] [Google Scholar]
- 85.Malavolta EA, Gracitelli MEC, Ferreira Neto AA, et al. Platelet-rich plasma in rotator cuff repair: a prospective randomized study. Am J Sports Med 2014; 42: 2446–2454. [DOI] [PubMed] [Google Scholar]
- 86.Weber SC, Kauffman JI, Parise C, et al. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med 2013; 41: 263–270. [DOI] [PubMed] [Google Scholar]
- 87.Bergeson AG, Tashjian RZ, Greis PE, et al. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med 2012; 40: 286–293. [DOI] [PubMed] [Google Scholar]
- 88.Randelli P, Arrigoni P, Ragone V, et al. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg 2011; 20: 518–528. [DOI] [PubMed] [Google Scholar]
- 89.Barber FA, Hrnack SA, Snyder SJ, et al. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy 2011; 27: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 90.Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med 2011; 39: 258–265. [DOI] [PubMed] [Google Scholar]
- 91.Castillo TN, Pouliot MA, Kim HJ, et al. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med 2011; 39: 266–271. [DOI] [PubMed] [Google Scholar]
- 92.Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med 2011; 39: 2135–2140. [DOI] [PubMed] [Google Scholar]
- 93.Würgler-Hauri CC, Dourte LM, Baradet TC, et al. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg 2007; 16: S198–S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mishra A, Tummala P, King A, et al. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods 2009; 15: 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vavken P, Sadoghi P, Palmer M, et al. Platelet-rich plasma reduces retear rates after arthroscopic repair of small- and medium-sized rotator cuff tears but is not cost-effective. Am J Sports Med 2015; 43: 3071–3076. [DOI] [PubMed] [Google Scholar]
- 96.Connell D, Datir A, Alyas F, et al. Treatment of lateral epicondylitis using skin-derived tenocyte-like cells. Br J Sports Med 2009; 43: 293–298. [DOI] [PubMed] [Google Scholar]
- 97.Gomes JLE, da Silva RC, Silla LMR, et al. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc 2011; 20: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop 2014; 38: 1811–1818. [DOI] [PubMed] [Google Scholar]
- 99.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 2007; 13: 1219–1227. [DOI] [PubMed] [Google Scholar]
- 100.Tsai C-C, Huang T-F, Ma H-L, et al. Isolation of mesenchymal stem cells from shoulder rotator cuff: a potential source for muscle and tendon repair. Cell Transplant 2013; 22: 413–422. [DOI] [PubMed] [Google Scholar]
- 101.Randelli P, Conforti E, Piccoli M, et al. Isolation and characterization of 2 new human rotator cuff and long head of biceps tendon cells possessing stem cell-like self-renewal and multipotential differentiation capacity. Am J Sports Med 2013; 41: 1653–1664. [DOI] [PubMed] [Google Scholar]
- 102.Mazzocca AD, McCarthy MBR, Chowaniec DM, et al. Rapid isolation of human stem cells (connective tissue progenitor cells) from the proximal humerus during arthroscopic rotator cuff surgery. Am J Sports Med 2010; 38: 1438–1447. [DOI] [PubMed] [Google Scholar]
- 103.Beitzel K, McCarthy MBR, Cote MP, et al. Comparison of mesenchymal stem cells (osteoprogenitors) harvested from proximal humerus and distal femur during arthroscopic surgery. Arthroscopy 2013; 29: 301–308. [DOI] [PubMed] [Google Scholar]
- 104.Mazzocca AD, McCarthy MBR, Chowaniec D, et al. Bone marrow-derived mesenchymal stem cells obtained during arthroscopic rotator cuff repair surgery show potential for tendon cell differentiation after treatment with insulin. Arthroscopy 2011; 27: 1459–1471. [DOI] [PubMed] [Google Scholar]
- 105.Centeno CJ, Al-Sayegh H, Freeman MD, et al. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop 2016; 40: 1755–1765. [DOI] [PubMed] [Google Scholar]
- 106.Hernigou P and Flouzat Lachaniette CH. Revision rotator cuff repair with mesenchymal stem cells decreases subsequent revision risk. In: Paper presented at AAOS 2016 annual meeting, Orlando, FL, USA, 1 March 2016.
- 107.Hernigou P, Merouse G, Duffiet P, et al. Reduced levels of mesenchymal stem cells at the tendon-bone interface tuberosity in patients with symptomatic rotator cuff tear. Int Orthop 2015; 39: 1219–1225. [DOI] [PubMed] [Google Scholar]
- 108.Greenspoon JA, Moulton SG, Millett PJ, et al. The role of platelet rich plasma (PRP) and other biologics for rotator cuff repair. Open Orthop J 2016; 10: 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]