Abstract
Background:
Gamma-ray irradiation could significantly induce widespread apoptosis in corneas and reduced the allogenicity of donor cornea. And the X-rays may have similar biological effects. The feasibility and effects of X-ray-irradiated corneal lamellae have not been assessed yet.
Methods
Different doses (10 gray unit (Gy), 20 Gy, 50 Gy, 100 Gy) of X-ray irradiated corneal lamellae were collected from SMILE surgery. These corneal lamellae were assessed by physical characterization, hematoxylin and eosin (H-E) staining, Masson’s staining, TdT-mediated dUTP nick end labeling (TUNEL), cell viability assay and transmission electron microscopy (TEM). We selected the optimum dose (100Gy) to treat the corneal lamellae to be the grafts. The human grafts and fresh allogeneic monkey corneal lamellae were implanted into rhesus monkeys via the small incision femtosecond laser-assisted surgery, respectively. Clinical examinations and the immunostaining were performed after surgery.
Results
There were no significant changes in the transparency of the corneal lamellae, but the absorbency of the corneal lamellae was increased. According to the H-E and Masson’s staining results, irradiation had little impact on the corneal collagen. The TUNEL assay and cell viability assay results showed that 100Gy X-ray irradiation resulted in complete apoptosis in the corneal lamellae, which was also confirmed by TEM observations. In the following animal model study, no immune reactions or severe inflammatory responses occurred, and the host corneas maintained transparency for 24 weeks of observation. And the expression of CD4 and CD8 were negative in the all host corneas.
Conclusion
X-ray irradiated corneal lamellae could serve as a potential material for xenogeneic inlay, and the small incision femtosecond laser-assisted implantation has the potential to become a new corneal transplantation surgical approach.
Keywords: X-ray-irradiated, SMILE, femtosecond laser, xenotransplantation, rhesus monkey
1. Introduction
Keratopathy is one of the most common eye diseases worldwide [2]. Currently, there are more than 10 million patients with binocular corneal blindness worldwide [1], and there is an increase trend that 1.5–2 million new cases of corneal blindness emerged annually [3]. Corneal transplantation has been the most effective solution in decades. However, the insufficient corneal materials donation and severe postoperative complications restrict the application of transplantation [4].
In recent years, novel corneal processing methods could help address the worldwide insufficient of corneal tissue by increasing the supply. In 1963, flint glass and some other artificial synthetic materials [5] were used for the grafts. However, there were serious compli-cations, such as corneal necrosis, toxic parenchy-matous keratitis, and implant discharge [6-8]. And the application of xenogeneic biomaterials has been considered. A recent study indicated that materials with low antigenicity, such as decellularized corneal materials, can be used as supporting materials for corneal tissue regeneration [9]. However, how to effectively remove the cellular components from the transplanted tissues while maintaining the inherent tissue structure is difficult to accomplish simultaneously [10]. Many decellularization methods have been used to solve this problem by reducing the antigenicity, such as lyophilization and irradiation, chemical, and physical methods [11]. Lyophilization could reduce the protein denaturation [12, 13], but it will influence the keratocyte migration and growth [11]. The implantation of corneal materials decellularized via chemical or physical methods may failed because of the poor material flexibility [14] and the change of the microenvironment of the host cornea [15].
Although the gamma-ray irradiation could significantly induce widespread apoptosis in corneas, and the allogenicity of donor cornea, but not elicit T-cell-mediated alloimmunity [16]. It has been shown that X-rays have a greater biological effect than gamma-rays [17], which means the X-rays also could induce apoptosis in tissues to a certain extent [1]. The energies and occurrence mechanisms of X-rays are different from the gamma rays. The amplitude of X-rays would decrease with distance, but the gamma-rays would not change [18]. The X-rays is a safer and more controllable technology as compared with gamma rays. And the X-rays could be easier to protect within the clinical application. The X-rays is also used in radiotherapies widely. Current studies on ocular X-rays have mostly been limited to treating ocular tumors [19]. Thus, X-irradiated corneal processed material is a newly area of study.
Known as an “all-in-one” technique, the small incision lenticule extraction (SMILE) is safer, more effective and more precise than other refractive surgical techniques [20, 21]. Another approach of the femtosecond laser is additive keratoplasty. This involves the insertion of a synthetic or biologic material into the cornea to alter the curvature of the cornea or replace the corneal partial stroma [22-26]. Our previous study has confirmed that implant rejection or any severe inflammatory responses were not observed after small incision femtosecond laser-assisted monkey corneal allotransplantation [27], which suggested that this surgical procedure avoids the vivid parts of ocular immunoreaction medium and could be applied in xenotransplantation. The lenticule extracted from the corneal stroma in SMILE surgery is a regular concave lens, but always abandoned intra-operatively. These lenticules are convenientto be collected and have the potential to be the inlay materials.
We applied X-rays to irradiate human corneal lamellae and explore the optimum irradiation dose. We adopted this X-ray dose to irradiate human corneal lamellae as xenogeneic grafts. The human xenogeneic grafts and fresh monkey allogeneic grafts were implanted into rhesus monkeys via small incision femtosecond laser-assisted surgery, respectively. The rejection reactions, inflammatory responses and graft stability were monitored for 24 weeks.
2. Materials and Methods
2.1. Study Design
Corneal lamellae were obtained from SMILE surgery. Corneal lamellae were decellularized via different dose X-ray irradiation and denoted as 10 gray unit (Gy) group, 20 Gy group, 50 Gy group and 100 Gy group. After irradiation, physical characterization, immunostaining and TEM were examined. And we chose the corneal lamellae with the optimum irradiated dose to be the inlay xenogenic grafts. Xenogenic grafts and fresh allogeneic grafts were inlayed into rhesus monkey corneas by the SMILE assisted surgery. After surgery, we observed the ocular appearance of the monkeys every day and examined 8 monkeys that received grafts at 1, 4, 12 and 24 weeks after implantation.
2.2. Animals
All experiments were approved by the Ethics Committee of the Zhongshan Ophthalmic Center, Sun Yat-Sen University (Acceptance NO. 2015-010). 12 five-year-old healthy adult rhesus monkeys (4.5–5.5 kg) obtained from Landao Biotechnology Co., Ltd., (Guangzhou, China) were used in this study. All the monkeys were housed per cage in clean environmentally controlled rooms in an Association for the Assessment and Accreditation of Laboratory Animal Care-accredited facility (Animal Experiment Center of the Zhongshan Ophthalmic Center) and provided free access to food and water throughout the study.
2.3. Corneal Lamellae Collection & Procedure of X-ray Irradiation
Human corneal lamellae were collected from SMILE surgery for the treatment of myopia at the Zhongshan Ophthalmic Center (Guangzhou, China). Patients had a spherical equivalent ranging from -3.00 to -5.00 diopters (D). All patients provided written consent to participate in this study.
Corneal lamellae were preserved temporarily at 4°C in a plate with a small amount of phosphate-buffered saline (PBS; Boshide, China). The RS2000 X-ray irradiation (Cancer Hospital, Sun Yat-Sen University) was used. The total doses of irradiation tested were 10 Gy, 20 Gy, 50 Gy, and 100 Gy. The irradiation parameters were 160 kV and 25 mA with 0.3 mm copper filter and a 50 cm range. About 50 corneal lamellae were used in each radiation dosage group.
2.4. Physical Characterization Examination
2.4.1. Optical Property
The corneal lamellae were placed in a 96-well culture dish with a small amount of PBS. The light transparency was measured at an average wavelength of 450 nm using a spectral photometer (Synergy H1; BioTek, USA) [28]. After dehydration in 100 v/v% glycerol for 1 hour, the transparency was measured again.
2.4.2. Water Content
The corneal lamellae were weighed after excess fluid removed. The ability to retain water was assessed using the following formula: (W2–W1)/W2 × 100%. W1 is the weight of the initial corneal lamellae sample, and W2 is the weight of the sample after being dried for 1 hour at 65°C [29].
2.5. Hematoxylin and Eosin (H-E) Staining and Masson’s Staining
Corneal lamellae were fixed in optimal cutting temperature compound (O.C.T. Compound, SAKURA, Japan) and frozen at -20°C overnight. The sections were prepared at a thickness of 7 μm, and the standard procedures for H-E staining and Masson’s staining were performed.
2.6. TdT-Mediated DUTP Nick End Labeling (TUNEL) Assay
TUNEL was performed using a commercially available kit (In Situ Cell Death Detection Kit; Roche Diagnostics Corp., Indianapolis, IN). The sections were fixed in 4% paraformaldehyde for 20 minutes and washed in PBS for 30 minutes at room temperature. Samples were permeabilized on ice for 2 minutes in a solution containing 0.1% Triton X-100 and 0.1% sodium citrate. TUNEL and labeling solutions were prepared as recommended, and the treated samples were incubated in a humidified chamber at 37°C for 60 minutes in the dark. The corneal lamellae were washed in PBS and mounted using mounting media with 4,6-diamidino-2-phenylindole (DAPI). The stained corneal sections were examined at 40x magnification by confocal microscopy (Leica CM1850; Leica Microsystems, Buffalo Grove, IL).
2.7. Cell Viability Assay
Cell viability was assessed with a commercially available kit (LIVE/DEAD viability/cytotoxicity kit for mammalian cells; Invitrogen, Carlsbad, CA). Fresh human corneal lamellae were washed 3 times in sterile PBS for 10 minutes. A solution containing 2.5μL calcein AM (4 mM in dimethyl sulfoxide [DMSO]) and 10μL ethidium homodimer-1 (2 mM in 1:4 DMSO/H2O) in 5mL PBS was prepared before staining. Corneal lamellae were incubated in this solution for 40 minutes in the dark at room temperature and subsequently washed one time in PBS for 5 minutes. And corneal lamellae were mounted using mounting media with DAPI. Samples were examined using a fluorescence microscopy (OLYMPUS BX53, JP) at 40× magnification.
2.8. Transmission Electron Microscopy (TEM) Observation
Corneal lamellae were fixed in 2.5% glutaraldehyde, embedded in resin, and cut into ultrathin sections. The sections were then analyzed by TEM (H600; Hitachi, Japan).
2.9. Formation of the Rhesus Monkey Allografts
Four selected rhesus monkeys were anesthetized with an intramuscular injection of Zoletil 50 (5 mg/kg, Virbac, Carros, France) and underwent SMILE surgery in one random eye with a myopic treatment correction of -4.00 D. The femtosecond laser parameters were as follows: power, 135 nJ; cap thickness, 120 μm; and cap diameter, 7.5 mm. The small incision (3 mm) was located at the 140° position. The corneal lamellae were separated as the allograft.
2.10. Small Incision Femtosecond Laser-Assisted Transplantation Surgical Procedure in Rhesus Monkeys
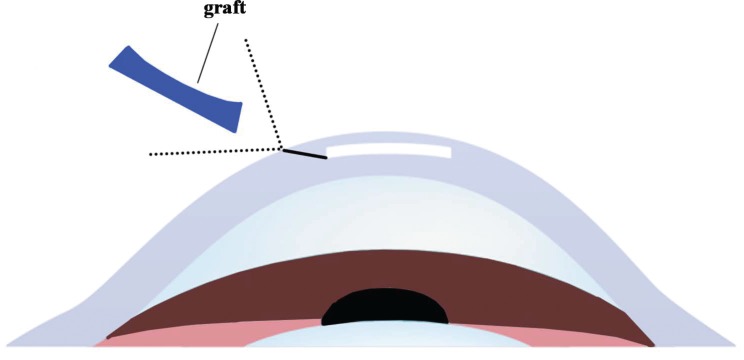
The remaining eight rhesus monkeys were anesthetized with an intramuscular injection of Zoletil 50 (5 mg/kg, Virbac, Carros, France) and underwent SMILE surgery in one random eye with a myopic correction of -0.75 D. The femtosecond laser parameters were as follows: power, 135 nJ; cap thickness, 160 μm; cap diameter, 7.8 mm; and corneal lamellae diameter, 7.8 mm. The small incision (3 mm) was located at the 140° position, with a side cut angle of 90°. The lenticule (28 μm in thickness) was separated out, and that produced a space similar to a “stromal pocket” (Fig. 1). Use forceps to implant the graft into the “stromal pocket.”
Fig. (1).
Use forceps to inlayed the graft into the “stromal pocket”.
Fore human corneal lamellae treated with X-ray irradiation were implanted into the monkeys, respectively, as the xenotransplantation group. Other four monkeys received the allogeneic grafts, as the allotransplantation group.
2.11. Postoperative Treatment and Clinical Examinations
Following the surgery, all 12 monkeys were administered tobramycin and dexamethasone (Alcon Laboratories) eye drop 4 times a day for 1 week. The ocular examinations were followed by a slit-lamp examination to assess corneal transparency, corneal edema and neovascularization.
2.12. Immunohistochemistry
At 24 weeks after the surgery, the monkeys were euthanized with an overdose intramuscular injection of Zoletil 50 (50 mg/kg). The corneas were dehydrated with dehydrated alcohol and were embedded in paraffin. Then, samples were cut into 7μm-thickness sections using a cryostat. A standard procedure for H-E staining was performed.
After paraffin removal and microwave treated antigen retrieval, the sections were treated with 0.3% H2O2 in PBS to quench endogenous peroxidase activity and then incubated with 5% goat serum to block the non-specific sites. CD4 (1:50) or CD8 (1:200). Antibody was applied and incubated at 4°C overnight, followed by incubation with secondary antibody at room temperature for 30 minutes.
2.13. Statistical Analysis
Values are expressed as the mean ± SD (standard deviation). Statistically significant differences are indicated as *P < 0.05, compared with the non-irradiate group, respectively.
Statistical analysis was conducted using SPSS 16.0 statistical software. Optical property and water content data (Fig. 2B & C) were expressed as mean±standard deviation (SD) from five separate experiments. All the data above were assessed using one-way ANOVA. For pairwise comparisons within the group, we used either the least significant difference test (LSD-t) or Tamhane’s T2. P<0.05 was considered significant.
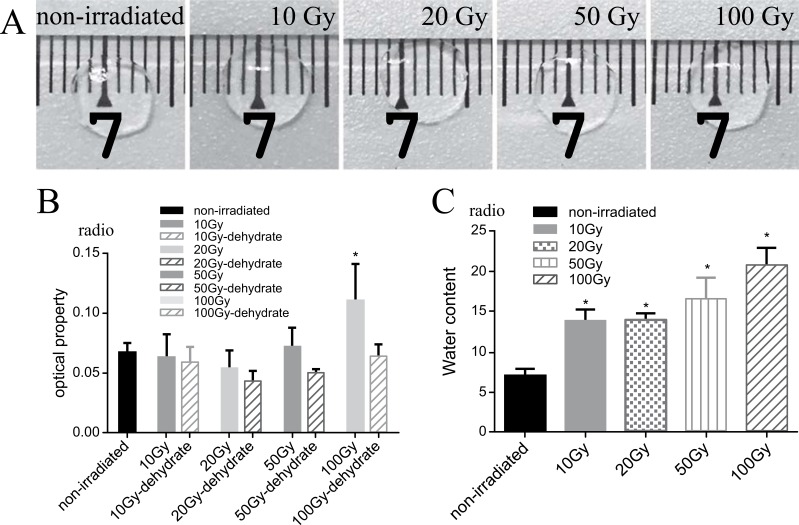
Fig. (2).
(A) Representative images of corneal lamellae. Though different dose of irradiation, corneal lamellae still stayed same transparency compare to the non-irradiated one. (B) The optical property at an average of 450 nm in the visible region of the corneal lamellae treated with two conditions. The optical property decreased in the 100Gy group (P=0.001), and those exposed to X-ray irradiation with dehydration showed no significant differences P10Gy=0.515,P20 Gy =0.052,P50 Gy =0.150,P100 Gy =0.820). (C) The radio of water content was calculated by the equation. The water content of corneal lamellae increased after X-ray irradiation (P10Gy=0.001,P20 Gy =0.001,P50 Gy =0.001,P100 Gy =0.001).
3. Results
3.1. Physical and Mechanical Characterization of Corneal Lamellae
The corneal lamellae remained transparent after the X-ray irradiation, as revealed by gross observation (Fig. 2A). Comparisons in terms of the transparency of non-irradiated corneal lamellae (control group) and those exposed to X-ray irradiation with dehydration showed no significant differences (Fig. 2B). However, the corneal lamellae from the control group showed a significantly lower level of water content ratio than those of the other groups (Fig. 2C).
3.2. H-E Staining and Masson’s Staining
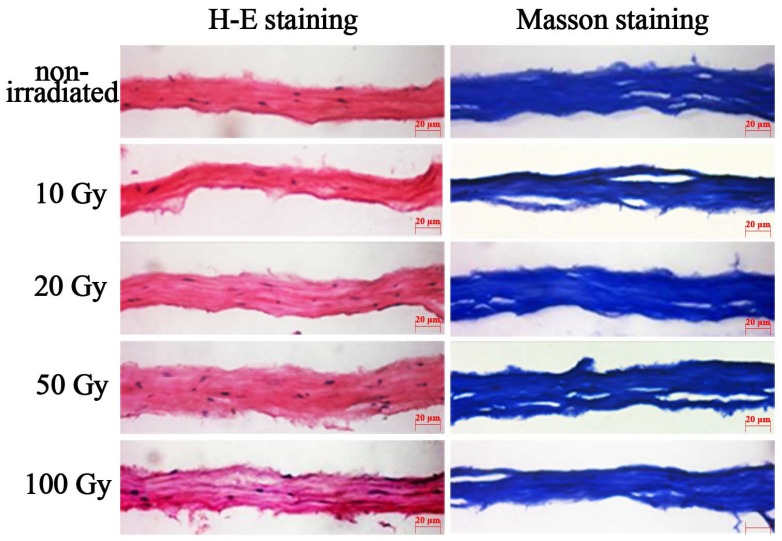
H-E staining and Masson’s staining revealed no obvious changes in collagen (Fig. 3). In addition, H-E staining revealed that blue-stained cell nuclei and cell debris remained present in the corneal stroma.
Fig. (3).
H-E staining and Masson staining corneal lamellae. After 10 Gy, 20 Gy, 50 Gy, 100 Gy irradiation, H-E staining of lamellar cornea (original magnification, X40), blue-stain cell nucleus and cell debris were found in the matrix. The collagens were lined up similarly to the non-irradiated corneal lamellae.
3.3. TUNEL Assay & Cell Viability Assay
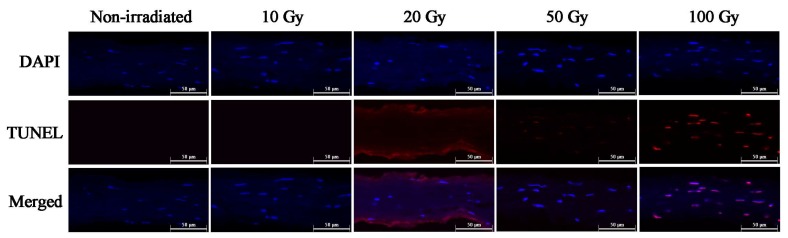
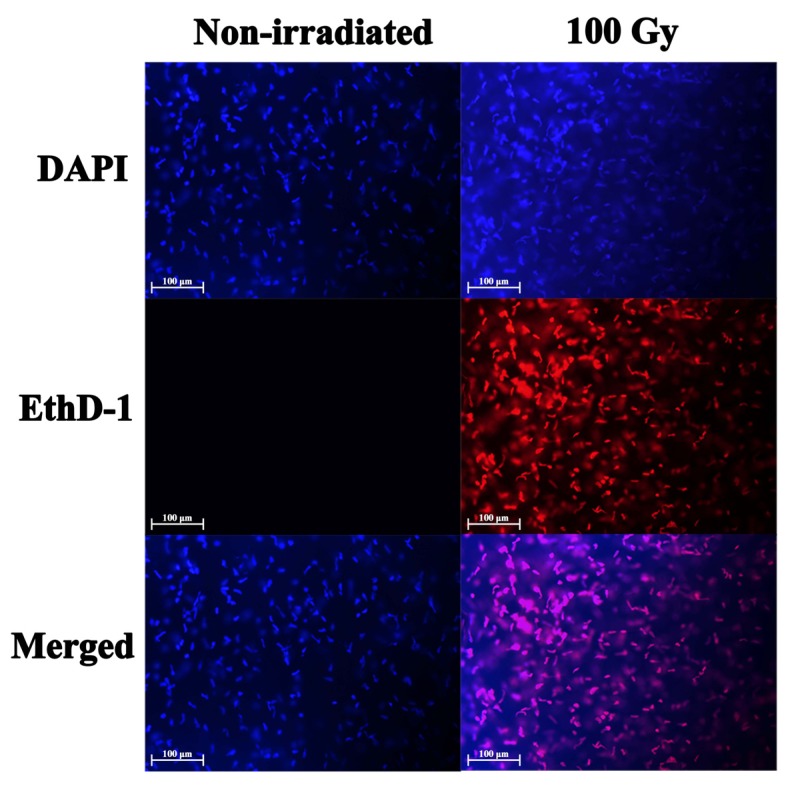
The TUNEL assay was used to evaluate cell viability by analyzing the irradiated corneal lamellae and non-irradiated corneas (Fig. 4). TUNEL was performed on corneal sections to identify DNA strand breaks generated by apoptosis. Apoptosis is a widespread occurrence in high dose irradiation corneal lamellae. These results were associated with the cell viability assay that allowed for the detection of live and dead cells. The intracellular esterase activity of live cells was analyzed by staining with calcein AM, while dead cells with disrupted cell membranes were stained with ethidium homodimer-1. In 100Gy X-ray irradiated corneal lamellae, viable cells were absent universally and all cells were positive for ethidium homodimer-1, indicating widespread cell death (Fig. 5).
Fig. (4).
Representative TUNEL staining. The apoptosis was relatively rare occurrence in non-irradiated corneas and corneal lamellae treated with 10 Gy or 20 Gy X-ray irradiation. However, the TUNEL assay revealed that apoptosis in 100 Gy X-ray irradiated corneal lamellae was remarkably widespread, and the apoptosis also occurred in 50 Gy X-ray irradiated ones (DAPI=blue, TUNEL= red).
Fig. (5).
Representative cell viability staining. After 100 Gy X-ray irradiation, all of the corneal cells in corneal lamellae were positive for ethidium homodimer-1 (EthD-1), indicating widespread plasma membrane disruption and cell death (DAPI=blue, EthD-1=red).
3.4. TEM
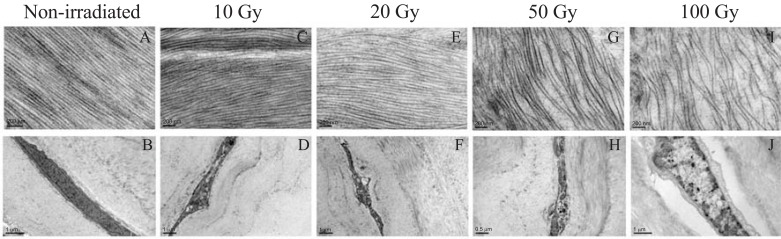
There were significant alterations in the shape of keratocytes and the arrangement of collagen after exposed to high-dose (50 Gy or 100 Gy) X-ray irradiation (Fig. 6G, H, I, J).
Fig. (6).
Representative images of TEM. The collagen in the non-irradiated corneal lamellae (A) arranged in a compact structure. Keratocytes in the non-irradiated one were long spindle (B). After 10 Gy or 20 Gy X-ray irradiation, collagens were arranged in a loose formation that was still similar to the normal corneal matrix fiber. (C, E), and keratocytes nuclear membrane turn to shrivel (D, F). After 50 Gy or 100 Gy irradiation, the collagen arranged in an obvious loose formation (G, I). After 100 Gy irradiation, keratocytes nuclear membrane remarkably disrupted, and autophagic vacuoles were observed (J). Moreover, keratocytes nuclear membrane disruption also occurred in 50 Gy X-irradiated the corneal lamellae (H).
3.5. Clinical Examinations
After 100Gy X-ray irradiation, significant apoptosis was observed, and the corneal lamellae remain transparency. We chose the corneal lamellae exposed to 100Gy X-ray irradiation to be the xenogenic grafts.
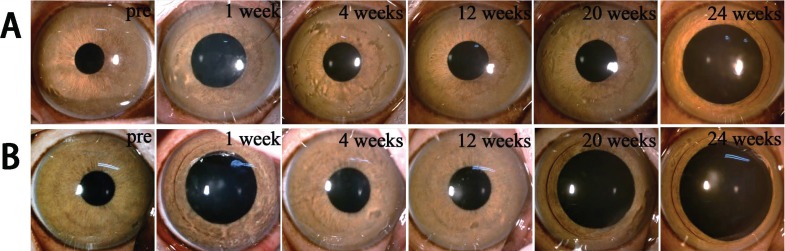
Throughout the observation period, all rhesus monkeys in both allotransplantation group (Fig. 7A) and xenotransplantation group (Fig. 7A) survived without infectious or hemorrhagic complications. No rejection reactions, severe inflammation responses, corneal neovascularization, or graft degradation were observed within 24 weeks after implantation. All the host cornea maintained transparency (Fig. 7).
Fig. (7).
Representative images of preoperation and postoperation. (A) Allotransplantation group (B) Xenotransplantation group.
3.6. Immunohistochemistry Staining
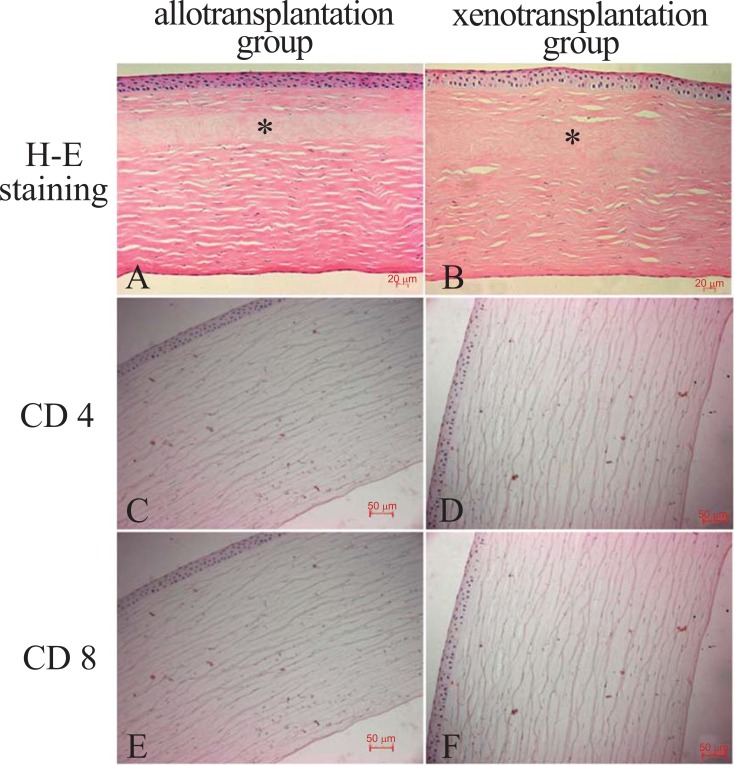
The grafts could be recognized in the monkey corneas at 24 weeks after surgery. There were no signs of neovascularization, inflammation cells or implant disposition, as demonstrated by H-E staining (Fig. 8A & Fig. 8B). And in the both groups, there was no expression of CD4 (Fig. 8C & Fig. 8D) and CD8 (Fig. 8E & Fig. 8F) in the corneas, as demonstrated by immunohistochemical staining.
Fig. (8).
In both groups, H-E staining showed implanted corneal lamellae material (asterisk) and no cell inside them (A&B). And there was no expression of CD4 (C&D) and CD8 (E&F), as demonstrated by immunohistochemical staining.
4. Discussion
100Gy X-ray irradiated human corneal inlays were well tolerated by all rhesus monkey corneas over the 24 weeks of this trial, demonstrating that it is possible to successfully maintain an inlay under a corneal flap. In these animals, the implants were centered under the corneal flap, and the corneas remained clear apart from low levels of interfacial debris with no signs of infiltration or neovascularization in the implanted corneas for the duration of the study period. In each case, the tear film was normal, the epithelium was intact and healthy, and there was no obvious stromal thinning. The small incision femtosecond laser technique achieved a corneal flap of consistent depth and thickness on every occasion gave a good long-term outcome in the present study. And the X-ray-irradiated corneal lamellae might be a potential inlay xenogeneic material.
In this study, we chose human corneal lamellae collected from SMILE surgery, for the acquirement were easy and convenient [30]. The thickness of these collected corneal lamellae ranged from 100µm to 150µm. In the radiation therapy, the normal X-ray dose for the human eye was 10–20 Gy [31]. In our preliminary study, low levels doses of X-ray irradiation were used (2 Gy, 4 Gy, 6 Gy, 8 Gy, 10 Gy). Physical characters of corneal lamellae were not significantly changed, neither the apoptosis was observed. Therefore, these levels of X-ray doses (10 Gy, 20 Gy, 50 Gy, 100 Gy) were selected to form different irradiation dose groups, and non-irradiated corneal lamellae were used as the control group. We chose the one dose with the optimum change to be used to from xenogenic grafts by exploring different aspects of the corneal lamellae after irradiation.
The transparency of corneal lamellae in each group changed after radiation, while there were no significant differences in transparency after the glycerol dehydration. This result may be related to the change in the absorbency of the materials, for the increase of the water content in the materials after irradiation could result in temporary edema and opaqueness. A normal cornea stroma structure and intact collagen tissue arrangement are necessary conditions for maintaining the corneal transparency [32]. When the structure is irreversibly changed, the transparency of the cornea cannot be recovered [33, 34]. The water content increased in a dose-dependent manner after X-ray irradiation. The Masson’s staining showed that the collagen structure was maintained, but changes in the collagen structure were still observed by TEM. The increased water content may have been caused by loose collagen matrix tissues of the corneal lamellae. Although there were changes in the collagen structure of the corneal lamellae (the collagen structure became sparse), the transparency of the corneal lamellae was not influenced. This outcome was consistent with the results from a previous study conducted by Chae et al. [35], who indicated that smaller collagen fibril diameters increase transparency, which compensates for decreased corneal transparency by increasing interfibrillar spacing.
Gotoh [36] and Stevenson et al. [37, 38] found that high doses of radiation can reduce the immunological transplant rejection of islet cells. Stevenson W et. [16] findings suggested that the inability of irradiated corneal grafts to remain optically clear was not immune-mediated. Cellular immunity is the most important rejection process in corneal transplantation [39]. External DNA and keratocytes can activate histocompatibility class I molecules and cause immunological rejection [40-42]. Cell processed techniques that devitalize cells have demonstrated immunosuppressive effects in a variety of experimental models [43]. The reduced allogenicity of irradiated corneas is likely related to the devitalization of potentially antigenic corneal cells, including the resident antigen-presenting cells. Therefore, gamma-ray irradiation can reduce the antigenicity of corneal implants [16]. X-rays and gamma rays have specific abilities for penetrating tissues and inducing apoptosis. In the present study, significant apoptosis was found in 100Gy X-ray irradiated corneal lamellae and the physical characters of these processed corneal lamellae still remained. Therefore, the 100Gy X-ray irradiation is the optimal process for inlay grafts.
In recent years, the femtosecond laser provides a novel approach for corneal transplantation due to its accuracy and predictability [44-46]. In this small incision femtosecond laser-assisted corneal intrastromal implantation, grafts were implanted into the corneal “stromal pocket” without being affected by aqueous humor or tears rich in inflammatory mediators [47]. This greatly reduced the incidence of implant rejection. The negative expression of CD4 and CD8 also confirms that the immunoreaction is negligible after implantation. The implantation used no sutures, and increased the degree of postoperative comfort. Twenty-four weeks after implantation, cell components were not found in the grafts of both groups and the grafts maintained their collagen structure.
Therefore, the host keratocytes still need further study to observe their migratory influence. And with small incision femtosecond-laser assisted, X-ray irradiated xenogeneic graft might be a potential inlay material. The small incision femtosecond laser-assisted surgery applied in this study also provides a new treatment method for aphakia, high hyperopia, and keratoconus.
Conclusion
This pilot study showed that the application of 100Gy X-ray irradiation can result in complete apoptosis in corneal lamellae and exert minimal impact on collagen. The 100Gy X-ray-irradiated corneal lamellae maintained transparency, presented no immunological rejection, and possess high stability. The small incision femtosecond laser implantation was highly efficient and safe treatment method. Thus, this study presents a new method for manufacturing inlay xenogeneic grafts and provides a new treatment for keratoconus, corneal ectasia, and presbyopia, among other diseases. Additionally, we will conduct more studies on the potential immune mechanisms involved and the changes in the corneal refraction state after implantation to support future clinical applications.
ACKNOWLEDGEMENTS
We acknowledge the American Journal Experts (AJE) for providing Premium Editing Service for this manuscript. The funding is provided by the Science and Technology Planning Project of Guangdong Province (2013B090200057), the National Natural Science Foundation of China (81371046), and Science and Technology Planning Project of Hainan Province (ZDYF2016111).
LIST OF ABBREVIATIONS
- SMILE
small incision lenticule extraction
- H-E staining
hematoxylin and eosin staining
- TUNEL
TdT-mediated dUTP nick end labeling
- TEM
transmission electron microscopy
- PBS
phosphate-buffered saline
Ethics Approval and Consent to Participate
All experiments were approved by the Ethics Committee of Hainan Eye Hospital of the Zhongshan Ophthalmic Center, Sun Yat-sen University (Acceptance number: 2015-010).
Human and Animal Rights
Humans were not used in this research. All animal research procedures followed were in accordance with the standards set forth in the eighth edition of Guide for the Care and Use of Laboratory Animals published by the National Academy of Sciences, The National Academies Press, Washington, D.C.).
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
He Jin and Liangping Liu contributed equally to this paper. All authors made substantial contributions to conception and design. He Jin, Liangping Liu and Miao He collected the data. Hui Ding and Chi Zhang made substantial contributions to analysis and interpretation of data. He Jin wrote the first draft of the manuscript. All authors were involved in revising the manuscript critically for important intellectual content. And Xingwu Zhong has the given final approval of the version to be published. All authors read and approved the final manuscript.
References
- 1.Dana MR. Corneal antigen-presenting cells: diversity, plasticity, and disguise: the Cogan lecture. 2004. [DOI] [PubMed]
- 2.Haber M., Cao Z., Panjwani N., et al. Effects of growth factors (EGF, PDGF-BB and TGF-beta 1) on cultured equine epithelial cells and keratocytes: implications for wound healing. Vet. Ophthalmol. 2003;6:211–217. doi: 10.1046/j.1463-5224.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 3.King W.J., Comer R.M., Hudde T., et al. Cytokine and chemokine expression kinetics after corneal transplantation. Transplantation. 2000;70:1225. doi: 10.1097/00007890-200010270-00017. [DOI] [PubMed] [Google Scholar]
- 4.Tan D.T., Dart J.K., Holland E.J., Kinoshita S. Corneal transplantation. Lancet. 2012;379:1749. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y.J. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 6.Treseler P.A., Foulks G.N., Sanfilippo F. The expression of HLA antigens by cells in the human cornea. Am. J. Ophthalmol. 1984;98:763–772. doi: 10.1016/0002-9394(84)90696-2. [DOI] [PubMed] [Google Scholar]
- 7.Williams K.A., Ash J.K., Coster D.J. Histocompatibility antigen and passenger cell content of normal and diseased human cornea. Transplantation. 1985;39:265–269. doi: 10.1097/00007890-198503000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Ismail M.M. Correction of hyperopia with intracorneal implants. J. Cataract Refract. Surg. 2002;28:527–530. doi: 10.1016/s0886-3350(01)01128-2. [DOI] [PubMed] [Google Scholar]
- 9.Fontana L., Errani P.G., Zerbinati A., et al. Frequency of positive donor rim cultures after penetrating keratoplasty using hypothermic and organ-cultured donor corneas. Cornea. 2007;26:552–556. doi: 10.1097/ICO.0b013e3180415d7e. [DOI] [PubMed] [Google Scholar]
- 10.Hanada K., Igarashi S., Muramatsu O., Yoshida A. Therapeutic keratoplasty for corneal perforation. Cornea. 2008;27:1219–1220. doi: 10.1097/ICO.0b013e31818200d2. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson W., Cheng S.F., Emami-Naeini P., et al. Gamma-irradiation reduces the allogenicity of donor corneas. Invest. Ophthalmol. Vis. Sci. 2012;53:7151–7158. doi: 10.1167/iovs.12-9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Killian D., Reichard M., Knueppel A., et al. Distribution changes of epithelial dendritic cells in canine cornea and mucous membranes related to hematopoietic stem cell transplantation. In Vivo. 2013;27:761–771. [PubMed] [Google Scholar]
- 13.Jiang S., Nail S.L. Effect of process conditions on recovery of protein activity after freezing and freeze-drying. Eur. J. Pharm. Biopharm. 1998;45:249–257. doi: 10.1016/s0939-6411(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 14.Li J., Shi S., Zhang X., et al. Comparison of different methods of glycerol preservation for deep anterior lamellar keratoplasty eligible corneas. Invest. Ophthalmol. Vis. Sci. 2012;53:5675–5685. doi: 10.1167/iovs.12-9936. [DOI] [PubMed] [Google Scholar]
- 15.Hamrah P., Liu Y., Zhang Q., Dana M.R. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch. Ophthalmol. 2003;121:1132–1140. doi: 10.1001/archopht.121.8.1132. [DOI] [PubMed] [Google Scholar]
- 16.Caporossi A., Mazzotta C., Baiocchi S., Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am. J. Ophthalmol. 2010;149:585–593. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Williams J.P., Brown S.L., Georges G.E., et al. Animal models for medical countermeasures to radiation exposure. Radiat. Res. 2010;173:557–578. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haines J., Dunford R., Moody J., et al. Loss of heterozygosity in spontaneous and X-ray-induced intestinal tumors arising in F1 hybrid min mice: evidence for sequential loss of APC(+) and Dpc4 in tumor development. Genes Chromosomes Cancer. 2000;28:387–394. doi: 10.1002/1098-2264(200008)28:4<387::aid-gcc4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Hadden O.B., Patel D., Gray T.B., et al. Multifocal lamellar keratitis following laser in situ keratomileusis. J. Cataract Refract. Surg. 2007;33:144–147. doi: 10.1016/j.jcrs.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 20.Stonecipher K., Ignacio T.S., Stonecipher M. Advances in refractive surgery: microkeratome and femtosecond laser flap creation in relation to safety, efficacy, predictability, and biomechanical stability. Curr. Opin. Ophthalmol. 2006;17:368–372. doi: 10.1097/01.icu.0000233957.88509.2d. [DOI] [PubMed] [Google Scholar]
- 21.Blum M., Täubig K., Gruhn C., et al. Five-year results of Small Incision Lenticule Extraction (ReLEx SMILE). Br. J. Ophthalmol. 2016 doi: 10.1136/bjophthalmol-2015-306822. [DOI] [PubMed] [Google Scholar]
- 22.Belau P.G., Dyer J.A., Ogle K.N., Henderson J.W. Correction of Ametropia With Intracorneal Lenses. An Exprimental Study. Arch. Ophthalmol. 1964;72:541–547. doi: 10.1001/archopht.1964.00970020541020. [DOI] [PubMed] [Google Scholar]
- 23.McCarey B.E., Andrews D.M. Refractive keratoplasty with intrastromal hydrogel lenticular implants. Invest. Ophthalmol. Vis. Sci. 1981;21:107–115. [PubMed] [Google Scholar]
- 24.Lane S.L., Lindstrom R.L., Cameron J.D., et al. Polysulfone corneal lenses. J. Cataract Refract. Surg. 1986;12:50–60. doi: 10.1016/s0886-3350(86)80057-8. [DOI] [PubMed] [Google Scholar]
- 25.Dupont D., Gravagna P., Albinet P., et al. Biocompatibility of human collagen type IV intracorneal implants. Cornea. 1989;8:251–258. [PubMed] [Google Scholar]
- 26.McCarey B.E. Current status of refractive surgery with synthetic intracorneal lenses: Barraquer lecture. Refract. Corneal Surg. 1990;6:40–46. [PubMed] [Google Scholar]
- 27.Liu L., Wang Y., He M., et al. Preliminary Investigation Femtosecond Laser-assisted Refractive Lenticule Transplantation in Rhesus Monkeys. J SunYat-Sen University. 2015;36:449–455. [Medical Sciences]. [Google Scholar]
- 28.Beems E.M., Van Best J.A. Light transmission of the cornea in whole human eyes. Exp. Eye Res. 1990;50:393–395. doi: 10.1016/0014-4835(90)90140-p. [DOI] [PubMed] [Google Scholar]
- 29.Friedman M.H., Green K. Swelling rate of corneal stroma. Exp. Eye Res. 1971;12:239–250. doi: 10.1016/0014-4835(71)90144-8. [DOI] [PubMed] [Google Scholar]
- 30.Moller-Pedersen T., Cavanagh H.D., Petroll W.M., Jester J.V. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: a 1-year confocal microscopic study. Ophthalmology. 2000;107:1235–1245. doi: 10.1016/s0161-6420(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 31.Hattori T., Takahashi H., Dana R. Novel Insights Into the Immunoregulatory Function and Localization of Dendritic Cells. Cornea. 2016;35(Suppl. 1):S49–S54. doi: 10.1097/ICO.0000000000001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moffatt S.L., Cartwright V.A., Stumpf T.H. Centennial review of corneal transplantation. Clin. Exp. Ophthalmol. 2005;33:642–657. doi: 10.1111/j.1442-9071.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- 33.Shah A., Brugnano J., Sun S., et al. The development of a tissue-engineered cornea: biomaterials and culture methods. Pediatr. Res. 2008;63:535–544. doi: 10.1203/PDR.0b013e31816bdf54. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto Y., Funamoto S., Sasaki S., et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials. 2010;31:3941–3948. doi: 10.1016/j.biomaterials.2010.01.122. [DOI] [PubMed] [Google Scholar]
- 35.Wedner S., Dineen B. Refractive errors. Trop. Doct. 2003;33:207–209. doi: 10.1177/004947550303300406. [DOI] [PubMed] [Google Scholar]
- 36.Gotoh M., Kanai T., Dono K., et al. Gamma-irradiation as a tool to reduce immunogenicity of islet allo- and xenografts. Horm. Metab. Res. Suppl. 1990;25:89–96. [PubMed] [Google Scholar]
- 37.Mamalis N., Anderson C.W., Kreisler K.R., et al. Changing trends in the indications for penetrating keratoplasty. Arch. Ophthalmol. 1992;110:1409–1411. doi: 10.1001/archopht.1992.01080220071023. [DOI] [PubMed] [Google Scholar]
- 38.Feng Y., Wang W. In vivo confocal microscopic observation of lamellar corneal transplantation in the rabbit using xenogenic acellular corneal scaffolds as a substitute. Chin. Med. J. (Engl.) 2015;128:933–940. doi: 10.4103/0366-6999.154301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishii K.J., Akira S. Innate immune recognition of, and regulation by, DNA. Trends Immunol. 2006;27:525–532. doi: 10.1016/j.it.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Netto M.V., Mohan R.R., Medeiros F.W., et al. Femtosecond laser and microkeratome corneal flaps: comparison of stromal wound healing and inflammation. J. Refract. Surg. 2007;23:667–676. doi: 10.3928/1081-597x-20070901-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Brien T.P., Li Q., Ashraf M.F., et al. Inflammatory response in the early stages of wound healing after excimer laser keratectomy. Arch. Ophthalmol. 1998;116:1470–1474. doi: 10.1001/archopht.116.11.1470. [DOI] [PubMed] [Google Scholar]
- 42.Takaoka A., Wang Z., Choi M.K., et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 43.Yu H.B., Shen G.F., Wei F.C. Effect of cryopreservation on the immunogenicity of osteoblasts. Transplant. Proc. 2007;39:3030–3031. doi: 10.1016/j.transproceed.2007.03.108. [DOI] [PubMed] [Google Scholar]
- 44.McNAIR JN KING JH. Preservation of cornea by dehydration; a preliminary report. AMA Arch. Opthalmol. 1954;53:519–521. doi: 10.1001/archopht.1955.00930010525009. [DOI] [PubMed] [Google Scholar]
- 45.Chen W., Lin Y., Zhang X., et al. Comparison of fresh corneal tissue versus glycerin-cryopreserved corneal tissue in deep anterior lamellar keratoplasty. Invest. Ophthalmol. Vis. Sci. 2010;51:775–781. doi: 10.1167/iovs.09-3422. [DOI] [PubMed] [Google Scholar]
- 46.Mathew J.H., Goosey J.D., Bergmanson J.P. Quantified histopathology of the keratoconic cornea. Optom. Vis. Sci. 2011;88:988–997. doi: 10.1097/OPX.0b013e31821ffbd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dana M.R., Qian Y., Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000;19:625–643. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]