Abstract
Background:
The pregnancy pathology preeclampsia is still among the leading causes of ma-ternal and perinatal morbidity and mortality. At the same time, its etiology is far from being identified and remains obscure in a number of facets.
A number of hypotheses have been developed to explain the altered interplay between placenta and mother leading to the clinical symptoms of preeclampsia. However, none of them offers the opportunity to explain the variability of cases with late-onset versus early-onset, mild versus severe and with or with-out additional fetal growth restriction.
Conclusion:
This paper identifies the weaknesses of the most important current hypothesis and at the same time offers a set of new elucidations including maternal susceptibility, and villous/extravillous trophoblast differentiation to explain the development of preeclampsia. Such elucidations allow following new scientific routes and pathways to untangle the etiology of preeclampsia.
Keywords: Aponecrosis, extravillous trophoblast, fetal growth restriction, trophoblast invasion, placental oxygenation, preeclampsia, syncytiotrophoblast, villous trophoblast
1. Introduction
The growth of a healthy fetus during pregnancy strongly depends on the genetic profile of the fetus, while factors derived from the maternal as well as the placental/fetal systems maintain communication between the two individuals to maintain pregnancy. At the same time, the functionality of the placenta is crucial to allow optimal support of nutrients and gases for the normal development of the fetus. Growth and differentiation of the placenta have to be directly linked to the physiological adaptations of the mother to ensure proper crosstalk between the two individuals. Major alterations of this crosstalk may further develop into pregnancy pathologies such as fetal growth restriction and especially preeclampsia.
2. Description, Definition and Subtypes of Preeclampsia
2.1. Historical view on Eclampsia/Preeclampsia
First descriptions of preeclampsia/eclampsia can be dated back to 200 BC and the identification of the relation between fits (of eclampsia) and protein in the urine (proteinuria) dates back to 1843. On this historical background it is fascinating to see that even with enormous scientific and clinical investments, the etiology of preeclampsia is still a mystery. Quite a variety of aspects are still obscure, including how and when preeclampsia develops, where are the main tissues contributing to the changes in factors, which tissues are mainly affected and whether it is mostly placenta-derived factors or whether maternal factors contribute to the syndrome as well. There is a general consensus that the placenta rather than fetus or mother is the initial source of factors leading to the development of preeclampsia. Of course, manifold other factors from both individuals increase or reduce the symptoms of preeclampsia, most of which are still under discussion.
2.2. Definition of Preeclampsia
Similar to fetal growth restriction, preeclampsia is one of the major syndromes leading to fetal and neonatal as well as maternal morbidity and mortality. A recent report displays that 2-8% of all pregnant women worldwide suffer from preeclampsia [1]. Additionally, preeclampsia has been shown to put another burden on mother and child as it induces long-term effects on both, such as a higher risk to progress towards cardiovascular diseases later in life [2].
If a so far normotensive pregnant woman is diagnosed with hypertension and proteinuria after 20 weeks of gestation, she – by definition – suffers from preeclampsia [1]. Hypertension is diagnosed with a systolic blood pressure of ≥140mm Hg, and/or a diastolic blood pressure of ≥90mm Hg, measured two times at least 4h apart. Proteinuria is diagnosed with protein in the urine of ≥0.3g protein in 24h urine, or a protein to creatinine ratio of ≥0.3. These are the two features that have represented the typical criteria for the definition of preeclampsia. In recent times, the exclusive definition of preeclampsia using the above two criteria has been widened and especially in the absence of proteinuria other criteria have been included, such as elevated levels of serum creatinine (indicating kidney insufficiency), elevated levels of liver transaminases in blood, thrombocytopenia, and/or pulmonary edema or visual and cerebral disturbances [3]. This clinically relevant adaptation of the definition has led to challenges in setting up respective cohorts for research as the specific definition of preeclampsia (hypertension and proteinuria) can no longer be used.
Preeclampsia is a pure maternal syndrome, exemplified by the fact that more than 80% of all preeclampsia cases only show clinical symptoms of the mother, while the baby shows a seemingly normal development and growth. At the same time, there are cases of preeclampsia, especially the early-onset cases prior to 34 weeks of gestation, where a combination of preeclampsia and growth restriction of the fetus can be observed. It will be discussed further in this paper, how this joint occurrence may develop.
Preeclampsia is pregnancy-specific and based on the presence of a placenta. The clinical symptoms of preeclampsia are based on the combination of factors released from the placenta into the maternal circulation and the maternal response and susceptibility to these factors. The combination of all the above defines the severity of the symptoms, time of onset of the syndrome, progression of preeclampsia and involvement of the fetus. Hence, all individuals and organs involved (mother, fetus and placenta) need to be taken into account when appraising the source and etiology of the syndrome preeclampsia.
2.3. Subtypes of Preeclampsia
Although the etiology of preeclampsia is still a mystery, attempts have been made to define specific subtypes of the syndrome. One of the most clinically important definitions of subtypes is related to the time of clinical symptoms and hence the time of delivery. Since the time of onset of symptoms is very hard to judge, the gestational age at delivery has been taken as a surrogate to define early-onset preeclampsia (delivery prior to 34 weeks of gestation) versus late-onset preeclampsia (delivery after 34 weeks of gestation). Sometimes, preterm preeclampsia (delivery between 34 and 37 weeks of gestation) is added, then late-onset preeclampsia refers to the deliveries at term (delivery after 37 weeks).
The subdivision of preeclampsia into an early and a late-onset subtype is justified not only because of the different gestational ages. Also, other differing characteristics justify this separation. In contrast to the late-onset type of preeclampsia, the early-onset type of preeclampsia only occurs in 5% to 20% of all preeclampsia cases and is mostly associated with:
Shallow invasion of extravillous trophoblasts,
Reduced transformation of uterine spiral arteries,
Changes in placental perfusion with maternal blood (higher flow velocities of maternal blood entering the intervillous space from inadequately transformed spiral arteries),
Changes in fetal perfusion of essential organs including the placenta, and
Fetal growth restriction.
Interestingly, all the above characteristics of the early-onset type of preeclampsia can be found in pure severe fetal growth restriction as well. At the same time, the two subtypes of preeclampsia (early and late) do not show differences in hypertension and proteinuria, the characteristics that directly define preeclampsia [4]. In the light of the new definition of preeclampsia, the ISSHP (International Society for the Study of Hypertension in Pregnancy) noted that distinctions between early and late onset are only useful for research purposes [5].
2.3.1. The Subtype of Late-onset Preeclampsia
Most of the preeclampsia research performed today is directed towards those cases suffering from a combination of preeclampsia and fetal growth restriction. Interestingly, this combination can only be found far in less than 20% of all preeclampsia cases. The vast majority of cases belong to the late-onset subtype of preeclampsia, comprising more than 80% and up to 95% of all preeclampsia cases worldwide. By definition, delivery in such cases takes place after 34 weeks of pregnancy. Looking at the growth trajectories of the fetuses of such cases, they mostly show normal growth curves and do not show any signs of growth restriction. Some studies, especially looking at preeclampsia at term even point to heavier babies in cases of late-onset preeclampsia. These findings go in line with the flow patterns of the maternal uterine and the fetal umbilical arteries. In all of these vessels no indications for alterations of flow can be detected, including the absence of an increase of the pulsatility indices.
The above description of features of the late-onset subtype of preeclampsia clearly depicts that this syndrome is a pure maternal syndrome with very little to no effects on the baby. There is still debate what role the placenta plays in this scenario. There are three options that may induce the maternal symptoms:
A defect of the placenta affects the maternal system and leads to maternal symptoms without affecting the fetus.
The placenta has been shown to have a very high compensatory capacity. Hence, even if the villous trophoblast is affected in some or larger parts, the other parts of the placenta may still be effective enough to enable proper growth and development of the fetus. However, the affected part may well induce systemic alterations of the maternal vasculature resulting in preeclampsia and its symptoms.
A normal release of factors from the placenta and a highly susceptible mother lead to maternal symptoms.
It has been calculated that in the third trimester about 2g to 3g of apoptotic trophoblast material is released into the maternal circulation per day [6, 7]. This corpuscular material is normally engulfed in the first capillary system behind the placenta, the lungs. If the defense systems of the mother cannot cope with this amount of non-self material, the apoptotic trophoblast material may become secondary necrotic and thus may result in systemic activation and defects of the maternal vascular system.
An increased release of factors from an enlarged surface of the placenta (as in multiple pregnancies, high altitude, anemia or diabetes mellitus) may even lead to maternal symptoms in a normally susceptible mother. Here, the same route of effects will then take place as in scenario number 2.
2.3.2. The Subtype of Early-onset Preeclampsia
As discussed above, only a small subset of cases (5-20% of all preeclampsia cases) ends in the delivery of the baby prior to 34 weeks of gestation and hence is referred to as early-onset preeclampsia. Clinically, this is the most important subtype of preeclampsia as it is associated with the majority of preeclampsia-related morbidities and mortalities of mother and child in the developed world [8].
Both subtypes, early- and late-onset preeclampsia, share the maternal features and symptoms of preeclampsia, while in clinical routine there are additional features that mostly occur in the early-onset type. Features of specific interest relate to alterations of blood flow in the uterine arteries of the mother as well as the umbilical arteries of the fetus. Interestingly, both features are not specific for early-onset preeclampsia but are typical features of severe fetal growth restriction. This finding reveals that the features of the early-onset subtype of preeclampsia may not be related to a single syndrome but may well be a mixture of two syndromes, preeclampsia and fetal growth restriction [4]. This has generated major speculations and hypotheses during the last decade.
As most cases of early-onset preeclampsia are linked with fetal growth restriction, the typical features of severe growth restriction of the fetus (high resistance and other changes in blood flow of uterine and umbilical arteries) have simply been taken to clinically define early-onset preeclampsia. This combination of two syndromes has raised doubts as to whether the features of early-onset preeclampsia (excluding hypertension and proteinuria) are based on the etiology of preeclampsia or are based on the etiology leading to growth restriction of the fetus [4]. Besides this scientific approach, it needs to be stated that the combination of fetal growth restriction and preeclampsia makes these cases highly dangerous for mothers and their preterm babies and thus central for clinical intervention.
3. Rebuttal of the Current Hypothesis on how Preeclampsia develops
Since early studies on preeclampsia cases in the mid 1960ies, a single hypothesis on how preeclampsia develops still exists in the literature. This theory focuses on the failure of extravillous trophoblast to sufficiently invade into uterine spiral arteries supposed to lead to placental hypoperfusion and placental hypoxia. This, in turn, is hypothesized to result in altered release of factors from the villous trophoblast and thus is supposed to lead to the clinical phenotype of preeclampsia. Although a number of data have accumulated over time that clearly disprove this hypothesis, it is still present and one of the most cited and accepted theories. This clearly points to the need for a more serious handling of hypotheses and the acceptance of their scientific falsification.
Since the above hypothesis is still used as a major basis for new studies on preeclampsia, below a short summary of the chronology of events of this theory is listed:
1st trimester: An unknown harmful event affects the extravillous trophoblast.
1st and 2nd trimester: Extravillous trophoblast invasion is impaired resulting in a reduced infiltration and transformation of uterine spiral arteries in the placental bed.
2nd trimester: Blood flow from the mother to the placental intervillous space is reduced leading to placental hypoperfusion.
2nd and 3rd trimester: Placental hypoperfusion results in placental hypoxia associated with fluctuations of oxygenation with hypoxia followed by reoxygenation.
2nd and 3rd trimester: Placental hypoxia results in damage of the villous trophoblast;
2nd and 3rd trimester: Altered release of placenta-specific factors from the syncytiotrophoblast into the maternal circulation.
2nd and 3rd trimester: Maternal inflammatory reactions due to placental factors and development of clinical symptoms of the mother.
During the last decade, a number of items of the list above have been rebutted. Below two of these items will be discussed in more detail associated with a critical assessment of their scientific value today.
3.1. Shallow Trophoblast Invasion versus Preeclampsia
Clinically, early-onset preeclampsia is more challenging than late-onset preeclampsia due to the following reasons: (1) If symptoms urge an immediate delivery, this is easy in late-onset preeclampsia but may be really difficult in early-onset preeclampsia at an gestational age of less than 34 weeks. (2) Since fetal growth restriction is mostly present in early-onset cases, two patients need to be taken into consideration, mother and fetus. Due to the greater importance of early-onset preeclampsia in clinical practice, this type of preeclampsia has gained more attraction and thus more studies have focused on this type of the syndrome. Over time, impreciseness of the definitions of cohorts and subtypes of preeclampsia has led to the transfer of the clinical symptoms of early-onset preeclampsia to all cases of the syndrome.
Although in more than 80% of all preeclampsia cases, shallow invasion of trophoblast does not occur, this feature is still called one of the major events in the etiology of preeclampsia in general. However, it may explain less than 20% of all preeclampsia cases, namely those associated with severe fetal growth restriction. The remaining majority of cases remain excluded and unexplained.
It is crucial for the understanding of preeclampsia that the cascade of events starting with shallow trophoblast invasion and described for early-onset preeclampsia is exactly the cascade of events leading to pure growth restriction of the fetus without any symptoms of the mother. Thus, a critical appraisal is needed to identify whether shallow trophoblast invasion is indeed a specific feature of (early-onset) preeclampsia or occurs in parallel as a feature of fetal growth restriction in such cases.
Studies on the usefulness of uterine artery Doppler assessment for the prediction of preeclampsia supported the idea of the co-occurrence of symptoms of two different syndromes. Two studies assessed blood flow in the uterine arteries at 11 to 14 weeks of gestation using Doppler ultrasound [9, 10]. In the first study the detection rate for early-onset preeclampsia was 40% at a 10% false positive rate [9]. In the second study the sensitivity to predict early-onset preeclampsia was 33.3% and to predict all cases of preeclampsia was 21%. In the same group of women, early-onset fetal growth restriction was detected with a sensitivity of 100% [10]. This data is supported by data from a study of more than 21,000 pregnant women correlating the presence of a high resistance index in the uterine arteries with fetal growth restriction [11]. The authors showed that there is a direct correlation between high resistance and growth restriction independent on the presence of preeclampsia. The numbers of both (high resistance and growth restriction) are highest <34 weeks and decrease in parallel until term without any changes in the presence or absence of preeclampsia.
The above studies and a number of other studies do not support a direct causal connection between failure in trophoblast invasion and the onset of preeclampsia. It seems as if the failure of trophoblast to invade spiral arteries is directly related to idiopathic fetal growth restriction rather than any type of preeclampsia. If this is shown to be the case, the specific clinical characteristics of early-onset preeclampsia are not related to preeclampsia but due to the parallel presence of fetal growth restriction. This would further mean that the only specific feature of early-onset preeclampsia differentiating this subtype from late-onset preeclampsia is the time of delivery - and the co-occurrence of fetal growth restriction.
3.2. Placental Hypoperfusion and Placental Hypoxia versus Preeclampsia
3.2.1. Placental Flow of Maternal Blood Early in Pregnancy
It is general knowledge today that during the first trimester of pregnancy the human placenta is not perfused with maternal blood. Rather, it is a combination of blood plasma and secretion products of uterine glands that can be found in the intervillous space of a first trimester placenta allowing histiotrophic nutrition of the embryo at low oxygen concentrations [12, 13]. During this period of pregnancy only physically solved oxygen in blood plasma is present in the placenta, leading to oxygen concentrations of <20mm Hg [14]. Untimely early opening of the spiral arteries and hence an early rise in oxygen levels prior to 10-12 weeks may result in loss of placental mass, fetal growth restriction or even spontaneous abortion [15]. Normal flow of maternal blood into the placenta and thus hemotrophic nutrition starts at the beginning of the second trimester leading to oxygen concentrations of about 60mm Hg [14]. Slightly lower levels between 35mm and 50mm Hg are maintained until the end of pregnancy [16-18]. This data shows that reduced flow of maternal blood (if hypoperfusion occurs at all) can only start after the onset of maternal blood flow into the placenta, hence after the beginning of the second trimester.
A continuous flow of maternal blood into the placenta is achieved by erosion of spiral arteries leading to reorganization of the vessel walls to reduce/eliminate vasomotor control of these vessels by the mother as well as to widening of these vessels at their very distal ends towards the placenta. This funnel-shaped opening of the arteries is an important feature of normal trophoblast invasion and allows normal flow of maternal blood into the placenta.
3.2.2. Funnel-shaped Openings of Spiral Arteries versus Placental Hypoperfusion
The velocity of maternal blood flowing through uterine spiral arteries remains constant during the second half of pregnancy with values between 33 cm/s and 50 cm/s [19]. According to Burton et al. (2009) the funnel-shaped opening of the arteries towards the intervillous space reduces the velocity of maternal blood flowing into the placenta to values of about 10 cm/s [20]. At the same time, it is interesting to note that these changes at the distal ends of such vessels do not change the total volume of blood flowing into the placenta [20]. The reduced velocity of maternal blood flowing into the placenta is crucial for (1) the maintenance of the fragile villous trees, (2) the distribution of maternal blood through the narrow passages between villi and (3) the maintenance of the adhesion of anchoring villi to the basal plate.
In the presence of shallow trophoblast invasion and failure of transforming spiral arteries, the funnel-shaped opening of such vessels does not properly occur. Rather, the arteries remain at their normal diameter until they connect to the intervillous space. In such cases, the blood flow into the placenta was calculated to be 1 to 2 m/s, hence 10 to 20 times faster than with funnel-shaped endings of vessels [20]. The massively increased blood flow velocities have major and deleterious effects on the placental villous trees, not related to changes in oxygen levels. Increased maternal blood flow velocities in the intervillous space will (1) damage the villous surface, (2) lead to more fibrin-type fibrinoid deposition, (3) detach anchoring villi from the basal plate, and (4) due to (3) decrease the pool of extravillous trophoblasts invading into uterine tissues. All of this will culminate in lower gaseous and nutritional exchange and thus growth restriction of the fetus.
3.2.3. Maternal Perfusion of the Placenta versus Placental Hypoxia
Do the changes in blood flow velocity described above lead to placental hypoperfusion and placental hypoxia? Although only little data has been published on oxygen levels during pregnancy and especially in preeclampsia and fetal growth restriction, a huge number of publications have based their studies on placental hypoxia. Interestingly, the few data that have been published on placental oxygenation do not support any changes towards reduced placental oxygen levels.
In 2002 Sibley et al. have analyzed blood samples of the uterine veins taken during cesarean section prior to delivery of the baby and used the oxygen levels as surrogate for placental oxygen [17]. This is a valid method as Schaaps et al. (2005) have shown that there is a fixed association between the pO2 in the placenta and the pO2 in the uterine vein behind the placenta [16]. The venous pO2 was calculated to be 1.5 times higher than the placental pO2 [16]. Sibley et al. (2002) focused on early-onset cases of fetal growth restriction with and without preeclampsia and compared the values with preterm controls with matched gestational age [17]. Taking the data from both publications into account, the mean pO2 of the preterm control group was 33mm Hg, while the mean pO2 in the group of fetal growth restriction (with and without preeclampsia) was 42mm Hg. The cases suffering from growth restriction and preeclampsia were not different in the pO2 values compared to pure growth restriction. The difference between cases and controls was a 1.3-fold increase in the placental pO2 of cases. Hence, this data clearly points to placental hyperoxia rather than hypoxia. This is supported by the data of oxygen extraction from the placenta, i.e. the pO2 in the umbilical arteries. This value was reduced in cases compared to controls pointing to a hypoxic fetus in such cases [17]. Hence, with the same amount of blood and thus the same amount of oxygen flowing into the placenta, a reduced extraction of oxygen to the fetus has two consequences: (1) a hypoxic fetus suffering from a low oxygen supply, and (2) a hyperoxic placenta suffering from a high oxygen environment.
Another surrogate of placental oxygenation is the assessment of the concentration of oxygenated hemoglobin by near-infrared spectroscopy (termed tissue oxygenation index; [18, 21]). In healthy pregnant women the tissue oxygenation index of the placenta was 71% between 25 and 40 weeks of gestation. In cases with pure fetal growth restriction the index increased to 78% and in cases with early-onset preeclampsia and fetal growth restriction the index increased to 80% [18, 21]. Transferring these values to pO2 values of the placenta reveals a pO2 of 30mm Hg for healthy pregnant women, a pO2 of 49mm Hg for cases with fetal growth restriction and a pO2 of 54mm Hg for cases with early-onset preeclampsia and fetal growth restriction.
In summary, the few studies looking at the intraplacental pO2 in cases with shallow trophoblast invasion revealed higher placental pO2 values in cases with severe fetal growth restriction with and without preeclampsia. To date, there is no publication presenting any measurements proving hypoxia in the human placenta. Thus, combining the calculations on flow velocities and shape of arterial openings with the surrogate measurements of placental pO2 provide evidence for an increased oxygenation level in cases with shallow trophoblast invasion. So far, there is no basis to use placental hypoxia for any hypothesis.
4. Updated Hypothesis of the Etiology of Preeclampsia
For preeclampsia to develop it does not only need the placenta. It also needs the mother as it is a maternal syndrome. Hence, the placenta and the factors released from this organ need to be put into a larger context and need to be analyzed taking the maternal environment into account as well. This environment of the mother also includes factors such as family history plus her genetic predisposition and her cardiovascular status. Only the interplay between the mother and her susceptibility on one hand and the placenta with its particles and factors defines whether or not the pregnant woman will develop the syndrome of preeclampsia [4].
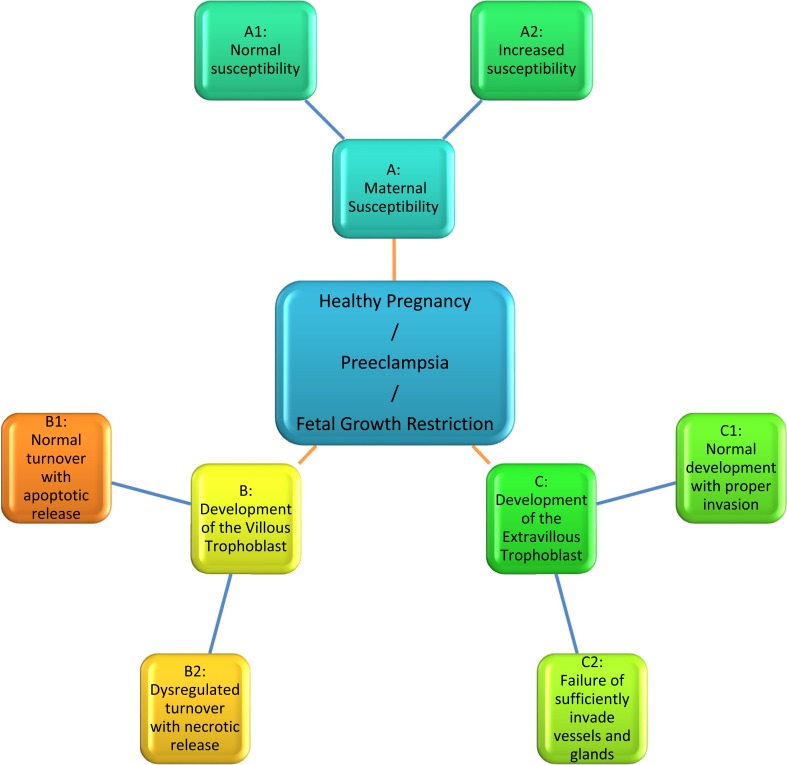
Based on the current knowledge, a hypothesis has been developed taking the following three variables into account (Fig. 1):
Fig. (1).
The newly developed hypothesis described in the text, takes into account three variables: maternal susceptibility, development of the villous trophoblast and development of the extravillous trophoblast. Each of the three variables is further divided into two opposite pathways.
-
Maternal susceptibility
A normal healthy pregnant woman versus,
A pregnant woman who is highly susceptible for factors/particles released from the placenta (including women with risk factors such as previous preeclampsia, pre-pregnancy hypertension, coagulation diseases etc.);
-
Development of the villous trophoblast
A normal development of the villous trophoblast with release of apoptotic syncytial knots not inducing an inflammatory response of the mother versus,
A dysregulated turnover of villous trophoblast resulting in aponecrotic and necrotic release of particles [6] and systemic activation/damage of the maternal vascular system;
-
Development of the extravillous trophoblast
4.1. Options with a Pregnant Woman and a Normal Placenta
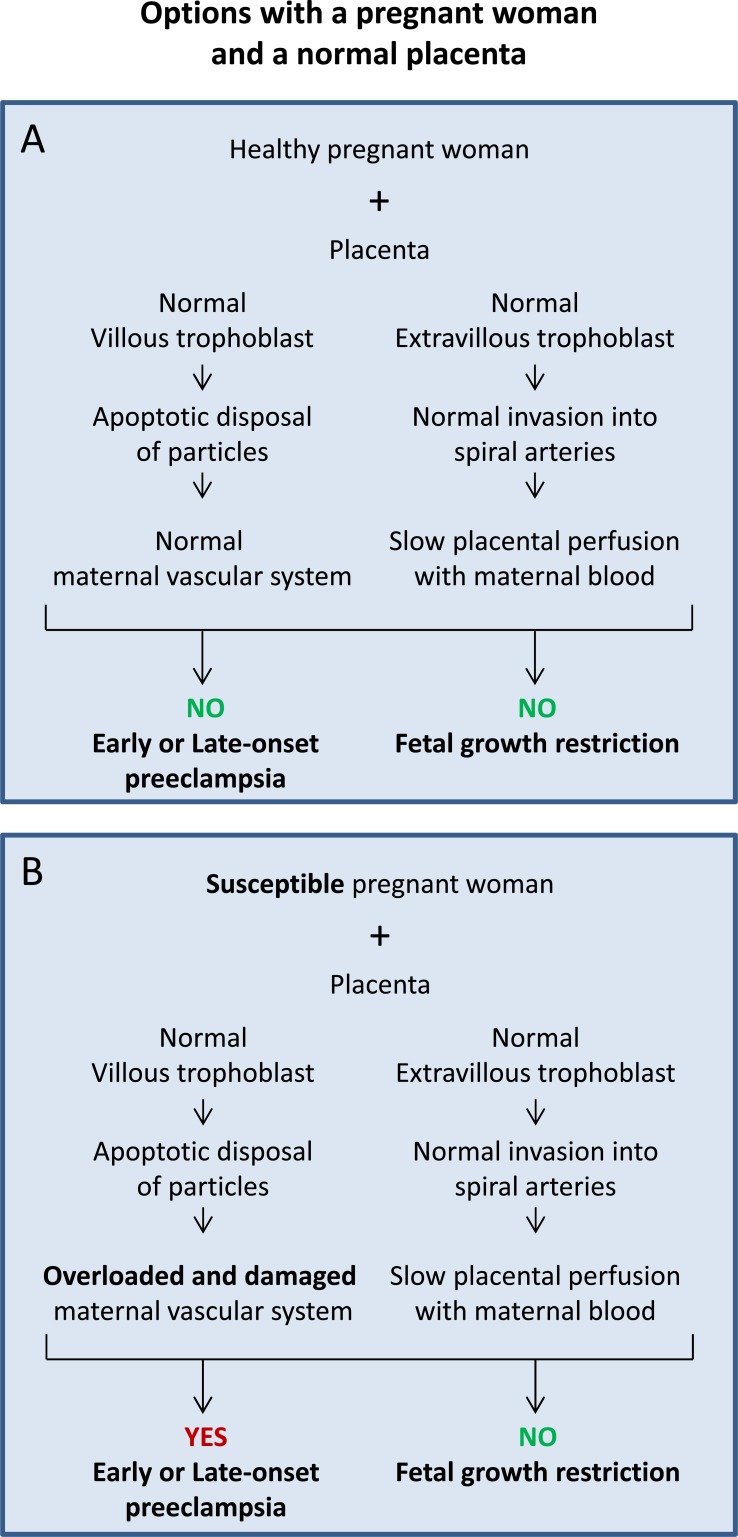
Based on the list above, the variables A1 and A2 are combined with the variables B1 and C1.
Fig. (2A) displays the scenario during normal healthy pregnancy with a healthy pregnant woman (A1) and normal development of the placenta including villous (B1) as well as extravillous (C1) trophoblast. This pregnancy should lead to a healthy baby and should not result in the development of preeclampsia or fetal growth restriction.
Fig. (2).
Sequence of events in cases with a healthy (A) or a susceptible woman (B) and a normally developing placenta. The option in (A) is the basis for a healthy pregnancy, while the option in (B) may well lead to early or late preeclampsia without fetal growth restriction.
Fig. (2B) displays the scenario in which a susceptible pregnant woman (A2) is confronted by a normally developing placenta including normal development of villous (B1) as well as extravillous (C1) trophoblast. This pregnancy may well lead to the development of preeclampsia, while development of fetal growth restriction is very unlikely.
These options are characterized by a normal release of particles and factors from the syncytiotrophoblast of a normal placenta by late apoptosis and controlled secretion [6]. The defect of the maternal defense system of a susceptible woman (Fig. 2B) may only be present in the scavenging systems, may be present in specific signaling pathways of e.g. kidney and/or liver, may be present in the endothelium or in leukocytes or may comprise up to all of them and more. Here, the pregnant woman may already be aware of predisposing factors such as chronic hypertension or renal disease or may have alterations on a subclinical level that have not become apparent prior to the stress test of pregnancy.
The options with a susceptible woman (Fig. 2B) also include cases with normal placentas presenting a larger mass and/or surface such as in diabetes, large-for-gestational-age babies, in multiple pregnancies or at high altitude. Characteristic for all these cases is a larger surface and hence an increased number of particles/factors released from the syncytiotrophoblast. The mother may easily cope with this increased amount or her defense and scavenging systems may be overloaded. In such a case the apoptotic syncytial knots may stay in blood for too long and may become secondary necrotic. Then again a systemic effect of this material may arise, ending up in the clinical symptoms of preeclampsia.
Depending on the degree of the maternal susceptibility, damage of parts of the vascular system and/or overload of the defense and scavenging systems will mostly result in late-onset preeclampsia. Looking at placentas with more surface/mass, the overload and subsequent damage depends on the quantity and quality of particles released from the syncytiotrophoblast. This option may result in early-onset preeclampsia as well.
Since the placenta with all its cell populations develops normal in these options (Fig. 2), the fetus should develop normal as well and should not show any signs of growth restriction.
4.2. Options with a Healthy Pregnant Woman and a Partly Affected Placenta
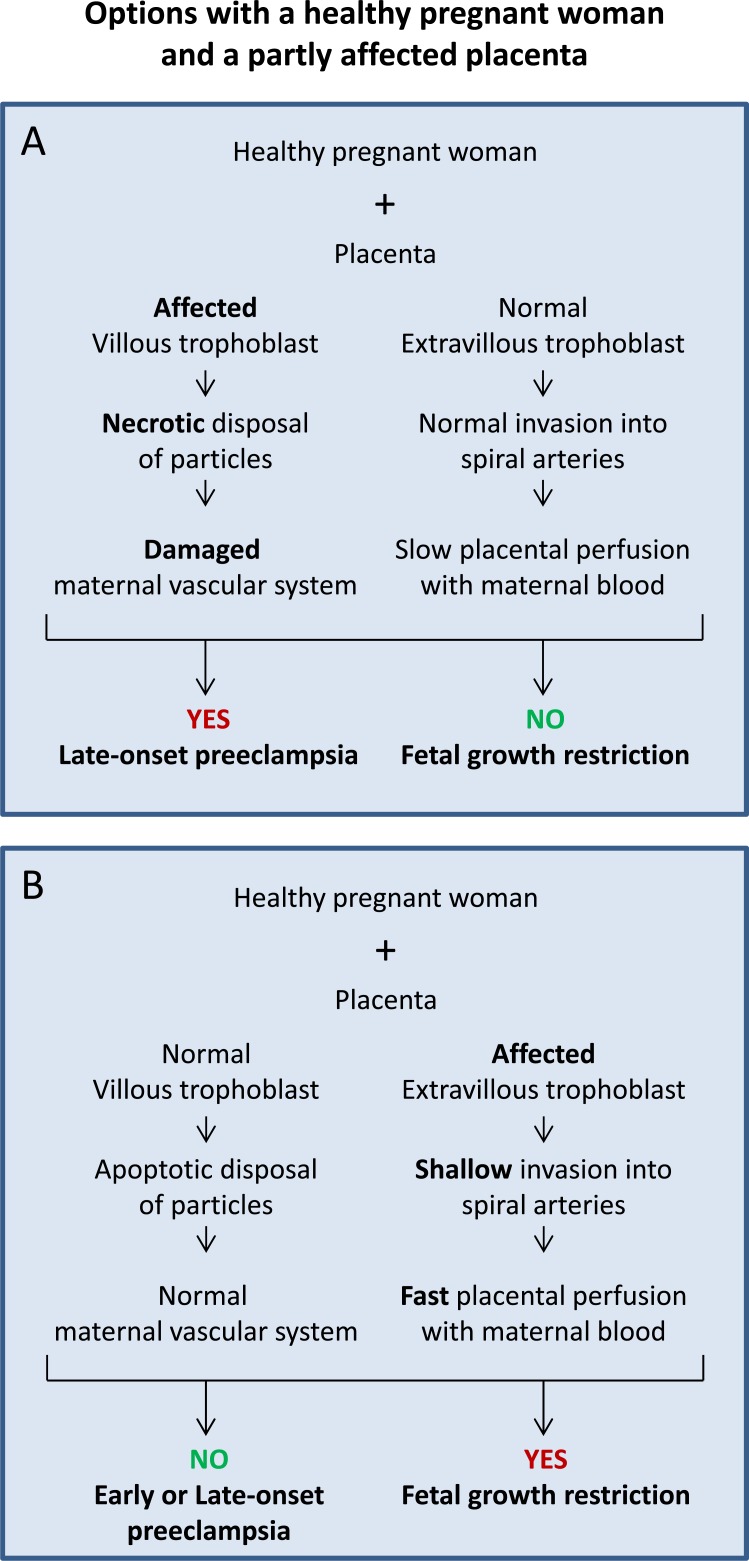
Based on the list above, the variable A1 is combined with the variables B2 or C2.
Fig. (3A) displays the scenario with a healthy pregnant woman (A1), normal development of the extravillous trophoblast (C1) but defective development of the villous (B2) trophoblast. This pregnancy should lead to a healthy baby with normal growth but may well result in the development of preeclampsia.
Sequence of events in cases with a healthy woman and a placenta with a dysfunctioning villous (A) or extravillous (B) trophoblast. The option in (A) may result in late-onset preeclampsia without growth restriction, while the option in (B) may lead to growth restriction without preeclampsia.
Fig. (3B) displays the scenario in which a healthy pregnant woman (A1) is confronted by a normally developing villous trophoblast (B1) but a defectively developing extravillous (C2) trophoblast. This pregnancy may well lead to the development of fetal growth restriction, while development of preeclampsia is very unlikely.
The option in Fig. (3A) includes maldevelopment of the villous trophoblast. Additionally to the release of apoptotic syncytial knots into maternal blood [4, 6, 26], in this option also subcellular fragments are released including necrotic and aponecrotic particles as well as free molecules such as proteins and DNA [4, 26]. Such subcellular micro- and nanoparticles [27] systemically activate the maternal endothelium and may finally result in damage of the maternal vascular system [28]. The maldeveloped villous trophoblast continuously and increasingly releases factors and non-apoptotic particles activating and injuring the maternal endothelium and the whole vascular system finally leading to an inflammatory response of the mother and the clinical symptoms of preeclampsia. Since the extravillous trophoblast is not affected and shows a normal development, perfusion of the placenta and nutritional support of the fetus are normal; hence, growth of the fetus should be normal as well (Fig. 3A).
The option in Fig. (3B) includes maldevelopment of the extravillous trophoblast. This option is characterized by a normal release of apoptotic particles from the villous trophoblast. However, due to a defect in the development of the extravillous trophoblast it is characterized by an inadequate invasion/transformation of uterine luminal structures (Fig. 3B).
The last few years have seen a dramatic change in our view of how trophoblast invades into uterine tissues and which type of luminal structures are eroded [13, 22]. For the last fifty years the focus was on the invasion and transformation of uterine spiral arteries by endovascular trophoblast. This has very recently been extended and it has become clear that extravillous trophoblasts invade into all luminal structures of the placental bed, i.e. uterine arteries and veins, lymph vessels as well as uterine glands [22].
Invasion into uterine glands by endoglandular trophoblasts has been identified to take place as early as during the first days of implantation [29]. Thereafter, invasion into uterine veins takes place by endovenous trophoblasts to allow backflow of secretion products of the uterine glands into the maternal system [22, 23, 24, 25]. Shortly afterwards, invasion and transformation of spiral arteries is established by endoarterial trophoblasts [22]. Invasion into lymph vessels by endolymphatic trophoblast [22] has been reported with so far unclear functional aspects [23, 25].
Today, there are no studies on the effect of deficient trophoblast invasion into glands, veins and lymph vessels. Inadequate invasion of endoglandular trophoblasts into uterine glands could lead to diminished histiotrophic nutrition of the embryo during the first trimester of pregnancy. The result could be growth restriction or even spontaneous abortion very early in pregnancy. Additionally, inadequate invasion of endovenous trophoblasts into uterine veins could lead to diminished draining of maternal plasma and secretion products from the placenta back into the maternal system early in pregnancy. It may also affect draining of maternal blood later in pregnancy. This reduced draining will negatively influence flow of maternal plasma/blood through the placenta and will thus negatively affect supply of the embryo/fetus.
No information on inadequate invasion of glands and veins is available. So far, only the effects of defective invasion and transformation of spiral arteries by endoarterial trophoblasts has been extensively studied. As has already been outlined above, inadequate invasion of spiral arteries does not at all result in reduced blood flow into the placenta and hence is not associated with placental hypoxia [4, 20]. Rather, the augmented velocities of maternal blood flowing into the placenta damage the fragile villous trees of the placenta and affect blood flow and thus peripheral resistance within placental vessels. Secondary effects of the increased peripheral resistance could be alterations of placental perfusion and hence fetal undernutrition.
4.3. Options with a Susceptible Pregnant Woman and a Partly Affected Placenta
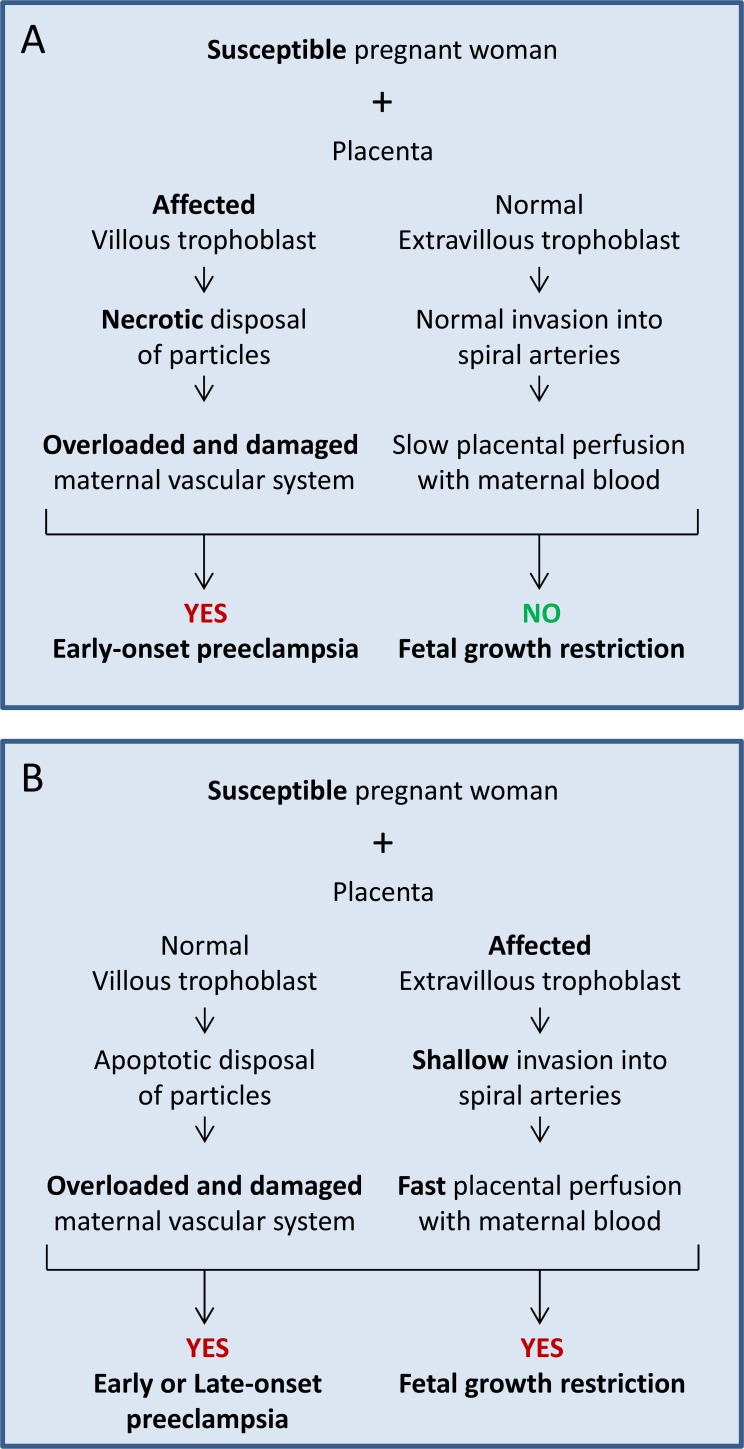
Based on the list above, the variable A2 is combined with the variables B2 or C2.
Fig. (4A) displays the scenario with a susceptible pregnant woman (A2), normal development of the extravillous trophoblast (C1) but defective development of the villous (B2) trophoblast. This pregnancy should lead to a healthy baby with no fetal growth restriction but will most likely result in the development of preeclampsia.
Fig. (4).
Sequence of events in cases with a susceptible woman and a placenta with a dysfunctioning villous (A) or extravillous (B) trophoblast. The option in (A) may result in early-onset preeclampsia without growth restriction, while the option in (B) may lead to growth restriction plus preeclampsia.
Fig. (4B) displays the scenario in which a susceptible pregnant woman (A2) is confronted by a normally developing villous trophoblast (B1) but a defectively developing extravillous (C2) trophoblast. This pregnancy may well lead to the development of fetal growth restriction, while development of preeclampsia is likely as well.
The option displayed in Fig. (4A) is an extension of the option in Fig. (1B), where a susceptible pregnant woman had an increased risk to develop preeclampsia even with a normally developing placenta. In the current option (Fig. 4A) the villous trophoblast is affected and will definitively activate and damage the maternal vascular system. Hence, it is very likely that preeclampsia will occur early during the third trimester.
The option displayed in Fig. (4B) is based on the option in Fig. (1B), where a susceptible pregnant woman had an increased risk to develop preeclampsia with a normally developing placenta. In the current option (Fig. 4B) the extra villous trophoblast is affected and will result in the inappropriate flow of maternal blood to the placenta and hence may result in fetal growth restriction.
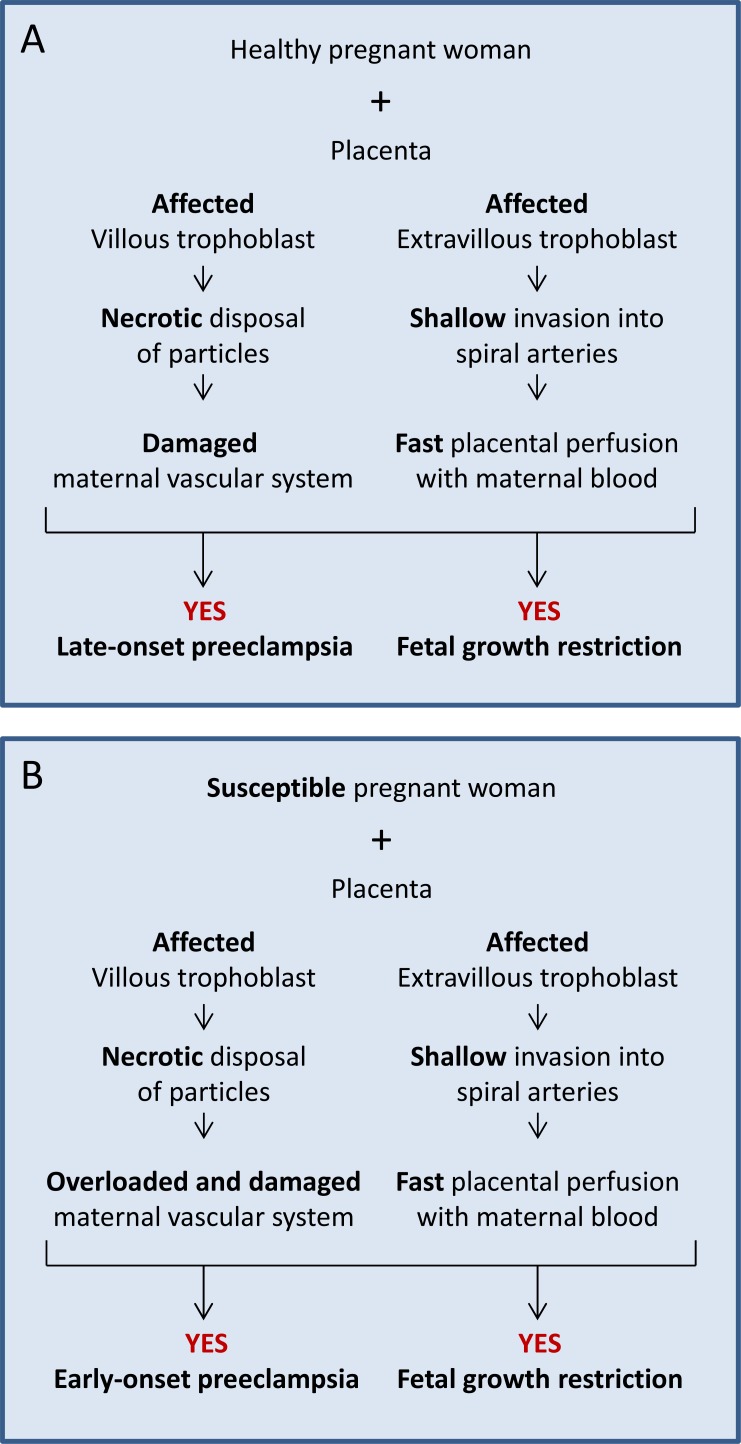
4.4. Options with a Pregnant Woman and a Fully Affected Placenta
Based on the list above, the variables A1 and A2 are combined with the variables B2 and C2.
Fig. (5A) displays the scenario in which a healthy pregnant woman (A1) is confronted by defective development of villous (B2) as well as extravillous (C2) trophoblast. This pregnancy will most probably result in a growth restricted baby and a mother suffering from late-onset preeclampsia.
Sequence of events in cases with a healthy (A) or susceptible (B) woman with a fully affected placenta (villous and extravillous trophoblast are affected). The option in (A) may result in late-onset preeclampsia with growth restriction. The option in (B) is the worst case scenario with early-onset preeclampsia and fetal growth restriction.
Fig. (5B) displays the worst scenario in which a susceptible pregnant woman (A2) is confronted by a defective placenta with abnormal development of villous (B2) as well as extravillous (C2) trophoblast. Such pregnancies are those of highest clinical relevance as the fetus will be growth restricted and the woman will most likely develop early-onset preeclampsia [30].
Conclusion
The developmental processes leading to preeclampsia are still mostly unclear and obscure, pointing to the necessity of more impartial studies and hypotheses. The above list of new options to explain the etiologies of preeclampsia as well as fetal growth restriction clearly shows the need of open-minded discussions of the topic and a new set of studies to identify the origins of both syndromes. Of course, the above hypothesis puts the placenta and the mother into specific groups, while in real life there are ranges of normality and ranges of susceptibilities that allow blurring of the borders between the options described above.
The recently identified new pathways and structures of trophoblast invasion open the way to newly designed studies and respective new perspectives. Today, a holistic approach needs to be chosen that takes into account the close interaction between maternal, fetal and placental factors and cells since a single factors/partner cannot explain the whole interactive scenario.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Conflict of Interest
The author declares no conflict of interest, financial or otherwise.
References
- 1.Townsend R., O’Brien P., Khalil A. Current best practice in the management of hypertensive disorders in pregnancy. Integr. Blood Press. Control. 2016;9:79–94. doi: 10.2147/IBPC.S77344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellamy L., Casas J.P., Hingorani A.D., Williams D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: A systematic review and meta-analysis. BMJ. 2007;335:974–977. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Task Force on Hypertension in Pregnancy Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Am. College Obstet. Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 4.Huppertz B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension. 2008;51:970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- 5.Tranquilli A.L., Dekker G., Magee L., Roberts J., Sibai B.M., Steyn W., Zeeman G.G., Brown M.A. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014;4:97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Huppertz B., Frank H.G., Kingdom J.C., Reister F., Kaufmann P. Villous cytotrophoblast regulation of the syncytial apoptotic cascade in the human placenta. Histochem. Cell Biol. 1998;110:495–508. doi: 10.1007/s004180050311. [DOI] [PubMed] [Google Scholar]
- 7.Benirschke K., Kaufmann P., Baergen R.N. Pathology of the Human Placenta. 5th ed. New York: Springer; 2006. [Google Scholar]
- 8.Chappell L.C., Enye S., Seed P., Briley A.L., Poston L., Shennan A.H. Adverse perinatal outcomes and risk factors for preeclampsia in women with chronic hypertension: A prospective study. Hypertension. 2008;51:1002–1009. doi: 10.1161/HYPERTENSIONAHA.107.107565. [DOI] [PubMed] [Google Scholar]
- 9.Nicolaides K.H., Bindra R., Turan O.M., Chefetz I., Sammar M., Meiri H., Tal J., Cuckle H.S. A novel approach to first-trimester screening for early pre-eclampsia combining serum PP-13 and Doppler ultrasound. Ultrasound Obstet. Gynecol. 2006;27:13–17. doi: 10.1002/uog.2686. [DOI] [PubMed] [Google Scholar]
- 10.Pilalis A., Souka A.P., Antsaklis P., Basayiannis K., Benardis P., Haidopoulos D., Papantoniou N., Mesogitis S., Antsaklis A. Screening for pre-eclampsia and small for gestational age fetuses at the 11-14 weeks’ scan by uterine artery Dopplers. Acta Obstet. Gynecol. Scand. 2007;86:530–534. doi: 10.1080/00016340601155056. [DOI] [PubMed] [Google Scholar]
- 11.Verlohren S., Melchiorre K., Khalil A., Thilaganathan B. Uterine artery Doppler, birth weight and timing of onset of pre-eclampsia: Providing insights into the dual etiology of late-onset pre-eclampsia. Ultrasound Obstet. Gynecol. 2014;44:293–298. doi: 10.1002/uog.13310. [DOI] [PubMed] [Google Scholar]
- 12.Burton G.J., Watson A.L., Hempstock J., Skepper J.N., Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J. Clin. Endocrinol. Metab. 2002;87:2954–2959. doi: 10.1210/jcem.87.6.8563. [DOI] [PubMed] [Google Scholar]
- 13.Moser G., Gauster M., Orendi K., Glasner A., Theuerkauf R., Huppertz B. Endoglandular trophoblast, an alternative route of trophoblast invasion? Analysis with novel confrontation co-culture models. Hum. Reprod. 2010;25:1127–1136. doi: 10.1093/humrep/deq035. [DOI] [PubMed] [Google Scholar]
- 14.Jauniaux E., Watson A.L., Hempstock J., Bao Y.P., Skepper J.N., Burton G.J. Onset of maternal arterial blood flow and placental oxidative stress; a possible factor in human early pregnancy failure. Am. J. Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jauniaux E., Greenwold N., Hempstock J., Burton G.J. Comparison of ultrasonographic and Doppler mapping of the intervillous circulation in normal and abnormal early pregnancies. Fertil. Steril. 2003;79:100–106. doi: 10.1016/s0015-0282(02)04568-5. [DOI] [PubMed] [Google Scholar]
- 16.Schaaps J.P., Tsatsaris V., Goffin F., Brichant J.F., Delbecque K., Tebache M., Collignon L., Retz M.C., Foidart J.M. Shunting the intervillous space: New concepts in human uteroplacental vascularization. Am. J. Obstet. Gynecol. 2005;192:323–332. doi: 10.1016/j.ajog.2004.06.066. [DOI] [PubMed] [Google Scholar]
- 17.Sibley C.P., Pardi G., Cetin I., Todros T., Piccoli E., Kaufmann P., Huppertz B., Bulfamante G., Cribiu F.M., Ayuk P., Glazier J., Radaelli T. Pathogenesis of intrauterine growth restriction (IUGR)-conclusions derived from a European Union Biomed 2 Concerted Action project ‘Importance of Oxygen Supply in Intrauterine Growth Restricted Pregnancies’-a workshop report. Placenta. 2002;23(Suppl. A):S75–S79. doi: 10.1053/plac.2002.0796. [DOI] [PubMed] [Google Scholar]
- 18.Kakogawa J., Sumimoto K., Kawamura T., Minoura S., Kanayama N. Noninvasive monitoring of placental oxygenation by near-infrared spectroscopy. Am. J. Perinatol. 2010;27:463–468. doi: 10.1055/s-0030-1247600. [DOI] [PubMed] [Google Scholar]
- 19.Bahlmann F., Fittschen M., Reinhard I., Wellek S., Steiner E. Reference values for blood flow velocity in the uterine artery in normal pregnancies from 18 weeks to 42 weeks of gestation calculated by automatic Doppler waveform analysis. Ultraschall Med. 2012;33:258–264. doi: 10.1055/s-0031-1281647. [DOI] [PubMed] [Google Scholar]
- 20.Burton G.J., Woods A.W., Jauniaux E., Kingdom J.C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamura T., Kakogawa J., Takeuchi Y., Takani S., Kimura S., Nishiguchi T., Sugimura M., Sumimoto K., Kanayama N. Measurement of placental oxygenation by transabdominal near-infrared spectroscopy. Am. J. Perinatol. 2007;24:161–166. doi: 10.1055/s-2006-958155. [DOI] [PubMed] [Google Scholar]
- 22.Moser G., Huppertz B. Implantation and extravillous trophoblast invasion: From rare archival specimens to modern biobanking. Placenta. 2017;56:19–26. doi: 10.1016/j.placenta.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He N., van Iperen L., de Jong D., Szuhai K., Helmerhorst F.M., van der Westerlaken L.A., Chuva de Sousa Lopes S.M. Human extravillous trophoblasts penetrate decidual veins and lymphatics before remodeling spiral arteries during early pregnancy. PLoS One. 2017;12(1):e0169849. doi: 10.1371/journal.pone.0169849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moser G., Weiss G., Sundl M., Gauster M., Siwetz M., Lang-Olip I., Huppertz B. Extravillous trophoblasts invade more than uterine arteries: Evidence for the invasion of uterine veins. Histochem. Cell Biol. 2017;147:353–366. doi: 10.1007/s00418-016-1509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Windsperger K., Dekan S., Pils S., Golletz C., Kunihs V., Fiala C., Kristiansen G., Knöfler M., Pollheimer J. Extravillous trophoblast invasion of venous as well as lymphatic vessels is altered in idiopathic, recurrent, spontaneous abortions. Hum. Reprod. 2017;32:1208–1217. doi: 10.1093/humrep/dex058. [DOI] [PubMed] [Google Scholar]
- 26.Huppertz B. Biology of the placental syncytiotrophoblast - Myths and facts. Placenta. 2010;31(Suppl.):S75–S81. doi: 10.1016/j.placenta.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Johansen M., Redman C.W., Wilkins T., Sargent I.L. Trophoblast deportation in human pregnancy - its relevance for pre-eclampsia. Placenta. 1999;20:531–539. doi: 10.1053/plac.1999.0422. [DOI] [PubMed] [Google Scholar]
- 28.Goswami D., Tannetta D.S., Magee L.A., Fuchisawa A., Redman C.W., Sargent I.L., von Dadelszen P. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27:56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Moser G., Weiss G., Gauster M., Sundl M., Huppertz B. Evidence from the very beginning: Endoglandular trophoblasts penetrate and replace uterine glands in situ and in vitro. Hum. Reprod. 2015;30:2747–2757. doi: 10.1093/humrep/dev266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huppertz B. Trophoblast differentiation, fetal growth restriction and preeclampsia. Pregnancy Hypertens. 2011;1:79–86. doi: 10.1016/j.preghy.2010.10.003. [DOI] [PubMed] [Google Scholar]