Abstract
Backgrounds:
We recently reported that Naoxintong (NXT), a China Food and Drug Administration (FDA)-approved cardiac medicine, could reduce the plaque size, but the underlying mechanism remains elusive now.
Objective:
In this study, we investigated the effects of NXT on foam cell accumulation both in vivo and in vitro and explored related mechanisms.
Method:
THP-1 cells and bone marrow-derived macrophages were incubated with oxidized low-density lipoprotein (ox-LDL) with/without Naoxintong. ApoE-/- mice fed an atherogenic diet were administered to receive NXT for eight weeks. Macrophage-derived foam cell formation in plaques was measured by immunohistochemical staining. Expression of proteins was evaluated by Western blot. Lentivirus was used to knockdown PPARα in THP-1 cells.
Results:
After NXT treatment, foam cell accumulation was significantly reduced in atherosclerotic plaques. Further investigation revealed that oxidized low-density lipoprotein (ox-LDL) uptake was significantly decreased and expression of scavenger receptor class A (SR-A) and class B (SR-B and CD36) was significantly downregulated post-NXT treatment. On the other hand, NXT increased cholesterol efflux and upregulated ATP-binding cassette (ABC) transporters (ABCA-1 and ABCG-1) in macrophages. Above beneficial effects of NXT were partly abolished after lentiviral knockdown of PPARα.
Conclusion:
Our findings suggest that NXT could retard atherosclerosis by inhibiting foam cell formation through reducing ox-LDL uptake and enhancing cholesterol efflux and above beneficial effects are partly mediated through PPARα pathway.
Keywords: Naoxintong, atherosclerosis, macrophage, foam cell, PPARα
1. INTRODUCTION
Atherosclerosis is a progressive chronic disease of the arteries [1-5]. The formation of foam cells in vessel walls, which is the result of excessive ox-LDL uptake by monocyte macrophages, is the hallmark event of early atherosclerosis. Lipid metabolism in monocyte-derived macrophages is a dynamic equilibrium process [6]. Excess ox-LDL uptake and reduced cholesterol efflux in monocyte-derived macrophages are known to accelerate plaque formation in atherosclerosis and are key processes that will finally determine the size of the atherosclerotic plaque [7].
Scavenger receptor class A (SR-A) and scavenger receptor class B (SR-B and CD36) could stimulate the lipid uptake capability of macrophages [8, 9]. The uptaken ox-LDL is digested in lysosomes, forming free cholesterol and accumulating in cells. Free cholesterol further stimulates related proteins to upregulate the expression of ABCA-1 and ABCG-1, which are representative members of ATP-binding cassette (ABC) transporters, and promote cholesterol efflux [10, 11]. Extracellular free cholesterol is then transferred to APOA-I to form nascent high-density lipoprotein (HDL). Finally, cholesterol is stored as mature HDL. When the balance is broken, especially in case of pumped SR-A and CD36 and dampened ABCA-1 and ABCG-1, the foam-cells formation will be accelerated [12]. Our previous study has shown that Lanatoside C promoted the formation of atherosclerosis by increasing the expression of SR-A and CD36 [13, 14]. In addition, Rousselle and colleagues reported that CXCL5 could reduce the macrophages foam cell formation in atherosclerosis by increasing the expression of ABCA-1 [15]. Therefore, it is of importance to define the integral factor that is involved in regulating the expression of these lipid metabolic proteins.
Naoxintong (NXT), as a China Food and Drug Administration (FDA)-approved cardiac medicine, has been widely used for patients with cardiovascular diseases in China [16-18]. Our previous study reported the protective effect of NXT in attenuating atherosclerosis, however, detailed underlying mechanisms remain elusive now [16, 19].
The PPAR family belongs to the steroid hormone receptor superfamily and plays crucial roles in various respects of cell metabolism such as peroxisome proliferation, carbohydrate metabolism and lipoprotein metabolism [20, 21]. PPARα has thus received extensive attention as a potential target of lipid metabolism regulation [11, 22, 23]. In this study, we tested the hypothesis that NXT might attenuate foam-cell formation through modulating PPARα pathway.
2. MATERIALS AND METHODS
2.1. Animals
Male ApoE-/- mice on the C57BL/6 background (Beijing Vital River Laboratory Animal Technology Co. Beijing, China) were bred in a house in a pathogen-free facility. Eight weeks old mice fed an atherogenic diet were randomly grouped to receive normal saline (0.1 mL/day, i.g.) or NXT (0.7g/kg/day, i.g.) treatment [24]. The animals were housed individually in the specific pathogen-free barrier facility at constant temperature (20-22°C) and humidity (45%-55%) with a 12-hour light-dark cycle. Eight weeks later, mice were euthanized for further analysis.
2.2. Reagents and Cell Culture
Human monocytic cell line THP-1 was cultured and maintained in complete RPMI 1640 (Gibco, Life Technologies), supplemented with 10% (v/v) heat-inactivated FBS, penicillin (100 U/ml), streptomycin (100 mg/ml), at 37˚C in a humidified atmosphere containing 5% (v/v) CO2. THP-1 cells were differentiated into macrophages by treatment with 100nM phorbol myristate acetate (PMA; Sigma–Aldrich, Poland) for 24h, and then the adherent macrophages were washed three times with PBS for further experiments [25-27]. NXT, which was generously provided by Buchang Co (Shandong, China), was added into 6-well dish at a final concentration of 200μg/mL. Fenofibrate (ab120832, Abcam), which is one of the agonists of PPARα, was added at a final concentration of 400ng/mL and GW6471 (Sigma–Aldrich, Poland), which is one of the antagonists of PPARα, was added at a final concentration of 625ng/mL [10, 22, 28, 29]. Cells were treated with these different agents for an additional 24 hrs.
Anti-ABCA-1 (ab18180), anti-ABCG-1 (ab52617), anti-CD36 (ab133625), anti-PPARα (ab8934), anti-phospho-PPARα Ser12 (ab3484), anti-SR-A (ab183725), anti-LXR alpha (ab176323), and anti-SR-BI antibodies (ab217318) were obtained from Abcam (Cambridge, MA), and anti-phospho-PPARα Ser21 was purchased from Sigma (St. Louis, Missouri, USA). Anti-β-actin, and secondary antibody (HRP-goat anti-mouse IgG and HRP-goat anti-rabbit IgG) were purchased from Weiao (Shanghai, China).
Human oxidized low-density lipoprotein (ox-LDL) and 1,19-dioctadecyl-3,3,39,39-tetramethylindocarbo-cyanine perchlorate (DiI) medium ox-LDL were purchased from Yiyuan Biotech (Guangdong, China). NBD-cholesterol (N1148) was purchased from Thermo Scientific (Rockford, IL, USA).
2.3. Isolation and Cultivation of Mouse Bone Marrow-derived Macrophages (BMDM)
After cervical vertebra resection, the mice were dropped into 75% ethanol for 20 minutes. At the same time, 1×Erythrocyte lysate (Biolegend) was prepared. Thigh of the mouse was cut and the legs were placed in 75% ethanol for 2 minutes. Then the muscles were removed from the femur and tibia. After cutting off both ends of the femur bone, a 23-gauge needle fulfilled with sufficient amount of PBS was inserted into either side of the bone to flush out the bone marrow cells into a 50 mL tube. Similar procedures were performed on tibia bone. Bone marrow cells were spun down at 1000rpm, for 5 minutes at 4°C and supernatant was discarded. 5 mL of 1× Erythrocyte lysate was added into the cells to lyse red blood cells, 2 minutes later 20 mL PBS was added to stop the reaction and cells were spun down again. Finally, cells were resuspended in complete RPMI 1640 medium supplemented with 10 ng/mL MCSF and seeded into 6-well plate at a density of 1×106 cells/mL. The culture medium was replaced every 2 days until day 7 when macrophages could be harvested.
2.4. DiI-ox-LDL Uptake Assays
THP-1 or BMDM cells were serum starved for 6 hrs before being co-incubated with NXT (200ug/mL), fenofibrate (400ng/mL), or GW6471 (625ng/mL) for another 6 hrs. Then DiI-labeled oxidized LDL (DiI-Ox-LDL) (50 mg/mL) was added in the medium. Cells were fixed in phosphate-buffered 4% formalin for 10 min and detected by Leica fluorescence microscope with laser excitation at 458 nm.
2.5. Cholesterol Efflux Assay
THP-1 or BMDM cells were treated with NXT (200μg/mL), fenofibrate (400ng/mL), or GW6471 (625μg/mL) for 12 hrs before being equilibrated with NBD-cholesterol (1 ug/mL) for an additional 6 hrs. The NBD-cholesterol–labelled macrophages were washed three times with cold PBS, then incubated in no phenol red DMEM containing 0.2% (w/v) fatty acid-free BSA or ApoA-I (10 ug/mL) for 6 hrs, and finally rinsed twice with PBS. The level of fluorescence-labelled cholesterol in the medium was measured using a multi-label counter (PerkinElmer, Waltham, MA, USA). Cholesterol efflux was expressed as a percentage of fluorescence in the medium relative to the total amount of fluorescence previously added.
2.6. Oil Red O Staining
THP-1 or BMDM cells (3× 105 cells/mL) were cultured overnight on chamber slides (Nalge Nunc International) in DMEM containing 10% FBS and 100 U/mL penicillin/streptomycin. The cells were serum-starved for 12 hrs and subsequently treated with NXT (200ug/mL), fenofibrate (400ng/mL), GW6471 (625ng/mL) or ox-LDL (100ug/mL) for an additional 24 hrs. Upon fixation with paraformaldehyde (4%), the cells were stained with 0.3% oil red O for 20 minutes. Hematoxylin was used as a counterstain, and cells were evaluated on Leica microscope. The stained cells were also eluted with isopropanol, and the supernatant was collected; the OD of the extracts was measured at 540 nm [14].
2.7. Immunoblotting
Total cell lysates of THP-1 cells and BMDM were separated by 8-12% SDS-polyacrylamide gels and then transferred to nitrocellulose membranes. The membranes were blocked for 2 hrs in 5% BSA dissolved in Tween/PBS buffer, then incubated with different dilutions of the indicated primary antibodies at 4°C overnight. Membranes were then incubated with either anti-mouse or anti-rabbit HRP-conjugated IgG at room temperature for 2 hrs prior to detection by ECL substrate (Thermo Scientific). Protein expression levels were semi-quantitatively analyzed using densitometry analysis. Above antibodies were diluted with the antibody solution before use (Millipore).
2.8. Lentivirus Infection
The PPARα knockdown lentivirus was purchased from Genechem Co., LTD (Shanghai, China). An empty vector was constructed in the same manner as a negative control. All the experiments were conducted according to the manufacturer’s protocol. THP-1 derived macrophages were 60–80% confluent and the cells were washed twice with 1 mL PBS. In our preliminary experiment, different doses of GFP-labelled lentivirus were added to the cells and infection efficiency was determined. The obtained optimal multiplicity of infection (MOI) was used for further lentivirus infection experiments. Infected cells were washed with new culture media for subsequent experiments.
2.9. Quantification of Foam Cells in Plaques
F4/80 staining was performed to mark macrophages in plaques, and then macrophage-derived foam cells were quantified. Plaque size and the mean number of foam cells with droplets in plaques were also determined for further calculation. Plaque foam cell content was calculated with the help of ImageJ. Foam cell numbers were counted in five different visual areas for each mouse (from each group, n = 8–9 mice).
2.10. Statistical Analysis
The data are presented as the mean ± standard error of the mean. Statistical significance was determined using one-way analysis of variance, followed by post hoc tests (Newman–Keuls) where appropriate. Student's t-test was used to evaluate two-tailed levels of significance. A P-value <0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism 5.0 (Graph Pad Prism Software Inc, San Diego, CA, USA) and SPSS software (version 17.0, SPSS Inc., Chicago, IL, USA) for Windows.
3. Results
3.1. NXT Reduces Macrophage Foam Cell Accumulation in vivo
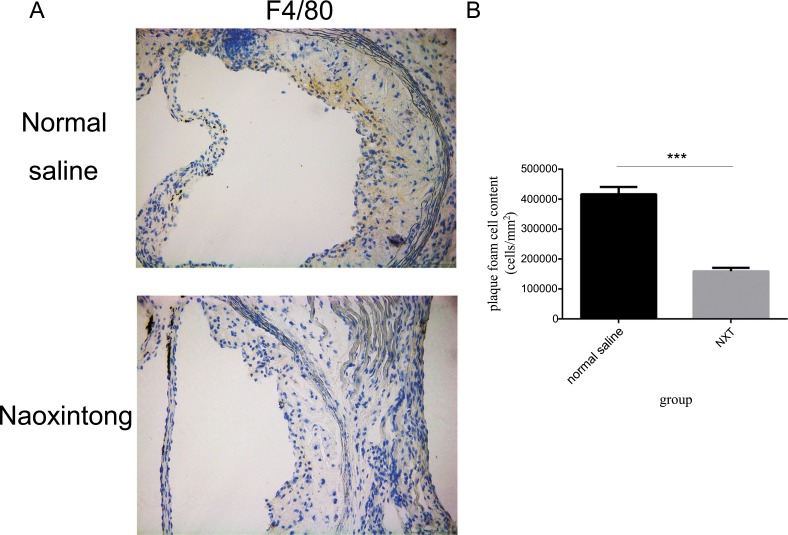
Our previous experiments showed that NXT significantly reduced atherosclerosis in LDLR−/− Mouse [16]. To identify whether NXT could also affect the accumulation of macrophage-derived foam cells in plaques, foam cells were counted on sections of the thoracic artery from ApoE−/− mouse. As shown in Fig. 1, the number of F4/80+ macrophages containing lipid droplets was significantly lower in NXT group than that in the Normal saline group, and NXT reduced the number of F4/80+ macrophage foam cells by 28%. These results demonstrated that NXT significantly reduced the percentage of macrophage-derived foam cells residing in atherosclerotic plaques in ApoE−/− mice.
Fig. (1).
NXT reduced accumulation of foam cells in ApoE-/- mouse. Representative images of atherosclerotic plaques from ApoE-/- mouse treated with Normal saline or Naoxintong. After F4/80+ staining, foam cell accumulation was observed under 400 fold magnification microscopy in 5 random vision fields. (n=8–9 mice). B Plaque foam cell content comparison between Normal saline and NXT group. All data were expressed as mean ± SEM. *p <0.05 by Student t test.
3.2. NXT Reduces ox-LDL Uptake Both in THP-1 and in BMDM in vitro
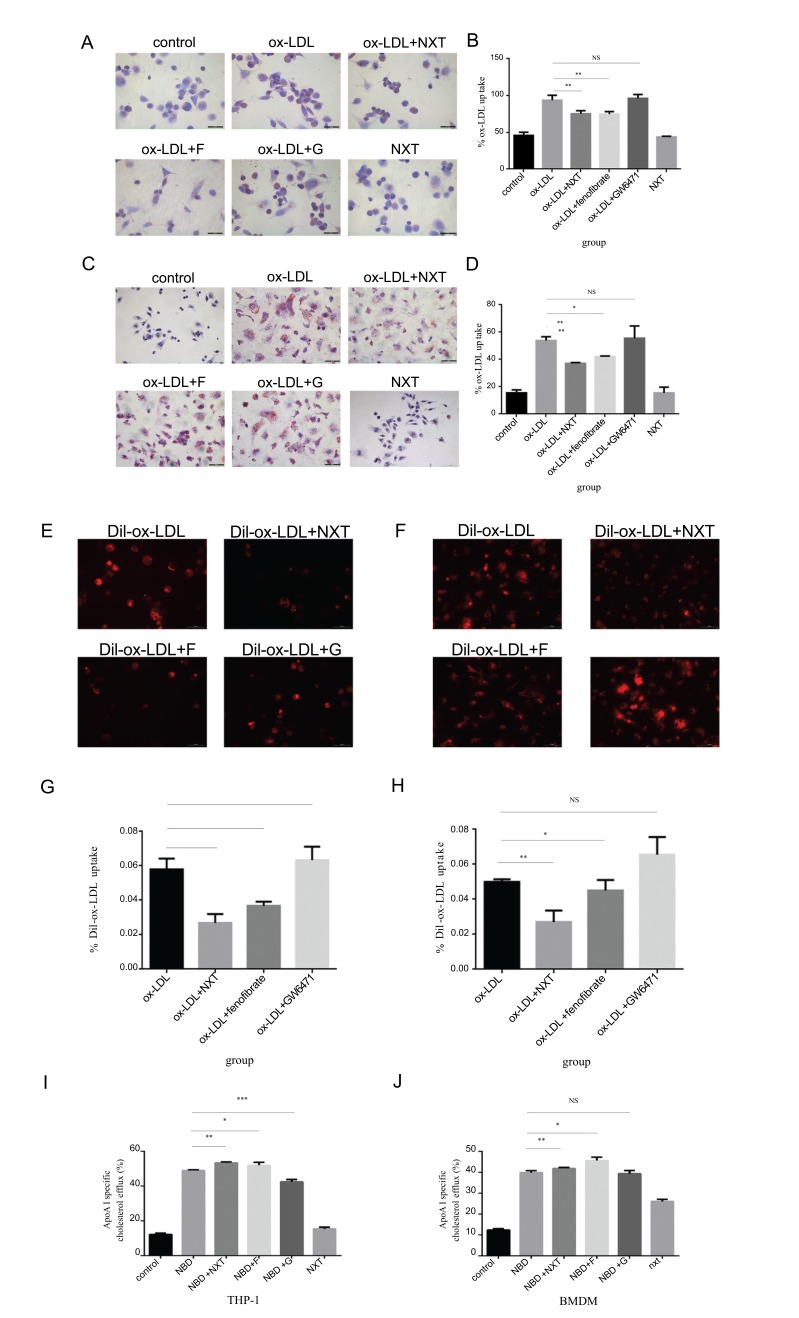
It is well-known that the imbalance between the uptake and efflux of ox-LDL is the major cause to accelerate foam cell formation in macrophages. So we chose the THP-1 and BMDM as models for exploring whether NXT could regulate ox-LDL uptake in macrophages. Cells were divided into 6 groups: ox-LDL, ox-LDL+NXT, ox-LDL+fenofebrate, ox-LDL+GW6471 or NXT.
As shown in Fig. 2A-2D, after 24 hours incubation, NXT significantly reduced the foam cell formation both in THP-1 and BMDMs. To investigate the underlying mechanism of NXT-induced macrophage functional change, fenofibrate, a well-known hypolipidemic drug and PPARα agonist, was used as a positive control and GW6471, a well-known PPARα antagonist, was used as a negative control. As expected, fenofibrate could also remarkably decrease foam cell formation, but GW6471 highly promoted foam cell formation. In order to further confirm our results in NXT-induced
Fig. (2).
Effects of NXT on ox-LDL cellular uptake and cholesterol efflux both in THP-1 derived macrophage and BMDM (F represents for fenofibrate, a well-known hypolipidemic drug and PPARα agonist. G represents for GW6471, a well-known PPARα antagonist). A - D After 6h starvation, macrophages were exposed to different treatments for another 24h. Finally, cells were stained with Oil Red O for detecting. A is THP-1 derived macrophages and C is bone marrow derived macrophages (n=5). B and D Statistical quantification of Oil Red O staining in macrophages. B is THP-1 derived macrophages and D is bone marrow derived macrophages. E and F DiI-ox-LDL cellular uptake in macrophages. E is THP-1 derived macrophages and F is bone marrow derived macrophages (n=5). G and H Statistical quantification of DiI-ox-LDL uptaking in macrophages. G is THP-1 derived macrophages and H is bone marrow-derived macrophages. All data were expressed as mean ± SEM. *p <0.05, compared with treatment of ox-LDL. I and J Cells were exposed to different treatments for 12h, after 3 times washing with cold PBS, NBD-cholesterol was added into the medium and cells were incubated for another 6h. Finally, cells were cultured in DMEM (no phenol red) medium containing 0.2% (w/v) fatty acid-free BSA or ApoA-I (10ug/mL) for 6 hrs (n=5). The supernatant was collected for detection. I NBD-cholesterol efflux in THP-1. J NBD-cholestrol efflux in BMDM. All data were expressed as mean ± SEM. *p <0.05, compared with treatment with ox-LDL.
ox-LDL cellular uptake reduction, we labeled ox-LDL with the DiI dye, which has a strong red fluorescence. As highlighted by our fluorescent microscopy images, the cellular uptake of these ox-LDL was found to be inhibited by NXT (Fig. 2E-2H). Similar results were also noticed after fenofibrate treatment, while GW6471 significantly increased cellular uptake in macrophages. These data indicated that NXT could significantly reduce ox-LDL uptake in macrophages and these effects might be mediated by the activation of PPARα.
3.3. NXT Enhances Cholesterol Efflux Both in THP-1 and BMDM
To demonstrate whether NXT could enhance cholesterol efflux ability in recipient cells, THP-1 and BMDM were subjected to cholesterol efflux assays. As shown in Fig 2I and 2J, NXT remarkably increased cholesterol efflux in foam cells derived from both THP-1 macrophages and BMDMs. Similar results were also noticed in cells treated with fenofibrate. However, cholesterol efflux in macrophage derived foam cells was significantly inhibited by GW6471. Taken together, these results indicated that NXT remarkably enhanced cholesterol efflux in macrophage derived foam cells and these effects might be due to PPARα.activation.
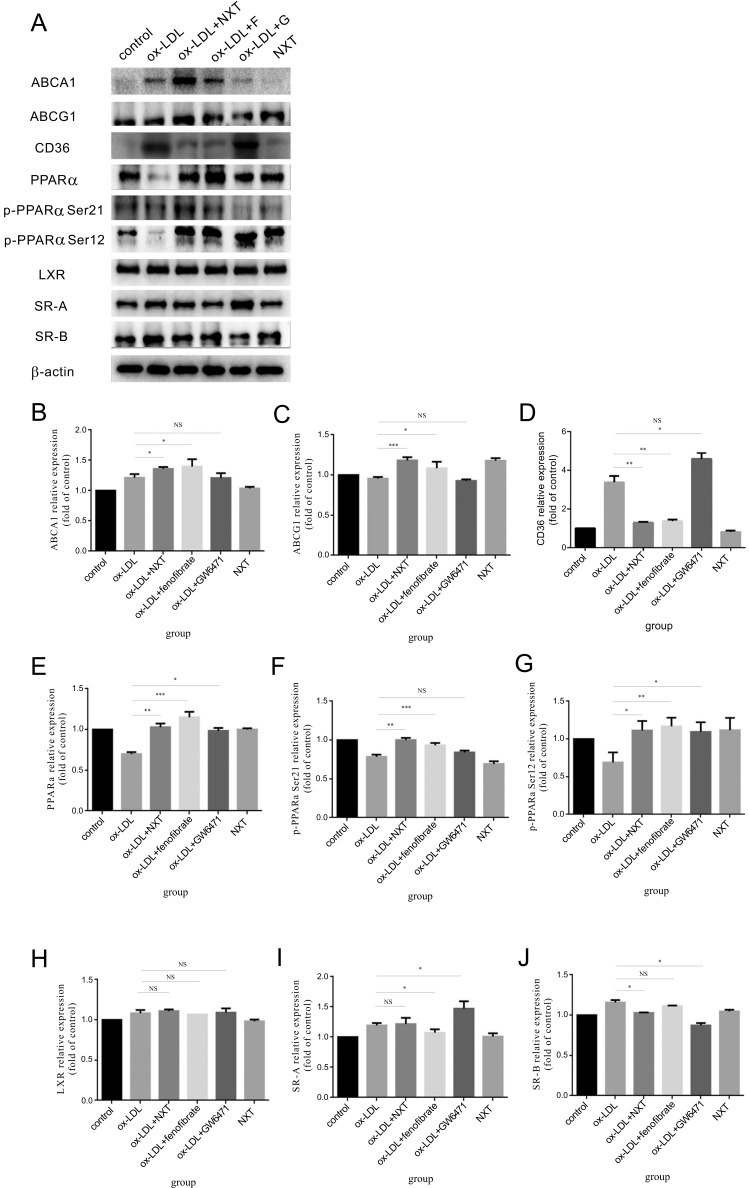
3.4. The Expression of Key Proteins Implicated in ox-LDL Uptake and Cholesterol Efflux were Altered by NXT Both in Human Macrophage (THP-1) and BMDM
Above results have shown that NXT could reduce macrophage-derived foam cell formation by decreasing ox-LDL cellular uptake and increasing cholesterol efflux, and these effects might be mediated by the activation of PPARα.
To determine the specific molecular mechanism by which NXT regulated macrophage-derived foam cell formation, we investigated the expression changes of key proteins implicated in lipoprotein metabolism in macrophages. As shown in Figs. 3 and 4, NXT signi-ficantly reduced the expression of proteins involved in ox-LDL cellular uptake in macrophages such as SR-A, SR-B and CD36. In contrast,
Fig. (3).
Changes of key protein expression implicated in the ox-LDL uptake and cholesterol efflux in THP-1 macrophages (F represents for fenofibrate, a well-known hypolipidemic drug and PPARα agonist. G represents for GW6471, a well-known PPARα antagonist). A. After PMA-induced differentiation, THP-1 derived macrophages were exposed to different treatments. Cell lysates of THP-1 were extracted for western blot to determine the protein levels of ABCA-1, ABCG-1, SR-A, CD36, SR-BI, PPARα, p-PPARα Ser21, p-PPARα Ser12, LXR and β-actin (n=5). B-J Relative expression of different proteins. The control group was set to 1. All data were expressed as mean ± SEM. *p <0.05, compared with treatment with ox-LDL.
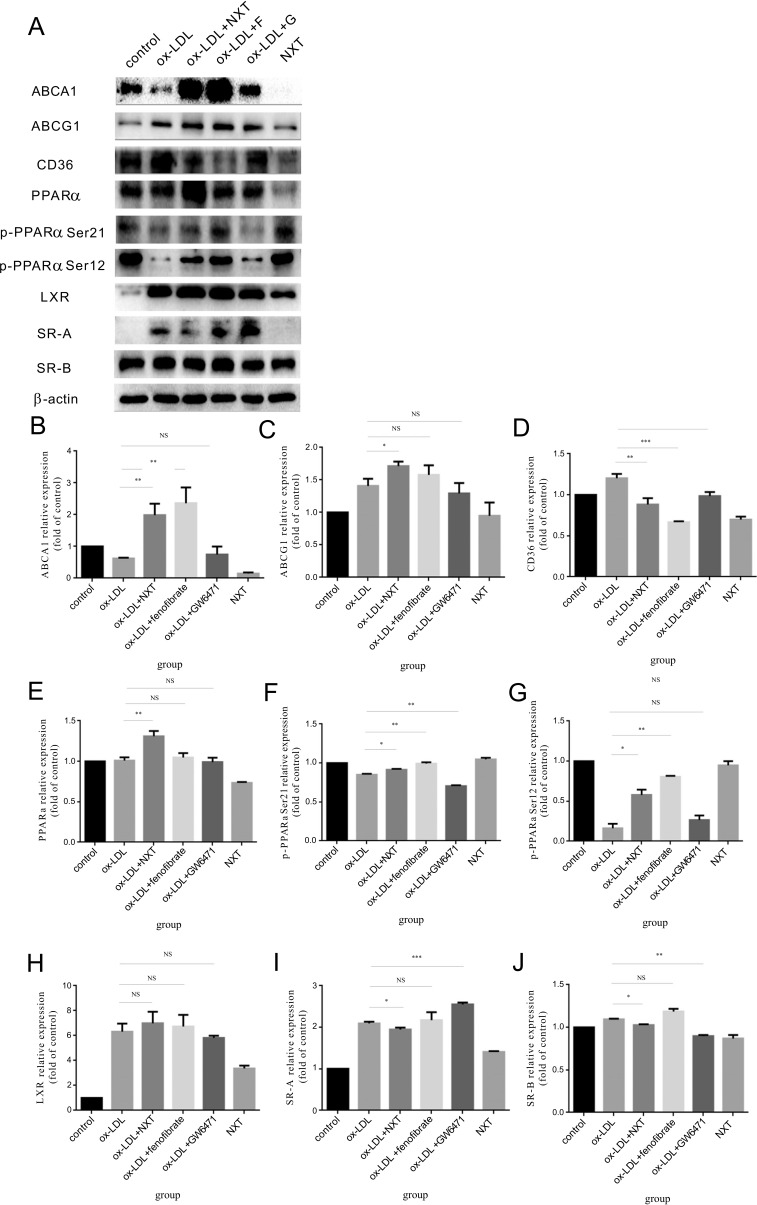
Fig. (4).
Changes of key protein expression implicated in the ox-LDL uptake and cholesterol efflux in BMDM (F represents for fenofibrate, a well-known hypolipidemic drug and PPARα agonist. G represents for GW6471, a well-known PPARα antagonist). A Cell lysates of BMDM were subjected to western blot to determine the protein levels of ABCA-1, ABCG-1, SR-A, CD36, SR-BI, PPARα, p-PPARα Ser21, p-PPARα Ser12, LXR and β-actin (n=5). B-J Relative expression of different proteins. The control group was set to 1. All data were expressed as mean ± SEM. *p <0.05, compared with treatment with ox-LDL.
NXT up-regulated the expression of cholesterol efflux-promoting proteins such as ABCA-1 and ABCG-1. Similar results were also observed in macrophages treated with fenofibrate. However, GW6471 showed the totally opposite effects.
These protein expression changes further led us to ask whether PPARα played the key role in NXT-induced foam cell formation reduction. So, we determined the PPARα expression and activation changes in the presence of NXT. Our results showed that NXT effectively up-regulated the expression of PPARα and activated the protein at two sites at the same time (phosphorylated-PPARα Ser 12 and phosphorylated-PPARα Ser 21). Interestingly, these results were further confirmed in fenofibrate group which is a commonly used clinic hypolipidemic drug and PPARα activator. But these effects were significantly abolished in the presence of GW6471 which is a well-known PPARα inhibitor. Surprisingly, we also observed that NXT has no effect on the expression of these proteins in the absence of ox-LDL. Collectively, these results suggest that NXT might regulate the expression of key proteins implicated in macrophage lipoprotein metabolism through activating PPARα pathway, thereby regulates ox-LDL uptake and cholesterol efflux in macrophages.
3.5. PPARα Plays an Integral Role in NXT Mediated Regulation of Macrophage-derived Foam Cell Formation
In order to further confirm the specific contribution of PPARα in NXT on foam cell formation regulation, a PPARα knockdown treatment was introduced in THP-1 derived macrophages (PPARα-/- macrophages).
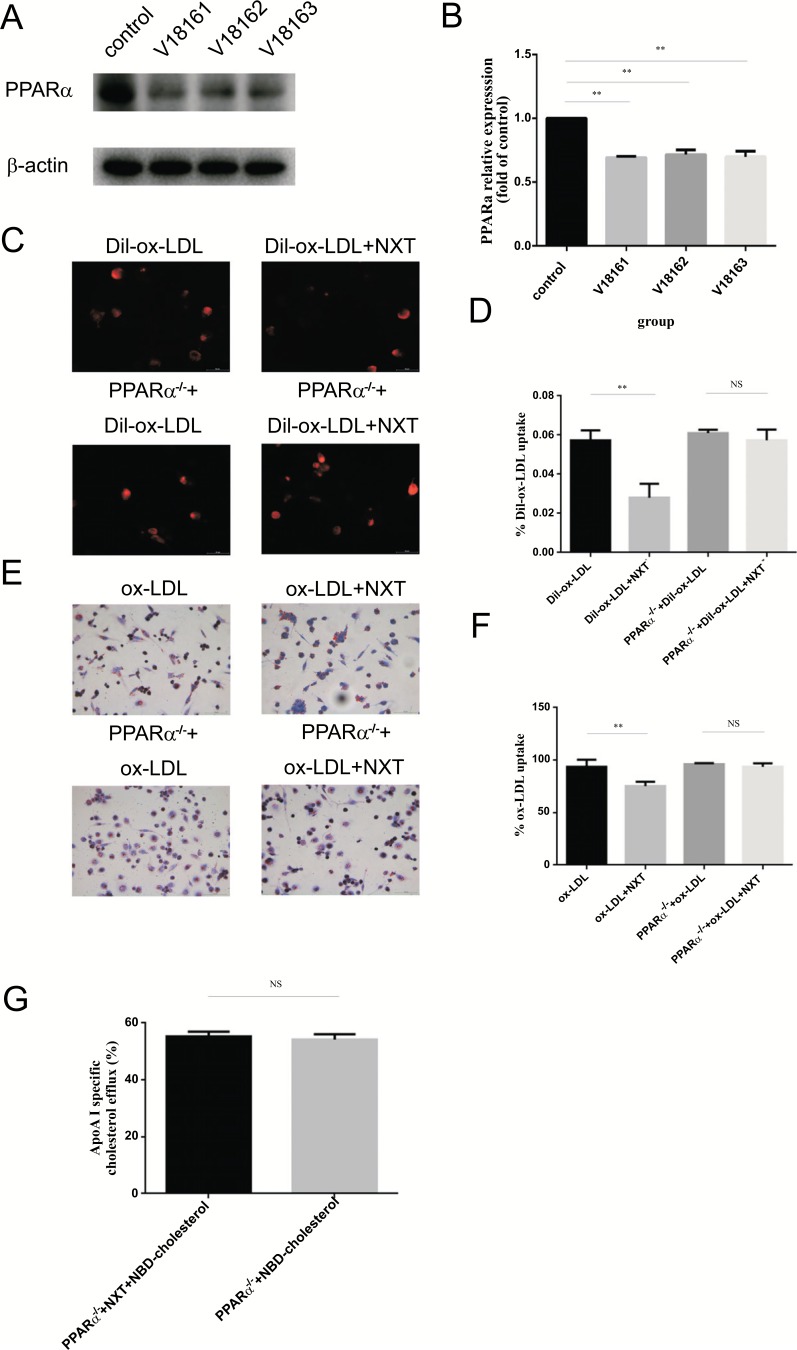
To investigate the lentivirus knockdown efficiency, THP-1 derived macrophages were exposed to three different lentiviruses: V18161, V18162 and V18163. Finally, V18162 was selected for its high PPARα knockdown efficiency and low cytotoxicity and this lentivirus was used subsequently for all future experiments (Fig. 5A).
Fig. (5).
PPARα knockdown eliminates the function of NXT in macrophage-derived foam formation (PPARα-/- represents for PPARα knockdown macrophages). A and B PPARα knockdown efficiency in protein level by different lentiviruses (n=5). C and D DiI-ox-LDL cellular uptake in PPARα-/- macrophages (n=5). E and F After 6h starvation, PPARα-/- macrophages were exposed to different treatments for another 24h. Finally, cells were stained with Oil Red O for detecting. All data were expressed as mean ± SEM. *p <0.05, compared with treatment of ox-LDL. G NBD-cholesterol efflux in PPARα-/- macrophages. Student t test was used in ApoA-I-mediated cholesterol efflux. All data were expressed as mean ± SEM. *p <0.05, compared with treatment with ox-LDL.
In cellular uptake assays, the results showed PPARα knockdown treatment remarkably promoted the malignant phenotypes cured by NXT (Fig. 5C-5F). More importantly, with the treatment of PPARα knockdown, no significance was detected between PPARα-/-+ox-LDL and PPARα-/-+ox-LDL+NXT group. In cholesterol efflux assays, PPARα-/- abolished the cholesterol efflux promoting effects of NXT in macrophages (Fig. 5G). Taken together, all these data addressed this issue and indicated that PPARα might be the integral component in NXT-induced foam cell formation reduction.
3.6. Protein Expression Changes in Human Macrophages After PPARα Lentivirus Knockdown
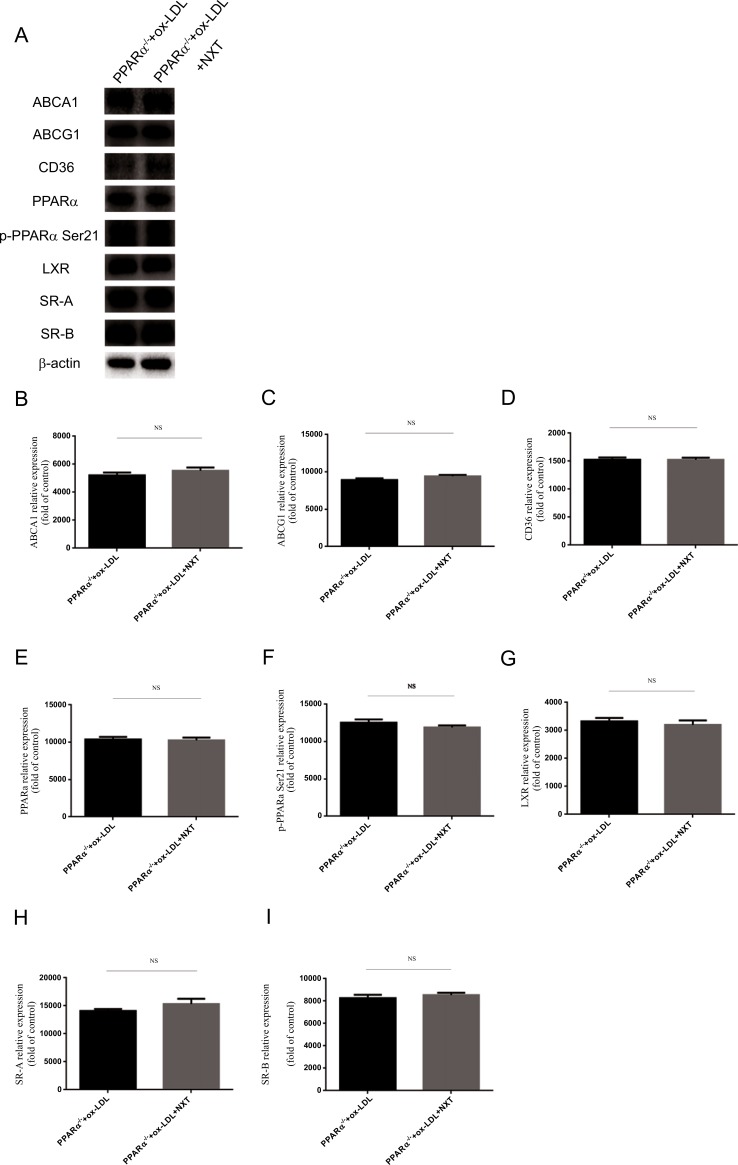
To validate the data obtained above, we investigated protein expression changes after NXT treatment in PPARα-/- cell models. After PPARα knockdown models were established, cells were treated with ox-LDL and NXT. 24 hours after treatment, immunoblotting against different antibodies demonstrated the loss of function of NXT on foam cell formation. Neither proteins involved in ox-LDL cellular uptake nor cholesterol efflux could be regulated by NXT in PPARα-/- cells (Fig. 6A-6I). More importantly, no significant difference was observed in the presence of NXT after PPARα knockdown, and PPARα could not be effectively activated either. To conclude, our results indicated that NXT could reduce macrophage-derived foam cell formation and these effects were partly mediated by PPARα pathway.
Fig. (6).
Key proteins expression change implicated in the uptake and cholesterol efflux after PPARα knockdown in macrophages (PPARα-/- represents for PPARα knockdown macrophages). A PPARα knockdown abolished NXT effects on macrophage-derived foam cell formation. Cell lysates of PPARα-/- macrophages treated with ox-LDL and NXT were subjected to western blot to determine the protein levels of ABCA-1, ABCG-1, SR-A, CD36, SR-BI, PPARα, p-PPARα Ser21, LXR and β-actin (n=5). B-I The control group was set to 1, and all the other prorein levels fold change were compared with the control. All data were expressed as mean ± SEM. *p <0.05 by Student t test.
4. DISCUSSION
Monocyte-derived macrophage foam cell formation is a hallmark of atherosclerosis [30, 31]. In view of the complexity of macrophage-derived foam cell formation,
it is becoming increasingly important to explore the dynamic regulatory mechanism of lipid metabolism. The combined regulation of lipid uptake and cholesterol efflux opened an unprecedented opportunity for drugs to inhibit the formation of foam cells [32]. To our knowledge, this is the first study to show that NXT inhibits foam cell formation by dynamically regulating the lipid metabolism in macrophages. As shown in results, NXT plays an important role in reducing macrophage-derived foam cell accumulation in vivo and decreasing macrophage-derived foam cell formation in vitro. In ApoE−/− mouse model, our results showed that NXT significantly decreased the number of foam cells in plaques. And these results were further confirmed by studies of THP-1 cell line and bone marrow-derived macrophages. These data served as direct evidence that NXT inhibits the foam cells formation in atherosclerosis (Fig. 7).
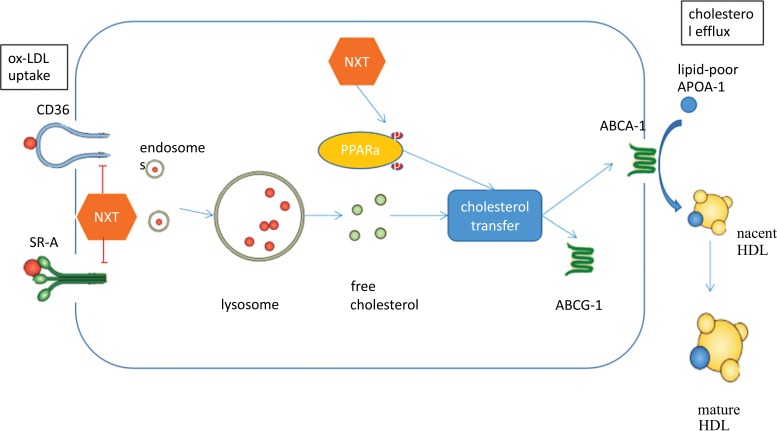
Fig. (7).
Schematic diagram of NXT intracellular stimulation in macrophages.
Due to species differences between animals and humans, animal studies alone are not sufficient to adequately reflect the specific mechanism of a drug in humans. In order to better study the effects of NXT on macrophage-derived foam cell formation and promote the medical application translation, we chose THP-1 and BMDM as models for our study. THP-1, a stable human monocytic cell line, was used to evaluate the effects of NXT on the human macrophages. BMDM, a primary macrophage derived from bone marrow, was used to evaluate the effects of NXT on the macrophages in mice. Our results showed that NXT could exhibit similar effects both in human cell lines and animals. So, the results we demonstrated here have successfully built a bridge between the molecular mechanisms study in animals and pharmaceutical efficacy evaluation in human.
The dynamic regulation process of lipid metabolism consists of two parts: lipid uptake and cholesterol efflux. SR-A and CD36, which are the central proteins implicated in the process of lipid-uptake, were detected in our study. Previous studies have shown that up-regulation of SR-A and CD36 significantly increases ox-LDL uptake in macrophages and promotes foam cell formation. But in our results, the expression of SR-A and CD36 was significantly reduced in macrophages co-cultured with NXT, and these changes were especially obvious in bone marrow-derived macro-phages. Our observations suggested that NXT-induced SR-A and CD36 expression reduction in the presence of ox-LDL may be responsible for decreased ox-LDL uptake and foam cell formation [33, 34].
Cholesterol efflux is another vital way in maintaining lipid homeostasis in cells. ABCA-1 and ABCG-1 are two major regulators in intracellular free cholesterol transfer [35]. Previous studies have shown that overexpression of ABCA1 and ABCG1 effectively enhanced cholesterol efflux in foam cell. On the contrary, the down-regulation of ABCA-1 and ABCG-1 will further promote foam cells formation. Therefore, we investigated the regulatory role of NXT on protein expression of ABCA-1 and ABCG-1 and we observed a remarkable upregulation of these two proteins. And these changes could be better supporting evidence for the observations in increased ApoA-I-mediated cholesterol efflux after NXT treatment.
By exploring the molecular mechanism, the protective effects of NXT appeared to be mediated, at least in part, by activating PPARα pathway [36]. PPARα could promote lipid degradation by regulating the expression of lipolytic genes [23]. A previous study has reported that dietary magnesium could reduce lipid accumulation in yellow catfish through PPARα pathway. Some other studies indicated that fenofibrate, a drug used for the treatment of hyperlipidemia, could promote the cellular cholesterol efflux by phos-phorylating PPARα [37, 38]. Earlier studies would fit nicely with the present results regarding the downregulation of SR-A and CD36 and upregulation of ABCA-1 and ABCG-1 by NXT, accompanied by increased PPARα protein expression. We further detected the phosphorylated level of PPARα Ser12 and PPARα Ser21, both of them were significantly increased in the group of NXT and fenofibrate [39, 40]. This finding indicated that NXT not only increases the expression of PPARα but also plays an important role as an agonist in reducing foam cell formation. In order to better explore the role of PPARα pathway in NXT induced foam cell formation reduction, we used GW6471, a PPARα specific inhibitor, as a negative agent. It was not surprising to observe that foam cell formation was more serious when macrophages were co-cultured with ox-LDL and GW6471 [29]. Compared with NXT group, the expression of ABCA-1 and ABCG-1 was significantly decreased, while the expression of CD36 and SR-A increased remarkably. Significantly decreased protein expression and phosphorylation level further confirmed the integral role of PPARα in NXT induced reduction of foam cell formation from the opposite side. Taken together, our study used both positive validation and negative validation to show the mechanism linked with the effect of NXT on foam cell reduction is mediated through PPARα pathway.
Lentivirus gene knockdown has become an important way to study the function of a specific gene. With the help of establishment of PPARα-/- macrophages, we further deepened our understanding of the potential integral role of PPARα in NXT-induced foam cell formation reduction. In the functional analysis, NXT has no effect on ox-LDL uptake or cholesterol efflux post-PPARα knockdown. As for proteins implicated in ox-LDL uptake or cholesterol efflux, no significant expression changes were detected on cells exposed to NXT post lentivirus PPARα knockdown. All these results suggested that NXT reduces foam-cell formation by upregulating and activating PPARα pathway.
conclusion
In summary, our results provide convincing evidence that NXT reduces foam cell formation both in vivo and in vitro. These effects appear to be mediated by upregulation and activation of PPARα pathway. And future studies should employ more molecule-specific methods for building a more complete intermolecular network.
ACKNOWLEDGEMENTS
This study was supported by National Natural Science Foundation of China (81570224 to Aijun Sun; 81521001 to Junbo Ge; 81700384 to Huairui Shi) and Postdoctoral Science Foundation of China (2017M621368 to Huairui Shi). Z.W., H.R.S. and J.B.G. designed the experiments. Z.D., H.Z. and B.C.Z. performed the experiments. W.X.Y., R.L.L. and X.L. analysed the data. Y.Z.Z., K.H. and A.J.S. prepared the figures. Z.W. wrote the main text. Correspondence: Junbo Ge and Aijun Sun. First author: Zeng Wang. Co-first authors: Huairui Shi, Huan Zhao and Zhen Dong. All authors reviewed the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All mice were maintained at the Zhongshan Experimental Animal Center, Fudan University, Shanghai. The animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996) and were approved by the Animal Care and Use Committee of Fudan University. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
HUMAN AND ANIMAL RIGHTS
No humans were used for studies that are the basis of this research. The reported experiments on animals are in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Consent for Publication
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors confirm that this article content has no conflicts of interest.
References
- 1.Parks B.W., Lusis A.J. Macrophage Accumulation in Atherosclerosis. N. Engl. J. Med. 2013;369(24):2352–2353. doi: 10.1056/NEJMcibr1312709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paramo F.J. Atherosclerosis and clonal hematopoyesis: A new risk factor. Clin. Investig. Arterioscler. 2018 doi: 10.1016/j.arteri.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Windecker S., Piccolo R., Ueki Y. Long-Term Assessment of Bioresorbable Coronary Scaffolds: Disappearing Stents, Reappearing Atherosclerosis. J. Am. Coll. Cardiol. 2018;71(17):1894–1896. doi: 10.1016/j.jacc.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Bogiatzi C., Gloor G., Allen-Vercoe E., et al. Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis. 2018;273:91–97. doi: 10.1016/j.atherosclerosis.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Hara T., Phuong P.T., Fukuda D., et al. Protease-Activated Receptor-2 Plays a Critical Role in Vascular Inflammation and Atherosclerosis in Apolipoprotein E-Deficient Mice. Circulation. 2018 doi: 10.1161/CIRCULATIONAHA.118.033544. [DOI] [PubMed] [Google Scholar]
- 6.Moore K.J., Tabas I. Macrophages in the Pathogenesis of Atherosclerosis. Cell. 2011;145(3):341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore K.J., Sheedy F.J., Fisher E.A. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 2013;13(10):709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortese R., Gileles-Hillel A., Khalyfa A., et al. Aorta macrophage inflammatory and epigenetic changes in a murine model of obstructive sleep apnea: Potential role of CD36. Sci Rep-Uk. 2017;7:43648. doi: 10.1038/srep43648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sini S., Deepa D., Harikrishnan S., Jayakumari N. High-density lipoprotein from subjects with coronary artery disease promotes macrophage foam cell formation: role of scavenger receptor CD36 and ERK/MAPK signaling. Mol. Cell. Biochem. 2017;427(1-2):23–34. doi: 10.1007/s11010-016-2895-7. [DOI] [PubMed] [Google Scholar]
- 10.Kammerer I., Ringseis R., Biemann R., Wen G., Eder K. 13-hydroxy linoleic acid increases expression of the cholesterol transporters ABCA1, ABCG1 and SR-BI and stimulates apoA-I-dependent cholesterol efflux in RAW264.7 macrophages. Lipids Health Dis. 2011;10:222. doi: 10.1186/1476-511X-10-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He X., Yu D., Li W., et al. Anti-atherosclerotic potential of baicalin mediated by promoting cholesterol efflux from macrophages via the PPARγ-LXRα-ABCA1/ABCG1 pathway. Biomed. Pharmacother. 2016;83:257–264. doi: 10.1016/j.biopha.2016.06.046. [DOI] [PubMed] [Google Scholar]
- 12.Li Y., Jiang B., Liang P., et al. Nucleolin protects macrophages from oxLDL-induced foam cell formation through up-regulating ABCA1 expression. Biochem. Biophys. Res. Commun. 2017;486(2):364–371. doi: 10.1016/j.bbrc.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 13.Shi H., Mao X., Zhong Y., et al. Digoxin reduces atherosclerosis in apolipoprotein E-deficient mice. Br. J. Pharmacol. 2016;173(9):1517–1528. doi: 10.1111/bph.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi H, Mao X, Zhong Y, et al. 2016.
- 15.Rousselle A., Qadri F., Leukel L., et al. CXCL5 limits macrophage foam cell formation in atherosclerosis. J. Clin. Invest. 2013;123(3):1343–1347. doi: 10.1172/JCI66580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J., Zhu H., Wang S., et al. Naoxintong protects against atherosclerosis through lipid-lowering and inhibiting maturation of dendritic cells in LDL receptor knockout mice fed a high-fat diet. Curr. Pharm. Des. 2013;19(33):5891. doi: 10.2174/1381612811319330008. [DOI] [PubMed] [Google Scholar]
- 17.Xu H., Jin J., Chen L., et al. Naoxintong/PPARα Signaling Inhibits H9c2 Cell Apoptosis and Autophagy in Response to Oxidative Stress. Evid-Based Compl Alt. 2016;2016:1–10. doi: 10.1155/2016/4370381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan S., Jin J., Chen L., Hou Y., Wang H. Naoxintong/PPARγ Signaling Inhibits Cardiac Hypertrophy via Activation of Autophagy. Evid-Based Compl Alt. 2017;2017:1–9. doi: 10.1155/2017/3801976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Yan X., Mi S., et al. Naoxintong attenuates Ischaemia/reperfusion Injury through inhibiting NLRP3 inflammasome activation. J. Cell. Mol. Med. 2017;21(1):4–12. doi: 10.1111/jcmm.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nan Y.M., Kong L.B., Ren W.G., et al. Activation of peroxisome proliferator activated receptor alpha ameliorates ethanol mediated liver fibrosis in mice. Lipids Health Dis. 2013;12:11. doi: 10.1186/1476-511X-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez-Calvo R., Vázquez-Carrera M., Masana L., Neumann D. AICAR Protects against High Palmitate/High Insulin-Induced Intramyocellular Lipid Accumulation and Insulin Resistance in HL-1 Cardiac Cells by Inducing PPAR-Target Gene Expression. PPAR Res. 2015;2015:1–12. doi: 10.1155/2015/785783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu G., Hou T., Yuan Y., et al. Fenofibrate inhibits atrial metabolic remodelling in atrial fibrillation through PPAR-α/sirtuin 1/PGC-1α pathway. Br. J. Pharmacol. 2016;173(6):1095–1109. doi: 10.1111/bph.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei C., Wu K., Gao Y., Zhang L., Li D., Luo Z. Magnesium Reduces Hepatic Lipid Accumulation in Yellow Catfish (Pelteobagrus fulvidraco) and Modulates Lipogenesis and Lipolysis via PPARA, JAK-STAT, and AMPK Pathways in Hepatocytes. J. Nutr. 2017;147(6):1070–1078. doi: 10.3945/jn.116.245852. [DOI] [PubMed] [Google Scholar]
- 24.Wang H., Qiu L., Ma Y., et al. Naoxintong inhibits myocardial infarction injury by VEGF/eNOS signaling-mediated neovascularization. J. Ethnopharmacol. 2017;209:13–23. doi: 10.1016/j.jep.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 25.Ledda A., González M., Gulfo J., et al. Decreased OxLDL uptake and cholesterol efflux in THP1 cells elicited by cortisol and by cortisone through 11β-hydroxysteroid dehydrogenase type 1. Atherosclerosis. 2016;250:84–94. doi: 10.1016/j.atherosclerosis.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Gutowska I., Baranowska-Bosiacka I., Goschorska M., et al. Fluoride as a factor initiating and potentiating inflammation in THP1 differentiated monocytes/macrophages. Toxicol. In Vitro. 2015;29(7):1661–1668. doi: 10.1016/j.tiv.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 27.Zheng H., Cui D., Quan X., et al. Lp-PLA2 silencing protects against ox-LDL-induced oxidative stress and cell apoptosis via Akt/mTOR signaling pathway in human THP1 macrophages. Biochem. Biophys. Res. Commun. 2016;477(4):1017–1023. doi: 10.1016/j.bbrc.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Tsai M.Y., Ordovas J.M., Li N., et al. Effect of fenofibrate therapy and ABCA1 polymorphisms on high-density lipoprotein subclasses in the Genetics of Lipid Lowering Drugs and Diet Network. Mol. Genet. Metab. 2010;100(2):118–122. doi: 10.1016/j.ymgme.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding L., Liang X.G., Lou Y.J. Time-dependence of cardiomyocyte differentiation disturbed by peroxisome proliferator-activated receptor alpha inhibitor GW6471 in murine embryonic stem cells in vitro. Acta Pharmacol. Sin. 2007;28(5):634–642. doi: 10.1111/j.1745-7254.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 30.Miteva K., Madonna R., De Caterina R., Van Linthout S. Innate and adaptive immunity in atherosclerosis. Vascul. Pharmacol. 2018 doi: 10.1016/j.vph.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Zuiderwijk M., Geerts M., van Rhijn C.J., et al. Leucocyte dynamics during the evolution of human coronary atherosclerosis: conclusions from a 7-fold, chromogen-based immunohistochemical evaluation. Am. J. Pathol. 2018 doi: 10.1016/j.ajpath.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Quinlivan V.H., Farber S.A. Lipid Uptake, Metabolism, and Transport in the Larval Zebrafish. Front. Endocrinol. (Lausanne) 2017;8:319. doi: 10.3389/fendo.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glatz J., Luiken J. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J. Lipid Res. 2018 doi: 10.1194/jlr.R082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cifarelli V., Abumrad N.A. Intestinal CD36 and Other Key Proteins of Lipid Utilization: Role in Absorption and Gut Homeostasis. Compr. Physiol. 2018;8(2):493–507. doi: 10.1002/cphy.c170026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machado-Lima A., Iborra R.T., Pinto R.S., et al. Advanced glycated albumin isolated from poorly controlled type 1 diabetes mellitus patients alters macrophage gene expression impairing ABCA-1-mediated reverse cholesterol transport. Diabetes Metab. Res. Rev. 2013;29(1):66–76. doi: 10.1002/dmrr.2362. [DOI] [PubMed] [Google Scholar]
- 36.Ding Z., Fan Y., Deng X. Concentration polarization of oxidative modification of low-density lipoproteins: its effect on oxidative modification of low-density lipoprotein uptake and apoptosis of the endothelial cells. ASAIO J. 2010;56(5):468–474. doi: 10.1097/MAT.0b013e3181e7be08. [DOI] [PubMed] [Google Scholar]
- 37.de Keyser C.E., Becker M.L., Uitterlinden A.G., et al. Genetic variation in the PPARA gene is associated with simvastatin-mediated cholesterol reduction in the Rotterdam Study. Pharmacogenomics. 2013;14(11):1295–1304. doi: 10.2217/pgs.13.112. [DOI] [PubMed] [Google Scholar]
- 38.Lima L.O., Bruxel E.M., Hutz M.H., et al. Influence of PPARA, RXRA, NR1I2 and NR1I3 gene polymorphisms on the lipid-lowering efficacy and safety of statin therapy. Arq. Bras. Endocrinol. Metabol. 2013;57(7):513–519. doi: 10.1590/s0004-27302013000700003. [DOI] [PubMed] [Google Scholar]
- 39.Arai H., Yamashita S., Yokote K., Araki E., Suganami H., Ishibashi S. Efficacy and Safety of Pemafibrate Versus Fenofibrate in Patients with High Triglyceride and Low HDL Cholesterol Levels: A Multicenter, Placebo-Controlled, Double-Blind, Randomized Trial. J. Atheroscler. Thromb. 2018 doi: 10.5551/jat.44412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi T., Lu K., Shen S., et al. Fenofibrate decreases the bone quality by down regulating Runx2 in high-fat-diet induced Type 2 diabetes mellitus mouse model. Lipids Health Dis. 2017;16(1):201. doi: 10.1186/s12944-017-0592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]