Abstract
The National Brain Injury Rescue and Rehabilitation Project was established as a preliminary study to test the safety and practicality of multi-center hyperbaric oxygen administration for the post-concussive symptoms of chronic mild traumatic brain injury as a precursor to a pivotal, independent, multi-center, controlled clinical trial. This report presents the results for 32 subjects who completed a preliminary trial of hyperbaric oxygen several years before the passage of the 21st Century Cures Act. This study anticipated the Act and its reassessment of clinical research. Subjects received 40–82 one-hour treatments at 1.5 atmospheres absolute 100% oxygen. Outcome measures included repeated self-assessment measures and automated neurocognitive tests. The subjects demonstrated improvement in 21 of 25 neurocognitive test measures observed. The objective neurocognitive test components showed improvement in 13 of 17 measures. Earlier administration of hyperbaric oxygen post injury, younger age at the time of injury and hyperbaric oxygen administration, military status, and increased number of hyperbaric oxygen administrations were characteristics associated with improved outcomes. There were no adverse events. Hyperbaric oxygen was found to be safe, inexpensive and worthy of clinical application in the 21st Century model of facile data collection provided by recent research regulatory shifts in medicine. The study was approved by the ethics review committee of the Western Institutional Review Board (WIRB® Protocol #20090761).
Keywords: hyperbaric oxygen, traumatic brain injury, post-concussion syndrome, post-traumatic stress disorder, 21st Century Cures Act, HBOT, mTBI, PTSD, concussion
INTRODUCTION
Untreated brain insult is among the most expensive public health problems in the world. An argument can be made that untreated brain insults are the most expensive public health problem in the world when you take into account brain injuries from war, falls, strokes, accidents, violence, blasts, sports injuries, near-drownings, and a myriad of other wounds to the brain brought on by substance abuse, etc. The systematic use of hyperbaric oxygen therapy (HBOT) for brain injury pre-dates World War II.1 Neurological injury is the first indication ever approved for HBOT and today the approved indications include repair of five acute/chronic neurological injuries, and three chronic non-healing wounds.2 Non-healing wounds in the brain are a logical progression as demonstrated by research conducted since 1976.1 Thousands of patients in hundreds of locations have been successfully treated since that date. An estimated 2000 HBOT treatments for these neurological conditions occur on any one day across the United States.3 The difficulty has been that while significant "off-label" treatment happens across all medical disciplines, there has been no systematic way to gather and collate those data to scientifically present the findings to effect third-party reimbursement.
A collaboration between William Duncan and John Eisenberg, founder and first Director of the Agency for Healthcare Research and Quality (AHRQ), initiated a track that led to the National Brian Injury, Rescue & Rehabilitation (NBIRR-01) observational study methodology to validate orphaned therapies. Though HBOT is far less expensive to society than the cost of living with the human tragedy of untreated brain insults, there has been no commercial pathway to development and thus, there was no corporate sponsor, because the treatment cannot be protected by patents. A few peer-reviewed scientific articles have reported on studies that document beneficial effects from hyperbaric oxygen (HBO2) in subjects with chronic residual effects of moderate to severe traumatic brain injury (TBI).4,5,6,7 And within the last half decade, a sizeable literature has developed that explores the safety and effectiveness of HBO2 with subjects diagnosed with TBI, post traumatic stress disorder (PTSD), post-concussion syndrome (PCS) and and/or persistent post-concussion syndrome (PPCS).8 Based on the mounting evidence that service member suicides were increasing, along with the toll extracted on service members by improvised explosive devices and blast injuries, the NBIRR was started to study the safety and practicality of a multicenter study of HBO2 administration at 1.5 atmospheres absolute (ATA, 1 atm = 1 × 105 Pa) for post-concussive symptoms from mild TBI (mTBI) with or without co-existing PTSD and PTSD without TBI. Study sites were the Oklahoma State University Center for Aerospace & Hyperbaric Medicine, Tulsa, OK, USA; San Francisco Institute for Hyperbaric Medicine, San Francisco, CA, USA; Hyperbaric Medicine, Inc., Fort Walton Beach, FL, USA; Restorix Health, Issaquah, WA, USA; and Life Force Therapies, Minneapolis, MN, USA. The study was designed to enroll 1000 subjects over a multi-year period in the expectation that improvement of symptoms could be offered to a large number of subjects, many of whom may have been injured in the "Global War on Terror."
The non-DoD/VA medical community, particularly National Institutes of Health (NIH) and the Food and Drug Administration (FDA), have not been immune to the need for faster responses to life-threatening emergencies that have become epidemics. In December 2016, the 21st Century Cures Act (the Act) was signed.9 A major component of the law is an effort to expedite approval of breakthrough medical technologies for patients with life-threatening illnesses and limited treatment options. Without specifically focusing on brain injuries, the Act called out several "requirements" that were already enabled in NBIRR, namely:
-
1)
recording the effect of a current therapeutic option on brain injury;
-
2)
capturing and assessing patient-reported outcomes (i.e., a measurement based on a report from a patient regarding the status of the patient's health condition without amendment or interpretation of the patient's report by a clinician or any other person);
-
3)
accumulating data regarding the usage, or the potential benefits or risks, of a drug derived from an observational study;
-
4)
capturing data in a repository and making it freely available to participants via the Cloud;
-
5)
subjecting clinical experience data collected nationwide to Bayesian analysis (anticipating adaptive trial designs in follow-on studies using other alternative therapies);
-
6)
tying results data to economic consequences, based on the separate or aggregated clinical consequences of the represented health outcomes;
-
7)
developing, reporting and making available for future use the patient-experience data necessary for enhanced structured risk-benefit assessment.
One of the purposes of NBIRR was to evaluate the safety of the HBO2 1.5 ATA protocol for mTBI post-concussive symptoms and PTSD. While HBO2 is generally accepted as safe, potential adverse effects resulting from HBO2 administration in brain injured patients include seizures and worsening of psychiatric conditions. The decades-long use of HBO2 1.5 ATA in hundreds of patients with multiple sclerosis in millions of sessions in the United Kingdom has established fully the safety of HBO2 1.5 ATA for neurologic conditions, yet at the time this study was designed the safety of HBO2 in mTBI subjects was being actively questioned.10,11 Further, in August 2013, the FDA issued a Consumer Update12 that was widely interpreted by some, wrongly, as a warning about the safety of HBO2. The Update was an FDA advisory to consumers not to believe excessive marketing material on the Web about the use of HBO2 for such things as Autism, Cancer and several other diseases. Our research into the use of HBO2 in treating TBI was never in question. The Study Director took the opportunity to terminate this pro-bono study due to lack of funding. The study had met its primary objectives: to ascertain in a large, multicenter cohort if there was a long-term benefit for a new use of a known safe treatment; to provide nationwide access to a safe treatment for a new indication of an FDA-cleared Medical device for mild-moderate chronic TBI and/or PTSD, while ascertaining broader efficacy under controlled conditions; and to ascertain the optimal number of treatments in the range from 40–80 HBOT sessions.
HBO2 has been used to treat a variety of brain injuries for decades. It was described as a treatment for carbon monoxide poisoning in 1960,13 and it was soon recognized that hyperbaric oxygen had effects impacting brain healing separate from the action to remove carbon monoxide from hemoglobin molecules.14 Since the adoption of oxygen treatment tables in 1967, the brain injuries of decompression sickness and arterial gas embolism have been effectively treated by HBO2.15 In 1969, clinical improvement from stroke was demonstrated with HBO2 administered three months after the stroke.16 Late hypoxic injury to the brain had also been treated successfully with HBO2.17 Significant changes in cerebral blood flow with simultaneous symptomatic, physical quality of life, and cognitive improvements have recently been demonstrated after HBO2 in a population of blast-induced mild-moderate TBI military veterans with PCS and PTSD. That experience replicated the findings in a controlled animal model of chronic traumatic brain injury incorporating an earlier version of the 1.5 ATA protocol where persistent changes in brain vasculature as well as spatial learning and memory were observed.18 The lack of high quality controlled clinical trials demonstrating the efficacy of HBO2 in brain injury had been a valid criticism for a decade.19 At the end of 2008, that began to change after a DoD-sponsored Consensus Conference on the use of HBO2 for brain injury. Over a dozen studies and analyses have been conducted since then. The data and worldwide analyses point to the safety and efficacy of HBOT when used to help treat brain injury.7
NBIRR IN CONTEXT: CONFRONTING THE ALLEGED BIAS IN STUDIES USING AN HBO2 SHAM
NBIRR was designed, and carried out, as an observational study. Throughout the research, other more extensive and expensive studies were conducted. As with all science, disagreements occurred about outcomes and interpretations of data. Where there was little disagreement, however, was the dire need to do a better job of making treatments available that actually did something to relieve the suffering of those with brain injuries. Beginning in 2008, suspicions arose about the futility of much of the research into treatments for what was then thought of as PTSD. Congressional doubts led to an Institute of Medicine review that found the expenditure of $9.3 billion to treat PTSD from 2010 through 2012 could not tell whether this staggering sum resulted in effective or adequate care.20
Despite those findings, research continued into a multitude of potential interventions. Despite the efforts and the large sums of dollars expended, senior analysts could say years later that "...no new treatments for persistent blast- or impact-related post-concussion symptoms have been identified, despite the extensive investment to date. The evidence remains weak and inconsistent for both pharmacological…and nonpharmacological…interventions [e.g., cognitive psychotherapy]…" Concerns have been raised that current screening approaches, combined with a specialty-driven structure of concussion care in the Veterans Health Administration (VHA) and DoD may inadvertently promote negative, rather than positive, recovery expectations.21 The NBIRR study came into being for precisely that need: to help overcome the paucity of effective and safe treatments that appeared to be affecting the health, safety, well-being and even the lives of the brain injured and their families.
Researchers into brain injuries can certainly disagree about many aspects of science, but the validity of the basic laws of physics and mathematics should not be flexible. Since NBIRR was intended to inform the design of further research studies to investigate the use of HBO2 in brain-injured service members and others, it is important to dwell on a worldwide controversy about the validity of the findings and conclusions in the DoD/VA/Army research.
Though it did not affect the NBIRR study, serious disagreements were raised very early – and continue to exist – about what Army studies characterize as the "sham" employed in their so-called randomized controlled trials. The first Army study was a "single-center, double-blind, randomized, shamcontrolled, prospective trial at the US Air Force School of Aerospace Medicine" where "the effects of 2.4 ATA HBO2 on post-concussion symptoms in 50 military service members with at least 1 combat-related, mild traumatic brain injury were examined. Each subject received 30 sessions of either a ‘sham’ compression (room air at 1.3 ATA) or HBO2 treatments at 2.4 ATA over an 8-week period."22
The challenges from around the world came quickly: the claimed "sham" in this DoD/VA/Army study, and others that followed, were not without medicinal properties (30% higher pO2 than in air at sea level) and therefore had to be considered a treatment and could not be considered a "sham". The DoD/VA/Army studies should accurately be characterized as dose-response studies, not sham-controlled RCTs. In a Letter to the Editor in JAMA by noted Canadian Dr. Pierre Marois, he explains:
"We have much more knowledge about the physiology of hyperbaric therapy in neurological conditions (ref) and this should help us understand and accept the impact of small increases of pressure on brain function. By definition ‘sham’ is ‘something false or empty′. Hyperbaric treatments at 1.2 ATA substantially increase the amount of dissolved oxygen in the blood and simultaneously induce cascades of metabolic changes and genes activation. Therefore, the claimed sham treatment of Miller's study [and its predecessors] is not close to being a placebo. An increase of just 0.2 ATA is an effective treatment and is used to save lives in patients with mountain sickness. It has to be considered as a treatment arm. Always... If there were no preconceived ideas on the amount of pressure needed to induce a positive response on post-concussion patients, the only scientific conclusion we could draw from the significant results described in Miller's controlled study is that, even at small pressures HBOT seems to be effective. This could have a significant impact on the quality of life of thousands of military personnel."23
Army researchers have not sufficiently explained their reasoning and the science behind justifying a sham of air at 1.3 ATA, despite it being a contradiction in terms. Their lack of transparency around their sham plagues on-going and proposed research. A worldwide outpouring of challenges arose when the Wolf-Cifu study was published. Not all of the commentary saw its way into print,24 but the gist of challenges can be summarized: the claimed DoD/VA/Army definition of "sham" and even "hyperbaric gas" are both wrong. Continuing the canard that their sham is legitimate are peer-reviewers who do not challenge the fallacy. Extensive exposition of the laws of physics, bio-chemistry, and physiology was delivered by Prof. Philip James.25 Dr. Paul Harch summarizes the science: "A reconsideration of the science of hyperbaric therapy reveals that the study by Wolf and colleagues is neither a sham nor placebo-controlled study. Rather, it is a Phase II study of two composite doses of hyperbaric therapy that demonstrated significant improvements in PCS and PTSD symptoms at the 2.4 atmospheres absolute (ATA) pure oxygen dose as well as the low-pressure 1.3 ATA air/oxygen dose."26
Other significant research published during NBIRR includes findings of an army team investigating blast injury.31 Enormous resources have been expended seeing to differentiate between PTSD and TBI. Extreme confusion about diagnoses have resulted due to the difficulty in understanding invisible wounds and the lack of definitive diagnostics. We can say from our own experience in this study that most subjects who had been diagnosed with "only PTSD" upon careful history-taking could be assumed to have been subjected to blasts and probably had incurred mild to moderate TBIs. From the clinical standpoint, NBIRR did not differentiate between PTSD and TBI diagnoses; all subjects received the same treatment under the protocol. Yet the research on Blast is instructive for anyone seeking to conduct research on invisible wounds, particularly using HBO2. The Perl Team from Walter Reed explains: "Our findings suggest, for the first time, that there might be a predictable pattern of physical damage to human brain after blast exposure…Additionally, the neuroanatomical locations of the interface astroglial scarring seen in our study support the concept that persistent symptoms of blast-exposed individuals may correlate with damage to particular structures with potential interference or alteration of their functions. We anticipate reconsideration about pathophysiology underlying the neuropsychiatric sequelae that follow blast exposure and also innovative approaches to diagnosis and treatment."27
These "neuropsychiatric sequelae" are further linked to PTSD since recent data suggest an association between combat blast TBI and PTSD.28 These findings about blast are relevant for this study and further research into TBI/PTSD/PCS/PPCS since we are dealing with a complex of injuries and symptoms that currently do not have definitive diagnoses and markers. Current on-label and off-label interventions without the use of HBO2 tend to compartmentalize each symptom and work on any number of symptoms in isolation. Evidence that the VA is aiming at symptom identification and resolution on a symptom-by-symptom basis – as opposed to wholistic, integrated, patient-centered, precision medicine – can be found in the latest update to VA and DoD Clinical Practice Guideline for the Management of Concussion-Mild Traumatic Brain Injury.29 Evolving treatment protocols are turning toward isolating individual symptoms and treating those symptoms of brain injury as opposed to a focus on the cause – the underlying brain injury.30
SUBJECTS AND METHODS
NBIRR was a multi-center trial conducted under the auspices of the International Hyperbaric Medical Foundation. HBO2 administration was performed on a voluntary basis by each treating center. The protocol at each participating center was approved by the Western Institutional Review Board [WIRB® Protocol #20090761], each site had its own Principal Investigator, and all subjects provided written informed consent. No effort was made to exclude subjects based on the etiology of injury. A control non-treatment group was not included in this initial observational study; however, all participants in this report had mTBI with post-concussive symptoms and a history of at least 3 months of clinical non-improvement or deterioration at the time of enrollment.
Male and female subjects 18 to 65 years of age were eligible if they had a diagnosis of mTBI with post-concussive symptoms and/or PTSD. Inclusion criteria for subjects in this report were: any 18–65-year-old subject with a history of mild TBI a) with post-concussive symptoms or PTSD, b) a diagnosis of mild TBI with post-concussive symptoms and/or PTSD made by a neurologist or neuropsychologist, c) negative pregnancy test in females, and d) current symptoms or functional impairment attributable to TBI and/or PTSD.
Exclusion criteria were: a) pulmonary disease that precludes HBO2 administration, b) unstable medical conditions that are contraindicated in HBO2 administration, c) severe confinement anxiety, d) pregnancy, d) a neurological diagnoses other than TBI or PCS, e) participation in another experimental trial with active intervention, f) high probability of inability to complete the experimental protocol, g) insufficient mental or physical capacity to complete the required tests, h) pre- or post-TBI history of systemic illness with impact on central nervous system, i) pre-existing mental illness, and j) any pre-existing chronic infection not related to battlefield injuries or government service.
A new online data entry system (CareVectorTM) was created to deploy study Case Report Forms to a network of clinics participating in clinical research. The CareVectorTM Platform (CVPTM) is the repository for all data collected on individual patients in NBIRR. It compiled a web-enabled electronic research record from multiple data sources available from HBO2 sessions, tests, laboratory tests and clinician-patient interactions. The platform also allowed for oversight with a built-in auditor role to supports analysis and data and safety monitoring functions. Security procedures are a built-in aspect of the CVP, with multi-role and multi-site access-controls. To avoid any conflict or bias of analysis, the CVP did not process or analyze any data. All collected data were analyzed independently by the biostatistician.
The self-assessment measures were a "percent back to normal" assessment, the PHQ-15 (Patient Health Questionnaire-15), a measure of somatic symptoms associated with mental disorders, the PHQ-9 (Patient Health Questionnaire-9), a measure of depression symptoms, a quality of life assessment, and the Rivermead Post-concussion Symptoms Questionnaire.31
Subjects who met the inclusion criteria underwent a battery of pre-HBO2 administration evaluations and testing including medical history, neurological examination, Automated Neuropsychological Assessment Metrics (ANAM4TM), Central Nervous System Vital Signs® (CNSVS), and a variety of self-assessment tests. The neurocognitive test results form the basis of this report. No imaging studies were included in this protocol. A flow diagram of subject participation and inclusion in this report is shown in Figure 1.
Figure 1.
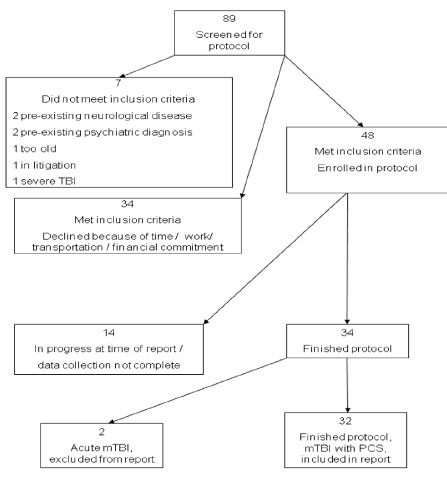
Flow diagram of study participants included in this report.
Note: mTBI: Mild traumatic brain injury; PCS: post-concussion syndrome.
ANAM4TM is a library of more than thirty computer-based test modules designed for a wide variety of clinical and research applications and is the direct outgrowth of more than twenty years of computer-based test development across all service branches within the Department of Defense.32 It is a neurocognitive assessment tool that can be used to identify changes in a service member's cognitive function and mood state as a result of some debilitating event. The ANAM4TM test battery used in this study has been tailored to provide an instrument that is sensitive to cognitive changes that often accompany mTBI.33,34 The ANAM4TM tests included the mood scores of sleepiness, vigor, restlessness, depression, anger, happiness, fatigue, and anxiety. The ANAM4TM neurocognitive measures were simple reaction time, code substitution – learning, procedural reaction time, mathematical processing, matching to sample, code substitution – delayed and simple reaction time (R), a measure of basic neural processing speed and efficiency. In order to minimize the effect of test-retest improvement the ANAM4TM test system automatically detects when an individual has been previously assessed and will iterate to a new stimulus set.35
CNSVSTM is a battery that evaluates verbal and visual memory, psychomotor speed, complex attention, reaction time, and cognitive flexibility through application of the verbal and visual memory test, finger tapping test, symbol digit coding, the Stroop test, the shifting attention test, and the continuous performance test.36 CNSVSTM was added as a secondary, objective neuropsychological assessment tool, and has been validated as a repetitive assessment measure for brain injury.37 The NBIRR study was designed at a time when ANAMTM and CNSVSTM were relatively non-controversial test instruments for capturing data about symptoms and monitoring progress toward end-points. They had the added advantage that they could be administered via computer over the worldwide web, and they were made available to the project for a reduced rate. Because this study was self-funded, we were without funds to perform brain imaging, though that would have been preferable. In addition to the subjective questionnaires and the scientifically-validated instruments of ANAMTM and CNSVSTM, objective brain scans are becoming standard instruments in civilian research.38 It would be useful in any further research that brain scans [functional MRI or Diffusion Tensor Imaging show great promise, though are expensive] are part of the protocol to avoid any misunderstandings about how HBO2 as the control is helping or hurting improvement in the health and well-being of subjects. HBO2 was delivered according to the 1.5 ATA protocol developed by Harch, Gottlieb, and Van Meter in the early 1990s based on the 1.5 ATA dose used by Neubauer in chronic brain injury.39 All subjects received 100% oxygen at 1.5 ATA in monoplace or multiplace chambers. Monoplace chambers were Sechrist 2500, Sechrist 3200, or Perry Sigma 40 monoplace chambers and 100% oxygen was delivered in the chamber ambient environment. The multiplace chamber was a 12-person Gulf Coast Hyperbarics chamber with oxygen delivered via hood or aviation non-rebreather mask.
Pressurization time was 3–7 minutes and decompression time was 3–7 minutes. The time at 1.5 ATA was 45 minutes for monoplace chambers and 50 minutes for the multiplace chamber. This difference was planned in order to give some equivalence to the amount of oxygen delivered in the two types of chambers since descent and ascent were conducted with 100% oxygen in the monoplace chambers and air in the multiplace chamber. After initial testing, the subjects were to receive 40 HBO2 and were tested again. If, in the opinion of the subject and the site principal investigator, maximum benefit was received evidenced by the initial improvement and then stabilization of symptoms without continued improvement, HBO2 administration was stopped at 40 HBO2 sessions. All subjects receiving 40 HBO2 sessions reported they had benefitted from the HBO2 administration. If possible, further benefit was anticipated, another 20 or 40 HBO2 sessions were administered and testing performed again when the HBO2 sessions were complete.
Statistical analysis was performed using R (Version 2.15.1; http://www.R-project.org). And post-HBO2 administration changes in ANAM Mood Scores, ANAM Cognitive Scores and CNSVSTM Cognitive Scores were compared using Paired t-test or Wilcoxon signed-rank test for not-normally distributed scores. Mean and standard deviation of the differences between the scores and its corresponding 95% confidence interval and P-value were reported. Differences in the neurocognitive scores between the groups were compared by independent t-test or Mann-Whitney U test for not normally distributed data. Average improvement in each subject's neurocognitive test scores were compared to number of HBO2 sessions received by correlation and regression analysis. Slopes, correlation coefficients (r and r2), and P-values were reported. Significance level was set at 0.05, and no adjustment for multiple endpoints was applied.
RESULTS
A total of 48 subjects with mTBI with post-concussive symptoms with or without PTSD were enrolled in the NBIRR study at 5 centers; 32 eventually completed the study. Twenty-nine subjects were males and three were females. Of the 32 subjects in this report, seven subjects (22%) were active duty military at the time of participation, 12 (37%) were veterans and 13 (41%) had no military service. Fifteen (47%) received their injury because of a blast and 17 (53%) were injured because of a blow (Table 1).
Table 1.
Study population characteristics
| Population characteristics | Value |
|---|---|
| Military status [n(%)] | |
| Active duty | 7 (22) |
| Veteran | 12 (37) |
| Civilian | 13 (41) |
| Etiology of injury [n(%)] | |
| Blast | 15 (47) |
| Blow | 17 (53) |
| Total | 32 (100) |
| Age at injury (years) | |
| Mean ± SD | 30.5±12.0 |
| Median (Range) | 28.5 (7–60) |
| Delay from injury to HBO2 Start (years) | |
| Mean ± SD | 9.5 ± 12.7 |
| Median (range) | 3.99 (0.36–45.6) |
| Duration of HBO2 (days) | |
| Mean ± SD | 114 ± 63 |
| Median (range) | 116 (26–304) |
| No. of HBO2 | |
| Mean ± SD | 55.8 ± 18.5 |
| Median (range) | 41 (35–82) |
| Diagnosis [n(%)] | |
| mTBI only | 25 (78) |
| mTBI + PTSD | 7 (22) |
Note: n = 32. HBO2: Hyperbaric oxygen; mTBI: mild traumatic brain injurt; PTSD: post traumatic stress disorder.
The delay from injury to protocol enrollment for the subject population was distributed from 0.37 to 46 years after injury. Two subjects could not remember their injury well enough to establish a clear date of injury (Figure 2). There were 25 potential outcome measures per subject at each time of measurement (pre-HBO2 and post-HBO2) calculated for this report. For each participant the measures were compared pre and post-HBO2 administration (Table 2). The results of the ANAMTM mood scores are expressed as subjective scales (0–100) and the objective neurocognitive screening measures are expressed as percentile placement compared to the peer populations (Figures 3 and 4).
Figure 2.
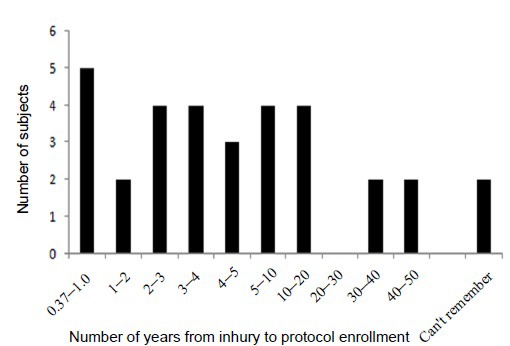
Distribution of subject population time from injury to protocol enrollment.
Table 2.
Outcome measures
| Measure | Number of outcomes |
|---|---|
| ANAM4TM mood scores | 8 |
| ANAM4TM neurocognitive tests | 7 |
| CNSVS neurocognitive tests | 10 |
| Total number of measures | 25 |
Note: CNSVS: Central Nervous System Vital Signs; ANAM4™: Automated Neuropsychological Assessment Metrics.
Figure 3.
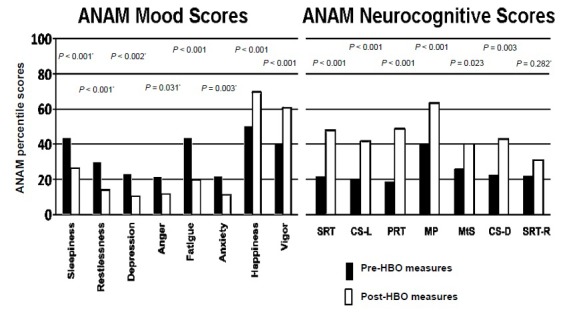
ANAM4™ Mood (left) & Neurocognitive (right) Test Scores.
Note: Average values from test subjects are displayed. Prior to each ANAM4™ assessment, subjects were asked to rate eight mood areas (left) before undertaking a battery of neurocognitive test (right). *nonparametric. SRT: Simple reaction time; CS-L: code substitutionlearning; PRT: procedural reaction time; MP: mathematical processing; MtS: matching to sample; CS-D: code substitutiondelayed; SRT-R: simple reaction time (R); ANAM4™: Automated Neuropsychological Assessment Metrics.
Figure 4.
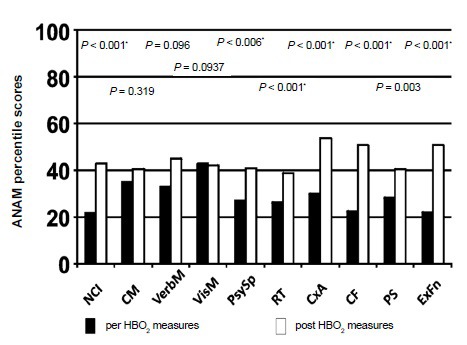
CNSVS Neurocognitive test scores.
Note: Average values from test subjects are displayed above. *denotes nonparametric. NCI: Neurocognitive index; CM: composite memory; VerbM: verbal memory; VisM: visual memory; PsySp: psychomotor speed. RT: reaction time; CxA: complex attention; CF: cognitive flexibility; PS: processing speed; ExFn: executive functioning; HBO2: hyperhabic oxygen; CNSVS: Central Nervous System Vital Signs; ANAM: Automated Neuropsychological Assessment Metrics.
The changes in subjects on active duty, veterans, or civilian status (no history of military service) were compared to each other as groups (active duty, veteran, civilian). In general, the active duty group showed more improvement than the veteran and civilian groups and the civilian group showed the least improvement (Table 3). In comparing subjects with blast injury (n = 15) to those receiving injury from a blow (n = 17) 4 measures showed post HBO2 administration differences between the two groups with the individuals with blast injury demonstrating more improvement. The measures showing significance were ANAM4TM Fatigue (Mean = –23.8 (percentile change), ± 25.1 (–23.8 ± 25.1), CI (confidence interval): –32.9 to –14.7, P = 0.034), ANAM4TM Code Substitution Learning (21.2 ± 27.2, CI: 11.2 – 31.2, P = 0.006), ANAM4TM Procedural reaction Time (30 ± 38, CI: 16 – 44, P < 0.001), and ANAM4TM simple reaction time (R) (8 ± 36, CI: –6 to 22, P = 0.025).
Table 3.
Changes in ANAM4™ test values from start to end of HBOT, within each Military Status (active duty/veteran/civilian), and for all subjects combined
| Active duty (n = 7) | Veteran (n = 12) | Civilian (n = 13) | Total (n = 32) | P value (ANOVA) | |
|---|---|---|---|---|---|
| Sleepiness | –1.86±1.46, P = 0.040 | –1.18±1.25, P = 0.022 | –0.69±1.25, P = 0.089 | –1.13±1.34, P < 0.001 | 0.156 |
| CI: –3.21 to –0.50 | CI: –2.02 to –0.34 | CI: –1.45 to 0.06 | CI: –1.62 to –0.64 | ||
| Vigor | 32.7±15.2, P = 0.001 | 27.4±23.9, P = 0.002 | 8.3±24.0, P = 0.236 | 20.8±24.2, P < 0.001 | 0.043 |
| CI: 18.7 to 46.7 | CI: 12.2 to 42.6 | CI: –6.2 to 22.8 | CI: 12.1 to 29.5 | ||
| Restlessness | –27.0±19.4, P = 0.016 | –18.3±27.0, P = 0.029 | –6.0±14.4, P = 0.182 | –15.2±21.9, P < 0.001 | 0.066 |
| CI: –44.9 to –9.1 | CI: –35.5 to –1.2 | CI: –14.7 to 2.7 | CI: –23.1 to –7.3 | ||
| Depression | –13.7±17.4, P = 0.058 | –18.7±27.6, P = 0.025 | –6.1±16.1, P = 0.184 | –12.5±21.5, P = 0.002 | 0.512 |
| CI: –29.8 to 2.4 | CI: –36.2 to –1.1 | CI: –15.8 to 3.7 | CI: –20.2 to –4.7 | ||
| Anger | –25.4±21.3, P = 0.058 | –9.3±27.4, P = 0.398 | –2.0±9.6, P = 0.720 | –9.8±21.7, P = 0.031 | 0.109 |
| CI: –45.1 to –5.8 | CI: –26.7 to 8.2 | CI: –7.8 to 3.8 | CI: –17.7 to –2.0 | ||
| Happiness | 25.0±25.7, P = 0.042 | 27.6±21.5, P < 0.001 | 8.5±20.5, P = 0.158 | 19.3±23.2, P < 0.001 | 0.089 |
| CI: 1.2 to 48.8 | CI: 13.9 to 41.3 | CI: –3.8 to 20.9 | CI: 10.9 to 27.6 | ||
| Fatigue | –35.9±19.1, P = 0.003 | –34.1±26.0, P < 0.001 | –7.8±19.1, P = 0.164 | –23.8±25.1, P < 0.001 | 0.008 |
| CI: –53.5 to –18.2 | CI: –50.6 to –17.5 | CI: –19.4 to 3.7 | CI: –32.9 to –14.7 | ||
| Anxiety | –16.7±17.8, P = 0.059 | –12.6±24.6, P = 0.052 | –5.4±17.8, P = 0.294 | –10.6±20.5, P = 0.003 | 0.342 |
| CI: –33.2 to –0.2 | CI: –28.2 to 3.0 | CI: –16.1 to 5.4 | CI: –17.9 to –3.2 | ||
| Simple | 64±30, P = 0.001 | 17±30, P = 0.068 | 13±19, P = 0.027 | 26±32, P < 0.001 | < 0.001 |
| reaction time | CI: 36 to 92 | CI: –2 to 36 | CI: 2 to 25 | CI: 14 to 38 | |
| Code substitution | 40.7±29.5, P = 0.011 | 16.8±27.4, P = 0.057 | 14.2±21.9, P = 0.047 | 21.2±27.2, P < 0.001 | 0.092 |
| learning | CI: 13.4 to 68.0 | CI: –0.6 to 34.3 | CI: 0.2 to 28.1 | CI: 11.2 to 31.2 | |
| Procedural | 67±18, P < 0.001 | 29±41, P = 0.033 | 10±30, P = 0.267 | 30±38, P < 0.001 | 0.004 |
| reaction time | CI: 50 to 84 | CI: 3 to 55 | CI: –9 to 29 | CI: 16 to 44 | |
| Mathematical | 37.3±31.8, P = 0.021 | 17.4±26.0, P = 0.041 | 21.5±20.5, P = 0.004 | 23.5±25.8, P < 0.001 | 0.262 |
| processing | CI: 7.8 to 66.7 | CI: 0.9 to 33.9 | CI: 8.5 to 34.5 | CI: 14.0 to 33.0 | |
| Matching to | 33±28, P = 0.021 | 6±31, P = 0.539 | 10±31, P = 0.296 | 13±31, P = 0.023 | 0.167 |
| sample | CI: 7 to 58 | CI: –14 to 25 | CI: –10 to 29 | CI: 2 to 25 | |
| Code substitution | 34±32, P = 0.029 | 17±29, P = 0.069 | 15±40, P = 0.220 | 20±34, P = 0.003 | 0.474 |
| delayed | CI: 5 to 63 | CI: –2 to 35 | CI: –10 to 41 | CI: 8 to 33 | |
| Simple | 73±14 (4), P = 0.098 | –10±29, P = 0.540 | 3±18, P = 0.755 | 8±36 (27), P = 0.282 | 0.007 |
| reaction time (R) | CI: 51 to 95 | CI: –29 to 10 | CI: –9 to 14 | CI: –6 to 22 |
Note: Final column indicates the significance of differences in ANAM4™ changes between the three groups. Values are expressed as mean change ± standard deviation of changes; confidence interval (CI) indicates the 95% CI around the mean change; P indicates the P value from a paired test for a significant mean within-group change. ANAM4™: Automated Neuropsychological Assessment Metrics; HBOT: hyperbaric oxygen therapy.
Age at the time of injury impacted improvement in CNSVSTM processing speed (slope = 0.642, P = 0.043, r = 0.445, r2 = 0.198), with earlier age at the time of injury resulting in greater test improvement after HBO2 administration. Age at the start of HBO2 administration affected improvement in ANAM4TM code substitution – learning (slope = –0.815, P = 0.017, r = –0.427. r2 = 0.182), and ANAM4TM procedural reaction time (slope = –1.567, P = 0.001, r = –0.584, r2 = 0.341) with earlier age at the start of HBO2 administration resulting in greater test improvement after HBO2 (Figure 5).
Figure 5.
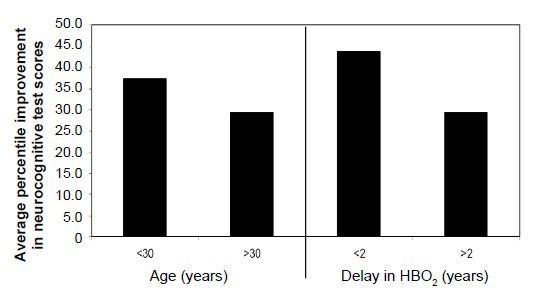
Effect of younger age at time of protocol enrollment and delay in HBO2 administration from time of injury on neurocognitive test score improvement.
Note: Neurocognitive scores are expressed as the sum of average improvement in tests administered. HBO2: Hyperbaric oxygen.
A delay in initiation of HBO2 from the time of injury affected four neurocognitive percentile scores – ANAM4TM code substitution – learning (slope = –0.0201, P = 0.008, r = –0.486, r2 = 0.286), ANAM4TM procedural reaction Time (slope = –0.02984, P = 0.004, r = –0.518, r2 = 0.269), ANAM4TM mathematical processing (slope = –0.01487, P = 0.037, r = –0.389, r2 = 0.152), ANAM4TM R (slope = –0.02398, P = 0.022, r = –0.455, r2 = 0.207), and CNSVS processing speed (slope = –0.01271, P = 0.019, r = –0.506, r2 = 0.256). A delay in HBO2 administration from the time of injury resulted in reduced improvement in test scores (Figure 5).
The trend toward improved neurocognitive test scores relative to number of HBO2 sessions received is reflected in Figure 6. The average neurocognitive scores improved approximately ½% with each HBO2 received (slope = 0.554, P = 0.038, r = 0.368, r2 = 0.136). The number of HBO2 received was associated with improvement in the percentile performance scores in 4 neurocognitive measures: ANAM4TM mathematical processing (slope = 0.782, P = 0.001, r = 0.562, r2 = 0.316), CNSVS psychomotor speed (slope = 0.509, P = 0.044, r = 0.424, r2 = 0.18), CNSVS reaction time (slope = 0.447, P =0.048, r = 0.417, r2 = 0.174), and CNS vs Executive Function (slope = 0.612, P =0.03, r = 0.453, r2 = 0.206). In each case a larger number of HBO2 received resulted in improved scores.
Figure 6.
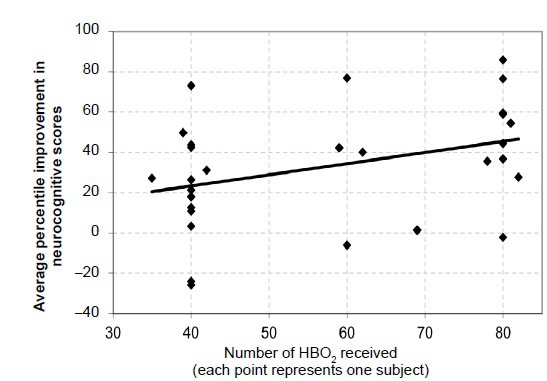
Average improvement in each subject's neurocognitive test scores bases on number of HBO2 sessions received.
Note: Each point in the graph represents one subject. The line through the points is a best-fit, linear regression (y=0.5539x + 1.1863) of individual subjects who have undergone 35 and up to 82 hyperbaric oxygen treatments. The trend line slope is 0.55; r = 0.3685, P = 0.038. HBO2: Hyperbaric oxygen.
Seven subjects (22%) had a diagnosis of PTSD in addition to mTBI with post-concussive symptoms. The subjects with a diagnosis of PTSD had more improvement in the ANAM4TM fatigue mood scale (mean change = –23.8 ± 25.1, CI: –32.9 to –14.7, P = 0.012), and the ANAM4TM Matching to Sample neurocognitive test (mean change = 13 ± 31, CI: 2 – 25, P = 0.028). A diagnosis of PTSD did not adversely affect any of the outcome measures. No adverse effects or complications were reported in any of the subjects enrolled in the NBIRR study and the administration of HBO2 at 1.5 AIAin this population of brain injury subjects was safe.
DISCUSSION
When the NBIRR observational study was initiated it was thought that it would be a means to gather evidence that could provide justification for and inform a much larger pivotal clinical trial. An observational study, while not the highest grade of evidence, is typically the first step in understanding a treatment when it is already in use using FDA-approved devices. Such a study provides clinical evidence that can establish the potential for and inform the design of a deeper, and more expensive, scientific investigation. NBIRR was begun with no outside funding. Throughout the course of the study, small donations and the patriotism of individual clinicians allowed the study to continue. The hope was that the network of clinics could treat some of the large number of active duty and veterans returning from war with post-concussive symptoms from mTBI when funding became available. The bulk of the study was done pro bono or at dramatically reduced costs by private HBO2 clinics. As the study progressed, funding was insufficient to continue. Additionally, to obtain an FDA study indication for HBO2 treatment (newly regulated as a drug by the FDA) for post concussive symptoms, a redesigned study with appropriate controls would be required. The preliminary results of the NBIRR study are potentially useful in designing an FDA sanctioned study seeking a new indication and they are presented here in an effort to speed the effort for interested researchers. This summary is a report on the 32 subjects with post-concussive symptoms who completed the protocol. At the time of the current data analysis the long-term follow-up was not available and is not part of this report. No adverse effects since conclusion of NBIRR have been reported. This report is offered in the expectation that the information learned can be put to use in an independent, well-designed, controlled clinical trial.
The DoD/VA/Army response to the need for effective treatments has been less than rapid. After several years ramping up for a study -- and after expenditure of many millions of dollars without start to a study -- the US Government commenced their own studies. Contemporaneously, the sense of urgency that NBIRR participants brought to the research was grounded in the nation's awakening to what even DoD called a suicide epidemic among service members. The rules for dealing with an epidemic are fairly straightforward: early detection, early response. In just the last few years, the US has confronted three potentially catastrophic epidemics: meningitis, Ebola and Zika. Over one billion dollars was spent on research, treatments and interventions that may or may not have contributed to the small number of infections and deaths. Yet the suicide epidemic and hundreds of thousands of veterans and active duty suffering from brain injuries, coupled with a suicide rate of 20 per day, for a total estimated at over 48,000, (nearly five combat divisions) has caused no sense of urgency and immediate use of alternative therapies that satisfy the criteria set by Doctors Without Borders during the last Ebola epidemic scare: "Even if the sample size is quite small and more research and analysis is needed, the enormity of the public health emergency should lead us to continue using this vaccine right now... Replication of a targeted approach focusing on those most at risk of infection should therefore happen immediately and we urge governments in affected countries to start using this vaccine as soon as they can within the framework of the existing trial."
The NBIRR study demonstrated that a protocol of 40-80 treatments, once a day, 5 days per week of 1.5 ATA, 60-minute HBO2 sessions (treatments) was safe in this cohort of subjects with mild blast and non-blast TBI with post-concussive symptoms with or without PTSD. The lack of complications in the study is consistent with traditional applications of HBO2.40 The once a day, 5 days per week schedule was well tolerated, at least to 80 sessions, in this study.
When the NBIRR study was designed, the researchers postulated that mTBI with post-concussive symptoms, and PTSD, were dynamic conditions capable of improvement years after diagnosis. For this reason, no upper limit on the number of years since injury was imposed on subjects. The results of the study demonstrated that improvement was possible years after injury, though not as great as for subjects who were treated sooner after injury.
The study also tested the new online data collection system and the research capabilities of a network of hyperbaric clinics. The online data entry system, the CareVectorTM Platform developed to support multicenter clinical observational studies, was successful in capturing patient, HBO2 administration, computer-generated tests, and case report data from multiple centers. Clinics, and the brain-injured and cognitively impaired subjects, were able to successfully manage the computer-based systems necessary to conduct this trial. This portends well for future larger studies of this type as well as other pilot trials with different brain-injured populations. Even though many of the mTBI subjects had lives in disarray, it was remarkable that they were able to commit such a large block of their time and resources to complete the study. It is also notable that each center paid all of their own administrative costs and Institutional Review Board fees as well as generally treating the subjects pro bono, demonstrating that small pilot HBO trials can be successfully conducted without outside funding.
The Hawthorne effect is a change in the outcome of an experiment affected through the inclusion in the experiment and thought to be influenced by the increased attention given to subjects and the knowledge among subjects that they are being studied.41 In any uncontrolled study placebo and Hawthorne effects cannot be excluded as the source of observed effects. Changes in outcome might be attributed, at least in part, to a placebo effect. The placebo effect in this type of study is a psychobiological phenomenon capable of altering the subject's brain and producing subjective improvement. The increased attention and schedule of appointments for subjects have the capability of altering subject perceptions of one's self and the added structure to personal schedules and commitment to study participation can be perceived as having therapeutic value.42 Placebo effects have the capability to change the brain and in neurobiology can be considered a form of treatment in some circumstances.43 Without proper controls, it is impossible to determine the cause of the improvement seen in the subjects whether it be from the Hawthorne effect, placebo effect, or hyperbaric oxygen, or some combination of the three.
When TBI patients have stabilized symptoms and have shown no improvement for several months or years, the likelihood of spontaneous improvement is low. A recent study at the University of Oklahoma Veterans Hospital in veterans of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) screening positive for chronic TBI indicated that "for all of the parameters measured, there was no difference in occurrence or intensity (of symptoms) between the subjects who were within 2 years of the TBI and those who suffered the TBI 3-8 years earlier."44 The Congressional Budget Office report on the Veterans Health Administration treatment of PTSD and TBI in recent combat veterans released in February 2012 showed that the health care costs of veterans with all types of TBI remained relatively constant and 67% of TBI patients, 76% of PTSD patients, and 96% of TBI/PTSD patients continued to use VA health care services after four years of treatment.45 Prior to enrollment in the study, the NBIRR subjects had failed to improve, sometimes for decades, and only improved after inclusion in the protocol.
The Samueli Institute was hired by the Army to "provide an independent, objective, and transparent analysis of the research conducted to date on HBO2 for TBI."46 They concluded, based on the DoD/VA/Army research invalidated by their sham that "HBOT does not work" but also stated that "HBOT is a healing environment." They also noted in Summary Conclusions that "improvements in outcomes .... cannot be ignored.... HBO may be of value and could benefit these patients [moderate-severe TBI] as a relatively safe adjunctive therapy if feasible."
Army and Samueli Institute researchers noted in an invited commentary that attention had to be paid to what they termed "the ritual" of hyperbarics."47 They went so far as to remind readers that routine post-concussion care may have negative effects that contribute to symptom persistence.48
We are aware of no other treatment demonstrating the degree of improvement for stable or deteriorating mTBI with post-concussive symptoms as seen in HBO2 studies conducted independent of DoD/VA/Army.49 The magnitude of these effects would have to rely on well-considered controls in a clinical study. Future studies should be designed to separate the possible Hawthorne and placebo effects from that experienced through administration of hyperbaric oxygen. One could argue that in the meantime, HBO2 should be considered as the safe and effective treatment that it is, and as an intervention to help overcome "iatrogenic effects that contribute to symptom persistence" in over 800,000 brain injured service members.
A diagnosis of PTSD did not appear to have a negative effect on outcomes and appeared to contribute to better cognitive improvement. For simplicity it would have been useful to exclude subjects with the diagnosis of only PTSD and evaluate these subjects in a separate study. Army research looking at the use of HBO2 for subjects with only PTSD is reportedly in pre-publication. Preliminary reporting on outcomes is that HBO2 is safe and efficacious.
A significant study was published in 2016 that throws a new light on the difficulty of differentiating between brain injuries caused by either PTSD or TBI.50 In what is being called a breakthrough study, Dr. Daniel P. Perl and his team at the Uniformed Services University of the Health Sciences in Bethesda, MO, USA [the medical school run by the Department of Defense], have found evidence of tissue damage caused by blasts alone, not by concussions or other injuries. The New York Times calls it the medical explanation for shell shock: preliminary proof of what medicine has been saying without proof for nearly 100 years -- blasts cause physical damage, and this physical damage leads to psychological problems, i.e., PTSD.51 The importance of this admission cannot be overstated: this is a DoD discovery with documented evidence that blast injury [improvised explosive devices, breeching, whether in training or combat, enemy and/or friendly fire] can lead directly to physical brain damage and the accompanying effects, many of which have been heretofore diagnosed as "only PTSD". We now know that TBI/PTSD is a type of nonhealing wound. And, it should be noted that HBOT is already approved for all other forms of non-healing wounds.
It is hoped that these results will prove useful in planning future randomized controlled studies of HBO2 for traumatic brain injury with post-concussive symptoms and post-traumatic stress disorder. Future randomized controlled studies should include imaging, objective neurocognitive tests as well as objective self-assessment measures. Objective measures of functionality in daily living would be useful; these might include employment status, earnings reports, education status, grade point averages, changes in marital status, medication records and changes, as well as medical visits and hospitalization for mTBI associated conditions. To allow time for subjects to demonstrate these results and to establish the durability of HBO2 associated improvement, future studies should incorporate long-term follow-up.
Based on clinical observations and multi-year experience, a few other suggestions should be made about the protocol for any further studies.
The study population should include only subjects who have proven neuroimaging abnormalities.
To the extent practicable, subjects should not live at or be treated at elevations that complicate analysis of the effects of HBO2.
Service members who participate should have guarantees that their compensation will not be reduced, or their service records used negatively if their medical condition is improved with the treatment. Too many arguments have been raised for years about the negative inducements voiced to subjects in prior government studies.
All drugs being ingested by subjects, as well as any and all additional therapies and interventions, should be carefully recorded throughout the entire study.
Funding should be provided for expanded end points: Brain perfusion MRI+DTI, brain SPECT and/or PET-CT, along with agreed-on computerized neuro-cognitive tests.
Additional end points should be considered: cost effectiveness, quality of life as reported by subjects, families and care givers, and documented sustainability of HBO2 treatment at six, twelve and twenty-four months.
SUMMARY
The HBO21.5 ATA protocol for mTBI with post-concussive symptoms with or without PTSD was safe and could be used in multiple centers. The neurocognitive test scores for subjects improved in 21 of 25 measures. Earlier HBO2 post-injury, younger age at time of injury and HBO2 administration, and 80 rather than 40 administrations of HBO2 were associated with greater improvement in neurocognitive scores. Similarly, active duty or veteran military status was associated with improved outcomes. Inclusion in the protocol with HBO2 administration was accompanied by reductions in symptoms and improvement in neurocognitive test scores even several years after sustaining mTBI. These results are consistent with the findings in other recent, much more well-funded peer-reviewed research which finds HBO2 both safe and effective. Figueroa and Wright summarize what many others have written over the last half52 decade: sufficient scientific evidence exists to approve HBOT use in mTBI and PTSD.
Note on the methodology and funding for this observational study
This study can be viewed as a citizen response in 2008 to the lack of urgency in DoD/VA/Army medical circles about treatments for the invisible wounds of war. It is a tribute to the clinics and researchers and volunteers who participated in this boot-strapped effort that the effort was successfully concluded with quality data and no adverse events. The results add to the evidence-based record speaking to the safety and efficacy of HBO2 for brain injuries. The participants take some pride in the fact that research funding has materialized and that data from this and government-funded studies – as opposed to editorial conclusions – demonstrate that all subjects receiving HBO2 improved.
Further research
The Act explicitly calls for the use of "real world evidence" instead of just hard clinical trial evidence when weighing the approval of existing drugs for new uses. Oxygen certainly qualifies. Due to the Act, for example, the FDA now must consider "patient experience" and anecdotal data in its review process. The FDA must also expand its programs for expedited approval of breakthrough medical technologies for patients with life-threatening diseases that have limited treatment options. TBI certainly qualifies. Further, the Act in general calls for priority review for breakthrough devices that provide for more effective treatment of life-threatening or irreversibly debilitating human diseases or conditions. TBI and the suicide epidemic certainly qualify for both research and expedited review of the data from a device like the combination drug/device, HBOT. The Government is required to establish a program to provide priority review of research and data for devices that: represent breakthrough technologies; for which no approved alternatives exist; offer significant advantages over existing approved or cleared alternatives, including the potential to, compared to existing approved or cleared alternatives, reduce or eliminate the need for hospitalization, improve patient quality of life, facilitate patients’ ability to manage their own care or establish long-term clinical efficiencies; or when the availability of which is in the best interest of patients. The Act requires that steps are taken to ensure that the design of clinical trials is as efficient as practicable, such as through adoption of shorter or smaller clinical trials, application of surrogate endpoints, and use of adaptive trial designs and Bayesian statistics, to the extent scientifically appropriate. HBOT under NBIRR and the contemplated follow-on use of alternative therapies in combination with HBOT certainly fall within the ambit of research and treatment contemplated in the Act.
Acknowledgements
The contributions of Drs. Paul Harch and James Wright, MD, along with Steve Reimers, MS are gratefully acknowledged. Karen Lawson, MD, ABIHM, NBC-HWC, Paul Rock, PhD, DO, Latisha Smith, MD, Julie Stapleton, MD, Enrico Versace, MD, Ladonna Lacey BS, Lisa Terry, MS, Gayle Link, RN, Ryan Fulmer, CHT, Eddie Gomez, CHT, Michelle Potpan, CHT, Brian Wolfe, CHT, Shayne Harmsen, BS, Franklin Brightwater, CHT and Dr. Xavier Figueroa were instrumental in operations, treatments and data collection. Special mention belongs to John C. Pezzullo, PhD, Georgetown University Medical Center, who died before publication. He was instrumental in statistical analysis of data.
Footnotes
Funding: The study was funded through pro-bono clinical care provided by the investigators and involved clinics, augmented by philanthropic resources of the International Hyperbaric Medical Foundation and multiple community hyperbaric clinics.
Conflicts of interest
Drs. Beckman and Mozayeni have an interest in CareVector LLC, the developer of the case report form data collection software used to support this study provided pro bono to support the study. The other authors declare no conflict of interest.
Financial support
The study was funded through pro-bono clinical care provided by the investigators and involved clinics, augmented by philanthropic resources of the International Hyperbaric Medical Foundation and multiple community hyperbaric clinics. Pro-bono clinical research has an important role in orphaned conditions such as mild traumatic brain injury where no grant money is available, and no pharmaceuticals or devices are commercially labeled for treatment.
Institutional review board statement
The study was approved by the ethics review committee of the Western Institutional Review Board (WIRB® Protocol #20090761).
Declaration of patient consent
The authors certify that they have obtained patient consent forms. In the form, patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement
This study follows the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Biostatistics statement
The statistical methods of this study were reviewed by the biostatistician of Oklahoma State University Center for Aerospace & Hyperbaric Medicine, Tulsa, OK, USA.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices). Study protocol and informed consent form will be available immediately following publication, without end date. Results will be disseminated through presentations at scientific meetings and/or by publication in a peer-reviewed journal. Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
REFERENCES
- 1.James Philip B. Oxygen and the Brain: The Journey of Our Lifetime. North Palm Beach, FL: Best Publishing Co; 2014. [Google Scholar]
- 2.Weaver LK. Carbon Monoxide poisoning, Neurological Decompression Sickness (Navy Dive Table 6A), Intercranial Abscess, Acute Hearing Loss, Radiation Necrosis of the Brain, compromised flaps and graphs, and arterial insufficiency. 13th edition. Durham, NC: Undersea and Hyperbaric Medical Society; 2014. Hyperbaric Oxygen Therapy: Indications. [Google Scholar]
- 3.Jain, KK . The Textbook of Hyperbaric Medicine, Fifth edition. Cambridge, MA: Hogrefe & Huber Publishers; 2009. X. [Google Scholar]
- 4.Golden ZL, Neubauer R, Golden CJ, Greene L, Marsh J, Mleko A. Improvement in cerebral metabolism in chronic brain injury after hyperbaric oxygen therapy. Int J Neurosci. 2002;112:119–131. doi: 10.1080/00207450212027. [DOI] [PubMed] [Google Scholar]
- 5.Hardy P, Johnston KM, De Beaumont L, et al. Pilot case study of the therapeutic potential of hyperbaric oxygen therapy on chronic brain injury. J Neurol Sci. 2007;253:94–105. doi: 10.1016/j.jns.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer RA, Gottlieb SF, Pevsner NH. Hyperbaric oxygen for treatment of closed head injury. South Med J. 1994;87:933–936. doi: 10.1097/00007611-199409000-00015. Neubauer RA, Gottlieb SF. Hyperbaric oxygen for brain injury. J Neurosurg. 1993;78:687-688. [DOI] [PubMed] [Google Scholar]
- 7.Golden Z, Golden CJ, Neubauer RA. Improving neuropsychological function after chronic brain injury with hyperbaric oxygen. Disabil Rehabil. 2006;28:1379–1386. doi: 10.1080/09638280600638364. [DOI] [PubMed] [Google Scholar]
- 8.Wright JK, Zant E, Groom K, Schlegel RE, Gilliland K. Case report: treatment of mild traumatic brain injury with hyperbaric oxygen. Undersea Hyperb Med. 2009;36:391–399. [PubMed] [Google Scholar]
- 9.H. R. 6, 114th Congress, 1st Session. The 21st Century Cures Act. Signed into law December 13, 2016. Washington DC: Government Printing Office; 2016. To accelerate the discovery, development, and delivery of 21st century cures, and for other purposes. [Google Scholar]
- 10.Perrins DJ, James PB. Hyperbaric oxygen therapy and multiple sclerosis. Undersea Hyperb Med. 2002;29:236–238. [PubMed] [Google Scholar]
- 11.Adamides AA, Winter CD, Lewis PM, Cooper DJ, Kossmann T, Rosenfeld JV. Current controversies in the management of patients with severe traumatic brain injury. ANZ J Surg. 2006;76:163–174. doi: 10.1111/j.1445-2197.2006.03674.x. [DOI] [PubMed] [Google Scholar]
- 12.Washington, DC: FDA Consumer Health Information, U.S. Food and Drug Administration, August 2013; Hyperbaric Oxygen Therapy: Don’t Be Misled. [Google Scholar]
- 13.Smith G, Sharp GR. Treatment of carbon-monoxide poisoning with oxygen under pressure. Lancet. 1960;2:905. [Google Scholar]
- 14.Sluiter ME. The treatment of carbon monoxide poisoning by the administration of oxygen at high atmospheric pressure. Proc R Soc Med. 1963;56:1002–1008. doi: 10.1177/003591576305601129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duff JH, Shibata HR, Vanschaik L, Usher R, Wigmore RA, MacLean LD. Hyperbaric oxygen: a review of treatment in eighty-three patients. Can Med Assoc J. 1967;97:510–515. [PMC free article] [PubMed] [Google Scholar]
- 16.Hart GB, Thompson RE. The treatment of cerebral ischemia with hyperbaric oxygen (OHP) Stroke. 1971;2:247–250. doi: 10.1161/01.str.2.3.247. [DOI] [PubMed] [Google Scholar]
- 17.Neubauer RA, James P. Cerebral oxygenation and the recoverable brain. Neurol Res. 1998;20:S33–S36. doi: 10.1080/01616412.1998.11740606. [DOI] [PubMed] [Google Scholar]
- 18.Harch PG, Kriedt C, Van Meter KW, Sutherland RJ. Hyperbaric oxygen therapy improves spatial learning and memory in a rat model of chronic traumatic brain injury. Brain Res. 2007;1174:120–129. doi: 10.1016/j.brainres.2007.06.105. [DOI] [PubMed] [Google Scholar]
- 19.Paris JJ, Schreiber MD, Reardon FE. Hyperbaric oxygen therapy for a neurologically devastated child: whose decision is it? J Perinatol. 2003;23:250–253. doi: 10.1038/sj.jp.7210889. [DOI] [PubMed] [Google Scholar]
- 20.Golden Z, Golden CJ, Neubauer RA. Improving neuropsychological function after chronic brain injury with hyperbaric oxygen. Disabil Rehabil. 2006;28:1379–1386. doi: 10.1080/09638280600638364. [DOI] [PubMed] [Google Scholar]
- 21.Washington, DC: Institute of Medicine; 2014. Jun, Institute of Medicine of the National Academies, Treatment for Posttraumatic Stress Disorder in Military and Veteran Populations: Final Assessment. [PubMed] [Google Scholar]
- 22.Hoge CW, Jonas WB. The ritual of hyperbaric oxygen and lessons for the treatment of persistent postconcussion v symptoms in military personnel. JAMA Intern Med. 2014;175:53–54. doi: 10.1001/jamainternmed.2014.3375. [DOI] [PubMed] [Google Scholar]
- 23.Wolf G, Cifu D, Baugh L, Carne W, Profenna L. The effect of hyperbaric oxygen on symptoms after mild traumatic brain injury. J Neurotrauma. 2012;29:2606–2612. doi: 10.1089/neu.2012.2549. [DOI] [PubMed] [Google Scholar]
- 24.Collet JP, Vanasse M, Marois P, et al. Hyperbaric oxygen for children with cerebral palsy: a randomised multicentre trial. Lancet. 2001;357:582–586. doi: 10.1016/s0140-6736(00)04054-x. [DOI] [PubMed] [Google Scholar]
- 25.Harch PG. Hyperbaric oxygen therapy for post-concussion syndrome: contradictory conclusions from a study mischaracterized as sham-controlled. J Neurotrauma. 2013;30:1995–1999. doi: 10.1089/neu.2012.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collet JP, Vanasse M, Marois P, et al. Hyperbaric oxygen for children with cerebral palsy: a randomised multicentre trial. Lancet. 2001;357:582–586. doi: 10.1016/s0140-6736(00)04054-x. [DOI] [PubMed] [Google Scholar]
- 27.Harch PG. Letters to the Editor. Journal of Neurotrauma. Hyperbaric Oxygen Therapy for Post-Concussion Syndrome: Contradictory Conclusions From a Study Mischaracterized as Sham-Controlled. J Neurotrauma. 2013;30:1995–1999. doi: 10.1089/neu.2012.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shively SB, Horkayne-Szakaly I, Jones RV, Kelly JP, Armstrong RC, Perl DP. Characterisation of interface astroglial scarring in the human brain after blast exposure: a post-mortem case series. Lancet Neurol. 2016;15:944–953. doi: 10.1016/S1474-4422(16)30057-6. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld JV, McFarlane AC, Bragge P, Armonda RA, Grimes JB, Ling GS. Blast-related traumatic brain injury. Lancet Neurol. 2013;12:882–893. doi: 10.1016/S1474-4422(13)70161-3. [DOI] [PubMed] [Google Scholar]
- 30.Grammer GG, DeGraba TJ, Picon LM. va/dod clinical practice guideline for the management of concussion-mild traumatic brain injury, 2016 update. VA HSR&D Cyberseminars, September 29, 2016 [Google Scholar]
- 31.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242:587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- 33.Vincent AS, Bleiberg J, Yan S, et al. Reference data from the Automated Neuropsychological Assessment Metrics for use in traumatic brain injury in an active duty military sample. Mil Med. 2008;173:836–852. doi: 10.7205/milmed.173.9.836. [DOI] [PubMed] [Google Scholar]
- 34.Cernich A, Reeves D, Sun W, Bleiberg J. Automated Neuropsychological Assessment Metrics Sports Medicine Battery. Arch Clin Neuropsychol. 2007;22:S101–114. doi: 10.1016/j.acn.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Segalowitz SJ, Mahaney P, Santesso DL, MacGregor L, Dywan J, Willer B. Retest reliability in adolescents of a computerized neuropsychological battery used to assess recovery from concussion. Neuro Rehabilitation. 2007;22:243–251. [PubMed] [Google Scholar]
- 36.Johnson DR, Vincent AS, Johnson AE, Gilliland K, Schlegel RE. Reliability and construct validity of the Automated Neuropsychological Assessment Metrics (ANAM) mood scale. Arch Clin Neuropsychol. 2008;23:73–85. doi: 10.1016/j.acn.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Gualtieri CT, Johnson LG. A computerized test battery sensitive to mild and severe brain injury. Medscape J Med. 2008;10:90. [PMC free article] [PubMed] [Google Scholar]
- 38.Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006;21:623–643. doi: 10.1016/j.acn.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Neubauer RA, Gottlieb SF, Kagan RL. Enhancing "idling" neurons. Lancet. 1990;35:542. doi: 10.1016/0140-6736(90)90777-3. [DOI] [PubMed] [Google Scholar]
- 40.Clark J. Gesell LB. Hyperbaric Oxygen Therapy Indications, 12th ed. Durham, NC: Undersea and Hyperbaric Medical Society; 2009. Side Effects; pp. 215–220. [Google Scholar]
- 41.Parsons HM. What Happened at Hawthorne?: New evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science. 1974;183:922–932. doi: 10.1126/science.183.4128.922. [DOI] [PubMed] [Google Scholar]
- 42.Miller FG, Colloca L, Kaptchuk TJ. The placebo effect: illness and interpersonal healing. Perspect Biol Med. 2009;52:518–539. doi: 10.1353/pbm.0.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benedetti F, Carlino E, Pollo A. How placebos change the patient's brain. Neuropsychopharmacology. 2011;36:339–354. doi: 10.1038/npp.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Couch JR, Stewart KE, Wisdom PJ. Persistence of symptoms of post-concussion syndrome (PCSS) for up to 8 years in veterans of OEF/OIF campaigns of the wars in Iraq and Afghanistan. Headache. 2012;52:907. [Google Scholar]
- 45.Washington, DC: Congressional Budget Office, Pub No. 4097; 2012. The Veterans Health Administration's Treatment of PTSD and Traumatic Brain Injury Among Recent Combat Veterans. [Google Scholar]
- 46.Samueli Institute. "Is Hyperbaric Oxygen Therapy Effective for Traumatic Brain Injury? Preliminary Report.". Prepared for the Hyperbaric Oxygen Research Program, USAMRMC, USAMMDA. 2015 Feb 18; [Google Scholar]
- 47.Miller RS, Weaver LK, Bahraini N, et al. Effects of hyperbaric oxygen on symptoms and quality of life among service members with persistent postconcussion symptoms: a randomized clinical trial. JAMA Intern Med. 2015;175:43–52. doi: 10.1001/jamainternmed.2014.5479. [DOI] [PubMed] [Google Scholar]
- 48.Hoge CW, Jonas WB. The ritual of hyperbaric oxygen and lessons for the treatment of persistent postconcussion symptoms in military personnel. JAMA Intern Med. 2014;175:53–54. doi: 10.1001/jamainternmed.2014.3375. [DOI] [PubMed] [Google Scholar]
- 49.Harch PG, Andrews SR, Fogarty EF, Lucarini J, Van Meter KW. Case control study: hyperbaric oxygen treatment of mild traumatic brain injury persistent post-concussion syndrome and post-traumatic stress disorder. Med Gas Res. 2017;7:156–174. doi: 10.4103/2045-9912.215745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shively SB, Horkayne-Szakaly I, Jones RV2, Kelly JP, Armstrong RC, Perl DP. Characterisation of interface astroglial scarring in the human brain after blast exposure: a post-mortem case series. Lancet Neurol. 2016;15:944–953. doi: 10.1016/S1474-4422(16)30057-6. [DOI] [PubMed] [Google Scholar]
- 51.Robert F. Worth. "What if PTSD is More Physical Than Psychological?". The New York Times Magazine. 2016 Jun 10; http://nyti.ms/1TYYp6U . [Google Scholar]
- 52.Figueroa XA, Wright JK. Hyperbaric oxygen: B-level evidence in mild traumatic brain injury clinical trials. Neurology. 2016;87:1400–1406. doi: 10.1212/WNL.0000000000003146. [DOI] [PubMed] [Google Scholar]


