Abstract
Background:
Androgenetic alopecia (AGA) is the most common cause of hair loss in men with limited treatment options. Platelet-rich plasma (PRP) therapy is one of the newer treatment options in the management of AGA which has shown promising results.
Aims and Objectives:
This study was aimed at comparing the clinical efficacy of PRP therapy with minoxidil therapy.
Materials and Methods:
In the study, patients were randomized into two groups – Group A (given PRP therapy) and Group B (given minoxidil therapy). Both groups were followed up over a period of 6 months, and final analysis was done with the help of global photography, hair pull test, standardized hair growth questionnaire, patient satisfaction score; in addition, a comparison of platelet counts in PRP was done, to know that if a clinical correlation exists between platelet concentration and clinical improvement. A total of 40 patients clinically diagnosed with AGA were enrolled in the study with 20 patients in each group. Four patients from Group A (PRP) and six patients from Group B (minoxidil) could not complete the treatment for 6 months and were eventually excluded.
Results:
At the end of 6 months, 30 patients were evaluated to compare the efficacy of intradermal PRP and topical minoxidil therapy. On global photography, Group A (PRP) was found to have a comparatively better outcome than Group B (minoxidil). In hair pull test, hair growth questionnaire, and patient satisfaction score, Group A was found to be better than Group B. Mean platelet count at baseline was 3.07 ± 0.5 lac/mm, 3 while platelet count in final PRP prepared was 12.4 ± 1.7 lac/mm, and patients with a higher platelet count in PRP had a much better clinical improvement compared to patients with a low platelet count in PRP. Side effects with PRP therapy were minimal with better results which may improve the compliance of the patient.
Conclusion:
PRP therapy can be a valuable alternative to topical minoxidil therapy in the treatment of AGA.
Key words: Androgenetic alopecia, minoxidil, platelet-rich plasma
INTRODUCTION
Androgenetic alopecia (AGA) is a hereditary and androgen-dependent dermatological disorder characterized by miniaturization of scalp hair in a defined pattern.[1] There is an alteration in the hair cycle dynamics leading to vellus transformation of terminal hair follicles.[2] Due to the limited treatment options, it is a growing concern for dermatologist worldwide.
The US Food and Drug Administration (FDA)-approved medical treatment options for the management of AGA are topical minoxidil and oral finasteride. Various other treatments have been tried beside FDA-approved options.[3] Most of the existing treatment options are relatively slow to act because of which there has been a continuous search for newer modality of treatment among which platelet-rich plasma (PRP) therapy has shown promising results. PRP is autologous preparation of platelets in concentrated plasma.[4] It contains more than 20 growth factors (GF), of which most important GFs include platelet-derived GFs, transforming GF-ß, vascular endothelial GF, and insulin-like GF-1 along with their isoforms.[4] In AGA, PRP induces differentiation of stem cells, prolongs survival of dermal papilla cells, prolongs anagen phase of hair cycle, and increases perifollicular vascular plexus by multiple mechanisms through various GFs.[5,6]
This study was aimed at comparing the clinical efficacy of PRP therapy and minoxidil therapy which may help us to know that which is more efficacious, acceptable, and safe form of therapy in the management of AGA.
MATERIALS AND METHODS
The study was conducted over 1 year with effect from July 1, 2016 to June 30, 2017. This prospective study was aimed at comparing the clinical efficacy of intradermal PRP and topical minoxidil therapy for the treatment of AGA patients attending the outdoor patient Department of Dermatology, Venereology, and Leprosy, Indira Gandhi Medical College, Shimla (H. P).
A detailed history was taken to rule out other causes of hair loss such as telogen effluvium, history of any drugs, or history of any systemic disease. The patients were asked about lifestyle-related factors such as smoking and ultraviolet exposure which can aggravate AGA. Patients were asked for family history of AGA. A thorough clinical examination was performed in eligible patients to rule out any local dermatological disorder of the scalp, and a diagnosis of AGA was made; grade of AGA was assigned as per the Modified Norwood–Hamilton criteria[2] [Annexure I].
Patients were counseled regarding the benefits, side effects, and limitations of both the treatment modalities. An informed written consent was then obtained after explaining the procedure. Enrolled patients were randomized into two groups – Groups A and B using sealed opaque envelope which contained a computer-generated random number. Group A underwent monthly injections of PRP for 4 months while Group B was given treatment with topical minoxidil therapy alone which was applied as 1 ml twice daily for 6 months.
Inclusion and exclusion criteria of PRP and minoxidil therapy are mentioned below (Table 1 and Table 2).
Table 1.
Inclusion and Exclusion criteria for PRP therapy
| PRP Therapy | |
|---|---|
| Inclusion Criteria | Exclusion Criteria |
| Age 20 to 49 years | Dermatological disease of the scalp |
| Patient with AGA grade I-V of modified Hamilton-Norwood or grade I-II of Ludwig scale | Patient who has tendency to form keloid |
| Platelet count more than or equal to 1.5 lacs/µl | Patient who is taking finasteride for the treatment of AGA |
| Person who has not taken medical treatment for AGA in any form or took treatment for less than 6 months | |
Table 2.
Inclusion and Exclusion criteria for Minoxidil therapy
| Minoxidil Therapy | |
|---|---|
| Inclusion criteria | Exclusion criteria |
| Age 20 to 49 years | Patient who is taking finasteride or other medications for the treatment of AGA |
| Patient with AGA grade I-V of modified Hamilton-Norwood or grade I-II of Ludwig scale | Dermatological disease of scalp |
| Patient with a tendency to form keloids and cannot be given PRP | Pregnancy or lactation |
| Patient of platelet function disorder or on anticoagulation therapy | History of use of PRP in the past |
| Person who has not taken medical treatment for AGA in any form or took treatment for less than 6 months | |
Groups A and B were assessed with the help of global photography,[3] hair pull test,[7] patient standardized hair growth questionnaire [Annexure II (1.2MB, tif) ],[8] patient satisfaction score,[9] and platelet count in PRP. In global photography, a baseline and post treatment photographs at the end of the study period were taken and reviewed by blinded evaluator. Multiple images were taken to cover all areas of the scalp. Four specific views particularly focused were the vertex, mid-pattern, frontal, and temporal views. Hair pull test was done to assess the severity of disease at the start of treatment and then at 6 months. Patients were advised to withhold shampooing for 24 h before a hair pull test. In the hair pull test, about 60 hairs were grasped between the thumb and the index and middle fingers. The hairs were then gently but firmly pulled. More than six hairs or 10% of the total hairs obtained was taken as “positive” hair pull test while six or less hairs or <10% of the total hairs was taken as a “negative” hair pull test. Furthermore, patients assessed their scalp hair using a validated, self-administered hair growth questionnaire developed by Barber et al.[8] which included four questions in the patient's language on treatment efficacy and three questions on satisfaction with appearance. Each question was found to have a statistical significance in clinical trials in terms of patient perception of hair loss, satisfaction with hair appearance, and hair counts. Thus, it had certain reliability in monitoring response to treatment. However, we finally included only 6 questions out of total 7, omitting the second question of the questionnaire as it was difficult to be interpreted by most of our patients which could possibly result in interpretation error. Patient satisfaction score: patients were asked to interpret their satisfaction with either of the treatment provided at the end of treatment on a scale of 1–10. The mean of score was calculated in either of the group, and results were then statistically analyzed. Platelet count in PRP: final platelet count in PRP prepared was also calculated before injecting. The mean platelet count of four sessions of PRP was calculated in each patient in Group A to evaluate any correlation between platelet count and response to treatment.
Patient standardized hair growth questionnaire
Method to prepare PRP: manual double-spin technique was used. About 25–35 ml of venous blood was drawn into a tube-containing anticoagulant sodium citrate, to avoid platelets activation and degranulation. Blood was processed for the first centrifugation (soft spin) at approximately 1500 rpm for 5 min, which separated blood into three layers, namely, bottom-most red blood cell (RBC) layer (around 55% of total volume), topmost acellular plasma layer (platelet poor plasma [PPP]) which is around 40% of total volume, and an intermediate PRP layer (around 5% of total volume) called the buffy coat [Figure 1]. Using a sterile syringe PPP, PRP and some RBCs were transferred into another tube without an anticoagulant. This tube underwent a second centrifugation, which was longer and faster than the first spin (hard spin). Hard spin was performed at around 2500 rpm for 15 min. This allowed the platelets (PRP) to settle at the bottom of the tube with a very few RBCs. PPP (80% of the volume) was formed at the top [Figure 2]. Most of the PPP was removed with a syringe and discarded, and the remaining PRP was shaken well and platelet count in PRP was performed using automated device. The PRP formed was collected into an insulin syringe, already containing calcium gluconate which acted as an activator by nullifying action of anticoagulant [Figure 3]. Ratio of calcium gluconate and PRP in insulin syringe was 1:9. PRP thus formed was injected intradermally in a dose of 0.1–0.2 ml per injection approximately 1 cm apart in interfollicular areas, at a monthly interval for 4 months [Figure 4].
Figure 1.

After soft spin
Figure 2.
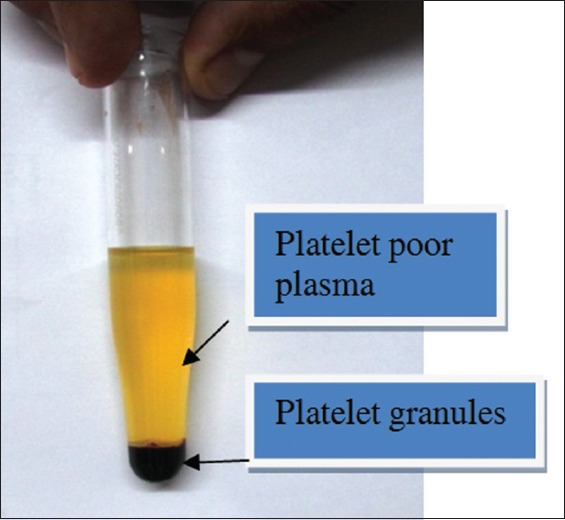
After hard spin
Figure 3.

Final platelet-rich plasma prepared
Figure 4.
After injecting platelet-rich plasma
In Group B, the patient was advised topical minoxidil in a concentration of 5% for men in a dose of 1 ml which has to be applied twice daily.
Patients in Group A were followed up at every month for 6 months, while patients in Group B were followed up at every 3 months for 6 months. The improvement from either of the groups was assessed by global photography, hair pull test, patient standardized hair growth questionnaire, and patient satisfaction score. Quantitative variables were analyzed by mean ± standard deviation, using Epi-info version 7 (Developer: Centre of Disease control and prevention, Atlanta, Georgia, USA) and unpaired t-test was applied. P < 0.05 was considered as statistically significant.
RESULTS
A total of 40 male suffering from AGA were enrolled in our study with 20 patients in each group. Four patients from Group A left the treatment in between because of the pain at injection site during the procedure while six patients from Group B left the treatment in between because they could not find any results even at 3–4 months of treatment. Thus, four patients from Group A and six patients from Group B were excluded from the study [Figure 5].
Figure 5.
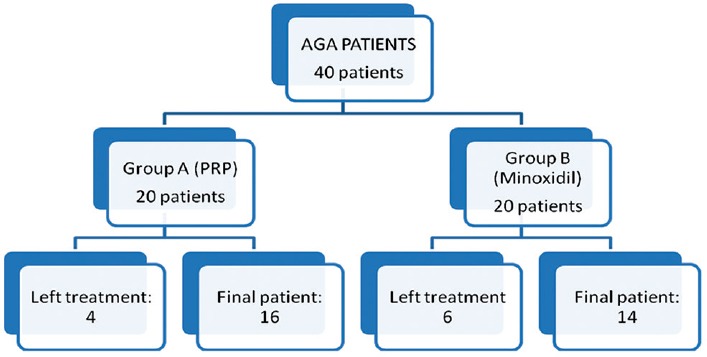
Patient distribution
Patient's demographic and hair loss features at baseline are shown in Table 3. The treatment groups were not significantly different at baseline with respect to the age of the patients, the age of onset of hair loss, grade of hair loss, and family history.
Table 3.
Characteristics of all patients in the study
| GROUP A [PRP] | GROUP B [MINOXIDIL] | |
|---|---|---|
| Number of Pts | 16 | 14 |
| Age (mean±SD) | 25.7±3.8 | 25.07±4.5 |
| Age of onset of AGA (mean±SD) | 21.3±1.7 | 21.1±3.3 |
| Family H/O AGA | 11 | 10 |
Patients were divided from Grade I-V as per the modified Norwood–Hamilton scale. Most of the patients were classified into Grades II and IV in either of the group with not much significant difference among the two groups [Table 4].
Table 4.
Grade of AGA (As per modified Norwood-Hamilton scale)
| Grade | Group A [PRP] | Group B [Minoxidil] |
|---|---|---|
| Grade I | 0 | 0 |
| Grade II | 6 | 7 |
| Grade III | 3 | 0 |
| Grade IV | 7 | 6 |
| Grade V | 0 | 1 |
| Total | 16 | 14 |
Global photography
-
Group A was found to have a comparatively better outcome than Group B
Figure 6.
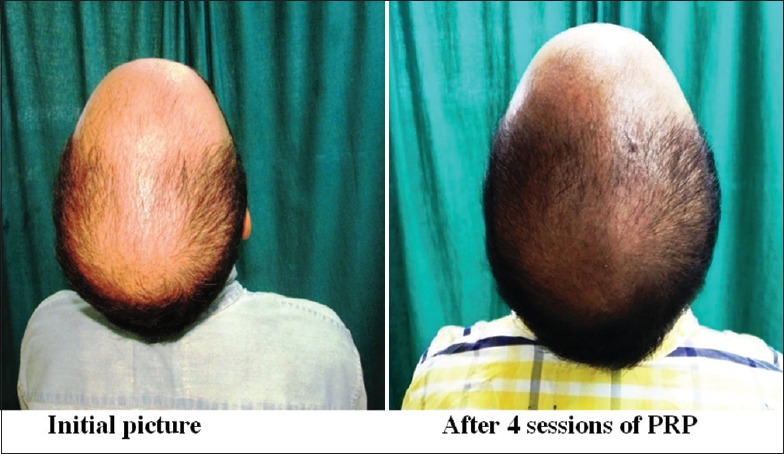
Group A: Patient 1
Figure 9.
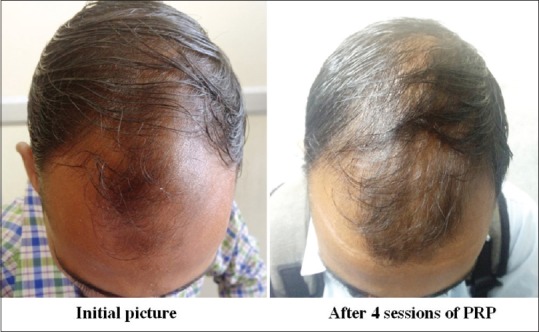
Group A: Patient 4
Figure 10.
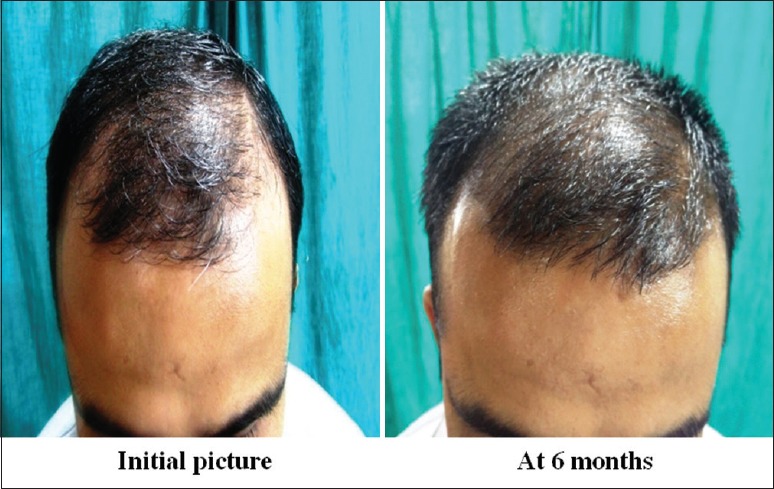
Group B: Patient 1 (frontal view)
Figure 12.
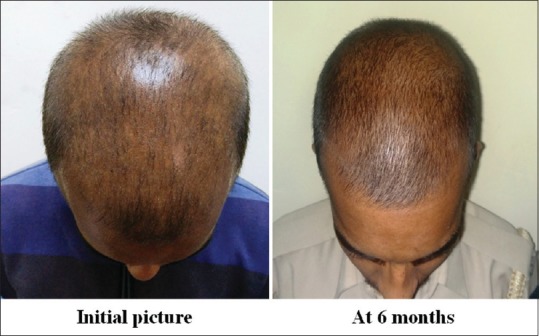
Group B: Patient 2
Figure 7.
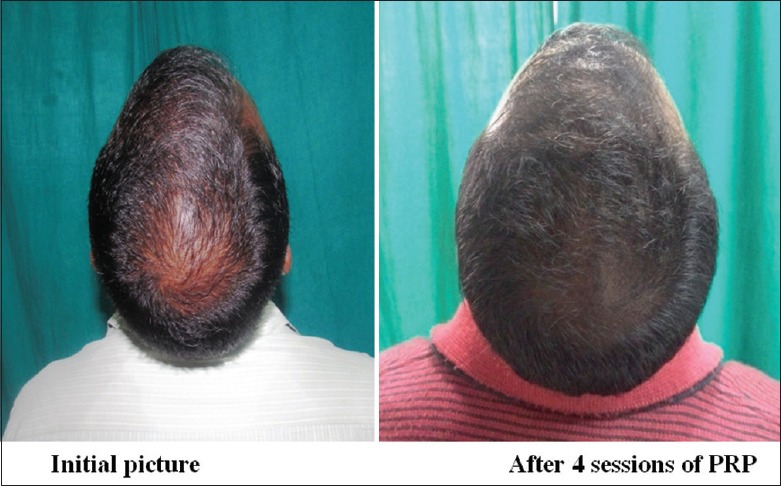
Group A: Patient 2
Figure 8.
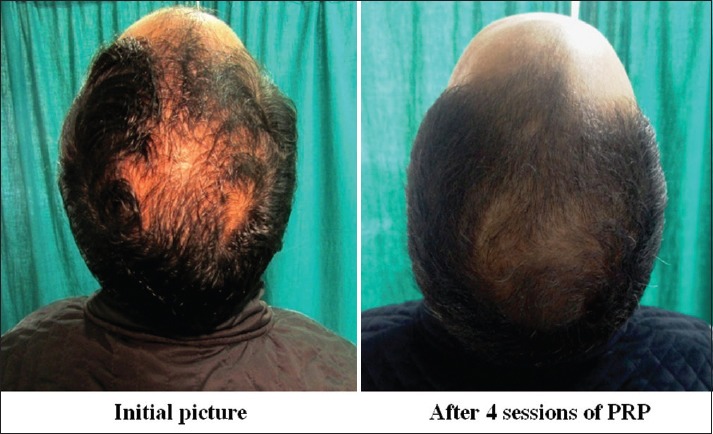
Group A: Patient 3
Figure 11.
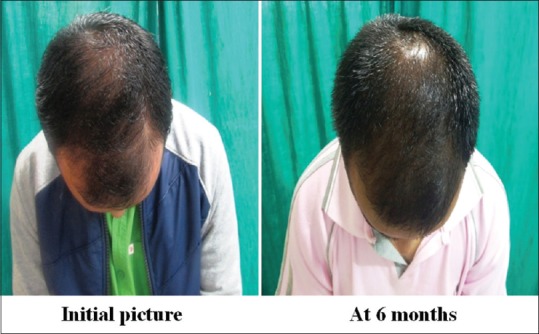
Group B: Patient 1 (mid-pattern view)
Hair pull test
It was done at the beginning of treatment and then at the end of 6 months. Twelve patients in Group A (75%) had a negative hair pull test compared to only six patients in Group B (42.8%); however, P value was not significant [Table 5].
Table 5.
Hair pull test
| Group A | Group B | p | |
|---|---|---|---|
| Negative hair pull test | 12 (75%) | 6 (42.8%) | 0.135 |
Patient standardized hair growth questionnaire
-
Question 1: Since the start of the study, I can see my bald spot getting smaller
- Results: Ten patients in Group A (62.5%) agreed that their bald spot was getting smaller while seven patients in Group B (50%) agreed that their bald spot was getting smaller; however, P value was not significant (0.5195) [Table 6]
-
Question 2: Since the start of the study, how would you describe the growth of your hair?
- Results: Eight patients in Group A (50%) reported a moderate increase in hair growth compared to only one patient in Group B (7.1%), P value of which was statistically significant (0.014) [Table 7]
-
Question 3: Since the start of the study, how effective do you think the treatment has been in slowing down your hair loss?
- Results: Six patients in Group A (37.5%) agreed that PRP therapy was very effective in slowing down hair loss compared to two patients in Group B (14.2%); however, P value was not significant (0.183) [Table 8]
-
Question 4: Compared to the beginning of the study, which statement best describes your satisfaction with the appearance of?
- Front of the head: Three patients in Group A were satisfied with the appearance of hairs at the front of the head in Group A compared to four patients in Group B. Results were almost comparable in both the groups, and P value was not significant [Table 9]
- Top of the head: Fourteen patients (87.5%) in Group A were satisfied with the appearance of hair on the top of the head compared to five patients (37.71%) in Group B. P value was found to be highly significant (0.004) [Table 10]
- Your hair overall: Ten patients (62.5%) in Group A were satisfied in Group A compared to five patients (35.76%) in Group B; however, P value was not significant between the two groups [Table 11].
Table 6.
Patients response when asked about their bald spot getting smaller since the start of the study:
| Group A (No of patients) | Group B (No of patients) | p | |
|---|---|---|---|
| Strongly Agree | 1 (6.25%) | 0 | Not applicable |
| Agree | 10 (62.5%) | 7 (50%) | 0.5195 |
| Neutral | 5 (31.25%) | 4 (28.5%) | 0.854 |
| Disagree | 0 | 3 (21.42%) | Not applicable |
| Strongly disagree | 0 | 0 | Not applicable |
Table 7.
Patients response when asked about the growth of their hair since the start of the study:
| Group A( No of patients) | Group B( No of patients) | p | |
|---|---|---|---|
| Greatly increased | 1 (6.25%) | 1 (7.1%) | 0.93 |
| Moderately increased | 8 (50%) | 1 (7.1%) | 0.014 |
| Slightly increased | 7 (43.75%) | 8 (57.14%) | 0.49 |
| No change | 0 | 0 | Not applicable |
Table 8.
Patients response when asked about how effective the treatment was in slowing down their hair loss:
| Group A(No of patients) | Group B(No of patients) | p | |
|---|---|---|---|
| Very effective | 6 (37.5%) | 2 (14.2%) | 0.183 |
| Somewhat effective | 9 (56.25%) | 9 (64.42%) | 0.67 |
| Not very effective | 1 (6.25%) | 3 (21.42%) | 0.285 |
Table 9.
Patients response when asked about the satisfaction with the appearance of hair on front of the head:
| Group A( No of patients) | Group B( No of patients) | P | |
|---|---|---|---|
| Very satisfied | 0 | 0 | N/A |
| Satisfied | 3 (18.75%) | 4 (28.5%) | 0.56 |
| Neutral | 12 (75%) | 8 (57.14%) | 0.33 |
| Dissatisfied | 1 (6.25%) | 2 (14.2%) | 0.53 |
Table 10.
Patients response when asked about the satisfaction with the appearance of hair on top of the head:
| Group A( No of patients) | Group B( No of patients) | p | |
|---|---|---|---|
| Very satisfied | 0 | 0 | N/A |
| Satisfied | 14 (87.5%) | 5 (35.71%) | 0.004 |
| Neutral | 2 (2.5%) | 7 (50%) | 0.03 |
| Dissatisfied | 0 | 2 (14.2%) | Non-applicable |
Table 11.
Patients response when asked about the satisfaction with the appearance of hair overall:
| Group A( No of patients) | Group B( No of patients) | p | |
|---|---|---|---|
| Very satisfied | 0 | 0 | N/A |
| Satisfied | 10 (62.5%) | 5 (35.76%) | 0.16 |
| Neutral | 5 (31.25%) | 7 (50%) | 0.32 |
| Dissatisfied | 1 (6.25%) | 2 (14.2%) | 0.53 |
Patient satisfaction score
Patients in Group A had a mean satisfaction score of 6.56 with standard deviation of 1.09 while patients in Group B had a mean satisfaction score of 4.85 with a standard deviation of 1.46. P value was found to be highly significant [Figure 13 and Table 12].
Figure 13.
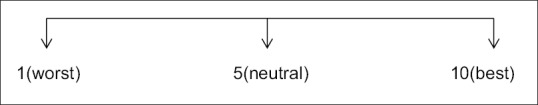
Patient satisfaction score
Table 12.
Patient satisfaction score:
| Group A [Mean score±SD] | Group B [Mean score±SD] | p |
|---|---|---|
| 6.56±1.09 | 4.85±1.46 | 0.001(highly significant) |
Mean platelet counts and platelet count in platelet-rich plasma
An initial platelet count was done in all the patients of Group A using automated device, and the mean platelet count was 3.07 ± 0.5 lac/mm3. Platelet count was again done in the final PRP prepared using the same automated device, and the mean PRP count was 12.4 ± 1.7 lac/mm3 [Table 13].
Table 13.
Mean platelet count and platelet count in PRP:
| INITIAL PLATELET COUNT (MEAN±SD) | PLATELET COUNT IN PRP (MEAN±SD) |
|---|---|
| 3.07±0.5 lac/mm3 | 12.4±1.7 lac/mm3. |
Thus, platelet count in PRP was increased to four times the baseline platelet count which is considered optimal in most of the studies.
DISCUSSION
AGA is one of the most common causes of hair loss encountered in general practice. Due to the limited treatment options available, there has been a continuous search for newer treatment options in AGA among which PRP therapy has shown promising results in the recent past.
Although many studies have been done in the past demonstrating the efficacy of minoxidil therapy and PRP therapy independently, there has not been much in the literature about the comparison of both forms of therapies. Hence, in this study, we compared already established minoxidil therapy with relatively newer PRP therapy which could help us to conclude that which is more safer, convenient, and efficient form of therapy in the treatment of AGA.
We used 25–35 ml of venous blood to prepare PRP, which was slightly higher as compared to studies by Betsi et al.,[9] Cervelli et al.,[6] Khatu et al.,[10] and Gkini et al.[11] who used 16 ml, 18 cc, 20 ml, and 16 ml of blood, respectively. In some studies such as Ubel et al.,[12] Greco and Brandt et al.,[13] and Kang et al.,[14] blood volume used was much higher as compared to our study, that is, 80 cc, 60 cc, and 60 ml, respectively.
PRP can be prepared by manual double-spin technique as well as with the help of automated devices. PRP was prepared by manual double-spin method in the present study, and similar methods of PRP preparation were also adopted by Ubel et al.[12] and Khatu et al.[10] PRP preparation through automated devices was preferred in studies such as Betsi et al.,[9] Cervelli et al.,[6] and Gkini et al.,[11] and average time taken by automated devices to separate PRP from blood was 5–10 min in a single spin at 1100–1500 g.
Regarding the use of activator in PRP, we used calcium gluconate which was also used by Gkini et al.[11] while other studies such as Khatu et al.[10] and Ubel et al.[12] used calcium chloride as an activator. Some studies such as Betsi et al.[9] and 'Greco and Brandt' et al.[13] have not mentioned the use of activator in their study.
In this study, the mean baseline platelet count was around 3 lac/mm3 which was increased to 12 lac/mm3 in PRP after centrifugation. The findings were similar to Ubel et al.[12] where the final PRP concentration was 4–6 times the baseline. In studies by Cervelli et al.,[6] the platelet concentration in PRP mentioned was 14.84 lac/mm3 while PRP concentration in the study by Gkini et al.[11] was 11.02 lac/mm3. In other studies such as Greco and Brandt et al.,[13] Betsi et al.,[9] and Khatu et al.,[10] the final concentration in PRP was not mentioned [Table 14].
Table 14.
Summary of various studies showing mode of PRP preparation, activator used, platelet concentration, volume of PRP and sample size
| Study | Blood volume | Mode of Preparation | Activator used | Mean baseline platelet concentration | Mean platelet concentration in PRP | Volume of PRP | Sample size |
|---|---|---|---|---|---|---|---|
| Ubel et al[12] | 80 cc | Manual double spin | 10% calcium chloride | Not mentioned | X4-6 | 2 cc | 20 |
| Greco and Brandt et al[13] | 60 cc | Not mentioned | Not mentioned | Not mentioned | Not mentioned | 10 cc | 10 |
| Kang et al[14] | 60 ml | Smart PreP2+TM | Not mentioned | Not mentioned | X5.9 | 4 ml | 26 |
| Betsi et al[9] | 16 ml | ACR-C Extra kits | Not mentioned | Not mentioned | Not mentioned | 8-12 ml | 42 |
| Cervelli et al[6] | 18 cc | Cascade- Selphyl-Esforax kit | Ca2+ | Not mentioned | 14.84 platelets/µl | 9 ml | 10 |
| Khatu et al[10] | 20 cc | Manual double spin | Calcium chloride | Not mentioned | Not mentioned | 2-3 cc | 11 |
| Gkini et al[11] | 16 ml | BCT-3 kits | Calcium gluconate | Not mentioned | 11.02 platelets/µl | 6 ml | 20 |
| Present study | 25-35 ml | Manual double spin | Calcium gluconate (1:9 dilution) | 3.07±0.5 lac/mm3 | 12.4±1.7 lac/mm3 | 4-6 ml | 16 |
Even though PRP therapy has been used for over a decade in AGA, there are still no standard treatment protocols regarding number of sessions and interval between the two sessions for giving PRP therapy. We gave PRP therapy at a monthly interval for 4 months, that is, 4 sessions 1 month apart which was similar to Cervelli et al.[6] who gave PRP therapy at monthly interval for 3 months, that is, 3 sessions 1 month apart.
Evaluation methods used in our study were global photography, hair pull test, patient standardized hair growth questionnaire, and patient satisfaction score for both Groups A and B. Apart from this, mean platelet count and platelet counts in PRP were also calculated to clinically correlate with the improvement in the patients of PRP group. Similar evaluation methods were also used by Betsi et al.[9] which included clinical examination, digital images, hair pull test, and patient satisfaction score. In their study, all the patients (100%) had a negative hair pull test after third treatment, and patient satisfaction score was 7.0 on a scale of 1–10. In this study, negative hair pull test was in 12 out of 16 (75%) patients in Group A and mean patient satisfaction score was 6.56 ± 1.09 at the end of treatment. Khatu et al.[10] used global photography, clinical examination, and phototrichogram to evaluate response to treatment in PRP patients, and in their study, 81.81% of patients had negative hair pull test at 3 months (four sessions) of treatment which is slightly higher as compared to our study, that is, 75% at 6 months (four sessions), while patient satisfaction score was 7 on a scale of 1–10 which was almost comparable to our study [Table 15].
Table 15.
Treatment protocols, evaluation methods, and results of various studies in comparison to the present study for Group A (platelet-rich plasma group)
| Study | Sessions | Interval between sessions | Method of application | Evaluation methods | Results |
|---|---|---|---|---|---|
| Ubel et al[12] | 1 | Nil | Embedment of follicular units inPRP solution before surgery | Manual counting with magnifying glass | 15.1% better graft survival |
| Greco and Brandt et al[13] | 1 | Nil | Injection and microneedling | Hair diameter measures by Starret micrometer | Increase in average hair shaft diameterAt 4 months: 9.7%At 8 months: 6.1% |
| Kang et al[14] | 2 | 3 months | Interfollicular injections of CD34+cell containing PRP of 0.05-0.1 ml/cm2 | Digital imagesPhototrichogram | Improvement in the mean number of hairs and in mean hair thickness, respectivelyAt 3 months: 20.5±17.0% and 31.3±30.1%At 6 months: 29.2±17.8% and 46.4±37.5% |
| Betsi et al.[9] | 5 | 12 days | Injections | Clinical examDigital imagesHair pull testPatient’s satisfaction | All patients after third treatment had negative hair pull test and an 25% decrease of hair lossPatients’ satisfaction: 7.0 on a scale of 1-10 |
| Khatu et al[10] | 4 | 2 weeks | Injections (nappage technique) | Global photographsClinical examPhototrichogram(trichoscan)Patient’s assessment scale | At 3 monthsNegative hair pull test in 81.81% of patientsModerate improvement in hair volume and coverageMean increase in hair density: 22.09 follicular units/cm2 |
| Gkini et al[11] | 4 | 3 weeks and last session at 6 months | Injections | Macroscopic photographsClinical examHair pull testDermoscopicmicrophotographsPatient’s assessment scale | Increase in hair density compared to that of baselineAt 3 months<0.001Negative hair pull testHair density: 170.70±37.81 (maximum value)At 6 months<0.001Hair density: 156.25±37.75At 1-year<0.001Hair density: 153.70±39.92Patients’ satisfaction: 7.1 on a scale of 1-10. |
| Present study | 4 | 1 month | Intradermal injections | Global photographsHair pull testStandardized hair growth questionnairePatient satisfaction score | On Global photography: Better outcome in PRP group as compared to minoxidil groupHair pull test negative in 75% in PRP compared to 42.8% in group B6.56±1.09 in PRP group v/s 4.85±1.46 (p-value: 0.001) |
Multiple randomized control trials as well as case series have already established the effectiveness of topical minoxidil therapy for the management of AGA.
In 2002, Olsen et al.[15] conducted a randomized clinical trial of 5% topical minoxidil solution versus 2% topical minoxidil solution and placebo in the treatment of AGA. Patients were given treatment for 48 weeks, and after 48 weeks of therapy, 5% topical minoxidil was significantly superior to 2% minoxidil and placebo in terms of change from baseline in nonvellus hair count (18.6 ± 25.4 in 5% minoxidil vs. 12.7 ± 20.7 in 2% minoxidil vs. 3.9 ± 21.7 in placebo group). Evaluation methods used included hair counts which were obtained from computer-assisted scans of macrophotographs of clipped hair in a 1 cm2 target evaluation area in the balding vertex scalp, patient self-assessment, and investigator assessment of hair growth using validated hair growth questionnaires. In this study, a different standardized hair growth questionnaire was used for evaluating efficacy of both forms of treatments along with other variables such as global photography, hair pull test, and patient satisfaction score which were not included in the study by Olsen et al.
A randomized study was done by Tsuboi et al.[16] which compared 5% and 1% minoxidil solution for the treatment of AGA in Japanese male patients. They found that after 18 weeks, the mean change in hair count was 22.3 in Group 1 (5% minoxidil) and 17.2 in Group 2 (1% minoxidil) yielding a P value of 0.009 which was statistically significant. Evaluation methods used were photography of hair with CCD microscope system, investigator assessment, and patient self-assessment using a 5-point scale: (i) markedly improved; (ii) moderately improved; (iii) slightly improved; (iv) unchanged; and (v) worsened. In this study, a different standardized hair growth questionnaire was used for evaluating efficacy of both forms of treatments along with other variables discussed in results which were not included in the study by Tsuboi et al.
Recently, Navarro et al.[17] retrospectively compared plasma rich in growth factors (PRGFs) and topical minoxidil therapy for the treatment of AGA. Evaluation methods used were diagnostic trichograms to analyze the anagen/telogen hair change improvement. PRGF-treated patients showed higher anagen hair increase compared to minoxidil-treated ones (6.9% ± 0.4% and 4.6% ± 0.5%, respectively) (P < 0.05). Telogen hair decrease was also higher in PRGF group (5.7% ± 0.3% and 2.6% ± 0.5%, respectively) (P < 0.05). Differences for both anagen/telogen improvement percentages between PRGF and minoxidil protocols proved to be statistically significant (P < 0.05). Global photographs showed an overall volume and quality hair improvement for both treatments. In this study also, intradermal PRP therapy was found to be superior to topical minoxidil therapy for the treatment of AGA although the evaluation method was different except for global photography.
To the best of our knowledge, this was the first prospective study comparing the efficacy of PRP and minoxidil therapy for the treatment of AGA where we concluded that intradermal PRP therapy (Group A) was better than topical minoxidil therapy (Group B). PRP patients had a better outcome on global photography, hair pull test, patient satisfaction score, and patient standardized hair growth questionnaire. Side effects in patients in PRP group were mainly pain at injection site because of which four patients left the treatment and were excluded. No significant side effects were observed in Group B patients except for mild scaling but six patients in this group left medication because they found it to be ineffective even at 3–4 months of treatment and were eventually excluded from the study.
CONCLUSION
PRP therapy can be a valuable adjuvant to topical minoxidil therapy in the treatment of AGA. Side effects with PRP therapy were minimal with better results which may improve the compliance of the patient. It can be especially preferred in those patients who are unsatisfied or are noncompliant to regular use of minoxidil or oral finasteride therapy. Thus, both forms of therapies can be combined together for improved compliance and better outcome. Major limitation of this study was a small sample size, study period, and no use of unit area trichoscopic analysis for hair density.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ANNEXURES
ANNEXURE I.
MODIFIED NORWOOD-HAMILTON SCALE
| Type | Clinical Definition |
|---|---|
| I | Minimal recession of the hairline along the anterior border in the frontotemporal (FT) region |
| II | The anterior border of the hair in the FT region has triangular areas of recession that tend to be symmetrical. These areas extend no further posterior than approximately 2 cm anterior to a line drawn in a coronal plane between the external auditory meatus on both sides. Hair is either lost or sparse along the mid-frontal border of the scalp |
| III | Characterized by deep FT hair recession, usually symmetrical and either bald or sparsely covered with hair. These areas of hair recession extend further posterior than a point that lies approximately 2 cm anterior to a line drawn in a coronal plane between the external auditory meatus on either side |
| IIIv (vertex) | Hair is mainly lost in the vertex. There may be some frontal recession but it does not exceed than that seen in type III |
| IV | The frontal and FT recession is more severe than type III. There is also sparseness or absence of hair in the vertex area. These bald areas are extensive, but separated from each other by a band of moderately dense hair that joins the fully haired fringe on each side of the head. |
| V | The hair loss over the vertex and FT areas is larger than in type IV and the band of hair between them is narrower and sparser |
| VI | The hair loss over the FT and vertex regions is confluent and the bridge of hair that crosses the crown is absent |
| VII | There is only a narrow horseshoe-shaped band of hair that begins laterally just anterior to the ear and extends posteriorly on the sides and fairly low on the occipital area |
| Variants(type variants-‘a’) | Constitutes 3% of all cases of AGA: (i) the entire anterior border of the hairline progresses posteriorly without the normal island of hair in the mid-frontal region and (ii) there is no simultaneous development of a bald area on the vertex. Instead, the anterior recession just advances posterior to the vertex |
| IIa | The entire anterior border of the hairline lies high on the forehead. The usual mid-frontal island of hair is represented by only a few sparse hairs. The area of denudation extends no farther than 2 cm from the frontal line |
| IIIa | The area of denudation reaches the mid-coronal line |
| Iva | The area of denudation extends beyond the mid-coronal line and there may be considerable thinning of hair posterior to the actual hair line |
| Va | Most advanced degree of alopecia; however, the bald area does not reach the vertex |
REFERENCES
- 1.Tosti A, Piraccini BM, Iorizzo M, Voudouris S. The natural history of androgenetic alopecia. J Cosmet Dermatol. 2005;4:41–3. doi: 10.1111/j.1473-2165.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaliyadan F, Nambiar A, Vijayaraghavan S. Androgenetic alopecia: An update. Indian J Dermatol Venereol Leprol. 2013;79:613–25. doi: 10.4103/0378-6323.116730. [DOI] [PubMed] [Google Scholar]
- 3.Blumeyer A, Tosti A, Messenger A, Reygagne P, Del Marmol V, Spuls PI, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;9(Suppl 6):S1–57. doi: 10.1111/j.1610-0379.2011.07802.x. [DOI] [PubMed] [Google Scholar]
- 4.Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297–300. doi: 10.1016/s0278-2391(00)90058-2. [DOI] [PubMed] [Google Scholar]
- 5.Li ZJ, Choi HI, Choi DK, Sohn KC, Im M, Seo YJ, et al. Autologous platelet-rich plasma: A potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38:1040–6. doi: 10.1111/j.1524-4725.2012.02394.x. [DOI] [PubMed] [Google Scholar]
- 6.Cervelli V, Garcovich S, Bielli A, Cervelli G, Curcio BC, Scioli MG, et al. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: Clinical and histomorphometric evaluation. Biomed Res Int. 2014;2014:760709. doi: 10.1155/2014/760709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillmann K, Blume-Peytavi U. Diagnosis of hair disorders. Semin Cutan Med Surg. 2009;28:33–8. doi: 10.1016/j.sder.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Barber BL, Kaufman KD, Kozloff RC, Girman CJ, Guess HA. A hair growth questionnaire for use in the evaluation of therapeutic effects in men. J Dermatol Treat. 1998;9:181–6. [Google Scholar]
- 9.Betsi EE, Germain E, Kalbermatten DF, Tremp M, Emmenegger V. Platelet-rich plasma injection is effective and safe for the treatment of alopecia. Eur J Plast Surg. 2013;36:407–12. [Google Scholar]
- 10.Khatu SS, More YE, Gokhale NR, Chavhan DC, Bendsure N. Platelet-rich plasma in androgenic alopecia: Myth or an effective tool. J Cutan Aesthet Surg. 2014;7:107–10. doi: 10.4103/0974-2077.138352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gkini MA, Kouskoukis AE, Tripsianis G, Rigopoulos D, Kouskoukis K. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213–9. doi: 10.4103/0974-2077.150743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uebel CO, da Silva JB, Cantarelli D, Martins P. The role of platelet plasma growth factors in male pattern baldness surgery. Plast Reconstr Surg. 2006;118:1458–66. doi: 10.1097/01.prs.0000239560.29172.33. [DOI] [PubMed] [Google Scholar]
- 13.Greco J, Brandt R. The effects of autologous platelet rich plasma and various growth factors on non-transplanted miniaturized hair. Hair Transplant Forum Int. 2009;19:49–50. [Google Scholar]
- 14.Kang JS, Zheng Z, Choi MJ, Lee SH, Kim DY, Cho SB, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: A preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72–9. doi: 10.1111/jdv.12062. [DOI] [PubMed] [Google Scholar]
- 15.Olsen EA, Dunlap FE, Funicella T, Koperski JA, Swinehart JM, Tschen EH, et al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Invest Dermatol. 2002;47:377–85. doi: 10.1067/mjd.2002.124088. [DOI] [PubMed] [Google Scholar]
- 16.Tsuboi R, Arano O, Nishikawa T, Yamada H, Katsuoka K. Randomized clinical trial comparing 5% and 1% topical minoxidil for the treatment of androgenetic alopecia in Japanese men. J Dermatol. 2009;36:437–46. doi: 10.1111/j.1346-8138.2009.00673.x. [DOI] [PubMed] [Google Scholar]
- 17.Navarro MR, Asín M, Martínez MA, Martínez AM, Molina C, Moscoso L, et al. Management of androgenetic alopecia: A comparative clinical study between plasma rich in growth factors and topical minoxidil. Eur J Plast Surg. 2016;39:173–80. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient standardized hair growth questionnaire


