Abstract
Background
Older adults with cancer experience negative long‐term functional effects of both cancer and treatments. Exercise may minimize their age‐related and cancer‐related functional decline.
Methods
We conducted a multicentre open‐label 12 month randomized clinical trial with two parallel arms including participants aged ≥70 years with lymphoma or carcinoma requiring curative treatment. The study started at the beginning of any phase of cancer treatment (surgery, chemotherapy, or radiotherapy). The usual care group (UCG) received the current national recommendations in physical activity (a guideline without specific counselling). The intervention group (IG) received 1 year phoned physical activity advice individually adapted to physical assessment (twice a month during the first 6 months and then monthly). The primary outcome was the proportion of subjects with a 1 year decreased short physical performance battery (SPPB) score of 1 point or more. Physical, cognitive, and clinical secondary outcomes were also investigated.
Results
We allocated 301 participants (age 76.7 ± 5.0, female 60.6%) to each group. At baseline, the median SPPB was 10/12 in both groups. Breast was the most frequent tumour site (35.7%). After 1 year, 14.0% of participants in the UCG and 18.7% in the IG had a decrease in SPPB score of 1 point or more (P = 0.772). At 2 years, there was no difference in SPPB, gait speed, International Physical Activity Questionnaire score, and verbal fluency. Subgroup analyses after 2 years showed a decline in SPPB for 29.8% of UCG and 5.0% of IG breast cancer participants (P = 0.006), in 21.7% of UCG and 6.2% of IG female participants (P = 0.019), and in 24.5% of UCG and 11.1% of IG normal nutritional status participants (P = 0.009). Falls, hospitalization, institutionalization, and death rates were similar in both groups.
Conclusions
Personalized phoned physical activity advice had not reduced functional decline at 1 year but provided preliminary evidence that may prevent physical performance decline at 2 years in older adults with breast cancer.
Keywords: Adapted physical activity, Onco‐geriatrics, Nutrition, Breast cancer, Frailty
Introduction
Cancer is frequent in older adults. By 2040, nearly three out of four cancer survivors will be 65 years and older.1 Cancer survivors are known to experience long‐term negative effects of treatment such as fatigue, pain, cognitive disturbance, depression, anxiety, and reduced health‐related quality of life.2, 3 Compared with other older adults, they are at greater risk of other cancers, cardiovascular disease, osteoporosis, diabetes, and accelerated functional decline.4 However, most survivorship studies have focused on childhood cancer or cancer in young adults but not in older adults.5
The long‐term outcome of successfully treated cancer patients is relatively unknown except for their vital prognosis.6, 7 Furthermore, the functional consequences of cancer may be greater in elderly than in younger adults.6 It is important to encourage older cancer survivors to adopt a healthy lifestyle with physical activity together with a health‐care plan that includes a dynamic conversation focused on education and motivation between patients and their health‐care providers.8
Frailty is associated with a high risk of dependency, falls, cognitive decline, infections, hospitalization, disability, institutionalization, and death.9, 10, 11 The most frequently used criteria to define frailty are the set proposed by Fried and colleagues that includes five conditions: exhaustion, slowness, weight loss, low physical activity, and muscle weakness.12 A growing body of evidence indicates that frailty is a dynamic syndrome characterized by frequent transitional stages that can be modified.11, 13 Thus, an intervention that could prevent frailty or reduce its severity would be of great interest for older patients with cancer.
The National Cancer Institute and the National Academy of Medicine of the USA have highlighted the need to address functional outcomes during and after oncology interventions more robustly.14 Physical performance measures can predict onset of disability, and gait speed alone15 or in combination with other measures like the short physical performance battery (SPPB) is a strong predictor of adverse outcomes like mortality, hospitalization, or disability.16 Nevertheless, few studies have established standardized and objective measures of physical function that are highly associated with an increased risk of disability, nursing home admission, and mortality.16
Older cancer survivors report low physical activity levels, and few meet recommended health promotion guidelines.17 Successfully engaging older adults in a regular and consistent physical activity programme can be challenging. The latter may face obstacles that make participation less likely: inability to travel to centres with supervised programmes, health concerns related to unsupervised physical activity, and lack of knowledge regarding appropriate exercise activities.18 Although home‐based programmes are associated with reduced effects on change in health‐related quality of life and physical function outcomes relative to centre‐based initiatives, home‐based programmes have the potential to reach a broader segment of the population at considerably lower cost.18 Furthermore, reference centres for cancer treatment may be far from older patients' homes, making participation in supervised programmes even more difficult. Thus, physical activity programmes that offer advice and support at regular intervals by phone may provide a necessary bridge for successful home‐based exercise in older adults with cancer. Telephone‐based physical activity interventions have been proposed to allow for greater outreach to patients and minimize the high number of participants who refuse to attend supervised exercise sessions after cancer diagnosis due to the lack of interest in exercise, living too far away to travel, or because they are too busy.19
The aim of this study was to compare individualized adapted phone advice for 1 year to usual care in patients aged 70 and older with good‐prognosis cancer and undergoing curative treatment. We tested the hypothesis that providing phone advice on exercising including strength, balance, proprioception, flexibility, and aerobic training for 1 year could prevent physical performance loss at 1 year as compared with a control arm. The prevention of physical performance loss at 2 years and better evolution of gait speed, physical activity level, and cognition (verbal fluency) at 1 and 2 years after the start of cancer treatment were analysed as secondary outcomes.
Methods
Study design
CAPADOGE (Conseils en Activité physique pour la Prévention de la perte d'Autonomie Des patients d'Onco‐GEriatrie) was a multicentre open‐label 12 month randomized clinical trial with two parallel arms conducted in 12 recruiting centres in France. The intervention took place between October 2011 and May 2016. The University Hospital of Bordeaux coordinated the study and performed data management, analysis, and quality control. The subjects received the intervention from the start of any phase of their cancer treatment that was surgery, chemotherapy, or radiotherapy.
Patient population
Details of the methods were published previously.20 Briefly, the population consisted of men and women aged ≥70 years with histological confirmation of lymphoma or carcinoma requiring treatment by surgery, chemotherapy, hormonotherapy, radiotherapy, or targeted therapy with a curative intention as estimated by oncologists. The locations included colon, rectum, anal canal, breast, oesophagus, ear, nose and throat, kidney, prostate, bladder, lung, stomach, biliary ducts, ovary, womb, endometrium, and pancreas ( Table S1). Were also included hepatocellular carcinomas, all large diffused B‐cell lymphomas, all T peripheral lymphomas, and all low‐grade lymphomas: lymphocytic, lymphoplasmacytic, follicular, mantle, marginal zone (mucosa‐associated lymphoid tissue and others), and primary unknown adenocarcinomas. Exclusion criteria were Eastern Cooperative Oncology Group test score >2, serious psychiatric or cognitive problems, no basic fluency in the French language, and functional disability leading to a total inability to walk. Finally, patients participating in concurrent studies containing physical activity, those in palliative care, and those under legal protection were also ineligible. The trial was approved by the institute and ethical committees.
Recruitment and randomization
All potentially eligible patients were selected and included before starting treatment in the oncology department. If the patient agreed orally to participate, he or she was subsequently randomized to the usual care group (UCG) or intervention group (IG) and centralized via Internet. Randomization was stratified on the centres and used allocation by random blocks of four or six. Patients were equally randomized to the UCG or the IG (Figure 1). The allocation sequence was centralized via Internet and concealed from the project team. Only the oncology team was aware of the group allocation of each patient via Internet and was able to deliver the CAPADOGE booklet to the patients in the IG at V1. Thus, no double or simple blind was possible.
Figure 1.
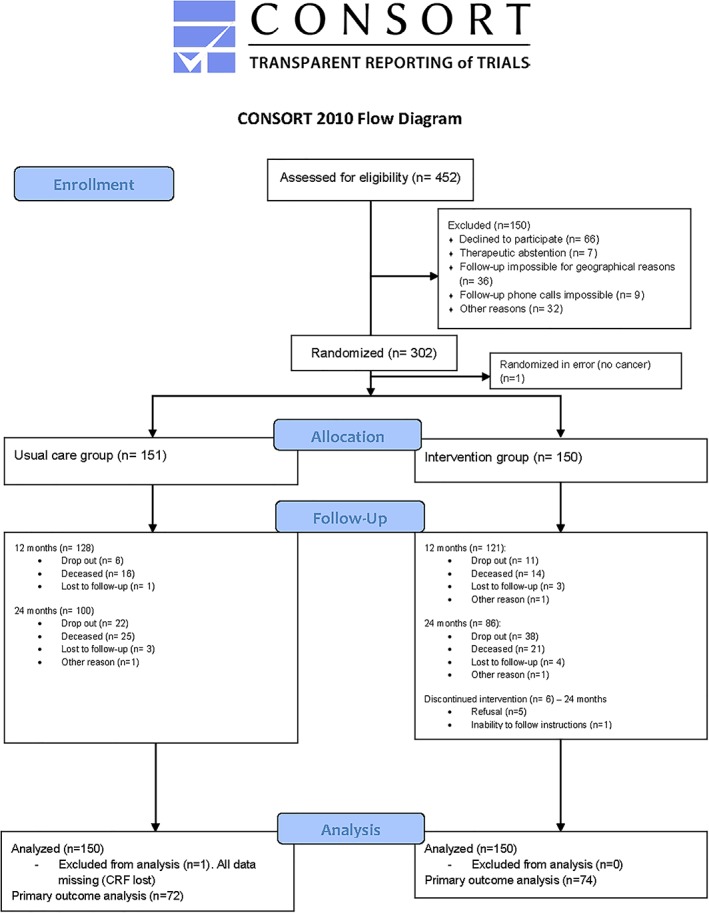
Study flow diagram.
Usual care group
The UCG received without comments the ‘PNNS booklet’ (French National Nutrition Health Program), which provides the current national recommendations for physical activity for people of this age group, that is, half an hour a day of any type of physical activity.
Intervention group
The physical activity intervention involved strength, balance, proprioception, flexibility, and aerobic training.20 The aim of the programme was to maintain fitness. Exercise sessions were conducted twice a week at the beginning of the intervention but more were proposed according to the patients' motivation and capabilities. Strength training comprised upper and lower body exercises: arm‐curl, squats, hip abduction, hip adduction, and more. First instruction was to perform 10 repetitions of each exercise without load to ensure an appropriate adaptation to resistance exercise. Thereafter, if they were well tolerated, loads were included, and number of repetitions was increased for additional benefit. The CAPADOGE booklet (which included images of different strength, balance, proprioception, flexibility, and aerobic exercises) was used so that the patients, apart from the advice they received over the phone, had a visual aid to be able to correctly carry out the exercises. The intensities that were proposed to the patients ranged from low to high and focused on avoiding pain and exhaustion. The instructor received patients' previous SPPB and International Physical Activity Questionnaire (IPAQ) assessment results to know their functional performance and habitual physical activity level. Then, the choice of intensity was based on the patient's feedback at each phone call to reach the highest intensity possible for each subject. Balance and proprioception training included exercises difficulty progression starting in sitting position or standing with two arms support. Then patients were invited to increase the complexity of movements according to their feedback: with one hand and finally without hands support if possible. Sessions finished with stretching exercises.
In addition to the exercise sessions, aerobic training was implemented through individualized recommendations regarding time and intensity to perform on their own. At first phone call, the instructor asked the patients about their daily physical activity (e.g. walking, shopping, and stair climbing). The instructor took this information into account together with the SPPB and IPAQ scores to encourage them to increase their daily amount of physical activity.
Advice was given by the same professional instructor with a degree in physical activity and sport sciences and trained in providing adapted physical activity to older adults. Phone calls were made twice a month during the first 6 months and then monthly until 1 year. The instructor reported all the exercises that the patients said that they had performed from the last phone call, in order to review each exercise and suggest individualized physical activity advice until the next phone call.
Study design and measurements
Participants were assessed immediately before the cancer treatment (visit 1, V1) and at 3 (V2), 6 (V3), 12 (V4), 18 (V5), and 24 (V6) months. Assessments were performed in each centre by clinical research assistants blind for group allocation. Data were anonymized by use of an identification code. Outcome measures were keyed in by data managers. The main baseline assessment included body mass index, Mini‐Mental State Examination,21 nutritional status [Mini Nutritional Assessment (MNA)—Short Form],22 hand grip strength, SPPB,16 Eastern Cooperative Oncology Group performance status, level of physical activity measured using the self‐reported IPAQ,23 Quality of Life Questionnaire (QLQ‐C30),24 verbal fluency,25 Cumulative Index Rating Scale–Geriatric,26 and C‐reactive protein dosage (mg/L). Frailty status was measured with a slightly modified version of the Fried criteria12 ( Table S2). We used a hand‐held dynamometer (Micro‐FET‐2®) to assess the grip strength criterion, the QLQ‐C30 fatigue symptom subscore24, 27 for the self‐reported exhaustion criterion, the MNA22 weight loss item for the weight loss criterion, and the IPAQ score for the decreased physical activity criterion.23
Outcomes
The primary outcome was the proportion of subjects with a 1 year decreased SPPB16 score of 1 point or more as compared with baseline. A 1‐point change in total SPPB score has been demonstrated to be of clinical relevance and to represent a substantial meaningful change.28 It was reported that 1‐point change can identify changes in the ability to walk one block, ability to climb one flight of stairs, or any self‐perceived change in mobility.28 The SPPB includes tests of gait speed, standing balance, and rising from a chair. For tests of standing balance, participants attempted to maintain the side‐by‐side, semi‐tandem, and tandem positions for 10 s. Usual pace during a 4 m walk was timed from a standing start, and participants were scored according to the time taken. Lastly, the third SPPB test consists of the time to rise from a chair as quickly as possible five times. Overall, the maximal SPPB score is 12 (4 points in each test). The secondary outcomes included the proportion of subjects with a 2 year decreased SPPB score of 1 point or more, self‐reported physical activity, cognitive assessments (verbal fluency), and evolution during the 2 year follow‐up, and the occurrence of clinical outcomes: hospitalization, institutionalization, number of self‐reported falls since the previous visit, and mortality.
Statistical analyses
Considering the recruitment capacity of the study, a sample size of 300 patients was required to provide 80% statistical power and a two‐sided type I error rate of 5% to detect a minimal difference in the proportion of patients with a lower SPPB, assuming less than 15% in the IG compared with 30% in the UCG and a 10% loss of follow‐up.
Analysis was performed on an intention‐to‐treat basis in a conventional manner: the patients were allocated to their randomization group whatever the intervention they actually received in order to keep the benefits of the randomization. For the main outcome, a missing = failure strategy was used: missing data were replaced by failure value, that is, loss of 1 SPPB point, being in the least favourable situation for the innovative intervention. The maximal bias strategy completed the sensitivity analysis.
Categorical variables such as 1 or 2 year 1 point SPPB decrease and clinical outcomes were analysed with logistic regression with adjustment for centre, gender, age (>80 years or not), and chemotherapy (yes/no). Some models for secondary outcomes could not be fully adjusted owing to convergence issues (see footnotes in tables). Continuous variables were described as mean, median, standard deviation, minimum, maximum, and interquartile range values when appropriate. Linear mixed models were used to estimate between‐group differences accounting for repeated measurements during the 2 year follow‐up, with subject‐specific random intercept. All analyses for secondary criteria were done with available data. Subgroup analyses were specified a priori by gender and breast cancer.
A P‐value less than 0.05 was considered statistically significant. The data were analysed with SAS® base 9.3 (SAS Institute, Cary, NC, USA). Compliance was assessed by dividing the number of phone calls made by the number of planned phone calls for people with a complete follow‐up. Efficiency of advice was measured by dividing the number of items of physical activity advice declared as effectively performed by the number of phone calls made.
Results
Study participants
From October 2011 to May 2014, we screened 452 participants. Of these, 301 were eligible, agreed to participate, and were allocated to either the UCG or the IG (Figure 1). The mean age was 76.7 years, and 60.0% were women (Table 1). Of 300 individuals analysed, 249 (83%) completed the 12 month follow‐up. Attrition was higher than the projected rate (17% at 1 year) used for sample size calculations. Dropout reasons are listed in Figure 1. Among the 300 participants who began the study, 186 (62%) completed the 24 month assessment (Figure 1). Breast was the most frequent tumour site (Table 1). Chemotherapy was used alone or in association in 91 (60.7%) subjects from the IG and 89 (59.3%) in the UCG (Table 1).
Table 1.
Baseline characteristics of patients
| Characteristics | Usual care group (n = 150) | Intervention group (n = 150) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Age (years) | ||||
| Mean | 76.6 | 76.8 | ||
| SD | 5.0 | 5.1 | ||
| Female gender | 83 | 55.3 | 97 | 64.7 |
| Body mass index (kg/m2) | ||||
| Mean | 26.1 | 26.2 | ||
| SD | 4.6 | 4.4 | ||
| MMSE, 0–30 | ||||
| Mean | 26.8 | 26.8 | ||
| SD | 2.9 | 2.9 | ||
| MNA questionnaire, 0–14 | ||||
| Good nutrition, score >11 | 49 | 33.8 | 54 | 38.6 |
| At risk/poor nutrition, score ≤11 | 96 | 66.2 | 86 | 61.4 |
| SPPB, 0–12 | ||||
| Mean | 9.2 | 8.9 | ||
| SD | 2.3 | 2.6 | ||
| ECOG performance status, n (%) | ||||
| 0 | 86 | 65.6 | 74 | 56.9 |
| 1 | 40 | 30.5 | 49 | 37.7 |
| 2 | 5 | 3.8 | 7 | 5.4 |
| IPAQ, n (%) | ||||
| <600 MET | 29 | 20.1 | 26 | 19.1 |
| 600–2999 MET | 52 | 36.1 | 47 | 34.6 |
| ≥3000 MET | 63 | 43.8 | 63 | 46.3 |
| Fatigue (QLQ‐C30) (0–100) | ||||
| Mean | 30.5 | 26.6 | ||
| SD | 28.9 | 27.1 | ||
| Physical activity (QLQ‐C30) (0–100) | ||||
| Mean | 23.3 | 16.7 | ||
| SD | 23.1 | 19.0 | ||
| Frailty status, n (%) | ||||
| Not frail | 90 | 74.4 | 83 | 72.8 |
| Frail | 31 | 25.6 | 31 | 27.2 |
| Verbal fluency, 15 s | ||||
| Mean | 6.6 | 6.8 | ||
| SD | 2.3 | 2.2 | ||
| Verbal fluency, 15–60 s | ||||
| Mean | 9.3 | 9.2 | ||
| SD | 6.6 | 4.1 | ||
| Co‐morbidity, CIRS‐G | ||||
| Normal, no grade 3–4 co‐morbidities | 115 | 76.7 | 121 | 80.7 |
| 1 co‐morbidity grade 3–4 | 28 | 18.7 | 24 | 16.0 |
| ≥2 co‐morbidities grade 3–4 | 7 | 4.7 | 5 | 3.3 |
| C‐reactive protein (mg/L) | ||||
| Median | 3 | 4 | ||
| IQR | 1–9 | 2–8 | ||
| Cancer origin | ||||
| Breast | 47 | 31.3 | 60 | 40.0 |
| Colon | 20 | 13.3 | 15 | 10.0 |
| Other | 83 | 55.3 | 75 | 50.0 |
| Treatment | ||||
| Surgery | 45 | 30.0 | 53 | 35.3 |
| Chemotherapy | 90 | 60.0 | 91 | 60.7 |
| Radiation | 58 | 38.7 | 59 | 39.3 |
| Hormone therapy | 32 | 21.3 | 29 | 19.3 |
| Targeted therapy | 2 | 1.3 | 3 | 2.0 |
CIRS‐G, Cumulative Index Rating Scale–Geriatric; ECOG, Eastern Cooperative Oncology Group; IPAQ, International Physical Activity Questionnaire; IQR, interquartile range; MMSE, Mini‐Mental State Examination; MNA‐SF, Mini Nutritional Assessment—Short Form; QLQ‐C30, Quality of Life Questionnaire; SD, standard deviation; SPPB, short physical performance battery.
Intervention adherence
The compliance and efficiency rates for the 12 month period were as follows: 81.1% of the 18 planned phone calls were actually made, and 70.1% of physical activity advice was declared as effectively performed by participants with a complete follow‐up.
Functional outcomes
After 1 year, a decline of 1 point or more on the SPPB was experienced by 14.0% of participants in the UCG and 18.7% in the IG (P = 0.772; Table 2). Robustness analysis with the maximal bias strategy was also performed, but given the proportion of missing data, the results were hardly interpretable. The odds ratio varied from 0.1 to 16.6 according to the orientation of the bias. At 2 years, 18.0% of UCG participants and 9.3% of IG participants had declined on the SPPB (P = 0.057; Table 2). Linear mixed models showed a similar evolution of both the UCG and IG for SPPB, gait speed, IPAQ score, and verbal fluency after 3, 6, 12, 18, and 24 months (NS; Tables 3 and S3).
Table 2.
Short physical performance battery change from baseline
| n (%) |
P*
12 months |
P*
24 months |
||||
|---|---|---|---|---|---|---|
| Usual care group | Intervention group | |||||
| 12 months | 24 months | 12 months | 24 months | |||
| Main outcome | n = 72 | n = 67 | n = 74 | n = 58 | ||
| SPPB change vs. baseline | ||||||
| No change or improvement | 51 (70.8) | 40 (59.7) | 46 (62.2) | 44 (75.9) | ||
| Decline ≥1 point | 21 (29.2) | 27 (40.3) | 28 (37.8) | 14 (24.1) | 0.772a | 0.057b |
| Breast cancer subgroup | n = 27 | n = 31 | n = 31 | n = 28 | ||
| SPPB change vs. baseline | ||||||
| No change or improvement | 20 (74.1) | 17 (54.8) | 18 (58.1) | 25 (89.3) | ||
| Decline ≥1 point | 7 (25.9) | 14 (45.2) | 13 (41.9) | 3 (10.7) | 0.119b | 0.006b |
| Male subgroup | n = 33 | n = 23 | n = 28 | n = 21 | ||
| SPPB change vs. baseline | ||||||
| No change or improvement | 22 (66.7) | 14 (60.9) | 20 (71.4) | 13 (61.9) | ||
| Decline ≥1 point | 11 (33.3) | 9 (39.1) | 8 (28.6) | 8 (38.1) | 0.622b | 0.943b |
| Female subgroup | n = 39 | n = 44 | n = 46 | n = 37 | ||
| SPPB change vs. baseline | ||||||
| No change or improvement | 29 (74.4) | 26 (59.1) | 26 (56.5) | 31 (83.8) | ||
| Decline ≥1 point | 10 (25.6) | 18 (40.9) | 20 (43.5) | 6 (16.2) | 0.068b | 0.019b |
| Normal nutritional status subgroup | n = 25 | n = 24 | n = 34 | n = 31 | ||
| SPPB change vs. baseline | ||||||
| No change or improvement | 17 (68.0) | 12 (50.0) | 22 (64.7) | 25 (80.6) | ||
| Decline ≥1 point | 8 (32.0) | 12 (50.0) | 12 (35.3) | 6 (19.4) | — | 0.009a |
P*, P for overall groupwise difference; SPPB, short physical performance battery.
Adjusted for centre, gender, age, and treatment.
Adjusted for centre, age, and treatment.
Table 3.
Effects of physical activity programme on physical function, physical activity and cognition
| Usual care group | Intervention group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Months | 24 Months | Baseline | 12 Months | 24 Months | P* | |||||||
| n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | ||
| Physical function | |||||||||||||
| SPPB score, 0–12 | 146 | 10 (8–11) | 73 | 10 (9–11) | 69 | 10 (8–12) | 140 | 10 (8–11) | 76 | 10 (7.5–12) | 60 | 11 (8–12) | 0.999a |
| Gait Speed (m/s) | 150 | 0.80 | 69 | 0.92 | 62 | 0.80 | 150 | 0.78 | 66 | 0.91 | 55 | 0.84 | 0.171a |
| Physical activity | |||||||||||||
| IPAQ score (MET) | 144 | 2280 | 80 | 2772 | 76 | 2946 | 136 | 2640 | 76 | 2133 | 64 | 2369 | 0.856a |
| Cognition | |||||||||||||
| Verbal fluency, 15 s | 132 | 6 (5–8) | 67 | 7 (6–8) | 122 | 6 (5–8) | 66 | 6 (5–8) | 0.920b | ||||
| Verbal fluency, 15–60s | 132 | 9 (6–11) | 67 | 9 (7–12) | 122 | 9 (7–11) | 66 | 9 (7–12) | 0.945b | ||||
Abbreviations: IQR, interquartile range; SPPB, Short Physical Performance Battery; IPAQ, International Physical Activity Questionnaire.
P*, P for Overall Groupwise Difference.
Adjusted for center, gender, age and treatment.
Adjusted for center.
In subgroup analyses, proportions of decline of 1 or more points on the SPPB did not significantly differ after 1 year when participants were categorized by breast cancer (P = 0.119; Table 2) and gender (P = 0.068; Table 2). However, after 2 years, 29.8% of UCG and 5.0% of IG breast cancer participants (P = 0.006; Table 2) had declined on the SPPB. In women, 21.7% of UCG and 6.2% of IG participants (P = 0.019; Table 2) had declined on the SPPB. The differences between both groups were not significant in men at 1 (P = 0.622; Table 2) and 2 years (P = 0.943; Table 2). We performed additional analyses to explore the role of nutritional status according to MNA categories. At the 2 year visit, among participants with a normal nutritional status, 24.5% of UCG and 11.1% of IG participants (P = 0.009; Table 2) had declined on the SPPB. There were no differences in SPPB decline for other MNA categories.
Clinical outcomes
Incidence of falls (UCG: 7% and IG: 7%), hospitalization (UCG: 16% and IG: 18%), institutionalization (UCG: 4% and IG: 9%), and death (UCG: 11% and IG: 10%) did not differ significantly between UCG and IG participants at 1 year. At 2 years, results for falls (UCG: 11% and IG: 10%), hospitalization (UCG: 29% and IG: 25%), institutionalization (UCG: 6% and IG: 9%), and death (UCG: 20% and IG: 20%) were also similar in both groups.
Discussion
The CAPADOGE study showed that both personalized physical activity advice given by phone and usual care based on the current national recommendations led to a similar proportion of patients whose SPPB score decreased by ≥1 point after 1 year. Due to the good prognosis with curative intention treatment of the participants included, we expected to recruit participants with a high SPPB score, that is, little likelihood of improvement but a potential decline. Indeed, this study confirmed our hypothesis of a high proportion of subjects with a 1 year decrease of 1 or more points on the SPPB. The proportion was around 15% at 1 year as compared with 30% in a geriatric frail population.29
In women with breast cancer, the intervention had efficiently slowed the functional decline after 2 years. This was not the case after 1 year possibly owing to their low muscle mass, strength, and physical activity level during and after chemotherapy treatment and their association with functional decline.30, 31, 32, 33 However, the significant results at 2 years despite the fact that we had 62% participation, and thus a lack of power, strongly suggest the positive effect of physical activity at 2 years. We added a subgroup analysis to explore the role of nutritional status in the response to advice on physical activity. At the 2 year visit, only the subgroup of subjects with normal nutritional status had benefited functionally from the intervention. Thus, a multimodal intervention including nutrition, which has been demonstrated to be feasible and safe, should be considered in any future trials with cancer patients.34 Interestingly, participants with breast cancer, women with any cancer and normal nutritional status, showed lower refusal rates compared with men and participants who had other types of cancer (data not shown). Because of the attrition observed at 24 months, this was still the case for participants still followed at that time. Indeed, the effect observed in those with breast cancer was strong enough to remain significant after correction for test multiplicity (e.g. Bonferroni correction). However, the difference by gender in the effect of the intervention may be because breast cancer concerns only women and because the number of patients with breast cancer was large. The low number of patients with other cancer sites did not allow other subgroup analyses to be performed.
Another study in younger participants and after completion of all surgery, chemotherapy, and/or radiation therapy showed that phone‐based physical activity advice was feasible and effective in improving functional capacity in breast cancer survivors,35 which was the most frequent cancer among women included. Thus, phone‐based physical activity interventions might be efficient in improving objectively measured physical functioning among older adults with cancer. As stated earlier, the phone‐based physical activity intervention was carried out to obtain greater outreach to patients, because our hypothesis was that those with lower physical function would not take part in a supervised exercise programme. However, in the present study, even though the compliance and the efficiency rates of the programme were high, the intensity of the exercises may have been too low, so the advice was not sufficient to prevent functional decline after 1 year. Therefore, due to the low effect of this intervention at 1 year, adding a digital virtual supervised session could be a key factor in getting patients to understand that the training programme is complementary to medical treatment. In addition, being part of the current study may have increased awareness in the UCG of the health benefits associated with physical activity and may have resulted in maintaining physical function during the programme. It is also possible that the effects of cancer treatment were still strong enough at 1 year to block the benefits of physical activity in older adults with cancer. The anti‐anabolic consequences of cancer cachexia may explain this lack of effect at 1 year. MNA is currently used as a marker of cancer cachexia and has a strong prognostic value, mainly for appetite and sarcopenia.36 A correlation between the biochemical markers of cachexia and MNA score has been shown in older patients with cancer.37 In addition, although exercise could be an attractive therapy for cancer cachexia, there is still a lack of studies focusing on the efficacy of exercise programmes in older cancer population with regard to functional issues.38
These findings are consistent with previous reports that did not find any self‐reported functional benefits at 1 year.4 However, other studies showed slowing down of self‐reported functional decline after older adults undergoing and recovering from cancer treatment had received physical activity advice, even though the IG still showed functional decline.17 Thus, the phone‐based physical activity intervention seems to be a suitable solution to prevent or delay physical performance decline in older adults with cancer. Our hypothesis should have concerned functional status 2 years after the start of the treatment rather than after the first year, because the effects of the treatments that patients have undergone still seemed to be strong enough to mask the beneficial effects of physical activity. However, in the CAPADOGE trial, the assessment schedule was adapted to cancer treatment follow‐up visits to minimize loss to follow‐up, and we expected high attrition rates in the second year. Even the number of participants who dropped out was slightly higher in the IG, so the number of patients lost to follow‐up in both groups is a major limitation of the study. Caution is also required in generalizing our results to patients whose cancer carries a poor prognosis, to the cognitively impaired, and to frail older adults. In addition, type of cancer, prognosis, and treatment may have influenced participation in the physical activity programme, and together with the high death rate of the study, participants may have confounded our analysis. However, the good prognosis as subjectively assessed by oncologists of all participants included and the adequate randomization in the two groups depending on the type of cancer ( Table S1) suggest that the aforementioned factors may not have influenced participation in the physical activity programme and thus the outcomes. We planned our subgroup analysis according to cancer type, but due to the small number in each cancer subgroup, only those with breast cancer were analysed separately. Indeed, the rate of cachexia may have been different according to cancer type. The subgroup analysis according to the MNA was performed to explore this possibility. Another limitation is that the secondary outcome measure of physical activity together with the measure of compliance with the exercise prescription was not objectively assessed via accelerometers. This point gives less weight and credibility to the physical activity level assessed in this study in comparison with other studies in which accelerometers were used.39 Responses may have reflected wishful thinking more than the actual level of physical activity. For these reasons, our physical activity data may be inaccurate. However, acceptance of using an accelerometer for such a long duration would probably have been low. The number of clinical trials using objective monitors in cancer patients and survivors is increasing,40 but accelerometers are normally used only for a few days and not in the long term. Thus, the lack of effect of intervention at 1 year may have been due to a lack of effect to achieve a sufficient level of physical activity, as advocated over the phone.
The study also has strengths. First, the physical activity intervention was simple to implement, low‐cost, practical, and broadly applicable to older patients treated for cancer. Second, the findings provide preliminary evidence that physical activity may prevent long‐term functional loss in older adults with breast cancer. Third, although poor physical function is known to be a strong predictor of adverse outcomes like disability, hospitalization, and mortality,16 this is the first long‐term study with a large sample to focus on objectively measured functional decline among older patients treated for cancer, instead of measuring it subjectively.41 Fourth, compliance was similar to that in other studies focusing on interventions in older participants with cancer.42
As in other research investigating physical activity in older participants, hospitalization rate and death were similar in both groups.43 However, given the small number of hospitalizations, these data are inconclusive, so further studies are needed to assess the effects of physical activity advice on hospitalization rates in older patients with cancer. To date, the CAPADOGE study is the largest and longest randomized trial assessing the effects of physical activity advice that focused on objectively measured functional decline in older patients treated for cancer. Future studies should focus on digital virtual supervised training in order to design a highly efficient programme for the recovery of older patients treated for cancer and designed for a main outcome measured at 2 years.
Conclusions
Compared with usual care given according to the current national recommendations in France, personalized advice on physical activity given over the phone did not reduce functional decline at 1 year although significant differences were observed after 2 years in women and in the breast cancer subgroup. Thus, these results highlight the potential for providing efficient personalized physical activity advice over the phone in older adults with cancer. Future work should focus on comparing the effects of digital virtual supervised training and its combination with the currently recommended usual care in older adults with cancer.
Funding
This work was funded by the National Hospital Program of Clinical Research (Programme Hospitalier de Recherche Clinique 2010) and sponsored by the University Hospital of Bordeaux (CHU Bordeaux). Haritz Arrieta was supported by a fellowship from University of the Basque Country (UPV/EHU).
Ethical issues
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.44
Conflict of interest
All authors declare that they have no conflict of interest.
Collaborators list
D Smith (CHU Bordeaux, France); A Sa‐Cunha (CHU Bordeaux, France); S Pesque (CHU Bordeaux, France); N Milpied (CHU Bordeaux, France); T Leguay Thibaut (CHU Bordeaux, France); G Harvet (CHU Bordeaux, France); S Vigouroux (CHU Bordeaux, France); A Pigneux (CHU Bordeaux, France); K K Bouabdallah (CHU Bordeaux, France); M‐S Dilhuydy (CHU Bordeaux, France); A Schmitt (CHU Bordeaux, France); A Cadier‐Lagnes (CHU Bordeaux, France); E Rullier (CHU Bordeaux, France); C Foucaud (CHU Bordeaux, France); C Duclos (CHU Bordeaux, France); A Lascaux (CHU Bordeaux, France); D Collet (CHU Bordeaux, France); C Subtil (CHU Bordeaux, France); A Guilngar (CHU Bordeaux, France); Q Denost (CHU Bordeaux, France); S Duc (CHU Bordeaux, France); P Soubeyran (Institut Bergonié, France); N Madranges (Institut Bergonié, France); A Floquet (Institut Bergonié, France); M Sire (Institut Bergonié, France); N Houede (Institut Bergonié, France); Y Bécouarn (Institut Bergonié, France); D Béchade (Institut Bergonié, France); C Mertens (CHU Bordeaux, France); C Tunon de Lara (Institut Bergonié, France); M Debled (Institut Bergonié, France); N Quenel‐Tueux (Institut Bergonié, France); H Rousselot (Centre Alexis Vautrin, France); P Feugier (CHU Nancy, France); J‐Y Niemer (CHU Nancy, France); S Périn (Institut jean Godinot, France); O Dubroeucq (Institut jean Godinot, France); A Prévost (Institut jean Godinot, France); A‐M Savoye (Institut jean Godinot, France); G Yazbek (Institut jean Godinot, France); C Jouannaud (Institut jean Godinot, France); A Fadin (Institut jean Godinot, France); B Billemont (Institut jean Godinot, France); P Guilbert (Institut jean Godinot, France); H Curé (Institut jean Godinot, France); B Costa (Institut jean Godinot, France); T D Nguyen (Institut jean Godinot, France); C Murariu (Institut jean Godinot, France); V Ceccato (Institut jean Godinot, France); J Nicolas (Institut jean Godinot, France);S Maillard (Institut jean Godinot, France); J‐C Eymard (Institut jean Godinot, France); C Mariette (CHRU Lille, France); J‐P Triboulet (CHRU Lille, France); G Piessen (CHRU Lille, France); N Briez (CHRU Lille, France); L‐G Depuydt (Centre Oscar Lambret, France); D Pasquier (Centre Oscar Lambret, France); S Dewas (Centre Oscar Lambret, France); A Mailliez (Centre Oscar Lambret, France); L Gras (Centre Oscar Lambret, France); L Vanlemmens (Centre Oscar Lambret, France); S Maillard (Centre Oscar Lambret, France); X Mirabel (Centre Oscar Lambret, France); E Carola (CH Senlis, France); S Trager‐Maury (CH Senlis, France); O Gasnier (CHU Limoges, France); V Le Brun‐Ly (CHU Limoges, France); S Falkowski (CHU Limoges, France); A Giraud (CHU Limoges, France); E Angellier (CH Mont de Marsan, France); J‐L Perie (CH Dax, France); L Halard (CH Dax, France); P Remuzon (CH Dax, France); N Pontier (CH Dax, France).
Supporting information
Table S1. Cancer origin of the participants.
Table S2. Modified version of the Fried criteria.
Table S3. Effects of physical activity program on physical function, physical activity and cognition
Arrieta, H. , Astrugue, C. , Regueme, S. , Durrieu, J. , Maillard, A. , Rieger, A. , Terrebonne, E. , Laurent, C. , Maget, B. , Servent, V. , Lavau‐Denès, S. , Dauba, J. , Fonck, M. , Thiébaut, R. , and Bourdel‐Marchasson, I. (2019) Effects of a physical activity programme to prevent physical performance decline in onco‐geriatric patients: a randomized multicentre trial. Journal of Cachexia, Sarcopenia and Muscle, 10: 287–297. 10.1002/jcsm.12382.
References
- 1. Swartz MC, Lewis ZH, Lyons EJ, Jennings K, Middleton A, Deer RR, et al. Effect of home and community‐based physical activity interventions on physical function among cancer survivors: a systematic review and meta‐analysis. Arch Phys Med Rehabil 2017;98:1652–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang IC, Hudson MM, Robison LL, Krull KR. Differential impact of symptom prevalence and chronic conditions on quality of life in cancer survivors and non‐cancer individuals: a population study. Cancer Epidemiol Biomarkers Prev 2017;26:1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agasi‐Idenburg SC, Thong MSY, Punt CJA, Stuiver MM, Aaronson NK. Comparison of symptom clusters associated with fatigue in older and younger survivors of colorectal cancer. Support Care Cancer 2017;25:625–632. [DOI] [PubMed] [Google Scholar]
- 4. Demark‐Wahnefried W, Clipp EC, Morey MC, Pieper CF, Sloane R, Snyder DC, et al. Lifestyle intervention development study to improve physical function in older adults with cancer: outcomes from Project LEAD. J Clin Oncol 2006;24:3465–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Extermann M, Leeuwenburgh C, Samiian L, Sehovic M, Xu J, Cubitt C, et al. Impact of chemotherapy on medium‐term physical function and activity of older breast cancer survivors, and associated biomarkers. J Geriatr Oncol 2017;8:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Avis NE, Deimling GT. Cancer survivorship and aging. Cancer 2008;113:3519–3529. [DOI] [PubMed] [Google Scholar]
- 7. Halpern MT, Urato MP, Lines LM, Cohen JB, Arora NK, Kent EE. Healthcare experience among older cancer survivors: analysis of the SEER‐CAHPS dataset. J Geriatr Oncol. 2018;9:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rowland JH, Bellizzi KM. Cancer survivorship issues: life after treatment and implications for an aging population. J Clin Oncol 2014;32:2662–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sacha J, Sacha M, Soboń J, Borysiuk Z, Feusette P. Is it time to begin a public campaign concerning frailty and pre‐frailty? A review article. Front Physiol 2017;8:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trevisan C, Veronese N, Maggi S, Baggio G, Toffanello ED, Zambon S, et al. Factors influencing transitions between frailty states in elderly adults: the Progetto Veneto Anziani longitudinal study. J Am Geriatr Soc 2017;65:179–184. [DOI] [PubMed] [Google Scholar]
- 11. Cesari M, Prince M, Thiyagarajan JA, De Carvalho IA, Bernabei R, Chan P, et al. Frailty: an emerging public health priority. J Am Med Dir Assoc 2016;17:188–192. [DOI] [PubMed] [Google Scholar]
- 12. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M157. [DOI] [PubMed] [Google Scholar]
- 13. Freiberger E, Kemmler W, Siegrist M, Sieber C. Frailty and exercise interventions. Z Gerontol Geriatr 2016;49:606–611. [DOI] [PubMed] [Google Scholar]
- 14. Duan‐Porter W, Cohen HJ, Demark‐Wahnefried W, Sloane R, Pendergast JF, Snyder DC, et al. Physical resilience of older cancer survivors: an emerging concept. J Geriatr Oncol. 2016;7:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pamoukdjian F, Lévy V, Sebbane G, Boubaya M, Landre T, Bloch‐Queyrat C, et al. Slow gait speed is an independent predictor of early death in older cancer outpatients: results from a prospective cohort study. J Nutr Health Aging 2017;21:202–206. [DOI] [PubMed] [Google Scholar]
- 16. Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221–M231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, et al. Effects of home‐based diet and exercise on functional outcomes among older, overweight long‐term cancer survivors: RENEW: a randomized controlled trial. JAMA 2009;301:1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cowper PA, Peterson MJ, Pieper CF, Sloane RJ, Hall KS, McConnell ES, et al. Economic analysis of primary care‐based physical activity counseling in older men: the VA‐LIFE trial. J Am Geriatr Soc 2017;65:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adamsen L, Quist M, Andersen C, Møller T, Herrstedt J, Kronborg D, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ 2009;b3410:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Durrieu J, Doussau A, Rieger A, Terrebonne E, Bouabdallah K, Zwolakowski MD, et al. Design of a physical activity program to prevent functional decline in onco‐geriatric patients (CAPADOGE): a randomized multicenter trial. J Frailty Aging 2012;1:138–143. [DOI] [PubMed] [Google Scholar]
- 21. Derouesne C, Poitreneau J, Hugonot L, Kalafat M, Dubois B, Laurent B, et al. Mini‐Mental State Examination: a useful method for the evaluation of the cognitive status of patients by the clinician. Consensual French version. Presse Med 1999;28:1141–1148. [PubMed] [Google Scholar]
- 22. Kaiser M, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Validation of the Mini Nutritional Assessment Short‐Form (MNA®‐SF): a practical tool for identification of nutritional status. J Nutr Health Aging 2009;13:782–788. [DOI] [PubMed] [Google Scholar]
- 23. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International Physical Activity Questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 24. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 25. Raoux N, Le Goff M, Auriacombe S, Dartigues JF, Amieva H. Semantic and letter fluency tasks: normative data in an elderly population of 70 years old and over from the PAQUID cohort. Rev Neurol 2010;166:594–605. [DOI] [PubMed] [Google Scholar]
- 26. Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the cumulative illness rating scale. Psychiatry Res 1992;41:237–248. [DOI] [PubMed] [Google Scholar]
- 27. Giesinger JM, Kuijpers W, Young T, Tomaszewski KA, Friend E, Zabernigg A, et al. Thresholds for clinical importance for four key domains of the EORTC QLQ‐C30: physical functioning, emotional functioning, fatigue and pain. Health Qual Life Outcomes 2016;14:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006;54:743–749. [DOI] [PubMed] [Google Scholar]
- 29. Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralnik JM, et al. Effects of a physical activity intervention on measures of physical performance: results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE‐P) study. J Gerontol A Biol Sci Med Sci 2006;61:1157. [DOI] [PubMed] [Google Scholar]
- 30. Klassen O, Schmidt ME, Ulrich CM, Schneeweiss A, Potthoff K, Steindorf K, et al. Muscle strength in breast cancer patients receiving different treatment regimes. J Cachexia Sarcopenia Muscle 2017;8:305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Demark‐Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 2001;19:2381–2389. [DOI] [PubMed] [Google Scholar]
- 32. Huy C, Schmidt ME, Vrieling A, Chang‐Claude J, Steindorf K. Physical activity in a German breast cancer patient cohort: one‐year trends and characteristics associated with change in activity level. Eur J Cancer 2012;48:297–304. [DOI] [PubMed] [Google Scholar]
- 33. Bye A, Sjøblom B, Wentzel‐Larsen T, Grønberg BH, Baracos VE, Hjermstad MJ, et al. Muscle mass and association to quality of life in non‐small cell lung cancer patients. J Cachexia Sarcopenia Muscle 2017;8:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solheim TS, Laird BJA, Balstad TR, Stene GB, Bye A, Johns N, et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle 2017;8:778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ligibel JA, Meyerhardt J, Pierce JP, Najita J, Shockro L, Campbell N, et al. Impact of a telephone‐based physical activity intervention upon exercise behaviors and fitness in cancer survivors enrolled in a cooperative group setting. Breast Cancer Res Treat 2012;132:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bourdel‐Marchasson I, Diallo A, Bellera C, Blanc‐Bisson C, Durrieu J, Germain C, et al. One‐year mortality in older patients with cancer: development and external validation of an MNA‐based prognostic score. PLoS One 2016;11:e0148523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gioulbasanis I, Georgoulias P, Vlachostergios PJ, Baracos V, Ghosh S, Giannousi Z, et al. Mini Nutritional Assessment (MNA) and biochemical markers of cachexia in metastatic lung cancer patients: interrelations and associations with prognosis. Lung Cancer 2011;74:516–520. [DOI] [PubMed] [Google Scholar]
- 38. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 39. Rogers LQ. Objective monitoring of physical activity after a cancer diagnosis: challenges and opportunities for enhancing cancer control. Phys Ther Rev 2010;15:224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schrack JA, Gresham G, Wanigatunga AA. Understanding physical activity in cancer patients and survivors: new methodology, new challenges, and new opportunities. Cold Spring Harb Mol Case Stud 2017;3:a001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kenzik KM, Morey MC, Cohen HJ, Sloane R, Demark‐Wahnefried W. Symptoms, weight loss, and physical function in a lifestyle intervention study of older cancer survivors. J Geriatr Oncol. 2015;6:424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, et al. Randomized trial of a telephone‐based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol 2014;32:2231–2239. [DOI] [PubMed] [Google Scholar]
- 43. Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014;311:2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cancer origin of the participants.
Table S2. Modified version of the Fried criteria.
Table S3. Effects of physical activity program on physical function, physical activity and cognition


