Abstract
Background
Conventional definitions of sarcopenia based on lean mass may fail to capture low lean mass relative to higher fat mass, that is, relative sarcopenia. The objective of this study is to determine the associations of sarcopenia and relative sarcopenia with mortality independent of co‐morbidities, and whether chronic kidney disease (CKD) and adiposity alter these associations.
Methods
Dual energy X‐ray absorptiometry‐derived appendicular lean mass index (ALMI, kg/m2) and fat mass index (FMI, kg/m2) were assessed in 14 850 National Health and Nutrition Examination Survey participants from 1999 to 2006 and were linked to death certificate data in the National Death Index with follow‐up through 2011. Sarcopenia was defined using sex‐specific and race/ethnicity‐specific standard deviation scores compared with young adults (T‐scores) as an ALMI T‐score < −2 and relative sarcopenia as fat‐adjusted ALMI (ALMIFMI) T‐score < −2. Glomerular filtration rate (GFR) was estimated using creatinine‐based (eGFRCr) and cystatin C‐based (eGFRCys) regression equations.
Results
Three (3.0) per cent of National Health and Nutrition Examination Survey participants met criteria for sarcopenia and 8.7% met criteria for relative sarcopenia. Sarcopenia and relative sarcopenia were independently associated with mortality (HR sarcopenia 2.20, 95% CI 1.69 to 2.86; HR relative sarcopenia 1.60, 95% CI 1.31 to 1.96). The corresponding population attributable risks were 5.2% (95% CI 3.4% to 6.4%) and 8.4% (95% CI 4.8% to 11.2%), respectively. Relative sarcopenia remained significantly associated with mortality (HR 1.32, 95% CI 1.08 to 1.61) when limited to the subset who did not meet the criteria for sarcopenia. The risk of mortality associated with relative sarcopenia was attenuated among persons with higher FMI (P for interaction <0.01) and was not affected by CKD status for either sarcopenia or relative sarcopenia.
Conclusions
Sarcopenia and relative sarcopenia are significantly associated with mortality regardless of CKD status. Relative sarcopenia is nearly three‐fold more prevalent amplifying its associated mortality risk at the population level. The association between relative sarcopenia and mortality is attenuated in persons with higher FMI.
Keywords: Sarcopenia, Relative sarcopenia, Chronic kidney disease, Obesity
Introduction
A reduction in skeletal muscle mass and strength, sarcopenia, can gradually occur with age1, 2 and is associated with mortality across many populations3, 4 including patients with end stage renal disease,5, 6 among whom more than 20% have sarcopenia.7, 8 Patients with chronic kidney disease (CKD) not requiring dialysis also have a high prevalence of sarcopenia9, 10, 11 although previous studies evaluating associations between sarcopenia and mortality in CKD have shown inconsistent results.12, 13, 14
Muscle mass is directly correlated with fat mass such that with higher fat mass, there is generally a compensatory increase in muscle mass.15 Conventional definitions of sarcopenia do not detect individuals with a deficit in muscle mass relative to fat mass; that is, relative sarcopenia.
We recently developed cutpoints to define sarcopenia and relative sarcopenia using dual energy X‐ray absorptiometry (DXA) data from 14 850 participants in the US National Health and Nutrition Examination Survey (NHANES).16 Because of the known sex and race/ethnicity differences in muscle mass,17, 18 our definitions are sex and race/ethnicity specific. Compared with sarcopenia, we found a higher prevalence of relative sarcopenia in an NHANES cohort.9 Further, compared with sarcopenia, relative sarcopenia demonstrated stronger correlations with physical function in patients with rheumatoid arthritis19 and better discriminated those with poor physical functioning and a higher risk of incident disability in the general elderly population.20 The association between relative sarcopenia and mortality is unknown.
We used DXA measures of lean and fat mass from NHANES 1999 to 2006 to determine the associations of sarcopenia and relative sarcopenia with mortality and whether these associations differed among participants with and without CKD. We hypothesized that both sarcopenia and relative sarcopenia are associated with mortality regardless of CKD status. Given the known higher prevalence of relative sarcopenia compared with sarcopenia in NHANES participants,9 we hypothesized that relative sarcopenia contributes to a higher proportion of deaths at the population level [population attributable risk (PAR)]. A number of prior studies have observed a survival advantage of overweight and mildly or moderately obese persons compared with those with normal weight among the elderly and chronic disease states (often termed the ‘obesity paradox’).21, 22, 23, 24, 25, 26 Therefore, we further hypothesized that adiposity attenuates the association between relative sarcopenia and mortality.
Methods
Study population
The study was conducted using NHANES data from 1999 to 2006, as these years included DXA measures of body composition. NHANES was designed to represent the non‐institutionalized, US civilian population using a complex, multistage probability sampling method including oversampling of non‐Hispanic Blacks and Hispanics to produce reliable race‐specific and ethnicity‐specific statistics. We included in our analyses the 14 850 participants 20 or more years of age with body composition data. All procedural manuals and survey contents are indexed and publically accessible online.27
Kidney disease and co‐morbidities
Laboratory assays for serum creatinine and cystatin C and urine albumin and creatinine were standardized and calibrated in accordance with established methods. Serum creatinine values were available in 13 980 participants, and values from 1999 to 2000 were calibrated to the Cleveland Clinic laboratory standard by multiplying by 1.013 and then adding 0.147. Serum cystatin C was measured in a subset of 3 754 participants including all of those ≥60 years of age, and a random 25% sample of those <60 years of age, supplemented with those with a serum creatinine >1.2 mg/dL in men and 1.0 in women. Estimated glomerular filtration rate (eGFR) was calculated using the appropriate creatinine or cystatin C age‐specific, sex‐specific, and race‐specific (Black vs. non‐Black) Chronic Kidney Disease Epidemiology Collaboration equations (eGFRCr and eGFRCys, respectively).28, 29 Albuminuria was defined as urine albumin:creatinine ratio ≥ 25 mg/g for women and ≥ 17 mg/g for men.30
We defined CKD using both creatinine (CKDCr) and cystatin C (CKDCys). Normal or near normal kidney function, hereinafter referred to as ‘non‐CKD’, was defined as eGFR ≥60 mL/min/1.73 m2 without albuminuria. CKD was defined as albuminuria or eGFR <60 mL/min/1.73 m2.
Diabetes was defined by an affirmative response to whether the participant had been told by a doctor that he or she had ‘diabetes or sugar diabetes’ while not pregnant, the current use of insulin or oral hypoglycaemic medications, or a glycohaemoglobin level >6.5%. Cardiovascular disease was defined by self‐report of a physician diagnosis of congestive heart failure, coronary heart disease, angina, heart attack (also called myocardial infarction), or stroke. Cancer was defined by an affirmative response to the question ‘have you ever been told by a doctor or other health professional that you had cancer or malignancy of any kind?’ Smoking status was defined by self‐report of smoking >100 cigarettes/lifetime. Liver disease was defined by an affirmative response to ‘Has a doctor or other health professional ever told you that you had any kind of liver condition?’
Leisure time physical activity information was obtained and given a MET score according to the Compendium of Physical Activities.31 Participants were categorized as meeting the minimum goal (<450 MET/min/week), more than meeting the minimum goal (450 to <750 MET/min/week) and exceeding the recommended goal (≥750 MET/min/week).32
Body composition
Whole body DXA scans were acquired using Hologic QDR 4500A fan‐beam densitometers (Hologic, Inc, Bedford, MA) in NHANES participants 8 years of age and older. DXA exclusion criteria included pregnancy, weight >300 pounds (136 kg, because of the weight limit of the scanner), height >77 inches (195 cm), recent nuclear medicine scan, or exposure to radioactive contrast. To account for potential biases of non‐random missing data, multiple imputation was performed by the National Center for Health Statistics for 3477 participants with invalid or missing data (with the exception of pregnant women) using over 50 non‐DXA variables and 32 DXA variables.33, 34, 35 The DXA measures of body composition included appendicular lean mass index (ALMI, kg/m2) and whole body fat mass index (FMI, kg/m2).
Mortality
The National Center for Health Statistics has linked mortality data from NHANES to death certificate data in the National Death Index with follow‐up through 31 December 2011.36 The National Death Index matches individuals on personal and demographic criteria, such as social security number and date of birth, and provides mortality status and months of follow‐up.37
Statistics
The following describes the generation of ALMI and FMI standard deviation scores and definitions of sarcopenia and relative sarcopenia, as previously reported in our study of NHANES participants.9 Sex‐specific and race/ethnicity‐specific curves for ALMI and FMI relative to age were previously published using NHANES data.17 The Lambda, Mu, Sigma (LMS) method38, 39 addresses skew, non‐linearity, and heteroscedasticity and is the standard method for expressing body composition results as standard deviation score. We used these curves to convert the ALMI and FMI results to sex‐specific and race/ethnicity‐specific Z‐scores relative to age and T‐scores based on LMS values in a 25 year‐old. The magnitude of the race differences in ALMI are illustrated within this dataset: among the Black men (mean age 42.5 years), the mean ALMI T‐score was −0.47 when compared with young Black men and +0.47 when compared with young White men. Similar patterns were observed among the women. Failure to employ race/ethnicity specific definitions would result in significant misclassification of Black individuals with low muscle mass for sex and race as normal. We defined sarcopenia as an ALMI T‐score < −2.0.
We generated fat‐adjusted ALMI (ALMIFMI) T‐scores by obtaining residuals based on the regression of ALMI T‐scores on FMI T‐scores within sex and race/ethnicity categories among 20 to 40 year‐olds, consistent with conventional definitions of sarcopenia based on comparisons with young adults. The relations between ALMI and FMI T‐scores were non‐linear; therefore, we included a significant FMI2 term in prediction models. We previously demonstrated that failure to adjust for this relation resulted in an overestimate of residuals at the extremes of adiposity. ALMIFMI Z‐scores were determined within age (by decade), sex, and race/ethnicity categories.15 We defined relative sarcopenia as an ALMIFMI T‐score < −2.0, or more than 2 SD below the mean for a population of 25 year‐olds of the same sex, race/ethnicity, and FMI.
Obesity defined by FMI (ObeseFMI) was defined based on sex‐specific and race/ethnicity‐specific FMI cutpoint values developed by Kelly et al. in order to generate a prevalence of obesity that was the same as observed using a body mass index (BMI) cutpoint of 30 kg/m2 in 25 year‐old participants in NHANES.17
We used descriptive weighted statistics to characterize the study population, with categorical characteristics summarized as counts/percentages and continuous characteristics summarized as mean with standard error or median with 25th to 75th percentile range. We calculated Kaplan–Meier product limit estimates and produced survival curves for participants with and without sarcopenia and relative sarcopenia.
Because of the known effects of muscle mass/creatinine generation on the serum creatinine concentration and the known effects of fat mass on the serum cystatin C concentration (more fat mass is associated with higher cystatin C concentrations),40, 41 we ran parallel analyses using CKD defined by eGFRCr and eGFRCys. Cystatin C measures were collected in all participants aged 60 and older from 1999 to 2000 and a 25% random sample of participants aged 12–59 years supplemented with all individuals with high serum creatinine 1.2 mg/dL in men and >1.0 mg/dL in women. Therefore, we repeated CKDCr analyses limited to individuals with both creatinine and cystatin C measures.
We performed unadjusted and multivariable proportional hazards (Cox) regression analyses, adjusting for variables determined to be significantly associated with mortality on univariate screen, including age, sex, and race/ethnicity. Compared with White individuals, Black individuals have a higher mortality rate and are more likely to be considered sarcopenic at a given muscle mass when evaluated using race/ethnicity specific definitions. Adjustment for race did not result in appreciable changes in the HR, indicating that race did not confound the associations between sarcopenia (or relative sarcopenia) and mortality in these models. In order to address potential effect modification by race, we tested multiplicative interaction terms between race and sarcopenia or relative sarcopenia. A subset of individuals met criteria for sarcopenia and relative sarcopenia; therefore, we also examined the association of mortality in those individuals who only met criteria for relative sarcopenia.
We tested the associations of sarcopenia and relative sarcopenia with mortality stratified by CKD status and evaluated for ‘effect’ modification using CKD status × sarcopenia or CKD status × relative sarcopenia interaction terms. We conducted sensitivity analyses using alternative definitions of CKD (definition 1: eGFR <30 mL/min/1.73 m2; definition 2: eGFR <30 mL/min/1.73 m2 and/or urine albumin:creatinine ratio ≥3500 mg/g).
The mortality risk of relative sarcopenia may differ according to degree of adiposity, that is, low muscle mass relative to fat mass may confer less risk when fat mass is high. We tested whether adiposity modified the associations of sarcopenia or relative sarcopenia with mortality using multiplicative interaction terms between sarcopenia or relative sarcopenia and FMI.
The burden of sarcopenia and relative sarcopenia on a population level is a function of both the strength of the association and the prevalence of each factor in the population. Using adjusted relative risks and corresponding weighted prevalences of sarcopenia and relative sarcopenia among the decedents (pd), we calculated the PARs of sarcopenia and relative sarcopenia using the following formula: pd (RR − 1/RR).42 Conceptually, the PAR represents the proportion of deaths in the total population that can be attributed to the exposure.
We considered two‐tailed P‐values <0.05 statistically significant. We performed all analyses using survey procedures with SAS version 9.4 for Unix (SAS Institute, Cary, North Carolina) to account for the complex sampling design of NHANES and appropriately weighted participants in statistical models.
Results
Participant characteristics
Participant characteristics including body composition measures are displayed in Table 1. Overall, 534 (3.0%) individuals met criteria for sarcopenia and 1299 (8.7%) for relative sarcopenia. Of the 1299 individuals with relative sarcopenia, 856 (66%) did not meet criteria for sarcopenia. Of the 534 individuals with sarcopenia, 91 (17%) did not meet criteria for relative sarcopenia. This subset had markedly lower FMI T‐scores (mean ± standard error T‐score = −1.64 ± 0.06) such that the ALMI was not low when considered in the context of low fat mass. Adjustment for the low FMI resulted in an ALMIFMI T‐score greater than −2.0 in these participants. The participant characteristics in those with sarcopenia only, relative sarcopenia, both and neither are summarized in Table S1.
Table 1.
Participant characteristics of 14 850 participants with body composition data in the National Health and Nutrition Examination Survey 1999–2006
| Sarcopenia | Relative sarcopenia | |||
|---|---|---|---|---|
| Present | Absent | Present | Absent | |
| N (unweighted) (%, weighted) | 534 (3.0%) | 14 316 (97.0%) | 1299 (8.7%) | 13 551 (50.3%) |
| Age (median, 25th–75th percentile) | 53.6 (38.9–66.2) | 44.5 (33.2–56.0) | 59.4 (47.3–70.7) | 43.7 (32.6–54.8) |
| Female (%) | 53.5 | 50.4 | 52.8 | 50.3 |
| Race/ethnicity | ||||
| Non‐Hispanic White (%) | 80.9 | 79.2 | 83.8 | 78.9 |
| Non‐Hispanic Black (%) | 12.0 | 12.3 | 11.6 | 12.4 |
| Mexican American (%) | 7.1 | 8.5 | 4.6 | 8.7 |
| eGFR cystatin (n = 3754) | 73 ± 3 | 82 ± 1 | 70 ± 1 | 83 ± 1 |
| eGFR creatinine (n = 13 980) | 92 ± 1 | 93 ± 0.4 | 86 ± 1 | 94 ± 0.4 |
| Physical activity | ||||
| <450 MET/min/week (%) | 80.1 | 67.4 | 78.7 | 66.9 |
| 450–750 MET/min/week (%) | 8.9 | 13.8 | 11.3 | 13.8 |
| >750 MET/min/week (%) | 11.0 | 18.8 | 10.0 | 19.3 |
| Smoker (%) | 62.4 | 50.5 | 58.3 | 50.2 |
| Diabetes (%) | 8.5 | 8.8 | 12.0 | 8.5 |
| Cancer (%) | 15.6 | 7.7 | 15.4 | 7.3 |
| CVD (%) | 13.9 | 7.7 | 17.1 | 7.2 |
| Liver disease (%) | 5.2 | 2.1 | 5.3 | 1.9 |
| Education | ||||
| <12 years (%) | 32.5 | 21.8 | 29.4 | 21.6 |
| 12+ years (%) | 67.5 | 78.2 | 70.6 | 78.4 |
| Income | ||||
| <$20 000 (%) | 35.0 | 21.3 | 29.1 | 21.1 |
| $20 000–$45 000 (%) | 29.4 | 29.5 | 35.1 | 29.1 |
| $45 000–$75 000 (%) | 18.3 | 23.4 | 19.0 | 23.5 |
| $75 000+ (%) | 17.3 | 25.9 | 16.8 | 26.3 |
| Low vitamin D (<50 nmol/L) (%) | 51.3 | 47.8 | 50.6 | 47.7 |
| Serum albumin (g/dL) | 4.3 ± 0.02 | 4.3 ± 0.01 | 4.3 ± 0.01 | 4.3 ± 0.01 |
| Serum CRP (mg/dL) | 0.48 ± 0.06 | 0.42 ± 0.01 | 0.54 ± 0.03 | 0.41 ± 0.01 |
| Bicarbonate (mEq/L) | 25 ± 0.2 | 24 ± 0.1 | 25 ± 0.1 | 24 ± 0.1 |
| BMI (kg/m2) | ||||
| Male | 20.45 ± 0.24 | 28.42 ± 0.10 | 24.97 ± 0.27 | 28.43 ± 0.11 |
| Female | 19.94 ± 0.21 | 28.74 ± 0.14 | 24.18 ± 0.21 | 28.81 ± 0.15 |
| FMI (kg/m2) | ||||
| Male | 5.34 ± 0.18 | 8.32 ± 0.06 | 8.19 ± 0.17 | 8.24 ± 0.06 |
| Female | 7.33 ± 0.19 | 11.99 ± 0.10 | 10.47 ± 0.16 | 11.95 ± 0.10 |
| FMI T‐score | −0.66 ± 0.05 | 0.41 ± 0.02 | 0.27 ± 0.03 | 0.38 ± 0.02 |
| FMI Z‐score | −1.25 ± 0.05 | 0.05 ± 0.02 | −0.28 ± 0.03 | 0.03 ± 0.02 |
| ObeseFMI (%) | 3.2 | 36.9 | 30.1 | 36.3 |
| % Body fat | ||||
| Male | 25.25 ± 0.56 | 28.35 ± 0.11 | 31.62 ± 0.34 | 28.02 ± 0.11 |
| Female | 35.97 ± 0.58 | 40.32 ± 0.15 | 42.24 ± 0.33 | 40.02 ± 0.16 |
| ALMI (kg/m2) | ||||
| Male | 6.06 ± 0.03 | 8.70 ± 0.02 | 6.73 ± 0.04 | 8.77 ± 0.02 |
| Female | 4.68 ± 0.02 | 6.85 ± 0.03 | 5.16 ± 0.03 | 6.91 ± 0.03 |
| ALMI T‐score | −2.48 ± 0.02 | 0.03 ± 0.01 | −1.73 ± 0.03 | 0.09 ± 0.01 |
| ALMI Z‐score | −2.26 ± 0.03 | 0.07 ± 0.02 | −1.49 ± 0.03 | 0.12 ± 0.02 |
| ALMIFMI T‐score | −2.86 ± 0.05 | −0.28 ± 0.02 | −2.67 ± 0.03 | −0.18 ± 0.01 |
| ALMIFMI Z‐score | −2.08 ± 0.06 | 0.06 ± 0.02 | −1.49 ± 0.03 | 0.14 ± 0.01 |
Data presented as mean+/− SE or % unless otherwise stated. Group categorization is not exclusive; that is, there is some overlap between groups. ALMI, appendicular lean mass index; BMI, body mass index; eGFR, estimated glomerular filtration rate; FMI, fat mass index.
Compared with participants without sarcopenia, participants with sarcopenia were more likely to be smokers, had higher prevalences of cancer, cardiovascular disease, and liver disease, and experienced less education, lower income, lower physical activity, and lower BMI, FMI, and ALMI. Similar results were seen when comparing participants with and without relative sarcopenia; additionally, a greater proportion of the group with relative sarcopenia had diabetes, compared with those without relative sarcopenia.
Sarcopenia and relative sarcopenia
Figure 1A and B illustrates the survival probability according to sarcopenia, and relative sarcopenia status. Both sarcopenia and relative sarcopenia were associated with mortality (P < 0.01) in unadjusted analyses and were independently associated with mortality after adjustment for age, sex, race/ethnicity, physical activity, smoking status, diabetes, cancer, liver disease, cardiovascular disease, education, and income (HR sarcopenia 2.20, 95% CI 1.69 to 2.86; HR relative sarcopenia 1.60, 95% CI 1.31 to 1.96). Race did not modify the associations between sarcopenia or relative sarcopenia and mortality.
Figure 1.
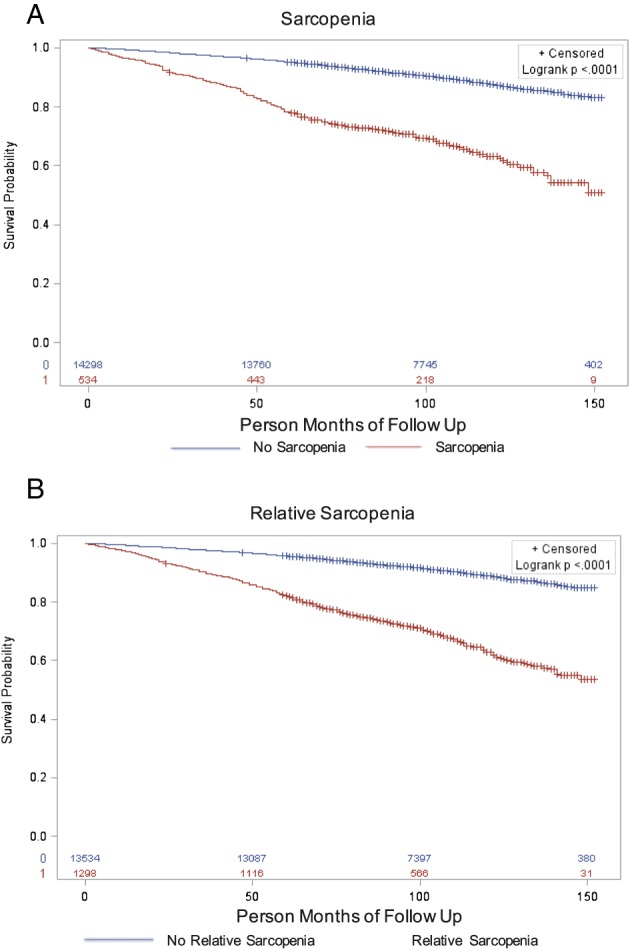
Kaplan–Meier survival curves of 14 850 National Health and Nutrition Examination Survey participants by sarcopenia (A) and relative sarcopenia (B) status.
The cohort included 1299 participants with relative sarcopenia. Among these, 443 (34%) also met the criteria for sarcopenia. When the analyses were limited to the 856 without sarcopenia (described in Table S1), the HR for mortality in the relative sarcopenia group decreased from 1.60 to 1.32 (95% CI 1.08 to 1.61).
In the overall cohort, the PARs for sarcopenia and relative sarcopenia were 5.2% (95% CI 3.4% to 6.4%) and 8.4% (95% CI 4.8% to 11.2%), respectively.
Association with chronic kidney disease
In multivariate models adjusted for age, sex, race/ethnicity, physical activity, smoking status, diabetes, cancer, liver disease, cardiovascular disease, education, and income, CKDCr was associated with increased risk of mortality (HR 3.70, 95% CI 2.98 to 4.60).
In participants with and without CKDCr, independent associations were found between mortality and sarcopenia (HR CKD 1.97, 95% CI 1.36 to 2.87; HR non‐CKD 2.41, 95% CI 1.62 to 3.57) and relative sarcopenia (HR CKD 1.49, 95% CI 1.14 to 1.93; HR non‐CKD 1.78, 95% CI 1.26 to 2.51). While the hazard ratios for sarcopenia and relative sarcopenia with mortality were lower among participants with CKD, tests for interaction were not statistically significant (sarcopenia P = 0.17; relative sarcopenia P = 0.10).
When limited to individuals with both cystatin C and creatinine measures (n = 3754), CKDCr and CKDCys status significantly modified the associations between sarcopenia and relative sarcopenia with mortality; the HRs were greater in those without CKD, compared with those with CKD (CKDCr by sarcopenia P = 0.01; all other interaction terms P < 0.01).
Sensitivity analyses demonstrated that the association of mortality with sarcopenia was comparable in those with and without CKD when a more stringent definition of CKD was applied. For example, when CKD was defined as eGFR <30 mL/min/1.73 m2, the HR for mortality was 2.41 (95% CI 0.98 to 5.96), compared with 2.41 (95% CI 1.62 to 3.57) noted above in non‐CKD. In contrast, the finding that the association of relative sarcopenia with mortality was lower among participants with CKD, compared with those without CKD, was observed consistently across all CKD definitions, although tests for interaction were not significant. The primary and sensitivity models are provided in Table S2.
Effect (association) modification by obesity
Fat mass index significantly attenuated the association between relative sarcopenia with mortality (β = −0.09, P < 0.01). When stratified by obeseFMI status, relative sarcopenia was more strongly associated with mortality in non‐obese participants (HR obese = 1.25, 95% CI 0.94 to 1.67; HR non‐obese = 1.76, 95% CI 1.34 to 2.31; interaction term P = 0.01). In contrast, neither FMI nor obeseFMI status modified the association between sarcopenia and mortality (P = 0.39 and 0.13 for FMI and obeseFMI, respectively).
Only 18 individuals (0.001%) met criteria for sarcopenia and obeseFMI; their mean ALMIFMI T‐score was −4.80, consistent with severe relative sarcopenia in the context of sarcopenia despite obesity.
Figure 2 summarizes the adjusted HR for sarcopenia and relative sarcopenia in all participants, and stratified according to CKD status and obeseFMI status.
Figure 2.
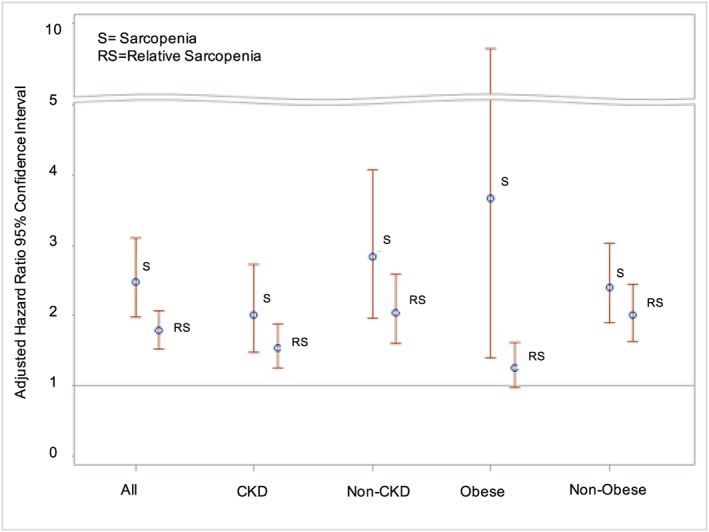
Risk of death with sarcopenia and relative sarcopenia in all, CKD, non‐CKD, obese, and non‐obese participants of the National Health and Nutrition Examination Survey 1999–2006.
Discussion
Using body composition data from NHANES, we found that both sarcopenia and relative sarcopenia were associated with mortality. Higher FMI attenuated the association between relative sarcopenia and mortality. While both constructs (sarcopenia and relative sarcopenia) are associated with mortality, the construct of relative sarcopenia captures a larger proportion of the participants and thus represents a more disturbing threat to public health. Had we not considered fat mass when evaluating muscle mass, we would have missed the significant mortality risk among the substantial proportion with relative sarcopenia but not sarcopenia.
Similar to other studies in the general population, we found a significant association between low muscle mass and mortality.3, 4, 12, 13, 43, 44 Numerous definitions have been used to define sarcopenia and to our knowledge, none is race‐specific45 and most use a relatively small reference population of 229 participants from New Mexico in 1986–1992.46 Our study updated these reference curves using more than 4500 young adults in the 1999–2004 NHANES dataset, providing a more representative sample of the current US population.17
Consideration of race is particularly important in these analyses, as skeletal muscle mass and life expectancy are known to vary by race and ethnicity.47, 48, 49 In the NHANES dataset, non‐Hispanic Blacks have higher mean muscle mass as compared with other races (e.g. ALMI White female = 6.61 ± 0.03 kg/m2; ALMI Black female = 7.89 ± 0.04 kg/m2). Therefore, if non‐race/ethnicity‐specific definitions of sarcopenia are used, many non‐Hispanic Blacks and Mexican American participants are categorized as having normal muscle mass despite a low measurement for their race/ethnicity. In this analysis, we defined sarcopenia and relative sarcopenia as 2 SD below these race/ethnicity‐specific means to provide a more relevant determination of low muscle mass in these groups. As an illustration of the magnitude of this effect, Androga, et al. reported that non‐Hispanic Blacks comprised 3% of NHANES participants with sarcopenia,13 while they comprised 12.3% in our study.
During the period of this study (1999–2011), the prevalence of CKD stages 1–4 was highest in non‐Hispanic Blacks followed by Mexican Americans and lastly non‐Hispanic Whites. The shorter life expectancy and misclassification of sarcopenia among non‐Hispanic Black persons49 with CKD may explain why our study observed an association of sarcopenia with mortality in the CKD population that was not detected in previous studies that did use race‐specific definitions of sarcopenia.12, 13, 43
Given the nearly 40% prevalence of obesity in the general population of the United States,50 traditional estimates of muscle mass that do not account for body size fail to appreciate relatively low muscle mass in a substantial proportion of the population. This issue is particularly problematic in disease states such as CKD with a higher prevalence of obesity compared with the general population.9 Our study is the first to examine and detect a significant association of relative sarcopenia with mortality. Given the much higher prevalence of relative sarcopenia compared with sarcopenia, relative sarcopenia exerts a more significant impact on public health.
Previous studies in elderly persons and various disease states showed a survival advantage of BMI 25–35 kg/m2 21, 22, 23, 24, 25, 26 suggesting an ‘obesity paradox’. Because BMI does not distinguish fat and muscle mass, these studies are unable to determine whether the survival advantage relates to higher adipose tissue or muscle mass (or both). In our study, higher FMI attenuated the association between relative sarcopenia and mortality, potentially supporting the paradox and providing a context to interpret the risks of relative sarcopenia. FMI did not modify the relation between sarcopenia and mortality, likely because sarcopenic patients almost universally have low FMI and excess adiposity is rare in this group.
Sarcopenic obesity is defined as the presence of both obesity and sarcopenia in an individual. The prevalence varies based on the criteria used to define sarcopenia and obesity.45, 51 Most studies use % body fat (%BF) to define obesity.13, 44, 45, 51 This definition of obesity is problematic in the context of sarcopenia, as lower muscle mass results in a greater %BF in two individuals with the same fat mass. In our study, only 18 individuals met criteria for both sarcopenia and obesity when FMI was used to define obesity. In contrast, when obesity was defined based on %BF, an additional 108 individuals meet criteria for sarcopenic obesity despite the fact that their FMI T‐scores were no greater than the average in the NHANES participants. These data illustrate that %BF is not a reliable measure of adiposity in individuals with low muscle mass.
The primary limitation of this study is the measurement of body composition at a single point in time. As such, we cannot infer a causal relation between mortality and altered body composition. Further, 3477 individuals (23%) in our analyses did not have 100% complete DXA data (e.g. one or more body regions could not be analysed accurately); therefore, multiple imputation was used for missing values. NHANES uses self‐reported race and does not capture differences in an individual's racial ancestry. Any misclassification of sarcopenia based on ancestry would bias our results to the null and further substantiate the significant results found in this study. In contrast, excess extracellular fluid (e.g. oedema in individuals with CKD) results in an overestimate of lean (body cell) mass by DXA.52 This misclassification would result in an overestimate of the association between sarcopenia and relative sarcopenia with mortality among those with CKD. Finally, we previously reported that stage 5 CKD was associated with lower FMI compared with those with less advanced CKD.9 The NHANES cohort used for this study contains few individuals with eGFR <15 mL/min/1.73 m2 thereby limiting the generalizability to those with advanced CKD.9
The strengths include the use of a novel way to account for body size when estimating the presence of muscle deficits that is particularly important in obese populations. Further, our race‐specific cutpoints for sarcopenia and relative sarcopenia provide more relevant assessments of muscle mass in these groups.
An additional strength of these analyses is the use of serum cystatin C‐based formulas for eGFR. The analyses of CKDcys were likely subject to less misclassification of CKD status because of the sampling strategy (enriched for those with greater serum creatinine) and avoidance of the confounding effects of low muscle mass to underestimate GFR. Although limited in power, these analyses suggested that sarcopenia and relative sarcopenia were associated with a greater risk of mortality among those without CKD, compared with those with CKD. There are two possible explanations for this finding. First, the impact of relative sarcopenia on mortality among those with CKD may appear less pronounced given the nearly four‐fold greater mortality risk in the CKD group compared with the non‐CKD group. Second, the presence of relative sarcopenia among those without CKD may represent co‐morbidities that have a greater relative impact on mortality, compared with the excess risk of mortality because of relative sarcopenia attributed to CKD.
In summary, sarcopenia and relative sarcopenia are significantly associated with mortality regardless of CKD status. Higher FMI attenuates the association between relative sarcopenia and mortality. Relative sarcopenia is nearly three‐fold more prevalent amplifying its associated mortality risk at the population level and should be recognized as a threat to the public health. Our published sex‐specific and race‐specific prediction equations for the generation of ALMI and ALMIFMI T‐score and Z‐score will facilitate the adoption of these methods in future studies.15
Conflict of interest
S.Z., J.L., J.B., G.C., and M.L. declare that they have no conflict of interest.
Funding
This research was supported by National Institutes of Health grants F32 DK111083 (S.Z.) and K24 DK076808 (M.B.L.).
Supporting information
Table S1 Participant characteristics of 14,850 participants with body composition data in the National Health and Nutrition Examination Survey 1999–2006.
Table S2 Hazard Ratios for Mortality using Alternative Definitions of CKD
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.53 All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Ziolkowski, S. L. , Long, J. , Baker, J. F. , Chertow, G. M. , and Leonard, M. B. (2019) Relative sarcopenia and mortality and the modifying effects of chronic kidney disease and adiposity. Journal of Cachexia, Sarcopenia and Muscle, 10: 338–346. 10.1002/jcsm.12396.
References
- 1. von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle 2010;1:129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta‐analysis of general population studies. J Diabetes Metab Disord 2017;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bunout D, de la Maza MP, Barrera G, Leiva L, Hirsch S. Association between sarcopenia and mortality in healthy older people. Australas J Ageing 2011;30:89–92. [DOI] [PubMed] [Google Scholar]
- 4. Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr 2007;86:1339–1346. [DOI] [PubMed] [Google Scholar]
- 5. Kittiskulnam P, Chertow GM, Carrero JJ, Delgado C, Kaysen GA, Johansen KL. Sarcopenia and its individual criteria are associated, in part, with mortality among patients on hemodialysis. Kidney Int 2017;92:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kakiya R, Shoji T, Tsujimoto Y, Tatsumi N, Hatsuda S, Shinohara K, et al. Body fat mass and lean mass as predictors of survival in hemodialysis patients. Kidney Int 2006;70:549–556. [DOI] [PubMed] [Google Scholar]
- 7. Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Barany P, Heimburger O, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol: CJASN 2014;9:1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim JK, Choi SR, Choi MJ, Kim SG, Lee YK, Noh JW, et al. Prevalence of and factors associated with sarcopenia in elderly patients with end‐stage renal disease. Clin Nutr (Edinburgh, Scotland) 2014;33:64–68. [DOI] [PubMed] [Google Scholar]
- 9. Ziolkowski SL, Baker J, Simard J, Chertow G, Leonard MB. Sarcopenia, relative sarcopenia and excess adiposity in chronic kidney disease. J Cachexia, Sarcopenia Muscle‐ Clin Rep 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma D, Hawkins M, Abramowitz MK. Association of sarcopenia with eGFR and misclassification of obesity in adults with CKD in the United States. Clin J Am Soc Nephrol: CJASN 2014;9:2079–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Souza VA, Oliveira D, Barbosa SR, Correa J, Colugnati FAB, Mansur HN, et al. Sarcopenia in patients with chronic kidney disease not yet on dialysis: analysis of the prevalence and associated factors. PLoS One 2017;12:e0176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Navaneethan SD, Kirwan JP, Arrigain S, Schold JD. Adiposity measures, lean body mass, physical activity and mortality: NHANES 1999‐2004. BMC Nephrol 2014;15:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Androga L, Sharma D, Amodu A, Abramowitz MK. Sarcopenia, obesity, and mortality in US adults with and without chronic kidney disease. Kidney Int Rep 2017;2:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pereira RA, Cordeiro AC, Avesani CM, Carrero JJ, Lindholm B, Amparo FC, et al. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant 2015;30:1718–1725. [DOI] [PubMed] [Google Scholar]
- 15. Weber D, Long J, Leonard MB, Zemel B, Baker JF. Development of novel methods to define deficits in appendicular lean mass relative to fat mass. PLoS One 2016;11:e0164385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baker JF, Davis M, Alexander R, Zemel BS, Mostoufi‐Moab S, Shults J, et al. Associations between body composition and bone density and structure in men and women across the adult age spectrum. Bone 2013;53:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X‐ray absorptiometry body composition reference values from NHANES. PLoS One 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silva AM, Shen W, Heo M, Gallagher D, Wang Z, Sardinha LB, et al. Ethnicity‐related skeletal muscle differences across the lifespan. Am J Hum Biol: Off J Hum Biol Counc 2010;22:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baker JF, Giles JT, Weber D, Leonard MB, Zemel BS, Long J, et al. Assessment of muscle mass relative to fat mass and associations with physical functioning in rheumatoid arthritis. Rheumatology (Oxford) 2017;56:981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baker JF, Long J, Leonard MB, Harris T, Delmonico MJ, Santanasto A, et al. Estimation of skeletal muscle mass relative to adiposity improves prediction of physical performance and incident disability. J Gerontol A Biol Sci Med Sci 2017;73:946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, et al. Obesity paradox in end‐stage kidney disease patients. Prog Cardiovasc Dis 2014;56:415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oreopoulos A, Padwal R, Kalantar‐Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta‐analysis. Am Heart J 2008;156:13–22. [DOI] [PubMed] [Google Scholar]
- 23. Oreopoulos A, Kalantar‐Zadeh K, Sharma AM, Fonarow GC. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr Med 2009;25:643–659, viii. [DOI] [PubMed] [Google Scholar]
- 24. Costanzo P, Cleland JG, Pellicori P, Clark AL, Hepburn D, Kilpatrick ES, et al. The obesity paradox in type 2 diabetes mellitus: relationship of body mass index to prognosis: a cohort study. Ann Intern Med 2015;162:610–618. [DOI] [PubMed] [Google Scholar]
- 25. Auyeung TW, Lee JS, Leung J, Kwok T, Leung PC, Woo J. Survival in older men may benefit from being slightly overweight and centrally obese—a 5‐year follow‐up study in 4,000 older adults using DXA. J Gerontol A Biol Sci Med Sci 2010;65:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu JL, Kalantar‐Zadeh K, Ma JZ, Quarles LD, Kovesdy CP. Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol 2014;25:2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/index.htm.
- 28. Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 2008;51:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Boer IH, Astor BC, Kramer H, Palmas W, Rudser K, Seliger SL, et al. Mild elevations of urine albumin excretion are associated with atherogenic lipoprotein abnormalities in the Multi‐Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2008;197:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:S498–S504. [DOI] [PubMed] [Google Scholar]
- 32. Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007;39:1423–1434. [DOI] [PubMed] [Google Scholar]
- 33. Schenker N, Borrud LG, Burt VL, Curtin LR, Flegal KM, Hughes J, et al. Multiple imputation of missing dual‐energy X‐ray absorptiometry data in the National Health and Nutrition Examination Survey. Stat Med 2011;30:260–276. [DOI] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey, 1999‐2006 DXA multiple imputation files. https://wwwn.cdc.gov/Nchs/Nhanes/Dxa/Dxa.aspx.
- 35. Centers for disease control and prevention National Health and Nutrition Examination Survey: Technical Documentation for the 1999–2004 Dual Energy X‐Ray Absorptiometry (DXA) Multiple Imputation Data files February 2008.
- 36. Centers for Disease Control and Prevention . NCHS data linked to NDI mortality files. https://www.cdc.gov/nchs/data‐linkage/mortality.htm.
- 37. Fillenbaum GG, Burchett BM, Blazer DG. Identifying a national death index match. Am J Epidemiol 2009;170:515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Flegal KM, Cole TJ. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. Natl Health Stat Rep 2013;1–3. [PubMed] [Google Scholar]
- 39. Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr 1990;44:45–60. [PubMed] [Google Scholar]
- 40. Chew‐Harris JS, Florkowski CM, George PM, Elmslie JL, Endre ZH. The relative effects of fat versus muscle mass on cystatin C and estimates of renal function in healthy young men. Ann Clin Biochem 2013;50:39–46. [DOI] [PubMed] [Google Scholar]
- 41. S‐w K, Jung H‐W, Kim C‐H, K‐i K, Chin HJ, Lee H. A new equation to estimate muscle mass from creatinine and cystatin C. PLoS One 2016;11:e0148495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health 1998;88:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abramowitz MK, Hall CB, Amodu A, Sharma D, Androga L, Hawkins M. Muscle mass, BMI, and mortality among adults in the United States: a population‐based cohort study. PLoS One 2018;13:e0194697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Batsis JA, Mackenzie TA, Emeny RT, Lopez‐Jimenez F, Bartels SJ. Low lean mass with and without obesity, and mortality: results from the 1999‐2004 National Health and Nutrition Examination Survey. Journals of Gerontology Series A, Biological Sciences and Medical Sciences 2017;72:1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez‐Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual‐energy X‐ray absorptiometry data from the National Health and Nutrition Examination Survey 1999‐2004. J Am Geriatr Soc 2013;61:974–980. [DOI] [PubMed] [Google Scholar]
- 46. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 47. Rahman M, Berenson AB. Racial difference in lean mass distribution among reproductive‐aged women. Ethn Dis 2010;20:346–352. [PMC free article] [PubMed] [Google Scholar]
- 48. Wagner DR, Heyward VH. Measures of body composition in blacks and whites: a comparative review. Am J Clin Nutr 2000;71:1392–1402. [DOI] [PubMed] [Google Scholar]
- 49. Kochanek KD, Murphy SL, Xu J, Tejada‐Vera B. Deaths: final data for 2014. Natl Vital Stat Rep: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System 2016;65:1–122. [PubMed] [Google Scholar]
- 50. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015‐2016. NCHS Data Brief 2017;1–8. [PubMed] [Google Scholar]
- 51. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 2018;14:513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Georgiou E, Virvidakis K, Douskas G, Lambrinoudaki I, Voudiklari S, Katsoudas S, et al. Body composition changes in chronic hemodialysis patients before and after hemodialysis as assessed by dual‐energy x‐ray absorptiometry. Metab: Clin Exp 1997;46:1059–1062. [DOI] [PubMed] [Google Scholar]
- 53. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Participant characteristics of 14,850 participants with body composition data in the National Health and Nutrition Examination Survey 1999–2006.
Table S2 Hazard Ratios for Mortality using Alternative Definitions of CKD


