Abstract
Background
Muscle atrophy (MA) and muscle strength decline are important clinical features in chronic liver disease (CLD) patients. An easy to perform MA screening method without need for special equipment would be helpful. We evaluated the usefulness of the previously reported finger‐circle test as screening for MA in CLD patients.
Methods
We retrospectively enrolled 358 Japanese CLD outpatients (70.8 ± 10.2 years, male/female = 234/124) who had undergone a computed tomography examination from December 2017 to March 2018, of whom 137 had chronic hepatitis, 169 had liver cirrhosis Child–Pugh A, and 52 had liver cirrhosis Child–Pugh B/C. Bilateral psoas muscle area at the middle of the third lumber vertebra (L3) was evaluated with computed tomography findings, which was performed as a screening of hepatocellular carcinoma, using a previously reported parameter for MA [psoas index (PI): total psoas muscle area (cm2)/height (m)2] [mean PI ± standard deviation (SD) of male patients: 6.50 ± 1.13 cm2/m2 and those of female patients: 4.30 ± 0.90 cm2/m2]. We then evaluated the correlation between MA and finger‐circle test results in these patients.
Results
The mean PI values for finger‐circle test results Bigger, Just‐fits, and Smaller were 5.64 ± 1.34, 5.00 ± 1.25, and 4.83 ± 1.46 cm2/m2, respectively, in male patients (P < 0.001) and 4.31 ± 1.06, 3.93 ± 0.97, and 3.42 ± 0.94 cm2/m2, respectively, in female patients (P = 0.001). We found that a finger‐circle test result in male patients other than Bigger (Just‐fits and Smaller) predicted a decline in psoas muscle area of L3 to PI 5.25 cm2/m2 (sensitivity/specificity 0.619/0.667, area under the curve 0.654, 95% confidence interval 0.583–0.724), which was approximately mean minus 1 SD (5.37 cm2/m2). On the other hand, a Smaller test result in female patients predicted a decline in psoas muscle area of L3 to PI 3.33 cm2/m2 (sensitivity/specificity 0.740/0.583, area under the curve 0.698, 95% confidence interval 0.583–0.813), approximately mean minus 1 SD (3.40 cm2/m2).
Conclusions
The finger‐circle test is an easy to perform and effective screening method for predicting earlier stage of MA in CLD patients without the need for special equipment.
Keywords: Muscle atrophy, Sarcopenia, Chronic liver disease, Finger‐circle test, CT
Introduction
Rosenberg in 1989 proposed sarcopenia as a condition that features age‐related muscle volume and function decline.1 Since that study, secondary sarcopenia, including muscle atrophy (MA) and muscle strength decline (MSD), has been reported to occur in chronic liver disease (CLD) patients, including those with chronic hepatitis (CH).2, 3 The diagnostic trees of the European Working Group on Sarcopenia in Older People4 and Asian Working Group for Sarcopenia5 for elderly people, as well as the criteria of the Japan Society of Hepatology (JSH) for CLD patients,6 note that assessment of muscle volume or MA requires findings from such examinations as dual‐energy X‐ray absorptiometry (DEXA), bioelectrical impedance analysis (BIA), or computed tomography (CT). However, CLD patients being treated at a local clinic cannot undergo such testing, because of the high cost of the devices for family physicians. Furthermore, X‐ray exposure is unavoidable in DEXA and CT examinations. Thus, development of an easy and economical method for surveillance for MA and screening of large numbers of CLD patients without the need for a special device is anticipated.
Recently, the finger‐circle (yubi‐wakka) test,7 which uses the patient's own fingers and does not require a special machine, has been proposed. The aim of the present study was to evaluate the usefulness of findings obtained with the finger‐circle test for surveillance of MA in CLD patients.
Materials/methods
Patients
For the present study, we enrolled 358 Japanese outpatients with CLD who underwent CT for screening of hepatocellular carcinoma (HCC) from December 2017 to March 2018. All were self‐reliant in regard to activities of daily living. Those with oesophageal‐gastric varices, collateral vessel formation, pathological findings, and/or a platelet count under 10 × 104 cells/μL were considered to have liver cirrhosis (LC) and classified using the Child–Pugh (CP) classification. Twelve patients with obesity [body mass index (BMI) 30 kg/m2 or more] (3.4%) were included (male:female = 4:8). Patients with past history of treatment against chronic heart failure and/or chronic renal failure, which may result extreme lower oedema, were excluded from this study. Tumour node metastasis stage was determined based on the criteria of the Liver Cancer Study Group of Japan, 6th edition8 and used for evaluation of HCC.
Finger‐circle test
The finger‐circle test was developed to determine whether the maximum non‐dominant calf circumference is larger than the individual subject's finger‐circle circumference, formed with the index finger and thumbs of both hands. Finger‐circle testing was carried out with the patient in a seated position, with turning up a hem. Dominant foot was determined as a usual first step side at the starting to walk. Based on the results of the finger‐circle test, the present patients were divided into three groups: Bigger, Just‐fits, and Smaller, as shown in Figure 1.
Figure 1.
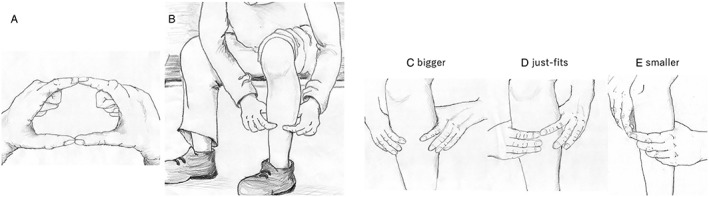
Finger‐circle (yubi‐wakka) test.
Evaluation of muscle atrophy and muscle strength
Muscle atrophy was defined using the previously reported psoas index (PI), which was calculated based on the psoas muscle area at the middle of the third lumber vertebra (L3) level (cm2), shown by CT, and height (m) (total bilateral psoas muscle area/height2: cm2/m2). For this retrospective study, we used results from a screening or follow‐up enhanced CT examination performed for HCC. Values for bilateral psoas muscle at the middle of L3 level obtained with CT were evaluated2 (Figure S1). The area of psoas muscle was manually traced and calculated on CT images using personal computer software (Centricity Web DX, ver. 3.7.3.6417: GE Healthcare Japan, Tokyo). The PI values for healthy young male normal subjects (45.6 ± 5.7 years, BMI 25.3 ± 3.1 kg/m2) and female normal subjects (47.0 ± 6.1 years, BMI 21.7 ± 3.0 kg/m2) used were 6.50 ± 1.13 and 4.30 ± 0.90 cm2/m2, respectively, and were calculated from previous findings of CT examinations of young normal control subjects, who were proven to have no obvious diseases (e.g. chronic renal failure, chronic heart failure, CLD, and diabetes mellitus) and past history in medical check‐up procedures with interview, electrocardiogram, urine and blood examinations, and positron emission tomography/CT as part of a medical check‐up procedure.2 A mean PI value lower than −2 standard deviation (SD) was defined as MA (male patients: 4.24 cm2/m2, female patients: 2.50 cm2/m2), while a mean PI value lower than −1 SD was defined as pre‐MA (male patients: 5.37 cm2/m2, female patients: 3.40 cm2/m2).2
Handgrip strength was measured using a hand dynamometer (TL110; TOEI LIGHT CO., LTD., Saitama, Japan) with the subject in a standing position. The highest values for both right and left handgrip strength from two measurements were averaged and then used for analysis. For assessment of MSD, the JSH criteria for handgrip strength decline (cut‐off value in male patients: 26 kg, in female patients: 18 kg) were used.6 In the present analysis, positive for both MA and MSD was provisionally defined as sarcopenia.
We evaluated the ability of detection of abnormal MA status using finger‐circle test results in CLD patients in a retrospective manner.
The study was conducted in compliance with the Helsinki Declaration, and the protocol was approved by the Institutional Ethics Committee of Ehime Prefectural Central Hospital (No. 27‐26).
Statistical analysis
Values are expressed as the mean ± SD. Statistical analyses were performed using Student's t‐test, Mann–Whitney's U‐test, one‐way analysis of variance, a Kruskal–Wallis test, Spearman's test, receiver operator characteristic curve (ROC) analysis, or area under the curve (AUC) analysis, as appropriate. Bonferroni's method was used for multiple comparisons among the three groups with EZR version 1.29,9 a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). P values less than 0.05 were considered to indicate statistical significance.
Results
Of the 358 patients with CLD, 234 (65.4%) were male, and the average age of all was 70.8 ± 10.2 years. Furthermore, CH was noted in 137, while 169 had LC and were classified as CP‐A, and 52 had LC and were classified as CP‐B/C (B: 43, C: 9) (Tables 1 and 2). The average age of those with CH was 70.8 ± 10.8 years, with LC and CP‐A was 71.1 ± 9.3 years, and with LC and CP B/C was 69.8 ± 11.2 years (P = 0.724).
Table 1.
Clinical characteristics of all patients
| All (n = 358) | CH (n = 137) | LC CP‐A (n = 169) | LC CP‐B/C (n = 52) | |
|---|---|---|---|---|
| Age, yearsa (SD) | 70.8 (10.2) | 70.8 (10.8) | 71.1 (9.3) | 69.8 (11.2) |
| Gender, male:female* | 234:124 | 99:38 | 107:62 | 28:24 |
| BMI, kg/m2 a (SD) | 23.4 (21.0–25.6) | 23.4 (3.5) | 23.6 (3.3) | 23.6 (3.8) |
| Aetiology, HCV:HBV:HBV&HCV:Alcohol:others | 192:49:1:54:62 | 72:30:1:15:19 | 93:17:0:28:31 | 27:2:0:11:12 |
| AST, IU/La (SD)** , **** | 40.0 (27.8) | 33.1 (22.6) | 40.1 (25.5) | 58.2 (37.9) |
| ALT, IU/La (SD)* | 30.6 (24.2) | 26.8 (20.4) | 31.5 (26.4) | 37.3 (24.9) |
| Platelets, 104/μLa (SD)** , *** | 14.1 (6.0) | 18.5 (5.0) | 11.8 (4.7) | 10.3 (5.0) |
| Total bilirubin, mg/dLa (SD)** , **** | 0.98 (0.93) | 0.7 (0.3) | 0.9 (0.4) | 2.0 (2.0) |
| Albumin, g/dLa (SD)** , *** , **** | 3.99 (0.61) | 4.28 (0.33) | 4.05 (0.52) | 3.08 (0.62) |
| Prothrombin time, %a (SD)** , *** , **** | 84.7 (15.3) | 92.3 (10.6) | 84.3 (13.7) | 69.0 (16.5) |
| History of HCC (%)**** | 228 (63.7) | 84 (61.3) | 121 (71.6) | 23 (44.2) |
| TNM stage in HCC patients, none:I:II:III:IV | 266:25:37:14:16 | 102:11:12:4:8 | 124:12:18:8:7 | 40:2:7:2:1 |
| HU by CTa (SD)** | 47.1 (5.0) | 47.5 (5.0) | 47.3 (4.8) | 45.4 (5.2) |
| Frequency of finger‐circle test results, Bigger:Just‐fits:Smaller | 192:104:62 | 78:35:24 | 90:54:25 | 24:15:13 |
| Frequency of MA, none:pre‐MA:MA | 179:121:58 | 68:47:22 | 87:57:25 | 24:17:11 |
| Positive for handgrip strength decline (%)* | 152 (42.5) | 48 (35.0) | 75 (44.4) | 29 (55.8) |
| With sarcopenia (%) | 30 (8.4) | 8 (5.8) | 14 (8.3) | 8 (15.4) |
ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index; CH, chronic hepatitis; CP, Child–Pugh classification; CT, computed tomography; HBV, hepatitis B virus; HCC, hepatitis C virus; HCV, hepatitis C virus; HU, Hounsfield units; LC, liver cirrhosis; MA, muscle atrophy.
Mean value.
P < 0.05 between CH and LC CP‐B/C.
P < 0.01 between CH and LC CP‐B/C.
P < 0.01 between CH and LC CP‐A.
P < 0.01 between LC CP‐A and CP‐B/C (Bonferroni's method).
Table 2.
Clinical characteristics divided by gender
| Male patients (n = 234) | Female patients (n = 124) | P value | |
|---|---|---|---|
| Age, yearsa (SD) | 70.3 (10.4) | 71.8 (9.6) | 0.179 |
| CLD stage, CH:LC CP‐A:LC CP‐B:LC CP‐C | 100:106:22:6 | 37:63:21:3 | 0.009 |
| BMI, kg/m2 a (SD) | 23.4 (3.1) | 23.6 (4.0) | 0.548 |
| Aetiology, HCV:HBV:HBV&HCV:NBNC | 115:35:1:47:36 | 77:14:7:26 | 0.103 |
| AST, IU/La (SD) | 38.7 (28.2) | 42.5 (27.0) | 0.221 |
| ALT, IU/La (SD) | 30.4 (24.6) | 30.9 (23.9) | 0.858 |
| Platelets, 104/μLa (SD) | 14.7 (5.7) | 13.1 (6.3) | 0.012 |
| Total bilirubin, mg/dLa (SD) | 0.99 (0.82) | 0.98 (1.1) | 0.988 |
| Albumin, g/dLa (SD) | 4.0 (0.6) | 3.9 (0.7) | 0.019 |
| Prothrombin time, %a (SD) | 85.3 (15.8) | 83.6 (14.1) | 0.335 |
| History of HCC (%) | 163 (69.7) | 65 (52.4) | 0.001 |
| TNM stage in patients with HCC, none:I:II:III:IV | 164:19:27:11:13 | 102:6:10:3:3 | 0.011 |
| HU by CTa (SD) | 47.1 (5.2) | 47.0 (4.4) | 0.737 |
| Frequency of finger‐circle test results, Bigger:Just‐fits:Smaller | 126:70:38 | 66:34:24 | 0.736 |
| Frequency of MA, none:pre‐MA:MA | 99:83:52 | 80:38:6 | <0.001 |
| Handgrip strength decline (%) | 88 (37.6) | 64 (51.6) | 0.011 |
ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index; CH, chronic hepatitis; CLD, chronic liver disease; CP, Child–Pugh classification; CT, computed tomography; HBV, hepatitis B virus; HCC, hepatitis C virus; HCV, hepatitis C virus; HU, Hounsfield units; LC, liver cirrhosis; MA, muscle atrophy; NBNC, both without HBV and HCV.
Mean value.
Finger‐circle test results and clinical characteristics
Male patients
For male patients with finger‐test results of Bigger, Just‐fits, and Smaller, the mean ages were 69.7 ± 11.0, 70.9 ± 8.6, and 71.0 ± 11.4 years, respectively (P = 0.676). Sarcopenia was noted in 8 (8.0%) with CH, 12 (11.3%) with LC CP‐A, and 7 (25.0%) with LC CP‐B/C (P = 0.047).
The average PI values for male patients with Bigger, Just‐fits, and Smaller results were 5.64 ± 1.34, 5.00 ± 1.25, and 4.83 ± 1.46 cm2/m2, respectively (P < 0.001) (Figure 2A), with the frequency of pre‐MA and MA increasing in accordance with worse results (P < 0.001) (Figure 3A). Also, results of the finger‐circle test showed a significant correlation with degree of muscle area of L3 decline (r = 0.274, P < 0.001). There were no significant differences observed regarding frequency of finger‐circle test results among the present male patients divided by CH, LC CP‐A, and CP‐B/C classification (P = 0.893) (Figure 4A).
Figure 2.
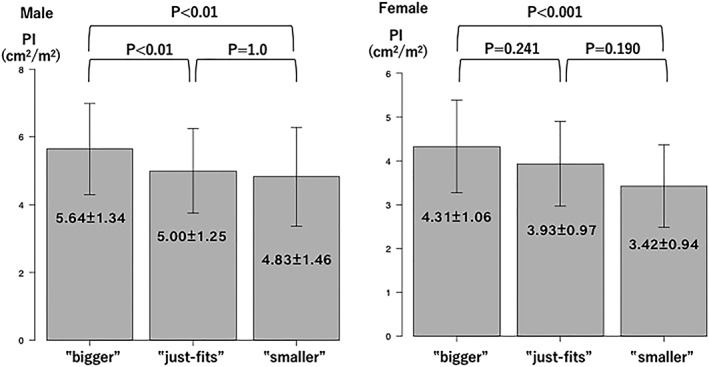
Average psoas index values for finger‐circle test results. The average psoas index value (cm2/m2) was reduced in association with smaller calf circumference in both genders (male patients: P < 0.001 and female patients: P = 0.001). Multiple comparisons using Bonferroni's method revealed significant differences between Bigger and Just‐fits and Bigger and Smaller in male patients (both P < 0.01) and between Bigger and Smaller in female patients (P < 0.001). PI, psoas index.
Figure 3.
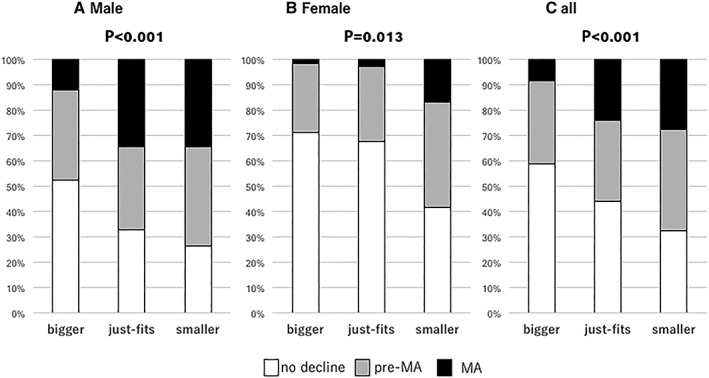
Distribution of muscle status (no decline, pre‐MA, MA) with finger‐circle test results. Muscle status became worse as calf circumference became smaller in male patients, female patients, and all patients (male patients: P < 0.001, female patients: P = 0.013, and all: P < 0.001). Multiple comparisons using Bonferroni's method revealed significant differences between Bigger and Just‐fits and between Bigger and Smaller in male patients (both, P = 0.002) and between Bigger and Smaller in female patients (P = 0.013). MA, muscle atrophy.
Figure 4.
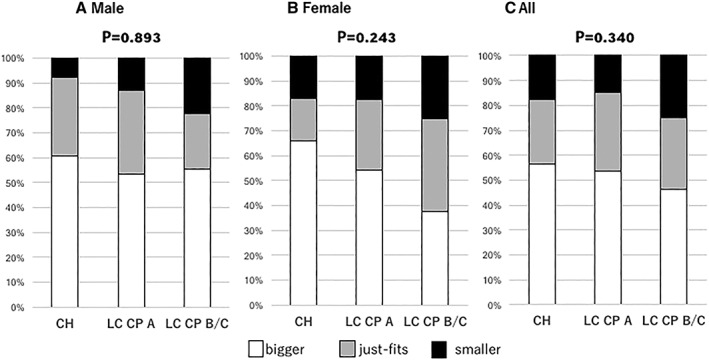
Distribution of finger‐circle test results for each Child–Pugh classification grade [(A) male patients, (B) female patients, and (C) all patients]. There were no significant differences among male patients, female patients, and all patients (P = 0.893, P = 0.243, P = 0.340, respectively). CH, chronic hepatitis; CP, Child–Pugh classification; LC, liver cirrhosis.
Using ROC analysis, the predictive PI value for a finger‐circle test result in male patients other than Bigger (Just‐fits and Smaller) was 5.25 cm2/m2 [sensitivity/specificity 0.619/0.667, AUC 0.654, 95% confidence interval (CI) 0.583–0.724]. When the same analysis was performed with Smaller as compared with the others (Bigger and Just‐fits), the predictive PI value was 5.40 cm2/m2 (sensitivity/specificity 0.444/0.763, AUC 0.612, 95% CI 0.508–0.715).
Handgrip strength decline was observed in 88 of the male patients, of whom 44 had Bigger, 29 had Just‐fits, and 15 had Smaller results (P = 0.645). The mean values for handgrip strength in male patients with CH, LC CP‐A, and LC CP‐B/C were 31.2 ± 8.3, 29.9 ± 8.7, and 26.4 ± 9.1 kg, respectively (P = 0.034).
Female patients
For female patients with finger‐test results of Bigger, Just‐fits, and Smaller, the mean ages were 70.6 ± 10.3, 75.4 ± 7.5, and 70.0 ± 9.4 years, respectively (P = 0.036). Sarcopenia was noted in zero (0%) with CH, two (3.2%) with LC CP‐A, and one (4.2%) with LC CP‐B/C (P = 0.504).
The average PI values for female patients with Bigger, Just‐fits, and Smaller results were 4.31 ± 1.06, 3.93 ± 0.97, and 3.42 ± 0.94 cm2/m2, respectively (P = 0.001) (Figure 2B), with the frequency of pre‐MA and MA increasing in accordance with worse results (P = 0.013) (Figure 3B). Also, there were no significant differences observed in regard to the frequency of finger‐circle test results among the present female patients with CH, LC CP‐A, and CP‐B/C classification (P = 0.243) (Figure 4B), while those results showed a significant correlation with degree of muscle area of L3 decline (r = 0.221, P = 0.014).
Using ROC analysis, the predictive PI value for a finger‐circle test result in female patients other than Bigger (Just‐fits and Smaller) was 4.26 cm2/m2 (sensitivity/specificity 0.530/0.776, AUC 0.655, 95% CI 0.559–0.752). When the same analysis was performed with Smaller as compared with the others (Bigger and Just‐fits), the predictive PI value was 3.33 cm2/m2 (sensitivity/specificity 0.740/0.583, AUC 0.698, 95% CI 0.583–0.813).
Handgrip strength decline was observed in 64 of the female patients, of whom 31 had Bigger, 21 had Just‐fits, and 12 had Smaller results (P = 0.371). The mean values for handgrip strength in female patients with CH, LC CP‐A, and CP‐B/C were 19.7 ± 5.6, 17.5 ± 4.5, and 16.4 ± 4.8 kg, respectively (P = 0.023).
All patients
Sarcopenia was noted in 8 (5.8%) with CH, 14 (8.3%) with LC CP‐A, and 8 (15.4%) with LC CP‐B/C (P = 0.110). For all of the present patients with finger‐test results of Bigger, Just‐fits, and Smaller, the mean ages were 70.0 ± 10.8, 72.4 ± 8.5, and 70.6 ± 10.7 years, respectively (P = 0.161). The frequency of pre‐MA and MA was increased in accordance with worse results (P < 0.001) (Figure 3C). There were no significant differences observed in regard to the frequency of finger‐circle test results among the patients with CH, LC CP‐A, and CP‐B/C classification (P = 0.340) (Figure 4C), while those results showed a significant correlation with degree of muscle area of L3 decline (r = 0.243, P < 0.001).
The frequency of MSD was increased in association with progression of CLD grade (CH 48/137, LC CP‐A 75/169, LC CP‐B 29/52; P = 0.033). In multiple comparison analysis, the only significant difference found for mean handgrip strength was between CH and LC CP‐B/C (P = 0.033, Bonferroni's method). However, there was no relationship between MSD and the results of finger‐circle test (P = 0.321). In addition, although Hounsfield units (HU) shown by CT10 in all patients with Bigger, Just‐fits, and Smaller results were 46.6 ± 5.1, 47.6 ± 4.6, and 47.6 ± 5.2, respectively (P = 0.187), there was a tendency of becoming lower HU level with progression of CLD (CH vs. LC CP‐A vs. LC CP‐B/C = 47.5 ± 5.0 vs. 47.3 ± 4.8 vs. 45.4 ± 5.2) (CH vs. LC CP‐B/C: P = 0.034 and LC CP‐A vs. LC CP‐B/C: P = 0.063, in Bonferroni's method, respectively).
Discussion
Muscle atrophy has been reported to be a prognostic risk factor for survival of HCC patients treated with living donor liver transplantation,11 resection,12, 13, 14 and sorafenib,15 as well as LC patients classified as CP‐A or CP‐B.16 Furthermore, muscle function loss has been reported to have a close relationship with cognitive function.14 It has been reported that percentage of muscle abnormality became larger and larger statistically with progression of CLD stage in past analyses with large number of CLD patients over 800 individuals.2, 3 Both conditions are related to lower quality of life and worse survival; thus, it is important for CLD patients to maintain muscle volume and function.
Although muscle function can be easily assessed by handgrip strength or walking speed, BIA and/or DEXA are not common in clinical practice of liver disease, and access to such devices for surveillance of MA is difficult for most CLD patients and family physicians. On the other hand, CT examination has been performed as a very common and objective screening modality for HCC in CLD patients in Japan, and it has been reported that a significant positive correlation was observed between skeletal muscle area of L3 level measured by CT and skeletal muscle volume by BIA in liver related hospital patients (r 2 = 0.575, P < 0.0001).17 In addition, Tanaka recently reported the finger‐circle (Japanese, yubi‐wakka) test, which compares the size of the circle made with the bilateral thumb and index fingers of the patient with that of the calf of their leg.7 Based on these reasons and these past reports, we selected CT examination as a method of evaluation of muscle in the present study to establish an easier and less expensive surveillance method without the need for special equipment for preventing progression of MA.
As noted in a previous report,3 the present results noted gender differences in regard to the test results. We found that a finger‐circle test result in male patients other than Bigger (Just‐fits and Smaller) predicted a decline in muscle area of L3 to PI 5.25 cm2/m2, which was approximately mean minus 1 SD (5.37 cm2/m2). On the other hand, a Smaller test result in female patients predicted a decline in muscle area of L3 to PI 3.33 cm2/m2, approximately mean minus 1 SD (3.40 cm2/m2). In the present analysis, a finger‐circle test result other than Bigger in male patients and Smaller in female patients predicted early occurrence of MA. Daily physical activity has been reported to be lower in CLD patients as compared with healthy subjects.18, 19 To confirm the relationships among total muscle volume or area of L3, maximum calf circumference, and level of daily physical activity, an additional study is necessary. In addition, number of obese individuals was too small in the present cohort (3.4%) for confirming the predictive value of finger‐circle test in obese individuals with CLD. Moreover, there might be influences of not only advanced obesity but also lower oedema due to low levels of serum albumin because of advanced LC on the present results of AUC (<0.7) of finger‐circle test for predictive values of mean minus 1 SD of each gender (male: 0.654, female 0.698, respectively). These might be weak points of this examination, and further examinations with larger number of CLD patients including those with advanced obesity or advanced LC will be needed.
Muscle volume assessment is recommended for patients with MSD as part of the diagnostic strategies for sarcopenia presented by European Working Group on Sarcopenia in Older People,4 Asian Working Group for Sarcopenia,5 and JSH.6 However, it is important to keep in mind that there are discrepancies between actual clinical practice and reported guidelines. There might be gender differences concerning to progressions of MA and MSD.2, 3 Moreover, although there was no significance in increasing the percentage of sarcopenia in each stage of CLD in the present cohort (n = 358; CH vs. LC CP‐A, vs. LC CP‐B/C = 5.8% vs. 8.3% vs. 15.4%; P = 0.110), progression of CLD stage is an important risk factor for existence of muscle abnormality.2, 3 Establishment of a simple assessment method for MA is needed. Even without MSD, assessment of muscle area or volume in affected patients should be considered when the finger‐circle test result is abnormal. Of course, the present results are based on cross‐sectional area of bilateral psoas muscle. From the view point of muscle composition, detailed analysis focused for not only muscle density (HU) but also myosteatosis should be also performed in future.
The present study has some limitations. First, all patients were treated at a single institution, and a validation study is needed. Second, numbers of obese individual and advanced LC patients were small. Efficacy of finger‐circle test in large number of those patients should be validated. Furthermore, the present results were obtained from observations of CT findings, while findings obtained from examinations of muscle throughout the body using BIA are needed, as there may be discrepancies among body parts (e.g. psoas, legs, and arms) due to individual patient lifestyle factors.
Assessments of MA and function are important for improving prognosis and quality of life in patients at high risk for progression of sarcopenia. Although the sensitivity of the finger‐circle test was not high in the present study, it is an easy and simple screening method that can be performed by family physicians to determine MA without the need for specialized equipment.
Conflict of interest
None declared.
Supporting information
Figure S1. Total muscle area was manually calculated with bilateral psoas muscle at the level of the middle of L3 (cm2) using personal computer software.
Acknowledgements
We are thankful for the cooperation of Miho Oonishi and Satsuki Koyama with the finger‐circle tests and handgrip strength measurements and for drawing the figures of yubi‐wakka test of Hiroko Hiraoka. All co‐authors in the present manuscript comply with the Ethical Guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.20
Hiraoka, A. , Izumoto, H. , Ueki, H. , Yoshino, T. , Aibiki, T. , Okudaira, T. , Yamago, H. , Suga, Y. , Iwasaki, R. , Tomida, H. , Mori, K. , Miyata, H. , Tsubouchi, E. , Kishida, M. , Ninomiya, T. , Hirooka, M. , Abe, M. , Matsuura, B. , Hiasa, Y. , and Michitaka, K. (2019) Easy surveillance of muscle volume decline in chronic liver disease patients using finger‐circle (yubi‐wakka) test. Journal of Cachexia, Sarcopenia and Muscle, 10: 347–354. 10.1002/jcsm.12392.
References
- 1. Rosenberg I. Summary comments and methodological problems in determining nutritional status of older persons. Am J Clin Nutr 1989;50:1231–1233. [PubMed] [Google Scholar]
- 2. Hiraoka A, Aibiki T, Okudaira T, Toshimori A, Kawamura T, Nakahara H, et al. Muscle atrophy as pre‐sarcopenia in Japanese patients with chronic liver disease: computed tomography is useful for evaluation. J Gastroenterol 2015;50:1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hiraoka A, Michitaka K, Ueki H, Kaneto M, Aibiki T, Okudaira T, et al. Sarcopenia and two types of presarcopenia in Japanese patients with chronic liver disease. Eur J Gastroenterol Hepatol 2016;28:940–947. [DOI] [PubMed] [Google Scholar]
- 4. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 6. Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 2016;46:951–963. [DOI] [PubMed] [Google Scholar]
- 7. Tanaka T, Takahashi K, Akishita M, Tsuji T, Iijima K. “Yubi‐wakka” (finger‐ring) test: a practical self‐screening method for sarcopenia, and a predictor of disability and mortality among Japanese community‐dwelling older adults. Geriatr Gerontol Int 2018;18:224–232. [DOI] [PubMed] [Google Scholar]
- 8. The Liver Cancer Study Group of Japan . The General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 6th ed. Tokyo: Kanehara; 2015. p 26. [Google Scholar]
- 9. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tachi Y, Kozuka A, Hirai T, Ishizu Y, Honda T, Kuzuya T, et al. Impact of myosteatosis on skeletal muscle volume loss in patients with chronic liver disease. J Gastroenterol Hepatol 2018;33:1659–1666. 10.1111/jgh.14133. Published online ahead of print 27 February 2018. [DOI] [PubMed] [Google Scholar]
- 11. Masuda T, Shirabe K, Ikegami T, Harimoto N, Yoshizumi T, Soejima Y, et al. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl 2014;20:401–407. [DOI] [PubMed] [Google Scholar]
- 12. Higashi T, Hayashi H, Taki K, Sakamoto K, Kuroki H, Nitta H, et al. Sarcopenia, but not visceral fat amount, is a risk factor of postoperative complications after major hepatectomy. Int J Clin Oncol 2016;21:310–319. [DOI] [PubMed] [Google Scholar]
- 13. Harimoto N, Yoshizumi T, Shimokawa M, Sakata K, Kimura K, Itoh S, et al. Sarcopenia is a poor prognostic factor following hepatic resection in patients aged 70 years and older with hepatocellular carcinoma. Hepatol Res 2016;46:1247–1255. [DOI] [PubMed] [Google Scholar]
- 14. Hiraoka A, Otsuka Y, Kawasaki H, Izumoto H, Ueki H, Kitahata S, et al. Impact of muscle volume and muscle function decline in patients undergoing surgical resection for hepatocellular carcinoma. J Gastroenterol Hepatol 2018;33:1271–1276. [DOI] [PubMed] [Google Scholar]
- 15. Hiraoka A, Hirooka M, Koizumi Y, Izumoto H, Ueki H, Kaneto M, et al. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol Res 2017;47:558–565. [DOI] [PubMed] [Google Scholar]
- 16. Hiraoka A, Kitahata S, Izumoto H, Ueki H, Aibiki T, Okudaira T, et al. Muscle volume loss a prognostic factor for death in liver cirrhosis patients and special relationship to portal hypertension. Hepatol Res 2017;48:E354–E359. [DOI] [PubMed] [Google Scholar]
- 17. Itoh S, Shirabe K, Yoshizumi T, Takeishi K, Harimoto N, Ikegami T, et al. Skeletal muscle mass assessed by computed tomography correlates to muscle strength and physical performance at a liver‐related hospital experience. Hepatol Res 2016;46:292–297. [DOI] [PubMed] [Google Scholar]
- 18. Hayashi F, Momoki C, Yuikawa M, Simotani Y, Kawamura E, Hagihara A, et al. Nutritional status in relation to lifestyle in patients with compensated viral cirrhosis. World J Gastroenterol 2012;18:5759–5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hiraoka A, Michitaka K, Kiguchi D, Izumoto H, Ueki H, Kaneto M, et al. Efficacy of branched‐chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 2017;29:1416–1423. [DOI] [PubMed] [Google Scholar]
- 20. Hiraoka A, Michitaka K, von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Total muscle area was manually calculated with bilateral psoas muscle at the level of the middle of L3 (cm2) using personal computer software.


