Abstract
Background
Pancreatic cancer (PC) patients have multiple risk factors for sarcopenia and loss of skeletal muscle mass (LSMM), which may cause greater treatment toxicities, reduced response to cancer therapy, prolonged hospitalization, impaired quality of life, and worse prognosis.
Methods
This is a retrospective study on advanced PC patients treated at the Department of Oncology of Udine, Italy, from January 2012 to November 2017. Among 162 patients who received chemotherapy, 94 consecutive patients with an available computed tomography (CT) scan were retrospectively analyzed. The primary objective of our study was to explore if an early LSMM ≥ 10% (measured at first radiological evaluation and compared with baseline) and/or baseline sarcopenia may impact prognosis. Baseline sarcopenia was defined according to Prado's criteria. Skeletal muscle area was measured as cross‐sectional areas (cm2) using CT scan data through the Picture archiving and communication system (PACS) image system.
Results
In the whole cohort, 48% of patients were ≤70 years old, and 50% had metastatic disease.
At baseline, 73% of patients had sarcopenia, and 16% presented a visceral fat area ≥ 44 cm2/m2. Overall, 21% experienced an early LSMM ≥ 10%. Approximately 33% of sarcopenic patients at baseline and ~35% of patients with early LSMM ≥ 10% had a body mass index > 25 kg/m2. Of note, 71% of patients were evaluated by a nutritionist, and 56% received a dietary supplementation (oral and/or parenteral). After a median follow‐up of 30.44 months, median overall survival (OS) was 11.28 months, whereas median progression‐free survival (PFS) was 5.72 months. By multivariate analysis, early LSMM ≥ 10% was significantly associated with worse OS [hazard ratio (HR): 2.16; 95% confidence interval (CI) 1.23–3.78; P = 0.007] and PFS (HR: 2.31; 95% CI 1.30–4.09; P = 0.004). Moreover, an exploratory analysis showed that inflammatory indexes, such as neutrophil–lymphocyte ratio variation, impact early LSMM ≥ 10% (odds ratio 1.31, 95% CI 1.06–1.61, P = 0.010).
Conclusions
Early LSMM ≥ 10% has a negative prognostic role in advanced PC patients. Further prospective investigations are needed to confirm these preliminary data.
Keywords: Sarcopenia, Pancreatic cancer, Muscle loss, Muscle depletion
Introduction
Pancreatic cancer (PC) is a very rapidly progressive disease characterized by several genetic and molecular alterations, as well as poor prognosis. Despite being a relatively rare type of cancer, recent estimates predict an increase in its incidence over the next years.1 Unfortunately, effective therapies capable of changing the disease's natural history are still lacking. PC patients have shorter survival and an increased risk of distant metastases.2, 3 Furthermore, the prognosis of these patients is conditioned by a higher incidence of malnutrition or cachexia, present in 70–80% of PC patients, responsible for at least 20% of all deaths.4, 5, 6, 7 In fact, multiple risk factors for loss of skeletal muscle mass (LSMM) due to cancer‐related factors and medical treatment concur to cause more treatment toxicities, asthenia, fatigue, reduced response to cancer therapy, prolonged hospitalization, impaired quality of life, and, therefore, a worse prognosis.2, 6, 8, 9 However, the identification of patients with muscle loss has become increasingly difficult, because ~40–60% of cancer patients are overweight or obese, even in the metastatic setting.3 Sarcopenia was defined by Evans as LSMM, which results in decreased strength and aerobic capacity and, thus, functional capacity. The pathogenesis of sarcopenia includes a systemic inflammatory reaction, involving anabolic and catabolic pathways responsible for skeletal muscle physiology.10 Firstly, inflammatory mediators such as cytokines [tumour necrosis factor (TNF‐α), interleukins (ILs)] and C‐reactive protein are released from the liver into the bloodstream, increasing the lipid and protein catabolism and, therefore, inhibiting the anabolic pathways. Moreover, they act on the central nervous system and visceral fat [visceral adipose tissue (VAT)], causing anorexia and fatigue.11
The best way to diagnose sarcopenia is by measuring the lean mass by either dual‐energy x‐ray absorptiometry (DXA) or computed tomography (CT) scan. Although DXA scans produce highly accurate results, they lack the ability to discriminate among lean and adipose tissue sub‐compartments. Conversely, CT image analysis at the third lumbar vertebra allows to distinguish adipose tissue (including visceral, subcutaneous, and intramuscular) from muscle tissue,6, 7, 8 and it has been validated as a reliable method for whole‐body composition assessment.
Recently, the use of gold standard methods for body composition assessment, including CT, has simplified the diagnosis of cancer‐related sarcopenia, thus better defining its prevalence. However, the impact survival of cachexia, including weight loss and/or sarcopenia, in PC patients has still been poorly studied.
Based on these premises, the aim of this study was to evaluate the prognostic impact of body composition among PC patients.
Material and methods
Study design
This is an observational, retrospective study that examined data of 165 advanced PC patients treated at the Oncology Department of Udine, Italy, from January 2012 to November 2017. A cohort of 94 consecutive patients with the availability of CT scan has been analysed. All patients had confirmed PC and the consent to the use of clinical data, rendered anonymous, for purposes of clinical research, epidemiology, training, and study of diseases for patients who have died and informed consent of the study for patients who are alive. The study was approved by the Departmental Review Board and by the Ethics Committee (approved in May 2018, Protocol Number 16662). Data have been obtained from electronic medical records according to strict privacy standards. The study aimed to characterize the prognostic impact of LSMM in advanced PC patients during first‐line treatment (i.e. baseline and after 12 weeks) analysing clinical outcome. Moreover, we evaluated the association among LSMM and inflammation index [neutrophil–lymphocyte ratio (NLR), lactate dehydrogenase (LDH), albumin] and with early dietary supplementation (defined as dietary implementation in the first 3 months of first‐line chemotherapy). Progression‐free survival (PFS) was defined as time from first‐line chemotherapy until progression of disease or death from any cause. Overall survival (OS) was defined as the time between treatment start and death from any cause.
Skeletal muscle mass assessment
Computed tomography scans, performed within 30 days since first‐line treatment start, were retrospectively evaluated. Skeletal muscle area was measured as a cross‐sectional area in cm2 using routine CT scan data through the PACS image system. Skeletal muscle index (SMI) was calculated as a cross‐sectional area of muscle (cm2) at the L3 level divided by the square of the height (m2). In particular, psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal oblique muscles of the abdomen, and rectus abdominis muscle were evaluated. Sarcopenia has been defined according to Prado's criteria [SMI < 53 cm2/m2 for men with body mass index (BMI) > 25, SMI < 43 cm2/m2 for men with BMI < 25, SMI < 41 cm2/m2 for women],2, 12, 13, 14 and early LSMM during chemotherapy has been defined as a decrease of SMI > 10% at first CT evaluation (approximately after 3 months).15
Blood sample analysis
Lactate dehydrogenase, albumin and C‐reactive protein levels were classified according to a cut‐off of 480 U/L, 35 g/L, and 5 mg/L, respectively. NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count. Full blood count data were eligible for analysis if performed within 1 month before the start of first‐line chemotherapy.
Statistical analysis
Patients' clinico‐pathological characteristics were summarized through descriptive analysis. Categorical variables were described through frequency distribution, whereas continuous variables will be reported through median range. Clinico‐pathological variables were compared between sarcopenic and non‐sarcopenic patients, and between patients who had an LSMM ≥ 10% and <10%.
The association of early LSMM with inflammation index was explored by Wilcoxon rank‐sum test or Kruskal–Wallis test, as statistically appropriate. The impact of clinico‐pathological features determining early LSMM and early nutritional intervention was analysed with logistic regression. Prognostic factors in terms of OS were tested in both univariate and multivariate models by Cox regression with 95% CI. Differences in survival were tested by log‐rank test and represented by Kaplan–Meier survival curves.
A two‐sided P < 0.05 was considered statistically significant. Statistical analysis was performed using STATA [StataCorp (2015) Stata Statistical Software: Release 14.2, College Station, TX, USA: StataCorp LP].
Results
This study included 94 patients with a diagnosis of advanced PC (patients' characteristics are shown in Table 1). In the whole cohort, 48% of patients were younger than 70 years, and 32% of patients had an Eastern Cooperative Oncology Group performance status (PS) of 0, and 50% had metastatic disease. Approximately 88% of patients had tumour histology or tumour cytology available showing a diagnosis of adenocarcinoma. The remaining 12% presented with clinical, radiological, and/or bio‐humoral features suggestive of pancreatic adenocarcinoma. Only 2% of patients received radiotherapy in multiple fractions. In addition, most of the patients (70%) did not receive surgery.
Table 1.
Patients' characteristics
| Variable | No. (n = 94) | % |
|---|---|---|
| Age | ||
| <70 years | 45 | 48 |
| ≥70 years | 49 | 52 |
| Gender | ||
| Male | 52 | 55 |
| Female | 42 | 45 |
| Site of primary tumour | ||
| Head | 51 | 54 |
| Body–tail | 43 | 46 |
| ECOG PS at first‐line chemotherapy start | ||
| 0 | 30 | 32 |
| ≥1 | 64 | 68 |
| Stage at first‐line chemotherapy start | ||
| Locally advanced | 47 | 50 |
| Metastatic disease | 47 | 50 |
| Metastatic site at first‐line chemotherapy start | ||
| Bone | 2 | 2 |
| Liver | 43 | 46 |
| Lymph nodes | 21 | 22 |
| Lung | 12 | 13 |
| Peritoneum | 7 | 8 |
| BMI (kg/m2) | ||
| 18.5–25 | 60 | 64 |
| >25 | 34 | 36 |
| Visceral fat (cm3/h2) | ||
| <44 | 79 | 84 |
| ≥44 | 15 | 16 |
| Sarcopenia at first‐line chemotherapy start | ||
| Yes | 69 | 73 |
| No | 25 | 27 |
| Nutritional assessment (since diagnosis) | ||
| Yes | 67 | 71 |
| No | 27 | 29 |
| Nutritional supplementation (since diagnosis) | ||
| Yes | 53 | 56 |
| No | 41 | 44 |
| Type of first‐line chemotherapy | ||
| Single agent | 25 | 27 |
| Gemcitabine | ||
| Capecitabine | ||
| Combination regimen | 53 | 56 |
| Gemcitabine plus nab‐paclitaxel | ||
| Gemcitabine plus cisplatin | ||
| Fluorouracil, irinotecan, and oxaliplatin | ||
| Other | 16 | 17 |
| Lines of therapy for metastatic disease | ||
| 1 | 51 | 54 |
| 2 | 27 | 29 |
| 3 | 16 | 17 |
| Laboratory parameter at first‐line chemotherapy start | ||
| LDH (UI/L) | ||
| ≤480 | 60 | 64 |
| >480 | 14 | 15 |
| Missing | 20 | 21 |
| Albumin (g/L) | ||
| ≤35 | 20 | 21 |
| >35 | 63 | 67 |
| Missing | 11 | 12 |
| C‐reactive protein (mg/L) | ||
| ≤5 | 18 | 19 |
| >5 | 40 | 43 |
| Missing | 36 | 38 |
| CA 19‐9 (UI/L) | ||
| ≤60 | 18 | 19 |
| >60 | 74 | 79 |
| Missing | 2 | 2 |
ECOG, Eastern Cooperative Oncology Group.
Overall, after a median follow‐up of 30.44 months, median OS was 11.28 months (25th–75th percentiles: 4.90–22.06 months), whereas median PFS was 5.72 months (25th–75th percentiles: 2.79–9.6 months).
As for anthropometric characteristics, median body weight of the whole cohort was 68.5 kg and median BMI was 24.1 kg/m2. Overall, 16% of patients presented a VAT ≥ 44 cm2/m2 (defined as visceral area divided by the square of the height), and 73% were sarcopenic at baseline (characteristics of sarcopenic patients were displayed in Table 2). Overall, 21% of population experienced an early LSMM ≥ 10% at first radiological assessment. On the other hand, 5% of population experienced a weight loss ≥ 10%. Characteristics of patients experiencing an early LSMM ≥ 10% are shown in Table 2. Among sarcopenic individuals, 12 patients experienced an early LSMM ≥ 10%. Approximately 33% of sarcopenic individuals at baseline and ~35% of patients with early LSMM had a BMI > 25 kg/m2.
Table 2.
Characteristic of sarcopenic patients and patients with Δ SMI ≥ 10%
| Variable |
Sarcopenic patients n = 69 |
Patients with Δ SMI ≥ 10% n = 20 |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age | ||||
| <70 years | 30 | 43 | 10 | 50 |
| ≥70 years | 39 | 57 | 10 | 50 |
| Gender | ||||
| Male | 39 | 57 | 11 | 55 |
| Female | 30 | 43 | 9 | 45 |
| Site of primary tumour | ||||
| Head | 38 | 55 | 8 | 40 |
| Body–tail | 31 | 45 | 12 | 60 |
| Previous surgery | ||||
| No | 50 | 72 | — | — |
| Yes | 19 | 28 | ||
| Diabetes | ||||
| No | 51 | 74 | — | — |
| Yes | 18 | 26 | ||
| ECOG PS at first‐line chemotherapy start | ||||
| 0 | 20 | 29 | 6 | 30 |
| ≥1 | 49 | 71 | 14 | 70 |
| Stage at first‐line chemotherapy start | ||||
| Locally advanced | 32 | 46 | 11 | 55 |
| Metastatic disease | 37 | 54 | 9 | 45 |
| Metastatic site at first‐line chemotherapy start | ||||
| Bone | 2 | 3 | 1 | 5 |
| Liver | 34 | 49 | 9 | 45 |
| Lymph nodes | 15 | 21 | 6 | 25 |
| Lung | 10 | 14 | 3 | 15 |
| Peritoneum | 5 | 7 | 1 | 5 |
| BMI (kg/m2) | ||||
| 18.5–25 | 46 | 67 | 13 | 65 |
| >25 | 23 | 33 | 7 | 35 |
| Visceral fat (cm3/h2) | ||||
| <44 | 59 | 86 | 17 | 85 |
| ≥44 | 10 | 14 | 3 | 15 |
| Nutritional assessment (since diagnosis) | ||||
| Yes | 50 | 72 | 17 | 85 |
| No | 19 | 28 | 3 | 15 |
| Nutritional supplementation (since diagnosis) | ||||
| Yes | 40 | 58 | 13 | 65 |
| No | 29 | 42 | 7 | 35 |
| Type of first‐line chemotherapy | ||||
| Single agent | 20 | 29 | 5 | 25 |
| Combination regimen | 38 | 55 | 9 | 45 |
| Other | 11 | 16 | 6 | 30 |
| Lines of therapy for metastatic disease | ||||
| 1 | 37 | 53 | 13 | 65 |
| 2 | 21 | 30 | 4 | 20 |
| 3 | 11 | 17 | 3 | 15 |
| Laboratory parameter at first‐line chemotherapy start | ||||
| LDH (UI/L) | ||||
| ≤480 | 41 | 59 | 17 | 85 |
| >480 | 11 | 16 | 1 | 5 |
| Missing | 17 | 25 | 2 | 10 |
| Albumin (g/L) | ||||
| ≤35 | 14 | 20 | 6 | 30 |
| >35 | 48 | 70 | 14 | 70 |
| Missing | 7 | 10 | — | — |
| C‐reactive protein (mg/L) | ||||
| ≤5 | 13 | 19 | 6 | 30 |
| >5 | 32 | 46 | 6 | 30 |
| Missing | 24 | 35 | 8 | 40 |
| CA 19‐9 (UI/L) | ||||
| ≤60 | 12 | 17 | 4 | 20 |
| >60 | 55 | 80 | 14 | 70 |
| Missing | 2 | 3 | 2 | 10 |
Δ, from baseline to first radiological assessment; ECOG, Eastern Cooperative Oncology Group.
Through univariate and multivariate analyses, we found that metastatic disease at diagnosis, VAT ≥ 44 cm2/m2, and an early LSMM ≥ 10% were negative prognostic factors in terms of both OS and PFS (Tables 3 and 4). More specifically, the median OS was 8.78 months for patients with an early LSMM ≥ 10% vs. 12.85 months for patients with an early LSMM < 10% [hazard ratio (HR): 2.16; 95% CI 1.23–3.78; P = 0.007, Figure 1]. Furthermore, median PFS was 4.14 months for the first group vs. 7.27 months for the second group (HR: 2.31; 95% CI 1.30–4.09; P = 0.004; Figure 2).
Table 3.
Univariate and multivariate analyses for overall survival
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | ||||||
| ≥70 vs. <70 years | 1.40 | 0.89–2.20 | 0.141 | |||
| Stage at diagnosis | ||||||
| Metastatic disease vs. locally advanced | 2.11 | 1.36–3.28 | 0.001 | 2.25 | 1.35–3.76 | 0.002 |
| Diabetes | ||||||
| Yes vs. no | 1.05 | 0.65–1.69 | 0.838 | |||
| ECOG PS | ||||||
| ≥1 vs. 0 | 1.44 | 0.88–2.34 | 0.141 | |||
| BMI | ||||||
| >25 vs. 18.5–25 kg/m2 | 1.00 | 0.97–1.57 | 0.973 | |||
| Visceral fat | ||||||
| ≥44 vs. <44 cm3/h2 | 2.19 | 1.23–3.91 | 0.008 | 2.77 | 1.31–5.87 | 0.008 |
| Site of primary tumour | ||||||
| Body–tail vs. head | 1.36 | 0.88–2.12 | 0.160 | |||
| Δ SMI | ||||||
| ≥10% vs. <10% | 1.92 | 1.12–3.31 | 0.018 | 2.16 | 1.23–3.78 | 0.007 |
| Δ Karnofsky PS | ||||||
| ≥20% vs. <20% | 1.87 | 1.02–3.44 | 0.042 | 1.18 | 0.52–2.69 | 0.686 |
| Nutritional assessment | ||||||
| Yes vs. no | 1.03 | 0.63–1.68 | 0.905 | |||
| Nutritional supplementation | ||||||
| Yes vs. no | 1.43 | 0.91–2.23 | 0.112 | |||
| Δ Body weight | ||||||
| ≥10% vs. <10% | 2.46 | 0.97–6.25 | 0.057 | |||
| Sarcopenia | ||||||
| Yes vs. no | 1.16 | 0.69–1.94 | 0.565 | |||
| Early nutritional assessment | ||||||
| Yes vs. no | 1.23 | 0.79–1.92 | 0.342 | |||
| Early nutritional supplementation | ||||||
| Yes vs. no | 1.49 | 0.95–2.33 | 0.077 | |||
The bold values are variables statistically significant in univariate and multivariate analysis.
Δ, from baseline to first radiological assessment; ECOG, Eastern Cooperative Oncology Group.
Table 4.
Univariate and multivariate analyses for progression‐free survival
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | ||||||
| ≥70 vs. <70 years | 1.19 | 0.77–1.84 | 0.420 | |||
| Stage at diagnosis | ||||||
| Metastatic disease vs. locally advanced | 1.88 | 1.22–2.90 | 0.004 | 1.84 | 1.11–3.04 | 0.017 |
| Diabetes | ||||||
| Yes vs. no | 0.98 | 0.62–1.56 | 0.961 | |||
| ECOG PS | ||||||
| ≥1 vs. 0 | 1.40 | 0.89–2.22 | 0.143 | |||
| BMI | ||||||
| >25 vs. 18.5–25 kg/m2 | 0.84 | 0.53–1.32 | 0.460 | |||
| Site of primary tumour | ||||||
| Body–tail vs. head | 1.06 | 0.69–1.63 | 0.762 | |||
| Δ SMI | ||||||
| ≥10% vs. <10% | 1.82 | 1.06–3.13 | 0.029 | 2.31 | 1.30–4.09 | 0.004 |
| Visceral fat | ||||||
| ≥44 vs. <44 cm3/h2 | 2.45 | 1.34–4.49 | 0.003 | 2.98 | 1.41–6.28 | 0.004 |
| Δ Karnofsky PS | ||||||
| ≥20% vs. <20% | 1.97 | 1.08–3.60 | 0.027 | 1.43 | 0.63–3.23 | 0.383 |
| Nutritional assessment | ||||||
| Yes vs. no | 0.77 | 0.48–1.24 | 0.294 | |||
| Nutritional supplementation | ||||||
| Yes vs. no | 1.06 | 0.68–1.63 | 0.791 | |||
| Δ Body weight | ||||||
| ≥10% vs. <10% | 2.60 | 1.03–6.59 | 0.043 | |||
The bold values are variables statistically significant in univariate and multivariate analysis.
Δ, from baseline to first radiological assessment; ECOG, Eastern Cooperative Oncology Group.
Figure 1.
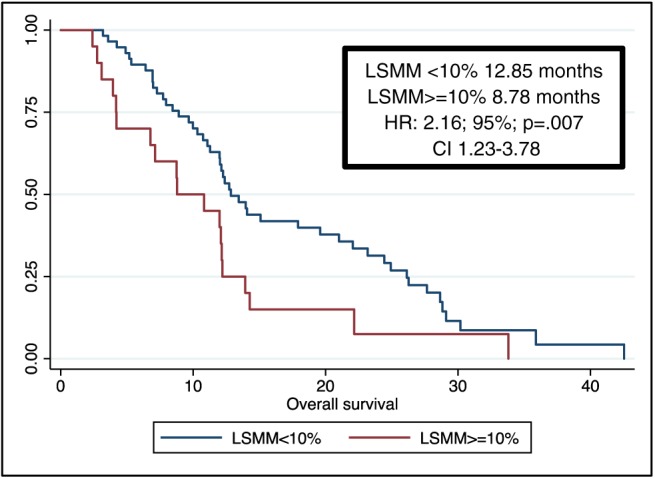
Overall survival. CI, confidence interval; HR, hazard ratio; LSMM, loss of skeletal muscle mass.
Figure 2.
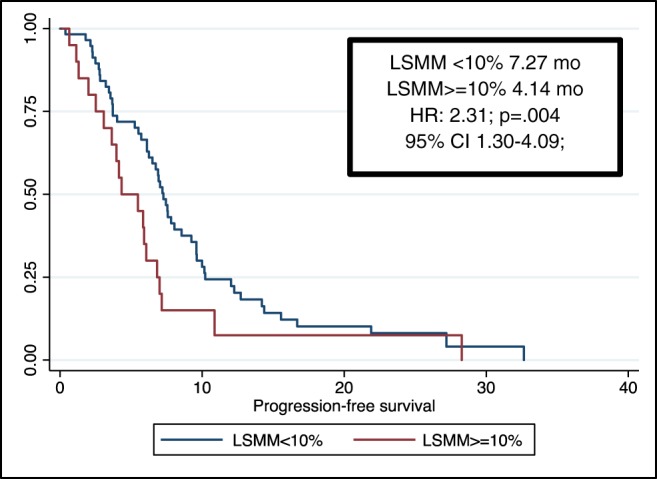
Progression‐free survival. CI, confidence interval; HR, hazard ratio; LSMM, loss of skeletal muscle mass.
To explore possible parameters that could predict an early LSMM superior or inferior to 10%, we performed a logistic regression revealing an association with NLR variation [odds ratio (OR) 1.31, 95% CI 1.06–1.61, P = 0.010] and LDH variation (OR 1.01, 95% CI 1.00–1.01, P = 0.020) (Figure 3). No association was observed with C‐reactive protein levels, Lymphocyte‐to‐monocytes ratio (LMR) variation, CA 19‐9 variation, albumin variation, BMI variation, or a body weight change after 3 months since diagnosis.
Figure 3.
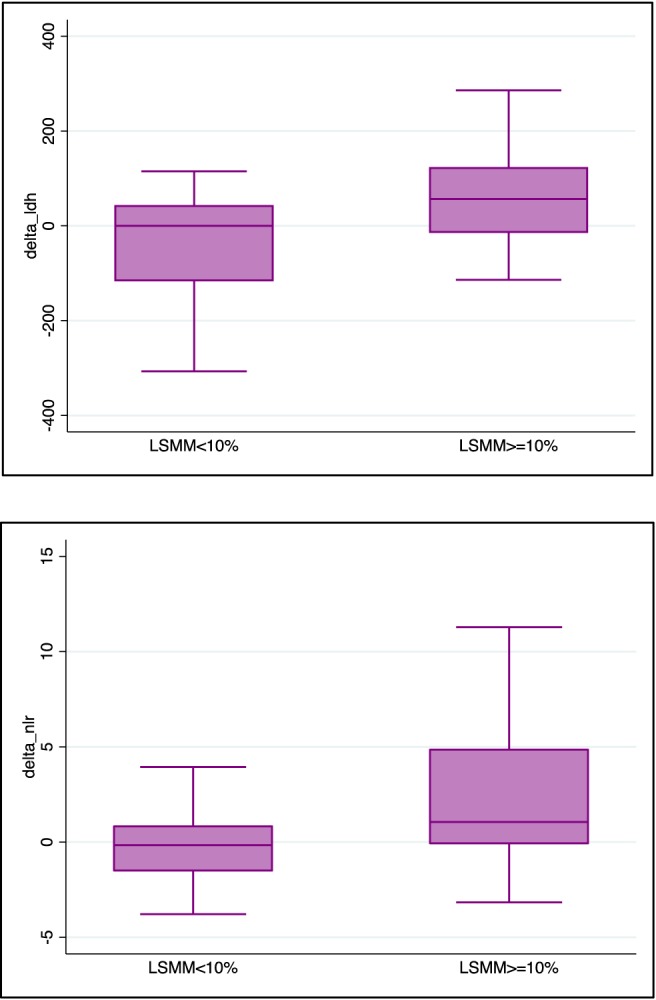
Association of inflammatory indexes with early nutritional intervention. LSMM, loss of skeletal muscle mass.
Overall, 71% of patients were evaluated by a nutritionist, and 56% of patients received a dietary supplementation (oral and/or parenteral). To explore possible factors associated with an earlier supplementation, we found an association only with body weight variation (OR 1.11, 95% CI 1.01–1.22, P = 0.024). No association was observed with a BMI variation, PS Karnofsky variation, baseline BMI, SMI variation, site of primary tumour, or age (Table 5, Figure 4). Only body weight variation at first radiological assessment during chemotherapy showed an association with the decision to prescribe an early supplementation.
Table 5.
Logistic regression—univariate for early dietary supplementation
| Variable | Univariate analysis | ||
|---|---|---|---|
| HR | 95% CI | P | |
| Δ: Body weight | 1.10 | 1.01–1.18 | 0.035 |
| ≤10% vs. >10% | |||
| BMI at first‐line chemotherapy start (kg/m2) | 0.74 | 0.53–1.04 | 0.091 |
| >25 vs. ≤25 | |||
| Δ SMI | 0.53 | 0.16–1.66 | 0.277 |
| ≤10% vs. >10% | |||
| Site of primary tumour | 0.99 | 0.43–2.31 | 0.996 |
| Body–tail vs. head | |||
| Age | 0.79 | 0.34–1.84 | 0.595 |
| ≥70 vs. <70 years | |||
| Δ Karnofsky PS | 1.00 | 0.97–1.03 | 0.796 |
| ≤20% vs. >20% | |||
The bold values are variables statistically significant in univariate and multivariate analysis.
Δ, from baseline to first radiological assessment.
Figure 4.
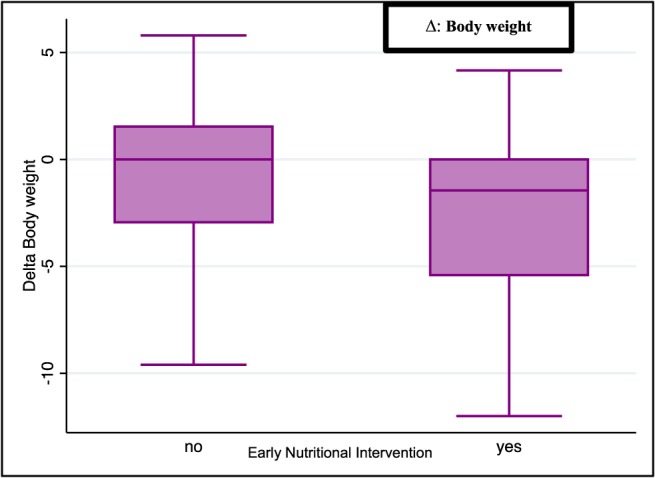
Association of body weight variation with early nutritional intervention.
Discussion
Recent literature has focused on the prognostic impact of sarcopenia and its post‐operative role in respect of complication.1 However, most of these studies included patients who received surgery, and, unfortunately,7, 16, 17 few data are available for advanced PC patients.
In the present study, we examined a cohort of 94 advanced PC patients, exploring in a real‐world scenario the prognostic impact of baseline sarcopenia (at the diagnosis of advanced PC), and of an early LSMM ≥ 10% (after 3 months). Additional anthropometric measures were evaluated (BMI, weight loss, and baseline VAT).
Approximately 73% of patients were sarcopenic at baseline according to previous data,2, 6, 7 and most of them had normal BMI (median BMI: 24.1 kg/m2). Furthermore, 33% of sarcopenic patients and 35% of patients with early LSMM had a BMI > 25 kg/m2. Of note, sarcopenic obesity may be potentially misleading because sarcopenia is also present among normal and obese patients.2, 18, 19 Therefore, in these cases, a skeletal muscle wasting may be masked, leading to greater toxicity and potentially serious adverse events to anticancer treatment.5, 20
Therefore, the use of BMI only is not reliable for nutritional assessment, because LSMM could be independent from weight loss and patients with similar weight or BMI may have a significant difference in body composition.21, 22, 23, 24 In our cohort, 21% of PC patients experienced an early LSMM ≥ 10%. Among sarcopenic patients, ~21% were subject to further loss of muscle mass ≥ 10%.
By univariate analysis, metastases at diagnosis, early PS loss according Karnofsky ≥ 20%, VAT ≥ 44 cm2/m2, and early LSMM ≥ 10% had a prognostic role. In multivariate analysis, in addition to the stage of diagnosis, only early LSMM ≥ 10% and VAT ≥ 44 cm2/m2 was statistically relevant and associated with worse prognosis in term of OS and PFS.
More recently, a retrospective analysis conducted on 782 PC patients found no significant prognostic association with survival outcomes for muscle attenuation.25 The considerable number of patients with early‐stage disease included in the study, ~35% of the whole cohort, may explain the discrepancy with our results, considering the possible differences in prognosis, the potential metabolic differences between advanced and early muscle wasting, and the recovering from muscle wasting after curative surgery.
Moreover, in contrast to Danai and colleagues who defined muscle attenuation as the average Hounsfield attenuation of all pixels in the muscle area, we evaluated the early LSMM as a decrease of SMI > 10% at first CT evaluation analysed in advanced PC.25
Cachexia in cancer patients is a well‐established negative prognostic factor,2 and sarcopenia may be one of its components, despite that the two conditions are very different. Cachexia is defined as a body weight loss over 12 months of 5% or more26; conversely, sarcopenia defines metabolic disorders that include LSMM, atrophy, fatigue, and reduction of muscular strength. During this process, muscle fibres are replaced by fibrotic tissue, resulting in neuromuscular junction degeneration, increased levels of reactive oxygen species alterations, and altered muscle metabolism.27, 28 Nowadays, the best index to identify sarcopenic patients is still under debate, as well as the nutritional follow‐up.4
Many studies are trying to clarify the molecular pathways underlying muscle wasting. The effect of systemic inflammation on muscle wasting could play a crucial role, because pro‐inflammatory factors such as cytokines (TNF‐α, IL‐1, IL‐6, and interferon γ), acute phase protein (C‐reactive protein), and metabolic factors impact metabolism, contributing to LSMM.29
In our study, we exploratorily evaluated the association between the early LSMM and systemic inflammation indexes and their impact on early LSMM considering NLR, LDH, C‐reactive protein, and albumin variation at 3 months after first‐line treatment. We found a significant association with NLR variations and LDH variations, although the LDH variation was not clinically relevant. No association was observed with other inflammatory indexes or with body weight loss. Notably, markers of systemic inflammation, such as NLR, are correlated with high levels of cytokines resulting in modulation of several factors (IGF‐1/Akt/mTOR axis, FoxOs, NF‐κB, and MAPK) favouring a catabolic state.27 More specially, IGF‐1 stimulates protein synthesis and promotes muscle hypertrophy; Akt/protein kinase B and FoxO are responsible for muscle homeostasis by regulating cell metabolism, growth, and survival. However, the main inhibitor of muscle growth is myostatin. It is produced by skeletal myocytes and negatively impact muscle growth through the interaction with IGF‐1–Akt signalling, mediated by the transcription factors small mothers against decapentaplegic 2 and 3.10, 30 Moreover, TNF‐α and IL‐6 inhibit myocyte differentiation promoting muscle atrophy and insulin resistance, leading to muscle wasting and increasing lipid deposition in skeletal muscle tissue.11, 28 LSMM could contribute to local inflammation, leading to further breakdown and enhancing systemic inflammation.11, 29, 31 Previous studies have confirmed the association of many of these inflammatory biomarkers with sarcopenia.27, 32, 33 In our study, we exploratorily evaluate the variation of these inflammatory indexes, but some of them were not associated with early LSMM, probably owing to the small sample size of the study and its retrospective nature.
In our cohort, 71% of patients received a nutritional assessment during any anticancer treatment line, and ~56% were evaluated in the first 3 months of first‐line chemotherapy. Moreover, 56% received a nutritional supplementation (oral and/or parenteral), and only 37% received an early nutritional intervention, although many of them were already sarcopenic. Overall, 85% of patients with early LSMM received a nutritional assessment, and only 55% received an early nutritional assessment. However, 30% received a dietary supplementation, and 25% received an early nutritional intervention (Table 2).
Surprisingly, early nutritional assessment and early dietary intervention did not impact prognosis in term of OS. Apparently, this finding was discordant with previous data.34, 35, 36 Therefore, we evaluated which factors were associated with early dietary supplementation, and only body weight loss was associated with an early dietary supplementation. However, as weight loss and BMI do not impact patient survival, nor does LSMM, it is useless to consider only these factors to perform a nutritional evaluation and implementation. Therefore, only a radiologic evaluation of LSMM provides an objective parameter that every clinician can use to monitor the nutritional status of PC patients.
Conclusions
Pancreatic cancer is a very rapidly progressive disease characterized by metabolic disorders that can easily lead to muscle mass depletion. The present study showed that an early LSMM ≥ 10% has a negative prognostic impact on advanced PC patients. On the other hand, baseline sarcopenia, weight loss, and BMI did not impact prognosis. Moreover, some inflammatory biomarkers, such as NLR variation, have an impact on an early LSMM ≥ 10%. Contrarily, early nutritional assessment and intervention were not associated with prognosis, and we showed that only body weight loss was associated with early nutritional implementation. Considering that weight loss and BMI did not impact patient survival, nor does LSMM, it is useless to consider only weight loss to perform a nutritional evaluation. In conclusion, to our knowledge, this is the first work to demonstrate the prognostic value of SMI loss during anticancer therapy independent from weight loss or gain. Therefore, to further evaluate the prognostic impact of an early nutritional treatment, we will provide a prospective Phase II study.
Funding
No funding was necessary for this work.
Conflict of Interest
The authors declare no competing financial interests.
Acknowledgements
The authors thank Federica Majer for her assistance. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.37 This manuscript has not been published previously or considered for publication concurrently in another publication.
Basile, D. , Parnofiello, A. , Vitale, M. G. , Cortiula, F. , Gerratana, L. , Fanotto, V. , Lisanti, C. , Pelizzari, G. , Ongaro, E. , Bartoletti, M. , Garattini, S. K. , Andreotti, V. J. , Bacco, A. , Iacono, D. , Bonotto, M. , Casagrande, M. , Ermacora, P. , Puglisi, F. , Pella, N. , Fasola, G. , Aprile, G. , and Cardellino, G. G. (2019) The IMPACT study: early loss of skeletal muscle mass in advanced pancreatic cancer patients. Journal of Cachexia, Sarcopenia and Muscle, 10: 368–377. 10.1002/jcsm.12368.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 3. Tan BHL, Birdsell LA, Martin L, Baracos VE, Fearon KCH. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res Off J Am Assoc Cancer Res 2009;15:6973–6979. [DOI] [PubMed] [Google Scholar]
- 4. Ozola Zalite I, Zykus R, Francisco Gonzalez M, Saygili F, Pukitis A, Gaujoux S, et al. Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: a systematic review. Pancreatol Off J Int Assoc Pancreatol IAP Al 2015;15:19–24. [DOI] [PubMed] [Google Scholar]
- 5. Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla Ē, Prado CM. Cancer‐associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc 2016;75:199–211. [DOI] [PubMed] [Google Scholar]
- 6. Ninomiya G, Fujii T, Yamada S, Yabusaki N, Suzuki K, Iwata N, et al. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: a retrospective cohort study. Int J Surg Lond Engl 2017;39:45–51. [DOI] [PubMed] [Google Scholar]
- 7. Choi Y, Oh D‐Y, Kim T‐Y, Lee K‐H, Han S‐W, Im S‐A, et al. Skeletal muscle depletion predicts the prognosis of patients with advanced pancreatic cancer undergoing palliative chemotherapy. Independent of Body Mass Index PloS One 2015;10:e0139749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evans WJ, Campbell WW. Sarcopenia and age‐related changes in body composition and functional capacity. J Nutr 1993;123:465–468. [DOI] [PubMed] [Google Scholar]
- 9. Naito T, Okayama T, Aoyama T, Ohashi T, Masuda Y, Kimura M, et al. Unfavorable impact of cancer cachexia on activity of daily living and need for inpatient care in elderly patients with advanced non‐small‐cell lung cancer in Japan: a prospective longitudinal observational study. BMC Cancer 2017;17:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ziaaldini MM, Marzetti E, Picca A, Murlasits Z. Biochemical pathways of sarcopenia and their modulation by physical exercise: a narrative review. Front Med 2017;4:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fearon KCH, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153–166. [DOI] [PubMed] [Google Scholar]
- 12. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol Off J Am Soc Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 13. Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab Physiol Appl Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 14. Montano‐Loza AJ, Meza‐Junco J, Baracos VE, Prado CMM, Ma M, Meeberg G, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc 2014;20:640–648. [DOI] [PubMed] [Google Scholar]
- 15. Sugiyama K, Narita Y, Mitani S, Honda K, Masuishi T, Taniguchi H, et al. Impact of sarcopenia on survival outcomes in patients (pts) with metastatic gastric cancer (mGC). J Clin Oncol 2017;35:141–141.28056202 [Google Scholar]
- 16. Sandini M, Bernasconi DP, Fior D, Molinelli M, Ippolito D, Nespoli L, et al. A high visceral adipose tissue‐to‐skeletal muscle ratio as a determinant of major complications after pancreatoduodenectomy for cancer. Nutr Burbank Los Angel Cty Calif 2016;32:1231–1237. [DOI] [PubMed] [Google Scholar]
- 17. Cloyd JM, Nogueras‐González GM, Prakash LR, Petzel MQB, Parker NH, Ngo‐Huang AT, et al. Anthropometric changes in patients with pancreatic cancer undergoing preoperative therapy and pancreatoduodenectomy. J Gastrointest Surg Off J Soc Surg Aliment Tract 2017;22:703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, et al. Explaining the obesity paradox: the Association Between Body Composition and Colorectal Cancer Survival (C‐SCANS Study). Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 2017;26:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gioulbasanis I, Martin L, Baracos VE, Thézénas S, Koinis F, Senesse P. Nutritional assessment in overweight and obese patients with metastatic cancer: does it make sense? Ann Oncol Off J Eur Soc Med Oncol 2015;26:217–221. [DOI] [PubMed] [Google Scholar]
- 20. Ongaro E, Buoro V, Cinausero M, Caccialanza R, Turri A, Fanotto V, et al. Sarcopenia in gastric cancer: when the loss costs too much. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc 2017;20:563–572. [DOI] [PubMed] [Google Scholar]
- 21. Gurney H. Dose calculation of anticancer drugs: a review of the current practice and introduction of an alternative. J Clin Oncol Off J Am Soc Clin Oncol 1996;14:2590–2611. [DOI] [PubMed] [Google Scholar]
- 22. Prado CMM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5‐fluorouracil‐based chemotherapy toxicity. Clin Cancer Res Off J Am Assoc Cancer Res 2007;13:3264–3268. [DOI] [PubMed] [Google Scholar]
- 23. Prado CMM, Lima ISF, Baracos VE, Bies RR, McCargar LJ, Reiman T, et al. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol 2011;67:93–101. [DOI] [PubMed] [Google Scholar]
- 24. Prado CMM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res Off J Am Assoc Cancer Res 2009;15:2920–2926. [DOI] [PubMed] [Google Scholar]
- 25. Danai LV, Babic A, Rosenthal MH, Dennstedt EA, Muir A, Lien EC, et al. Altered exocrine function can drive adipose wasting in early pancreatic cancer. Nature 2018;558:600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 27. Zhang G, Li X, Sui C, Zhao H, Zhao J, Hou Y, et al. Incidence and risk factor analysis for sarcopenia in patients with cancer. Oncol Lett 2016;11:1230–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meng S‐J, Yu L‐J. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci 2010;11:1509–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baracos VE. Cancer‐associated cachexia and underlying biological mechanisms. Annu Rev Nutr 2006;26:435–461. [DOI] [PubMed] [Google Scholar]
- 30. Romanick M, Thompson LV, Brown‐Borg HM. Murine models of atrophy, cachexia, and sarcopenia in skeletal muscle. Biochim Biophys Acta 1832;2013:1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim EY, Kim YS, Seo J‐Y, Park I, Ahn HK, Jeong YM, et al. The relationship between sarcopenia and systemic inflammatory response for cancer cachexia in small cell lung cancer. PloS One 2016;11:e0161125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS Study. JAMA Oncol 2017;3:e172319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cox S, Powell C, Carter B, Hurt C, Mukherjee S, Crosby TDL. Role of nutritional status and intervention in oesophageal cancer treated with definitive chemoradiotherapy: outcomes from SCOPE1. Br J Cancer 2016;115:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cereda E, Cappello S, Colombo S, Klersy C, Imarisio I, Turri A, et al. Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol 2017;126:81–88. [DOI] [PubMed] [Google Scholar]
- 36. Caccialanza R, Cereda E, Pinto C, Cotogni P, Farina G, Gavazzi C, et al. Awareness and consideration of malnutrition among oncologists: insights from an exploratory survey. Nutr Burbank Los Angel Cty Calif 2016;32:1028–1032. [DOI] [PubMed] [Google Scholar]
- 37. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


