Abstract
Objective
The purposes of this study were three‐fold: (i) to describe handgrip strength in older individuals aged ≥60 years in Colombia; (ii) to identify sex‐specific and age‐specific muscle weakness cut‐off points in older adults; and (iii) to determine the odds of adverse events for each of the intrinsic capacity domains for individuals with handgrip strength greater than the muscle weakness cut‐off points, as compared with their weaker counterparts.
Methods
A cross‐sectional study was conducted in Colombia, among 5237 older adults aged ≥60 years old (58.5% women, 70.5 ± 7.8 years), according to ‘SABE Survey 2015’. Handgrip strength data were obtained with a Takei dynamometer. Sociodemographic variables, five domains of intrinsic capacity (i.e. locomotion, vitality, cognition, psychological, and sensory), and medical conditions were assessed and analyzed. Adjustments variables were age, ethnicity, socio‐economic status, urbanicity, body mass index, smoking status, alcohol intake, drug use, physical activity, and co‐morbid chronic diseases. Sex‐stratified analyses were conducted with logistic regression models.
Results
Handgrip strength was greater among men than among women (26.7 ± 8.5 vs. 16.7 ± 5.7 kg, respectively, P < 0.001) at all ages. Weak handgrip strength cut‐off points ranged from 17.4 to 8.6 and from 10.1 to 4.9 in men and women, respectively. Overall, participants with optimal handgrip strength had better intrinsic capacity [in men, odds ratio (OR) = 0.62, 95% confidence interval (CI) 0.53 to 0.71; P < 0.001; and in women, OR = 0.79, 95% CI 0.68 to 0.92; P = 0.002] than their weaker counterparts. Also, men with optimal handgrip strength had a lower risk of hospitalization (OR = 0.47, 95% CI 0.29 to 0.78; P = 0.004) than their weaker counterparts.
Conclusions
This study is the first to describe handgrip strength values and cut‐off points for muscle weakness among a nationally representative sample of Colombian older adults by age and sex. After categorizing older adults as weak or not weak based on the handgrip cut‐off points, non‐weakness was associated with a decreased odds of intrinsic capacity impairments. These cut‐off points may be good candidates for clinical assessment of risks to physical and mental health in older Colombian adults.
Keywords: Skeletal muscle, Handgrip, Older adults, Locomotion, Vitality, Cognition, Mental health
Introduction
Colombia is a country with approximately 48 million inhabitants, with some 5.2 million inhabitants aged 60 years and older.1 Currently, the life expectancy in Colombia is 72.3 years, and by 2025, life expectancy is expected to be 77.6 years for women and 69.8 years for men. In this context, the World Health Organization has published the World Report on Aging and Health,2 which describes healthy ageing as the result of the interaction between the physical and mental capacity of an individual (the intrinsic capacity) and the context of each individual's life (the environment).3 Accordingly, healthy ageing depends upon intrinsic capacity, socio‐economic status, and physical environment, and the interactions between these factors.4 In this vein, the World Health Organization has proposed five domains—locomotion, vitality, cognition, psychological, and sensory—that can be used to evaluate an individual's intrinsic capacity.
Muscular strength as evaluated by handgrip strength has predictive value for assessing declines in physical and mental capacities in older adults,5 which are both components of the intrinsic capacity construct. Recent studies have shown that greater handgrip muscular strength is associated with lower all‐cause6 and cancer mortality.7
The world's population is rapidly getting older8; thus, the preservation of muscle strength and power with advancing age is of considerable clinical significance. Against this background, the development of clinically viable screening tools for detecting individuals at heightened risk for functional limitations is warranted.7 Indeed, it has been reported that handgrip strength reference values and muscular weakness cut‐off points for older adults are needed,9 but no data have yet been described for the Colombian elderly population. Additionally, several cross‐sectional studies have presented different handgrip strength cut‐off points in Italian,10 Finnish,11 Chinese,12 and American13, 14 older adults, thus suggesting that different ethnicities may have different muscle weakness cut‐off points for identifying clinically relevant health outcomes.
The results of the Survey on Health, Well‐Being, and Aging in Latin America and the Caribbean (SABE, from the initials in Spanish SAlud, Bienestar, and Envejecimiento) have increased our understanding of the ageing process and have helped to create public policies aimed at improving the well‐being of the Latin American and Caribbean populations. They have also provided a framework for performing a second set of studies in the region.
The purposes of this study were three‐fold: (i) to describe handgrip strength in older individuals aged ≥60 years in Colombia; (ii) to identify sex‐specific and age‐specific muscle weakness cut‐off points in older adults; and (iii) to determine the odds of adverse events for each of the intrinsic capacity domains for individuals with handgrip strength greater than the muscle weakness cut‐off points, as compared with their weaker counterparts.
Materials and methods
Study design
This study is part of the 2015 SABE study Survey on Health, Well‐Being, and Aging in Latin America and the Caribbean. Of the initial 23 694 elderly Colombians who took part in SABE Colombia, a total of 5237 were included in the present analysis after excluding participants without handgrip strength results (n = 18 457). There were no differences in the study key characteristics (i.e. age groups, body mass, height, body mass index, and sex distribution) between the current study sample and the original SABE study sample (all P > 0.05). The 5237 elderly Colombians constituted the final analytical sample of the non‐institutionalized population. Institutional review boards at the two universities involved in developing the SABE Colombia study (University of Caldas, ID protocol CBCS‐021‐14, and University of Valle, ID protocol 09‐014 and O11‐015) reviewed and approved the study protocol, and written informed consent was obtained from each individual before inclusion and completion of the first examination (including permission to use secondary data and blood samples). The study protocol to the secondary analysis was approved by The Human Subjects Committee at the Pontificia Universidad Javeriana (ACTA ID 20/2017‐2017/180, FM‐CIE‐0459‐17).
Details of background and design methods (i.e. characteristics of participants, sample calculation, outcomes, and analysis plan) of the SABE study have been previously published elsewhere1; nevertheless, the most relevant information is briefly described in the succeeding texts. All information collected was obtained through face‐to‐face interviews conducted at each site on mobile capture devices (e.g. tablets) or with printed versions of the questionnaire. During the personal interviews, direct physical measurements were taken, including handgrip strength [absolute and relative; i.e. handgrip strength (kg)/body mass (kg)], measured with a Takei dynanometer (Takei Scientific Instruments Co., Tokyo, Japan), height (standing and sitting), and weight. Five domains of the intrinsic capacity (locomotion, vitality, cognition, psychological, and sensory) were assessed as follows: (i) the cognition domain was assessed by the modified version of the mini‐mental state examination15, 16; (ii) the locomotion domain was defined according to five definitions—sarcopenia,17 prevalence of falls, functional impairments assessed with an activities of daily living scale,18 mobility/disability,19 and physical performance assessed by the validated Spanish version of the short physical performance battery20; (iii) the psychological domain was assessed by the Yesavage Geriatric Depression Scale and mental problems; (iv) the sensory domain was assessed as hearing and vision problems21; and (v) the vitality domain was assessed as loss of appetite22 and weight loss.
The self‐reported co‐morbidities or medical conditions category was assessed by asking the participants if they had been diagnosed by a physician with hypertension, diabetes, respiratory diseases, cardiovascular diseases, cancer, or osteoporosis. Drug use was evaluated with the following question: ‘Do you currently take or use any prescription medication’? For the lifestyle domain, personal habits regarding alcohol consumption and cigarette smoking were recorded. A ‘proxy physical activity’ was also evaluated. Finally, hospitalization >24 h in the last year was recorded. Details of the methods are available in the Supporting Information.
Data analysis
We used SPSS v24.0 software for Windows (SPSS, Chicago, IL, USA), except for the LMS method calculations (see the succeeding texts). The optimal power to obtain normality was calculated for each of a series of age groups and the trend summarized by a smooth (L) curve. Trends in the mean (M) and coefficient of variation (S) were similarly smoothed. The resulting L, M, and S curves contain the information to produce any centile curve and to convert measurements (even extreme values) into exact standard deviation (SD) scores.23 Anthropometric and handgrip characteristics from the study sample are presented as the mean and SD. Normality for the selected variables was verified using histograms and Q–Q plots. Differences were analyzed by two‐way analysis of variance or the χ2 test to compare sex and age differences.
The LMS method assumes that the outcome variable has a normal distribution after a Box–Cox power transformation is applied, according to the LMS method implemented in the LMS Chart Maker Pro Version 2.54 (Medical Research Council, London, UK). Smoothed and specific curves for each age were obtained via a penalized maximum likelihood with the following abbreviations: M (median), L (Box–Cox transformation), and S (coefficient of variation).24 The appropriate number of degrees of freedom was selected on the basis of the deviance, Q‐tests, and worm plots, following the suggestions of Royston and Wright.25 The P3, P10, P25, P50, P75, P90, and P97 smoothing centiles were chosen as age‐specific and sex‐specific reference values. Statistical significance was assessed with a two‐tailed α level of 0.05.
Finally, logistic regression models were used to compare the prevalence of adverse events in the five domains of the intrinsic capacity according to the cut‐off for handgrip strength. The analysis was adjusted for age, ethnicity, socio‐economic status, urbanicity, body mass index, smoking status, alcohol intake, drug use, physical activity, and medical conditions (presence or absence of osteoporosis, cardiovascular diseases, hypertension, diabetes, cancer, or respiratory diseases).
Results
The characteristics of the sample are summarized in Table 1. Age ranged from 60 to 85 years, with a mean of 70.5 ± 7.8 years. Absolute and relative handgrip strength was higher among men than among women (P < 0.001). In the total sample, self‐reported co‐morbidities were presented in the following proportions of cases: cancer (4.9%), respiratory diseases (10.8%), osteoporosis (12.3%), cardiovascular diseases (14.3%), diabetes (16.6%), and hypertension (55.9%). According to the intrinsic capacity domains, overall, women had more problems with locomotion, sensory, and psychological parameters than men (P < 0.01), except in terms of hearing problems.
Table 1.
Characteristics of the study participants (n = 5237)
| Characteristics | Men (n = 2172) | Women (n = 3065) | Overall (n = 5237) | P for group |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Anthropometric and handgrip strength | ||||
| Age (years) | 70.8 ± 8.0 | 70.2 ± 7.7 | 70.5 ± 7.8 | 0.004 |
| Height (cm) | 162.9 ± 6.9 | 150.9 ± 6.3 | 156.0 ± 8.8 | <0.001 |
| Weight (kg) | 67.8 ± 12.5 | 62.7 ± 13.2 | 64.8 ± 13.1 | <0.001 |
| BMI (kg/m2) | 25.6 ± 4.0 | 27.7 ± 5.3 | 26.8 ± 4.9 | <0.001 |
| Handgrip strength (kg) | 26.7 ± 8.5 | 16.7 ± 5.7 | 20.9 ± 8.6 | <0.001 |
| Handgrip (kg)/weight (kg) | 0.40 ± 0.12 | 0.27 ± 0.10 | 0.33 ± 0.12 | <0.001 |
| n (%) | n (%) | n (%) | ||
| Sociodemographic outcomes | ||||
| Socio‐economic status | ||||
| Level I | 807 (37.2) | 986 (32.2) | 1793 (34.2) | 0.088 |
| Level II | 897 (41.3) | 1295 (42.3) | 2192 (41.9) | 0.397 |
| Level III | 417 (19.2) | 662 (21.6) | 1079 (20.6) | 0.709 |
| Level IV | 38 (1.7) | 90 (2.9) | 128 (2.4) | <0.001 |
| Level V–VI | 13 (0.6) | 32 (1.0) | 45 (0.9) | 0.763 |
| Urbanicity | ||||
| Urban | 1602 (73.8) | 2453 (80.0) | 4055 (77.4) | 0.589 |
| Rural | 570 (26.2) | 612 (20.0) | 1182 (22.6) | 0.016 |
| Ethnic group | ||||
| Indigenous | 171 (7.9) | 142 (4.6) | 313 (6.0) | <0.001 |
| Black ‘mulato’ or Afro‐Colombian | 202 (9.3) | 226 (7.4) | 428 (8.2) | 0.016 |
| White | 540 (24.9) | 848 (27.7) | 1388 (26.5) | 0.671 |
| Othersa | 955 (44.0) | 1351 (44.1) | 2306 (44.0) | 0.342 |
| Cognition outcome | ||||
| Cognitive impairment | 304 (14.0) | 498 (16.2) | 802 (15.3) | 0.014 |
| Locomotion outcomes | ||||
| Sarcopenia | 445 (20.5) | 796 (26.0) | 1241 (23.7) | <0.001 |
| Falls | 324 (14.9) | 560 (18.3) | 884 (16.9) | <0.001 |
| Functional impairment < 90 points | 126 (5.8) | 229 (7.4) | 335 (6.3) | <0.001 |
| Difficulty walking 400 m | 274 (12.6) | 423 (13.8) | 697 (13.3) | <0.001 |
| SPPB < 6 points | 455 (20.9) | 1015 (33.1) | 1470 (28.1) | <0.001 |
| Psychological outcomes | ||||
| Depression | 1073 (49.4) | 1605 (52.4) | 2678 (51.1) | <0.001 |
| Mental problems | 123 (5.7) | 342 (11.2) | 465 (8.9) | <0.001 |
| Sensory outcomes | ||||
| Visual problems | 1187 (54.7) | 1715 (56.0) | 2902 (55.4) | <0.001 |
| Hearing problems | 618 (28.5) | 682 (22.3) | 1300 (24.8) | <0.001 |
| Vitality | ||||
| Weight loss | 335 (15.4) | 582 (19.0) | 582 (11.1) | <0.001 |
| Appetite loss | 403 (18.6) | 868 (28.3) | 1271 (24.3) | <0.001 |
| Co‐morbid chronic diseases | ||||
| Hypertension | 1040 (47.9) | 1889 (61.6) | 2929 (55.9) | <0.001 |
| Diabetes | 306 (14.1) | 562 (18.3) | 868 (16.6) | <0.001 |
| Respiratory diseases | 217 (10.0) | 351 (11.5) | 568 (10.8) | 0.050 |
| Cardiovascular disease | 297 (13.7) | 452 (14.7) | 749 (14.3) | 0.145 |
| Osteoporosis | 101 (4.7) | 543 (17.7) | 644 (12.3) | <0.001 |
| Cancer | 98 (4.5) | 161 (5.3) | 259 (4.9) | 0.122 |
| Clinical outcomes | ||||
| Hospitalized >24 h last year | 249 (11.5) | 378 (12.3) | 627 (12.0) | 0.187 |
| Drug use | 1360 (62.6) | 2424 (79.1) | 3784 (72.3) | <0.001 |
| Lifestyle outcomes | ||||
| Alcohol | 494 (22.7) | 151 (4.9) | 645 (12.3) | <0.001 |
| Smoking | 329 (15.1) | 219 (7.1) | 548 (10.5) | <0.001 |
| Meeting PA recommendations | 1683 (77.5) | 2630 (85.8) | 4313 (82.4) | <0.001 |
Data are presented as mean ± SD or no. (percentage) of participants. Significant differences between men and women group were analyzed by Student's t‐test or χ2 test.
BMI, body mass index; PA, physical activity; SD, standard deviation; SPPB, short physical performance battery.
Others (mestizo, gitano and gypsy, etc.).
Table 2 and Figure 1 show smoothed age‐specific and sex‐specific percentiles of handgrip strength in men (panel A) and women (panel B). The data showed that men performed better in the test at all ages than women. In men, the 50th centile of handgrip strength ranged from 17.6 to 30.5 kg and in women from 12.4 to 18.5 kg. There was a decrease in muscle strength across the age range in both sexes.
Table 2.
Smoothed age‐specific and sex‐specific percentile of handgrip strength (kg) in men and women
| Sex/age group | n | L | S | P3 | P10 | P25 | P50 (M) | P75 | P90 | P97 |
|---|---|---|---|---|---|---|---|---|---|---|
| Men (n = 2172) | ||||||||||
| 60–64 | 562 | 1.07 | 0.26 | 14.0 | 19.6 | 25.1 | 30.5 | 35.9 | 41.2 | 46.4 |
| 65–69 | 535 | 1.04 | 0.28 | 12.6 | 18.0 | 23.3 | 28.6 | 33.9 | 39.1 | 44.3 |
| 70–74 | 423 | 0.95 | 0.30 | 11.0 | 16.0 | 21.2 | 26.3 | 31.6 | 36.8 | 42.1 |
| 75–79 | 304 | 0.81 | 0.33 | 9.4 | 13.9 | 18.7 | 23.8 | 29.1 | 34.6 | 40.3 |
| 80–84 | 201 | 0.69 | 0.36 | 7.5 | 11.4 | 15.7 | 20.4 | 25.5 | 30.9 | 36.6 |
| 85+ | 147 | 0.60 | 0.40 | 6.0 | 9.3 | 13.2 | 17.6 | 22.5 | 27.8 | 33.6 |
| Women (n = 3065) | ||||||||||
| 60–64 | 873 | 1.00 | 0.32 | 6.8 | 10.7 | 14.6 | 18.5 | 22.4 | 26.3 | 30.1 |
| 65–69 | 774 | 1.10 | 0.32 | 6.1 | 9.8 | 13.6 | 17.3 | 21.1 | 24.8 | 28.6 |
| 70–74 | 563 | 1.02 | 0.35 | 5.0 | 8.7 | 12.4 | 16.1 | 19.8 | 23.5 | 27.2 |
| 75–79 | 427 | 0.99 | 0.36 | 4.0 | 7.6 | 11.1 | 14.7 | 18.3 | 21.8 | 25.4 |
| 80–84 | 271 | 1.14 | 0.43 | 1.9 | 5.8 | 9.6 | 13.4 | 17.2 | 21.0 | 24.8 |
| 85+ | 157 | 0.98 | 0.44 | 1.4 | 5.1 | 8.8 | 12.4 | 16.1 | 19.8 | 23.5 |
L, power in the Box–Cox transformation for ‘correcting’ the skewness; M, median; P, percentile; S, coefficient of variation.
Figure 1.
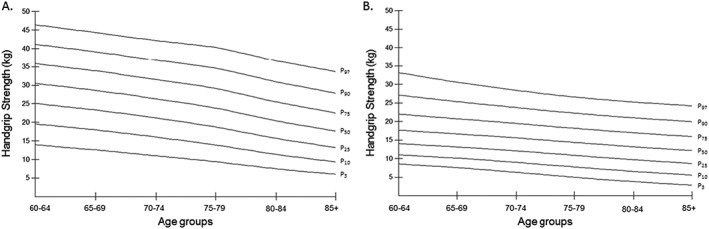
Absolute strength smoothed centile curves for men (A) and women (B) for Colombian aged 60+ years.
Weak handgrip cut‐off values using <1 SD by sex and age group are shown in Table 3. These cut‐off points ranged from 17.4 to 8.6 and from 10.1 to 4.9 in men and women, respectively.
Table 3.
Weak handgrip cut point values using <1 SD by sex and age group
| Sex/age group | Cut point (kg) |
|---|---|
| Men | Mean |
| 60–64 | 17.4 |
| 65–69 | 15.7 |
| 70–74 | 14.3 |
| 75–79 | 12.3 |
| 80–84 | 10.1 |
| 85+ | 8.6 |
| Women | |
| 60–64 | 10.1 |
| 65–69 | 8.9 |
| 70–74 | 8.2 |
| 75–79 | 6.7 |
| 80–84 | 5.3 |
| 85+ | 4.9 |
SD, standard deviation.
Finally, Figure 2 shows the association between optimal handgrip strength (kg) with the domains of intrinsic capacity. Overall, participants with optimal handgrip strength had better intrinsic capacity than weak older adults, including both men [odds ratio (OR) = 0.62, 95% confidence interval (CI) 0.53 to 0.71; P < 0.001] and women (OR = 0.79, 95% CI 0.68 to 0.92; P = 0.002). Regarding the intrinsic capacity domains, older men with optimal handgrip strength had lower odds of having cognition (OR = 0.50, 95% CI 0.29 to 0.85; P = 0.010), locomotion (OR = 0.47, 95% CI 0.37 to 0.59; P < 0.001), and vitality (OR = 0.68, 95% CI 0.47 to 0.98; P = 0.040) problems than their weaker counterparts. Older women with optimal handgrip strength also had lower odds of having cognition (OR = 0.44, 95% CI 0.24 to 0.80; P = 0.007), locomotion (OR = 0.66, 95% CI 0.51 to 0.85; P = 0.001), and psychological (OR = 0.57, 95% CI 0.40 to 0.82; P = 0.002) problems than their weaker counterparts (Figure 2 ). Additionally, older men with handgrip strength greater than the muscle weakness cut‐off points had lower odds of hospitalization than their weaker counterparts (OR = 0.47, 95% CI 0.29 to 0.78; P = 0.004).
Figure 2.
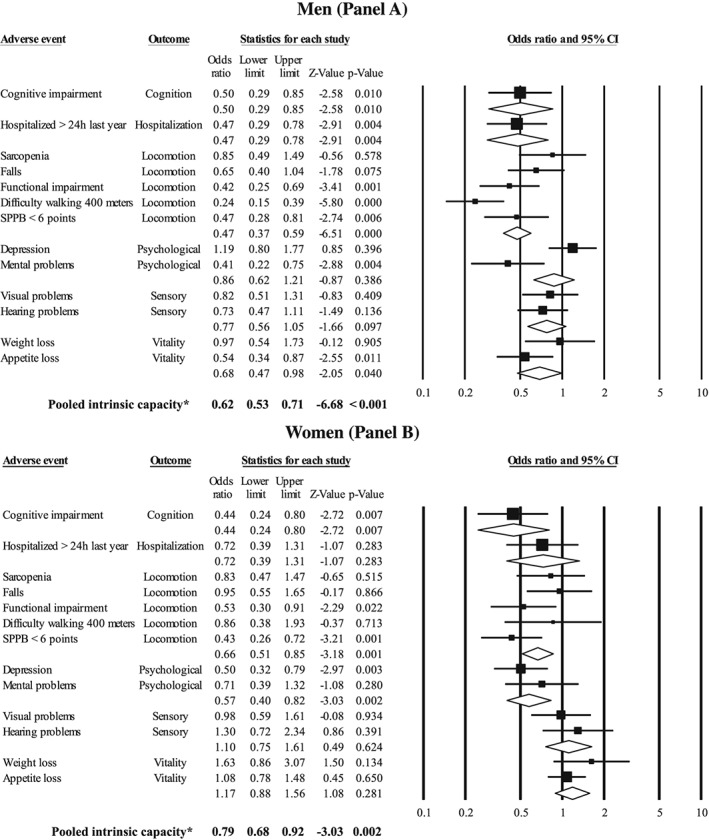
Association between healthy handgrip strength (kg) with the components of the intrinsic capacity and hospitalization. The analysis was adjusted for age, ethnicity, socio‐economic status, urbanicity, body mass index, smoking status, alcohol intake, drug use, physical activity, and medical conditions (presence or absence of osteoporosis, cardiovascular diseases, hypertension, diabetes, cancer, or respiratory diseases). *Pooled intrinsic capacity was calculated without hospitalization outcome values. CI, confidence interval; SPPB, short physical performance battery.
Discussion
Using a nationally representative sample of older Colombian adults, this study presents normative data for handgrip strength, identifies sex‐specific and age‐specific muscle weakness cut‐off points, and determines the odds of adverse events for each intrinsic capacity domain for individuals with handgrip strength greater than the muscle weakness cut‐off points. Overall, older adults with handgrip strength greater than the muscle weakness cut‐off points had lower odds of adverse events in most of the intrinsic capacity domains (especially in cognition and locomotion domains) and hospitalization (only in men) than their weaker peers. Our results may inform intervention strategies aiming to increase muscle strength and promote healthy ageing.
Several handgrip strength normative ranges for older adults from populations with different nationalities and ethnicities have been published in the last several years26, 27, 28, 29, 30; however, to our knowledge, normal handgrip strength values have never been described for the Colombian population. We found that, overall, older Colombian adults have lower handgrip strength values than their peers in other populations.26, 27, 28, 29 Our results showing that gender and age affect handgrip strength are in accordance with the findings of previous studies.26, 27, 28, 29 Thus, the results of this study contribute to the current body of literature by presenting sex‐specific and age‐specific weakness cut‐off points among the Colombian elderly.
Different handgrip strength mean values have been observed in different countries, as reported previously,20 but the nature of these differences is not known. Differences in handgrip strength mean values between Colombian older individuals from a less‐developed country (this study) and individuals in developed countries may be due to a number of factors, although it is uncertain which of the three factors, genetic, environment, or biological, are more decisive for handgrip strength results.31 For instance, biological or environment factors such as health status, lifestyle, and demographic and socio‐economic characteristics vary greatly between countries with different handgrip strength levels. Along this line, education and socio‐economic status are factors that might explain differences in handgrip strength ranges among countries.32 Also, beyond ethnic differences in height and in skeletal muscle mass and function,33 there are well‐recognized differences in dietary protein intake between different countries, and this variation might also explain differences in muscle strength.34 Absolute strength has also been related to nutrition status and is reported to have positive influence on individuals' grip strength. Another possible reason for the divergence between studies might be methodological differences (i.e. variability in the equipment used and the protocol for measuring handgrip strength).35
Several studies have determined absolute10, 11, 14 and relative handgrip strength cut‐off points12, 13 that are overall higher than our cut‐off points. For example, Duchowny et al.14 determined cut‐off points for weakness associated with gait speed (<0.8 m/s) in older American adults, with values of <40 and <31 kg in men and women, respectively. Similarly, Cruz‐Jentoft et al.10 found that among community‐dwelling older adults in Italy, a handgrip strength <30 kg in men and <20 kg in women was associated with slow gait speed and an inability to walk 1 km without difficulty. Therefore, different cut‐off points are observed in different countries; however, while the nature of these differences is unclear,31 as mentioned, heterogeneous designs, genetic, and environmental factors may explain some of the differences among the studies.
The handgrip strength cut‐off points reported in the present study are useful for determining who among older adults may benefit from lifestyle modifications to preserve muscle strength and reduce the odds of physical and mental limitations. In this context, our results reveal that older men with handgrip strength greater than the muscle weakness cut‐off points had a lower odds of hospitalization for >24 h than their weaker counterparts. However, the cross‐sectional design of the present study did not allow us to determine whether the measured handgrip strength differed from habitual strength or if the handgrip was impaired as a result of the presenting condition (see limitations in the succeeding texts). Our results partially confirm those of previous studies that analyzed the prospective association between customary handgrip strength and hospital admission, which showed varying results9, 36 The Hertfordshire Cohort Study9 provided evidence that handgrip strength among community‐dwelling men and women in the UK was associated with risk of hospital admission over the following decade. In contrast, a prospective study of 279 older adults aged ≥70 years from Germany who were followed up for 18 months found no association between handgrip strength and the risk of hospital admission.36 The differences between these studies may be explained by the thresholds for admission, which likely differ between healthcare systems and their cross‐sectional design.
A recent narrative review reported that weak handgrip strength was associated with reduced cognitive performance over time; therefore, greater handgrip strength may be protective against cognitive decline in older adults.37 Indeed, recent evidence has shown that weak handgrip strength is associated with an increased risk of psychological problems such as depression.38, 39 These findings were confirmed by the results of our analysis, which suggested that older adults with handgrip strength greater than the muscle weakness cut‐off points had lower odds of cognitive impairment and depression than their weaker peers. Overall, weak handgrip strength, cognition, and depression share some risk and pathogenic factors (particularly an increased rate of oxidative stress40 and inflammation,41 decreased sex hormone levels,42 and maximal voluntary contraction43) that may influence the onset of depression and cognitive impairment.44 Given the strength of the evidence in this area, handgrip strength and our cut‐off points are easy to utilize and are clinically useful biomarkers of cognitive decline and mental health across the lifespan of individuals from the Colombian population.
Physical decline, in terms of decreasing muscle weakness and poor mobility, has been repeatedly included as an additional vital sign45 and a key instrument for the functional assessment of older patients.46 The present results are also in agreement with those of previous research and suggest that handgrip weakness is associated with measures of functional limitations in older adults.47 For example, Ishizaki et al.48 determined that older adults with weak handgrip strength had difficulty performing many tasks, such as shopping for groceries, preparing meals, and performing housework. Our lower cut‐off points appear to have the ability to identify older adults of both sexes with locomotion problems. Therefore, it seems that it is especially important for older adults to preserve muscle strength to avoid functional limitations, such as by participating in muscle strengthening activities to preserve function. Additionally, our results suggest that optimal handgrip strength is associated with a lower odds of experiencing weight loss in older men, and so muscle strength could favour the maintenance of optimal homeostasis.7
Finally, the only intrinsic capacity domain that did not appear to be related to handgrip strength was hearing impairment. Hearing loss in the elderly is of increasing importance as the global population ages. Disabling hearing negatively affects communication and social engagement and can lead to reduced quality of life in adults49 and may also underlie cardiovascular risk and diseases (i.e. diabetes, hypertension, and history of cerebrovascular accident).50 These associations are mainly related to the compromised blood supply to the cochlea under vascular disease conditions,51 as well as other sensor neural, nutritional status, and medical co‐morbidity factors that may accumulate with age and are not a result of normal ageing.51 Peripheral age‐related hearing loss is also a possible biomarker and modifiable risk factor for cognitive decline, cognitive impairment, and dementia.50, 51, 52 Our results, however, revealed that an individual with low levels of handgrip strength might not necessarily have a worse hearing status and suggest that different age‐related mechanisms could be underlying this.
The strengths of this study include the large sample size of older adults with a nationally representative proportion of persons aged ≥60 and the objective assessment of muscle strength. This study does, however, have some limitations. First, the cross‐sectional design did not allow a definitive conclusion that weak muscle strength precedes adverse events. Second, several measures, such as falls, history of chronic disease, and drug use, were self‐reported by participants; therefore, different types of response biases may have been introduced.
Conclusions
This study provides reference values for handgrip strength in Colombian individuals 60 years and older. After categorizing participants as weak or not weak based on the handgrip cut‐off points, non‐weakness was associated with a decreased odds of intrinsic capacity impairments, especially in the cognition and locomotion domains, and of hospitalization (only in men). Therefore, a simple handgrip strength test with the appropriate cut‐off points may be a good candidate for clinical assessment of risks to physical and mental health in older Colombian adults.
Conflict of interest
None declared.
Supporting information
Data S1. Electronic Supplementary Material
Acknowledgements
The SABE study is supported by a fund (2013, no. 764) from the Colciencias y Ministerio de Salud y la Protección Social de Colombia. Mikel Izquierdo is funded by ISCIII and Fondos FEDER (PI17/01814). The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.53
Ramírez‐Vélez, R. , Correa‐Bautista, J. E. , García‐Hermoso, A. , Cano, C. A. , and Izquierdo, M. (2019) Reference values for handgrip strength and their association with intrinsic capacity domains among older adults. Journal of Cachexia, Sarcopenia and Muscle, 10: 278–286. 10.1002/jcsm.12373.
References
- 1. Gomez F, Corchuelo J, Curcio CL, Calzada MT, Mendez F. SABE Colombia: Survey on Health, Well‐Being, and Aging in Colombia—study design and protocol. Curr Gerontol Geriatr Res 2016;2016:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . World Report on Ageing and Health: World Health Organization 2015. [Google Scholar]
- 3. Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel JP, et al. The world report on ageing and health: a policy framework for healthy ageing. The Lancet 2016;387:2145–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cesari M, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, Cooper C, Martin FC, Reginster JY, et al. Evidence for the domains supporting the construct of intrinsic capacity. J Gerontol: Series A 2018; gly011. [DOI] [PubMed] [Google Scholar]
- 5. Rijk JM, Roos PR, Deckx L, van den Akker M, Buntinx F. Prognostic value of handgrip strength in people aged 60 years and older: a systematic review and meta‐analysis. Geriatr Gerontol Int 2016;16:5–20. [DOI] [PubMed] [Google Scholar]
- 6. García‐Hermoso A, Cavero‐Redondo I, Ramírez‐Vélez R, Ruiz J, Ortega FB, Lee DC, et al. Muscular strength as a predictor of all‐cause mortality in apparently healthy population: a systematic review and meta‐analysis of data from approximately 2 million men and women. Arch Phys Med Rehabil 2018;99:2100–2113. [DOI] [PubMed] [Google Scholar]
- 7. Celis‐Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, et al. Associations of grip strength with cardiovascular, respiratory and cancer outcomes, and all‐cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 2018;361:k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amaral TF, Santos A, Guerra RS, Sousa AS, Álvares L, Valdiviesso R, et al. Nutritional strategies facing an older demographic: the nutrition UP 65 study protocol. JMIR research protocols 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simmonds SJ, Syddall HE, Westbury LD, Dodds RM, Cooper C, Aihie Sayer A, et al. Grip strength among community‐dwelling older people predicts hospital admission during the following decade. Age Ageing 2015;44:954–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sallinen J, Stenholm S, Rantanen T, Heliövaara M, Sainio P, Koskinen S, et al. Hand‐grip strength cut points to screen older persons at risk for mobility limitation. J Am Geriatr Soc 2010;58:1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dong R, Guo Q, Wang J. Optimal cutoffs of grip strength for definition as weakness in the elderly. J Biosci Med 2014;2:14–18. [Google Scholar]
- 13. McGrath RP, Ottenbacher KJ, Vincent BM, Kraemer WJ, Peterson MD. Muscle weakness and functional limitations in an ethnically diverse sample of older adults. Ethn Health 2017;1–12. [DOI] [PubMed] [Google Scholar]
- 14. Duchowny KA, Peterson MD, Clarke PJ. Cut points for clinical muscle weakness among older Americans. Am J Prev Med 2017;53:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borda MG, Ruíz de Sánchez C, Gutiérrez S, Samper‐Ternent R, Cano‐Gutiérrez C. Relationship between cognitive impairment and instrumental activities of daily living (IADL): SABE‐Bogotá, Colombia Study. Acta Neurol Colomb 2016;32:27–34. [Google Scholar]
- 16. Murphy SL, Dubin JA, Gill TM. The development of fear of falling among community‐living older women: predisposing factors and subsequent fall events. J Gerontol A Biol Sci Med Sci 2003;58:M943–M947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rolland Y, Lauwers‐Cances V, Cournot M, Nourhashémi F, Reynish W, Rivière D, et al. Sarcopenia, calf circumference, and physical function of elderly women: a cross‐sectional study. J Am Geriatr Soc 2003;51:1120–1124. [DOI] [PubMed] [Google Scholar]
- 18. Batzán JJ, Pérez del Molino J, Alarcón T, San Cristóbal E, Izquierdo G, Manzarbeitia J. Índice de Barthel: instrumento válido para la valoración functional de pacientes con enfermedad cerebrovascular. Rev Esp Geriatr Gerontol 1993;28:32–40. [Google Scholar]
- 19. Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc 1976;54:439–467. [PubMed] [Google Scholar]
- 20. Gómez JF, Curcio CL, Alvarado B, Zunzunegui MV, Guralnik J, et al. Validity and reliability of the short physical performance battery (SPPB): a pilot study on mobility in the Colombian Andes. Colo Med 2013;44:165–171. [PMC free article] [PubMed] [Google Scholar]
- 21. Yueh B, Shapiro N, MacLean CH, Shekelle PG. Screening and management of adult hearing loss in primary care: scientific review. JAMA 2003;289:1976–1985. [DOI] [PubMed] [Google Scholar]
- 22. Thomas DR, Ashmen W, Morley JE, Evans WJ. Nutritional management in long‐term care: development of a clinical guideline. J Gerontol A Biol Sci Med Sci 2000;55:M725–M734. [DOI] [PubMed] [Google Scholar]
- 23. Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr 1990;44:45–60. [PubMed] [Google Scholar]
- 24. Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 1992;11:1305–1319. [DOI] [PubMed] [Google Scholar]
- 25. Royston P, Wright E. Goodness‐of‐fit statistics for age‐specific reference intervals. Stat Med 2000;19:2943–2962. [DOI] [PubMed] [Google Scholar]
- 26. Mendes J, Amaral TF, Borges N, Santos A, Padrão P, Moreira P, et al. Handgrip strength values of Portuguese older adults: a population based study. BMC Geriatr 2017;17:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ribom EL, Mellström D, Ljunggren Ö, Karlsson MK. Population‐based reference values of handgrip strength and functional tests of muscle strength and balance in men aged 70–80 years. Arch Gerontol Geriatr 2011;53:e114–e117. [DOI] [PubMed] [Google Scholar]
- 28. Pedrero‐Chamizo R, Gomez‐Cabello A, Delgado S, Rodríguez‐Llarena S, Rodríguez‐Marroyo JA, Cabanillas E, et al. Physical fitness levels among independent non‐institutionalized Spanish elderly: the elderly EXERNET multi‐center study. Arch Gerontol Geriatr 2012;55:406–416. [DOI] [PubMed] [Google Scholar]
- 29. Kenny RA, Coen RF, Frewen J, Donoghue OA, Cronin H, et al. Normative values of cognitive and physical function in older adults: findings from the Irish Longitudinal Study on Ageing. J Ame Geria Soc 2013;61:S279–S290. [DOI] [PubMed] [Google Scholar]
- 30. Wang YC, Bohannon RW, Li X, Yen SC, Sindhu B, Kapellusch J. Summary of grip strength measurements obtained in the 2011–2012 and 2013–2014 National Health and Nutrition Examination Surveys. J Hand Ther 2018. 10.1016/j.jht.2018.03.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31. Leong DP, Teo KK, Rangarajan S, Kutty VR, Lanas F, Hui C, et al. Reference ranges of handgrip strength from 125,462 healthy adults in 21 countries: a prospective urban rural epidemiologic (PURE) study. J Cachexia Sarcopenia Muscle 2016;7:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Lima TR, Silva DAS, de Castro JAC, Christofaro DGD. Handgrip strength and associated sociodemographic and lifestyle factors: a systematic review of the adult population. J Bodyw Mov Ther 2017;21:401–413. [DOI] [PubMed] [Google Scholar]
- 33. Silva AM, Shen W, Heo M, Gallagher D, Wang Z, Sardinha LB, et al. Ethnicity‐related skeletal muscle differences across the lifespan. Am J Hum Biol 2010;22:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McLean RR, Mangano KM, Hannan MT, Kiel DP, Sahni S. Dietary protein intake is protective against loss of grip strength among older adults in the Framingham Offspring Cohort. J Gerontol A Biol Sci Med Sci 2016;71:356–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ong HL, Abdin E, Chua BY, Zhang Y, Seow E, Vaingankar JA, et al. Hand‐grip strength among older adults in Singapore: a comparison with international norms and associative factors. BMC Geriatr 2017;17:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nikolaus T, Bach M, Oster P, Schlierf G. Prospective value of self‐report and performance‐based tests of functional status for 18‐month outcomes in elderly patients. Aging Clin Exp Res 1996;8:271–276. [DOI] [PubMed] [Google Scholar]
- 37. Fritz NE, McCarthy CJ, Adamo DE. Handgrip strength as a means of monitoring progression of cognitive decline—a scoping review. Ageing Res Rev 2017;35:112–123. [DOI] [PubMed] [Google Scholar]
- 38. Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Is sarcopenia associated with depression? A systematic review and meta‐analysis of observational studies. Age Ageing 2017;46:738–746. [DOI] [PubMed] [Google Scholar]
- 39. Veronese N, Stubbs B, Trevisan C, Bolzetta F, De Rui M, Solmi M, et al. Poor physical performance predicts future onset of depression in elderly people: Progetto Veneto Anziani Longitudinal Study. Phys Ther 2017;97:659–668. [DOI] [PubMed] [Google Scholar]
- 40. Solmi M, Veronese N, Luchini C, Manzato E, Sergi G, Favaro A, et al. Oxidative stress and antioxidant levels in patients with anorexia nervosa after oral re‐alimentation: a systematic review and exploratory meta‐analysis. Eur Eat Disord Rev 2016;24:101–105. [DOI] [PubMed] [Google Scholar]
- 41. Solmi M, Veronese N, Favaro A, Santonastaso P, Manzato E, Sergi G, et al. Inflammatory cytokines and anorexia nervosa: a meta‐analysis of cross‐sectional and longitudinal studies. Psychoneuroendocrinology 2015;51:237–252. [DOI] [PubMed] [Google Scholar]
- 42. Bowen RS, Turner MJ, Lightfoot JT, . Sex hormone effects on physical activity levels. Sports Med 2011;41:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petersen NT, Taylor JL, Butler JE, Gandevia SC. Depression of activity in the corticospinal pathway during human motor behavior after strong voluntary contractions. J Neurosci 2003;23:7974–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE, et al. Interleukin‐6 and risk of cognitive decline MacArthur Studies of Successful Aging. Neurology 2002;59:371–378. [DOI] [PubMed] [Google Scholar]
- 45. Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc 2003;51:314–322. [DOI] [PubMed] [Google Scholar]
- 46. Applegate WB, Blass JP, Williams TF. Instruments for the functional assessment of older patients. N Engl J Med 1990;322:1207–1214. [DOI] [PubMed] [Google Scholar]
- 47. Vermeulen J, Neyens JC, van Rossum E, Spreeuwenberg MD, de Witte LP. Predicting ADL disability in community‐dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr 2011;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ishizaki T, Watanabe S, Suzuki T, Shibata H, Haga H. Predictors for functional decline among nondisabled older Japanese living in a community during a 3‐year follow‐up. J Am Geriatr Soc 2000;48:1424–1429. [DOI] [PubMed] [Google Scholar]
- 49. Wilson BS, Tucci DL, Merson MH, O'Donoghue GM. Global hearing health care: new findings and perspectives. Lancet 2017;390:2503–2515. [DOI] [PubMed] [Google Scholar]
- 50. Wattamwar K, Qian ZJ, Otter J, Leskowitz Mj, Caruana FF, Siedlecki B, et al. Association of cardiovascular comorbidities with hearing loss in the older old. JAMA Otolaryngology–Head & Neck Surgery 2018;144:623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Helzner EP, Patel AS, Pratt S, Sutton‐Tyrrell K, Cauley JA, Talbott E, et al. Hearing sensitivity in older adults: associations with cardiovascular risk factors in the health, aging and body composition study. J Am Geriatr Soc 2011;59:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673–2734. [DOI] [PubMed] [Google Scholar]
- 53. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Electronic Supplementary Material


