Abstract
Atherosclerotic plaque rupture is the primary mechanism responsible for myocardial infarction and stroke, the top two killers worldwide. Despite being potentially fatal, the ubiquitous prevalence of atherosclerosis amongst the middle aged and elderly renders individual events relatively rare. This makes the accurate prediction of MI and stroke challenging. Advances in imaging techniques now allow detailed assessments of plaque morphology and disease activity. Both CT and MR can identify certain unstable plaque characteristics thought to be associated with an increased risk of rupture and events. PET imaging allows the activity of distinct pathological processes associated with atherosclerosis to be measured, differentiating patients with inactive and active disease states. Hybrid integration of PET with CT or MR now allows for an accurate assessment of not only plaque burden and morphology but plaque biology too. In this review, we discuss how these advanced imaging techniques hold promise in redefining our understanding of stable and unstable coronary artery disease beyond symptomatic status, and how they may refine patient risk-prediction and the rationing of expensive novel therapies.
Keywords: Unstable plaque, Computed tomography, Positron emission tomography
1. Introduction
Atherosclerotic plaque rupture is the primary mechanism responsible for two of the biggest killers worldwide: myocardial infarction and stroke [1]. In 2015, 423 million people were estimated to be living with cardiovascular disease, and it caused an estimated 18 million deaths. Whilst the clinical effects of atherosclerotic plaque rupture can be devastating, the development of atheromatous plaque is itself a silent and for many a benign process. Indeed, atherosclerosis is an almost ubiquitous finding in older patients the majority of whom will never suffer a cardiovascular event. Perhaps, the major challenge facing contemporary cardiovascular researchers is therefore to develop methods of accurate risk prediction without over medicalizing the population as a whole. In this review, we will briefly discuss the pathophysiology of atherosclerosis before investigating novel non-invasive imaging methods aimed at detecting unstable atherosclerotic plaque and measuring disease activity in the coronary arteries and large vessels. These advanced imaging techniques hold promise in redefining our understanding of stable and unstable coronary artery disease beyond a patient’s symptomatic status with the potential to improve our pathological understanding, to refine patient risk-prediction and to appropriately target expensive novel therapies.
1.1. Pathophysiology of atherosclerosis
Atherosclerosis is a smouldering immunoinflammatory disease fuelled by lipids [2]. It is characterised by focal thickening of the arterial intima (plaque formation) in medium and large sized arteries. Within the plaques lipid, inflammatory infiltrates, smooth muscle cells and connective tissues are found. An injury to the plaque cap known as a plaque rupture results in exposure of its core contents to the blood, causing acute thrombus formation and either partial or complete occlusion of the vessel lumen [3]. Atherothrombosis from plaque rupture is the most common cause of fatal myocardial infarction, accounting for approximately three quarters of cases, with plaque erosion accounting for the remaining quarter [4]. However, the majority of coronary plaque rupture events appear to be clinically silent, resulting in plaque growth rather than myocardial infarction.
Atherosclerosis begins in a hypercholesterolaemic state where low-density lipoproteins (LDL) infiltrate the endothelial wall. Subsequent oxidation of LDL molecules causes an inflammatory response with infiltration of T-lymphocytes and macrophages that consume LDL and form foam cells. This is initially protective, but with further LDL accumulation, macrophage cell death is ultimately triggered, contributing to further inflammation and the development of a necrotic core of soft unstable atheroma. Plaque inflammation also triggers smooth muscle cell loss and the production of matrix metalloproteinases (MMP) that weaken the fibrous cap, predisposing it to plaque rupture [2,5]. The thick necrotic acellular lipid core becomes increasingly hypoxic, stimulating angiogenesis, with the formation of immature microvessels prone to intra-plaque haemorrhage (IPH) [6–8].
Similar to tuberculosis [9], calcification of atherosclerotic plaque is thought to be a healing response to intense necrotic plaque inflammation characterised by two distinct stages. In the latter stage of macrocalcification, the healing process is complete and the plaque stabilised [10–12]. By contrast, the earlier stage of microcalcification is a common feature of ruptured and unstable plaques where healing is incomplete, inflammation remains active and the fibrous cap weakened by the tiny calcific deposits [13–15].
Unstable plaques at risk of rupture, therefore, have certain pathological features, including a large necrotic core, thin fibrous cap, inflammation, hypoxia, haemorrhage and microcalcification. By contrast, stable plaques at low risk of rupture have different characteristics, including a thick fibrous cap and macroscopic calcification. Advanced imaging now allows us to identify these plaque characteristics in vivo and determine whether patients predominantly have stable or unstable atheroma. Development of hybrid molecular imaging allows us to measure disease activity in the coronary arteries, directly. These developments hold promise in altering how we define stable and unstable atherosclerosis and in refining risk prediction beyond standard approaches. However, it should be noted that the plaque characteristics and pathophysiology underlying plaque erosion remain poorly understood, representing an important limitation of this approach.
1.2. Atherosclerotic plaque imaging
Direct imaging of coronary atherosclerotic plaque is now possible with CT calcium scoring (CACs) and coronary computerised tomography angiography (CCTA). This has permitted more accurate determination of coronary plaque burden, the presence of both obstructive and non-obstructive disease and assessments of plaque composition. Magnetic resonance (MR) whilst not as advanced as CT can provide similar information without radiation exposure, whilst novel PET approaches allow, for the first time, assessment of coronary disease activity. These plaque-imaging techniques are developing rapidly and, in the case of CT, starting to enter routine clinical practice. They are discussed in greater detail below and illustrated in Fig. 1.
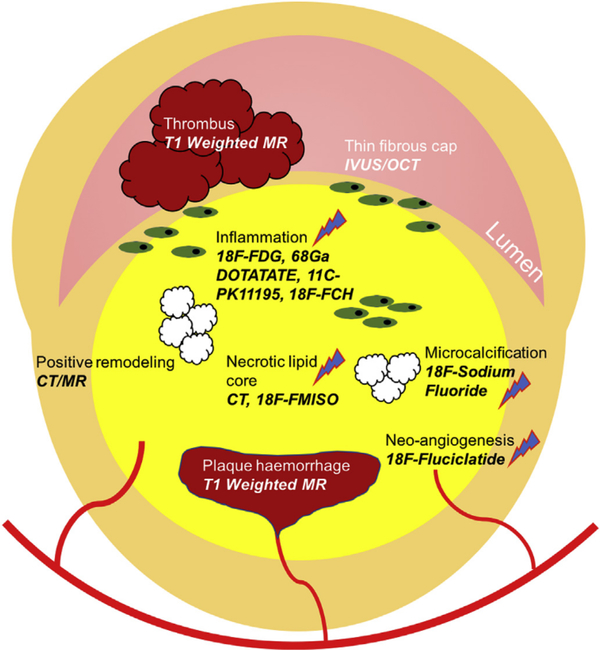
Fig. 1.
Schematic representation of morphological and biological targets for unstable plaque imaging.
1.3. Measures of plaque burden
Atherosclerotic plaque burden can be measured in different vascular beds using multiple different modalities, including ultrasound, CT and MR. Regardless of the methodology, plaque burden assessments provide powerful prognostic information, based upon the rationale that the greater number of plaques, the more likely a plaque is to rupture and cause an event.
CT calcium scoring is the best studied technique having been incorporated in to clinical guidelines [16] and providing prognostic information of incremental value to standard risk factor assessments [17]. However, most patients with high CT calcium scores will never suffer a clinical event. This may be because whilst CT calcium provides a surrogate of global plaque volume, it actually targets stable macrocalcific plaques, not the unstable plaques at highest risk of rupture. Moreover, CT calcium scoring cannot differentiate stable burnt-out disease from active unstable atheroma. Methods that can directly quantify unstable plaque and assess disease activity are therefore required.
1.4. Plaque morphological characteristics
Culprit plaques that have ruptured and caused an event have certain histological characteristics. Indeed, many retrospective and pathological studies have demonstrated the thin capped, fibro-fatty atheromatous (TCFA) plaque as the cause for the majority of myocardial infarctions and strokes [15,18–20]. Other recognised features of potentially unstable plaques are microcalcification, positive remodelling, inflammation and plaque haemorrhage [21] each representing a potential imaging target to improve the identification of high risk patients. As with plaque burden, multiple imaging modalities have been employed to better characterise plaque morphology.
IVUS can assess plaque burden, positive remodelling and lipid core. Moreover, virtual histology IVUS (VH-IVUS) allows direct detection of the VH-IVUS TCFAs in the coronary vasculature [22–24]. However, in the PROSPECT study of the 695 patients, whilst 595 VH-IVUS TCFAs were identified, only 6 MIS were observed over a 3-year period [25]. Comparable findings were reported in the VIVA study [26], suggesting low predictive value of these supposedly high-risk plaques [27,28]. We, therefore, prefer the term unstable plaque characteristics.
The other main invasive assessment of unstable plaque is OCT. This technique is particularly good at imaging the fibrous plaque, with the ability to identify thin caps and both plaque rupture and erosion [29,30]. Emerging OCT techniques offer assessment of further unstable plaque characteristics including plaque macrophages and angiogenesis [31–33]. However, similar to VH-IVUS, OCT-defined unstable plaques only rarely cause clinical events, so that plaque directed therapies cannot be recommended [34]
Contrast coronary CT angiography (CCTA) offers major advantages compared to CT calcium scoring. First, it can identify obstructive stenosis [35], providing improved diagnostic accuracy and clinical outcomes in the assessment of patients presenting with chest pain [36,37]. Second, it can inform about plaque morphology. Unlike CACs, CCTA can identify non-calcified as well as calcified plaque and can identify multiple unstable plaque characteristics. These include low attenuation as a marker of necrotic core, positive remodelling and spotty calcification (early macrocalcific deposits). Multiple studies have demonstrated these features in culprit plaque post-myocardial infarction [38–41]. Moreover, Motoyama recently demonstrated that patients with these unstable CT plaques are at an elevated risk of subsequent myocardial infarction [42].
MR coronary angiography is a developing technology that remains inferior to CCTA. However, MR can also identify unstable coronary plaque characteristics, with Tl weighted imaging holding particular promise. This approach makes use of the high Tl signal associated with methaemoglobin, a key constituent of fresh thrombus. On this basis, TI-weighted imaging can identify both intraplaque haemorrhage and intraluminal thrombosis [43,44] with increased signal localising to culprit carotid and coronary plaques. Moreover, increased carotid and coronary signal identifies patients at increased risk of subsequent stroke and MI, respectively [45–47].
Several common themes appear to be emerging across these vulnerable plaque studies. The first is that in prospective studies, so-called vulnerable plaques in fact only rarely go on themselves to cause clinical events. The majority will likely heal without consequence whilst others may rupture sub-clinically without prompting myocardial infarction. Only a tiny minority will go on to cause a clinical event. In these circumstances, invasive imaging and therapies aimed at individual plaques do not make sense. However, the CT and MRI studies discussed above suggest that unstable plaque detection still holds promise in improving risk stratification at the patient level and in directing the use of systemic therapies. Unstable plaques rarely exist in isolation and their identification can highlight patients with an active disease process and an on-going propensity to develop plaques with an unstable phenotypes [48] Whilst most of the individual plaques will heal, with on-going formation there is an increased probability of one such unstable plaque eventually rupturing and causing an MI. Further work is required to demonstrate the clinical utility of advanced coronary plaque characterisation. In particular, it remains unclear whether unstable plaque identification can improve patient risk stratification over and above more simple assessments of plaque burden.
1.5. Imaging disease activity in atherosclerosis
The emergence of hybrid PET/CT scanners with advanced imaging capability has for the first time allowed assessment of atherosclerotic disease activity. In principle, the activity of any disease process can be assessed, however, in practice this is dependent on the availability of PET tracers. To date, vascular PET imaging has focused upon 18F-FDG: a marker of glucose utilisation and a non-specific marker of inflammation that is limited to imaging of the carotid arteries, femoral arteries and aorta. However, novel tracers are quickly emerging specific to multiple different pathological processes. Moreover, technological developments aimed at improving the (currently limited) spatial resolution of PET now allow quantification of certain tracers in the coronary arteries. Below, we discuss the current status of atherosclerotic plaque imaging using PET, SPECT, and a variety of different tracers.
1.6. Imaging inflammation with 18F-flurodeoxyglucose (FDG)
As described above, atherosclerosis is a chronic inflammatory disease characterised by lipid deposition and macrophage infiltration. The recent CANTOS RCT of 10,061 patients with previous MI and elevated CRP levels demonstrated, for the first time, that an anti-inflammatory agent (150 mg Canikinumab a monoclonal antibody targeting Interleukin lβ) can reduce hard clinical end points when compared to placebo. This trial therefore confirmed the close relationship between inflammation and cardiovascular events independent of lipid levels [49], and established inflammation as a key target for therapeutic and novel molecular imaging approaches.
18F-FDG PET has in fact been used to image inflammation for over a decade, based upon the high glucose consumption of vascular macrophages [50].18F-FDG competes physiologically with glucose to enter the cell, where it becomes trapped and unable to undergo further metabolism. FDG, therefore, accumulates in metabolically active cells at a rate proportional to their glycolytic activity [51].
The association between 18F-FDG activity and unstable atherosclerotic plaque was first demonstrated by Rudd et al., who observed increased uptake in symptomatic carotid plaques ipsilateral to a recent stroke compared to the asymptomatic contralateral plaque. Autoradiography of excised plaques confirmed greatest uptake in macrophage rich plaques [52]. Here was the first human evidence that active inflammation assessed by FDG PET could accurately identify clinical plaque rupture. Other studies have confirmed the close association between FDG uptake and macrophage burden on histology [53] as well as upregulated gene expression of CD68 (a macrophage specific marker) [54]. Association with other markers of plaque vulnerability have also been established, including circulating MMP-I levels [55], whilst an elegant mechanistic study demonstrated that hypoxia potentiates 18F-FDG uptake by macrophages [56].
Recent studies have explored vascular 18F-FDG activity in systemic inflammatory conditions, providing mechanism for the increased cardiovascular event rates observed in these patients. In particular patients with psoriasis have demonstrated increased vascular 18F-FDG uptake beyond that explained by their cardiovascular risk factors [57], with similar results described for subjects with both HIV and rheumatoid arthritis [58,59]. Moreover, Tahara et al. reported increased carotid 18F-FDG uptake in patients with metabolic syndrome [60], whilst Kim described increased activity in diabetics and those with impaired glucose tolerance independent of blood glucose [61].
Tawakol et al. recently elucidated the link between emotional stress, increased vascular inflammation and cardiovascular events. This elegant study embraced FDG’s lack of specificity, indeed, it harnessed it to investigate the relationship between processes occurring in disparate organ system. The authors used resting 18FFDG uptake in the amygdala as a marker of emotional stress and demonstrated an association with both FDG uptake in the bone marrow (as a marker of hemopoietic activiation) and the vasculature (as a marker of vascular inflammation). They also demonstrated that increased 18F-FDG uptake in these tissues identified patients with an increased risk of cardiovascular events, proposing that stress caused macrophage mobilisation from the bone marrow, increased vascular inflammation and clinical events [62]. Other retrospective studies have also linked increased vascular 18F-FDG activity to subsequent cardiovascular events. In a study of over 500 individuals devoid of a previous history of cardiovascular disease, uptake in the ascending aorta strongly predicted the development of cardiovascular disease independent of conventional risk factors [63]. However, prospective outcome studies in non-biased patient populations are now required to confirm the prognostic capability of 18F-FDG PET.
18F-FDG PET is emerging as a novel method for assessing the anti-inflammatory effects of both established and novel atherosclerotic medication. The clinical efficacy of statins is well established [64], it is, therefore, reassuring that statins consistently reduce the arterial FDG signal in a dose dependent manner [64–66]. Similarly, pioglitazone, which is associated with lower rates of adverse cardiovascular outcomes in diabetics [67], has been shown to attenuate vascular 18F-FDG uptake, further supporting links between diabetes and vascular inflammation independent of blood glucose levels [68,69].
By comparison, Dalcetrapib, a CETP inhibitor which increases high density lipoprotein (HDL) levels, did not have any effect of vascular 18F-FDG activity; an effect that was mirrored by a similar inability to reduce adverse cardiovascular events [70,71]. Similarly, the lipoprotein-associated phospholipase A2 (implicated in atherosclerosis progression) inhibitor Rilapladib failed to reduce both 18F-FDG uptake [72] and cardiovascular events in the STABILITY trial of 15,000 patients [73]. Finally, the P38 mitogen activated protein kinase (MAP) failed to have a positive impact on either the arterial 18F-FDG activity [74,75] or cardiovascular events [76].
To summarise, large vessel FDG PET is a marker of vascular inflammation, whose uptake acts a surrogate of plaque macrophage burden and is associated with conventional cardiovascular risk factors and systemic inflammatory conditions. Importantly, FDG uptake can be attenuated with medication, leading to its adoption as an endpoint in trials assessing the anti-inflammatory effects of novel therapies. With the development of increasingly expensive atherosclerosis drugs, FDG may also prove useful for identifying patients most likely to gain clinical benefit, although prospective outcome trials are first required to assess whether it provides independent prognostic information.
Whilst FDG’s lack of specificity has allowed investigators to assess the links between vascular inflammation and both the brain and bone marrow, it has limited the application of 18F-FDG PET to the coronary arteries. Indeed, physiological uptake of 18F-FDG by the myocardium means that coronary interpretation is challenging, even despite meticulous dietary preparations [77]. The desire to assess disease activity in the coronary arteries, the vascular bed responsible for the majority of clinical events, has prompted investigation of multiple novel tracers discussed below [78] (Fig. 2).
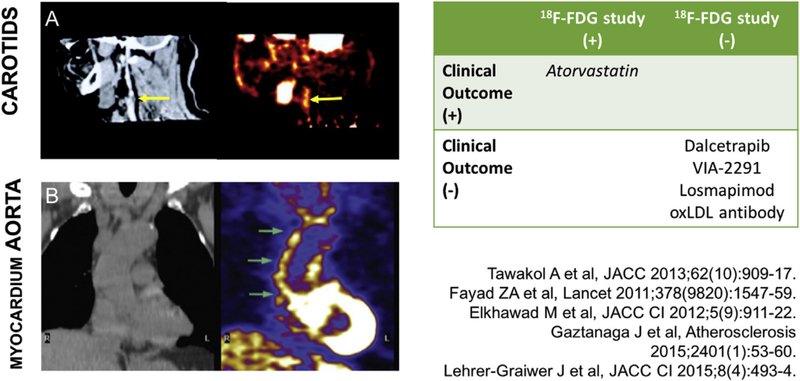
Fig. 2.
FDG performance in early phase clinical trials.
(A) CT on the left with corresponding PET image on the right demonstrating FDG uptake in culprit carotid plaque (B) How FDG PET CT imaging can be applied to the aorta and myocardium.
1.7. Other PET tracers targeting inflammation
1.7.1. 68 Gallium-dotatate
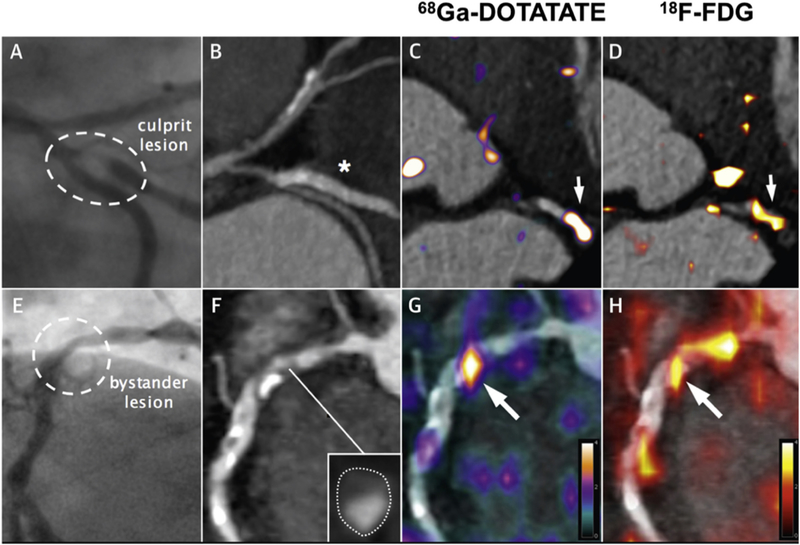
Gallium 68 labelled DOTATATE targets the somatostatin receptor subtype 2 (SSTR2) found on the surface of macrophages [79]. Pre-clinical studies have confirmed the superiority of 68GaDOTATATE over FDG in terms of macrophage specificity in atherosclerotic inflammation [80] [81]. Early retrospective clinical studies in cancer patients supported the pre-clinical findings and interestingly found some discordance between FDG and DOTATATE activity provoking further research [81–83]. Recently, a prospective clinical study pitched DOTATATE versus FDG in the setting of established aortic, carotid and coronary atherosclerosis. DOTATATE demonstrated increased uptake in culprit coronary and carotid arteries and outperformed FDG in discriminating between unstable and stable coronary plaque as defined by CT. Histological validation confirmed that DOTATATE uptake occurred in CD68-positive, macrophage-rich carotid plaques. Specific binding to proinflammatory Ml macrophages was demonstrated through exclusive expression of the SSTR2 receptor [78]. These findings poise DOTATATE PET as an exciting alternative to FDG, providing more specific information about Ml macrophages in atherosclerotic plaque that can be translated in the coronary arteries. Further studies are on going in this area (Fig. 3).
Fig. 3.
Detection of coronary atherosclerotic inflammation by 68Ga DOTATATE.
The panels in the top row show the culprit lesion in the circumflex artery with corresponding angiogram (A), CT (B), and PET CT with 68GA DOTATATE (C) and FDG (D). The bottom row of panels illustrates a bystander lesion in the proximal right coronary artery which is appears of moderate stenosis on angiography (A), appears non-calcified and positively remodeled on CT angiography (F) and has a clear correlation of DOTATATE (G) and FDG (H) uptake [78].
1.7.2. 18F-fluoroch01ine (18F-FCH)
Like many inflammatory tracers, 18F-FCH was first developed for use in oncology. Via specific transport mechanisms, choline is taken up into the cell, phosphorylated and metabolized to phosphatidylcholine and eventually incorporated into the cell membrane. Increased choline uptake has been shown in tumor cells and activated macrophages [84]. Its potential for human atherosclerotic imaging was confirmed by ex vivo and in vivo mouse model studies of atherosclerosis where uptake was significantly higher in atherosclerotic versus healthy aorta [85–87]. A strong association between large vessel uptake and atherosclerotic changes in the arterial wall has been demonstrated in a cohort of prostate cancer patients with an apparent inverse relationship with calcification [88]. This inverse relationship was confirmed in a larger study of 93 prostate cancer patients where only 6% of tracer uptake colocalised with macrocalcification calcification and in all calcified lesions only 1% had tracer uptake [89]. Further prospective research is now required.
1.7.3. 11C-PK11195
11C-PK11195 is a ligand of the 18-kDa translocator protein (TSPO), which is highly expressed in human macrophages [90]. It has been most widely used for neuroinflammatory imaging owing to high microglial and low neuronal uptake and was first explored in the cardiovascular system in patients with large vessel vasculitis [91]. In atherosclerosis, 11C-PK11195 demonstrates increased uptake in culprit carotid plaques following stroke, and a close association with inflammatory cell burden in carotid endarterectomy specimens [92]. However, significant challenges with data interpretation remain due to genotypic differences that govern the metabolism of this tracer and have a profound effect on tracer activity. Moreover, the short half-life (20 min) of the tracer necessitates an on-site cyclotron, limiting its availability and use. Other next generation TSPO tracers are in development that seek to use 18F as a more convenient radiolabel and to avoid the genetic influences on metabolism.
1.8. Other novel atherosclerotic tracers
1.8.1. 18F-fluoromisonidazole (18F-FMISO)-hypoxia
FMISO targets hypoxia, an important process linked to atherosclerotic progression. As a plaque grows its core becomes progressively hypoxic promoting inflammatory cell infiltration and further oxygen consumption, thereby further worsening hypoxic conditions. Micro vessel formation ensues and is the necessary substrate for intraplaque haemorrhage; a key marker of plaque vulnerability. In a rabbit model of aortic atherosclerosis, 18F-FMISO was compared with 18F-FDG. In vivo and ex vivo PET imaging demonstrated strong accumulation of 18F-FMISO accumulation in areas of aortic atheroma, co-localising to regions of hypoxia detected on immunohistochemistry with pimonidazole [93]
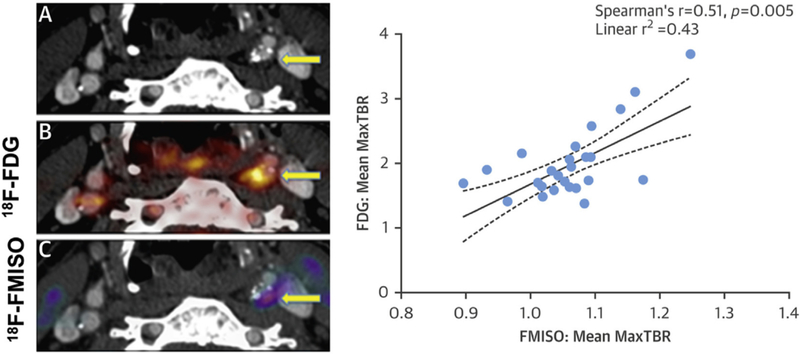
A recent study by Joshi et al. (Fig. 4) investigated FMISO PET in patients with recent TIA or stroke (n = 16). FMISO uptake was slightly higher in symptomatic plaques than contralateral lesions (TBR 1.11 ± 0.07 vs. 1.05 ± 0.06; p < 0.05) and demonstrated a correlation with FDG activity, suggesting once again that hypoxia contributes to the inflammatory response and FDG uptake [78,94]. This tracer appears to hold future promise for atherosclerotic plaque imaging promoting greater understanding of the relationship between hypoxia and inflammation.
Fig. 4.
FMISO quantifies hypoxia in carotid plaques.
(A) Plaque in left internal carotid on contrast enhanced cr. Co-registered PET images with FDG (B) and FMISO (C) demonstrate a strong correlation of tracer uptake.
1.8.2. 18F-fluciclatide-angiogenesis
18F-fluciclatide is a novel αvβ3 selective radiotracer and has been investigated as a marker of angiogenesis following MI with encouraging results. In a study of 37 patients, uptake was significantly increased at sites of recent MI thereby acting as a biomarker of cardiac repair and importantly predicting regions most likely to recover contractile function [95]. Data regarding arterial uptake of this tracer has not yet been published.
1.8.3. Annexin V (SPECT tracer) – apoptosis
99mTc labelled Annexin V has a high affinity for phosphatidylserine which is predominantly found on the plasma membrane of apoptotic cells. It has primarily been used in oncology but has also been studied in heart failure, cardiac transplant recipients and atherosclerosis [96]. In a small study of those undergoing carotid endarterectomy, uptake was shown to strongly correlate with high risk plaque characteristics (macrophage infiltration and intraplaque haemorrhage) suggesting a potential role for unstable plaque imaging [97].
1.8.4. 18F-sodium fluoride-microcalcification
The PET tracer 18F-sodium fluoride ( 18F-NaF) has been used for many decades as a maker of increased bone metabolism. It works via the exchange of 18F-NaF with hydroxyl groups on hydroxyapatite: a key structural component of both bone and vascular calcification [98]. More recently, 18F-fluoride has been used to investigate developing microcalcification in the vasculature, emerging as a promising new PET radiotracer in the field of cardiovascular medicine.
The intimal calcification observed in atherosclerosis is thought to occur as a healing response to intense necrotic inflammation within the plaque. At the outset, small crystals of hydroxyapatite begin to coalesce into organised areas of microcalcification. The natural continuation of this process is the formation of dense sheets of macroscopic calcification that stabilize the plaque, effectively walling off the necrotic core from the lumen. Whilst the end-stages of macroscopic calcification impart stability, the early stages of microcalcification are instead consistently associated with culprit and unstable plaque phenotypes and an increased propensity to rupture. In part, this may reflect the ongoing plaque inflammation that is yet to heal and in part the increased mechanical stress caused by microcalcific deposits in the fibrous cap that weaken its tensile strength and predispose it to rupture [13].
Whilst CT is able to detect macroscopic calcification, including early spotty calcification it is unable to resolve microcalcification. However, this has now become possible with 18F-fluoride PET. Irkle et al. demonstrated that 18F-fluoride binds preferentially to regions of developing microcalcification in carotid atheroma, with little or no binding to non-calcific tissue types [99]. Certainly, 18F-fluoride PET provides different information to CT. In carotid atheroma, coronary atheroma, aortic atheroma, aortic stenosis and AAA, 18F-fluoride has consistently demonstrated a different pattern of uptake to the macroscopic calcium observed on CT [98,100–102]. In aortic stenosis, areas of increased 18F-fluoride activity predict where novel deposits of macroscopic calcium will deposit, providing excellent prediction of progression in CT calcium scores of the valve [103,104]. Similar results have also been described in atheroma, with a recent prospective study demonstrating the ability of 18F-fluoride PET to predict progression in the CT calcium score over 1 year in 34 patients [105]. This is potentially important as fast calcium score progression is known to offer powerful prognostic information of incremental value to single baseline measurements [106].
Dweck et al. first demonstrated that coronary 18F-fluoride uptake was associated with cardiovascular risk, demonstrating significant associations between coronary arterial NaF uptake and prior coronary events, angina status and Framingham risk scores (p =.016, p = 0.023 and p = 0.011, respectively) [96] This was supported by a study of 89 healthy adults, in whom 10-year risk scores for the development of cardiovascular disease were 2.4 times higher amongst adults in the highest quartile of coronary artery 18F-NaF uptake compared with those in the lowest (8.0 vs. 3.3%, p < 0.001) [107].
At the plaque level, 18F-fluoride localises to plaques with multiple unstable features, including inflammation, positive remodelling, necrotic core and cell death as assessed by histology, VH-IVUS, OCT and CT imaging [15,108]. Moreover, increased 18F-fluoride uptake has been demonstrated in both culprit coronary and carotid plaques after acute ischemic events. In a study of 40 patients with recent MI, increased uptake localised to the culprit plaque in 93% of patients. Similar findings were observed in a small MR/PET study [109], whilst in the carotid arteries increased 18F-fluoride uptake was observed in the culprit carotid plaque of patients with recent stroke/TIA) versus controls [110]. This finding was confirmed in a case control study by Vesey et al. (n = 26), who also demonstrated increased 18F-fluoride uptake in ipsilateral versus contralateral carotid plaques and that once again was associated with unstable plaque features, plaque burden and cardiovascular risk factors [111 ] (Fig. 5).
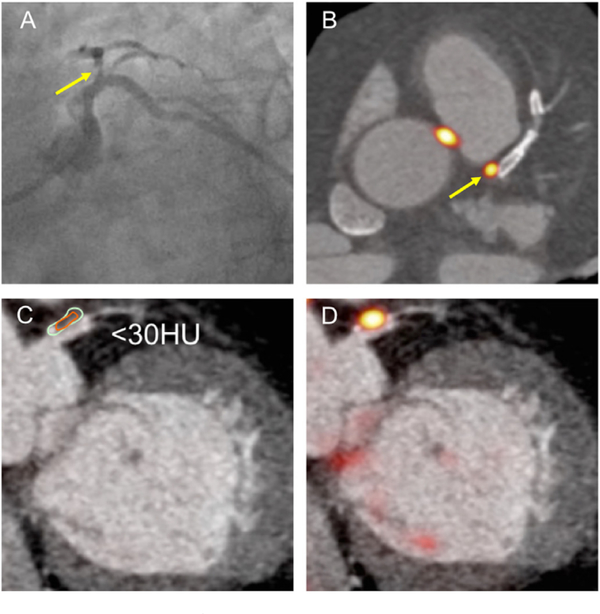
Fig. 5.
CT characteristics of 18F-NaF uptake.
Coronary angiography in LAO caudal view showing severe proximal LAD lesion (A, arrowed). Note co-localised PET uptake in (B, arrowed). CT allows assessment of plaque density in (C) (<30 Hounsfield units). Hybrid PET CT in (D) shows co-localisation of 18-fluoride uptake.
Together, this data suggests that 18F-fluoride allows detection of both unstable and culprit plaque with increased coronary uptake being observed in patients with increased disease activity and at increased cardiovascular risk. Whether it provides incremental prospective prediction of cardiovascular events compared to conventional risk scores and CT imaging will be investigated in the prospective, multicenter PREFFIR trial (NCT02278211 ). Unlike FDG, drug trials evaluating the effects of cardiovascular medicines on 18F-fluoride uptake are lacking, although the on going SALTIRE 2 study is assessing the effects of bisphosphonates and Denosumab on vascular calcification activity in aortic stenosis (NCT 02132026). It should provide proof of concept as to whether the vascular 18F-fluoride signal is modifiable.
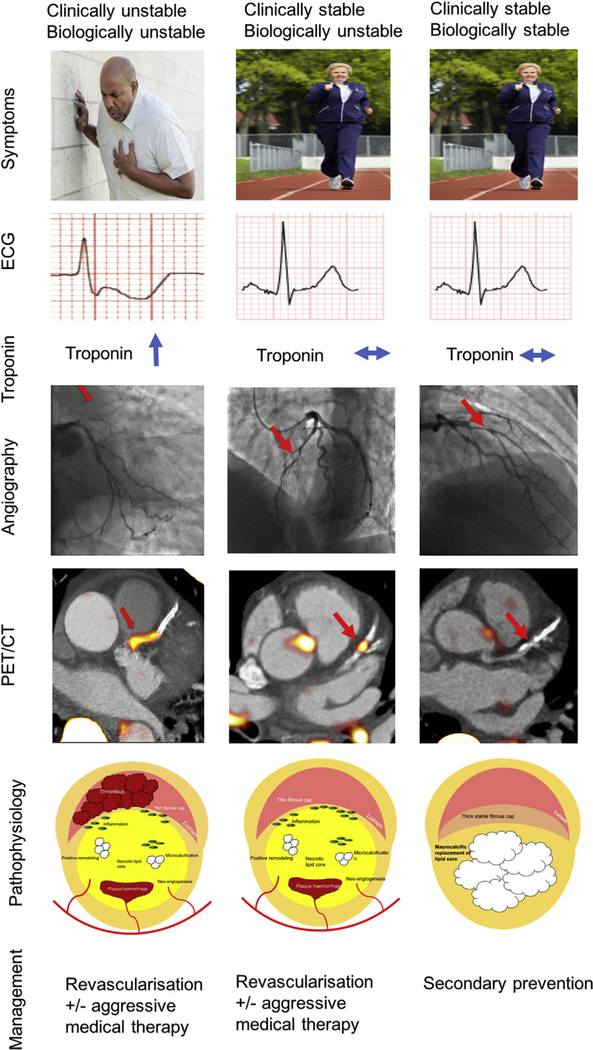
1.9. A novel definition of stable and unstable coronary artery disease
Patients with coronary atherosclerosis have traditionally been divided into two groups depending on their symptom pattern. Patients with stable coronary artery disease are classically either asymptomatic or experience predictable exertional angina [112,113]. The assumption has been that, like their symptoms, these patients have stable inactive atherosclerosis at low risk of rupture. By contrast, patients with unstable coronary artery disease describe rapid and unpredictable symptom escalation that reflects a highly active and unstable underlying disease process. Modern imaging technology now allows us to examine coronary plaque characteristics and disease activity directly. These indicate that some apparently stable patients in fact also have an active underlying disease process characterised by the dynamic formation and resolution of multiple unstable coronary plaques. Early data suggest that these clinically stable but biologically active patients may be at increased cardiovascular risk and therefore may benefit from aggressive medical therapy and potentially even revascularisation [107]. Equally, patients with disease that is both clinically and biologically stable could have therapy tailored back potentially avoiding expensive and intrusive revascularisation strategies. Further studies are required to investigate this hypothesis and to investigate whether it is cost-effective before adoption in to clinical practice (Fig. 6).
Fig. 6.
A potential novel definition of stable and unstable coronary artery disease.
Note: this proposal would require extensive research and investigation before it could be recommended in routine clinical practice. Pending this current guideline, recommendations for revascularsation and the prescription of optimal medical therapy should be followed.
2. Conclusion
Modern non-invasive imaging using CT, MR and PET now allows identification of unstable characteristics and the direct measurement of disease activity in the coronary arteries and large vessels. This advance appears set to improve our pathological understanding of atherosclerosis and has the potential to redefine our understanding of what stable and unstable atherosclerosis truly represents. Indeed, ultimately, we may be able to track disease activity and the transition between stable and unstable disease states with time and in response to therapy. Further work is now required to validate these early findings and to investigate whether these novel imaging approaches provide incremental prognostic information.
Acknowledgments
The authors would like to thank Dr Alastair Moss and Dr Mhairi Doris for their assistance with developing figures 2, 5 and 6.
Financial support
JPMA is supported by BHF Clinical Research Training Fellowship no. FS/17/51/33096. ZAF by NIH NHLBI POI HL131478; NHLBI ROI HL071021; NHLBI ROI HL128056; ROIHL135878; NBIB ROI EB009638; and AHA 14SFRN20780005. MRD is supported by the Sir Jules Thorn Biomedical Research Award 2015 (15/JTA) and by the British Heart Foundation (FS/14/78/31020).
Footnotes
Conflicts of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
References
- [1].Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. , Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015, J. Am. Coll. Cardiol 70 (1) (2017. July 4) 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Falk E, Pathogenesis of atherosclerosis, J. Am. Coll. Cardiol 47 (8 Suppl) (2006. April 18) C7–C12. [DOI] [PubMed] [Google Scholar]
- [3].Schaar JA, Muller IE, Falk E, Virmani R, Fuster V, Serruys PW, et al. , Terminology for High-risk and Vulnerable Coronary Artery Plaques Report of a Meeting on the Vulnerable Plaque, June 17 and 18, 2003, Santorini, Greece, 2004, pp. 1077–1082. [DOI] [PubMed] [Google Scholar]
- [4].Falk E, Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi, Heart 50 (2) (1983. August 1) 127–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jones CB, Sane DC, Herrington DM, Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome, Cardiovasc. Res 59 (4) (2003. October 1) 812–823. [DOI] [PubMed] [Google Scholar]
- [6].Michel J-B, Virmani R, Arbustini E, Pasterkamp G, Intraplaque haemorrhages as the trigger of plaque vulnerability, Eur. Heart J 32 (16) (2011. August) 1977–1985, 1985a–1985b–1985c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Parma L, Baganha F, Quax PHA, de Vries MR, Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis, Eur. J. Pharmacol (2017. April 2) [DOI] [PubMed] [Google Scholar]
- [8].Haasdijk RA, Dekker Den WK, Cheng C, Tempel D, Szulcek R, Bos FL, et al. , THSDI preserves vascular integrity and protects against intraplaque haemorrhaging in ApoE−/− mice, Cardiovasc. Res 110 (1) (2016. May 1) 129–139. [DOI] [PubMed] [Google Scholar]
- [9].Ordonez A-A, DeMarco VP, Klunk MH, Pokkali S, Jain SK, Imaging chronic tuberculous lesions using sodium [918)F] fluoride positron emission tomography in mice, Mol Imaging Biol 17 (5) (2015. October) 609–614, 8 ed. Springer US. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen W, Dilsizian V, Targeted PET/CI’ imaging of vulnerable atherosclerotic plaques: microcalcification with sodium fluoride and inflammation with fluorodeoxyglucose, Curr Cardiol Rep. Current Science Inc 15 (6) (2013. June) 364. [DOI] [PubMed] [Google Scholar]
- [11].New SEP, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, et al. , Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques, Circ Res American Heart Association, Inc 113 (1) (2013. June 21) 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Beckman JA, Ganz J, Creager MA, Ganz P, Kinlay S, Relationship of clinical presentation and calcification of culprit coronary artery stenoses, Arterioscler. Thromb. Vasc. Biol 21 (10) (2001. October) 1618–1622. [DOI] [PubMed] [Google Scholar]
- [13].Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, et al. , A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps, Proc Natl Acad Sci USA. National Acad Sciences 103 (40) (2006. October 3) 14678–14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vengrenyuk Y, Cardoso L, Weinbaum S, Micro-CT based analysis of a new paradigm for vulnerable plaque rupture: cellular microcalcifications in fibrous caps, Mol. cell. BioMech 5 (1) (2008. March) 37–47. [PubMed] [Google Scholar]
- [15].Joshi N.v., Vesey AT, Williams M.c., Shah ASV, Calvert PA, Craighead FHM, et al. , 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial, Lancet 383 (9918) (2014. February 22) 705–713. [DOI] [PubMed] [Google Scholar]
- [16].Stone NJ, Robinson JC, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. , ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, J. Am. coll. Cardiol 63 (2013) 2889–2934, 2014. [DOI] [PubMed] [Google Scholar]
- [17].Detrano A, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. , Coronary calcium as a predictor of coronary events in four racial or ethnic groups, N EnglJ Med. Massachusetts Medical Society 358 (13) (2008. March 27) 1336–1345. [DOI] [PubMed] [Google Scholar]
- [18].Rogers IS, Nasir K, Figueroa AL, Cury RC, Hoffmann U, Vermylen DA, et al. , Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina, JACC Cardiovasc Imaging 3 (4) (2010. April) 388–397. [DOI] [PubMed] [Google Scholar]
- [19].Finn AV, Kolodgie FD, Virmani R, Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. arteriosclerosis, thrombosis, and vascular biology, American Heart Association, Inc 30 (2) (2010. February) 177–181. [DOI] [PubMed] [Google Scholar]
- [20].Libby P, Mechanisms of acute coronary syndromes, N Engl J Med. Massachusetts Medical Society 369 (9) (2013. August 29) 883–884. [DOI] [PubMed] [Google Scholar]
- [21].Ylä-Herttuala S, Bentzon JF, Daemen M, Falk E, Garcia-Garcia HM, Herrmann J, et al. , Stabilization of atherosclerotic plaques: an update, Eur. Heart J 34 (42) (2013. November) 3251–3258. [DOI] [PubMed] [Google Scholar]
- [22].Nair A, Margolis MP, Kuban BD, Vince DC, Automated coronary plaque characterisation with intravascular ultrasound backscatter: ex vivo validation, Eurolntervention 3 (1) (2007. May) 113–120. [PubMed] [Google Scholar]
- [23].Diethrich EB, Pauliina Margolis M, Reid DB, Burke A, Ramaiah V, Rodriguez-Lopez JA, et al. , Virtual histology intravascular ultrasound assessment of carotid artery disease: the Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study, J Endovasc Ther 14 (5) (2007. October) 676–686. SAGE Publications-sage CA: Los Angeles, CA. [DOI] [PubMed] [Google Scholar]
- [24].Brugaletta S, Cola C, Martin-Yuste V, Vilahur G, Oriol J, Padro T, et al. , Qualitative and quantitative accuracy of ultrasound-based virtual histology for detection of necrotic core in human coronary arteries, Int J Cardiovasc Imaging. Springer Netherlands 30 (3) (2014. March) 469–476. [DOI] [PubMed] [Google Scholar]
- [25].Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. , A prospective natural-history study of coronary atherosclerosis, N Engl J Med. Massachusetts Medical Society 364 (3) (2011. January 20) 226–235. [DOI] [PubMed] [Google Scholar]
- [26].Calvert PA, Obaid DR, O’Sullivan M, Shapiro LM, McNab D, Densem CC, et al. , Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study, JACC Cardiovasc Imaging 4 (8) (2011. August) 894–901. [DOI] [PubMed] [Google Scholar]
- [27].Cheng JM, Garcia-Garcia HM, de Boer SPM, Kardys I, Heo JH, Akkerhuis KM, et al. , In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: resuits of the ATHEROREMO-IVUS study, Eur. Heart J 35 (10) (2014. March) 639–647. [DOI] [PubMed] [Google Scholar]
- [28].Sanidas EA, Maehara A, Mintz GS, Kashiyama T, Guo J, Pu J, et al. , Angioscopic and virtual histology intravascular ultrasound characteristics of Culprit lesion morphology underlying coronary artery thrombosis, Am. J. Cardiol 107 (9) (2011. May 1) 1285–1290. [DOI] [PubMed] [Google Scholar]
- [29].Schroeder AP, Falk E, Vulnerable and dangerous coronary plaques, Atherosclerosis 118 (Suppl) (1995. December). S141–9. [PubMed] [Google Scholar]
- [30].Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, et al. , In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography, J. Am. Coll. Cardiol 62 (19) (2013. November 5) 1748–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kubo T, Matsuo Y, Ino Y, Tanimoto T, Ishibashi K, Komukai K, et al. , Optical coherence tomography analysis of attenuated plaques detected by intravascular ultrasound in patients with acute coronary syndromes, Cardiol Res Pract. Hindawi 2011 (2) (2011) 687515–687517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Taruya A, Tanaka A, Nishiguchi T, Matsuo Y, Ozaki Y, Kashiwagi M, et al. , Vasa vasorum restructuring in human atherosclerotic plaque vulnerability: a clinical optical coherence tomography study, J. Am. Coll. Cardiol 65 (23) (2015. June 16) 2469–2477. [DOI] [PubMed] [Google Scholar]
- [33].Hoang V, Grounds J, Pham D, Virani S, Hamzeh l., Qureshi AM, et al. , The role of intracoronary plaque imaging with intravascular ultrasound, optical coherence tomography, and near-infrared spectroscopy in patients with coronary artery disease, Curr Atheroscler Rep. Springer US 18 (9) (2016. September) 57. [DOI] [PubMed] [Google Scholar]
- [34].Nakano M, Yahagi K, Yamamoto H, Taniwaki M, Otsuka F, Ladich ER, et al. , Additive value of integrated backscatter IVUS for detection of vulnerable plaque by optical frequency domain imaging: an ex vivo autopsy study of human coronary arteries, JACC Cardiovasc Imaging 9 (2) (2016. February) 163–172. [DOI] [PubMed] [Google Scholar]
- [35].Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R, Decramer I, Van Hoe LR, Wijns W, et al. , Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis, Radiology 244 (2) (2007. August) 419–428. [DOI] [PubMed] [Google Scholar]
- [36].SCOT-HEART investigators, CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial, Lancet 385 (9985) (2015. June 13) 2383–2391. Elsevier. [DOI] [PubMed] [Google Scholar]
- [37].Williams MC, Hunter A, Shah ASV, Assi V, Lewis S, Smith J, et al. , Use of coronary computed tomographic angiography to guide management of patients with coronary disease, J. Am. Coll. Cardiol 67 (15) (2016. April 19) 1759–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. , Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome, J. Am. Coll. Cardiol 54 (1 ) (2009. June 30) 49–57. [DOI] [PubMed] [Google Scholar]
- [39].Maurovich-Horvat P, Hoffmann U, Vorpahl M, Nakano M, Virmani R, Alkadhi H, The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc Imaging 3 (4) (2010. April) 440–444. [DOI] [PubMed] [Google Scholar]
- [40].Otsuka K, Fukuda S, Tanaka A, Nakanishi K, Taguchi H, Yoshikawa J, et al. , Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome, JACC Cardiovasc Imaging 6 (4) (2013. April) 448–457. [DOI] [PubMed] [Google Scholar]
- [41].Kitagawa T, Yamamoto H, Horiguchi J, Ohhashi N, Tadehara F, Shokawa T, et al. , Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography, JACC Cardiovasc Imaging 2 (2) (2009. February) 153–160. [DOI] [PubMed] [Google Scholar]
- [42].Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, et al. , Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up, J. Am. Coll. Cardiol 66 (4) (2015. July 28) 337–346. [DOI] [PubMed] [Google Scholar]
- [43].Matsumoto K, Ehara S, Hasegawa T, Sakaguchi M, Otsuka K, Yoshikawa J, et al. , Localization of coronary high-intensity signals on T I-weighted MR imaging: relation to plaque morphology and clinical severity of angina pectoris, JACC Cardiovasc Imaging 8 (10) (2015. October) 1143–1152. [DOI] [PubMed] [Google Scholar]
- [44].Xie Y, Kim Y-J, Pang J, Kim J-S, Yang Q, Wei J, et al. , Coronary atherosclerosis T I-weighed characterization with integrated anatomical reference: comparison with high-risk plaque features detected by invasive coronary imaging, JACC Cardiovasc Imaging 10 (6) (2017. June) 637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Larose E, Yeghiazarians Y, Libby P, Yucel EK, Aikawa M, Kacher DF, et al. , Characterization of human atherosclerotic plaques by intravascular magnetic resonance imaging, Circulation. American Heart Association, Inc 112 (15) (2005) 2324–2331. [DOI] [PubMed] [Google Scholar]
- [46].Noguchi T, Kawasaki T, Tanaka A, Yasuda S, Goto Y, Ishihara M, et al. , High-intensity signals in coronary plaques on noncontrast Tl-weighted magnetic resonance imaging as a novel determinant of coronary events, J. Am. Coll. Cardiol 63 (10) (2014. March 18) 989–999. [DOI] [PubMed] [Google Scholar]
- [47].Noguchi T, Yamada N, Higashi M, Goto Y, Naito H, High-intensity signals in carotid plaques on Tl-weighted magnetic resonance imaging predict coronary events in patients with coronary artery disease, J. Am. Coll. Cardiol 58 (4) (2011. July 19) 416–422. [DOI] [PubMed] [Google Scholar]
- [48].Dweck MR, Aikawa E, Newby DE, Tarkin JM, Rudd JHF, Narula J, et al. , Noninvasive molecular imaging of disease activity in atherosclerosis, Circ Res. American Heart Association, Inc 119 (2) (2016. July 8) 330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ridker PM, Everett BM, Thuren T, MacFadyen JC, Chang WH, Ballantyne C, et al. , Antiinflammatory therapy with canakinumab for atherosclerotic disease, N Engl J Med. Massachusetts Medical Society (2017. August 27). NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- [50].Tarkin JM, Joshi FR, Rudd JHF, PET imaging of inflammation in atherosclerosis, Nat. Rev. Cardiol 11 (8) (2014. August) 443–457. [DOI] [PubMed] [Google Scholar]
- [51].Rudd JHF, Hyafil F, Fayad ZA, Inflammation imaging in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology, American Heart Association, Inc 29 (7) (2009. July 1) 1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rudd JHF, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. , Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography, Circulation 105 (23) (2002. June 11) 2708–2711. [DOI] [PubMed] [Google Scholar]
- [53].Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. , In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients, J. Am. Coll. Cardiol 48 (9) (2006. November 7) 1818–1824. [DOI] [PubMed] [Google Scholar]
- [54].Graebe M, Pedersen SF, Borgwardt L, Højgaard L Sillesen H, Kjaer A, Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET), Eur. J. Vasc. Endovasc. surg 37 (6) (2009. June) 714–721. [DOI] [PubMed] [Google Scholar]
- [55].wu Y-W, Kao H-L, Chen M-F, Lee B-C, Tseng W-YI, Jeng J-S, et al. , Characterization of plaques using 18F-FDG PET/CT in patients with carotid atherosclerosis and correlation with matrix metalloproteinase-I, J. Nucl. Med 48 (2) (2007. February) 227–233. [PubMed] [Google Scholar]
- [56].Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, et al. , Hypoxia but not inflammation augments glucose uptake in human macrophages: implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-D-glucose positron emission tomography, J. Am. Coll. Cardiol 58 (6) (2011. August 2) 603–614. [DOI] [PubMed] [Google Scholar]
- [57].Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, et al. , Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective Observational study. Arteriosclerosis, thrombosis, and vascular biology, American Heart Association, Inc 35 (12) (2015. December) 2667–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tawakol A, Lo J, Zanni MV, Marmarelis E, lhenachor EJ, MacNabb M, et al. , Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients, J. Acquir. Immune Defic. Syndr 66 (2) (2014. June 1) 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rose S, Sheth NH, Baker JF, Ogdie A, Raper A, Saboury B, et al. , A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study, Am J Cardiovasc Dis. e-Century Publishing Corporation 3 (4) (2013) 273–278. [PMC free article] [PubMed] [Google Scholar]
- [60].Tahara N, Kai H, Yamagishi S.-l., Mizoguchi M, Nakaura H, Ishibashi M, et al. , Vascular inflammation evaluated by [18F]-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome, J. Am. Coll. Cardiol 49 (14) (2007. April 10) 1533–1539. [DOI] [PubMed] [Google Scholar]
- [61].Kim TN, Kim S, Yang s.J., Yoo HJ, seo JA, Kim SG, et al. , Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: analysis with 18F-fluorodeoxyglucose positron emission tomography, Circ Cardiovasc Imaging. American Heart Association, Inc 3 (2) (2010. March) 142–148. [DOI] [PubMed] [Google Scholar]
- [62].Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, et al. , Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study, Lancet 389 (10071) (2017. February 25) 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, et al. , Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events, JACC Cardiovasc Imaging 6 (12) (2013. December) 1250–1259. [DOI] [PubMed] [Google Scholar]
- [64].Naci H, Brugts JJ, Fleurence R, Tsoi B, Toor H, Ades AE, Comparative benefits of statins in the primary and secondary prevention of major coronary events and all-cause mortality: a network meta-analysis of placebo-controlled and active-comparator trials, Eur J Prev Cardiol 20 (4) (2013. August) 641–657. [DOI] [PubMed] [Google Scholar]
- [65].Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, et al. , Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography, J. Am. Coll. Cardiol 48 (9) (2006. November 7) 1825–1831. [DOI] [PubMed] [Google Scholar]
- [66].Singh P, Emami H, Subramanian S, Maurovich-Horvat P, Marincheva-Savcheva G, Medina HM, et al. , Coronary plaque morphology and the anti-inflammatory impact of atorvastatin: a multicenter 18F-fluorodeoxyglucose positron emission tomographic/computed tomographic study, Circ Cardiovasc Imaging. American Heart Association, Inc 9 (12) (2016. December) e004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yanai H, Adachi H, The low-dose (7.5 mg/day) pioglitazone therapy, J. Clin. Med. Res 9 (10) (2017. October) 821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mizoguchi M, Tahara N, Tahara A, Nitta Y, Kodama N, Oba T, et al. , Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes a prospective, randomized, comparator-controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta, JACC Cardiovasc Imaging 4 (10) (2011. October) 1110–1118. [DOI] [PubMed] [Google Scholar]
- [69].Nitta Y, Tahara N, Tahara A, Honda A, Kodama N, Mizoguchi M, et al. , Pioglitazone decreases coronary artery inflammation in impaired glucose tolerance and diabetes mellitus: evaluation by FDG-PET/CT imaging, JACC Cardiovasc Imaging 6 (11) (2013. November) 1172–1182. [DOI] [PubMed] [Google Scholar]
- [70].Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, et al. , Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (Dal-PLAQUE): a randomised clinical trial, Lancet 378 (9802) (2011. October 29) 1547–1559. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. , Effects of dalcetrapib in patients with a recent acute coronary syndrome, N Engl J Med. Massachusetts Medical Society 367 (22) (2012. November 29) 2089–2099. [DOI] [PubMed] [Google Scholar]
- [72].Tawakol A, Singh P, Rudd JHF, Soffer J, Cai G, Vucic E, et al. , Effect of treatment for 12 weeks with rilapladib, a lipoprotein-associated phospholipase A2 inhibitor, on arterial inflammation as assessed with 18F-fluorodeoxyglucose-positron emission tomography imaging, J. Am. Coll. Cardiol 63 (1) (2014. January) 86–88. [DOI] [PubMed] [Google Scholar]
- [73].White HD, Held C, Stewart R, Tarka E, Brown R, et al. , Stability investigators darapladib for preventing ischemic events in stable coronary heart disease, N Engl J Med. 370 (18) (2014. May 1) 1702–1711. [DOI] [PubMed] [Google Scholar]
- [74].Elkhawad M, Rudd JHF, Sarov-Blat L, Cai G, Wells L Davies C, et al. , Effects of p38 mitogen-activated protein kinase inhibition on vascular and systemic inflammation in patients with atherosclerosis, JACC Cardiovasc Imaging 5 (9) (2012. September) 911–922. [DOI] [PubMed] [Google Scholar]
- [75].Emami H, Vucic E, Subramanian S, Abdelbaky A, Fayad ZA, Du S, et al. , The effect of BMS-582949, a P38 mitogen-activated protein kinase (P38 MAPK) inhibitor on arterial inflammation: a multicenter FDG-PET trial, Atherosclerosis 240 (2) (2015. June) 490–496. [DOI] [PubMed] [Google Scholar]
- [76].O’Donoghue ML, Glaser R, Cavender MA, Aylward PE, Bonaca MP, Budaj A, et al. , Effect of losmapimod on cardiovascular outcomes in patients hospitalized with acute myocardial infarction: a randomized clinical trial, J. Am. Med. Assoc 315 (15) (2016. April 19) 1591–1599. [DOI] [PubMed] [Google Scholar]
- [77].Joshi N.v., Toor I, Shah ASV, Carruthers K, Vesey AT, Alam SR, et al. , Systemic atherosclerotic inflammation following acute myocardial infarction: myocardial infarction begets myocardial infarction, J Am Heart Assoc. American Heart Association, Inc 4 (9) (2015. August 27) e001956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tarkin JM, Joshi FR, Evans NR, Chowdhury MM, Figg NL, Shah AV, et al. , Detection of atherosclerotic inflammation by 68Ga-DOTATATE PET compared to [18F]FDG PET imaging, J Am Coll Cardiol. Journal of the American College of Cardiology 69 (14) (2017. April 3) 1774–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Armani C, Catalani E, Balbarini A, Bagnoli P, Cervia D, Expression, pharmacology, and functional role of somatostatin receptor subtypes 1 and 2 in human macrophages, J Leukoc Biol. Society for Leukocyte Biology 81 (3) (2007. March) 845–855. [DOI] [PubMed] [Google Scholar]
- [80].Rinne P, Hellberg S, Kiugel M, Virta J, Li X-G, Käkelä M, et al. , Comparison of somatostatin receptor 2-targeting PET tracers in the detection of mouse atherosclerotic plaques, Mol Imaging Biol. Springer US 18 (1) (2016. February) 99–108. [DOI] [PubMed] [Google Scholar]
- [81].Li X, Samnick S, Lapa C, Israel l., Buck AK, Kreissl MC, et al. , 68 Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: correlation with 18 F-FDG, calcium burden and risk factors, EJNMMI Res. Springer Berlin Heidelberg 2 (1) (2012. September 27) 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rominger A, Saam T, Vogl E, Ubleis C, la Fougére C, Förster S, et al. , In vivo imaging of macrophage activity in the coronary arteries using 68Ga-DOTATATE PET/CT: correlation with coronary calcium burden and risk factors, J Nucl Med. Society of Nuclear Medicine 51 (2) (2010. February) 193–197. [DOI] [PubMed] [Google Scholar]
- [83].Schatka l., Wollenweber T, Haense C, Brunz F, Gratz KF, Bengel FM, Peptide receptor-targeted radionuclide therapy alters inflammation in atherosclerotic plaques, J. Am. Coll. Cardiol 62 (24) (2013. December 17) 2344–2345. [DOI] [PubMed] [Google Scholar]
- [84].Boggs KP, Rock CO, Jackowski S, Lysophosphatidylcholine and 1–0-octa-decyl-2-O-methyl-rac-glycero-3-phosphocholine inhibit the CDP-choline pathway of phosphatidylcholine synthesis at the CTP:phosphocholine cyti-dyiyltransferase step, J. Biol. Chem 270 (13) (1995. March 31) 7757–7764. [DOI] [PubMed] [Google Scholar]
- [85].Matter CM, Wyss MT, Meier P, Späth N, Lukowicz von T, Lohmann C, et al. , 18F-choline images murine atherosclerotic plaques ex vivo. arteriosclerosis, thrombosis, and vascular biology, American Heart Association, Inc 26 (3) (2006. March) 584–589. [DOI] [PubMed] [Google Scholar]
- [86].Laitinen IEK, Luoto P, Nägren K, Marjamäki PM, Silvola JMU, Hellberg S, et al. , Uptake of 1 IC-choline in mouse atherosclerotic plaques, J Nucl Med. Society of Nuclear Medicine 51 (5) (2010. May) 798–802. [DOI] [PubMed] [Google Scholar]
- [87].Hellberg S, Silvola JMU, Kiugel M, Liljenbäck H, Metsälä O, Viljanen T, et al. , Type 2 diabetes enhances arterial uptake of choline in atherosclerotic mice: an imaging study with positron emission tomography tracer 18F-fluoromethylcholine, Cardiovasc Diabetol. BioMed Central 15 (1) (2016. February 6) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bucerius J, Schmaljohann J, Böhm l., Palmedo H, Guhlke S, Tiemann K, et al. , Feasibility of 18F-fluoromethylcholine PET/CT for imaging of vessel wall alterations in humans–first results, Eur J Nucl Med Mol Imaging. Springer Berlin Heidelberg 35 (4) (2008. April) 815–820. [DOI] [PubMed] [Google Scholar]
- [89].Kato K, Schober O, Ikeda M, Schäfers M, Ishigaki T, Kies P, et al. , Evaluation and comparison of 1 IC-choline uptake and calcification in aortic and common carotid arterial walls with combined PET/Cr, Eur J Nucl Med Mol Imaging. Springer-Verlag 36 (10) (2009. October) 1622–1628. [DOI] [PubMed] [Google Scholar]
- [90].Bird JLE, Izquierdo-Garcia D, Davies JR, Rudd JHF, Probst KC, Figg N, et al. , Evaluation of translocator protein quantification as a tool for characterising macrophage burden in human carotid atherosclerosis, Atherosclerosis 210 (2) (2010. June) 388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Pugliese F, Gaemperli O, Kinderlerer AR, Lamare F, Shalhoub J, Davies AH, et al. , Imaging of vascular inflammation with [1 ICI-PKI 1195 and positron emission tomography/computed tomography angiography, J. Am. Coll. Cardiol 56 (8) (2010. August 17) 653–661. [DOI] [PubMed] [Google Scholar]
- [92].Gaemperli O, Shalhoub J, Owen DRJ, Lamare F, Johansson S, Fouladi N, et al. , Imaging intraplaque inflammation in carotid atherosclerosis with 1 1CPKI 1195 positron emission tomography/computed tomography, Eur. Heart J 33 (15) (2012. August) 1902–1910. [DOI] [PubMed] [Google Scholar]
- [93].Mateo J, Izquierdo-Garcia D, Badimon JJ, Fayad ZA, Fuster V, Noninvasive assessment of hypoxia in rabbit advanced atherosclerosis using I T-fluoromisonidazole positron emission tomographic imaging, Circ Cardiovasc Imaging. American Heart Association, Inc 7 (2) (2014. March) 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Joshi FR, Mannavaki R, Fryer T, Figg NL, Sluimer JC, Franklin LA, et al. , Vascular imaging with 18F-fluorodeoxyglucose positron emission tomography is influenced by hypoxia, J Am Coll Cardiol. The Authors 69 (14) (2017. April 11) 1873–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Jenkins WSA, Vesey AT, Stirrat C, Connell M, Lucatelli C, Neale A, et al. , Cardiac α Vβ 3integrin expression following acute myocardial infarction in humans, Heart 103 (8) (2017. March 28) 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wang X, Feng H, Zhao S, xu J, wu X, cui J, et al. , SPECr and PET radio-pharmaceuticals for molecular imaging of apoptosis: from bench to clinic, Oncotarget 8 (12) (2017) 20476–20495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kietselaer BLJH, Reutelingsperger CPM, Heidendal GAK, Daemen MJAP, Mess WH, Hofstra L, Narula J, Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis, NEJM 350 (14) (2014) 1472–1473. [DOI] [PubMed] [Google Scholar]
- [98].Dweck MR, Chow MWL, Joshi NV, Williams MC, Jones C, Fletcher AM, et al. , Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology, J. Am. coll. Cardiol 59 (17) (2012. April 24) 1539–1548. [DOI] [PubMed] [Google Scholar]
- [99].Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JLE, Dweck MR, et al. , Identifying active vascular microcalcification by 18F-sodium fluoride positron emission tomography, Nature Communications. Nature Publishing Group 6 (2015. July 7) 7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Derlin T, Toth Z, Papp L, Wisotzki C, Apostolova I, Habermann CR, et al. , Correlation of inflammation assessed by 18F-FDG PET, active mineral deposition assessed by 18F-fluoride PET, and vascular calcification in atherosclerotic plaque: a dual-tracer PET/CI study, J Nucl Med. Society of Nuclear Medicine 52 (7) (2011. July) 1020–1027. [DOI] [PubMed] [Google Scholar]
- [101].Morbelli S, Fiz F, Piccardo A, Picori L, Massollo M, Pestarino E, et al. , Divergent determinants of 18F-NaF uptake and visible calcium deposition in large arteries: relationship with Framingham risk score, Int J Cardiovasc Imaging. Springer Netherlands 30 (2) (2014. February) 439–447. [DOI] [PubMed] [Google Scholar]
- [102].Derlin T, Janssen T, Salamon J, Veldhoen S, Busch JD, Schön G, et al. , Age-related differences in the activity of arterial mineral deposition and regional bone metabolism: a 18F-sodium fluoride positron emission tomography study, Osteoporos. Int 26 (1) (2015. January) 199–207. [DOI] [PubMed] [Google Scholar]
- [103].Dweck MR, Jenkins WSA, Vesey AT, Pringle MAH, Chin CWL, Malley TS, et al. , 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis, Circ Cardiovasc Imaging. American Heart Association, Inc 7 (2) (2014. March) 371–378. [DOI] [PubMed] [Google Scholar]
- [104].Jenkins WSA, Vesey AT, Shah ASV, Pawade TA, Chin CWL, White A-c., et al. , Valvular (18)F-fluoride and (18)F-fluorodeoxyglucose uptake predict disease progression and clinical outcome in patients with aortic stenosis, J. Am. Coll. Cardiol 66 (10) (2015. September 8) 1200–1201. [DOI] [PubMed] [Google Scholar]
- [105].Ishiwata Y, Kaneta T, Nawata S, Hino-Shishikura A, Yoshida K, Inoue T, Quantification of temporal changes in calcium score in active atherosclerotic plaque in major vessels by (18)F-sodium fluoride PET/CT, Eur J Nucl Med Mol Imaging 15 (2017. March 27) 364. [DOI] [PubMed] [Google Scholar]
- [106].McEvoy J.w., Blaha MJ, Defilippis AP, Budoff MJ, Nasir K, Blumenthal RS, et al. , Coronary artery calcium progression: an important clinical measurement? A review of published reports, J. Am. Coll. Cardiol 56 (20) (2010. November ) 1613–1622. [DOI] [PubMed] [Google Scholar]
- [107].Blomberg BA, Thomassen A, de Jong PA, Lam MGE, Diederichsen A-CP, Olsen MH, et al. , Coronary fluorine-18-sodium fluoride uptake is increased in healthy adults with an unfavorable cardiovascular risk profile: results from the CAMONA study, Nucl Med Commun. Nuclear Medicine Communications (2017. September 4). Publish Ahead of Print: 1. [DOI] [PubMed] [Google Scholar]
- [108].Lee Joo Myung, Bang Ji-ln, Koo Bon-Kwon, Hwang Doyeon, Park Jonghanne, Zhang Jinlong, et al. , Clinical relevance of 18F-sodium fluoride positron emission tomography inNon-invasive identification of high risk plaque in patients with coronary artery disease, Circulation Imaging (2017) epub. [DOI] [PubMed] [Google Scholar]
- [109].Robson P, Dweck MR, Trivieri M, Abgral R, Karakatsanis NA, Contreras J, et al. , Coronary artery PET/MR imaging: feasibility, limitations, and solutions, JACC Cardiovasc Imaging (2017). 10.1016/j.jcmg.2016.09.029. [DOI] [PMC free article] [PubMed]
- [110].Quirce R, Martinez-Rodriguez L, De Arcocha Torres M, Jiménez-Bonilla JF, Banzo L, Rebollo M, et al. , Contribution of 18F-sodium fluoride PET/CT to the study of the carotid atheroma calcification, Rev Esp Med Nucl Imagen Mol. 32 (1) (2013. January) 22–25. [DOI] [PubMed] [Google Scholar]
- [111].Vesey AT, Jenkins WSA, Irkle A, Moss A, Sng G, Forsythe RO, et al. , (18)F-Fluoride and (18)F-fluorodeoxyglucose positron emission tomography after transient ischemic attack or minor ischemic stroke: case-control study, Circ Cardiovasc Imaging 10 (3) (2017. March) e004976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Campeau L, Letter: grading of angina pectoris, Circulation 54 (3) (1976. September) 522–523. [PubMed] [Google Scholar]
- [113].Hamm CW, Braunwald E, A classification of unstable angina revisited, Circulation 102 (1) (2000. July 4) 118–122. [DOI] [PubMed] [Google Scholar]