Abstract
Rationale:
Metabolic remodeling in hypertrophic hearts includes inefficient glucose oxidation via increased anaplerosis fueled by pyruvate carboxylation. Pyruvate carboxylation to malate through elevated ME1 (malic enzyme 1) consumes NADPH necessary for reduction of glutathione and maintenance of intracellular redox state.
Objective:
To elucidate upregulated ME1 as a potential maladaptive mechanism for inefficient glucose oxidation and compromised redox state in hypertrophied hearts.
Methods and Results:
ME1 expression was selectively inhibited, in vivo, via non-native miR-ME1 (miRNA specific to ME1) in pressure-overloaded rat hearts. Rats subjected to transverse aortic constriction (TAC) or Sham surgery received either miR-ME1 or PBS. Effects of ME1 suppression on anaplerosis and reduced glutathione (GSH) content were studied in isolated hearts supplied 13C-enriched substrate: palmitate, glucose, and lactate. Human myocardium collected from failing and nonfailing hearts during surgery enabled RT-qPCR confirmation of elevated ME1 gene expression in clinical heart failure versus nonfailing human hearts (P<0.04). TAC induced elevated ME1 content, but ME1 was lowered in hearts infused with miR-ME1 versus PBS. Although Sham miR-ME1 hearts showed no further reduction of inherently low anaplerosis in normal heart, miR-ME1 reduced anaplerosis in TAC to baseline: TAC miRME1=0.034±0.004; TAC PBS=0.081±0.005 (P<0.01). Countering elevated anaplerosis in TAC shifted pyruvate toward oxidation in the tricarboxylic acid cycle. Importantly, via the link to NADPH consumption by pyruvate carboxylation, ME1 suppression in TAC restored GSH content, reduced lactate production, and ultimately improved contractility.
Conclusions:
A maladaptive increase in anaplerosis via ME1 in TAC is associated with reduced GSH content. Suppressing increased ME1 expression in hypertrophied rat hearts, which is also elevated in failing human hearts, reduced pyruvate carboxylation thereby normalizing anaplerosis, restoring GSH content, and reducing lactate accumulation. Reducing ME1 induced favorable metabolic shifts for carbohydrate oxidation, improving intracellular redox state and enhanced cardiac performance in pathological hypertrophy.
Keywords: glucose, heart failure, hypertrophy, metabolism, microRNAs
The pathologically hypertrophied heart has been long characterized as energy starved, a condition that is, in part, a consequence of maladaptive shifts in energy metabolism.1–4 One well-reported metabolic shift that contributes to this energy deficit in the hypertrophied heart is an uncoupling between glycolysis and the oxidation of glycolytic end products.5,6 Pyruvate decarboxylation to form acetyl coenzyme A (acetyl-CoA), mediated by the pyruvate dehydrogenase complex (PDC), does not keep pace with increased glycolytic flux in response to pathological stress on the heart. The diversion of carbon flux away from PDC was first accounted for by experiments in our laboratory, demonstrating that hypertrophied hearts shift carbohydrate oxidation away from PDC-mediated entry into the tricarboxylic acid (TCA) cycle and toward anaplerosis, not only because of inactivation of PDC but also via increased expression and activity of cytosolic NADPH-dependent ME1 (malic enzyme 1).7,8 ME1 carboxylates pyruvate, forming malate, which can enter mitochondria and the second span of the TCA cycle as anaplerotic carbon influx:
Compared with the oxidative decarboxylation of pyruvate via PDC, the alternative route for carbon entry into the TCA cycle facilitated by pyruvate carboxylation is inefficient for generation of energy-yielding metabolism, bypassing numerous reactions that would otherwise generate NADH for oxidative production of ATP.8,9 In addition, pyruvate carboxylation by ME1 consumes NADPH for glutathione reduction that is necessary to maintain intracellular redox state. In adipocytes, ME1 expression increases with high-fat feeding.10 In this context, ME1 is a lipogenic enzyme catalyzing NADPH production through the conversion of malate into pyruvate. In hypertrophied hearts, the reverse flux through ME1 (pyruvate to malate) would consume NADPH, and we hypothesize that ME1 activity then limits content of reduced glutathione (GSH) in the heart.11,12
Increased conversion of pyruvate to malate by ME1 in heart may hold consequences for other cellular processes that require NADPH such as maintenance of cellular redox state. NADPH is used to maintain glutathione in its reduced form (GSH), and the ratio of reduced:oxidized glutathione (GSH/GSSG) reflects the overall redox state of the cell.13,14 Imbalanced cellular redox state causes contractile dysfunction and is reported to be a contributing factor to the pathogenesis of declining function in heart failure.15 Increased ME1-mediated consumption of NADPH in hypertrophied myocardium then holds potential to compromise GSH content, which is necessary for maintenance of redox state in the cardiomyocyte, linking upregulated ME1 to myocardial oxidative stress in hypertrophied hearts.
Interestingly, our laboratory has shown that increased anaplerosis in hypertrophy can be attenuated through pharmacological activation of PDC.8 By treating intact perfused hypertrophied hearts with dichloroacetate, an inhibitor of pyruvate dehydrogenase kinase, anaplerotic flux was partially reduced, coinciding with improved left ventricle (LV) contractility. These findings of beneficial, short-term pharmacological intervention in the hypertrophic heart provide the impetus for the current study investigating potential benefit of selective knockdown of cardiac ME1 expression in vivo in rats subjected to transverse aortic constriction (TAC).
To accomplish this aim, we used a technique developed in our laboratory to specifically suppress gene expression of the NADPH-dependent ME1 enzyme without affecting expression of the ME2 and ME3 isoforms, via cardiac-specific adenoviral-mediated delivery of non-native miR-ME1 (microRNA specific to ME1), restricting ME1 knockdown specifically to the heart.16 Whereas we have demonstrated that dichloroacetate treatment enhanced flux through PDC in hypertrophied hearts leading to improved function,8 here we hypothesized that suppressing ME1 expression, via miR-ME1, will reduce anaplerotic flux through ME1, thereby providing NADPH for glutathione reduction and pyruvate for decarboxylation and oxidation via PDC. The findings show consistency in ME1 gene activation between failing human hearts and hypertrophied rats hearts, while elucidating a mechanism for inefficient carbohydrate utilization in response to pathological stress. As demonstrated, increased expression of ME1 induces anaplerotic flux via pyruvate carboxylation, compromising glucose oxidation, redox state, and ultimately contractile function.
Methods
All data and supporting materials have been provided with the published article. An expanded Methods Section is available in the Online Data Supplement.
Human Study Protocol
The human study protocol for surgical collection of myocardial samples from clinical patients was approved by the Florida Hospital Institutional Review Board. All patients provided written informed consent before inclusion in the study. LV myocardium samples were collected from end-stage failing hearts of nondiabetic, male patients (n=6, 54–75 years of age, median age=66) during LV assist device implantation or cardiac transplant and from nonfailing hearts of non-diabetic, male patients (n=7, 43–77 years of age, median age=66) undergoing elective valve repair or replacement with ejection fractions ≥60% (ejection fraction range 60%–79%). Myocardium was not collected from failing hearts after assist device explantation as bridged to transplant nor with coronary bypass graft.
Myocardial samples were surgically excised during the clinical procedure and immediately snap frozen with liquid nitrogen-cooled tongs. Samples were then stored at −80°C for subsequent quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis of ME1 gene expression.
Animal Model of Pressure-Overload Hypertrophy
Cardiac hypertrophy by chronic pressure overload was induced by constricting the transverse aorta (hemoclip) of 3-week-old male Sprague–Dawley rats, as previously described.7,11,17,18 An initial dose of buprenorphine (0.05 mg/kg SC) was given at the time of anesthesia induction and before conducting surgery. Weanling rats (3 weeks age) were anesthetized with intraperitoneal pentobarbital (65 mg/kg IP). Buprenex (0.1 mg/kg) was administered after surgery (8–12 hours) and twice daily for 3 days and as needed thereafter. This banding procedure relies on the natural growth of the animal to produce a gradually increasing degree of aortic constriction. The rats develop a concentric hypertrophy and increased heart weight, heart weight:body weight ratio, and heart weight:tibia length ratio associated with short-term improvement in the systolic function of the heart.11,19–21 At 12 weeks post-banding, the animals enter a decompensated stage with depressed LV developed pressure and rate of pressure development (dP/dt). In this model of LV hypertrophy, no systemic activation of the sympathetic nervous system or of the renin–angiotensin–aldosterone system occurs.21 Consequently, there are no signs of cardiac lesions, peripheral arteritis, myocardial necrosis, or extensive fibrosis. The rats progress to a dilated cardiac hypertrophy with acute end-stage heart failure at 4 to 6 months post-banding. The sham groups underwent similar surgery without placement of the aortic band. Rats had free access to food and water while being housed under controlled temperature and lighting. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Chicago and Sanford Burnham Prebys Medical Discovery Institute.
Adenovirus Production
Synthesis of a non-native miR-RNA sequence was designed to target cytoplasmic rat ME1 mRNA, as we previously reported.16 The miRRNA sequence was cloned into pcDNA6.2-GW/miR expression vector (Invitrogen) with a cytomegalovirus promoter. The miR-RNA sequence was then recombined into a pAd/CMV/V5-DEST vector (Invitrogen) and transfected into human embryonic kidney 293 cells for amplification as previously described.16,22 The virus was harvested and purified using cesium chloride density gradient centrifugation as previously described.22
Adenoviral Delivery of Micro-RNA Targeted to ME1 to the Myocardium
The in vivo effectiveness in the heart of adenovirus containing miRME1 (Adv.miR-ME1) has been previously characterized by our laboratory and shown to specifically suppress the ME1 isoform in comparison to either PBS or vector containing scrambled code, neither of which induced any effect on the expression of ME isoforms.16 Thus, Adv.miRME1 was administered versus PBS administration to the heart, in vivo by perfusion of the coronary arteries as previously described.16,22
Our prior studies demonstrated that the Adv.miR-ME1 induced no additional off-target effects in comparison to vector containing scrambled code with minimal delivery and expression of code in peripheral organs such as liver, lung, and skeletal muscle.16 However, there is no ideal control group for adenoviral-based gene transfer studies. Whether a scrambled, PBS, GFP (green fluorescent protein), luciferase, or even empty virus strategy is included as a control group, expression levels of some endogenous proteins are altered relative to hearts receiving no treatment.16,23,24 As an example of this phenomenon, in both isolated cardiomyocytes and whole intact heart receiving adenovirus to over-express green fluorescent protein, beta-galactosidase or a small hairpin RNA of green fluorescent protein, the expression of SERCA2 (sarcoplasmic reticulum calcium ATPase2) was downregulated compared with nontreated groups, whereas CALSEQ (calsequestrin) was unchanged.23,24 Green fluorescent protein and beta-galactosidase were the target, not SERCA2. It is beyond the scope of this initial study to identify and explain potential complications from content changes of all nontarget proteins which are not specific to the processes under evaluation in this study. Indeed, there is much to be learned with this emerging and powerful methodology. Nevertheless, as reported in our earlier report, whether we compared the Adv.miR-ME1–treated animals to the untreated excised heart group, the PBS group, or the Adv.miR-scrambled group, ME1 protein expression was reduced significantly by 5 to 6 days via this Adv.miRNA interference strategy.16 For this reason, we selected the PBS strategy as our control group in this study.
At 12 weeks post-surgery, hypertrophied (TAC) or sham-operated rats were anesthetized and intubated. An initial dose of buprenorphine (0.1 mg/kg SC) was given at the time of anesthesia induction and before conducting surgery. Initially, anesthesia was induced in adult rats using a small anesthetic chamber with 4% isoflurane (Minrad, Inc, Bethlehem, PA) and 1% to 2% maintenance. Core body temperature was monitored via rectal thermometer and lowered to 30°C using an ice pad. The chest was opened at the second or third intercostal space, and all vessels leading to and from the heart were cross-clamped simultaneously. A catheter for fluid delivery was inserted into the LV at the apex and advanced into the aortic root, and a second catheter was positioned in the right ventricle for fluid efflux. The coronary vessels were perfused for 7 minutes with calcium-free Tyrode solution, followed by delivery of 0.2 mL of either Adv.miR-ME1 (1013 viral particles U/mL in PBS) or virus-free PBS. After a 90-s incubation period, unsequestered virus was flushed from the coronary vessels with Krebs buffer containing 1.5 mmol/L calcium, thereby eliminating adenoviral delivery to all other organs. The cross-clamp was removed, the chest was closed, and the rats were allowed to recover from anesthesia in an oxygen chamber.
Isolated Perfused Hearts
Six days after infusion of either adenovirus or PBS, animals were heparinized (1000 IU, intraperitoneal injection) and anesthetized (100 mg/kg pentobarbital, intraperitoneal injection). Hearts were excised and retrogradely perfused with modified Krebs-Henseleit buffer (in mmol/L: 116 NaCl, 4 KCl, 1.5 CaCl2, 1.2 MgSO4, and 1.2 NaH2PO4) equilibrated with 95% O2/5% CO2 and containing 0.4 mmol/L 12C palmitate complexed to bovine serum albumin in a 3:1 molar ratio, 5 mmol/L 12C glucose, and 1 mmol/L 12C sodium lactate. Buffer temperature was maintained at 37°C.
A water-filled latex balloon, connected to a force transducer, was fitted into the LV and set to a diastolic pressure of 5 mm Hg. LV developed pressure data were continuously acquired during perfusion with Powerlab (ADInstruments, Dunedin, New Zealand). Rate pressure product was calculated as heart rate×LV developed pressure, and mean peak rates of pressure development and relaxation (+dP/dt and −dP/dt) were calculated from the first derivative of the LV developed pressure trace. Functional data are reported at midpoint of perfusion and were determined to be not significantly different over the entire protocol using repeated measures ANOVA.
For determinations of anaplerosis, isotopic enrichment was initiated by switching the perfusate supply to buffer containing 0.4 mmol/L [2,4,6,8,10,12,14,16-13C8] palmitate, 5 mmol/L unlabeled glucose, and 1 mmol/L unlabeled sodium lactate: Sham PBS, n=7; Sham miR-ME1, n=4; TAC PBS, n=8; and TAB miR-ME1, n=6. Enrichment continued for 40 minutes. For determinations of carbohydrate oxidation, isotopic enrichment was initiated by switching the perfusate supply to buffer containing 0.4 mmol/L palmitate, 5 mmol/L [1,6-13C2] glucose, and 1 mmol/L [3-13C] sodium lactate: Sham PBS, n=5; Sham miR-ME1, n=5; TAC PBS, n=8; TAB miR-ME1, n=4. Again, enrichment continued for 40 minutes. At the end point of each enrichment protocol, oxygen consumption was determined from pulmonary artery effluent with a blood gas analyzer (GEM Premier 300, Instrumentation Laboratory) before freeze-clamping hearts with liquid N2-cooled tongs for subsequent in vitro biochemical analysis. Tissue lactate concentration was determined by spectrophotometric kit (R-Biopharm).
In Vitro NMR
Perchloric acid extracts of frozen LV tissue from perfused hearts were lyophilized and reconstituted in 0.5 mL deuterium oxide. High-resolution proton-decoupled 13C NMR spectra were acquired from in vitro samples with a 5 mm 13C probe (Bruker Instruments, Billerica, MA). The relative contribution of anaplerosis (y) to the 4-carbon intermediate pool of the TCA cycle (ratio of anaplerotic flux:citrate synthase flux) was determined by isotopomer analysis of the gluta-mate 3- and 4-carbon 13C resonances as previously described.25,26 Enrichment of glutamate from 13C glucose and 13C lactate was used to index the relative extent of carbohydrate oxidation and incorporation into the TCA cycle. The fractional 13C enrichment of glutamate was quantified by signal intensity relative to an NMR spectrum of a standard glutamate solution and quantification of glutamate in the tissue extract by spectrophotometry.27,28
Reduced to Oxidized Glutathione Assay
Reduced (GSH) and oxidized (GSSG) glutathione content was determined using a commercially available kit (Millipore). Frozen LV was homogenized, deproteinated, and centrifuged. Supernatant was incubated with 5,5′-dithio-bis(2-nitrobenzoic acid), which oxidizes glutathione to form the derivative 5′-thio-2-nitrobenzoic acid, measurable at 412 nm.29 GSH and GSSG content were normalized to protein content, as determined by BCA protein assay kit (Thermo Scientific).
NADP+ and NADPH Content
NADP+ and NADPH content was determined by the Sanford Burnham Prebys metabolomics core (Orlando, FL) as described previously.30 In brief, pulverized frozen heart samples (≈50 mg per sample) were homogenized in 500 μL of 0.5 mol/L perchloric acid. For NADP+ extraction, a 100-μL aliquot of heart homogenate was spiked with a 10-μL aliquot of heavy isotope-labeled internal standards (18O2-labeled NAD+; synthesized by the Sanford Burnham Prebys Medicinal Chemistry Core). This was followed by the addition of 100 μL of 1 mol/L ammonium formate. Samples were vortexed thoroughly and centrifuged at 18 000g for 5 minutes at 10°C. The clarified homogenates were passed through an AcroPrep Advance 3K Omega Filter Plate (Pall Corporation) before liquid chromatography–tandem mass spectrometry analysis.
For NADPH extraction, a 100-μL aliquot of heart homogenate was spiked with a 10-μL aliquot of heavy isotope-labeled internal standard (18O2-labeled NADH; synthesized by the Sanford Burnham Prebys Medicinal Chemistry Core). Samples were vortexed thoroughly and centrifuged at 18 000g for 5 minutes at 10°C. The clarified homogenates were passed through an AcroPrep Advance 3K Omega Filter Plate (Pall Corporation) before liquid chromatography–tandem mass spectrometry analysis. All pyridine nucleotides were separated on a 2.1×50 mm, 3-μm Thermo Hypercarb column (T=30°C) using a 5.8-minute linear gradient with 10 mmol/L ammonium acetate, pH 9.5, and acetonitrile at a flow rate of 0.5 mL/min. Quantification of pyridine nucleotides was achieved using multiple reaction monitoring on a Dionex UltiMate 3000 HPLC/Thermo Scientific Quantiva triple quadrupole mass spectrometer (Thermo Scientific). Data were normalized to the mass of lyophilized heart powder.
Western Blot and Quantitative RT-qPCR Analysis
ME1 (Abcam) expression in perfused hearts was measured in whole-tissue lysates, with CALSEQ (Thermal Scientific) as a loading control.16 Western band intensity, normalized to loading control, was analyzed by NIH Image software.
Total RNA was extracted from frozen human heart tissue by using an RNeasy Fibrous Tissue kit (Qiagen). RNA quantity was determined at 260 nm (NanoDrop 1000 Spectrometer; Thermal Scientific). Single-stranded cDNA was synthesized from the prepared RNA, and gene products were determined by quantitative real-time polymerase chain reaction, using a Taqman 1-step master mix (Applied Biosystems) with an ABI ViiA7 instrument. The cycle profile was 1 cycle at 50°C for 5 minutes, 1 cycle at 95°C for 20 s, and 40 cycles at 95°C for 15 s, and 60°C for 60 s. The mRNA levels were determined using a standard curve method, normalized to GAPDH. Human primer sequences for the gene ME1 were obtained from Thermo Fisher Life Technologies reference ID Hs00159110_m1.
Statistical Analysis
Results are presented as mean±SEM. Comparisons of 2 means were performed using Student unpaired t test. For human, data sets comparison of means was also performed with the Welch test to account for any potential variance in the groups. Comparisons of >2 means were performed using ANOVA and Tukey–Kramer Multiple Comparisons post hoc test. Significant differences between means were determined at the 5% probability level (P<0.05).
Results
Pressure-Overload Hypertrophy in Rat Hearts
TAC similarly induced elevated heart mass in hearts in groups of hearts that were exposed to either PBS or Adv.miRME1 infusion by 31% to 35% (P<0.01). Heart weight:tibia length ratios (g/cm) were Sham PBS=0.75±0.04; Sham miRME1=0.73±0.02; TAC PBS=0.98±0.5 (P<0.01 versus both Sham groups); and TAC miR-ME1=0.99±0.03 (P<0.01 versus both Sham groups).
Adenovirus-Mediated Reduction in ME1 Protein Expression
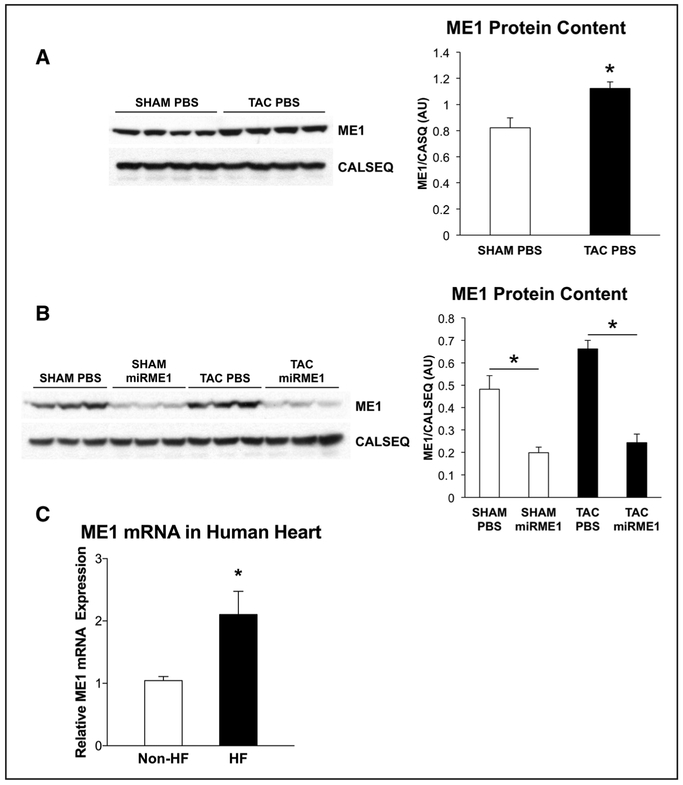
Consistent with previous reports,8 TAC caused an elevation in cytosolic ME1 content (P<0.01; Figure 1). Delivery of Adv.miR-ME1 resulted in dramatically reduced expression of ME1 in both sham-operated and TAC hearts 6 days after injection: 69% decrease in sham operated, 85% decrease in TAC (P<0.01).
Figure 1. Protein levels of ME1 (malic enzyme 1) in pressure-overloaded rat hearts and ME1 message levels in failing human hearts.
A, Protein expression of ME1 was increased in transverse aortic constriction (TAC) PBS compared with sham-operated (SHAM) PBS (n=4 for both groups). B, Delivery of adenovirus containing miR-ME1 (miRNA specific to ME1) vector reduced protein expression of ME1 in both groups (n=3 for each group). White bar, PBS injected; black bar, miR-ME1 injected. *P<0.01. Graphs represent the quantitative analysis of each corresponding blot using NIH ImageJ software. C, mRNA levels of the gene encoding ME1 were elevated in failing human hearts (HF) versus that of nonfailing human hearts (non-HF). *P<0.02 with Students t test and P<0.04 with Welch t test. CALSEQ indicates calsequestrin
ME1 Gene Activation in Failing Human Hearts
Elevated ME1 message in pathologically hypertrophied rats hearts, as previously reported,8,9 is consistent with the data from clinical heart failure in humans (Figure 1C). As shown, ME1 gene expression was significantly elevated in myocardium from failing human hearts over that from nonfailing human myocardium.
Suppressing the Elevation of ME1 Expression in Cardiac Hypertrophy Decreases Anaplerosis, Augments Glucose Oxidation, and Increases GSH Content
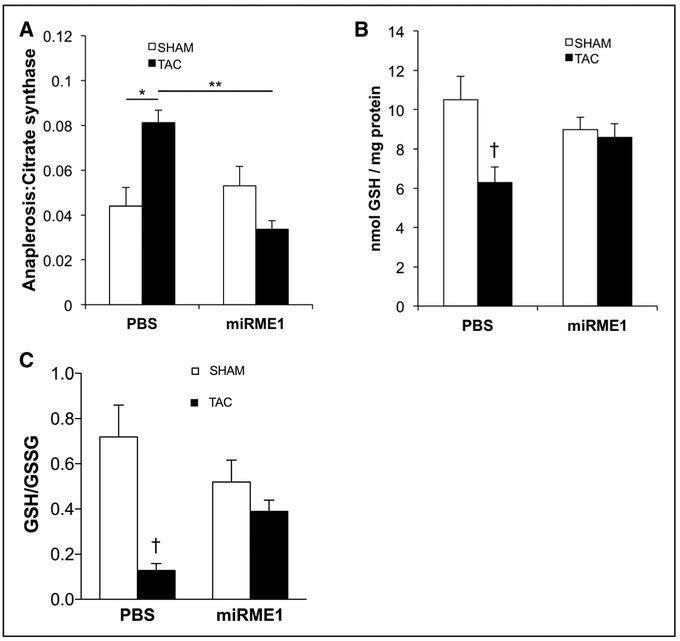
Although sham-operated miR-ME1 hearts showed no further reduction of inherently low baseline ratios of anaplerosis:citrate synthase flux from normal hearts, miR-ME1 reduced anaplerosis from pyruvate carboxylation in TAC hearts down to baseline levels: TAC miRME1=0.034±0.004; TAC PBS=0.081±0.005 (P<0.001; Figure 2A).
Figure 2. Anaplerosis and reduced glutathione content (GSH) in transverse aortic constriction (TAC) hearts.
A, Anaplerosis, expressed as a ratio to citrate synthase activity. In the PBS group, anaplerotic flux was increased nearly 100% with TAC. Knockdown of ME1 expression significantly reduced anaplerosis in TAC to normal levels. *P<0.01;**P<0.001 by ANOVA and Tukey–Kramer test. B, Compared with sham operated (SHAM), TAC hearts demonstrated significantly lower GSH content, as expected. Suppression of ME1 (malic enzyme 1) expression (miRME1 [miRNA specific to ME1]) restored GSH content in TAC to levels comparable to SHAM. C, GSH/GSSG ratios were also improved in the miR-ME1 TAC group in comparison to reduced GSH/GSSG in the Sham-infused TAC hearts. White bar, SHAM; black bar, TAC. †P<0.05 vs SHAM PBS by ANOVA and Tukey–Kramer test.
Importantly, ME1 suppression restored GSH in TAC (P<0.05; Figure 2B), consistent with decreased ME1-mediated carboxylation of pyruvate into malate, a reaction that consumes NADPH used for maintaining glutathione in its reduced form. Although variability exists among published values for the ratio of GSH/GSSG, the functional component for maintaining redox regulation of the heart remains the primary measure of reduced glutathione that is available and was restored because of suppression of ME1 and, thus, inhibition of the otherwise elevated anaplerosis in TAC hearts. As expected from previously published findings, the ratio of GSH/GSSG was reduced in TAC heart.31 Consistent with the GSH content data, the ratio of GSH/GSSG was also improved in TAC hearts with suppression of ME1 (Figure 2C).
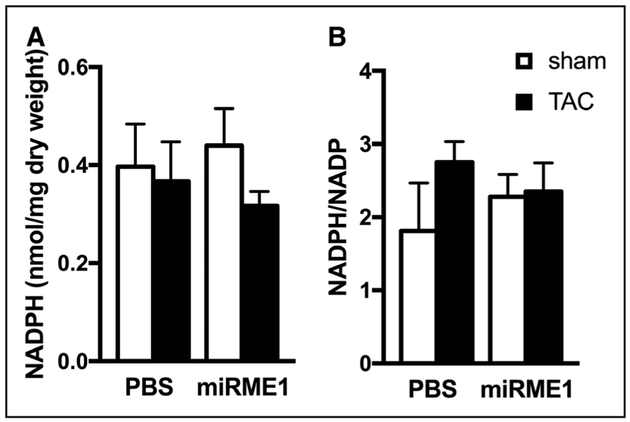
Suppression of ME1 Affected NADPH Utilization in Hypertrophied Hearts
No obvious effect of either TAC-induced hypertrophy or ME1 suppression on the static NADPH level or the ratio of NADPH/NADP was observed (Figure 3). The reduction in ME1 expression could be expected to lead to a net increase in NADPH in the hypertrophied heart; however, additional pathways that consume NADPH, such as glutathione reduction, described above, were also increased by miR-ME1 delivery. Therefore, ME1 suppression facilitated a redistribution of NADPH utilization rather than a change in total NADPH content.
Figure 3. NADPH (A) and NADPH/NADP (B) ratio were not significantly affected in response to ME1 (malic enzyme 1) suppression.
Consumption of NADPH to maintain reduced glutathione (GSH) levels in transverse aortic constriction (TAC) hearts with ME1 suppression is reflected in lack of differences in the values among experimental groups. White bars, Sham operated (SHAM); Black bars, TAC. miR-ME1 indicates miRNA specific to ME1.
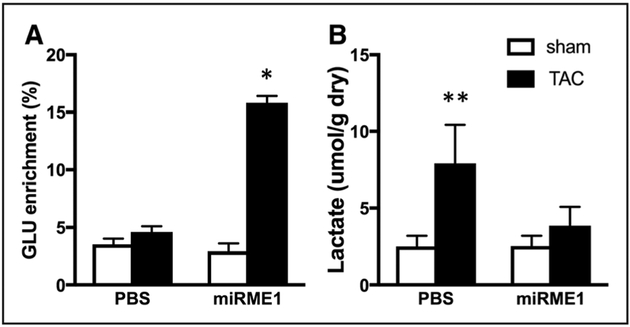
Glucose and Lactate Oxidation in Response to ME1 Suppression
Evidence of increased glucose and lactate oxidation in response to suppression of ME1 in TAC hearts is shown in the isotopic enrichment of glutamate within each group of hearts, as represented in Figure 4A.7 Reduced pyruvate carboxylation via ME1 suppression resulted in increased availability of substrate for PDC, with consequential increases in pyruvate decarboxylation and progressive oxidation in the TCA cycle. Therefore, eliminating the upregulation of ME1 in the response to pathological stress on the heart counteracts the well-reported inefficiencies in glucose oxidation that are associated with elevated glycolysis but limited oxidation of glycolytic end products. Consistent with our previous study of activation of PDC in the hypertrophied heart, suppression of ME1-mediated carboxylation of the pyruvate to form malate enables increased glucose oxidation to support oxidative ATP production. Improving the efficiency of glucose oxidation through ME1 suppression also prevented the accumulation of lactate in post-TAC hypertrophied hearts (Figure 4B).
Figure 4. Increased carbohydrate oxidation in hearts with miR ME1 (miRNA specific to malic enzyme 1) suppression and attenuated lactate accumulation after transverse aortic constriction (TAC).
A, Glutamate (GLU) 13C enrichment from 13C glucose and 13C lactate reflecting oxidation of pyruvate from glycolysis and lactate. Note increased isotopic enrichment of GLU in TAC hearts receiving miR-ME1 to suppress pyruvate carboxylation and enhance oxidation via pyruvate decarboxylase. B, Tissue lactate levels. ME1 suppression prevented the accumulation of lactate in TAC hearts. White bars, Sham operated (SHAM); Black bars, TAC. *P<0.05 vs all other experimental groups; **P<0.05 vs sham hearts by ANOVA and Tukey–Kramer test.
Functional Response to Reduced ME1 and Anaplerosis in Hypertrophied Hearts
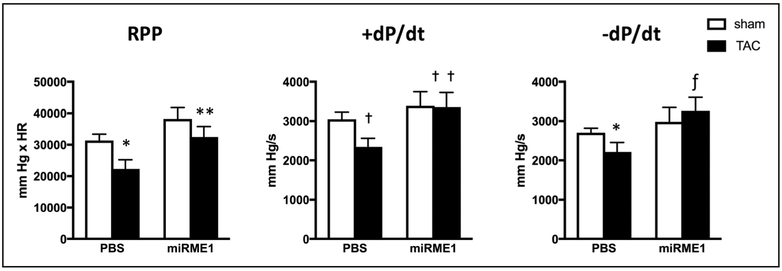
As anticipated, mechanical work was compromised in the isolated hearts post-TAC, as assessed by rate pressure product.7,8,11,32 Contractility, as assessed by both positive and negative dP/dt, was also reduced in hypertrophied hearts. Direct comparison of mean values indicated that suppression of ME1 produced functional improvements (both RPP and dP/dt) in isolated hearts after TAC and ME1 suppression compared with that in Sham hearts subjected to TAC (Figure 5). These data are consistent with previously reported improvements in dP/dt in hypertrophied hearts with reduced anaplerosis because of increased competition against ME1 via activation of PDC by dichloroacetate.8
Figure 5. Contractile response to ME1 (malic enzyme 1) suppression in Sham operated (Sham) and transverse aortic constriction (TAC) hearts.
Direct comparison between mean values reveals improved dP/dt in TAC hearts receiving miR-ME1 (miRNA specific to ME1) vs TAC. White bars, SHAM; Black bars, TAC. *P<0.05, Sham ME1 vs TAC PBS by ANOVA and Tukey–Kramer test; **P<0.05, TAC PBS vs TAC miR-ME1 by Student t test; †P<0.05, Sham PBS vs TAC PBS by ANOVA and Tukey–Kramer test; ††P=0.05, TAC PBS vs TAC miRME1 by Student t test; ƒP<0.05 TAC PBS vs TAC miR-ME1 by Student t test. RPP indicates rate pressure product.
Discussion
This current study probed the mechanism of ME1-mediated anaplerosis in pressure-overloaded hearts, by countering the otherwise elevated expression of ME1 in the pressure-overloaded, hypertrophied rat heart. Samples from failing human myocardium also demonstrated elevated ME1 gene activation over that from nonfailing human myocardium, and these data support the translational relevance of the current study to elucidate the maladaptive metabolic response of the pathological heart. To target ME1 as the potential source of increased anaplerosis in hypertrophied rat hearts, we used an in vitro gene transfer technique developed in our laboratory to induce acute knockdown of ME1 in heart using non-native miRNA specifically targeted to ME1 mRNA without affecting the ME2 and ME3 isoforms.16 Having established the protocol and demonstrating that the Adv.miR-ME1 induced no additional off-target effects in comparison to vector containing scrambled code or PBS, we were careful to eliminate the potential detrimental effects of scrambled code on cardiac function and metabolism by using PBS infusion as the control for the diseased heart. The infusion technique has been shown to specifically limit delivery to the myocardium,22 which is not a trivial concern because of the active role of ME1 in lipogenesis in liver and adipose tissue. Thus, we were able to investigate the effects of ME1 knockdown on heart metabolism without any potentially confounding changes induced by altered ME1 expression in other tissues. As discussed below, the current study provides new evidence that the upregulation of a metabolic enzyme rather than downregulation poses a significant metabolic challenge to the heart with consequences beyond ATP production, that are here shown to include the capacity for homeostasis of intracellular redox state via the availability of reduced glutathione (GSH). Importantly, the findings demonstrate that the changes in ME1 expression affect cardiac function (Figure 6), revealing the maladaptive component of metabolic remodeling in response to pathological stress.
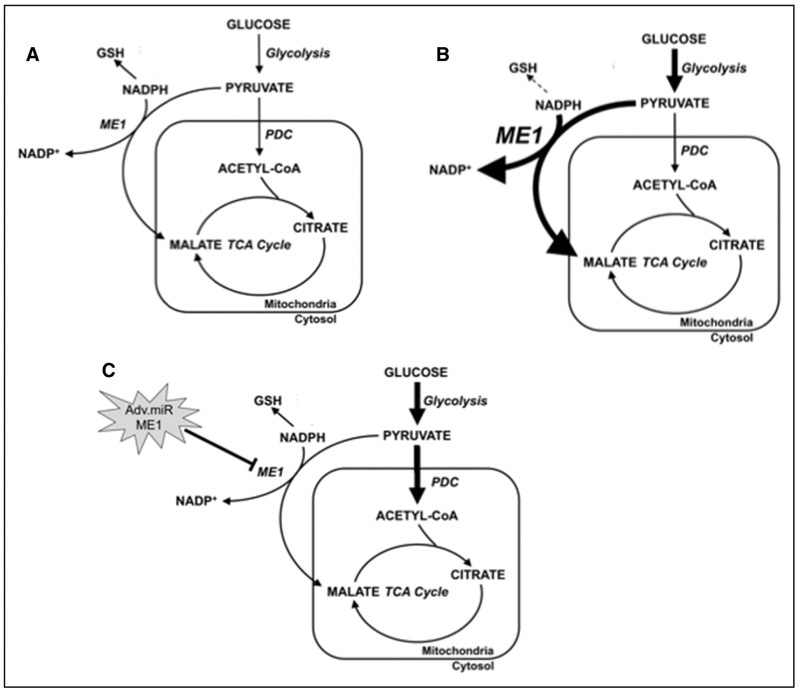
Figure 6. Summary scheme depicting the effects of ME1 (malic enzyme 1) knockdown on glucose and pyruvate metabolism in hypertrophied hearts.
Metabolic fate of glucose and glycolytic end products in a (A) normal heart; (B) pathological heart with increased carbon flux through glycolysis and ME1-mediated entry of carbon into transverse aortic constriction (TCA) cycle via anaplerosis; (C) failing heart treated with virus that suppresses ME1 expression. Increased carbon flux into TCA cycle via pyruvate dehydrogenase complex (PDC) and restoring NADPH-mediated glutathione reduction. Increased metabolic activity is represented by thick arrows. Increased ME1 expression is represented by enlarged text. Decreased reduced glutathione (GSH) formation is represented by dashed line. Adv.miR-ME1 indicates adenovirus containing miR-ME1.
The hypertrophied heart has been well characterized as having both reduced fatty acid oxidation and increased glucose utilization, a reversion to the so-called fetal metabolic profile.20 However, such broad characterization of shifting substrate utilization by hypertrophied and failing hearts ignores the overall balance of influx and efflux of carbon to and from the TCA cycle. Hypertrophied hearts demonstrate increased glycolytic rate without a corresponding increase in glucose oxidation, mediated by PDC that converts pyruvate into acetyl-CoA.5,6 This discrepancy was accounted for by studies in our laboratory demonstrating that hypertrophied hearts have increased protein content of the cytosolic enzyme ME1.7 In the hypertrophied heart, this carboxylation redirects pyruvate metabolism away from oxidative decarboxylation by PDC. Alternatively, ME1 carboxylates pyruvate, forming ma-late, which is then available for intermediate exchange across the mitochondrial membrane to enter the second span of the TCA cycle as anaplerotic carbon influx.8
Compared with oxidation via PDC, the alternative route for carbon entry into the TCA cycle facilitated by ME1 is inefficient, bypassing numerous reactions that would otherwise generate reducing equivalents for oxidative production of ATP and fails to match the flux of glucose through the glycolytic pathway leading to lactate accumulation. Importantly, the augmented oxidation of pyruvate that resulted from ME1 suppression also diverted pyruvate metabolism away from lactate accumulation in TAC hearts. This reduction in tissue lactate is likely a consequence of the improved coupling between glycolysis and the oxidation of glycolytic pyruvate and is consistent with a general restoration of cytosolic redox state (NADH/NAD+) which is perhaps also linked to the benefits of preserving NADPH through reduced ME1. Nevertheless, the improved efficiency of glucose metabolism in the heart averted the negative effects of lactate accumulation on contractility.33–35 In addition, enhancement of contractile function has been previously associated with increases in pyruvate oxidation in various TAC models of disease,8,36 which is consistent with the currently observed improvements in contractility induced by ME1 suppression in TAC hearts.
In addition, carboxylation of pyruvate by ME1 consumes NADPH, a cofactor for glutathione reduction to maintain cellular redox state. The resulting 3- to 5-fold increase in the oxidation of pyruvate, derived from glucose and lactate, that occurred in the hypertrophied heart during suppression of ME1 upregulation, over that of all other experimental groups, is further evidence of the significant level of uncoupling between the increased glycolysis and limited glucose oxidation that has been previously reported in the literature.5,6 What is newly realized by these data are the considerable extent to which maladaptive pyruvate carboxylation in the hypertrophied heart limits its availability as substrate for oxidative ATP production via PDC and the TCA cycle. These findings support the emerging concept that the increase in ME1 expression and anaplerotic flux does not seem to be necessary adaptations that help maintain carbon flux into the TCA cycle in response to chronic pressure overload, but rather are mal-adaptive responses that participate directly in the uncoupling of glycolysis and glucose oxidation. Suppressing ME1 does not limit carbon entry into the TCA cycle from carbohydrate sources, but rather increases the contribution of pyruvate to the acetyl-CoA formation, thereby restoring NADH generation from pyruvate oxidation.8
The findings demonstrate that the maladaptive increase in anaplerosis via ME1 with TAC can be completely attenuated by knocking down expression of the enzyme. In contrast, enzyme–substrate competition induced by activating PDC with dichloroacetate only partially reduced the anaplerotic response to pathological challenge in hypertrophied hearts.8 Importantly, this study provides direct evidence that suppressing the upregulation of ME1 and consequential carboxylation of pyruvate contributes to the maintenance of the reduced form of glutathione. GSH has been reported to be reduced in hypertrophied myocardium because of impaired redox state.31 We have now linked GSH content and impaired redox state, as indexed by GSH/GSSG, to the increased expression and activity of ME1 in the carboxylation reaction that essentially limits the availability of NADPH for reduction of glutathione. Imbalanced cellular redox state causes contractile dysfunction and reportedly contributes to the pathophysiology of heart failure,15 potentially linking increased ME1-mediated consumption of NADPH with myocardial oxidative stress in hypertrophy.
Surprisingly, very little is known about the concentration of NADPH in the hypertrophied and failing heart and the ratio of NADP/NADPH,37–39 whereas almost nothing is known concerning the turnover of NADPH at either baseline or in response to pathological stress. As shown here, the maladaptive increase in anaplerosis via ME1 in TAC is associated with the known observation of reduced glutathione content in hypertrophied hearts.31 NADPH was then presumably expended in the process of restoring NADPH-dependent GSH. These processes would be consistent with the observed levels of each of these respective metabolites. Suppressing increased ME1 expression in hypertrophied hearts, and thus consumption of NADPH for the production of malate produced favorable metabolic shifts, including improved GSH content. Although the reductions in ME1 content in the hypertrophied heart improved cardiac work performance and contractility, we are unable to discern whether the functional benefits were the results of either a singular restoration of GSH or induction of the more efficient oxidation of glucose to produce ATP, or whether the contractile response is the consequence of multiple metabolic responses.
The current mainstays of treatment for chronic heart failure, including the so-called guideline-directed medical therapies40 and the various mechanical assist devices, exert beneficial effects, at least in part, by reducing energy requirements of the failing heart. However, the current therapeutic stratagems fail to address underlying inefficiencies in metabolism that are increasingly appreciated as contributing to chronic mismatch between energy supply and demand. In this report, we show that correcting maladaptive upregulation of ME1 expression through targeted gene suppression induces favorable shifts in myocardial metabolism, improves redox state, and supports improved contractile function. Clearly, the contractile benefit that resulted from the upstream suppression of ME1 is likely to be a multifactorial consequence of overlapping mechanisms associated with normalization of the intracellular environment. Importantly, the data from human myocardial samples demonstrate that ME1 gene activation does occur in the failing human heart. Whether ME1 can be manipulated, either pharmacologically or with gene therapy, in human heart failure patients or in patients with pathological hypertrophy or decompensated cardiomyopathy, poses an exciting avenue of future investigation.
Supplementary Material
Novelty and Significance.
What Is Known?
The hypertrophic myocardium exhibits remodeled metabolic phenotype of impaired glucose oxidation, in part because of high levels of anaplerosis.
NADPH-dependent ME1 (malic enzyme 1) expression is increased in hypertrophied rats heart, impairing glucose oxidation through pyruvate dehydrogenase.
What New Information Does This Article Contribute?
ME1 gene activation occurs in failing human hearts.
Targeted delivery code for a novel, non-native miR-ME1 (microRNA specific to ME1) reduced expression and content of ME1 in pathologically hypertrophied rat hearts, normalizing anaplerotic flux.
Suppressing the maladaptive increase in ME1 expression and in hypertrophied hearts increased glucose oxidation, improved intra-cellular redox state, and enhanced cardiac function.
This study elucidates a novel mechanism producing both inefficient glucose oxidation for ATP production and impaired redox state in the pathologically hypertrophied heart that is dependent on the maladaptive upregulation of the NADP+-dependent ME1. The findings confirm increased gene activation of ME1 in failing human hearts, while elucidating the specific mechanism for elevated anaplerosis that is linked to inefficient glucose metabolism and reduced glutathione content in hypertrophied rat hearts. Cardiac-specific, adenoviral-mediated delivery of exogenous code for a novel, non-native microRNA, specific to ME1 to the rat heart in vivo, eliminated increased ME1 expression in hypertrophied hearts, thereby normalizing anaplerosis. With the induction of normal anaplerosis in hypertrophied hearts, both glucose oxidation and the NADPH-dependent content of reduced glutathione were increased. Significantly, these improvements in the metabolic state of the diseased heart enhanced contractile function. The novel finding that maladaptive expression of ME1 is responsible for the underlying mechanisms of inefficient glucose oxidation and impaired redox state in pathological cardiac hypertrophy identifies a potential therapeutic target to combat maladaptive metabolic remodeling in the heart.
Sources of Funding
This research was supported by the following grants: National Institutes of Health, National Heart, Lung and Blood Institute R01HL132525, R01HL049244 (E.D. Lewandowski); American Heart Association, Midwest Affiliate, Predoctoral Fellowship 12PRE11780022 (R. Lahey). Human tissue studies were supported by institutional funds from Sanford Burnham Prebys Medical Discovery Institute and the Translational Research Institute for Metabolism and Diabetes.
Nonstandard Abbreviations and Acronyms
- Adv
adenovirus
- CALSEQ
calsequestrin
- GFP
green fluorescent protein
- GSH
reduced form of glutathione
- GSSG
oxidized form of glutathione
- LV
left ventricle
- ME
malic enzyme
- miR
microRNA
- PDC
pyruvate dehydrogenase complex
- SERCA
sarcoplasmic reticulum calcium ATPase
- TAC
transverse aortic constriction
- TCA
tricarboxylic acid
Footnotes
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA. 118.312660/-/DC1.
Disclosures
None.
References
- 1.Carley AN, Taegtmeyer H, Lewandowski ED. Matrix revisited: mechanisms linking energy substrate metabolism to the function of the heart. Circ Res. 2014;114:717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. 2013;113:709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolwicz SC Jr, Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res. 2013;113:603–616. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 5.Lydell CP, Chan A, Wambolt RB, Sambandam N, Parsons H, Bondy GP, Rodrigues B, Popov KM, Harris RA, Brownsey RW, Allard MF. Pyruvate dehydrogenase and the regulation of glucose oxidation in hypertrophied rat hearts. Cardiovasc Res. 2002;53:841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leong HS, Brownsey RW, Kulpa JE, Allard MF. Glycolysis and pyruvate oxidation in cardiac hypertrophy–why so unbalanced? Comp Biochem Physiol A Mol Integr Physiol. 2003;135:499–513. [DOI] [PubMed] [Google Scholar]
- 7.Sorokina N, O’Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation. 2007;115:2033–2041. [DOI] [PubMed] [Google Scholar]
- 8.Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, Lanoue KF, Taegtmeyer H, O’Donnell JM, Lewandowski ED. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ Res. 2009;104:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sack MN. Innate short-circuiting of mitochondrial metabolism in cardiac hypertrophy: identification of novel consequences of enhanced anaplerosis. Circ Res. 2009;104:717–719. doi: 10.1161/CIRCRESAHA.109.195495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zabala A, Churruca I, Fernández-Quintela A, Rodríguez VM, Macarulla M, Martínez JA, Portillo MP. trans-10,cis-12 Conjugated linoleic acid inhibits lipoprotein lipase but increases the activity of lipogenic enzymes in adipose tissue from hamsters fed an atherogenic diet. Br J Nutr. 2006;95:1112–1119. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell JM, Fields AD, Sorokina N, Lewandowski ED. The absence of endogenous lipid oxidation in early stage heart failure exposes limits in lipid storage and turnover. J Mol Cell Cardiol. 2008;44:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chokshi A, Drosatos K, Cheema FH, et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–2853. doi: 10.1161/CIRCULATIONAHA.111.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain M, Brenner DA, Cui L, Lim CC, Wang B, Pimentel DR, Koh S, Sawyer DB, Leopold JA, Handy DE, Loscalzo J, Apstein CS, Liao R. Glucose-6-phosphate dehydrogenase modulates cytosolic redox status and contractile phenotype in adult cardiomyocytes. Circ Res. 2003;93:e9–e16. doi: 10.1161/01.RES.0000083489.83704.76. [DOI] [PubMed] [Google Scholar]
- 14.Jain M, Cui L, Brenner DA, Wang B, Handy DE, Leopold JA, Loscalzo J, Apstein CS, Liao R. Increased myocardial dysfunction after ischemia-reperfusion in mice lacking glucose-6-phosphate dehydrogenase. Circulation. 2004;109:898–903. doi: 10.1161/01.CIR.0000112605.43318.CA. [DOI] [PubMed] [Google Scholar]
- 15.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI24408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Donnell JM, Kalichira A, Bi J, Lewandowski ED. In vivo, cardiac-specific knockdown of a target protein, malic enzyme-1, in rat via adenoviral delivery of DNA for non-native miRNA. Curr Gene Ther. 2012;12:454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Donnell JM, Fields A, Xu X, Chowdhury SA, Geenen DL, Bi J. Limited functional and metabolic improvements in hypertrophic and healthy rat heart overexpressing the skeletal muscle isoform of SERCA1 by adenoviral gene transfer in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H2483–H2494. doi: 10.1152/ajpheart.01023.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewandowski ED, Fischer SK, Fasano M, Banke NH, Walker LA, Huqi A, Wang X, Lopaschuk GD, O’Donnell JM. Acute liver carnitine palmitoyltransferase I overexpression recapitulates reduced palmitate oxidation of cardiac hypertrophy. Circ Res. 2013;112:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vatner DE, Ingwall JS. Effects of moderate pressure overload cardiac hypertrophy on the distribution of creatine kinase isozymes. Proc Soc Exp Biol Med. 1984;175:5–9. [DOI] [PubMed] [Google Scholar]
- 20.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro HB, Okoshi K, Cicogna AC, Bregagnollo EA, Rodrigues MA, Padovani CR, Aragon FF, Jamas E, Okoshi MP. Follow-up study of morphology and cardiac function in rats undergoing induction of supravalvular aortic stenosis. Arq Bras Cardiol. 2003;81:569, 562–575, 562. [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell JM, Lewandowski ED. Efficient, cardiac-specific adenoviral gene transfer in rat heart by isolated retrograde perfusion in vivo. Gene Ther. 2005;12:958–964. [DOI] [PubMed] [Google Scholar]
- 23.Suckau L, Fechner H, Chemaly E, et al. Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation. 2009;119:1241–1252. doi: 10.1161/CIRCULATIONAHA.108.783852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisser-Thomas J, Dieterich E, Janssen PM, Schmidt-Schweda S, Maier LS, Sumbilla C, Pieske B. Method-related effects of adenovirus-mediated LacZ and SERCA1 gene transfer on contractile behavior of cultured failing human cardiomyocytes. J Pharmacol Toxicol Methods. 2005;51:91–103. doi: 10.1016/j.vascn.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Malloy CR, Sherry AD, Jeffrey FM. Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by 13C NMR spectroscopy. J Biol Chem. 1988;263:6964–6971. [PubMed] [Google Scholar]
- 26.Lewandowski ED, Doumen C, White LT, LaNoue KF, Damico LA, Yu X. Multiplet structure of 13C NMR signal from glutamate and direct detection of tricarboxylic acid (TCA) cycle intermediates. Magn Reson Med. 1996;35:149–154. [DOI] [PubMed] [Google Scholar]
- 27.Yu X, White LT, Doumen C, Damico LA, LaNoue KF, Alpert NM, Lewandowski ED. Kinetic analysis of dynamic 13C NMR spectra: metabolic flux, regulation, and compartmentation in hearts. Biophys J. 1995;69:2090–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson JR, Corkey BE. Assay of citric acid cycle intermediates and related compounds–update with tissue metabolite levels and intracellular distribution. Methods Enzymol. 1979;55:200–222. [DOI] [PubMed] [Google Scholar]
- 29.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 30.Horton JL, Martin OJ, Lai L, Riley NM, Richards AL, Vega RB, Leone TC, Pagliarini DJ, Muoio DM, Bedi KC, Margulies KB, Coon JJ, Kelly DP. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2016;2:e84897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheeran FL, Rydström J, Shakhparonov MI, Pestov NB, Pepe S. Diminished NADPH transhydrogenase activity and mitochondrial redox regulation in human failing myocardium. Biochim Biophys Acta. 2010;1797:1138–1148. doi: 10.1016/j.bbabio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Lahey R, Wang X, Carley AN, Lewandowski ED. Dietary fat supply to failing hearts determines dynamic lipid signaling for nuclear receptor activation and oxidation of stored triglyceride. Circulation. 2014;130:1790–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewandowski ED, White LT. Pyruvate dehydrogenase influences postischemic heart function. Circulation. 1995;91:2071–2079. [DOI] [PubMed] [Google Scholar]
- 34.Rovetto MJ, Whitmer JT, Neely JR. Comparison of the effects of anoxia and whole heart ischemia on carbohydrate utilization in isolated working rat hearts. Circ Res. 1973;32:699–711. [DOI] [PubMed] [Google Scholar]
- 35.Liu B, Clanachan AS, Schulz R, Lopaschuk GD. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ Res. 1996;79:940–948. [DOI] [PubMed] [Google Scholar]
- 36.Wambolt RB, Lopaschuk GD, Brownsey RW, Allard MF. Dichloroacetate improves postischemic function of hypertrophied rat hearts. J Am Coll Cardiol. 2000;36:1378–1385. [DOI] [PubMed] [Google Scholar]
- 37.Hecker PA, Lionetti V, Ribeiro RF Jr, Rastogi S, Brown BH, O’Connell KA, Cox JW, Shekar KC, Gamble DM, Sabbah HN, Leopold JA, Gupte SA, Recchia FA, Stanley WC. Glucose 6-phosphate dehydrogenase deficiency increases redox stress and moderately accelerates the development of heart failure. Circ Heart Fail. 2013;6:118–126. doi: 10.1161/CIRCHEARTFAILURE.112.969576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupte SA, Levine RJ, Gupte RS, Young ME, Lionetti V, Labinskyy V, Floyd BC, Ojaimi C, Bellomo M, Wolin MS, Recchia FA. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J Mol Cell Cardiol. 2006;41:340–349. doi: 10.1016/j.yjmcc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Xu J, Wang Q, Brainard RE, Watson LJ, Jones SP, Epstein PN. Reduced cardiac fructose 2,6 bisphosphate increases hypertrophy and decreases glycolysis following aortic constriction. PLoS One. 2013;8:e53951. doi: 10.1371/journal.pone.0053951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yancy CW, Jessup M, Bozkurt B, et al. ; WRITING COMMITTEE MEMBERS; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.