Abstract
BACKGROUND:
Congenital syphilis (CS) results when an infected pregnant mother transmits syphilis to her unborn child prior to or at delivery. The severity of infection can range from a delivery at term without signs of infection to stillbirth or death after delivery.
OBJECTIVE:
We sought to describe CS morbidity and mortality during 1999 through 2013.
STUDY DESIGN:
National CS case data reported to Centers for Disease Control and Prevention during 1999 through 2013 were analyzed. Cases were classified as dead (stillbirths and deaths up to 12 months after delivery), morbid (cases with strong [physical, radiographic, and/or non-serologic laboratory] evidence of CS), and nonmorbid (cases with a normal physical examination reported, without strong evidence of infection). Annual rates of these cases were calculated. Cases were compared using selected maternal and infant criteria.
RESULTS:
During 1999 through 2013, 6383 cases of CS were reported: 6.5% dead, 33.6% morbid, 53.9% nonmorbid, and 5.9% unknown morbidity; 81.8% of dead cases were stillbirths. Rates of dead, morbid, and nonmorbid cases all decreased over this time period, but the overall proportions that were dead or morbid cases did not significantly change. The overall case fatality ratio during 1999 through 2013 was 6.5%. Among cases of CS, maternal race/ethnicity was not associated with increased morbidity or death, although most cases (83%) occurred among black or Hispanic mothers. No or inadequate treatment for maternal syphilis, <10 prenatal visits, and maternal nontreponemal titer ≥1:8 increased the likelihood of a dead case; risk of a dead case increased with maternal nontreponemal titer (χ2 for trend P < .001). Infants with CS born alive at <28 weeks’ gestation (relative risk, 107.4; P < .001) or born weighing <1500 g (relative risk, 43.9; P < .001) were at greatly increased risk of death.
CONCLUSION:
CS remains an important preventable cause of perinatal morbidity and mortality, with comparable case fatality ratios during 1999 through 2013 (6.5%) and 1992 through 1998 (6.4%). Detection and treatment of syphilis early during pregnancy remain crucial to reducing CS morbidity and mortality.
Keywords: congenital syphilis, infant mortality, prenatal care, sexually transmitted disease surveillance, stillbirth
Introduction
Congenital syphilis (CS) is the transmission of syphilis from an infected mother to her infant at or prior to delivery. Syphilis that is untreated or treated late in gestation can lead to CS in >50% of affected pregnancies,1,2 resulting in stillbirth, neonatal death, or other morbidity, including visceral or neurologic damage in surviving infants. CS is preventable if syphilis in the mother is treated early in pregnancy with penicillin,3 and prenatal syphilis screening and treatment are effective interventions.4 Because CS is related to both community prevalence of syphilis among reproductive-aged women and prenatal intervention, CS case rates reflect a health system’s effectiveness.
Effective prevention of CS in the United States is challenging. Diagnosis remains difficult, and lack of provider awareness about CS still exists.5 Social determinants of health and health systems’ issues affect access to and quality of prenatal care. Many of these issues affect racial/ethnic minority women,6-8 leading to disparities in diseases,9-13 including syphilis and CS.14
A previous analysis by Gust et al15 reviewed national case report data during 1992 through 1998, following a revision to the CS surveillance case definition that increased sensitivity to increase treatment among affected infants. The analysis of Gust et al15 showed declines in both overall cases of CS and cases of CS that died, with no decline in the proportion of CS cases that died. Here, we describe trends in CS infant mortality and morbidity during 1999 through 2013, and associated maternal and infant factors.
Materials and Methods
Case data for CS are reported to local and state health departments; states voluntarily transmit that data to the Centers for Disease Control and Prevention (CDC). We reviewed CS case data from all 50 states and Washington, DC, reported to CDC as of Aug. 1, 2014, for cases born during 1999 through 2013; cases were reported based on the surveillance case definition for CS used during this time period.14 Cases were classified by disease severity. A dead case was defined as a case of CS that died, either as a stillbirth or as a live birth that died within 12 months of delivery. A morbid case was defined as a case of CS reported as alive, with strong evidence of infection based on ≥1 of the following situations: (1) findings on darkfield microscopy or direct fluorescent antibody examination of lesions (on the infant), placenta, or umbilical cord consistent with syphilis, a reactive cerebrospinal fluid (CSF) Venereal Disease Research Laboratory test, or changes on long bone radiographs consistent with syphilis; (2) physical signs or symptoms of syphilis (hepatosplenomegaly, rash, condyloma lata, snuffles, jaundice [nonviral hepatitis], pseudoparalysis, anemia, edema [nephrotic syndrome and/or malnutrition]); or (3) an elevated CSF white blood cell count (WBC) and/or CSF protein concentration absent other causes, and the case’s mother was not treated or inadequately treated for syphilis (“adequate” defined as penicillin therapy appropriate for maternal stage of infection, administered ≥30 days before delivery). A nonmorbid case was defined as a case of CS reported as alive, without the strong evidence of infection described for a morbid case (either because such tests or procedures were negative or not performed), who had a normal physical examination reported. A case of unknown morbidity was defined as a case of CS reported as alive, without the strong evidence of infection described for a morbid case (either because such tests or procedures were negative or not performed), and without a normal physical examination reported. To understand how testing (or lack of it) influenced case classification, the number of morbid and nonmorbid cases with CSF testing (CSF protein, WBC count, and/or CSF Venereal Disease Research Laboratory test) and long bone radiographic examination were calculated.
Annual rates of dead, morbid, and nonmorbid cases of CS were calculated with the annual count of dead, morbid, or nonmorbid cases of CS as the numerator and live births for the corresponding year as the denominator, using natality data matched for maternal race/ethnicity. Population denominators for 2012 (the most recent data available at time of analysis) were used to calculate rates for 2013.
Cases of CS (dead, morbid, non-morbid, and unknown) were described by maternal and infant characteristics. Maternal characteristics analyzed were age (<25 or ≥25 years); marital status (married; single, never married; separated/divorced; or other); race/ethnicity (black, Hispanic, white, Asian/Pacific Islander, Native American/Alaska Native, or other); census region (Midwest, Northeast, South, or West); number of prenatal care visits (none, 1–4, 5–9, or ≥10 visits); trimester of first prenatal visit (first, second, or third trimester); treatment for syphilis (adequate or inadequate); and nontreponemal titer closest to delivery (≤1:4, 1:8–1:32, 1:64–1:256, or >1:256). Infant characteristics analyzed were gestational age at time of delivery (<28, 28–31, 32–36, or ≥37 weeks) and birthweight (<1500, 1500-2499, or ≥2500 g); because prematurity and low birthweight are common among stillbirths,16 analysis of gestational age and birthweight was limited to cases of CS that were born alive. Race/ethnicity was defined using the National Center for Health Statistics bridged-race categories,17 while US regions were defined using the US Census geographic regions.
A χ2 test for linear trend was used to determine if significant overall trends in proportions of dead or morbid cases of CS were present during 1999 through 2013. To determine if maternal or infant characteristics were associated with negative outcomes (a morbid or dead case), relative risks (RR) and 95% confidence intervals (CIs) were calculated, comparing morbid cases and dead cases as a group to nonmorbid cases. To determine if maternal or infant characteristics were associated with the most extreme negative outcome (ie, death), RR and 95% CIs were calculated, comparing dead cases to morbid cases and nonmorbid cases as a group. A sensitivity analysis was performed to explore how missing information (ie, cases classified as unknown morbidity) might affect these analyses: RR and 95% CIs were also calculated with cases of unknown morbidity reclassified as: (1) all nonmorbid cases, and (2) all morbid cases. Data were analyzed using software (SAS, Version 9.3; SAS Institute Inc, Cary, NC). CS data were collected as part of routine public health surveillance and were thus exempt from institutional review board review.
Results
During 1999 through 2013, a total of 6383 cases of CS were reported to CDC, with the highest proportions among black or Hispanic mothers; single, never-married mothers; and mothers in the South (Table 1): 6.5% dead, 33.6% morbid, 53.9% nonmorbid, and 5.9% of unknown morbidity. Of the 2145 morbid cases, 1960 (91.4%) had some combination of CSF testing (CSF protein, WBC, and/or CSF Venereal Disease Research Laboratory test) and/or long bone radiographic examination, with 1197 (61.1%) having both tests performed. Of the 3443 nonmorbid cases, 511 (14.8%) had both CSF testing and long bone radiographic examination performed.
TABLE 1.
Selected demographic and clinical characteristics of infants with congenital syphilis and their mothers, by severity of illness–United States, 1999 through 2013
| Dead (%) |
Morbid (%) |
Nonmorbid (%) |
Unknown (%) |
Total (%) |
|
|---|---|---|---|---|---|
| n = 418 | n = 2145 | n = 3443 | n = 377 | N = 6383 | |
| Maternal characteristics–demographics | |||||
| Maternal age, y | |||||
| ≥25 | 188 (45) | 1176 (55) | 1981 (58) | 222 (59) | 3567 (56) |
| ≤24 | 228 (55) | 954 (44) | 1424 (41) | 146 (39) | 2752 (43) |
| Blank | 2 (0) | 15 (1) | 38 (1) | 9 (2) | 64 (1) |
| Marital status | |||||
| Married | 41 (10) | 298 (14) | 574 (17) | 59 (16) | 972 (15) |
| Single, never married | 310 (74) | 1506 (70) | 2328 (68) | 230 (61) | 4374 (69) |
| Separated/divorced | 8 (2) | 80 (4) | 91 (3) | 11 (3) | 190 (3) |
| Other | 1 (0) | 8 (0) | 18 (1) | 7 (2) | 34 (1) |
| Unknown/missing | 58 (14) | 253 (12) | 432 (13) | 70 (19) | 813 (13) |
| Race/ethnicity | |||||
| White | 38 (9) | 262 (12) | 377 (11) | 47 (12) | 724 (11) |
| Black | 218 (52) | 1125 (52) | 1749 (51) | 211 (56) | 3303 (52) |
| Hispanic | 141 (34) | 642 (30) | 1083 (31) | 96 (25) | 1962 (31) |
| Asian/Pacific Islander | 7 (2) | 46 (2) | 95 (3) | 7 (2) | 155 (2) |
| American Indian/Alaska Native | 5 (1) | 26 (1) | 33 (1) | 3 (1) | 67 (1) |
| Other | 1 (0) | 9 (0) | 27 (1) | 4 (1) | 41 (1) |
| Unknown | 8 (2) | 35 (2) | 79 (2) | 9 (2) | 131 (2) |
| Regiona | |||||
| Midwest | 75 (18) | 306 (14) | 437 (13) | 106 (28) | 924 (14) |
| Northeast | 30 (7) | 253 (12) | 394 (11) | 13 (3) | 690 (11) |
| South | 213 (51) | 1083 (50) | 1812 (53) | 207 (55) | 3315 (52) |
| West | 100 (24) | 503 (23) | 800 (23) | 51 (14) | 1454 (23) |
| Maternal characteristics–detection of maternal syphilis | |||||
| Prenatal visits, no. | |||||
| 0/No prenatal care | 199 (48) | 690 (32) | 794 (23) | 100 (27) | 1783 (28) |
| 1–4 | 84 (20) | 377 (18) | 551 (16) | 52 (14) | 1064 (17) |
| 5–9 | 39 (9) | 335 (16) | 570 (17) | 42 (11) | 986 (15) |
| ≥10 | 18 (4) | 284 (13) | 583 (17) | 37 (10) | 922 (14) |
| Unknown | 78 (19) | 459 (21) | 945 (27) | 146 (39) | 1628 (26) |
| Prenatal visits, trimester | |||||
| No prenatal care | 199 (48) | 690 (32) | 794 (23) | 100 (27) | 1783 (28) |
| First | 75 (18) | 472 (22) | 830 (24) | 63 (17) | 1440 (23) |
| Second | 68 (16) | 355 (17) | 680 (20) | 55 (15) | 1158 (18) |
| Third | 11 (3) | 253 (12) | 399 (12) | 27 (7) | 690 (11) |
| Unknown | 65 (16) | 375 (17) | 740 (21) | 132 (35) | 1312 (21) |
| Maternal characteristics–treatment and nontreponemal titers | |||||
| Treatmentb | |||||
| None | 304 (73) | 1433 (67) | 1814 (53) | 255 (68) | 3844 (60) |
| Adequate | 17 (4) | 82 (4) | 684 (20) | 34 (9) | 809 (13) |
| Inadequate | 67 (16) | 630 (29) | 916 (27) | 86 (23) | 1697 (27) |
| Unknown | 30 (7) | 0 (0) | 29 (1) | 2 (1) | 33 (1) |
| Nontreponemal titerc | |||||
| ≤1:4 | 45 (11) | 553 (26) | 1273 (37) | 127 (34) | 1998 (31) |
| 1:8–1:32 | 146 (35) | 844 (39) | 1426 (41) | 144 (38) | 2560 (40) |
| 1:64–1:256 | 200 (48) | 660 (31) | 587 (17) | 78 (21) | 1525 (24) |
| >1:256 | 25 (6) | 76 (4) | 123 (4) | 21 (6) | 245 (4) |
| Unknown | 2 (0) | 12 (1) | 34 (1) | 7 (2) | 55 (1) |
| Infant characteristics | |||||
| Gestational age at birth, wk | |||||
| ≥37 | 43 (10) | 1353 (63) | 2504 (73) | 202 (54) | 4102 (64) |
| 32–36 | 103 (25) | 535 (25) | 542 (16) | 71 (19) | 1251 (20) |
| 28–31 | 114 (27) | 117 (5) | 81 (2) | 12 (3) | 324 (5) |
| <28 | 132 (32) | 37 (2) | 56 (2) | 8 (2) | 233 (4) |
| Unknown | 26 (6) | 103 (5) | 260 (8) | 84 (22) | 473 (7) |
| Birthweight, g | |||||
| ≥2500 | 45 (11) | 1408 (66) | 2592 (75) | 218 (58) | 4263 (67) |
| 1500–2499 | 111 (27) | 528 (25) | 513 (15) | 65 (17) | 1217 (19) |
| <1500 | 164 (39) | 116 (5) | 125 (4) | 18 (5) | 423 (7) |
| Unknown | 98 (23) | 93 (4) | 213 (6) | 76 (20) | 480 (8) |
In descending order of severity: stillborn infants and infants who died up to 12 mo after birth (dead); infants with strong evidence of syphilis, based on testing indicative of syphilis, physical signs or symptoms of syphilis, and/or (in mother untreated or inadequately treated for syphilis) elevated cerebrospinal fluid (CSF) white blood cell count or CSF protein concentration absent other causes (see “Materials and Methods”) (morbid); infants lacking evidence of infection present in morbid cases, with normal physical examination reported (nonmorbid); and infants lacking evidence of infection present in morbid cases, without normal physical examination reported (unknown).
US regions as defined by Census Bureau;
“Adequate” treatment was defined as penicillin treatment appropriate for mother’s stage of infection administered ≥30 d before delivery;
Nontreponemal titer nearest to delivery.
Dead cases, overall case fatality ratio, and neonatal mortality rate
Of the 418 dead cases of CS reported during 1999 through 2013, 342 (81.8%) were stillbirths, 70 died <28 days after delivery (ie, were neonatal deaths), and 6 died 1-12 months after birth. Of the 70 neonatal deaths, 29 were within 1 day of birth, 23 between 2-7 days after birth, and 18 between 8-27 days after birth. The overall case fatality ratio was 6.5% (418 deaths among 6383 cases of CS); overall neonatal mortality among reported cases of CS was 11.6 per 1000 live births (70 neonatal deaths per 6041 live-born cases of CS).
Trends in rates of dead, morbid, and nonmorbid cases of CS
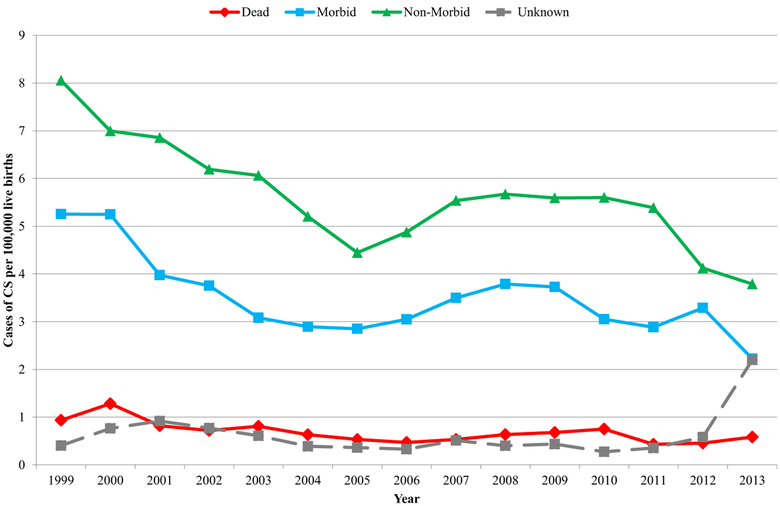
Overall, rates of dead, morbid, and nonmorbid cases of CS all declined during 1999 through 2013 (Figure 1). The rate of dead cases declined slightly and very gradually throughout 1999 through 2013, while rates of morbid and nonmorbid cases declined the most during 1999 through 2005. Dead cases comprised a median of 6.5% (range 4.7–9.0%) cases of CS annually. Morbid cases comprised a median of 34.7% (range 25.3–38.9%) cases of CS annually. No significant overall trends in proportions of either dead or morbid cases of CS were observed.
FIGURE 1. Rates of congenital syphilis (CS), by severity of disease–United States, 1999 through 2013.
In descending order of severity: stillborn infants and infants who died up to 12 months after birth (dead); infants with strong evidence of syphilis, based on testing indicative of syphilis, physical signs or symptoms of syphilis, and/or (in mother untreated or inadequately treated for syphilis) elevated cerebrospinal fluid (CSF) white blood cell count or CSF protein concentration absent other causes (see “Materials and Methods”) (morbid); infants lacking evidence of infection present in morbid cases, with normal physical examination reported (nonmorbid); and infants lacking evidence of infection present in morbid cases, without normal physical examination reported (unknown). Overall, rates of dead, morbid, and nonmorbid cases of CS all declined during 1999 through 2013. While rate of dead cases declined slightly and very gradually throughout 1999 through 2013, rates of morbid and nonmorbid cases declined most during 1999 through 2005.
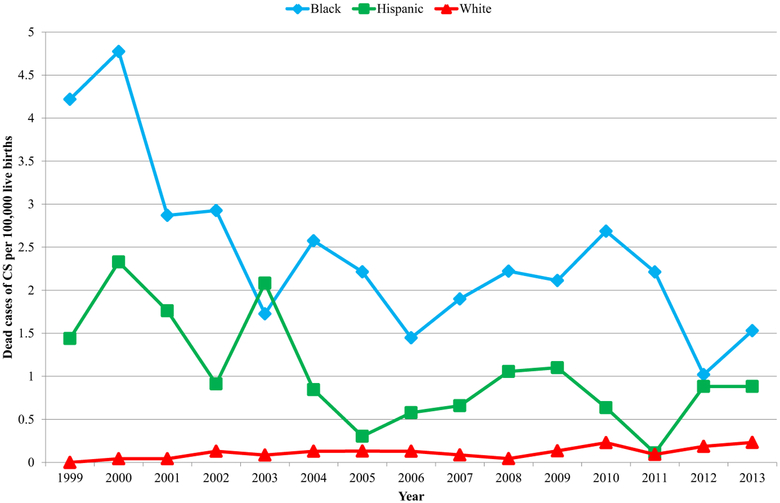
During 1999 through 2013, 5989 (94%) cases of CS occurred among black, Hispanic, and white mothers. Rates for dead cases of CS among black mothers and Hispanic mothers decreased during this time period, but remained relatively unchanged among white mothers (Figure 2). In 2013, the rate of dead, morbid, and nonmorbid cases of CS among black mothers was 7.5, 9.0, and 12.7 times the rate among white mothers, respectively; the rate of dead, morbid, and nonmorbid cases of CS among Hispanic mothers was 4.5, 3.5, and 2.9 times the rate among white mothers, respectively. Despite these racial/ethnic disparities, black mothers and Hispanic mothers of cases of CS were no more likely to bear dead or morbid cases of CS than white mothers of cases of CS (Table 2).
FIGURE 2. Rates of dead cases of congenital syphilis (CS), by race/ethnicity of mother–United States, 1999 through 2013.
Because cases of CS born to non-Hispanic black (black), non-Hispanic white (white), and Hispanic mothers accounted for 94% of reported cases of CS, this chart focuses on these 3 races/ethnicities. During 1999 through 2013, rates of dead cases of CS decreased among black mothers and Hispanic mothers, but remained relatively unchanged among white mothers. In 2013, rate of dead cases of CS among black mothers and Hispanic mothers was 7.5 and 4.5 times rate among white mothers, respectively.
TABLE 2.
Selected demographic and clinical characteristics of infants with congenital syphilis and their mothers and relative risks, by severity of illness–United States, 1999 through 2013
| Maternal characteristics | ||||||
|---|---|---|---|---|---|---|
| Morbid and dead cases(%) |
Nonmorbid cases (%) |
Dead cases (%) |
Morbid and nonmorbid cases (%) |
|||
| n = 2563 | n = 3443 | RR (95% CI) | n = 418 | n = 5588 | RR (95% CI) | |
| Race/ethnicity | ||||||
| White | 300 (12) | 377 (11) | Ref | 38 (9) | 639 (11) | Ref |
| Black | 1343 (52) | 1749 (51) | 1.0 (0.9–1.1), P = .676 | 218 (52) | 2874 (51) | 1.3 (0.9–1.8), P = .178 |
| Hispanic | 783 (31) | 1083 (31) | 0.9 (0.9–1.0), P = .289 | 141 (34) | 1725 (31) | 1.3 (1.0–1.9), P = .090 |
| Asian/Pacific Islander | 53 (2) | 95 (3) | 0.8 (0.6–1.0), P = .058 | 7 (2) | 141 (3) | 0.8 (0.4–1.8), P = .668 |
| American Indian/Alaska Native | 31 (1) | 33 (1) | 1.1 (0.8–1.4), P = .526 | 5 (1) | 59 (1) | 1.4 (0.6–3.4), P = .472 |
| Other | 10 (0) | 27 (1) | 0.6 (0.4–1.0), P = .039 | 1 (0) | 36 (1) | 0.5 (0.1–3.4), P = .448 |
| Unknown | 43 (2) | 79 (2) | 8 (2) | 114 (2) | ||
| Prenatal visits, no. | ||||||
| 0/No prenatal care | 889 (35) | 794 (23) | 1.5 (1.4–1.7), P < .001 | 199 (48) | 1484 (27) | 5.8 (3.6–9.4), P < .001 |
| 1–4 | 461 (18) | 551 (16) | 1.3 (1.2–1.5), P < .001 | 84 (20) | 928 (17) | 4.1 (2.5–6.7), P < .001 |
| 5–9 | 374 (15) | 570 (17) | 1.2 (1.0–1.3), P = .015 | 39 (9) | 905 (16) | 2.0 (1.2–3.5), P = .010 |
| ≥10 | 302 (12) | 583 (17) | Ref | 18 (4) | 867 (16) | Ref |
| Unknown | 537 (21) | 945 (27) | 78 (19) | 1404 (25) | ||
| Treatmenta | ||||||
| None | 1775 (69) | 1814 (53) | 4.2 (3.5–5.1), P < .001 | 342 (82) | 3247 (58) | 8.2 (4.3–15.8), P < .001 |
| Adequate | 91 (4) | 684 (20) | Ref | 9 (2) | 766 (14) | Ref |
| Inadequate | 695 (27) | 916 (27) | 3.7 (3.0–4.5), P < .001 | 65 (16) | 1546 (28) | 3.5 (1.7–6.9), P < .001 |
| Unknown | 2 (0) | 29 (1) | 2 (0) | 29 (1) | ||
| Nontreponemal titerb | ||||||
| ≤1:4 | 598 (23) | 1273 (37) | Ref | 45 (11) | 1826 (33) | Ref |
| 1:8–1:32 | 990 (39) | 1426 (41) | 1.3 (1.2–1.4), P < .001 | 146 (35) | 2270 (41) | 2.5 (1.8–3.5), P < .001 |
| 1:64–1:256 | 860 (34) | 587 (17) | 1.9 (1.7–2.0), P < .001 | 200 (48) | 1247 (22) | 5.7 (4.2–7.9), P < .001 |
| >1:256 | 101 (4) | 123 (4) | 1.4 (1.2–1.7), P < .001 | 25 (6) | 199 (4) | 4.6 (2.9–7.4), P < .001 |
| Unknown | 14 (1) | 34 (1) | 2 (0) | 46 (1) | ||
| Infant characteristics | ||||||
| Morbid and dead cases(%) |
Nonmorbid cases (%) |
Dead cases (%)c |
Morbid and nonmorbid cases (%) |
|||
| n = 2221 | n = 3443 | RR (95% CI) | n = 76 | n = 5588 | RR (95% CI) | |
| Gestational age at birth, wk | ||||||
| ≥37 | 1362 (61) | 2504 (73) | Ref | 9 (12) | 3857 (69) | Ref |
| 32–36 | 550 (25) | 542 (16) | 1.4 (1.3–1.5), P < .001 | 15 (20) | 1077 (19) | 5.9 (2.6–13.4), P < .001 |
| 28–31 | 135 (6) | 81 (2) | 1.8 (1.6–2.0), P < .001 | 18 (24) | 198 (4) | 35.8 (16.3–78.7), P < .001 |
| <28 | 68 (3) | 56 (2) | 1.6 (1.3–1.8), P < .001 | 31 (41) | 93 (2) | 107.4 (52.3–220.7), P < .001 |
| Unknown | 106 (5) | 260 (8) | 3 (4) | 36 (6) | ||
| Birthweight, g | ||||||
| ≥2500 | 1421 (64) | 2592 (75) | Ref | 13 (17) | 4000 (72) | Ref |
| 1500–2499 | 544 (24) | 513 (15) | 1.5 (1.4–1.6), P < .001 | 16 (21) | 1041 (19) | 4.7 (2.3–9.7), P < .001 |
| <1500 | 156 (7) | 125 (4) | 1.6 (1.4–1.8), P < .001 | 40 (53) | 241 (4) | 43.9 (23.8–81.2), P < .001 |
| Unknown | 100 (5) | 213 (6) | 7 (9) | 306 (5) | ||
In descending order of severity: stillborn infants and infants who died up to 12 mo after birth (dead); infants with strong evidence of syphilis, based on testing indicative of syphilis, physical signs or symptoms of syphilis, and/or (in mother untreated or inadequately treated for syphilis) elevated cerebrospinal fluid (CSF) white blood cell count or CSF protein concentration absent other causes (see “Materials and Methods”) (morbid); infants lacking evidence of infection present in morbid cases, with normal physical examination reported (nonmorbid). CI, confidence interval; RR, relative risk.
“Adequate” treatment was defined as penicillin treatment appropriate for mother’s stage of infection administered ≥30 days before delivery;
Nontreponemal titer nearest to delivery;
Only dead cases that were born alive, but later died, were included (ie, stillbirths were excluded).
Maternal characteristics and risk of infant morbidity and mortality
Maternal characteristics associated with risk of morbidity and mortality from CS included number of prenatal care visits, adequacy of treatment, and non-treponemal titer (Table 2). Compared to mothers with ≥10 prenatal visits, mothers with fewer visits were more likely to bear a morbid or dead case. The likelihood of bearing a dead case increased as prenatal visits decreased (χ2 for trend P < .001); this trend remained significant after limiting consideration to dead cases that were live-born but later died (eg, no stillbirths) (data not shown). Compared to mothers receiving adequate treatment (penicillin appropriate for stage of infection, administered ≥30 days before delivery), mothers receiving inadequate treatment or no treatment were more likely to bear a morbid or dead case. Compared to mothers with a nontreponemal titer of ≤1:4, mothers with titers of ≥1:8 were more likely to bear a morbid or dead case. The likelihood of bearing a morbid or dead case increased with titer (χ2 for trend P < .001).
Infant characteristics associated with mortality
Most infants with CS (64%) were born at ≥37 weeks’ gestation (Table 1); most infants (67%) were born weighing ≥2500 g. Because prematurity and low birthweight are common among stillbirths,16 birthweights and gestational ages of dead cases that were not stillbirths (ie, that were born alive but later died), morbid cases, and nonmorbid cases were compared (Table 2). Compared to infants born at ≥37 weeks’ gestation, infants born at ≤36 weeks’ gestation were more likely to be dead cases; as gestational age decreased, risk of death increased (χ2 for trend P < .001). Compared to infants weighing ≥2500 g at birth, infants weighing <2500 g at birth were more likely to be dead cases; as birthweight decreased, risk of death increased (χ2 for trend P < .001).
Among live-born cases of CS born at <37 weeks’ gestation, the neonatal mortality rate of CS was 38.1 per 1000 live births (58 neonatal deaths/1522 live-born cases of CS born at <37 weeks’ gestation). Among live-born cases of CS born at <2500 g at birth, the neonatal mortality rate of CS was 35.2 per 1000 live births (50 neonatal deaths/1419 live-born cases of CS born at <2500 g at birth).
Sensitivity analysis to explore effect of infants of unknown morbidity
After reclassifying all cases of CS of unknown morbidity as nonmorbid, the associations with maternal characteristics and infant characteristics in Table 2 remained unchanged. After reclassifying all cases of CS of unknown morbidity as morbid, the RR of delivering a morbid or dead case among mothers with no (RR, 3.4) or inadequate (RR, 3.0) treatment for syphilis (compared to mothers with adequate treatment) were reduced, but remained statistically significant. There were no other changes in significance of association.
Comment
Reducing CS (and infant death associated with it) in the United States remains a challenge. Overall case fatality ratios for CS during 1999 through 2013 (6.5%) and 1992 through 1998 (6.4%)15 were comparable, as were overall neonatal mortality rates (11.6 per 1000 live births during 1999 through 2013, 11.3 per 1000 births during 1992 through 1998).15 Most cases of CS (83%) during 1999 through 2013 still occurred among black or Hispanic women, with over half of cases of CS reported from the South in 2013.14 Almost half of cases of CS with normal physical examinations (eg, nonmorbid cases) are infected with CS based on the presence of treponemal IgM antibodies18; treatment guidelines recommend treating infants at risk for, but without symptoms of, CS.19 Continued efforts to prevent CS are warranted, including early detection and treatment of syphilis during pregnancy, particularly among women of color.
The decreases in rates of dead, morbid, and nonmorbid cases during 1999 through 2013 (Figure 1) reflect the decreased overall rate of CS during this time period.14 Similarly, the racial/ethnic disparities in rates of dead cases and morbid cases reported here approximate the racial/ethnic disparities in the overall rates of CS in 2013.14 However, the lack of association between maternal race/ethnicity of mothers bearing cases of CS and risk of a morbid case or dead case of CS (Table 2) indicate other characteristics (eg, lack of adequate treatment, low birthweight) are more strongly associated with the risk of a morbid or dead case of CS.
Many maternal and infant characteristics associated with dead cases of CS during 1992 through 199815 were associated with dead cases of CS during 1999 through 2013 (Table 2). Current guidelines recommend a minimum of 10 visits for prenatal care in a 38-week gestation.20 The data here demonstrate that the likelihood of bearing a morbid or dead case of CS increased as the number of prenatal visits fell below this minimum (Table 2). Likewise, the likelihood of bearing a morbid or dead case of CS increased with inadequate or no treatment, and with increasing maternal nontreponemal titer. These observations underscore the importance of early access to prenatal care (ideally during first trimester), screening of all pregnant mothers for syphilis early in pregnancy, and timely and appropriate treatment of maternal syphilis to prevent infant morbidity and death from CS.
Extreme prematurity (<28 weeks’ gestation) and extremely low birth-weight (<1500 g) were the strongest risks for a dead case of CS among cases of CS born alive (Table 2). Previous studies demonstrate that CS is associated with prematurity and low birthweight in infants.2,21 Controlled studies have shown that, compared to pregnant women with nonreactive nontreponemal tests, both prematurity and reduced birthweight occurred more frequently in pregnant women with untreated syphilis22-24; with appropriate treatment of syphilis during pregnancy, frequencies of prematurity and reduced birthweight were comparable to pregnant women with nonreactive nontreponemal tests.24 The data here indicate that the likelihood of death in a live-born infant with CS increases considerably with both the severity of prematurity and the severity of reduced birthweight. These data further highlight the need for detection and treatment of syphilis early in pregnancy.
A complex network of overlapping systems, social structures, and economic conditions influence population health outcomes, causing gaps in care.7,9,11,12 In 2009, among 29 states of the United States, 1 in 4 women delivering an infant lacked health insurance when they became pregnant.25 Prenatal care providers can be unaware of both the (high) local prevalence of syphilis, and screening and treatment recommendations for syphilis.12 Single-parent, female-headed households are likely to have young, undereducated mothers, social and familial instability, gender power imbalances, and poverty.11 Any of these factors could complicate attending prenatal visits, or referral for treatment.
Prenatal care among mothers bearing cases of CS is dramatically different from the norm. In 2010, among all US women giving birth, 57% had at least 10 prenatal visits and only 1.2% had no prenatal care26; 88% of infants were at ≥37 weeks’ gestation, and 92% weighed ≥2500 g at birth.27 In 2010, the neonatal mortality rate for infants born in the United States was 4.1 per 1000 live births.27 In stark contrast, >20 times as many cases of CS during 1999 through 2013 had no prenatal care, with a neonatal mortality rate almost 3 times the national rate in 2010 (Table 1). Detecting infection with syphilis in preconception or early pregnancy could avert many cases of, and most deaths from, CS.
This analysis has limitations. Because surveillance for CS is a passive reporting system,14 cases of CS in the United States might be underreported. Only 15% of nonmorbid cases had both CSF and long bone radiographic examinations. With further laboratory and radiographic examination, some nonmorbid cases might have had positive findings, and been classified as morbid cases. If laboratory and radiographic examination is differentially associated with maternal or infant characteristics, the estimated risks associated with morbidity might be biased. However, the sum of morbid and nonmorbid cases would remain unchanged, and risks associated with infant death would also remain unchanged. Morbidity was unknown for 377 (6%) cases, but reclassifying all of these cases as either morbid or non-morbid led to almost no change in associated risks. Lastly, case report data do not include social factors pertinent to understanding maternal risk and quality of health care.7,9,11,12
As rates of syphilis among females approach historic lows, so do rates of CS, with rates of CS in the United States in 2013 near historic lows.14 Nonetheless, each CS case is a preventable cause of infant morbidity and death in the United States. Detecting and treating pregnant mothers with syphilis by 21 weeks’ gestation could avert 70% of all mortality from CS.15 Prenatal and public health providers in communities with high rates of CS (and high rates of syphilis among reproductive-aged women) should be aware of CS and screen and treat patients early in pregnancy in accordance with treatment guidelines.19 Efforts to prevent syphilis among women, particularly of reproductive age, should continue. Local barriers to accessing quality prenatal care (eg, early screening and treatment for syphilis), and to broader sexually transmitted disease and reproductive health interventions, should be identified and addressed in these same communities.
Acknowledgments
Dr Brooks was supported by the Centers for Disease Control and Prevention (CDC) Experience Fellowship, which had no involvement in this analysis. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Footnotes
The authors report no conflict of interest.
Contributor Information
John R. Su, Division of Sexually Transmitted Disease Prevention, Centers for Disease Control and Prevention, Atlanta, GA.
Lesley C. Brooks, Sunrise Monfort Community Health Center Greeley, CO; North Colorado Health Alliance, Greeley, CO.
Darlene W. Davis, Division of Sexually Transmitted Disease Prevention, Centers for Disease Control and Prevention, Atlanta, GA
Elizabeth A. Torrone, Division of Sexually Transmitted Disease Prevention, Centers for Disease Control and Prevention, Atlanta, GA.
Hillard S. Weinstock, Division of Sexually Transmitted Disease Prevention, Centers for Disease Control and Prevention, Atlanta, GA.
Mary L. Kamb, Division of Sexually Transmitted Disease Prevention, Centers for Disease Control and Prevention, Atlanta, GA.
References
- 1.Gomez GB, Kamb ML, Newman LM, Mark J, Broutet N, Hawkes SJ. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ 2013;91:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingraham NR Jr. The value of penicillin alone in the prevention and treatment of congenital syphilis. Acta Derm Venereol Suppl (Stockh) 1950;31(Suppl):60–87. [PubMed] [Google Scholar]
- 3.Alexander JM, Sheffield JS, Sanchez PJ, Mayfield J, Wendel GD Jr. Efficacy of treatment for syphilis in pregnancy. Obstet Gynecol 1999;93:5–8. [DOI] [PubMed] [Google Scholar]
- 4.Calonge N US Preventive Services Task Force. Screening for syphilis infection: recommendation statement. Ann Fam Med 2004;2:362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.St Lawrence JS, Montano DE, Kasprzyk D, Phillips WR, Armstrong K, Leichliter JS. STD screening, testing, case reporting, and clinical and partner notification practices: a national survey of US physicians. Am J Public Health 2002;92:1784–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeNavas-Walt C, Proctor BD. US Census Bureau, current population reports, P60-249, income and poverty in the United States: 2013. Washington (DC): US Government Printing Office; 2014. [Google Scholar]
- 7.Frieden TR; Centers for Disease Control and Prevention. CDC health disparities and inequalities report–United States, 2013. MMWR Surveill Summ 2013;62(Suppl):1–2. [PubMed] [Google Scholar]
- 8.Harling G, Subramanian S, Barnighausen T, Kawachi I. Socioeconomic disparities in sexually transmitted infections among young adults in the United States: examining the interaction between income and race/ethnicity. Sex Transm Dis 2013;40:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marmot M Social determinants of health inequalities. Lancet 2005;365:1099–104. [DOI] [PubMed] [Google Scholar]
- 10.Risser WL, Hwang LY. Congenital syphilis in Harris County, Texas, USA, 1990-92: incidence, causes and risk factors. Int J STD AIDS 1997;8:95–101. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe TT, Voute C, Rose MA, Cleveland J, Dean HD, Fenton K. Social determinants of HIV/AIDS and sexually transmitted diseases among black women: implications for health equity. J Womens Health (Larchmt) 2012;21:249–54. [DOI] [PubMed] [Google Scholar]
- 12.Warner L, Rochat RW, Fichtner RR, Stoll BJ, Nathan L, Toomey KE. Missed opportunities for congenital syphilis prevention in an urban southeastern hospital. Sex Transm Dis2001;28:92–8. [DOI] [PubMed] [Google Scholar]
- 13.Williams PB, Ekundayo O. Study of distribution and factors affecting syphilis epidemic among inner-city minorities of Baltimore. Public Health 2001;115:387–93. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2013. Atlanta (GA): US Department of Health and Human Services; 2014. [Google Scholar]
- 15.Gust DA, Levine WC, St Louis ME, Braxton J, Berman SM. Mortality associated with congenital syphilis in the United States, 1992-1998. Pediatrics 2002;109 E79–9. [DOI] [PubMed] [Google Scholar]
- 16.MacDorman MF, Kirmeyer SE, Wilson EC. Fetal and perinatal mortality, United States, 2006. Natl Vital Stat Rep 2012;60:1–22. [PubMed] [Google Scholar]
- 17.National Vital Statistics System. U.S. Census Populations With Bridged Race Categories. Available at: http://www.cdc.gov/nchs/nvss/bridged_race.htm#suggested. Accessed December 2, 2015.
- 18.Sanchez PJ, Wendel GD Jr, Grimprel E, et al. Evaluation of molecular methodologies and rabbit infectivity testing for the diagnosis of congenital syphilis and neonatal central nervous system invasion by Treponema pallidum. J Infect Dis 1993;167:148–57. [DOI] [PubMed] [Google Scholar]
- 19.Workowski KA, Berman S; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep 2010;59:1–110. [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics, American College of Obstetrics and Gynecology. Guidelines for perinatal care. 7th ed. Elk Grove Village (IL); 2012. [Google Scholar]
- 21.Lago EG, Vaccari A, Fiori RM. Clinical features and follow-up of congenital syphilis. Sex Transm Dis 2013;40:85–94. [DOI] [PubMed] [Google Scholar]
- 22.Gichangi P, Renterghem LV, Karanja J, Bwayo J, Kiragu D, Temmerman M. Congenital syphilis in a Nairobi maternity hospital. East Afr Med J 2004;81:589–93. [PubMed] [Google Scholar]
- 23.Temmerman M, Gichangi P, Fonck K, et al. Effect of a syphilis control program on pregnancy outcome in Nairobi, Kenya. Sex Transm Infect 2000;76:117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson-Jones D, Oliff M, Terris-Prestholt F, et al. Antenatal syphilis screening in sub-Saharan Africa: lessons learned from Tanzania. Trop Med Int Health 2005;10:934–43. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Pregnancy Risk Assessment Monitoring System (PRAMS) Report on CDC’s Winnable Battles: Collecting Data in Order to Improve the Health of Mothers and Infants. Available at: http://www.cdc.gov/prams/pramsreport.html. Accessed December 2, 2015. [Google Scholar]
- 26.Centers for Disease Control and Prevention. National Center for Health Statistics. VitalStats. Available at: http://www.cdc.gov/nchs/data_access/vitalstats/VitalStats_Births.htm. Accessed December 2, 2015.
- 27.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2009 period linked birth/infant death data set. Natl Vital Stat Rep 2013;61:1–27. [PubMed] [Google Scholar]