Abstract
OBJECTIVE
Comorbid depression is associated with increased health care utilization and cost. We examined the effects of peer support on acute care (AC) and hospital utilization in individuals with diabetes with or without depressive symptoms.
RESEARCH DESIGN AND METHODS
This was a cluster-randomized controlled trial conducted in 2010–2012, with the clusters being practices and their surrounding communities. Adults with type 2 diabetes who wanted help with self-management were eligible to participate. Those without a doctor, with limited life expectancy, with plans to move within the next year, and with an unwillingness to work with a peer advisor were excluded. Intervention participants received 1 year of peer support. Control participants received usual care. The Patient Health Questionnaire (PHQ-8) (range 0–24; 5 indicates mild and 10 indicates moderate depressive symptoms) assessed depressive symptoms. AC and hospital utilization were measured by self-report. Data were collected at baseline, 6 months, and 12 months. Quasi-Poisson regression using generalized estimating equations examined differences in utilization per year attributable to the intervention for those with and without mild depressive symptoms (and separately, moderate depressive symptoms), controlling for imbalance across treatment arms.
RESULTS
At baseline, half of the sample reported mild depressive symptoms (52% intervention and 48% control, P = 0.37), a quarter reported moderate depressive symptoms (25% intervention and 26% control, P = 1.0), and there were no significant differences in utilization. A total of 168 intervention (six clusters) and 187 control (five clusters) participants had follow-up data. In individuals with mild depressive symptoms, the incident rate ratio (IRR) for hospitalization among intervention compared with control was 0.26 (95% CI 0.08–0.84) per 10 patient-years. The IRR for AC was 0.55 (95% CI 0.28–1.07) per 10 person-years. Findings were similar for individuals with moderate depressive symptoms.
CONCLUSIONS
Peer support lowered AC visits and hospitalizations for individuals with depressive symptoms but not for those without depressive symptoms; these findings can guide resource allocation for population health management.
Introduction
Recent and impending changes related to reimbursement for health care services in the U.S. have led to an increasing interest in population health, defined as the health outcomes of a group of individuals (1). With bundled payments, the emergence of accountable care organizations, and risk sharing, health systems are seeking disseminatable, cost-effective strategies to improve outpatient chronic disease management and reduce preventable acute care (AC) utilization and hospitalizations (2,3). There are currently 29.1 million individuals living with diabetes in the U.S. (4), and the prevalence, disease-related complications, and costs continue to rise (4,5). The annual cost attributed to diabetes is $245 billion, with $176 billion in direct costs (4). A majority of these dollars are spent on AC visits, hospitalizations, and diabetes-related complications rather than prevention and self-management education and support (4). Utilization rates and costs are often higher for those with comorbid mental health disorders, including depression (6,7). Peer support and coaching can be an important bridge from clinic to community that could be used as a strategy for population health management, particularly for high-risk individuals (8), but few data report on the impact of peer support on health services utilization for people living with diabetes. The data that do exist suggest that peer support interventions are cost-effective, in part through reductions in high-cost health care utilization (9,10). However, these studies do not examine the impact on high-risk subgroups with diabetes, such as those with comorbid depression.
Peer support includes instrumental, emotional, and ongoing support from people living with a chronic condition such as diabetes, cancer, cardiovascular disease, mental illness, and HIV and has been shown to be important for sustaining healthy behaviors and self-care (11). In peer support interventions, support is provided by trained, lay individuals (often referred to as peer advisors, community health workers, or promotoras) who are trusted members of the community, know their communities’ needs and strengths, and can help translate evidence-based disease management strategies for implementation in community settings (12,13). In 2015, the American Diabetes Association (ADA) recognized emotional support as a critical component of comprehensive diabetes management, based on mounting evidence of its impact on health behavior, health outcomes, and cost and utilization (14). Recent standards of care published by the ADA recommend that patients be provided self-management support from lay health coaches, navigators, or community health workers when possible (15). Emerging data further suggest that peer support may be most potent for those at highest risk, such as individuals suffering from comorbid depression and stress (16,17).
In the context of a peer support trial conducted with individuals with type 2 diabetes living in rural southern Alabama, we describe the impact of peer support on AC and hospitalization for individuals with and without depressive symptoms.
Research Design and Methods
Setting and Participants
This study was conducted in eight counties (Choctaw, Dallas, Lowndes, Marengo, Perry, Pickens, Sumter, and Wilcox) located in southern rural Alabama, otherwise known as the Alabama Black Belt region. Alabama has a disproportionately high prevalence of diabetes, ranking first in 2016, with a rate of diagnosed diabetes just over 16% (18). The diabetes mortality rate in the Black Belt region in 2004 was ∼31.6 per 100,000 compared with 24.9 per 100,000 nationally (19). Despite the high burden, diabetes-related resources are scarce; at the time the study was implemented, there was a single certified diabetes educator covering all eight counties. Details of participant recruitment, intervention development, and the main study findings are detailed elsewhere (20–22). In brief, adults (>18 years of age) who had been told by a doctor or nurse they had diabetes and who wanted help with self-management were eligible to participate. Exclusions included not having a primary care provider, advanced illness with limited life expectancy, plans to move out of the area within the next year, and unwillingness to work with a peer advisor over the telephone. All participants provided written informed consent, and the University of Alabama at Birmingham Institutional Review Board approved the study protocol prior to initiation of study activities.
Study Design
The Evaluating Community Peer Advisors and Diabetes Outcomes in Rural Alabama (ENCOURAGE) study was a 1-year cluster-randomized community-based trial, conducted in 2010–2012 in the rural Alabama counties described above. The clusters were practices and their immediate surrounding communities, with participants nested within practice/community. The study findings are published elsewhere. In brief, 424 participants with diabetes were recruited in eight partnering communities via respondent-driven sampling (20). The trial was designed to provide 80% power to detect a clinically meaningful difference in HbA1c (0.4%) (23). Sample size calculations included a variance inflation factor to account for the cluster-randomized design and 20% attrition. Because of the nature of the study, participants and peer advisors were not blinded to trial arm assignment. The total number of reported contacts between a peer advisor and participant over the intervention period ranged from 0 to 58 contacts. The mean number of contacts was 13.3 (SD 8.1); 54 (32.1%) patients had 17 or more contacts, which was the number specified in the protocol. One hundred and fifteen (68.5%) had 10 or more contacts, and 14 (8.2%) had zero contacts. The analysis that included the 75% (n = 270) of participants followed up for 15 months showed a significant increase in patient activation in the intervention group. The analysis that included all participants who eventually completed follow-up (n = 360) revealed that intervention arm participants had significant differences in changes in systolic blood pressure (P = 0.047), BMI (P = 0.02), quality of life (QoL) (P = 0.003), diabetes distress (P = 0.004), and patient activation (P = 0.03), but not in HbA1c (P = 0.14) or LDL cholesterol (P = 0.97).
A separate set of analyses examined the impact of peer support on depressive symptoms and whether or not PS was more potent among individuals with higher levels of depressive symptoms (17). In analyses of participants followed up for 15 months, depressive symptoms improved for both intervention and control participants; however, after 15 months of follow-up, there was a significant but nonlinear difference between intervention participants and control participants (P = 0.01). In stratified analyses for additional outcomes, those with Patient Health Questionnaire (PHQ-8) ≥5 lost weight (P = 0.03) and had improved QoL (P = 0.04) but had unchanged HbA1c. Those with PHQ-8 <5 also had unchanged HbA1c and lost weight but did not improve QoL (P = 0.06).
Peer Support Intervention
Participants randomized to the intervention met with a peer advisor for an initial 45- to 60-min session in person or over the telephone to get to know each other, to go over the participant’s personalized diabetes report card, and to select a personal self-management goal. All peer advisors were recruited from the same communities as participants, had to have type 2 diabetes themselves, or had to care for someone in their family who had type 2 diabetes. Peer advisors completed a 2-day training that included information on the basics of diabetes, healthy eating, physical activity, motivational interviewing and communication, community resources, ethics of research, and the study protocol and expectations. The study-specific training program is described elsewhere and was developed with input from community partners and stakeholders (22). The number of participants paired with a peer advisor depended on the peer’s preference and availability and ranged from 2 to 14 participants. On average, peer coaches were paired with six to seven participants. Once paired with a study participant, peer advisors called participants on the telephone weekly for the first 2 months and then at least monthly for an additional 8 months to coach the participant to work toward their goal and to provide social and emotional support. Because the intervention was designed to enhance patient activation, contacts were largely unstructured and highly individualized to focus on the goal selected by the participant. Peer advisors kept a one-page contact log that included who initiated the call, the length of the contact, and if the contact was scheduled in advance. The form also had questions to assess if the patient had medication problems that were discussed during the contact, a review of previously set goals, and a section to set new goals around exercise, diet, stress management, and talking to the doctor. Intervention fidelity was monitored through review of the contact forms as well as weekly phone calls with the peer advisors and a random selection of intervention participants.
Participants in both the intervention and control arms received a 1-h group diabetes education class at the time of enrollment. The class covered diabetes basics, healthy eating, stress reduction, physical activity, social support, and how to get the most out of their doctor visit.
Measures
At baseline and follow-up (12 months), all participants also completed a detailed in-person interview. The interviews were administered by trained, certified, and quality-controlled staff.
Dependent Variables
Baseline diabetes, AC, and hospital utilization were measured by participant self-report using the following questions: During the last 6 months, 1) how many times have you visited a diabetes doctor or nurse practitioner, 2) how many times have you visited other doctors or nurse practitioners, 3) how many times have you visited the emergency room/AC, and 4) how many overnight stays have you had in a hospital (related to your diabetes)? Participants were asked to provide a number for each. At 12 months, the stem was changed to read “since your last study visit….” Although the questions are self-reported, self-reported measures of utilization have been shown to have good agreement with administrative data, particularly for AC utilization (24).
Independent Variables
We tested sociodemographic characteristics of study participants by study arm for statistically significant differences, and those with a P value <0.10 were included in the models. Sociodemographic variables included age (operationalized as a continuous variable), race/ethnicity (self-reported; black or white), sex, education (less than high school, high school or equivalency, or more than high school), annual household income (< and >$40,000), the total number of medications taken as assessed by pill bottle review, and whether the participant used insulin.
Depressive symptoms were assessed with PHQ-8 (range 0–24; 5 indicates mild and 10 indicates moderate depressive symptoms). Reliability and validity have been well established (25). The PHQ-8 eliminates the question on suicidal ideation that is included in the PHQ-9, but psychometric evaluations demonstrate that this elimination does not reduce the sensitivity or specificity of the screening score (26). As per the original validation work with the measure, scores of 1–4 are interpreted to indicate no depressive symptoms, 5–9 indicate mild severity, and 10 or greater indicate moderate to severe depressive symptoms (25).
Analysis
Unadjusted differences in utilization were tested using independent-sample Student t tests. Quasi-Poisson regression was used to estimate incident rate ratios (IRRs) for utilization while allowing for overdispersion. For the main study, data 6 months, and 12 months. For the current study, we compared utilization reported at baseline with utilization reported at the final data collection. The time between follow-up and the previous study visit was included as an offset in the regression models. To control for variable lengths of time during which utilization could be reported, the time between follow-up and the previous study visit was included as an offset in the regression models so that results can be interpreted as rates of utilization per unit time. When available, this interval was the time between the 1-year and 6-month follow-up. For patients who did not have in-person data collection at 6 months, the interval was from baseline to follow-up. Models controlled for income and education, and the intervention effect was tested by the significance of the coefficient for study group (intervention vs. control) at the P < 0.05 level. Models including adjustment for race and sex failed to converge. Separate models including all participants without stratification by depressive symptoms included terms to test for an interaction between treatment group and PHQ-8 ≥5 and for an interaction between treatment group and PHQ-8 ≥10. There was a significant interaction with the quasi-Poisson regression for AC usage by PHQ-8 ≥10 (P = 0.047). Using generalized estimating equation (GEE) models, there were significant interactions for AC usage by PHQ-8 ≥10 (P = 0.042), inpatient by PHQ-8 ≥5 (P = 0.02), and inpatient by PHQ-8 ≥10 (P = 0.095). Although the intraclass correlation among randomization clusters was not significant at the P < 0.05 level, sensitivity analysis used Poisson regression with GEEs to account for the clustered sample. Results from the GEE modeling had a narrower CI and were less conservative than the quasi-Poisson models. Sensitivity analyses considered additional methods to operationalize the time between follow-up and last study visit as well as the inclusion of additional covariates to explain the associations observed in the main analysis.
Results
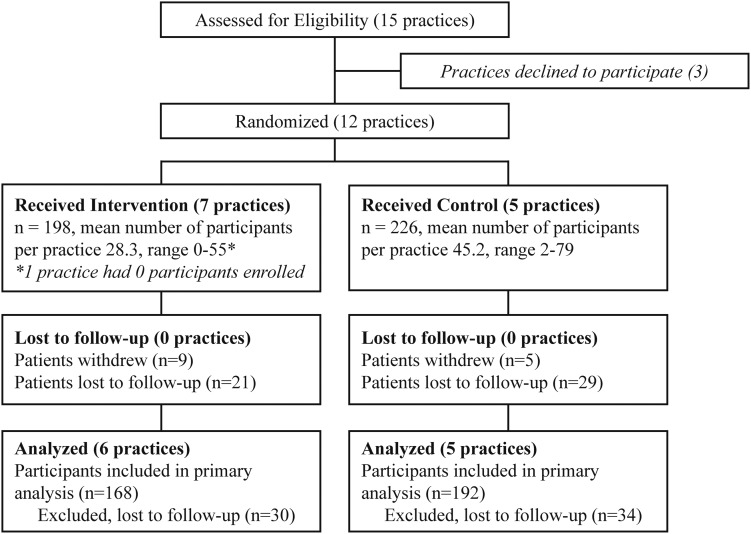
Of 424 participants enrolled in the study, 360 completed follow-up with an attrition rate of 15.1% (Fig. 1). There were no significant differences at baseline in sociodemographics or baseline measures of study outcomes between those with and without follow-up, although there was a trend toward greater depressive symptoms for those without follow-up (21). Follow-up data were available for 360 participants (168 intervention and 187 control) (Table 1). Mean age was 60 years (SD 12), and participants were mostly African American (87%) and female (75%). Mean HbA1c was 7.9%, and participants reported an average of 13.3 years living with diagnosed diabetes. Half of participants had PHQ >5 (52% of intervention and 48% of control participants, P = 0.37) and one-fourth had PHQ >10 (25% of intervention and 26% of control participants, P = 1.0) (data not shown). At baseline, there were few differences between intervention and control group participants. For those with PHQ-8 <5, intervention participants were younger (59.5 vs. 63.2 years, P = 0.046) and more likely to be African American (93.7% vs. 77.6%, P = 0.003) compared with control participants. Among those with PHQ-8 >5, intervention participants were more likely to be African American (95.5% vs. 84.3%, P = 0.01) compared with control participants. There were no significant differences in utilization at baseline between intervention and control participants above or below PHQ-8 of 5 (Table 1). Comparing all individuals (intervention plus control) with PHQ-8 >5 at baseline with individuals with PHQ-8 <5, those with PHQ-8 >5 reported more AC visits compared with those with PHQ-8 <5 (0.7 vs. 0.3, respectively, P < 0.001). However, there were no significant differences between individuals with PHQ-8 >5 versus those with PHQ-8 <5 for hospitalizations or other doctor visits (data not shown).
Figure 1.
Consort diagram.
Table 1.
Baseline characteristics by depressive symptoms (ENCOURAGE study)
| Overall |
Depressed (PHQ-8 ≥5) |
Not depressed (PHQ-8 <5) |
|||||
|---|---|---|---|---|---|---|---|
| n = 360 | Intervention (n = 88) | Control (n = 89) | P value | Intervention (n = 80) | Control (n = 98) | P value | |
| Black, n (%) | 313 (87.4) | 84 (95.5) | 75 (84.3) | 0.01 | 74 (93.7) | 76 (77.6) | 0.003 |
| Female, n (%) | 271 (75.3) | 69 (78.4) | 66 (74.2) | 0.51 | 62 (77.5) | 71 (72.5) | 0.44 |
| Education less than high school, n (%) | 111 (31.2) | 32 (37.2) | 29 (32.6) | 0.52 | 21 (26.3) | 27 (27.8) | 0.81 |
| Insulin therapy, n (%) | 142 (39.6) | 39 (44.2) | 43 (48.3) | 0.59 | 28 (35.0) | 29 (29.6) | 0.44 |
| Age, mean (SD), years | 60.2 (12.1) | 59.0 (11.3) | 58.8 (11.7) | 0.91 | 59.5 (12.4) | 63.2 (12.4) | 0.046 |
| HbA1c, mean (SD), % | 7.9 (2.0) | 8.0 (2.0) | 8.1 (1.9) | 0.76 | 8.0 (2.1) | 7.7 (1.8) | 0.24 |
| Time with diabetes, mean (SD), years | 13.3 (11.9) | 12.9 (11.6) | 12.5 (10.6) | 0.84 | 12.9 (11.4) | 13.8 (13.0) | 0.63 |
| BMI, mean (SD), kg/m2 | 36.3 (8.5) | 36.6 (6.8) | 37.2 (9.8) | 0.85 | 36.4 (8.6) | 35.0 (8.3) | 0.27 |
| Diastolic blood pressure, mean (SD), mmHg | 83.0 (12.9) | 85.0 (12.0) | 83.0 (12.6) | 0.27 | 82.2 (11.8) | 81.7 (14.7) | 0.81 |
| Systolic blood pressure, mean (SD), mmHg | 135.2 (21.4) | 136.9 (22.4) | 133.6 (21.1) | 0.32 | 132.1 (20.8) | 137.6 (21.0) | 0.08 |
| EuroQol Index, mean (SD) | 0.8 (0.2) | 0.6 (0.2) | 0.7 (0.2) | 0.05 | 0.8 (0.1) | 0.8 (0.1) | 0.82 |
| Health care utilization at baseline (in past 6 months), mean (SD) | |||||||
| How many times have you visited a diabetes clinician? | 1.5 (1.9) | 1.6 (2.1) | 1.5 (1.9) | 0.72 | 1.2 (1.6) | 1.5 (1.8) | 0.35 |
| How many times have you visited other clinicians? | 1.5 (2.1) | 1.4 (2.4) | 1.8 (2.0) | 0.28 | 1.2 (2.0) | 1.6 (2.2) | 0.22 |
| How many times have you visited the emergency room/AC? | 0.5 (1.0) | 0.7 (1.0) | 0.7 (1.3) | 0.83 | 0.2 (0.6) | 0.4 (0.7) | 0.22 |
| How many overnight stays have you had in hospital (related to your diabetes)? | 0.3 (1.5) | 0.2 (0.8) | 0.5 (1.6) | 0.16 | 0.3 (2.4) | 0.2 (0.7) | 0.64 |
Boldface type denotes significant differences between groups.
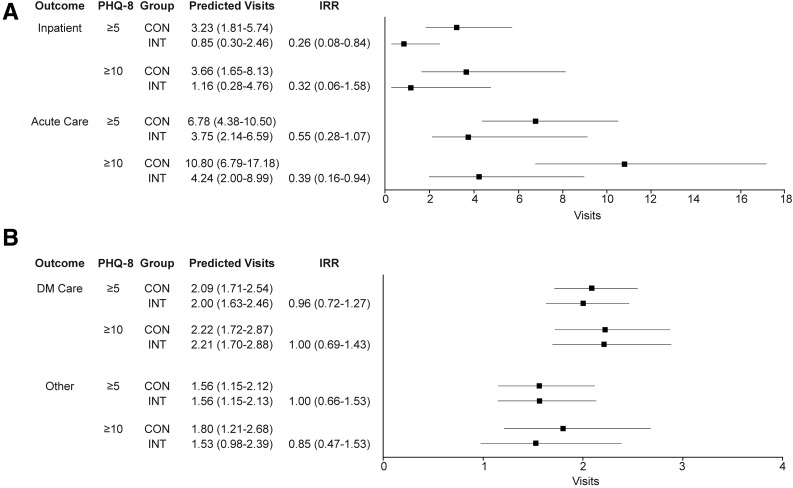
For intervention participants compared with control participants, the IRRs were 0.505 (0.21–1.21, P = 0.128) for hospitalizations, 0.699 (0.386–1.27, P = 0.238) for AC visits, and 0.983 (0.802–1.21, P = 0.87) for other visits (data not shown). For participants with PHQ-8 >5, compared with control participants, the IRR for hospitalizations among intervention participants was 0.26 (95% CI 0.08–0.84) per 10 patient-years, indicating 74% fewer visits at follow-up (Figs. 1 and 2A). The predicted number of hospitalizations for intervention participants was 0.85 (95% CI 0.30–2.46) per 10 person-years vs. 3.23 (95% CI 1.81–5.74) for control participants. This result corresponds to one hospitalization prevented on average per year for every 4.2 participants enrolled in the intervention. Compared with control participants, the IRR for AC visits by intervention participants was 0.55 (95% CI 0.28–1.07) per 10 person-years, again indicating fewer visits for intervention participants at follow-up. The predicted number of AC visits for intervention participants was 3.75 (95% CI 2.14–6.59) vs. 6.78 (95% CI 4.38–10.50) for control participants, or one AC visit prevented per year per three participants enrolled. Results were similar for participants with PHQ-8 >10, with intervention participants experiencing fewer hospitalizations and AC visits compared with control participants. No difference was seen between intervention and control participants among those with PHQ-8 <5 (IRR 1.04 [0.45–2.38] for AC and IRR 0.97 [0.26–3.65] for hospitalization). For diabetes-specific clinic visits or other clinic visits, there were no significant differences between intervention and control for participants with PHQ-8 >5 (Fig. 2B).
Figure 2.
Predicted usage per 10 patient-years. A: Hospitalizations and AC. B: Diabetes-specific or other clinic visits. CON, control; DM, diabetes; INT, intervention.
In an attempt to identify potential mechanisms for these findings, we also examined whether there was a dose-response relationship exposure to peer-coaching and utilization. Using linear regression, we found no association between peer-coaching contacts and utilization either overall or in groups stratified by PHQ-8 (all P > 0.20). We also considered whether change in PHQ-8 could explain the change in utilization. Although this was potentially problematic given that the utilization outcomes preceded the report of PHQ-8 at follow-up, which could confuse any causal relationship, we added change in PHQ-8 to our adjusted models for utilization. In each case where we had found a significant effect of the intervention on utilization, the effect remained significant after adjusting for change in PHQ-8. In fact, in every case (for each outcome, overall, and stratified by PHQ-8), the estimated effect of the intervention was slightly larger after adjustment for change in PHQ-8.
Conclusions
In this study of 360 mostly African American individuals living with type 2 diabetes in a rural low-income setting, individuals with minimal or greater depressive symptoms (PHQ-8 >5) randomized to a peer support intervention experienced fewer AC visits and hospitalizations compared with those with similar symptoms in the control condition. These trends were similar when examined using a PHQ-8 cut point of >10. There were no differences between intervention and control participants for diabetes clinic visits or other routine clinic visits. These findings are notable since almost half (49%) of the sample reported mild or greater symptoms of depression at baseline. Individuals scoring 5 or greater on the PHQ-8 (mild or greater depressive symptoms) were more likely to have experienced an AC visit in the last 6 months than those scoring <5 on PHQ-8.
In general, individuals with diabetes are more likely to use AC services. In 2012, Eby et al. (27) reported that more than a quarter of all hospitalizations in the U.S. were incurred by individuals with diabetes. Eby et al. (27) found that the presence of comorbid depression in the setting of chronic illness increases the likelihood that a patient will incur one or more emergency room visits and also increases the rate of hospitalizations and readmissions. In fact, patients with diabetes and comorbid depression have 4.5 times higher costs compared with individuals without comorbid depression in part due to increased health care utilization (28). These trends may underestimate the true impact since individuals with comorbid depression often go undiagnosed (29). And although studies have documented a positive correlation between depressive symptom severity and emergency department use, particularly for vulnerable populations such as low-income and elderly populations (6,30), the current study demonstrates that even individuals experiencing mild to moderate depression may have higher rates of utilization. This is not surprising since subclinical depression has been associated with worse diabetes self-care, worse medication adherence, and cost (6,31).
In the current study, reductions in hospitalizations and AC visits were noted for individuals with mild to moderate depressive symptoms despite the fact that the peer coaching was focused on diabetes self-management. Other studies examining peer support interventions for people with mental health disorders have also documented reductions in health services utilization. For example, Chan et al. (16) found similar results in a study of 628 Chinese adults with type 2 diabetes recruited from three publicly funded diabetes centers in Hong Kong. Chan et al. tested the impact of a telephone-based peer support intervention in the context of an ongoing integrated care quality improvement program. They found that the addition of peer support resulted in fewer hospitalizations and readmissions for individuals with high levels of distress. In another study, Sledge et al. (32) demonstrated a marked reduction in rehospitalizations and hospital days as a result of providing peer support to individuals with recurrent psychiatric hospitalizations. In the current study, the reduction in AC visits and hospitalizations was not associated with a concomitant reduction in depressive symptoms, and it was not dose dependent. This raises questions as to what the underlying mechanism might be. Mental health diagnoses are often seen in conjunction with low levels of family and social support, so it is plausible that the provision of emotional and instrumental support would have a direct beneficial effect on outcomes for individuals with diabetes and depression even if it does not improve depression itself. Future studies should incorporate measures of each component of peer support (emotional vs. instrumental, etc.) to better understand how peer support works to decrease AC utilization.
Nearly half of participants in the current study had mild to moderate depressive symptoms, falling within the range of rates seen in other studies (roughly 30–60%) (33). Because identification and treatment of comorbid depression is associated with better health outcomes and reduced costs, the ADA recommends routine psychosocial assessments and appropriate treatments and referral for individuals with diabetes (34). Unfortunately, individuals with chronic illness and comorbid depression often go undiagnosed and untreated, with one study finding 45% of patients with diabetes and depression to be undiagnosed for depression (35). The current study focused on a sample of mostly African American rural-dwelling individuals with type 2 diabetes. Studies show that racial minorities are less likely to be diagnosed with depression and, once diagnosed, less likely to be treated (36,37). Similarly, rural residents suffer a high burden related to depression. Although the prevalence of depression is only modestly higher in rural areas compared with urban areas, the rates of suicide are markedly higher (38,39). Further, the majority of rural-dwelling Americans live in areas with a shortage of mental health professionals, and evidence to date suggests that depressive symptoms often go unrecognized in primary care (39,40). Although published results from the current trial did not demonstrate a linear reduction in depressive symptoms as a result of peer support (17), other studies have done so. In a recent meta-analysis, Pfeiffer et al. (41) found that peer support interventions for depression were superior to usual care and as effective as group cognitive behavioral therapy. In one study of depressed patients with continued symptoms or functional impairment, Travis et al. (42) demonstrated a reduction in depressive symptoms using a peer-based telephone support intervention. If peer coaching can effectively treat mild to moderate depression in the context of chronic disease management, a door will be opened to the treatment of depression as an unintended consequence. This effect may be particularly useful in communities where mental health resources are sparse and where the stigma associated with mental health disorders and depression is a barrier to treatment-seeking behaviors, including rural African American communities (43).
Limitations and Strengths
This study did not include medical chart abstraction; thus, primary outcome measures of AC utilization and hospitalization were obtained through participant self-report. However, a recent systematic review found that self-reported measures of utilization generally had good agreement with administrative data, particularly for AC utilization (24). The study was also limited to a largely African American sample living in the rural south, and thus the results may not be generalizable to other populations. This limitation is also a strength, because African Americans and individuals living in rural communities are at higher risk for diabetes and its complications.
Conclusion
In the current study, the addition of peer support to community-based diabetes education resulted in a potent reduction in AC visits and emergency room utilization for individuals with depressive symptoms. As the U.S. population ages and the number of individuals with diabetes and comorbid depression increases, the burden on the health care system to provide care for these individuals also grows (5). Health care systems and providers must consider new strategies that simultaneously improve health outcomes and attend to the patient experience while managing costs (3). Peer support represents one strategy that has the potential to achieve each of these aims in the setting of diabetes and comorbid depression.
Article Information
Funding. Funding for this research was provided by the American Academy of Family Physicians Foundation through the Peers for Progress program with support from the Eli Lilly and Company Foundation and by the University of Alabama at Birmingham Diabetes Research Center by award number P30-DK-079626 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.L.C. researched data and wrote, reviewed, and edited the manuscript. Y.K. wrote, reviewed, and edited the manuscript. J.S.R. reviewed and edited the manuscript. S.J.A., C.G., and M.M.S. researched data and reviewed and edited the manuscript. A.L.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT02460718, clinicaltrials.gov.
References
- 1.Kindig D, Stoddart G. What is population health? Am J Public Health 2003;93:380–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casalino LP. Accountable care organizations: the risk of failure and the risks of success. N Engl J Med 2014;371:1750–1751 [DOI] [PubMed] [Google Scholar]
- 3.Stiefel M, Nolan K. Measuring the triple aim: a call for action. Popul Health Manag 2013;16:219–220 [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med 2000;160:3278–3285 [DOI] [PubMed] [Google Scholar]
- 7.Shim RS, Druss BG, Zhang S, et al. Emergency department utilization among Medicaid beneficiaries with schizophrenia and diabetes: the consequences of increasing medical complexity. Schizophr Res 2014;152:490–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokol R, Fisher E. Peer support for the hardly reached: a systematic review. Am J Public Health 2016;106:e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viswanathan M, Kraschnewski JL, Nishikawa B, et al. Outcomes and costs of community health worker interventions: a systematic review. Med Care 2010;48:792–808 [DOI] [PubMed] [Google Scholar]
- 10.Gillespie P, O’Shea E, Paul G, O’Dowd T, Smith SM. Cost effectiveness of peer support for type 2 diabetes. Int J Technol Assess Health Care 2012;28:3–11 [DOI] [PubMed] [Google Scholar]
- 11.Webel AR, Okonsky J, Trompeta J, Holzemer WL. A systematic review of the effectiveness of peer-based interventions on health-related behaviors in adults. Am J Public Health 2010;100:247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherrington A, Ayala GX, Elder JP, Arredondo EM, Fouad M, Scarinci I. Recognizing the diverse roles of community health workers in the elimination of health disparities: from paid staff to volunteers. Ethn Dis 2010;20:189–194 [PMC free article] [PubMed] [Google Scholar]
- 13.Swider SM. Outcome effectiveness of community health workers: an integrative literature review. Public Health Nurs 2002;19:11–20 [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association 4. Foundations of care: education, nutrition, physical activity, smoking cessation, psychosocial care, and immunization. In Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(Suppl. 1):S20–S30 [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association 1. Promoting health and reducing disparities in populations. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(Suppl. 1):S6–S10 [DOI] [PubMed] [Google Scholar]
- 16.Chan JC, Sui Y, Oldenburg B, et al.; JADE and PEARL Project Team . Effects of telephone-based peer support in patients with type 2 diabetes mellitus receiving integrated care: a randomized clinical trial. JAMA Intern Med 2014;174:972–981 [DOI] [PubMed] [Google Scholar]
- 17.Khodneva Y, Safford MM, Richman J, Gamboa C, Andreae S, Cherrington A. Volunteer peer support, diabetes, and depressive symptoms: results from the ENCOURAGE trial. J Clin Transl Endocrinol 2016;4:38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallup-Sharecare State of American Well-Being: 2017 State Well-Being Rankings. Available from https://wellbeingindex.sharecare.com/wp-content/uploads/2018/02/Gallup-Sharecare-State-of-American-Well-Being_2017-State-Rankings_FINAL.pdf?t=1518473023878. Accessed 4 October 2018
- 19.Alabama Department of Public Health. Selected Indicators of Health Status in Alabama. Montgomery, AL, Office of Primary Care and Rural Health, 2007 [Google Scholar]
- 20.Andreae SJ, Halanych JH, Cherrington A, Safford MM. Recruitment of a rural, southern, predominantly African-American population into a diabetes self-management trial. Contemp Clin Trials 2012;33:499–506 [DOI] [PubMed] [Google Scholar]
- 21.Safford MM, Andreae S, Cherrington AL, et al. Peer coaches to improve diabetes outcomes in rural Alabama: a cluster randomized trial. Ann Fam Med 2015;13(Suppl. 1):S18–S26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherrington A, Martin MY, Hayes M, et al. Intervention mapping as a guide for the development of a diabetes peer support intervention in rural Alabama. Prev Chronic Dis 2012;9:E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garber AJ. Treat-to-target trials: uses, interpretation and review of concepts. Diabetes Obes Metab 2014;16:193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leggett LE, Khadaroo RG, Holroyd-Leduc J, et al. Measuring resource utilization: a systematic review of validated self-reported questionnaires. Medicine (Baltimore) 2016;95:e2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64:258–266 [DOI] [PubMed] [Google Scholar]
- 26.Kroenke K, Spitzer RL, Williams JB, Löwe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry 2010;32:345–359 [DOI] [PubMed] [Google Scholar]
- 27.Eby E, Hardwick C, Yu M, et al. Predictors of 30 day hospital readmission in patients with type 2 diabetes: a retrospective, case-control, database study. Curr Med Res Opin 2015;31:107–114 [DOI] [PubMed] [Google Scholar]
- 28.Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care 2002;25:464–470 [DOI] [PubMed] [Google Scholar]
- 29.Hermanns N, Caputo S, Dzida G, Khunti K, Meneghini LF, Snoek F. Screening, evaluation and management of depression in people with diabetes in primary care. Prim Care Diabetes 2013;7:1–10 [DOI] [PubMed] [Google Scholar]
- 30.Choi NG, Marti CN, Bruce ML, Kunik ME. Relationship between depressive symptom severity and emergency department use among low-income, depressed homebound older adults aged 50 years and older. BMC Psychiatry 2012;12:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care 2007;30:2222–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sledge WH, Lawless M, Sells D, Wieland M, O’Connell MJ, Davidson L. Effectiveness of peer support in reducing readmissions of persons with multiple psychiatric hospitalizations. Psychiatr Serv 2011;62:541–544 [DOI] [PubMed] [Google Scholar]
- 33.Andreoulakis E, Hyphantis T, Kandylis D, Iacovides A. Depression in diabetes mellitus: a comprehensive review. Hippokratia 2012;16:205–214 [PMC free article] [PubMed] [Google Scholar]
- 34.Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2126–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C, Ford ES, Zhao G, Ahluwalia IB, Pearson WS, Mokdad AH. Prevalence and correlates of undiagnosed depression among U.S. adults with diabetes: the Behavioral Risk Factor Surveillance System, 2006. Diabetes Res Clin Pract 2009;83:268–279 [DOI] [PubMed] [Google Scholar]
- 36.Akincigil A, Olfson M, Siegel M, Zurlo KA, Walkup JT, Crystal S. Racial and ethnic disparities in depression care in community-dwelling elderly in the United States. Am J Public Health 2012;102:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alegría M, Chatterji P, Wells K, et al. Disparity in depression treatment among racial and ethnic minority populations in the United States. Psychiatr Serv 2008;59:1264–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh GK, Siahpush M. Increasing rural-urban gradients in US suicide mortality, 1970-1997. Am J Public Health 2002;92:1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Probst JC, Laditka SB, Moore CG, Harun N, Powell MP, Baxley EG. Rural-urban differences in depression prevalence: implications for family medicine. Fam Med 2006;38:653–660 [PubMed] [Google Scholar]
- 40.Jameson JP, Blank MB. Diagnosis and treatment of depression and anxiety in rural and nonrural primary care: national survey results. Psychiatr Serv 2010;61:624–627 [DOI] [PubMed] [Google Scholar]
- 41.Pfeiffer PN, Heisler M, Piette JD, Rogers MA, Valenstein M. Efficacy of peer support interventions for depression: a meta-analysis. Gen Hosp Psychiatry 2011;33:29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Travis J, Roeder K, Walters H, et al. Telephone-based mutual peer support for depression: a pilot study. Chronic Illn 2010;6:183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wells A, Lagomasino IT, Palinkas LA, Green JM, Gonzalez D. Barriers to depression treatment among low-income, Latino emergency department patients. Community Ment Health J 2013;49:412–418 [DOI] [PubMed] [Google Scholar]