Abstract
Transcriptional and epigenetic embryonic programs can be reactivated in cancer cells. As result, a specific subset of undifferentiated cells with stem-cells properties emerges and drives tumorigenesis. Recent findings have shown that ectoderm- and endoderm-derived tissues continue expressing stem-cells related transcription factors of the SOX-family of proteins such as SOX2 and SOX9 which have been implicated in the presence of cancer stem-like cells (CSCs) in tumors. Currently, there is enough evidence suggesting an oncogenic role for SOX9 in different types of human cancers. This review provides a summary of the current knowledge about the involvement of SOX9 in development and progression of cancer. Understanding the functional roles of SOX9 and clinical relevance is crucial for developing novel treatments targeting CSCs in cancer.
1. Introduction
Recently, there has been a growing interest in the study of mechanisms leading to the expression of genes involved in developmental and cell differentiation, since they are related to the presence of a special type of tumor cells with a stemness phenotype dubbed as cancer stem-like cells (CSCs). Stem cells regulatory proteins are now being recognized as potential oncogenes because of their ability to regulate CSCs phenotype and maintenance in tumors of diverse types of cancer. Moreover, it has been well accepted that CSCs are the main driving force behind tumor formation and metastasis [1]. CSCs exhibit diverse cell properties including self-renewal, differentiation capacity, and resistance to apoptosis. Moreover, CSCs are usually resistant to chemotherapy and eventually give rise to recurrence [1, 2]. Sex-determining region Y (SRY)-box 9 protein (SOX9) is a member of the SOX family of transcription factors (TFs) which are developmental regulators that possess high mobility group (HMG) box DNA binding and transactivation domains [3]. It participates in a variety of functions, such as lineage restriction and terminal differentiation, through precise temporal and spatial expression patterns that differ between particular cell types and tissues [4]. SOX9 gene has been implicated in different types of cancer as an oncogene; however, it also may behave as a tumor suppressor [5, 6]. Recent findings have shown that ectoderm- and endoderm-derived tissues continue to express SOX9 in stem cell pools [7] and evidence also suggests that it may regulate CSCs [8–10]. However, detailed mechanisms need to be elucidated. In this review we aim to condense the knowledge about the involvement of SOX9 in the initiation and progression of different types of cancer and to highlight its potential as a clinical biomarker.
2. SOX Family of Transcription Factors
SOX family of proteins comprise a group of transcriptional regulators containing a highly conserved HMG domain that was first discovered in SRY protein, a transcription factor involved in mammalian male sex determination [11]. SOX9 is located in chromosome 17 in a 3 Mb region devoid of other protein-coding genes and its expression is complex with individual enhancers directing tissue-specific expression [12]. In general, proteins containing an HMG domain which consists of three α helices with 50% or higher amino acid similarity to the HMG are referred to as SOX proteins (SRY-related HMG box). Around 20 related SOX proteins have been identified in humans, and they have been grouped based on the structural homology of regions outside of their conserved HMG boxes [7]. SOX proteins bind to ATTGTT consensus or related sequence motifs through their HMG domain [13]. SOX9 belongs to the SoxE subgroup, and its HMG domain induces significant bending at the consensus-binding motif (A/TA/TCAAA/TG) by forming an L-shaped complex in the minor groove of DNA [7]. Members of the SOXE subgroup share regions of significant homology outside the HMG domain and constitute two additional functional domains: a self-dimerization domain and a transactivation domain at the C-terminus [14]. One current model suggests that dimerization is achieved through cooperative binding between the dimerization domain of one SOXE protein and the HMG box of its partner SOXE protein [15]. SOX protein is subject to posttranslational modifications which alter its nuclear import (phosphorylation and acetylation) and its rate of degradation (ubiquitination and sumoylation) [16]. Individual SOX members within a group share biochemical properties and thus have overlapping functions. Conversely, SOX factors from different groups have acquired distinct biological functions despite recognizing the same DNA consensus motif [4]. Target gene selectivity by different SOX factors can be achieved through differential affinity for particular flanking sequences next to consensus SOX sites, homo- or heterodimerization among Sox proteins, posttranslational modifications of SOX factors, or interaction with other cofactors [4]. The expression of SOX genes is frequently subject to autoregulation or control by other SOX members. SOX is also regulated post-transcriptionally by microRNAs [17]. Furthermore, SOX-dependent regulations intersect with signaling networks such as the sonic hedgehog (Shh) [18], Wnt signaling pathways, in which SOX-Gli and SOX-β-catenin interactions are implicated [19]. SOX factors respond to different extracellular signals and interact with a host of intracellular cofactors to control different sets of genes in distinct cell types [20]. Additionally, SOX compete with other transcriptional factors regulating alternative lineages to achieve different cell fates during development. At molecular level, this is often accomplished by directly activating genes that promote their own lineage and repressing genes of alternative lineages [20]. In summary, SOX factors have profound implication in cell fate determination during development, even though recent findings reveal their crucial role in establishing and maintaining stem and progenitor cells [21].
3. Role of SOX9 in Human Cancer
SOX9 has been studied from a developmental point of view, particularly during chondrogenesis and male gonad genesis. Nevertheless, recent molecular and functional analyses have elucidated an important role in stem cell biology of mesoderm-, ectoderm-, and endoderm-derived tissues and organs [7]. Importantly, SOX9 maintains both adult stem and progenitor cells with high turnover, as in intestine and hair follicles, and it is crucial for postnatal injury repair in endodermic and ectodermic organs [7]. Remarkably, dysregulation of tissue differentiation pathways and stem cell homeostasis contributed to the initiation and progression of cancer. Experimental and clinical data demonstrated an important role for SOX9 in tumorigenesis since it is overexpressed in a wide range of human cancers where its expression correlated with tumor progression and clinical data (Table 1) [73]. In addition, SOX9 interacted with different transcription factors and exhibited several pro-oncogenic characteristics including promotion of proliferation, senescence inhibition, and neoplastic transformation in collaboration with other oncogenes [73]. COSMIC analysis showed that, from a total of 46,601 unique cancer samples, 572 samples have mutations in SOX9 and the most frequent mutation type is missense substitution (38.81%) of which 113 (33.63%) are C>T transitions. Copy number variations (CNV) gain was reported in 108 unique samples and overexpression was present in 509 samples [74, 75]. The versatility of SOX9 may be explained by a combination of posttranscriptional modifications, binding partners, and the tissue type in which it is expressed [7].
Table 1.
SOX9 expression and functions in human cancers.
| Type of cancer | Status of SOX9 | Sox9 participation | References |
|---|---|---|---|
| Hepatocellular carcinoma | overexpression | Related whit poor prognosis Related with poor disease free survival Related with poor overall survival |
[22, 23] |
|
| |||
| Breast cancer | overexpression | Promotes proliferation, tumorigenesis and metastasis Related with poor overall survival | [24] |
|
| |||
| Bladder cancer | overexpression | Promotes tumorigenesis Related with poor overall survival |
[25] |
|
| |||
| Gastric cancer | overexpression | Promotes chemoresistance Related with poor disease free survival |
[26] |
|
| |||
| Prostate cancer | overexpression | Promotes cell proliferation and apoptosis resistance Related with high clinical stage Related with poor relapse free survival Related with poor overall survival |
[27] |
|
| |||
| Prostate cancer | downregulation | Promotes metastasis Related with advanced clinical stage Related with EGR-positive tumors |
[28] |
|
| |||
| Ovarian cancer | overexpression | Its coexpression with HIF-2α induces the expression of TUBB3 which is related with poor overall survival | [29] |
|
| |||
| Pancreatic cancer | overexpression | Promotes chemoresistance | [30] |
|
| |||
| colorectal cancer | overexpression | Promotes cell proliferation, senescence inhibition and chemoresistance | [31–33] |
3.1. SOX9 Alterations in Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy. Its genetic complexity relies on interaction between several somatic genomic alterations and diverse etiologies linked to liver diseases by the concerted action of passenger and driver cancer genes. HCC progression is a complex process that implicates accumulative genomic alteration that includes aberrant gene expression, oncogene upregulation, and tumor suppressor downregulation providing favorable conditions for the development of HCC [76–78]. These mechanisms have been associated to several alterations in some important cellular signaling pathways relevant to a therapeutic perspective, such as RAF/MEK/ERK pathway, phosphatidylinositol-3 kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway, and Wnt/β-catenin pathway [79]. Nonetheless, the molecular pathogenesis of HCC is still unclear.
SOX9 regulated by Notch determines the timing and structure of bile duct morphogenesis during liver embryogenesis. Besides, not only during development, but also in the adult organs, SOX9 expression levels appear to be crucial for controlling the cell status of the duct cells [80]. In vitro analysis has shown that SOX9 expression in HCC cell lines was upregulated in comparison to normal hepatic cell lines; furthermore, it was expressed at higher level in highly metastatic cells lines relative to low metastatic cells. Moreover, downregulation of SOX9 in HCC cell lines decreased invasiveness and migration [81]. Recent studies using SOX9 chromatin immunoprecipitation combined with deep sequencing (ChIP-seq) analysis indicated that SOX9 can activate canonical Wnt/β-catenin signaling in HCC endowing stemness features through Frizzled-7 [82]. Besides, the results of a genome-wide transcriptional analysis indicated that TGFβ and Wnt/β-catenin signaling pathways were activated in hepatocholangiocarcinoma (cHCC-CC) [83]. Furthermore, integrative genomics revealed that cHCC-CC shares characteristics of poorly differentiated HCC with stem cell traits and poor prognosis [83]. Interestingly, early biomarkers of biliary commitment such as SOX9, as well as master genes of signaling pathways, which regulate the differentiation of hepatoblasts to cholangiocytes, were induced in cHCC-CC (e.g., TGFβ, Wnt, and Notch) [83]. SOX9 overexpression was commonly observed in HCC with high tumor stage and tumor grade tissues. Also, the high expression of SOX9 was linked to a significant trend toward both poorer disease-free survival and poorer overall survival [22]. Besides, poor prognosis of HCC patients has been linked with high SOX9 expression independent of the presence of cirrhosis [23].
3.2. Role of SOX9 in Breast Cancer
Breast cancer is a complex and heterogeneous disease that includes morphological and molecular different entities. Clinical parameters such as tumor size, lymph node involvement, histological grade, age and the expression of estrogen receptor (ER), progesterone receptor (PGR), and epidermal growth factor receptor 2 (HER2) biomarkers are responsible of its high clinical heterogeneity [84]. Mammary glands contain a small subpopulation of cells with a stem cells activity and it is also known that several TFs play pivotal roles in the establishment of cellular states. SLUG and SOX9 play essential roles in induction and maintenance of tumor initiating capacity in breast cancer cells [58]. In breast tumors, SOX9 expression was higher in comparison to normal mammary tissues, which was associated with an increased proliferation and Ki67 and p53 expression [59–61]. There is also evidence that upregulation of SOX9 affected metastasis and tumorigenesis in breast cancer cells by 5-fold and 40-fold, respectively [9]. Primary tumors with high expression levels of SLUG and SOX9 had a significant lower overall survival rate.
On the other hand, knockout studies have demonstrated that SOX9 was essential for the function of mammary stem/progenitor cell populations. Knockdown of SOX9 resulted in decreased proliferation of mammary stem cells [85]. On the other hand, higher expression of cytoplasmic-SOX9 in breast tumors was significantly associated with ER-status and decreased overall survival [24]. Altogether, these data indicate that cytoplasmic location of SOX9 was directly related with increased proliferation in breast cancer cell lines. Similarly, cytoplasmic SOX9 expression was directly related to neoplastic progression and its nuclear expression was more common in early stages of differentiation [24].
3.3. The Importance of SOX9 in Bladder Cancer
Bladder cancer (BC) is the ninth most common malignant disease and the thirteenth most frequent cause of cancer death worldwide. Men are more affected than women (3.2:0.9 ratio) and disease incidence increases with age [86]. In previous studies using biopsies of BC, 75% positive immunostaining of SOX9 was observed in the nucleus of cancer cells and the expression was significantly associated with the advanced pathological grade and clinical stage. However, SOX9 immunostaining in the normal bladder tissues occurred mainly in the cytoplasm and nucleus. These findings could indicate that SOX9 may play a promotive role in the progression of BC [25]. On the other hand, epigenetic changes of SOX9 were associated with the aggressiveness of bladder cancer [87]. Methylation of Sox9 promoter gene was identified in a study of 101 BC samples and it was significantly associated with shorter overall survival. Besides, in vitro analyses demonstrated that the expression of SOX9 was aberrantly silenced by CpG island promoter hypermethylation in BC [88]. However, Sun et al. (2009) found a hypermethylated state of SOX9 in only 3/82 (3.7%) cases of BC and 2/15 (13.3%) cases of the control in a Chinese cohort [89]. These results indicated the necessity to further compare the methylation profiles between populations, given the discrepancies in this disease as proposed previously [90].
3.4. SOX9 Aberrant Expression in Gastric Cancer
Gastric cancer (GC) is one of the most aggressive malignant tumors worldwide with a high mortality rate, preceded only by lung cancer [91]. Globally, GC is the fourth most common cancer and second leading cause of cancer related mortality with a 5-year overall survival rate less than 25% [86]. SOX9 expression has been found in epithelial cells at the proliferative zone of the normal gastric mucosa and bottom area of the intestinal metaplasia of the stomach. Many tumor cells of type I GC are positive for SOX9 [92, 93]. Ectopic expression of β-catenin in AGS and MKN-1 cells induced increased expression of SOX9 [94], whereas its suppression by PPARγ decreased SOX9 expression in MKN-28, SGC-7901, and BGC-823 cells [95]. Gastrokine 1 (GKN1), a tumor suppressor like protein which expression is lost in gastric tumors (including adenoma and cancer) [96], was responsible for decreased SOX9 expression in AGS and MKN-1 cells. Nevertheless, in GC tissues, nuclear expression of SOX9 was closely associated with GKN1 immuno-negativity suggesting that aberrant SOX9 expression by GKN1 inactivation may be involved in the development of sporadic GC as an early event [94].
Additionally, SOX9 overexpression was correlated with lymph node metastasis and advanced tumoral stages of GC, indicating that it is related to tumor progression by promoting invasion and metastasis [97] and with reduced disease-free survival [26].
Moreover, elevated SOX9 levels were associated with resistance to cisplatin in MKN45 and KATO III cells [26] whereas miR-524-5p inhibited SOX9 expression conferring sensitivity to cisplatin-resistant GC cells by targeting its 3`UTR. This is because resistance to cisplatin in GC cells was associated with the expression of miR-524-5p. Thus, overexpression of miR-524-5p in GC cells enhanced their sensitivity to cisplatin and it depends on the downregulating SOX9 [98]. Also, SOX9 has been correlated with the carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1). Their coexpression was detected in normal gastric mucosa, hyperplastic polyp, intestinal metaplasia, gastric intraepithelial neoplasia, and adenocarcinoma, showing highly elevated expression from benign proliferative lesions to malignant lesions, suggesting that SOX9 could change CEACAM1 expression patterns, which might promote the tumor progression [99].
Helicobacter pylori infection has a very important role in GC. In animal model, histopathological changes such as metaplasia of the gastric mucosa after H. pylori infection show increased expression of CD44 and SOX9 dependent on IL-1 signaling, suggesting the participation of SOX9 in gastric carcinogenesis [100]. In humans, the risk of developing GC was higher in individuals infected with cytotoxin-associated gene A (cagA)—positive strains or some vacuolating cytotoxin gene A (vacA) allelic combinations causing the loss of crucial features of epithelial differentiation in gastric cells, leading to transformation and tumor formation [101–103]. In this regard, SOX9 was transcriptionally activated following H. pylori infection in GC cell and its silencing resulted in an increase of phospho-histone H3- (p-H3-) proliferative cells and spheres formation ability promoted by bacteria. β-catenin-silenced cells also presented a marked reduction in p-H3-positive cells when infected with both strains [26]. Conversely, downregulation of SOX9 by promoter methylation was related to GC progression in Epstein Barr Virus-positive biopsies and infected MKN7 cells. SOX9 methylation was detected in 47% of GCs and correlated with low levels of SOX9 protein. Besides, the rate of methylated SOX9 tumors increased and SOX9 expression gradually decreased through the depth of GC invasion. These data strongly suggested that the decrease of SOX9 expression in advanced GC was related with the epigenetic suppression of SOX9 during tumor invasion [104].
3.5. SOX9 Is Involved in Different Types of Pancreatic Cancer
SOX9 regulated by Notch is involved in the maintenance of pancreatic progenitor pools [105]. Furthermore, SOX9 is essential for pancreas development. At the early stages of mouse pancreas development it is expressed in all epithelial cells and its expression is confined to the ductal cells and centroacinar cells as development progresses [80]. Genetic lineage-tracing studies showed that all types of pancreatic epithelial cells including endocrine, acinar, and duct cells express Sox9, suggesting that the all Sox9-expressing cells are a common progenitor of pancreatic epithelial cells [106].
Recently, it was shown that Sox9 is expressed during premalignant and malignant lesions such as mucinous cystic neoplasias (MCNs), intraductal papillary mucinous neoplasias (IPMNs), pancreatic intraepithelial neoplasias (PanINs), and pancreatic ductal adenocarcinoma (PDAC) (Table 2) [34, 107], which is the most common pancreatic cancer and develops from cells lining pancreatic ducts [108]. The evidences suggested that a phenotypic switch converting pancreatic acinar cells to duct-like cells can lead to PanIN [109] and eventually PDAC. Studies about the expression of SOX9 and Hepatocyte Nuclear Factor 6 (HNF6) show that these TFs were expressed in acinar cells. HNF6 induced SOX9 expression, indicating that SOX9 is downstream of HNF6. In acinar-to-ductal metaplasia (ADM), SOX9 was predominantly found in metaplastic cells that displayed duct-like characteristics and it was also found in PanIN [34, 35].
Table 2.
Sox9 is expressed during premalignant and malignant lesions in pancreatic cancer.
| Lesions | Model | Status of SOX9 | Effects | References |
|---|---|---|---|---|
| Acinar-to-ductal metaplasia | Mice | Overexpressed | HNF6 induces Sox9 expression, which is characteristic of ADM in humans | [34, 35] |
|
| ||||
| Acinar-to-ductal metaplasia | Mice | Overexpressed | Aberrant expression of p27 induces the nuclear expression of Sox9 | [36] |
|
| ||||
| Mucinous cystic neoplasias, intraductal papillary mucinous neoplasias, pancreatic intraepithelial neoplasias and pancreatic ductal adenocarcinoma | Mice | Overexpressed | SOX9 and Kras co-expression is associated with PDAC initiation | [37] |
|
| ||||
| Pancreatic ductal adenocarcinoma | 88 tumors samples of PDAC | Overexpressed | Sox9 and p-Akt double-positive expression is related with an unfavorable prognosis, high TNM and distant metastasis | [38] |
|
| ||||
| Pancreatic ductal adenocarcinoma | Patient-derived tumor organoids with PDAC | Overexpressed in cytoplasm | High expression of Sox9 in cytoplasm is related with a poor DFS, OS, higher tumor grade and worse disease-specific survival compared to patients with nuclear Sox9 expression | [39] |
|
| ||||
| Pancreatic cancer | PANC1 and HPAC cell lines of pancreatic cancer | Overexpressed | Sox9 is highly expressed in pancreatic CSCs. Moreover, NF-κB subunit p65 positively regulates SOX9 | [40] |
|
| ||||
| Pancreatic ductal adenocarcinoma | HD3 colon cancer cells | Overexpressed | ST6Gal-I induces expression of Sox9, promoting stem-like cell properties | [41] |
|
| ||||
| Pancreatic cancer | PANC-1, Capan-1, BxPC-3, MiaPaCa-2 cell lines of pancreatic cancer | Expression depending on chemoresistance | High level of Sox9 is related to stronger chemoresistance to Gemcitabine | [42] |
|
| ||||
| Pancreatic ductal adenocarcinoma | HPDE cell line of PDAC | Overexpressed | GLI1 induces the transcription of Sox9 promoting stem cell properties | [43] |
|
| ||||
| Pancreatic cancer | PANC1 cell line of pancreatic cancer | Downregulated | Induction of EMT with TGF-β results in low levels of SOX9, FOXA2, and GATA4 | [44] |
|
| ||||
| Pancreatic cancer | Mice | Overexpressed | p53-/- mice enhanced sphere formation, increased expression of the stemness regulator Bmi1 and Klf4, and pancreatic multipotent progenitor markers as Ptf1a, Pdx1, Cpa1, c-myc, Hnf1b and Sox9 | [45] |
|
| ||||
| Pancreatic cancer | FG and L3.6pl cell lines of pancreatic cancer under hypoxia | Overexpressed | Under hypoxia conditions, FG cell line expresses high levels of Sox9 and L3.6pl. Besides, WNT, CXCR4, retinoic acid, and FAK signaling pathways are regulated by Sox9 in L3.6pI | [46] |
|
| ||||
| Pancreatic ductal adenocarcinoma and anaplastic pancreatic cancer | 6 APC patients and 53 PDAC patients | Overexpressed | PDAC and APC have high expression of Sox9, being the expression of proteins related with CSCs and EMT process higher in APC samples than PDAC | [30] |
|
| ||||
| Intraductal papillary mucinous neoplasm | 19 IPMN cases | Overexpressed | SOX9-positive cells were confined to the lower portions of the papillary structures of IPMN | [47] |
|
| ||||
| Solid pseudopapillary tumor | 8 samples of SPT | Overexpressed | PDX1 and Sox9 are both expressed in the cytoplasm of SPT cell | [48] |
Epidermal growth factor (EGFR) in the PDAC promotes expression of SOX9 as an early event. In this context, pancreatic metaplasia could be also caused by loss of p27 function, a negative regulator of proliferation and a tumor suppressor that inhibits cyclin-CDK activity in the nucleus [110–112]. Besides, K-RAS activation, the earliest known event in pancreatic carcinogenesis [113, 114], may induce p27 mislocalization producing loss of nuclear p27 expression and as a result derepression of SOX9, triggering ADM [36]. The formation of acinar-derived premalignant lesions depends on ectopic induction of SOX9, a ductal gene [115]. Moreover, when it is concomitantly expressed with oncogenic K-RAS, SOX9 accelerated the formation of premalignant PDAC lesions [37]. Furthermore, SOX9 and p-AKT double-positive expression was related with an unfavorable prognosis, high TNM, and distant metastasis in PDAC [38, 116].
Studies using patient-derived tumor organoids with PDAC show that in normal pancreas SOX9 is expressed in the nucleus, while, in the organoids with TP53-mutated (R175H) PDAC, it was expressed in cytoplasm. Clinically, high expression of SOX9 in cytoplasm could be related with a poor disease-free survival (DFS), overall survival, higher tumor grade, and worse disease-specific survival compared to patients with nuclear SOX9 [39]. Emerging evidence suggests that CSCs are exclusively tumorigenic and essential drivers for tumor progression and metastasis [117]. Pancreatic CSCs have been identified and characterized using different surface markers: CD44, CD24, EpCAM, CD133, CXCR4, c-Met, and Aldehyde Dehydrogenase-1a1 (ALDH1) [118–121]. SOX9 has been found expressed in the pancreatic CSCs isolated from PANC1 and HPAC cell lines of pancreatic cancer. This population was more capable of initiating tumors in NOD/SCID xenograft model than the population no-invasive (no-CSCs) [40].
Demethylated SOX9 is found in CSCs and plays a crucial role in the invasion process. Also, NF-κB subunit p65 positively regulates SOX9 expression by directly binding to the SOX9 promoter [40], suggesting that the NF-κB pathway is one of the most activated pathways in pancreatic CSCs. Another important regulator of SOX9 is the glycosyltransferase ST6Gal-I which adds α2-6-linked sialic acids to substrate glycoproteins and it is known that its upregulation in cancer cells confers stemness characteristics [41, 122]. Modulating ST6Gal-I expression in pancreatic cancer cells directly altered CSC spheroid growth. In this regard, ST6Gal-I knockdown decreased the levels of SOX9 [41], suggesting that SOX9 expression was regulated by a specific glycosyltransferase, and tumor glycosylation could be a mechanism for functionally shifting cells to a less differentiated, stem-like state.
Interestingly, SOX9 expression in different pancreatic cell lines (PANC-1, Capan-1) was related to stronger chemoresistance to gemcitabine than cells with low SOX9 expression (BxPC-3, MiaPaCa-2). Conversely, SOX9 repression using siRNA recovers the chemosensitivity, affected spheres formation rate, and the proportion of CD44high and CD24high cells. This indicates that the expression of Sox9 plays an important role in chemoresistance by the induction of stemness in pancreatic cancer cells [42].
On the other hand, GLI1, a member of the GLI family of zinc finger transcription factors, is a central regulator of cell fate that is deregulated in diverse tumor types [62–66]. GLI1 signaling impacts multiple cancer-relevant cellular processes, promoting dedifferentiation, the generation of CSCs, tumor progression, and metastasis. GLI1 directly induced the transcription of SOX9 and a positive feedback promoting SOX9-dependent cancer stem cell properties was observed [43].
Epithelial-to-mesenchymal transition (EMT) process is a critical regulator of the CSC phenotype [123, 124]. Tumor growth factor β (TGFβ) induces EMT, promoting cancer cell invasion and metastasis [124]. PANC1 cell line stimulated with TGFβ1 showed a significant downregulation of SOX9, FOXA2, and GATA4 master genes [44].
Furthermore, p53-/- mice enhanced sphere formation, increased expression of the stemness regulator BMI1 and KLF4 and pancreatic multipotent progenitor markers such as PTF1A, PDX1, CPA1, c-MYC, HNF1B, and SOX9 [45]. These results can be relevant to understand the relationship between p53 and SOX9 and their importance in the acquisition of EMT characteristics in pancreatic cancer.
Microarray analysis demonstrated that another important issue related with cancer and the CSCs was hypoxia, which induced expression of Sox9 in low metastatic cell line FG, whereas in high metastatic cell line L3.6pl it was found constitutively expressed and was not more inducible under hypoxic conditions [46]. Besides, a subset of transcripts related different networks including WNT, CXCR4, retinoic acid, and FAK signaling pathways were also regulated by SOX9 in the aggressive-metastatic cells, but driven by HIF-1α in low metastatic cells [46].
Opposite to the oncogenic role of SOX9 in pancreas carcinogenesis studies in tissues corresponding to later stages of tumor development have found downregulation of SOX9 and other master regulators of embryonic development such as GATA4, PDX1, PTF1a, and HNF1b [123, 125–127].
Even though PDAC is the most common type of pancreatic cancer, there are other types of pancreatic tumors with less incidence but more aggressive behavior such as the anaplastic pancreatic cancer (APC), which has been considered a variant of PDAC [67, 68]. There was evidence that PDAC and APC have high expression of Sox9. Also, the expression of proteins related with CSCs and EMT process is higher in APC samples than PDAC, which correlated with aggressiveness of APC [30].
Another type of pancreatic cancer called intraductal papillary mucinous neoplasm (IPMN) is less aggressive than PDAC and APC [47, 128, 129] and exhibits a characteristic expression of SOX9 confined to the lower portions of IPMN which is lost once the neoplasms advance to high-grade dysplasia carcinoma. Cells in the upper portions of IPMN may be, albeit speculative at this point, supplied by the SOX9-positive cells in the lower portions of the neoplasm [47].
Finally, solid pseudopapillary tumor (SPT) is an uncommon type of pancreatic tumor of undetermined origin present especially in children [130]. Studies on samples from pediatric patients with SPT showed that PDX1 and SOX9 were both expressed in the cytoplasm of SPT cells, supporting the hypothesis that tumor cells originate from pancreatic stem cells persisting after the embryonic period [48]. This is relevant since both transcription factors are crucial for pancreatic organogenesis and linked to Wnt/β-catenin signaling pathway [105, 131–133].
3.6. SOX9 Is Required for Prostate Cancer Initiation
The human prostate is composed of prostatic glands with well-defined basal and luminal epithelial cell layers. Cells within the luminal epithelium have a very low rate of proliferation and express high levels of androgen receptor (AR) [69]. In contrast, basal cells have a higher rate of proliferation, express low or undetectable levels of AR, and are not androgen dependent, playing critical roles in prostate organogenesis, homeostasis, support, and a barrier for the luminal cells. This barrier becomes discontinuous in prostatic intraepithelial neoplasia, which is believed to be a precancerous lesion. The complete loss of the basal cell layer is a defining feature of prostate cancer (PCa) [134].
SOX9 protein was expressed in adult prostate basal epithelium and at the initial stages of bud outgrowth from the urogenital sinus and could play a role in maintaining the committed stem cell phenotype, differentiation, and supporting the overlying luminal epithelium [134–136].
In vivo studies showed that SOX9 was highly expressed during fetal prostate development by epithelial cells expanding into the mesenchyme, suggesting that it may contribute to invasive growth in PCa [137]. Besides, SOX9 expression in prostate cancer cells was regulated by Wnt/β-catenin signaling, being AR one identified downstream target [134]. In turn, SOX9 positively regulated multiple Wnt pathway genes, including encoded Wnt receptors (frizzled [FZD] and lipoprotein receptor-related protein [LRP] family members) and the downstream β-catenin effector TCF4 [70]. Microarray analysis showed that SOX9 was overexpressed in PCa tissues when compared with noncancerous prostate tissues. Also, SOX9 overexpression was found in PCa tissues with higher clinical stage and was related to lower biochemical recurrence-free survival and overall survival rates [138]. When the SOX9 expression was correlated with overexpressed HIVEP3 (human immunodeficiency virus type I enhancer binding protein 3), the patients also exhibited significantly shorter biochemical recurrence-free survival [139].
Some tumor suppressors have been related with SOX9 participation in PCa. Its overexpression in adult mouse prostate epithelia gives rise to an increase in proliferation and induced early high-grade prostate intraepithelial neoplasia lesions when mice are heterozygous for PTEN (phosphatase and tensin homolog deleted on chromosome 10). This study shows that high levels of SOX9 contributed to regulate proliferation within the prostate epithelia and can cooperate with PTEN loss to accelerate prostate neoplasia [140]. Zbtb7a (also known as pokemon) has been recently reported as an oncosuppressor in PCa since it is lost in a subset of human advanced prostate cancer and facilitates the oncogenic activity of SOX9 during prostate tumorigenesis favoring senescence bypass, increase of proliferation rate, apoptosis resistance, and invasive potential [71].
Interestingly, in human castration resistant PCa samples, nMET was remarkably increased. Androgen deprivation induced endogenous nMET which activates SOX9 and promoted cell proliferation and stem-like cell self-renewal in androgen-nonresponsive PCa cells. This indicates that coupregulation of endogenous nMET and SOX9 upon androgen deprivation may activate cell reprogramming to promote transformation and androgen nonresponsive growth [141].
Fusion genes have a very important role in PCa. SOX9 is a critical downstream effector of ERG in TMPRSS2: ERG fusion-positive PCa, and ERG stimulates SOX9 expression by redirecting AR to a cryptic AR-regulated enhancer in the SOX9 gene [142]. Besides, association between TMPRSS2, ERG positive PCa, and rs1859962 at 17q24 has been demonstrated suggesting a molecular mechanism linking the risk region to the ERG pathway where SOX9 is a downstream target. There is also evidence of a positive correlation between SOX9 gene expression and the rs1859962 risk allele in TMPRSS2: ERG positive tumor tissue [143].
Analysis of tissue microarrays of prostate biopsies samples of patient with metastatic castration-resistant PCa shows 18.3% and 87.3% of patients with positive ERG and SOX9 expression, respectively [27]. Besides, ERG and SOX9 are significant risk factors for lower prostate-specific antigen-Progression Free Survival (PFS), Clinical/Radiological-PFS, and Overall Survival after docetaxel treatment suggesting that ERG and SOX9 are potential biomarkers for prediction to docetaxel treatment in mCRPC patients [27]. Conversely, a gradual decrease of SOX9 has been related to a progression to advanced stage, high Gleason grade, and metastatic growth in ERG-positive cancers and these effects were strictly limited to the subset of prostate cancers harboring PTEN deletions [28].
3.7. Oncogenic Role of SOX9 in Ovarian Cancer
Ovarian cancer is the most lethal gynecological cancer [144]. Carcinomas of the ovarian surface epithelium correspond to 90% of ovarian malignancies and are classified into four main histological subtypes, which have distinct characteristics regarding genetic abnormalities and specific signaling pathways [144]. Sertoli-Leydig cell tumors, ovarian sex-cord stromal tumors, granulosa cell tumors, and primary ovarian tumors constitute carcinomas in ovary [145].
In normal ovarian development, SOX9 has different expression levels, as wells as roles in comparison to other tissues. During the follicular development in early pre-antral follicles there is not expression of SOX9, but the cells surrounding the developing follicles present nuclear expression. These are very important since they have a participation in the production of collagen or laminin fibers which constructs follicular lamina [146].
Little is known about SOX9 role in ovarian cancer. Nonetheless, higher expression of SOX9 has been found in human Sertoli tumor biopsies coexpressed with BCL-2 and Ki-67, being the last the less expressed in well-differentiated cells [147]. This suggests that apoptotic and proliferative properties depend on tumor differentiation stage. Besides, it is known that hypoxia conditions promote Tubulin Beta 3 (TUBB3) expression through HIF-2α and SOX9. High expression of these genes correlates with shorter overall survival in women with ovarian cancer [29].
3.8. The Role of SOX9 in Colorectal Cancer
Colorectal cancer (CRC) is a major cause of morbidity and mortality throughout the world [148]. It accounts for over 9% of all cancer incidences [149, 150] and it is the third most common cancer worldwide and the fourth most common cause of death [150]. In most patients, death is not caused by the primary tumor, but rather by its metastasis in other organs and associated complications [151].
Paneth cells are a highly specialized population of intestinal epithelial cells located into the crypts [152]; these cells are critical to the control of the intestinal stem cell (ISC) niche and the intestinal barrier [153, 154]. The function of SOX9 in Paneth cells has not been clarified but in vitro studies suggest a role in the control of cell differentiation in the intestinal epithelium [155]. In vitro and in vivo data indicate that Sox9 gene is a transcriptional target of Wnt signaling; this pathway is involved in the regulation of intestinal epithelium homeostasis [156].
Sox9 expression is regulated by TCF4, the main Wnt pathway TF in the intestinal epithelium [155]. This is relevant since mutations in components of the Wnt pathway, including the tumor suppressor APC and β-catenin protein, result in stabilization of β-catenin, which then continuously interacts with TCF4, leading to constitutive activation of target genes [72]. Moreover, targeted mutations of APC or β-catenin are sufficient to initiate tumorigenesis in mouse [157–159], highlighting the importance of the Wnt pathway in the development of cancer.
Recent studies of CRC have found that overexpression of SOX9 in vitro and in vivo was related to several pro-oncogenic properties, including the ability to promote proliferation, inhibit senescence, and collaborate with other oncogenes in neoplastic transformation [6, 49, 50, 73, 160]. The overexpression of SOX9 is related with recurrent distal truncating mutations as frameshift mutations and nonsense mutation in approximately 11% of CRCs; also, SOX9 mutation is strongly associated with coexistent mutant K-RAS and wild type TP53 [49, 50].
Nevertheless, in the particular case of DLD-1 CRC cell line, which has a heterozygous L142P inactivating mutation of SOX9, the restoration of wild type SOX9 expression results in an oncoprotective activity which inhibits cell growth, clonal capacity, and colonosphere formation while decreasing both the activity of the oncogenic Wnt/ß-catenin signaling pathway and the expression of the c-MYC oncogene [6].
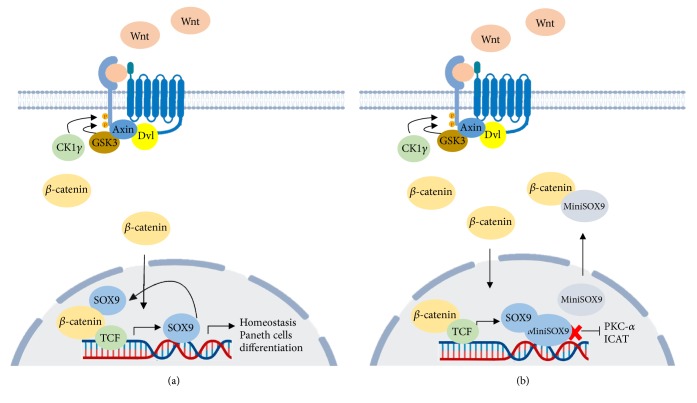
Besides, a truncated version of SOX9 devoid of transactivation domain as a result of retention of the second intron called MiniSOX9 has been discovered in human tumor samples of CRC; this version is expressed at high levels in CRC but it is undetectable in the surrounding healthy tissue. The possible mechanism of MiniSOX9 could be through activation of canonical Wnt target genes and repression of PKCα (Figure 1), two features in favor of oncogenic properties [51].
Figure 1.
MiniSOX9 has an oncogenic behavior in CRC. (a) In normal conditions canonical Wnt/β-catenin pathway triggers SOX9 expression resulting in regulation of differentiation and homeostasis in intestinal epithelium. (b) Truncated version of SOX9, MiniSOX9, accumulates in the nucleus to inhibit SOX9 DNA-binding-dependent transcriptional activity and PKC-alpha expression.
It has been stablished that overexpression of SOX9 in CRC is associated with β-catenin activation; however, the largest clinical study on SOX9 expression over 188 primary CRC specimens from a Chinese population shows that it does not present significant correlation between SOX9 and β-catenin [31].
SOX9 upregulation is common in colorectal adenoma and cancer and is an independent indicator for an adverse prognosis in CRC [31]. Conversely, low levels of SOX9 at the invasive front of the primary tumor have been shown as an independent predictor of relapse in stage II colon cancer patients (Table 3) [32]. Studies over African Americans CRC cases, diagnosed at earlier ages compared to non-Hispanic withes, have found that SOX9, GATA6, TET1, GLIS1, and FAT1 were differentially hypermethylated in APC mutation-negative CRC; this lack of APC mutation is associated with the early-onset CRC [33].
Table 3.
SOX9 roles in CRC as oncogene and tumor suppressor.
| Model | Status of SOX9 | Effects | References |
|---|---|---|---|
| 353 tumors samples of CRC | SOX9 mutated and WT are overexpressed | Truncating SOX9 mutations are associated with SOX9 overexpression, KRAS mutation, and TP53 wild type | [49, 50] |
|
| |||
| DLD-1 cell line of CRC | Loss of SOX9 transcriptional activity by L142P mutation | Restoration of wild type SOX9 expression inhibits cell growth, clonal capacity and colonosphere formation; besides, the expression of the c-MYC and the activity of Wnt/ß-catenin signaling pathway are affected | [6] |
|
| |||
| 17 tumors samples of CRC | High levels of SOX9 and MiniSOX9 | Overexpression of MiniSOx9 is found in CCR tissues whereas SOX9 is also expressed in normal and adjacent tissues | [51] |
|
| |||
| 188 tumors samples of CRC from Chinese population | Overexpressed | Does not show significant correlation between SOX9 and β-catenin | [31] |
|
| |||
| 144 primary tumors from patients diagnosed in stage II CRC | Downregulated | Low levels of SOX9 have been shown as an independent predictor of relapse in stage II colon cancer patients | [32] |
|
| |||
| 45 tumors samples of CRC from African Americans population | Hypermethylated | SOX9, GATA6, TET1, GLIS1, and FAT1 are differentially hypermethylated in APC-negative CRC | [33] |
|
| |||
| CaCo2, SW480, HCT116 and HT29 cell lines of CRC | Overexpressed | The synthetic PPARγ ligand rosiglitazone induces changes of SOX9 and β-catenin expression and subcellular localization | [52] |
|
| |||
| HT29 and HCT116 cell lines of CRC | Cofactor of NF-Y | SOX9 is necessary for the function of NF-Y in activating expression of cyclin B1, cyclin B2, cyclin dependent kinase 1 and topoisomerase II α | [53] |
|
| |||
| HCT116, SW480, SW620, DLD-1 cell lines of CRC | Overexpressed | Sox9 promotes proliferation through FOXK2 | [54] |
|
| |||
| HCT116 cell line of CRC | Overexpressed | Sox9 promotes invasiveness and metastasis in CRC through S100P | [55] |
|
| |||
| CCD 841 CoN, DLD-1, HCT-116, and HT-29 cell lines of CRC under hypoxia | Overexpressed | Sox9 upregulates the expression of USP47 promoting EMT under hypoxia | [56] |
|
| |||
| SW620 and SW480 cell lines of CRC | Overexpressed | SOX9 mediates the acquisition and maintenance of CR-CSCs | [57] |
A recent study about the role and association between SOX9, β-catenin, and PPARγ in CRC tissues showed that SOX9 and β-catenin were overexpressed whereas PPARγ was downregulated. Treatment with the synthetic PPARγ ligand rosiglitazone induced different changes of SOX9 and β-catenin expression and subcellular localization in the colon cancer cell lines Caco2, SW480, HCT116, and HT29. All this data indicated that SOX9, β-catenin, and PPARγ expression levels were deregulated in the CRC tissue, and in colon cancer cell lines ligand-dependent PPARγ activation unevenly influences SOX9 and β-catenin expression and subcellular localization, suggesting a variable mechanistic role in colon carcinogenesis [52].
In HT29 and HCT116 cell lines of CRC, SOX9 was recruited by NF-Y to the target genes and interacted with NF-Y on CCAAT promoter sequences. Besides, SOX9 is necessary for the function of NF-Y in activating expression of some cell-cycle regulatory gene expressions such as cyclin B1, cyclin B2, cyclin dependent kinase 1, and topoisomerase II α [53].
Multiples targets of SOX9 have been described. One of these is FOXK2, a transcription factor which promotes the cell proliferation in samples tissues of CRC [54]. Another important target of SOX9 is S100P; both were coexpressed in CRC and the knockdown of SOX9 expression downregulates S100P expression resulting in reduced invasiveness and metastasis of colon cancer cells by inhibiting the activation of receptor for advanced glycation end products (RAGE)/ERK signaling and EMT [55].
Interestingly, hypoxia induced EMT and SOX9 overexpression in CRC cells. SOX9 was able to migrate to nucleus and upregulated the expression of USP47, a deubiquitinating enzyme [56]. Another way to enhance the EMT by SOX9 is the loss of ZFP36 expression, a tumor suppressor [161]. On the other hand, it was demonstrated that SOX9 levels were higher in metastatic SW620 cell line than in primary CRCs SW480 cell line isolated from the same patient. SOX9 is sufficient and necessary for the acquisition and maintenance of CR-CSCs and metastatic traits, properties linked to transcriptional and post-transcriptional regulation. Finally, SOX9-mediated self-renewal and growth were impaired by the mTOR inhibitor rapamycin [57].
4. Clinical Relevance of SOX9 in Cancer
SOX9 has proven its functional role in various aspects of cancer biology. Besides, research on SOX9 has also investigated its importance in the clinic regarding disease prognosis, relapse, and therapy resistance. For instance, SOX9 overexpression was commonly observed in those HCC high tumor stage and tumor grade tissues. Also, the high expression of SOX9 was linked to a significant trend toward both poorer disease-free survival and poorer overall survival [22]. Moreover, poor prognosis of HCC patients has been linked with high SOX9 expression independent of the presence of cirrhosis [23]. In breast cancer, primary tumors that exhibit high expression levels of both SLUG and SOX9 had a significantly lower overall survival rate than the rest of the patients. Thus, SOX9 expression in carcinogenesis and malignity in breast cancer tumors is relevant. Besides, higher expression of cytoplasmic-SOX9 in human breast tumors is significantly associated to ER-status and to decreased overall survival [24]. Previous studies using biopsies of BC have shown that 75% positive immunostaining of SOX9 is observed in the nucleus of cancer cells and this expression is significantly associated with the advanced pathological grade and clinical stage [25]. In the case of GC, SOX9 overexpression has been correlated with lymph node metastasis and advanced tumoral stages, indicating that it is related to tumor progression by promoting invasion and metastasis [97] and with reduced disease-free survival [26]. Analysis of tissue microarrays of prostate biopsies samples of patient with metastatic castration-resistant PCa (mCRPC) showed 18.3% and 87.3% of patients with positive ERG and SOX9 expression, respectively [27]. Besides, ERG and SOX9 are significant risk factors for lower prostate-specific antigen-progression free survival (PFS), Clinical/radiological-PFS, and overall survival after docetaxel treatment suggesting that ERG and SOX9 were potential biomarkers for prediction to docetaxel treatment in mCRPC patients [27]. Conversely, a gradual decrease of SOX9 has been related to a progression to advanced stage, high Gleason grade, and metastatic growth in ERG-positive cancers, and these effects were strictly limited to the subset of prostate cancers harboring PTEN deletions [28].
Clinical relevance of SOX9 in ovarian cancer relies on its coexpression with HIF-2α under hypoxia conditions, promoting TUBB3 expression. The combined presence of high TUBB3/SOXn levels is associated with a relevant reduction of PFS and overall survival in women with ovarian cancer [29]. In pancreatic cancer, there is evidence that PDAC and APC have high expression of Sox9. Also, the expression of proteins related with CSCs and EMT process is higher in APC samples than PDAC, which correlates with aggressiveness of APC [30]. SOX9 upregulation is common in colorectal adenoma and cancer and is an independent indicator for an adverse prognosis in CRC [31]. Conversely, low levels of SOX9 at the invasive front of the primary tumor have been shown as an independent predictor of relapse in stage II colon cancer patients [32]. Studies over African Americans CRC cases, diagnosed at earlier ages compared to non-Hispanic withes, have found that SOX9, GATA6, TET1, GLIS1, and FAT1 are differentially hypermethylated in APC mutation-negative CRC; this lack of APC mutation was associated with the early-onset CRC [33].
5. Concluding Remarks
Nowadays, we have a solid background about SOX9 function in normal embryonic and adult tissues, and a whole network of regulatory mechanisms depends on and influences SOX9 expression and activity. However, SOX9 expression is also a common characteristic of CSCs. Emerging evidence suggests that CSCs play a crucial role in the development and progression of malignancies. It is already known that SOX9 has an adaptable role since it participates in different steps of cancer progression. For instance, SOX9 is very important in the initiation of pancreatic, gastric, and prostate cancer. Conversely, in bladder and colorectal cancer, SOX9 participates in the progression of the disease, whereas it is correlated to metastasis in breast, gastric, pancreatic, and colorectal cancer. Moreover, SOX9 is clinically relevant as it may contribute in diagnosis, prognosis, and therapeutic among diverse types of cancer. This is because its expression levels and location could be cytoplasmic or nuclear depending on the stage, place, and aggressiveness. Thus, it could serve as a potential biomarker. Besides, SOX9 expression levels are related with chemoresistance in gastric, pancreatic, and colorectal cancer and high expression of SOX9 in several solid tumors is related to poor overall survival, biochemical recurrence-free survival, disease-specific survival, and DFS. Even though SOX9 has a pivotal role in different types of cancer, it has been described as an oncogene and as tumor suppressor. In this regard, it is remarkably important to consider that differences in cell lines, animal models, and populations may cause diverse outcomes. Therefore, more work is needed to study SOX9 participation in Wnt/β-catenin and other pathways, including its relationship with other TFs (Table 4) related with stem-cell maintenance in different types of cancer, in order to elucidate additional mechanisms through which it may function. This is specially required for gaining a better understanding of SOX9 roles in normal and disease states to developing novel cancer therapeutic strategies.
Table 4.
Role of SOX9 and associated transcription factors in diverse types of cancer.
| Type of cancer | TF | Effects | References |
|---|---|---|---|
| Breast cancer | SLUG (SNAI2) | Induction and maintenance of tumor initiating capacity in breast cancer cells | [58] |
|
| |||
| Breast cancer | TP53 | Increased proliferation | [59–61] |
|
| |||
| Pancreatic cancer | HNF6 (ONECUT1) | Produces ectopic expression of Sox9 in acinar cells converting them in ductal cells | [34, 35] |
|
| |||
| Pancreatic cancer | NF-κB | NF-κB subunit p65 positively regulates SOX9 expression by directly binding to the SOX9 promoter | [40] |
|
| |||
| Pancreatic cancer | GLI1 | Induces the transcription of SOX9 | [62–66] |
|
| |||
| Pancreatic cancer | PDX1 | Co-expressed in the cytoplasm with Sox9 in solid pseudopapillary tumors | [67, 68] |
|
| |||
| Prostate cancer | AR | Downstream target of SOX9 | [69] |
|
| |||
| Prostate cancer | TCF4 | It is positively regulated by SOX9 | [70] |
|
| |||
| Prostate cancer | ZBTB7A (POKEMON) | It is lost in advance prostate cancer facilitating the oncogenic activity of SOX9 | [71] |
|
| |||
| Ovarian cancer | HIF2A (EPAS1) | Hif-2α and Sox9 promote TUBB3 expression. High expression of TUBB3 and SOX9 correlates with shorter overall survival | [29] |
|
| |||
| Colorectal cancer | TCF4 | Positively regulates SOX9 | [72] |
|
| |||
| Colorectal cancer | PPARγ | In cell lines ligand-dependent PPARγ activation unevenly influences SOX9 and β-catenin expression and subcellular localization, suggesting a variable mechanistic role in colon carcinogenesis | [52] |
|
| |||
| Colorectal cancer | NF-Y | SOX9 is necessary for the function of NF-Y in activating expression of cyclin B1, cyclin B2, cyclin dependent kinase 1 and topoisomerase II α | [53] |
|
| |||
| Colorectal cancer | FOXK2 | Is a SOX9 target and promotes proliferation | [54] |
Acknowledgments
The authors acknowledge Consejo Nacional de Ciencia y Tecnología CONACYT (grant 290311) for funding. Mariana Avendaño-Félix (575985), Erik Lizárraga-Verdugo (304939), and Mercedes Bermúdez (220327) received CONACYT fellowships.
Contributor Information
Maribel Aguilar-Medina, Email: maribelaguilar@uas.edu.mx.
César López-Camarillo, Email: genomicas@yahoo.com.mx.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Beck B., Blanpain C. Unravelling cancer stem cell potential. Nature Reviews Cancer. 2013;13(10):727–738. doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]
- 2.Kuşoğlu A., Biray Avcı Ç. Cancer stem cells: a brief review of the current status. Gene. 2019;681:80–85. doi: 10.1016/j.gene.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 3.Sekido R., Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends in Genetics. 2009;25(1):19–29. doi: 10.1016/j.tig.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Wegner M. All purpose sox: the many roles of sox proteins in gene expression. The International Journal of Biochemistry & Cell Biology. 2010;42(3):381–390. doi: 10.1016/j.biocel.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Wang H.-Y., Lian P., Zheng P.-S. SOX9, a potential tumor suppressor in cervical cancer, transactivates p21WAF1/CIP1 and suppresses cervical tumor growth. Oncotarget . 2015;6(24):20711–20722. doi: 10.18632/oncotarget.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prévostel C., Rammah-Bouazza C., Trauchessec H., et al. SOX9 is an atypical intestinal tumor suppressor controlling the oncogenic Wnt/ss-catenin signaling. Oncotarget. 2016;7(50):82228–82243. doi: 10.18632/oncotarget.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jo A., Denduluri S., Zhang B., et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes & Diseases. 2014;1(2):149–161. doi: 10.1016/j.gendis.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luanpitpong S., Li J., Manke A., et al. SLUG is required for SOX9 stabilization and functions to promote cancer stem cells and metastasis in human lung carcinoma. Oncogene. 2016;35(22):2824–2833. doi: 10.1038/onc.2015.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo W., Keckesova Z., Donaher J. L., et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148(5):1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voronkova M. A., Luanpitpong S., Rojanasakul L. W., et al. SOX9 regulates cancer stem-like properties and metastatic potential of single-walled carbon nanotube-exposed cells. Scientific Reports. 2017;7(1):p. 11653. doi: 10.1038/s41598-017-12037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinclair A. H., Berta P., Palmer M. S., et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346(6281):240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 12.Symon A., Harley V. SOX9: A genomic view of tissue specific expression and action. The International Journal of Biochemistry & Cell Biology. 2017;87:18–22. doi: 10.1016/j.biocel.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Badis G., Berger M. F., Philippakis A. A., et al. Diversity and complexity in DNA recognition by transcription factors. Science. 2009;324(5935):1720–1723. doi: 10.1126/science.1162327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Research. 1999;27(6):1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y.-H., Jankowski A., Cheah K. S. E., Prabhakar S., Jauch R. SOXE transcription factors form selective dimers on non-compact DNA motifs through multifaceted interactions between dimerization and high-mobility group domains. Scientific Reports. 2015;5:10398–10398. doi: 10.1038/srep10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sim H., Argentaro A., Harley V. R. Boys, girls and shuttling of SRY and SOX9. Trends in Endocrinology & Metabolism. 2008;19(6):213–222. doi: 10.1016/j.tem.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Peng C., Li N., Ng Y.-K., et al. A unilateral negative feedback loop between miR-200 microRNAs and Sox2/E2F3 controls neural progenitor cell-cycle exit and differentiation. The Journal of Neuroscience. 2012;32(38):13292–13308. doi: 10.1523/jneurosci.2124-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oosterveen T., Kurdija S., Alekseenko Z., et al. Mechanistic differences in the transcriptional interpretation of local and long-range shh morphogen signaling. Developmental Cell. 2012;23(5):1006–1019. doi: 10.1016/j.devcel.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Bernard P., Harley V. R. Acquisition of SOX transcription factor specificity through protein-protein interaction, modulation of Wnt signalling and post-translational modification. The International Journal of Biochemistry & Cell Biology. 2010;42(3):400–410. doi: 10.1016/j.biocel.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Sarkar A., Hochedlinger K. The SOX family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12(1):15–30. doi: 10.1016/j.stem.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott C. E., Wynn S. L., Sesay A., et al. SOX9 induces and maintains neural stem cells. Nature Neuroscience. 2010;13(10):1181–1189. doi: 10.1038/nn.2646. [DOI] [PubMed] [Google Scholar]
- 22.Guo X., Xiong L., Sun T., et al. Expression features of SOX9 associate with tumor progression and poor prognosis of hepatocellular carcinoma. Diagnostic Pathology. 2012;7(1, article 44) doi: 10.1186/1746-1596-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richtig G., Aigelsreiter A., Schwarzenbacher D., et al. SOX9 is a proliferation and stem cell factor in hepatocellular carcinoma and possess widespread prognostic significance in different cancer types. PLoS ONE. 2017;12(11):p. e0187814. doi: 10.1371/journal.pone.0187814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakravarty G., Rider B., Mondal D. Cytoplasmic compartmentalization of SOX9 abrogates the growth arrest response of breast cancer cells that can be rescued by Trichostatin A treatment. Cancer Biology & Therapy. 2011;11(1):71–83. doi: 10.4161/cbt.11.1.13952. [DOI] [PubMed] [Google Scholar]
- 25.Wan Y.-P., Xi M., He H.-C., et al. Expression and clinical significance of SOX9 in renal cell carcinoma, bladder cancer and penile cancer. Oncology Research and Treatment. 2017;40(1-2):15–20. doi: 10.1159/000455145. [DOI] [PubMed] [Google Scholar]
- 26.Santos J. C., Carrasco-Garcia E., Garcia-Puga M., et al. SOX9 elevation acts with canonical WNT signaling to drive gastric cancer progression. Cancer Research. 2016;76(22):6735–6746. doi: 10.1158/0008-5472.CAN-16-1120. [DOI] [PubMed] [Google Scholar]
- 27.Song W., Kwon G. Y., Kim J. H., et al. Immunohistochemical staining of ERG and SOX9 as potential biomarkers of docetaxel response in patients with metastatic castration-resistant prostate cancer. Oncotarget . 2016;7(50):83735–83743. doi: 10.18632/oncotarget.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdelski C., Bujupi E., Tsourlakis M. C., et al. Loss of SOX9 expression is associated with PSA recurrence in ERG-positive and pten deleted prostate cancers. PLoS ONE. 2015;10(6) doi: 10.1371/journal.pone.0128525.e0128525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raspaglio G., Petrillo M., Martinelli E., et al. Sox9 and Hif-2α regulate TUBB3 gene expression and affect ovarian cancer aggressiveness. Gene. 2014;542(2):173–181. doi: 10.1016/j.gene.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 30.Miura K., Kimura K., Amano R., et al. Analysis of the origin of anaplastic pancreatic cancer and the mechanism of its dedifferentiation. Journal of Hepato-Biliary-Pancreatic Sciences. 2017;24(3):176–184. doi: 10.1002/jhbp.429. [DOI] [PubMed] [Google Scholar]
- 31.Lü B., Fang Y., Xu J., et al. Analysis of SOX9 expression in colorectal cancer. American Journal of Clinical Pathology. 2008;130(6):897–904. doi: 10.1309/AJCPW1W8GJBQGCNI. [DOI] [PubMed] [Google Scholar]
- 32.Marcker Espersen M. L., Linnemann D., Christensen I. J., Alamili M., Troelsen J. T., Høgdall E. SOX9 expression predicts relapse of stage II colon cancer patients. Human Pathology. 2016;52:38–46. doi: 10.1016/j.humpath.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 33.Xicola R. M., Manojlovic Z., Augustus G. J., et al. Lack of APC somatic mutation is associated with early-onset colorectal cancer in African Americans. Carcinogenesis. 2018;39(11):1331–1341. doi: 10.1093/carcin/bgy122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prévot P.-P., Simion A., Grimont A., et al. Role of the ductal transcription factors HNF6 and Sox9 in pancreatic acinar-to-ductal metaplasia. Gut. 2012;61(12):1723–1732. doi: 10.1136/gutjnl-2011-300266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hessmann E., Zhang J.-S., Chen N.-M., et al. NFATc4 Regulates Sox9 gene expression in acinar cell plasticity and pancreatic cancer initiation. Stem Cells International. 2016;2016:11. doi: 10.1155/2016/5272498.5272498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeannot P., Callot C., Baer R., et al. Loss of p27Kip1 promotes metaplasia in the pancreas via the regulation of Sox9 expression. Oncotarget . 2015;6(34):35880–35892. doi: 10.18632/oncotarget.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kopp J. L., von Figura G., Mayes E., et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22(6):737–750. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia S., Feng Z., Qi X., et al. Clinical implication of Sox9 and activated Akt expression in pancreatic ductal adenocarcinoma. Medical Oncology. 2015;32(1):p. 358. doi: 10.1007/s12032-014-0358-0. [DOI] [PubMed] [Google Scholar]
- 39.Huang L., Holtzinger A., Jagan I., et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nature Medicine. 2015;21(11):p. 1364. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun L., Mathews L. A., Cabarcas S. M., et al. Epigenetic regulation of SOX9 by the NF-κB signaling pathway in pancreatic cancer stem cells. Stem Cells. 2013;31(8):1454–1466. doi: 10.1002/stem.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultz M. J., Holdbrooks A. T., Chakraborty A., et al. The tumor-associated glycosyltransferase ST6Gal-I regulates stem cell transcription factors and confers a cancer stem cell phenotype. Cancer Research. 2016;76(13):3978–3988. doi: 10.1158/0008-5472.CAN-15-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higashihara T., Yoshitomi H., Nakata Y., et al. Sex determining region y box 9 induces chemoresistance in pancreatic cancer cells by induction of putative cancer stem cell characteristics and its high expression predicts poor prognosis. Pancreas. 2017;46(10):1296–1304. doi: 10.1097/MPA.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 43.Deng W., Vanderbilt D. B., Lin C., Martin K. H., Brundage K. M., Ruppert J. M. SOX9 inhibits beta-TrCP-mediated protein degradation to promote nuclear GLI1 expression and cancer stem cell properties. Journal of Cell Science. 2015;128(6):1123–1138. doi: 10.1242/jcs.162164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kondratyeva L. G., Sveshnikova A. A., Grankina E. V., et al. Downregulation of expression of mater genes SOX9, FOXA2, and GATA4 in pancreatic cancer cells stimulated with TGFβ1 epithelial–mesenchymal transition. Doklady Biochemistry and Biophysics. 2016;469(1):257–259. doi: 10.1134/S1607672916040062. [DOI] [PubMed] [Google Scholar]
- 45.Pinho A. V., Rooman I., Real F. X. p53-dependent regulation of growth, epithelial-mesenchymal transition and stemness in normal pancreatic epithelial cells. Cell Cycle. 2011;10(8):1312–1321. doi: 10.4161/cc.10.8.15363. [DOI] [PubMed] [Google Scholar]
- 46.Camaj P., Jackel C., Krebs S., et al. Hypoxia-independent gene expression mediated by SOX9 promotes aggressive pancreatic tumor biology. Molecular Cancer Research. 2014;12(3):421–432. doi: 10.1158/1541-7786.MCR-13-0351. [DOI] [PubMed] [Google Scholar]
- 47.Meng F., Takaori K., Ito T., et al. Expression of SOX9 in intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2014;43(1):7–14. doi: 10.1097/MPA.0b013e3182a70b2f. [DOI] [PubMed] [Google Scholar]
- 48.Galmiche L., Sarnacki S., Verkarre V., et al. Transcription factors involved in pancreas development are expressed in paediatric solid pseudopapillary tumours. Histopathology. 2008;53(3):318–324. doi: 10.1111/j.1365-2559.2008.03108.x. [DOI] [PubMed] [Google Scholar]
- 49.Javier B. M., Yaeger R., Wang L., et al. Recurrent, truncating Sox9 mutations are associated with sox9 overexpression, KRAS mutation, and TP53 wild type status in colorectal carcinoma. Oncotarget . 2016;7(32):50875–50882. doi: 10.18632/oncotarget.9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Kuraya K. S. Editorial: KRAS and TP53 mutations in colorectal carcinoma. Saudi Journal of Gastroenterology. 2009;15(4):217–219. doi: 10.4103/1319-3767.56087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdel-Samad R., Zalzali H., Rammah C., et al. MiniSOX9, a dominant-negative variant in colon cancer cells. Oncogene. 2011;30(22):2493–2503. doi: 10.1038/onc.2010.621. [DOI] [PubMed] [Google Scholar]
- 52.Panza A. Interplay between SOX9, beta-catenin and PPARgamma activation in colorectal cancer. Biochimica et Biophysica Acta. Aug 2013;1833(8):1853–65. doi: 10.1016/j.bbamcr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Shi Z., Chiang C.-I., Labhart P., et al. Context-specific role of SOX9 in NF-Y mediated gene regulation in colorectal cancer cells. Nucleic Acids Research. 2015;43(13):6257–6269. doi: 10.1093/nar/gkv568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qian Y., Xia S., Feng Z. Sox9 mediated transcriptional activation of FOXK2 is critical for colorectal cancer cells proliferation. Biochemical and Biophysical Research Communications. 2017;483(1):475–481. doi: 10.1016/j.bbrc.2016.12.119. [DOI] [PubMed] [Google Scholar]
- 55.Shen Z., Deng H., Fang Y., et al. Identification of the interplay between SOX9 and S100P in the metastasis and invasion of colon carcinoma. Oncotarget. 2015;6(24):20672–20684. doi: 10.18632/oncotarget.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi B., Park S., Lee S., Cha Y. N., Surh Y. Hypoxia induces epithelial-mesenchymal transition in colorectal cancer cells through ubiquitin-specific protease 47-mediated stabilization of Snail: A potential role of Sox9. Scientific Reports. 2017;7(1) doi: 10.1038/s41598-017-15139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carrasco-Garcia E., Lopez L., Aldaz P., et al. SOX9-regulated cell plasticity in colorectal metastasis is attenuated by rapamycin. Scientific Reports. 2016;6(1):p. 32350. doi: 10.1038/srep32350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fazilaty H., Gardaneh M., Akbari P., Zekri A., Behnam B. SLUG and SOX9 cooperatively regulate tumor initiating niche factors in breast cancer. Cancer Microenvironment. 2016;9(1):71–74. doi: 10.1007/s12307-015-0176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chakravarty G., Moroz K., Makridakis N. M., et al. Prognostic significance of cytoplasmic SOX9 in invasive ductal carcinoma and metastatic breast cancer. Experimental Biology and Medicine. 2011;236(2):145–155. doi: 10.1258/ebm.2010.010086. [DOI] [PubMed] [Google Scholar]
- 60.Wang Q.-Y., et al. MiR-133b targets Sox9 to control pathogenesis and metastasis of breast cancer. Cell Death & Disease. 2018;9(7):752–2018. doi: 10.1038/s41419-018-0715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lei B., Zhang Y., Liu T., Li Y., Pang D. Sox9 upregulation in breast cancer is correlated with poor prognosis and the CD44+/CD24-/low phenotype. International Journal of Clinical and Experimental Pathology. 2016;9(7) [Google Scholar]
- 62.Lauth M., Toftgård R. Non-canonical activation of GLI transcription factors: Implications for targeted anti-cancer therapy. Cell Cycle. 2007;6(20):2458–2463. doi: 10.4161/cc.6.20.4808. [DOI] [PubMed] [Google Scholar]
- 63.Ruiz i Altaba A., Mas C., Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends in Cell Biology. 2007;17(9):438–447. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stecca B., Ruiz I Altaba A. Context-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG signals. Journal of Molecular Cell Biology. 2010;2(2):84–95. doi: 10.1093/jmcb/mjp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morris J. P., Wang S. C., Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nature Reviews Cancer. 2010;10(10):683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hui C.-C., Angers S. Gli proteins in development and disease. Annual Review of Cell and Developmental Biology. 2011;27:513–537. doi: 10.1146/annurev-cellbio-092910-154048. [DOI] [PubMed] [Google Scholar]
- 67.Strobel O., Hartwig W., Bergmann F., et al. Anaplastic pancreatic cancer: presentation, surgical management, and outcome. Surgery. 2011;149(2):200–208. doi: 10.1016/j.surg.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 68.Clark C. J., Graham R. P., Arun J. S., Harmsen W. S., Reid-Lombardo K. M. Clinical outcomes for anaplastic pancreatic cancer: a population-based study. Journal of the American College of Surgeons. 2012;215(5):627–634. doi: 10.1016/j.jamcollsurg.2012.06.418. [DOI] [PubMed] [Google Scholar]
- 69.Evans G. S., Chandler J. A. Cell proliferation studies in rat prostate I. the proliferative role of basal and secretory epithelial cells during normal growth. The Prostate. 1987;10(2):163–178. doi: 10.1002/pros.2990100208. [DOI] [PubMed] [Google Scholar]
- 70.Ma F., Ye H., He H. H., et al. SOX9 drives WNT pathway activation in prostate cancer. The Journal of Clinical Investigation. 2016;126(5):1745–1758. doi: 10.1172/JCI78815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang G., Lunardi A., Zhang J., et al. Zbtb7a suppresses prostate cancer through repression of a Sox9-dependent pathway for cellular senescence bypass and tumor invasion. Nature Genetics. 2013;45(7):739–746. doi: 10.1038/ng.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Korinek V., Barker N., Morin P. J., et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275(5307):1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 73.Matheu A., Collado M., Wise C., et al. Oncogenicity of the developmental transcription factor Sox9. Cancer Research. 2012;72(5):1301–1315. doi: 10.1158/0008-5472.CAN-11-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Institute W. S. Catalogue of somatic mutations in cancer. https://cancer.sanger.ac.uk/cosmic.
- 75.Forbes S. A., Beare D., Boutselakis H., et al. COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Research. 2017;45(1):D777–D783. doi: 10.1093/nar/gkw1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Balogh J., Victor D., Asham E. H., et al. Hepatocellular carcinoma: a review. Journal of Hepatocellular Carcinoma. 2016;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin W., Chen Y.-L., Jiang L., Chen J.-K. Reduced expression of chemerin is associated with a poor prognosis and a lowed infiltration of both dendritic cells and natural killer cells in human hepatocellular carcinoma. Clinical Laboratory. 2011;57(11-12):879–885. [PubMed] [Google Scholar]
- 78.Dettmer M., Itin P., Miny P., Gandhi M., Cathomas G., Willi N. Giant ectopic liver, hepatocellular carcinoma and pachydermia-a rare genetic syndrome? Diagnostic Pathology. 2011;6(1, article no. 75) doi: 10.1186/1746-1596-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Whittaker S., Marais R., Zhu A. X. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29(36):4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 80.Kawaguchi Y. Sox9 and programming of liver and pancreatic progenitors. The Journal of Clinical Investigation. 2013;123(5):1881–1886. doi: 10.1172/JCI66022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xue T.-C., Zhang L., Ren Z.-G., et al. Sex-determination gene SRY potentially associates with poor prognosis but not sex bias in hepatocellular carcinoma. Digestive Diseases and Sciences. 2015;60(2):427–435. doi: 10.1007/s10620-014-3377-y. [DOI] [PubMed] [Google Scholar]
- 82.Leung C. O.-N., Mak W.-N., Kai A. K. L., et al. Sox9 confers stemness properties in hepatocellular carcinoma through Frizzled-7 mediated Wnt/β-catenin signaling. Oncotarget . 2016;7(20):29371–29386. doi: 10.18632/oncotarget.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coulouarn C., Cavard C., Rubbia-Brandt L., et al. Combined hepatocellular-cholangiocarcinomas exhibit progenitor features and activation of Wnt and TGFβ signaling pathways. Carcinogenesis. 2012;33(9):1791–1796. doi: 10.1093/carcin/bgs208. [DOI] [PubMed] [Google Scholar]
- 84.Cleere D. W. Triple-negative breast cancer: a clinical update. Community Oncology. 2010;7(5):203–211. doi: 10.1016/S1548-5315(11)70394-1. [DOI] [Google Scholar]
- 85.Malhotra G. K., Zhao X., Edwards E., et al. The role of Sox9 in mouse mammary gland development and maintenance of mammary stem and luminal progenitor cells. BMC Developmental Biology. 2014;14(1):p. 47. doi: 10.1186/s12861-014-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferlay J., Soerjomataram I., Dikshit R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 2014;136(5):E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 87.Vallot C., Stransky N., Bernard-Pierrot I., et al. A novel epigenetic phenotype associated with the most aggressive pathway of bladder tumor progression. Journal of the National Cancer Institute. 2011;103(1):47–60. doi: 10.1093/jnci/djq470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aleman A., Adrien L., Lopez-Serra L., et al. Identification of DNA hypermethylation of SOX9 in association with bladder cancer progression using CpG microarrays. British Journal of Cancer. 2008;98(2):466–473. doi: 10.1038/sj.bjc.6604143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun J., Chen Z., Zhu T., et al. Hypermethylated SFRP1, but none of other nine genes “informative” for western countries, is valuable for bladder cancer detection in Mainland China. Journal of Cancer Research and Clinical Oncology. 2009;135(12):1717–1727. doi: 10.1007/s00432-009-0619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu J., Zhu T., Wang Z., et al. A novel set of DNA methylation markers in urine sediments for sensitive/specific detection of bladder cancer. Clinical Cancer Research. 2007;13(24):7296–7304. doi: 10.1158/1078-0432.CCR-07-0861. [DOI] [PubMed] [Google Scholar]
- 91.Son H. S., Shin Y. M., Park K. K., et al. Correlation between HER2 overexpression and clinicopathological characteristics in gastric cancer patients who have undergone curative resection. Gastric Cancer. 2014;14(3):180–186. doi: 10.5230/jgc.2014.14.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yasui W., Oue N., Sentani K., Sakamoto N., Motoshita J. Transcriptome dissection of gastric cancer: Identification of novel diagnostic and therapeutic targets from pathology specimens. Pathology International. 2009;59(3):121–136. doi: 10.1111/j.1440-1827.2009.02329.x. [DOI] [PubMed] [Google Scholar]
- 93.Sashikawa Kimura M., Mutoh H., Sugano K. SOX9 is expressed in normal stomach, intestinal metaplasia, and gastric carcinoma in humans. Journal of Gastroenterology. 2011;46(11):1292–1299. doi: 10.1007/s00535-011-0443-5. [DOI] [PubMed] [Google Scholar]
- 94.Choi Y. J., Song J. H., Yoon J. H., et al. Aberrant expression of SOX9 is associated with gastrokine 1 inactivation in gastric cancers. Gastric Cancer. 2014;17(2):247–254. doi: 10.1007/s10120-013-0277-3. [DOI] [PubMed] [Google Scholar]
- 95.Ren X., Zheng D., Guo F., et al. PPARγ suppressed Wnt/β-catenin signaling pathway and its downstream effector SOX9 expression in gastric cancer cells. Medical Oncology. 2015;32(4):p. 91. doi: 10.1007/s12032-015-0536-8. [DOI] [PubMed] [Google Scholar]
- 96.Yoon J. H., Song J. H., Zhang C., et al. Inactivation of the Gastrokine 1 gene in gastric adenomas and carcinomas. The Journal of Pathology. 2011;223(5):618–625. doi: 10.1002/path.2838. [DOI] [PubMed] [Google Scholar]
- 97.Zhou C., Guo J., Zhu K., et al. Elevated expression of SOX9 is related with the progression of gastric carcinoma. Diagnostic Cytopathology. 2011;39(2):105–109. doi: 10.1002/dc.21348. [DOI] [PubMed] [Google Scholar]
- 98.Wang J., Xue X., Hong H., et al. Upregulation of microRNA-524-5p enhances the cisplatin sensitivity of gastric cancer cells by modulating proliferation and metastasis via targeting SOX9. Oncotarget. 2017;8(1):574–582. doi: 10.18632/oncotarget.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J.-N., Shang Guan Y.-M., Qi Y.-Z., Wang H.-B., Zhang T.-G., Zhou C.-J. The evaluation of SOX9 expression and its relationship with carcinoembryonic antigen-related cell adhesion molecule 1 in gastric neoplastic and nonneoplastic lesions. Annals of Diagnostic Pathology. 2012;16(4):235–244. doi: 10.1016/j.anndiagpath.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 100.Serizawa T., Hirata Y., Hayakawa Y., et al. Gastric metaplasia induced by Helicobacter pylori is associated with enhanced SOX9 expression via interleukin-1 signaling. Infection and Immunity. 2015;84(2):562–572. doi: 10.1128/IAI.01437-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amieva M., Peek R. M., Jr. Pathobiology of helicobacter pylori-induced gastric cancer. Gastroenterology. 2016;150(1):64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wadhwa R., Song S., Lee J., Yao Y., Wei Q., Ajani J. A. Gastric cancer—molecular and clinical dimensions. Nature Reviews Clinical Oncology. 2013;10(11):643–655. doi: 10.1038/nrclinonc.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sigal M., Rothenberg M. E., Logan C. Y., et al. Helicobacter pylori activates and expands Lgr5+ stem cells through direct colonization of the gastric glands. Gastroenterology. 2015;148(7):1392–1404.e21. doi: 10.1053/j.gastro.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 104.Sun M., Uozaki H., Hino R., et al. SOX9 expression and its methylation status in gastric cancer. Virchows Archiv. 2012;460(3):271–279. doi: 10.1007/s00428-012-1201-7. [DOI] [PubMed] [Google Scholar]
- 105.Seymour P. A., Freude K. K., Tran M. N., et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proceedings of the National Acadamy of Sciences of the United States of America. 2007;104(6):1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Furuyama K., Kawaguchi Y., Akiyama H., et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nature Genetics. 2011;43(1):34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 107.Morris J. P. T., IV, Cano D. A., Sekine S., Wang S. C., Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. The Journal of Clinical Investigation. 2010;120(2):508–520. doi: 10.1172/jci40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gnoni A., Licchetta A., Scarpa A., et al. Carcinogenesis of pancreatic adenocarcinoma: precursor lesions. International Journal of Molecular Sciences. 2013;14(10):19731–19762. doi: 10.3390/ijms141019731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hruban R. H., Adsay N. V., Albores-Saavedra J., et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. The American Journal of Surgical Pathology. 2001;25(5):579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 110.Fero M. L., Rivkin M., Tasch M., et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient Mice. Cell. 1996;85(5):733–744. doi: 10.1016/S0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 111.Kiyokawa H., Kineman R. D., Manova-Todorova K. O., et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of P27Kip1. Cell. 1996;85(5):721–732. doi: 10.1016/S0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 112.Nakayama K., Ishida N., Shirane M., et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85(5):707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 113.Navas C., Hernández-Porras I., Schuhmacher A. J., Sibilia M., Guerra C., Barbacid M. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22(3):318–330. doi: 10.1016/j.ccr.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bardeesy N., DePinho R. A. Pancreatic cancer biology and genetics. Nature Reviews Cancer. 2002;2(12):897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 115.Delous M., Yin C., Shin D., et al. sox9b is a key regulator of pancreaticobiliary ductal system development. PLoS Genetics. 2012;8(6) doi: 10.1371/journal.pgen.1002754.e1002754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vivanco I., Sawyers C. L. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nature Reviews Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 117.Balic A., Dorado J., Alonso-Gómez M., Heeschen C. Stem cells as the root of pancreatic ductal adenocarcinoma. Experimental Cell Research. 2012;318(6):691–704. doi: 10.1016/j.yexcr.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 118.Li C., Heidt D. G., Dalerba P., et al. Identification of pancreatic cancer stem cells. Cancer Research. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.can-06-2030. [DOI] [PubMed] [Google Scholar]
- 119.Hermann P. C., Huber S. L., Herrler T., et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 120.Li C., Wu J., Hynes M., et al. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141(6):2218.e5–2227.e5. doi: 10.1053/j.gastro.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 121.Rasheed Z. A., Yang J., Wang Q., et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. Journal of the National Cancer Institute. 2010;102(5):340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Swindall A. F., Londoño-Joshi A. I., Schultz M. J., Fineberg N., Buchsbaum D. J., Bellis S. L. ST6Gal-I protein expression is upregulated in human epithelial tumors and correlates with stem cell markers in normal tissues and colon cancer cell lines. Cancer Research. 2013;73(7):2368–2378. doi: 10.1158/0008-5472.CAN-12-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kondratyeva L. G., Chernov I. P., Zinovyeva M. V., Kopantzev E. P., Sverdlov E. D. Expression of master regulatory genes of embryonic development in pancreatic tumors. Doklady Biochemistry and Biophysics. 2017;475(1):250–252. doi: 10.1134/S1607672917040020. [DOI] [PubMed] [Google Scholar]
- 124.Heldin C.-H., Vanlandewijck M., Moustakas A. Regulation of EMT by TGFβ in cancer. FEBS Letters. 2012;586(14):1959–1970. doi: 10.1016/j.febslet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 125.Reichert M., Rustgi A. K. Pancreatic ductal cells in development, regeneration, and neoplasia. The Journal of Clinical Investigation. 2011;121(12):4572–4578. doi: 10.1172/jci57131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arda H. E., Benitez C. M., Kim S. K. Gene regulatory networks governing pancreas development. Developmental Cell. 2013;25(1):5–13. doi: 10.1016/j.devcel.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Handorf A. M., Li W.-J. Fibroblast growth Factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis. PLoS ONE. 2011;6(7) doi: 10.1371/journal.pone.0022887.e22887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Klöppel G., Basturk O., Schlitter A. M., Konukiewitz B., Esposito I. Intraductal neoplasms of the pancreas. Seminars in Diagnostic Pathology. 2014;31(6):452–466. doi: 10.1053/j.semdp.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 129.Kimura W., Sasahira N., Yoshikawa T., Muto T., Makuuchi M. Duct-ectatic type of mucin producing tumor of the pancreas - new concept of pancreatic neoplasia. Hepato-Gastroenterology. 1996;43(9):692–709. [PubMed] [Google Scholar]
- 130.Bosman F. T., Organization W. H., Cancer I. A. f. R. o. WHO Classification of Tumours of the Digestive System. International Agency for Research on Cancer; 2010. [Google Scholar]
- 131.Cano D. A., Hebrok M., Zenker M. Pancreatic development and disease. Gastroenterology. 2007;132(2):745–762. doi: 10.1053/j.gastro.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 132.Heiser P. W., Lau J., Taketo M. M., Herrera P. L., Hebrok M. Stabilization of beta-catenin impacts pancreas growth. Development. 2006;133(10):2023–2032. doi: 10.1242/dev.02366. [DOI] [PubMed] [Google Scholar]
- 133.Offield M. F., et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122(3):983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 134.Wang H., McKnight N. C., Zhang T., Lu M. L., Balk S. P., Yuan X. SOX9 is expressed in normal prostate basal cells and regulates androgen receptor expression in prostate cancer cells. Cancer Research. 2007;67(2):528–536. doi: 10.1158/0008-5472.CAN-06-1672. [DOI] [PubMed] [Google Scholar]
- 135.Huang Z., Hurley P. J., Simons B. W., et al. Sox9 is required for prostate development and prostate cancer initiation. Oncotarget. 2012;3(6):651–663. doi: 10.18632/oncotarget.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thomsen M. K., Francis J. C., Swain A. The role of Sox9 in prostate development. Differentiation. 2008;76(6):728–735. doi: 10.1111/j.1432-0436.2008.00293.x. [DOI] [PubMed] [Google Scholar]
- 137.Wang H., Leav I., Ibaragi S., et al. SOX9 is expressed in human fetal prostate epithelium and enhances prostate cancer invasion. Cancer Research. 2008;68(6):1625–1630. doi: 10.1158/0008-5472.CAN-07-5915. [DOI] [PubMed] [Google Scholar]
- 138.Zhong W., Qin G., Dai Q., et al. SOXs in human prostate cancer: implication as progression and prognosis factors. BMC Cancer. 2012;12(1):p. 248. doi: 10.1186/1471-2407-12-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qin G.-Q., He H.-C., Han Z.-D., et al. Combined overexpression of HIVEP3 and SOX9 predicts unfavorable biochemical recurrence-free survival in patients with prostate cancer. OncoTargets and Therapy. 2014;7:137–146. doi: 10.2147/OTT.S55432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thomsen M. K., Ambroisine L., Wynn S., et al. SOX9 elevation in the prostate promotes proliferation and cooperates with PTEN loss to drive tumor formation. Cancer Research. 2010;70(3):979–987. doi: 10.1158/0008-5472.CAN-09-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xie Y., Lu W., Liu S., et al. Crosstalk between nuclear MET and SOX9/β-catenin correlates with castration-resistant prostate cancer. Molecular Endocrinology. 2014;28(10):1629–1639. doi: 10.1210/me.2014-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cai C., Wang H., He H. H., et al. ERG induces androgen receptor-mediated regulation of SOX9 in prostate cancer. The Journal of Clinical Investigation. 2013;123(3):1109–1122. doi: 10.1172/JCI66666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Luedeke M., et al. Prostate cancer risk regions at 8q24 and 17q24 are differentially associated with somatic TMPRSS2:ERG fusion status. Human Molecular Genetics. 2016;25(24):5490–5499. doi: 10.1093/hmg/ddw349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ricciardelli C., Oehler M. K. Diverse molecular pathways in ovarian cancer and their clinical significance. Maturitas. 2009;62(3):270–275. doi: 10.1016/j.maturitas.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 145.Kurman R. J., Ellenson L. H., Ronnettm B. M. Blaustein’s Pathology of the Female Genital Tract. Springer; 2011. [DOI] [Google Scholar]
- 146.Irving-Rodgers H. F., Rodgers R. J. Extracellular matrix of the developing ovarian follicle. Seminars in Reproductive Medicine. 2006;24(4):195–203. doi: 10.1055/s-2006-948549. [DOI] [PubMed] [Google Scholar]
- 147.Papanastasopoulos P., Repanti M., Damaskou V., Bravou V., Papadaki H. Investigating differentiation mechanisms of the constituent cells of sex cord-stromal tumours of the ovary. Virchows Archiv. 2008;453(5):465–471. doi: 10.1007/s00428-008-0677-7. [DOI] [PubMed] [Google Scholar]
- 148.Parkin D. M., Whelan S. L., Ferlay J., Teppo L., Thomas D. B. Cancer incidence in five continents. IARC Scientific Publications. 2002;VIII(155):1–781. [PubMed] [Google Scholar]
- 149.Boyle P., Langman J. S. ABC of colorectal cancer epidemiology. British Medical Journal. 2000;321(7264):805–808. doi: 10.1136/bmj.321.7264.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Testa U., Pelosi E., Castelli G. Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Medical Sciences. 2018;6(2):p. 31. doi: 10.3390/medsci6020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Steele G., Jr., Ravikumar T. S. Resection of hepatic metastases from colorectal cancer: biologic perspectives. Annals of Surgery. 1989;210(2):127–138. doi: 10.1097/00000658-198908000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van der Flier L. G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annual Review of Physiology. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 153.Adolph T. E., Tomczak M. F., Niederreiter L., et al. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503(7475):272–276. doi: 10.1038/nature12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Clevers H. C., Bevins C. L. Paneth cells: Maestros of the small intestinal crypts. Annual Review of Physiology. 2013;75:289–311. doi: 10.1146/annurev-physiol-030212-183744. [DOI] [PubMed] [Google Scholar]
- 155.Blache P., Van De Wetering M., Duluc I., et al. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. The Journal of Cell Biology. 2004;166(1):37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bastide P., Darido C., Pannequin J., et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. The Journal of Cell Biology. 2007;178(4):635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fodde R., Edelmann W., Yang K., et al. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proceedings of the National Acadamy of Sciences of the United States of America. 1994;91(19):8969–8973. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shibata H., Toyama K., Shioya H., et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science. 1997;278(5335):120–133. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- 159.Harada N., Tamai Y., Ishikawa T.-O., et al. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO Journal. 1999;18(21):5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Candy P. A., Phillips M. R., Redfern A. D., et al. Notch-induced transcription factors are predictive of survival and 5-fluorouracil response in colorectal cancer patients. British Journal of Cancer. 2013;109(4):1023–1030. doi: 10.1038/bjc.2013.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Montorsi L., Guizzetti F., Alecci C., et al. Loss of zfp36 expression in colorectal cancer correlates to wnt/β-catenin activity and enhances epithelial-to-mesenchymal transition through upregulation of zeb1, sox9 and macc1. Oncotarget . 2016;7(37):59144–59157. doi: 10.18632/oncotarget.10828. [DOI] [PMC free article] [PubMed] [Google Scholar]