Abstract
Bariatric surgery procedures, such as Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG), are the most effective interventions available for sustained weight loss and improved glucose metabolism. Bariatric surgery alters the enterohepatic bile acid circulation, resulting in increased plasma bile levels as well as altered bile acid composition. While it remains unclear why both VSG and RYGB can alter bile acids, it is possible that these changes are important mediators of the effects of surgery. Moreover, a molecular target of bile acid synthesis, the bile acid–activated transcription factor FXR, is essential for the positive effects of VSG on weight loss and glycemic control. This Perspective examines the relationship and sequence of events between altered bile acid levels and composition, FXR signaling, and gut microbiota after bariatric surgery. We hypothesize that although bile acids and FXR signaling are potent mediators of metabolic function, unidentified downstream targets are the main mediators behind the benefits of weight-loss surgery. One of these targets, the gut-derived peptide FGF15/19, is a potential molecular and therapeutic marker to explain the positive metabolic effects of bariatric surgery. Focusing research efforts on identifying these complex molecular mechanisms will provide new opportunities for therapeutic strategies to treat obesity and metabolic dysfunction.
Bariatric Surgery: The New Era of Obesity and Diabetes Treatment
While obesity rates continue to rise, most treatment options such as dietary interventions are hampered by limited long-term efficacy. In contrast, bariatric surgeries, such as Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG), have proven to provide effective long-term weight loss and glycemic improvements in obese patients with type 2 diabetes (T2D) (1). Clinical data demonstrate that patients who have undergone RYGB or VSG experience increased satiety and major glycemic improvements prior to significant weight loss, suggesting that metabolic changes as result of these surgeries are essential to the weight loss and glycemic benefits (2). Therefore, it is important to identify the mediators that play a role in promoting the benefits of these surgeries with the goal of improving current surgical approaches and developing less invasive therapies that harness these effects.
The effectiveness of bariatric surgery to reduce body weight and improve glucose metabolism highlights the important role the gastrointestinal tract plays in regulating a wide range of metabolic processes. In addition to changes in traditional gut hormones, bariatric surgery also alters both the levels and composition of bile acids in rodents (3,4) and humans (5,6). Accordingly, clinical studies have shown that exogenous bile acid administration can alter a number of metabolic parameters, including decreased appetite (7), improved glucose homeostasis by increased insulin and GLP-1 levels (8,9), and increased energy expenditure and brown adipose tissue activity (10). While it remains unclear why bariatric surgery can alter bile acids, it is possible that these changes in bile acid levels and composition are important mediators of the effects of surgery.
Bile acids are well known as potent detergents that are essential for the absorption of dietary fat. Synthesized from cholesterol in the liver, bile acids are stored in the gallbladder and secreted into bile. The ingestion of fatty foods causes the gallbladder to contract and bile to be released into the proximal duodenum through the common bile duct. Upon reaching the ileum, 95% of bile acids are reabsorbed via specific transport proteins back into portal circulation, and the remaining 5% are excreted through the stool (Fig. 1). Beyond their traditional role as surfactants that enable lipid absorption, a wide range of evidence points toward bile acids acting as hormones by interacting with two receptors: a cell surface receptor termed TGR5 and nuclear ligand-activated nuclear receptor FXR. The bile acid–activated transcription factor FXR is essential for the positive effects of VSG on weight loss and glycemic control, as FXR-deficient mice show diminished effects of VSG to reduce body weight and improve glucose tolerance (11). TGR5-deficient mice also show reduced glucose improvements after VSG but normal weight loss (12). While TGR5 is an important component of bile acid physiology, the remainder of this review will focus on the multiple roles for FXR in the regulation of metabolism.
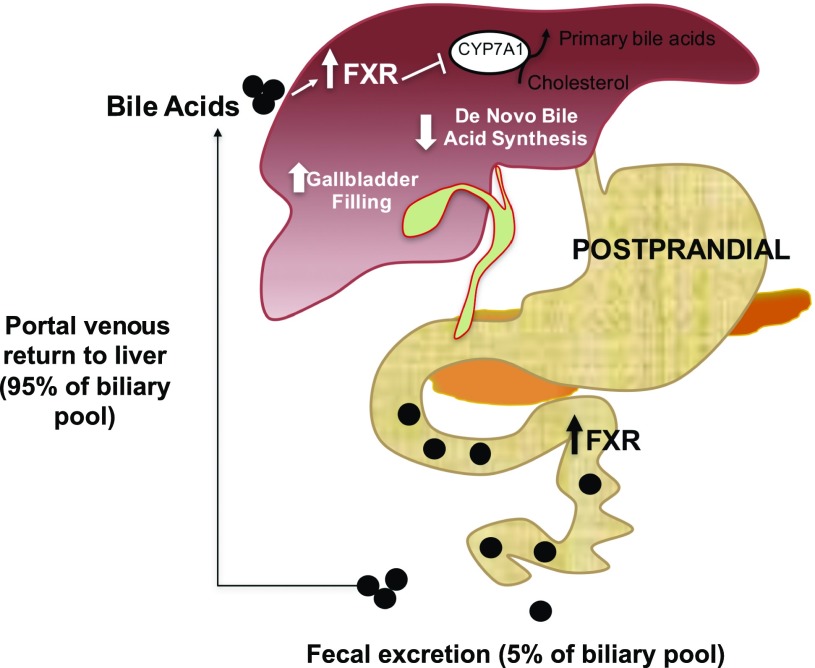
Figure 1.
Bile acid and FXR signaling. The ingestion of food causes bile acids to be released from the gallbladder into the duodenum through the common bile duct. Bile acids aid the absorption of lipids in the small intestine and activate signaling pathways to regulate the bile acid synthesis or gallbladder filling through the repression of CYP7A1, the cholesterol 7a-hydroxylase, an enzyme that governs the rate-limiting step in converting cholesterol to bile acids. Upon reaching the ileum, 95% of bile acids are recycled back through the reabsorption via specific transport proteins back into portal circulation and the remaining 5% are excreted through the stool.
Currently, it remains unclear why bile acid levels and composition are altered and whether downstream targets of FXR signaling mediate the potent benefits of bariatric surgery. In this Perspective, we evaluate on the impact of bile acids and bile acid signaling in mediating the positive metabolic effects of bariatric surgery. We examine the current knowledge and the gaps in the field about the role of bile acids and FXR signaling in regulating metabolism to highlight the potential of FXR and downstream target FGF15/19 as novel therapeutic approaches for treating metabolic disorders.
Bariatric Surgery Alters Enterohepatic Circulation of Bile Acids
Bile acid levels are increased in response to bariatric surgery and have been suggested to mediate weight loss and metabolic improvements after bariatric surgery in rodents (3,4) and humans (5,6). In concert with this, surgical bile acid diversion into the ileum of high-fat diet (HFD)-induced obese rats and mice results in comparable improvements in body weight and fat mass loss, glucose tolerance, insulin sensitivity, and liver steatosis to those observed in mice after RYGB (13,14). In RYGB, bile acids do not mix with food until the latter part of the jejunum. Increasing the circulating conjugated and total bile acid levels through surgically diverting the bile directly into jejunum of obese rats also resulted in weight loss and improvement in glucose tolerance in a weight-independent manner (14). Notably, VSG also results in increased bile levels in rodents (3) and humans (15). VSG’s ability to increase circulating bile acids implies that this profound alteration in bile acid handling is not simply the product of rerouting of bile acid and chyme as occurs with RYGB. The key point is that at least in the case of VSG, the effect is likely an alteration in the regulation of bile acids in a physiological manner rather than simply physically displacing the bile acids.
Bile Acids as Metabolic Targets in Bariatric Surgery
We have highlighted that bariatric surgical procedures cause a significant alteration of enterohepatic circulation of bile acids, which provides us with a unique model to further understand the role that bile acids play in metabolism. Conjugated and total bile acid levels are increased in T2D patients who received RYGB but decreased in T2D patients after a hypocaloric diet that resulted in similar weight loss, suggesting that the increase in bile acids after bariatric surgery is weight independent (16). Despite several lines of evidence that point to a causal role for bile acids in mediating the effects of bariatric surgery, not all the data are consistent with this hypothesis. For example, in a clinical study of T2D and normoglycemic patients who received RYGB, glucose metabolism (including insulin and postprandial GLP-1 secretion) improved shortly after surgery, but the total bile levels did not increase until 3 months postsurgery (17). Another study found decreased bile acid levels shortly after surgery and an increase at 2 years postsurgery (18). At the same time, murine models of bariatric surgery present much more consistent temporal changes in bile acid levels. We and others have reported that RYGB and VSG in obese rats and mice lead to increased bile acid levels as early as 2 weeks postsurgery (3,19). The differences observed in the timing and direction of changes in bile acids levels after surgery differs between clinical and rodent studies also point to the differences between rodent and human gut physiology in response to bariatric surgery. These data reveal the possibility that the relationship between the clinically relevant effects of bariatric procedures and the changes in bile acid levels and signaling may be more complex. Therefore, we aimed to review bile acid signaling pathways in an attempt to elucidate mechanisms responsible for altered bile acid metabolism following bariatric surgery.
The Role of Bile Acid Signaling in the Mechanisms Underlying Bariatric Surgery
FXR Signaling and Glucose Metabolism
The nuclear ligand-activated farnesoid X receptor (FXR) controls the enterohepatic cycling of bile acids by inhibiting hepatic bile acid synthesis and intestinal absorption, rendering FXR as a major regulator of bile acid signaling in both the liver and intestine. (Fig. 1). Bile acids serve as a ligand for FXR and appear to be involved in regulating glucose metabolism via FXR-related pathways, thus greatly expanding their molecular repertoire as targets for glucose and lipid control. Not surprisingly, FXR−/− (total body knockout) mice display elevated serum cholesterol and triglyceride levels and excessive fat accumulation in the liver (20,21). In addition to its pleiotrophic effects on lipid metabolism, FXR plays an important role in glucose metabolism. FXR−/− mice are insulin resistant and have reduced glucose disposal characterized by decreased liver and adipose tissue insulin signaling when mice are maintained on standard chow (20,22). Although FXR is not expressed in skeletal muscle, FXR−/− mice also display skeletal muscle insulin insensitivity contributing to their overall insulin resistance (20). At the same time, these mice have reduced adipocyte size and are protected against HFD- and genetically induced obesity (20,23). Thus, the physiological role of FXR appears to be dependent on the nutritional milieu on which the mouse is maintained (summarized in Fig. 2). Intriguingly, overexpression of FXR in db/db mice or treatment with the FXR agonist GW4064 (activating both hepatic and intestinal FXR) in db/db and ob/ob mice has positive metabolic effects, suggesting that activating FXR is a potential therapeutic strategy for metabolic disease (Fig. 2) (20,22).
Figure 2.
Comparison of genetic and pharmacological mouse models examining liver and intestinal FXR signaling in glucose metabolism and weight management. FXR controls the enterohepatic cycling of bile acids by inhibiting hepatic bile acid synthesis and intestinal absorption. Bile acids serve as a ligand for FXR and are involved in regulating glucose metabolism via FXR-related pathways. Genetic and pharmacological mouse models have revealed differential roles of liver and intestinal FXR signaling in glucose metabolism and weight management.
Recent rodent studies from our laboratory reported that bile acids are increased after VSG and that FXR is essential for the positive effects of bariatric surgery on weight loss and glycemic control (3,11). Ryan et al. (11) showed that unlike wild-type mice, FXR−/− mice did not maintain body weight loss and did not have improved glucose tolerance after VSG and while maintained on HFD (Fig. 2). A caveat of these studies is that FXR−/− mice are resistant to HFD-induced obesity and glucose intolerance, and, therefore, the window for improvement in these parameters is small (23). The key point is that it is likely that FXR’s role in metabolic regulation is different in various tissues. Consequently, this may be a critical component for mediating the beneficial effects of bile acid metabolism following bariatric surgery.
Tissue-Specific FXR Signaling
We have identified FXR as a potential link for mediating the beneficial effects of elevated bile acids following bariatric surgery. Therefore, we next aimed to elucidate the tissue-specific actions of FXR to gain a better understanding of FXR biology. Jiang and colleagues (24,25) found that mice selectively lacking intestinal expression of FXR have decreased insulin resistance and fatty livers in response to HFD. The same group showed that glycine-β-muricholic acid (Gly-MCA), a selective high-affinity intestinal FXR inhibitor that can be administered orally, prevents and reverses obesity, insulin resistance, glucose intolerance, and hepatic steatosis in HFD- and genetically induced obese mice (25). Interestingly, FXR expression levels in ileum biopsies from obese patients were positively correlated with BMI (25). These recent reports would implicate that increased FXR signaling in the ileum negatively impacts glucose metabolism and body weight. However, Fang et al. (26) reported that treatment with the gut-selective FXR agonist fexaramine reduced body weight and insulin resistance, improved glucose metabolism, increased energy expenditure, and promoted adipose tissue browning. Another recent study also showed that fexaramine improved hepatic glucose and lipid metabolism and induced adipose tissue browning (27). Examining the contribution of hepatic FXR signaling through the liver-specific FXR−/− mice showed increased plasma triglycerides and that these mice are not protected from HFD-induced obesity and insulin resistance (23). Further complicating the contribution of hepatic FXR are data showing that hepatic expression of constitutively active FXR (FXR-VP16) resulted in lower plasma glucose levels in nondiabetic mice and reduced hyperglycemia in db/db mice (22). These data (summarized in Fig. 2) represent a complex circuitry and propose new challenges in the development of pharmaceutical strategies when it comes to targeting intestinal, liver, or systemic FXR for treatment of obesity and impaired metabolic conditions.
Studies in the literature have largely focused on the function of FXR in liver and intestine, and few studies have examined how FXR signaling directly regulates the physiological action of the pancreas, adipose tissue, and other metabolic tissues involved in lipid or glucose handling. White adipose tissue expression of FXR is downregulated in HFD- and genetically induced obese mouse models (20). However, the direct contribution of FXR signaling in adipose tissue in the obese state is not known.
FXR is also expressed in pancreatic β-cells, and FXR−/− mice have reduced insulin levels (20,23) and islet size (25) However, studies exploring the direct role of FXR signaling on insulin synthesis and function are currently lacking. A recent study showed that treatment of primary cultured islets with the FXR agonist GW4064 and FXR ligand taurochenodeoxycholic acid increased calcium concentrations and electrical activity, leading to increased insulin secretion (28). The authors attributed these results to FXR-induced inhibition of KATP channel activity and not regulation of insulin synthesis. Recent studies have also tapped into the role of the microbiome in regulating β-cell mass and function through an FXR-dependent mechanism (29). Parséus et al. (29) demonstrated that FXR−/− mice from both germ-free and conventionally raised backgrounds were similarly resistant to HFD-induced β-cell dysfunction. It remains unclear whether bile acids are the primary stimulants of FXR signaling in pancreatic β-cells leading to functional changes in insulin levels. Therefore, mechanism-driven studies exploring the β-cell–specific role of FXR are needed. As a whole, these data indicate that more research is required to fully understand tissue-specific FXR biology. Gaining this knowledge may provide us with a deeper understanding of which tissues contribute to the bile acid–mediated metabolic improvements following bariatric surgery.
Gut Microbiota: Target for Enterohepatic Bile Acid/FXR Signaling
As we have discussed, bile acid signaling through FXR has been reported to be a critical mediator of the beneficial effects of bariatric surgery. This has led researchers to investigate the potential downstream targets responsible for these effects. One hypothesis is that bile acid signaling regulates metabolism via alterations in gut microbiota. Gut microbiota have previously been shown to modulate metabolism and have been implicated in the development of obesity. However, the magnitude and relevance of gut microbiota in regulating metabolism is currently an ongoing debate. The primary bile acids (chenodeoxycholic acid and cholic acid) are actively reabsorbed in the ileum, but those that escape reabsorption are deconjugated to deoxycholic acid and lithocholic acid by colonic bacteria and reabsorbed through the portal system. The colonic bacteria involved in the deconjugaton of bile acids are mostly Bacteroides species, which studies have found to be decreased in bariatric surgery patients (30,31) and rodents (11), and this change is correlated with decreased adiposity and improved glucose control.
Therefore, one target for FXR signaling in modulating the metabolic outcomes of bariatric surgery is the gut microbiota community. Sayin et al. (32) compared germ-free and conventionally raised FXR−/− mice and showed that the gut microbiota regulates CYP7A1 and FXR-downstream target Fgf15 in the ileum through an FXR-dependent pathway, thus directly regulating bile acid synthesis. The group also showed that germ-free mice have higher levels of muricholic acids and lower expression of ileum FXR-dependent genes, thus identifying tauro-conjugated β- and α-muricholic acids as FXR antagonists (32). Follow-up studies by the same group showed that FXR alters bile acid and gut microbiota composition and diversity (29). However, FXR−/− mice raised in a germ-free background had lower fasting blood glucose and improved glucose tolerance, suggesting that the microbiome improves these parameters in an FXR-independent matter (29). Transfer of the cecal microbiota from HFD-fed FXR−/− mice to germ-free wild-type mice resulted in less fat mass gain and improved glucose metabolism compared with mice that were colonized with microbiota from HFD-fed wild-type mice, suggesting that the altered gut microbiota in HFD-fed FXR−/− mice may at least partly contribute to the lean phenotype of these mice (29). Additional studies by Jiang et al. (24) similarly showed that HFD-induced obese mice treated with tempol or antibiotics had altered bile acid composition, reduced FXR signaling, reduced plasma and ileum ceramide levels, and decreased hepatic lipids. These reports suggest that gut microbiota influences glucose and lipid metabolism via bile acid–dependent modulation of FXR signaling.
FGF15/19 as Target Gene for FXR Signaling in the Metabolic Effects of Bariatric Surgery
We have discussed that the metabolic improvements that result from bariatric surgery may be partially attributed to changes in gut microbiota induced by bile acid signaling through FXR. Another FXR target gene of interest that has been identified as a potential mediator of the beneficial effects of bariatric surgery is the gut-derived hormone fibroblast growth factor 15, FGF15 (in mouse and human ortholog FGF19).
FGF15/19 has potent effects to reduce bile acid secretion at the level of both the liver and the gallbladder (33,34) and has potent effects on body weight and glucose maintenance (35). Bile acids aid the absorption of lipids in the ileum of the small intestine and activate signaling pathways to regulate the bile acid synthesis or gallbladder filling through the repression of CYP7A1, an enzyme that governs the rate-limiting step in converting cholesterol to bile acids. In the ileum, bile acids activate intestinal FXR and its downstream target FGF15/19. FGF15/19 is expressed in ileal enterocytes of the small intestine and is released postprandially in response to bile acid absorption (34). Once released from the ileum, FGF15/19 enters the portal venous circulation and travels to the liver where FGF15/19 binds to its receptor FGFR4 and represses de novo bile acid synthesis through suppression of cholesterol 7a-hydroxylase (CYP7A1) and gallbladder filling. Therefore, bile acids and FGF15/19 act as ligands to regulate bile acid synthesis and facilitate communication between the liver and small intestine. The actions of FGF15/19 resemble that of insulin in stimulating protein and glycogen synthesis and reducing gluconeogenesis. However, unlike insulin, FGF15/19 decreases hepatic triglycerides and reduces cholesterol. This notable difference has made FGF19 an attractive therapeutic target to aid insulin’s actions while avoiding some pitfalls of insulin therapy.
Circulating FGF19 levels are reduced in individuals with metabolic disorders and nonalcoholic fatty liver disease (NAFLD). Most importantly, plasma FGF19 levels increase in bariatric surgery patients, pointing to FGF15/19 as a potential target to mediate the effects of weight-loss surgeries (6). However, recent data have suggested that FGF15/19 may not provide a simple answer. Similar to FXR, FGF19 expression in ileum biopsies from obese patients also show a positive correlation to BMI (25). These data suggest that intestinal FXR and FGF19 expression in the ileum are increased in response to obesity; however, circulating levels of FGF19 are lower in obese individuals. This discrepancy in expression and circulating levels of FGF19 in obesity is unclear and requires further research efforts.
FGF15/19 Signaling in Metabolic Tissues
The mechanisms underlying the physiological and pharmacological actions of FGF15/19 or the metabolic tissues targeted by FGF15/19 are still not well understood. FGF15/19 signaling is mediated through membrane-bound FGF receptors. There are four FGF receptors (1–4) and two splice variants (FGFR1b and FGFR1c), and their expression and activation by FGF15/19 varies between tissues. FGFR4 is highly expressed in liver and gallbladder and believed to be exclusively activated by FGF15/19 in liver to suppress bile acid synthesis, gluconeogenesis, and lipogenesis and increase glycogen synthesis. The physiological role of FGF15 in metabolism was revealed through the phenotypes of FGF15 and FGFR4 total-body knockout mice and mice constitutively overexpressing FGF19. FGF19 transgenic mice are hyperphagic but have lower body weight and fat mass along with increased energy expenditure (36). Global ablation of FGF15 in FGF15−/− mice resulted in impaired glucose tolerance and elevated postprandial hepatic glycogen levels, but these mice are surprisingly protected against HFD-induced obesity. FGFR4−/− mice fed a standard diet present with metabolic syndrome phenotype, including increased adipose tissue, hyperlipidemia, and impaired glucose tolerance (37). This discrepancy in the differential body weight phenotype between FGF15−/− and FGFR4−/− mice inspired further studies looking at the signaling receptors and target tissues responsible for FGF15’s metabolic actions. One of these studies showed that liver-specific expression of a constitutively active FGFR4 variant in FGFR4−/− mice rescued lipid and cholesterol levels but did not improve the impaired glucose and insulin sensitivity of FGFR4−/− mice (37). These data have been supported by other studies showing that FGF15–FGFR4 signaling does not play a role in FGF15/19’s regulation of body weight and glucose metabolism (35,38,39).
Another FGF15/19 receptor, FGFR1, is widely expressed in most tissues, with high expression in brain and adipose tissue. Since both FGF21 and FGF15/19 can signal through FGFR1c, recent studies have explored tissue-specific mechanism of FGF19 and FGF21 in reducing body weight, blood glucose, insulin, and hepatic triglycerides. Recent studies by Lan et al. (35) ablated β-Klotho (a key component for signaling of endocrine FGFs) specifically in hepatocytes, adipose tissue, and neurons. Their findings concluded that the nervous system, and not adipose tissue or liver, is required for the weight-loss effects of FGF19. Potent effects of FGF15/19 in the central nervous system on reducing food intake and improving glucose homeostasis have been supported by several studies (35,40–42). However, acute FGF19 actions may also be partly dependent on adipose tissue to increase insulin sensitivity and whole-body glucose uptake. These findings do not eliminate the possibility of additional FGFRs and additional tissues in contributing to FGF15/19’s role in glucose homeostasis and body weight. Further research is required to evaluate the reciprocal communication between FGFRs in different metabolic tissues (liver, brain, and adipose tissue) in response to the potent metabolic effects of pharmacological administration of FGF15/19.
FGF15/19 Physiology: What Questions Remain Unanswered?
Our analysis of the literature highlights a beneficial role of FGF15/19 and FGFR1/4 signaling. However, many questions in the field remain unanswered with regard to FGF15/19 tissue-specific actions as well as its link to the beneficial effects of bariatric surgery (outlined in Fig. 3). A recent study showed that increasing FGF19 can increase skeletal muscle mass and strength, identifying muscle as a previously unrecognized site of FGF19 action. Further studies could examine whether FGF15/19 levels increase after weight-loss surgery as an adaptive mechanism to prevent muscle loss (43). Many unanswered questions also remain regarding the role of FGF15/19 signaling in pancreatic α- and β-cells and its effect on insulin and glucagon levels after bariatric surgery. FGFRs are expressed on β-cells, but there is limited research evidence as to whether FGF15/19 plays a role in pancreatic islet cell mass and function.
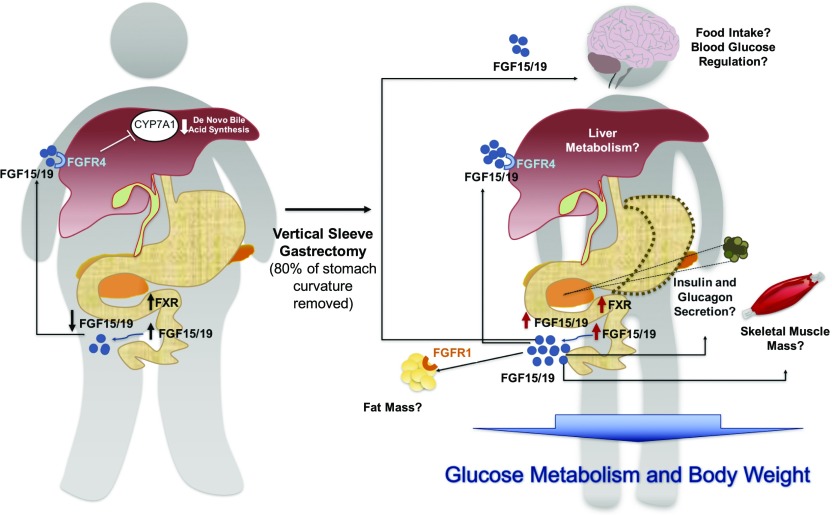
Figure 3.
Endocrine actions of FGF15/19 after VSG. Bile acids aid the absorption of lipids in the small intestine and activate FXR’s downstream target FGF15 (in mouse and human ortholog FGF19). FGF15/19 is expressed in ileal enterocytes of the small intestine and is released postprandially in response to bile acid absorption. Once released from the ileum, FGF15/19 enters the portal venous circulation and travels to the liver where FGF15/19 binds to its receptor FGFR4 and represses de novo bile acid synthesis through suppression of cholesterol 7a-hydroxylase (CYP7A1) and gallbladder filling. Circulating FGF19 levels are reduced in individuals with metabolic disorders and NAFLD. Most importantly, circulating FGF19 levels increase after bariatric surgery, pointing to FGF15/19 as a potential target to mediate the positive effects of weight-loss surgeries. Future studies are needed to examine whether increased FGF15/19 levels after VSG play a role on regulating food intake, adipose mass, muscle mass, insulin and glucagon levels, and liver metabolism.
Consistent reports demonstrate that elevating endogenous FGF15/19 levels in preclinical models of metabolic disease results in multiple metabolic benefits including increased energy expenditure, reduced adiposity, and improved lipid and glucose homeostasis (36,40,41,44,45). However, how the increased levels of FGF19 in patients following bariatric surgery directly mediate the beneficial effects of the surgery is still unclear (Fig. 3). Future studies that apply bariatric surgery in combination with animal models with tissue-specific deletion of FGF15 or FGFR1/4 may provide further insight into understanding the direct role of FGF15/19 signaling in mediating the effects of bariatric surgery. As a whole, the data in the literature suggest that more studies are required to fully understand the plethora of FGF15/19-mediated actions. Understanding these complex actions may help researchers to directly link the elevation of FGF15/19 with specific metabolic benefits of bariatric surgery.
Clinical Implications for FXR and FGF15/19 Pharmacotherapies
The beneficial effects of bile acid signaling on energy, glucose, and lipid metabolism described above have motivated pharmaceutical companies to invest in developing drugs that target these signaling pathways to treat complications associated with metabolic syndrome (summarized in Table 1). One of the complications associated with metabolic syndrome is the development of liver diseases including nonalcoholic steatohepatitis (NASH) and NAFLD. FXR agonists are promising treatments for both NASH and NAFLD, which is evident by multiple pharmaceutical companies advancing compounds through clinical development for these indications (Table 1). The FXR agonist obeticholic acid (INT-747) is an orally active analog of the natural human bile acid CDCA that improves the histological features in patients with NASH (46). Coagonist strategies that aim to activate both bile acid receptors (TGR5 and FXR) have also been developed (47). HFD-induced obese mice treated with the TGR5/FXR coagonist INT-767 had decreased body weight and improved glucose tolerance and hepatic metabolism (48). These metabolic improvements were more pronounced in mice treated with the coagonist as compared with FXR or TGR5 monotherapies, suggesting that targeting both FXR and TGR5 may have added benefit for treating obesity, diabetes, and NASH/NAFLD.
Table 1.
FXR agonists and FGF19 analogs currently under clinical development
| Compound | Company | Biological target | Route of administration | Most advanced phase | Indications |
|---|---|---|---|---|---|
| INT-747 (obeticholic acid) | Intercept Pharmaceuticals | FXR agonist | Oral | Phase II | NASH |
| Px-104 | Gilead (Phenex Pharmaceuticals) | FXR agonist | Oral | Phase II | NAFLD and NASH |
| LJN-452 | Novartis | FXR agonist | Oral | Phase II | NASH and PBC |
| GS-9674 | Gilead (Phenex Pharmaceuticals) | FXR agonist | Oral | Phase II | NASH and PBC |
| INT-767 | Intercept Pharmaceuticals | FXR/TGR5 agonist | Oral | Phase I | Liver fibrosis |
| LMB-763 | Novartis | FXR agonist | Oral | Phase II | NASH |
| EDP-305 | Enanta | FXR agonist | Oral | Phase I | NASH and PBC |
| NGM-282 | NGM Biopharmaceuticals | FGFR4 agonist | Subcutaneous injection | Phase II | T2D, PBC, NASH |
| NGM-313 | NGM Biopharmaceuticals | β-Klotho (KLB)/FGFR1c receptor agonist | Subcutaneous injection | Phase I | Obesity |
PBC, primary biliary cirrhosis.
The downstream effector of FXR, FGF19, has also gained much attention for treating metabolic diseases. A challenge that has hindered the progress of FGF19 therapeutics is the mitogenic actions of FGF19, which are mediated through the activation of FGFR4. A study comparing the structures of FGF19 and FGF21 determined the regions that were critical for mediating FGFR4-induced tumorigenic action. Based on these data, FGF19 variants were generated which lacked the ability to induce hepatocyte proliferation but still retained the ability to reduce blood glucose in obese mice (49). In another study, the FGF19 variant M70 (now NGM282) caused weight loss in obese mice but did not promote tumor development (50). Together, these preclinical data provided evidence that the metabolic and mitogenic actions of FGF19 can be separated from each other. This has reinvigorated the pursuit of FGF19 as a target for obesity and diabetes.
Studies in humans have helped solidify the preclinical evidence that the metabolic benefits of FGF19 therapies can be dissected from its unwanted mitogenic action. The FGF19 variant M70 (NGM282) reduces liver fat content and improves liver function without causing tumorigenesis in humans (51). Another compound currently in development is NGM313, which aims to avoid the tumorigenesis mediated by FGFR4 by selectively targeting the β-Klotho (KLB)/FGFR1c complex. Phase I clinical trials are currently ongoing to evaluate the safety and efficacy of NGM313 in healthy overweight and obese adults (ClinicalTrials.gov identifier NCT02708576). More studies will be required to evaluate the long-term safety and efficacy of FGF19 analogs. Importantly, understanding the potent role of FGF19 in the nervous system to mediate effects on body weight and glycemia will require determining whether these FGF19-based drugs will need to cross the blood-brain barrier to access the central nervous system (35). Taken as a whole, the data in the literature and data from clinical trials suggest that targeting bile acid signaling pathways and downstream mediators has immense potential to successfully treat patients with obesity, T2D, or NASH/NAFLD.
Article Information
Acknowledgments. The authors thank Dr. Mette Guldbrandt (Novo Nordisk) and Dr. Simon Evers (University of Michigan) for critical review of the manuscript.
Funding. R.J.S. is supported by National Institutes of Health grant 5R01DK107652. N.B. is supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grant 5T32DK071212-12.
Duality of Interest. R.J.S. has received research support and/or worked as a consultant for Novo Nordisk, Ethicon Endo-Surgery/Johnson & Johnson, Orexigen, Daiichi Sankyo, Janssen/Johnson & Johnson, Novartis, Paul Hastings Law Firm, Kallyope, Zafgen, MedImmune, Sanofi, and Scohia. K.M.H. is a paid employee of Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
References
- 1.Schauer PR, Bhatt DL, Kirwan JP, et al.; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 2017;376:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.le Roux CW, Bueter M. The physiology of altered eating behaviour after Roux-en-Y gastric bypass. Exp Physiol 2014;99:1128–1132 [DOI] [PubMed] [Google Scholar]
- 3.Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring) 2014;22:390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohli R, Kirby M, Setchell KD, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol 2010;299:G652–G660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pournaras DJ, Glicksman C, Vincent RP, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 2012;153:3613–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray GA, Gallagher TF Jr. Suppression of appetite by bile acids. Lancet 1968;1:1066–1067 [DOI] [PubMed] [Google Scholar]
- 8.Wu T, Bound MJ, Standfield SD, et al. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes Metab 2013;15:474–477 [DOI] [PubMed] [Google Scholar]
- 9.Adrian TE, Gariballa S, Parekh KA, et al. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia 2012;55:2343–2347 [DOI] [PubMed] [Google Scholar]
- 10.Broeders EP, Nascimento EB, Havekes B, et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab 2015;22:418–426 [DOI] [PubMed] [Google Scholar]
- 11.Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014;509:183–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGavigan AK, Garibay D, Henseler ZM, et al. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut 2017;66:226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn CR, Albaugh VL, Cai S, et al. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun 2015;6:7715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohli R, Setchell KD, Kirby M, et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology 2013;154:2341–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism 2009;58:1400–1407 [DOI] [PubMed] [Google Scholar]
- 16.Jahansouz C, Xu H, Hertzel AV, et al. Bile acids increase independently from hypocaloric restriction after bariatric surgery. Ann Surg 2016;264:1022–1028 [DOI] [PubMed] [Google Scholar]
- 17.Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. Improvements in glucose metabolism early after gastric bypass surgery are not explained by increases in total bile acids and fibroblast growth factor 19 concentrations. J Clin Endocrinol Metab 2015;100:E396–E406 [DOI] [PubMed] [Google Scholar]
- 18.Dutia R, Embrey M, O’Brien CS, et al. Temporal changes in bile acid levels and 12α-hydroxylation after Roux-en-Y gastric bypass surgery in type 2 diabetes. Int J Obes 2015;39:806–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhutta HY, Rajpal N, White W, et al. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PLoS One 2015;10:e0122273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cariou B, van Harmelen K, Duran-Sandoval D, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem 2006;281:11039–11049 [DOI] [PubMed] [Google Scholar]
- 21.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 2000;102:731–744 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Lee FY, Barrera G, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A 2006;103:1006–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prawitt J, Abdelkarim M, Stroeve JH, et al. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 2011;60:1861–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang C, Xie C, Li F, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest 2015;125:386–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang C, Xie C, Lv Y, et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun 2015;6:10166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med 2015;21:159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pathak P, Xie C, Nichols RG, et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 27 February 2018 [Epub ahead of print]. DOI: 10.1002/hep.29857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Düfer M, Hörth K, Wagner R, et al. Bile acids acutely stimulate insulin secretion of mouse β-cells via farnesoid X receptor activation and K(ATP) channel inhibition. Diabetes 2012;61:1479–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parséus A, Sommer N, Sommer F, et al. Microbiota-induced obesity requires farnesoid X receptor. Gut 2017;66:429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damms-Machado A, Mitra S, Schollenberger AE, et al. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res Int 2015;2015:806248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewulf EM, Cani PD, Claus SP, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013;62:1112–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayin SI, Wahlström A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 2013;17:225–235 [DOI] [PubMed] [Google Scholar]
- 33.Kir S, Beddow SA, Samuel VT, et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 2011;331:1621–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potthoff MJ, Boney-Montoya J, Choi M, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab 2011;13:729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lan T, Morgan DA, Rahmouni K, et al. FGF19, FGF21, and an FGFR1/β-Klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metab 2017;26:709–718.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomlinson E, Fu L, John L, et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 2002;143:1741–1747 [DOI] [PubMed] [Google Scholar]
- 37.Huang X, Yang C, Luo Y, Jin C, Wang F, McKeehan WL. FGFR4 prevents hyperlipidemia and insulin resistance but underlies high-fat diet induced fatty liver. Diabetes 2007;56:2501–2510 [DOI] [PubMed] [Google Scholar]
- 38.Wu AL, Coulter S, Liddle C, et al. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLoS One 2011;6:e17868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X, Ge H, Lemon B, et al. Selective activation of FGFR4 by an FGF19 variant does not improve glucose metabolism in ob/ob mice. Proc Natl Acad Sci U S A 2009;106:14379–14384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan KK, Kohli R, Gutierrez-Aguilar R, Gaitonde SG, Woods SC, Seeley RJ. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology 2013;154:9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morton GJ, Matsen ME, Bracy DP, et al. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest 2013;123:4799–4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry RJ, Lee S, Ma L, Zhang D, Schlessinger J, Shulman GI FGF1 and FGF19 reverse diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat Commun 2015;6:6980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benoit B, Meugnier E, Castelli M, et al. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat Med 2017;23:990–996 [DOI] [PubMed] [Google Scholar]
- 44.Fu L, John LM, Adams SH, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 2004;145:2594–2603 [DOI] [PubMed] [Google Scholar]
- 45.Miyata M, Sakaida Y, Matsuzawa H, Yoshinari K, Yamazoe Y. Fibroblast growth factor 19 treatment ameliorates disruption of hepatic lipid metabolism in farnesoid X receptor (Fxr)-null mice. Biol Pharm Bull 2011;34:1885–1889 [DOI] [PubMed] [Google Scholar]
- 46.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al.; NASH Clinical Research Network . Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rizzo G, Passeri D, De Franco F, et al. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol 2010;78:617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pathak P, Liu H, Boehme S, et al. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J Biol Chem 2017;292:11055–11069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu X, Ge H, Lemon B, et al. Separating mitogenic and metabolic activities of fibroblast growth factor 19 (FGF19). Proc Natl Acad Sci U S A 2010;107:14158–14163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou M, Wang X, Phung V, et al. Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Cancer Res 2014;74:3306–3316 [DOI] [PubMed] [Google Scholar]
- 51.Harrison SA, Rinella ME, Abdelmalek MF, et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2018;391:1174–1185 [DOI] [PubMed] [Google Scholar]