Abstract
Background
High dose interferon induction treatment of hepatitis C viral infection blocks viral production over 95%. Since dose reduction is often performed due to clinical considerations, the effect of dose reduction on hepatitis C virus kinetics was studied.
Methods
A new model that allowed longitudinal changes in the parameters of viral dynamics was used in a group of genotype-1 patients (N = 15) with dose reduction from 10 to 3 million units of interferon daily in combination with ribavirin, in comparison to a control group (N = 9) with no dose reduction.
Results
Dose reduction gave rise to a complex viral kinetic pattern, which could be only explained by a decrease in interferon effectiveness in blocking virion production. The benefit of the rapid initial viral decline following the high induction dose is lost after dose reduction. In addition, in some patients also the second phase viral decline slope, which is highly predictive of success of treatment, was impaired by the dose reduction resulting in smaller percentage of viral clearance in the dose reduction group.
Conclusions
These findings, while explaining the failure of many induction schedules, suggest that for genotype-1 patients induction therapy should be continued till HCVRNA negativity in serum in order to increase the sustained response rate for chronic hepatitis C.
Background
The hepatitis C virus (HCV) causes a slowly progressive liver disease, which may lead to cirrhosis, liver failure and liver cancer. Currently, about 10,000 patients die in the US from HCV related disease yearly and this number is expected to triple in the next 2–3 decades [1] Anti-viral therapy is successful in arresting the progression of the disease in those patients who reach a sustained clearance of the virus, currently only 40% of treated patients [2]. Response to therapy with alpha Interferon injections thrice a week with or without additional Ribavirin is thought to occur gradually over time, and research has focused on improving efficacy by prolonging treatment up to 1 to 2 years [2-4]. However, reports on viral dynamics analysis show that response to interferon is very fast and that a 10 to 1000 fold decrease in viral load can be reached within 24 hours of treatment. [5-7] The pattern of viral decline seems to be biphasic, with a rapid viral decline within the first 24–48 hours followed by a much slower second phase of viral decline. This biphasic decline is hypothetically caused by a direct anti-viral effect of interferon in blocking virion production from infected cells [5].
A strong dependence of the viral decline in the first phase on the dose of interferon used has been described [5,8]. Nevertheless, it has been shown that it is the second slope which is the best predictor for response to treatment [5,9]. This slower second phase slope of viral decline has large variability between patients, and therefore cross sectional analysis of its dose dependence is hindered. Instead, here we investigated the longitudinal changes in viral dynamics in patients going through a dose reduction in order to asses the effect of dose on the second slope. The current model for HCV dynamics, in which the dynamical parameters are fixed during treatment, fits the observed biphasic viral decline in patients treated with fixed Interferon dosages [5]. However, in this study we adapted the model such that the dynamical parameters can change over time due to a change in dose. We now report that early dose reduction is followed by a rise in viral load, that can only be explained by a decrease in interferon effectiveness; so the potential benefit of a rapid viral decline following high dose induction is often lost.
Methods
A case-control study was performed in an university-based tertiary referral center. Informed consent was obtained from all patients, and the human experimentation guidelines of the University Hospital Rotterdam were followed in the conduct of clinical research.
Study population
All 24 HCV genotype-1 patients enrolled in our high-dose induction studies were evaluated. All patients met the inclusion and exclusion criteria previously described [10]. Note that all patients in these studies were considered "difficult-to-treat", either because they were non-responders to previous treatment or had cirrhosis and/or high baseline viral load. Group 1 patients (N = 9) received 10 million units (MU) of Interferon-α-2b (Intron-A, Schering-Plough) daily for 4 weeks. Group 2 patients (N = 15) received 10 MU of interferon daily for the first 3 days only, followed by 3 MU interferon daily from day 3 until day 28. In both groups, ribavirin was given orally in divided doses of 1,000–1,200 mg daily (according to weight >75 kg). Subsequently, all patients received a maintenance treatment of minimally 3 MU interferon daily for 52 weeks. The patients' baseline characteristics (Table 1) were well balanced, except for a trend for larger number of cirrhotic patients in group 1.
Table 1.
Patient baseline characteristics.
| Patient characteristics | Group 1 (1) | Group 2 (1) |
| Number of patients | 9 | 15 |
| Median age | 44 | 47 |
| Male/Female | 7 / 2 | 11 / 4 |
| Race (Caucasian / Asian) | 7 / 2 | 15 / 0 |
| Pre-treatment RNA HCV | 5.5. × 106 | 7.5 × 106 |
| Genotype 1 | All | All |
| Median ALT at baseline | 89 | 122 |
| Cirrhosis/No-cirrhosis | 4 / 5 | 1 / 14 |
| Previous NR / other * | 6 / 3 | 11 / 4 |
*) Non-sustained responder to previous therapy or previously untreated patients with cirrhosis and genotype 1.
Detection of Serum HCV RNA
Plasma samples were collected frequently during the first 4 weeks of treatment for HCV RNA detection. Blood samples were collected in Vacutainer PPT tubes (Becton-Dickinson) which were spun directly after collection in order to avoid RNA breakdown. The spun PPT tubes [11] were then transported to the virology department where plasma was aliquotted in 5 separate tubes that were stored at -80°C. Plasma samples were obtained at day 0 (0, 4, 8, 12, 16 hours), day 1 (24, 32 and 40 hours), day 2 (48 and 56 hours), day 3 (72 and 80 hours) day 4, 5, 6 7, 10, 14, 17, 21 and 28 after treatment initiation. Viral load was quantified using the Cobas Amplicor Monitor™ version 2 (Roche Molecular Systems). Since the linearity of quantitative assays for high numbers of viral copies has been questionable [12] we routinely diluted samples and re-tested, if the early quantification of that sample was higher than 106 copies/ml.
Mathematical Modeling
Viral kinetics were analyzed using a modification of a previously described mathematical model for viral dynamics [5], for which the analytical solution is,
V(t) = V0 {A exp[-λ1(t - t0)] + (1 - A) exp[-λ2(t - t0)]} for (t > t0) (Eq. 1)
Where
λ1,2 = ½ {(c + δ) ± [(c - δ)2 + 4(1 - ε)(1 - Η) cδ]½} (Eq. 2)
A = (εc - λ2)/(λ1 - λ2) (Eq. 3)
This formula contains several dynamical parameters (c, δ, Η and ε) which may vary per patient according to the best fit of the actual data, but are constant over time. c describes the clearance rate of free virus, with the corresponding virus half-life of ln(2)/c. δ describes the loss rate of productively infected cells, with the corresponding cellular half-life of ln(2)/δ. The effect of interferon can be modeled here either by a block of de-novo cell infection with effectiveness Η (0 ≤ Η ≤ 1), or block of virion production with effectiveness ε (0 ≤ ε ≤ 1). The logarithmic drop in viral decline during the first phase (24–48 hours) of treatment can be approximated by log(1-ε). The 2nd phase slope can be approximated by ε times δ when Η << 1, or by δ alone when Η ≅ 1.
To investigate the effect of reducing treatment dose, all the above dynamical parameters were allowed to change over time in the solution, e.g. for interferon effectiveness in blocking virion production, ε, we use the function ε (t) :
for t ≤ t1 : ε(t) = ε1 (Eq. 4)
for t > t1 : ε (t) = (ε1 - ε2) exp(-k(t - t1)) + ε2
where t1 is the time of dose change and k is a exponential rate representing how rapid does the change in interferon dose effect the change in the parameter. Thus, the blocking effectiveness starts at ε1 (for t ≤ t1), and changes with an exponential transition to ε2 (after (t-t1) >>1/k). The same functional form was used to investigate changes in Η (from Η1 to Η2), δ (from δ1 to δ2), and c (from c1 to c2).
It is important to note that we do not explicitly model in Eq. 1 the dynamics of viral replication after the dose reduction, but rather replace the fixed parameters by time dependent parameters in the original analytical solution obtained with fixed parameters. Nevertheless, we have tested this approximation by simulating a modification of the original differential equation model [5] where changes in the dynamical parameters were allowed to change at the time of dose reduction. We found no significant difference between the simulation of the full modified differential equation model and the modified analytical solution. Since we only have 2–3 viral measurements immediately after dose reduction, we can not estimate the appropriate replication parameters and thus chose to use the simple approximation given in Eq 1.
To estimate HCV viral kinetic parameters for each patient, the logarithm of V(t) in Eq. 1 (using ε (t) from Eq. 4) was fit to the logarithm of the viral load data by a non-linear least squares method using the Madonna software (R.I. Macey and G.F. Oster, Berkeley, CA, USA). Two patients in group 2 were missing viral load data during the first week of treatment and one patient had a null response (less than 3 fold change in viral load during treatment) and therefore their viral kinetics could not be fitted.
Statistical analysis
The Fisher-exact test (2 × 2 tables) and the Chi-square test (N × N tables) were used to determine the statistical significance of the distribution of categorical variables between groups. The non-parametric independent (or related) Mann-Whitney rank sum test was used to determine the statistical significance of differences in continuous variables between the two groups (or of changes in the parameters within the same patients). Correlation among parameters, or between parameters and baseline values, was evaluated using the Spearman non-parametric test. Significance was established at P < 0.05.
Results
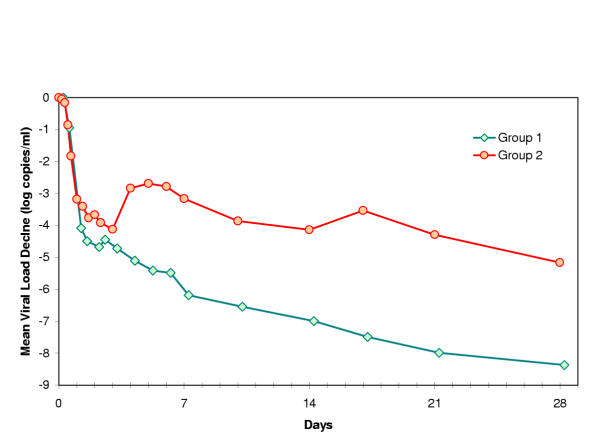
The biphasic decline previously reported [5-7,13] describes the viral kinetics during the first month for all patients in group 1 (Fig 1, Fig 2a), for whom interferon dose was kept constant. In contrast, we observed a complex dynamic pattern for 11 out of 12 evaluable patients in group 2 (Fig 1, Fig 2b,c,d). In these patients, a rapid decline occurred during the first day, followed by a slower decline on the second and third days, at which point a rapid increase in viral load (mean 0.8 [range 0–1.3] log copies/ml) is observed within 24–48 hours after the reduction in interferon dose. Thereafter, viral load again declined with a mean exponential slope comparable to the second phase slope in group 1.
Figure 1.
Group 1 (N = 9) received a continues dose of 10 MU INTERFERON daily for 28 days. Group 2 (dose reduction group, N = 15) reduced from 10 MU INTERFERON daily to 3 MU INTERFERON daily after 3 days of treatment. After the viral load data was log-transformed, the geometric means were calculated for the 2 groups for each time-point of viral load measurement. The created mean viral for group 1 clearly shows a biphasic decline in viral load as previously described for chronic HCV. Viral decline in the patients with a INTERFERON dose reduction (line Group 2) is more complex: a rebound in viral load is observed coinciding with the time of INTERFERON dose reduction.
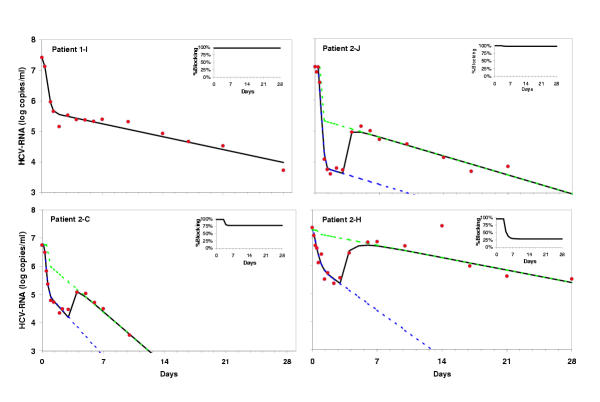
Figure 2.
In figure 2a the actual viral load measurements (red dots) and the fitted viral decline curve using Eq. 1 (black line) is presented of one of the patients (No 1-I) who receive 10 MU INTERFERON daily for 28 days. The actual decline in viral load in this patient is best described by the original bi-phasic model. In figure 2b,c,d the actual viral load (red dots) and the fitted viral decline using the modified model allowing for a change in INTERFERON efficacy as described in Eq.5 is presented (black line). The actual viral decline could only be correctly fitted by using our modified model. The red lines represent the calculated bi-phasic decline curve for a continues 10 MU INTERFERON regimen in the patients of group 2 by fitting only the observed viral load during the treatment period of 10 MU daily in the original model. The green line represents the calculated viral decline curve as if a continues treatment of 3 MU INTERFERON daily was given from the start of treatment (only the viral load during 3 MU INTERFERON are fitted). Note that the difference in the slope between the calculated decline curves for 10 MU versus 3 MU (red versus green lines) is dependent on the change in interferon efficacy after dose reduction (inserts). Patients 2-H was calculated to become HCV RNA negative within 14 days if 10 MU INTERFERON was continued (red line figure 2-d), but after dose reduction the actual viral decline curved shifted dramatically due to a strong decline in INTERFERON efficacy (green line).
We have tried to fit the viral kinetics of the patients in group 2 with several models, in each one of them allowing to change one parameter (ε, Η, c or δ) at the time of dose reduction. The only model that was able to qualitatively reproduce the observed kinetics was the one which allowed a longitudinal change in the interferon effectiveness in blocking virion production (ε) as function of the interferon dose (Eq. 4). By only allowing to change the interferon effectiveness in blocking de-novo infection (Η), death rate of infected cells (δ) or the clearance rate of free virions (c), it was not possible to fit the observed data. When assuming the major effect of interferon is to block virion production in a dose dependent way (1 > ε1 > ε2 > 0), it was possible to fit the data both with Η = 0 or Η = 1. Thus it was not possible to determine if interferon also blocks de-novo infection, in addition to blocking virion production, or not. Varying Η between 0 and 1 only gives rise to minimal changes in the estimate of ε and c, while somewhat affecting the estimate of δ when ε is smaller than 0.98 (minimal estimate of δ obtained for Η = 1, and maximal estimate for Η = 0). Moreover, when allowing ε to change at the time of dose reduction, it was not possible to rule out that the other parameters also change at the same time.
For simplicity, and since our data only implies minute effects due to changes in the other parameters, we have assumed a change occurs only in ε when estimating the dynamical parameters. In addition, we needed to estimate the transition rate k (Eq. 4) from ε1 to ε2. It was not possible to get a unique estimate of k for each patient individually, with only 2–3 measurements during the rebound. Since using k = 1 up to 20 did not significantly affect the estimate of the other parameters, we have assumed k = 2 for all patients such that the effect of ε1 vanishes within 24–48 hours after the dose reduction in accordance to the observed data.
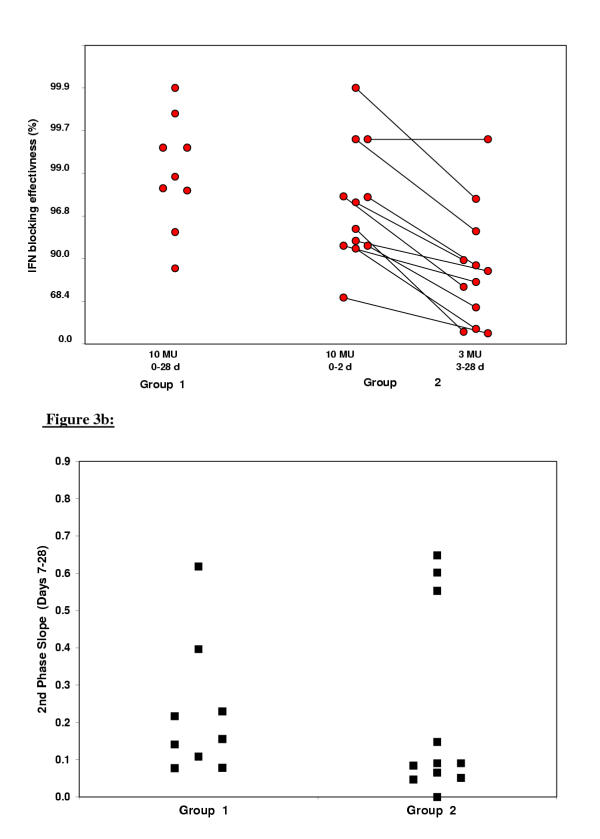
The estimates obtained by non-linear fitting of each patient's viral kinetics individually are given in Table 2. As expected the baseline viral load, the half-life of free virions and the initial effectiveness in blocking virion production, all related to the first phase decline, were similar between the 2 groups. Mean half-life of free virions for all patients was 3.0 hours ± standard deviation of 1.6 hours, similar to that derived in previous studies for interferon-α monotherapy (2.7 hours) [5]. The mean effectiveness in blocking virion production for all patients was 95.6% ± 6.5% for the 10 MU interferon dose. For group 1 the non-linear fit (with Eq. 4) did not give rise to a significant change in ε over time, in accordance to the constant daily interferon dose used in these patients (insert Fig 2a). For group 2, however, a significant (p < 0.005) decrease in effectiveness of blocking production was observed (inserts Fig 2b,c,d, Fig 3a) from an average of ε1 = 94% ± 8% to ε2 = 69% ± 27%. The effectiveness of interferon with 10 MU (ε1) and with 3 MU (ε2) were pair wise correlated (R = 0.8, P < 0.001).
Table 2.
Results of non-linear fitting of viral dynamics
| Patient (1) | Initial viral load (log copies/ml) | % blocking production | Half-life(2) of free virions (hours) | Half-life of infected cells (days) | |
| During 10 MU qd | After reduction to 3 MU qd | minimal and maximal estimates (3) | |||
| 1-A | 6.9 | 98.4 | 2.3 | 8.7–8.8 | |
| 1-B | 6.9 | 99.9 | 2.5 | 3.0–3.0 | |
| 1-C | 6.7 | 99.5 | 2.2 | 1.7–1.7 | |
| 1-D | 6.1 | 86.9 | 1.9 | 7.8–9.0 | |
| 1-E | 5.8 | 99.5 | 5.3 | 4.4–4.5 | |
| 1-F | 6.4 | 98.5 | 6.4 | 3.2–3.2 | |
| 1-G | 7.0 | 95.1 | 1.8 | 6.1–6.4 | |
| 1-H | 7.1 | 99.8 | 1.7 | 1.1–1.1 | |
| 1-I | 7.4 | 98.9 | 3.3 | 4.9–4.9 | |
| Group 1(4) mean (std) | 6.7 (0.5) | 97.4 % (0.04) | 3.0 hours (1.7) | 4.5–4.7 days (2.6–2.8) | |
| 2-A | 7.3 | 93.0 | 62.5 | 1.8 | 4.8–7.7 |
| 2-C | 6.8 | 98.1 | 78.5 | 3.3 | 0.9–1.2 |
| 2-E | 6.7 | 93.8 | 86.0 | 3.3 | 7.1–8.2 |
| 2-F | 6.5 | 99.6 | 95.2 | 1.9 | 1.0–1.0 |
| 2-G | 7.2 | 97.8 | 85.0 | 3.3 | 4.0–4.7 |
| 2-H | 7.3 | 95.5 | 27.8 | 3.9 | 1.2–4.7 |
| 2-I | 6.1 | 98.1 | 88.0 | 1.1 | 11.8–13.5 |
| 2-J | 7.1 | 99.9 | 98.0 | 1.6 | 3.4–3.4 |
| 2-K | 7.1 | 71.4 | 25.2 | 2.1 | 2.6–2.7 |
| 2-L | 6.3 | 99.6 | 99.6 | 6.9 | 2.2–2.3 |
| 2-M | 7.4 | 93.0 | 81.1 | 2.1 | 12.0–14.8 |
| 2-N | 6.8 | 92.4 | 33.2 | 3.6 | 2.5–7.7 |
| Group 2(4) mean (std) | 6.9 (0.4) | 94.3% (0.08) | 69.1% (**) (26.6) | 2.9 hours (1.6) | 4.4–6.7 days (3.9–4.6) |
1) Fitting was not done for: patients 2-B (non-detectable at day 2 on), 2-D (Null-response), 2-O (missing data and rebound). 2) For group 1 half-life of free virions is only a maximal estimate because only samples from 0, 8 and 24 hours were available. 3) Minimal and maximal estimates of the half-life of infected cells was estimated assuming Η = 1 and Η = 0 respectively in Eq 2. 4) No statistically significant differences in any parameter between the two groups. **) Statistically significant (p < 0.002) difference in percentage blocking production before and after dose reduction in group 2.
Figure 3.
Figure 3a. Interferon efficacy in blocking production (%) is displayed for group 1 and group 2 (logarithmic scale). For the patients that received a dose reduction after 3 days of treatment (group 2), both the efficacy for the initial, 10 MU INTERFERON daily period, is displayed as well as the reduced efficacy after the dose reduction to 3 MU daily. Note that the initial interferon efficacy between group 1 and 2 does not differ. After the dose reduction the interferon efficacy is reduced in 11/12 patients in group 2 (connected black dots).Figure 3b. The slopes of the second phase decline (days-1) is represented per patient (black squares) for both groups. No differences between both groups could be observed in this study.
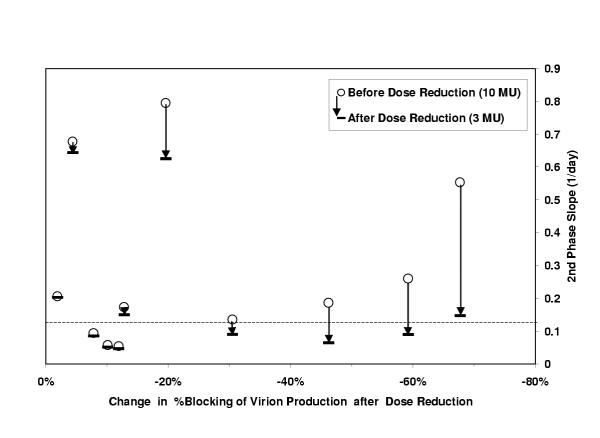
Cross-sectionally there was no difference observed between the 2 groups in the death rate of infected cells (Table 2) or in the second phase slope (Fig 3b). However, longitudinal analysis of group 2 patients revealed a significant decrease from the predicted second phase slope, which would have occurred without a dose reduction, to the actual slope after dose reduction in 4 out of 12 patients (Fig 4a). These are the patients with the largest decrease (range 20% to 68%) from ε1 to ε2, since the second slope is a linear function of the interferon effectiveness in blocking production (ε).
Figure 4.
The effect of the change in interferon efficacy after the dose reduction (x-axis) for the patients in group 2 is related to the change in slope of the second phase viral decline (open circles: second phase slope before dose reduction; horizontal lines: second phase slope after dose reduction). In 4 patients the slope of the second phase reduces drastically, note that 3/4 of these patients have the largest reduction in interferon efficacy. The dashed line reflects the threshold of 0.13 days-1 below which no sustained response was observed.
The clinical consequences of the viral dynamics data relate to the predicted time of HCV RNA negativity, which is strongly dependent on the second phase slope. The predicted time to HCV-negativity with the biphasic model (median 3.5 weeks) was confirmed by the observed individual data of group 1 patients (median 4 weeks), and the dose-reduction model (with the reduced 3 MU dose) predicted the time to HCV-negativity in group 2 patients (medians 12 and 14.5 weeks, Table 3). Reducing the interferon dose to 3 MU daily decreased the predicted number of patients that would become HCV-negative within 12 weeks from 12/15 with the 10 MU dose to only 7/15 with the reduced 3 MU dose, as indeed confirmed by the actual individual data (8/15).
Table 3.
Predicted and observed time to HCV RNA negativity
| Patient | Predicted time (weeks) to HCV RNA negativity(1) with 10 MU/daily | Predicted time (weeks) to HCV RNA negativity(1) with 3 MU/daily | First observedHCV RNA negativity(1) (weeks) |
| 1-A | 11 | 12 (2) | |
| 1-B | 2 | 2 | |
| 1-C | 1.5 | 2 | |
| 1-D | 12 | 4–8 | |
| 1-E | 2.5 | 2 | |
| 1-F | 3.5 | 4 | |
| 1-G | 10.5 | 4–8 | |
| 1-H | 1 | 1 | |
| 1-I | 9 | Positive at 24 weeks (3)(4) | |
| Group 1: HCV neg before 12 weeks | 9 / 9 patients | 8 / 9 patients | |
| 2-A | 9 | 16 | Positive at 24 weeks (3) |
| 2-B | 1 | 1 | 1 |
| 2-C | 1 | 2 | 1.5 |
| 2-D | Never | Never | Positive at 24 weeks (3) |
| 2-E | 11 | 13 | 4–8 |
| 2-F | 1 | 1.5 | 1.5 |
| 2-G | 5.5 | 7 | 4–8 |
| 2-H | 3 | 10 | 12–16 |
| 2-I | 11 | 18 | Positive at 24 weeks (3) |
| 2-J | 3 | 5 | 4 |
| 2-K | 7.5 | 24 | Positive at 24 weeks (3) |
| 2-L | 1.5 | 1.5 | 1.5 |
| 2-M | 23 | 30 | 8–12 |
| 2-N | 4 | 16 | Positive at 24 weeks (3) |
| 2-O | Never | Never | Positive at 24 weeks (3) |
| Group 2: HCV neg before 12 weeks | 12 / 15 patients | 7 / 15 patients | 8 / 15 patients |
Detection level for HCV RNA negativity is at < 500 copies/ml. 2) Patient 1-D had a viral breakthrough after 12 weeks and was HCV RNA positive at 24 weeks of treatment. 3) Patients with positive HCV RNA at 24 weeks stopped treatment according to protocol. 4) Patient 1-I was non-compliant from 8 weeks on according to self-report
Discussion
Our results indicate that the effect of interferon dose reduction on viral dynamics can be completely attributed to decrease in the effectiveness of interferon in blocking virion production (ε). Changes in other parameters, such as blocking de-novo infection (Η) loss rate of infected cells (δ) and clearance rate of free virions (c), without a change in blocking production, can not reproduce the observed kinetics. The longitudinal dose dependence of the interferon anti-viral effectiveness observed here corroborates the dose dependence of interferon effectiveness previously described only cross-sectionally [5]. The strength of the current results is that the dose dependence of the effectiveness cannot be attributed to baseline differences between the patients. Since longitudinal changes in the other parameters of this model (such as Η, δ and c) do not give rise to significant differences in the kinetics, we can not rule out combined effects of changes in the other parameters concomitantly with the change in effectiveness of blocking production (ε).
In turn, the dose dependency of the interferon effectiveness determines the result of both the 1st phase and the 2nd phase kinetics. The effectiveness in blocking virion production with 10 MU before dose reduction (mean 95.6% and a mean viral decline of 1.8 log cp/ml) was similar to that of a previous study with 10 MU interferon (96%) [5]. However, we show that the benefit of the initial viral decline due to 3 days of high induction dose was lost after the dose reduction in almost all patients (Fig 1, 3). The effectiveness in blocking virion production with 3 MU after the dose reduction (mean 69.1% and a mean viral decline of 0.7 log cp/ml) was similar to that estimated in a previous study with 3 MU interferon initially (70%) [8]. As a consequence, the viral kinetics after the dose reduction in group 2 patients is similar to the kinetics that would have been obtained if the patients had started with the reduced dose to begin with (green line Fig 2).
In contrast to the 1st phase viral decline, which exponentially depends on the interferon effectiveness, the 2nd phase slope is a linear function of the effectiveness in blocking virion production. This slope is the most predictive parameter for treatment outcome, with a threshold of 0.13 days-1 below which no sustained response was observed [5] While the increase observed in viral load immediately after dose reduction can delay the time to negativity by several weeks at the most (patients 2-J and 2-C Fig 2), the decrease in the second phase slope can radically reduce the chance for HCV-RNA negativity (patient 2-H Fig 2). Therefore the decrease in the second slope could be crucial for their success of treatment. Indeed, the number of patients predicted to become HCV-negative within 12 weeks with the high induction dose was drastically reduced due to the dose reduction (compare the 10 MU column versus 3 MU column in Table 3).
Interestingly, the results obtained here are for interferon and ribavirin combination treatment, while the results from previous studies [5,8] are for interferon monotherapy. On the other hand, in this study most patients are non-responders or cirrhotic rather than normal naïve patients as in the previous studies [5,8]. Thus, we can not conclude if ribavirin has an additive effect on initial viral decline or not.
Is induction treatment beneficial at all considering that following dose reduction the virus rebounds back to the level it would reach anyway with the reduced dose? Previous studies with a longer period of induction treatment (14 days) do not show a consistent viral rebound as observed here [14]. Moreover, studies of prolonged induction treatment (> 28 days) do not show any re-emergence of virus production [15]. Therefore, it could be suggested that an induction period of 3 days is too short, but longer induction periods, which continue until HCVRNA negativity in serum, might give rise to continuous suppression of viral replication. In all likelihood this concept may partly explain the increased efficacy of pegylated interferon [16,17] since the rather constant interferon levels with this type of interferon result in a constant block of virus production.
Conclusions
Dose reduction to 3 MU daily after 3 days of 10 MU of interferon daily in HCV genotype-1 patients negated the extra virus suppressive effectiveness of the induction dose. These observations, while explaining the failure of many induction schedules suggests that induction therapy should be continued till HCV RNA negativity in serum in order to increase the sustained response rate in chronic hepatitis C.
Competing interests
Frank Bekkering: none declared
Avidan Neumann: none declared
Johannes Brouwer: received grant support from Schering Plough International
Rachel Levi-Drummer: none declared
Solko Schalm: received grant support from Schering Plough International
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgments
We thank Dr. Raj Reddy for fruitful discussions.
Presented in part: 51st annual meeting of the American Association for the Study of Liver Diseases, Dallas, 27–31 October 2000 (Bekkering FC, Neumann AU, Levi-Drummer R, Brouwer JT, Schalm. SW. In-vivo longitudinal changes in anti-viral effectiveness of interferon after dose reduction in chronic hepatitis C patients. Hepatology 2000;32:367A).
Financial support: Schering Plough International, Kenilworth, NJ.
Contributor Information
Frank C Bekkering, Email: fcbekkering@hotmail.com.
Avidan U Neumann, Email: neumann@mail.biu.ac.it.
Johannes T Brouwer, Email: brouwer@mdl.azr.nl.
Rachel S Levi-Drummer, Email: drummer@mail.biu.ac.it.
Solko W Schalm, Email: schalm@mdl.azr.nl.
References
- Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26:62S–65S. doi: 10.1016/S0168-8278(97)80010-4. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- Lin R, Roach E, Zimmerman M, Strasser S, Farrell GC. Interferon alfa-2b for chronic hepatitis C: effects of dose increment and duration of treatment on response rates. Results of the first multicentre Australian trial. Australia Hepatitis C Study Group. J Hepatol. 1995;23:487–496. doi: 10.1016/0168-8278(95)80052-2. [DOI] [PubMed] [Google Scholar]
- Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, Albrecht J. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998;352:1426–1432. doi: 10.1016/S0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, Perelson AS. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- Bekkering FC, Brouwer JT, Leroux-Roels G, Van Vlierberghe H, Elewaut A, Schalm SW. Ultrarapid hepatitis C virus clearance by daily high-dose interferon in non-responders to standard therapy. J Hepatol. 1998;28:960–964. doi: 10.1016/S0168-8278(98)80343-7. [DOI] [PubMed] [Google Scholar]
- Zeuzem S, Schmidt JM, Lee JH, Ruster B, Roth WK. Effect of interferon alfa on the dynamics of hepatitis C virus turnover in vivo. Hepatology. 1996;23:366–371. doi: 10.1002/hep.510230225. [DOI] [PubMed] [Google Scholar]
- Lam NP, Neumann AU, Gretch DR, Wiley TE, Perelson AS, Layden TJ. Dose-dependent acute clearance of hepatitis C genotype 1 virus with interferon alfa. Hepatology. 1997;26:226–231. doi: 10.1002/hep.510260130. [DOI] [PubMed] [Google Scholar]
- Bekkering FC, Stalgis C, McHutchison JG, Brouwer JT, Perelson AS. Estimation of early hepatitis C viral clearance in patients receiving daily interferon and ribavirin therapy using a mathematical model. Hepatology. 2001;33:419–423. doi: 10.1053/jhep.2001.21552. [DOI] [PubMed] [Google Scholar]
- Brouwer JT, Nevens F, Kleter B, Elewaut A, Adler M, Brenard R, Chamuleau RA, Michielsen PP, Pirotte J, Hautekeete ML, Weber J, Bourgeois N, Hansen BE, Bronkhorst CM, ten Kate FJ, Heijtink RA, Fevery J, Schalm SW. Efficacy of interferon dose and prediction of response in chronic hepatitis C: Benelux study in 336 patients. J Hepatol. 1998;28:951–959. doi: 10.1016/S0168-8278(98)80342-5. [DOI] [PubMed] [Google Scholar]
- Holodniy M, Mole L, Yen-Lieberman B, Margolis D, Starkey C, Carroll R, Spahlinger T, Todd J, Jackson JB. Comparative stabilities of quantitative human immunodeficiency virus RNA in plasma from samples collected in VACUTAINER CPT, VACUTAINER PPT, and standard VACUTAINER tubes. J Clin Microbiol. 1995;33:1562–1566. doi: 10.1128/jcm.33.6.1562-1566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor J, Hawkins A, Simmonds P. Genotype dependence of hepatitis C virus load measurement in commercially available quantitative assays. J Clin Microbiol. 1999;37:2525–2532. doi: 10.1128/jcm.37.8.2525-2532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuzem S, Lee JH, Franke A, Ruster B, Prummer O, Herrmann G, Roth WK. Quantification of the initial decline of serum hepatitis C virus RNA and response to interferon alfa. Hepatology. 1998;27:1149–1156. doi: 10.1002/hep.510270433. [DOI] [PubMed] [Google Scholar]
- Iino S, Hino K, Kuroki T, Suzuki H, Yamamoto S. Treatment of chronic hepatitis C with high-dose interferon alpha-2b. A multicenter study. Dig Dis Sci. 1993;38:612–618. doi: 10.1007/BF01316789. [DOI] [PubMed] [Google Scholar]
- Bekkering FC, Brouwer JT, Hansen BE, Schalm SW. Hepatitis C viral kinetics in difficult to treat patients receiving high dose interferon and ribavirin. J Hepatol. 2001;34:435–440. doi: 10.1016/S0168-8278(00)00033-7. [DOI] [PubMed] [Google Scholar]
- Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, O'Grady J, Reichen J, Diago M, Lin A, Hoffman J, Brunda MJ. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343:1666–1672. doi: 10.1056/NEJM200012073432301. [DOI] [PubMed] [Google Scholar]
- Heathcote EJ, Shiffman ML, Cooksley WG, Dusheiko GM, Lee SS, Balart L, Reindollar R, Reddy RK, Wright TL, Lin A, Hoffman J, De Pamphilis J. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N Engl J Med. 2000;343:1673–1680. doi: 10.1056/NEJM200012073432302. [DOI] [PubMed] [Google Scholar]