Abstract
OBJECTIVE
We compared the risk of cardiovascular disease (CVD) and all-cause mortality in subgroups of prediabetes defined by fasting plasma glucose (FPG), 2-h plasma glucose (2hPG), or HbA1c.
RESEARCH DESIGN AND METHODS
In the Whitehall II cohort, 5,427 participants aged 50–79 years and without diabetes were followed for a median of 11.5 years. A total of 628 (11.6%) had prediabetes by the World Health Organization (WHO)/International Expert Committee (IEC) criteria (FPG 6.1–6.9 mmol/L and/or HbA1c 6.0–6.4%), and 1,996 (36.8%) by the American Diabetes Association (ADA) criteria (FPG 5.6–6.9 mmol/L and/or HbA1c 5.7–6.4%). In a subset of 4,730 individuals with additional measures of 2hPG, 663 (14.0%) had prediabetes by 2hPG. Incidence rates of a major event (nonfatal/fatal CVD or all-cause mortality) were compared for different definitions of prediabetes, with adjustment for relevant confounders.
RESULTS
Compared with that for normoglycemia, incidence rate in the context of prediabetes was 54% higher with the WHO/IEC definition and 37% higher with the ADA definition (P < 0.001) but declining to 17% and 12% after confounder adjustment (P ≥ 0.111). Prediabetes by HbA1c was associated with a doubling in incidence rate for both the IEC and ADA criteria. However, upon adjustment, excess risk was reduced to 13% and 17% (P ≥ 0.055), respectively. Prediabetes by FPG or 2hPG was not associated with an excess risk in the adjusted analysis.
CONCLUSIONS
Prediabetes defined by HbA1c was associated with a worse prognosis than prediabetes defined by FPG or 2hPG. However, the excess risk among individuals with prediabetes is mainly explained by the clustering of other cardiometabolic risk factors associated with hyperglycemia.
Introduction
In 1979–1980, the World Health Organization (WHO), the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), and other diabetes organizations made a common agreement regarding the diagnostic criteria for diabetes and impaired glucose tolerance (IGT) based on the oral glucose tolerance test (OGTT) (1,2). Since then, the diagnostic criteria for diabetes and prediabetes have changed several times, and there is currently no consensus on the definition of prediabetes between the different organizations worldwide (3–5). It is generally accepted that diabetes and prediabetes can be diagnosed on the basis of measures of fasting plasma glucose (FPG), 2-h plasma glucose (2hPG) after an OGTT, or hemoglobin A1c (HbA1c). In clinical practice, FPG and HbA1c are preferred over the OGTT, which is inconvenient, less reproducible, and more costly. However, the cut points for FPG and HbA1c vary by the different organizations. In 2003, ADA suggested to lower the cut point for impaired fasting glycemia (IFG) from 6.1 mmol/L (110 mg/dL) to 5.6 mmol/L (100 mg/dL) (6) in order to capture more individuals with IGT without performing an OGTT. The sensitivity for identifying IGT was increased with the new lower criterion for fasting glucose, but it also resulted in a two- to fourfold increase in the prevalence of IFG across countries (7). Furthermore, with the lower cutoff, the overall incidence rate for diabetes among people with IFG was greatly reduced (8). This observation, together with a lack of evidence for a reduction in adverse outcomes among these newly defined individuals with IFG, have led the WHO not to adopt the lower cutoff for IFG (3).
More recently, HbA1c was recommended for the diagnosis of diabetes by both ADA and WHO (9,10). However, in terms of identifying individuals with prediabetes or intermediate hyperglycemia, the two organizations again differed in their recommendations. While ADA now recommends using HbA1c in the range of 5.7–6.4% (39–47 mmol/mol) for defining prediabetes, WHO has not yet adopted HbA1c for diagnosing intermediate hyperglycemia/prediabetes (9). The International Expert Committee (IEC), in turn, acknowledges the elevated risk of progression to diabetes associated with increasing HbA1c levels and recommends initiation of prevention strategies in individuals with HbA1c levels in a narrower range of 6.0–6.4% (42–47 mmol/mol) (11).
In addition to increasing risk of diabetes, both fasting glucose and HbA1c levels in the range of prediabetes are associated with increased risk of cardiovascular disease (CVD) and mortality (12,13). However, large inconsistencies between studies have been observed due to the use of different cut points and reference groups (14), and direct comparisons between the associations of different glucose and HbA1c criteria with development of CVD and/or mortality within the same population are sparse (15). In the ongoing Whitehall II study we therefore compared the risk of fatal or nonfatal CVD or all-cause mortality in individuals with prediabetes identified by FPG, 2hPG, or HbA1c using the cut points suggested by ADA versus WHO/IEC. Additionally, we examined the associations of continuous prediabetes levels of FPG, 2hPG, or HbA1c with the 10-year risk of CVD or mortality.
Research Design and Methods
Study Design and Participants
The prospective cohort study is based on participants from the Whitehall II study, which is an occupational cohort of 10,308 British civil servants (6,896 men and 3,412 women) initially recruited in 1985. The study population has been followed with clinical examinations every 5 years. (Whitehall II data, protocols, and other metadata are available to the scientific community. Please refer to the Whitehall II data sharing policy at https://www.ucl.ac.uk/whitehallII/data-sharing.) This study is based on phase 7 (2002–2004) and phase 9 (2007–2009), where FPG, 2hPG, and HbA1c were measured, excluding participants with known diabetes. The study population consists of the 5,427 participants with complete information on both HbA1c and FPG (87% of whom also had 2hPG measured). All the included participants had been fasting ≥8 h.
Ethics
The University College London Ethics Committee reviewed and approved the study. Written informed consent was obtained from all participants at each study phase. The Whitehall II study has previously been described in detail (16,17).
Definition of Prediabetes
At each study phase the participants underwent a standard 75-g OGTT with measurement of plasma glucose in the fasting state and after 120 min. HbA1c was also measured. Prediabetes was defined according to the WHO/IEC criteria as FPG 6.1–6.9 mmol/L and/or HbA1c 6.0–6.4% (42–47 mmol/mol) and according to the ADA criteria as FPG 5.6–6.9 mmol/L and/or HbA1c 5.7–6.4% (39–47 mmol/mol). For 2hPG, we defined prediabetes as 7.8–11.0 mmol/L according to the definition by WHO and ADA. Normoglycemia was defined as values below the cut points for prediabetes for each diagnostic criterion.
Assessment of Clinical Characteristics
At all clinical examinations, measurements of anthropometry and handling of blood samples were carried out according to standard protocols (16). Plasma glucose concentrations were measured by the glucose oxidase method (17). HbA1c was measured in whole blood, drawn into EDTA Monovette tubes, using the validated (18) Tosoh G8 high-performance ion-exchange liquid chromatography platform (Tosoh Bioscience, Tessenderlo, Belgium). Information on medication, family history of diabetes, smoking, and alcohol intake was obtained from questionnaire.
Outcome Ascertainment
Outcome was defined as a composite end point of CVD or death. The participants’ unique National Health Service (NHS) identification numbers were linked to the NHS Hospital Episode Statistics database (19). Incidence of CVD was assessed over the follow-up period from 2002–2004 to the end of follow-up (30 June 2015) and included fatal and nonfatal coronary heart disease (defined by ICD-9 codes 410–414 or ICD-10 codes I20–25) and stroke. Nonfatal myocardial infarction was determined using data from questionnaires, study electrocardiograms, hospital acute electrocardiograms, cardiac enzymes, and physician records (16). In the definition of stroke, cases identified by self-report only were excluded. Stroke included first subarachnoid hemorrhage, cerebral infarction, intracerebral hemorrhage, not specified stroke (ICD-10 codes I60–I64), and transient cerebral ischemic attacks (ICD-10 code G45). Cases of stroke were ascertained from participants’ general practitioners, by information extracted from hospital medical records, or from the NHS Hospital Episode Statistics database. Cardiovascular event ascertainment in the Whitehall II study has recently been validated (20). All-cause mortality was assessed from 2002–2004 to end of follow-up by flagging participants at the NHS Central Registry, which provided information on the cause and date of death.
Statistical Analysis
Participants were followed from the date of their 2002–2004 (or 2007–2009) clinical examination until first registered event or to the end of follow-up (30 June 2015). When relevant, prediabetes status was allowed to change from normoglycemia in phase 7 (2002–2004) to prediabetes in phase 9 (2007–2009). Poisson regression analysis with log-person time as offset was used to estimate crude incidence rates of an event and adjusted incidence rate ratios for subgroups of prediabetes defined by different criteria (WHO/IEC and ADA) and by different glycemic measures (FPG, 2hPG, and HbA1c). Rate ratios were adjusted in a stepwise approach; first, with adjustment for age, sex, and ethnicity, and second, with further adjustment for previous CVD and the CVD risk factors identified in the Framingham Heart Study (21): smoking, total cholesterol, HDL cholesterol, systolic blood pressure, and use of antihypertensive treatment. To account for the nonconstant effect of age over time on CVD risk and mortality, we split the follow-up period of each participant into 1-year age bands prior to analysis.
We performed two sensitivity analyses: 1) we repeated the analyses using only fatal and nonfatal CVD events as outcome (constituting 65% of the composite events) and censoring the study participants at time of death, and 2) in a subset with complete information on FPG, HbA1c, and 2hPG levels (n = 4,730), we expanded the analyses to include prediabetes by 2hPG (i.e., IGT).
We further calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value for the 10-year risk of an event for each prediabetes subgroup. We also estimated the 10-year risk of an event across the prediabetes range of glycemia by the ADA criteria using Poisson regression analysis with models fitted separately for FPG, 2hPG, and HbA1c. In the models, the glycemic measure was specified with natural cubic splines with three knots to facilitate detection of a potential inflection point in the associations.
Statistical analyses were performed in R, version 3.2.3 (R Foundation for Statistical Computing [www.r-project.org]), and SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Prediabetes by Different Definitions
With use of the WHO/IEC criteria, 402 (7.4%) of the 5,427 participants in the study population had prediabetes by the FPG criterion and 288 (5.3%) by the HbA1c criterion (n = 628 in total [11.6%]). With the ADA criteria, 1,418 (26.1%) had prediabetes by FPG levels and 940 (17.3%) by HbA1c (n = 1,996 in total [36.8%]). (See Supplementary Fig. 1 for further details.) Thus, the proportion of individuals with prediabetes was more than threefold higher with the ADA criteria compared with the WHO/IEC criteria. With application of the ADA criteria in the subset of the 4,730 individuals with full data on FPG, 2hPG, and HbA1c, 26.6% had prediabetes by FPG, 15.8% by HbA1c, and 14.2% by 2hPG.
For both WHO/IEC- and ADA-defined prediabetes, individuals identified by FPG levels only were more likely to be men and to report a higher amount of alcohol consumption than those identified by HbA1c (Table 1). Those identified by HbA1c only were more likely to be of nonwhite ethnicity, were older, and had lower levels of total cholesterol, LDL cholesterol, and blood pressure than those identified by FPG levels only. However, those identified by HbA1c were more likely to have had a previous CVD event and to be receiving antihypertensive and/or lipid-lowering treatment (Table 1).
Table 1.
Baseline characteristics by normal glycemia and subgroups of prediabetes defined by WHO/IEC and ADA
| Normal glycemia | Prediabetes |
P | |||
|---|---|---|---|---|---|
| FPG only | HbA1c only | Both | |||
| WHO/IEC | |||||
| N | 4,799 | 340 | 226 | 62 | |
| Men (%) | 71.6 (70.3; 72.9) | 85.6 (81.4; 89.1)a | 67.3 (60.7; 73.3)b | 66.1 (53.0; 77.7)b | <0.001 |
| White ethnicity (%) | 93.6 (92.9; 94.3) | 90.6 (87.0; 93.5)a | 80.1 (74.3; 85.1)ab | 83.9 (72.3; 92.0)a | <0.001 |
| Age (years) | 61.2 (6.1) | 61.8 (6.1) | 65.5 (6.4)ab | 64.2 (5.7)ab | <0.001 |
| BMI (kg/m2) | 26.4 (4.1) | 28.2 (4.4)a | 27.7 (4.7)a | 30.4 (6.2)abc | <0.001 |
| Total cholesterol (mmol/L) | 5.7 (1.0) | 5.7 (1.0) | 5.3 (1.1)ab | 5.6 (1.3) | <0.001 |
| HDL cholesterol (mmol/L) | 1.6 (0.4) | 1.5 (0.5)a | 1.5 (0.4)a | 1.4 (0.3)a | <0.001 |
| Systolic blood pressure (mmHg) | 126.6 (16.2) | 134.4 (17.3)a | 128.5 (17.8)b | 136.9 (19.3)ac | <0.001 |
| FPG (mmol/L) | 5.2 (0.4) | 6.3 (0.2)a | 5.5 (0.9)ab | 6.4 (0.2)ac | <0.001 |
| 2hPG (mmol/L) | 6.1 (1.5) | 7.4 (2.0)a | 7.5 (2.5)a | 9.5 (2.3)abc | <0.001 |
| HbA1c (%) | 5.2 (0.4) | 5.5 (0.5)a | 6.1 (0.1)ab | 6.1 (0.1)ab | <0.001 |
| HbA1c (mmol/mol) | 34 (4) | 37 (5)a | 43 (1)ab | 44 (2)ab | <0.001 |
| Previous CVD (%) | 9.7 (8.9; 10.6) | 12.1 (8.8; 16.0) | 18.1 (13.3; 23.8)ab | 24.2 (14.2; 36.7)ab | <0.001 |
| Current smoker (%) | 7.9 (7.2; 8.7) | 5.9 (3.6; 8.9) | 10.2 (6.6; 14.9) | 9.7 (3.6; 19.9) | 0.282 |
| Antihypertensive treatment (%) | 21.9 (20.7; 23.1) | 36.2 (31.1; 41.5)a | 42.0 (35.5; 48.8)a | 45.2 (32.5; 58.3)a | <0.001 |
| ADA | |||||
| N | 3,431 | 1,056 | 578 | 362 | |
| Men (%) | 69.4 (67.8; 70.9) | 83.9 (81.5; 86.1)a | 66.8 (62.8; 70.6)b | 75.1 (70.4; 79.5)abc | <0.001 |
| White ethnicity (%) | 94.3 (93.5; 95.1) | 94.5 (93.0; 95.8) | 83.4 (80.1; 86.3)ab | 87.0 (83.1; 90.3)ac | <0.001 |
| Age (years) | 60.8 (6.0) | 61.1 (6.0) | 64.8 (6.6)ab | 63.6 (6.1)abc | <0.001 |
| BMI (kg/m2) | 26.1 (4.1) | 27.4 (4.2)a | 27.2 (4.5)a | 28.5 (4.5)abc | <0.001 |
| Total cholesterol (mmol/L) | 5.7 (1.0) | 5.8 (1.0) | 5.5 (1.1)ab | 5.6 (1.2)b | <0.001 |
| HDL cholesterol (mmol/L) | 1.6 (0.5) | 1.5 (0.4)a | 1.6 (0.4)a | 1.5 (0.4)abc | <0.001 |
| Systolic blood pressure (mmHg) | 125.7 (16.2) | 130.9 (16.5)a | 127.3 (16.9)ab | 131.2 (16.6)ac | <0.001 |
| FPG (mmol/L) | 5.0 (0.3) | 5.9 (0.3)a | 5.2 (0.7)ab | 6.0 (0.3)abc | <0.001 |
| 2hPG (mmol/L) | 5.9 (1.5) | 6.5 (1.7)a | 6.7 (2.1)a | 7.8 (2.1)abc | <0.001 |
| HbA1c (%) | 5.1 (0.3) | 5.3 (0.4)a | 5.9 (0.2)ab | 5.9 (0.2)ab | <0.001 |
| HbA1c (mmol/mol) | 33 (3) | 34 (4)a | 41 (2)ab | 41 (2)ab | <0.001 |
| Previous CVD (%) | 9.5 (8.5; 10.5) | 10.5 (8.7; 12.5) | 18.0 (14.9; 21.4)ab | 19.9 (15.9; 24.4)ab | <0.001 |
| Current smoker (%) | 8.0 (7.1; 8.9) | 6.8 (5.4; 8.5) | 9.3 (7.1; 12.0) | 8.6 (5.9; 11.9) | 0.312 |
| Antihypertensive treatment (%) | 19.5 (18.2; 20.9) | 27.1 (24.4; 29.9)a | 33.7 (29.9; 37.8)ab | 41.7 (36.6; 47.0)abc | <0.001 |
Data are means (SD) or percentage (95% CI). WHO/IEC: normal glycemia, FPG <6.1 mmol/L and HbA1c <6.0%, and for prediabetes by FPG only, FPG 6.1–6.9 mmol/L and HbA1c <6.0%; HbA1c only, HbA1c <6.0–6.4% and FPG <6.1 mmol/L; and both, FPG 6.1–6.9 mmol/L and HbA1c 6.0–6.4%. ADA: normal glycemia, FPG <5.6 mmol/L and HbA1c <5.7%, and for prediabetes by FPG only, FPG 5.6–6.9 mmol/L and HbA1c <5.7%; HbA1c only, HbA1c <5.7–6.4% and FPG <5.6 mmol/L; and both, FPG 5.6–6.9 mmol/L and HbA1c 5.7–6.4%. P is the level of significance for the overall unadjusted test of difference between groups of normal glycemia and prediabetes subgroups, using t tests for difference in means or log(means) and χ2 tests for difference in proportions.
aVersus normal glycemia, P < 0.05.
bVersus FPG only, P < 0.05.
cVersus HbA1c only, P < 0.05.
Event Rates in Individuals With Prediabetes by Different Definitions
Overall Event Rates
Median follow-up time for a CVD or mortality event was 11.5 (interquartile range 8.9; 12.1) years. During follow-up, 134 (21.3%) individuals with prediabetes by WHO/IEC FPG or HbA1c criteria developed CVD or died. The corresponding number was 370 (18.5%) in the ADA prediabetes group. With the WHO/IEC criteria, the incidence rate of an event in those with prediabetes was 22.7/1,000 person-years (PY), which was 54% higher than in individuals with normoglycemia (Table 2). The higher incidence rate in the prediabetes group was unaffected by adjustment for age, sex, and ethnicity but decreased to 17% and became nonsignificant after adjustment for previous CVD, smoking, total cholesterol, HDL cholesterol, systolic blood pressure, and use of antihypertensive treatment. With use of the ADA criteria, the incidence rate for the prediabetes group was somewhat lower at 18.9/1,000 PY, which was 37% higher compared with the normoglycemic group, and decreased to only 12% higher (nonsignificant) in the fully adjusted model (Table 2).
Table 2.
Rates and rate ratios with 95% CI of an event (CVD or mortality) for prediabetes subgroups, using the WHO/IEC criteria or the ADA criteria
| Definition | Rate per 1,000 PY | RR | RRadj1 | RRadj2 | |
|---|---|---|---|---|---|
| WHO/IEC | |||||
| Overall | |||||
| Normal glycemia | FPG <6.1 mmol/L and HbA1c <6.0% | 14.8 (13.7; 15.9) | ref. | ref. | ref. |
| Prediabetes | FPG 6.1–6.9 mmol/L or HbA1c 6.0–6.4% | 22.7 (19.2; 26.9) | 1.54 (1.28; 1.85) | 1.51 (1.25; 1.81) | 1.17 (0.97; 1.41) |
| By HbA1c | |||||
| Normal glycemia | HbA1c <6.0% | 14.9 (13.9; 15.9) | ref. | ref. | ref. |
| Prediabetes | HbA1c 6.0–6.4% | 29.5 (23.4; 37.3) | 1.99 (1.55; 2.53) | 1.52 (1.19; 1.95) | 1.13 (0.88; 1.46) |
| By FPG | |||||
| Normal glycemia | FPG <6.1 mmol/L | 15.3 (14.2; 16.4) | ref. | ref. | ref. |
| Prediabetes | FPG 6.1–6.9 mmol/L | 19.4 (15.6; 24.2) | 1.27 (1.01; 1.60) | 1.17 (0.93; 1.48) | 1.00 (0.79; 1.26) |
| By 2hPG | |||||
| Normal glycemia | 2hPG <7.8 mmol/L | 13.4 (12.4; 14.6) | ref. | ref. | ref. |
| Prediabetes | 2hPG 7.8–11.0 mmol/L | 19.3 (16.3; 23.0) | 1.44 (1.19; 1.75) | 1.14 (0.94; 1.39) | 1.00 (0.82; 1.22) |
| ADA | |||||
| Overall | |||||
| Normal glycemia | FPG <5.6 mmol/L and HbA1c <5.7% | 13.8 (12.7; 15.1) | ref. | ref. | ref. |
| Prediabetes | FPG 5.6–6.9 mmol/L or HbA1c 5.7–6.4% | 18.9 (17.1; 21.0) | 1.37 (1.20; 1.57) | 1.34 (1.17; 1.54) | 1.12 (0.97; 1.28) |
| By HbA1c | |||||
| Normal glycemia | HbA1c <5.7% | 13.7 (12.7; 14.8) | ref. | ref. | ref. |
| Prediabetes | HbA1c 5.7–6.4% | 26.0 (22.6; 29.8) | 1.89 (1.62; 2.22) | 1.49 (1.27; 1.75) | 1.17 (1.00; 1.38) |
| HbA1c 5.7–5.9% | 24.6 (20.8; 29.0) | 1.79 (1.50; 2.15) | 1.43 (1.19; 1.72) | 1.18 (0.98; 1.42) | |
| HbA1c 6.0–6.4% | 29.5 (23.4; 37.3) | 2.15 (1.68; 2.76) | 1.62 (1.26; 2.08) | 1.13 (0.87; 1.46) | |
| By FPG | |||||
| Normal glycemia | FPG <5.6 mmol/L | 15.2 (14.1; 16.5) | ref. | ref. | ref. |
| Prediabetes | FPG 5.6–6.9 mmol/L | 16.5 (14.5; 18.7) | 1.08 (0.93; 1.25) | 1.00 (0.86; 1.16) | 0.93 (0.80; 1.08) |
| FPG 5.6–6.0 mmol/L | 15.4 (13.2; 17.9) | 1.01 (0.85; 1.20) | 0.94 (0.79; 1.12) | 0.91 (0.76; 1.08) | |
| FPG 6.1–6.9 mmol/L | 19.4 (15.6; 24.2) | 1.27 (1.01; 1.61) | 1.16 (0.91; 1.46) | 0.98 (0.77; 1.24) | |
| By 2hPG | |||||
| Normal glycemia | 2hPG <7.8 mmol/L | 13.4 (12.4; 14.6) | ref. | ref. | ref. |
| Prediabetes | 2hPG 7.8–11.0 mmol/L | 19.3 (16.3; 23.0) | 1.44 (1.19; 1.75) | 1.14 (0.94; 1.39) | 1.00 (0.82; 1.22) |
ref., reference; RR, crude rate ratio; RRadj1, rate ratio adjusted for age, sex, and ethnicity; RRadj2, rate ratio adjusted for age, sex, ethnicity, previous CVD, smoking, total cholesterol, HDL cholesterol, systolic blood pressure, and antihypertensive treatment.
Event Rates by Glycemic Criteria
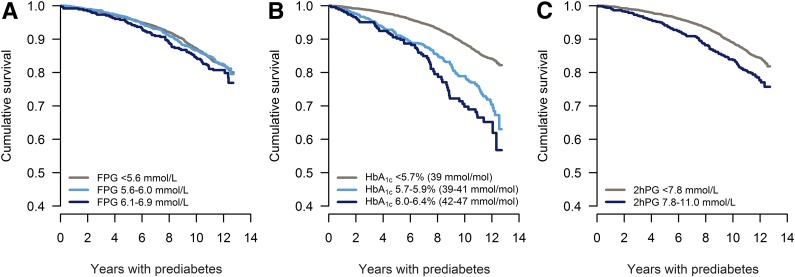
Kaplan-Meier survival curves for an event for individuals with prediabetes versus normoglycemia by different glycemic criteria are shown in Fig. 1, whereas rates and rate ratios are shown in Table 1. In individuals defined as having prediabetes by FPG levels (without taking the HbA1c level into account), the rate of an event was 19.4 for FPG levels 6.1–6.9 mmol/L (WHO criteria) and 16.5 for FPG levels 5.6–6.9 mmol/L (ADA criteria) (Fig. 1A and Table 2). In the fully adjusted model, the incidence rates were at the same level as that of the normoglycemic group for both the WHO and ADA criteria (Table 2).
Figure 1.
Kaplan-Meier survival curves for an event (CVD or mortality) for individuals with prediabetes (light blue and dark blue) versus normal glycemia (gray), using FPG, n = 5,427 (A); HbA1c, n = 5,427 (B); or 2hPG, n = 4,730 (C).
Among individuals with prediabetes by HbA1c levels (without taking the FPG level into account), the incidence rate was 29.5 for HbA1c levels 6.0–6.4% (IEC criteria) and 26.0 for HbA1c levels 5.7–6.4% (ADA criteria) (Fig. 1B and Table 2), which was approximately twice that of the rate in the normoglycemic group for both the WHO and ADA criteria. Adjustment for age, sex, and ethnicity decreased the excess in incidence rate to ∼50%, and additional adjustment reduced it further to an excess of 13–17% (nonsignificant) (Table 2).
Analyses limiting the outcome to only include CVD-related events confirmed the associations reported above (Supplementary Table 1).
In the sensitivity analysis including only individuals with 2hPG measurements, the rate of an event for individuals with prediabetes by 2hPG was 19.3, which was 44% higher than in the normoglycemic group (<7.8 mmol/L). Upon confounder adjustment there was no excess risk associated with prediabetes (Table 2 and Supplementary Fig. 2).
Comparing Event Rates Between Nonoverlapping Groups
The incidence rate of an event in individuals with prediabetes by FPG levels 5.6–6.0 mmol/L but normal HbA1c levels (<5.7%) was low and comparable with that of the normoglycemic group (FPG <5.6 mmol/L and HbA1c <5.7%) (∼13/1,000 PY [Supplementary Fig. 3]). In contrast, the incidence rate in people with prediabetes by HbA1c 5.7–5.9% but normal FPG (<5.6 mmol/L) was twice as high at 26.0/1,000 PY (Supplementary Fig. 3). After adjustment for age, sex, and ethnicity, the rate was still 64% (95% CI 25; 117) higher in the group with HbA1c 5.7–5.9% but normal FPG (<5.6 mmol/L) compared with the group with FPG levels 5.6–6.0 mmol/L but normal HbA1c levels (<5.9%). Further adjustment for previous CVD, smoking, total cholesterol, HDL cholesterol, systolic blood pressure, and antihypertensive treatment reduced this to 33% (95% CI 0; 77) (P = 0.046).
Performance of the Different Glycemic Criteria
The sensitivity and PPV for the 10-year risk of an event were low for all the prediabetes subgroups (Supplementary Table 2). Use of the ADA criteria for FPG and HbA1c more than doubled the sensitivity but decreased the specificity compared with the results with use of the WHO/IEC criteria. The PPV was higher for prediabetes defined by HbA1c than by FPG or 2hPG, whereas the negative predictive value was similar across all the prediabetes subgroups.
Exploring Event Rates by Increasing Levels of Glycemia
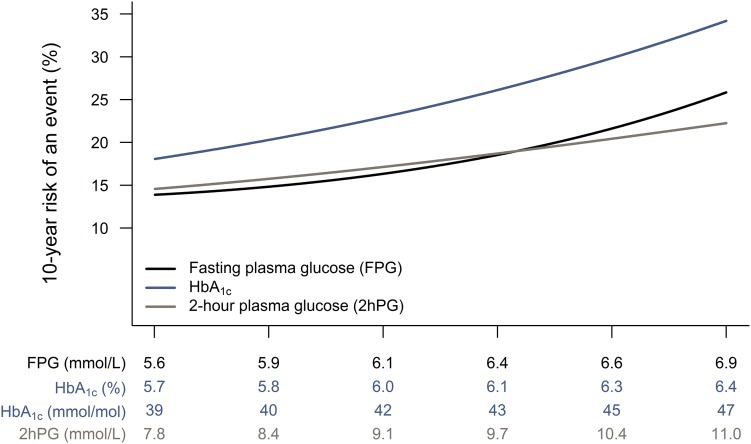
The 10-year absolute risk of an event across the prediabetes range by the ADA criteria for FPG, 2hPG, and HbA1c is shown in Fig. 2. The risk was higher for all levels of HbA1c, whereas for FPG and 2hPG the risk across the prediabetes range was somewhat comparable. There was no indication of an inflection point for any of the glycemic measures.
Figure 2.
Association between glycemia in the prediabetes range and 10-year risk of an event (CVD or mortality).
Conclusions
In the present large prospective Whitehall II cohort study of adults aged 50–80 years with simultaneous measures of different glycemic measures, we found that the prevalence of prediabetes defined by FPG and/or HbA1c was three times higher when the ADA criteria were used than when the WHO/IEC definitions were used. Individuals with prediabetes defined by HbA1c had substantially higher risk of CVD and mortality than those defined by the FPG or 2hPG criteria irrespective of whether the cut point of 5.7% (39 mmol/mol) or 6.0% (42 mmol/mol) was used. For none of the glycemic measures did we find an inflection point for risk of CVD and mortality over the prediabetes range. Furthermore, the excess risk of CVD and death associated with having prediabetes was greatly reduced in HbA1c-defined prediabetes and null in FPG- and 2hPG-defined prediabetes after adjustment for demographic and cardiovascular risk factors. These findings indicate that there is no obvious optimal glycemic cutoff for risk stratification, and the higher risk for CVD and death among individuals with prediabetes is mainly explained by its clustering with other risk factors associated with hyperglycemia. This challenges the use of the prediabetes classification as a stand-alone tool for risk stratification among older adults.
Only a few previous studies have compared different definitions of prediabetes in relation to CVD and mortality in the same population (15,22). Warren et al. (15) found in the prospective Atherosclerosis Risk in Communities (ARIC) study that HbA1c was more specific and provided better risk discriminating regarding future major events compared with FPG or 2hPG concentrations. After adjustment for cardiovascular risk factors, the risk of all-cause mortality was reduced but still significantly elevated in those with HbA1c-defined prediabetes. However, incidence rates for cardiovascular mortality became nonsignificantly elevated for all prediabetes subgroups after adjustment for cardiovascular risk factors (15), which is in accordance with the findings from our analysis and underscores the importance of focusing on nonglycemic risk factors in individuals with prediabetes. In contrast to the ARIC study, we also reported absolute 10-year risk estimates over the prediabetes range of all the three glycemic measures, and these showed a higher absolute risk for HbA1c than for FPG and 2hPG concentrations. We found no inflection point of risk in the association, which is in line with the findings from the population-based Australian Diabetes, Obesity and Lifestyle (AusDiab) study (13). We further evaluated the risk associated with different combinations of HbA1c and FPG levels, which enabled us to show that prediabetes HbA1c levels are associated with elevated risk of CVD and death even when FPG levels are normal, while the opposite is not the case (i.e., prediabetes FPG and normal HbA1c levels). In support of this finding, data from the ADDITION study (Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen Detected Diabetes in Primary Care) showed that among individuals with normal glucose tolerance on an OGTT, those with HbA1c levels in the range 6.0–6.4% had 21% higher risk of all-cause mortality than those with HbA1c levels <6.0% (23), again suggesting that HbA1c predicts mortality beyond fasting and 2-h glucose levels. In relation to the use of FPG for diagnosis of prediabetes, results from meta-analyses have shown that prediabetes defined by the WHO IFG criterion, but not the ADA IFG criterion, is associated with increased risk of cardiovascular and all-cause mortality (24). Similar results were found in relation to the risk of stroke (25). A recent meta-analysis, however, concluded that IFG defined by the ADA criterion is associated with an increased risk of all-cause mortality, cardiovascular mortality, coronary heart disease, and stroke (14). However, a subgroup analysis revealed that among individuals aged 55 years or older, ADA-defined IFG was not associated with all-cause mortality (14). Combined with our results, these findings suggest that the increased CVD and mortality risk associated with prediabetes FPG levels may decrease with age. It is thus likely that FPG is better for risk stratification in younger adults, whereas HbA1c is a better and more stable measure for health status in older adults, but this hypothesis needs to be tested in study populations with a wide age range. Part of the stronger association found between HbA1c and incident CVD may be explained by the capacity of HbA1c to reflect average glycemia, but HbA1c may also indirectly capture information about other important pathophysiological processes such as iron metabolism and low-grade inflammation (26,27). However, their causal effects need to be examined in more detail.
A major strength of the Whitehall II cohort is that the measures of FPG, 2hPG, and HbA1c were obtained simultaneously and can be linked to validated measures of morbidity and mortality over a long follow-up period (20). Deaths attributable to causes other than CVD were included in the analysis to avoid bias from competing risk. However, two out of three of the composite events were related to CVD, and sensitivity analyses with only CVD as outcome were consistent with our conclusions. Another strength of the current analysis is the application of all the different definitions for prediabetes. Most previous studies have only focused on a single definition of prediabetes (either WHO/IEC or ADA) and thereby used different reference groups for defining normoglycemia (13,23,28). Accordingly, event rates cannot be compared directly across studies to derive solid evidence on the association of prediabetes with morbidity and mortality. This could also explain why previous meta-analyses on the relationship of prediabetes with future morbidity and mortality have shown conflicting results (12,13,24,25). In the Whitehall II study, some individuals with prediabetes may develop diabetes during follow-up and subsequently receive treatment to reduce CVD risk. This could potentially have biased the results in the sense that the calculated event rates for WHO/IEC-defined prediabetes are underestimated relative to the rates in ADA-defined prediabetes, which have lower levels of glycemia and therefore are less likely to convert to diabetes during follow-up. It is also possible that the way diabetes has been diagnosed by general practitioners between the study visits during follow-up may have introduced bias. Before 2012, diabetes was mostly diagnosed by measurement of FPG levels, but after 2012 there has been a shift from FPG to HbA1c for diagnosis of diabetes in clinical practice in the U.K. (9). However, given that end of follow-up in our study was 30 June 2015, we expect these effects to even out. Therefore, it is reasonable to believe that the differential associations of FPG versus HbA1c with CVD and mortality are not caused by diagnosis and treatment of diabetes and CVD risk in individuals identified by one specific diagnostic criterion over another during follow-up.
During the last decades there has been an increased focus on identifying high-risk individuals in order to prevent future disease and premature mortality. As a result, the diagnostic thresholds have been lowered for many diseases (29), which has increased sensitivity at the cost of specificity. With the adoption of HbA1c as a diagnostic criterion for diabetes (9,30), the possibility of also using HbA1c to risk stratify individuals for diabetes prevention is obvious, but the challenge is to choose the optimal cut point. As shown in this and other studies (13,28), there does not seem to be an inflection point in the nondiabetes range for HbA1c in the association with CVD or mortality. Accordingly, when deciding on the diagnostic test and thresholds used to guide preventive interventions one needs to consider the effectiveness of interventions as well as the health and economic consequences of false positives and false negatives (5,31). The major diabetes prevention trials performed thus far have included individuals with IGT and not people identified as having high risk by FPG or HbA1c (32–34). Despite the limited evidence for prevention in these groups of individuals, the current recommendations from ADA suggest that all individuals with prediabetes (IFG, IGT, or HbA1c 5.7–6.4%) should be targeted for diabetes preventive efforts (lifestyle modification or metformin) (35). Because of the poor concordance between the different diagnostic criteria, it is questionable whether results from trials in IGT will apply to individuals identified by slightly elevated FPG or HbA1c levels. Thus, intervention studies among individuals identified by FPG or HbA1c aiming at reducing risk for diabetes and CVD are warranted in order to improve and modify the current recommendations (36). More recent research also suggests that intermediate time points or different glucose curve patterns during an OGTT may be relevant to use for risk stratification purposes (37,38). In addition, it will be important to evaluate prediabetes in the context of overall CVD risk because of the close relationship of glycemia with other cardiovascular risk factors. Thus, future risk prediction models should study whether easily measured risk factors and/or cheap biomarkers can jointly predict future diabetes, CVD, and mortality.
In conclusion, our study showed that individuals with prediabetes defined using the ADA criteria have a lower risk of CVD and all-cause mortality than individuals with prediabetes identified by the WHO/IEC criteria. This difference was mainly driven by the lower incidence of CVD or death among individuals with IFG but normal levels of HbA1c. Our results showed a high incidence rate of CVD and death in those with HbA1c levels 5.7–5.9%, which advocates for lowering the cut point for prediabetes to <6.0% for CVD preventive interventions. That said, our study also shows that a substantial part of the excess risk in prediabetes is explained by other CVD risk factors, suggesting that the use of prediabetes as an independent factor for risk stratification is questionable.
Supplementary Material
Article Information
Acknowledgments. The authors thank all participating women and men in the Whitehall II study, as well as all Whitehall II research scientists, study and data managers, and clinical and administrative staff who make the study possible.
Funding. The U.K. Medical Research Council (K013351 to M.K.), British Heart Foundation (RG/13/2/30098 to M.K.), and the U.S. National Institutes of Health (R01-HL-36310 and R01-AG-013196 to M.K.) have supported collection of data in the Whitehall II study. D.R.W. is supported by the Danish Diabetes Academy, which is funded by an unrestricted grant from the Novo Nordisk Foundation. M.K. is supported by NordForsk and the Academy of Finland (311492). K.F. is supported by a grant from the Novo Nordisk Foundation.
The funders of the study had no role in study design, data collection, analysis, interpretation, or writing of the manuscript.
Duality of Interest. M.E.J. has received research grants from AstraZeneca (investigator-initiated research). No other potential conflicts of interest relevant to this article were reported.
Author Contributions. D.V. and K.F. contributed to the study concept and design, planned the statistical analyses, and drafted the manuscript. D.V. conducted the statistical analysis. E.J.B., M.K., and A.T. provided data. D.V. and K.F. had final responsibility for the decision to submit for publication. All authors provided intellectual input and read and approved the final version of the manuscript. D.V. and K.F. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-2530/-/DC1.
References
- 1.National Diabetes Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Expert Committee on Diabetes Mellitus: Second Report. Geneva, World Health Org., 1980 (Tech. Rep. Ser., no. 646) [PubMed]
- 3.World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia. Geneva, World Health Org., 2006
- 4.American Diabetes Association Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetes—2017 Diabetes Care 2017;40(Suppl. 1):S11–S2427979889 [Google Scholar]
- 5.Makaroff LE. The need for international consensus on prediabetes. Lancet Diabetes Endocrinol 2017;5:5–7 [DOI] [PubMed] [Google Scholar]
- 6.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 7.Borch-Johnsen K, Colagiuri S, Balkau B, et al. Creating a pandemic of prediabetes: the proposed new diagnostic criteria for impaired fasting glycemia. Diabetologia 2004;47:1396–1402 [DOI] [PubMed] [Google Scholar]
- 8.Balkau B, Hillier T, Vierron E, et al. doi: 10.1007/s00125-005-1695-5. Comment to: Borch-Johnson K, Colagiuri S, Balkau B, et al. (2004) Creating a pandemic of prediabetes: the proposed new diagnostic criteria for impaired fasting glycaemia. Diabetologia 47:1396-1402 (Letter). Diabetologia 2005;48:801–802. [DOI] [PubMed]
- 9.World Health Organization. Use of Glycated Hemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Geneva, World Health Org., 2011 [PubMed]
- 10.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care 2014;37(Suppl. 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 11.The International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol 2010;55:1310–1317 [DOI] [PubMed] [Google Scholar]
- 13.Barr EL, Boyko EJ, Zimmet PZ, Wolfe R, Tonkin AM, Shaw JE. Continuous relationships between non-diabetic hyperglycemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia 2009;52:415–424 [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 2016;355:i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren B, Pankow JS, Matsushita K, et al. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol 2017;5:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol 2005;34:251–256 [DOI] [PubMed] [Google Scholar]
- 17.Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 2009;373:2215–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapelle JP, Teixeira J, Maisin D, et al. Multicentre evaluation of the Tosoh HbA1c G8 analyser. Clin Chem Lab Med 2010;48:365–371 [DOI] [PubMed] [Google Scholar]
- 19.Hinnouho GM, Czernichow S, Dugravot A, et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J 2015;36:551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kivimäki M, Batty GD, Singh-Manoux A, Britton A, Brunner EJ, Shipley MJ. Validity of cardiovascular disease event ascertainment using linkage to UK hospital records. Epidemiology 2017;28:735–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care. Circulation 2008;117:743–753 [DOI] [PubMed] [Google Scholar]
- 22.Yudkin JS. “Prediabetes”: are there problems with this label? Yes, the label creates further problems! Diabetes Care 2016;39:1468–1471 [DOI] [PubMed] [Google Scholar]
- 23.Skriver MV, Borch-Johnsen K, Lauritzen T, Sandbek A. HbA1c as predictor of all-cause mortality in individuals at high risk of diabetes with normal glucose tolerance, identified by screening: a follow-up study of the Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen-Detected Diabetes in Primary Care (ADDITION), Denmark. Diabetologia 2010;53:2328–2333 [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Cai X, Chen P, et al. Associations of prediabetes with all-cause and cardiovascular mortality: a meta-analysis. Ann Med 2014;46:684–692 [DOI] [PubMed] [Google Scholar]
- 25.Lee M, Saver JL, Hong K-S, Song S, Chang K-H, Ovbiagele B. Effect of pre-diabetes on future risk of stroke: meta-analysis. BMJ 2012;344:e3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S, Hempe JM, McCarter RJ, Li S, Fonseca VA. Association between inflammation and biological variation in hemoglobin A1c in U.S. nondiabetic adults. J Clin Endocrinol Metab 2015;100:2364–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herder C, Ferch K, Carstensen-Kirberg M, et al. Biomarkers of subclinical inflammation and increases in glycemia, insulin resistance and beta-cell function in non-diabetic individuals: the Whitehall II study. Eur J Endocrinol 2016;175:367–377 [DOI] [PubMed] [Google Scholar]
- 28.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickering TG. Lowering the thresholds of disease—are any of us still healthy? J Clin Hypertens 2004;6:672–674 [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2011;34(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregg EW, Geiss L, Zhang P, Zhuo X, Williamson DF, Albright AL. Implications of risk stratification for diabetes prevention: the case of hemoglobin A1c. Am J Prev Med 2013;44(Suppl. 4):S375–S380 [DOI] [PubMed] [Google Scholar]
- 32.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 33.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 34.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Diabetes Association Prevention or delay of type 2 diabetes. Sec. 5. In Standards of Medical Care in Diabetes—2017 Diabetes Care 2017;40(Suppl. 1):S44–S47 [DOI] [PubMed] [Google Scholar]
- 36.Ferch K, Vistisen D. Is there a need for new diabetes prevention trials? BMJ 2017;356:j1003. [DOI] [PubMed] [Google Scholar]
- 37.Hulman A, Vistisen D, Glumer C, Bergman M, Witte DR, Ferch K. Glucose patterns during an oral glucose tolerance test and associations with future diabetes, cardiovascular disease and all-cause mortality rate. Diabetologia 2018;61:101–107 [DOI] [PubMed] [Google Scholar]
- 38.Pareek M, Bhatt DL, Nielsen ML, et al. Enhanced predictive capability of a 1-hour oral glucose tolerance test: a prospective population-based cohort study. Diabetes Care 2018;41:171–177 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.