Abstract
OBJECTIVE
The MiniMed 670G System is the first commercial hybrid closed-loop (HCL) system for management of type 1 diabetes. Using data from adolescent and young adult participants, we compared insulin delivery patterns and time-in-range metrics in HCL (Auto Mode) and open loop (OL). System alerts, usage profiles, and operational parameters were examined to provide suggestions for optimal clinical use of the system.
RESEARCH DESIGN AND METHODS
Data from 31 adolescent and young adult participants (14–26 years old) at three clinical sites in the 670G pivotal trial were analyzed. Participants had a 2-week run-in period in OL, followed by a 3-month in-home study phase with HCL functionality enabled. Data were compared between baseline OL and HCL use after 1 week, 1 month, 2 months, and 3 months.
RESULTS
Carbohydrate-to-insulin (C-to-I) ratios were more aggressive for all meals with HCL compared with baseline OL. Total daily insulin dose and basal-to-bolus ratio did not change during the trial. Time in range increased 14% with use of Auto Mode after 3 months (P < 0.001), and HbA1c decreased 0.75%. Auto Mode exits were primarily due to sensor/insulin delivery alerts and hyperglycemia. The percentage of time in Auto Mode gradually declined from 87%, with a final use rate of 72% (−15%).
CONCLUSIONS
In transitioning young patients to the 670G system, providers should anticipate immediate C-to-I ratio adjustments while also assessing active insulin time. Users should anticipate occasional Auto Mode exits, which can be reduced by following system instructions and reliably bolusing for meals. Unique 670G system functionality requires ongoing clinical guidance and education from providers.
Introduction
Diabetes technology has progressed in the past decade from separate continuous subcutaneous insulin infusion pumps and continuous glucose monitors (CGMs) to integrated systems that use continuous glucose feedback from CGMs and historical insulin delivery information to calculate insulin dosing to be delivered by the pump. This progression toward “artificial pancreas” systems (AP) (1,2) started with systems that suspend insulin delivery for low and predicted low sensor glucose levels (3) and evolved to more fully integrated closed-loop systems that calculate all continuous insulin delivery with and without meal bolus delivery. Many inpatient and outpatient studies have been completed on these closed-loop systems and consistently show improved glycemic outcomes and reduction in hypoglycemia in both adults and children, especially in the overnight period (4–7).
The MiniMed 670G System from Medtronic (Northridge, CA) is the first commercial hybrid closed-loop (HCL) system available globally. The system uses a proprietary proportional-integral-derivative controller with insulin feedback to calculate insulin doses continually based on CGM levels (8–10). The system works in two modes: open-loop (OL) “Manual Mode” (insulin pump with or without sensor) and HCL “Auto Mode,” in which the system algorithmically calculates background “auto-basal” insulin delivery based on CGM glucose values, with predefined insulin delivery limits adapted daily. When the system operates in OL, all insulin dosing parameters common to continuous subcutaneous insulin infusion are modifiable, including programmed basal rates, carbohydrate-to-insulin (C-to-I) ratios, insulin sensitivity factors, active insulin time, and glucose targets. When the HCL Auto Mode feature is enabled, the system automates insulin delivery, although it still requires user-initiated boluses for carbohydrates and optional correction doses. The user can modify the HCL system by adjusting C-to-I ratios, active insulin time, and the rate of bolus insulin delivery (standard or quick bolus). The glucose target can be temporarily increased from 120 to 150 mg/dL for exercise or driving. All other parameters are system determined based on the previous days of total daily insulin and fasting glycemic control. This system has been studied in inpatient, hotel, camp, and outpatient settings with favorable glycemic outcomes (10–14). The pivotal study of the 670G system involved 124 participants with type 1 diabetes aged 14–75 years wearing the system for 3 months in an outpatient setting. Overall mean HbA1c decreased during the study from 7.4 to 6.9% (57 to 52 mmol/mol) with a concomitant increase in the time spent in range, defined as 71–180 mg/dL, from a mean of 66.7% before system use to 72.2% by the end of the 3-month study (15,16). Notably, a slightly greater decrease in HbA1c, from 7.7 to 7.1% (61 to 54 mmol/mol), occurred in those aged 14–21 years (15).
Although the main trial data were subanalyzed for ages 14–21, the more clinically important young age-group includes emerging adults up to age 26. This precise population demonstrates significantly elevated HbA1c levels compared with older adults (17). These patients are often monitored by their pediatric providers until insurance or life changes dictate transition to adult clinical care. The physiological and developmental changes that continue through the third decade of life require unique attention on the part of the clinician. It is this adolescent and emerging adult population that may have the greatest benefit from HCL systems. By achieving more targeted control during this stage of life, patients may be set on a course for more stable glycemic control throughout adulthood (17). Furthermore, data from the Epidemiology of Diabetes Interventions and Complications (EDIC) study have demonstrated that the persistent benefits of targeted glycemic control earlier in life lead to metabolic memory and reduction of long-term complications even if glycemic control later deteriorates (18), highlighting the importance of improving care for this difficult-to-manage population.
There is scant guidance available, however, on how to optimize use of the MiniMed 670G, and no data are available on how to fine-tune system operation. These data are essential for clinicians to competently manage patients using the 670G and guide insulin therapy with the best chance for clinical success (19). The purpose of this present analysis was 1) to analyze 670G system insulin delivery settings, time in range, and patterns of HCL use to recommend how clinicians can optimize parameters for system use, and 2) to accomplish this in the context of adolescent and emerging adults (ages 14–26 years old), the population most likely to benefit from fine-tuned HCL insulin delivery and for whom achievement of ideal glucose control is most difficult.
Research Design and Methods
Participants and Study Design
The results presented here represent a subanalysis of 31 adolescent and young adult participants (14–26 years old) at three clinical sites (Barbara Davis Center, Stanford University, and Yale University) in the larger pivotal trial of the MiniMed 670G HCL system. Full methods of the main trial have been published (16). Briefly, the trial enrolled participants 14–75 years old with type 1 diabetes for ≥2 years, baseline HbA1c <10% (<86 mmol/mol), who had used an insulin pump for >6 months, with or without CGM experience. The 670G system consists of the MiniMed 670G insulin pump with incorporated HCL algorithm, the Guardian Sensor 3 CGM system, and the CONTOUR NEXT Link blood glucose meter (Ascensia Diabetes Care, Parsippany, NJ). The study was conducted under a U.S. Food and Drug Administration investigation device exemption, and Institutional Review Board approval was obtained at each clinical site. All participants, or their guardians, provided informed consent, and all minors provided written assent. The trial was registered with clinicaltrials.gov (NCT02463097).
Participants had a 2-week at home run-in period that involved using the 670G system in OL Manual Mode (insulin pump with CGM). During this run-in period, the study investigators made routine insulin dose adjustments as necessary. Data obtained during this run-in period served as the basis of comparison for efficacy subanalysis in the main trial (15,16). Insulin dosing from the last 6 days of the OL period provided the basis for the internally calculated, personalized HCL algorithm parameters for Auto Mode (8). Once personalized parameters were derived, Auto Mode was enabled, and participants were monitored for a 3-month home study phase. Participants were instructed to upload their pump data via the Clinical CareLink website every day during the first 2 weeks of the study and then weekly thereafter. Uploaded data were reviewed by the research team to verify compliance with system usage and to periodically tune system parameters to optimize HCL performance.
Site investigators evaluated and fine-tuned the initial Manual Mode programming of basal rates, C-to-I ratios, insulin sensitivity, blood glucose target, and active insulin time. When the system was used in Auto Mode, only the C-to-I ratios and active insulin time remained adjustable for meal bolusing. The remaining parameters, including basal rates, sensitivities, and targets, were replaced by Auto Mode algorithmic function and were only operational if a participant reverted to Manual Mode. During Auto Mode use, the basal rates were dynamically determined by the system every 5 min as previously described. The target glucose was fixed at 120 mg/dL, with an optional temporary target of 150 mg/dL, which could be used for exercise. The insulin sensitivity factor was also determined by the system during Auto Mode by dividing the total daily dose (TDD) into 1,800 and correcting to 150 mg/dL. Correction boluses were only delivered if the blood glucose was >150 mg/dL and a user entered this information into the pump. More complete descriptions of the MiniMed 670G HCL algorithm have been previously published (8,13,20). Participants could choose to exit Auto Mode or the system could force Auto Mode exit for several reasons. Prolonged hyperglycemia (>300 mg/dL for 1 h or >250 mg/dL for 3 h), which may be due to pump or infusion set malfunction, caused direct system exit from Auto Mode into Manual Mode, whereas other conditions caused the system to enter a transitional “Safe Basal” Mode. Safe Basal is when the system automatically calculated basal delivery but did not modulate the rate based on sensor glucose levels. The 670G system triggered Safe Basal when it detected sensor issues (e.g., no calibration, lost sensor signal, sensor replacement, poor sensor readings) or anomalous insulin delivery (e.g., auto-basal delivery above or below internally calculated safety levels for a prolonged period of time). The Safe Basal condition could resolve on its own or request a blood glucose entry from the user to return to Auto Mode. If the user did not respond by entering a blood glucose within 90 min, the Safe Basal reverted to Manual Mode after the 90-min “Safe Basal time-out.”
Statistical Analysis
For analysis, we split the 670G pivotal trial data into five 1-week time periods: 7 days before Auto Mode initiation, Auto Mode enabled days 1–7, Auto Mode enabled days 22–28 (1 month’s use), Auto Mode enabled days 50–56 (2 months’ use), and Auto Mode enabled days 78–84 (3 months’ use) with the reported data averaged over the given week. The aim of these periods was to capture settings in Manual Mode as well as settings for the 1st week of Auto Mode use and then at the end of each month of the 3-month study phase.
Insulin usage was analyzed as TDD, auto-basal insulin delivery in units per day, bolus units per day, average active insulin time, and programmed total daily basal rate for Manual Mode. The breakfast C-to-I ratio was reported based on 8 a.m. programming, lunch as 12 p.m., and dinner as 6 p.m. Target range was considered to be 70–180 mg/dL as per AP consensus reporting guidelines (21). Reasons for participants being exited from Auto Mode were grouped into system alarms (e.g., overt prolonged hyperglycemia or other system failures), Safe Basal time-outs (e.g., sensor or insulin delivery concerns that were not resolved in a 90-min time period as described above), and user-initiated exits. All data are presented as mean ± SD or as a percentage. Group-wise comparisons were conducted via ANOVA, and head-to-head comparisons were conducted by paired t tests. No correction was made for multiple comparisons. Linear regression was used to evaluate the effect of time in Auto Mode and other predictors on time in target range. An α value of <0.05 was considered statistically significant. Statistical analysis was conducted using SAS 9.4 software (SAS Institute, Cary, NC).
Results
The analysis included 31 (15 female) adolescent and young adult participants who completed the 3-month pivotal trial. Mean age was 17.8 ± 3.9 years, average duration of type 1 diabetes was 9.3 ± 5.5 years, and mean HbA1c at enrollment was 7.8 ± 0.9% (62 mmol/mol) (Supplementary Table 1).
Overall HbA1c improvement was 0.75 ± 0.69% from baseline to the end of the 3-month study phase (P < 0.0001). During the trial, TDD did not change significantly from baseline to the end of the 3-month study phase in which Auto Mode was enabled (P = 0.49) or in any interim period (Table 1). The HCL auto-basal delivery during the study phase was similar during all 7-day time points to the preprogrammed total daily basal insulin during the baseline OL period. Likewise, bolus insulin total and ratio of basal-to-bolus insulin did not change from baseline to end of trial. During the study phase, participants averaged 1.05 ± 0.61 correction boluses per day with an average amount of insulin of 1.39 ± 0.89 units, with all other bolus insulin being delivered with carbohydrate consumption.
Table 1.
Insulin delivery characteristics and time in range at baseline, week 1, month 1, month 2, and month 3 of the trial period
| Period | Mean ± SD | P value change from baseline | |
|---|---|---|---|
| Mean TDD of insulin (units/day) | Baseline | 58.6 ± 19.1 | N/A |
| Days 1–7 | 58.2 ± 20.1 | 0.83 | |
| Days 22–28 | 60.6 ± 21.5 | 0.41 | |
| Days 50–56 | 58.9 ± 22.3 | 0.91 | |
| Days 78–84 | 60.3 ± 21.5 | 0.49 | |
| Mean basal/auto-basal insulin dose (units/day) | Baseline | 25.8 ± 8.3 | N/A |
| Days 1–7 | 27.1 ± 10.6 | 0.22 | |
| Days 22–28 | 27.0 ± 9.6 | 0.31 | |
| Days 50–56 | 26.0 ± 9.1 | 0.86 | |
| Days 78–84 | 26.7 ± 9.3 | 0.37 | |
| Mean bolus insulin (units/day) | Baseline | 32.6 ± 13.7 | N/A |
| Days 1–7 | 31.1 ± 12.9 | 0.27 | |
| Days 22–28 | 33.8 ± 14.4 | 0.44 | |
| Days 50–56 | 32.9 ± 15.8 | 0.86 | |
| Days 78–84 | 33.5 ± 15.1 | 0.59 | |
| Percentage basal/bolus split | Baseline | 45.1 ± 9.2 | N/A |
| Days 1–7 | 46.9 ± 10.3 | 0.25 | |
| Days 22–28 | 44.9 ± 9.0 | 0.87 | |
| Days 50–56 | 45.1 ± 10.8 | 0.99 | |
| Days 78–84 | 44.8 ± 10.3 | 0.82 | |
| Mean 24-h basal program for OL (units/day) | Baseline | 26.7 ± 8.8 | N/A |
| Days 1–7 | 26.8 ± 8.7 | 0.10 | |
| Days 22–28 | 26.6 ± 8.7 | 0.74 | |
| Days 50–56 | 26.9 ± 8.9 | 0.21 | |
| Days 78–84 | 27.0 ± 9.1 | 0.18 | |
| C-to-I ratio | |||
| 8 a.m. | Baseline | 7.9 ± 2.7 | N/A |
| Days 1–7 | 7.4 ± 2.5 | 0.017 | |
| Days 22–28 | 7.0 ± 2.7 | 0.0014 | |
| Days 50–56 | 6.9 ± 2.7 | <0.0001 | |
| Days 78–84 | 6.8 ± 2.8 | 0.0002 | |
| 12 p.m. | Baseline | 8.9 ± 3.3 | N/A |
| Days 1–7 | 8.4 ± 3.1 | 0.0085 | |
| Days 22–28 | 7.8 ± 2.9 | <0.0001 | |
| Days 50–56 | 7.6 ± 3.0 | <0.0001 | |
| Days 78–84 | 7.6 ± 3.1 | <0.0001 | |
| 6 p.m. | Baseline | 8.7 ± 3.4 | N/A |
| Days 1–7 | 8.1 ± 3.1 | 0.0004 | |
| Days 22–28 | 7.4 ± 2.7 | <0.0001 | |
| Days 50–56 | 7.3 ± 2.7 | <0.0001 | |
| Days 78–84 | 7.2 ± 2.6 | <0.0001 | |
| Insulin action time (min) | Baseline | 174.2 ± 51.6 | N/A |
| Days 1–7 | 171.6 ± 49.2 | 0.16 | |
| Days 22–28 | 168.4 ± 46.9 | 0.083 | |
| Days 50–56 | 169.2 ± 47.3 | 0.15 | |
| Days 78–84 | 168.4 ± 46.9 | 0.083 | |
| Percentage time in range 70–180 mg/dL | |||
| Overall | Baseline | 55.3 ± 14.9 | N/A |
| Days 1–7 | 68.4 ± 11.5 | <0.0001 | |
| Days 22–28 | 67.4 ± 10.1 | <0.0001 | |
| Days 50–56 | 70.2 ± 8.9 | <0.0001 | |
| Days 78–84 | 69.0 ± 12.0 | <0.0001 | |
| HCL Mode | Baseline | N/A | N/A |
| Days 1–7 | 69.7 ± 10.6 | N/A | |
| Days 22–28 | 69.5 ± 8.5 | N/A | |
| Days 50–56 | 71.9 ± 8.1 | N/A | |
| Days 78–84 | 71.5 ± 10.3 | N/A | |
| OL | Baseline | 55.3 ± 14.9 | N/A |
| Days 1–7 | 56.6 ± 28.1 | 0.80 | |
| Days 22–28 | 56.8 ± 25.9 | 0.75 | |
| Days 50–56 | 60.3 ± 19.7 | 0.16 | |
| Days 78–84 | 57.4 ± 28.8 | 0.67 |
N/A, not applicable.
The C-to-I ratios during the Auto Mode–enabled study phase were made more aggressive regardless of the meal period compared with baseline (Table 1). Breakfast (8 a.m.) C-to-I ratios decreased significantly from 7.9 at baseline to 7.4 at week 1 and 7.0 at month 1 and leveled off at 6.9 and 6.8 for months 2 and 3, respectively (P ≤ 0.001 for all time points from baseline). The interval changes were significant from baseline and week 1 and likewise from week 1 to month 1 (P = 0.01), with no significant interval change from month 1 to month 2 and month 2 to 3. This was similarly observed in the lunch (12 p.m.) C-to-I ratio, which decreased from 8.9 at baseline to 8.4 at week 1, 7.8 at month 1, 7.6 at month 2, and 7.6 at month 3 (P < 0.001 for all time points). The dinner (6 p.m.) C-to-I ratio likewise decreased from 8.7 at baseline to 8.1, 7.4, 7.3, and 7.2 at week 1 and months 1, 2, and 3, respectively (P < 0.001 for all time points). Across all three C-to-I doses, the significant changes were found from baseline to week 1 and week 1 to month 1, with no appreciable differences in the intervals from month 1 to 2 and 3.
Overall percentage of time in range (defined as 70–180 mg/dL) significantly increased with Auto Mode use (Table 2). At baseline during OL insulin delivery, the percentage of time in range was 55.3%. Within the first 7 days of Auto Mode use, the percentage in range increased to 68.4% (P < 0.001). This ∼15% increase in time in range was sustained for the remainder of the trial, with the percentage of time in range at months 1, 2, and 3 being 67.4%, 70.2%, and 69%, respectively (P < 0.001 at all time points). When the 3-month trial period was subdivided by Manual Mode versus Auto Mode use, there was a significant increase in time in range during Auto Mode compared with Manual Mode for all 7-day time points (week 1: 69.7% vs. 56.6%, P = 0.009; month 1: 69.5% vs. 56.8%, P = 0.006; month 2: 71.9% vs. 60.3%, P = 0.001; month 3: 71.5% vs. 57.4%, P = 0.005).
Table 2.
Reasons for exit from Auto Mode into OL Manual Mode during 3-month trial phase
| Period | Mean ± SD | P value* | |
|---|---|---|---|
| Percentage time in HCL | Days 1–7 | 87.0 ± 10.8 | |
| Days 22–28 | 80.2 ± 15.5 | 0.019 | |
| Days 50–56 | 76.0 ± 14.2 | 0.25 | |
| Days 78–84 | 71.8 ± 23.3 | 0.26 | |
| Mean number of events removing participant from HCL | |||
| Total (events/week) | Days 1–7 | 5.3 ± 2.7 | |
| Days 22–28 | 5.4 ± 2.5 | ||
| Days 50–56 | 5.8 ± 1.9 | ||
| Days 78–84 | 5.8 ± 2.6 | ||
| System initiated (events/week) | Days 1–7 | 5.0 ± 2.9 | |
| Days 22–28 | 5.2 ± 2.6 | ||
| Days 50–56 | 5.5 ± 2.1 | ||
| Days 78–84 | 5.6 ± 2.8 | ||
| User initiated (events/week) | Days 1–7 | 0.3 ± 0.7 | |
| Days 22–28 | 0.2 ± 0.6 | ||
| Days 50–56 | 0.3 ± 0.6 | ||
| Days 78–84 | 0.2 ± 0.5 | ||
| Percentage of events removing participant from HCL | |||
| Safe Basal time-outs | Days 1–7 | 64.0 ± 23.5 | |
| Days 22–28 | 76.9 ± 21.3 | ||
| Days 50–56 | 69.8 ± 25.7 | ||
| Days 78–84 | 65.8 ± 28.5 | ||
| Hyperglycemia and other alerts | Days 1–7 | 28.3 ± 23.4 | |
| Days 22–28 | 19.1 ± 18.5 | ||
| Days 50–56 | 23.3 ± 20.1 | ||
| Days 78–84 | 27.2 ± 25.0 | ||
| User initiated | Days 1–7 | 7.7 ± 17.8 | |
| Days 22–28 | 4.0 ± 13.1 | ||
| Days 50–56 | 6.9 ± 15.2 | ||
| Days 78–84 | 7.0 ± 20.3 |
*Comparison of change from previous period.
During each 7-day interval measured in the 3-month study phase, the percentage of time using Auto Mode fluctuated. In the first 7 days, Auto Mode was active 87% of the time. This decreased to 80% at the end of month 1, 76% at the end of month 2, and 71.8% at the end of month 3. Auto Mode usage decreased significantly between days 1 and 7 and the end of month 1 (87.0 ± 10.8% vs. 80.2 ± 15.5%, P = 0.019) but not between month 1 and month 2 (80.2 ± 15.5% vs. 76.0 ± 14.2%, P = 0.248) or month 2 and month 3 (76.0 ± 14.2% vs. 71.8 ± 23.3%, P = 0.257). For each 7-day analysis period, there was an average of 5.3–5.8 Auto Mode exits/week. At all given time periods, the overwhelming number of Auto Mode exits were initiated by the system. Most of the system-initiated Auto Mode exits were due to time-out from the Safe Basal condition: 64% in the first week of use, 76.9% at the end of month 1, 69.8% at the end of month 2, and 65.8% at the end of month 3. Other system alerts, such as overt hyperglycemia, caused 28.3%, 19.1%, 23.3%, and 27.2% of Auto Mode exits, respectively. Less than 8% of all Auto Mode exits were related to the user initiating the exit, accounting for 0.2–0.3 exits/week.
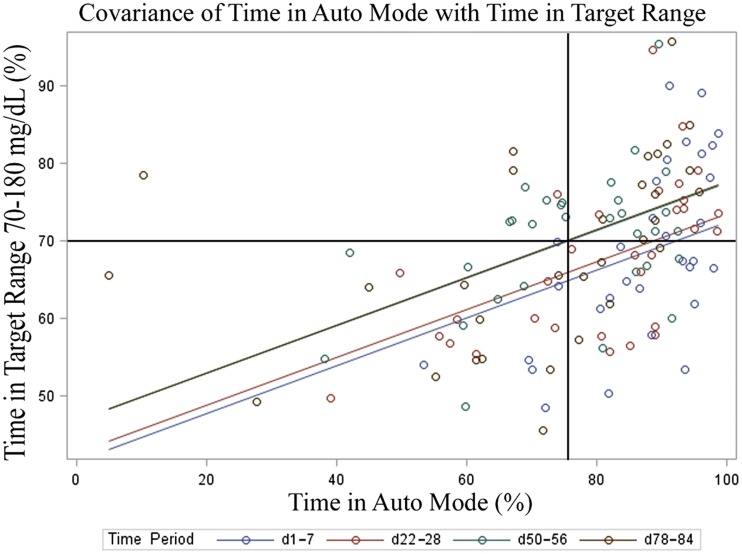
Linear regression analysis (Fig. 1) showed that time in Auto Mode had a modest but statistically significant correlation with time in range in all time periods (r2 = 0.19; P < 0.0001). By the last week of the 3-month study phase, 70% time in range was achieved by using Auto Mode ∼75% of the time. The percentage of change in the C-to-I ratio from baseline to 3 months was also a significant predictor of time in range at 3 months for breakfast (−13.9% change; P = 0.0024) and lunch (−14.6% change; P = 0.0054), but not for dinner (−17.2% change; P = 0.58). The overall changes in C-to-I ratio accounted for 44% of the variance in time in range. Time in target range during the 3rd month only was also a significant predictor of improvement in HbA1c (r2 = 0.16; P = 0.024).
Figure 1.
Linear regression of time in HCL and time in range (70–180 mg/dL), with crosshairs indicating 70% time in range correlated to ∼75% time in Auto Mode during the final week of study phase. d, day.
Conclusions
This is the first report analyzing MiniMed 670G insulin dose parameters and characteristics of Auto Mode use in the adolescent and emerging adult population. Health care providers can capitalize on this information when implementing 670G therapy in clinical care. The findings of this study indicate that C-to-I ratios should be reviewed with Auto Mode initiation and reexamined over the 1st month’s time, because these ratios will likely need to be strengthened by 10–20% at all times of day upon initiation of Auto Mode. Providing realistic expectations to patients may allow a smoother transition process, and our data suggest the 1st month may require closer follow-up, followed by recommended quarterly visits. Although this study reports no change in active insulin time, this should be evaluated as well, considering that active insulin time contributes to the overall aggressiveness of the HCL algorithm and is one of two modifiable parameters in Auto Mode.
It is further important that patients understand they are likely to encounter frequent exits from Auto Mode. With provision of this information at the time of the initial training, patients will have realistic expectations for how the system will function. Our adolescent and young adult participants experienced 5–6 exits per week from Auto Mode, with the most common reasons being Safe Basal alerts and overt hyperglycemia. Knowing this, clinicians can proactively counsel patients about the importance of premeal bolusing to reduce hyperglycemia and maximize the time spent in Auto Mode. By the end of 3 months of HCL use, 70% time in range was achieved with ∼75% Auto Mode use, providing clinicians with a standard they can seek to achieve with their patients.
This 3-month pivotal trial and the subsequent year-long continuation of 670G use in young adults and adolescents with type 1 diabetes have contributed to our competency with the 670G system due to extensive longitudinal use. Table 3 presents important teaching points learned from the trial that may be useful in implementing 670G into clinical care. These include an emphasis on modifiable and nonmodifiable parameters with the HCL, which are system specific and may differ in future AP iterations (22). Further, C-to-I ratios and active insulin parameters should be used to optimize Auto Mode function after HCL therapy is initiated. As with most diabetes self-management, providers must prioritize setting appropriate expectations and educating patients on crucial aspects of the system.
Table 3.
Summary of clinical recommendations and teaching points developed from MiniMed 670G System use during pivotal trial and continuation phase
| Modifiable parameters for 670G | Basal rates |
| OL Manual Mode use | C-to-I ratios |
| Sensitivity/correction dose | |
| Active insulin time | |
| Temporary basal rates | |
| Bolus delivery speed | |
| Modifiable parameters for 670G HCL Auto Mode use | C-to-I ratios |
| Active insulin time | |
| Temporary target for exercise (from 120 to 150 mg/dL) | |
| Bolus delivery speed | |
| Insulin dose adjustments | C-to-I ratios may be reduced (made more aggressive) due to postbolus algorithmic auto-basal suppression, which can decrease insulin immediately after a delivery but also can decrease insulin to prevent hypoglycemia in the late postprandial period. |
| Active Insulin time may need to be adjusted to 2–3 h for optimal use. | |
| If running in OL for prolonged period of time, consider underestimating carbohydrates or increasing C-to-I ratio (to be less aggressive). | |
| Programmed basal rates are difficult to assess if running in Auto Mode most of the time. Consider checking programmed total basal against average auto-basal delivery and making sure they are comparable. If prolonged period in OL, adjust basal rates as per usual care. | |
| Education | System uses 2–6 days of insulin delivery to determine how to tune the algorithm. Expect system to optimize over a period of days, not hours. |
| The number of 90-min Safe Basal time-out exits from Auto Mode can be reduced by following messaging on insulin pump to perform a blood glucose or calibration. | |
| Direct exits from Auto Mode due to hyperglycemia can be reduced by changing C-to-I ratios and active insulin time and decreasing the number of missed meal boluses. | |
| Expectations | Patient can expect to be in Auto Mode most of the time (if wearing CGM sensor consistently), but will still revert to OL 20–30% of the time. |
| Frequent sensor calibration will optimize ability to stay in Auto Mode. Calibrating 3–4 times/day will yield the best sensor accuracy (27). | |
| Consider using OL Manual Mode for temporary conditions where overall daily dose may change such as illness, camp week, sports tournament week, steroid burst, etc. | |
| Staying in Auto Mode is a virtuous circle: the fewer missed meal boluses and prolonged high postprandial glucose values, the more time the patient remains in Auto Mode, which is working to bring the glucose to target of 120 mg/dL. |
The decision to analyze adolescents and emerging adults ages 14–26 years revealed a greater reduction in HbA1c (−0.75%) than previously described (15,16) and a similar increase in the percentage of time in range to that described by Garg et al. (16). The glycemic improvement in this cohort is encouraging considering the T1D Exchange data showing that elevated HbA1c levels do not stabilize to “adult” levels until age 26, making young adults more similar to adolescents than older adults (17). The potential for glycemic benefit of HCL is more evident with this stratification than when the young adults are lumped into the older adult categories, as was done with previous analyses (15,16).
Although Auto Mode use required stronger C-to-I ratios than Manual Mode use, total average insulin delivery did not significantly change throughout the study. Insulin dose adjustments were made in the run-in period and the trial follow-up. Therefore, it is possible the C-to-I ratio changes were necessary because of how the 670G system delivers insulin and not simply due to increased vigilance from the research team, although this cannot be stated definitively without a randomized controlled trial. This increased up-front insulin delivery was likely due to the HCL algorithm’s reliance on insulin feedback, which suppresses auto-basal delivery for a period of time after a bolus is administered, whereas a traditional insulin pump would have delivered preprogrammed basal insulin throughout the meal. It was also safer to give more insulin up front with the meal, because after the meal, when blood glucose levels are decreasing, the 670G algorithm will reduce or stop basal insulin delivery to avoid impending hypoglycemia. Despite this phenomenon, TDD, total basal and bolus delivery, and basal/bolus split did not significantly change. It is possible that fewer or smaller correction boluses were needed due to the dynamic auto-basal insulin (as evidenced by the approximately one correction bolus per day in the study period), which may have mitigated the relative increase in carbohydrate bolus amounts with more aggressive C-to-I ratios. This may have also contributed to increased time in range. Strengthening C-to-I ratios may be necessary for some (23) but not all HCL systems and is dependent on specifics of the algorithm (19,24,25).
Time spent using Auto Mode did change throughout the trial, decreasing from 80% in the 1st week of use to 71% in the final week of the 3-month period. This is the first report of Auto Mode usage behavior at varying time points in the pivotal trial. Interestingly, the reasons for Auto Mode exits were consistent across time points: most were due to Safe Basal time-outs (64–70%), followed by hyperglycemia and direct exit conditions (19–28%) and by user-initiated exits (4–8%). As mentioned previously, Safe Basal time-outs occur after a 90-min “troubleshooting” period in which the 670G system tries to resolve sensor performance issues or an insulin delivery concern (e.g., maximum or minimum insulin delivery for a prolonged period of time). The devices used in this investigational trial provided ambiguous messaging related to the Safe Basal condition, resulting in inconsistent troubleshooting performed by the user. This has been improved in the subsequent commercial version of the device. To reduce the number of Safe Basal time-outs, clinicians can educate patients to follow messaging on the insulin pump related to performing a calibration or blood glucose entry to resolve the condition. System exits due to prolonged hyperglycemia (>300 mg/dL for 1 h or >250 mg/dL for 3 h) can be reduced by changing C-to-I ratios and active insulin time parameters and by encouraging patients to take correction doses of insulin for blood glucose levels >150 mg/dL. In addition, it is important to remind patients not to miss meal boluses and to bolus before eating. Finally, user-initiated exits to Manual Mode were protocol dictated if the participant had ketones or illness with vomiting. In clinical care, these recommendations may still be appropriate. Manual Mode should also be considered for other conditions that dramatically change insulin sensitivity such as steroid bursts or a camp week.
As in previous studies (15,16), time in range was significantly improved using HCL compared with OL insulin delivery. In regression analysis, time spent in Auto Mode significantly contributed to this finding, as did the C-to-I ratio changes at breakfast and lunch. A frequent barrier to patients maintaining consistent Auto Mode use was the system exiting Auto Mode for prolonged hyperglycemia. In this population, this was often seen when a meal bolus was missed, contributing to prolonged hyperglycemia and system-initiated exit from Auto Mode. The 670G requires the user to manually restart Auto Mode after a hyperglycemia exit, and this was not always performed immediately by this population, possibly producing more hyperglycemia in Manual Mode.
It is also interesting to note that although users only remained in Auto Mode 71% of time by the end of the study period, this is relatively high compared with how this age-group has incorporated other new technologies in previous studies. In the JDRF CGM trial, the overall mean sensor use at 3 months was ∼51% of time for the population aged 15–24 years (26). The increased usage of the HCL above CGM use in the JDRF CGM trial suggests that current HCL technology may be more readily adopted by this challenging population. Longer follow-up studies will be needed to assess whether Auto Mode use stabilizes or declines over time.
This analysis of insulin delivery, time in range, and Auto Mode use provides significant insight and context necessary for clinical use of the 670G system. Strengths of this analysis include the first month-by-month comparison of HCL use, the first description of Auto Mode discontinuation reasons, the targeted adolescent/emergent adult population most likely to see changes to glycemic control with HCL use, and the consolidated experience of three clinical centers.
The results must also be considered within the context of the study limitations. The 670G pivotal trial design was not a randomized controlled trial, but rather a single-arm longitudinal intervention trial (15,16). The comparison of a control population with the intervention population is not possible; rather, participant data can only be analyzed in a pre-to-post fashion. Future randomized controlled trials are planned to test superiority of HCL compared with standard insulin therapy (sensor-augmented pump, insulin pump only, and multiple daily injections). It is possible, therefore, that the changes to C-to-I ratios, increased time in range, and HbA1c change are due to participants faring better within the context of a clinical trial rather than due to device intervention. A further limitation is the small sample size in this analysis, although this age-group was chosen deliberately to represent the critical adolescent and emergent adult population.
This report on the month-by-month breakdown of the 670G HCL system use in adolescent and young adult patients during the MiniMed 670G pivotal trial highlights the first crucial months on the novel HCL system. It increases our understanding of expected changes to parameter settings that diabetes providers and educators may encounter in clinical practice. Although our study focused specifically on adolescent and young adult participants, the attention to insulin dosing parameters and system function will remain significant to all 670G system users. Future studies are needed to further characterize optimal insulin dosing strategies over long-term use of the 670G system and in a variety of settings and patients.
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge the contributions of the following individuals: from the Barbara Davis Center for Childhood Diabetes, G. Todd Alonso (co-investigator), Emily Westfall, Emily Jost, Cari Berget, Lindsey Towers, Maninder Sethi, Katelin Thivener, Samantha Lange, Michele Clay, Jason Genzler, Sofia Meier, and David Swaschnig; from Stanford University, Trang Ly (co-investigator), Ellen Ambers, Jasmine Doiev, Hojin Min, and Sarah Loebner; from Yale University, Eda Cengiz (co-investigator), Lori Carria, Melinda Zgorski, Kate Weyman, Eileen Tichy, and Amy Steffens; and from Medtronic MiniMed, Benny Grosman, Natalie Kurtz, Scott W. Lee, John Shin, Toni Cordero, Mark Faillace, Xiaoxiao Chen, and Margaret Liu.
Duality of Interest. This study was made possible by a research grant from Medtronic MiniMed. G.P.F. has received research grant funding from Medtronic MiniMed, Insulet Corp., Tandem Diabetes Care, and Dexcom, has been a consultant for Abbott Diabetes Care, and has served on the advisory board for Dexcom. J.L.S. has been a consultant for Medtronic MiniMed and served on the advisory board for Bigfoot Biomedical and Insulet Corp. R.P.W. has received research funding from Bigfoot Biomedical, Dexcom, Novo Nordisk, Lexicon Pharmaceuticals, and Xeris Pharmaceuticals and has been a consultant for Eli Lilly and Co. and Novo Nordisk. B.A.B. has received research grant funding from Medtronic MiniMed, Insulet Corp., Bigfoot Biomedical, Tandem Diabetes Care, and Dexcom and has been a consultant for Novo Nordisk, Sanofi, Becton Dickinson, ConvaTec, and Tandem Diabetes Care. S.A.W. has received research grant funding from Medtronic MiniMed and has been a consultant for Medtronic MiniMed and Insulet Corp. D.M.M. has received research grant funding from Medtronic MiniMed, Dexcom, Roche Diabetes Care, and Bigfoot Biomedical and has been a consultant for Insulet Corp. and Abbott Diabetes Care. R.H.S. has received research grant funding from Medtronic MiniMed. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. L.H.M. and G.P.F. researched data and wrote the manuscript. J.L.S., B.A.B., S.A.W., and D.M.M. reviewed and edited the manuscript. R.P.W. and R.H.S. researched data and reviewed and edited the manuscript. G.P.F. is the guarantor of this work, and as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of these data were presented as an oral abstract at the 10th International Conference on Advanced Technologies & Treatments for Diabetes, Paris, France, 15–18 February 2017.
Footnotes
Clinical trial reg. no. NCT02463097, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1682/-/DC1.
References
- 1.Kowalski AJ. Can we really close the loop and how soon? Accelerating the availability of an artificial pancreas: a roadmap to better diabetes outcomes. Diabetes Technol Ther 2009;11(Suppl. 1):S113–S119 [DOI] [PubMed] [Google Scholar]
- 2.Kowalski A. Pathway to artificial pancreas systems revisited: moving downstream. Diabetes Care 2015;38:1036–1043 [DOI] [PubMed] [Google Scholar]
- 3.Weiss R, Garg SK, Bode BW, et al. Hypoglycemia reduction and changes in hemoglobin A1c in the ASPIRE in-home study. Diabetes Technol Ther 2015;17:542–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forlenza GP, Buckingham B, Maahs DM. Progress in diabetes technology: developments in insulin pumps, continuous glucose monitors, and progress towards the artificial pancreas. J Pediatr 2016;169:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kropff J, DeVries JH. Continuous glucose monitoring, future products, and update on worldwide artificial pancreas projects. Diabetes Technol Ther 2016;18(Suppl. 2):S253–S263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah VN, Shoskes A, Tawfik B, Garg SK. Closed-loop system in the management of diabetes: past, present, and future. Diabetes Technol Ther 2014;16:477–490 [DOI] [PubMed] [Google Scholar]
- 7.Thabit H, Hovorka R. Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia 2016;59:1795–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz JL, Sherr JL, Cengiz E, et al. Effect of insulin feedback on closed-loop glucose control: a crossover study. J Diabetes Sci Technol 2012;6:1123–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steil GM. Algorithms for a closed-loop artificial pancreas: the case for proportional-integral-derivative control. J Diabetes Sci Technol 2013;7:1621–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008;31:934–939 [DOI] [PubMed] [Google Scholar]
- 11.Ly TT, Weinzimer SA, Maahs DM, et al. Automated hybrid closed-loop control with a proportional-integral-derivative based system in adolescents and adults with type 1 diabetes: individualizing settings for optimal performance. Pediatr Diabetes 2016;18:348–355 [DOI] [PubMed] [Google Scholar]
- 12.Forlenza GP, Nathan BM, Moran A, et al. Accuracy of continuous glucose monitoring in patients after total pancreatectomy with islet autotransplantation. Diabetes Technol Ther 2016;18:455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grosman B, Ilany J, Roy A, et al. Hybrid closed-loop insulin delivery in type 1 diabetes during supervised outpatient conditions. J Diabetes Sci Technol 2016;10:708–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Grady MJ, Retterath AJ, Keenan DB, et al. The use of an automated, portable glucose control system for overnight glucose control in adolescents and young adults with type 1 diabetes. Diabetes Care 2012;35:2182–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 16.Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller KM, Foster NC, Beck RW, et al.; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 18.Nathan DM, Zinman B, Cleary PA, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group . Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005). Arch Intern Med 2009;169:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messer LH, Forlenza GP, Wadwa RP, et al. The dawn of automated insulin delivery: a new clinical framework to conceptualize insulin administration. Pediatr Diabetes 2018;19:14–17 [DOI] [PubMed] [Google Scholar]
- 20.Steil GM, Palerm CC, Kurtz N, et al. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab 2011;96:1402–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maahs DM, Buckingham BA, Castle JR, et al. Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care 2016;39:1175–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messer L, Jost E, Berget C, et al. Automated insulin delivery (artificial pancreas) on the horizon: educational considerations for youth with type 1 diabetes. J Pediatr Nurs 2017;34:104–105 [Google Scholar]
- 23.Dassau E, Pinsker JE, Kudva YC, et al. Twelve-week 24/7 ambulatory artificial pancreas with weekly adaptation of insulin delivery settings: effect on hemoglobin A1c and hypoglycemia. Diabetes Care 2017;40:1719–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tauschmann M, Allen JM, Wilinska ME, et al. Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2016;39:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyle FJ 3rd, Huyett LM, Lee JB, Zisser HC, Dassau E. Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care 2014;37:1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamborlane WV, Beck RW, Bode BW, et al.; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 27.Diabetes Daily. Medtronic's New 670G: Answers to Your Frequently Asked Questions [article online], 2016. Available from https://www.diabetesdaily.com/blog/medtronics-new-670g-answers-to-your-frequently-asked-questions-314306/. Accessed 14 June 2017
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.