Abstract
Background
Combination antiretroviral therapy (cART) initiation in hospital settings, where individuals often present with undiagnosed, untreated, advanced HIV disease, is not well understood.
Methods
A cross-sectional study was conducted to determine a period prevalence of cART initiation within two weeks of eligibility, as determined at hospitalization. Using a pretested and precoded data extraction tool, data on cART initiation status and reason for not initiating cART was collected. Phone calls were made to patients that had left the hospital by the end of the two-week period. Delayed cART initiation was defined as failure to initiate cART within two weeks. Sociodemographic characteristics, WHO clinical stage, CD4 count, cART initiation status, and reasons for delayed cART initiation were extracted and analyzed.
Results
Overall, 386 HIV-infected adults were enrolled, of whom 289/386 (74.9%) had delayed cART initiation, 77/386 (19.9%) initiated cART, and 20/386 (5.2%) were lost-to-follow-up, within two weeks of cART eligibility. Of 289 with delayed ART initiation, 94 (32.5%) died within two weeks of cART eligibility. Patients with a CD4 cell count≥ 50 cells/μl and who resided in ≥8 kilometers from the hospital were more likely to have delayed cART initiation [adjusted odds ratio (AOR) 2.34, 95% CI: 1.33-4.10, p value 0.003; and AOR 1.92, 95% CI: 1.09-3.40, p value 0.025; respectively].
Conclusion
Up to 75% of hospitalized HIV-infected, cART-naïve, cART-eligible patients did not initiate cART and had a 33% pre-ART mortality rate within two weeks of eligibility for cART. Hospital based strategies to hasten cART initiation during hospitalization and electronic patient tracking systems could promote active linkage to HIV treatment programs, to prevent HIV/AIDS-associated mortality in resource-limited settings.
1. Background
There is global commitment to fast-track the end of the HIV/AIDS epidemic through the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 campaign to test 90% of all people living with HIV, initiate and sustain combination antiretroviral therapy (cART) for 90% of all those diagnosed HIV-infected, and have sustained undetectable viral load among 90% of cART-treated individuals [1]. Access to life saving cART has been achieved by the worlds' most-affected regions of eastern and southern Africa where over 10.3 million people are receiving cART and AIDS-related deaths decreased by 36% since 2010 [2]. Many sub-Saharan African countries, including Uganda, have updated their HIV national guidelines to reflect the World Health Organization's (WHO) Universal Test and Treat guidelines, 2016, to initiate cART as soon as HIV is diagnosed irrespective of CD4 count [3–7]. Recent clinical trial data (HPTN 052) demonstrated that earlier initiation of cART results in near complete interruption of HIV transmission, with a 96% reduction in HIV transmission in sero-discordant couples [8]. However, up to 33% of adults living with HIV and 53% of children living with HIV had not received cART by the end of 2016 [9]. Effectiveness of “test and treat” approaches however remains limited by poor engagement of HIV-infected adults within the national HIV care program.
Consequently, challenges of delayed cART initiation persist in resource-limited settings. Data from cohorts in sub-Saharan Africa showed that most ART-treated adults initiated cART at a median CD4 count range of 87 to 212 cells/μL [10, 11]. Delayed initiation of cART continues to drive morbidity, mortality, and onward transmission of HIV. Causes of late initiation of cART include late diagnosis due to low uptake of HIV testing [10], and limited capacity of clinics to absorb the numbers of all in need of cART, which have been described in ambulatory, community, and observational cohort settings [12–14]. Little has been documented about cART initiation in hospital settings where majority of patients present with advanced HIV disease and opportunistic infections [15, 16], with a potentially high risk of mortality after discharge [17].
In the past, delayed cART initiation has been defined as untreated advanced HIV disease at WHO stage 4 or CD4 ≤200 cells/μl [11, 18, 19]. We defined delayed cART initiation as failure to initiate cART among HIV-infected hospitalized individuals within two weeks of determination of HIV-infection at hospitalization. Our definition of “two weeks” cut-off was based on evidence from the Strategic Timing of Antiretroviral Therapy (START) and Early Initiation of Antiretroviral Therapy for HIV (TEMPRANO) trials which demonstrated profound impact of immediate cART initiation among asymptomatic HIV-infected patients with CD4+ counts 500 cells/μl and over [20–22]. Similarly, the AIDS Clinical Trials Group (ACTG) trials showed remarkable benefit of immediate cART initiation among individuals with advanced disease and opportunistic infections [17, 23]. Our results inform the development of strategies to reach hosptalised HIV-infected adults who are most-at-risk of morbidity and mortality, amidst the wider scale of “test and treat” strategy in many ambulatory HIV care settings.
2. Methods
2.1. Study Setting
This study was conducted at Mulago hospital, a 1500-bed hospital that serves referred patients from Kampala, the capital city, and outside Kampala. Patients were recruited from three medical wards, where adults with nonsurgical and nonobstetric/gynaecological conditions are admitted. HIV tests and CD4 count measurement for HIV-infected individuals are offered as part of routine medical care. During hospitalization, patients are investigated to obtain confirmatory diagnosis and subsequently treated. HIV infected patients are treated for any opportunistic infection and prepared for cART initiation, except patients with cryptococcal meningitis (CM) in whom cART is deferred for 5 weeks for better outcomes [24]. Upon discharge, cART eligible patients who are not yet ready to initiate cART are routinely given referral notes and appointments to attend the HIV treatment clinic at Mulago or one nearest to their home, depending on the patients' preference. There is no specific follow-up mechanism to determine whether patients honor the referrals or whether they attend HIV clinics different from those indicated on the referral notes.
2.2. Study Design and Participants
A cross-sectional study was conducted to determine a period prevalence of cART initiation status within two weeks of diagnosis, as determined by the attending physician during hospitalization. From December 2012 to March 2013, charts of HIV-infected, cART-eligible, cART-naïve patients 13 years and older were consecutively reviewed for cART-initiation status within two weeks of hospitalization. cART initiation status data, CD4 cell count and WHO HIV clinical stage III/IV (determined upon hospitalization at Mulago hosptals' medical wards), were extracted from patients' charts for those still hospitalized for two weeks and more, and through phone calls for those that left hospital within the two-week period. Phone call interviews included preset and precoded questions to determine whether patient was alive, sick/well, had initiated cART, place where cART was initiated (for those that had initiated), and reasons for not initiating (for those that had not initiated). Charts of patients with suspected or confirmed cryptococcal meningitis (CM) were excluded because the Cryptococcal Optimal ART Timing (COAT) trial had shown increased mortality in patients who initiated cART within two weeks of being treated for CM [24].
2.3. Measurements
Using a pretested precoded data extraction tool, data extracted included sociodemographic characteristics (age, gender, district of residence, distance (in kilometers) to nearest health center, level of education, occupation, religion, and marital status), and medical history including HIV status, date and place of prior HIV test, use of co-trimoxazole prophylaxis or alternative medicine, prior to hospitalization (for those with known HIV status), previous ambulatory clinic consultations, hospitalization admission in the preceding year, and stage of HIV disease (WHO clinical stage/CD4 count). The main outcome was cART initiation status during hospitalization within two weeks of eligibility (as determined at hospitalization). We also extracted data on date of admission, inpatient diagnosis, CD4 cell count and date of most recent CD4 count whenever available, opportunistic infections in past and present, Karnofsky performance score, comorbidities, and reasons for delayed cART initiation (for those that did not initiate cART during hospitalization). For HIV-infected patients that were no longer in hospital by the end of the two-week period, phone calls were made to them (or the provided next of kin) to ascertain the outcome (dead/alive), cART initiation status, date of cART initiation (for those that had initiated), and reasons for not initiating cART (for those that had not initiated cART). Patients whose calls were picked by neither patient nor the next of kin or other relative provided were considered lost to follow-up.
2.4. Ethics Approval and Consent to Participate
Ethical approval was obtained from the School of Medicine Research and Ethics Committee, Makerere University College of Health Sciences. Written informed consent was obtained from all study participants at admission on the medical wards, and assent obtained from the guardian/parent/ next of kin if the patient was below 16 years of age.
2.5. Data Analysis
Data was double-entered into EpiData (version 3.1) software, cleaned, and exported to Stata version 12 for analysis. The primary outcome variable was the proportion of hospitalized HIV-infected, cART eligible- cART-naïve, patients who had delayed initiation of cART, defined as failure to initiate cART within two weeks of cART eligibility during hospitalization. Continuous variables such as age, distance to nearest health center, duration of hospitalization, Karnofsky performance status score, and CD4 cell count were summarized using mean and standard deviation, median, and interquartile range. Bivariate analysis using Student's t-test was used for continuous variables that followed a normal distribution. Wilcoxon rank sum test was used for predictors that were not normally distributed. The Chi-square test was used to compare categorical variables among patients with and without delayed cART initiation. Multivariate analysis was used to determine factors associated with delayed cART initiation. Factors with p value < 0.2 after the bivariate analysis were considered for a multivariate analysis. Statistical significance was considered at a p value <0.05. Diagnosis at admission and reasons for not initiating cART were recorded and grouped into frequencies and proportions.
3. Results
3.1. Sociodemographic and Clinical Characteristics of Hospitalized HIV-Infected Adults
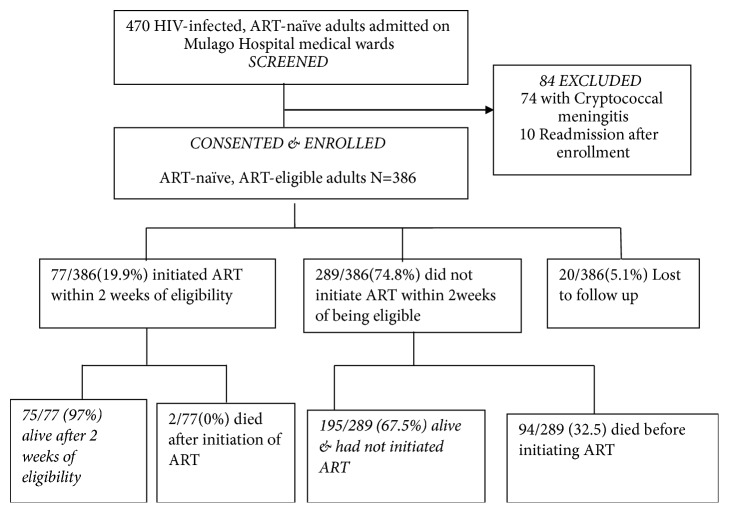
Between December 2012 and March 2013, a total of 470 HIV-infected, ART-naïve patients were identified and screened for eligibility to participate in the study. Of these, 386 (81.6%) patients were enrolled into the study, and 84 patients were excluded (74 had cryptococcal meningitis and 10 were re-admissions that had already been enrolled), as shown in Figure 1. Of 386 individuals recruited, 193/386 (50.0%) were females, 219/386 (56.7%) were residents of the two neighboring districts of the hospital, 211/386 (54.7%) of the patients had attained primary education, and 384/386 (99.5%) had WHO stage III/IV disease. All HIV-infected adults were eligible for cART initiation, according to the national guidelines at the time. The median distance from home to the nearest health centre was 3 km (IQR 1.6-5km), median age was 32 years (IQR 27-40 years), and median CD4 cell count was 68 (IQR 18-195) cells/μl. Patients who initiated cART had significantly lower CD4 counts than those that delayed, p value 0.005 (Table 1).
Figure 1.
Study profile of HIV-infected adults hospitalized on medical ward at Mulago in December 2012-March 2013.
Table 1.
Characteristics of HIV-infected adults hospitalized at Mulago hospital and their antiretroviral therapy (ART) initiation status within two weeks of eligibility.
| Variable | Initiated ART | Delayed ART initiation∞ | P-value |
|---|---|---|---|
| N=75 | N=195 | ||
| n (%) | n (%) | ||
| Gender | 0.95 | ||
| Female | 37 (49) | 97 (49.7) | |
| Male | 38 (51) | 98 (50.2) | |
|
| |||
| Age | 0.51 | ||
| ≤ 40 years | 63 (66) | 157 (80.5) | |
| >40years | 12 (16) | 38 (19.5) | |
|
| |||
| District of residence Kampala | 0.108 | ||
| Kampala | 47 (83) | 101 (51.8) |
|
| Outside | 28 (37) | 94 (48.2) | |
|
| |||
| Level of education | 0.22 | ||
| No formal & Primary | 38 (51) | 115 (58.9) | |
| Secondary & Tertiary | 37 (49) | 80 (41.0) | |
|
| |||
| Distance to nearest health Centre | 0.44 | ||
| ≤3km | 42 (56) | 99 (50.7) | |
| >3km | 33 (44) | 96 (49.2) | |
|
| |||
| Newly diagnosed HIV positive | 60 (80) | 161 (82.5) | 0.62 |
| Known HIV positive | 15 (20) | 34 (17.4) | |
|
| |||
| Disclosure | 60 (80) | 149 (76.4) | 0.53 |
| No disclosure | 15 (20) | 46 (23.5) | |
|
| |||
| Social Support | 72 (96) | 182 (93.3) | 0.41 |
| No social support | 3 (4) | 13 (6.6) | |
|
| |||
| Karnofsky score | 0.15 | ||
| ≤40 | 66 (85) | 157 (80.5) | |
| >40 | 9 (12) | 38 (19.5) | |
|
| |||
| ¥OP clinics attended in year | 0.15 | ||
| <5 | 30 (40) | 97 (49.7) | |
| ≥5 | 45 (60) | 98 (50.3) | |
|
| |||
| WHO HIV clinical stage | 0.07 | ||
| stage II&III∗ | 30 (40) | 97 (49.7) | |
| stage IV | 45 (60) | 98 (50.1) | |
|
| |||
| CD4 cell count | 0.005 | ||
| ≤50 | 37 (49) | 56 (28.7) | |
| >50 | 38 (51) | 126 (64.6) | |
∞ Delayed ART initiation referred to failure to initiate ART within two weeks of eligibility, as determined during the current hospitalization.
¥OP clinics, outpatient clinics.
Note: 94 died before ART initiation, 2 died after ART initiation, and 20 were lost to follow-up.
∗Only 2/386 patients were stage II.
3.2. Delayed cART Initiation among Hospitalized Patients
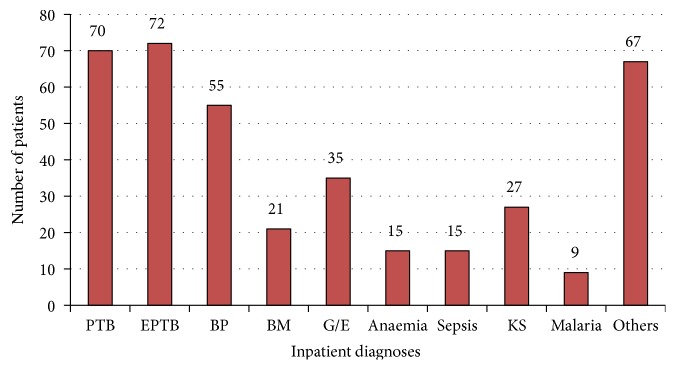
Overall, 289/386 (74.8%) had delayed cART initiation, since they did not initiate cART within two weeks of eligibility. Only 77/386 (19.9%) initiated cART within two weeks of eligibility, [22/386 (5.7%) initiated during hospitalization, 55/386 (14.2%) initiated after leaving hospital], and 20/386 (5.2%) were lost to follow-up. Of patients with delayed cART initiation, 94/289 (32.5%) died (76 died in hospital and 18 died after discharge) within two weeks of being found cART eligible. The median hospital stay was 8 (IQR 4-13) days, and the commonest causes of hospitalization were tuberculosis (pulmonary and extrapulmonary), followed by bacterial pneumonia (Figure 2). The leading causes of death during hospitalization were tuberculosis [31/94 (33%)], Kaposi's sarcoma 10/94 (11%), and bacterial meningitis 9/94 (10%). The main reason not to initiate cART for the patients that died was refusal of patient/relative to initiate cART, despite doctors' recommendation, because patients had been too sick (as expressed by the relatives).
Figure 2.
Histogram showing the most common diagnoses among HIV-infected adults hospitalized at Mulago hospital. PTB, pulmonary tuberculosis; EPTB, extra pulmonary tuberculosis; BP, bronchopneumonia; BM, bacterial meningitis; G/E, gastroenteritis; KS, Kaposis sarcoma, and others included PCP, HIV associated nephropathy, toxoplasmosis, acute hepatitis, and recurrent pleural effusions. 74 patients with Cryptococcal meningitis were excluded from the study.
3.3. Poor Linkage to cART before Hospitalization
Prior to the current hospitalization, majority [295/386 (76.4%)] of HIV-infected patients were aware of their HIV-infected status, yet only 132/295 (44.7%) had been enrolled into an HIV care program, yet 291/295 (98.6%) had received cotrimoxazole prophylaxis. This was the first admission within the year for 258/386 (66.8%) patients, although more than half had visited outpatient clinics five times or more during the year prior to this study.
3.4. Factors Associated with Delayed ART Initiation
Patients with CD4 counts >50 cells/μl and patients living outside Kampala were more likely to have delayed cART initiation [OR 2.34 (95%CI 1.33-4.10); p value 0.003] and [1.92 (95% CI 1.09-3.40); p value 0.025], respectively (Table 2). The leading reasons for delay of cART initiation, as extracted from patient records and responses to phone interviews, were long period (> two weeks) of preparation of cART 54/195 (27.6%) and failure to honor referral appointments 51/195 (26.1%). Other reasons mentioned were related to patients being too weak to initiate cART (Table 3).
Table 2.
Predictors of delayed initiation of cART among HIV-infected adults hospitalized at Mulago hospital.
| Effect | Odds ratio | P- value | |
|---|---|---|---|
| OR (95% CI) | |||
| CD4 cell count/µl | ≤50 | 1 | |
| >50 | 2.34 (1.33 – 4.10) | 0.003 | |
|
| |||
| District of residence | Kampala | 1 | |
| Outside Kampala | 1.92 (1.09 – 3.40) | 0.025 | |
|
| |||
| Karnofsky Performance status score | ≤40 | 1 | |
| >40 | 1.92 (1.07 – 3.45) | 0.67 | |
|
| |||
| Outpatient clinic attendances /year | <5 | 1 | |
| ≥5 | 0.63 (0.35−1.12) | 0.11 | |
Table 3.
Reasons for delayed initiation of cART among HIV-infected cART-naïve adults hospitalized at Mulago hospital.
| Reason for not initiating cART in 2 weeks of eligibility | Frequency N=195 |
|---|---|
| n (%) | |
| Referral systems | |
| Period of preparation for cART initiation >2weeks | 54 (27.7) |
| Did not honor referral date given | 51 (26.1) |
| Review date given more than 2 weeks after discharge | 16 (8.2) |
| Told CD4 above 200mg/µl | 9 (4.7) |
| Attending OPD clinic not integrated with cART | 3 (1.5) |
| Given wrong clinic day | 1 (0.5) |
| Advanced disease considered too weak to initiate cART | |
| Patient readmitted | 18 (9.2) |
| Very sick & being treated for opportunistic infection | 18 (9.2) |
| Very sick and weak to start cART | 12 (6.1) |
| Patient taken to village gave up on life | 5 (2.5) |
| Patient social support | |
| No social support | 7 (3.6) |
| Feared drug reaction | 1 (0.5) |
4. Discussion
We found a high prevalence of delayed cART initiation among hospitalized patients. Up to 75% of HIV-infected adults did not initiate cART within two weeks of eligibility. Only 20% initiated cART within two weeks, majority (77%) of whom initiated cART after leaving hospital. Of patients with delayed cART initiation, 94/289 (33%) died within two weeks. Up to 80% of patients who died before cART initiation were still in hospital and the main reason for not initiating cART despite physicians' recommendations was postponement (by the patients and relatives) because patients were too sick. Similarly, high mortality was reported among patients that initiated cART during hospitalization in Tanzania, where the main causes of death within the first month of cART were anemia, thrombocytopenia, and severe malnutrition [25]. It remains unclear whether mortality would have been different if cART had been initiated during hospitalization, given that most of the patients that died had Kaposi's sarcoma, tuberculosis, and bacterial meningitis. Important to note too is the role of patients' and relatives' perceptions of the benefits of initiating cART in very sick patients, which was not assessed during this study.
Our results are comparable to previous reports from an HIV testing trial on the same wards where only 62% of surviving HIV-infected participants were linked to HIV care, only 15% received cART, and 35% died, within six months of discharge from hospital [17]. A study in Tanzania showed that less than 1% of HIV-infected patients initiated cART during hospitalization [26]. Despite advances in national guidelines to include the WHO “test and treat” guidelines for initiating cART among HIV-infected adults irrespective of CD4 counts [6], which is largely adhered to in ambulatory clinics and community programs [4], hospital settings continue to present the challenge of advanced untreated HIV disease that is associated with a high postdischarge mortality rate. In addition to scale up the “test and treat” strategy to meet the 90-90-90 targets for 2020, there is a need to fast-track cART initiation among hospitalized patients who continue to present with advanced untreated HIV disease.
Patients with CD4 counts above 50 cells/μl and patients living outside Kampala were more likely to have delayed cART initiation. Similarly, lengthy preparation for cART [54/195 (28%)] and failure to honor referral appointments [51/195 (28%)] were mentioned by individuals that did not initiate cART. Our data from hospitalized patients was comparable to reports from a review of observational cART cohorts in sub-Saharan Africa where patients presented to the health care system with advanced untreated HIV diseases with CD4 cell count <50 cells/μL and WHO stage IV [27]. A recent review of from 27 studies from 18 African countries reported a range of 87-212 cells/μL at cART initiation [10, 11]. Limited access to HIV testing, cART centers, and CD4 counts were previously highlighted as a barrier to timely initiation of cART, particularly in ambulatory HIV/AIDS care settings [28, 29], and indeed increased availability of these services has increased cART initiation rates [17, 30, 31]. Hospital settings, however, present a unique challenge of patients with advanced untreated HIV disease despite large strides that have been taken to test and treat HIV in several ambulatory and community settings [4]. In our study, tuberculosis, Kaposi's sarcoma, and bacterial meningitis were the three leading causes of mortality among HIV-infected adults that did not initiate cART during hospitalization. Delayed diagnosis and treatment of opportunistic infections (OI) continue to delay cART initiation in hospital settings in sub-Saharan Africa [10, 24, 32], although national treatment guidelines encourage concurrent treatment of OI and cART with the exception of cryptococcal meningitis.
We found that 76% of hospitalized HIV-infected patients were aware of their HIV-infection status prior to current hospitalization and had received cotrimoxazole prophylaxis but not cART, and only 45% of them had been enrolled into an HIV care program. Of the patients that did not initiate cART within two weeks of eligibility, 28% were still under preparation for cART, 26% had not honored their referral notes to ART clinics, and another 24% were still considered too weak to initiate cART. Health system challenges such as poor access to diagnostic services, prolonged patient preparation for cART, and limited tracking of referrals continue to affect the quality of health care [17, 28]. In a stepped-wedge cluster randomized trial at lower-level health care facilities in Southwestern Uganda, 38% initiated cART within two weeks after eligibility, although cART initiation rate increased with implementation of an intervention comprising training and coaching of front-line health workers, a point-of-care CD4 cell count testing platform, a revised counselling approach without mandatory multiple preinitiation sessions, and feedback to the facilities on their cART initiation rates [33]. A similar intervention might be useful to support front-line health care providers to handle hospitalized cART eligible individuals, particularly those considered too weak to initiate cART due to ongoing treatment for opportunistic infections. Implementation studies are clearly needed to support the scale-up of already proven interventions of immediate cART for patients, such as immediate initiation of ART among patients receiving anti-tuberculosis treatment [23], and other comorbidities such as bacterial meningitis and Kaposi's sarcoma [34]. There is a need for comprehensive strategies to mitigate the known health system delays to cART initiation, and specific interventions to reach individuals that slip through the ambulatory ART initiation efforts, only to present to hospital settings with untreated advanced HIV disease with a high risk of mortality.
cART initiation was simply by patient self-report, but could not be confirmed via any tracking system. Similarly it was unclear whether patients recorded as “lost to follow-up” had been enrolled into other HIV care facilities in or outside urban Kampala. We recommend development of a national HIV care database with unique identifiers to track patients between different HIV treatment sites. Our definition of delayed cART initiation was limited to two weeks after eligibility, as determined during hospitalization, mainly because of the evidence that early cART initiation within two weeks of eligibility reduced the rate of severe illnesses in clinical trial settings [21, 34].
5. Conclusion
Up to 75% of hospitalized HIV-infected, ART-naïve, ART-eligible patients did not initiate cART and had a 33% pre-ART mortality rate within two weeks of eligibility for cART. Hospital based strategies to hasten cART initiation during hospitalization and electronic patient tracking tools could improve linkage to HIV treatment programs and prevent HIV/AIDS-associated mortality in resource-limited settings.
Acknowledgments
The authors acknowledge the staff and patients on Mulago hospital's medical wards where the study was conducted. The authors also acknowledge MUII-plus for Damalie Nakanjako's group leader award from Wellcome Trust grant number 107743/z/15/z. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)'s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust and the UK government. This work was funded by GILEAD Sciences at the Infectious Diseases Institute, Makerere University College of Health Sciences, and the Uganda HIV/TB COHRE Training Program based at Joint Clinical Research Center (JCRC).
Abbreviations
- ART:
Antiretroviral therapy
- cART:
Combined antiretroviral therapy
- WHO:
World Health Organization
- START:
Strategic Timing of Antiretroviral Therapy
- TEMPRANO:
Early Initiation of Antiretroviral Therapy for HIV
- ACTG:
AIDS Clinical Trials Group
- CM:
Cryptococcal meningitis
- COAT:
Cryptococcal Optimal ART Timing.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
Ethical approval was obtained from the School of Medicine Research and Ethics Committee, Makerere University College of Health Sciences.
Consent
Written informed consent was obtained from all study participants, and assent obtained from the guardian/parent/next of kin if the patient was below 16 years of age.
Disclosure
The views expressed in this publication are those of the authors and not necessarily those of AAS, NEPAD Agency, Wellcome Trust, or the UK government.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Prossie Merab Ingabire, Fred Semitala, Moses R. Kamya, and Damalie Nakanjako made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; Prossie Merab Ingabire and Damalie Nakanjako drafted the manuscript, Fred Semitala and Moses R. Kamya reviewed the manuscript critically for important intellectual content. Prossie Merab Ingabire, Fred Semitala, Moses R. Kamya, and Damalie Nakanjako reviewed and approved the manuscript for publication.
References
- 1.UNAIDS. HIV Treatment Bulletin. 2014. UNAIDS sets 90-90-90 target for 2020 to end AIDS by 2030. http://i-base.info/htb/27174. [Google Scholar]
- 2.UNAIDS. Global AIDS update- 2016. 2016, http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016.
- 3.Gardner E. M., McLees M. P., Steiner J. F., Del Rio C., Burman W. J. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical Infectious Diseases. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thirumurthy H., Jakubowski A., Camlin C., et al. Expectations about future health and longevity in Kenyan and Ugandan communities receiving a universal test-and-treat intervention in the SEARCH trial. AIDS Care Psychological and Socio-medical Aspects of AIDS/HIV. 2016;28(Supplement 3):90–98. doi: 10.1080/09540121.2016.1178959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams I., Churchill D., Anderson J., et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012 (Updated November 2013. All changed text is cast in yellow highlight.) HIV Medicine. 2014;15(supplement 1):1–85. doi: 10.1111/hiv.12119. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Guidelines on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. Geneva, Switzerland: 2015. http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ [PubMed] [Google Scholar]
- 7.WHO. Treat All People Living with HIV, Offer Antiretrovirals as Additional Prevention Choice for People at "Substantial" Risk. 2015. http://www.who.int/mediacentre/news/releases/2015/hiv-treat-all-recommendation/en/ [Google Scholar]
- 8.Cohen M. S., Chen Y. Q., McCauley M., et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England Journal of Medicine. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UNAIDS. UNAIDS Data 2017. 2017, http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf.
- 10.Lahuerta M., Ue F., Hoffman S., et al. The problem of late ART initiation in sub-Saharan Africa: A Transient aspect of scale-up or a long-term phenomenon? Journal of Health Care for the Poor and Underserved. 2013;24(1):359–383. doi: 10.1353/hpu.2013.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawn S. D., Campbell L., Kaplan R., Little F., Morrow C., Wood R. Delays in starting antiretroviral therapy in patients with HIV-associated tuberculosis accessing non-integrated clinical services in a South African township. BMC Infectious Diseases. 2011;11, article 258 doi: 10.1186/1471-2334-11-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Govindasamy D., Ford N., Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26(16):2059–2067. doi: 10.1097/qad.0b013e3283578b9b. [DOI] [PubMed] [Google Scholar]
- 13.Plazy M., Newell M.-L., Orne-Gliemann J., Naidu K., Dabis F., Dray-Spira R. Barriers to antiretroviral treatment initiation in rural KwaZulu-Natal, South Africa. HIV Medicine. 2015;16(9):521–532. doi: 10.1111/hiv.12253. [DOI] [PubMed] [Google Scholar]
- 14.Rosen S., Fox M. P., Larson B. A., et al. Accelerating the uptake and timing of antiretroviral therapy initiation in sub-saharan africa: an operations research agenda. PLoS Medicine. 2016;13(8) doi: 10.1371/journal.pmed.1002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakanjako D., Kamya M., Daniel K., et al. Acceptance of routine testing for HIV among adult patients at the medical emergency unit at a national referral hospital in Kampala, Uganda. AIDS and Behavior. 2007;11(5):753–758. doi: 10.1007/s10461-006-9180-9. [DOI] [PubMed] [Google Scholar]
- 16.Nakanjako D., Kyabayinze D. J., Mayanja-Kizza H., Katabira E., Kamya M. R. Eligibility for HIV/AIDS treatment among adults in a medical emergency setting at an urban hospital in Uganda. African Health Sciences. 2007;7(3):124–128. doi: 10.5555/afhs.2007.7.3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanyenze R. K., Hahn J. A., Liechty C. A., et al. Linkage to HIV care and survival following inpatient HIV counseling and testing. AIDS and Behavior. 2011;15(4):751–760. doi: 10.1007/s10461-010-9704-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotily M., Bentz L., Pradier C., Obadia Y., Cavailler P. Factors related to delayed diagnosis of HIV infection in southeastern France. International Journal of STD & AIDS. 2000;11(8):531–535. doi: 10.1258/0956462001916272. [DOI] [PubMed] [Google Scholar]
- 19.Wolbers M., Bucher H. C., Furrer H., et al. Delayed diagnosis of HIV infection and late initiation of antiretroviral therapy in the Swiss HIV Cohort Study. HIV Medicine. 2008;9(6):397–405. doi: 10.1111/j.1468-1293.2008.00566.x. [DOI] [PubMed] [Google Scholar]
- 20.The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic hiv infection. The New England Journal of Medicine. 2015;373(9):795–807. doi: 10.1056/nejmoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.TEMPRANO ANRS Study Group, Danel C., Moh R. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. The New England Journal of Medicine. 2015;373(9):808–822. doi: 10.1056/nejmoa1507198. [DOI] [PubMed] [Google Scholar]
- 22.Cohen M. S., Chen Y. Q., McCauley M., et al. Antiretroviral therapy for the prevention of HIV-1 transmission. The New England Journal of Medicine. 2016;375(9):830–839. doi: 10.1056/nejmoa1600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havlir D. V., Kendall M. A., Ive P., et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. The New England Journal of Medicine. 2011;365(16):1482–1491. doi: 10.1056/nejmoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boulware D. R., Meya D. B., Muzoora C., et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. The New England Journal of Medicine. 2014;370(26):2487–2498. doi: 10.1056/NEJMoa1312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johannessen A., Naman E., Ngowi B. J., et al. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infectious Diseases. 2008;8(1, article 52) doi: 10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wajanga B. M., Webster L. E., Peck R. N., et al. Inpatient mortality of HIV-infected adults in sub-Saharan Africa and possible interventions: a mixed methods review. BMC Health Services Research. 2014;14(1) doi: 10.1186/s12913-014-0627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawn S. D., Harries A. D., Anglaret X., Myer L., Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–1908. doi: 10.1097/qad.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakanjako D., Colebunders R., Coutinho A. G., Kamya M. R. Strategies to optimize HIV treatment outcomes in resource-limited settings. AIDS Reviews. 2009;11(4):179–189. [PubMed] [Google Scholar]
- 29.Wanyenze R. K., Kamya M. R., Fatch R., et al. Missed opportunities for HIV testing and late-stage diagnosis among HIV-infected patients in Uganda. PLoS ONE. 2011;6(7) doi: 10.1371/journal.pone.0021794.e21794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wanyenze R. K., Kamya M. R., Fatch R., et al. Abbreviated HIV counselling and testing and enhanced referral to care in Uganda: A factorial randomised controlled trial. The Lancet Global Health. 2013;1(3):E137–E145. doi: 10.1016/S2214-109X(13)70067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wanyenze R. K., Kyaddondo D., Kinsman J., Makumbi F., Colebunders R., Hardon A. Client-provider interactions in provider-initiated and voluntary HIV counseling and testing services in Uganda. BMC Health Services Research. 2013;13(1):p. 423. doi: 10.1186/1472-6963-13-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wajanga B. M., Webster L. E., Peck R. N., et al. Inpatient mortality of HIV-infected adults in sub-Saharan Africa and possible interventions: a mixed methods review. BMC Health Services Research. 2014;14(1):p. 627. doi: 10.1186/s12913-014-0627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amanyire F. C. S. G., Namusobya J., Katuramu R., et al. Streamlining antiretroviral therapy uptake: a stepped-wedge cluster randomized trial. Proceedings of the Conference On Retroviruses and Opportunistic Infections; 2016. [Google Scholar]
- 34.Zolopa A. R., Andersen J., Komarow L., et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: A multicenter randomized strategy trial. PLoS ONE. 2009;4(5) doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.