Abstract
Ovaries represent one of the primary steroidogenic organs, producing estrogen and progesterone under the regulation of gonadotropins during the estrous cycle. Gonadotropins fluctuate the expression of various steroidogenesis-related genes, such as those encoding steroidogenic enzymes, cholesterol deliverer, and electronic transporter. Steroidogenic factor-1 (SF-1)/adrenal 4-binding protein (Ad4BP)/NR5A1 and liver receptor homolog-1 (LRH-1) play important roles in these phenomena via transcriptional regulation. With the aid of cAMP, SF-1/Ad4BP and LRH-1 can induce the differentiation of stem cells into steroidogenic cells. This model is a useful tool for studying the molecular mechanisms of steroidogenesis. In this article, we will provide insight into the transcriptional regulation of steroidogenesis-related genes in ovaries that are revealed from stem cell-derived steroidogenic cells. Using the cells derived from the model, novel SF-1/Ad4BP- and LRH-1-regulated genes were identified by combined DNA microarray and promoter tiling array analyses. The interaction of SF-1/Ad4BP and LRH-1 with transcriptional regulators in the regulation of ovarian steroidogenesis was also revealed.
1. Introduction
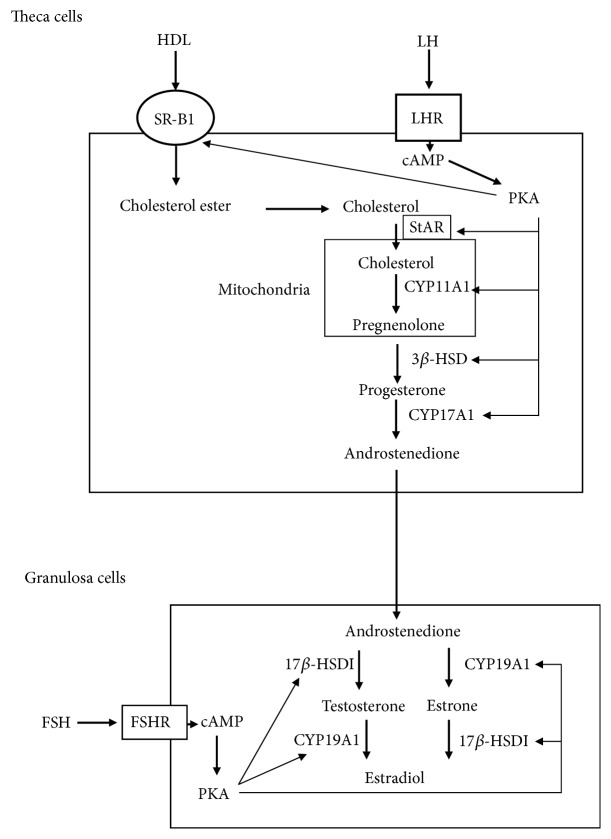
In mammal, gonads and adrenal glands are primary organs that produce steroid hormones from cholesterol. Steroid hormones are involved in various physiological phenomena for the maintenance of homeostasis. Adrenal glucocorticoid and mineralocorticoid are essential for glucose metabolism, stress response, immunity, and fluid/electrolyte balance. Gonadal androgen and estrogen are important for sex differentiation and reproduction. These steroid hormones are produced from cholesterol through a series of reactions catalyzed by steroid cytochrome P450 (CYP) hydroxylases and hydroxysteroid dehydrogenases [1, 2]. The source of cholesterol for steroidogenesis primarily depends on cholesterol ester uptake from plasma proteins by lipoprotein receptors, such as scavenger receptor class B member 1 (SR-BI) [3, 4], although de novo synthesis and intracellular store also contribute to this process. Cholesterol transport from the outer to the inner mitochondria membrane by steroidogenic acute regulatory protein (StAR) represents a rate-limiting step of steroidogenesis [5]. Then, steroidogenesis begins with conversion of cholesterol into pregnenolone in mitochondria by the P450 side chain cleavage enzyme (P450scc/CYP11A1/Cyp11a1), an essential enzyme in the synthesis of all steroid hormones. Thereafter, various hormones are synthesized by tissue-specific CYP enzymes and hydroxysteroid dehydrogenases [1, 6, 7]. Earlier studies have demonstrated that ovaries secrete multiple steroid hormones such as pregnenolone, progesterone, 17α-progesterone, dehydroepiandrosterone, androstenedione, testosterone, estrone, and estradiol that depend on the estrous cycle [8]. Two types of somatic cells, follicular granulosa cells and surrounding theca cells, are responsible for ovarian steroidogenesis. Theca cells autonomously synthesize progesterone and androgen, whereas immature granulosa cells only convert theca cell-produced androgens into estrogens. These processes are finely regulated by two gonadotropins, follicle stimulating hormone (FSH) and luteinizing hormone (LH). During follicle development, LH stimulates the production of androgens in theca cells, which are converted to estrogens by FSH-inducible aromatase (CYP19A1/Cyp19a1) in granulosa cells (Figure 1). Such ovarian estrogen synthetic pathway represents the two-cell–two-gonadotropin theory [9, 10]. However, LH induces the differentiation of granulosa cells into autonomous progesterone-producing luteal cells during the ovulatory stage.
Figure 1.
Schematic diagram of two-cell–two-gonadotropin theory in ovarian steroidogenesis. Theca cells autonomously produce androstenedione from cholesterol via the positive regulation of steroidogenic enzymes by LH/cAMP/PKA pathway. Granulosa cells convert androstenedione into estradiol. It is promoted by FSH/cAMP/PKA pathway.
Transcriptional regulation of steroidogenesis-related genes, including those that encode steroidogenic enzymes, is an important step for regulating the aforementioned gonadotropin-dependent ovarian steroidogenesis. In general, gonadal steroidogenesis is activated by gonadotropin/cAMP/ cAMP-dependent protein kinase (PKA) pathway via induction of steroidogenic genes (Figure 1). These phenomena have been investigated using various in vitro culture systems, including follicle culture [11], primary cultures of theca and granulosa cells [12, 13], and established cell lines [14, 15]. Among them, granulosa cells collected from estrogen-primed immature rodents represent one of the most valuable models, as they can easily recapitulate the differentiation of nonsteroidogenic granulosa cells into steroidogenic luteal-like cells by FSH stimulation (even though LH is the physiological inducer of luteinization in vivo) [16, 17]. Human theca cells in primary and long-term culture are also valuable models for examining the theca cell differentiation, steroidogenesis, and pathology [12]. In addition to such current models, we have recently developed model systems to induce steroidogenic cells from nonsteroidogenic stem cells [18–22]. This model provides another useful tool to study the molecular mechanisms of steroidogenesis, since it can circumvent the limitation of cell number, lifespan, and stability of differentiation conditions [22, 23]. In this article, we will review the novel insights into transcriptional regulation of ovarian steroidogenesis-related genes that are obtained from this induction system, following the description of the roles of steroidogenic factor-1 (SF-1)/adrenal 4-binding protein (Ad4BP) in developing steroidogenic organs and differentiation of stem cells.
2. NR5A1/SF-1/Ad4BP: A Master Regulator of Organogenesis in Ovaries and Other Steroidogenic Organs
Although ovaries show different steroid hormone production profiles compared with testis and adrenal during adult life, they have a common developmental origin, a so-called adrenogonadal primordium (AGP) that mainly originates from the intermediate mesoderm and is localized on the coelomic epithelia of the developing urogenital ridge [24–26]. As embryonic development proceeds, AGP separates into two distinct populations, adrenocortical and gonadal primordia, characterized by the existence of chromaffin cell precursors and primordial germ cells (PGCs), respectively, which originate and migrate from other germ layers. SF-1/Ad4BP is one of the earliest markers of the appearance of AGP [24, 27]. Its expression is detectable within primitive urogenital ridges from the stage, when the AGP is not discernible based on morphological criteria (accession of PGCs) [27–29]. After separation into primitive gonads and adrenal, expression levels of SF-1/Ad4BP increased along with steroidogenesis initiation, although there are some species-specific differences in gonads. In rodent and pig, fetal testicular SF-1/Ad4BP is upregulated with the differentiation from bipotential gonads, whereas it is transiently downregulated in fetal ovaries until birth [28, 30, 31]: such sexual dimorphic gene expression reflects the difference in steroidogenic activity between fetal testes and ovaries. However, such sexually dimorphic expressions are never observed in humans [29] and other animals [32, 33]. SF-1/Ad4BP is important for steroidogenesis by regulating the transcription of various steroidogenesis-related genes. It belongs to the nuclear receptor (NR) superfamily and is very similar to liver receptor homolog-1 (LRH-1), as will be described in later sections. These factors constitute one of the NR subfamilies, officially termed as NR5A family proteins (SF-1/Ad4BP is NR5A1 and LRH-1 is NR5A2) [34]. Consistent with its role, ovarian SF-1/Ad4BP expression is detected in theca and granulosa cells, as well as in the corpus lutea to a lesser extent. In addition to steroidogenic enzymes, diverse groups of SF-1/Ad4BP target genes have been identified in ovaries and other steroidogenic organs [35–37].
Total Nr5a1/SF-1/Ad4BP-knockout (KO) mice die shortly after birth due to glucocorticoid deficiency and exhibit male-to-female sex reversal in external genitalia [35, 38, 39]. These phenotypes are caused by the complete loss of gonads and adrenals. Although the initial stages of gonadal and adrenal development occur in the absence of SF-1/Ad4BP, these organs regress and disappear during the following developmental stage, possibly due to the abnormality of glycolysis and pentose phosphate pathway [40]. This KO mice model demonstrated that SF-1/Ad4BP is crucial in determining steroidogenic organ fate in vivo and represents a master regulator for the development of these organs. A granulosa cell-specific KO (GCKO) model has shown that SF-1/Ad4BP plays important roles in steroidogenesis following the ovarian development [41]. In GCKO mice, the ovaries are hypoplastic with reduced oocytes and lack the corpora lutea [42]. Gonadotropin-induced steroid hormone production is also markedly reduced in this model. Thus, SF-1/Ad4BP is necessary not only for ovary development, but also for its physiological role throughout life.
3. Differentiation of MSCs into Steroidogenic Cells by SF-1/Ad4BP
In an early study, Milbrandt and colleagues attempted to induce steroidogenic cells from embryonic stem (ES) cells [43]. Ectopic expression of SF-1/Ad4BP was shown to direct differentiation of ES cells toward the steroidogenic lineage, and then Cyp11a1 mRNA was expressed after the addition of cAMP and retinoic acid (RA). However, these cells do not undergo de novo synthesis because supplementation of 20α-hydroxycholesterol, a membrane-permeable substrate, is necessary to produce progesterone, which is the only steroid produced by these cells. Major differences between these differentiated cells and natural steroidogenic cells are noted in cholesterol delivery and the steroidogenic pathway, including deficiencies of steroidogenic acute regulatory protein (StAR, a cholesterol delivery protein from the outer to the inner mitochondrial membrane in steroidogenic cells) and steroidogenic enzymes except for Cyp11a1 and 3β-HSD [18, 43, 44]. In addition, it is also very difficult to isolate clones expressing SF-1/Ad4BP from pluripotent cells such as ES cells and induced pluripotent stem cells [18, 21, 22], because SF-1/Ad4BP overexpression affects the survival and self-renewal of these pluripotent stem cells. These studies clearly indicate that SF-1/Ad4BP initiates the fate-determination program of the steroidogenic lineage in stem cells, even though it is not completed in pluripotent stem cells.
Based on this work, recent studies have focused on mesenchymal stem cells (MSCs) derived from bone marrow [18, 45]. MSCs are multipotent adult stem cells that differentiate into cells of mesodermal origin, such as adipocytes, chondrocytes, osteoblasts, and hematopoietic-supporting stroma both in vivo and ex vivo [46, 47]. Furthermore, MSCs are capable of generating cells of all three germ layers at least in vitro. Although MSCs were originally isolated from bone marrow (BM-MSCs) [48], they have also been derived from fat, placenta, umbilical cord blood and other tissues. Because MSCs are, like steroidogenic cells, of mesodermal origin, it was expected they are prone to execute their differentiation program.
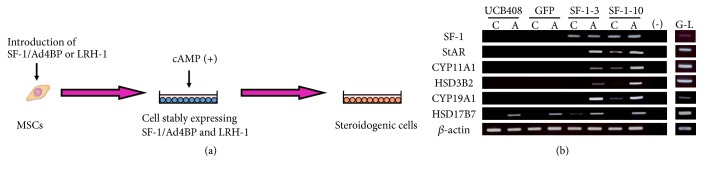
Indeed, MSCs have been completely differentiated into steroidogenic cells following stable expression of SF-1/Ad4BP and cAMP-treatment (Figure 2(a)) [18–22, 49]. While SF-1/Ad4BP induces morphological changes in murine MSCs, such as the accumulation of numerous lipid droplets, these cells hardly express steroidogenic enzymes or produce steroid hormones at detectable levels. However, SF-1/Ad4BP-expressing cells become markedly more positive for CYP11A1/Cyp11a1 after cAMP-treatment. These cells express many other steroidogenesis-related genes (SR-BI, StAR, 3β-HSD, and other P450 steroid hydroxylases) and autonomously produce steroid hormones including androgen, estrogen, progestin, glucocorticoid, and aldosterone. This approach differentiates human (h)BM-MSCs into high cortisol-producing cells in response to ACTH, which are very similar to fasciculata cells in the adrenal cortex. Adenovirus-mediated transient expression of SF-1/Ad4BP also differentiates BM-MSCs into steroidogenic cells with the capacity of de novo synthesis of various steroid hormones [45, 50–53]. In addition to BM-MSCs, various MSC types have been differentiated into steroidogenic cells via this method. For example, human umbilical cord blood- (hUCB-) derived MSCs are differentiated into progesterone-producing luteal-like cells (Figure 2(b)). However, as mentioned above, these methods are not applicable to pluripotent stem cells and embryonal carcinoma cells [18, 21, 45]. These results indicate that MSCs are suitable stem cells for the induction of steroidogenic cells. This hypothesis is supported by the fact that after predifferentiation into MSCs, ES cells can be subsequently differentiated into steroidogenic cells using SF-1/Ad4BP [21, 54]. It is also conclusive that SF-1/Ad4BP represents the master regulator of steroidogenesis. In fact, recent reports showed that SF-1/Ad4BP can reprogram some terminally differentiated cells, such as fibroblasts and endothelial cells [55].
Figure 2.
Differentiation of MSCs into steroidogenic cells. (a) Schematic diagram of induction of steroidogenic cells from MSCs by introduction of SF-1/Ad4BP or LRH, and cAMP-treatment. (b) Differentiation of UCB-MSCs into luteal-like cells by SF-1/Ad4BP. RT-PCR analysis of each gene in each cell with or without 8-bromo-cAMP for 2 d. Lanes G–L are granulosa-luteal cells from women undergoing oocyte retrieval for in vitro fertilization.
4. Differentiation of MSCs into Steroidogenic Cells by Liver Receptor Homolog-1 (LRH-1) and Its Involvement in Luteal Steroidogenesis
The structural characteristics of SF-1/Ad4BP are very similar to liver receptor homolog-1 (LRH-1), which represents another NR5A family member and is designated as NR5A2. LRH-1 was originally identified in the liver and serves in various metabolic pathways, as well as cholesterol and bile acid homeostasis by regulating transcription of numerous genes [56–58]. In addition to the liver, LRH-1 is highly expressed in tissues of endodermal origin, such as the pancreas and intestine. It is also expressed in gonads, most abundantly in ovaries, and localized on granulosa cells and luteal cells [19, 20, 59–61]. LRH-1 shares various common characteristics with SF-1/Ad4BP, such as binding sequences, target genes, and cofactors, which can be applied to transcriptional regulation of steroidogenic genes. LRH-1 also activates the transcription of steroidogenesis-related genes [21, 62, 63]. Nr5a2/LRH-1 KO mice exhibit abnormalities in steroidogenesis. Although total Nr5a2/LRH-1 KO embryos die around E6.5–7.5 days [64, 65], heterozygous and GCKO models revealed the importance of LRH-1 in steroidogenesis [55, 66–70]. In heterozygous Nr5a2/LRH-1-deficient male mice, testicular testosterone production is decreased along with the expression of steroidogenic enzymes and the development of sexual characteristics [71]. GCKO mice are infertile because of anovulation with impaired progesterone production [66]. These results strongly suggest that LRH-1 can also induce differentiation of MSCs into steroidogenic cells.
As for SF-1/Ad4BP, introduction of LRH-1 into BM-MSCs with the aid of cAMP induced expression of steroidogenic enzymes and differentiation into steroid hormone-producing cells [19, 21]. Expression of SF-1/Ad4BP was never induced in LRH-1-transduced cells, and vice versa. Therefore, LRH-1 could act as another master regulator for determining the fate of MSCs into the steroidogenic lineage. This phenomenon is likely to represent a situation where active progesterone production occurs in the ovarian corpus luteum, where LRH-1 is highly expressed, while SF-1/Ad4BP is expressed at very low levels [72]. Consistent with this hypothesis, Murphy and colleagues demonstrated using conditional KO mice models that Nr5a2/LRH-1 deletion in the corpus luteum reduced steroidogenesis, causing luteal insufficiency [67]. They also showed that depletion of Nr5a2/LRH-1 from granulosa cells impaired progesterone production and luteinization, even though SF-1/Ad4BP is expressed to some extent [66, 68]. Of note, intestinal glucocorticoid synthesis is also induced by LRH-1 [57, 73, 74]. When considered together, LRH-1 in addition to SF-1/Ad4BP can be another master regulator for steroidogenesis, as represented by luteal cells.
5. The Role of Transcriptional Coactivator PGC-1α in Progesterone Production by Granulosa Cells
Steroidogenic properties of MSCs-derived cells vary markedly and depend on the tissues and species from which they are derived [18, 20, 52, 53, 72]. As mentioned above, BM-MSCs differentiated into cortisol-producing adrenocortical-like cells, and UCB-MSCs differentiated into granulosa-luteal-like cells, which produced high levels of progesterone [18, 20]. To determine why UCB-MSCs differentiate into luteal cells, gene expression profiles were compared with those of BM-MSCs using DNA microarray technology [20]. Among the identified genes, peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) was expressed only in UCB-MSCs at relatively high levels. PGC-1α was originally discovered by Spiegelman and colleagues as coactivator of PPARγ by conferring transcriptional regulation of its brown fat-specific target genes to PPARγ [75]. It can also activate other NRs and transcription factors [76–78], as well as being an important regulator of metabolism and cell fate determination in a variety of tissues [77, 79–81].
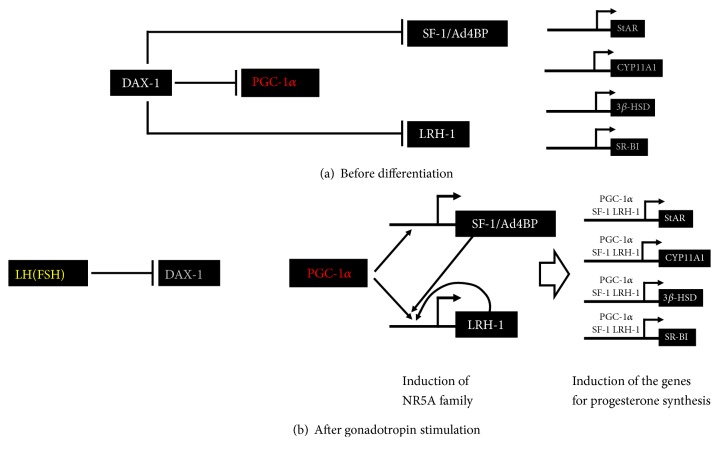
Consistent with the results in MSCs, ovarian PGC-1α was expressed in granulosa cells and colocalizes with SF-1/Ad4BP and LRH-1. PGC-1α acts as a very strong coactivator for SF-1/Ad4BP and LRH-1 via direct binding to their ligand-binding domains; its coactivation occurred in much higher levels compared to other coactivators (SRC-1, CBP, and P300) for SF-1/Ad4BP and LRH-1. Reporter assays revealed that PGC-1α activated the promoter activities of SF-1/Ad4BP and LRH-1 target genes, such as StAR, CYP11A1, and HSD3B2. Overexpression of PGC-1α induced expression of steroidogenic genes and progesterone production in both rat primary granulosa cells and human granulosa cell tumor–derived KGN cells. In addition to steroidogenic genes, PGC-1α also induced the expression of SF-1/Ad4BP and LRH-1. These results indicate that PGC-1α is involved in progesterone production in ovarian granulosa cells not only via potentiating transcriptional activities of the NR5A family proteins, but also via inducing their expression. Although PGC-1α induced the expression of both NR5A family genes, induction of LRH-1 occurred at a much higher level. As mentioned above, LRH-1 is highly expressed in the corpus luteum and SF-1/Ad4BP is expressed at very low levels [72]. Thus, PGC-1α may be a key factor promoting differentiation of granulosa cells into progesterone-producing luteal cells by acting on LRH-1 along multiple steps (Figure 3). Regarding the essential roles of LRH-1 in ovulation, recent reports suggest PGC-1α polymorphisms are associated with polycystic ovary syndrome (PCOS) [82]. PCOS is the most common endocrinopathy in women and the most common cause of anovulatory infertility, affecting 5%-10% of the population. It may be possible that dysfunction of PGC-1α in granulosa cells is one cause for anovulation in these patients.
Figure 3.
Regulation of granulosa cell differentiation by DAX-1 and PGC-1α via SF-1/Ad4BP and LRH-1. (a) During follicular development, the activities of SF-1/Ad4BP, LRH-1, and PGC-1α are repressed by DAX-1 in undifferentiated granulosa cells. Therefore, transcription of steroidogenic genes hardly occurred. (b) Gonadotropin induces the differentiation of granulosa cells into progesterone-producing cells. At this time, PGC-1α is released from DAX-1-mediated suppression via its reduction. This PGC-1α activation induces SF-1/Ad4BP and LRH-1. In particular, LRH-1 is highly induced by positive-feedback loop. Then, NR5A family proteins couple with PGC-1α to induce steroidogenic genes, such as StAR, CYP11A1, 3β-HSD, and SR-BI.
6. Regulation of NR0B1/DAX-1 (Dosage Sensitive Sex Reversal, Adrenal Hypoplasia Congenital Critical Region on the X Chromosome, Gene 1) Expression by Gonadotropin
Nonsteroidogenic granulosa cells from preantral follicles differentiate into steroidogenic cells by FSH treatment under culture conditions, after which FSH rapidly induces various steroidogenic genes. However, the expression of SF-1/Ad4BP and LRH-1 is unaffected by FSH during earlier stages [83, 84]. In addition, PGC-1α mRNA and protein levels are relatively high even before FSH stimulation and are barely influenced by FSH [20]. This indicates that transactivation of SF-1/Ad4BP and LRH-1 via PGC-1α is repressed by other factor(s). Orphan nuclear receptor, DAX-1 (the encoded gene is officially termed NR0B1), is one of the most plausible candidates as a repressor. In ovaries, DAX-1 is localized on granulosa and theca cells [85]. The human NR0B1 gene is located on the X chromosome at p21 and gives rise to 46,XY DSD/testicular dysgenesis through duplication of this region [86, 87]. NR0B1/DAX-1 mutations are associated with pathogenesis of adrenal hypoplasia congenita and hypogonadotropic hypogonadism. DAX-1 represents an unusual nuclear receptor, as it lacks DNA-binding domain [88, 89]. Instead, the N-terminal domain contains three LXXLL-like motifs (NR boxes) that interact with the ligand-binding domain of NRs. DAX-1 represses the transcriptional activities of both SF-1/Ad4BP and LRH-1 via these NR boxes [20, 90]. Tissue localization of DAX-1 is very similar to SF-1/Ad4BP [85, 91], probably due to the fact that SF-1/Ad4BP regulates DAX-1 expression by direct binding to the promoter and enhancer regions at least during fetal life [85, 91, 92]. Therefore, ovarian DAX-1 is detectable in both granulosa and theca cells [85, 93].
In cultured granulosa cells, DAX-1 expression is acutely downregulated by FSH within 2h [20, 83]. This is consistent with the time for initiating induction of various SF-1/Ad4BP and LRH-1 target genes, including steroidogenic genes. Overexpression of DAX-1 inhibited promoter activities of SF-1/Ad4BP and LRH-1 target genes induced by FSH. In addition, DAX-1 suppressed not only the transactivation of NR5A proteins, but also PGC-1α dependent coactivation in a dose-dependent manner. LXXLL motifs of DAX-1 have much stronger affinities to SF-1/Ad4BP and LRH-1 than that of PGC-1α [20]. Therefore, a release from the DAX-1-mediated transcriptional repression could be an important mechanism for the transactivation of many SF-1/Ad4BP and LRH-1 target genes induced by gonadotropins (Figure 3). This should also be an important event during the differentiation of granulosa cells into steroidogenic luteal cells, because DAX-1 expression is further suppressed in luteal stage. Consistent with this hypothesis, Zeleznik and colleagues showed that overexpression of DAX-1 repressed the FSH-induced progesterone and estradiol production in granulosa cells [94]. Furthermore, LH and ACTH stimulate the steroidogenesis with a reduction of DAX-1 expression in theca, Leydig, and adrenocortical cells [95, 96]. Therefore, downregulation of DAX-1 expression is an important mechanism for promoting the steroidogenesis by pituitary hormones in primary steroidogenic organs.
7. Transcriptional Regulation of Electron Transporter That Transfers Electron to Steroidogenic Enzymes
To identify novel SF-1/Ad4BP and LRH-1 target genes, genome-wide analyses by a promoter tiling array (chip-on-chip) and DNA microarray were performed using MSCs-derived steroidogenic cells [97, 98]. In these studies, multiple genes were considered as possible targets of NR5A family proteins, including some electron transporter genes.
Steroidogenesis is catalyzed by various cytochrome P450 enzymes, which have to receive electrons from nicotinamide adenine dinucleotide (NADPH) through their redox partners for the catalytic reactions [99]. Therefore, the activities of P450 enzymes are determined not only by their own expression levels, but also by the rate of electron transfer from redox partners. It is then probable that expression of these redox genes is also regulated by pituitary hormones and NR5A family proteins, as in the case of P450 enzymes. We investigated this possibility using stem cell-derived steroidogenic cells.
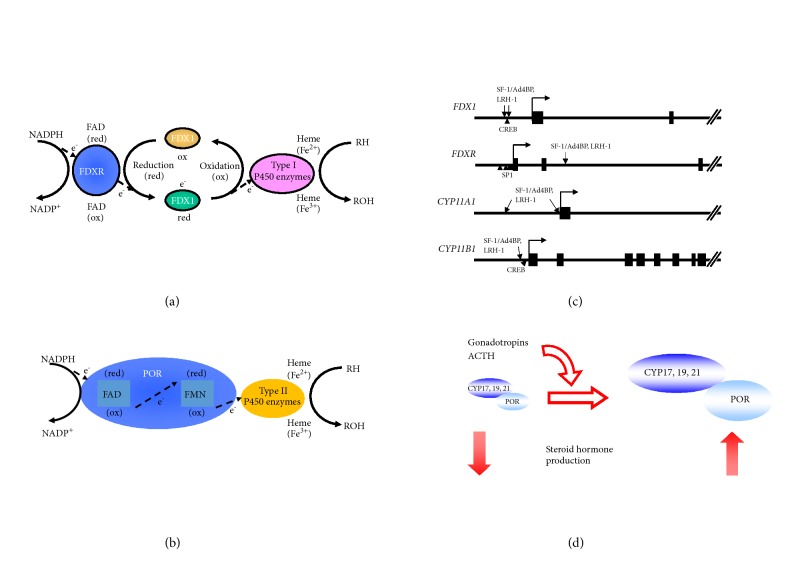
The human genome contains 57 P450 genes categorized into two classes based on their properties: type I and II enzymes. Each class occurs on different intracellular localizations and is coupled with different redox partners. Type I enzymes are localized on mitochondria and receive electrons from NADPH mediated by ferredoxin (FDX) and ferredoxin reductase (FDXR: Figure 4(a)). Conversely, type II enzymes are located in the endoplasmic reticulum and receive electrons from NADPH mediated by P450 oxidoreductase (POR) (Figure 4(b)). The results from genome-wide analyses suggest that SF-1/Ad4BP can control both pathways.
Figure 4.
P450 enzymes and their redox partners in steroidogenesis. (a) Schematic diagram of electron transfer from NADPH to mitochondrial type I P450 enzymes by FDXR and FDX1. At first, NADPH passes the electrons to the nonreduced (ox) FAD moiety of FDXR. Then, reduced (red) FDXR passes them to FDX1, followed by the transfer to P450 enzymes and hydroxylation of cholesterol and its metabolites. (b) Schematic diagram of electron transfer from NADH to microsomal type II P450 enzymes by POR. POR receives the electrons from NADPH by using its FAD moiety, and red FAD passes them to its FMN. Then, electrons are transferred to P450 enzymes to activate the steroid hormone production. (c) The binding sites of NR5A family and other transcription factors, which are essential for the transcription of FDX, FDXR, and ovarian type I P450 genes. (d) Pituitary hormones promote the steroidogenesis by enhancing the transcription of both mitochondrial P450 enzymes and POR.
7.1. Transcriptional Regulation of FDX1 and FDXR by NR5A Family Proteins
Among type I P450 (CYP) enzymes, P450scc (CYP11A1) and CYP11B1 use FDX and FDXR as redox partners for receiving electron from NADPH. Although CYP11B1 is known to represent glucocorticoid-synthesizing enzyme in the adrenal gland, we have shown it is induced by LH/hCG pathway in ovarian theca cells and testicular Leydig cells [49, 100]. To pass electrons to these enzymes, NADPH initially binds to FDXR, flavoprotein localized on the mitochondrial inner membrane (Figure 4(a)) [99]. Using its flavin adenine dinucleotide (FAD) moiety, FDXR transfers electrons derived from NADPH to an iron sulfur (Fe2S2) cluster of FDX1. FDX1 then interacts with P450 enzymes and donates electrons to progress steroid hormone synthetic reactions. Chromatin immunoprecipitation (ChIP) analyses suggested that both FDX1 and FDXR genes are targets of SF-1/Ad4BP and LRH-1 [101, 102]. DNA microarray analyses also reveal that FDX1 is upregulated by SF-1/Ad4BP and cAMP during differentiation.
Consistent with the results of stem cell-derived steroidogenic cells, FDX1 is rapidly induced by FSH in cultured granulosa cells [101, 103, 104]. ChIP analyses using stem cell-derived cells revealed that the binding of SF-1/Ad4BP is enriched in the region of about 1kb upstream to transcription start site (TSS) of the FDX1 gene. Reporter and gel mobility shift assays indicate that there are two SF-1/Ad4BP-binding sites within in this region, which are important for the FDX1 promoter activity under 8Br-cAMP stimulation. LRH-1 can also bind to these sites and activate the transcription. In addition to NR5A family proteins, cAMP response element binding protein (CREB), which binds to CRE-like sequence adjacent to the both SF-1/Ad4BP-binding sites, was critical for the transcription of FDX1 by the stimulation of cAMP. Taken together, it is probable that the synergistic action of SF-1/Ad4BP and CREB is responsible for the acute induction of FDX1 gene by FSH/cAMP pathway in ovarian granulosa cells. Such regulation can be applicable to other FSH-inducible genes [83, 105–107].
In contrast to FDX1, the expression of FDXR is largely unaffected by FSH and cAMP in granulosa cells and stem cell–derived steroidogenic cells [102, 108]. FDXR is ubiquitously expressed in various tissues, despite being highly expressed in steroidogenic tissues including gonads and adrenal glands [108]. Therefore, it is reasonable that there are powerful basal elements near TSS; this region contains six elements closely resembling the consensus sequences for SP1 [109]. On the other hand, it was revealed by our ChIP analysis that SF-1/Ad4BP binds to the intronic region of FDXR gene [102, 110]. This region enhanced the activity of its own proximal promoter, containing SP1-binding sites. It was also shown that SF-1/Ad4BP- binding to this site induces dynamic changes of chromatin architectures. Introduction of SF-1/Ad4BP into MSCs clearly increased the enrichment of active chromatin marks, acetylated lysine 27 on histone 3 (H3K27ac) and dimethylated lysine 4 on histone 3 (H3K4me2) of intronic region of FDXR, compared with control cells. These data demonstrated that the intronic SF-1/Ad4BP binding region of FDXR functions as an enhancer to cause the higher expression of FDXR in primary steroidogenic organs including ovary.
Ovarian type I P450 enzymes are target genes of SF-1/Ad4BP and LRH-1 [105, 111, 112]. Thus, SF-1/Ad4BP and LRH-1 control the steroidogenesis by regulating not only the transcription of steroidogenic enzymes, but also the transcription of FDX and FDXR (Figure 4(c)).
7.2. Regulation of POR Expression by Gonadotropins
P450c17 (CYP17A1 and CYP19A1) represents ovarian type II enzymes, which use POR as redox partners for receiving electron from NADPH. POR is a membrane-bound flavoprotein, expressed ubiquitously with more or less variable expression levels among different tissues [99]. It contains not only FAD moiety, but also flavin mononucleotide (FMN), electron acceptor from FAD as Fe2S2 cluster of FDX1. Therefore, POR can transfer NADPH-derived electrons directly to P450 enzymes (Figure 4(b)) [99]. However, the microsomal P450 component is found in a great molar excess to POR, especially in steroidogenic tissues [113]. This is possible to cause a profound influence on steroidogenesis, when steroidogenic enzymes are acutely induced by pituitary hormones. This hypothesis might be supported by the fact that POR deficient patients exhibit skeletal dysplasia referred to as Antley-Bixler syndrome (ABS) that is accompanied with impaired steroidogenesis, resulting in adrenal dysfunction, disorders of sexual development, and maternal virilization during pregnancy [114–117].
DNA microarray analyses demonstrated that POR expression is increased with the differentiation of MSCs into steroidogenic cells by SF-1/Ad4BP and cAMP [97]. As in the case of MSCs-derived steroidogenic cells, POR was induced by FSH and hCG with CYP19A1 in rat granulosa cells. POR expression was also increased by LH in theca cells. In the transfection experiments, expression of POR enhanced the aromatase activity in dose-dependent manner, even though aromatase protein levels were constant. On the other hand, knockdown of endogenous POR proteins in KGN cells led to the reduction of estrogen production. These results indicate that POR is one of the gonadotropin-regulatable genes in ovarian granulosa cells, and this regulation should cause the augmentation of estrogen production by gonadotropins. It was also reported in adrenocortical cell lines that ACTH or cAMP increases the expression of POR [118, 119]. In addition, Hall and colleagues showed in vitro that the activity of purified CYP17A1 was increased by the addition of POR proteins in a dose-dependent manner, resulting in increased androgen production [120–122]. Therefore, it is conceivable that POR expression is regulated by the pituitary hormones with steroidogenic enzymes in steroidogenic cells (Figure 4(d)).
Although the mechanisms of transcriptional regulation by pituitary hormones remained unknown yet, Ogata and colleagues have analyzed the pivotal elements of the proximal promoter of human POR [123]. The POR gene spans more than 70 kb, including coding 1-15 exons and an untranslated exon 1 (exon 1U) residing about 38.8 kb upstream of the coding exon 1 [124]. The region around exon 1U was completely unmethylated CpG-rich region in various cells [123]. In addition, in silico analysis suggests that this region exhibits promoter-associated histone marks. Reporter, electron mobility shift assays and ChIP analyses demonstrated that SP1 proteins bind to at least three binding sites within this region and markedly activate the promoter activities. Promoter activities were undetectable in SP family-deficient cells. These results indicate that SP1-binding sites represent an essential element for the transcription of POR gene. Consistent with these experimental results, some ABS patients had a deletion of this region, and POR gene was never transcribed from this allele [123, 125].
8. Conclusion
We have shown that MSCs-derived steroidogenic cells provide an important tool for investigating the transcriptional regulation of ovarian steroidogenesis-related genes. Especially, it contributes to understand the function of SF-1/Ad4BP and LRH-1 by revealing the regulation of their transcriptional activity and their novel target genes. This system is also useful for studying other steroidogenic phenomena. Ovarian-specific LRH-1 isoform was also identified based on above studies [126, 127]. However, many mysteries remained in ovarian steroidogenesis, yet. Among them, the cause of the difference between granulosa and theca cells on steroidogenic properties is not clear, which is the essence of ovarian steroidogenesis, two-cell–two-gonadotropin theory. The difference of SF-1/Ad4BP and LRH-1 function in steroidogenesis is also unknown. Further studies will be necessary for the comprehensive elucidation of such mysteries.
Acknowledgments
This study was performed under the cooperative research program of Institute of Nature and Environmental Technology, Kanazawa University (Acceptance No. 17020). This work was supported in part by JSPS Grant Number 18K15971 (Grant-in-Aid for Scientific Research (C)), 17K11214 (Grant-in-Aid for Scientific Research (C)), and 16K19012 (Grant-in-Aid for Young Scientists (B)) granted by Japan Society for the Promotion of Science, the Smoking Research Foundation of Japan, and by the Grant of National Center for Child Health and Development.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Miller W. L., Auchus R. J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine Reviews. 2011;32(1):81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller W. L. Steroidogenesis: Unanswered Questions. Trends in Endocrinology & Metabolism. 2017;28(11):771–793. doi: 10.1016/j.tem.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Connelly M. A., Williams D. L. SR-BI and cholesterol uptake into steroidogenic cells. Trends in Endocrinology & Metabolism. 2003;14(10):467–472. doi: 10.1016/j.tem.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Connelly M. A. SR-BI-mediated HDL cholesteryl ester delivery in the adrenal gland. Molecular and Cellular Endocrinology. 2009;300(1-2):83–88. doi: 10.1016/j.mce.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Stocco D. M., Zhao A. H., Tu L. N., Morohaku K., Selvaraj V. A brief history of the search for the protein(s) involved in the acute regulation of steroidogenesis. Molecular and Cellular Endocrinology. 2017;441:7–16. doi: 10.1016/j.mce.2016.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller W. L. Molecular biology of steroid hormone synthesis. Endocrine Reviews. 1988;9(3):295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- 7.Waterman M. R., Simpson E. R. Regulation of steroid hydroxylase gene expression is multifactorial in nature. Recent Progress in Hormone Research. 1990;45(1):533–563. doi: 10.1016/b978-0-12-571145-6.50016-9. [DOI] [PubMed] [Google Scholar]
- 8.Adashi E. Y. Endocrinology of the ovary. Human Reproduction. 1994;9(5):815–827. doi: 10.1093/oxfordjournals.humrep.a138602. [DOI] [PubMed] [Google Scholar]
- 9.Ryan K. J., Petro Z., Kaiser J. Steroid formation by isolated and recombined ovarian granulosa and tehcal cells. The Journal of Clinical Endocrinology & Metabolism. 1968;28(3):355–358. doi: 10.1210/jcem-28-3-355. [DOI] [PubMed] [Google Scholar]
- 10.Ryan K. J., Petro Z. Steroid biosynthesis by human ovarian granulosa and thecal cells. The Journal of Clinical Endocrinology & Metabolism. 1966;26(1):46–52. doi: 10.1210/jcem-26-1-46. [DOI] [PubMed] [Google Scholar]
- 11.Devine P. J., Rajapaksa K. S., Hoyer P. B. In vitro ovarian tissue and organ culture: a review. Frontiers in bioscience : a journal and virtual library. 2002;7:d1979–1989. doi: 10.2741/devine. [DOI] [PubMed] [Google Scholar]
- 12.Wickenheisser J. K., Nelson-DeGrave V. L., McAllister J. M. Human ovarian theca cells in culture. Ttrends in Endocrinology and Metabolism. 2006;17(2):65–71. doi: 10.1016/j.tem.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Azhar S., Tsai L., Medicherla S., Chandrasekher Y., Giudice L., Reaven E. Human granulosa cells use high density lipoprotein cholesterol for steroidogenesis. The Journal of Clinical Endocrinology & Metabolism. 1998;83(3):983–991. doi: 10.1210/jcem.83.3.4662. [DOI] [PubMed] [Google Scholar]
- 14.Havelock J. C., Rainey W. E., Carr B. R. Ovarian granulosa cell lines. Molecular and Cellular Endocrinology. 2004;228(1-2):67–78. doi: 10.1016/j.mce.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Rainey W. E., Sawetawan C., Mccarthy J. L., et al. Human ovarian tumor cells: A potential model for thecal cell steroidogenesis. The Journal of Clinical Endocrinology & Metabolism. 1996;81(1):257–263. doi: 10.1210/jc.81.1.257. [DOI] [PubMed] [Google Scholar]
- 16.Wan-Kyng L., Burleigh B. D., Ward D. N. Steroid and plasminogen activator production by cultured rat granulosa cells in response to hormone treatment. Molecular and Cellular Endocrinology. 1981;21(1):63–73. doi: 10.1016/0303-7207(81)90031-9. [DOI] [PubMed] [Google Scholar]
- 17.Trzeciak W. H., Waterman M. R., Simpson E. R. Synthesis of the cholesterol side-chain cleavage enzymes in cultured rat ovarian granulosa cells: Induction by follicle-stimulating hormone and dibutyryl adenosine 3′,5′-monophosphate. Endocrinology. 1986;119(1):323–330. doi: 10.1210/endo-119-1-323. [DOI] [PubMed] [Google Scholar]
- 18.Yazawa T., Mizutani T., Yamada K., et al. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology. 2006;147(9):4104–4111. doi: 10.1210/en.2006-0162. [DOI] [PubMed] [Google Scholar]
- 19.Yazawa T., Inanoka Y., Mizutani T., Kuribayashi M., Umezawa A., Miyamoto K. Liver receptor homolog-1 regulates the transcription of steroidogenic enzymes and induces the differentiation of mesenchymal stem cells into steroidogenic cells. Endocrinology. 2009;150(8):3885–3893. doi: 10.1210/en.2008-1310. [DOI] [PubMed] [Google Scholar]
- 20.Yazawa T., Inaoka Y., Okada R., et al. PPAR-γ Coactivator-1α Regulates Progesterone Production in Ovarian Granulosa Cells with SF-1 and LRH-1. Molecular Endocrinology. 2010;24(3):485–496. doi: 10.1210/me.2009-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yazawa T., Kawabe S., Inaoka Y., et al. Differentiation of mesenchymal stem cells and embryonic stem cells into steroidogenic cells using steroidogenic factor-1 and liver receptor homolog-1. Molecular and Cellular Endocrinology. 2011;336(1-2):127–132. doi: 10.1016/j.mce.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Yazawa T., et al. Differentiation of mesenchymal stem cells into gonad and adrenal steroidogenic cells. World Journal of Stem Cells. 2014;6:203–212. doi: 10.4252/wjsc.v6.i2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyamoto K., Yazawa T., Mizutani T., et al. Stem cell differentiation into steroidogenic cell lineages by NR5A family. Molecular and Cellular Endocrinology. 2011;336(1-2):123–126. doi: 10.1016/j.mce.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Morohashi K. The ontogenesis of the steroidogenic tissues. Genes to Cells: Devoted to Molecular & Cellular Mechanisms. 1997;2:95–106. doi: 10.1046/j.1365-2443.1997.1060304.x. [DOI] [PubMed] [Google Scholar]
- 25.Val P., Martinez-Barbera J.-P., Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development. 2007;134(12):2349–2358. doi: 10.1242/dev.004390. [DOI] [PubMed] [Google Scholar]
- 26.Bandiera R., Vidal V. P. I., Motamedi F. J., et al. WT1 maintains adrenal-gonadal primordium identity and marks a population of AGP-like progenitors within the adrenal gland. Developmental Cell. 2013;27(1):5–18. doi: 10.1016/j.devcel.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatano O., Takakusu A., Nomura M., Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes to Cells: Devoted to Molecular and Cellular Mechanisms. 1996:663–671. doi: 10.1046/j.1365-2443.1996.00254.x. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda Y., Shen W.-H., Ingraham H. A., Parker K. L. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Molecular Endocrinology. 1994;8(5):654–662. doi: 10.1210/me.8.5.654. [DOI] [PubMed] [Google Scholar]
- 29.Hanley N. A., Ball S. G., Clement-Jones M., et al. Expression of steroidogenic factor 1 and Wilms' tumour 1 during early human gonadal development and sex determination. Mechanisms of Development. 1999;87:175–180. doi: 10.1016/s0925-4773(99)00123-9. [DOI] [PubMed] [Google Scholar]
- 30.Hatano O., Takayama K., Imai T., et al. Sex-dependent expression of a transcription factor, Ad4BP, regulating steroidogenic P-450 genes in the gonads during prenatal and postnatal rat development. Development. 1994;120(10):2787–2797. doi: 10.1242/dev.120.10.2787. [DOI] [PubMed] [Google Scholar]
- 31.Pilon N., Behdjani R., Daneau I., Lussier J. G., Silversides D. W. Porcine steroidogenic factor-1 gene (pSF-1) expression and analysis of embryonic pig gonads during sexual differentiation. Endocrinology. 1998;139(9):3803–3812. doi: 10.1210/endo.139.9.6193. [DOI] [PubMed] [Google Scholar]
- 32.Quirke L. D., Juengel J. L., Tisdall D. J., Lun S., Heath D. A., McNatty K. P. Ontogeny of steroidogenesis in the fetal sheep gonad. Biology of Reproduction. 2001;65(1):216–228. doi: 10.1095/biolreprod65.1.216. [DOI] [PubMed] [Google Scholar]
- 33.Whitworth D. J., Pask A. J., Shaw G., Marshall Graves J. A., Behringer R. R., Renfree M. B. Characterization of steroidogenic factor 1 during sexual differentiation in a marsupial. Gene. 2001;277(1-2):209–219. doi: 10.1016/S0378-1119(01)00677-1. [DOI] [PubMed] [Google Scholar]
- 34.Yazawa T., Imamichi Y., Miyamoto K., et al. Regulation of steroidogenesis, development, and cell differentiation by steroidogenic factor-1 and liver receptor homolog-1. Zoological Science. 2015;32(4):323–330. doi: 10.2108/zs140237. [DOI] [PubMed] [Google Scholar]
- 35.Parker K. L., Schimmer B. P. Steroidogenic factor 1: A key determinant of endocrine development and function. Endocrine Reviews. 1997;18(3):361–377. doi: 10.1210/er.18.3.361. [DOI] [PubMed] [Google Scholar]
- 36.Parker K. L., et al. Steroidogenic factor 1: An essential mediator of endocrine development. Recent Progress in Hormone Research. 2002;57:19–36. doi: 10.1210/rp.57.1.19. [DOI] [PubMed] [Google Scholar]
- 37.Schimmer B. P., White P. C. Minireview: Steroidogenic factor 1: Its roles in differentiation, development, and disease. Molecular Endocrinology. 2010;24(7):1322–1337. doi: 10.1210/me.2009-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo X., Ikeda Y., Parker K. L. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 39.Sadovsky Y., Crawford P. A., Woodson K. G., et al. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proceedings of the National Acadamy of Sciences of the United States of America. 1995;92(24):10939–10943. doi: 10.1073/pnas.92.24.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baba T., Otake H., Sato T., et al. Glycolytic genes are targets of the nuclear receptor Ad4BP/SF-1. Nature Communications. 2014;5(1) doi: 10.1038/ncomms4634. [DOI] [PubMed] [Google Scholar]
- 41.Jeyasuria P., Ikeda Y., Jamin S. P., et al. Cell-Specific Knockout of Steroidogenic Factor 1 Reveals Its Essential Roles in Gonadal Function. Molecular Endocrinology. 2004;18(7):1610–1619. doi: 10.1210/me.2003-0404. [DOI] [PubMed] [Google Scholar]
- 42.Pelusi C., Ikeda Y., Zubair M., Parker K. L. Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells. Biology of Reproduction. 2008;79(6):1074–1083. doi: 10.1095/biolreprod.108.069435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crawford P. A., Sadovsky Y., Milbrandt J. Nuclear receptor steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Molecular and Cellular Biology. 1997;17(7):3997–4006. doi: 10.1128/MCB.17.7.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jadhav U., Jameson J. L. Steroidogenic factor-1 (SF-1)-driven differentiation of murine embryonic stem (ES) cells into a gonadal lineage. Endocrinology. 2011;152(7):2870–2882. doi: 10.1210/en.2011-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanase T., Gondo S., Okabe T., et al. Differentiation and regeneration of adrenal tissues: An initial step toward regeneration therapy for steroid insufficiency. Endocrine Journal. 2006;53(4):449–459. doi: 10.1507/endocrj.KR-74. [DOI] [PubMed] [Google Scholar]
- 46.Gojo S., Umezawa A. Plasticity of mesenchymal stem cells--regenerative medicine for diseased hearts. Human Cell: Official Journal of Human Cell Research Society. 2003;16(1):23–30. doi: 10.1111/j.1749-0774.2003.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 47.Prockop D. J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 48.Friedenstein A. J., Gorskaja J. F., Kulagina N. N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Experimental Hematology. 1976;4(5):267–274. [PubMed] [Google Scholar]
- 49.Yazawa T., Uesaka M., Inaoka Y., et al. Cyp11b1 is induced in the murine gonad by luteinizing hormone/human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen: Conservation and evolution of the androgen metabolic pathway. Endocrinology. 2008;149(4):1786–1792. doi: 10.1210/en.2007-1015. [DOI] [PubMed] [Google Scholar]
- 50.Gondo S., et al. SF-1/Ad4BP transforms primary long-term cultured bone marrow cells into ACTH-responsive steroidogenic cells. Genes to Cells: Devoted to Molecular & Cellular Mechanisms. 2004:1239–1247. doi: 10.1111/j.1365-2443.2004.00801.x. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka T., Gondo S., Okabe T., et al. Steroidogenic factor 1/adrenal 4 binding protein transforms human bone marrow mesenchymal cells into steroidogenic cells. Molecular Endocrinology. 2007;39(5-6):343–350. doi: 10.1677/JME-07-0076. [DOI] [PubMed] [Google Scholar]
- 52.Gondo S., Okabe T., Tanaka T., et al. Adipose tissue-derived and bone marrow-derived mesenchymal cells develop into different lineage of steroidogenic cells by forced expression of steroidogenic factor 1. Endocrinology. 2008;149(9):4717–4725. doi: 10.1210/en.2007-1808. [DOI] [PubMed] [Google Scholar]
- 53.Wei X., Peng G., Zheng S., Wu X. Differentiation of umbilical cord mesenchymal stem cells into steroidogenic cells in comparison to bone marrow mesenchymal stem cells. Cell Proliferation. 2012;45(2):101–110. doi: 10.1111/j.1365-2184.2012.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonoyama T., Sone M., Honda K., et al. Differentiation of human embryonic stem cells and human induced pluripotent stem cells into steroid-producing cells. Endocrinology. 2012;153(9):4336–4345. doi: 10.1210/en.2012-1060. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz-Babot G., Balyura M., Hadjidemetriou I., et al. Modeling congenital adrenal hyperplasia and testing interventions for adrenal insufficiency using donor-specific reprogrammed cells. Cell Reports. 2018;22:1236–1249. doi: 10.1016/j.celrep.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee Y.-K., Moore D. D. Liver receptor homolog-1, an emerging metabolic modulator. Frontiers in Bioscience. 2008;13(15):5950–5958. doi: 10.2741/3128. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez-Marcos P. J., Auwerx J., Schoonjans K. Emerging actions of the nuclear receptor LRH-1 in the gut. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2011;1812(8):947–955. doi: 10.1016/j.bbadis.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fayard E., Auwerx J., Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends in Cell Biology. 2004;14(5):250–260. doi: 10.1016/j.tcb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 59.Falender A. E., Lanz R., Malenfant D., Belanger L., Richards J. S. Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology. 2003;144(8):3598–3610. doi: 10.1210/en.2002-0137. [DOI] [PubMed] [Google Scholar]
- 60.Bookout A. L., Jeong Y., Downes M., Yu R. T., Evans R. M., Mangelsdorf D. J. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126(4):789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sierens J., Jakody I., Poobalan Y., et al. Localization and regulation of aromatase liver receptor homologue-1 in the developing rat testis. Molecular and Cellular Endocrinology. 2010;323(2):307–313. doi: 10.1016/j.mce.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z.-N., Bassett M., Rainey W. E. Liver receptor homologue-1 is expressed in the adrenal and can regulate transcription of 11 beta-hydroxylase. Molecular Endocrinology. 2001;27(2):255–258. doi: 10.1677/jme.0.0270255. [DOI] [PubMed] [Google Scholar]
- 63.Camats N. LRH-1 may rescue SF-1 deficiency for steroidogenesis: An in vitro and in vivo study. Sexual Development : Genetics, Molecular Biology, Evolution, Endocrinology, Embryology, and Pathology of Sex Determination and Differentiation. 2015:144–154. doi: 10.1159/000381575. [DOI] [PubMed] [Google Scholar]
- 64.Gu P., Goodwin B., Chung A. C.-K., et al. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Molecular and Cellular Biology. 2005;25(9):3492–3505. doi: 10.1128/MCB.25.9.3492-3505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paré J.-F., Malenfant D., Courtemanche C., et al. The fetoprotein transcription factor (FTF) gene is essential to embryogenesis and cholesterol homeostasis and is regulated by a DR4 element. The Journal of Biological Chemistry. 2004;279(20):21206–21216. doi: 10.1074/jbc.M401523200. [DOI] [PubMed] [Google Scholar]
- 66.Duggavathi R., et al. Liver receptor homolog 1 is essential for ovulation. Genes & Development. 2008;22:1871–1876. doi: 10.1101/gad.472008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C., et al. Liver receptor homolog-1 is essential for pregnancy. Nature Medicine. 2013;19:1061–1066. doi: 10.1038/nm.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bertolin K., Gossen J., Schoonjans K., Murphy B. D. The orphan nuclear receptor Nr5a2 is essential for luteinization in the female mouse ovary. Endocrinology. 2014;155(5):1931–1943. doi: 10.1210/en.2013-1765. [DOI] [PubMed] [Google Scholar]
- 69.Meinsohn M. C., et al. The Orphan Nuclear Receptor Liver Homolog Receptor-1 (Nr5a2) Regulates Ovarian Granulosa Cell Proliferation. Journal of the Endocrine Society. 2018;2:24–41. doi: 10.1210/js.2017-00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Labelle-Dumais C., Paré J.-F., Bélanger L., Farookhi R., Dufort D. Impaired progesterone production in Nr5a2+/- mice leads to a reduction in female reproductive function. Biology of Reproduction. 2007;77(2):217–225. doi: 10.1095/biolreprod.106.059121. [DOI] [PubMed] [Google Scholar]
- 71.Volle D. H., Duggavathi R., Magnier B. C., et al. The small heterodimer partner is a gonadal gatekeeper of sexual maturation in male mice. Genes and Development. 2007;21:303–315. doi: 10.1101/gad.409307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng N., Kim J. W., Rainey W. E., Carr B. R., Attia G. R. The Role of the Orphan Nuclear Receptor, Liver Receptor Homologue-1, in the Regulation of Human Corpus Luteum 3β-Hydroxysteroid Dehydrogenase Type II. The Journal of Clinical Endocrinology & Metabolism. 2003;88(12):6020–6028. doi: 10.1210/jc.2003-030880. [DOI] [PubMed] [Google Scholar]
- 73.Mueller M., Cima I., Noti M., et al. The nuclear receptor LRH-1 critically regulates extra-adrenal glucocorticoid synthesis in the intestine. The Journal of Experimental Medicine. 2006;203(9):2057–2062. doi: 10.1084/jem.20060357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coste A., Dubuquoy L., Barnouin R., et al. LRH-1-mediated glucocorticoid synthesis in enterocytes protects against inflammatory bowel disease. Proceedings of the National Acadamy of Sciences of the United States of America. 2007;104(32):13098–13103. doi: 10.1073/pnas.0702440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Puigserver P., Wu Z., Park C. W., Graves R., Wright M., Spiegelman B. M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/S0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 76.Huss J. M., Kopp R. P., Kelly D. P. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and –gamma: identification of novel Leucine-rich interaction motif within PGC-1alpha. The Journal of Biological Chemistry. 2002;277(43):40265–40274. doi: 10.1074/jbc.m206324200. [DOI] [PubMed] [Google Scholar]
- 77.Puigserver P., Spiegelman B. M. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocrine Reviews. 2003;24(1):78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 78.Mootha V. K., et al. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proceedings of the National Academy of Sciences of the United States of America 101; 2004; pp. 6570–6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Handschin C., Spiegelman B. M. Peroxisome proliferator-activated receptor γ coactivator 1 coactivators, energy homeostasis, and metabolism. Endocrine Reviews. 2006;27(7):728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 80.Mootha V. K., Lindgren C. M., Eriksson K.-F., et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature Genetics. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 81.Chan M. C., Arany Z. The many roles of PGC-1α in muscle—recent developments. Metabolism - Clinical and Experimental. 2014;63(4):441–451. doi: 10.1016/j.metabol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reddy T. V., Govatati S., Deenadayal M., Shivaji S., Bhanoori M. Polymorphisms in the TFAM and PGC1-α genes and their association with polycystic ovary syndrome among South Indian women. Gene. 2018;641:129–136. doi: 10.1016/j.gene.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 83.Yazawa T., Mizutani T., Yamada K., et al. Involvement of cyclic adenosine 5′-monophosphate response element-binding protein, steroidogenic factor 1, and Dax-1 in the regulation of gonadotropin-inducible ovarian transcription factor 1 gene expression by follicle-stimulating hormone in ovarian granulosa cells. Endocrinology. 2003;144(5):1920–1930. doi: 10.1210/en.2002-221070. [DOI] [PubMed] [Google Scholar]
- 84.Weck J., Mayo K. E. Switching of NR5A proteins associated with the inhibin α-subunit gene promoter after activation of the gene in granulosa cells. Molecular Endocrinology. 2006;20(5):1090–1103. doi: 10.1210/me.2005-0199. [DOI] [PubMed] [Google Scholar]
- 85.Kawabe K., Shikayama T., Tsuboi H., et al. Dax-1 as one of the target genes of Ad4BP/SF-1. Molecular Endocrinology. 1999;13(8):1267–1284. doi: 10.1210/mend.13.8.0325. [DOI] [PubMed] [Google Scholar]
- 86.Muscatelli F., Strom T. M., Walker A. P., et al. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature. 1994;372(6507):672–676. doi: 10.1038/372672a0. [DOI] [PubMed] [Google Scholar]
- 87.Zanaria E., Muscatelli F., Bardoni B., et al. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994;372(6507):635–641. doi: 10.1038/372635a0. [DOI] [PubMed] [Google Scholar]
- 88.Iyer A. K., McCabe E. R. B. Molecular mechanisms of DAX1 action. Molecular Genetics and Metabolism. 2004;83(1-2):60–73. doi: 10.1016/j.ymgme.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 89.Zhang H., Thomsen J. S., Johansson L., Gustafsson J.-Å., Treuter E. DAX-1 functions as an LXXLL-containing corepressor for activated estrogen receptors. The Journal of Biological Chemistry. 2000;275(51):39855–39859. doi: 10.1074/jbc.C000567200. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki T., Kasahara M., Yoshioka H., Umesono K., Morohashi K.-I. LXXLL motifs in DAX-1 have target specificity for the orphan nuclear receptors Ad4BP/SF-1 and LRH-1. Endocrine Research. 2002;28(4):p. 537. doi: 10.1081/ERC-120016835. [DOI] [PubMed] [Google Scholar]
- 91.Ikeda Y., et al. Steroidogenic factor 1 and Dax-1 colocalize in multiple cell lineages: potential links in endocrine development. Molecular Endocrinology. 1996;10:1261–1272. doi: 10.1210/mend.10.10.9121493. [DOI] [PubMed] [Google Scholar]
- 92.Hoyle C., Narvaez V., Alldus G., Lovell-Badge R., Swain A. Dax1 expression is dependent on steroidogenic factor 1 in the developing gonad. Molecular Endocrinology. 2002;16(4):747–756. doi: 10.1210/mend.16.4.0802. [DOI] [PubMed] [Google Scholar]
- 93.Sato Y., Suzuki T., Hidaka K., et al. Immunolocalization of nuclear transcription factors, DAX-1 and COUP-TF II, in the normal human ovary: Correlation with adrenal 4 binding protein/steroidogenic factor-1 immunolocalization during the menstrual cycle. The Journal of Clinical Endocrinology & Metabolism. 2003;88(7):3415–3420. doi: 10.1210/jc.2002-021723. [DOI] [PubMed] [Google Scholar]
- 94.Saxena D., Escamilla-Hernandez R., Little-Ihrig L., Zeleznik A. J. Liver receptor homolog-1 and steroidogenic factor-1 have similar actions on rat granulosa cell steroidogenesis. Endocrinology. 2007;148(2):726–734. doi: 10.1210/en.2006-0108. [DOI] [PubMed] [Google Scholar]
- 95.Jo Y., Stocco D. M. Regulation of steroidogenesis and steroidogenic acute regulatory protein in R2C cells by DAX-1 (dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene-1) Endocrinology. 2004;145(12):5629–5637. doi: 10.1210/en.2004-0941. [DOI] [PubMed] [Google Scholar]
- 96.Ragazzon B., Lefrançois-Martinez A.-M., Val P., et al. Adrenocorticotropin-dependent changes in SF-1/DAX-1 ratio influence steroidogenic genes expression in a novel model of glucocorticoid-producing adrenocortical cell lines derived from targeted tumorigenesis. Endocrinology. 2006;147(4):1805–1818. doi: 10.1210/en.2005-1279. [DOI] [PubMed] [Google Scholar]
- 97.Inaoka Y., Yazawa T., Mizutani T., et al. Regulation of P450 oxidoreductase by gonadotropins in rat ovary and its effect on estrogen production. Reproductive Biology and Endocrinology. 2008;6(1):p. 62. doi: 10.1186/1477-7827-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ju Y., Mizutani T., Imamichi Y., et al. Nuclear receptor 5A (NR5A) family regulates 5-aminolevulinic acid synthase 1 (ALAS1) gene expression in steroidogenic cells. Endocrinology. 2012;153(11):5522–5534. doi: 10.1210/en.2012-1334. [DOI] [PubMed] [Google Scholar]
- 99.Miller W. L. Minireview: Regulation of steroidogenesis by electron transfer. Endocrinology. 2005;146(6):2544–2550. doi: 10.1210/en.2005-0096. [DOI] [PubMed] [Google Scholar]
- 100.Yazawa T., Kawabe S., Kanno M., et al. Androgen/androgen receptor pathway regulates expression of the genes for cyclooxygenase-2 and amphiregulin in periovulatory granulosa cells. Molecular and Cellular Endocrinology. 2013;369(1-2):42–51. doi: 10.1016/j.mce.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 101.Imamichi Y., Mizutani T., Ju Y., et al. Transcriptional regulation of human ferredoxin 1 in ovarian granulosa cells. Molecular and Cellular Endocrinology. 2013;370(1-2):1–10. doi: 10.1016/j.mce.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 102.Imamichi Y., Mizutani T., Ju Y., et al. Transcriptional regulation of human ferredoxin reductase through an intronic enhancer in steroidogenic cells. Biochimica et Biophysica Acta - Gene Regulatory Mechanisms. 2014;1839(1):33–42. doi: 10.1016/j.bbagrm.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 103.Voutilainen R., Picado-Leonard J., Diblasio A. M., Miller W. L. Hormonal and developmental regulation of adrenodoxin messenger ribonucleic acid in steroidogenic tissues. The Journal of Clinical Endocrinology & Metabolism. 1988;66(2):383–388. doi: 10.1210/jcem-66-2-383. [DOI] [PubMed] [Google Scholar]
- 104.Golos T. G., Miller W. L., Strauss 3rd. J. F. Human chorionic gonadotropin and 8-bromo cyclic adenosine monophosphate promote an acute increase in cytochrome P450scc and adrenodoxin messenger RNAs in cultured human granulosa cells by a cycloheximide-insensitive mechanism. The Journal of Clinical Investigation. 1987;80(3):897–899. doi: 10.1172/JCI113149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fitzpatrick S. L., Richards J. S. Identification of a cyclic adenosine 3′, 5′-monophosphate-response element in the rat aromatase promoter that is required for transcriptional activation in rat granulosa cells and R2C leydig cells. Molecular Endocrinology. 1994;8(10):1309–1319. doi: 10.1210/mend.8.10.7854348. [DOI] [PubMed] [Google Scholar]
- 106.Ito M., et al. Synergistic activation of the inhibin alpha-promoter by steroidogenic factor-1 and cyclic adenosine 3', 5'-monophosphate. Molecular Endocrinology. 2000;14:66–81. doi: 10.1210/mend.14.1.0410. [DOI] [PubMed] [Google Scholar]
- 107.Zheng W., Jefcoate C. R. Steroidogenic factor-1 interacts with cAMP response element-binding protein to mediate cAMP stimulation of CYP1B1 via a far upstream enhancer. Molecular Pharmacology. 2005;67(2):499–512. doi: 10.1124/mol.104.005504. [DOI] [PubMed] [Google Scholar]
- 108.Solish S. B., Picado-Leonard J., Morel Y., et al. Human adrenodoxin reductase: Two mRNAs encoded by a single gene on chromosome 17cen→q25 are expressed in steroidogenic tissues. Proceedings of the National Acadamy of Sciences of the United States of America. 1988;85(19):7104–7108. doi: 10.1073/pnas.85.19.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brentano S. T., Black S. M., Dong L., Miller W. L. cAMP post-transcriptionally diminishes the abundance of adrenodoxin reductase mRNA. Proceedings of the National Acadamy of Sciences of the United States of America. 1992;89(9):4099–4103. doi: 10.1073/pnas.89.9.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leclerc D., Odièvre M.-H., Wu Q., et al. Molecular cloning, expression and physical mapping of the human methionine synthase reductase gene. Gene. 1999;240(1):75–88. doi: 10.1016/S0378-1119(99)00431-X. [DOI] [PubMed] [Google Scholar]
- 111.Hu M.-C., Hsu N.-C., Pai C.-I., Leo Wang C.-K., Chung B.-C. Functions of the upstream and proximal steroidogenic factor 1 (SF-1)-binding sites in the CYP11A1 promoter in basal transcription and hormonal response. Molecular Endocrinology. 2001;15(5):812–818. doi: 10.1210/mend.15.5.0636. [DOI] [PubMed] [Google Scholar]
- 112.Wang X.-L., Bassett M., Zhang Y., et al. Transcriptional regulation of human 11β-hydroxylase (hCYP11B1) Endocrinology. 2000;141(10):3587–3594. doi: 10.1210/endo.141.10.7689. [DOI] [PubMed] [Google Scholar]
- 113.Estabrook R. W., et al. Biochemical and genetic factors influencing drug metabolism. Influence of hepatic microsomal mixed function oxidation reactions on cellular metabolic control. Metabolism: Clinical and Experimental. 1971;20:187–199. doi: 10.1016/0026-0495(71)90091-6. [DOI] [PubMed] [Google Scholar]
- 114.Fluck C. E., et al. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nature Genetics. 2004;36:228–230. doi: 10.1038/ng1300. [DOI] [PubMed] [Google Scholar]
- 115.Arlt W., Walker E. A., Draper N., et al. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: Analytical study. The Lancet. 2004;363(9427):2128–2135. doi: 10.1016/S0140-6736(04)16503-3. [DOI] [PubMed] [Google Scholar]
- 116.Fukami M., Horikawa R., Nagai T., et al. Cytochrome P450 oxidoreductase gene mutations and antley-bixler syndrome with abnormal genitalia and/or impaired steroidogenesis: Molecular and clinical studies in 10 patients. The Journal of Clinical Endocrinology & Metabolism. 2005;90(1):414–426. doi: 10.1210/jc.2004-0810. [DOI] [PubMed] [Google Scholar]
- 117.Miller W. L. P450 oxidoreductase deficiency: A disorder of steroidogenesis with multiple clinical manifestations. Science Signaling. 2012;5(247):p. 11. doi: 10.1126/scisignal.2003318. [DOI] [PubMed] [Google Scholar]
- 118.Meng K. T., Dong Q., Miller W. L. Pathways leading to phosphorylation of P450c17 and to the posttranslational regulation of androgen biosynthesis. Endocrinology. 2008;149(5):2667–2677. doi: 10.1210/en.2007-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schimmer B. P., Cordova M., Cheng H., et al. Global profiles of gene expression induced by adrenocorticotropin in Y1 mouse adrenal cells. Endocrinology. 2006;147(5):2357–2367. doi: 10.1210/en.2005-1526. [DOI] [PubMed] [Google Scholar]
- 120.Nakajin S., Hall P. F. Microsomal cytochrome P-450 from neonatal pig testis. Purification and properties of A C21 steroid side-chain cleavage system (17 alpha-hydroxylase-C17,20 lyase). The Journal of Biological Chemistry. 1981;256(8):3871–3876. [PubMed] [Google Scholar]
- 121.Nakajin S., Shinoda M., Haniu M. C21 steroid side chain cleavage enzyme from porcine adrenal microsomes. Purification and characterization of the 17α-hydroxylase/C17,20-lyase cytochrome P-450. The Journal of Biological Chemistry. 1984;259(6):3971–3976. [PubMed] [Google Scholar]
- 122.Yanagibashi K., Hall P. F. Role of electron transport in the regulation of the lyase activity of C21 side-chain cleavage P-450 from porcine adrenal and testicular microsomes. The Journal of Biological Chemistry. 1986;261(18):8429–8433. [PubMed] [Google Scholar]
- 123.Soneda S., Yazawa T., Fukami M., et al. Proximal promoter of the cytochrome P450 oxidoreductase gene: Identification of microdeletions involving the untranslated exon 1 and critical function of the SP1 binding sites. The Journal of Clinical Endocrinology & Metabolism. 2011;96(11):E1881–E1887. doi: 10.1210/jc.2011-1337. [DOI] [PubMed] [Google Scholar]
- 124.Scott R. R., Gomes L. G., Huang N., Van Vliet G., Miller W. L. Apparent manifesting heterozygosity in P450 oxidoreductase deficiency and its effect on coexisting 21-hydroxylase deficiency. The Journal of Clinical Endocrinology & Metabolism. 2007;92(6):2318–2322. doi: 10.1210/jc.2006-2345. [DOI] [PubMed] [Google Scholar]
- 125.Fukami M., Nishimura G., Homma K., et al. Cytochrome P450 oxidoreductase deficiency: Identification and characterization of biallelic mutations and genotype-phenotype correlations in 35 Japanese patients. The Journal of Clinical Endocrinology & Metabolism. 2009;94(5):1723–1731. doi: 10.1210/jc.2008-2816. [DOI] [PubMed] [Google Scholar]
- 126.Kawabe S., Yazawa T., Kanno M., et al. A novel isoform of liver receptor homolog-1 is regulated by steroidogenic factor-1 and the specificity protein family in ovarian granulosa cells. Endocrinology. 2013;154(4):1648–1660. doi: 10.1210/en.2012-2008. [DOI] [PubMed] [Google Scholar]
- 127.Imamichi Y., Yuhki K.-I., Orisaka M., et al. 11-ketotestosterone is a major androgen produced in human gonads. The Journal of Clinical Endocrinology & Metabolism. 2016;101(10):3582–3591. doi: 10.1210/jc.2016-2311. [DOI] [PubMed] [Google Scholar]