Abstract
TFCP2L1 (transcription factor CP2-like 1) is a transcriptional regulator critical for maintaining mouse and human embryonic stem cell (ESC) pluripotency. However, the direct TFCP2L1 target genes are uncharacterized. Here, using gene overexpression, immunoblotting, quantitative real-time PCR, ChIP, and reporter gene assays, we show that TFCP2L1 primarily induces estrogen-related receptor β (Esrrb) expression that supports mouse ESC identity and also selectively enhances Kruppel-like factor 4 (Klf4) expression and thereby promotes human ESC self-renewal. Specifically, we found that in mouse ESCs, TFCP2L1 binds directly to the Esrrb gene promoter and regulates its transcription. Esrrb knockdown impaired Tfcp2l1's ability to induce interleukin 6 family cytokine (leukemia inhibitory factor)–independent ESC self-renewal and to reprogram epiblast stem cells to naïve pluripotency. Conversely, Esrrb overexpression blocked differentiation induced by Tfcp2l1 down-regulation. Moreover, we identified Klf4 as a direct TFCP2L1 target in human ESCs, bypassing the requirement for activin A and basic fibroblast growth factor in short-term human ESC self-renewal. Enforced Klf4 expression recapitulated the self-renewal–promoting effect of Tfcp2l1, whereas Klf4 knockdown eliminated these effects and caused loss of colony-forming capability. These findings indicate that TFCP2L1 functions differently in naïve and primed pluripotency, insights that may help elucidate the different states of pluripotency.
Keywords: embryonic stem cell, STAT3, pluripotency, Wnt pathway, differentiation, naive, Tfcp2l1
Introduction
Embryonic stem cells (ESCs)3 are derived from the inner cell mass of the preimplantation blastocyst (1–3). Under appropriate in vitro culture conditions, ESCs proliferate indefinitely without differentiation while retaining the capacity to generate cell lineages derived from all three primary germ layers (4). To date, although ESC-like cells from many species have been established, only ESCs derived from mice and rats possess the ability to generate germline-competent chimeric offspring and thus represent a “naïve” pluripotent state (1, 2, 5, 6). Interestingly, the available human ESCs (hESCs) are more similar to mouse postimplantation epiblast-derived stem cells (EpiSCs) than to mouse ESCs (mESCs) in their self-renewal requirements and morphology and thus represent a “primed” pluripotency state (3, 7, 8). mESC self-renewal can be maintained in two distinct culture systems: serum-containing medium supplemented with leukemia inhibitor factor (LIF) (9, 10) and serum-free N2B27 medium supplemented with two small molecule inhibitors (2i), CHIR99021 and PD0325901 (11). LIF supports self-renewal by inducing activation of signal transducer and activator of transcription 3 (STAT3) (12). CHIR99021 and PD0325901 maintain self-renewal through inhibition of glycogen synthase kinase 3 (GSK3) and mitogen-activated protein kinase kinase (MEK) (11), respectively. However, hESCs requires the activin A and basic fibroblast growth factor (bFGF) cytokines to maintain their identity (3). The addition of Wnt/β-catenin signaling inhibitors can further enable robust hESC propagation (13, 14). Understanding how these growth factors mediate intracellular signaling pathways controlling the unique pluripotent state and the similarities and differences between naïve and primed pluripotency are hot spots in current stem cell research.
Despite the difference in growth factor requirements between mESCs and hESCs, the core transcription factors governing pluripotency are similar, such as the master pluripotency genes Oct4, Nanog, and Sox2 (15). Recently, transcription factor CP2-like 1 (Tfcp2l1) has been identified as an important pluripotent factor and has become one of the core markers to identify ESCs generated from many species (16–20). We and other groups reported that Tfcp2l1 expression is high in the inner cell mass and mESCs, down-regulated in primed stem cells, and further reduced in differentiated cells (16, 17, 21, 22). Tfcp2l1 plays an essential role in maintaining ESC identity. In mESCs, it is a critical target in LIF- and 2i-mediated self-renewal (16, 17, 23). To date, only knockdown of Tfcp2l1, but not other STAT3 targets, is able to weaken the LIF/STAT3-mediated mESC self-renewal and EpiSC reprograming (16, 17). In addition, overexpression of Tfcp2l1 can compensate for the function of 2i when combined with Klf2, another pluripotency gene (23). In contrast to naïve-type stem cells, Tfcp2l1 is expressed highly in early human embryos, while it declines in vitro in established primed hESCs (21, 24). However, overexpression of Tfcp2l1 cannot reprogram hESCs into the naïve pluripotency state (18). Remarkably, enforced expression of Tfcp2l1 promotes self-renewal, whereas its suppression leads to hESC differentiation toward endoderm and mesoderm specification (22, 24). Taken together, these findings suggest that the self-renewal–promoting function of Tfcp2l1 is conserved in mESCs and hESCs.
Tfcp2l1 has been proposed to act partially through repression of multiple lineage commitments (25). However, it is unclear whether Tfcp2l1 functions through direct activation of a selective pluripotent factor. To resolve this issue, we sought to identify genes directly regulated by Tfcp2l1 in mouse and human ESCs mainly based on gain- and loss-of-function analyses. These analyses identified Esrrb and Klf4 as two direct targets of Tfcp2l1 that are capable of mediating the self-renewal–promoting effects of Tfcp2l1 in mESCs and hESCs, respectively.
Results
Esrrb is a direct target of Tfcp2l1 in mESCs
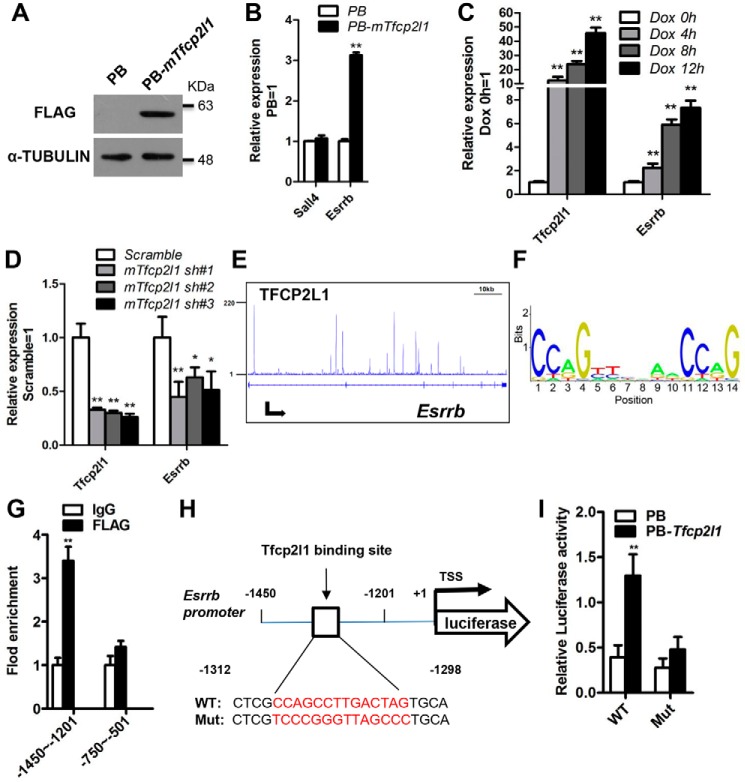
Previously, Smith and co-workers (26) developed a data-constrained, computational method and defined the simplest essential cassette for maintaining naïve pluripotency. This minimal set comprises 3 inputs (2i/LIF), 12 transcription factors (Oct4, Sox2, Nanog, Klf2, Esrrb, Tfcp2l1, Klf4, Sall4, Gbx2, STAT3, TCF3, and MEK), and 16 interactions, in which Esrrb and Sall4 are two potential direct targets of Tfcp2l1 (26). To validate this predicted relationship of Tfcp2l1, we designed five different approaches. First, we generated one mESC line that overexpressed FLAG-tagged mouse Tfcp2l1 using a PiggyBac vector (PB-mTfcp2l1) in which Tfcp2l1 expression was efficiently enhanced (Fig. 1A). Quantitative real-time PCR (qRT-PCR) analysis revealed that overexpression of Tfcp2l1 resulted in up-regulation of the Esrrb transcript but not Sall4 (Fig. 1B). Second, to further validate that Esrrb is regulated by Tfcp2l1, we examined Esrrb transcription under different Tfcp2l1 expression levels by using one mESC line that contains a doxycycline (Dox)-inducible mouse Tfcp2l1 transgene (17), in which Tfcp2l1 transcription was efficiently induced by Dox treatment (Fig. 1C). Esrrb has a similar expression pattern to Tfcp2l1 following Dox addition (Fig. 1C). Third, to investigate whether Tfcp2l1 down-regulation attenuates Esrrb expression, we constructed lentiviral vectors expressing mouse Tfcp2l1-specific shRNA sequences (mTfcp2l1 sh#1, mTfcp2l1 sh#2, and mTfcp2l1 sh#3). Stable knockdown (70–80%) of Tfcp2l1 transcript was observed following drug selection (Fig. 1D). As expected, the Esrrb transcript decreased in Tfcp2l1 shRNA cells when compared with scramble control cells (Fig. 1D). Fourth, to explore how Tfcp2l1 regulates the expression of Esrrb, we mapped the binding sites of Tfcp2l1 by analyzing the deep sequencing (ChIP-seq) date of Tfcp2l1 (27). As shown in Fig. 1E, the Tfcp2l1 protein has many binding sites in the Esrrb locus (Fig. 1E). Finally, to further determine whether Esrrb is a direct target of Tfcp2l1, we analyzed the Tfcp2l1-binding consensus motifs (Fig. 1F) and predicted two potential binding sites within the Esrrb promoter region (from −3000 ∼ +1) from the JASPAR CORE database (motif 1, −1312CCAGCCTTGACTAG−1298; and motif 2, −574GCAGACTGGCCCAG−561). We then performed ChIP–qRT-PCR in PB-mTfcp2l1 46C mESCs and found a direct interaction between Tfcp2l1 and the Esrrb promoter region containing the motif 1 sequence (Fig. 1G). This suggested an interaction between Tfcp2l1 and the Esrrb promoter. To further determine whether Tfcp2l1 is a functional activator of the Esrrb promoter, Tfcp2l1 was co-transfected with reporter vectors that drive the expression of luciferase under the control of Esrrb promoter fragments (pGL3-Esrrb), containing motif 1 or mutated motif 1 sequences (Mut) (Fig. 1H). Under these conditions, we observed a 2.7-fold increase in the WT promoter activity relative to the mutant sequence in PB-mTfcp2l1 cells (Fig. 1I). Collectively, these results suggest that Esrrb is positively regulated and activated by Tfcp2l1 in mESCs.
Figure 1.
Mouse Tfcp2l1 directly stimulates Esrrb expression in mESCs. A, Western blotting analysis of the FLAG protein in FLAG-tagged mouse Tfcp2l1 (PB-mTfcp2l1) 46C mESCs cultured in LIF/serum. α-Tubulin was used as a loading control. B, qRT-PCR analysis of Sall4 and Esrrb expression levels in PB and PB-mTfcp2l1 46C mESCs cultured in the presence of LIF. The data are presented as the means ± S.D. of three biological replicates. **, p < 0.01 versus PB. C, qRT-PCR analysis of Esrrb expression in mouse Tfcp2l1-inducible mESCs treated with Dox for different times. The data are presented as the means ± S.D. of three biological replicates. **, p < 0.01 versus 0 h. D, qRT-PCR analysis of Esrrb expression in 46C mESCs infected with scramble or mouse Tfcp2l1 shRNA lentivirus (mTfcp2l1 sh#1, mTfcp2l1 sh#2, and mTfcp2l1 sh#3). The data are presented as the means ± S.D. of three biological replicates. *, p < 0.05; **, p < 0.01 versus scramble. E, gene tracks represent Tfcp2l1 binding at the indicated gene loci. The x axis represents the linear sequence of genomic DNA, and the y axis represents the total number of mapped reads. F, predicted consensus binding motif of Tfcp2l1 target loci from the JASPAR CORE database. G, Tfcp2l1 binds to the Esrrb promoter. ChIP assays were performed using anti-Flag and control IgG antibodies, and fold enrichment in the indicated regions of the Esrrb promoter was examined using qRT-PCR. Two fragments of the Esrrb promoter are indicated by −1450 to −1201 and −750 to −501. The data are presented as the means ± S.D. of three biological replicates. **, p < 0.01 versus IgG. H, the positions of one putative Tfcp2l1 binding site in Esrrb promoter. TSS, transcription start site. I, luciferase activity analysis of PB cells overexpressing the WT Esrrb promoter plasmid or FLAG-mTfcp2l1 cells transfected with the WT or mutant Esrrb promoter reporter plasmid. The data are presented as the means ± S.D. of three biological replicates. **, p < 0.01 versus PB.
Esrrb mediates the self-renewal–promoting effects of Tfcp2l1 in mESCs
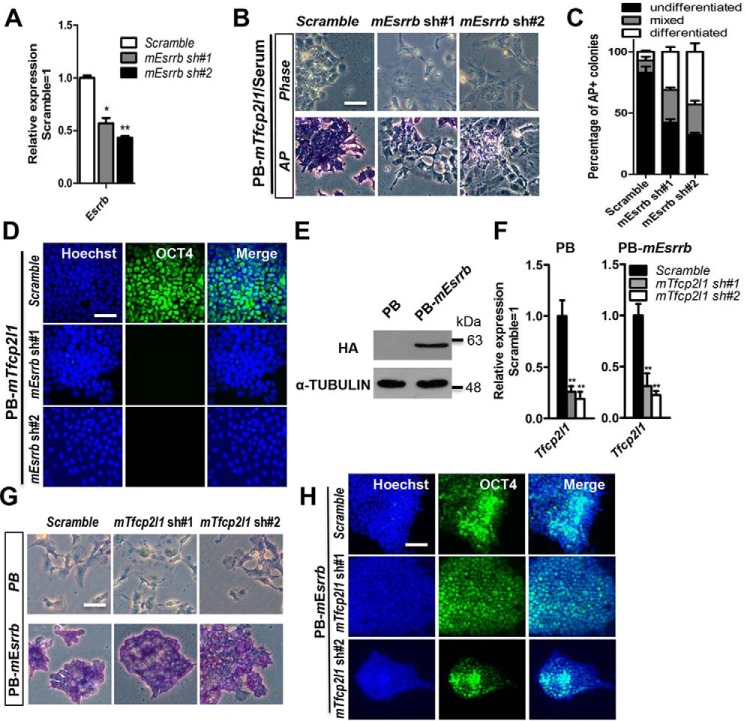
Tfcp2l1 overexpression can substitute for LIF or CHIR99021 to support mESC self-renewal (17, 23, 28). To determine whether Tfcp2l1 functions in maintaining mESC pluripotency depend on Esrrb in serum or serum-free conditions, we first decreased Esrrb expression in PB-mTfcp2l1 mESCs with two shRNAs specific to mouse Esrrb mRNA (mEsrrb sh#1 and mEsrrb sh#2). qRT-PCR analysis confirmed that the Esrrb transcript levels were decreased (40–60%) in these cells (Fig. 2A). Next, we cultured these cells in serum-containing medium without LIF for 8 days and found that scramble control mESCs retained normal mESC morphology, positive AP activity, and expression of the pluripotency marker OCT4 (Fig. 2, B–D), whereas knockdown of Esrrb induced differentiation in PB-mTfcp2l1 mESCs (Fig. 2, B–D). To further verify this observation, we seeded these cell lines in serum-free N2B27 medium supplemented with PD0325901. After three passages, we found that down-regulation of Esrrb extinguished the pluripotency supported by PB-mTfcp2l1, as indicated by the flat cell morphology and decreased AP activity (Fig. S1, A and B). Overall, these data suggest that Tfcp2l1 requires Esrrb to maintain the naïve pluripotency of mESCs.
Figure 2.
Esrrb is critical for mouse Tfcp2l1 to support the ground state of mESCs. A, qRT-PCR analysis of Esrrb expression in 46C mESCs infected with scramble or mouse Esrrb shRNA lentivirus (mEsrrb sh#1 and mEsrrb sh#2). The data are presented as the means ± S.D. of three biological replicates. *, p < 0.05; **, p < 0.01 versus scramble. B, phase-contrast images and AP staining of scramble control and mEsrrb shRNA mESCs that are overexpressing PB-mTfcp2l1 and cultured in serum without LIF for 8 days. Bar, 100 μm. C, quantification of AP positive colonies in Fig. 2B. The data are presented as the means ± S.D. of three biological replicates. D, immunofluorescence staining of OCT4 in PB-mTfcp2l1/mEsrrb shRNA 46C mESCs. The nuclei were counterstained with Hoechst 33342 (Hoechst). Bar, 100 μm. E, Western blotting analysis of the HA protein in HA-tagged mouse Esrrb (PB-mEsrrb)-expressing 46C mESCs cultured in LIF/serum. α-Tubulin was used as a loading control. F, qRT-PCR analysis of Tfcp2l1 expression in PB and PB-mEsrrb mESCs infected with scramble or mTfcp2l1 shRNA lentivirus. The data are presented as the means ± S.D. of three biological replicates. **, p < 0.01 versus scramble. G, AP staining of the scramble control and mTfcp2l1 shRNA mESCs overexpressing PB or PB-mEsrrb and cultured in serum without LIF for 8 days. Bar, 100 μm. H, immunofluorescence staining of OCT4 in PB-mEsrrb/mTfcp2l1 shRNA 46C mESCs. Bar, 100 μm.
Because knockdown of Tfcp2l1 impairs the undifferentiated state of mESCs under LIF conditions, we consequently wanted to test whether elevated expression of Esrrb diminishes this phenotype. First, we overexpressed HA-tagged mouse Esrrb using a PB vector (PB-mEsrrb), which caused significant increases in ESRRB (Fig. 2E). Second, we decreased Tfcp2l1 expression in PB and PB-mEsrrb mESCs with two shRNAs specific to mouse Tfcp2l1 mRNA (mTfcp2l1 sh#1 and mTfcp2l1 sh#2). qRT-PCR analysis confirmed that the Tfcp2l1 transcript levels were decreased (40–60%) in these cells (Fig. 2F). After being cultured in serum-containing medium without LIF for 8 days, only the PB-mEsrrb mESCs infected with the scramble or Tfcp2l1 shRNA lentivirus displayed ESC morphological characteristics and expressed the pluripotency marker OCT4, whereas PB mESCs infected with the scramble or Tfcp2l1 shRNA lentivirus differentiated (Fig. 2, G and H). Notably, Tfcp2l1 protein and Esrrb protein also interact in mESCs (29). Therefore, Tfcp2l1 promotes mESC self-renewal via Esrrb at the protein and transcriptional levels.
Esrrb is required for Tfcp2l1 to induce naïve pluripotency in EpiSCs
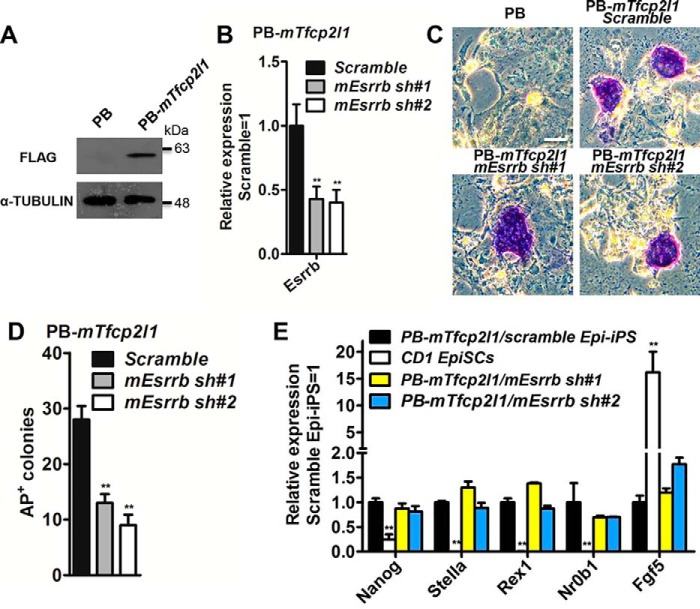
Both Esrrb and Tfcp2l1 can reprogram postimplantion embryo derived EpiSCs to naïve pluripotency (16, 17, 23, 28). To assess whether Esrrb modulates the ability of Tfcp2l1 to reprogram EpiSCs, CD1 mouse EpiSCs, isolated from embryonic day 5.5 embryos (14), were transfected with a PB construct containing a FLAG-tagged mouse Tfcp2l1 (PB-mTfcp2l1) (Fig. 3A) and then infected with the scramble control or mouse Esrrb shRNA lentivirus (mEsrrb sh#1 and mEsrrb sh#2) (Fig. 3B). After exposure to reprograming conditions including 2i/LIF for 12 days, the EpiSCs transfected with empty vector PB differentiated or died, whereas an average of 28 AP-positive colonies were generated from 1 × 105 PB-mTfcp2l1/scramble transfectants in three independent replicates. In parallel experiments, an average of 13 and 9 colonies were obtained from PB-mTfcp2l1/mEsrrb sh#1 and PB-mTfcp2l1/mEsrrb sh#2 transfectants, respectively (Fig. 3, C and D). These ESC-like colonies tolerated single-cell dissociation and could be continuously expanded in LIF/serum. At the same time, they acquired the mESC-specific transcriptional program and expressed high levels of Nanog, Stella, Rex1, and Nr0b1, but a low level of the EpiSC-specific marker Fgf5 (Fig. 3E); this suggests that they had been reprogrammed into a naïve state. Collectively, these results indicate that augmented Esrrb expression is likely important for Tfcp2l1 to convert mouse EpiSCs to the ESC state.
Figure 3.
Esrrb plays an important role in the induction of naïve pluripotency downstream of mouse Tfcp2l1. A, FLAG-tagged mouse Tfcp2l1 (PB-mTfcp2l1) was introduced into CD1 EpiSCs, and the protein level of FLAG was determined by Western blotting. The cells were cultured in activin A, basic FGF, and IWR1 conditions. B, qRT-PCR analysis of Esrrb expression levels in mEsrrb knockdown EpiSCs overexpressed PB-mTfcp2l1. The data are presented as the means ± S.D. of three biological replicates. **, p < 0.01 versus scramble. C, AP staining of colonies generated from scramble control and mEsrrb knockdown EpiSCs transfected with PB-mTfcp2l1. The cells were cultured in serum medium in the presence of LIF/2i for 12 days. Bar, 100 μm. D, quantification of the AP-positive colonies generated from scramble and mEsrrb knockdown EpiSCs carrying the mTfcp2l1 transgene. The cells were cultured in serum medium supplemented with LIF/2i for 12 days. The data are presented as the means ± S.D. of three biological replicates. E, comparison of marker gene expression in the indicated Epi-iPS cells and EpiSCs. Nanog, Stella, Rex1, and Nr0b1 are mESC markers, whereas Fgf5 is an EpiSC marker. The data are presented as the means ± S.D. of three biological replicates. **, p < 0.01 versus CD1 EpiSCs. Epi-iPS, induced pluripotent stem cells generated from EpiSCs.
KLF4 is critical for TFCP2L1 to maintain hESC identity
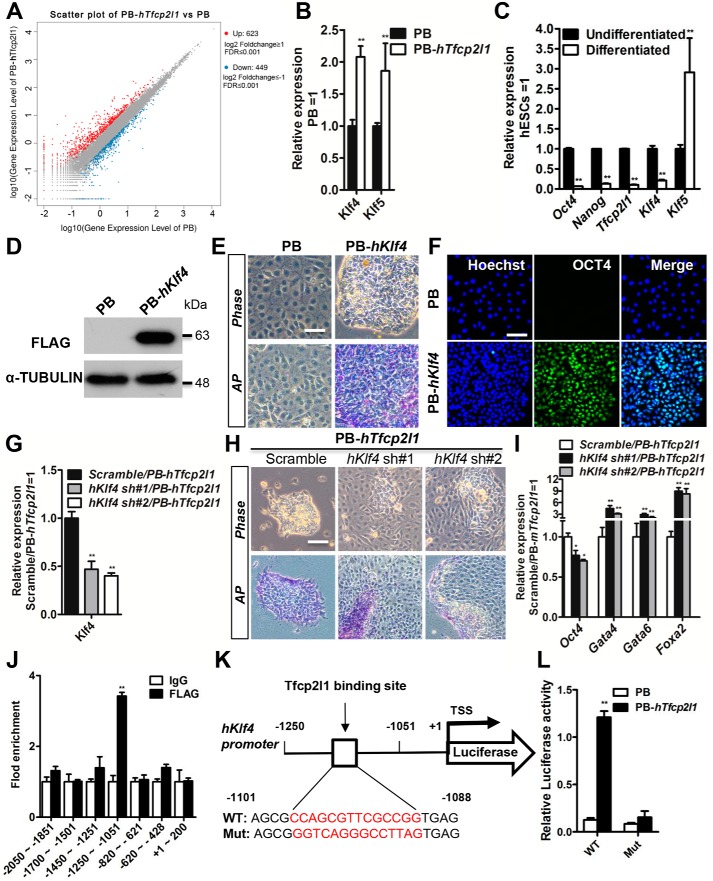
Ectopic expression of TFCP2L1 can bypass the requirement for activin A and bFGF in short-term self-renewal of hESCs, whereas suppression of TFCP2L1 abolishes hESC pluripotency (22, 24). To screen the functional targets of human TFCP2L1, we performed an RNA-Seq analysis. We identified 623 genes that were up-regulated and 449 genes that were down-regulated at least 2-fold by human TFCP2L1 overexpression (PB-hTfcp2l1) compared with empty vector PB (GEO accession no GSE115075) (Fig. 4A). Among these increased genes, we focused on pluripotency genes and identified KLF4 and KLF5. qRT-PCR was then used to confirm the mRNA levels of these two candidates in PB and PB-hTfcp2l1 cells (Fig. 4B). Subsequently, to examine whether the expression of the two candidates decreased during hESC differentiation, such as TFCP2L1, we performed monolayer differentiation. As shown in Fig. 4C, KLF5 is expressed in both pluripotent hESCs and differentiated cells, whereas the Klf4 transcript is down-regulated as hESCs differentiate (Fig. 4C). In particular, the change in KLF4 expression is similar to that seen for TFCP2L1, as well as other pluripotency markers, such as OCT4 and NANOG (Fig. 4C), which implies that KLF4 contributes to hESC maintenance and that there is association between KLF4 and TFCP2L1.
Figure 4.
KLF4 mediates the function of human TFCP2L1 in HES2 hESCs. A, scatter plot of the genes modified by human Tfcp2l1 overexpression (PB-hTfcp2l1). A Benjamini Hochberg false discovery rate of 0.01 was applied. B, qRT-PCR analysis of Klf4 and Klf5 expression in PB and PB-hTfcp2l1 hESCs. The data are presented as the means ± S.D. of three biological replicates. **, p < 0.01 versus PB. C, comparison of Oct4, Nanog, Tfcp2l1, Klf4, and Klf5 expression between undifferentiated hESCs and differentiated cells cultured in basal medium for 8 days. The data are presented as the means ± S.D. of three biological replicates. **, p < 0.01 versus undifferentiated. D, Western blotting analysis of the FLAG protein in FLAG-tagged human Klf4 (PB-hKlf4) hESCs. α-Tubulin was used as a loading control. E, phase-contrast images and AP staining of PB and PB-hKlf4 hESCs cultured in basal medium for 6 days. Bar, 100 μm. F, immunofluorescence analysis of OCT4 expression in PB and PB-hKlf4 cells. OCT4 is a marker of undifferentiated hESCs. The nuclei were counterstained with Hoechst. Bar, 100 μm. G, qRT-PCR analysis of Klf4 expression in PB-hTfcp2l1 hESCs infected with scramble or human Klf4 shRNA lentivirus (hKlf4 sh#1 and hKlf4 sh#2). The data are presented as the means ± S.D. of three biological replicates. **, p < 0.01 versus scramble/PB-hTfcp2l1. H, phase-contrast images and AP staining of PB-hTfcp2l1 hESCs infected with scramble or hKlf4 shRNA lentivirus and cultured in basal medium for 6 days. Bar, 100 μm. I, qRT-PCR analysis of Oct4, Gata4, Gata6, and FoxA2 expression in PB-hTfcp2l1 hESCs infected with scramble or hKlf4 shRNA lentivirus. The data are presented as the means ± S.D. of three biological replicates. *, p < 0.05; **, p < 0.01 versus scramble/PB-hTfcp2l1. J, Tfcp2l1 binds to the Klf4 promoter. ChIP assays were performed using anti-Flag and control IgG antibodies and fold enrichment within the indicated regions of the Klf4 promoter was examined using qRT-PCR. The data are presented as the means ± S.D. of three biological replicates. *, p < 0.05; **, p < 0.01 versus IgG. K, the position of one putative Tfcp2l1 binding site in Klf4 promoter. L, PB or FLAG-hTfcp2l1 cells were transfected with the indicated Klf4 promoter reporter plasmids for 48 h, and then the luciferase activity was measured. The data are presented as the means ± S.D. of three biological replicates. **, p < 0.01 versus PB.
To evaluate the ability of KLF4 to promote hESC self-renewal, we constructed a human KLF4-overexpressing hESC line with the PB vector (PB-hKlf4) (Fig. 4D). After cultured in hESC basal medium without activin A and bFGF for 6 days, PB cells differentiated, whereas PB-hKlf4 cells maintained an undifferentiated morphology and sustained AP-positive activity (Fig. 4E). Accordingly, immunofluorescence showed high expression of the pluripotency marker OCT4 (Fig. 4F). However, similar to TFCP2L1 overexpressing hESCs (22), PB-hKlf4 ESCs exhibited some evidence of differentiation at the edge of each colony and could not been maintained for long-term cultures. Therefore, overexpression of KLF4 enables short-term self-renewal of hESCs.
To demonstrate that Klf4 is a key downstream mediator of Tfcp2l1, we knocked down the human KLF4 transcript (hKlf4 sh#1 and hKlf4 sh#2) in PB-hTfcp2l1 transfectants (Fig. 4G). Knockdown of KLF4 resulted in PB-hTfcp2l1 hESC differentiation in the absence of activin A and bFGF, as indicated by the flat cell morphology, decreased AP activity and low expression of OCT4 (Fig. 4, H and I). Conversely, they highly expressed differentiation genes, such as GATA4, GATA6, and FOXA2 (Fig. 4I), suggesting that knockdown of KLF4 eliminates the ability of TFCP2L1 to promote self-renewal. To determine whether TFCP2L1 directly binds to the KLF4 locus, we analyzed the TFCP2L1-binding consensus motifs (Fig. 1F) and investigated the role of TFCP2L1 in the regulation of KLF4 expression. To detect whether TFCP2L1 binds to the KLF4 promoter, we predicted several potential binding sites within the KLF4 gene (from −2500 to +200) from the JASPAR CORE database. Then we used ChIP–qRT-PCR in PB-hTfcp2l1 hESCs and found the direct interaction of TFCP2L1 with the KLF4 promoter (Fig. 4J). To further characterize the role of TFCP2L1 in KLF4 expression, we first constructed WT and mutant pGL3-Klf4 cells (Fig. 4K) and transfected the modified mESCs with the TFCP2L1 gene. Under these conditions, we observed that TFCP2L1 overexpression enhanced the luciferase activity in pGL3-Klf4 cells. TFCP2L1 overexpression did not increase the KLF4 promoter activity in mutant pGL3-Klf4 cells (Fig. 4L), indicating that KLF4 is a direct target of TFCP2L1. To further investigate whether TFCP2L1 protein and KLF4 protein interact in hESCs, we performed co-immunoprecipitation in flag-tagged TFCP2L1 and HA-tagged KLF4 overexpressing hESCs. We found that the TFCP2L1 and KLF4 proteins also interact (Fig. S2). Taken together, these results suggest that KLF4 is a direct target of TFCP2L1 and that the function of the latter in hESC self-renewal is largely dependent on KLF4. It will be of great interest to investigate whether KLF4 regulates its own expression via TFCP2L1.
To verify that ESRRB is not essential for human TFCP2L1 to promote hESC self-renewal and that Klf4 is dispensable for mouse Tfcp2l1 to support mESC stemness, we repressed human ESRRB in PB-hTfcp2l1 hESCs (Fig. S3A) and decreased mouse Klf4 in PB-mTfcp2l1 mESCs (Fig. S4, A and B), respectively. As expected, all of these ESCs remained undifferentiated (Figs. S3, B and C, and S4, C and D). These observations further demonstrate that the molecular mechanism by which Tfcp2l1 promotes self-renewal is different in naïve and primed pluripotency states.
Discussion
Tfcp2l1 plays an important role in maintaining mouse and human ESC identity. Although many modulators that regulate Tfcp2l1 expression have been explored, such as the LIF/STAT3 signaling pathway (16, 17), Wnt/β-catenin signaling pathway (17, 23, 28), JMJD1A (30) and Oct4 (31), it remains unclear how Tfcp2l1 safeguards ESC pluripotency. It may act as either an activator or as a repressor of transcription in ESCs. To resolve these issues, we sought to determine the direct targets of Tfcp2l1 and identified the Esrrb and Klf4 transcription factors. Surprisingly, our findings suggest that Tfcp2l1 preferentially activates Esrrb to reinforce the pluripotent status of mESCs, whereas Tfcp2l1 largely depends on Klf4 to promote hESC self-renewal (Fig. 5). To the best of our knowledge, this may be the first report demonstrating that one transcription factor (Tfcp2l1) maintains the undifferentiated state of naïve and primed pluripotency through induction of different downstream targets.
Figure 5.
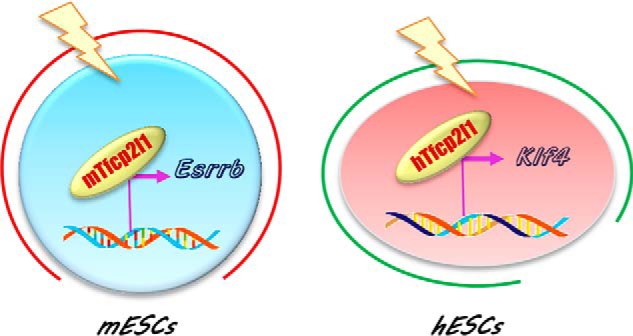
Diagram of the Tfcp2l1 function in mESC and hESC maintenance. Tfp2l1 functions differently in naïve and primed state ESCs. Specifically, Tfcp2l1 supports mESC stemness mainly though induction of Esrrb, whereas it promotes hESC self-renewal largely via up-regulation of Klf4.
Although we previously reported that pluripotency gene Nanog is important for Tfcp2l1 to promote mESC self-renewal, it is unclear whether Nanog is directly controlled by Tfcp2l1 (17). In this study, we determined that Esrrb is a direct target of Tfcp2l1 and could mediate the influence of Tfcp2l1 on maintaining and inducing naïve pluripotency (Figs. 1–3). This is unsurprising, given that both Tfcp2l1 and Esrrb share many similar and associated features in mESCs. First, both genes are specific markers of pluripotency that are negatively regulated by the GSK3/TCF3 axis, and overexpression of either phenocopies the GSK3 inhibitor effect if supporting mESC pluripotency (17, 23, 28). Second, their expression levels both are dramatically reduced upon mESC differentiation into EpiSCs (16, 17, 32). Thus, overexpression of either factor not only renders mESCs independent of LIF but also reprograms EpiSCs to enter a naïve pluripotency state (16, 17, 32, 33). Third, LIF/STAT3 signaling is necessary for the up-regulation of Esrrb expression induced by Nanog and the GSK3 inhibitor in partially reprogrammed cells (pre-iPSCs) (34), although Esrrb was shown to be a direct downstream target of Nanog and Wnt/β-catenin signaling in mESCs (28, 32). Importantly, overexpression of Esrrb can resume the reprogramming halted by inhibition of LIF/STAT3 signaling (34). Esrrb thus serves as an LIF activity–dependent downstream effector for the establishment of complete pluripotency establishment (34). Nevertheless, Esrrb is not a direct target of STAT3 (16, 17, 33). Tfcp2l1, as one of the most important direct targets of STAT3 (16, 17), may bridge the indirect interactions between the LIF/STAT3 pathway and Esrrb. Of note, LIF/serum conditions allow self-renewal of Esrrb-null mESCs (28, 32), whereas other groups found that knockdown of Esrrb impairs LIF-mediated mESC self-renewal (35, 36). These inconsistencies may be due to the cross-compensatory functions between Esrrb and other genes and may be triggered by complete loss but not down-regulation of Esrrb. For example, Esrrg can replace Esrrb to induce the generation of iPSCs (37). It will be of great interest to identify the gene redundant with Esrrb in Esrrb-null mESCs.
In disagreement with the role in mESCs, elevated expression of TFCP2L1 does not induce ESRRB expression in human ESCs (Fig. 4A). We further explored this and demonstrated that KLF4 is a direct target of TFCP2L1, and it can mediate TFCP2L1-induced self-renewal in hESCs (Fig. 4, E–I). In fact, great progress has been made around the roles of Klf4 and Tfcp2l1 in mESCs, but little was known about their roles in hESCs until now. In mESCs, both genes are direct targets of the LIF/STAT3 signaling pathway and up-regulate Nanog expression (16, 17, 38). Meanwhile, they maintain and induce naïve pluripotency (16, 17, 39). In hESCs, the KLF4 transcript level was markedly reduced upon hESC differentiation, which is similar to TFCP2L1 (22, 40). Elevated expression of KLF4 transcription inhibits neural differentiation of hESCs and enhances pluripotency marker expression (41). On the other hand, KLF4 is a Yamanaka factor that can induce iPSCs from human fibroblasts (42). All of these data highlight an important characteristic of KLF4 in hESCs and imply that the interaction between TFCP2L1 and KLF4 is likely a key contribution of Tfcp2l1 to promote hESC self-renewal. Interestingly, in mESCs, Klf4 has overlapping functions with Klf2 and Klf5 in LIF/serum medium (43, 44). However, in hESCs, we found that knockdown of KLF4 is enough to trigger differentiation in TFCP2L1-overexpressing hESCs (Fig. 4, G–I). These may be caused by the different culture conditions. In addition, KLF proteins exhibit unequal potency (44). For example, depletion of Klf2 is sufficient to abolish mESC self-renewal in 2i conditions (45). Furthermore, no single Klf is sufficient to restore self-renewal of triple-knockout mESCs (44). Further studies are necessary to address the detailed mechanism of TFCP2L1 and KLF4 in maintaining hESC pluripotency.
In summary, we found two distinct transcription factors downstream of Tfcp2l1 that are functionally important for the self-renewal of mouse and human ESCs. These results provide new insights into the fully understanding of the regulatory circuitry that maintains naïve and primed pluripotency and will help to derive ESCs from different species and ultimately to safely use ESCs for future translational applications.
Experimental procedures
Cell culture
For maintenance of mESCs, 46C mESCs (46), provided by Qi-Long Ying (University of Southern California), were cultured in 0.1% gelatin-coated dishes at 37 °C in 5% carbon dioxide. The medium used for routine maintenance was Dulbecco's modified Eagle's medium (TransGen Biotech) supplemented with 10% fetal bovine serum (FND500, ExCell Bio), 1× minimal essential medium nonessential amino acids (11140-050, Invitrogen), 2 mm GlutaMAX (21051024, Invitrogen), 0.1 mm β-mercaptoethanol (M3148, Sigma), and 1000 units/ml LIF (LIF1010, Millipore). For serum-free culture, mESCs were maintained in N2B27 medium (11) supplemented with PD0325901 (1 μm, Sigma) and CHIR99021 (3 μm, Sigma).
For maintenance of CD1 mouse EpiSCs and HES2 hESCs, EpiSCs were seed in 0.1% gelatin-coated dishes, whereas hESCs were cultured on plates precoated with Matrigel (BD Biosciences). hESCs were cultured in N2B27 medium supplemented with 10% KSR (Invitrogen), activin A (10 ng/ml; 120–14E, Peprotech), bFGF (10 ng/ml; AF-100–18B, Peprotech), and 4 μm IWR1 (I0161, Sigma). EpiSCs were cultured in mESC medium supplemented with Activin A, bFGF and IWR1. Y27632 (1 μm; Tocris) was added when they were passaged. For passaging, the cells were dissociated with a calcium trypsin KSR solution every 3–5 days (47, 48).
Plasmid construction
The coding regions of the Tfcp2l1, Esrrb, and Klf4 genes were inserted into the BglII and XhoI sites of the PiggyBac transposon vectors carrying a Flag or HA tag. The shRNA-expressing plasmids were constructed according to the pLKO.1-TRC protocol (Addgene plasmid no. 10878). The target-specific shRNA sequences for Tfcp2l1, Esrrb, and Klf4 were inserted into the AgeI and EcoRI sites. The shRNA sequences are as follows: mEsrrb sh#1, CGATTCATGAAATGCCTCAAA; mEsrrb sh#2, GCCGAGGACTATATCATGGAT; hKlf4 sh#1: GCCAGAATTGGACCCGGTGTA; and hKlf4 sh#2, GCCTTACACATGAAGAGGCAT. Details of the mouse and human Tfcp2l1 shRNA vectors have been reported in our previous studies (17, 22). The target specific shRNA sequences for Tfcp2l1, Esrrb, and Klf4 used in this study are listed in Table S1.
Western blotting analysis
Western blotting was performed according to a standard protocol. Briefly, the cells were lysed in radioimmune precipitation assay buffer (P0013B, Beyotime Biotechnology, China). The protein concentrations of the samples were estimated with a BCA kit (PA115, Tiangen Biotech), and then the samples were separated on 10% PAGE gels and electrotransferred onto polyvinylidene difluoride membranes. Probing was performed with specific primary antibodies and horseradish peroxidase-conjugated secondary antibodies. We used the following primary antibodies: Flag (F1804, Sigma, 1:2000), HA (3724S, Cell Signaling Technology, 1:2000), and α-tubulin (32-2500, Invitrogen, 1:5000). Signals were detected using a Pro-Light horseradish peroxidase kit (PA112, Tiangen Biotech).
qRT-PCR
Total RNA was extracted using the TRIzol Up Plus RNA kit (ER501-01, TransGen Biotech). cDNA was synthesized from 1 μg of total RNA using the TransScript all-in-one first-strand cDNA synthesis SuperMix for qPCR (One-Step gDNA removal, AT341-02, TransGen Biotech) according to the manufacturer's instructions. qRT-PCR was carried out with Top Green qPCR SuperMix (AQ131-04, TransGen Biotech) in a PikoReal real-time PCR machine (Thermo Scientific). The relative expression level was determined by the 2-ΔCq method and normalized to β-actin expression. The primers used are listed in Table S2.
Immunostaining
Immunostaining was performed via standard protocols. Briefly, the cells were fixed in 4% paraformaldehyde for 20 min and incubated at 37 °C in blocking buffer (PBS containing 5% BSA and 0.2% Triton X-100). The cells were then incubated with the Oct4 primary antibody at 4 °C overnight. After three times washes with PBS, the cells were then incubated with secondary antibody for 1 h at 37 °C. The nuclei were stained with Hoechst 33342 (H3570, Invitrogen, 1:10,000) for 30 s.
AP activity assay
The cells were fixed in 4% paraformaldehyde for 2 min at room temperature, washed in PBS, and incubated in AP staining reagent (85L2-1KT, Sigma) for 30 min at room temperature in the darkroom. After two washes with PBS, the cells were visualized under a Leica DMI8 microscope.
EpiSC reprogramming
A total of 1×105 transfectants were plated in 0.1% gelatin-coated 6-well plates and cultured in mESC medium supplemented with LIF and 2i for 12 days. Then the number of AP-positive clones generated from the CD1 EpiSCs was counted under a Leica DMI8 microscope.
ChIP assay
ChIP was performed using the ChIP assay kit (P2078; Beyotime Biotechnology) according to the manufacturer's protocol. Anti-FLAG antibody (F1804, Sigma) was used for immunoprecipitation, and IgG was used as a control antibody. ChIP enrichment was determined by qRT-PCR. The primer sequences and locations within the Esrrb and Klf4 genes are listed in Tables S3 and S4, respectively.
Luciferase assay
The promoter regions of Esrrb (from −1450 to −1201) and Klf4 (from −1250 to −1051) were cloned into pGL3-base plasmid; the new constructs were named pGL3-Esrrb and pGL3-Klf4, respectively. The primers sequences are listed in Tables S5 and S6. They were co-transfected into 46C mESCs or hESCs, along with the Tfcp2l1-overexpressed construct, or empty vector, with a Renilla luciferase plasmid. After 48 h, luciferase activities were measured with the dual luciferase assay kit. (FR201, TransGen Biotech).
Co-immunoprecipitation
Cell extracts were prepared using Nonidet P-40 lysis buffer (50 mm Tris-HCl, 150 mm NaCl, 0.5% Nonidet P-40, and protease inhibitors). The supernatant was collected and incubated with 10 μl of anti-FLAG affinity gel (SG4110-16, GNI, Tokyo, Japan) for 2 h at 4 °C. The beads were then washed six times with lysis buffer and resuspended in 100 μl of 1× SDS sample buffer for Western blotting analysis.
Accession number
RNA-seq data have been deposited to the NCBI Gene Expression Omnibus under the accession number GSE115075.
Statistical analysis
All data are reported as the means ± S.D. Student's t test was used to determine the significance of differences using GraphPad Prism 6. p < 0.05 was considered statistically significant. Sample size and p values have been included in the figure legends.
Author contributions
Xiaohu Wang, S. Z., and H. S. investigation; Xiaohu Wang, Xiaoxiao Wang, S. Z., and H. D. methodology; Xiaoxiao Wang and Y. Y. formal analysis; H. S. and S.L. validation; X. Z. resources; S.-D. Y. project administration.
Supplementary Material
This work was supported by Grant 31671535 from the Natural Science Foundation of China; Grant J01006068 from the Scientific Research Startup Fund of Anhui University; Grant S01003106 from the Open Fund for Discipline Construction, Institute of Physical Science and Information Technology, Anhui University; and Grant KYXL2017034 from the Undergraduate Research Training Program of Anhui University. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Tables S1–S6 and Figs. S1–S4.
The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE115075.
- ESC
- embryonic stem cell
- LIF
- leukemia inhibitory factor
- STAT3
- signal transducer and activator of transcription 3
- Esrrb
- estrogen-related receptor β
- Tfcp2l1
- transcription factor CP2-like 1
- Klf
- Kruppel-like factor
- PB
- PiggyBac system
- AP
- alkaline phosphatase
- qRT-PCR
- quantitative real-time PCR
- shRNA
- short hairpin RNA
- mESC
- mouse ESC
- hESC
- human ESC
- EpiSC
- epibiast stem cell
- GSK
- glycogen synthase kinase
- MEK
- mitogen-activated protein kinase kinase
- bFGF
- basic fibroblast growth factor
- Dox
- doxycycline.
References
- 1. Martin G. R. (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. U.S.A. 78, 7634–7638 10.1073/pnas.78.12.7634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans M. J., and Kaufman M. H. (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 10.1038/292154a0 [DOI] [PubMed] [Google Scholar]
- 3. Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., and Jones J. M. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- 4. Smith A. G. (2001) Embryo-derived stem cells: of mice and men. Annu. Rev. Cell Dev. Biol. 17, 435–462 10.1146/annurev.cellbio.17.1.435 [DOI] [PubMed] [Google Scholar]
- 5. Buehr M., Meek S., Blair K., Yang J., Ure J., Silva J., McLay R., Hall J., Ying Q. L., and Smith A. (2008) Capture of authentic embryonic stem cells from rat blastocysts. Cell 135, 1287–1298 10.1016/j.cell.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 6. Li P., Tong C., Mehrian-Shai R., Jia L., Wu N., Yan Y., Maxson R. E., Schulze E. N., Song H., Hsieh C. L., Pera M. F., and Ying Q. L. (2008) Germline competent embryonic stem cells derived from rat blastocysts. Cell 135, 1299–1310 10.1016/j.cell.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tesar P. J., Chenoweth J. G., Brook F. A., Davies T. J., Evans E. P., Mack D. L., Gardner R. L., and McKay R. D. (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 10.1038/nature05972 [DOI] [PubMed] [Google Scholar]
- 8. Brons I. G., Smithers L. E., Trotter M. W., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S. M., Howlett S. K., Clarkson A., Ahrlund-Richter L., Pedersen R. A., and Vallier L. (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191–195 10.1038/nature05950 [DOI] [PubMed] [Google Scholar]
- 9. Smith A. G., Heath J. K., Donaldson D. D., Wong G. G., Moreau J., Stahl M., and Rogers D. (1988) Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 336, 688–690 10.1038/336688a0 [DOI] [PubMed] [Google Scholar]
- 10. Williams R. L., Hilton D. J., Pease S., Willson T. A., Stewart C. L., Gearing D. P., Wagner E. F., Metcalf D., Nicola N. A., and Gough N. M. (1988) Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 336, 684–687 10.1038/336684a0 [DOI] [PubMed] [Google Scholar]
- 11. Ying Q. L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., and Smith A. (2008) The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 10.1038/nature06968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niwa H., Burdon T., Chambers I., and Smith A. (1998) Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 12, 2048–2060 10.1101/gad.12.13.2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davidson K. C., Adams A. M., Goodson J. M., McDonald C. E., Potter J. C., Berndt J. D., Biechele T. L., Taylor R. J., and Moon R. T. (2012) Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc. Natl. Acad. Sci. U.S.A. 109, 4485–4490 10.1073/pnas.1118777109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim H., Wu J., Ye S., Tai C. I., Zhou X., Yan H., Li P., Pera M., and Ying Q. L. (2013) Modulation of β-catenin function maintains mouse epiblast stem cell and human embryonic stem cell self-renewal. Nat. Commun. 4, 2403 10.1038/ncomms3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang G., Ye S., Zhou X., Liu D., and Ying Q. L. (2015) Molecular basis of embryonic stem cell self-renewal: from signaling pathways to pluripotency network. Cell Mol. Life Sci. 72, 1741–1757 10.1007/s00018-015-1833-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martello G., Bertone P., and Smith A. (2013) Identification of the missing pluripotency mediator downstream of leukaemia inhibitory factor. EMBO J. 32, 2561–2574 10.1038/emboj.2013.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ye S., Li P., Tong C., and Ying Q. L. (2013) Embryonic stem cell self-renewal pathways converge on the transcription factor Tfcp2l1. EMBO J. 32, 2548–2560 10.1038/emboj.2013.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takashima Y., Guo G., Loos R., Nichols J., Ficz G., Krueger F., Oxley D., Santos F., Clarke J., Mansfield W., Reik W., Bertone P., and Smith A. (2014) Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 158, 1254–1269 10.1016/j.cell.2014.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo G., von Meyenn F., Santos F., Chen Y., Reik W., Bertone P., Smith A., and Nichols J. (2016) Naive pluripotent stem cells derived directly from isolated cells of the human inner cell mass. Stem Cell Reports 6, 437–446 10.1016/j.stemcr.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo G., von Meyenn F., Rostovskaya M., Clarke J., Dietmann S., Baker D., Sahakyan A., Myers S., Bertone P., Reik W., Plath K., and Smith A. (2017) Epigenetic resetting of human pluripotency. Development 144, 2748–2763 10.1242/dev.146811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Leary T., Heindryckx B., Lierman S., van Bruggen D., Goeman J. J., Vandewoestyne M., Deforce D., de Sousa Lopes S. M., and De Sutter P. (2012) Tracking the progression of the human inner cell mass during embryonic stem cell derivation. Nat. Biotechnol. 30, 278–282 10.1038/nbt.2135 [DOI] [PubMed] [Google Scholar]
- 22. Sun H., You Y., Guo M., Wang X., Zhang Y., and Ye S. (2018) Tfcp2l1 safeguards the maintenance of human embryonic stem cell self-renewal. J. Cell Physiol. 233, 6944–6951 10.1002/jcp.26483 [DOI] [PubMed] [Google Scholar]
- 23. Qiu D., Ye S., Ruiz B., Zhou X., Liu D., Zhang Q., and Ying Q. L. (2015) Klf2 and Tfcp2l1, two Wnt/β-catenin targets, act synergistically to induce and maintain naive pluripotency. Stem Cell Reports 5, 314–322 10.1016/j.stemcr.2015.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J., Xie G., Singh M., Ghanbarian A. T., Raskó T., Szvetnik A., Cai H., Besser D., Prigione A., Fuchs N. V., Schumann G. G., Chen W., Lorincz M. C., Ivics Z., Hurst L. D., et al. (2014) Primate-specific endogenous retrovirus-driven transcription defines naive-like stem cells. Nature 516, 405–409 10.1038/nature13804 [DOI] [PubMed] [Google Scholar]
- 25. Liu K., Zhang Y., Liu D., Ying Q. L., and Ye S. (2017) TFCP2L1 represses multiple lineage commitment of mouse embryonic stem cells through MTA1 and LEF1. J. Cell Sci. 130, 3809–3817 10.1242/jcs.206532 [DOI] [PubMed] [Google Scholar]
- 26. Dunn S. J., Martello G., Yordanov B., Emmott S., and Smith A. G. (2014) Defining an essential transcription factor program for naive pluripotency. Science 344, 1156–1160 10.1126/science.1248882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen X., Xu H., Yuan P., Fang F., Huss M., Vega V. B., Wong E., Orlov Y. L., Zhang W., Jiang J., Loh Y. H., Yeo H. C., Yeo Z. X., Narang V., Govindarajan K. R., et al. (2008) Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133, 1106–1117 10.1016/j.cell.2008.04.043 [DOI] [PubMed] [Google Scholar]
- 28. Martello G., Sugimoto T., Diamanti E., Joshi A., Hannah R., Ohtsuka S., Göttgens B., Niwa H., and Smith A. (2012) Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell 11, 491–504 10.1016/j.stem.2012.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van den Berg D. L., Snoek T., Mullin N. P., Yates A., Bezstarosti K., Demmers J., Chambers I., and Poot R. A. (2010) An Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell 6, 369–381 10.1016/j.stem.2010.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loh Y. H., Zhang W., Chen X., George J., and Ng H. H. (2007) Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev. 21, 2545–2557 10.1101/gad.1588207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kelly K. F., Ng D. Y., Jayakumaran G., Wood G. A., Koide H., and Doble B. W. (2011) β-Catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell 8, 214–227 10.1016/j.stem.2010.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Festuccia N., Osorno R., Halbritter F., Karwacki-Neisius V., Navarro P., Colby D., Wong F., Yates A., Tomlinson S. R., and Chambers I. (2012) Esrrb is a direct Nanog target gene that can substitute for Nanog function in pluripotent cells. Cell Stem Cell 11, 477–490 10.1016/j.stem.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang X., Zhang J., Wang T., Esteban M. A., and Pei D. (2008) Esrrb activates Oct4 transcription and sustains self-renewal and pluripotency in embryonic stem cells. J. Biol. Chem. 283, 35825–35833 10.1074/jbc.M803481200 [DOI] [PubMed] [Google Scholar]
- 34. Huang D., Wang L., Duan J., Huang C., Tian X. C., Zhang M., and Tang Y. (2018) LIF-activated Jak signaling determines Esrrb expression during late-stage reprogramming. Biol. Open 7, pii: bio029264 10.1242/bio.029264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ivanova N., Dobrin R., Lu R., Kotenko I., Levorse J., DeCoste C., Schafer X., Lun Y., and Lemischka I. R. (2006) Dissecting self-renewal in stem cells with RNA interference. Nature 442, 533–538 10.1038/nature04915 [DOI] [PubMed] [Google Scholar]
- 36. Loh Y. H., Wu Q., Chew J. L., Vega V. B., Zhang W., Chen X., Bourque G., George J., Leong B., Liu J., Wong K. Y., Sung K. W., Lee C. W., Zhao X. D., Chiu K. P., et al. (2006) The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 38, 431–440 10.1038/ng1760 [DOI] [PubMed] [Google Scholar]
- 37. Feng B., Jiang J., Kraus P., Ng J. H., Heng J. C., Chan Y. S., Yaw L. P., Zhang W., Loh Y. H., Han J., Vega V. B., Cacheux-Rataboul V., Lim B., Lufkin T., and Ng H. H. (2009) Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nat. Cell Biol. 11, 197–203 10.1038/ncb1827 [DOI] [PubMed] [Google Scholar]
- 38. Zhang P., Andrianakos R., Yang Y., Liu C., and Lu W. (2010) Kruppel-like factor 4 (Klf4) prevents embryonic stem (ES) cell differentiation by regulating Nanog gene expression. J. Biol. Chem. 285, 9180–9189 10.1074/jbc.M109.077958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo G., Yang J., Nichols J., Hall J. S., Eyres I., Mansfield W., and Smith A. (2009) Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136, 1063–1069 10.1242/dev.030957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chan K. K., Zhang J., Chia N. Y., Chan Y. S., Sim H. S., Tan K. S., Oh S. K., Ng H. H., and Choo A. B. (2009) KLF4 and PBX1 directly regulate NANOG expression in human embryonic stem cells. Stem Cells 27, 2114–2125 10.1002/stem.143 [DOI] [PubMed] [Google Scholar]
- 41. Kim H., Lee G., Ganat Y., Papapetrou E. P., Lipchina I., Socci N. D., Sadelain M., and Studer L. (2011) miR-371–3 expression predicts neural differentiation propensity in human pluripotent stem cells. Cell Stem Cell 8, 695–706 10.1016/j.stem.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 42. Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., and Yamanaka S. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- 43. Jiang J., Chan Y. S., Loh Y. H., Cai J., Tong G. Q., Lim C. A., Robson P., Zhong S., and Ng H. H. (2008) A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat. Cell Biol. 10, 353–360 10.1038/ncb1698 [DOI] [PubMed] [Google Scholar]
- 44. Yamane M., Ohtsuka S., Matsuura K., Nakamura A., and Niwa H. (2018) Overlapping functions of Kruppel-like factor family members: targeting multiple transcription factors to maintain the naive pluripotency of mouse embryonic stem cells. Development 145, pii: dev162404 10.1242/dev.162404 [DOI] [PubMed] [Google Scholar]
- 45. Yeo J. C., Jiang J., Tan Z. Y., Yim G. R., Ng J. H., Göke J., Kraus P., Liang H., Gonzales K. A., Chong H. C., Tan C. P., Lim Y. S., Tan N. S., Lufkin T., and Ng H. H. (2014) Klf2 is an essential factor that sustains ground state pluripotency. Cell Stem Cell 14, 864–872 10.1016/j.stem.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 46. Ying Q. L., Stavridis M., Griffiths D., Li M., and Smith A. (2003) Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 21, 183–186 10.1038/nbt780 [DOI] [PubMed] [Google Scholar]
- 47. Sun H., Wang X., Liu K., Guo M., Zhang Y., Ying Q. L., and Ye S. (2017) β-Catenin coordinates with Jup and the TCF1/GATA6 axis to regulate human embryonic stem cell fate. Dev. Biol. 431, 272–281 10.1016/j.ydbio.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 48. Hasegawa K., Fujioka T., Nakamura Y., Nakatsuji N., and Suemori H. (2006) A method for the selection of human embryonic stem cell sublines with high replating efficiency after single-cell dissociation. Stem Cells 24, 2649–2660 10.1634/stemcells.2005-0657 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.