Abstract
Objective
Leg length discrepancy (LLD) could be a predisposing factor for early degeneration of lumbar intervertebral discs (IVD). The purpose of this study was to elucidate the molecular effect of LLD on IVDs.
Methods
IVDs of Eleven patients (25.6 ± 4.3years) with LLD (>10 mm) and 14 control subjects (23.9 ± 3.5years) were compared using a 3T-MR scanner. Morphological T2-weighted and glycosaminoglycan-chemical-exchange-saturation-transfer (gagCEST) sequences were performed.
Results
No differences in morphological Pfirrmann grading were found (p > 0.05). In contrast, nucleus-pulposus-gagCEST-values of L5/S1 were significantly lower (p = 0.0008).
Conclusion
Our results suggest that LLD is a predisposing factor for molecular IVD alterations, which are detectable even before morphological pathologies could be found.
Keywords: Leg length discrepancy, Intervertebral disc degeneration, Preventive medicine, Glycosaminoglycan (GAG), Magnetic resonance imaging (MRI), Chemical exchange saturation transfer (CEST)
1. Introduction
Leg length discrepancy (LLD) is a frequent finding during orthopedic physical examination. LLD can be caused by a disproportion of bony structures or altered mechanics of the lower extremities due to muscle tightness or contractures. Mostly, LLD is a cause of structural and functional spine changes.1 In daily clinical practice, only physical examination is frequently used to determine the clinical LLD, and only in severe cases, radiological examination is mandatory.2,3 Recent studies suggest that chronic low back pain (LBP) correlates with LLD.4,5 However, LLD can be found in about two-thirds of the population without physical complaints, and the amount of LLD that has to be treated is still discussed controversially.6,7 LLD leads to pelvic obliquity and a lateral tilt of the lumbar spine; therefore, it could be responsible for asymmetric loading of the intervertebral discs (IVDs) of the lower back and functional changes in the facet joints.8 Asymmetric loading of the IVD causes higher tension on the convex side and higher compression on the concave side.9 This functional change could become structural over time.10 Some studies propose that LLD could be a predisposing factor for lumbar disc herniation.11 A well-established and sensitive method for detecting morphological IVD changes is magnetic resonance imaging (MRI).12 Glycosaminoglycan chemical exchange saturation transfer (gagCEST) imaging is a noninvasive tool to assess the matrix components of IVDs, glycosaminoglycans (GAGs).13 Previous findings suggest, that a decrease of GAG content correlates with degeneration of the lumbar discs.14 Thus, with gagCEST imaging, it could be possible to detect early molecular changes in the lumbar discs in patients with LLD, before they could be visible in standard MRI. Therefore, the purpose of this prospective study was to elucidate the effect of LLD greater than 10 mm on the GAG content of lumbar discs.
2. Materials and methods
2.1. Subjects
Patients were screened for LLD prospectively for six months in the Department of Trauma and Hand Surgery. Eleven patients aged from 20 to 33 years (25.6 ± 4.3 years, five female, six male) with LLD greater than 10 mm were compared with 14 control subjects aged from 21 to 30 years (23.9 ± 3.5 years, seven female, seven male) without LLD. Only subjects without a history of trauma or musculoskeletal diseases of the lower back, hips, or lower limbs were included. In order to identify possible exclusion criteria and to match control subjects and patients according to risk factors for lumbar disc herniation15, 16, 17 (age, gender, height, weight and body mass index (BMI)), a customized questionnaire and written informed consent from each subject was obtained prior to the procedure. After checking for any excluding criteria (musculoskeletal or systemic diseases, chronic drug or alcohol abuse, or treatment of LLD from other health professionals), physical examination was followed by MRI. This study was approved by the institutional review board (3980).
2.2. Physical examination
All patients had the initial diagnosis of LLD based on physical examinations and a referral from an orthopedic specialist with 30 years of experience. One single experienced orthopedic surgeon (six years of experience), who was blinded to the initial diagnosis, examined each patient and each control subject. Two clinical methods were used for LLD measurement: 1) an indirect method visualizing the pelvic level using a spirit level (Beckenwasserwaage, Schein Orthopädie Service KG, Remscheid, Germany), which was clipped on the anterior superior iliac spine (ASIS). The degree of LLD was quantified using small heel lifts under the shorter leg and 2) a direct method measuring from bony landmarks with measuring tape. In this study, the distance from the ASIS to the medial malleolus (MM) and the ASIS to the lateral malleolus (LM) were evaluated. Furthermore, to exclude an apparent LLD caused by an asymmetric hypoplastic iliac bone, the distance from the greater trochanter (GT) to MM and GT to LM were determined. To exclude measurement errors caused by contractures, the range of motion (ROM) of the cervical, thoracic, and lumbar spine and of the lower limbs (hip, knee, and upper/lower ankle joints), using a double-armed goniometer (Winkelmesser Goniometer, Kirchner & Wilhelm GmbH + Co. KG, Asperg, Germany), was obtained.3,18
2.3. Magnetic resonance imaging, T2-weighted and gagCEST
All participants were examined with a whole body 3T MR system (Magnetom Trio, A Tim System, Siemens Healthineers, Forchheim, Germany) in supine position. For signal reception, four channel body matrix coils and a 24 channel spine matrix coil were used. The protocol included a localizer, T2-weighted imaging in sagittal and transversal orientation.
Biochemical imaging was performed with a novel gagCEST sequence, chemical exchange saturation transfer imaging using the spin-lock technique (CESL), and the WASABI (water saturation and B1) method for B0 and B1 field inhomogeneity correction.19 For gagCEST imaging, several images were acquired with presaturation pulses at different offset frequencies around the bulk water resonance and one reference image without saturation. The spin-lock saturation block was comprised of three radiofrequency (RF) pulses. The first RF pulse flips the magnetization away from the direction of the main magnetic field to the effective field at a specific frequency offset. The second RF pulse is a rectangular pulse, during which the chemical exchange saturation transfer process takes place. The third RF pulse flips the magnetization back to the direction of the main magnetic field. In this study, the presaturation module of CESL was comprised of 10 Gaussian or spin-lock pulses with a pulse duration of 100 ms, B1 amplitude of 1.0, 1.5, and 2.0 μT and a duty cycle of 50%. The entire Z-spectrum was acquired with 33 frequency offsets in intervals of 0.3 ppm from −4.8–4.8 ppm. In addition, one reference scan with a frequency offset of −300 ppm was acquired for CESL-spectrum normalization. The WASABI-Z-spectrum was obtained using 49 frequency offsets in a frequency range from −2.4–2.4 ppm with one rectangular-shaped RF pulse (B1 = 4 μT and PD = 5 ms) for the presaturation module.20,21
Table 1 and Table 2 gives detailed information about the sequence parameters. To suppress artifacts caused by the abdominal wall or movement of the bowels, a saturation band was applied anterior to the spine.
Table 1.
Detailed sequence parameters of T2-weighted images.
| CEST | WASABI | ||
|---|---|---|---|
| TR/TE | [ms]/[ms] | 14/3.64 | 14/3.64 |
| Field of view | [mm2] | 300 × 300 | 300 × 300 |
| In-plane resolution | [mm2] | 2.3 × 2.3 | 2.3 × 2.3 |
| Slice thickness | [mm] | 5 | 5 |
| Flip angle | [°] | 10 | 10 |
| Averages | 1 | 1 | |
| Basic resolution | 128 × 128 | 128 × 128 | |
| Number of slices | 1 | 1 | |
| Acquisition duration | [min:sec] | 9:51 | 3:10 |
Table 2.
Detailed sequence parameters for spin-lock CEST (three pulses with B1 amplitude of 1.0, 1.5, and 2.0 μT) and B0-/B1-field inhomogeneity correction (WASABI).
| T2-weighted imaging (sagittal) | T2-weighted imaging (transversal) | |
|---|---|---|
| Sequence type | Turbo spin echo | Turbo spin echo |
| Turbo factor | 31 | 18 |
| TR/TE [ms] | 3100/105 | 4510/113 |
| Field of View (FOV) [mm2] | 300 × 300 | 240 × 240 |
| In-pane resulution [mm2] | 1.2 × 1.2 | 0.8 × 0.6 |
| Slice thickness [mm] | 3.0 | 3.0 |
| Flip angle [°] | 160 | 140 |
| Averages | 2 | 1 |
| Basic resolution | 256 × 256 | 384 × 307 |
| Number of slices | 15 | 54 |
| Acquisition duration [min:sec] | 3:39 | 5:13 |
2.4. Data analysis
One board certified radiologist with six years of experience in musculoskeletal radiology, who was blinded to the gagCEST values, scored all lumbar IVDs according to the Pfirrmann scoring system.12 A region-of-interest (ROI) analysis was performed for SLRasym evaluation of the nucleus pulposus (NP) and annulus fibrosus (AF). All ROIs were selected by an in-house developed automatic image processing algorithm based on MATLAB software (The Mathworks, Inc., Natick, MA, R2012b).22 The disc segmentation to divide bone and ligament from disc tissue of the lumbar spine was based on Bayes classification. Every automatically positioned ROI was visually checked by one radiologist with six years of experience in IVD segmentation, blinded to Pfirrmann classification analysis and clinical information. None of the ROIs was repositioned. For data analysis, an in-house developed MATLAB software23 was generated. A reduction of image noise was performed using an in-plane 3 × 3 Gaussian filter. Z-spectra of the WASABI B0 and B1 maps were shifted pixel-wise according to the obtained frequency offset maps.23 SLRasym maps were calculated by averaging the asymmetry effect in the offset frequency range of GAG resonances (0.9–1.9 ppm).24 Pixels with an absolute value of SLRasym >15% were excluded from further analysis.
2.5. Statistical analysis
Statistical analysis was performed using MATLAB. The mean and standard deviations for risk factors for lumbar disc herniation15, 16, 17 (age, gender, height, and weight), physical examination, NP-gagCEST, and AF-gagCEST were calculated. Morphological IVD grading was illustrated according to Pfirrmann score.12 Kolmogorow-Smirnow-Lilliefors tests were used to assess normal distribution. Univariate analysis of variance (ANOVA) and Kruskal-Wallis tests were performed to assess statistical differences of the means of the gagCEST values of the different groups and subgroups. P values < 0.05 were assumed to be statistically significant.
3. Results
3.1. Subjects
Of 25 participants, no one had previously received treatment for LLD from other health professionals for at least one year. There were no significant differences with regard to typical risk factors for developing a lumbar disc herniation15, 16, 17 between patients with LLD and controls: age (patients with LLD: 25.6 ± 4.3 years vs. controls: 23.9 ± 3.5 years, p > 0.05), gender (patients with LLD: 5 female, 6 male vs. controls: 7 female, 7 male), height (patients with LLD: 177.0 ± 8.6 cm vs. controls: 174.5 ± 9.0 cm, p > 0.05), weight (patients with LLD: 73.6 ± 15.5 kg vs. controls: 67.9 ± 11.3 kg, p > 0.05) and BMI (patients with LLD: 23.3 ± 3.1 kg/m2 vs. controls: 22.2 ± 2.2 kg/m2, p > 0.05).
3.2. Physical examination
The average LLD was 12 mm (range 10–20 mm). No participant had contractures or a loss of ROM of the cervical, thoracic, or lumbar spine or of the lower limbs (hip, knee, or upper/lower ankle joints).
3.3. Magnetic resonance imaging, T2-weighted and gagCEST
Of 11 patients with LLD and 14 control subjects without LLD, 125 lumbar IVDs (L1–S1) were successfully imaged with biochemical imaging. Subjects with bulging or herniated discs were excluded. No IVD had to be excluded due to motion artifact.
Morphological IVD grading was performed according to the Pfirrmann12 classification. Of 125 IVDs, 37 IVDs were scored Pfirrmann grade 1, 86 IVDs Pfirrmann grade 2, and 2 IVDs Pfirrmann grade 3. No degenerated discs, Pfirrmann grade 4 and 5, were scored. In the level L1/2, three discs were scored Pfirrmann grade 1 and 22 discs grade 2. In level L2/3 and in level L3/4, eight discs were graded Pfirrmann score 1 and 17 IVDs Pfirrmann score 2. In level L4/5, eight IVDs were scored Pfirrmann grade 1, 15 discs Pfirrmann grade 2, and 2 IVDs Pfirrmann score 3. In level L5/S1, 10 IVDs were graded Pfirrmann score 1 and 15 discs Pfirrmann grade 2.
No significant differences in morphological Pfirrmann grading were found between LLD patients and healthy controls (p > 0.05).
The mean NP-gagCEST value of the L5/S1 IVD in patients with LLD greater than 10 mm was 1.57 ± 1.19, while for controls, it was 4.46 ± 2.24. NP-gagCEST values of L5/S1 IVDs in patients with LLD greater than 10 mm were significantly lower when compared to subjects without LLD (p = 0.0008; Fig. 1, Fig. 2, Fig. 3).
Fig. 1.

Comparison of NP-gagCEST values of L5/S1 IVD between patients with LLD >10 mm and control subjects.
Fig. 2.
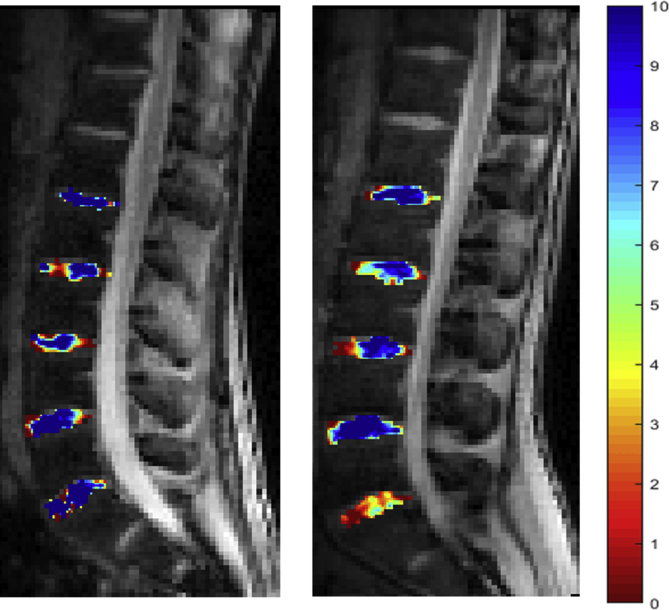
Color-coded gagCEST map with high GAG content in blue and low GAG content in red of the lumbar spine (L1–S1). On the left side, a participant without leg length discrepancy (LLD) with high GAG content (blue IVDs), and on the right side, a patient with LLD demonstrating molecular alteration of the lumbar disc on level L5/S1 with low GAG content in red and orange. Pfirrmann grading illustrates no morphological disc degeneration with scores of 1 or 2.
Fig. 3.
Color-coded gagCEST map with high GAG content in blue and low GAG content in red of the lumbar spine (L1–S1). Another example of a participant without LLD on the left side compared to a patient with LDD on the right side. On level L5/S1, the patient with LLD (right side) showed significantly lower GAG content compared to control participant. All discs revealed no morphological disc degeneration with Pfirrmann score 1 or 2 (not shown in this picture).
All other disc levels showed no significant difference between participants with LLD and controls without LLD (p > 0.05). Additionally, no significant difference between the two groups was found for AF.
4. Discussion
NP-gagCEST values of L5/S1 IVDs in patients with LLD were significantly lower compared to healthy subjects. In contrast, no morphologically significant differences could be found. Our results indicate that LLD correlates with molecular alterations of the L5/S1 lumbar disc in asymptomatic healthy young adults with MRI morphologically inconspicuous IVDs and facet joints. It is known that the rhythmic load on the spine supports the metabolism of the IVD,25 whereas a permanent static one-sided compressive load worsens the disc's nutrition.26 LLD leads to pelvic obliquity and a lateral tilt of the lumbar spine; therefore, it could be responsible for a static one-sided compressive load of the IVD.8,10 A poor nutrient supply to the IVD correlates with a loss of matrix production and matrix degradation and could lead to early disc degeneration.27,28 Young adults with uncorrected scoliosis are at higher risk of recurrent lumbar disc herniation.29 Additionally, there is evidence about a significant correlation of the side of radiculopathy to the shorter leg in patients with lumbar disc herniation and LLD.30 Our findings are in line with previous studies that suggest a loss of GAG in the NP before morphological IVD degeneration occurs24 and with increasing grade of morphological degeneration.13,31 Additionally, it is known that degeneration of the IVD occurs before degeneration of the facet joints.32 The assessment of IVD via gagCEST is a highly sensitive method to detect the early stages of disc degeneration.33 GAG loss is a central part of disc degeneration.34 Thus, with gagCEST, detection of early and potentially reversible degeneration of the IVD may be possible.35, 36, 37 The lower NP-gagCEST values of L5/S1 IVDs in patients with LLD could be interpreted as a predisposing factor for lumbar disc protrusion or herniation because of the direct correlation between the compression resistance of the IVD and the number of GAG molecules bound to the core protein38. So far, it is unknown if treatment of LLD, for example with shoe inserts, could prevent lumbar disc herniation or could change potentially reversible lower NP-gagCEST values. Noteworthy, IVD herniation of L5/S1 occurs at younger ages, whereas IVD herniation of L4/L5 and especially L3/L4 occur at older ages.39 Some studies suggest that degeneration of the lumbar spinal column progresses from caudal to rostral.32 Tayler et al. made the conclusion that the proteoglycans of L5/S1 turned over faster than the proteoglycans of the adjacent lumbar discs because of its proximity to the rigid segment of the sacrum.40 Arguably, this could be a reason why a compensation for LLD and pelvic obliquity is affecting L5/S1 first. However, there is no consensus regarding the amount of LLD that should be treated by physical therapy and shoe inserts.7 Some studies have shown that patients with LLD >25 mm functioned well athletically and without any complaints.41 In contrast, newer studies suggest that mild LLD (<20 mm) causes compensatory changes during gait. Furthermore, these authors pointed out that these compensatory changes during gait were unable to prevent the effects of mild LLD on pelvic obliquity.42 Harvey et al. demonstrated that LLD of 10 mm or more is associated with prevalent and symptomatic knee osteoarthritis in the shorter leg43. In line with that, Defrin et al. showed that shoe inserts can significantly reduce pain intensity and functional disability in patient with LLD <10 mm.44
This study has several limitations. One limitation is the limited number of participants. Nevertheless, the results of this study seem to be promising for further evaluation in a larger population. The second limitation is that LLD was determined only by clinical and not radiological examination. We minimized measurement errors by using two clinical methods for LLD assessment (indirect and direct methods). Furthermore, when using the direct method, the average value of two separate tape measurements were used.45 In daily clinical practice, physical examination is frequently used to determine the clinical LLD, and only in severe cases, radiological examination is mandatory. The tape measurement method has equal validity with radiological examination (Pearson product moment correlation 0.98, intertester reliability 0.99).2 Therefore, previous studies suggest that the physical exam may be more clinically relevant in daily routine than radiological examination.46 In this study, we tried to elucidate the effect of LLD on GAG content in lumbar IVDs as a predisposing factor for degeneration or even herniation. Therefore, a relatively homogeneous, young patient-collective was examined, since lumbar IVD herniations are uncommon in the first two decades of life, with a peak of prevalence in the fourth decade.47
For gagCEST and Pfirrmann classification no intra- and inter-observer agreement was performed. However, gagCEST analysis was performed automatically with an established segmentation algorithm, and Pfirrmann classification is known to enable excellent intra- and inter-reader agreement.22,23
5. Conclusion
This study supports the hypothesis that LLD could be a predisposing factor for early molecular alterations of the lumbar disc of L5/S1. Furthermore, lower gagCEST values of the lumbar disc of L5/S1 caused by LLD were observed before any morphological pathologies were detectable. So far, it is unknown if these molecular alterations of the lumbar disc of L5/S1 are reversible. The effect of shoe inserts and physical therapy on the gagCEST values of the lumbar discs of patients with LLD could be the basis for a prospective, long-term follow-up study.
Declarations
Ethics approval and consent to participate
There was a positive vote from the ethics committee of the Medical Faculty (3980).
Consent for publication
Written informed consent from each subject was obtained prior to the procedure.
Conflicts of interest
The authors declare that they have no conflict of interests.
Funding
Not applicable.
Authors’ contributions
All authors were major contributors concerning the management of the patients, review of the articles and manuscript preparation. All authors read and approved the final manuscript.
Acknowledgements
The last and 2nd-last author contributed equally.
Abbreviations
- LLD
Leg length discrepancy
- LBP
Low back pain
- IVD
Intervertebral disc
- MRI
Magnetic resonance imaging
- gagCEST
Glycosaminoglycan chemical exchange saturation transfer
- GAG
Glycosaminoglycan
- MM
Medial malleolus
- LM
Lateral malleolus
- GT
Greater trochanter
- ROM
Range of motion
- CEST
Chemical exchange saturation transfer
- CESL-
Chemical exchange imaging with spin-lock technique
- WASABI
Water saturation and B1
- RF
Radiofrequency
- NP-
Nucleus pulposus
- AF
Annulus fibrosus
- BMI
Body mass index
Contributor Information
David Latz, Email: David.Latz@med.uni-duesseldorf.de.
Miriam Frenken, Email: Miriam.Frenken@med.uni-duesseldorf.de.
Erik Schiffner, Email: Erik.Schiffner@med.uni-duesseldorf.de.
Maxime Knautz, Email: Maxime.knautz@uni-duesseldorf.de.
Wolfgang Alois Quante, Email: Waq@live.de.
Joachim Windolf, Email: Windolf@uni-duesseldorf.de.
Jan Peter Grassmann, Email: Jan.Grassmann@med.uni-duesseldorf.de.
Pascal Jungbluth, Email: Pascal.Jungbluth@med.uni-duesseldorf.de.
Christoph Schleich, Email: Christoph.Schleich@med.uni-duesseldorf.de.
References
- 1.Morscher E. Hungerford DS, Hrsg. Leg Length Discrepancy the Injured Knee. Springer Berlin Heidelberg; Berlin, Heidelberg: 1977. Etiology and pathophysiology of leg length discrepancies; pp. 9–19. [Google Scholar]
- 2.Gogia P.P., Braatz J.H. Validity and reliability of leg length measurements. J Orthop Sport Phys Ther. 1986;8:185–188. doi: 10.2519/jospt.1986.8.4.185. [DOI] [PubMed] [Google Scholar]
- 3.Thomann K.-D., Schröter F., Grosser V. Elsevier Health Sciences; 2013. Orthopädisch-unfallchirurgische Begutachtung: Praxis der klinischen Begutachtung. [Google Scholar]
- 4.Giles L., Taylor J. Low-back pain associated with leg length inequality. Spine. 1981;6:510–521. doi: 10.1097/00007632-198109000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Kendall J.C., Bird A.R., Azari M.F. Foot posture, leg length discrepancy and low back pain – their relationship and clinical management using foot orthoses – an overview. Foot. 2014;24:75–80. doi: 10.1016/j.foot.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Woerman A.L., Binder-Macleod S.A. Leg length discrepancy assessment: accuracy and precision in five clinical methods of evaluation. J Orthop Sport Phys Ther. 1984;5:230–239. doi: 10.2519/jospt.1984.5.5.230. [DOI] [PubMed] [Google Scholar]
- 7.Subotnick S.I. Limb length discrepancies of the lower extremity (the short leg syndrome) J Orthop Sport Phys Ther. 1981;3:11–16. doi: 10.2519/jospt.1981.3.1.11. [DOI] [PubMed] [Google Scholar]
- 8.Giles L.G., Singer K.P. Elsevier Health Sciences; 1997. Clinical Anatomy and Management of Low Back Pain. [Google Scholar]
- 9.Li Z., Zhang Y., Straumann L. Effects of asymmetric dynamic loading on intervertebral disc–towards a scoliosis mimicking organ culture model. Glob Spine J. 2016;6 s-0036-1582636-s-1580036-1582636. [Google Scholar]
- 10.Giles L.G., Taylor J.R. Lumbar spine structural changes associated with leg length inequality. Spine. 1982;7:159–162. doi: 10.1097/00007632-198203000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Balik M.S., Kanat A., Erkut A. Inequality in leg length is important for the understanding of the pathophysiology of lumbar disc herniation. J Craniovertebral Junction Spine. 2016;7:87–90. doi: 10.4103/0974-8237.181829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfirrmann C.W., Metzdorf A., Zanetti M. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 13.Haneder S., Apprich S.R., Schmitt B. Assessment of glycosaminoglycan content in intervertebral discs using chemical exchange saturation transfer at 3.0 Tesla: preliminary results in patients with low-back pain. Eur Radiol. 2013;23:861–868. doi: 10.1007/s00330-012-2660-6. [DOI] [PubMed] [Google Scholar]
- 14.Muller-Lutz A., Schleich C., Pentang G. Age-dependency of glycosaminoglycan content in lumbar discs: a 3t gagcEST study. J Magn Reson Imaging : JMRI. 2015;42:1517–1523. doi: 10.1002/jmri.24945. [DOI] [PubMed] [Google Scholar]
- 15.Ma D., Liang Y., Wang D. Trend of the incidence of lumbar disc herniation: decreasing with aging in the elderly. Clin Interv Aging. 2013;8:1047–1050. doi: 10.2147/CIA.S49698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y-g, Sun Z., Zhang Z. Risk factors for lumbar intervertebral disc herniation in Chinese population: a case-control study. Spine. 2009;34:E918–E922. doi: 10.1097/BRS.0b013e3181a3c2de. [DOI] [PubMed] [Google Scholar]
- 17.Ala-Kokko L. Genetic risk factors for lumbar disc disease. Ann Med. 2002;34:42–47. doi: 10.1080/078538902317338634. [DOI] [PubMed] [Google Scholar]
- 18.Konrads C., Plumhoff P. Klinische Tests und Untersuchung in Orthopädie und Unfallchirurgie. Springer; 2018. Neutral-0-Methode; pp. 175–181. [Google Scholar]
- 19.Schuenke P., Windschuh J., Roeloffs V. Simultaneous mapping of water shift and B1 (WASABI)—application to field‐Inhomogeneity correction of CEST MRI data. Magn Reson Med. 2017;77:571–580. doi: 10.1002/mrm.26133. [DOI] [PubMed] [Google Scholar]
- 20.Muller-Lutz A., Cronenberg T., Schleich C. Comparison of glycosaminoglycan chemical exchange saturation transfer using Gaussian-shaped and off-resonant spin-lock radiofrequency pulses in intervertebral disks. Magn Reson Med. 2017;78:280–284. doi: 10.1002/mrm.26362. [DOI] [PubMed] [Google Scholar]
- 21.Stabinska J., Cronenberg T., Wittsack H.-J. Quantitative pulsed CEST-MRI at a clinical 3T MRI system. Magnetic Resonance Materials in Physics, Biology and Medicine. 2017;30:505–516. doi: 10.1007/s10334-017-0625-0. [DOI] [PubMed] [Google Scholar]
- 22.Schleich C., Müller-Lutz A., Eichner M. Glycosaminoglycan chemical exchange saturation transfer of lumbar intervertebral discs in healthy volunteers. Spine. 2016;41:146–152. doi: 10.1097/BRS.0000000000001144. [DOI] [PubMed] [Google Scholar]
- 23.Schleich C., Müller‐Lutz A., Matuschke F. Glycosaminoglycan chemical exchange saturation transfer of lumbar intervertebral discs in patients with spondyloarthritis. J Magn Reson Imaging. 2015;42:1057–1063. doi: 10.1002/jmri.24877. [DOI] [PubMed] [Google Scholar]
- 24.Schleich C., Muller-Lutz A., Blum K. Facet tropism and facet joint orientation: risk factors for the development of early biochemical alterations of lumbar intervertebral discs. Osteoarthritis Cartilage. 2016;24:1761–1768. doi: 10.1016/j.joca.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Q., Jackson A.R., Gu W.Y. Cell viability in intervertebral disc under various nutritional and dynamic loading conditions: 3d finite element analysis. J Biomech. 2012;45:2769–2777. doi: 10.1016/j.jbiomech.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams M.A., Hutton W.C. The effect of posture on the fluid content of lumbar intervertebral discs. Spine. 1983;8:665–671. doi: 10.1097/00007632-198309000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Urban J.P.G., Smith S., Fairbank J.C.T. Nutrition of the intervertebral disc. Spine. 2004;29:2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Q., Gao X., Levene H.B. Influences of nutrition supply and pathways on the degenerative patterns in human intervertebral disc. Spine. 2016;41:568–576. doi: 10.1097/BRS.0000000000001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang H.-K., Chang H.-C., Wu J.-C. Scoliosis may increase the risk of recurrence of lumbar disc herniation after microdiscectomy. J Neurosurg Spine. 2016;24:586–591. doi: 10.3171/2015.7.SPINE15133. [DOI] [PubMed] [Google Scholar]
- 30.ten Brinke A., van der Aa H.E., van der Palen J. Is leg length discrepancy associated with the side of radiating pain in patients with a lumbar herniated disc? Spine. 1999;24:684–686. doi: 10.1097/00007632-199904010-00013. [DOI] [PubMed] [Google Scholar]
- 31.Kim M., Chan Q., Anthony M.P. Assessment of glycosaminoglycan distribution in human lumbar intervertebral discs using chemical exchange saturation transfer at 3 T: feasibility and initial experience. NMR Biomed. 2011;24:1137–1144. doi: 10.1002/nbm.1671. [DOI] [PubMed] [Google Scholar]
- 32.Butler D., Trafimow J., Andersson G. Discs degenerate before facets. Spine. 1990;15:111–113. doi: 10.1097/00007632-199002000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Saar G., Zhang B., Ling W. Assessment of glycosaminoglycan concentration changes in the intervertebral disc via chemical exchange saturation transfer. NMR Biomed. 2012;25:255–261. doi: 10.1002/nbm.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urban J.P., Winlove C.P. Pathophysiology of the intervertebral disc and the challenges for MRI. J Magn Reson Imaging. 2007;25:419–432. doi: 10.1002/jmri.20874. [DOI] [PubMed] [Google Scholar]
- 35.Kalson N.S., Richardson S., Hoyland J.A. 2008. Strategies for Regeneration of the Intervertebral Disc. [DOI] [PubMed] [Google Scholar]
- 36.Ciavarro C., Caiani E.G., Brayda-Bruno M. Mid-term evaluation of the effects of dynamic neutralization system on lumbar intervertebral discs using quantitative molecular MR imaging. J Magn Reson Imaging. 2012;35:1145–1151. doi: 10.1002/jmri.23525. [DOI] [PubMed] [Google Scholar]
- 37.Vaga S., Raimondi M.T., Caiani E.G. Quantitative assessment of intervertebral disc glycosaminoglycan distribution by gadolinium‐enhanced MRI in orthopedic patients. Magn Reson Med. 2008;59:85–95. doi: 10.1002/mrm.21433. [DOI] [PubMed] [Google Scholar]
- 38.Scott J.E., Bosworth T.R., Cribb A.M. The chemical morphology of age-related changes in human intervertebral disc glycosaminoglycans from cervical, thoracic and lumbar nucleus pulposus and annulus fibrosus. J Anat. 1994;184:73. [PMC free article] [PubMed] [Google Scholar]
- 39.Dammers R., Koehler P.J. Lumbar disc herniation: level increases with age. Surg Neurol. 2002;58:209–212. doi: 10.1016/s0090-3019(02)00797-8. [DOI] [PubMed] [Google Scholar]
- 40.Taylor T.K., Melrose J., Burkhardt D. Spinal biomechanics and aging are major determinants of the proteoglycan metabolism of intervertebral disc cells. Spine. 2000;25:3014–3020. doi: 10.1097/00007632-200012010-00008. [DOI] [PubMed] [Google Scholar]
- 41.Gross R.H. Leg length discrepancy: how much is too much? Orthopedics. 1978;1:307–310. doi: 10.3928/0147-7447-19780701-08. [DOI] [PubMed] [Google Scholar]
- 42.Resende R.A., Kirkwood R.N., Deluzio K.J. Biomechanical strategies implemented to compensate for mild leg length discrepancy during gait. Gait Posture. 2016;46:147–153. doi: 10.1016/j.gaitpost.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Harvey W.F., Yang M., Cooke T.V. Association of leg-length inequality with knee osteoarthritis: a cohort study. Ann Intern Med. 2010;152:287–295. doi: 10.1059/0003-4819-152-5-201003020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Defrin R., Benyamin S.B., Aldubi R.D. Conservative correction of leg-length discrepancies of 10mm or less for the relief of chronic low back pain. Arch Phys Med Rehabil. 2005;86:2075–2080. doi: 10.1016/j.apmr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Beattie P., Isaacson K., Riddle D.L. Validity of derived measurements of leg-length differences obtained by use of a tape measure. Phys Ther. 1990;70:150–157. doi: 10.1093/ptj/70.3.150. [DOI] [PubMed] [Google Scholar]
- 46.Harris I., Hatfield A., Walton J. Assessing leg length discrepancy after femoral fracture: clinical examination or computed tomography? ANZ J Surg. 2005;75:319–321. doi: 10.1111/j.1445-2197.2005.03349.x. [DOI] [PubMed] [Google Scholar]
- 47.Jordon J., Konstantinou K., O'Dowd J. Herniated lumbar disc. BMJ Clin Evid. 2009;2009:1118. [PMC free article] [PubMed] [Google Scholar]