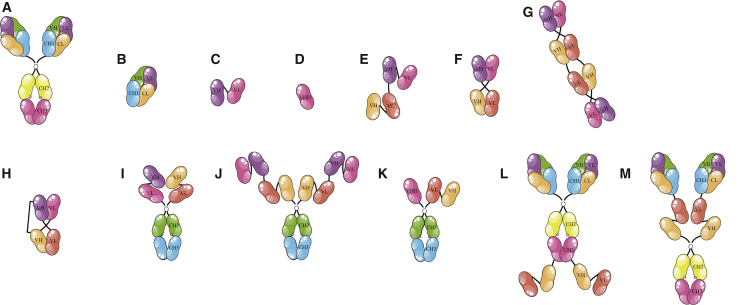Abstract
Despite the success of monoclonal antibodies (mAbs) to treat some disorders, the monospecific molecular entity of mAbs as well as the presence of multiple factors and pathways involved in the pathogenesis of disorders, such as various malignancies, infectious diseases, and autoimmune disorders, and resistance to therapy have restricted the therapeutic efficacy of mAbs in clinical use. Bispecific antibodies (bsAbs), by concurrently recognizing two targets, can partly circumvent these problems. Serial killing of tumor cells by bsAb-redirected T cells, simultaneous blocking of two antigens involved in the HIV-1 infection, and concurrent targeting of the activating and inhibitory receptors on B cells to modulate autoimmunity are part of the capabilities of bsAbs. After designing and developing a large number of bsAbs for years, catumaxomab, a full-length bsAb targeting EpCAM and CD3, was approved in 2009 to treat EpCAM-positive carcinomas besides blinatumomab, a bispecific T cell engager antibody targeting CD19 and CD3, which was approved in 2014 to treat relapsed or refractory acute lymphoblastic leukemia. Furthermore, approximately 60 bsAbs are under investigation in clinical trials. The current review aims at portraying different formats of the single-chain variable fragment (scFv)-based bsAbs and shedding light on the scFv-based bsAbs in preclinical development, different phases of clinical trials, and the market.
Keywords: monoclonal antibody, single-chain variable fragment, bispecific antibody, cancer, HIV-1, bacteria, ESKAPE, autoimmune diseases
Main Text
High-affinity and specific binding to an antigen and effector functions, both resulting from a particular structure, make the antibody as one of the most compelling components of the immune system.1, 2, 3 Besides, good drug-like properties, such as solubility, stability, and prolonged half-life, led to the success of the therapeutic monoclonal antibodies (mAbs) in the clinics; therefore, a large number of antibodies or related products are either approved or under investigation in clinical trials.1, 4
Antibodies encompass two identical heavy chains (∼55 kDa) and two identical light chains (∼25 kDa) linked by disulfide and noncovalent bonds, representing a Y-shaped molecule with a molecular weight of 150 kDa (Figure 1A).3 Both light and heavy chains are divided into the variable (V) and constant (C) regions. The light chain has one variable domain (VL) and one constant domain (CL), and the heavy chain has one variable domain (VH) and at least three constant domains (CH1-CH2-CH3). Based on function, the structure of these glycoproteins is divided into two parts, including the antigen binding sites and the Fc region. The N terminus parts of both light and heavy chains form the former, and the C terminus domains of the two heavy chains form the latter.3 Each antibody has two Fab (fragment antigen binding) arms, consisting of the light chains and the VH and CH1 domains of the heavy chains, carrying the antigen-binding sites (each 50 kDa; Figure 1B). It is noteworthy that the Fc region is involved in antibody effector functions, including antibody-dependent-cell-mediated cytotoxicity (ADCC), antibody-dependent-cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC).3
Figure 1.
The Schematic Representation of Various scFv-Based bsAb Formats
These bsAbs either employing immune cells, such as T cells and NK cells, and bringing them close to the target cell or targeting two vital markers on the cell(s) could exert their therapeutic functions in a range of disorders, such as cancers, autoimmune diseases, and infectious diseases. (A) Whole antibody, (B) antigen-binding fragment (Fab), (C) single-chain variable fragment (scFv), (D) single-domain antibody (e.g., VHH), (E) tandem scFv (e.g., bispecific T cell engager), (F) diabody, (G) tandem antibody (TandAb), (H) dual-affinity retargeting (DART), (I) scFv2-Fc, (J) scFv2-scFv2-Fc, (K) scFv-VHH-Fc, (L) anti-IGF-1R IgG-scFv2, and (M) Fab2-scFv2-Fc (e.g., BiS4aPa) are shown.
Valency, specificity, affinity, and avidity are four underlying definitions with great effects on the functional properties of antibodies.5 The valency is the number of antigen-binding sites on an antibody molecule.6 The ability to discriminate a particular epitope from other epitopes by an antibody is defined as the specificity.3 Affinity (intrinsic affinity) is defined as the strength of the interaction between an epitope on the antigen and a paratope on the antibody, measured by the equilibrium dissociation constant (KD).5, 6 Avidity (functional affinity) describes the combined strength of multiple antigen-antibody interactions.5, 6
Thanks to genetic engineering and the emergence of methods, such as phage display, a group of constructs, including the Fab, single-chain variable fragment (scFv) (Figure 1C), and single-domain antibody (sdAb) (Figure 1D), are designed and developed.7, 8 The scFv consists of two variable domains connected by a flexible linker that is generally the (G4S)3 sequence (25 kDa; VLmAbA-VHmAbA; derived from the parental mAbA).9 The sdAb only contains a single variable domain (12–15 kDa), such as nanobodies (VHHs) derived from camelid heavy-chain antibodies (HCAbs).10 Following the clinical and commercial success of mAbs and antibody fragments, attention was drawn to the development of products with multiple capabilities, leading to the next generation of antibodies, such as bispecific antibodies (bsAbs).11 Chemical conjugation of two different mAbs, generation of a quadroma cell line (a hybrid hybridoma secreting a bsAb), and genetic engineering are the most routine methods used to produce the bsAbs.12 Particularly, the latter is the strategy thoroughly employed to generate fully human bsAbs, owing to the immunogenicity of mouse-origin antibodies.13
The dual targeting is a particular concept linked with bsAbs, enabling them to target two different antigens on two different cells, two different antigens on the same cell, or two different epitopes on the same antigen.13, 14 Compared with conventional mAbs, the bsAbs can bring immune effector cells, such as T cells and natural killer (NK) cells, alongside the tumor cell, resulting in the efficient tumor cell killing.15, 16 Simultaneous binding of bsAbs to two different antigens on the surface of the target cell causes the enhanced binding specificity and the concurrent blockade of two different pathways involved in the disease pathogenesis.17, 18, 19 As single molecules, bsAbs have a low production cost in comparison with those of the two mAbs needed for combination therapy.20
The bsAbs are classified into two groups of immunoglobulin G (IgG)-like and non-IgG-like molecules based on containing the Fc region or not.12, 20 The former ones, encompassing the Fc region, have effector functions (ADCC, ADCP, and CDC), easy purification, and a prolonged serum half-life due to their size above the renal clearance threshold and the neonatal Fc receptor (FcRn)-mediated recycling.9, 21 On the contrary, although the non-IgG-like molecules, devoid of the Fc region, have a short half-life due to their low molecular weight, they benefit from superior tumor penetration, better epitope accessibility, less immunogenicity, and less complicated production compared with the IgG-like molecules.9, 21 Therefore, different strategies are employed to improve the serum half-life of these molecules, including fusion with human serum albumin (HSA) or the Fc part of an IgG molecule (IgG-like molecules), and polyethylene glycolylation.22, 23 This group includes tandem scFvs, diabody (single-chain and tandem diabodies), dual-affinity retargeting molecules (DARTs), etc.
The scFv-Based bsAbs
The scFv is one of the attractive fragments used as the building block of most bsAbs.11 This molecule has advantages, such as preservation of the binding activity of the parental antibody, efficient expression in a wide range of hosts (e.g., bacteria and mammalian cells), and great tumor tissue penetration.17, 24, 25 On the other hand, the small size of the scFv causes a short in vivo half-life owing to the rapid blood clearance and poor retention time in the target tissues.9, 24 According to these limitations, tandem scFv molecules (ta-scFvs) consisting of two scFvs fused by a peptide linker (VLmAbA-VHmAbA–VLmAbB-VHmAbB) were generated (50–60 kDa; Figure 1E).11 These bivalent molecules contain one binding site for each individual antigen.21 The long linker between the two scFvs can enhance the flexibility of antigen binding sites of the ta-scFv, leading to better binding to two different targets.20 The bispecific T cell engager (BiTE) is one type of ta-scFv that consists of two scFvs, one binds to CD3 on T cells and the second one binds to an antigen on the tumor cell.11, 27 Concurrent binding of the BiTE to the T cell and the tumor cell triggers a cascade of events, including T cells activation, the release of cytokines engaging other immune cells, and the secretion of perforin and granzyme B, leading to the tumor cell apoptosis.28, 29, 30 Nevertheless, BiTEs also have a short half-life; hence, the continuous intravenous (cIV) infusion is required to provide the optimal serum concentration.31
A diabody consists of two polypeptide chains, one of which contains the VH of the antibody A connected with the VL of the antibody B, and the other one contains the VH of the antibody B connected with the VL of the antibody A (VHmAbA-VLmAbB/VHmAbB-VLmAbA; Figure 1F).9, 11 Furthermore, this heterodimeric molecule can be designed with a different configuration (VLmAbA-VHmAbB/VLmAbB-VHmAbA).21 Each variable domain is connected to another one by a short peptide linker (five amino acids).21 Similar to BiTEs, diabodies have two different antigen-binding sites.21 Owing to the incorrect dimers generated in the cell and instability of diabodies, different formats of diabody, including double-chain diabody and single-chain diabody, are developed to enhance the stability of the construct.9, 21 In the former, a disulfide bond is introduced between the domains of one chain, and in the latter, two polypeptide chains are fused by a flexible peptide linker (15 amino acids).21 Based on the single-chain diabody format, a dimeric molecule with four binding sites, the tandem diabody (TandAb), is generated (Figure 1G).12, 21 The TandAb molecules with molecular weight of about 114 kDa and bivalent binding for each antigen exhibit a prolonged half-life and higher binding affinity to the targets compared with ta-scFvs and diabodies.9 The other diabody-based bsAb is a DART containing two polypeptide chains (VLmAbA-VHmAbB/VLmAbB-VHmAbA) linked by a disulfide bond, causing more stability and easy manufacturability (Figure 1H).21, 27, 32
The scFv-Based bsAbs in Preclinical Development
In vivo production and in situ secretion of bsAbs by genetically engineered cells is one of the attractive strategies employed to circumvent problems of short half-life, low penetration into tumor sites, production costs, and severe adverse events, such as cytokine release syndrome observed in patients after a systemic administration of bsAbs.33, 34 In this regard, different studies demonstrated that genetically modified human primary peripheral blood lymphocytes or endothelial cells could secrete a functionally active carcinoembryonic antigen (CEA)CD3 diabody redirecting T cells toward CEA-positive tumor cells, resulting in significant tumor growth inhibition in vivo.33, 34, 35 Furthermore, two different bsAb formats, including a ta-scFv and a diabody, constructed with variable domains of mAbs targeting CEA and CD3, were compared based on their in vivo secretion and ability to activate T cells.36 The results unveiled that, although both proteins were secreted from engineered human cells with similar yields, the ta-scFv had a superior tendency to form aggregates resulting in TCR/CD3 cross-linking and thereby target-independent T cell activation.36 Together, it was proposed that, although the noncovalent connection between the two chains of diabody might lead to decreased binding capacity (based on the amount of assembled diabody), the diabody format is preferred due to the lack of aggregation leading to adverse events.36
To increase the production of functional assembled diabodies and reduce free diabody chain that might disrupt the binding of the assembled diabody to the antigen, a self-cleaving 2A peptide derived from a foot-and-mouth disease virus was incorporated into the two-chain CEACD3 diabody gene.37 The co-translational “cleavage” of diabody chains by the 2A self-cleaving peptide and subsequently augmented production and secretion of assembled diabodies by genetically engineered human cells led to the improved binding of the bsAb to the antigen (CEA) and enhanced T cell cytotoxicity against CEA-positive tumor cells.37
To augment the antitumor activity of ta-scFv bsAbs, Ahmed et al.38 constructed an engineered ta-scFv molecule composing of a scFv antibody targeting the carbohydrate epitope on disialoganglioside antigen GD2 (GD2) and a scFv antibody targeting CD3 on T cells. This molecule contained a human hepatocyte nuclear factor 1 (HNF1) dimerization domain (HDD) added to the C terminus of the molecule that was distal to the GD2-specific scFv placed at the N terminus.38 As the CD3-specific scFvs located proximal to the HDD, forming tightly anti-parallel helices, were sterically restricted, monovalency to CD3 was maintained in the HDD-dimeric bsAb. This event prohibited excessive cytokine release. Furthermore, the dimeric GD2CD3 ta-scFv (∼118 kDa) exhibited enhanced avidity to GD2, increased T cell cytotoxicity against GD2-positive tumor cells, prolonged half-life, and significant antitumor activity in mouse xenograft models.38
Despite the significant reduction in AIDS-related mortality since 2004, HIV-1 is still one of the major public health challenges, with 36.9 million infected people (1.8 million children <15 years) and 940,000 AIDS-related deaths worldwide in 2017.39 Among various strategies employed up to now to eradicate HIV-1,40, 41 passive immunotherapy with potent and broadly neutralizing antibodies (bNAbs) seems as an effective strategy to prevent or cure the HIV-1 infection.41, 42 Due to the inherent plasticity of HIV-1, it is demonstrated that the employment of two bNAbs targeting non-overlapping epitopes or bsAbs simultaneously binding to targets involved in the HIV-1 infection is more efficacious than using a single bNAb.43 Indeed, the latter leads to the development of a group of bsAbs with different formats.19, 42, 43, 44, 45, 46 In this regard, Mouquet et al.19 generated three scFv2-Fc IgG-like molecules (immunoadhesins) consisting of one scFv against a non-neutralizing epitope on gp41 and the other derived from one of the three different antibodies targeting neutralizing epitopes on gp120 (Figure 1I). To promote the heterodimer formation, the “knobs-into-holes” strategy was used.19 Indeed, the “knobs-into-holes” strategy is the introduction of a “knob,” substituting a small amino acid with a larger one in the CH3 domain of one heavy chain and a “hole,” substituting a large amino acid with a smaller one in the CH3 domain of the other heavy chain.45, 47, 48 Simultaneous binding of anti-gp41/120 bsAbs to both antigens led to increased viral neutralization in comparison with the parental anti-gp120 IgG antibodies. Indeed, their results demonstrated that the enhanced HIV-1 neutralization resulted from the increased avidity of the anti-gp41/120 bsAb.19
In order to cover the cell surface of CD4+ T cells with bsAbs (blockade of virus entry) and boost their binding affinity for targets,19, 45 Wu et al.44 generated a single-gene-encoded tandem bispecific immunoadhesin molecule (BiIA-SG) containing two scFvs for gp120 and two scFvs for CD4 (Figure 1J). It was constructed with the scFv of an anti-gp120 antibody (PGT128) and the scFv of an anti-CD4 antibody (Hu5A8) fused to the CH2-CH3 domains of human IgG in tandem (Figure 1J). Due to the two gp120-specific scFvs and the subsequent improved binding to gp120, BiIA-SG exhibited significantly enhanced breadth and potency (IC50 value of 0.073 μg/mL against 124 HIV-1 strains). Moreover, the administration of a single dose of BiIA-SG led to sterile protection against divergent HIV-1 challenges in humanized mice. In a short-term treatment setting (for 3 weeks), BiIA-SG plus combination antiretroviral therapy (ART) could delay viral rebound. Consequently, they reported that the administration of a single high dose of the adeno-associated virus-transferred BiIA-SG gene resulted in the elevated half-life of BiIA-SG and thereby eliminating infected cells in humanized mice.44
To improve the T-cell-mediated eradication of HIV-1-infected cells, Sung et al.46 designed two HIVCD3 DARTs containing an anti-HIV-1 binding arm (derived from mAb A32 specific to gp120 or mAb 7B2 specific to gp41) linked to an anti-CD3 binding arm. These molecules comprised two polypeptide chains, one constructed with the VL of the anti-CD3 antibody combined with the VH of the anti-HIV antibody and the other one constructed with the VL of the anti-HIV antibody combined with the VH of the anti-CD3.46 Two HIVCD3 DARTs could redirect cytolytic T cells to kill a panel of cells expressing HIV-1 envelope (infected CD4+ T cells). In an autologous system, the DARTs could recruit CD8+ T cells isolated from patients with HIV-1 infection receiving suppressive ART to eradicate infected CD4+ T cells.46
CD47/SIRPa signaling (“don’t eat me” signal) is one of the pathways harnessed by tumor cells to escape from phagocytosis by immune cells.49, 50, 51 CD47 is a checkpoint receptor overexpressed in different hematological (e.g., acute myelogenous leukemia [AML] and non-Hodgkin lymphoma [NHL]) and solid malignancies (e.g., colon, bladder, and brain).49, 50 To interfere with the CD47/SIRPa interaction, different therapeutic agents are developed, including anti-CD47 mAbs, SIRPa blocking agents, SIRPa-Fc fusion proteins, and bsAbs targeting CD47 and the tumor target. Although anti-CD47 mAbs (Hu5F9-G4 and CC-90002) and a SIRPa-Fc fusion protein (TTI-621) could recently enter phase I clinical trials, it is stated that the ubiquitous expression of CD47, as well as the Fc region, likely affects the therapeutic efficacy, safety, and tolerability of these agents in patients with solid tumors and blood malignancies.49 In this regard, Celgene halted a phase I, open-label dose-escalation and expansion trial of CC-90002 in patients with AML and myelodysplastic syndrome (MDS) (ClinicalTrials.gov: NCT02641002). In fact, the broad expression of CD47 throughout the human body causes toxicity and adverse events in patients receiving CD47 blocking agents and creates an antigen sink that avoids agents from reaching the target site.51 To circumvent these limitations, bsAbs with low affinity for CD47 but high affinity for the tumor antigen (e.g., CD19 and CD20) were designed.50 In this regard, two bsAbs were constructed, including a CD47CD20 dual variable-domain Ig (DVD-Ig) molecule targeting CD47 and CD20 and a kλ-body targeting CD47 and CD19.49, 50 The latter was a fully human IgG1 with two different light chains, including a kappa light chain binding to CD47 and a lambda light chain binding to CD19.49 Although both bsAbs could efficiently kill tumor cells, the binding of their Fc part to Fc receptors (FcRs) on phagocytes might lead to the toxicity and decreased efficacy in vivo (low free antibodies can reach the targets on tumor cells).49, 50, 51 Therefore, van Bommel et al.51 constructed a novel ta-scFv bsAb (RTX-CD47) comprising two scFvs derived from rituximab and from an anti-CD47 mAb. Due to the monovalency of RTX-CD47 to each antigen, it was essential that RTX-CD47 initially bound to CD20, promoting binding to CD47, and thereby inhibiting CD47/SIRPa signaling. In fact, CD20-restricted blocking of CD47/SIRPa interaction through RTX-CD47 prevented toxicity resulting from blocking of CD47 on normal cells. Due to the lack of the Fc part, RTX-CD47 was devoid of off-target activation of phagocytic cells. In vitro, RTX-CD47 could potentiate phagocytic removal of CD20+/CD47+ cells by human phagocytes and synergistically augmented phagocytosis of malignant B cells when combined with obinutuzumab (anti-CD20 mAb), daratumumab (anti-CD38 mAb), or alemtuzumab (anti-CD52 mAb).51
The epidermal growth factor receptor variant III (EGFRvIII) is a truncated receptor resulted from the in-frame deletion of 801 base pairs of exons 2–7 in the EGFR gene.52 Due to this deletion, EGFRvIII is constitutively active in a ligand-independent fashion, and its aberrant signaling is associated with glioma growth and progression.53 Two different groups individually developed TandAb and BiTE molecules targeting EGFRvIII and CD3.54, 55 The particular feature of both bsAbs is their EGFRvIII-specific scFvs. By the phage display technology and fully human antibody libraries, Ellwanger et al.54 isolated a low-affinity scFv antibody, Li3G30, identifying EGFRvIII. Following affinity maturation, high-affinity scFvs (KDs in the range of 0.25–6.50 nM) were used to construct TandAbs that could completely discriminate EGFRvIII from the wild-type EGFR. In vitro, highly potent EGFRvIIICD3 TandAbs by engaging T cells could kill different EGFRvIII-positive cells with EC50 values ranging from 1 to 10 pM, without any cytotoxic effects on EGFR-positive cells or EGFRvIII-negative cells. Furthermore, they demonstrated that EGFRvIIICD3 TandAbs with high-affinity binding to CD3 did not activate T cells co-cultured with EGFRvIII-negative cells. In a dose-dependent manner, the TandAbs could inhibit the tumor growth in a tumor xenograft model derived from the EGFRvIII-positive cell line.54
In another study, Gedeon et al.55 generated a library of ta-scFvs (BiTEs) consisting of variable domains derived from human anti-EGFRvIII (mAb clone 139) and human anti-CD3 (foralumab; 28F11-AE; NI-0401) antibodies. Following multiple screening and assessments, one fully human bsAb, EGFRvIIICD3 BiTE molecule, was selected for further evaluation. By recruiting human T cells, the selected BiTE molecule potently and selectively lysed EGFRvIII-positive cells (malignant glioma cell lines and patients-derived glioma cells). Both in orthotopic and subcutaneous glioma models, the intravenous (i.v.) administration of EGFRvIIICD3 BiTE molecule led to the regression of established tumors and prolonged survival in patient-derived glioma xenografts and could cure established, highly aggressive, syngeneic glioma.55
Overexpression of human epidermal growth factor receptor 2 (HER2) (two million molecules on the surface of a tumor cell) in 20%–30% of breast cancers and its correlation with disease progression and poor prognosis in patients result in the design and development of a bunch of HER2-targeted therapeutic agents, including trastuzumab (4D5; Herceptin), ado-trastuzumab emtansine (T-DM1; Kadcyla), pertuzumab (2C4; Omnitarg), and lapatinib (Tykerb/Tyverb).10, 56, 57, 58, 59 Nanobodies (VHHs) are sdAbs derived from functional HCAbs.10, 60, 61 The small size (15 kDa) and single domain entity endow nanobodies particular features, including binding ability to “hard to reach” epitopes, high-affinity binding, high stability, easy cloning, and easy expression and purification from Escherichia coli.10, 60, 61 Hence, Xing et al.60 constructed an IgG-like bsAb with the mutated Fc, BiHC (a bispecific HER2-CD3 antibody), comprising an anti-HER2 nanobody and an anti-CD3 scFv (derived from a humanized UCHT1 antibody) linked to CH2 and CH3 domains (Figure 1K). Moreover, they employed the knobs-into-holes strategy to facilitate the Fc heterodimerization.60 The BiHC exhibited cytotoxicity against HER2-positive cell lines in a target- and dose-dependent fashion. This molecule could also effectively inhibit the tumor growth in a cell-line-derived xenograft mouse model.60 The scFv-based bsAbs in preclinical development are summarized in Table 1.
Table 1.
The scFv-Based bsAbs in Preclinical Development
| bsAb | Format | Biological Activity | References |
|---|---|---|---|
| Anti-CEAanti-CD3 | diabody | redirecting T cells to CEA-positive tumor cells | 36, 37 |
| Anti-GD2anti-CD3 | BiTE | redirecting T cells to GD2-positive tumor cells | 38 |
| Anti-gp41anti-gp120 | scFv2-Fc | enhanced HIV-1 neutralization | 19 |
| Anti-gp120anti-CD4 (BiIA-SG) | scFv2-scFv2-Fc | elimination of HIV-1-infected cells | 44 |
| Anti-gp120anti-CD3 | DART | redirecting T cells to kill a panel of Env-expressing cells | 46 |
| Anti-gp41anti-CD3 | |||
| Anti-CD47anti-CD20 | ta-scFv | inhibition of “don’t eat me signaling” and phagocytic removal of CD20+/CD47+ cells | 51 |
| Anti-EGFRvIIIanti-CD3 | TandAb | redirecting T cells to EGFRvIII-positive cells | 54 |
| Anti-EGFRvIIIanti-CD3 | BiTE | redirecting T cells to EGFRvIII-positive cells | 55 |
| Anti-HER2anti-CD3 (BiHC) | scFv-VHH-Fc | redirecting T cells to HER2-positive tumor cells | 60 |
BiHC, bispecific HER2-CD3 antibody; BiIA-SG, single-gene-encoded tandem bispecific immunoadhesin; BiTE, bispecific T cell engager; CEA, carcinoembryonic antigen; DART, dual-affinity retargeting molecule; EGFRvIII, epidermal growth factor receptor variant III; Env, envelope; GD2, disialoganglioside GD2; gp41, glycoprotein 41; gp120, glycoprotein 120; HER2, human epidermal growth factor receptor 2; scFv, single-chain fragment variable; TandAb, tandem antibody; ta-scFv, tandem scFv; VHH, the variable domain of camel heavy-chain antibodies.
The scFv-Based bsAbs in Clinical Trials
Overexpression in a wide range of carcinomas, such as lung, breast, colorectal, gastric, pancreas, and ovarian cancers; restricted expression in normal epithelial cells; and involvement in cancer progression make the epithelial cell adhesion molecule (EpCAM) an ideal target for immunotherapy-based approaches, including mAb therapy and adoptive T cell therapy.62, 63, 64 Catumaxomab (Removab) is the first bispecific trifunctional antibody composed of one mouse light and heavy chains specific for human EpCAM and one rat light and heavy chains specific for human CD3, as well as the Fc region preferentially binding to Fc activation receptors (Fc gamma receptor [FcR]I, FcRIIa, and FcRIII) on macrophage, NK, and dendritic cells.65 Therefore, catumaxomab by harnessing immune effector cells potentiates the immune system of patients, leading to the eradication of tumor cells through cytolytic activation of T cells, phagocytosis, and ADCC.65 Cytokine-release-related events and hepatotoxicity are the common adverse events reported with catumaxomab.65 Catumaxomab was approved in 2009 by the European Medicines Agency (EMA) to treat patients with malignant ascites and withdrawn in 2017 in the European Union market due to commercial reasons.65, 66
MT110 (also known as AMG 110 or solitomab) is an EpCAMCD3 BiTE molecule.16, 62 MT110 benefits from an EpCAM-specific scFv with a moderate affinity isolated by the phage display technology.16 Because identification of EpCAM on normal tissues by high-affinity anti-EpCAM mAbs (KD of 1 nM) leads to severe adverse events, such as acute pancreatitis in patients, the moderate affinity of MT110 for EpCAM (ranging from 10 to 100 nM) enables it to discriminate EpCAM-overexpressing tumor cells from normal epithelial cells with low levels of accessible EpCAM.16 It also exhibited good manufacturability and lower immunogenicity (using a de-immunized anti-CD3 scFv) compared with MT102, another anti-EpCAM BiTE molecule.16 In vitro, MT110 exhibited a potent activity in killing EpCAM-overexpressing tumor cells by redirected unstimulated human peripheral T cells (CD4+ and CD8+).16, 62 MT110 was efficacious in three in vivo models (tumor growth inhibition and elimination of established tumors).16 Consequently, a multicenter phase I study assessed the tolerability, pharmacokinetics (PKs), pharmacodynamics (PDs), and antitumor activity of MT110 in patients with relapsed and/or refractory (R/R) EpCAM-positive solid tumors not amenable to standard therapy (ClinicalTrials.gov: NCT00635596). Patients received MT110 (1–96 g/day) as cIV infusion (due to a half-life of 4.5 h) for 28 days. The maximum tolerated dose (MTD) was reported 24 g/day. Despite the antitumor effects in two patients with ovarian cancer, dose-limiting toxicities (DLTs), such as severe diarrhea and elevated liver enzymes, impeded dose escalation to therapeutic levels.63 Conclusively, they attributed these adverse events to the EpCAM expression in the bile duct and gastrointestinal (GI) epithelial cells and mode of BiTEs action.63
Overexpression in tumor cells and the correlation between the elevated serum levels of soluble CEA (sCEA) shedding from tumor cells with disease progression made CEA as one of the most important biomarkers in cancers, such as colorectal cancer (CRC).67, 68, 69 On the other hand, sCEA may interfere with immunotherapy approaches targeting membrane-bound CEA.67, 68, 69 To that end, Lutterbuese et al.67 constructed a series of BiTE antibody constructs, consisting of CD3- and CEA-specific scFvs that their subsets were not competitively inhibited by sCEA. They assessed the ability of CEACD3 BiTE molecules in redirecting of T cells to lyse CEA-positive tumor cells, demonstrating the specificity and potent cytotoxic activity of the molecules in both in vitro and in vivo assays.67 In another study, Osada et al.68 reported that the low concentration (0.1–1 ng/mL) of MEDI-565 (also known as AMG 211), developed from the mentioned constructs, could engage patients’ T cells to kill CEA-positive CRC specimens derived from patients that previously received conventional chemotherapy. Moreover, they showed that both CD4+ and CD8+ T cells were involved in T-cell-mediated killing of CEA-positive tumor cells in agreement with previous data published by Kischel et al.68, 69, 70, 71 MEDI-565 is evaluated in three phase I studies in patients with GI adenocarcinomas (ClinicalTrials.gov: NCT01284231, NCT02291614, and NCT02760199). The results from the first one, an open-label, dose-escalation study, showed DLTs, such as hypoxia (in two patients) and cytokine release syndrome (in one patient), linear PK, a short half-life as expected, no objective responses, and stable disease in 11 patients (28%) receiving 5 mg MEDI-565 as an intermittent 3-h infusion for 5 consecutive days (ClinicalTrials.gov: NCT01284231).71 The second study assessed the safety, PK, PD, and antitumor activity of MEDI-565 in patients with R/R GI adenocarcinoma (ClinicalTrials.gov: NCT02291614). In order to reduce adverse events and achieve a better therapeutic index, patients received a cIV administration of MEDI-565 (0.2–12.8 mg/day).72 More than 20% of the patients showed adverse events related to the GI system, and anti-MEDI-565 antibodies were observed in all patients receiving doses of more than 3.2 mg of MEDI-565. Conclusively, a therapeutic window was not defined for MEDI-565 due to immunogenicity limiting adequate exposure for objective responses.72 The third study evaluated the usefulness of Zirconium-89-labeled MEDI-565 positron-emission tomography (PET) scan in patients with R/R GI adenocarcinoma before and during treatment with MEDI-565 (ClinicalTrials.gov: NCT02760199). The former could help determine the uptake and distribution of radiolabeled MEDI-565 in primary and metastatic tumor lesions and normal organs, and the latter could help assess the impact of prolonged MEDI-565 exposure on tumor and tissue uptake. The results of the last study are not yet published.
Second to lung cancer, prostate cancer is the most life-threatening cancer among American males, with an estimate of 29,430 deaths in 2018.73, 74 The selection of appropriate targets is a critical step in the development of BiTEs; prostate-specific membrane antigen (PSMA) is one of the ideal candidates, which is highly expressed in prostate adenocarcinoma and plays an underlying role in the progression of prostate cancer.75, 76 Despite the different PSMA-specific bsAbs developed up to now, the particular features of BAY2010112 (also known as MT111, AMG 212, or pasotuxizumab), an anti-PSMA BiTE antibody designed by Friedrich et al.73 distinguishes it from other previous constructs, such as diabodies constructed by Buhler et al.77, 78 and Fortmuller et al.79 These diabodies have drawbacks, including the murine origin of their components and the anti-CD3 scFv derived from OKT-3 lacking the ability to react with cynomolgus monkey T cells.73, 77, 78, 79, 80 On the contrary, the amino acid sequence of BAY2010112 is very close to the variable gene segments of human Ig, leading to less immunogenicity.73 BAY2010112 consists of scFvs that can react with both human and monkey PSMA and CD3 antigens, providing a nonclinical safety evaluation of BAY2010112 in the cynomolgus monkey. In a target-dependent fashion, BAY2010112 could induce T-cell-mediated cytolysis of prostate cancer cell lines with EC50 values ranging from 0.1 to 4 ng/mL, depending on the cell line.73 This is while, in the two abovementioned studies, the PSMACD3 diabodies showed EC50 values of 1.479 and 15 ng/mL.77, 78 In addition to remarkable inhibition of tumor formation similar to the mentioned diabodies, BAY2010112 could cause complete remissions in mice with established prostate cancer xenografts.73 BAY2010112 is now investigated in a phase I, open-label, dose-escalation study to determine its MTD, safety, tolerability, and PK in patients with castration-resistant prostate cancer (ClinicalTrials.gov: NCT01723475).
AML is one of the two most common types of leukemia in adults with 10,670 deaths in the US in 2018.74, 81 As AML progresses quickly, patients need prompt treatment, including chemotherapy, allogeneic stem cell transplantation, and targeted therapy.82, 83 Although mAb-based immunotherapy is a treatment option for patients with AML, it needs AML-specific targets to obtain promising results.84 The broad expression of CD33 on AML blasts in a large population of AML patients (90%), in addition to no expression in normal hemopoietic stem cells, qualify CD33 as a suitable target for AML immunotherapy.85, 86 Although antibody-based immunotherapies have great impacts on the treatment of B cell malignancies, AML has not significantly benefited until now, especially after withdrawal of gemtuzumab ozogamicin (an anti-CD33 antibody-drug conjugate) from the market of the US and no further clinical development of lintuzumab (an anti-CD33 mAb) and vadastuximab talirine (an anti-CD33 antibody-drug conjugate).84, 87 Among different antibody-related therapeutics (e.g., camidanlumab tesirine and actinium-225-lintuzumab) in AML clinical trials, AMG 330 is a novel CD33CD3 BiTE molecule currently in a phase I clinical trial to evaluate its therapeutic efficacy in patients with R/R AML (ClinicalTrials.gov: NCT02520427).84, 85, 87, 88 Soluble CD33 (sCD33), which inhibits binding of AMG 330 to its target on AML cells, and de novo expression of CD33 on activated T cells, turning them to target cells for AMG 330, are the two critical points affecting the efficacy of AMG 330.87 Nonetheless, Friedrich et al.87 reported that AMG-330-mediated lysis was scarcely influenced by sCD33 at concentrations up to 100 ng/mL, and CD33-positive T cells activated by blinatumomab only constituted 6% of all T cells. In vitro, AMG-330-redirected T cells could potently lyse AML cell lines expressing different levels of CD33 (14,400 and 56,700 molecules/cell) with EC50 values ranging 18–149 pg/mL.87 In an autologous system, AMG 330 could recruit T cells derived from patients with AML to deplete AML blasts.89 According to the ability of AMG 330 to interact with CD33 and CD3 of both human and nonhuman primates, AMG 330 could redirect autologous T cells against CD33-positive cells isolated from monkey bone marrow samples in an ex vivo assay.87 Furthermore, AMG 330 exhibited the antitumor activity in vivo, leading to the elevated survival in a mouse xenograft model of leukemia.87 In a phase I dose-escalation study to evaluate the safety, tolerability, PK, PD, and efficacy of AMG 330 in patients with R/R AML, AMG 330 was administrated as a cIV infusion at doses up to 480 μg/day (ClinicalTrials.gov: NCT02520427). Serious treatment-associated adverse events were seen in 15 out of 35 patients. At the dose of 480 μg/day, DLTs were cytokine release syndrome (grade 2) and ventricular fibrillation (grade 4), leading to the reduction of the target dose to 240 μg/day. A complete response was seen in two patients who were on 240 μg/day. In conclusion, they suggested that the BiTE molecules could be suitable therapeutics for the eradication of CD33-positive cells.90
Multiple myeloma (MM) is one of the most common hematological malignancies, featured by the unrestrained proliferation of plasma cells in the bone marrow.91, 92 Aside from advances in the treatment of MM and primary remission, relapse is the main factor threatening patients.91 Thus, there is still an unmet medical need for finding therapies with more efficacies in patients not responding to standard treatments.28 Among MM-related antigens, B cell maturation antigen (BCMA) has characteristics distinguishing it from others, including overexpression in MM cells, stimulation of MM cells proliferation, and involvement in the upregulation of antiapoptotic proteins and drug resistance.93 AMG 420 (formerly BI836909) is a BiTE molecule targeting BCMA and CD3.28 In vitro, AMG 420 by harnessing and stimulating both CD4+ and CD8+ T cell subpopulations could specifically lyse BCMA-positive MM cells, in agreement with data from an EpCAM/CD3 bsAb revealing that both T cell subsets play a role in this process.16, 28 Furthermore, Hipp et al.28 demonstrated that the pathological levels of soluble BCMA or a proliferation-inducing ligand (APRIL) could not exert great effects on the activity of AMG 420. In addition to a potent activity in vivo, including tumor cells elimination and elevated survival, AMG 420, by retargeting T cells toward MM cells (both autologous and obtained from newly diagnosed and relapsed and refractory MM patients), could exhibit remarkable antitumor activity in ex vivo assays. The potential of AMG 420 was also assessed in cynomolgus monkeys, leading to the depletion of BCMA-positive plasma cells in the bone marrow.28 The preclinical results motivated Boehringer Ingelheim to launch an open-label, phase I study for measuring the MTD (the primary objective) and the safety, PK, PD, and efficacy (the secondary objectives) of AMG 420 in patients with R/R MM who experienced progression after equal or more than two prior treatment lines (ClinicalTrials.gov: NCT02514239). Based on the results, AMG 420 exhibited a favorable antitumor activity, and the objective response rate was 83% at the dose of 400 μg/day. Treatment-related serious adverse events were cytokine release syndrome and peripheral polyneuropathy observed at the dose of 800 μg/day, although no DLTs were reported up to 400 μg/day. Consequently, PK data revealed that patients who responded to treatment had superior free-drug exposure levels compared to those who did not.94
To eliminate EGFRvIII-expressing cells by bsAb-redirected T cells, Amgen launched a phase I, open-label, sequential-dose-escalation study to assess the tolerability, safety, PK, and PD of AMG 596, an EGFRvIIICD3 BiTE molecule, in patients with glioblastoma expressing mutant EGFRvIII (ClinicalTrials.gov: NCT03296696). This study is conducted with two groups of patients based on disease stage and recurrent disease (the first group) and maintenance treatment after standard of care (SOC) in newly diagnosed disease (the second group).
Although the underlying role of CD19 in the activation and differentiation of B cells is undeniable and its highly conserved expression is observed in most B cell malignancies, such as acute lymphoblastic leukemias (ALL), chronic lymphocytic leukemias, and lymphomas, it is still unknown whether CD19 is directly involved in B cell carcinogenesis or not.95 Nevertheless, CD19 is a fascinating substitution for CD20 in the development of therapeutic antibodies used for the treatment of patients with B cell malignancies.96 To deplete CD19-positive leukemia and lymphoma cells by retargeted T cells, Reusch et al.96 developed a TandAb, AFM11, possessing the same target specificity with blinatumomab. However, the particular structure of AFM11 endows distinct features that discriminate it from BiTE molecules, including a bivalent format and subsequently a greater binding affinity for each target (about 5- and 100-fold higher for CD19 and CD3, respectively) and a molecular weight of about 105 kDa, causing an increased half-life up to 22.9 h.96 In vitro, AFM11 exhibited a potent cytolytic activity against B cells with little dependency upon the effector: target ratio and induced tumor growth inhibition in a non-obese diabetic (NOD)/severe combined immunodeficiency (SCID) xenograft model in a dose-dependent manner.96 In this regard, two phase I studies were launched separately to assess the safety and activity of AFM11 in patients with relapsed or refractory adult B-precursor ALL (ClinicalTrials.gov: NCT02848911) and the ones with R/R CD19-positive B cell NHL (ClinicalTrials.gov: NCT02106091). Nevertheless, both trials were stopped due to serious adverse events, including one death and two life-threatening events in patients with ALL and NHL enrolled in the highest dose cohorts of each study, respectively.97
The other anti-CD19 bsAb in the clinical development is a humanized CD19CD3 DART molecule, designated MGD011 (also known as JNJ64052781 or duvortuxizumab).98, 99 To shun the target-independent T cell activation, MGD011 was engineered with a mutated Fc domain with no binding ability to FcγRs and complement C1q.98 Due to a superior affinity for CD19, compared with CD3, MGD011 initially bound to CD19 on target cells, diminishing the target-independent activation of T cells.98 Notably, the engineered MGD011 could bind to the FcRn, increasing its half-life to 161 h. The other key feature of MGD011 was its cross-reaction with CD19 and CD3 antigens in cynomolgus monkeys, providing a preclinical safety evaluation, PK assessment, and dose escalation in this species. In this regard, in addition to the potent antitumor activity in vitro and in mice models of lymphoma and leukemia, a weekly administration of MGD011 led to the profound and long-lasting B cells elimination in cynomolgus monkeys.98 Furthermore, no cytokine storm (due to monovalent binding) and no infection-related adverse events were observed in monkeys treated with MGD011. Notably, neurotoxicity was not observed in toxicological studies of MGD011 (the predictive value of the cynomolgus monkey to neurologic toxicity associated with CD19CD3 bsAbs is unknown). Although Janssen, in collaboration with MacroGenics, initiated dosing of MGD011 in a phase I study in patients with relapsed or refractory B cell malignancies (ClinicalTrials.gov: NCT02454270), the former terminated the enrollment of the trial due to clinical concerns for neurotoxicity observed in a number of patients receiving treatment.100, 101
Overexpression in various tumors, such as ovarian, pancreatic, CRC, and breast cancers, and its connection with invasiveness and poor prognosis in patients with related cancers made P-cadherin a great therapeutic target for cancer therapy.30, 102 An affinity-optimized scFv with picomolar affinity to P-cadherin, as well as an engineered Fc region in the structure of a DART molecule, led to the generation of PF-06671008 with a prolonged circulation half-life (105.7 h), high stability, high expression, no ADCC activity, and great antitumor activity against P-cadherin-positive cell lines.30, 102 Of note, in the latter, a significant correlation was observed between cytotoxic activity (EC50 values) of PF-06671008-redirected T cells and the surface expression of P-cadherin.102 Moreover, PF-06671008, by activating human T cells engrafted to mice, could cause the regression of established tumors in the cell-line- and patient-derived tumor xenograft models.102 To assess the safety, PK, and PD of PF-06671008, a phase I dose-escalation study sponsored by Pfizer is being conducted on patients with P-cadherin expressing triple-negative breast cancer, CRC, or non-small-cell lung cancer (ClinicalTrials.gov: NCT02659631).
Overexpression in a large proportion of AML patients has translated CD123 to one of the attractive antigens in the development of CD123-based targeted immunotherapy.103, 104 The overexpression of CD123, the chain of interleukin-3 receptor (IL-3R), and thereby IL-3R signaling overactivity are correlated with increased proliferation and enhanced tumor cell viability.105 To eliminate CD123-positive AML cells by redirecting T cells, Al-Hussaini et al.104 designed a humanized CD123CD3 bispecific DART molecule. They demonstrated that this molecule, MGD006 (also known as S80880 or flotetuzumab), by simultaneously binding to human CD123 and CD3 induced T-cell-mediated killing of CD123-positive AML cells in vitro and in vivo.104 Similar to MGD011, MGD006 was also able to recognize cynomolgus monkey CD123 and CD3, and its continuous administration for up to 4 weeks demonstrated the well tolerability of MGD006.104, 106 Based on these promising results, a phase I, open-label, dose-escalation study is ongoing to establish the MTD and to evaluate the safety profile and preliminary anti-leukemic activity of MGD006 (at doses between 3 and 1,000 ng/kg/day) in patients with relapsed or refractory AML or intermediate-2 or high-risk MDS (ClinicalTrials.gov: NCT02152956). The preliminary data revealed that MGD006 was well tolerable, and drug-related adverse events (≥grade 3) observed in 36% of patients were alleviated by the early administration of tocilizumab. MGD006 exhibited anti-leukemic activity in patients at doses of 500 ng/kg/day.107 Furthermore, MGD006 is currently in a phase II trial study to assess its toxicity profile and antitumor activity in patients with CD123-positive advanced ALL and other hematological malignancies (ClinicalTrials.gov: NCT03739606).
The B-cell antigen receptor (BCR) signaling, triggered by the recognition of an immune complex, in connection with other downstream signaling pathways, leads to B cell proliferation, differentiation, and activation through Ig production.108 To prevent the hyperactivation of BCR signaling pathway, the Fc domain of the complex-bound IgG binds to FcγRIIb (CD32B), and as a result, an inhibitory loop is triggered.109 To couple the activation signals, such as BCR signaling pathway, involved in autoimmune diseases, with the inhibition one, a DART molecule consisting of humanized variable domains specifically recognizing CD32B and CD79B (CD79B is the Igβ in the signal-transducing part of the BCR complex) was designed by Veri et al.109, 110 The CD32BCD79B DART benefits from the two particular characteristics; the first is its CD32B-specific variable domains that show superior affinities for their target compared with the CD79B-specific variable domains, favoring CD32B recognition. The other one is that each component binds to its target in a monovalent manner, making the molecule lack intrinsic activation potential.109 They showed that the CD32BCD79B DART molecule inhibited BCR-induced proliferation and Ig production in activated B cells in vitro.109, 111 The therapeutic effect of the molecule was assessed in a mouse model of collagen-induced arthritis, proving its efficacy in the inhibition of the disease.109 To assess the safety, tolerability, and PK of this DART molecule, designated MGD010, as well as its effect on humoral and cell-mediated immune responses in healthy volunteers, a phase I study was conducted on subjects who received a single dose of MGD010 (3 or 10 mg/kg) or placebo, followed by a single-dose administration of hepatitis A vaccine (HAV) (∼50 U; ClinicalTrials.gov: NCT02376036). No serious adverse events were observed in the enrolled subjects. MGD010 exhibited linear PK and dose-dependent binding to peripheral B cells with no B cells elimination but with diminished surface expression of BCR and CD40. In comparison with the placebo group, decreased HAV seroconversion rates with remarkably lower HAV-specific IgG levels were seen in subjects receiving MGD010. Together, the results exhibited that MGD010 had an immunomodulatory activity with a satisfactory safety profile, making it as a suitable candidate for further development as an immunomodulator in patients with autoimmune diseases.111, 112
CD30 is a member of the tumor necrosis factor receptor superfamily expressed in activated B cells and T cells (both CD4 and CD8 subtypes).113 CD30 has emerged as an attractive therapeutic target because of its overexpression in certain malignancies, such as anaplastic large cell lymphoma (ALCL), Hodgkin lymphoma (HL), testicular embryonal carcinoma, on the one hand, and its correlation with cell survival, on the other hand.113, 114, 115, 116 Brentuximab vedotin (Adcetris) is an anti-CD30 antibody conjugated with an antineoplastic agent monomethyl auristatin E.117 This antibody-drug conjugate was initially approved in 2011 for the treatment of patients with relapsed or refractory117 HL and the ones with systemic and primary cutaneous ALCL, not responding to other regimens. Furthermore, in 2018, the US Food and Drug Administration (FDA) approved brentuximab vedotin for the treatment of adult patients with previously untreated stage III or IV classical HL in combination with chemotherapy.115 NK cells have always been attractive candidates for cancer immunotherapy due to their high cytotoxic activities. To engage NK cells toward CD30-positive tumor cells, a TandAb molecule, AFM13, with two binding sites for CD16A and two for CD30 was constructed.15, 118 This TandAb consisted of the affinity-matured human anti-CD16A and murine anti-CD30 variable domains. The former specifically bound to CD16A with no binding to the CD16B isoform, which prevented the elimination of the molecule from the circulation by CD16B+ granulocytes.15, 118 The specific and strong binding to CD30 and CD16A due to the bivalent entity and the resultant reduced Koff made AFM13 as a potent and efficacious agent lacking off-target NK cell activation and destroying only CD30-positive tumor cells in vitro (with an IC50 value of 35.8 nM).15, 118 Furthermore, no significant cytokine release was observed in toxicological studies either in cynomolgus monkeys or in a phase I clinical study.119 A phase I dose-escalation study assessed the safety, tolerability, PK, PD, and antitumor activity of AFM13 (one cycle; once weekly for 4 weeks) in patients with heavily pretreated R/R HL (ClinicalTrials.gov: NCT01221571).118 Notably, as no suitable in vivo model did exist to exhibit the safety and efficacy of AFM13-mediated NK cell activation against HL cells and there was no previous experience with CD16A-specific antibodies, the dosing schedule of AFM13 was chosen with the focus on the safety of patients rather than efficacy.118 Therefore, patients initially received very low doses of AFM13 that subsequently increased by 700 folds.118 The infusions of AFM13 at doses ranging from 0.01 to 7 mg/kg showed mild to moderate adverse events.15, 118 The only DLT in the study was hemolytic anemia in a patient receiving three infusions at 0.5 mg/kg. Due to the murine part of AFM13, anti-drug antibodies were also observed partly in 50% of the patients, and half of the antibodies showed neutralizing potential. Moreover, based on the PK data, the half-life of AFM13 was 19 h.15 Despite the activated NK cells and decreased soluble CD30 in the sera of patients, only partial remission was observed in 3 out of 26 evaluable patients (11.5%). The overall response rate and the overall disease control rate in 13 heavily pretreated patients, receiving AFM13 at a dose of ≥1.5 mg/kg, were 23% and 77%, respectively.118 Furthermore, AFM13 could be active in brentuximab-vedotin-refractory patients.15, 118 Taken together, clinical results from this study confirmed the safety, tolerability, and therapeutic activity of AFM13 in patients with R/R HL, providing a proper condition to launch a phase II study for demonstrating the efficacy of AFM13 with an optimized treatment schedule.118 In this regard, University of Cologne, in collaboration with Affimed, sponsored an open-labeled, randomized, multicenter, phase II trial in relapsed or refractory HL patients pretreated with both brentuximab vedotin and anti-PD-L1 or anti-PD-1 antibodies (GHSG-AFM13; ClinicalTrials.gov: NCT02321592). Preliminary results reported from the company showed that AFM13 has efficacy as monotherapy in a subset of heavily pretreated subjects.120 Zhao et al.121 indicated that the combination AFM13 with a PD-1 blockade led to an enhanced antitumor activity due to the cross-talk between NK cells and T cells and suggested that this combination might improve the clinical outcomes in patients with relapsed or refractory HL. Additionally, based on the clinical data demonstrating high response rates in relapsed or refractory HL patients receiving pembrolizumab (Keytruda)122, 123 and the clinical activity of AFM13 in R/R HL patients in a previous phase I study,118 a phase Ib dose-escalation study was launched to assess the safety, tolerability, and preliminary efficacy of a combination of AFM13 and pembrolizumab in such patients not responding to standard treatment, including brentuximab vedotin (KEYNOTE-206; ClinicalTrials.gov: NCT02665650).124 Thirty patients were divided into dose-escalation cohorts (cohorts I, II, and III) and the extension cohort (12 and 18 patients, respectively). The most common adverse events were infusion-related reactions (80%).124 The overall response rate and complete response rate in the patients treated with the dose and schedule determined for expansion (cohort III and extension cohort) were 87% and 35%, respectively. Taken together, the clinical results of this study demonstrated that the combination of AFM13 and pembrolizumab was a well-tolerated treatment in relapsed or refractory HL patients.124 The fourth clinical study is an open-label phase Ib/IIa study evaluating the biological activity of AFM13 in the elimination of CD30-positive tumor cells in patients with relapsed or refractory cutaneous lymphomas (ClinicalTrials.gov: NCT03192202). The preliminary results confirmed that AFM13 as monotherapy had a satisfactory safety profile and therapeutic activity in enrolled patients (the overall response rate of 66%).125
The ineffectiveness of HER2-targeted therapy due to the ligand-induced activation of the HER2/HER3 signaling pathway has fortified the hypothesis that the concurrent inhibition of HER2 and HER3 in HER2-overexpressing tumors may be more beneficial than individual targeting of HER2 and HER3.17, 126 In this regard, based on computation modeling, MM-111, consisting of fully human anti-HER2 scFv (KD of 0.3 nM) and anti-HER3 scFv (KD of 16 nM) linked to the modified HSA, was designed by McDonagh et al.17 This molecule, by binding to HER2 on HER2-overexpressing cells and then binding to HER3, prevented the ligand-activated signaling triggered from the HER2/HER3/heregulin complex.17 In fact, the antitumor activity of MM-111 was dependent on HER2 overexpression, and its binding to HER3 on cells expressing normal levels of HER2 significantly decreased, indicating the high selectivity and no off-target activity of MM-111.17 Of note, the incorporation of HSA between two anti-HER2 and anti-HER3 scFvs led to the prolonged serum half-life of MM-111 in mice (up to 20 h) and cynomolgus monkeys (up to 99 h).17 Moreover, the combination of MM-111 and either lapatinib or trastuzumab led to the significant tumor growth inhibition in vivo.17, 127 Based on the promising preclinical data, three phase I open-label studies were conducted to assess the safety, tolerability, and clinical activity of MM-111 as a monotherapy or in combination with trastuzumab, lapatinib, or chemotherapy in patients with advanced HER2-positive cancers (ClinicalTrials.gov: NCT00911898, NCT01097460, and NCT01304784).127, 128, 129, 130 In a multi-arm, dose-escalation, phase I study, the safety, tolerability, PK, and antitumor activity of MM-111 in combination with SOC regimens were evaluated in patients with advanced HER2-positive solid tumors (ClinicalTrials.gov: NCT01304784).126 The results proved the clinical activity of MM-111 and SOC HER2-directed regimens in patients with an overall clinical benefit rate (defined as the complete response, partial response, and stable disease for at least 4 months) of 55%.126, 131 Adverse effects were also similar to the adverse effects reported for the regimens alone (e.g., diarrhea, fatigue, decreased appetite, and neutropenia).126, 131 In order to compare the efficacy of MM-111 plus trastuzumab and paclitaxel with that of trastuzumab and paclitaxel alone, a randomized, open-label, phase II study was carried out in patients with advanced gastric, esophagus, and gastroesophageal junction cancers, terminated due to the low efficacy of the former combination (ClinicalTrials.gov: NCT01774851).
Despite the role of the insulin-like growth factor (IGF) pathway in the proliferation and survival of cancer cells, anti-IGF-1 receptor (IGF-1R) antibodies showed limited clinical efficacy in non-stratified patient populations.132 It was indicated that HER3, an underlying driver of phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR signaling, is involved in the resistance to anti-IGF-1R therapies.132, 133 Fitzgerald et al.133 reported that, not only was the IGF-1R signaling pathway co-activated with HER3, but also the IGF-1R inhibition caused the HER3 signaling pathway overactivity.133 Hence, the concurrent blockade of IGF-1R and HER3 seems to be compelling because, indeed, the PI3K/AKT/mTOR signaling and related survival pathways are inhibited.133 In this regard, MM-141 (also known as istiratumab) was developed by Merrimack Pharmaceuticals, consisting of two anti-HER3 scFvs linked to the C terminus of heavy chains of a fully human anti-IGF-1R antibody (Figure 1L).133 MM-141 by simultaneous targeting of IGF-1R and HER3 prevented ligands binding (IGF-1 and IGF-2 to IGF-1R and heregulin to HER3) and decreased the levels of IGF-1R and HER3 on the cell surface, resulting in the inhibition of IGF-1R/ErbB3/PI3K/AKT/mTOR signaling.132, 133, 134 Markedly, in in vivo models, MM-141 through the cross-linking ability derived from its tetravalent entity could more decrease the receptor levels compared with the combination of anti-IGF-1R and anti-HER3 antibodies.133 Based on preclinical models in which MM-141 could augment docetaxel, gemcitabine, everolimus, and nab-paclitaxel (Abraxane), in an open-label, phase I dose-escalation study, the safety, tolerability, PK, and PD of MM-141 in patients with advanced solid tumors were evaluated in three arms, including arm A (MM-141 alone as a monotherapy), arm B (MM-141 with everolimus), and arm C (MM-141 with Abraxane and gemcitabine; ClinicalTrials.gov: NCT01733004).132, 133, 134 Although, in more than 20% of the patients, nausea, vomiting, decreased appetite, and a headache were observed; patients could tolerate MM-141 (as a monotherapy or in combination with chemotherapy). Notably, the assessment of tumor specimens from treated patients exhibited the low cell surface levels of IGF-1R and HER3, demonstrating receptor internalization due to MM-141 activity. Furthermore, this study revealed that there might be a correlation between the serum levels of IGF-1 in patients before treatment and prolonged antitumor activity.132 Based on these results, a randomized, double-blind, phase II study was conducted to compare the efficacy of the combination of MM-141 plus nab-paclitaxel and gemcitabine with that of nab-paclitaxel and gemcitabine alone in metastatic pancreatic cancer patients with high serum levels of free IGF-1 (CARRIE; ClinicalTrials.gov: NCT02399137). The results showed that this combination was not more effective than nab-paclitaxel and gemcitabine alone in enrolled patients.135
The antibiotic resistance in the “ESKAPE” group consisting of Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species, as well as less tendency of pharmaceutical companies toward developing new antibiotics and toxicity of the existing antibiotics, such as colistin and vancomycin, highlight the need to find novel therapeutic agents reclaiming immunocompromised patients in hospitals.136, 137 Among ESKAPE pathogens, the large genome coding capacity and intricate regulatory systems have endowed P. aeruginosa high adaptation ability to environmental stress.18 One of the best strategies to target such pathogens and reduce their resistance is to employ agents with different mechanisms of actions.18 In this regard, multifunctional antibodies simultaneously targeting two virulence factors have demonstrated a promising platform in the eradication of pathogens like P. aeruginosa. Bs4Ab, a particular platform designed by Bezabeh et al.,138 was used to generate a tetravalent bispecific molecule, designated MEDI3902 (formerly, BiS4aPa), consisting of two Fabs against type III secretion protein PcrV and two scFvs against Psl exopolysaccharide, inserted into the hinge domain of a full-length IgG1 mAb (Figure 1M).18, 137 The virulence factor PcrV involved in the type III secretion system (T3S injectisome) and the persistence factor Psl exopolysaccharide involved in the immune evasion and biofilm formation of P. aeruginosa have striking roles in the establishment of acute and persistent infections associated with P. aeruginosa.18, 139 Interestingly, the anti-Psl scFv was inserted into the upper hinge region of MEDI3902 that, by providing an intermediate distance between paratopes, makes an ideal format for concurrent targeting of PcrV and Psl.18 Owing to the pervasiveness of PcrV and Psl in global clinical isolates, it was implied that MEDI3902 is able to cover various P. aeruginosa strains and related infections.139 In this way, BiS4aPa showed remarkable activity against various clinical strains, such as multi-drug-resistant strains in multiple in vivo models, including pneumonia, bacteremia, and thermal injury, in both prophylactic and therapeutic regimens.18, 140 Besides, it exhibited potent synergy when used with multiple antibiotic classes.18 Notably, MEDI3902 maintained the integrity of the lung, decreased bacterial burden, and shunned the dissemination of bacteria into the spleen and kidneys.18, 140 The safety, PK, and anti-drug antibody responses of MEDI3902 were assessed in a phase I dose-escalation study with healthy adults (ClinicalTrials.gov: NCT02255760). Following a single i.v. infusion, no severe treatment-emergent adverse events were observed other than infusion-associated reactions (e.g., redness and skin rashes).140 The small sample size was the major point of the current study, due to which, some less common safety events might not have been recognized.140 MEDI3902 is currently in a phase IIb clinical trial evaluating the efficacy and safety of MEDI3902 in 286 mechanically ventilated patients for the prevention of nosocomial pneumonia caused by P. aeruginosa (EVADE; ClinicalTrials.gov: NCT02696902). The preliminary results from the current trial showed that a single i.v. infusion of 1,500 mg MEDI3902 provided a mean serum concentration beyond the target level through day 22. Although anti-drug antibodies were detected in some subjects, they had no obvious effect on MEDI3902 PK. Consequently, the Data Monitoring Committee had no concerns with the safety data, and the results supported more MEDI3902 development (Guo et al., 2018, ECCMID, abstract). The scFv-based bsAbs in clinical trials are summarized in Table 2.
Table 2.
The scFv-Based bsAbs in Clinical Trials and the Market
| bsAb | Format | Targets | Biological Activity | Status (ClinicalTrials.gov) | Indication | Sponsor | Comments |
|---|---|---|---|---|---|---|---|
| MT110 | BiTE | EpCAM/CD3 | redirecting T cells to EpCAM-positive tumor cells | phase I | – | – | – |
| NCT00635596 | R/R EpCAM-positive solid tumors | Amgen | Despite the antitumor effects in two patients with ovarian cancer, DLTs, such as severe diarrhea and elevated liver enzymes, impeded dose escalation to therapeutic levels. | ||||
| MEDI-565 | BiTE | CEA/CD3 | redirecting T cells to CEA-positive tumor cells | phase I | – | – | – |
| NCT01284231 | GI adenocarcinoma | MedImmune | DLTs, such as hypoxia (in two patients) and cytokine release syndrome (in one patient), no objective responses, and stable disease (28%) were observed in patients. | ||||
| NCT02291614 | R/R GI adenocarcinoma | Amgen | A therapeutic window was not defined for MEDI-565, due to immunogenicity limiting adequate exposure for objective responses. | ||||
| NCT02760199 | R/R GI adenocarcinoma | University Medical Center Groningen | – | ||||
| BAY2010112 | BiTE | PSMA/CD3 | redirecting T cells to PSMA-expressing cells | phase I | – | – | – |
| NCT01723475 | prostate cancer | Bayer | – | ||||
| AMG 330 | BiTE | CD33/CD3 | redirecting T cells to CD33-positive AML cells | phase I | – | – | – |
| NCT02520427 | R/R AML | Amgen | A complete response was seen in two patients who were on 240 μg/day. | ||||
| AMG 420 | BiTE | BCMA/CD3 | redirecting T cells to BCMA-positive MM cells | phase I | – | – | – |
| NCT02514239 | R/R MM | Boehringer Ingelheim | It exhibited a favorable antitumor activity, and the objective response rate was 83% at the dose of 400 μg/day. Treatment-related serious adverse events were cytokine release syndrome and peripheral polyneuropathy observed at the dose of 800 μg/day, and no DLTs were reported up to 400 μg/day. | ||||
| AMG 596 | BiTE | EGFRvIII/CD3 | redirecting T cells to EGFRvIII-expressing cells | phase I | – | – | |
| NCT03296696 | glioblastoma expressing mutant EGFRvIII | Amgen | – | ||||
| AFM11 | TandAb | CD19/CD3 | redirecting T cells to CD19-positive tumor cells | phase I | – | – | Both trials were stopped due to serious adverse events, including one death and two life-threatening events in patients with ALL and NHL enrolled in the highest dose cohorts of each study, respectively. |
| NCT02848911 | RR adult B-precursor ALL | Affimed | |||||
| NCT02106091 | R/R CD19-positive B cell NHL | Affimed | |||||
| MGD011 | DART-Fc protein | CD19/CD3 | redirecting T cells to CD19-positive cells | phase I | – | – | – |
| NCT02454270 | RR B cell malignancies | Janssen Research & Development | Janssen terminated the enrollment of the trial due to clinical concerns for neurotoxicity observed in a number of patients receiving treatment. | ||||
| PF-06671008 | DART-Fc protein | P-cadherin/CD3 | redirecting T cells to P-cadherin-positive tumor cells | phase I | – | – | – |
| NCT02659631 | P-cadherin expressing TNBC, CRC, or NSCLC | Pfizer | – | ||||
| MGD006 | DART | CD123/CD3 | redirecting T cells to CD123-positive AML cells | phase I | – | – | – |
| NCT02152956 | RR AML or intermediate-2 or high-risk MDS | MacroGenics | The preliminary data revealed that MGD006 was well tolerable and exhibited anti-leukemic activity in patients at doses of 500 ng/kg/day. | ||||
| phase II | – | – | – | ||||
| NCT03739606 | CD123-positive advanced ALL and other hematological malignancies | City of Hope Medical Center | – | ||||
| MGD010 | DART | CD32B/CD79B | inhibition of BCR-induced proliferation and Ig production in activated B cells | phase I | – | – | – |
| NCT02376036 | healthy subjects | MacroGenics | The results exhibited that MGD010 had an immunomodulatory activity with a satisfactory safety profile. | ||||
| AFM13 | TandAb | CD30/CD16A | redirecting NK cells to CD30-positive tumor cells | phase I | – | – | – |
| NCT01221571 | R/R classical HL | Affimed | Clinical results from this study confirmed the safety, tolerability, and therapeutic activity of AFM13 in R/R HL patients. | ||||
| phase II | – | – | – | ||||
| NCT02321592 | RR HL | University of Cologne | AFM13 had efficacy as monotherapy in a subset of heavily pretreated subjects. | ||||
| phase Ib | – | – | |||||
| NCT02665650 | RR HL | Affimed | The clinical results of this study demonstrated that the combination of AFM13 and pembrolizumab was a well-tolerated treatment in RR HL patients. | ||||
| phase Ib/IIa | |||||||
| NCT03192202 | RR cutaneous Lymphoma | Ahmed Sawas | AFM13 as monotherapy had a satisfactory safety profile and therapeutic activity in enrolled patients. | ||||
| MM-111 | scFv2-HSA | HER2/HER3 | blocking HER2 and HER3 on HER2-overexpressing tumor cells | phase I | – | – | |
| NCT00911898 | advanced HER2-positive cancers | Merrimack Pharmaceuticals | – | ||||
| NCT01097460 | advanced HER2-positive cancers | Merrimack Pharmaceuticals | – | ||||
| NCT01304784 | advanced HER2-positive cancers | Merrimack Pharmaceuticals | The results proved the clinical activity of MM-111 and SOC HER2-directed regimens in patients with an overall clinical benefit rate of 52%. | ||||
| phase II | – | – | – | ||||
| NCT01774851 | HER2-positive carcinomas of the distal esophagus, gastroesophageal junction, and stomach | Merrimack Pharmaceuticals | This study was terminated due to the low efficacy of MM-111 plus trastuzumab and paclitaxel in enrolled patients. | ||||
| MM-141 | anti-IGF-1R-IgG-scFv2 | HER3/IGF-1R | blocking of IGF-1R and HER3, leading to IGF-1R-mediated growth inhibition | phase I | – | – | – |
| NCT01733004 | advanced solid tumors | Merrimack Pharmaceuticals | Patients could tolerate MM-141 (as a monotherapy or in combination with chemotherapy). The assessment of tumor specimens from treated patients exhibited the low cell surface levels of IGF-1R and HER3, demonstrating receptor internalization due to MM-141 activity. | ||||
| phase II | – | – | – | ||||
| NCT02399137 | metastatic pancreatic cancer | Merrimack Pharmaceuticals | Results showed that the combination of MM-141 plus nab-paclitaxel and gemcitabine was not more effective than nab-paclitaxel and gemcitabine alone in enrolled patients. | ||||
| MEDI3902 | Fab2-scFv2-Fc | PcrV/Ps1 | killing of P. aeruginosa | phase I | – | – | – |
| NCT02255760 | healthy adults | MedImmune | Following a single i.v. infusion, no severe treatment-emergent adverse events were observed other than infusion-related reactions, such as skin rashes with or without pruritus. | ||||
| phase IIb | – | – | – | ||||
| NCT02696902 | nosocomial pneumonia caused by P. aeruginosa | MedImmune | The Data Monitoring Committee had no concerns with safety data, and the results supported more MEDI3902 development. | ||||
| Blinatumomab | BiTE | CD19/CD3 | redirecting T cells to CD19-positive B cells | approved | RR Philadelphia chromosome (Ph)-negative and Ph-positive precursor B cell ALL | Amgen | – |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BCMA, B cell maturation antigen; BCR, B-cell antigen receptor; BiTE, bispecific T cell engager; bsAbs, bispecific antibodies; CEA, carcinoembryonic antigen; CRC, colorectal cancer; DART, dual-affinity retargeting molecule; DLTs, dose-limiting toxicities; EGFRvIII, epidermal growth factor receptor variant III; EpCAM, epithelial cell adhesion molecule; Fab, fragment antigen binding; GI, gastrointestinal; HER2, human epidermal growth factor receptor 2; HER3, human epidermal growth factor receptor 3; HL, Hodgkin lymphoma; HSA, human serum albumin; Ig, immunoglobulin; IGF-1R, insulin-like growth factor-1 receptor; i.v., intravenous; MDS, myelodysplastic syndromes; MM, multiple myeloma; NHL, non-Hodgkin’s lymphoma; NK cells, natural killer cells; NSCLC, non-small-cell lung cancer; P. aeruginosa, Pseudomonas aeruginosa; PcrV, type III secretion system protein PcrV; PK, pharmacokinetics; PSMA, prostate-specific membrane antigen; R/R, relapsed and/or refractory; RR, relapsed or refractory; scFv, single-chain fragment variable; SOC, standard of care; TandAb, tandem antibody; TNBC, triple-negative breast cancer.
The scFv-Based bsAbs in the Market
Blinatumomab (Blincyto; developed by Amgen) is a first-in-class BiTE antibody recruiting cytotoxic T cells to kill CD19-positive ALL blasts.142 This BiTE antibody was approved by the US FDA for relapsed or refractory Philadelphia chromosome (Ph)-negative and Ph-positive precursor B cell ALL and by the EMA just for the treatment of relapsed or refractory Ph-negative precursor B cell ALL (Table 2).143
By evaluating data from six phase I and II trials in patients with relapsed or refractory ALL, minimal residual disease-positive ALL, and NHL, Zhu et al.29 demonstrated that blinatumomab has linear PK under cIV infusion over 4–8 weeks and fast clearance due to the lack of the Fc part and glycosylation. They also proposed that the loss of the Fc region, as well as the prevention of B cell differentiation into plasma cells (as the source of generation of anti-drug antibodies), might be the key causes leading to low immunogenicity of blinatumomab in patients. Their results showed that the type of cancer (ALL or NHL) or patients’ demographics had no clinically meaningful effects on blinatumomab PK. Furthermore, they highlighted that blinatumomab PD is involved in B cell depletion and dose-dependent cytokine elevation through T cell redistribution and activation.29 Aside from side effects, such as the cytokine release syndrome and neurological toxicities, not only do some patients not respond to blinatumomab, but some patients also experience disease progression during treatment second to the primary response.144 The escape of CD19-negative leukemia cells, overexpression of PD-L1 on leukemia cells, and increased numbers of regulatory T cells in combination with an elevated level of lactic dehydrogenase are the possible mechanisms causing the inefficiency of treatment with blinatumomab.144
More than 30 clinical trials (four phase III, two phase II/III, 21 phase II, four phase I/II, and seven phase I) are evaluating blinatumomab on different populations of patients with ALL, NHL, MM, Richter’s transformation, etc., as alone or in combination with chemotherapy or drugs, such as pembrolizumab, nivolumab, ipilimumab, ibrutinib, lenalidomide, etc. (clinicaltrials.gov).
Conclusions
During the two recent decades, the bsAb technology, grabbing pharmaceutical companies’ attentions, has extensively progressed, particularly following the successful clinical use of blinatumomab in humans. Efficiently penetrating into tumor tissues and recruiting T cells and NK cells to kill tumor cells or blocking ligands involved in the pathogenesis of various disorders are some of the brilliant properties of bsAbs. To improve the PK profile of this generation of antibodies, different combinations of scFvs with other molecules, such as Fab, HSA, and the Fc part of IgG, were generated, resulting in the development of bsAbs with an extended half-life, potent activity, and more stability compared with other ones.
Therapeutic agents, such as rituximab, which not only is prescribed for patients with cancer but also is used in patients with autoimmune disorders, are inspirational in the development of antibodies used in a group of diseases.145 In this regard, finding targets, such as IL-1, IL-6, IL-17, IL-4, etc., which may be involved in both cancers and autoimmune diseases, can help design valuable agents used in a broad population of patients.145, 146, 147, 148 As the role of inflammation in the pathogenesis of cancer was established, designing more scFv-based bsAbs, such as SAR156597 (a DVD-IgG bsAb), which concurrently blocks IL-4 and IL-13 and is under investigation in two phase II trials (ClinicalTrials.gov: NCT02921971 and NCT02345070), can be a promising strategy to get rid of inflammation-related disorders.148, 149 The efficacy and safety of bsAbs were proven in patients with cancer and partly in patients with autoimmune diseases. However, due to the high rate of mortality in patients with infectious diseases, it is mandatory to focus on developing bsAbs targeting life-threatening bacteria. The development of bsAbs blocking vital proteins in the bacteria of the ESKAPE group or BiTE antibodies recruiting T cells against these bacteria can circumvent many problems in the health care system. The last, but not the least, the “cytokine storm” is one of the most dangerous side effects threatening patients receiving the BiTE antibody, such as blinatumomab. Therefore, the development of agents debilitating this life-threatening outcome can reduce concerns about BiTE therapy.
Author Contributions
F.R.J. wrote the manuscript with support from all other authors.
Conflicts of Interest
The authors declare no competing interests.
References
- 1.Wang X., Mathieu M., Brezski R.J. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018;9:63–73. doi: 10.1007/s13238-017-0473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sela-Culang I., Kunik V., Ofran Y. The structural basis of antibody-antigen recognition. Front. Immunol. 2013;4:302. doi: 10.3389/fimmu.2013.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipman N.S., Jackson L.R., Trudel L.J., Weis-Garcia F. Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources. ILAR J. 2005;46:258–268. doi: 10.1093/ilar.46.3.258. [DOI] [PubMed] [Google Scholar]
- 4.Kaplon H., Reichert J.M. Antibodies to watch in 2018. MAbs. 2018;10:183–203. doi: 10.1080/19420862.2018.1415671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudnick S.I., Adams G.P. Affinity and avidity in antibody-based tumor targeting. Cancer Biother. Radiopharm. 2009;24:155–161. doi: 10.1089/cbr.2009.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuesta A.M., Sainz-Pastor N., Bonet J., Oliva B., Alvarez-Vallina L. Multivalent antibodies: when design surpasses evolution. Trends Biotechnol. 2010;28:355–362. doi: 10.1016/j.tibtech.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Chames P., Baty D. Bispecific antibodies for cancer therapy: the light at the end of the tunnel? MAbs. 2009;1:539–547. doi: 10.4161/mabs.1.6.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arezumand R., Alibakhshi A., Ranjbari J., Ramazani A., Muyldermans S. Nanobodies as novel agents for targeting angiogenesis in solid cancers. Front. Immunol. 2017;8:1746. doi: 10.3389/fimmu.2017.01746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan G., Wang Z., Hao M., Li J. Bispecific antibodies and their applications. J. Hematol. Oncol. 2015;8:130. doi: 10.1186/s13045-015-0227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamnani F.R., Rahbarizadeh F., Shokrgozar M.A., Ahmadvand D., Mahboudi F., Sharifzadeh Z. Targeting high affinity and epitope-distinct oligoclonal nanobodies to HER2 over-expressing tumor cells. Exp. Cell Res. 2012;318:1112–1124. doi: 10.1016/j.yexcr.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Spiess C., Zhai Q., Carter P.J. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol. Immunol. 2015;67(2 Pt A):95–106. doi: 10.1016/j.molimm.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Kontermann R.E., Brinkmann U. Bispecific antibodies. Drug Discov. Today. 2015;20:838–847. doi: 10.1016/j.drudis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Kontermann R.E. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4:182–197. doi: 10.4161/mabs.4.2.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyakatura E.K., Soare A.Y., Lai J.R. Bispecific antibodies for viral immunotherapy. Hum. Vaccin. Immunother. 2017;13:836–842. doi: 10.1080/21645515.2016.1251536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J., Fu J., Zhang M., Liu D. AFM13: a first-in-class tetravalent bispecific anti-CD30/CD16A antibody for NK cell-mediated immunotherapy. J. Hematol. Oncol. 2015;8:96. doi: 10.1186/s13045-015-0188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brischwein K., Schlereth B., Guller B., Steiger C., Wolf A., Lutterbuese R., Offner S., Locher M., Urbig T., Raum T. MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol. Immunol. 2006;43:1129–1143. doi: 10.1016/j.molimm.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 17.McDonagh C.F., Huhalov A., Harms B.D., Adams S., Paragas V., Oyama S., Zhang B., Luus L., Overland R., Nguyen S. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol. Cancer Ther. 2012;11:582–593. doi: 10.1158/1535-7163.MCT-11-0820. [DOI] [PubMed] [Google Scholar]
- 18.DiGiandomenico A., Keller A.E., Gao C., Rainey G.J., Warrener P., Camara M.M., Bonnell J., Fleming R., Bezabeh B., Dimasi N. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci. Transl. Med. 2014;6:262ra155. doi: 10.1126/scitranslmed.3009655. [DOI] [PubMed] [Google Scholar]
- 19.Mouquet H., Warncke M., Scheid J.F., Seaman M.S., Nussenzweig M.C. Enhanced HIV-1 neutralization by antibody heteroligation. Proc. Natl. Acad. Sci. USA. 2012;109:875–880. doi: 10.1073/pnas.1120059109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., Yang Y., Fan D., Xiong D. The development of bispecific antibodies and their applications in tumor immune escape. Exp. Hematol. Oncol. 2017;6:12. doi: 10.1186/s40164-017-0072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkmann U., Kontermann R.E. The making of bispecific antibodies. MAbs. 2017;9:182–212. doi: 10.1080/19420862.2016.1268307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann S., Perera R., Grimm H.P., Sam J., Colombetti S., Fauti T., Fahrni L., Schaller T., Freimoser-Grundschober A., Zielonka J. In vivo fluorescence imaging of the activity of CEA TCB, a novel T-cell bispecific antibody, reveals highly specific tumor targeting and fast induction of T-cell-mediated tumor killing. Clin. Cancer Res. 2016;22:4417–4427. doi: 10.1158/1078-0432.CCR-15-2622. [DOI] [PubMed] [Google Scholar]
- 23.Weidle U.H., Kontermann R.E., Brinkmann U. Tumor-antigen-binding bispecific antibodies for cancer treatment. Semin. Oncol. 2014;41:653–660. doi: 10.1053/j.seminoncol.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Monnier P.P., Vigouroux R.J., Tassew N.G. In vivo applications of single chain Fv (variable domain) (scFv) fragments. Antibodies. 2013;2:193–208. [Google Scholar]
- 25.Wang S., Zhang P., He F., Wang J.G., Sun J.Z., Li Z.L., Yi B., Xi J., Mao Y.P., Hou Q. Combination of specific single chain antibody variable fragment and siRNA has a synergistic inhibitory effect on the propagation of avian influenza virus H5N1 in chicken cells. Virol. J. 2014;11:208. doi: 10.1186/s12985-014-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pishko A., Nasta S.D. The role of novel immunotherapies in non-Hodgkin lymphoma. Transl. Cancer Res. 2017;6:93–103. doi: 10.21037/tcr.2017.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hipp S., Tai Y.-T., Blanset D., Deegen P., Wahl J., Thomas O., Rattel B., Adam P.J., Anderson K.C., Friedrich M. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia. 2017;31:1743–1751. doi: 10.1038/leu.2016.388. [DOI] [PubMed] [Google Scholar]
- 29.Zhu M., Wu B., Brandl C., Johnson J., Wolf A., Chow A., Doshi S. Blinatumomab, a bispecific T-cell engager (BiTE(®)) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin. Pharmacokinet. 2016;55:1271–1288. doi: 10.1007/s40262-016-0405-4. [DOI] [PubMed] [Google Scholar]
- 30.Root A.R., Cao W., Li B., LaPan P., Meade C., Sanford J., Jin M., O’Sullivan C., Cummins E., Lambert M. Development of PF-06671008, a highly potent anti-P-cadherin/anti-CD3 bispecific DART molecule with extended half-life for the treatment of cancer. Antibodies. 2016;5:6. doi: 10.3390/antib5010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thakur A., Lum L.G. “NextGen” biologics: bispecific antibodies and emerging clinical results. Expert Opin. Biol. Ther. 2016;16:675–688. doi: 10.1517/14712598.2016.1150996. [DOI] [PubMed] [Google Scholar]
- 32.Walseng E., Nelson C.G., Qi J., Nanna A.R., Roush W.R., Goswami R.K., Sinha S.C., Burke T.R., Jr., Rader C. Chemically programmed bispecific antibodies in diabody format. J. Biol. Chem. 2016;291:19661–19673. doi: 10.1074/jbc.M116.745588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Compte M., Blanco B., Serrano F., Cuesta A.M., Sanz L., Bernad A., Holliger P., Alvarez-Vallina L. Inhibition of tumor growth in vivo by in situ secretion of bispecific anti-CEA x anti-CD3 diabodies from lentivirally transduced human lymphocytes. Cancer Gene Ther. 2007;14:380–388. doi: 10.1038/sj.cgt.7701021. [DOI] [PubMed] [Google Scholar]
- 34.Compte M., Alonso-Camino V., Santos-Valle P., Cuesta A.M., Sánchez-Martín D., López M.R., Vicario J.L., Salas C., Sanz L., Alvarez-Vallina L. Factory neovessels: engineered human blood vessels secreting therapeutic proteins as a new drug delivery system. Gene Ther. 2010;17:745–751. doi: 10.1038/gt.2010.33. [DOI] [PubMed] [Google Scholar]
- 35.Blanco B., Holliger P., Vile R.G., Alvarez-Vallina L. Induction of human T lymphocyte cytotoxicity and inhibition of tumor growth by tumor-specific diabody-based molecules secreted from gene-modified bystander cells. J. Immunol. 2003;171:1070–1077. doi: 10.4049/jimmunol.171.2.1070. [DOI] [PubMed] [Google Scholar]
- 36.Compte M., Alvarez-Cienfuegos A., Nuñez-Prado N., Sainz-Pastor N., Blanco-Toribio A., Pescador N., Sanz L., Alvarez-Vallina L. Functional comparison of single-chain and two-chain anti-CD3-based bispecific antibodies in gene immunotherapy applications. OncoImmunology. 2014;3:e28810. doi: 10.4161/onci.28810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mølgaard K., Compte M., Nuñez-Prado N., Harwood S.L., Sanz L., Alvarez-Vallina L. Balanced secretion of anti-CEA × anti-CD3 diabody chains using the 2A self-cleaving peptide maximizes diabody assembly and tumor-specific cytotoxicity. Gene Ther. 2017;24:208–214. doi: 10.1038/gt.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed M., Cheng M., Cheung I.Y., Cheung N.-K.V. Human derived dimerization tag enhances tumor killing potency of a T-cell engaging bispecific antibody. OncoImmunology. 2015;4:e989776. doi: 10.4161/2162402X.2014.989776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joint United Nations Programme on HIV/AIDS (UNAIDS). (2018). UNAIDS Data 2018. http://www.unaids.org/en/resources/documents/2018/unaids-data-2018. [PubMed]
- 40.Pham H.T., Mesplède T. The latest evidence for possible HIV-1 curative strategies. Drugs Context. 2018;7:212522. doi: 10.7573/dic.212522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimata J.T., Rice A.P., Wang J. Challenges and strategies for the eradication of the HIV reservoir. Curr. Opin. Immunol. 2016;42:65–70. doi: 10.1016/j.coi.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bournazos S., Gazumyan A., Seaman M.S., Nussenzweig M.C., Ravetch J.V. Bispecific anti-HIV-1 antibodies with enhanced breadth and potency. Cell. 2016;165:1609–1620. doi: 10.1016/j.cell.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagh K., Seaman M.S., Zingg M., Fitzsimons T., Barouch D.H., Burton D.R., Connors M., Ho D.D., Mascola J.R., Nussenzweig M.C. Potential of conventional & bispecific broadly neutralizing antibodies for prevention of HIV-1 subtype A, C & D infections. PLoS Pathog. 2018;14:e1006860. doi: 10.1371/journal.ppat.1006860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X., Guo J., Niu M., An M., Liu L., Wang H., Jin X., Zhang Q., Lam K.S., Wu T. Tandem bispecific neutralizing antibody eliminates HIV-1 infection in humanized mice. J. Clin. Invest. 2018;128:2239–2251. doi: 10.1172/JCI96764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Y., Yu J., Lanzi A., Yao X., Andrews C.D., Tsai L., Gajjar M.R., Sun M., Seaman M.S., Padte N.N., Ho D.D. Engineered bispecific antibodies with exquisite HIV-1-neutralizing activity. Cell. 2016;165:1621–1631. doi: 10.1016/j.cell.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sung J.A.M., Pickeral J., Liu L., Stanfield-Oakley S.A., Lam C.-Y.K., Garrido C., Pollara J., LaBranche C., Bonsignori M., Moody M.A. Dual-affinity re-targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J. Clin. Invest. 2015;125:4077–4090. doi: 10.1172/JCI82314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei H., Cai H., Jin Y., Wang P., Zhang Q., Lin Y., Wang W., Cheng J., Zeng N., Xu T., Zhou A. Structural basis of a novel heterodimeric Fc for bispecific antibody production. Oncotarget. 2017;8:51037–51049. doi: 10.18632/oncotarget.17558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunasekaran K., Pentony M., Shen M., Garrett L., Forte C., Woodward A., Ng S.B., Born T., Retter M., Manchulenko K. Enhancing antibody Fc heterodimer formation through electrostatic steering effects: applications to bispecific molecules and monovalent IgG. J. Biol. Chem. 2010;285:19637–19646. doi: 10.1074/jbc.M110.117382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dheilly E., Moine V., Broyer L., Salgado-Pires S., Johnson Z., Papaioannou A., Cons L., Calloud S., Majocchi S., Nelson R. Selective blockade of the ubiquitous checkpoint receptor CD47 is enabled by dual-targeting bispecific antibodies. Mol. Ther. 2017;25:523–533. doi: 10.1016/j.ymthe.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piccione E.C., Juarez S., Liu J., Tseng S., Ryan C.E., Narayanan C., Wang L., Weiskopf K., Majeti R. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs. 2015;7:946–956. doi: 10.1080/19420862.2015.1062192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Bommel P.E., He Y., Schepel I., Hendriks M.A.J.M., Wiersma V.R., van Ginkel R.J., van Meerten T., Ammatuna E., Huls G., Samplonius D.F. CD20-selective inhibition of CD47-SIRPα “don’t eat me” signaling with a bispecific antibody-derivative enhances the anticancer activity of daratumumab, alemtuzumab and obinutuzumab. OncoImmunology. 2017;7:e1386361. doi: 10.1080/2162402X.2017.1386361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gan H.K., Cvrljevic A.N., Johns T.G. The epidermal growth factor receptor variant III (EGFRvIII): where wild things are altered. FEBS J. 2013;280:5350–5370. doi: 10.1111/febs.12393. [DOI] [PubMed] [Google Scholar]
- 53.An Z., Aksoy O., Zheng T., Fan Q.W., Weiss W.A. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. 2018;37:1561–1575. doi: 10.1038/s41388-017-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellwanger K., Reusch U., Fucek I., Knackmuss S., Weichel M., Gantke T., Molkenthin V., Zhukovsky E.A., Tesar M., Treder M. Highly specific and effective targeting of EGFRvIII-positive tumors with TandAb antibodies. Front. Oncol. 2017;7:100. doi: 10.3389/fonc.2017.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gedeon P.C., Schaller T.H., Chitneni S.K., Choi B.D., Kuan C.T., Suryadevara C.M., Snyder D.J., Schmittling R.J., Szafranski S.E., Cui X. A rationally designed fully human EGFRvIII:CD3-targeted bispecific antibody redirects human T cells to treat patient-derived intracerebral malignant glioma. Clin. Cancer Res. 2018;24:3611–3631. doi: 10.1158/1078-0432.CCR-17-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin Q., Esteva F.J. Cross-talk between the ErbB/HER family and the type I insulin-like growth factor receptor signaling pathway in breast cancer. J. Mammary Gland Biol. Neoplasia. 2008;13:485–498. doi: 10.1007/s10911-008-9107-3. [DOI] [PubMed] [Google Scholar]
- 57.Weigelt B., Lo A.T., Park C.C., Gray J.W., Bissell M.J. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res. Treat. 2010;122:35–43. doi: 10.1007/s10549-009-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moasser M.M. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Araki K., Fukada I., Yanagi H., Kobayashi K., Shibayama T., Horii R., Takahashi S., Akiyama F., Ohno S., Ito Y. First report of eribulin in combination with pertuzumab and trastuzumab for advanced HER2-positive breast cancer. Breast. 2017;35:78–84. doi: 10.1016/j.breast.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 60.Xing J., Lin L., Li J., Liu J., Zhou C., Pan H., Shu R., Dong B., Cao D., Li Q., Wang Z. BiHC, a T-cell–engaging bispecific recombinant antibody, has potent cytotoxic activity against Her2 tumor cells. Transl. Oncol. 2017;10:780–785. doi: 10.1016/j.tranon.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharifzadeh Z., Rahbarizadeh F., Shokrgozar M.A., Ahmadvand D., Mahboudi F., Rahimi Jamnani F., Aghaee Bakhtiari S.H. Development of oligoclonal nanobodies for targeting the tumor-associated glycoprotein 72 antigen. Mol. Biotechnol. 2013;54:590–601. doi: 10.1007/s12033-012-9601-0. [DOI] [PubMed] [Google Scholar]
- 62.Ferrari F., Bellone S., Black J., Schwab C.L., Lopez S., Cocco E., Bonazzoli E., Predolini F., Menderes G., Litkouhi B. Solitomab, an EpCAM/CD3 bispecific antibody construct (BiTE®), is highly active against primary uterine and ovarian carcinosarcoma cell lines in vitro. J. Exp. Clin. Cancer Res. 2015;34:123. doi: 10.1186/s13046-015-0241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kebenko M., Goebeler M.E., Wolf M., Hasenburg A., Seggewiss-Bernhardt R., Ritter B., Rautenberg B., Atanackovic D., Kratzer A., Rottman J.B. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE®) antibody construct, in patients with refractory solid tumors. OncoImmunology. 2018;7:e1450710. doi: 10.1080/2162402X.2018.1450710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trzpis M., McLaughlin P.M., de Leij L.M., Harmsen M.C. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am. J. Pathol. 2007;171:386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sedykh S.E., Prinz V.V., Buneva V.N., Nevinsky G.A. Bispecific antibodies: design, therapy, perspectives. Drug Des. Devel. Ther. 2018;12:195–208. doi: 10.2147/DDDT.S151282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eskander R.N., Tewari K.S. Epithelial cell-adhesion molecule-directed trifunctional antibody immunotherapy for symptom management of advanced ovarian cancer. Clin. Pharmacol. 2013;5(Suppl 1):55–61. doi: 10.2147/CPAA.S45885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lutterbuese R., Raum T., Kischel R., Lutterbuese P., Schlereth B., Schaller E., Mangold S., Rau D., Meier P., Kiener P.A. Potent control of tumor growth by CEA/CD3-bispecific single-chain antibody constructs that are not competitively inhibited by soluble CEA. J. Immunother. 2009;32:341–352. doi: 10.1097/CJI.0b013e31819b7c70. [DOI] [PubMed] [Google Scholar]
- 68.Osada T., Hsu D., Hammond S., Hobeika A., Devi G., Clay T.M., Lyerly H.K., Morse M.A. Metastatic colorectal cancer cells from patients previously treated with chemotherapy are sensitive to T-cell killing mediated by CEA/CD3-bispecific T-cell-engaging BiTE antibody. Br. J. Cancer. 2010;102:124–133. doi: 10.1038/sj.bjc.6605364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oberst M.D., Fuhrmann S., Mulgrew K., Amann M., Cheng L., Lutterbuese P., Richman L., Coats S., Baeuerle P.A., Hammond S.A. CEA/CD3 bispecific antibody MEDI-565/AMG 211 activation of T cells and subsequent killing of human tumors is independent of mutations commonly found in colorectal adenocarcinomas. MAbs. 2014;6:1571–1584. doi: 10.4161/19420862.2014.975660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kischel R., Hausmann S., Baeuerle P., Kufer P. Abstract #3252: Effector memory T cells make a major contribution to redirected target cell lysis by T cell-engaging BiTE antibody MT110. Cancer Res. 2009;69:3252. [Google Scholar]
- 71.Pishvaian M., Morse M.A., McDevitt J., Norton J.D., Ren S., Robbie G.J., Ryan P.C., Soukharev S., Bao H., Denlinger C.S. Phase 1 dose escalation study of MEDI-565, a bispecific T-cell engager that targets human carcinoembryonic antigen, in patients with advanced gastrointestinal adenocarcinomas. Clin. Colorectal Cancer. 2016;15:345–351. doi: 10.1016/j.clcc.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Moek K.L., Fiedler W.M., von Einem J.C., Verheul H.M., Seufferlein T., de Groot D.J., Heinemann V., Kebenko M., Menke-van der Houven van Oordt C.W., Ettrich T.J. Phase I study of AMG 211/MEDI-565 administered as continuous intravenous infusion (cIV) for relapsed/refractory gastrointestinal (GI) adenocarcinoma. Ann. Oncol. 2018;29 mdy279.414. [Google Scholar]
- 73.Friedrich M., Raum T., Lutterbuese R., Voelkel M., Deegen P., Rau D., Kischel R., Hoffmann P., Brandl C., Schuhmacher J. Regression of human prostate cancer xenografts in mice by AMG 212/BAY2010112, a novel PSMA/CD3-bispecific BiTE antibody cross-reactive with non-human primate antigens. Mol. Cancer Ther. 2012;11:2664–2673. doi: 10.1158/1535-7163.MCT-12-0042. [DOI] [PubMed] [Google Scholar]
- 74.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 75.Bravaccini S., Puccetti M., Bocchini M., Ravaioli S., Celli M., Scarpi E., De Giorgi U., Tumedei M.M., Raulli G., Cardinale L., Paganelli G. PSMA expression: a potential ally for the pathologist in prostate cancer diagnosis. Sci. Rep. 2018;8:4254. doi: 10.1038/s41598-018-22594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.von Eyben F.E., Baumann G.S., Baum R.P. PSMA diagnostics and treatments of prostate cancer become mature. Clin. Transl. Imaging. 2018;6:145–148. doi: 10.1007/s40336-018-0270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bühler P., Wolf P., Gierschner D., Schaber I., Katzenwadel A., Schultze-Seemann W., Wetterauer U., Tacke M., Swamy M., Schamel W.W., Elsässer-Beile U. A bispecific diabody directed against prostate-specific membrane antigen and CD3 induces T-cell mediated lysis of prostate cancer cells. Cancer Immunol. Immunother. 2008;57:43–52. doi: 10.1007/s00262-007-0348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bühler P., Molnar E., Dopfer E.P., Wolf P., Gierschner D., Wetterauer U., Schamel W.W., Elsässer-Beile U. Target-dependent T-cell activation by coligation with a PSMA x CD3 diabody induces lysis of prostate cancer cells. J. Immunother. 2009;32:565–573. doi: 10.1097/CJI.0b013e3181a697eb. [DOI] [PubMed] [Google Scholar]
- 79.Fortmüller K., Alt K., Gierschner D., Wolf P., Baum V., Freudenberg N., Wetterauer U., Elsässer-Beile U., Bühler P. Effective targeting of prostate cancer by lymphocytes redirected by a PSMA × CD3 bispecific single-chain diabody. Prostate. 2011;71:588–596. doi: 10.1002/pros.21274. [DOI] [PubMed] [Google Scholar]
- 80.Kipriyanov S.M., Moldenhauer G., Martin A.C., Kupriyanova O.A., Little M. Two amino acid mutations in an anti-human CD3 single chain Fv antibody fragment that affect the yield on bacterial secretion but not the affinity. Protein Eng. 1997;10:445–453. doi: 10.1093/protein/10.4.445. [DOI] [PubMed] [Google Scholar]
- 81.The Leukemia & Lymphoma Society. (2018). Facts 2017-2018. https://www.lls.org/sites/default/files/file_assets/PS80_Facts2017-2018.pdf.
- 82.Showel M.M., Levis M. Advances in treating acute myeloid leukemia. F1000Prime Rep. 2014;6:96. doi: 10.12703/P6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perl A.E. The role of targeted therapy in the management of patients with AML. Hematology (Am. Soc. Hematol. Educ. Program) 2017;2017:54–65. doi: 10.1182/asheducation-2017.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schürch C.M. Therapeutic antibodies for myeloid neoplasms-current developments and future directions. Front. Oncol. 2018;8:152. doi: 10.3389/fonc.2018.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laing A.A., Harrison C.J., Gibson B.E.S., Keeshan K. Unlocking the potential of anti-CD33 therapy in adult and childhood acute myeloid leukemia. Exp. Hematol. 2017;54:40–50. doi: 10.1016/j.exphem.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 86.Ehninger A., Kramer M., Röllig C., Thiede C., Bornhäuser M., von Bonin M., Wermke M., Feldmann A., Bachmann M., Ehninger G., Oelschlägel U. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014;4:e218. doi: 10.1038/bcj.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Friedrich M., Henn A., Raum T., Bajtus M., Matthes K., Hendrich L., Wahl J., Hoffmann P., Kischel R., Kvesic M. Preclinical characterization of AMG 330, a CD3/CD33-bispecific T-cell-engaging antibody with potential for treatment of acute myelogenous leukemia. Mol. Cancer Ther. 2014;13:1549–1557. doi: 10.1158/1535-7163.MCT-13-0956. [DOI] [PubMed] [Google Scholar]
- 88.Laszlo G.S., Gudgeon C.J., Harrington K.H., Dell’Aringa J., Newhall K.J., Means G.D., Sinclair A.M., Kischel R., Frankel S.R., Walter R.B. Cellular determinants for preclinical activity of a novel CD33/CD3 bispecific T-cell engager (BiTE) antibody, AMG 330, against human AML. Blood. 2014;123:554–561. doi: 10.1182/blood-2013-09-527044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aigner M., Feulner J., Schaffer S., Kischel R., Kufer P., Schneider K., Henn A., Rattel B., Friedrich M., Baeuerle P.A. T lymphocytes can be effectively recruited for ex vivo and in vivo lysis of AML blasts by a novel CD33/CD3-bispecific BiTE antibody construct. Leukemia. 2013;27:1107–1115. doi: 10.1038/leu.2012.341. [DOI] [PubMed] [Google Scholar]
- 90.Ravandi F., Stein A.S., Kantarjian H.M., Walter R.B., Paschka P., Jongen-Lavrencic M., Ossenkoppele G.J., Yang Z., Mehta B., Subklewe M. A phase 1 first-in-human study of AMG 330, an anti-CD33 bispecific T-cell engager (BiTE®) antibody construct, in relapsed/refractory acute myeloid leukemia (R/R AML) Blood. 2018;132:25. [Google Scholar]
- 91.Carpenter R.O., Evbuomwan M.O., Pittaluga S., Rose J.J., Raffeld M., Yang S., Gress R.E., Hakim F.T., Kochenderfer J.N. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin. Cancer Res. 2013;19:2048–2060. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li J., Wang N., Tesfaluul N., Gao X., Liu S., Yue B. Prognostic value of circulating plasma cells in patients with multiple myeloma: A meta-analysis. PLoS ONE. 2017;12:e0181447. doi: 10.1371/journal.pone.0181447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cho S.F., Anderson K.C., Tai Y.T. Targeting B cell maturation antigen (BCMA) in multiple myeloma: potential uses of BCMA-based immunotherapy. Front. Immunol. 2018;9:1821. doi: 10.3389/fimmu.2018.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Topp M.S., Duell J., Zugmaier G., Attal M., Moreau P., Langer C., Kroenke J., Facon T., Einsele H., Munzert G. Treatment with AMG 420, an anti-B-cell maturation antigen (BCMA) bispecific T-cell engager (BiTE®) antibody construct, induces minimal residual disease (MRD) negative complete responses in relapsed and/or refractory (R/R) multiple myeloma (MM) patients: results of a first-in-human (FIH) phase I dose escalation study. Blood. 2018;132:1010. [Google Scholar]
- 95.Wang K., Wei G., Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp. Hematol. Oncol. 2012;1:36. doi: 10.1186/2162-3619-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reusch U., Duell J., Ellwanger K., Herbrecht C., Knackmuss S.H., Fucek I., Eser M., McAleese F., Molkenthin V., Gall F.L. A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19(+) tumor cells. MAbs. 2015;7:584–604. doi: 10.1080/19420862.2015.1029216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.GlobeNewswire (2018). Affimed places AFM11 (CD19/CD3-targeting T cell engager) phase 1 program on clinical hold. https://www.globenewswire.com/news-release/2018/10/08/1618171/0/en/Affimed-Places-AFM11-CD19-CD3-Targeting-T-cell-Engager-Phase-1-Program-on-Clinical-Hold.html.
- 98.Liu L., Lam C.K., Long V., Widjaja L., Yang Y., Li H., Jin L., Burke S., Gorlatov S., Brown J. MGD011, a CD19 x CD3 dual-affinity retargeting bi-specific molecule incorporating extended circulating half-life for the treatment of B-cell malignancies. Clin. Cancer Res. 2017;23:1506–1518. doi: 10.1158/1078-0432.CCR-16-0666. [DOI] [PubMed] [Google Scholar]
- 99.Liu L., Lam A., Alderson R., Yang Y., Li H., Long V., Gorlatov S., Burke S., Ciccarone V., Nordstrom A. MGD011, humanized CD19 × CD3 DART® protein with enhanced pharmacokinetic properties, demonstrates potent T-Cell mediated anti-tumor activity in preclinical models and durable B-cell depletion in cynomolgus monkeys following once-a-week dosing. Blood. 2014;124:1775. [Google Scholar]
- 100.House, D.W. (2017). Janssen bails on duvortuxizumab development deal with MacroGenics after neurotoxicity observed in early-stage study. https://seekingalpha.com/news/3293160-janssen-bails-duvortuxizumab-development-deal-macrogenics-neurotoxicity-observed-early-stage.
- 101.MacroGenics (2017). MacroGenics announces termination of duvortuxizumab collaboration and license agreement with Janssen. http://ir.macrogenics.com/news-releases/news-release-details/macrogenics-announces-termination-duvortuxizumab-collaboration.
- 102.Fisher T.S., Hooper A.T., Lucas J., Clark T.H., Rohner A.K., Peano B., Elliott M.W., Tsaparikos K., Wang H., Golas J. A CD3-bispecific molecule targeting P-cadherin demonstrates T cell-mediated regression of established solid tumors in mice. Cancer Immunol. Immunother. 2018;67:247–259. doi: 10.1007/s00262-017-2081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Testa U., Pelosi E., Frankel A. CD 123 is a membrane biomarker and a therapeutic target in hematologic malignancies. Biomark. Res. 2014;2:4. doi: 10.1186/2050-7771-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Al-Hussaini M., Rettig M.P., Ritchey J.K., Karpova D., Uy G.L., Eissenberg L.G., Gao F., Eades W.C., Bonvini E., Chichili G.R. Targeting CD123 in acute myeloid leukemia using a T-cell-directed dual-affinity retargeting platform. Blood. 2016;127:122–131. doi: 10.1182/blood-2014-05-575704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wittwer N.L., Brumatti G., Marchant C., Sandow J.J., Pudney M.K., Dottore M., D’Andrea R.J., Lopez A.F., Ekert P.G., Ramshaw H.S. High CD123 levels enhance proliferation in response to IL-3, but reduce chemotaxis by downregulating CXCR4 expression. Blood Adv. 2017;1:1067–1079. doi: 10.1182/bloodadvances.2016002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chichili G.R., Huang L., Li H., Burke S., He L., Tang Q., Jin L., Gorlatov S., Ciccarone V., Chen F. A CD3xCD123 bispecific DART for redirecting host T cells to myelogenous leukemia: preclinical activity and safety in nonhuman primates. Sci. Transl. Med. 2015;7:289ra82. doi: 10.1126/scitranslmed.aaa5693. [DOI] [PubMed] [Google Scholar]
- 107.Vey N., Davidson-Moncada J., Uy G.L., Foster M., Rizzieri D., Godwin J., Topp M., Ciceri F., Carrabba M., Martinelli G. Interim results from a phase 1 first-in-human study of flotetuzumab, a CD123 x CD3 bispecific DART molecule, in AML/MDS. Ann. Oncol. 2017;28 mdx373.001. [Google Scholar]
- 108.He X., Kläsener K., Iype J.M., Becker M., Maity P.C., Cavallari M., Nielsen P.J., Yang J., Reth M. Continuous signaling of CD79b and CD19 is required for the fitness of Burkitt lymphoma B cells. EMBO J. 2018;37:e97980. doi: 10.15252/embj.201797980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Veri M.C., Burke S., Huang L., Li H., Gorlatov S., Tuaillon N., Rainey G.J., Ciccarone V., Zhang T., Shah K. Therapeutic control of B cell activation via recruitment of Fcgamma receptor IIb (CD32B) inhibitory function with a novel bispecific antibody scaffold. Arthritis Rheum. 2010;62:1933–1943. doi: 10.1002/art.27477. [DOI] [PubMed] [Google Scholar]
- 110.Stohl W. Future prospects in biologic therapy for systemic lupus erythematosus. Nat. Rev. Rheumatol. 2013;9:705–720. doi: 10.1038/nrrheum.2013.136. [DOI] [PubMed] [Google Scholar]
- 111.Pandya N., Chen W., Lohr J., Yao X.-T., Burns R., Li H., Li H., Muth J., Goldwater R., Bonvini E. Safety, tolerability, and functional activity of MGD010, a Dart® molecule targeting CD32B and CD79B, following a single dose administration in healthy volunteers. Ann. Rheum. Dis. 2016;75:132–133. [Google Scholar]
- 112.Chen W., Shankar S., Lohr J., Yao X.-T., Li H., Chen X., Muth J., Gal-Edd N., Bonvini E., Johnson S. SAT0027 Immunomodulatory effects of MGD010, a dart® molecule targeting human B-cell CD32B and CD79B. Ann. Rheum. Dis. 2017;76:777–778. [Google Scholar]
- 113.Blazar B.R., Levy R.B., Mak T.W., Panoskaltsis-Mortari A., Muta H., Jones M., Roskos M., Serody J.S., Yagita H., Podack E.R., Taylor P.A. CD30/CD30 ligand (CD153) interaction regulates CD4+ T cell-mediated graft-versus-host disease. J. Immunol. 2004;173:2933–2941. doi: 10.4049/jimmunol.173.5.2933. [DOI] [PubMed] [Google Scholar]
- 114.Hu S., Xu-Monette Z.Y., Balasubramanyam A., Manyam G.C., Visco C., Tzankov A., Liu W.M., Miranda R.N., Zhang L., Montes-Moreno S. CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: a report from the International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2013;121:2715–2724. doi: 10.1182/blood-2012-10-461848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van der Weyden C.A., Pileri S.A., Feldman A.L., Whisstock J., Prince H.M. Understanding CD30 biology and therapeutic targeting: a historical perspective providing insight into future directions. Blood Cancer J. 2017;7:e603. doi: 10.1038/bcj.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jacobsen E.D., Sharman J.P., Oki Y., Advani R.H., Winter J.N., Bello C.M., Spitzer G., Palanca-Wessels M.C., Kennedy D.A., Levine P. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125:1394–1402. doi: 10.1182/blood-2014-09-598763. [DOI] [PubMed] [Google Scholar]
- 117.Bhatt S., Ashlock B.M., Natkunam Y., Sujoy V., Chapman J.R., Ramos J.C., Mesri E.A., Lossos I.S. CD30 targeting with brentuximab vedotin: a novel therapeutic approach to primary effusion lymphoma. Blood. 2013;122:1233–1242. doi: 10.1182/blood-2013-01-481713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rothe A., Sasse S., Topp M.S., Eichenauer D.A., Hummel H., Reiners K.S., Dietlein M., Kuhnert G., Kessler J., Buerkle C. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;125:4024–4031. doi: 10.1182/blood-2014-12-614636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reusch U., Burkhardt C., Fucek I., Le Gall F., Le Gall M., Hoffmann K., Knackmuss S.H., Kiprijanov S., Little M., Zhukovsky E.A. A novel tetravalent bispecific TandAb (CD30/CD16A) efficiently recruits NK cells for the lysis of CD30+ tumor cells. MAbs. 2014;6:728–739. doi: 10.4161/mabs.28591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.CNBC. (2018). Affimed reports financial results for fourth quarter and year end 2017. https://www.cnbc.com/2018/03/20/globe-newswire-affimed-reports-financial-results-for-fourth-quarter-and-year-end-2017.html.
- 121.Zhao X., Rajasekaran N., Reusch U., Marschner J.-P., Treder M., Kohrt H. CD30/CD16A Tandab AFM13-induced target cell lysis by NK-cells is enhanced by CD137 co-stimulation and blocking PD-1. Blood. 2015;126:2747. [Google Scholar]
- 122.Armand P., Shipp M.A., Ribrag V., Michot J.M., Zinzani P.L., Kuruvilla J., Snyder E.S., Ricart A.D., Balakumaran A., Rose S., Moskowitz C.H. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J. Clin. Oncol. 2016;34:3733–3739. doi: 10.1200/JCO.2016.67.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen R., Zinzani P.L., Fanale M.A., Armand P., Johnson N.A., Brice P., Radford J., Ribrag V., Molin D., Vassilakopoulos T.P., KEYNOTE-087 Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J. Clin. Oncol. 2017;35:2125–2132. doi: 10.1200/JCO.2016.72.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bartlett N.L., Chen R.W., Domingo-Domenech E., Forero-Torres A., Garcia-Sanz R., Armand P., Devata S., Rodriguez Izquierdo A., Lossos I.S., Reeder C.B. A phase 1b study investigating the combination of the tetravalent bispecific NK cell engager AFM13 and pembrolizumab in patients with relapsed/refractory Hodgkin lymphoma after brentuximab vedotin failure: updated safety and efficacy data. Blood. 2018;132:1620. [Google Scholar]
- 125.GlobeNewswire. (2018). Affimed reports new data for AFM13 from two separate clinical trials in Hodgkin and CD30-positive lymphomas. https://www.globenewswire.com/news-release/2018/02/01/1330095/0/en/Affimed-Reports-New-Data-for-AFM13-from-Two-Separate-Clinical-Trials-in-Hodgkin-and-CD30-Positive-Lymphomas.html.
- 126.Richards D.A., Braiteh F.S., Garcia A.A., Denlinger C.S., Conkling P.R., Edenfield W.J., Anthony S.P., Hellerstedt B.A., Raju R.N., Becerra C. A phase 1 study of MM-111, a bispecific HER2/HER3 antibody fusion protein, combined with multiple treatment regimens in patients with advanced HER2-positive solid tumors. J. Clin. Oncol. 2014;32:651. [Google Scholar]
- 127.Kirouac D.C., Du J.Y., Lahdenranta J., Overland R., Yarar D., Paragas V., Pace E., McDonagh C.F., Nielsen U.B., Onsum M.D. Computational modeling of ERBB2-amplified breast cancer identifies combined ErbB2/3 blockade as superior to the combination of MEK and AKT inhibitors. Sci. Signal. 2013;6:ra68. doi: 10.1126/scisignal.2004008. [DOI] [PubMed] [Google Scholar]
- 128.Yu S., Liu Q., Han X., Qin S., Zhao W., Li A., Wu K. Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment. Exp. Hematol. Oncol. 2017;6:31. doi: 10.1186/s40164-017-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang B., Lahdenranta J., Du J., Kirouac D., Nguyen S., Overland R., Paragas V., Kudla A., Nielsen U., McDonagh C. Abstract 4633: MM-111, a bispecific HER2 and HER3 antibody, synergistically combines with trastuzumab and paclitaxel in preclinical models of gastric cancer. Cancer Res. 2013;73:4633. [Google Scholar]
- 130.Zhang B., Nguyen S., Huhalov A., Nielsen U.B., Niyikiza C., McDonagh C.F., Kudla A.J., Onsum M. Abstract 1888: MM-111, a bispecific HER2 and HER3 antibody, inhibits trastuzumab-resistant tumor cell growth. Cancer Res. 2012;72:1888. [Google Scholar]
- 131.Merrimack (2014). Merrimack Pharmaceuticals presents phase 1 clinical data supporting four novel antibody therapeutic programs at the 2014 ASCO Annual Meeting. http://investors.merrimackpharma.com/news-releases/news-release-details/merrimack-pharmaceuticals-presents-phase-1-clinical-data.
- 132.Isakoff S., Bahleda R., Saleh M., Bordoni R., Shields A., Dauer J., Curley M., Baum J., McClure T., Louis C.U. A phase 1 study of MM-141, a novel tetravalent monoclonal antibody targeting IGF-1R and ErbB3, in relapsed or refractory solid tumors. Eur. J. Cancer. 2016;69:S137–S138. [Google Scholar]
- 133.Fitzgerald J.B., Johnson B.W., Baum J., Adams S., Iadevaia S., Tang J., Rimkunas V., Xu L., Kohli N., Rennard R. MM-141, an IGF-IR- and ErbB3-directed bispecific antibody, overcomes network adaptations that limit activity of IGF-IR inhibitors. Mol. Cancer Ther. 2014;13:410–425. doi: 10.1158/1535-7163.MCT-13-0255. [DOI] [PubMed] [Google Scholar]
- 134.Isakoff S.J., Saleh M.N., Lugovskoy A., Mathews S., Czibere A.G., Shields A.F., Bahleda R., Soria J.-C., Arnedos M. First-in-human study of MM-141: a novel tetravalent monoclonal antibody targeting IGF-1R and ErbB3. J. Clin. Oncol. 2015;33:384. [Google Scholar]
- 135.Ko A.H., Cubillo A., Kundranda M., Zafar S.F., Meiri E., Bendell J., Alguel H., Rivera Herrero F., Ahn E., Watkins D. CARRIE: a randomized, double-blind, placebo-controlled phase II study of istiratumab (MM-141) plus nab-paclitaxel and gemcitabine versus nab-paclitaxel and gemcitabine in front-line metastatic pancreatic cancer. Ann. Oncol. 2018;29 doi: 10.1016/j.annonc.2019.09.004. mdy424.031. [DOI] [PubMed] [Google Scholar]
- 136.Irani N., Basardeh E., Samiee F., Fateh A., Shooraj F., Rahimi A., Shahcheraghi F., Vaziri F., Masoumi M., Pazhouhandeh M. The inhibitory effect of the combination of two new peptides on biofilm formation by Acinetobacter baumannii. Microb. Pathog. 2018;121:310–317. doi: 10.1016/j.micpath.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 137.Thanabalasuriar A., Surewaard B.G.J., Willson M.E., Neupane A.S., Stover C.K., Warrener P., Wilson G., Keller A.E., Sellman B.R., DiGiandomenico A., Kubes P. Bispecific antibody targets multiple Pseudomonas aeruginosa evasion mechanisms in the lung vasculature. J. Clin. Invest. 2017;127:2249–2261. doi: 10.1172/JCI89652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bezabeh B., Fleming R., Fazenbaker C., Zhong H., Coffman K., Yu X.-Q., Leow C.C., Gibson N., Wilson S., Stover C.K. Insertion of scFv into the hinge domain of full-length IgG1 monoclonal antibody results in tetravalent bispecific molecule with robust properties. MAbs. 2017;9:240–256. doi: 10.1080/19420862.2016.1270492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tabor D.E., Oganesyan V., Keller A.E., Yu L., McLaughlin R.E., Song E., Warrener P., Rosenthal K., Esser M., Qi Y. Pseudomonas aeruginosa PcrV and Psl, the molecular targets of bispecific antibody MEDI3902, are conserved among diverse global clinical isolates. J. Infect. Dis. 2018;218:1983–1994. doi: 10.1093/infdis/jiy438. [DOI] [PubMed] [Google Scholar]
- 140.Ali S.O., Yu X.Q., Robbie G.J., Wu Y., Shoemaker K., Yu L., DiGiandomenico A., Keller A.E., Anude C., Hernandez-Illas M. Phase 1 study of MEDI3902, an investigational anti-Pseudomonas aeruginosa PcrV and Psl bispecific human monoclonal antibody, in healthy adults. Clin. Microbiol. Infect. 2018 doi: 10.1016/j.cmi.2018.08.004. Published online August 11, 2018. [DOI] [PubMed] [Google Scholar]
- 142.Kantarjian H., Stein A., Gökbuget N., Fielding A.K., Schuh A.C., Ribera J.M., Wei A., Dombret H., Foà R., Bassan R. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 2017;376:836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Larson, R.A. (2018). Treatment of relapsed or refractory acute lymphoblastic leukemia in adults. https://www.uptodate.com/home/linking-policy?&redirect=true.
- 144.Wei G., Wang J., Huang H., Zhao Y. Novel immunotherapies for adult patients with B-lineage acute lymphoblastic leukemia. J. Hematol. Oncol. 2017;10:150. doi: 10.1186/s13045-017-0516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ajorloo F., Vaezi M., Saadat A., Safaee S.R., Gharib B., Ghanei M., Siadat S.D., Vaziri F., Fateh A., Pazhouhandeh M. A systems medicine approach for finding target proteins affecting treatment outcomes in patients with non-Hodgkin lymphoma. PLoS ONE. 2017;12:e0183969. doi: 10.1371/journal.pone.0183969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pazhouhandeh M., Samiee F., Boniadi T., Khedmat A.F., Vahedi E., Mirdamadi M., Sigari N., Siadat S.D., Vaziri F., Fateh A. Comparative network analysis of patients with non-small cell lung cancer and smokers for representing potential therapeutic targets. Sci. Rep. 2017;7:13812. doi: 10.1038/s41598-017-14195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pazhouhandeh M., Sahraian M.A., Siadat S.D., Fateh A., Vaziri F., Tabrizi F., Ajorloo F., Arshadi A.K., Fatemi E., Piri Gavgani S. A systems medicine approach reveals disordered immune system and lipid metabolism in multiple sclerosis patients. Clin. Exp. Immunol. 2018;192:18–32. doi: 10.1111/cei.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lyman M., Lieuw V., Richardson R., Timmer A., Stewart C., Granger S., Woods R., Silacci M., Grabulovski D., Newman R. A bispecific antibody that targets IL-6 receptor and IL-17A for the potential therapy of patients with autoimmune and inflammatory diseases. J. Biol. Chem. 2018;293:9326–9334. doi: 10.1074/jbc.M117.818559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mullard A. Bispecific antibody pipeline moves beyond oncology. Nat. Rev. Drug Discov. 2017;16:666–668. doi: 10.1038/nrd.2017.187. [DOI] [PubMed] [Google Scholar]