Abstract
Strong associations between HLA alleles and infectious and autoimmune diseases are well established. Although obesity is also associated with these diseases, the relationship between HLA and obesity has not been systematically investigated in a large cohort. In the current study, we analyzed the association of HLA alleles with BMI using data from 1.3 million healthy adult donors from the Chinese Marrow Donor Program (CMDP). We found 23 HLA alleles, including 12 low-resolution and 11 high-resolution alleles, were significantly associated with BMI after correction for multiple testing. Alleles associated with high BMI were enriched in haplotypes that were common in both Chinese and European populations, whereas the alleles associated with low BMI were enriched in haplotypes common only in Asians. Alleles B*07, DRB1*07, DRB1*12, and C*03:02 provided the strongest associations with BMI (P = 6.89 × 10−10, 1.32 × 10−9, 1.52 × 10−9, and 4.45 × 10−8, respectively), where B*07 and DRB1*07 also had evidence for sex-specific effects (Pheterogeneity = 0.0067 and 0.00058, respectively). These results, which identify associations between alleles of HLA-B, DRB1, and C with BMI in Chinese young adults, implicate a novel biological connection between HLA alleles and obesity.
Introduction
Being overweight (BMI of 25–29.9 kg/m2) or obese (BMI ≥30 kg/m2) is well known to be a risk factor for several chronic diseases, including type 2 diabetes (T2D), cardiovascular disease, hypertension, and cancer. Because BMI is heritable, many groups have sought to identify its genetic determinants. Genome-wide association studies (GWAS) in individuals of European and Asian descent have identified >100 loci that associate with BMI or an obesity-related measure (1–6). However, commonly used GWAS platforms do not accurately capture genotypic variation within the HLA region due to its sequence complexity. In contrast, focused analyses of the HLA region have identified several alleles that have notable effects on disease susceptibility, including infectious diseases, autoimmune diseases, cancer, schizophrenia, and T2D (7–11). Despite obesity being a major risk factor for several of these diseases, no large-scale study published to date has interrogated the HLA region to determine whether HLA alleles associate with BMI. Among the few studies that have investigated HLA in relation to obesity in small cohorts, the results have been inconsistent (12–15).
The HLA region, also known as the MHC, encompasses ∼3.6 Mb on chromosome 6p21.3 and includes five classic transplantation HLA genes: HLA-A, HLA-C, HLA-B, HLA-DRB1, and HLA-DQB1. There are two classes of MHC proteins, MHC class I (HLA-A, -B, and -C), which present peptides from inside the cell to T cells (endogenous antigens), and MHC class II (HLA-DP, -DM, -DOA, -DOB, -DQ, and -DR), which present antigens from outside of the cell (exogenous antigens) (16). HLA alleles affect the response to many infectious and autoimmune diseases due to their essential role in activation of both natural killer cells and T cells (17,18). Response to pathogens, such as human and nonhuman viruses, scrapie agents, bacteria, and gut microflora, has been reported to cause or exacerbate obesity in various experimental models (19). For example, the gut microbiota has been linked to multiple human diseases, including obesity (20,21), and human adenoviruses (Ad) 36, Ad-37, and Ad-5 have been shown to stimulate enzymes and transcription factors that cause accumulation of triglycerides and affect the differentiation of preadipocytes into mature adipocytes (22,23). Therefore, the diversity in HLA alleles may differentially alter the gut microbiota as well as differentially affect response to pathogens or infection, which in turn could have a subtle effect on obesity.
To determine whether a relationship exists between HLA alleles and BMI, we used the statistical power of data obtained from 1.3 million healthy donors aged 18–50 years. Data were derived from the Data Bank of Chinese Hematopoietic Stem Cell Donors, also known as the Chinese Marrow Donor Program (CMDP), which has information on low-resolution and high-resolution HLA genotypes and anthropometric measures on >1.8 million donors through 31 branch registries.
Research Design and Methods
Study Participants and Data Collection
The CMDP began in the year 2001 and is operated under the guidance of the Red Cross Society of China. Through this program, anthropometric data on >1.8 million donors from 31 branch registries have been collected. These branches cover the major geographic areas of all 31 provinces, autonomous regions, and municipalities in mainland China (24).
In the current study, basic information on the donor (age, sex, ethnicity/race) and anthropometric measures (weight, height) of 1,816,443 subjects with unique CMDP identification numbers (from dates 2 October 2001 to 2 October 2014) were obtained from the CMDP. Excluded were individuals with missing data on weight, height, age, or sex (n = 600,352) and individuals who were outliers for body weight (>216 kg or <30.8 kg) or height (>194.1 cm or <132.2 cm), as defined by the Survey on the Status of Nutrition and Health of the Chinese People conducted in 2002 (25) (n = 11,871). Also excluded were individuals aged <18 years or >50 years (n = 12,338) because relatively few individuals were in these age-groups in this database. All individuals examined from 2001 to 2003 (n = 23,847) were further excluded because of the small sample size and uneven distribution across different provinces. After these exclusions, data for 1,377,047 adults were available for analysis.
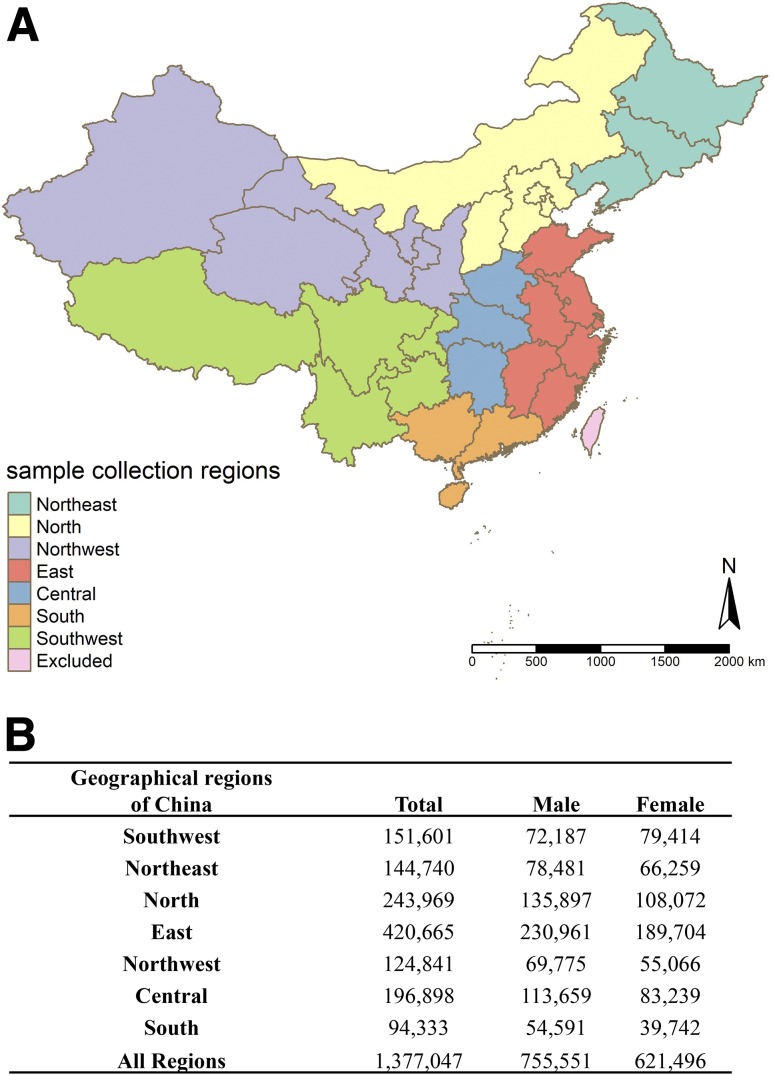
Individuals from the different provinces were grouped into seven geographical regions and analyzed separately. The seven regions are as follows: 1) Northeast China (Heilongjiang, Jilin, and Liaoning); 2) North China (Beijing, Tianjin, Hebei, Shanxi, and Inner Mongolia); 3) Northwest China (Xinjiang, Qinghai, Gansu, Ningxia, and Shaanxi); 4) East China (Shanghai, Shandong, Jiangsu, Anhui, Zhejiang, Jiangxi, and Fujian); 5) Central China (Henan, Hubei, Hunan); 6) South China (Guangdong, Guangxi, Hainan); and 7) Southwest China (Chongqing, Tibet, Yunnan, Sichuan, Guizhou). The locations of the seven geographical regions and the sample size from each region are shown in Fig. 1A and B. Taiwan, Hong Kong, and Macau were not included in this study.
Figure 1.
Seven geographical regions in China map (A) and sample size from each region (B). Individuals from the different provinces were grouped into seven geographical regions and analyzed separately. The seven regions are as follows: 1) Northeast China (Heilongjiang, Jilin, and Liaoning); 2) North China (Beijing, Tianjin, Hebei, Shanxi, and Inner Mongolia); 3) Northwest China (Xinjiang, Qinghai, Gansu, Ningxia, and Shaanxi); 4) East China (Shanghai, Shandong, Jiangsu, Anhui, Zhejiang, Jiangxi, and Fujian); 5) Central China (Henan, Hubei, Hunan); 6) South China (Guangdong, Guangxi, Hainan); and 7) Southwest China (Chongqing, Tibet, Yunnan, Sichuan, Guizhou). Taiwan, Hong Kong, and Macau were not included in this study.
The Institutional Review Board of the First Affiliated Hospital within Nanjing Medical University and the CMDP Ethics Review Board approved the study protocol.
Study Outcome Definitions
Anthropometric measurements were made by trained technicians in mobile examination centers at each of the 31 CMDP branch registries. Height (cm) and weight (kg) were measured using metal weighing scales with height rods. Subjects were required to stand straight on the instrument, barefoot, and at ease. BMI was calculated as weight in kilograms divided by height in meters squared and rounded to the nearest tenth. Birth date and sex were obtained from the individual’s identification card, and age was calculated using the sampling date and birth date.
HLA Genotyping
HLA typing was performed at 31 CMDP-accredited HLA typing laboratories. HLA typing was done by serological methods from 2001 to 2005. In 2005, high-resolution HLA genotyping was performed using three methods: PCR–reverse sequence-specific oligonucleotide probes method (26), Sanger sequencing-based typing, and next-generation high-throughput sequence-based typing (27). HLA data, including HLA-A, -B, -C, -DRB1, and -DQB1, were submitted to the CMDP database annually by each HLA typing laboratory. HLA high-resolution genotypes are given a four-digit code representing protein level assignment, and HLA low-resolution genotypes are given a two-digit code.
Statistical Analysis
For each HLA locus, multiple alleles were expanded as multiple biallelic markers for each testing allele by custom python scripts (https://github.com/zhenyisong/guo.project). Homozygotes for the testing allele (T/T), heterozygotes (T/O), and homozygotes for the other allele combined (O/O) were coded as a continuous numeric variable for genotype (2, 1, and 0 copies of the testing allele). Association analysis with BMI was performed using multiple linear regression with adjustment for age, sex, residential province, and sampling year in each of the seven geographical regions separately, and then meta-analyzed using the inverse variance method, which is based on β values or the odds ratio (OR) and SE from each geographical region. Cochran’s Q statistics were used to assess consistency of effect and to quantify heterogeneity among the seven regions. The regression analysis and meta-analysis were performed in Golden Helix SNP & Variation Suite 8.1 software (Bozeman, MT). A Bonferroni multiple testing correction was used to account for the number of common low-resolution or high-resolution HLA alleles.
Multiple linear regression, as described above, was repeated for men and women separately with correction for age, residential province, and sampling year in each of the seven geographical regions separately. A meta-analysis was performed to combine a sex-differentiated test of association and a test of heterogeneity in allelic effects between men and women implemented in GWAMA (Genome-Wide Association Meta-Analysis) software (28).
The variance explained by a single HLA allele was calculated by 2 × MAF × (1 − MAF) × β2, where MAF is the minor allele frequency, and β is the HLA allele effect estimate, assuming an additive model, computed by meta-analysis (29,30).
Multilocus haplotypes were constructed, and their frequencies were calculated using the expectation–maximization algorithm based on a subset of the samples in this study using the Arlequin software (31).
The estimated allele frequencies in Chinese from the current study were compared with the corresponding allele frequencies of Europeans obtained from the Allele Frequency Net Database (http://www.allelefrequencies.net/). The allele count calculated from frequency and total number of the samples was used for χ2 or the Fisher exact test. Testing the difference in the proportions of alleles associated with high BMI between alleles that have significantly higher allele frequencies in European and those in Chinese was performed by the Fisher extract test. The significance level of variable difference used a value of P < 0.05.
Results
Characteristics of the Participants
A total of 1,377,047 subjects (755,551 men and 621,496 women) aged 18–50 years were included in the association analysis for BMI. The age distribution for men and women was asymmetric due to an overrepresentation of younger people who typically participate in the CMDP. The mean age of subjects included in the final analysis was 27.2 for men and 25.6 years for women (Supplementary Fig. 1).
Association Between HLA Alleles and BMI
Approximately 99% of the individuals had low-resolution HLA genotypic data, where 125 different alleles were detected, and ∼67% of the individuals had both low- and high-resolution HLA genotypic data, where 1,455 different alleles were detected (allele frequencies reported in Supplementary Table 1). To preserve statistical power and reduce the multiple testing burden, we initially focused on 72 low-resolution alleles (57% of total) with a minor allele frequency >0.001 and performed a multiple linear regression analysis for BMI with adjustment for appropriate covariates in each geographical region separately. We then performed a meta-analysis for the seven geographical regions. Significance for the low-resolution alleles was defined as a P value of ≤0.0006 (0.05/72) based on a stringent Bonferroni correction, which assumes 72 independent tests, although linkage disequilibrium (LD) within the HLA region has been well documented. We then performed multiple linear regression analysis and meta-analysis for BMI on the 163 high-resolution alleles (11% of total) with a minor allele frequency >0.001. Significance for the high resolution alleles was defined as a P value of ≤0.0003 (0.05/163).
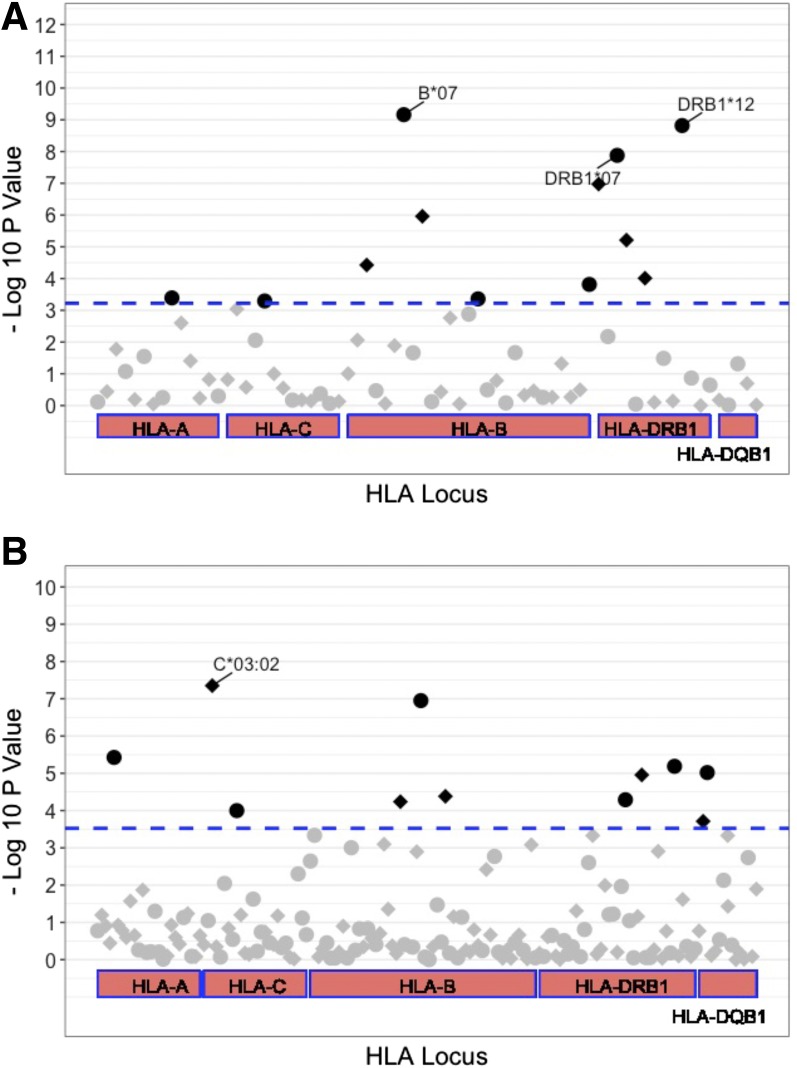
Meta-analysis of the results from the seven geographical regions showed that 12 low-resolution alleles (A*02, B*07, B*08, B*13, B*46, B*58, C*07, DRB1*03, DRB1*04, DRB1*07, DRB1*09, and DRB1*12) and 11 high-resolution alleles (A*02:01, B*07:02, B*27:04, B*40:01, C*01:03, C*03:02, DQB1*03:02, DQB1*06:02, DRB1*03:01, DRB1*07:01, and DRB1*12:02) were significantly associated with BMI (Table 1). Performance of a genotype pairwise correlation analysis among these alleles identified strong correlations (r2 > 0.3) between high-resolution alleles (A*02:01, B*07:02, DRB1*03:01, DRB1*07:01, and DRB1*12:02) and their respective low resolution alleles (A*02, B*07, DRB1*03, DRB1*07, and DRB1*12) (Fig. 5A and Supplementary Table 4), suggesting these high-resolution alleles are almost identical to their respective low-resolution alleles. Therefore, 19 unique HLA alleles were found to be associated BMI.
Table 1.
Meta-analysis results of 23 HLA alleles that associated with BMI in CMDP
| Allele | Gene | Frequency | P value | β ± SE | Explained variance (%) |
|---|---|---|---|---|---|
| A*02 | HLA-A | 0.306 | 4.07E-04 | 0.015 ± 0.004 | 0.010 |
| B*07 | HLA-B | 0.030 | 6.89E-10 | 0.071 ± 0.011 | 0.029 |
| B*08 | HLA-B | 0.009 | 1.53E-04 | 0.079 ± 0.021 | 0.011 |
| B*13 | HLA-B | 0.110 | 4.37E-04 | 0.022 ± 0.006 | 0.009 |
| B*46 | HLA-B | 0.100 | 1.10E-06 | 0.042 ± 0.009 | 0.032 |
| B*58 | HLA-B | 0.057 | 3.75E-05 | 0.086 ± 0.021 | 0.080 |
| C*07 | HLA-C | 0.176 | 5.09E-04 | 0.038 ± 0.011 | 0.042 |
| DRB1*03 | HLA-DRB1 | 0.048 | 1.06E-07 | 0.048 ± 0.009 | 0.021 |
| DRB1*04 | HLA-DRB1 | 0.113 | 6.15E-06 | 0.028 ± 0.006 | 0.016 |
| DRB1*07 | HLA-DRB1 | 0.093 | 1.32E-08 | 0.041 ± 0.007 | 0.028 |
| DRB1*09 | HLA-DRB1 | 0.145 | 9.76E-05 | 0.024 ± 0.006 | 0.014 |
| DRB1*12 | HLA-DRB1 | 0.121 | 1.52E-09 | 0.047 ± 0.008 | 0.047 |
| A*02:01 | HLA-A | 0.125 | 3.75E-06 | 0.035 ± 0.007 | 0.027 |
| B*07:02 | HLA-B | 0.022 | 1.12E-07 | 0.089 ± 0.017 | 0.034 |
| B*27:04 | HLA-B | 0.010 | 4.15E-05 | 0.113 ± 0.028 | 0.025 |
| B*40:01 | HLA-B | 0.099 | 5.78E-05 | 0.038 ± 0.009 | 0.026 |
| C*01:03 | HLA-C | 0.006 | 1.00E-04 | 0.182 ± 0.047 | 0.042 |
| C*03:02 | HLA-C | 0.059 | 4.45E-08 | 0.085 ± 0.016 | 0.081 |
| DQB1*03:02 | HLA-DQB1 | 0.057 | 1.94E-04 | 0.066 ± 0.018 | 0.047 |
| DQB1*06:02 | HLA-DQB1 | 0.076 | 9.55E-06 | 0.122 ± 0.028 | 0.210 |
| DRB1*03:01 | HLA-DRB1 | 0.048 | 1.11E-05 | 0.049 ± 0.011 | 0.022 |
| DRB1*07:01 | HLA-DRB1 | 0.093 | 6.49E-06 | 0.040 ± 0.009 | 0.027 |
| DRB1*12:02 | HLA-DRB1 | 0.082 | 5.13E-05 | 0.047 ± 0.012 | 0.033 |
Bold font indicates the HLA alleles with the strongest associations (P < 5.0 × 10−8). Significance is defined as P ≤ 0.0006 (0.05/72) for low-resolution alleles and P ≤ 0.0003 (0.05/163) for high-resolution HLA alleles based on a stringent Bonferroni correction. Explained variance (%) was estimated based on the effect sizes (β) and allele frequencies in CMDP. β ± SE is the regression slope and its SE.
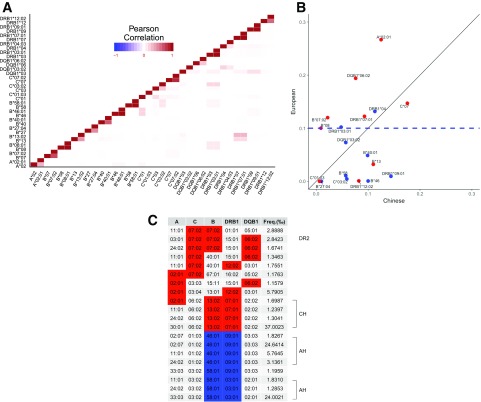
Figure 5.
BMI-associated HLA alleles and haplotype. A: Pairwise correlation matrix heat map of BMI-associated HLA alleles. The heat map grid represents the square of the Pearson correlation coefficient (r2). Three high-resolution alleles (DRB1*03:01, DRB1*07:01, and DRB1*09:01) are nearly perfectly correlated (r2 > 0.99) with their respective low-resolution alleles (DRB1*03, DRB1*07, and DRB1*09). In addition, B*07:02 is strongly correlated with B*07 (r2 = 0.73), and A*02:01 is strongly correlated with A*02 (r2 = 0.32). Among alleles associated with higher BMI, DRB1*07:01 and B*13:02 are strongly correlated (r2 = 0.31). Among alleles associated with lower BMI, B*46:01 is correlated with DRB1*09:01 (r2 = 0.028), and B*58:01 is correlated with DRB1*03:01 (r2 = 0.051). B: Allele frequency between European and Chinese. The allele frequencies in Europeans were derived from the Allele Frequency Net Database (http://www.allelefrequencies.net/), and the frequencies in Chinese are from the current study. Eighteen had significantly different allele frequencies between Chinese and European populations (χ2 or Fisher exact test P < 0.05). HLA alleles highlighted in red are the alleles associated with higher BMI, and the HLA alleles highlighted in blue are the alleles associated with lower BMI. The alleles around the diagonal line y = x are similar in frequency between European and Chinese. The blue horizontal dashed line represents the allele frequency of 0.1 in Europeans. Six (A*02:01, DQB1*06:02, DRB1*07:01, B*07:02, B*08, and C*07) of nine alleles associated with a higher BMI have an allele frequency >0.10 in Caucasians, whereas six (B*27:04, C*03:02, B*58, B*46, DRB1*09:01, and B*40:01) of nine alleles associated with lower BMI have an allele frequency <0.10 in Caucasians. There is no significant difference in the proportions of alleles associated with high BMI between alleles that have significantly higher allele frequencies in Caucasian and those in Chinese (Fisher exact test P > 0.05). C: Assignment of BMI-associated HLA alleles into five locus haplotypes. Blue box: alleles associated with lower BMI; red box: alleles associated with high BMI. The first row contains the gene name, A = HLA-A, C = HLA-C, B = HLA-B, DRB1 = HLA-DRB1, DQB1 = HLA-DQB1. Each row contains one common haplotype with at least two alleles associated with lower or higher BMI. The last column (Freq. [‰]) is the haplotype frequency reported in the Chinese population. Most of the 19 BMI-associated alleles mapped onto one of these common haplotypes and several alleles mapped to multiple haplotypes. Three alleles associated with high BMI (A*02:01, B*13:02, and DRB1*07:01) are in a four-locus haplotype (CH: C*06:02-B*13:02-DRB1*07:01-DQB1*02:02), two alleles associated with high BMI (B*07:02 and DQB1*06:02) are in the five-locus haplotype (DR2: A*03:01-C*07:02-B*07:02-DRB1*15:01-DQB1*06:02). Both of these haplotypes are common in Chinese and European populations. The latter is also called ancestral DR2 haplotype. A four-locus haplotype (AH) C*03:02-B*58:01-DRB1*03:01-DQB1*02:01 contains two alleles associated with lower BMI and has a higher frequency in Asians than Africans or Caucasians. Most of the haplotypes contained more than one risk or protective allele, suggesting high LD among these alleles.
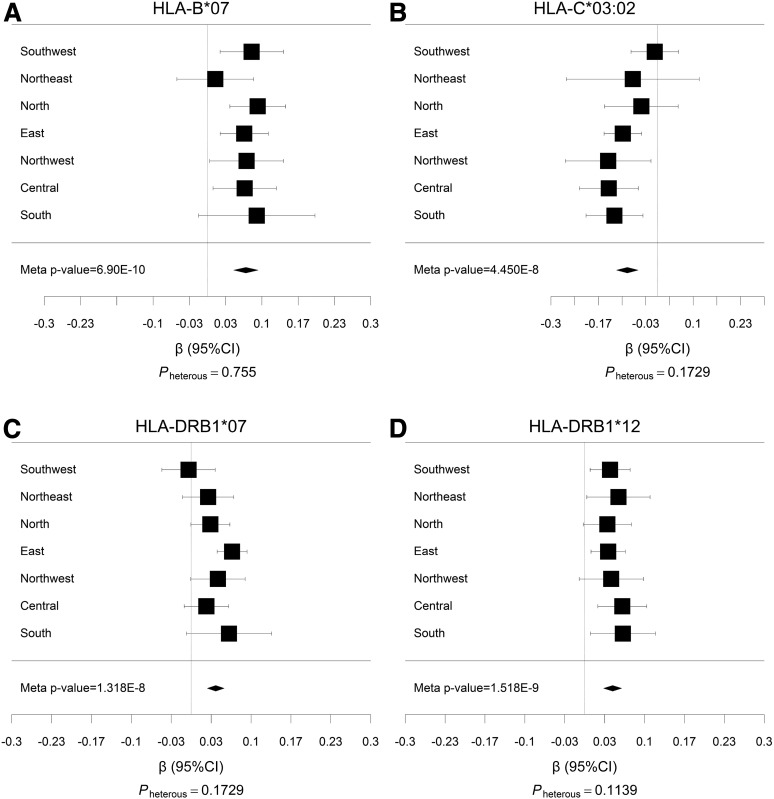
Three low-resolution alleles and one high-resolution allele had the strongest evidence for association with BMI (P < 5 × 10−8) (Fig. 2). These alleles are B*07 (P = 6.89 × 10−10) from the HLA-B locus, C*03:02 (P = 4.45 × 10−8) from the HLA-C locus, and DRB1*07 (P = 1.32 × 10−8) and DRB1*12 (P = 1.52 × 10−9) from the HLA-DRB1 locus. Heterogeneity in the direction of the effect was not detected among the different geographical regions (heterogeneity P > 0.05) (forest plots shown in Fig. 3). These alleles were also assessed for their association with categories of BMI, namely, overweight (BMI 25.0–30.0 kg/m2; n = ∼236,000 case subjects and ∼850,000 control subjects) and obese (BMI ≥30 kg/m2; n = ∼40,000 case subjects and ∼850,000 control subjects) (Supplementary Table 3). Notably, B*07, DRB1*07, and C*03:02, which had strong evidence for association with BMI as a quantitative trait, also associated with overweight (P = 7.80 × 10−5, 4.70 × 10−12, and 2.08 × 10−5, respectively) and obesity (P = 4.79 × 10−4, 0.03, and 4.05 × 10−3, respectively) (Supplementary Table 2).
Figure 2.
Scatter plots of association results of low-resolution (A) and high-resolution (B) HLA alleles with BMI in CMDP. The –log10 P values were plotted against their positions in each gene. HLA alleles associated with higher BMI are indicated by circles and HLA alleles associated with lower BMI are indicated by diamonds. The HLA alleles with the strongest association (P < 0.00000005) are labeled with their allele name. The horizontal dashed line represents the threshold for the statistical significance after Bonferroni correction (P = 0.0006 for low-resolution allele and P = 0.0003 for high-resolution alleles).
Figure 3.
Meta-analysis forest plots for HLA-B*07 (A), HLA-C*03:02 (B), HLA-DRB1*07 (C), and HLA-DRB1*12 (D), HLA alleles with the strongest associations with BMI in Chinese adults. Plots show the study-specific association estimates (β) and 95% CI for the seven geographical regions presented as bars. The scale is the β value. The association estimate and CI for the meta-analysis combining the seven geographical regions is shown as a diamond. Pheterous is the P value for heterogeneity among seven regions.
Sex-Stratified Analyses
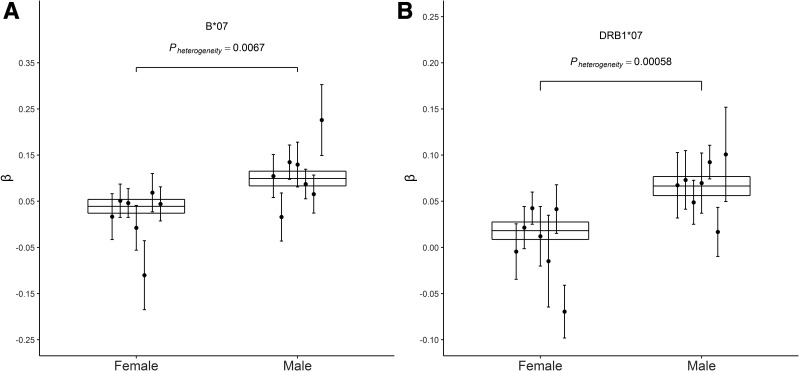
We performed sex-specific meta-analyses to test for an association of each allele with BMI in men and women separately, which identified nominal evidence for heterogeneity (P < 0.05) for the DRB1*07 and B*07 alleles. The sex-specific effects of DRB1*07 (Pheterogeneity = 0.00058) met Bonferroni significance (significance requires Pheterogeneity ≤ 0.002; 0.05/23 alleles tested), where the association was much stronger in men (β = 0.066, P = 1.54 ×10−10) than in women (β = 0.018, P = 0.056) (Fig. 4). The association between B*07 and BMI was also stronger in men (β = 0.099, P = 1.50 ×10−9) than in women (β = 0.039, P = 0.009), although the evidence for heterogeneity for this allele was statistically nominal (Pheterogeneity = 0.0067).
Figure 4.
HLA alleles that associate with BMI in a sex-dependent manner. Each of the seven geographic study-specific association estimates (β; represented by a circle) and SE (represented by a vertical line) are shown in males and females separately for B*07 (A) and DRB1*07 (B). The meta-analysis mean and SE (represented by the horizontal lines) for all seven studies was calculated using the GWAMA software. Pheterogeneity is the P value for heterogeneity between male and female.
Comparison of HLA Allele Frequencies in Chinese and Europeans
Among the 19 unique HLA alleles that associated with BMI in our Chinese cohort, 18 had significantly different allele frequencies between Chinese and European populations (χ2 or Fisher exact test, P < 0.05). Of the eight HLA alleles with higher frequency in Europeans, five associated with a higher BMI (A*02:01, B*08, B*07:02, DRB1*07:01, and DQB1*06:02) and three associated with lower BMI (DRB1*04, DRB1*03:01, and DQB1*03:02) in our study of the Chinese cohort. Of the 10 HLA alleles with higher frequency in Chinese, 4 associated with a high BMI (B*13, C*01:03, C*07, and DRB1*12:02) and 6 associated with a lower BMI (B*27:04, B*58, B*46, B*40:01, C*03:02, and DRB1*09:01). Tests of the difference in the proportions of alleles associated with high BMI between alleles that have significantly higher allele frequencies in Caucasians (n = 8) and those in Chinese (n = 10) show no statistical significance (P > 0.05). Interestingly, six (A*02:01, DQB1*06:02, DRB1*07:01, B*07:02, B*08, and C*07) of nine alleles associated with a higher BMI have an allele frequency >0.10 in Caucasians, whereas six (B*27:04, C*03:02, B*58, B*46, DRB1*09:01, and B*40:01) of nine alleles associated with lower BMI have an allele frequency <0.10 in Caucasians (Fig. 5B).
Assignment of BMI-Associated Alleles to Common Haplotypes
The extremely high LD within the HLA region can confound genetic association studies. Therefore, haplotypes were constructed based on 169,995 volunteers, which represents a subset of the samples in this study (27). The BMI-associated alleles were mapped onto 105 five-locus haplotypes with allele frequencies >0.001. For the BMI-associated low-resolution alleles, we used r2 to identify high-resolution alleles (B*46:01, B*58:01, and DRB1*04:03), which could be used to represent them.
Among alleles associated with high BMI, B*13:02 and DRB1*07:01 are strongly correlated (r2 = 0.31) and were observed in multiple common haplotypes “CH” that contain C*06:02-B*13:02-DRB1*07:01-DQB1*02:02 (Fig. 5C). These haplotypes are common in both Chinese and European populations (32). The A*02:01 allele was observed on haplotype “CH” (A*02:01-C*06:02-B*13:02-DRB1*07:01-DQB1*02:02) and also on other haplotypes containing C*07:02, DRB1*12:02, or DQB1*06:02. The three alleles C*07:02, B*07:02, and DQB1*06:02 were frequently carried on the same haplotypes. Most often, these are preceded by A*03:01 or A*24:02 (Fig. 5C).
Among alleles associated with lower BMI, B*46:01 correlated with DRB1*09:01 (r2 = 0.028) and B*58:01 correlated with DRB1*03:01 (r2 = 0.051). These alleles have been observed on two common four-locus haplotypes (“AH”), C*03:02-B*58:01-DRB1*03:01-DQB1*02:01 and C*01:02-B*46:01-DRB1*09:01-DQB1*03:03, which have higher frequencies in Asians compared with Africans or Caucasians (Fig. 5C) (32).
Discussion
In this study, we examined 72 low-resolution HLA alleles and 163 high-resolution HLA alleles in 1.3 million individuals who had been HLA typed as part of the CMDP repository. We identified 19 unique HLA alleles that associated with BMI after correction for multiple testing. However, the effect size of these alleles (β is defined as change in kg/m2 per allele) ranged from −0.024 to −0.11 for alleles associated with low BMI and from 0.01 to 0.12 for alleles associated with high BMI. These effects are smaller than those reported in prior GWAS of Caucasians (4) (βs ranged from 0.06 to 0.39 kg/m2 per allele); therefore, the variance explained by a single HLA allele in our study of Chinese is very small (ranging from 0.009 to 0.21%).
The HLA alleles with the strongest evidence for association with BMI were B*07, DRB1*07, DRB1*12, and C*03:02, where B*07, DRB1*07, and DRB1*12 were associated with higher BMI, and C*03:02 was associated with lower BMI. Several of these alleles have been previously implicated in response to viral infection. For example, HLA-DRB1*07 has been associated with viral persistence in both hepatitis C and B virus infection (33–35) and hepatitis B vaccine failure (36–39). Chronic infection with hepatitis C or B virus is the most common risk factor for hepatocellular carcinoma. HLA-B*07 has been associated with increased risk of cervical cancer (40–42), which is often caused by human papillomavirus. Two of the HLA alleles, B*07 and DRB1*07, had evidence for a sex-specific effect on BMI in our study. Sex specificity in response to viral infection has been reported, where males mount more vigorous immune and behavioral responses to influenza viral infection than females (43).
Prior studies in mice and humans have also suggested an impact of classic HLA alleles on the gut microbiota composition (44–46), and the role of the gut microbiota on obesity is an area of current investigation. Therefore, further studies are needed to investigate the role of HLA-B*07, -DRB1*07, and -C*03:02 on gut microbiota in Chinese populations. Infectious pathogens are arguably among the strongest selective forces that act on human populations (47).
During migrations of modern humans, immune response genes, in particular the HLA system, have been under strong selective pressure to respond and adapt to pathogens. Many pathogens have been reported to cause or exacerbate obesity in animals by damaging the central nervous system (48). In our haplotype analysis, we found that three risk alleles are carried in the haplotype A*02:01-C*06:02-B*13:02-DRB1*07:01-DQB1*02:02, and three risk alleles are carried in the haplotype of A*03:01-C*07:02-B*07:02-DRB1*15:01-DQB1*06:02. These haplotypes are both common in Chinese and European populations. The later haplotype is the “ancestral DR2” haplotype, which is considered to be the longest of the widely distributed ancestral haplotypes (49). These common haplotypes, which could increase survival by providing immune and/or stored energy advantage, are thought to have occurred around the time of the “out of Africa” and expansion into Europe migration (50,51). Four HLA alleles, which we found were protective of obesity, are carried on the most common haplotypes in Asians. These haplotypes are much less common in Africans and Caucasians (52) and therefore are likely to have occurred after the “out of Africa” migration, allowing Asians to gain resistance for “new” pathogens in Asia (53,54).
Future studies are needed to investigate the role of these BMI-associated HLA alleles on T2D. The prevalence of T2D in China has escalated during the past two decades. In 2010, the prevalence of T2D was comparable in China and the U.S. (both ∼11.3%), yet among the Chinese population, T2D occurred at a significantly lower BMI (mean BMI 23 kg/m2) compared with the U.S. population (mean BMI 28.7 kg/m2) (55). A prior study of individuals living in southern China, a region that is experiencing the world’s fastest economic development, reported that increases in BMI and overall obesity have been leveling off, while increases in waist circumference and abdominal obesity have continued to rise (56). Waist circumference has been proven to be a better predictor of T2D than BMI among the Chinese adult population (57). Therefore, the increased prevalence of T2D in China may possibly be due to increases in abdominal obesity, even though HLA alleles predictive of lower BMI are more common in this population. In addition, the small effect sizes of HLA alleles on BMI suggest that the effect of HLA-protective alleles on T2D is minimal. Thus, environmental factors, gene-gene, and gene-environmental interaction likely also play important roles.
Our study has three major strengths. First, to our knowledge, this study is the largest genetic association study for BMI in the world, affording >80% power to detect variants with an allele frequency of 0.001. Second, HLA genotypes were not imputed (58,59), but rather directly typed. Third, phenotypic data were all derived from a single source.
A weakness of our study is that it only identifies associations, and the strong LD across the HLA region makes it difficult to define the exact location of etiological variants. For example, TNFA, which encodes tumor necrosis factor-α (TNF-α) is among the 200 genes in the HLA locus. TNF-α is a key regulator of the inflammatory response and can cause necrosis of tumors (60). Dysregulation of TNF-α production has been implicated in a variety of human diseases such as obesity, T2D, autoimmune diseases, and cancers (61–63). Therefore, variation in the TNFA gene or other genes in this locus could possibly account for some of the association between HLA and obesity observed in our study.
In summary, the results of this study suggest that specific HLA alleles are associated with higher or lower BMI in human populations and that these alleles may cause alterations in obesity risk through their roles in immune responses.
Supplementary Material
Article Information
Acknowledgments. The authors thank Dr. Bernice Morrow, Department of Genetics, Albert Einstein College of Medicine, and Dr. Enpeng Zhao, Department of Medicine, Albert Einstein College of Medicine, for critical comments and suggestions for discussion. All of the data are from the CMDP.
Funding. This work was supported by grants from the CMDP (Grant No. 2011001), the Jiangsu Province Administration of Traditional Chinese Medicine (Grant No. LZ1320), and the Foundation of Jiangsu Health Committee and the State Key Laboratory of Natural Medicines of China Pharmaceutical University (Grant No. SKLNMZZ 20210F).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.S. and T.G. conceived the study and contributed to the study design and analysis strategy. J.S., T.G., T.W., Y.Z., and Y.W. conducted all analyses. J.S., T.G., and L.J.B. wrote the manuscript. X.M., Z.-X.Z., J.-P.C., W.M., F.-M.Z., J.-P.L., Z.-L.W., D.-M.Z., M.-L.L., X.-Y.S., B.-W.Z., C.-F.Z., Z.-H.D., W.-J.Y., Q.C., G.-L.L., T.Y., S.L., Q.-Q.P., S.F., Xia.-Y.W., X.Z., X.-Y.B., Y.-H.Q., P.-C.S., R.L., G.-Y.L., H.-C.L., B.P., L.-X.J., G.S., J.L., Z.-H.F., Y.-P.S., Y.-B.X., W.-Y.D., Xin-Y.W., X.L., X.-P.Z., D.D., Q.L., and Y.H. contributed primarily to sample collection and/or HLA genotyping. J.-W.C. and M.G. contributed to critical writing and review of the manuscript. All authors contributed to the final manuscript. J.S. and T.G. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0852/-/DC1.
References
- 1.Willer CJ, Speliotes EK, Loos RJ, et al.; Wellcome Trust Case Control Consortium; Genetic Investigation of ANthropometric Traits Consortium . Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 2009;41:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen W, Cho YS, Zheng W, et al.; Genetic Investigation of ANthropometric Traits (GIANT) Consortium . Meta-analysis identifies common variants associated with body mass index in east Asians. Nat Genet 2012;44:307–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorleifsson G, Walters GB, Gudbjartsson DF, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 2009;41:18–24 [DOI] [PubMed] [Google Scholar]
- 4.Speliotes EK, Willer CJ, Berndt SI, et al.; MAGIC; Procardis Consortium . Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okada Y, Kubo M, Ohmiya H, et al.; GIANT consortium . Common variants at CDKAL1 and KLF9 are associated with body mass index in east Asian populations. Nat Genet 2012;44:302–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradfield JP, Taal HR, Timpson NJ, et al.; Early Growth Genetics Consortium . A genome-wide association meta-analysis identifies new childhood obesity loci. Nat Genet 2012;44:526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott RA, Scott LJ, Mägi R, et al.; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium . An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes 2017;66:2888–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams RC, Muller YL, Hanson RL, et al. HLA-DRB1 reduces the risk of type 2 diabetes mellitus by increased insulin secretion. Diabetologia 2011;54:1684–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purcell SM, Wray NR, Stone JL, et al.; International Schizophrenia Consortium . Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009;460:748–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bei JX, Li Y, Jia WH, et al. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat Genet 2010;42:599–603 [DOI] [PubMed] [Google Scholar]
- 11.Tse KP, Su WH, Chang KP, et al. Genome-wide association study reveals multiple nasopharyngeal carcinoma-associated loci within the HLA region at chromosome 6p21.3. Am J Hum Genet 2009;85:194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabsitz RR, Nam JM, Gart J, Stunkard A, Price RA, Wilson PW. HLA associations with obesity. Hum Hered 1989;39:156–164 [DOI] [PubMed] [Google Scholar]
- 13.Bouchard C, Pérusse L, Rivest J, et al. HLA system, body fat and fat distribution in children and adults. Int J Obes 1985;9:411–422 [PubMed] [Google Scholar]
- 14.Beck MD, Svejcar J, Kühnl P, Baur MP. HLA and obesity. Biomed Pharmacother 1984;38:209–211 [PubMed] [Google Scholar]
- 15.Fumeron F, Apfelbaum M. Association between HLA-B18 and the familial-obesity syndrome. N Engl J Med 1981;305:645. [DOI] [PubMed] [Google Scholar]
- 16.Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev 2014;13:981–1000 [DOI] [PubMed] [Google Scholar]
- 17.Gough SC, Simmonds MJ. The HLA region and autoimmune disease: associations and mechanisms of action. Curr Genomics 2007;8:453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackwell JM, Jamieson SE, Burgner D. HLA and infectious diseases. Clin Microbiol Rev 2009;22:370–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasarica M, Dhurandhar NV. Infectobesity: obesity of infectious origin. Adv Food Nutr Res 2007;52:61–102 [DOI] [PubMed] [Google Scholar]
- 20.Cotillard A, Kennedy SP, Kong LC, et al.; ANR MicroObes consortium . Dietary intervention impact on gut microbial gene richness. Nature 2013;500:585–588 [DOI] [PubMed] [Google Scholar]
- 21.Le Chatelier E, Nielsen T, Qin J, et al.; MetaHIT consortium . Richness of human gut microbiome correlates with metabolic markers. Nature 2013;500:541–546 [DOI] [PubMed] [Google Scholar]
- 22.Atkinson RL. Viruses as an etiology of obesity. Mayo Clin Proc 2007;82:1192–1198 [DOI] [PubMed] [Google Scholar]
- 23.Ponterio E, Gnessi L. Adenovirus 36 and obesity: an overview. Viruses 2015;7:3719–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong J. The historical development and current status of the China Marrow Donor Program. Clin Transpl 2011;63–66 [PubMed] [Google Scholar]
- 25.Jin Shueigao. Survey Report for the Status of Nutrition and Health About Chinese. Beijing, People’s Medical Publishing House, 2002 [Google Scholar]
- 26.Pan Q, Fan S, Wang X, et al. The distribution of HLA-A, -B, and -DRB1 alleles and haplotypes in inhabitants of Guizhou Province of China. J Biomed Res 2011;25:328–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou XY, Zhu FM, Li JP, et al. High-resolution analyses of human leukocyte antigens allele and haplotype frequencies based on 169,995 volunteers from the China Bone Marrow Donor Registry Program. PLoS One 2015;10:e0139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magi R, Lindgren CM, Morris AP. Meta-analysis of sex-specific genome-wide association studies. Genet Epidemiol 2010;34:846–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers AJ, Gibbs JR, Webster JA, et al. A survey of genetic human cortical gene expression. Nat Genet 2007;39:1494–1499 [DOI] [PubMed] [Google Scholar]
- 30.Shungin D, Winkler TW, Croteau-Chonka DC, et al.; ADIPOGen Consortium; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GEFOS Consortium; GENIE Consortium; GLGC; ICBP; International Endogene Consortium; LifeLines Cohort Study; MAGIC Investigators; MuTHER Consortium; PAGE Consortium; ReproGen Consortium . New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015;518:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Excoffier L, Lischer HE. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 2010;10:564–567 [DOI] [PubMed] [Google Scholar]
- 32.Okada Y, Momozawa Y, Ashikawa K, et al. Construction of a population-specific HLA imputation reference panel and its application to Graves’ disease risk in Japanese. Nat Genet 2015;47:798–802 [DOI] [PubMed] [Google Scholar]
- 33.Wawrzynowicz-Syczewska M, Underhill JA, Clare MA, Boron-Kaczmarska A, McFarlane IG, Donaldson PT. HLA class II genotypes associated with chronic hepatitis C virus infection and response to alpha-interferon treatment in Poland. Liver 2000;20:234–239 [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Pyo CW, Koh DK, Hur JK, Kang JH, Kim TG. Alteration of the influences of HLA classes I and II alleles on the perinatal hepatitis B virus infection after immunoprophylaxis in Korean children. Hepatol Res 2006;35:118–126 [DOI] [PubMed] [Google Scholar]
- 35.Chu RH, Ma LX, Wang G, Shao LH. Influence of HLA-DRB1 alleles and HBV genotypes on interferon-alpha therapy for chronic hepatitis B. World J Gastroenterol 2005;11:4753–4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh R, Kaul R, Kaul A, Khan K. A comparative review of HLA associations with hepatitis B and C viral infections across global populations. World J Gastroenterol 2007;13:1770–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian Y, Zhang L, Liang XM, Hou JL, Luo KX. Association of immune response to hepatitis B vaccine with HLA-DRB1*02, 07, 09 genes in the population of Han nationality in Guangdong Province. Di Yi Jun Yi Da Xue Xue Bao 2002;22:67–69 [PubMed] [Google Scholar]
- 38.McDermott AB, Zuckerman JN, Sabin CA, Marsh SG, Madrigal JA. Contribution of human leukocyte antigens to the antibody response to hepatitis B vaccination. Tissue Antigens 1997;50:8–14 [DOI] [PubMed] [Google Scholar]
- 39.Amirzargar AA, Mohseni N, Shokrgozar MA, et al. HLA-DRB1, DQA1 and DQB1 alleles and haplotypes frequencies in Iranian healthy adult responders and non-responders to recombinant hepatitis B vaccine. Iran J Immunol 2008;5:92–99 [DOI] [PubMed] [Google Scholar]
- 40.Qiu X, Zhang F, Chen D, et al. HLA-B*07 is a high risk allele for familial cervical cancer. Asian Pac J Cancer Prev 2011;12:2597–2600 [PubMed] [Google Scholar]
- 41.Ellis JR, Keating PJ, Baird J, et al. The association of an HPV16 oncogene variant with HLA-B7 has implications for vaccine design in cervical cancer. Nat Med 1995;1:464–470 [DOI] [PubMed] [Google Scholar]
- 42.Wang SS, Wheeler CM, Hildesheim A, et al. Human leukocyte antigen class I and II alleles and risk of cervical neoplasia: results from a population-based study in Costa Rica. J Infect Dis 2001;184:1310–1314 [DOI] [PubMed] [Google Scholar]
- 43.Avitsur R, Mays JW, Sheridan JF. Sex differences in the response to influenza virus infection: modulation by stress. Horm Behav 2011;59:257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomez A, Luckey D, Yeoman CJ, et al. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS One 2012;7:e36095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Palma G, Capilla A, Nadal I, et al. Interplay between human leukocyte antigen genes and the microbial colonization process of the newborn intestine. Curr Issues Mol Biol 2010;12:1–10 [PubMed] [Google Scholar]
- 46.Toivanen P, Vaahtovuo J, Eerola E. Influence of major histocompatibility complex on bacterial composition of fecal flora. Infect Immun 2001;69:2372–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fumagalli M, Sironi M, Pozzoli U, Ferrer-Admetlla A, Pattini L, Nielsen R. Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution [published correction appears in PLoS Genet 2011;7]. PLoS Genet 2011;7:e1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhurandhar NV. Contribution of pathogens in human obesity. Drug News Perspect 2004;17:307–313 [DOI] [PubMed] [Google Scholar]
- 49.Traherne JA. Human MHC architecture and evolution: implications for disease association studies. Int J Immunogenet 2008;35:179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moalem S, Storey KB, Percy ME, Peros MC, Perl DP. The sweet thing about type 1 diabetes: a cryoprotective evolutionary adaptation. Med Hypotheses 2005;65:8–16 [DOI] [PubMed] [Google Scholar]
- 51.Distante S, Robson KJ, Graham-Campbell J, Arnaiz-Villena A, Brissot P, Worwood M. The origin and spread of the HFE-C282Y haemochromatosis mutation. Hum Genet 2004;115:269–279 [DOI] [PubMed] [Google Scholar]
- 52.Gragert L, Madbouly A, Freeman J, Maiers M. Six-locus high resolution HLA haplotype frequencies derived from mixed-resolution DNA typing for the entire US donor registry. Hum Immunol 2013;74:1313–1320 [DOI] [PubMed] [Google Scholar]
- 53.Abi-Rached L, Jobin MJ, Kulkarni S, et al. The shaping of modern human immune systems by multiregional admixture with archaic humans. Science 2011;334:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrer-Admetlla A, Bosch E, Sikora M, et al. Balancing selection is the main force shaping the evolution of innate immunity genes. J Immunol 2008;181:1315–1322 [DOI] [PubMed] [Google Scholar]
- 55.Xu Y, Wang L, He J, et al.; 2010 China Noncommunicable Disease Surveillance Group . Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–959 [DOI] [PubMed] [Google Scholar]
- 56.Lao XQ, Ma WJ, Sobko T, et al. Overall obesity is leveling-off while abdominal obesity continues to rise in a Chinese population experiencing rapid economic development: analysis of serial cross-sectional health survey data 2002-2010. Int J Obes 2015;39:288–294 [DOI] [PubMed] [Google Scholar]
- 57.Smith SC Jr, Haslam D. Abdominal obesity, waist circumference and cardio-metabolic risk: awareness among primary care physicians, the general population and patients at risk--the Shape of the Nations survey. Curr Med Res Opin 2007;23:29–47 [DOI] [PubMed] [Google Scholar]
- 58.Pillai NE, Okada Y, Saw WY, et al. Predicting HLA alleles from high-resolution SNP data in three Southeast Asian populations. Hum Mol Genet 2014;23:4443–4451 [DOI] [PubMed] [Google Scholar]
- 59.Jia X, Han B, Onengut-Gumuscu S, et al. Imputing amino acid polymorphisms in human leukocyte antigens. PLoS One 2013;8:e64683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waters JP, Pober JS, Bradley JR. Tumour necrosis factor and cancer. J Pathol 2013;230:241–248 [DOI] [PubMed] [Google Scholar]
- 61.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev 2006;25:409–416 [DOI] [PubMed] [Google Scholar]
- 62.Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett 2008;582:117–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bradley JR. TNF-mediated inflammatory disease. J Pathol 2008;214:149–160 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.