Abstract
Dietary supplementation with thymol (2-isopropyl-5-methylphenol) has been proposed as a strategy to improve modern intensive poultry production. Moreover, its antioxidant properties and potential beneficial influence on lipid metabolism have fostered current research focusing on enhancing nutritional quality of meat and egg products. In general, studies have focused on the overall effects of dietary supplementation once the supplementation protocol has finished and using only one potential dose, without actually measuring bioactive compounds' concentration in the diet supplied or target tissues. Herein, we provide a unique dataset of the dynamics of thymol bioavailability and biological action, optimal dosage and duration of supplementation needed to achieve meaningful effects, as well as persistence of induced changes after chronic supplement withdrawal. Specifically, during a month-long supplementation period, 5 sampling points were evaluated separated by at least 1 week. Then, a last sampling point was studied after a 3-week withdrawal period. Three increasing doses of dietary thymol were used, and approximately 80 variables assessed. The measured variables were associated with free thymol concentration in feed, egg yolk and droppings, feed and egg yolk fatty acids profile (saturated, unsaturated, and polyunsaturated fatty acids), performance traits (body weight, feed intake, egg laying rate, egg physical characteristics), general welfare quality assessment (plumage state) and liver histopathology. The data can provide insights on the link between the dynamics of free thymol concentration and the changes in fatty acids profile in quail egg yolk, both during chronic thymol dietary supplementation and after supplement withdrawal. The comprehensive approach used herein for studying thymol supplementation outcome could help understanding the scope of its effects on a whole organism level.
Keywords: Natural products, Feed additives, PUFA, MUFA, SFA, Liver histopathology, Healthy, Eggs, Poultry nutrition
Specifications table
| Subject area | Animal Science and Zoology; Agricultural and Biological Sciences (General) |
| More specific subject area | Poultry metabolism and nutrition; Feed additives; Natural Products; Healthy table eggs; Fatty acids profile |
| Type of data | Tables |
| How data was acquired | Thymol concentration was assessed using head-space solid phase microextraction followed by gas chromatography-mass spectrometry (HS-SPME/GC-MS in a Perkin Elmer Clarus® 600). Fatty acid methyl esters were analyzed by gas chromatography mass-spectrometry (GC-MS in a Perkin Elmer Clarus® 600). Body weight and feed intake were assessed using a balance (OHAUS Scout-Pro® SP601), egg physical characteristics with an analytical balance (OHAUS Adventurer® AR2140 and a digital caliper) and general welfare quality with a procedure adapted Welfare Quality consortium protocol. Histopathological alterations were recorded with a light microscope (Olympus X-785) coupled to a digital camera (Moticam Camera 2300, 3 Megapixels). |
| Data format | Raw and processed data |
| Experimental factors | Husbandry under standard laboratory protocol for quail |
| Experimental features | A bi-factorial design combining the effects of the diet supplied (five levels) and time of sampling (five levels) was established for the variables studied: >Five experimental diets (two controls: the basal diet and the basal diet with vehicle solution; and three thymol doses: 2, 4 and 6.25 g/kg of feed, respectively). >Five times of sampling (one initial sample point; three sample points along the supplementation period and one at the end of the post-supplementation period). |
| Data source location | Instituto de Investigaciones Biológicas y Tecnológicas (IIByT), Córdoba, Argentina. |
| Data accessibility | Data are presented in excel format file and stored in the public repository figshare: DOI: https://doi.org/10.6084/m9.figshare.7589066.v1 |
| Related research article | M.E. Fernandez, R.H. Marin, A. Luna, M.P. Zunino, M.C. Labaque, Thymol feed supplementation in quail alters the percentages of nutritionally relevant egg yolk fatty acids: effects throughout incubation, J. Sci. Food Agric. 97 (2017) 5233–40. https://doi.org/10.1002/jsfa.8407 |
Value of the data
|
1. Data
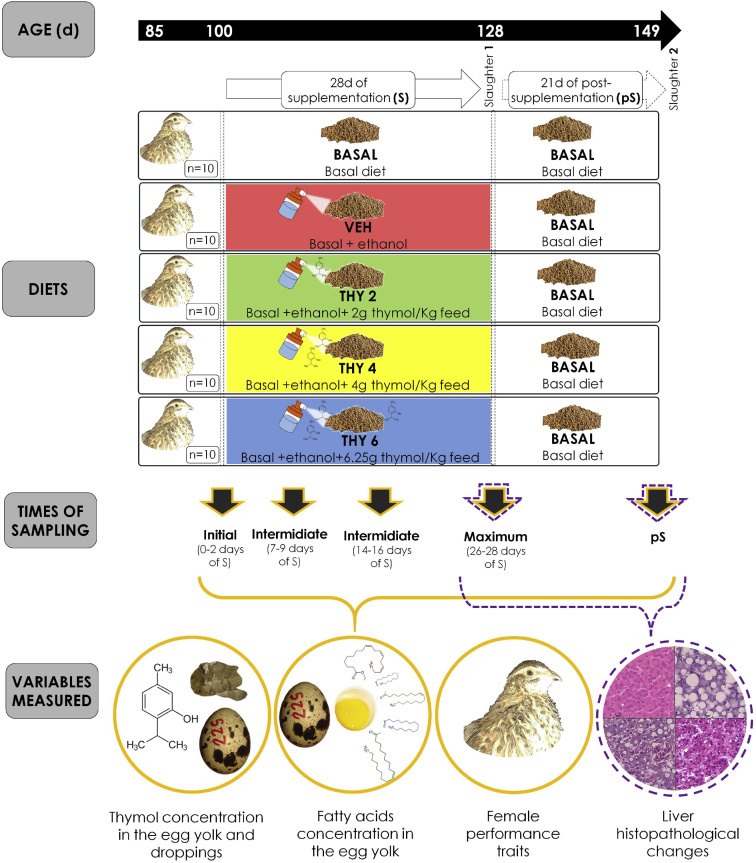
Data on quantitative changes promoted by three increasing doses of dietary thymol (2-isopropyl-5-methylphenol, at 2, 4 and 6.25 g/Kg of feed) during a month-long supplementation period and after a 3-week withdrawal period were assed (i.e. 5 times of sampling separated by at least 1 week). For schematic representation of experimental design see Fig. 1.
Fig. 1.
Timeline scheme. The days of age (d) of the birds is indicated by the time line in the upper box. All birds received basal diet between 85 and 100 days of age. From 100 to 128 days of age (Supplementation (S) period), females were provided a diet according to their dietary treatment assignment. Five diets were administrated (10 individuals randomly assigned to each one): two controls (BASAL and VEHICLE) and three increasing doses of thymol (THY2, THY4 and THY6, corresponding to 2, 4 and 6.25 g of THY/kg feed, respectively). Administered diets are indicated with boxes of rounded edges. Vertical dotted lines indicate changes in the diet of each experimental group. Once finished the supplementation period, half of the quail were slaughtered for histological analysis (Slaughter 1). The other half of the quail was subjected to a post-supplementation period of 21 days (pS) during which the basal diet was reestablished. Finished de pS period, the remaining birds were slaughtered for the same purpose as the first group (Slaughter 2). To assess changes in the variables studied, five times of sampling were defined along S and pS periods: one initial sample point; three sample points along the S period and one at the end of the pS period. Thymol concentration in the egg yolk and droppings, fatty acids concentration in the egg yolk and female performance traits were evaluated along the five sample points (horizontal bracket and circles of solid line). Histopathological changes of liver were assessed at the sample point of maximum Sand in the point of pS (horizontal bracket and a circle of dotted line).
Data corresponding to the approximately 80 variables assessed are presented as raw data in 11 excel files and stored in the public repository figshare, at https://doi.org/10.6084/m9.figshare.7589066.v1. In each file, the headers of the columns representing the variable analyzed for each animal (rows). In this article, information describing the registered variables is summarized in four tables (see data records section Table 1, Table 2, Table 3, Table 4). In Table 1, information on free thymol concentration (in the feed, egg yolk and droppings), fatty acids concentrations (i.e. saturated (SFA), monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acids in the feed and egg yolk) and performance traits (weekly body weight, feed intake, egg laying and egg physical characteristics) are shown. Table 2, summarizes information regarding variables related to the general welfare assessment (female skin lesions and plumage state). Finally, Table 3, Table 4 show information regarding liver histopathological alterations and histological indices, respectively.
Table 1.
Description of variable column headers for excel files corresponding to data on thymol (2-isopropyl-5-methylphenol) concentration in feed, egg yolk and droppings, concentration of feed and egg yolk total fatty acids, weekly body weight and feed intake, egg laying and egg physical characteristics publicly available [18].
| File name | Variable | Description |
|---|---|---|
| Fernandez 2019 Thymol in feed.xls | Concentration (ng/g) | Thymol concentration (ng/g) in quail feed |
| Fernandez 2019 Thymol in egg yolk.xls | Concentration (ng/g) | Thymol concentration (ng/g) in quail egg yolk |
| Fernandez 2019 Thymol in droppings.xls | Concentration (ng/g) | Thymol concentration (ng/g) in quail droppings |
| Fernandez 2019 Concentration of egg yolk total fatty acids.xls | Concentration (g/100 g FAME) | Concentration of fatty acids (g/100 g of FAME) in quail egg yolk. Column headers indicate the name of fatty acid analyzed |
| Fernandez 2019 Concentration of feed total fatty acids.xls | Concentration (g/100 g FAME) | Concentration of fatty acids (g/100 g of FAME) in quail feed. Column headers indicate the name of fatty acid analyzed |
| Fernandez 2019 Weekly body weight and feed intake.xls | Weekly body weight (g) | Weekly body weight of quail |
| Weekly feed intake (g) | Weekly feed intake (WFI) of quail. For example, WFI at the time of sampling “7 days of S” correspond to the summation of individual daily feed intake between 1 and 7 days of S; of WFI at the time of sampling “21 days of pS” correspond to the summation of individual daily feed intake between 15 and 21 days of pS. | |
| Fernandez 2019 Cumulative number of eggs.xls | Cumulative number of eggs | Cumulative number of eggs laid from the beginning of S until the end pS. |
| Fernandez 2019 Egg physical characteristics.xls | Egg weight (g) | Quail egg weight (g) |
| Egg shell (%) | Percentage of the total weight of the egg that corresponds to the shell | |
| Egg albumen (%) | Percentage of the total weight of the egg that corresponds to the albumen. | |
| Egg yolk (%) | Percentage of the total weight of the egg that corresponds to yolk | |
| Egg shape index | Ratio between the egg width and the egg length, expressed as a percentage |
S = supplementation period, pS = post-supplementation period.
Table 2.
Description of variable column headers for excel file “Fernandez 2019 General welfare.xls” [18] regarding welfare assessment variables in adult female quail supplemented with dietary thymol (2-isopropyl-5-methylphenol).
| Variable name | Description |
|---|---|
| Lesions legs | Legs skin lesions score (range 0–2) |
| Lesions rear end | Rear end skin lesions score (range 0–2) |
| Lesions chest | Chest skin lesions score (range 0–2) |
| Lesions cloacae | Cloacae skin lesions score (range 0–2) |
| Lesions wings | Wing skin lesions score (range 0–2) |
| Plumage neck | Neck plumage damage score (range 0–2) |
| Plumage head | Head plumage damage score (range 0–2) |
| Plumage wings | Wings plumage damage score (range 0–2) |
| Plumage rear end | Rear end plumage damage score (range 0–2) |
| Plumage chest | Chest plumage damage score (range 0–2) |
| Foot Pad dermatitis | Score assigned to lesion observed in the foot (range 0–2) |
| Eye pathologies | Presence or absence of eye pathologies (1 or 0, respectively) |
| Dirt legs | Presence or absence of dirt in the legs (1 or 0, respectively) |
| Dirt cloacae | Presence or absence of dirt in the cloacae (1 or 0, respectively) |
| Dirt belly | Presence or absence of dirt in the belly (1 or 0, respectively) |
Table 3.
Description of histopathological variable recorded in the liverof in adult female quail supplemented with dietary thymol (2-isopropyl-5-methylphenol) presented in the columns of the excel file “Fernandez 2019 Frequency of liver histological alterations.xls” [18].
| Variable | Description |
|---|---|
| Hemorrhage | Extent and severity of hemorrhage (a*w). RP1 |
| Dilation of sinusoids | Extent and severity of dilation of sinusoids (a*w). RP1 |
| Vascular congestion | Extent and severity of vascular (a*w). RP1 |
| Hydropic degenerat. | Extent and severity of hydropic degeneration (a*w). RP2 |
| Steatosis, fatty change | Extent and severity of steatosis or fatty change (a*w). RP2 |
| Pycnotic hepatocytes | Extent and severity of pycnotic hepatocytes (a*w). RP2 |
| Fibrosis | Extent and severity of fibrosis (a*w). RP2 |
| Necrosis | Extent and severity of necrosis (liquefactive or focal) (a*w). RP2 |
| Oval cells | Extent and severity of the area occupied by oval cells (a*w). RP3 |
| Hypertrophy | Extent and severity of the hypertrophy (acute cellular swelling) (a*w). RP3 |
| Hyaline degeneration | Extent and severity of hyaline degeneration (Intracellular eosinophilic bodies). RP3 |
| Leucocyte infiltration | Extent and severity of the leucocyte infiltration (a*w). RP4 |
| Average hemorrhage | Average of “a*w” values for hemorrhage within a treatment. RP1 |
| Av. Dilation sinusoids | Average of “a*w” values for dilation of sinusoids within a treatment. RP1 |
| Av. Vascular congest. | Average of “a*w” values for vascular congestion within a treatment. RP1 |
| Av.hydropic degen. | Average of “a*w” values for hydropic degeneration within a treatment. RP2 |
| Av. Steatosis, fatty ch. | Average of “a*w” values for steatosis or fatty change within a treatment. RP2 |
| Av. Pycnotichepatoc. | Average of “a*w” values for pycnotic hepatocytes within a treatment. RP2 |
| Av.Fibrosis | Average of “a*w” values for fibrosis within a treatment. RP2 |
| Av. Necrosis | Average of “a*w” values for necrosis within a treatment. RP2 |
| Av. Oval cells | Average of “a*w” values for area occupied by oval cells within a treatment. RP3 |
| Av. Hypertrophy | Average of “a*w” values for hypertrophy within a treatment. RP3 |
| Av. Hyaline nfiltrate. | Average of “a*w” values for hyaline degeneration within a treatment. RP3 |
| Av. Leucocyte nfiltrate. | Average of “a*w” values for leucocyte infiltration within a treatment. RP4 |
| Rel. hemorrhage | Relativized “a*w” values for hemorrhage within a treatment. RP1 |
| Rel. dilation of sinus. | Relativized “a*w” values for dilation of sinusoids within a treatment. RP1 |
| Rel. vascular congest. | Relativized “a*w” values for vascular congestion within a treatment. RP1 |
| Rel.hydropic degener. | Relativized “a*w” values for hydropic degeneration within a treatment. RP2 |
| Rel. steatosis, fatty | Relativized “a*w” values for steatosis or fatty change within a treatment. RP2 |
| Rel.pycnotichepatoc. | Relativized “a*w” values for pycnotic hepatocytes within a treatment. RP2 |
| Rel.fibrosis | Relativized “a*w” values for fibrosis within a treatment. RP2 |
| Rel. necrosis | Relativized “a*w” values for necrosis within a treatment. RP2 |
| Rel. oval cells | Relativized “a*w” values for area occupied by oval cells within a treatment. RP2 |
| Rel.hypertrophy | Relativized “a*w” values for hypertrophy within a treatment. RP3 |
| Rel. hyaline degener. | Relativized “a*w” values for hyaline degeneration within a treatment. RP3 |
| Rel. leucocyte infiltr. | Relativized “a*w” values for leucocyte infiltration within a treatment. RP4 |
Av = Average, Rel. = Relativized. RP = Contributes to specified reaction pattern.
Table 4.
Description of variable column headers for excel file “Fernandez 2019 Liver histopathological indices.xls” regarding liver histopathology indices in adult female quail supplemented with dietary thymol (2-isopropyl-5-methylphenol) [18].
| Variable | Description |
|---|---|
| HILiv.Rp1 | Liver histopathological index related to reaction pattern 1 |
| HILiv.Rp2 | Liver histopathological index related to reaction pattern 2 |
| HILiv.Rp3 | Liver histopathological index related to reaction pattern 3 |
| HILiv.Rp4 | Liver histopathological index related to reaction pattern 4 |
| HILiv | Total liver histopathological index |
2. Experimental design, materials, and methods
2.1. Animals and husbandry
All experimental procedures were in compliance with the Guide for the Care and Use of Laboratory Animals issued by the National Institute of Health [1]. The experimental protocol was approved by the Institutional Committee for the Care and Use of Laboratory Animals (Comité Institucional para el Cuidado y Uso de Animales de Laboratorio (CICUAL)) of the Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba (ACTA 4/2015 Resolución 571-HCD-2014).
Adult female Japanese quail (Coturnix japonica) taken from a population of a single 230-bird hatch were used in this study. Chicks were brooded from day 1 in mixed-sex groups of ∼40 within each of 6 brooder boxes, each measuring 90 cm × 90 cm × 60 cm (length × width × height). Each box had two feeders covering the front part of the box and 16 automatic nipple drinkers. A wire mesh floor (1 cm grid) was raised 5 cm to allow the passage of droppings and a lid prevented the birds from escaping. Brooding temperature was 37.5 °C during the first week of life, with a weekly decline of 3 °C until room temperature (24–27 °C) was achieved. At 28 days of age, females were sexed by plumage coloration, individually weighted and wing banded to identify each bird. At this age, female birds were individually housed in cages measuring 20 cm × 45 cm × 25 cm (length × width × height), allowing them to establish visual and auditory contact with each other, while permitting individual measurements of feed consumption and egg production. An individual feeder and an automatic nipple drinker were positioned in each cage. From hatch to 28 d of age, all birds were fed a starter ration (24% of Crude Protein and 2900 kcal of Metabolizable Energy kg diet-1). From this age on and until feed supplementation was initiated (100 d of age), birds were fed with the BASAL diet, which was a layer ration (20% of Crude Protein and 2900 kcal of Metabolizable Energy kg diet-1). At all stages feed and water were provided ad libitum. The photoperiod was 14 h light and 10 h dark (0600–2000 h; approximately 300–320 lx). The environmental temperature was maintained at 24 ± 2 °C.
2.2. General procedure
A total of 50 eighty-five days-old female quail of homogeneous body weights (251 ± 1 g; Mean ± SE) and in their egg-laying peak were selected from an initial group of 96 female individuals. All birds continued receiving a basal diet between 85 and 100 days of age and all variables (see below for details) were monitored along this period (Fig. 1). From 100 to 128 days of age (Supplementation period, S; Fig. 1), females were randomly assigned to either a chronic dietary supplementation with one of 3 THY doses (THY2, THY4 and THY6; 10 females each) or to one of 2 control groups (basal diet, BASAL, and basal diet with vehicle solution, VEHICLE; 10 females each). Once finished the S period, half of the quail were slaughtered for histological analysis (n = 25; Slaughter 1, Fig. 1) and the other half were subjected to a post-supplementation (pS) period of 21 days, during which all females received the basal diet -BASAL- (pS period, Fig. 1). After this period, the remaining 25 animals were also slaughtered for histological analysis (Slaughter 2, Fig. 1). In this way, we assessed whether THY induced changes in variables indicative of tissue damage and if so, whether the potential damages could reverse after 3 weeks of supplement withdrawal.
To assess changes in the variables studied, five times of sampling were defined along S and pS periods: one initial sample point (at 0 days of S); three sample points during the S period (at 7, 14 and 28 days of S); and one sample point at the end of the pS period (21 days of pS). It should be noted that for determinations of THY concentration, eggs and quail droppings were collected offset from the other variables measured by 2 days, thus were collected on days 2, 9, 16, and 26 days of S, and 17 days of pS, respectively.
For the variables THY concentration, FA concentration, feed intake, body weight, morphometric variables and percentage of egg constituents, the experimental design contemplates that data could be analyzed using a general linear mixed model including the diet supplied (five levels: BASAL, VEHICLE, THY2, THY4 and THY6), time of sampling (five levels: initial, three sample points during the S period and one sample point at the end of the pS period) and their interaction as the fixed effects, and female identity (categorical variable with levels = N) could be included in the model as a random effect. For each one of the above mentioned variables separately, considering a normal distribution, the mixed model can be represented as:
| Y = Xβ + Zμ + ε | (1) |
where,
Y is the vector of observations of the response variable Y with mean E(Y) = Xβ; β is an unknown vector of the fixed effects Diet supplied, Time of sampling and their interaction.
μ is an unknown vector of the random effect female identity, with mean E(μ) = 0 and variance-covariance matrix var (μ) = G; μ ∼ N (0, G).
ε is an unknown vector of random errors of the random variable female identity, with mean E(ε) = 0 and variance var (ε) = R, which reflect Y variation non explicated by the fixed effects Diet and Time of sampling nor the random effect female identity; ε ∼ N (0, R).
Z is a known design matrix that denotes belonging of each Y observation to a level of the random effect female identity (μ).
Thus, Zμ are the random effects resulting from female identity that determine Y covariance.
X is a known design matrix that denotes belonging of each y observation to a combination of levels of the fixed effects Diet and Time of sampling (β).
Thus, X and Z are known design matrices relating the observations Y to β and u, respectively.
For histological variables, experimental design would imply fixed effects of diet supplied (the five levels above-mentioned), the time of sampling (including two levels: 28 days of S or 21 days of pS), and their interaction. For each one of the histological variables, considering a normal distribution and in linear notation, the model can be represented as:
| Yijk = μ + αi + βj + αβij + εijk | (2) |
Yijk is the output of an individual k observation of the response variable for i level of the fixed effect Diet and j level of the fixed effect Time of sampling.
μ is the global mean for response variable Y.
αi denotes the fixed effect Diet (i.e. μi-μ), i = BASAL, VEHICLE, THY2, THY4, THY6.
βj denotes the fixed effect Time of sampling (i.e. μj-μ), j = 28S and 21pS.
αβij denotes the interactive fixed effect of the factors Diet and Time of Sampling (i.e. μij-μ).
εijk residual errors ∼ N (0, σ2).
Finally, for egg-laying (as cumulative number of eggs) only Diet could be considered as a fixed effect. Considering a normal distribution and in linear notation, the model can be represented as:
| Yij = μ + αi + εij | (3) |
Yij is the output of an individual j observation of the response variable for i level of the fixed effect Diet.
μ is the global mean for response variable Y.
αi denotes the fixed effect Diet (i.e. μi-μ), i = BASAL, VEHICLE, THY2, THY4, THY6.
εij residual errors ∼ N (0, σ2).
2.3. Dietary supplementation
Thymol was commercially obtained from Sigma-Aldrich (SAFC®, ⩾99%; FCC, Saint Louis, MO, USA). Thymol was prepared in a 2.4, 4.8 and 7.5% w/v ethanolic solution and uniformly sprayed on fresh feed in order to obtain 2, 4 or 6.25 g of THY per kg of feed [2]. Although THY volatilization in the feed supplied to the quail has not yet been characterized, the estimated Henry's Law constant for THY (3.5 × 10-6 atm-cu m/mole, derived from its vapor pressure, 0.016 mm Hg at 25 °C, and water solubility, 900 mg/L) together with experimental evidence, indicate that this compound volatilizes or could be degraded from water and moist soil surfaces at rates which greatly vary (from days to months) depending on specific conditions of the matrix (For details, please see: [3]). As THY volatilization and actual concentration in the feed supplied potentially affects its transference and activity in target tissues, herein feed was prepared and supplied daily (in order to minimize THY volatilization from feed before its consumption by females) and THY concentration in feed (sampled immediately after being prepared) was determined for the three doses by HS-SPME (see following section).
Nutrient and FA compositions of the diets supplied were identical between each other and FA concentrations are reported in excel file “Fernandez 2019 Concentration of feed total fatty acids.xls”.
2.4. Thymol quantification in egg yolk, quail droppings and feed
Thymol concentration was immediately measured in egg yolks and droppings obtained at each sample point and in feed by head space-solid phase microextraction followed by gas chromatography-mass spectrometry (HS-SPME/GC-MS) according to Fernandez et al. (2017) [4]. SPME was performed with a manual holder with 100 μm polydimethylsiloxane (PDMS) fiber. An aliquot of egg yolk (3 g), droppings (5 g) or feed (5 g) was put in a sealed 20 mL glass vial, spiked at the corresponding level with the standard m-cresol (Sigma-Aldrich; ≥99% (GC); Saint Louis, MO, USA) stock solution, and vortexed for 5 min. The vials were placed in a water bath and the PDMS fiber was exposed to the headspace for 30 min at 60 °C and 5 min at 40 °C for egg yolk and droppings or feed, respectively. The fiber was then inserted directly into the GC injector for desorption at 250 °C for 10 min in splitless mode. Chromatography analysis was carried out in a Perkin Elmer Clarus 600 equipped with a PSSI injector and a quadrupole MS detector (Perkin Elmer, USA). Turbo Mass 5.4.2 software was used to control and acquire data from GC–MS. All the separations were performed using a Perkin Elmer fused silica DB 5 MS capillary column (60 m, 0.25 mm ID, 0.25 μm film thickness), with High-purity helium (99.998%) as a carrier gas (49.6 psi). The splitless injection mode was selected. Electron-impact Ionization was carried out in the mass spectrometer under vacuum with 70-eV ionization energy. Samples were analyzed under the following chromatographic and MS detection conditions: initial oven temperature was set at 100 °C (held for 2 min), and then raised to 230 at 10 °C/min rate. A column head pressure of 14.99 psi and an injector temperature of 280 °C were set. The GC transfer line was maintained at 250 °C. The fiber was desorbed in the GC injector port for 10 min. Chromatograms were acquired in scan mode, which scans the quadrupole from m/z = 50 to m/z = 300 (scan time: 0.20 s, inter-scan time: 0.10 s). All quantitative analyses were performed in TIC mode. The compounds were identified by comparing their mass spectra with those of the libraries of the NIST MS search 2.0. The main components were further identified by co-injection of commercial standards.
2.5. Total fatty acid analysis in egg yolks
Dataset includes the estimated concentration of saturated (i.e. myristic, pentadecanoic, palmitic and stearic acids), monounsaturated (i.e. palmitoleic and oleic acids) and polyunsaturated (i.e. linoleic, linolenic, arachidonic, docosahexaenoic, eicosapentaenoic and docosapentaenoic acids) fatty acids of quail egg yolk. Eggs obtained at each time of sampling were stored at −20 °C until yolk total fatty acids analysis. Lipids were extracted from 1 g of yolk following homogenization in a suitable excess of chloroform/methanol (2:1 v/v) [5]. The solvents were removed under reduced pressure in a rotary evaporator. Lipids were subjected to alkaline saponification (1 mol/L potassium hydroxide in methanol), and the unsaponifiable matter was extracted with n-hexane. The fatty acid methyl esters (FAMEs) were prepared by transmethylation through treatment with 1 mol/L sulfuric acid in methanol and analyzed by gas chromatography/mass spectrometry (GC/MS) [2], [6]. All chemicals used in this study were reagent-grade commercial products. FAMEs were analyzed by gas chromatography on a 60 m fused capillary column with an internal diameter of 0.25 mm (PerkinElmer Elite-WAX Polyethylene Glycol). The analysis was performed on a PerkinElmer Clarus® 600 GC/MS system equipped with a flame ionization detector (Waltham, MA, USA). Helium was used as carrier gas (constant flow of 49.6 psi). The injection port temperature was 250 °C and the detector temperature was 250 °C. The oven temperature was initially held at 180 °C for 5 min, then increased at 4 °C/min to 200 °C and held for 5 min and finally increased at 3 °C/min to 230 °C and held for 25 min. Peak identification was carried out by comparing the known retention times for the fatty acids reported with the temperature program and the chromatographic system used. A solution of known concentration of nonadecanoic acid methyl ester (Sigma Aldrich, ≥98.0% (GC); Saint Louis, MO, USA) as was used internal standard to estimate the content of each fatty acid in the sample.
2.6. Female performance traits
Female performance was evaluated through the measurement of body weight, feed intake, egg laying rate, egg physical characteristics and a general welfare quality assessment. Body weight was measured once a week throughout the experimental period. Daily feed intake (DFI) was estimated as the difference between the amount of feed supplied to each animal (60 g) and the rest that remained in the feeders the next day (g of feed/day/quail) [7], [8]. Egg laying was registered daily and the weekly laying rate was calculated as: (number of eggs/7 days) x 100. Egg weight, quality characteristics (such as intact, membranous, soft shell and broken shell), morphometric characteristics (egg shape index= (egg width/egg length) x 100) and percentage of constituents (egg yolk, albumen and shell) were registered at each time of sampling [9], [10].
A welfare assessment based on observations of physical characteristics was made. Female skin lesions and plumage status were evaluated following a procedure proposed by our colleagues [11] that is an adapted version of the protocol developed by the Welfare Quality consortium [12]. Briefly, skin lesions, which include wounds that have not healed in the legs, rear end, chest, cloacae and wings were determined using a score scale from 0 to 2, where “0” represents no lesions or scratches, “1” represents at least one lesion <0.5 cm diameter or less than 3 pecks (punctiform damage ∼ 0.1 cm of diameter) or scratches, and “2” reflects one lesion ≥0.5 cm of diameter or more than 3 pecks or scratches. Plumage damage was also determined using a score scale from 0 to 2 as follows: “0” represents individuals with no plumage damage or slight wear (only single feathers lacking), “1” represent individuals with one or more body parts that have moderate wear (i.e. damaged feathers worn or deformed) or one or more featherless areas <1.5 cm in diameter at the larger extent and “2” corresponded to individuals that have at least one featherless area >1.5 cm in diameter at the largest extent. Foot pad dermatitis, for which both feet were analyzed and the foot with the worst condition was scored according to the following: “0” representing feet intact, no or minimal proliferation of epithelium, “1” corresponded to necrosis or proliferation of epithelium or chronic bumble foot with no or moderate swelling, and “2” indicated swollen dorsally visible. Eye pathologies, which include swelling of the eyelids and the skin around the eyes, closure of the eye/eyes and discharge from the eyes were classified as “0” when no evidence of eye pathologies were observed or “1” if a there were eye pathologies. Additionally, dirt from the legs, cloacae and belly was examined for signs of diarrhea potentially caused by dietary supplementation.
2.7. Liver histological analysis
At each slaughter point birds were euthanized by decapitation [13] and livers were removed and stored in buffered formalin at 10%, processed routinely and stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) as described elsewhere [14], [15]. Each slide was examined blinded, with a light microscope (Olympus X-785) and photographed with a digital camera (Moticam Camera 2300, 3 Megapixels). To evaluate histological alterations of liver, tissue was divided into 8 random equal areas to ensure there was no overlapping of the studied areas. For each area, the extension and number of each alteration were recorded with a microscope at 40× magnitude. Histopathological index of liver (HIliv) were estimated using a semi-quantitative protocol following [16], modified by our colleagues [17]. This procedure has advantages for analysis and interpretation of the data, since it performs a standardized quantification of the different alterations. Briefly, alterations were classified into four major reaction patterns each one of those including alterations that concern to different functional units of the liver or to the whole organ: RP1, circulatory disturbances (result from a pathological condition of blood and tissue fluid flow, such as dilatation of sinusoids, vascular congestion, hemorrhage); RP2, regressive changes (include processes leading to a reduction or total loss of organ function, such as steatosis or fatty degeneration, hydropic degeneration, nuclear alteration, fibrosis, necrosis); RP3, progressive changes (refer to processes that lead to an increased activity of cells or tissues, such as cell hypertrophy and hyperplasia, and presence of oval cells); and RP4, liver inflammation (include processes generally associated to alterations belonging to other reaction patterns, such as leucocyte infiltration, oedema, etc.). The relevance of each lesion depends on its pathological importance, that is, how much it affects the functionality of the organ or the survival of the bird. This is taken into account through a factor of importance (W) that is assigned to each histological alteration. This factor (W) can take values of:
W = 1: minimal pathological importance (the lesion is easily reversible when the toxic exposure ends);
W = 2: moderate pathological importance (the lesion may or may not be reversible depending on the severity and extent of the injury);
W = 3: marked pathological importance (the lesion is normally irreversible, leading to a partial or total loss of the organ function).
On the other hand, each observed pathological damage is assigned an Occurrence Value (a), whose values oscillate between 0 (zero) and 8 (eight), depending on the degree and extent of the damage expressed in percentage. Thus, each possible value of the occurrence value (a) indicates:
0: absence of alteration;
2: mild occurrence;
4: moderate occurrence;
6: severe occurrence;
8: extreme occurrence.
Then, for each RP, an index was calculated based on the two factors: the pathological importance of the lesions (factor of importance, W; range 1–3) and the extension of pathological change (occurrence value, a; range 0–8). Finally, a total liver histopathological index (HIliv) was calculated by adding the single RP liver indices of each individual quail. A greater value of (HIliv) reflects the most severely affected individual.
3. Data records
The data corresponding to the variables assessed are presented in excel format file and stored in the public repository figshare [18] with the headers of the columns representing the variable analyzed for each animal (rows). In all excel files the first 3 columns prior to variable records correspond to animal identification and treatment group. In the first column the animal identification number is stated, in the second the diet supplied (BASAL, VEHICLE, THY2, THY4, THY6) and in the third the time of sampling (0–2 days of S, 7–9 days of S, 14–16 days of S, 26–28 days of S and 17–21 days of pS). In excel files “Fernandez 2019 Thymol in feed.xls” and “Fernandez 2019 Concentration of feed total fatty acids” only the columns feed identification and diet supplied are indicated prior to variable records, due to feed was measured only once immediately after it was prepared. Table 1, Table 2, Table 3, Table 4 indicate the information referred to each excel file, column header abbreviation of variable and its corresponding description.
Acknowledgments
We thank Marcela Palacio and Guillermo Blanco for technical assistance with gas chromatography analysis and Maria Julia Ortiz and Pablo Prokopiuk for technical assistance with animal husbandry.
This research was supported by grants from CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas), FONCyT (Fondo para la Investigación Científica y Tecnológica, Préstamo BID-PICT 2014–2764) and SECyT, UNC (Secretaria de Ciencia y Tecnología – Universidad Nacional de Córdoba, grant number 33820180100125CB), Argentina.
Footnotes
Transparency document associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2019.103884.
Contributor Information
Jackelyn M. Kembro, Email: jkembro@unc.edu.ar.
Jorge M. Caliva, Email: martincaliva@unc.edu.ar.
Maria C. Labaque, Email: maria.carla.labaque@unc.edu.ar.
Transparency document
The following is the transparency document related to this article:
Multimedia Component 1
References
- 1.National Institue of Health . eighth ed. 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 2.Fernandez M.E., Marin R.H., Luna A., Zunino M.P., Labaque M.C. Thymol feed supplementation in quail alters the percentages of nutritionally relevant egg yolk fatty acids: effects throughout incubation. J. Sci. Food Agric. 2017;97:5233–5240. doi: 10.1002/jsfa.8407. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Biotechnology Information. PubChem Compound Database; CID=6989, http://pubchem.ncbi.nlm.gov/compound/6989. (accessed 13 February 2019).
- 4.Fernandez M.E., Palacio M.A., Labaque M.C. Thymol detection and quantitation by solid-phase microextraction in faeces and egg yolk of Japanese quail. J. Chromatogr. B. 2017;1044–1045:39–46. doi: 10.1016/j.jchromb.2016.12.042. https://doi: 10.1016/j.jchromb.2016.12.042 [DOI] [PubMed] [Google Scholar]
- 5.Folch J., Less M., Sloane Stanley G. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 6.Labaque M.C., Martella M., Maestri D., Navarro J. The influence of diet composition on egg and chick traits in captive Greater Rhea females. Br. Poult. Sci. 2013;54:374–380. doi: 10.1080/00071668.2013.791965. [DOI] [PubMed] [Google Scholar]
- 7.Vercese F., Garcia E.A., Sartori J.R., Silva A. de P.I., Faitarone A.B.G.I., Berto D.A.I., Molino A. de B.I., Pelícia K.I.V. Performance and egg quality of Japanese quails submitted to cyclic heat stress. Brazilian J. Poult. Sci. 2012;14:37–41. doi: 10.1590/S1516-635X2012000100007. [DOI] [Google Scholar]
- 8.Vali N., Mottaghi S. The effect of using different levels of cinnamon and thyme powder on egg characteristics and fatty acids profile in Japanese quail. CIBTech. J. Zool. 2016;5:2319–3883. [Google Scholar]
- 9.Luna A., Dambolena J.S., Zygadlo J.A., Marin R.H., Labaque M.C. Effects of thymol and isoeugenol feed supplementation on quail adult performance, egg characteristics and hatching success. Br. Poult. Sci. 2012;53:631–639. doi: 10.1080/00071668.2012.721536. [DOI] [PubMed] [Google Scholar]
- 10.Nikolova N., Kocevski D. Forming egg shape index as influenced by ambiente temperatures and age of hens. Biotechnol. Anim. Husb. 2006;22:119–125. [Google Scholar]
- 11.Pellegrini S., Condat L., Marin R., Guzman D. Can Japanese quail male aggressions toward a female cagemate predict aggressiveness toward unknown conspecifics? Poultry Sci. 2017;96:133. [Google Scholar]
- 12.Blokhuis Harry J., editor. Welfare Quality® Consortium. Welfare Quality® Assessment Protocol for Poultry (Broilers, Laying Hens) Consortium. 2009. Collection of data for laying hens on farm; pp. 76–98. Lelystad, Netherlands. [Google Scholar]
- 13.AVMA Guidelines for the Euthanasia of Animals. ed. S3.4. 2013. p. 62. N. Meacham Road Schaumburg. [Google Scholar]
- 14.Jacobsen M.L., Jaspers V.L.B., Ciesielski T.M., Jenssen B.M., Løseth M.E., Briels N., Eulaers I., Leifsson P.S., Rigét F.F., Gomez-Ramirez P., Sonne C. Japanese quail (Coturnix japonica) liver and thyroid gland histopathology as a result of in ovo exposure to the flame retardants tris(1,3-dichloro-2-propyl) phosphate and Dechlorane Plus. J. Toxicol. Environ. Health A. 2017;80:525–531. doi: 10.1080/15287394.2017.1336414. [DOI] [PubMed] [Google Scholar]
- 15.Lefkowitch J.H. Special stains in diagnostic liver pathology. Semin. Diagn. Pathol. 2006;23:190–198. doi: 10.1053/j.semdp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Bernet D., Schmidt H., Meier W., Burkhardt-Holm P., Wahli T. Histopathology in fish: proposal for a protocol to assess aquatic pollution. J. Fish Dis. 2001;22:25–34. [Google Scholar]
- 17.Rautenberg G.E., Amé M.V., Monferrán M.V., Bonansea R.I., Hued A.C. A multi-level approach using Gambusia affinis as a bioindicator of environmental pollution in the middle-lower basin of Suquía River. Ecol. Indicat. 2015;48:706–720. [Google Scholar]
- 18.M.E. Fernandez, J.M.K. Kembro, M.L. Ballesteros, J.M. Caliva, R.H. Marin, M.C. Labaque, Data of thymol dietary supplementation in quail (Coturnix japonica): linking bioavailability, effects on egg yolk total fatty acids and performance traits, DOI: 10.6084/m9.figshare.7589066.v1. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia Component 1