Abstract
Inhibition of notch signaling is known to induce differentiation of endocrine cells in zebrafish and mouse. After performing an unbiased in vivo screen of ∼2,200 small molecules in zebrafish, we identified an inhibitor of Cdk5 (roscovitine), which potentiated the formation of β-cells along the intrapancreatic duct during concurrent inhibition of notch signaling. We confirmed and characterized the effect with a more selective Cdk5 inhibitor, (R)-DRF053, which specifically increased the number of duct-derived β-cells without affecting their proliferation. By duct-specific overexpression of the endogenous Cdk5 inhibitors Cdk5rap1 or Cdkal1 (which previously have been linked to diabetes in genome-wide association studies), as well as deleting cdk5, we validated the role of chemical Cdk5 inhibition in β-cell differentiation by genetic means. Moreover, the cdk5 mutant zebrafish displayed an increased number of β-cells independently of inhibition of notch signaling, in both the basal state and during β-cell regeneration. Importantly, the effect of Cdk5 inhibition to promote β-cell formation was conserved in mouse embryonic pancreatic explants, adult mice with pancreatic ductal ligation injury, and human induced pluripotent stem (iPS) cells. Thus, we have revealed a previously unknown role of Cdk5 as an endogenous suppressor of β-cell differentiation and thereby further highlighted its importance in diabetes.
Introduction
Apart from proliferation (1,2) and transdifferentiation (3–5), neogenesis (differentiation of new β-cells from endocrine progenitors or stem cells) is one of the major mechanisms in β-cell regeneration (6–10). Recent studies in mice have shown that pancreatic ductal ligation (PDL) or overexpression of the transcription factor Pax4 in α-cells induces neogenesis of endocrine cells originating from the pancreatic duct (8–10). In humans, acinar-associated neogenesis was promoted in obese donors without diabetes whereas duct-associated neogenesis was increased in both lean and obese donors with type 2 diabetes (6). Despite being widely reported, some studies show that neogenesis of β-cells rarely occurs or even does not happen in certain experimental conditions (11,12). This discrepancy suggests that β-cell neogenesis is a precisely controlled event and it is likely limited endogenously. Identifying new factors and signaling pathways that promote β-cell neogenesis could reveal a new route of exploiting potential β-cell progenitors, and they could serve as targets for future therapeutic strategies against diabetes.
Inhibition of notch signaling was first shown to promote endocrine cell differentiation in mice (13), a finding that was later confirmed in zebrafish (14). Although sustained inhibition of notch generates predominantly glucagon-producing α-cells in mice, it generates several different endocrine cell types in zebrafish. Therefore, we used notch inhibition simply as a starting point, i.e., we used it to initiate differentiation toward a variety of endocrine cells in zebrafish, enabling us to then screen for small molecules that can promote differentiation specifically to β-cells. After testing ∼2,200 small molecules, we found an inhibitor of Cdk5 that increased β-cell neogenesis in the presence of notch inhibition. We then confirmed the role of Cdk5 by genetic means and translated our findings using mouse embryonic pancreatic explants, adult mice with PDL, and human induced pluripotent stem (iPS) cells, indicating that the role of Cdk5 in β-cell formation is conserved in mice and humans. Together, our work suggests that inhibiting Cdk5 specifically stimulates β-cell neogenesis, and hence regeneration, which could represent a future curative approach for diabetes.
Research Design and Methods
Ethical Approval
All studies involving stem cells and animals were performed in accordance with local guidelines and regulations and were approved by regional authorities.
Zebrafish
The following previously published transgenic zebrafish lines were used: Tg(ins:GFP)zf5 , Tg(tp1:H2BmCherry)s939 , Tg(tp1:GFP)um14 , Tg(sst2:dsRed2)gz19 , Tg(ins:Flag-NTR)s950 , Tg(ins:H2B-GFP;ins:dsRed)s960, Tg(ins:Kaede)s949, and Tg(ins:CFPNTR)s892.
The overexpression models Tg(tp1:cdk5rap1;cmlc2:eGFP)KI108 and Tg(tp1:cdkal1;cmlc2:eGFP)KI109 were generated by the Tol2 transposon system similarly to our previous report (3), with the following modifications. The constructs were generated by MultiSite Gateway cloning (Invitrogen) with forward primers 5′- GGGGACAAGTTTGTACAAAAAAGCAGGCTCTgccaccATGAACAGAATTAGTACTTTCA-3′ for cdk5rap1 and 5′- GGGGACAAGTTTGTACAAAAAAGCAGGCTCTgccaccATGATGGCGTTGGTGTGTG-3′ for cdkal1, together with reverse primers 5′- GGGGACCACTTTGTACAAGAAAGCTGGGTCTCAGGCTGTTTTTCTGTCAT-3′ for cdk5rap1 and 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCTCAGAGCAGTTTCTCCATC-3′ for cdkal1 in the PCR, resulting in an amplicon for the BP reaction. Subsequently, p5E-tp1 together with the middle-entry vector containing cdk5rap1 or cdkal1 were used in the LR reaction.
The mutant cdk5KI110 was generated by CRISPR/Cas9. We acquired customized plasmids encoding single guide RNA targeting cdk5 and Cas9 protein from the University of Utah Mutation Generation and Detection Core. We coinjected 200 pg of single guide RNA and 750 pg of Cas9 protein into one-cell-stage zebrafish embryos. The founder was identified by genotyping according to the shape of melting curves after quantitative PCR (as described in genotyping below). The PCR product from the genotyping was sent for sequencing to confirm the mutagenesis and define the 25–base pair deletion (Supplementary Fig. 2). Although appearing overtly normal during the first week of development, zebrafish with homozygous mutation of Cdk5 did not survive to adulthood, correlating with Cdk5 deletion in mice (15).
Real-time PCR
Total RNA extraction and real-time PCR were performed according to our previous report (3) with the following primers: 5′-AGCGGGCTAGCAATGTCTTA-3′ with 5′-TTATCACAGCCACGCATGAT-3′ for cdk5rap1, 5′-ATGCGGAGGGAGTACTGCTG-3′ with 5′-CCTGGAACCCTCTCCTTCAGGTA-3′ for cdkal1, and 5′-CGAGCAGGAGATGGGAACC-3′ with 5′-CAACGGAAACGCTCATTGC-3′ for the housekeeping gene actb1. The relative mRNA levels of cdk5rap1 and cdkal1 were normalized to that of actb1.
Chemical Screening With Zebrafish Larvae
Four 3-days-postfertilization (3 dpf) larvae were transferred to each well of 96-well plates in a volume of 400 μL with 10 μmol/L of LY-411575 plus 0.1–10 μmol/L or 0.5–5 μg/mL of the compounds from the chemical libraries (Selleckchem Bioactive Compound Library and ENZO SCREEN-WELL ICCB Known Bioactives Library). At 5 dpf, the larvae were anesthetized with Tricaine, and the number of β-cells in the pancreatic tail was counted using a wide-field Leica DMI4000 B inverted microscope.
Immunofluorescence and EdU Staining of Zebrafish Larvae
Immunohistochemistry and 5-ethynyl-2′-deoxyuridine (EdU) staining were performed according to our previous report (3) and manufacturers’ protocols. The following primary antibodies were used: anti-GFP (1:500, catalog no. GFP-1020; Aves Laboratories), anti-dsRed (1:500, catalog no. 632496; Clontech Laboratories), anti-insulin (1:200, catalog no. I8510; Sigma-Aldrich), anti-glucagon (1:200, catalog no. G6254; Sigma-Aldrich), anti-somatostatin (1:200, catalog no. A0566; Dako), and anti-ghrelin (1:200, catalog no. 55529; AnaSpec).
Genotyping
Whole zebrafish larvae, clipped fins of juveniles, or posterior part of the larvae was transferred individually to each well of a 96-well PCR plate containing 30 μL of genomic DNA lysis buffer and lysed with standard procedures. The lysate containing genomic DNA was spun down, and the top layer was used for genotyping by real-time PCR with the cdk5 primers 5′-GGCTGAAACCATGCAAAAGT-3′ and 5′-ATTCAGGCCAGACAGTGCTT-3′. We genotyped the genomic DNA based on the shape of the melting curve compared with that of wild-type (WT) genomic DNA (Supplementary Fig. 2A).
Chemical Treatments and β-Cell Ablation
Zebrafish larvae were incubated in E3 medium containing different chemicals for various periods of time according to the experiments. The chemicals included LY-411575 (10 μmol/L; Sigma-Aldrich), roscovitine (5 μg/mL; Sigma-Aldrich), and (R)-DRF053 (DRF) (5 μmol/L; Sigma-Aldrich). We ablated β-cells in Tg(ins:kaede);Tg(ins:CFP-NTR) or Tg(ins:Flag-NTR) zebrafish larvae by incubating the larvae in E3 medium supplemented with 10 mmol/L metronidazole (Sigma-Aldrich), 1% of DMSO (VWR), and 0.2 mmol/L 1-phenyl-2-thiourea (Acros Organics) from 3 to 4 dpf. Free glucose levels were determined by lysing groups of seven larvae and analyzing the lysates using the Glucose Colorimetric/Fluorometric Assay Kit (BioVision).
Mouse Embryonic Pancreatic Explants
Mouse dorsal pancreata were explanted at embryonic day 12.5 (E12.5) based on a previous report (16). The explants were cultured on fibronectin-coated chamber slides (Corning) for 2 days (day 0–2) and then treated with the notch inhibitor N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) (10 μmol/L; Sigma-Aldrich) with or without DRF (2.5 μg/mL; Calbiochem) for 1 day (day 2–3). After allowing the explants to grow and differentiate for one more day without treatment, explants were fixed with 4% paraformaldehyde on day 4 for immunohistochemistry with guinea pig anti-insulin (1:800, catalog no. A0564; Dako) and rat anti-Cdh1 (1:1,000, catalog no. ECCD2; Novo Nordisk) antibodies. Five optical sections were captured at 6-μm intervals throughout each explant. Area of insulin+ (INS+) and CDH1+ immunopositive areas were analyzed by ImageJ (NIH).
Adult Mouse PDL
Mouse manipulation, PDL, and immunohistochemistry were performed according to previous reports (8,17,18). After PDL, mice received intraperitoneal osmotic pump (ALZET) containing roscovitine (5 μg/μL, resulting in a dosage of 2 mg/kg/day; Sigma-Aldrich) or PBS for 2 weeks. The following primary antibodies were used: anti-insulin (Diabetes Research Center, Vrije Universiteit Brussel), anti-Ki67 (eBioscience), and anti-CK19 (Developmental Studies Hybridoma Bank, University of Iowa). In the proliferation analysis, at least 5,000 β-cells were quantified per sample by the ICTN plugin in ImageJ (NIH). Quantification of the total β-cell and CK19+ cell volumes in the morphometry analysis was performed as described previously (8,17).
Human iPS Cells
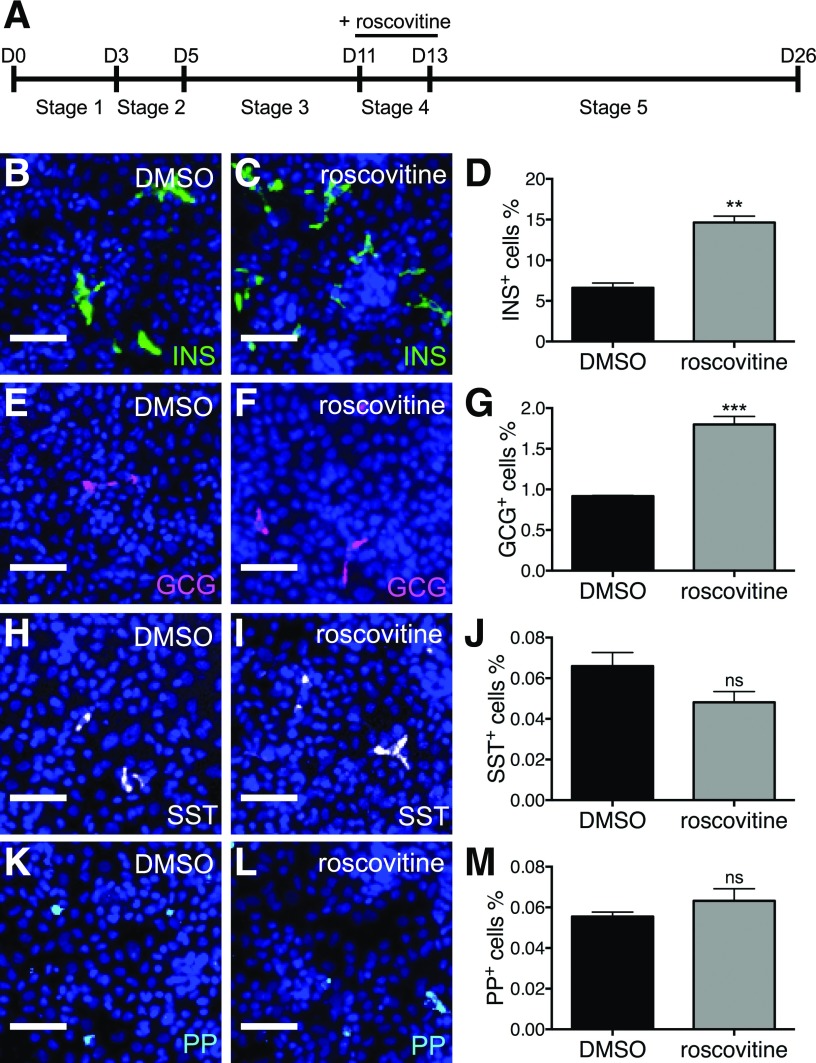
The human iPS cell line, named Toe, was originally derived from fetal lung cells (MRC-5) infected with retroviruses expressing Oct3/4, Sox2, Klf4, and c-Myc, and has the designation JCRB1338. The human iPS cells were induced to differentiate to insulin-producing cells according to a five-stage protocol (Fig. 8A) with modifications (19,20). The cells were treated with DMSO or roscovitine (1 μmol/L, unless specified differently) at stage 4 or stage 5, during which pancreatic progenitor cells differentiate to endocrine progenitor cells.
Figure 8.
Roscovitine stimulates differentiation of human iPS cells to β-cells. A: Schema for differentiating human iPS cells to insulin-producing cells. Human iPS cells were differentiated through sequential stages using defined media, medium A (stage 1), medium B (stage 2), medium C (stage 3), medium D (stage 4), and medium E (stage 5). Representative images of human iPS cells treated with DMSO or roscovitine at stage 4 and stained for insulin (B and C), glucagon (GCG) (E and F), somatostatin (SST) (H and I), and pancreatic polypeptide (PP) (K and L), displaying INS+ cells in green, GCG+ cells in magenta, SST+ in white, PP+ in cyan, and DAPI in blue. Scale bar = 50 μm. Percentages of differentiated human iPS cells expressing insulin (D), glucagon (G), somatostatin (J), and pancreatic polypeptide (M) after treatment with DMSO or roscovitine at stage 4. **P ≤ 0.01; ***P ≤ 0.001. n = 3.
Human iPS cells were fixed with 4% paraformaldehyde for immunocytochemistry with primary antibodies anti-insulin (1:1,000; Dako), anti-glucagon (1:300; Sigma-Aldrich), anti-somatostatin (1:500; Santa Cruz Biotechnology), and anti-pancreatic polypeptide (1:300; Dako). Images were quantified by the MetaXpress cellular image analysis software (Molecular Devices).
Statistical Analysis
Data were analyzed with GraphPad Prism. Results are presented as mean values ± SEM. P values ≤0.05 are considered statistically significant. Statistical analyses were carried out by Student t tests when two groups were analyzed and by ANOVA when more than two groups were analyzed. Similar experiments were performed at least two times, and representative data are presented in the figures.
Results
Chemical Screening in Zebrafish Identifies a Cdk5 Inhibitor That Promotes β-Cell Neogenesis
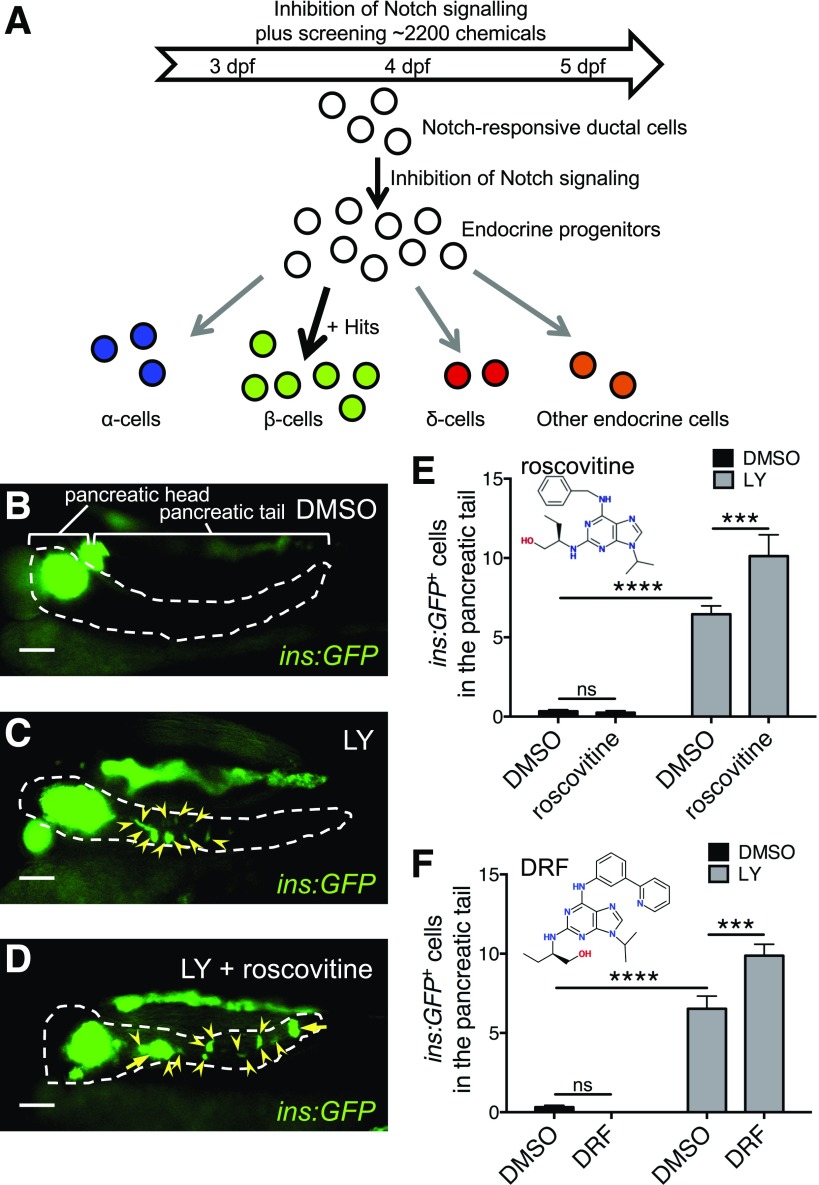
We inhibited notch signaling in Tg(ins:GFP) zebrafish larvae to stimulate the formation of endocrine progenitors along the pancreatic duct (14) for screening small molecules that potentiate the differentiation of these duct-derived endocrine progenitors to β-cells. We treated Tg(ins:GFP) with LY-411575 (LY), a notch inhibitor, together with different compounds from chemical libraries from 3 to 5 dpf and analyzed the number of ins:GFP+ cells along the pancreatic tail (Fig. 1A).
Figure 1.
Chemical screening in zebrafish identifies a Cdk5 inhibitor that promotes β-cell neogenesis. A: Scheme of the chemical screening. Zebrafish larvae Tg(ins:GFP) were cotreated with a compound from chemical libraries and the notch inhibitor LY from 3 to 5 dpf to screen for hits that promote/divert the stochastic endocrine cell differentiation induced by notch inhibition to a more specific β-cell differentiation, i.e., from the progenitors in the intrapancreatic duct. B–D: Representative images of the pancreas of Tg(ins:GFP) larvae at 5 dpf treated with DMSO as control, LY, or LY plus roscovitine, displaying β-cells in green. Arrowheads indicate GFP-positive β-cells. Arrows point to clusters of GFP-positive β-cells. Dashed lines show the outline of the pancreas, and the region of pancreatic tail is indicated in B. Scale bar = 100 μm. E: Quantification of ins:GFP+ cells in the pancreatic tail per larva at 5 dpf. After screening for ∼2,200 chemicals, roscovitine was identified as an enhancer of β-cell neogenesis in the presence of LY. ***P ≤ 0.001; ****P ≤ 0.0001. n = 12. F: The selective Cdk5 inhibitor DRF mimicked roscovitine to promote β-cell formation. The number of ins:GFP+ cells in the pancreatic tail per larva was quantified after treatment with DMSO, LY, or LY plus DRF from 3 to 5 dpf. ***P ≤ 0.001; ****P ≤ 0.0001. n = 7–18.
In the DMSO control group, there were very few ins:GFP+ cells posterior to the primary islet, i.e., along the pancreatic tail (Fig. 1B). Similar to another inhibitor of notch signaling DAPT (14), LY induced a limited number of insulin-expressing cells along the pancreatic tail (Fig. 1C). After testing ∼2,200 small molecules, we found that roscovitine, an inhibitor preferentially targeting Cdk5, synergized with LY to increase the number of ins:GFP+ cells in the pancreatic tail (Fig. 1D and E). We then further confirmed the effect of roscovitine by a more potent and selective Cdk5 inhibitor, DRF (Fig. 1F), and continued to use DRF in the subsequent experiments.
Inhibition of Cdk5 Enhances β-Cell Neogenesis From Notch-Responsive Ductal Cells Without Affecting β-Cell Proliferation
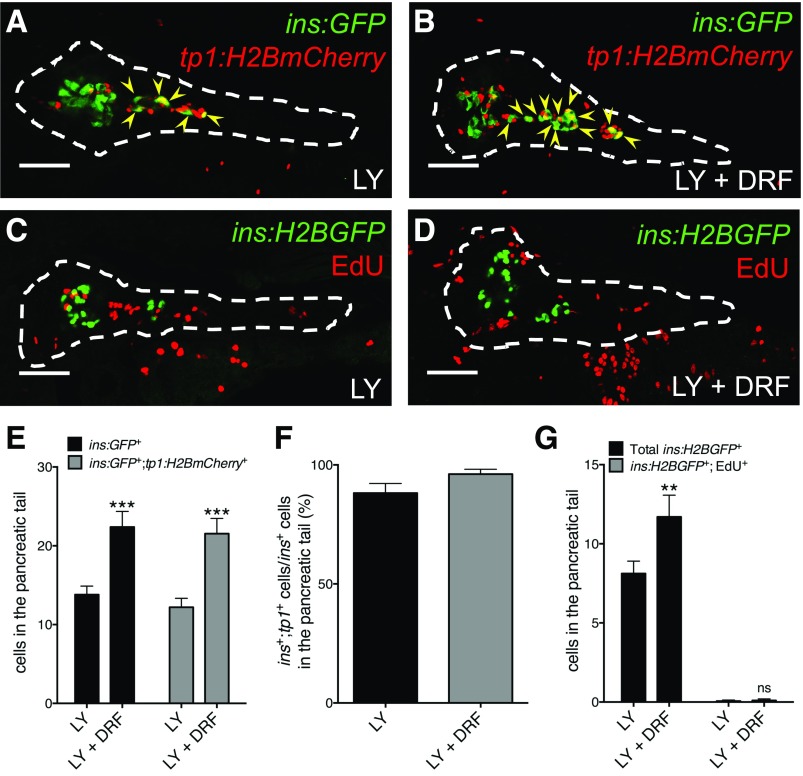
To examine the origin of new β-cells, we performed lineage tracing as well as assessed proliferation. We traced the lineage of the β-cells induced by Cdk5 inhibition using Tg(ins:GFP);Tg(tp1:H2BmCherry) zebrafish larvae, which not only mark the insulin-expressing cells in green but also stably label the nucleus of notch-responsive ductal cells in red. DRF potentiated LY to expand the population of cells expressing ins:GFP and cells coexpressing ins:GFP and tp1:H2BmCherry (Fig. 2A, B, and E). Interestingly, the vast majority of β-cells coexpressed both ins:GFP and tp1:H2BmCherry (Fig. 2F), indicating that notch-responsive progenitors in the pancreatic duct are the major source of β-cell neogenesis in the tail of the pancreas.
Figure 2.
Inhibition of Cdk5 promotes differentiation of duct-derived β-cells. A, B, E, and F: The notch-responsive ductal cells are the major source of β-cell differentiation induced by DRF. A and B: Representative confocal images of the pancreas of Tg(ins:GFP);Tg(tp1:H2BmCherry) zebrafish larvae at 6 dpf treated with LY or LY plus DRF from 3 to 6 dpf, displaying β-cells in green and β-cells of a notch-responsive ductal lineage in yellow overlap (arrowheads). Dashed lines show the outline of the pancreas. Scale bar = 50 μm. E: Quantification of ins:GFP+ and ins:GFP+;tp1:H2BmCherry+ cells in the pancreatic tail per larva at 6 dpf. ***P ≤ 0.001; n = 13–15. F: Percentage of ins:GFP+;tp1:H2BmCherry+ coexpressing cells among the total number of ins:GFP+ cells in the pancreatic tail at 6 dpf. n = 13–15. C, D, and G: DRF did not expand the β-cell population by increasing its proliferation. C and D: Representative confocal images of the pancreas of Tg(ins:H2BGFP) larvae at 6 dpf treated with LY or LY plus DRF from 3 to 6 dpf and incubated with EdU from 5 to 6 dpf, displaying β-cells in green and all cells that had incorporated EdU in red. Dashed lines show the outline of the pancreas. Scale bar = 50 μm. G: Quantification of ins:H2BGFP+ cells and ins:H2BGFP+cells that had incorporated EdU in the pancreatic tail per larva at 6 dpf. **P ≤ 0.01; n = 10–16.
To determine whether Cdk5 inhibition affects β-cell proliferation, we treated Tg(ins:H2BGFP) larvae with LY and DRF from 3 to 6 dpf while exposing them to EdU from 5 to 6 dpf. There were very few ins:H2BGFP+ cells that incorporated EdU in the zebrafish treated only with LY, despite several neighboring cells in the pancreatic tail being marked by EdU (Fig. 2C). Moreover, despite significantly increasing β-cell numbers in the pancreatic tail, DRF did not alter β-cell proliferation (Fig. 2D and G) or apoptosis (data not shown).
Cdk5 Inhibition Preferentially Stimulates β-Cell Neogenesis
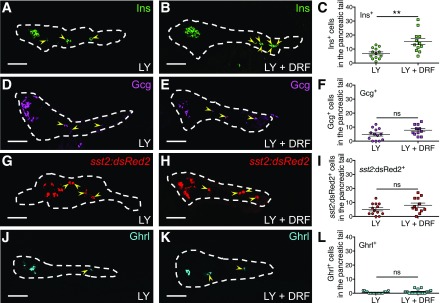
We examined whether Cdk5 inhibition exerted a specific effect on β-cell neogenesis or a general effect on the neogenesis of various endocrine cells by treating Tg(sst2:dsRed2) larvae with LY and DRF, staining for insulin, glucagon, and ghrelin, and quantifying the number of each cell type. Similar to the effect on Tg(ins:GFP) (Figs. 1 and 2), DRF expanded the population of insulin-expressing β-cells in the pancreatic tail (Fig. 3A–C). On the contrary, DRF did not significantly alter the number of glucagon-expressing α-cells (Fig. 3D–F), sst2:dsRed2+ δ-cells (Fig. 3G–I), or ghrelin-expressing ε-cells (Fig. 3J–L) in the pancreatic tail. Moreover, there were no significant changes in the number of these endocrine cells in the pancreatic head (Supplementary Fig. 1), indicating that DRF preferentially stimulates β-cell neogenesis in the pancreatic tail.
Figure 3.
Cdk5 inhibition preferentially stimulates β-cell neogenesis. A–L: Inhibition of Cdk5 preferentially promoted β-cell differentiation. Representative confocal images of the pancreas of zebrafish larvae at 6 dpf treated with LY or LY plus DRF from 3 to 6 dpf and stained for insulin (Ins) (A and B), glucagon (Gcg) (D and E), Tg(sst2:dsRed2) (G and H), and ghrelin (Ghrl) (J and K), displaying β-cells in green, α-cells in magenta, δ-cells in red, and ε-cells in cyan (arrowheads). Dashed lines show the outline of the pancreas. Scale bar = 50 μm. C, F, I, and L: Quantification of insulin-positive, glucagon-positive, sst2:dsRed2−, and ghrelin-positive cells in the pancreatic tail per larva at 6 dpf. **P ≤ 0.01; n = 11–19.
Overexpression of Endogenous Inhibitors of Cdk5 Promotes β-Cell Neogenesis
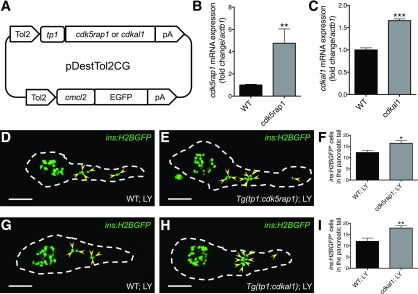
To validate our findings from the experiments using chemical inhibitors of Cdk5, we established stable transgenic zebrafish lines overexpressing endogenous inhibitors of Cdk5 signaling (21,22) specifically in notch-responsive cells, thus generating Tg(tp1:cdk5rap1) and Tg(tp1:cdkal1) lines (Fig. 4A). Data from quantitative PCR confirmed the overexpression (Fig. 4B and C). Next, we tested the effect of overexpressing Cdk5rap1 or Cdkal1 on β-cell neogenesis during inhibition of notch. Similar to the effect of roscovitine or DRF, overexpressing Cdk5rap1 or Cdkal1 in the notch-responsive ductal cells increased the number of ins:H2BGFP+ cells along the pancreatic tail (Fig. 4D–I), indicating that roscovitine and DRF indeed act directly through inhibition of Cdk5 in ductal cells (and not through an indirect effect on another cell type or an off-target effect) to increase the number of β-cells.
Figure 4.
Overexpression of Cdk5rap1 or Cdkal1 increases the number of β-cells. A: Scheme of constructs used for overexpressing cdk5rap1 or cdkal1 under the control of tp1 promoter and expressing GFP in the heart (as an internal control of genomic integration). Relative mRNA levels of cdk5rap1 (B) or cdkal1 (C) normalized to the housekeeping gene actb1 in WT larvae and stable transgenic larvae overexpressing cdk5rap1 (B) or cdkal1 (C) at 6 dpf. **P ≤ 0.01; ***P ≤ 0.001. n = 5–8. D–I: Overexpressing Cdk5rap1 or Cdkal1 promoted β-cell neogenesis. Representative confocal images of the pancreas of Tg(ins:H2BGFP) larvae with or without the overexpression transgene, Tg(tp1:cdk5rap1) (D and E) or Tg(tp1:cdkal1) (G and H), at 6 dpf treated with LY from 3 to 6 dpf, displaying β-cells in green (arrowheads mark β-cells along the pancreatic tail). Dashed lines show the outline of the pancreas. Scale bar = 50 μm. F and I: Quantification of ins:H2BGFP+ cells in the pancreatic tail per larva at 6 dpf. *P ≤ 0.05; **P ≤ 0.01. n = 19–20.
Deletion of cdk5 Promotes Both Formation and Regeneration of β-Cells
Previous studies have shown that Cdk5rap1 and Cdkal1 may function as methylthiotransferases or inhibitors of Cdk5 activity depending on the context (23,24). Therefore, we also established a mutant of cdk5 (cdk5−/−) (Supplementary Fig. 2) to specifically study the effect of Cdk5 on β-cell formation, without any involvement of alternative effects of Cdk5rap1 and Cdkal1 or potential off-target effects of small molecule inhibitors.
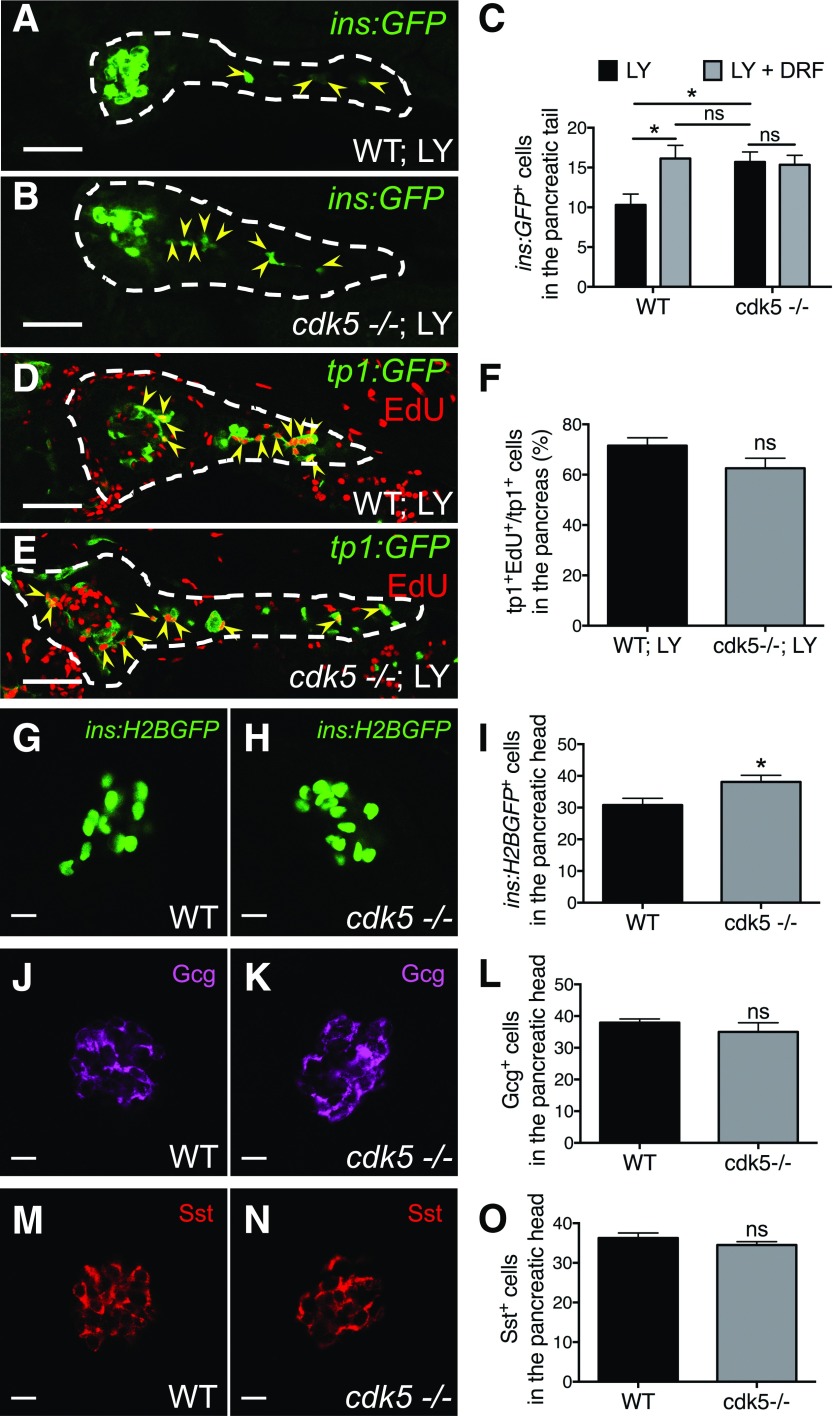
Concordant with the experiments described earlier in this study using chemical inhibition of Cdk5, there were more ins:GFP+ cells along the pancreatic tail in cdk5−/− larvae compared with WT siblings after notch inhibition (Fig. 5A–C), whereas there was no significant change in cdk5+/− larvae (data not shown). DRF did not exert any additional effect in the cdk5−/− larvae, indicating that its effect on the WT is mediated exclusively via Cdk5. Deleting cdk5 did not alter EdU labeling of tp1+ pancreatic duct cells (Fig. 5D–F), suggesting that inhibiting the Cdk5 pathway does not significantly affect the proliferation of the notch-responsive ductal source of endocrine cell progenitors.
Figure 5.
Deletion of cdk5 promotes formation of β-cells. A–C: Deficiency of cdk5 increased the number of β-cells in the pancreatic tail. A and B: Representative confocal images of the pancreas of WT and cdk5−/− Tg(ins:GFP) larvae at 6 dpf treated with LY from 3 to 6 dpf, displaying β-cells in green (arrowheads). Dashed lines show the outline of the pancreas. Scale bar = 50 μm. C: Quantification of ins:GFP+ cells in the pancreatic tail per larva at 6 dpf. *P ≤ 0.05; n = 15–17. D and E: Representative confocal images of the pancreas of WT and cdk5−/− Tg(tp1:GFP) larvae at 6 dpf treated with LY from 3 to 6 dpf and incubated with EdU from 5 to 6 dpf, displaying notch-responsive cells in green and notch-responsive cells that had incorporated EdU in yellow overlap (arrowheads). Dashed lines show the outline of the pancreas. Scale bar = 50 μm. F: Percentage of tp1:GFP+ cells that had incorporated EdU among the total number of tp1:GFP+ cells in the pancreas at 6 dpf. n = 13–16. G–O: Deficiency of cdk5 preferentially expanded the population of β-cells. Representative confocal images of the pancreatic head of WT and cdk5−/− zebrafish larvae carrying Tg(ins:H2BGFP) (G and H), stained for glucagon (Gcg) (J and K) or somatostatin (Sst) (M and N) at 6 dpf, displaying β-cells in green, α-cells in magenta, and δ-cells in red. Scale bar = 10 μm. I, L, and O: Quantification of ins:H2BGFP+, glucagon-positive, and somatostatin-positive cells in the head of the pancreas per larva at 6 dpf. *P ≤ 0.05; n = 8–18.
Interestingly, even without the inhibition of notch, there was an increased number of β-cells, but not α- or δ-cells, in the pancreatic head in cdk5 mutants compared with their WT siblings (Fig. 5G–O). In contrast to the β-cells induced in the pancreatic tail, the majority of induced β-cells in the pancreatic head did not originate from the tp1+ duct because very few of them were lineage traced by tp1:H2BmCherry (Supplementary Fig. 3).
In addition to the basal state (Fig. 5G–I), deletion of cdk5 promoted regeneration of ins:H2BGFP+ cells independent of notch inhibition (Supplementary Fig. 4), i.e., β-cells regenerating in the pancreatic head. Increased β-cell regeneration was also observed when we treated zebrafish larvae with DRF during notch inhibition (Supplementary Fig. 5A–C). These β-cells originated from the tp1+ duct and are likely functional because DRF facilitated the restoration of glycemia after β-cell ablation (Supplementary Fig. 5D).
DRF Promotes Differentiation of β-Cells in Mouse Embryonic Pancreatic Explants
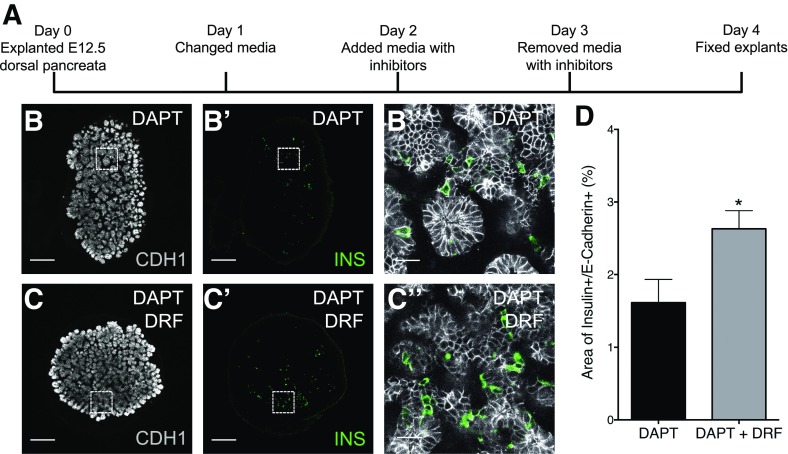
Using mouse embryonic (E12.5) pancreatic explants (Fig. 6A), we determined whether Cdk5 inhibition facilitates the differentiation of β-cells in mammalian tissue in vitro. After treatment with DRF in the presence of DAPT, there was an increase in the area of INS+ cells in the pancreatic explants (Fig. 6B–D), whereas neither DAPT nor DRF had any effect on their own (data not shown). This indicates that Cdk5 inhibition can promote β-cell differentiation in the developing mouse pancreas.
Figure 6.
DRF promotes differentiation of β-cells in mouse embryonic pancreatic explants. A: Schema for culturing and treating mouse embryonic pancreatic explants. On day 0, mouse dorsal pancreata were explanted at E12.5. After culturing for 2 days, the explants were treated with DAPT (notch inhibitor) in the presence or absence of DRF (Cdk5 inhibitor) from day 2 to 3. The explants were allowed to differentiate for one more day before fixation on day 4. Representative confocal images of mouse pancreatic explants treated with DAPT only (B, B′, and B′′) or DAPT together with DRF (C, C′, and C′′), displaying INS+ cells in green and CDH1+ cells in white. B′′ and C′′ were magnified eight times from the selected area in B′ and C′, respectively. Scale bar = 200 μm (B, B′, C, and C′) or 25 μm (B′′ and C′′). D: Percentage of area of INS+/CDH1+ cells after treatment with DAPT or DAPT plus DRF. *P ≤ 0.05; n = 5 (5 explants; 5 sections per explant; 25 sections in total in each group).
Roscovitine Promotes β-Cell Formation in Adult Mouse Pancreas After Ductal Ligation
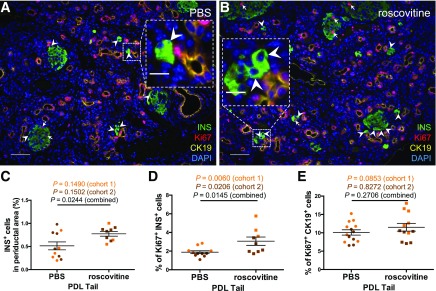
To validate the effects of Cdk5 in adult mammals in vivo, we studied the effects of roscovitine in mice with PDL injury, which activates endocrine cell progenitors (8). Roscovitine was used in these studies since its effective dose in mice is known, contrary to DRF. After treatment for 2 weeks, roscovitine increased the number of INS+ cells in the periductal area, that is within 30 μm from the CK19+ ductal cells, in the ligated tail part of the pancreas (the procedure was performed in two cohorts of mice that were analyzed both individually and combined) (Fig. 7A–C). Roscovitine also promoted proliferation of β-cells but not of CK19+ ductal cells (Fig. 7D and E). The enhanced formation of β-cells by roscovitine did not significantly alter body weight, glycemia, glucose tolerance, β-cell volume, or duct volume (data not shown). Together, these data suggest that roscovitine enhanced both β-cell proliferation and β-cell neogenesis from the duct, although lineage tracing would be needed to formally define the origin of the periductal β-cells.
Figure 7.
Roscovitine promotes β-cell formation in adult mouse pancreas after ductal ligation. A and B: Representative images of the duct-ligated part of pancreas (PDL tail) after treatment with PBS or roscovitine via osmotic pumps for 2 weeks, displaying INS+ cells in green, Ki67+ cells in red, CK19+ cells in yellow, and DAPI in blue. Insets show magnified representative INS+ cells in the periductal area. Arrowheads and arrows indicate INS+ cells in the periductal area and proliferating INS+ cells, respectively. Scale bar of main figures = 100 μm. Scale bar of insets = 25 μm. Quantification of INS+ cells in the periductal area (C), percentage of Ki67+ INS+ cells (D), and percentage of Ki67+ CK19+ cells in the PDL tail (E). P values from cohort 1, cohort 2, and combined are shown; n = 9–14.
Roscovitine Stimulates Differentiation of Human iPS Cells to β-Cells
To further examine whether the effect of Cdk5 inhibition in zebrafish and mice holds true in human cells, we differentiated human iPS cells into β-cells by a five-stage differentiation protocol (19,20) in the presence of roscovitine at stage 4 (day 11–13) (Fig. 8A) or stage 5 (day 13–26) (Supplementary Fig. 7A), during which pancreatic progenitors differentiate to endocrine progenitors and finally mature endocrine cells. No notch inhibitor was added since the cultures already contain endocrine progenitors at stage 4 and 5. Moreover, DRF was excluded from this analysis since it appeared toxic at the concentrations under examination. Suppressing Cdk5 signaling by roscovitine at stage 4 facilitated the differentiation of insulin- and glucagon-expressing cells, but not somatostatin- or pancreatic polypeptide–expressing cells (Fig. 8B–M). The promoting effect of roscovitine on INS+ cell differentiation was dose dependent (Supplementary Fig. 6), and treatment with roscovitine at stage 5 was still effective but less potent (Supplementary Fig. 7). These data suggest that the role of Cdk5 in β-cell differentiation is conserved in zebrafish, mice, and humans.
Discussion
In the current study, we demonstrated that inhibition of Cdk5 promotes β-cell differentiation from notch-responsive progenitors in the pancreas. Using an unbiased chemical in vivo screen with zebrafish larvae, we found that the Cdk5 inhibitor roscovitine enhances neogenesis of β-cells along the pancreatic tail, which was also confirmed by another more potent Cdk5 inhibitor, DRF. Via lineage tracing, cell proliferation assay, and confocal microscopy, we found that Cdk5 inhibition stimulates β-cell neogenesis from notch-responsive ductal cells in the basal state, as well as during regeneration of β-cells. The critical role of Cdk5 inhibition was further validated by overexpressing endogenous Cdk5 inhibitors and deleting cdk5. Translating these findings from zebrafish to mammals using mouse embryonic pancreatic explants, adult mice with PDL injury, and human iPS cells, we showed that the role of Cdk5 in β-cell differentiation is conserved in mice and humans.
Cdk5 is a unique Cdk family member that is activated by the noncyclins p35 and p39 (25). In contrast to other Cdk members, Cdk5 is not believed to play a large role in cell cycle regulation. Cdk5 is highly implicated in neuronal development and functions, and dysregulation of Cdk5 signaling is correlated with Alzheimer disease (25).
Apart from neurodegenerative diseases, studies in recent decades have suggested a crucial role of Cdk5 in diabetes. Cdk5 and its activators p35 and p39 are expressed in the β-cells of rodents (26,27). A few studies with data mainly obtained from in vitro experiments and cell lines have shown that Cdk5 may be important for insulin expression and secretion and β-cell survival and proliferation (26–28). Nevertheless, numerous recent studies, in line with our findings, have demonstrated that inhibition of Cdk5 activity may help to maintain glucose homeostasis. First, pharmacological inhibition of Cdk5 in isolated rodent islets and genetic knockout of p35 in mice enhanced glucose-stimulated insulin secretion, and performance in glucose tolerance tests was improved in the p35-knockout mice (29). Second, strengthening Cdk5 signaling by knocking out Cdkal1 (the endogenous Cdk5 inhibitor) in mice suppressed glucose-stimulated insulin secretion and increased blood glucose levels in the glucose tolerance test (30). Concordantly, Cdk5 inhibition also plays a role in the mechanism of several antidiabetic drugs targeting peroxisome proliferator–activated receptor γ (PPAR-γ), i.e., rosiglitazone, MRL24, and UHC, which reduce Cdk5-mediated phosphorylation of PPAR-γ in adipocytes and increase insulin sensitivity (31). These studies all highlight the importance of Cdk5 in glucose control by affecting various cell types, including β-cells and adipocytes, and our current report adds a role for Cdk5 in ductal cells; we show that duct-specific overexpression of Cdk5 inhibitors potentiates β-cell differentiation in a ductal-autonomous manner. Interestingly, recent studies, including genome-wide association studies, have extensively reported that genetic variations in CDKAL1 are strongly associated with increased risk of type 2 diabetes and glucose-related traits across different ethnic populations (32–36), implicating the involvement of CDK5 in the progression of diabetes. Furthermore, two recent studies, despite focusing on the cell cycle and CDKs other than CDK5, have demonstrated that roscovitine inhibits hyperphosphorylation of NEUROG3. This stabilizes NEUROG3 and hence promotes pancreatic endocrine cell differentiation (37,38). As Cdk5 has a different phosphorylation motif than other Cdks, and Neurog3 is not expressed in the zebrafish pancreas (39), Cdk5 is affecting a different downstream mechanism. Nevertheless, it is possible that we, when using roscovitine or DRF, have a combined effect of inhibiting Cdk5 and other Cdks. In future studies, it would be interesting to genetically test whether the other Cdks influence the effects of Cdk5 or play a role in regulating β-cell formation in mammals. However, in zebrafish we see an equally potent effect with chemical inhibition of Cdk5, as in cdk5 mutants, which specifically affects Cdk5 and not ductal cell proliferation or other Cdks. Thus, our current study is the first to report that Cdk5 inhibition promotes β-cell differentiation from ductal cells.
In our findings, we have shown that the ductal cells represent the major source of β-cell neogenesis induced by Cdk5 inhibition. It is known that these ductal cells are important for the formation of secondary islets and regeneration of β-cells in zebrafish (14,40–44), but apart from notch (14) and retinoic acid (45), the signals regulating their differentiation remain poorly characterized. Moreover, Bonner-Weir et al. (7) have reviewed various studies providing evidence of in vivo neogenesis of endocrine islets, especially insulin-positive cells, in mammals including humans. For instance, PDL of the pancreas in mice induced expression of Neurog3 in and adjacent to pancreatic ducts (8). Similarly, ductal ligation also resulted in insulin-positive cells deriving from the ductal lineage, which was traced by inducible Cre recombinase driven by the carbonic anhydrase II promoter (9). Furthermore, a recent study on human pancreatic samples has revealed that duct-associated neogenesis of insulin-positive cells was more abundant in the samples from the donors with type 2 diabetes compared with those from donors without diabetes (6). Another study on adult mice with ectopic expression of Pax4, a transcription factor for β-/δ-cell fate, in glucagon-expressing α-cells induced pancreatic endocrine hormone expression in cells from the ductal lineage (10). These studies have indicated that the ductal cells can constitute endocrine progenitors in the pancreas, although the factors that can stimulate neogenesis of endocrine cells from the ductal progenitors are ill defined. Here, our current study makes a conceptual advance by not only showing that inhibition of Cdk5 promotes endocrine differentiation from the ductal lineage but also demonstrating that its effect is preferential to β-cell differentiation. In addition, apart from the Tp1-positive intrapancreatic duct, we observed notch-independent enhanced β-cell formation from Tp1-negative progenitors in the cdk5 mutants. This suggests that Tp1-negative extrapancreatic ductal cells close to the pancreatic head could be an alternative source for Cdk5-dependent β-cell neogenesis.
Cdk5rap1, previously named C42, was cloned and identified as a binding protein of the Cdk5 activator p35 by a yeast two-hybrid screen (46). The interaction between Cdk5rap1 and p35, plus the inhibitory effect of Cdk5rap1 on Cdk5 activity were subsequently shown (21). Because of the similarity in the protein domains between Cdk5rap1 and Cdkal1, Cdkal1 was proposed as another endogenous inhibitor of Cdk5 activity (22). However, a study identified Cdkal1 as a methylthiotransferase (24). Interestingly, recent reports have also shown that Cdk5rap1 can act as a methylthiotransferase (23,47), suggesting that both Cdk5rap1 and Cdkal1 may indeed play dual roles as endogenous Cdk5 inhibitors and methylthiotransferases. In our current study, overexpression models of Cdk5rap1 and Cdkal1 both mimicked the Cdk5 mutant in potentiating β-cell neogenesis along the pancreatic tail, indicating that both Cdk5rap1 and Cdkal1 function as Cdk5 inhibitors to enhance β-cell differentiation and providing a potential mechanistic explanation for the genetic linkage between CDKAL1 and diabetes.
Current Cdk5 inhibitors are well known for playing crucial roles in the nervous system and would lead to neural side effects, which perhaps could be limited in the future by the development of Cdk5 inhibitors that do not cross the blood-brain barrier. Moreover, further studies on the molecular mechanisms of the induced β-cell formation could potentially identify downstream factors of the Cdk5 pathway to provide additional insights and targets for the development of more specific therapeutic strategies. Nevertheless, our data from human iPS cells indicate that Cdk5 inhibitors are promising candidates for advancing β-cell differentiation protocols in vitro for β-cell replacement therapies.
In summary, we have shown that Cdk5 inhibition promotes differentiation of β-cells from pancreatic ductal cells in zebrafish and mice, as well as from human iPS cells. Further studies on this pathway may facilitate the development of potential regenerative therapies, based on either endogenous β-cell progenitors or stem cell cultures, for the management of diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank the University of Utah Mutation Generation and Detection Core for assistance in generating mutants, Takuya Sakaguchi (Cleveland Clinic) for exchanging unpublished information regarding Cdk5, and Yves Heremans (Vrije Universiteit Brussel) for discussion and additional in vitro studies.
Funding. This work was supported by grants from the Ragnar Söderberg Foundation, the Swedish Research Council, the Swedish Foundation for Strategic Research, the Wenner-Gren Foundation, the Novo Nordisk Foundation, the Strategic Research Programme in Diabetes, and Stem Cell Research and Regenerative Medicine at the Karolinska Institutet to O.A. This work was also supported by a European Foundation for the Study of Diabetes/Lilly Fellowship awarded to K.-C.L.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.-C.L. designed, performed, and analyzed the zebrafish experiments; analyzed data for the mouse pancreatic explant experiments; and wrote the manuscript with help from all authors. G.L., W.S., Y.V., and H.H. performed mouse PDL experiments and analyzed the data. D.S. and S.K. performed human iPS cell experiments and analyzed the data. P.A.S. and P.S. performed mouse pancreatic explant experiments and analyzed the data. C.L.M. worked on translation. L.R. designed, performed, and analyzed the zebrafish experiments. O.A. designed, performed, and analyzed the zebrafish experiments and wrote the manuscript with help from all authors. O.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-1587/-/DC1.
References
- 1.Andersson O, Adams BA, Yoo D, et al. . Adenosine signaling promotes regeneration of pancreatic β cells in vivo. Cell Metab 2012;15:885–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by β cell regeneration. J Clin Invest 2007;117:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu J, Liu KC, Schulz N, et al. . IGFBP1 increases β-cell regeneration by promoting α- to β-cell transdifferentiation. EMBO J 2016;35:2026–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorel F, Népote V, Avril I, et al. . Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 2010;464:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baeyens L, Lemper M, Leuckx G, et al. . Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat Biotechnol 2014;32:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Hanley SC, Austin E, Assouline-Thomas B, et al. . β-Cell mass dynamics and islet cell plasticity in human type 2 diabetes. Endocrinology 2010;151:1462–1472 [DOI] [PubMed] [Google Scholar]
- 7.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. β-Cell growth and regeneration: replication is only part of the story. Diabetes 2010;59:2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, D’Hoker J, Stangé G, et al. . β cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008;132:197–207 [DOI] [PubMed] [Google Scholar]
- 9.Inada A, Nienaber C, Katsuta H, et al. . Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA 2008;105:19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Hasani K, Pfeifer A, Courtney M, et al. . Adult duct-lining cells can reprogram into β-like cells able to counter repeated cycles of toxin-induced diabetes. Dev Cell 2013;26:86–100 [DOI] [PubMed] [Google Scholar]
- 11.Xiao X, Chen Z, Shiota C, et al. . No evidence for β cell neogenesis in murine adult pancreas. J Clin Invest 2013;123:2207–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopp JL, Dubois CL, Hao E, Thorel F, Herrera PL, Sander M. Progenitor cell domains in the developing and adult pancreas. Cell Cycle 2011;10:1921–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apelqvist A, Li H, Sommer L, et al. . Notch signalling controls pancreatic cell differentiation. Nature 1999;400:877–881 [DOI] [PubMed] [Google Scholar]
- 14.Parsons MJ, Pisharath H, Yusuff S, et al. . Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev 2009;126:898–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohshima T, Ward JM, Huh CG, et al. . Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA 1996;93:11173–11178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Percival AC, Slack JM. Analysis of pancreatic development using a cell lineage label. Exp Cell Res 1999;247:123–132 [DOI] [PubMed] [Google Scholar]
- 17.Van de Casteele M, Leuckx G, Baeyens L, et al. . Neurogenin 3+ cells contribute to β-cell neogenesis and proliferation in injured adult mouse pancreas. Cell Death Dis 2013;4:e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang RN, Klöppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 1995;38:1405–1411 [DOI] [PubMed] [Google Scholar]
- 19.Nakashima R, Morooka M, Shiraki N, et al. . Neural cells play an inhibitory role in pancreatic differentiation of pluripotent stem cells. Genes Cells 2015;20:1028–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahjalal HM, Shiraki N, Sakano D, et al. . Generation of insulin-producing β-like cells from human iPS cells in a defined and completely xeno-free culture system. J Mol Cell Biol 2014;6:394–408 [DOI] [PubMed] [Google Scholar]
- 21.Ching YP, Pang AS, Lam WH, Qi RZ, Wang JH. Identification of a neuronal Cdk5 activator-binding protein as Cdk5 inhibitor. J Biol Chem 2002;277:15237–15240 [DOI] [PubMed] [Google Scholar]
- 22.Stancáková A, Pihlajamäki J, Kuusisto J, et al.; EUGENE2 Consortium . Single-nucleotide polymorphism rs7754840 of CDKAL1 is associated with impaired insulin secretion in nondiabetic offspring of type 2 diabetic subjects and in a large sample of men with normal glucose tolerance. J Clin Endocrinol Metab 2008;93:1924–1930 [DOI] [PubMed] [Google Scholar]
- 23.Reiter V, Matschkal DM, Wagner M, et al. . The CDK5 repressor CDK5RAP1 is a methylthiotransferase acting on nuclear and mitochondrial RNA. Nucleic Acids Res 2012;40:6235–6240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arragain S, Handelman SK, Forouhar F, et al. . Identification of eukaryotic and prokaryotic methylthiotransferase for biosynthesis of 2-methylthio-N6-threonylcarbamoyladenosine in tRNA. J Biol Chem 2010;285:28425–28433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol 2001;2:749–759 [DOI] [PubMed] [Google Scholar]
- 26.Lilja L, Yang SN, Webb DL, Juntti-Berggren L, Berggren PO, Bark C. Cyclin-dependent kinase 5 promotes insulin exocytosis. J Biol Chem 2001;276:34199–34205 [DOI] [PubMed] [Google Scholar]
- 27.Ubeda M, Kemp DM, Habener JF. Glucose-induced expression of the cyclin-dependent protein kinase 5 activator p35 involved in Alzheimer’s disease regulates insulin gene transcription in pancreatic β-cells. Endocrinology 2004;145:3023–3031 [DOI] [PubMed] [Google Scholar]
- 28.Daval M, Gurlo T, Costes S, Huang CJ, Butler PC. Cyclin-dependent kinase 5 promotes pancreatic β-cell survival via Fak-Akt signaling pathways. Diabetes 2011;60:1186–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei FY, Nagashima K, Ohshima T, et al. . Cdk5-dependent regulation of glucose-stimulated insulin secretion. Nat Med 2005;11:1104–1108 [DOI] [PubMed] [Google Scholar]
- 30.Wei FY, Suzuki T, Watanabe S, et al. . Deficit of tRNA(Lys) modification by Cdkal1 causes the development of type 2 diabetes in mice. J Clin Invest 2011;121:3598–3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi SS, Kim ES, Koh M, et al. . A novel non-agonist peroxisome proliferator-activated receptor γ (PPARγ) ligand UHC1 blocks PPARγ phosphorylation by cyclin-dependent kinase 5 (CDK5) and improves insulin sensitivity. J Biol Chem 2014;289:26618–26629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott LJ, Mohlke KL, Bonnycastle LL, et al. . A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007;316:1341–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinthorsdottir V, Thorleifsson G, Reynisdottir I, et al. . A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet 2007;39:770–775 [DOI] [PubMed] [Google Scholar]
- 34.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007;447:661–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeggini E, Weedon MN, Lindgren CM, et al.; Wellcome Trust Case Control Consortium (WTCCC) . Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007;316:1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxena R, Voight BF, Lyssenko V, et al.; Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research . Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007;316:1331–1336 [DOI] [PubMed] [Google Scholar]
- 37.Krentz NAJ, van Hoof D, Li Z, et al. Phosphorylation of NEUROG3 links endocrine differentiation to the cell cycle in pancreatic progenitors. Dev Cell 2017;41:129.e6–142.e6 [DOI] [PMC free article] [PubMed]
- 38.Azzarelli R, Hurley C, Sznurkowska MK, et al. Multi-site neurogenin3 phosphorylation controls pancreatic endocrine differentiation. Dev Cell 2017;41:274.e5–286.e5 [DOI] [PMC free article] [PubMed]
- 39.Flasse LC, Pirson JL, Stern DG, et al. . Ascl1b and Neurod1, instead of Neurog3, control pancreatic endocrine cell fate in zebrafish. BMC Biol 2013;11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delaspre F, Beer RL, Rovira M, et al. . Centroacinar cells are progenitors that contribute to endocrine pancreas regeneration. Diabetes 2015;64:3499–3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghaye AP, Bergemann D, Tarifeño-Saldivia E, et al. . Progenitor potential of nkx6.1-expressing cells throughout zebrafish life and during beta cell regeneration. BMC Biol 2015;13:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manfroid I, Ghaye A, Naye F, et al. . Zebrafish sox9b is crucial for hepatopancreatic duct development and pancreatic endocrine cell regeneration. Dev Biol 2012;366:268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalgin G, Prince VE. Differential levels of Neurod establish zebrafish endocrine pancreas cell fates. Dev Biol 2015;402:81–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ninov N, Borius M, Stainier DY. Different levels of Notch signaling regulate quiescence, renewal and differentiation in pancreatic endocrine progenitors. Development 2012;139:1557–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang W, Wang G, Delaspre F, Vitery Mdel C, Beer RL, Parsons MJ. Retinoic acid plays an evolutionarily conserved and biphasic role in pancreas development. Dev Biol 2014;394:83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ching YP, Qi Z, Wang JH. Cloning of three novel neuronal Cdk5 activator binding proteins. Gene 2000;242:285–294 [DOI] [PubMed] [Google Scholar]
- 47.Wei FY, Zhou B, Suzuki T, et al. . Cdk5rap1-mediated 2-methylthio modification of mitochondrial tRNAs governs protein translation and contributes to myopathy in mice and humans. Cell Metab 2015;21:428–442 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.