Abstract
A 10-month-old, domestic shorthaired, spayed female cat was presented to the Veterinary Medical Centre, Western College of Veterinary Medicine, for evaluation of an oral mucosal mass. Histopathology and polymerase chain reaction with sequencing allowed for a definitive diagnosis of bovine papillomavirus type-14-associated oral sarcoid. The mass resolved spontaneously following incisional biopsy and was still grossly absent 10 months later.
Résumé
Sarcoïde oral chez une chatte. Une chatte stérilisée âgée de 10 mois a été présentée au Veterinary Medical Centre du Western College of Veterinary Medicine, pour l’évaluation d’une masse orale. L’histopathologie et une amplification en chaîne par polymérase avec le séquençage ont permis de poser un diagnostic définitif de sarcoïde oral associé au papillomavirus bovin de type-14. La masse s’est résorbée spontanément après une biopsie incisionelle et n’était toujours pas visuellement détectable 10 mois plus tard.
(Traduit par Isabelle Vallières)
A sarcoid is a neoplasm that affects cats and horses. In both species, sarcoids have a high risk of recurrence following surgical excision, do not metastasize, and are typically comprised of both spindle and epithelial components. Both feline and equine sarcoids are caused by aberrant infection with a Bos taurus papillomavirus (BPVs). These viruses are double-stranded DNA, non-enveloped viruses (1). BPV types 1, 2, and 13 have been identified in equine sarcoids (2). BPV nucleic acids have only been shown in fibroblasts of feline sarcoids (3), whereas in equids, viral infection can be documented in both keratinocytes and fibroblasts, depending on the stage of tumor development (4). A putative BPV infection had long been suspected as the cause of feline sarcoids based on the sequencing of a small fragment of papillomavirus (PV) DNA, referred to as feline sarcoid-associated papillomavirus (FeSarPV). Recently, the entire papillomavirus genome that contained the FeSarPV sequence was sequenced and classified as BPV-14, a delta-PV, closely related to BPV types 1, 2, and 13 (5). According to a single study, which evaluated 120 oral and cutaneous samples [including swabs and formalin-fixed paraffin embedded (FFPE) biopsies taken from healthy and diseased-non-sarcoid cats], BPV-14 nucleic acids are only present in sarcoids and not in non-sarcoid felid tissues (6). BPV-14 has been identified in both normal (5) and diseased bovid tissues [fibropapillomas (5,7), bladder carcinomas (8)] but not equine sarcoids.
Little is known about the transmission or how this PV causes feline sarcoid development. Due to the small number of reported cases, particularly those affecting mucosal epithelium, treatment recommendations are lacking (9). This report describes a case of oral mucosal-associated sarcoid in a cat, confirms the identification of lesion-associated BPV-14 DNA, and presents current knowledge on this disease entity.
Case description
A 3.5 kg, 10-month-old, spayed female, domestic shorthaired cat was presented to the Veterinary Medical Centre (VMC), Western College of Veterinary Medicine (WCVM) oncology service for a second opinion and evaluation of an oral mass which had recurred following surgical excision performed 2 mo earlier at another institution. The initial, incompletely resected mass had been tentatively diagnosed as a sarcoid based on histologic evaluation alone and was described as a firm mass comprised of densely packed spindle cells near the overlying, non-hyperplastic mucosal epithelium and lacking the maturation, inflammatory cell infiltration, or angiogenesis expected of granulation tissue. In the 2 mo following attempted excision, the mass recurred and became larger than it had been pre-surgically, causing the cat discomfort and difficulty eating.
The animal had originally been acquired as a kitten from a dairy barn at 7 wk old and subsequently restricted to the indoors and kept current on deworming and vaccination protocols. The exact period between adoption (7 wk old) and the first recognition of the oral mass by the owner is unclear but would have been no later than at 7 mo of age.
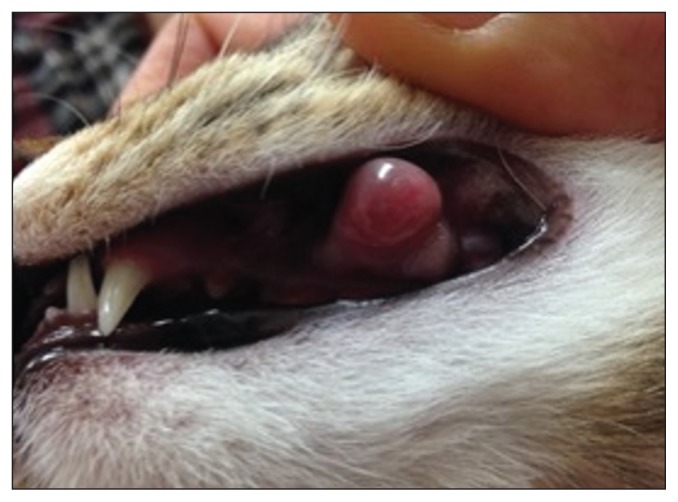
On physical examination, the animal was in good body condition and the only abnormality noted was a 1-cm diameter oral mass on the left maxillary buccal mucosa, near the gingival margin associated with teeth 207 and 208 (Figure 1). The mass was well-circumscribed, soft, and focally ulcerated.
Figure 1.
Gross appearance of feline oral mucosal sarcoid at the level of teeth 207 and 208 at time of presentation to Western College of Veterinary Medicine, Veterinary Medical Centre.
Routine pre-anesthetic blood analysis was completed before sedation. The cat had a normal packed cell volume [36%; reference range (RR): 28.5% to 47.7%] and total solids (6.5 g/L; RR: 5.6 to 8.4 g/L), was hyperglycemic (13.1 mmol/L; RR: 3.5 to 8.4 mmol/L) and had a normal blood urea nitrogen concentration (15 to 26 mg/dL on Azostix, RR: 5 to 40 mg/dL). Tests for feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV) were negative (SNAP FIV/FeLV Combo Test; IDEXX Laboratories, Westbrook, Maine, USA).
An incisional wedge biopsy was collected under sedation using dexmedetomidine (Dexdomitor 0.5 mg/mL; Orion Pharma, Zoetis Canada, Kirkland, Quebec) and butorphanol (Torbugesic 10 mg/mL; Orion Pharma) and reversed with atipamezole (Antisedan 5 mg/mL; Orion Pharma). The cat recovered uneventfully from the surgery and was sent home with oral buprenorphine (Vetergesic 0.3 mg/mL; Champion Alstoe Animal Health, Whitby, Ontario), 0.2 mL, PO, q8h for 3 d for pain relief.
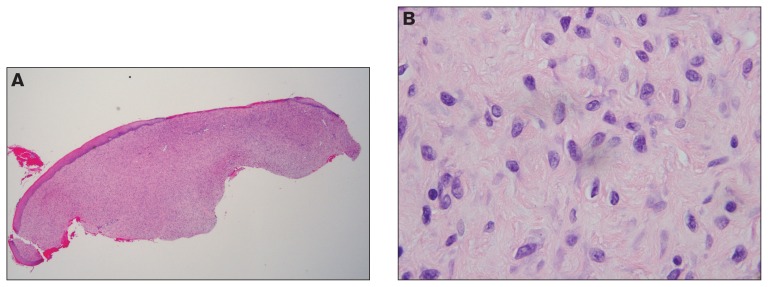
Tissues included stratified squamous epithelium with associated underlying connective tissue and were submitted to Prairie Diagnostic Services, Saskatoon, Saskatchewan for histopathologic evaluation. A highly cellular mass which extended to all margins was observed within the oral submucosa (Figure 2A). This mass was comprised of numerous plump, spindle cells arranged either haphazardly or in loose streams. Cells had indistinct borders, scant, slightly vacuolated basophilic cytoplasm, with 1.5- to 2-fold anisocytosis and anisokaryosis (Figure 2B). Nuclei were round to oblong with chromatin that was either stippled or marginated and nucleoli were variably distinct. Mitotic count was low (< 1 per 10 high power fields). Occasional apoptotic cells were present. Multifocally, variably sized congested vessels with hypertrophic endothelial cells were present, as were ectatic lymphatics. The overlying mucosal epithelium was variably pigmented and generally hyperplastic; rete pegs were not present. Focally, there was full-thickness loss of epithelium (ulceration) that was covered by a thin mat of fibrin and red blood cells. Granulation tissue, typified by mature fibroblasts, admixed with small, perpendicularly oriented blood vessels and occasional multinucleated macrophages, was present at the edges of the ulcerated mucosa. Numerous neutrophils, both degenerate and non-degenerate, were present throughout the mass but highest in areas of ulceration where they were occasionally admixed with colonies of both rod-shaped and coccal bacteria.
Figure 2.
Histology of incisional biopsies of a feline oral mucosal sarcoid. Hematoxylin and eosin. A — Hyperplastic mucosal epithelium overlies a highly cellular submucosal mass comprised of spindle cells arranged in short arcs and intersecting bundles (200×). There are focal areas of ulceration. B — Spindle cells have variably defined cell-borders, scant cytoplasm, are arranged into loose streams, have 2-fold anisocytosis and anisokaryosis, round to oblong nuclei with marginated chromatin and low mitotic counts 100× (< 1 in 10; 400× high power fields).
DNA was extracted from FFPE tissue for polymerase chain reaction (PCR) and sequencing. Briefly, four 25 μm sections were de-paraffinized with xylene and washed with ethanol before overnight incubation in lysis buffer with proteinase K. This was followed by separation with sequential phenol-chloroform treatments and precipitation with acetic acid. DNA pellets were washed, dried, and re-suspended in TrisEDTA buffer. DNA concentrations were measured using spectrophotometry (NanoDrop; Thermo Fisher Scientific, Wilminton, Delaware, USA). Conventional PCR was performed using degenerate consensus primer pairs, MY 09 and MY 11, designed to target the highly conserved major capsid protein (L1) gene of mucosa-adapted papillomaviruses (10). A known EcPV-2 positive equine cutaneous biopsy was used as a positive control. Internal controls included a negative template control, blank block, and extraction blank. Housekeeping gene beta-actin was amplified to confirm the presence of amplifiable DNA. Amplicons were visualized using ethidium bromide-stained 1.0% agarose gel, viewed under UV light. Amplicons were sequenced (Macrogen, Seoul, South Korea). Sequence analysis, using Staden Package programs Pregap4 and Gap4 (Staden Package, Medical Research Council Laboratory of Molecular Biology, Cambridge, England) confirmed a 98% to 99% sequence identity between the patient sample and 2 GenBank entries representing the same virus: bovine papillomavirus type 14 (GenBank accession numbers KP276343.1 and KR868288) and feline associated sarcoid virus (GenBank accession numbers FJ977616.1 and JQ071449.1).
Immunohistochemistry was performed using rabbit anti-BPV-1 antibodies (Prairie Diagnostic Services) with omission of the primary antibody serving as a negative control and a known-BPV-1 positive bovid sample as a positive control. No immunostaining was present in any of the sample’s tumor cells or overlying epithelium.
The owner reported marked regression of the mass as early as 3 wk after biopsy and this was confirmed by physical examination 5 mo after biopsy. Ten months following incisional biopsy, the owner reported no concerns and only a small amount of pigmentation was present at the tumor site.
Discussion
Oral neoplasms constitute 3% of all cancers in the cat; the most common being squamous cell carcinomas (70% to 80%) and fibrosarcomas (13% to 17%), which affect middle-aged cats (10 to 12 y) predominantly (11). In younger cats, oral masses are less frequent; differential diagnoses include feline inductive odontogenic tumor, giant cell epulis, eosinophilic granuloma, and exuberant granulation tissue (12). Feline sarcoids are most common in young cats, with preferential sites including the skin of the head, neck, digits, and ventral abdomen (13). The anatomic distribution has been suggested to reflect trauma from inter-cat aggression, whereby bites and scratches enable the virus to access the underlying dermis (13). There is 1 reported case of cutaneous sarcoids in captive lions and in this case, the source of BPV was postulated to be the feeding of infected bovid tissues (14).
There is little published information on oral sarcoids in cats. One of the two references identified by the authors is a conference proceeding abstract regarding a 9-month-old, male, European shorthaired cat with a gingival mass covering the maxillary incisors that was diagnosed as a sarcoid by histopathology and BPV-specific PCR and in situ hybridization. There was recurrence 4 mo following surgical excision (15). The second reference is a case series describing multifocal oral fibropapillomas in a 15-month-old Persian cat from Norway and an 18-month-old domestic shorthaired cat from the Channel Islands (16), for which only histopathology was performed but the authors indicated they suspected a viral etiology. One of the two cats had recurrence of lesions 3 mo following surgical excision. In both cats, histologic descriptions note that fibroblasts were immature and proliferative and that a lesser degree of mixed inflammatory cell infiltration and ulceration was present. In both studies, the overlying mucosal epithelium was described as hyperplastic with rete formation.
The scarcity of published case reports of mucosal sarcoids in cats may reflect the true rarity of the disease. Alternatively, the entity may be under-reported given that the afflicted cats are typically young cats exposed to cattle (e.g., barn cats, that may receive less regular veterinary care). Finally, the potential for sarcoids to be a self-limiting entity in a subset of cases, whereby spontaneous regression, as seen in the current case, could further dilute the frequency of diagnosis of this disease in cats.
The best-studied PVs are the HPVs of the alpha genus which include high risk HPVs such as HPV-16 and -18, that cause cervical cancer in women and whose major oncogenes are early (E) E6 and E7, which induce and propagate unregulated cell proliferation through various cellular pathways including inhibition of host tumor suppressor p53 and retinoblastoma proteins, respectively (17). In veterinary medicine, most oncogenic PVs fall outside the alpha genus. For example, BPVs of the delta genus are associated with benign [such as fibropapillomas (18–20)] and malignant diseases [such as bladder urothelial carcinomas (8,18)], in their natural host. Delta BPVs can cause abortive (non-productive) infections of mesenchymal cells, leading to sarcoids, in non-host species such as equids (BPV-1, 2, 13) (2,19,21) and felids (BPV-14) (6,22).
In contrast to the high risk-HPVs of the alpha genus, the major oncoprotein of delta PVs is E5 (18), which binds and activates platelet derived growth factor beta receptor (PDGFB-R), leading to mesenchymal cell proliferation (18,23). Binding of E5 protein to PDGFB-R causes dimerization, leading to activation of several kinases including those of the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (Akt) signalling pathway involved in cell cycle regulation. PDGFR-B activation also contributes to the recruitment of pericytes required for angiogenesis (24). E5 proteins also promote immune evasion by sequestering MHC-II heavy chains and inhibiting MHC-1 and cyclooxygenase expression (18,24). Finally, E5 proteins inhibit host cells from forming gap junctions required for cell-to-cell communications and focal adhesions required for differentiation (18,24).
Although the oncogenic mechanism of BPV-14 has not been definitively determined in feline sarcoids, whole genome sequencing shows the presence of putative oncogenes E5, E6, and E7. However, sequence analysis shows that the BPV-14 E6 lacks a PDZ binding motif and E7 lacks a retinoblastoma binding site; in contrast, the BPV-14 E5 gene does contain the residues necessary to bind PDGFB-R (5).
Of interest in the current case are the anatomical location of the sarcoid and the lack of classic epithelial changes typically described in sarcoids, such as the presence of rete pegs (25). The current case highlights the importance of not discounting a histologic diagnosis of a mucosal sarcoid in a cat with a spindle mass that lacks the “classical” overlying epithelial features. Differential diagnoses in the current case included fibrosarcoma or exuberant granulation tissue. Sarcoids are frequently ulcerated and granulation tissue or secondary bacterial infection may be superimposed, making diagnosis challenging. These diagnoses were ruled out by the histologic appearance of the mass combined with the identification of BPV-14 nucleic acid. Given that BPV-14 DNA has only been identified in sarcoids in cats and not non-sarcoid tissues (6), PCR that targets papillomaviruses, as performed here, could serve as a useful diagnostic tool to differentiate between sarcoids and other processes.
The normal, productive papillomaviral life cycle is tightly linked to epithelial cell proliferation and maturation. L1 major capsid proteins are not synthesised until the final stages of viral replication in superficial epithelial cells in preparation for release of fully assembled viral particles via desquamation (26). When PVs such as BPVs infect non-host species such as cats or horses, the normal productive lifecycle is thought not to occur, although recently, the final steps of L1 capsid protein synthesis in equine sarcoids has been demonstrated but only in early lesions (27).
Immunohistochemistry was not found to be diagnostically helpful herein. This may reflect a lack of cross-reactivity between the anti-BPV-1 L1 capsid protein antibodies used and BPV-14 L1 proteins, or that the presence of BPV-14 DNA in feline sarcoids does not equate to productive, virion-producing infection. The latter is assumed given a prior study of cutaneous feline sarcoids that showed PV DNA can be identified by in situ hybridization within neoplastic mesenchymal cells but not the overlying epithelium and that IHC against BPV L1 proteins was negative (3).
Clinical regression of the oral mass followed the second incisional biopsy in our study, whereas rapid recurrence followed the first attempt at excisional biopsy. To the authors’ knowledge, there is no information pertaining to the frequency of spontaneous regression of sarcoids in cats. In contrast, spontaneous regression, in either the absence of any treatment, or incomplete excision, occurs in equine sarcoids (28).
To the authors’ knowledge, there are no published case reports of oral sarcoids in horses and only 1 in cats (16). The occasional finding of such a lesion in cats may be more a reflection of the behavior of this species such as the consumption of raw cow’s milk, fastidious self-grooming, and inter-cat aggression causing puncture lesions rather than a true viral tissue tropism.
BPV-14-associated feline sarcoid, both cutaneous and oral, should be considered as a differential diagnosis for a spindle cell mass, with or without the presence of an overlying hyperplastic epithelium with rete pegs, particularly in a young cat with known exposure to cattle. Although exaggerated recurrence following attempts at excision is common in both feline and equine sarcoids, the current case serves as a reminder that spontaneous regression may occur (28), although close monitoring for an extended time is advised as recurrence may be delayed.
Acknowledgments
The authors thank Betty Lockerbie and Bruce Wobeser for their assistance with PCR, sequencing and editorial revisions, Kristen Peters for provision of additional photographs and historical information and Dale Godson for his assistance with immunohistochemistry. Funding for this publication was provided by the Western College of Veterinary Medicine Graduate Student Research Fund. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.zur Hausen H. Papillomavirus infections — A major cause of human cancers. Biochim Biophys Acta Rev Cancer. 1996;1288:F55–78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]
- 2.Lunardi M, De Alcântara BK, Otonel RAA, Rodrigues WB, Alfieri AF, Alfieri AA. Bovine papillomavirus type 13 DNA in equine sarcoids. J Clin Microbiol. 2013;51:2167–2171. doi: 10.1128/JCM.00371-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teifke JP, Kidney BA, Löhr CV, Yager JA. Detection of papillomavirus-DNA in mesenchymal tumour cells and not in the hyperplastic epithelium of feline sarcoids. Vet Dermatol. 2003;14:47–56. doi: 10.1046/j.1365-3164.2003.00324.x. [DOI] [PubMed] [Google Scholar]
- 4.Bogaert L, Martens A, Kast WM, Van Marck E, De Cock H. Bovine papillomavirus DNA can be detected in keratinocytes of equine sarcoid tumors. Vet Microbiol. 2010;146:269–275. doi: 10.1016/j.vetmic.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 5.Munday JS, Thomson N, Dunowska M, Knight CG, Laurie RE, Hills S. Genomic characterisation of the feline sarcoid-associated papillomavirus and proposed classification as Bos taurus papillomavirus type 14. Vet Microbiol. 2015;177:289–295. doi: 10.1016/j.vetmic.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Munday JS, Knight CG, Howe L. The same papillomavirus is present in feline sarcoids from North America and New Zealand but not in any non-sarcoid feline samples. J Vet Diagn Investig. 2010;22:97–100. doi: 10.1177/104063871002200119. [DOI] [PubMed] [Google Scholar]
- 7.Da Silva MAR, Carvalho CCR, Coutinho LCA, et al. Co-infection of bovine papillomavirus and feline-associated papillomavirus in bovine cutaneous warts. Transbound Emerg Dis. 2012;59:539–543. doi: 10.1111/j.1865-1682.2012.01307.x. [DOI] [PubMed] [Google Scholar]
- 8.Roperto S, Munday JS, Corrado F, Goria M, Roperto F. Detection of bovine papillomavirus type 14 DNA sequences in urinary bladder tumors in cattle. Vet Microbiol. 2016;190:1–4. doi: 10.1016/j.vetmic.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Head KW, Else RW, Dubielzig R, Meuten D. In: Tumors in Domestic Animals. 5th ed. Meuten, editor. Ames, Iowa: Wiley-Blackwell; 2016. pp. 148–149.pp. 511 [Google Scholar]
- 10.Chan S-Y, Delius H, Halpern AL, Bernard H-U. Analysis of genomic sequences of 95 papillomavirus types: Uniting typing, phylogeny, and taxonomy. J Virol. 1995;69:3074–3083. doi: 10.1128/jvi.69.5.3074-3083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Withrow S, Vail D, Rodney P. In: Withrow and MacEwen’s Small Animal Clinical Oncology. 5th ed. Vail D, editor. Philadelphia, Pennsylvania: Elsevier Saunders; 2013. pp. 335–355. [Google Scholar]
- 12.Head KW, Cullen JM, Dubielzig RR, et al. In: Histological Classification of Tumors of the Alimentary System of Domestic Animals. Schulman FY, editor. Washington, DC: Armed Forces Institute of Pathology; 2003. pp. 27–57. (Second Ser.). [Google Scholar]
- 13.Schulman FY, Krafft AE, Janczewski T. Feline cutaneous fibropapillomas: Clinicopathologic findings and association with papillomavirus infection. Vet Pathol. 2001;38:291–296. doi: 10.1354/vp.38-3-291. [DOI] [PubMed] [Google Scholar]
- 14.Orbell GMB, Young S, Munday JS. Cutaneous sarcoids in captive African lions associated with feline sarcoid-associated papillomavirus infection. Vet Pathol. 2011;48:1176–1179. doi: 10.1177/0300985810391111. [DOI] [PubMed] [Google Scholar]
- 15.Brandes K, Lendl C, Teifke JP. A case of a gingival feline sarcoid in a young cat. J Comp Pathol. 2014;150:104. [Google Scholar]
- 16.Rest JR, Gumbrell RC, Heim P, Rushton-Taylor P. Oral fibropapillomas in young cats. Vet Rec. 1997;141:528. [PubMed] [Google Scholar]
- 17.Yim E-K, Park J-S. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat. 2005;37:319–324. doi: 10.4143/crt.2005.37.6.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munday JS. Bovine and human papillomaviruses: A comparative review. Vet Pathol. 2014;51:1063–1075. doi: 10.1177/0300985814537837. [DOI] [PubMed] [Google Scholar]
- 19.Munday JS, Kiupel M. Papillomavirus-associated cutaneous neoplasia in mammals. Vet Pathol. 2010;47:254–264. doi: 10.1177/0300985809358604. [DOI] [PubMed] [Google Scholar]
- 20.Campo MS. Bovine papillomavirus and cancer. Vet J. 1997;154:175–188. doi: 10.1016/s1090-0233(97)80019-6. [DOI] [PubMed] [Google Scholar]
- 21.Finlay M, Yuan Z, Morgan IM, Campo MS, Nasir L. Equine sarcoids: Bovine papillomavirus type 1 transformed fibroblasts are sensitive to cisplatin and UVB induced apoptosis and show aberrant expression of p53. Vet Res. 2012;43:1. doi: 10.1186/1297-9716-43-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munday JS, Dunowska M, Hills SF, Laurie RE. Genomic characterization of Felis catus papillomavirus-3: A novel papillomavirus detected in a feline Bowenoid in situ carcinoma. Vet Microbiol. 2013;165:319–325. doi: 10.1016/j.vetmic.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Borzacchiello G, Mogavero S, De Vita G, Roperto S, Della Salda L, Roperto F. Activated platelet-derived growth factor beta receptor expression, PI3K-AKT pathway molecular analysis, and transforming signals in equine sarcoids. Vet Pathol. 2009;46:589–597. doi: 10.1354/vp.08-VP-0191-B-FL. [DOI] [PubMed] [Google Scholar]
- 24.Araldi RP, Assaf SMR, de Carvalho RF, et al. Papillomaviruses: A systematic review. Gen Mol Biol. 2017;40:1–21. doi: 10.1590/1678-4685-GMB-2016-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauldin EA, Peters-Kennedy J. Integumentary system. In: Maxie G, editor. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals. 6th ed. Vol. 1. St Louis, Missouri: Elsevier; 2016. p. 709.p. 712. [Google Scholar]
- 26.Hamid NA, Brown C, Gaston K. The regulation of cell proliferation by the papillomavirus early proteins. Cell Mol Life Sci. 2009;66:1700–1717. doi: 10.1007/s00018-009-8631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaynor AM, Zhu KW, Cruz FND, Affolter VK, Pesavento PA. Localization of bovine papillomavirus nucleic acid in equine sarcoids. Vet Pathol. 2015;53:567–573. doi: 10.1177/0300985815594852. [DOI] [PubMed] [Google Scholar]
- 28.Knottenbelt D. Pascoe’s Principles & Practice of Equine Dermatology. 2nd ed. St. Louis, Missouri: Elsevier; 2009. pp. 387–407. [Google Scholar]