Abstract
This retrospective study investigated predisposing factors and ultrasound features in dogs (n = 36) and cats (n = 2) with emphysematous cystitis (EC). Urinary tract infection was present in 25 patients (65.8%), impaired immune system in 10 (26.3%), bladder stones in 9 (23.7%) and neurologic bladder in 7 (18.4%). Diabetes mellitus was present in only 4 patients (10.5%). Most patients had positive urine culture (n = 35; 92.1%), with elevated concentration of Escherichia coli in 25 patients (71.4%). The most common ultrasound features were diffuse thickening of the urinary bladder (n = 15; 39.5%), polyps (n = 9; 23.7%), and focal thickening (n = 4; 10.5%). In 13 patients (34.2%) the bladder wall was not assessable, due to excessive bladder gas. Bladder gas was mostly identified in the lumen (n = 18; 47.4%), followed by the bladder wall (n = 11; 28.9%), and wall and lumen (n = 9; 23.7%).
Résumé
Cystite emphysémateuse : évaluation rétrospective des facteurs prédisposants et des caractéristiques des échographies chez 36 chiens et 2 chats. Cette étude rétrospective a examiné les facteurs prédisposants et les caractéristiques chez des chiens (n = 36) et des chats (n = 2) atteints d’une cystite emphysémateuse (CE). Une infection des voies urinaires était présente chez 25 patients (65,8 %), un système immunitaire affaibli chez 10 patients (26,3 %), des calculs vésicaux chez 9 patients (23,7 %) et une vessie neurologique chez 7 patients (18,4 %). Le diabète sucré était présent chez seulement 4 patients (10,5 %). La plupart des patients avaient une culture d’urine positive (n = 35; 92,1 %), avec une concentration élevée d’Escherichia coli chez 25 patients (71,4 %). Les caractéristiques les plus courantes à l’échographie étaient un épaississement diffus de la vessie urinaire (n = 15; 39,5 %), des polypes (n = 9; 23,7 %) et l’épaississement concentrique (n = 4; 10,5 %). Chez 13 patients (34,2 %) la vessie n’a pas pu être évaluée en raison de gaz excessifs dans la vessie. Les gaz de la vessie ont surtout été identifiés dans la lumière (n = 18; 47,4 %), suivie de la paroi de la vessie (n = 11; 28,9 %) et de la paroi et la lumière de la vessie (n = 9; 23,7 %).
(Traduit par Isabelle Vallières)
Introduction
Emphysematous cystitis (EC) is a rare disease in both human and veterinary patients, which is characterized by the presence of gas within the urinary bladder wall, bladder lumen, or both (1,2). Diagnostic imaging, such as ultrasonographic and radiographic examination of the urinary tract, is pivotal for a definitive diagnosis of EC. Small amounts of gas may be difficult to identify radiographically, especially when intestinal loops are superimposed on the urinary bladder. Ultrasonography is considered more sensitive than radiology to detect early stages of EC (3).
Although the pathogenesis is not well-understood, the combination of high tissue glucose levels and impaired immune system has been suggested to facilitate infections with glucose-fermenting bacteria or yeast. Fermentation of glucose and albumin leads to formation of H2 and CO2 gas within the lumen and mucosa of the urinary tract (1).
Historically, EC has been reported mainly in diabetic dogs, as a consequence of a combination of infection with glucose-fermenting bacteria and impaired immune system (1). In a recent review of 27 dogs with EC, diabetes mellitus was present in 33% of dogs (4). In cats, EC was diagnosed in association with diabetes in 1 cat (2) and with urinary tract infection in another cat (3). In human medicine, non-diabetic cases of EC have been reported mostly in elderly and debilitated patients, in association with urinary tract obstructions, structural abnormalities of the bladder, prolonged use of indwelling urinary catheters, and long-term therapy with glucocorticoids (5). In non-glycosuric veterinary patients, EC has been reported in association with chronic urinary tract infections, bladder trigone diverticulum, and long-term administration of steroids (3,6). The aim of the present retrospective study was to investigate predisposing factors and ultrasound features in dogs (n = 36) and cats (n = 2) with naturally acquired EC.
Materials and methods
Records of client-owned dogs and cats of various breeds, gender, ages, and weights, referred to the Mario Modenato Veterinary Teaching Hospital, between December 2010 and December 2016, with an ultrasonographic diagnosis of EC were reviewed. For each dog, data regarding history, results of biochemical analyses and urinalysis, urine culture for aerobic and anaerobic agents, abdominal ultrasound, and abdominal radiography in right lateral projection were collected from the medical record. Only patients with complete history, biochemical profile, urinalysis, urine culture, and abdominal ultrasound consistent with EC were eligible for the study. The ultrasonographic evidence of gas within the urinary bladder wall, bladder lumen, or both was considered suggestive of EC. Medical records of patients which received urinary catheterization 24 to 48 h before imaging and had free gas in the bladder lumen as the only urologic alteration were excluded from the study. In these cases, free gas in the bladder lumen was likely iatrogenic. Medical records were reviewed for co-morbidities (diabetes mellitus, chronic urinary tract infections, immunosuppression, neurological bladder, bladder stones), symptoms and physical examination findings [abdominal pain, nausea, vomiting, fever, hematuria, pneumaturia, pollakiuria, stranguria, urinary incontinence, polyuria/polydipsia (PU/PD)], laboratory findings (bacteriuria, positive urine culture, leukocyturia, hematuria, leucocytosis, serum creatinine > 123.8 μmol/L, hyperglycemia, glycosuria), and imaging findings. Data regarding ultrasound appearance and stratigraphy of the urinary bladder, appearance of urinary content, and localization of gas were recorded for each patient. Ultrasonography was performed using a Toshiba Aplio 400 (Canon Medical Systems Europe B.V., Zoetermeer, The Netherlands), with a 7.5 MHz microconvex probe and a 12 MHz linear probe, with patients in lateral recumbency or in a standing position.
The D’Agostino and Pearson normality test was used to test data for normality using Graphpad Prism 4 (Graph Pad, San Diego, California, USA).
Results
Of 40 dogs and 2 cats diagnosed with EC between December 2010 and December 2016, 36 dogs and 2 cats met the inclusion criteria. Four dogs were excluded from review for incomplete medical record. Dogs were represented by the following breeds: mixed breed (n = 12), Epagneul Breton (n = 3), 2 each of boxer, golden retriever, English setter, and 1 each of schnauzer, cocker spaniel, doberman, rough fox terrier, Pomeranian, American Staffordshire, border collie, shepherd Maremma, chow chow, greyhound, pitbull, Rottweiler, Maltese, Labrador retriever, and German hound. Dogs had a mean age of 9.5 ± 3.3 y, a mean body weight of 20.5 ± 2.4 kg. Twenty dogs were female and 16 were male. Both cats were castrated male domestic shorthairs. The cats were 10 y old and 3 y old and had body weights of 4.5 kg and 6 kg, respectively. Clinical, laboratory, and ultrasonographic findings at time of diagnosis are reported in Table 1.
Table 1.
Clinical, laboratory, and ultrasonographic findings at time of diagnosis.
| Parameters | Number affected among 38 patients | Percentage (%) |
|---|---|---|
| Co-morbidities | ||
| Chronic UTI | 25 | 65.7 |
| Impaired immune system | 10 | 26.3 |
| Bladder stones | 9 | 23.6 |
| Neurological bladder | 7 | 18.4 |
| Diabetes mellitus | 4 | 10.5 |
| Clinical findings | ||
| Hematuria | 17 | 44.7 |
| Pollakiuria | 5 | 13.1 |
| Urinary incontinence | 4 | 10.5 |
| PU/PD | 4 | 10.5 |
| Stranguria | 3 | 7.8 |
| Vomiting | 3 | 7.8 |
| Pneumaturia | 1 | 2.6 |
| Abdominal pain | 1 | 2.6 |
| Nausea | 1 | 2.6 |
| Fever | 1 | 2.6 |
| Laboratory findings | ||
| Positive urine culture | 35 | 92.1 |
| Bacteriuria | 26 | 68.4 |
| Leukocyturia | 18 | 47.3 |
| Serum creatinine > 1.4 mg/dL | 10 | 26.3 |
| Leukocytosis | 9 | 23.6 |
| Hyperglycemia | 5 | 13.1 |
| Glycosuria | 5 | 13.1 |
| Imaging findings | ||
| Bladder content | ||
| Hyperechoic | 29 | 76.3 |
| Anechoic | 9 | 23.6 |
| Bladder wall | ||
| Diffuse thickening | 15 | 39.4 |
| NA | 13 | 34.2 |
| Polyps | 9 | 23.6 |
| Focal thickening | 4 | 10.5 |
| Air location | ||
| Bladder lumen | 18 | 47.3 |
| Bladder wall | 11 | 28.9 |
| Bladder wall and lumen | 9 | 23.6 |
NA — bladder wall not assessable due to large amount of free gas in the lumen.
UTI — Urinary tract infection.
PU/PD — Polyuria/Polydipsia.
In this cohort of patients EC was always associated with co-morbidities. In 19 patients (50%), EC was associated with only 1 co-morbidity, while in the others there were 2 or more co-morbidities. The most prevalent co-morbidity was chronic urinary tract infection (n = 25; 65.8%), followed by impaired immune system (n = 10; 26.3%), bladder stones (n = 9; 23.7%), and neurologic bladder (n = 7; 18.4%). Diabetes mellitus was present in only 4 patients (10.5%). Of the 10 patients with impaired immune system, 3 dogs had Cushing’s disease, 2 dogs were on chemotherapy, 1 dog was on long-term glucocorticoids, and 4 dogs had chronic kidney disease (CKD). Both cats had a history of repeated urinary catheterization due to recurrent episodes of urethral obstruction over the previous 4 to 5 mo. All 7 dogs with neurologic bladder had a history of at least 1 urinary catheterization over the previous 4 to 5 mo.
The most prevalent clinical sign was hematuria (n = 17; 44.7%), followed by pollakiuria (n = 5; 13.1%), urinary incontinence (n = 4; 10.5%), PU/PD (n = 4; 10.5%), stranguria (n = 3; 7.9%), vomiting (n = 3; 7.9%). Pneumaturia, fever, nausea, and abdominal pain were found in 1 patient (2.6%). Urine culture isolates of the 35 patients with positive urine culture are reported in Table 2. Four patients with Escherichia coli infection and 1 patient with Klebsiella pneumoniae had multi-drug resistant infections. Isolates from all other patients showed susceptibility to the main classes of antibiotics. Three patients had negative urine culture and had been on antibiotics at the time ultrasound examination was performed.
Table 2.
Urine isolates from 35 patients with positive urine culture.
| Bacteria | Number of patients | Percentage (%) |
|---|---|---|
| Escherichia coli | 25 | 71.4 |
| Proteus mirabilis | 3 | 8.6 |
| Enterococcus + K. pneumoniae | 3 | 8.6 |
| Staph + K. pneumoniae | 3 | 8.6 |
| K. pneumoniae | 1 | 2.8 |
Enterococcus — Enterococcus faecalis; K. pneumoniae — Klebsiella pneumoniae; Staph — Staphylococcus aureus .
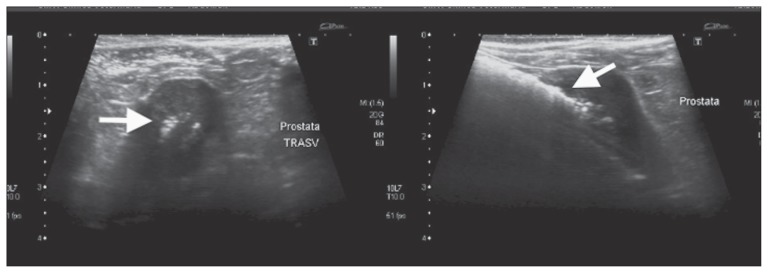
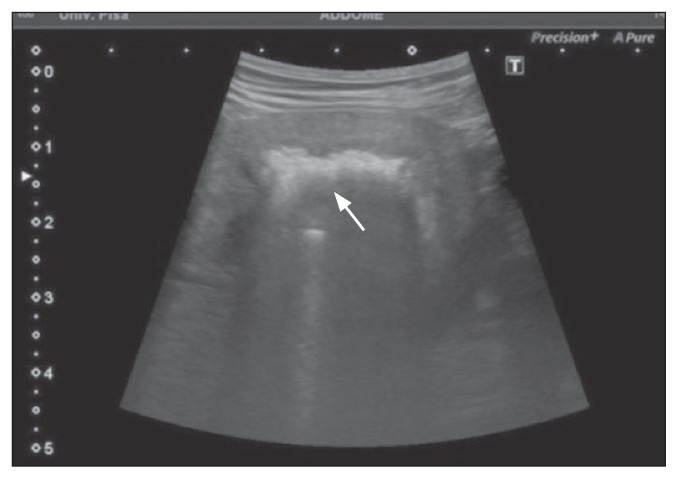
Ultrasound of the urinary system showed a series of subtle hyperechomic stripes, very close to each other, almost fused, in the thickness of the wall, which were attenuated by the increase in depth; this was attributed to a reverberation artifact, typical of gas. If the gas was in the lumen, the intraluminal hyperechoic specks were mobile and shifted to a nondependent location with postural changes. Dogs with air in the bladder lumen (n = 18) showed at least 1 abnormality of the bladder content and bladder wall associated with free intraluminal gas. Hyperechoic bladder content was present in 18/18 dogs, diffuse bladder wall thickening in 11/18, focal bladder wall thickening in 1/18, and bladder polyps in 6/18. Among 5 dogs with ultrasonographic signs of prostatitis, 1 dog showed gas within the prostate gland (Figure 1). One dog showed ultrasonographic signs of bilateral emphysematous pyelonephritis (Figure 2).
Figure 1.
Ultrasonographic image of transverse (left) and longitudinal (right) sections of the prostate gland, showing hyperechoic foci (arrows), consistent with gas, in a dog with EC and prostatitis.
Figure 2.
Ultrasonographic image of a sagittal section of the left kidney, showing reverberation artifacts (arrow) within the renal parenchyma, consistent with gas in a dog with bilateral emphysematous pyelonephritis.
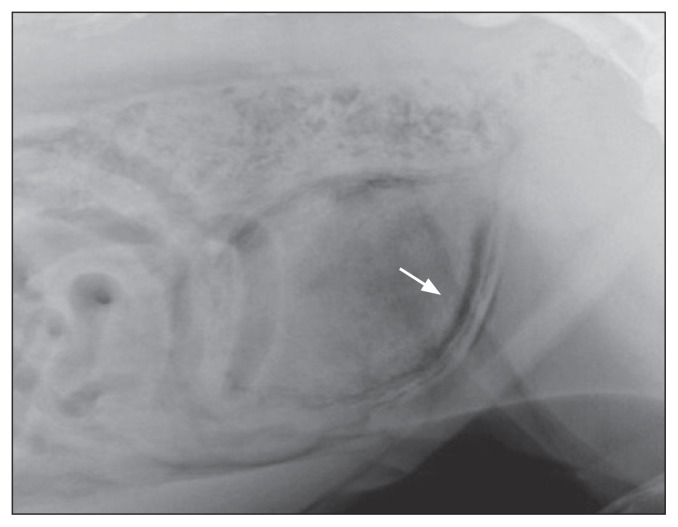
Three dogs with a large amount of gas in the bladder wall and classified as not assessable (NA) showed bladder polyps at the following ultrasound (3 to 4 d later). Abdominal radiographs were available for 5/38 patients. Abdominal radiographs showed roundish small areas of increased radiolucency, with an aspect defined as “cobblestoned,” demonstrating the gas localization at the wall of the bladder (Figure 3). Four patients showed radiologic signs consistent with EC, while 1 patient showed no radiologic abnormalities of the urinary system.
Figure 3.
Survey abdominal radiograph, showing mottled gas opacities (arrow) within the bladder wall and lumen of the bladder, in a dog with EC.
Discussion
In our study, EC did not seem to have any gender predisposition as similar prevalences were found for males and females. By contrast, in diabetic humans EC occurs 2.2 times more frequently in females than males (7). In humans, EC is more often observed in elderly diabetic women between 60 and 70 y old (2). The higher prevalence of EC in women is suggested to result from the higher incidence of urinary tract infections (UTI) (7). The mean age of affected dogs was 9.5 y. This may reflect a higher prevalence of the disease in middle- to old-aged dogs, similar to humans. Emphysematous cystitis is a form of complicated UTI (8) and it is plausible that older dogs are more likely to have co-morbidities, such as hyperadrenocorticism and chronic kidney disease (9,10), which may predispose them to UTI.
Diabetes mellitus is the strongest risk factor for EC in humans; approximately 70% of human patients with EC have diabetes, which is often poorly controlled. Elevated concentrations of urinary glucose represent an ideal substrate for bacterial fermentation and gas production (8). In humans, the prevalence of diabetes mellitus among patients affected by EC is significantly higher than that found in the general population (62.2% versus 8.6%) (11). However, diabetes mellitus was present in only 10.5% of patients in our population of dogs. This percentage was markedly lower than the 33% previously reported in dogs by Merkel et al (8). In our population, EC was always associated with at least 1 co-morbidity. Similarly, co-morbid diseases were found in all but 1 case reported by Merkel et al (8). In our study, chronic UTI and impaired immune system were the most prevalent co-morbidities, with prevalences of 65.7% and 31.5%, respectively. This is not surprising considering that EC may result as a complication of a chronic UTI. It is therefore plausible that pathological conditions causing impairment of the immune system may predispose patients to develop EC. Impairment of host defence mechanism is probably the most significant factor predisposing the patient to recurrent complicated UTI (12). It is likely that patients in our study, with a history of chronic UTI, developed EC because of a disorder in their immune system. However, the lack of a well-documented history before presentation to our hospital makes it impossible to verify our hypothesis. In our study EC seems to occur in combination with conditions of immunological host defence impairment. Among the causes of impaired immune system, chronic kidney disease (CKD) played an important role, as it affected over half of the patients (6/10). Uremic toxin retention, malnutrition, and chronic inflammation have been proven to cause impairment of the immune system in humans (13). Therefore, CKD might predispose our patients to develop EC. Also, impairment of the immune system is a possible predisposing factor for EC in the 3 patients with hyperadrenocorticism and in the dog on long-term therapy with steroids. A similar mechanism has also been hypothesized for the 2 dogs of our cohort, which were on chemotherapy. Emphysematous cystitis has, in fact, been found in association with prolonged chemotherapy in a non-diabetic dog (5).
Most patients (92.1%) had positive urine culture at the time of examination. Escherichia coli was responsible for > 70% of UTIs. Similarly, in human EC E. coli was involved in approximately 60% of UTIs, with E. coli and K. pneumoniae being the major organisms isolated in urine cultures. Both bacteria ferment glucose and lactate to produce gases, such as carbon dioxide, nitrogen, hydrogen, and oxygen (8). In our patients, bacterial species other than E. coli were markedly less frequent, with 8.6% Proteus mirabilis, Enterococcus faecalis, and Staphyloccus aureus, and 2.8% K. pneumoniae. Human data reported a prevalence of Proteus infections in EC of 5% to 8%, while infections with Enterococcus and Staphylococcus tended to be rare (9). Infections with non-gas-producing agents, such as Enterococcus, may be the result of mixed infections (14). This observation seems to reflect our findings, in which the 3 patients with E. faecalis infection were also positive for K. pneumoniae. We hypothesized that the finding of negative urine culture in 3 patients was due to antibiotic treatment, which had been started before presentation to our center. Unfortunately, no urine culture was run at time of diagnosis by the referring veterinarian.
As there was a relatively low number of diabetic and/or glycosuric patients in our cohort, it is likely that fermentation pathways other than glucose were involved. In non-diabetic patients, urinary lactose or tissue proteins have been hypothesized as substrate for gas formation (11). The low prevalence (13.1%) of glycosuria in our patients might reflect the low prevalence of diabetic subjects. However, it is also possible that the low prevalence of glycosuria reflects active glucose consumption by bacteria in the urine. As urinary bacteria can ferment glucose, glycosuria may be an inaccurate way to monitor glycemia in diabetic patients (13).
In our study, EC seemed to be associated mostly with nonspecific, mild, signs of lower urinary tract disease. This is similar to humans (11), in whom hematuria was a consistent finding: present in 44.7% of patients. Pollakiuria was present in 13.1% of patients, while stranguria was less common (7.8%). Urinary incontinence was found in 10.5% of patients and was associated with neurologic bladder. Pneumaturia is a highly specific sign, which was present in only 1 patient of our cohort. This finding is in agreement with human literature, in which pneumaturia is generally a rare patient complaint when a urinary catheter is not present. However, when bladder catheterization was used, the prevalence of pneumaturia increased to 70% (8). More severe signs of discomfort, such as abdominal pain, fever, and nausea were rarely seen in our patients. This finding differs significantly from that in human medicine, in which abdominal pain has been reported in more than 80% of patients (14).
In agreement with a previous report (3), the bladder content was hyperechoic in most patients (76.3%). This is not surprising, as many patients showed signs of inflammation of the urinary tract, such as bacteriuria and leukocyturia. The bladder wall was often diffusely thickened (39.4%) and showed focal thickening and bladder polyps (10.5% and 15.7%, respectively) less frequently. The bladder wall could not be assessed by ultrasound in 34.12% of patients, due to excessive free gas in the lumen, which made it difficult to evaluate the bladder wall and to diagnose bladder thickening or polyps. Therefore, the use of serial ultrasound evaluations should be encouraged to rule out bladder wall abnormalities. Interestingly, the prevalence of gas location in our cohort differed from that reported by Merkel et al (8). Many patients (47.3%) showed free gas in the bladder lumen in our study, compared with 14.8% in the study by Merkel et al (8). As urinary catheterization was not done immediately before ultrasound, we hypothesized a massive production of gas or an advanced stage of the disease. In fact, gas tends to collect in small bubbles within the bladder wall and lumen. These vesicles coalesce and rupture as cystitis extends (3). Three radiographic stages of severity have been described for EC. Free gas in the bladder lumen is typical of a more advanced stage of EC in humans (15).
The 2 cats in our study were nondiabetic patients with a history (at least 4 to 5 mo) of recurrent episodes of urethral obstruction and frequent catheterization. Both cats had a recent history of urethral catheterization for removal of urethral plugs 1 to 2 wk before presentation. Urinary stasis due to urine outlet obstruction or recurrent urethral catheterization might predispose these cats to EC. In 1 report, development of EC resulted from unblocking an obstructed urethra with a urinary catheter (16). Both urine cultures were positive for E. coli.
Ultrasonography of the urinary tract is a more sensitive way of diagnosing EC, compared to radiology, due to the finding of pathognomonic artifacts (13). Limited volumes of gas, especially if intramural, may be difficult to be diagnosed or to be differentiated from intestinal gas by radiology. In our study, ultrasonography was confirmed to be a sensitive technique for diagnosing EC. Ultrasonography was also able to identify predisposing factors of UTI, such as polyps and stones, and to diagnose emphysematous involvement of other organs, such as kidneys and prostate.
The present study has some limitations. Due to the retrospective nature of the study, cases with missing or incomplete data were excluded. Although all patients had abdominal ultrasonography consistent with EC, radiography of the urinary tract was available in only 5 patients. Consequently, no comparison of sensitivity and specificity between ultrasonography and radiology was possible. As fungal cultures were not performed, we could not rule out mixed infections with yeasts.
In our cohort of patients, EC seemed to occur more often in association with chronic UTIs and conditions of impaired immune system, rather than diabetes mellitus. In the 2 cats, EC occurred in connection with urethral unblocking and catheterization. This connection should be further investigated, and attention should be given to sterile catheterization, in order to prevent iatrogenic contamination of the bladder. Chronic cystitis and recurrent infections may represent a potential predisposing factor for EC. Emphysematous cystitis was characterized by mild to moderate clinical signs and was more often associated with lower urinary tract signs, such as hematuria and pollakiuria. Positive urine culture was a consistent finding in our patients, although negative cultures may occur as result of a recent antibiotic course. Severe clinical signs and involvement of kidneys and prostate tended to be uncommon. Ultrasonography can be considered a pivotal technique in the diagnosis of veterinary EC. In patients with EC, serial abdominal ultrasounds should be encouraged to rule out bladder wall abnormalities. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Aizenberg I, Aroch I. Emphysematous cystitis due to Escherichia coli associated with prolonged chemotherapy in a non-diabetic dog. J Vet Med. 2003;B50:396–398. doi: 10.1046/j.1439-0450.2003.00692.x. [DOI] [PubMed] [Google Scholar]
- 2.Amano M, Shimizu T. Emphysematous cystitis: A review of the literature. Intern Med. 2014;53:79–82. doi: 10.2169/internalmedicine.53.1121. [DOI] [PubMed] [Google Scholar]
- 3.Davis NL, Williams JH. Emphysematous cystitis in a non-diabetic cat. J South Afr Vet Assoc. 1993;64:162–164. [PubMed] [Google Scholar]
- 4.Fabbi M, Manfredi S, Bianchi E, Gnudi G, Miduri F, Volta A. Emphysematous pyelitis and cystitis associated with vesicoureteral reflux in a diabetic dog. Can Vet J. 2016;57:382–386. [PMC free article] [PubMed] [Google Scholar]
- 5.Guidi G, Rossini C, Cinelli C, Meucci V, Lippi I. Canine chronic kidney disease: Retrospective study of a 10-year period of clinical activity. Vet Sci. 2012:115–118. [Google Scholar]
- 6.Kato S, Chmielewski M, Honda H, Pecoits-Filho R, et al. Aspects of immune dysfunction in end stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foxman B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am J Med. 2002;113:5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 8.Merkel LK, Lulich J, Polzin D, Ober C, Westropp J, Sykes J. Clinicopathologic and microbiologic findings associated with emphysematous cystitis in 27 dogs. J Am Anim Hosp Assoc. 2017;53:313–320. doi: 10.5326/JAAHA-MS-6722. [DOI] [PubMed] [Google Scholar]
- 9.Miceli DD, Pignataro OP, Castillo VA. Concurrent hyperadrenocorticism and diabetes mellitus in dogs. Res Vet Sci. 2017;115:425–431. doi: 10.1016/j.rvsc.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Mokabberi R, Ravakhah K. Emphysematous urinary tract infections: Diagnosis, treatment and survival (case review series) Am J Med Sci. 2007;333:111–116. doi: 10.1097/00000441-200702000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Moon R, Biller DS, Smee NM. Emphysematous cystitis and pyelonephritis in a nondiabetic dog and a diabetic cat. J Am Anim Hosp Assoc. 2014;50:124–129. doi: 10.5326/JAAHA-MS-5972. [DOI] [PubMed] [Google Scholar]
- 12.Ney C, Friedenberg RM. Inflammation of the bladder. In: Ney C, Friedenberg RM, editors. Radiographic Atlas of the Genitourinary System. 2nd ed. Philadelphia, Pennsylvania: Lippincott; 1981. pp. 1394–1395. [Google Scholar]
- 13.Petite A, Busoni V, Heinen MP, Billen F, Snaps F. Radiographic and ultrasonographic findings of emphysematous cystitis in four nondiabetic female dogs. Vet Radiol Ultrasound. 2006;47:90–93. doi: 10.1111/j.1740-8261.2005.00112.x. [DOI] [PubMed] [Google Scholar]
- 14.Schicho A, Stroszczynski C, Wiggermann P. Emphysematous cystitis: Mortality, risk factors, and pathogens of a rare disease. Clin Pract. 2017;7:930. doi: 10.4081/cp.2017.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherding RG, Chew DJ. Nondiabetic emphysematous cystitis in 2 dogs. J Am Vet Med Assoc. 1979;174:1105–1109. [PubMed] [Google Scholar]
- 16.Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis illustrative case report and review of the literature. Medicine. 2007;86:47–53. doi: 10.1097/MD.0b013e3180307c3a. [DOI] [PubMed] [Google Scholar]