Abstract
A 1-year-old, female, domestic shorthair cat with a history of cyanotic mucous membranes for several months was referred for ovariohysterectomy. Blood samples exhibited a noticeably brownish discoloration, while laboratory screening revealed mild-to-moderate erythrocytosis and near normal partial arterial oxygen pressure. Blood methemoglobin content was 41% of total hemoglobin concentration, and erythrocytic methemoglobin reductase activity was < 1% compared with control samples. A diagnosis of hereditary methemoglobinemia was established. After an intravenous injection of methylene blue, the cat’s mucous membranes became transiently pink, and the ovariohysterectomy was uneventful. Methylene blue may have improved safety during anesthesia and surgery. Hereditary methemoglobinemia should be considered in persistently cyanotic cats with normal partial arterial oxygen pressure and lack of evidence of cardiopulmonary disease, anemia, or toxin exposure.
Résumé
Méthémoglobinémie héréditaire chez une chatte cyanotique présentée pour une ovariohystérectomie. Une chatte domestique âgée de 1 an avec une anamnèse de muqueuses cyanotiques pendant plusieurs mois a été recommandée pour l’ovariohystérectomie. Des prélèvements sanguins présentaient une décoloration brune manifeste tandis que les tests de laboratoire ont révélé une érythrocytose de légère à modérée et une pression d’oxygène artérielle partielle presque normale. Le contenu de méthémoglobine sanguine était de 41 % de la concentration totale des hémoglobines et l’activité de la réductase de la méthémoglobine érythrocytaire était < 1 % comparativement aux prélèvements témoins. Un diagnostic de méthémoglobinémie héréditaire a été posé. Après une injection intraveineuse de bleu de méthylène, les muqueuses du chat sont devenues provisoirement roses et l’ovariohystérectomie a été réalisée sans complications. Le bleu de méthylène peut avoir amélioré l’innocuité durant l’anesthésie et la chirurgie.
(Traduit par Isabelle Vallières)
Normal hemoglobin contains iron in the reduced ferrous (Fe2+) form, whereas methemoglobin (MetHb) contains iron in an oxidized ferric (Fe3+) state. Methemoglobin cannot bind to and carry oxygen (1,2). Small amounts of ferrous hemoglobin (< 3%) are normally oxidized to MetHb daily in dogs and humans (1–3). This occurs due to low levels of continual auto-oxidation and as a result of exposure to environmental or endogenous oxidants (2). Methemoglobin is kept in low levels by an efficient redox system within red blood cells. Most reduction occurs by methemoglobin reductase (MR), currently recognized as a 2-enzyme system (cytochrome b5 and cytochrome b5 reductase); a negligible amount of MetHb is reduced by an alternative pathway driven by the enzyme nicotinamide adenine dinucleotide phosphate (NADPH) dependent methemoglobin reductase (1,4–6). The latter pathway usually proceeds slowly because it requires an electron carrier, but it can be accelerated 5- to 10-fold if a carrier such as the redox dye methylene blue (MB) is present (6). Methemoglobin accumulation can occur following exposure to excess oxidants or an inability to reduce MetHb. It may lead to cyanosis (> 20% MetHb), impaired oxygenation of tissues, metabolic acidosis, and death (1). Hereditary methemoglobinemia (HM) due to MR deficiency, is a rare hematological disorder that has been reported in humans, several canine breeds, and a few domestic shorthair cats (2–4). It is characterized by a persistently elevated MetHb concentration in the blood (2,5,6). Presurgical treatment with MB has been reported to afford transient resolution of methemoglobinemia in dogs, and long-term administration of MB may decrease MetHb concentration and ameliorate clinical signs associated with HM (1,6). This report discusses the clinical presentation and diagnostic approach of a cat with HM due to MR deficiency and the prophylactic use of MB prior to elective surgery.
Case description
A 1-year-old, female, domestic shorthair cat with cyanotic mucous membranes was referred for ovariohysterectomy to the Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki, Greece. The cat had been kept indoors and was given a regular feline diet and no medications or supplements. The cat’s family and prior medical history were unknown. Three months earlier the cat was presented for ovariohysterectomy to the primary care clinician, but surgery was delayed due to the presence of cyanotic mucous membranes at the time of induction of anesthesia. Complete blood (cell) count (CBC) and a comprehensive serum biochemistry panel at the time were unremarkable, except for a moderate erythrocytosis [hematocrit: 0.58 L/L; reference interval (RI): 0.30 to 0.45 L/L]. The cat tested negative for feline leukemia virus antigen and feline immunodeficiency virus antibodies. At the time of referral, the owner reported the animal was healthy, active, and playful.
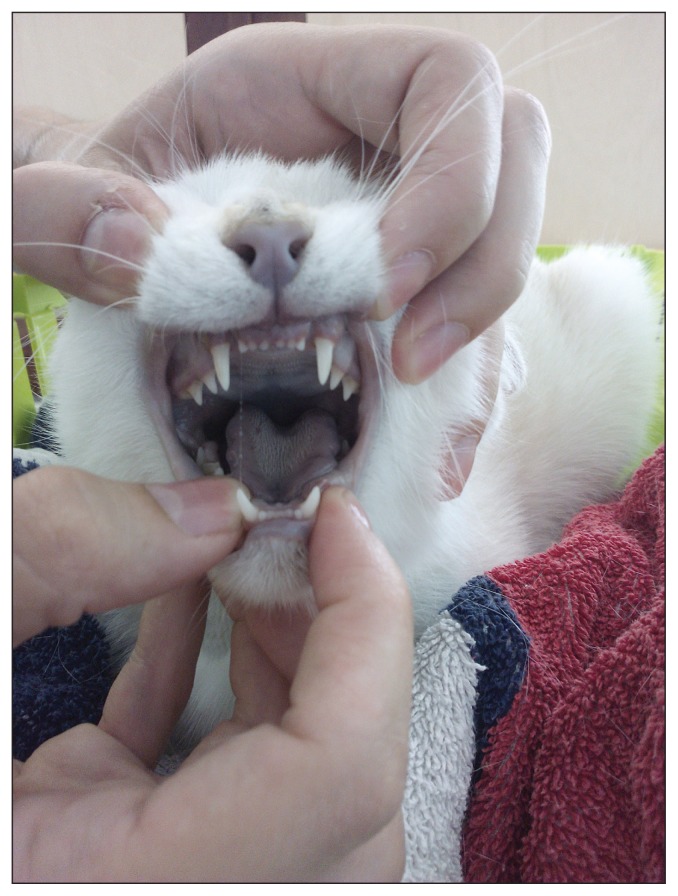
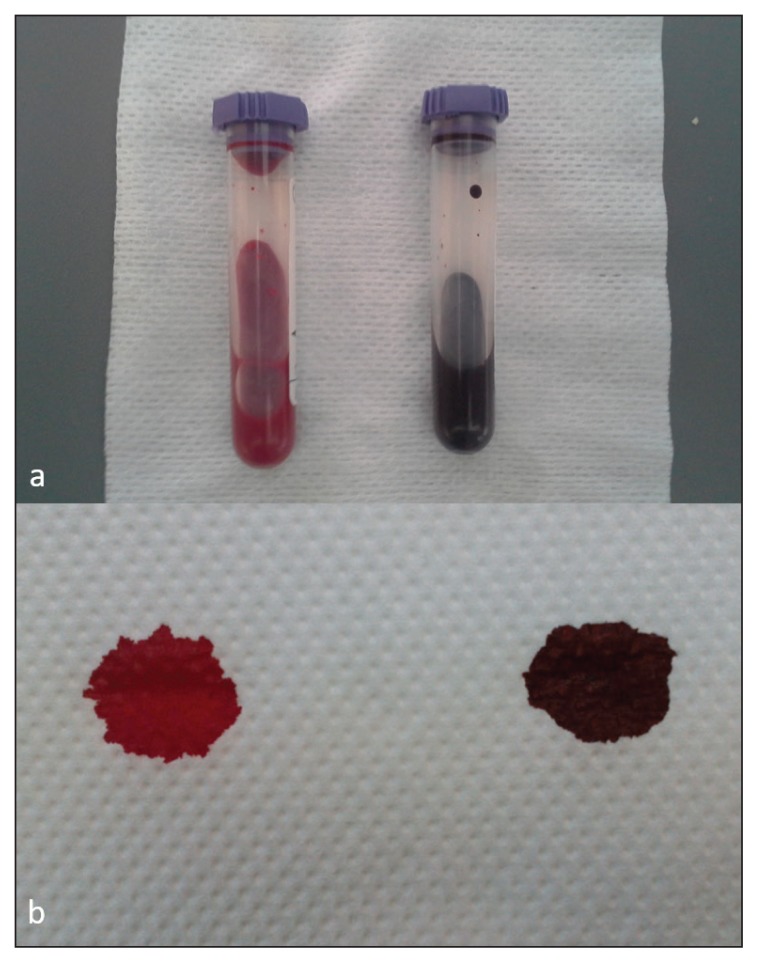
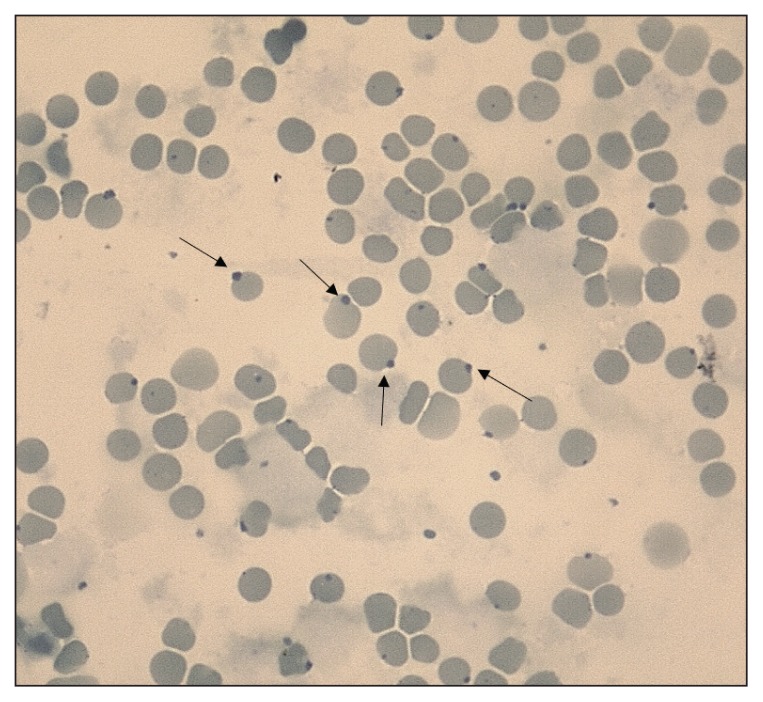
Clinical examination revealed generalized mucosal cyanosis (Figure 1) in a cat that was bright, alert, and responsive, with a normal temperature, and normal pulse, respiratory and cardiac rates. A blood sample collected for CBC was noticeably brownish compared to the bright red color of a control feline sample (spot test, Figure 2), suggestive of methemoglobinemia. An arterial blood gas analysis (Rapidpoint 400; Siemens Healthcare Diagnostics, Athens, Greece) revealed a near normal partial oxygen pressure (pO2: 94.4 mmHg, RI: 95 to 115 mmHg) and normal oxygen saturation (SaO2: 96.6%, reference value: > 95%). Cardiopulmonary evaluation (thoracic radiography, electrocardiography, and echocardiography) did not detect any abnormalities. A CBC (ADVIA 120; Siemens Healthcare Diagnostics) performed on admission, revealed a mild erythrocytosis (hematocrit: 0.54 L/L) and pseudothrombocytopenia (150 × 109/L; RI: 300 to 800 × 109/L) with many large platelet aggregates. Heinz bodies were visualized in 25% of the erythrocytes (Figure 3) in blood smears stained by new methylene blue.
Figure 1.
Clinical signs of methemoglobinemia in a cat. Markedly cyanotic buccal mucosa and nasal planum on admission.
Figure 2.
Venous anticoagulated blood sample in tube and on absorbent paper from a healthy cat (a and b, left) compared with blood from a cat with hereditary methemoglobinemia (a and b, right).
Figure 3.
Heinz bodies (arrows) in a cat with hereditary methemoglobinemia. New methylene blue stain, ×100 objective lens.
Anticoagulated blood samples (EDTA treated) from the patient with methemoglobinemia and 2 healthy control cats were shipped chilled to the PennGen Laboratory, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, USA. Established procedures (1,7–10) were used to determine a blood MetHb concentration of 41.3% (2.7% and 3.8% in the controls) and an erythrocytic MR activity of < 1% (compared with 83% and 100% in the controls), establishing the diagnosis of HM due to MR deficiency. For the MR measurement, a pellet of washed red blood cells was suspended in an equal volume of 0.9% saline, and stored at −20°C. The hemoglobin concentration (g/L) of the suspension was read with a HemoCue Hb 201+ (HemoCue; Brea, California, USA) and diluted to 10 to 30 g/L with purified water. A solution containing 200 μL of 2 mM potassium ferricyanide and 20 μL of the hemolysate was incubated at 30°C for 10 min along with a blank containing only 200 μL of 2 mM potassium ferricyanide. Another solution containing 100 μL of Tris-HCl, 100 μL of 2 mM NADH, and 690 μL of purified water (700 μL for the blank) was prepared in a separate tube. Then, 110 μL of the incubated potassium ferricyanide-hemolysate mixture (100 μL of the incubated potassium ferricyanide for the blank) was added to this solution. In triplicate, 300 μL of the resulting solution was added to an untreated, flat-bottom 96-well microplate (Evergreen Scientific, Rancho Dominguez, California, USA). The plate was run on a VersaMax microplate reader (CIMTEC, Charlotte, North Carolina, USA) at 30°C for 20 min, with readings taken every 20 s. Data between 4 and 10 min were used to calculate the MR enzyme activity in units per gram of hemoglobin (10).
Dexmedetomidine (Dextomidor; Orion Pharma, Espoo, Finland) 5 μg/kg body weight (BW), IM, pethidine (Pethidine; Martindale Pharmaceuticals, Essex, UK) 3 mg/kg BW, IM, and robenacoxib (Onsior; Elanco, Larchwood, Iowa, USA), 1 mg/kg BW, SC, were given as preanesthetics for ovariohysterectomy. Anesthesia was induced with propofol (Propofol; Hospira Enterprises B.V. Almere, The Netherlands), 1.5 mg/kg BW, IV, and maintained with isoflurane (Isoflo, ~2% in oxygen; Abbott Laboratories, Abbott Park, Illinois, USA) inhalation. Following the administration of preanesthetics and prior to ovariohysterectomy, a single intravenous infusion of methylene blue (Methylthioninium chloride Proveblue; Provepharm SAS, Marseille, France), 1 mg/kg BW, as previously used in dogs (1,6) was administered over 15 min. The mucous membranes became pink during surgery, which was completed uneventfully. The cat was hospitalized for 1 d during which its mucous membranes retained a pink coloration. This coloration continued the next day at home, as witnessed by the owners.
Follow-up
As of this writing, the cat is 3.5 y old, clinically continues to do well and is experiencing an excellent quality of life according to the owners. The cat, however, still shows cyanosis, persistent erythrocytosis (hematocrit range: 0.54 to 0.59 L/L), pseudothrombocytopenia (platelet range: 30 to 130 × 109/L, always with platelet clumping), and Heinz bodies ranging from 20% to 40% in serial CBCs performed at 5, 6, 24, and 30 mo after initial hospital admission. A cause for the increased amount of Heinz bodies could not be identified. Measurement of the blood MetHb concentration was repeated at 30 mo post-admission at the same institution and found to be 44.5% of total hemoglobin concentration (10.7% in the control cat); the shipment of the samples to PennGen unexpectedly took several days causing lysis and hemoglobin oxidation.
Discussion
Hereditary methemoglobinemia was suspected in the cat of this report based upon the persistence and degree of cyanosis, with blood remaining dark following exposure to air, and the lack of evidence of cardiopulmonary disease or a known toxin exposure. The diagnosis of HM was established by direct measurement of a high blood MetHb concentration and a severe deficiency of erythrocytic MR activity. The intravenous administration of a single dose of MB before surgery provided a short-term apparent resolution of severe methemoglobinemia, presumably improving oxygenation and safety of the cat during anesthesia and surgery. This was followed by an uneventful recovery. While the indoor cat continues to do clinically well, it has developed an absolute, secondary, appropriate polycythemia (erythrocytosis) due to persistent hypoxia which may need to be addressed with occasional phlebotomy in the future if hyperviscosity occurs. Similarly, any potential comorbidity (e.g., anemia, cardiorespiratory disease, any source of inflammation or toxin exposure) that may increase MetHb should be treated promptly before dangerously decompensating the cat.
Domestic shorthair cats have been previously described with HM (2,3,11). In agreement with the cat described herein, affected animals remain apparently healthy, until the problem is recognized serendipitously during routine physical examination or surgery, or they may experience intermittent exercise intolerance, lethargy, or syncope upon strenuous exercise or stressful situations (1,2,5,6,12). Due to the docile indoor life-style of this and other affected cats, clinical signs may not be overt except for cyanosis. Previously reported cats with HM tended to have a slightly higher erythrocytic MetHb content (44% to 52%) compared to dogs (13% to 41%) (2), and MetHb levels of > 60% are likely to be fatal (1).
Hereditary methemoglobinemia should not be confused with acute toxicity causing methemoglobinemia and Heinz body anemia. Many cases of acquired methemoglobinemia have been reported, usually associated with exposure to oxidizing agents including acetaminophen, benzocaine and lidocaine spray, or ingestion of onions and garlic. Cats are especially vulnerable to oxidants due to limited drug metabolism (2). Cats with acetaminophen toxicity tend to be overtly ill and anemic on admission and not just cyanotic compared with cats with HM which have normal to high hematocrits (5,6).
An important clinical clue is that venous blood containing significant amounts of MetHb (Fe3+) is darker than venous blood containing deoxyhemoglobin (Fe2+). If MetHb content is > 10%, the blood remains noticeably brown in color, as seen in the spot test applied in this case (Figure 1) (5). Absolute erythrocytosis has been previously reported in cats, dogs, and humans with HM, due to the resultant impaired tissue oxygenation, although to the authors’ knowledge, erythrocytosis necessitating phlebotomy has not been reported in cats despite the hematocrit in affected cats often being greater than the normal feline hematocrit (25% to 45%) but less than 60% (3,6,13). Arterial blood gas analysis-based pO2 and SaO2 measurements are misleading in cases of methemoglobinemia. Specifically, pO2 is normal as it indicates oxygen dissolved in blood plasma, not bound to hemoglobin, which is why it remains unchanged despite methemoglobinemia. The dissociation between pO2 and cyanosis is therefore a diagnostically useful clue in methemoglobinemia. SaO2 is also falsely normal because it is based on a calculation that assumes a normal oxygen dissociation curve and absence of dyshemoglobins. It can alternatively be measured by pulse oximetry, which is spectrophotometric, and will give a reduced value in these cases, but the reduction doesn’t correlate with the severity of methemoglobinemia. The latter method measures 2 wavelengths and assumes that only oxy- and deoxy-hemoglobin are present (11,14). Co-oximetry is the only reliable method of measuring MetHb and is also more reliable for SaO2. Like pulse oximetry, it is spectrophotometric, but measures multiple wavelengths instead of just 2, thus identifying and quantifying all hemoglobin species (11,14). However, co-oximetry was not available at the clinic at the time this case was presented.
Healthy cats may have up to 10% Heinz bodies due to the inherent susceptibility of this species’ hemoglobin to denaturation, and increased numbers of Heinz bodies may occur in diseases such as lymphoma, hyperthyroidism, and diabetes mellitus (15). In this case these conditions that predispose to Heinz body formation could be excluded, and no known toxin was identified. The persistently high percentages of Heinz bodies (20% to 40% of the erythrocytes) over several months is not a typical feature of HM because the latter implies an enzyme shortage to reduce heme iron and not a toxicity affecting the hemoglobin. Therefore, the cause of Heinz bodies in this cat remains elusive, although an oxidative comorbidity cannot definitely be ruled out and Heinz bodies may theoretically develop spontaneously in unstable hemoglobin variants that occur with the conversion of ferrous to ferric heme, such as in HM (16). Propofol and MB may potentially induce transient formation of Heinz bodies in cats, but in this case, they were used only once prior to surgery and no temporal association could be inferred with the excessive Heinz body formation (17,18). Moreover, propofol was recently shown to facilitate intubation and afford a rapid and smooth recovery, without any clinically relevant Heinz body formation (18). The clinical impact of the oxidative injury was apparently minimal throughout the follow-up period in this cat, as accelerated erythrocyte destruction with signs of hemolysis and/or anemia was never observed.
No evidence-based management recommendations are currently available for cats with HM. Methylene blue appears to be the most promising medical option, as it has been used successfully in humans and dogs with severe manifestations associated with HM and in cats with acetaminophen associated methemoglobinemia (1,6,17,19,20). Methylene blue dramatically decreases MetHb concentrations by potentiating the alternative NADPH MR pathway (1). In 2 published canine cases, intravenous administration of a single MB dose of 1 mg/kg (in 1 dog before surgery), objectively decreased the concentration of MetHb within 60 to 90 min (1,6). In 1 of the latter cases, MB given orally, daily, or every other day, was potentially beneficial on a long-term basis in reducing the MetHb concentration and ameliorating clinical signs, as opposed to the oral administration of general antioxidants such as riboflavin or ascorbic acid (1). The latter 2 treatments had been given on the rationale that in some human patients, methemoglobinemia could be controlled with oral administration of these agents (19,20). It seems, therefore, that the potential utility of MB in cats with HM may include the intravenous prophylactic use in the preoperative setting in cats displaying clinical signs, and possibly the oral MB administration post-surgery to ameliorate clinical signs and polycythemia. However, the clinical benefit and safety of chronic oral MB for the cats has yet to be established. Since this cat as well as others appear clinically healthy (except for cyanosis and any crisis situations due to secondary oxidative damage), long-term treatment was not considered. Moreover, prospective clinical trials and pharmacokinetics/pharmacodynamics studies would be needed to establish safe dosage recommendations, because MB has a narrow therapeutic index and too much MB can readily cause oxidative damage and paradoxically worsen methemoglobinemia, if the NADPH system is oversaturated (1). In the case reported here the effect of MB after treatment was not objectively assessed (no MetHb measured) but was inferred based on the observation that the cat retained a pink mucosal color during the surgery and for the next 2 d. In an experimental setting, a similar dose of intravenous MB has been used successfully in nitrite- or acetaminophen-induced methemoglobinemia in cats (17).
In conclusion, HM should be considered in persistently cyanotic cats with normal arterial pO2, lack of cardiorespiratory disease, and dark blood after exposure to air. Life quality and expectancy may not be substantially affected, while MB may improve safety during surgery or crisis.
Acknowledgment
This study was supported in part by a grant NIH #010939 from PennGen Laboratory, School of Veterinary Medicine, University of Pennsylvania, USA. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Jaffey JA, Harmon MR, Villani NA, et al. Long-term treatment with methylene blue in a dog with hereditary methemoglobinemia caused by cytochrome b5 reductase deficiency. J Vet Intern Med. 2017;31:1860–1865. doi: 10.1111/jvim.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey JW. Pathogenesis, laboratory diagnosis, and clinical implications of erythrocyte enzyme deficiencies in dogs, cats, and horses. Vet Clin Pathol. 2006;35:144–156. doi: 10.1111/j.1939-165x.2006.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 3.Harvey JW, Dahl M, High ME. Methemoglobin reductase deficiency in a cat. J Am Vet Med Assoc. 1994;205:1290–1291. [PubMed] [Google Scholar]
- 4.Shino H, Otsuka-Yamasaki Y, Sato T, et al. Familial congenital methemoglobinemia in Pomeranian dogs caused by a missense variant in the NADH-cytochrome B5 reductase gene. J Vet Intern Med. 2018;32:165–171. doi: 10.1111/jvim.15031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey JW. Hematology procedures. In: Harvey JW, editor. Veterinary Hematology: A Diagnostic Guide and Color Atlas. 2nd ed. St. Louis, Missouri: Elsevier Saunders; 2012. pp. 12–14. [Google Scholar]
- 6.McKenna J, Sacco J, Son TT, et al. Congenital methemoglobinemia in a dog with a promoter deletion and a nonsynonymous coding variant in the gene encoding cytochrome b5. J Vet Intern Med. 2014;28:1626–1631. doi: 10.1111/jvim.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnaud F, Higgins A, McCarron R, Moon-Massat PF. Determination of methemoglobin and hemoglobin levels in small volume samples. Artif Cells Nanomed Biotechnol. 2017;45:58–62. doi: 10.3109/21691401.2016.1138490. [DOI] [PubMed] [Google Scholar]
- 8.Board PG. NADH-ferricyanide reductase, a convenient approach to the evaluation of NADH-methaemoglobin reductase in human erythrocytes. Clin Chim Acta. 1981;109:233–237. doi: 10.1016/0009-8981(81)90340-5. [DOI] [PubMed] [Google Scholar]
- 9.Beutler E, editor. Red Cell Metabolism: A Manual of Biochemical Methods. 3rd ed. Orlando, Florida: Grune & Stratton; 1984. pp. 10–11. [Google Scholar]
- 10.Beutler E, editor. Red Cell Metabolism: A Manual of Biochemical Methods. 3rd ed. Orlando, Florida: Grune & Stratton; 1984. pp. 81–83. [Google Scholar]
- 11.Giger U, Wang P, Boyden M. Familial methemoglobin reductase deficiency in domestic shorthair cats. Feline Pract Suppl. 1999;31:14. [abstract] [Google Scholar]
- 12.Love L, Singer M. Anesthesia case of the month. J Am Vet Med Assoc. 2013;242:753–756. doi: 10.2460/javma.242.6.753. [DOI] [PubMed] [Google Scholar]
- 13.Soliman DS, Yassin M. Congenital methemoglobinemia misdiagnosed as polycythemia vera: Case report and review of literature. Hematol Rep. 2018;10:7221. doi: 10.4081/hr.2018.7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahilly LG, Mandell DC. Methemoglobinemia. In: Silverstein DC, Hopper K, editors. Small Animal Critical Care Medicine. 2nd ed. St. Louis, Missouri: Elsevier Saunders; 2015. pp. 580–585. [Google Scholar]
- 15.Christopher MM. Relation of endogenous Heinz bodies to disease and anemia in cats: 120 cases (1978–1987) J Am Vet Med Assoc. 1989;194:1089–1095. [PubMed] [Google Scholar]
- 16.Layton DM, Roper DR. Erythroenzyme disorders. In: Porwit A, McCullough J, Erber W, editors. Blood and Bone Marrow Pathology. 2nd ed. St Louis, Missouri: Elsevier Churchill Livingstone; 2011. pp. 121–129. [Google Scholar]
- 17.Rumbeiha WK, Oehme FW. Methylene blue can be used to treat methemoglobinemia in cats without inducing Heinz body hemolytic anemia. Vet Hum Toxicol. 1992;34:120–122. [PubMed] [Google Scholar]
- 18.Bley CR, Roos M, Price J, et al. Clinical assessment of repeated propofol-associated anesthesia in cats. J Am Vet Med Assoc. 2007;231:1347–1353. doi: 10.2460/javma.231.9.1347. [DOI] [PubMed] [Google Scholar]
- 19.Gokalp S, Unuvar E, Oguz F, Kilic A, Sidal M. A case with quadriparetic cerebral palsy and cyanosis: Congenital methemoglobinemia. Pediatr Neurol. 2005;33:131–133. doi: 10.1016/j.pediatrneurol.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Cooper MS, Randall M, Rowell M, Charlton M, Greenway A, Barnes C. Congenital methemoglobinemia type II-clinical improvement with short-term methylene blue treatment. Pediatr Blood Cancer. 2016;63:558–560. doi: 10.1002/pbc.25791. [DOI] [PubMed] [Google Scholar]