Abstract
Background
Nasal continuous positive airway pressure (NCPAP) is a strategy for maintaining positive airway pressure throughout the respiratory cycle through the application of bias flow of respiratory gas to an apparatus attached to the nose. Treatment with NCPAP is associated with decreased risk of mechanical ventilation and might be effective in reducing chronic lung disease. Nasal intermittent positive pressure ventilation (NIPPV) is a form of noninvasive ventilation during which patients are exposed intermittently to higher levels of airway pressure, along with NCPAP through the same nasal device.
Objectives
To examine the risks and benefits of early NIPPV versus early NCPAP alone for preterm infants at risk of or in respiratory distress within the first hours after birth.
Primary endpoints are respiratory failure and the need for intubated ventilatory support during the first week of life. Secondary endpoints include chronic lung disease (CLD) (oxygen therapy at 36 weeks' postmenstrual age), air leaks, duration of respiratory support, duration of oxygen therapy, intraventricular hemorrhage, and incidence of mortality.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 9), MEDLINE via PubMed (1966 to September 28, 2015), Embase (1980 to September 28, 2015), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to September 28, 2015). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomized controlled trials and quasi‐randomized trials. A member of the Cochrane Neonatal Review Group handsearched abstracts from the European Society of Pediatric Research (ESPR). We contacted the authors of ongoing clinical trials to ask for information.
Selection criteria
We considered all randomized and quasi‐randomized controlled trials. Studies selected compared NIPPV versus NCPAP treatment, starting at birth or shortly thereafter in preterm infants (< 37 weeks' gestational age).
Data collection and analysis
We performed data collection and analysis using the recommendations of the Cochrane Neonatal Review Group.
Main results
Ten trials, enrolling a total of 1061 infants, met criteria for inclusion in this review. Meta‐analyses of these studies showed significantly reduced risk of meeting respiratory failure criteria (typical risk ratio (RR) 0.65, 95% confidence interval (CI) 0.51 to 0.82; typical risk difference (RD) ‐0.09, 95% CI ‐0.13 to ‐0.04) and needing intubation (typical RR 0.78, 95% CI 0.64 to 0.94; typical RD ‐0.07, 95% CI ‐0.12 to ‐0.02) among infants treated with early NIPPV compared with early NCPAP. The meta‐analysis did not demonstrate a reduction in the risk of CLD among infants randomized to NIPPV (typical RR 0.78, 95% CI 0.58 to 1.06). Investigators observed no evidence of harm. Review authors graded the quality of the evidence as moderate (unblinded studies).
Authors' conclusions
Early NIPPV does appear to be superior to NCPAP alone for decreasing respiratory failure and the need for intubation and endotracheal tube ventilation among preterm infants with respiratory distress syndrome. Additional studies are needed to confirm these results and to assess the safety of NIPPV compared with NCPAP alone in a larger patient population.
Plain language summary
Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants
Review question: Does NIPPV confer greater short‐term and long‐term benefits without harm to preterm infants with or at risk of respiratory distress compared with NCPAP?
Background: Some evidence suggests that nasal intermittent positive pressure ventilation (NIPPV) increases the effectiveness of nasal continuous positive airway pressure (NCPAP) in preterm babies who have respiratory difficulties or are at risk of such difficulties. Preterm babies with breathing problems often require help from a machine (ventilator) that provides regular breaths through a tube in the windpipe. Pediatricians caring for these preterm infants try to avoid use of ventilators, as they can damage the growing lung. NCPAP and NIPPV are ways of supporting babies' breathing in a less invasive way ‐ the tubes are shorter and go only to the back of the nose, thereby causing less damage to the lungs. NCPAP and NIPPV may be used early after birth to reduce the number of babies needing to go on a ventilator. NCPAP provides steady pressure to the back of the nose that is transmitted to the lungs, helping the baby breathe more comfortably. NIPPV provides the same support but also adds some breaths through the ventilator.
Study characteristics: We searched scientific databases for studies comparing NCPAP with NIPPV in preterm infants (born before 37 completed weeks of pregnancy) who need respiratory support shortly after birth. We looked at breathing problems, the need for a breathing tube and ventilator, and side effects. The evidence is current to September 2015.
Key results: We found nine trials comparing NCPAP with NIPPV. When analyzing all trials, we found that NIPPV reduces the risk for respiratory failure and the need for a ventilator. Additional studies are needed to determine how NIPPV can be best delivered to infants.
Quality of the evidence: The overall quality of the studies included in this review was good.
Summary of findings
Summary of findings for the main comparison. NIPPV versus NCPAP (by population).
| NIPPV versus NCPAP (by population) | ||||||
| Patient or population: preterm infants Setting: neonatal intensive care units Intervention: NIPPV Comparison: NCPAP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with NCPAP | Risk with NIPPV | |||||
| Respiratory failure | Study population | RR 0.62 (0.47 to 0.82) | 876 (9 RCTs) | Moderatea | Risk of bias: unblinded intervention Meets optimal information size (OIS) (N = 377) |
|
| 251 per 1000 | 155 per 1000 (120 to 200) | |||||
| Moderate | ||||||
| 175 per 1000 | 109 per 1000 (84 to 140) | |||||
| Need for intubation | Study population | RR 0.79 (0.64 to 0.97) | 766 (8 RCTs) | Moderatea | Risk of bias: unblinded intervention Does not meet OIS (N = 838) |
|
| 300 per 1000 | 237 per 1000 (192 to 291) | |||||
| Moderate | ||||||
| 175 per 1000 | 138 per 1000 (112 to 170) | |||||
| Pneumothorax | Study population | RR 0.69 (0.35 to 1.34) | 876 (9 RCTs) | Lowa,b | Risk of bias: unblinded intervention Imprecision: wide confidence intervals | |
| 43 per 1000 | 29 per 1000 (15 to 57) | |||||
| Moderate | ||||||
| 44 per 1000 | 30 per 1000 (15 to 58) | |||||
| Severe intraventricular hemorrhage (grade III/IV) | Study population | RR 1.26 (0.53 to 3.01) | 430 (4 RCTs) | Very lowa,b | Risk of bias: unblinded intervention Imprecision: extremely wide confidence intervals | |
| 37 per 1000 | 46 per 1000 (19 to 110) | |||||
| Moderate | ||||||
| 49 per 1000 | 61 per 1000 (26 to 147) | |||||
| Chronic lung disease | Study population | RR 0.67 (0.47 to 0.94) | 727 (8 RCTs) | Moderatea | Risk of bias: unblinded intervention Does not meet OIS (N = 1250) |
|
| 179 per 1000 | 120 per 1000 (84 to 168) | |||||
| Moderate | ||||||
| 170 per 1000 | 114 per 1000 (80 to 160) | |||||
| Mortality during study period | Study population | RR 0.77 (0.51 to 1.17) | 876 (9 RCTs) | Lowa,b | Risk of bias: unblinded intervention Imprecision: wide confidence intervals | |
| 89 per 1000 | 69 per 1000 (46 to 105) | |||||
| Low | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 26 per 1000 | 20 per 1000 (13 to 30) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aUnblinded intervention.
bImprecision: wide confidence intervals.
Background
Description of the condition
Chronic lung disease (CLD), also known as bronchopulmonary dysplasia (BPD), is the most common serious morbidity among preterm infants. BPD has been defined historically as the need for supplemental oxygen therapy at 28 days of life; however, over time, this definition evolved to describe the need for supplemental oxygen therapy at 36 weeks' postgestational age, and it has been further standardized to include the need for oxygen therapy at 36 weeks' postgestational age to achieve oxygen saturation of 88% for 60 minutes or longer (Bancalari 2001).
The major risk factor for chronic lung disease is prematurity necessitating treatment with oxygen and mechanical ventilation (Avery 1987; Van Marter 2000), both of which are potentially modifiable. The pathogenesis of CLD involves barotrauma or volutrauma (due to assisted ventilation) that results in airway injury, smooth muscle hypertrophy, and parenchymal lung fibrosis and emphysematous changes, often complicated by inflammation, genetic predisposition, and concomitant abnormalities such as patent ductus arteriosus (PDA). CLD is also associated with increased risk of many cardiovascular abnormalities such as progressive pulmonary hypertension (due to hypoxia and vasoconstriction in the pulmonary vasculature), systemic hypertension, and left ventricular hypertrophy. It is important to note that whereas the survival of extremely low birth weight infants has increased over the years, rates of CLD have stayed relatively constant despite improving technology. CLD remains a serious problem; the incidence of CLD may be on the rise because of increased survival among the most vulnerable infants (Stoelhorst 2005).
Neonatologists have looked for noninvasive ways to support the breathing of preterm infants to avoid the need for mechanical ventilation.
Description of the intervention
Continuous distending airway pressure with the application of nasal constant positive airway pressure (NCPAP) has been used as a strategy for avoiding endotracheal tube ventilation, thereby minimizing the risk of CLD.
Historical analyses of nasal ventilation demonstrate a decreased incidence of BPD and reduced need for intubation in infants treated with noninvasive respiratory support. A study by Avery et al in 1987 demonstrated very different rates of CLD at eight institutions in the United States. The institution with the lowest rate of CLD, Columbia University, was also the institution with the highest use of nasal prong respiratory support in preterm infants with birth weight between 700 and 1500 grams. Infants showing signs of respiratory distress received nasal positive pressure support in the delivery room or within three hours of life. These infants were less likely than infants at other institutions to need mechanical ventilation, and they were less likely to develop BPD (Avery 1987).
Unfortunately, although at some centers NCPAP is a successful therapy for preterm infants, this success is not replicated at all centers. "Failures" of NCPAP are common, and many infants born at less than 28 weeks' gestation ultimately require endotracheal intubation and mechanical ventilation (Meyer 2004). In a randomized trial by Finer et al in 2004, infants who received NCPAP in the delivery room were no less likely than infants without NCPAP to need endotracheal intubation in the delivery room or in the subsequent first week of life. This study demonstrated instead a profound effect of gestational age on the need for intubation (virtually all infants born at 23 weeks required intubation vs only 14% of infants born at 27 weeks), regardless of the cohort to which they had been randomized (Finer 2004).
Nasal intermittent positive pressure ventilation (NIPPV) is a simple, effective mode of respiratory support. NIPPV augments continuous positive airway pressure (CPAP) with superimposed inflations to a set peak pressure. NIPPV may be delivered by nasal mask or prongs, which may be short or long, single or binasal. Some devices attempt to synchronize inflations with the infant's respiratory efforts.
How the intervention might work
NIPPV reduces asynchronous thoracoabdominal motion, perhaps as a result of reduced tube resistance or better stabilization of the chest wall, or both (Kiciman 1998). NIPPV results in improved tidal and minute volumes and decreased inspiratory effort required by neonates when compared with NCPAP (Moretti 1999). NIPPV may decrease the need for intubation and endotracheal tube ventilation and their associated risks. To evaluate the efficacy of NIPPV, these potential benefits should be weighed against the risks associated with delivery of positive pressure to the upper airway, pharynx, and esophagus. Potential risks of NIPPV include nasal septal injury and gastrointestinal perforation (Garland 1985).
Why it is important to do this review
Currently, two Cochrane reviews are comparing NCPAP versus NIPPV ‐ one for treatment of infants with apnea of prematurity (Lemyre 2002), and the other for prevention of extubation failure (Lemyre 2014).
The review by Lemyre et al titled "Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation" reveals a decreased need for reintubation among infants treated with NIPPV versus NCPAP. The review authors concluded that use of NIPPV may prevent extubation failure more effectively than use of NCPAP.
Thus, previous studies have shown that NIPPV may be superior to NCPAP in other clinical scenarios (eg, prevention of extubation failure, prevention of apnea), and it remains to be shown whether early NIPPV may provide similar benefit in preventing the primary need for intubation and mechanical ventilation when compared with NCPAP. This review seeks to determine whether NIPPV or NCPAP is a better primary mode of nasal ventilation for preventing respiratory failure, the need for intubation, and development of CLD, without causing harm such as air leak or intraventricular hemorrhage (IVH).
Objectives
To examine the risks and benefits of early NIPPV versus early NCPAP alone for preterm infants at risk of or in respiratory distress within the first hours after birth.
Primary endpoints are respiratory failure and the need for intubated ventilatory support during the first week of life. Secondary endpoints include chronic lung disease (oxygen therapy at 36 weeks' postmenstrual age), air leaks, duration of respiratory support, duration of oxygen therapy, IVH, and incidence of mortality.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomized and quasi‐randomized trials for inclusion. We excluded cross‐over trials.
Types of participants
We included studies that enrolled newly born preterm infants. For this protocol, we defined newly born infants as infants less than six hours old, and preterm infants as those born at less than 37 weeks' gestational age. We included infants who received surfactant therapy if the duration of endotracheal intubation was short, and if application of NIPPV or NCPAP occurred before six hours of life.
Types of interventions
Intermittent positive pressure ventilation provided by a ventilator or a bilevel device and administered via the nasal route through short nasal prongs or nasopharyngeal tubes versus NCPAP delivered by the same methods. NIPPV included noninvasive support delivered by a mechanical ventilator or a bilevel device in a synchronized or nonsynchronized way.
Types of outcome measures
Primary outcomes
Respiratory failure: defined by respiratory acidosis, increased oxygen requirement, or apnea that was frequent or severe, leading to additional ventilatory support during the first week of life
Need for endotracheal tube (ETT) ventilation (intermittent positive pressure ventilation (IPPV) through an endotracheal tube)
Secondary outcomes
Mortality (neonatal and before discharge)
Major neurodevelopmental disability (cerebral palsy, developmental delay (Bayley or Griffith assessment greater than two standard deviations (SDs) below the mean) or intellectual impairment (intelligence quotient (IQ) greater than two SDs below the mean), blindness (vision < 6/60 in both eyes), sensorineural deafness requiring amplification)
Chronic lung disease (oxygen therapy at 36 weeks' postmenstrual age)
Pneumothorax
Patent ductus arteriosus
IVH (all grades) (Papile 1978)
Severe IVH (grade III/IV) (Papile 1978)
Necrotizing enterocolitis (Bell's ≥ stage 2) (Bell 1978)
Sepsis
Retinopathy of prematurity (≥ stage 3) (ICCROP 2005)
Duration of ETT ventilation (any)
Duration of oxygen dependence (days)
Duration of hospital stay (days)
Nasal septal injury
Gastrointestinal perforation
Search methods for identification of studies
Electronic searches
Please see study flow diagram (Figure 1).
1.
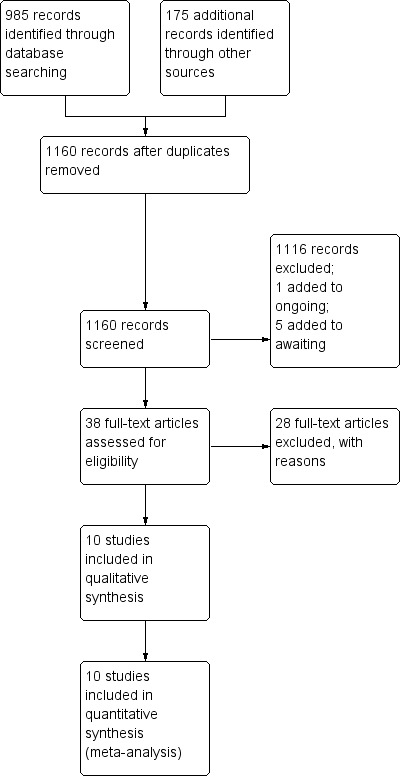
Study flow diagram.
We used the criteria and standard methods of the Cochrane Collaboration and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register). We conducted a comprehensive search of the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 9) in the Cochrane Library; MEDLINE via PubMed (1966 to September 28, 2015); Embase (1980 to September 28, 2015); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to September 28, 2015) using the following search terms: (nasal continuous positive airway pressure OR NCPAP OR nasal intermittent positive pressure ventilation OR NIPPV OR nasal intermittent mandatory ventilation OR NIMV OR nasal distending pressure OR nasal positive pressure OR nasal ventilation OR non‐invasive positive pressure ventilation OR synchronized intermittent mandatory ventilation OR SIMV OR nasopharyngeal synchronized intermittent mandatory ventilation OR bilevel CPAP OR BiCPAP OR BiPAP OR SiPAP), plus database‐specific limiters for RCTs and neonates (see Appendix 2 for the full search strategies for each database). We applied no language restrictions. We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/); the ISRCTN Registry).
Searching other resources
We searched abstracts from the Pediatric Academic Society meetings (2011 to 2014) through abstract archives on the website (www.pas‐meeting.org). Members of the Cochrane Neonatal Review Group handsearched abstracts from the European Society of Pediatric Research (ESPR). We consulted experts in the field of neonatology in reference to other published articles.
Data collection and analysis
We used the standard method of conducting a systematic review, as described in the Cochrane Handbook for Systematic Reviews of Interventions, which can be found online at http://www.cochrane.org/training/cochrane‐handbook.
Selection of studies
Two review authors checked titles and abstracts identified through database searches. Review authors were not masked to authorship, journal, or results. Both review authors obtained the full text of all studies of possible relevance for independent assessment.
Data extraction and management
Two review authors independently extracted data. We contacted trial authors to request missing data if needed.
Assessment of risk of bias in included studies
We assessed risk of bias using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011) and presented this information in "Risk of bias tables" for the following domains.
Selection bias.
Performance bias.
Attrition bias.
Reporting bias.
Any other bias.
We resolved disagreements by discussion or by consultation with a third assessor. See Appendix 3 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
For individual studies, we expressed results as risk ratios (RRs) and risk differences (RDs) with 95% confidence intervals (CIs) for dichotomous outcomes, and as mean differences (MDs and 95% CIs) for continuous outcomes.
Assessment of heterogeneity
We calculated the I2 statistic across trials using the Cochrane statistical package (RevMan 5.1) to test for significant heterogeneity. We explored possible sources of heterogeneity if the treatment effect showed moderate to high heterogeneity between studies, as described in the Cochrane Handbook for Systematic Reviews of Interventions, as an I2 statistic of > 50% heterogeneity.
Data synthesis
We synthesized data by using the standard method of the Cochrane Neonatal Review Group. We expressed results as typical risk ratios (RRs) and typical risk differences (RDs) with 95% confidence intervals (CIs) for dichotomous outcomes, and as weighted mean differences (WMDs and 95% CIs) for continuous outcomes, by using a fixed‐effect "assumption free" model.
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: respiratory failure, need for endotracheal tube ventilation (IPPV through an endotracheal tube), chronic lung disease (oxygen therapy at 36 weeks' postmenstrual age), pneumothorax, severe IVH (grade III/IV), and mortality (neonatal and before discharge) (post hoc).
Two review authors independently assessed the quality of evidence for each of the outcomes above. We considered evidence from RCTs as high quality but downgraded evidence one level for serious (or two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro 2008 Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence by one of four grades.
High: We are very confident that the true effect lies close to that of the estimate of effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
Planned subgroup analyses included gestational age (< 28 weeks vs ≥ 28 weeks), birth weight (< 1000 grams vs ≥ 1000 grams), whether infants received surfactant before randomization (via intubation‐surfactant‐extubation (INSURE) or prophylactic), whether NIPPV was delivered by a ventilator or by a bilevel device, and whether or not NIPPV was synchronized.
Results
Description of studies
Please see the Characteristics of included studies table.
Results of the search
The initial literature search returned 1160 potential articles or abstracts, of which 37 were of particular interest (full‐text review). See Figure 1.
Included studies
We identified 10 studies that met our inclusion criteria: Armanian 2014, Bisceglia 2007, Kirpalani 2013, Sai Sunil Kishore 2009, Kugelman 2007, Lista 2009, Meneses 2011, Ramanathan 2012, Salama 2015, and Wood 2013.
Armanian 2014 performed a quasi‐randomized trial that enrolled 98 infants (< 1501 grams and/or < 35 weeks) with respiratory distress syndrome (RDS) and a compatible chest x‐ray requiring noninvasive respiratory support after birth. Investigators randomized 44 infants to NIPPV and 54 to NCPAP, according to their medical chart number (odd or even number). All infants received aminophylline. A ventilator (unspecified) in the nonsynchronized mode provided NIPPV. The control group received bubble CPAP. Both groups used short binasal prongs. The primary outcome measure was respiratory failure with need for intubation within 48 hours. Secondary outcomes included need for surfactant, duration of oxygen, CLD, time to full feeds, length of hospital stay, pneumothorax, IVH, and PDA.
Bisceglia 2007 enrolled 88 preterm infants (28 to 34 weeks' gestational age) with mild to moderate RDS in the first four hours of birth. Investigators randomized 46 infants to NCPAP alone and 42 to NIPPV. They did not treat infants with aminophylline or caffeine, and they performed ventilation of both groups via nasal cannulae with the Bear Infant Ventilator CUB 750 (Ackrad Laboratories, Cranford, NJ, USA). Study authors did not synchronize NIPPV with spontaneous breathing. The primary outcome measured was number of infants needing intubation, and secondary outcomes included total duration of respiratory support, number of apneic episodes, and variation in blood gas levels. Upon receiving correspondence, study authors provided data for mortality at 28 days; presence of BPD, IVH, and necrotizing enterocolitis; and duration of hospital stay.
Kirpalani 2013 enrolled 1009 preterm infants (< 1000 grams and < 30 weeks). Researchers provided NIPPV by ventilator or bilevel device. Synchronization was allowed but was not mandatory. The primary outcome was death or moderate to severe BPD at 36 weeks. Among studied infants, 185 received noninvasive respiratory support from birth and were randomized in their first 24 hours (95 infants received NIPPV; 90 received NCPAP). We obtained data from study authors about these 185 infants for inclusion in this review.
Sai Sunil Kishore 2009 enrolled 76 infants (28 to 34 weeks' gestational age) with RDS in an RCT. Investigators randomized 37 infants to NIPPV and 39 NCPAP alone. They provided nasal respiratory support for infants by using VI P Bird‐R Sterling (Viasys Health Care, Conshohocken, PA, USA) and Drager Babylog 8000 (Drager Medical Inc, Lubeck, Germany) ventilators. NIPPV was nonsynchronized. Primary outcomes measured were failure of noninvasive respiratory support and need for intubation within the first 48 hours of randomization. Secondary outcomes included failure of nasal respiratory support within the first seven days, duration of respiratory support, duration of oxygen, duration of hospitalization, time to full feeds, air leaks, septicemia, upper airway injury, feed intolerance, abdominal distention, and bowel perforation.
Kugelman 2007 performed an RCT of 84 infants (24 to 34 weeks' gestational age) with RDS. Study authors randomized 41 infants to NCPAP alone and 43 to NIPPV. They administered both modes of respiratory support using the SLE 2000 (SLE Ltd, Surrey, UK) via nasal prongs and synchronized NIPPV with spontaneous breathing and with the infants' breathing. The primary outcome measured was need for mechanical ventilation, and secondary outcomes included blood pressure, heart rate, respiratory rate, blood gases, time needed to stop respiratory support, incidence of IVH, incidence of BPD, time to full feeds, and length of hospital stay.
Lista 2009 conducted an RCT of 40 infants (28 to 34 weeks' gestational age) with RDS diagnosed in the first hour of life. Researchers randomized 20 infants to NIPPV and 20 to NCPAP. They administered both treatments using Infant Flow (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) devices, and they synchronized NIPPV. The primary outcome measured was cytokine levels, and secondary outcomes included heart rate, blood pressure, oxygen saturation, blood gas levels, length of ventilation, incidence of PDA, treatment with ibuprofen, treatment with surfactant, pneumothorax, IVH, and oxygen dependency at 28 days (or at 36 weeks' gestational age).
Meneses 2011 enrolled 200 infants (26 to 33 and 6/7 weeks' gestational age) with RDS in an RCT. Investigators randomized 100 infants to NIPPV and an equal number to NCPAP alone. They performed nasal respiratory support of infants using the InterMed continuous‐flow and bubble CPAP systems (InterMed Inc, São Paulo, Brazil) via binasal prongs. NIPPV was nonsynchronized. The primary outcome measured was need for intubation in the first 72 hours of life. Secondary outcomes included total duration of ETT ventilation, total duration on NCPAP, total duration on supplemental oxygen, pneumothorax, BPD, PDA, necrotizing enterocolitis, IVH (grade III/IV), retinopathy of prematurity (ROP) (≥ stage 3), time to full feeds, and length of hospital stay.
Ramanathan 2012 performed a randomized controlled multicenter trial. Within 120 minutes of delivery, they enrolled 110 infants born between 26 + 0 and 29 + 6 weeks who required intubation for respiratory distress. They randomized infants at the time they received surfactant to NIPPV, nonsynchronized, via a ventilator or a bilevel device, or to NCPAP (bubble CPAP, synchronized inspiratory positive airway pressure (SiPAP), or ventilator). The primary outcome was the need for mechanical ventilation at seven days. Secondary outcomes included number of doses of surfactant, days on a ventilator, days on CPAP, days on oxygen, mortality, air leak, pulmonary hemorrhage, PDA, IVH grade III/IV, spontaneous intestinal perforation, ROP stage 3 or higher, length of stay, and CLD.
Salama 2015 performed a quasi‐randomized trial that enrolled 60 infants (28 to 34 weeks with RDS). The population was mixed at randomization: Some infants had received prophylactic surfactant (those < 29 weeks) and others had not. Researchers randomized 30 infants to NIPPV, provided by a Neoport E100M ventilator (DRE Medical, Louisville, KY, USA) in the synchronized mode, and 30 infants to NCPAP (bubble CPAP). Randomization was based on admission numbers. The primary outcome was failure of noninvasive respiratory support with defined criteria. Secondary outcomes included duration of mechanical ventilation, nasal injury, abdominal distention, gastrointestinal perforation, pneumothorax, CLD, sepsis, and IVH.
Wood 2013 (abstract) performed a two‐center RCT that enrolled 120 infants at 28 + 0 to 31 + 6 weeks and less than 6 hours of life with respiratory distress. Infants did not receive surfactant before randomization. Study authors provided NIPPV (n = 60) using a SiPAP in the synchronized mode (Trigger); the control group received CPAP (n = 60) without additional settings. The primary outcome was failure of noninvasive support.
Excluded studies
See Characteristics of excluded studies.
Studies that evaluated infants with apnea included Gizzi 2015,Lin 1998,Lin 2011,Pantalitschka 2009, and Ryan 1989.
Studies that evaluated infants after extubation included Barrington 2001,Friedlich 1999,Gao 2010,Jasani 2016, Kahramaner 2014,Khalaf 2001,Khorana 2008,Moretti 2008, and O'Brien 2012.
Studies that were not RCTs (or quasi‐randomized trials) included Aghai 2006,Herber‐Jonat 2006,Liu 2003,Manzar 2004, and Migliori 2005.
Other studies included Bhandari 2007 (compared synchronized NIPPV vs mechanical ventilation), Baneshi 2014 (no report on primary outcome), Chen 2015 (enrolled twins only), Kugelman 2014a (compared high‐flow nasal cannula vs NIPPV), Salvo 2015 (compared two methods of providing NIPPV), Santin 2004 (compared NIPPV vs conventional ventilation), Shi 2010 (included term infants), Shi 2014 (included term infants), and Zhou 2015 (examined DuoPAP (Hamilton Medical, Bonaduz, Switzerland)).
Ongoing studies and studies awaiting classification included Chen 2013,Fu 2014,Gao 2014,Sasi 2013, and Silveira 2015.
Risk of bias in included studies
We assessed methodological quality using the criteria of the Cochrane Neonatal Review Group. See risk of bias descriptions in the Characteristics of included studies table and Figure 2 for details.
2.
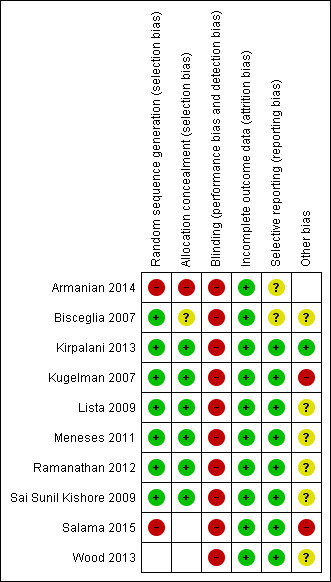
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
See: Table 1
NIPPV versus NCPAP (by population) (Comparison 1)
PRIMARY OUTCOME
Respiratory failure: defined by respiratory acidosis, increased oxygen requirement, or apnea that was frequent or severe, leading to additional ventilatory support during the first week of life (Outcome 1.1)
Overall, included trials enrolled 1061 preterm infants. Three of the 10 trials (Ramanathan 2012; Kugelman 2007; Sai Sunil Kishore 2009 showed statistically significant benefit for infants initially treated with NIPPV in terms of respiratory failure in the first week of life. Meta‐analysis showed that the effect was clinically important (typical risk ratio (RR) 0.65, 95% confidence interval (CI) 0.51 to 0.82; typical risk difference (RD) ‐0.09, 95% CI ‐0.13 to ‐0.04), with 11 infants (95% CI 8 to 25) needing to be treated with NIPPV to prevent one respiratory failure. We graded evidence for this outcome as moderate quality (unblinded intervention) (Analysis 1.1; Figure 3). Heterogeneity was low (for all outcomes).
1.1. Analysis.
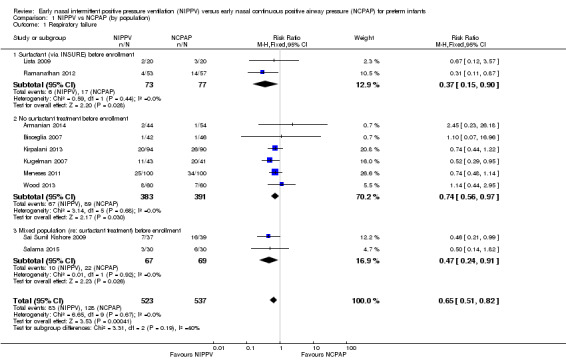
Comparison 1 NIPPV vs NCPAP (by population), Outcome 1 Respiratory failure.
3.
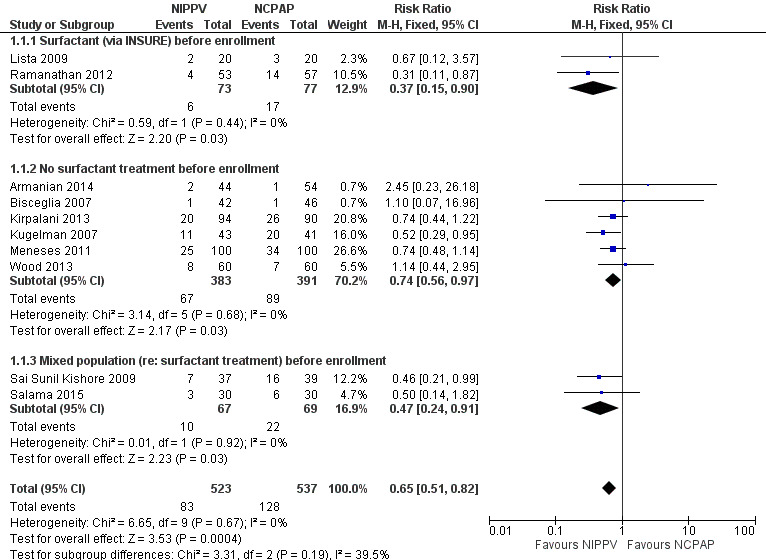
Forest plot of comparison: 1 NIPPV vs NCPAP (by population), outcome: 1.1 Respiratory failure.
Two studies (Lista 2009; Ramanathan 2012) included infants who received surfactant before randomization via INSURE. Six studies (Armanian 2014; Bisceglia 2007; Kirpalani 2013; Kugelman 2007; Meneses 2011; Wood 2013) included infants who did not receive surfactant, and two studies (Sai Sunil Kishore 2009; Salama 2015) included a mixed population of infants. The effect on respiratory failure was statistically significant in the three populations of infants studied.
Need for endotracheal tube ventilation (Outcome 1.2)
Nine trials reported on this outcome (n = 950), which could not be ascertained in one trial (Ramanathan 2012). Meta‐analysis showed statistically significant benefit for infants initially treated with NIPPV (typical RR 0.78, 95% CI 0.64 to 0.94; typical RD ‐0.07, 95% CI ‐0.12 to ‐0.02), with 17 infants (95% CI 8 to 50) needing to be treated with NIPPV to prevent one respiratory failure. We graded evidence for this outcome as moderate quality (unblinded intervention) (Analysis 1.2; Figure 4).
1.2. Analysis.
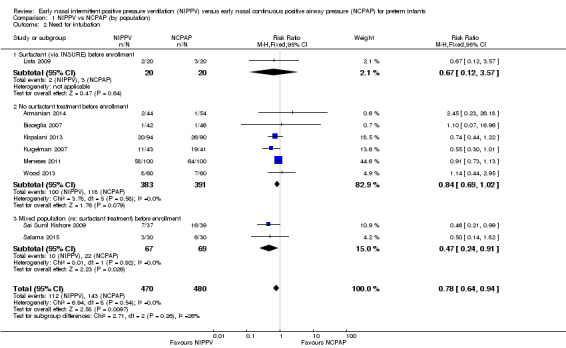
Comparison 1 NIPPV vs NCPAP (by population), Outcome 2 Need for intubation.
4.
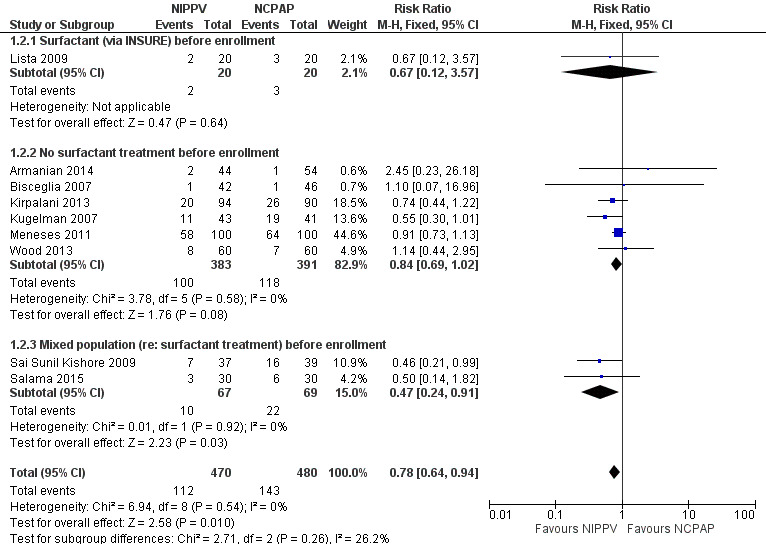
Forest plot of comparison: 1 NIPPV vs NCPAP (by population), outcome: 1.2 Need for intubation.
SECONDARY OUTCOMES
Mortality during study period (Outcome 1.3)
All trials reported this outcome. Overall, investigators noted no statistically significant reduction in mortality during neonatal intensive care unit (NICU) admission (typical RR 0.77, 95% CI 0.51 to 1.15). We graded evidence for this outcome as moderate quality (unblinded intervention) (Analysis 1.3; Figure 5).
1.3. Analysis.
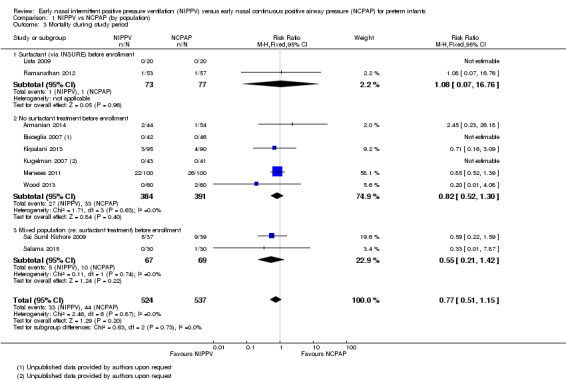
Comparison 1 NIPPV vs NCPAP (by population), Outcome 3 Mortality during study period.
5.
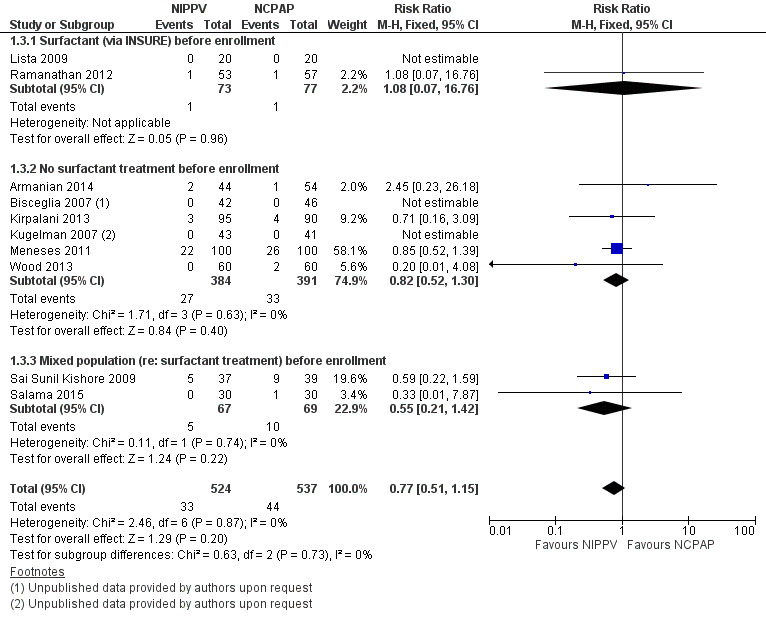
Forest plot of comparison: 1 NIPPV vs NCPAP (by population), outcome: 1.3 Mortality during study period.
Chronic lung disease (need for oxygen at 36 weeks in surviving infants) (Outcome 1.4)
All trials except Armanian 2014 reported oxygen need at 36 weeks' corrected gestational age (CLD). Only one trial (Ramanathan 2012), in which infants received surfactant before randomization, reported a decrease in CLD. Meta‐analysis did not show a reduction in CLD (typical RR 0.78, 95% CI 0.58 to 1.06). We graded evidence for this outcome as moderate quality (unblinded intervention) (Analysis 1.4; Figure 6).
1.4. Analysis.
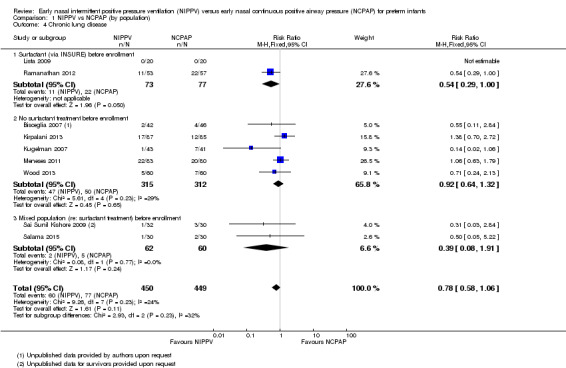
Comparison 1 NIPPV vs NCPAP (by population), Outcome 4 Chronic lung disease.
6.
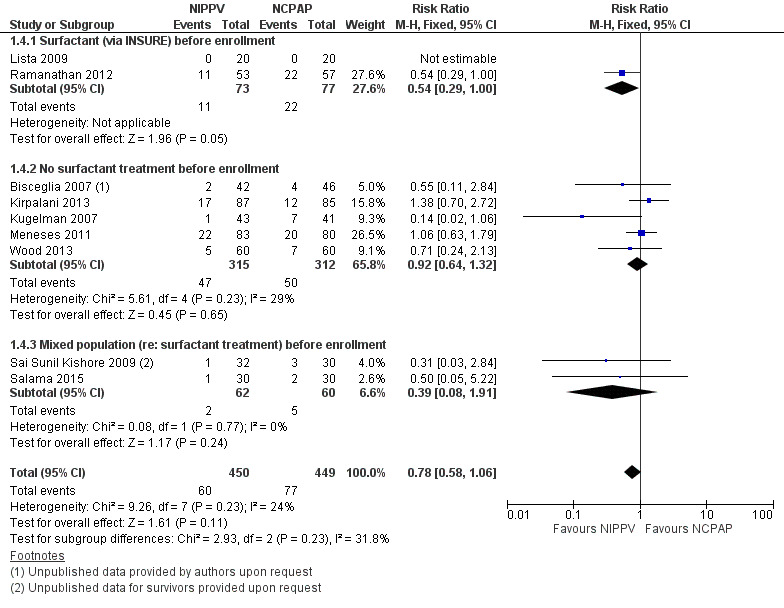
Forest plot of comparison: 1 NIPPV vs NCPAP (by population), outcome: 1.4 Chronic lung disease.
Pneumothorax (Outcome 1.5)
All 10 trials reported this outcome. Regardless of the population (surfactant or not before randomization), results showed no difference in the incidence of pneumothorax between infants randomized to NIPPV and those randomized to NCPAP (typical RR 0.79, 95% CI 0.42 to 1.48). We graded evidence for this outcome as moderate quality (unblinded intervention) (Analysis 1.5).
1.5. Analysis.
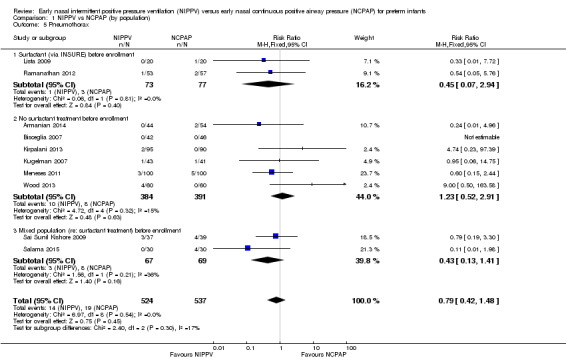
Comparison 1 NIPPV vs NCPAP (by population), Outcome 5 Pneumothorax.
Intraventricular hemorrhage, all grades (Outcome 1.6)
Five trials (Armanian 2014; Bisceglia 2007; Kugelman 2007; Lista 2009; Salama 2015) reported on this outcome. Sai Sunil Kishore 2009 reported a combined outcome of IVH and periventricular leukomalacia. No trial showed a difference in IVH between treatment groups (typical RR 0.79, 95% CI 0.54 to 1.16; typical RD ‐0.05, 95% CI ‐0.13 to 0.03). We graded evidence for this outcome as moderate quality (unblinded intervention) (Analysis 1.6).
1.6. Analysis.
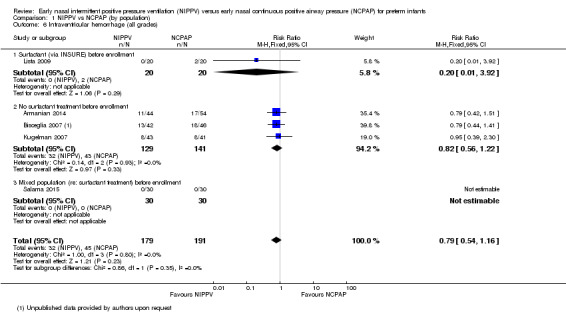
Comparison 1 NIPPV vs NCPAP (by population), Outcome 6 Intraventricular hemorrhage (all grades).
Severe intraventricular hemorrhage, grade III/IV (Outcome 1.7)
Only four trials (n = 430) (Bisceglia 2007 (study authors provided on request); Kugelman 2007; Meneses 2011; Ramanathan 2012) reported on this outcome. No trial showed a reduction in IVH in one treatment group compared with the other (typical RR 1.26, 95% CI 0.53 to 3.01; typical RD 0.01, 95% CI ‐0.03 to 0.05). We graded evidence for this outcome as low quality (unblinded intervention and wide CI) (Analysis 1.7).
1.7. Analysis.
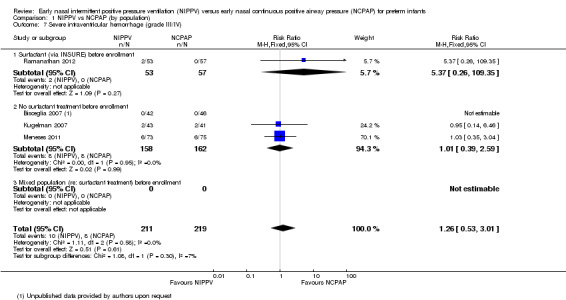
Comparison 1 NIPPV vs NCPAP (by population), Outcome 7 Severe intraventricular hemorrhage (grade III/IV).
Necrotizing enterocolitis ≥ Bell's stage 2 (Outcome 1.8)
Seven trials (Bisceglia 2007; Sai Sunil Kishore 2009; Kugelman 2007; Lista 2009; Meneses 2011; Ramanathan 2012; Wood 2013) reported on this outcome. No trial showed a reduction in necrotizing enterocolitis stage 2 or greater in one treatment group compared with the other (typical RR 0.67, 95% CI 0.34 to 1.31; typical RD ‐0.02, 95% CI ‐0.05 to 0.01). We graded evidence for this outcome as moderate quality (unblinded intervention) (Analysis 1.8).
1.8. Analysis.
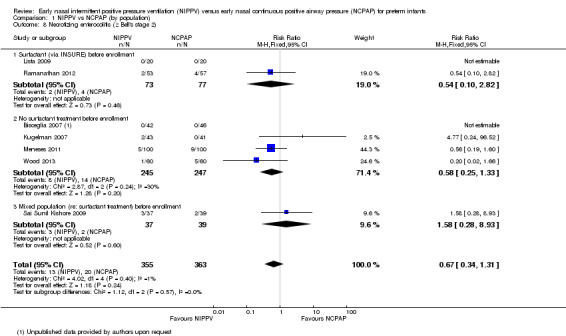
Comparison 1 NIPPV vs NCPAP (by population), Outcome 8 Necrotizing enterocolitis (≥ Bell's stage 2).
Sepsis (Outcome 1.9)
Only two studies (Sai Sunil Kishore 2009; Salama 2015) (n = 136) reported on this outcome. Results of meta‐analysis showed no difference between groups (typical RR 0.78, 95% CI 0.36 to 1.70; typical RD ‐0.04, 95% CI ‐0.16 to 0.08). We graded evidence for this outcome as moderate quality (unblinded intervention) (Analysis 1.9).
1.9. Analysis.
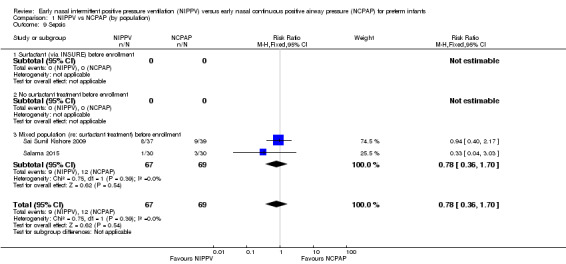
Comparison 1 NIPPV vs NCPAP (by population), Outcome 9 Sepsis.
Retinopathy of prematurity (≥ stage 3) (Outcome 1.10)
Only two studies (Meneses 2011; Ramanathan 2012) (n = 245) reported on this outcome. Meta‐analysis showed no difference between groups (typical RR 1.50, 95% CI 0.65 to 3.44; typical RD 0.03, 95% CI ‐0.04 to 0.10). We graded evidence for this outcome as moderate quality (unblinded intervention) (Analysis 1.10).
1.10. Analysis.
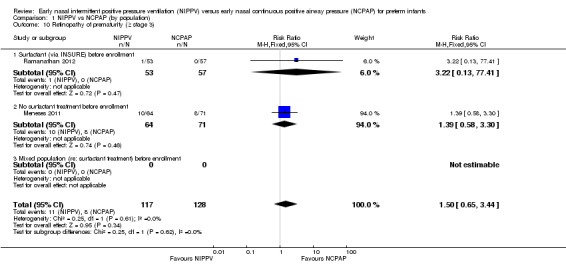
Comparison 1 NIPPV vs NCPAP (by population), Outcome 10 Retinopathy of prematurity (≥ stage 3).
Duration of endotracheal tube intubation
Three studies reported on this outcome; all included infants who did not receive surfactant before randomization. Duration of intubation and mechanical ventilation varied significantly between trials, with one trial reporting mean duration of seven to 12 hours (Bisceglia 2007) and the other two trials reporting mean duration between 10 and 13 days (Kugelman 2007; Meneses 2011). This heterogeneity precludes a meaningful meta‐analysis. Overall, no trial reported a clinically significant reduction in time required for mechanical ventilation in infants who received NIPPV. We graded evidence for this outcome as low quality (unblinded intervention and inconsistency).
Duration of oxygen dependence
Four studies reported this outcome (Sai Sunil Kishore 2009; Lista 2009; Meneses 2011; Ramanathan 2012) for 412 participants. These studies showed a high degree of heterogeneity, precluding meaningful meta‐analysis. Only one study (Ramanathan 2012), in which all infants received surfactant before randomization, demonstrated a reduction in the number of days on oxygen among infants who received NIPPV (29 days vs 38 days). We graded evidence for this outcome as low quaity (unblinded intervention and inconsistency).
Duration of hospital stay
Five trials (Armanian 2014; Sai Sunil Kishore 2009Kugelman 2007; Meneses 2011; Ramanathan 2012) reported this outcome (n = 554). Hospital stay ranged between 21 and 71 days with a high degree of heterogeneity between studies; therefore, we did not perform a meta‐analysis. Only one study (Armanian 2014) demonstrated a reduction in duration of stay (22 days in the NIPPV group vs 29 days in the CPAP group). We graded evidence for this outcome as low quality (unblinded intervention and inconsistency).
Upper airway injury (Outcome 1.11)
Sai Sunil Kishore 2009 reported two infants in each group with local upper airway injury. Study authors did not specify the nature of the injury and did not state whether it was nasal septal injury. Salama 2015 reported 20 cases of nasal injury in the NCPAP group (out of 30 infants) and none in the NIPPV group, without further description. We graded evidence for this outcome as low quality (unblinded intervention and imprecision) (Analysis 1.11).
1.11. Analysis.
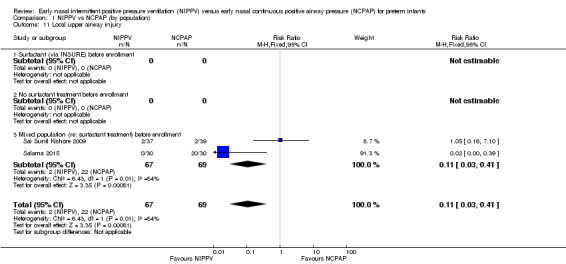
Comparison 1 NIPPV vs NCPAP (by population), Outcome 11 Local upper airway injury.
NIPPV versus NCPAP (by device) (Comparison 2)
Six trials used NIPPV delivered via ventilator (Bisceglia 2007; Sai Sunil Kishore 2009; Kugelman 2007; Meneses 2011; Armanian 2014; Salama 2015), two used bilevel devices (Lista 2009; Wood 2013), and two used both ventilator‐driven and bilevel devices (Kirpalani 2013; Ramanathan 2012). Two of the six trials using a ventilator to generate NIPPV showed benefit of NIPPV in preventing respiratory failure post extubation. Meta‐analysis of these six trials showed a reduction in rate of respiratory failure in the NIPPV group (typical RR 0.63, 95% CI 0.47 to 0.86) (Analysis 2.1). Results showed no evidence of benefit in the two trials using bilevel devices. One trial using both ventilators and bilevel devices (Ramanathan 2012) showed a reduction in respiratory failure at seven days (typical RR 0.31, 95% CI 0.11 to 0.87). Study authors provided no information on the relative proportion of infants who received NIPPV via ventilator or bilevel device (Analysis 2.1). Infants who received NIPPV via ventilator were intubated less often than those who received CPAP (typical RR 0.77, 95% CI 0.63 to 0.95; typical RD ‐0.08, 95% CI ‐0.14 to ‐0.02; number needed to treat for an additional beneficial outcome (NNTB) 13 (95% CI 7 to 50)) (Analysis 2.2).
2.1. Analysis.
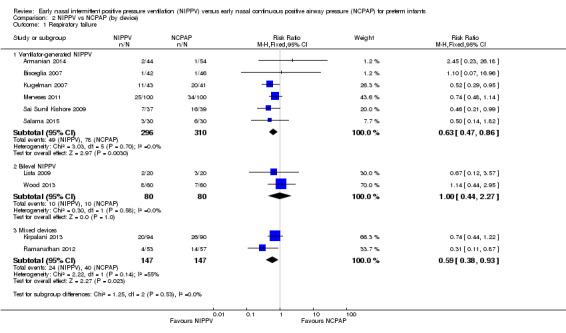
Comparison 2 NIPPV vs NCPAP (by device), Outcome 1 Respiratory failure.
2.2. Analysis.
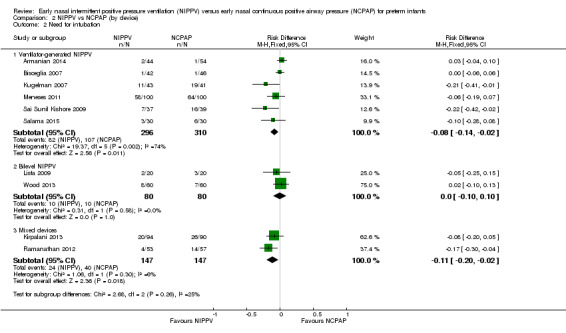
Comparison 2 NIPPV vs NCPAP (by device), Outcome 2 Need for intubation.
When review authors examined CLD by device delivering NIPPV, we observed no reduction in CLD in any of the subgroups (Analysis 2.4). One trial using mixed devices showed a possible reduction in CLD in the NIPPV group, with the confidence interval including 1.0 (typical RR 0.54, 95% CI 0.29 to 1.0). We noted no difference in the rate of pneumothoraces between groups (Analysis 2.5) and no difference in mortality or severe IVH within subgroups (Analysis 2.3; Analysis 2.6).
2.4. Analysis.
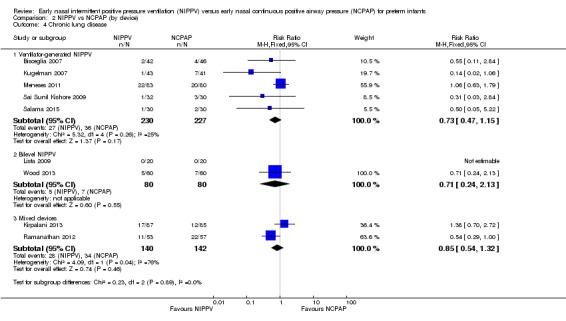
Comparison 2 NIPPV vs NCPAP (by device), Outcome 4 Chronic lung disease.
2.5. Analysis.
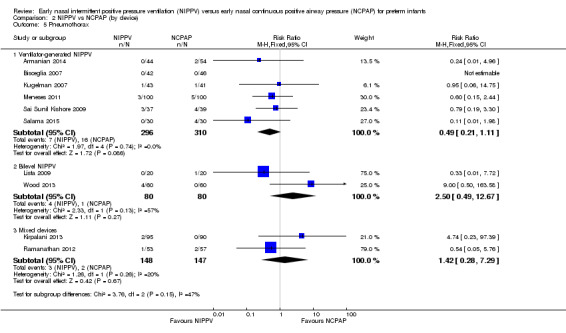
Comparison 2 NIPPV vs NCPAP (by device), Outcome 5 Pneumothorax.
2.3. Analysis.
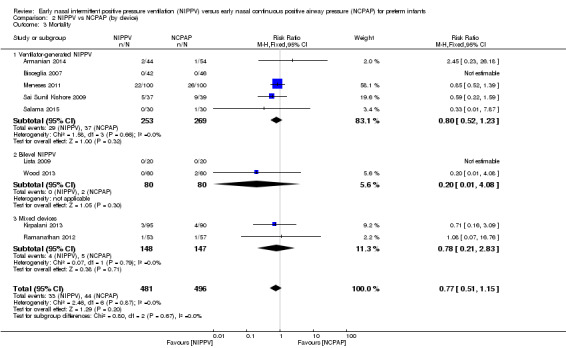
Comparison 2 NIPPV vs NCPAP (by device), Outcome 3 Mortality.
2.6. Analysis.
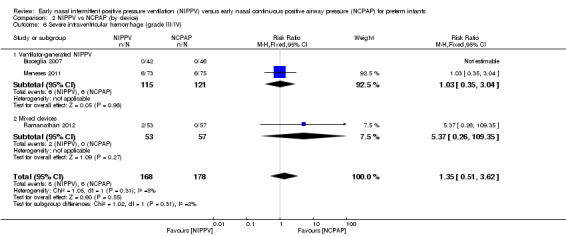
Comparison 2 NIPPV vs NCPAP (by device), Outcome 6 Severe intraventricular hemorrhage (grade III/IV).
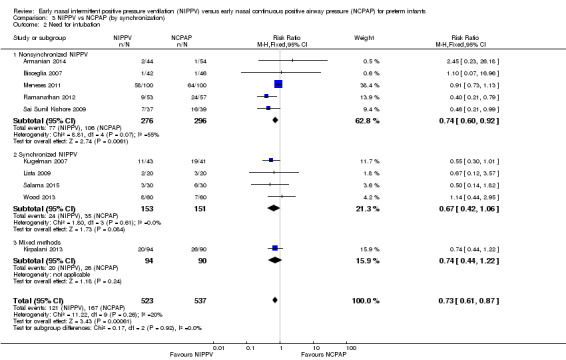
Synchronized versus nonsynchronized NIPPV (Comparison 3)
Four studies used synchronized NIPPV (Kugelman 2007; Lista 2009; Salama 2015; Wood 2013), and five did not (Armanian 2014; Bisceglia 2007; Sai Sunil Kishore 2009; Meneses 2011; Ramanathan 2012). One study allowed both methods (Kirpalani 2013). Nonsynchronized studies (n = 572) showed overall benefit in reducing respiratory failure (typical RR 0.60, 95% CI 0.44 to 0.83), and synchronized studies (n = 304) did not demonstrate benefit (typical RR 0.65, 95% CI 0.41 to 1.02) (Analysis 3.1). Findings were similar when we examined the need for intubation: Nonsynchronized studies showed benefit (typical RR 0.74, 95% CI 0.60 to 0.92), and synchronized studies showed a trend toward benefit (typical RR 0.67, 95% CI 0.42 to 1.06) (Analysis 3.2). However, the subgroup difference was not statistically significant.
3.1. Analysis.
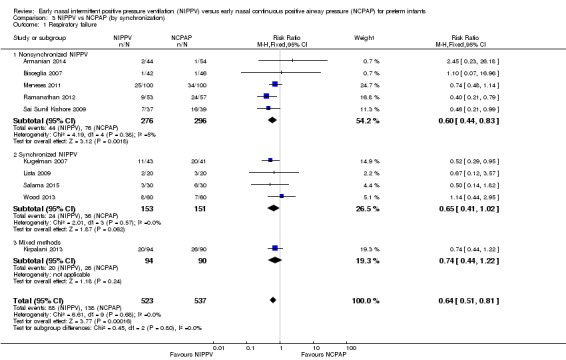
Comparison 3 NIPPV vs NCPAP (by synchronization), Outcome 1 Respiratory failure.
3.2. Analysis.
Comparison 3 NIPPV vs NCPAP (by synchronization), Outcome 2 Need for intubation.
We noted no difference in mortality within subgroups (Analysis 3.3). When we examined CLD by synchronization, we found that no method showed statistically significant benefit (Analysis 3.4). We observed no difference in the rate of pneumothoraces between groups (Analysis 3.5). No study using synchronized NIPPV reported on severe IVH (Analysis 3.6).
3.3. Analysis.
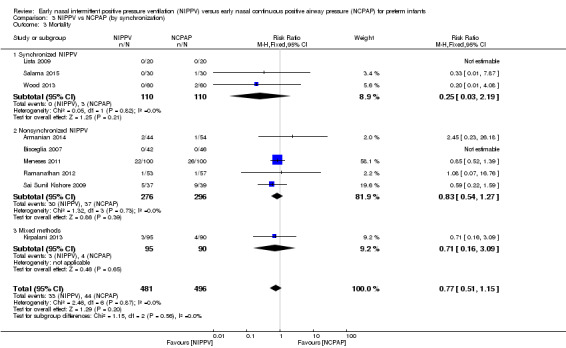
Comparison 3 NIPPV vs NCPAP (by synchronization), Outcome 3 Mortality.
3.4. Analysis.
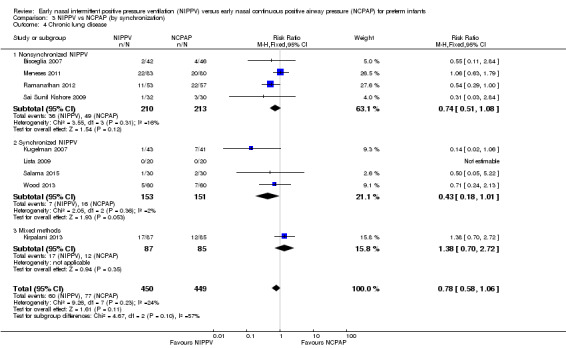
Comparison 3 NIPPV vs NCPAP (by synchronization), Outcome 4 Chronic lung disease.
3.5. Analysis.
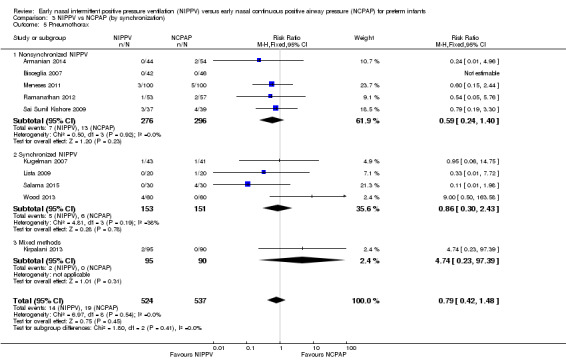
Comparison 3 NIPPV vs NCPAP (by synchronization), Outcome 5 Pneumothorax.
3.6. Analysis.
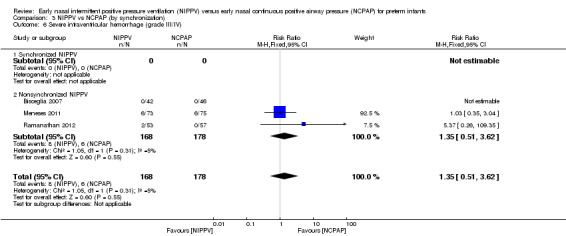
Comparison 3 NIPPV vs NCPAP (by synchronization), Outcome 6 Severe intraventricular hemorrhage (grade III/IV).
Post hoc analysis
NIPPV versus NCPAP: high‐quality studies (by device) (Comparison 4)
Respiratory failure: high‐quality studies only (by device) (Outcome 4.1)
Overall, meta‐analysis of 10 studies enrolling 1061 infants revealed a clinically important reduction in respiratory failure (defined by respiratory acidosis, increased oxygen requirement, or apnea that was frequent or severe, leading to additional ventilatory support during the first week of life) (typical RR 0.65, 95% CI 0.51 to 0.82; typical RD ‐0.09, 95% CI ‐0.13 to ‐0.04), with 11 infants (95% CI 8 to 25) needing to be treated with NIPPV to prevent one respiratory failure. We graded evidence for this outcome as moderate quality (unblinded intervention).
Owing to methodological limitations (risk of selection bias) in two studies (Armanian 2014; Salama 2015), we performed a post hoc analysis for the outcome respiratory failure post extubation, including only higher‐quality studies in the analysis (Analysis 4.1). Results were largely unchanged (typical RR 0.64, 95% CI 0.50 to 0.82; typical RD ‐0.10, 95% CI ‐0.15 to ‐0.04).
4.1. Analysis.
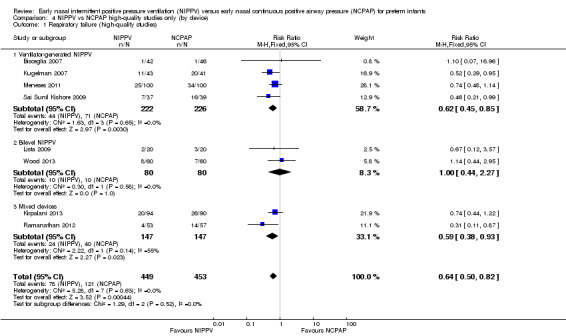
Comparison 4 NIPPV vs NCPAP high‐quality studies only (by device), Outcome 1 Respiratory failure (high‐quality studies).
Discussion
The meta‐analysis performed in this review showed a strong effect of nasal intermittent positive pressure ventilation (NIPPV) on prevention of respiratory failure or need for intubation the first few days after birth in infants with respiratory distress. Results showed no reduction in risk of chronic lung disease (CLD) overall but revealed a reduction in one trial, in which infants received surfactant before randomization. We identified no other short‐term benefit and no harm in any trials. Most included studies were small, single‐center trials. Eight of the 10 trials identified in this review had no major methodological limitations. Two trials were quasi‐randomized and had potential selection bias (Armanian 2014; Salama 2015), and one was reported as an abstract with clearly presented data (Wood 2013). Because of the nature of the interventions, it has been impossible to blind caregivers, and bias may have arisen through uneven use of co‐interventions. Investigators dealt with potential confounders, such as methylxanthine usage and indications for intubation, by having management protocols in place and by using objective respiratory failure criteria, enhancing confidence in study findings.
Because of the promising results of another related systematic review on NIPPV versus nasal continuous positive airway pressure (NCPAP) to aid with extubation (Lemyre 2014), it is reasonable to conclude that NIPPV might be more effective than NCPAP alone in providing respiratory support to preterm infants and in preventing the need for intubation. Results may have revealed differences in the effectiveness of different devices used to provide NIPPV (ventilator and bilevel); however, additional studies are needed to delineate this. We could not determine benefits for a subgroup of (smaller and more immature) infants included in this review. However, researchers should further assess benefits as well as relatively uncommon risks such as pneumothorax and severe intraventricular hemorrhage because occasionally large trials can disagree with the results of meta‐analysis of smaller trials (Cappelleri 1996; Villar 1995).
Authors' conclusions
Implications for practice.
Early NIPPV does appear to be superior to NCPAP alone for the treatment of preterm infants with respiratory distress syndrome (RDS) in decreasing respiratory failure and the need for intubation and endotracheal tube ventilation. Additional studies are needed to confirm these results and to assess the safety of NIPPV compared with NCPAP alone in a larger patient population.
Implications for research.
Future research should assess the efficacy and safety of early NIPPV compared with NCPAP for treatment of preterm infants with RDS, with specific focus on devices used and on synchronization. Clinically relevant outcomes, such as long‐term survival and incidence of neurodevelopmental impairment, are important to explore.
What's new
| Date | Event | Description |
|---|---|---|
| 6 February 2017 | Amended | Added external source of support |
Acknowledgements
We would like to acknowledge the assistance of Drs Bisceglia, Kugelman, Dutta, Lista, and Meneses, who assisted us by providing supplementary, unpublished information.
Appendices
Appendix 1. Abbreviations used in this review
NIPPV: nasal intermittent positive pressure ventilation.
NCPAP: nasal continuous positive pressure ventilation.
RDS: respiratory distress syndrome.
BPD: bronchopulmonary dysplasia.
IVH: intraventricular hemorrhage.
Appendix 2. Standard search methods
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial) CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Appendix 3. Risk of bias tool
The following issues were evaluated and entered into the risk of bias table: 1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated? For each included study, we categorized the method used to generate the allocation sequence as: a. low risk (any truly random process, eg, random number table, computer random number generator); b. high risk (any nonrandom process, eg, odd or even date of birth, hospital or clinic record number); or c. unclear risk. 2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed? For each included study, we categorized the method used to conceal the allocation sequence as: a. low risk (eg, telephone or central randomization, consecutively numbered sealed opaque envelopes); b. high risk (open random allocation, eg, unsealed or nonopaque envelopes, alternation, date of birth); or c. unclear risk. 3. Blinding (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment? For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as: a. low risk, high risk or unclear risk for participants; b. low risk, high risk or unclear risk for personnel; or c. low risk, high risk or unclear risk for outcome assessors. 4. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed? For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by trial authors, we re‐included missing data in the analyses. We categorized the methods as: a. low risk (< 20% missing data); b. high risk (≥ 20% missing data); or c. unclear risk. 5. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting? For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as: a. low risk (when it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported); b. high risk (when not all of the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported); or c. unclear risk. 6. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias? For each included study, we described any important concerns that we had about other possible sources of bias (eg, whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as: a. low risk; b. high risk; or c. unclear risk. If needed, we planned to explore the impact of the level of bias by undertaking sensitivity analyses.
Data and analyses
Comparison 1. NIPPV vs NCPAP (by population).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Respiratory failure | 10 | 1060 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.51, 0.82] |
| 1.1 Surfactant (via INSURE) before enrollment | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.15, 0.90] |
| 1.2 No surfactant treatment before enrollment | 6 | 774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.56, 0.97] |
| 1.3 Mixed population (re: surfactant treatment) before enrollment | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.24, 0.91] |
| 2 Need for intubation | 9 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.64, 0.94] |
| 2.1 Surfactant (via INSURE) before enrollment | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.57] |
| 2.2 No surfactant treatment before enrollment | 6 | 774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.69, 1.02] |
| 2.3 Mixed population (re: surfactant treatment) before enrollment | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.24, 0.91] |
| 3 Mortality during study period | 10 | 1061 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.51, 1.15] |
| 3.1 Surfactant (via INSURE) before enrollment | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.07, 16.76] |
| 3.2 No surfactant treatment before enrollment | 6 | 775 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.52, 1.30] |
| 3.3 Mixed population (re: surfactant treatment) before enrollment | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.21, 1.42] |
| 4 Chronic lung disease | 9 | 899 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.58, 1.06] |
| 4.1 Surfactant (via INSURE) before enrollment | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.29, 1.00] |
| 4.2 No surfactant treatment before enrollment | 5 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.64, 1.32] |
| 4.3 Mixed population (re: surfactant treatment) before enrollment | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 1.91] |
| 5 Pneumothorax | 10 | 1061 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.42, 1.48] |
| 5.1 Surfactant (via INSURE) before enrollment | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.07, 2.94] |
| 5.2 No surfactant treatment before enrollment | 6 | 775 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.52, 2.91] |
| 5.3 Mixed population (re: surfactant treatment) before enrollment | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.13, 1.41] |
| 6 Intraventricular hemorrhage (all grades) | 5 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.54, 1.16] |
| 6.1 Surfactant (via INSURE) before enrollment | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| 6.2 No surfactant treatment before enrollment | 3 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.56, 1.22] |
| 6.3 Mixed population (re: surfactant treatment) before enrollment | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Severe intraventricular hemorrhage (grade III/IV) | 4 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.53, 3.01] |
| 7.1 Surfactant (via INSURE) before enrollment | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.37 [0.26, 109.35] |
| 7.2 No surfactant treatment before enrollment | 3 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.39, 2.59] |
| 7.3 Mixed population (re: surfactant treatment) before enrollment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Necrotizing enterocolitis (≥ Bell's stage 2) | 7 | 718 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.31] |
| 8.1 Surfactant (via INSURE) before enrollment | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.10, 2.82] |
| 8.2 No surfactant treatment before enrollment | 4 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.25, 1.33] |
| 8.3 Mixed population (re: surfactant treatment) before enrollment | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.28, 8.93] |
| 9 Sepsis | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.36, 1.70] |
| 9.1 Surfactant (via INSURE) before enrollment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 No surfactant treatment before enrollment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Mixed population (re: surfactant treatment) before enrollment | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.36, 1.70] |
| 10 Retinopathy of prematurity (≥ stage 3) | 2 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.65, 3.44] |
| 10.1 Surfactant (via INSURE) before enrollment | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.22 [0.13, 77.41] |
| 10.2 No surfactant treatment before enrollment | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.58, 3.30] |
| 10.3 Mixed population (re: surfactant treatment) before enrollment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Local upper airway injury | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.41] |
| 11.1 Surfactant (via INSURE) before enrollment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 No surfactant treatment before enrollment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Mixed population (re: surfactant treatment) before enrollment | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.41] |
Comparison 2. NIPPV vs NCPAP (by device).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Respiratory failure | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Ventilator‐generated NIPPV | 6 | 606 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.86] |
| 1.2 Bilevel NIPPV | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.44, 2.27] |
| 1.3 Mixed devices | 2 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.93] |
| 2 Need for intubation | 10 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Ventilator‐generated NIPPV | 6 | 606 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.08 [‐0.14, ‐0.02] |
| 2.2 Bilevel NIPPV | 2 | 160 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
| 2.3 Mixed devices | 2 | 294 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.11 [‐0.20, ‐0.02] |
| 3 Mortality | 9 | 977 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.51, 1.15] |
| 3.1 Ventilator‐generated NIPPV | 5 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.23] |
| 3.2 Bilevel NIPPV | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.08] |
| 3.3 Mixed devices | 2 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.21, 2.83] |
| 4 Chronic lung disease | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Ventilator‐generated NIPPV | 5 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.15] |
| 4.2 Bilevel NIPPV | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.24, 2.13] |
| 4.3 Mixed devices | 2 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.54, 1.32] |
| 5 Pneumothorax | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Ventilator‐generated NIPPV | 6 | 606 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.21, 1.11] |
| 5.2 Bilevel NIPPV | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.49, 12.67] |
| 5.3 Mixed devices | 2 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.28, 7.29] |
| 6 Severe intraventricular hemorrhage (grade III/IV) | 3 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.51, 3.62] |
| 6.1 Ventilator‐generated NIPPV | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.35, 3.04] |
| 6.2 Mixed devices | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.37 [0.26, 109.35] |
Comparison 3. NIPPV vs NCPAP (by synchronization).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Respiratory failure | 10 | 1060 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.51, 0.81] |
| 1.1 Nonsynchronized NIPPV | 5 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.44, 0.83] |
| 1.2 Synchronized NIPPV | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.41, 1.02] |
| 1.3 Mixed methods | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.44, 1.22] |
| 2 Need for intubation | 10 | 1060 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.87] |
| 2.1 Nonsynchronized NIPPV | 5 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.60, 0.92] |
| 2.2 Synchronized NIPPV | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.42, 1.06] |
| 2.3 Mixed methods | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.44, 1.22] |
| 3 Mortality | 9 | 977 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.51, 1.15] |
| 3.1 Synchronized NIPPV | 3 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.19] |
| 3.2 Nonsynchronized NIPPV | 5 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.54, 1.27] |
| 3.3 Mixed methods | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.16, 3.09] |
| 4 Chronic lung disease | 9 | 899 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.58, 1.06] |
| 4.1 Nonsynchronized NIPPV | 4 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.51, 1.08] |
| 4.2 Synchronized NIPPV | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.18, 1.01] |
| 4.3 Mixed methods | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.70, 2.72] |
| 5 Pneumothorax | 10 | 1061 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.42, 1.48] |
| 5.1 Nonsynchronized NIPPV | 5 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.40] |
| 5.2 Synchronized NIPPV | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.43] |
| 5.3 Mixed methods | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.74 [0.23, 97.39] |
| 6 Severe intraventricular hemorrhage (grade III/IV) | 3 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.51, 3.62] |
| 6.1 Synchronized NIPPV | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Nonsynchronized NIPPV | 3 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.51, 3.62] |
Comparison 4. NIPPV vs NCPAP high‐quality studies only (by device).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Respiratory failure (high‐quality studies) | 8 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.50, 0.82] |
| 1.1 Ventilator‐generated NIPPV | 4 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.45, 0.85] |
| 1.2 Bilevel NIPPV | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.44, 2.27] |
| 1.3 Mixed devices | 2 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.93] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Armanian 2014.
| Methods | Study design: single‐center RCT Setting: tertiary NICU Duration: March 2013 to January 2014 |
|
| Participants | Infants with birth weight ≤ 1500 grams and/or gestational age ≤ 34 weeks with respiratory distress, including x‐ray diagnosis. Infants with major congenital anomalies, asphyxia, cyanotic heart defects, cardiovascular instability, and orofacial anomalies were excluded. | |
| Interventions | NIPPV was provided by a ventilator (exact ventilator not specified) and short binasal prongs. Settings: rate 40‐50, PIP 16‐20 cmH2O, PEEP 5‐6 cmH2O; nonsynchronized CPAP was provided via bubble CPAP, 5‐6 cmH2O. |
|
| Outcomes | Primary outcomes: need for intubation due to respiratory failure (defined) within 48 hours of life, duration of noninvasive respiratory support Secondary outcomes: need for INSURE, oxygen days, chronic lung disease, length of hospital stay, air leaks |
|
| Notes | IRCT2014021410026N4; quasi‐randomized trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Assigned by "file" number |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | Intervention cannot be blinded. All outcomes with the exception of long‐term follow‐up would be potentially biased. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/98 lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Stated secondary outcome of need for INSURE surfactant treatment; chronic lung disease not reported |
Bisceglia 2007.
| Methods | Study design: prospective randomized controlled trial Setting: single‐center trial at San Giovanni Di Dio Hospital in Italy Duration of study: January 2001‐January 2004 |
|
| Participants | Inclusion criteria: infants between 24 and 37 weeks' gestational age with mild to moderate RDS, defined as the need for FiO2 < 0.4 to keep oxygen saturation between 90% and 96%, as well as
a chest x‐ray positive for early hyaline membrane disease Exlusion criteria: pneumothorax, pneumomediastinum, surgical or cardiac disease, intraventricular hemorrhage, major congenital abnormalities Infants were not treated with aminophylline or caffeine. Number randomized: 88 infants total (46 males, 42 females) |
|
| Interventions | Both interventions were performed with use of the Bear Infant Ventilator CUB 750 (Ackrad Laboratories, Cranford, NJ, USA) via silicone binasal prongs (Ginevri, Rome, Italy). NCPAP (n = 46) was administered at 4‐6 cmH2O. NIPPV (n = 42) was administered with PIP 14‐20 cmH2O at 40 breaths per minute and end expiratory pressure 4‐6 cmH2O. NIPPV was nonsynchronized. | |
| Outcomes | Primary outcome: number of infants in each group who needed endotracheal intubation (i.e. failure of nasal ventilatory support) Secondary outcomes: total duration of respiratory support, number of apneic episodes, variation in blood O2 and CO2 partial pressures (evaluated at 4 hours after study enrollment) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors used EPISTAT, an online statistical program, to generate the sequence of interventions. |
| Allocation concealment (selection bias) | Unclear risk | Investigators concealed the sequence from practitioners before each participant assignment (information obtained upon personal correspondence with study authors). It is unclear, however, which method of concealment was used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was impossible owing to the nature of the interventions used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Unclear from information provided |
| Other bias | Unclear risk | Unclear from information provided |
Kirpalani 2013.
| Methods | Randomized controlled multicenter trial | |
| Participants | Infants with birth weight less than 1000 grams and gestational age less than 30 weeks were assigned to 1 of 2 forms of noninvasive respiratory support ‐ nasal intermittent positive pressure ventilation (IPPV) or nasal continuous positive airway pressure (CPAP) ‐ at the time of first use of noninvasive respiratory support during the first 28 days of life. | |
| Interventions | NIPPV (ventilator or bilevel, synchronized or not) vs NCPAP | |
| Outcomes | Death or BPD at 36 weeks, air leaks and necrotizing enterocolitis, duration of respiratory support, time to full feedings | |
| Notes | Obtained data from study authors regarding infants enrolled in the study and randomized within first 24 hours who were never on intubated respiratory support and never received surfactant (before randomization) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded outcomes. BPD status determined by oxygen reduction test |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Kugelman 2007.
| Methods | Study design: randomized controlled prospective clinical trial Setting: single‐center study at Bnai Zion Medical Center in Israel Duration of study: September 2004‐April 2006 |
|
| Participants | Inclusion criteria: infants between 24 and 34 6/7 weeks' gestational age with RDS, which study authors defined as clinical features such as tachypnea, grunting, nostril flaring, and retractions, as well as a positive chest x‐ray Exclusion criteria: cardiac disease, congenital malformation, sepsis, anemia, severe IVH, refusal of consent, ventilatory unavailability Number randomized: 84 infants total (53 males, 31 females) |
|
| Interventions | Both modes of respiratory support were administered via nasal prongs (INCA, Ackrad Laboratories, Berlin, Germany) by the SLE 2000 Ventilator (Specialized Laboratory Equipment, Croydon, UK). NCPAP (n = 41) was administered at 6‐7 cmH2O, and NIPPV (n = 43) was given with PIP 14‐22 cmH2O (adjusted according to chest excursion and birth weight), positive end expiratory pressure 6‐7 cmH2O, and 12‐30 breaths per minute. FiO2 was adjusted to keep oxygen saturation (measured by pulse oximetry) between 88% and 92%. NIPPV was synchronized. | |
| Outcomes | Primary outcome: failure of nasal respiratory support (i.e. need for endotracheal intubation). Criteria for failure: worsened RDS in conjunction with at least 1 of the following: pH < 7.2, PCO2 > 60 mmHg, PO2 < 50 mmHg, arterial oxygen saturation SpO2 < 88% on FiO2 > 50%, recurrent significant apnea needing stimulation or bag and mask ventilation Secondary outcomes: blood pressure, heart rate, respiratory rate, pulse oximetry saturation, respiratory status before mechanical ventilation, time to stop nasal support (stopped if FiO2 < 30% and no clinical signs of respiratory distress), incidence of IVH, duration of mechanical ventilation, incidence of BPD, time until full feeds, and length of hospital stay |
|
| Notes | Two infants were switched from NCPAP to NIPPV treatment group after randomization. Upon request, study authors provided raw data for outcomes of need for intubation and chronic lung disease for performance of a sensitivity analysis excluding those 2 infants. Results remained significant upon sensitivity analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors stated that they generated a random sequence order and performed block randomization to provide similar numbers of infants in each treatment group for the subgroup of birth weights above and below 1500 grams. |
| Allocation concealment (selection bias) | Low risk | Study authors placed individual cards with sequence designations in sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was impossible owing to the nature of the interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes listed in the protocol were published in this manuscript or in a subsequent manuscript in the journal Acta Paediatrica. |
| Other bias | High risk | Two infants were switched from NCPAP to NIPPV in violation of the study protocol. |
Lista 2009.
| Methods | Study design: randomized controlled prospective clinical trial Setting: single‐center study in Milan, Italy Duration of study: 2007‐2008 |
|
| Participants | Inclusion criteria: infants from 28‐34 weeks' gestational age with moderate RDS, defined in the first hour of life as PO2 ratio of 0.3‐0.35 and signs on chest x‐ray Exclusion criteria: infants with lethal congenital anomalies, infants requiring muscle relaxants, severe intraventricular hemorrhage, chorioamnionitis, sepsis, or suspected infection Number randomized: 40 infants total (gender not reported) |
|
| Interventions | Interventions were performed with the Infant Flow CPAP and Infant Flow SiPAP ventilators (Viasys Health Care, Conshohocken, PA, USA) via binasal prongs. NCPAP (n = 20) was administered at 6 cmH2O, and NIPPV (n = 20) at a lower CPAP level of 4.5 cmH2O and an upper CPAP level of 8 cmH2O with an initial rate of 30 breaths per minute. NIPPV was synchronized. | |
| Outcomes | Primary outcome: cytokine levels (IL‐6, IL‐8, and TNF‐alpha) Secondary outcomes: heart rate, systemic blood pressure, oxygen saturation, arterial blood gases, length of ventilation (total duration of respiratory support), PDA, need for treatment with ibuprofen or surfactant, incidence of air leaks, severe IVH, oxygen dependency at day 28 and/or at 36 weeks' postconceptional age, survival |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers with stratified randomization for gestational age |
| Allocation concealment (selection bias) | Low risk | Random number (representing allocation) was disclosed during phone call after enrollment in the trial. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was impossible owing to the nature of the interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported no incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported in the published article corresponded with outcomes listed in the original protocol. Information provided by study authors |
| Other bias | Unclear risk | Unclear from the information provided |
Meneses 2011.
| Methods | Study design: randomized controlled prospective clinical trial Setting: single‐center study in Brazil Duration of study: 2007‐2009 |
|
| Participants | Inclusion criteria: infants from 26‐33 and 6/7 weeks' gestational age with clinical evidence of RDS Exclusion criteria: infants with major congenital anomalies, cardiovascular instability, intubation at admission to NICU, refusal of consent, or unavailability of ventilator Number randomized: 200 infants total (100 males, 100 females) |
|
| Interventions | Interventions were performed with the continuous‐flow neonatal ventilator and Bubble CPAP systems (InterMed Inc, São Paulo, Brazil) via binasal prongs. NCPAP (n = 100) was administered at 5‐6 cmH2O, and NIPPV (n = 100) was administered at PEEP 4‐6 cmH2O and PIP 15‐20 cmH2O with initial flow rate of 8‐10 L/min and rate of 20‐30 breaths/min. NIPPV was nonsynchronized. | |
| Outcomes | Primary outcome: need for intubation in the first 72 hours of life Secondary outcomes: total duration of ETT ventilation, total duration on NCPAP, total duration on supplemental oxygen, pneumothorax, BPD, PDA, necrotizing enterocolitis, IVH (grade III/IV), ROP (≥ stage 3), time to full feeds, length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number computer‐generated protocol |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes containing intervention assignments |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was impossible owing to the nature of the interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors reported no incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes originally listed on ClinicalTrials.gov were the same as those reported in the published study. |
| Other bias | Unclear risk | Unclear from information provided |
Ramanathan 2012.
| Methods | Study design: multicenter randomized controlled trial Setting: 2 tertiary NICUs Duration of study: October 2006‐November 2008 |
|
| Participants | Infants 26 + 0 to 29 + 6 weeks' gestation, intubated for respiratory distress soon after birth. Planned INSURE procedure with Curosurf and early extubation. Excluded infants at greater than 120 minutes of life, < 600 grams, those not requiring intubation and surfactant within 60 minutes of life, with Apgar of 0 at 1 minute, outborn infants and those with major congenital malformations | |
| Interventions | NIPPV delivered via ventilator or bilevel device, by nasal prongs or nasopharyngeal prongs. Nonsynchronized mode. Settings: PIP 10‐15 cmH2O above PEEP, PEEP 5 cmH2O, Ti 0.5 seconds, rate 30‐40. NCPAP delivered via short binasal prongs, bubble CPAP, SiPAP or ventilator, PEEP of 5‐8 cmH2O | |
| Outcomes | Need for mechanical ventilation at 7 days (primary outcome). Secondary outcomes: number of doses of surfactant, days on ventilator, days on CPAP, days on oxygen, mortality, air leaks, pulmonary hemorrhage, PDA, IVH grade 3 or worse, spontaneous intestinal perforation, ROP stage 3 or worse, length of stay, use of postnatal steroids, BPD | |
| Notes | Infants randomized to NIPPV were extubated earlier than those randomized to NCPAP (randomization occurred after surfactant was received). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Timing of extubation different between 2 study groups (earlier in NIPPV group) |
Sai Sunil Kishore 2009.
| Methods | Study design: randomized controlled prospective trial Setting: single‐center study at a tertiary care neonatal unit in Northern India Duration of study: January 2007‐April 2008 |
|
| Participants | Inclusion criteria: infants between 28 and 34 weeks' gestational age, weighting ≥ 750 grams, with Downe's score ≥ 4, and showing signs of RDS (defined as 2 or more of the following: respiratory rate > 60 breaths/min, retractions, or grunting) within 6 hours of birth Exclusion criteria: major malformations, upper airway anomalies, severe cardiovascular instability or poor respiratory efforts, ventilator unavailability, or refusal of parental consent Number randomized: 76 infants total (50 males, 26 females) |
|
| Interventions | Interventions were performed with the VIP Bird‐R Sterling (Viasys Health Care, Conshohocken, PA, USA) or the Drager Babylog 8000 (Drager Medical Inc, Lubeck, Germany). NCPAP (n = 39) was administered at 5 cmH2O with flow set at 6‐7 litres/min. NIPPV (n = 37) was administered at about 50 breaths per minute, with PIP 15‐16 cmH2O, PEEP 5 cmH2O. Settings in both groups were adjusted on the basis of arterial blood gases and clinical parameters. Maximum permissible settings were CPAP 7 cmH2O and FiO2 0.7. NIPPV was nonsynchronized. | |
| Outcomes | Primary outcome: failure of noninvasive respiratory support within first 48 hours of randomization. Criteria for failure included at least 1 of the following: PaCO2 ≥ 60 mmHg with pH < 7.2, more than 2 apneic episodes per hour, any episode of apnea that did not respond to tactile stimulation and needed bag and mask ventilation, episodes of desaturation (SpO2 ≤ 85%) ≥ 3 per hour, or not responding to maximum settings for the allocated intervention. Secondary outcomes: failure of nasal respiratory support within first 7 days, duration of respiratory support, duration of oxygen, duration of hospitalization, time to full spoon feeds, air leaks, any grade IVH or periventricular leukomalacia (PVL), BPD, septicemia, upper airway injury, feed intolerance, abdominal distention, and bowel perforation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A study author not involved in recruitment generated a random sequence order using the online program www.randomizer.org. |
| Allocation concealment (selection bias) | Low risk | Treatment designations were placed in sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | A separate study author assessed outcomes, but blinding was impossible owing to the nature of the interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data were noted. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported in the published article corresponded with outcomes listed in the original protocol. Information was provided by study authors. |
| Other bias | Unclear risk | Unclear from information provided |
Salama 2015.
| Methods | Study design: quasi‐randomised controlled trial Setting: single‐center NICU in Jordania Duration of study: January 2011 to December 2011 |
|
| Participants | Preterm infants born at gestational age 28‐34 weeks with RDS, including positive chest x‐ray (Downe's score) | |
| Interventions | NIPPV delivered via ventilator (Neoport E100M; DRE Medical, Louisville, KY, USA) by nasal cannula. Synchronized mode. Settings: PIP 5‐12 cmH2O, PEEP 4‐6 cmH2O, Ti 0.3‐0.5 seconds. Bubble CPAP delivered via nasal cannula, PEEP 6 cmH2O | |
| Outcomes | Primary outcome: failure of noninvasive ventilation Secondary outcomes: duration of mechanical ventilation, nasal injury, abdominal distention, GI perforation, air leak, BPD, sepsis, IVH |
|
| Notes | Mixed population: Some infants received prophylactic surfactant via INSURE (< 29 weeks) or rescue surfactant if needed, before randomization. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomized trial; allocation based on admission number (odd or even) |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | High risk | No flow diagram |
Wood 2013.
| Methods | Study design: randomized controlled trial Setting: 2 centers Duration of study: unspecified |
|
| Participants | Infants with GA 28 + 0 to 31 + 6; inborn; < 6 hours old; no prior intubation; no major congenital disorders | |
| Interventions | SiPAP (BiPhasic Tr); settings unspecified CPAP delivered by the Infant Flow SiPAP device |
|
| Outcomes | Primary outcome was predefined failure of noninvasive respiratory support, necessitating intubation and ventilation, in the first 72 hours of treatment. | |
| Notes | Abstract only. Manuscript unpublished but ongoing (communication with study author) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Abstract |
BPD: bronchopulmonary dysplasia. CPAP: continuous positive airway pressure. ETT: endotracheal tube. FiO2: fraction of inspired oxygen. GA: gestational age. GI: gastrointestinal. IL: interleukin. INSURE: intubation‐surfactant‐extubation. IPPV: intermittent positive pressure ventilation. IVH: intraventricular hemorrhage. NCPAP: nasal continuous positive airway pressure. NICU: neonatal intensive care unit. NIPPV: nasal intermittent positive pressure ventilation. PCO2: partial pressure of carbon dioxide. PDA: patent ductus arteriosus. PEEP: positive end expiratory pressure. PIP: peak inspiratory pressure. PO2: partial pressure of oxygen. PVL: periventricular leukomalacia. RCT: randomized controlled trial. RDS: respiratory distress syndrome. ROP: retinopathy of prematurity. SiPAP: synchronized inspiratory positive airway pressure. SNIPPV: synchronized nasal intermittent positive airway pressure. SpO2: peripheral oxygen saturation. TNF: tumor necrosis factor.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aghai 2006 | Cross‐over trial design |
| Baneshi 2014 | Did not report on primary outcome or secondary outcomes |
| Barrington 2001 | Postextubation trial |
| Bhandari 2007 | SNIPPV vs conventional ventilation |
| Chen 2015 | Single‐center paired design; randomized controlled trial enrolling only preterm twins with RDS. One of a pair was randomized to NIPPV, another to NCPAP. |
| Friedlich 1999 | Postextubation trial |
| Gao 2010 | Postextubation trial |
| Gizzi 2015 | NIPPV for apnea of prematurity |
| Herber‐Jonat 2006 | Cross‐over trial design |
| Jasani 2016 | Postextubation trial |
| Kahramaner 2014 | Postextubation trial |
| Khalaf 2001 | Postextubation trial |
| Khorana 2008 | Postextubation trial |
| Kugelman 2014a | RCT comparing heated, humidified high‐flow nasal cannula (HHHFNC) with nasal intermittent positive pressure ventilation (NIPPV) on the requirement for endotracheal ventilation in preterm infants with respiratory distress syndrome (RDS) |
| Lin 1998 | NIPPV vs NCPAP for apnea of prematurity |
| Lin 2011 | NIPPV for apnea of prematurity |
| Liu 2003 | Variable‐flow nasal CPAP only |
| Manzar 2004 | NIPPV only |
| Migliori 2005 | Cross‐over trial design; post extubation |
| Moretti 2008 | Postextubation trial |
| O'Brien 2012 | Postextubation trial |
| Pantalitschka 2009 | NIPPV to reduce apnea of prematurity; cross‐over design |
| Ramanathan 2009 | Immediate extubation to NIPPV vs continued intubation followed by extubation to NCPAP |
| Ryan 1989 | Cross‐over trial design |
| Salvo 2015 | Comparison between nonsynchronized NIPPV delivered via ventilator and NIPPV delivered by a bilevel device. No CPAP arm |
| Santin 2004 | SNIPPV vs conventional ventilation |
| Shi 2010 | Included term infants (outside of our age range for inclusion) |
| Shi 2014 | Included term infants (outside of our age range for inclusion) |
| Zhou 2015 | This study examined the usefulness of nasal Duo positive airway pressure (DuoPAP) in the treatment of very low birth weight preterm infants with neonatal respiratory distress syndrome (NRDS). |
DuoPAP: Duo positive airway pressure. HHHFNC: heated, humidified, high‐flow nasal cannula. NCPAP: nasal continuous positive airway pressure. NIPPV: nasal intermittent positive pressure ventilation. NRDS: neonatal respiratory distress syndrome. RCT: randomized controlled trial. RDS: respiratory distress syndrome. SNIPPV: synchronized nasal intermittent positive airway pressure.
Characteristics of studies awaiting assessment [ordered by study ID]
Chen 2013.
| Methods | Prospective randomized controlled single‐center study performed on 67 preterm infants with NRDS between March 2011 and May 2012 |
| Participants | Preterm infants were randomly assigned to receive NIPPV and NCPAP. Oxygenation index (OI), pH, PaCO2, duration of respiratory support, complications, success rate, hospital mortality, and incidence of bronchopulmonary dysplasia (BPD) were compared between the 2 groups. |
| Interventions | Preterm infants were randomly assigned to receive NIPPV and NCPAP. |
| Outcomes | Preterm infants were randomly assigned to receive NIPPV and NCPAP. Oxygenation index (OI), pH, PaCO2, duration of respiratory support, complications, success rate, hospital mortality, and incidence of bronchopulmonary dysplasia (BPD) were compared between the 2 groups. |
| Notes | Study requires translation. |
Fu 2014.
| Methods | Randomized controlled study |
| Participants | One hundred neonates with neonatal RDS |
| Interventions | NIPPV or NCPAP |
| Outcomes | Reduced CO2 retention, improved oxygenation, reduced second endotracheal intubation and second use of pulmonary surfactant (PS), reduced duration of invasive respiratory support, reduced duration of oxygen use; reduced incidence of air leak, abdominal distention, and ventilator‐associated pneumonia |
| Notes | PMID: 24856992 (requires translation from Chinese) |
Gao 2014.
| Methods | Randomized controlled trial |
| Participants | Preterm infants with RDS |
| Interventions | NIPPV (synchronized bilevel) vs NCPAP |
| Outcomes | FiO2 at 24 hours, respiratory index (RI) at 12‐24 hours post ventilation, abdominal distention, period of noninvasive ventilation, ratio of intubation for invasive ventilation if failed noninvasive ventilation, air leak syndrome, neonatal necrotizing enterocolitis, periventricular‐intraventricular hemorrhage, bronchopulmonary dysplasia, retinopathy of prematurity, mortality rate after 36 hours of age, rate of abandon for discharge |
| Notes | PMID: 24680406 (requires translation from Chinese) |
Kong 2012.
| Methods | Single‐center randomized controlled trial |
| Participants | Preterm neonates (30‐35 weeks) with RDS |
| Interventions | DuoPAP or NCPAP |
| Outcomes | Total invasive respiratory support rates within 48 and 72 hours after birth; BPD; OI at 1, 12, 24, 48, and 72 hours |
| Notes |
Sasi 2013.
| Methods | Randomized controlled trial |
| Participants | Preterm infants at 28‐36 weeks with RDS |
| Interventions | Nonsynchronized NIMV vs NCPAP |
| Outcomes | Failure of allocated mode within 48 hours |
| Notes | PSANZ 2013 17th Annual Congress. Journal of Paediatrics and Child Health 2013;49(Suppl 2):10–58 |
Silveira 2015.
| Methods | Randomized controlled trial |
| Participants | Preterm infants at less than 37 weeks and weighing less than 2500 grams; with RDS and early use of noninvasive respiratory support or post extubation |
| Interventions | NIPPV (unspecified) vs NCPAP |
| Outcomes | Failure of allocated mode within 48 hours |
| Notes | Contact with study authors initiated to extract infants who were treated early for possible inclusion in a future update of this review |
BPD: bronchopulmonary dysplasia. DuoPAP: Duo positive airway pressure. NCPAP: nasal continuous positive airway pressure. NIPPV: nasal intermittent positive pressure ventilation. NRDS: neonatal respiratory distress syndrome. OI: oxygenation index. PaCO2: partial pressure of carbon dioxide. PS: pulmonary surfactant. PSANZ: Perinatal Society of Australia and New Zealand. RDS: respiratory distress syndrome. RI: respiratory index.
Characteristics of ongoing studies [ordered by study ID]
Sabzehei 2015.
| Trial name or title | Early nasal intermittent positive pressure ventilation vs continuous positive airway pressure in preterm infants with respiratory distress syndrome |
| Methods | Randomized controlled trial |
| Participants | Infants at 28‐34 weeks, single center, with respiratory distress; birth weight 1000‐2000 grams |
| Interventions | NIPPV vs NCPAP |
| Outcomes | Need for mechanical ventilation, death, pneumothorax, PDA, pulmonary hemorrhage, DIC, sepsis (all within 7 days) |
| Starting date | |
| Contact information | |
| Notes | IRCT2014072618598N1 |
DIC: disseminated intravascular coagulation. NCPAP: nasal continuous positive airway pressure. NIPPV: nasal intermittent positive pressure ventilation. PDA: patent ductus arteriosus.
Differences between protocol and review
Because the technology and the terminology of these interventions have evolved in recent years, we expanded our search terms to include nasal intermittent mandatory ventilation, NIMV, nasal distending pressure, nasal positive pressure, nasal ventilation, non‐invasive positive pressure ventilation, synchronized intermittent mandatory ventilation, SIMV, nasopharyngeal synchronized intermittent mandatory ventilation, bilevel CPAP, BiCPAP, BiPAP, and SiPAP. We amended the search dates to include articles written between protocol publication and the official search day for the review. Because we found the original requirement of infant enrollment in studies before the age of six hours to be too stringent, we relaxed the criteria to include studies in which nasal ventilation was described as "prophylactic" or "early."
We added methods and plans for "Summary of findings" tables and GRADE recommendations, which were not included in the original protocol.
Contributions of authors
ML, CB, and BL prepared the protocol for this review.
ML and CB performed a preliminary review and meta‐analysis; BL updated the literature search, made independent quality assessments, and extracted data before comparing results and resolving differences for the final review.
All review authors participated in data analysis and interpretation of results of the updated review.
Sources of support
Internal sources
No sources of support supplied
External sources
This work was supported, in part, by NIH grant number T35DK007386, USA.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
Review authors acknowledge no implied or actual potential conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Armanian 2014 {published data only}
- Armanian AM, Badiee Z, Heidari G, Feizi A, Salehimehr N. Initial treatment of respiratory distress syndrome with nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure: a randomized controlled trial. International Journal of Preventive Medicine 2014;5(12):1543‐51. [PUBMED: 25709790] [PMC free article] [PubMed] [Google Scholar]
Bisceglia 2007 {published and unpublished data}
- Bisceglia M, Belcastro A, Poerio V, Raimondi F, Mesuraca L, Crugliano C, et al. A comparison of nasal intermittent versus continuous positive pressure delivery for the treatment of moderate respiratory distress syndrome in preterm infants. Minerva Pediatrica 2007;59(2):91‐5. [PubMed] [Google Scholar]
Kirpalani 2013 {unpublished data only}
- Kirpalani H, Millar D, Lemyre B, Yoder BA, Chiu A, Roberts RS. NIPPV Study Group. A trial comparing noninvasive ventilation strategies in preterm infants. New England Journal of Medicine 2013;369(7):611‐20. [DOI] [PubMed] [Google Scholar]
Kugelman 2007 {published data only}
- Kugelman A, Feferkorn I, Riskin A, Chistyakov I, Kaufman B, Bader D. Nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure for respiratory distress syndrome: a randomized, controlled, prospective study. Journal of Pediatrics 2007;150(5):521‐6. [DOI] [PubMed] [Google Scholar]
Lista 2009 {published data only}
- Lista G, Castoldi F, Fontana P, Daniele I, Cavigioli F, Rossi S, et al. Nasal continuous positive airway pressure (CPAP) versus bi‐level nasal CPAP in preterm babies with respiratory distress syndrome: a randomised control trial. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2009;95(2):F85‐9. [DOI] [PubMed] [Google Scholar]
Meneses 2011 {published and unpublished data}
- Meneses J, Bhandari V, Alves JG, Herrmann D. Noninvasive ventilation for respiratory distress syndrome: a randomized controlled trial. Pediatrics 2011;127(2):300‐7. [DOI] [PubMed] [Google Scholar]
Ramanathan 2012 {published data only}
- Ramanathan R, Sekar KC, Rasmussen M, Bathia J, Soll RF. Nasal intermittent positive pressure ventilation after surfactant treatment for respiratory distress syndrome in preterm infants under 30 weeks gestation: a randomized controlled trial. Journal of Perinatology 2012;32(5):336‐43. [DOI] [PubMed] [Google Scholar]
Sai Sunil Kishore 2009 {published data only}
- Sai Sunil Kishore M, Dutta S, Kumar P. Early nasal intermittent positive pressure ventilation versus continuous positive airway pressure for respiratory distress syndrome. Acta Paediatrica 2009;98(9):1412‐5. [DOI] [PubMed] [Google Scholar]
Salama 2015 {published data only}
- Salama GS, Ayyash FF, Al‐Rabadi AJ, Alquran ML, Shakkoury AG. Nasal‐IMV versus nasal‐CPAP as an initial mode of respiratory support for premature infants with RDS: a prospective randomized clinical trial. Rawal Journal Medical 2015;40(2):197‐202. [Google Scholar]
Wood 2013 {published data only}
- Wood FE, Gupta S, Tin W, Sinha S. Randomised controlled trial of synchronised intermittent positive airway pressure (SiPAP) versus continuous positive airway pressure (CPAP) as a primary mode of respiratory support in preterm infants with respiratory distress syndrome. Archives of Disease in Childhood 2013;98(Suppl 1):A1‐117. [Google Scholar]
References to studies excluded from this review
Aghai 2006 {published data only}
- Aghai ZH, Saslow JG, Nakhla T, Milcarek BB, Hart J, Lawrysh‐Plunkett R, et al. Synchronized nasal intermittent positive pressure ventilation (SNIPPV) decreases work of breathing (WOB) in premature infants with respiratory distress syndrome (RDS) compared to nasal continuous positive airway pressure (NCPAP). Pediatric Pulmonology 2006;41(9):875‐81. [DOI] [PubMed] [Google Scholar]
Baneshi 2014 {published data only}
- Baneshi MR, Bahmanbijari B, Mahdian R, Haji‐Maghsoodi S, Nikbakht R. Comparison of nasal intermittent positive pressure ventilation and nasal continuous positive airway pressure treatments using parametric survival models. Iranian Journal of Pediatrics 2014;24(2):207‐13. [PUBMED: 25535541] [PMC free article] [PubMed] [Google Scholar]
Barrington 2001 {published data only}
- Barrington KJ, Bull D, Finer NN. Randomized trial of nasal synchronized intermittent mandatory ventilation compared with continuous positive airway pressure after extubation of very low birth weight infants. Pediatrics 2001;107(4):638‐41. [PUBMED: 11335736] [DOI] [PubMed] [Google Scholar]
Bhandari 2007 {published data only}
- Bhandari V, Gavino RG, Nedrelow JH, Pallela P, Salvador A, Ehrenkranz RA, et al. A randomized controlled trial of synchronized nasal intermittent positive pressure ventilation in RDS. Journal of Perinatology 2007;27(11):697‐703. [DOI] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen L, Wang L, Li J, Wang N, Shi Y. Noninvasive ventilation for preterm twin neonates with respiratory distress syndrome: a randomized controlled trial. Scientific Reports 2015;5:14483. [PUBMED: 26399752] [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedlich 1999 {published data only}
- Friedlich P, Lecart C, Posen R, Ramicone E, Chan L, Ramanathan R. A randomized trial of nasopharyngeal‐synchronised intermittent mandatory ventilation versus nasopharyngeal continuous positive airway pressure in very low birth weight infants following extubation. Journal of Perinatology 1999;19(6 Pt 1):413‐8. [DOI] [PubMed] [Google Scholar]
Gao 2010 {published data only}
- Gao WW, Tan SZ, Chen YB, Zhang Y, Wang Y. Randomized trial of nasal synchronized intermittent mandatory ventilation compared with nasal continuous positive airway pressure in preterm infants with respiratory distress syndrome. Zhongguo Dang Dai Er Ke Za Zhi (Chinese Journal of Contemporary Pediatrics) 2010;12(7):524‐6. [PUBMED: 20637147] [PubMed] [Google Scholar]
Gizzi 2015 {published data only}
- Gizzi C, Montecchia F, Panetta V, Castellano C, Mariani C, Campelli M, et al. Is synchronised NIPPV more effective than NIPPV and NCPAP in treating apnoea of prematurity (AOP)? A randomised cross‐over trial. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2015;100(1):F17‐23. [PUBMED: 25318667] [DOI] [PubMed] [Google Scholar]
Herber‐Jonat 2006 {published data only}
- Herber‐Jonat S, Rieger‐Fackeldey E, Hummler H, Schulze A. Adaptive mechanical backup ventilation for preterm infants on respiratory assist modes ‐ a pilot study. Intensive Care Medicine 2006;32(2):302‐8. [DOI] [PubMed] [Google Scholar]
Jasani 2016 {published data only}
- Jasani B, Nanavati R, Kabra N, Rajdeo S, Bhandari V. Comparison of non‐synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as post‐extubation respiratory support in preterm infants with respiratory distress syndrome: a randomized controlled trial. Journal of Maternal and Fetal Medicine 2016;29(10):1546‐51. [DOI] [PubMed] [Google Scholar]
Kahramaner 2014 {published data only}
- Kahramaner Z, Erdemir A, Turkoglu E, Cosar H, Sutcuoglu S, Ozer EA. Unsynchronized nasal intermittent positive pressure versus nasal continuous positive airway pressure in preterm infants after extubation. Journal of Maternal and Fetal Neonatal Medicine 2014;27(9):926‐9. [DOI] [PubMed] [Google Scholar]
Khalaf 2001 {published data only}
- Khalaf MN, Brodsky N, Hurley J, Bhandari V. A prospective randomised controlled trial comparing synchronized nasal intermittent positive pressure ventilation (SNIPPV) versus nasal continuous positive airway pressure (NCPAP) as mode of extubation. Pediatric Research 1999;45:204a. [DOI] [PubMed] [Google Scholar]
- Khalaf MN, Brodsky N, Hurley J, Bhandari V. A prospective randomized, controlled trial comparing synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as modes of extubation. Pediatrics 2001;108(1):13‐7. [PUBMED: 11433048] [DOI] [PubMed] [Google Scholar]
Khorana 2008 {published data only}
- Khorana M, Paradeevisut H, Sangtawesin V, Kanjanapatanakul W, Chotigeat U, Ayutthaya JK. A randomized trial of non‐synchronized nasopharyngeal intermittent mandatory ventilation (nsNIMV) vs. nasal continuous positive airway pressure (nCPAP) in the prevention of extubation failure in preterm under 1500 grams. Journal of the Medical Association of Thailand 2008;91(3):S136‐42. [PubMed] [Google Scholar]
Kugelman 2014a {published data only}
- Kugelman A, Riskin A, Said W, Shoris I, Mor F, Bader D. A randomized pilot study comparing heated humidified high‐flow nasal cannulae with NIPPV for RDS. Pediatric Pulmonology 2015;50(6):576‐83. [PUBMED: 24619945] [DOI] [PubMed] [Google Scholar]
Lin 1998 {published data only}
- Lin CH, Wang ST, Lin YJ, Yeh TF. Efficacy of nasal intermittent positive pressure ventilation in treating apnea of prematurity. Pediatric Pulmonology 1998;26(5):349‐53. [DOI] [PubMed] [Google Scholar]
Lin 2011 {published data only}
- Lin XZ, Zheng Z, Lin YY, Lai JD, Li YD. [Nasal synchronized intermittent positive pressure ventilation for the treatment of apnea in preterm infants]. Zhongguo Dang Dai Er Ke Za Zhi (Chinese Journal of Contemporary Pediatrics) 2011;13(10):783‐6. [PUBMED: 22000430] [PubMed] [Google Scholar]
Liu 2003 {published data only}
- Liu T, Tong F, Du LZ, Shi LP. Clinical observation of variable‐flow nasal continuous positive airway pressure in preterm neonates with respiratory failure. Zhonghua Er Ke Za Zhi (Chinese Journal of Pediatrics) 2003;41(6):473‐4. [PubMed] [Google Scholar]
Manzar 2004 {published data only}
- Manzar S, Nair AK, Pai MG, Paul J, Manikoth P, Georage M, et al. Use of nasal intermittent positive pressure ventilation to avoid intubation in neonates. Saudi Medical Journal 2004;25(10):1464‐7. [PubMed] [Google Scholar]
Migliori 2005 {published data only}
- Migliori C, Motta M, Angeli A, Chirico G. Nasal bilevel vs. continuous positive airway pressure in preterm infants. Pediatric Pulmonology 2005;40(5):426‐30. [DOI] [PubMed] [Google Scholar]
Moretti 2008 {published data only}
- Moretti C, Giannini L, Fassi C, Gizzi C, Papoff P, Colarizi P. Nasal flow‐synchronized intermittent positive pressure ventilation to facilitate weaning in very low‐birthweight infants: unmasked randomized controlled trial. Pediatrics International 2008;50(1):85‐91. [PUBMED: 18279212] [DOI] [PubMed] [Google Scholar]
O'Brien 2012 {published data only}
- O'Brien K, Campbell C, Havlin L, Wenger L, Shah V. Infant flow biphasic nasal continuous positive airway pressure (BP‐ NCPAP) vs. infant flow NCPAP for the facilitation of extubation in infants' ≤ 1,250 grams: a randomized controlled trial. BMC Pediatrics 2012;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pantalitschka 2009 {published data only}
- Pantalitschka T, Sievers J, Urschitz MS, Herberts T, Reher C, Poets CF. Randomised crossover trial of four nasal respiratory support systems for apnoea of prematurity in very low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2009;94(4):F245‐8. [PUBMED: 19131432] [DOI] [PubMed] [Google Scholar]
Ramanathan 2009 {unpublished data only}
- Ramanathan R, Sekar K, Rasmussen M, Bhatia J, Soll R. Nasal intermittent positive pressure ventilation (NIPPV) versus synchronized intermittent mandatory ventilation (SIMV) after surfactant treatment for respiratory distress syndrome (RDS) in preterm infants <30 weeks' gestation: multicenter, randomized, clinical trial. Pediatric Academic Society Annual Meeting. 2009; Vol. Oral Presentation:3212.6.
Ryan 1989 {published data only}
- Ryan CA, Finer NN, Peters KL. Nasal intermittent positive‐pressure ventilation offers no advantages over nasal continuous positive airway pressure in apnea of prematurity. American Journal of Diseases of Children 1989;143(10):1196‐8. [DOI] [PubMed] [Google Scholar]
Salvo 2015 {published data only}
- Salvo V, Lista G, Lupo E, Ricotti A, Zimmermann LJ, Gavilanes AW, et al. Noninvasive ventilation strategies for early treatment of RDS in preterm infants: an RCT. Pediatrics 2015;135(3):444‐51. [DOI] [PubMed] [Google Scholar]
Santin 2004 {published data only}
- Santin R, Brodsky N, Bhandari V. A prospective observational pilot study of synchronized nasal intermittent positive pressure ventilation (SNIPPV) as a primary mode of ventilation in infants ≥ 28 weeks with respiratory distress syndrome (RDS). Journal of Perinatology 2004;24(8):487‐93. [DOI] [PubMed] [Google Scholar]
Shi 2010 {published data only}
- Shi Y, Tang S, Zhao J, Hu Z, Li T. Efficiency of nasal intermittent positive pressure ventilation vs nasal continuous positive airway pressure on neonatal respiratory distress syndrome: a prospective, randomized, controlled study. Acta Academiae Medicinae Militaris Tertiae 2010;32(18):1991‐3. [Google Scholar]
Shi 2014 {published data only}
- Shi Y, Tang S, Zhao J, Shen J. A prospective, randomized, controlled study of NIPPV versus nCPAP in preterm and term infants with respiratory distress syndrome. Pediatric Pulmonology 2014;49(7):673‐8. [DOI] [PubMed] [Google Scholar]
Zhou 2015 {published data only}
- Zhou B, Zhai JF, Jiang HX, Liu Y, Jin B, Zhang YY, et al. Usefulness of DuoPAP in the treatment of very low birth weight preterm infants with neonatal respiratory distress syndrome. European Review for Medical and Pharmacological Sciences 2015;19(4):573‐7. [PUBMED: 25753873] [PubMed] [Google Scholar]
References to studies awaiting assessment
Chen 2013 {published data only}
- Chen X, Peng WS, Wang L, Xu JL, Dong HF, Pan JH. [A randomized controlled study of nasal intermittent positive pressure ventilation in the treatment of neonatal respiratory distress syndrome].. Zhongguo Dang Dai Er Ke Za Zhi (Chinese Journal of Contemporary Pediatrics) 2013;15(9):713‐7. [PubMed] [Google Scholar]
Fu 2014 {published data only}
- Fu CH, Xia SW. Clinical application of nasal intermittent positive pressure ventilation in initial treatment of neonatal respiratory distress syndrome. Zhongguo Dang Dai Er Ke Za Zhi (Chinese Journal of Contemporary Pediatrics) 2014;16(5):460‐4. [PUBMED: 24856992] [PubMed] [Google Scholar]
Gao 2014 {published data only}
- Gao X, Yang B, Hei M, Cui X, Wang J, Zhou G, et al. Application of three kinds of non‐invasive positive pressure ventilation as a primary mode of ventilation in premature infants with respiratory distress syndrome: a randomized controlled trial. Zhonghua Dang Er Ke Za Zhi (Chinese Journal of Contemporary Pediatrics) 2014;52(1):34‐40. [PUBMED: 24680406] [PubMed] [Google Scholar]
Kong 2012 {published data only}
- Kong LK, Kong XY, Li LH, Dong JY, Shang MX, Chi JH, et al. Comparative study on application of Duo positive airway pressure and continuous positive airway pressure in preterm neonates with respiratory distress syndrome. Zhongguo Dang Dai Er Ke Za Zhi (Chinese Journal of Contemporary Pediatrics) 2012;14(12):888‐92. [PUBMED: 23234771] [PubMed] [Google Scholar]
Sasi 2013 {published data only}
- Sasi A, Skariah T, Lewis L. Early nasal intermittent mandatory ventilation (NIMV) versus nasal continuous positive airway pressure (NCPAP) for respiratory distress syndrome (RDS) in infants 28 to 36 weeks gestation ‐ a randomized controlled trial. Journal of Paediatrics and Child Health 2013;49(Suppl 2):34‐5. [Google Scholar]
Silveira 2015 {published data only}
- Silveira CS, Leonardi KM, Melo AP, Zaia JE, Brunherotti MA. Response of preterm infants to 2 noninvasive ventilatory support systems: nasal CPAP and nasal intermittent positive‐pressure ventilation. Respiratory Care 2015;60(12):1772‐6. [PUBMED: 26374907] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Sabzehei 2015 {published data only}
- Early nasal intermittent positive pressure ventilation vs continuous positive airway pressure in preterm infants with respiratory distress syndrome. Ongoing study Starting date of trial not provided. Contact author for more information.
Additional references
Avery 1987
- Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics 1987;79(1):26‐30. [PubMed] [Google Scholar]
Bancalari 2001
- Bancalari E, Claure N. Changes in the pathogenesis and prevention of chronic lung disease of prematurity. American Journal of Perinatology 2001;18(1):1‐9. [DOI] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cappelleri 1996
- Cappelleri JC, Ioannidis JP, Schmid CH, Ferranti SD, Aubert M, Chalmers TC, et al. Large trials vs meta‐analysis of smaller trials: how do their results compare?. Journal of the American Medical Association 1996;276(16):1332‐8. [PubMed] [Google Scholar]
Finer 2004
- Finer NN, Carlo WA, Duara S, Fanaroff AA, Donovan EF, Wright LL, et al. Delivery room continuous positive airway pressure/positive end‐expiratory pressure in extremely low birth weight infants: a feasibility trial. Pediatrics 2004;114(3):651‐7. [DOI] [PubMed] [Google Scholar]
Garland 1985
- Garland JS, Nelson DB, Rice T, Neu J. Increased risk of gastrointestinal perforations in neonates mechanically ventilated with either face mask or nasal prongs. Pediatrics 1985;76(3):406‐10. [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro [Version 3.2 for Windows]. The GRADE Working Group, 2008.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
ICCROP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991‐9. [DOI] [PubMed] [Google Scholar]
Kiciman 1998
- Kiciman NM, Andréasson B, Bernstein G, Mannino FL, Rich W, Henderson C, et al. Thoracoabdominal motion in newborns during ventilation delivered by endotracheal tube or nasal prongs. Pediatric Pulmonology 1998;25(3):175‐81. [DOI] [PubMed] [Google Scholar]
Lemyre 2002
- Lemyre B, Davis PG, Paoli AG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for apnea of prematurity. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD002272] [DOI] [PubMed] [Google Scholar]
Lemyre 2014
- Lemyre B, Paoli AG, Kirpalani H, Davis PG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD003212] [DOI] [PubMed] [Google Scholar]
Meyer 2004
- Meyer M, Mildenhall L, Wong M. Outcomes for infants weighing less than 1000 grams cared for with a nasal continuous positive airway pressure‐based strategy. Journal of Paediatrcs and Child Health 2004;40(1):38‐41. [DOI] [PubMed] [Google Scholar]
Moretti 1999
- Moretti C, Gizzi C, Papoff P, Lampariello S, Capoferri M, Calcagnini G, et al. Comparing the effects of nasal synchronized intermittent positive pressure ventilation (nSIPPV) and nasal continuous positive airway pressure (nCPAP) after extubation in very low birth weight infants. Early Human Development 1999;56(2‐3):166‐77. [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GWG. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. www.guidelinedevelopment.org/handbook Updated October 2013.
Stoelhorst 2005
- Stoelhorst GM, Rijken M, Martens SE, Brand R, Ouden AL, Wit JM, et al. Changes in neonatology: comparison of two cohorts of very preterm infants (gestational age <32 weeks): the Project On Preterm and Small for Gestational Age Infants 1983 and the Leiden Follow‐Up Project on Prematurity 1996‐1997. Pediatrics 2005;115(2):396‐405. [DOI] [PubMed] [Google Scholar]
Van Marter 2000
- Marter LJ, Allred EN, Pagano M, Sanocka U, Parad R, Moore M, et al. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network. Pediatrics 2000;105(6):1194‐201. [DOI] [PubMed] [Google Scholar]
Villar 1995
- Villar J, Carroli G, Belizán JM. Predictive ability of meta‐analyses of randomised controlled trials. Lancet 1995;345(8952):772‐6. [DOI] [PubMed] [Google Scholar]


