Abstract
Background
Oxygen (O2) is widely used in people with acute myocardial infarction (AMI). Previous systematic reviews concluded that there was insufficient evidence to know whether oxygen reduced, increased or had no effect on heart ischaemia or infarct size. Our first Cochrane review in 2010 also concluded there was insufficient evidence to know whether oxygen should be used. Since 2010, the lack of evidence to support this widely used intervention has attracted considerable attention, prompting further trials of oxygen therapy in myocardial infarction patients. It is thus important to update this Cochrane review.
Objectives
To assess the effects of routine use of inhaled oxygen for acute myocardial infarction (AMI).
Search methods
We searched the following bibliographic databases on 6 June 2015: the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE (OVID), Embase (OVID), CINAHL (EBSCO) and Web of Science (Thomson Reuters). LILACS (Latin American and Caribbean Health Sciences Literature) was last searched in September 2016. We also contacted experts to identify eligible studies. We applied no language restrictions.
Selection criteria
Randomised controlled trials in people with suspected or proven AMI (ST‐segment elevation myocardial infarction (STEMI) or non‐STEMI) within 24 hours after onset, in which the intervention was inhaled oxygen (at normal pressure) compared to air, regardless of co‐therapies provided to participants in both arms of the trial.
Data collection and analysis
Two authors independently reviewed the titles and abstracts of identified studies to see if they met the inclusion criteria and independently undertook the data extraction. We assessed the quality of studies and the risk of bias according to guidance in the Cochrane Handbook for Systematic Reviews of Interventions. The primary outcome was death. The measure of effect used was the risk ratio (RR) with a 95% confidence interval (CI). We used the GRADE approach to evaluate the quality of the evidence and the GRADE profiler (GRADEpro) to import data from Review Manager 5 and create 'Summary of findings' tables.
Main results
The updated search yielded one new trial, for a total of five included studies involving 1173 participants, 32 of whom died. The pooled risk ratio (RR) of all‐cause mortality in the intention‐to‐treat analysis was 0.99 (95% CI 0.50 to 1.95; 4 studies, N = 1123; I2 = 46%; quality of evidence: very low) and 1.02 (95% CI 0.52 to 1.98; 4 studies, N = 871; I2 = 49%; quality of evidence: very low) when only analysing participants with confirmed AMI. One trial measured pain directly, and two others measured it by opiate usage. The trial showed no effect, with a pooled RR of 0.97 for the use of opiates (95% CI 0.78 to 1.20; 2 studies, N = 250). The result on mortality and pain are inconclusive. There is no clear effect for oxygen on infarct size (the evidence is inconsistent and low quality).
Authors' conclusions
There is no evidence from randomised controlled trials to support the routine use of inhaled oxygen in people with AMI, and we cannot rule out a harmful effect. Given the uncertainty surrounding the effect of oxygen therapy on all‐cause mortality and on other outcomes critical for clinical decision, well‐conducted, high quality randomised controlled trials are urgently required to inform guidelines in order to give definitive recommendations about the routine use of oxygen in AMI.
Plain language summary
Routine use of oxygen in people who have had a heart attack
Background
Many people who are having a heart attack are routinely given oxygen to breathe.
Review question
We looked for the evidence to support this longstanding practice by searching for randomised controlled trials that compared the outcomes for people given oxygen versus normal air to breathe. We were primarily interested in seeing whether there was a difference in the number of people who died, but we also looked at whether administering oxygen reduced pain or other adverse outcomes.
Key results
We found five randomised controlled trials that compared people with suspected or proven heart attack who were given oxygen to a similar group of people who were given air (evidence is current to June 2016). These trials involved a total of 1173 participants, 32 of whom died. There were similar death rates in both groups, suggesting oxygen neither helps nor harms, but the trials are not big enough to know for sure. Moreover, it is possible that more heart muscle might be damaged in people given oxygen than in people given air.
Conclusion
Since there is no evidence whether the oxygen is good or harmful in this clinical condition, it is important to test oxygen in a big trial as soon as possible to be sure that this common treatment is doing more good than harm in people who are having a heart attack.
Summary of findings
Summary of findings for the main comparison. Oxygen versus air for acute myocardial infarction.
| Oxygen versus air for acute myocardial infarction | ||||||
|
Patient or population: people with acute myocardial infarction
Settings: pre‐hospital and hospital
Intervention: oxygen Comparison: air | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Air (or titrated oxygen) | Oxygen | |||||
| All‐cause mortality in hospital for participants with AMI Follow‐up: 4 weeks | Study population | RR 1.02 (0.52 to 1.98) | 871 (4 studies) | ⊕⊝⊝⊝ Very lowa | — | |
| 36 per 1000 | 37 per 1000 (19 to 71) | |||||
| Moderate population | ||||||
| 34 per 1000 | 35 per 1000 (18 to 67) | |||||
|
All‐cause mortality in hospital for all participants (including those without confirmed AMI) Follow‐up: 4 weeks |
Study population | RR 0.99 (0.50 to 1.95) | 1123 (4 studies) | ⊕⊝⊝⊝ Very lowb | — | |
| 28 per 1000 | 28 per 1000 (14 to 55) | |||||
| Moderate population | ||||||
| 29 per 1000 | 29 per 1000 (15 to 57) | |||||
| All‐cause mortality in hospital for all participants (including those without confirmed AMI) trials done in the revascularisation era Follow‐up: 4 weeks | Study population population | RR 0.58 (0.24 to 1.39) | 923 (3 studies) | ⊕⊕⊝⊝ Lowc | — | |
| 27 per 1000 | 16 per 1000 (7 to 38) | |||||
| Moderate population | ||||||
| 26 per 1000 | 15 per 1000 (6 to 36) | |||||
| Opiate use (as a proxy measure for pain) for all participants on ITT (including those without confirmed AMI) Follow‐up: 4 weeks | Study population | RR 0.97 (0.78 to 1.20) | 250 (2 studies) | ⊕⊕⊝⊝ Lowd | ||
| 583 per 1000 | 566 per 1000 (455 to 700) | |||||
| Moderate population | ||||||
| 634 per 1000 | 615 per 1000 (495 to 761) | |||||
|
Recurrent myocardial infarction (or ischaemia) Follow‐up 4 weeks |
Study population | RR 1.67 (0.94 to 2.99) | 578 (2 studies) | ⊕⊕⊕⊝ Lowe | — | |
| 64 per 1000 | 87 per 1000 (50 to 152) | |||||
| Moderate population | ||||||
| 140 per 1000 | 190 per 1000 (109 to 333) | |||||
| Infarct size CK and other enzymes | See comment | See comment | Not estimable | 0 (2 studies) | ⊕⊝⊝⊝ Low | The are slight inconsistencies between 2 trials with respect to the effect of oxygen on CK levels (Ukholkina 2005 and Stub 2015). There are inconsistency in the effect of oxygen on CK levels and the effect on troponin I in Stub 2015 (whitin study inconsistency). |
|
Infarct size by MRI (estimated 6 months after AMI) |
See comment | See comment | Not estimable | 0 (2 studies) | ⊕⊝⊝⊝ Very low | The evidence for this outcome comes from 2 randomised trials but in 'selected' groups of patients,. As the data comes from with non‐randomised comparisons and was performed 6 months after AMI, we considered them unsuitable for quantitative synthesis. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AMI: acute myocardial infarction; CI: confidence interval; MRI: magnetic resonance imaging; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aThe evidence for this outcome has very serious limitations due to incomplete outcomes data in 2 of the 4 included studies (Ranchord 2012; Ukholkina 2005); downgraded 2 levels. An additional level is applied for imprecision. bDowngraded 2 levels for very serious limitations due to incomplete data outcomes in 2 of 4 studies (Ranchord 2012; Ukholkina 2005). An additional point deducted for imprecision. cThe evidence for this outcome has serious limitations due to incomplete data in 2 of the 3 studies (Ranchord 2012; Ukholkina 2005), but the other one study with low risk of bias is the most weighted in meta analysis (82.3%) (Stub 2015), so the quality is downgraded 1 level. An additional point deducted for imprecision. dThe evidence for pain comes from a blinded study with unclear risk of bias and another unblinded study with high risk of bias for a subjective outcome; downgraded one level. An additional point deducted for indirectness (opiate is used as proxy for pain). eDowngraded for imprecision and for inconsistency
Background
Description of the condition
Coronary heart disease (CHD) is an important cause of death worldwide. Over 7 million people every year die from CHD, accounting for 12.8% of all deaths (WHO 2011). It is the single most common cause of death before the age of 75 in Europe (Townsend 2015), and in the USA it accounted for around one of every seven deaths in 2011 (Mozaffarian 2015), although deaths from cardiovascular disease and CHD in men and women have fallen in most developed countries. For example, rates of CHD deaths per million in men without diabetes in England fell by more than half between 1995 and 2010 (Ecclestone 2015). According to the Euro Heart Survey of acute myocardial infarction (AMI) in 47 countries (Puymirat 2013), in‐hospital mortality was 6.2%. Approximately 45% of the reduction in CHD mortality is attributable to improvement in medical therapies for coronary disease (Capewell 2000).
A common manifestation of CHD, often the first, is AMI. The third Global MI Task Force defines AMI as "any evidence of myocardial necrosis in a clinical setting consistent with acute myocardial ischaemia" (Thygesen 2012).
Myocardial ischaemia is usually the result of spontaneous complications of atherosclerosis (plaque rupture, ulceration, fissuring, erosion or dissection) resulting in coronary thrombosis (type 1 AMI). Other categories of AMI include: those produced by underlying CHD with an ischaemic imbalance attributable to a wide range of factors including endothelial dysfunction, coronary spasm, coronary embolism, tachy‐/brady‐arrhythmias and hypo‐ and hypertension (type 2 AMI); sudden cardiac death induced by myocardial ischaemia (type 3 AMI); and AMI occurring in the context of invasive coronary procedures such as percutaneous coronary intervention (PCI), in‐stent thrombosis, or coronary artery bypass grafting (CABG), categorised as subtypes 4a, 4b and 5 of AMI. By far the most common types of AMI are types 1 and 2, to such an extent that their incidence may be used as proxy variables to estimate the prevalence of CHD in the general population. Hereafter we will use the term 'AMI' to refer the type 1 and type 2 AMI.
Myocardial injury may be detected through: highly sensitive biochemical markers such as troponin (I or T), or the MB fraction of the creatine kinase (CK‐MB); electrocardiographic changes; or imaging techniques such as echocardiography, magnetic resonance imaging (MRI) or radionuclide imaging. Necessary criteria to diagnose AMI in a clinical context include a change (rise and/or fall) in cardiac biomarker values, together with at least one of the following: ischaemic symptoms, typical electrocardiographic changes, or abnormalities in the structure or wall motion of the heart identified by imaging techniques.
Moreover, the recognition that acute coronary syndromes represent a spectrum of pathophysiological processes rather than a uniform type of 'heart attack' has led to publication of separate guidelines with different therapeutic options for AMI presenting with persistent ST‐segment elevation (STEMI) and non‐STEMI (NSTEMI) presentations.
The in‐hospital mortality rate of unselected STEMI patients according to the Euro Heart Survey, published by the European Society of Cardiology, varies between 6% and 14% (Mandelzweig 2006). The most serious complications of AMI are cardiogenic shock, heart failure, ventricular fibrillation and recurrent ischaemia. Around 8% of people with AMI develop cardiogenic shock (Babaev 2005), but this remains present in 29% of those people on admission to hospital. The Global Registry of Acute Coronary Events (GRACE) reported that heart failure occurred in 15.6% of people with STEMI and 15.7% of those with NSTEMI, but heart failure was present in only 13% of these patients on admission to hospital (Steg 2004). Ventricular fibrillation occurred in 1.9% of people with AMI (Goldberg 2008), and 21% of those with acute coronary syndromes presented with recurrent ischaemia (Yan 2010), about half of whom experienced this outcome in the first 24 hours. Other possible complications of AMI include pericarditis, mitral insufficiency, arrhythmias and conduction disturbances.
The cornerstone of contemporary management of people with STEMI is reperfusion therapy, with either primary percutaneous coronary intervention (PCI) or thrombolytic treatment if less than 12 hours has elapsed from the onset of symptoms. Other recommended treatments in international guidelines include morphine, oxygen (O2), nitrates and aspirin (MONA) (O'Connor 2010; O'Gara 2013; Steg G 2012). Some of these treatments have a well‐established research base, while others do not (Nikolaou 2012; O'Driscoll 2008; SIGN 2010).
Description of the intervention
Inhaled oxygen at normal pressure delivered by face mask or nasal cannula, at any concentration.
How the intervention might work
Myocardial infarction occurs when the flow of oxygenated blood in the heart is interrupted for a sustained period of time. The rationale for providing supplemental oxygen to a person with AMI is that it may improve the oxygenation of the ischaemic myocardial tissue and reduce ischaemic symptoms (pain), infarct size and consequent morbidity and mortality.
Why it is important to do this review
Although it is biologically plausible that oxygen is helpful, it is also biologically plausible that it may be harmful. Potentially harmful mechanisms include the paradoxical effect of oxygen in reducing coronary artery blood flow and increasing coronary vascular resistance, measured by intracoronary Doppler ultrasonography (McNulty 2005; McNulty 2007); reduced stroke volume and cardiac output (Milone 1999); other adverse haemodynamic consequences, such as increased vascular resistance from hyperoxia; and reperfusion injury from increased oxygen free radicals (Rousseau 2005), which may also have adverse electrophysiological effects, triggering lethal arrhythmias (Xie 2009).
A systematic review of human studies that included non‐randomised studies did not confirm that oxygen administration diminishes acute myocardial ischaemia (Nicholson 2004). Indeed, some evidence suggested that oxygen may increase myocardial ischaemia (Nicholson 2004). Another narrative review of oxygen therapy also sounded a cautionary note (Beasley 2007). It referenced a randomised controlled trial (RCT) conducted in 1976 showing that the risk ratio (RR) of death was 2.89 (95% confidence interval (CI) 0.81 to 10.27) in participants receiving oxygen compared to those breathing air (Rawles 1976). While this suggested that oxygen may be harmful, the increased risk of death could easily have been a chance finding. A systematic review looked at the effect of oxygen on infarct size in people with AMI and concluded that "[t]here is little evidence by which to determine the efficacy and safety of high flow oxygen therapy in MI. The evidence that does exist suggests that the routine use of high flow oxygen in uncomplicated AMI may result in a greater infarct size and possibly increase the risk of mortality" (Wijesinghe 2009).
Despite this lack of robust evidence of effectiveness prior to the publication of our 2010 Cochrane review of the evidence, international guidelines widely recommended oxygen administration (AARC 2002; AHA 2005; Anderson 2007; Antman 2002; ILCOR 2005; Van de Werf 2008). Some guidelines were more cautious; for example, the European guideline did not recommend routine oxygen use in acute coronary syndrome (ACS) (Bassand 2007), and the Scottish Intercollegiate Guidelines Network (SIGN) guidance only recommended oxygen use in hypoxaemia (< 90% saturation), noting that there was no clinical evidence for its effectiveness and referring to animal models that showed a reduction in infarct size (SIGN 2007).
Guidelines published since the 2010 Cochrane review have tended to move to a more cautious position reflecting the lack of evidence. In 2010, for example, the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular care stated that: "EMS providers administer oxygen during the initial assessment of patients with suspected ACS. However, there is insufficient evidence to support its routine use in uncomplicated ACS. If the patient is dyspnoeic, hypoxaemic, or has obvious signs of heart failure, providers should titrate therapy, based on monitoring of oxyhaemoglobin saturation, to 94% (class I, level of evidence: C). Updated SIGN guidance states, "A Cochrane review found no conclusive evidence from randomised controlled trials to support the routine use of inhaled oxygen in patients with AMI. There is no evidence that routine administration of oxygen to all patients with the broad spectrum of acute coronary syndromes improves clinical outcome or reduces infarction size" (SIGN 2010).
In 2011 an addendum to the National Heart Foundation of Australia/Cardiac Society of Australia and New Zealand Guidelines for the Management of Acute Coronary Syndromes (ACS), authors stated that "There is currently insufficient evidence to formulate clear recommendations about oxygen therapy . . . Definitive trials are needed to answer this question" (Chew 2011).
Similarly, the 2012 ESC guidelines for STEMI, citing the Cochrane review, now state: "Oxygen (by mask or nasal prongs) should be administered to those who are breathless, hypoxic, or who have heart failure. Whether oxygen should be systematically administered to patients without heart failure or dyspnoea is at best uncertain. Noninvasive monitoring of blood oxygen saturation greatly helps when deciding on the need to administer oxygen or ventilator support" (Steg G 2012).
The 2013 ACCF/AHA Guideline for the Management of ST Elevation Myocardial Infarction shows a similar change in emphasis: "Few data exist to support or refute the value of the routine use of oxygen in the acute phase of STEMI, and more research is needed. A pooled Cochrane analysis of 3 trials showed a 3‐fold higher risk of death for patients with confirmed AMI treated with oxygen than for patients with AMI managed on room air. Oxygen therapy is appropriate for patients who are hypoxaemic (oxygen saturation < 90%) and may have a salutary placebo effect in others. Supplementary oxygen may, however, increase coronary vascular resistance. Oxygen should be administered with caution to patients with chronic obstructive pulmonary disease and carbon dioxide retention". (O'Gara 2013).
The British Heart Foundation (BHF), in response to the doubts about oxygen use raised by Beasley 2007, originally stated in an article in The Guardian in 2007 that "[t]he current practice of giving high‐flow oxygen is an important part of heart attack treatment. Best practice methods have been developed and refined over the years to ensure the best possible outcome for patients. There is not enough evidence to change the current use of oxygen therapy in heart attacks". Five years after the publication of the first Cochrane Review, the use of oxygen in AMI and across the spectrum of coronary acute syndromes is still controversial (Shuvy 2013). We think that, given the evidence cited, it would have been more appropriate to conclude that despite decades of use there is inadequate clinical trial evidence to unequivocally support routine administration of oxygen. The BHF subsequently stated that the 2010 Cochrane review "highlights the need for more research into the effects of oxygen when it is given during a heart attack. Until recently, heart attack patients were routinely treated with oxygen but we simply do not have enough evidence to know if that treatment is beneficial or harmful" (BHF 2010).
Despite the attention given to the uncertainty around the role of oxygen since our 2010 Cochrane review, practice appears to vary, possibly because the evidence base informing current guideline recommendations remains uncertain. A survey of 231 cardiac care units in the UK undertaken shortly after the 2010 review reported that only a third adhered to guideline recommendations to titrate oxygen to saturation rather than administer routinely, and practice was no different in hospitals that had formal oxygen therapy policies versus those that did not (Ripley 2012).
With the lack of collective certainty about the use of oxygen, a number of clinical trials are now underway or have recently been reported to reassess this treatment. In general, practice should not be based on tradition but on proven benefit and safety. Given that the 1976 trial was suggestive of potential harm from oxygen in suspected AMI (Rawles 1976), it is important to systematically review and update the evidence base for current and future guidance regarding the role of oxygen therapy in heart attack patients, and if necessary, to undertake further research to clarify whether this intervention does more harm than good. If the only robust evidence is suggestive of potentially serious harm, even if the result is not statistically significant, it reinforces our opinion that this intervention should not be routinely used, however sound the pathophysiological reasoning.
Objectives
To assess the effects of routine use of inhaled oxygen for acute myocardial infarction (AMI). Primary outcomes include death and pain.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of parallel or cluster design, in any language, with any length of follow‐up, and with any publication status (full publication, abstract only or unpublished).
Types of participants
Adults of any age treated, in a pre‐hospital or a hospital setting, for suspected or proven AMI (STEMI or NSTEMI), within 24 hours of symptoms onset, regardless of any co‐therapy (for example a reperfusion therapy) provided to both arms of the trial.
Types of interventions
The intervention is routinely given inhaled oxygen administered by any device at normal pressure for one hour or more within 24 hours of AMI symptoms onset. The comparator is air, or air with titrated oxygen in the event of desaturation.
Excluded interventions are hyperbaric oxygen or aqueous oxygen therapy (unless the studies include arms with air or oxygen at normal pressure).
Types of outcome measures
This review is primarily focused on clinically important outcomes. To facilitate the assessment of the clinical importance of outcomes we used the nine‐point scale suggested by GRADE (Guyatt 2008), which classifies the outcomes into three levels of importance. The outcomes included in the review are type I ("critical for decision‐making"‐ ratings 9, 8, 7) and also type 2 ("important but not critical for decision‐making"‐ ratings 6, 5, 4). We did not include the type 3 outcomes: ("not important for decision‐making, of lower importance to patients" ‐ ratings 3, 2, 1). We pre‐specified mortality as the primary outcome. We agreed the point on the GRADE scale for each outcome through discussion within the review team, where we easily reached a consensus. We showed our proposed classifications to cardiologist colleagues to see whether they agreed with them. Although there were one‐point differences in some of their assessments of the importance of particular outcomes, none of these affected the level of importance into which we classified an outcome.
We classified the following outcomes as type I (the review group's consensus score is given in brackets).
All‐cause mortality (9).
Cardiac mortality (9).
Cardiac failure (8).
Stroke (8).
Recurrence of myocardial infarction or ischaemia (8).
Major bleeding (8).
Pain (7).
Revascularisation (7).
Pericarditis (7).
Arrhthymias (7).
We classified the following outcomes as type 2 outcomes.
Left ventricular function (global and segmentary) (6).
Infarct size, whether estimated using biological methods (electrocardiogram (ECG), enzymes CK, CK‐MB, troponin T or troponin I, brain natriuretic peptide (BNP)) or imaging techniques such as magnetic resonance imaging (MRI), or echocardiography (5).
We classified the following outcomes as type 3 outcomes.
ECG changes (4).
Platelet aggregation (3).
Biomarkers of oxidative stress (2).
Apoptosis (2).
Inflammation (2).
Although these outcomes may prove useful for helping understand the disease process, they currently have little implication for decision‐making or prognosis.
We used standard direct measures for all types of outcomes. For the case of pain, when the direct measurement was not available we used the opiate dosage as a proxy for pain. This approach (response to treatment) is classically used when validating pain scales. We have included type 2 outcomes because they may be used to make clinical decisions or recommendations.
Search methods for identification of studies
Electronic searches
We searched the following bibliographic databases (from inception to 6 June 2016).
Cochrane Central Register of Controlled Trials (CENTRAL, 2016, Issue 5) in the Cochrane Library.
MEDLINE In‐Process & Other Non‐Indexed Citations and MEDLINE Ovid (1946 to 6 June 2016).
Embase Ovid (1980 to 2016 week 23).
PubMed (2012 to 4 June 2015).
CINAHL Plus (EBSCO, 1937 to 6 June 2016).
Web of Science Core Collection (Thomson Reuters, 1970 to 6 June 2016).
We also searched LILACS (Latin American and Caribbean Health Sciences Literature) in BIREME (Centro Latinoamericano y del Caribe de Información en Ciencias de la Salud) from 2012 to 22 September 2016. (lilacs.bvsalud.org).
We applied the sensitivity‐maximising version of the Cochrane RCT search filter to the MEDLINE searches and its adaptations to Embase, CINAHL Plus and Web of Science (Lefebvre 2011).
We searched the following databases for ongoing trials using the search terms "(Acute myocardial infarction AND oxygen as search strategy)" (12 September 2016).
Current Controlled Trials metaRegister (www.controlled‐trials.com/mrct).
The European Union Clinical Trials Register (www.clinicaltrialsregister.eu/about.html).
International Clinical Trials Registry Platform (ICTRP), World Health Organization (www.who.int/ictrp/network/en/)
Details of the database search strategies are in Appendix 1 (for 2010), Appendix 2 (for 2012), Appendix 3 (for 2015) and Appendix 4 (for 2016).
Searching other resources
We searched proceedings of annual meetings and conferences of professional bodies (American Heart Association, British Cardiovascular Society, European Society of Cardiology and American College of Cardiology) for relevant abstracts (from August 2013 to 4 June 2015).
We contacted experts in the field to locate any unpublished studies and checked citations from key references.
We applied no date or language restrictions to the searches.
Data collection and analysis
We used the standard methods of Cochrane as described in the Cochrane Handbook for Systematic Reviews of Interventions so that the review methods are consistent with current recommendations (Higgins 2011). We used Review Manager 5 (RevMan 5) for the analysis (RevMan 2014).
Selection of studies
Two authors independently reviewed the titles and abstracts of studies identified in the searches to see if they met the above inclusion criteria. We obtained study reports in full text when inclusion could not be decided from the title or abstract.
Data extraction and management
Two authors independently evaluated the methodological quality and undertook independent data extraction using an agreed data extraction form. We resolved differences by discussion. One review author entered the data into RevMan 2014, and two others checked them.
Assessment of risk of bias in included studies
Risk of bias in individual studies
We used the two‐part tool described in section 8.5 of Higgins 2011. We explored the six specific domains: sequence generation, allocation concealment, blinding (participants, personnel and outcome assessors), incomplete outcome data, selective outcome reporting, and other potential threats to validity.
For each trial, two review authors first independently described the design characteristics relating to each domain and then judged the risk of bias associated with the main outcome. We used a nominal scale for the judgement: low, high or unclear risk of bias.
Risk of bias across studies
We did an overall assessment of risk of bias for every outcome within the review for each domain, using a similar scale: low risk of bias in all domains, unclear risk of bias for one or more domains, and high risk of bias for one or more domains.
When we undertook meta‐analysis, we summarised the risk of bias for the main outcomes across studies. We resolved disagreements between review authors in the description or in the judgement by consensus, without the need for recourse to a third review author.
Measures of treatment effect
We looked at the risk ratio (RR) of death and reported this rather than the risk difference. We also looked for differences in mean pain scores; if studies did not report these scores, we used the RR of opiate use as a proxy measure for pain intensity. We used the differences in mean for continuous measurement of infarct size such as cardiac enzymes, troponin T, BNP or MRI.
Unit of analysis issues
The earliest trial randomised 200 participants, but authors only analysed the results for the 157 who were later confirmed to have had an AMI (Rawles 1976). Ranchord 2012 also excluded five participants in whom AMI was not confirmed and seven withdrawn participants from the analysis. In the newly included trial involving 638 participants with suspected AMI, randomised by paramedic personnel in the ambulance, investigators excluded 50 for different reasons and assessed 588 for STEMI upon hospital arrival (Stub 2015). Angiography was indicated (and performed) in 470 participants with clinical diagnosis of AMI. Physicians ruled out STEMI in 29 participants (17 in the oxygen group and 12 in the air) and confirmed it in only 441 patients, in whom investigators measured the primary outcome (infarct size estimated by troponin peak cTnI and CK). The other patients were excluded from the primary analysis, but many clinical data including mortality are available.
It is legitimately open for debate whether people who did not have an AMI should be included in a study of the benefits of oxygen in AMI. Theoretically, diagnosis may be more certain today, but not at symptoms onset, and of course a hospital physician will be able to more accurately diagnose AMI than paramedics in the ambulance. On the other hand, we treat suspected MIs, and these represent some of the people to whom a treatment would be given in practice.
We have therefore performed two analyses: one in participants who had confirmed AMI in Rawles 1976, Ukholkina 2005, Ranchord 2012 and Stub 2015, and a second that also covered all participants from the trials in a strict intention‐to‐treat (ITT) analysis that included the 43 participants from Rawles 1976 who did not have an AMI confirmed, the 12 withdrawn participants from Ranchord 2012, and the 197 (of the 638 randomised participants) from Stub 2015 in which STEMI was ruled out. This was to preserve the strict randomisation process and to minimise selection bias.
Dealing with missing data
We contacted study authors for missing data.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of the outcomes tables of the different analysis and using the I2 statistic (where I2 > 50% was considered substantial or considerable heterogeneity) (Higgins 2003).
Assessment of reporting biases
As there were only five studies that met the inclusion criteria, it was not possible to explore reporting bias using funnel plots or the Begg and Egger tests (Begg 1994; Egger 1997).
Data synthesis
We undertook meta‐analyses where data were available and it was clinically sensible to do so, using both fixed‐effect and random‐effects models. We reported the results using both models because we recognise that readers may have different perspectives (for example preconceptions, values or contexts) and different people may wish to see the results with the different mathematical assumptions.
Subgroup analysis and investigation of heterogeneity
The data were too sparse to permit adequate exploration of all the subgroups that had been pre‐specified for analysis (such as timing and duration of oxygen therapy, pre‐existing levels of hypoxaemia or other measures of severity of infarction). We undertook an analysis including only the trials undertaken during the reperfusion era, as these reflect today's clinical practice. We define 'reperfusion era' as the period in which thrombolysis, PCI or CABG were generalised as the main treatment for AMI (since 1985).
Sensitivity analysis
Similarly, our intention to explore the effect of trial quality in a sensitivity analysis was limited by the number of trials and the quality of reporting. We undertook separate analyses using the confirmed AMI population and the ITT population, and undertook 'best‐case' and 'worst‐case' scenarios in sensitivity analysis for the missing data on deaths (Wilson 1997).
Summary of findings table
We created a 'Summary of findings' table for the outcomes all‐cause mortality in hospital for participants with AMI, all‐cause mortality in hospital for all participants, all‐cause mortality in hospital for all participants in trials done in the revascularisation era, opiate use as a proxy measure for pain, recurrent myocardial infarction, infarct size by CK and other enzymes, and infarct size by MRI. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes (Guyatt 2008). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions using GRADEpro software (GRADEpro; Higgins 2011). We justified all decisions to down‐ or upgrade the quality of studies using footnotes, and we made comments to aid readers' understanding of the review where necessary.
Results
Description of studies
Results of the search
We identified 204 new records with the updated search in June 2016. The removal of duplicates left 136 new records for screening. Based on title and abstract, we excluded 111 papers and retrieved 25. Ten were reviews, editorials or non‐randomised studies, three RCTs were not relevant to our purpose, and five were references for ongoing trials. The remaining seven records all reported one new randomised controlled trial that was eligible for inclusion (Stub 2015): three were conference abstracts, one was the protocol, one was the main study, and the remaining two were respectively a sub‐group study and re‐analysis of the same study. We describe the process in Figure 1.
1.
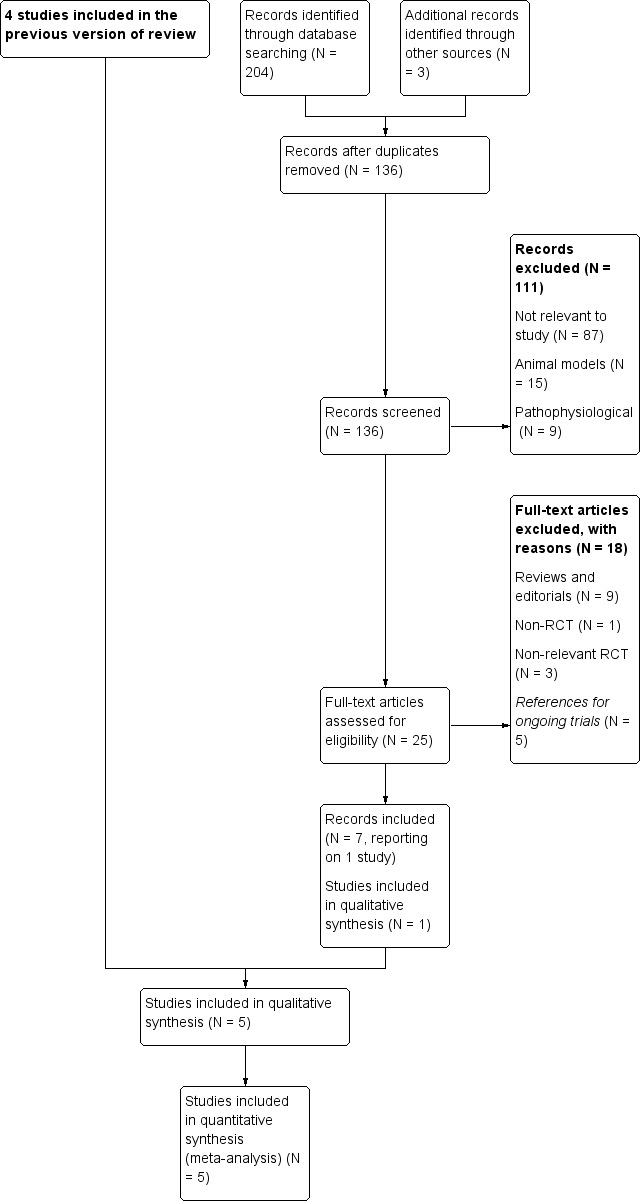
Study flow diagram with previous included studies incorporated into the results of the updated literature search.
Including the papers identified in the previous version of the review, we retrieved a total of 2892 records and screened 2442 unique records (Figure 2). Based on title and abstract, we excluded 2268 and retrieved 174 full papers retrieved. We excluded a further 162 articles, as 138 were not RCTs or were RCTs not related to our review, 16 were excluded for various other reasons, 5 were references for ongoing studies (NCT01787110; NCT02290080), and 3 were ongoing trials identified in the previous version of this review (old ongoing trials). This left 12 papers reporting five trials that met the inclusion criteria (Ranchord 2012; Rawles 1976; Stub 2015; Ukholkina 2005; Wilson 1997). We describe the process with reasons for exclusion in Figure 2 and the list of excluded trials in Characteristics of excluded studies.
2.
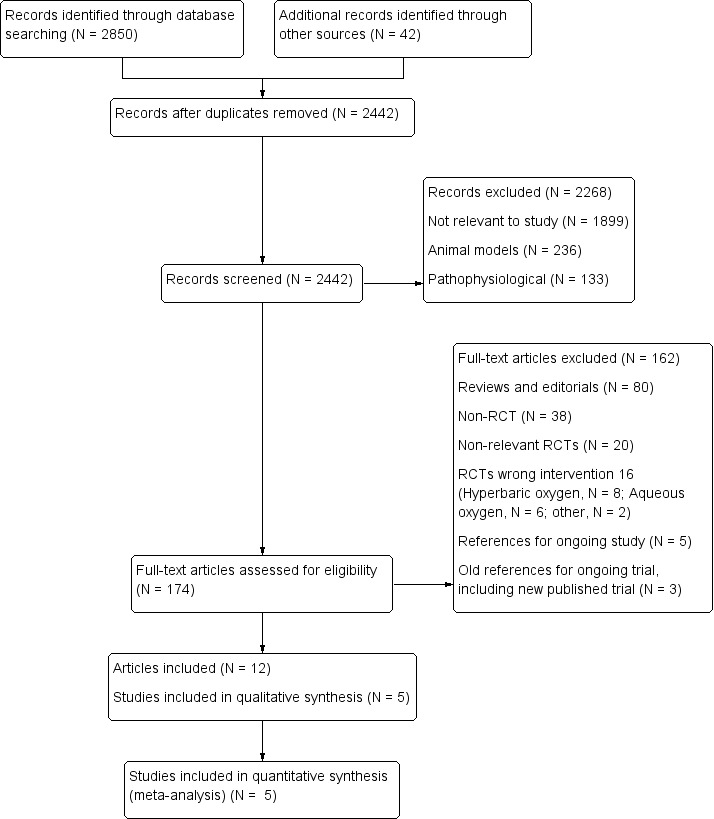
Study flow diagram (cumulative searches)
With respect to the three ongoing trials identified in the previous version of this review, we included one of them in this review (Stub 2015). The protocol of a second study has been published as paper but the final report has not (NCT01423929). The third protocol, identified exclusively by trials register, has not started, and the register had reported no activity as of September 2016 (ACTRN12609000466246). We therefore maintained these two last trials as ongoing trials in this version of the review.
In total, we identified four ongoing trials as of September 2016 (see Characteristics of ongoing studies). All four are parallel designs to compare oxygen (O2) versus air in people with suspected acute myocardial infarction (AMI). In the first study, the main outcome is in‐hospital mortality (this study, despite having been registered in 2009, has not yet commenced recruitment (ACTRN12609000466246). In the second study, the primary outcome is infarct size estimated by magnetic resonance imaging (MRI) and myocardial salvage index by MRI (NCT01423929); in the third study, the main outcome is one year all‐cause mortality, while secondary outcomes are 30‐days mortality as well as major adverse cardiac events (MACE) at 30 days and one year, including reinfarction and hospitalisations for cardiac failure (NCT01787110). This third study has nested a fourth trial with a slightly different architecture and oriented exclusively to biochemical outcomes (NCT02290080).
Included studies
The five included trials took place between 1976 and 2015 (Ranchord 2012; Rawles 1976; Stub 2015; Ukholkina 2005; Wilson 1997). Two were conducted in the UK (Rawles 1976; Wilson 1997), one in Russia (Ukholkina 2005), one in New Zealand (Ranchord 2012), and one in Australia (Stub 2015). All five studies were parallel‐design, randomised controlled trials. Rawles 1976 was double‐blind, and the other four were open‐label.
Population: a total of 1173 participants were involved, of whom 75.3% were men. Three studies recruited participants with suspected AMI (Ranchord 2012; Rawles 1976; Stub 2015), and the other two included only people with confirmed AMI (Ukholkina 2005; Wilson 1997). The mean ages in years (and standard errors where given) of the included participants in each group were as follows: Rawles 1976: air, 50.8 years (SE 2.4); O2, 51.3 years (SE 1.7); Wilson 1997: air, 64 years; O2, 65 years; Ukholkina 2005: air, 53.5 years (SE 1.06); O2, 55.6 years (SE 1.33); Ranchord 2012: air, 60 years (SE 12.8); O2, 62.1 years (SE 12.5). In Stub 2015, the median and interquartile range were 62 years (IQR 53.0 to 71.0) and 63.5 years (IQR 54.0 to 73.0) for the air and oxygen groups, respectively.
Intervention: in all five included trials the intervention was inhaled oxygen at 4 L/min to 8 L/min. Administration was by mask in four studies and by a nasal cannula in the other study (Ukholkina 2005). The comparator was air in four studies, breathed normally in the two open‐label studies and given at 4 L/min to 6 L/min by facial mask in the double‐blind study. In the remaining study, the comparison was titrated oxygen delivered by nasal prongs or mask adjusting the flow‐rate to achieve an oxygen saturation of 93% to 96% (Ranchord 2012).
Outcomes: all five studies reported death. Stub 2015 explicitly measured pain, while Rawles 1976 and Wilson 1997 reported opiate usage (as a proxy for pain). Four studies included infarct size estimated by electrocardiogram mapping (ECG), biochemical markers such as creatine kinase (CK), troponin (I or T) or BNP. Finally, two studies estimated infarct size by MRI (Ranchord 2012; Stub 2015).
The main characteristics of the included studies are in Characteristics of included studies.
Excluded studies
Of the 162 excluded articles, 80 did not report original data, 38 were not RCTs, 20 were RCTs of interventions that were not relevant to our study; and 16 papers reported studies that had a different oxygen intervention (8 used hyperbaric oxygen; 6, aqueous oxygen; 1, oxygen associated with haemoglobin; and 1, oxygen combined with nitric oxide versus placebo for pain control). Three records were related to previously identified ongoing trials. Of the five remaining papers, four were related to an ongoing trial (register, protocol, and two proceedings of congress of NCT01787110), and the other one was the protocol of a nested ongoing trial (NCT02290080). The main characteristics of the excluded studies are in the table Characteristics of excluded studies.
Risk of bias in included studies
Allocation
Three studies provided no description of randomisation sequence generation (Rawles 1976; Ukholkina 2005; Wilson 1997), and we therefore judged this domain to be at unclear risk of bias. In Ranchord 2012, a random number sequence was generated by a computer programme. This study was undertaken in two centres and randomisation was not stratified by centre; nevertheless we judged this as being at low risk of bias. In Stub 2015, a computer‐generated code into blocks of 10 was used (low risk of bias).
In four studies, allocation was concealed using numbered sealed envelopes (Ranchord 2012; Rawles 1976; Stub 2015; Wilson 1997), so we judged them as being at low risk of bias. Ukholkina 2005 did not report the method of allocation concealment, so we judged it as being at unclear risk of bias. In Ranchord 2012 (two centres) there is no description of how the envelopes were distributed to each centre, but we judged it to carry a low risk. In Stub 2015 trial allocation concealment was accomplished with externally numbered sealed envelopes (each block of 10). Three of these envelopes were carried in each ambulance and were replaced with the remainder envelops from the block. When the block was completed, a new block of 10 envelopes was allocated to the ambulance by the study coordinator. In terms of randomisation this may be seen as a strata for each ambulance (we judged this as being at low risk of bias).
Blinding
Only Rawles 1976 was double‐blinded. Blinding was done by using shrouded cylinders, but there is no information about how effective this was. Nursing staff were not aware that the record of opiate administration would be used as a proxy measure of pain. The use of shrouded cylinders left blinding potentially compromised, so we could not rule out performance and observer bias and judged this domain as being at unclear risk of bias. However, while this could affect the assessment of the surrogate outcomes for pain, it is much less likely to have affected the primary outcome of this review, which was death (Wood 2008). We have no clear information whether infarct size measurement (through ECG, CK, troponin I, troponin T or BNP) was done blindly. In Ranchord 2012, the cardiologist who measured the infarct size through MRI was blinded to treatment received by the participant and to biomarker data. Finally in Stub 2015, there is no clear information on how investigators measured pain, but as this trial was open label, both patient and rater are unblinded; therefore we judged this to be at high risk of bias. Blind observers performed measurement of MRI offline on dedicated workstations; the statistician who analysed the data was blinded to the allocation, and a central coordinator blinded to treatment allocation performed the six‐month clinical follow‐up.
Performance and observer biases were possible in the four unblinded studies, which may have affected the direct measurement of pain in Stub 2015 and the surrogate outcome for pain in Wilson 1997, so we judged this as carrying a high risk of bias. Neither Ukholkina 2005 nor Ranchord 2012 reported this outcome. The assessment of the primary outcome (death) and the other secondary outcome of complications such as recurrent ischaemia or AMI, heart failure, arrhythmias and pericarditis were less likely to be subject to significant observer bias (we judged this as being at low risk of bias). On the other hand, the methods used for infarct size estimation (ECG, creatine kinase, troponin T, or MRI) are theoretically robust to observer bias, so these measures may be considered free of observer bias (low risk).
Incomplete outcome data
All participants were followed to discharge in Rawles 1976, but randomisation took place before confirming the diagnosis. AMI was not confirmed in 21.5% of participants with suspected AMI. Although this may appear high, it is not inconsistent with diagnostic techniques in the 1970s. Of the 105 people randomised to oxygen and the 95 to air, AMI was not confirmed in 25 and 18 participants, respectively. The characteristics of those in whom AMI was not confirmed were similar in both groups, and there were no deaths among the excluded individuals.
In Wilson 1997, it was unclear for how long participants were followed up. The analysis excluded eight people: one death, one stroke, four who withdrew consent and two because data were incomplete. This is 16% of the participants, and the expected effect on the results for the primary event was very low; the risk of bias was therefore high, but its direction is unknown.
In Ukholkina 2005, investigators measured the outcomes for 10 days and lost no participants to follow‐up. However, trials provided no explicit data about the participants who were excluded postrandomisation because of failed revascularisation or the relative number of failed revascularisations in each group. The mismatch between the numbers reported in the tables and the text suggest that two participants may have been excluded from the air group and four from the oxygen group, but we cannot be certain. Consequently, we could not include these participants in the intention‐to‐treat analysis, and we think there is a high risk of bias for the outcomes we measured.
In Ranchord 2012, 12 participants were excluded after randomisation (four in the experimental group and eight in the control group). The published study did not report these participants' outcomes, which were excluded from the analysis. The reasons for withdrawal were: absence of formal consent (n = 5), incorrect initial diagnosis of STEMI (n = 2 acute pericarditis and n = 3 with normal coronary arteries), and cardiogenic shock (n = 2), which was an exclusion criterion for the study. The group to which these participants had been allocated was not reported.
We contacted authors to try and find out to which groups the 12 withdrawn participants had been allocated and their vital status, so that we could include them in an intention‐to‐treat (ITT) analysis. Although the authors replied, the information provided was contradictory and of limited value. Initially we were told that five people had been withdrawn because they did not consent and that the other seven had not been randomised. When we enquired further about this because it contradicted the published report, we were told that these seven had been randomised. Of concern to us was the fact that the distribution of their allocation to groups subsequently provided was not consistent with the numbers in the published trial report. The authors declined to provide the mortality outcomes for the participants who had alternative diagnoses, stating that "[a]lthough they are described as 'randomised and withdrawn' in the manuscript, they received no study treatment. For these reasons we are firmly of the view that these subjects should not be included in the mortality analysis." This failure to appreciate the nature of ITT analysis compounded our concerns raised by the inconsistencies in the allocation information. The authors felt unable to tell us the mortality status of the five participants who did not consent on the grounds that "if they have not consented then we can collect no further details about them". While we understand that trial‐specific data could not be collected on these people, mortality can be known by public methods. However, we appreciate that others may judge this differently. The only information of use was that three participants who withdrew because they had normal coronary arteries were alive at the end of the study period.
The two cases excluded from the analysis by cardiogenic shock merit special comment. While cardiogenic shock was an exclusion criterion of the study, it is important to recognise that this is a dynamic clinical condition that is present on admission to hospital in only 29% of those who go on to develop this complication. The paper does not report whether the participants had cardiogenic shock when they arrived at the hospital or not. If cardiogenic shock developed after randomisation but before treatment, then the exclusion of these participants could bias the results since people with cardiogenic shock have a higher mortality rate. This illustrates the importance of ITT analysis.
As we were unable to include these participants in the ITT analysis because mortality data were withheld, we undertook a sensitivity analysis with a 'worst‐case' scenario in which we tested the robustness of the current estimate by assuming that both participants received oxygen but died.
In Stub 2015, all the participants (N = 638) were followed to hospital discharge. However, as randomisation occurred in the ambulance, and informed consent was obtained verbally and then provided in writing at hospital, 14 people refused to participate in the study (6 in the oxygen arm and 8 in the air) after randomisation. Data about mortality at discharge are available from all randomised participants except those who refused to give informed consent (N = 624). We contacted authors to include this information in the analysis assuming that mortality is information that may be known by public methods (consistent with the above‐mentioned argument).
In addition, 35 participants were excluded after randomisation for "protocol violations", but these violations are not specified in the publication, and one repeated enrollment was also excluded. We contacted authors, who informed us of these causes: non‐study hospital (n = 28), chest pain for more than 12 hours (n = 12), oxygen given prior contact paramedics (n = 2), and hypoxaemia before enrolment (n = 3). In total, 588 participants were assessed for STEMI in emergency department: 470 of them were eligible for angiography, but STEMI was only confirmed in 441. The primary analysis was performed exclusively in confirmed STEMI. The 29 patients who underwent angiography but had other diagnoses were excluded from the analysis (17 in the oxygen arm and 12 in the air). There is no published information about the final diagnosis of these excluded patients. We contacted the author who informed us of the final diagnoses in the O2 group: 5 NSTEMI, 3 pericarditis, 2 apical ballooning syndrome (takotsubo) and 6 other diagnoses; and in the air group: 2 NSTEMI, 2 pericarditis, 1 aortic dissection, 4 apical ballooning syndrome, and 3 other.
Of a total of 624 randomised participants, AMI (STEMI or NSTEMI) was confirmed in 471. This implies that in 24.5% of randomised patients, AMI was not confirmed. This may appear to be a high rate of misdiagnosis in contemporary practice but could be explained, at least partially, by initial assessment undertaken by paramedics rather than physicians – data for 'false' activation of the cardiac catheter laboratory for STEMI varies, but some studies report 28% to 36% of misdiagnoses for STEMI (Barnes 2013; McCabe 2012), suggesting diagnosis remains a challenge in the pre‐hospital phase where exposure of paramedics to STEMI is infrequent. There is no specific information about mortality in the AMI group (STEMI and NSTEMI). We contacted the authors, and all‐cause mortality in AMI (STEMI or NSTEMI) at discharge and at six months was available and is discussed below. Regarding six‐month follow‐up, 11 participants each were lost in the oxygen group and air group.
For the analysis of primary outcome in this trial, data of the peak troponin I were available in 200 of the 218 in the oxygen group and in 205 of 223 participants in the air group (data were missing in 18 participants in each group, or 8.3% and 8.7% of the total, respectively). Data on CK is reported for 217 of 218 participants in the oxygen arm and for 222 of 223 in the air group (0.45% and 0.44%, respectively). The impact of these lost data on effect estimation is probably low in the case of CK (low risk of bias) but unknown in the case of troponin I (high risk of bias). The loss of these data may be related to the absence of a central 'core lab' for enzymes in this multicentre study.This last point may also induce some doubts about the quality control of these variables, which are the primary outcome in the Stub 2015 trial.
There is no detailed information about missing values of the biomarkers (cTnI and CK) that were used to elaborate the respective area under curve (AUC). The study report states that when one or more values were missing, authors addressed it through a strategy of trapezoidal integration with multiple imputation using a Markov Chain‐Monte Carlo simulation and sensitivity analysis. We judged this domain to be at unclear risk of bias.
Selective reporting
Protocols were unavailable for older studies. Rawles 1976 was the best‐quality trial, and we believe that the report probably included all the prespecified variables. In Wilson 1997, the primary purpose was to look at the incidence and degree of hypoxaemia and the effect of oxygen on hypoxaemia, rather than this review's primary outcome of death; the participant who died was excluded from the analysis. Despite contacting the authors, we were unable to establish in which group the death occurred, and we could not include this study in the meta‐analysis. We carried out a sensitivity analysis to assess the potential risk of bias.
In Ukholkina 2005, ECGs were mapped to estimate the surrogate outcome of infarct size, but only in a subset of 31 participants in the oxygen group; there was no information for the air group. We therefore believe that it is not possible to draw meaningful conclusions about infarct size. We do not think the pain and death outcomes were subject to selective reporting.
In Ranchord 2012 the infarct size, estimated by MRI, was undertaken in a small subgroup of 71 participants (selective reporting of subgroup). In addition, neither the protocol nor the trial report give any defined criteria on whether or not to perform MRI, so this analysis should be considered a non‐randomised comparison. On the other hand, given that MRI was performed four to six weeks after AMI, this specific subgroup represents a cohort of survivors, which also needs to be taken into account in the infarct size comparison. We judged this study to be at high risk of selective reporting bias.
In Stub 2015, all patients "who were agreeable to travel to a core site for scanning" were invited for MRI, which was therefore performed in a self‐selected subgroup of 139 participants: 65 in the oxygen group and 74 in the air group (selective reporting of subgroup).The self‐selection implies that randomisation was broken and therefore the comparison of infarct size estimated by MRI is a non‐randomised comparison, very sensitive to selection bias. On the other hand, MRI was performed six months after the STEMI; consequently the infarct size was estimated in a cohort of survivors. If we accept an association between infarct size and mortality, the comparison between oxygen and air will be biased towards the null hypothesis. We judged this study to be at high risk of selective reporting bias.
Other potential sources of bias
We did not identify any other biases in Rawles 1976 or Wilson 1997.
Ukholkina 2005 reported differences in infarct size between the two interventions, but the authors did not specify the time after symptoms onset when creatine phosphokinase M and B isoenzymes (MB‐CPK) were measured; they were not measured at the same time in all participants. In addition, no information was provided about the consistency and validity of the method used to map myocardial damage (number and blinding of observers; reliability and repeatability of their measurements; whether there were disagreements and, if so, how these were resolved). While these methodological weaknesses call into question the reliability of the estimation of myocardial damage, they do not affect the main outcomes of this review. Only Ukholkina 2005 reported complications, but there was an inconsistency between the data in the table and the text. We recalculated complication rates and used these data in our analysis.
In Ranchord 2012, prior to randomisation both the experimental and control groups received pre‐hospital oxygen (86.8% and 63.0%, respectively). If the effect of oxygen truly determines the outcome, then this pre‐randomisation intervention could have produced a bias in effect estimation toward the null hypothesis (i.e. a reduction of the study power).
In Stub 2015, we detected some differences between the final paper and the published protocol. Firstly, the study population in the protocol is "suspicion of STEMI" but in the final paper the analysis was performed in confirmed STEMI (normoxic patients with STEMI). Secondly, there are differences in the sample size calculations despite using similar assumptions: in the protocol the estimated sample size was 490 (245 patients in each arm) while in the paper the sample size calculation was 600 participants, and 638 were enrolled. Finally, the protocol reported planning an interim analysis after randomisation of 100 participants in each arm, while in the paper the interim analysis was performed after 405 participants were recruited. In both cases justification for these number of patients to make the interim analysis is unclear, and there is no reflection about implications for the statistical analysis.
It is difficult to know the possible impact on validity of these discrepancies between the paper and the protocol (unclear risk of bias). However it is clear that authors changed some decisions regarding the conduct of the study after commencing, and they did not adequately explain these decisions in the paper. This suggests that some decisions could have been 'data‐induced' or motivated by post hoc hypotheses.
Baseline characteristics
Overall, the two groups appeared similar after randomisation in Rawles 1976 and Wilson 1997. In Ukholkina 2005 the two groups appeared similar in age, smoking, hypertension, unstable angina and cholesterol. There was a (non‐significant) difference in the Killip stage, with more Killip II in the oxygen group than in the air group. Time to revascularisation was 41 minutes shorter in the air group (P = 0.052), which even if due to chance may have important clinical implications for our outcomes of interest. In Ranchord 2012 the two groups appear similar in age, sex, body mass index, diabetes, hyperlipidaemia, hypertension and previous coronary artery bypass grafting. There were differences in the number of previous percutaneous coronary interventions (PCIs), and in the infarct territory, with less anterior infarction in the experimental group than in the control group (18% versus 31%).
In Stub 2015 baseline characteristics are reported for 441 participants with STEMI confirmed by angiography. There was no clear difference between oxygen and air regarding important clinical characteristics. Surprisingly, there is no description of the baseline characteristics of all randomised patients according to table 1 of the CONSORT statement. Therefore we cannot make a judgement on whether the randomisation process worked, given that is not possible to explore the differences in potential confounding factors between randomised groups.
Summary of risk of bias
Death as an outcome had a low risk of bias in Rawles 1976 and Stub 2015, was not adequately reported in Wilson 1997, and had a high risk of bias in Ukholkina 2005. There are also the 'withdrawn' participants from Ranchord 2012, for whom we had no outcome data and do not know their vital status. We therefore consider the overall risk of bias for mortality in the meta‐analyses to be high. For pain, we consider the risk of bias to be unclear in Rawles 1976 and high in Wilson 1997 and Stub 2015. Consequently we consider the risk of bias in the meta‐analysis for pain to be high. For ischaemia recurrence there are low risks of bias in both Ukholkina 2005 and Stub 2015 (Figure 3).
3.
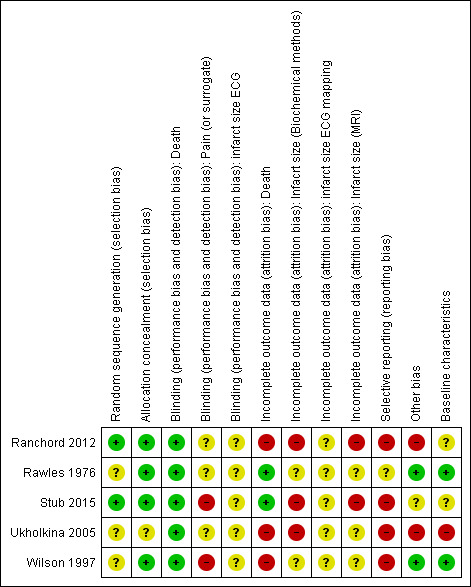
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
For infarct size estimated by CK and MB, there is high risk of bias in Ukholkina 2005 and a low risk of bias in Stub 2015. For infarct size by different troponins the risks are unclear in Ranchord 2012 and Stub 2015. Finally, for infarct size estimated by MRI there is high risk of bias in both Ranchord 2012 and Stub 2015.
Effects of interventions
See: Table 1
All‐cause mortality
All five trials reported the observed mortality at hospital discharge. Rawles 1976 found more deaths in the group randomised to oxygen than in the air group, both for all randomised participants with suspected AMI (N = 200) and for those with confirmed AMI (N = 157). Wilson 1997 described one death but did not report in which group it occurred. We contacted both of the authors of the original paper, who confirmed that they no longer had the trial data and did not remember in which arm the death and the stroke had occurred; however, they stated that 25 participants had been randomised into each group. In Ukholkina 2005, only 1 person out of 58 died in the oxygen group and none out of 79 participants died in the air group. In Ranchord 2012 ,1 participant out of 68 died in the high oxygen group and 2 out 68 in the titrated group. Twelve participants (4 in the high oxygen group and 8 in the titrated group) were withdrawn after randomisation, with the mortality data for these 12 people not reported in the paper. We contacted the authors of the trial, but they were unable to provide the missing data for these cases. In Stub 2015 the all‐cause mortality at discharge was 4 out 218 and 10 out 223, respectively, for the oxygen and air groups in confirmed STEMI, and 5 out 231 and 11 out 240 respectively for the AMI (STEMI or NSTEMI).
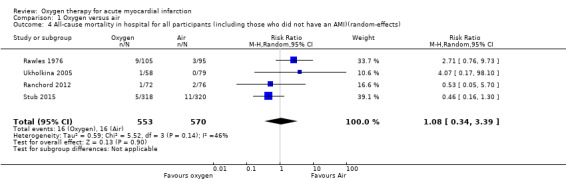
We could only combine results from four of the five studies (Ranchord 2012; Rawles 1976; Stub 2015; Ukholkina 2005). In contrast with previous versions of this review, in the meta‐analysis of the current version, the same number of people died (n = 16) in each group . This suggests oxygen effect is not good, but not harmful. The complete results are given numerically below together with the GRADE assessment in the Table 1 (Guyatt 2008).We also present the sensitivity analysis for the missing data from Wilson 1997 and Ranchord 2012 .
Meta‐analysis for mortality in participants with confirmed AMI: risk ratio (RR) 1.02 (95% CI 0.52 to 1.98); I2 = 49%, fixed‐effect model; 4 trials, N = 871, quality of evidence: very low (Analysis 1.1). The effect does not change when applying a random‐effects model (Analysis 1.2).
1.1. Analysis.
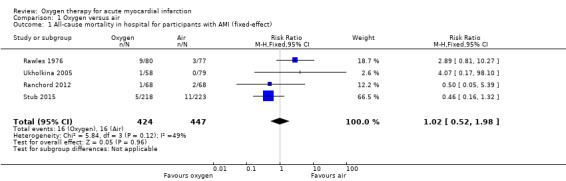
Comparison 1 Oxygen versus air, Outcome 1 All‐cause mortality in hospital for participants with AMI (fixed‐effect).
1.2. Analysis.
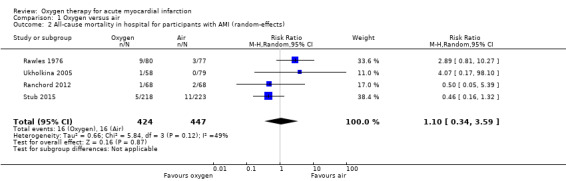
Comparison 1 Oxygen versus air, Outcome 2 All‐cause mortality in hospital for participants with AMI (random‐effects).
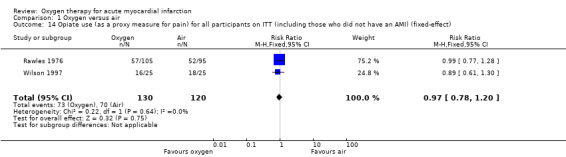
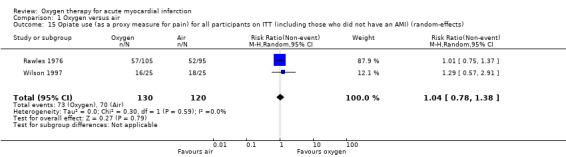
Meta‐analysis for mortality in an ITT population, including those who did not have AMI showed an RR of 0.99 (95% CI 0.50 to 1.95; I2 = 46%, fixed‐effect model; 4 trials, N = 1123, quality of evidence very low; Analysis 1.3). The effect does not change when applying a random‐effects model (Analysis 1.4).
1.3. Analysis.
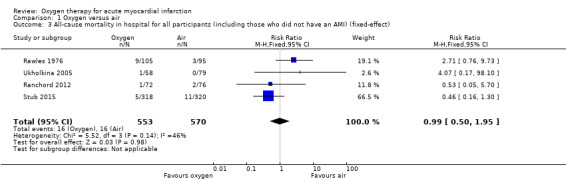
Comparison 1 Oxygen versus air, Outcome 3 All‐cause mortality in hospital for all participants (including those who did not have an AMI) (fixed‐effect).
1.4. Analysis.
Comparison 1 Oxygen versus air, Outcome 4 All‐cause mortality in hospital for all participants (including those who did not have an AMI)(random‐effects).
Sensitivity analysis for missing information about the arm in which the death occurred in Wilson 1997 (ITT analysis): a 'worst‐case' scenario assuming that the participant who died was in the oxygen arm gave an RR for death of 1.05 (95% CI 0.54 to 2.02; I2 = 33%; fixed‐effect model; 5 trials, N = 1173; Analysis 1.5). A 'best‐case' scenario assuming that the participant who died was in the air arm gave an RR for death of 0.94 (95% CI 0.49 to 1.80; I2 = 32%; 5 trials, N = 1173; Analysis 1.6). In both cases we used a fixed‐effect model. Sensitivity analysis for missing information about the group in which the two participants of Ranchord 2012 with cardiogenic shock were allocated: assuming that both participants died, a 'worst‐case' scenario in which both were in the oxygen arm gave an RR of 1.11 (95% CI 0.58 to 2.15; I2 = 45%; 4 trials, N = 1123; Analysis 1.7) and a 'best‐case' assuming that the participants were in the control arm gave a RR of 0.89 (95% CI 0.046 to 1.71; I2=54%; 4 trials, N = 1123; Analysis 1.8).
1.5. Analysis.
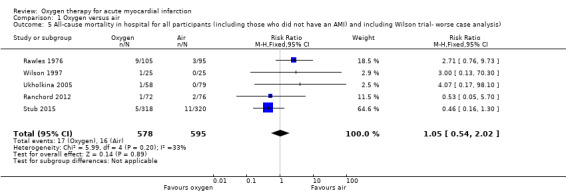
Comparison 1 Oxygen versus air, Outcome 5 All‐cause mortality in hospital for all participants (including those who did not have an AMI) and including Wilson trial‐ worse case analysis).
1.6. Analysis.
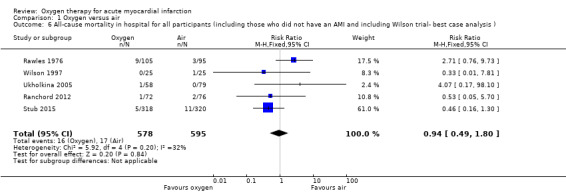
Comparison 1 Oxygen versus air, Outcome 6 All‐cause mortality in hospital for all participants (including those who did not have an AMI and including Wilson trial‐ best case analysis ).
1.7. Analysis.
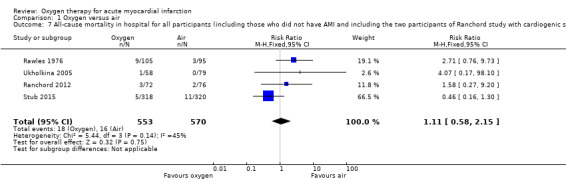
Comparison 1 Oxygen versus air, Outcome 7 All‐cause mortality in hospital for all participants (including those who did not have AMI and including the two participants of Ranchord study with cardiogenic shock who died). Worse‐case analysis..
1.8. Analysis.
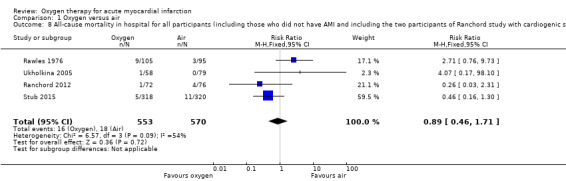
Comparison 1 Oxygen versus air, Outcome 8 All‐cause mortality in hospital for all participants (including those who did not have AMI and including the two participants of Ranchord study with cardiogenic shock who died). Best‐case analysis.
The subgroup analysis, including only the three most recent trials, all which were performed in the reperfusion era (Analysis 1.9), gave an RR for death of 0.58 (95% CI 0.24 to 1.39; I2 = 0% fixed‐effect model; 3 trials, N = 923, quality of evidence: low). Despite being recent, two of these three studies did not meet current standards of trial design and conduct and are at high risk of bias (see Risk of bias in included studies).
1.9. Analysis.
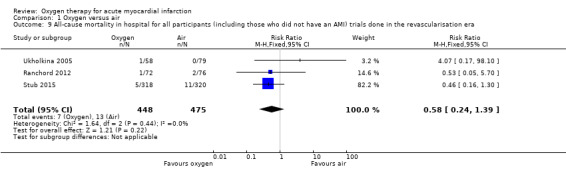
Comparison 1 Oxygen versus air, Outcome 9 All‐cause mortality in hospital for all participants (including those who did not have an AMI) trials done in the revascularisation era.
Only Stub 2015 reported all‐cause mortality at six months: 9 participants out 318 died in oxygen group versus 13 out 320 in the air group (RR 0.39, 95% IC 0.14 to 1.07; 1 trial, N = 628).
Cardiac mortality
Only Stub 2015 reported cardiac mortality, with 4 out 318 and 7 out 320 participants dying in the oxygen and air groups, respectively (RR 0.58, 95% CI 0.17 to 1.95; 1 trial, N = 628).
Cardiac failure
Two studies reported cardiac failure (Rawles 1976; Wilson 1997). In Ranchord 2012, cardiogenic shock was an exclusion criterion for the study, so the two cases that occurred postrandomisation were excluded from the analysis. In Ukholkina 2005, cardiogenic shock and cardiac failure at hospital arrival were also considered criteria for exclusion from the trial. In included participants, cardiac failure was reported in one and five participants in the oxygen and air groups, respectively. In Stub 2015, 20 participants in each group presented cardiogenic shock. The meta‐analysis for cardiac failure showed no significant difference between groups (RR 0.88, 95% CI 0.50 to 1.55; I2 = 27%, 2 trials, N = 775; Analysis 1.10).
1.10. Analysis.
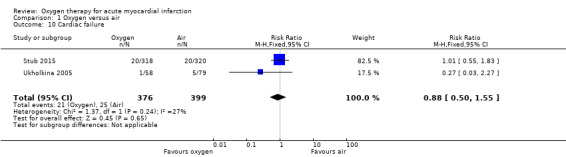
Comparison 1 Oxygen versus air, Outcome 10 Cardiac failure.
Stroke
Only one trial reported stroke or transitory ischaemic attack, which occurred in 3 out 218 participants in the oxygen group and and 1 out 223 in the air group (Stub 2015).
Recurrence of myocardial infarction or ischaemia
Recurrence of ischaemia was similar in both groups in Ukholkina 2005: it occurred in 12 participants in the oxygen group (N=58) and 16 (N= 79) in the air group (RR 1.02, 95% CI 0.52 to 1.99; 1 trial, N = 137). Conversely in Stub 2015, recurrence of myocardial infarction or ischaemia at hospital discharge occurred in 12 out 218 participants in the oxygen group and in 2 out 223 in the air group (RR 6.14, 95% CI 1.39 to 27.1), which suggests a negative effect for oxygen. The effect estimate from both studies suggests a disadvantage for oxygen, but this is not significant: meta‐analysis of the two trials shows an RR of 1.67 (95% CI 0.94 to 2.99; I2= 80%, 2 trials, N = 578, quality of evidence: low; Analysis 1.11). This substantial heterogeneity could be due to different causes. In Ukholkina 2005, inclusion criteria were uncomplicated AMI, exclusion criteria included cardiogenic shock, and failure in revascularisation was considered cause for study withdrawal. Moreover, the study setting was clearly different: participants in Ukholkina 2005 were recruited in hospital, versus pre‐hospital in Stub 2015 (methodological sources of heterogeneity). On the other hand, part of the observed heterogeneity may be related to technological and procedural advances in percutaneous intervention (stenting, thromboaspiration, etc.) and with progress in the use of adjuvant antiplatelet medication in the decade separating the two trials (clinical sources of heterogeneity). Finally, some heterogeneity may be statistical.
1.11. Analysis.
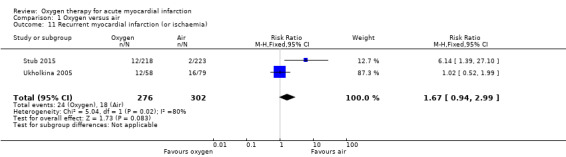
Comparison 1 Oxygen versus air, Outcome 11 Recurrent myocardial infarction (or ischaemia).
Recurrence of myocardial infarction at six months in Stub 2015 occurred in 16 (out 218) and 8 (out 223) participants in the oxygen and air groups respectively (RR 2.05 [95% CI 0.89 to 4.68] 1 trial, N=441)
Major bleeding
Stub 2015 was the only trial to report major bleeding: 9 and 6 cases were reported in the oxygen (n = 218) and air groups (n = 223), respectively (RR 1.53, 95% CI 0.56 to 4.24, 1 trial, N = 441). In two cases in the air group, this outcome was the cause of death.
Pain
Stub 2015 directly measured pain at two time points: on arrival of paramedics and on arrival at hospital. The median pain scores were exactly the same for both the oxygen and air groups in both measurements: median 6.0 (IQR 4.8 to 8.0) versus 6.0 (IQR 4.0 to 8.0) on arrival of paramedics, and 2.0 (IQR 0.0 to 4.0) versus 2.0 (IQR 0.5 to 3.5) on arrival at hospital.There is not explicit description of the used tool for this measurement (probably a 10‐point VAS scale). In two other studies, the authors reported diamorphine use as a proxy for pain: in Rawles 1976, a similar proportion of participants from both groups received analgesia. The total dosage was similar: 54.3% of randomised participants (71.3% of those with confirmed AMI) in the oxygen group received analgesia, with an average of 2.1 doses (standard deviation (SD) 1.5), but it was not clear whether the denominator was participants who used diamorphine or all participants; 54.7% of randomised participants (67.5% of those with confirmed AMI) in the air group received analgesia, with an average of 2.0 doses (SD 1.4), but again the denominator population was not clearly defined. In Wilson 1997, the authors reported opiate use as a proxy for pain. Although 50 people were randomised, authors reported results for just 42, as follows: 16 of 22 participants (72.7%) in the oxygen group used opiates; 18 of 20 participants (90%) in the air group used opiates. Ukholkina 2005 did not measure pain or opiates use.Thus, we can only combine results from two studies (Rawles 1976; Wilson 1997), which showed no difference in opiate use between the oxygen and the air groups. Meta‐analysis for opiate use in confirmed AMI showed the following results (fixed‐effect model): RR 0.99 (95% CI 0.83 to 1.18; I2 = 54%, 2 trials, N = 190, quality of evidence: low; Analysis 1.12). Applying a random‐effects model slightly altered these results: RR 0.94 (95% CI 0.72 to 1.23; I2 = 54%, 2 trials, N = 190; Analysis 1.13). Meta‐analysis for opiate use in the ITT population including those who did not have an AMI (fixed‐effect model): RR 0.97 (95% CI 0.78 to 1.20; I2 = 0%, 2 trials, N = 250, quality of evidence: low; Analysis 1.14). This remained unchanged using a random‐effects model: RR 1.04 (95% CI 0.78 to 1.38; I2 = 0%; Analysis 1.15).
1.12. Analysis.
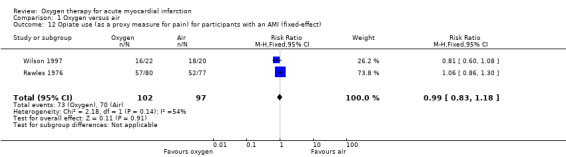
Comparison 1 Oxygen versus air, Outcome 12 Opiate use (as a proxy measure for pain) for participants with an AMI (fixed‐effect).
1.13. Analysis.
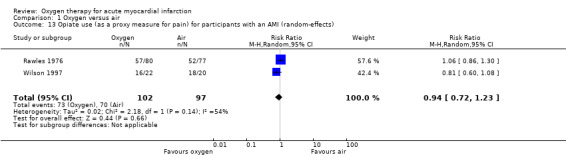
Comparison 1 Oxygen versus air, Outcome 13 Opiate use (as a proxy measure for pain) for participants with an AMI (random‐effects).
1.14. Analysis.
Comparison 1 Oxygen versus air, Outcome 14 Opiate use (as a proxy measure for pain) for all participants on ITT (including those who did not have an AMI) (fixed‐effect).
1.15. Analysis.
Comparison 1 Oxygen versus air, Outcome 15 Opiate use (as a proxy measure for pain) for all participants on ITT (including those who did not have an AMI) (random‐effects).
Revascularisation
Revascularisation (as the current standard of the treatment in AMI) was a criteria for inclusion in the three most recent trials (Ranchord 2012; Stub 2015; Ukholkina 2005). Only Stub 2015 reported 'new' revascularisation as an outcome. Twenty‐three of 218 participants in oxygen group and 16 of 223 in the air group underwent revascularisation. There is no information about the causes of new revascularisation nor of the techniques used for it.
Pericarditis
Only Ukholkina 2005 reported pericarditis as a complication of AMI: 1 participant in the oxygen group (n = 58) and 6 participants in the air group (n = 79) experienced this outcome. Stub 2015 randomised and included 15 cases of acute pericarditis in the study as AMI (9 in the oxygen and 6 in the air group). The true diagnosis was made after catheterisation, leading to these participants' exclusion from the study analysis. However, we have included these participants in the ITT analysis.
Arrhythmia
Four trials reported different types of arrhythmias, but the information is not detailed enough to make a qualitative syntheses (Rawles 1976; Stub 2015; Ukholkina 2005; Wilson 1997).
Left ventricular function
Three studies estimated ventricular function using different imaging techniques (echocardiography, MRI) (Ranchord 2012; Stub 2015; Ukholkina 2005). Investigators performed measurements at different points of AMI clinical evolution, and given the dynamic condition of this outcome in AMI, the variability in considered time points may be an important source of variability in the results. Thus, we did not attempt any synthesis for this outcome.
Infarct size estimation
Four of the five studies explored the effect of oxygen on infarct size using different methods (Ranchord 2012; Rawles 1976; Stub 2015; Ukholkina 2005).
In the oldest trial (Rawles 1976), investigators estimated infarct size by means of maximum serum aspartate aminotransferase levels. In two other studies, authors used CK or CK‐MB (peak or AUC): in Ukholkina 2005, CPK and MB‐CPK activity was significantly higher in the oxygen arm at 6 hours and 24 hours of symptoms onset, while at other time points of the clinical evolution (between 12 and 18 hours, as well as at 36 and 48 hours after onset) the levels of MB‐CPK and CPK were significantly lower in the oxygen group. The authors considered this ambiguous result to be favourable to oxygen but provided no coherent explanation for these data. Stub 2015 found a significant increase in the geometric mean peak of creatine kinase in the oxygen group compared with the non‐oxygen group (1948 U/L versus 1543 U/L; geometric means ratio 1.27, 95% CI 1.04 to 1.52). There was a similar result when using the AUC: ratio of geometric means of AUC 1.19 (95% CI 1.01 to 1.40). Given the huge differences in the timing of blood sampling, in laboratory methods and in mathematical expression, it was not possible to make quantitative syntheses of infarct size by CK. On the other hand, the two more recent trials measured different subtypes of troponin. In Ranchord 2012, the mean ratio of troponin T in the oxygen versus air group was 0.74 (95% CI 0.50 to 1.10), and in Stub 2015, the mean ratio of troponin I between oxygen and air was 1.20 (95% CI 0.92 to 1.56); no significant differences were apparent in either case. It was not possible to undertake meta‐analysis of infarct size by troponin. The quality of evidence for this outcome was low.
In two studies, MRI was used to estimate infarct size (Ranchord 2012; Stub 2015). In Ranchord 2012, the mean infarct size was 15.6 g (SD 15.6) in the oxygen group and 16.3 g (SD 11.7) in the air group. The mean difference of infarct mass between oxygen and air groups was −0.8 g (95% CI −7.6 to 6.1). When expressed as a percentage of left ventricular mass, mean infarct mass was 12.5% ( SD 10.9) in oxygen versus 13.1% (SD 9.7) in the air group, which suggests a positive (but not significant) effect for oxygen. However, in Stub 2015 the geometric mean of infarct mass was 14.6 g (IQR 11.3 to 18.3) in the oxygen group versus 10.2 g (IQR 7.7 to 13.4) in the air group, and the ratio of geometric means of infarct size was 1.43 (95% CI 0.99 to 2.07), which suggests an increase of infarct size in the oxygen group on the border of statistical significance. When expressed as a percentage of infarct mass, the geometric mean was 12.6% (IQR 6.7 to 19.2) in the oxygen group and 9.0% (IQR 4.1 to 16.3) in the air group. It is clear that these indices have different mathematical properties (we contacted authors). Nevertheless, considering that these comparisons come from biased subgroups of patients of the trials, we considered them unsuitable to make quantitative synthesis of infarct size estimated by MRI. The quality of evidence for this outcome is very low.
Discussion
Summary of main results
We identified five studies meeting our inclusion criteria, involving a total of 1173 participants, 32 of whom died. The quality of the evidence, according to GRADE, ranged from low to very low (Guyatt 2008). There were a similar number of deaths in people receiving oxygen versus air, so for all‐cause mortality at hospital discharge there is neither evidence of benefit nor harm for oxygen treatment in patients with AMI. This finding is consistent in the intention‐to‐treat meta‐analysis and the confirmed AMI meta‐analysis. Interestingly, the inclusion of the recent Stub 2015 trial changed the RR from our previous review, where the ITT analysis gave an RR of 2.05 (95% CI 0.75 to 5.58), compared to 0.99 (95% CI 0.50 to 1.95) in the present review. In confirmed AMI, the RR changed from 2.11 (CI 0.78 to 5.68) to 1.02 (CI 0.52 to 1.98).
Regarding pain, there was no effect for oxygen on pain relief when pain was directly measured nor when trials measured opiate use as a surrogate for pain. With regard to complications following AMI, there was no clear effect for oxygen on a range of complications, except for recurrent ischaemia, which was higher (but not significantly so) in the oxygen group compared to the air group.
Based on outcomes that were 'important but not critical for decision‐making', particularly infarct size, there was partial evidence that oxygen may increase infarct size as estimated by CK. However, this evidence is not consistent with CK in other trials and is also inconsistent when infarct size is estimated through troponin I or T (even when CK and troponin I were measured in the same trial). Finally, there is no evidence of effect for oxygen on infarct size as estimated by MRI.
Overall completeness and applicability of evidence
Regarding the applicability of the evidence, three aspects are worth noting.
Firstly, Rawles 1976 took place before the reperfusion era (primary percutaneous coronary intervention (PPCI) or thrombolysis) and also before the routine use of treatments such as beta‐blockers, aspirin, angiotensin‐converting enzyme inhibitors or modern antiplatelet therapies, so their results may have been different in today's context. The sensitivity analysis for death in the reperfusion era trials gave moderate quality of evidence; nevertheless, readers should consider the resulting risk ratio (RR) of 0.58 (95% CI 0.24 to 1.39; I2 = 0%; 3 studies, N = 913) when planning future studies (for example, in calculating sample sizes).
Moreover, the reported case fatality rates from AMI have fallen in recent decades (Koopman 2013; Schmidt 2012; Smolina 2012; Yeh 2010). In the studies included in this review, hospital mortality among control participants was only 3.8%. This rate is lower than that observed in routine surveillance data (Babaev 2005; Movahed 2009), and it is half that of the recent Euro Heart Survey in 47 countries, where hospital mortality was 6.2% (Puymirat 2013). A possible explanation is that these trials only recruited lower‐risk participants, but it there could have also been a chance deficit of deaths in the control arm, which would have contributed to the apparent difference between the oxygen and control groups. This aspect should inform participant selection criteria in future studies.
The the most recent trial had a large impact on our estimation of all‐cause mortality. However, all cases of non‐cardiac mortality were in the air group (3 out 11: 27%), and three deaths were due to complications presumably unrelated to oxygen: major bleeding in two cases and sepsis in the other. In a sensitivity analysis, if these 3 cases had occurred in the oxygen group, the RR for the ITT meta analysis would have been 1.43 (95% CI 0.72 to 2.84), instead of 0.99 (95% CI 0.50 to 1.95). Similar change is apparent in the subgroup of participants in the reperfusion era: from RR 0.58 (95% CI 0.24 to 1.39) to RR 1.04 (95% CI 0.45 to 2.41).
Secondly, despite the longstanding beliefs of health professionals, there is no evidence that oxygen has a beneficial effect on pain, whether measured directly or by proxy variables. This result is consistent with a new trial undertaken with oxygen versus air during percutaneous coronary intervention for balloon‐induced angina (Zughaft 2013), which we considered but excluded from this review.
A further issue to consider when assessing the contemporary relevance and applicability of the earlier studies in our review is that the definition of AMI has evolved over the years to reflect increasing understanding of underlying pathophysiological processes as well as developments in diagnostic techniques such as the high sensitivity troponins. Furthermore, there is now recognition that acute coronary syndromes represent a spectrum of pathophysiological processes rather than a uniform type of 'heart attack'. Notably, there are now separate guidelines for STEMI and NSTEMI presentations, reflecting the different therapeutic options. Future studies should consider this spectrum of ACS, as mortality varies by phenotype (at least in the hospital stay), but in clinical terms the common scenario for decision‐making about oxygen administration is at the point where AMI is suspected, regardless of final diagnosis following biomarker of coronary angiography.
Bearing these considerations in mind, future studies of oxygen in (suspected) AMI should enrol participants in the pre‐hospital phase. Given the challenges of pre‐hospital diagnosis, this probably implies (as in Stub 2015) that trials will recruit a significant proportion of participants without a subsequent confirmed diagnosis of AMI. To minimise this problem, some ongoing trials are using wireless ECG transmission with interpretation by cardiology staff and additional discussion of the patient. In any case, for mortality and some other variables, it is possible to make a true intention‐to‐treat (ITT) analysis, but assessing other clinical outcomes such as infarct size or complications may not be practical or appropriate for all randomised patients. The optimal time for estimating infarct size for clinical research using MRI is worthy of special consideration.
Quality of the evidence
The (published and unpublished) evidence in support of such a widespread practice is surprisingly scant and scattered. We used the GRADE approach to assess the quality of evidence and the GRADE profiler (GRADEpro) to import data from RevMan to create 'Summary of findings' tables (GRADEpro; RevMan 2014).The quality of evidence for the outcomes judged critical for decision‐making was very low for death and low for pain and for recurrent ischaemia. Therefore, with regard to these outcomes, readers should interpret results with caution (Table 1).
Oxygen trials have rarely investigated – and poorly reported – different complications such as cardiac failure, bleeding, stroke, recurrence of ischaemia, pericarditis, etc. Despite being hard variables that are robust to observed bias, these outcomes are not totally free of performance bias, and quality of the evidence is low or very low, meriting caution upon interpretation.
Finally, for other outcomes judged 'important but not critical for decision‐making', such as infarct size measured by biological markers, the quality of evidence is low, and very low in the case of infarct size estimated by MRI.
Potential biases in the review process
We were unable to determine if there was any publication bias using formal methods, as we found only five studies for inclusion. We cannot rule out the possibility that there are unpublished or ongoing studies, especially in languages other than English, that were not indexed in the electronic databases we searched.
Regarding heterogeneity, in the meta‐analysis for opiate use in confirmed AMI, we found moderate heterogeneity (I2 = 54%), which disappeared in the ITT analysis. While the two studies used in the meta‐analysis had differences in their design (for example, blinded versus open‐label) and attrition rates (much higher in Wilson 1997), it was not possible to investigate the heterogeneity further with only two trials.
Agreements and disagreements with other studies or reviews
The findings of our updated review are not consistent with prior reviews (Cabello 2010; Cabello 2013), and the inclusion of a new trial has altered the evidence (point estimate) of the previous version of this review, which suggested an excess of mortality in the oxygen group compared to air (Stub 2015). Nevertheless, the results of this updated review are too imprecise to definitively determine whether oxygen is helpful or harmful in AMI.
Authors' conclusions
Implications for practice.
The evidence available about the effect of the oxygen on all‐cause mortality in patients with AMI is inconclusive, and we cannot rule out a possible harmful effect. This lack of evidence is consistent across different outcomes critical for clinical decisions. However, the evidence in this area is sparse, of low or very low quality, and partially predates the advances in reperfusion techniques and trial methods of recent years. Finally, the evidence about the effects of oxygen on other type 2 outcomes such as infarct size (estimated through different methods) is also inconsistent and of very low quality.
Therefore, current evidence neither supports nor clearly refutes the routine use of oxygen in people with AMI. The implication for clinical practice is that pending new evidence, practitioners should only give oxygen to patients with suspected or confirmed AMI with a blood oxygen saturation < 90% or in cases of patients with respiratory distress.
Implications for research.
As early as 1950, studies demonstrated that the administration of pure oxygen via a facial mask not only failed to reduce the duration of angina pain but also prolonged the electrocardiographic changes indicative of an AMI (Russek 1950). In 1975, other authors explicitly called for further research on the topic (Salzman 1975). Given that Rawles 1976 subsequently suggested possible harm, it is surprising that no research groups have undertaken a definitive study to rule out the possibility that oxygen may do more harm than good. The recently published Stub 2015 has reactivated clinical and scientific interest in the possible harmful effects of oxygen (Nedeljkovic 2015).
Part of the reason for the failure to fund such an essential study until recently may be the strong assumption (Cabello 2009; Danchin 2009), based on pathophysiological reasoning, that oxygen administration reduces both the oxygen deficit in ischaemic myocardial tissue and consequent tissue death. Indeed, both the medical profession and the public have become so familiar with the use of oxygen that the general attitude may have been that even if oxygen does no good, it is at least not harmful. However, recent years have seen the recognition of oxygen as a 'vasoactive substance' (Farquhar 2009). In summary, while there are pathophysiological reasons to believe that oxygen may have the potential to reduce tissue damage, it is also biologically plausible that oxygen is doing harm (see Why it is important to do this review).
Given the widespread use of oxygen for AMI, the inconsistencies in recommendations about when and to whom it should be given, and the fact that the best current evidence is not conclusive regarding benefit or harm, we maintain the belief expressed in our previous reviews that there is an urgent need for an adequately powered randomised controlled trial to establish the effects of administering oxygen to people with AMI. That trial must incorporate contemporary standards in design, conduct, analysis and reporting of trials and address the spectrum, population and sample size mentioned above to reflect contemporary diagnosis and care of the patient with AMI.
Three of the identified ongoing trials are currently recruiting participants (NCT01787110; NCT02290080; NCT01423929). The first is an ambitious trial (DETOX‐AMI) based on the national AMI registry in Sweden, focused on mortality and recruiting in the pre‐hospital phase of AMI, with a planned sample size of 6000 participants. This trial has a nested sub‐study focused on biological markers. A third nested study is focused on the effect of oxygen on infarct size estimated by biochemical markers and MRI in patients with STEMI. The MRI study will be performed at two to six days of AMI to determine the myocardium at risk and to calculate the myocardial salvage index (MSI) by PCI.
What's new
| Date | Event | Description |
|---|---|---|
| 12 October 2016 | New citation required but conclusions have not changed | The addition of one new trial changed the results of this review but not the conclusions. This version includes a 'Summary of findings' table with GRADE assessment, unlike the previous version. |
| 3 August 2015 | New search has been performed | We conducted a new search in June 2016 and identified one new trial for inclusion along with four ongoing trials. |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 6, 2010
| Date | Event | Description |
|---|---|---|
| 7 April 2013 | New citation required but conclusions have not changed | One new study included |
| 7 April 2013 | New search has been performed | The updated search was conducted in May 2013, and identified one new trial for inclusion and three ongoing trials. |
Acknowledgements
We are grateful to the Cochrane Heart Group for their help and comments on the protocol. We would like to thank Marimar Ubeda and Eukene Ansuategui for her help and advice on the electronic searches. We are also grateful to Pascual Bordes and Teresa Lozano for helping us to classify the importance of clinical outcomes.
Appendices
Appendix 1. Search strategies 2010
CENTRAL in the Cochrane Library
#1 MeSH descriptor Myocardial Infarction explode all trees #2 myocardial next infarct* #3 heart next infarct* #4 (acute near/3 coronary ) #5 (coronary near/3 syndrome* ) #6 heart next attack* #7 MeSH descriptor Coronary Thrombosis this term only #8 coronary near/3 thrombosis #9 ami #10 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9) #11 MeSH descriptor Oxygen Inhalation Therapy explode all trees #12 oxygen #13 (#10 and #12)
MEDLINE (OVID)
1 exp Myocardial Infarction/ 2 myocardial infarct$.tw. 3 heart attack$.tw. 4 heart infarct$.tw. 5 (coronary adj3 syndrome$).tw. 6 acute coronary.tw. 7 Coronary Thrombosis/ 8 coronary thrombosis.tw. 9 ami.tw. 10 or/1‐9 11 Oxygen Inhalation Therapy/ 12 (oxygen adj3 (therapy or treat$ or effect$ or admin$ or inhal$)).tw.13 oxygen.ti. or Oxygenotherapy/ 14 or/11‐13 15 10 and 14 16 randomized controlled trial.pt. 17 controlled clinical trial.pt. 18 randomized controlled trials.sh. 19 random allocation.sh. 20 double blind method.sh. 21 single‐blind method.sh. 22 or/16‐21 23 (animals not humans).sh. 24 22 not 23 25 clinical trial.pt. 26 exp clinical trials/ 27 (clin$ adj25 trial$).ti,ab. 28 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 29 placebos.sh. 30 placebo$.ti,ab. 31 random$.ti,ab. 32 research design.sh. 33 or/25‐32 34 33 not 23 35 34 not 24 36 comparative study.sh. 37 exp evaluation studies/ 38 follow up studies.sh. 39 prospective studies.sh. 40 (control$ or prospectiv$ or volunteer$).ti,ab. 41 or/36‐40 42 41 not 23 43 42 not (24 or 35) 44 24 or 35 or 43 45 15 and 44
Embase (OVID)
1 exp Heart Infarction/ 2 Coronary Artery Thrombosis/ 3 myocardial infarct$.tw. 4 heart attack$.tw. 5 heart infarct$.tw. 6 (coronary adj3 syndrome$).tw. 7 acute coronary.tw. 8 coronary thrombosis.tw. 9 ami.tw. 10 or/1‐9 11 oxygen therapy/ 12 (oxygen adj3 (therapy or treat$ or effect$ or admin$ or inhal$)).tw. 13 oxygen.ti. 14 or/11‐13 15 10 and 14
Pascal
1 oxygen.mp. [mp=abstract, descriptors ‐ english, descriptors ‐ french, descriptors ‐ spanish, heading words, identifiers ‐ english, identifiers ‐ french, identifiers ‐ spanish, title, translated title] 2 myocardial infarction.mp. [mp=abstract, descriptors ‐ english, descriptors ‐ french, descriptors ‐ spanish, heading words, identifiers ‐ english, identifiers ‐ french, identifiers ‐ spanish, title, translated title] 3 acute coronary syndrome.mp. [mp=abstract, descriptors ‐ english, descriptors ‐ french, descriptors ‐ spanish, heading words, identifiers ‐ english, identifiers ‐ french, identifiers ‐ spanish, title, translated title] 4 2 or 3 5 1 and 4 6 random$.mp. [mp=abstract, descriptors ‐ english, descriptors ‐ french, descriptors ‐ spanish, heading words, identifiers ‐ english, identifiers ‐ french, identifiers ‐ spanish, title, translated title] 7 5 and 6
CINAHL (EBSCO)
(heart attack* or MI or AMI or heart infarct* or myocardial infarct* or coronary syndrome or coronary thrombosis) AND ((oxygen) AND (random* or control* or trial*)
LILACS (BIREME)
(heart or MI or AMI or myocardial or coronary) AND (oxygen) AND (random* or control* or trial*)
ISI Proceedings (Web of Knowledge)
(heart or MI or AMI or myocardial or coronary) AND (oxygen) AND (random* or control* or trial*)
Appendix 2. Search strategies 2012
CENTRAL
#1 MeSH descriptor Myocardial Infarction explode all trees #2 (myocardial infarct*) #3 (heart attack*) #4 (heart infarct*) #5 (coronary near/3 syndrome*) #6 "acute coronary" #7 MeSH descriptor Coronary Thrombosis, this term only #8 "coronary thrombosis" #9 (ami) #10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9) #11 MeSH descriptor Oxygen Inhalation Therapy, this term only #12 (oxygen near/3 (therapy or treat* or effect* or admin* or inhal*)) #13 (oxygen):ti #14 (oxygenotherapy) #15 (#11 OR #12 OR #13 OR #14) #16 (#10 AND #15), from 2010 to 2012
MEDLINE (OVID)
1 exp Myocardial Infarction/ 2 myocardial infarct$.tw. 3 heart attack$.tw. 4 heart infarct$.tw. 5 (coronary adj3 syndrome$).tw. 6 acute coronary.tw. 7 Coronary Thrombosis/ 8 coronary thrombosis.tw. 9 ami.tw. 10 or/1‐9 11 Oxygen Inhalation Therapy/ 12 (oxygen adj3 (therapy or treat$ or effect$ or admin$ or inhal$)).tw. 13 oxygen.ti. or Oxygenotherapy.tw. 14 or/11‐13 15 10 and 14 16 randomized controlled trial.pt. 17 controlled clinical trial.pt. 18 randomized.ab. 19 placebo.ab. 20 drug therapy.fs. 21 randomly.ab. 22 trial.ab. 23 groups.ab. 24 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25 exp animals/ not humans.sh. 26 24 not 25 27 15 and 26 28 limit 27 to yr="2010 ‐Current"
Embase (OVID)
1 exp Myocardial Infarction/ 2 myocardial infarct$.tw. 3 heart attack$.tw. 4 heart infarct$.tw. 5 (coronary adj3 syndrome$).tw. 6 acute coronary.tw. 7 Coronary Thrombosis/ 8 coronary thrombosis.tw. 9 ami.tw. 10 or/1‐9 11 Oxygen Inhalation Therapy/ 12 (oxygen adj3 (therapy or treat$ or effect$ or admin$ or inhal$)).tw. 13 oxygen.ti. or Oxygenotherapy.tw. 14 or/11‐13 15 10 and 14 16 random$.tw. 17 factorial$.tw. 18 crossover$.tw. 19 cross over$.tw. 20 cross‐over$.tw. 21 placebo$.tw. 22 (doubl$ adj blind$).tw. 23 (singl$ adj blind$).tw. 24 assign$.tw. 25 allocat$.tw. 26 volunteer$.tw. 27 crossover procedure/ 28 double blind procedure/ 29 randomized controlled trial/ 30 single blind procedure/ 31 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 32 (animal/ or nonhuman/) not human/ 33 31 not 32 34 15 and 33 35 limit 34 to yr="2010 ‐Current"
CINAHL
S19 S14 and S17 Limiters ‐ Published Date from: 20100101‐20120731 S18 S14 and S17 S17 S15 or S16 S16 (MH "Randomized Controlled Trials") S15 random* or blind* or allocat* or trial* or placebo* or crossover* or cross‐over* S14 S10 and S13 S13 S11 or S12 S12 oxygen or oxygenotherapy S11 (MH "Oxygen Therapy+") S10 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 S9 ami S8 coronary N3 thrombosis S7 (MH "Coronary Thrombosis") S6 (heart attack*) S5 (coronary N3 syndrome* ) S4 (acute N3 coronary ) S3 (heart infarct*) S2 (myocardial infarct*) S1 (MH "Myocardial Infarction+")
Web of Science
#14 #13 AND #12 AND #8 #13 Topic=((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)) #12 #11 OR #10 OR #9 #11 Topic=(oxygenotherapy) #10 Title=((oxygen near/3 (therapy or treat* or effect* or admin* or inhal*))) #9 Title=(oxygen) #8 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #7 Topic=(ami) #6 Topic=(coronary near/3 thrombosis) #5 Topic=((heart attack*)) #4 Topic=((coronary near/3 syndrome* )) #3 Topic=((acute near/3 coronary )) #2 Topic=((heart infarct*)) #1 Topic=((myocardial infarct*))
Appendix 3. Search strategies 2015
CENTRAL
#1 MeSH descriptor: [Myocardial Infarction] explode all trees
#2 myocardial infarct*
#3 heart attack*
#4 heart infarct*
#5 (coronary near/3 syndrome*)
#6 "acute coronary"
#7 MeSH descriptor: [Coronary Thrombosis] this term only
#8 "coronary thrombosis"
#9 ami
#10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9
#11 MeSH descriptor: [Oxygen Inhalation Therapy] this term only
#12 (oxygen near/3 (therapy or treat* or effect* or admin* or inhal*))
#13 oxygen:ti
#14 oxygenotherapy
#15 #11 or #12 or #13 or #14
#16 #10 and #15 Publication Year from 2012 to 2015
MEDLINE OVID
1 exp Myocardial Infarction/
2 myocardial infarct$.tw.
3 heart attack$.tw.
4 heart infarct$.tw.
5 (coronary adj3 syndrome$).tw.
6 acute coronary.tw.
7 Coronary Thrombosis/
8 coronary thrombosis.tw.
9 ami.tw.
10 or/1‐9
11 Oxygen Inhalation Therapy/
12 (oxygen adj3 (therapy or treat$ or effect$ or admin$ or inhal$)).tw.
13 oxygen.ti. or Oxygenotherapy.tw.
14 or/11‐13
15 10 and 14
16 randomized controlled trial.pt.
17 controlled clinical trial.pt.
18 randomized.ab.
19 placebo.ab.
20 drug therapy.fs.
21 randomly.ab.
22 trial.ab.
23 groups.ab.
24 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
25 exp animals/ not humans.sh.
26 24 not 25
27 15 and 26
28 limit 27 to yr="2012 ‐Current"
Embase OVID
1 exp Myocardial Infarction/
2 myocardial infarct$.tw.
3 heart attack$.tw.
4 heart infarct$.tw.
5 (coronary adj3 syndrome$).tw.
6 acute coronary.tw.
7 Coronary Thrombosis/
8 coronary thrombosis.tw.
9 ami.tw.
10 or/1‐9
11 Oxygen Inhalation Therapy/
12 (oxygen adj3 (therapy or treat$ or effect$ or admin$ or inhal$)).tw.
13 oxygen.ti. or Oxygenotherapy.tw.
14 or/11‐13
15 10 and 14
16 random$.tw.
17 factorial$.tw.
18 crossover$.tw.
19 cross over$.tw.
20 cross‐over$.tw.
21 placebo$.tw.
22 (doubl$ adj blind$).tw.
23 (singl$ adj blind$).tw.
24 assign$.tw.
25 allocat$.tw.
26 volunteer$.tw.
27 crossover procedure/
28 double blind procedure/
29 randomized controlled trial/
30 single blind procedure/ (16122)
31 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30
32 (animal/ or nonhuman/) not human/
33 31 not 32
34 15 and 33
35 limit 34 to yr="2012 ‐ 2015"
CINAHL Plus (EBSCO)
S19 S14 and S17 Limiters ‐ Published Date from: 20120701‐20150603
S18 S14 and S17
S17 S15 or S16
S16 (MH "Randomized Controlled Trials")
S15 random* or blind* or allocat* or trial* or placebo* or crossover* or cross‐over*
S14 S10 and S13
S13 S11 or S12
S12 oxygen or oxygenotherapy
S11 (MH "Oxygen Therapy+")
S10 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9
S9 ami
S8 coronary N3 thrombosis
S7 (MH "Coronary Thrombosis")
S6 (heart attack*)
S5 (coronary N3 syndrome* )
S4 (acute N3 coronary )
S3 (heart infarct*)
S2 (myocardial infarct*)
S1 (MH "Myocardial Infarction+")
Web of Science (Thomson Reuters)
RCT filter adapted from Cochrane RCT filter.
#14 #13 AND #12 AND #8
#13 TS=((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*))
#12 #11 OR #10 OR #9
#11 TS=(oxygenotherapy)
#10 TS=((oxygen near/3 (therapy or treat* or effect* or admin* or inhal*)))
#9 TS=(oxygen)
#8 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#7 TS=(ami)
#6 TS=(coronary near/3 thrombosis)
#5 TS=((heart attack*))
#4 TS=((coronary near/3 syndrome* ))
#3 TS=((acute near/3 coronary ))
#2 TS=((heart infarct*))
#1 TS=((myocardial infarct*))
PubMed
Search ((((((publisher[sb] NOT pubstatusnihms)))) AND ( "2012/01/01"[PDat] : "2015/12/31"[PDat] ))) AND (((((((((Oxygen Inhalation Therapy[MeSH Major Topic]) OR ((oxygen n3 (therapy or treat* or effect* or admin* or inhal*)))) OR oxygen[Title]) OR oxygenotherapy)) AND (((myocardial infarct* or heart attack* or heart infarct* or (coronary n3 syndrome) or "acute coronary" or "coronary thrombosis" or ami)) OR Coronary Thrombosis[MeSH Major Topic]))) AND ((((((((((randomized controlled trial[Publication Type]) OR controlled clinical trial[Publication Type]) OR randomized[Title/Abstract]) OR placebo[Title/Abstract]) OR drug therapy[MeSH Subheading]) OR randomly[Title/Abstract]) OR trial[Title/Abstract]) OR groups[Title/Abstract])) NOT ((animals[MeSH Terms]) NOT humans[MeSH Terms]))) AND ( "2012/01/01"[PDat] : "2015/12/31"[PDat] )) Filters: Publication date from 2012/01/01 to 2015/12/31
LILACS. Latin American and Caribbean Health Sciences Literature (BIREME)
(tw:(myocardial infarction OR Coronary Disease OR Myocardium OR Heart Failure)) AND (tw:(Oxygen$” OR Oxygen Consumption OR Oxygen Inhalation Therapy)) AND (year_cluster:(“2013” OR “2014” OR “2015”)
Appendix 4. Search strategies 2016
CENTRAL
#1 MeSH descriptor: [Myocardial Infarction] explode all trees
#2 myocardial infarct*
#3 heart attack*
#4 heart infarct*
#5 (coronary near/3 syndrome*)
#6 "acute coronary"
#7 MeSH descriptor: [Coronary Thrombosis] this term only
#8 "coronary thrombosis"
#9 ami
#10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9
#11 MeSH descriptor: [Oxygen Inhalation Therapy] this term only
#12 (oxygen near/3 (therapy or treat* or effect* or admin* or inhal*))
#13 oxygen:ti
#14 oxygenotherapy
#15 #11 or #12 or #13 or #14
#16 #10 and #15 Publication Year from 2012 to 2016
MEDLINE OVID
1 exp Myocardial Infarction/
2 myocardial infarct$.tw.
3 heart attack$.tw.
4 heart infarct$.tw.
5 (coronary adj3 syndrome$).tw.
6 acute coronary.tw.
7 Coronary Thrombosis/
8 coronary thrombosis.tw.
9 ami.tw.
10 or/1‐9
11 Oxygen Inhalation Therapy/
12 (oxygen adj3 (therapy or treat$ or effect$ or admin$ or inhal$)).tw.
13 oxygen.ti. or Oxygenotherapy.tw.
14 or/11‐13
15 10 and 14
16 randomized controlled trial.pt.
17 controlled clinical trial.pt.
18 randomized.ab.
19 placebo.ab.
20 drug therapy.fs.
21 randomly.ab.
22 trial.ab.
23 groups.ab.
24 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
25 exp animals/ not humans.sh.
26 24 not 25
27 15 and 26
28 limit 27 to yr="2012 ‐Current"
EMBASE OVID
1 exp Myocardial Infarction/
2 myocardial infarct$.tw.
3 heart attack$.tw.
4 heart infarct$.tw.
5 (coronary adj3 syndrome$).tw.
6 acute coronary.tw.
7 Coronary Thrombosis/
8 coronary thrombosis.tw.
9 ami.tw.
10 or/1‐9
11 Oxygen Inhalation Therapy/
12 (oxygen adj3 (therapy or treat$ or effect$ or admin$ or inhal$)).tw.
13 oxygen.ti. or Oxygenotherapy.tw.
14 or/11‐13
15 10 and 14
16 random$.tw.
17 factorial$.tw.
18 crossover$.tw.
19 cross over$.tw.
20 cross‐over$.tw.
21 placebo$.tw.
22 (doubl$ adj blind$).tw.
23 (singl$ adj blind$).tw.
24 assign$.tw.
25 allocat$.tw.
26 volunteer$.tw.
27 crossover procedure/
28 double blind procedure/
29 randomized controlled trial/
30 single blind procedure/ (16122)
31 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30
32 (animal/ or nonhuman/) not human/
33 31 not 32
34 15 and 33
35 limit 34 to yr="2012 ‐ 2016"
Cinahl Plus (EBSCO)
S19 S14 and S17 Limiters ‐ Published Date from: 20120701‐20160606
S18 S14 and S17
S17 S15 or S16
S16 (MH "Randomized Controlled Trials")
S15 random* or blind* or allocat* or trial* or placebo* or crossover* or cross‐over*
S14 S10 and S13
S13 S11 or S12
S12 oxygen or oxygenotherapy
S11 (MH "Oxygen Therapy+")
S10 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9
S9 ami
S8 coronary N3 thrombosis
S7 (MH "Coronary Thrombosis")
S6 (heart attack*)
S5 (coronary N3 syndrome* )
S4 (acute N3 coronary )
S3 (heart infarct*)
S2 (myocardial infarct*)
S1 (MH "Myocardial Infarction+")
Web of Science (ISI)
RCT filter adapted from Cochrane RCT filter.
#14 #13 AND #12 AND #8
#13 TS=((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*))
#12 #11 OR #10 OR #9
#11 TS=(oxygenotherapy)
#10 TS=((oxygen near/3 (therapy or treat* or effect* or admin* or inhal*)))
#9 TS=(oxygen)
#8 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#7 TS=(ami)
#6 TS=(coronary near/3 thrombosis)
#5 TS=((heart attack*))
#4 TS=((coronary near/3 syndrome* ))
#3 TS=((acute near/3 coronary ))
#2 TS=((heart infarct*))
#1 TS=((myocardial infarct*))
PubMed
(publisher[sb] NOT pubstatusnihms) AND ( "2015/01/01"[PDat] : "2016/12/31"[PDat]) AND
(Oxygen Inhalation Therapy[MeSH Major Topic]) OR (oxygen n3 (therapy or treat* or effect* or admin* or inhal*)) OR (oxygen[Title]) OR oxygenotherapy) AND
(myocardial infarct* or heart attack* or heart infarct* or (coronary n3 syndrome) or "acute coronary" or "coronary thrombosis" or ami) OR Coronary Thrombosis[MeSH Major Topic]) AND
(((randomized controlled trial[Publication Type]) OR (controlled clinical trial[Publication Type]) OR randomized[Title/Abstract] OR placebo[Title/Abstract] OR drug therapy[MeSH Subheading] OR randomly[Title/Abstract] OR trial[Title/Abstract] OR groups[Title/Abstract]) NOT ((animals[MeSH Terms]) NOT humans[MeSH Terms])) AND ( "2015/01/01"[PDat] : "2016/12/31"[PDat])
LILACS. Latin American and Caribbean Health Sciences Literature (BIREME)
(tw:(myocardial infarction OR Coronary Disease OR Myocardium OR Heart Failure)) AND (tw:(Oxygen$” OR Oxygen Consumption OR Oxygen Inhalation Therapy)) AND (year_cluster:(“2013” OR “2014” OR “2015” OR "2016")
Data and analyses
Comparison 1. Oxygen versus air.
1.16. Analysis.
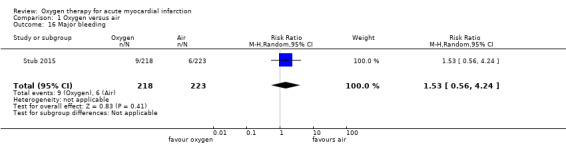
Comparison 1 Oxygen versus air, Outcome 16 Major bleeding.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ranchord 2012.
| Methods | Open‐label randomised controlled trial (6 weeks) | |
| Participants | People with ischaemic symptoms + ST‐segment elevation (0.1 mV) in 2 contiguous leads STEMI or elevation (0.2 mV) in more than 2 precordial leads (STEMI), or with ischaemic symptoms + new onset left bundle branch block N randomised = 148. N analysed = 136. Dropouts: withdrew consent (n = 5), alternative diagnosis (n = 5): 2 cases with pericarditis and 3 cases with normal coronary arteries. 2 cases of cardiogenic shock (an exclusion criterion) Mean age (SD): oxygen 60 years (12.5), air 60 years (12.8). Sex: 77.9% men in oxygen and 70.6% men in air group |
|
| Interventions | Intervention: oxygen high flow 6 L/min by concentration mask Comparator: oxygen titrated delivered by nasal prongs or mask adjusting the flow‐rate to achieve an oxygen saturation of 93%‐96% |
|
| Outcomes | 30 days mortality, complications, infarct size estimated by troponin T level measured 66 h to 78 h after randomisation, infarct mass (absolute and as percentage) documented by MRI (measured at 4‐6 weeks after AMI in a subset of participants), pro‐BNP measured 24 h after randomisation. As composite variable, major cardiac event (death, reinfarction, target vessel revascularisation) at 30 days | |
| Exclusions | Previous myocardial infarction, COPD, type II respiratory failure, cardiogenic shock, oxygen desaturation below 85%, pregnancy, bleomycin treatment or participation in another trial | |
| Length of follow‐up | 30 days for mortality, troponin T and BNP, 4‐5 weeks after AMI for MRI | |
| Clinical Context and parallel care | The study was undertaken exclusively in inpatients, therefore the pre‐hospital phase of AMI was not considered. Primary percutaneous coronary intervention (PPCI) was the first‐choice treatment in one centre, while in the other, PPCI or thrombolysis was the treatment, depending on the hour of hospital admission. |
|
| Notes | The study was conducted in two centres. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence was undertaken by a computer programme. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) Death | Low risk | There is no threat for this outcome |
| Blinding (performance bias and detection bias) Pain (or surrogate) | Unclear risk | Not applicable in this trial |
| Blinding (performance bias and detection bias) infarct size ECG | Unclear risk | Not applicable in this trial |
| Incomplete outcome data (attrition bias) Death | High risk | There are 12 postrandomisation exclusions for which there are no 30‐day mortality data reported. |
| Incomplete outcome data (attrition bias) Infacrt size (Biochemical methods) | High risk | There are 12 postrandomisation exclusions in which there are no reported biochemical data |
| Incomplete outcome data (attrition bias) infarct size ECG mapping | Unclear risk | Not applicable in this trial |
| Incomplete outcome data (attrition bias) Infarct size (MRI) | High risk | By definition, the primary outcome (30 days mortality) implies that MRI was not performed (by protocol performed 4‐5 weeks after AMI). Data therefore not available. |
| Selective reporting (reporting bias) | High risk | MRI was performed only in a subgroup of participants (selective reporting of subgroup) |
| Other bias | High risk | Pre‐randomisation oxygen was administered in experimental and control group (86.8% and 63% respectively). This prerandomisation intervention may have produced a bias in effect estimation towards the null hypothesis. The comparison of infarct size measured by MRI between the two groups should be considered a non‐randomised comparison, therefore prone to the bias of observational studies |
| Baseline characteristics | Unclear risk | There were differences in previous PCI, and in the infarct territory: anterior infarction was less frequent in the experimental group (18%) than in the control group (31%) |
Rawles 1976.
| Methods | Double‐blind, randomised controlled trial (In‐Hospital period) | |
| Participants | People with suspected AMI presenting within 24 h of symptoms onset Clinical setting: single site coronary care unit in the UK N randomised = 200: 105 oxygen and 95 air. N analysed: 80 oxygen and 77 air Excluded (non‐confirmed AMI): 25 oxygen and 18 air Mean age (SD): oxygen 51.3 years (1.7), air 50.8 years (2.4) Sex: 60% men in oxygen and 64% in air group |
|
| Interventions | Oxygen or compressed air administered by MC mask at 6 L/min over 24 h Comparator: air at normal pressure given at 6 L/min by MC mask |
|
| Outcomes | Death in Hospital, arrhythmias in 24 hours, use of opiates, maximum serum aspartate aminotransferase levels, length of stay, systolic ejection time, hypoxaemia (first day) | |
| Exclusions | People with heart failure, bronchitis, emphysema, or other respiratory problems | |
| Length of follow‐up | Discharge | |
| Clinical Context and parallel care | Prethrombolysis period | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no description of how the sequence was generated |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes |
| Blinding (performance bias and detection bias) Death | Low risk | Double‐blinded using shrouded cylinders (but likely that the blinding could have been compromised) |
| Blinding (performance bias and detection bias) Pain (or surrogate) | Unclear risk | Double‐blinded using shrouded cylinders (but likely that the blinding could have been compromised, and this may affect the assessment of this outcome: pain or surrogate) |
| Blinding (performance bias and detection bias) infarct size ECG | Unclear risk | Not applicable in this trial |
| Incomplete outcome data (attrition bias) Death | Low risk | There were postrandomisation exclusions due to unconfirmed AMI (19% air group and 24% O2 group) |
| Incomplete outcome data (attrition bias) Infacrt size (Biochemical methods) | Unclear risk | Not applicable in this trial |
| Incomplete outcome data (attrition bias) infarct size ECG mapping | Unclear risk | Not applicable in this trial |
| Incomplete outcome data (attrition bias) Infarct size (MRI) | Unclear risk | Not applicable in this trial |
| Selective reporting (reporting bias) | Unclear risk | There was no protocol published, but we judged that there was no bias in reporting the primary outcome. |
| Other bias | Low risk | We did not identify other biases. |
| Baseline characteristics | Low risk | Consecutive participants, similar age, sex |
Stub 2015.
| Methods | Multicentre, open‐label, randomised controlled trial (6 months) | |
| Participants | Patients ≥18 year old with ischaemic symptoms of < 12 h duration, with evidence of STEMI defined by ST elevation > 0.1 mm in 2 contiguous limb leads, > 0.2 mm in 2 contiguous chest leads, or new left bundle branch block. In the published protocol the participants were defined as those with suspected STEMI. The recruitment was made in the pre‐hospital setting (by paramedics in the ambulance) and the informed consent was obtained after the arrival at hospital (delayed consent) N randomised = 638. N analysed = 441: 14 refused consent, 35 exclusions for protocol violations, 1 repeated enrollment (see text). Elegible for angiography: 470 (471 for authors), 29 other diagnosis. Mean age (SD): oxygen 13.0 years ( 11.9), air 62.6 years (13.0) Sex: 79.8% men in oxygen, 78.0% men in air group |
|
| Interventions | Intervention: oxygen by mask 8 L/min Comparator: air If the oxygen saturation fell below 94% then titrated oxygen was administered by cannula or face mask to achieve an oxygen saturation of 94%. |
|
| Outcomes | Infarct size estimated by cTnl and CK (in both cases geometric mean peak and geometric mean of AUC) at 72 h of reperfusion Pain score (measured on arrival of paramedics and on arrival at hospital). The scale for measuring pain is not described in the protocol nor in the paper. Survival at hospital discharge, mortality and major adverse cardiac events (MACE: death, recurrent myocardial infarction, repeat revascularisation, and stroke) were assessed at 6 months Infarct size was estimated by MRI at 6 months after hospital discharge. An MRI was undertaken in a subset of self‐selected participants (non‐random comparison) *All primary efficacy and safety outcomes (mortality , cardiac arrest and unplanned intubation) were explored by a monitoring committee in an interim analysis made after 450 randomisations. In the protocol this interim analysis was planned after 100 were randomised to each arm. This planned analysis is not reported, and no adjustments for multiple comparisons were undertaken. |
|
| Exclusions | Oxygen desaturation < 94% (pulse oximeter); bronchospasm requiring salbutamol nebulised; altered conscious state; oxygen administration prior to randomisation | |
| Length of follow‐up | 6 months | |
| Clinical Context and parallel care | Primary percutaneous coronary intervention (PPCI) was the first‐choice treatment in all the participant hospitals. All the patients received aspirin 300 mg in ambulance and antiplatelet therapy according to interventional cardiologist criteria. | |
| Notes | This was a multicentre study undertaken in 9 metropolitan hospitals that provide 24 h percutaneous coronary intervention services. The participant were included, randomised and treated (with oxygen or air) before the arrival at hospital (in the ambulance), and therefore the trial was designed to cover the pre‐hospital phase of STEMI. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence was computer‐generated code in blocks of ten. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes in each ambulance participating in the study: 3 envelopes of the block of 10, and replaced with other of the block, and after new block by coordinator.?? |
| Blinding (performance bias and detection bias) Death | Low risk | There was no information in the paper about participants who refuse to give informed consent and we contacted the authors for more information. |
| Blinding (performance bias and detection bias) Pain (or surrogate) | High risk | This was an open label trial, although pain scores are shown, there is no description of the scale and methods used to measure the pain. |
| Blinding (performance bias and detection bias) infarct size ECG | Unclear risk | Not applicable in this trial |
| Incomplete outcome data (attrition bias) Death | Low risk | Mortality at discharge and six month following the patient's randomisation |
| Incomplete outcome data (attrition bias) Infacrt size (Biochemical methods) | High risk | Peak of creatin kinase is reported in 217 of 218 participants in oxygen arm and in 222 of 223 in the air (loss of 0.45% and 0.44% respectively). Low risk of bias Peak of troponin I is reported in 200 of the 218 in the oxygen group and 205 of 223 participants in the air (loss of 8.3% and 8.7% respectively). High risk of bias The missing values in serial enzyme estimation to built the AUC are unknown (a method is described as a Markov method was used to performed the estimation of AUC. Unclear risk of bias |
| Incomplete outcome data (attrition bias) infarct size ECG mapping | Unclear risk | Not applicable in this trial |
| Incomplete outcome data (attrition bias) Infarct size (MRI) | High risk | The MRI was done in a self‐selected group of study population, this implies the existence of high risk of selection bias and the nullification of the balancing effect of randomisation. |
| Selective reporting (reporting bias) | High risk | MRI was performed only in a subgroup of 'self‐selected' participants (selective reporting of subgroup) In addition, the MRI was undertaken 6 months after the STEMI and the population represents a cohort of survivors. |
| Other bias | Unclear risk | Protocol deviation, sample size, interim analysis |
| Baseline characteristics | Unclear risk | There is a table with the description of key data in both groups with confirmed STEMI, but there is no table comparing the baseline characteristics of all randomised patients (table 1 of CONSORT) |
Ukholkina 2005.
| Methods | Randomised, open‐label, controlled trial (10 days) | |
| Participants | Confirmed AMI within 12 h of onset of symptoms. Clinical setting: single‐site coronary care unit in Russia N randomised = 137. No explicit data were provided about the participants who were excluded postrandomisation Mean age (SD): oxygen 55.6 years (1.33), air 53.5 years (1.06) Sex: 45% men in oxygen and 70% in air group |
|
| Interventions | Oxygen for 3 h after intervention administered via nasal cannulae 3‐6 L/min (FiO2 30%‐40%) and 30 min before the PCA in a subgroup of 30 participants Comparator: air |
|
| Outcomes | Death, arrhythmias within 1 h of reperfusion, surgery during hospital stay, recurrent AMI, postinfarction angina, hypoxaemia, heart failure, pericarditis Area of tissue damage measured by ECG mapping and cardiac enzymes (CK‐MB) |
|
| Exclusions | People with complicated AMI, congestive heart failure, pulmonary disease, COPD or anaemia | |
| Length of follow‐up | 10 days | |
| Clinical Context and parallel care | Context of primary PCI | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Death | Low risk | This was an open‐label trial (but absence of blinding unlikely to introduces bias in this outcome) |
| Blinding (performance bias and detection bias) Pain (or surrogate) | Unclear risk | Not applicable in this trial (pain was not a variable evaluated in the study) |
| Blinding (performance bias and detection bias) infarct size ECG | Unclear risk | This was an open‐label trial (but the absence of blinding unlikely to introduce bias in this outcome) |
| Incomplete outcome data (attrition bias) Death | High risk | While mortality was adequately reported for included participants, there was inadequate description of exclusion postrandomisation in each group (e.g. failed revascularisation) |
| Incomplete outcome data (attrition bias) Infacrt size (Biochemical methods) | High risk | There was inadequate description of exclusion postrandomisation in each group |
| Incomplete outcome data (attrition bias) infarct size ECG mapping | Unclear risk | Inadequate description of exclusion postrandomisation in each group (e.g. failed revascularisation). Consequently, these participants are not included in the infarct size comparison. There were problems of consistency in the measurement process of ECG mapping done to estimate infarct size |
| Incomplete outcome data (attrition bias) Infarct size (MRI) | Unclear risk | Not applicable in this trial |
| Selective reporting (reporting bias) | High risk | We have no information about the protocol, but the infarct size estimation was only reported in 31 patients in the oxygen group and no information in the air group |
| Other bias | High risk | See baseline imbalances |
| Baseline characteristics | High risk | The groups were different at baseline in two important variables:
|
Wilson 1997.
| Methods | Randomised, open‐label, controlled trial | |
| Participants | People with confirmed AMI presenting within 24 h of onset of symptoms Clinical setting: single‐site coronary care unit in the UK N randomised = 50. N analysed = 42 (dropouts: 1 death, 1 stroke, 4 withdrew consent, 2 incomplete data collection) Mean age: oxygen 65 years, air 64 years Sex: 59% men |
|
| Interventions | Oxygen by face mask at 4 L/min versus normal air over 24 h | |
| Outcomes | 24 hours for Hypoxaemia, arrhythmias, cardiac enzymes. | |
| Exclusions | People with heart failure, cyanosis central or pulmonary disease requiring O2 | |
| Length of follow‐up | Discharge | |
| Clinical Context and parallel care | Thrombolysis period | |
| Notes | The primary purpose of this trial was to look at the effect of oxygen on hypoxaemia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes for randomisation |
| Blinding (performance bias and detection bias) Death | Low risk | This was an open‐label trial (but the absence of blinding is unlikely to introduce bias in this outcome) |
| Blinding (performance bias and detection bias) Pain (or surrogate) | High risk | This was an open‐label trial, therefore the risk of bias in this outcome cannot be ruled out |
| Blinding (performance bias and detection bias) infarct size ECG | Unclear risk | Not relevant to this study |
| Incomplete outcome data (attrition bias) Death | High risk | 8 out of 50 missing data (group not specified); 1 death, 1 stroke, 4 withdrew consent, 2 incomplete data |
| Incomplete outcome data (attrition bias) Infacrt size (Biochemical methods) | Unclear risk | Not relevant in this study |
| Incomplete outcome data (attrition bias) infarct size ECG mapping | Unclear risk | Not relevant in this study |
| Incomplete outcome data (attrition bias) Infarct size (MRI) | Unclear risk | Not relevant in this study |
| Selective reporting (reporting bias) | High risk | The main variables of the study were incidence and degree of hypoxaemia and the effect of oxygen administration. The main outcome of this review (death) was not reported, and in fact the only participant who died was not included in the analysis |
| Other bias | Low risk | Other biases were not identified |
| Baseline characteristics | Low risk | Consecutive participants, similar age, smoking and diabetes |
AMI: myocardial infarction; AUC: area under the curve; BNP: brain natriuretic peptide; COPD: chronic obstructive pulmonary disease; ECG: electrocardiogram; MC: medium concentration: MRI: magnetic resonance imaging; PCI: percutaneous coronary intervention; PCA:percutaneous coronary angioplaty. SD: standard deviation; STEMI: ST‐segment elevation myocardial infarction.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AMIHOT 2003 | Wrong intervention: aqueous oxygen therapy in STEMI |
| Dekleva 2004 | Wrong intervention: hyperbaric oxygen versus air in participants after thrombolysis in AMI |
| Dotsenko 2007 | Wrong intervention: hyperbaric oxygen versus air in conventionally treated participants with AMI |
| Haude 2007 | Wrong intervention: supersaturated oxygen therapy after percutaneous coronary intervention in AMI |
| Kerr 1975 | Different intervention: nitrous oxide 50% with or without oxygen 50% versus air in participants with AMI |
| Shandling 1997 | Wrong intervention: hyperbaric oxygen |
| Slagboom 2005 | Wrong intervention: haemoglobin‐based oxygen therapeutics in elective PCI |
AMI: acute myocardial infarction; PCI: percutaneous coronary intervention; PCA: percuaneous coronary angioplasty; STEMI: ST‐segment elevation myocardial infarction.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12609000466246.
| Trial name or title | A randomised controlled trial comparing controlled oxygen therapy versus high flow oxygen therapy for acute myocardial infarctions in the pre‐hospital setting (no specific name available) |
| Methods | Randomised controlled trial, parallel design with open label and allocation concealment |
| Participants | People with chest pain and suspicion of acute coronary syndrome attended by Tasmanian ambulance service in the Launceston region |
| Interventions | High flow oxygen 8‐15 L/min by non‐breather mask compared to oxygen therapy to maintain oxygen saturation between 92%‐96% |
| Outcomes | Primary outcome: mortality during ambulance or in the hospital stay Secondary outcomes:
|
| Starting date | Theoretically January 2012 |
| Contact information | Dr Michael Austin, Menzies Research Institute (Private Bag 23) Hobart TAS 7001. maaustin@utas.edu.au |
| Notes | Not recruiting yet (register visited last time 30 June 2016) |
NCT01423929.
| Trial name or title | Supplemental oxygen in catheterization coronary emergency reperfusion (SOCCER) |
| Methods | Multicentre single‐blind randomised controlled trial, parallel design and allocation concealment |
| Participants | Normoxic STEMI ambulance patients with symptom duration less than 6 hours (bypassing the emergency department) |
| Interventions | Oxygen 10 L/min by oxymask versus room air |
| Outcomes | Primary outcomes: infarct size estimated by MRI at day 4, myocardial salvage index by MRI Secondary outcomes: TIMI flow during PCI, echocardiography (acute and 6 months after AMI), pro‐BNP, and dose of opioids |
| Starting date | January 2012 |
| Contact information | Mahin Akbarzadeh (Skåne University Hospital at Lund) |
| Notes | 2 hospitals with PPCI capabilities. Verbal informed consent in ambulance and delayed written informed consent |
NCT01787110.
| Trial name or title | An efficacy and outcome study of supplemental oxygen treatment in patients with suspected myocardial infarction (DETO2X‐AMI) |
| Methods | Multicentre open label registry‐based randomised clinical trial |
| Participants | Normoxic patients (saturation > 90%) in emergency medical service or emergency departments with suspicion of acute myocardial infarction (STEMI and non STEMI) within the last 6 h |
| Interventions | Oxygen 6 L/min by oxymask started as soon as possible and maintained for 13 h vs no oxygen except if saturation < 90% (repeatedly checked) |
| Outcomes | Primary outcome: 1 year all‐cause mortality. Secondary outcomes: 30‐days mortality, major cardiac coronary events (MACE) in 30 days and 1 year including reinfarction and hospitalisations for cardiac failure. In sub‐studies, echocardiograpy and cardiac magnetic resonance will be used to assess infarct size and cardiac function. |
| Starting date | April 2013 |
| Contact information | Leif Svensson and Robin Hofmann (Södersjukhuset. Stockholm, Sweden, 11883) |
| Notes | The protocol and feasibility studies have been published (see ongoing trials) |
NCT02290080.
| Trial name or title | Determination of the role of oxygen in suspected acute myocardial infarction by biomarkers (DETO2X‐bio) |
| Methods | Single blind (outcomes assessor) randomised clinical trial |
| Participants | Normoxic patients (saturation > 90%) in emergency medical service or emergency departments with suspicion of acute myocardial infarction (STEMI and non STEMI) within the last 6 h |
| Interventions | Oxygen 6 L/min by oxymask started as soon as possible and maintained for 13 h vs no oxygen except if saturation < 90% (repeatedly checked) |
| Outcomes | Plasma concentration levels over time of biomarkers of oxidative stress, apoptosis, inflammation and platelet aggregation |
| Starting date | October 2014 (estimated study completion October 2015) |
| Contact information | Leif Svensson & Robin Hofmann (Södersjukhuset. Stockholm, Sweden, 11883 |
| Notes | This study is a substudy of DETO2X‐AMI |
AMI: acute myocardial infarction; BNP: brain natriuretic peptide; MRI: magnetic resonance imaging; PCI: percutaneous coronary intervention; TIMI: thrombolysis in myocardial infarction.
Differences between protocol and review
Data were too sparse to permit adequate analysis of the subgroups that had been prespecified for exploration.
We made three changes:
One minor change in the search strategy to improve the sensitivity, i.e. the inclusion of the text word 'oxygenotherapy' in the title (the original search failed to pick up the Russian article and we looked to see if it was in MEDLINE and, if so, why the search strategy had missed it);
After the protocol was published, a new version of the Cochrane Handbook for Systematic Reviews of Interventions recommended a new approach to assessment of risk of bias, so we changed our method of assessment to be consistent with the recommendations (Higgins 2011).
In this version of the review, we have used the nine‐point scale suggested by GRADE to classify the clinical importance of the outcomes (Guyatt 2008).
In this version, we include the 'Summary of findings' table with GRADE assessment, unlike the previous version.
Contributions of authors
Juan Cabello provided expert advice, co‐wrote the protocol and helped with quality assessment, contacted authors for further information, data extraction, analysis, writing the discussion and entering data into RevMan.
Amanda Burls co‐wrote the protocol, contacted authors for further information and contributed to quality assessment, data extraction, analysis, writing the discussion, and entering data into RevMan.
Sue Bayliss undertook the electronic searches, helped obtain papers and proofread the review.
Jose Emparanza Knorr co‐wrote the protocol and contributed to quality assessment, data extraction, analysis and writing of the discussion.
Tom Quinn provided expert advice, contacted experts to find unpublished studies and contributed to quality assessment, data extraction, revision of the draft paper and writing of the discussion.
Sources of support
Internal sources
No sources of support supplied
External sources
-
None, Not specified.
No financial support was received for this review
Declarations of interest
None on starting this review. After starting this systematic review two of the authors (AB and TQ) have put together, with other clinical colleagues, a proposal for a randomised controlled trial in the UK of oxygen for AMI in the pre‐hospital setting. This proposal was not supported, and therefore the protocol was cancelled.
Tom Quinn was a member of the steering group for the STREAM Trial (Boehringer Ingelheim) and the EUROMAX trial (Medicines Company), and he was a local collaborator/principal investigator for the ATLANTIC trial (Astra Zeneca). All of these were studies of pre‐hospital management of patients with acute coronary syndrome.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ranchord 2012 {published data only (unpublished sought but not used)}
- Ranchord AM, Argyle R, Beynon R, Perrin K, Sharma V, Weatherall M, et al. High‐concentration versus titrated oxygen therapy in ST‐elevation myocardial infarction: a pilot randomized controlled trial. American Heart Journal 2012;163(2):168‐75. [DOI] [PubMed] [Google Scholar]
Rawles 1976 {published data only}
- Rawles J, Kenmure ACF. Controlled trial of oxygen in uncomplicated myocardial infarction. British Medical Journal 1976;1:1121‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stub 2015 {published data only (unpublished sought but not used)}
- Nehme Z, Stub D, Bernard S, Stephenson M, Bray JE, Cameron P, et al. Effect of supplemental oxygen exposure on myocardial injury in ST‐elevation myocardial infarction. Heart 2016;102(6):441‐51. [DOI: 10.1136/heartjnl-2015-308636] [DOI] [PubMed] [Google Scholar]
- Smith K, Bernard S, Bray JE, Stephenson M, Cameron P, Meredith I, Kaye DM. A randomized controlled trial of oxygen therapy in acute myocardial infarction Air Verses Oxygen In myocarDial infarction study (AVOID Study). American Heart Journal 2012;169:339‐45. [DOI] [PubMed] [Google Scholar]
- Smith K, Bray J, Stub D, Stephenson M, Bernard S. Avoid methods and experience of initial randomisations. Academic Emergency Medicine 2012;19:762‐3. [Google Scholar]
- Smith K, Nehme Z, Stub D, Stephenson M, Bernard S. Air Versus Oxygen In myocarDial infarction (AVOID) study: trial methods and experience from initial randomisation. Australasian Journal of Paramedicine 2012;10:24. [Google Scholar]
- Stub D, Smith K, Bernard S. A randomised controlled trial of oxygen therapy in acute ST‐segment elevation myocardial infarction: the air versus oxygen in myocardial infarction (AVOID) study. Circulation 2014;130:2111. [Google Scholar]
- Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray J, et al. Air versus oxygen in myocardial infarction (AVOID) trial sub‐study: time‐dependent effect of oxygen administration on myocardial injury. Heart Lung and Circulation 2015;24(Suppl 3):S374. [DOI: 10.1016/j.hlc.2015.06.609] [DOI] [Google Scholar]
- Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST‐segment elevation myocardial infarction. Circulation 2015;131:2143‐50. [DOI: 10.1161/CIRCULATIONAHA.114.014494] [DOI] [PubMed] [Google Scholar]
- Stub D. [pers comm]. RE: AVOID study. Juan Cabello 21 de July de 2015.
Ukholkina 2005 {published data only}
- Ukholkina GB, Kostianov II, Kuchkina NV, Grendo EP, Gofman I. Effect of oxygen therapy used in combination with reperfusion in patients with acute myocardial infarction. Kardiologiia 2005;45(5):59. [PubMed] [Google Scholar]
- Ukholkina GB, Kostyanov I, Kuchkina NV, Grendo EP, Gofman I. Oxygen therapy in combination with endovascular reperfusion during the first hours of acute myocardial infarction:clinical and laboratory findings. International Journal of Interventional Cardioangiology 2005;9:45‐51. [Google Scholar]
Wilson 1997 {published data only}
- Wilson AT, Channer KS. Hypoxaemia and supplemental oxygen therapy in the first 24 hours after myocardial infarction: the role of pulse oximetry. Journal of the Royal College of Physicians of London 1997;31:657‐61. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
AMIHOT 2003 {published data only}
- Martin J, Shapiro T, Dixon S, Pensyl C, Oemrawsingh PV, Atsma D, et al. Aqueous oxygen therapy for ST‐segment elevation myocardial infarction; AMIHOT trial safety report and enrolment completion. European Heart Journal 2004;25:422. [Google Scholar]
- Martin JL, Day F, Dixon S, Atsma D, Oemrawsingh P, Pensyl C, et al. Aqueous oxygen therapy for ST segment elevation myocardial infarction: AMIHOT trial safety report and enrolment completion. Journal of the American College of Cardiology 2004;43(5):304A. [Google Scholar]
- Martin JL, Day F, Dixon S, David S, Pensyl C, Hermiller J, et al. Aqueous oxygen therapy for ST‐segment elevation myocardial infarction; AMIHOT trial design and interim report. American Journal of Cardiology 2003;92(6A):3L. [Google Scholar]
- Martin JL, Dixon S, David S, Pensyl C, Lindsay B, O'Neill W. Aqueous oxygen therapy for ST‐segment elevation myocardial infarction: AMIHOT trial design and preliminary results. Journal of the American College of Cardiology 2003;41(6):72A. [Google Scholar]
- Martin JL, Oemrawsingh PV, Bartorelli AB, Dixon SD, Krukoff MW, Lindsay BS, et al. Aqueous oxygen therapy for ST segment elevation myocardial infarction; Final results and one year follow up of the AMIHOT trial. Journal of the American College of Cardiology 2005;45(3):242A. [Google Scholar]
- O'Neill WW, Martin JL, Dixon SR, Bartorelli AL, Trabattoni D, Oemrawsingh PV, et al. Acute myocardial infarction with hyperoxemic therapy (AMIHOT) ‐ a prospective, randomized trial of intracoronary hyperoxemic reperfusion after percutaneous coronary intervention. Journal of the American College of Cardiology 2007;50(5):397‐405. [DOI] [PubMed] [Google Scholar]
- Stone GW, Martin JL, Boer MJ, Margheri M, Bramucci E, Blankenship JC, et al. Effect of supersaturated oxygen delivery on infarct size after percutaneous coronary intervention in acute myocardial infarction. Circulation. Cardiovascular interventions 2009;2(6):366‐75. [DOI] [PubMed] [Google Scholar]
Dekleva 2004 {published data only}
- Dekleva M, Neskovic A, Vlahovic A, Putnikovic B, Beleslin B, Ostojic M. Adjunctive effect of hyperbaric oxygen treatment after thrombolysis on left ventricular function in patients with acute myocardial infarction. American Heart Journal 2004;148(4):E14. [DOI] [PubMed] [Google Scholar]
Dotsenko 2007 {published data only}
- Dotsenko EA, Salivonchik DP, Kozyro VI. Long‐term results of the use of hyperbaric oxygenation in patients with acute myocardial infarction. Kardiologiia 2007;47(12):53‐6. [PubMed] [Google Scholar]
Haude 2007 {published data only}
- Haude M. AMIHOT‐II: A prospective, randomized evaluation of supersaturated oxygen therapy after percutaneous coronary intervention in acute anterior myocardial infarction. Herz 2007;32(8):669. [Google Scholar]
Kerr 1975 {published data only}
- Kerr F, Brown MG, Irving JB, Hoskins MR, Ewing DJ, Kirby BJ. A double‐blind trial of patient‐controlled nitrous‐oxide/oxygen analgesia in myocardial infarction. Lancet 1975;1(7922):1397‐400. [DOI] [PubMed] [Google Scholar]
Shandling 1997 {published data only}
- Laden G. HOT MI pilot study. Hyperbaric oxygen and thrombolysis in myocardial infarction. American Heart Journal 1998;136(4 Pt 1):749. [PubMed] [Google Scholar]
- Shandling AH, Ellestad MH, Hart GB, Crump R, Marlow D, Natta B, et al. Hyperbaric oxygen and thrombolysis in myocardial infarction: the "HOT MI" pilot study. American Heart Journal 1997;134(3):544‐50. [DOI] [PubMed] [Google Scholar]
- Stavitsky Y, Shandling AH, Ellestad MH, Hart GB, Natta B, Messenger JC, et al. Hyperbaric oxygen and thrombolysis in myocardial infarction: the 'HOT MI' randomized multicenter study. Cardiology 1998;90(2):131‐6. [DOI] [PubMed] [Google Scholar]
Slagboom 2005 {published data only}
- Slagboom T, Winter R, Regar E, Schuler G, Thiele H, Laarman GJ, et al. Hemoglobin‐based oxygen therapeutics in (elective) percutaneous (coronary) revascularization ‐ The HEMOPURE trial. American Journal of Cardiology 2005;96(7A):51H. [Google Scholar]
References to ongoing studies
ACTRN12609000466246 {unpublished data only}
- ACTRN12609000466246. A randomized controlled trial comparing controlled oxygen therapy versus high flow oxygen therapy for acute myocardial infarctions in the pre‐hospital setting. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609000466246 first received 23 May 2013.
NCT01423929 {published and unpublished data}
- Khoshnood A, Carlsson A, Akbarzadeh M, Bhiladvala P, Roijer A, Hoglund P, et al. The effects of oxygen therapy on myocardial salvage in ST elevation myocardial infarction treated with acute percutaneous coronary intervention: the Supplemental Oxygen in Catheterized Coronary Emergency Reperfusion (SOCCER) study. Cardiology 2015;132:16‐21. [DOI: 10.1159/000398786] [DOI] [PubMed] [Google Scholar]
- NCT01423929. Supplemental Oxygen in Catheterized Coronary Emergency Reperfusion (SOCCER). clinicaltrials.gov/show/NCT01423929 first received 23 May 2013.
NCT01787110 {published and unpublished data}
- Hofmann R, James K, Svensson L, Witt N, Frick M, Lindahl B, et al. DETermination of the role of OXygen in Acute Myocardial Infarction (DETO2X‐AMI trial). Scandinavian Cardiovascular Journal 2013;47:28. [Google Scholar]
- Hofmann R, James K, Svensson L, Witt N, Frick M, Lindahl B, et al. DETermination of the role of OXygen in suspected Acute Myocardial Infarction trial. American Heart Journal 2014;167:322‐8. [DOI] [PubMed] [Google Scholar]
- Hofmann R, Svensson L, Frick M, Jemberg T, James SK, Lindahl B, Ekelund U, Omerovic E, Herlitz J, Witt N. Determination of the role of oxygen in acute myocardial infarction (deto2x‐ami) trial): safety and feasibility pilot study. Scandinavian Cardiovascular Journal. 2013; Vol. 47:28‐9.
- NCT01787110. DETermination of the Role of OXygen in Suspected Acute Myocardial Infarction (DETO2X‐AMI) Based on the SWEDEHEART Registry. clinicaltrials.gov/show/NCT01787110, 2013.
NCT02290080 {published data only}
- NCT02290080. DETermination of the Role of OXygen in Suspected Acute Myocardial Infarction by Biomarkers (DETO2X‐bio). clinicaltrials.gov/show/NCT02290080.
Additional references
AARC 2002
- Kallstrom TJ. American Association for Respiratory Care (AARC). AARC Clinical Practice Guideline: oxygen therapy for adults in the acute care facility‐‐2002 revision & update. Respiratory Care 2002;47(6):717‐20. [PubMed] [Google Scholar]
AHA 2005
- American Heart Association. American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care: part 8: stabilization of the patient with acute coronary syndromes. Circulation 2005;112 Suppl IV(24):89‐110. [Google Scholar]
Anderson 2007
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. ACC/AHA2007 guidelines for the management of patients with unstable angina/non ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2007;116:e148‐e304. [DOI] [PubMed] [Google Scholar]
Antman 2002
- Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association. Circulation 2004;110(5):588‐636. [DOI] [PubMed] [Google Scholar]
Babaev 2005
- Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS, NRMI Investigators. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2005;294:448‐54. [DOI] [PubMed] [Google Scholar]
Barnes 2013
- Barnes GD1, Katz A, Desmond JS, Kronick SL, Beach J, Chetcuti SJ, et al. False activation of the cardiac catheterization laboratory for primary PCI. American Journal of Managed Care 2013;19(8):671‐5. [PubMed] [Google Scholar]
Bassand 2007
- Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez‐Aviles F, et al. Guidelines for the diagnosis and treatment of non‐ST‐segment elevation acute coronary syndromes. European Heart Journal 2007;28:1598‐660. [DOI] [PubMed] [Google Scholar]
Beasley 2007
- Beasley R, Aldington S, Weatherall M, Robinson G, McHaffie D. Oxygen therapy in myocardial infarction: an historical perspective. Journal of the Royal Society of Medicine 2007;100(3):130‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of rank correlation test for publication bias. Biometrics 1994;50:1088‐101. [PubMed] [Google Scholar]
BHF 2010
- British Heart Foundation. Oxygen should not be routinely given to heart attack patients. www.bhf.org.uk/default.aspx?page=11843 (accessed 21 May 2013).
Cabello 2009
- Cabello JB, Emparanza JI, Ruiz‐Garcia V, Burls A. Oxygen therapy for acute myocardial infarction: a web‐based survey of physicians' practices and beliefs [Oxigenoterapia en el infarto agudo de miocardio:una encuesta‐web sobre la práctica y las creencias de los clínicos]. Emergencias 2009;21:422‐8. [Google Scholar]
Capewell 2000
- Capewell S, Beaglehole R, Seddon M, McMurray J. Explanation for the decline in coronary heart disease mortality rates in Auckland, New Zealand, between 1982 and 1993. Circulation 2000;102(13):1511‐6. [DOI] [PubMed] [Google Scholar]
Chew 2011
- Chew DP, Aroney CN, Aylward PE, Kelly AM, White HD, Tideman PA, et al. 2011 Addendum to the National Heart Foundation of Australia/Cardiac Society of Australia and New Zealand Guidelines for the management of acute coronary syndromes (ACS). Heart, Lung & Circulation 2011;20:487‐502. [DOI] [PubMed] [Google Scholar]
Danchin 2009
- Danchin N, Chemla D. Challenging doctors' lifelong habits may be good for their patients: oxygen therapy in acute myocardial infarction. Heart 2009;95(3):176‐7. [DOI] [PubMed] [Google Scholar]
Ecclestone 2015
- Ecclestone TC1, Yeates DG, Goldacre MJ. Fall in population‐based mortality from coronary heart disease negated in people with diabetes mellitus: data from England. Diabetic Medicine 2015;32(10):1329‐34. [DOI: 10.1111/dme.12770] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Shneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farquhar 2009
- Farquhar H, Weatherall M, Wijesinghe M, Perrin K, Ranchord A, Simmonds M, Beasley R. Systematic review of studies of the effect of hyperoxia on coronary blood flow.. Am Heart J 2009 Sep;158(3):371‐7. [DOI: 10.1016/j.ahj.2009.05.037] [DOI] [PubMed] [Google Scholar]
Goldberg 2008
- Goldberg RJ, Yarzebski J, Spencer FA, Zevallos JC, Lessard D, Gore JM. Thirty‐year trends (1975‐2005) in the magnitude, patient characteristics, and hospital outcomes of patients with acute myocardial infarction complicated by ventricular fibrillation. American Journal of Cardiology 2008;102:1595‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- GRADE Working Group, McMaster University. GRADEpro. Version accessed prior to 12 December 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ, et al. GRADE Working Group. Rating quality of evidence and strength of recommendations: What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SJ, Deeks JJ, Altman DJ. Measuring inconsistencies in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ILCOR 2005
- International Liaison Committee on Resuscitation. International Liaison Committee on Resuscitation Part 5: acute coronary syndromes. Resuscitation 2005;67(2‐3):249‐69. [DOI] [PubMed] [Google Scholar]
Koopman 2013
- Koopman C, Bots ML, Oeffelen AA, Dis I, Verschuren WM, Engelfriet PM, et al. Population trends and inequalities in incidence and short‐term outcome of acute myocardial infarction between 1998 and 2007. International Journal of Cardiology 2013;168:993‐8. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Mandelzweig 2006
- Mandelzweig L, Battler A, Boyko V, Beuno H, Danchin N, Fillipatos G, et al. The second Euro Heart Survey on acute coronary syndromes: characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. European Heart Journal 2006;27(19):2285‐93. [DOI] [PubMed] [Google Scholar]
McCabe 2012
- McCabe JM1, Armstrong EJ, Kulkarni A, Hoffmayer KS, Bhave PD, Garg S, et al. Prevalence and factors associated with false‐positive ST‐segment elevation myocardial infarction diagnoses at primary percutaneous coronary intervention–capable centers: a report from the Activate‐SF registry. Archives of Internal Medicine 2012;172(11):864‐71. [DOI: 10.1001/archinternmed.2012.945] [DOI] [PubMed] [Google Scholar]
McNulty 2005
- McNulty PH, King N, Scott S, Hartman G, McCann J, Kozak M, et al. Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. American Journal of Physiology. Heart and Circulation Physiology 2005;288(3):H1057‐62. [DOI] [PubMed] [Google Scholar]
McNulty 2007
- McNulty PH, Robertson BJ, Tulli MA, Hess J, Harach LA, Scott S, et al. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischaemic heart disease. Journal of Applied Physiology 2007;102(5):2040‐5. [DOI] [PubMed] [Google Scholar]
Milone 1999
- Milone SD, Newton GE, Parker JD. Hemodynamic and biochemical effects of 100% oxygen breathing in humans. Canadian Journal of Physiology and Pharmacology 1999;77(2):124‐30. [PubMed] [Google Scholar]
Movahed 2009
- Movahed MR, John J, Hashemzadeh M, Jamal MM, Hashemzadeh M. Trends in the age adjusted mortality from acute ST segment elevation myocardial infarction in the United States (1988‐2004) based on race, gender, infarct location and co morbidities. American Journal of Cardiology 2009;104:1030. [DOI] [PubMed] [Google Scholar]
Mozaffarian 2015
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics‐‐2015 update: a report from the American Heart Association. Circulation 2015;131(4):e29‐322. [DOI: 10.1161/CIR.0000000000000152] [DOI] [PubMed] [Google Scholar]
Nedeljkovic 2015
- Nedeljkovic ZS, Jacobs AK. O2 for STEMI: still up in the air. Circulation 2015;131(24):2101‐3. [DOI: 10.1161/CIRCULATIONAHA.115.017072] [DOI] [PubMed] [Google Scholar]
Nicholson 2004
- Nicholson C. A systematic review of the effectiveness of oxygen in reducing acute myocardial ischaemia. Journal of Clinical Nursing 2004;13(8):996‐1007. [DOI] [PubMed] [Google Scholar]
Nikolaou 2012
- Nikolaou NI, Vrints CJ. Initial treatment of acute coronary syndromes. Is there a future for MONA acronym after the 2010 guidelines?. Resuscitation. 2012;83(1):e7. [DOI] [PubMed] [Google Scholar]
O'Connor 2010
- O'Connor RE, Brady W, Brooks SC, Diercks G, Egan J, Ghaemmaghami C, et al. Part 10: acute coronary syndromes: 2010 American Heart Association. Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010;122(18 Suppl 3):S787‐817. [DOI] [PubMed] [Google Scholar]
O'Driscoll 2008
- O'Driscoll BR, Howard LS, Davison AG. BTS guideline for emergency oxygen use in adult patients. Thorax 2008;63 Suppl 6:1‐68. [DOI] [PubMed] [Google Scholar]
O'Gara 2013
- O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, Lemos JA, et al. 2013 ACCF/AHA Guideline for the Management of ST‐Elevation Myocardial Infarction. A report of the American College of Cardiology Foundation/American Heart Assoiation Task Force on Practice Guidelines. Journal of the American College of Cardiology 2013;61(4):e78‐140. [DOI] [PubMed] [Google Scholar]
Puymirat 2013
- Puymirat E1, Battler A, Birkhead J, Bueno H, Clemmensen P, Cottin Y, et al. Euro Heart Survey 2009 snapshot: regional variations in presentation and management of patients with AMI in 47 countries. European Heart Journal Acute Cardiovascular Care 2013;2(4):359‐70. [DOI: 10.1177/2048872613497341] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ripley 2012
- Ripley DP1, Riley SJ, Shome JS, Awan MA, McCloskey MC, Murphy JJ, et al. Oxygen use for chest pain in coronary care units across the UK. QJM 2012;105(9):855‐60. [DOI] [PubMed] [Google Scholar]
Rousseau 2005
- Rousseau A, Bak Z, Janerot‐Sjoberg B, Sjoberg F. Acute hyperoxemia‐induced effects on regional blood flow, oxygen consumption and central circulation in man. Acta Physiologica Scandinavica 2005;183(3):231‐40. [DOI] [PubMed] [Google Scholar]
Russek 1950
- Russek HI, Regan FD, Naegele CF. One hundred percent oxygen in the treatment of acute myocardial infarction and severe angina pectoris. Journal of the American Medical Association 1950;50:2299‐301. [DOI] [PubMed] [Google Scholar]
Salzman 1975
- Salzman HA. Efficacy of oxygen enriched gas mixtures in the treatment of acute myocardial infarction. Circulation 1975;52:357‐9. [DOI] [PubMed] [Google Scholar]
Schmidt 2012
- Schmidt M, Jacobsen JB, Lash TL, Bøtker HE, Sørensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ 2012;344:e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shuvy 2013
- Shuvy M, Atar D, Gabriel Steg P, Halvorsen S, Jolly S, Yusuf S, et al. Oxygen therapy in acute coronary syndrome: are the benefits worth the risk?. European Heart Journal 2013;34(22):1630‐5. [DOI] [PubMed] [Google Scholar]
SIGN 2007
- Scottish Intercollegiate Guidelines Network. Acute coronary syndrome. A national clinical guideline. www.sign.ac.uk/pdf/sign93.pdf (accessed prior to 12 December 2016).
SIGN 2010
- Scottish Intercollegiate Guidelines Network (SIGN). Acute coronary syndromes (SIGN publication no. 93). www.sign.ac.uk 2010 (accessed prior to 28 February 2013).
Smolina 2012
- Smolina K, Wright FL, Rayner M, Goldacre MJ. Determinants of the decline in mortality from acute myocardial infarction in England between 2002 and 2010: linked national database study. BMJ 2012;344:d8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steg 2004
- Steg PG, Dabbous OH, Feldman LJ, Cohen‐Solal A, Aumont MC, López‐Sendón J, et al. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation 2004;109:494‐9. [DOI] [PubMed] [Google Scholar]
Steg G 2012
- Steg G, James SK, Atar D, Badano LP, Blömstrom‐Lundqvist C, Borger MA, et al. Task force on the management of ST‐elevation acute myocardial infarction of the European Cardiology Society (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. European Heart Journal 2012;33(20):2569‐619. [DOI] [PubMed] [Google Scholar]
Thygesen 2012
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. European Heart Journal 2012;33(20):2551‐67. [DOI] [PubMed] [Google Scholar]
Townsend 2015
- Townsend N, Nichols M, Scarborough P, Rayner M. Cardiovascular disease in Europe — epidemiological update 2015. European Heart Journal 2015;36(40):2696‐705. [DOI: ] [DOI] [PubMed] [Google Scholar]
Van de Werf 2008
- Werf F, Bax J, Betriu A, Blömstrom‐Lundqvist C, Crea F, Falk V, et al. Management of acute myocardial infarction in patients presenting with persistent ST‐segment elevation: the Task Force on the Management of ST‐Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. European Heart Journal 2008;29(23):2909‐45. [DOI] [PubMed] [Google Scholar]
WHO 2011
- WHO 2011. Fact sheet N8310. www.who.int/mediacentre/factsheets/fs310/en/index.html (accessed prior to 30 June 2011).
Wijesinghe 2009
- Wijesinghe M, Perrin K, Ranchord A, Simmonds M, Weatherall M, Beasley R. Routine use of oxygen in the treatment of myocardial infarction: systematic review. Heart 2009;95(3):198‐202. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xie 2009
- Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative‐stress‐induced after depolarizations and calmodulin kinase II signalling. Circulation Research 2009;104:79‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2010
- Yan AT, Steg PG, Fitzgerald G, Feldman LJ, Eagle KA, Gore JM, et al. Recurrent ischemia across the spectrum of acute coronary syndromes: Prevalence and prognostic significance of (Re‐)infarction and ST‐segment changes in a large contemporary registry. International Journal of Cardiology 2010; Vol. 145, issue 1:15‐20. [DOI] [PubMed]
Yeh 2010
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. New England Journal of Medicine 2010;362(23):2155‐65. [DOI] [PubMed] [Google Scholar]
Zughaft 2013
- Zughaft D, Bhiladvala P, Dijkman A, Harnek J, Madsen Hardig B, Bjork J, et al. The analgesic effect of oxygen during percutaneous coronary intervention (the OXYPAIN Trial). Acute Cardiology Care 2013;15:63‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Burls 2011
- Burls A, Cabello JB, Emparanza JI, Bayliss S, Quinn T. Oxygen therapy for acute myocardial infarction: a systematic review and meta‐analysis. Emergency Medicine Journal 2011;28(11):917‐23. [DOI] [PubMed] [Google Scholar]
Cabello 2010
- Cabello JB, Burls A, Emparanza JI, Bayliss S, Quinn T. Oxygen therapy for acute myocardial infarction. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD007160.pub2] [DOI] [PubMed] [Google Scholar]
Cabello 2013
- Cabello JB, Burls A, Emparanza JI, Bayliss S, Quinn T. Oxygen therapy for acute myocardial infarction. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD007160.pub3] [DOI] [PubMed] [Google Scholar]


