Abstract
Background
Pain on propofol injection is an untoward effect and this condition can reduce patient satisfaction. Intravenous lidocaine injection has been commonly used to attenuate pain on propofol injection. Although many studies have reported that lidocaine was effective in reducing the incidence and severity of pain, nevertheless, no systematic review focusing on lidocaine for preventing high‐intensity pain has been published.
Objectives
The objective of this review was to determine the efficacy and adverse effects of lidocaine in preventing high‐intensity pain on propofol injection.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 10), Ovid MEDLINE (1950 To October 2014), Ovid Embase (1988 to October 2014), LILACS (1992 to October 2014) and searched reference lists of articles.
We reran the search in November 2015. We found 11 potential studies of interest, those studies were added to the list of ‘Studies awaiting classification' and will be fully incorporated into the formal review findings when we update the review.
Selection criteria
We included randomized controlled trials (RCTs) using intravenous lidocaine injection as an intervention to decrease pain on propofol injection in adults. We excluded studies without a placebo or control group.
Data collection and analysis
We collected selected studies with relevant criteria. We identified risk of bias in five domains according to the following criteria: random sequence generation, allocation concealment, adequacy of blinding, completeness of outcome data and selective reporting. We performed meta‐analysis by direct comparisons of intervention versus control. We estimated the summary odds ratios (ORs) and 95% confidence intervals using the random‐effects Mantel‐Haenszel method in RevMan 5.3. We used the I2 statistic to assess statistical heterogeneity. We assessed overall quality of evidence using the GRADE approach.
Main results
We included 85 studies, 82 of which (10,350 participants) were eligible for quantitative analysis in the review. All participants, aged 13 years to 89 years, were American Society of Anesthesiologists (ASA) I‐III patients undergoing elective surgery. Each study was conducted in a single centre in high‐ , middle‐ and low‐income countries worldwide. According to the risk of bias assessment, all except five studies were identified as being of satisfactory methodological quality, allowing 82 studies to be combined in the meta‐analysis. Five of the 82 studies were assessed as high risk of bias: one for participant and personnel blinding, one for incomplete outcome data, and three for other potential sources of bias.
The overall incidence of pain and high‐intensity pain following propofol injection in the control group were 63.7% (95% CI 60% to 67.9%) and 37.9% (95% CI 33.4% to 43.1%), respectively while those in the lidocaine group were 30.2% (95% CI 26.7% to 33.7%) and 11.8% (95% CI 9.7% to 13.8%). Both lidocaine admixture and pretreatment were effective in reducing pain on propofol injection (lidocaine admixture OR 0.19, 95% CI 0.15 to 0.25, 31 studies, 4927 participants, high‐quality evidence; lidocaine pretreatment OR 0.13, 95% CI 0.10 to 0.18, 41 RCTs, 3918 participants, high‐quality evidence). Similarly, lidocaine administration could considerably decrease the incidence of pain when premixed with the propofol (OR 0.19, 95% CI 0.15 to 0.24, 36 studies, 5628 participants, high‐quality evidence) or pretreated prior to propofol injection (OR 0.14, 95% CI 0.11 to 0.18, 50 studies, 4722 participants, high‐quality evidence). Adverse effects of lidocaine administration were rare. Thrombophlebitis was reported in only two studies (OR not estimated, low‐quality evidence). No studies reported patient satisfaction.
Authors' conclusions
Overall, the quality of the evidence was high. Currently available data from RCTs are sufficient to confirm that both lidocaine admixture and pretreatment were effective in reducing pain on propofol injection. Furthermore, there were no significant differences of effect between the two techniques.
Keywords: Adolescent; Adult; Aged; Aged, 80 and over; Humans; Middle Aged; Anesthesia; Anesthesiology; Anesthetics, Intravenous; Anesthetics, Intravenous/administration & dosage; Anesthetics, Intravenous/adverse effects; Anesthetics, Local; Anesthetics, Local/administration & dosage; Lidocaine; Lidocaine/administration & dosage; Pain; Pain/chemically induced; Pain/prevention & control; Propofol; Propofol/administration & dosage; Propofol/adverse effects
Plain language summary
Lidocaine for reducing propofol‐induced pain on anaesthesia in adults
Review question
Is intravenous, (directly into a vein), lidocaine injection effective in reducing the pain caused by the injection of propofol, given to induce anaesthesia in adults undergoing general anaesthesia?
Background
Propofol is an anaesthetic drug (an induction agent) which is given to induce and maintain anaesthesia in adults undergoing surgery. Propofol is a popular induction agent because it provides a smooth induction and faster recovery than other drugs such as thiopental. The main disadvantage of propofol is that it often causes people severe pain. This is because propofol is usually injected into a hand vein and can cause skin irritation. This can make the anaesthesia experience unpleasant. One method for preventing propofol‐induced pain is to give lidocaine either before the propofol injection or mixed in with the propofol. Lidocaine is a commonly used low‐cost local anaesthetic drug. The objective of this review was to determine how effective lidocaine was in reducing the high pain levels caused by the injection of propofol.
Study characteristics
We searched the databases until October 2014. We included 85 studies, 82 of which (10,350 participants) were eligible for quantitative analysis. The study participants were randomly selected to receive either intravenous lidocaine injection or normal saline (placebo) at the same time as the propofol injection.
We reran the search in November 2015. We found 11 potential studies of interest, those studies were added to the list of ‘Studies awaiting classification' and will be fully incorporated into the formal review findings when we update the review.
Study funding sources
Three out of the 85 studies were funded by either a pharmaceutical manufacturer with a commercial interest in the results of the studies or the company which supplied the propofol. Eight studies were supported by government hospital or university funds and one study was funded by a charitable grant.
Key results
We found that the injection of lidocaine into a vein, either mixing lidocaine with propofol or injecting lidocaine before propofol, could effectively reduce the incidence and the high levels of pain associated with the injection of propofol. Adverse effects such as inflammation (redness, swelling) of the vein at the injection site were rare and in two studies were not more frequent with the use of lidocaine. No study reported on patient satisfaction.
Statistics
Based on these results we would expect that out of 1000 patients receiving intravenous propofol, about 384 who did not also receive intravenous lidocaine, would experience moderate to severe pain, compared to only 89 patients who also received intravenous lidocaine.
Quality of the evidence
The overall quality of evidence was high with a very large beneficial effect obtained by the administration of lidocaine to reduce painful propofol injections.
Summary of findings
Summary of findings for the main comparison. Lidocaine for propofol‐induced pain on induction of anaesthesia in adults.
| Lidocaine for propofol‐induced pain on induction of anaesthesia in adults | ||||||
| Patient or population: Adult participants receiving propofol for induction of anaesthesia Settings: Patients undergoing elective surgery Intervention: Lidocaine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Lidocaine | |||||
| High‐intensity pain with lidocaine admixture Pain scales Follow‐up: 1‐5 minutes | Study population | OR 0.19 (0.15 to 0.25) | 4927 (31 studies) | ⊕⊕⊕⊕ high | ||
| 305 per 1000 | 77 per 1000 (62 to 99) | |||||
| Moderate | ||||||
| 329 per 1000 | 85 per 1000 (69 to 109) | |||||
| High‐intensity pain with lidocaine pretreatment Pain scales Follow‐up: 1‐5 minutes | Study population | OR 0.13 (0.1 to 0.18) | 3918 (41 studies) | ⊕⊕⊕⊕ high | ||
| 463 per 1000 | 101 per 1000 (79 to 134) | |||||
| Moderate | ||||||
| 457 per 1000 | 99 per 1000 (78 to 132) | |||||
| Incidence of pain with lidocaine admixture Pain scales Follow‐up: 1‐5 minutes | Study population | OR 0.19 (0.15 to 0.24) | 5628 (36 studies) | ⊕⊕⊕⊕ high | ||
| 582 per 1000 | 209 per 1000 (173 to 250) | |||||
| Moderate | ||||||
| 679 per 1000 | 287 per 1000 (241 to 337) | |||||
| Incidence of pain with lidocaine pretreatment Pain scales Follow‐up: 1‐5 minutes | Study population | OR 0.14 (0.11 to 0.18) | 4722 (50 studies) | ⊕⊕⊕⊕ high | ||
| 698 per 1000 | 244 per 1000 (203 to 294) | |||||
| Moderate | ||||||
| 743 per 1000 | 288 per 1000 (241 to 342) | |||||
| Adverse effects Events Follow‐up: 0‐7 days | An adverse effect, thrombophlebitis, was observed in two out of 32 studies (Ganta 1992; Smith 1996). One study (Ganta 1992) reported thrombophlebitis 4/85 participants in lidocaine pretreatment group compared with 8/85 cases in saline group. Another study (Smith 1996) reported thrombophlebitis within seven days postoperatively 9/22 participants in lidocaine pretreatment group, compared to 4/29 in saline group. However, there were no statistically significant differences in lidocaine and saline groups. | Not estimated | 4007 (32 studies) | ⊕⊕⊝⊝ low1 | ||
| *The basis for the assumed risk is the median control group risk across studies, or the average risk for pooled data; and the control group risk for single studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; HR: Hazard ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The quality of evidence was downgraded by two levels due to serious imprecision and inconsistency.
Background
Propofol is used intravenously for the induction and maintenance of anaesthesia. The main disadvantage of propofol is pain on injection, however it is a popular induction agent for ambulatory surgery as it provides smoother induction and faster recovery than other agents such as thiopental, which is considered to be the standard induction agent (Kevin 2003; McCluskey 2003).
Description of the condition
Propofol is a phenol compound that causes skin and mucous membrane irritation, leading to pain at the injection site in 80% to 90% of people (Kwak 2007a). Although the underlying mechanisms are still not fully understood, pain immediately after injection of propofol may be caused either by direct stimulation of nociceptors and free nerve endings in the venous wall or indirectly by the release of mediators, such as bradykinin and prostaglandin E2, which stimulate afferent nerve endings, leading to a delayed onset of pain (Brooker 1985; Eriksson 1997; Wong 2001).
Description of the intervention
Many different physicopharmacological interventions have been proposed to reduce the incidence and severity of this adverse effect of propofol (Appendix 2; Jalota 2011; Picard 2000). Lidocaine in various dosages and concentrations, or combined with other interventions for reducing pain on propofol injection, has been extensively evaluated and seems to be the most promising drug for this condition. To reduce pain on propofol injection, lidocaine is administered either by mixing with the propofol and injecting both together, or by injecting separately, as an intravenous pretreatment prior to the propofol injection.
Concerning the physicochemical reaction of propofol‐lidocaine mixtures, there have not been any reports of adverse reactions in vivo. However in vitro there was a report of coalescence of oil droplets (diameters ≥ 5 micron) 30 minutes after the addition of 40 mg lidocaine to propofol. This reaction is time‐ and dose‐dependent and may cause pulmonary embolism, depending on the dose of lidocaine. In addition, propofol concentrations in the mixture with 40 mg of lidocaine decreased linearly and significantly from 4 hours to 24 hours after preparation, while those combined with 20 mg or less of lidocaine were unchanged (Masaki 2003).
How the intervention might work
Lidocaine appears to provide good results in preventing pain on injection by propofol. The mechanisms of action are still unclear. A preceding injection of lidocaine caused a reduction of pain, probably due to the direct effect of local anaesthetics on vascular smooth muscle (Nicol 1991). In addition, the discomfort of pain on injection could be reduced by mixing a small amount of lidocaine to the propofol. This might be because lidocaine hydrochloride is a weak free base cation solution, which would lower the pH after mixing it with propofol (Brooker 1985; Eriksson 1997). However, the right dose and concentration are required to demonstrate good efficacy (Adachi 2002).
Why it is important to do this review
Although people suffer from pain only temporarily after injection of propofol, the level of pain may be severe (Michael 1996). This results in them having a terrible anaesthesia experience. Therefore, many anaesthesiologists are concerned about this issue and a great amount of research has been conducted in order to prevent the pain from propofol injection. Among all the drugs and interventions that can decrease pain on propofol injection, lidocaine is a very common drug, being both effective and easily available worldwide. The cost of lidocaine is also relatively low. Therefore, lidocaine is of particular interest to us. Even though Picard 2000 produced a quantitative systematic review on this subject, and Jalota 2011 produced a systematic review, , there have been a significant number of trials using lidocaine to prevent pain from propofol injection since these reviews were published. Also, no systematic review focusing on lidocaine for preventing high‐intensity pain has been published. Therefore, the aim of this systematic review was to evaluate, with the best available evidence, the efficacy of lidocaine treatment for preventing high‐intensity pain following propofol injection.
Objectives
The objective of this review was to determine the efficacy and adverse effects of lidocaine in preventing high‐intensity pain on propofol injection.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) that compared the use of lidocaine intervention with placebo or other interventions in order to reduce the severity of pain in patients receiving intravenous propofol injection.
Types of participants
We included participants aged 13 years and older who were administered propofol intravenously.
We excluded studies in children (below 13 years of age) due to differences in children's pain‐scale rating methods, and difficulty in classifying high and low doses in children and adults; for example, 20 mg lidocaine may be a high dose in small children but not in adults.
Types of interventions
Lidocaine in various regimens and dosages versus placebo or control group, which were those without lidocaine. Both lidocaine and control groups might similarly receive some active adjunct, for example remifentanil, ketamine, etc.
Types of outcome measures
Primary outcomes
The incidence of high‐intensity pain on propofol injection.
'High‐intensity pain’ was defined as the combination of moderate and severe pain levels. For different scales in each included study, we defined ‘low‐ and high‐intensity pain’ according to the definition of the scoring system of each included study. ‘Low‐intensity pain’ included mild pain, pain mentioned only when questioned, without any behavioural signs. ‘High‐intensity pain’ included moderate pain, severe pain, pain mentioned when questioned and accompanied by behavioural signs, or pain reported spontaneously not as a result of questioning, pain associated with grimacing, withdrawal movement of forearm, tears. In case the definition of the pain score was not clear, we categorized one‐third of the lower range of pain scores as ‘low‐intensity pain’ and two‐thirds of the upper range of pain scores as ‘high‐intensity pain’.
Secondary outcomes
Incidence of pain
Adverse effects (such as thrombophlebitis, cardiac arrhythmia, allergic reaction or local anaesthetic toxicity)
Patient satisfaction
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL2014, Issue 10); Ovid MEDLINE; (1950 to October 2014); Ovid Embase (1988 to October 2014); and LILACS (1992 to October 2014).
We developed a specific strategy for each database. We based each search strategy on that developed for MEDLINE (see Appendix 3 (CENTRAL); Appendix 4 (MEDLINE); Appendix 5 (EMBASE); Appendix 6 (LILACS)).
We combined the MEDLINE search strategy with the Cochrane highly sensitive search strategy phases one and two as described in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
We also looked for trials by manually searching abstracts of relevant conference proceedings. We checked the reference lists of relevant articles. We contacted relevant trial authors to identify any additional studies.
We did not apply language or publication restrictions.
We reran the search in November 2015. We found 11 potential studies of interest, those studies were added to the list of ‘Studies awaiting classification' and will be fully incorporated into the formal review findings when we update the review.
Searching other resources
We searched for relevant ongoing trials in www.controlled‐trials.com. The last search of this database was conducted in November 2015.
Data collection and analysis
Selection of studies
Two authors (PE and SM) independently scanned the titles and abstracts of reports identified by searching the electronic databases and handsearching journals. We obtained the full texts of trials that appeared to be eligible RCTs, and inspected them to assess their relevance, based on a pre‐planned checklist. A third author (WS) settled any disagreements.
Data extraction and management
Three authors (PE, SD and SM) independently extracted the data using a specific data extraction forms (see Appendix 7)and assessed trial quality using a standardized checklist. We resolved any disagreement through consultation with a fourth author (WS).
Assessment of risk of bias in included studies
We judged study quality on the basis of the following methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Random sequence generation (selection bias)
We described the allocation method used in each study, whether study authors provided adequate information to allow assessment in comparable groups or not.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator, shuffling envelopes);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number, judgement by clinicians);
unclear risk of bias.
Allocation concealment (selection bias)
We described the method used to conceal allocation to interventions that prevented participants and investigators seeing assignment in advance, during recruitment or changing allocation after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone randomization, consecutively numbered sealed opaque envelopes);
high risk of bias (e.g. a list of random numbers, unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
Blinding of participants and personnel (performance bias)
We described the methods used to blind study participants and personnel from knowledge of which intervention a participant received in each study. If studies were blinded, we regarded them as a low risk of bias. We assessed blinding separately in each outcome. We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel
Blinding of outcome assessment (detection bias)
We described the methods used to blind the investigator from knowledge of which intervention a participant received in each study. If studies were blinded, we regarded them as a low risk of bias. We assessed blinding separately in each outcome. We assessed the methods as:
low, high or unclear risk of bias for outcome assessment.
Incomplete outcome data (attrition bias)
We described the completeness of outcome data including attrition and exclusions from the analysis in each study. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook. We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as‐treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
Selective reporting (reporting bias)
We described the possibility of selective outcome reporting bias in each study. We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias
Other bias
We described any important concerns about other possible sources of bias in each study. We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias (the study appears to be free of other sources of bias);
high risk of other bias (e.g. had a potential bias from a study design, baseline imbalance, early stopping);
unclear whether there is risk of other bias.
We discussed the impact of the methodological quality on the results.
Measures of treatment effect
We expressed results as odds ratio (OR) with 95% confidence intervals (CI) for all categorical outcomes of the individual studies.
Unit of analysis issues
We included RCTs with two or more intervention groups (multi‐arm studies). For multi‐arm studies, we either included the directly relevant arms only, or divided the shared group into two or more groups, so that the total number of events and the total number of participants added up to the original size of the shared group.
Dealing with missing data
We extracted the information that was available from the published reports. Denominators for each group in each study were extracted based on all participants that allocated to that group. Some studies presented numerical data as graphs so we were not able to extract numerical data directly, but estimated them from the graphs.
Assessment of heterogeneity
We assessed the heterogeneity among trials by the Chi2 test for heterogeneity with a 10% level of statistical significance and by determining the I2 statistic (Higgins 2003). When interpreting the I2 statistic, we used the following recommendations as stated in (Deeks 2011).
0% to 40% might not be important.
30% to 60% may represent moderate heterogeneity.
50% to 90% may represent substantial heterogeneity.
75% to 100%: represents considerable heterogeneity.
Assessment of reporting biases
We assessed the possibility of publication bias in a meta‐analysis including at least 10 trials by visual inspection of funnel plots (Sterne 2011).
Data synthesis
We performed meta‐analyses using a random‐effects model for assessment of treatment effects because this approach is widely used and tends to give a more conservative estimate of treatment effects with wider confidence intervals than the fixed‐effect model (Deeks 2011). All analyses were done by using Review Manager 5.3 (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
In order to perform a subgroup analysis and also to estimate the treatment effects for each subgroup as well as overall studies, we presented the results according to doses of lidocaine pretreatment or admixture, and the application of venous occlusion above injection site (Deeks 2011). Moreover, we also planned to perform subgroup analysis regarding age groups, speed of propofol injection and site of propofol injection, but data were not available.
Sensitivity analysis
We carried out sensitivity analyses for the primary outcome, in order to explore the robustness of the results, by keeping studies at low risk of selection bias (random sequence generation and allocation concealment) and removing studies at unclear or high risk of selection bias from the analysis.
Summarizing and interpreting results
We used the GRADE approach to interpret findings (Schünemann 2011) and we used GRADE profiler software (GRADEpro GDT 2015) to import data from Review Manager (RevMan) (RevMan 2014) to create 'Summary of findings' tables using information on quality of evidence, magnitude of effects of the interventions examined, and sums of available data on all important outcomes from each study included in the comparison. The GRADE approach (Schünemann 2011) considers ‘quality’ to be a judgement of the extent to which we can be confident that the estimates of effect are correct. We initially graded evidence from RCTs as high, it was downgraded by one or two levels on each of five domains after full consideration of any limitations in the design of studies including risk of bias, indirectness of the evidence, inconsistency and imprecision of results and the possibility of publication bias. We upgraded the quality of evidence by one or two levels on three domains, including large magnitude of the effect, the influence of all possible residual confounding and the dose‐response gradient. A GRADE quality level of 'high' reflects confidence that the true effect lies close to the estimate of the effect for a given outcome. A judgement of 'moderate' quality indicates that the true effect is likely to be close to the estimate of the effect, but acknowledges that it could be substantially different. Evidence of 'low' and 'very low' quality limits our confidence in the effect estimate. The outcomes selected for the 'Summary of findings' tables include the following.
High‐intensity pain with low dose lidocaine admixture ‐ lidocaine ≤ 20 mg or ≤ 0.2 mg/kg admixture
High‐intensity pain with high dose lidocaine admixture ‐ lidocaine > 20 mg or > 0.2 mg/kg admixture
High‐intensity pain with low dose lidocaine pretreatment ‐ lidocaine ≤ 20 mg or ≤ 0.2 mg/kg pretreatment alone
High‐intensity pain with high dose lidocaine pretreatment ‐ lidocaine > 20 mg or > 0.2 mg/kg pretreatment alone
High‐intensity pain with low dose lidocaine pretreatment ‐ lidocaine ≤ 20 mg or ≤ 0.2 mg/kg pretreatment with venous occlusion
High‐intensity pain with high dose lidocaine pretreatment ‐ lidocaine > 20 mg or > 0.2 mg/kg pretreatment with venous occlusion
Results
Description of studies
See the Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
In October 2014, the search retrieved 5014 articles. We judged 116 studies to be potentially eligible and retrieved the full texts; 85 studies met our inclusion criteria and 31 studies were excluded, 82 studies were eligible for quantitative analysis. We reran the search in November 2015. 11 potential new studies of interest were added to the list of ‘Studies awaiting classification' and will be incorporated into the formal review findings during the review update. No disagreement between review authors regarding inclusion of studies was observed. See PRISMA study flow diagram (Figure 1) for further details (Liberati 2009).
1.
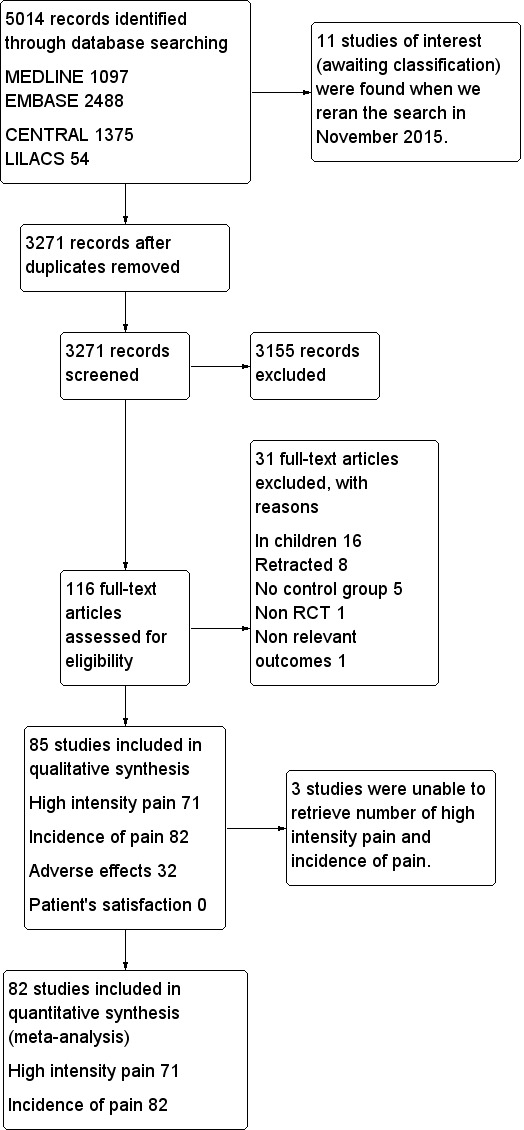
PRISMA study flow diagram
Included studies
Study design
We included 85 parallel‐design RCTs in this review; 82 studies were eligible for quantitative analysis.
Sample size
The number of participants analysed in the 82 included studies ranged from 36 to 464.
Setting
All of the included studies were single‐centre studies conducted in high‐, middle‐ and low‐income countries worldwide. Most of the included studies (59/85) were performed in Asia. There were 18 studies from Europe (Bachmann‐Mennenga 2007; Barker 1991; Eriksson 1997; Gajraj 1996; Gehan 1991; Harmon 2003; Helbo‐Hansen 1988; Johnson 1990; Lyons 1996; Mallick 2007; McCluskey 2003; McCulloch 1985; McDonald 1996; Niazi 2005; Nicol 1991; Nathanson 1996; Scott 1988; Smith 1996); seven studies from North America (Aldrete 2010; Ahmad 2013; Ganta 1992; Haugen 1995; Minogue 2005; O'Hara 1997; Walker 2011); two studies from Australia (Lee 1994; Newcombe 1990); and one study from Africa (Saadawy 2007).
Funding
Eight studies (Asik 2003; Bachmann‐Mennenga 2007; Ho 1999; Jeon 2012; Krobbuaban 2008; Kwak 2007b; Kwon 2012; Walker 2011) were supported by government hospital or university funds and one study (Helbo‐Hansen 1988) was funded by a charitable grant while three out of 85 studies (Aldrete 2010; Bachmann‐Mennenga 2007; McCulloch 1985) were funded or supplied propofol by the pharmaceutical manufacturer with a commercial interest in the results of the studies.
Participants
This review included 10,350 participants; 5597 of whom were in the lidocaine group and 4753 in the control group. All participants were American Society of Anesthesiologists (ASA) I‐III patients undergoing elective surgery and administered propofol for induction of anaesthesia. The age of participants ranged from 13 years to 89 years.
Intervention
All studies compared lidocaine versus placebo. Twelve of the 85 studies used an adjunct with lidocaine versus an adjunct with placebo: remifentanil (Aouad 2007; Han 2010; Kwak 2007a; Kwak 2007b), nitroglycerine ointment (O'Hara 1997), 50%N2O (Kim 2013a; Niazi 2005; Sinha 2005), sevoflurane (DeSousa 2011), dexamethasone (Kwak 2008), ketamine (Hwang 2010), dexmedetomidine (Ayoglu 2007). In our review, there were some included studies with multiple intervention groups (DeSousa 2011; Gajraj 1996; Gehan 1991; Ho 1999; Johnson 1990; Kim 2010; Massad 2006; Scott 1988), therefore, we split the shared group into two or more groups with a smaller sample size and included two or more (reasonably independent) comparisons, as mentioned in 'Unit of analysis issues'. The dose of lidocaine and injection techniques were classified into six subgroups as follows:
23/85 studies used a dose of ≤ 20 mg or ≤ 0.2 mg/kg of lidocaine‐propofol admixture (Bachmann‐Mennenga 2007; Barker 1991; Gajraj 1996; Gehan 1991; Harmon 2003; Helbo‐Hansen 1988; Ho 1999; Johnson 1990; Kim 2010; King 1992; Krobbuaban 2008; Kwak 2007b; McDonald 1996; Minogue 2005; Newcombe 1990; O'Hara 1997; Parmar 1998; Scott 1988; Sethi 2009; Tariq 2006; Tariq 2010; Tham 1995; Yew 2005).
19/85 studies used a dose of > 20 mg or > 0.2 mg/kg of lidocaine‐propofol admixture (Aldrete 2010; Aouad 2007; Gajraj 1996; Gehan 1991; Han 2010; Ho 1999; Johnson 1990; Karasawa 2000; Kim 2010; Krobbuaban 2005; Mallick 2007; Massad 2006; McCluskey 2003; Nakane 1999; Nathanson 1996; Sinha 2005; Tham 1995; Walker 2011; Yokota 1997).
7/85 studies used a dose of ≤ 20 mg or ≤ 0.2 mg/kg lidocaine pretreatment alone (Ganta 1992; Lee 1994; Lyons 1996; McCulloch 1985; Nicol 1991; Scott 1988; Smith 1996).
14/85 studies used a dose of > 20 mg or > 0.2 mg/kg lidocaine pretreatment alone (Agarwal 2004b; Agarwal 2004d; Azma 2004; Cheong 2002; DeSousa 2011; Haugen 1995; Honarmand 2008; Koo 2006; Lu 2013; Massad 2006; Nishiyama 2005; Salman 2011; Shimizu 2005; Zahedi 2009).
9/85 studies used a dose of ≤ 20 mg or ≤ 0.2 mg/kg lidocaine pretreatment with venous occlusion (Asik 2003; Batra 2005; Burimsittichai 2006; Jeon 2012; Johnson 1990; Kwak 2007a; Kwak 2008; Kwon 2012; Scott 1988).
25/85 studies used a dose of > 20 mg or > 0.2 mg/kg lidocaine pretreatment with venous occlusion (Ayoglu 2007; Agarwal 2004a; Agarwal 2004c; Ahmad 2013; Akgun 2013; Apiliogullari 2007; Borazan 2010; Canbay 2008; DeSousa 2011; Dubey 2003; El‐Radaideh 2007; Hwang 2010; Johnson 1990; Kim 2013a; Kim 2013b; Liaw 1999; Massad 2006; Niazi 2005; Ozgul 2013; Pang 1998; Pang 1999; Reddy 2001; Saadawy 2007; Walker 2011; Wong 2001).
Outcomes
All studies assessed the pain intensity by different pain scales and reported in different ways as follow.
71/85 studies reported both incidence of high‐intensity pain and incidence of pain.
11/85 studies reported only incidence of pain (El‐Radaideh 2007; Haugen 1995; Johnson 1990; Kim 2013a; Liaw 1999; Mallick 2007; McCulloch 1985; Pang 1998; Scott 1988; Tham 1995; Walker 2011).
2/85 studies reported only mean and standard deviation of pain‐intensity which were not included in meta‐analysis (Ayoglu 2007; Eriksson 1997).
1/85 studies reported only percentage of pain reduction which was not included in meta‐analysis (Polat 2012).
32/85 studies reported adverse effects (Agarwal 2004a; Agarwal 2004b; Agarwal 2004c; Agarwal 2004d; Akgun 2013; Apiliogullari 2007; Ayoglu 2007; Borazan 2010; Canbay 2008; Cheong 2002; Dubey 2003; Ganta 1992; Han 2010; Honarmand 2008; Jeon 2012; Johnson 1990; Kim 2013a; Koo 2006; Krobbuaban 2005; Krobbuaban 2008; Kwak 2007a; Kwak 2008; Kwon 2012; Liaw 1999; McCulloch 1985; Nakane 1999; Ozgul 2013; Pang 1999; Saadawy 2007; Smith 1996; Tham 1995; Zahedi 2009).
None of the studies reported patient satisfaction levels.
See Characteristics of included studies table for more details.
Excluded studies
We excluded 31 studies for the following reasons.
16/31 were studies in paediatric patients (Barbi 2003; Beh 2002; Cameron 1992; Cheng 1998; Depue 2013; Hiller 1992; Kaabachi 2007; Kwak 2009; Lembert 2002; Morton 1990; Nyman 2005; Nyman 2006; Pollard 2002; Rahman 2007; Rochette 2008; Valtonen 1989).
8/31 were retracted after publishing (Fujii 2004; Fujii 2005a; Fujii 2005b; Fujii 2006; Fujii 2008; Fujii 2009; Fujii 2011; Roehm 2003).
5/31 had no placebo‐controlled group (Brock 2010; Chaudhary 2013; Fujii 2007; Lee 2004; Massad 2008).
1/31 reported non‐relevant outcomes (So 2013).
1/31 was letter‐to‐editor and non randomized controlled trial (Ewart 1990).
see Characteristics of excluded studies for more details.
Studies awaiting classification
Eleven studies (Alipour 2014; Byon 2014; Galgon 2015; Gharavi 2014; Goktug 2015; Kim 2014a; Kim 2014b; Le Guen 2014; Lee 2011; Singh 2014; Terada 2014) are awaiting classification.
See the Characteristics of studies awaiting classification for more details.
Ongoing studies
There are no ongoing studies.
Risk of bias in included studies
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
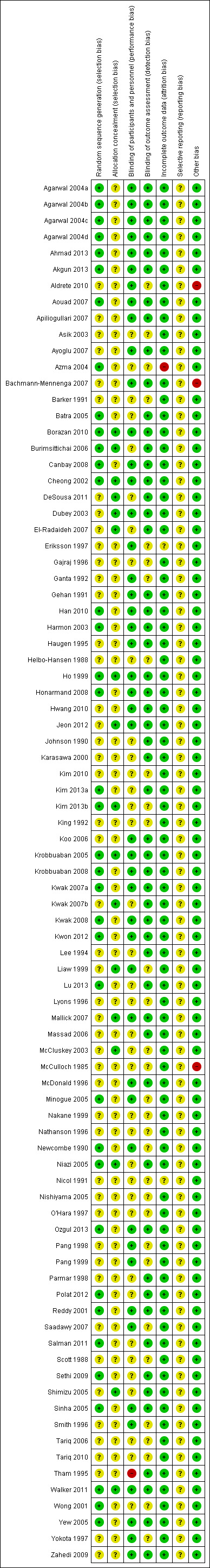
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All of the studies reported that the study was randomized but only 37 of 85 studies (43.5%) provided adequate sequence generation. Also, 17 of 85 (20%) mentioned adequate method of allocation concealment. No studies were assessed as high risk of selection bias.
Blinding
Most of the studies blinded participants, personnel and outcome assessors. The injected solution was prepared by an another person not involved in the investigation. Forty‐seven of 85 studies (55.3%) reported adequate blinding of participants and personnel. Only one study (Tham 1995) was judged as high risk of performance bias as the study mentioned that propofol was administered by the same person who prepared the mixture. However, 54 studies (63.5%) reported adequate blinding of outcome assessor.
Incomplete outcome data
Sixty‐five studies (76.5%) reported no withdrawal of participants after randomization. Seventeen studies (20%) reported that fewer than 15% of recruited participants dropped out and provided the reasons for their exclusions (Bachmann‐Mennenga 2007; Harmon 2003; Hwang 2010; Kim 2013a; Kim 2013b; King 1992; Krobbuaban 2005; Krobbuaban 2008; Kwak 2007a; Kwak 2007b; Kwak 2008; Kwon 2012; Mallick 2007; McDonald 1996; Newcombe 1990; Ozgul 2013; Sinha 2005). Only one study was assessed as high risk of attrition bias (Azma 2004). This study excluded 43 out of 180 patients (more than 15%) but reported only 20 excluded patients due to difficulty in cephalic venous catheterization and seven due to incidence of pain and severity beyond the expectations of the investigator, but other exclusions were not described. Also most of the excluded participants were in the control group. Another study (Nicol 1991) was judged as unclear risk of attrition bias. This is because the study reported that ten of 283 patients were excluded and provided the reason that more operations in one group were cancelled after randomization than in the other two groups. However, there was no information regarding the number of exclusions in each group. Moreover, a study by Eriksson 1997, which was not included in meta‐analysis, did not report some missing data. Total injections in this study were 88 injections (44 participants were injected both hands) but the study reported only 71 injections. No explanation of missing data were described.
Selective reporting
The assessment of selective reporting outcome was unclear in all studies because we could not access the study protocols.
Other potential sources of bias
Most of the included studies demonstrated a low risk of other potential sources of bias. Only three studies (Aldrete 2010; Bachmann‐Mennenga 2007; McCulloch 1985) were funded or supplied propofol by the pharmaceutical company and therefore were judged as high risk of other sources of bias.
Effects of interventions
See: Table 1
See Table 1 Lidocaine for reducing propofol‐induced pain on induction of anaesthesia in adults.
Primary outcome: high‐intensity pain
There were 71 studies including a total of 8845 participants. Most of the studies assessed pain intensity by using 4‐point scales, although seven studies used 3‐point scales (Apiliogullari 2007; Asik 2003; DeSousa 2011; Massad 2006; Newcombe 1990; Nishiyama 2005; Wong 2001), one study, Azma 2004 used a 6‐point scale, and two studies, Kim 2013b; Pang 1999 used 11‐point scales. The overall incidence of high‐intensity pain in the control group was 37.9% (95% CI 33.4% to 43.1%) whereas in the lidocaine group it was only 11.8% (95% CI 9.7% to 13.8%). The number needed to treat for an additional harmful outcome (NNTH) was 3.8.
Subgroup analysis
High‐intensity pain with lidocaine admixture (Analysis 1.1)
According to 31 of 71 studies, the percentage of high‐intensity pain in the control group was 30.5%, compared to 11.3% in the lidocaine admixture group. In other words, the magnitude (risk ratio reduction) of lidocaine admixture to reduce high‐intensity pain following propofol injection was 63%.
The overall effect of the random‐effects meta‐analysis favoured lidocaine admixture with a statistically significant benefit for high‐intensity pain (OR 0.19, 95% CI 0.15 to 0.25, 31 RCTs, 4927 participants, I2 = 45%, Tau2 = 0.27, high‐quality evidence, Analysis 1.1. The direction of the result was similar when we conducted the sensitivity analysis of studies with low risk of selection bias (OR 0.20, 95% CI 0.05 to 0.83, two RCTs (Ho 1999; Krobbuaban 2005), 627 participants, I2 = 87%, Tau2 = 1.87). The visual inspection of the funnel plot presented roughly symmetrical results around the overall effect, OR 0.19, this indicated that there might be no publication bias (Figure 4).
1.1. Analysis.
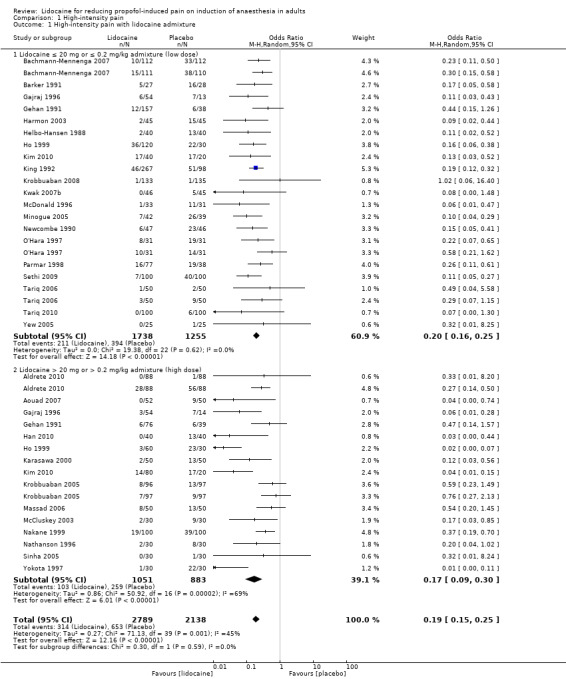
Comparison 1 High‐intensity pain, Outcome 1 High‐intensity pain with lidocaine admixture.
4.
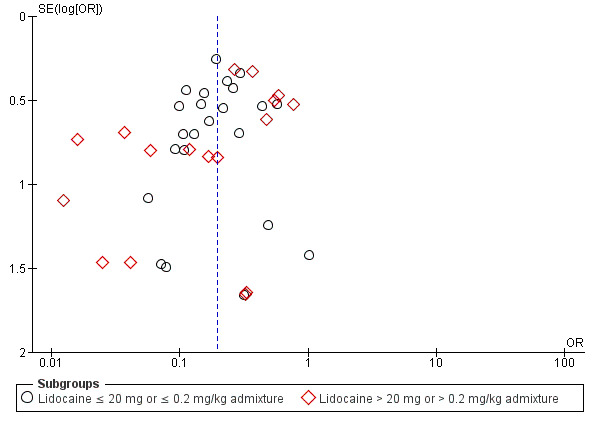
Funnel plot of outcome: High‐intensity pain with lidocaine admixture
Low dose lidocaine admixture (≤ 20 mg or ≤ 0.2 mg/kg): 20 studies (Bachmann‐Mennenga 2007; Barker 1991; Gajraj 1996; Gehan 1991; Harmon 2003; Helbo‐Hansen 1988; Ho 1999; Kim 2010; King 1992; Krobbuaban 2008; Kwak 2007b; McDonald 1996; Minogue 2005; Newcombe 1990; O'Hara 1997; Parmar 1998; Sethi 2009; Tariq 2006; Tariq 2010; Yew 2005), 2993 participants were analysed with OR 0.20, 95% CI 0.16 to 0.25, I2 = 0%.
High dose lidocaine admixture (> 20 mg or > 0.2 mg/kg): 15 studies (Aldrete 2010; Aouad 2007; Gajraj 1996; Gehan 1991; Han 2010; Ho 1999; Karasawa 2000; Kim 2010; Krobbuaban 2005; Massad 2006; McCluskey 2003; Nakane 1999; Nathanson 1996; Sinha 2005; Yokota 1997), 1934 participants were analysed with OR 0.17, 95% CI 0.09 to 0.30, I2= 69%.
There was no significant difference between these two subgroups (Analysis 1.1; Chi² test = 0.30, df = 1 (P = 0.59), I² = 0%).
High‐intensity pain with lidocaine pretreatment (Analysis 1.2)
For lidocaine pretreatment, the statistically significant benefit was also observed (OR 0.13, 95% CI 0.10 to 0.18, 41 RCTs, 3918 participants, I2 statistic = 57%, Tau2 = 0.50, high‐quality evidence, Analysis 1.2. The sensitivity analysis which excluded studies with unclear and high risk of selection bias did not affect the direction of the result nor the statistical significance of high‐intensity pain (OR 0.18, 95% CI 0.11 to 0.29, five RCTs (Borazan 2010; Burimsittichai 2006; Cheong 2002; Kim 2013b; Niazi 2005), 466 participants, I2 = 0%, Tau2 = 0.00, Figure 5). We found the funnel plot to be asymmetrical (the absence of negative studies) (Figure 6), demonstrating the likelihood of publication bias.
1.2. Analysis.
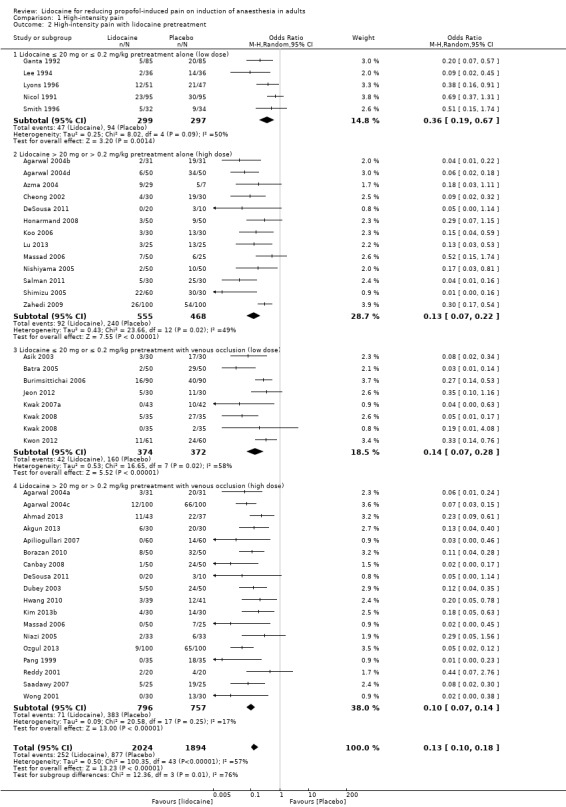
Comparison 1 High‐intensity pain, Outcome 2 High‐intensity pain with lidocaine pretreatment.
5.
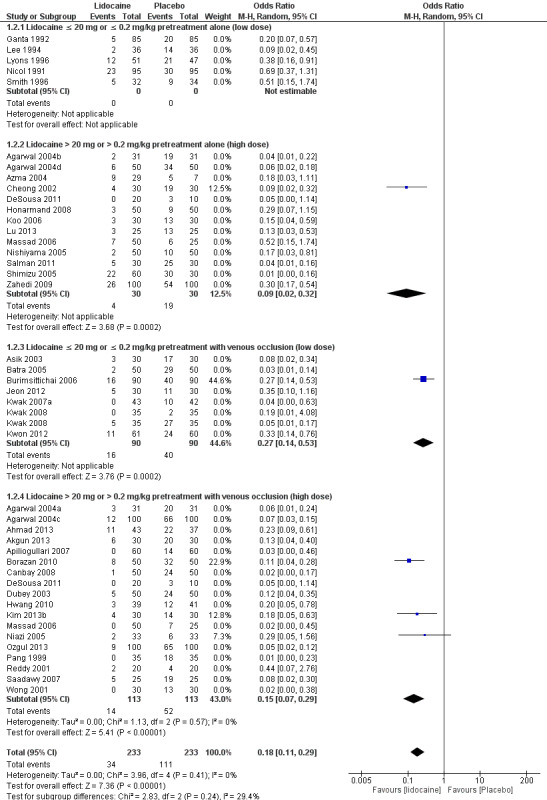
Sensitivity analysis for low risk of selection bias of outcome: High‐intensity pain with lidocaine pretreatment
6.
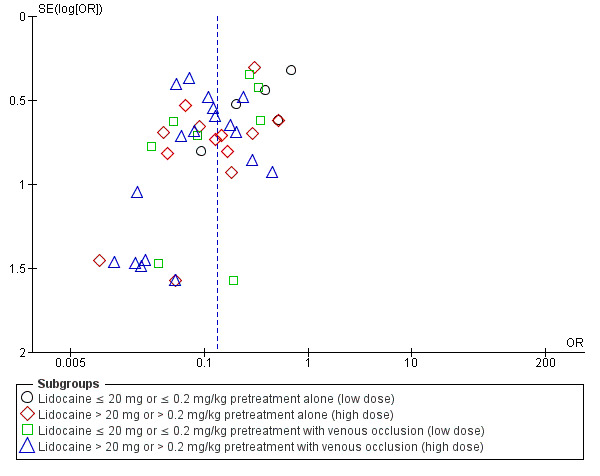
Funnel plot of outcome: High‐intensity pain with lidocaine pretreatment
This category comprised two doses of lidocaine regimen (low dose: ≤ 20 mg or ≤ 0.2 mg/kg lidocaine and high dose: > 20 mg or > 0.2 mg/kg lidocaine) with two different techniques of administration (with or without venous occlusion). A total of 41 studies were included with 3918 patients. Similar to lidocaine admixture, the percentage of patients with high‐intensity pain decreased from 46.3% in the control group to 12.5% in the lidocaine pretreatment group, which is 73% risk ratio reduction. Furthermore, all of the four different techniques of lidocaine administration in subgroup analysis showed a very good result in reducing pain on propofol injection. Accordingly, low dose lidocaine pretreatment without venous occlusion seemed to be the least effective treatment (OR 0.36, 95% CI 0.19 to 0.67, I2 = 50%).
Low dose lidocaine pretreatment alone (≤ 20 mg or ≤ 0.2 mg/kg): five studies (Ganta 1992; Lee 1994; Lyons 1996; Nicol 1991; Smith 1996), 596 participants were analysed with OR 0.36, 95% CI 0.19 to 0.67, I2 = 50%.
High dose lidocaine pretreatment alone (> 20 mg or > 0.2 mg/kg): 13 studies (Agarwal 2004b; Agarwal 2004d; Azma 2004; Cheong 2002; DeSousa 2011; Honarmand 2008; Koo 2006; Lu 2013; Massad 2006; Nishiyama 2005; Salman 2011; Shimizu 2005; Zahedi 2009), 1023 participants were analysed with OR 0.13, 95% CI 0.07 to 0.22, I2 = 49%.
Low dose lidocaine pretreatment with venous occlusion (≤ 20 mg or ≤ 0.2 mg/kg): seven studies (Asik 2003; Batra 2005; Burimsittichai 2006; Jeon 2012; Kwak 2007a; Kwak 2008; Kwon 2012), 746 participants were analysed with OR 0.14, 95% CI 0.07 to 0.28, I2 = 58%.
High dose lidocaine pretreatment with venous occlusion (> 20 mg or > 0.2 mg/kg): 18 studies (Agarwal 2004a; Agarwal 2004c; Ahmad 2013; Akgun 2013; Apiliogullari 2007; Borazan 2010; Canbay 2008; DeSousa 2011; Dubey 2003; Hwang 2010; Kim 2013b; Massad 2006; Niazi 2005; Ozgul 2013; Pang 1999; Reddy 2001; Saadawy 2007; Wong 2001), 1553 participants were analysed with OR 0.10, 95% CI 0.07 to 0.14, I2 = 17%.
There was a significant difference among these subgroups (Analysis 1.2; Chi² test = 12.36, df = 3 (P = 0.006), I² = 75.7%).
Both the lidocaine pretreatment subgroup (OR 0.13, 95% CI 0.10 to 0.18, I2 = 57%, high‐quality evidence) and the lidocaine admixture subgroup (OR 0.19, 95% CI 0.15 to 0.25, I2 = 45%, high‐quality evidence) demonstrated a very large effect size for reducing pain on propofol injection.
Secondary outcomes
Incidence of pain
All 82 studies (total of 10,350 participants) reported incidence of pain. The pain intensity was assessed using 2‐point scales in one study (El‐Radaideh 2007), 3‐point scales in ten studies (Apiliogullari 2007; Asik 2003; DeSousa 2011; Johnson 1990; Massad 2006; McCulloch 1985; Newcombe 1990; Nishiyama 2005; Tham 1995; Wong 2001), 4‐point scales in 64 studies, 6‐point scales in one study (Azma 2004), and 11‐point scales in eight studies (Haugen 1995; Kim 2013a; Kim 2013b; Liaw 1999; Mallick 2007; Pang 1998; Pang 1999; Walker 2011). The overall incidence of pain was significantly decreased from 63.7% (95% CI 60% to 67.9%) in the control group to about 30.2% (95% CI 26.7% to 33.7%) in the lidocaine group. The number needed to treat for an additional harmful outcome (NNTH) was 3.0. Both the admixture and pretreatment with lidocaine groups saw a reduction in the incidence of pain, with OR varying from 0.11 to 0.40.
Subgroup analysis
Incidence of pain with lidocaine admixture (Analysis 2.1)
There were 36 studies included in this subgroup with a total of 5628 participants (Aldrete 2010; Aouad 2007; Bachmann‐Mennenga 2007; Barker 1991; Gajraj 1996; Gehan 1991; Han 2010; Harmon 2003; Helbo‐Hansen 1988; Ho 1999; Johnson 1990; Karasawa 2000; Kim 2010; King 1992; Krobbuaban 2005; Krobbuaban 2008; Kwak 2007b; Mallick 2007; Massad 2006; McCluskey 2003; McDonald 1996; Minogue 2005; Nakane 1999; Nathanson 1996; Newcombe 1990; O'Hara 1997; Parmar 1998; Scott 1988; Sethi 2009; Sinha 2005; Tariq 2006; Tariq 2010; Tham 1995; Walker 2011; Yew 2005; Yokota 1997).
The overall effect of the random effects meta‐analysis favoured lidocaine admixture with a statistically significant reduction of incidence of pain (OR 0.19, 95% CI 0.15 to 0.24, 36 RCTs, 5628 participants, I2 = 66%, Tau2 = 0.44, high‐quality evidence). A premixed high dose of lidocaine > 20 mg or > 0.2 mg/kg with propofol (OR 0.15, 95% CI 0.09 to 0.24, 19 RCTs, 2495 participants, high‐quality evidence) or premixed low dose regimen (lidocaine ≤ 20 mg or ≤ 0.2mg/kg; OR 0.22, 95% CI 0.17 to 0.28, 23 RCTs, 3133 participants, high‐quality evidence) was comparably effective in preventing propofol‐induced pain.
Low dose lidocaine admixture (≤ 20 mg or ≤ 0.2 mg/kg): 23 studies (Bachmann‐Mennenga 2007; Barker 1991; Gajraj 1996; Gehan 1991; Harmon 2003; Helbo‐Hansen 1988; Ho 1999; Johnson 1990; Kim 2010; King 1992; Krobbuaban 2008; Kwak 2007b; McDonald 1996; Minogue 2005; Newcombe 1990; O'Hara 1997; Parmar 1998; Scott 1988; Sethi 2009; Tariq 2006; Tariq 2010; Tham 1995; Yew 2005), 3133 participants were analysed with OR 0.22, 95% CI 0.17 to 0.28, I2= 32%.
High dose lidocaine admixture (> 20 mg or > 0.2 mg/kg): 19 studies (Aldrete 2010; Aouad 2007; Gajraj 1996; Gehan 1991; Han 2010; Ho 1999; Johnson 1990; Karasawa 2000; Kim 2010; Krobbuaban 2005; Mallick 2007; Massad 2006; McCluskey 2003; Nakane 1999; Nathanson 1996; Sinha 2005; Tham 1995; Walker 2011; Yokota 1997), 2495 participants were analysed with OR 0.15, 95% CI 0.09 to 0.24, I2 = 79%.
There was no significant difference between the two subgroups (Analysis 2.1; Chi² test = 2.00, df = 1 (P = 0.16), I² = 49.9%).
2.1. Analysis.
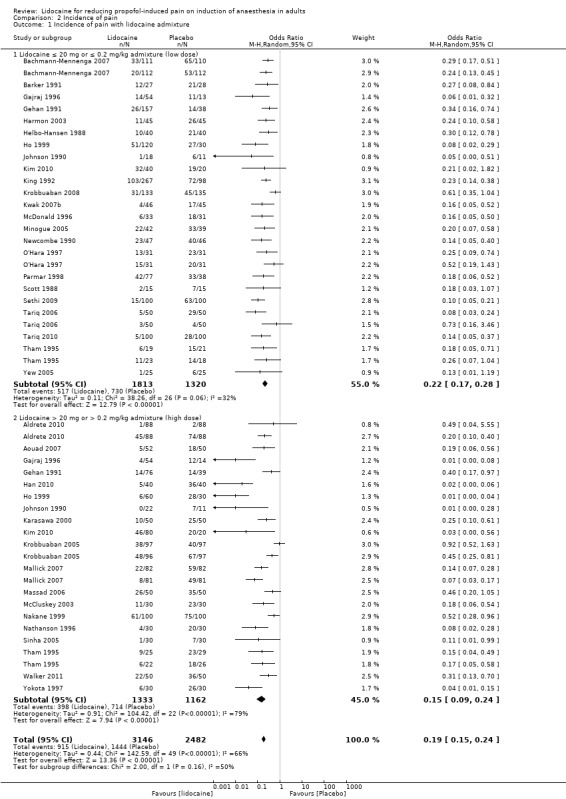
Comparison 2 Incidence of pain, Outcome 1 Incidence of pain with lidocaine admixture.
Incidence of pain with lidocaine pretreatment (Analysis 2.2)
There were 50 studies with 4722 participants included in this outcome. A statistically significant benefit was also demonstrated with lidocaine pretreatment (OR 0.14, 95% CI 0.11 to 0.18, 50 RCTs, 4722 participants, I2 = 62%, Tau2 = 0.49, high‐quality evidence). For the subgroup analysis, The high or low dose lidocaine, with or without venous occlusion, similarly demonstrated a good efficacy for decreasing the incidence of pain following propofol injection. However, the low dose lidocaine pretreatment alone (OR 0.40, 95% CI 0.29 to 0.55, seven studies, 713 participants, high‐quality evidence) appeared to be the least effective technique.
Low dose lidocaine pretreatment alone (≤ 20 mg or ≤ 0.2 mg/kg): seven studies (Ganta 1992; Lee 1994; Lyons 1996; McCulloch 1985; Nicol 1991; Scott 1988; Smith 1996), 713 participants were analysed with OR 0.40, 95% CI 0.29 to 0.55, I2 = 0%.
High dose lidocaine pretreatment alone (> 20 mg or > 0.2 mg/kg): 14 studies (Agarwal 2004b; Agarwal 2004d; Azma 2004; Cheong 2002; DeSousa 2011; Haugen 1995; Honarmand 2008; Koo 2006; Lu 2013; Massad 2006; Nishiyama 2005; Salman 2011; Shimizu 2005; Zahedi 2009), 1083 participants were analysed with OR 0.13, 95% CI 0.08 to 0.20, I2 = 40%.
Low dose lidocaine pretreatment with venous occlusion (≤ 20 mg or ≤ 0.2 mg/kg): nine studies (Asik 2003; Batra 2005; Burimsittichai 2006; Jeon 2012; Johnson 1990; Kwak 2007a; Kwak 2008; Kwon 2012; Scott 1988), 801 participants were analysed with OR 0.13, 95% CI 0.05 to 0.29, I2= 79%.
High dose lidocaine pretreatment with venous occlusion (> 20 mg or > 0.2 mg/kg): 24 studies (Agarwal 2004a; Agarwal 2004c; Ahmad 2013; Akgun 2013; Apiliogullari 2007; Borazan 2010; Canbay 2008; DeSousa 2011; Dubey 2003; El‐Radaideh 2007; Hwang 2010; Johnson 1990; Kim 2013a; Kim 2013b; Liaw 1999; Massad 2006; Niazi 2005; Ozgul 2013; Pang 1998; Pang 1999; Reddy 2001; Saadawy 2007; Walker 2011; Wong 2001), 2125 participants were analysed with OR 0.11, 95% CI 0.09 to 0.15, I2 = 26%.
There was a significant difference between these four subgroups (Analysis 2.2; Chi² test = 40.05, df = 3 (P < 0.00001), I² = 92.5%).
2.2. Analysis.

Comparison 2 Incidence of pain, Outcome 2 Incidence of pain with lidocaine pretreatment.
Adverse effects
32 studies (Agarwal 2004a; Agarwal 2004b; Agarwal 2004c; Agarwal 2004d; Akgun 2013; Apiliogullari 2007; Ayoglu 2007; Borazan 2010; Canbay 2008; Cheong 2002; Dubey 2003; Ganta 1992; Han 2010; Honarmand 2008; Jeon 2012; Johnson 1990; Kim 2013a; Koo 2006; Krobbuaban 2005; Krobbuaban 2008; Kwak 2007a; Kwak 2008; Kwon 2012; Liaw 1999; McCulloch 1985; Nakane 1999; Ozgul 2013; Pang 1999; Saadawy 2007; Smith 1996; Tham 1995; Zahedi 2009), 4007 participants were analysed for adverse effects (OR not estimated, low‐quality evidence). An adverse effect, thrombophlebitis, was observed in two studies (Ganta 1992; Smith 1996).
One study (Ganta 1992) reported thrombophlebitis: 4/85 participants in the lidocaine 10 mg pretreatment group compared with 8/85 cases in the saline group.
Another study (Smith 1996) reported thrombophlebitis within seven days postoperatively by self‐assessment questionnaire. The incidence of thrombophlebitis was 9/22 participants in the lidocaine 20 mg pretreatment group, compared to 4/29 in the saline group. However, there were no statistically significant differences in the two groups.
According to the GRADE approach to consider the quality of the evidence, we downgraded the quality of evidence by two levels due to serious imprecision and inconsistency.
Patient's satisfaction
None of the studies described patient satisfaction in the reports.
Discussion
Summary of main results
Lidocaine, both administered by admixture and pretreatment (Table 1) could significantly reduce high‐intensity pain levels and decrease the incidence of pain. In subgroup analyses, there were no significant differences in the efficacy (with very large effect size) among two different techniques of lidocaine admixture administration for reducing propofol injection pain. However, there was a significant difference in the efficacy among four different techniques of lidocaine pre‐treatment administration for reducing propofol injection pain. Low dose lidocaine (≤ 20 mg or ≤ 0.2 mg/kg) pretreatment alone appeared to provide the least efficacy (with large effect size) in reducing and preventing propofol‐induced pain. Thrombophlebitis was an adverse effect reported in only two studies (Ganta 1992; Smith 1996) which was not significantly different between lidocaine and placebo groups.
Overall completeness and applicability of evidence
Our review included studies conducted worldwide with low risk of bias in all except two domains, allocation concealment and selective reporting bias. Participants of the included studies were adults up to 89 years old. The level of pain intensity was reported in 71 of 82 studies while 33 studies reported adverse effects. Interestingly, no study reported patient satisfaction. This might be because there are many factors which influence the level of patient satisfaction, such as discomfort from sore throat after intubation, or postoperative pain, making it difficult to measure. Regrettably, patient satisfaction is a key outcome to present: it is important whether patients considered such pain was significant. In addition, there were many scoring systems for pain assessment in the different studies. However, most of the included studies used 4‐point scales as an outcome measure rather than a validated pain scale, such as visual analogue score or numerical rating score. This was probably because 11‐point scales might have been too complicated for patients under the induction process.
Generally, application of the review’s findings to clinical practice is possible since lidocaine is a cheap and easily available drug throughout the world. Premixed lidocaine with propofol has been well‐accepted (Kim 2010; Scott 1988; Tariq 2006). Subgroup analysis of the dose of lidocaine suggested that a higher dose is more effective for reducing and preventing propofol‐induced pain than a lower dose in both admixture and pretreatment groups. The most common high dose used in the included studies was 40 mg, double that in the low dose lidocaine group. The maximum dose was 100 mg (5 ml of 2% lidocaine) in Aldrete 2010, which is common for attenuation of haemodynamic response to intubation in clinical practice; however, there were no adverse effects detected.
Picard 2000 suggested that the combination of high dose lidocaine pretreatment and venous occlusion is more effective than the other lidocaine administration techniques to reduce the incidence of propofol injection pain. This is because venous occlusion with a tourniquet allowed high concentrations of the drug to be retained locally, extending the analgesic time. The procedure of pretreatment with venous occlusion involves more steps to start the anaesthesia, however and the efficacy of pretreatment with venous occlusion and without venous occlusion was not significantly different. Therefore it is not a popular strategy for many anaesthesiologists. Another reason is that, in some circumstances, such as in rapid sequence induction, it may not be appropriate to perform pretreatment with venous occlusion. A subsequent systematic review (Jalota 2011) recommended using the antecubital vein instead of a vein in the hand, as it was an equally effective method, and accessing the antecubital vein was relatively simple. However, it was quite unpopular because it is easy to dislodge intravenous lines when patients flex their elbows, and the hand vein proves more comfortable to the patient than the antecubital vein does.
Regarding our review, the result also showed that there were no significant differences among six subgroups. Therefore, we would recommend lidocaine administration by any method (low dose/high dose; premixed/pretreatment; with/without venous occlusion), depending on the anaesthesiologists’ circumstances and appropriateness, to provide effective pain reduction following propofol injection.
Quality of the evidence
We included 82 studies, with 10,350 participants for quantitative analysis. The overall risk of bias of most individual studies ranged from 'low' to 'unclear'. In terms of blinding, 48 studies were described as randomized, double blinded, controlled trials. However in the studies described as single blinded (four studies: Azma 2004; Barker 1991; DeSousa 2011; Tham 1995) or only randomized controlled trials (30 studies), the authors provided explicit detail about investigator blinding. Nevertheless the participants were likely to be blinded, as the injection of study drugs was done in the same manner for both the lidocaine and placebo groups. There was unclear risk of selection bias from inadequate information about sequence generation in 38 studies, and only 19 studies mentioned allocation concealment (see Figure 3). However the result was similar when sensitivity analysis of studies with low risk of selection bias was done. Only one study (Tham 1995) had high risk of performance bias, since propofol was injected by the same person who prepared the study drug (n = 183). We judged the risk of attrition bias as high in one study (Azma 2004) (n = 137) since more than 15% of participants were excluded without clear reasons reported for all excluded cases. There was also unclear risk of attrition bias in another study (Nicol 1991) (n = 283) as the number of excluded participants was not reported per group. The risk of reporting bias in all studies was unclear as the study protocols could not be accessed. There were also three studies (Aldrete 2010; Bachmann‐Mennenga 2007; McCulloch 1985) with high risk of other potential sources of bias as the studies were funded or supplied propofol by a pharmaceutical company. Furthermore the benefit of lidocaine pretreatment was interpreted with caution since there might be evidence of publication bias due to small study effect (Figure 6). Co‐treatment such as remifentanil was found in some studies with lidocaine pretreatment (Aouad 2007; Han 2010; Kwak 2007a; Kwak 2007b). This particular co‐treatment possibly confounded the levels of pain. However, when we tried to exclude studies with co‐treatments to confirm the applicability of the data, the results showed that the efficacy of the intervention groups was not changed.
Overall, the quality of the evidence for high‐intensity pain and incidence of pain outcomes with lidocaine admixture and pretreatment was high when using the GRADE approach. Considering 'lidocaine ≤ 20 mg or ≤ 0.2 mg/kg pretreatment alone' and 'lidocaine ≤ 20 mg or ≤ 0.2 mg/kg pretreatment with venous occlusion' subgroups, although the number of events was lower than 300, 95% confidence intervals around absolute effects were narrow. Therefore the quality of evidence for imprecision was not downgraded. Nevertheless, the quality of the evidence for adverse effects outcomes was low due to serious imprecision and inconsistency.
Potential biases in the review process
Despite using comprehensive and systematic searching we may have missed trials that were not indexed in CENTRAL, MEDLINE, EMBASE and LILACS, or websites of ongoing trials that remain unpublished in journals. We reran the search strategy in November 2015. We found 11 studies of interest. Those studies were added to a list of ‘Studies awaiting classification' and will be incorporated into the formal review findings during the review update.
There might be a possibility of publication bias as shown in Figure 6 for the effect of lidocaine pretreatment on high‐intensity pain. However, published evidence comprised a considerable number of trials, therefore, we would not consider publication bias in this subgroup.
We clearly stated in our protocol that participants aged over 15 years could be included, however, there was one study (DeSousa 2011) which enrolled participants aged 13 years to 65 years. On the agreement of two authors, we included this study in our review, because patients aged more than 12 years could certainly report pain rating scales (von Baeyer 2006) and body weight was not much different to adults. We were concerned about selective bias and misclassification bias regarding this decision, however the results of the forest plot that included or excluded DeSousa 2011 were no different.
There were eight studies excluded from this review due to retraction (Fujii 2004; Fujii 2005a; Fujii 2005b; Fujii 2006; Fujii 2008; Fujii 2009; Fujii 2011; Roehm 2003). This is very uncommon in this area and seven of the eight retracted papers were from one author. The reason for retraction was fabrication of data detected by journals. The findings of these retracted papers should not have any implications on the current findings. We think that the validity of our findings will be strengthened by excluding fabricated papers.
We were aware that deviating from or changing the protocol may cause potential biases. However, reducing the scope of the interventions and changing subgroup analyses in this review were considered as low potential bias.
Agreements and disagreements with other studies or reviews
There were two published systematic reviews and meta analyses exploring interventions for reducing pain on injection induced by propofol (Jalota 2011; Picard 2000). Without any intervention the incidence of pain in our review (63.7%) was similar to the results of those two previously reported (60% from Jalota 2011 and 70% from Picard 2000). We could not find any other studies or reviews investigating the effect of lidocaine on the prevention of high‐intensity pain to compare against our review. We found the incidence of high‐intensity pain in the lidocaine group was only 11.8% compared with 37.9% in the control group (NNTH 3.8).
Both systematic reviews identified pretreatment with lidocaine in conjunction with venous occlusion, using a tourniquet above the injection site, to be the most efficacious intervention. With the combination of intravenous lidocaine 40 mg pretreatment and a tourniquet 30 seconds to 120 seconds before injection of propofol, the NNTH to prevent any pain was 1.6 in Picard 2000. Jalota 2011 reported RR 0.29 with the same technique. Our systematic review also confirmed the efficacy of these previous reports. The conjunction between high dose lidocaine (> 20 mg or > 0.2 mg/kg) pretreatment and venous occlusion showed a very large effect size in our review (OR 0.1, 95% CI 0.07 to 0.14). The duration of venous occlusion varied from a period of three seconds to three minutes before propofol injection, which was similar to other reports (Johnson 1990; Picard 2000).
Comparing lidocaine admixture to pretreatment, Picard 2000 reported NNTH was 2.4 in lidocaine admixture, compared with 1.6 in pretreatment with venous occlusion. Conversely, Lee 2004 demonstrated that high dose lidocaine admixture was modestly more effective in reducing incidence of pain than high dose lidocaine pretreatment, and recommended that lidocaine should be added to propofol for induction rather than given before induction. Regarding the findings in our review, however, there was no significant difference between lidocaine pretreatment and admixture for reducing and preventing propofol injection pain.
Adverse effects were rare in our review. Only thrombophlebitis was observed in two studies (Ganta 1992; Smith 1996), which were comparable to previous reviews (Jalota 2011; Picard 2000).
Authors' conclusions
Implications for practice.
Regarding our results, lidocaine admixture and pretreatment were effective in reducing and preventing pain on propofol injection. As a consequence, lidocaine administration by any method (low dose/high dose; premixed/pretreatment; with/without venous occlusion), depending on the anaesthesiologists’ circumstances and on the appropriateness of the procedure, is beneficial for reducing propofol‐induced pain in adults. The venous occlusion technique may not be suitable for some situations, for example in cases requiring rapid induction, as the procedure takes more time to carry out. In such cases, premixed or pretreatment alone with high dose lidocaine (> 20 mg or > 0.2 mg/kg) may be a preferred option.
Implementation decisions should balance the high degree of certainty in the reduction in pain, low cost and wide availability of lidocaine against the lack of evidence for harms and patient satisfaction identified by this review.
Implications for research.
To date, there are a large number of randomized controlled trials regarding interventions for reducing propofol‐induced pain. Therefore, we would suggest that systematic reviews should be conducted in other aspects, for example, lidocaine for reducing pain caused by propofol injection in children, or opioids for reducing propofol‐induced pain.
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2016 | Amended | Two recently retracted studies (Fujii 2008; Fujii 2009), were excluded from the review |
Notes
December 2016: two recently retracted studies (Fujii 2008; Fujii 2009), were excluded from the review
Acknowledgements
We would like to thank Karen Hovhannisyan for his help in writing the search strategy and search process, Miss Boonruan Kanlayanamatee, Siriraj medical librarian, for her assistance on full article access. Our appreciation also goes to Jane Cracknell (Managing Editor, Cochrane Anaesthesia, Critical and Emergency Care) for co‐ordinating the protocol and review processes.
We thank Andrew Smith (content editor); Andrew Moore, Yoshitaka Fujii, and Stefan Soltesz (peer reviewers); and Anne Lyddiatt (Cochrane Consumer Network) for their help and editorial advice during the preparation of the protocol (Euasobhon 2009) for the systematic review.
We also would like to thank Andrew Smith (content editor), Jing Xie (statistical editor), Yvonne Nyman, Chi Wai Cheung (peer reviewers), Janet Wale (consumer editor), Denise Mitchell (copy editor) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. Glossary
| Terms | Definition |
| Afferent nerve ending | The distal end of nerve fibre of an sensory neuron that carries nerve impulses from sensory receptors or sense organs toward the central nervous system. |
| American Society of Anesthesiologists (ASA) classification | ASA I = A normal healthy patient ASA II = A patient with mild systemic disease ASA III = A patient with severe systemic disease ASA IV = A patient with severe systemic disease that is a constant threat to life ASA V = A moribund patient who is not expected to survive without the operation ASA VI = A declared brain‐dead patient whose organs are being removed for donor purposes |
| Bioclusive dressing | A thin, polyurethane, acrylic adhesive‐coated dressing, which is permeable to water and O2, but not bacteria; it prevents scabbing and facilitates epidermal regeneration, compared to wounds treated with dry dressings. |
| Bradykinin | A potent endothelium‐dependent vasodilator, leading to a drop in blood pressure. It also causes contraction of non‐vascular smooth muscle in the bronchus and gut, increases vascular permeability and is also an inflammatory mediator involved in the mechanism of pain. |
| Mallampati classification | Class 0: Ability to see any part of the epiglottis upon mouth opening and tongue protrusion Class I: Soft palate, fauces, uvula, pillars visible Class II: Soft palate, fauces, uvula visible Class III: Soft palate, base of uvula visible Class IV: Soft palate not visible at all |
| Prostaglandins | A group of physiologically active lipid compounds having diverse hormone‐like effects and also involved in inflammatory process. |
Appendix 2. Physicopharmacological interventions
| Pretreatment | Admixtured with propofol | Miscellaneous |
| Lidocaine | Lidocaine | Cold temperature |
| Venous occlusion with lidocaine | Thiopental | Warm temperature |
| Lidocaine + metoclopramide | Ketamine | pH adjusted |
| Epidural anaesthesia with lidocaine | 5% dextrose in Ringer's acetate solution | Bacteriostatic saline containing benzyl alcohol |
| Fentanyl | MCT/LCT propofol | |
| Alfentanyl | 0.5% diluted propofol | |
| Remifentanil | Lipid‐free propofol | |
| Remifentanyl + lidocaine | Microfiltation | |
| Meperidine | Aspiration of blood | |
| Ketamine | Target‐controlled propofol | |
| Thiopental | Double lumen intravenous set | |
| Butorphanol | Low‐dose propofol | |
| Flurbiprofen | ||
| Venous occlusion with flurbiprofen axetil | ||
| Ondansetron | ||
| Granisetron | ||
| Dolasetron | ||
| Ephedrine | ||
| Clonidine‐ephedrine | ||
| Oral clonidine | ||
| Metoclopramide | ||
| Dexmedetomidine | ||
| Magnesium sulphate | ||
| Ketolorac | ||
| Diclofenac | ||
| Metoprolol | ||
| Topical nitroglycerine | ||
| Cold saline | ||
| Acetaminophen + lidocaine | ||
| Diphenhydramine | ||
| Nitrous oxide | ||
| Lidocaine and nitrous oxide in oxygen | ||
| Neostigmine | ||
| Tramadol | ||
| Nafamostat mesylate | ||
| Prilocaine |
Appendix 3. Search strategy for CENTRAL, The Cochrane Library
#1 propofol* and pain*
#2 Dolasetron or Remifentanil or Lidocaine or Fentanylor Alfentanil or Pethidineor KETAMINE or THIOPENTAL or Butorphanol or ONDANSETRON or GRANISETRON or EPHEDRINE or CLONIDINE or METOCLOPRAMIDE or DEXMEDETOMIDINE or Magnesium Sulfate or Ketorolac or DICLOFENAC or METOPROLOL or Glyceryl Trinitrate or Cold saline or Acetaminophen or Paracetamol or DIPHENHYDRAMINE or Nitrous Oxide or Neostigmine or Tramadol or Nafamostat mesilate or Prilocaine or PRILOCAINE
#3 temperature near (cold or warm)
#4 (#1 AND ( #2 OR #3 ))
Appendix 4. Search strategy for Ovid MEDLINE (1950 to present)
#1 exp Propofol/ or propofol*.mp.
#2 (propofol adj6 (induc* or relat*)).mp.
#3 #1 or #2
#4 pain.mp. or exp Pain/
#5 #4 and #3
#6 exp Lidocaine/ or Lidocain*.mp.
#7 Fentanyl.mp. or Fentanyl/
#8 Alfentanyl.mp. or Alfentanil/
#9 Meperidine.mp. or Meperidine/
#10 Ketamine.mp. or Ketamine/
#11 Thiopental.mp. or Thiopental/
#12 Butorphanol.mp. or Butorphanol/
#13 Flurbiprofen.mp. or Flurbiprofen/
#14 Ondansetron.mp. or Ondansetron/
#15 Granisetron.mp. or Granisetron/
#16 Ephedrine.mp. or Ephedrine/
#17 clonidine.mp. or Clonidine/
#18 Metoclopramide.mp. or Metoclopramide/
#19 Dexmedetomidine.mp. or Dexmedetomidine/
#20 Magnesium sulfate.mp. or Magnesium Sulfate/
#21 Ketorolac/
#22 Diclofenac.mp. or Diclofenac/
#23 Metoprolol.mp. or Metoprolol/
#24 Nitroglycerin/ or Topical nitroglycerine.mp.
#25 Cold saline.mp.
#26 Acetaminophen.mp. or Acetaminophen/
#27 Diphenhydramine.mp. or Diphenhydramine/
#28 Nitrous oxide.mp. or Nitrous Oxide/
#29 Neostigmine.mp. or Neostigmine/
#30 Tramadol.mp. or Tramadol/
#31 Nafamostat mesilate.mp.
#32 Prilocaine.mp. or Prilocaine/
#33 (temperature adj3 (cold or warm)).mp.
#34 (Dolasetron or Remifentanil).mp.
#35 or/6‐34
#36 #35 and #5
#37 randomised controlled trial.pt.
#38 controlled clinical trial.pt.
#39 randomized.ab.
#40 placebo.ab.
#41 drug therapy.fs.
#42 randomly.ab.
#43 trial.ab.
#44 groups.ab.
#45 or/37‐44
#46 humans.sh.
#47 #45 and #46
#48 #36 and #47
mp = title, original title, abstract, name of substance word, subject heading word
ti = title
ab = abstract
pt = publication type
fs = floating subject
sh = Medline subject heading (MeSH)
Appendix 5. Search strategy for EMBASE (OvidSP) (1988 to present)
#1 exp PROPOFOL/ or propofol*.mp.
#2 (propofol adj6 (induc* or relat*)).mp.
#3 #1 or #2
#4 PAIN/ or pain.ti,ab.
#5 #4 and #3
#6 LIDOCAINE/ or Lidocaine.mp.
#7 FENTANYL/ or Fentanyl.mp.
#8 Alfentanyl.mp. or Alfentanil/
#9 Meperidine.mp. or Pethidine/
#10 Ketamine.mp. or KETAMINE/
#11 Thiopental.mp. or THIOPENTAL/
#12 BUTORPHANOL/ or Butorphanol.mp.
#13 Flurbiprofen.mp. or FLURBIPROFEN/
#14 Ondansetron.mp. or ONDANSETRON/
#15 Granisetron.mp. or GRANISETRON/
#16 Ephedrine.mp. or EPHEDRINE/
#17 Clonidine.mp. or CLONIDINE/
#18 Metoclopramide.mp. or METOCLOPRAMIDE/
#19 Dexmedetomidine.mp. or DEXMEDETOMIDINE/
#20 Magnesium sulfate.mp. or Magnesium Sulfate/
#21 KETOROLAC/ or Ketorolac.mp.
#22 Diclofenac.mp. or DICLOFENAC/
#23 Metoprolol.mp. or METOPROLOL/
#24 Nitroglycerin.mp. or Glyceryl Trinitrate/
#25 Cold saline.mp.
#26 Acetaminophen.mp. or Paracetamol/
#27 Diphenhydramine.mp. or DIPHENHYDRAMINE/
#28 Nitrous Oxide.mp. or Nitrous Oxide/
#29 NEOSTIGMINE/ or Neostigmine.mp.
#30 TRAMADOL/ or Tramadol.mp.
#31 Nafamostat mesilate.mp.
#32 Prilocaine.mp. or PRILOCAINE/
#33 (temperature adj3 (cold or warm)).mp.
#34 (Dolasetron or Remifentanil).mp.
#35 or/6‐34
#36 #35 and #5
#37 Randomized Controlled Trial/
#38 RANDOMIZATION/
#39 Controlled Study/
#40 Multicenter Study/
#41 Phase 3 Clinical Trial/
#42 Phase 4 Clinical Trial/
#43 Double Blind Procedure/
#44 Single Blind Procedure/
#45 (RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*).ti,ab.
#46 ((SINGL* or DOUBL* or TREBL* or TRIPL*) adj3 (BLIND* or MASK*)).ti,ab.
#47 or/37‐46
#48 "human*".ec,hw,fs.
#49 #48 and #47
#50 #49 and #36
Appendix 6. Search strategy for LILACS (1992 to present)
("PROPOFOL/" or "propofol$") and ("PAIN" or "pain$")
Appendix 7. Study Selection, Quality Assessment and Data Extraction Form
First author Journal/Conference Proceedings etc Year
Study eligibility
RCT/Quasi/CCT (delete as appropriate) Yes/No/Unclear
Relevant participants Yes/No/Unclear
Relevant interventions Yes/No/Unclear
Relevant outcomes Yes/No*/Unclear
* Issue relates to selective reporting when authors may have taken measurements for particular outcomes, but not reported these within the paper(s). Review authors should contact trialists for information on possible non‐reported outcomes & reasons for exclusion from publication. Study should be listed in ‘Studies awaiting assessment’ until clarified. If no clarification is received after three attempts, study should then be excluded.
Do not proceed if any of the above answers are ‘No’. If study to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into ‘Table of excluded studies’.
Freehand space for comments on study design and treatment:
References to trial
Code each paper Author(s) Journal/Conference Proceedings etc Year
Participants and trial characteristics
Participant characteristics
Age (mean, median, range, etc)
Sex of participants (numbers/%, etc)
Disease status/type, etc (if applicable)
Other
Trial characteristics
See Section 1, usually just completed by one review author
Methodological quality
State here method used to generate allocation and reasons for grading Grade (circle)
Adequate (random)
Inadequate (e.g. alternate)
Unclear
Concealment of allocation
Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from blinding
State here method used to conceal allocation and reasons for grading Grade (circle)
Adequate
Inadequate
Unclear
Blinding
Person responsible for participants' care Yes/No
Participant Yes/No
Outcome assessor Yes/No
Other (please specify) Yes/No
Intention‐to‐treat
An intention‐to‐treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not.
All participants entering trial Yes/No
Participants were excluded 15% or fewer/More than 15%
Analysed as intention to treat/per protocol/unspecified
Were withdrawals described? Yes? No? Not clear?
Discuss if appropriate
Data extraction
Outcomes relevant to your review
Copy and paste from ‘Types of outcome measures’ reported in paper (circle)
Pain score (VAS / NRS / VRS) Yes/No
Adverse effects Yes/No
Patients' satisfaction score Yes/No
Others Yes/No
For continuous data
Code of paper
Outcomes
Unit of measurement
Treatment group 1 N/Mean (SD)
Treatment group 2 N/Mean (SD)
Details if outcome only described in text
For dichotomous data
Code of paper
Outcomes
Character
Treatment group 1 n/%
Treatment group 2 n/%
Details if outcome only described in text
Other information which you feel is relevant to the results
Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review.
Freehand space for writing actions such as contact with study authors and changes
References to other trials
Did this report include any references to published reports of potentially eligible trials not already identified for this review?
First author
Journal/Conference
Year of publication
Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details.
Section 1
Trial characteristics further details
Single centre/multicentre
Country/countries
How many people were randomized?
Number of participants in each intervention group
Number of participants who received intended treatment
Number of participants who were analysed
Drug treatment(s) used
Dose of administration
Duration of treatment (state weeks/months, etc, if cross‐over trial give length of time in each arm)
Time points when measurements were taken during the study
Time points reported in the study
Time points you are using in RevMan
Trial design (e.g. parallel/cross‐over*)
Other
* If cross‐over design, please refer to the Cochrane Editorial Office for further advice on how to analyse these data
Data and analyses
Comparison 1. High‐intensity pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 High‐intensity pain with lidocaine admixture | 31 | 4927 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.15, 0.25] |
| 1.1 Lidocaine ≤ 20 mg or ≤ 0.2 mg/kg admixture (low dose) | 20 | 2993 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.16, 0.25] |
| 1.2 Lidocaine > 20 mg or > 0.2 mg/kg admixture (high dose) | 15 | 1934 | Odds Ratio (M‐H, Random, 95% CI) | 0.17 [0.09, 0.30] |
| 2 High‐intensity pain with lidocaine pretreatment | 41 | 3918 | Odds Ratio (M‐H, Random, 95% CI) | 0.13 [0.10, 0.18] |
| 2.1 Lidocaine ≤ 20 mg or ≤ 0.2 mg/kg pretreatment alone (low dose) | 5 | 596 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.19, 0.67] |
| 2.2 Lidocaine > 20 mg or > 0.2 mg/kg pretreatment alone (high dose) | 13 | 1023 | Odds Ratio (M‐H, Random, 95% CI) | 0.13 [0.07, 0.22] |
| 2.3 Lidocaine ≤ 20 mg or ≤ 0.2 mg/kg pretreatment with venous occlusion (low dose) | 7 | 746 | Odds Ratio (M‐H, Random, 95% CI) | 0.14 [0.07, 0.28] |
| 2.4 Lidocaine > 20 mg or > 0.2 mg/kg pretreatment with venous occlusion (high dose) | 18 | 1553 | Odds Ratio (M‐H, Random, 95% CI) | 0.10 [0.07, 0.14] |
Comparison 2. Incidence of pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of pain with lidocaine admixture | 36 | 5628 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.15, 0.24] |
| 1.1 Lidocaine ≤ 20 mg or ≤ 0.2 mg/kg admixture (low dose) | 23 | 3133 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.17, 0.28] |
| 1.2 Lidocaine > 20 mg or > 0.2 mg/kg admixture (high dose) | 19 | 2495 | Odds Ratio (M‐H, Random, 95% CI) | 0.15 [0.09, 0.24] |
| 2 Incidence of pain with lidocaine pretreatment | 50 | 4722 | Odds Ratio (M‐H, Random, 95% CI) | 0.14 [0.11, 0.18] |
| 2.1 Lidocaine ≤ 20 mg or ≤ 0.2 mg/kg pretreatment alone (low dose) | 7 | 713 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.29, 0.55] |
| 2.2 Lidocaine > 20 mg or > 0.2 mg/kg pretreatment alone (high dose) | 14 | 1083 | Odds Ratio (M‐H, Random, 95% CI) | 0.13 [0.08, 0.20] |
| 2.3 Lidocaine ≤ 20 mg or ≤ 0.2 mg/kg pretreatment with venous occlusion (low dose) | 9 | 801 | Odds Ratio (M‐H, Random, 95% CI) | 0.13 [0.05, 0.29] |
| 2.4 Lidocaine > 20 mg or > 0.2 mg/kg pretreatment with venous occlusion (high dose) | 24 | 2125 | Odds Ratio (M‐H, Random, 95% CI) | 0.11 [0.09, 0.15] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwal 2004a.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 33.6 ± 14.8, Group II = 31.8 ± 14.8, Group III = 35.6 ± 13.5, Group IV = 34.2 ± 15.4 Gender (M:F): Group I = 15:16, Group II = 17:14, Group III = 14:17, Group IV = 16:15 Inclusion criteria: patients aged 18 yr to 50 yr, ASA I‐II, undergoing elective surgical procedures lasting between 1 hour to 2 hours. Exclusion criteria: patients having problems communicating Recruitment: 124 adults randomly assigned into four groups of 31 each group Setting: India |
|
| Interventions |
Pretreatment with venous occlusion Group I (NS) received normal saline Group II (L) received lidocaine 2% (40 mg) Group III (T25) received thiopental 0.25 mg/kg Group IV (T50) received thiopental 0.5 mg/kg All study drugs were made into 2 ml with NS and were administered over 5 sec in a dedicated IV line (18‐gauge) in a vein on the dorsum of the nondependent hand while the venous drainage was occluded manually at the middle of the forearm just before the administration of the study drug and was maintained for 1 min. Patients then received 1/4 of the total calculated dose of propofol over 5 sec. The induction dose of propofol (propofol 1% W/V in lipid base; Claris Lifesciences Limited, Ahmedabad, India) was 2.5 mg/kg. Fentanyl was administered only after induction of anaesthesia. |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "An anaesthesiologist not involved in the study prepared pretreatment drugs." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A second, independent anaesthesiologist who was unaware of group assignments, assessed the level of pain. Within 24 hours after operation, the injection site was checked for pain, edema, wheal, and flare response by an anaesthesiologist who was unaware which drug was administered." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Agarwal 2004b.
| Methods | Prospective, double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 33.6 ± 14.8, Group II = 34.3 ± 16.2, Group III = 35.5 ± 12.6 Gender (M:F): Group I 15:16, Group II 13:18, Group III 16:15 Inclusion criteria: 18 years to 50 years, ASA I‐II, elective laparoscopic surgery Exclusion criteria: patients having difficulty in communication, anticipated difficulty in intubation or those who could not be intubated at the first attempt. Recruitment: 93 adults randomly assigned (31 in each group) Setting: India |
|
| Interventions |
Pretreatment alone Group 1 (NS): normal saline Group 2 (L): 2% lidocaine (40 mg) Group 3 (E): ephedrine 30 mcg/kg |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "An independent anaesthetist prepared the pretreatment solutions and the investigators were blinded to the contents." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An independent anaesthetist who was unaware of the group to which the patient had been allocated assessed the level of pain." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | This paper seems to be free of other bias |
Agarwal 2004c.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 33.6 ± 15.4, Group II = 31.8 ± 14.8, Group III = 34.5 ± 16.2 Gender (M:F): Group I = 46:54, Group II = 49:51, Group III = 44:56 Inclusion criteria: age 18 years to 50 years, ASA I‐II, undergoing elective surgery Exclusion criteria: patients with neuromuscular disease, difficulty in communication, cardiac rhythm other than sinus rhythm, a history of angina or myocardial infarction and endocrine or metabolic disease Recruitment: 300 adults randomly assigned into three groups (100 in each group) Setting: India |
|
| Interventions |
Pretreatment with venous occlusion Group I received magnesium sulphate 1 g Group II received lidocaine 2% (40 mg) Group III received normal saline all in a volume of 2 ml and accompanied by venous occlusion for one minute. Induction with propofol 2.5 mg/kg was accomplished following the release of venous occlusion. |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned into three groups of 100 each using a table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "An anaesthesiologist not involved in the study prepared pretreatment solutions and the investigator was blinded to study drugs." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An anaesthesiologist not involved in the study prepared pretreatment solutions and the investigator was blinded to study drugs." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Agarwal 2004d.
| Methods | A double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 36.5 ± 12.6, Group II = 31.8 ± 14.8, Group III = 33.6 ± 15.4 Gender (M:F): Group I = 25:25, Group II = 22:28, Group III = 24:26 Inclusion criteria: age 18 years to 50 years, ASA I–II adults,elective surgery Exclusion criteria: patients having difficulty in communication or with history of allergy to study drugs Recruitment: 150 adults randomly assigned into three groups (50 in each group) Setting: India |
|
| Interventions |
Pretreatment alone Group I (NS) received normal saline Group II (L) received lidocaine 2% (40 mg) Group III (B) received butorphanol 2 mg All patients received pretreatment solutions made in 2 ml with normal saline administered over 5 sec. One min after pretreatment patients received one‐fourth of the total calculated dose of propofol (2.5 mg/kg) over 5 sec. Assessment of pain with IV propofol was done using a 4‐point scale. |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "An independent anaesthesiologist prepared pretreatment solutions and the investigator did not know the contents of solutions." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An independent anaesthesiologist prepared pretreatment solutions and the investigator did not know the contents of solutions." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Ahmad 2013.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): N/A Gender: 100% female Inclusion criteria: aged older than 18 years, ASA I & II, outpatient gynaecologic surgery Exclusion criteria: patients had a hypersensitivity to propofol or soy bean oil, glycerol, egg lecithin, or sodium oleate, if they had small calibre veins on the dorsum of the hands, if they required intravenous drug administration prior to induction of anaesthesia, or if they required a rapid sequence induction of anaesthesia. Pregnant or lactating patients and those with a history of chronic pain, with neurologic, psychiatric, significant cardiac, renal, or liver disease, or taking sedatives or analgesics preoperatively Recruitment: 122 adults randomly assigned (114 were analysed) Setting: USA |
|
| Interventions |
Pretreatment with venous occlusion 114 female subjects received (n = 37) 5 ml of preservative‐free saline, (n = 43) 0.5 mg/kg of 1% lidocaine hydrochloride or (n = 34) 0.25 mg/kg of dexamethasone pretreatment, intravenously, following exsanguination and occlusion of the veins of the arm. This was followed by a 0.5 mg/kg−1 injection of propofol. Pain scores, facial grimacing, arm withdrawal, and vocalization were recorded prior to and at 15 and 30 seconds following the injection of propofol |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Subjects were randomly assigned (computer‐generated table). |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The study medication was prepared by a single investigator (Paul C. Fitzgerald), and the investigator who administered the study drugs was blind to the study group." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The study medication was prepared by a single investigator (Paul C. Fitzgerald), and the investigator who administered the study drugs was blind to the study group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eight subjects (< 15%) were excluded from the study prior to randomization: one due to a changed anaesthetic plan four due to cancellation of the procedure two due to pain prior to the study drug administration at the intravenous site one because a non‐study drug was administered |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Akgun 2013.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group E = 39.1 ± 13.7, Group L = 41.8 ± 11.8, Group S = 40.2 ± 2.8 Gender (M:F): Group E = 11:19, Group L = 12:18, Group S = 12:18 Inclusion criteria: aged 18 years to 60 years, ASA I or II, elective surgical procedures, lasting 1 to 3 hours, Exclusion criteria: obesity (body mass index > 30 kg/m2), pregnancy, risk of aspiration of gastric contents, suspected or known difficult airway, presence of severe neurologic deficits or psychiatric disorders, use of medications likely to affect central nervous system, use of NSAIDs and opioids, significant cardiac and liver dysfunction, hypersensitivity to study drugs. Recruitment: 90 adult patients randomly assigned (30 in each group) Setting: Turkey |
|
| Interventions |
Pretreatment with venous occlusion A 20 G cannula was inserted into the dorsum of the nondependent hand. After venous occlusion for one minute, Groups E, L and S were pretreated with 5 mg/ml (total 2 ml) esmolol, 40 mg lidocaine and 2 ml saline IV respectively. After release of venous occlusion, one fourth of the total propofol dose was administered at a rate of 0.5 ml/sec |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All pretreatment drugs were prepared in 2 ml and coded by an anaesthesiologist who was not involved directly in the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A blinded anaesthesiologist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Aldrete 2010.
| Methods | A comparative, double‐blind randomized controlled trial | |
| Participants | Age: N/A Gender: N/A Inclusion criteria: N/A Exclusion criteria: N/A Recruitment: 22 adult patients undergoing pain relief procedures Setting: USA |
|
| Interventions |
Admixture Propofol 1.7 mg/kg from Baxter Laboratories, premixed with either 5 ml of 2% lidocaine or 5 ml of NaCl 0.9%, was compared with propofol Laboratorios Dr Gray, which was similarly mixed. Baxter premixed with 2% lidocaine 5 ml Baxter premixed with NSS 5 ml Gray premixed with 2% lidocaine 5 ml Gray premixed with NSS 5 ml 16 injections were randomly administered four times each, blindly, to each of 22 patients (total 352 injections). |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = None 1 = Verbal complaint 2 = Moved arm 3 = Moved body (Moved arms or body was considered as moderate to severe pain) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The patients and the physician (J. A. A.) were blinded as to what preparation of propofol was to be administered at each treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Evaluation of the pain response and related events were recorded by a trained observer (F. H. M.)." (but N/A about blinding) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | N/A but assuming from the table all patients were included |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | High risk | Jose Alcover is the chief pharmacist at ‘Laboratories Dr Gray’. The Propofol used for this study was donated by ‘Laboratories Dr Gray’ to the senior author (J. A. A.) |
Aouad 2007.
| Methods | Prospective, double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 37.9 ± 12.4, Group II = 38.8 ± 15.1, Group III = 36.7 ± 13.7 Gender (M:F): Group I = 24:30, Group II = 18:32, Group III = 24:28 Inclusion criteria: ASA I‐III, elective surgery Exclusion criteria: indication for rapid sequence induction of anaesthesia, known allergy to any of the study drugs, ASA physical status IV, haemodynamic instability, psychiatric disorders, severe neurological deficits, and patients receiving opioids as long‐term treatment Recruitment: 156 adult patients were randomly assigned. Setting: Lebanon |
|
| Interventions |
Admixture Participants in the lidocaine group (Group I) (n = 54) received 2% lidocaine premixed with propofol (40 mg lidocaine in 180 mg propofol). Participants in the remifentanil group (Group II) (n = 50), received pretreatment with remifentanil 2 mcg/kg IV over 30 sec. Participants in the combination group (Group III) (n= 52) received both lidocaine premixed with propofol and pretreatment with remifentanil |
|
| Outcomes | Pain intensity assessed on 4‐point scale 1 = no pain 2 = mild pain, if only report after questioning patient 3 = moderate pain, if spontaneous verbal expression of pain without grimacing or withdrawal of arm occurred 4 = severe pain, if spontaneous strong vocal response with facial grimacing or withdrawal of arm occurred during the injection of propofol Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomly assigned according to a computer‐generated random table to one of three groups." |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All the syringes used were prepared by the resident and their identity was concealed." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The incidence and severity of pain were assessed during the injection of the study dose of propofol by an anaesthesiologist who was blinded to the group to which the patient was assigned." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Apiliogullari 2007.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 36.16 ± 10.7, Group II = 35.36 ± 9.6, Group III = 33.46 ± 10.7 Gender (M:F): Group I = 11:49, Group II = 21:39, Group III = 20:40 Inclusion criteria: ASA I or II, elective surgery Exclusion criteria: patients with difficulties in communication or with a history of allergy to diphenhydramine or amide group drugs, diabetes mellitus or cardiac problems and patients who received analgesics or sedative drugs within the 24 hours before surgery Recuitment: 180 adult patients randomly assigned (60 in each group) Setting: Turkey |
|
| Interventions |
Pretreatment with venous occlusion Group I (placebo) received normal saline 2 ml, Group II received lidocaine 2 ml (40 mg) (Aritmals 2%; Biosel, Istanbul) and Group III received diphenhydramine hydrochloride 2 ml (20 mg) (Benisons; Biosel, Istanbul) A 1 min venous occlusion, followed by propofol into a cephalic forearm vein of the antecubital fossa |
|
| Outcomes | Pain intensity assessed on 3‐point scale 0 = no pain 1 = mild pain 2 = severe pain (strong vocal response accompanied by facial grimacing, arm withdrawal or tears) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identical syringes containing study drugs were prepared and labelled by a pharmacist not involved in this study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An independent anaesthetist, who was unaware of group assignments, assessed the intensity of the pain the patients experienced." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | N/A but assuming from the table all patients were included |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Asik 2003.
| Methods | Randomized controlled trial | |
| Participants | Age: N/A Gender: N/A Inclusion criteria: ASA I‐II, elective surgery Exclusion criteria: N/A Recruitment: 90 adult patients randomly assigned (30 in each group) Setting: Turkey |
|
| Interventions |
Pretreatment with venous occlusion Patients were premedicated one hour before surgery with atropine 0.5 mg and meperidine 50 mg intramuscularly. Ninety patients scheduled for elective surgery under general anaesthesia were randomly allocated to one of three groups to receive pretreatment with venous occlusion, either metoprolol 2 mg, lidocaine 20 mg or saline 2 ml before any propofol was injected. Each patient was given one of these agents intravenously via a 20‐G cannula on the dorsum of the hand whilst the venous drainage was occluded manually, at the middle of the forearm, for 45 sec. After the occlusion was released, propofol 2.0 mg/kg to 2.5 mg/kg, at room temperature, was injected at 2 ml (20 mg) every 4 sec. |
|
| Outcomes | Pain intensity assessed on 3‐point scale 0 = no pain 1 = mild pain 2 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Pain was assessed verbally and scored as none (0), mild (1) or severe (2). Every 4 sec during the injection of propofol, an independent investigator – blinded to the pretreatment solution used – asked the patients if they had any discomfort or pain in their arm." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | This study was conducted entirely from departmental resources. The study appears to be free of other sources of bias. |
Ayoglu 2007.
| Methods | Prospective double‐blind randomized placebo‐controlled study | |
| Participants | Age (years, mean ± SD): Group 1 = 40.0 ± 13.2, Group 2 = 42.1 ± 12.4, Group 3 = 42.9 ± 13.5, Group 4 = 37.1 ± 11.9, Group 5 = 43.2 ± 12.9 Gender (M:F): Group 1 = 18:12, Group 2 = 10:20, Group 3 = 13:17, Group 4 = 12:18, Group 5 = 16:14 Inclusion criteria:18 years to 65‐years old, ASA I–II, scheduled to undergo minor elective surgery Exclusion criteria: the presence of neurological or psychiatric diseases, difficulty with communication, history of renal or hepatic insufficiency and hypersensitivity to the study drugs Recruitment: 150 adult patients randomly assigned (30 in each group) Setting: Turkey |
|
| Interventions |
Pretreatment with venous occlusion All participants had a 20‐G intravenous cannula inserted into a vein on the dorsum of the hand for administration of study drugs. Another cannula was placed in the other hand for infusion of. IV fluids. Following the elevation of the arm for 15 sec, a tourniquet was applied on the forearm up to 70 mmHg. Then, all participants were assigned to receive one of the following: Group 1, n = 30 saline (3 ml) Group 2, n = 30 dexmedetomidine 0.25 mg/kg Group 3, n = 30 lidocaine 0.5 mg/kg Group 4, n = 30 dexmedetomidine 0.25 mg/kg plus lidocaine 0.25 mg/kg Group 5, n = 30 dexmedetomidine 0.25 mg/kg plus lidocaine 0.5 mg/kg All study drugs were diluted into 3 ml of saline and injected at a rate of 0.5 ml/s. The tourniquet was released after 1 min and 5 ml of propofol was injected over 20 sec. Pretreatment with venous occlusion The participants were observed and asked immediately if they had pain in the arm, and their responses were assessed |
|
| Outcomes | Pain intensity was assessed on an 11‐point verbal rating scale (VRS). Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported. This study was not included in meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All pretreatment drugs were prepared in 3 ml saline in a 5‐ml syringe that was covered by red tape, and the investigator did not know the contents of the solutions." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An independent anaesthesiologist prepared the pretreatment solutions, and the investigator did not know the contents of the solutions." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Azma 2004.
| Methods | Prospective single‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): N/A Gender: N/A Inclusion criteria: aged 20 years to 65 years, elective surgery Exclusion criteria: patients with asthma, liver or renal dysfunction and cardiac diseases Recruitment: 180 adult patients randomly assigned (137 were analysed) Setting: Japan |
|
| Interventions |
Pretreatment alone Participants were allocated into six groups: saline (n = 7) thiopental 25 mg (n = 25) thiopental 50 mg (n = 28) thiopental 75 mg (n = 25) thiopental 100 mg (n = 23) lidocaine 40 mg (n = 29) pretreated 30 sec prior to propofol 1 mg/kg injection |
|
| Outcomes | Pain intensity assessed on 6‐point scale 1 = patient was asleep before the interview 2 = no complaint of pain according to the interview 3 = complaint of pain according to the interview 4 = complaint of pain spontaneous before the interview 5 = complaint of pain with an agonized face 6 = complaint of pain with an agonized movement of the injected arm Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly sorted case‐cards |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 43 of 180 patients (more than 15%) were excluded but described only "20 excluded due to difficulty in cephalic catheterization, 7 were excluded due to incidence of pain and severity beyond the expectation of investigator", but other exclusions were not described. Also, most of excluded participants were in control group |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Bachmann‐Mennenga 2007.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, median (range)): Group I = 52 (18 to 82), Group II = 57 (21 to 83), Group III = 52 (19 to 89), Group IV = 52 (19 to 89) Gender (M:F): Group I = 56:56, Group II = 56:55, Group III = 58:54, Group IV = 57:53 Inclusion criteria: age 18 years to 89 years old, ASA I‐II, elective surgery under regional anaesthesia Exclusion criteria: emergency, pregnancy, renal, hepatic, cardiac disease, ASA Grade III, age under 18 years, alcohol or drug abuse, chronic pain, cancer patients with previous radiation therapy, neuroleptic, antidepressant or pain medication and insufficient command of the German language. Patients where no venous access could be obtained Recruitment: 464 adult patients randomly assigned, 116 in each group (445 patients were analysed) Setting: Germany |
|
| Interventions |
Lidocaine admixture All patients received oral midazolam (7.5 mg) as premedication All patients were assigned to receive one of the following four options: Group 1 (MCT/LCT + Li) (n = 112) received propofol MCT/LCT premixed with 1% lidocaine at a ratio of 20:1 Group 2 (LCT + Li) (n = 111) received propofol LCT premixed with 1% lidocaine at a ratio of 20:1 Group 3 (MCT/LCT) (n = 112) received propofol MCT/LCT Group 4 (LCT) (n = 110) received propofol LCT |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients and the attending anaesthesiologist remained blind to the randomization." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The medication was prepared immediately before injection by a study nurse who did not participate in the outcome assessment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "19 (4%) patients were excluded from the data analysis due to various reasons, including withdrawal of informed consent before starting anaesthesia (n= 10), failure of the regional anaesthetic technique (n = 3), change of the operative procedure (n = 3), or failure to get venous access on the dorsum of the hand (n = 3)." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | High risk | This study was supported by a grant from B. Braun Melsungen, Germany and in part by departmental funds |
Barker 1991.
| Methods | Single‐blind, randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group 1 = 41.4 ± 2.6, Group 2 = 47.7 ± 2.7, Group 3 = 46.6 ± 3.3, Group 4 = 41.9 ± 2.95 Gender (M:F): Group 1 = 14:14, Group 2 = 14:13, Group 3 = 10:17, Group 4 = 12:15 Inclusion criteria: age 19 years to 80 years old, ASA I‐II, elective surgery Exclusion criteria: N/A Recruitment: 109 adult patients randomly assigned Setting: England |
|
| Interventions |
Admixture Patients were allocated randomly to receive: Group 1 (n = 28): unmodified propofol Group 2 (n = 27): propofol premixed with lidocaine to concentration 0.05% (= lidocaine 10 mg in propofol 20 ml) Group 3 (n = 27): propofol at 4°C Group 4 (n = 27): unmodified propofol preceded by 10 ml of 0.9% saline at 4°C |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no discomfort 1 = uncomfortable 2 = painful 3 = very painful Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Batra 2005.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): N/A Gender: N/A Inclusion criteria: age 20 years to 60 years old, ASA I‐II, elective surgery Exclusion criteria: patients taking regular analgesics or sedative or suffering from acute and chronic pain syndromes or any neurological diseases, thrombophlebitis, known allergy to local anaesthetics, propofol or ketamine Recruitment: 150 adult patients randomly assigned (50 in each group) Setting: Kuwait |
|
| Interventions |
Pretreatment with venous occlusion Group saline (n = 50) received physiological saline 2 ml with venous occlusion for 1 min Group lidocaine (n = 50) received lidocaine 20 mg in 2mL saline with venous occlusion for 1 min Group ketamine (n = 50) received ketamine 10 mg in 2mL saline with venous occlusion for 1 min Venous occlusion was performed using a rubber tourniquet placed on the upper arm after elevating the arm for 30 sec for gravity drainage of venous blood. Sixty seconds after the pretreatment bolus, the occlusion was released and propofol 2.5mg/kg was administered through the same 20‐G catheter at the rate of 1mL/sec. Fifteen seconds after injection of 25% of the dose of propofol, patients were asked to grade their pain. After assessment of the pain intensity, the rest of the dose of propofol was given and anaesthesia was continued as planned |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = none 1 = mild; complaint of pain only when asked for 2 = moderate; spontaneous complaint of pain 3 = severe; spontaneous complaint of pain associated with grimacing or withdrawal of hand during injection Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned using a sealed envelope technique to one of three groups." |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients were asked by an independent, second anaesthesiologist to grade their pain." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | There is no patient’s characteristic information. |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Borazan 2010.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group P0.5 = 45.18 ± 12.44, Group P1 = 45.24 ± 14.36, Group P2 = 43.54 ± 15.01, Group L = 41.28 ± 14.12, Group C = 44.06 ± 13.62 Gender (M:F): Group P0.5 = 26:24, Group P1 = 33:17, Group P2 = 31:19, Group L = 36:14, Group C = 26:24 Inclusion criteria: age 20 years to 60 years old, ASA I‐II, elective surgery Exclusion criteria: patients who experienced difficulty in communication, those with body weight exceeding 75 kg, those who had cardiac, renal and hepatic failure, those who were taking antianxiety drugs for psychiatric or neurological disorders and those who had a known allergy to the study drugs Recruitment: 250 adult patients randomly assigned into five groups (50 in each group) Setting: Turkey |
|
| Interventions |
Pretreatment with venous occlusion Group P0.5, group P1 and group P2 received 0.5, 1 and 2 mg/kg paracetamol respectively Group L: received 0.5 mg/kg lidocaine Group C: received isotonic saline pretreatment in the dorsum of the hand A rubber tourniquet was placed on the forearm for 1 min to produce a venous occlusion; the patients were pretreated over a period of 15 seconds followed by propofol 1 min later |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐conducted randomization in which the code was sealed until the arrival of the patient in the operating room." |
| Allocation concealment (selection bias) | Low risk | "A computer‐conducted randomization in which the code was sealed until the arrival of the patient in the operating room." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The drugs were prepared by one of the investigators, with both the patient and an independent observer (a trainee anaesthesiologist)." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The drugs were prepared by one of the investigators, with both the patient and an independent observer (a trainee anaesthesiologist)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Burimsittichai 2006.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 46.8 ± 13.2, Group II = 47.1 ± 14.9, Group III = 47.3 ± 13.5, Group IV = 48.2 ± 15.3 Gender (M:F): Group I = 32:58, Group II = 23:67, Group III = 29:61, Group IV = 37:53 Inclusion criteria: ASA I‐III, elective surgery Exclusion criteria: patients with history of hypersensitivity to propofol or any of the constituents of the emulsion, patients with haemodynamic instability, ASA IV, pregnancy Recruitment: 360 adult patients randomly assigned (90 in each group) Setting: Thailand |
|
| Interventions |
Pretreatment with venous occlusion All patients were randomly allocated into 4 groups: Group I (L+LCT) propofol LCT 2 mg/kg after pretreatment of 1% lidocaine 2 ml IV Group II (L + MCT/LCT) propofol MCT/LCT 2 mg/kg after pretreatment with 1% lidocaine 2 ml IV Group III (P + MCT/LCT) propofol MCT/LCT 2 mg/kg after 0.9% NaCl 2 ml IV, Group IV (P + mixed L + LCT) propofol LCT 2 mg/kg premixed with lidocaine 1% after 0.9% NaCl 2 ml IV All groups received pretreatment under venous occlusion for 60 sec |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generation |
| Allocation concealment (selection bias) | Low risk | Randomized opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded investigator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Canbay 2008.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean (range)): Group I = 35.02 (22 to 60), Group II = 30.7 (20 to 54), Group III = 39.3 (20 to 60) Gender (M:F): Group I = 20:30, Group II = 30:20, Group III = 26:24 Inclusion criteria: age 20 years to 60 years old, ASA I or II, undergoing general anaesthesia Exclusion criteria: patients with vascular diseases, habituation to analgesics, sedatives or anti‐anxiety drugs; allergic diseases or sensitivity to lidocaine, propofol or acetaminophen, and infection on the dorsum of their left hands Recruitment: 150 adult patients randomly assigned (50 in each group) Setting: Turkey |
|
| Interventions |
Pretreatment with venous occlusion A 20‐gauge catheter was inserted into a superficial radial vein of the left hand, and after the occlusion of venous drainage, Groups I, II, and III were pretreated with 40 mg of lidocaine in saline, 50 mg of IV acetaminophen, and 5 ml of saline, respectively. The occlusion was released after two minutes and one‐fourth of the total propofol dose was injected into the vein over a period of 5 sec No other analgesics or sedatives were administered before propofol injection. |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain (negative response to questioning) 1 = mild pain (pain reported only in response to questioning with no behavioural signs) 2 = moderate pain (pain reported in response to questioning and accompanied by a behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "An independent anaesthetist prepared the solutions and the investigator was blind to the contents of the solutions." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An independent anaesthetist prepared the solutions and the investigator was blind to the contents of the solutions." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Cheong 2002.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group P = 38.2 ± 14.0, Group L = 41.5 ± 12.4, Group E30 = 35.7 ± 14.1, Group E70 = 37.1 ± 16.1, Group E110 = 33.7 ± 8.7, Group E150 = 39.8 ± 10.4 Gender (M:F): Group P = 9:21, Group L = 9:21, Group E30 = 11:17, Group E70 = 10:20, Group E110 = 10:20, Group E150 = 11:17 Inclusion criteria: age 19 years to 59 years old, ASA I‐II, elective surgery Exclusion criteria: patients taking sedatives or analgesics and those with allergic, neurologic, or cardiovascular disease Recruitment: 176 adult patients randomly assigned (28 in group E30 and E150, while 30 in each other remaining groups) Setting: Korea |
|
| Interventions |
Pretreatment alone Patients were randomly allocated into six study groups to compare the incidence of propofol‐induced pain after pretreatment with different doses of ephedrine as compared with lidocaine Participants in Group P (n = 30) received saline placebo Participants in Group L (n = 30) received 2% lidocaine 40 mg Participants received ephedrine 30 mcg/kg (Group E30, n = 28) 70 mcg/kg (Group E70, n = 30) 110 mcg/kg (Group E110, n = 30) 150 mcg/kg (Group E150, n = 28) followed 30 sec later by propofol 2.5 mg/kg |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain associated with grimacing, withdrawal movement of forearm, or both Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was based on computer‐generated codes that were maintained in sequentially numbered opaque envelopes." |
| Allocation concealment (selection bias) | Low risk | "Randomization was based on computer‐generated codes that were maintained in sequentially numbered opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All syringes of test solution were prepared by another investigator and covered so that the investigator who assessed the patient response was unaware of the nature of the solution." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Also, a blinded anaesthesiologist asked the patient to evaluate the pain score." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
DeSousa 2011.
| Methods | Single‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group SLT = 31.9 ± 10.9, Group SL = 32 ± 8, Group LT = 30.4 ± 10, Group L = 29.7 ± 12.7, Group S = 32.7 ± 12.1 Gender (M:F): Group SLT = 3:17, Group SL = 6:14, Group LT = 8:12, Group L = 3:17, Group S = 4:16 Inclusion criteria: age 13 years to 65 years old, ASA I‐II, elective bariatric, general, orthopedic, urological, gynaecological, or ENT surgery Exclusion criteria: N/A Recruitment: 100 adult patients randomly assigned into five equal groups (20 in each group) Setting: Kuwait (Singapore, India, Egypt) |
|
| Interventions |
Pretreatment alone P retreatment with venous occlusion 100 patients were randomly allocated equally into five groups: sevoflurane–lidocaine–tourniquet (SLT) sevoflurane–lidocaine (SL) lidocaine–tourniquet (LT) lidocaine (L) sevoflurane (S) Approximately 10 min before the induction of anaesthesia, midazolam 1 mg to 2 mg was administered intravenously to all patients. All patients received fentanyl 1 µg/kg as pretreatment and a full induction dose of propofol. A blinded anaesthesia nurse assessed pain and hand movements throughout the injection of propofol |
|
| Outcomes | Pain intensity assessed on 3‐point scale 0 = no pain: no complaints, no grimacing, and denial on direct questioning 1 = mild pain pain: minimal grimacing or complaint on direct questioning, no self‐reporting 2 = moderate pain: severe grimacing, shouting, or complaining, or self‐reporting Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Low risk | "The patients were randomly allocated, using sealed envelopes, to one of following five groups of 20 each." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The pain on injection of propofol was evaluated throughout the injection, by a blinded anaesthesia nurse." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Dubey 2003.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 38.6 ± 10.0, Group II = 36.5 ± 12.3, Group III = 35.5 ± 8.6 Gender (M:F): Group I = 18:32, Group II = 16:34, Group III = 19:31 Inclusion criteria: ASA I‐II, elective surgery (day‐care laparoscopic procedures using general anaesthesia) Exclusion criteria: known sensitivity to lidocaine, propofol or granisetron; concomitant analgesic or sedative medication; presence of infection on the dorsum of the left hand; indications for rapid sequence intubation; presence of cardiac conduction defects; epilepsy; and use of antiarrhythmic medications Recruitment: 150 adult patients randomly assigned (50 in each group) Setting: India |
|
| Interventions |
Pretreatment with venous occlusion 150 adult patients were randomly assigned to one of three groups: Group 1 (who received 5 ml of 0.9% saline pretreatment) Group 2 (who received 5 ml lidocaine [40 mg in 0.9% saline] pretreatment) Group 3 (who received 5 ml granisetron [2 mg in 0.9% saline] pretreatment) Injections were given in the largest vein on the dorsum of the hand. After two minutes, the tourniquet was released and one fourth of the total calculated dose of propofol (2.5 mg/kg body weight) was administered and pain assessment was made |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "By use of sealed envelopes, patients were allocated randomly to one of three groups." |
| Allocation concealment (selection bias) | Low risk | "By use of sealed envelopes, patients were allocated randomly to one of three groups." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "An independent anaesthetist prepared the solutions, and the investigator did not know the contents of the solutions." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An independent anaesthetist prepared the solutions, and the investigator did not know the contents of the solutions." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
El‐Radaideh 2007.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group P = 47.9 ± 13.8, Group L = 48.7 ± 13.7, Group LF = 46.0 ± 14.2, Group R = 44.5 ± 13.4 Gender (M:F): Group P = 23:27, Group L = 21:29, Group LF = 18:32, Group R = 24:26 Inclusion criteria: age 21 years to 73 years old, ASA I‐III, elective gynaecological, urological or general surgical procedures Exclusion criteria: refusal of consent, heart failure, renal failure and liver dysfunction. Patients taking sedatives, analgesics, central nervous system (CNS) depressants or anti‐seizure medication, or with a history of intolerance or adverse reactions to the medications used in the study Recruitment: 200 adult patients randomly assigned (50 in each group) Setting: Jordan |
|
| Interventions |
Pretreatment with venous occlusion A total of 200 patients (50 patients each group) were randomized by a sealed envelope system to be pretreated with either 4 ml lidocaine 1% (40 mg) (Group L) 2 ml lidocaine 2% (40 mg) mixed with 2 ml fentanyl (100 mcg) (Group LF) 4 ml IV paracetamol 40 mg (Group R) 4 ml isotonic sodium chloride solution as placebo (Group P) followed by propofol 2.5 mg/kg after 60 seconds of venous occlusion. |
|
| Outcomes | Pain intensity assessed on 2‐point scale 0 = no pain 1 = any pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Low risk | "A total of 200 patients (50 patients each group) were randomized by a sealed envelope system." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The anaesthesiologist, who was blind to the content of the study syringe, assessed the pain on injection associated with propofol." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Eriksson 1997.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean (range)): 43 (18 to 72) Gender (M:F): 18:26 Inclusion criteria: ASA I‐II, undergoing elective ENT surgery Exclusion criteria: N/A Recruitment: 44 adults Setting: Sweden |
|
| Interventions |
Admixture All patients were premedicated with ketobemidon 2.5 mg to 5 mg and atropine 0.5 mg or glycopyrronium 0.2 mg IM, 30 minutes before induction. A 20‐gauge IV cannula was inserted into dorsal vein of each hand and 1% propofol (Diprivan) 10 ml at room temperature was randomly premixed with 1 ml of one of these Group 1: 1% lidocaine (10 mg) (n= 25) Group 2: Sterile hydrochloric acid 0.064 mole/litre (n = 24) Group 3: Saline (n = 22) Patients were requested to name which of two propofol injections, one in each hand, at its maximum caused most discomfort and in addition to grade the pain. All mixtures were prepared immediately before injection. |
|
| Outcomes | Pain intensity assessed on 11‐point scale 0 = no pain 1 = hardly recognizable 10 = extreme pain Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported. This study was not included in meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "all injections were made in a double‐blind manner." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Total injections in this study were 88 injections (44 participants were injected both hands) but the study reported only 71 injections. No missing data were reported. |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Gajraj 1996.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group A = 35 ± 10, Group B = 41 ± 12.9, Group C = 37.6 ± 12.3, Group D = 38.5 ± 9.7, Group E = 35.2 ± 11.9 Gender : 100% female Inclusion criteria: ASA I and II, minor outpatient surgery Exclusion criteria: N/A Recruitment: 135 adult patients randomly assigned (27 in each group) Setting: United Kingdom |
|
| Interventions |
Admixture Patients were randomly allocated to one of five groups: Group A (control), no lidocaine Group B, lidocaine 10 mg Group C, lidocaine 20 mg Group D, lidocaine 30 mg Group E, lidocaine 40 mg |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ganta 1992.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean): Group 1 = 47, Group 2 = 51, Group 3 = 48 Gender (M:F): Group 1 = 44:41, Group 2 = 43:42, Group 3 = 46:39 Inclusion criteria: Age 16 years to 70 years old, ASA I‐II, elective surgery Exclusion criteria: patients with a history of Parkinsonism or those who had poor veins Recruitment: 255 adult patients randomly assigned Setting: USA |
|
| Interventions |
Pretreatment alone Compare the efficacy of pretreated lidocaine and metoclopramide in minimizing the pain of injection of IV propofol. When administered immediately before propofol into a dorsal hand vein compared with placebo. Group 1 (n = 85) normal saline 1 ml Group 2 (n = 85) 1% lidocaine 1 ml (10 mg) Group 3 (n = 85) metoclopramide 5 mg Premedication with diazepam 10 mg |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Ampoules were prepared and coded by hospital pharmacy." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Gehan 1991.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean): N/A Gender (M:F): Group A = 40:37, Group B = 44:42, Group C = 37:34, Group D = 41:35 Inclusion criteria: Age 18 years to 80 years old, ASA I‐II, diagnostic and minor elective surgery Exclusion criteria: N/A Recruitment: 310 adult patients were randomly assigned Setting: France |
|
| Interventions |
Admixture Patients were allocated to four groups according to the lidocaine dosage: group A (control), no lidocaine (n = 77) group B, lidocaine 0.1 mg/kg (n = 86) group C, lidocaine 0.2 mg/kg (n = 71) group D, lidocaine 0.4 mg/kg (n = 76) Admixture with propofol 2.5 mg/kg |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (patient complained of pain when asked after injection 50% of total dose of propofol) 2 = moderate pain (spontaneous complaint by patient before injection of 50% of total dose of propofol) 3 = severe pain (reported pain was associated with grimacing, withdrawal movement of the forearm, or both) Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Three different members of the anaesthesia team took responsibility for anaesthesia, preparation of the mixture, and recording of pain on injection." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Three different members of the anaesthesia team took responsibility for anaesthesia, preparation of the mixture, and recording of pain on injection." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Han 2010.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group 1 = 47 ± 14.5, Group 2 = 51 ± 13.5, Group 3 = 50 ± 14.4 Gender (M:F): Group 1 = 20:20, Group 2 = 20:20, Group 3 = 19:21 Inclusion criteria: age 20 years to 65 years old, ASA I‐II, elective surgery Exclusion criteria: patients with self‐confirming allergies to opioids, local anaesthetics, asthma, neurological deficits and those who had received analgesics or sedatives within the previous 24 hours Recruitment: 120 adult patients randomly assigned (40 in each group) Setting: Korea |
|
| Interventions |
Admixture Patients were allocated randomly into one of three groups (n = 40, in each) The patients in the remifentanil group (Group 1) received remifentanil 0.5 mcg/kg IV pretreated for 30 seconds before a micro emulsion propofol injection The patients in the lidocaine group (Group 2) received propofol 2 mg/kg premixed with 40 mg lidocaine over a 60 second period. The patients in the combination group (Group 3) received both remifentanil and lidocaine |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (a minor verbal/facial response or motor reaction to the injection) 2 = moderate pain (a clear verbal/facial response or motor reaction to the injection) 3 = severe pain (the patient both complained of pain and withdrew their arm) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were allocated randomly to one of three groups using a computer generated randomization list manipulated by a statistician in a sealed envelope." |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The patients, anaesthesia providers and investigators who scored the movements were blinded to the treatment group." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The patients, anaesthesia providers and investigators who scored the movements were blinded to the treatment group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Harmon 2003.
| Methods | Observer‐blind randomized controlled trial | |
| Participants | Age (years, mean (range)): Group C = 40 (16 to 77), Group n = 39 (19 to 65), Group L = 45 (17 to 79) Gender (M:F): Group C = 18:27, Group n = 13:32, Group L = 16:29 Inclusion criteria: age 16 years to 79 years old, ASA I–II, elective surgery Exclusion criteria: patients in ASA III–V and those who suffered from cardiac conduction defects, epilepsy, those taking antidysrrhythmic drugs or receiving analgesic drugs in the previous 24 hours Recruitment: 140 were recruited, but 135 adult patients were randomly assigned (45 patients in each group) Setting: Ireland |
|
| Interventions |
Admixture C = preoxygenated with 100% oxygen (120 sec) N = preoxygenated with 50% nitrous oxide in oxygen (120 sec) Both C and N groups, anaesthesia was induced with propofol with no added lidocaine L = preoxygenated with 100% oxygen and anaesthesia was induced with 1% propofol 18 ml premixed with 1% lidocaine 2 ml (20 mg) (lidocaine concentration of 1 mg/ml) The speed of injection was carefully controlled by hand Propofol was injected at 1 ml/sec, the injection was stopped at 5 sec and then continued after pain scores were assessed at 10 sec On initial injection (after 5 sec), the degree of pain experienced by the patient was scored by observation of any verbal response and behavioural signs such as facial grimacing or arm withdrawal. At 10 sec, if there was no observed pain response, the patient was asked a standard question about comfort at the injection site |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain reported in response to questioning only) 2 = moderate pain (pain reported in response to questioning and accompanied by behavioural signs, or pain reported spontaneously) 3 = severe pain (strong verbal response or a response accompanied by behavioural signs) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was conducted by a computer with the code sealed until arrival of the patient in operating room." |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A member of the anaesthesia team took responsibility for anaesthesia was unblinded." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The investigator recording the pain scores was blinded to the drugs given and the gas mixture administered to the patients (flowmeters were covered by cardboard)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Five were excluded before randomization due to difficulty with venous cannulation." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Haugen 1995.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean (range)): Group I = 26.5 (16 to 54), Group II = 25.5 (15 to 49), Group III = 25.5 (17 to 48) Gender: 100% female Inclusion criteria: Age 15 years to 34 years old, gynaecologic surgery Exclusion criteria: N/A Recruitment: 90 adult patients randomly assigned (30 in each group) Setting: Canada |
|
| Interventions |
Pretreatment alone Ninety women were allocated to receive one of three treatments prior to induction of anaesthesia with propofol Patients in Group C: received 2 ml normal saline Group L: 2 ml lidocaine 2% (40 mg) pretreatment Group T: 2 ml thiopentone 2.5% (50 mg) pretreatment venous discomfort was assessed 5 sec to 15 sec after commencing propofol administration using an infusion pump (rate 1000 μg/kg/min). |
|
| Outcomes | Pain intensity assessed on 11‐point scale The VAS ruler had a scale from 0 to 10 cm on one side and no markings on the patient side. Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patient recruitment and preparation of blinded treatment syringes were done by one investigator. The syringes were blinded with pink opaque tape." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients were taken to the operating room where an anaesthetist, blinded to the group assignment, applied monitors and familiarized the patient with use of a Visual Analog Scale (VAS) ruler." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Helbo‐Hansen 1988.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 35 ± 10, Group II = 36 ± 12 Gender: 100% female Inclusion criteria: age 18 years to 55 years old, ASA I‐II, dilatation and curettage of the uterus or termination of pregnancy Exclusion criteria: N/A Recruitment: 80 adult patients randomly assigned (40 in each group) Setting: Denmark |
|
| Interventions |
Admixture 80 patients were randomly assigned to two groups of 40 patients: In the study group, 10 mg of lidocaine hydrochloride and 7 mg of sodium chloride in 1 ml of water were mixed with 19 ml of propofol emulsion less than 3 min before induction of anaesthesia (Group I). In the control group, 1 ml of isotonic saline was mixed with 19 ml of propofol (Group II). |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = slight pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study was supported by grants from Direktor E. Danielsen og Hustrus Fond. The study appears to be free of other sources of bias |
Ho 1999.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group A = 41.07 ± 9.11, Group B = 43.82 ± 11.42, Group C = 42.80 ± 9.65, Group D = 41.58 ± 9.56 Gender: 100% female Inclusion criteria: Age 21 years to 65 years old, ASA I‐II, dilatation and curettage surgery Exclusion criteria: any patient with a history of epilepsy, or allergy to either egg protein, soya bean emulsion, or lidocaine, patients who had received analgesics 24 hours prior to anaesthesia or had taken antiarrhythmic drugs Recruitment: 240 adult patients randomly assigned (60 in each group) Setting: Taiwan |
|
| Interventions |
Admixture Patients, who were not premedicated, were randomly allocated to receive one of the four treatments (n = 60 per group): Group A (control), propofol 18 ml (10 mg /ml) mixed with normal saline (NS) 2 ml Group B (propofol containing 0.05% lidocaine), propofol 18 ml mixed with 0.5 ml 2% lidocaine in 1.5 ml NS Group C (propofol containing 0.1% lidocaine), propofol 18 ml mixed with 1 ml 2% lidocaine in 1 ml NS Group D (propofol containing 0.2% lidocaine), propofol 18 ml mixed with 2 ml 2% lidocaine |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (soreness or slight pain when asked) 2 = moderate pain (subjective complaint of a tolerable painful sensation) 3 = severe pain (a pain rendering the patient to flex her arm to deny injection) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patient randomization was performed by computer, with each code sealed in an envelope to be opened on the patient’s arrival in the operating room." |
| Allocation concealment (selection bias) | Low risk | "Patient randomization was performed by computer, with each code sealed in an envelope to be opened on the patient’s arrival in the operating room." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The patient and the anaesthesiologist were unaware of the study solution used." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Pain on injection of the mixture was determined by a nurse‐anaesthetist, who was blinded as to the study groups." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | N/A but assuming from the table all patients were included. |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | Supported by Research Grant VGH‐88‐A217 from the Veterans General Hospital‐Taipei, Taiwan, R.O.C. The study appears to be free of other sources of bias |
Honarmand 2008.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group K = 24.6 ± 3.1, Group L = 25.5 ± 3.0, Group M = 25.5 ± 3.1, Group C = 24.3 ± 2.9 Gender (M:F): Group K = 32:18, Group L = 23:27, Group M = 28:22, Group C = 31:19 Inclusion criteria: Age 18 years to 86 years old, ASA status I—III, scheduled for elective operations in the departments of ophthalmology Exclusion criteria: patients with neuromuscular disease, difficulty in communication, patients with ischaemic heart disease, cardiac rhythm other than sinus rhythm, recent convulsions, a history of endocrine or metabolic disease, severe neurological deficits and psychiatric disorders, patients with physical status ASA IV—V and emergency admission, and those with thin dorsal hand veins, suspected or known pregnancy, lactating women, disorders of pancreas or liver (glutamate—pyruvate—transaminase or glutamate—oxalate—transaminase > 40 U /L; lipase > 30 U/L), renal problems, thrombophlebitis, or chronic pain for which they were taking sedative or analgesic medication, received an analgesic within 24 hours before surgery, and those with known hyper‐sensitivity to propofol or to any of the constituents of the emulsion and those patients requiring a rapid‐sequence induction Recruitment: 200 adult patients randomly assigned (50 in each group) Setting: Iran |
|
| Interventions |
Pretreatment alone Group M (n = 50) were given magnesium sulphate 2.48 mmol (2 ml) diluted in 5mL of saline IV Group K (n = 50) were given ketamine 10 mg in a total volume of 5mL with 0.9% saline. Group L (n = 50) were given 1% lidocaine (3 ml = 30 mg) diluted in 2 ml of saline IV Group C (n = 50) received 5mL of saline IV The pretreatment drug was prepared in an unlabelled syringe at room temperature (21°C to 23°C) and randomly handed for pretreatment to the anaesthesiologist who was unaware of the identity of the drug Propofol administration followed a standardized protocol with a dose of 2 mg/kg for patients aged 18 years to 69 years and 1.5 mg/kg for patients aged > 70 years. |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain in response to questioning only without any behavioural signs) 2 = moderate pain (pain in response to questioning and accompanied by behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to one of four groups by computer‐generated randomization." |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The pretreatment drug was prepared in an unlabelled syringe at room temperature (21°C to 23°C) and randomly handed for pretreatment to the anaesthesiologist who was unaware of the identity of the drug." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients were asked repeatedly, until loss of consciousness, whether they felt any pain during the administration of propofol by an independent, second anaesthetist who classified the patients’ response" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | N/A but assuming from the table all patients were included |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | This paper seems to be free of other sources of bias |
Hwang 2010.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group L = 44.8 ± 12.6, Group K = 46.8 ± 16.5, Group LK = 44.1 ± 11.8 Gender (M:F): Group L = 21:21, Group K = 22:19, Group LK = 21:18 Inclusion criteria: age 18 years to 70 years old, ASA I‐II, elective surgery Exclusion criteria: patients showed neurological disorders, a negative effect in communication, or hypersensitivity towards these drugs Recruitment: 130 adult patients randomly assigned (122 were analysed) Setting: Korea |
|
| Interventions |
Pretreatment with venous occlusion The patients were premedicated with IM midazolam 3 mg and glycopyrrolate 0.2 mg 30 minutes prior to surgery. The patients received intravenous lidocaine 40 mg (Group L, n = 42) ketamine 25 mg (Group K, n = 41) lidocaine 40 mg plus ketamine 25 mg (Group LK, n = 39) pretreatment with a rubber tourniquet on the forearm 1 min before the injection of micro emulsion propofol. The pain score was assessed at 10 seconds after injection of micro emulsion propofol 30 mg and during the injection of the remaining total dose |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain in response to questioning only without any behavioural signs) 2 = moderate pain (pain in response to questioning and accompanied by behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The nurse mixed all the pretreated drugs with normal saline so that they would be identically 3 ml." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded investigator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "4 patients in Group K and 4 patients in Group LK fell asleep before receiving the remaining total dose of micro emulsion propofol, making it impossible to assess their pain. They were excluded from the study, leaving 122 patients in the study." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Jeon 2012.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group 1 = 48 ± 14.5, Group 2 = 50 ± 13.5, Group 3 = 45 ± 14.5 Gender (M:F): Group 1 = 13:17, Group 2 = 14:16, Group 3 = 14:16 Inclusion criteria: Age 19 years to 60 years old, ASA I‐II, elective plastic surgery Exclusion criteria: patients with cardiovascular, hepatic, or renal problems; patients who had received analgesic or sedative medications within 24 hours before the surgery; patients with neurological deficits or psychiatric disorders; and patients requiring a rapid sequence induction Recruitment: 90 adult patients randomly assigned (30 in each group) Setting: Korea |
|
| Interventions |
Pretreatment with venous occlusion Patients were allocated randomly to three groups, to receive lidocaine 20 mg, Group 1 (n = 30), a combination of lidocaine 20 mg and nitroglycerin 0.1 lg/kg Group 2 (n = 30), normal saline as a placebo Group 3 (n = 30), with venous occlusion for 1 min followed by the administration of 25 % of the total calculated dose of propofol (2 mg/kg) into a dorsal hand vein |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain in response to questioning only without any behavioural signs) 2 = moderate pain (pain in response to questioning and accompanied by behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Low risk | a sealed envelope technique |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "An anaesthesiologist not involved in this study prepared identically coded syringes." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Blinded investigator for any complications such as pain, edema, or a wheal and flare response." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | This research was supported by Kyungpook National University Research Fund, 2010. The study appears to be free of other sources of bias |
Johnson 1990.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): N/A Gender (M:F): N/A Inclusion criteria: Elective surgery Exclusion criteria: ASA III‐V, cardiovascular disease, epilepsy, taking antiarrhythmic drugs Recruitment: 103 adult patients randomly assigned (22, 21, 20, 18, 22 in groups A‐E, respectively) Setting: England |
|
| Interventions |
Pretreatment with venous occlusion Admixture Patients were allocated randomly to one of five groups. Each patient received 2 ml of a pretreatment solution with venous occlusion followed by an induction mixture. Group A (n = 22) pretreatment with saline and propofol 200 mg + saline 2 ml Group B (n = 21) pretreatment with 1% lidocaine 2 ml (20 mg) and propofol 200 mg + saline 2 ml Group C (n = 20) pretreatment with 2% lidocaine 2 ml (40 mg) and propofol 200 mg + saline 2 ml Group D (n = 18) pretreatment with saline and propofol 200 mg + lidocaine 20 mg Group E (n = 22) pretreatment with saline and propofol 200 mg + lidocaine 40 mg |
|
| Outcomes | Pain intensity assessed on 3‐point scale 0 = neutral (no pain) 1 = discomfort 2 = pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were allocated randomly to one of five groups |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All drugs were prepared by anaesthetist but their contents were not known to the investigating anaesthetist." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Karasawa 2000.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group S = 51 ± 3, Group F = 58 ± 2, Group L = 49 ± 3 Gender (M:F): Group S = 28:22, Group F = 25:25, Group L = 27:23 Inclusion criteria: ASA I‐II, elective surgery Exclusion criteria: N/A Recruitment: 150 adult patients randomly assigned (50 in each group) Setting: Japan |
|
| Interventions |
Admixture Group S received 5 ml of NSS followed by propofol mixed with 0.4 ml of NSS Group F received 5 ml of LFP 50 mg followed by propofol mixed with 0.4 ml of NSS Group L received 5 ml of NSS followed by propofol premixed with 0.4 ml of 10% lidocaine (40 mg) |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (uncomfortable or a little painful) 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated into three groups." |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Pain score were asked by anaesthesiologist who was unaware of the group of patient." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kim 2010.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group A = 40.9 ± 13.0, Group B = 37.9 ± 14.7, Group C = 42.2 ± 13.4, Group D = 40.45 ± 14.7 Gender (M:F): Group A = 25:15, Group B = 23:17, Group C = 20:20, Group D = 25:15 Inclusion criteria: age 16 years to 65 years old, ASA I‐II, elective surgery Exclusion criteria: patients with allergies to any drugs or renal, hepatic, or cardiac problems, neurologic deficits or psychiatric disorder Recruitment: 160 adult patients randomly assigned (40 in each group) Setting: Korea |
|
| Interventions |
Admixture Patients were randomly allocated to four groups: admixture of Group A, saline Group B, 20 mg lidocaine Group C, 30 mg lidocaine Group D, 40 mg lidocaine None of the patients was premedicated before entering the operation room |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (tolerable soreness or slight pain) 2 = moderate pain (subjective complaint between mild and severe pain) 3 = severe pain (pain causing the patient to flex his/her arm to deny injection) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kim 2013a.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group C = 38.32 ± 15.73, Group L = 35.52 ± 15.57, Group N = 41.86 ± 15.67, Group LN = 47.26 ± 13.97 Gender (M:F): Group C = 25:25, Group L = 20:30, Group N = 29:21, Group LN = 20:30 Inclusion criteria: Age 18 years to 68 years old, ASA I‐II, undergoing elective surgery Exclusion criteria: if patients met any of the following criteria: the regular use of sedatives or analgesics; an allergy to lidocaine; a pre‐existing movement disorder; pre‐existing drug abuse; inability to co‐operate or give informed consent; any anticipated difficulty in obtaining an airway; thrombophlebitis (or any other pain‐causing lesion); the presence of chronic obstructive pulmonary disease (COPD); or any contraindication to the administration of N2O (e.g. pneumothorax Recruitment: 205 adult patients randomly assigned (200 were analysed) Setting: Korea |
|
| Interventions |
Pretreatment with venous occlusion A total of 205 adult patients received one of the following combinations: NaCl and 100% O2 (Group C; n = 50); 0.5 mg/kg pretreated lidocaine and 100 % O2 (Group L; n = 50); NaCl and a mixture of 67% N2O/O2 (Group N; n = 50); 0.5 mg/kg lidocaine and a mixture of 67% N2O/O2 (Group LN; n = 50). Vein occlusion was released after 1 min, and 5 ml propofol was injected over 10 sec. The remainder of the induction dose of propofol (with a 3 ml bolus of normal saline and 0.6 mg/kg rocuronium) was then injected. The response to the rocuronium injection was assessed with a 4‐point scale (0–3) |
|
| Outcomes | Pain intensity assessed on 11‐point scale using a 0‐10, visually enlarged, laminated, Numeric Rating Scale Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomization table |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The investigator recording the pain scores was blinded to the drugs given and to the gas mixture administered to the patients." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "A total of 205 patients were initially recruited into the study. Three patients were excluded because of difficulty with venous cannulation on the dorsum of the hand. Two patients in group N could not complete the study (one developed excitement and laughing and the other was oversedated)." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kim 2013b.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group A = 45.3 ± 2.5, Group B = 47.8 ± 2.5, Group C = 43.2 ± 1.9, Group D = 45.3 ± 3.0 Gender (M:F): Group A = 14:16, Group B = 17:12, Group C = 11:19, Group D = 13:15 Inclusion criteria: age 18 years to 65 years, ASA I‐II, elective surgery Exclusion criteria: patients had known hypersensitivity to lidocaine or microemulsion propofol; impaired communication; a renal, hepatic, cardiac, or neurologic problem; or a hypovolaemic state and who refused to provide informed consent Recruitment: 140 patients were enrolled (nine were excluded due to severe pain during ringer lactated solution injection) 131 adult patients were randomly allocated (33, 33, 33 and 32 patients were assigned into group A, B, C and D respectively), 117 patients were analysed Setting: Korea |
|
| Interventions |
Pretreatment with venous occlusion Patients were randomly divided into four groups Group A (n = 30) received MP (2 mg/kg) after lidocaine pretreatment (0.6mg/kg) with a tourniquet with arm down (venous engorgement) Group B (n = 29) received MP after lidocaine with a tourniquet with arm up (venous gravity drainage) Group C (n = 30) received MP with a tourniquet with arm down Group D (n = 28) (control group) received MP only (with no tourniquet) In groups A and C, the tourniquet was released after MP; in group B, the tourniquet was released before MP. |
|
| Outcomes | Pain intensity assessed on verbal pain score (VPS) with 11‐point scale by 0 being no pain and 10 being the most excruciating pain Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was accomplished by using numbered, sealed envelopes that had a computer‐generated assignment." |
| Allocation concealment (selection bias) | Low risk | "Randomization was accomplished by using numbered, sealed envelopes that had a computer‐generated assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "14 patients were excluded (12 patients due to protocol violation, 2 patients due to lost follow up)." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
King 1992.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): group 1 = 31 ± 10, group 2 = 31 ± 10, group 3 = 33 ± 10, group 4 = 31 ± 10 Gender: 100% female Inclusion criteria: ASA I‐II, minor gynaecologic surgery Exclusion criteria: N/A Recruitment: 400 adult patients randomly assigned (368 were analysed) Setting: New Zealand |
|
| Interventions |
Admixture Patients were allocated to one of four groups group 1 (n = 98): patient received 19 ml of propofol premixed with 1 ml of 0.9% saline group 2 (n = 91): patient received 19 ml of propofol premixed with 1 ml of 0.5% (5 mg) lidocaine group 3 (n = 90): patient received 19 ml of propofol premixed with 1 ml of 1 % (10 mg) lidocaine group 4 (n = 89): patient received 19 ml of propofol premixed with 1 ml of 2 % (20 mg) lidocaine |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "32 of 400 (less than 15%) were excluded due to incomplete data,leaving 368 patients for analysis." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Koo 2006.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group S = 40.8 ± 11.2, Group L = 41.3 ± 9.9, Group K10 = 40.3 ± 11.3, Group K50 =45 ± 10.5, Group K100 = 39.5 ± 11.7, Group KP 43.4 ± 11.8, Group Pre 37.3 ± 11.8, Group M = 40.4 ± 11.4 Gender (M:F): Group S = 11:19, Group L = 5:25, Group K10 = 13:17, Group K50 = 10:20, Group K100 = 12:18, Group KP = 13:17, Group Pre = 18:12, Group M = 22:8 Inclusion criteria: age 19 years to 59 years old, ASA I‐II, elective surgery Exclusion criteria: patients taking sedatives or analgesics, and those with allergic, neurologic, or cardiovascular disease Recruitment: 240 adult patients randomly assigned (30 in each group) Setting: Korea |
|
| Interventions |
Pretreatment alone Randomly allocated into eight groups; five groups during the first part of the study and three groups during the second part In Part 1, patients received pretreatment saline 2 ml (Group S) 2% lidocaine 2 ml (40 mg) (Group L) ketamine 10 mcg/kg (Group K10) ketamine 50 mcg/kg (Group K50) ketamine 100 mcg/kg (Group K100), respectively, immediately followed by propofol 2.5 mg/kg In Part 2, ketamine (100 mcg/kg) was administered 3 min before propofol (Group Pre) ketamine (100 mcg/kg) mixed with propofol solution and administered immediately after 2 ml saline (Group KP) ketamine (100 mcg/kg) administered just before injection of propofol in patients premedicated with midazolam (7.5 mg orally) 90 min before arrival in the operating room (Group M) |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain associated with grimacing, withdrawal movement of forearm, or both Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All syringes of test solution were prepared by a doctor not involved in induction of anaesthesia and covered so that the investigator who assessed the patient response was unaware of the nature of the solution." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An anaesthesiologist blinded to the study group monitored each patient’s pain score at 5 sec intervals. All syringes of test solution were prepared by a doctor not involved in induction of anaesthesia and covered so that the investigator who assessed the patient response was unaware of the nature of the solution." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Krobbuaban 2005.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 43.9 ± 15.9, Group II = 44.5 ± 16.1, Group III = 46.1 ± 16.0, Group IV = 42.3 ± 15.9 Gender (M:F): Group I = 43:54, Group II = 38:58, Group III = 39:58, Group IV = 43:54 Inclusion criteria: age 18 years to 50 years old, ASA I‐II, elective surgery Exclusion criteria: patients with neurologic or cardiovascular disorder, history of drug abuse, or egg lecithin or soybean oil allergies, as well as patients breast feeding at the time of surgery, taking sedatives or analgesics within 24 hours preceding surgery or requesting anxiolysis Recruitment: 388 adult patients randomly assigned (387 were analysed) Setting: Thailand |
|
| Interventions |
Admixture Patients were allocated randomly to receive either a small particle size lipid emulsion of propofol (Anepol: average particle size 140.5 nm), or standard propofol (propofol: average particle size 193.3 nm), by dividing into four groups. Group I (n = 97) received 2 ml of 0.9% NaCl and propofol admixture Group II (n = 96) received 2 ml of 2% lidocaine (40 mg) and propofol, Group III (n = 97) received 2 ml of 0.9% NaCl and Anepol Group IV (n = 97) received 2 ml of 2% lidocaine (40 mg) and Anepol into a dorsal vein of the hand |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain in response to questioning only without any behavioural signs) 2 = moderate pain (pain in response to questioning and accompanied by behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Low risk | "The propofol solution was prepared by a nurse anaesthetist in unlabeled syringes according to group allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "As the physical appearance of the two study drugs were identical, the anaesthesia providers and the investigators recording the data were unaware of the formulation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "As the physical appearance of the two study drugs were identical, the anaesthesia providers and the investigators recording the data were unaware of the formulation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One patient in group II (LP) was excluded from the analysis due to protocol violation." (midazolam given before induction) |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Krobbuaban 2008.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 43 ± 17, Group II = 44 ± 16 Gender (M:F): Group I = 62:73, Group II = 66:67 Inclusion criteria: age 18 years to 60 years, ASA I or II, and undergoing an elective surgical procedure with general anaesthesia Exclusion criteria: patients with a neurological or cardiovascular disorder, history of drug abuse, or egg lecithin or soybean oil allergies, as well as patients breast feeding at the time of surgery, taking sedatives or analgesics within 24 hrs preceding surgery or requesting anxiolysis Recruitment: 270 adult patients randomly assigned (268 were analysed) Setting: Thailand |
|
| Interventions |
Admixture Patients were allocated randomly into two groups to receive either propofol‐MCT/LCT alone (n = 135) or propofol‐MCT/LCT plus 20 mg lidocaine admixture (n = 133) The study solution was injected at 1 ml/second by one anaesthesiologist and patients graded any associated pain |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain in response to questioning only without any behavioural signs) 2 = moderate pain (pain in response to questioning and accompanied by behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "As the physical appearances of the two study drugs were identical, the anaesthesia providers and an investigator recording were unaware of the propofol formulation. The propofol solutions were prepared by a nurse anaesthetist in unlabeled syringes as per the patient’s group allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "As the physical appearances of the two study drugs were identical, the anaesthesia providers and an investigator recording were unaware of the propofol formulation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two patients from the propofol‐MLC/LCT plus lidocaine group were excluded from the analysis due to protocol violation (midazolam given before induction)." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study was financially supported by Chaiyaphum (government) Hospital. The study appears to be free of other sources of bias |
Kwak 2007a.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 42.1 ± 14.7, Group II = 42.9 ± 15.2, Group III = 41.2 ± 13 Gender (M:F): Group I = 27:15, Group II = 20:22, Group III = 28:15 Inclusion criteria: age 18 years to 65 years old, ASA I‐II, elective surgery Exclusion criteria: known sensitivity to propofol, neurological and psychiatric disease, concomitant analgesic or sedative medication or the presence of infection on the dorsum of the left hand Recruitment: 129 adult patients were randomly assigned (127 were analysed) Setting: Korea |
|
| Interventions |
Pretreatment with venous occlusion 127 patients were allocated to one of three groups receiving lidocaine 20 mg (n = 42) remifentanil 0.3 μg/kg (n = 42) lidocaine 20 mg plus remifentanil 0.3 μg/kg (n = 43) as pretreatment, venous occlusion was then maintained for 1 min, after relief of venous occlusion, followed by injection of 5 ml of 1% propofol |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated table |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "An anaesthesiologist not involved in this study prepared identical coded syringes." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A study‐blinded anaesthesiologist evaluated pain during propofol injection." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two were excluded due to failure to cannulate dorsal vein. |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kwak 2007b.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group A = 45.0 ± 16.0, Group B = 42.0 ± 12.4, Group C = 46.2 ± 11.9 Gender (M:F): Group A = 13:32, Group B = 14:31, Group C = 16:30 Inclusion criteria: ASA I‐II, elective surgery Exclusion criteria: known sensitivity to propofol, neurological and psychiatric disease, concomitant analgesic or sedative medication or the presence of infection on the dorsum of the left hand. Recruitment: 141 adult patients randomly assigned (136 were analysed) Setting: South Korea |
|
| Interventions |
Admixture Group A (n = 45) received saline 0.9% (0.035 ml/kg/min, same volume as remifentanil in order to obtain blinding) via a syringe pump 30 sec before the injection of propofol premixed with lidocaine. Group B (n = 45) received remifentanil (0.35 mg/kg/min) 30 sec before the injection of propofol. Group C (n = 46) received remifentanil (0.35 mg/kg/min) 30 sec before the injection of propofol premixed with lidocaine. For propofol premixed with lidocaine, 1% propofol (Diprivan; AstraZeneca, Italy) and 1% lidocaine were mixed in a 10:1 volume ratio. General anaesthesia was induced with propofol 2mg/kg. During the induction of anaesthesia, propofol was administered at a rate of 990 ml/hr in Group A, C and 900 ml/hr in Group B (equivalent to 2.5 mg/sec) by an infusion pump. |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain in response to questioning only without any behavioural signs) 2 = moderate pain (pain in response to questioning and accompanied by behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Low risk | "Patients allocated randomly to one of three groups (n = 47 each) using sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A study‐blinded investigator evaluated the level of pain on injection of propofol." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Five patients had to be excluded for a technical reason such as a defect of the infusion pump or difficulty with venous cannulation." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | This research was supported by Kyungpook National University Research Team fund, 2002 The study appears to be free of other sources of bias |
Kwak 2008.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group L = 48 ± 14.5, Group D = 50 ± 13.5, Group LD = 48 ± 14.4, Group N = 45 ± 14.5 Gender (M:F): Group L = 15:20, Group D = 13:22, Group LD = 11:24, Group N = 10:25 Inclusion criteria: age 16 years to 73 years old, ASA I‐II, elective surgery Exclusion criteria: patients had received sedative or analgesic medication within 24 hours before surgery; had known allergy to any drugs; had renal, hepatic, or cardiac problems; had neurologic deficits and psychiatric disorders; or required a rapid sequence induction Recruitment: 142 adult patients randomly assigned (140 were analysed, 35 in each group) Setting: Korea |
|
| Interventions | Pretreatment with venous occlusion No premedication was given Patients were randomized to receive pretreated lidocaine 20 mg (Group L), dexamethasone 6 mg (Group D), combination lidocaine 20 mg and dexamethasone 6 mg (Group LD), or normal saline (Group N) with venous occlusion for 1 minute, followed by administration of 25% of the total calculated dose of propofol (2.5 mg/kg) into a dorsal hand vein |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain in response to questioning only without any behavioural signs) 2 = moderate pain (pain in response to questioning and accompanied by behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table was used |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "An anaesthesiologist who was not involved in this study prepared identically coded syringes." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A study‐blinded anaesthesiologist evaluated the intensity and incidence of pain." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Of the 142 patients enrolled, 2 were excluded due to difficulty with venous cannulation." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kwon 2012.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Placebo = 56.9 ± 12.3, Lidocaine = 60.4 ± 11.7 Gender (M:F): Placebo = 1:0.64, Lidocaine = 1:0.87 Inclusion criteria: ASA I‐II, sedative diagnostic upper GI endoscopy. Exclusion criteria: patients who were pregnant, allergic to soybeans, eggs or any drugs, who received sedatives or analgesics within 24 hours before the procedure, patients with a poor general condition who had an American Society of Anesthesiology classification III‐V, patients with psychiatric disorders or arrhythmia, and those with difficult venous cannulation Recruitment: 126 adult patients randomly assigned (121 were analysed) Setting: South Korea |
|
| Interventions |
Preatreatment with venous occlusion Subjects were randomly assigned to lidocaine (n = 61) and placebo (n = 60) groups Pretreatment with a bolus of 1% lidocaine 2 ml or normal saline 2 ml was injected through the cannula of the dorsal hand for 10 sec with venous occlusion by a tourniquet. The occlusion was released after 1 min and the bolus of propofol 0.5 mg/kg was administered for 10 sec. |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain in response to questioning only without any behavioural signs) 2 = moderate pain (pain in response to questioning and accompanied by behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated to two groups according to a computerized table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identical syringes were used to maintain blinding of the investigators and were arranged by medical professionals who were not involved in the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Pain intensity was estimated by an examiner blinded to the group assignment using a four point verbal rating scale." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Among the 126 subjects screened, five were excluded from the study due to underlying arrhythmia and difficult venous cannulation." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | This research was conducted under the Bisa Research Grant of Keimyung University in 2009 The study appears to be free of other sources of bias |
Lee 1994.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 34 ± 14, Group II = 36 ± 15, Group III = 29 ± 9 Gender (M:F): Group I = 6:30, Group II = 10:26, Group III = 8:35 Inclusion criteria: age 16 years to 70 years old, ASA I‐II, minor surgical procedures Exclusion criteria: patients who suffered from cardiac conduction defects or epilepsy or who were taking anti‐arrhythmic or analgesic drugs Recruitment: 115 adult patients randomly assigned (36, 36 and 43 in saline, lidocaine and thiopentone group respectively) Setting: Australia |
|
| Interventions |
Pretreatment alone Compare the prior administration of intravenous pretreatment saline 4 ml (Group I) (n = 36), lidocaine 20 mg (Group II) (n = 36) thiopentone 100 mg (Group III) (n = 43) on the pain produced by intravenous injection of propofol |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Syringes contained 4 ml of the test solution and were covered to ensure that the investigator who assessed the patient's response was unaware of the nature of the solution." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Liaw 1999.
| Methods | Semi‐double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group A = 44 ± 20, Group B = 39 ± 19, Group C = 40 ± 16, Group D = 42 ± 7, Group E = 40 ± 15 Gender (M:F): Group A = 13:22, Group B = 17:18, Group C = 19:16, Group D = 12:23, Group E = 20:15 Inclusion criteria: ASA I‐II, elective surgery Exclusion criteria: N/A Recruitment: 175 adult patients randomly assigned (35 in each group) Setting: Taiwan |
|
| Interventions |
Pretreatment with venous occlusion Patients were allocated into 5 groups Group A received 10 mg (2 ml) of metoclopramideIV immediately before 2 mg/kg propofol induction. Group B was induced with the same dose of propofol (2 mg/kg) to which metoclopramide (10 mg or 2 ml) was added. Group C the IV infusion was stopped and the arm with the IV line was elevated for 15 sec for gravity drainage of venous blood. A rubber tourniquet was placed on the upper arm to produce a venous occlusion. Metoclopramide 10 mg (2 ml) was injected intravenously and retained in the veins for 1 min, followed by tourniquet release and propofol induction (2 mg/kg). Group D was identical to group C but instead of metoclopramide, 2 ml of 2% lidocaine (40 mg) was injected. Group E was identical to groups C and D but received 2 ml of normal saline. |
|
| Outcomes | Pain intensity assessed on 11‐point scale (VPS) with 0 being no pain and 10 being the most excruciating pain Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Low risk | "Before induction, one of the investigators opened a sealed envelope and prepared an appropriate solution for the injection of propofol." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "One of the investigators opened a sealed envelope and prepared an appropriate solution for the injection of propofol. Groups A and B (iv bolus) were treated differently from C, D and E (iv retention); otherwise the administration was double blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Lu 2013.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group CON = 50 ± 12, Group LID = 47 ± 13, Group DEZ = 47 ± 13 Gender (M:F): Group CON = 12:13, Group LID = 13:12, Group DEZ = 11:14 Inclusion criteria: age 16 years to 65 years, ASA I and II, scheduled for elective surgery under general anaesthesia were recruited for the study Exclusion criteria: patients with a history of renal or hepatic insufficiency, hypersensitivity to the study drugs, neurological or cardiovascular disease and patients with obesity, difficult airway, pregnant patients and patients on medication with pain modifying drugs Recruitment: 75 adult patients were randomly assigned (25 patients in each group) Setting: China |
|
| Interventions |
Pretreatment alone A total of 75 patients were randomly assigned to one of the three groups, thus Group CON, received 2 ml of normal saline Group LID, received 2 ml of 2% lidocaine (40 mg) Group DEZ, received 2 ml of dezocine 2 mg as pretreatment Propofol was injected 1 min later |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group randomization was done according to the computer software generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A written only double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An anaesthesiologist blinded to the intervention evaluated the pain level using a four‐point verbal rating scale (VRS) during injection of propofol." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Lyons 1996.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group 1 = 41.1 ± 18.1, Group 2 = 38.3 ± 16.9, Group 3 = 40.6 ± 17.3 Gender (M:F): Group 1 = 33:19, Group 2 = 32:19, Group 3 = 29:18 Inclusion criteria: age 16 years to 70 years old, ASA I‐II, a variety of elective orthopaedic surgery Exclusion criteria: patients with difficult venous access and those requiring a rapid sequence induction Recruitment: 150 adult patients randomly assigned (52, 51 and 47 in pethidine, lidocaine and saline group respectively) Setting: Ireland |
|
| Interventions |
Pretreatment alone Patients were randomly allocated into 3 groups: pethidine 25 mg (n = 52), Group 1 lidocaine 10 mg (n = 51), Group 2 0.9% saline 1 ml (n = 47), Group 3 pretreatment 10 sec before propofol 5 ml injection over 15 sec Premed with diazepam 0.15 mg/kg 90 min before surgery |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Mallick 2007.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group DP = 44 ± 16.4, Group LP = 42 ± 17.8, Group DL = 41 ± 16.9, Group LL = 41 ± 16.9 Gender (M:F): Group DP = 21:61, Group LP = 25:56, Group DL = 26:56, Group LL = 25:56 Inclusion criteria: ASA I‐II, elective surgery Exclusion criteria: N/A Recruitment: 328 adult patients randomly assigned (326 patients were analysed) Setting: England |
|
| Interventions |
Admixture Group DP (n = 82) received propofol (Diprivan) 1% Group LP (n = 81) Lipuro propofol 1% Group DL (n = 82) propofol (Diprivan) 1% with lidocaine 2% 2 ml (40 mg) admixture Group LL (n = 81) Lipuro propofol 1% with lidocaine 2% 2 ml (40 mg) admixture No patients received pre‐medication or intravenous opioids prior to propofol induction |
|
| Outcomes | Pain intensity assessed on 11‐point scale (VAS) with 0 being no pain and 10 being the worst imaginable pain Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly allocated, using the ‘sealed envelope method’, to one of four groups." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All drugs were drawn up independently and out of view, so that anaesthetic staff and the operator performing the assessments were blinded to group allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All drugs were drawn up independently and out of view, so that anaesthetic staff and the operator performing the assessments were blinded to group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two patients were randomized but excluded from analysis owing to cannulation problems." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Massad 2006.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 38.2 ± 14.7, Group II = 40.3 ± 16.6, Group III = 43.6 ± 17.4, Group IV = 43.0 ± 16.7 Gender (M:F): Group I = 25:25, Group II = 16:35, Group III = 18:32, Group IV = 20:30 Inclusion criteria: age 15 years to 90 years old, ASA I‐III, minor elective surgery demanding laryngeal mask Exclusion criteria: patients with difficulty in communication, not co‐operative, below 14 years, received any type of analgesia before arriving operating room including local anaesthesia, positive past history of hypersensitivity to anaesthetic agents or decompensated heart failure Recruitment: 200 adult patients randomly assigned (50 in each group) Setting: Jordan |
|
| Interventions |
Admixture Pretreatment alone Pretreatment with venous occlusion 200 patients, divided into four groups: Group I the control group, 1% propofol alone Group II 1% propofol premixed with 40 mg lidocaine Group III 1% propofol 60 sec after 40 mg of lidocaine pretreatment Group IV lidocaine 40 mg pretreatment venous occlusion Pain was graded during induction |
|
| Outcomes | Pain intensity assessed on 3‐point scale 0 = no pain 1 = pain 2 = pain with behavioural changes Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Blinded by resident doctor as an outcome assessor." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
McCluskey 2003.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Control group = 36.7 ± 11.3, EMLA group = 37.1 ± 11.2, Lidocaine group = 41.4 ± 10.7 Gender (M:F): 100% female Inclusion criteria: Age 18 years to70 years old, ASA I‐II, gynaecological day case‐surgery Exclusion criteria: N/A Recruitment: 90 adult patients randomly assigned (30 in each group) Setting: United Kingdom |
|
| Interventions |
Admixture Control group: placebo cream applied over a wide area to the dorsum of the nondominant hand and distal forearm 60 min before surgery and anaesthesia induced with 18 ml of 1% propofol (Diprivan®) mixed with 2 ml of 0.9% saline EMLA group: EMLA cream applied as described above and anaesthesia induced with 18 ml of 1% propofol mixed with 2 ml of 0.9% saline Lidocaine group: placebo cream applied as described above and anaesthesia induced with 18 ml of 1% propofol premixed with 2 ml of 2% lidocaine (40 mg). |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Low risk | "The patients were randomly allocated by sealed envelope into three groups." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The nurses who insert IV cannula were the investigator assessing pain on propofol injection". Possible bias as nurses might suspect patient had had EMLA if they did not have pain when IV cannula inserted. However, the nurses would not know whether patient was in control or lidocaine group which were the groups whose results we analysed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
McCulloch 1985.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean (range)): Group A = 35 (19 to 54), Group B = 37 (19 to 65), Group C = 35 (16 to 65), Group D = 32 (18 to 65) Gender (M:F): Group A = 6:34, Group B = 5:35, Group C = 9:31, Group D = 1:39 Inclusion criteria: age 16 years to 65 years old, ASA I‐II, minor elective surgery Exclusion criteria: pregnancy, weight more than 10% above expected body weight Recruitment: 160 adult patients randomly assigned (40 in each group) Setting: United Kingdom |
|
| Interventions |
Pretreatment alone Group A (n = 40): IV at dorsum of hand, propofol 2.5 mg/kg Group B (n = 40): IV at forearm/antecubital fossa, propofol 2.5 mg/kg Group C (n = 40): IV at dorsum of hand, lidocaine 10 mg pretreated followed by propofol 2.5 mg/kg Group D (n = 40): IV at dorsum of hand, thiopental 4.5mg/kg (without propofol) |
|
| Outcomes | Pain was assessed on 3‐point scale 0 = no pain 1 = other sensations e.g.. tingling, numbness, cold and warmth 2 = pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The position of IV in group B were different. The appearance of thiopental and propofol were also different. However, only the result of group A and group C were analyzed, where the position of venous access and the appearance of drug solution were similar." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | High risk | The authors would like to thank I.C.I. pharmaceuticals for supplying propofol |
McDonald 1996.
| Methods | Randomized controlled trial | |
| Participants | Age: N/A Gender: N/A Inclusion criteria: ASA I‐II, minor surgery Exclusion criteria: patients taking analgesics or suffered from epilepsy or cardiac arrhythmia Recruitment: 100 adult patients randomly assigned (96 were analysed) Setting: United Kingdom |
|
| Interventions |
Admixture Patients were randomly allocated to one of three groups. group 1 (n = 31): NSS 2 ml in propofol 18 ml group 2 (n = 33): 1% lidocaine 2 ml (20 mg) in propofol 18 ml group 3 (n = 32): blood aspirate 2 ml in propofol 18 ml All patients' arms were kept hidden from the assessor |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Every effort was made to ensure that neither patients nor assessor was aware of the patient group allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "in all three groups the arm used for induction was kept hidden from the assessor. All questions were asked by an independent investigator." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Four patients were excluded from the trial; one with severe eczema, one with no obvious veins on the back of her hand, one who did not understand what was asked of him, and one in blood group from whom it was possible to aspirate only 1 ml of blood." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Minogue 2005.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Benzyl alcohol group (BA) = 44 ± 16, Placebo group (PL) = 41 ±12, Lidocaine group (LI) = 41 ± 12 Gender (M:F): BA group = 8:31, PL group = 18:21, LI group = 7:35 Inclusion criteria: ASA I‐II, elective surgery Exclusion criteria: N/A Recruitment: 120 adult patients randomly assigned Setting: Canada |
|
| Interventions |
Admixture The benzyl alcohol (BA; n = 39) group received 10 ml of bacteriostatic saline followed by 5 ml (50 mg) of propofol. The lidocaine (LI; n = 42) group, 1 ml of 2% lidocaine (20 mg) was premixed with 19 ml of propofol (190 mg). Ten ml of preservative‐free normal saline was then administered followed by 5 ml of the propofol and lidocaine mixture The placebo (PL; n = 39) group received 10 ml of preservative‐free normal saline followed by 5 ml of propofol |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a randomly‐generated computer assignment |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The agents were prepared by the investigator and given to the attending anaesthesiologist who was blinded as to the contents." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Nakane 1999.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean (range)): Control group = 78 (19 to 78), Lidocaine group = 53 (19 to 80), FUT group = 53 (16 to 81) Gender: N/A Inclusion criteria: ASA I‐II, elective surgery Exclusion criteria: N/A Recruitment: 300 adult patients randomly assigned (100 in each group) Setting: Japan |
|
| Interventions |
Admixture 300 patients allocated randomly to one of three groups 1. Control group: 20 ml of 1% propofol 2. Lidocaine group: 20 ml of 1% propofol premixed with 2 ml of 2% lidocaine (40 mg) 3. FUT group: 20 ml of 1% propofol mixed with FUT 10 mcg |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Nathanson 1996.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean (range)): Lidocaine group (L) = 57 (19 to 75), alfentanil group (A) = 55 (18 to ‐72), placebo group (P) = 53.5 (3 to 74) Gender (M:F): Lidocaine group = 18:12, alfentanil group = 14:15, placebo group = 17:13 Inclusion criteria: age 18 years to 75 years old, elective surgery Exclusion criteria: a history of chronic pain syndromes, thrombophlebitis, neurological disease, and analgesic administration at the time of the study Recruitment: 89 adult patients randomly assigned (30, 29, 30 in group L, A, P respectively) Setting: United Kingdom |
|
| Interventions |
Admixture Patients were randomly allocated to one of three groups Group L (lidocaine; n = 30) received 2 ml of normal saline followed 30 sec later by premixed propofol 180 mg (18 ml) and lidocaine 40 mg (2 ml of lidocaine 2%) Group A (alfentanil; n = 29) received pretreatment with alfentanil 1 mg followed 30 sec later by propofol and normal saline (propofol 180 mg mixed with 2 ml normal saline) Group P (placebo; n = 30) receive 2 ml normal saline followed 30 sec later by propofol and normal saline (as for Group A) |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Newcombe 1990.
| Methods | Double‐blind, randomized controlled trial | |
| Participants | Age (years, mean (range)): Saline group = 29 (17 to 57), lidocaine group = 28 (16 to 56) Gender: N/A Inclusion criteria: age 16 years to 57 years, ASA I, day case surgery Exclusion criteria: required premedication 100 patients were randomly assigned (93 patients were analysed) Setting: Australia |
|
| Interventions |
Admixture Patients were assigned to receive either propofol and 1 ml of normal saline (n = 46) propofol and 1 ml of 1% lidocaine (10 mg) admixture (n = 47) |
|
| Outcomes | Pain intensity assessed on 3‐point scale 0 = no pain 1 = pain on questioning 2 = spontaneous complaint of pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a table of random numbers and numbered envelopes |
| Allocation concealment (selection bias) | Unclear risk | Using a table of random numbers and numbered envelopes. (sealed?) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The solution was made up by an anaesthetist or registered nurse who was not involved with the case." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "7 patients were excluded from the study: 2 patients refusal to participate, 2 patients no venous access, 1 patient, language difficulties, 1 patient mixture left too long, 1 patient incomplete documentation." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Niazi 2005.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean (range)): Group A = 32.7 (20 to 48), Group B = 35.5 (18 to 53), Group C = 33.9 (18 to 55) Gender (M:F): Group A 17:16, Group B = 15:18, Group C = 15:18 Inclusion criteria: age 18 years to 55 years old, ASA I‐II, elective surgery Exclusion criteria: patients of ASA grades III–V, airway Mallampati Class III–IV, history of cardiac conduction defects, antidysrhythmic medications, allergies to local anaesthetics and propofol, abnormalities of lipid metabolism, epilepsy, pregnancy and analgesic drug use in the previous 24 hours. Recruitment: 102 adult patients randomly assigned (99 were analysed, 33 in each group) Setting: Ireland |
|
| Interventions |
Pretreatment with venous occlusion Group assignment • A: IV lidocaine 0.5 mg/kg pretreatment, 50% O2 in air mixture • B: 0.9% normal saline pretreatment, 50% N2O in O2 mixture • C: IV lidocaine 0.5mg/kg pretreatment, 50% N2O in O2 mixture All patients had a rubber tourniquet applied to the forearm with the sited IV cannula |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain in response to questioning only without any behavioural signs) 2 = moderate pain (pain in response to questioning and accompanied by behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated to one of three groups by a computer‐conducted randomization." |
| Allocation concealment (selection bias) | Low risk | "With the code sealed until arrival of the patient in the operating room." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The investigator measuring pain scores was blinded to the drugs given as all drug syringes were labelled as ‘study drug’." "The investigator was blinded to the gas mixture administered." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "A total of 102 patients were recruited; three of whom were excluded due to difficulty with venous cannulation." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Nicol 1991.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean (range)): control group = 50 (19 to 86), lidocaine group = 52 (18 to 85), procaine group = 52 (15 to 83) Gender (M:F): N/A Inclusion criteria: age 15 years to 86 years, ASA I‐II Exclusion criteria: pregnancy, any patients requiring rapid sequence induction, difficult intubation or allergy to local anaesthetics Recruitment: 283 adult patients randomly assigned (273 were analysed) Setting: England |
|
| Interventions |
Pretreatment alone Patients were allocated at random to receive either 0.5 ml isotonic saline (n = 95) 0.5 ml 2% lidocaine (10 mg) (n = 95) 0.5 ml 2% procaine (10 mg) (n = 83) followed by propofol 2.5 mg/kg |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10 of 283 patients were excluded. "More operations in procaine group were cancelled after randomization than in the other two groups." However, there was no information regarding the number of exclusion in each group and the reasons of withdrawal. |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Nishiyama 2005.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): LCT group = 53 ± 14, Lidocaine group = 50 ± 16, Flurbiprofen group = 48 ± 15, Flurbiprofen 1 group = 46 ± 14, MCT/LCT group = 51 ± 15 Gender (M:F): LCT group = 8:42, Lidocaine group = 11:39, Flurbiprofen group = 10:40, Flurbiprofen 1 group = 11:39, MCT/LCT group = 9:41 Inclusion criteria: age 20 years to 70 years old, ASA I‐II, body surface, spine and shoulder surgery Exclusion criteria: patients had vascular diseases, habituation of analgesics, sedatives, or antianxiety drugs, or allergic diseases Recruitment: 250 adult patients randomly assigned (50 in each group) Setting: Japan |
|
| Interventions |
Pretreatment alone Anaesthesia was induced with intravenous administration of flurbiprofen 50 mg followed immediately by propofol LCT 2 mg/kg (Flurbiprofen group, n = 50), flurbiprofen 50 mg followed by propofol LCT 2 mg/kg 1 min later (Flurbiprofen 1 group, n = 50), 2% lidocaine 40 mg pretreated followed by propofol LCT 2 mg/kg (Lidocaine group, n =50), propofol LCT 2 mg/kg alone (LCT group, n = 50), propofol MCT/LCT 2mg/kg (MCT/LCT group, n =50) |
|
| Outcomes | Pain assessed on 3‐point scale 0 = no pain 1 = mild 2 = severe Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
O'Hara 1997.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Placebo group = 44.0 ± 13.4, Lidocaine group = 40.0 ± 12.5, NTG group = 37.9 ± 16.2, NTG+Lidocaine group = 46.4 ± 15.6 Gender (M:F): Placebo group = 11:20, Lidocaine group = 14:17, NTG group = 22:9, NTG + lidocaine group = 13:18 Inclusion criteria: ASA I‐III, elective ambulatory surgery Exclusion criteria: N/A Recruitment: 124 adult patients randomly assigned (31 in each group) Setting: USA |
|
| Interventions |
Admixture Nitroglycerin or placebo ointments were applied to the back of the hand over the skin area overlying the IV catheter tip. Lidocaine was or was not added to the propofol solution: placebo ointment and plain propofol placebo ointment and propofol premixed with 1% lidocaine 2 ml nitroglycerin ointment 7.5 mg and plain propofol nitroglycerin ointment 7.5 mg and propofol premixed with 1% lidocaine 2 ml |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ozgul 2013.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group L = 32 ± 10, Group A = 33 ± 11, Group S = 30 ± 10 Gender (M:F): Group L = 34:66, Group A = 37:68, Group S = 35:65 Inclusion criteria: age 18 years to 60 years old, ASA I‐II, elective surgery Exclusion criteria: difficulty in communication, allergy to study drugs, difficult airway, use of opioid or NSAIDS in the past week Recruitment: 305 adult patients were randomly assigned (300 patients were analysed with 100 in each group) Setting: Turkey |
|
| Interventions |
Pretreatment with venous occlusion Randomized into three groups, Group L received lidocaine 0.05 ml/kg = 0.5 mg/kg (5 ml of NSS + 2% lidocaine 5 ml), Group A received 0.05 ml/kg of alkalinised lidocaine (5 ml of 2% lidocaine + 1 ml 8.4% NaHCO3 + 4 ml NSS) Group S control received NSS same amount All drugs were given as a bolus over 20 sec before propofol injection |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded investigator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Five patients were excluded: three in group L, one in group A, one in group S due to difficulty in cannula insertion." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Pang 1998.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group A = 45.6 ± 18.0, Group B = 37.5 ± 13.6, Group C = 40.9 ± 13.4, Group D = 49.1 ± 18.6, Group E = 39.6 ± 19.0 Gender (M:F): Group A = 20:15, Group B = 19:16, Group C = 18:17, Group D = 11:24, Group E = 20:15 Inclusion criteria: age 41.5 ± 17.6 years, ASA I‐II, elective surgery Exclusion criteria: patients with communication difficulties Recruitment: 175 adult patients randomly assigned (35 in each group) Setting: Taiwan |
|
| Interventions |
Pretreatment with venous occlusion Group A received fentanyl 150 mcg, Group B received morphine 4 mg, Group C received meperidine 40 mg, Group D received 2% lidocaine 3 ml (60 mg), and Group E received 3 ml normal saline and served as the control group followed by propofol 100 mg |
|
| Outcomes | Pain intensity assessed on 11‐point scale (VAS) with 0 being no pain and 10 being most excruciating pain Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The drug was randomly prepared in an unlabeled syringe for pretreatment and handed to the anaesthesiologist who was blind to the identity of the drug." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Pang 1999.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group T = 38.3 ± 15.2, Group L = 42.3 ± 18.7, Group NS = 39.6 ± 19.0 Gender (M:F): Group T = 19:16, Group L = 17:18, Group NS = 15:20 Inclusion criteria: ASA I‐II, elective surgery Exclusion criteria: patients with communication difficulties Recruitment: 105 adult patients randomly assigned (35 in each group) Setting: Taiwan |
|
| Interventions |
Pretreatment with venous occlusion Group T received 50 mg tramadol (Tramal ®, Grunenthal, Germany) in NS 3mL Group L received 60 mg lidocaine (3 ml of 2% solution) Group NS received 3 ml normal saline, serving as the control Propofol 10 ml was injected at rate 0.5 ml/sec |
|
| Outcomes | Pain intensity assessed on 11‐point scale (VAS) with 0 being no pain and 10 being most excruciating pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The pretreatment drug was prepared in an unlabeled syringe and randomly handed for pretreatment to the anaesthesiologist who was unaware of the identity of the drug." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Parmar 1998.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group A = 32.9 ± 13.1, Group B = 36.5 ± 14.1, Group C = 33.8 ± 11.4, Group D = 37.4 ± 14.0 Gender (M:F): Group A = 22:17, Group B = 24:14, Group C = 22:16, Group D = 20:18 Inclusion criteria: age 18 years to 65 years old, ASA I‐II, elective surgery Exclusion criteria: pregnant/lactating mother, patients with epilepsy or cardiac conduction defects, on antiarrhythmic drugs or analgesics, patients with disorders of lipid metabolism, past history of allergy to propofol or lidocaine Recruitment: 153 adult patients randomly assigned Setting: Singapore |
|
| Interventions |
Admixture Patients were randomly allocated to one of four groups Group A (n = 39) propofol + 1% lidocaine 0.1 mg/kg admixture Group B (n = 38) propofol + 1% lidocaine 0.2 mg/kg admixture Group C (n = 38) cold propofol Group D (n = 38) control group (propofol mixed with normal saline) General anaesthesia was induced with propofol 2.5 mg/kg |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain (no pain when asked 15 sec after start of injection) 1 = mild pain (complaint of pain when asked 15 sec after start of injection) 2 = moderate pain (spontaneous complaint of pain by patient) 3 = severe pain (spontaneous complaint of pain by patient associated with grimacing or withdrawal of hand during injection) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A second, independent anaesthetist, blinded to the mixture given, noted and recorded the presence of pain." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Polat 2012.
| Methods | Prospective randomized double‐blind trial | |
| Participants | Age (years, mean ± SD): Group R = 45.64 ± 13.89, Group L = 45.22 ± 16.4, Group M = 49.3 ± 16.2, Group K = 47.2 ± 14.6, Group N = 46.2 ± 16.1 Gender (M:F): Group R = 19:31, Group L = 28:22, Group M = 23:27, Group K = 20:30, Group N = 27:23 Inclusion criteria: ASA physical status I or II undergoing elective surgery with general anaesthesia Exclusion criteria: history of allergy, renal, hepatic problems, thrombophlebitis, chronic pain taking sedative or analgesic medication, weight < 50 kg Recruitment: 250 adult patients randomly assigned Setting: Turkey |
|
| Interventions |
Pretreatment with venous occlusion All patients were pre‐medicated with 3 mg midazolam IM 45 min before induction of anaesthesia. A 20 gauge Teflon catheter was inserted into a vein on the dorsum of the patient’s non‐dominant hand and an infusion of Ringer’s lactate solution was started at a rate of 5 ml/kg/h. A rubber tourniquet was used to perform 1 minute of venous occlusion before administration of the study drugs and then 25% of total calculated dose of propofol (2 mg/kg) was injected into the dorsal vein of the hand through a 20‐gauge IV cannula a rate of 1 ml/s. During a 10‐second pause before the induction of anaesthesia, patients were questioned by a blinded investigator about the pain intensity on injection. Patients were randomly divided into 5 groups receive either: 2 ml (0.02 mg) of remifentanil (n = 50, Group R) 2 ml (40 mg) of lidocaine (n = 50, Group L) 2 ml (10 mg) of metoclopramide (n = 50, Group M) 2 ml (100 μg/kg) of ketamine (n = 50, Group K) 2 ml of saline (n = 50, Group N) |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported This study was not included in meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using the table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients were questioned by a blinded investigator about the pain intensity on injection." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Reddy 2001.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group 1 = 33.5 ± 13.5, Group 2 = 39.8 ± 14.2, Group 3 = 41.4 ± 18.3 Gender (M:F): Group 1 = 12:8, Group 2 = 9:11, Group 3 = 10:10 Inclusion criteria: ASA I‐II, elective orthopaedic and gastrointestinal procedures Exclusion criteria: N/A Recruitment: 60 adult patients randomly assigned (20 in each group) Setting: Singapore |
|
| Interventions |
Pretreatment with venous occlusion Group 1 received 5 ml 0.9% sodium chloride solution intravenously as the control Group 2 received ondansetron 4 mg (2 mg/ml) diluted with water into a 5‐ml solution Group 3 received 50 mg lidocaine (5 ml of 1% solution) intravenously |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain in response to questioning only without any behavioural signs) 2 = moderate pain (pain in response to questioning and accompanied by behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly allocated to one of three groups by the drawing of lots." |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All syringes of test solution were prepared by another investigator and covered so that the investigator who assessed the patient's response was unaware of the nature of the solution." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All syringes of test solution were prepared by another investigator and covered so that the investigator who assessed the patient's response was unaware of the nature of the solution." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Saadawy 2007.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group K = 39.9 ± 13.6, Group T = 40.1 ± 14.1, Group M = 47.7 ± 20.1, Group L = 46.2 ± 26.7, Group S = 39.9 ± 13.6 Gender (M:F): Group K = 14:11, Group T = 13:12, Group M = 13:12, Group L = 14:11, Group S = 12:13 Inclusion criteria: ASA I‐II, elective surgery lasting 1 hr to 2 hr Exclusion criteria: N/A Recruitment: 125 adult patients randomly assigned (25 in each group) Setting: Egypt (Turkey, Saudi Arabia) |
|
| Interventions |
Pretreatment with venous occlusion Group K, ketamine 0.4 mg/kg Group T, thiopental 0.5 mg/kg Group M, meperidine 0.4 mg/kg Group L, lidocaine 1 mg/kg Group S, saline 3 ml All pretreatment drugs were made into 4 ml solutions and were accompanied by manual venous occlusion for 1 min, followed by tourniquet release and slow IV administration of propofol |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain in response to questioning only without any behavioural signs) 2 = moderate pain (pain in response to questioning and accompanied by behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The study drugs were randomly prepared in an unlabeled syringe for pretreatment and handed to the anaesthesiologist who was blind to the identity of the drug." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Salman 2011.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group I = 37.7 ± 10.3, Group II = 41.0 ± 10.5, Group III = 37.4 ± 11.3 Gender (M:F): Group I = 9:21, Group II = 7:23, Group III = 8:22 Inclusion criteria: ASA I‐II, elective surgery Exclusion criteria: patients having problems with communication; those with a known allergy to local anaesthetics or propofol, severe neurological deficits, or psychiatric disorder; those of ASA physical status III or higher, severe cardiac impairment, suspected or known pregnancy; or those who received opioids or nonsteroidal anti‐inflammatory drugs within the preoperative week Recruitment: 90 adult patients randomly assigned (30 in each group) Setting: Turkey |
|
| Interventions |
Pretreatment alone Patients were randomly allocated to one of three groups of 30 patients each Group I received 50 mg of methylene blue Group II received 40 mg of lidocaine Group III, the control group, was given normal saline All drugs were pretreated as a 2.0 ml bolus 45 seconds before propofol administration |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain in response to questioning only without any behavioural signs) 2 = moderate pain (pain in response to questioning and accompanied by behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | With a computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Scott 1988.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): N/A Gender (M:F): N/A Inclusion criteria: age 16 years to 70 years old, ASA I‐II, day‐case surgery Exclusion criteria: N/A Recruitment: 120 adult patients randomly assigned (15 in each group) Setting: United Kingdom |
|
| Interventions |
Pretreatment alone Admixture Pretreatment with venous occlusion Group 1 propofol bolus only at rate 2 ml/sec Group 2 1% lidocaine 1 ml intravenously, propofol bolus 30 seconds later (pretreatment) Group 3 1% lidocaine 1 ml intravenously, propofol bolus 120 seconds later (pretreatment) Group 4 1% lidocaine 1 ml mixed with propofol 200 mg intravenously (admixture) Group 5 propofol bolus through 23 G butterfly needle antecubital fossa Group 6 venous tourniquet at wrist, 1% lidocaine 1 ml intravenously, propofol bolus and release of tourniquet 120 seconds later (pretreatment with venous occlusion) Group 7 16 G intravenous catheter, dorsum of hand, propofol bolus in fast‐flowing intravenous infusion Group 8 propofol only, slow (75 second) injection |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A the techniques of propofol administration were different and that could be detected by personnel who assessed pain. This could cause bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sethi 2009.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group A = 42.02 ± 14.12, Group B = 40.63 ± 13.71, Group C = 41.5 ± 14.63 Gender (M:F): Group A = 59:41, Group B = 61:39, Group C = 49:51 Inclusion criteria: age 18 years to 65 years old, ASA I‐III, elective surgery Exclusion criteria: patients with ischaemic heart disease and neurological problems, pregnant or lactating patients, those who were taking any analgesics before surgery, or those with known hypersensitivity to propofol or to any of the constituents of the emulsion (soy‐bean oil, MCT, glycerol, egg lecithin, sodium oleate or water for injection) Recruitment: 300 adult patients were randomly assigned (100 in each group) Setting: India |
|
| Interventions |
Admixture The patients were assigned to 3 groups (100 each), using computer generated randomization Group A received propofol‐MCT/LCT premixed with normal saline (1 ml of normal saline added to 19 ml propofol‐lipuro) Group B received propofol‐MCT/LCT premixed with lidocaine (1 ml of 2% lidocaine added to 19 ml propofol‐lipuro) Group C received propofol‐LCT premixed with lidocaine (1 ml of 2% lidocaine added to 19 ml propofol) The investigators and patients were blinded to the study preparation being used |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain or grimacing, or both, or withdrawal of limb Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No data about blind methods for personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The investigators and patients were blinded to the study preparation being used." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | N/A but assuming from the table all patients were included |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Shimizu 2005.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group A = 48 ± 5, Group B = 46 ± 5, Group C = 48 ± 4, Group D = 47 ± 4 Gender (M:F): N/A Inclusion criteria: age 18 years to 70 years old, ASA I‐II, elective surgery Exclusion criteria: patients were allergic to propofol, had communication difficulties, a history of cardiovascular or neurological disease, a body mass index 30 kg/m2 or were unsuitable for intravenous induction Recruitment: 120 adult patients randomly assigned (30 in each group) Setting: Japan (Australia) |
|
| Interventions |
Pretreatment alone In Group A, patients were pretreated with normal saline 5 ml and then given propofol 2 mg/kg at a rate of 3.3 mg/sec In Group B, patients were pretreated with preservative‐free lidocaine 0.5 mg/kg adjusted to a volume of 5 ml and then given propofol 2 mg/kg at a rate of 3.3 mg/sec In Group C, patients were pretreated with preservative‐free lidocaine 1.0 mg/kg adjusted to a volume of 5 ml and then given propofol 2 mg/kg at a rate of 3.3 mg/sec In Group D, patients were pretreated with normal saline 5 ml then given propofol 2 mg/kg at a rate of 50 mg/sec |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain (no verbal pain or movement) 1 = mild pain (verbal pain, but no movement) 2 = moderate pain (verbal pain and movement of wrist) 3 = severe pain (verbal pain and movement of elbow or shoulder) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Low risk | "The treatment group determined by opening an opaque envelope." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A second anaesthesiologist, blinded to the type of pre‐treatment and rate of propofol infusion, evaluated the response." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sinha 2005.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group NL = 36.8 ± 14.7, Group N = 41.7 ± 15.0, Group L = 36.4 ± 15 Gender (M:F): Group NL = 15:15, Group N = 18:12, Group L = 17:13 Inclusion criteria: age 16 years to 55 years old, ASA I‐II, elective surgery Exclusion criteria: patients taking regular sedatives or analgesics, allergy to lidocaine, a history of movement disorder, history of drug abuse, uncooperative, anticipated difficult airway, thrombophlebitis, contraindication to nitrous oxide Recruitment: 90 adult patients randomly assigned (89 were analysed) Setting: India |
|
| Interventions |
Admixture Group NL (n = 30) received inhalation of 50% nitrous oxide in oxygen for 3 min along with 2% lidocaine 2ml premixed in propofol Group N (n = 29) inhalation of 50% nitrous oxide in oxygen for 3 min along with 2 ml NSS mixed in propofol Group L (n = 30) inhalation of 50% oxygen in air along with 2% lidocaine 2ml mixed in propofol |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain or grimace 2 = moderate pain or grimace and cry 3 = severe pain or cry and withdrawal of arm Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated to one of the three treatments by drawing of lots." |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients were blinded about the group to which they had been assigned, and the authors who held the mask were blinded to the group assigned." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "During induction, a screen was placed in front of the flowmeters in such a fashion that the first author who collected the data, and second author who was assisting in holding the mask were blinded to the group assigned." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One patient in Group N developed excitement, agitation and tremor during 50% nitrous oxide in oxygen inhalation." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Smith 1996.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group 1 = 37.6 ± 12.6, Group 2 = 34.2 ± 15.5, Group 3 35.4 ± 11.8 Gender (M:F): Group 1 = 7:28, Group 2 = 2:30, Group 3 = 9:25 Inclusion criteria: aged 16 years and older, ASA I‐II, day case surgery Exclusion criteria: patients taking sedative or analgesic medication Recruitment: 101 adult patients randomly assigned Setting: United Kingdom |
|
| Interventions |
Pretreatment alone Group 1 (n = 35) received ketorolac 10 mg in 2 ml of NSS pretreatment Group 2 (n = 32) received 1% lidocaine 2 ml (20 mg) pretreatment Group 3 (n = 34) received 2 ml of NSS followed by propofol 2.5 mg/kg |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The patient and observer were unaware of which drug was given." "All drugs were drawn up and given by an anaesthetist who was not part of the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Tariq 2006.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean (range)): Group A = 22 (15 to 36), Group B = 23 (16 to 34), Group C = 22 (16 to 37), Group D = 24 (18 to 35) Gender (M:F): Group A = 25:25, Group B = 24:26, Group C = 26:24, Group D = 25:25 Inclusion criteria: age 15 years to 37 years old, ASA I patients, routine tonsillectomy Exclusion criteria: N/A Recruitment: 200 adult patients randomly assigned (50 in each group) Setting: Pakistan |
|
| Interventions |
Admixture Group A: induction dose of propofol 2.5 mg /kg was administered in dorsum vein Group B: Lidocaine 10 mg (1 ml of 1% solution) and propofol admixture was administered in dorsum vein Group C Propofol was administered in a large vein in the antecubital vein Group D Lidocaine 10 mg was added to propofol administered in the antecubital vein |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain in response to questioning only without any behavioural signs) 2 = moderate pain (pain in response to questioning and accompanied by behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated into 4 groups." |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The position ofIV catheter was different in each group. However, both participants and personnel were not aware that the solution in syringe was normal saline or lidocaine." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The position ofIV catheter was different in each group. However, the outcome assessor was not aware that the solution in syringe was normal saline or lidocaine." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Tariq 2010.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group A = 46 ± 19, Group B = 50 ± 16, Group C = 48 ± 17 Gender (M:F): Group A = 58:42, Group B = 53:47, Group C = 56:44 Inclusion criteria: ASA I‐II, various surgical procedures Exclusion criteria: the presence of neurological or psychiatric diseases, difficulty with communication, history of renal or hepatic insufficiency, suspected or known difficult airway intake of any analgesics before surgery and hypersensitivity to the study drugs Recruitment: 300 adult patients randomly assigned (100 in each group) Setting: Pakistan |
|
| Interventions |
Admixture Patients were randomized to three groups Group A received propofol (Diprivan®) (LCT‐propofol) premixed with lidocaine (i.e. 2 ml of 1% lidocaine in 20 ml of propofol) Group B received Propofol‐Lipuro® (MCT/LCT‐propofol) premixed with 2 ml normal saline Group C received Propofol‐Lipuro® (MCT/LCT‐propofol) premixed with lidocaine (2 ml of 1% lidocaine in 20 ml of propofol) Anaesthesia was standardized in all the three groups Undiluted Diprivan® (LCT‐propofol) and Propofol‐Lipuro® (MCT/LCT‐propofol) were used for induction of anaesthesia and subjects were questioned about discomfort until contact was lost |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain (pain in response to questioning only without any behavioural signs) 2 = moderate pain (pain in response to questioning and accompanied by behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Tham 1995.
| Methods | Single‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group PL1 = 31.8 ± 10.3, Group PL2 = 34.6 ± 11.3, Group PL3 = 36.1 ± 11.7, Group PL4 = 31.0 ± 8.9. Group PS1 = 32.9 ± 9.3, Group PS2 = 31.5 ± 10.3, Group PS3 = 36.2 ± 10.9, Group PS4 = 29 ± 9.3 Gender (M:F):Group PL1 = 7:12, Group PL2 = 9:14, Group PL3 = 11:14, Group PL4 = 7:15, Group PS1 = 6:15, Group PS2 = 10:8, Group PS3 = 8:21, Group PS4 = 7:19 Inclusion criteria: ASA I or II , aged 15 years to 65 years undergoing elective surgery Exclusion criteria: N/A Recruitment: 183 patients were randomly assigned. Setting: Singapore |
|
| Interventions |
Admixture Patients were randomly allocated to one of eight groups. The first four group (PL1 to 4) received mixtures of propofol with varying amounts of 1% lidocaine and the other four groups (PS1 to 4) receiving mixtures of propofol with equal volumes of saline PL1 (n = 19) propofol 19 ml + 1% lidocaine 1 ml (10 mg) PL2 (n = 23) propofol 18 ml + 1% lidocaine 2 ml (20 mg) PL3 (n = 25) propofol 17 ml + 1% lidocaine 3 ml (30 mg) PL4 (n = 22) propofol 16 ml + 1% lidocaine 4 ml (40 mg) PS1 (n = 21) propofol 19 ml + saline 1 ml PS2 (n = 18) propofol 18 ml + saline 2 ml PS3 (n = 29) propofol 17 ml + saline 3 ml PS4 (n = 26) propofol 16 ml + saline 4 ml |
|
| Outcomes | Pain intensity assessed on 3‐point scale 1 = no pain (no pain when asked 15 sec after start of injection) 2 = mild pain (complaint of pain when asked 15 sec after start of injection) 3 = severe pain (spontaneous complaint of pain by patient associated with grimacing or withdrawal of hand during injection) Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated to one of eight groups." |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The propofol was administered by the same person who prepared the mixture." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A separate investigator, blinded to the mixture given, noted the presence of excitation and pain." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Walker 2011.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Control group = 53 ± 14, Pretreatment group = 52 ± 17, Admixture group = 54 ± 14 Gender (M:F): Control group = 21:29, Pretreatment group = 25:26, Admixture group = 28:22 Inclusion criteria: age 18 years to 80 years old, ASA I‐II, ambulatory surgery with propofol induction of general anaesthesia Exclusion criteria: weight < 40 kg or > 100 kg; history of opioid, benzodiazepine, nonsteroidal anti‐inflammatory drug, or acetaminophen use within the past month; allergy to propofol, bisulphite, eggs, or lidocaine; or the presence of pre‐existing, potentially distracting pain Recruitment: 199 adult patients were randomly assigned (151 patients were analysed) Setting: USA |
|
| Interventions |
Pretreatment with venous occlusion Admixture A control group (saline pretreatment/saline admixture; n = 50) A pretreatment group (lidocaine 50 mg pretreatment/saline admixture; n = 51) An admixture group (saline pretreatment/lidocaine 50 mg admixture; n = 50) |
|
| Outcomes | Pain intensity assessed on 11‐point VPS (0 = no pain, 10 = worst pain imaginable) Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sequentially numbered sealed envelopes that contained a computer‐generated assignment." |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered sealed envelopes that contained a computer‐generated assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "An anaesthesiologist not involved with the study prepared 2 syringes, thereby blinding both subject and investigator to syringe contents." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An anaesthesiologist not involved with the study prepared 2 syringes, thereby blinding both subject and investigator to syringe contents." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "After initial allocation of 43 subjects, our hospital formulary changed from 1% propofol solution with bisulphite preservative to 1% propofol solution with benzyl alcohol, which has mild local anaesthetic properties. Although the nearly equal randomisation of these 43 subjects might have mitigated substantive effect on our overall results, the uncertainty of benzyl alcohol’s effect, particularly on the control group, led us to exclude these subjects from further analysis. A new randomisation was created for subsequent subject recruitment and five patients were excluded due to protocol violation. Ultimately, 151 subjects (all having received propofol with benzyl alcohol) completed analysis (51 in the pretreatment group and 50 each in the control and admixture groups)." |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | This study was supported by departmental funds The study appears to be free of other sources of bias |
Wong 2001.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Placebo = 39.3 ± 15.8, Tramadol = 37.4 ± 15.1, Lidocaine = 36.2 ± 12.9 Gender (M:F): Placebo = 14:16, Tramadol = 15:15, Lidocaine = 17:13 Inclusion criteria: age 18 years to 60 years old, ASA I‐II Exclusion criteria: N/A Recruitment: 90 adult patients randomly assigned (30 in each group) Setting: Singapore |
|
| Interventions |
Pretreatment with venous occlusion Group 1 received 5 ml of normal saline as placebo Group 2 received 5 ml (50 mg) of tramadol as the test drug Group 3 was administered 5 ml (50 mg) of lidocaine pretreatment with venous occlusion Propofol 2.5mg/kg was then administered as a bolus dose over 30 seconds |
|
| Outcomes | Pain intensity assessed on 3‐point scale 0 = none 1 = mild pain 2 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomly selected using a coded syringe method into three groups of 30 each." |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | N/A |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Yew 2005.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group A = 37 ± 16, Group B = 38 ± 10, Group C = 39 ± 10 Gender (M:F): Group A = 8:17, Group B = 9:16, Group C = 7:18 Inclusion criteria: Aae 18 years to 60 years old, ASA I‐II, elective surgery Exclusion criteria: patients with ischaemic heart disease and neurological problems, pregnant or lactating patients, those who were taking any analgesics before surgery, or those with known hypersensitivity to propofol or to any of the constituents of the emulsion (soy‐bean oil, MCT, glycerol, egg lecithin, sodium oleate, water for injection) Recruitment: 75 adult patients randomly assigned (25 in each group) Setting: Singapore |
|
| Interventions |
Admixture Group A received propofol formulated with LCT premixed with lidocaine (i.e., 2 ml of 1% lidocaine in 20 ml of propofol) Group B received propofol‐LCT/MCT premixed with 2 ml normal saline Group C received propofol‐LCT/MCT premixed with lidocaine (2 ml of 1% lidocaine in 20 ml of propofol) |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = none 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were assigned to 3 groups by computer‐generated randomization." |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The drugs were prepared by one of the investigators, with both the patient and an independent observer (a trainee anaesthesiologist) blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The drugs were prepared by one of the investigators, with both the patient and an independent observer (a trainee anaesthesiologist) blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Yokota 1997.
| Methods | Randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group A = 54 ± 12, Group B = 44 ± 19, Group C = 52 ± 21 Gender (M:F): Group A = 17:13, Group B = 11:19, Group C = 13:17 Inclusion criteria: ASA I‐II, elective surgery Exclusion criteria: N/A Recruitment: 90 adult patients randomly assigned (30 in each group) Setting: Japan |
|
| Interventions |
Admixture Patients were randomly assigned to one of three groups: Group A, pretreatment with a bioclusive dressing on the dorsum of hand 120 min before induction + received propofol mixed NSS 2 ml Group B, pretreatment with 60% lidocaine tape on the dorsum of hand 120 min before induction + received propofol mixed NSS 2 ml Group C pretreatment with a bioclusive dressing on the dorsum of hand 120 min before induction + received propofol premixed with 2% lidocaine 2 ml (40 mg) |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = none 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were allocated randomly to one of three groups." |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The anaesthesiologists in the theatre did not know the group of the patients." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Zahedi 2009.
| Methods | Double‐blind randomized controlled trial | |
| Participants | Age (years, mean ± SD): Group NS = 28.9 ± 7.4, Group L = 29.7 ± 6.8, Group K50 = 29.4 ± 7.8, Group K75 = 29.7 ± 8.8, Group K100 = 30.7 ± 6.6 Gender (M:F): Group NS = 55:45, Group L = 51:49, Group K50 = 48:52, Group K75 = 56:44, Group K100 = 49:51 Inclusion criteria: age 18 years to 40 years old, ASA I‐II, elective strabismus surgery Exclusion criteria: patients taking sedatives or analgesics in the past 24 hours before surgery and those with history of allergic reactions to anaesthetic drugs, neurologic or cardiovascular disease and pregnant patients Recruitment: 500 adult patients randomly assigned (100 in each group) Setting: Iran |
|
| Interventions |
Pretreatment alone Patients were randomly allocated into five groups: patients received normal saline (Group NS) lidocaine 1 mg/kg (Group L) different doses of ketamine, 50 μg/kg, 75 μg/kg or 100 μg/kg (Group K50, K75, K100 respectively), pretreated immediately before the injection of 2.5 mg/kg propofol Each patient’s pain scores were measured at five‐second intervals by a blinded anaesthesiologist |
|
| Outcomes | Pain intensity assessed on 4‐point scale 0 = none 1 = mild pain 2 = moderate pain 3 = severe pain Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes | Period of the study: dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | N/A |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Study drugs were diluted with NS 0.9% up to 5cc and were prepared by an investigator not involved in drug injection or assessment of patients' responses." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A blinded anaesthesiologist before the administration of propofol asked the patient to rate any sensation of pain." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | N/A |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Abbreviations used in tables: ASA = American Society of Anesthesiologists physical status classification ENT = Ear Nose Throat hr = hour kg = kilograms LCT = Long‐chain triglyceride MCT = Medium‐chain triglyceride μg = micrograms mg = milligrams ml = millilitres min = minutes n o = number N/A = not available NSAIDs = Non‐steroidal antiinflammatory drugs sec = seconds yr = years
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barbi 2003 | The participants were children aged 1 year to 18 years |
| Beh 2002 | The participants were children aged 3 years to 12 years |
| Brock 2010 | No placebo‐controlled group |
| Cameron 1992 | The participants were children aged 1 year to 10 years |
| Chaudhary 2013 | No placebo‐controlled group |
| Cheng 1998 | The participants were children aged three years to six years |
| Depue 2013 | The participants were children aged two years to seven years |
| Ewart 1990 | Letter to editor, non RCT |
| Fujii 2004 | The study has been retracted |
| Fujii 2005a | The study has been retracted |
| Fujii 2005b | The study has been retracted |
| Fujii 2006 | The study has been retracted |
| Fujii 2007 | No placebo‐controlled group |
| Fujii 2008 | The study has been retracted |
| Fujii 2009 | The study has been retracted |
| Fujii 2011 | The study has been retracted |
| Hiller 1992 | The participants were children with a mean age of 4.3 +/‐ 0.6 years |
| Kaabachi 2007 | The participants were children aged 1 year to 12 years |
| Kwak 2009 | The participants were children aged 3 years to 10 years |
| Lee 2004 | No placebo‐controlled group Participants were divided into two treatment groups of 50 participants each: 4 ml 1% lidocaine pre‐treatment followed by propofol and 2 ml saline, or 4 ml saline followed by propofol and 2 ml 2% lidocaine |
| Lembert 2002 | The participants were children aged more than five years |
| Massad 2008 | No placebo‐controlled group The participants were divided into three groups. All three groups had propofol 1% infusion at a constant rate after applying venous occlusion with lidocaine. The occlusion was applied for 15 seconds (group I, n = 50), 30 seconds (group II, n = 50) and 60 seconds (group III, n = 50) |
| Morton 1990 | The participants were children aged 1 year to 10 years |
| Nyman 2005 | The participants were children aged 2 years to 18 years |
| Nyman 2006 | The participants were children aged 2 years to 16 years |
| Pollard 2002 | The participants were children aged 1 year to 10 years |
| Rahman 2007 | The participants were children aged 5 years to 12 years |
| Rochette 2008 | The participants were children aged less than seven years |
| Roehm 2003 | The study has been retracted since 2011 |
| So 2013 | The study reported non‐relevant outcomes |
| Valtonen 1989 | The participants were children aged 3 years to 10 years. |
Abbreviations used in tables: n = number
Characteristics of studies awaiting assessment [ordered by study ID]
Alipour 2014.
| Methods | Randomized controlled trial |
| Participants | Age 20 years to 50 years, ASA class I and II, elective orthopedic surgery Exclusion criteria: allergy to propofol, thin veins in back of the hand, severe mental and neurological disorders, neuromuscular diseases, heart disease, convulsion, pregnancy and breast‐feeding, and BMI above 30 Recruitment: 336 participants were randomly assigned (56 in each group) Setting: Iran |
| Interventions |
Pretreatment with venous occlusion The studied drug was prepared and encoded in 5 cc volume by the nurse who was blinded to the study groups. Afterwards the studied drugs including Paracetamol 2 mg/kg (group P) Magnesium sulfate 2 mmol (group M) Ondansetron 4 mg (group O) Granisetron 2 mg (group G) Lidocaine 40 mg (group L) Saline solution (group S) were injected with an equal volume of 5 cc, before propofol injection. After 60 seconds, the tourniquet was opened and one quarter of the total dose of propofol 2.5 mg/kg (Germany model, Hamburg, Fresenius Kabi) was injected with a flow rate of 4 mg/sec. |
| Outcomes | Pain intensity assessed on 4‐point scale 0 = none 1 = low pain 2 = moderate pain 3 = severe pain Other outcomes: incidence of pain |
| Notes |
Byon 2014.
| Methods | Double‐blind randomized controlled trial |
| Participants | Age 20 years to 65 years, ASA I or II, injected with propofol for general anaesthesia Exclusion criteria: ischaemic heart diseases, neurologic problems, pregnancy, breast‐feeding women, administration of analgesics within 24 hours before surgery, allergic to propofol or its contents (soy‐bean oil, MCT, glycerol, egg lecithin, and sodium oleate), and allergic to lidocaine. Recruitment: 234 participants were randomly assigned (117 participants in each group) Setting: Korea |
| Interventions |
Pretreatment alone Admixture The pretreatment group was injected with a 40 mg solution of 2% lidocaine at the distal‐most part of the intravenous line, flushed with 3 ml of normal saline. One minute later, propofol 2 mg/kg with 2 ml of normal saline solution was injected The premixed group was pretreated with a tourniquet with 2 ml of normal saline, then flushed with 3 ml of normal saline. Then one minute later, propofol 2 mg/kg with a 40 mg solution of 2% lidocaine was injected All of the agents were injected at the same rate of 1 ml/sec, as the drug infusion rate may affect the incidence of propofol injection pain |
| Outcomes | Pain intensity assessed on 3‐point scale No pain Minor pain criteria included moaning and frowning Major pain criteria included verbal expressions of pain and movement of hand with a look of withdrawing from pain Other outcomes: pain incidence, adverse effects |
| Notes | There was no control group |
Galgon 2015.
| Methods | prospective, double‐blind, placebo‐controlled, randomized trial |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes |
Gharavi 2014.
| Methods | Randomized controlled trial |
| Participants | Age 4 years to 8 years, ASA I‐II, elective surgery Exclusion criteria: contraindication to use propofol or lidocaine, participants with thrombophlebitis, analgesics administration 24 hours prior to the operation and severe mental and neurological disease and neuromuscular disease Recruitment: 100 participants were randomly assigned (50 participants in each group) Setting: Iran |
| Interventions |
Pretreatment with venous occlusion Subjects were divided randomly into two equal groups A and B including 50 participants in each group, who were injected with lidocaine solutions 2% and 0.4% respectively. Dose of lidocaine was 1 mg/kg diluted with normal saline. All participants received their shots in their left antecubital vein with a tourniquet tied on their upper arm, which was removed 30 seconds following the lidocaine solution injection. We then started to administer propofol 1 mg/kg in 5 – 10 seconds, (Don Kook, Korea). Only 1/4 of the entire drug solution was initially administered and the rest was given after participant's pain evaluation based on VSD (verbal descriptor scale) and NRS (Numeric Rating Scale) using participant's verbal reaction and behaviour namely fretting, hand drag and tearing |
| Outcomes | Pain intensity assessed on NRS (Numeric Rating Scale) 1‐3 = mild pain 4‐6 = moderate pain 7‐10 = severe pain Other outcomes: pain incidence |
| Notes | The participants were children There was no control group |
Goktug 2015.
| Methods | Randomized controlled trial |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | The article is in Spanish. Abstract is in English. |
Kim 2014a.
| Methods | Double‐bind, randomized controlled trial |
| Participants | Age 20 years to 60 years, ASA I‐II, elective surgery Exclusion criteria: participants with a history of neurological problems or allergies and those who had taken medications including sedatives and analgesics within 24 hours of surgery, participants with a body weight of < 55 kg Recruitment: 69 participants were randomly assigned (68 participants were analysed) Setting: Korea |
| Interventions |
Pretreatment with venous occlusion Group L40: 40 mg lidocaine (n = 22) Group L60: 60 mg lidocaine (n = 23) Group L80: 80 mg lidocaine (n = 23) The tourniquet was released after 1 min and microemulsion propofol was administered through the same venous cannula to achieve a target effect‐site concentration of 5.2 mg/ml at a maximum flow rate of 750 ml/h, which represented a bolus of 120 mg over 60 sec for a 66 kg participant (the mean weight of the study participants) approximating to an infusion rate of 2 mg/kg per min. A placebo group was not included for ethical reasons. To preserve blinding, normal saline (0.9%) was added to give a total volume of study medication of 4 ml in groups L40 and L60. |
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Other outcomes: pain incidence, adverse effects |
| Notes | There was no control group |
Kim 2014b.
| Methods | Double‐blind randomized controlled trial |
| Participants | Age 18 years to65 years, ASA I‐II, elective surgery Exclusion criteria: study refusal; allergy to propofol or egg; Mallampati class III–IV; limited neck mobility; history of difficult intubation; history of cardiovascular, respiratory, neurological, neuromuscular or psychiatric disease. Recruitment: 200 participants were randomly assigned (50 participants each group) Setting: Korea |
| Interventions |
Pretreatment with venous occlusion A venous tourniquet was applied just above the elbow and the pretreatment drug was administered in a double‐blind manner Control group, pretreatment with normal saline Lidocaine group, pretreatment with 0.5 mg/kg preservative‐free lidocaine Cisatracurium 0.03 mg/ kg group, pretreatment with 0.03 mg/kg cisatracurium Cisatracurium 0.15 mg/kg group, pretreatment with 0.15 mg/kg cisatracurium The tourniquet was released after 30 sec, then 0.5 mg/kg propofol was delivered via the intravenous cannula |
| Outcomes | Pain intensity assessed on 4‐point scale 0 = none 1 = mild pain (pain reported only in response to questioning and without behavioural signs) 2 = moderate pain (pain reported in response to questioning and with behavioural signs, or pain reported without questioning) 3 = severe pain (strong vocal or behavioural response) Other outcomes: pain incidence, adverse effects |
| Notes |
Le Guen 2014.
| Methods | Multicenter, double‐blind, randomized controlled trial |
| Participants | ASA I‐III, elective surgery Exclusion criteria: undergo cardiac surgery or cranial neurosurgery, used psychotropic drugs, had supraspinal neurologic disorders, or used a pacemaker Recruitment: 227 participants were randomly assigned (217 participants were analysed) Setting: France |
| Interventions |
Admixture Participants were randomly allocated to one of six groups 45 ml of three different propofol 1% formulations, Diprivan®, Propoven®, or Lipuro®, were compared during induction of anaesthesia premixed with either 5 ml of placebo (saline solution) or 1% lidocaine (50 mg) Diprivan® with saline (n = 39) Diprivan® with lidocaine (n = 40) Propoven® with saline (n = 34) Propoven® with lidocaine (n = 36) Lipuro® with saline (n = 36) Lipuro® with lidocaine (n = 32) |
| Outcomes | Pain intensity assessed on 7‐point scale by the sum of these three clinical parameters (range, 0–6). facial expression (0: none, 1: frowning, or 2: grimacing) verbal response (0: none, 1: groan, or 2: clear verbal pain expression) attempt to withdraw the infused arm (0: none, 1: moderate, or 2: strong) Other outcomes: adverse effects |
| Notes | Pain intensity was presented in mean and SD No data of incidence of pain |
Lee 2011.
| Methods | Randomized controlled trial |
| Participants | Age 20 years to 60 years, ASA I‐II, elective surgery Exclusion criteria: experience of hypersensitivity to local anaesthetics and antiemetics; participants who had asthma, neurological disorders, or took analgesics or sedatives within 24 hours before surgery; and who had weak or thin blood vessels into which drug is injected were excluded. Recruitment: 200 participants were randomly assigned Setting: Korea |
| Interventions |
Pretreatment with venous occlusion normal saline (Group N, n = 50) lidocaine 20 mg (Group L, n = 50) ramosetron 0.3 mg (Group R, n = 50) lidocaine 20 mg plus ramosetron 0.3 mg (Group LR, n = 50) diluted into a 5 ml solution. The occlusion was released after 30 seconds and one‐fourth of microemulsion propofol 2 mg/kg was injected over 10‐15 seconds |
| Outcomes | Pain intensity assessed on 4‐point scale 0 = none 1 = mild pain (mild movement or oral/facial response during injection) 2 = moderate pain (clear movement or oral/facial response during injection) 3 = severe pain (complaint of pain and withdrawal response of the upper extremities) Other outcomes: incidence of pain |
| Notes |
Singh 2014.
| Methods | Double‐blind, randomized controlled trial |
| Participants | ASA I‐II, elective surgery Exclusion criteria: participants having problems in communication and history of allergic response to either propofol or 5HT3 antagonists Recruitment: 120 participants were randomly assigned (40 participants in each group) Setting: India |
| Interventions |
Pretreatment with venous occlusion Group N received 2 ml of 0.9% saline Group L received 2 ml of 2% lidocaine Group R received 2 ml of ramosetron 0.3 mg Mid forearm was occluded manually before injection and released after 1 min and then one‐fourth of the total calculated dose of propofol‐LCT was injected over 5 sec |
| Outcomes | Pain intensity assessed on 4‐point scale 0 = none 1 = mild pain (pain reported only in response to questioning without any behavioural signs) 2 = moderate pain (pain reported in response to questioning and accompanied by a behavioural sign or pain reported spontaneously without questioning) 3 = severe pain (i.e. strong vocal response or response accompanied by facial grimacing, arm withdrawal, or tears) Other outcomes: pain incidence |
| Notes |
Terada 2014.
| Methods | Randomized controlled trial |
| Participants | Elective surgery Exclusion criteria: N/A Recruitment: 226 participants were randomly assigned Setting: Japan |
| Interventions |
Pretreatment alone A control group receiving no prophylactic intervention A cooling group receiving topical cooling A lidocaine group receiving 1 mg/kg lidocaine pretreatment A lidocaine plus cooling group receiving topical cooling and 1 mg/kg lidocaine pretreatment |
| Outcomes | Pain intensity assessed on 4‐point scale 0 = no pain 1 = mild pain 2 = moderate pain 3 = severe pain Other outcomes: pain incidence |
| Notes | The article is in Japanese. Abstract is in English |
Abbreviations used in tables: ASA = American Society of Anesthesiologists physical status classification BMI = body mass index cc = cubic centimetre hr = hour kg = kilograms LCT = Long‐chain triglyceride MCT = Medium‐chain triglyceride mg = milligrams ml = millilitres min = minutes Mmol = millimols N/A = not available NRS = Numeric Rating Scale SD = standard deviation sec = seconds 5HT3 antagonists = 5‐hydroxytryptamine receptor antagonists
Differences between protocol and review
We have changed the title from "Physicopharmacological interventions for reducing propofol‐induced pain on induction of anaesthesia in adults" (Euasobhon 2009) to "Lidocaine for reducing propofol‐induced pain on induction of anaesthesia in adults". The title was changed due to the changing of the scope of the review.
Types of participants: participants' age was changed from 15 years and above to 13 years and above because one of the included studies (DeSousa 2011) included participants aged 13 to 65, who were able to understand and rate the pain score according to the scoring system of the study. This makes this review more generalized, to cover adults and adolescents.
Types of interventions: In the protocol, we planned to retrieve all data containing physicopharmacological and other interventions versus placebo or no treatment for reducing pain on propofol injection. However, many of those methods had limitations in the number of studies, or the methods of administration were relatively different, making it difficult to make a comparison. The data were relatively large and could possibly be an overwhelming amount of information for readers. Therefore, we reduced the scope of the meta‐analysis to lidocaine versus placebo or no treatment, as lidocaine is popular, effective and widely available throughout the world for propofol‐induced pain prophylaxis. We found a significant number of studies using lidocaine to reduce propofol‐induced pain that can demonstrate strong evidence. Also, the studies on high‐intensity pain have never been reviewed. Therefore, this meta‐analysis will provide essential information on how to administer lidocaine and the dosage necessary for reducing propofol injection pain.
Regarding the search terms for the review: we have still used the same search terms we proposed in the protocol (Euasobhon 2009) and manually excluded the studies that did not use lidocaine. Our goal was to retrieve all data including lidocaine treatment for prevention of propofol injection pain. This is because the old search terms provided more studies for inclusion and prevented us from missing data, which can occur when some studies mainly presented the efficacy of interventions other than lidocaine for reducing propofol‐induced pain in their title. But we found lidocaine as a comparing group in the abstract of such studies.
Subgroup analysis: There is a limitation of data regarding pain intensity in different genders or age groups, speed of propofol injection and site of propofol injection. Therefore, subgroup analyses were changed to doses of pretreatment or admixture drugs and the application of a tourniquet above the injection site. This information is also helpful for practice
The primary outcome has changed from 'the intensity of pain on propofol injection' to 'the incidence of high‐intensity pain on propofol injection', as it explains our intention more clearly. We also changed the secondary outcomes from 'number of patients who had any adverse effect' to 'adverse effects' and from 'patient satisfaction score' to 'patient satisfaction'.
New authors have been added to the review team since the protocol was published (Euasobhon 2009): Sukanya Dej‐arkom joined in 2013; Arunotai Siriussawakul and Pisake Lumbiganon joined in early 2014.
Contributions of authors
Pramote Euasobhon (PE), Sukanya Dej‐arkom (SD), Arunotai Siriussawakul (AS), Saipin Muangman (SM), Wimonrat Sriraj (WS), Porjai Pattanittum (PP), Pisake Lumbiganon (PL)
Conceiving the review: PE, SM Co‐ordinating the review: PE Undertaking manual searches: PE, WS Screening search results: PE, SM Organizing retrieval of papers: PE, SM Screening retrieved papers against inclusion criteria: PE, SM Appraising quality of papers: PE, SM, SD Extracting data from papers: PE, SM, SD Writing to authors of papers for additional information: PE Providing additional data about papers: PE, SD Obtaining and screening data on unpublished studies: PE, SM Data management for the review: PE, SD Entering data into Review Manager (RevMan) (RevMan 2014): PE, SD RevMan statistical data: PE, PP Other statistical analysis not using RevMan: PE, PP Double entry of data: (data entered by person one: PE; data entered by person two:SD) Interpretation of data: PE, WS, PP, AS Statistical inferences: PE, PP, AS Writing the review: PE, WS, PP, AS, PL Securing funding for the review: none Performing previous work that was the foundation of the present study: none Guarantor for the review (one author): PE Person responsible for reading and checking review before submission: PE, WS, AS, PL
Sources of support
Internal sources
Faculty of Medicine, Siriraj Hospital, Mahidol University, Thailand.
Faculty of Public Health, Khon Kaen University, Thailand.
Faculty of Medicine, Khon Kaen University, Thailand.
External sources
Thai Cochrane Network, Thailand.
Declarations of interest
Pramote Euasobhon: none known
Sukanya Dej‐arkom: none known
Arunotai Siriussawakul: none known
Saipin Muangman: none known
Wimonrat Sriraj: none known
Porjai Pattanittum: none known
Pisake Lumbiganon: none known
Edited (no change to conclusions)
References
References to studies included in this review
Agarwal 2004a {published data only}
- Agarwal A, Ansari MF, Gupta D, Pandey R, Raza M, Singh PK, et al. Pretreatment with thiopental for prevention of pain associated with propofol injection. Anesthesia and Analgesia 2004;98:683‐6. [PUBMED: 14980919] [DOI] [PubMed] [Google Scholar]
Agarwal 2004b {published data only}
- Agarwal A, Dhiraaj S, Raza M, Singhal V, Gupta D, Ranjan R, et al. Pain during injection of propofol: the effect of prior administration of ephedrine. Anaesthesia and Intensive Care 2004;32(5):657‐60. [PUBMED: 15535489] [DOI] [PubMed] [Google Scholar]
Agarwal 2004c {published data only}
- Agarwal A, Dhiraj S, Raza M, Pandey R, Pandey CK, Singh PK, et al. Vein pretreatment with magnesium sulfate to prevent pain on injection of propofol is not justified. Canadian Journal of Anaesthesia 2004;51(2):130‐3. [PUBMED: 14766688] [DOI] [PubMed] [Google Scholar]
Agarwal 2004d {published data only}
- Agarwal A, Raza M, Dhiraaj S, Pandey R, Gupta D, Pandey CK, et al. Pain during injection of propofol: the effect of prior administration of butorphanol. Anesthesia and Analgesia 2004;99(1):117‐9. [PUBMED: 15281515] [DOI] [PubMed] [Google Scholar]
Ahmad 2013 {published data only}
- Ahmad S, Oliveira GS, Fitzgerald PC, McCarthy RJ. The effect of intravenous dexamethasone and lidocaine on propofol‐induced vascular pain: a randomized double‐blinded placebo‐controlled trial. Pain Research and Treatment 2013;2013:734531. [PUBMED: 23956857] [DOI] [PMC free article] [PubMed] [Google Scholar]
Akgun 2013 {published data only}
- Akgun SE, Titiz L, Akpek E, Arslan G. Pretreatment with a very low dose of intravenous esmolol reduces propofol injection pain. Agri 2013;25:13‐8. [PUBMED: 23588865] [DOI] [PubMed] [Google Scholar]
Aldrete 2010 {published data only}
- Aldrete JA, Otero P, Alcover J, Parietti A, Johnson SC, Montpetit FH, et al. Pain on injection from propofol may be avoided by changing its formulation. Acta Anaesthesiologica Scandinavica 2010;54(4):442‐6. [PUBMED: 20002361] [DOI] [PubMed] [Google Scholar]
Aouad 2007 {published data only}
- Aouad MT, Siddik‐Sayyid SM, Al‐Alami AA, Baraka AS. Multimodal analgesia to prevent propofol‐induced pain: pretreatment with remifentanil and lidocaine versus remifentanil or lidocaine alone. Anesthesia and Analgesia 2007;104:1540–4. [PUBMED: 17513655] [DOI] [PubMed] [Google Scholar]
Apiliogullari 2007 {published data only}
- Apiliogullari S, Keles B, Apiliogullari B, Balasar M, Yilmaz H, Duman A. Comparison of diphenhydramine and lidocaine for prevention of pain after injection of propofol: a double‐blind, placebo‐controlled, randomized study. European Journal of Anaesthesiology 2007;24(3):235‐8. [PUBMED: 17202008] [DOI] [PubMed] [Google Scholar]
Asik 2003 {published data only}
- Asik I, Yorukoglu D, Gulay I, Tulunay M. Pain on injection of propofol: comparison of metoprolol with lidocaine. European Journal of Anaesthesiology 2003;20(6):487‐9. [PUBMED: 12803269] [DOI] [PubMed] [Google Scholar]
Ayoglu 2007 {published data only}
- Ayoglu H, Altunkaya H, Ozer Y, Yapakci O, Cukdar G, Ozkocak I. Does dexmedetomidine reduce the injection pain due to propofol and rocuronium?. European Journal of Anaesthesiology 2007;24(6):541‐5. [PUBMED: 17241503] [DOI] [PubMed] [Google Scholar]
Azma 2004 {published data only}
- Azma T, Kawai K, Tamura H, Okada K, Okida M. Comparative benefit of preemptively applied thiopental for propofol injection pain: the advantage over lidocaine. Hiroshima Journal of Medical Sciences 2004;53(1):13‐6. [PUBMED: 15274426] [PubMed] [Google Scholar]
Bachmann‐Mennenga 2007 {published data only}
- Bachmann‐Mennenga B, Ohlmer A, Boedeker RH, Mann M, Muhlenbruch B, Heesen M. Preventing pain during injection of propofol: effects of a new emulsion with lidocaine addition. European Journal of Anaesthesiology 2007;24(1):33‐8. [PUBMED: 16824248] [DOI] [PubMed] [Google Scholar]
Barker 1991 {published data only}
- Barker P, Langton JA, Murphy P, Rowbotham DJ. Effect of prior administration of cold saline on pain during propofol injection. A comparison with cold propofol and propofol with lignocaine. Anaesthesia 1991;46(12):1069‐70. [PUBMED: 1781537] [DOI] [PubMed] [Google Scholar]
Batra 2005 {published data only}
- Batra YK, Al Qattan AR, Marzouk HM, Smilka M, Agzamov A. Ketamine pretreatment with venous occlusion attenuates pain on injection with propofol. European Journal of Anaesthesiology 2005;22(1):69‐70. [PUBMED: 15816578] [DOI] [PubMed] [Google Scholar]
Borazan 2010 {published data only}
- Borazan H, Erdem TB, Kececioglu M, Otelcioglu S. Prevention of pain on injection of propofol: a comparison of lidocaine with different doses of paracetamol. European Journal of Anaesthesiology 2010;27:253‐7. [PUBMED: 19696679] [DOI] [PubMed] [Google Scholar]
Burimsittichai 2006 {published data only}
- Burimsittichai R, Kumwilaisuk K, Charuluxananan S, Tingthanathikul W, Premsamran P, Sathapanawath N. Pain on injection of propofol: propofol LCT vs propofol MCT/LCT with or without lidocaine pretreatment. Journal of the Medical Association of Thailand 2006;89 Suppl 3:S86‐91. [PUBMED: 17722306] [PubMed] [Google Scholar]
Canbay 2008 {published data only}
- Canbay O, Celebi N, Arun O, Karagoz AH, Saricaoglu F, Ozgen S. Efficacy of intravenous acetaminophen and lidocaine on propofol injection pain. British Journal of Anaesthesia 2008;100(1):95‐8. [PUBMED: 17959585] [DOI] [PubMed] [Google Scholar]
Cheong 2002 {published data only}
- Cheong MA, Kim KS, Choi WJ. Ephedrine reduces the pain from propofol injection. Anesthesia and Analgesia 2002;95(5):1293‐6. [PUBMED: 12401613] [DOI] [PubMed] [Google Scholar]
DeSousa 2011 {published data only}
- DeSousa K, Ali MS. Sevoflurane to alleviate pain on propofol injection. Journal of Anesthesia 2011;25:879‐83. [PUBMED: 21881932] [DOI] [PubMed] [Google Scholar]
Dubey 2003 {published data only}
- Dubey PK, Prasad SS. Pain on injection of propofol: the effect of granisetron pretreatment. The Clinical Journal of Pain 2003;19(2):121‐4. [PUBMED: 12616182] [DOI] [PubMed] [Google Scholar]
El‐Radaideh 2007 {published data only}
- El‐Radaideh KM. Effect of pretreatment with lidocaine, intravenous paracetamol and lidocaine‐fentanyl on propofol injection pain. Comparative study [Efectos del tratamiento previo con lidocaína, paracetamol y lidocaína‐fentanil intravenosos en el dolor causado por la inyección de propofol. Estudio comparativo]. Revista Brasileira de Anestesiologia 2007;57(1):32‐8. [PUBMED: 19468616] [DOI] [PubMed] [Google Scholar]
Eriksson 1997 {published data only}
- Eriksson M, Englesson S, Niklasson F, Hartvig P. Effect of lignocaine and pH on propofol‐induced pain. British Journal of Anaesthesia 1997;78(5):502‐6. [PUBMED: 9175962] [DOI] [PubMed] [Google Scholar]
Gajraj 1996 {published data only}
- Gajraj NM, Nathanson MH. Preventing pain during injection of propofol: the optimal dose of lidocaine. Journal of Clinical Anesthesia 1996;8(7):575‐7. [PUBMED: 8910180] [DOI] [PubMed] [Google Scholar]
Ganta 1992 {published data only}
- Ganta R, Fee JP. Pain on injection of propofol: comparison of lignocaine with metoclopramide. British Journal of Anaesthesia 1992;69(3):316‐7. [PUBMED: 1389851] [DOI] [PubMed] [Google Scholar]
Gehan 1991 {published data only}
- Gehan G, Karoubi P, Quinet F, Leroy A, Rathat C, Pourriat JL. Optimal dose of lignocaine for preventing pain on injection of propofol. British Journal of Anaesthesia 1991;66(3):324‐6. [PUBMED: 2015149] [DOI] [PubMed] [Google Scholar]
Han 2010 {published data only}
- Han YK, Jeong CW, Lee HG. Pain reduction on injection of microemulsion propofol via combination of remifentanil and lidocaine. Korean Journal of Anesthesiology 2010;58(5):435‐9. [PUBMED: 20532050] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harmon 2003 {published data only}
- Harmon D, Rozario C, Lowe D. Nitrous oxide/oxygen mixture and the prevention of pain during injection of propofol. European Journal of Anaesthesiology 2003;20(2):158‐61. [PUBMED: 12622502] [DOI] [PubMed] [Google Scholar]
Haugen 1995 {published data only}
- Haugen RD, Vaghadia H, Waters T, Merrick PM. Thiopentone pretreatment for propofol injection pain in ambulatory patients. Canadian Journal of Anaesthesia 1995;42(12):1108‐12. [PUBMED: 8595686] [DOI] [PubMed] [Google Scholar]
Helbo‐Hansen 1988 {published data only}
- Helbo‐Hansen S, Westergaard V, Krogh BL, Svendsen HP. The reduction of pain on injection of propofol: the effect of addition of lignocaine. Acta Anaesthesiologica Scandinavica 1988;32(6):502‐4. [PUBMED: 3262980] [DOI] [PubMed] [Google Scholar]
Ho 1999 {published data only}
- Ho CM, Tsou MY, Sun MS, Chu CC, Lee TY. The optimal effective concentration of lidocaine to reduce pain on injection of propofol. Journal of Clinical Anesthesia 1999;11(4):296‐300. [PUBMED: 10470630] [DOI] [PubMed] [Google Scholar]
Honarmand 2008 {published data only}
- Honarmand A, Safavi M. Magnesium sulphate pretreatment to alleviate pain on propofol injection: A comparison with ketamine or lidocaine. Acute Pain 2008;10:23‐9. [DOI: 10.1016/j.acpain.2008.01.001] [DOI] [Google Scholar]
Hwang 2010 {published data only}
- Hwang I, Noh JI, Kim SI, Kim MG, Park SY, Kim SH, et al. Prevention of pain with the injection of microemulsion propofol: a comparison of a combination of lidocaine and ketamine with lidocaine or ketamine alone. Korean Journal of Anesthesiology 2010;59(4):233‐7. [PUBMED: 21057611] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeon 2012 {published data only}
- Jeon Y. Reduction of pain on injection of propofol: combination of nitroglycerin and lidocaine. Journal of Anesthesia 2012;26:728‐31. [PUBMED: 22526437] [DOI] [PubMed] [Google Scholar]
Johnson 1990 {published data only}
- Johnson RA, Harper NJ, Chadwick S, Vohra A. Pain on injection of propofol. Methods of alleviation. Anaesthesia 1990;45:439‐42. [PUBMED: 2200299] [DOI] [PubMed] [Google Scholar]
Karasawa 2000 {published data only}
- Karasawa F, Ehata T, Okuda T, Satoh T. Propofol injection pain is not alleviated by pretreatment with flurbiprofen axetil, a prodrug of a nonsteroidal antiinflammatory drug. Journal of Anesthesia 2000;14:135‐7. [PUBMED: 14564579] [DOI] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim HS, Cho KR, Lee JH, Kim YH, Lim SH, Lee KM, et al. Prevention of pain during injection of microemulsion propofol: application of lidocaine mixture and the optimal dose of lidocaine. Korean Journal of Anesthesiology 2010;59(5):310‐3. [PUBMED: 21179291] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2013a {published data only}
- Kim E, Kim CH, Kim HK, Kwon JY, Lee do W, Kim HY. Effect of nitrous oxide inhalation on pain after propofol and rocuronium injection. Journal of Anesthesia 2013;27:868‐73. [PUBMED: 23982855] [DOI] [PubMed] [Google Scholar]
Kim 2013b {published data only}
- Kim K, Sung Kim Y, Lee DK, Lim BG, Kim HZ, Kong MH, et al. Reducing the pain of microemulsion propofol injections: a double‐blind, randomized study of three methods of tourniquet and lidocaine. Clinical Therapeutics 2013;35:1734‐43. [PUBMED: 24161288] [DOI] [PubMed] [Google Scholar]
King 1992 {published data only}
- King SY, Davis FM, Wells JE, Murchison DJ, Pryor PJ. Lidocaine for the prevention of pain due to injection of propofol. Anesthesia and Analgesia 1992;74(2):246‐9. [PUBMED: 1731545] [DOI] [PubMed] [Google Scholar]
Koo 2006 {published data only}
- Koo SW, Cho SJ, Kim YK, Ham KD, Hwang JH. Small‐dose ketamine reduces the pain of propofol injection. Anesthesia and Analgesia 2006;103(6):1444‐7. [PUBMED: 17122220] [DOI] [PubMed] [Google Scholar]
Krobbuaban 2005 {published data only}
- Krobbuaban B, Diregpoke S, Kumkeaw S, Tanomsat M. Comparison of pain on injection of a small particle size‐lipid emulsion of propofol and standard propofol with or without lidocaine. Journal of the Medical Association of Thailand 2005;88(10):1401‐5. [PUBMED: 16519386] [PubMed] [Google Scholar]
Krobbuaban 2008 {published data only}
- Krobbuaban B, Siriwan D, Kumkeaw S, Tanomsat M, Jamjamrat G, Thanetses K, et al. Does addition of lidocaine to medium‐ and long‐chain triglyceride propofol emulsions significantly reduce pain on injection?. Journal of the Medical Association of Thailand 2008;91(3):383‐7. [PUBMED: 18575293] [PubMed] [Google Scholar]
Kwak 2007a {published data only}
- Kwak K, Chung H, Lim C, Han C, Choi G, Lim D, et al. A combination of lidocaine (lignocaine) and remifentanil reduces pain during propofol injection. Clinical Drug Investigation 2007;27(7):493‐7. [PUBMED: 17563129] [DOI] [PubMed] [Google Scholar]
Kwak 2007b {published data only}
- Kwak K, Kim J, Park S, Lim D, Kim S, Baek W, et al. Reduction of pain on injection of propofol: combination of pretreatment of remifentanil and premixture of lidocaine with propofol. European Journal of Anaesthesiology 2007;24(9):746‐50. [PUBMED: 17261216] [DOI] [PubMed] [Google Scholar]
Kwak 2008 {published data only}
- Kwak KH, Ha J, Kim Y, Jeon Y. Efficacy of combination intravenous lidocaine and dexamethasone on propofol injection pain: a randomized, double‐blind, prospective study in adult Korean surgical patients. Clinical Therapeutics 2008;30(6):1113‐9. [PUBMED: 18640467] [DOI] [PubMed] [Google Scholar]
Kwon 2012 {published data only}
- Kwon JS, Kim ES, Cho KB, Park KS, Park WY, Lee JE, et al. Incidence of propofol injection pain and effect of lidocaine pretreatment during upper gastrointestinal endoscopy. Digestive Diseases and Sciences 2012;57:1291‐7. [PUBMED: 22160549] [DOI] [PubMed] [Google Scholar]
Lee 1994 {published data only}
- Lee TW, Loewenthal AE, Strachan JA, Todd BD. Pain during injection of propofol. The effect of prior administration of thiopentone. Anaesthesia 1994;49(9):817‐8. [PUBMED: 7978145] [PubMed] [Google Scholar]
Liaw 1999 {published data only}
- Liaw WJ, Pang WW, Chang DP, Hwang MH. Pain on injection of propofol: the mitigating influence of metoclopramide using different techniques. Acta Anaesthesiologica Scandinavica 1999;43:24‐7. [PUBMED: 9926183] [DOI] [PubMed] [Google Scholar]
Lu 2013 {published data only}
- Lu Y, Ye Z, Wong GT, Dong C, Yu J. Prevention of injection pain due to propofol by dezocine: a comparison with lidocaine. Indian Journal of Pharmacology 2013;45:619‐21. [PUBMED: 24347773] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lyons 1996 {published data only}
- Lyons B, Lohan D, Flynn C, McCarroll M. Modification of pain on injection of propofol. A comparison of pethidine and lignocaine. Anaesthesia 1996;51:394‐5. [PUBMED: 8686833] [DOI] [PubMed] [Google Scholar]
Mallick 2007 {published data only}
- Mallick A, Elliot SC, Krishnan K, Vucevic M. Lidocaine is more efficient than the choice of propofol formulations to reduce incidence of pain on induction. European Journal of Anaesthesiology 2007;24:403‐7. [PUBMED: 17376253] [DOI] [PubMed] [Google Scholar]
Massad 2006 {published data only}
- Massad IM, Abu‐Ali HM, Abu‐Halaweh SA, Badran IZ. Venous occlusion with lidocaine for preventing propofol induced pain. A prospective double‐blind randomized study. Saudi Medical Journal 2006;27:997‐1000. [PUBMED: 16830018] [PubMed] [Google Scholar]
McCluskey 2003 {published data only}
- McCluskey A, Currer BA, Sayeed I. The efficacy of 5% lidocaine‐prilocaine (EMLA) cream on pain during intravenous injection of propofol. Anesthesia and Analgesia 2003;97:713‐4. [PUBMED: 12933391] [DOI] [PubMed] [Google Scholar]
McCulloch 1985 {published data only}
- McCulloch MJ, Lees NW. Assessment and modification of pain on induction with propofol (Diprivan). Anaesthesia 1985;40:1117‐20. [PUBMED: 3878103] [DOI] [PubMed] [Google Scholar]
McDonald 1996 {published data only}
- McDonald DS, Jameson P. Injection pain with propofol. Reduction with aspiration of blood. Anaesthesia 1996;51:878‐80. [PUBMED: 8882257] [DOI] [PubMed] [Google Scholar]
Minogue 2005 {published data only}
- Minogue SC, Sun DA. Bacteriostatic saline containing benzyl alcohol decreases the pain associated with the injection of propofol. Anesthesia and Analgesia 2005;100:683‐6. [15728052] [DOI] [PubMed] [Google Scholar]
Nakane 1999 {published data only}
- Nakane M, Iwama H. A potential mechanism of propofol‐induced pain on injection based on studies using nafamostat mesilate. British Journal of Anaesthesia 1999;83:397‐404. [PUBMED: 10655909] [DOI] [PubMed] [Google Scholar]
Nathanson 1996 {published data only}
- Nathanson MH, Gajraj NM, Russell JA. Prevention of pain on injection of propofol: a comparison of lidocaine with alfentanil. Anesthesia and Analgesia 1996;82:469‐71. [PUBMED: 8623944] [DOI] [PubMed] [Google Scholar]
Newcombe 1990 {published data only}
- Newcombe GN. The effect, on injection pain, of adding lignocaine to propofol. Anaesthesia and Intensive Care 1990;18:105‐7. [PUBMED: 2186654] [DOI] [PubMed] [Google Scholar]
Niazi 2005 {published data only}
- Niazi A, Galvin E, Elsaigh I, Wahid Z, Harmon D, Leonard I. A combination of lidocaine and nitrous oxide in oxygen is more effective in preventing pain on propofol injection than either treatment alone. European Journal of Anaesthesiology 2005;22:299‐302. [PUBMED: 15892409] [DOI] [PubMed] [Google Scholar]
Nicol 1991 {published data only}
- Nicol ME, Moriarty J, Edwards J, Robbie DS, A'Hern RP. Modification of pain on injection of propofol ‐ a comparison between lignocaine and procaine. Anaesthesia 1991;46:67‐9. [PUBMED: 1996763] [DOI] [PubMed] [Google Scholar]
Nishiyama 2005 {published data only}
- Nishiyama T. How to decrease pain at rapid injection of propofol: effectiveness of flurbiprofen. Journal of Anesthesia 2005;19:273‐6. [PUBMED: 16261462] [DOI] [PubMed] [Google Scholar]
O'Hara 1997 {published data only}
- O'Hara JR, Sprung J, Laseter JT, Maurer WG, Carpenter T, Beven M, et al. Effects of topical nitroglycerin and intravenous lidocaine on propofol‐induced pain on injection. Anesthesia and Analgesia 1997;84(4):865‐9. [PUBMED: 9085972] [DOI] [PubMed] [Google Scholar]
Ozgul 2013 {published data only}
- Ozgul U, Begec Z, Erdogan MA, Aydogan MS, Sanli M, Colak C. Effect of alkalinisation of lignocaine for propofol injection pain: a prospective, randomised, double‐blind study. Anaesthesia and Intensive Care 2013;41:501‐4. [PUBMED: 23808510] [DOI] [PubMed] [Google Scholar]
Pang 1998 {published data only}
- Pang WW, Mok MS, Huang S, Hwang MH. The analgesic effect of fentanyl, morphine, meperidine, and lidocaine in the peripheral veins: a comparative study. Anesthesia and Analgesia 1998;86(2):382‐6. [PUBMED: 9459253] [DOI] [PubMed] [Google Scholar]
Pang 1999 {published data only}
- Pang WW, Huang PY, Chang DP, Huang MH. The peripheral analgesic effect of tramadol in reducing propofol injection pain: a comparison with lidocaine. Regional Anesthesia and Pain Medicine 1999;24:246‐9. [PUBMED: 10338176] [DOI] [PubMed] [Google Scholar]
Parmar 1998 {published data only}
- Parmar AK, Koay CK. Pain on injection of propofol. A comparison of cold propofol with propofol premixed with lignocaine. Anaesthesia 1998;53:79‐83. [PUBMED: 9505748] [DOI] [PubMed] [Google Scholar]
Polat 2012 {published data only}
- Polat R, Aktay M, Ozlu O. The effects of remifentanil, lidocaine, metoclopramide, or ketamine pretreatment on propofol injection pain. Middle East Journal of Anesthesiology 2012;21:673‐7. [PUBMED: 23265029] [PubMed] [Google Scholar]
Reddy 2001 {published data only}
- Reddy MS, Chen FG, Ng HP. Effect of ondansetron pretreatment on pain after rocuronium and propofol injection: a randomised, double‐blind controlled comparison with lidocaine. Anaesthesia 2001;56:902‐5. [PUBMED: 11531681] [DOI] [PubMed] [Google Scholar]
Saadawy 2007 {published data only}
- Saadawy I, Ertok E, Boker A. Painless injection of propofol: pretreatment with ketamine vs thiopental, meperidine, and lidocaine. Middle East Journal of Anesthesiology 2007;19:631‐44. [PUBMED: 18044291] [PubMed] [Google Scholar]
Salman 2011 {published data only}
- Salman AE, Salman MA, Saricaoglu F, Akinci SB, Aypar U. Pain on injection of propofol: a comparison of methylene blue and lidocaine. Journal of Clinical Anesthesia 2011;23:270‐4. [PUBMED: 21663809] [DOI] [PubMed] [Google Scholar]
Scott 1988 {published data only}
- Scott RP, Saunders DA, Norman J. Propofol: clinical strategies for preventing the pain of injection. Anaesthesia 1988;43:492‐4. [PUBMED: 3261547] [DOI] [PubMed] [Google Scholar]
Sethi 2009 {published data only}
- Sethi N, Jayaraman L, Sethi M, Sharma S, Sood J. Prevention of propofol pain: a comparative study. Middle East Journal of Anesthesiology 2009;20(1):71‐4. [PUBMED: 19266829] [PubMed] [Google Scholar]
Shimizu 2005 {published data only}
- Shimizu T, Inomata S, Kihara S, Toyooka H, Brimacombe JR. Rapid injection reduces pain on injection with propofol. European Journal of Anaesthesiology 2005;22:394‐6. [PUBMED: 15918392] [DOI] [PubMed] [Google Scholar]
Sinha 2005 {published data only}
- Sinha PK, Neema PK, Rathod RC. Effect of nitrous oxide in reducing pain of propofol injection in adult patients. Anaesthesia and Intensive Care 2005;33:235‐8. [PUBMED: 15960407] [DOI] [PubMed] [Google Scholar]
Smith 1996 {published data only}
- Smith AJ, Power I. The effect of pretreatment with ketorolac on pain during intravenous injection of propofol. Anaesthesia 1996;51:883‐5. [PUBMED: 8882259] [DOI] [PubMed] [Google Scholar]
Tariq 2006 {published data only}
- Tariq MA, Kamran M. Incidence of pain on propofol injection and efficacy of addition of lignocaine or selecting big vein or both combined in reducing it: a randomized control trial. Journal of Postgraduate Medical Institute 2006;20(1):8‐11. [Google Scholar]
Tariq 2010 {published data only}
- Tariq MA, Wadood R, Kamran M. The effects of intravenous lignocaine on pain during injection of medium and long‐chain triglyceride propofol emulsions. Journal of Postgraduate Medical Institute 2010;24(3):222‐5. [Google Scholar]
Tham 1995 {published data only}
- Tham CS, Khoo ST. Modulating effects of lignocaine on propofol. Anaesthesia and Intensive Care 1995;23:154‐7. [PUBMED: 7793583] [DOI] [PubMed] [Google Scholar]
Walker 2011 {published data only}
- Walker BJ, Neal JM, Mulroy MF, Humsi JA, Bittner RC, McDonald SB. Lidocaine pretreatment with tourniquet versus lidocaine‐propofol admixture for attenuating propofol injection pain: a randomized controlled trial. Regional Anesthesia and Pain Medicine 2011;36:41‐5. [PUBMED: 21455088] [DOI] [PubMed] [Google Scholar]
Wong 2001 {published data only}
- Wong WH, Cheong KF. Role of tramadol in reducing pain on propofol injection. Singapore Medical Journal 2001;42:193‐5. [PUBMED: 11513054] [PubMed] [Google Scholar]
Yew 2005 {published data only}
- Yew WS, Chong SY, Tan KH, Goh MH. The effects of intravenous lidocaine on pain during injection of medium‐ and long‐chain triglyceride propofol emulsions. Anesthesia and Analgesia 2005;100:1693‐5. [PUBMED: 15920197] [DOI] [PubMed] [Google Scholar]
Yokota 1997 {published data only}
- Yokota S, Komatsu T, Komura Y, Nishiwaki K, Kimura T, Hosoda R, et al. Pretreatment with topical 60% lidocaine tape reduces pain on injection of propofol. Anesthesia and Analgesia 1997;85:672‐4. [PUBMED: 9296429] [DOI] [PubMed] [Google Scholar]
Zahedi 2009 {published data only}
- Zahedi H, Nikooseresht M, Seifrabie M. Prevention of propofol injection pain with small‐dose ketamine. Middle East Journal of Anesthesiology 2009;20(3):401‐4. [PUBMED: 19950734] [PubMed] [Google Scholar]
References to studies excluded from this review
Barbi 2003 {published data only}
- Barbi E, Marchetti F, Gerarduzzi T, Neri E, Gagliardo A, Sarti A, et al. Pretreatment with intravenous ketamine reduces propofol injection pain. Paediatric Anaesthesia 2003;13(9):764‐8. [PUBMED: 14617116] [DOI] [PubMed] [Google Scholar]
Beh 2002 {published data only}
- Beh T, Splinter W, Kim J. In children, nitrous oxide decreases pain on injection of propofol mixed with lidocaine. Canadian Journal of Anaesthesia 2002;49:1061‐3. [PUBMED: 12477679] [DOI] [PubMed] [Google Scholar]
Brock 2010 {published data only}
- Brock MF, Grace BE, Morley B, Hillegass G, Houle TT, Groban L. Does lidocaine more effectively prevent pain upon induction with propofol or etomidate when given preemptively than when mixed with the drug?. Journal of Clinical Anesthesia 2010;22(7):505‐9. [PUBMED: 21056806] [DOI] [PubMed] [Google Scholar]
Cameron 1992 {published data only}
- Cameron E, Johnston G, Crofts S, Morton NS. The minimum effective dose of lignocaine to prevent injection pain due to propofol in children. Anaesthesia 1992;47:604‐6. [PUBMED: 1626674] [DOI] [PubMed] [Google Scholar]
Chaudhary 2013 {published data only}
- Chaudhary K, Gupta P, Gogia AR. A prospective, randomized, double‐blind study to compare the efficacy of lidocaine + metoclopramide and lidocaine + ketamine combinations in preventing pain on propofol injection. Journal of Anesthesia 2013;27:402‐6. [PUBMED: 23233136] [DOI] [PubMed] [Google Scholar]
Cheng 1998 {published data only}
- Cheng KI, Tang CS, Chiu SL, Chen TI, Wang CJ, Fan KT, et al. Injection pain with propofol: the effectiveness of thiopentone on induction. The Kaohsiung Journal of Medical Sciences 1998;14:480‐5. [PUBMED: 9780597] [PubMed] [Google Scholar]
Depue 2013 {published data only}
- Depue K, Christopher NC, Raed M, Forbes ML, Besunder J, Reed MD. Efficacy of intravenous lidocaine to reduce pain and distress associated with propofol infusion in pediatric patients during procedural sedation. Pediatric Emergency Care 2013;29:13‐6. [PUBMED: 23283255] [DOI] [PubMed] [Google Scholar]
Ewart 1990 {published data only}
- Ewart MC, Whitwam JG. Prevention of pain during injection of propofol. Lancet 1990;335(8692):798‐9. [PUBMED: 1969548] [DOI] [PubMed] [Google Scholar]
Fujii 2004 {published data only}
- Fujii Y, Nakayama M. Reduction of Propofol‐Induced Pain through Pretreatment with Lidocaine and/or Flurbiprofen. Clinical Drug Investigation 2004;24:749‐53. [PUBMED: 17523738] [DOI] [PubMed] [Google Scholar]
Fujii 2005a {published data only}
- Fujii Y, Nakayama M. Efficacy of Lignocaine plus Ketamine at Different Doses in the Prevention of Pain Due to Propofol Injection. Clinical Drug Investigation 2005;25:537‐42. [PUBMED: 17532697] [DOI] [PubMed] [Google Scholar]
Fujii 2005b {published data only}
- Fujii Y, Nakayama M. A lidocaine/metoclopramide combination decreases pain on injection of propofol. Canadian Journal of Anaesthesia 2005;52:474‐7. [PUBMED: 15872124] [DOI] [PubMed] [Google Scholar]
Fujii 2006 {published data only}
- Fujii Y, Shiga Y. Influence of aging on lidocaine requirements for pain on injection of propofol. Journal of Clinical Anesthesia 2006;18:526‐9. [PUBMED: 17126782] [DOI] [PubMed] [Google Scholar]
Fujii 2007 {published data only}
- Fujii Y, Nakayama M. Prevention of pain due to injection of propofol with IV administration of lidocaine 40 mg + metoclopramide 2.5, 5, or 10 mg or saline: a randomized, double‐blind study in Japanese adult surgical patients. Clinical Therapeutics 2007;29:856‐61. [PUBMED: 17697904] [DOI] [PubMed] [Google Scholar]
Fujii 2008 {published data only}
- Fujii Y, Itakura M. Comparison of lidocaine, metoclopramide, and flurbiprofen axetil for reducing pain on injection of propofol in Japanese adult surgical patients: a prospective, randomized, double‐blind, parallel‐group, placebo‐controlled study. Clinical Therapeutics 2008;30:280‐6. [PUBMED: 18343266] [DOI] [PubMed] [Google Scholar]
Fujii 2009 {published data only}
- Fujii Y, Itakura M. A comparison of pretreatment with fentanyl and lidocaine preceded by venous occlusion for reducing pain on injection of propofol: a prospective, randomized, double‐blind, placebo‐controlled study in adult Japanese surgical patients. Clinical Therapeutics 2009;31:2107‐12. [PUBMED: 19922881] [DOI] [PubMed] [Google Scholar]
Fujii 2011 {published data only}
- Fujii Y, Itakura M. Efficacy of the lidocaine/flurbiprofen axetil combination for reducing pain during the injection of propofol. Minerva Anestesiologica 2011;77:693‐7. [PUBMED: 21364503] [PubMed] [Google Scholar]
Hiller 1992 {published data only}
- Hiller A, Saarnivaara L. Injection pain, cardiovascular changes and recovery following induction of anaesthesia with propofol in combination with alfentanil or lignocaine in children. Acta Anaesthesiologica Scandinavica 1992;36:564‐8. [PUBMED: 1514343] [DOI] [PubMed] [Google Scholar]
Kaabachi 2007 {published data only}
- Kaabachi O, Chettaoui O, Ouezini R, Abdelaziz AB, Cherif R, Kokki H. A ketamine‐propofol admixture does not reduce the pain on injection compared with a lidocaine‐propofol admixture. Paediatric Anaesthesia 2007;17:734‐7. [PUBMED: 17596218] [DOI] [PubMed] [Google Scholar]
Kwak 2009 {published data only}
- Kwak HJ, Min SK, Kim JS, Kim JY. Prevention of propofol‐induced pain in children: combination of alfentanil and lidocaine vs alfentanil or lidocaine alone. British Journal of Anaesthesia 2009;103:410‐2. [PUBMED: 19542104] [DOI] [PubMed] [Google Scholar]
Lee 2004 {published data only}
- Lee P, Russell WJ. Preventing pain on injection of propofol: a comparison between lignocaine pre‐treatment and lignocaine added to propofol. Anaesthesia and Intensive Care 2004;32:482‐4. [15675207] [DOI] [PubMed] [Google Scholar]
Lembert 2002 {published data only}
- Lembert N, Wodey E, Geslot D, Ecoffey C. Prevention of pain on injection with propofol in children: comparison of nitrous oxide with lidocaine. Annales Francaises d'Anesthesie et de Reanimation 2002;21:263‐70. [PUBMED: 12033094] [DOI] [PubMed] [Google Scholar]
Massad 2008 {published data only}
- Massad IM, Abu‐Ali HM, Al‐Ghanem SA, Badran IZ, Ammari BA, Daradkeh SS. Duration of venous occlusion with lidocaine for preventing propofol induced pain. Saudi Medical Journal 2008;29:971‐4. [PUBMED: 18626523] [PubMed] [Google Scholar]
Morton 1990 {published data only}
- Morton NS. Abolition of injection pain due to propofol in children. Anaesthesia 1990;45:70. [PUBMED: 2316858] [DOI] [PubMed] [Google Scholar]
Nyman 2005 {published data only}
- Nyman Y, Hofsten K, Georgiadi A, Eksborg S, Lonnqvist PA. Propofol injection pain in children: a prospective randomized double‐blind trial of a new propofol formulation versus propofol with added lidocaine. British Journal of Anaesthesia 2005;95:222‐5. [PUBMED: 15894560] [DOI] [PubMed] [Google Scholar]
Nyman 2006 {published data only}
- Nyman Y, Hofsten K, Palm C, Eksborg S, Lonnqvist PA. Etomidate‐Lipuro is associated with considerably less injection pain in children compared with propofol with added lidocaine. British Journal of Anaesthesia 2006;97:536‐9. [PUBMED: 16914464] [DOI] [PubMed] [Google Scholar]
Pollard 2002 {published data only}
- Pollard RC, Makky S, McFadzean J, Ainsworth L, Goobie SM, Montgomery CJ. An admixture of 3 mg x kg(‐1) of propofol and 3 mg x kg(‐1) of thiopentone reduces pain on injection in pediatric anesthesia. Canadian Journal of Anaesthesia 2002;49:1064‐9. [PUBMED: 12477680] [DOI] [PubMed] [Google Scholar]
Rahman 2007 {published data only}
- Rahman AA, Al‐Mujadi H, Petrova IM, Marzouk HM, Batra YK, Al‐Qattan AR. Prevention of pain on injection of propofol: a comparison of remifentanil with alfentanil in children. Minerva Anestesiologica 2007;73:219‐23. [PUBMED: 17159759] [PubMed] [Google Scholar]
Rochette 2008 {published data only}
- Rochette A, Hocquet AF, Dadure C, Boufroukh D, Raux O, Lubrano JF, et al. Avoiding propofol injection pain in children: a prospective, randomized, double‐blinded, placebo‐controlled study. British Journal of Anaesthesia 2008;101:390‐4. [PUBMED: 18567678] [DOI] [PubMed] [Google Scholar]
Roehm 2003 {published data only}
- Roehm KD, Piper SN, Maleck WH, Boldt J. Prevention of propofol‐induced injection pain by remifentanil: a placebo‐controlled comparison with lidocaine. Anaesthesia 2003;58:165‐70. [PUBMED: 12625310] [DOI] [PubMed] [Google Scholar]
So 2013 {published data only}
- So SY, Kim YH, Ko YK, Park SI, Pak HJ, Jung WS. Effect of lidocaine (40 mg) mixed to prevent injection pain of propofol on the intubating conditions and onset time of rocuronium. Korean Journal of Anesthesiology 2013;64:29‐33. [PUBMED: 23372883] [DOI] [PMC free article] [PubMed] [Google Scholar]
Valtonen 1989 {published data only}
- Valtonen M, Iisalo E, Kanto J, Rosenberg P. Propofol as an induction agent in children: pain on injection and pharmacokinetics. Acta Anaesthesiologica Scandinavica 1989;33:152‐5. [PUBMED: 2646847] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Alipour 2014 {published data only}
- Alipour M, Tabari M, Alipour M. Paracetamol, ondansetron, granisetron, magnesium sulfate and lidocaine and reduced propofol injection pain. Iranian Red Crescent Medical Journal 2014;16:e16086. [PUBMED: 24829787] [DOI] [PMC free article] [PubMed] [Google Scholar]
Byon 2014 {published data only}
- Byon HJ, Lee KW, Shim HY, Song JH, Jung JK, Cha YD, et al. Comparison of the preventive effects of pretreatment of lidocaine with a tourniquet and a premixed injection of lidocaine on propofol‐LCT/MCT injection pain. Korean Journal of Anesthesiology 2014;66:95‐8. [PUBMED: 24624265] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galgon 2015 {published data only}
- Galgon RE, Strube P, Heier J, Groth J, Wang S, Schroeder KM. Magnesium sulfate with lidocaine for preventing propofol injection pain: a randomized, double‐blind, placebo‐controlled trial. Journal of Anesthesia 2015;29:206‐11. [PUBMED: 25097088] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gharavi 2014 {published data only}
- Gharavi M, Sabzevari A, Ghorbanian E, Sajadi R, Akhondi M. Effect of lidocaine volume and concentration on preventing incidence and severity of propofol injection pain. Iranian Red Crescent Medical Journal 2014;16:e16099. [PUBMED: 24829788] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goktug 2015 {published data only}
- Goktug A, Gulec H, Takmaz SA, Turkyilmaz E, Basar H. Lidocaine alleviates propofol related pain much better than metoprolol and nitroglycerin. Brazilian Journal of Anesthesiology 2015;65:338‐42. [PUBMED: 26323730] [DOI] [PubMed] [Google Scholar]
Kim 2014a {published data only}
- Kim DH, Chae YJ, Chang HS, Kim JA, Joe HB. Intravenous lidocaine pretreatment with venous occlusion for reducing microemulsion propofol induced pain: comparison of three doses of lidocaine. The Journal of International Medical Research 2014;42:368‐75. [PUBMED: 24595146] [DOI] [PubMed] [Google Scholar]
Kim 2014b {published data only}
- Kim YH, Namgung J, Lim CH. Cisatracurium pretreatment with tourniquet reduces propofol injection pain: a double‐blind randomized controlled trial. The Journal of International Medical Research 2014;42:360‐7. [PUBMED: 24573971] [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee HY, Kim SH, So KY. Prevention of microemulsion propofol injection pain: a comparison of a combination of lidocaine and ramosetron with lidocaine or ramosetron alone. Korean Journal of Anesthesiology 2011;61:30‐4. [PUBMED: 21860748] [DOI] [PMC free article] [PubMed] [Google Scholar]
Le Guen 2014 {published data only}
- Guen M, Grassin‐Delyle S, Cornet C, Genty A, Chazot T, Dardelle D, et al. Comparison of the potency of different propofol formulations: a randomized, double‐blind trial using closed‐loop administration. Anesthesiology 2014;120:355‐64. [PUBMED: 24051391] [DOI] [PubMed] [Google Scholar]
Singh 2014 {published data only}
- Singh D, Jagannath S, Priye S, Shivaprakash, Kadli C, Reddy D. Prevention of propofol injection pain: Comparison between lidocaine and ramosetron. Journal of Anaesthesiology, Clinical Pharmacology 2014;30:213‐6. [PUBMED: 24803760] [DOI] [PMC free article] [PubMed] [Google Scholar]
Terada 2014 {published data only}
- Terada N, Takubo I, Fujinaka W, Takatori M. Effectiveness of local cooling and lidocaine administration for prevention of pain upon injection of propofol. Masui. The Japanese Journal of Anesthesiology 2014;63:836‐40. [PUBMED: 25199313] [PubMed] [Google Scholar]
Additional references
Adachi 2002
- Adachi H, Inagaki Y, Harada T, Tsubokura H, Otsuki A, Hirosawa J, et al. Effects of concentration and dosage of lidocaine on preventing the pain on injection of propofol.. Masui ‐ The Japanese Journal of Anesthesiology 2002;51(9):983‐7. [PUBMED: 12382386] [PubMed] [Google Scholar]
Brooker 1985
- Brooker J, Hull CJ, Stafford M. Effect of lignocaine on pain caused by propofol injection. Anesthesia 1985;55:41‐7. [PUBMED: 3871594] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc) available from www.gradepro.org. GRADEpro guideline development tool. McMaster University (developed by Evidence Prime, Inc) available from www.gradepro.org, 2015.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jalota 2011
- Jalota L, Kalira V, George E, Shi YY, Hornuss C, Radke O, et al. Prevention of pain on injection of propofol: systematic review and meta‐analysis. BMJ 2011;342:d1110. [PUBMED: 21406529] [DOI] [PubMed] [Google Scholar]
Kevin 2003
- Kevin JC, Eugene M, Charles V. Comparison of the effects of propofol versus thiopental induction of postoperative outcomes following surgical procedures longer than 2 hours. American Association of Nurse Anesthetists Journal 2003;71:215‐22. [PUBMED: 12847946] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Masaki 2003
- Masaki Y, Tanaka M, Nishikawa T. Physicochemical compatibility of propofol‐lidocaine mixture. Anesthesia and Analgesia 2003;97:1646‐51. [PUBMED: 14633535] [DOI] [PubMed] [Google Scholar]
Michael 1996
- Michael HN, Noor MG, John AR. Prevention of pain on injection of propofol: a comparison of lidocaine with alfentanil. Anesthesia and Analgesia 1996;82:469‐71. [PUBMED: 8623944] [DOI] [PubMed] [Google Scholar]
Picard 2000
- Picard P, Tramèr MR. Prevention of pain on injection with propofol: a quantitative systematic review. Anesthesia and Analgesia 2000;90:963–9. [PUBMED: 10735808 ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
von Baeyer 2006
- Baeyer CL. Children's self‐reports of pain intensity: scale selection, limitations and interpretation. Pain Research & Management 2006;11(3):157‐62. [PUBMED: 16960632] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Euasobhon 2009
- Euasobhon P, Muangman S, Sriraj W, Pattanittum P. Physicopharmacological interventions for reducing propofol‐induced pain on induction of anaesthesia in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007874] [DOI] [PMC free article] [PubMed] [Google Scholar]


