Abstract
Background
Supportive interventions such as serving meals in a dining room environment or the use of assistants to feed patients are frequently recommended for the management of nutritionally vulnerable groups. Such interventions are included in many policy and guideline documents and have implications for staff time but may incur additional costs, yet there appears to be a lack of evidence for their efficacy.
Objectives
To assess the effects of supportive interventions for enhancing dietary intake in malnourished or nutritionally at‐risk adults.
Search methods
We identified publications from comprehensive searches of the Cochrane Library, MEDLINE, Embase, AMED, British Nursing Index, CINAHL, SCOPUS, ISI Web of Science databases, scrutiny of the reference lists of included trials and related systematic reviews and handsearching the abstracts of relevant meetings. The date of the last search for all databases was 31 March 2013. Additional searches of CENTRAL, MEDLINE, ClinicalTrials.gov and WHO ICTRP were undertaken to September 2016. The date of the last search for these databases was 14 September 2016.
Selection criteria
Randomised controlled trials of supportive interventions given with the aim of enhancing dietary intake in nutritionally vulnerable adults compared with usual care.
Data collection and analysis
Three review authors and for the final search, the editor, selected trials from titles and abstracts and independently assessed eligibility of selected trials. Two review authors independently extracted data and assessed risk of bias, as well as evaluating overall quality of the evidence utilising the GRADE instrument, and then agreed as they entered data into the review. The likelihood of clinical heterogeneity amongst trials was judged to be high as trials were in populations with widely different clinical backgrounds, conducted in different healthcare settings and despite some grouping of similar interventions, involved interventions that varied considerably. We were only able, therefore, to conduct meta‐analyses for the outcome measures, 'all‐cause mortality', 'hospitalisation' and 'nutritional status (weight change)'.
Main results
Forty‐one trials (10,681 participants) met the inclusion criteria. Trials were grouped according to similar interventions (changes to organisation of nutritional care (N = 13; 3456 participants), changes to the feeding environment (N = 5; 351 participants), modification of meal profile or pattern (N = 12; 649 participants), additional supplementation of meals (N = 10; 6022 participants) and home meal delivery systems (N = 1; 203 participants). Follow‐up ranged from ‘duration of hospital stay’ to 12 months.
The overall quality of evidence was moderate to very low, with the majority of trials judged to be at an unclear risk of bias in several risk of bias domains. The risk ratio (RR) for all‐cause mortality was 0.78 (95% confidence interval (CI) 0.66 to 0.92); P = 0.004; 12 trials; 6683 participants; moderate‐quality evidence. This translates into 26 (95% CI 9 to 41) fewer cases of death per 1000 participants in favour of supportive interventions. The RR for number of participants with any medical complication ranged from 1.42 in favour of control compared with 0.59 in favour of supportive interventions (very low‐quality evidence). Only five trials (4451 participants) investigated health‐related quality of life showing no substantial differences between intervention and comparator groups. Information on patient satisfaction was unreliable. The effects of supportive interventions versus comparators on hospitalisation showed a mean difference (MD) of ‐0.5 days (95% CI ‐2.6 to 1.6); P = 0.65; 5 trials; 667 participants; very low‐quality evidence. Only three of 41 included trials (4108 participants; very low‐quality evidence) reported on adverse events, describing intolerance to the supplement (diarrhoea, vomiting; 5/34 participants) and discontinuation of oral nutritional supplements because of refusal or dislike of taste (567/2017 participants). Meta‐analysis across 17 trials with adequate data on weight change revealed an overall improvement in weight in favour of supportive interventions versus control: MD 0.6 kg (95% CI 0.21 to 1.02); 2024 participants; moderate‐quality evidence. A total of 27 trials investigated nutritional intake with a majority of trials not finding marked differences in energy intake between intervention and comparator groups. Only three trials (1152 participants) reported some data on economic costs but did not use accepted health economic methods (very low‐quality evidence).
Authors' conclusions
There is evidence of moderate to very low quality to suggest that supportive interventions to improve nutritional care results in minimal weight gain. Most of the evidence for the lower risk of all‐cause mortality for supportive interventions comes from hospital‐based trials and more research is needed to confirm this effect. There is very low‐quality evidence regarding adverse effects; therefore whilst some of these interventions are advocated at a national level clinicians should recognise the lack of clear evidence to support their role. This review highlights the importance of assessing patient‐important outcomes in future research.
Plain language summary
Supportive interventions for improving dietary intake in nutritionally vulnerable groups
Review question
Are supportive interventions for improving dietary intake in nutritionally vulnerable groups (malnourished or nutritionally at‐risk individuals) effective?
Background
Serving meals in a dining room, or the use of assistance to help feed people in need and other similar methods are often recommended to help especially sick and elderly people who have lost or are likely to lose weight (nutritionally vulnerable groups). Such supportive interventions are implemented in the health care in many countries but their effects are not well investigated.
Study characteristics
We included 41 randomised controlled studies (clinical studies where people are randomly put into one of two or more treatment groups) with a total of 10,681 people in our review. There were five different interventions which we call 'supportive interventions': changes to the organisation of nutritional care (13 studies, 3456 people), changes to the feeding environment (5 studies, 351 people), modification of the meal profile or pattern (12 studies, 649 people), additional supplementation of meals (10 studies, 6022 people) and home meal delivery systems (1 study, 203 people). Monitoring participants over time (follow‐up) ranged from ‘duration of hospital stay’ to 12 months. The comparator groups received 'usual' care. More than half of all participants took part in studies investigating the additional supplementation of meals (for example a protein‐energy oral nutritional supplement in addition to the usual diet).
Key results
It is possible that supportive interventions for enhancing dietary intake in nutritionally vulnerable groups reduce death from any cause (approximately 23 fewer cases of death per 1000 participants in favour of supportive interventions). However, this has to be confirmed by more evidence from high‐quality randomised controlled studies. The number of participants experiencing any medical complication did not differ substantially between the supportive interventions and the comparator groups. The same was found for health‐related quality of life (which is physical, mental, emotional and social health attributed to health), patient satisfaction, nutritional or energy intake and days spent in hospital. Economic costs were not well investigated.
Only three studies reported on side effects, describing intolerance to the nutritional supplement (such as diarrhoea or vomiting in 5 of 34 participants) and discontinuation of oral nutritional supplements because of refusal or dislike of taste (567 of 2017 participants).
After analysing 15 studies in 1945 participants we found a beneficial effect of supportive interventions compared with comparators on weight: on average people in the supportive interventions groups increased their weight 0.6 kg more than people in the comparator groups.
This evidence is up to date as of September 2016.
Quality of evidence
The overall quality of evidence ranged between moderate to very low, mainly because for most of our outcomes there was only a small number of studies and participants to achieve reliable information, or because risk of bias made results uncertain. However, if some randomised controlled studies with low risk of bias for our patient‐important outcomes and a good number of participants were performed, this review could quickly provide good guidance for better health care.
Summary of findings
Summary of findings for the main comparison. Supportive interventions for enhancing dietary intake versus comparators in malnourished or nutritionally at‐risk adults.
| Supportive interventions compared with usual care for malnourished or nutritionally at‐risk adults | ||||||
| Population: malnourished or nutritionally at‐risk adults Settings: residential care (21 trials), hospital (15 trials), outpatients (5 trials) Intervention: supportive interventions for enhancing dietary intake (changes to the organisation of nutritional care, changes to the feeding environment, modification of meal profile or pattern, additional supplementation of meals, congregate and home meal delivery systems) Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Usual care | Supportive interventions | |||||
| All‐cause mortality Follow‐up: duration of hospital stay to 12 months | 133 per 1000 | 107 per 1000 (92 to 124) | RR 0.78 (0.66 to 0.92) | 6683 (12) | ⊕⊕⊕⊝ moderatea | ‐ |
|
Morbidity/complications (number of participants with any medical complication) Follow‐up: duration of hospital stay to 6 months |
See comment | See comment | See comment | 4015 (5) | ⊕⊝⊝⊝ very lowb | No summary effect size calculated because of high inconsistency; RR ranged from 0.59 in favour of supportive interventions to 1.42 in favour of usual care |
|
Health‐related quality of life and patient satisfaction Follow‐up: duration of hospital stay to 12 months |
See comment | See comment | See comment | 4451 (5) | ⊕⊕⊝⊝ lowc | 5/41 trials investigated health‐related quality of life using different instruments in participants from a wide range of different clinical backgrounds; overall we noted no substantial differences between intervention and comparator groups 2/41 trials investigated patient satisfaction by means of an unvalidated questionnaire |
| Hospitalisation and institutionalisation (days) Follow‐up: 8 days to 4 months | The mean hospitalisation ranged across control groups from 10 days to 40 days | The mean hospitalisation in the intervention groups was 0.5 days shorter (2.6 days shorter to 1.6 days longer) | ‐ | 667 (5) | ⊕⊝⊝⊝ very lowd | 3/5 trials with data on hospitalisation were in the group of trials of 'Changes to the organisation of nutritional care' |
|
Adverse events Follow‐up: 8 days to 6 months |
See comment | See comment | See comment | 4108 (3) | ⊕⊝⊝⊝ very lowe | Only 3/41 trials reported on adverse events (all evaluating the impact of supplementation of meals with oral nutritional supplements); 1 trial reported intolerance to the supplement (diarrhoea, vomiting) in 3/34 (15%) of participants. In another large trial 565/2017 (28%) of stroke patients stopped taking the oral nutritional supplements because of refusal or dislike of taste |
| Nutritional status (weight change in kg) Follow‐up: 8 days to 12 months | The mean weight change ranged across control groups from ‐3.0 kg to +0.3 kg | The mean weight change in the intervention groups was +0.6 kg higher (0.2 kg to 1.0 kg higher) | ‐ | 2024 (17) | ⊕⊕⊕⊝ moderatef | ‐ |
|
Economic costs Follow‐up: duration of hospital stay to 12 months |
See comment | See comment | See comment | 1152 (3) | ⊕⊝⊝⊝ very lowg | 3/41 trials evaluated and 2/41 trials reported some data on economic costs; none of the trials used accepted health economic methods and the reported data on both costs and effectiveness were generally poor |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
*aAssumed risk was derived from the event rates in the comparator groups (usual care)
aDowngraded by one level because of risk of bias in several risk of bias domains bDowngraded by three levels because of risk of bias in several risk of bias domains, serious inconsistency and imprecision cDowngraded by two levels because of risk of bias in several risk of bias domains, indirectness and few trials investigating health‐related quality of life in substantially diverse trial populations dDowngraded by three levels because of risk of performance bias and serious imprecision eDowngraded by three levels because of risk of bias in several risk of bias domains, imprecision and general substandard reporting of adverse events in included trials fDowngraded by one level because of imprecision gDowngraded by three levels because of risk of bias in several risk of bias domains, imprecision and few trials investigating economic costs with poor reporting, not using accepted health economic methods
Background
Malnutrition in patients admitted to hospital was initially recognised in the 1970s (Butterworth 1974;McWhirter 1994). In recent years, malnutrition in the community has also been reported (Elia 2009). Whether in the hospital or the community, malnutrition is associated with poor clinical outcome, decreased health‐related quality of life and increased mortality (Kubrak 2007; Norman 2008; Stratton 2003).
Malnutrition is both a cause and consequence of ill health (Lean 2008) and its aetiology is complex. It predisposes to illness but is also a consequence of illness (NCCAC 2006), creating a vicious, self‐perpetuating cycle of malnutrition and infection (Scrimshaw 2003). People who are undernourished on admission to hospital, who do not receive adequate nutritional care, experience decline in their nutritional status (McWhirter 1994). While in hospital, the reasons for further poor intakes and subsequent weight loss may include temporary starvation for medical procedures, difficulty in feeding, lack of nursing supervision during mealtimes, depression, unpalatable foods and disease‐ or drug‐induced anorexia (Kelly 2000; Lennard‐Jones 1992). At home, in addition to the effects of illness and its management, sub‐optimal nutritional status may be due to practical challenges, such as lack of transport, difficulties in grocery shopping, or difficulties utilising cooking facilities, resulting in diets of poor nutritional quality. Social and psychological issues also have a significant impact. The factors that contribute to malnutrition in hospital and community patients have been described extensively elsewhere (Lennard‐Jones 1992; NCCAC 2006).
Nutrition intervention and treatment of malnutrition has been recommended in clinical guidelines from many countries based on associations between improved dietary intake and nutritional status, health‐related quality of life and functional outcomes (Mueller 2011; NCCAC 2006). Therefore, it is recommended that at the first sign of malnutrition or risk of malnutrition, a full nutritional assessment and appropriate nutritional intervention should follow (Mueller 2011; NCCAC 2006). As the causes of malnutrition are multifactorial, the interventions designed to treat malnutrition are likely to be complex. This merits an understanding of the multidimensional causes of malnutrition and the complex support strategies needed across a range of healthcare services from the strategic policy level down to the individual feeding of a patient (Weekes 2009).
Description of the condition
Despite the absence of universally accepted diagnostic criteria, a widely quoted definition describes malnutrition as the nutritional state in which an energy, protein or nutrient deficiency, excess or imbalance leads to adverse effects on body or tissue form (body shape, size and composition) and function, as well as clinical outcome (Elia 2003). The recently convened International Guideline Consensus Committee categorised malnutrition as, "starvation‐related malnutrition" in cases of chronic starvation in the absence of inflammation, "chronic disease‐related malnutrition" where there is chronic but mild‐to‐moderate inflammation and, "acute disease or injury‐related malnutrition" where there is acute severe inflammation (Jensen 2010). While this provides a useful aetiological classification of malnutrition and recognises the effect of illness on nutritional status, there remain no clear criteria for how each category might be identified in practice. Nutrition screening is often used to detect risk factors known to be associated with nutritional complications (McMahon 2000) such as recent, unintentional weight loss; inadequate food intake; disease‐related anorexia; low body weight, body mass index (BMI) or lean body mass; in order to decide whether a full nutritional assessment is indicated (Elia 2003). Nutrition screening tools commonly employ a standard pro forma to determine nutritional risk. The included parameters are intended to determine whether an individual is nutritionally at risk on the basis of a score, which determines the course of action (Green 2006; Jones 2002). Many tools suggest suitable action plans that may involve nutritional intervention. Nutritional assessment is a more comprehensive investigation including anthropometric measurements, biochemical tests, clinical examination and dietary intake monitoring, used to determine whether an individual is malnourished or likely to become malnourished (at risk of malnutrition) (Corish 2000a; McMahon 2000). Nutritional assessment is usually followed by appropriate nutritional intervention (Corish 2000a; McMahon 2000).
The absence of clear and universally accepted criteria for the diagnosis of malnutrition further complicates the interpretation of prevalence data and intervention trials. Major classic and more recent trials that assessed the prevalence of malnutrition in hospitals have estimated a prevalence of between 11% and 50% depending on the criteria used (Bistrian 1974; Corish 2000a; Corish 2000b; Edington 2000; Hill 1977; Kelly 2000; McWhirter 1994; Naber 1997). The variation in reports of prevalence result largely from differences in the definitions used to identify malnutrition across trials. In 2008, the nutrition screening week carried out by the British Association for Parenteral and Enteral Nutrition (BAPEN), which uses a standardised tool to assess nutritional risk status, demonstrated that malnutrition was present in nearly a third of people admitted to hospital, in just over a third of people admitted to care homes and in a fifth of people admitted to mental health units (Elia 2009). Furthermore, it has been estimated that at any given time over three million people in the UK are thought to be malnourished or at risk of malnutrition with the vast majority of these (93%) living at home (Elia 2009). In Australia, a survey that used a different nutrition screening tool to screen 3122 participants in the acute hospital setting, revealed that 41% of participants were "at risk" of malnutrition, with an overall prevalence of malnutrition of 32% (Agarwal 2011).
The clinical consequences of malnutrition are believed to include reduced muscle strength; failure of the respiratory, thermoregulatory, pancreatic, gastrointestinal, mental, endocrine, and cardiovascular systems; as well as impaired wound healing and poor clinical outcomes from surgical procedures or illness (Allison 2000; Corish 2000a; Lennard‐Jones 1992). Wounds that heal more slowly become much more vulnerable to infection. Immune function is impaired, compounding constraints on the body from other disease states, constituting a much reduced resistance to infection (Corish 2000a). Respiratory muscle wasting may also predispose to infections if patients are unable to cough and expectorate effectively (Lennard‐Jones 1992). Pressure sores may develop as mobility is reduced (Lennard‐Jones 1992) and as the body becomes thinner and wasted. Arguably, the effects of malnutrition on the musculoskeletal system extend beyond the gain or loss of lean body tissue, but may incur metabolic changes in cellular electrolytes including calcium accumulation, which may prevent optimal muscle function (Jeejeebhoy 1986). Furthermore, excretory systems may fail to regulate body sodium‐water balance efficiently and may result in excess fluid retention and oedema (Allison 2000), which has reportedly been detected in 17% of malnourished people admitted to hospital (Weekes 1999). As disease further impinges on appetite (Allison 2000), malnutrition will progress and the clinical implications aforementioned will occur much more quickly in ill people than in healthy individuals (Corish 2000a).
In addition to the clinical and social consequences, the economic impact of malnutrition is considerable. The increasing costs have become an economic burden for healthcare systems in many countries. Recent data from the UK suggest that malnutrition costs in excess of GBP 7.3 billion each year (EURO 8.74 billion/year ‐ December 2011 conversion) (DOH 2007; Russell 2007). Poor clinical outcomes, such as extended hospital stays, increased medical complications, reduced health‐related quality of life and slow disease recovery, all contribute to rising hospital and home care costs (Gallagher 1996; Russell 2007; Stratton 2003). Malnourished patients stay in hospital for longer, are three times more likely to develop complications during surgery and have a higher mortality than adequately nourished patients (DOH 2007). Furthermore, those considered at risk of malnutrition are much more likely to require home healthcare services after discharge from hospital than those considered not at risk (Chima 1997). Malnutrition in the community has also been shown to increase the need for healthcare resources such as general practitioner (GP) visits, hospital admissions and new prescriptions, in addition to contributing to an increased risk of mortality (Martyn 1998). Therefore, if healthcare economics is considered, an undernourished patient imposes a greater economic burden on health services than a patient whose nutritional status is well maintained (Lennard‐Jones 1992).
Description of the intervention
This review seeks to determine whether effective clinical management of malnutrition in both hospital and community settings requires more than just the provision of nutrients, dietary advice, or a combination, and whether additional strategies to support these existing approaches to ensure overall nutritional care is optimal, is worthy of consideration. The specific types of interventions considered are listed in Table 2. Related interventions include the sole use of oral nutritional supplements, dietary counselling or strategies, or a combination to manage malnutrition.
1. Intervention subcategories.
|
Supportive nutritional care intervention Broad intervention category |
Examples |
| 1. Changes to the organisation of nutritional care |
|
| 2. Changes to the feeding environment |
|
| 3. Modification of meal profile or pattern |
|
| 4. Additional supplementation of meals |
|
| 5. Congregate and home meal delivery systems |
|
Guidelines exist for the identification, regular monitoring and initiation of nutritional support in individuals who may be malnourished or at nutritional risk. These include UK clinical guidelines for nutritional screening and support in adults (NCCAC 2006), Essence of Care benchmarks for food and nutrition from the UK Department of Health (DOH 2003), and the American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines on nutrition screening, assessment and intervention in adults (Mueller 2011).
The strategies most frequently used to treat malnutrition in individuals requiring nutritional support aim to increase energy and nutrient intake by means of the following.
Dietary counselling – provision of nutritional advice to increase nutrient intake, requiring an individual to understand and act upon instructions given. This approach may include providing advice on food fortification, to increase the energy density of foods without increasing quantity, or dietary fortification, to increase the energy density of the diet by adding extra snacks or drinks between meals.
Oral nutritional supplements – available in either liquid or solid forms. These usually provide a mixture of macro‐ and micronutrients and may be nutritionally complete in a specified volume and are often available in the form of commercial supplement products.
Artificial nutrition support ‐ includes enteral tube feeds and parenteral nutrition that are used when oral intake is not possible.
The efficacy of nutritional support interventions has been the subject of much previous research but so far has focused primarily on the use of oral nutritional supplements, which may be applicable to only a minority of people (Weekes 2009). There are more than 20 systematic reviews in the literature of oral nutritional supplement‐based interventions in the management of malnutrition (Stratton 2007). The findings are variable with some reviews showing clinical and nutritional benefits (Stratton 2007). However, these findings are by no means consistent and the patient groups most likely to benefit from this type of intervention remain to be characterised (Stratton 2007). Despite this, there has been a consistent trend to use oral nutritional supplements in clinical practice but the high cost implications of this approach, especially in the community as recently highlighted in a UK report (LPP 2009), makes the consideration of alternative approaches worthwhile. There has been an increased focus on the routine provision of food and drink as part of nutritional care since the 10 key characteristics of good nutritional care in hospital were published (COE 2003). Forty‐five trials have examined the role of food‐based interventions with or without oral nutritional supplements in the management of poor dietary intake (Baldwin 2011). The findings suggested that although dietary counselling may result in improvements in weight, body composition and muscle function, trials were heterogeneous and of variable quality with no evidence of benefit on mortality (Baldwin 2011). These trials have concentrated on interventions that rely on the patient receiving and acting on instructions to enhance their nutritional intake (i.e. dietary counselling). Despite the body of clinical evidence supporting the appropriate use of oral nutritional supplements and previous research around dietary counselling, whether additional supportive interventions are clinically effective in the management of malnutrition or the risk of malnutrition, remains unknown.
The Council of Europe and the UK Department of Health highlighted the importance of overall nutritional care including, among other supportive initiatives: mandatory nutritional screening, adequate provision of food and drink, oral supplements, modified diets, assistance with feeding and changes to the dining environment (COE 2003; DOH 2007). Such interventions have been incorporated into guidelines and healthcare policies and aim to improve nutritional intake by modifying aspects of food provision (e.g. the use of protected mealtimes, red tray initiatives (to identify those requiring mealtime assistance) and feeding assistance) or by adjusting the portion size and nutrient content of foods and enhancing the flavour, however, evidence of benefit of such initiatives is lacking.
Adverse effects of the intervention
The possible adverse effects of the supportive nutritional care interventions considered in this review may include but are not limited to the following events: provision of incorrect nutritional supplement, provision of incorrect between‐meal snacks, gastrointestinal effects due to intolerance of supplements/extra snacks/drinks (e.g. bloating, vomiting or diarrhoea), potential accidents occurring as a result of the intervention such as a patient falling on the way to a dining area in a change of dining environment intervention, inappropriate moving and handling by untrained staff trying to obtain a weight or height measure, inappropriate screening or intervention (e.g. during end of life).
How the intervention might work
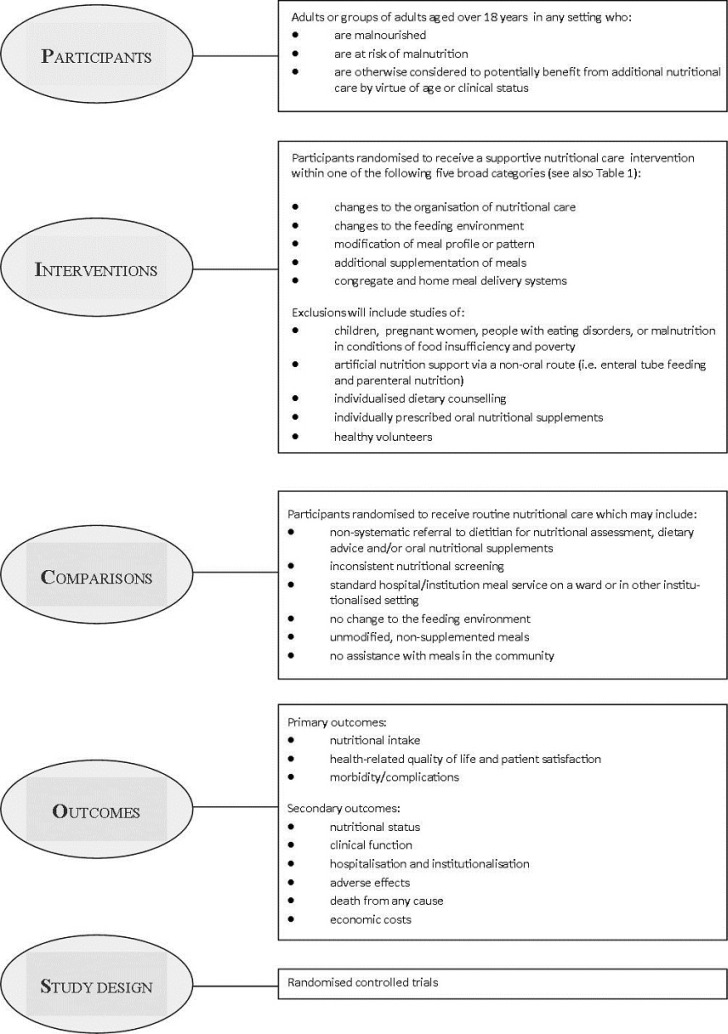
As recommended in the PRISMA statement (Liberati 2009), a conceptual framework highlighting the participants, interventions, comparisons, outcomes and trial design (PICOS) considered for this review, is illustrated (Figure 1).
1.
The treatment of malnutrition aims to reverse its effects, including the physical and functional impairments, and the provision of appropriate nutritional care may involve several approaches. The factors that influence our experiences with food are complex and nutritional care interventions aimed at the management of malnutrition or nutritional risk may need to address more than the provision of energy (calories). The biological and symbolic dimensions of food are inseparable and a socio‐anthropological perspective suggests an intimate yet dynamic relationship between consumption of food and perceptions of self (Lupton 1996). The meaning of food extends beyond its mere nutritive value as it can have a tremendous impact on a person's sense of independence, self‐esteem, well‐being and health‐related quality of life, especially in elderly people (Donini 2003). Indeed, experiences with food have important implications for the emotional and psychological well‐being of an individual that sit within a traditional, cultural, socioeconomic and religious context and ultimately determines our food preferences (Donini 2003; Khan 1981; Lupton 1996). In severe illness, coping mechanisms, sense of body image, value of social networks and support, and personal symbolism may all be affected and food may take on new meaning (McQuestion 2011). Overall, this represents a challenge to health professionals and merits a deeper understanding of what really impacts on our experiences with food. Taking this into account, interventions that enhance the food experiences of malnourished individuals or those at risk of malnutrition by supporting their ability to take the intervention, thereby improving compliance, should theoretically result in greater dietary intakes and improved outcomes. Furthermore, the benefits of such interventions may extend beyond the conventional clinical, nutritional or functional outcomes and could conceivably also improve patient‐satisfaction and perceived health‐related quality of life. Indeed, following improvements in nutritional intake there may also be psychological and social benefits in individuals who are malnourished or at risk of malnutrition (NCCAC 2006). To summarise the mode of action, supportive nutritional care interventions should theoretically increase intake of micro‐ and macro‐nutrients and, in turn, improve the nutritional status and clinical function of nutritionally at‐risk individuals. By this, mortality, morbidity and hospitalisation are expected to be lowered. Considering the beneficial effects on physical health and the symbolic dimensions of food, health‐related quality of life should also improve.
Why it is important to do this review
A Cochrane systematic review of protein and energy supplementation in individuals over 65 years at risk from malnutrition contains 62 trials with a total of 10,187 randomised participants and the authors concluded that supplementation led to small but consistent weight gain in older people, and reductions in mortality in those who were undernourished (Milne 2009). There was no evidence of benefit to complications, functional status or length of hospital stay (Milne 2009). Interventions considered focused primarily on dietary supplementation with commercial sip feeds, milk‐based supplements and via the fortification of normal food sources (Milne 2009), rather than the array of supportive nutritional care interventions of interest to this review. In addition, the review included both randomised and quasi‐randomised trials (e.g. allocation by alternation, day of week, date of birth) (Milne 2009). It is acknowledged that the complex nature of the interventions in this area may result in trials that lack robust design and their inclusion may best represent the body of evidence available. However, meaningful conclusions may be more difficult to decipher, and therefore this systematic review of purely randomised controlled trials will better highlight the research needs and knowledge gaps in this area. Furthermore, a wider range of interventions and trials including adults of all ages have been considered in this review.
There is an urgent need to identify effective strategies for the management for malnourished people in hospitals and other health and social care settings. Not only has this been highlighted in reports from the Council of Europe (COE 2003) and within the UK by the Department of Health (DOH 2007), but also by professional bodies such as the Royal College of Nursing, the British Association for Parenteral and Enteral Nutrition (BAPEN) and patient‐focused organisations such as Age UK (BAPEN 2009; RCON 2008). Numerous strategies aimed at influencing nutritional management and improving the provision of nutritional care in hospitals, care homes and other health and social care settings, have been adopted and incorporated into national policies and international guidelines. Additionally, in the UK, protected mealtimes and the use of red trays have been rolled out across the National Health Service very recently, and interventions applicable across a range of healthcare settings, such as the use of feeding assistance, adjusting the portion size and nutrient content of foods and enhancing food flavours, are increasingly being used. Such service developments have received widespread support by local and national organisations and government. There has been a consistent trend to recommend the implementation of policies designed to influence nutritional care and the environment in which nutrition is provided, without a synthesis of the evidence of potential benefits or harms of such interventions. Crucially, the incorporation of such initiatives into usual care has implications for the staffing and funding of healthcare as well as the potential need for additional training across services. As yet there has been no synthesis of evidence to support the potential benefits of their implementation. Furthermore, a supportive multidisciplinary team approach is necessary in the provision of adequate nutritional care (Jefferies 2011). Given the widespread prevalence of malnutrition and with so many at risk, the potential impact of this systematic review in terms of informing the nutritional management of patients is considerable and therefore, the need for this review was paramount.
Two literature reviews examined various supportive nutritional care interventions (Silver 2009; Weekes 2009) but neither was systematic and both presented a narrative synthesis without meta‐analysis. Furthermore, the review by Weekes and colleagues (Weekes 2009) included non‐randomised trials and searched only electronic sources, while the review by Silver (Silver 2009) considered only trials in older adults. Despite their usefulness in presenting some of the available literature in this area, the true effect of supportive interventions to improve dietary intake by modifying the nutrient content of foods served or aspects of the food service system or environment remains unknown. Therefore, this review represents a first systematic attempt to bring together evidence on the impact of supportive interventions on nutritional, clinical, economic and patient‐centred outcomes.
Objectives
To assess the effects of supportive interventions for enhancing dietary intake in malnourished or nutritionally at‐risk adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled clinical trials (RCTs).
Types of participants
Adults (aged over 18 years) who were malnourished, judged to be at nutritional risk or otherwise would potentially benefit from improved nutritional care. The population is therefore described as nutritionally vulnerable.
Diagnostic criteria (malnourished or nutritionally at‐risk adults)
The term malnutrition used in this review refers to under‐nutrition, considered to be the state of poor nutritional status as a result of inadequate nutrient intake or metabolic impairment as well as the state of increased nutritional risk and imminent malnutrition (Corish 2000a; Reilly 1995).
The Malnutrition Universal Screening Tool (MUST) published by BAPEN (Elia 2003), as well as clinical guidelines in the UK and Europe published by the European Society for Parenteral and Enteral Nutrition (ESPEN) (Volkert 2006) and the National Institute for Health and Care Excellence (NICE) (NCCAC 2006), allow identification of malnourished individuals and those at risk of malnutrition in clinical practice and may be used to classify trial participants. These criteria are:
Malnourished
NICE (NCCAC 2006)
Body mass index (BMI) below 18.5 kg/m²
Unintentional weight loss greater than 10% within the last three to six months
BMI below 20 kg/m² and unintentional weight loss greater than 5% within the last three to six months
ESPEN (Volkert 2006)
5% unintentional weight loss in last three months and BMI below 20 kg/m²
10% unintentional weight loss in last six months and BMI below 20 kg/m²
At risk of malnutrition
NICE (NCCAC 2006)
Have eaten little or nothing for more than five days, are likely to eat little or nothing for the next five days or longer, or both
Have a poor absorptive capacity, have high nutrient losses, have increased nutritional needs from causes such as catabolism, or a combination
ESPEN (Volkert 2006)
Loss of appetite
Reduced dietary intake
Physical or psychological stress
MUST (Elia 2003)
Current acute illness plus no (or likely to be no) nutritional intake for more than five days
In the absence of clear, internationally accepted diagnostic criteria for clinical malnutrition, in many instances a health professional's decision to initiate dietetic referral for nutritional assessment or a clinician's decision to commence nutritional intervention is based on subjective criteria and clinical judgement (McCarron 2010). It was assumed therefore, that participants recruited to intervention trials were judged by the researcher to be malnourished or at risk of malnutrition, or otherwise had the potential to benefit from improved nutritional care on the basis of their clinical background or age.
Types of interventions
Intervention
Interventions that aimed to enhance food intake by improving either the meal itself (e.g. food fortification), aspects of the mealtime environment (e.g. enhancement of the eating environment), aspects of meal delivery, supplementation of meals or indirect supportive strategies (e.g. training of staff or carers). The strategies anticipated prior to searching included the examples listed within the five categories shown in Table 2. However, we recognised that it may become necessary to create additional categories as necessary following searching.
A previous systematic review (Baldwin 2011) included trials of interventions based on dietary counselling that required a person to receive instruction on food modification, oral nutritional supplements or both and have the ability and willingness to act on the instructions in order to enhance their nutritional intake. Although this review is closely related to the previous review, we planned to exclude trials where dietary counselling or oral nutritional supplements, or both were offered on an individualised basis. This review only considered food‐based or oral nutritional supplement interventions when they were provided as an institution‐led intervention without the patient needing to understand and act on instructions to take the additional items (e.g. offering snacks or supplements routinely to frail elderly people in an institutional setting, or the use of organisational structures to support the delivery of oral nutritional supplements). The inevitable overlap with reviews of oral nutritional supplements in the management of malnutrition is noted, but the inclusion of such trials in this review contributes to a more precise understanding of the benefits to be derived from these products.
Comparator
All interventions were compared with usual care.
Summary of specific exclusion criteria
We excluded the following intervention trials from this review.
Trials in children, pregnant women, people with eating disorders or malnutrition in conditions of food insufficiency and poverty. We have excluded these trials as malnutrition in such cases results from different aetiology, and the types of interventions and responses to such interventions also differ.
Trials of artificial nutrition support via a non‐oral route (i.e. enteral tube feeding and parenteral nutrition).
Trials of individualised nutritional support including either dietary counselling (i.e. where the individual was required to understand and act upon specific nutritional advice, which is most likely to occur in the outpatient setting). In cases where dietary advice was provided in combination with a supportive intervention, we have only included the trial if it was possible to evaluate the impact of the supportive intervention separately.
Trials of individually prescribed oral nutritional supplements.
Trials in healthy volunteers.
Types of outcome measures
We recorded the following outcome measures as change from baseline to end of intervention unless otherwise stated.
Primary outcomes
Nutritional intake (actual or percentage change in macro‐ and micronutrient intake)
Health‐related quality of life (evaluated by validated scores) and patient satisfaction
Morbidity/complications (number of participants with medical complications)
Secondary outcomes
Nutritional status (change in weight, body mass index (BMI), mid‐upper arm circumference (MUAC), triceps skin‐fold thickness (TSF) or as otherwise reported)
Clinical function (change in clinical functional status (e.g. skeletal muscle strength), respiratory and cardiac function, cognitive and behavioural function, activities of daily living)
Hospitalisation and institutionalisation
Adverse events
All‐cause mortality
Economic costs
Timing of outcome measurement
We extracted data on outcomes measured in each trial from baseline to the end of the intervention period. For trials with follow‐up periods that extended beyond the end of the intervention, we also extracted data at the end of intervention to the point of final follow‐up. From experience of a previous review of dietary advice with or without oral nutritional supplements for disease‐related malnutrition in adults (Baldwin 2011) we anticipated that the length, intensity and type of intervention would vary considerably in this current review, given its wider scope. We did not, therefore, establish lengths of intervention and only grouped interventions by time point if a sufficient number of trials was identified to permit this.
Summary of findings
We have presented a 'Summary of findings' table to report the following outcomes, listed according to priority.
All‐cause mortality
Morbidity/complications
Health‐related quality of life and patient satisfaction
Hospitalisation and institutionalisation
Adverse events
Nutritional status
Economic costs
Because of lack of data and substantial clinical and methodological heterogeneity we only performed meta‐analyses on all‐cause mortality, number of participants with complications and nutritional status (weight change).
Search methods for identification of studies
Electronic searches
We searched the following sources from inception of each database to the specified date and placed no restrictions on the language of publication.
Cochrane Library (14 September 2016).
Ovid Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 14 September 2016).
Embase (to March 2013).
AMED (to March 2013).
British Nursing Index (to March 2013).
CINAHL (to March 2013).
SCOPUS (to May 2013).
ISI Web of Science (to March 2013).
ClinicalTrials.gov (14 September 2016).
World Health Organization (WHO) ICTRP (International Clinical Trials Registry Platform ‐ http://apps.who.int/trialsearch/) (14 September 2016)
During the first round of electronic searches, we searched databases for all trials published up until the end of October 2011. During the second round of electronic searches, we searched databases for trials published between November 2011 and the end of March 2013 (May 2013 for SCOPUS only). We used identical search strategies in both the first and second round of searches. We carried out a third round of electronic searches prior to publication, when we used a revised search strategy to search the Cochrane Library, Ovid MEDLINE, ClinicalTrials.gov and WHO ICTRP. We carried out revised searches of the Cochrane Libary and Ovid MEDLINE from 1 January 2013 to 14 September 2016. We searched ClinicalTrials.gov and the ICTRP from inception to 14 September 2016.
For detailed search strategies please see Appendix 1 and Appendix 2.
Searching other resources
We searched the references lists of included trials and (systematic) reviews, and meta‐analyses to identify additional trials. We also searched the conference proceedings of relevant professional bodies and associations (British Dietetic Association, BAPEN and Royal College of Nursing) for the 10‐year period 2001 to 2011.
Data collection and analysis
Selection of studies
In order to identify trials to be assessed further, two review authors (MG and CEW) independently scanned the abstract, title or both for every record retrieved according to the inclusion criteria for the first round of searches. For the second round of searches, MG and CB independently scanned the abstract, title or both for every record retrieved according to the inclusion criteria, as before. For the third round of searching, CB and Bernd Richter (The review group editor) scanned titles and abstracts. We obtained all potentially relevant articles as full text and the three review authors (MG, CB and CEW) independently assessed their eligibility using a standardised trial eligibility form. Where there were differences in opinion, we resolved them by discussion among the three authors and made a decision by consensus. If resolving disagreement was not possible, we added the article to those 'awaiting assessment' and contacted the trial authors for clarification. We marked trials where we had not reached a primary consensus and if we included them later on, we planned to subject them to a sensitivity analysis. We listed excluded trials in the 'Characteristics of excluded studies' table along with the reasons for their exclusion. We present an adapted PRISMA flow‐diagram of trial selection (Liberati 2009).
Data extraction and management
For trials that fulfilled the inclusion criteria, two review authors (CB, CEW) abstracted relevant population and intervention characteristics using modified versions of standard data extraction sheets from the CMED Group which incorporated some adaptations from the data collection form used in a previous review by two of the review authors (Baldwin 2011). Data are reported as shown in Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18; Table 19; Table 20; Table 21; Table 22; Table 23; Table 24; Table 25; Table 26; Table 27 and Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10. The third review author acted as an arbiter in case of disagreement.
2. Overview of study populations.
| Intervention(s) and comparator(s) | Screened/eligible (N) | Randomised (N) | ITT (N) | Finishing trial (N) | Randomised finishing trial (%) | Follow‐up | |
| Barton 2000a2 (modification of meal profile or pattern) | I1: reduced portion size, fortified menu | ‐ | 13 | ‐ | b | 70c | 56 days |
| I2: cooked breakfast | (8 not randomised) | ||||||
| C: normal hospital diet with usual portion size | 14 | ||||||
| total: | 27a | ‐ | ‐ | ||||
| Beck 2002a1 (additional supplementation of meals) | I1: homemade oral supplement (A) | ‐ | ‐ | ‐ | ‐ | ‐ | 2 months |
| I2: homemade oral supplement (B) | |||||||
| C: usual diet | |||||||
| total: | 36 | ‐ | ‐ | ||||
| Bouillane 2013a1 (modification of meal profile or pattern) | I: 78% protein at lunch | ‐ | 30 | ‐ | 30 | 88 | 6 weeks |
| C: usual diet (protein distributed between meals) | 36 | 23 | 79 | ||||
| total: | 66 | 63 | 96 | ||||
| Bourdel‐Marchasson 2000a3 (additional supplementation of meals) | I: 2 oral nutritional supplements | 295 | ‐ | ‐ | ‐ | 15 days or until hospital discharge | |
| C: usual care | 377 | ||||||
| total: | 672 | ‐ | ‐ | ||||
| Brouillette1991a1 (changes to the feeding environment) | I: osmotherapy + activities | ‐ | 10 | ‐ | 9 | 90 | 4 weeks |
| C: activities only | 10 | 7 | 70 | ||||
| total: | 20 | 16 | 80 | ||||
| Castellanos 2009a2 (modification of meal profile or pattern) | I1: fortified breakfast and lunch menu | 39 | d | e | 2 days of the study | ||
| I2: fortified lunch menu | 39 | ||||||
| C: usual menu | 39 | ||||||
| total: | 39a | 33 | 85 | ||||
| Chang 2005a3 (changes to the organisation of nutritional care) | I: training in feeding skills | ‐ | 31 | ‐ | 12 | 60 | Quote: "Data collection was from February 2004 to May 2004" Comment: implies 4 months of data collection, following training but not clearly stated |
| C: no training | 36 | 8 | 50 | ||||
| total: | 67 | 20f | 56 | ||||
| Dennis 2005a1 (additional supplementation of meals) | I: oral nutritional supplement + normal diet | 2016 | ‐ | ‐ | ‐ | 6 months | |
| C: normal hospital diet | 2007 | ||||||
| total: | 4023 | ‐ | ‐ | ||||
| Duncan 2006a1 (changes to the organisation of nutritional care) | I: dietetic assistant | 363 | 153 | ‐ | 145 | 95 | 4 months |
| C: usual care | 165 | 157 | 95 | ||||
| total: | 318 | 302 | 95 | ||||
| Essed 2007a4 (modification of meal profile or pattern) | I1: monosodium glutamate | ‐ | ‐ | ‐ | 19 | N/A | 16 weeks |
| I2: flavour | 19 | ||||||
| I3: monosodium glutamate + flavour | 22 | ||||||
| C: maltodextrin (placebo) | 23 | ||||||
| total: | 97 | 83 | 86 | ||||
| Essed 2009a2 (modification of meal profile or pattern) | I: monosodium glutamate + NaCl | ‐ | 59 | ‐ | 53 | 90 | 4 weeks |
| C: usual hot meal | 59 | 53 | 90 | ||||
| total: | 59a | 53 | 90 | ||||
| Faxen‐Irving 2011a1 (additional supplementation of meals) | I: 30 mL of fat emulsion 3 x per day | 107 | 34 | ‐ | 24 | 71 | Median 8 days |
| C: usual care | 37 | 27 | 73 | ||||
| total: | 71 | 51 | 72 | ||||
| Gaskill 2009a3 (changes to the organisation of nutritional care) | I: nutrition education programme | 377 | ‐ | ‐ | ‐ | ‐ | 6 months |
| C: usual care | |||||||
| total: | 352 | ‐ | ‐ | ||||
| Germain 2006a1 (modification of meal profile or pattern) | I: re‐formed foods | 93 | 8 | ‐ | 7 | 88 | 12 weeks |
| C: usual diet | 9 | 8 | 89 | ||||
| total: | 17 | 15 | 88 | ||||
| Hankey 1993a1 (additional supplementation of meals) | I: supplemented with nutritionally complete drink in addition to normal hospital diet | ‐ | 10 | ‐ | 7 | 70 | 8 weeks |
| C: standard hospital food | 10 | 7 | 70 | ||||
| total: | 20 | 14 | 70 | ||||
| Hickson 2004a1 (changes to the organisation of nutritional care) | I: feeding assistance | 1776 | 292 | 292 | 250 | 86 | Duration of hospital stay |
| C: usual care | 300 | 300 | 259 | 86 | |||
| total: | 592 | 592 | 509 | 86 | |||
| Holyday 2012a1 (changes to the organisation of nutritional care) | I: malnutrition care plan | ‐ | 71 | 71 | 71 | 100 | Duration of hospital stay |
| C: usual care | 72 | 72 | 72 | 100 | |||
| total: | 143 | 143 | 143 | 100 | |||
| Johansen 2004a1 (changes to the organisation of nutritional care) | I: nutrition team | 7468 | ‐ | ‐ | 108 | N/A | Duration of hospital stay |
| C: usual care | 104 | ||||||
| total: | 215 | 212 | 99 | ||||
| Kraft 2012a1 (changes to the organisation of nutritional care) | I: oral nutritional supplement + telemedicine monitoring | 87/50 | 13 | 5 | 1 | 8 | 6 months |
| C: usual care | 13 | 9 | 4 | 31 | |||
| total: | 26 | 14 | 5 | 19 | |||
| Kretser 2003a1 (congregate and home meal delivery systems) | I: modified meals on wheels | 324 | 102 | ‐ | ‐ | ‐ | 26 weeks |
| C: traditional meals on wheels | 101 | ||||||
| total: | 203 | 60 | 30 | ||||
| Larsson 1990a1 (additional supplementation of meals) | I: oral nutritional supplement + normal hospital diet | ‐ | 197 | ‐ | ‐ | ‐ | 26 weeks |
| C: normal hospital diet | 238 | ||||||
| total: | 435 | ‐ | ‐ | ||||
| Leslie 2012a3 (modification of meal profile or pattern) | I: energy enriched usual meals | 445 | 22 | 16 | 73 | 12 weeks | |
| C: usual care | 19 | 16 | 84 | ||||
| total: | 41 | ||||||
| Lin 2010a3 (changes to the organisation of nutritional care) | I1: spaced‐retrievalg | ‐ | 32 | ‐ | ‐ | ‐ | 8 weeks |
| I2: Montessorih | 29 | ||||||
| C: usual care | 24 | ||||||
| total: | 85 | 82 | 97 | ||||
| Lin 2011a2, a3 (changes to the organisation of nutritional care) | I: Montessori | ‐ | ‐ | ‐ | ‐ | 8 weeks | |
| C: usual care | |||||||
| total: | 29a | 29 | 100 | ||||
| Mathey 2001aa3 (changes to the feeding environment) | I: improved meal ambiance | 60 | 21 | ‐ | 12 | 57 | 12 months |
| C: usual care | 17 | 10 | 59 | ||||
| total: | 38 | 22 | 58 | ||||
| Mathey 2001ba1 (changes to the feeding environment) | I: flavour enhancement | ‐ | ‐ | ‐ | 31 | N/A | 16 weeks |
| C: usual care | 36 | ||||||
| total: | 71 | 67 | 94 | ||||
| Munk 2014a1 (modification of meal profile or pattern) | I: energy and protein enriched foods provided via a la carte menu in addition to hospital food | 44 | 41 | 96 | Duration of hospital stay | ||
| C: usual care | 40 | 40 | |||||
| total: | 84 | ||||||
| Nijs 2006a3 (changes to the feeding environment) | I: family‐style meals | 282 | 133 | ‐ | 95 | 71 | 6 months |
| C: usual care | 112 | 83 | 74 | ||||
| total: | 245 | 178 | 73 | ||||
| Olofsson 2007a1 (changes to the organisation of nutritional care) | I: multi‐component intervention (including nutrition) | 353 | 102 | ‐ | 83 | 81 | 4 months |
| C: usual care | 97 | 74 | 76 | ||||
| total: | 199 | 157 | 79 | ||||
| Pivi 2011a1 (changes to the organisation of nutritional care) | I1: nutrition education | ‐ | ‐ | ‐ | 25 | N/A | 6 months |
| I2: oral nutritional supplements | 26 | ||||||
| C: usual care | 27 | ||||||
| total: | 90 | 78 | 87 | ||||
| Potter 2001a1 (additional supplementation of meals) | I: oral nutritional supplement + normal hospital diet | 618 | 186 | ‐ | 186 | 100 | Duration of hospital stay |
| C: normal hospital diet | 195 | 195 | 100 | ||||
| total: | 381 | 381 | 100 | ||||
| Remsburg 2001a1 (changes to the feeding environment) | I: buffet‐style meals | 62 | 20 | ‐ | 20 | 100 | 3 months |
| C: usual care | 20 | 19 | 95 | ||||
| total: | 40 | 39 | 98 | ||||
| Salva 2011a3 (changes to the organisation of nutritional care) | I: teaching and training | ‐ | 448 | 448 | 300 | 67 | 12 months |
| C: usual care | 498 | 498 | 368 | 74 | |||
| total: | 946 | 946 | 668 | 71 | |||
| Silver 2008a2 (modification of meal profile or pattern) | I: fortified home‐delivered lunch | ‐ | ‐ | ‐ | ‐ | ‐ | 7 months |
| C: usual home‐delivered lunch | |||||||
| total: | 52 | 45 | 87 | ||||
| Simmons 2008a2, a3 (additional supplementation of meals) | I: feeding assistance and/or snacks | 173 | 30 | ‐ | 28 | 88 | 24 weeks |
| C: usual diet | 34 | 32 | 94 | ||||
| total: | 64a | ‐ | 60 | 94 | |||
| Simmons 2010a1 (additional supplementation of meals) | I1: snacks | 280 | ‐ | ‐ | 25 | N/A | 6 weeks |
| I2: additional supplementation of meals | 18 | ||||||
| C: usual care | 20 | ||||||
| total: | 86 | 63 | 73 | ||||
| Smolliner 2008a3 (modification of meal profile or pattern) | I: fortified meals and snacks | 295/92 | ‐ | ‐ | 22 | N/A | 12 weeks |
| C: usual diet | 30 | ||||||
| total: | 65 | 52 | 80 | ||||
| Splett 2003a3 (changes to the organisation of nutritional care) | I: medical nutrition therapy | 394 | 223 | ‐ | 200 | 90 | 19‐180 days |
| C: usual care | 171 | 164 | 96 | ||||
| total: | 394 | 364 | 92 | ||||
| Taylor 2006a2 (modification of meal profile or pattern) | I: 5‐meal menu | 66 | ‐ | ‐ | ‐ | ‐ | 2 periods of 4 days |
| C: usual (3‐meal menu) | |||||||
| total: | 31a | 31 | 100 | ||||
| Van den Berg 2015a1 (additional supplementation of meals | I1: offered 125 mL ONS daily with medication rounds | 885 | 88 | 75 | 85 | Maximum period 30 days | |
| I2: offered 62 mL ONS daily with medication rounds | 66 | 51 | 77 | ||||
| C: offered 125 mL ONS twice daily in between meals | 80 | 66 | 83 | ||||
| total: | 234 | ||||||
| Van Ort 1995a1 (changes to the feeding environment) | I: contextual and behavioural intervention | 8 | ‐ | ‐ | ‐ | ‐ | 1 month to 6 weeks |
| C: usual care | |||||||
| total: | 8 | 7 | 88 | ||||
| Grand total | All interventionsj | ||||||
| All controlsj | |||||||
| All interventions and controls | 10,681 | ||||||
a1Parallel RCT; a2cross‐over RCT; a3cluster RCT; a4 factorial RCT bData presented on 19 participants who had at least 3 days on each menu cOf those randomised to normal or fortified menu, not stated for those receiving cooked breakfast dData analysed for 26 participants with complete data eData were reported on 67% of those who consented fData on knowledge and attitude of staff to nutrition available on all 67 staff. Data on actual practice at mealtimes from observation available on 20 staff gMethod to enhance learning, retention and recall of information hMethod capable of stopping or reducing residents' problem behaviours iAssmumed 30 per group, two groups included in this review jNo details because of substantial number of trials not providing data
C: comparator; I: intervention; ITT: intention‐to‐treat
3. Summary of outcomes reported in intervention category 1: changes to the organisation of nutritional care.
| Outcome measure | No. of studies reporting outcome | No. of participants | Studies potentially with data for meta‐analysis |
| Energy intake | 5 | 666 | 1 |
| Health‐related quality of life | 1 | 220 | 0 |
| Patient satisfaction | 2 | 1105 | 0 |
| Complications | 4 | 1263 | 3 |
| Nutritional status: weight | 10 | 2184 | 9 |
| BMI | 7 | 1537 | 6 |
| TSF | 3 | 536 | 3 |
| MAC | 3 | 568 | 3 |
| Length of stay | 5 | 1256 | 3 |
| Hospital admission | 1 | 143 | 1 |
| Mortality | 5 | 2182 | 5 |
| Costs | 2 | 1089 | 0 |
BMI: body mass index; MAC: mid‐arm circumference; TSF: triceps skinfold thickness
4. Summary of outcomes reported in intervention category 2: changes to the feeding environment.
| Outcome measure | No. of studies reporting outcome | No. of participants (treatment/control) | Studies with data for meta‐analysis |
| Energy intake | 3 | 216 | 3 |
| Health‐related quality of life | 2 | 200 | 0 |
| Nutritional status: weight | 3 | 239 | 3 |
| MAC | 1 | 178 | 1 |
| Clinical function | 3 | 1664 | 2 |
| Mortality | 3 | 236 | 3 |
MAC: mid‐arm circumference
5. Summary of outcomes reported in intervention category 3: modification of meal profile or pattern.
| Outcome measure | No. of studies reporting outcome | No. of participants | Studies potentially with data for meta‐analysis |
| Energy intake | 11 | 506 | 7 |
| Health‐related quality of life | 1 | 52 | 0 |
| Complications | 1 | 66 | 1 |
| Nutritional status: weight | 7 | 387 | 7 |
| BMI | 3 | 98 | 3 |
| MAC | 1 | 32 | 1 |
| Clinical function | 3 | 200 | 3 |
| Length of stay | 1 | 81 | 1 |
| Mortality | 4 | 243 | 4 |
BMI: body mass index; MAC: mid‐arm circumference
6. Summary of outcomes reported in intervention category 4: additional supplementation of meals.
| Outcome measure | No. of studies reporting outcome | No. of participants | Studies potentially with data for meta‐analysis |
| Energy intake | 8 | 1469 | 7 |
| Health‐related quality of life | 1 | 4023 | 0 |
| Complications | 2 | 4695 | 1 |
| Nutritional status: weight | 7 | 605 | 4 |
| BMI | 2 | 102 | 1 |
| TSF | 2 | 0 | |
| MAC | 3 | 1 | |
| Clinical function | 2 | 618 | 0 |
| Length of stay | 4 | 4689 | 1 |
| Mortality | 5 | 5745 | 5 |
| Costs | 1 | 63 | 0 |
BMI: body mass index; MAC: mid‐arm circumference; TSF: triceps skinfold thickness
7. Summary of outcomes reported in all interventions.
| Outcome measure | No. of studies reporting outcome | No. of participants (treatment/control) | Studies included in the meta‐analysis |
| Energy intake | 27 | 2857 | 0 |
| Health‐related quality of life | 5 | 4495 | 0 |
| Patient satisfaction | 2 | 1105 | 0 |
| Complications | 7 | 6024 | 5 |
| Nutritional status: weight | 28 | 3618 | 24 |
| BMI | 12 | 1737 | 0 |
| TSF | 5 | ‐ | 0 |
| MAC | 8 | ‐ | 0 |
| Clinical function | 9 | 2746 | 0 |
| Length of hospital stay | 10 | 6026 | 5 |
| Hospital admissions | 2 | 389 | 0 |
| Mortality | 18 | 8690 | 17 |
| Economic costs | 3 | 1152 | 0 |
BMI: body mass index; MAC: mid‐arm circumference; TSF: triceps skinfold thickness
8. Reasons for contacting authors, and outcomes of contact with authors.
| Outcome | Reason the data were not usable | Contact with author | Outcome of contact with author | Action taken | |
| 1. Organisational change | |||||
| Chang 2005 | Energy intake | Data reported as amount eaten in ¼, ½, ¾ | Yes | No response | Data reported in structured narrative summary |
| Duncan 2006 | Complications | Reported as a median and IQR | Yes | Data provided | Data used |
| Length of stay | Reported as median and IQR | Yes | Confirmed data skewed | Data reported in structured narrative summary | |
| Gaskill 2009 | Measured prevalence of malnutrition with SGA | Not an outcome of interest for this review | Yes, to request weight data (a component of SGA) | Unable to provide data | Data not reported |
| Hickson 2004 | Energy intake | Not measured at baseline, only at follow‐up | Yes, to confirm interpretation of data | Data not measured at baseline | Data reported in structured narrative summary |
| Complications (antibiotic prescription) | Reported as median and IQR | Yes, to request complications according to group allocation | No. complications according to group allocation was provided | Data reported in structured narrative summary | |
| Hospital admission | States in protocol these are collected, but not reported | Yes, to request data | Author unable to recall what happened with data | Data not reported | |
| Holyday 2012 | Costs | An estimate based on local prices, not a complete cost analysis | No, judged unlikely to be available | N/A | Data not reported |
| Hospital admission | Presented as a frequency | Yes, to request total number of readmissions | Data provided | Data reported in structured narrative summary | |
| Johansen 2004 | Energy intake | Reported as kJ/kg/day | Yes, for mean change | No response | Data not reported |
| Kraft 2012 | BMI | Presented as mean and SD at baseline and follow‐up, but no mean change | Yes | No response | Data not reported |
| Lin 2010 | Energy intake | 'Amount of each meal consumed' was reported as % eaten | Yes | No response | Data reported in structured narrative summary |
| Weight | Reported as mean and SD pre and post intervention/control | Yes, to request mean change | No response | Calculated mean change, and imputed the SD of change from Salva 2011 | |
| BMI | Reported as mean and SD pre and post intervention/control | Yes, to request mean change | No response | Calculated mean change, and imputed the SD of change from Salva 2011 | |
| Olofsson 2007 | Weight | Reported as mean and SD pre and post intervention/control | Yes, to request mean change and SD | Data provided | Data reported in structured narrative summary |
| BMI | Reported as mean and SD pre and post intervention/control | Yes, to request mean change and SD | Data provided | Data reported in structured narrative summary | |
| Complications | Reported as no. falls in men and women | Yes, to request total complications per group | Data provided | Data reported in structured narrative summary | |
| Pivi 2011 | Weight | Reported as mean and SD pre and post intervention/control | Yes, to request mean change | No response | Calculated mean change, and imputed the SD of change using the P value |
| BMI | Reported as mean and SD pre and post intervention/control | Yes, to request mean change | No response | Calculated mean change, and imputed the SD of change from Salva 2011 | |
| TSF | Reported as mean and SD pre and post intervention/control | Yes, to request mean change | No response | Calculated mean change, and imputed the SD of change from Salva 2011 | |
| MAC | Reported as mean and SD pre and post intervention/control | Yes, to request mean change | No response | Calculated mean change, and imputed the SD of change | |
| Salva 2011 | MAC | Methodology reported this was an outcome measured, but not reported in results | Yes | No response | Data not used |
| Costs | Described as data to be collected, but reported that analysis was not undertaken | No | Not reported | ||
| Splett 2003 | Intake | Food intake is documented as a nutrition assessment activity | Yes, to request mean energy intake per group | Unable to provide data | Not reported |
| Weight | Methodology reports this was an outcome measured, but reported in a format not usable | Yes | Unable to provide data | Not reported | |
| 2. Feeding environment | |||||
| Brouilette 1991 | Energy | Reported pre and post intervention data, but no SD of change | No, as no author contact details and study published in 1991 | N/A | Imputed the SD from Nijs 2006 |
| Van Ort 1995 | Weight change | No figures reported | Yes, to request data on mean and SD of change for each group | Waiting response | Not used |
| Intervention group clarification | Were the behavioural and contextual intervention received at the same time | Yes, to request this detail | Waiting response | Assumed the two interventions were given at the same time | |
| 3. Meal modification | |||||
| Bouillanne 2013 | Weight | Did not report weight, but assumed they had the data as Full Body Composition was used | Yes, to request data | Data provided | Data reported |
| Energy intake | Reported as kcal/kg/day | Yes, to request data | Data provided | Data reported | |
| Hand grip strength | Reported data as mean/median and 95% CI of the median | Yes, to request data | Provided mean and SD of change | Data reported | |
| ADL | Reported data as mean/median and 95% CI of the median | Yes, to request data | Data provided | Data reported | |
| Castellanos 2009 | Energy intake | Results were not analysed according to groups randomised, but regrouped subjects into small eaters and large eaters | Yes, to ask for data on mean and SD of change for each group | No response | Data reported |
| Germain 2006 | BMI | They reported the mean BMI rather than mean change | Yes, for mean and SD of change | Data provided | Data reported |
| Smolliner 2008 | Weight change | Reported mean and SD at baseline and end of intervention | Yes, for mean change and SD | Data provided | Data reported |
| BMI | Reported mean and SD at baseline and end of intervention | Yes, for mean change and SD | Data provided | Data reported | |
| Handgrip strength | Reported mean and SD at baseline and end of intervention | Yes, for mean change and SD | Data provided | Data reported | |
| health‐related quality of life | Reported mean and SD at baseline and end of intervention | Yes, for mean change and SD | Data provided | Data reported | |
| 4. Supplementation of meals | |||||
| Beck 2002 | Weight | Reported as median change with 95% CI | Yes, for mean change and SD | Response received but data not available | Data reported in structured narrative summary |
| Energy intake | Reported as median change with 95% CI | Yes, for mean change and SD | Response received but data not available | Data reported in structured narrative summary | |
| Bourdel‐ Marchasson 2000 | Pressure ulcers | Data given as percentage per group | Yes, for number per group | Data provided | Data reported in structured narrative summary |
| Weight | Data given for baseline only | Yes, for change in weight from baseline to follow‐up | Yes, author stated she did not find the analysis of discharge weight, probably due to the low quality of this data (too many missing data) | Data not reported | |
| Dennis 2005 | Complications | Data given as percentages | Yes for data on total complications per group | Data provided | Data reported in structured narrative summary |
| Health‐related quality of life score | Differences between means provided | Yes, to request mean and SD of changes | Unable to provide data, as EuroQol was only measured at follow‐up | Data reported in structured narrative summary | |
| Faxen‐Irving 2011 | Energy intake | Data given in a graph, no numbers available | Yes, for mean and SD of change in energy intake, between the control and intervention groups from baseline to the 2nd registration | Data provided | Data reported in structured narrative summary |
| Length of stay | Data provided at baseline, not follow‐up | Yes, for mean and SD | Data provided | Data reported in structured narrative summary | |
| Infection | Data provided at baseline, not follow‐up | Yes, for mean and SD | Unable to provide data | Data not reported | |
| BMI | Data provided at baseline, not follow‐up | Yes, for mean and SD | Data provided | Not reported in the summary because few studies measured this outcome | |
| ADL | Data provided at baseline, not follow‐up | Yes, for mean and SD | Data provided | Not reported in the summary because few studies measured this outcome | |
| Hankey 1993 | Weight | Presented in graphs, no numbers given | Yes, for mean and SD | Unable to provide data but suggested using data from the review by Milne 2009 which included these data | Data obtained from systematic review by Milne 2009 |
| MAC | Presented in graphs, no numbers given | Yes, for mean and SD | Unable to provide data but suggested using data from the review by Milne 2009 which included these data | Data obtained from systematic review by Milne 2009 but not reported as few studies measured this outcome | |
| TSF | Presented in graphs, no numbers given | Yes, for mean and SD | Unable to provide data but suggested using data from the review by Milne 2009 which included these data | Not reported in the summary because few studies measured this outcome | |
| Energy and protein intake | Presented in graphs, no numbers given | Yes, for mean and SD | Unable to provide data | Data not reported | |
| Larsson 1990 | Energy intake | Data included in Modified Norton Scale | Yes, data for change in energy intake between groups (mean and SD) | No response | Data not reported |
| Weight | Data provided as ‘weight index’ | Yes, for change in weight between groups (mean and SD) | No response | Data not reported | |
| TSF | Data provided as differences between men and women, and non‐PEM and PEM groups | Yes, for change between groups (mean and SD) | No response | Data not reported | |
| MAC | Data provided as differences between men and women, and non‐PEM and PEM groups | Yes, for change between groups (mean and SD) | No response | Data not reported | |
| Length of stay | Not given | Yes, for mean and SD between groups | No response | Data not reported | |
| Total number of eligible participants | Unclear across all 4 duplicates of this study | Yes, for a clear number of randomised participants, no finishing study, and deaths | No response | Data not reported | |
| Potter 2001 | Length of stay | Provided as median with a range | Yes, for mean and SD between groups | No response | Data reported in structured narrative summary |
| ADL | Stated as an outcome measure in methodology, then not reported in results | Yes, for mean and SD between groups | No response | Not reported in the summary because few studies measured this outcome | |
| BMI | Stated as an outcome measure in methodology, then not reported in results | Yes, for mean and SD between groups | No response | Not reported in the summary because few studies measured this outcome | |
| TSF | Stated as an outcome measure in methodology, then not reported in results | Yes, for mean and SD between groups | No response | Not reported in the summary because few studies measured this outcome | |
| Simmons 2008 | Weight | Data presented as phase 1 and 2 cross‐over combined. The data from phase 1 was needed for this review | Yes, for the phase 1 data | Yes, responded but unable to provide data | Data reported in structured narrative summary |
| BMI | Data presented as phase 1 and 2 cross‐over combined. The data from phase 1 was needed for this review | Yes, for the phase 1 data | Yes, responded but unable to provide data | Not reported in the summary because few studies measured this outcome | |
| Energy intake | Presented as pre‐ and post intervention | Yes, for mean and SD of change | Yes, responded but unable to provide data | Imputed SD from Nijs 2006 | |
| Simmons 2010 | Energy | Reported as mean difference without the SD | Yes, requested SD for mean change | Yes, responded but unable to provide data | Imputed SD from Nijs 2006 |
| 5. Home meal delivery systems | |||||
| Kretser 2003 | Weight | Reported separately for participants at risk of malnutrition, and those malnourished | No, failed to find contact information for the author | N/A | Combined the mean change data using the formulae for combining groups |
ADL: activities of daily living; BMI: body mass index; CI: confidence interval; EuroQol: European Quality of Life Scale; IQR: interquartile range; MAC: midarm muscle circumference; N/A: not applicable; PEM: protein‐energy malnutrition; SD: standard deviation; SGA: subjective global assessment; TSF: triceps skinfold thickness
9. No. participants identified in each setting from included studies.
| Setting | No. participants [N/N (%)] | No. studies |
| Hospital | 7591/10,681 (71.1) | 15 |
| Residential care home | 1731/10,681 (16.2) | 21 |
| Free‐living/outpatient setting | 1305/10,681 (12.2) | 5 |
10. Effects of changes to the organisation of nutritional care on nutritional intake.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Dietetic assistants (Hospital) | |||||
| Duncan 2006 | Mean (SD) energy intake (kcal/day) | 275 (total N = 302) | 1105 (361) | 756 (399) | < 0.001 |
| Hickson 2004 | Between‐group difference (kcal) | 37 (total N = 592) | 89 | 0.538 | |
| Specialist training (residential care settings) | |||||
| Chang 2005 | % (SD) meals consumed | 67 | Pre: 90 % (22) Post: 85 (25) |
Pre: 78 % (34) Post: 94 % (18) |
0.49 |
| Lin 2010 | % (SD) meals consumed | 85 | Spaced retrieval (SR) Pre: 85 % (11) Post: 91 % (9) Montessori (MON) Pre: 75 % (23) Post 78 % (10) |
Pre: 79 % (19) Post: 88 % (18) |
SR vs control = NS MON vs control < 0.05 |
| Multi‐disciplinary team (hospital) | |||||
| Johansen 2004 | kcal/kg body weight per day (SE) | 202 (total N = 212) | 30 (SE 1) | 25 (SE 1) | < 0.005 |
kcal: kilocalorie; SD: standard deviation; SE: standard error
11. Effects of changes to organisation of nutritional care on health‐related quality of life, patient satisfaction and morbidity and complications.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Patient satisfaction | |||||
| Dietetic assistants (hospital) | |||||
| Duncan 2006 | Median score (IQR) | 159 | 6.5 (2) | 3.0 (4) | 0.0001 |
| Health‐related quality of life | |||||
| Multi‐disciplinary team (hospital) | |||||
| Johansen 2004 | Change in physical score (SF‐36) | 110 | 2.4 (1.3) | 0.2 (1.5) | NS |
| Change in mental score (SF‐36) | 110 | 2.2 (2.5) | 3.3 (2) | NS | |
| Number of complications | |||||
| Dietetic assistants (hospital) | |||||
| Duncan 2006 | Total number of participants with complications | 302 | 84/125 (67%) | 79/130 (61%) | 0.29 |
| Hickson 2004 | Number of participants receiving oral antibiotics | 592 | 142/292 (49%) | 150/300 (50%) | 0.67 |
| Multi‐disciplinary team (hospital) | |||||
| Johansen 2004 | Total number of participants with complications | 212 | 34/108 (31%) | 23/104 22%) | NS |
| Olofsson 2007 | Total number of participants with complications | 157 | 81/83 (98%) | 74/74 (100%) | |
IQR: interquartile range; NS: not significant; SF‐36: short form‐36
12. Effects of changes to organisation of nutrition care on nutritional status.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Dietetic assistants (hospital) | |||||
| Duncan 2006 | Mean change (SD) Weight (kg) MAC (cm) TSF (mm) |
(total N = 302) 170 230 205 |
‐0.36 (3.3) ‐0.9 (2.2) ‐0.88 (2.6) |
‐1.0 (2.8) ‐1.3 (1.5) ‐1.23 (3.2) |
0.16 0.002 0.087 |
| Hickson 2004 | Mean change (SD) Weight (kg) MAC (cm) TSF (mm) Median (IQR) MAMC BMI (kg/m²) |
(total N = 592) 191 286 279 429 254 |
‐0.92 (2.71) ‐0.3 (1) ‐0.4 (1.8) ‐0.1 (‐0.8‐0.4) ‐0.04 (1.1) |
‐0.9 (3) ‐0.3 (1) ‐0.4 (1.7) ‐0.1 (‐0.5‐0.3) ‐0.25 (1.18) |
0.23 0.65 0.86 0.84 0.68 |
| Specialist training (residential care settings) | |||||
| Lin 2010 | Mean change (SD) Weight (kg) BMI (kg/m²) |
85 | Spaced retrieval ‐0.07 (0.57) Montessori ‐0.15 (0.57) Spaced retrieval 0.1 (1.0) Montessori ‐0.06 (1.0) |
‐0.09 (0.57) ‐0.03 (1) |
NS NS |
| Lin 2011 | BMI | 29 | ‐0.26 (0.73) | ‐0.09 (0.85) | 0.245 |
| Specialist training (free‐living individuals) | |||||
| Pivi 2011 | Mean change (SD) Weight (kg) MAC (cm) TSF (mm) BMI (kg/m²) |
52 | 1.19 (imputed SD: 3.3) 1.87 (2) 2.3 (5.4) 1.19 (1) |
‐2.2 (imputed SD: 3.3) ‐0.4 (0.46) 2.2 (5.3) ‐2.21 (1) |
Reported as between‐group differences for 4 groups |
| Salva 2011 | Mean change (SD) Weight (kg) BMI (kg/m²) |
946 | 0.26 (0.7) ‐0.01 (2.2) |
0.09 (0.5) ‐0.06 (3.2) |
0.598 0.843 |
| Multi‐disciplinary team (hospital) | |||||
| Johansen 2004 | Mean change (SD) Weight (kg) |
(total N = 212) 95 |
‐0.22 (3.9) | 0.1 (2) | NS |
| Olofsson 2007 | Mean change (SD) Weight (kg) BMI (kg/m²) |
(total N = 199) 157 157 |
‐1.1 (3.6) ‐0.45 (1.3) |
‐0.7 (3.8) ‐0.3 (1.5) |
0.05 0.05 |
| Protocol‐driven pathway (hospital) | |||||
| Holyday 2012 | Mean change (SD) Weight (kg) |
(total N = 143) 69 |
‐0.9 (3.6) | ‐0.9 (2.3) | 0.98 |
| Protocol‐driven pathway (residential care settings) | |||||
| Splett 2003 | Weight | 364 | No wt loss at baseline: 95% maintained wt. Wt loss at baseline: 48% maintained or gained wt. |
No wt loss at baseline: 58% maintained wt. Wt loss at baseline: 57% maintained or gained wt. |
|
| Telemedicine (free‐living individuals) | |||||
| Kraft 2012 | Mean change (SD) Weight (kg) BMI (kg/m²) |
26 14 |
‐4.5 (7.9) Baseline 24.5 (5.1) Follow‐up 23.0 (4.2) |
‐3 (6.2) Baseline 23.9 (4.4) Follow‐up 22.8 (4.3) |
NS NS |
BMI: body mass index; IQR: interquartile range; MAC: mid‐arm circumference; MAMC: mid‐arm muscle circumference; NS: not significant; SD: standard deviation; TSF: triceps skinfold thickness; wt: weight
13. Effects of changes to the organisation of nutritional care on handgrip strength.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Handgrip strength | |||||
| Dietetic assistants (Hospital) | |||||
| Duncan 2006 | Mean change (SD) | 126 (total N = 302) | 2.2 (10.7) | 0.16 (11.8) | 0.32 |
| Hickson 2004 | Median change (IQR) (kg) | (total N = 592) | 0.8 (‐1.4 to 2.5) | 0.7 (‐1.5 to 3) | 0.85 |
IQR: interquartile range; SD: standard deviation
14. Effects of changes to the organisation of nutritional care on hospitalisation, institutionalisation and death from any cause.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Mortality | |||||
| Dietetic assistants (Hospital) | |||||
| Duncan 2006 | 4‐month mortality | (total N = 302) | 19/145 (13%) | 36/157 (23%) | 0.036 |
| Hickson 2004 | In‐hospital mortality | (total N = 592) | 31/292 (11%) | 35/300 (12%) | 0.69 |
| Specialist training (free‐living individuals) | |||||
| Salva 2011 | 12‐month mortality | 946 | 43/448 (10%) | 29/498 (6%) | NR |
| Multi‐disciplinary team (hospital) | |||||
| Olofsson 2007 | 4‐month mortality | 199 | 9/102 (9%) | 13/97 (13%) | NR |
| Protocol‐driven pathway (hospital) | |||||
| Holyday 2012 | Not reported | 143 | 1/72 (1%) | 4/71 (6%) | 0.21 |
| Length of stay in hospital | |||||
| Dietetic assistants (hospital) | |||||
| Duncan 2006 | Median (IQR) (days) | 167 | 34 (48) | 32 (49) | 0.81 |
| Hickson 2004 | Median (IQR) (days) | 592 | 21(13‐36) | 23(14‐39) | 0.41 |
| Multi‐disciplinary team (hospital) | |||||
| Johansen 2004 | Mean (SD) LOS to 28 days |
197 | 11.6 (8) | 11.5( 8) | NS |
| Olofsson 2007 | Mean (SD) (days) | 157 | 27.4 (15.9) | 39.8 (41.9) | < 0.05 |
| Protocol‐driven pathway (hospital) | |||||
| Holyday 2012 | Mean (SD) (days) | 143 | 13.7 (11.8) | 13.5 (11) | 0.85 |
| Hospital readmissions | |||||
| Protocol‐driven pathway (hospital) | |||||
| Holyday 2012 | Number of readmissions at 6 months | 30/71 | 37/72 | NR | |
IQR: interquartile range; LOS: length of stay; SD: standard deviation
15. Effects of changes to the feeding environment on nutritional intake.
| Outcome | (N) | Results | P Value | |||
| Intervention | Control | |||||
| Changes to the dining room environment | ||||||
| Mathey 2001 | Mean change (SD) energy intake (kcal) | 22 | 199 (406) | 185( 247) | NR | |
| Nijs 2006 | Mean change (SD) energy intake (kcal) | 178 | 116 (456) | ‐100 (357) | ||
| Mean difference (95% CI) | 178 | 235 (83‐268) | Described as significantly different but no P value reported |
|||
| Remsburg 2001 | NR | |||||
| Sensory stimulation | ||||||
| Brouillette 1991 | Mean change (SD) in intake of lunch meal (kcal) |
16 | ‐1.6 (450) | 11.14 (360) | 0.49 | |
CI: confidence interval; NR: not reported; SD: standard deviation
16. Effects of changes to the feeding environment on health related quality of life.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Changes to the dining room environment | |||||
| Mathey 2001a | Sickness Impact Profile, mean change (SD) in score | 16/2 | ‐2 (11) | ‐13 (12) | NR |
| Nijs 2006 | Overall QOL mean change (95% CI) in score | 178 | 0.4 (‐1.8 to 2.5) | ‐5 (‐9.4 to ‐0.6) | NR |
| Mean difference (95% CI) | 178 | 6.1 (2.1 to 10.3) | Described as significantly different but no P value reported |
||
| Physical performance, mean change (95% CI) in score | 178 | 0.2 (‐2.3 to 2.7) | ‐2.2 (‐4.1 to ‐0.4) | NR | |
| Mean difference (95% CI) | 178 | 3.2 0.9 to 5.5) | Described as significantly different but no P value reported |
||
CI: confidence interval; NR: not reported; QOL: quality of life; SD: standard deviation
17. Effects of changes to the feeding environment on nutritional status.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Weight | |||||
| Changes to the dining room environment | |||||
| Mathey 2001a | Mean change (SD) (kg) | 22 | 3.3 (5) | ‐0.4 (4) | I: < 0.05; C: 0.78 |
| Nijs 2006 | Mean change (SD) (kg) | 178 | 0.5 (3.9) | ‐1.1 (3.7) | NR |
| Mean difference (95% CI) | 178 | 1.5 (0.6 to 2.4) | Described as significantly different but no P value reported |
||
| Remsburg 2001 | Mean change (SD) (kg) | 39 | ‐0.11 (3.1) | 0.32 (2.2) | 0.638 |
C: control; I: intervention; NR: not recorded; SD: standard deviation
18. Effects of changes to the feeding environment on death from any cause.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Changes to the dining room environment | |||||
| Mathey 2001a | Mortality | 38 | 7/21 (33%) | 5/17 (29%) | NR |
| Nijs 2006 | Mortality | 178 | 18/112 (16%) | 16/133 (12%) | NR |
| Sensory stimulation | |||||
| Brouillette 1991 | Mortality | 20 | 1/10 (10%) | 0/10 (0%) | NR |
NR: not reported
19. Effects of modification to meals on nutritional intake.
| Outcome | (N) | Results | P Value | ||||
| Intervention | Control | ||||||
| Fortification of food (studies in hospital) | |||||||
| Barton 2000 | Total energy intake (kcal/d) | 36 | 1711 (195) | 1425 (136) | < 0.001 | ||
| Munk 2014 | Mean (SD) intake (kj/d) | 81 | 5843 (1660) | 5149 (1832) | |||
| Mean (95% CI) difference between groups | 693 (‐80 to 1466) | 0.08 | |||||
| Fortification of food (studies in residential care homes) | |||||||
| Leslie 2012 | mean (SEM) change in energy intake (baseline to week 12) (kcal/d) |
16 | 133 (89) | ‐36 (84) | 0.154 | ||
| Food fortification (studies in free‐living individuals) | |||||||
| Silver 2008 | Total energy intake (kcal/d) | 45 | 1876 (543) | 1423 (422) | < 0.001 | ||
| Modifications to meal composition (studies in intermediate care) | |||||||
| Bouillane 2013 | Change in energy intake (kcal) | 63 | 50.9 (458) | 39.2 (401) | NR | ||
| Modifications to meal delivery (studies in residential care homes) | |||||||
| Germain 2006 | Change in energy intake (kcal) | 15 | 611 (408) | 81 (169) | 0.03 | ||
| Taylor 2006 | Total energy intake (kcal/d) | 31 | 1342 (177) | 1325 (207) | 0.565 | ||
| Modifications to flavour (studies in residential care homes) | |||||||
| Essed 2007 | Change in energy intake (kcal) | 83 | Flavour: ‐17 (445) Flavour + MSG: 78 (352) MSG: ‐32 (28) |
102 (452) | NR | ||
| Essed 2009 | Energy intake from modified meal (kcal) | 53 | 420 (211) | 424 (216) | 0.896 | ||
| Mathey 2001b | Change in energy intake (kcal) | 67 | ‐50 (267) | ‐115 (298) | Baseline to end of intervention I: NR, C: < 0.05 | ||
C: control; I: intervention; MSG: monosodium glutamate; NR: not recorded; SD standard deviation; SEM standard error of the mean; CI confidence interval
20. Effects of modifications to meals on nutritional status.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Weight and BMI (mean change (SD)) | |||||
| Fortification of food (studies in hospital) | |||||
| Munk 2014 | Mean (SD) within‐group change in weight (kg) |
66 | 0.4 (2.6) | ‐0.4 (1.8) | 0.17 |
| Mean (95% CI) between‐group difference in weight (kg) |
‐0.8 (‐1.9 to 0.3) | ||||
| Fortification of food (studies in residential care homes) | |||||
| Leslie 2012 | Mean (SD) within‐group weight change (kg) | 31 | 1.3 (0.53)* | ‐0.2 (1.5)** | *0.03 **0.536 |
| Mean (SD) within‐group change in BMI (kg/m2) | 31 | 0.5 (0.25)* | ‐0.1 (0.4)** | *0.042 **0.517 |
|
| Mean (SD) within‐group change in MUAC (mm) | 32 | 0.4 (0.16)* | ‐0.1 (0.3)** | *0.019 **0.691 |
|
| Smolliner 2008 | Mean (SD) change weight (kg) | 52 | 2 (2.1) | 1.6 (2) | NS |
| BMI change (kg/m²) | 52 | 0.77 (1.5) | 0.45 (1.1) | Between‐group difference NS |
|
| Modifications to meal composition (studies in intermediate care) | |||||
| Bouillanne 2013 | Mean (SD) change weight (kg) | 63 | 0.4 (2.3) | ‐0.7 (3.1) | NR |
| Modifications to meal delivery (studies in residential care homes) | |||||
| Germain 2006 | Mean (SD) change weight (kg) | 15 | 3.9 (2.3) | ‐0.8 (4.2) | 0.02 |
| BMI change (kg/m²) | 15 | 1.51 (1.16) | 0.27 (1.46) | Data provided by study author P value NR |
|
| Modifications to flavour (studies in residential care homes) | |||||
| Essed 2007 | Mean (SD) change weight (kg) | 83 | Flavour: 0.1 (2.4) Flavour + MSG: ‐ 0.8 (3.3) MSG: ‐ 0.7 (3.6) |
0.1 (3.8) | NR |
| Mathey 2001b | Mean (SD) change weight (kg) | 67 | 1.1 (1.3) | ‐0.3 (1.6) | < 0.05 |
BMI: body mass index; CI: confidence interval; MSG: monosodium glutamate; MUAC: mid‐upper arm circumference; NR: not reported; NS: not significant; SD: standard deviation
21. Effects of modifications to meals on clinical function, hospitalisation and death from any cause.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Mortality | |||||
| Fortification of food (studies in hospital) | |||||
| Munk 2014 | Mortality | 81 | 1/44 | 1/40 | NR |
| Fortification of food (studies in residential care homes) | |||||
| Leslie 2012 | Mortality | 32 | 2/19 | 5/22 | NR |
| Smolliner 2008 | Mortality | 65 | 2/31 | 1/34 | NR |
| Modifications to meal composition (studies in intermediate care) | |||||
| Bouillane 2013 | Mortality | 66 | 1/30 (3%) | 1/36 (3%) | NR |
| Length of hospital stay | |||||
| Fortification of food (studies in hospital) | |||||
| Munk 2014 | Days from study inclusion to discharge | 81 | 10 (8) | 10 (8) | 0.73 |
| Handgrip strength | |||||
| Fortification of food (studies in hospital) | |||||
| Munk 2014 | Mean change (SD) baseline to day 3 (kg) | 76 | ‐0.1 (2.9) | ‐0.4 (4.3) | 0.76 |
| Mean difference (95% CI) between I & C | ‐0.3 (‐1.9 to ‐1.4) | 0.95 | |||
| Fortification of food (studies in residential care homes) | |||||
| Smolliner 2008 | Mean change (SD) (kg) | 61 | ‐0.81 (3.12) | ‐1.29 (3) | NR |
| Modifications to meal composition (studies in intermediate care) | |||||
| Bouillane 2013 | Mean change (SD) (N) | 63 | ‐0.5 (41.7) | 14 (45.1) | 0.411 (ANCOVA 0.271) |
| Bouillane 2013 | Change in ADL score (mean (SD) | 63 | ‐0.02 (1.6) | 0.54 (1.7) | 0.125 (ANCOVA 0.118) |
ADL: activities of daily living; ANCOVA: analysis of covariance; N: Newtons; NR: not reported; SD: standard deviation
I: intervention; C: control
22. Effects of supplementation of meals on nutritional intake.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Supplementation with food (residential care homes) | |||||
| Beck 2002 | Change in energy intake (kcal/d) (median 95% CI) | 16 | ‐24 (‐454 to 860) | 24 (‐167 to 478) | NS |
| Simmons 2008 | Change in energy intake kcal/ (mean SD) | 64 | 302 (450) | 127 (360) | Baseline to 6 months I: = 0.000; C: NS |
| Simmons 2010 | Change in energy intake (mean SD) | 43 | ‐65 (450) | 67 (360) | NS |
| Supplementation with ONS (in hospital) (reported as mean (SD) | |||||
| Bourdel‐Marchasson 2000 | Total energy intake (kcal/d) | 672 | 1188 (613) | 1102 (503) | 0.13 |
| Faxen‐Irving 2011 | Change in energy intake (kcal/d) | 38 | 94 (350) | 6.5 (358) | NR |
| Potter 2001 | Total energy intake (kcal/d) | 381 | 1409 (448) | 1090 (417) | S |
| Van den Berg 2015 | Mean (SD) energy intake from ONS (kcal/d) | 192 | I1:343 (172)* I2: 469 (111)** |
389 (162) | *0.289 **0.006 |
| Supplementation with ONS (long‐term/residential care settings) | |||||
| Hankey 1993 | Total energy intake (kcal/d) | 21 | 1747 (273) | 1147 (310) | Baseline to wk 8, I: 0.01; C: NS |
| Simmons 2010 | Change in energy intake | 42 | 28 (450) | 67 (360) | 0.14 |
C: control; CI: confidence interval; I: intervention; NS: not significant; NR: not reported; ONS: oral nutritional supplement; S: significant; SD: standard deviation; wk: week
23. Effects of supplementation of meals on health‐related quality of life, morbidity/complications.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Incidence of pressure ulcers | |||||
| Supplementation with ONS (in hospital) | |||||
| Bourdel‐Marchasson 2000 | Cumulative incidence at end of follow‐up (%) Number of participants with pressure ulcers at day 15 |
672 | 40 101/295 |
48 164/37 |
NR NR |
| Dennis 2005 | Number of participants with pressure ulcers | 4023 | 15/2016 | 26/2007 | 0.0507 |
| Total complications | |||||
| Supplementation with ONS (in hospital) | |||||
| Dennis 2005 | All in‐hospital complications | 4023 | 515/2014 (26%) | 448/2001 (22%) | NR |
| Health‐related quality of life | |||||
| Supplementation with ONS (in hospital) | |||||
| Dennis 2005 | Utilitiy (median (IQR)) (EUROQoL) | 3086 | Median group difference 0.52 (0.03 to 0.74) | 0.96 | |
EUROQol: European Quality of Life Scale; IQR: interquartile range; NR: not reported; ONS: oral nutritional supplement
24. Effects of supplementation of meals on nutritional status.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Supplementation with food (residential care homes) | |||||
| Beck 2002 | Change in weight (median 95% CI) | 16 | 1.3 (‐1 to 3) | 1.5 (‐2.3 to 9) | NS |
| Simmonds 2008 | Mean change (SD) weight (kg) Mean (SD) change in BMI |
64 | The intervention group gained 4 lbs more The intervention group gained 0.72 kg/m2 than the usual care |
NR NR |
0.009 0.009 |
| Simmonds 2010 | Mean change (SD) weight (kg) | 43 | 0.02 (1.1) | 0.21 (1.7) | NS |
| Supplementation with ONS (in hospital) | |||||
| Faxen‐Irving 2011 | Mean change (SD) weight (kg) Mean (SD) BMI at follow‐up (kg/m2) |
38 38 |
0.13 (2.2) 20.4 (3.7) |
‐0.95 (2.3) 20.4 (3.7) 21.9 (3.8) |
NR 0.17 |
| Potter 2001 | Mean change in weight (kg) Mean change (SD) MAC (cm) |
381 381 |
0.4 (2.6) ‐0.1 (1.3) |
‐0.5 (2.9) ‐0.4 (1.2) |
0.003 NS |
| Supplementation with ONS (long‐term care settings) | |||||
| Hankey 1993 | Mean change (SD) weight (kg) Mean change (SD) MAC |
21 21 |
2.83 (10) ‐1 (10) |
‐0.53 (10) 0.6 (10) |
NR ‐ data from Milne 2009 NR data from Milne 2009 |
| Simmons 2010 | Mean change in weight (kg) | 42 | 0.91 (2.3) | 0.24 (1.96) | NS |
BMI: body mass index; CI: confidence interval; MAC: mid‐arm circumference; NR: not reported; NS: not significant; ONS: oral nutritional supplement; SD: standard deviation
25. Effects of supplementation of meals on hospitalisation, institutionalisation and death from any cause.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Mortality | |||||
| Supplementation with ONS (in hospital) | |||||
| Bourdel‐Marchasson 2000 | Mortality | 672 | 25/295 (8%) | 22/377 (6%) | 0.18 |
| Dennis 2005 | Mortality | 4023 | 241/2016 (12%) | 253/2007 (13%) | 0.7 |
| Potter 2001 | Mortality | 381 | 21/186 (11%) | 33/195 (17%) | 0.117 |
| Supplementation with ONS (long‐term care settings) | |||||
| Larsson 1990 | Mortality | 435 | 29/197 (15%) | 56/238 (24%) | 0.13 |
| Length of stay | |||||
| Supplementation with ONS (in hospital) | |||||
| Faxen‐Irving 2011 | Length of hospital stay (days) | 51 | 10.5 (SD 5.6) | 10.3 (SD 4.9) | NS |
| Dennis 2005 | Length of hospital stay (days) Median (IQR) |
4023 | 16 (IQR 7–44) | 16 (IQR 7–41) | NS |
| Potter 2001 | Length of hospital stay (median (range)) | 381 | 16 (3‐141) | 18 (2‐76) | 0.31 |
| Van den Berg 2015 | Length of hospital stay (median (range)) | 234 | I1: 10 (3‐63) I2: 10 (3‐27) |
11 (4‐71) | NR |
| Hospital readmissions & discharge destination | |||||
| Supplementation with ONS (in‐hospital) | |||||
| Potter 2001 | Discharge to home Discharge to institution |
381 381 |
131/186 31/186 |
127/195 33/195 |
NS |
| Van den Berg 2015 | Hospital readmissions | 246 | I1: 13 I2: 24 |
15 | NR |
IQR: interquartile range; NR not reported; NS: not significant; ONS: oral nutritional supplement
26. Effects of home meal delivery systems on nutritional status and death from any cause.
| Outcome | (N) | Results | P Value | ||
| Intervention | Control | ||||
| Weight change | |||||
| Kretser 2003 | Mean change in weight (kg) | 163 | 1.86 (5.3) | ‐1,04 (5.2) | 0.0062 |
| Mortality | |||||
| Kretser 2003 | Mortality | 203 | 3/102 (3%) | 9/101 (9%) | NR |
NR: not reported
We sent an email request to authors of included trials to enquire whether they were willing to answer questions regarding their trials. Appendix 11 shows the results of this survey. Thereafter, we sought relevant missing information on the trial from the trial authors of the article, if required.
Dealing with duplicate publications
In the case of duplicate publications and companion papers of a primary trial, we have tried to maximise yield of information by inclusion of and simultaneous evaluation of all available data.
Assessment of risk of bias in included studies
Two review authors (CB and CEW) assessed each trial independently. We resolved possible disagreements by discussion amongst the three authors and made a judgement based on consensus.
We assessed risk of bias using the Cochrane tool for assessing risk of bias (Higgins 2011a; Higgins 2011b). We used the following risk of bias criteria.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding (performance bias and detection bias), separated for blinding of participants and personnel and blinding of outcome assessment
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other bias
We assessed risk of bias for each component of each trial as 'low risk', 'high risk' or 'unclear risk' as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Measures of treatment effect
We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs) and continuous data as mean differences (MDs) with 95% CIs.
Unit of analysis issues
We planned to take into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome. For cross‐over trials data had to be available from baseline to the end of phase 1 of the cross‐over trial to be included in meta‐analyses. The cross‐over design as such was not feasible for our research question because of anticipated substantial carryover effects.
We could not recalculate data taking into account the design effect for cluster‐RCTs because we did not have reliable information about intracluster correlation coefficients for our substantial heterogeneous populations in the included trials. Therefore, we did not establish meta‐analyses by using both parallel and cluster‐RCTs but excluded the cluster‐RCTs from all meta‐analyses.
Dealing with missing data
Where feasible, we obtained relevant missing data from study authors. We investigated attrition rates, for example number of dropouts, losses to follow‐up and withdrawals, and critically appraised issues of missing data and imputation methods (e.g. last‐observation‐carried‐forward (LOCF)).
Assessment of heterogeneity
In the event of substantial clinical, methodological or statistical heterogeneity, we did not report trial results as the pooled effect estimate in a meta‐analysis. We identified heterogeneity (inconsistency) through visual inspection of the forest plots and by using a standard Chi² test with a significance level of α = 0.1. In view of the low power of this test, we also considered the I² statistic, which quantifies inconsistency across trials to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003); where an I² statistic of 75% or more indicates a considerable level of heterogeneity (Deeks 2011). When we found heterogeneity, we attempted to determine possible reasons for it by examining individual trial and subgroup characteristics.
Assessment of reporting biases
If we included 10 trials or more investigating a particular outcome and intervention, we planned to use funnel plots to assess small study effects. Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias. Therefore we interpreted results carefully (Sterne 2011).
Data synthesis
Prior to undertaking any data synthesis, two authors (CB, CEW) considered the clinical heterogeneity of the trials. The likelihood of clinical heterogeneity amongst trials was judged to be high in many cases, as trials were in populations with widely different clinical backgrounds, conducted in different healthcare settings, and despite some grouping of similar interventions, involved interventions that varied considerably. We undertook data synthesis, therefore, for some outcome measures only, by means of a random‐effects model.
Quality of evidence
We presented the overall quality of the evidence for each outcome according to the GRADE approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity such as directness of results. We presented a summary of the evidence in Table 1. This provides key information about the best estimate of the magnitude of the effect, in relative terms and absolute differences, for each relevant comparison of alternative management strategies, numbers of participants and trials addressing each important outcome and the rating of the overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011) by means of the Review Manager (RevMan) table editor (RevMan 2014). We included the Appendix 11 'Checklist to aid consistency and reproducibility of GRADE assessments' (Meader 2014) to help with standardisation of the 'Summary of findings' tables. We presented the results for the outcomes as described in the Types of outcome measures section. If meta‐analysis was not possible, we presented results in a narrative format in the 'Summary of findings' table. We justified all decisions to downgrade the quality of trials using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We undertook the following subgroup analysis.
Intervention category (e.g. changes to the organisation of nutritional care, changes to the feeding environment, modification of meal profile or pattern, additional supplementation of meals, congregate and home meal delivery systems)
Insufficient data were available to undertake the following subgroup analyses.
Intervention format (e.g. interventions given to individuals or groups of individuals)
Baseline nutritional status (e.g. judged to be malnourished or at risk of malnutrition)
Mean age of participants (e.g. below 65 years and 65 years or over)
Intervention setting (e.g. home, hospital, long‐term care facility, other community setting)
Intervention duration (e.g. short term (less than 3 months), medium term (3 to 6 months) or long term (above 6 months))
Intensity of intervention (e.g. number of visits/consults; considerations will be given to a post hoc analysis if sufficient data are available, as the intensity of intervention is very likely to differ according to care setting)
Effects beyond the cessation of intervention (e.g. maintenance of weight gain, continued improvements in health‐related quality of life)
Change in outcome versus no change in outcome for nutritional status and intake
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes by restricting the analysis to the following.
Published trials
Taking into account risk of bias, as specified in the Assessment of risk of bias in included studies section
Very long or large trials to establish the extent to which they dominate the results
Trials using the following filters: diagnostic criteria, imputation, language of publication, source of funding (industry versus other), or country
We also planned to test the robustness of the results by repeating the analysis using different measures of effect size (RRs, ORs etc.) and different statistical models (fixed‐effect and random‐effects models).
Due to lack of data we only performed sensitivity analyses on some risk of bias.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
The electronic searches identified 29,155 records. An additional 1107 records were identified from searches of conference abstracts/proceedings, systematic reviews and reference lists of included trials. We screened a total of 30,262 records after removal of duplicates. Three review authors (MG, CEW and CB) independently scanned titles and abstracts from the first two searches and the Co‐ordinating Editor (Bernd Richter (BR)) and one review author (CB) screened titles and abstracts from the third search and fourth search. We did not identify any ongoing trials.
Three review authors (CB, CEW and MG) and the Co‐ordinating Editor (BR) assessed eligibility of trials against the inclusion criteria and grouped trials according to similar intervention categories. We identified a total of 41 randomised controlled trials (RCTs) for inclusion in the review (see Characteristics of included studies). The number of trials identified for each intervention category were as follows.
Changes to the organisation of nutritional care (N = 13)
Changes to the feeding environment (N = 5)
Modification of meal profile or pattern (N = 12)
Additional supplementation of meals (N = 10)
Congregate and home meal delivery systems (N = 1)
A PRISMA flow‐diagram of trial selection is shown in Figure 2.
2.
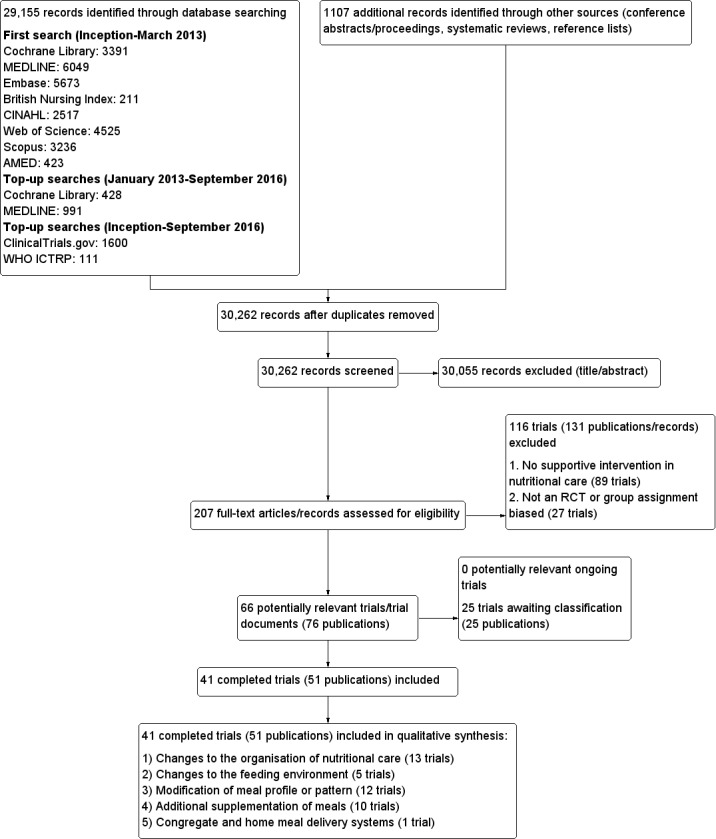
Study flow diagram
Contact with authors
Of the 41 included trials, we requested additional information on outcomes of interest and quality from the authors of 31 trials, and obtained it for 15 (Barton 2000; Beck 2002, Bouillanne 2013; Bourdel‐Marchasson 2000; Dennis 2005; Duncan 2006; Faxen‐Irving 2011; Gaskill 2009; Germain 2006; Hickson 2004; Holyday 2012; Olofsson 2007; Simmons 2008; Simmons 2010; Smoliner 2008). For six of the 15 trials where the study authors responded, they were unable to provide the data requested, or the data were not usable in a meta‐analysis (Barton 2000; Beck 2002; Bourdel‐Marchasson 2000; Gaskill 2009; Simmons 2008; Simmons 2010). The authors of the remaining 16 trials did not respond (Castellanos 2009; Chang 2005; Essed 2007; Essed 2009; Hankey 1993; Johansen 2004; Kraft 2012; Larsson 1990; Lin 2010; Mathey 2001a; Mathey 2001b; Pivi 2011; Potter 2001; Salva 2011; Splett 2003; Van Ort 1995).
Missing data
Despite the comprehensive search strategies used to identify trials in this review, it is possible that we have missed additional trials (e.g. unpublished trials, those published in obscure places, or those inappropriately indexed in databases).
The largest source of missing data in this review arose from data on outcomes that were measured but reported in such a way that they were unusable for entry into a meta‐analysis, because the data were reported as a median and interquartile range or were expressed as kcal/kg or the standard deviation (SD ) of change was not reported. The details of the amount of missing data according to intervention group are given in Table 4; Table 5; Table 6; Table 7 and Table 8. We contacted study authors in an attempt to obtain any missing data. The reasons for contacting authors and the outcome of contacts are described in Table 9 and Appendix 11.
Where it was not possible to obtain original data from study authors, we either imputed data, for example, standard deviations, using methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c), or used formulae for combining groups as outlined in Table 9.
The majority of included trials did not report intention‐to‐treat analyses.
Dealing with duplicate publications/companion papers
Six trials included in this review had duplicate or companion publications (Essed 2007; Hickson 2004; Larsson 1990; Lin 2010; Nijs 2006; Potter 2001).
Included studies
This systematic review identified 41 randomised controlled trials, with a total of 10,681 randomised participants (ranging from 8 (Van Ort 1995) to 4023 (Dennis 2005)). One included trial is awaiting clarification of participant numbers from the study authors (Larsson 1990). This trial had several publications, which stated varying numbers of participants (435 to 501). The primary reference reported data on 435 participants and this is the number that we would use in any meta‐analysis (Larsson 1990).
Participants were from a variety of countries including Australia, Brazil, CanadaDenmark, France, Germany, Netherlands, Spain, Sweden, Taiwan, , UK, and USA. Approximately 70% of participants were female (no information was provided for gender in three trials (Chang 2005; Larsson 1990; Simmons 2008). In those trials that reported ages in the intervention and usual care groups separately (N = 23), the mean age ranged from 62 to 87 years. Where the age of participants was reported for intervention and comparison groups separately, the mean age ranged from 75.2 to 87.3 (N = 11) (no data were provided for mean age in three trials (Kretser 2003; Potter 2001; Simmons 2008).
Altogether seven of the 41 included RCTs had a cross‐over design (Barton 2000; Castellanos 2009; Essed 2009; Lin 2011; Silver 2008; Simmons 2008; Taylor 2006), 12 a cluster‐randomised design (Bourdel‐Marchasson 2000; Chang 2005; Gaskill 2009; Leslie 2012; Lin 2010; Lin 2011; Mathey 2001a; Nijs 2006; Salva 2011; Simmons 2008; Smoliner 2008; Splett 2003) and one was a factorial RCT (Essed 2007). Two trials had both a cluster‐randomised and a cross‐over design (Lin 2011; Simmons 2008). One large trial investigating a normal hospital diet plus oral nutritional supplements versus a normal hospital diet in participants with a recent stroke randomised 38% participants (4023/10,681) of all individuals in the 41 included trials (Dennis 2005).
Interventions were carried out in the hospital setting (described as elderly rehabilitation wards, intermediate care units, geriatric units, acute trauma wards, geriatric acute wards, geriatric orthopaedic wards, medicine for the elderly units and acute medical admissions) (N = 15), residential care homes (N = 21) and free‐living or outpatient settings (N = 5) including neurology outpatients, and those enrolled at hospital discharge (see Table 10).
Nutritional status was reported in 27 trials, either because it was assessed at baseline or it was one of the criteria for inclusion in the trial (Beck 2002; Bouillanne 2013; Essed 2007; Essed 2009; Faxen‐Irving 2011; Gaskill 2009; Germain 2006; Hickson 2004; Holyday 2012; Johansen 2004; Kraft 2012; Kretser 2003; Larsson 1990; Leslie 2012; Lin 2010; Lin 2011; Munk 2014; Nijs 2006; Mathey 2001b; Olofsson 2007; Potter 2001; Remsburg 2001; Salva 2011; Silver 2008; Smoliner 2008; Taylor 2006; Van den Berg 2015). The remaining trials did not assess nutritional status at trial inclusion but we judged them appropriate to be included in this review as the clinical background of trial participants meant that they could be considered to be at risk of malnutrition or the patients were described as frail or vulnerable. Ten of 16 trials used a score from the Mini Nutritional Assessment (MNA) tool of 17 to 23.5 or less than 17 (Beck 2002; Essed 2007; Essed 2009; Holyday 2012; Kretser 2003; Nijs 2006; Olofsson 2007; Salva 2011; Smoliner 2008; Taylor 2006), to indicate risk of malnutrition, one trial used the Subjective Global Assessment score (SGA) (Gaskill 2009), two used the Nutritional Risk Screening 2002 (NRS‐2002) tool (Johansen 2004; Munk 2014), eight used only body mass index (BMI) (Faxen‐Irving 2011; Hickson 2004; Leslie 2012; Lin 2010; Lin 2011; Mathey 2001b; Remsburg 2001; Silver 2008), four used a combination of indices with variable cut‐offs (Bouillanne 2013; Germain 2006; Kraft 2012; Larsson 1990) and one used their own classification scoring system (Potter 2001). The average BMI measurements, in the trials that clearly reported BMI in all participants, ranged from less than 18.5 kg/m² (Kretser 2003) to 28.7 kg/m² (Nijs 2006)
The most commonly reported outcomes of interest to this review were nutritional intake (predominantly energy and protein), weight and mortality. These were reported in 27, 28 and 18 trials respectively. The three primary outcomes in the review, nutritional intake, health‐related quality of life and morbidity and complications, were reported in 27, 5, and 5 trials respectively. Patient satisfaction, hospital admission and costs were reported for a limited number of trials (2, 2 and 3 respectively). Six trials reported no usable data for potential combination in a meta‐analysis (Beck 2002; Castellanos 2009; Chang 2005; Gaskill 2009; Splett 2003; Van Ort 1995). We contacted the study authors who either were unable to provide the data requested, or failed to respond (see Table 9 and Appendix 11).
The outcomes reported in all intervention groups and those of use in this review, are summarised in Table 8.
Length of intervention and follow‐up
Length of intervention and follow‐up ranged from ‘length of hospital stay’ to 12 months in the included trials. In one trial, the length of intervention was unclear (Gaskill 2009). In 7 of 38 trials (Brouillette 1991; Dennis 2005; Duncan 2006; Gaskill 2009; Holyday 2012; Johansen 2004; Olofsson 2007) the follow‐up period extended beyond the intervention from two weeks to six months.
Further results of the included trials are given in their individual intervention categories (see Appendix 3 for description of interventions).
Changes to the organisation of nutritional care
We identified 13 trials for this category (Chang 2005; Duncan 2006; Gaskill 2009; Hickson 2004; Holyday 2012; Johansen 2004; Kraft 2012; Lin 2010; Lin 2011; Olofsson 2007; Pivi 2011; Salva 2011; Splett 2003), (N = 3426, 32.4% of review participants). Participants either had dementia, hip fractures or were from a range of clinical backgrounds, living in residential care homes, hospital or their own homes. Interventions consisted of the use of dietetic assistants (Duncan 2006; Hickson 2004), multidisciplinary team care (Johansen 2004), specialised teaching and training (Chang 2005; Gaskill 2009; Lin 2010; Lin 2011; Pivi 2011; Salva 2011), protocol‐driven nutrition care pathways (Holyday 2012; Splett 2003), multicomponent intervention (Olofsson 2007) and monitoring by telemedicine (Kraft 2012). Duration ranged from a few days of hospital stay to 12 months, and follow‐up from 28 days to 12 months. We have summarised the outcomes reported, and those usable for this review, Table 5.
Changes to the feeding environment
We identified five trials for this category (Brouillette 1991; Mathey 2001a; Nijs 2006; Remsburg 2001; Van Ort 1995), (N = 351, 3.3% of review participants). All trials were conducted in elderly participants living in residential care homes. Interventions consisted of the use of osmotherapy (pre‐meal sensory stimulation) (Brouillette 1991), improving mealtime ambience (Mathey 2001a), using family style meals (Nijs 2006), a buffet‐style meal service (Remsburg 2001), and a contextual/behavioural intervention (Van Ort 1995). Duration of intervention ranged from 3 weeks to 12 months, and follow‐up ranged from 4 weeks to 12 months. We have summarised the outcomes reported, and those usable for this review, in Table 5.
Modification of meal profile or pattern
We identified 12 trials for this category (Barton 2000; Bouillanne 2013; Castellanos 2009; Essed 2007; Essed 2009; Germain 2006; Leslie 2012; Mathey 2001b; Munk 2014; Silver 2008; Smoliner 2008; Taylor 2006), (N = 649, 6% of review participants). The trial by Barton 2000 included three groups, two of which were randomised to treatment or control and one other where it was unclear whether there was randomisation. Data have therefore only been included for those participants who were randomised to the treatment and usual care groups (N = 27). The trials included people from a range of clinical backgrounds who were in hospital (Barton 2000; Bouillanne 2013; Munk 2014), residential care homes (Castellanos 2009; Essed 2007; Essed 2009; Germain 2006; Leslie 2012; Mathey 2001b; Smoliner 2008; Taylor 2006), and free‐living participants in receipt of home‐delivered lunch meals (Silver 2008). Interventions consisted of altering portion sizes or fortifying meals, or both (Barton 2000; Castellanos 2009; Leslie 2012; Silver 2008), providing 78% of daily protein requirements at the lunch time meal, rather than spread evenly throughout the day (Bouillanne 2013), modifying the taste of foods previously identified as preferred (Essed 2007; Essed 2009; Mathey 2001b), modification of the appearance and presentation of pureed foods, thickened beverages, and dietary supplements (Germain 2006), the provision of an a la carte menu of enriched meals (Munk 2014) and altering meal pattern (Taylor 2006). We have summarised the outcomes reported, and those of use in this review, in Table 6.
Additional supplementation of meals
We identified 10 trials for this category (Beck 2002; Bourdel‐Marchasson 2000; Dennis 2005; Faxen‐Irving 2011; Hankey 1993; Larsson 1990; Potter 2001; Simmons 2008; Simmons 2010; Van den Berg 2015) (N = 6022, 56.4% of review participants). One trial did not state clearly the number of participants as additional publications appeared to include different numbers (Larsson 1990). As stated in the primary reference, 435 participants were therefore included in this review. The trial by Simmons 2008 was a two‐phase crossover and cluster‐randomised trial where residents were randomised only if they had a low oral food and fluid intake and were responsive to one of two feeding‐assistance interventions. This randomised sub‐group of intervention and control participants were then crossed over. We used data from the intervention and comparison groups prior to cross‐over in this review, as additional participants were added to the trial at the crossover.
One trial (Dennis 2005) included only people who had had a stroke . Other trials included either mixed participants, or did not report diagnoses. The majority of participants were from the hospital setting (Bourdel‐Marchasson 2000; Dennis 2005; Faxen‐Irving 2011; Hankey 1993; Larsson 1990; Potter 2001; Van den Berg 2015), and only 168 were from residential care homes (Beck 2002; Simmons 2008; Simmons 2010). In nine RCTs participants were offered between 400 kcal/day to 685 kcal/day in the form of a protein‐energy oral nutritional supplement, in addition to usual diet. In the other RCT participants were offered up to 420 kcal extra using 90 mL of fat emulsion/day (Faxen‐Irving 2011). We have summarised the outcomes reported, and those of use in this review, in Table 7.
Congregate and home meal delivery systems
We identified one trial for this category (Kretser 2003), including 203 free‐living participants (2% of review participants). Participants were offered modified home‐delivered meals with a daily follow‐up phone call. The outcomes of interest reported in this review included weight, clinical function, Activities of Daily Living score and number of deaths.
Excluded studies
Of the 182 trials/trial records after eligibility assessment, we excluded 27 trials as they were non‐randomised controlled trials or the group assignment was made after randomisation, and 89 trials that did not describe supportive interventions in nutritional care. It was necessary for all four review authors to participate in discussion about the reasons for exclusion of trials from intervention category four, ‘additional supplementation of meals’. Trials were excluded in this group for the following reasons.
Participants were not from an institutionalised setting; therefore it was considered that they would have been given individualised advice on taking oral nutritional supplements.
No clear organisational component to the intervention was described (for example when supplements were given without a clear description of delivery (i.e. administered at the same time as medication, or in place of usual morning/afternoon tea), or frequency of delivery).
Trials with multi component interventions where it was not possible to extract data relating to the specific effect of nutritional intervention.
Twenty‐four trials are awaiting assessment.
Risk of bias in included studies
The judgements made about risk of bias for individual trials are detailed in the 'risk of bias' section (Characteristics of included studies). A ‘Risk of bias summary’, and ‘Risk of bias graph’ are shown in Figure 3 and Figure 4. We judged the majority of criteria used in the assessment of risk of bias as unclear, indicating insufficient information to permit a full assessment of the risk of bias. The exceptions were attrition bias and reporting bias, where we judged the majority of trials (61% and 76% respectively) as being at low risk of bias (Figure 4).
3.
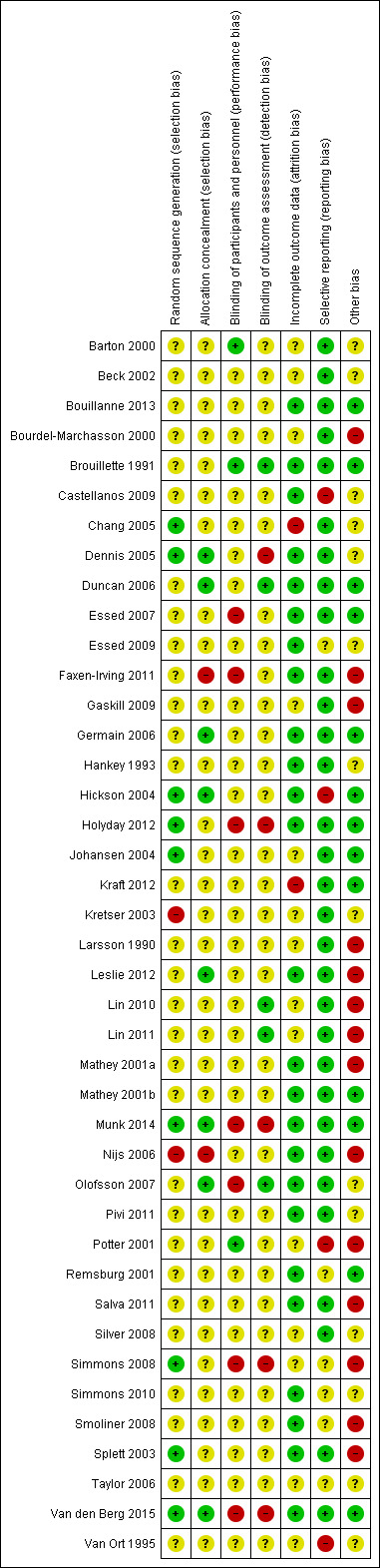
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
4.
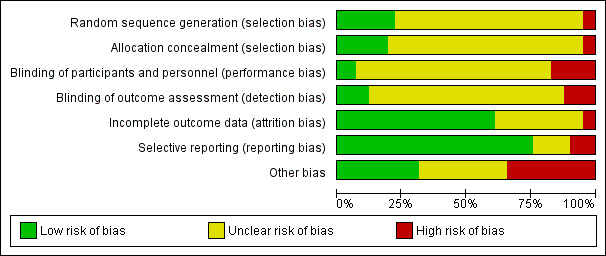
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
Allocation
Generation of sequence
We assessed nine of 41 trials (Chang 2005; Dennis 2005; Hickson 2004; Holyday 2012; Johansen 2004; Munk 2014; Simmons 2008; Splett 2003; Van den Berg 2015), as being at low risk of bias for the method of random sequence generation. Two of these trials used the toss of a coin as a method of randomisation (Chang 2005; Simmons 2008), one used a sequence generated by a member of staff not involved in the trial (Munk 2014) and another used a random number table (Splett 2003). The other trials in this group used computer‐generated randomisation methods.
Two of 41 trials ( Kretser 2003; Nijs 2006) used inadequate methods of randomisation and we consequently gave them a high risk of bias. In another trial (Kretser 2003) the authors stated "randomised treatment assignment was followed with a few exceptions". When the participants were randomised to receive the new meals on wheels and refused, they were automatically placed on the traditional meals on wheels model. We therefore considered that allocation was made by preference of the participant. In the trial by Nijs 2006 the investigators described a non‐random component in the sequence generation process, based on the name of the ward. This was therefore given a high risk of bias score.
One trial did not detail whether the third intervention group was randomised, and subsequently received an unclear risk of bias (Barton 2000). The remaining trials in the review provided insufficient information about the sequence generation process to permit judgement of low or high risk of bias. We therefore categorised them as unclear risk of bias.
Allocation concealment
We assessed eight of 41 trials (Dennis 2005; Duncan 2006; Germain 2006; Hickson 2004; Leslie 2012; Munk 2014; Olofsson 2007; Van den Berg 2015), as being at low risk of bias for allocation concealment , as they used sequentially numbered or opaque sealed envelopes opened by a member of staff not involved in the trial, or allocation was made by a statistician having no other contact with the participants. The trial by Faxen‐Irving 2011 was considered to be at a high risk of allocation concealment, as they used sealed envelopes without describing the appropriate safeguards, for example, not sequentially numbered, or opaque. This suggested that participants, or investigators enrolling participants, could predict assignments, and thus introduce selection bias. Another trial used no concealment and therefore we judged it to be at a high risk of bias (Nijs 2006). The remaining trials included in the review we categorised as unclear risk of bias, as they provided insufficient information to permit a full assessment of the risk of bias.
Blinding
Blinding of participants and personnel (performance bias)
We judged three of 41 trials (Barton 2000; Brouillette 1991; Potter 2001) to be at a low risk of bias, as the trial participants were blind to group allocation or to what treatment they were receiving. We also judged that blinding was unlikely to have been broken throughout the trials. To give examples, in the trial by Barton 2000 the participants and staff were blinded to which menu they were following. In the trial by Brouillette 1991, the research assistant was unaware of group assignment. We awarded Potter 2001 a low risk of bias score, as researchers who knew the randomisation codes were not involved in outcome data collection or data entry.
We judged seven of 41 trials (Essed 2007; Faxen‐Irving 2011; Holyday 2012; Munk 2014; Olofsson 2007; Simmons 2008; Van den Berg 2015) to be at high risk of bias, predominantly due to a lack of blinding of key trial personnel. In the trial by Essed 2007 there was incomplete blinding, as participants were blinded but the research personnel were not. In the trial by Faxen‐Irving 2011, study nurses opened sealed envelopes, therefore would have been aware of group allocation. In the trial by Holyday 2012, the authors stated it was not possible to blind the clinical dietitian to group allocation. We therefore judged that the outcome was likely to be influenced by a lack of blinding of key trial personnel. Additionally, the trial by Olofsson 2007 stated that staff on the usual care ward were aware of a programme being implemented on another ward in the hospital. It was therefore judged that outcome assessment was likely to be influenced by lack of blinding to these key trial personnel. The remaining trials in the review we categorised as unclear risk of bias, as insufficient information was provided to permit judgement.
Blinding of outcome assessment (detection bias)
We judged five of 41 trials (Brouillette 1991; Duncan 2006; Lin 2010; Lin 2011; Olofsson 2007) to be at low risk of bias. Researchers assessing outcomes were unaware of treatment allocation; therefore we judged that the blinding was unlikely to have been broken. We judged five of 41 trials (Dennis 2005; Holyday 2012; Munk 2014; Simmons 2008; Van den Berg 2015) as at high risk of bias, as outcome assessment was not blinded, and the outcome measurement was likely to be influenced by the lack of blinding. One trial stated, “as the outcomes are primarily objective measures, they are mostly not open to the influence of bias” (Holyday 2012). Additionally, the trial by Dennis 2005 stated “follow up was masked to treatment allocation except when patients or carers inadvertently divulged it to an interviewer, which was usually, but not systematically recorded”. In the trial by Simmons 2008 outcomes were not assessed blinded to treatment and the outcomes were judged to be susceptible to detection bias. In the trial by Van Ort 1995, the research staff who observed videotapes were unaware of the trial hypothesis, but were aware of group allocation. We gave this trial, and the remaining 28 trials, an unclear risk of bias, as insufficient information was provided to permit judgement of the risk of bias.
Incomplete outcome data
The numbers of participants excluded from trials, along with reasons, were fully reported in 25 out of 41 trials and we judged these to have a low risk of bias. The number of participant exclusions ranged from 0% to 81%. The trial by Chang 2005 we judged to be at high risk of bias, because data were presented on only 20 of the 36 participants, without explanation. We judged another trial as high risk due to the high attrition rate in the intervention group (Kraft 2012). Here, eight participants out of 13 in the intervention group withdrew, and three out of 13 in the usual care group withdrew.
We included a total of 14 trials in the unclear risk of bias category. Three trials did not report exclusions (Barton 2000; Beck 2002; Simmons 2008). One of these is awaiting clarification from the trial author (Beck 2002), and another only reported participant exclusions in one of the intervention groups (Barton 2000). In a further three trials, the numbers of exclusions were unclear (Bourdel‐Marchasson 2000; Gaskill 2009; Larsson 1990). Six trials only reported a total number finishing the trial, rather than a breakdown for the intervention and usual care groups separately (Johansen 2004; Kretser 2003; Lin 2010; Silver 2008; Taylor 2006; Van Ort 1995). Each of these trials stated why participants dropped out, however it was unclear which group they were allocated to. Simmons 2008 reported dropouts from each group, however only described mortality as the primary reason (58%). One trial did not describe attrition (Lin 2011), and another trial reported outcome in relation to BMI and triceps skinfold thickness (TSF), but not BMI and TSF alone (Potter 2001).
Selective reporting
Thirty‐one of the 41 trials reported all outcomes as stated in the trial methodology, and we therefore judged them to be at low risk of bias. We categorised four trials as high risk of bias (Castellanos 2009; Hickson 2004; Potter 2001; Van Ort 1995). In the trial by Potter 2001, one or more outcomes of interest to the review were described as collected but were incompletely reported. In another trial, results for the whole group were not reported according to the initial randomisation (Castellanos 2009). In the trial by Hickson 2004, no data were reported on: use of service questionnaires, referral rate to therapists, readmission within six months, laxative use, pressure sores and economic analysis. In the trial by Van Ort 1995, outcomes were described in the methodology, however no quantitative data were reported. We categorised the remaining six trials as unclear risk of bias (Essed 2009; Remsburg 2001; Simmons 2008; Simmons 2010; Smoliner 2008; Taylor 2006), as insufficient information was provided in order to make a judgement on risk of bias.
Other potential sources of bias
We judged 13 of the 41 trials as low risk of bias, as intervention and usual care groups were comparable at baseline (Bouillanne 2013; Brouillette 1991; Duncan 2006; Essed 2007; Germain 2006; Hickson 2004; Holyday 2012; Johansen 2004; Kraft 2012; Mathey 2001b; Munk 2014; Remsburg 2001; Van den Berg 2015). In Hickson 2004, there were significantly more women in the intervention compared with the usual care group, but otherwise groups were comparable. Three parallel RCTs were judged at high risk of bias (Faxen‐Irving 2011; Larsson 1990; Potter 2001). Faxen‐Irving 2011 provided data only from those who completed the trial, potentially missing valuable data for those who dropped out. In the trial by Larsson 1990, there were significant differences between groups at baseline. TSF and weight index in men, and mid‐arm circumference (MAC) in women were significantly lower in the intervention group than the control. The intervention group also had a significantly poorer mental condition as assessed using the modified Norton score on admission. In the trial by Potter 2001, only half of those in the ‘well nourished’ group were randomised, therefore bias was likely to have occurred. We categorised 14 trials as unclear risk of bias, as there was insufficient information to assess whether an important risk of bias existed.
We considered the following risk of bias criteria for the 12 cluster‐RCTs (Bourdel‐Marchasson 2000; Chang 2005; Gaskill 2009; Leslie 2012; Lin 2010; Lin 2011; Mathey 2001a; Nijs 2006; Salva 2011; Simmons 2008; Smoliner 2008; Splett 2003): (a) recruitment bias, (b) baseline imbalance, (c) loss of clusters, (d) incorrect analysis, and (e) comparability with individually randomised trials or different types of clusters as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). If any of the aforementioned criteria applied, we assigned a high risk of 'other bias'. Consequently, all included cluster RCTs had a high risk of bias. In the trial by Chang 2005 it was unclear whether randomisation occurred at the unit level (more probable) or the individual level. We therefore judged this trial to be an unclear risk of other bias.
Effects of interventions
See: Table 1
We could not recalculate data taking into account the design effect for the 12 cluster RCTs (Bourdel‐Marchasson 2000; Chang 2005; Gaskill 2009; Leslie 2012; Lin 2010; Lin 2011; Mathey 2001a; Nijs 2006; Salva 2011; Simmons 2008; Smoliner 2008; Splett 2003) because we did not have reliable information about intracluster correlation coefficients for our substantial heterogeneous populations in the included trials. Therefore, we did not establish meta‐analyses by using both parallel and cluster RCTs but excluded the cluster RCTs from all meta‐analyses. Also, cross‐over trials did not contribute to the effect estimates established by meta‐analyses.
Overview of all trials combined
Primary Outcomes
Nutritional intake
Data on this outcome were reported in 27 of 41 trials (Barton 2000; Beck 2002; Bouillanne 2013; Bourdel‐Marchasson 2000; Brouillette 1991; Castellanos 2009; Chang 2005; Duncan 2006; Essed 2007; Essed 2009; Faxen‐Irving 2011; Germain 2006; Hankey 1993; Hickson 2004; Johansen 2004; Leslie 2012; Lin 2010; Mathey 2001a; Mathey 2001b; Munk 2014; Nijs 2006; Potter 2001; Silver 2008; Simmons 2008; Simmons 2010; Taylor 2006; Van den Berg 2015).
The trials reporting on change in energy intake were in participants from a range of clinical backgrounds and healthcare settings and there were differences between trials in how energy intake was assessed (from observations of amounts eaten to detailed weighing and analysis). The majority of trials found no marked difference in energy intake between groups. One trial of assistance at mealtimes in hospitalised patients with hip fracture (Duncan 2006) reported a greater energy intake in the intervention group than in the usual care group (1105 kcal (SD 361) versus 759 (SD 399), P < 0.001) and a trial of a multidisciplinary team intervention in hospitalised patients (Johansen 2004) reported a higher intake in the intervention group than in the control group (Table 11). Two trials of fortification of meals (Barton 2000; Silver 2008) reported greater energy intakes in participants receiving the fortification than those receiving usual care (Table 16) and one trial of modifications to the appearance and presentation of foods to individuals with dysphagia (Germain 2006) reported a greater energy intake in the participants receiving the intervention (Table 16). Two of 10 trials of supplementation of meals with oral nutritional supplements (Hankey 1993; Van den Berg 2015) reported a higher energy intake in groups receiving the supplement, however the between‐group differences were not reported (Table 20).
Health‐related quality of life and patient satisfaction
Data on health‐related quality of life were reported in five of 41 trials (Dennis 2005; Johansen 2004; Mathey 2001a; Nijs 2006; Smoliner 2008). Data were collected using different quality‐of‐life instruments; two trials used the Short Form‐36 (SF‐36) (Johansen 2004; Smoliner 2008), one trial used the Dutch quality of life of somatic nursing home residents questionnaire (Nijs 2006), one used the European Quality of Life Scale (EuroQOL‐5D or EQ‐5D) (Dennis 2005) and the final trial (Mathey 2001a) used the Sickness Impact Profile (SIP) and Philadelphia Geriatric Center Morale Scale (PGCMS, 17 items). The trials reporting on health‐related quality of life included participants from a wide range of different clinical backgrounds. No marked differences between groups were found in four trials (Dennis 2005; Johansen 2004; Mathey 2001a; Smoliner 2008) (Table 12; Table 17; Table 24), the overall quality of evidence was low and two trials were cluster‐randomised trials and therefore at high risk of bias (Mathey 2001a; Smoliner 2008). Nijs 2006 assessed health‐related quality of life using a validated Dutch questionnaire (Van Campen 1998). This questionnaire consists of five sub‐scales, each representing a quality‐of‐life dimension: sensory functioning (focusing on pain); physical functioning (perceived performance and self care); psychosocial functioning (depression or loneliness); perceived autonomy (freedom of movement); and perceived safety (feeling at home in the institution). The number of statements in the five sub‐scales is not equal. The questionnaire consists of 50 statements, scored on a dichotomous scale (yes or no). Each sub‐scale and the total questionnaire is computed to achieve a score from 0 to 100. A high score represents a high quality of life. The results were presented as difference in changes in overall quality of life between the groups and were reported as statistically significant (6.1 units, 95% confidence interval (CI) 2.1 to 10.3). The intervention group remained stable (0.4 units, 95% CI 1.8 to 2.5), whereas the usual care group declined (‐0.5 units, 95% CI ‐9.4 to 0.6), although the overall changes were small and it is unclear if the observed differences were likely to be noticeable to participants (Table 17). Moreover, this trial was at high risk of bias. Therefore, all reported outcome measures of this trial must be interpreted with caution.
Data on patient satisfaction were reported in two trials (Duncan 2006; Salva 2011). Duncan 2006 assessed patient satisfaction using an unvalidated questionnaire with 10 questions about aspects of meals, diet and feeding. Participants answered yes or no, where yes = 1, no = ‐1 and NA = 0. Those participants who had received the support of the dietetic assistants showed greater satisfaction, with a median score of 6.5 (interquartile range (IQR) 2) compared to 3 (IQR 4) for participants receiving usual care (P < 0.0001) (Table 12). In the trial by Salva 2011 satisfaction of participants and their families was assessed by an unvalidated questionnaire which asked about the use of and perceived usefulness of five aspects of the overall programme. Families and carers were asked to indicate whether they had used the service and whether they had found it very useful, useful or not very useful. Information cards were used by 94.5% of families and rated the service as very useful (26%) or useful (67%). The nutrition course was used by 66% of families and rated as very useful (24%) and useful (65%). Weight curves were sent to 88% of families and rated as very useful (13%) and useful (78%). Information sessions were attended by 75% of families and rated as very useful (32%) and useful (61.5%). The hot line was used by 33% of families and rated as very useful (17%) and useful (51%).
Morbidity/complications
Data on this outcome were reported in seven of 41 trials (Bouillanne 2013; Bourdel‐Marchasson 2000; Dennis 2005; Duncan 2006; Hickson 2004; Johansen 2004; Olofsson 2007). Complications were reported as either the number of participants experiencing any complication (Bouillanne 2013; Dennis 2005; Duncan 2006; Johansen 2004; Olofsson 2007), number of participants with pressure ulcers (Bourdel‐Marchasson 2000; Dennis 2005) or the number of participants needing oral antibiotics (Hickson 2004). Trials were in participants from different clinical backgrounds, in different healthcare settings and receiving interventions that aimed to be supportive of improved nutritional intake, and varied widely. There were no marked differences in complication rates between groups reported in any trial (Table 12).
Meta‐analysis of trials reporting number of participants experiencing any complication showed considerable inconsistency (I² = 91%). Risk ratios ranged between 0.59 indicating benefit for supportive interventions, to 1.42 indicating benefit of control interventions (5 trials; 4015 participants; very low‐quality evidence; Analysis 1.1).
1.1. Analysis.
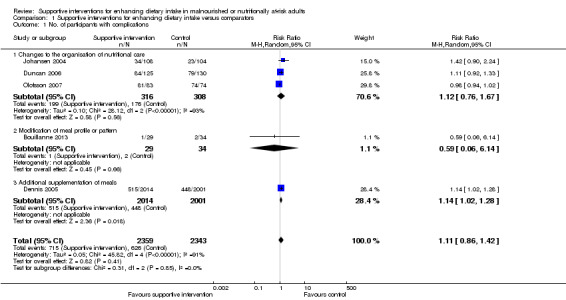
Comparison 1 Supportive interventions for enhancing dietary intake versus comparators, Outcome 1 No. of participants with complications.
Secondary Outcomes
Nutritional status
Weight change
Data on this outcome were reported in 28 of 41 trials (Beck 2002; Bouillanne 2013; Chang 2005; Duncan 2006; Essed 2007; Faxen‐Irving 2011; Germain 2006; Hankey 1993; Hickson 2004; Holyday 2012; Johansen 2004; Kraft 2012; Kretser 2003; Larsson 1990; Leslie 2012; Lin 2010; Mathey 2001a; Mathey 2001b; Munk 2014; Nijs 2006; Olofsson 2007; Pivi 2011; Potter 2001; Remsburg 2001; Salva 2011; Simmons 2008; Simmons 2010; Smoliner 2008). Trials were in participants from different clinical backgrounds, in different healthcare settings and receiving interventions which, although aiming to support improved nutritional intake, varied from one another in the nature of the intervention.
Meta‐analysis across 17 trials with adequate data on weight change revealed an overall improvement in weight in favour of supportive interventions versus control: mean difference (MD) 0.6 kg (95% CI 0.21 to 1.02); P = 0.003; 2024 participants; moderate‐quality evidence; Analysis 1.2. However, heterogeneity was moderate (I² = 51%). We excluded the trial by Pivi 2011 from this meta‐analysis because missing SDs for weight change could not be reliably imputed. Trial authors reported a significant difference between intervention groups using a P value < 0.001. Using a P value of 0.0005 for imputation of SDs resulted in an SD of 3.3. Using these data did not substantially alter the effect estimate. Some other trials showed bias from different sources, however, exclusion of these trials did not substantially change the overall effect estimate. Also, elimination of any subtype of supportive intervention did not change the overall effect estimate in a substantial way. The body of evidence for this outcome consisted mainly of trials on change to the organisation of nutritional care (6 trials). However, the interaction test for subgroup differences was significant indicating the need to further investigate the various types of supportive interventions in future trials (Figure 5).
1.2. Analysis.
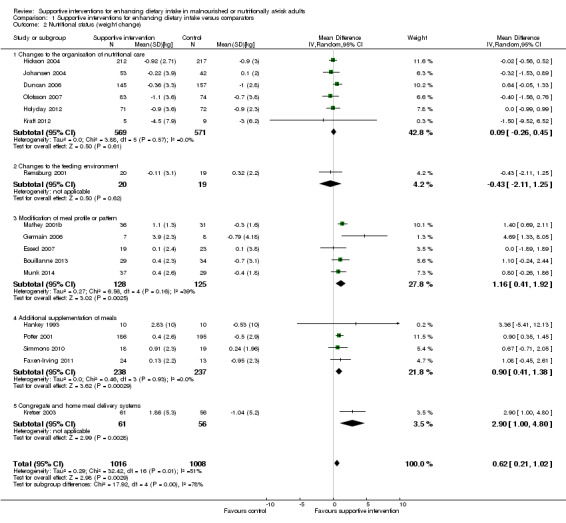
Comparison 1 Supportive interventions for enhancing dietary intake versus comparators, Outcome 2 Nutritional status (weight change).
5.
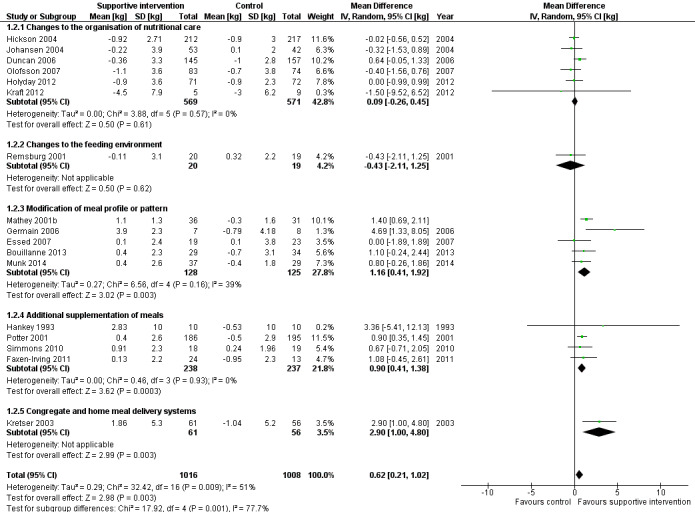
Forest plot of comparison: 1 Supportive interventions for enhancing dietary intake versus comparators, outcome: 1.2 Nutritional status (weight change) (kg)
Change in BMI
Data on change in BMI were reported in 12 of 41 trials (Faxen‐Irving 2011; Germain 2006; Hickson 2004; Kraft 2012; Leslie 2012; Lin 2010; Lin 2011; Olofsson 2007; Pivi 2011; Salva 2011; Simmons 2008; Smoliner 2008). Trials were in participants from different clinical backgrounds, in different healthcare settings and receiving interventions that aimed to support improved nutritional intake but varied from one another. The majority of trials reported no marked difference in BMI between groups. In the trial by Pivi 2011 participants receiving specialist training experienced an increase in BMI (1.2 kg/m² (SD 1)) and participants in the usual care group experienced a reduction in BMI (‐2.2 kg/m² (SD 1)). However, the between‐group difference and statistical tests were not reported. The trial by Germain 2006, which examined the effects of modifications to the presentation of meals to participants with dysphagia, and in the trial by Leslie 2012 of food fortification in residential care homes, the intervention group had a greater gain in BMI than the usual care group (Table 18). However, between‐group differences with statistical tests were not reported. In the trial by Faxen‐Irving 2011 BMI was reported according to group at the end of the intervention and there was no marked difference between groups, change from baseline and between‐group differences were not reported. In the trial by Simmons 2008 the intervention group gained 0.7 kg/m² more than the usual care group (P < 0.009) (Table 25).
Change in TSF
Data on this outcome were reported in five of 41 trials (Duncan 2006; Hankey 1993; Hickson 2004; Larsson 1990; Pivi 2011). Trials were in participants receiving assistance during mealtimes (Duncan 2006; Hickson 2004), specialist training (Pivi 2011) and supplementation with oral nutritional supplement (Hankey 1993; Larsson 1990) in different healthcare settings. There were no marked differences in TSF reported between groups in the trials by Duncan 2006, Hickson 2004 and Pivi 2011. In the trials by Hankey 1993 and Pivi 2011 data were presented in figures with minimal description in the text. In the trial by Hankey 1993 the intervention group was described as experiencing a smaller decrease in TSF than the usual care group (6.6% versus 15.8%). In the trial by Larsson 1990 TSF decreased over the 26 weeks of follow‐up in both groups with the greatest decrease occurring in the usual care group.
Change in MAC
Data on this outcome were reported in eight of 41 trials (Duncan 2006; Hankey 1993; Hickson 2004; Larsson 1990; Leslie 2012; Nijs 2006; Pivi 2011; Potter 2001). Trials were in participants from different clinical backgrounds, in different healthcare settings and receiving interventions which aimed to support improved nutritional intake but varied from one another. Three trials reported no marked difference in MAC between groups (Hickson 2004; Nijs 2006; Potter 2001). In the trial by Duncan 2006, the group that received assistance with eating had a smaller reduction in MAC of ‐0.9 cm (SD 2.2) compared with the group that received usual care, ‐1.3 (SD 1.5) (P = 0.002). One trial evaluating the impact of specialist training in free‐living individuals (Pivi 2011) reported improvements in MAC in the intervention group of 1.9 cm (SD 2) compared with a reduction of ‐0.4 cm (SD 0.5) in the group receiving usual care. In the trial by Leslie 2012 of food fortification in residential care homes, participants in the intervention group had a greater improvement in MUAC than those in the control group but the between‐group differences and statistical tests were not reported (Table 21) In the trial by Hankey 1993, the data were unavailable from the original trial report but we obtained them from a systematic review by Milne 2009. We read the figures for change from a graph, and we assumed the SD of change to be 10 cm for each group. MAC was described as improving in the intervention group (P < 0.05) but remaining unchanged in the usual care group. The changes were small and no between‐group differences were reported (Table 25). In the trial by Larsson 1990 the data are presented in a figure with some description in the text, participants who were well nourished at the start of the trial and received supplementation of meals experienced less decrease in MAC at 26 weeks (P < 0.05) than those receiving usual care. In participants who were malnourished at the start of the trial both groups experienced a decrease in MAC at 26 weeks.
Clinical function
Data on this outcome were reported in nine of 41 trials (Bouillanne 2013; Duncan 2006; Faxen‐Irving 2011; Hickson 2004; Kretser 2003; Munk 2014; Potter 2001; Salva 2011; Smoliner 2008). Trials were in participants from a variety of different clinical backgrounds, in different healthcare settings and were assessed using a variety of methods including handgrip strength, Barthel score, Activities of Daily Living (ADL), instrumental ADL (iADL) and peak flow.
Three trials assessed functional recovery using the Barthel score (Hickson 2004; Smoliner 2008; Potter 2001). The Barthel index consists of 10 items that measure a person's daily functioning, specifically the activities of daily living and mobility (Mahoney 1965). The items include feeding, moving from wheelchair to bed and return, grooming, transferring to and from a toilet, bathing, walking on level surface, going up and down stairs, dressing, continence of bowels and bladder. The items are weighted according to a scheme developed by the authors. The person receives a score based on whether they have received help while doing the task. The scores for each of the items are summed to create a total score. The higher the score the more 'independent' the person. Independence means that the person needs no assistance with any part of the task. There were no marked differences between groups in any trial. In the trial by Potter 2001 there was no marked difference in numbers achieving functional recovery assessed using the Barthel index in the group receiving supplementation compared with the usual care group (102/149 intervention versus 100/157 control, P = 0.38). However, more participants classified as severely undernourished experienced an improvement in their Barthel scores on supplementation compared with those that received usual care (17/25 intervention versus 11/28 control, P < 0.04).
Four trials assessed clinical function using the ADL and iADL scores (Bouillanne 2013; Faxen‐Irving 2011; Kretser 2003; Salva 2011). Two main types of abilities are measured by these functional assessment scales. Basic ADL consist of activities that are performed daily, habitually and universally, such as dressing, bathing, and eating. In contrast, iADL requires organisation and planning, and includes such tasks as shopping, using transportation, preparing meals, handling finances, keeping the house, and using a telephone. The scores range from 0 to 100 and amount of functional impairment is then rated as ‘‘none to mild’’ (0 to 33), ‘‘moderate’’ (34 to 66), or ‘‘severe’’ (> 66). All trials reported no marked differences in ADL between the intervention and usual care groups. One trial used the iADL (Kretser 2003) to measure clinical function. There was a greater decline in iADL in those receiving traditional meals on wheels compared with those receiving modified meals on wheels at six months (P = 0.0494).
Five trials assessed clinical function using handgrip strength (Bouillanne 2013; Duncan 2006; Hickson 2004; Munk 2014; Smoliner 2008), and there were no marked differences in any trial between the groups receiving the intervention and those receiving usual care (Table 14; Table 22).
In the trial by Smoliner 2008 clinical function was also measured using peak flow. Peak expiratory flow is the maximum flow generated during expiration performed with maximal force and started after a full inspiration. A decrease in peak flow rates indicates a deterioration in clinical function and vice versa. The peak flow in the intervention group increased from baseline to follow‐up (12 weeks) (mean 152 mL/min (SD 105) to 186 mL/min (SD 140) whereas the usual care showed a decline (151 mL/min (SD90) to 150 mL/min (SD 67). The between‐group difference was statistically significant (P = 0.039).
Hospitalisation and institutionalisation
Data on length of hospital stay were reported in 10 of 41 trials (Dennis 2005; Duncan 2006; Faxen‐Irving 2011; Hickson 2004; Holyday 2012; Johansen 2004; Munk 2014; Olofsson 2007; Potter 2001; Van den Berg 2015). The trials were either of changes to the organisation of nutritional care (Duncan 2006; Hickson 2004; Holyday 2012; Johansen 2004; Olofsson 2007 ), fortification of meals in hospital (Munk 2014) or of supplementation of meals with oral nutritional supplements (Dennis 2005; Faxen‐Irving 2011; Potter 2001: Van den Berg 2015 ). Nine trials reported no marked difference in length of hospital stay between groups (Dennis 2005; Duncan 2006; Faxen‐Irving 2011; Hickson 2004; Holyday 2012; Johansen 2004; Munk 2014; Potter 2001; Van den Berg 2015). In the trial by Olofsson 2007 groups receiving a multidisciplinary team intervention had a shorter mean length of hospital stay (27.4 days (SD 15.9)) than groups receiving usual care (39.8 days (SD 41.9)) (P < 0.05) (Table 15).
Meta‐analysis across five trials with adequate data on length of hospital stay showed a MD between intervention and comparator groups of ‐0.5 days (95% CI ‐2.6 to 1.6); P = 0.56; 667 participants; very low‐quality evidence; Analysis 1.3.
1.3. Analysis.
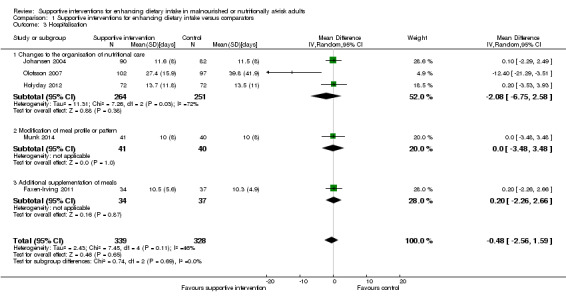
Comparison 1 Supportive interventions for enhancing dietary intake versus comparators, Outcome 3 Hospitalisation.
Data on hospital readmissions were reported in two of 41 trials (Holyday 2012; Van den Berg 2015). In the trial by Holyday 2012 the groups receiving a protocol‐driven pathway for the management of nutrition whilst in hospital had fewer hospital readmissions than the group receiving usual care (30/71 versus 37/72 respectively). However the between‐group difference was not statistically significant. In the trial by Van den Berg 2015 there were more hospital readmissions in the group receiving an oral nutritional supplement four times daily than the groups receiving the supplement twice daily or the usual care group (24 versus 13 versus 15 respectively).
The trial by Potter 2001 reported the destination of participants at discharge according to group allocation. There was no marked difference between groups in the numbers of participants returning to their own home and those being discharged to an institution (Table 26).
Adverse events
Three of 41 trials (Dennis 2005; Faxen‐Irving 2011; Hankey 1993) reported on adverse events, all trials evaluating the impact of supplementation of meals with oral nutritional supplements. The overall quality of the evidence was very low. The trial by Faxen‐Irving 2011 reported that 5 of 34 (15%) participants experienced intolerance to the supplement assessed as diarrhoea and vomiting. In the trial by Dennis 2005 565 of 2017 (28%) of participants stopped taking the oral nutritional supplement due to individuals' refusal or dislike of taste, unwanted weight gain, or feelings of nausea. The trials by Potter 2001 and Van den Berg 2015 reported that no adverse events occurred.
All‐cause mortality
Adequate data were reported on this outcome in 12 out of 41 trials (Bouillanne 2013; Brouillette 1991; Dennis 2005; Duncan 2006; Hickson 2004; Holyday 2012; Kretser 2003; Larsson 1990; Munk 2014; Olofsson 2007; Potter 2001; Van den Berg 2015). Six cluster‐RCTs could not be included in the meta‐analysis (Bourdel‐Marchasson 2000; Leslie 2012; Mathey 2001a; Nijs 2006; Salva 2011; Smoliner 2008).
Trials were in participants from a variety of clinical backgrounds and in a range of different healthcare settings, receiving interventions which were all supportive of improved nutritional intake but varied widely. Meta‐analysis showed a RR of 0.78 (95% CI 0.66 to 0.92); P = 0.004; 12 trials; 6683 participants; moderate‐quality evidence; Analysis 1.4 in favour of supportive interventions (Figure 6). The test for subgroup differences of the various supportive interventions did not indicate interaction. Subgroup analysis of longer‐term trials (four months to one year) showed a RR of 0.73 (95% CI 0.55 to 0.98); 6 trials; 5200 participants. The sensitivity analysis after exclusion of the biggest trial, Dennis 2005, showed a RR of 0.67 (95% CI 0.54 to 0.82); 11 trials; 2660 participants.
1.4. Analysis.
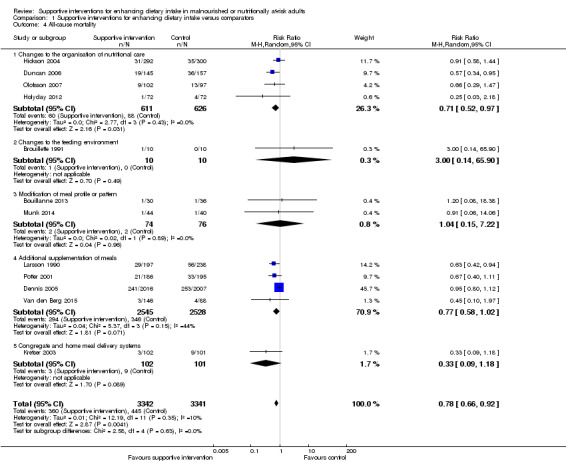
Comparison 1 Supportive interventions for enhancing dietary intake versus comparators, Outcome 4 All‐cause mortality.
6.
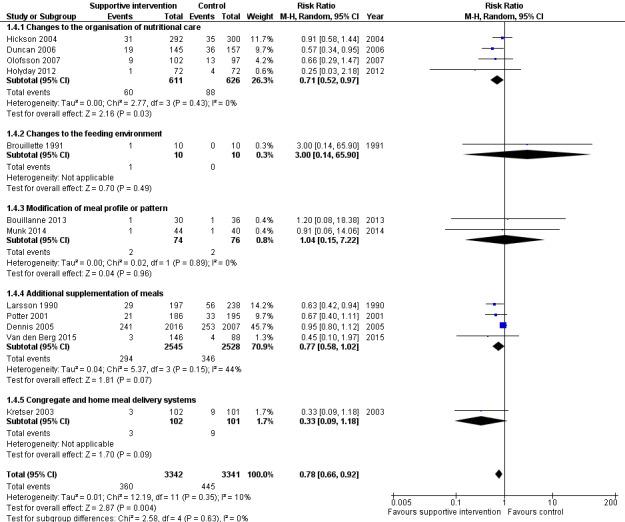
Forest plot of comparison: 1 Supportive interventions for enhancing dietary intake versus comparators, outcome: 1.4 All‐cause mortality
Economic costs
Data on this outcome were reported in three of 41 trials (Holyday 2012; Salva 2011; Simmons 2010). The overall quality of the evidence was very low. The trial by Holyday 2012 evaluated the impact of a protocol‐driven pathway for the management of nutritional care in hospital patients and the trial by Salva 2011 evaluated the impact of specialist training for carers of free‐living individuals with dementia. In the trial by Holyday 2012 the data on cost savings were based on reductions in the length of hospital stay. There was no marked difference in overall length of stay between groups. There was a shorter length of stay by eight days in the subgroup of 32 malnourished participants (12 days in the intervention group and 20 days in the usual care group). These data were used to estimate a cost saving of AUD 63,360 from treating malnutrition in the group of 12 malnourished participants based on the cost per hospital bed per day, the cost of the dietitians' time and the average cost of a commercial oral nutritional supplement. The trial by Salva 2011 collected data on resource utilisation but the data were not reported. The trial by Simmons 2010 evaluated the impact of a food‐based and oral nutritional supplement‐based intervention. In this trial a formal cost effectiveness analysis was not undertaken and reporting of the impact of the interventions on costs was limited to a report of the cost per serving of the oral nutritional supplement or food provided and an estimate of staff time required to encourage and assist consumption. The average costs (per person per day in USD) were significantly higher in groups receiving supplements and snacks compared with those in the usual care group (USD 2.10 versus, USD 2.06). None of the trials used accepted health economic methods and the reported data on both costs and effectiveness were generally poor.
Subgroup analyses
We carried out the first planned subgroup analysis 'intervention category'. Trials were grouped according to similar interventions into five categories. There were insufficient data to undertake further subgroup analyses.
Sensitivity analyses
We did not do any sensitivity analyses because of insufficient data.
Changes to the organisation of nutritional care
Primary outcomes
Nutritional intake
Data on energy intake were reported in five of 13 trials (Chang 2005; Duncan 2006; Hickson 2004; Johansen 2004; Lin 2010) (Table 11). Two trials used dietetic assistants in a hospital setting: one found a greater energy intake in groups receiving assistance than those receiving usual care (1105 kcal (SD 361) versus 759 kcal (SD 399), P < 0.001) (Duncan 2006), whereas in the other trial (Hickson 2004), which assessed between‐group difference in intake in 37 of 592 participants, the difference in energy intake between the groups was 89 kcal, P < 0.538. Of the four trials that implemented specialist training in long‐term care facilities, two reported data on energy intake as percentage of meals consumed (Chang 2005;Lin 2010). In one trial (Chang 2005), the intervention group experienced a reduction in percentage of meals consumed and the group receiving usual care increased their intake (P < 0.49). In the other trial (Lin 2010) there were small increases in percentage of meals consumed in all groups (Table 11). One trial providing multi‐disciplinary team care in a hospital setting reported a greater energy intake in the intervention group compared with usual care (30 kcal/kg/d (standard error (SE) 1) versus 25 kcal/kg/d (SE 1) (Johansen 2004).
Health‐related quality of life and patient satisfaction
Data on health‐related quality of life were reported in one of 13 trials (Johansen 2004). Quality of life was assessed using the SF36 questionnaire (Ware 1992) which was completed by 57% participants. A dropout analysis showed responders and non‐responders were similar in terms of baseline characteristics. There were no marked differences between the groups in both the physical and mental summary scores from baseline to follow‐up (physical score mean 2.4 (SE 1.3) in the intervention versus mean 0.2 (SE 1.5) in the control; mental score mean 2.2 (SE 2.5) in the intervention versus mean 3.3 (SE 2) in the usual care) (Table 12).
Data on patient satisfaction were reported in two of 13 trials (Duncan 2006; Salva 2011). In the trial by Duncan 2006 patient satisfaction was assessed using an unvalidated questionnaire with 10 questions about aspects of meals, diet and feeding. Patients answered yes or no where yes = 1, no = ‐1 and NA = 0. Those participants who had received the support of the dietetic assistants showed greater satisfaction with a median score of 6.5 (IQR 2) compared to 3 (IQR 4) for participants receiving usual care (P < 0.0001) (Table 12). In the trial by Salva 2011 satisfaction of participants and their families was assessed using an unvalidated questionnaire which asked about the use of and perceived usefulness of five aspects of the overall programme. Families and carers were asked to indicate whether they had used the service and whether they had found it very useful, useful or not very useful. Information cards were used by 94.5% of families and rated as very useful (26%) and useful (67%). The nutrition course was used by 66% of families and rated as very useful (24%) and useful (65%). Weight curves were sent to 88% of families and rated as very useful (13%) and useful (78%). Information sessions were attended by 75% of families and rated as very useful (32%) and useful (62%). The hot line was used by 33% of families and rated as very useful (17%) and useful (51%).
Morbidity/complications
Data on complications were reported in four of 13 trials (Duncan 2006; Hickson 2004; Johansen 2004; Olofsson 2007), three of which reported the number of participants experiencing any complications (Dennis 2005; Johansen 2004; Olofsson 2007) and one trial (Hickson 2004) reported the number of participants receiving oral antibiotics. There were no marked between‐group differences in any of the trials (Table 12).
Secondary outcomes
Nutritional status
Weight change
Data on this outcome were reported in 10 of 13 trials (Duncan 2006; Hickson 2004; Holyday 2012; Johansen 2004; Kraft 2012; Lin 2010; Olofsson 2007; Pivi 2011; Salva 2011; Splett 2003) (Table 13).
Two trials evaluated the impact of dietetic assistants in a hospital setting (Duncan 2006; Hickson 2004) and there were no marked differences in mean weight change between groups in either trial. One trial used specialist training in a residential care setting (Lin 2010) and there was no marked difference in mean weight change between the two groups. Two trials looked at specialist training for carers of free‐living individuals with dementia (Pivi 2011; Salva 2011). In one trial the intervention group experienced a small weight gain of 1.2 kg whereas the usual care experienced a small weight loss of 2.2 kg (Pivi 2011). In the other trial (Salva 2011) there was no marked difference between the two groups in mean weight change. Two trials reported weight change for interventions consisting of a multi‐disciplinary team approach to nutritional care (Johansen 2004; Olofsson 2007) and reported no marked differences between groups receiving intervention and those receiving usual care in either trial. One trial described a protocol‐driven pathway of nutritional care in hospital (Holyday 2012) and reported no marked differences in weight change between the groups receiving the intervention and usual care. Another trial reported data using a protocol‐driven care in a care home setting (Splett 2003). The authors did not report mean weight change but provided a narrative description of the proportions of participants maintaining or gaining weight. The percentage of participants maintaining or gaining weight during the trial was greater in the usual care group (57%) than in the intervention group (48%). One trial evaluated the impact of telemedicine in free‐living individuals and reported no marked difference between the groups in mean weight change (Kraft 2012).
Change in BMI
Data on this outcome were reported in seven of 13 trials (Hickson 2004; Kraft 2012; Lin 2010; Lin 2011; Olofsson 2007; Pivi 2011; Salva 2011): two trials of specialist training in a residential care setting (Lin 2010; Lin 2011), two of specialist training of free‐living individuals (Pivi 2011; Salva 2011), one of additional nutritional care from a trained health care assistant (Hickson 2004), one of multi‐disciplinary team care in hospital (Olofsson 2007) and one of telemedicine (Kraft 2012). There were no marked differences in BMI change between groups in six of the seven trials (Table 13). In one trial (Pivi 2011) participants receiving specialist training experienced an increase in BMI (1.2 kg/m² (SD 1) and participants in the usual care group experienced a reduction in BMI (‐2.2 kg/m2 (SD 1). However, the between‐group difference and statistical tests were not reported.
Change in TSF, MAMC and MUAC
Data on this outcome were reported in three of 13 trials (Duncan 2006; Hickson 2004; Pivi 2011). In the two trials that assessed the effects of using dietetic assistants in hospital (Duncan 2006; Hickson 2004) there were no marked differences in either TSF or MAMC between groups. In one trial (Hickson 2004) there was no marked difference in MAC between groups receiving assistance with eating and those receiving usual care, whereas in the other trial (Duncan 2006) the group that received assistance with eating had a smaller reduction in MAC (‐0.9 cm (SD 2.2)) compared with the group that received usual care (‐1.3 (SD 1.5), P < 0.002). One trial used specialist training in free‐living individuals (Pivi 2011) and reported improvements in MAC in the intervention group of 1.9 cm (SD 2) compared with a reduction of 0.4 cm (SD 0.5) in the group receiving usual care, and no marked difference between the groups in TSF.
Overall the data across all interventions suggest that there is minimal impact on weight change and body composition from changes to the organisation of nutritional care across different healthcare settings.
Clinical function
Data on this outcome were reported in three of 13 trials (Duncan 2006; Hickson 2004; Salva 2011). The trials by Duncan 2006 and Hickson 2004 both assessed the effect of assistance with eating in people in hospital on handgrip strength. There were no marked differences in handgrip strength between the intervention and usual care groups in either trial (Table 14). The trial by Hickson 2004 also assessed functional recovery in participants using the Barthel score. There was no marked difference between groups' initial assessment to discharge from hospital (median score 2.0 (IQR 0 to 5) in the group receiving feeding assistance and 1.0 (IQR 0 to 4), P = 0.23 in the group receiving usual care). The trial by Salva 2011 measured change in ADL (Katz 1963), and iADL (Lawton 1969) in free‐living individuals with dementia who had received specialist training on nutrition. There were no marked differences between the groups in either ADL or iADL at six and 24 months' follow‐up.
Hospitalisation and institutionalisation
Data were reported on length of hospital stay in five of 13 trials (Duncan 2006; Hickson 2004; Holyday 2012; Johansen 2004; Olofsson 2007). Two trials evaluated the impact of dietetic assistants in a hospital setting (Duncan 2006; Hickson 2004), two evaluated a multi‐disciplinary team intervention in hospital (Olofsson 2007; Johansen 2004) and one evaluated a protocol‐driven pathway in hospital (Holyday 2012). There were no marked differences between groups in length of hospital stay in four trials (Duncan 2006; Hickson 2004; Holyday 2012; Johansen 2004). In the other trial (Olofsson 2007) the group receiving a multidisciplinary team intervention had a shorter mean length of hospital stay than the group receiving usual care (27.4 days (SD 15.9) in the intervention group and 39.8 days (SD 41.9) in the usual care group (P < 0.05) (Table 15). Data on hospital readmissions were reported in one of 13 trials (Holyday 2012). The group receiving a protocol‐driven pathway for the management of nutrition whilst in hospital had fewer hospital readmissions than the group receiving usual care (30/71 (42%) versus 37/72 (51%) respectively) but the difference between the groups was not statistically significant.
Adverse events
No trial reported data on this outcome.
All‐cause mortality
Data were reported on this outcome in five of 13 trials (Duncan 2006; Hickson 2004; Holyday 2012; Olofsson 2007; Salva 2011). Two trials evaluated the impact of dietetic assistants in a hospital setting (Duncan 2006; Hickson 2004), one evaluated specialist training for free‐living individuals with dementia (Salva 2011), one evaluated a multi‐disciplinary team intervention in hospital (Olofsson 2007) and one evaluated a protocol‐driven pathway in hospital (Holyday 2012). There were no marked differences between groups in mortality in four trials (Hickson 2004; Holyday 2012; Olofsson 2007; Salva 2011), whereas in the other trial (Duncan 2006) there was a lower mortality at four months in the group receiving the intervention from dietetic assistants compared with the group receiving usual care (19/145 (13%) versus 36/157 (23%), P = 0.036) (Table 15).
Economic costs
Data on this outcome were reported in two of 13 trials (Holyday 2012; Salva 2011). One trial (Holyday 2012) evaluated the impact of a protocol‐driven pathway for the management of nutritional care in hospital patients and the other trial (Salva 2011) evaluated specialist training for carers of free‐living individuals with dementia. In one trial (Holyday 2012) the data on cost savings are based on reductions in length of stay achieved. There was no marked difference in length of stay overall between groups. There was a shorter length of stay by eight days in the subgroup of 32 malnourished participants (12 in the intervention group and 20 in the usual care group). These data were used to estimate a cost savings of AUD 63,360 from treating malnutrition in the group of 12 malnourished participants based on the cost per hospital bed per day, the cost of the dietitians' time and the average cost of a commercial oral nutritional supplement. The trial by Salva 2011 collected data on resource utilisation but the data were not reported. Neither trial used accepted health economic methods and the reported data on both costs and effectiveness were generally poor.
Changes to the feeding environment
Primary outcomes
Nutritional intake
Data were reported on energy intake in three of five trials (Brouillette 1991; Mathey 2001a; Nijs 2006). Two trials evaluated the impact of changes to the dining room environment (Mathey 2001a; Nijs 2006) and one evaluated a pre‐meal sensory stimulation intervention (Brouillette 1991). All trials assessed energy intake and were conducted in people in residential care. There were no marked between‐group differences in energy intake in any trial (Table 16).
Health‐related quality of life and patient satisfaction
Data were reported on health‐related quality of life in two of five trials (Mathey 2001a; Nijs 2006). One trial (Mathey 2001a) used the Sickness Impact Profile (SIP) (Gilson 1975), and Philadelphia Geriatric Center Morale Scale (PGCMS, 17 items) (Lawton 1972) to assess health‐related quality of life.The SIP is a validated generic health status measure of change in behaviour as a consequence of illness . It includes 136 items describing activities of daily living (ADL), divided into 12 categories: sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behaviour, emotional behaviour, and communication. Patients endorse statements that best describe them that day and are related to their health. Items are scored on a numeric scale, with higher scores reflecting greater dysfunction. The mean SIP score in the usual care declined more (‐13% (SD 12), P < 0.05) than in the experimental group (‐2% (SD 11)). The PGCMS is a multidimensional approach to assessing the state of psychological well‐being of older people. It measures perceived morale in elderly people through three factors: agitation, attitude toward own aging and 'lonely satisfaction'. Each high‐morale response receives a score of '1' and each low‐morale response a score of '0', so that the total score ranges from 0 to17. As a general guideline, scores between 13 to17 would be considered high scores on the morale scale, 10 to 12 fall within the mid‐range and scores under 9 are at the lower end. Mean changes in the PGCMS scores were relatively stable for both groups with ‐2% (SD 19) for the usual care, and ‐3% (SD 20) for the experimental group. In the trial by Nijs 2006, health‐related quality of life was assessed in a face‐to‐face interview using the Dutch health‐related quality of life of somatic nursing home residents questionnaire which is a validated questionnaire consisting of five sub‐scales, each representing a quality of life dimension: sensory functioning (focusing on pain); physical functioning (perceived performance and self‐care); psychosocial functioning (depression or loneliness); perceived autonomy (freedom of movement); and perceived safety (feeling at home in the institution). The number of statements in the five sub‐scales is not equal. The questionnaire consists of 50 statements, scored on a dichotomous scale (yes or no). Each sub‐scale and the total questionnaire is computed to achieve a score from 0 to 100. A high score represents a high quality of life. There was a difference between groups in overall quality of life (6.1 units, 95% CI 2.1 to 10.3). The intervention group remained stable (0.4 units, 95% CI 1.8 to 2.5), whereas the usual care declined (‐0.5 units, 95% CI ‐9.4 to 0.6), although the overall changes were small (Table 17).
No trial reported data on patient satisfaction.
Morbidity/complications
No trial reported data on this outcome.
Secondary outcomes
Nutritional status
Weight change
Data were reported on this outcome in three of five trials (Mathey 2001a; Nijs 2006; Remsburg 2001), all of which were trials evaluating the impact of changes to the dining environment. There were no marked differences between intervention and usual care groups in mean weight change in any of the trials (Table 18).
Change in BMI
No trial reported data on this outcome.
Change in TSF
No trial reported data on this outcome.
Change in MAC
Data were reported on this outcome in one of five trials (Nijs 2006). The trial evaluated the impact of providing family‐style meals in residential care homes. There was no marked difference in change in MAC between the groups, MD between groups was 0.5 cm (95% CI ‐0.2 to 1.3)
Clinical function
No trial reported data on this outcome.
Hospitalisation and institutionalisation
No trial reported data on this outcome.
Adverse events
No trial reported data on this outcome.
All‐cause mortality
Data were reported on this outcome in three of five trials (Brouillette 1991; Mathey 2001a; Nijs 2006). Two evaluated the impact of changes to the dining room environment (Mathey 2001a; Nijs 2006) and one of pre‐meal sensory stimulation (Brouillette 1991). There were no marked differences between groups in death from any cause in any trial (Table 19).
Economic costs
No trial reported data on this outcome.
Modification of meal profile or pattern
Primary outcomes
Nutritional intake
Data were reported on energy intake in 11 of 12 trials (Barton 2000; Bouillanne 2013; Castellanos 2009; Essed 2007; Essed 2009; Germain 2006; Leslie 2012; Mathey 2001b; Munk 2014; Silver 2008; Taylor 2006). Four trials evaluated the impact of food fortification, two in hospital (Barton 2000; Munk 2014), one in a care home (Leslie 2012) and one in free‐living individuals receiving home‐delivered meals (Silver 2008), one trial evaluated the impact of modifications to meal delivery in an intermediate care home (Bouillanne 2013), two trials evaluated modifications to meal delivery in residential care homes (Germain 2006; Taylor 2006), and three evaluated flavour modification in residential care homes (Essed 2007; Essed 2009; Mathey 2001b). There were no marked differences in mean change in energy intake between groups in five trials (Bouillanne 2013; Essed 2007; Essed 2009; Mathey 2001b; Taylor 2006). Three trials reported higher energy intakes in the intervention group of between 300 to 500 kcal/day, two of which were trials of food fortification in either hospital or in free‐living individuals (Barton 2000; Silver 2008) and one was of a modification to meal delivery involving improved presentation of pureed foods to participants with dysphagia (Germain 2006). In the randomised cross‐over trial by Castellanos 2009, between‐group differences were not reported however data were presented for a post hoc analysis of 'big' eaters (overall intake 1150 kcal or more a day) and 'small' eaters (overall intake less than 1150 kcal a day) (data not reported in the table). Data were presented as mean intake from both fortified and non‐fortified food items at each meal under each of three menu conditions (Table 20).
Health‐related quality of life and patient satisfaction
Data on health‐related quality of life were reported in one trial (Smoliner 2008). The physical functioning component of the validated medical outcomes Study 36‐item Short Form (SF‐36 ) were reported (Ware 1992). The SF‐36 is a participant‐completed validated questionnaire to assess eight different domains of health (vitality, physical functioning, bodily pain, general health perception, physical function, emotional role function, social role function and mental health). The SF‐36 consists of eight scaled scores, which are the weighted sums of the questions in their section. Each scale is directly transformed into a 0 to 100 scale on the assumption that each question carries equal weight. The lower the score the poorer the quality of life. The higher the score the better the quality of life, that is, a score of zero is equivalent to poorest quality of life and a score of 100 is equivalent to optimal quality of life. Baseline to follow‐up (12 weeks) score in the intervention group receiving the fortified diet changed from a mean of 17.1 (SD 22.7) at baseline to a mean of 10.7 (SD 15.6) at 12 weeks (P = 0.047), and in the usual care from 24 (SD 24.3) at baseline to 13.6 (SD 13.9) at 12 weeks (P < 0.0001), however the between‐group differences were not statistically significant.
No trial reported data on patient satisfaction.
Morbidity/complications
Data on the number of participants experiencing complications were reported in one of twelve trials (Bouillanne 2013) which evaluated the impact of modifications to meal composition in people in intermediate care. There was no marked difference between the intervention and usual care in the number of infectious complications experienced by participants included in the intention‐to‐treat analysis (1 of 29 participants in the intervention group and 2 of 34 participants in the usual care group).
Secondary outcomes
Nutritional status
Weight change
Data on this outcome were reported in seven of 12 trials (Bouillanne 2013; Essed 2007; Germain 2006; Leslie 2012; Mathey 2001b; Munk 2014; Smoliner 2008). Three trials evaluated the impact of food fortification, one in hospital (Munk 2014) and two in a residential care home (Leslie 2012; Smoliner 2008), one evaluated modification to meal composition in an intermediate care setting (Bouillanne 2013), one evaluated modifications to the presentation of food in a residential care home (Germain 2006) and two evaluated flavour modifications in residential care homes (Essed 2007; Mathey 2001b). There were no marked differences in mean weight change between groups reported in three trials (Bouillanne 2013; Essed 2007; Smoliner 2008). Three trials reported higher weight gain in the intervention group compared with the usual care. One was a trial of food fortification in residential care (Leslie 2012) (1.3 kg (SE 0.53) in the intervention group versus ‐0.2 kg (SE 1.5) in the control group, P = 0.03. The second was a trial of modification to meal presentation (Germain 2006) (3.9 kg (SD 2.3) in the intervention group versus ‐0.8 kg (SD 4.2) in the usual care. The other trial evaluated the impact of flavour enhancement in people in a residential care home (Mathey 2001b) (1.1 kg (SD 1.3) in the intervention group versus ‐0.3 (1.6) in the usual care, P < 0.05) (Table 21).
Change in BMI
Data on this outcome were reported in three of 12 trials (Germain 2006; Leslie 2012; Smoliner 2008). One evaluated the impact of modification to meal presentation in people in residential care (Germain 2006) and the others evaluated food fortification in people in residential care (Leslie 2012; Smoliner 2008). In one trial (Smoliner 2008) there was no marked difference between the groups in change in BMI. The group receiving modification to the presentation of meals in Germain 2006 and the group receiving fortified meals in Leslie 2012 experienced a greater increase in BMI than those receiving usual care but the between‐group difference was not reported (Table 21).
Change in TSF
No trial reported data on this outcome.
Change in MAC
One trial of meal fortification in people in residential care reported data on this outcome (Leslie 2012). Participants in the intervention group experienced a greater improvement in MUAC than those in the control group (mean change 0.4 mm (SE 0.16) in the intervention group and ‐0.1 mm (SE 0.3) in the control group, P = 0.019.
Clinical function
Data on handgrip strength were reported in three of 12 trials (Bouillanne 2013; Munk 2014; Smoliner 2008). One trial evaluated the impact of modification to meal composition in people in intermediate care (Bouillanne 2013) and the others evaluated food fortification in people in hospital (Munk 2014) and in residential care (Smoliner 2008). There were no differences between the intervention and usual care groups in either trial (Table 22). The trial by Bouillanne 2013 also assessed change in ADL score (Sonn 1996) and there was no marked difference between the groups (Table 22). In the trial by Smoliner 2008 clinical function was also assessed by peak flow and the Barthel index .The peak flow (L/min) in the intervention group increased from baseline to follow‐up (12 weeks) in the intervention group (mean 152 (SD 105) to 186 (SD 140)) whereas the usual care group showed a decline (mean 151 (SD 90) to 150 (SD 67)). The differences observed between groups were statistically significant (P = 0.039). The mean change in Barthel score was ‐15.2 (SD 18.5) in the group receiving fortification of food and ‐7.5 (SD 10.4) in the group receiving usual care. The between‐group differences were not statistically significant.
Hospitalisation and institutionalisation
One trial of food fortification of menu items provided via an a la carte menu reported data on length of hospital stay (Munk 2014). There were no differences in mean length of stay between groups in from trial inclusion to discharge from hospital (mean 10 days (SD 8) in the intervention group and mean 10 days (SD 8) in the control group, between‐group difference, 0.6 days (95% CI ‐3 to 4, P = 0.73).
Adverse events
No trial reported data on this outcome.
All‐cause mortality
Data on this outcome were reported in four of 12 trials (Bouillanne 2013; Leslie 2012; Munk 2014; Smoliner 2008). The number of deaths were small in each trial and there were no marked differences between groups (Table 22).
Economic costs
No trial reported data on this outcome.
Additional supplementation of meals
Primary outcomes
Nutritional intake
Data were reported on energy intake in eight of 10 trials (Beck 2002; Bourdel‐Marchasson 2000; Faxen‐Irving 2011; Hankey 1993; Potter 2001; Simmons 2008; Simmons 2010; Van den Berg 2015). Three trials evaluated the impact of supplementation with food in residential care homes (Beck 2002; Simmons 2008; Simmons 2010), four evaluated supplementation with oral nutritional supplements in hospital (Bourdel‐Marchasson 2000; Faxen‐Irving 2011; Potter 2001; Van den Berg 2015) and two evaluated supplementation with oral nutritional supplements in residential care homes (Hankey 1993; Simmons 2010). One trial provided both a food‐based intervention and oral nutritional supplements in participants in residential care homes (Simmons 2010). There were no marked differences reported in energy intake between groups in either the trials of food‐based interventions or the trials of oral nutritional supplement‐based interventions (Table 23). In the trial by (Hankey 1993) the group receiving oral nutritional supplements had an energy intake 600 kcal greater than the usual care group (1747 kcal (SD 273) versus 1147 kcal (SD 310) respectively), However, between‐group statistical tests were not reported. In the trial by Van den Berg 2015 participants receiving oral nutritional supplements in four 62 mL portions during the drug round had a significantly higher energy intake than those receiving supplements in the conventional, between‐meal style.
Health‐related quality of life and patient satisfaction
Data on health‐related quality of life were reported in one trial (Dennis 2005) undertaken in people with stroke supplemented with oral nutritional supplements during hospitalisation. Health‐related quality of life was measured in 77% (N = 3086) of participants using EUROQoL score (EQ‐5D) (EuroQol group 1990). The questionnaire comprises five questions on mobility, self‐care, pain, usual activities and psychological status with three possible answers for each item (1 = no problems, 2 = moderate problems, 3 = severe problems). An overall utility score is calculated based on these domains, with a range score from 0 (worse health scenario) to a maximum of 1.0 (best health scenario). An additional visual analogue scale (VAS, scale 0 to 100) was used to assess general health status with 100 indicating the best health status. No marked differences were identified between the intervention and usual care groups (Table 24).
No trial reported data on patient satisfaction.
Morbidity/complications
The incidence of, and number of people with, pressure ulcers was reported in two trials (Bourdel‐Marchasson 2000; Dennis 2005) and the total number of complications was reported in one trial (Dennis 2005). Both trials were of supplementation of participants with oral nutritional supplements in hospital. There was no marked difference between groups in cumulative incidence of, or number of participants with, pressure ulcers in either trial (Table 24). In the trial by Dennis 2005 there was no marked difference in total complications between groups (Table 24).
Secondary outcomes
Nutritional status
Weight change
Data on this outcome were reported in seven of 10 trials (Beck 2002; Faxen‐Irving 2011; Hankey 1993; Larsson 1990; Potter 2001; Simmons 2008; Simmons 2010). Three trials evaluated the impact of supplementation with food in residential care settings (Beck 2002; Simmons 2008; Simmons 2010), two evaluated supplementation with oral nutritional supplements in hospital (Faxen‐Irving 2011; Potter 2001) and three evaluated supplementation with oral nutritional supplements in long‐term care settings (Hankey 1993; Larsson 1990; Simmons 2010), with the trial by Simmons 2010 providing data on both food and oral nutritional supplements. There were no marked differences in weight change between groups receiving food‐based or oral nutritional supplement‐based interventions in six trials (Beck 2002; Faxen‐Irving 2011; Hankey 1993; Larsson 1990; Potter 2001; Simmons 2010). In two trials (Faxen‐Irving 2011; Hankey 1993), the groups receiving oral nutritional supplements gained weight and the usual care group lost weight overall. However, the between‐group differences and the results of statistical tests were not reported. In one trial (Simmons 2008) the intervention group gained 4 lbs more in weight than the group receiving usual care (P = 0.009) (Table 25).
Change in BMI
Data on this outcome were reported in two of 10 trials (Faxen‐Irving 2011; Simmons 2008), both trials evaluated the impact of supplementation with oral nutritional supplements in hospital. In one trial (Faxen‐Irving 2011) BMI was reported according to group at the end of the intervention and there was no marked difference between groups. Change from baseline and between‐group differences were not reported. In the other trial by (Simmons 2008) the intervention group gained 0.72 kg/m² more than the group receiving usual care (P < 0.009) (Table 25).
Change in TSF
Data on this outcome were reported in two of 10 trials (Hankey 1993; Larsson 1990), both of which evaluated the impact of supplementation with oral nutritional supplements in long‐term care settings. In each trial data were presented in figures with minimal description in the text. In one trial (Hankey 1993) the intervention group was described as experiencing a smaller decrease in TSF than the usual care group (6.6% versus 15.8%). In the other trial (Larsson 1990) TSF decreased over the 26 weeks of follow‐up with the greatest decrease occurring in the usual care group. In another trial (Potter 2001) TSF is described as an outcome but the data were not reported.
Change in MACe
Data on this outcome were reported in three of 10 trials (Hankey 1993; Larsson 1990; Potter 2001), all of which evaluated the impact of supplementation with oral nutritional supplements in either hospital or long‐term care settings. In one trial (Hankey 1993), the data were unavailable from the original trial report but we have obtained them from a systematic review by Milne 2009. We read the figures for change from a graph and assumed SD of change to be 10 cm for each group. MAC is described as improving statistically significantly in the intervention group (P < 0.05) but remaining unchanged in the usual care group. The changes are small and no between‐group differences were reported (Table 25). In the trial by Larsson 1990 the data were presented in a figure with some description in the text, participants who were well nourished at the start of the trial and received supplementation of meals experienced less of a decrease in MAC at 26 weeks (P < 0.05) than those receiving usual care. In participants who were malnourished at the start of the trial both groups experienced a decrease in MAC to 26 weeks. In the final trial (Potter 2001), there was no marked difference between groups in MAC (Table 25).
Clinical function
Data on clinical function were reported in two of ten trials (Faxen‐Irving 2011; Potter 2001), both evaluating the impact of supplementation with oral nutritional supplements in hospital. In one trial (Faxen‐Irving 2011) the group receiving oral nutritional supplements changed from being dependent in all five functions to being dependent in only one function as assessed by ADL (Katz 1963). However, no marked change was identified in those receiving usual care (P = 0.011). Mean change (SD) in ADL score according to group was not markedly different between groups (2.95 (SD 2.2) intervention and 4.1 (SD 2.2) control, P = 0.09). In the other trial (Potter 2001) there was no statistically significant difference in numbers achieving functional recovery assessed using the Barthel index in the group receiving supplementation compared with the usual care group (102/149 (68%) intervention versus 100/157 (64%) control, P = 0.38). However, significantly more participants classified as severely undernourished experienced an improvement in their Barthel scores on supplementation compared with those who received usual care (17/25 (68%) intervention versus 11/28 (39%) control, P < 0.04).
Hospitalisation and institutionalisation
Data on length of hospital stay were reported in four of 10 trials (Dennis 2005; Faxen‐Irving 2011; Potter 2001; Van den Berg 2015) all of which evaluated the impact of supplementation of meals with oral nutritional supplements in hospital. There were no marked differences in length of hospital stay between groups in any trial (Table 26).
One trial of supplementation with oral nutritional supplements in hospital reported data on hospital re‐admissions (Van den Berg 2015). The number of re‐admissions to hospital were higher in intervention group 2, but these data were not commented on by the trial authors (13 participants in intervention group 1, 24 participants in intervention group 2 and 15 participants in the control group being readmitted to hospital). One trial reported on the destination of participants at discharge according to group allocation (Potter 2001). There was no marked difference between groups in numbers of participants returning to their own home and those being discharged to an institution (Table 26).
Adverse events
Data on this outcome were reported in three of nine trials (Faxen‐Irving 2011; Hankey 1993; Dennis 2005), one of which reported intolerance to the oral nutritional supplement (e.g. diarrhoea or vomiting, N = 5) (Faxen‐Irving 2011). Another trial (Dennis 2005) reported that 28% stopped taking the oral nutritional supplement due to participant refusal or because of dislike of taste, unwanted weight gain, or feelings of nausea. The trials by Potter 2001 and Van den Berg 2015 reported no adverse events.
All‐cause mortality
Data on this outcome were reported in five of 10 trials (Bourdel‐Marchasson 2000; Dennis 2005; Larsson 1990; Potter 2001: Van den Berg 2015). Four trials evaluated the impact of supplementation with oral nutritional supplements in hospital (Bourdel‐Marchasson 2000; Dennis 2005; Potter 2001; Van den Berg 2015 ) and one evaluated supplementation with oral nutritional supplements in a long‐term care setting (Larsson 1990;). There was no marked difference in death from any cause between groups in any of the trials (Table 26).
Economic costs
Data on this outcome were reported in one trial (Simmons 2010). The cost effectiveness of the intervention was determined from data on cost per serving of the oral nutritional supplement or food provided and staff time to encourage and assist consumption. The average costs (per person per day) were significantly higher in groups receiving supplements and snacks compared with those in the usual care group (USD 2.10 versus USD 2.06 versus USD ‐0.03 respectively). The trial did not use accepted health economic methods and the reported data on both costs and effectiveness were generally poor.
Home meal delivery systems
Primary outcomes
Nutritional intake
No trial data were reported on this outcome.
Health‐related quality of life and patient satisfaction
No trial data were reported on this outcome.
Morbidity/complications
No trial data were reported on this outcome.
Secondary outcomes
Nutritional status
Weight change
Data on this outcome were reported in the one trial in this group (Kretser 2003). The group receiving modified meals‐on‐wheels experienced a weight gain of 1.6 kg (SD 4.6) compared to the group receiving standard meals‐on‐wheels who had an overall weight gain of 0.7 kg (SD 3.3) (Table 27). No statistical tests were conducted on the between‐group differences.
Change in BMI
No trial data were reported on this outcome.
Change in TSF
No trial data were reported on this outcome.
Change in MAC
No trial data were reported on this outcome.
Clinical function
The one trial in this group reported data on ADL and iADL (Kretser 2003). No marked differences were identified in the number experiencing a decline (4/22 versus 8/24) or improvement (3/22 versus 2/24) in ADL between groups receiving modified meals‐on‐wheels, and groups receiving traditional meals‐on‐wheels. However, there was a greater number of participants experiencing a decline in iADL in those receiving traditional meals on wheels (16/24 ) compared with those receiving modified meals on wheels (8/22) at six months (P = 0.0494).
Hospitalisation and institutionalisation
No trial data were reported on this outcome.
Adverse events
No trial data were reported on this outcome.
All‐cause mortality
Data on this outcome were reported in the one trial in this group (Kretser 2003). The number of deaths from any cause were similar in each group (Table 27 ). No statistical tests were conducted on the between‐group differences.
Economic costs
No trial reported data on this outcome.
Discussion
Summary of main results
The aim of this review was to look for an effect of supportive interventions to enhance dietary intake in nutritionally vulnerable adults on patient‐centred, nutritional, clinical and economic outcome. We identified 41 trials and categorised them into five broadly similar types of intervention. Meta‐analysis was only possible for the outcome measures all‐cause mortality, hospitalisation and nutritional status (weight change) showing a possible effect in favour of supportive dietary interventions for all‐cause mortality and nutritional status. These findings should be interpreted with caution as few trials reported data on the outcomes of interest, and the quality of the evidence was between moderate to very low, depending on the outcome measurement. A number of patient‐important outcomes were measured by just a few trials, for example, health‐related quality of life and patient satisfaction. With regard to health‐related quality of life only one of the five trials that reported this outcome suggested benefits associated with the intervention. Although the two trials that measured patient satisfaction reported benefits in those receiving the intervention it should be noted that both trials used unvalidated questionnaires and are potentially subject to the limitations inherent in collecting these types of data, for example, participants need to be literate to complete the questionnaire, blinding may not be possible.
Until there are more large trials of higher methodological quality, evaluating the impact of similar interventions in similar patient groups, the effects of supportive interventions on nutritional, clinical, patient‐centred and healthcare outcomes cannot be fully evaluated.
Overall completeness and applicability of evidence
The trials identified for this review represent a wide range of interventions given with the aim of improving intake in nutritionally vulnerable individuals. Interventions took place in a variety of settings, residential care, hospital and outpatients. Although 21 of 41 included trials took place in residential care, the results of the meta‐analyses were dominated by large trials conducted in hospitals. It is particularly important to consider that the relevance of different outcomes are likely to differ between settings; most of the data for the outcome of all‐cause mortality came from trials recruiting hospital inpatients. Many of the interventions identified were similar to those recommended in policy and guideline documents on the prevention and management of malnutrition (BAPEN 2012; RCON 2008; The Malnutrition Task Force 2013). Despite the comprehensive range of interventions identified in this review, no RCTs were found for some widely used interventions, specifically protected meal times and the use of red trays to identify those requiring mealtime assistance. Examples of good practice reported in these key documents (BAPEN 2012; RCON 2008; The Malnutrition Task Force 2013) are frequently justified on the basis of their potential impact on patient experience and on staff awareness and motivation. These sorts of outcomes are rarely reported in trials, and therefore are not included in systematic reviews and meta‐analyses. The key finding of this review is that there is a lack of evidence to support these interventions and good quality RCTs are urgently needed to inform the widespread implementation of these initiatives. While there is limited evidence on adverse events, nutritional interventions are generally assumed to be safe. However, the impact of implementing and maintaining such interventions at an organisational and unit level has not been evaluated. For example, there are likely to be significant costs in terms of finance, time and resources associated with setting up and maintaining a staff training programme, yet these data are rarely reported. In this review we found very limited data on costs and no formal health economic analyses from which to draw conclusions.
During searching for this review a number of trials were identified that met the inclusion criteria for types of participants and interventions, however they were non‐randomised trials. The reasons for the weaker methodology used in many trials can only be speculated on, and may result from lack of funding, lack of research expertise, concern about the ethics of not providing all participants with an intervention perceived as 'beneficial', and practicalities related to the care setting. This underlines the need for adequate funding of trials with more robust designs (e.g. cluster‐randomised controlled trials with adequate planning, analysis and data especially on intracluster correlation coefficients) to enable a fuller understanding of the potential impact of supportive interventions.
Quality of the evidence
The quality of evidence in this review is between moderate to very low, depending on the outcome measurement. The main issue regarding risk of bias was that although attrition was usually reported clearly and there was little evidence of selective reporting, random sequence generation, concealment of allocation and blinding were frequently unclear. Most trials were small and inadequately powered to answer the question. Although there was significant performance bias, the nature of the included interventions and the settings in which they were undertaken, primarily care homes and hospital wards, means that it is unlikely that participants in the usual care arms were able to get access to the intervention. The possible exceptions to this are the trials by Pivi 2011 and Salva 2011, where a training intervention was provided to carers of people with Alzheimers disease living at home. In this case, it might have been possible for the carers allocated to the usual care group to seek out the information provided to those in the intervention group. Interestingly, the effect size in the trial by Pivi 2011, was significantly different from others in that grouping.
A meta‐analysis and GRADE approach was only possible for the outcome measures all‐cause mortality, length of hospital stay and weight change. These outcomes showed moderate‐quality evidence (all‐cause mortality, nutritional status) and very low‐quality evidence (hospitalisation), mainly because of the small number of included trials and issues of imprecision and indirectness, as well as inconsistency.
Potential biases in the review process
The protocol developed prior to undertaking this review was followed closely, throughout the process and particularly during the trial selection stage when three review authors were involved in detailed discussion. The original search strategy for this review was comprehensive in that we searched 10 databases, including databases other than those most commonly used (Avenell 2001) and we did not place any language restrictions on searches. We undertook additional searching, for example hand searching of the abstracts of meetings, reference lists of identified trials and extensive searching of the reference lists of relevant systematic reviews. In addition, we made considerable efforts to contact authors of included studies, where clarification of data or methodology were required. However, we did not survey study authors to identify additional reports of trials that may have been missed, which has to be acknowledged as a potential source of bias.
There was considerable clinical heterogeneity across all trials contributing to the findings in this review. At the trial selection stage and during categorisation of trials into sub‐groups, care was taken to group trials with similar interventions and populations together. It is possible that interventions judged to be similar, varied according to factors that are currently impossible to identify. For example, the trials evaluating the training of carers or dietetic assistants to deliver improved nutritional care resulted in different effects which may be attributable to a number of factors such as the quality of training, the level of attention provided by individual carers, constraints of the care setting, or indeed to the clinical characteristics of the trial populations. It was not possible to undertake many of the proposed subgroup analyses due to an absence of data. In addition, 12 of 41 (30%) trials included in this review were cluster‐randomised trials. Inadequate analysis methods used in these trials, which failed to account for the likelihood of similarity of participants within clusters and correlation of observations within clusters meant that these trials were excluded from the meta‐analyses. We cannot rule out the possibility that inclusion of data from these 12 trials in the meta‐analyses might change the overall findings.
Agreements and disagreements with other studies or reviews
The authors are aware of four published reviews of similar interventions (Cole 2012; Lambert 2010; Silver 2009; Weekes 2009), two of which employed systematic search strategies to identify trials (Cole 2012; Weekes 2009). All of the reviews looked at similar groupings of interventions (e.g. feeding assistance, changes to eating environment, staff training) and indeed included some of the trials identified in this review. They also included trials of weaker methodological quality (e.g. non‐randomised controlled trials), excluded from this review.
One review (Weekes 2009) arrived at a similar conclusion to this one, that there was a serious lack of evidence to support interventions designed to improve nutritional care. The other three focused on positive results from individual trials.
To the review authors’ knowledge, this is the first attempt at a systematic review with meta‐analyses, the results of which reveals lack of good evidence for supportive interventions. While the protocol specified outcome measures that are frequently assessed in nutrition intervention trials, the review authors question whether these are the most appropriate outcomes to assess the benefits of supportive interventions. Existing reports of supportive interventions similar to the ones identified in this review, have speculated on their benefits in terms of patient experience, staff awareness and motivation. These may be more relevant outcome measures for interventions of this type, which may explain the lack of trials for interventions such as the use of red trays, or protected meal times, since the primary intention was to improve the patient experience.
The review authors note however, that the explicit aim of all the trials included in this review was to increase dietary intake, and thus influence clinical outcome.
Authors' conclusions
Implications for practice.
There is moderate‐quality evidence that supportive interventions to improve nutritional care improve nutritional status such as minimal weight gain or energy intake. Moderate‐quality evidence shows that supportive interventions can reduce the risk of all‐cause mortality, based mainly on studies recruiting hospital inpatients. There was very low‐quality evidence to suggest adverse effects maybe associated with the interventions. Therefore, whilst some of these interventions are advocated at a national level, clinicians should recognise the lack of clear evidence to support their role across different settings.
Implications for research.
This review revealed a lack of good quality randomised controlled trials evaluating the effect of supportive interventions. However, even small effects such as a potential reduction in all‐cause mortality could result in relevant public health effects given the number of affected malnourished or nutritionally at‐risk individuals. As these interventions remain in common use and are actively promoted at a national level, research is urgently needed. This review has identified a range of interventions that may benefit nutritionally vulnerable individuals and highlights the importance of assessing patient‐important outcomes in different healthcare settings in future research.
The nature of the interventions being examined in the studies included in this review means that cluster‐randomised trials are likely to be the method of choice because of the need to study the effects of interventions in groups of patients rather than individuals. Attention should be given to the reporting of cluster‐randomised trials to take into consideration the correlation of observations within clusters and authors should account for the potential bias inherent in these trials when analysing and reporting results. Cluster level analyses, analyses of individual level data that are adjusted for the design effect, or regression analyses of individual level data using methods for clustered data are all valid approaches (McKenzie 2014).
Notes
Portions of the methods sections, the appendices, additional tables and figures 1 to 3 of this review are based on a standard template established by Cochrane Metabolic and Endocrine Disorders.
Acknowledgements
We wish to thank Professor Peter Emery of King's College London, UK for his time and input into this review. We would also like to thank Karen Poole, Biomedical & Health Information Specialist at King's College London, UK for her useful introduction to database searching in the protocol's early stages.
Additionally we are grateful to Dr Rafael Perera of the Department of Primary Healthcare Science, University of Oxford for his advice on statistical methods. We wish to thank all the staff and Editor of Cochrane Metabolic and Endocrine Disorders for their assistance in the conduct of this review. Particular thanks to the Co‐ordinating Editor, Professor Bernd Richter and Maria‐Inti Metzendorf, the Information Specialist of Cochrane Metabolic and Endocrine Disorders, who have made substantial contributions to identifying and interpreting the trials for this review.
Appendices
Appendix 1. Search strategies (inception to March 2013)
| Cochrane Library |
| #1 food* OR meal* OR snack* OR drink* OR feed*: ti,ab #2 nutri* OR diet*: ti,ab #3 dining*: ti,ab #4 screening OR monitoring: ti,ab #5 documentation OR communication: ti,ab #6 time* OR timing OR pattern OR style OR arrangement* OR environment*: ti,ab #7 staff* OR train*: ti,ab #8 nurs*: ti,ab #9 healthcare OR health care: ti,ab #10 cater*: ti,ab #11 flavo?r* OR taste: ti,ab #12 content OR composition OR density: ti,ab #13 appear* OR presentation:ti,ab #14 size OR portion OR amount: ti,ab #15 protected meal*: ti,ab #16 red tray*: ti,ab #17 fortif*:ti,ab #18 supplement*: ti,ab #19 ((supportive OR nutrition* OR diet*) NEAR/3 intervention):ti,ab #20 (assist* OR help* OR support*):ti,ab #21 (add* OR extra):ti,ab #22 (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*):ti,ab #23 (food* OR meal* OR snack* OR drink* OR feed*) NEAR/3 ((time* OR timing OR pattern OR style OR arrangement* OR environment*) OR (flavour* OR flavor* OR taste) OR (content OR composition OR density) OR (appear* OR presentation) OR (size OR portion OR amount) OR (fortif*) OR (supplement*) OR (assist* OR help* OR support*) OR (add* OR extra) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*)):ti,ab #24 (nutri* OR diet*) NEAR/4 ((content OR composition OR density) OR (fortif*) OR (supplement*) OR (add* OR extra) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*)):ti,ab #25 dining* NEAR/4 ((time* OR timing OR pattern OR style OR arrangement* OR environment*) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*)):ti,ab #26 (screening OR monitoring) NEAR/4 ((nutri* OR diet*) OR (assist* OR help* OR support*) OR (add* OR extra) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*)):ti,ab #27 (documentation OR communication) NEAR/4 (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*):ti,ab #28 (staff* OR train*) NEAR/4 ((nurs*) OR (healthcare OR health care) OR (cater*) OR (assist* OR help* OR support*) OR (add* OR extra) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*)):ti,ab #29 supplement* NEAR/5 (add* OR extra):ti,ab #30 (assist* OR help* OR support*) NEAR/4 ((nurs*) OR (healthcare OR health care) OR (cater*)):ti,ab #31 (#15 OR #16 OR #19) #32 (#23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31) #33 (low BMI OR low body mass index):ti,ab #34 (low weight OR underweight OR under‐weight):ti,ab #35 (maln*):ti,ab #36 (nutritional risk OR (risk NEAR/4 maln*)):ti,ab #37 (poor nutr* OR undernourish* OR under‐nourish*):ti,ab #38 ((poor OR inadequate OR suboptimal) NEAR/5 intake*):ti,ab #39 (institutionali?ed):ti,ab #40 (elderly):ti,ab #41 (homebound OR home‐bound OR housebound OR house‐bound):ti,ab #42 ((extended OR longterm OR long‐term OR community) NEAR/1 care):ti,ab #43 ((nursing OR care OR residential) NEAR/1 home):ti,ab #44 (inpatient* OR hospitali?* OR hospital patient*):ti,ab #45 exp Nutritional Status/ #46 exp Nutrition Disorders/ #47 exp Nutrition Assessment/ #48 exp Nutritional Support/ #49 exp Nutrition Policy/ #50 exp Malnutrition/ #51 diet/ #52 dietetics/ #53 hospital food service/ #54 energy intake/ #55 fortified food/ #56 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 #57 32 AND 56 #58 exp Pregnancy/ #59 pregnan*:kw,ti #60 #58 OR #59 #61 #57 NOT #60 #62 (child* OR infant OR paediatric OR pediatric):kw,ti #63 #61 NOT #62 #64 (animal OR rat OR mouse OR guinea pig OR primate OR monkey OR cat OR dog):kw,ti #65 #63 NOT #64 |
| MEDLINE + OLDMEDLINE |
| #1 (food* OR meal* OR snack* OR drink* OR feed*).ab,ti. #2 (nutri* OR diet*).ab,ti. #3 "dining*".ab,ti. #4 (screening OR monitoring).ab,ti. #5 (documentation OR communication).ab,ti. #6 (time* OR timing OR pattern OR style OR arrangement* OR environment).ab,ti. #7 (staff* OR train*).ab,ti. #8 "nurs*".ab,ti. #9 (healthcare OR health care).ab,ti. #10 "cater*".ab,ti. #11 (flavo?r* OR taste).ab,ti. #12 (content OR composition OR density).ab,ti. #13 (appear* OR presentation).ab,ti. #14 (size OR portion OR amount).ab,ti. #15 "protected meal*".ab,ti. #16 "red tray*".ab,ti. #17 "fortif*".ab,ti. #18 "supplement*".ab,ti. #19 ((supportive OR nutrition* OR diet*) ADJ3 intervention).ab,ti. #20 (assist* OR help* OR support*).ab,ti. #21 (add* OR extra).ab,ti. #22 (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*).ab,ti. #23 1 ADJ3 (6 OR 11 OR 12 OR 13 OR 14 OR 17 OR 18 OR 20 OR 21 OR 22) #24 2 ADJ4 (12 OR 17 OR 18 OR 21 OR 22) #25 3 ADJ4 (6 OR 22) #26 4 ADJ4 (2 OR 21 OR 22) #27 5 ADJ4 22 #28 7 ADJ4 (8 OR 9 OR 10 OR 20 OR 21 OR 22) #29 18 ADJ5 21 #30 20 ADJ4 (8 OR 9 OR 10) #31 15 OR 16 OR 19 #32 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 #33 (low bmi OR low body mass index).ab,ti. #34 (low weight OR underweight OR under‐weight).ab,ti. #35 "maln*".ab,ti. #36 (nutritional risk OR (risk ADJ4 maln*)).ab,ti. #37 (poor nutr* OR undernourish* OR under‐nourish*).ab,ti. #38 ((poor OR inadequate OR suboptimal) adj5 intake*).ab,ti. #39 institutionali?ed.ab,ti. #40 elderly.ab,ti. #41 (homebound OR home‐bound OR housebound OR house‐bound).ab,ti. #42 ((extended OR longterm OR long‐term OR community) ADJ1 care).ab,ti. #43 ((nursing OR care OR residential) ADJ1 home).ab,ti. #44 (inpatient* OR hospitali?* OR hospital patient*).ab,ti. #45 exp Nutritional Status/ #46 exp Nutrition Disorders/dh, th [Diet Therapy, Therapy] #47 nutrition assessment.sh. #48 nutritional support.sh. #49 nutrition policy.sh. #50 exp Malnutrition/dh, th [Diet Therapy, Therapy] #51 *diet/ #52 *dietetics/ #53 *food service, hospital/ #54 *energy intake/ #55 *food, fortified/ #56 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45 OR 46 OR 47 OR 48 OR 49 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55 #57 32 AND 56 #58 randomized controlled trial.pt. #59 controlled clinical trial.pt. #60 randomi?ed.ab. #61 placebo.ab. #62 clinical trials as topic.sh. #63 randomly.ab. #64 trial.ti. #65 58 OR 59 OR 60 OR 61 OR 62 OR 63 OR 64 #66 meta‐analysis.pt #67 exp technology assessment, biomedical/ #68 exp meta‐analysis/ #69 exp meta‐analysis as topic/ #70 hta.tw, ot. #71 (health technology ADJ6 assessment$).tw,ot. #72 (meta analy$ OR metaanaly$ or meta?analy$).tw,ot. #73 ((review$ OR search$) ADJ10 (literature$ OR medical database$ OR medline OR pubmed OR embase OR cochrane OR cinahl OR psycinfo OR psyclit OR healthstar OR biosis OR current content$ OR systemat$)).tw,ot. #74 66 OR 67 OR 68 OR 69 OR 70 OR 71 OR 72 OR 73 #75 65 OR 74 #76 (comment OR editorial OR historical‐article).pt. #77 75 NOT 76 #78 57 AND 77 #79 (animals NOT (animals AND humans)).sh. #80 78 NOT 79 #81 exp Pregnancy/ #82 pregnan*.tw,ot. #83 81 OR 82 #84 80 NOT 83 #85 limit 84 to "all adult (19 plus years)" |
| MEDLINE in‐process & other non‐indexed citations |
| #1 (food* OR meal* OR snack* OR drink* OR feed*).ab,ti. #2 (nutri* OR diet*).ab,ti. #3 "dining*".ab,ti. #4 (screening OR monitoring).ab,ti. #5 (documentation OR communication).ab,ti. #6 (time* OR timing OR pattern OR style OR arrangement* OR environment).ab,ti. #7 (staff* OR train*).ab,ti. #8 "nurs*".ab,ti. #9 (healthcare OR health care).ab,ti. #10 "cater*".ab,ti. #11 (flavo?r* OR taste).ab,ti. #12 (content OR composition OR density).ab,ti. #13 (appear* OR presentation).ab,ti. #14 (size OR portion OR amount).ab,ti. #15 "protected meal*".ab,ti. #16 "red tray*".ab,ti. #17 "fortif*".ab,ti. #18 "supplement*".ab,ti. #19 ((supportive OR nutrition* OR diet*) ADJ3 intervention).ab,ti. #20 (assist* OR help* OR support*).ab,ti. #21 (add* OR extra).ab,ti. #22 (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*).ab,ti. #23 1 ADJ3 (6 OR 11 OR 12 OR 13 OR 14 OR 17 OR 18 OR 20 OR 21 OR 22) #24 2 ADJ4 (12 OR 17 OR 18 OR 21 OR 22) #25 3 ADJ4 (6 OR 22) #26 4 ADJ4 (2 OR 21 OR 22) #27 5 ADJ4 22 #28 7 ADJ4 (8 OR 9 OR 10 OR 20 OR 21 OR 22) #29 18 ADJ5 21 #30 20 ADJ4 (8 OR 9 OR 10) #31 15 OR 16 OR 19 #32 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 #33 (low bmi OR low body mass index).ab,ti. #34 (low weight OR underweight OR under‐weight).ab,ti. #35 "maln*".ab,ti. #36 (nutritional risk OR (risk ADJ4 maln*)).ab,ti. #37 (poor nutr* OR undernourish* OR under‐nourish*).ab,ti. #38 ((poor OR inadequate OR suboptimal) adj5 intake*).ab,ti. #39 institutionali?ed.ab,ti. #40 elderly.ab,ti. #41 (homebound OR home‐bound OR housebound OR house‐bound).ab,ti. #42 ((extended OR longterm OR long‐term OR community) ADJ1 care).ab,ti. #43 ((nursing OR care OR residential) ADJ1 home).ab,ti. #44 (inpatient* OR hospitali?* OR hospital patient*).ab,ti. #45 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 #46 32 AND 45 #47 (random* OR rct*).tw,ot. #48 “single blind*”.tw, ot. #49 “double blind*”.tw, ot. #50 ((triple OR treble) AND blind*).tw,ot. #51 ((control* AND trial*) OR (clinical ADJ4 trial*) OR trial*).tw,ot. #52 (systematic* review*).tw,ot. #53 hta.tw, ot. #54 (health technology ADJ6 assessment$).tw,ot. #55 (meta analy$ OR metaanaly$ or meta?analy$).tw,ot. #56 ((review$ OR search$) ADJ10 (literature$ OR medical database$ OR medline OR pubmed OR embase OR cochrane OR cinahl OR psycinfo OR psyclit OR healthstar OR biosis OR current content$ OR systemat$)).tw,ot. #57 47 OR 48 OR 49 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55 OR 56 #58 (comment OR editorial OR historical‐article).pt. #59 57 NOT 58 #60 46 AND 59 #61 pregnan*.tw,ot. #62 60 NOT 61 |
| Embase + Embase classic |
| #1 (food* OR meal* OR snack* OR drink* OR feed*).ab,ti. #2 (nutri* OR diet*).ab,ti. #3 "dining*".ab,ti. #4 (screening OR monitoring).ab,ti. #5 (documentation OR communication).ab,ti. #6 (time* OR timing OR pattern OR style OR arrangement* OR environment).ab,ti. #7 (staff* OR train*).ab,ti. #8 "nurs*".ab,ti. #9 (healthcare OR health care).ab,ti. #10 "cater*".ab,ti. #11 (flavo?r* OR taste).ab,ti. #12 (content OR composition OR density).ab,ti. #13 (appear* OR presentation).ab,ti. #14 (size OR portion OR amount).ab,ti. #15 "protected meal*".ab,ti. #16 "red tray*".ab,ti. #17 "fortif*".ab,ti. #18 "supplement*".ab,ti. #19 ((supportive OR nutrition* OR diet*) ADJ3 intervention).ab,ti. #20 (assist* OR help* OR support*).ab,ti. #21 (add* OR extra).ab,ti. #22 (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*).ab,ti. #23 1 ADJ3 (6 OR 11 OR 12 OR 13 OR 14 OR 17 OR 18 OR 20 OR 21 OR 22) #24 2 ADJ4 (12 OR 17 OR 18 OR 21 OR 22) #25 3 ADJ4 (6 OR 22) #26 4 ADJ4 (2 OR 21 OR 22) #27 5 ADJ4 22 #28 7 ADJ4 (8 OR 9 OR 10 OR 20 OR 21 OR 22) #29 18 ADJ5 21 #30 20 ADJ4 (8 OR 9 OR 10) #31 15 OR 16 OR 19 #32 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 #33 (low bmi OR low body mass index).ab,ti. #34 (low weight OR underweight OR under‐weight).ab,ti. #35 "maln*".ab,ti. #36 (nutritional risk OR (risk ADJ4 maln*)).ab,ti. #37 (poor nutr* OR undernourish* OR under‐nourish*).ab,ti. #38 ((poor OR inadequate OR suboptimal) adj5 intake*).ab,ti. #39 institutionali?ed.ab,ti. #40 elderly.ab,ti. #41 (homebound OR home‐bound OR housebound OR house‐bound).ab,ti. #42 ((extended OR longterm OR long‐term OR community) ADJ1 care).ab,ti. #43 ((nursing OR care OR residential) ADJ1 home).ab,ti. #44 (inpatient* OR hospitali?* OR hospital patient*).ab,ti. #45 exp Nutritional Status/ #46 exp Nutritional Disorder/dh, th [Therapy] #47 nutrition assessment.sh. #48 nutritional support.sh. #49 health care policy.sh. #50 exp Malnutrition/dh, th [Therapy] #51 *diet/ #52 *dietetics/ #53 *food service, hospital/ #54 *energy intake/ #55 *food, fortified/ #56 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45 OR 46 OR 47 OR 48 OR 49 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55 #57 32 AND 56 #58 (random* OR rct*).tw,ot. #59 “single blind*”.tw, ot. #60 “double blind*”.tw, ot. #61 ((triple OR treble) AND blind*).tw,ot. #62 ((control* AND trial*) OR (clinical ADJ4 trial*) OR trial*).tw,ot. #63 (systematic* review*).tw,ot. #64 hta.tw, ot. #65 (health technology ADJ6 assessment$).tw,ot. #66 (meta analy$ OR metaanaly$ or meta?analy$).tw,ot. #67 ((review$ OR search$) ADJ10 (literature$ OR medical database$ OR medline OR pubmed OR embase OR cochrane OR cinahl OR psycinfo OR psyclit OR healthstar OR biosis OR current content$ OR systemat$)).tw,ot. #68 58 OR 59 OR 60 OR 61 OR 62 OR 63 OR 64 OR 65 OR 66 OR 67 #69 (comment OR editorial OR historical‐article).pt. #70 68 NOT 69 #71 57 AND 70 #72 exp Pregnancy/ #73 pregnan*.tw,ot. #74 72 OR 73 #75 71 NOT 74 #76 limit 75 to (human and (adult <18 to 64 years> or aged <65+ years>)) |
| AMED |
| #1 (food* OR meal* OR snack* OR drink* OR feed*).ti,ab #2 (nutri* OR diet*).ti,ab #3 "dining*".ti,ab #4 screening OR monitoring).ti,ab #5 documentation OR communication).ti,ab #6 time* OR timing OR pattern OR style OR arrangement* OR environment).ti,ab #7 staff* OR train*).ti,ab #8 nurs*".ti,ab #9 healthcare OR “health care”).ti,ab #10 cater*".ti,ab #11 flavo?r* OR taste).ti,ab #12 content OR composition OR density).ti,ab #13 appear* OR presentation).ti,ab #14 size OR portion OR amount).ti,ab #15 protected meal*".ti,ab #16 red tray*".ti,ab #17 fortif*".ti,ab #18 "supplement*".ti,ab #19 ((supportive OR nutrition* OR diet*) ADJ3 intervention).ti,ab #20 (assist* OR help* OR support*).ti,ab #21 (add* OR extra).ti,ab #22 alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*).ti,ab #23 1 DJ3 (6 OR 11 OR 12 OR 13 OR 14 OR 17 OR 18 OR 20 OR 21 OR 22) #24 2 ADJ4 (12 OR 17 OR 18 OR 21 OR 22) #25 3 ADJ4 (6 OR 22) #26 4 ADJ4 (2 OR 21 OR 22) #27 5 ADJ4 22 #28 7 ADJ4 (8 OR 9 OR 10 OR 20 OR 21 OR 22) #29 18 ADJ5 21 #30 20 ADJ4 (8 OR 9 OR 10) #31 15 OR 16 OR 19 #32 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 #33 (“low bmi” OR “low body mass index”).ti,ab #34 (“low weight” OR underweight OR under‐weight).ti,ab #35 "maln*".ti,ab #36 (“nutritional risk” OR (risk ADJ4 maln*)).ti,ab #37 (“poor nutr*” OR undernourish* OR under‐nourish*).ti,ab #38 ((poor OR inadequate OR suboptimal) ADJ5 intake*).ti,ab #39 institutionali?ed.ti,ab #40 elderly.ti,ab #41 (homebound OR home‐bound OR housebound OR house‐bound).ti,ab #42 ((extended OR longterm OR long‐term OR community) ADJ1 care).ti,ab #43 ((nursing OR care OR residential) ADJ1 home).ti,ab #44 (inpatient* OR hospitali?* OR hospital patient*).ti,ab #45 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 #46 2 AND 45 #47 (random* OR rct*).ti,ab #48 “single blind*”.ti,ab #49 “double blind*”.ti,ab #50 ((triple OR treble) AND blind*).ti,ab #51 ((control* AND trial*) OR (clinical ADJ4 trial*) OR trial*).ti,ab #52 (systematic* review*).ti,ab #53 (“health technology” ADJ6 assessment$).ti,ab #54 hta.ti,ab #55 (“meta analy$” OR metaanaly$ or meta?analy$).ti,ab #56 47 OR 48 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55 #57 46 AND 56 #58 (comment OR editorial OR historical‐article).pt. #59 57 NOT 58 #60 (animal OR rat OR mouse OR guinea pig OR primate OR monkey OR cat OR dog).ti,ab #61 9 NOT 60 #62 regnan*.ti,ab #63 61 NOT 62 #64 (child* OR infant OR paediatric OR pediatric).ti,ab #65 63 NOT 64 |
| British Nursing Index |
| #1 (food* OR meal* OR snack* OR drink* OR feed*).ab,ti. #2 (nutri* OR diet*).ab,ti. #3 "dining*".ab,ti. #4 (screening OR monitoring).ab,ti. #5 (documentation OR communication).ab,ti. #6 (time* OR timing OR pattern OR style OR arrangement* OR environment).ab,ti. #7 (staff* OR train*).ab,ti. #8 "nurs*".ab,ti. #9 (healthcare OR health care).ab,ti. #10 "cater*".ab,ti. #11 (flavo?r* OR taste).ab,ti. #12 (content OR composition OR density).ab,ti. #13 (appear* OR presentation).ab,ti. #14 (size OR portion OR amount).ab,ti. #15 "protected meal*".ab,ti. #16 "red tray*".ab,ti. #17 "fortif*".ab,ti. #18 "supplement*".ab,ti. #19 ((supportive OR nutrition* OR diet*) ADJ3 intervention).ab,ti. #20 (assist* OR help* OR support*).ab,ti. #21 (add* OR extra).ab,ti. #22 (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*).ab,ti. #23 1 ADJ3 (6 OR 11 OR 12 OR 13 OR 14 OR 17 OR 18 OR 20 OR 21 OR 22) #24 2 ADJ4 (12 OR 17 OR 18 OR 21 OR 22) #25 3 ADJ4 (6 OR 22) #26 4 ADJ4 (2 OR 21 OR 22) #27 5 ADJ4 22 #28 7 ADJ4 (8 OR 9 OR 10 OR 20 OR 21 OR 22) #29 18 ADJ5 21 #30 20 ADJ4 (8 OR 9 OR 10) #31 15 OR 16 OR 19 #32 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 #33 (low bmi OR low body mass index).ab,ti. #34 (low weight OR underweight OR under‐weight).ab,ti. #35 "maln*".ab,ti. #36 (nutritional risk OR (risk ADJ4 maln*)).ab,ti. #37 (poor nutr* OR undernourish* OR under‐nourish*).ab,ti. #38 ((poor OR inadequate OR suboptimal) adj5 intake*).ab,ti. #39 institutionali?ed.ab,ti. #40 elderly.ab,ti. #41 homebound OR home‐bound OR housebound OR house‐bound).ab,ti. #42 ((extended OR longterm OR long‐term OR community) ADJ1 care).ab,ti. #43 ((nursing OR care OR residential) ADJ1 home).ab,ti. #44 (inpatient* OR hospitali?* OR hospital patient*).ab,ti. #45 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 #46 32 AND 45 #47 (random* OR rct*).tw,ot. #48 “single blind*”.tw, ot. #49 “double blind*”.tw, ot. #50 ((triple OR treble) AND blind*).tw,ot. #51 ((control* AND trial*) OR (clinical ADJ4 trial*) OR trial*).tw,ot. #52 (systematic* review*).tw,ot. #53 hta.tw, ot. #54 (health technology ADJ6 assessment$).tw,ot. #55 (meta analy$ OR metaanaly$ or meta?analy$).tw,ot. #56 (review$ OR search$) ADJ10 (literature$ OR medical database$ OR medline OR pubmed OR embase OR cochrane OR cinahl OR psycinfo OR psyclit OR healthstar OR biosis OR current content$ OR systemat$)).tw,ot. #57 47 OR 48 OR 49 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55 OR 56 #58 (comment or editorial or historical‐article).pt. #59 57 NOT 58 #60 46 AND 59 #61 pregnan*.tw,ot. #62 60 NOT 61 |
| CINAHL |
| #1 (TI (food* OR meal* OR snack* OR drink* OR feed*)) OR (AB (food* OR meal* OR snack* OR drink* OR feed*)) #2 (TI (nutri* OR diet*)) OR (AB (nutri* OR diet*)) #3 (TI dining*) OR (AB dining*) #4 (TI (screening OR monitoring)) OR (AB (screening OR monitoring)) #5 (TI (documentation OR communication)) OR (AB (documentation OR communication)) #6 (TI (time* OR timing OR pattern OR style OR arrangement* OR environment*)) OR (AB (time* OR timing OR pattern OR style OR arrangement* OR environment*)) #7 (TI (staff* OR train*)) OR (AB (staff* OR train*)) #8 (TI nurs*) OR (AB nurs*) #9 (TI (healthcare OR health care)) OR (AB (healthcare OR health care)) #10 (TI cater*) OR (AB cater*) #11 (TI (flavo?r* OR taste)) OR (AB (flavo?r* OR taste)) #12 (TI (content OR composition OR density)) OR (AB (content OR composition OR density)) #13 (TI (appear* OR presentation)) OR (AB (appear* OR presentation)) #14 (TI (size OR portion OR amount)) OR (AB (size OR portion OR amount)) #15 (TI (protected meal*)) OR (AB (protected meal*)) #16 (TI (red tray*)) OR (AB (protected meal*)) #17 (TI fortif*) OR (AB fortif*) #18 (TI supplement*) OR (AB supplement*) #19 (TI ((supportive OR nutrition* OR diet*) N3 intervention)) OR (AB ((supportive OR nutrition* OR diet*) N3 intervention)) #20 (TI (assist* OR help* OR support*)) OR (AB (assist* OR help* OR support*)) #21 (TI (add* OR extra)) OR (AB (add* OR extra)) #22 (TI (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*)) OR (AB (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*)) #23 (TI (food* OR meal* OR snack* OR drink* OR feed*) N3 ((time* OR timing OR pattern OR style OR arrangement* OR environment*) OR (flavour* OR flavor* OR taste) OR (content OR composition OR density) OR (appear* OR presentation) OR (size OR portion OR amount) OR (fortif*) OR (supplement*) OR (assist* OR help* OR support*) OR (add* OR extra) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*))) OR (AB (food* OR meal* OR snack* OR drink* OR feed*) N3 ((time* OR timing OR pattern OR style OR arrangement* OR environment*) OR (flavour* OR flavor* OR taste) OR (content OR composition OR density) OR (appear* OR presentation) OR (size OR portion OR amount) OR (fortif*) OR (supplement*) OR (assist* OR help* OR support*) OR (add* OR extra) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*))) #24 (TI (nutri* OR diet*) N4 ((content OR composition OR density) OR (fortif*) OR (supplement*) OR (add* OR extra) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*))) OR (AB (nutri* OR diet*) N4 ((content OR composition OR density) OR (fortif*) OR (supplement*) OR (add* OR extra) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*))) #25 (TI dining* N4 ((time* OR timing OR pattern OR style OR arrangement* OR environment*) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*))) OR (AB dining* N4 ((time* OR timing OR pattern OR style OR arrangement* OR environment*) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*))) #26 (TI (screening OR monitoring) N4 ((nutri* OR diet*) OR (assist* OR help* OR support*) OR (add* OR extra) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*))) OR (AB (screening OR monitoring) N4 ((nutri* OR diet*) OR (assist* OR help* OR support*) OR (add* OR extra) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*))) #27 (TI (documentation OR communication) N4 ((alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*))) OR (AB (documentation OR communication) N4 ((alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*))) #28 (TI (staff* OR train*) N4 ((nurs*) OR (healthcare OR health care) OR (cater*) OR (assist* OR help* OR support*) OR (add* OR extra) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*))) OR (AB (staff* OR train*) N4 ((nurs*) OR (healthcare OR health care) OR (cater*) OR (assist* OR help* OR support*) OR (add* OR extra) OR (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*))) #29 (TI (supplement* N5 (add* OR extra))) OR (AB (supplement* N5 (add* OR extra))) #30 (TI (assist* OR help* OR support*) N4 ((nurs*) OR (healthcare OR health care) OR (cater*))) OR (AB (assist* OR help* OR support*) N4 ((nurs*) OR (healthcare OR health care) OR (cater*))) #31 (S15 OR S16 OR S19) #32 S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 #33 (TI (low BMI OR low body mass index)) OR (AB (low BMI OR low body mass index)) #34 (TI (low weight OR underweight OR under‐weight)) OR (AB (low weight OR underweight OR under‐weight)) #35 (TI maln*) OR (AB maln*) #36 (TI (nutritional risk OR (risk N4 maln*))) OR (AB (nutritional risk OR (risk N4 maln*))) #37 (TI (poor nutr* OR undernourish* OR under‐nourish*)) OR (AB (poor nutr* OR undernourish* OR under‐nourish*)) #38 TI (poor OR inadequate OR suboptimal) N5 intake*) OR (AB (poor OR inadequate OR suboptimal) N5 intake*) #39 (TI (institutionali?ed)) OR (AB (institutionali?ed)) #40 (TI elderly) OR (AB elderly) #41 (TI (homebound OR home‐bound OR housebound OR house‐bound)) OR (AB (homebound OR home‐bound OR housebound OR house‐bound)) #42 (TI ((extended OR longterm OR long‐term OR community) N1 care)) OR (AB ((extended OR longterm OR long‐term OR community) N1 care)) #43 (TI ((nursing OR care OR residential) N1 home)) OR (AB ((nursing OR care OR residential) N1 home)) #44 (TI (inpatient* OR hospitali?* OR hospital patient*)) OR (AB (inpatient* OR hospitali?* OR hospital patient*)) #45 SU Nutritional Status #46 SU Nutrition Disorders #47 SU Nutritional Assessment #48 SU Nutritional Support #49 SU Nutrition Policy #50 SU Malnutrition #51 SU Diet #52 SU Dietetics #53 SU Hospital Food Service #54 SU Energy Intake #55 SU Fortified Food #56 S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 #57 S32 AND S56 #58 (TI (random* OR rct*)) OR (TX (random* OR rct*)) #59 (TI single blind*) OR (TX single blind*) #60 (TI double blind*) OR (TX double blind*) #61 (TI ((triple OR treble) AND blind*)) OR (TX ((triple OR treble) AND blind*)) #62 (TI ((control* AND trial*) OR (clinical N4 trial*) OR trial*)) OR (TX ((control* AND trial*) OR (clinical N4 trial*) OR trial*)) #63 (TI systematic* review*) OR (TX systematic* review*) #64 (TI hta) OR (TX hta) #65 (TI (health technology N6 assessment*)) OR (TX (health technology N6 assessment*)) #66 (TI (meta analy* OR metaanaly* or meta?analy*)) OR (TX (meta analy* OR metaanaly* or meta?analy*)) #67 (TI ((review* OR search*) N10 (literature* OR medical database* OR medline OR pubmed OR embase OR cochrane OR cinahl OR psycinfo OR psyclit OR healthstar OR biosis OR current content* OR systemat*))) OR (TX ((review* OR search*) N10 (literature* OR medical database* OR medline OR pubmed OR embase OR cochrane OR cinahl OR psycinfo OR psyclit OR healthstar OR biosis OR current content* OR systemat*))) #68 S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 #69 PT (comment OR editorial OR historical‐article) #70 S68 NOT S69 #71 SU Pregnancy #72 (TI pregnan*) OR (TX pregnan*) #73 S71 OR S72 #74 S70 NOT S73 #75 S57 AND S74 Limiters ‐ Human; Age Groups: Adult: 19‐44 years, Aged: 65+ years |
| SCOPUS |
| #1 ((TITLE‐ABS‐KEY((food* W/3 time*) OR (food* W/3 timing) OR (food* W/3 pattern) OR (food* W/3 style) OR (food* W/3 arrangement*)OR (food*W/3 environment) OR (food* W/3 flavour) OR (food* W/3 taste) OR (food* W/3 content) OR (food* W/3 composition) OR (food* W/3 density) OR (food* W/3 appear*) OR (food* W/3 presentation) OR (food* W/3 size) OR (food* W/3 portion) OR (food* W/3 amount) OR (food* W/3 fortifi*) OR (food* W/3 supplement*) OR (food* W/3 assist*) OR (food* W/3 help*) OR (food* W/3 support*) OR (food* W/3 add*) OR (food* W/3 extra) OR (food* W/3 alter*) OR (food* W/3 chang*) OR (food* W/3 new) OR (food* W/3 enhance*) OR (food* W/3 modif*) OR (food* W/3 increas*) OR (food W/3 decreas*) OR (food* W/3 improv*) OR (food* W/3 reduc*) OR (food* W/3 target*))) OR (TITLE‐ABS‐KEY((meal* W/3 time*) OR (meal* W/3 timing) OR (meal* W/3 pattern) OR (meal* W/3 style) OR (meal* W/3 arrangement*)OR (meal*W/3 environment) OR (meal* W/3 flavour) OR (meal* W/3 taste) OR (meal* W/3 content) OR (meal* W/3 composition) OR (meal* W/3 density) OR (meal* W/3 appear*) OR (meal* W/3 presentation) OR (meal* W/3 size) OR (meal* W/3 portion) OR (meal* W/3 amount) OR (meal* W/3 fortifi*) OR (meal* W/3 supplement*) OR (meal* W/3 assist*) OR (meal* W/3 help*) OR (meal* W/3 support*) OR (meal* W/3 add*) OR (meal* W/3 extra) OR (meal* W/3 alter*) OR (meal* W/3 chang*) OR (meal* W/3 new) OR (meal* W/3 enhance*) OR (meal* W/3 modif*) OR (meal* W/3 increas*) OR (food W/3 decreas*) OR (meal* W/3 improv*) OR (meal* W/3 reduc*) OR (meal* W/3 target*))) OR (TITLE‐ABS‐KEY((snack* W/3 time*) OR (snack* W/3 timing) OR (snack* W/3 pattern) OR (snack* W/3 style) OR (snack* W/3 arrangement*)OR (snack*W/3 environment) OR (snack* W/3 flavour) OR (snack* W/3 taste) OR (snack* W/3 content) OR (snack* W/3 composition) OR (snack* W/3 density) OR (snack* W/3 appear*) OR (snack* W/3 presentation) OR (snack* W/3 size) OR (snack* W/3 portion) OR (snack* W/3 amount) OR (snack* W/3 fortifi*) OR (snack* W/3 supplement*) OR (snack* W/3 assist*) OR (snack* W/3 help*) OR (snack* W/3 support*) OR (snack* W/3 add*) OR (snack* W/3 extra) OR (snack* W/3 alter*) OR (snack* W/3 chang*) OR (snack* W/3 new) OR (snack* W/3 enhance*) OR (snack* W/3 modif*) OR (snack* W/3 increas*) OR (food W/3 decreas*) OR (snack* W/3 improv*) OR (snack* W/3 reduc*) OR (snack* W/3 target*))) OR (TITLE‐ABS‐KEY((drink* W/3 time*) OR (drink* W/3 timing) OR (drink* W/3 pattern) OR (drink* W/3 style) OR (drink* W/3 arrangement*)OR (drink*W/3 environment) OR (drink* W/3 flavour) OR (drink* W/3 taste) OR (drink* W/3 content) OR (drink* W/3 composition) OR (drink* W/3 density) OR (drink* W/3 appear*) OR (drink* W/3 presentation) OR (drink* W/3 size) OR (drink* W/3 portion) OR (drink* W/3 amount) OR (drink* W/3 fortifi*) OR (drink* W/3 supplement*) OR (drink* W/3 assist*) OR (drink* W/3 help*) OR (drink* W/3 support*) OR (drink* W/3 add*) OR (drink* W/3 extra) OR (drink* W/3 alter*) OR (drink* W/3 chang*) OR (drink* W/3 new) OR (drink* W/3 enhance*) OR (drink* W/3 modif*) OR (drink* W/3 increas*) OR (food W/3 decreas*) OR (drink* W/3 improv*) OR (drink* W/3 reduc*) OR (drink* W/3 target*))) OR (TITLE‐ABS‐KEY((feed* W/3 time*) OR (feed* W/3 timing) OR (feed* W/3 pattern) OR (feed* W/3 style) OR (feed* W/3 arrangement*) OR (feed* W/3 environment) OR (feed* W/3 flavour) OR (feed* W/3 taste) OR (feed* W/3 content) OR (feed* W/3 composition) OR (feed* W/3 density) OR (feed* W/3 appear*) OR (feed* W/3 presentation) OR (feed* W/3 size) OR(feed* W/3 portion) OR(feed* W/3 amount) OR(feed* W/3 fortifi*) OR (feed* W/3 supplement*) OR (feed* W/3 assist*) OR(feed* W/3 help*) OR (feed* W/3 support*) OR (feed* W/3 add*) OR (feed* W/3 extra) OR (feed* W/3 alter*) OR(feed* W/3 chang*) OR (feed* W/3 new) OR (feed* W/3 enhance*) OR (feed* W/3 modif*) OR (feed* W/3 increas*) OR (food W/3 decreas*) OR (feed* W/3 improv*) OR (feed* W/3 reduc*) OR (feed* W/3 target*)))) #2 OR ((TITLE‐ABS‐KEY((nutri* W/3 content) OR (nutri* W/3 composition)OR (nutri* W/3 density))) OR (TITLE‐ABS‐KEY((diet* W/3 content) OR (diet* W/3 composition)OR (diet* W/3 density))) OR (TITLE‐ABS‐KEY(nutri* W/3 fortifi*)) OR (TITLE‐ABS‐KEY(diet* W/3 fortifi*)) OR (TITLE‐ABS‐KEY(nutri* W/3 supplement*)) OR (TITLE‐ABS‐KEY(diet* W/3 supplement*)) OR (TITLE‐ABS‐KEY(nutri* W/3 add*) OR (nutri* W/3 extra)) OR (TITLE‐ABS‐KEY((diet* W/3 add*) OR (diet* W/3 extra)))) #3 OR ((TITLE‐ABS‐KEY((dining* W/3 time*) OR (dining* W/3 timing) OR (dining* W/3 pattern) OR (dining* W/3 style) OR (dining* W/3 arrangement*)OR (dining* W/3 environment))) OR (TITLE‐ABS‐KEY((dining* W/3 alter*) OR (dining* W/3 chang*) OR (dining* W/3 new) OR (dining* W/3 enhance*) OR (dining* W/3 modif*) OR (dining*W/3 increas*) OR (food W/3 decreas*) OR (dining* W/3 improv*) OR (dining* W/3 reduc*) OR (dining* W/3 target*)))) #4 OR ((TITLE‐ABS‐KEY((screening W/3 nutri*) OR (screening W/3 diet*))) OR (TITLE‐ABS‐KEY((monitoring W/3 nutri*) OR (monitoring W/3 diet*))) OR (TITLE‐ABS‐KEY((screening W/3 add*) OR (screening W/3 extra))) OR (TITLE‐ABS‐KEY((monitoring W/3 add*) OR (monitoring W/3 extra))) OR (TITLE‐ABS‐KEY((screeningW/3 alter*) OR (screening W/3 chang*) OR (screening W/3 new) OR (screening W/3 enhance*) OR (screeningW/3 modif*) OR (screening W/3 increas*) OR (food W/3 decreas*) OR (screening W/3 improv*) OR (screening W/3 reduc*) OR (screening W/3 target*))) OR (TITLE‐ABS‐KEY((monitoring W/3 alter*)OR (monitoringW/3 chang*)OR (monitoringW/3 new)OR (monitoringW/3 enhance*)OR (monitoringW/3 modif*)OR (monitoringW/3 increas*)OR (foodW/3 decreas*)OR (monitoringW/3 improv*)OR (monitoringW/3 reduc*)OR (monitoringW/3 target*)))) #5 OR ((TITLE‐ABS‐KEY((documentation W/3 alter*) OR (documentation W/3 chang*) OR (documentation W/3 new) OR (documentation W/3 enhance*) OR (documentation W/3 modif*) OR (documentation W/3 increas*) OR (food W/3 decreas*) OR (documentation W/3 improv*)OR (documentation W/3 reduc*)OR (documentation W/3 target*))) OR (TITLE‐ABS‐KEY((communication W/3 alter*)OR (communicationW/3 chang*) OR (communication W/3 new) OR (communication W/3 enhance*) OR (communication W/3 modif*) OR (communication W/3 increas*) OR (food W/3 decreas*) OR (communication W/3 improv*) OR (communication W/3 reduc*) OR (communication W/3 target*)))) #6 OR ((TITLE‐ABS‐KEY((staff* W/3 nurs*)OR (train* W/3 nurs*))) OR (TITLE‐ABS‐KEY((staff* W/3 healthcare)OR (train*W/3 healthcare)OR (staff* W/3 health care) OR (train* W/3 healthcare))) OR (TITLE‐ABS‐KEY(staff* W/3 cater*)) OR (TITLE‐ABS‐KEY((staff* W/3 assist*) OR (train* W/3 assist) OR (staff* W/3 help*) OR (train* W/3 help*) OR (staff* W/3 support*) OR (train*W/3 support*) OR (staff* W/3 add*) OR (train* W/3 add*) OR (staff* W/3 extra) OR (train* W/3 extra))) OR (TITLE‐ABS‐KEY((staff* W/3 alter*) OR (staff* W/3 chang*) OR (staff* W/3 new) OR (staff* W/3 enhance*)OR (staff* W/3 modif*)OR (staff* W/3 increas*)OR (food W/3 decreas*)OR (staff* W/3 improv*)OR (staff* W/3 reduc*)OR (staff* W/3 target*))) OR (TITLE‐ABS‐KEY((train* W/3 alter*) OR (train* W/3 chang*) OR (train* W/3 new) OR (train* W/3 enhance*)OR (train* W/3 modif*)OR (train* W/3 increas*)OR (food W/3 decreas*)OR (train* W/3 improv*)OR (train* W/3 reduc*)OR (train* W/3 target*)))) #7 OR (TITLE‐ABS‐KEY((supplement* W/3 add*) OR (supplement* W/3 extra))))) #8 (((((((TITLE‐ABS‐KEY((assist* W/3 nurs*) OR (assist* W/3 healthcare) OR (assist* W/3 healthcare) OR(assist* W/3 cater*))) OR (TITLE‐ABS‐KEY((help* W/3 nurs*) OR (help* W/3 healthcare)OR (help* W/3 health care) OR (help* W/3 cater*))) OR (TITLE‐ABS‐KEY((support* W/3 nurs*) OR (support* W/3 healthcare) OR (support* W/3 health care) OR (support* W/3 cater*)))) #9 OR (TITLE‐ABS‐KEY(("protected meal" OR "red tray")) OR ((supportive W/3 intervention) OR (nutrition* W/3intervention) OR (diet* W/3intervention))) #10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 #11 OR (TITLE‐ABS‐KEY("low BMI" OR "low body mass index")) #12 OR (TITLE‐ABS‐KEY("low weight" OR underweightOR "under‐weight")) #13 OR (TITLE‐ABS‐KEY(maln*)) #14 OR (TITLE‐ABS‐KEY(("nutritional risk") OR (risk W/3 maln*))) #15 OR (TITLE‐ABS‐KEY("poor nutr*"OR undernourish* OR "under‐nourish*")) #16 OR (TITLE‐ABS‐KEY((poor W/3 intake*) OR (inadequateW/3 intake*) OR (suboptimal W/3 intake*))) #17 OR (TITLE‐ABS‐KEY(institutionali?ed OR elderly)) #18 OR (TITLE‐ABS‐KEY(homebound OR "home bound" OR housebound OR "house bound")) #19 OR (ABS((extended W/1care) OR(longterm W/1care) OR("long term" W/1 care) OR (community W/1 care))) #20 OR (ABS((nursing W/1 home)OR (care W/3 home) OR (residential W/3 home))) #21 OR (ABS(inpatient* OR hospitali?* OR "hospital patient*")) #22 OR (ABS("nutritional status" OR "nutrition disorder*" OR "nutrition assessment*" OR "nutritional support*" OR "nutrition policy")) #23 OR (ABS(diet* OR "food service" OR "energy intake" OR "fortified food")) #24 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 #25 #18 OR #19 OR #20 OR #21 OR #22 OR #23 #26 #24 OR #25 #27 #10 AND #26 #28 ((ABS("controlled trial*" OR "controlled clinical trial*" OR "clinical trial*")) OR (ABS(random* ORplacebo)) OR (ABS("meta‐analys*" OR metaanalys* OR hta OR "health technology assessment")) OR (ABS(literature* OR "medical database*" OR medline OR pubmed OR embase OR cochrane OR cinahl OR psycinfo OR psyclit OR healthstar OR biosis OR "current content*" OR "systematic review*"))) #29 #27 AND #28 #30 (ABS(adult*)) #31 #29 AND #30 #32 (ABS(pregnan*)) #33 #31 AND NOT #32 #34 (ABS(animal*)) #35 #33 AND NOT #34 |
| ISI Web of Science |
| #1 TS=((food* OR meal* OR snack* OR drink* OR feed*) NEAR/3 (time* OR timing OR pattern OR style OR arrangement* OR environment OR flavor OR taste OR content OR composition OR density OR appear* OR presentation OR size OR portion OR amount OR fortifi* OR supplement* OR assist* OR help* OR support* OR add* OR extra OR alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*)) # 2 TS=((nutri* OR diet*) NEAR/3 (content OR composition OR density OR fortfi* OR supplement* OR add* OR extra OR alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target)) # 3 TS=((dining*) NEAR/3 (time* OR timing OR pattern OR style OR arrangement* OR environment OR alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*)) # 4 TS=((screening OR monitoring) NEAR/3 (nutri* OR diet* OR add* OR extra OR alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*)) # 5 TS=((documentation OR communication) NEAR/3 (alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*)) # 6 TS=((staff* OR train*) NEAR/3 (nurs* OR healthcare OR "health care" OR cater* OR assist* OR help* OR support* OR add* OR extra OR alter* OR chang* OR new OR enhance* OR modif* OR increas* OR decreas* OR improv* OR reduc* OR target*)) # 7 TS=((supplement*) NEAR/6 (add* OR extra)) # 8 TS=((assist* OR help* OR support*) NEAR/3 (nurs* OR healthcare OR "health care" OR cater*)) # 9 TS=(("protected meal" OR "red tray") OR ((supportive OR nutrition* OR diet*) NEAR/3 intervention*)) # 10 #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 # 11 TS=(("low bmi" OR "low body mass index")) # 12 TS=(("low weight" OR underweight OR "under‐weight")) # 13 TS=(maln*) # 14 TS=("nutritional risk" OR (risk NEAR/3 maln*)) # 15 TS=(("poor nutr*" OR undernourish* OR "under nourish*")) # 16 TS=((poor OR inadequate OR suboptimal) NEAR/6 intake*) # 17 TS=((institutionali?ed OR elderly)) # 18 TS=((homebound OR "home bound" OR housebound OR "house bound")) # 19 TS=((extended OR longterm OR "long term" OR community) NEAR/1 care) # 20 TS=((nursing OR care OR residential) NEAR/1 home) # 21 TS=((inpatient* OR hospitali?* OR "hospital patient*")) # 22 TS=(nutritional status) # 23 TS=(nutrition disorder*) # 24 TS=(("nutrition assessment*" OR "nutritional support*"OR "nutrition policy")) # 25 TS=((diet* OR "food service" OR "energy intake" OR "fortified food")) # 26 #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 # 27 #26 AND #10 # 28 TS=("controlled trial*" OR "controlled clinical trial*" OR "clinical trial*") # 29 TS=(random* OR placebo) # 30 TS=("meta‐analys*" OR metaanalys* OR hta OR "health technology assessment") # 31 TS=((literature* OR "medical database*" OR medline OR pubmed OR embase OR cochrane OR cinahl OR psycinfo OR psyclit OR healthstar OR biosis OR "current content*" OR "systematic review*")) # 32 #31 OR #30 OR #29 OR #28 # 33 #32 AND #27 # 34 TS=(adult*) # 35 #34 AND #33 # 36 TS=(pregnan*) # 37 #35 NOT #36 # 38 TS=(animal*) # 39 #37 NOT #38 |
Appendix 2. Search strategies (January 2013 to September 2016)
| Cochrane Library (Wiley) |
|
I: Population 1. [mh ^Stroke] or stroke:ti,ab 2. [mh ^"Alzheimer Disease"] or alzheimer:ti,ab 3. [mh ^Dementia] or dement*:ti,ab 4. [mh ^"Mild Cognitive Impairment"] or "cognitive impairment":ti,ab 5. [mh "Hip Fractures"] or ("hip fracture*" or "femoral neck fracture*"):ti,ab 6. [mh ^"Nursing Homes"] or [mh ^"Homes for the Aged"] or ("nursing home*"):ti,ab 7. (residents or residential):ti,ab 8. [mh ^"Aged"] or [mh ^"Aging"] or aged:ti,ab 9. [mh ^"Frail Elderly"] or (elder or elders or elderly):ti,ab 10. (older or geriatric):ti,ab 11. [mh ^"Inpatients"] or inpatients:ti,ab 12. [mh ^"Outpatients"] or outpatients:ti,ab 13. [mh ^"Institutionalization"] or institutionali*:ti,ab 14. [mh ^"Hospitalization"] or (hospitali?ed or hospitali?ation):ti,ab 15. {or #1‐#14} II: Condition 16. [mh ^"Malnutrition"] or [mh ^"Protein‐Energy Malnutrition"] 17. (malnourish* or malnutrition):ti,ab 18. [mh ^"Nutrition Assessment"] 19. [mh ^"Nutritional Status"] or "nutritional status":ti,ab 20. [mh ^"Nutritional Requirements"] 21. [mh ^"Nutrition Disorders"] 22. [mh ^"Nutritional Support"] 23. ((nutritional or nutrition or nutritionally) near/2 risk):ti,ab 24. ((unintentional or risk) near/2 "weight loss"):ti,ab 25. (undernutrition or undernourished or hyponutrition):ti,ab 26. [mh ^"Elder Nutritional Physiological Phenomena"] 27. [mh ^"Energy Intake"] 28. [mh ^"Feeding Behavior"] or [mh ^"Feeding Methods"] 29. ("Mini Nutritional Assessment" or "Eating Behaviour Scale" or "Edinburgh Feeding Evaluation" or "Malnutrition Universal Screening Tool"):ti,ab 30. ((improve* or increase* or inadequate) near/3 ("nutrient intake" or "energy intake" or "dietary intake" or "food intake")):ti,ab 31. {or #16‐#30} 32. #15 and #31 33. #32 not (child* or infant* or pregnan*):ti,ab,kw 34. Publication Year from 2013 to 2016 |
| MEDLINE (Ovid SP) |
|
I: Population 1. Stroke/ or stroke.tw. 2. Alzheimer Disease/ or alzheimer.tw. 3. Dementia/ or dement*.tw. 4. Mild Cognitive Impairment/ or cognitive impairment.tw. 5. exp Hip Fractures/ or (hip fracture? or femoral neck fracture?).tw. 6. Nursing Homes/ or Homes for the Aged/ or (nursing home?).tw. 7. (residents or residential).tw. 8. Aged/ or Aging/ or aged.tw. 9. Frail Elderly/ or (elder or elders or elderly).tw. 10. (older or geriatric).tw. 11. Inpatients/ or inpatients.tw. 12. Outpatients/ or outpatients.tw. 13. Institutionalization/ or institutionali*.tw. 14. Hospitalization/ or (hospitali?ed or hospitali?ation).tw. 15. or/1‐14 II: Condition 16. Malnutrition/ or Protein‐Energy Malnutrition/ 17. (malnourish* or malnutrition).tw. 18. Nutrition Assessment/ 19. Nutritional Status/ or nutritional status.tw. 20. Nutritional Requirements/ 21. Nutrition Disorders/ 22. Nutritional Support/ 23. ((nutritional or nutrition or nutritionally) adj2 risk).tw. 24. ((unintentional or risk) adj2 weight loss).tw. 25. (undernutrition or undernourished or hyponutrition).tw. 26. Elder Nutritional Physiological Phenomena/ 27. Energy Intake/ 28. Feeding Behavior/ or Feeding Methods/ 29. (Mini Nutritional Assessment or Eating Behaviour Scale or Edinburgh Feeding Evaluation or Malnutrition Universal Screening Tool).tw. 30. ((improve* or increase? or inadequate) adj3 (nutrient intake or energy intake or dietary intake or food intake)).tw. 31. or/16‐30 32. 15 and 31 III. [Cochrane Handbook 2008 RCT filter ‐ sensitivity and precision max. version] 33. randomized controlled trial.pt. 34. controlled clinical trial.pt. 35. randomi?ed.ab. 36. placebo.ab. 37. clinical trials as topic/ 38. randomly.ab. 39. trial.ti. 40. or/33‐39 41. exp animals/ not humans/ 42. 40 not 41 43. 32 and 42 44. 43 not (child* or infant* or pregnan*).tw. 45. limit 44 to yr="2013‐Current" |
| ClinicalTrials.gov (Advanced search) |
|
Search Terms: malnourished OR malnutrition OR undernourished OR undernutrition OR "under nutrition" OR "poor nutritional status" OR "nutritional risk" OR "inadequate nutrient intake" Study Type: Interventional Studies Age Group: Adult (18–65), Senior (66+) |
| WHO ICTRP (Standard search) |
| malnourished AND elder* OR malnutrition AND elder* OR undernourished AND elder* OR undernutrition AND elder* OR malnourished AND aged OR malnutrition AND aged OR undernourished AND aged OR undernutrition AND aged OR malnourished AND geriatric OR malnutrition AND geriatric OR undernourished AND geriatric OR undernutrition AND geriatric |
Appendix 3. Description of interventions
| Intervention(s) | Type of intervention(s)a | Comparator(s) | |
| Barton 2000 | I1: portion size decreased by 20% but fortified to achieve overall daily energy provision increased by 200 kcal (randomised) | Modification of meal profile or pattern | Normal hospital menu (randomised group) |
| I2: normal hospital menu plus cooked breakfast (not randomised group) | |||
| Beck 2002 | I1: homemade oral supplement (group A, not randomised) | Additional supplementation of meals | Usual diet |
| I2: homemade oral supplement (group B) | |||
| Bouillanne 2013 | 'Pulse diet': 78% of daily protein requirements provided at lunch (no change to energy and protein) | Modification of meal profile or pattern | 'Spread diet': usual diet (daily protein requirements distributed between meals) |
| Bourdel‐Marchasson 2000 | Oral supplementation in addition to standard diet | Additional supplementation of meals | Standard diet |
| Brouillette 1991 | Osmotherapy (use of aromas to stimulate appetite) + activities | Changes to the feeding environment | Activities only |
| Castellanos 2009 | I1: two breakfast and two lunch foods fortified to improve energy and protein content (hot cereal and juice breakfast, soup and side dish at lunch) | Modification of meal profile or pattern | Routine care, no meals enhanced |
| I2: two lunch foods only fortified versus normal menu | |||
| Chang 2005 | Training in feeding skills (feeding skills training programme for nursing assistants) | Changes to the organisation of nutritional care | No training |
| Dennis 2005 | Normal hospital diet plus oral nutritional supplements | Additional supplementation of meals | Normal hospital diet |
| Duncan 2006 | Additional personal attention of a dietetic assistant e.g. checking personal food preferences, assisting with food choice, provision of appropriate feeding aids, feeding assistance and collecting information to aid nutritional assessment | Changes to the organisation of nutritional care | Routine care |
| Essed 2007 | Food sprinkled with 1 g (+ 0.2 g) of intervention + maltodextrin carrier I1: monosodium glutamate |
Modification of meal profile or pattern | Maltodextrin (placebo) |
| I2: flavour | |||
| I3: monosodium glutamate + flavour | |||
| Essed 2009 | Three foods (previously identified as preferred), i.e. mashed potato (0.2 g NaCl/100 g + 0.5% monosodium glutamate), mince meat (0.37 g NaCl/100 g + 2% monosodium glutamate and spinach (0.25 g NaCl/100 g + 2% monosodium glutamate) | Modification of meal profile or pattern | Usual hot meal |
| Faxen‐Irving 2011 | A daily dose of 3 x 30 mL fat emulsion distributed at the same time as pharmaceutical prescriptions | Additional supplementation of meals | Standard care |
| Gaskill 2009 | Nutrition education programme | Changes to the organisation of nutritional care | Usual care |
| Germain 2006 | Re‐formed foods, thickened beverages and dietary supplements as necessary | Modification of meal profile or pattern | Traditional modified texture diet |
| Hankey 1993 | Supplements in addition to their normal hospital diet | Additional supplementation of meals | Standard hospital food |
| Hickson 2004 | Additional nutritional care from a trained health care assistant | Changes to the organisation of nutritional care | Usual care |
| Holyday 2012 | Malnutrition care plan; screening, assessment and intervention tailored to individuals requirements (including texture modification, fortification, oral nutritional supplements, snacks, assistance) | Changes to the organisation of nutritional care | Usual care |
| Johansen 2004 | Nutrition team (dietitian + nurse) | Changes to the organisation of nutritional care | Usual care |
| Kraft 2012 | Oral nutritional supplements + monitoring using telemedicine | Changes to the organisation of nutritional care | Usual care |
| Kretser 2003 | Modified meals on wheels system (21 meals + 14 snacks) and daily phone call | Congregate and home meal delivery systems | Traditional meals on wheels (one hot meal delivered five days a week at lunch) |
| Larsson 1990 | Oral nutritional supplements plus normal hospital diet | Additional supplementation of meals | Normal hospital diet |
| Leslie 2012 | Energy enriched usual meals | Modification of meal profile or pattern | Usual care |
| Lin 2010 | I1: spaced‐retrieval ‐ a method to enhance learning, retention and recall of information | Changes to the organisation of nutritional care | Usual care |
| I2: Montessori intervention ‐ a method capable of stopping or reducing residents problem behaviours | |||
| Lin 2011 | Montessori intervention ‐ designed to manage eating difficulties | Changes to the organisation of nutritional care | Usual care |
| Mathey 2001a | Improved meal ambiance comprising improvements to physical environment, meal service and organisation of assistance | Changes to the feeding environment | Usual care |
| Mathey 2001b | Creating a better ambience during food consumption | Changes to the feeding environment | Usual care |
| Munk 2014 | Energy and protein enriched foods provided in addition to the hospital food via an a la carte menu | Modification of meal profile or pattern | Usual care |
| Nijs 2006 | Family‐style meals comprising table dressing, food service, staff protocols, residents protocol and a meal‐time protocol, meal choice at the time of meal | Changes to the feeding environment | Individual pre‐plated meal service, meal chosen 2 weeks in advance. |
| Olofsson 2007 | Multi‐component intervention (including nutrition) | Changes to the organisation of nutritional care | Usual care |
| Pivi 2011 | I1: nutrition education for caregivers and participants | Changes to the organisation of nutritional care | Usual care |
| I2: oral nutritional supplements (two cartons daily for six months) | |||
| Potter 2001 | Oral nutritional supplement + normal hospital diet | Additional supplementation of meals | Normal hospital diet |
| Remsburg 2001 | Buffet style dining programme for supper only | Changes to the feeding environment | Usual care, tray‐style meal served by nursing home staff |
| Salva 2011 | Teaching and training intervention to improve nutrition care | Changes to the organisation of nutritional care | Usual care |
| Silver 2008 | Home‐delivered fortified lunch once weekly for 7 months | Modification of meal profile or pattern | Home delivered usual lunch once weekly for 7 months |
| Simmons 2008 | Mealtime feeding assistance and/or between meal snacks | Additional supplementation of meals | Usual care |
| Simmons 2010 | I1: snacks between meal snacks | Additional supplementation of meals | Usual care |
| Smolliner 2008 | I2: oral nutritional supplements | ||
| Protein and energy‐enriched soups and sauces and two additional snacks high in protein and energy | Modification of meal profile or pattern | Usual diet | |
| Splett 2003 | Medical nutrition therapy (protocol‐driven nutritional assessment and intervention activities carried by dietitians) | Changes to the organisation of nutritional care | Usual care by dietitians |
| Taylor 2006 | 5‐meal menu pattern with energy content similar to existing 3‐meal menu | Modification of meal profile or pattern | 3‐meal menu (usual care) |
| Van den Berg 2015 | I1: offered 125 mL ONS twice daily with medication rounds | Additional supplementation of meals | Usual care (125 mL ONS offered in between meals) |
| I2: offered 62 mL ONS four times daily with medication rounds | |||
| Van Ort 1995 | I2: offered 62 mL ONS daily with medication rounds | Changes to the feeding environment | Usual care |
|
aNumbers refer to intervention sub‐categories: (1) changes to the organisation of nutritional care, (2) changes to the feeding environment, (3) modification of meal profile or pattern, (4) additional supplementation of meals, (5) congregate and home meal delivery systems ‐ see Table 2 C: comparator; I: intervention; ONS: oral nutritional supplement | |||
Appendix 4. Baseline characteristics (I)
| Intervention(s) and comparator(s) | Participants (N) | Description of participants (trial design) | Country | Setting | Sex N (female %) | Age mean years (SD)/range) | |
| Barton 2000 | I1: reduced portion fortified menu | 13 | Elderly hospitalised individuals (cross‐over RCT) |
UK | Elderly rehab ward | 6 (46) | 77 (8) |
| I2: normal menu plus cooked breakfast | 8 (non‐randomised) | 5 (63) (non‐randomised) | 78 (9) (non‐randomised) | ||||
| C: normal hospital menu | 14 | 11 (79) | 75 (11) | ||||
| Beck 2002 | I1: homemade oral supplement | 36 | Nursing home residents > 65 years (parallel RCT) |
Denmark | Residential care home | 22 (61) | 81 (76‐86) |
| I2: homemade oral supplement (B) | |||||||
| C: usual diet | |||||||
| Bouillanne 2013 | I: pulse diet (78% protein at lunch) | 66 | Hospitalised elderly individuals (parallel RCT) | France | Intermediate care unit | 46 (70) | 84.1 (6) |
| C: usual diet (protein distributed between meals) | 85.7 (6.3) | ||||||
| Bourdel‐Marchasson 2000 | I: oral supplementation + standard diet | 295 | Critically ill elderly participants (cluster‐RCT) |
France | Hospital wards & geriatric units | 199 (67.5) | 83.6 (7.3) |
| C: standard diet | 377 | 238 (63.1) | 83.0 (7.1) | ||||
| Brouillette 1991 | I: osmotherapy + activities | 10 | Nursing home residents (parallel RCT) | USA | Residential care home | 14 (88) of those that completed the trial | 80 (6.4) |
| C: activities only | 10 | 87 (6.8) | |||||
| Castellanos 2009 | I1: fortified breakfast and lunch menu | 39 | Nursing home residents (cross‐over RCT) |
USA | Residential care home | 23/33 finishing (70) | 87.3 (8.6) |
| I2: fortified lunch menu | 39 | ||||||
| C: usual menu | 39 | ||||||
| Chang 2005 | I: training in feeding skills | 20 | Nursing assistants and nursing home residents with dementia (cluster RCT) | Taiwan | Residential care home | ‐ | ‐ |
| C: no training | 16 | ||||||
| Dennis 2005 | I: nutritional supplement + normal hospital diet | 2016 | Participants with recent stroke (parallel RCT) | 15 different countries | Hospital | 945 (47) | 71 (12) |
| C: normal hospital diet | 2007 | 929 (46) | 71 (13) | ||||
| Duncan 2006 | I. dietetic assistant | 153 | Women > 65 years admitted with acute hip fracture (parallel RCT) | UK | Acute trauma ward | 318 (100) | 83.6 |
| C: usual care | 165 | 83.5 | |||||
| Essed 2007 | I1: monosodium glutamate | 19 | Residents of nursing home ≥ 65 years (factorial RCT) |
Netherlands | Residential care home | 58 (70) | 84.9 (5.7) |
| I2: flavour | 19 | 85.4 (6.7) | |||||
| I3: monosodium glutamate + flavour | 22 | 84.9 (6.2) | |||||
| C: maltodextrin | 23 | 85.6 (8.5) | |||||
| Essed 2009 | I: monosodium glutamate + NaCl | 53 | Nursing home residents > 65 years (cross‐over RCT) |
Netherlands | Residential care home | 40 (76) | 85.8 (6.2) |
| C: usual hot meal | 53 | ||||||
| Faxen‐Irving 2011 | I: 3 x 30 mL of fat emulsion daily | 34 | Recently admitted geriatric persons > 65 years (parallel RCT) | Sweden | Geriatric acute ward | (61) | 82.7 (7.5) ‐ data from those who completed the trial only (N = 24) |
| C: usual care | 37 | (49) | 85.1 (6.7) ‐ data from those who completed the trial only (N = 27) | ||||
| Gaskill 2009 | I: nutrition education programme | 352 | Nursing home residents (cluster‐RCT) | Australia | Residential care home | 245 (70) | 84.2 (8.7) |
| C: usual care | |||||||
| Germain 2006 | I: re‐formed foods | 8 | Frail institutionalised elderly people with dysphagia (parallel RCT) | Canada | Residential care home | 5 (63) | 82.5 (4.4) |
| C: usual diet | 9 | 5 (56) | 84.6 (3.8) | ||||
| Hankey 1993 | I: oral nutritional supplement | 7 | Frail elderly persons in continuing care (parallel RCT) | UK | Hospital | 11 (79) | 81 (1.6) |
| C: standard hospital diet | 7 | ||||||
| Hickson 2004 | I: feeding assistance | 292 | Acutely ill elderly inpatients (parallel RCT) | UK | Elderly medicine ward | 200 (69) | 82 (76 ‐ 86) |
| C: usual care | 300 | 173 (58) | 82 (77 ‐ 87) | ||||
| Holyday 2012 | I: malnutrition care plan | 71 | Acutely ill elderly inpatients (parallel RCT) | Australia | Acute geriatric medicine ward | 43 (61) | 83.7 (6.7) |
| C: usual care | 72 | 39 (54) | 83.4 (7.6) | ||||
| Johanssen 2004 | I: nutrition team | 108 | Nutritional risk score 2000 > 3 on admission to hospital (parallel RCT) | Denmark | Hospital, three different levels | 54 (50) | 62 (1.6) |
| C: usual care | 104 | 56 (54) | 62.4 (1.7) | ||||
| Kraft 2012 | I: ONS + telemedicine monitoring | 13 | Malnourished geriatric home‐dwelling persons (parallel RCT) | Germany | Hospital discharge and tele‐medicine monitoring | 7 (54) | 80.7 (5.6) |
| C: usual care | 13 | 9 (69) | 78.8 (8.8) | ||||
| Kretser 2003 | I: modified meals on wheels | 102 | Homebound older adults at nutritional risk (parallel RCT) | USA | Home care | 70 (69) | (60‐90) |
| C: traditional meals on wheels | 101 | 74 (73) | |||||
| Larsson 1990 | I: ONS plus normal hospital diet | 435 | Older people admitted to a long‐term medical care clinic (parallel RCT) | Sweden | Hospital | Unclear ‐ varies between papers, authors to be contacted | 80.1 (8.5) |
| C: normal hospital diet | |||||||
| Leslie 2012 | I: energy enriched meals | 22 | People living in residential care homes (cluster‐RCT) |
UK | Residential care home | 36 (88%) | 90.9 (77‐105) |
| C; usual care | 19 | 90.3 (70‐100) | |||||
| Lin 2010 | I1: spaced‐retrieval | 32 | Residents with dementia (cluster‐RCT) | Taiwan | Residential care home | 18 (56) | 76.7 (6.1) |
| I2: Montessori | 29 | 12 (41) | 82.9 (6.0) | ||||
| C: usual care | 24 | 15 (63) | 81.1 (7.0) | ||||
| Lin 2011 | I: Montessori | 29 | Residents with dementia (cluster RCT & cross‐over RCT) |
Taiwan | Residential care home | 12 (41) | 82.9 (6.0) |
| C: usual care | |||||||
| Mathey 2001a | I: improved meal ambiance | 21 | Nursing home residents > 65 years (cluster‐RCT) | Netherlands | Residential care home | 25 (66) | 82.2 (7.9) |
| C: usual care | 17 | ||||||
| Mathey 2001b | I: flavour enhancement | 36 | Nursing home residents > 65 years (parallel RCT) | Netherlands | Residential care home | 29 (74) | 84.6 (6.1) |
| C: usual care | 31 | 25 (81) | 83.0 (5.5) | ||||
| Munk 2014 | I: energy and protein enriched foods provided via a la carte menu in addition to hospital food | 41 (number completing the trial) | New admissions to hospital ward (oncology, orthopaedics or urology) (parallel RCT) | Denmark | Hospital | 25 (61) | 75 (10 |
| C: usual care | 40 | 22 (55) | 74 (11) | ||||
| Nijs 2006 | I: family‐style meals | 94 | Nursing home residents (cluster‐RCT) |
Netherlands | Residential care home | 70 (74) | 78 (11.1) |
| C: usual care | 84 | 55 (65) | 75 (9.9) | ||||
| Olofsson 2007 | I: multi‐component intervention (including nutrition) | 102 | People > 70 years with femoral neck fracture (parallel RCT) | Sweden | Geriatric orthopaedic ward | 62 (75) | 82.1 (6.8) |
| C: usual care | 97 | 57 (77) | 82.2 (5.6) | ||||
| Pivi 2011 | I1: nutrition education | 25 | Individuals > 65 years old with Alzheimer's disease (parallel RCT) | Brazil | Neurology outpatients | 53 (68) | 75.2 |
| I2: oral nutritional supplements | 26 | ||||||
| C: usual care | 27 | ||||||
| Potter 2001 | I: oral nutritional supplement + normal hospital diet | 186 | Unwell elderly people (parallel RCT) | Scotland, UK | Medicine for the elderly unit | 140 (75) | Median 83 (61‐79) |
| C: normal hospital diet | 195 | 139 (71) | |||||
| Remsburg 2001 | I: buffet‐style meals | 20 | Nursing home residents > 65 years (parallel RCT) | USA | Residential care home | 19 (95) | 80 (6) |
| C: usual care | 20 | 13 (65) | 80 (8) | ||||
| Salva 2011 | I: teaching and training | 448 | People with dementia (cluster RCT) |
Spain | Home care | 300 (67) | 79.4 (7) |
| C: usual care | 498 | 344 (69) | 78.6 (7.5) | ||||
| Silver 2008 | I: fortified home delivered lunch | ‐ | Adults > 60 years receiving home‐delivered lunch meals (cross‐over RCT) |
USA | Home care | 31(69) of those who completed the trial (N = 45) | 84.4 (1) of those who completed the trial (N = 45) |
| C: usual home delivered lunch | |||||||
| Simmons 2008 | I: feeding assistance and/or snacks | 35 | Nursing home residents (cluster‐RCT & cross‐over RCT) |
USA | Residential care home | Reported for the total group, and not the subgroup to be used in this review | Reported for the total group, and not the subgroup to be used in this review |
| C: usual diet | 34 | ||||||
| Simmons 2010 | I1: snacks | 25 | Nursing home residents (parallel RCT) | USA | Residential care home | 39 (62) | 86.9 (11.3) |
| I2: supplements | 18 | ||||||
| C: usual care | 20 | ||||||
| Smolliner 2008 | I: fortified meals and snacks | Elderly nursing home residents (cluster RCT) |
Germany | Residential care home | 17 (77) | 82.2 (9.5) | |
| C: usual diet | 21 (70) | 84.7 (9.5) | |||||
| Splett 2003 | I: medical nutrition therapy | 223 | Frail elderly nursing home residents (cluster‐RCT) |
USA | Residential care home | 143 (67) | Male 79.2 (9.7); Female 82.8 (8.7) |
| C: usual care | 171 | 125 (73) | |||||
| Taylor 2006 | I: 5‐meal menu | 31 | Elderly nursing home residents with dysphagia (cross‐over RCT) |
Canada | Residential care home | 26 (84) | 85 (6.4) |
| C: usual (3‐meal menu) | |||||||
| Van den Berg 2015 | I1: 125 mL ONS twice daily with medication round | Patients newly admitted to medical and surgical wards (parallel RCT) | The Netherlands | Hospital | 34 (52) | 70.5 (15) | |
| I2: 62 mL ONS four times daily with medication round | 37 (46) | 72.6 (10) | |||||
| C: usual care (125 mL ONS offered in between meals) | 34 (39) | 70.4 (13) | |||||
| Van Ort 1995 | I: contextual and behavioural intervention | 4 | Nursing home residents requiring feeding assistance (parallel RCT) | USA | Residential care home | 6 (75) | (65‐93) |
| C: usual care | 4 | ||||||
| ‐ denotes not reported C: comparator; I: intervention; ONS: oral nutritional supplement; RCT: randomised controlled trial | |||||||
Appendix 5. Baseline characteristics (II)
| Intervention(s) and comparator(s) | Ethnic groups | Baseline nutritional status (N (%)) | BMI (mean kg/m² (SD), range) | Duration of intervention (duration of follow‐up) | Comedications/cointerventions | Comorbidities (N or %) | |
| Barton 2000 | I1: reduced portion fortified menu | ‐ | ‐ | ‐ | Maximum of 56 d | ‐ | ‐ |
| I2: normal menu plus cooked breakfast | |||||||
| C: normal hospital menu | |||||||
| Beck 2002 | I1: homemade oral supplement (A) | ‐ | Mini Nutritional Assessment score 17‐23.5 (increased risk of malnutrition) | 22.8 (21.3 ‐ 26.1) | 2 mo (2 mo) | ‐ | ‐ |
| I2: homemade oral supplement (B) | |||||||
| C: usual diet | |||||||
| Bouillanne 2013 | I: pulse diet (78% protein at lunch) | ‐ | Albumin 25‐35 g/L; BMI < 22 kg/m² and/or weight loss > 10% in 6 months and/or MNA < 23.5 | Median 20.7 (95% CI 20‐23.2) | 6 wk (6 wk) | ‐ | ‐ |
| C: usual diet (protein distributed between meals) | ‐ | Median 20.9 (95% CI 20‐25) | |||||
| Bourdel‐Marchasson 2000 | I: oral supplementation + standard diet | ‐ | ‐ | ‐ | 15 d or until discharge (15 d or until discharge) | ‐ | ‐ |
| C: standard diet | |||||||
| Brouillette 1991 | I: osmotherapy + activities | ‐ | ‐ | ‐ | 3 wk (4 wk) | ‐ | ‐ |
| C: activities only | |||||||
| Castellanos 2009 | I1: fortified breakfast and lunch menu | ‐ | ‐ | 2 d (‐) | ‐ | ‐ | |
| I2: fortified lunch menu | |||||||
| C: usual menu | |||||||
| Chang 2005 | I: training in feeding skills | ‐ | ‐ | ‐ | Intervention: 3 hours "in‐service" within 2 days + 1 h "hands‐on" instruction (‐) | ‐ | ‐ |
| C: no training | |||||||
| Dennis 2005 | I: nutritional supplement + normal hospital diet | ‐ | ‐ | ‐ | Duration of hospital stay (6 mo) | ‐ | ‐ |
| C: normal hospital diet | |||||||
| Duncan 2006 | I. dietetic assistant | ‐ | ‐ | ‐ | Duration of hospital stay (4 mo) | ‐ | ‐ |
| C: usual care | |||||||
| Essed 2007 | I1: monosodium glutamate | ‐ | 22 (27) at increased risk of malnutrition by MNA | ‐ | 16 wk (16 wk) | ‐ | ‐ |
| I2: flavour | |||||||
| I3: monosodium glutamate + flavour | |||||||
| C: maltodextrin | |||||||
| Essed 2009 | I: monosodium glutamate + NaCl | ‐ | 8 (15) at increased risk of malnutrition by MNA | 26.5 (4.2) | 4 wk (4 wk) | ‐ | ‐ |
| C: usual hot meal | |||||||
| Faxen‐Irving 2011 | I: 3 x 30 mL of fat emulsion daily | ‐ | ‐ | 20.4 (3.5) | Median 8 d (median 8 d) | ‐ | Comorbidities related to anorexia were cancer (N = 6), liver disease (N = 1) and renal failure (N = 1) ‐ in both groups |
| C: usual care | 22.2 (3.7) | ||||||
| Gaskill 2009 | I: nutrition education programme | ‐ | 171 (49) moderately or severely malnourished by SGA | ‐ | 6 mo (6 mo) | ‐ | ‐ |
| C: usual care | |||||||
| Germain 2006 | I: re‐formed foods | ‐ | 17 (100) unintentional weight loss > 7.5% in previous 3 mo or BMI < 24 kg/m² | 22.4 (3.9) | 12 wk (12 wk) | ‐ | ‐ |
| C: usual diet | 21.2 (2.3) | ||||||
| Hankey 1993 | I: ONS | ‐ | ‐ | ‐ | 8 wk (8 wk) | ‐ | ‐ |
| C: standard hospital diet | |||||||
| Hickson 2004 | I: feeding assistance | 282 (96.6) white ethnic group | 21.7 (18.6‐25.3) | Duration of hospital stay (duration of hospital stay) | ‐ | ‐ | |
| C: usual care | 286 (95.3) white ethnic group | 21.8 (19.1‐25.7) | |||||
| Holyday 2012 | I: malnutrition care plan | ‐ | 119 (83) malnourished or at risk of malnutrition by MNA score | 23.8 (5.9) | Duration of hospital stay (duration of hospital stay) | ‐ | ‐ |
| C: usual care | 23.3 (5.9) | ||||||
| Johansen 2004 | I: nutrition team | ‐ | 212 (100) ESPEN 2002 NRS (score > 3 nutritionally at risk) | 21.2 (0.5) | Duration of hospital stay (duration of hospital stay) | ‐ | ‐ |
| C: usual care | ‐ | 21.8 (0.5) | |||||
| Kraft 2012 | I: ONS + telemedicine monitoring | ‐ | 26 (100) weight loss > 10% in 6 months, BMI < 21 kg/m², albumin < 35 g/L | 23.4 (3.7) | 6 mo (6 mo) | Number of medications: 7.5 (SD 4.2) | ‐ |
| C: usual care | 23.4 (4.5) | Number of medications: 8.2 (SD 3.4) | |||||
| Kretser 2003 | I: modified meals on wheels | 45 (44) white | 97 (96) at risk or malnourished according to MNA | 14 (14%) BMI < 18.5 | 26 wk (26 wk) | ‐ | A variety of self‐reported health problems reported |
| C: traditional meals on wheels | 38 (58) white | 95 (95) at risk or malnourished | 9 (9%) BMI < 18.5 | ||||
| Larsson 1990 | I: ONS plus normal hospital diet | ‐ | (28.5) malnourished | ‐ | 26 wk (26 wk) | ‐ | ‐ |
| C: normal hospital diet | |||||||
| Leslie 2012 | I: energy enriched meals | ‐ | 100% malnourished (BMI < 18.5 kg/m2 | 17.1 (1.5) | 12 wk (12 wk) | ‐ | 6 participants with dementia |
| C; usual care | 17.3 (1.4) | ||||||
| Lin 2010 | I1: spaced‐retrieval | ‐ | ‐ | 24.7 (4.3) | 8 wk (8 wk) | ‐ | ‐ |
| I2: Montessori | 21.2 (3.4) | ||||||
| C: usual care | 23.1 (2.7) | ||||||
| Lin 2011 | I: Montessori | ‐ | ‐ | 21.4 (3.5) | 8 wk (8 wk) | ‐ | ‐ |
| C: usual care | |||||||
| Mathey 2001a | I: improved meal ambiance | ‐ | ‐ | ‐ | 12 mo (12 mo) | ‐ | ‐ |
| C: usual care | |||||||
| Mathey 2001b | I: flavour enhancement | ‐ | ‐ | 28.4 (7.1) | 16 wk (16 wk) | Number of medicines/day 2.1 (1.8) | ‐ |
| C: usual care | 28.1 (7.0) | Number of medicines/day 2.1 (1.6) | |||||
| Munk 2014 | I: energy and protein enriched foods provided via a la carte menu in addition to hospital food | ‐ | NRS score 0 = 1, 1 = 10, 2 = 18, 3 = 12 | 21(4) | Duration of hospital stay (duration of hospital stay) | ‐ | Disease severity score: 0 = 2; 1 = 30; 2 = 8; 3 = 1 |
| C: usual care | NRS score 0 = 0, 1 = 15, 2 = 17, 3 = 8 | 22(4) | Disease severity score: 0 = 3; 1 = 34; 2 = 3; 3 = 0 | ||||
| Nijs 2006 | I: family‐style meals | ‐ | 17 (18) MNA score < 17 | 28.7 (6.8) | 6 mo (6 mo) | ‐ | CVA: 57% |
| C: usual care | 13 (13) MNA score < 17 | 28.4 (5.8) | CVA: 50% | ||||
| Olofsson 2007 | I: multi‐component intervention (including nutrition) | ‐ | 48 (58 ) malnourished or at risk by MNA score | 25.1 (4.1) | Duration of hospital stay (4 mo) | Staff education; team work, individual care planning; prevention and treatment of delirium and complications; nutrition; rehabilitation; secondary prevention of falls and fractures; osteoporosis prophylaxis | ‐ |
| C: usual care | 47 (57 ) malnourished or at risk by MNA score | 23.3 (4.0) | ‐ | ||||
| Pivi 2011 | I1: nutrition education | ‐ | ‐ | ‐ | 6 mo (6 mo) | ‐ | ‐ |
| I2: ONS | |||||||
| C: usual care | |||||||
| Potter 2001 | I: ONS + normal hospital diet | ‐ | Adequately nourished: 62/186 (33); moderately malnourished: 90/186 (48); severely malnourished: 34/186 (18) |
‐ | Duration of hospital stay (duration of hospital stay) | ‐ | ‐ |
| C: normal hospital diet | Adequately nourished: 68/195 (35); moderately malnourished: 87/195 (45); severely malnourished: 40/195 (21) |
||||||
| Remsburg 2001 | I: buffet‐style meals | 17 (85) white ethnic group | ‐ | 24.4 (6.1) | 3 mo (3 mo) | ‐ | CVA: 6 (30%) CVD: 13 (65%) |
| C: usual care | 15 (75) white ethnic group | 24.3 (5.8) | CVA: 10 (50%) CVD: 12 (60%) |
||||
| Salva 2011 | I: teaching and training | ‐ | (7.8) malnourished (51.5) or at risk by MNA | 26.6 (4.4) | 12 mo (12 mo) | Number of comorbidities 4.6 (SD 2.2) | ‐ |
| C: usual care | (2.8) malnourished (34.5) or at risk by MNA | 27.3 (4.6) | Number of comorbidities 4.2 (SD 2.6) | ||||
| Silver 2008 | I: fortified home‐delivered lunch | ‐ | ‐ | 24.2 (7) | 7 mo (7 mo) | ‐ | ‐ |
| C: usual home‐delivered lunch | |||||||
| Simmons 2008 | I: feeding assistance and/or snacks | ‐ | ‐ | ‐ | 2 x/d for 5 days/week and 24 wk (24 wk) | ‐ | ‐ |
| C: usual diet | |||||||
| Simmons 2010 | I1: snacks | ‐ | ‐ | ‐ | 6 wk (6 wk) | ‐ | ‐ |
| I2: supplements | |||||||
| C: usual care | |||||||
| Smolliner 2008 | I: fortified meals and snacks | ‐ | 22 (100) by MNA score indicating at risk or malnourished | 21.6 (3.6) | 12 wk (12 wk) | Number of prescriptions median 4 (IQR 2‐6.5) | GDS: 6.7 (SD 2.9) |
| C: usual diet | 30 (100) by Mini Nutritional Assessment score indicating at risk or malnourished | 22.5 (3.4) | Number of prescriptions median 6 (IQR 3.8‐7) | GDS: 7.5 (SD 3) | |||
| Splett 2003 | I: medical nutrition therapy | ‐ | ‐ | ‐ | 19‐180 d (19‐180 d) | ‐ | Dementia: 24%
congestive heart failure: 25%
depression: 19%
Alzheimer’s disease: 14%
bone/hip fracture: 15% chronic obstructive: 10% pulmonary disease cancer: 5% pneumonia: 4% dehydration: 1% |
| C: usual care | Dementia: 34% congestive heart failure: 26% depression: 32% Alzheimer’s disease: 21% bone/hip fracture: 19% chronic obstructive: 17% pulmonary disease cancer: 12% pneumonia: 6% dehydration: 4% | ||||||
| Taylor 2006 | I: 5‐meal menu | ‐ | 31 (100) mean MNA score 16.3 | 20.4 (3.4) | 2 x 4 d, separated by 4 wk) (2 x 4 d, separated by 4 wk) | ‐ | ‐ |
| C: usual (3‐meal menu) | |||||||
| Van den Berg 2015 | I1: 125 mL ONS twice daily with medication round | ‐ | SNAQ score 1 or 2 = 6, 3 = 13, 4 or 5= 6, 6 or 7 = 41 | 25 (4.3) | median 5 (range 1‐17) | ‐ | ‐ |
| I2: 62 mL ONS four times‐ daily with medication round | SNAQ score 1 or 2 = 6, 3 = 13, 4 or 5= 12, 6 or 7 = 49 | 23.8 (3.9) | median 5 (range 1‐15) | ‐ | ‐ | ||
| C: usual care (125 mL ONS offered in between meals) | SNAQ score 1 or 2 = 5, 3 = 13, 4 or 5 = 18, 6 or 7 = 52 | 24.3 (4.7) | median 6 (range 1‐30) | ‐ | ‐ | ||
| Van Ort 1995 | I: contextual and behavioural intervention | ‐ | ‐ | ‐ | 2 wk (6 wk, 1 mo after intervention) | ‐ | ‐ |
| C: usual care | |||||||
| '‐' denotes not reported BMI: body mass index; C: comparator; CI: confidence interval; CVA: cerebrovascular accident; CVD: cardiovascular diagnosis; d: days; GDS: geriatric depression scale; I: intervention; IQR: interquartile range; mo: month(s); MNA: mini nutritional assessment; NRS: nutritional risk score; ONS: oral nutritional supplement; SD: standard deviation; SGA: subjective global assessment; SNAQ: Simplified Nutritional Appetite Questionnaire; wk: week(s) | |||||||
Appendix 6. Matrix of study endpoints (publications and trial documents)
| Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a | Trial results/ publications available in trials register | Endpoints quoted in publication(s)b,c | Endpoints quoted in abstract of publication(s)b,c | |
| Bouillanne 2013 |
Source:NCT00135590 Primary outcome measure(s):
|
No/Yes (last verified: November 2004) |
Primary outcome measure(s):
|
Primary outcome measure(s):
|
Secondary outcome measure(s):
|
Secondary outcome measure(s):
|
Secondary outcome measure(s):
|
||
| Other outcome measure(s): ‐ |
Other outcome measure(s):
|
Other outcome measure(s): ‐ | ||
| History of changes: 6 documented changes | ||||
| Faxen‐Irving 2011 |
Source:NCT01042340 Primary outcome measure(s):
|
No/Yes (last verified: December 2009) |
Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ |
Secondary outcome measure(s):
|
Secondary outcome measure(s):
|
Secondary outcome measure(s): ‐ | ||
| Other outcome measure(s): ‐ |
Other outcome measure(s):
|
Other outcome measure(s):
|
||
| History of changes: 1 documented change | ||||
| Holyday 2012 |
Source:NCT01179321 Primary outcome measure(s): length of stay |
No/No (last verified: March 2006) |
Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | ||
| Other outcome measure(s): ‐ |
Other outcome measure(s):
|
Other outcome measure(s):
|
||
| History of changes: 0 documented changes | ||||
| Munk 2014 |
Source:NCT01415635 Primary outcome measure(s):
|
No/No (last verified: December 2012) |
Primary outcome measure(s):
|
Primary outcome measure(s):
|
Secondary outcome measure(s):
|
Secondary outcome measure(s):
|
Secondary outcome measure(s):
|
||
| Other outcome measure(s): none provided | Other outcome measure(s): number of participants receiving ONS | Other outcome measure(s): ‐ | ||
| History of changes: 1 documented change | ||||
| Nijs 2006 |
Source:NCT00114582 Primary outcome measure:
|
No/Yes (last verified: February 2009) |
Primary outcome measure(s):
|
Primary outcome measure(s): ‐ |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | ||
| Other outcome measure(s): ‐ |
Other outcome measure(s):
|
Other outcome measure(s):
|
||
| History of changes: 4 documented changes | ||||
| Salva 2011 |
Source:NCT00479843 Primary outcome measure(s):
|
No/Yes (last verified: January 2014) |
Primary outcome measure(s): ‐ |
Primary outcome measure(s):
|
Secondary outcome measure(s):
(time frame: baseline, 6 months, 12 months) |
Secondary outcome measure(s):
|
Secondary outcome measure(s):
|
||
| Other outcome measure(s): ‐ |
Other outcome measure(s):
|
Other outcome measure(s)‐ | ||
| History of changes: 2 documented changes | ||||
| Van den Berg 2015 |
Source:NTR2535 Primary outcome measure(s): proportion of participants who received their treatment goal. The treatment goal was to receive at least 75% of the prescribed volume of ONS during admission |
No (last verified 19 Nov 2010) | Primary outcome measure(s):the percentage of participants who reached the treatment objective of at least 75% of the prescribed volume of ONS during admission |
Primary outcome measure(s): The percentage of participants who consumed at least 75% of the prescribed volume of ONS |
|
Secondary outcome measure(s): intake (mL of ONS) (nurses and food assistants read the amount of ONS left in the bottle) |
Secondary outcome measure(s): Mean intake of ONS per day in mL and energy and protein |
Not stated | ||
| Other outcome measure(s):‐ | Other outcome measure(s): length of hospital stay, hospital readmissions, time to intervention, duration of intervention, mortality | Median time of taking ONS | ||
| History of changes: No documented changes | ||||
| '‐' denotes not reported aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trials registers) bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents or multiple reports of a primary trial) cOther outcome measures refer to all outcomes not specified as primary or secondary outcome measures ADL: activities of daily living; BMI: body mass index; EMA: European Medicines Agency; FDA: Food and Drug Administration (US); mo: month(s); N/A: not applicable; N/T: no trial document available; yr: year(s); wk: week(s); ONS oral nutritional supplement | ||||
Appendix 7. High risk of outcome reporting bias according to ORBIT classification
| Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d | |
| Barton 2000 | Energy intake | Yes | |||
| Food wastage | Yes | ||||
| Protein intake | Yes | ||||
| Beck 2002 | N/D | ||||
| Bouillanne 2013 | N/D | ||||
| Bourdel‐Marchasson 2000 | Energy intake | Yes | |||
| Incidence of death | Yes | ||||
| Pressure ulcer developments | Yes (40% in intervention group, 48% in control; no further analysis) | ||||
| Castellanos 2009 | 3 meal energy intake | Yes | |||
| 3 meal protein intake | Yes | ||||
| Chang 2005 | N/D | ||||
| Dennis 2005 | Death or poor outcome | Yes | |||
| Death | Yes | ||||
| Complications: pneumonia, UTI, pressure sores | Yes | ||||
| Length of stay | Yes | ||||
| Discharge destination | Yes | ||||
| EUROQoL | Yes | ||||
| Duncan 2006 | N/D | ||||
| Essed 2007 | Pleasantness | Yes | |||
| Olfactory sensitivity | Yes (analysed but reported as correlation with energy intake) | ||||
| Appetite, hunger and sensory perception | Yes | ||||
| GDS | Yes | ||||
| Essed 2009 | N/D | ||||
| FaYesen‐Irving 2011 | Energy intake | Yes | |||
| Body mass index | Yes | ||||
| Activities of Daily Living | Yes | ||||
| Length of stay | Yes | ||||
| Appetite | |||||
| Fatty acid profiles (myristic acid, margarinic acid, stearic acid, oleic acid, alpha‐linoleic acid, eicosapentaenoic acid) | Yes | ||||
| Pentadecanoic acid | Yes | ||||
| Gaskill 2009 | Subjective global assessment | Yes | |||
| Germain 2006 | N/D | ||||
| Hankey 1993 | Anthropometry: TSF, MAC weight | Yes | |||
| Serum albumin | Yes | ||||
| Fiber intake | Yes | ||||
| Hickson 2004 | Serum albumin | Yes | |||
| Barthel score | Yes | ||||
| Cognition and depression score (BASDEC) | Yes | ||||
| Pressure sore incidence | Yes | ||||
| Laxative use | Yes | ||||
| Artificial nutrition use | Yes | ||||
| Economic analysis | Yes | ||||
| Dietary intake | |||||
| In‐hospital mortality | |||||
| Grip strength | |||||
| Holyday 2012 | N/D | ||||
| Johansen 2004 | N/D | ||||
| Kraft 2012 | N/D | ||||
| Kretser 2003 | Satisfaction with programme | Yes | |||
| Larsson 1990 | Nutritional assessment (TSF, MAC) | Yes | |||
| Serum protein analysis | Yes | ||||
| Acute phase reactants (antitrypsin, orosomucoid) | Yes | ||||
| Length of stay | Yes | ||||
| Leslie 2012 | N/D | ||||
| Lin 2010 | Eating time | Yes | |||
| Lin 2011 | N/D | ||||
| Mathey 2001a | Health‐related quality of life | Yes (P < 0.05 stated for intervention but no P value for control) | |||
| Philadelphia Geriatric Center Morale Scale | Yes (no P values reported) | ||||
| Mathey 2001b | N/D | ||||
| Munk 2014 | N/D | ||||
| Nijs 2006 | N/D | ||||
| Olofsson 2007 | N/D | ||||
| Pivi 2011 | N/D | ||||
| Potter 2001 | Anthropometry: TSF, BMI | Yes | |||
| Arm muscle circumference | Yes | ||||
| Mortality | Yes (significant result when severely undernourished analysed in isolation) | ||||
| Functional recovery (Barthel ADL index) | Yes (significant result when severely undernourished analysed in isolation) | ||||
| Discharge placement | Yes | ||||
| Length of hospital stay | Yes | ||||
| Remsburg 2001 | N/D | ||||
| Salva 2012 | Health and social care costs (Resource Utilisation in Dementia (RUD) scale) | Yes | |||
| Silver 2008 | Confounding effect of age, sex and BMI on meal treatment order, total energy, energy density and macronutrients | Yes | |||
| Simmons 2008 | N/D | ||||
| Simmons 2010 | Weight | Yes | |||
| Smolliner 2008 | N/D | ||||
| Splett 2003 | N/D | ||||
| Taylor 2006 | N/D | ||||
| Van den Berg 2015 | N/D | ||||
| Van Ort 1995 | Nutritional status (weight change) | Yes | |||
| Feeding related interpersonal contact between residents and feeder | Yes | ||||
| Functional ability of subject, and level of assistance offered by feeder | Yes | ||||
|
aClear that outcome was measured and analysed; trial report states that outcome was analysed but only reports that result was not significant
(Classification 'A', table 2, Kirkham 2010)
bClear that outcome was measured and analysed; trial report states that outcome was analysed but no results reported
(Classification 'D', table 2, Kirkham 2010)
cClear that outcome was measured; clear that outcome was measured but not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results
(Classification 'E', table 2, Kirkham 2010)
dUnclear whether the outcome was measured; not mentioned but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results
(Classification 'G', table 2, Kirkham 2010) ADL: activities of daily living; BMI: body mass index; EuroQol: European Quality of Life Scale; GDS: geriatric depression scale; mo: months; N/D: none detected; ORBIT: Outcome Reporting Bias In Trials; TSF: triceps skinfold thickness | |||||
Appendix 8. Definition of endpoint measurement (I)
| Nutritional intake | Health‐related quality of life/patient satisfaction | Mortality | Morbidity/complications | Nutritional status | |
| Barton 2000 | Energy intake (kcal), protein intake (g), food wastage (%) | ‐ | ‐ | ‐ | ‐ |
| Beck 2002 | Energy intake (MJ) | ‐ | ‐ | ‐ | Weight (kg) |
| Bouillanne 2013 | ‐ | ‐ | Yes | Infections | Body composition |
| Bourdel‐Marchasson 2000 | Energy intake (kcal), protein intake (g) | ‐ | Yes | ‐ | ‐ |
| Brouillette 1991 | Energy intake (kcal), % food consumed | ‐ | Yes | ‐ | ‐ |
| Castellanos 2009 | Energy intake (kcal), protein intake (g) | ‐ | ‐ | ‐ | |
| Chang 2005 | % meal eaten | ‐ | ‐ | ‐ | ‐ |
| Dennis 2005 | ‐ | Quality of life (EUROQoL) | Yes | Incidence of pneumonia, UTI and pressure sores | ‐ |
| Duncan 2006 | Dietary intake records on day 3‐6 | ‐ | ‐ | Records of medical and surgical complications | Weight, MAMC, TSF, HGS |
| Essed 2007 | Energy intake (kJ), protein, fat and CHO (g) | ‐ | ‐ | ‐ | Weight (kg), BMI, body composition |
| Essed 2009 | Energy intake (kJ) | ‐ | ‐ | ‐ | ‐ |
| Faxen‐Irving 2011 | Energy intake (kcal/kg body weight/day) | ‐ | Yes | ‐ | Weight, appetite, BMI |
| Gaskill 2009 | SGA | ‐ | ‐ | ‐ | ‐ |
| Germain 2006 | Dietary intake, energy (kcal), other nutrients (g/mg) | ‐ | Yes | ‐ | Weight, BMI |
| Hankey 1993 | Energy intake (kj/24hours), protein intake (g/24hours) | ‐ | ‐ | ‐ | Weight, TSF, MAC, AMC, serum albumin |
| Hickson 2004 | Energy intake (J), protein (g) | EQ‐5D | Yes | Antibiotics prescribed (N), days on antibiotics | Weight, BMI, MAC, TSF, MAMC, |
| Holyday 2012 | ‐ | ‐ | Yes | ‐ | Weight |
| Johansen 2004 | Energy intake (kJ/kg and % requirements), protein intake (g/kg and % requirements) | SF‐36 | Yes | Infectious and other complications graded into major and minor (using Buzby et al 1988 and CDC definitions) | Weight change (kg) |
| Kraft 2012 | _ | ‐ | ‐ | ‐ | Weight change (kg) |
| Kretser 2003 | ‐ | ‐ | Yes | ‐ | Weight, weight change (lb) |
| Larsson 1990 | Encompassed in the Modified Norton Scale | ‐ | Yes | ‐ | Weight index, TSF, MAC, AMC |
| Leslie 2012 | Dietary intake, 3 day weighed records | ‐ | Yes | ‐ | Weight change, change in BMI & MUAC |
| Lin 2010 | Eating amount (unit unclear) | ‐ | No | ‐ | MNA and BMI |
| Lin 2011 | ‐ | ‐ | No | ‐ | MNA and BMI |
| Mathey 2001a | Macro‐ and micronutrient intakes | SIP, PGCMS | Yes | No | Weight |
| Mathey 2001b | Energy intake | _ | No | no | Weight |
| Munk 2014 | Percent of participants meeting > 75% of their energy and protein requirements. Mean daily energy and protein intake | ‐ | Yes | ‐ | Weight |
| Nijs 2006 | Energy intake (kcal), macronutrient (g) | Dutch QOL nursing home residents questionnaire | Yes | ‐ | Weight (kg, calf circumference (cm), MAC (cm), MNA score |
| Olofsson 2007 | ‐ | ‐ | Yes | Infectious and non‐infectious complications during hospital stay | Weight, BMI, MNA |
| Pivi 2011 | ‐ | ‐ | Yes | ‐ | Weight, BMI, MAC, MAMC, TSF |
| Potter 2001 | Total energy intake (kcal) | ‐ | Yes | ‐ | Weight, AMC, TSF, BMI |
| Remsburg 2001 | ‐ | ‐ | Yes | ‐ | Weight (kg) |
| Salva 2011 | ‐ | ‐ | Yes | ‐ | Weight (kg), BMI, MNA |
| Silver 2008 | Total energy (kcal), energy density (kcal/g), macronutrient's (g), micronutrients | ‐ | ‐ | ‐ | ‐ |
| Simmons 2008 | Total energy (kcal) | ‐ | ‐ | ‐ | Weight (lb), BMI |
| Simmons 2010 | Energy intake (kcal) | ‐ | Yes | ‐ | Weight (lb) |
| Smolliner 2008 | Energy (kcal and kcal/kg body weight), protein (g and g/kg body weight) | ‐ | Yes | ‐ | Weight, BMI, MNA score, fat‐free mass |
| Splett 2003 | ‐ | ‐ | Yes | ‐ | Weight |
| Taylor 2006 | Energy intake (kcal/day), fluid intake (mL/day) | ‐ | ‐ | ‐ | ‐ |
| Van den Berg 2015 | Energy intake from ONS (kcal/day) | ‐ | Yes | ||
| Van Ort | ‐ | ‐ | ‐ | ‐ | Weight |
| ADL: activities of daily living; AMC: arm muscle circumference; BMI: body mass index; CDC: Centre for Disease Control; CDR: clinical dementia rating scale; CHO: carbohydrate; d: day; EQ‐5D/EuroQol: European Quality of Life Scale; HGS: handgrip strength; iADL: instrumental Activities of Daily Living; MAC: mid‐arm circumference; MAMC: mid‐arm muscle circumference; MJ: mega joules; MMSE: Mini Mental State Examination; MNA: Mini Nutritional Assessment; NPIQ: Neuropyschiatric Inventory Question; PGCMS: Philadelphia Geriatric Centre Morale Scale; QOL: quality of life; RUD: reduction in use of health and social care scale; SF‐36: Short Form ‐ 36; SGA: subjective global assessment; SIP: sickness impact profile; TSF: triceps skinfold thickness | |||||
Appendix 9. Definition of endpoint measurement (II)
| Functional status | Clinical function | Hospitalisation/ institutionalisation | Severe/serious adverse events | Economic costs | |
| Barton 2000 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Beck 2002 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Bouillanne 2013 | Handgrip strength, ADL score | Biochemical data | ‐ | ‐ | ‐ |
| Bourdel‐Marchasson 2000 | ‐ | Pressure ulcer development | ‐ | ‐ | ‐ |
| Brouillette 1991 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Castellanos 2009 | ‐ | ‐ | ‐ | ‐ | |
| Chang 2005 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Dennis 2005 | ‐ | ‐ | Discharge destination | ‐ | ‐ |
| Duncan 2006 | ‐ | ‐ | Length of stay in acute unit and in hospital (days) | ‐ | ‐ |
| Essed 2007 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Essed 2009 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Faxen‐Irving 2011 | ‐ | Serum/plasma proteins, serum lipids, fatty acid profiles, ADLs | Length of stay | ‐ | ‐ |
| Gaskill 2009 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Germain 2006 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Hankey 1993 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Hickson 2004 | Grip strength | ‐ | Length of stay (d), volume of fluids given | ‐ | ‐ |
| Holyday 2012 | ‐ | ‐ | Length of stay, readmissions | Estimated | |
| Johansen 2004 | ‐ | ‐ | Length of stay (LOS28) = LOS from admission to inclusion + LOS from inclusion to discharge (maximum 28 days) LOS NDI = LOS 28 ‐ number of final days with NDI = 3) NDI = index of mobility, infections and complications |
‐ | ‐ |
| Kraft 2012 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Kretser 2003 | iADL, ADL, dependence | ‐ | ‐ | ‐ | ‐ |
| Larsson 1990 | Encompassed in the Modified Norton Scale | ‐ | ‐ | ‐ | ‐ |
| Leslie 2012 | ‐ | ‐ | Yes | ‐ | ‐ |
| Lin 2010 | Eating function (need for verbal and/or physical assistance or feeding + eating time) | ‐ | ‐ | ‐ | ‐ |
| Lin 2011 | Eating function (need for verbal and/or physical assistance or feeding + eating time) | ‐ | ‐ | ‐ | ‐ |
| Mathey 2001a | ‐ | Biochemical data | _ | _ | _ |
| Mathey 2001b | Hunger, appetite and sensory perception | ‐ | ‐ | ‐ | ‐ |
| Munk 2014 | Handgrip strength | Length of hospital stay | ‐ | ‐ | |
| Nijs 2006 | Motor function (nursing home physical performance test) | ‐ | ‐ | ‐ | ‐ |
| Olofsson 2007 | ‐ | ‐ | Length of hospital stay | ‐ | ‐ |
| Pivi 2011 | ‐ | Biochemical data | ‐ | ‐ | ‐ |
| Potter 2001 | Functional recovery (20‐point Barthel ADL index) | ‐ | Length of hospital stay, discharge placement | ‐ | ‐ |
| Remsburg 2001 | ‐ | Biochemical status | ‐ | ‐ | ‐ |
| Salva 2011 | ADL, iADL scores | MMSE, CDR, NPIQ | ‐ | ‐ | RUD score |
| Silver 2008 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Simmons 2008 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Simmons 2010 | ‐ | ‐ | ‐ | ‐ | Cost‐effectiveness |
| Smolliner 2008 | Handgrip strength, peak flow, Barthel score, SF‐36 (physical function only) | ‐ | ‐ | ‐ | ‐ |
| Splett 2003 | ‐ | ‐ | Hospital admissions | ‐ | ‐ |
| Taylor 2006 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Van den Berg 2015 | Length of stay | Stated as none but not defined | ‐ | ||
| Van Ort | Functional ability of participant | ‐ | ‐ | ‐ | ‐ |
| ADL: activities of daily living; AMC: arm muscle circumference; BMI: body mass index; CDC: Centre for Disease Control; CDR: clinical dementia rating scale; CHO: carbohydrate; d: day; EQ‐5D/EuroQol: European Quality of Life Scale; HGS: handgrip strength; iADL: instrumental Activities of Daily Living; MAC: mid‐arm circumference; MAMC: mid‐arm muscle circumference; MJ: mega joules; MMSE: Mini Mental State Examination; MNA: Mini Nutritional Assessment; NPIQ: Neuropyschiatric Inventory Question; PGCMS: Philadelphia Geriatric Centre Morale Scale; QOL: quality of life; RUD: reduction in use of health and social care scale; SF‐36: Short Form ‐ 36; SGA: subjective global assessment; SIP: sickness impact profile; TSF: triceps skinfold thickness | |||||
Appendix 10. Adverse events
| Intervention(s) and comparator(s) | Deaths (N/N (%)) | Participants with adverse events (N/N (%)) | Participants with severe/serious adverse events (N/N (%)) | Participants discontinuing trial due to adverse event (N/N (%)) | |
| Barton 2000 | I1: reduced portion fortified menu | ‐ | ‐ | ‐ | ‐ |
| I2: normal menu plus cooked breakfast | ‐ | ‐ | ‐ | ||
| C: normal hospital menu | ‐ | ‐ | ‐ | ‐ | |
| Beck 2002 | I1: homemade oral supplement (A) | ‐ | ‐ | ‐ | ‐ |
| I2: homemade oral supplement (B) | |||||
| C: usual diet | ‐ | ‐ | ‐ | ‐ | |
| Bouillanne 2013 | I: pulse diet (78% protein at lunch) | 1/30 (3.3) | ‐ | ‐ | ‐ |
| C: usual diet (protein distributed between meals) | 1/36 (2.8) | ‐ | ‐ | ‐ | |
| Bourdel‐Marchasson 2000 | I: oral supplementation + standard diet | 25/295 (8.5) | ‐ | ‐ | ‐ |
| C: standard diet | 22/377 (5.8) | ‐ | ‐ | ‐ | |
| Brouillette 1991 | I: osmotherapy + activities | 1/10 (10) | ‐ | ‐ | ‐ |
| C: activities only | 0/10 (0) | ‐ | ‐ | ‐ | |
| Castellanos 2009 | I1: fortified breakfast and lunch menu | ‐ | ‐ | ‐ | ‐ |
| I2: fortified lunch menu | ‐ | ‐ | ‐ | ‐ | |
| C: usual menu | ‐ | ‐ | ‐ | ||
| Chang 2005 | I: training in feeding skills | ‐ | ‐ | ‐ | ‐ |
| C: no training | ‐ | ‐ | ‐ | ‐ | |
| Dennis 2005 | I: nutritional supplement + normal hospital diet | 241/2016 (12) | 138/4023 (3.4) | ‐ | ‐ |
| C: normal hospital diet | 253/2007 (12.6) | ‐ | ‐ | ||
| Duncan 2006 | I. dietetic assistant | 19/145 (13.1) | ‐ | ‐ | ‐ |
| C: usual care | 36/157 (22.9) | ‐ | ‐ | ‐ | |
| Essed 2007 | I1: monosodium glutamate | ‐ | ‐ | ‐ | ‐ |
| I2: flavour | ‐ | ‐ | ‐ | ‐ | |
| I3: monosodium glutamate + flavour | ‐ | ‐ | ‐ | ‐ | |
| C: maltodextrin | ‐ | ‐ | ‐ | ‐ | |
| Essed 2009 | I: monosodium glutamate + NaCl | ‐ | ‐ | ‐ | ‐ |
| C: usual hot meal | ‐ | ‐ | ‐ | ‐ | |
| Faxen‐Irving 2011 | I: 3 x 30 mL of fat emulsion daily | ‐ | 5/34 (14.7) | ‐ | 5/34 (14.7) |
| C: usual care | 2/37 (5.4) | ‐ | ‐ | ‐ | |
| Gaskill 2009 | I: nutrition education programme | ‐ | ‐ | ‐ | ‐ |
| C: usual care | ‐ | ‐ | ‐ | ‐ | |
| Germain 2006 | I: re‐formed foods | ‐ | ‐ | ‐ | ‐ |
| C: usual diet | ‐ | ‐ | ‐ | ‐ | |
| Hankey 1993 | I: oral nutritional supplement | ‐ | 3/10 (30) | ‐ | ‐ |
| C: standard hospital diet | ‐ | 3/10 (30) | ‐ | ‐ | |
| Hickson 2004 | I: feeding assistance | 31/292 (10.6) | ‐ | ‐ | |
| C: usual care | 35/300 (11.7) | ‐ | ‐ | ‐ | |
| Holyday 2012 | I: malnutrition care plan | 1/72 (1.4) | ‐ | ‐ | ‐ |
| C: usual care | 4/72 (5.6) | ‐ | ‐ | ‐ | |
| Johansen 2004 | I: nutrition team | ‐ | ‐ | ‐ | ‐ |
| C: usual care | ‐ | ‐ | ‐ | ‐ | |
| Kraft 2012 | I: oral nutritional supplement + telemedicine monitoring | ‐ | ‐ | ‐ | 2/13 (15.4) |
| C: usual care | ‐ | ‐ | ‐ | ‐ | |
| Kretser 2003 | I: modified meals on wheels | 3/102 (2.9) | ‐ | ‐ | ‐ |
| C: traditional meals on wheels | 9/101 (8.9) | ‐ | ‐ | ‐ | |
| Larsson 1990 | I: oral nutritional supplement plus normal hospital diet | 29/197 (14.7) | ‐ | ‐ | ‐ |
| C: normal hospital diet | 56/238 (23.5) | ‐ | ‐ | ‐ | |
| Leslie 2012 | I: energy enriched meals | 2/19 (10.5) | ‐ | ‐ | |
| C: usual care | 5/22 (22.7) | ‐ | ‐ | ‐ | |
| Lin 2010 | I1: spaced‐retrieval | ‐ | ‐ | ‐ | ‐ |
| I2: Montessori | ‐ | ‐ | ‐ | ‐ | |
| C: usual care | ‐ | ‐ | ‐ | ‐ | |
| Lin 2011 | I: Montessori | ‐ | ‐ | ‐ | ‐ |
| C: usual care | ‐ | ‐ | ‐ | ‐ | |
| Mathey 2001a | I: improved meal ambiance | 7/21 (33.3) | ‐ | ‐ | ‐ |
| C: usual care | 5/17 (29.4) | ‐ | ‐ | ‐ | |
| Mathey 2001b | I: flavour enhancement | ‐ | ‐ | ‐ | ‐ |
| C: usual care | ‐ | ‐ | ‐ | ‐ | |
| Munk 2014 | I: energy and protein enriched foods provided via a la carte menu in addition to hospital food | 1/44 (2.2) | ‐ | ||
| C: usual care | 1/40 (2.5) | ‐ | |||
| Nijs 2006 | I: family‐style meals | 18/112 (16.1) | ‐ | ‐ | |
| C: usual care | 16/133 (12.0) | ‐ | ‐ | ‐ | |
| Olofsson 2007 | I: multi‐component intervention (including nutrition) | 9/102 (8.8) | ‐ | ‐ | ‐ |
| C: usual care | 13/97 (13.4) | ‐ | ‐ | ‐ | |
| Pivi 2011 | I1: nutrition education | ‐ | ‐ | ‐ | ‐ |
| I2: oral nutritional supplements | ‐ | ‐ | ‐ | ‐ | |
| C: usual care | ‐ | ‐ | ‐ | ‐ | |
| Potter 2001 | I: oral nutritional supplement + normal hospital diet | 21/186 (11.3) | Reported "no serious adverse events" | ‐ | ‐ |
| C: normal hospital diet | 33/195 (16.9) | ‐ | ‐ | ||
| Remsburg 2001 | I: buffet‐style meals | ‐ | ‐ | ‐ | ‐ |
| C: usual care | ‐ | ‐ | ‐ | ‐ | |
| Salva 2011 | I: teaching and training | 43/448 (9.6) | ‐ | ‐ | ‐ |
| C: usual care | 29/498 (5.8) | ‐ | ‐ | ‐ | |
| Silver 2008 | I: fortified home‐delivered lunch | ‐ | ‐ | ‐ | ‐ |
| C: usual home‐delivered lunch | ‐ | ‐ | ‐ | ||
| Simmons 2008 | I: feeding assistance and/or snacks | ‐ | ‐ | ‐ | ‐ |
| C: usual diet | ‐ | ‐ | ‐ | ‐ | |
| Simmons 2010 | I1: snacks | ‐ | ‐ | ‐ | ‐ |
| I2: supplements | ‐ | ‐ | ‐ | ‐ | |
| C: usual care | ‐ | ‐ | ‐ | ‐ | |
| Smolliner 2008 | I: fortified meals and snacks | 2/31 (6.5) | ‐ | ‐ | ‐ |
| C: usual diet | 1/34 (2.9) | ‐ | ‐ | ‐ | |
| Splett 2003 | I: medical nutrition therapy | ‐ | ‐ | ‐ | ‐ |
| C: usual care | ‐ | ‐ | ‐ | ‐ | |
| Taylor 2006 | I: 5‐meal menu | ‐ | ‐ | ‐ | ‐ |
| C: usual (3‐meal menu) | ‐ | ‐ | ‐ | ‐ | |
| V an den Berg 2015 | I1: 125 mL ONS twice daily with medication round | 1/66 (1.5) | Reported "no serious adverse events" | 11/88 (12.5) (refused further ONS) | |
| I2: 62 mL ONS four times daily with medication round | 2/80 (2.5) | 9/66 (13.6) (refused further ONS) | |||
| C: usual care (125 mL ONS offered in between meals) | 4/88 (4.5) | 11/80 (13.8) (refused further ONS) | |||
| Van Ort 1995 | I: contextual and behavioural intervention | ‐ | ‐ | ‐ | ‐ |
| C: usual care | ‐ | ‐ | ‐ | ‐ | |
| C: comparator, I: intervention; ONS: oral nutritional supplement | |||||
Appendix 11. Survey of authors' providing information on trials
| Trial author contacted | Trial author replied | Trial author provided data | Comments | |
| Barton 2000 | Yes | Yes | Yes | Additional data not used |
| Beck 2002 | Yes | Yes | Yes | Additional data not used |
| Bourdel‐Marchasson 2000 | Yes | Yes | Yes | Not used, and unable to provide data requested on weight |
| Bouillanne 2013 | Yes | Yes | Yes | Data received on weight and energy intake |
| Brouillette 1991 | No | N/A | N/A | |
| Castellanos 2009 | Yes | No | N/A | |
| Chang 2005 | Yes | No | N/A | |
| Dennis 2005 | Yes | Yes | Yes | Information used on complication rates |
| Duncan 2006 | Yes | Yes | Yes | Awaiting data on length of stay |
| Essed 2007 | Yes | No | N/A | |
| Essed 2009 | Yes | No | N/A | |
| Faxen‐Irving 2011 | Yes | Yes | Yes | Data on energy intake, length of stay, BMI and ADLs provided. No data available on infections |
| Gaskill 2009 | Yes | Yes | No | Assume unable to provide data |
| Germain 2006 | Yes | Yes | Yes | Data provided for BMI mean and SD of change |
| Hankey 1993 | Yes | No | N/A | |
| Hickson 2004 | Yes | Yes | Yes | Author unable to provide this data on energy intake and hospital readmission as it was not measured, therefore not usable. Data provided on complications as requested |
| Holyday 2012 | Yes | Yes | Yes | Data obtained and used for hospital readmission rates |
| Johansen 2004 | Yes | No | N/A | Data not used |
| Kraft 2012 | Yes | No | N/A | |
| Kretser 2003 | No | N/A | N/A | Unable to find contact for author |
| Larsson 1990 | Yes | No | N/A | Data not used |
| Lin 2010 | Yes | No | N/A | |
| Lin 2011 | No | N/A | N/A | |
| Mathey 2001a | Yes | No | N/A | |
| Mathey 2001b | Yes | No | N/A | |
| Nijs 2006 | No | N/A | N/A | |
| Olofsson 2007 | Yes | Yes | Yes | Data used for BMI, weight and complications |
| Pivi 2011 | Yes | No | N/A | |
| Potter 2001 | Yes | No | N/A | |
| Remsburg 2001 | No | N/A | N/A | |
| Salva 2011 | Yes | No | N/A | |
| Silver 2008 | No | N/A | N/A | |
| Simmons 2008 | Yes | Yes | No | Data not available |
| Simmons 2010 | Yes | Yes | No | Data not available |
| Smolliner 2008 | Yes | Yes | Yes | Data provided for mean and SD of change for weight, BMI, handgrip, and QoL |
| Splett 2003 | Yes | No | N/A | |
| Taylor 2006 | No | N/A | N/A | |
| Van Ort 1995 | Yes | No | N/A | |
| Leslie 2012 | No | N/A | N/A | |
| Munk 2014 | No | N/A | N/A | |
| V an den Berg 2015 | Yes | Yes | Yes | The clinical trial register number did not allow the trial to be identified within the register. The authors provided a link to the trial protocol via the WHO International Clinical Trials Registry Platform. |
| ADL: activities of daily living; BMI: body mass index; N/A: not applicable; QoL: (health‐related) quality of life; SD: standard deviation: WHO World Health Organisation | ||||
Appendix 12. Checklist to aid consistency and reproducibility of GRADE assessments
| (1) All‐cause mortality | (2) Morbidity/complications: number of participants with complications (any/pressure ulcers/needing oral antibiotics) | (3) Health‐related quality of life and patient satisfaction | (4) Hospitalisation and institutionalisation | (5) Adverse events | (6) Nutritional status (weight change) | (7) Economic costs | ||
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Yes | Unclear | Unclear | Unclear |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Unclear | Unclear | Unclear | No (↓) | Unclear | Unclear | No (↓) | |
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Unclear | Unclear | No (↓) | Unclear | No (↓) | Unclear | No (↓) | |
| Was an objective outcome used? | Yes | No (↓) | No (↓) | Yes | No (↓) | Yes | Yes | |
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | |
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | No (↓) | Yes | Yes | Yes | No (↓) | |
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Inconsistencyb | Point estimates did not vary widely? | Yes | No (↓) | N/A | Yes | N/A | Yes | N/A |
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlapped but not all overlapped at least 1 point estimate; no: at least 1 outlier: where the confidence interval of some of the studies did not overlap with those of most included studies)? | Substantial | Some | N/A | Substantial | N/A | Substantial | N/A | |
| Was the direction of effect consistent? | Yes | No (↓) | N/A | Yes | N/A | Yes | N/A | |
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40%‐60%), high I² > 60%)? | Low | High (↓) | N/A | Moderate | N/A | Moderate | N/A | |
| Was the test for heterogeneity statistically significant (P < 0.1)? | Not statistically significant | Statistically significant (↓) | N/A | Not statistically significant | N/A | Statistically significant (↓) | N/A | |
| Indirectnessa | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable |
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | |
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes and unclear | Yes | Yes | Yes | Yes | |
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | |
| Were the conclusions based on direct comparisons? | Yes | Yes | N/A | Yes | Yes | Yes | Yes | |
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | Yes | No (↓) | N/A | No (↓) | N/A | Yes | N/A |
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100‐300 participants, low: < 100 participants)?e | Intermediate to high | Intermediate | Intermediate | Intermediate | Intermediate | Low | Intermediate | |
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5‐10 studies, small: < 5 studies)?e | Large | Moderate | Moderate | Moderate | Small (↓) | Large | Small (↓) | |
| Was the outcome a common event (e.g. occurs more than 1/100)? | Yes | Yes | N/A | N/A | Yes | N/A | N/A | |
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was grey literature searched? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| There was no industry influence on studies included in the review? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| There was no evidence of funnel plot asymmetry? | Unclear | Unclear | N/A | Unclear | Unclear | Unclear | N/A | |
| There was no discrepancy in findings between published and unpublished trials? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I² cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials eDepends on the context of the systematic review area (↓): key item for possible downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); GRADE: Grading of Recommendations Assessment, Development and Evaluation; N/A: not applicable | ||||||||
Data and analyses
Comparison 1. Supportive interventions for enhancing dietary intake versus comparators.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. of participants with complications | 5 | 4702 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.86, 1.42] |
| 1.1 Changes to the organisation of nutritional care | 3 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.76, 1.67] |
| 1.2 Modification of meal profile or pattern | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.06, 6.14] |
| 1.3 Additional supplementation of meals | 1 | 4015 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.02, 1.28] |
| 2 Nutritional status (weight change) | 17 | 2024 | Mean Difference (IV, Random, 95% CI) | 0.62 [0.21, 1.02] |
| 2.1 Changes to the organisation of nutritional care | 6 | 1140 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.26, 0.45] |
| 2.2 Changes to the feeding environment | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐2.11, 1.25] |
| 2.3 Modification of meal profile or pattern | 5 | 253 | Mean Difference (IV, Random, 95% CI) | 1.16 [0.41, 1.92] |
| 2.4 Additional supplementation of meals | 4 | 475 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.41, 1.38] |
| 2.5 Congregate and home meal delivery systems | 1 | 117 | Mean Difference (IV, Random, 95% CI) | 2.90 [1.00, 4.80] |
| 3 Hospitalisation | 5 | 667 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐2.56, 1.59] |
| 3.1 Changes to the organisation of nutritional care | 3 | 515 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐6.75, 2.58] |
| 3.2 Modification of meal profile or pattern | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐3.48, 3.48] |
| 3.3 Additional supplementation of meals | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐2.26, 2.66] |
| 4 All‐cause mortality | 12 | 6683 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.66, 0.92] |
| 4.1 Changes to the organisation of nutritional care | 4 | 1237 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.52, 0.97] |
| 4.2 Changes to the feeding environment | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.14, 65.90] |
| 4.3 Modification of meal profile or pattern | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.15, 7.22] |
| 4.4 Additional supplementation of meals | 4 | 5073 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.58, 1.02] |
| 4.5 Congregate and home meal delivery systems | 1 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.09, 1.18] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barton 2000.
| Methods |
Cross‐over randomised controlled clinical trial: this trial included 3 groups, 2 of which were randomised to treatment or control and one other where it was unclear whether there was randomisation Randomisation ratio: not stated but appears to be 1:1 Superiority design |
|
| Participants | 35 participants (27 randomised to intervention or control, 8 received cooked breakfast), 13 male, 22 female, mean age 75‐78 depending on group; no details of nutritional status at baseline Inclusion criteria: not stated Exclusion criteria: not stated Diagnostic criteria: elderly hospitalised patients in a rehabilitation ward, 19 of 35 had had a stroke |
|
| Interventions | Portion size decreased by 20% but fortified to achieve overall daily energy provision increased by 200 kcal versus normal hospital menu. An additional group given normal hospital menu plus cooked breakfast Number of trial centres: 1 Treatment before trial: not stated but assume normal hospital diet |
|
| Outcomes | Outcomes reported in abstract of publication: food wastage, energy and protein intake | |
| Study details |
Location: Nottingham, UK Year: unclear Setting: 22‐bedded rehab ward Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: not stated Publicaton status: peer review journal |
|
| Stated aim for study | Quote from publication: "To compare food wastage and intake between the normal hospital menu and one where more energy dense, but smaller portions were provided" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "randomly allocated to receive either normal menu or reduced portion fortified menu". Comment: no details whether the third group was included in the randomisation & insufficient detail provided of randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from publication: "patients and staff were blind as to which menu each patient was following" Comment: those receiving the cooked breakfast rather than cereal could not have been blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no detailed information provided. Data on 19 of 27 randomised participants provided but no information on attrition |
| Selective reporting (reporting bias) | Low risk | Comment: data presented on all three stated outcomes |
| Other bias | Unclear risk | Comment: no information on baseline characteristics of populations apart from age and gender |
Beck 2002.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1:1 Superiority design |
|
| Participants | 36 care home residents; 14 male; 22 female; mean age 81 (range 76‐86) years Inclusion criteria: resident in a care home; aged > 65 years Exclusion criteria: in terminal condition Diagnostic criteria: not specified |
|
| Interventions | Home‐made oral supplement (240 kcal/serving) provided in the evening Number of trial centres: 1 Treatment before trial: none |
|
| Outcomes | Outcomes reported in abstract of publication: energy intake and body weight | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding ‐ Health Insurance Foundation Grant Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To examine the effect of a home‐made oral supplement on body weight and energy intake of old people residing in a nursing home with MNA scores less than or equal to 23.5" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "Participants....were randomly allocated (block randomization) to two groups" Comment: insufficient detail of method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not fully described |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | Unclear risk | Comment: not fully described |
Bouillanne 2013.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 73 hospitalised elderly; 46 female; 27 male; mean age intervention 84.1 (95% CI 82 to 86); control 85.7 (95% CI 84 to 88) years Inclusion criteria: albumin 25‐35 g/L; BMI < 22 kg/m2 and/or weight loss > 10% in 6 months and/or MNA < 23.5 Exclusion criteria: not specified Diagnostic criteria: admitted to geriatric intermediate care unit |
|
| Interventions |
Number of trial centres: 1 Treatment before trial: none Pulse diet i.e. 78% daily protein requirements provided at noon meal |
|
| Outcomes | Outcomes reported in abstract of publication: body composition, handgrip strength and ADL score | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding ‐ French Ministry of Health Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To evaluate the efficacy of a new nutritional strategy, termed protein pulse feeding" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "A randomization procedure was used (EXCEL 2003....." Comment: insufficient detail of the method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants fully accounted for |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes fully reported |
| Other bias | Low risk | Comment: baseline characteristics fully compared; serum albumin higher and body cell mass index and skeletal muscle mass index are lower in the pulse diet group |
Bourdel‐Marchasson 2000.
| Methods |
Cluster‐randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 672 critically ill elderly participants; N = 295 intervention (199 female, 96 male); N = 377 control (238 female, 139 male). Mean age in intervention group = 83.6 yrs (SD 7.3) and mean age in the intervention group = 83.0 yrs (SD 7.1) Inclusion criteria: wards inclusion: > 40% of participants over age 65 yrs and nurses able to guarantee significant involvement in the trial. Older than 65 yrs, in acute phase of a critical illness, unable to move by themselves, unable to eat independently on admission Exclusion criteria: pressure ulcers at admission Diagnostic criteria: critically ill inpatients |
|
| Interventions | Intervention group received standard diet of 1800 kcal/day plus 2 oral nutritional supplements of 200 kcal each, one with breakfast and the other mid afternoon. Control group received standard diet of 1800 kcal/day Number of trial centres: unclear Treatment before trial: none |
|
| Outcomes | Outcomes reported in abstract of publication: energy and protein intakes; incidence of pressure ulcers; serum albumin; Kuntzmann score; Norton score; lower limb fracture | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial/other funding ‐ Projet Hospitalier de Recherche Clinique, Ministere de la Sante et de l'Action Humanitaire, Direction Generale de la Sante and the Direction des Hopitaux Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "The purpose of the present study was to assess the effects of nutritional supplementation (400 kcal/day) for 15 days on dietary intake and on pressure ulcer development in critically ill older patients; 672 subjects older than 65 years were included" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "Nineteen wards were then selected and stratified according their speciality....These wards were then randomised in two groups according to the nutritional intervention Comment: insufficient detail of method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 25 deaths in intervention group and 22 in the usual care. Other attrition not described |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | High risk |
Assessment of risk of bias in cluster‐randomised trials (1) Recruitment bias: unclear (2) Baseline imbalance: yes (serum albumin at baseline, weight, Norton score, Kuntzmann mean score) (3) Loss of clusters: unclear (4) Incorrect analysis: no (5) Comparability with individually randomised trials/different types of clusters: different types of clusters |
Brouillette 1991.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 16 participants; 14 female; 2 male; mean age Intervention 80 (SD 6.4); control 87 (SD 6.8) years Inclusion criteria: care home residents Exclusion criteria: cancer; severe GI disorder and/or oral disorder; extreme dietary restriction or other conditions that affect ability to eat or feed themselves Diagnostic criteria: not specified |
|
| Interventions | Exposure to olfactory stimuli prior to meals + other activities Number of trial centres: 1 Treatment before trial: none |
|
| Outcomes | Outcomes reported in abstract of publication: olfactory acuity and attention level | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: not stated Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To test whether odours can influence the desire to eat and therefore increase caloric intake" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "From the remaining pool, 20 subjects were selected for the research ... The 20 subjects were assigned randomly to either the experimental or control group" Comment: no details on randomisation procedure |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: during the research period it is stated that "the research assistant was unaware of group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: during the research period it is stated that "the research assistant was unaware of group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all dropouts fully accounted for |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes fully reported |
| Other bias | Low risk | Comment: baseline characteristics reported and groups comparable |
Castellanos 2009.
| Methods |
Cross‐over randomised controlled clinical trial: each individual was tested under three menu conditions (2 different interventions and 1 control) Randomisation ratio: not stated Superiority design |
|
| Participants | 39 participants (4 died and 2 withdrew before inclusion, complete data on 26 following attrition). 10 male, 23 female, mean age 87.3 (SD 8.6) years, mean BMI 25.1 (SD 3.6) Inclusion criteria: nursing home residents Exclusion criteria: < 60 years, hospice patients, on tube feeding, renal diet, pureed diet, thickened liquids, ate only in their room, required feeding assistance. Diagnostic criteria: nursing home residents |
|
| Interventions | 2 breakfast and 2 lunch foods fortified to improve energy and protein content (hot cereal and juice breakfast, soup and side dish at lunch) versus 2 lunch foods only fortified versus normal menu Number of trial centres: 1 Treatment before trial: not stated, assume usual menu |
|
| Outcomes | Outcomes reported in abstract of publication: energy and protein intake | |
| Study details |
Location: Florida, USA Year: mid 2000's Setting: nursing home Run‐in period: not stated Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial ‐ Retirement Research Foundation. Other funding: Juice drinks donated by Lyons Magnus, Fresno CA Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "the study objective was to determine whether energy and protein enhancement of a small number of menu items would result in increased 3‐meal (breakfast, lunch and supper) calorie and protein intakes in long term care residents" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "Using a single blind randomised cross over design, each subject was tested under three menu conditions" Comment: insufficient details of method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: described as single blind, unclear whether residents or staff were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: complete data included in figure 1 |
| Selective reporting (reporting bias) | High risk | Comment: results for the whole group are not reported according to initial randomisation. Only data for post hoc separation of the whole group into large (> 1150 kcal in 3 meals) and small eaters (< 1150 kcal in 3 meals) were reported. This excludes 7 participants with incomplete data |
| Other bias | Unclear risk | Comment: baseline characteristics reported in table 1 for large and smaller eaters but not for the whole group |
Chang 2005.
| Methods |
Cluster‐randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 67 nursing assistants randomised, 36 nursing assistants took part in the observation of mealtimes part of the study; N = 20 intervention (all female); N = 16 control (14 female and 2 male) and 36 care home residents with dementia (mean age 84.2 (SD 4) intervention and 72 (SD 5.8) years in control) Inclusion criteria: nursing assistants had to have worked at least 6 months in the same long‐term care facility and able to communicate in either Mandarin, Taiwanese or English. Residents diagnosed with dementia, having an eating problem and needing assistance Exclusion criteria: not stated Diagnostic criteria: dementia |
|
| Interventions | Feeding skills training programme for nursing assistants versus usual care Number of trial centres: 1 Treatment before trial: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: knowledge, attitude and behaviour of nursing assistants, Edinburgh Feeding Evaluation in Dementia Score; food intake and eating time of participants | |
| Study details |
Run‐in period: not stated Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial and non‐commercial ‐ Sigma Theta Tau International‐Alpha Mu Chapter and the Alumni Association of the FPB School of Nursing and National health Research Institute Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "to provide a feeding skills training programme for nursing assistants in a Taiwanese dementia‐specialised long term care facility and to test the effects of this feeding skills training programme on the outcomes of nursing assistants and dementia patients". | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from paper: "Two convenience‐chosen, dementia‐specialised, long‐term care facilities in North Taiwan were randomly assigned into either a control or treatment group by flipping a coin" Comment: implies that the study may be cluster randomised but not clear from the information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: data not presented on 16/36 individuals with no reasons why |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | Unclear risk | Comment: insufficient data on baseline characteristics of nursing assistants and of participants; nursing assistants in usual care had significantly longer work experience than in treatment group; intervention group participants were older than usual care. This trial probably was a cluster randomised trial |
Dennis 2005.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 4023 participants; N = 2016 intervention (47% female; mean age 71 (SD 12)); N = 2007 usual care (46% female; mean age 71 (SD 13)) Inclusion criteria: people admitted with recent stroke, (first or recurrent stroke no more than 7 days before admission), if they passed swallow screen, the responsible clinician was uncertain whether to use oral nutritional supplements and the participant or relative consented to enrolment Exclusion criteria: subarachnoid haemorrhage Diagnostic criteria: stroke patients |
|
| Interventions | Intervention group received normal hospital diet plus oral protein energy supplements (360 mL) prescribed on drug administration charts; usual care received normal hospital diet until discharge Number of trial centres: 125 hospitals in 15 different countries Treatment before trial: none |
|
| Outcomes | Outcomes reported in abstract of publication: death, poor outcome (modified Rankin scale grade 3‐5) | |
| Study details |
Run‐in period: not stated Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial/other funding‐ Health Technology Assessment Board of NHS Research and Development in the UK, the Stroke Association, the Chief Scientist Office of the Scottish Executive, and Chest, Heart and Stroke, Scotland Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "to establish whether routine oral nutritional supplements improve outcome after stroke" | |
| Notes | The FOOD (feed or ordinary diet) trials consisted of three RCTs, sharing the same randomisation, data collection, and follow‐up systems, allowed co‐enrolment, and aimed to compare the outcomes of stroke patients in hospital. Dysphagic patients were enrolled into one or both of two trials: (1) early enteral tube feeding versus avoidance of tube feeding for at least 7 days; and (2) tube feeding via percutaneous endoscopic gastrostomy versus nasogastric tube. For this systematic review we describe the outcomes of participants who were able to swallow | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "the computer allocated the feeding regimen". Also, "A computer generated minimisation algorithm" was used |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "the computer allocated the feeding regimen" Comment: central allocation method ensured treatment allocation was concealed until intervention was given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from publication: "Neither the randomising clinician, the clinical team, nor patients were unaware of treatment allocation; doing so would have been very difficult" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from publication: "Follow‐up was masked to treatment allocation (except when patients or carers inadvertently divulged it to an interviewer, which was unusual but not systematically recorded)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: study attrition presented in a figure and all randomised participants accounted for |
| Selective reporting (reporting bias) | Low risk | Comment: all of the outcomes specified were reported |
| Other bias | Unclear risk | Comment: insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
Duncan 2006.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: not stated but assume 1:1 Superiority design |
|
| Participants | 318 participants; 100% female; mean age intervention 83.5 and control 83.6 years Inclusion criteria: women > 65 years admitted with acute hip fracture Exclusion criteria: not stated Diagnostic criteria: acute non‐pathological hip fracture |
|
| Interventions |
Intervention: additional personal attention of a dietetic assistant Control: usual care Number of trial centres: 1 Treatment before trial: none |
|
| Outcomes |
Outcomes reported in abstract of publication: Primary: post‐operative mortality in the acute trauma unit Secondary: post‐operative mortality at 4 months; length of hospital stay, energy intake and nutritional status |
|
| Study details |
Location: Wales, UK Year: May‐August 2003 Setting: acute trauma ward Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding ‐ Womens Royal Volunteer Service + British Dietetic Association, Innovations in Care Shire Pharmaceuticals, Wales Office of Research & Development Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To examine how improved attention to nutrition status and dietary intake achieved through the employment of dietetic assistants will affect post‐operative clinical outcome among elderly women with hip fracture" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Quote from paper: "sequentially numbered opaque sealed envelope method in blocks of 10, prepared by a member of staff not involved in the trial" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: assessments made by member of trial team blind to treatment allocation and independent of dietitian and dietetic assistants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: fully described |
| Selective reporting (reporting bias) | Low risk | Comment: all specified outcomes reported |
| Other bias | Low risk | Comment: baseline characteristics show groups are comparable |
Essed 2007.
| Methods |
Factorial randomised controlled clinical trial Randomisation ratio: 1:1:1:1 Superiority design |
|
| Participants | 97 participants (83 completed); mean age 84.9‐85.6 years (SD 5.7‐8.5); 58 female and 25 male Inclusion criteria: aged 65 years or older; resident of nursing home for more than 3 months; no terminal disease; no allergy to MSG; consuming meals provided by the nursing home at least 5 days/week Exclusion criteria: Diagnostic criteria: not stated |
|
| Interventions | Four arms; food sprinkled with 1. MSG 2. Flavour 3. MSG + Flavour 4. Maltodextrin (placebo) Number of trial centres: 3 care homes in the Netherlands Treatment before trial: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: energy intake and body weight | |
| Study details |
Run‐in period: two weeks Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To determine whether daily addition of flavour and/or MSG to the animal protein part of a cooked meal for 16 weeks leads to an increase in energy intake of the cooked meal and an increase in body weight" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: participants were reported as being "randomly assigned" Comment: insufficient detail of the method provided |
| Allocation concealment (selection bias) | Unclear risk |
Quote from paper: "The residents were unaware to which group they were assigned". Comment: insufficient detail of the method provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: single‐blind i.e. participants were blinded but not research personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants accounted for |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes specified in the methods are reported in the results |
| Other bias | Low risk | Comment: baseline characteristics comparable and reported in Table 1 |
Essed 2009.
| Methods |
Cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 53 nursing home residents (13 male: 40 female); aged 85.8 (SD 5.2) years Inclusion criteria: > 65 years old; able to participate; good eyesight Exclusion criteria: allergy to MSG; on sodium restricted diet; on anti‐depressants; terminal illness Diagnostic criteria: not stated |
|
| Interventions |
Intervention: hot meal including three foods with added salt and MSG Control: usual hot meals Number of trial centres: 1 Treatment before trial: usual diet |
|
| Outcomes | Outcomes reported in abstract of publication: dietary intake | |
| Study details |
Run‐in period: none Was study terminated early: not stated |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To determine whether or not an optimal preferred MSG concentration in several foods increases intake in elderly people" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: described as: " .. in a random order" Comment: insufficient detail of the method provided |
| Allocation concealment (selection bias) | Unclear risk |
Quote from paper: "The studies were carried out single blind" Comment: insufficient detail of the method provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not stated who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: fully described |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge |
| Other bias | Unclear risk | Comment: baseline characteristics reported |
Faxen‐Irving 2011.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 71 recently admitted geriatric patients; N = 34 intervention; N = 37 control. Mean age of all participants = 84 (SD 7.1) Inclusion criteria: likelihood of hospital stay more than one week, > 65 years old and able to give informed consent Exclusion criteria: pancreatitis, fat malabsorption, BMI > 30 kg/m2, and non‐consent for participation Diagnostic criteria: not stated |
|
| Interventions | Intervention group received a daily dose of 3 x 30 mL fat emulsion at the same time as pharmaceutical prescriptions. The usual care received usual care Number of trial centres: 1 Treatment before trial: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: food intake, self‐rated appetite, NRS, serum lipids, fatty acid profiles | |
| Study details |
Run‐in period: no Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial/commercial funding‐ SHS International & Nutricia (Sweden) and the Regional Agreement on Medical Training & Clinical Research between Stockholm County Council and the Karolinska Institutet Publicaton status: peer review journal |
|
| Stated aim for study | Quote from publication: "the effects on an oleic acid rich formula on energy intake and appetite were studied" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "an open randomised controlled trial. Permutted blocks of 10 were employed for the randomisation. No stratification was used". Comment: insufficient detail of method provided |
| Allocation concealment (selection bias) | High risk |
Quote from publication: "Sealed envelopes, opened by the study nurses after acceptance from the patients was received, were used to allocate individuals to intervention or control" Comment: sealed envelopes may have been used without appropriate safeguards, e.g. not sequentially numbered, nor opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from publication: "Sealed envelopes, opened by study nurses", therefore personnel aware of allocation. The study was also unblinded "open randomised controlled trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | High risk | Comment: data provided from only those who completed the study (rather than all those initially randomised) ‐ page 207 |
Gaskill 2009.
| Methods |
Cluster randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 352 nursing home residents (245 female; 107 male); mean age 84.2 (SD 8.7) years Inclusion criteria: not stated Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions | Nutrition education programme by nutrition coordinators compared with usual care Number of trial centres: 8 Treatment before trial: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: nutritional status (SGA) | |
| Study details |
Run‐in period: no Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To investigate the impact of implementing a train‐the‐trainer nutrition programme on the nutritional status of older adults residing in residential care." | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "Four of the eight Residential Aged Care Facilities were selected at random.." Comment: method used not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient reporting of attrition data |
| Selective reporting (reporting bias) | Low risk | Comment: reported all outcomes |
| Other bias | High risk |
Comment: baseline characteristics reported for whole group rather than for intervention and control separately Assessment of risk of bias in cluster‐randomised trials (1) Recruitment bias: unclear (2) Baseline imbalance: number of diagnoses (3) Loss of clusters: unclear (4) Incorrect analysis: yes (5) Comparability with individually randomised trials/different types of clusters: unclear |
Germain 2006.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 17 participants (10 female; 7 male); mean age 82.5 (SD 4.4) years intervention 84.6 (SD 3.8) years control Inclusion criteria: 60‐95 years old; resident > 3 months in the centre; unintentional weight loss > 7.5% in previous 3 months or BMI < 24 kg/m2 Exclusion criteria: active cancer or chronic intestinal disease or terminally ill Diagnostic criteria: dysphagia |
|
| Interventions | Re‐formed foods, thickened beverages and dietary supplements as necessary compared with traditional modified texture diet (control) Number of trial centres: 1 Treatment before trial: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: macro and micronutrient intake, weight and BMI | |
| Study details |
Run‐in period: no Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: not stated Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To evaluate to nutrient intake of frail institutionalised elderly persons with dysphagia, and to assess the impact of Sainte‐Anne's Hospital Advanced Nutritional Care Programme, on dietary intake and weight" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "Eligible subjects were randomly allocated....... using a blocked allocation strategy." Comment: insufficient detail of method provided |
| Allocation concealment (selection bias) | Low risk | Quote from paper: "sealed opaque envelopes indicating subject assignment were prepared off‐site" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants fully accounted for |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | Low risk | Comment: groups comparable at baseline; data reported in table 1 and text |
Hankey 1993.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 20 frail elderly people; N = 10 intervention; N = 10 control Inclusion criteria: frail elderly Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions | The intervention group received Build Up drink (1 unit) daily during routine drug prescription, in addition to their normal hospital diet. The usual care received the standard hospital diet Number of study centres: 1 Treatment before trial: none described |
|
| Outcomes | Outcomes reported in abstract of publication: food intake, glucose polymer intake, anthropometric measurements (TSF, MAMC) | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: not stated Publicaton status: peer review journal |
|
| Stated aim for study | Quote from publication: "the effectiveness of dietary supplements for frail elderly subjects in continuing care" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "...subjects were randomised to control or supplemented groups" Comment: insufficient detail of method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Comment: insufficient information to assess whether an important risk of bias exists |
Hickson 2004.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: > 65 years old; admitted to medicine for the elderly wards Exclusion criteria: unable to take food orally; not expected to survive the admission; planned discharge within 4 days; readmitted if already recruited into the trial Diagnostic criteria: acutely ill with a range of clinical conditions |
|
| Interventions |
Number of trial centres: 1 Treatment before trial: none |
|
| Outcomes | Outcomes reported in abstract of publication: nutritional status, mortality, length of stay, grip strength, Barthel score, intravenous antibiotic prescription | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "to examine whether healthcare assistants trained to provide additional support with feeding will improve acutely ill elderly inpatients clinical outcomes" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from paper: stratified by ward and achieved using "computer generated random numbers tables" |
| Allocation concealment (selection bias) | Low risk | Quote from paper: "using sealed, opaque envelopes prepared by an independent group" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: fully described |
| Selective reporting (reporting bias) | High risk | Comment: no data reported on the following outcomes: use of services questionnaire, referral rate to therapists, readmission within 6 months, laxative use, pressure sores, economic analysis |
| Other bias | Low risk | Comment: significantly more women in the intervention group otherwise both groups comparable at baseline |
Holyday 2012.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 143 hospitalised patients (61 male: 82 female); age intervention 83.7 (SE 0.8) control 83.4 (SE 0.9) years Inclusion criteria: all patients admitted under the care of a Geriatrician to an acute geriatric medicine ward Exclusion criteria: expected length of stay < 72 h; palliative care; unable to be nutritionally assessed; not speaking English; severe dementia or confusion; non‐cooperation Diagnostic criteria: acute geriatric medicine |
|
| Interventions | Malnutrition Care Pathway (modification of hospital meals; prescription of nutritional supplements or snacks; flagging for feeding assistance; education of participants and carers; referral to other health professionals and discharge planning) versus usual care Number of trial centres: 1 Treatment before trial: not specified |
|
| Outcomes | Outcomes reported in abstract of publication: length of hospital stay; readmissions; weight change; number of malnourished participants identified without routine nutrition screening | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial and non‐commercial funding ‐ The Gut Foundation + Pharmatel Fresenius Kabi PTY Ltd. Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To examine the prevalence of malnutrition in acutely ill older patients and to assess the impact of malnutrition screening and early dietetic intervention on weight, length of hospital stay, hospital costs and subsequent emergency presentations and hospital readmissions in geriatric patients at risk of malnutrition using a randomised controlled trial" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from paper: "randomly allocated by computerised random number generator" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from publication: "not possible to blind the clinical dietitian to group allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from publication: "as the outcomes are primarily objective measures they are mostly not open to the influence of bias" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all deaths and dropouts fully accounted for |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes fully reported |
| Other bias | Low risk | Comment: baseline characteristics similar between groups |
Johansen 2004.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: NRS‐2000 score > 3 on admission to hospital Exclusion criteria: predicted admission < 4 days; < 18 years old; < 1 month expected survival; ability to understand Danish; previously included participants; patients next to another participant; pregnant and lactating; psychiatric disorder; haemodialysis; patients receiving or planned to receive EN or PN Diagnostic criteria: varied |
|
| Interventions | Received daily attention from the nutrition team (nurse and dietitian); motivation of participant and staff; daily monitoring and adjustment of nutrition care plan; secured supply of ordered food Number of trial centres: 3 hospitals in Denmark Treatment before trial: not described |
|
| Outcomes | Outcomes reported in abstract of publication: length of stay; nutrition discharge index; health‐related quality of life (Short Form ‐36 health survey) | |
| Study details |
Run‐in period: no Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding ‐ Danish Ministry of Health + participating Hospitals Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To evaluate the clinical benefits of nutritional intervention in a random sample of all patients at nutritional risk according to Nutritional Risk Score ‐2002 from three different hospital levels" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from paper: participants selected "by a random numbers system" Comment: suggests that random sequence appropriate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: assessment of complications undertaken by a member of the investigation team blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: clearly described in the results; intention‐to‐treat analysis undertaken, however they do not report which group participants dropped out of |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes specified in the methods fully reported |
| Other bias | Low risk | Comment: baseline characteristics fully described; intervention and usual cares comparable |
Kraft 2012.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 26 participants; mean age 79.8 (SD 7.3) years; 10 male; 16 female Inclusion criteria: weight loss > 10% in previous 6 months; BMI < 21 kg/m2; albumin < 35g/L Exclusion criteria: malignancy, dementia, liver cirrhosis, dialysis‐dependent kidney insufficiency; insufficient cognitive ability; receiving professional care at home or living in a nursing home Diagnostic criteria: malnourished on discharge from hospital |
|
| Interventions | Intervention group received an oral nutritional supplement and telemedicine monitoring comprising daily assessment of weight, compliance with supplement prescription and state of health. Responses triggered a range of nutritional management actions by a nurse employed by the tele‐medicine centre Number of trial centres: 1 Treatment before trial: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: weight and BMI | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial and commercial funding ‐ Ministry of Social Affairs and Health, Western Pomerania, Germany and Nutricia (Germany) Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To evaluate the feasibility and explore the patients acceptance of the tele‐medical concept" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "Patients were randomized consecutively into the intervention and control group" Comment: insufficient details of the method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: high risk because of high attrition rate in the intervention group i.e. intervention (N = 13) 8 withdrew; control (N = 13) 3 withdrew; all withdrawals accounted for |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures reported |
| Other bias | Low risk | Comment: baseline characteristics fully described; intervention and usual cares comparable |
Kretser 2003.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 203 participants; 144 female: 59 male Inclusion criteria: people on a waiting list or referred on hospital discharge for meals on wheels or responding to local advertisements Exclusion criteria: MNA score > 22.5; self‐reported terminal illness; medical conditions that precluded the meal being adequate or food allergy; previously received meals on wheels Diagnostic criteria: not stated |
|
| Interventions | 21 meals and 14 snacks consisting of frozen meals, nutritional supplements and shelf‐stable and frozen food items. Menus provided 100% macro and micronutrient requirements for people over the age of 50 years. Daily phone call from older adult volunteer to provide safety and socialisation. Control = one hot meal five days a week at lunchtime Number of trial centres: not relevant (all at home) Treatment before trial: none |
|
| Outcomes | Outcomes reported in abstract of publication: weight, MNA, functional status | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Commercial/non‐commercial/other funding: not stated Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To compare a traditional meals on wheels (MoW) programme consisting of one hot meal delivered daily, Monday through Friday, versus a new MoW programme consisting of 21 meals and 14 snacks that required some preparation delivered weekly" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote from publication: "Randomized treatment assignment was followed with a few exceptions...Participants who were offered the new MoW model and refused, were placed in the traditional MoW model" Comment: insufficient detail of method provided as well as patients moving between groups as above |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: overall attrition reported but not from which groups they dropped out |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes fully reported |
| Other bias | Unclear risk | Comment: traditional MoW group had significantly lower functional ability (instrumental ADL) and lower education attainment |
Larsson 1990.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with an expected hospital stay of more than 3 weeks, admitted consecutively to a long‐term medical care clinic Exclusion criteria: not stated Diagnostic criteria: diagnosis of participants included: malignancy, endocrine, neurological, heart, vascular, respiratory, musculoskeletal, fracture |
|
| Interventions | Intervention group received nutritional supplementation (400 kcal) in the morning and afternoon between meals, when all patients on the ward were routinely supplied with drinks, as well as standard hospital diet. The usual care received standard hospital diet alone Number of trial centres: 1 Treatment before trial: none described |
|
| Outcomes | Outcomes reported in abstract of publication: anthropometry, serum protein analysis, delayed hypersensitivity skin test, mobility, general physical condition, food intake | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial/other funding ‐ Swedish Medical Research Council, Research Fund of the County of Ostergotland, Kabi Nutrition Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "to investigate the relationship between nutritional state and the development and healing of pressure sores in patients in a long term care clinic" (page 245). Larsson: "to evaluate the effect of dietary supplements on clinical outcome and nutritional status in a large group of geriatric patients" (Ek 1991) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Randomisation was carried out by means of sealed envelopes containing group designation". Comment: insufficient information provided about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: number of study dropouts presented in figure but unclear which group they belong to and the reason |
| Selective reporting (reporting bias) | Low risk | Comment: data not reported at all time points for all outcomes |
| Other bias | High risk | Comment: significant differences between groups at baseline in TSF and weight index in men, and AMC in women were significantly lower in experimental than the usual care. The supplemented group also had a significantly lower mental score on admission (Unosson 1992) |
Leslie 2012.
| Methods |
Cluster‐randomised controlled trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 41 people living in residential care homes, 36 female, 5 male, mean age 91(SD 7) years Inclusion criteria: BMI < 18.5 kgm2, without acute disease Exclusion criteria: not described Diagnostic criteria: mixed diagnoses, people living in residential care homes |
|
| Interventions | Provision of energy enriched meals vs usual care Number of trial centres: 21 residential care homes Treatment before trial: not described |
|
| Outcomes | Outcomes reported in abstract of publication: energy intake, weight and BMI | |
| Study details |
Run‐in period: no Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial funding ‐ GlaxoSmithKline Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To examine whether the nutritional status of aged undernourished residents in care could be improved through dietary modification to increase energy intake but not portion size" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Random permuted block design, stratified by home type (dementia/no dementia) by a statistician who had no contact with the homes" Comment: insufficient detail of method provided |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Allocation made post recruitment and baseline screening by a statistician who had no contact with the homes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not mentioned. As energy enrichment was of usual meals it would have been possible to blind participants to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not mentioned. Assessment of weight and food intake might have been influenced by knowing the study group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: the number of participants that dropped out and the reasons are given |
| Selective reporting (reporting bias) | Low risk | Comment: all specified outcomes are reported |
| Other bias | High risk |
Assessment of risk of bias in cluster‐randomised trials (1) Recruitment bias: no (2) Baseline imbalance: unclear (3) Loss of clusters: unclear (4) Incorrect analysis: no (5) Comparability with individually randomised trials/different types of clusters: different types of clusters |
Lin 2010.
| Methods |
Cluster‐randomised controlled clinical trial Randomisation ratio: 1:1:1 Superiority design |
|
| Participants |
Inclusion criteria: diagnosis of dementia; EdFed score > 2; able to stay in institution for duration of study; Mini Mental State Examination score 10‐23 Exclusion criteria: not described Diagnostic criteria: diagnosis of dementia |
|
| Interventions |
Number of trial centres: 3 Treatment before trial: not described |
|
| Outcomes | Outcomes reported in abstract of publication: EdFed score; frequency of physical and verbal assistance provided; nutritional status | |
| Study details |
Run‐in period: no Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding ‐ National Health Research Insitute Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To investigate the effectiveness of training of spaced‐retrieval and Montessori‐based activities in decreasing feeding difficulty and nutritional status for residents with dementia" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "To avoid confounding, the three institutes were randomly assigned ...." Comment: insufficient detail of method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: nature of blinding not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from publication: "The data collectors did not know which group the subjects belonged to". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: state reason for dropouts, but unclear which groups they dropped out of |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes fully reported |
| Other bias | High risk |
Comment: baseline characteristics reported; significant difference in ADL observed. Assessment of risk of bias in cluster‐randomised trials (1) Recruitment bias: no (2) Baseline imbalance: frail status (3) Loss of clusters: no (4) Incorrect analysis: no (5) Comparability with individually randomised trials/different types of clusters: different types of clusters |
Lin 2011.
| Methods |
Cluster‐ and cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 29 participants; mean age 82.9 (SD 6.0) years; 17 male: 12 female with dementia in care home. Appear to be identical to participants in Group 2 in Lin 2010; No response from study author Inclusion criteria: diagnosis of dementia ; > 2 Edinburgh Feeding Evaluation in Dementia scale (EdFed); MMSE score = 10‐23 Exclusion criteria: not stated Diagnostic criteria: dementia |
|
| Interventions | Montessori intervention including sensory stimulation, procedural movements (e.g. hand eye co‐ordination) and extension and conclusion activities Number of trial centres: 2 Treatment before trial: none |
|
| Outcomes | Outcomes reported in abstract of publication: EdFed score; Eating Behaviours score; MNA score; self‐feeding frequency and self‐feeding time | |
| Study details |
Run‐in period: 2‐week wash out between cross‐over Was study terminated early: no |
|
| Publication details | Language of publication: English Funding: non‐commercial funding ‐ National Health Research Inistitute (Taiwan) Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To investigate the efficacy of a Montessori intervention in improving the eating ability and nutritional status of residents with dementia in long term care facilities" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "To avoid contamination among participants ......the two demential special care units were randomly assigned....." Comment: insufficient information provided to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: described as not blinded, lack of blinding therefore may have influenced participant responses |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors blind to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not described |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes fully reported |
| Other bias | High risk |
Comment: baseline data suggest considerable variation in length of institutionalisation and length of time diagnosed with dementia Assessment of risk of bias in cluster‐randomised trials (1) Recruitment bias: no (2) Baseline imbalance: frail status (3) Loss of clusters: no (4) Incorrect analysis: no (5) Comparability with individually randomised trials/different types of clusters: different types of clusters |
Mathey 2001a.
| Methods |
Cluster randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: > 65 years old; resident in nursing home for > 3 months Exclusion criteria: parenteral nutrition; terminal phase of disease; severe anaemia Diagnostic criteria: varied |
|
| Interventions |
Number of trial centres: 1 Treatment before trial: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: weight; dietary intake; biochemical indicators; health‐related quality of life (SIP); life satisfaction score (PGCMS) | |
| Study details |
Run‐in period: no Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: not stated Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To determine the effect of an improved ambience of food consumption on health and nutritional status of Dutch nursing home elderly residents" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "Four wards, each with 15 residents and comparable for diseases and treatment were randomly assigned to be in either the control (two wards) or the experimental group (two wards)." Comment: insufficient detail of method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition fully reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes fully reported |
| Other bias | High risk |
Comment: baseline characteristics fully reported (including dropouts); control and intervention groups comparable at baseline Assessment of risk of bias in cluster‐randomised trials (1) Recruitment bias: no (2) Baseline imbalance: frail status (3) Loss of clusters: no (4) Incorrect analysis: no (5) Comparability with individually randomised trials/different types of clusters: different types of clusters |
Mathey 2001b.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 67 elderly care home residents; mean age intervention 84.6 (SD 6.1) years; control 83 (SD 5.5) years; 54 female: 13 male Inclusion criteria: > 65 years, resided in care home > 3 months and consuming cooked meal provided by care home kitchen at least 5 days/week Exclusion criteria: dementia, hospitalised, depression; in terminal phase; allergy to MSG Diagnostic criteria: not specified |
|
| Interventions | Four flavour powders to enhance the cooked meal (chicken, beef, turkey or lemon) using 1 (+ 0.2) g flavour powder Number of trial centres: 1 Treatment before trial: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: body weight, energy intake and hunger | |
| Study details |
Run‐in period: one Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial funding ‐ flavours donated by IFF BV; funding from Friesland Coberco Research and the Suikerstichting Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To determine whether the addition of flavour enhancers to the cooked meals over 16 weeks would lead to an increase in food consumption and thereby provide nutritional benefits to elderly nursing home residents" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "subjects were randomly assigned to be in the control group ..or the flavour group.." Comment: insufficient detail of the method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all dropouts fully accounted for |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes specified in the methods fully reported |
| Other bias | Low risk | Comment: baseline characteristics fully reported; control and intervention groups comparable at baseline |
Munk 2014.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 84 people newly admitted to hospital; mean age intervention 75 (SD 10) years; control 74 (SD 11) years; 47 female, 34 male (data on those that completed the study) Inclusion criteria:new admissions to hospital, > 18 years old and at nutritional risk according to NRS‐2002, able to eat orally, anticipated length of stay > 3 days, sufficient language proficiency Exclusion criteria: dysphagia, food allergy or intolerance, anatomical obstruction preventing food intake, receiving enteral or parenteral nutrition, judged to be terminally ill Diagnostic criteria: admitted to oncology, orthopaedics or urology wards |
|
| Interventions | An a la carte menu of small dishes enriched with natural energy‐dense ingredients and supplemented with protein powder Number of trial centres: 1 Treatment before trial: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: percent reaching their calculated energy and protein requirements, mean energy and protein intake, body weight, handgrip strength, LOS, mortality | |
| Study details |
Run‐in period: 5 weeks to ensure optimal staff training. Recruitment started at the end of the run‐in Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial funding ‐ protein powder donated by Toft Care System, Copenhagen, Denmark. Study funded by Herlev University Hospital Research Unit Publication status: peer review journal |
|
| Stated aim for study | Quote "to investigate whether a novel food service concept with protein supplementation would increase protein and energy intake in hospitalised patients at nutritional risk". | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from paper: "The allocation sequence was generated by a secretary who was not otherwise involved in the trial" |
| Allocation concealment (selection bias) | Low risk | Quote from paper: "using sealed opaque envelopes, with a total of 9 blocks each with 10 envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and study personnel were not blinded to group allocation. Blinding of participants would not be possible due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: data assessors were not blinded to group allocation. Blinding of the assessors was judged by the authors to be difficult as participants were likely to reveal their group allocation. The analyses were conducted blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: three participants did not receive the intervention and so not included in the study |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes specified in the methods reported |
| Other bias | Low risk | Comment: baseline characteristics fully reported; control and intervention groups comparable at baseline |
Nijs 2006.
| Methods |
Cluster‐randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: nursing homes: medium sized, with a general population, two wards for people with chronic somatic diseases, long‐term or permanent stay, located in different parts of the country, similar for staff numbers, disciplines, education levels of carers, newness of infrastructure, location and residents' activities Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions |
Number of trial centres: 5 Treatment before trial: none |
|
| Outcomes | Outcomes reported in abstract of publication: dietary intake, MNA score | |
| Study details |
Run‐in period: 2‐month run‐in to allow nurses to accommodate the change in organisation Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "to investigate the effect of family‐style meals on energy intake and the risk of malnutrition in Dutch nursing home residents" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote from paper: "The wards' name with the initial letter occurring first in the alphabet became the intervention ward". Comment: the randomisation was based on the ward name and therefore predictable |
| Allocation concealment (selection bias) | High risk | Comment: no concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not done, but probably not possible to do |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants are fully accounted for |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | High risk |
Assessment of risk of bias in cluster‐randomised trials (1) Recruitment bias: unclear (2) Baseline imbalance: age, sex (3) Loss of clusters: unclear (4) Incorrect analysis: no (5) Comparability with individually randomised trials / different types of clusters: unclear |
Olofsson 2007.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: femoral neck fracture; > 70 years old; admitted to orthopaedic wards Exclusion criteria: severe rheumatoid arthritis, hip osteoarthritis or renal failure or metastatic fracture and bed‐ridden before the injury Diagnostic criteria: femoral neck fracture |
|
| Interventions |
Number of trial centres: 1 Treatment before trial: none Complex intervention: staff education; team work, individual care planning; prevention and treatment of delirium and complications; nutrition; rehabilitation; secondary prevention of falls and fractures; osteoporosis prophylaxis |
|
| Outcomes | Outcomes reported in abstract of publication: days of delirium; decubitus ulcers; length of stay; BMI, body weight; MNA score | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding ‐ Borgerskapt in Umea Research Foundation; the Dementia Fund; the Vardal foundation; the Joint committee of the Northern Health Region of Sweden; the JC Kempe Memorial Foundation; the Foundation for the Medical Faculty, University of Umea, local councils and the Swedish Research Council Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To investigate whether a nutritional intervention which was part of a multi‐factorial intervention programme for old women and men with a femoral neck fracture had any effect on post‐operative complications during hospitalisation and on nutritional status at four months follow‐up" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "Patients were randomised to post op care in a geriatric ward with a special intervention programme or to conventional care in the orthopaedic department. All participants received an envelope while in the emergency room, but it was not opened until immediately before surgery...." Comment: insufficient detail of method provided |
| Allocation concealment (selection bias) | Low risk | Comment: "sealed opaque envelopes stratified by operation"; envelopes opened by a nurse not involved in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded; staff on the control ward knew that a new programme was being implemented on another ward in the hospital |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: assessments on the intervention ward were carried out by a nurse on the control ward and vice versa. A specialist in geriatric medicine who was not working in either of the two departments, and did not know which groups the patients were randomised to, analysed all the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: fully described |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes fully reported |
| Other bias | Unclear risk | Comment: baseline characteristics reported; groups comparable apart from prevalence of heart failure |
Pivi 2011.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1:1 Superiority design |
|
| Participants |
Inclusion criteria: > 65 years old with probable Alzheimer's disease (AD) Exclusion criteria: other forms of dementia; receiving tube feeding; diabetes or renal disease Diagnostic criteria: Alzheimer's disease |
|
| Interventions |
Number of trial centres: 1 Treatment before trial: no |
|
| Outcomes | Outcomes reported in abstract of publication: weight; BMI; MAC and ;MAMC; TSF; total protein; total lymphocyte count | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial/non‐commercial funding ‐ Ministry of Education; Abbott Laboratories Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To determine if there is any difference between oral nutritional supplementation and nutrition education on the nutritional status of patients with AD" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "subjects were randomised into three groups....." Comment: insufficient detail of method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: fully described |
| Selective reporting (reporting bias) | Low risk | Comment: fully reported |
| Other bias | Unclear risk | Comment: baseline characteristics reported; groups comparable |
Potter 2001.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: emergency admissions to medicine for the elderly unit (aged over 60), emergency admissions from home, ability to gain consent from participants or relatives, no known malignancy, ability to swallow, non obesity, BMI < 75th percentile. Exclusion criteria: overweight (BMI >75th percentile), in terminal stage of illness, or had swallow difficulty preventing oral intake Diagnostic criteria: unwell elderly people |
|
| Interventions | Intervention group received 120 mL sip feed 3 x daily throughout hospitalisation. The usual care received normal ward diet Number of trial centres: 1 ‐ medicine for the elderly unit in a Scottish Hospital Treatment before trial: none |
|
| Outcomes | Outcomes reported in abstract of publication: anthropometry, mortality, length of hospital stay, functional recovery, rates of institutionalisation, patient compliance with supplement, total energy intake, nursing staff views of the method | |
| Study details |
Run‐in period: no Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial/other funding ‐ Chief Scientist's Office of Scottish Office, and Frusenius UK Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "to assess whether prescription of oral sip‐feed supplements in small quantities in the medicine prescription chart and distribution at medication rounds could increase total energy intake and provide sufficient energy to prevent nutritional decline" (Roberts 2003) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "Patients were assigned to the intervention arm randomly..." Comment: not described in sufficient detail |
| Allocation concealment (selection bias) | Unclear risk |
Quote from paper: "using sealed envelopes containing allocation specification" Comment: insufficient detail provided of sequential numbering or whether envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from publication: "Supplement prescription was done by researchers who knew the randomisation codes, and were not involved in outcome data collection, nor data entry to allow blinding" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote from publication: "The researchers who performed the anthropometry and assessed the clinical outcomes, were blinded to the intervention status of the patients" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: outcomes reported in relation to BMI and TSF, but not BMI and TSF data alone |
| Selective reporting (reporting bias) | High risk | Comment: one or more outcomes of interest to the review were reported incompletely, so they could not be entered into the meta‐analysis |
| Other bias | High risk | Comment: in the well‐nourished group, only 1/2 were sequentially randomised |
Remsburg 2001.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1.1 Superiority design |
|
| Participants |
Inclusion criteria: older than 65 years, on a soft or normal diet Exclusion criteria: medically unstable, active malignancy or HIV, creatinine > 260 micromols/L, complex dietary needs Diagnostic criteria: nursing home residents |
|
| Interventions |
Number of trial centres: 1 Treatment before trial: none specific |
|
| Outcomes | Outcomes reported in abstract of publication: no abstract | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding ‐ Johns Hopkins University Fund for Geriatric Medicine and Nursing Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To determine the feasibility of implementing a comprehensive buffet‐style dining program and to determine the impact of the program on weight and biochemical indicators of nutritional status among nursing home residents..." | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: described as "subjects were randomised to participate" Comment: no details of procedure provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detail |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: reported in footnotes of table 2 |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge |
| Other bias | Low risk | Comment: baseline characteristics comparable. Significantly more men in the usual care |
Salva 2011.
| Methods |
Cluster‐randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants | 946 participants with dementia; mean age 79 (SD 7.3) years; 644 female: 302 male Inclusion criteria: diagnosis of mild‐moderate dementia; MMSE < 26; living at home; ambulatory with identified care giver Exclusion criteria: MMSE > 26; residents in an institution; nasogastric feeding; terminal care; already participating in a nutrition intervention study Diagnostic criteria: dementia (diagnosed using DSM4 criteria) |
|
| Interventions | A standardised protocol for feeding and nutrition comprising 5 components; personalised information pack handed to participants and carers, 4 training sessions given by a dietitian to families and care‐givers, support in monitoring weight, periodic information for families, standardised action protocols Number of trial centres: 11 outpatient clinics and day hospital units (intervention N = 6; control N = 5) Treatment before trial: none |
|
| Outcomes | Outcomes reported in abstract of publication: ADL; MNA; Caregiver Burden Scale | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial funding ‐ Nestec Limited Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To assess the effectiveness of a health and nutrition programme (NurtiALZ) versus usual care on functional level in elderly people with dementia living at home, as well as on clinical practice related to nutrition and on the caregivers burden" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "The unit of randomisation was the medical centres..." Comment: insufficient detail of the method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants and dropouts fully accounted for |
| Selective reporting (reporting bias) | Low risk | Comment: all fully reported |
| Other bias | High risk |
Assessment of risk of bias in cluster‐randomised trials (1) Recruitment bias: no (2) Baseline imbalance: frail status (3) Loss of clusters: no (4) Incorrect analysis: no (5) Comparability with individually randomised trials/different types of clusters: different types of clusters |
Silver 2008.
| Methods |
Cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: greater than 60 years and receiving home‐delivered lunch meals Exclusion criteria: chewing or swallowing dysfunction, need for feeding assistance, an eating disorder, depression, impaired functional status, dementia, BMI < 30 kg/m2, medically‐restricted diet on oral nutritional supplements, on orexigenic aids, regularly skip meals, smoke, more than 1 alcoholic drink per day Diagnostic criteria: not stated |
|
| Interventions |
Number of trial centres: not applicable, participants are free‐living Treatment before trial: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: energy intake, key macro‐nutrients and micronutrients are mentioned but data not presented | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding ‐ Retirement research Foundation, Chicago, Illinois Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To determine whether enhancing the energy density of food items regularly served in a home delivered meals programme would increase lunch and 24 hour energy and nutrient intakes" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "The experiment used a randomized crossover within‐subjects design" Comment: insufficient detail of method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detail |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 7 participants dropped out but reasons not given, and unclear from which group they dropped out |
| Selective reporting (reporting bias) | Low risk | Comment: the outcomes specified in the methods are reported in the results |
| Other bias | Unclear risk | Comment: no table of baseline characteristics. The information on need for assistance with shopping and preparation of food and recent weight loss suggests heterogeneity in the population |
Simmons 2008.
| Methods |
Cluster‐ and cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: long‐stay residents in a care home; free of feeding tube, not receiving palliative care, not on planned weight loss diet Exclusion criteria: not explicitly stated Diagnostic criteria: not stated |
|
| Interventions |
Number of trial centres: 4 care homes Treatment before trial: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: energy intake, weight change; staff time to provide interventions | |
| Study details | At baseline all eligible participants were assessed for responsiveness (15% increase in energy intake) to one of 2 interventions (i.e. feeding assistance or between‐meal snacks). This was a 2‐phase cross‐over trial where residents not eligible in the first phase were re‐evaluated for possible inclusion in the second phase and residents included in the first phase were re‐evaluated and could become ineligible for the second phase (based on adequacy of energy intake) Run‐in period: not stated Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding ‐ National Institute of Aging and National Institute of Health, University of California, LA Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To evaluate the effect of two feeding assistance interventions (meal time assistants and between meal snack delivery) on residents oral food and fluid intake, BMI and weight status when maintained by research staff for 24 weeks" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Participants were randomised at the facility level..., the four nursing homes were identified as intervention or control (in pairs of two) using a toss of the coin...." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding and outcome likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding and outcome likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: numbers are described in text and appendix. Mortality given as a reason for most dropouts (58%), but the remaining reasons are not described. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge |
| Other bias | High risk |
Comment: baseline characteristics presented for total numbers of participants in each group (phase 1 and 2 combined) Assessment of risk of bias in cluster‐randomised trials (1) Recruitment bias: no (2) Baseline imbalance: frail status (3) Loss of clusters: no (4) Incorrect analysis: no (5) Comparability with individually randomised trials/different types of clusters: different types of clusters |
Simmons 2010.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1:1 Superiority design |
|
| Participants |
Inclusion criteria: long stay residents; free of feeding tube; not receiving hospice care; identified for nutritional supplementation Exclusion criteria: not stated Diagnostic criteria: |
|
| Interventions |
Number of trial centres: 3 Treatment before trial: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: energy intake, staff time and costs | |
| Study details |
Run‐in period: not stated Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding ‐ National Alzheimer's Association and National Institute for Aging Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To determine the cost effectiveness of supplements relative to offering residents snack foods and fluids between meals to increase caloric intake" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "Participants....were randomised into one of three groups" Comment: insufficient detail of method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detail provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: fully reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient detail to judge |
| Other bias | Unclear risk | Comment: baseline characteristics reported for whole study population and not according to group allocation |
Smoliner 2008.
| Methods |
Cluster‐randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: MNA score < 23.5 points Exclusion criteria: MNA >23.5 points, severe cognitive impairment, on enteral feeding, hospital stay > 6 days during the study period Diagnostic criteria: not stated |
|
| Interventions |
Number of trial centres: 3 care homes Treatment before trial: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: protein and energy intake, nutritional status and body composition, muscle function and physical function | |
| Study details |
Run‐in period: not stated Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial funding ‐ Schubert Holding Ag & Co, KG Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To evaluate the effect of a 12 week nutritional intervention with protein and energy enriched food and snacks on nutritional and functional status in elderly nursing home residents at risk of malnutrition" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "Randomisation was done according to ward...." Comment: insufficient detail of the method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detail |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: fully described and figure 1 |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available |
| Other bias | High risk |
Assessment of risk of bias in cluster‐randomised trials (1) Recruitment bias: unclear (2) Baseline imbalance: length of stay, number of medications, SF‐36 physical functioning score (3) Loss of clusters: unclear (4) incorrect analysis: yes (5) Comparability with individually randomised trials/different types of clusters: unclear |
Splett 2003.
| Methods |
Cluster‐randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people entering residential care facilities with service provided by a dietitian Exclusion criteria: people entering a hospice or respite care programme or those expected to have a stay < 30 days Diagnostic criteria: varied |
|
| Interventions |
Number of trial centres: 29 Treatment before trial: 57% intervention group and 61% usual care had previous dietary modification and 25% intervention and 35% control received help at mealtimes |
|
| Outcomes | Outcomes reported in abstract of publication: rate of unintentional weight loss, weight status 90 days after admission and weight status 90 days after identification of unintentional weight loss | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: not stated Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To assess the effectiveness of a new medical nutrition therapy protocol for the prevention and treatment of unintentional weight loss and describe nutrition assessment and intervention activities of dietitians" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: ".. facilities were randomly assigned to either the medical nutrition therapy protocol care group (MNTPC) or the usual care (UC) group using a random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: fully described |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | High risk |
Assessment of risk of bias in cluster‐randomised trials (1) Recruitment bias: unclear (2) Baseline imbalance: number of diagnoses (3) Loss of clusters: unclear (4) Incorrect analysis: yes (5) Comparability with individually randomised trials/different types of clusters: unclear |
Taylor 2006.
| Methods |
Cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: aged > 65 years; dysphagia (diagnosed by swallowing team); receiving a texture modified diet Exclusion criteria: tube‐fed; medically unstable; receiving a diabetic diet Diagnostic criteria: not stated |
|
| Interventions |
Number of trial centres: 1 Treatment before trial: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: energy and fluid intakes | |
| Study details |
Run‐in period: not stated Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding ‐ Canadian Foundation for Dietetic Research Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "To determine whether serving a 5 meal pattern versus a traditional 3 meal pattern would improve energy intake among elderly, extended care residents with dysphagia" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "Participants were randomly assigned to one of two groups." Comment: insufficient detail of method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: reason for dropouts reported, however unclear from which groups they dropped out |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge |
| Other bias | Unclear risk | Comment: baseline characteristics reported in the text; homogeneous population |
Van den Berg 2015.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 2:1 (2 intervention groups and 1 control) Superiority design |
|
| Participants | 834 people newly admitted to hospital; mean age intervention 1: 70.5 (SD 15) years, intervention 2: 72.6 (SD 10) years; control 70.4 (SD 13) years; 105 female: 129 male Inclusion criteria: new admissions to internal medicine and surgical wards, > 18 years old scoring > 3 on the SNAQ, who were advised to take ONS by the dietitian Exclusion criteria: < 18 years old, dysphagia, end‐stage renal disease, people receiving enteral or parenteral nutrition, or with an expected length of stay < 3 days Diagnostic criteria: internal medicine(oncology, nephrology, cardiology, pulmonary disease, internal gastroenterology, gynaecology, urology wards, neurology & geriatrics & surgical (orthopaedics, gastroenterology, vascular and trauma) |
|
| Interventions | ONS offered during the medication rounds either 125 mL twice a day or 62 mL four times a day vs usual care (125 mL ONS offered during meals) Number of trial centres: 1 Treatment before trial: none stated |
|
| Outcomes | Outcomes reported in abstract of publication: percentage of participants consuming at least 75% of prescribed ONS, mean intake (mL) of ONS | |
| Study details |
Run‐in period: none Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: Study funded by Deventer Hospital, the Netherlands. (No commercial funding and the ONS was not donated) Publication status: peer review journal |
|
| Stated aim for study | Quote "to investigate whether the distribution of ONS during medication rounds, either in 2 higher volumes or in 4 lower volumes, would increase the intake of the supplements and to evaluate its effects on patient compliance with consumption of the ONS | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Computerised random number system" |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "concealed blinded envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from publication: "it is not possible to perform a blinded study for nutritional support" Comment: the participants and personnel were not blinded to intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: outcomes were not assessed blinded to study group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition fully described, 31 patients refused the ONS during the study but were included in the analysis, 42 patients were discharged within 2 days of follow‐up and were excluded from analyses |
| Selective reporting (reporting bias) | Low risk | Comment: all specified outcomes were reported |
| Other bias | Low risk | Comment: baseline characteristics fully reported and groups similar at baseline |
Van Ort 1995.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: required feeding assistance by a caregiver (nurse and/or nursing assistant), were able to sit in a chair for feeding, were responsive to human interaction, were not usually restrained during feeding, were not usually combative Exclusion criteria: not given Diagnostic criteria: not stated |
|
| Interventions | 2 treatments were applied to the intervention group (contextual intervention and behavioural intervention). It was unclear whether interventions were given together, or given one after the other. 2 complete lunches and two dinners in week 1, and 3 lunches and dinners in week 2 were video tape‐recorded Number of trial centres: 1 Treatment before trial: none |
|
| Outcomes | Outcomes reported in abstract of publication: no abstract | |
| Study details |
Run‐in period: no Was trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding; "This study was supported by a 1991 Christian P. Voltz Memorial Pilot Grant Award from the Alzheimers Association" Publication status: peer review journal |
|
| Stated aim for study | Quote from publication: "the interventions were designed to first create a feeding context or environment that promoted function by being as "near normal" as possible and by removing barriers to function, and second to provide randomly selected patients with behavioural prompts, cues and reinforcements for self feeding approximations" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "Then four of the eight subjects were randomly selected to receive the intervention...." Comment: insufficient details of the procedure |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement ‐ study stated "the project research associates were blind to the specific study hypothesis", however their role in the study unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: the research associates were responsible for implementing the behavioural intervention. On analysing the video tapes, they were blinded to the study hypotheses, however no statement to say they were blinded to the study interventions |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient reporting of attrition/exclusions to permit judgement. The number of dropouts were stated, however it was unclear from which group |
| Selective reporting (reporting bias) | High risk | Comment: the study report failed to include results for key outcomes that would be expected to have been reported for such a study ‐ the study does not provide any data |
| Other bias | Unclear risk | Comment: no baseline characteristics reported, therefore insufficient information to assess whether an important risk of bias exists |
ADL: activities of daily living; BMI: body mass index; EdFED: Edinburgh Feeding Evaluation in Dementia; HIV: human immunodeficiency virus; MAC: mid‐arm circumference; MAMC: mid‐arm muscle circumference; MMSE: Mini Mental State Examination; MNA: Mini Nutritional Assessment; MoW: meals on wheels; MSG: monosodium glutamate; MUAC: mid upper‐arm circumference; NRS: Nutritional Risk Screening; ONS: oral nutritional supplement; PGCMS: Philadelphia Geriatric Centre Morale Scale; SD: standard deviation; SE: standard error; SGA: subjective global assessment; SIP: sickness impact profile; SNAQ: Simplified Nutritional Appetite Questionnaire; TSF: triceps skin fold
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aleman‐Mateo 2012 | Not a supportive intervention in nutritional care; intervention included individual advice on taking ONS as participants were free‐living |
| Allman 1990 | Not a supportive intervention in nutritional care; ONS prescribed on an individualised basis, as dietary advice was given, and participants had to follow instructions to take ONS at home |
| Arias 2008 | Not a supportive intervention; intervention is an ONS with no mention of supportive strategy to support administration |
| Asplund 2000 | Not a supportive intervention in nutritional care; looked at the effect of residence in an acute geriatrics‐based ward, outcomes not relevant to this review |
| Baldwin 2011 | Not a supportive intervention in nutritional care; individualised interventions therefore participants were required to understand and follow instructions |
| Banerjee 1978 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| Bauer 2005 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given. The intervention was also micronutrient‐specific |
| Beattie 2000 | Not a supportive intervention in nutritional care; no clear organisational component to the intervention was described, and the intervention was continued post hospital discharge, therefore participants would have been given individual advice on taking ONS |
| Beck 2008 | Not a supportive intervention in nutritional care; but a multicomponent intervention, therefore unable to extract specific effect of nutrition component |
| Benati 2001 | The intervention included supplementation with ONS but there was no indication that a supportive protocol was used to support the intervention |
| Bonjour 2011 | Not a supportive intervention in nutritional care; intervention involved calcium and vitamin D supplementation |
| Bonjour 2012 | Not a supportive intervention in nutritional care; unclear nutritional risk of participants |
| Bonnefoy 2003 | Not a supportive intervention in nutritional care but a multicomponent intervention, therefore unable to extract specific effect of nutrition component |
| Bos 2001 | Not a RCT |
| Botella‐Carretero 2008 | Not a supportive intervention in nutritional care; intervention continued post hospital discharge, therefore participants would have been given individual advice on taking ONS |
| Botella‐Carretero 2010 | Not a supportive intervention in nutritional care; ONS prescribed on an individualised basis, and tailored to texture and estimated nutritional requirements |
| Boudville 2003 | Not a supportive intervention in nutritional care; intervention given to outpatients, therefore participants would have been given individual advice on taking ONS |
| Bunout 1989 | Not a supportive intervention in nutritional care; ONS tailored to body weight/nutritional requirements, therefore prescribed on an individualised basis |
| Bunout 2001 | Not a randomised control trial; the nutritional intervention was not randomised but the exercise intervention was |
| Carlsson 2011 | Not a supportive intervention in nutritional care but a multicomponent intervention, therefore unable to extract specific effect of nutrition component |
| Carnaby 2006 | Not a supportive intervention in nutritional care; intervention specific to stroke participants with dysphagia hence scope not considered broad enough to be a supportive intervention in nutritional care |
| Charlin 2002 | Not a supportive intervention in nutritional care; intervention given to outpatients, therefore participants would have been given individual advice on taking ONS |
| Charras 2010 | Not a randomised controlled trial |
| Chernoff 1990 | Not a supportive intervention in nutritional care; artificial support was given via non oral route, enteral tube feeding |
| Chin 2001 | Not a supportive intervention in nutritional care; micronutrient supplementation study; usual care had non‐enriched 'product' |
| Collins 2005 | Not a supportive intervention in nutritional care; intervention given to outpatients, therefore participants would have been given individual advice on taking ONS |
| Dangour 2011 | Not a supportive intervention in nutritional care; intervention given to outpatients, therefore participants would have been given individual advice on taking an ONS |
| De Jong 1999 | Not a supportive intervention in nutritional care; a micronutrient enrichment intervention |
| de Sousa 2012 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| Delmi 1990 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| Dhanraj 1997 | Not a supportive intervention in nutritional care; artificial support was given via non oral route (nasogastric feeding); no usual care comparison; some participants < 18 yrs; individualised nutritional care given |
| Dillabough 2011 | Not a RCT; article describing a pilot quality improvement project |
| Edington 2004 | Not a supportive intervention in nutritional care; ONS tailored to individual estimated nutritional requirements, therefore prescribed on an individualised basis |
| Elkort 1981 | Not a supportive intervention in nutritional care; ONS tailored to individual estimated nutritional requirements, therefore prescribed on an individualised basis |
| Endevelt 2011 | Not a supportive intervention in nutritional care; intervention was individualised |
| Eneroth 2004 | Not a supportive intervention in nutritional care; intervention given to outpatients, therefore participants would have been given individual advice on taking ONS |
| Espaulella 2000 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| Fiatarone 1994 | Not a supportive intervention in nutritional care but a multicomponent intervention, therefore unable to extract specific effect of nutrition component |
| Forster 2005 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| Gall 1998 | Not a RCT; controlled trial |
| Gariballa 1998 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| Gazzotti 2003 | Not a supportive intervention in nutritional care; intervention continued post hospital discharge, therefore participants would have been given individual advice on taking ONS. |
| Gegerle 1986 | Not a RCT; a dietary survey |
| Gil Gregorio 2003 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given; unclear what the usual care received |
| Goris 2003 | Not a supportive intervention in nutritional care; intervention continued post hospital discharge, therefore participants would have been given individual advice on taking ONS. |
| Hogarth 1996 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| Hopkinson 2010 | Not a supportive intervention in nutritional care; study not aimed at increasing intake as related to psychological/coping mechanisms |
| Houles 2010 | Not a supportive intervention in nutritional care but a multicomponent intervention, therefore unable to extract specific effect of nutrition component |
| Hubbard 2008 | Not a supportive intervention in nutritional care; intervention was on dietary advice vs ONS, so no usual care comparison was given |
| Hubsch 1992 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| Huisman 2012 | Not a supportive intervention in nutritional care; dietary counselling intervention |
| Isenring 2003 | Not a supportive intervention in nutritional care; dietary counselling intervention |
| Isenring 2004 | Not a supportive intervention in nutritional care; dietary counselling intervention |
| Jahnavi 2010 | Not a supportive intervention in nutritional care; individualised intervention |
| James 2006 | Not a supportive intervention in nutritional care; participants consumed ONS at will, intervention not identical for all participants |
| Johnson 1993 | Not a RCT; retrospective case control study |
| Keele 1997 | Not a supportive intervention in nutritional care; intervention continued post hospital discharge, therefore participants would have been given individual advice on taking ONS |
| Kikutani 2006 | Not a supportive intervention in nutritional care; no usual care comparison was described; ONS intervention compared with oral functional training |
| Knowles 1988 | Not a supportive intervention in nutritional care; intervention given to outpatients, therefore participants would have been given individual advice on taking ONS; intervention was tailored and targeted at increasing intake by 50% above normal |
| Krondl 1999 | Not a supportive intervention in nutritional care; intervention given to outpatients, therefore participants would have been given individual advice on taking ONS |
| Kruizenga 2004 | Not a RCT |
| Kuhlmann 1997 | Not a supportive intervention in nutritional care; intervention given to outpatients, therefore participants would have been given individual advice on taking ONS |
| Kwok 2001 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| Kwok 2012 | Not a supportive intervention in nutritional care; examined whether dietary interventions promoting intakes of fruit, vegetable, fish and lower salt, intake were effective in preventing cognitive decline in older people |
| Lauque 2000 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given; intervention not identical for all participants, variety of oral nutritional support offered and dietitian visited sites regularly to direct product distribution and intake, hence likely tailoring |
| Lauque 2004 | Not a supportive intervention in nutritional care; intervention not identical for all participants, variety of ONS offered ranging between 300‐500 kcal therefore likely tailoring |
| Lawson 2000 | Not a RCT |
| Le Cornu 2000 | Not a supportive intervention in nutritional care; intervention given to outpatients, therefore participants would have been given individual advice on taking ONS |
| Lee 2013 | Participants were selected for the intervention after group allocation on the basis of their nutritional status rather than before intervention, or by restricting the inclusion to malnourished participants only |
| Leon 2001 | Not a supportive intervention in nutritional care; individualised intervention |
| Leon 2006 | Not a supportive intervention in nutritional care; individualised intervention |
| Locher 2011 | Not a supportive intervention in nutritional care; dietary advice intervention |
| MacFie 2000 | Not a supportive intervention in nutritional care; intervention given initially to outpatients, therefore participants would have been given individual advice on taking ONS |
| Mamhidir 2007 | Not an RCT |
| Manders 2006 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| McEvoy 1982 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| McMurdo 2009 | Not a supportive intervention in nutritional care; intervention given to participants on discharge from hospital, therefore would have been given individual advice on taking ONS |
| Moretti 2009 | Not a supportive intervention in nutritional care; intervention was given to outpatients, therefore participants would have been given individual advice on taking ONS |
| Navrátilová 2007 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| Nayel 1992 | Not a supportive intervention in nutritional care; ONS tailored/individually prescribed according to requirements (deficit between requirements and intake) |
| Olin 1996 | Not a RCT |
| Otte 1989 | Not a supportive intervention in nutritional care; intervention given to community‐dwelling participants, therefore would have been given individual advice on taking ONS |
| Payette 2002 | Not a supportive intervention in nutritional care; intervention included individualised dietary counselling |
| Price 2005 | Not a supportive intervention in nutritional care; intervention given to participants on discharge from hospital, therefore would have been given individual advice on taking ONS |
| Rana 1992 | Not a supportive intervention in nutritional care; intervention not identical for all participants; participants were allowed to consume ONSat will hence not provided in controlled, routine fashion |
| Richeson & Neil 2004 | Not a RCT; quasi‐experimental time series |
| Roberts 2013 | Not a RCT; the protocol for a controlled trial |
| Robinson 2002 | Not a RCT |
| Rosendahl 2006 | Not supportive intervention in nutritional care; but a multicomponent intervention, therefore unable to extract specific effect of nutrition component |
| Roy 2006 | Not randomised control trial; quasi experimental design with an untreated usual care |
| Rypkema 2004 | Not a RCT |
| Saudny‐Unterberger 1997 | Not supportive intervention in nutritional care; oral nutritional support tailored to nutritional requirements |
| Shinnar 1983 | Not a RCT; observational study |
| Simmons 2004 | Not a RCT; participants allocated according to ability to respond to individualised assistance |
| Smedley 2004 | Not a supportive intervention in nutritional care; intervention not the same for all participants; participants encouraged to consume oral nutritional supplements at will hence not provided in controlled, routine fashion |
| Somanchi 2011 | Not a RCT |
| Soneff 1994 | Not a supportive intervention in nutritional care; outcomes reported at facility level, not participant level |
| Southgate 2010 | Not a supportive intervention in nutritional care; personalised dietetic intervention |
| Starke 2011 | Not a supportive intervention in nutritional care; individualised intervention |
| Stauffer 1986 | Not a RCT: a prospective observational study |
| Steiner 2003 | Not a supportive intervention in nutritional care; intervention was given to outpatients, therefore participants would have been given individual advice on taking ONS |
| Stotts 2009 | Not a supportive intervention in nutritional care; intervention involved administration of supplemental fluid |
| Teixido‐Planas 2005 | Not a supportive intervention in nutritional care; intervention was given to outpatients, therefore participants would have been given individual advice on taking ONS |
| Tkatch 1992 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| Vetter 1992 | Not a supportive intervention in nutritional care; multicomponent intervention; difficult to extract specific effect of nutrition component; included dietary advice |
| Vlaming 2001 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| Watanabe 2010 | Not a RCT; appears to be a matched cohort |
| Williams 1989 | Not a RCT |
| Wong 2010 | Not a RCT |
| Woo 1994 | Not a supportive intervention in nutritional care; intervention was given on hospital discharge, therefore participants would have been given individual advice on taking ONS |
| Wouters‐Wesseling 2002 | Not a supportive intervention in nutritional care; intervention was described, however no clear organisational component to the intervention was given |
| Wright 2006 | Not a RCT; quasi‐experimental |
| WY Lin 2010 | Not a supportive intervention in nutritional care; multicomponent intervention; difficult to extract specific effect of nutrition component; the presence of a dietitian in the multidiciplinary team was the only difference between the two groups |
| Yamaguchi 1998 | Not a supportive intervention in nutritional care; intervention was given to outpatients, therefore participants would have been given individual advice on taking ONS |
| Young 2004 | Not a RCT |
| Ödlund Olin 2003 | Not a RCT |
ONS: oral nutritional supplement; RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Allen 2014.
| Methods | RCT |
| Participants | Participants with long‐standing cognitive impairment in hospital or living in a residential care home |
| Interventions | Oral nutritional supplement drink provided 3 times a day in a glass/beaker or consumed through a straw inserted directly into the container |
| Outcomes | Amount of nutritional supplement drink consumed |
| Notes | Full data extraction has not yet been undertaken and will be completed at the next update |
Borges 2003.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Requires translation, unable to locate abstract |
Burns 1998.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Requires translation, unable to locate abstract |
Deutz 2016.
| Methods | Randomised, placebo‐controlled, double‐blind trial |
| Participants | Older (> 65 years), malnourished adults hospitalised for congestive heart failure, acute myocardial infarction, pneumonia or chronic obstructive pulmonary disease |
| Interventions | Standard‐of‐care plus a high‐protein oral nutritional supplement or a placebo supplement |
| Outcomes | Primary composite endpoint: 90‐day postdischarge incidence of death or nonelective readmission; other endpoints: 30‐ and 60‐day postdischarge incidence of death or readmission, length of stay, malnourishment class ()SGA, body weight, and ADL |
| Notes | Full data extraction has not yet been undertaken and will be completed at the next update |
Ekinci 2016.
| Methods | RCT |
| Participants | Older female participants with a hip fracture |
| Interventions | The intervention group received an enteral product containing 3 g calcium beta‐hydroxy‐beta‐methylbutyrate, 1000 IU vitamin D and 36 g protein, in addition to standard postoperative nutrition. The control group received standard postoperative nutrition |
| Outcomes | Wound‐healing period, shortening of immobilisation period, muscle strength, BMI |
| Notes | Full data extraction has not yet been undertaken and will be completed at the next update |
ISRCTN04327195.
| Methods | RCT |
| Participants | Undernourished geriatric inpatients |
| Interventions | Intervention group: energy dense, small volume oral nutritional supplements; control group: fortified foods |
| Outcomes | Primary outcome measure: number of participants achieving an extra intake of 450 kcal per day; secondary outcome measures: recommended energy and protein intakes, length of hospital stay, antibiotic usage |
| Notes | Retrospectively registered; trial end date: 15 May 2010 |
ISRCTN96923961.
| Methods | RCT |
| Participants | Malnutrition in the elderly |
| Interventions | Standard dietary care versus a high‐energy supplement versus a high‐energy supplement plus micronutrients |
| Outcomes | Primary outcome measure: nutrient intake; secondary outcome measures: gastro‐intestinal tolerance, product compliance, appetite, anthropometry (weight and BMI), muscle function, measured by hand grip dynamometry, quality of life, measured using EuroQol EQ‐5D questionnaire, blood lipids and micronutrients, safety, falls assessment measured using Berg Balance Scale |
| Notes | Retrospectively registered; trial end date: 30 December 2007 |
Jobse 2015.
| Methods | RCT |
| Participants | Nursing home residents with malnutrition or at risk of malnutrition |
| Interventions | Intervention group received 2 x 125 mL oral nutritional supplements for 12 weeks, and the control group received usual care |
| Outcomes | Body weight change, BMI, upper arm and calf‐circumferences, MNA score |
| Notes | Full data extraction has not yet been undertaken and will be completed at the next update |
Lee 2015.
| Methods | RCT |
| Participants | Older people living in a nursing home |
| Interventions | Each participant in the intervention group received a 50 g/day soy‐protein‐based nutritional supplement when he/she was rated as undernourished; all participants including those who were in the control group received the same normal meals and a light afternoon snack daily |
| Outcomes | Handgrip strength, Barthel index, anthropometric and biochemical indicators |
| Notes | Full data extraction has not yet been undertaken and will be completed at the next update |
Leslie 2013.
| Methods | Cluster‐randomised trial in 21 residential care homes |
| Participants | Undernourished residents with a BMI <18.5 kg/m2 |
| Interventions | Enrichment of meals to increase energy density |
| Outcomes | Nutritional intake, body weight, MUAC, BMI, mortality |
| Notes | Full data extraction has not yet been undertaken and will be completed at the next update |
Luna‐Ramos 2016.
| Methods | RCT |
| Participants | Elderly fragile, hospitalised participants |
| Interventions | Polymeric diet versus standard diet |
| Outcomes | Nutritional status, BMI, body weight |
| Notes | Full data extraction has not yet been undertaken and will be completed at the next update |
Madigan 1994.
| Methods | Unclear |
| Participants | Elderly participants with fractured neck of femur |
| Interventions | Oral feed with protein and energy vs normal ward diet, followed up for 3 months post hospital discharge |
| Outcomes | Mortality, length of hospital stay, postoperative functional status, dietary intake, compliance |
| Notes | Unable to locate dissertation |
Moore 2010.
| Methods | RCT |
| Participants | Older people with dementia living in a residential care home and an assisted living facility |
| Interventions | A 25‐min activity offered 30 min before meal times (aiming to reduce apathy and agitation and to increase eating ability and intake |
| Outcomes | Apathy, agitation, eating ability, dietary intake |
| Notes | Full data extraction has not yet been undertaken and will be completed at the next update |
Parsons 2016.
| Methods | RCT |
| Participants | Malnourished, care home residents |
| Interventions | Oral nutritional supplements or dietary advice |
| Outcomes | Health‐related quality of life, nutritional intake |
| Notes | Full data extraction has not yet been undertaken and will be completed at the next update |
Pouyssegur 2015.
| Methods | A multicentre RCT |
| Participants | Malnourished older adults living in nursing homes |
| Interventions | In addition to usual meals, the provision of eight cookies (30 kcals and 1.44 g protein) throughout the day |
| Outcomes | Body weight, appetite, occurrence of pressure ulcers, diarrhoea |
| Notes | Full data extraction has not yet been undertaken and will be completed at the next update |
Scorer 1990.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Unable to locate paper |
Simmons 2013.
| Methods | RCT |
| Participants | People living in residential care homes |
| Interventions | Staff training to improve feeding assistance |
| Outcomes | Mealtime feeding assistance, body weight |
| Notes | Full data extraction has not yet been undertaken and will be completed at the next update |
Simmons 2015.
| Methods | RCT |
| Participants | Long‐stay residents with orders for nutrition supplementation |
| Interventions | Usual care control group verus an oral liquid nutrition supplement intervention group, or a snack intervention group |
| Outcomes | Body weight, food, beverage and supplement intake and the amount of staff time spent providing assistance, cost‐effectiveness |
| Notes | Full data extraction has not yet been undertaken and will be completed at the next update |
Stelten 2015.
| Methods | Single‐blind RCT |
| Participants | Acutely ill elderly participants admitted to hospital |
| Interventions | Protein‐enriched bread and drinking yoghourt |
| Outcomes | Protein intake |
| Notes | Full data extraction has not yet been undertaken and will be completed at the next update |
Stow 2015.
| Methods | Cluster‐RCT |
| Participants | Care home residents with or at risk of malnutrition |
| Interventions | Standard care, food‐based intervention or oral nutritional supplement intervention |
| Outcomes | Anthropometry, dietary intake, healthcare resource usage and participant‐reported outcome measures |
| Notes | Registered trial: ISRCTN38047922 Full data extraction has not yet been undertaken and will be completed at the next update |
Sutton 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Unable to locate paper |
Turano 1999.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Requires translation |
White 1999.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Unable to locate paper |
Zhong 2016.
| Methods | RCT and economic evaluation |
| Participants | Malnourished older hospitalised participants |
| Interventions | Nutrient‐dense ONS, containing high protein and beta‐hydroxy‐beta‐methylbutyrate versus placebo |
| Outcomes | Health‐care costs, measured as the product of resource use and per unit cost, quality‐adjusted life‐years (QALYs), life‐years saved and the incremental cost‐effectiveness ratio |
| Notes | Full data extraction has not yet been undertaken and will be completed at the next update |
ADL: activities of daily living; BMI: body mass index; MNA: Mini Nutritional Assessment; MUAC: mid upper‐arm circumference; ONS: oral nutritional supplement; SGA: subjective global assessment
Differences between protocol and review
Katherine Kimber began work on this review after publication of the protocol. At the protocol stage it was anticipated that searching of Greynet would be undertaken but this was not done and so the sections on electronic searching and searching other resources have been amended.
Since the publication of the protocol of this review and the final review draft a considerable time has elapsed which demanded a number of changes to the protocol such as specification of a number of additional secondary outcomes (which are mandatory within the CMED Group), specification of outcomes for the 'Summary of findings' table and specification of timing of outcome measurement. Also the updated search strategy was focused on major databases and differed slightly from the older versions mainly due to changes in the database structure over time.
We could not investigate a number of prespecified subgroup and sensitivity analyses because of lack of data. Also, cross‐over trials did not contribute to the effect estimates established by meta‐analyses because data were not available from baseline to the end of phase 1 of the cross‐over trials to be included in meta‐analyses.
Contributions of authors
All authors have read, commented and contributed to the preparation of review manuscripts.
Michelle Gibbs (MG): protocol draft, search strategy development, acquisition of copies of trials, trial selection, data extraction, and future review updates.
Katherine Kimber (KK): trial selection, data extraction, data analyses, data interpretation, and future review updates.
Christine Baldwin (CB): protocol draft, trial selection, data extraction, data analysis, data interpretation and completed revision of the review following peer review, and future review updates.
Christine Elizabeth Weekes (CEW): protocol draft, trial selection, data extraction, data analysis, data interpretation and completed revision of the review following peer review, and future review updates.
Sources of support
Internal sources
No sources of support supplied
External sources
-
British Dietetic Association, UK.
This review was part funded by a grant from the British Dietetic Association.
Declarations of interest
Michelle Gibbs: this work was financially supported by a grant from the British Dietetic Association.
Katherine Kimber: none known.
Christine Baldwin: some of the early work on this review was funded by an educational grant from the British Dietetic Association. The grant was used to support the salary of two research assistants who contributed to the searching, study selection and writing of the review.
Christine Elizabeth Weekes: none known.
New
References
References to studies included in this review
Barton 2000 {published data only}
- Barton AD, Beigg CL, Macdonald IA, Allison SP. A recipe for improving food intakes in elderly hospitalized patients. Clinical Nutrition 2000;19(6):451‐4. [DOI] [PubMed] [Google Scholar]
Beck 2002 {published data only}
- Beck AM, Ovesen L, Schroll M. Home‐made oral supplement as nutritional support of old nursing home residents, who are undernourished or at risk of undernutrition based on the MNA. A pilot trial. Mini Nutritional Assessment. Aging Clinical and Experimental Research 2002;14(3):212‐5. [DOI] [PubMed] [Google Scholar]
Bouillanne 2013 {published data only}
- Bouillanne O, Curis E, Hamon‐Vilcot B, Nicolis I, Chretien P, Schauer N, et al. Impact of protein pulse feeding on lean mass in malnourished and at‐risk hospitalized elderly patients: a randomized controlled trial. Clinical Nutrition 2013;32(2):186‐92. [DOI] [PubMed] [Google Scholar]
- Bouillanne O, Neveux N, Nicolis I, Curis E, Cynober L, Aussel C. Long‐lasting improved amino acid bioavailability associated with protein pulse feeding in hospitalized elderly patients: a randomized controlled trial. Nutrition 2014;30(5):544‐50. [PUBMED: 24355438] [DOI] [PubMed] [Google Scholar]
Bourdel‐Marchasson 2000 {published data only}
- Bourdel‐Marchasson I, Barateau M, Rondeau V, Dequae‐Merchadou L, Salles‐Montaudon N, Emeriau J, et al. A multi‐center trial of the effects of oral nutritional supplementation in critically ill older inpatients. Nutrition 2000;16(1):1‐5. [DOI] [PubMed] [Google Scholar]
Brouillette 1991 {published data only}
- Brouillette ME, White LW. The effects of olfactory stimulation on the appetites of nursing home residents. Physical & Occupational Therapy in Geriatrics 1991;10(1):1‐13. [Google Scholar]
Castellanos 2009 {published data only}
- Castellanos VH, Marra MV, Johnson P. Enhancement of select foods at breakfast and lunch increases energy intakes of nursing home residents with low meal intakes. Journal of the American Dietetic Association 2009;109(3):445‐51. [DOI] [PubMed] [Google Scholar]
Chang 2005 {published data only}
- Chang CC, Lin LC. Effects of a feeding skills training programme on nursing assistants and dementia patients. Journal of Clinical Nursing 2005;14(10):1185‐92. [DOI] [PubMed] [Google Scholar]
Dennis 2005 {published data only}
- Dennis M. Routine oral nutritional supplementation for stroke patients in hospital (FOOD): A multicentre randomised controlled trial. Lancet 2005;365(9461):755‐63. [DOI] [PubMed] [Google Scholar]
Duncan 2006 {published data only}
- Duncan DG, Beck SJ, Hood K, Johansen A. Using dietetic assistants to improve the outcome of hip fracture: a randomised controlled trial of nutritional support in an acute trauma ward. Age and Ageing 2006;35(2):148‐53. [DOI] [PubMed] [Google Scholar]
Essed 2007 {published data only}
- Essed NH, Staveren WA, Kok FJ, Graaf C. No effect of 16 weeks flavor enhancement on dietary intake and nutritional status of nursing home elderly. Appetite 2007;48(1):29‐36. [DOI] [PubMed] [Google Scholar]
Essed 2009 {published data only}
- Essed NH, Oerlemans P, Hoek M, Staveren WA, Kok FJ, Graaf C. Optimal preferred MSG concentration in potatoes, spinach and beef and their effect on intake in institutionalized elderly people. Journal of Nutrition, Health & Aging 2009;13(9):769‐75. [DOI] [PubMed] [Google Scholar]
Faxen‐Irving 2011 {published data only}
- Faxen‐Irving G, Cederholm T. Energy dense oleic acid rich formula to newly admitted geriatric patients‐‐feasibility and effects on energy intake. Clinical Nutrition 2011;30(2):202‐8. [DOI] [PubMed] [Google Scholar]
Gaskill 2009 {published data only}
- Gaskill D, Isenring EA, Black LJ, Hassall S, Bauer JD. Maintaining nutrition in aged care residents with a train‐the‐trainer intervention and nutrition coordinator. Journal of Nutrition, Health & Aging 2009;13(10):913‐7. [DOI] [PubMed] [Google Scholar]
Germain 2006 {published data only}
- Germain I, Dufresne T, Gray‐Donald K. A novel dysphagia diet improves the nutrient intake of institutionalized elders. Journal of the American Dietetic Association 2006;106(10):1614‐23. [DOI] [PubMed] [Google Scholar]
Hankey 1993 {published data only}
- Hankey CR, Summerbell J, Wynne HA. The effect of dietary supplementation in continuing‐care elderly people: nutritional, anthropometric and biochemical parameters. Journal of Human Nutrition and Dietetics 1993;6(4):317‐22. [Google Scholar]
Hickson 2004 {published data only}
- Edington J, Barnes R, Bryan F, Dupree E, Frost G, Hickson M, et al. A prospective randomised controlled trial of nutritional supplementation in malnourished elderly in the community: clinical and health economic outcomes. Clinical Nutrition 2004;23(2):195‐204. [PUBMED: 15030959] [DOI] [PubMed] [Google Scholar]
- Hickson M, Bulpitt C, Nunes M, Peters R, Cooke J, Nicholl C, Frost G. Does additional feeding support provided by health care assistants improve nutritional status and outcome in acutely ill older in‐patients? ‐ a randomised control trial. Clinical Nutrition 2004;23(1):69‐77. [DOI] [PubMed] [Google Scholar]
- Hickson M, Frost G. An investigation into the relationships between quality of life, nutritional status and physical function. Clinical Nutrition 2004;23:213‐21. [DOI] [PubMed] [Google Scholar]
- Hickson M, Nicholl C, Bulpitt C, Fry M, Frost G, Davies L. The design of the feeding support trial ‐ does intensive feeding support improved nutritional status and outcome in older in‐patients?. Journal of Human Nutrition & Dietetics 1999;12:53‐59. [Google Scholar]
Holyday 2012 {published data only}
- Holyday M, Daniells S, Bare M, Caplan GA, Petocz P, Bolin T. Malnutrition screening and early nutrition intervention in hospitalised patients in acute aged care: a randomised controlled trial. Journal of Nutrition, Health & Aging 2012;16(6):562‐8. [DOI] [PubMed] [Google Scholar]
Johansen 2004 {published data only}
- Johansen N, Kondrup J, Plum LM, Bak L, Norregaard P, Bunch E, et al. Effect of nutritional support on clinical outcome in patients at nutritional risk. Clinical Nutrition 2004;23(4):539‐50. [DOI] [PubMed] [Google Scholar]
Kraft 2012 {published data only}
- Kraft M, Berg N, Kraft K, Schmekel S, Gartner S, Kruger J, et al. Development of a telemedical monitoring concept for the care of malnourished geriatric home‐dwelling patients: a pilot study. Maturitas 2012;72(2):126‐31. [DOI] [PubMed] [Google Scholar]
Kretser 2003 {published data only}
- Kretser AJ, Voss T, Kerr WW, Cavadini C, Friedmann J. Effects of two models of nutritional intervention on homebound older adults at nutritional risk. Journal of the American Dietetic Association 2003;103(3):329‐36. [DOI] [PubMed] [Google Scholar]
Larsson 1990 {published data only}
- Ek AC, Unosson M, Larsson J, Schenck H, Bjurulf P. The development and healing of pressure sores related to the nutritional state. Clinical Nutrition 1991;10(5):245‐50. [DOI] [PubMed] [Google Scholar]
- Larsson J, Unosson M, Ek AC, Nilsson L, Thorslund S, Bjurulf P. Effect of dietary supplement on nutritional status and clinical outcome in 501 geriatric patients ‐ a randomised study. Clinical Nutrition 1990;9(4):179‐84. [DOI] [PubMed] [Google Scholar]
- Unosson M, Larsson J, Ek AC, Bjurulf P. Effects of dietary supplement on functional condition and clinical outcome measured with a modified Norton scale. Clinical Nutrition 1992;11(3):134‐9. [DOI] [PubMed] [Google Scholar]
Leslie 2012 {published data only}
- Leslie WS, Woodward M, Lean MEJ, Theobald H, Watson L, Hankey CR. Improving the dietary intake of under nourished older people in residential care homes using an energy‐enriching food approach: a cluster randomised controlled study. Journal of Human Nutrition and Dietetics 2012;26:387‐394. [DOI] [PubMed] [Google Scholar]
Lin 2010 {published data only}
- Lin L‐C, Huang Y‐J, Su S‐G, Watson R, Tsai BWJ, Wu S‐C. Using spaced retrieval and Montessori‐based activities in improving eating ability for residents with dementia. International Journal of Geriatric Psychiatry 2010;25(10):953‐9. [DOI] [PubMed] [Google Scholar]
Lin 2011 {published data only}
- Lin L‐C, Huang Y‐J, Watson R, Wu S‐C, Lee Y‐C. Using a Montessori method to increase eating ability for institutionalised residents with dementia: a crossover design. Journal of Clinical Nursing 2011;20(21):3092. [DOI] [PubMed] [Google Scholar]
Mathey 2001a {published data only}
- Mathey MFAM, Vanneste VGG, Graaf C, Groot LCPGM, Staveren WA. Health effect of improved meal ambiance in a Dutch nursing home: A 1‐year intervention study. Preventive Medicine 2001;32(5):416‐23. [DOI] [PubMed] [Google Scholar]
Mathey 2001b {published data only}
- Mathey M‐FAM, Siebelink Els, Graaf C, Staveren WA. Flavor enhancement of food improves dietary intake and nutritional status of elderly nursing home residents. Journal of Gerontology: Medical Sciences 2001;56A(4):M200‐M205. [DOI] [PubMed] [Google Scholar]
Munk 2014 {published data only}
- Munk T, Beck AM, Holst M, Rosenbom E, Rasmussen HH, Nielsen MA, et al. Positive effect of protein‐supplemented hospital food on protein intake in patients at nutritional risk: a randomised controlled trial. Journal of Human Nutrition and Dietetics 2014;27:122‐32. [DOI] [PubMed] [Google Scholar]
Nijs 2006 {published data only}
- Nijs K, Graaf C, Kok F. Effect of family style mealtimes on quality of life, physical performance, and body weight of nursing home residents: cluster randomised controlled trial. BMJ 2006;332(7551):1180‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijs KAND, Graaf C, Siebelink E, Blauw YH, Vannest V, Kok FJ, et al. Effect of family‐style meals on energy intake and risk of malnutrition in Dutch nursing home residents: a randomised controlled trial. Journal of Gerontology 2006;61A(9):935‐42. [DOI] [PubMed] [Google Scholar]
Olofsson 2007 {published data only}
- Olofsson B, Stenvall M, Lundstrom M, Svensson O, Gustafson Y. Malnutrition in hip fracture patients: an intervention study. Journal of Clinical Nursing 2007;16(11):2027‐38. [DOI] [PubMed] [Google Scholar]
Pivi 2011 {published data only}
- Pivi GAK, Silva RV, Juliano Y, Novo NF, Okamoto IH, Brant CQ, et al. A prospective study of nutrition education and oral nutritional supplementation in patients with Alzheimer's disease. Nutrition Journal 2011;10(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Potter 2001 {published data only}
- Potter JM, Roberts MA, McColl JH, Reilly JJ. Protein energy supplements in unwell elderly patients ‐ a randomized controlled trial. JPEN Journal of Parenteral and Enteral Nutrition 2001;25(6):323‐9. [DOI] [PubMed] [Google Scholar]
- Potter JM, Roberts MA, Reilly JJ, McColl JH. An evaluation of protein energy supplementation in medically ill admissions to a geriatric unit. Proceedings of the Nutrition Society 1998;3:88A. [Google Scholar]
- Roberts M, Potter J, McColl J, Reilly J. Can prescription of sip‐feed supplements increase energy intake in hospitalised older people with medical problems?. British Journal of Nutrition 2003;90(2):425‐9. [DOI] [PubMed] [Google Scholar]
Remsburg 2001 {published data only}
- Remsburg RE, Luking A, Bara P, Radu C, Pineda D, Bennett RG, et al. Impact of a buffet‐style dining program on weight and biochemical indicators of nutritional status in nursing home residents: a pilot study. Journal of the American Dietetic Association 2001;101(12):1460‐3. [DOI] [PubMed] [Google Scholar]
Salva 2011 {published data only}
- Roqué M, Salvà A, Vellas B. Malnutrition in community‐dwelling adults with dementia (NutriAlz Trial). Journal of Nutrition, Health & Aging 2013;17(4):295‐9. [PUBMED: 23538648] [DOI] [PubMed] [Google Scholar]
- Salva A, Andrieu S, Fernandez E, Schiffrin EJ, Moulin J, Decarli B, et al. Health and nutrition promotion program for patients with dementia (NutriAlz): cluster randomized trial. Journal of Nutrition Health & Aging 2011;15(10):822‐30. [DOI] [PubMed] [Google Scholar]
Silver 2008 {published data only}
- Silver HJ, Dietrich MS, Castellanos VH. Increased energy density of the home‐delivered lunch meal improves 24‐hour nutrient intakes in older adults. Journal of the American Dietetic Association 2008;108(12):2084‐9. [DOI] [PubMed] [Google Scholar]
Simmons 2008 {published data only}
- Simmons SF, Keeler E, Zhuo X, Hickey KA, Sato H‐W, Schnelle JF. Prevention of unintentional weight loss in nursing home residents: a controlled trial of feeding assistance. Journal of the American Geriatrics Society 2008;56(8):1466‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simmons 2010 {published data only}
- Simmons SF, Zhuo X, Keeler E. Cost‐effectiveness of nutrition interventions in nursing home residents: a pilot intervention. Journal of Nutrition Health & Aging 2010;14(5):367‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smoliner 2008 {published data only}
- Smoliner C, Norman K, Scheufele R, Hartig W, Pirlich M, Lochs H. Effects of food fortification on nutritional and functional status in frail elderly nursing home residents at risk of malnutrition. Nutrition 2008;24(11‐12):1139‐44. [DOI] [PubMed] [Google Scholar]
Splett 2003 {published data only}
- Splett PL, Roth‐Yousey LL, Vogelzang JL. Medical nutrition therapy for the prevention and treatment of unintentional weight loss in residential healthcare facilities. Journal of the American Dietetic Association 2003;103(3):352‐62. [DOI] [PubMed] [Google Scholar]
Taylor 2006 {published data only}
- Taylor KA, Barr SI. Provision of small, frequent meals does not improve energy intake of elderly residents with dysphagia who live in an extended‐care facility. Journal of the American Dietetic Association 2006;106(7):1115‐8. [DOI] [PubMed] [Google Scholar]
Van den Berg 2015 {published data only}
- Berg GH, Lindeboom R, Zwet WC. The effects of the administration of oral nutritional supplementation with medication rounds on the achievement of nutritional goals: a randomised controlled trial. Clinical Nutrition (Edinburgh, Scotland) 2015;34:15‐19. [DOI] [PubMed] [Google Scholar]
Van Ort 1995 {published data only}
- Ort S, Phillips LR. Nursing interventions to promote functional feeding. Journal of Gerontological Nursing 1995;10:6‐14. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aleman‐Mateo 2012 {published data only}
- Aleman‐Mateo H, Macias L, Esparza‐Romero J, Astiazaran‐Garcia H, Blancas AL. Physiological effects beyond the significant gain in muscle mass in sarcopenic elderly men: evidence from a randomized clinical trial using a protein‐rich food. Clinical Interventions in Aging 2012;7:225‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allman 1990 {published data only}
- Allman MA, Stewart PM, Tiller DJ, Horvath JS, Duggin GG, Truswell AS. Energy supplementation and the nutritional status of hemodialysis patients. American Journal of Clinical Nutrition 1990;51(4):558‐62. [DOI] [PubMed] [Google Scholar]
Arias 2008 {published data only}
- Arias S, Bruzzone I, Blanco V, Inchausti M, Garcia F, Casavieja G, et al. Identification and early nutritional support in hospitalized malnourished patients. Nutrición Hospitalaria 2008;23(4):348‐53. [PubMed] [Google Scholar]
Asplund 2000 {published data only}
- Asplund K, Gustafson Y, Jacobsson C, Bucht G, Wahlin A, Peterson J, et al. Geriatric‐based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources. Journal of the American Geriatrics Society 2000;48(11):1381‐8. [DOI] [PubMed] [Google Scholar]
Baldwin 2011 {published data only}
- Baldwin C, Spiro A, McGough C, Norman AR, Gillbanks A, Thomas K, et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non‐small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. Journal of Human Nutrition & Dietetics 2011;24(5):431‐40. [DOI] [PubMed] [Google Scholar]
Banerjee 1978 {published data only}
- Banerjee AK, Brocklehurst JC, Wainwright H, Swindell R. Nutritional status of long‐stay geriatric in‐patients: effects of a food supplement (Complan). Age and Ageing 1978;7(4):237‐43. [DOI] [PubMed] [Google Scholar]
Bauer 2005 {published data only}
- Bauer J, Capra S, Battistutta D, Davidson W, Ash S, Cancer Cachexia Study G. Compliance with nutrition prescription improves outcomes in patients with unresectable pancreatic cancer. Clinical Nutrition 2005;24(6):998‐1004. [DOI] [PubMed] [Google Scholar]
Beattie 2000 {published data only}
- Beattie AH, Prach AT, Baxter JP, Pennington CR. A randomised controlled trial evaluating the use of enteral nutritional supplements postoperatively in malnourished surgical patients. Gut 2000;46(6):813‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 2008 {published data only}
- Beck AM, Damkjaer K, Beyer N. Multifaceted nutritional intervention among nursing‐home residents has a positive influence on nutrition and function. Nutrition 2008;24(11‐12):1073‐80. [DOI] [PubMed] [Google Scholar]
- Beck AM, Damkjaer K, Sorbye LW. Physical and social functional abilities seem to be maintained by a multifaceted randomized controlled nutritional intervention among old (>65 years) Danish nursing home residents. Archives of Gerontology and Geriatrics 2010;50(3):351‐5. [DOI] [PubMed] [Google Scholar]
- Beck AM, Damkjaer K, Tetens I. Lack of compliance of staff in an intervention study with focus on nutrition, exercise and oral care among old (65+ yrs) Danish nursing home residents. Aging Clinical and Experimental Research 2009;21(2):143‐9. [DOI] [PubMed] [Google Scholar]
Benati 2001 {published data only}
- Benati G, Delvecchio S, Cilla D, Pedone V. Impact on pressure ulcer healing of an arginine‐enriched nutritional solution in patients with severe cognitive impairment. Archives of Gerontology and Geriatrics 2001;33(Supp 7):43‐7. [DOI] [PubMed] [Google Scholar]
Bonjour 2011 {published data only}
- Bonjour JP, Benoit V, Pourchaire O, Rousseau B, Souberbielle JC. Nutritional approach for inhibiting bone resorption in institutionalized elderly women with vitamin D insufficiency and high prevalence of fracture. Journal of Nutrition, Health & Aging 2011;15(5):404‐9. [DOI] [PubMed] [Google Scholar]
Bonjour 2012 {published data only}
- Bonjour JP, Benoit V, Rousseau B, Souberbielle JC. Consumption of vitamin D‐and calcium‐fortified soft white cheese lowers the biochemical marker of bone resorption TRAP 5b in postmenopausal women at moderate risk of osteoporosis fracture. Journal of Nutrition 2012;142(4):698‐703. [DOI] [PubMed] [Google Scholar]
Bonnefoy 2003 {published data only}
- Bonnefoy M, Cornu C, Normand S, Boutitie F, Bugnard F, Rahmani A, et al. The effects of exercise and protein‐energy supplements on body composition and muscle function in frail elderly individuals: a long‐term controlled randomised study. British Journal of Nutrition 2003;89(5):731‐9. [DOI] [PubMed] [Google Scholar]
Bos 2001 {published data only}
- Bos C, Benamouzig R, Bruhat A, Roux C, Valensi P, Ferriere F, et al. Nutritional status after short‐term dietary supplementation in hospitalized malnourished geriatric patients. Clinical Nutrition 2001;20(3):225‐33. [DOI] [PubMed] [Google Scholar]
Botella‐Carretero 2008 {published data only}
- Botella‐Carretero JI, Iglesias B, Balsa JA, Zamarron I, Arrieta F, Vazquez C. Effects of oral nutritional supplements in normally nourished or mildly undernourished geriatric patients after surgery for hip fracture: a randomized clinical trial. JPEN Journal of Parenteral & Enteral Nutrition 2008;32(2):120‐8. [DOI] [PubMed] [Google Scholar]
Botella‐Carretero 2010 {published data only}
- Botella‐Carretero JI, Iglesias B, Balsa JA, Arrieta F, Zamarron I, Vazquez C. Perioperative oral nutritional supplements in normally or mildly undernourished geriatric patients submitted to surgery for hip fracture: a randomized clinical trial. Clinical Nutrition 2010;29(5):574‐9. [DOI] [PubMed] [Google Scholar]
Boudville 2003 {published data only}
- Boudville N, Rangan A, Moody H. Oral nutritional supplementation increases caloric and protein intake in peritoneal dialysis patients. American Journal of Kidney Diseases 2003;41(3):658‐63. [DOI] [PubMed] [Google Scholar]
Bunout 1989 {published data only}
- Bunout D, Aicardi V, Hirsch S, Petermann M, Kelly M, Silva G, et al. Nutritional support in hospitalized patients with alcoholic liver disease. European Journal of Clinical Nutrition 1989;43(9):615‐21. [PubMed] [Google Scholar]
Bunout 2001 {published data only}
- Bunout B, Barrera G, Maza P, Avendano M, Gattas V, Petermann M, et al. Effects of nutritional supplementation and resistance training on muscle strength in free living elders. Results of one year follow.. Journal of Nutrition, Health & Aging 2004;8(2):68‐75. [PubMed] [Google Scholar]
- Bunout D, Barrera G, Maza P, Avendano M, Gattas V, Petermann M, et al. The impact of nutritional supplementation and resistance training on the health functioning of free‐living Chilean elders: results of 18 months of follow‐up. Journal of Nutrition 2001;131(9):2441S‐6S. [DOI] [PubMed] [Google Scholar]
Carlsson 2011 {published data only}
- Carlsson M, Littbrand H, Gustafson Y, Lundin‐Olsson L, Lindelof N, Rosendahl E, et al. Effects of high‐intensity exercise and protein supplement on muscle mass in ADL dependent older people with and without malnutrition‐A randomized controlled trial. Journal of Nutrition, Health & Aging 2011;15(7):554‐60. [DOI] [PubMed] [Google Scholar]
Carnaby 2006 {published data only}
- Carnaby G, Hankey GJ, Pizzi J. Behavioural intervention for dysphagia in acute stroke: a randomised controlled trial. Lancet Neurology 2006;5(1):31‐7. [DOI] [PubMed] [Google Scholar]
Charlin 2002 {published data only}
- Charlin V, Carrasco F, Sepulveda C, Torres M, Kehr J. Nutritional supplementation according to energy and protein requirements in malnourished HIV‐infected patients. Archivos Latinoamericanos de Nutricion 2002;52(3):267‐73. [PubMed] [Google Scholar]
Charras 2010 {published data only}
- Charras K, Fremontier M. Sharing meals with institutionalized people with dementia: a natural experiment. Journal of Gerontological Social Work 2010;53(5):436‐48. [DOI] [PubMed] [Google Scholar]
Chernoff 1990 {published data only}
- Chernoff RS, Milton KY, Lipschitz DA. The effect of a very high‐protein liquid formula on decubitus ulcers healing in long‐term tube‐fed institutionalized patients. Journal of the American Dietetic Association 1990;90:A–130. [Google Scholar]
Chin 2001 {published data only}
- Chin APMJM, Jong N, Schouten EG, Hiddink GJ, Kok FJ. Physical exercise and/or enriched foods for functional improvement in frail, independently living elderly: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2001;82(6):811‐7. [DOI] [PubMed] [Google Scholar]
Collins 2005 {published data only}
- Collins CE, Kershaw J, Brockington S. Effect of nutritional supplements on wound healing in home‐nursed elderly: a randomized trial. Nutrition 2005;21(2):147‐55. [DOI] [PubMed] [Google Scholar]
Dangour 2011 {published data only}
- Dangour AD, Albala C, Adeo C, Elbourne D, Grundy E, Walker D, et al. A factorial‐design cluster randomised controlled trial investigating the cost‐effectiveness of a nutrition supplement and an exercise programme on pneumonia incidence, walking capacity and body mass index in older people living in Santiago, Chile: the CENEX study protocol. Nutrition Journal 2007;6(14):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangour AD, Albala C, Allen E, Grundy E, Walker DG, Aedo C, et al. Effect of a nutrition supplement and physical activity program on pneumonia and walking capacity inChilean older people: a factorial cluster randomized trial. PLoS Medicine 2011;8(4):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Jong 1999 {published data only}
- Jong N, Chin A Paw MJ, Graaf C, Staveren WA. Effect of dietary supplements and physical exercise on sensory perception, appetite, dietary intake and body weight in frail elderly subjects. British Journal of Nutrition 2000;83(6):605‐13. [DOI] [PubMed] [Google Scholar]
- Jong N, Chin A Paw MJ, Groot LC, Graaf C, Kok FJ, et al. Functional biochemical and nutrient indices in frail elderly people are partly affected by dietary supplements but not by exercise. Journal of Nutrition 1999;129(11):2028‐36. [DOI] [PubMed] [Google Scholar]
- Jong N, Chin A Paw MJ, Groot LC, Hiddink GJ, Staveren WA. Dietary supplements and physical exercise affecting bone and body composition in frail elderly persons. American Journal of Public Health 2000;90(6):947‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delmi 1990 {published data only}
- Delmi M, Rapin CH, Bengoa JM, Delmas PD, Vasey H, Bonjour JP. Dietary supplementation in elderly patients with fractured neck of the femur. Lancet 1990;335(8696):1013‐6. [DOI] [PubMed] [Google Scholar]
de Sousa 2012 {published data only}
- Sousa OLV, Amaral TF. Three‐week nutritional supplementation effect on long‐term nutritional status of patients with mild Alzheimer disease. Alzheimer Disease and Associated Disorders 2012;26(2):119‐23. [DOI] [PubMed] [Google Scholar]
Dhanraj 1997 {published data only}
- Dhanraj P, Chacko A, Mammen M, Bharathi R. Hospital‐made diet versus commercial supplement in postburn nutritional support. Burns 1997;23(6):512‐4. [DOI] [PubMed] [Google Scholar]
Dillabough 2011 {published data only}
- Dillabough A, Mammel J, Yee J. Improving nutritional intake in post‐operative hip fracture patients: A quality improvement project. International Journal of Orthopaedic & Trauma Nursing 2011;15(4):196‐201. [Google Scholar]
Edington 2004 {published data only}
- Edington J, Barnes R, Bryan F, Dupree E, Frost G, Hickson M, et al. A prospective randomised controlled trial of nutritional supplementation in malnourished elderly in the community: clinical and health economic outcomes. Clinical Nutrition 2004;23(2):195‐204. [DOI] [PubMed] [Google Scholar]
Elkort 1981 {published data only}
- Elkort RJ, Baker FL, Vitale JJ, Cordano A. Long‐term nutritional support as an adjunct to chemotherapy for breast cancer. JPEN Journal of Parenteral and Enteral Nutrition 1981;5(5):385‐90. [DOI] [PubMed] [Google Scholar]
Endevelt 2011 {published data only}
- Endevelt R, Lemberger J, Bregman J, Kowen G, Berger‐Fecht I, Lander H, et al. Intensive dietary intervention by a dietitian as a case manager among community dwelling older adults: the edit study. Journal of Nutrition, Health and Aging 2011;15(8):624‐30. [DOI] [PubMed] [Google Scholar]
Eneroth 2004 {published data only}
- Eneroth M, Larsson J, Oscarsson C, Apelqvist J. Nutritional supplementation for diabetic foot ulcers: the first RCT. Journal of Wound Care 2004;13(6):230‐4. [DOI] [PubMed] [Google Scholar]
Espaulella 2000 {published data only}
- Espaulella J, Guyer H, Diaz‐Escriu F, Mellado‐Navas JA, Castells M, Pladevall M. Nutritional supplementation of elderly hip fracture patients. A randomized, double‐blind, placebo‐controlled trial. Age and Ageing 2000;29(5):425‐31. [DOI] [PubMed] [Google Scholar]
Fiatarone 1994 {published data only}
- Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. New England Journal of Medicine 1994;330(25):1769‐75. [DOI] [PubMed] [Google Scholar]
- Fiatarone Singh MA, Bernstein MA, Ryan AD, O'Neill EF, Clements KM, Evans WJ. The effect of oral nutritional supplements on habitual dietary quality and quantity in frail elders. Journal of Nutrition, Health & Aging 2000;4(1):5‐12. [PubMed] [Google Scholar]
Forster 2005 {published data only}
- Forster SE, Gariballa SE, Powers HJ. Function‐enabling diet (FED) study: a randomised, double‐blind, placebo‐controlled trial of the effects of energy, protein and micronutrient supplementation of hospitalised elderly patients. Proceedings of Nutrition Society 2005;64:25A. [Google Scholar]
- Gariballa S, Forster S. Associations between underlying disease and nutritional status following acute illness in older people. Clinical Nutrition 2007;26(4):466‐73. [DOI] [PubMed] [Google Scholar]
- Gariballa S, Forster S. Effects of dietary supplements on depressive symptoms in older patients: a randomised double‐blind placebo‐controlled trial. Clinical Nutrition 2007;26(5):545‐51. [DOI] [PubMed] [Google Scholar]
- Gariballa S, Forster S, Walters S, Powers H. A randomized, double‐blind, placebo‐controlled trial of nutritional supplementation during acute illness. American Journal of Medicine 2006;119(8):693‐9. [DOI] [PubMed] [Google Scholar]
- Gariballa S, Forster S. D 2007, 55(12):2030‐4. Dietary supplementation and quality of life of older patients: a randomized, double‐blind, placebo‐controlled trial. Journal of the American Geriatrics Society 2007;55(12):2030‐4. [DOI] [PubMed] [Google Scholar]
Gall 1998 {published data only}
- Gall MJ, Grimble GK, Reeve NJ, Thomas SJ. Effect of providing fortified meals and between‐meal snacks on energy and protein intake of hospital patients. Clinical Nutrition 1998;17(6):259‐64. [DOI] [PubMed] [Google Scholar]
Gariballa 1998 {published data only}
- Gariballa SE, Parker SG, Taub N, Castleden CM. A randomized, controlled, single‐blind trial of nutritional supplementation after acute stroke. JPEN Journal of Parenteral and Enteral Nutrition 1998;22(5):315‐9. [DOI] [PubMed] [Google Scholar]
Gazzotti 2003 {published data only}
- Gazzotti C, Arnaud‐Battandier F, Parello M, Farine S, Seidel L, Albert A, et al. Prevention of malnutrition in older people during and after hospitalisation: results from a randomised controlled clinical trial. Age and Ageing 2003;32(3):321‐5. [DOI] [PubMed] [Google Scholar]
Gegerle 1986 {published data only}
- Gegerle P, Bengoa JM, Delmi M, Rapin CH, Loizeau E, Vasey H. Dietary survey on the effect of an oral nutritional supplement after femoral neck fracture [Enquete alimentaire apres fracture du col du femur: effet d’un supplement dietetique sur les apports nutritonnels]. Schweizerische Rundschau fur Medizin Praxis 1986;75(32):933–5. [PubMed] [Google Scholar]
Gil Gregorio 2003 {published data only}
- Gil Gregorio P, Ramirez Diaz SP, Ribera Casado JM, Group D. Dementia and Nutrition. Intervention study in institutionalized patients with Alzheimer disease. Journal of Nutrition, Health & Aging 2003;7(5):304‐8. [PubMed] [Google Scholar]
Goris 2003 {published data only}
- Goris AH, Vermeeren MA, Wouters EF, Schols AM, Westerterp KR. Energy balance in depleted ambulatory patients with chronic obstructive pulmonary disease: the effect of physical activity and oral nutritional supplementation. The British Journal of Nutrition 2003;89(5):725‐31. [DOI] [PubMed] [Google Scholar]
Hogarth 1996 {published data only}
- Hogarth MB, Marshall P, Lovat LB, Palmer AJ, Frost CG, Fletcher AE, et al. Nutritional supplementation in elderly medical in‐patients: a double‐blind placebo‐controlled trial. Age and Ageing 1996;25(6):453‐7. [DOI] [PubMed] [Google Scholar]
Hopkinson 2010 {published data only}
- Hopkinson JB, Fenlon DR, Okamoto I, Wright DNM, Scott I, Addington‐Hall JM, et al. The deliverability, acceptability, and perceived effect of the Macmillan approach to weight loss and eating difficulties: a phase II, cluster‐randomized, exploratory trial of a psychosocial intervention for weight‐ and eating‐related distress in people with advanced cancer. Journal of Pain and Symptom Management 2010;40(5):684‐95. [DOI] [PubMed] [Google Scholar]
Houles 2010 {published data only}
- Houles M. Randomised study of a multi‐disciplinary programme focusing on geriatric syndromes in vulnerable, elderly individuals living at home (Dutch EASYcare Study). Cahiers de l'Annee Gerontologique 2010;2(3):166‐8. [Google Scholar]
Hubbard 2008 {published data only}
- Hubbard GP, Bolch R, Holdoway H, Beams A, Kerr A, Robertson D, et al. A randomised, controlled trial of the effects of an energy‐dense supplement on energy intake, appetite and blood lipids in malnourished community‐based elderly patients. Journal of Human Nutrition and Dietetics 2008;21(4):390‐1. [Google Scholar]
Hubsch 1992 {published data only}
- Hubsch S, Volkert D, Oster P, Schlierf G. Effect of dietary supplementation on weight and protein status in undernourished geriatric patients. Clinical Nutrition 1992;11:97. [Google Scholar]
Huisman 2012 {published data only}
- Huisman EJ, Hoek B, Soest H, Nieuwkerk KM, Arends JE, Siersema PD, et al. Preventive versus "on‐demand" nutritional support during antiviral treatment for hepatitis C: a randomized controlled trial. Journal of Hepatology 2012;57(5):1069‐75. [DOI] [PubMed] [Google Scholar]
Isenring 2003 {published data only}
- Isenring E, Capra S, Bauer J, Davies PSW. The impact of nutrition support on body composition in cancer outpatients receiving radiotherapy. Acta Diabetologica 2003;40(SUPPL. 1):S162‐S4. [DOI] [PubMed] [Google Scholar]
Isenring 2004 {published data only}
- Isenring E, Capra S, Bauer J. Patient satisfaction is rated higher by radiation oncology outpatients receiving nutrition intervention compared with usual care. Journal of Human Nutrition and Dietetics 2004;17(2):145‐52. [DOI] [PubMed] [Google Scholar]
Jahnavi 2010 {published data only}
- Jahnavi G, Sudha CH. Randomised controlled trial of food supplements in patients with newly diagnosed tuberculosis and wasting. Singapore Medical Journal 2010;51(12):957‐62. [PubMed] [Google Scholar]
James 2006 {published data only}
- James M, Stokes E, Bowling T, Smedley F, Jones P, Silk D. An economic assessment of peri‐operative oral nutritional supplements in patients undergoing elective moderate to major lower gastrointestinal surgery. Proceedings of the Nutrition Society 2006;65:4A. [Google Scholar]
Johnson 1993 {published data only}
- Johnson LE, Dooley PA, Gleick JB. Oral nutritional supplement use in elderly nursing home patients. Journal of the American Geriatrics Society 1993;41(9):947‐52. [DOI] [PubMed] [Google Scholar]
Keele 1997 {published data only}
- Keele AM, Bray MJ, Emery PW, Duncan HD, Silk DB. Two phase randomised controlled clinical trial of postoperative oral dietary supplements in surgical patients. Gut 1997;40(3):393‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kikutani 2006 {published data only}
- Kikutani T, Enomoto R, Tamura F, Oyaizu K, Suzuki A, Inaba S. Effects of oral functional training for nutritional improvement in Japanese older people requiring long‐term care. Gerodontology 2006;23(2):93‐8. [DOI] [PubMed] [Google Scholar]
Knowles 1988 {published data only}
- Knowles JB, Fairbarn MS, Wiggs BJ, Chan‐Yan C, Pardy RL. Dietary supplementation and respiratory muscle performance in patients with COPD. Chest 1988;93(5):977‐83. [DOI] [PubMed] [Google Scholar]
Krondl 1999 {published data only}
- Krondl M, Coleman PH, Bradley CL, Lau D, Ryan N. Subjectively healthy elderly consuming a liquid nutrition supplement maintained body mass index and improved some nutritional parameters and perceived well‐being. Journal of the American Dietetic Association 1999;99(12):1542‐8. [DOI] [PubMed] [Google Scholar]
Kruizenga 2004 {published data only}
- Kruizenga HM, Seidell J, Ader HJ, Bokhorst MAE. Early screening and treatment of malnutrition in hospital reduces length of stay. Clinical Nutrition 2004;23(764):04‐A‐228. [Google Scholar]
Kuhlmann 1997 {published data only}
- Kuhlmann MK, Schmidt F, Kohler H. Oral nutritional support in malnourished HD‐patients. Preliminary results of a randomised controlled study. Journal of the American Society of Nephrology 1997;8(SS):A0924. [Google Scholar]
Kwok 2001 {published data only}
- Kwok T, Woo J, Kwan M. Does low lactose milk powder improve the nutritional intake and nutritional status of frail older Chinese people living in nursing homes?. Journal of Nutrition, Health & Aging 2001;5(1):17‐21. [PubMed] [Google Scholar]
Kwok 2012 {published data only}
- Kwok TCY, Lam LCW, Sea MMM, Goggins W, Woo J. A randomized controlled trial of dietetic interventions to prevent cognitive decline in old age hostel residents. European Journal of Clinical Nutrition 2012;66(10):1135‐40. [DOI] [PubMed] [Google Scholar]
Lauque 2000 {published data only}
- Lauque S, Arnaud‐Battandier F, Mansourian R, Guigoz Y, Paintin M, Nourhashemi F, et al. Protein‐energy oral supplementation in malnourished nursing‐home residents. A controlled trial. Age and Ageing 2000;29(1):51‐6. [DOI] [PubMed] [Google Scholar]
Lauque 2004 {published data only}
- Lauque S, Arnaud‐Battandier F, Gillette S, Plaze J‐M, Andrieu S, Cantet C, et al. Improvement of weight and fat‐free mass with oral nutritional supplementation in patients with Alzheimer's disease at risk of malnutrition: a prospective randomized study. Journal of the American Geriatrics Society 2004;52(10):1702‐7. [DOI] [PubMed] [Google Scholar]
Lawson 2000 {published data only}
- Lawson RM, Doshi MK, Ingoe LE, Colligan JM, Barton JR, Cobden I. Compliance of orthopaedic patients with postoperative oral nutritional supplementation. Clinical Nutrition 2000;19(3):171‐5. [DOI] [PubMed] [Google Scholar]
Le Cornu 2000 {published data only}
- Cornu KA, McKiernan FJ, Kapadia SA, Neuberger JM. A prospective randomized study of preoperative nutritional supplementation in patients awaiting elective orthotopic liver transplantation. Transplantation 2000;69(7):1364‐9. [DOI] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee L‐C, Tsai AC, Want J‐Y, Hurng B‐S, Hsu J‐C, Tsai H‐J. Need‐based intervention is an effective strategy for improving the nutritional status of older people living in a nursing home: a randomized controlled trial. International Journal of Nursing Studies 2013;50:1580‐8. [DOI] [PubMed] [Google Scholar]
Leon 2001 {published data only}
- Leon JB, Majerle AD, Soinski JA, Kushner I, Ohri‐Vachaspati P, Sehgal AR. Can a nutrition intervention improve albumin levels among hemodialysis patients? A pilot study. Journal of Renal Nutrition 2001;11(1):9‐15. [DOI] [PubMed] [Google Scholar]
Leon 2006 {published data only}
- Leon JB, Albert JM, Gilchrist G, Kushner I, Lerner E, Mach S, et al. Improving albumin levels among hemodialysis patients: a community‐based randomized controlled trial. American Journal of Kidney Diseases 2006;48(1):28‐36. [DOI] [PubMed] [Google Scholar]
Locher 2011 {published data only}
- Locher JL, Bales CW, Ellis AC, Lawrence JC, Newton L, Ritchie CS, et al. A theoretically based behavioral nutrition intervention for community elders at high risk: The B‐NICE randomized controlled clinical trial. Journal of Nutrition in Gerontology and Geriatrics 2011;30(4):384‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacFie 2000 {published data only}
- MacFie J, Woodcock NP, Palmer MD, Walker A, Townsend S, Mitchell CJ. Oral dietary supplements in pre‐ and postoperative surgical patients: a prospective and randomized clinical trial. Nutrition 2000;16(9):723‐8. [DOI] [PubMed] [Google Scholar]
Mamhidir 2007 {published data only}
- Mamhidir A, Karlsson I, Norberg A. Weight increase in patients with dementia, and alteration in meal routines and meal environment after integrity promoting care. Journal of Clinical Nursing 2007;16(5):987‐96. [DOI] [PubMed] [Google Scholar]
Manders 2006 {published data only}
- Manders M. Nutritional care in old age: the effect of supplementation on nutritional status and performance [PhD thesis]. Wageningen: Wageningen University, 2006. [Google Scholar]
- Manders M, Groot CPGM, Blauw YH, Dhonukshe‐Rutten RAM, Hoeckel‐Prust L, Bindels JG, et al. Effect of a nutrient‐enriched drink on dietary intake and nutritional status in institutionalised elderly. European Journal of Clinical Nutrition 2009;63(10):1241‐50. [DOI] [PubMed] [Google Scholar]
- Manders M, Groot LCPGM, Hoefnagels WHL, Dhonukshe‐Rutten RAM, Wouters‐Wesseling W, Mulders AJMJ, et al. The effect of a nutrient dense drink on mental and physical function in institutionalized elderly people. Journal of Nutrition, Health & Aging 2009;13(9):760‐7. [DOI] [PubMed] [Google Scholar]
McEvoy 1982 {published data only}
- McEvoy AW, James OF. The effect of a dietary supplement (Build‐up) on nutritional status in hospitalized elderly patients. Human Nutrition Applied Nutrition 1982;36(5):374‐6. [PubMed] [Google Scholar]
McMurdo 2009 {published data only}
- McMurdo MET, Price RJG, Shields M, Potter J, Stott DJ. Should oral nutritional supplementation be given to undernourished older people upon hospital discharge? A controlled trial. Journal of the American Geriatrics Society 2009;57(12):2239‐45. [DOI] [PubMed] [Google Scholar]
Moretti 2009 {published data only}
- Moretti HD, Johnson AM, Keeling‐Hathaway TJ. Effects of protein supplementation in chronic hemodialysis and peritoneal dialysis patients. Journal of Renal Nutrition 2009;19(4):298‐303. [DOI] [PubMed] [Google Scholar]
Navrátilová 2007 {published data only}
- Navrátilová M, Jarkovský J, Cešková E, Leonard B, Sobotka L. Alzheimer disease: malnutrition and nutritional support. Clinical and Experimental Pharmacology and Physiology 2007;34:S11‐S3. [Google Scholar]
Nayel 1992 {published data only}
- Nayel H, el‐Ghoneimy E, el‐Haddad S. Impact of nutritional supplementation on treatment delay and morbidity in patients with head and neck tumors treated with irradiation. Nutrition 1992;8(1):13‐8. [PubMed] [Google Scholar]
Ödlund Olin 2003 {published data only}
- Ödlund Olin A, Armyr I, Soop M, Jerström S, Classon I, Cederholm T, et al. Energy‐dense meals improve energy intake in elderly residents in a nursing home. Clinical Nutrition (Edinburgh, Scotland) 2003;22(2):125‐31. [DOI] [PubMed] [Google Scholar]
Olin 1996 {published data only}
- Olin AO, Osterberg P, Hadell K, Armyr I, Jerstrom S, Ljungqvist O. Energy‐enriched hospital food to improve energy intake in elderly patients. JPEN Journal Parenteral & Enteral Nutrition 1996;20(2):93‐7. [DOI] [PubMed] [Google Scholar]
Otte 1989 {published data only}
- Otte KE, Ahlburg P, D'Amore F, Stellfeld M. Nutritional repletion in malnourished patients with emphysema. JPEN Journal Parenteral & Enteral Nutrition 1989;13(2):152‐6. [DOI] [PubMed] [Google Scholar]
Payette 2002 {published data only}
- Payette H, Boutier V, Coulombe C. Efficacy of nutritional intervention in the free‐living frail elderly. American Journal of Clinical Nutrition 2002;75(2):340S. [Google Scholar]
Price 2005 {published data only}
- Price R, Daly F, Pennington CR, McMurdo MET. Nutritional supplementation of very old people at hospital discharge increases muscle strength: a randomised controlled trial. Gerontology 2005;51(3):179‐85. [DOI] [PubMed] [Google Scholar]
Rana 1992 {published data only}
- Rana SK, Bray J, Menzies‐Gow N, Jameson J, Payne James JJ, Frost P, et al. Short term benefits of post‐operative oral dietary supplements in surgical patients. Clinical Nutrition 1992;11(6):337‐44. [DOI] [PubMed] [Google Scholar]
Richeson & Neil 2004 {published data only}
- Richeson NE, Neil DJ. Therapeutic recreation music intervention to decrease mealtime agitation and increase food intake in older adults with dementia. American Journal of Recreation Therapy 2004;3(1):37‐41. [Google Scholar]
Roberts 2013 {published data only}
- Roberts HC, Pilgrim AL, Elia M, Jackson AA, Cooper C, Sayer AA, et al. Southampton Mealtime Assistance Study: design and methods. BMC Geriatrics 2013;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 2002 {published data only}
- Robinson S, Clump D, Weitzel T, Henderson L, Lee K, Schwartz C, et al. The Memorial Meal Mates: a program to improve nutrition in hospitalized older adults. Geriatric Nursing (New York, NY) 2002;23(6):332‐5. [DOI] [PubMed] [Google Scholar]
Rosendahl 2006 {published data only}
- Rosendahl E, Lindelof N, Littbrand H, Yifter‐Lindgren E, Lundin‐Olsson L, Haglin L, et al. High‐intensity functional exercise program and protein‐enriched energy supplement for older persons dependent in activities of daily living: a randomised controlled trial. Australian Journal of Physiotherapy 2006;52(2):105‐13. [DOI] [PubMed] [Google Scholar]
Roy 2006 {published data only}
- Roy MA, Payette H. Meals‐on‐wheels improves energy and nutrient intake in a frail free‐living elderly population. Journal of Nutrition, Health & Aging 2006;10(6):554‐60. [PubMed] [Google Scholar]
Rypkema 2004 {published data only}
- Rypkema G, Adang E, Dicke H, Naber T, Swart B, Disselhorst L, et al. Cost‐effectiveness of an interdisciplinary intervention in geriatric inpatients to prevent malnutrition. Journal of Nutrition, Health & Aging 2004;8(2):122‐7. [PubMed] [Google Scholar]
Saudny‐Unterberger 1997 {published data only}
- Saudny‐Unterberger H, Martin JG, Gray‐Donald K. Impact of nutritional support on functional status during an acute exacerbation of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1997;156(3 Pt 1):794‐9. [DOI] [PubMed] [Google Scholar]
Shinnar 1983 {published data only}
- Shinnar SE. Use of adaptive equipment in feeding the elderly. Journal of the American Dietetic Association 1983;83(3):321‐2. [PubMed] [Google Scholar]
Simmons 2004 {published data only}
- Simmons SF, Schnelle JF. Individualized feeding assistance care for nursing home residents: staffing requirements to implement two interventions. Journals of Gerontology ‐ Series A Biological Sciences and Medical Sciences 2004;59(9):966‐73. [DOI] [PubMed] [Google Scholar]
Smedley 2004 {published data only}
- Smedley F, Bowling T, James M, Stokes E, Goodger C, O'Connor O, et al. Randomized clinical trial of the effects of preoperative and postoperative oral nutritional supplements on clinical course and cost of care. British Journal of Surgery 2004;91(8):983‐90. [DOI] [PubMed] [Google Scholar]
Somanchi 2011 {published data only}
- Somanchi M, Tao X, Mullin GE. The facilitated early enteral and dietary management effectiveness trial in hospitalized patients with malnutrition. JPEN. Journal of Parenteral and Enteral Nutrition 2011;35(2):209‐16. [DOI] [PubMed] [Google Scholar]
Soneff 1994 {published data only}
- Soneff R, McGeachy F, Davison K, McCargar L, Therien G. Effectiveness of two training methods to improve the quality of food service in small facilities for adult care. Journal of the American Dietetic Association 1994;94(8):869‐73. [DOI] [PubMed] [Google Scholar]
Southgate 2010 {published data only}
- Southgate KM, Keller HH, Reimer HD. Determining knowledge and behaviour change after nutrition screening among older adults. Canadian Journal of Dietetic Practice and Research 2010;71(3):128‐33. [DOI] [PubMed] [Google Scholar]
Starke 2011 {published data only}
- Starke J, Schneider H, Alteheld B, Stehle P, Meier R. Short‐term individual nutritional care as part of routine clinical setting improves outcome and quality of life in malnourished medical patients. Clinical Nutrition 2011;30(2):194‐201. [DOI] [PubMed] [Google Scholar]
Stauffer 1986 {published data only}
- Stauffer JL, Carbone JE, Bendoski MT. Effects of diet supplementation on anthropometric and laboratory nutritional parameters in malnourished ambulatory patients with severe Chronic Obstructive Pulmonary disease (COPD). American Review of Respiratory Disease 1986;133:A204. [Google Scholar]
Steiner 2003 {published data only}
- Steiner MC, Barton RL, Singh SJ, Morgan MD. Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: a randomised controlled trial. Thorax 2003;58(9):745‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stotts 2009 {published data only}
- Stotts NA, Hopf HW, Kayser‐Jones J, Chertow GM, Cooper BA, Wu H. Increased fluid intake does not augment capacity to lay down new collagen in nursing home residents at risk for pressure ulcers: a randomized, controlled clinical trial. Wound Repair and Regeneration 2009;17(6):780‐8. [DOI] [PubMed] [Google Scholar]
Teixido‐Planas 2005 {published data only}
- Teixido‐Planas J, Ortiz A, Coronel F, Montenegro J, Lopez‐Menchero R, Ortiz R, et al. Oral protein‐energy supplements in peritoneal dialysis: a multicenter study. Peritoneal Dialysis International 2005;25(2):163‐72. [PubMed] [Google Scholar]
Tkatch 1992 {published data only}
- Tkatch L, Rapin CH, Rizzoli R, Slosman D, Nydegger V, Vasey H, et al. Benefits of oral protein supplementation in elderly patients with fracture of the proximal femur. Journal of the American College of Nutrition 1992;11(5):519‐25. [DOI] [PubMed] [Google Scholar]
Vetter 1992 {published data only}
- Vetter NJ, Lewis PA, Ford D. Can health visitors prevent fractures in elderly people?. BMJ 1992;304(6831):888‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vlaming 2001 {published data only}
- Vlaming S, Biehler A, Hennessey EM, Jamieson CP, Chattophadhyay S, Obeid OA, et al. Should the food intake of patients admitted to acute hospital services be routinely supplemented? A randomized placebo controlled trial. Clinical Nutrition 2001;20(6):517‐26. [DOI] [PubMed] [Google Scholar]
Watanabe 2010 {published data only}
- Watanabe S, Kumagai S, Shibata H. An interventional trial to improve nutritional status in the community‐dwelling elderly. Nippon Ronen Igakkai Zasshi 2010;47(5):422‐5. [DOI] [PubMed] [Google Scholar]
Williams 1989 {published data only}
- Williams CM, Driver LT, Older J, Dickerson JW. A controlled trial of sip‐feed supplements in elderly orthopaedic patients. European Journal of Clinical Nutrition 1989;43(4):267‐74. [PubMed] [Google Scholar]
Wong 2010 {published data only}
- Wong SS, O'Driscoll J, Weldon M, Yau CY. Do regular oral nutritional supplements improve clinical outcomes in patients with Clostridium difficile infection: a pilot study. Proceedings of the Nutrition Society 2010;69(OCE2):E174. [Google Scholar]
Woo 1994 {published data only}
- Woo J, Ho SC, Mak YT, Law LK, Cheung A. Nutritional status of elderly patients during recovery from chest infection and the role of nutritional supplementation assessed by a prospective randomized single‐blind trial. Age and Ageing 1994;23(1):40‐8. [DOI] [PubMed] [Google Scholar]
Wouters‐Wesseling 2002 {published data only}
- Wouters‐Wesseling W, Rozendaal M, Snijder M, Graus Y, Rimmelzwaan G, Groot L, et al. Effect of a complete nutritional supplement on antibody response to influenza vaccine in elderly people. Journals of Gerontology Series A: Biological Sciences & Medical Sciences 2002;57(9):M563‐6. [DOI] [PubMed] [Google Scholar]
Wright 2006 {published data only}
- Wright L, Hickson M, Frost G. Eating together is important: using a dining room in an acute elderly medical ward increases energy intake. Journal of Human Nutiriton & Dietetics 2006;19(1):23‐6. [DOI] [PubMed] [Google Scholar]
WY Lin 2010 {published data only}
- Lin WY, Huang HY, Liu CS, Li CI, Lee SD, Lin CC, et al. A hospital‐based multidisciplinary approach improves nutritional status of the elderly living in long‐term care facilities in middle Taiwan. Archives of Gerontology and Geriatrics 2010;50 Suppl 1:S22‐6. [DOI] [PubMed] [Google Scholar]
Yamaguchi 1998 {published data only}
- Yamaguchi LY, Coulston AM, Lu NC, Dixon LB, Craig LD. Improvement in nutrient iIntake by elderly meals‐on‐wheels participants receiving a liquid nutrition supplement. Nutrition Today 1998;33(1):37. [Google Scholar]
Young 2004 {published data only}
- Parrott MD, Young KWH, Greenwood CE. Energy‐containing nutritional supplements can affect usual energy intake post supplementation in institutionalized seniors with probable Alzheimer's disease. Journal of the American Geriatrics Society 2006;54(9):1382‐7. [DOI] [PubMed] [Google Scholar]
- Young KWH, Greenwood CE, Reekum R, Binns MA. A randomized, crossover trial of high‐carbohydrate foods in nursing home residents with Alzheimer's disease: associations among intervention response, body mass index, and behavioral and cognitive function.. Journal of Gerontology: Medical Sciences 2005;60(8):1039‐45. [DOI] [PubMed] [Google Scholar]
- Young KWH, Greenwood CE, Reekum R, Binns MA. Providing nutrition supplements to institutionalized seniors with probable Alzheimer's disease is least beneficial to those with low body weight status. Journal of the American Geriatrics Society 2004;52(8):1305‐12. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Allen 2014 {published data only}
- Allen VJ, Methven L, Gosney M. Impact of serving method on the consumption of nutritional supplement drinks: randomized trial in older adults with cognitive impairment. Journal of Advanced Nursing 2014;70(6):1323‐33. [DOI] [PubMed] [Google Scholar]
Borges 2003 {published data only}
- Borges VC. [Repercussão Nutricional nas Doenças Neurológicas da Velhice: Alzheimer e Parkinson]. [texto na internet]. Nutrição Total SãoPaulo. Disponível em; [http://www.nutritotal.com.br] 2003.
Burns 1998 {published data only}
- Burns BL, Davis EMC. [Cuidado Nutricional nas Doenças do Sistema Nervoso]. In: Mahan LK, Stump SE. Alimentos, Nutrição e Dietoterapia. 9edition. São Paulo: Roca. 1998:896‐7. [Google Scholar]
Deutz 2016 {published data only}
- Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson Jl, et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clinical Nutrition (Edinburgh, Scotland) 2016;35(1):18‐26. [DOI] [PubMed] [Google Scholar]
Ekinci 2016 {published data only}
- Ekinci O, Yanik S, Terzioglu Bebitoglu B, Yilmaz Akyuz E, Dokuyucu A, Erdem S. Effect of calcium beta‐hydroxy‐beta‐methylbutyrate (CaHMB), vitamin D, and protein supplementation on postoperative immobilization in malnourished older adult patients with hip fracture: a randomized controlled study. Nutrition in Clinical Practice 2016 [Epub ahead of print]; Vol. 10. [DOI: 10.1177/0884533616629628] [DOI] [PubMed]
ISRCTN04327195 {unpublished data only}
ISRCTN96923961 {unpublished data only}
Jobse 2015 {published data only}
- Jobse I, Liao Y, Bartram M, Delantonio K, Uter W, Stehle P, et al. Compliance of nursing home residents with a nutrient‐ and energy‐dense oral nutritional supplement determines effects on nutritional status. Journal of Nutrition, Health & Aging 2015;19(3):356‐64. [DOI] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee Lc, Tsai Ac, Wang Jy. Need‐based nutritional intervention is effective in improving handgrip strength and Barthel Index scores of older people living in a nursing home: a randomized controlled trial. International Journal of Nursing Studies 2015;52(5):904‐12. [DOI] [PubMed] [Google Scholar]
Leslie 2013 {published data only}
- Leslie WS, Woodward M, Lean MEJ, Theobald H, Watson L, Hankey CR. Improving the dietary intake of under nourished older people in residential care homes using an energy‐enriching food approach: a cluster randomised controlled study. Journal of Human Nutrition and Dietetics 2013;26:387‐94. [DOI] [PubMed] [Google Scholar]
Luna‐Ramos 2016 {published data only}
- Luna‐Ramos GK, Pedraza‐Zarate MA, Franco‐Alvarez N, Gonzalez‐Velazquez F. Diet and polymer standard vs. standard in the nutritional status of elderly patients with fragility. Revista Medica del Instituto Mexicano del Seguro Social 2016;54(4):439‐45. [PubMed] [Google Scholar]
Madigan 1994 {published data only}
- Madigan C. Benefits of dietary supplementation in elderly patients with fractured neck of femur [MSc dissertation]. Sydney: University of Sydney, 1994. [Google Scholar]
Moore 2010 {published data only}
- Moore JR. Familiar physical activity to familiar music: The effects on apathy, agitation, eating ability, and dietary intake in institutionalised older adults with dementia [Dissertation]. Amherst: University of Massachusetts, 2010. [Google Scholar]
Parsons 2016 {published data only}
- Parsons EL, Stratton RJ, Cawood AL, Smith TR, Elia M. Oral nutritional supplements in a randomised trial are more effective than dietary advice at improving quality of life in malnourished care home residents. Clinical Nutrition 2016 [Epub ahead of print]; Vol. 11. [DOI: 10.1016/j.clnu.2016.01.002] [DOI] [PubMed]
Pouyssegur 2015 {published data only}
- Pouyssegur V, Brocker P, Schneider SM, Philip JL, Barat P, Reichert E, et al. An innovative solid oral nutritional supplement to fight weight loss and anorexia: open randomised controlled trial of efficacy in institutionalised, malnourished older adults. Age and Ageing 2015;44:245‐51. [DOI] [PubMed] [Google Scholar]
Scorer 1990 {published data only}
- Scorer HJM. The effect of nutritional supplementation with Ensure on the general health of undernourished elderly patients in the community. Abbott UK Medical, 1990. [Google Scholar]
Simmons 2013 {published data only}
- Simmons SF, Durkin DW, Shotwell MS, Erwin S, Schnelle JF. A staff training and management intervention in VA long‐term care: impact on feeding assistance care quality. Translational Behavioral Medicine 2013;13:189‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simmons 2015 {published data only}
- Simmons SF, Keeler E, An R, Liu X, Shotwell MS, Kuertz B, et al. Cost‐effectiveness of nutrition intervention in long‐term care. Journal of the American Geriatrics Society 2015; Vol. 63, issue 11:2308‐16. [DOI] [PMC free article] [PubMed]
Stelten 2015 {published data only}
- Stelten S, Dekker IM, Ronday EM, Thijs A, Boelsma E, Peppelenbos, et al. Protein‐enriched 'regular products' and their effect on protein intake in acute hospitalized older adults: a randomised controlled trial. Clinical Nutrition 2015;34:409‐14. [DOI] [PubMed] [Google Scholar]
Stow 2015 {published data only}
- Stow R, Ives N, Smith C, Rick C, Rushton A. A cluster randomised feasibility trial evaluating nutritional interventions in the treatment of malnutrition in care home adult residents. Trials 2015;16:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sutton 2006 {published data only}
- Sutton D, Hubbard GP, Bolch R, Cawood AL, Stevens L, Bircher G, et al. Evaluation of a renal‐specific oral nutritional supplement in the management of chronic renal failure in haemodialysis and peritoneal dialysis patients. Journal of Renal Care 2006;32:Abstracts: 34. [Google Scholar]
Turano 1999 {published data only}
- Turano W, Almeida CCC. [Educação Nutricional]. In: Gouveia ELC. Nutrição Saúde e Comunidade. 2 edition. Rio de Janeiro: Revinter 1999:57‐60.
White 1999 {published data only}
- White JV. In: The role of nutrition in chronic disease. Nutrition Screening Initiative. A project of American Academy of Family Physicians. The American Dietetic Association and National Council on the Ageing, USA 1997.
Zhong 2016 {published data only}
- Zhong Y, Cohen JT, Goates S, Luo M, Nelson J, Neumann PJ. The cost‐effectiveness of oral nutrition supplementation for malnourished older hospital patients. Applied Health Economics & Health Policy 2016 [Epub ahead of print]; Vol. 4. [DOI] [PMC free article] [PubMed]
Additional references
Agarwal 2011
- Agarwal E, Ferguson M, Banks M, Bauer J, Capra S, Isenring E. Nutritional status and dietary intake of acute care patients: results from the Nutrition Care Day Survey 2010. Clinical Nutrition 2012;31(1):41‐7. [DOI] [PubMed] [Google Scholar]
Allison 2000
- Allison SP. Malnutrition, disease, and outcome. Nutrition 2000;16(7‐8):590‐3. [DOI] [PubMed] [Google Scholar]
Avenell 2001
- Avenell A, Handoll HHG, Grant AM. Lessons for search strategies from a systematic review, in the Cochrane Library, of nutritional supplementation trials in patients after hip fracture. The American Journal of Clinical Nutrition 2001;73(3):505‐10. [DOI] [PubMed] [Google Scholar]
BAPEN 2009
- British Association for Parenteral and Enteral Nutrition (BAPEN). Combatting Malnutrition: Recommendations for Action. Redditch: BAPEN, 2009. [Google Scholar]
BAPEN 2012
- BAPEN Quality Group. Malnutrition Matters ‐ Meeting Quality Standards in Nutritional Care: A Toolkit for Commissioners and Providers in England. Redditch , Worcestershire: BAPEN Quality Group, 2012. [Google Scholar]
Bistrian 1974
- Bistrian BR, Blackburn GL, Hallowell E, Heddle R. Protein status of general surgical patients. Journal of the American Medical Association 1974;230(6):858‐60. [PubMed] [Google Scholar]
Butterworth 1974
- Butterworth CEJ. The skeleton in the hospital closet. Nutrition Today 1974;9(2):4‐8. [Google Scholar]
Chima 1997
- Chima CS, Barco K, Dewitt MLA, Maeda M, Teran JC, Mullen KD. Relationship of nutritional status to length of stay, hospital costs, and discharge status of patients hospitalized in the medicine service. Journal of the American Dietetic Association 1997;97(9):975‐8. [DOI] [PubMed] [Google Scholar]
COE 2003
- Council of Europe (COE). Food and nutritional care in hospitals: how to prevent undernutrition. Nutrition programmes in Hospitals Group for the Committee of experts on Nutrition, Food & Safety and Consumer Health. Strasbourg: Council of Europe, 2003. [Google Scholar]
Cole 2012
- Cole D. Optimising nutrition for older people with dementia. Nursing Standard 2012;26(20):41‐8. [DOI] [PubMed] [Google Scholar]
Corish 2000a
- Corish CA, Kennedy NP. Protein‐energy undernutrition in hospital in‐patients. British Journal of Nutrition 2000;83(6):575‐91. [DOI] [PubMed] [Google Scholar]
Corish 2000b
- Corish CA, Flood P, Mulligan S, Kennedy NP. Apparent low frequency of undernutrition in Dublin hospital in‐patients: should we review the anthropometric thresholds for clinical practice?. British Journal of Nutrition 2000;84(3):325‐35. [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
DOH 2003
- Department of Health (DOH). The essence of care: patient focused benchmarks for clinical governance. London, UK: Department of Health, 2003. [Google Scholar]
DOH 2007
- Department of Health (DOH). Improving nutrition care: a joint action plan from the Department of Health and the Nutrition Summit Stakeholders. London, UK: Department of Health, 2007. [Google Scholar]
Donini 2003
- Donini LM, Savina C, Cannella C. Eating habits and appetite control in the elderly: the anorexia of aging. International Psychogeriatrics 2003;15(1):73‐87. [DOI] [PubMed] [Google Scholar]
Edington 2000
- Edington J, Boorman J, Durrant ER, Perkins A, Giffin CV, James R, et al. Prevalence of malnutrition on admission to four hospitals in England. Clinical Nutrition 2000;19(3):191‐5. [DOI] [PubMed] [Google Scholar]
Elia 2003
- Elia M. The "MUST" report. Nutritional screening of adults: a multidisciplinary responsibility. Redditch, Worcs, UK: Malnutrition Advisory Group of the British Association for Parenteral and Enteral Nutrition, 2003. [Google Scholar]
Elia 2009
- Elia M, Russell CA. British Associaton for Parenteral, Enteral Nutrition (BAPEN). Nutrition screening survey in the UK in 2008. Hospitals, care homes and mental health units. www.bapen.org.uk (accessed 16 March 2012).
EuroQol group 1990
- EuroQol. EuroQol‐a new facility for the measurement of health related quality of life. Health Policy 1990;16(3):199‐208. [DOI] [PubMed] [Google Scholar]
Gallagher 1996
- Gallagher Allred CR, Coble Voss A, Finn SC, McCamish MA. Malnutrition and clinical outcomes: the case for medical nutrition therapy. Journal of the American Dietetic Association 1996;96(4):361‐9. [DOI] [PubMed] [Google Scholar]
Gilson 1975
- Gilson BS, Gilson JS, Bergner M, Bobbitt RA, Kressel S, Pollard WE, et al. The sickness impact profile: development of an outcome measure of healthcare. American Journal of Public Health 1975;65:1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2006
- Green S, Watson R. Nutritional screening and assessment tools for older adults: literature review. Journal of Advanced Nursing 2006;54:477‐90. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hill 1977
- Hill GL, Blackett RL, Pickford I, Burkinshaw L, Young GA, Warren JV, et al. Malnutrition in surgical patients. An unrecognised problem. Lancet 1977;1:689‐92. [DOI] [PubMed] [Google Scholar]
Jeejeebhoy 1986
- Jeejeebhoy KN. Muscle function and nutrition. Gut 1986;27(Suppl 1):25‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferies 2011
- Jefferies D, Johnson M, Ravens J. Nurturing and nourishing: the nurses' role in nutritional care. Journal of Clinical Nursing 2011;20(3‐4):317‐30. [DOI] [PubMed] [Google Scholar]
Jensen 2010
- Jensen GL, Mirtallo J, Compher C, Dhaliwal R, Forbes A, Grijalba RF, et al. Adult starvation and disease‐related malnutrition: a proposal for etiology‐based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. Clinical Nutrition 2010;29(2):151‐3. [DOI] [PubMed] [Google Scholar]
Jones 2002
- Jones JM. The methodology of nutritional screening and assessment tools. Journal of Human Nutrition and Dietetics 2002;15:59‐71. [DOI] [PubMed] [Google Scholar]
Katz 1963
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the index of ADL, a standardised measure of biological and psychological function. JAMA 1963;185:914‐19. [DOI] [PubMed] [Google Scholar]
Kelly 2000
- Kelly IE, Tessier S, Cahill A, Morris SE, Crumley A, McLaughlin D, et al. Still hungry in hospital: identifying malnutrition in acute hospital admissions. Quarterly Journal of Medicine 2000;93(2):93‐8. [DOI] [PubMed] [Google Scholar]
Khan 1981
- Khan MA, Hackler LR. Evaluation of food selection patterns and preferences. CRC Critical Reviews in Food Science and Nutrition 1981;15(2):129‐53. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Kubrak 2007
- Kubrak C, Jensen L. Critical evaluation of nutrition screening tools recommended for oncology patients. Cancer Nursing 2007;30(5):E1‐6. [DOI] [PubMed] [Google Scholar]
Lambert 2010
- Lambert MA, Potter JM, McMurdo MET. Nutritional Supplementation for older people. Reviews in Clinical Gerontology 2010;20:317‐26. [Google Scholar]
Lawton 1969
- Lawton MP, Brody EM. Assessment of older people: self maintaining and instrumental activities of daily living. The Gerontologist 1969;9(3):179‐86. [PubMed] [Google Scholar]
Lawton 1972
- Lawton MP (chapter). The Dimensions of Morale (chapter). Research planning and action for the elderly. Kent D, Kastenbaum R, Sherwood S. New York: Behavioural Publications, 1972. [Google Scholar]
Lean 2008
- Lean M, Wiseman M. Malnutrition in hospitals. BMJ 2008;336(7639):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lennard‐Jones 1992
- Lennard‐Jones JE. A positive approach to nutrition as treatment: report of a working party chaired by Professor JE Lennard‐Jones on the role of enteral and parenteral feeding in hospital and at home. London: King's Fund Centre, 1992. [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. Public Library of Science Medicine 1999;6(7):1‐28. [Google Scholar]
LPP 2009
- London Procurement Programme (LPP). Quality, Innovation, Productivity, Prevention (QIPP). Appropriate community oral nutritional supplementation, 2009. www.evidence.nhs.uk/qipp (accessed 16 March 2012).
Lupton 1996
- Lupton D. Food, the Body and the Self. London: Sage, 1996. [Google Scholar]
Mahoney 1965
- Mahoney FI, Bathel DW. Functional evaluation; the Barthel index. Maryland State Medical Journal 1965;14:61‐5. [PubMed] [Google Scholar]
Martyn 1998
- Martyn CN, Winter PD, Coles SJ, Edington J. Effect of nutritional status on use of health care resources by patients with chronic disease living in the community. Clinical Nutrition 1998;17(3):119‐23. [DOI] [PubMed] [Google Scholar]
McCarron 2010
- McCarron J, Baldwin C, Weekes CE. Subjective assessment rather than systematic screening to identify malnourished patients for referral to a dietitian. Proceedings of the Nutrition Society 2010;69:E570. [Google Scholar]
McKenzie 2014
- McKenzie J, Ryan R, Tanna GL. Cochrane Consumers and Communication Review Group: cluster randomised controlled trials. cccrg.cochrane.org March 2014.
McMahon 2000
- McMahon K, Brown JK. Nutritional screening and assessment. Seminars in Oncology Nursing 2000;16:106‐12. [DOI] [PubMed] [Google Scholar]
McQuestion 2011
- McQuestion M, Fitch M, Howell D. The changed meaning of food: physical, social and emotional loss for patients having received radiation treatment for head and neck cancer. European Journal of Oncology Nursing 2011;15(2):145‐51. [DOI] [PubMed] [Google Scholar]
McWhirter 1994
- McWhirter JP, Pennington CR. Incidence and recognition of malnutrition in hospital. BMJ 1994;308(6934):945‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milne 2009
- Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD003288.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mueller 2011
- Mueller C, Compher C, Druyan ME, the American Society for Parenteral and Enteral Nutrition (ASPEN) Board of Directors. (Clinical Guidelines) Nutrition screening, assessment and intervention in adults. Journal of Parenteral and Enteral Nutrition 2011;35(1):16‐24. [DOI] [PubMed] [Google Scholar]
Naber 1997
- Naber TH, Schermer T, Bree A, Nusteling K, Eggink L, Kruimel JW, et al. Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. American Journal of Clinical Nutrition 1997;66(5):1232‐9. [DOI] [PubMed] [Google Scholar]
NCCAC 2006
- National Collaborating Centre for Acute Care (NCCAC). Nutrition Support in Adults: Oral Nutrition Support, Enteral Tube Feeding and Parental Nutrition. NICE Clinical Guideline 32. London, UK: National Collaborating Centre for Acute Care, 2006. [PubMed] [Google Scholar]
Norman 2008
- Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease‐related malnutrition. Clinical Nutrition 2008;27(1):5‐15. [DOI] [PubMed] [Google Scholar]
RCON 2008
- Royal College of Nursing (RCON). Enhancing Nutritional Care. London: Royal College of Nursing, 2008. [Google Scholar]
Reilly 1995
- Reilly HM, Martineau JK, Moran A, Kennedy H. Nutritional screening ‐ evaluation and implementation of a simple Nutrition Risk Score. Clinical Nutrition 1995;14(5):269‐73. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Russell 2007
- Russell CA. The impact of malnutrition on healthcare costs and economic considerations for the use of oral nutritional supplements. Clinical Nutrition Supplements 2007;2(1):25‐32. [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Scrimshaw 2003
- Scrimshaw NS. Historical concepts of interactions, synergism and antagonism between nutrition and infection. Journal of Nutrition 2003;133(1):316S‐21S. [DOI] [PubMed] [Google Scholar]
Silver 2009
- Silver HJ. Oral strategies to supplement older adults' dietary intakes: comparing the evidence. Nutrition Reviews 2009;67(1):21‐31. [DOI] [PubMed] [Google Scholar]
Sonn 1996
- Sonn U. Longitudinal studies of dependence in daily life activities among elderly persons. Scandinavian Journal of Rehabilation Medicine Supplements 1996;34:1‐35. [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Stratton 2003
- Stratton RJ, Green CJ, Elia M. Disease related malnutrition: an evidence‐based approach to treatment. Wallingford, UK: CABI Publishing, 2003. [Google Scholar]
Stratton 2007
- Stratton RJ, Elia M. A review of reviews: a new look at the evidence for oral nutritional supplements in clinical practice. Clinical Nutrition Supplements 2007;2(1):5‐23. [Google Scholar]
The Malnutrition Task Force 2013
- The Malnutrition Task Force. Prevention and Early Intervention of Malnutrition in Later Life. Best Practice Principles and Implementation Guide: A Local Community Approach. London: The Malnutrition Task Force, 2013. [Google Scholar]
Van Campen 1998
- Campen C, Kerkstra A. Perceived quality of life of elderly somatic nursing‐home patients. Construction of a measuring instrument. Tijdschrift voor Gerenterologie en Geriatrie 1998;29:11‐18. [PubMed] [Google Scholar]
Volkert 2006
- Volkert D, Berner YN, Berry E, Cederholm T, Coti Bertrand P, Milne A, et al. ESPEN guidelines on enteral nutrition: geriatrics. Clinical Nutrition 2006;25(2):330‐60. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36 item short form health survey (SF‐36). Conceptual framework and item selection. Medical Care 1992;30(6):473‐483. [PubMed] [Google Scholar]
Weekes 1999
- Weekes CE. The incidence of malnutrition in medical patients admitted to a hospital in South London. Proceedings of Nutrition Society 1999;58:126A. [Google Scholar]
Weekes 2009
- Weekes CE, Spiro A, Baldwin C, Whelan K, Thomas JE, Parkin D, et al. A review of the evidence for the impact of improving nutritional care on nutritional and clinical outcomes and cost. Journal of Human Nutrition and Dietetics 2009;22(4):324‐35. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gibbs 2012
- Gibbs M, Baldwin C, Weekes CE. Supportive interventions for enhancing dietary intake in malnourished or nutritionally at‐risk adults. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009840] [DOI] [PMC free article] [PubMed] [Google Scholar]


