Abstract
Background
Peritonsillar abscess is a common infection presenting as a collection of pus in the peritonsillar area. The condition is characterised by a severe sore throat, difficulty in swallowing and pain on swallowing, fever and malaise, and trismus. Needle aspiration and incision and drainage are the two main treatment modalities currently used in the treatment of this condition. The effectiveness of one versus the other has not been clearly demonstrated and remains an area of debate.
Objectives
To assess the effectiveness and risks of needle aspiration versus incision and drainage for the treatment of peritonsillar abscess in older children (eight years of age or older), adolescents and adults.
Search methods
The Cochrane ENT Information Specialist searched the ENT Trials Register; Central Register of Controlled Trials (CENTRAL 2016, Issue 7); Ovid MEDLINE; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 25 August 2016.
Selection criteria
Randomised controlled trials comparing needle aspiration with incision and drainage.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. Our primary outcomes were recurrence rate (proportion of patients needing repeat intervention) and adverse effects associated with the intervention. Secondary outcomes were time to resumption of normal diet, complications of the disease process and symptom scores. We used GRADE to assess the quality of evidence for each outcome; this is indicated in italics.
Main results
We included 11 studies (674 participants). The risk of bias was high or unclear in all of the included studies. All studies compared needle aspiration to incision and drainage.
All but one of the 11 studies reported on the primary outcome of recurrence. When we pooled data from the 10 studies the recurrence rate was higher in the needle aspiration group compared with incision and drainage: risk ratio (RR) 3.74 (95% confidence interval (CI) 1.63 to 8.59; 612 participants). We detected moderate heterogeneity in this analysis (I2 = 48%). In interpreting the pooled result it is important to note that the evidence for this outcome was of very low quality.
None of the other outcomes (adverse effects of the intervention, time to resumption of normal diet, complications of the disease process and symptom scores) were consistently measured across all studies.
Only three studies reported on adverse effects/events associated with the intervention and only one such event in a single patient was reported (post‐procedure bleeding following incision and drainage: 1/28, 3.6%) (very low‐quality evidence).
Time to resumption of normal diet was compared in two studies; neither found an obvious difference between needle aspiration and incision and drainage (very low‐quality evidence).
Only three studies stated that they would report complications of the disease process. In these three studies, the only complication reported was admission to hospital for dehydration in two patients who underwent incision and drainage (2/13, 6.7%).
Symptom scores were measured in four studies; three evaluated pain using different scales and one other symptoms. The data could not be pooled in a meta‐analysis. Two studies evaluating procedural pain reported this to be lower in the needle aspiration groups. One study found comparable rates of pain resolution at five days post‐intervention between groups. The quality of the evidence for symptom scores was very low.
Authors' conclusions
Although a number of studies have sought to evaluate whether or not needle aspiration or incision and drainage is more effective in patients with peritonsillar abscess, there is no high‐quality evidence to allow a firm conclusion to be drawn and the answer remains uncertain. Very low‐quality evidence suggests that incision and drainage may be associated with a lower chance of recurrence than needle aspiration. There is some very low‐quality evidence to suggest that needle aspiration is less painful.
Plain language summary
Needle aspiration compared to incision and drainage for the treatment of peritonsillar abscess (quinsy)
Review question
This review compared the effectiveness of the two main treatment options for peritonsillar abscess: needle aspiration and incision and drainage.
Background
Peritonsillar abscesses are infections at the back of the throat in which a collection of pus (abscess) has formed next to the tonsil. The condition is characterised by a severe sore throat, difficulty in swallowing and pain on swallowing, fever and malaise, and trismus (inability to open the mouth completely). Treatment is usually by one of two methods. The first is needle aspiration (sucking the pus out using a syringe and needle) and the second 'incision and drainage' (putting a small knife into the abscess to let the pus drain out). It remains unclear whether one type of treatment is better than the other.
Study characteristics
We included 11 studies with a total of 674 participants. The participants in the studies were aged from 8 to 79. The studies were conducted in a number of countries (six from Pakistan, two from the USA, one from Taiwan and two from South Africa). All but one of the 11 studies reported the difference in recurrence rate between needle aspiration and incision and drainage. Four studies compared symptom scores associated with the procedure and two studies compared time to resumption of normal diet. Three studies reported adverse effects/events associated with the intervention. Two studies reported complications of the disease process itself.
The evidence is current to August 2016.
Key results
Ten studies reported on the recurrence of peritonsillar abscess (our main outcome). Most of them did not clearly define 'recurrence' and they varied in the timing of its assessment, however we were able to combine (pool) the data from these studies. When we pooled the data the recurrence rate was higher in the needle aspiration group compared with incision and drainage. It is important to note that the evidence for this outcome was of very low quality. Some studies found that patients had more pain when they had incision and drainage.
Quality of the evidence
We identified problems or potential problems in all of the included studies. The most important of these was that the studies did not all assess recurrence in the same way, at the same time, using the same criteria. The quality of the evidence for all of the outcomes that we looked at was very low.
Summary of findings
Summary of findings for the main comparison. Needle aspiration versus incision and drainage for the treatment of peritonsillar abscess.
| Needle aspiration versus incision and drainage for the treatment of peritonsillar abscess | ||||||
| Patient or population: patients older than 8 years with peritonsillar abscess Setting: inpatients and outpatients Intervention: incision and drainage Comparison: needle aspiration | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with incision and drainage | Risk with needle aspiration | |||||
| Primary outcome: recurrence rate | Study population | RR 3.74, 95% CI 1.63 to 8.59 | 612 (10 RCTs) |
⊕⊝⊝⊝ very low1,2 | — | |
| 47 per 1000 | 245 per 1000 | |||||
| Primary outcome: adverse effects/events associated with the interventions | One study reported post‐procedural bleeding in 1 patient (3.6%) in the incision and drainage group, with no adverse effects/events reported in the needle aspiration group. Two studies stated that no complications were seen in either group. | — | 226 (3 RCTs) |
⊕⊝⊝⊝ very low2,3 | Adverse effects/events were not mentioned as a pre‐specified outcome measure in any of the studies. | |
| Secondary outcome: time to resumption of normal diet | One study found no difference in the time to resumption of normal diet (mean 3.7 days in both groups, no confidence intervals provided). Another study found that a similar percentage of patients returned to solid food within 4 days (87%: needle aspiration, 88%: incision and drainage). | — | 124 (2 RCTs) |
⊕⊝⊝⊝ very low2,4 | — | |
| Secondary outcome: complications of the disease process | One study described a complication of 2 patients requiring admission to hospital for dehydration in the incision and drainage group and no complications in the needle aspiration group. One study stated that no complications were seen in either group. | — | 170 (2 RCTs) |
⊕⊝⊝⊝ very low2,5 | Complications of the disease process were not mentioned as a pre‐specified outcome measure in any of the studies. | |
| Secondary outcome: symptom scores (Multiple different outcome scales used) |
Procedural pain Study 1 Pain was less in the needle aspiration group: MD ‐0.8, 95% CI ‐1.16 to ‐0.44 (10‐point scale) Study 2 Reported less pain in the needle aspiration group Pain resolution Study 3 Pain resolution was similar between groups at 5 days post‐intervention Other symptoms Study 4 Reported comparable symptom scores between groups at presentation and 48 hours |
— | Study 1 110 participants Study 2 56 participants Study 3 62 participants Study 4 52 participants |
⊕⊝⊝⊝ very low2,6 | — | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded once due to serious risk of inconsistency (unexplained heterogeneity).
2Downgraded twice due to very serious risk of bias (limitations in study design).
3Adverse event (post‐procedural bleeding) was not well described.
4Incomplete data (no standard deviations or confidence intervals provided).
5Admission to hospital for rehydration is inherently subjective and depends on multiple clinical variables.
6Downgraded once due to imprecision and differences in data reporting.
Background
Description of the condition
Quinsy, better known as peritonsillar abscess, is one of the most common abscesses treated by otolaryngologists. It is one of the most common ear, nose and throat (ENT) emergencies seen in acute ENT clinics and emergency departments in hospitals. Peritonsillar abscesses are a collection of pus between the fibrous capsule of the tonsil and the superior constrictor muscles of the pharynx. A peritonsillar abscess tends to be unilateral (on one side) and is believed to arise from obstructed Weber glands within the superior pole of the tonsil or from acute tonsillitis (Johnson 2005). In the United States, the incidence is estimated to be 30 people per 100,000 per year, which accounts for about 45,000 cases per year (Herzon 1995). Peritonsillar abscess affects people of all ages (Herzon 1995), and has a significant resource cost (Johnson 2005; Powell 2012).
Patients with a peritonsillar abscess present with fever, dysphagia, trismus, otalgia, a change in the voice and ipsilateral throat pain. On examination there is a swelling in the oropharynx with medialisation of the tonsil. Physical examination usually reveals uvular deviation to the contralateral side, tonsillar exudate, trismus and jugulodigastric lymphadenopathy. Patients with peritonsillar abscesses are at risk of extension of the abscess into deeper neck spaces and airway obstruction. A combination of symptoms and signs is the accepted basis for diagnosis. Further diagnostic investigations are not generally used unless the peritonsillar abscess presents with neck involvement (Powell 2012). In these circumstances the most common diagnostic test is the computed tomography (CT) scan. CT scans can accurately diagnose peritonsillar abscesses with 100% sensitivity and help to determine the extent of the disease. Ultrasound is less useful, with a diagnostic accuracy of 89% to 95% sensitivity and 79% to 100% specificity, based on level 3 evidence (Powell 2012).
Description of the intervention
Treatment commonly involves drainage of the abscess together with antibiotics, but there is no agreement on the optimal technique for initial drainage of a peritonsillar abscess (Hall 1990; Herzon 1995; Johnson 2003). Physicians generally have a choice between needle aspiration and incision and drainage, although quinsy tonsillectomy and antibiotic therapy alone are also less commonly used treatment options. Needle aspiration uses a large‐bore needle inserted through the palatoglossus muscles into the abscess. Several insertions of the needle in different locations may be performed during a single treatment episode. The incision and drainage method uses a guarded scalpel to incise the palatoglossus muscle and enter the peritonsillar space/abscess. The peritonsillar space is then opened widely by dissection with blunt forceps to promote drainage of the abscess. A patient with a peritonsillar abscess is sometimes treated with a combination of these methods.
Why it is important to do this review
Patients with a quinsy often present 'out of hours' and in many healthcare settings are first assessed and managed by doctors in training. A survey in the UK showed that 60% of otolaryngologists would use needle aspiration as their primary method for draining a peritonsillar abscess. If the needle aspiration failed, 52% would then perform scalpel incision and drainage. The survey also showed geographic differences in the management of peritonsillar abscess (Mehanna 2002). In Singapore, a retrospective review showed that most patients were treated with scalpel incision and drainage (66%) (Ong 2004).
There is no consensus regarding the best drainage procedure and each method has risks and benefits. Needle aspiration may potentially be less painful, cheaper and technically easier to perform; it can also double as a diagnostic method. Incision and drainage that includes blunt dissection theoretically promotes more effective drainage of the abscess by dissecting through the tissue barriers (septations) that divide the abscess cavity into micro‐cavities or loculations. The resulting wide pathway to the oral cavity allows air to enter the depths of the abscess cavity, increasing the oxygen tension that in turn reduces the survival of anaerobic bacteria exposed to air. However, it is a more invasive method and it has been suggested that it may carry a risk of aspiration of purulent material or incisional injury to underlying structures (Khayr 2005; Spires 1987). The method of drainage used may be associated with varying degrees of abscess recurrence, pain and haemorrhage. A review performed by Johnson et al concluded that needle aspiration was the best initial treatment, followed by incision and drainage if needle aspiration failed (Johnson 2003). Since then, additional studies on this subject have been undertaken, making it important to re‐evaluate the available evidence. If there is an optimal drainage procedure for peritonsillar abscesses, then it should be adopted widely.
Objectives
To assess the effectiveness and risks of needle aspiration versus incision and drainage for the treatment of peritonsillar abscess in older children (eight years of age or older), adolescents and adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Adult or child patients (of eight years of age or older) with a clinical diagnosis of peritonsillar abscess.
Types of interventions
Needle aspiration versus incision and drainage.
We defined needle aspiration as either a single insertion or multiple insertions of a needle with aspiration during the same clinical procedure. We defined needle aspirations undertaken on subsequent days or during a separate attendance at a healthcare facility on the same day as repeat interventions.
We defined incision and drainage as incision of the pharyngeal mucosa with or without any additional wound exploration to promote drainage. We considered 'confirmatory' needle aspiration immediately prior to this procedure as part of the incision and drainage intervention.
We considered antibiotic therapy as part of the intervention (needle aspiration or incision and drainage), as long as it was available to both treatment groups.
Types of outcome measures
Primary outcomes
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
Recurrence rate (proportion of patients needing repeat intervention)
Adverse effects/events associated with the interventions
Secondary outcomes
Time to resumption of normal diet
Complications of the disease process
Symptom scores
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 25 August 2016.
Electronic searches
The Information Specialist searched:
Cochrane ENT Trials Register (searched 25 August 2016);
Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 7);
-
Ovid MEDLINE (1946 to 25 August 2016):
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations);
PubMed (as a top up to searches in Ovid MEDLINE);
Ovid Embase (1974 to 2016 week 34);
EBSCO CINAHL (1982 to 25 August 2016);
LILACS, lilacs.bvsalud.org (searched 25 August 2016);
KoreaMed (searched via Google Scholar 25 August 2016);
IndMed, www.indmed.nic.in (searched 25 August 2016);
PakMediNet, www.pakmedinet.com (searched 25 August 2016);
Web of Knowledge, Web of Science (1945 to 25 August 2016);
CNKI, www.cnki.com.cn (searched via Google Scholar 25 August 2016);
ClinicalTrials.gov (searched via the Cochrane Register of Studies 25 August 2016);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), www.who.int/ictrp (searched 25 August 2016);
ISRCTN, www.isrctn.com (searched 25 August 2016);
Google Scholar, scholar.google.co.uk (searched 25 August 2016);
Google, www.google.com (searched 25 August 2016).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched Ovid MEDLINE, the Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
Data collection and analysis
Selection of studies
Two authors (BAC and AT) independently reviewed all retrieved articles and determine eligibility based on the inclusion criteria. We resolved disagreements by consensus discussion.
Data extraction and management
Two authors (BAC and AT) independently extracted data from studies using standardised data forms. Briefly, extracted data items included information on study design, study participants, study characteristics, interventions and outcomes. We extracted data so as to allow an intention‐to‐treat analysis. Where data were missing, we wrote to the authors of the study to request further information.
Assessment of risk of bias in included studies
BAC, AT and DAN undertook assessment of the risk of bias of the trials being considered for inclusion independently, with the following taken into consideration, as guided by the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective outcome reporting; and
other sources of bias.
We used the Cochrane 'Risk of bias' tool in RevMan 5.3 (RevMan 2014), which involves describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias. Each review author independently determined whether a study was sufficiently free of bias in each domain and a majority decision determined the ratings given.
Measures of treatment effect
Recurrence (primary outcome) was measured as dichotomous data. We used risk ratios (RR) with 95% confidence intervals to compare data. We planned to use the mean difference to assess continuous data. We had planned to use hazard ratios to assess time‐to‐event data.
Dealing with missing data
We made attempts to contact original investigators to obtain any missing data. We had planned sensitivity analysis to assess the impact of data that could not be obtained.
Assessment of heterogeneity
We assessed heterogeneity using the I² statistic. Roughly, we used the following thresholds as a guide to heterogeneity (RevMan 2014):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
We planned meta‐analysis for outcomes in which heterogeneity was less than 50%.
Assessment of reporting biases
We had intended the use of funnel plots to assess the potential for reporting (publication) bias, if necessary. However, this was not possible.
Data synthesis
We had intended to use a fixed‐effect model if there was minimal heterogeneity in the included studies (less than 30%); otherwise we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We had planned no subgroup analysis.
Sensitivity analysis
If there was any ambiguity regarding whether studies would meet the inclusion criteria or if study quality was insufficient, then we had intended the use of sensitivity analysis (repetition of the analysis with the inclusion of different data or trials due to decisions that may have been arbitrary or unclear) (Handbook 2011).
GRADE and 'Summary of findings' table
Two authors independently used the GRADE approach to rate the overall quality of evidence. The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we applied this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high quality of evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of very low quality implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high quality. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision; and
publication bias.
We included a 'Summary of findings' table, constructed according to the recommendations described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). We included the following outcomes in the 'Summary of findings' table: recurrence rate, adverse effects/events associated with the intervention, time to resumption of normal diet, complications of the disease process and symptom scores.
Results
Description of studies
Results of the search
The literature search identified 370 potential records after duplicates were removed. Of these, we deemed 17 eligible for full‐text review. We identified one additional article from searching the reference lists of these publications, yielding 18 total studies that we reviewed in full.
Eleven publications fulfilled the review's inclusion criteria (Chi 2014; Khan 2011; Khan 2012; Khokhar 2015; Maharaj 1991; Nwe 2000; Rafi 2007; Sheikh 2012; Spires 1987; Stringer 1988; Younas 2015). We included these 11 studies in the review.
We excluded six studies (Fry 1987; Herzon 1995; Khayr 2005; Kulkarni 2013; Stringer 1986; Wolf 1994). See Excluded studies and Characteristics of excluded studies.
One study, Tyagi 2011, is awaiting classification (see Characteristics of studies awaiting classification). Currently, there are no ongoing studies identified.
See Figure 1 for a PRISMA flow diagram depicting the search and study selection process.
1.
Process for sifting search results and selecting studies for inclusion.
Included studies
See Characteristics of included studies.
Design
All of the included studies were randomised controlled trials; however, three of the studies were quasi‐randomised (Khan 2012; Maharaj 1991; Spires 1987). Maharaj 1991 and Khan 2012 allocated patients on an alternate basis. Spires 1987 only randomised a portion of the patients. None of the trials were obviously blinded in terms of the study personnel collecting follow‐up data. Intention‐to‐treat analysis was not specifically carried out.
Sample sizes
The numbers of participants in the included studies were as follows: 62 (Spires 1987), 52 (Stringer 1988), 60 (Maharaj 1991), 75 (only 50 patients were in groups compared in this review) (Nwe 2000), 50 (Rafi 2007), 62 (Khan 2011), 56 (Khan 2012), 50 (Sheikh 2012), 110 (Chi 2014), 62 (Younas 2015) and 60 (Khokhar 2015). Other than Khokhar 2015, no mention was made of sample size calculations in any of the studies to ensure that they were adequately powered.
Setting
Nwe 2000 did not specify the setting of the study, although it was published in South Africa. Otherwise, all included studies took place in a hospital setting. Khan 2011, Khan 2012, Khokhar 2015, Rafi 2007, Sheikh 2012 and Younas 2015 all took place in Pakistan and were undertaken with participants in an inpatient hospital setting. Chi 2014 took place in Taiwan and also had participants admitted to hospital in an inpatient setting. The remaining studies treated patients in an outpatient hospital setting: Maharaj 1991 (South Africa), Spires 1987 (USA) and Stringer 1988 (USA).
Participants
Spires 1987 included patients aged 12 to 53, Stringer 1988 included patients aged 13 to 60, Nwe 2000 included patients aged 15 to 43, Khan 2011 included patients aged 15 to 35, Khan 2012 included patients aged 16 to 50 and Chi 2014 included patients aged 12 to 79. Rafi 2007 included patients aged 22 to 43 and Sheikh 2012 included patients aged 18 to 51. Khokhar 2015 included patients aged 17 to 53. Younas 2015 included patients aged 8 to 57. Maharaj 1991 included patients ranging from "under 14" to "over 40", and did not state how many were male or female. Nwe 2000 had a population that was 67% female; all the other studies had more male than female participants.
Interventions
All 11 studies compared needle aspiration with incision and drainage (Chi 2014; Khan 2011; Khan 2012; Khokhar 2015Maharaj 1991; Nwe 2000; Rafi 2007; Sheikh 2012; Spires 1987; Stringer 1988, Younas 2015). One study also compared a third group treated only with intravenous antibiotics (Nwe 2000).
Outcomes
Primary outcomes
All studies except one (Younas 2015) reported on recurrence rate (Chi 2014; Khan 2011; Khan 2012; Khokhar 2015; Maharaj 1991; Nwe 2000; Rafi 2007; Sheikh 2012; Spires 1987; Stringer 1988). However, the definition of recurrence was not well described. This is summarised in Table 2. Less than half of the studies provided a specific definition for recurrence. There was also wide variability in the method and timing of assessment for recurrence.
1. Definition of recurrence and timing.
| Study ID | Definition of recurrence or criteria for re‐intervention described | Timing of assessment of recurrence |
| Spires 1987 | No | 2, 7 days (2x returned day 1) |
| Stringer 1988 | "Failure to improve symptom scale score; visual evidence of a persistent abscess" | 1, 2 days (24, 48 hours) |
| Maharaj 1991 | "reaccumulation of pus" | 1, 7 days |
| Nwe 2000 | "patients in whom the trismus and pyrexia persisted 48 hours after the initial treatment" | 2 days (48 hours) |
| Rafi 2007 | No | Not stated |
| Khan 2011 | No | Not stated |
| Khan 2012 | No | Not stated |
| Sheikh 2012 | Yes* | 0, 1, 2 days |
| Chi 2014 | No | Not stated |
| Khokhar 2015 | No | "during the course of the study", 7, 14 days |
| Younas 2015 | N/A | N/A |
* "Improvement in patients was determined by examining the patient the next day after the procedure, a reduction in supra tonsillar swelling along with decrease in pain and also improvement in odynophagia were taken as criteria of improvement and termination of surgical attempts."
N/A: not available
Only three studies made reference to plans to identify and report any adverse events/effects associated with the intervention (Chi 2014; Khan 2012; Khokhar 2015).
Secondary outcomes
Time to resumption of normal diet was reported by two studies (Spires 1987; Younas 2015). Complications of the disease process were mentioned in two studies, with Maharaj 1991 reporting two complications (dehydration requiring hospitalisation) and Chi 2014 reporting no complications. Symptom scores were reported by four studies (Chi 2014; Khan 2012; Stringer 1988; Younas 2015), each using different scales. Chi 2014 measured pain intensity one hour after the procedure using a visual analogue score. Khan 2012 had patients grade their score as mild, moderate or severe and measured proportions. Younas 2015 compared the percentage of patients who had resolution of pain by five days after the intervention. Stringer 1988 scored patients according to a scale out of 5 (1 = eating normally, minimal to moderate pain; 2 = eating impaired, moderate pain; 3 = unable to eat solids, moderate to severe pain; 4 = unable to eat solids or liquids, severe pain; 5 = unable to eat solids or liquids, severe pain, volume depletion).
Excluded studies
After initial screening six studies did not meet the inclusion criteria (see Characteristics of excluded studies). One was classified as a retrospective study (Wolf 1994). Two were classified as review papers (Herzon 1995; Khayr 2005). One study was classified as a commentary (Fry 1987). We considered one study (Stringer 1986) an earlier publication of data in one of the included studies (Stringer 1988). One study was found to be non‐randomised (Kulkarni 2013).
Risk of bias in included studies
The risk of bias of the included studies is shown in the Characteristics of included studies table and summarised in Figure 2.
2.
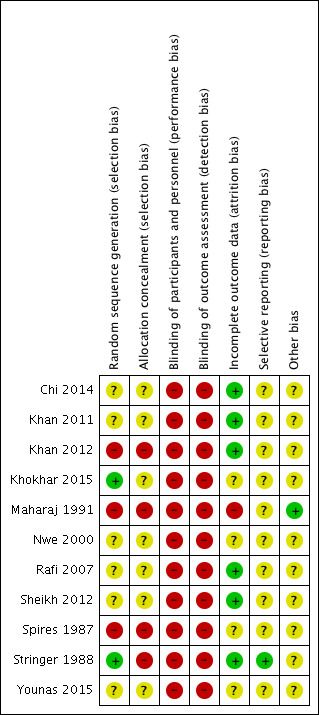
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All 11 included studies were reportedly randomised. However, it was not clear that proper random sequence generation had been used in any of the studies (Chi 2014; Khan 2011; Khan 2012; Maharaj 1991; Nwe 2000; Rafi 2007; Sheikh 2012; Spires 1987; Younas 2015), other than Stringer 1988 and Khokhar 2015 who used a random numbers table. Additionally, two studies allocated participants on an alternate basis (Khan 2012; Maharaj 1991), and one based on hospital number (Spires 1987). Allocation concealment was not specified to have been used in any of the studies (Chi 2014; Khan 2011; Khan 2012; Khokhar 2015; Maharaj 1991; Nwe 2000; Rafi 2007; Sheikh 2012; Spires 1987; Younas 2015). Stringer 1988 reported not using any allocation concealment.
Blinding
Blinding of participants or study personnel was either not used (Stringer 1988), or not specifically mentioned in any of the included studies (Chi 2014; Khan 2011; Khan 2012; Khokhar 2015; Maharaj 1991; Nwe 2000; Rafi 2007; Sheikh 2012; Spires 1987; Younas 2015).
Incomplete outcome data
Most studies did not specifically report losses to follow‐up. Several studies had patients admitted to hospital and we assumed in these cases that there were no losses unless specifically stated (Chi 2014; Khan 2011; Khan 2012; Rafi 2007; Sheikh 2012). Maharaj 1991 lost 18% of patients to follow‐up on day one and 37% at day seven. Patient losses were unclear in Spires 1987 and Nwe 2000. Khokhar 2015 excluded patients who failed to return for follow‐up. No mention of intention‐to‐treat analysis was made in any of the studies (Chi 2014; Khan 2011; Khan 2012; Khokhar 2015; Maharaj 1991; Nwe 2000; Rafi 2007; Sheikh 2012; Spires 1987; Stringer 1988; Younas 2015). In Younas 2015, there was a discrepancy between the number of patients reportedly randomised (62) and the number reported in the results (64); therefore, there is a possibility of incomplete or missing data.
Selective reporting
None of the included studies had published study protocols with pre‐specified outcomes. Stringer 1988 communicated that all measured outcomes were reported in the published manuscript and, therefore, we deemed it at low risk of bias in this category. We judged all the other studies to have an unclear risk of bias in this category (Chi 2014; Khan 2011; Khan 2012; Khokhar 2015; Maharaj 1991; Nwe 2000; Rafi 2007; Sheikh 2012; Spires 1987; Stringer 1988; Younas 2015).
Other potential sources of bias
We assessed all studies except one (Maharaj 1991) as having an unclear risk of bias in this category due to concerns about methodology. Specifically, five studies did not provide a definition for recurrence or criteria for re‐intervention (Chi 2014; Khan 2011; Khan 2012; Khokhar 2015; Rafi 2007; Spires 1987). Two studies had subjective definitions of recurrence, including persistent trismus (Nwe 2000), "failure to improve visual symptoms score", "visual evidence of a persistent abscess" (Stringer 1988) and "a reduction in supra tonsillar swelling along with decrease in pain and also improvement in odynophagia" (Sheikh 2012). Four studies did not specify the timing of assessment for recurrence (Chi 2014; Khan 2011; Khan 2012; Rafi 2007).
Effects of interventions
See: Table 1
See Table 1.
Primary outcomes
Recurrence rate (proportion of patients needing repeat intervention)
Ten out of 11 of the included studies reported recurrence rate following needle aspiration versus incision and drainage (Chi 2014; Khan 2011; Khan 2012; Khokhar 2015; Maharaj 1991; Nwe 2000; Rafi 2007; Sheikh 2012; Spires 1987; Stringer 1988). There was wide variability in the recurrence rate in both groups. Recurrence in the needle aspiration group ranged from 4.9% to 80.0%. In the incision and drainage group recurrence ranged from 0% to 20%. The studies that reported recurrence all reported a comparable or higher recurrence rate with needle aspiration.
Timing of assessment for recurrence was inconsistent. Specifically, timing of assessment for recurrence ranged from one day to seven days for initial post‐intervention assessment. The timing of follow‐up was not actually specified in four of the studies (Chi 2014; Khan 2011; Khan 2012; Rafi 2007). One study did not specifically report recurrence rate (Younas 2015). An "initial success rate" was reported; however, this was not defined.
When we pooled data from the 10 studies the recurrence rate was higher in the needle aspiration group compared with incision and drainage: risk ratio (RR) 3.74 (95% confidence interval (CI) 1.63 to 8.59; 612 participants). We detected moderate heterogeneity in this analysis (I2 = 48%). In interpreting the pooled result it is important to note that the quality of evidence for this outcome was very low.
Adverse effects/events associated with the interventions
Only one study reported an adverse outcome associated with the intervention (Khan 2012). In this study "reactionary haemorrhage" was described in one of 28 patients (3.6%) in the incision and drainage group. Chi 2014 and Khokhar 2015 stated that no complications were seen in either group. No other studies reported characterising adverse effects/events associated with the intervention.
Secondary outcomes
Time to resumption of normal diet
Two studies specifically compared time to resumption of normal diet (Spires 1987; Younas 2015). Spires 1987 found no difference in the average time to resumption of a normal diet between the needle aspiration versus the incision and drainage group (3.7 days, range 1 to 14 days in the needle aspiration group versus 3.7 days, range 1 to 10 days in the incision and drainage group; no standard deviation or statistics provided). Younas 2015 compared the percentage of patients that had returned to a semisolid or solid diet within a certain time frame. They found that 87% of patients treated with needle aspiration returned to semisolid food within two days and solid food within four days. Comparatively, they found that 88% of patients treated with needle aspiration returned to semisolid food by two days and solid food by four days. Two studies described "duration of symptoms" (Stringer 1988) or "period of recovery" (Rafi 2007), but did not specifically define these terms.
Complications of the disease process
Complications relating to the disease process were generally not reported. Only one study described a complication in two patients (6.7%) who required admission to hospital for dehydration (following incision and drainage) (Maharaj 1991). Chi 2014 stated that no complications were seen in either group. No other study specifically reported measuring complications of the disease process.
Symptom scores
Pain
Two studies reported on pain associated with the intervention. Chi 2014 reported pain intensity one hour after the procedure with visual analogue scores (out of 10) and found a statistically lower amount of pain in the needle aspiration group (4.5 ± 0.8 in the needle aspiration group versus 5.3 ± 1.1 in the incision and drainage group; mean difference (MD) ‐0.8, 95% CI ‐1.16 to ‐0.44, 10‐point scale). Khan 2012 compared postoperative pain as measured by a "mild/moderate/severe" subjective grade. They reported a statistically significant higher proportion of patients with higher pain scores in the incision and drainage group (needle aspiration: 50.0% mild, 28.6% moderate, 21.4% severe; incision and drainage: 17.9% mild, 21.4% moderate, 60.7% severe; P < 0.01). Outcome measures for these two studies were not comparable and, therefore, meta‐analysis was not possible. Younas 2015 reported on pain five days after the intervention: 75% of patients in the needle aspiration group had no pain, whereas 78% of patients treated with incision and drainage had no pain.
Other symptoms
One study reported symptom scores that were not directly related to procedural pain (Stringer 1988). This study reported symptom scores at presentation and 48 hours (1 = eating normally, minimal to moderate pain; 2 = eating impaired, moderate pain; 3 = unable to eat solids, moderate to severe pain; 4 = unable to eat solids or liquids, severe pain; 5 = unable to eat solids or liquids, severe pain, volume depletion). They found averages of 3.4 (needle aspiration) versus 3.3 (incision and drainage) at presentation and 1.3 (needle aspiration) versus 1.4 (incision and drainage), and complete resolution of symptoms at 10 days post‐treatment in all groups (no statistics or confidence intervals provided).
Discussion
Summary of main results
Peritonsillar abscess is one of the most common ear, nose and throat (ENT) emergencies presenting to acute ENT services and more often than not requires a procedural intervention as the mode of treatment. Despite this, there remains uncertainty about which technique ‐ needle aspiration or incision and drainage ‐ is more effective. There is an absence of high‐quality evidence to show whether or not one technique is superior to the other. There is very low‐quality evidence to suggest that there is a lower recurrence rate with incision and drainage compared with needle aspiration. At the same time, very low‐quality evidence also suggests that needle aspiration may be less painful than incision and drainage. There is an absence of evidence to answer questions about adverse effects/events associated with the intervention, time to resumption of normal diet, complications of the disease process and symptom scores for factors other than pain.
Overall completeness and applicability of evidence
Data from the studies identified are insufficient to allow us to address all of the objectives of this review and produce a clear and decisive answer. The included studies have enrolled the relevant types of participants. Whilst the studies do evaluate the interventions we sought to include, in some cases those interventions were part of a treatment plan involving periods of hospitalisation and courses of intravenous or oral antibiotics (or both) of varying length. This is relevant because in some healthcare settings patients are almost invariably managed as outpatients without being admitted to hospital. Not all outcomes that we felt were important had been investigated in the included studies. More significantly, the failure to define 'recurrence' and to include comprehensive information about the timing of any recurrence or its identification makes the evidence base incomplete. In all cases, for those outcomes for which we found data, the evidence was of very low quality.
There are likely to be geographic or institutional differences in the presentation of peritonsillar abscess. Six out of 11 studies originated from the same country (Pakistan) (see Characteristics of included studies). Different microbial patterns and choice of antimicrobial agents in different geographic areas could theoretically influence recurrence rates. The different settings in which patients were treated in the included studies highlights the potential for variation through different management protocols; over half of the included studies managed patients in an inpatient setting. Other factors of relevance include the likelihood of seeking medical attention, access to medical care and costs or thresholds for performing a repeat intervention.
In this review, we have not compared other treatment options for the management of patients with a peritonsillar abscess. So called 'quinsy tonsillectomy' or 'hot tonsillectomy' is another option available for treatment. We have not compared quinsy tonsillectomy, given that it is less commonly performed as a first‐line treatment (Qureshi 2015), and that different practical considerations guide the decision to perform it (such as patient age and operating room resources). We have also not compared non‐interventional options, such as antibiotic therapy alone. This was done in order to answer a specific clinical question of interest, as opposed to exploring all the available options for treating peritonsillar abscesses.
Quality of the evidence
Overall, the quality of evidence is very low for all the included outcomes (assessed using the GRADE criteria, see Table 1). The high risk of bias and flawed design of the studies reviewed is apparent when they are analysed using the Cochrane 'Risk of bias' tool (Figure 2). The descriptions of the methods used in most studies lacked the detail required to assess risk of bias completely. None of this review's predetermined outcome measures were well assessed by any of the trials. The specific criteria for determining recurrence were poorly defined in all of the studies.
A number of studies included in this review had recurrence rates higher than we might normally expect from day‐to‐day practice. In one study, the needle aspiration group had a recurrence rate as high as 80% (Rafi 2007). Khan 2012 had a recurrence rate as high as 64% in the needle aspiration group. Several studies had recurrence rates over 20% in the needle aspiration group (Khan 2011; Nwe 2000; Sheikh 2012). These recurrence rates differ significantly from the recurrence rates reported in other published studies and from our experience. In the review by Powell 2012, an overall recurrence rate for peritonsillar abscess of ˜10% was estimated with either needle aspiration or incision and drainage. This raises the question of the competence of the clinician or practitioner managing the condition and the criteria for defining recurrence, as well as the question of regional differences in peritonsillar abscess presentation and management. Ideally, recurrence should be confirmed by evidence of pus at a repeat incision or needle aspiration procedure. The absence of clearly stated diagnostic criteria for peritonsillar abscess recurrence in the studies makes it impossible to determine whether the same patients presenting to another clinician would be diagnosed as suffering from a recurrence or the more common post‐inflammatory oedema associated with a recent abscess.
The heterogeneity encountered in our assessment of recurrence rate, whilst only moderate (I2 = 48%), merits further discussion. As mentioned earlier, most of the studies did not clearly define 'recurrence' and varied widely in the timing of post‐intervention recurrence assessment. Only four of the 10 studies that measured recurrence provided a definition for what constituted 'recurrence' or specified criteria for re‐intervention. Definitions of recurrence included visual evidence of swelling (Stringer 1988), persistence of pyrexia/trismus (Nwe 2000), or persistent odynophagia (Sheikh 2012). Only one study defined recurrence with reference to the review's definition of an abscess, that is as "reaccumulation of pus" (Maharaj 1991). This study did not describe how the presence of pus was determined. Therefore, 'recurrence' in the studies reviewed could be anything along a spectrum from simply some residual symptoms to true reaccumulation of pus. Additionally, the timing of post‐treatment assessment for recurrence was not stated in four of the studies and was variable amongst the remaining studies (ranging from post‐procedure day 1 to 7). The inter‐study variation in the timing of post‐treatment review and the criteria used to diagnose peritonsillar abscess recurrence raises the question of whether 'recurrence' – unless much more clearly defined – is a valid outcome metric. Inter‐study methodological variation could account for the majority of the heterogeneity in recurrence rates. However, other factors may have contributed; it would be reasonable to postulate that clinical variability also contributed to the heterogeneity, given that there was wide inter‐study variation in treatment setting and adjunctive variables.
There is inherent operator variability in the delivery of the primary intervention itself. In many settings care for patients with quinsy is delivered by relatively junior doctors. We have defined the interventions as above (Types of interventions). However, differences in operator skill may partly explain differences in the outcome of the interventional studies. Variation in the timing of patient presentation for health care, co‐treatment factors such as antibiotic choice, and access and compliance with the prescribed antibiotic regimen in these studies, which are drawn from countries with divergent healthcare resources, may also account for heterogeneous outcomes. Limited methodological descriptions and our inability to gain further information from the authors make it difficult to explore these possible explanations.
One of the theoretical arguments against incision and drainage is that it is a more painful procedure for patients. Given that incision and drainage often involves a diagnostic needle aspiration in addition to further procedural intervention, it is understandable that this theory exists. In our review, two studies (n = 166) did suggest that subjective pain scores were higher in patients who underwent incision and drainage compared to needle aspiration (Chi 2014; Khan 2012). One study showed comparable resolution of pain at five days post‐treatment (Younas 2015). Given that this was assessed at five days after the procedure, it is difficult to separate the pain associated with the procedure from the resolution of pain associated with improvement of the disease process. Different measurement scales made pooled analysis impractical. However, the data are of limited quality for the reasons mentioned above and, therefore, this remains unclear.
Potential biases in the review process
We think it is unlikely that there have been significant biases in the review process itself. Whilst it is likely that all or most relevant studies have been identified, our failure to identify useable data from each included study has been universal. A few minor post hoc changes to the methods we had planned at protocol stage (Chang 2014) are described in Differences between protocol and review.
Authors' conclusions
Implications for practice.
Currently, only very low‐quality evidence is available to determine whether needle aspiration or incision and drainage is most effective for treating peritonsillar abscess. This suggests that incision and drainage is associated with a lower recurrence rate. The absence of high‐quality evidence results in a dilemma for practitioners and patients. Through shared and informed decision‐making, practitioners and patients must balance the potential ‐ but uncertain ‐ benefit of an incision and drainage procedure, which may be more painful, against needle aspiration, which may have of a greater chance of recurrence but be potentially less painful.
Implications for research.
A sufficiently powered, well‐designed, high‐quality study is required to compare the effectiveness of needle aspiration with incision and drainage for the treatment of peritonsillar abscesses. If a randomised controlled clinical trial were conducted, it would be useful to compare recurrence (in a strictly defined manner), adverse events/effects associated with the intervention, time until resumption of normal diet, complications of the disease process and symptom scores.
History
Protocol first published: Issue 4, 2006 Review first published: Issue 12, 2016
| Date | Event | Description |
|---|---|---|
| 30 June 2014 | New citation required and major changes | New authors. Withdrawn protocol redrafted, updated and republished. |
Notes
The original protocol was withdrawn from Issue 11, 2011 of theCochrane Library onwards as the authors were unable to continue with the review. A new protocol by new authors was published in 2014 (Chang 2014).
Acknowledgements
We would like to acknowledge Cochrane ENT for their helpful guidance and input.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Keir J, Almeyda R, Bowyer D and Wilbourn M were authors of the original protocol (withdrawn in 2011). New authors took over the review in 2014, publishing a new protocol (Chang 2014).
Appendices
Appendix 1. Search strategies
| CENTRAL | Ovid MEDLINE | EMBASE |
| #1 MeSH descriptor: [Peritonsillar Abscess] explode all trees #2 abscess* near tonsil* or abscess* near peritonsil* or abscess* near retrotonsil* or abscess* near peri‐tonsil* #3 suppurat* near tonsil* or suppurat* near peritonsil* or suppurat* near retrotonsil* or suppurat* near peri‐tonsil* #4 sepsis near tonsil* or sepsis near peritonsil* or sepsis near retrotonsil* or sepsis near peri‐tonsil* #5 septic near tonsil* or septic near peritonsil* or septic near retrotonsil* or septic near peri‐tonsil* #6 pus near tonsil* or pus near peritonsil* or pus near retrotonsil* or pus near peri‐tonsil* #7 infect* near peritonsil* or infect* near retrotonsil* or infect* near peri‐tonsil* #8 acute near peritonsil* or acute near retrotonsil* or acute near peri‐tonsil* #9 quinsy or "interval tonsil*" #10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 |
1 exp Peritonsillar Abscess/ 2 ((abscess* adj5 tonsil*) or (abscess* adj5 peritonsil*) or (abscess* adj5 retrotonsil*)).ab,ti. 3 ((suppurat* adj5 tonsil*) or (suppurat* adj5 peritonsil*) or (suppurat* adj5 retrotonsil*) or (suppurat* adj5 peri‐tonsil*)).ab,ti. 4 ((sepsis adj5 tonsil*) or (sepsis adj5 peritonsil*) or (sepsis adj5 retrotonsil*) or (sepsis adj5 peri‐tonsil*)).ab,ti. 5 ((septic adj5 tonsil*) or (septic adj5 peritonsil*) or (septic adj5 retrotonsil*) or (septic adj5 peri‐tonsil*)).ab,ti. 6 ((pus adj5 tonsil*) or (pus adj5 peritonsil*) or (pus adj5 retrotonsil*) or (pus adj5 peri‐tonsil*)).ab,ti. 7 ((infect* adj5 peritonsil*) or (infect* adj5 retrotonsil*) or (infect* adj5 peri‐tonsil*)).ab,ti. 8 ((acute adj5 peritonsil*) or (acute adj5 retrotonsil*) or (acute adj5 peri‐tonsil*)).ab,ti. 9 (quinsy or "interval tonsil*").ab,ti. 10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 |
1 exp Peritonsillar Abscess/ 2 ((abscess* adj5 tonsil*) or (abscess* adj5 peritonsil*) or (abscess* adj5 retrotonsil*)).ab,ti. 3 ((suppurat* adj5 tonsil*) or (suppurat* adj5 peritonsil*) or (suppurat* adj5 retrotonsil*) or (suppurat* adj5 peri‐tonsil*)).ab,ti. 4 ((sepsis adj5 tonsil*) or (sepsis adj5 peritonsil*) or (sepsis adj5 retrotonsil*) or (sepsis adj5 peri‐tonsil*)).ab,ti. 5 ((septic adj5 tonsil*) or (septic adj5 peritonsil*) or (septic adj5 retrotonsil*) or (septic adj5 peri‐tonsil*)).ab,ti. 6 ((pus adj5 tonsil*) or (pus adj5 peritonsil*) or (pus adj5 retrotonsil*) or (pus adj5 peri‐tonsil*)).ab,ti. 7 ((infect* adj5 peritonsil*) or (infect* adj5 retrotonsil*) or (infect* adj5 peri‐tonsil*)).ab,ti. 8 ((acute adj5 peritonsil*) or (acute adj5 retrotonsil*) or (acute adj5 peri‐tonsil*)).ab,ti. 9 (quinsy or "interval tonsil*").ab,ti. 10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 |
| CINAHL | Web of Science | ClinicalTrials.gov |
| S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 S9 quinsy or "interval tonsil*" S8 acute N5 peritonsil* or acute N5 retrotonsil* or acute N5 peri‐tonsil* S7 infect* N5 peritonsil* or infect* N5 retrotonsil* or infect* N5 peri‐tonsil* S6 pus N5 tonsil* or pus N5 peritonsil* or pus N5 retrotonsil* or pus N5 peri‐tonsil* S5 TX septic N5 tonsil* or septic N5 peritonsil* or septic N5 retrotonsil* or septic N5 peri‐tonsil* S4 TX sepsis N5 tonsil* or sepsis N5 peritonsil* or sepsis N5 retrotonsil* or sepsis N5 peri‐tonsil* S3 TX suppurat* N5 tonsil* or suppurat* N5 peritonsil* or suppurat*i N5 retrotonsil* or suppurat* N5 peri‐tonsil* S2 TX abscess* N5 tonsil* or abscess* N5 peritonsil* or abscess* N5 retrotonsil* or abscess* N5 peri‐tonsil* S1 (MH "Peritonsillar Abscess") |
#1 TOPIC: (abscess* near/5 tonsil* or abscess* near/5 peritonsil* or abscess* near/5 retrotonsil* or abscess* near/5 peri‐tonsil*) #2 TOPIC: (suppurat* near/5 tonsil* or suppurat* near/5 peritonsil* or suppurat* near/5 retrotonsil* or suppurat* near/5 peri‐tonsil*) #3 TOPIC: (sepsis near/5 tonsil* or sepsis near/5 peritonsil* or sepsis near/5 retrotonsil* or sepsis near/5 peri‐tonsil*) #4 TOPIC: (septic near/5 tonsil* or septic near/5 peritonsil* or septic near/5 retrotonsil* or septic near/5 peri‐tonsil*) #5 TOPIC: (pus near/5 tonsil* or pus near/5 peritonsil* or pus near/5 retrotonsil* or pus near/5 peri‐tonsil*) #6 TOPIC: (infect* near/5 peritonsil* or infect* near/5 retrotonsil* or infect* near/5 peri‐tonsil*) #7 TOPIC: (acute near/5 peritonsil* or acute near/5 retrotonsil* or acute near/5 peri‐tonsil*) #8 TOPIC: (quinsy or "interval tonsil*") #9 #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 |
((abscess OR sepsis OR septic OR pus OR infect OR acute) AND (tonsil* OR peritonsil* OR retrotonsil* OR per‐tonsil*)) OR quinsy OR "interval tonsil" |
Data and analyses
Comparison 1. Needle aspiration versus incision and drainage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of recurrence | 10 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 3.74 [1.63, 8.59] |
1.1. Analysis.
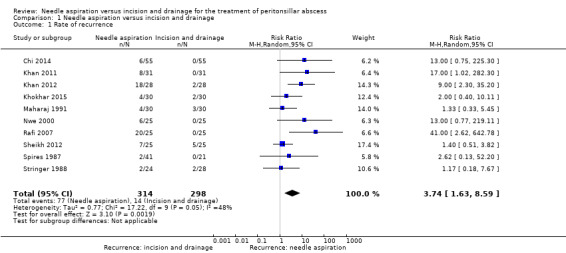
Comparison 1 Needle aspiration versus incision and drainage, Outcome 1 Rate of recurrence.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chi 2014.
| Methods |
Allocation: randomised trial Design: parallel‐group |
|
| Participants |
Number randomised: 110 Age range: 12 to 79 Gender: 89% males Setting: hospital, Taiwan Inclusion criteria: diagnosis of peritonsillar abscess Exclusion criteria: none stated Participant characteristics: mean age = 31.0 ± 15.0 years, days of symptoms prior to presentation = 4.7 ± 2.8 |
|
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 55 Comparator group: incision and drainage n = 55 Use of additional interventions: antibiotics, intravenous hydration |
|
| Outcomes | 1. Recurrence rate 2. Length of hospital stay 3. Pain score (Outcomes not specified as primary/secondary) |
|
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Participants lost to follow‐up: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly divided into two groups..." Comment: specific method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients presumably accounted for (admitted as inpatients); no dropouts stated |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias. Incomplete methodological description (definition of recurrence/criteria for re‐intervention not described, timing of assessment for recurrence not described). |
Khan 2011.
| Methods |
Allocation: randomised trial, alternation Design: parallel‐group |
|
| Participants |
Number randomised: 62 Age range: 15 to 35 Gender: 74% males Setting: hospital, Pakistan Inclusion criteria: not specifically stated Exclusion criteria: other associated illness Participant characteristics: mean age = 24.6 years |
|
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 31 Comparator group: incision and drainage n = 31 Use of additional interventions: antibiotics (injection benzyl penicillin and metronidazole), analgesics |
|
| Outcomes | 1. Recurrence rate 2. Length of hospital stay (Outcomes not specified as primary/secondary) |
|
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Needle aspiration failures treated with incision and drainage Participants lost to follow‐up: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients presumably accounted for (admitted as inpatients); no dropouts stated |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias. Incomplete methodological description (definition of recurrence/criteria for re‐intervention not described, timing of assessment for recurrence not described). |
Khan 2012.
| Methods |
Allocation: randomised trial, alternation Design: parallel‐group |
|
| Participants |
Number randomised: 56 Age range: 16 to 50 Gender: 71% males Setting: hospital, Pakistan Eligibility criteria: age > 15 years with peritonsillar abscess Exclusion criteria: patients with bleeding disorders, acute follicular tonsillitis Participant characteristics: mean age = 31.2 years |
|
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 28 Comparator group: incision and drainage n = 28 Use of additional interventions: antibiotics (intravenous amoxicillin/clavulanate and metronidazole), povidone‐iodine (Pyodine) mouth wash, intravenous crystalloid (if necessary) |
|
| Outcomes | 1. Recurrence rate 2. Symptom score 3. Length of hospital stay (Outcomes not specified as primary/secondary) |
|
| Funding sources | None stated | |
| Declarations of interest | None declared | |
| Notes | Alternate basis randomisation Participants lost to follow‐up: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate basis randomisation |
| Allocation concealment (selection bias) | High risk | Alternate basis randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients presumably accounted for (admitted as inpatients); no dropouts stated |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias. Incomplete methodological description (definition of recurrence/criteria for re‐intervention not described, timing of assessment for recurrence not described) |
Khokhar 2015.
| Methods |
Allocation: randomised trial Design: parallel‐group |
|
| Participants |
Number randomised: 70 (data reported on 60) Age range: 17 to 53 Gender: 76.7% males Setting: military hospital, Pakistan Inclusion criteria: "clinical diagnosis of PTA was made on clinical features of unitonsillar erythema, swelling, odynophagia (pain on swallowing), uvular deviation towards the opposite direction and trismus", age ≥ 15 Exclusion criteria: "patients with a history of bleeding disorders or diabetes mellitus, on anticoagulant drugs or diagnosed with immunodeficiency disorders or who refused to undergo the procedure under local anesthesia"; "Patients failing to follow up were excluded from this study" Participant characteristics: mean age 32.7 |
|
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 35 Comparator group: incision and drainage n = 35 Use of additional interventions: Use of additional interventions: antibiotics and analgesia ((a) co‐amoxiclav 1.2 g intravenously 8‐hourly and metronidazole 500 mg intravenously 8‐hourly for 3 days, followed by oral co‐amoxiclav 1 g twice daily and metronidazole 400 mg 3 times a day for the next 4 days, (b) paracetamol 1 g 8‐hourly orally "for fever and analgesia") |
|
| Outcomes | 1. Time of resolution of odynophagia (days) 2. Fever – time to resolution (days) Above information used to define "Recovery period" as "time taken to settle both odynophagia and fever" 3. Recurrence 4. Complications |
|
| Funding sources | Not specified | |
| Declarations of interest | None declared | |
| Notes | Participants lost to follow‐up: "Patients failing to follow up were excluded from this study" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a "random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | Not certain if random numbers table was open |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding; probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1. "Patients, who had cultured organisms resistant to [the] antibiotics [named above] were excluded from the study" 2. "Patients failing to follow up were excluded from this study" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias |
Maharaj 1991.
| Methods |
Allocation: randomised, alternation Design: parallel‐group |
|
| Participants |
Number randomised: 60 Age range: "under 14" to "over 40" Gender: not specified Setting: hospital, South Africa Inclusion criteria: positive needle aspiration Exclusion criteria: negative needle aspiration Participant characteristics: mean age not provided, "all patients presented with some degree of odynophagia and drooling of saliva", 47% had trismus and 27% had pyrexia |
|
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 30 Comparator group: incision and drainage n = 30 Use of additional interventions: antibiotics (penicillin), analgesics, mouthwash (unspecified) |
|
| Outcomes | 1. Recurrence rate (Outcomes not specified as primary/secondary) |
|
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Participants lost to follow‐up: 11 (18%) at day 1, 22 (37%) at day 7 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised on alternate basis |
| Allocation concealment (selection bias) | High risk | Randomised on alternate basis |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 82% of patients followed up on day 1 post‐treatment and 63% followed up on day 7 |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Low risk | No other sources of bias identified |
Nwe 2000.
| Methods |
Allocation: randomised trial Design: parallel‐group |
|
| Participants |
Number randomised: 75 (only 50 patients were in groups compared in this review) Age range: 15 to 43 Gender: 33% males Setting: South Africa Inclusion criteria: not described Exclusion criteria: none stated Participant characteristics: "unilateral swelling of the tonsil and soft palate, and medial displacement of the uvula", "all patients were pyrexial" |
|
| Interventions | Needle aspiration versus incision and drainage versus intravenous antibiotics alone Intervention group: intravenous antibiotics n = 25 Comparator group 1: needle aspiration n = 25 Comparator group 2: incision and drainage n = 25 Use of additional interventions: single dose of intravenous antibiotics |
|
| Outcomes | 1. Distance between upper and lower incisor teeth (degree of trismus) 2. Body temperature 3. Ability to drink water 4. Microbiology cultures 5. Treatment failures (recurrence) |
|
| Funding sources | None stated | |
| Declarations of interest | None declared | |
| Notes | Participants lost to follow‐up: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not clear, dropouts not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias |
Rafi 2007.
| Methods |
Allocation: randomised trial Design: parallel‐group |
|
| Participants |
Number randomised: 50 Age range: 22 to 43 Gender: 94% males Setting: hospital, Pakistan Inclusion criteria: "peritonsillar abscess" Exclusion criteria: none stated Participant characteristics: "all the patients were otherwise healthy and young with no immune compromising disease", no patients with previous peritonsillar abscess |
|
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 25 Comparator group: incision and drainage n = 25 Use of additional interventions: antibiotics (lincomycin) |
|
| Outcomes | 1. Recurrence rate 2. Length of hospital stay 3. "Period of recovery" (Outcomes not specified as primary/secondary) |
|
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Participants lost to follow‐up: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients presumably accounted for (admitted as inpatients); no dropouts stated |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias. Incomplete methodological description (definition of recurrence/criteria for re‐intervention not described, timing of assessment for recurrence not described) |
Sheikh 2012.
| Methods |
Allocation: randomised trial Design: parallel‐group |
|
| Participants |
Number randomised: 50 Age range: 18 to 51 Gender: 62% males Setting: hospital, Pakistan Inclusion criteria: "presented with peritonsillar abscess" Exclusion criteria: diabetics, less than 18 years old Participant characteristics: mean age = 32.7 years |
|
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 25 Comparator group: incision and drainage n = 25 Use of additional interventions: antibiotics, chlorhexidine mouth wash |
|
| Outcomes |
Primary outcome: recurrence rate Secondary outcomes: none |
|
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Needle aspiration failures treated with incision and drainage Participants lost to follow‐up: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients presumably accounted for (admitted as inpatients); no dropouts stated |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias. Subjective definition of recurrence/criteria for re‐intervention |
Spires 1987.
| Methods |
Allocation: randomised trial Design: parallel‐group |
|
| Participants |
Number randomised: 62 Age range: 12 to 53 Gender: "2:1 male predilection" Setting: hospital, USA Inclusion criteria: not specified Exclusion criteria: not specified Participant characteristics: median age = 24; 1 patient with bilateral abscesses |
|
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 41 Comparator group: incision and drainage n = 21 Use of additional interventions: analgesics, antibiotics (penicillin V or cephalexin or erythromycin) |
|
| Outcomes | 1. Recurrence rate 2. Time to resumption of normal diet (Outcomes not specified as primary/secondary) |
|
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Only partial randomisation Participants lost to follow‐up: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | First 15 participants treated with needle aspiration, then patients were subsequently randomised |
| Allocation concealment (selection bias) | High risk | Randomised by hospital number |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not clear, dropouts not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risk of bias. Incomplete methodological description (definition of recurrence/criteria for re‐intervention not described) |
Stringer 1988.
| Methods |
Allocation: randomised trial Design: parallel‐group |
|
| Participants |
Number randomised: 52 Age range: 13 to 60 Gender: 60% males Setting: hospital, USA Inclusion criteria: positive needle aspiration Exclusion criteria: negative needle aspiration Participant characteristics: mean age = 27 years average duration of symptoms prior to presentation = 5.3 days |
|
| Interventions | Needle aspiration versus incision and drainage Intervention group: needle aspiration n = 24 Comparator group: incision and drainage n = 28 Use of additional interventions: antibiotics (initial dose of intramuscular penicillin G followed by intramuscular penicillin G or oral penicillin V for 10 days), erythromycin/cephalosporin/clindamycin if allergic |
|
| Outcomes | 1. Recurrence rate 2. Symptom score (Outcomes not specified as primary/secondary) |
|
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Authors contacted and further information/clarification obtained Participants lost to follow‐up: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | High risk | No allocation concealment performed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding made; probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts mentioned |
| Selective reporting (reporting bias) | Low risk | All measured outcomes stated to have been reported |
| Other bias | Unclear risk | Not enough data to assess other risk of bias. Subjective definition of recurrence/criteria for re‐intervention. |
Younas 2015.
| Methods |
Allocation: randomised trial Design: parallel‐group |
|
| Participants |
Number randomised: 62 (discrepant total = 64 in results) Age range: 8 to 57 Gender: 61.3% male Setting: hospital, Pakistan Eligibility criteria: "clinically diagnosed for peritonsillar abscess" Exclusion criteria: none stated Participant characteristics: average duration of symptoms prior to presentation = 6 days; 84% treated with antibiotics prior to presentation |
|
| Interventions |
Intervention group: needle aspiration n = 32 Intervention group: incision and drainage n = 32 Use of additional interventions: not stated |
|
| Outcomes | 1. "Success" – not defined 2. Length of time to return to semisolid/solid food (days) 3. Proportion with "no pain by 05 days" (%) 4. Length of hospital stay (days) |
|
| Funding sources | Not specified | |
| Declarations of interest | Not specified | |
| Notes | Participants lost to follow‐up: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding; probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not clear; dropouts not mentioned; numbers in methods and results do not match |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement of high or low risk |
| Other bias | Unclear risk | Not enough data to assess other risks of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fry 1987 | Commentary |
| Herzon 1995 | Review paper |
| Khayr 2005 | Review paper |
| Kulkarni 2013 | Participants were not randomised: "All patients were divided in two groups according to surgical procedures carried out". While the study states that this was a prospective study, it seems that outcomes were assessed retrospectively. |
| Stringer 1986 | Considered a duplicate publication of Stringer 1988 |
| Wolf 1994 | Retrospective study |
Characteristics of studies awaiting assessment [ordered by study ID]
Tyagi 2011.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | Study not available at this time. This study was inaccessible by all attempted means, including contact with the journal editor |
Differences between protocol and review
We widened the age criteria for participants in included studies based on post hoc review. A number of studies included older child (generally > 8 years old), adolescent and adult participants. Stratified data by age group (adults versus adolescents) were not available in any of the studies. Given that adolescents and older children with peritonsillar abscess are often managed similarly to adults, we thought that the review would also be applicable to this age group. Younger patients typically require a general anaesthetic for management and, therefore, have different practical considerations for intervention. We therefore made a decision to include trials with older children (age > 8), adolescents and adults.
We added the methods for the creation of a 'Summary of findings' table and GRADE assessment to the review after the protocol was published.
We also added a standard statement to clarify the role of outcomes in the review (Types of outcome measures).
Contributions of authors
Brent A Chang: literature searching, study selection, data collection, data management, 'Risk of bias' assessment, data analysis, data interpretation, drafting the protocol and review.
Andrew Thamboo: literature searching, study selection, data collection, data management, 'Risk of bias' assessment, data analysis, data interpretation, drafting the protocol and review.
Chris Diamond: protocol design, content expertise, revising the protocol and review, critical appraisal and study selection.
Martin J Burton: content expertise, data interpretation, revising the review.
Desmond A Nunez: protocol design, 'Risk of bias' assessment, content expertise, revising the protocol and review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
Declarations of interest
Brent A Chang: none to declare.
Andrew Thamboo: none to declare.
Chris Diamond: none to declare.
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial sign‐off this review, which was carried out by Cochrane ENT's second Co‐ordinating Editor.
Desmond A Nunez: none to declare.
New
References
References to studies included in this review
Chi 2014 {published data only}
- Chi TH, Yuan CH, Tsao YH. Comparison of needle aspiration with incision and drainage for the treatment of peritonsillar abscess. WIMJ Open 2014;1(1):11‐3. [Google Scholar]
Khan 2011 {published data only}
- Khan Q, Hamza A, Haleem G, Wahid FI. Comparative study of management of peritonsillar abscess by needle aspiration versus incision and drainage. Pakistan Journal of Medical & Health Sciences 2011;5(4):699‐701. [Google Scholar]
Khan 2012 {published data only}
- Khan MI, Iqbal K, Muhammad. Peritonsillar abscess: comparison of outcome of incision and drainage versus needle aspiration. Gomal Journal of Medical Sciences 2012;10(2):205‐8. [Google Scholar]
Khokhar 2015 {published data only}
- Khokhar MR, Raza SN, Ayaz SB. Comparison of efficacy of needle aspiration versus incision and drainage in management of peritonsillar abscess. Pakistan Armed Forces Medical Journal 2015;65:172‐7. [Google Scholar]
Maharaj 1991 {published data only}
- Maharaj D, Rajah V, Hemsley S. Management of peritonsillar abscess. Journal of Laryngology and Otology 1991;105:743‐5. [DOI] [PubMed] [Google Scholar]
Nwe 2000 {published data only}
- Nwe TT, Singh B. Management of pain in peritonsillar abscess. Journal of Laryngology and Otology 2000;114:765‐7. [DOI] [PubMed] [Google Scholar]
Rafi 2007 {published data only}
- Rafi T, Farooq MU, Sheikh HU. Comparative study of management of peritonsillar abscess by needle aspiration versus incision and drainage. Journal of Surgery Pakistan 2007;12(3):126‐8. [Google Scholar]
Sheikh 2012 {published data only}
- Sheikh ZA, Najam A, Ahmed A. Needle aspiration an equal to incision and drainage in management of peritonsillar abscess. Pakistan Armed Forces Medical Journal 2012;62(2):online. [Google Scholar]
Spires 1987 {published data only}
- Spires JR, Owens JJ, Woodson GE, Miller RH. Treatment of peritonsillar abscess. Archives of Otolaryngology ‐ Head & Neck Surgery 1987;113:984‐6. [DOI] [PubMed] [Google Scholar]
Stringer 1988 {published data only}
- Stringer SP, Schaefer SD, Close LG. A randomized trial for outpatient management of peritonsillar abscess. Archives of Otolaryngology ‐ Head & Neck Surgery 1988;114(3):296‐8. [DOI] [PubMed] [Google Scholar]
Younas 2015 {published data only}
- Younas M. Satisfaction of patients with peritonsillar abscess with permucosal needle aspiration versus incision & drainage. Medical Forum Monthly 2015;26(3):37‐9. [Google Scholar]
References to studies excluded from this review
Fry 1987 {published data only}
- Fry TL. A randomized trial for outpatient management of peritonsillar abscess. Archives of Otolaryngology ‐‐ Head and Neck Surgery 1987;113(1):19. [DOI] [PubMed] [Google Scholar]
Herzon 1995 {published data only}
- Herzon FS. Peritonsillar abscess: incidence, current management practices, and a proposal for treatment guidelines. Laryngoscope 1995;105:1‐17. [DOI] [PubMed] [Google Scholar]
Khayr 2005 {published data only}
- Khayr W, Taepke J. Management of peritonsillar abscess: needle aspiration versus incision and drainage versus tonsillectomy. American Journal of Therapeutics 2005;12:344‐50. [PubMed] [Google Scholar]
Kulkarni 2013 {published data only}
- Kulkarni V, Patel T. Management of peritonsillar abscess: comparative prospective study of needle aspiration and incision & drainage in central Indian population. Asian Journal of Biomedical and Pharmaceutical Sciences 2013;3(18):61‐3. [Google Scholar]
Stringer 1986 {published data only}
- Stringer SP, Schaefer SD, Close LG. A randomized trial for outpatient management of peritonsillar abscess. Otolaryngology ‐ Head and Neck Surgery 1986;114(3):296‐8. [DOI] [PubMed] [Google Scholar]
Wolf 1994 {published data only}
- Wolf M, Even‐Chen I, Kronenberg J. Peritonsillar abscess: repeated needle aspiration versus incision and drainage. Annals of Otology, Rhinology & Laryngology 1994;103(7):554‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Tyagi 2011 {published data only}
- Tyagi V, Kaushal A, Garg D, De S, Nagpure P. Treatment of peritonsillar abscess: a prospective study of aspiration versus incision and drainage. Calcutta Medical Journal 2011;9(3):e3. [Google Scholar]
Additional references
Hall 1990
- Hall SF. Peritonsillar abscess: the treatment options. Journal of Otolaryngology 1990;19:226‐9. [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johnson 2003
- Johnson RF, Stewart MG, Wright CC. An evidence based review of the treatment of peritonsillar abscess. Otolaryngology ‐ Head and Neck Surgery 2003;128(3):332‐43. [DOI] [PubMed] [Google Scholar]
Johnson 2005
- Johnson RF, Stewart MG. The contemporary approach to diagnosis and management of peritonsillar abscess. Current Opinion in Otolaryngology & Head and Neck Surgery 2005;13:157‐60. [DOI] [PubMed] [Google Scholar]
Mehanna 2002
- Mehanna HM, Al‐Bahnasawi L, White A. National audit of the management of peritonsillar abscess. Postgraduate Medical Journal 2002;78(923):545‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ong 2004
- Ong YK, Goh YH, Lee YL. Peritonsillar infections: local experience. Singapore Medical Journal 2004;45(3):105‐9. [PubMed] [Google Scholar]
Powell 2012
- Powell J, Wilson JA. An evidence‐based review of peritonsillar abscess. Clinical Otolaryngology 2012;37(2):136‐45. [PUBMED: 22321140] [DOI] [PubMed] [Google Scholar]
Qureshi 2015
- Qureshi H, Ference E, Novis S, Pritchett CV, Smith SS, Schroeder JW. Trends in the management of pediatric peritonsillar abscess infections in the U.S., 2000‐2009. International Journal of Pediatric Otorhinolaryngology 2015;79(4):527‐31. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
References to other published versions of this review
Chang 2014
- Chang BA, Thamboo A, Diamond C, Nunez DA. Needle aspiration versus incision and drainage for the treatment of peritonsillar abscess. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD006287.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


