Abstract
Background
Behavioural and psychological symptoms in dementia (BPSD) form an important sub-syndrome of dementia. We assessed the frequency and severity of BPSD in a random sample of Hungarian treatment-naïve dementia patients. Furthermore, we examined the relationship between cognitive symptoms and BPSD and the pattern of BPSD in specific types of dementias.
Methods
Patients (n=131) were classified into 3 groups: Alzheimer’s (AD), vascular (VD), and mixed (MD) dementia. The Mini-Mental State Examination (MMSE), Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) and Neuropsychiatric Inventory (NPI) neuropsychological tests were employed.
Results
Mean age and MMSE score did not differ significantly among groups. BPSD was frequent (100% prevalence, NPI mean total Frequency score: 14.58, SD=7.55); abnormal motor behaviour showed the highest frequency. Hallucinations and delusions were related to the aetiology of dementia and were independent of the level of cognitive deterioration, whereas irritability, sleep-wake cycle dysfunctions, and eating-appetite change were associated with cognitive deterioration and were independent from aetiology. Both aberrant motor behaviour and disinhibition were significantly associated with aetiology and cognitive deterioration.
Conclusions
BPSD symptoms are significant constituents of dementia syndromes, affecting quality of life and substantially contributing to the caregiver’s burden. Specific symptom patterns can be identified in different types of dementia.
Keywords: Alzheimer’s disease, Behaviour and psychological symptoms in dementia (BPSD), Dementia, Neurocognitive disorder, Vascular dementia
1. Introduction
The 2010 WHO report drew attention to the importance of the increasing prevalence of dementia and to the urgent need for a solution to proper care of dementia patients, given its global burdens and dangers [1]. Based on the WHO report, 682 million people will probably suffer from Alzheimer’s disease or other old-age dementia by 2050 versus the present 36 million dementia patients.
Based on the DSM-IV [2], dementia is a gradually developing decline of cognitive functions that results in occupational and social dysfunctions. Beyond memory problems, at least one of the following symptoms must be present: aphasia (language disturbance), apraxia (impaired ability to carry out motor activities despite intact motor function), agnosia (failure to recognize or identify objects despite intact sensory function), disturbance in executive functioning (i.e., planning, organizing, sequencing, abstracting).
Given its possible stigmatizing effect, in DSM-5 the term dementia was replaced by neurocognitive disorder (NCD), which can be classified as mild or major and by its aetiology. NCD covers all types of diseases in which the core feature of the disease is cognitive dysfunction appearing as a decline from a previous level [3]. Mean-while, the ICD-10 [4] is still in use worldwide; in that system, the term and the diagnostic criteria for dementia remain unchanged. Because Hungarian clinical practice follows ICD-10 and our research work had started earlier than the publication of DSM-5, in this paper we use the diagnostic category of dementia, and the term according to ICD-10 [4].
The two most frequent aetiological forms of dementia are Alzheimer’s disease and vascular dementia; the occurrence of a mixed form is also common [5,6]. The most accepted categories for the clinical symptoms of dementias are cognitive, affective, and behavioural symptoms. The core feature of dementias are cognitive symptoms, whereas affective and behavioural symptoms are frequently reported as “behavioural and psychological symptoms of dementia”, or BPSD [7,8]. Differentiation, identification, and quantification of cognitive symptoms associated with dementia are more convenient for clinicians in everyday practice than identification and assessment of BPSD symptoms [5,8]. BPSD symptoms had been regarded as a ‘neglected area’ of geronto-psychiatry [9]. The International Psychiatric Association (IPA) introduced the term BPSD (IPA Complete Guides to Behavioural and Psychological Symptoms of Dementia [10]) in 1996. BPSD is considered a non-disease–specific clinical syndrome, a sub-syndrome that consists of heterogenic psychiatric symptoms. The interaction of biological, psychological, and social factors is assumed to be behind the development of BPSD, which consists of two subgroups of symptoms, psychological and behavioural. The psychological symptoms include delusion, hallucination, misidentification, depression, apathy, and anxiety. The behavioural symptoms include irritability, agitation, aggression, wandering or aberrant motor activity, disinhibition, sleep-wake cycle disturbance, and eating disorders [11,12]. Regarding the relevance of BPSD symptoms, relevant literature suggests that their role in the disease-related costs and other economic indexes far exceeds that of cognitive symptoms [13]. A recently published review explored the results of the last 30 years’ finding in dementia research and emphasized the importance of BPSD symptoms [14]. The central role of BPSD symptoms for both the patients with dementia and their caregivers was clearly pointed out in this summary. According to the results of that review, at least two BPSD symptoms appeared in more than 90% of dementia patients, indicating connection with longer medical care, medicine abuse, and increased healthcare costs. The study suggested that the significance of BPSD symptoms lies in their contribution to the process in which primary characteristics and identity of dementia patients become unavoidably lost [14]. These effects of BPSD symptoms play an important role in caregiver distress, which in turn is a fundamental factor in the decision of social welfare placement of dementia patients [15]. The effects of BPSD symptoms also play a key role in the increasing mortality rates of those patients [8].
To our knowledge, no data on the systematic evaluation of BPSD symptoms in dementia patients are available for Hungary. Thus, the first objective of this study was to evaluate the frequency and the severity of BPSD symptoms in a random sample of Hungarian dementia patients. We expected frequency and severity rates to be similar to those reported in the literature. The second objective of this study was to examine the relationship between cognitive and BPSD symptoms, and the potential for using BPSD symptoms to differentiate clinically distinct types of dementias, specifically Alzheimer’s type, vascular type, and mixed type of dementia. We hypothesized that the presentation of BPSD symptoms would show a specific pattern in different types of dementias.
2. Methods
2.1. Study sample
Our study sample was a random, treatment-naïve sample of dementia patients (n=131). We included patients who were referred by their GP for clinical evaluation and care because of the suspected diagnosis of dementia, and who appeared for the first time in a dementia specialist practice at the Psychiatry Clinic and Neurology Clinic of the Clinical Centre of the University of Debrecen between February 2013 and April 2015. Further inclusion criteria were that patients had to live in their own apartment and not in a social welfare home or other nursing facility, and that the patient had to arrive with their relatives. Exclusion criteria were ongoing treatment for depression or any other known mental disorder, vision, or/and hearing disorder that would have interfered with the completion of the tests in the study.
This investigation was carried out in accordance with the latest version of the Declaration of Helsinki. The study was approved by the local ethics committee and all subjects provided written informed consent.
2.2. Neuropsychological examination
After collecting demographic data and anamnesis based on a clinical interview, we performed a detailed neuropsychological examination focusing on the cognitive deterioration, BPSD symptoms, quality of life, illness intrusiveness, and the burden on relatives. The evaluation process took approximately one hour per patient. In this paper we focus on the results obtained from tests measuring cognitive functions and BPSD symptoms. The following tests were applied:
2.2.1. Mini-Mental State Examination (MMSE)
The MMSE is a brief standardized instrument that has been used extensively to assess individuals’ cognitive function. The MMSE score ranges from 0 to 30, with higher scores indicating better cognitive function. The MMSE assesses attention, orientation, language, ability to follow simple verbal and written commands, and immediate and short-term recall [16].
We used MMSE because it is a widely known and used test for screening dementia, and it is part of the clinical protocol of the assessment for dementia diagnoses. However, we did not include the results of the MMSE in our analyses: because the ADAS-Cog test (introduced in upcoming text) seemed to be more appropriate for evaluation of the cognitive functions of the patient from a broader perspective.
2.2.2. Alzheimer Disease Assessment Scale-Cognitive Test (ADAS-Cog)
This is a new rating instrument that was designed specifically to evaluate the severity of cognitive and noncognitive behavioural dysfunctions and characteristics of patients with Alzheimer’s disease. The original version of the ADAS-Cog consists of 11 items, including a Word Recall Task, Naming Objects and Fingers, Following Commands, Constructional Praxis, Ideational Praxis, Orientation, Word Recognition Task, Remembering Test Directions, Spoken Language, Comprehension, and Word-finding Difficulty. The ADAS-Cog score can vary between 0 and 70 (the greater the dysfunction, the higher the score). A usual score for someone who does not have any type of dementia is five [17,18].
2.2.3. Neuropsychiatric Inventory (NPI)
The NPI was developed by Cummings et al. (1994) [19] to assess dementia-related behavioural symptoms. The NPI originally examined 10 sub-domains of behavioural functioning: delusions, hallucinations, agitation/aggression, dysphoria, anxiety, euphoria, apathy, disinhibition, irritability/lability, and aberrant motor activity. Two more sub-domains have been added since its development: night-time behavioural disturbances, appetite and eating abnormalities. This wide variety of domains means that, unlike other dementia measures, the NPI is able to screen for BPSD symptoms in multiple types of dementia. The NPI is assessed based on caregiver report. A screening question is asked about each sub-domain. If the responses to these questions indicate that the patient has problems with the behaviour addressed by the particular sub-domain in question, the caregiver is then asked all the questions only about that domain. The NPI rates the frequency of the symptoms on a 4-point scale, the severity of the symptoms on a 3-point scale, and the distress the symptom causes for the caregiver on a 5-point scale. The maximum score is 144 [19].
2.3. Diagnosis of dementia: Assigning patients into diagnostic groups
Patients who received the clinical diagnosis of dementia in the outpatient clinic were further divided into 3 groups: Alzheimer’s-disease (AD), vascular-dementia (VD), and mixed-dementia (MD).
The diagnosis of dementia and assigning diagnosed patients to three different subtype groups were based on a routine clinical decision protocol that included detailed anamnesis, neuropsychiatric and somatic examination, laboratory tests, CT and/or MRI scans.
This protocol followed the usual clinical practice of the evaluation and diagnosis of dementia in the participating specialist centres.
2.4. Statistical analysis
For data management and analysis, we used Stata (Stat-aCorp. 2009. Stata Statistical Software: Release 11. College Station, TX StataC) statistical program. We considered p<0.05 as indicating statistical significance. For data analysis we applied descriptive statistics for presenting the main characteristics of the sample, calculating means and standard deviations. For inferential analysis we applied Pearson’s chi-squared test and Fisher’s exact test for examining between-group differences in categorical variables, whereas for examining associations between categorical and continuous variables we applied either ANOVA, or the Kruskal-Wallis test based on the normal versus non-normal distribution of the data.
3. Results
Altogether 131 patients were included in the study; all were Caucasians. Mean age was 77 years (SD=8.3). Fifty-five patients were assigned to the AD group, 33 patients were assigned to the VD group, and 43 patients were assigned to the MD group. There were no significant differences across groups regarding gender distribution and age. Demographic data is summarized in Table 1.
Table 1.
Demographic data of the study sample
| Study group | Sample size n (%) | Age (years) Mean (SD) | Gender male (%) |
|---|---|---|---|
| Alzheimer | 55 (41.9) | 75.1 (9.72) | 27.27 |
| Vascular | 33 (25.2) | 76.8 (7.59) | 39.39 |
| Mixed | 43 (32.8) | 79.5 (6.12) | 46.51 |
| Total | 131 (100) | 77 (8.31) | 36.64 |
3.1. Results of the evaluation of cognitive functions
Cognitive functions were evaluated with the MMSE and the ADAS-Cog. Mean score was 19 (SD=5.69) for the MMSE, and 39.47 (SD=14.18 range 8–66) for the ADAS-Cog. There was no significant difference between the diagnostic groups regarding severity of cognitive deterioration as measured by the two tests. Table 2 shows the results of the cognitive measures in the different study groups.
Table 2.
Results of the assessment of cognitive functions and results of evaluation of BPSD symptoms in the different study groups
| Alzheimer’s | Vascular | Mixed | p-value+ | |
|---|---|---|---|---|
| MMSE Mean (SD) | 16.72 (5.36) | 17.35 (4.51) | 18.102 (4.75) | 0.4155 |
| ADAS-Cog Mean (SD) | 38.75 (13.8) | 41.6 (14.66) | 38.73 (14.46) | 0.6943 |
| NPI total score Mean (SD) | 45.21 (23.82) | 42.63 (18.87) | 43.76 (24.38) | 0.87 |
| NPI frequency Mean (SD) | 14.85 (7.87) | 14.3 (6.4) | 14.4 (8.09) | 0.93 |
| NPI severity Mean (SD) | 13.29 (6.87) | 12.27 (5.36) | 12.79 (6.66) | 0.77 |
+: p-values based on ANOVA test
3.2. Results of the evaluation of BPSD symptoms
BPSD symptoms were measured using the NPI scale. We compared the study groups on mean total scores, and frequency and severity of the individual symptoms.
The mean NPI total score was 44.09 (SD=22.73; range 11–103), mean total Frequency score was 14.58 (SD=7.55; range 3–36), and mean total Severity score was 12.87 (SD=6.42; range 3–29). With respect to individual symptoms, based on the frequency scores, abnormal motor behaviours (2.79 [SD=1.2]), depression (1.86, [SD=1.2]), agitation (1.77 [SD=1.2]), and eating-appetite change (1.4 [SD=1.3]) were the most frequent in the total sample.
Table 2. Results of the assessment of cognitive functions and results of evaluation of BPSD symptoms in the different study groups
There were no significant differences across study groups in the mean total score, mean frequency score and mean severity score of NPI.
We found significant differences across study groups with regard to the frequency of individual BPSD symptoms. Specifically, frequency of delusions was significantly higher in both the MD and AD groups compared with the VD group. Hallucinations were the most frequent in the AD group, and the least frequent in the VD group, with significant differences across all groups. Between-group differences were significant also for the uninhibitedness symptom, showing the highest frequency in the VD group and the lowest frequency in the AD group, although this symptom was in general the third-less-frequent symptom among all the BPSD symptoms measured in the test. Abnormal motor behaviour, however, showed the highest prevalence in all study groups from the measured BPSD symptoms and also showed significant differences between the study groups. Specifically, abnormal motor behaviour was more frequent in both the AD and the MD group compared with the VD group.
Considering the severity of symptoms, the most severe symptoms turned out to be abnormal motor behaviours (mean 2.32 [SD=1.04]), agitation (mean 1.61 [SD=1.1]), depression (mean 1.59 [SD=0.99]), and eating-appetite change (mean 1.2 [SD=1.1]), (Table 3).
Table 3.
Associations between NPI item scores and severity of cognitive deterioration and dementia aetiology
| NPI scores | ADAS-Cog | 3 diagnostic groups | Mixed vs Vascular+++ | Alzheimer’s vs Vascular+++ | Alzheimer’s vs Mixed+++ |
|---|---|---|---|---|---|
| frequency total score | p<0.0001+ | p=0.9394++ | - | - | - |
| delusion: frequency | p=0.0526+ | p=0.038+++ | p=0.027 | p=0.351 | p=0.061 |
| hallucinations: frequency | p=0.1184+ | p<0.001+++ | p=0.218 | p=0.015 | p<0.001 |
| agitation: frequency | p=0.9609++ | p=0.091+++ | p=0.236 | p=0.038 | p=0.266 |
| depression: frequency | p=0.2107+ | p=0.116+++ | p=0.089 | p=0.018 | p=0.991 |
| anxiety: frequency | p=0.6039++ | p=0.606+++ | p=0.495 | p=0.556 | p=0.514 |
| euphoria: frequency | p=0.9513+ | p=0.060+++ | p=0.071 | p=0.045 | p=0.573 |
| apathy: frequency | p=0.0096+ | p=0.122+++ | p=0.655 | p=0.074 | p=0.123 |
| disinhibition: frequency | p<0.0001+ | p=0.032+++ | p=0.329 | p=0.005 | p=0.259 |
| irritability: frequency | p=0.0003+ | p=0.433+++ | p=0.743 | p=0.468 | p=0.211 |
| aberrant motor behaviour: frequency | p=0.0102+ | p=0.005+++ | p=0.004 | p=0.007 | p=0.350 |
| sleep and night-time behaviour: frequency | p=0.0015+ | p=0.930+++ | p=0.673 | p=0.814 | p=0.919 |
| appetite and eating changes: frequency | p=0.0027+ | p=0.123+++ | p=0.313 | p=0.185 | p=0.077 |
| severity total score | p=0.0002++ | p=0.7706++ | - | - | - |
| delusion: severity | p=0.0954++ | p=0.086+++ | p=0.082 | p=0.028 | p=0.735 |
| hallucinations: severity | p=0.1572+ | p=0.000+++ | p=0.004 | p=0.000 | p=0.016 |
| agitation: severity | p=0.9764++ | p=0.047+++ | p=0.124 | p=0.268 | p=0.029 |
| depression: severity | p=0.2995+ | p=0.083+++ | p=0.140 | p=0.013 | p=0.850 |
| anxiety: severity | p=0.9356++ | p=0.032+++ | p=0.617 | p=0.169 | p=0.007 |
| euphoria: severity | p=0.9513+ | p=0.060+++ | p=0.071 | p=0.045 | p=0.573 |
| apathy: severity | p=0.0074+ | p=0.161+++ | p=0.151 | p=0.054 | p=0.790 |
| disinhibition: severity | p<0.0001+ | p=0.009+++ | p=0.084 | p=0.001 | p=0.425 |
| irritability: severity | p<0.0001+ | p=0.568+++ | p=0.449 | p=0.308 | p=0.718 |
| aberrant motor behaviour: severity | p=0.0010+ | p=0.084+++ | p=0.143 | p=0.033 | p=0.445 |
| sleep and night-time behaviour: severity | p=0.0001+ | p=0,221+++ | p=0.228 | p=0.046 | p=0.830 |
| appetite and eating changes: severity | p=0.0010+ | p=0.449+++ | p=0.772 | p=0.230 | p=0.375 |
+: p-values based on Kruskal-Wallis test
++: p-values based on ANOVA test
+++: p-values based on Fisher’s exact test
Red colour highlights significant p-values for easier review of the data presented
Regarding the severity of the individual BPSD symptoms, we found significant differences among the groups. Specifically, hallucinations were the most severe in the AD group and the least severe in the VD group; agitation was significantly more severe in the VD group compared with the MD group; anxiety was significantly more severe in the AD group compared with the VD group; uninhibitedness was significantly more severe in the VD group compared with the AD group (Table 3).
3.3. Results of analysing the relationship between BPSD symptoms and cognitive functions
We examined the relationship between the level of cognitive deterioration as measured by the ADAS-Cog, and the frequency and severity of BPSD symptoms as measured by the NPI test.
NPI total score, frequency total score, and severity total score were significantly associated with the ADAS-Cog total score (p < 0.0001, p < 0.0001 based on Kruskal-Wallis test, and p= 0.0002 based on ANOVA).
Individual BPSD symptoms also showed association with the ADAS-Cog score, specifically apathy, irritability, sleep-wake cycle dysfunctions, eating-appetite change, aberrant motor behaviour, and disinhibition. Results indicated that lower level of cognitive functions were associated with higher frequency and more severe presentation of these BPSD symptoms. Please see Table 3 and Figures 1-3 for more details.
Figure 1.
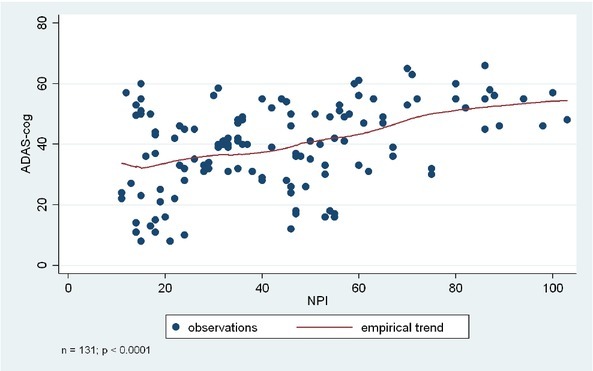
Association between cognitive functions (ADAS-Cog score) and BPSD symptoms (based on NPI total score).
Figure 3.
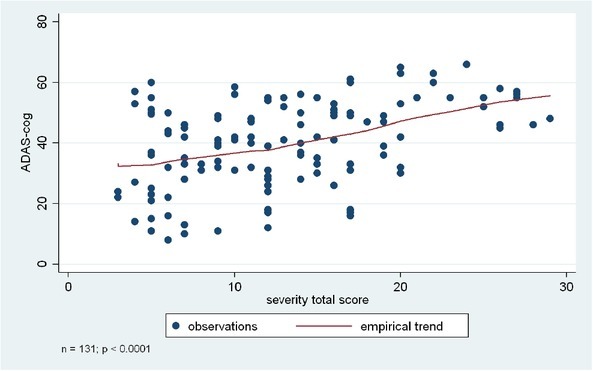
Association between cognitive functions (ADAS-Cog score) and BPSD symptoms (based on NPI severity total score).
Figure 1. Association between cognitive functions (ADAS-Cog score) and BPSD symptoms (based on NPI total score). Analyses were based on the Kruskal-Wallis test, or ANOVA, depending on the original normal vs non-normal distribution of the data.
Figure 2. Association between cognitive functions (ADAS-Cog score) and BPSD symptoms (based on in NPI frequency total score). Analyses were based on the Kruskal-Wallis test, or ANOVA, depending on the original normal vs non-normal distribution of the data.
Figure 2.
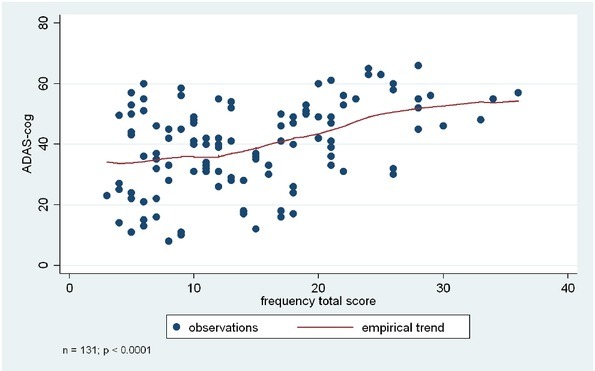
Association between cognitive functions (ADAS-Cog score) and BPSD symptoms (based on NPI frequency total score).
Figure 3. Association between cognitive functions (ADAS-Cog score) and BPSD symptoms (based on in NPI severity total score). Analyses were based on the Kruskal-Wallis test, or ANOVA, depending on the original normal vs non-normal distribution of the data.
4. Discussion
In this study, we examined the frequency and severity of BPSD symptoms in a random sample of treatment-naïve Hungarian dementia patients. Our sample on average showed medium severity of dementia (Mean ADAS-Cog score 39.46 points). Demographical data (gender distribution: 36.64% male, mean age: 77 years) of our sample was comparable to that of previously examined samples reported in the literature [20].
Regarding the frequency of BPSD symptoms, published data shows extreme differences in various research settings and in samples of dementia patients with different levels of severity [21]. In the case of medium-severity dementia, frequency of BPSD symptoms of different levels of severity was reported between 20% and 90% [22]. In our study, the prevalence of BPSD symptoms was 100%, meaning that there was no patient in our sample without at least one BPSD symptom [23]. The reason for this exceptionally high prevalence rate of BPSD symptoms may be that in our study the evaluation of the BPSD symptoms was thorough, was based on a very detailed BPSD specific interview, and that the questions were directed to the patient’s primary caregiver. In contrast, most of the studies reporting data on the presence of BPSD symptoms in patients used either less specific and less detailed measurements, or a different information source (self-evaluation, doctors rating, etc.) [24,25,26]. Furthermore, some studies evaluated only certain parts of the BPSD symptoms or did not target the thorough evaluation of the BPSD symptoms themselves but had a different focus (e.g., pharmacotherapy studies) [27,28].
Based on our results, the most frequent BPSD symptoms were aberrant motor behaviour (69.31%), depression (46.21%), agitation (44.12%), appetite and eating change (35.03%), anxiety (30.11%), sleep and night-time behaviour (28.78%), delusion (28.77%), irritability (24.62%), and hallucination (22.72%), whereas apathy, disinhibition, and euphoria were present in less than 10% of cases. These findings are different from previously reported findings in the literature regarding the prevalence of mood disorder symptoms and the possibly related appetite and sleep-wake cycle symptoms. In our study, these BPSD symptoms are less frequent compared to previously reported data [29, 30, 31]. A reason for this difference may be that in our study patients with previously diagnosed mental disorders – including major depression – were excluded to avoid overlapping symptom detection (e.g., affective symptoms being present not due to BPSD but because of a pre-existing mental condition).
Our findings suggest that BPSD as a symptom group requires medical attention, because in line with previous data [13], the most frequent symptoms in our sample were those that had been already reported as important risk factors for higher mortality rates and early nursing home placement.
As the second objective of this study, we examined whether a specific BPSD symptom pattern existed in different types of dementias. Our results showed that hallucination, abnormal motor behaviour, and anxiety were significantly more frequent in AD and MD compared with VD; hallucinations and delusions were also significantly more severe in AD and MD compared with VD. Disinhibition, however, was significantly more frequent and more severe, and agitation was significantly more severe among VD patients compared to those with AD and MD.
In a study that included more than 2000 demented patients from the 4 most frequent etiological groups, similar results were reported [32]. Specifically, in vascular dementia, mood-related BPSD symptoms were prominent, whereas in Alzheimer dementia, the psychotic-type BPSD symptoms were rather prevalent. Brain SPECT confirmed frontotemporal-perfusion abnormality in Alzheimer patients with BPSD symptoms. Based on post-mortem data, more neurofibrillary tangles were found in the brain of patients previously showing psychotic symptoms like hallucinations or delusions. This finding indeed connects the presence of these BPSD symptoms to Alzheimer-specific neuropathology. Thus, psychotic symptoms are less likely to be taken as generalizable BPSD symptoms in dementia [9,33]. The presence of mood disorders such as apathy, irritability, agitation, depression, distress, and euphoria, as well as delusions and disinhibition have been, however, emphasized in vascular dementia in previous reports [34,35].
Our results indicated that not only the dementia subtype, but also the level of cognitive deterioration was associated with the frequency and the severity of certain BPSD symptoms. Worse cognitive functions were related to higher frequency and greater severity of apathy, irritability, sleep-wake cycle dysfunctions, eating-appetite change, aberrant motor behaviour, and disinhibition.
Interestingly, our findings showed that hallucinations and delusions were related to the aetiology of dementia and were independent of the level of cognitive deterioration, whereas apathy, irritability, sleep-wake cycle dysfunctions, and eating-appetite change were associated with cognitive deterioration and were independent of aetiology. Although relevant scientific research focuses rather on identifying factors that may contribute to the more accurate clinical diagnosis regarding the aetiology of dementia to provide more specific therapeutic interventions, our findings point toward another important aspect of dementia. Specifically, cognitive deterioration is a leading symptom of dementia, but at the same time there is a broader symptomatology to be taken into consideration over the course of the illness that is connected to the gradual structural deterioration of the brain that results in declining functionality. Identifying those BPSD symptoms that become more frequent and severe along with the cognitive deterioration helps us understand the nature of the structural damage appearing over time during the course of the illness on one hand, and on the other hand, it helps us to exclude observable symptoms from aetiology-based clinical decisions or research targets.
We found two symptoms, aberrant motor behaviour and disinhibition, that were both associated with aetiology and cognitive deterioration. Aberrant motor behaviour was the most frequent BPSD symptom, so it might be an early appearing BPSD symptom, especially in AD, whereas disinhibition was one of the less frequent BPSD symptoms in this sample, thus it may appear in later phases (in severe cases – our sample was on average medium severity), and is typical especially for VD. These findings are in line with previous findings [14,32].
4.1. Limitations of the study
Our results have to be interpreted in light of certain limitations. This study was explorative in nature and thus, conclusions have to be drawn cautiously. Second, causative relationships cannot be judged because this was a cross sectional study. Third, sample sizes in the examined subgroups were small; that also prevents us from drawing firm conclusions from our results. Fourth, there was no neuropathological confirmation of the dementia subtype.
Using a randomly selected sample, however, brought a natural heterogeneity to the whole sample. All these features of the study allowed us to observe general trends in dementia patients with regard to the examined associations. These observed trends may help clinicians in their everyday practice and may suggest future research directions for more specific and rigorously designed experiments.
5. Conclusions
Our results, based on this explorative study on a randomly selected sample of treatment naïve dementia patients, supported previous findings in the literature concerning high prevalence of BPSD symptoms in dementia patients. This was the first systematic study collecting information on BPSD symptoms among Hungarian dementia patients. We identified specific differences in the presentation of BPSD symptoms in clinically distinct types of dementia, and a significant relationship between the level of cognitive deterioration and the presentation of BPSD symptoms. Although our findings are preliminary and future studies would be essential to understand these associations in detail, a possible clinical pattern of non-cognitive, behavioural, and psychological symptoms is suggested in different types of dementia. Such a pattern may aid clinicians in the diagnosis, as well as in informing and educating patients and relatives on expected outcome and course of illness. A more predictable course of illness may, in turn, help caregivers prepare practically and psychologically to the challenge they are facing in the long term, and thus, may significantly increase the nursing time at home instead of early placement of the patient in a nursing home.
The consistently reported high prevalence of BPSD symptoms in dementia patients, together with our current findings, emphasizes the clinical importance of this sub-syndrome of dementia that requires further study and clinical attention.
Acknowledgements
This work was supported by grants from the National Brain Research Program 2.0, Hungary (2017-1.2.1-NKP-2017-00002), SZTE ÁOK-KKA 2018/HorT, Hungary and Economic Development and Innovation Operation Programme (GINOP), Hungary (GINOP-2.3.2-15-2016-00043).
Abbreviations
- BPSD
Behavioural and psychological symptoms in dementia
- AD
Alzheimer’s disease
- VD
vascular-dementia
- MD
mixed-dementia
- NCD
neurocognitive disorder
- MMSE
Mini-Mental State Examination
- NPI
Neuropsychiatric Inventory
- ADAS-Cog
Alzheimer Disease Assessment Scale-Cognitive Test
- IPA
The International Psychiatric Association
Footnotes
Conflict of interest
Conflict of interest statement: The authors report and declare no conflicts of interest.
References
- [1].Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dementia. 2016;6:158. doi: 10.1016/j.jalz.2010.01.009. –. [DOI] [PubMed] [Google Scholar]
- [2].American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders, 4th Edition. Arlington: American Psychiatric Publishing; 2010. [Google Scholar]
- [3].American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- [4].World Health Organization (WHO). The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- [5].Penke B., Hortobágyi T., Fülöp L.. Aging and Alzheimer’s disease. (in Hungarian) Magyar Tudomány. 2016;177:573. –. [Google Scholar]
- [6].Kalaria R. N. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathologica. 2016;131:659. doi: 10.1007/s00401-016-1571-z. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gauthier S. Clinical diagnosis and management of Alzheimer’s disease (3rd ed.) London: Informa Healtcare; 2007. ISBN 9780415372992. [Google Scholar]
- [8].Kálmán J., Kálmán S., Pákáski M.. Recognition and treatment of behavioural and psychological symptoms of dementias: lessons from the CATIE-AD Study. Neuropsychopharmacologia Hungarica. 2008;10:233. –. [PubMed] [Google Scholar]
- [9].Bereczki E., Francis P.T., Howlett D., Pereira J. B.. Bogstedt A., Cedazo-Minguez A., Baek J.H., Hortobágyi T., Attems J., Ballard C.. Synaptic proteins predictor cognitive decline in Alzheimer’s disease and Lewy body dementia. Alzheimer’s Dementia. 2016;12:1149. doi: 10.1016/j.jalz.2016.04.005. Höglund K. Aarsland D. –. [DOI] [PubMed] [Google Scholar]
- [10].International Psychiatric Association (IPA) Complete Guides to Behavioural and Psychological Symptoms of Dementia. 1996. https://www.ipa-online.org/publications/guides-to-bpsd https://www.ipa-online.org/publications/guides-to-bpsd
- [11].Robert P. H.. Verhey F. R. J. Byrne E. J. Grouping for behavioural and psychological symptoms in dementia: clinical and biological aspects. Consensus paper of the European Alzheimer diseaseconsortium. European Psychiatry. 2005;20:490. doi: 10.1016/j.eurpsy.2004.09.031. –. [DOI] [PubMed] [Google Scholar]
- [12].Finkel S. I., Costa e Silva J., Cohen G., Miller S., Sartorius N.. Behavioral and psychological signs and symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. International Psychogeriatrics. 1996;8(Suppl. 3):497. doi: 10.1017/s1041610297003943. –. [DOI] [PubMed] [Google Scholar]
- [13].Finkel S. I., Burns A., Cohen G. Behavioural and psychological symptoms of dementia (BPSD): a clinical and research update, overview. International Psychogeriatrics. 2000;12:13. –. [Google Scholar]
- [14].Feast A., Orrell M., Charlesworth G., Melunsky N., Poland F., Moniz-Cook E. Behavioural and psychological symptoms in dementia and the challenges for family carers: systematic review. The British Journal of Psychiatry. 2016;208:429. doi: 10.1192/bjp.bp.114.153684. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Clare L., Rowlands J., Bruce E., Surr C., Downs M. The experience of living with dementia in residential care: an interpretative phenomenological analysis. The Gerontologist. 2008;48:711. doi: 10.1093/geront/48.6.711. –. [DOI] [PubMed] [Google Scholar]
- [16].Folstein M. F., Folstein S. E., McHugh P. R.. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research. 1975;12:189. doi: 10.1016/0022-3956(75)90026-6. –. [DOI] [PubMed] [Google Scholar]
- [17].Rosen W. G., Mobs R. C., Davis K. L. A new rating scale for Alzheimer’s Disease. American Journal of Psychiatry. 1984;141:1356. doi: 10.1176/ajp.141.11.1356. –. [DOI] [PubMed] [Google Scholar]
- [18].Pákáski M., Drótos G., Janka Z., Kálmán J.. Validation of the Hungarian version of Alzheimer’s Disease Assessment Scale-Cognitive Subscale. Orvosi Hetilap. 2012;153:461. doi: 10.1556/OH.2012.29332. –. [DOI] [PubMed] [Google Scholar]
- [19].Cummings J., Meg M.. Rosenberg-Thompson S., Carus D. A., Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308. doi: 10.1212/wnl.44.12.2308. Gray K. –. [DOI] [PubMed] [Google Scholar]
- [20].Hashimoto M., Yatabe Y., Ishikawa T. Relationship between Dementia Severity and Behavioural and Psychological Symptoms of Dementia in Dementia with Lewy Bodies and Alzheimer’s Disease Patients. Dementia and Geriatric Cognitive Disorders Extra. 2015;5:244. doi: 10.1159/000381800. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McKeith I., Cummings J. Behavioural changes and psychological symptoms in dementia disorders. Lancet Neurology. 2015;4:735. doi: 10.1016/S1474-4422(05)70219-2. –. [DOI] [PubMed] [Google Scholar]
- [22].Bassiony M. M., Steinberg M. S., Warren A., Rosenblatt A., Baker A. S., Lyketsos C., G.. Delusions and hallucinations in Alzheimer’s disease: Prevalence and clinical correlates. International Journal of Geriatric Psychiatry. 2000;15:99. doi: 10.1002/(sici)1099-1166(200002)15:2<99::aid-gps82>3.0.co;2-5. –. [DOI] [PubMed] [Google Scholar]
- [23].Rosdinom R.. Zarina M. Z. N. Zanariah M. S., Marhani M., Suzaly W. Behavioural and psychological symptoms of dementia, cognitive impairment and caregiver burden in patients with dementia. Preventive Medicine. 2013;57:67. doi: 10.1016/j.ypmed.2012.12.025. –. [DOI] [PubMed] [Google Scholar]
- [24].Vaingankar J. A., Chong S. A., Abdin E. Psychiatric morbidity and its correlates among informal caregivers of older adults. Comprehensive Psychiatry. 2016;68:178. doi: 10.1016/j.comppsych.2016.04.017. –. [DOI] [PubMed] [Google Scholar]
- [25].Miyamura T. Differences between family caregivers and people with dementia in recognizing the difficulties encountered in the lives of people with dementia. Japanese Journal of Public Health. 2016;63:202. doi: 10.11236/jph.63.4_202. –. [DOI] [PubMed] [Google Scholar]
- [26].Ferreira A. R, Dias C. C., Fernandes L. Needs in Nursing Homes and Their Relation with Cognitive and Functional Decline, Behavioural and Psychological Symptoms. Frontiers in Aging Neuroscience. 2016;8:72. doi: 10.3389/fnagi.2016.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kupeli N., Vickerstaff V., White N., Lord K., Scott S., Jones L., Sampson E. L. Psychometric evaluation of the Cohen-Mansfield Agitation Inventory in an acute general hospital setting. International Journal of Geriatric Psychiatry. 2018;33:158. doi: 10.1002/gps.4741. –. [DOI] [PubMed] [Google Scholar]
- [28].Ikarashi Y., Mizoguchi K. Neuropharmacological efficacy of the traditional Japanese Kampo medicine yokukansan and its active ingredients. Pharmacological Therapies. 2016;166:84. doi: 10.1016/j.pharmthera.2016.06.018. –. [DOI] [PubMed] [Google Scholar]
- [29].der Linde R. M., Dening T., Matthews F. E., Brayne C. Grouping of behavioural and psychological symptoms of dementia. International Journal of Geriatric Psychiatry. 2014;29:562. doi: 10.1002/gps.4037. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kang H., Zhao F., You L., Giorgetta C., Venkatesh D., Sarkhel S., Prakash R. Pseudo-dementia: A neuropsychological review. Annals of Indian Acadaemy of Neurology. 2014;17:147. doi: 10.4103/0972-2327.132613. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Egerhazi A., Balla P., Ritzl A., Varga Z., Frecska E., Berecz R. Automated neuropsychological test battery in depression-Preliminary data. Neuropsychopharmacology Hungarica. 2013;15:5. –. [PubMed] [Google Scholar]
- [32].Kazui H., Yoshiyama K., Kanemoto H., Suzuki Y., Sato S., Hashimoto M., Ikeda M., Tanaka H., Hatada Y., Matsushita M., Nishio Y., Mori E., Tanimukai S., Komori K., Yoshida T., Shimizu H., Matsumoto T., Mori T., Kashibayashi T., Yokoyama K., Shimomura T., Kabeshita Y., Adachi H., Tanaka T. Differences of Behavioral and Psychological Symptoms of Dementia in Disease Severity in Four Major Dementias. PLoS One. 2016;11(8):e0161092. doi: 10.1371/journal.pone.0161092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Farbe N. B., Rubin E H., Newcomer J. W., Kinscherf D. A., Miller J. P., Morris J. C., Olney J.W., McKeel Jr. D. W.. Increased neocortical neurofibrillary tangle density in subjects with Alzheimer disease and psychosis. Archives of General Psychiatry. 2000;57:1165. doi: 10.1001/archpsyc.57.12.1165. –. [DOI] [PubMed] [Google Scholar]
- [34].Nagata K., Yokoyama E., Yamazaki T. Effects of yokukansan on behavioural and psychological symptoms of vascular dementia: An open-label trial. Phytomedicine. 2012;19:524. doi: 10.1016/j.phymed.2012.02.008. –. [DOI] [PubMed] [Google Scholar]
- [35].Mulugeta E., Molina-Holgado F., Elliott M. S., Hortobagyi T., Perry R., Kalaria R.N., Ballard C.G., Francis P.T. Inflammatory mediators in the frontal lobe of patients with mixed and vascular dementia. Dementia and Geriatric Cognitive Disorders. 2008;25:278. doi: 10.1159/000118633. –. [DOI] [PubMed] [Google Scholar]


