Abstract
Background
Venous thromboembolism (VTE) is a common condition with potentially serious and life‐threatening consequences. The standard method of thromboprophylaxis uses an anticoagulant such as low molecular weight heparin (LMWH) or warfarin. In recent years, another type of anticoagulant, pentasaccharide, an indirect factor Xa inhibitor, has shown good anticoagulative effect in clinical trials. Three types of pentasaccharides are available: short‐acting fondaparinux, long‐acting idraparinux and idrabiotaparinux. Pentasaccharides cause little heparin‐induced thrombocytopenia and are better tolerated than unfractionated heparin, LMWH and warfarin. However, no consensus has been reached on whether pentasaccharides are superior or inferior to other anticoagulative methods.
Objectives
To assess effects of pentasaccharides versus other methods of thromboembolic prevention (thromboprophylaxis) in people who require anticoagulant treatment to prevent venous thromboembolism.
Search methods
The Cochrane Vascular Information Specialist (CIS) searched the Specialised Register (last searched March 2016) and the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 2). The CIS searched trial databases for details of ongoing and unpublished studies. Review authors searched LILACS (Latin American and Caribbean Health Sciences) and the reference lists of relevant studies and reviews identified by electronic searches.
Selection criteria
We included randomised controlled trials on any type of pentasaccharide versus other anticoagulation methods (pharmaceutical or mechanical) for VTE prevention.
Data collection and analysis
Two review authors independently selected trials, assessed methodological quality and extracted data in predesigned tables.
Main results
We included in this review 25 studies with a total of 21,004 participants. All investigated fondaparinux for VTE prevention; none investigated idraparinux or idrabiotaparinux. Studies included participants undergoing abdominal surgery, thoracic surgery, bariatric surgery or coronary bypass surgery; acutely ill hospitalised medical patients; people requiring rigid or semirigid immobilisation; and those with superficial venous thrombosis. Most studies focused on orthopaedic patients. We lowered the quality of the evidence because of heterogeneity between studies and a small number of events causing imprecision.
When comparing fondaparinux with placebo, we found less total VTE (risk ratio (RR) 0.24, 95% confidence interval (CI) 0.15 to 0.38; 5717 participants; 8 studies; I2 = 64%; P < 0.00001), less symptomatic VTE (RR 0.15, 95% CI 0.06 to 0.36; 6503 participants; 8 studies; I2 = 0%; P < 0.0001), less total DVT (RR 0.25, 95% CI 0.15 to 0.40; 5715 participants; 8 studies; I2 = 67%; P < 0.00001), less proximal DVT (RR 0.12, 95% CI 0.04 to 0.39; 2746 participants; 7 studies; I2 = 64%; P = 0.0004) and less total pulmonary embolism (PE) (RR 0.16, 95% CI 0.04 to 0.62; 6412 participants; 8 studies; I2 = 0%; P = 0.008) in the fondaparinux group. The quality of the evidence was moderate for total VTE, total DVT and proximal DVT, and high for symptomatic VTE and total PE.
When fondaparinux was compared with LMWH, analyses indicated that fondaparinux reduced total VTE and DVT (RR 0.55, 95% CI 0.42 to 0.73; 9339 participants; 11 studies; I2 = 64%; P < 0.0001; and RR 0.54, 95% CI 0.40 to 0.71; 9356 participants; 10 studies; I2 = 67%; P < 0.0001, respectively), and showed a trend toward reduced proximal DVT (RR 0.58, 95% CI 0.33 to 1.02; 8361 participants; 9 studies; I2 = 53%; P = 0.06). Symptomatic VTE (RR 1.03, 95% CI 0.65 to 1.63; 12240 participants; 9 studies; I2 = 35%; P = 0.90) and total PE (RR 1.24, 95% CI 0.65 to 2.34; 12350 participants; 10 studies; I2 = 0%; P = 0.51) indicated no difference between fondaparinux and LMWH. The quality of the evidence was moderate for total VTE, symptomatic VTE, total DVT and total PE, and low for proximal DVT.
We showed that fondaparinux increased major bleeding compared with both placebo and LWMH (RR 2.56, 95% CI 1.48 to 4.44; 6659 participants; 8 studies; I2 = 0%; P = 0.0008; moderate‐quality evidence; and RR 1.38, 95% CI 1.09 to 1.75; 12,501 participants; 11 studies; I2 = 24%; P = 0.008; high‐quality evidence, respectively). All‐cause mortality was not different between fondaparinux and placebo or LMWH (RR 0.76, 95% CI 0.48 to 1.22; 6674 participants; 8 studies; I2 = 14%; P = 0.26; moderate‐quality evidence; and RR 0.88, 95% CI 0.63 to 1.22; 12,400 participants; 11 studies; I2 = 0%; P = 0.44; moderate‐quality evidence, respectively).
One study compared fondaparinux with variable and fixed (1 mg per day) doses of warfarin after elective hip or knee replacement surgery and showed no difference in primary and secondary outcomes between fondaparinux and both variable and fixed doses of warfarin. The quality of the evidence was very low. One small study compared fondaparinux with edoxaban in patients with severe renal impairment undergoing lower‐limb orthopaedic surgery and reported no thromboembolic events, major bleeding events or deaths in either group. The quality of the evidence was very low. One small study compared fondaparinux with mechanical thromboprophylaxis. Results showed no difference in total VTE and total DVT between fondaparinux and mechanical thromboprophylaxis. This study reported no cases pertaining to the other outcomes of this review. The quality of the evidence was low.
There were insufficient studies to permit meaningful conclusions for subgroups of clinical conditions other than orthopaedic surgery.
Authors' conclusions
Moderate to high quality evidence shows that fondaparinux is effective for short‐term prevention of VTE when compared with placebo. It can reduce total VTE, DVT, total PE and symptomatic VTE, and does not demonstrate a reduction in deaths compared with placebo. Low to moderate quality evidence shows that fondaparinux is more effective for short‐term VTE prevention when compared with LMWH. It can reduce total VTE and total DVT and does not demonstrate a reduction in deaths when compared with LMWH. However, at the same time, moderate to high quality evidence shows that fondaparinux increases major bleeding when compared with placebo and LMWH. Therefore, when fondaparinux is chosen for the prevention of VTE, attention should be paid to the person's bleeding and thrombosis risks. Most data were derived from patients undergoing orthopaedic surgery. Therefore, the conclusion predominantly pertains to these patients. Data on fondaparinux for other clinical conditions are sparse.
Keywords: Humans; Anticoagulants; Anticoagulants/adverse effects; Anticoagulants/therapeutic use; Fondaparinux; Heparin, Low‐Molecular‐Weight; Heparin, Low‐Molecular‐Weight/therapeutic use; Polysaccharides; Polysaccharides/adverse effects; Polysaccharides/therapeutic use; Randomized Controlled Trials as Topic; Venous Thromboembolism; Venous Thromboembolism/prevention & control; Venous Thrombosis; Venous Thrombosis/prevention & control; Warfarin; Warfarin/therapeutic use
Plain language summary
Pentasaccharides for the prevention of venous blood clots
Background
Venous thromboembolism (VTE) is a condition in which people develop blood clots in their veins. It includes deep vein thrombosis (DVT) and the potentially fatal pulmonary embolism (PE). VTE occurs in more than 10% of patients in hospital and is the third most frequent cause of death among them. Therefore, effective prevention is necessary for people who are at high risk of VTE. The standard method of prevention involves use of an anticoagulant, for example, low molecular weight heparin (LMWH) or warfarin, among orthopaedic patients. In recent years, another type of anticoagulant, pentasaccharide, has shown good anticoagulative effect in clinical trials. Three types of pentasaccharides are available, namely, short‐acting fondaparinux, long‐acting idraparinux and idrabiotaparinux.
Key results
Our systematic review included 25 studies involving 21,004 participants (current until March 2016). We found no studies on long‐acting idraparinux nor idrabiotaparinux for prevention of VTE. Therefore, we included only studies on short‐acting fondaparinux for prevention of VTE.
Moderate to high quality evidence shows that fondaparinux is effective for short‐term prevention of VTE when compared with placebo. It can reduce total VTE, DVT, total PE and symptomatic VTE and shows no difference in the number of deaths when compared with placebo. Low to moderate quality evidence shows that fondaparinux is effective for short‐term prevention of VTE when compared with LMWH. It can reduce total VTE and total DVT and shows no difference in the number of deaths when compared with LMWH. At the same time, however, fondaparinux increases major bleeding when compared with placebo and LMWH. Therefore, when fondaparinux is chosen for the prevention of VTE, attention should be paid to the person's bleeding risk. Most of the data were obtained from studies on patients undergoing orthopaedic surgery. Therefore, the conclusion pertains predominantly to these patients. Data on fondaparinux for other medical conditions such as internal, medical and abdominal surgery are sparse.
Quality of evidence
We downgraded the quality of the evidence because of small numbers of events causing imprecision, and differences and inconsistency between studies. We need additional high‐quality clinical trials to confirm the efficacy and safety of fondaparinux.
Summary of findings
Summary of findings for the main comparison. Fondaparinux versus placebo for the prevention of venous thromboembolism.
| Fondaparinux versus placebo for the prevention of venous thromboembolism | ||||||
| Patient or population: people requiring prevention of venous thromboembolism Settings: hospital and outpatient Intervention: fondaparinux versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Fondaparinux | |||||
| Total VTE Follow‐up: 7‐45 days | Study population | RR 0.24 (0.15 to 0.38) | 5717 (8 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 91 per 1000 | 22 per 1000 (14 to 34) | |||||
| Moderate | ||||||
| 181 per 1000 | 43 per 1000 (27 to 69) | |||||
| Symptomatic VTE Follow‐up: 7‐45 days | Study population | RR 0.15 (0.06 to 0.36) | 6503 (8 studies) | ⊕⊕⊕⊕ high2,3 | ||
| 12 per 1000 | 2 per 1000 (1 to 4) | |||||
| Moderate | ||||||
| 7 per 1000 | 1 per 1000 (0 to 3) | |||||
| Total DVT Follow‐up: 7‐45 days | Study population | RR 0.25 (0.15 to 0.4) | 5715 (8 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 87 per 1000 | 22 per 1000 (13 to 35) | |||||
| Moderate | ||||||
| 173 per 1000 | 43 per 1000 (26 to 69) | |||||
| Proximal DVT Follow‐up: 7‐45 days | Study population | RR 0.12 (0.04 to 0.39) | 2746 (7 studies) | ⊕⊕⊕⊝ moderate1,2,3 | ||
| 60 per 1000 | 7 per 1000 (2 to 23) | |||||
| Moderate | ||||||
| 54 per 1000 | 6 per 1000 (2 to 21) | |||||
| Total PE Follow‐up: 7‐45 days | Study population | RR 0.16 (0.04 to 0.62) | 6412 (8 studies) | ⊕⊕⊕⊕ high2,3 | ||
| 5 per 1000 | 1 per 1000 (0 to 3) | |||||
| Moderate | ||||||
| 1 per 1000 | 0 per 1000 (0 to 1) | |||||
| Major bleeding Follow‐up: 7‐45 days | Study population | RR 2.56 (1.48 to 4.44) | 6659 (8 studies) | ⊕⊕⊕⊝ moderate2,4 | ||
| 5 per 1000 | 12 per 1000 (7 to 21) | |||||
| Moderate | ||||||
| 3 per 1000 | 8 per 1000 (4 to 13) | |||||
| All causes of death Follow‐up: 7‐45 days | Study population | RR 0.76 (0.48 to 1.22) | 6674 (8 studies) | ⊕⊕⊕⊝ moderate2,5 | ||
| 12 per 1000 | 9 per 1000 (6 to 15) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; RR: risk ratio; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Data were pooled with a random‐effects model owing to heterogeneity ‐ downgraded by one level. 2 All studies were published in English; most studies were organised by a pharmaceutical company and could indicate potential publication bias, but we did not deem this sufficient to downgrade the quality of the evidence. 3 Small number of events but no imprecision of effect estimate, therefore not downgraded. 4 Small number of events causing wide CI ‐ downgraded by one level. 5 Small number of events; many studies do not contribute to effect estimate ‐ downgraded by one level for imprecision.
Summary of findings 2. Fondaparinux versus LMWH for the prevention of venous thromboembolism.
| Fondaparinux versus LMWH for the prevention of venous thromboembolism | ||||||
| Patient or population: people requiring prevention of venous thromboembolism Settings: hospital Intervention: fondaparinux versus LMWH | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| LMWH | Fondaparinux | |||||
| Total VTE Follow‐up: 7‐45 days | Study population | RR 0.55 (0.42 to 0.73) | 9339 (11 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 108 per 1000 | 60 per 1000 (46 to 79) | |||||
| Moderate | ||||||
| 83 per 1000 | 46 per 1000 (35 to 61) | |||||
| Symptomatic VTE Follow‐up: 7‐45 days | Study population | RR 1.03 (0.65 to 1.63) | 12240 (9 studies) | ⊕⊕⊕⊝ moderate2,3 | ||
| 6 per 1000 | 6 per 1000 (4 to 9) | |||||
| Moderate | ||||||
| 3 per 1000 | 3 per 1000 (2 to 5) | |||||
| Total DVT Follow‐up: 7‐45 days | Study population | RR 0.54 (0.4 to 0.71) | 9356 (10 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 106 per 1000 | 57 per 1000 (42 to 75) | |||||
| Moderate | ||||||
| 86 per 1000 | 46 per 1000 (34 to 61) | |||||
| Proximal DVT Follow‐up: 7‐45 days | Study population | RR 0.58 (0.33 to 1.02) | 8361 (9 studies) | ⊕⊕⊝⊝ low1,2,3 | ||
| 23 per 1000 | 13 per 1000 (7 to 23) | |||||
| Moderate | ||||||
| 25 per 1000 | 14 per 1000 (8 to 25) | |||||
| Total PE Follow‐up: 7‐45 days | Study population | RR 1.24 (0.65 to 2.34) | 12350 (10 studies) | ⊕⊕⊕⊝ moderate2,3 | ||
| 3 per 1000 | 3 per 1000 (2 to 6) | |||||
| Moderate | ||||||
| 1 per 1000 | 1 per 1000 (1 to 2) | |||||
| Major bleeding Follow‐up: 7‐45 days | Study population | RR 1.38 (1.09 to 1.75) | 12501 (11 studies) | ⊕⊕⊕⊕ high2 | ||
| 18 per 1000 | 25 per 1000 (19 to 31) | |||||
| Moderate | ||||||
| 23 per 1000 | 32 per 1000 (25 to 40) | |||||
| All causes of death Follow‐up: 7‐45 days | Study population | RR 0.88 (0.63 to 1.22) | 12400 (11 studies) | ⊕⊕⊕⊝ moderate2,4 | ||
| 12 per 1000 | 10 per 1000 (7 to 15) | |||||
| Moderate | ||||||
| 3 per 1000 | 3 per 1000 (2 to 4) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; RR: risk ratio; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Data were pooled with the random‐effects model because of heterogeneity ‐ downgraded by one level. 2 All studies were written in English; most studies were funded by a pharmaceutical company, which could indicate potential publication bias but we did not deem this sufficient to downgrade the quality of the evidence. 3 Few events leading to wide confidence interval ‐ downgraded by one level. 4 Small number of events; many studies do not contribute to effect estimate ‐ downgraded by one level for imprecision.
Summary of findings 3. Fondaparinux versus variable dose warfarin for the prevention of venous thromboembolism.
| Fondaparinux versus variable dose warfarin for the prevention of venous thromboembolism | ||||||
| Patient or population: patients requiring prevention of venous thromboembolism Settings: hospital Intervention: fondaparinux versus variable dose warfarin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Variable dose warfarin | Fondaparinux | |||||
| Total VTE Follow‐up: mean 28 days | See comment | See comment | Not estimable | 236 (1 study) | ⊕⊝⊝⊝ very low1 | No VTE events recorded |
| Symptomatic VTE Follow‐up: mean 28 days | See comment | See comment | Not estimable | 236 (1 study) | ⊕⊝⊝⊝ very low1 | No VTE events recorded |
| Total DVT Follow‐up: mean 28 days | See comment | See comment | Not estimable | 236 (1 study) | ⊕⊝⊝⊝ very low1 | No DVT events recorded |
| Proximal DVT Follow‐up: mean 28 days | See comment | See comment | Not estimable | 236 (1 study) | ⊕⊝⊝⊝ very low1 | No proximal DVT events recorded |
| Total PE Follow‐up: mean 28 days | See comment | See comment | Not estimable | 236 (1 study) | ⊕⊝⊝⊝ very low1 | No PE events recorded |
| Major bleeding Follow‐up: mean 28 days | Study population | RR 7 (0.37 to 134.05) | 236 (1 study) | ⊕⊝⊝⊝ very low1 | ||
| No cases of bleeding (0/118) were reported in the variable warfarin group. Three cases (3/118) of bleeding were reported in the fondaparinux group. | ||||||
| All causes of death Follow‐up: mean 28 days | See comment | See comment | Not estimable | 236 (1 study) | ⊕⊝⊝⊝ very low1 | No deaths recorded |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; RR: risk ratio; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One study was included in this comparison; the study sample was small, and the events were rare ‐ downgraded by three levels.
Summary of findings 4. Fondaparinux versus 1 mg warfarin for the prevention of venous thromboembolism.
| Fondaparinux versus 1 mg warfarin for the prevention of venous thromboembolism | ||||||
| Patient or population: people requiring prevention of venous thromboembolism Settings: hospital Intervention: fondaparinux versus 1mg warfarin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 1 mg warfarin | Fondaparinux | |||||
| Total VTE Follow‐up: mean 28 days | Study population | RR 0.2 (0.01 to 4.12) | 236 (1 study) | ⊕⊝⊝⊝ very low1 | ||
| 17 per 1000 | 3 per 1000 (0 to 70) | |||||
| Moderate | ||||||
| 17 per 1000 | 3 per 1000 (0 to 70) | |||||
| Symptomatic VTE Follow‐up: mean 28 days | See comment | See comment | Not estimable | 236 (1 study) | ⊕⊝⊝⊝ very low1 | No systematic VTE events recorded |
| Total DVT Follow‐up: mean 28 days | Study population | RR 0.2 (0.01 to 4.12) | 236 (1 study) | ⊕⊝⊝⊝ very low1 | ||
| 17 per 1000 | 3 per 1000 (0 to 70) | |||||
| Moderate | ||||||
| 17 per 1000 | 3 per 1000 (0 to 70) | |||||
| Proximal DVT Follow‐up: mean 28 days | See comment | See comment | Not estimable | 236 (1 study) | ⊕⊝⊝⊝ very low1 | No proximal DVT events recorded |
| Total PE Follow‐up: mean 28 days | See comment | See comment | Not estimable | 236 (1 study) | ⊕⊝⊝⊝ very low1 | No PE events recorded |
| Major bleeding Follow‐up: mean 28 days | Study population | RR 3 (0.32 to 28.43) | 236 (1 study) | ⊕⊝⊝⊝ very low1 | ||
| 8 per 1000 | 25 per 1000 (3 to 241) | |||||
| Moderate | ||||||
| 9 per 1000 | 27 per 1000 (3 to 256) | |||||
| All causes of death Follow‐up: mean 28 days | See comment | See comment | Not estimable | 236 (1 study) | ⊕⊝⊝⊝ very low1 | No deaths recorded |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; RR: risk ratio; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One study was included in this comparison; the study sample was small, and the events were rare ‐ downgraded by three levels.
Summary of findings 5. Fondaparinux versus edoxaban for the prevention of venous thromboembolism.
| Fondaparinux versus edoxaban for the prevention of venous thromboembolism | ||||||
| Patient or population: people requiring prevention of venous thromboembolism Settings: hospital Intervention: fondaparinux versus edoxaban | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Edoxaban | Fondaparinux | |||||
| Total VTE Follow‐up: 25 to 35 days after treatment completion | See comment | See comment | Not estimable | 38 (1 study) | ⊕⊝⊝⊝ very low1 | No VTE events recorded |
| Symptomatic VTE Follow‐up: 25 to 35 days after treatment completion | See comment | See comment | Not estimable | 38 (1 study) | ⊕⊝⊝⊝ very low1 | No VTE events recorded |
| Total DVT Follow‐up: 25 to 35 days after treatment completion | See comment | See comment | Not estimable | 38 (1 study) | ⊕⊝⊝⊝ very low1 | No DVT events recorded |
| Proximal DVT Follow‐up: 25 to 35 days after treatment completion | See comment | See comment | 38 (1 study) | Proximal DVT not an outcome of this study | ||
| Total PE Follow‐up: 25 to 35 days after treatment completion | See comment | See comment | Not estimable | 38 (1 study) | ⊕⊝⊝⊝ very low1 | No PE events recorded |
| Major bleeding Follow‐up: 25 to 35 days after treatment completion | See comment | See comment | Not estimable | 38 (1 study) | ⊕⊝⊝⊝ very low1 | No major bleeding events recorded |
| All causes of death Follow‐up: 25 to 35 days after treatment completion | See comment | See comment | Not estimable | 38 (1 study) | ⊕⊝⊝⊝ very low1 | No deaths recorded |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; RR: risk ratio; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One study was included in this comparison; the study sample was small, and no events were recorded ‐ downgraded by three levels.
Summary of findings 6. Fondaparinux versus mechanical thromboprophylaxis for the prevention of venous thromboembolism.
| Fondaparinux versus mechanical thromboprophylaxis for the prevention of venous thromboembolism | ||||||
| Patient or population: people requiring prevention of venous thromboembolism Settings: hospital and outpatient Intervention: fondaparinux versus mechanical thromboprophylaxis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Mechanical thromboprophylaxis | Fondaparinux | |||||
| Total VTE Follow‐up: 4 to 8 days | Study population | RR 0.61 (0.22 to 1.67) | 99 (1 study) | ⊕⊕⊝⊝ low1 | ||
| 176 per 1000 | 108 per 1000 (39 to 295) | |||||
| Moderate | ||||||
| 177 per 1000 | 108 per 1000 (39 to 296) | |||||
| Symptomatic VTE Follow‐up: 4 to 8 days | See comment | See comment | Not estimable | 120 (1 study) | ⊕⊕⊝⊝ low1 | No cases of symptomatic VTE recorded |
| Total DVT Follow‐up: 4 to 8 days | Study population | RR 0.63 (0.23 to 1.72) | 100 (1 study) | ⊕⊕⊝⊝ low1 | ||
| 171 per 1000 | 108 per 1000 (39 to 295) | |||||
| Moderate | ||||||
| 171 per 1000 | 108 per 1000 (39 to 294) | |||||
| Proximal DVT Follow‐up: 4 to 8 days | See comment | See comment | Not estimable | 105 (1 study) | ⊕⊕⊝⊝ low1 | No cases of proximal DVT recorded |
| Total PE Follow‐up: 4 to 8 days | See comment | See comment | Not estimable | 120 (1 study) | ⊕⊕⊝⊝ low1 | No cases of PE recorded |
| Major bleeding Follow‐up: 4 to 8 days | See comment | See comment | Not estimable | 120 (1 study) | ⊕⊕⊝⊝ low1 | No cases of major bleeding recorded |
| All causes of death Follow‐up: 4 to 8 days | See comment | See comment | Not estimable | 120 (1 study) | ⊕⊕⊝⊝ low1 | No deaths recorded |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; RR: risk ratio; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One study included in this comparison, small study sample, funded by pharmaceutical company, very short follow‐up ‐ downgraded by two levels.
Background
Description of the condition
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is a prevalent disease with potentially serious consequences. A major life‐threatening complication for patients with VTE is that parts of the thrombus, called emboli, can break off and travel to the lungs, eventually leading to sudden collapse and death. Even in patients without such an acute condition, emboli can cause pulmonary hypertension and post‐thrombotic syndrome over the longer term, leading to chronic swelling and ulceration of the skin. Venous thromboembolism has become the third most frequent cause of death and the most common preventable cause of death in hospital (Agnelli 2000; Geerts 2008). The incidence of VTE is about seven per 10,000 person‐years among community residents (Heit 2001). The incidence of DVT is five per 10‐000 person‐years and rises exponentially with increasing age (Fowkes 2003). The relapse rate of DVT is variable and depends mainly on the risk factor profile of patients. For patients with symptomatic and proximal DVT, the recurrence rate is over 20% after five years of initial treatment (Hansson 2000; Pinede 2001). Among hospitalised patients, the incidence of VTE is more than 100 times greater than among community residents (Heit 2001), and the prevalence of fatal PE is approximately 1% (Geerts 2004). Venous thromboembolism has become the third most frequent cause of death among patients in hospital in the United States (Heit 2005). Therefore, prevention of VTE among those at risk is important for reducing morbidity and mortality (Geerts 2001).
Until relatively recently, the main pharmacological options for treatment and prevention of thromboembolic disorders were warfarin and heparin (unfractionated heparin or low molecular weight heparin). However, the principal disadvantage of using unfractionated heparin involves its unpredictable pharmacological effect, which requires close monitoring. Disadvantages of warfarin and other vitamin K antagonists include a narrow therapeutic window, numerous drug interactions and the need for close monitoring. Although low molecular weight heparins (LMWHs) could be superior to unfractionated heparins because monitoring of coagulation is not needed and LMWHs have a better safety record (Erkens 2010), problems associated with their use include bleeding, hypersensitivity and heparin‐induced thrombocytopenia (HIT). New oral anticoagulants ‐ direct factor X inhibitors and direct thrombin inhibitors ‐ may be as effective as traditional anticoagulants without the need for monitoring but may cause more bleeding (Southworth 2013) and are associated with other adverse effects (Uchino 2012); data are still insufficient to fully understand their effects (Metzger 2015; Salazar 2010). In addition, DVT may occur despite prophylaxis with LMWH or other agents (Geerts 2001). Other non‐pharmacological methods, including thromboembolic deterrent (TED) stockings and pneumatic calf compression devices, are safe but are not effective enough to be used alone (Geerts 2001). These unresolved issues have led to continued research into potentially effective and safe anticoagulants ‐ pentasaccharides (Koopman 2003; Turpie 2003).
Description of the intervention
Pentasaccharide are a new class of agents with a specific effect on the blood coagulation cascade; they may be more effective and may cause fewer side effects than LMWH (Bauer 2003; Koopman 2003). The structure of the pentasaccharide is modelled on the pentasaccharide sequence in heparin, which binds to antithrombin‐III, but it is modified to have higher affinity (Bauer 2003). Once bound, antithrombin‐III undergoes a conformational change and binds to factor Xa. This inhibits the coagulation cascade because factor Xa is positioned at the start of the common pathway of the intrinsic and extrinsic systems and results in effective inhibition of thrombin generation (Bauer 2003). As no direct interference with components of the coagulation cascade occurs, these agents have been proposed to be less likely to cause haemorrhagic complications whilst possessing equivalent or superior efficacy when compared with LMWH (Koopman 2003; Shoda 2015).
Currently, three types of pentasaccharides are used for VTE prophylaxis. One type is fondaparinux, which has a half‐life time of approximately 15 hours and can be administered subcutaneously once daily. A systematic review on fondaparinux for acute coronary syndrome showed a better anticoagulative effect, less major bleeding and reduced total mortality when compared with LMWH (Brito 2011). The second type is the long‐acting pentasaccharide idraparinux, which has a half‐life time of approximately 120 hours when administered for the first time, which increases to 60 days when metabolism has been in steady state (Veyrat‐Follet 2009). With its long half‐life time, idraparinux can be administered subcutaneously once weekly and can greatly improve patients' tolerance and quality of life. On the other hand, the anticoagulative effect may accumulate and bleeding risk may increase after long‐term administration. If acute major bleeding takes place during idraparinux injection, it will be difficult to reverse its anticoagulative effect because no specific rescuer is available. For this reason, a new medicine called idrabiotaparinux was developed by the pharmaceutical company Sanofi‐Aventis. It is a compound of idraparinux and biotin that can be neutralised immediately by the external agent avidin. When avidin is administered, it binds rapidly to idrabiotaparinux, and the avidin–idrabiotaparinux complex is rapidly cleared from plasma to tissues in which avidin normally resides, resulting in a rapid decrease in circulating anti‐factor Xa activity (Paty 2007; Savi 2008). No relevant toxicity or adverse effects of avidin have been reported in safety pharmacology and toxicology studies (Stoldt 1997). Paty and colleagues reported that avidin is effective in reversing the anticoagulative effect of idrabiotaparinux (Paty 2010).
Why it is important to do this review
Pentasaccharides have longer half‐life times with no need for monitoring coagulation time, and they cause little HIT so are more tolerable than the traditional unfractionated heparin, LMWH or warfarin. However, in terms of beneficial effects and adverse effects in VTE prevention, although many clinical trials have compared them with other anticoagulative methods (medical, mechanical or combined), no consensus has been reached on whether they are superior or inferior to other anticoagulative methods (Bauersachs 2005; Bergqvist 2006; Falck‐Ytter 2012; Prandoni 2008; Turpie 2003a). Therefore, we conducted this systematic review to assess beneficial and adverse effects of pentasaccharides for VTE prevention.
Objectives
To assess effects of pentasaccharides versus other methods of thromboembolic prevention (thromboprophylaxis) in people who require anticoagulant treatment to prevent venous thromboembolism.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing pentasaccharides with other methods of thromboprophylaxis. We included only trials involving adults (18 years of age and older) and excluded trials in which participants were randomised by a quasi‐random method of allocation (such as alternation, date of birth or case record number).
Types of participants
We included participants over 18 years of age who required anticoagulant treatment to prevent VTE and excluded people who had contraindications to anticoagulant therapy, or who were pregnant. For participants with a serum creatinine concentration above 180 µmol/L or a glomerular filtration rate less than 30 mL/min who were well hydrated, anticoagulant doses should have been adjusted according to renal function. We excluded participants undergoing haemodialysis and those who received any type of anticoagulant, fibrinolytic therapy or dextran within two days of planned administration of the first study drug.
Types of interventions
All participants had received pentasaccharide or another method of thromboprophylaxis (pharmacological or mechanical) for prevention of VTE. We included adjunctive measures such as compression hosiery, as long as they were used equally between groups and their use was not affected by randomisation.
Types of outcome measures
Primary outcomes
The primary efficacy and safety outcome measures were overall rate of VTE and rate of major bleeding.
Secondary outcomes
Symptomatic VTE
Total DVT
Proximal DVT
Total PE
Fatal PE
Non‐fatal PE
Symptomatic PE
Fatal bleeding
Myocardial infarction (MI) (non‐fatal and fatal)
All causes of death
Any other serious adverse effects
Participants should have been assessed postoperatively for the presence of DVT by ascending venography, 125‐I‐labelled fibrinogen uptake or Doppler ultrasonography. We did not accept clinical scoring or D‐dimer assay or both as a suitable confirmatory test of DVT in this context.
Pulmonary embolism should have been confirmed by pulmonary angiography, high‐probability ventilation/perfusion (V/Q) scan or computerised tomography (CT), or should have been evaluated post mortem.
We defined major bleeding as bleeding that was fatal, retroperitoneal, intracranial or intraspinal, or that involved any other critical organ; or bleeding that led to reoperation or intervention, declined haemoglobin levels by greater than 2 grams per decilitre, transfusion of 2 or more units of packed red blood cells and a bleeding index ≥ 2.0. We derived the bleeding index by adding the number of transfused units of packed red blood cells or whole blood to the difference in haemoglobin level measured in grams per decilitre before and after a bleeding event, as mentioned above.
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials.
Cochrane Vascular Specialised Register (March 2016).
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 2) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library (www.cochranelibrary.com).
In addition, the review authors searched Latin American and Caribbean Health Sciences (LILACS) (August 2016) using the search strategy provided in Appendix 2.
The CIS searched (7 March 2016) the following trial registries for details of ongoing and unpublished studies.
ClinicalTrials.gov (www.clinicaltrials.gov).
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch).
International Standard Randomised Controlled Trial Number (ISRCTN) Register (www.isrctn.com/).
Searching other resources
We checked the reference lists of relevant studies and reviews identified by the electronic searches.
Data collection and analysis
Selection of studies
Two review authors (YS, XL or KD) independently selected and assessed trials before they were included in the review. For trials without sufficient information, we contacted study authors and researchers to request needed details. We resolved differences between the two review authors about grading or inclusion of some studies by discussion. If agreement could not be reached, we asked JD to arbitrate.
Data extraction and management
Two review authors (YS, XL or KD) independently performed data extraction using a standard form and resolved inconsistencies and disagreements by discussion or with involvement of a third review author (JD).
We extracted the following data.
Number of participants allocated to each group to allow an intention‐to‐treat analysis.
Method used to assess outcome endpoints.
Number of participants excluded or lost to follow‐up.
Details of therapy employed such as dosage and timing.
Use of adjunctive prophylaxis methods such as mechanical methods, and whether they were applied equally between groups.
Details of outcome measures as stated above.
Assessment of risk of bias in included studies
Two review authors (YS and XL or KD) independently assessed the methodological quality of included trials and resolved discrepancies by discussion. If agreement could not be reached, JD arbitrated. We used the Cochrane recommended tool for assessing risk of bias (Higgins 2011). This tool comprises seven specific domains (namely, sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and 'other bias'). Each domain in the tool included one or more specific entries in a 'Risk of bias' table. Within each entry, the first part of the tool described what was reported to have happened in the study and whether sufficient detail was provided to support a judgement about risk of bias. The second part of the tool assigned a judgement related to risk of bias for that entry by assigning a judgement of 'low risk' of bias, 'high risk' of bias or 'unclear risk' of bias (Higgins 2011). We stratified studies into two groups ‐ a 'low risk' of bias group and a 'high risk' of bias group. We stratified in the 'low risk' of bias group studies that showed 'low risk' of bias in all of the sequence generation, allocation concealment and blinding of outcome assessment domains; if any of the three domains could not be achieved, we classified the study into the 'high risk' of bias group. We considered other domains as well. If other risks of bias would obviously affect the calculated intervention effect substantially, we classified the outcome into the 'high risk' of bias group. For outcomes with information insufficient for classification into the 'low risk' of bias or 'high risk' of bias group, we tried to contact study authors to obtain the original data, attempted to find additional information in other published articles of the same study or used other methods that would be helpful in obtaining sufficient data to clarify the bias. If information was still insufficient, we decided to classify studies into the 'low risk' of bias or 'high risk' of bias group through discussion. We pooled only data from 'low risk' of bias groups in the meta‐analyses.
Measures of treatment effect
We used risk ratio (RR) with a 95% confidence interval (CI) as the measure of treatment effect for dichotomous outcomes. YS, KD and XL carried out the analysis.
Dealing with missing data
For studies with incomplete data, we attempted to contact study authors to obtain the missing information needed.
Assessment of heterogeneity
We assessed heterogeneity using the Chi‐squared test and the I2 statistic. If we found evidence of heterogeneity, we explored the cause by subgroup analyses.
Assessment of reporting biases
We used a funnel plot to detect reporting bias for analyses that involved more than 10 studies and performed sensitivity analyses when necessary.
Data synthesis
We used the fixed‐effect model to combine studies with no significant heterogeneity (confirmed by P value of the Chi‐squared test > 0.10 and I2< 25%). With P value of the Chi‐squared test > 0.10 and 25% ≤ I2< 50%, we decided on heterogeneity through discussion. When the P value of the Chi‐squared test was ≤ 0.10 or I2 was ≥ 50%, we investigated for clinical heterogeneity. If we found clinical heterogeneity, we analysed the data using subgroups, then combined the included studies using the Mantel‐Haenszel random‐effects model analysis. However, because we included data from various clinical conditions with risk of clinical heterogeneity, we performed subgroup analyses on different clinical conditions for all outcomes to detect whether inter‐subgroup heterogeneity was present.
Subgroup analysis and investigation of heterogeneity
In our protocol, we had planned to analyse the short‐term acting pentasaccharides and the long‐term acting pentasaccharides as subgroups. However, our searches identified that short‐term acting pentasaccharides were used only for primary VTE prevention and were administered once daily for a period of approximately one month, but long‐term acting pentasaccharides were used only for VTE treatment and were administered once weekly for several months. Participants and treatment duration were different for the two kinds of pentasaccharide studies. We therefore analysed studies on short‐term acting pentasaccharides for VTE prevention and excluded studies on long‐term acting pentasaccharides from the meta‐analyses without performing tests for heterogeneity. We also performed subgroup analyses on different surgical clinical conditions.
Sensitivity analysis
We performed sensitivity analyses to confirm the effects of data from the 'unclear risk' of bias group on the intervention effect. We have presented the sensitivity analyses separately.
Summary of findings
We present the main findings of the review concerning quality of evidence, magnitude of effect of the interventions examined and the sum of available data on main outcomes (total VTE, symptomatic VTE, total DVT, proximal DVT, total PE, major bleeding, all causes of death) in a 'Summary of findings' table, according to the GRADE principles described by Atkins 2004 and Higgins 2011a. We assessed different intervention comparisons (e.g. fondaparinux compared with placebo, LMWH and mechanical thromboprophylaxis), so we developed a 'Summary of findings' table for each comparison included in the Effects of interventions section. We used GRADE software (GradePro) to assist in preparation of the 'Summary of findings' table.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
See Figure 1.
1.
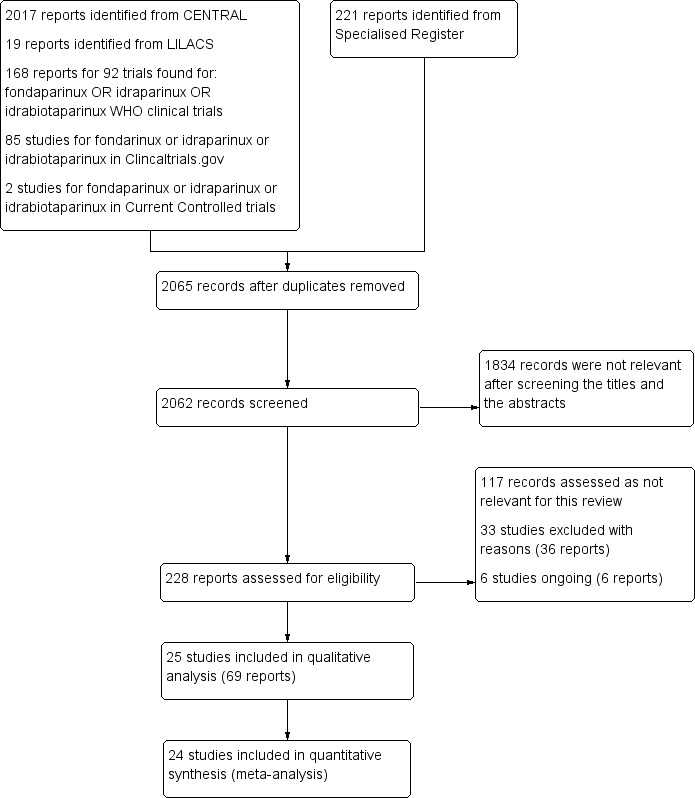
Study flow diagram.
Included studies
See Characteristics of included studies.
All 25 included studies (ACT2545; APOLLO; AR3106116; ARTEMIS; Bern 2015; CALISTO; Cho 2013; DRI4090; DRI4757; EFFORT; EPHESUS; FONDACAST; Kolluri 2016; L‐8541; L‐8635; Li 2015; PEGASUS; PENTAMAKS; PENTATHLON; PENTATHLON 2000; PENTHIFRA; PENTHIFRA PLUS; Shen 2014; Yokote 2011; Fuji 2015) investigated fondaparinux for short‐term VTE prevention. No studies examined idraparinux or idrabiotaparinux for VTE prevention. Seventeen studies (ACT2545; APOLLO; AR3106116; ARTEMIS; CALISTO; DRI4090; DRI4757; EPHESUS; FONDACAST; L‐8541; L‐8635; PEGASUS; PENTAMAKS; PENTATHLON; PENTATHLON 2000; PENTHIFRA; PENTHIFRA PLUS) were run by a pharmaceutical company, two (EFFORT; Fuji 2015) received support from a pharmaceutical company and six (Bern 2015; Cho 2013; Kolluri 2016; Li 2015; Shen 2014; Yokote 2011) were not supported by a pharmaceutical company.
Fifteen studies (ACT2545; Bern 2015; Cho 2013; DRI4090; DRI4757;EPHESUS;Fuji 2015;L‐8541;L‐8635; PENTAMAKS; PENTATHLON; PENTATHLON 2000; PENTHIFRA; PENTHIFRA PLUS; Yokote 2011) included participants undergoing orthopaedic procedures, three (APOLLO; AR3106116; PEGASUS) included participants undergoing abdominal surgery, one (Shen 2014) included participants following surgical resection of oesophageal cancer, one (ARTEMIS) acutely ill hospitalised medical patients, one (FONDACAST) participants requiring rigid or semirigid immobilisation (e.g. with a plaster cast or brace), one (Li 2015) intensive care unit (ICU) patients with hypercoagulability secondary to traumatic infection, one (EFFORT) people undergoing bariatric surgery, one (Kolluri 2016) patients undergoing coronary artery bypass graft surgery and one (CALISTO) participants with superficial venous thrombosis. Fuji 2015 involved participants with renal impairment with creatinine clearance ≥ 20 to < 30 mL/min undergoing orthopaedic procedures in the fondaparinux group and its comparator edoxaban. The other studies did not involve participants with renal impairment.
In most studies, the follow‐up period was less than 30 days, except for CALISTO and FONDACAST, both of which had a follow‐up period up to 45 days. All included studies were RCTs with comparable baseline demographic characteristics of participants in different treatment groups and low withdrawal rates (< 15%, with most studies showing a withdrawal rate < 10%). Most studies were carried out in Western countries, except for Cho 2013, DRI4090, DRI4757, Fuji 2015, Yokote 2011, Li 2015, L‐8541, L‐8635 and Shen 2014. The first five studies were carried out in Japan, and the last four in China.
Shen 2014 used effective methods to warrant the randomisation sequence, effective allocation concealment and blinding of the outcome assessor but reported VTE rates for fondaparinux and LMWH groups instead of numbers of VTE events. We were unable to obtain these details when we contacted the study authors. Therefore, we did not include this study in the meta‐analyses.
Reports of L‐8541 and Yokote 2011 did not contain sufficient information to clearly stratify them into low risk or high risk of bias groups, as discussed above. The L‐8541 report did not mention whether personnel who evaluated outcomes were independent or were blind to group treatment details. Yokote 2011 was a single‐centre study that reported no reliable randomisation method. Therefore, we included L‐8541 and Yokote 2011 in the sensitivity analyses only. Investigators in the EFFORT study administered 5 mg/d fondaparinux in the study arm and 40 mg enoxaparin twice daily in the control arm after participants had undergone bariatric surgery. Doses of fondaparinux and enoxaparin used by EFFORT were double those used for prevention of VTE in other included studies for other types of patients. Therfore, we performed sensitivity analyses excluding this study to demonstrate whether this factor would affect the results.
APOLLO, ARTEMIS, Bern 2015, CALISTO, Cho 2013, EFFORT, EPHESUS, Fuji 2015, Kolluri 2016, PENTAMAKS, PENTATHLON 2000, PENTHIFRA and PENTHIFRA PLUS reported using adjunctive anticoagulation methods. APOLLO used an intermittent pneumatic compression device (IPC) equally in different treatment groups. CALISTO, Cho 2013, EPHESUS and PENTAMAKS mentioned that they used compression stockings equally in different treatment groups as the adjunctive anticoagulation method. CALISTO also used a low dose of oral aspirin (≤ 325 mg per day) or other antiplatelet agents as an adjunctive anticoagulation method, and again this was done equally between treatment groups. ARTEMIS, Bern 2015, EFFORT, Kolluri 2016, PEGASUS, PENTATHLON 2000, PENTHIFRA, PENTHIFRA PLUS and Yokote 2011 mentioned that researchers used adjunctive anticoagulation methods (aspirin, thienopyridines and non‐steroidal anti‐inflammatory drugs were discouraged during the study; other antiplatelet agents, intermittent pneumatic leg compression, dextran and anticoagulant or thrombolytic agents were prohibited; graded‐pressure elastic stockings were permitted; and early mobilisation was strongly recommended) but did not report whether they were used equally in the different treatment groups. Because the adjunctive methods used by most participants in seven studies (ARTEMIS; EFFORT; Kolluri 2016; PEGASUS; PENTATHLON; PENTHIFRA; PENTHIFRA PLUS) were graduated compression stockings (GCS) and IPC, and no evidence suggests that these mechanical methods would substantially affect the symptomatic VTE rate, we decided to include these in the meta‐analysis. We used Yokote 2011 only for the sensitivity analysis, as described above. Another reason for including these studies was that they included large samples. If we excluded these from the meta‐analysis, the result would be biased. In Bern 2015, participants had early postoperative ambulation. All participants wore pneumatic compression stockings while in‐patients. Elastic compression stockings were prescribed to be used after discharge until follow‐up ultrasonography. Hydroxyethyl starch (HES) 6% was allowed intraoperatively for case‐specific reasons. Use of platelet function suppressive drugs, such as non‐steroidal anti‐inflammatory drugs (NSAIDs), was discouraged but not prohibited by the protocol. As Bern 2015 was the single study comparing fondaparinux and warfarin for VTE prevention, we included this study to present its results. Fuji 2015 allowed concomitant physiotherapy (intermittent pneumatic compression devices or elastic stockings) throughout the treatment period. AR3106116 compared the efficacy of fondaparinux versus IPC for VTE prevention in patients undergoing abdominal surgery who were at high risk of VTE, so the two groups in this study received fondaparinux injection and IPC treatment separately.
ACT2545, EFFORT, EPHESUS, FONDACAST, L‐8635, Li 2015, PEGASUS, PENTAMAKS, PENTATHLON, PENTATHLON 2000, PENTHIFRA and Shen 2014 compared the efficacy and safety of fondaparinux versus LMWH (approximately 100 anti‐factor Xa units per kilogram per day). ACT2545, EPHESUS and PENTATHLON 2000 compared the efficacy and safety of fondaparinux (2.5 mg subcutaneous (sc) once daily) versus LMWH after total hip replacement and hip fracture surgery. PENTATHLON was a dose‐ranging study involving five fondaparinux dose groups (0.75 mg, 1.5 mg, 3.0 mg, 6.0 mg, 8.0 mg) and a 60 mg enoxaparin control group of people undergoing elective primary hip replacement or revision of a primary procedure. We chose the 1.5 mg and 3.0 mg groups and combined their data for inclusion; we included the enoxaparin group as a comparator. FONDACAST compared the efficacy and safety of fondaparinux 2.5 mg sc once daily versus LMWH in participants requiring rigid or semirigid immobilisation for at least 21 days and up to 45 days because of isolated non‐surgical below‐knee injuries that were treated with a plaster cast. L‐8635 and PENTAMAKS compared the efficacy and safety of fondaparinux 2.5 mg sc once daily versus LMWH after major knee surgery, L‐8635 after elective knee replacement and PENTAMAKS for major knee surgery. PEGASUS compared the efficacy and safety of fondaparinux 2.5 mg sc once daily versus LMWH in participants after major abdominal surgery who were at high risk of VTE. PENTHIFRA compared the efficacy and safety of fondaparinux (2.5 mg sc once daily) versus LMWH in participants undergoing standard surgery for fracture of the upper third of the femur, including the femoral head and neck. Li 2015 compared the efficacy and safety of fondaparinux (2.5 mg sc once daily) versus LMWH in ICU patients with hypercoagulability accompanied by traumatic infection. EFFORT compared the efficacy and safety of fondaparinux (5 mg sc once daily) versus LMWH (40 mg twice daily) in bariatric surgical patients. Shen 2014 compared the efficacy and safety of fondaparinux (2.5 mg sc once daily) versus LMWH in participants undergoing surgical resection for oesophageal cancer.
APOLLO, AR3106116, ARTEMIS, Bern 2015, CALISTO, Cho 2013, DRI4090, DRI4757, Kolluri 2016 and PENTHIFRA PLUS compared the efficacy and safety of fondaparinux versus placebo or the mechanical method IPC for VTE prevention. CALISTO investigated the efficacy and safety of fondaparinux 2.5 mg sc once daily for prevention of venous thromboembolic complications for acute symptomatic isolated superficial thrombophlebitis of the lower limbs. DRI4090 and DRI4757 were dose‐ranging studies conducted to determine the dose‐response effect of fondaparinux on the prophylaxis of VTE after total hip replacement (THR) and total knee replacement (TKR), respectively. These studies included four fondaparinux dose groups (0.75 mg, 1.5 mg, 2.5 mg, 3.0 mg) and a placebo control group. We combined the 1.5 mg, 2.5 mg and 3.0 mg groups into a single group and dropped the data for the 0.75 mg group because the 0.75 mg dose of fondaparinux was too small and was not effective for routine VTE prevention and treatment (PENTATHLON). In PENTHIFRA PLUS, participants received open‐label fondaparinux 2.5 mg sc once daily postoperatively for 7 ± 1 days (day 1 = day of surgery) after hip fracture surgery. They were then randomised to receive double‐blind fondaparinux 2.5 mg sc once daily for 21 ± 2 days or placebo for three weeks. AR3106116 compared fondaparinux (2.5 mg sc once daily) with IPC for VTE prevention. Cho 2013 investigated fondaparinux (2.5 mg sc once daily) in participants after unilateral total knee arthroplasty (TKA), and ARTEMIS focused on the efficacy and safety of the anticoagulant fondaparinux (2.5 mg sc once daily) in older acutely ill medical in‐patients at moderate to high risk of VTE. Kolluri 2016 compared fondaparinux 2.5 mg sc once daily versus placebo for prevention of VTE after coronary artery bypass graft surgery. APOLLO compared fondaparinux 2.5 mg sc once daily combined with IPC versus IPC alone in participants at high risk of VTE after major abdominal surgery.
Bern 2015 compared fondaparinux 2.5 mg sc once daily versus variable (target international normalised ratio (INR) 2.0 to 2.5) and fixed (1 mg) doses of warfarin after elective hip or knee replacement surgery.
Fuji 2015 compared fondaparinux (1.5 mg sc once daily) versus edoxaban (15 mg oral once daily) in participants with serious renal impairment undergoing orthopaedic procedures.
We also identified six ongoing studies (EUCTR2007‐003746‐15‐DE; EUCTR2008‐001779‐31‐IT; JPRN‐UMIN000002444; JPRN‐UMIN000007005;JPRN‐UMIN000008435;PROTECT), which were recruiting participants and had reported no results at the time of the search for this review.
Excluded studies
See Characteristics of excluded studies.
In total, we excluded 33 studies (ACT1840; Amadeus 2008; AR3106206; AR3106333; AR3106335; Argun 2013; Bonneux 2006; Buller 2014; Cassiopea; Cohen 2007; EQUINOX; extended van Gogh; FLEXTRA; Kawaji 2012; Li 2013; MATISSE‐DVT; MATISSE‐PE; NCT00521885; NCT00539942; PENTATAK; PERSIST; Rembrandt; SAFE‐AF; Sasaki 2009; Sasaki 2011; Savi 2005; Tsutsumi 2012; van Gogh‐DVT; van Gogh‐PE; Xin 2013; Yamaoka 2014; Zhao 2013; Zhao 2015). Cassiopea, extended van Gogh, PERSIST, van Gogh‐DVT and van Gogh‐PE investigated idraparinux or idrabiotaparinux for long‐term VTE treatment, not for primary VTE prevention. Amadeus 2008 focused on idraparinux for prevention of thromboembolism in participants with atrial fibrillation. Because most thrombotic events in this study among participants with atrial fibrillation consisted of arterial embolism (stoke or non‐central nervous system systemic embolism), not venous thrombosis, published results did not distinguish the arterial embolism and venous thrombosis events; therefore, we excluded this study from the review. Buller 2014 focused on idrabiotaparinux for prevention of thromboembolism in participants with atrial fibrillation. Published results did not distinguish systemic embolism from venous thrombosis events; therefore, we excluded this study from the review. We excluded AR3106206, Rembrandt, MATISSE‐DVT and MATISSE‐PE because researchers investigated initial treatment of VTE, but not prophylaxis of VTE. In AR3106333, AR3106335, Cohen 2007, EQUINOX, FLEXTRA and PENTATAK, all study groups received pentasaccharides for VTE prevention, so investigators included no comparator. Kawaji 2012, Tsutsumi 2012 and Yamaoka 2014 were not RCTs, and Sasaki 2009 and Sasaki 2011 were quasi‐RCTs, so we excluded them. In ACT1840, the fondaparinux dose (3 mg sc twice daily) markedly extended the normal VTE prevention dose; therefore we excluded this study (PENTATHLON). NCT00539942 and NCT00521885 were terminated owing to problems with accrual and reported no results. Savi 2005 reported no VTE rate; as this was our primary outcome, we excluded this study. Argun 2013 and Bonneux 2006 reported no reliable primary efficacy and safety results. Li 2013, SAFE‐AF, Xin 2013, Zhao 2013 and Zhao 2015 investigated the efficacy and safety of fondaparinux for treatment of acute coronary syndrome and atrial fibrillation, not for VTE prevention.
Risk of bias in included studies
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
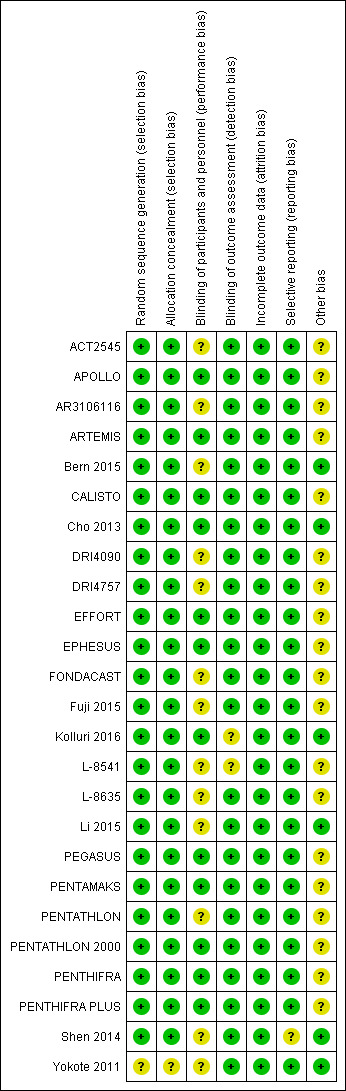
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Seventeen studies (ACT2545;APOLLO; AR3106116; ARTEMIS; CALISTO; DRI4090; DRI4757; EPHESUS; FONDACAST; L‐8541; L‐8635; PEGASUS; PENTAMAKS; PENTATHLON; PENTATHLON 2000; PENTHIFRA; PENTHIFRA PLUS) were multi‐centre RCTs organised by a pharmaceutical company. Five of them (ARTEMIS; CALISTO; PENTAMAKS; PENTATHLON 2000; PENTHIFRA PLUS) clearly described in their published articles the use of computerised or central randomisation at an independent centre and were assessed to be at low risk of selection bias. We assumed that the remaining 12 multi‐centre randomised studies organised by the same company (GlaxoSmithKline) (ACT2545; APOLLO; AR3106116; DRI4090; DRI4757; EPHESUS; FONDACAST; L‐8541; L‐8635; PEGASUS; PENTATHLON; PENTHIFRA) were also randomised centrally by reliable methods. In addition, we believed that the 17 studies had sufficient allocation concealment and assessed them as having low risk of selection bias. Another seven studies also used reliable randomisation methods (Bern 2015; Cho 2013; EFFORT; Fuji 2015; Kolluri 2016; Li 2015; Shen 2014); we therefore assessed them as having low risk of selection bias. Yokote 2011 was a single‐centre study without sufficient information to estimate whether its randomisation was reliable, and we assessed it as having unclear risk of selection bias. We did not include this study in the efficacy analysis but did include it in a sensitivity analysis.
Blinding
ACT2545, AR3106116, FONDACAST, Fuji 2015 and L‐8635 were open‐label studies. ACT2545, FONDACAST and L‐8635 compared fondaparinux versus LMWH. AR3106116 compared fondaparinux versus IPC, and this was impossible to blind to both participants and personnel. Although the five studies were open‐label studies, their outcome evaluators were independent and were blinded to the randomisation. Therefore, we believed that failure to realise blinding to participants and healthcare personnel might not cause serious bias, and we included these trials in the data analyses. We therefore assessed these five studies as having unclear risk of performance bias but low risk of detection bias. Bern 2015, DRI4090, DRI4757, PENTATHLON, Shen 2014 and Yokote 2011 did not clearly describe the method used to ensure blinding of participants and personnel. However, they did describe effective methods that they used to ensure that outcome evaluators were blinded to the randomisation. According to our protocol, we included these studies in the data analyses. We assessed Bern 2015, DRI4090, DRI4757, PENTATHLON, Shen 2014 and Yokote 2011 as having unclear risk of performance bias but low risk of detection bias. Kolluri 2016 did not clearly describe whether outcome assessors were blinded; we therefore judged this study to have unclear risk of detection bias. Li 2015 reported reliable blinding of participants and outcome assessors but not of healthcare personnel; we therefore judged this study to have unclear risk of performance bias but low risk of detection bias. L‐8541, a single‐blind study, did not mention whether investigators used methods to ensure that outcome assessors were blinded to the treatment that participants received. We assessed L‐8541 to have unclear risk of performance and detection bias. We did not include this study in the data analysis but included it in a sensitivity analysis.
The remaining included studies were all triple‐blind and reported use of effective blinding strategies; we judged these studies to have low risk of performance and detection bias.
Incomplete outcome data
More than 90% of participants in every treatment group in all but two studies (PENTATHLON; PENTHIFRA PLUS) completed treatment as planned in the protocol, and we included them in the analyses of study results. In PENTATHLON, 10.4% of participants in the comparator enoxaparin group withdrew from the study, and in PENTHIFRA PLUS, 12.3% and 13.9% of participants from the fondaparinux group and placebo group, respectively, discontinued studies midway. We deemed all studies to have low risk of attrition bias.
Selective reporting
All included studies reported the primary efficacy outcomes listed in the Methods section of this review. However, Shen 2014 reported VTE rates rather than numbers of events, and we could not include this study in the meta‐analysis. All but one study (Shen 2014) reported the primary safety outcome. Shen 2014 reported drainage volume rather than major bleeding; we therefore judged this study to have unclear risk of reporting bias.
We used the funnel plot to detect publication bias.
Other potential sources of bias
All studies except Bern 2015, Cho 2013, Kolluri 2016, Li 2015, Shen 2014 and Yokote 2011 were organised by pharmaceutical companies, and EFFORT was supported by a pharmaceutical company, which provided the study medication. Therefore, we judged all studies except Bern 2015, Cho 2013, EFFORT, Kolluri 2016, Li 2015, Shen 2014 and Yokote 2011 as having unclear risk of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Fondaparinux versus placebo
Eight studies (APOLLO; ARTEMIS; CALISTO; Cho 2013; DRI4090; DRI4757; Kolluri 2016; PENTHIFRA PLUS) compared the efficacy and safety of fondaparinux versus placebo for VTE prevention. As the outcomes total VTE, total DVT and proximal DVT showed significant heterogeneity, we analysed the data by using the random‐effects model. Remaining outcomes (symptomatic VTE, total PE, fatal PE, non‐fatal PE, major bleeding, fatal bleeding, MI, all causes of death, other serious adverse effects) showed low heterogeneity and were analysed with the fixed‐effect model. Our results showed less total VTE (RR 0.24, 95% CI 0.15 to 0.38; P < 0.00001; 8 studies; 5717 participants), less symptomatic VTE (RR 0.15, 95% CI 0.06 to 0.36; P < 0.0001; 8 studies; 6503 participants), less total DVT (RR 0.25, 95% CI 0.15 to 0.40; P < 0.00001; 8 studies; 5715 participants), less proximal DVT (RR 0.12, 95% CI 0.04 to 0.39; P = 0.0004; 7 studies; 2746 participants) and less total PE (RR 0.16, 95% CI 0.04 to 0.62; P = 0.008; 8 studies; 6412 participants) for fondaparinux compared with placebo. All PE events were symptomatic. Fondaparinux also showed less fatal PE and non‐fatal PE, but results from eight studies and 6412 participants were not significant compared with placebo (RR 0.14, 95% CI 0.02 to 1.17; P = 0.07; and RR 0.22, 95% CI 0.05 to 1.03; P = 0.05).
On the other hand, results showed that major bleeding was increased in the fondaparinux arm (RR 2.56, 95% CI 1.48 to 4.44; P = 0.0008; 8 studies; 6659 participants) versus the placebo arm. Only two out of six studies reporting on fatal bleeding reported participants suffered from fatal bleeding, and the rate was not different between study arms (RR 4.87, 95% CI 0.58 to 40.84; P = 0.14; 6 studies; 5993 participants).
Four out of eight studies (APOLLO; ARTEMIS; CALISTO; PENTHIFRA PLUS) reporting on all causes of death reported deaths. We combined results and showed that the all causes of death outcome was not different between fondaparinux and placebo arms (RR 0.76, 95% CI 0.48 to 1.22; P = 0.26; 8 studies; 6674 participants).
Our results did not show differences in MI (RR 0.25, 95% CI 0.03 to 2.19; P = 0.21; 5 studies; 5777 participants) or other serious adverse effects (RR 0.98, 95% CI 0.77 to 1.24; P = 0.85; 7 studies; 6581 participants) between fondaparinux and placebo. Most of the included studies reporting MI did not classify MI into fatal and non‐fatal events.
The quality of evidence for total VTE, total DVT and proximal DVT was moderate, and the quality of evidence for symptomatic VTE and total PE was high. The quality of evidence for major bleeding and all causes of death was moderate. See Table 1.
Sensitivity analysis and subgroup analysis
We added Yokote 2011 to the analysis to assess its effects on treatment effects. Results did not change for VTE, PE and bleeding outcomes. Yokote 2011 did not report on the other outcomes of this review. For details, see the Data and analyses section, Comparison 2.
The CALISTO study investigated fondaparinux for the treatment of patients with superficial venous thrombosis (SVT), as well as for VTE prevention. Considering that the clinical characteristics were different between participants with SVT and other participants included in the meta‐analyses, we performed another sensitivity analysis that excluded the CALISTO study. Results showed no significant differences. For details, see the Data and analyses section, Comparison 3.
We also performed subgroup analyses based on different clinical conditions (by dividing studies into surgery, superficial thrombophlebitis and medically ill participants subgroups). Subgroup analyses showed no differences between subgroups for total VTE, major bleeding, all causes of death and other serious adverse effects (for details, see the Data and analyses section, Comparison 4). The medically ill participants subgroup showed no significant differences between fondaparinux and placebo in total DVT and proximal DVT. For total PE, results revealed no significant differences between fondaparinux and placebo arms in every subgroup. The medically ill patients and thrombophlebitis subgroups exhibited no significant differences between fondaparinux and placebo in terms of major bleeding. However, we included only one study for each of the superficial thrombophlebitis and medically ill participants subgroups.
Fondaparinux versus LMWH
Twelve studies (ACT2545; EFFORT; EPHESUS; FONDACAST; L‐8635; Li 2015; PEGASUS; PENTAMAKS; PENTATHLON; PENTATHLON 2000; PENTHIFRA; Shen 2014) compared the efficacy and safety of fondaparinux versus LMWH for the prevention of VTE. We combined the first 11 studies into meta‐analyses. Total VTE, total DVT and proximal DVT outcomes showed significant heterogeneity; therefore, we used the random‐effects model. Outcomes of symptomatic VTE, total PE, fatal PE, non‐fatal PE, major bleeding, fatal bleeding, all causes of death, MI and other serious adverse effects showed low heterogeneity; therefore, we used the fixed‐effect model for analyses of these variables. The heterogeneity assessment of symptomatic VTE showed a P value for the Chi‐squared test of 0.16 and an I2 statistic of 35%; after discussion, we decided to analyse this outcome by using the fixed‐effect model. Results showed that fondaparinux reduced VTE and DVT (RR 0.55, 95% CI 0.42 to 0.73; P < 0.0001; 11 studies; 9339 participants; and RR 0.54, 95% CI 0.40 to 0.71; P < 0.0001; 10 studies; 9356 participants, respectively) and showed a trend towards reduced proximal DVT (RR 0.58, 95% CI 0.33 to 1.02; P = 0.06; 9 studies; 8361 participants). EPHESUS, PEGASUS, PENTAMAKS, PENTATHLON, PENTATHLON 2000, PENTHIFRA, Li 2015, EFFORT and FONDACAST reported symptomatic VTE. The combined result showed no differences between fondaparinux and LMWH in symptomatic VTE (RR 1.03, 95% CI 0.65 to 1.63; P = 0.90; 9 studies; 12,240 participants). Fondaparinux increased major bleeding (RR 1.38, 95% CI 1.09 to 1.75; P = 0.008; 11 studies; 12,501 participants) but did not increase fatal bleeding (RR 0.71, 95% CI 0.14 to 3.62; P = 0.68; 6 studies; 10,293 participants). Total PE, fatal PE and non‐fatal PE were not different (RR 1.24, 95% CI 0.65 to 2.34; P = 0.51; 10 studies; 12,350 participants; and RR 0.72, 95% CI 0.25 to 2.05; P = 0.54; 9 studies; 11,107 participants; and RR 1.40, 95% CI 0.63 to 3.11; P = 0.41; 9 studies; 11,107 participants, respectively) between fondaparinux and LMWH groups. All PE events were symptomatic events. Shen 2014 reported VTE rates of fondaparinux and LMMH groups as 7.02% and 8.47%, respectively (P = 0.957). However, because Shen 2014 did not report the number of events for each group, we did not include this study in the meta‐analysis.
EPHESUS, FONDACAST, PEGASUS, PENTAMAKS, PENTATHLON, PENTATHLON 2000 and PENTHIFRA reported deaths, and ACT2545, Li 2015, EFFORT and L‐8635 reported no cases of death. The pooled result showed no differences between fondaparinux and LMWH arms (RR 0.88, 95% CI 0.63 to 1.22; P = 0.44; 11 studies; 12,400 participants). EPHESUS, PENTATHLON and PENTHIFRA also reported deaths associated with VTE or bleeding. Pooled results did not show a difference (RR 0.89, 95% CI 0.38 to 2.07; P = 0.79; 5 studies; 4774 participants). Results showed no differences in MI (RR 1.28, 95% CI 0.69 to 2.37; P = 0.43; 6 studies; 10,720 participants) nor in other serious adverse effects (RR 1.06, 95% CI 0.94 to 1.19; P = 0.31; 10 studies; 12,465 participants) between the two types of medicine. Most included studies did not classify MI data into fatal and non‐fatal events.
The quality of evidence was moderate for total VTE, symptomatic VTE, total DVT, total PE and all causes of death, and was low for proximal DVT. The quality of evidence was high for major bleeding. See Table 2.
Sensitivity analysis and subgroup analysis
We subsequently added the studies L‐8541 and Yokote 2011 to the analysis to assess their effects. Results did not change significantly for total VTE, symptomatic VTE, total DVT, proximal DVT, total PE, fatal PE, non‐fatal PE, major bleeding, fatal bleeding, all causes of death and other serious adverse effects. For details, see the Data and analyses section, Comparison 6.
Because the doses of fondaparinux and enoxaparin used in the EFFORT study were double those used by other included studies, we excluded the EFFORT study in another sensitivity analysis to assess the effects of these different doses. Results were not significantly changed for any outcomes. For details, see the Data and analyses section, Comparison 7.
We performed subgroup analyses comparing fondaparinux versus LMWH on the basis of different clinical conditions (by dividing studies into orthopaedic, abdominal surgery, bariatric surgery and ICU patients subgroups). Subgroup analyses showed no differences between subgroups. Subgroup analyses revealed no differences between study arms in the orthopaedic subgroup in symptomatic VTE, proximal DVT, total DVT, total VTE, major bleeding, total PE, all causes of death and other serious adverse effects (for details, see the Data and analyses section, Comparison 8). In addition, we noted no differences between fondaparinux and LMWH arms in total VTE, total DVT and major bleeding outcomes in the other three subgroups. However, we included only one study in each of the other three subgroups.
Fondaparinux versus warfarin
Only one study (Bern 2015) compared fondaparinux versus variable dose warfarin (target INR 2.0 to 2.5) for VTE prevention after hip or knee replacement until day 28 ± 2 after operation. Investigators included 118 participants in each of the two treatment groups and reported no VTE events (VTE, DVT or PE) in either group. They reported three cases of major bleeding in the fondaparinux group and none in the variable dose warfarin groups, showing no differences between groups (RR 7.00, 95% CI 0.37 to 134.5; 236 participants). Study authors reported no fatal bleeding. The quality of the evidence for all outcomes was very low. For details, see the Data and analyses section, Comparison 9.
Bern 2015 also compared fondaparinux with the fixed dose 1 mg once daily warfarin for VTE prevention. They reported no VTE events in the fondaparinux group and two distal DVTs in the 1 mg warfarin group, showing no statistically significant differences (RR 0.2, 95% CI 0.01 to 4.12; 236 participants for VTE and total DVT analyses). Results showed three and one major bleeding events, respectively, in the fondaparinux and 1 mg warfarin groups (RR 3.00, 95% CI 0.32 to 28.43; 236 participants). The quality of the evidence for all outcomes was very low. For details, see the Data and analyses section, Comparison 10.
Bern 2015 reported no deaths and no other serious adverse events.
Fondaparinux versus edoxaban
Only one study (Fuji 2015) involving 43 participants compared fondaparinux (1.5 mg sc once daily) versus edoxaban (15 mg oral once daily) for patients with renal impairment undergoing lower‐limb orthopaedic surgery. Researchers reported no cases of thromboembolic and major bleeding events in the fondaparinux and edoxaban groups. In addition, they reported no deaths. The quality of the evidence for all outcomes was very low. For details, see the Data and analyses section, Comparison 11.
Fondaparinux versus mechanical thromboprophylaxis
Only one study (AR3106116) compared fondaparinux with the mechanical thromboprophylaxis method and intermittent pneumatic compression (IPC). This study showed no differences in total VTE (RR 0.61, 95% CI 0.22 to 1.67; 99 participants), DVT (RR 0.63, 95% CI 0.23 to 1.72; 100 participants) or other serious adverse effects (RR 0.18, 95% CI 0.02 to 1.67; 120 participants) between fondaparinux and IPC. Results included no cases of symptomatic VTE, proximal DVT, PE, major bleeding or death,although this is likely a result of the fact that the study lasted only up to eight days. The quality of the evidence for all outcomes was low. For details, see the Data and analyses section, Comparison 12.
Discussion
Summary of main results
We included in this review 25 studies with a total of 21,004 participants; all investigated fondaparinux for venous thromboembolism (VTE) prevention, and no studies examined idraparinux or idrabiotaparinux for prevention of VTE.
Fondaparinux versus placebo
Our results show that compared with placebo, fondaparinux reduced VTE events for people needing VTE prevention. Effects of fondaparinux were consistent in nearly all efficacy outcomes and in most of the included studies. Table 1 shows that the quality of the evidence was moderate for total VTE. Therefore, we can conclude that fondaparinux is effective for VTE prevention. Results also demonstrate that fondaparinux reduced symptomatic VTE, total deep venous thrombosis (DVT), proximal DVT and total pulmonary embolism (PE), and the quality of evidence for these analyses was moderate or high, indicating that fondaparinux is effective for VTE prevention. At the same time, however, our results show that fondaparinux increased major bleeding compared with placebo. The quality of the evidence was moderate. This finding is significant, as major bleeding is vital for assessing the safety of anticoagulants. As a result, we consider that caution is warranted when fondaparinux is used in people who are at high risk of bleeding. In the analyses of all causes of death, we did not show a difference in numbers of deaths between fondaparinux and placebo. Sensitivity analyses did not change these results.
In subgroup analyses for different clinical conditions, we included only one study in the medically ill and superficial thrombophlebitis subgroups, respectively. Therefore, information was insufficient to permit conclusions on the efficacy and safety of fondaparinux for different clinical conditions. In the medically ill patients subgroup, fondaparinux caused fewer deaths than were caused by placebo (P = 0.06). However, we included only one study (ARTEMIS), and, as a result, evidence was insufficient to show a real trend. The medically ill patients group is a special group of patients that usually consists of more seriously ill and bedridden patients. In such a clinical setting, fondaparinux may have a larger effect. Our analyses (Analysis 4.1) show that fondaparinux significantly reduced total VTE, with a P value of 0.02 compared with placebo, in the medically ill patients subgroup. However, according to available evidence, the reduced VTE rate failed to significantly improve the survival rate. In addition, the small number of included studies did not allow us to draw conclusions. We need additional randomised controlled trials (RCTs) to assess whether fondaparinux can reduce mortality among medically ill patients. In the surgery patients subgroup analyses, only APOLLO investigated patients undergoing abdominal surgery, only Kolluri 2016 investigated those undergoing bariatric surgery and the remaining four studies investigated orthopaedic patients. Consequently, it is suggested that fondaparinux is effective for VTE prevention among orthopaedic patients, although the evidence for patients undergoing other types of surgery remains insufficient. This may be the case because it is common for orthopaedic surgeons to give anticoagulation treatment to immobilised patients. At the same time, it is not usual for surgeons from other departments to administer anticoagulative medicine to prevent VTE in immobilised patients after surgery. We need additional high‐quality studies on fondaparinux for VTE prevention among people in clinical settings other than orthopaedics.
4.1. Analysis.
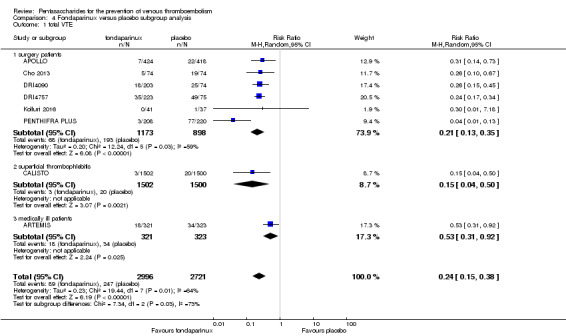
Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 1 total VTE.
Fondaparinux versus low molecular weight heparin (LMWH)
We demonstrated that total VTE was reduced in the fondaparinux group compared with the LMWH group, but symptomatic VTE was not different. Results indicate that fondaparinux decreased only non‐symptomatic VTE compared with LMWH. As the importance of non‐symptomatic isolated distal DVT remains unclear, most studies cannot show a significant increase in mortality or in the incidence of post‐thrombotic syndrome (Palareti 2012). In addition, the quality of evidence for the efficacy analyses was moderate or low. See Table 2. We showed a tendency for fondaparinux to reduce proximal DVT compared with LMWH (P = 0.06). According to Hansson 2000, asymptomatic proximal DVT causes significantly more deaths compared with both asymptomatic distal DVT and no DVT; therefore, targeting of asymptomatic proximal DVT is an appropriate endpoint in future clinical studies of venous thromboprophylaxis. Moreover, our results show that fondaparinux increased the major bleeding rate. In terms of safety, fondaparinux was inferior to LMWH. In summary, on the basis of available evidence, we conclude that fondaparinux may be more effective than LMWH for VTE prevention, but it increases the major bleeding rate, which limits its usefulness.
Similar to the fondaparinux versus placebo subgroup analysis, we included only one study in the abdominal surgery and ICU patients subgroups, respectively, and the subgroup result was not significantly different from the total result. Therefore, we cannot draw conclusions regarding the superiority or inferiority of fondaparinux compared with LMWH in different clinical conditions for VTE prophylaxis.
Fondaparinux versus other methods of thromboprophylaxis
Only one study (Bern 2015) compared fondaparinux versus variable warfarin (target INR 2 to 2.5) and 1 mg once daily warfarin for VTE prevention among patients who have undergone elective hip or knee replacement surgery. This study was small, and reported efficacy and safety outcomes were rare. Only one study (Fuji 2015) compared fondaparinux with edoxaban. Thus the evidence on fondaparinux compared with anticoagulants other than LMWH for VTE prevention was insufficient to allow any conclusions. Additional studies are needed.
Only one small study (AR3106116) with a short duration of eight days compared fondaparinux versus mechanical thromboprophylaxis. Evidence was insufficient to allow any conclusions. Additional studies are needed for this comparison.
Overall completeness and applicability of evidence
All included studies of fondaparinux for VTE prevention investigated total VTE and major bleeding as primary efficacy and safety outcomes.
No studies reported on fondaparinux versus other anticoagulation methods besides LMWH, warfarin, edoxaban and an intermittent pneumatic compression device (IPC), such as unfractionated heparin, aspirin, oral thrombin inhibitors and other direct factor X inhibitors; too few studies have examined fondaparinux versus LMWH, warfarin, edoxaban and IPC for VTE prevention. In addition, no study investigated long‐acting pentasaccharides (idraparinux and idrabiotaparinux) and pentasaccharides other than fondaparinux for primary VTE prevention.
Most studies of fondaparinux have focused on prevention of VTE among patients undergoing orthopaedic surgery. Therefore, our results mainly reflect fondaparinux for VTE prevention among these patients. Subgroup analyses did not include enough studies in subgroups other than orthopaedic patients, such as the medically ill patients subgroup and the superficial venous thrombosis subgroup; hence, the evidence is insufficient to reveal whether effects and adverse effects of fondaparinux are different for patients in different clinical settings. Therefore, we are in greater need of studies that focus on fondaparinux for VTE prevention in different clinical conditions, such as gynaecological surgery, neurological surgery, etc, and other medical conditions, such as malignant disease.
Quality of the evidence
See Table 1, Table 2, Table 3, Table 4 and Table 6.
Most of the studies included in this systematic review were high‐quality RCTs with large sample sizes and reliable randomisation methods. All outcome assessments were completed by independent adjudicated committees that were blind to randomisation, and most VTEs were diagnosed by objective reliable tests (venography, independent ultrasonography, etc, for DVT; spiral computed tomography (CT), venography, etc, for PE). These points enhanced the quality of the evidence. On the other hand, some included studies were open‐label or single‐blinded (ACT2545; AR3106116; FONDACAST; L‐8635; Li 2015) with risk affecting allocation (Hills 2009). Most of the included studies (except Bern 2015; Cho 2013; Kolluri 2016; and Li 2015) were organised by a pharmaceutical company, and the four studies that did not receive support from pharmaceutical companies included relatively small samples. These disadvantages could potentially introduce bias and lower the quality of the evidence.
Summary of findings tables show moderate‐quality evidence in the fondaparinux versus placebo meta‐analysis for total VTE, total DVT and proximal DVT, and high‐quality evidence for symptomatic VTE and total PE. At the same time, we graded the quality of the evidence for the efficacy of fondaparinux versus LMWH as moderate or low. The quality of the evidence for major bleeding in the fondaparinux versus both placebo and LMWH analyses was moderate and high, respectively. Reasons for lowering the quality of the evidence included small numbers of events causing imprecision, and heterogeneity or inconsistency between studies. In addition, a potential risk of bias was caused by studies organised by pharmaceutical companies and by lack of publication in languages other than English, although we did not consider these sufficient to downgrade the quality of evidence. The Summary of findings table showed very low‐quality evidence for fondaparinux versus both variable and 1 mg once daily fixed doses of warfarin. We included only one study comparing fondaparinux with warfarin, and events were rare, which lowered the grade of the evidence. The Summary of findings table showed low‐quality evidence for fondaparinux versus mechanical thromboprophylaxis; only one included study performed this comparison, and the study was only up to eight days long, resulting in many outcomes with no reported events, as follow‐up was too short. This study was organised by a pharmaceutical company. The Summary of findings table showed very low‐quality evidence for fondaparinux versus edoxaban. Only one small study assessed this comparison and recorded no events, which led to lowering of the grade of evidence.
Potential biases in the review process
This systematic review had some potential biases.
Most included studies analysed efficacy outcomes by using the per‐protocol strategy, not the intention‐to‐treat (ITT) strategy that was preplanned in our protocol, because most VTE and DVT events were diagnosed by venography, which is an invasive procedure that could not be used on every participant. It would be very difficult to arbitrate these participants without such efficacy evaluation results (about 10% in every study); therefore, we pooled and analysed the results presented by study reports, but not ITT data as planned. Furthermore, we included in the meta‐analysis all VTE events before study time endpoints. So we pooled per‐protocol data but not ITT data, as our protocol had outlined.
ARTEMIS, PEGASUS, PENTATHLON 2000, PENTHIFRA and PENTHIFRA PLUS mentioned that investigators used adjunctive anticoagulation methods but did not report whether they were used equally in the different treatment groups. Because adjunctive methods used by most participants in these studies consisted of graduated compression stockings (GCS), and evidence suggests that GCS would not substantially affect the symptomatic VTE rate (Falck‐Ytter 2012), we decided to include these in the meta‐analysis. Another reason for including these studies was that they included large samples, and excluding them from the meta‐analysis would bias the overall outcome.
Funnel plots for the outcome total VTE in the comparison of fondaparinux versus placebo and LMWH were asymmetrical (Figure 4; Figure 5). Most included studies (except Bern 2015, Cho 2013, Kolluri 2016, Li 2015, Shen 2014 and Yokote 2011) were conducted by pharmaceutical companies, and all were reported in English. Therefore, we considered risk of publication bias was possible. Cho 2013, Shen 2014, Yokote 2011, Li 2015, Bern 2015 and Kolluri 2016 were not conducted by pharmaceutical companies and included relatively small sample sizes. Yokote 2011 failed to report a reliable method used for randomisation; therefore, we included only this study in the sensitivity analysis. Shen 2014 reported VTE rates of fondaparinux and LMWH groups instead of numbers of VTE events, and we were unable to obtain these details when communicating with study authors. Therefore, we did not include Shen 2014 in the meta‐analyses. We included Bern 2015, Kolluri 2016, Li 2015 and Cho 2013 in analyses of primary and secondary outcomes.
4.
Funnel plot of comparison: 1 fondaparinux versus placebo, outcome: 1.1 total VTE.
5.
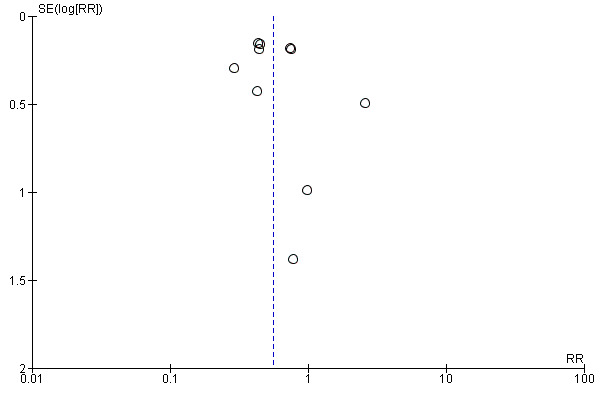
Funnel plot of comparison: 5 fondaparinux versus LMWH, outcome: 5.1 total VTE.
Finally, because we included all suitable trials on VTE prevention that included patients after orthopaedic and abdominal surgery, medically ill patients and those with superficial venous thrombosis, we noted large heterogeneity in some meta‐analyses. We chose the random‐effects model to analyse the data.
The reliability of this systematic review might be influenced by these factors, and results might need to be interpreted with caution. Although we had some limitations, we believe that our review is significant for evaluating the efficacy and safety of fondaparinux for VTE prevention.
Agreements and disagreements with other studies or reviews
Our results show that fondaparinux was more effective in VTE prevention than both placebo and LMWH. Results were consistent with those reported by the Bounameaux 2002, Nijkeuter 2004 and Falck‐Ytter 2012 reviews. In the Antithrombotic Therapy and Prevention of Thrombosis Guidelines (Falck‐Ytter 2012), review authors combined five large trials comparing fondaparinux 2.5 mg started six to eight hours after wound closure versus a routine prophylactic dose of LMWH for patients after surgery. Review authors showed that fondaparinux reduced the asymptomatic DVT rate significantly but did not show significant differences in symptomatic DVT and PE rates between the two types of anticoagulants. In our systematic review, we showed that fondaparinux significantly reduced total VTE and DVT rates but did not reduce symptomatic DVT and PE rates. On the other hand, our systematic review showed that fondaparinux might reduce proximal DVT by less than 50% compared with LMWH (risk ratio (RR) 0.56, 95% confidence interval (CI) 0.31 to 1.0; P = 0.06), although this finding was not statistically significant. In Falck‐Ytter 2012, review authors did not study this outcome. The proximal DVT analysis result might be helpful, as proximal DVT is associated with more recurrent VTE (symptomatic and non‐symptomatic) and more frequent deaths than distal DVT (Pinede 2001). We need additional data to confirm whether the result was caused by real efficacy of fondaparinux or occurred by chance. For bleeding risk, Falck‐Ytter 2012 demonstrated a substantial increase in bleeding requiring reoperation associated with the use of fondaparinux (RR 1.85, 95% CI 1.1 to 3.11) compared with LMWH, but showed no difference in the non‐fatal major bleeding rate (RR 1.35, 95% CI 0.89 to 2.05). In our analyses, we showed that fondaparinux increased overall major bleeding (Analysis 5.8) but did not increase fatal bleeding. Our review used a different definition of fatal bleeding than was used in Falck‐Ytter 2012. Also, participants in our study were different from those involved in the Falck‐Ytter 2012 study. We included all people requiring primary VTE prevention, but Falck‐Ytter 2012 included only people who had undergone surgery. In addition, participants included in our review were potentially at lower risk of haemorrhage. Nevertheless, fondaparinux increased the overall major bleeding rate compared with LMWH. Our systematic review and the Falck‐Ytter 2012 study suggest similar efficacy and safety of fondaparinux for VTE prevention in different patient populations.
5.8. Analysis.
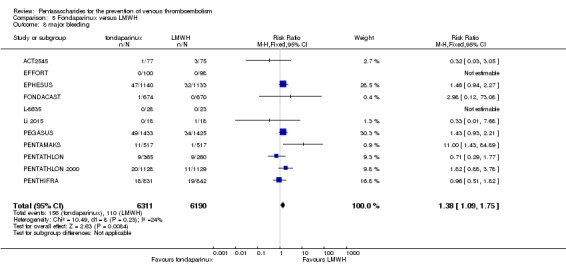
Comparison 5 Fondaparinux versus LMWH, Outcome 8 major bleeding.
Fondaparinux is the first agent in its class that was approved by the US Food and Drug Administration and the European Agency for the Evaluation of Medicinal Products. Compared with LMWH for short‐term VTE prevention, it is more effective and has predictable linear pharmacokinetics (PENTATHLON). Fondaparinux is rarely neutralised by platelet factor 4 and is highly unlikely to cause thrombocytopaenia (Amiral 1997). Fondaparinux highly selectively inhibits factor Xa with no direct effect against the thrombin molecule itself (Olson 1992). However, it causes more major bleeding, which limits its use. Therefore, we should be cautious when using fondaparinux for VTE prevention, especially in people who are at high risk of bleeding. Our results show that when fondaparinux is injected at a dose of 2.5 mg once daily, it not only reduces VTE but also increases bleeding significantly compared with the routine dose of LMWH for VTE prophylaxis. In a Cochrane systematic review (Brito 2011), review authors showed that fondaparinux was non‐inferior to LMWH in anticoagulative efficacy and lowered both major and minor bleeding rates compared with LMWH when used in the treatment of patients with acute coronary syndrome. In that research, the LMWH dose was approximately two times the dose used in our review, but the fondaparinux dose was the same as the one reported here. Therefore, we speculate that the higher major bleeding rate of fondaparinux might be caused by the relatively high dose used for VTE prevention. Dose‐response studies (DRI4090; DRI4757; PENTATHLON) have shown that when the fondaparinux dose is increased, bleeding risk is increased. Further research is warranted on whether the bleeding rate can be reduced by reducing the fondaparinux dose, for example, 2 mg or 1.5 mg sc once daily, without an effect on its efficacy in VTE prevention. Several recent studies on 1.5 mg once daily fondaparinux used for VTE prophylaxis for patients with renal dysfunction (Ageno 2012; Delavenne 2012; Mismetti 2012) showed that it was effective and would cause little bleeding. Pharmacokinetics studies (Delavenne 2010; Delavenne 2012) have reported interindividual variability with the same dose of fondaparinux between patients with different body weight, age and creatinine clearance. Currently, only one dose of 2.5 mg fondaparinux is used for VTE prevention. Perhaps this is not suitable for all people, and the fondaparinux dose may need to be adjusted according to body surface area, body weight, age, risk of thrombosis, renal function, etc, when used for VTE prevention.
Authors' conclusions
Implications for practice.
Evidence of moderate to high quality shows that fondaparinux is effective for short‐term prevention of VTE when compared with placebo. It can reduce total VTE, DVT, total PE and symptomatic VTE and shows no reduction in deaths when compared with placebo. Evidence of low to moderate quality shows that fondaparinux is more effective for short‐term prevention of VTE when compared with LMWH. It can reduce total VTE and total DVT and does not demonstrate a reduction in deaths when compared with LMWH. However, at the same time, evidence of moderate and high quality shows that fondaparinux increases major bleeding when compared with placebo and LMWH, respectively. Therefore, when fondaparinux is chosen for VTE prophylaxis, attention should be paid to the patient's bleeding and thrombosis risks. Most available data were derived from studies on patients undergoing orthopaedic surgery. Therefore, the conclusion predominantly pertains to these patients. Data on other clinical conditions such as internal medical and abdominal surgery are sparse.
Implications for research.
Based on the moderate quality of evidence, we suggest that additional RCTs are needed to assess effects of fondaparinux for VTE prevention compared with placebo and LMWH, especially in terms of outcomes such as proximal DVT and PE.
Additional RCTs are needed on the use of fondaparinux for VTE prevention among patients undergoing surgery other than orthopaedic surgery, such as abdominal surgery, thoracic surgery, gynaecological surgery, neurological surgery, etc, and with other medical conditions, such as malignant disease.
We retrieved no RCTs on the long‐acting pentasaccharides for primary VTE prevention. Therefore, RCTs are needed to assess the efficacy and safety of long‐acting pentasaccharides for primary prevention of VTE.
As only one RCT of small sample size compared fondaparinux versus mechanical thromboprophylaxis methods, additional RCTs are needed to evaluate the efficacy of mechanical thromboprophylaxis compared with pentasaccharides.
No RCTs have compared pentasaccharides with other anticoagulants, such as oral direct antithrombin inhibitors and oral direct factor X inhibitors. Therefore, we need additional studies to compare these different interventions.
History
Protocol first published: Issue 1, 2005 Review first published: Issue 10, 2016
| Date | Event | Description |
|---|---|---|
| 3 November 2008 | Amended | Converted to new review format |
Acknowledgements
The review authors thank the Cochrane Vascular editorial base for extentive help provided during the writing process.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MESH DESCRIPTOR Thrombosis | 1209 |
| #2 | MESH DESCRIPTOR Thromboembolism | 880 |
| #3 | MESH DESCRIPTOR Venous Thromboembolism | 220 |
| #4 | MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES | 1978 |
| #5 | (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*):TI,AB,KY | 16054 |
| #6 | MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES | 719 |
| #7 | (PE or DVT or VTE):TI,AB,KY | 4155 |
| #8 | ((vein* or ven*) near thromb*):TI,AB,KY | 5808 |
| #9 | (blood near3 clot*):TI,AB,KY | 2264 |
| #10 | (pulmonary near3 clot*):TI,AB,KY | 5 |
| #11 | (lung near3 clot*):TI,AB,KY | 4 |
| #12 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 | 20706 |
| #13 | MESH DESCRIPTOR Anticoagulants | 3199 |
| #14 | MESH DESCRIPTOR Polysaccharides WITH QUALIFIERS TU | 164 |
| #15 | (fondapar* or Arixtra ):TI,AB,KY | 243 |
| #16 | (Sanorg‐34006 or Sanorg34006 ):TI,AB,KY | 2 |
| #17 | (SSR‐126517* or SSR126517*):TI,AB,KY | 3 |
| #18 | pentasac*:TI,AB,KY | 39 |
| #19 | MESH DESCRIPTOR Factor X EXPLODE ALL TREES WITH QUALIFIERS AI | 23 |
| #20 | (Factor X* near4 (antag* or inhib* or block*) ):TI,AB,KY | 514 |
| #21 | (FX* near4 (antag* or inhib* or block*) ):TI,AB,KY | 43 |
| #22 | ( Factor 10* near4 (antag* or inhib* or block*) ):TI,AB,KY | 81 |
| #23 | *arinux:TI,AB,KY | 276 |
| #24 | ORG31540*:TI,AB,KY | 17 |
| #25 | #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 | 3863 |
| #26 | #12 AND #25 | 2017 |
Appendix 2. LILACS search strategy
| Search on |
(fondaparinux or idraparinux) [Words] or idrabiotaparinux [Words] or pentasac$ [Words] |
| References found | 19 |
Data and analyses
Comparison 1. Fondaparinux versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 total VTE | 8 | 5717 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.38] |
| 2 symptomatic VTE | 8 | 6503 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.06, 0.36] |
| 3 total DVT | 8 | 5715 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.40] |
| 4 proximal DVT | 7 | 2746 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.04, 0.39] |
| 5 total PE | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.62] |
| 6 fatal PE | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.17] |
| 7 non‐fatal PE | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.03] |
| 8 major bleeding | 8 | 6659 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.48, 4.44] |
| 9 fatal bleeding | 6 | 5993 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| 10 MI | 5 | 5777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.19] |
| 11 all causes of death | 8 | 6674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.22] |
| 12 other serious adverse effects | 7 | 6581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.24] |
1.1. Analysis.
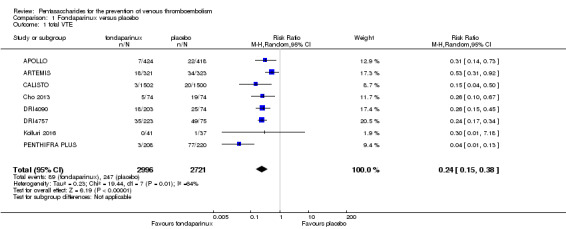
Comparison 1 Fondaparinux versus placebo, Outcome 1 total VTE.
1.2. Analysis.
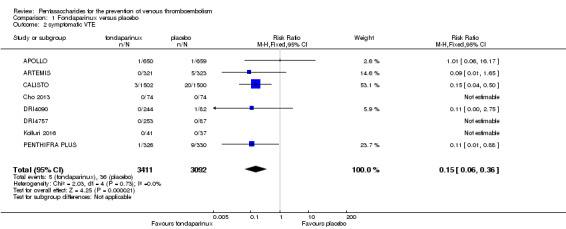
Comparison 1 Fondaparinux versus placebo, Outcome 2 symptomatic VTE.
1.3. Analysis.
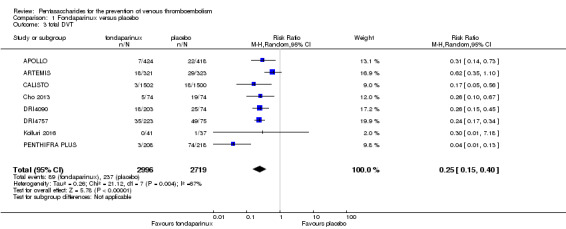
Comparison 1 Fondaparinux versus placebo, Outcome 3 total DVT.
1.4. Analysis.
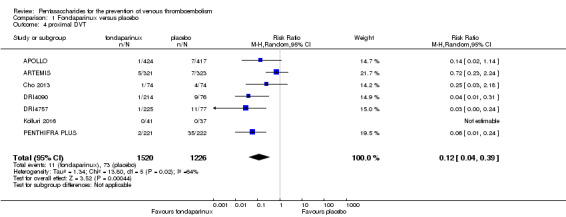
Comparison 1 Fondaparinux versus placebo, Outcome 4 proximal DVT.
1.5. Analysis.
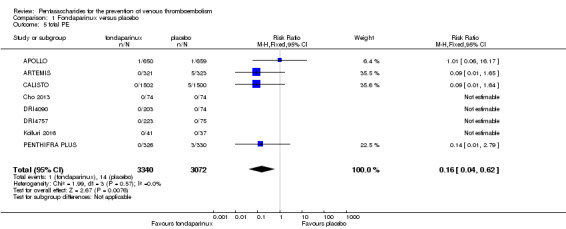
Comparison 1 Fondaparinux versus placebo, Outcome 5 total PE.
1.6. Analysis.
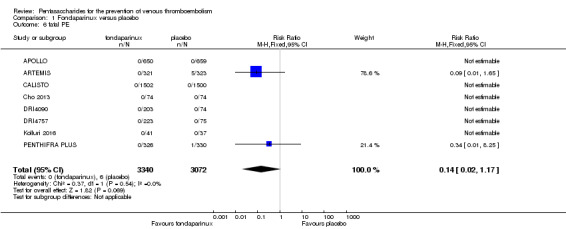
Comparison 1 Fondaparinux versus placebo, Outcome 6 fatal PE.
1.7. Analysis.

Comparison 1 Fondaparinux versus placebo, Outcome 7 non‐fatal PE.
1.8. Analysis.
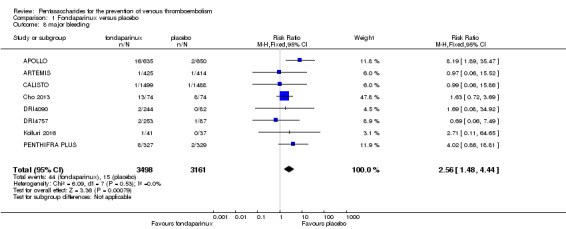
Comparison 1 Fondaparinux versus placebo, Outcome 8 major bleeding.
1.9. Analysis.
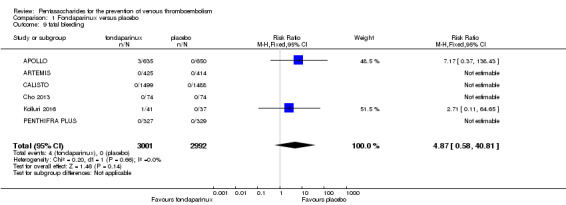
Comparison 1 Fondaparinux versus placebo, Outcome 9 fatal bleeding.
1.10. Analysis.
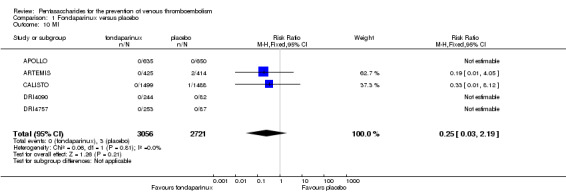
Comparison 1 Fondaparinux versus placebo, Outcome 10 MI.
1.11. Analysis.
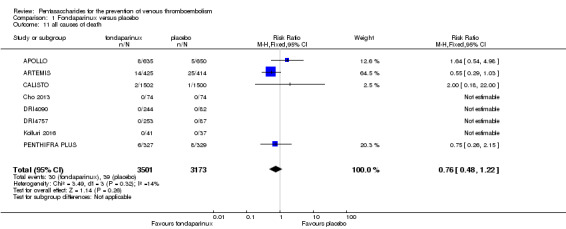
Comparison 1 Fondaparinux versus placebo, Outcome 11 all causes of death.
1.12. Analysis.
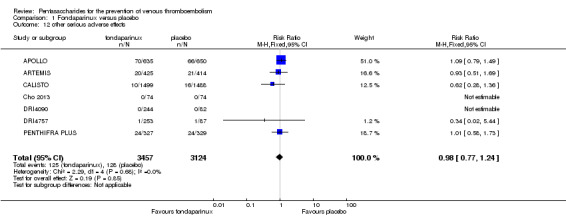
Comparison 1 Fondaparinux versus placebo, Outcome 12 other serious adverse effects.
Comparison 2. Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 total VTE | 9 | 5884 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.17, 0.43] |
| 2 symptomatic VTE | 9 | 6670 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.09, 0.42] |
| 3 total DVT | 9 | 5882 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.17, 0.45] |
| 4 proximal DVT | 8 | 2913 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.05, 0.51] |
| 5 total PE | 9 | 6579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.62] |
| 6 fatal PE | 9 | 6579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.17] |
| 7 non‐fatal PE | 9 | 6579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.03] |
| 8 major bleeding | 9 | 6829 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.48, 4.44] |
| 9 fatal bleeding | 7 | 6163 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| 10 all causes of death | 8 | 6766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.22] |
2.1. Analysis.
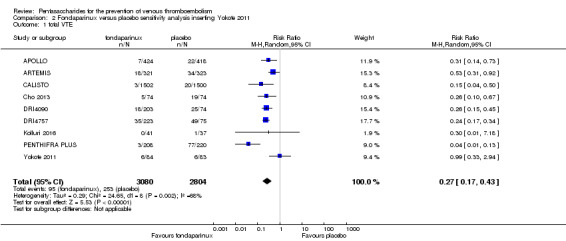
Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 1 total VTE.
2.2. Analysis.
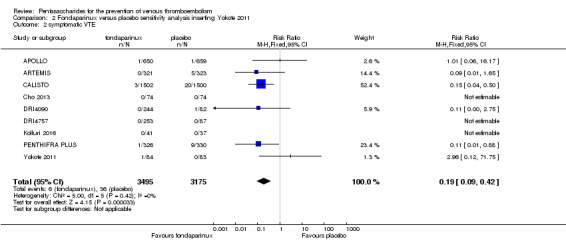
Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 2 symptomatic VTE.
2.3. Analysis.

Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 3 total DVT.
2.4. Analysis.
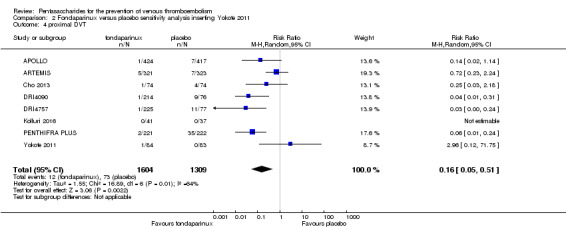
Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 4 proximal DVT.
2.5. Analysis.
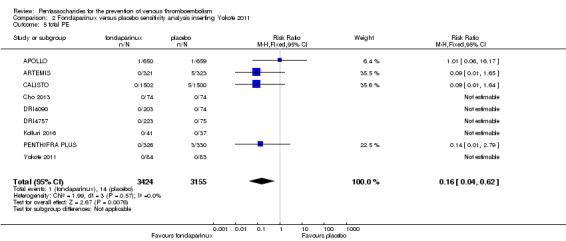
Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 5 total PE.
2.6. Analysis.
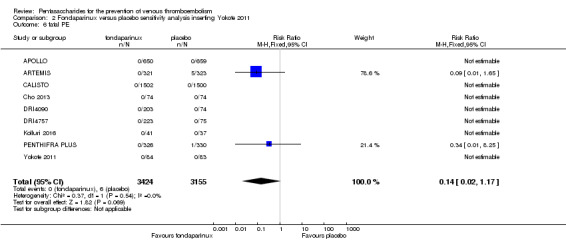
Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 6 fatal PE.
2.7. Analysis.
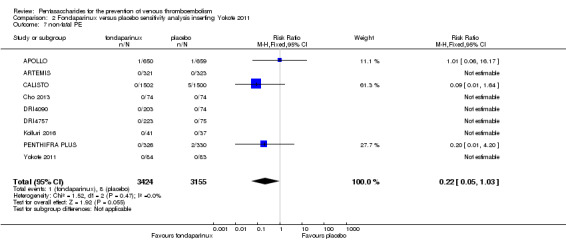
Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 7 non‐fatal PE.
2.8. Analysis.
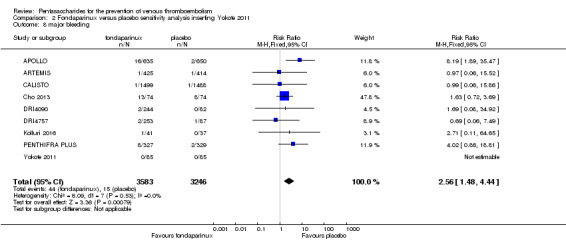
Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 8 major bleeding.
2.9. Analysis.

Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 9 fatal bleeding.
2.10. Analysis.
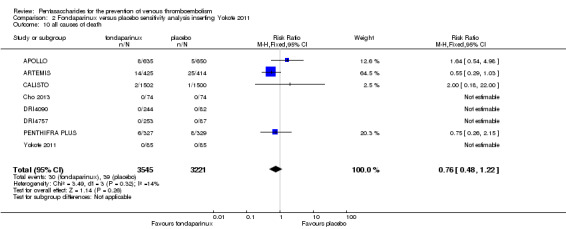
Comparison 2 Fondaparinux versus placebo sensitivity analysis inserting Yokote 2011, Outcome 10 all causes of death.
Comparison 3. Fondaparinux versus placebo sensitivity analysis excluding CALISTO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 total VTE | 7 | 2715 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.40] |
| 2 symptomatic VTE | 7 | 3501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.05, 0.54] |
| 3 total DVT | 7 | 2713 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.43] |
| 4 proximal DVT | 7 | 2746 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.04, 0.39] |
| 5 total PE | 7 | 3412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.92] |
| 6 fatal PE | 7 | 3410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.17] |
| 7 non‐fatal PE | 7 | 3410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.06, 2.93] |
| 8 major bleeding | 7 | 3672 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.52, 4.67] |
| 9 fatal bleeding | 5 | 3006 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| 10 MI | 4 | 2790 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.05] |
| 11 all causes of death | 7 | 3672 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.45, 1.18] |
| 12 other serious adverse effects | 6 | 3594 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.80, 1.32] |
3.1. Analysis.
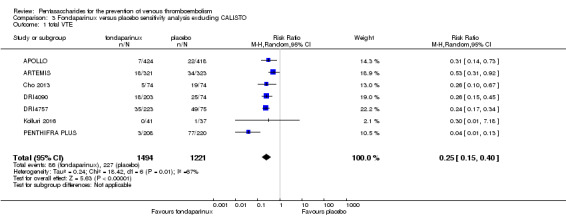
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 1 total VTE.
3.2. Analysis.
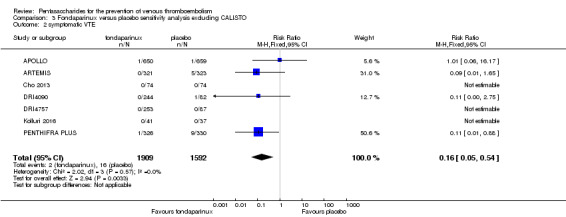
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 2 symptomatic VTE.
3.3. Analysis.
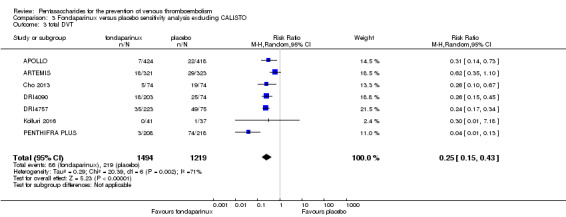
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 3 total DVT.
3.4. Analysis.
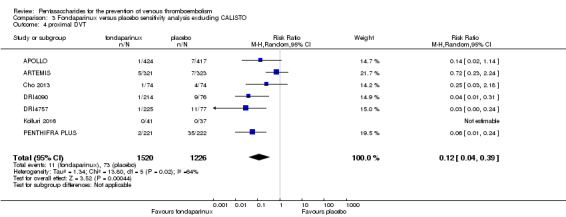
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 4 proximal DVT.
3.5. Analysis.
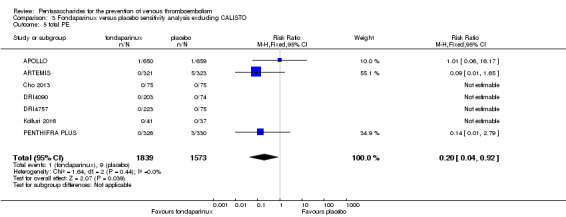
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 5 total PE.
3.6. Analysis.
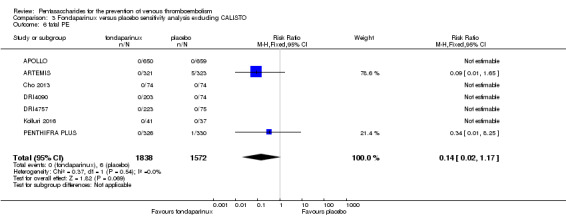
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 6 fatal PE.
3.7. Analysis.
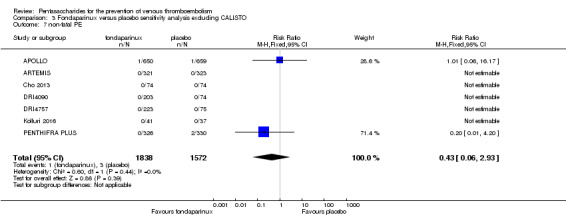
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 7 non‐fatal PE.
3.8. Analysis.
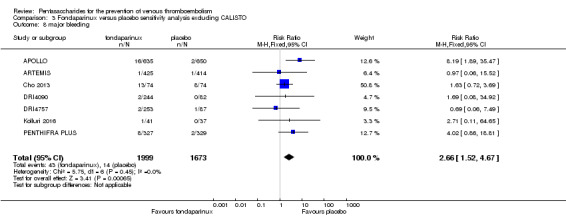
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 8 major bleeding.
3.9. Analysis.
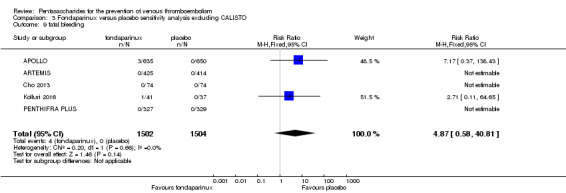
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 9 fatal bleeding.
3.10. Analysis.
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 10 MI.
3.11. Analysis.
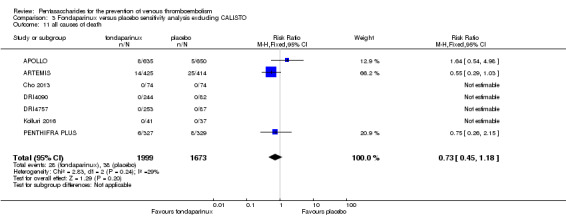
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 11 all causes of death.
3.12. Analysis.
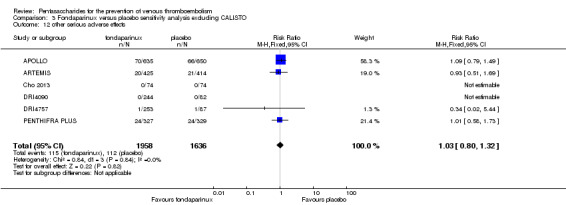
Comparison 3 Fondaparinux versus placebo sensitivity analysis excluding CALISTO, Outcome 12 other serious adverse effects.
Comparison 4. Fondaparinux versus placebo subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 total VTE | 8 | 5717 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.38] |
| 1.1 surgery patients | 6 | 2071 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.13, 0.35] |
| 1.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.04, 0.50] |
| 1.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.31, 0.92] |
| 2 symptomatic VTE | 8 | 6503 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.06, 0.36] |
| 2.1 surgery patients | 6 | 2857 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.05, 0.73] |
| 2.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.50] |
| 2.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.65] |
| 3 total DVT | 8 | 5715 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.40] |
| 3.1 surgery patients | 6 | 2069 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.13, 0.35] |
| 3.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.05, 0.56] |
| 3.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.35, 1.10] |
| 4 proximal DVT | 7 | 2745 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.07, 0.22] |
| 4.1 surgery patients | 6 | 2101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.03, 0.15] |
| 4.2 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.23, 2.24] |
| 5 total PE | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.62] |
| 5.1 surgery patients | 6 | 2766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.05, 2.14] |
| 5.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.64] |
| 5.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.65] |
| 6 fatal PE | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.17] |
| 6.1 surgery patients | 6 | 2766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.25] |
| 6.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.65] |
| 7 non‐fatal PE | 8 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.03] |
| 7.1 surgery patients | 6 | 2766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.06, 2.93] |
| 7.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.64] |
| 7.3 medically ill patients | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 major bleeding | 8 | 6659 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.48, 4.44] |
| 8.1 surgery patients | 6 | 2833 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [1.56, 4.95] |
| 8.2 superficial thrombophlebitis | 1 | 2987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.86] |
| 8.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 15.52] |
| 9 fatal bleeding | 6 | 5993 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| 9.1 surgery patients | 4 | 2167 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.87 [0.58, 40.81] |
| 9.2 superficial thrombophlebitis | 1 | 2987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 MI | 5 | 5777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.19] |
| 10.1 surgery patients | 3 | 1951 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 superficial thrombophlebitis | 1 | 2987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.12] |
| 10.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.05] |
| 11 all causes of death | 8 | 6674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.48, 1.22] |
| 11.1 surgery patients | 6 | 2833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.52, 2.31] |
| 11.2 superficial thrombophlebitis | 1 | 3002 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.18, 22.00] |
| 11.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.29, 1.03] |
| 12 other serious adverse effects | 7 | 6581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.24] |
| 12.1 surgery patients | 5 | 2755 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.80, 1.38] |
| 12.2 superficial thrombophlebitis | 1 | 2987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.28, 1.36] |
| 12.3 medically ill patients | 1 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.51, 1.69] |
4.2. Analysis.
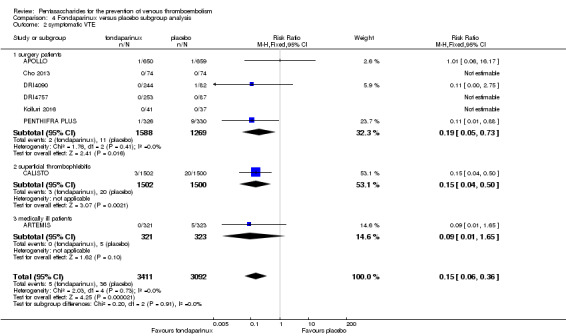
Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 2 symptomatic VTE.
4.3. Analysis.
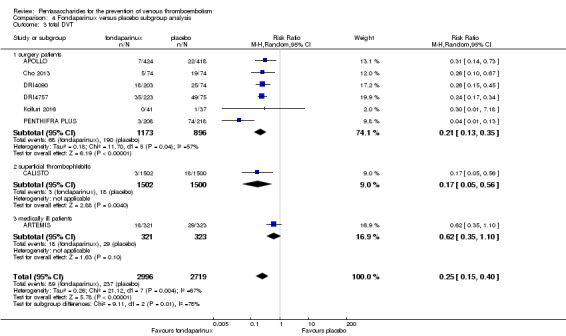
Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 3 total DVT.
4.4. Analysis.
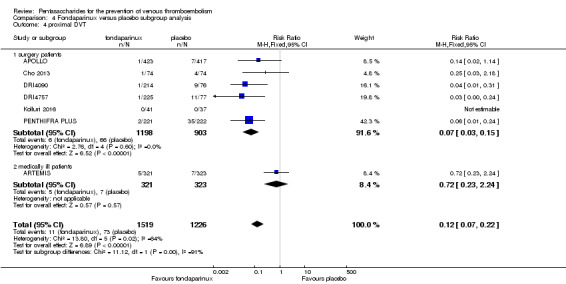
Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 4 proximal DVT.
4.5. Analysis.

Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 5 total PE.
4.6. Analysis.
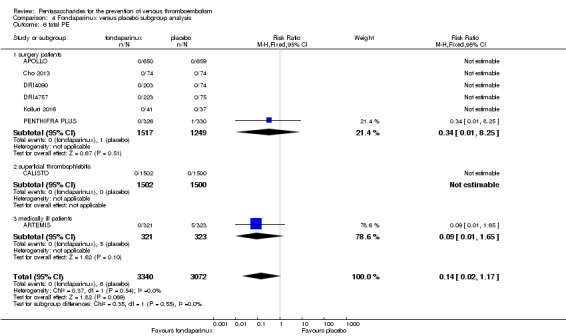
Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 6 fatal PE.
4.7. Analysis.
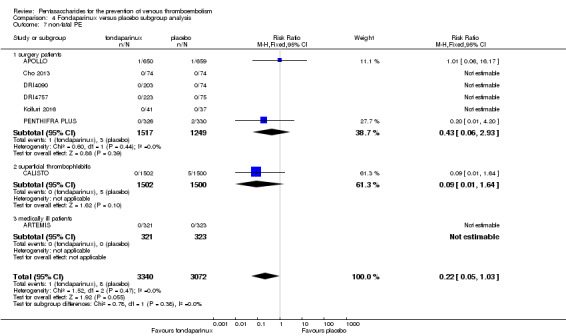
Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 7 non‐fatal PE.
4.8. Analysis.
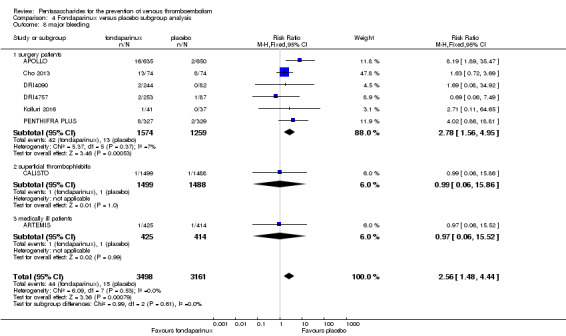
Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 8 major bleeding.
4.9. Analysis.
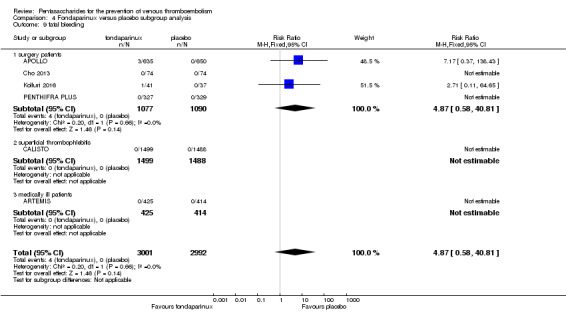
Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 9 fatal bleeding.
4.10. Analysis.
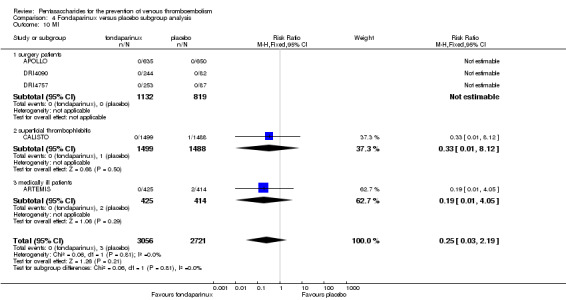
Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 10 MI.
4.11. Analysis.
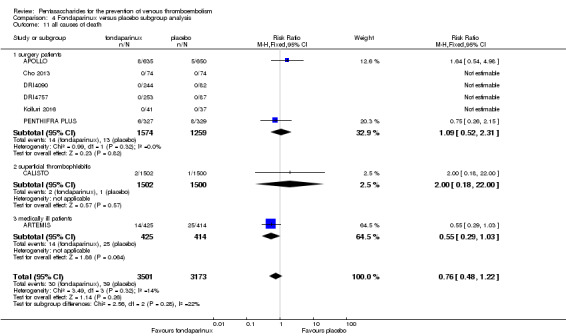
Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 11 all causes of death.
4.12. Analysis.
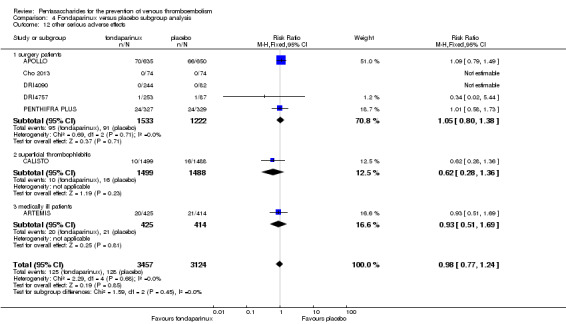
Comparison 4 Fondaparinux versus placebo subgroup analysis, Outcome 12 other serious adverse effects.
Comparison 5. Fondaparinux versus LMWH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 total VTE | 11 | 9339 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.42, 0.73] |
| 2 symptomatic VTE | 9 | 12240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.65, 1.63] |
| 3 total DVT | 10 | 9356 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.40, 0.71] |
| 4 proximal DVT | 9 | 8361 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.33, 1.02] |
| 5 total PE | 10 | 12350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.65, 2.34] |
| 6 fatal PE | 9 | 11107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.05] |
| 7 non‐fatal PE | 9 | 11107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.63, 3.11] |
| 8 major bleeding | 11 | 12501 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.09, 1.75] |
| 9 fatal bleeding | 6 | 10293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.62] |
| 10 MI | 6 | 10720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.69, 2.37] |
| 11 all causes of death | 11 | 12400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.22] |
| 12 death associated with VTE or bleeding | 5 | 4774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.38, 2.07] |
| 13 other serious adverse effects | 10 | 12465 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.94, 1.19] |
5.1. Analysis.
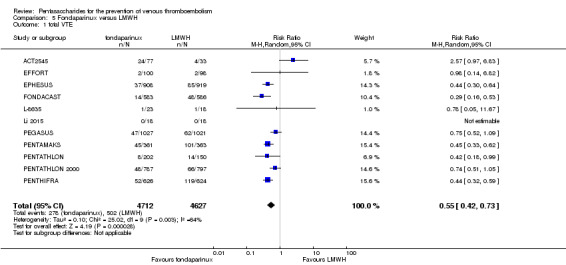
Comparison 5 Fondaparinux versus LMWH, Outcome 1 total VTE.
5.2. Analysis.
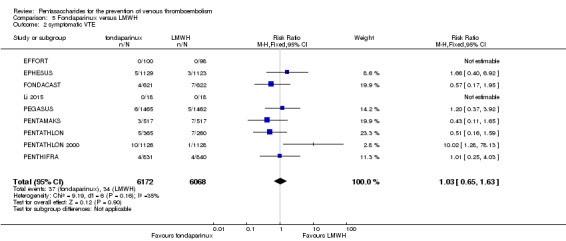
Comparison 5 Fondaparinux versus LMWH, Outcome 2 symptomatic VTE.
5.3. Analysis.
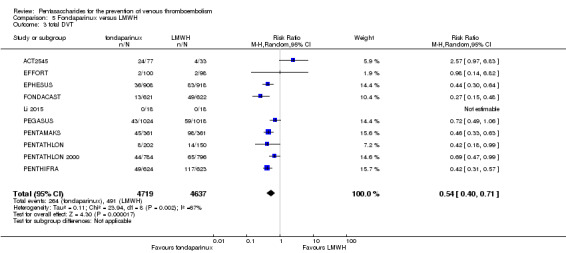
Comparison 5 Fondaparinux versus LMWH, Outcome 3 total DVT.
5.4. Analysis.
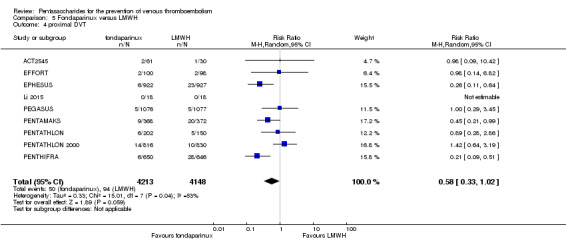
Comparison 5 Fondaparinux versus LMWH, Outcome 4 proximal DVT.
5.5. Analysis.
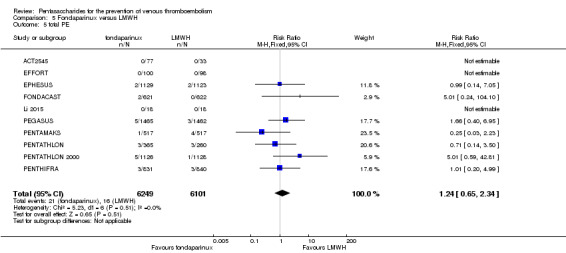
Comparison 5 Fondaparinux versus LMWH, Outcome 5 total PE.
5.6. Analysis.
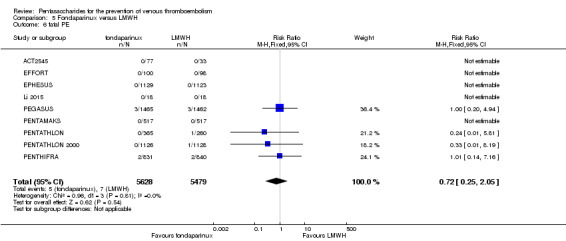
Comparison 5 Fondaparinux versus LMWH, Outcome 6 fatal PE.
5.7. Analysis.
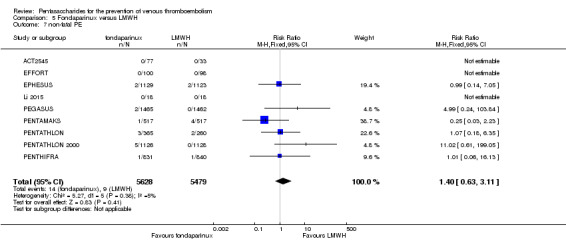
Comparison 5 Fondaparinux versus LMWH, Outcome 7 non‐fatal PE.
5.9. Analysis.
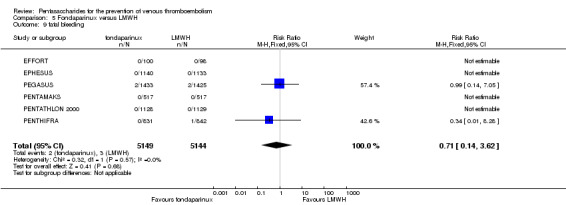
Comparison 5 Fondaparinux versus LMWH, Outcome 9 fatal bleeding.
5.10. Analysis.
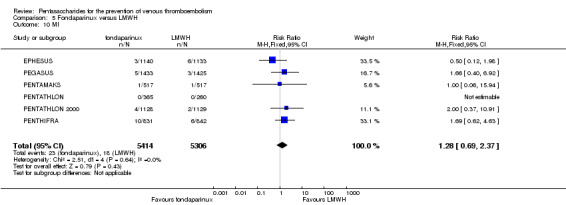
Comparison 5 Fondaparinux versus LMWH, Outcome 10 MI.
5.11. Analysis.
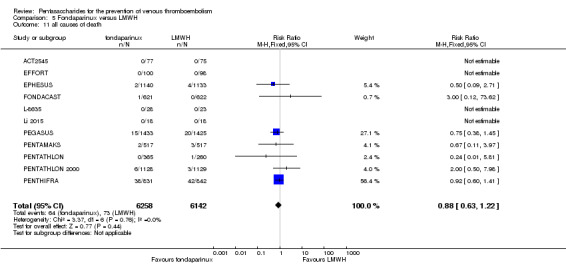
Comparison 5 Fondaparinux versus LMWH, Outcome 11 all causes of death.
5.12. Analysis.
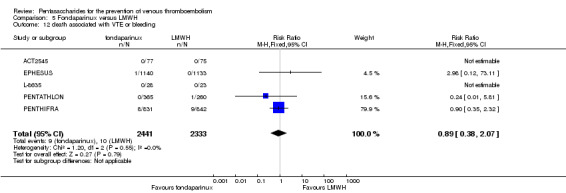
Comparison 5 Fondaparinux versus LMWH, Outcome 12 death associated with VTE or bleeding.
5.13. Analysis.
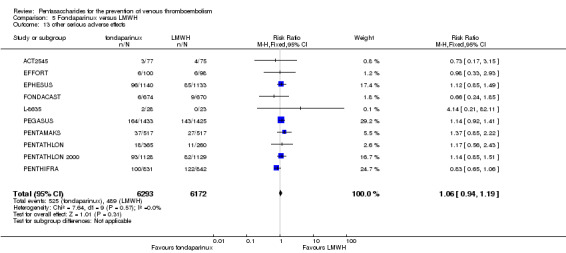
Comparison 5 Fondaparinux versus LMWH, Outcome 13 other serious adverse effects.
Comparison 6. Fondaparinux versus LMWH sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 total VTE | 13 | 9709 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.43, 0.74] |
| 2 symptomatic VTE | 11 | 12569 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.69, 1.70] |
| 3 total DVT | 12 | 9726 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.42, 0.72] |
| 4 proximal DVT | 10 | 8528 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.35, 1.06] |
| 5 total PE | 12 | 12720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.65, 2.34] |
| 6 fatal PE | 11 | 11477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.05] |
| 7 non‐fatal PE | 11 | 11486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.63, 3.11] |
| 8 major bleeding | 13 | 12874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.06, 1.68] |
| 9 fatal bleeding | 8 | 10499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.62] |
| 10 all causes of death | 12 | 12603 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.22] |
| 11 other serious adverse effects | 11 | 12707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.20] |
6.1. Analysis.
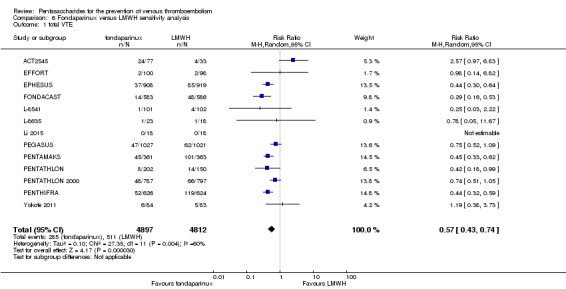
Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 1 total VTE.
6.2. Analysis.
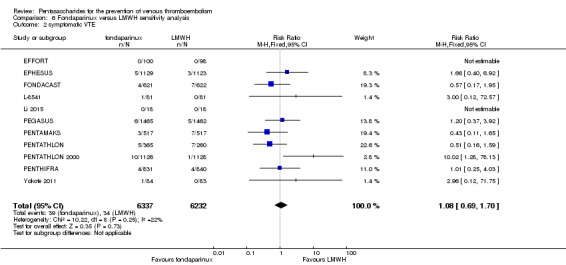
Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 2 symptomatic VTE.
6.3. Analysis.
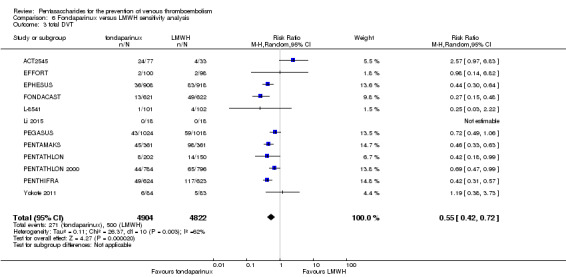
Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 3 total DVT.
6.4. Analysis.
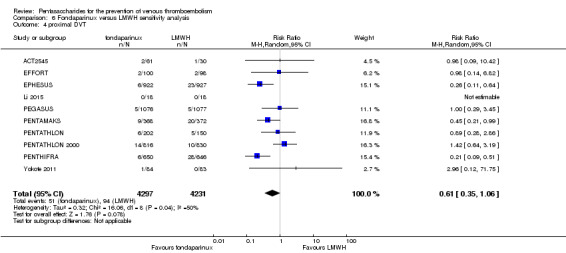
Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 4 proximal DVT.
6.5. Analysis.

Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 5 total PE.
6.6. Analysis.

Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 6 fatal PE.
6.7. Analysis.
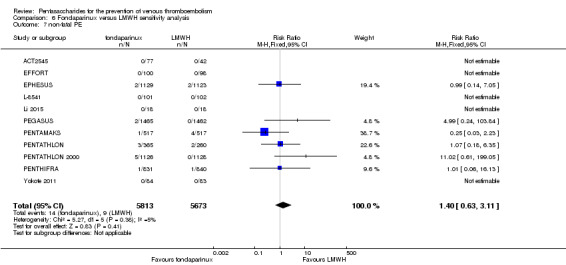
Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 7 non‐fatal PE.
6.8. Analysis.
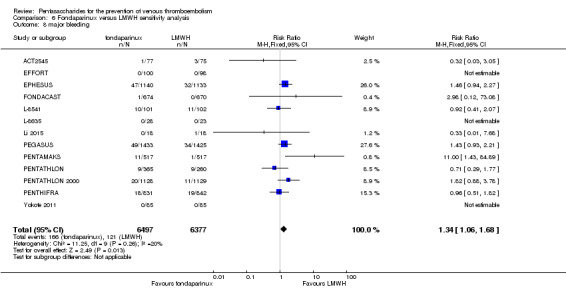
Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 8 major bleeding.
6.9. Analysis.
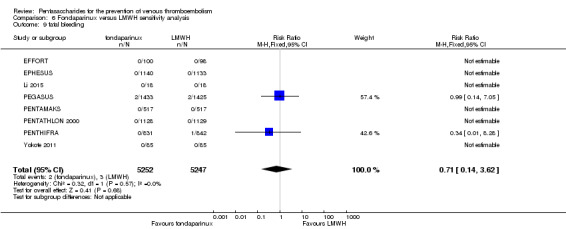
Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 9 fatal bleeding.
6.10. Analysis.
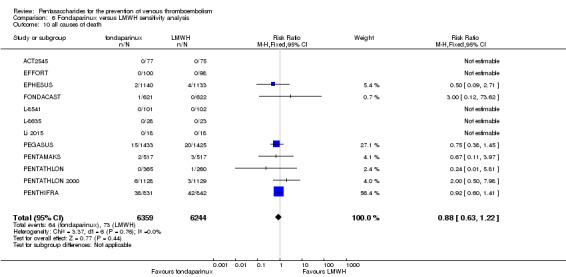
Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 10 all causes of death.
6.11. Analysis.
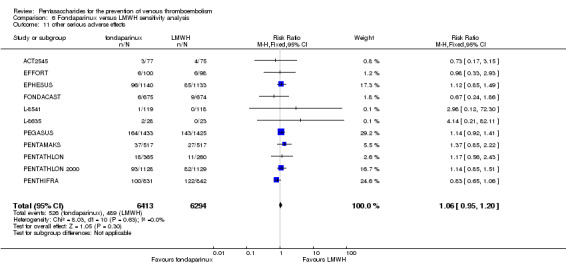
Comparison 6 Fondaparinux versus LMWH sensitivity analysis, Outcome 11 other serious adverse effects.
Comparison 7. Fondaparinux versus LMWH sensitivity analysis without EFFORT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 total VTE | 10 | 9141 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.73] |
| 2 symptomatic VTE | 8 | 12042 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.65, 1.63] |
| 3 total DVT | 9 | 9158 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.40, 0.71] |
| 4 proximal DVT | 8 | 8163 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.31, 1.03] |
| 5 total PE | 9 | 12152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.65, 2.34] |
| 6 fatal PE | 8 | 10909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.05] |
| 7 non‐fatal PE | 8 | 10909 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.63, 3.11] |
| 8 major bleeding | 10 | 12303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.09, 1.75] |
| 9 fatal bleeding | 5 | 10095 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.62] |
| 10 MI | 6 | 10720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.69, 2.37] |
| 11 all causes of death | 10 | 12202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.22] |
| 12 death associated with VTE or bleeding | 5 | 4774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.38, 2.07] |
| 13 other serious adverse effects | 9 | 12267 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.20] |
7.1. Analysis.
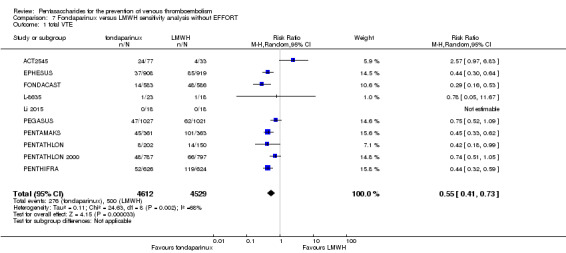
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 1 total VTE.
7.2. Analysis.
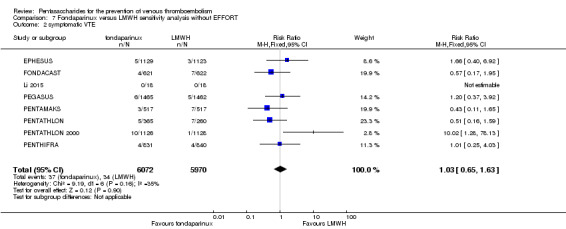
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 2 symptomatic VTE.
7.3. Analysis.
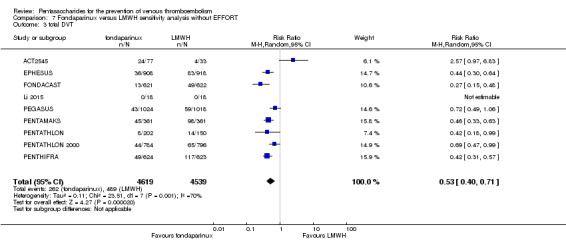
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 3 total DVT.
7.4. Analysis.
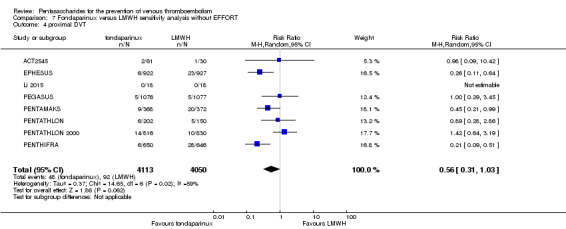
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 4 proximal DVT.
7.5. Analysis.
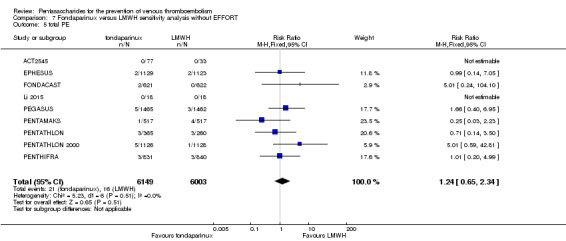
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 5 total PE.
7.6. Analysis.
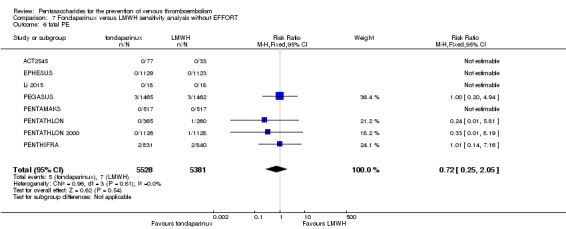
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 6 fatal PE.
7.7. Analysis.
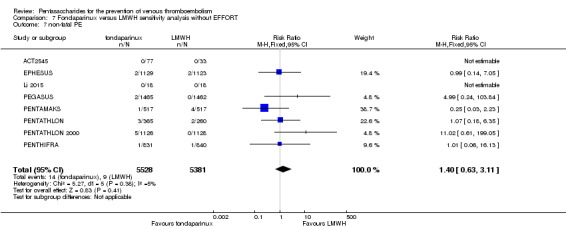
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 7 non‐fatal PE.
7.8. Analysis.
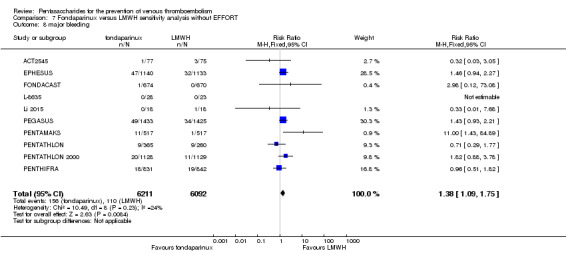
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 8 major bleeding.
7.9. Analysis.
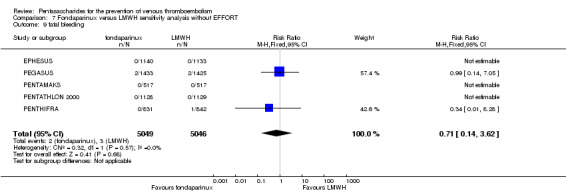
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 9 fatal bleeding.
7.10. Analysis.
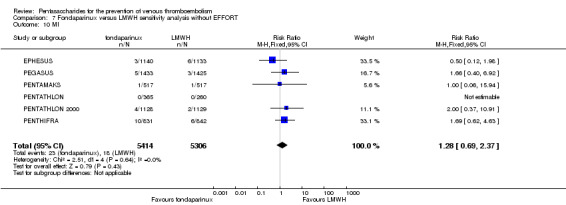
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 10 MI.
7.11. Analysis.
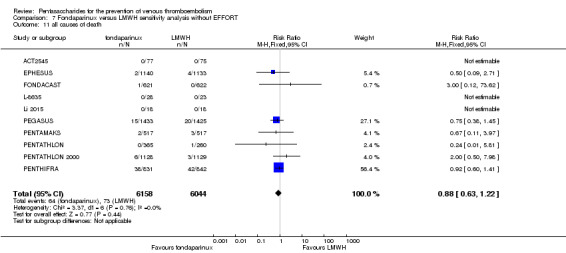
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 11 all causes of death.
7.12. Analysis.
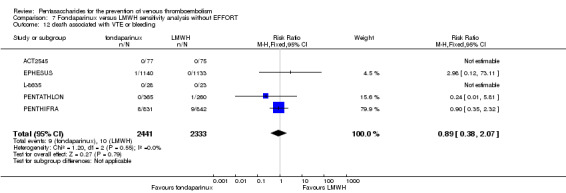
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 12 death associated with VTE or bleeding.
7.13. Analysis.
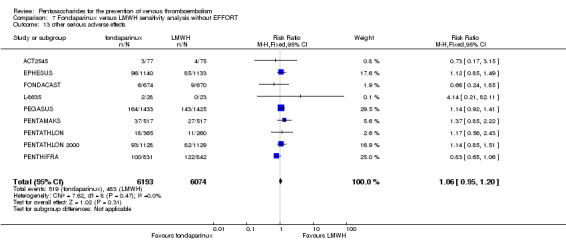
Comparison 7 Fondaparinux versus LMWH sensitivity analysis without EFFORT, Outcome 13 other serious adverse effects.
Comparison 8. Fondaparinux versus LMWH subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 total VTE | 11 | 9339 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.42, 0.73] |
| 1.1 orthopaedic patients | 8 | 7057 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.38, 0.70] |
| 1.2 abdominal surgery | 1 | 2048 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.52, 1.09] |
| 1.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.14, 6.82] |
| 2 symptomatic VTE | 9 | 12240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.65, 1.63] |
| 2.1 orthopaedic patients | 6 | 9079 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.61, 1.65] |
| 2.2 abdominal surgery | 1 | 2927 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.37, 3.92] |
| 2.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 total DVT | 10 | 9356 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.40, 0.71] |
| 3.1 orthopaedic patients | 7 | 7080 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.37, 0.69] |
| 3.2 abdominal surgery | 1 | 2042 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.49, 1.06] |
| 3.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.14, 6.82] |
| 4 proximal DVT | 9 | 8361 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.33, 1.02] |
| 4.1 orthopaedic patients | 6 | 5974 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.26, 1.02] |
| 4.2 abdominal surgery | 1 | 2153 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.29, 3.45] |
| 4.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.14, 6.82] |
| 5 total PE | 10 | 12350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.65, 2.34] |
| 5.1 orthopaedic patients | 7 | 9189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.56, 2.34] |
| 5.2 abdominal surgery | 1 | 2927 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.40, 6.95] |
| 5.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 fatal PE | 9 | 11107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.05] |
| 6.1 orthopaedic patients | 6 | 7946 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.14, 2.29] |
| 6.2 abdominal surgery | 1 | 2927 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.20, 4.94] |
| 6.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 non‐fatal PE | 9 | 11107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.63, 3.11] |
| 7.1 orthopaedic patients | 6 | 7946 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.53, 2.83] |
| 7.2 abdominal surgery | 1 | 2927 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.99 [0.24, 103.84] |
| 7.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 bariatric surgery | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 major bleeding | 11 | 12501 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.09, 1.75] |
| 8.1 orthopaedic patients | 8 | 9409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.03, 1.84] |
| 8.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.93, 2.21] |
| 8.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.68] |
| 8.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 fatal bleeding | 7 | 10329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.62] |
| 9.1 orthopaedic patients | 4 | 7237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.28] |
| 9.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 7.05] |
| 9.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 MI | 6 | 10720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.69, 2.37] |
| 10.1 orthopaedic patients | 5 | 7862 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.61, 2.39] |
| 10.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.40, 6.92] |
| 11 all causes of death | 11 | 12400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.22] |
| 11.1 orthopaedic patients | 8 | 9308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.64, 1.35] |
| 11.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.38, 1.45] |
| 11.3 ICU patients | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.4 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 other serious adverse effects | 10 | 12470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.19] |
| 12.1 orthopaedic patients | 8 | 9414 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.89, 1.19] |
| 12.2 abdominal surgery | 1 | 2858 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.92, 1.41] |
| 12.3 bariatric surgery patients | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.33, 2.93] |
8.1. Analysis.
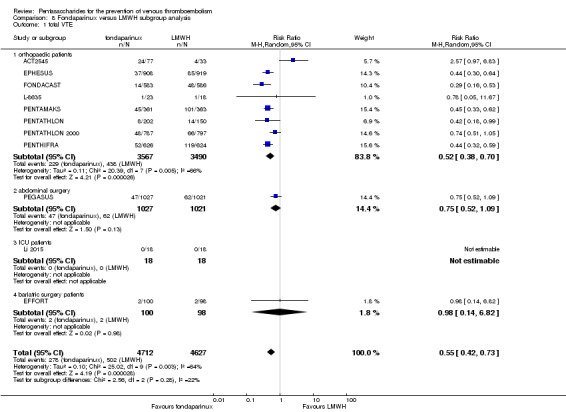
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 1 total VTE.
8.2. Analysis.
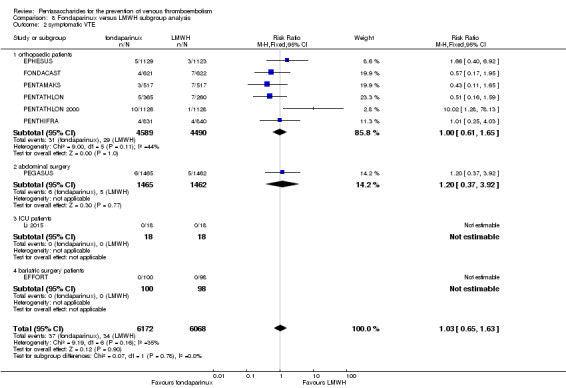
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 2 symptomatic VTE.
8.3. Analysis.
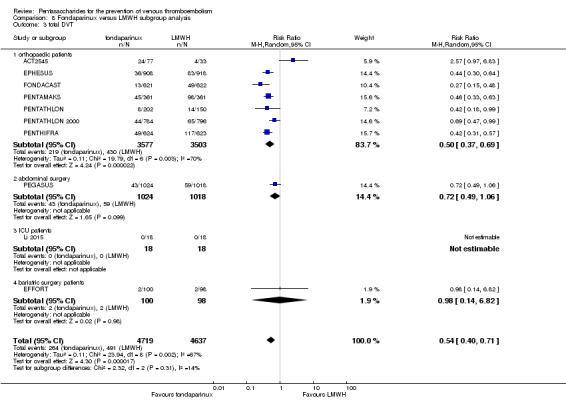
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 3 total DVT.
8.4. Analysis.
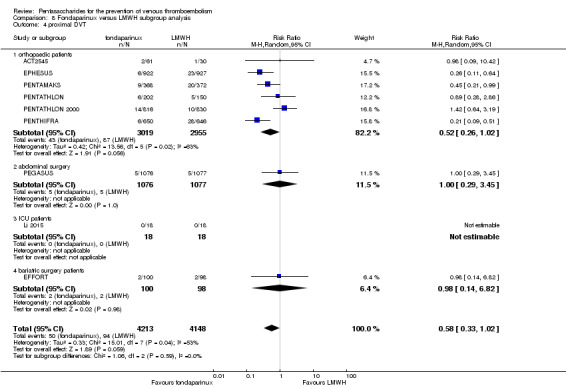
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 4 proximal DVT.
8.5. Analysis.
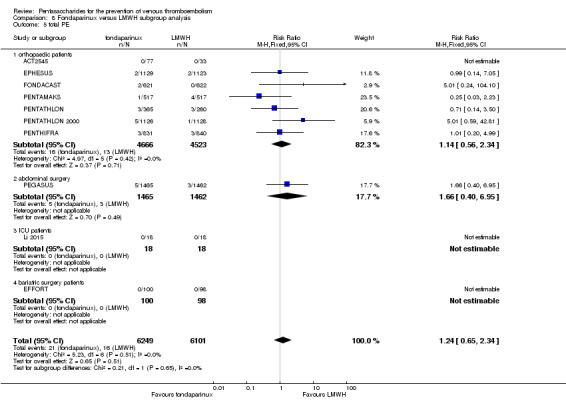
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 5 total PE.
8.6. Analysis.
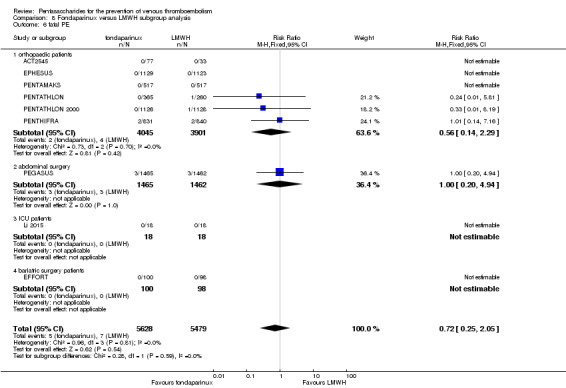
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 6 fatal PE.
8.7. Analysis.

Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 7 non‐fatal PE.
8.8. Analysis.
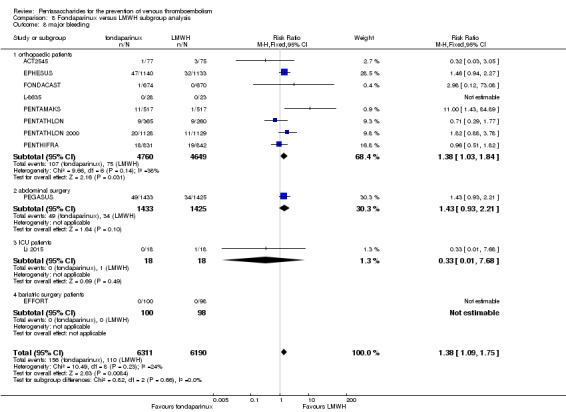
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 8 major bleeding.
8.9. Analysis.
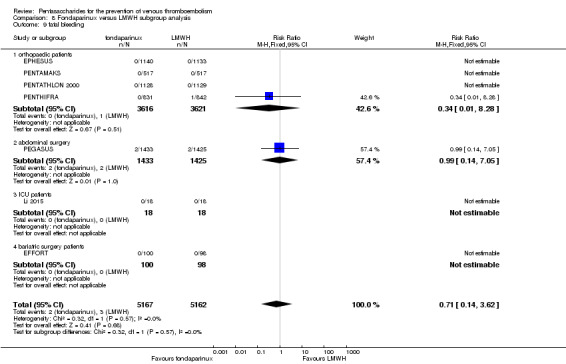
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 9 fatal bleeding.
8.10. Analysis.
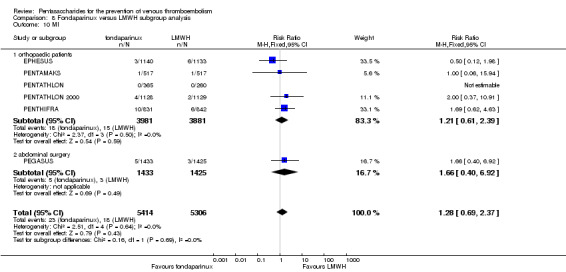
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 10 MI.
8.11. Analysis.
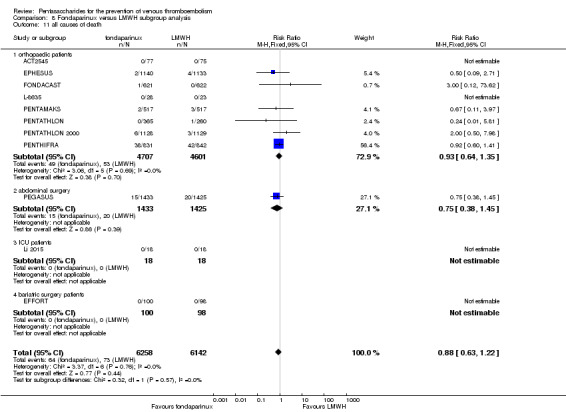
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 11 all causes of death.
8.12. Analysis.
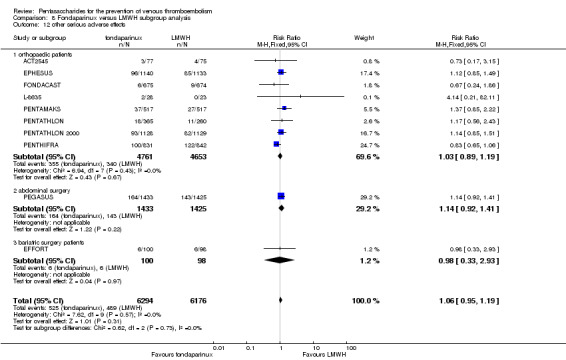
Comparison 8 Fondaparinux versus LMWH subgroup analysis, Outcome 12 other serious adverse effects.
Comparison 9. Fondaparinux versus variable dose warfarin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 total VTE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 symptomatic VTE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 total DVT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 proximal DVT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 total PE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 all causes of death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
9.1. Analysis.
Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 1 total VTE.
9.2. Analysis.
Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 2 symptomatic VTE.
9.3. Analysis.
Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 3 total DVT.
9.4. Analysis.
Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 4 proximal DVT.
9.5. Analysis.
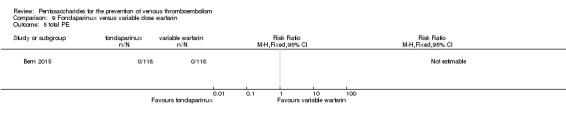
Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 5 total PE.
9.6. Analysis.
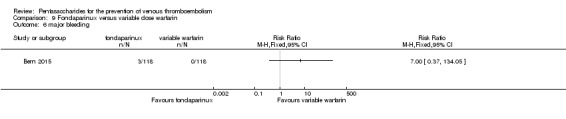
Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 6 major bleeding.
9.7. Analysis.
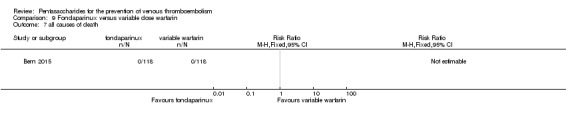
Comparison 9 Fondaparinux versus variable dose warfarin, Outcome 7 all causes of death.
Comparison 10. Fondaparinux versus 1 mg warfarin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 total VTE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 symptomatic VTE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 total DVT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 proximal DVT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 total PE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 all causes of death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
10.1. Analysis.
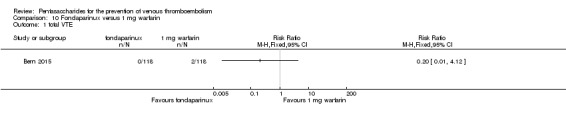
Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 1 total VTE.
10.2. Analysis.

Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 2 symptomatic VTE.
10.3. Analysis.
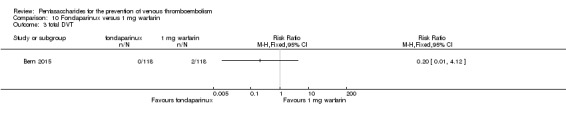
Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 3 total DVT.
10.4. Analysis.
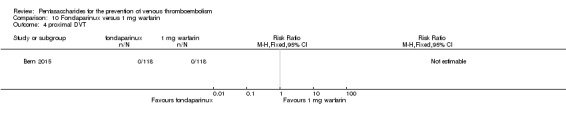
Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 4 proximal DVT.
10.5. Analysis.
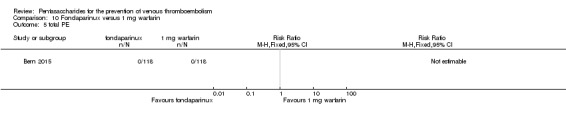
Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 5 total PE.
10.6. Analysis.
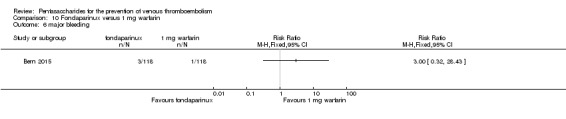
Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 6 major bleeding.
10.7. Analysis.
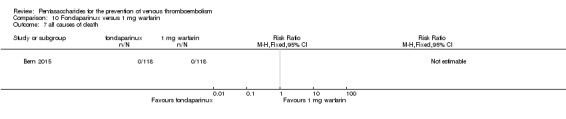
Comparison 10 Fondaparinux versus 1 mg warfarin, Outcome 7 all causes of death.
Comparison 11. Fondaparinux versus edoxaban.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 total VTE | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 major bleeding | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 all causes of death | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
11.1. Analysis.
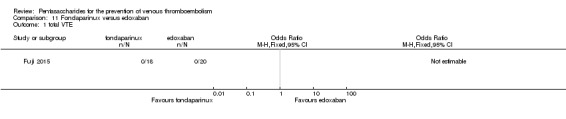
Comparison 11 Fondaparinux versus edoxaban, Outcome 1 total VTE.
11.2. Analysis.
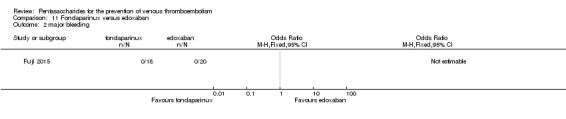
Comparison 11 Fondaparinux versus edoxaban, Outcome 2 major bleeding.
11.3. Analysis.
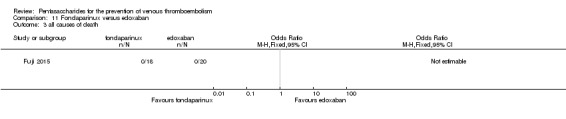
Comparison 11 Fondaparinux versus edoxaban, Outcome 3 all causes of death.
Comparison 12. Fondaparinux versus mechanical thromboprophylaxis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 total VTE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 symptomatic VTE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 total DVT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 proximal DVT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 total PE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 all causes of death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 other serious adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
12.1. Analysis.
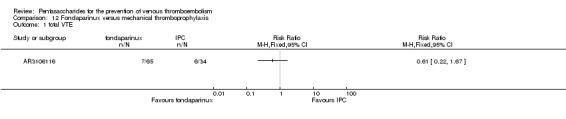
Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 1 total VTE.
12.2. Analysis.
Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 2 symptomatic VTE.
12.3. Analysis.

Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 3 total DVT.
12.4. Analysis.
Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 4 proximal DVT.
12.5. Analysis.
Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 5 total PE.
12.6. Analysis.
Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 6 major bleeding.
12.7. Analysis.
Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 7 all causes of death.
12.8. Analysis.
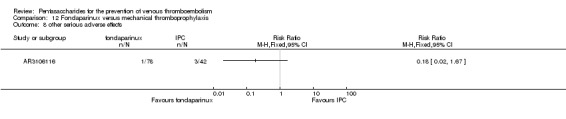
Comparison 12 Fondaparinux versus mechanical thromboprophylaxis, Outcome 8 other serious adverse effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACT2545.
| Methods | Multi‐centre, randomised, open‐label, dose‐finding study | |
| Participants |
Total number of participants: 243 Number of participants allocated to each group: fondaparinux (FX) 4 mg group: 86, FX 2 mg group: 78, enoxaparin (EN) 40 mg group: 79 Number of participants excluded and/or lost to follow‐up: FX 4 mg group: 4 (1 adverse event, 1 lack of efficacy, 2 other reasons), FX 2 mg group: 3 (1 adverse event, 0 lack of efficacy, 2 other reasons), EN 40 mg group: 3 (1 adverse event, 1 lack of efficacy, 1 other reasons) Inclusion: Men and postmenopausal women aged > 40 years with body weight 50 to 100 kg inclusive who were undergoing first single non‐revision total hip replacement (subsequently amended to non‐revision total hip replacement), with no contraindication to undergo phlebography on day 8 ± 1 Exclusion: Patients were excluded from study participation on the basis of their bleeding risk at the time of randomisation (e.g. known bleeding tendency, thrombocytes < 150 × 109/L, prothrombin time < 65%, APTT/control > 1.2 or other medical conditions associated with a bleeding risk), other significant conditions (e.g. history of PE or DVT, serum creatinine > 2.3 mg% (200 µmol/L), severe hepatic disease or uncontrolled severe high blood pressure (systolic blood pressure/diastolic blood pressure > 200/120 mmHg) or use of anticoagulant or fibrinolytic therapy within 1 week before randomisation. |
|
| Interventions |
FX: Phase I: 4 mg FX once daily. Phase II: 2 mg FX once daily; FX was administered for 7 days from day 2 (first injection planned 6 hours after surgery) to day 8. EN: Phase I: 40 mg EN once daily (first control group (CG). Phase II: 40 mg EN once daily (second CG). EN was administered for 8 days, from day 1 to day 8 (first injection planned 12 hours before surgery and first postoperative injection planned 6 hours after surgery). |
|
| Outcomes |
Primary efficacy outcome: incidence of any DVT; DVT was assessed on day 8 ± 1 by phlebography Primary safety outcome: major bleeding; major bleeding was defined as a clinically overt haemorrhage (except drain < 500 mL/d) in addition to 1 of the following criteria: haemoglobin (Hb) reduction to < 8 g/dL or Hb decrease > 2 g/dL over any 48‐hour period between day 3 and day 9 inclusive, or reoperation or intracranial bleeding or retroperitoneal or withdrawal |
|
| Notes | Use of adjunctive prophylaxis methods: No adjunctive method was used in this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multicentre, randomised, open‐label, dose finding study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multicentre, randomised, open‐label, dose finding study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mention that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Without clear description Comment: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A central evaluation was performed blindly by two independent experts" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 95.3%, 96.2%, 96.2% of participants in the 3 study groups finished treatment. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
APOLLO.
| Methods | Multi‐centre, randomised, double‐blind, parallel‐group trial | |
| Participants |
Total number of participants: 1309 Number of participants allocated to each group: fondaparinux (FX) group: 650, placebo group: 659 Number of participants excluded and/or lost to follow‐up: FX group: 57 (23 adverse events, 1 lack of efficacy, 33 other reasons), placebo group: 58 (17 adverse events, 2 lack of efficacy, 39 other reasons) Inclusion: Patients were eligible if they were undergoing abdominal surgery (defined as surgery between the diaphragm and the pelvic floor), lasting longer than 45 minutes (from anaesthesia induction to incision closure); over 40 years old Exclusion: Exclusion criteria were based on the labelling of LMWH in force at the time study was conducted (e.g. active clinically significant bleeding, presence or history of low platelet count (< 100 x 109/L), medical condition associated with a bleeding risk), criteria related to contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 μmol/L) or hypersensitivity to contrast media) or criteria related to trial methods (e.g. current or recent DVT, contraindication to heparin or oral anticoagulant, use of anticoagulant or fibrinolytic therapy during screening period). |
|
| Interventions |
FX: 2.5 mg FX sodium given sc starting 6 to 8 hours postoperatively, then once daily for 7 ± 2 days (day 1 was the day of surgery) or until the mandatory venography was obtained, whichever came first. Mandatory venography had to be performed between day 5 and day 10, but not more than 1 calendar day after the last study treatment administration. All participants were to receive IPC therapy concomitantly. Placebo: Placebo was given sc starting 6 to 8 hours postoperatively, then once daily for 7 ± 2 days (day 1 was the day of surgery) or until mandatory venography was performed, whichever came first. Mandatory venography had to be performed between day 5 and day 10, but not more than 1 calendar day after the last study treatment administration. All participants were to receive IPC therapy concomitantly. |
|
| Outcomes |
Primary efficacy outcome: cluster of 1 or more of the following VTE outcomes, evaluated (by an independent adjudicating committee) up to the first venography or up to day 10, whichever came first: venogram positive for DVT between day 5 and day 10, symptomatic DVT and/or non‐fatal PE, fatal PE Primary safety outcome: incidence of major bleeding (any investigator‐reported unusual bleeding) recorded during treatment period (between first injection of study drug and 2 calendar days after last injection) and adjudicated as a major bleeding event by the Central Adjudication Committee (CAC). Major bleeding was defined as: fatal bleeding, surgical bleeding leading to intervention; non‐surgical site bleeding: retroperitoneal or intracranial bleeding, or bleeding into a critical organ (eye, adrenal gland, pericardium, spine) or leading to intervention, and/or a bleeding index ≥ 2. |
|
| Notes | Use of adjunctive prophylaxis methods: Both groups received background mechanical prophylaxis with IPC. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multicenter, randomised, double‐blind, placebo‐controlled, parallel‐group study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multicenter, randomised, double‐blind, placebo‐controlled, parallel‐group study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Multicenter, randomised, double‐blind, placebo‐controlled, parallel‐group study……2.5 mg FX sodium (or FX placebo) given subcutaneously (s.c.)" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "evaluated (by an independent adjudicating committee)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% (9% and 8.9% of the 2 study groups) of participants withdrawn Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
AR3106116.
| Methods | Multi‐centre, randomised, open‐label study | |
| Participants |
Total number of participants: 127 Number of participants allocated to each group: fondaparinux (FX) group: 83, intermittent pneumatic compression (IPC) group: 44 Number of participants excluded and/or lost to follow‐up: FX group: 7 (2 adverse events, 0 lack of efficacy, 5 other reasons), IPC group: 1 (0 adverse events, 0 lack of efficacy, 1 other reasons) Inclusion: patients aged 40 years undergoing the following abdominal (area between diaphragm and pelvic floor) surgery under general anaesthesia lasting longer than 45 minutes: general or urological surgery, cancer surgery, gynaecological surgery, radical surgery for pelvic malignancy Exlcusion: Patients were excluded if any of the exclusion criteria based on contraindications and precautions for use of anticoagulants currently approved in Japan (e.g. active, clinically significant bleeding, bleeding tendency) or exclusion criteria related to venography (e.g. severe renal disorder, hypersensitivity to contrast media) were applied, or if any of the prohibited medications were used within 1 week before first study drug administration, or use of IPC was contraindicated or inappropriate. |
|
| Interventions |
FX: 2.5 mg FX was administered once daily by sc injection for 4 to 8 days. First injection of study drug was given 24 ± 2 hours after surgical closure. Second and subsequent injections of study drug were given at approximately the same time every day as far as possible (but longer than 12 hours after the first dose). IPC was prohibited during surgical and treatment periods. IPC: IPC was initiated before or after surgery and was continued until an appropriate time point. Procedures and methods usually employed at each individual study centre were followed as a rule. |
|
| Outcomes |
Primary efficacy outcome: rate of VTE (symptomatic PE and any DVT) during main efficacy period Primary safety outcomes: major bleeding Major bleeding defined as:
BI calculated as "number of units* transfused" within 48 hours of the bleed + prebleed Hb (g/dL) – postbleed Hb within 48 hours of the bleed (g/dL) * 450 mL of whole blood or red blood cells derived from 450 mL of whole blood is considered as 1 unit. |
|
| Notes | Use of adjunctive prophylaxis methods: No adjunctive prophylaxis method was used in this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A multicenter, randomised, open‐label study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "A multicenter, randomised, open‐label study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mention that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Without clear description Comment: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Rate of VTE (symptomatic PE and any DVT) during main efficacy period, adjudicated by Central Independent Adjudication Committee of Efficacy (CIACE)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 85.5% and 93.2% of randomised participants in the 2 study groups respectively finished their treatment. Comment: low risk of bias; most participants finished the study |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
ARTEMIS.
| Methods | Multi‐centre, multi‐national, randomised, double‐blind, placebo‐controlled study | |
| Participants |
Total number of participants: 849 Number of participants allocated to each group: fondaparinux group: 429; placebo group: 420 Number of participants excluded and/or lost to follow up: fondaparinux group: 4; placebo group: 6 Inclusion: Participants were acutely ill medical patients, aged ≥ 60 years and expected to require bed rest for at least 4 days at the moment of inclusion; hospitalised for congestive heart failure New York Heart Association (NYHA) class III/IV, and/or acute respiratory illness in the presence of chronic lung disease, and/or acute infectious or inflammatory disease Exclusion: Patients were excluded from study participation on the basis of their bleeding risk at the time of randomisation (e.g. active clinically significant bleeding or medical conditions associated with a bleeding risk) criteria related to contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 µmol/L) or hypersensitivity to contrast media) or use of anticoagulant or fibrinolytic therapy within 48 hours before randomisation. |
|
| Interventions |
Fondaparinux (FX): Administration of 2.5 mg FX (2.5 mg once daily as sc injection) started 2 hours after randomisation. Study treatment was to be given at least up to and including day 6 but not after day 14. Venography had to be performed within 1 day after cessation of treatment on days 6 to 15, or earlier in case of symptomatic VTE. Placebo: Placebo was started 2 hours after randomisation. Study treatment was to be given at least up to and including day 6 but not after day 14. Venography had to be performed within 1 day after cessation of treatment on days 6 to 15, or earlier in case of symptomatic VTE. |
|
| Outcomes |
Primary efficacy outcome: composite of the following VTE events recorded up to day 15 or up to the first venography, whichever came first: venogram positive for DVT, symptomatic DVT, non‐fatal PE or fatal PE. Venography and all other available diagnostic tests (ultrasonography, ventilation/perfusion lung scan, pulmonary angiography or spiral computed tomography scan, autopsy report, etc) were blindly adjudicated by experts of the Central Independent Adjudication Committee (CIAC). Primary safety outcome: major bleeding during treatment and 2 days thereafter, defined as fatal bleeding, bleeding in a critical location, bleeding leading to surgical intervention or overt bleeding associated with a drop in haemoglobin (Hb) concentration ≥ 20 g/L or leading to transfusion of 2 or more units of red blood cells Efficacy and safety outcomes were adjudicated by a central independent committee (CIAC), whose members were unaware of the treatment assignment. Accumulated safety data were regularly reviewed by an independent committee. |
|
| Notes | Use of adjunctive prophylaxis methods: Use of aspirin or non‐steroidal anti‐inflammatory drugs was discouraged. Graduated compression stockings and physiotherapy were allowed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was carried out using a predefined central randomisation list, balanced in blocks of four" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Multi‐centre, multi‐national, randomised, double‐blind, placebo‐controlled study Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study drugs were provided in identical boxes" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All venograms ……were blindly adjudicated by experts of the Central Independent Adjudication Committee (CIAC)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 89.4% and 88.7% of randomised participants in the 2 study groups, respectively, finished their treatment Comment: low risk of bias; most participants finished the study |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
Bern 2015.
| Methods | Prospective, randomised, 3‐arm study | |
| Participants |
Total number of participants: 355 Number of participants allocated to each group: variable‐dose warfarin group: 118, fondaparinux group: 118, 1 mg daily warfarin group: 119 Number of participants excluded and/or lost to follow‐up: 2 in variable‐dose warfarin group did not receive allocated intervention, 1 was not analysed; 10 in fondaparinux group did not receive allocated intervention, 6 were not analysed; 7 in 1 mg daily warfarin group did not receive allocated intervention, 6 were not analysed Inclusion criteria: Participants were recruited from among patients over 20 years of age planning elective primary unilateral total hip or knee replacement surgery at an orthopaedic specialty hospital. Exclusion criteria
|
|
| Interventions |
ARM A
Variable‐dose warfarin: 5.0 mg beginning the night before surgery, followed by 5.0 mg the night of surgery, then variable daily dose (target INR 2.0 to 2.5) until day 28 ± 2 of follow‐up
ARM B
Fondaparinux: 2.5 mg daily starting 6 or more hours after surgery, but no later than 6 AM the next day, or 6 to 8 hours after epidural catheter removal; continued until day 28 ± 2 of follow‐up
ARM C Fixed low‐dose warfarin 1.0 mg daily, beginning 7 days preoperatively, and continued at 1.0 mg daily until day 28 ± 2 of follow‐up |
|
| Outcomes | Primary endpoint was composite DVT, PE or death due to VTE. Secondary endpoints included frequency of proximal vs distal DVT, estimated blood loss (EBL) at surgery and haemorrhagic complications. | |
| Notes | Use of adjunctive anticoagulative methods: Patients had early postoperative ambulation. All patients wore pneumatic compression stockings while in‐patients. Elastic compression stockings were prescribed to be used after discharge until follow‐up ultrasonography. Hydroxyethyl starch (HES) 6% was allowed intraoperatively for case‐specific reasons. Use of platelet function suppressive drugs, such as non‐steroidal anti‐inflammatory drugs (NSAIDs), was discouraged but was not prohibited by the protocol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A member of the pharmacy department pulled randomized cards as prepared by the statisticians" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "A member of the pharmacy department pulled randomized cards as prepared by the statisticians" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Radiology technicians and the radiologists were blinded to patient randomization" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 99.1%, 94.9% and 94.9% of participants in 3 groups completed the study. Comment: probably done |
| Selective reporting (reporting bias) | Low risk | All endpoints listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Low risk | No risk of other bias identified |
CALISTO.
| Methods | Multi‐centre, randomised, double‐blind, placebo‐controlled, 2‐parallel‐group study | |
| Participants |
Total number of participants: 3002 Number of participants allocated to each group: fondaparinux (FX) group: 1502, placebo group: 1500 Number of participants excluded and/or lost to follow‐up: FX group: 21 (2 adverse events, 0 lack of efficacy, 19 other reasons), placebo group: 33 (1 adverse event, 0 lack of efficacy, 32 other reasons) Inclusion: Hospitalised and non‐hospitalised male and female patients 18 years of age or older with acute symptomatic isolated superficial venous thrombosis (SVT) of the lower limbs at least 5 cm long, documented by standard compression ultrasonography (CUS) were eligible to enter the study. Exclusion: Patients at high risk of VTE were excluded (e.g. those with DVT on the qualifying ultrasound exam and/or documented PE at inclusion, with SVT within 3 cm from the sapheno‐femoral junction (SFJ) requiring ligation of the SFJ or thrombectomy, with active cancer, or with documented DVT or PE within the previous 6 months). |
|
| Interventions |
FX: 2.5 mg FX sc once daily (self‐administered or not self‐administered). Treatment was presented as prefilled (0.5 mL) syringes. Duration of treatment was 45 days with 30‐day follow‐up. Placebo: matching placebo administered sc once daily (self‐administered or not self‐administered). Treatment was presented as prefilled (0.5 mL) syringes. Duration of treatment was 45 days with 30‐day follow‐up. |
|
| Outcomes |
Primary efficacy outcome: incidence of VTE and/or death from any cause recorded up to day 47. VTE was defined as a composite of symptomatic DVT, symptomatic PE, symptomatic extension of SVT or symptomatic recurrence of SVT. All VTEs were confirmed by objective tests and were then adjudicated by an independent central adjudication committee (CAC), whose members were blinded to treatment assignment. Primary safety outcome: major bleeding |
|
| Notes | Use of adjunctive methods: Participants were encouraged to use graduated compression stockings and were allowed to take acetaminophen or topical non‐steroidal anti‐inflammatory drugs as needed. Use of oral antiplatelet agents or aspirin at a low dose (≤ 325 mg per day) was discouraged. In total, 1131 participants in FX group used graduated stockings, and 347 participants used antiplatelet agents of all 1502 participants; 1147 participants in placebo group used graduated stockings, and 364 used antiplatelet agents of all 1500 participants, as adjunctive anticoagulative methods | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "With the use of a central telephone system and a computer‐generated randomisation list" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "With the use of a central telephone system and a computer‐generated randomisation list" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "to fondaparinux at a dose of 2.5 mg or matching placebo" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "adjudicated by an independent central adjudication committee (CAC)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1481 (98.6%) and 1467 (97.8%) participants in the 2 study groups finished the study. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
Cho 2013.
| Methods | Double‐blind, prospective, randomised, controlled trial | |
| Participants |
Total number of participants: 148 Number of participants allocated to each group: fondaparinux group: 74, placebo group: 74 Number of participants excluded and/or lost to follow‐up: FX group: 0, placebo group: 0 Inclusion: All adult patients with a diagnosis of primary osteoarthritis of the knee and undergoing elective unilateral primary TKA were considered for inclusion in the study. Exclusion: patients undergoing bilateral knee replacements; patients with diagnosed chronic or acute DVT preoperatively; patients with active bleeding or documented congenital or acquired bleeding disorders such as haemophilia, current ulcerative or angiodysplastic gastrointestinal disease; hemorrhagic stroke or brain, spinal or ophthalmologic surgery within the previous 3 months. Additional exclusion criteria were contraindication to anticoagulant therapy, serum creatinine concentration > 2 mg/dL in a well‐hydrated patient and platelet count < 100,000 per cubic millimetre. |
|
| Interventions |
FX: subcutaneous doses of 2.5 mg of fondaparinux (Arixtra; GlaxoSmith‐Kline, UK) once daily Placebo: 0.25 mL of isotonic saline once daily The first postoperative injection was administered 6 to 8 hours after surgery, and the second injection was given 24 hours after the first. The day of surgery was defined as day 1. Treatment was scheduled to continue with a daily single dose until day 5. |
|
| Outcomes |
Primary efficacy outcome: prevalence of DVT ‐ total, proximal and distal ‐ and symptomatic PE up to day 7 Primary safety outcome: incidence of major bleeding. Major bleeding included clinically overt bleeding requiring transfusion of ≥ 2 units of blood products (considering 450 mL of reinfused shed blood as 1 U), bleeding with a serious or life‐threatening clinical event or requiring surgical intervention, bleeding in retroperitoneal, intracranial or intraocular locations or bleeding resulting in death. |
|
| Notes | Use of adjunctive anticoagulative methods: Graduated compression stockings were applied in all participants. All were managed by the same rehabilitation protocol, which included range of motion, quadriceps, hamstring and calf pump exercises and straight leg raising on postoperative day 1, and bedside continuous passive mobilization on day 2. Participants were made to stand on day 1 and were allowed partial weight bearing on the operated leg from day 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes: "a double‐blind, prospective randomised controlled trial" and "Eligible patients were randomly assigned prior to the surgery through a computer‐derived randomisation table with block sizes of four to receive……" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation schedule was known to the research pharmacist who prepared the study medication but was not involved in any way with the care of the patients. The patients, surgeon, health care providers, and outcome assessors were blinded to the randomisation till the end of the study" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients, surgeon, health care providers, and outcome assessors were blinded to the randomisation till the end of the study" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The radiologist was blinded to the treatment assignment" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants in the 2 groups finished treatment, and primary efficacy and safety results were assessed. |
| Selective reporting (reporting bias) | Low risk | All results planned in the study design section were assessed. |
| Other bias | Low risk | No risk of other bias was identified. |
DRI4090.
| Methods | Multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group, dose‐response study | |
| Participants |
Total number of participants: 411 Number of participants allocated to each group: fondaparinux (FX) 0.75 mg group: 82, FX 1.5 mg group: 82, FX 2.5 mg group: 82, FX 3.0 mg group: 83, placebo group: 82 Number of participants excluded and/or lost to follow‐up: FX 0.75 mg group: 8 (6 adverse events, 0 lack of efficacy, 2 other reasons), FX 1.5 mg group: 6 (5 adverse events, 0 lack of efficacy, 1 other reasons), FX 2.5 mg group: 3 (3 adverse events, 0 lack of efficacy, 0 other reasons), FX 3.0 mg group: 5 (5 adverse events, 0 lack of efficacy, 0 other reasons), placebo group: 3 (2 adverse event, 0 lack of efficacy, 1 other reasons) Inclusion: Patients undergoing primary elective THR surgery or revision of a THR; ≥ 20 years of age Exclusion: Exclusion criteria were based on the Japanese labelling for anticoagulants in force at the time study was conducted (e.g. active, clinically significant bleeding; documented congenital or acquired bleeding tendency/disorders, other medical condition associated with a bleeding risk), or criteria related to use of contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 μmol/L) or hypersensitivity to contrast media) or use of anticoagulant or fibrinolytic therapy within 1 week before first dose of study medication. |
|
| Interventions |
FX: once daily sc dosing of FX 0.75, 1.5, 2.5 or 3.0 mg for at least 10 calendar days (maximum 14 days) from day 2 to day 11 or 15. The first dose of study drug was administered 24 ± 2 hours after surgical closure (day 1 was the day of surgery). Mandatory venography had to be performed between day 11 and day 17 but not later than 2 calendar days after the last study drug administration Placebo: once daily sc placebo for at least 10 calendar days (maximum 14 days) from day 2 to day 11 or 15. First dose of study drug was administered 24 ± 2 hours after surgical closure (day 1 was the day of surgery). Mandatory venography had to be performed between day 11 and day 17 but not later than 2 calendar days after the last study drug administration. |
|
| Outcomes |
Primary efficacy outcome: cluster of the following VTE outcomes recorded up to day 17 or to first venography, whichever occurred first: adjudicated mandatory venogram positive for DVT between day 11 and day 17; adjudicated symptomatic DVT; adjudicated positive fatal or non‐fatal PE. All venography sessions, scheduled or unscheduled, and other available diagnostic tests (ultrasonography, ventilation/perfusion lung scan, pulmonary angiography or spiral computed tomography scan, autopsy report, etc.) were adjudicated blindly by independent experts of the Central Independent Adjudication Committee of Efficacy (CIACE). Primary safety outcome: incidence of major bleeding (any investigator‐reported bleeding adjudicated as a major bleeding event by the Central Independent Adjudication Committee of Safety (CIACS)) recorded during \treatment period (i.e. from first injection of study drug to 2 days after last dose). Major bleeding was defined as fatal bleeding, or clinically overt bleeding including retroperitoneal or intracranial bleeding or bleeding into a critical organ (eye, adrenal gland, pericardium, spine); reoperation due to bleeding/hematoma at the operative site; clinically overt bleeding leading to Hb fall > 2 g/dL (1.6 mmol/L) within 48 hours of the bleed; clinically overt bleeding that required a transfusion of red blood cell or whole blood derived from > 900 mL of whole blood within 48 hours of the bleed (excluding autologous transfusion except for treatment of bleeding adverse event (AE)); clinically overt bleeding leading to bleeding index > 2 (within 48 hours of the bleed, calculated as "number of units transfused" + prebleed Hb (g/dL) – postbleed Hb (g/dL) |
|
| Notes | Use of adjunctive anticoagulative methods: no mention of use of any adjunctive anticoagulative method | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multicentre, randomised, double‐blind, placebo controlled, parallel group, dose response study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multicentre, randomised, double‐blind, placebo controlled, parallel group, dose response study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Without clear description Comment: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "were adjudicated blindly by independent experts of the Central Independent Adjudication Committee of Efficacy (CIACE)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 56 of 71 randomised participants, 62 of 82 randomised participants, 63 of 82 randomised participants, 67 of 82 randomised participants, 68 of 83 randomised participants in the 5 study groups, respectively, finished their treatment and were involved in the efficacy analysis. Comment: low risk of bias; most participants finished the study |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
DRI4757.
| Methods | Multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group, dose‐response study | |
| Participants |
Total number of participants: 432 Number of participants allocated to each group: fondaparinux (FX) 0.75 mg group: 86, FX 1.5 mg group: 87, FX 2.5 mg group: 86, FX 3.0 mg group: 86, placebo group: 87 Number of participants excluded and/or lost to follow‐up: FX 0.75 mg group: 4 (4 adverse events, 0 lack of efficacy, 0 withdrawn consent, 0 other reasons), FX 1.5 mg group: 7 (3 adverse events, 0 lack of efficacy, 4 withdrawn consents, 0 other reasons), FX 2.5 mg group: 6 (2 adverse events, 0 lack of efficacy, 4 withdrawn consents, 0 other reasons), FX 3.0 mg group: 5 (4 adverse events, 0 lack of efficacy, 0 withdrawn consent, 1 other reasons), placebo group: 7 (4 adverse events, 0 lack of efficacy, 1 withdrawn consent, 2 other reasons) Inclusion: patients who were undergoing elective primary TKR surgery or revision surgery of a TKR; ≥ 20 years of age Exclusion: Exclusion criteria were based on the Japanese labelling for anticoagulants in force at the time study was conducted (e.g. active, clinically significant bleeding; documented congenital or acquired bleeding tendency/disorders or other medical conditions associated with a bleeding risk), criteria related to use of contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 μmol/L) or hypersensitivity to contrast media) or use of anticoagulant or fibrinolytic therapy within 1 week before first dose of study medication. |
|
| Interventions |
FX: once daily sc dosing of FX 0.75, 1.5, 2.5 or 3.0 mg for at least 10 calendar days (maximum 14 days) from day 2 to day 11 or 15. First dose of study drug was administered 24 ± 2 hours after surgical closure (day 1 was the day of surgery). Mandatory venography had to be performed between day 11 and day 17 but not later than 2 calendar days after the last study drug administration. Placebo: once daily sc placebo for at least 10 calendar days (maximum 14 days) from day 2 to day 11 or 15. First dose of study drug was administered 24 ± 2 hours after surgical closure (day 1 was the day of surgery). Mandatory venography had to be performed between day 11 and day 17 but no later than 2 calendar days after last study drug administration. |
|
| Outcomes |
Primary efficacy outcome: cluster of the following VTE outcomes recorded up to day 17 or to first venography, whichever occurred first: adjudicated mandatory venogram positive for DVT between day 11 and day 17; adjudicated symptomatic DVT; adjudicated positive fatal or non‐fatal PE. All venography procedures, scheduled or unscheduled, and other available diagnostic tests (ultrasonography, ventilation/perfusion lung scan, pulmonary angiography or spiral computed tomography scan, autopsy report, etc.) were adjudicated blindly by independent experts of the Central Independent Adjudication Committee of Efficacy (CIACE). Primary safety outcome: incidence of major bleeding (any investigator‐reported bleeding adjudicated as a major bleeding event by the Central Independent Adjudication Committee of Safety (CIACS)). This was recorded during treatment period (i.e. from first injection of study drug to 2 days after the last dose). Major bleeding was defined as fatal bleeding, clinically overt bleeding including retroperitoneal or intracranial bleeding or bleeding into a critical organ (eye, adrenal gland, pericardium, spine); reoperation due to bleeding/haematoma at the operative site; clinically overt bleeding leading to Hb fall > 2 g/dL (1.6 mmol/L) within 48 hours of the bleed; clinically overt bleeding that required a transfusion of red blood cell or whole blood derived from > 900 mL of whole blood within 48 hours of the bleed (excluding autologous transfusion, except for treatment of bleeding adverse event (AE)); clinically overt bleeding leading to bleeding index > 2 (within 48 hours of the bleed, calculated as "number of units* transfused" + prebleed Hb (g/dL) – postbleed Hb (g/dL)). Other safety variables were minor bleeding (defined as clinically overt bleeding not meeting the criteria for major bleeding and considered more than expected in the clinical context), transfusion requirements, AEs/serious AEs (SAEs) and deaths. *450 mL of whole blood or red blood cell derived from 450 mL of whole blood is considered as 1 unit. |
|
| Notes | Use of adjunctive anticoagulative methods: no mention of use of any adjunctive anticoagulative method | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multicentre, randomised, double‐blind, PBO controlled, parallel group, dose response study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multicentre, randomised, double‐blind, PBO controlled, parallel group, dose response study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Without clear description Comment: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A central, independent adjudication committee reviewed both safety and efficacy outcomes" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 74 of 85 randomised participants, 74 of 85 randomised participants, 74 of 85 randomised participants, 74 of 85 randomised participants and 74 of 85 randomised participants in the 5 study groups, respectively, finished treatment and were involved in the efficacy analysis. Comment: low risk of bias; most participants finished the study |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
EFFORT.
| Methods | Randomised, double‐blind, pilot trial | |
| Participants |
Total number of participants: 198 Number of participants allocated to each group: fondaparinux group: 100, enoxaparin group: 98 Number of participants excluded and/or lost to follow‐up: Of the 198 randomised participants, 7 in the fondaparinux group and 7 in the enoxaparin group did not receive treatment according to protocol, but all randomised participants were analysed. Inclusion: Patients were eligible for the study if they were 18 years of age or older with a body mass index (BMI) of 35 to 59 kg/m2, and were undergoing laparoscopic vertical sleeve gastrectomy (VSG) or laparoscopic Roux‐en Y gastric bypass (LRYGB) Exclusion: Patients with BMI > 60 were excluded, as they may have required extended DVT prophylaxis. Patients with contraindications to low molecular weight heparin or selective antithrombin III agonists, previous history of DVT or PE, documented clotting/coagulation disorders, history of treatment for cancer within the past year, history of venous stasis or superficial thrombophlebitis, vein stripping or ligation, obesity hypoventilation syndrome or recent history of smoking (within the past year) were excluded. Patients with severe hepatic impairment, creatinine clearance < 30 mL per minute or platelet count < 100,000 per cubic millimetre were also excluded, as were women of childbearing age if they were pregnant or were taking oestrogen‐based birth control medication within 1 month of surgery |
|
| Interventions |
FX: The fondaparinux group received a placebo on call to the operating room. Six hours after surgery stop time, participants were given 5 mg fondaparinux subcutaneously. Beginning on postoperative day 1, participants received 5 mg of fondaparinux subcutaneously once daily in the morning and placebo (saline) injections subcutaneously once daily in the evening for the duration of their hospital stay. Enoxaparin: In accordance with current practice, the enoxaparin group received a dose of enoxaparin 40 mg subcutaneously on call to the operating room. To maintain blinding, participants randomised to enoxaparin received placebo (saline) injection 6 hours after surgery stop time. Beginning on postoperative day 1, 40 mg of enoxaparin was administered subcutaneously twice daily for the duration of the participant's hospital stay. |
|
| Outcomes | Primary outcome was the effect of preoperative enoxaparin vs postoperative fondaparinux prophylaxis on antifactor Xa concentrations in participants undergoing bariatric surgery. Attainment of a target antifactor Xa level was determined on the basis of blood samples drawn 3 hours after the drug was received on postoperative day 1. This cutoff was the standard for adequate prophylaxis used by our in‐patient haematology lab (Z 0.20 IU/mL for enoxaparin and Z 0.39 mg/L for fondaparinux). Secondary outcomes were asymptomatic DVT, defined as a positive MRV within 2 weeks after surgery, and symptomatic DVT. Safety outcomes included perioperative bleeding, perioperative complications and death. |
|
| Notes | Use of adjunctive anticoagulative methods: All participants had sequential compression devices and antiembolic stockings placed before induction of anaesthesia; 4 to 6 hours after surgery stop time, participants were ambulated in the hallways. Sequential compression devices were removed during ambulation. Use of aspirin, non‐steroidal anti‐inflammatory drugs and other antiplatelet agents was prohibited during participants' hospital stay. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a computer‐generated randomization scheme (Microsoft Excel 2007 data analysis tool pack)" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was performed by the pharmacy and was concealed from patients and study personnel" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "placebo doses were prepared to maintain the blind. Active and placebo syringes were prepared by our inpatient pharmacy and were not identifiable by external appearance" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "asymptomatic DVT, defined as a positive MRV within 2 weeks following surgery, and symptomatic DVT" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 83 of 98 in the enoxaparin group, 94 of 100 in the fondaparinux group had MRV results. Comment: low risk of bias; most participants finished the study |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company provided study material and additional financial support but was not involved in the design and procedure of the study. |
EPHESUS.
| Methods | Multi‐national, multi‐centre, randomised, double‐blind, double‐dummy, parallel‐group study | |
| Participants |
Total number of participants: 2309 Number of participants allocated to each group: fondaparinux (FX) group: 1155, enoxaparin (EN) group: 1154 Number of participants excluded and/or lost to follow‐up: FX group: 70 (18 adverse events, 7 lack of efficacy, 45 other reasons), EN group: 58 (15 adverse events, 5 lack of efficacy, 38 other reasons) Inclusion: Patients were eligible if they were undergoing an elective, primary THR surgery or a revision of at least 1 component of a THR; ≥ 18 years of age; men and women of non‐childbearing potential or of childbearing potential and having a negative pregnancy test within 48 hours before surgery or first study drug administration, whichever came first; written informed consent Exclusion: Exclusion criteria were based on the labelling of LMWH in force at the time of study conduct (e.g. active clinically significant bleeding, presence or history of low platelet count (< 100 x 109/L), medical condition associated with a bleeding risk), criteria related to contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 µmol/L) or hypersensitivity to contrast media) or use of anticoagulant or fibrinolytic therapy within 2 days before first dose of study medication. |
|
| Interventions |
FX: administration of FX (2.5 mg once daily sc injection) started postoperatively at 6 ± 2 hours after surgery closure EN: administration of EN (40 mg once daily as sc injection) started preoperatively at 12 ± 2 hours before the start of surgery, then postoperatively at least 12 hours after the preoperative dose but not longer than 24 hours after surgery Placebo: Respective placebo to each drug was administered to protect the double‐blind (double‐dummy method). Study treatment was given up to day 7 ± 2 (day 1 was the day of surgery) or until mandatory venography was performed, whichever came first. Mandatory venography had to be performed between day 5 and day 11, but not more than 2 calendar days after last study treatment administration. |
|
| Outcomes |
Primary efficacy outcome: cluster of the following VTE outcome results recorded up to day 11: adjudicated venogram positive for DVT or adjudicated symptomatic/asymptomatic DVT; adjudicated PE. All venography procedures, scheduled or unscheduled, and other available diagnostic tests (ultrasonography, ventilation/perfusion lung scan, pulmonary angiography or spiral computed tomography scan, autopsy report, etc) were adjudicated blindly by independent experts of the Central Independent Adjudication Committee (CIAC). Primary safety endpoint: incidence of major bleeding (any investigator‐reported unusual bleeding adjudicated as a major bleeding event by the CIAC) recorded between first injection of study drug (active drug or placebo) and day 11. Major bleeding was defined as fatal bleeding; clinically overt bleeding including retroperitoneal or intracranial bleeding, or bleeding into a critical organ (eye, spine, pericardium, adrenal gland); reoperation due to bleeding/haematoma at the operative site; clinically overt bleeding leading to a fall in Hb ≥ 2 g/dL (1.6 mmol/L) and/or transfusion of ≥ 2 units of packed red blood cells or whole blood AND for which the combined calculated index was ≥ 2. Other safety variables were minor bleeding (defined as clinically overt bleeding not meeting the criteria for major bleeding and considered more than expected in the clinical context), transfusion requirements, adverse events (AEs)/serious AEs (SAEs), deaths and changes in laboratory parameters recorded between first injection of study drug and day 11. In addition, all safety parameters were recorded between first injection and day 49. |
|
| Notes | Use of adjunctive anticoagulative methods: In FX group, 29 participants received prohibited treatment (intermittent pneumatic compression, dextran, thrombolytic treatment and any other anticoagulant agents), 483 received discouraged treatment (aspirin or non‐steroidal anti‐inflammatory drugs) and 649 wore graduated compression stockings; in EN group, 30 participants received prohibited treatment (intermittent pneumatic compression, dextran, thrombolytic treatment and any other anticoagulant agents), 493 received discouraged treatment (aspirin or non‐steroidal anti‐inflammatory drugs) and 654 wore graduated compression stockings. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multinational, multicenter, randomised, double‐blind, double‐dummy, parallel‐group study" Comment: probably done, as earlier reports from the same company clearly described use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multinational, multicenter, randomised, double‐blind, double‐dummy, parallel‐group study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Respective placebo to each drug was administered to protect the double‐blind (double‐dummy method)" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "were adjudicated blindly by independent experts of the Central Independent Adjudication Committee (CIAC)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 93.9% and 94.9% of participants in the 2 study groups, respectively, finished treatment. Comment: low risk of bias; most participants finished the study |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
FONDACAST.
| Methods | Multi‐centre, randomised, open‐label, controlled, 2‐parallel‐group, Phase III study | |
| Participants |
Total number of participants: 1243 Number of participants allocated to each group: fondaparinux (FX) group: 621, nadroparin (NA) group: 622 Number of participants excluded and/or lost to follow‐up: FX group: 14 (3 adverse events, 4 withdrawals by participant, 4 lost to follow‐up, 0 immobilisation, 1 investigator/orthopaedic surgeon decision, 2 orthopaedic surgery, 0 visits not performed, 0 deep vein thrombosis), NA group: 8 (0 due to adverse events, 0 due to lack of efficacy, 8 due to other reasons) Inclusion: requiring rigid or semirigid immobilisation (e.g. with a plaster cast or brace) for at least 21 days and up to 45 days because of isolated non‐surgical below‐knee injury, with a no weight‐bearing recommendation at the time of inclusion (partial weight bearing is permitted, e.g. crutches, walking cast, relief shoes); presenting at least 1 of the following risk factors for venous thromboembolism: below‐knee fracture or Achilles tendon rupture, age ≥ 40 years, body mass index > 30 kg/m2, oestrogen‐containing hormonal replacement therapy or oral contraception, active cancer (treatment ongoing or stopped for less than 1 year), history of VTE, congenital or acquired hypercoagulable stat; requiring thromboprophylaxis according to the investigator's judgement up to complete mobilisation (corresponding to cast or brace removal; able and willing to provide written informed consent) Exclusion: delay between injury and randomisation greater than 2 days; treatment with antithrombotic or anticoagulant therapy, including low‐dose anticoagulation, for longer than 2 days before randomisation; anticoagulant therapy required or likely to be required during the study period for another reason (e.g. planned surgery justifying pharmacological thromboprophylaxis, curative dose for treatment of VTE); shown hypersensitivity to fondaparinux or nadroparin or their excipient; known history of heparin‐induced thrombocytopenia; women of childbearing potential not using a reliable contraceptive method throughout the study period; women pregnant or breast‐feeding during the study period; active, clinically significant bleeding; clinically significant bleeding within past 6 months; major surgery within previous 3 months; intraocular (other than cataract), spinal and/or brain surgery within previous 12 months; haemorrhagic stroke within previous 12 months; severe head injury within previous 3 months; documented congenital or acquired bleeding tendency/disorder(s); previous (within 12 months) or active or currently treated peptic ulcer disease; uncontrolled arterial hypertension (systolic blood pressure over 180 mmHg or diastolic blood pressure over 110 mm Hg); treatment with more than 1 antiplatelet agent (e.g. clopidogrel and aspirin) at any dose; need for long‐term aspirin at doses ≥ 325 mg or long‐term NSAIDs; bacterial endocarditis; severe hepatic impairment; calculated creatinine clearance < 30 mL/min; thrombocytopenia (< 100 x 109/L); body weight < 50 kg; any condition that could prevent the patient from providing written informed consent or from adhering to study treatment; life expectancy < 6 months; participation in any study using an investigational drug during previous 3 months; patient in whom V3 is unlikely to be feasible (e.g. patient moving house); In France, a patient was not be eligible for inclusion in this study if not affiliated with or a beneficiary of a social security system. This is an additional exclusion criterion that applies only to individuals enrolled in France. |
|
| Interventions |
FX: 2.5 mg in 0.5 mL or 1.5 mg in 0.3 mL for at least 21 days and up to 45 days NA: 2850 anti‐Xa IU in 0.3 mL administered sc once daily. Treatments were presented as prefilled syringes for at least 21 days and up to 45 days |
|
| Outcomes |
Primary efficacy outcome: composite of VTE and death up to complete mobilisation, corresponding to cast or brace removal (plus 2 days). VTE was defined in this study as asymptomatic DVT detected by systematic compression ultrasonography, symptomatic DVT or symptomatic fatal or non‐fatal PE. Primary safety outcome: major bleeding, non‐major clinically relevant bleeding and minor bleeding up to complete mobilisation (V3) plus 4 days, and up to the final visit or contact |
|
| Notes | Use of adjunctive anticoagulative methods: No adjunctive anticoagulative method was used in this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "multicenter, randomised, open‐label, controlled, two‐parallel‐group, phase III study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "multicenter, randomised, open‐label, controlled, two‐parallel‐group, phase III study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Multi‐centre, randomised, open‐label, controlled, 2‐parallel‐group, phase III study Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All events were blindly adjudicated by an independent committee" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 97.7% and 98.7% of participants in the 2 study groups, respectively, finished treatment. Comment: low risk of bias; most participants finished the study |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
Fuji 2015.
| Methods | Randomised, open‐label, controlled study | |
| Participants |
Total number of participants: 43 Number of participants allocated to each group: fondaparinux (FX) group: 21, edoxaban group: 22 Number of participants excluded and/or lost to follow‐up: FX group: 3 participants discontinued, edoxaban group: 2 participants discontinued Inclusion: patients ≥ 20 years of age, with serious renal injury (creatinine clearance ≥ 20 mL/min to < 30 mL/min) who were undergoing unilateral TKA or THA (excluding revision surgeries) or hip fracture surgery for medial or lateral femoral neck fracture (trochanteric or subtrochanteric section of the femur) within 10 days of presurgical examination. Informed consent was obtained from all participants. Exclusion: Presurgical exclusion criteria included, but were not limited to, patients undergoing or possibly undergoing haemodialysis; risk of bleeding; risk of thromboembolism; and hepatic dysfunction. Postsurgical exclusion criteria included, but were not limited to, creatinine clearance < 15 mL/min; abnormal bleeding at the site of spinal anaesthesia; abnormal or excessive bleeding during or immediately after surgery; and inability to take oral medication. |
|
| Interventions |
Fondaparinux: subcutaneous fondaparinux 1.5 mg sc once daily Edoxaban: oral edoxaban 15 mg once daily |
|
| Outcomes |
Primary efficacy outcome: incidence of symptomatic VTE (composite of symptomatic DVT or PE) during treatment period Primary safety outcome: major bleeding defined as fatal bleeding; clinically overt bleeding accompanied by a decrease in haemoglobin > 2 g/dL or requiring a transfusion of > 4 units of blood (1 unit = ˜200 mL); retroperitoneal, intracranial, intraocular or intrathecal bleeding; or bleeding requiring repeat surgery |
|
| Notes | Use of adjunctive anticoagulative methods: Concomitant physiotherapy (intermittent pneumatic compression devices or elastic stockings) was permitted throughout the treatment period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, controlled study applied permuted block method with SAS software to generate random sequence. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Randomised, controlled study applied permuted block method with SAS software to generate random sequence. Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label study Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All thromboembolic events were assessed by a thromboembolic event assessor, who was blinded to treatment group, on the basis of imaging results. Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18/21 and 20/22 participants in the fondaparinux and edoxaban groups, respectively finished their treatment. Comment: probably done |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | This study was supported by a pharmaceutical company that was involved in the study design and analysis of the data and provided writing and editorial support. |
Kolluri 2016.
| Methods | Randomised, placebo‐controlled, double‐blind study | |
| Participants |
Total number of participants: 78 Number of participants allocated to each group: fondaparinux group: 41, placebo group: 37 Number of participants excluded and/or lost to follow‐up: 2 participants in the fondaparinux group and no participants in the placebo group withdrew; all randomised participants were analysed. Inclusion criteria: All patients scheduled to undergo a first or a repeat isolated CABG operation were considered for enrolment in the study. Exclusion criteria
|
|
| Interventions |
Fondaparinux (FX): Intervention group received 2.5 mg subcutaneous injections of fondaparinux sodium daily, starting at a mean of 12 ± 2 hours after wound closure or on the morning of the first postoperative day. Second dose was administered at a mean of 24 ± 2 hours after the first dose, and subsequent injections were administered once daily for 9 days or until the patient was discharged from the hospital, whichever happened first. Placebo: Control group received similar amounts of subcutaneous isotonic saline on the same schedule as the intervention group. |
|
| Outcomes |
Primary study endpoint: composite, up to day 11, of cumulative incidence of all VTE events, defined as symptomatic and asymptomatic DVT, and fatal and non‐fatal pulmonary embolisms Primary safety endpoint: cumulative incidence of major haemorrhages |
|
| Notes | Use of adjunctive anticoagulative methods: Both groups routinely received graduated compression stockings and/or intermittent pneumatic compression (mechanical antithrombotic prophylaxis). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This study was conducted in compliance with the Good Clinical Practice guidelines" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "This study was conducted in compliance with the Good Clinical Practice guidelines" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The control group received similar amounts of subcutaneous isotonic saline on the same schedule as the interventional group" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Patients who developed symptomatic DVT or VTE underwent DUS scan of the lower extremities. An independent Data and Safety Monitoring Board monitored the safety of the study." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients in the fondaparinux group, who withdrew their consent at 3 and 8 days
post enrollment, respectively, did not undergo DUS and were removed from the study. Clinical follow‐ups were complete in 76 patients (97.4%)" Comment: probably done |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Low risk | No other bias noted |
L‐8541.
| Methods | Multi‐centre, randomised, single‐blind, parallel‐group control study | |
| Participants |
Total number of participants: 237 Number of participants allocated to each group: fondaparinux (FX) group: 119, enoxaparin (EN) group: 118 Number of participants excluded and/or lost to follow‐up: FX group: 6 (5 adverse events, 0 lack of efficacy, 1 other reasons), EN group: 3 (2 adverse events, 0 lack of efficacy, 1 other reasons) Inclusion: Male/female patients (aged 18 to 75 years) who were to undergo an elective hip or knee replacement or revision, and who gave written informed consent, were included in the study. Exclusion: Exclusion criteria included the following: history of serious active bleeding in past 3 months; concurrent or history of thrombocytopenia (platelet count < 100 x 109/L); concurrent haemorrhagic cerebrovascular disease or surgical history in brain, spine or eye; abnormality in hepatic (> 1.5 x ULN), renal (CrCl < 30 mL/min) or cardiac function, uncontrolled hypertension or tumour; and concurrent need for hip and knee replacement or double hip or knee replacement. |
|
| Interventions |
FX: 2.5 mg for 7 ± 2 days (once daily sc injection) EN: 40 mg for 7 ± 2 days First treatment injection (placebo or enoxaparin) was administered at 12 ± 2 hours before surgery, then was continued for 7 ± 2 days post surgery, via daily sc injection. |
|
| Outcomes |
Primary efficacy outcome: overall DVT events as confirmed by colour ultrasound imaging conducted within 2 days after the last dose following orthopaedic surgery Primary safety outcome: major bleeding recorded between day 1 and day 9 post surgery |
|
| Notes |
Use of adjunctive anticoagulative methods: no mention of use of any adjunctive anticoagulative method Used for sensitivity analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multi‐centre, randomised, single‐blind, parallel control study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multi‐centre, randomised, single‐ blind, parallel control study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Multi‐centre, randomised, single‐blind, parallel control study" Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly described Comment: unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 94.96% and 97.46% of participants in both groups completed the study. Comment: probably done |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
L‐8635.
| Methods | Randomised, open label, evaluator‐blinded | |
| Participants |
Total number of participants: 51 Number of participants allocated to each group: fondaparinux (FX) group: 28, enoxaparin (EN) group: 23 Number of participants excluded and/or lost to follow‐up: FX group: 4 (0 adverse events, 0 lack of efficacy, 4 other reasons), EN group: 1 (1 adverse event, 0 lack of efficacy, 0 other reasons) Inclusion: Patients ≥ 20 years of age scheduled for primary elective total knee replacement surgery were included in the study. Exclusion: Patients were excluded if they had leg oedema, peripheral vascular disease, diabetes with peripheral neuropathy or any condition likely to increase the risk of bleeding. |
|
| Interventions | FX: 2.5 mg FX 2.5 sc once daily for 7 days. First postoperative dose was given ≥ 6 hours after closure of the surgical wound, and the second dose 18 to 24 hours after the first dose. Thereafter, daily at 8 PM ± 2 hours for 5 days. EN: 40 mg EN sc once daily for 7 days. First dose was given 12 hours before surgery, and thereafter daily for 7 days. | |
| Outcomes |
Primary efficacy outcome: incidence of occurrence of VTE events (DVT, as determined by clinical assessment and compression Doppler) up to day 10 Primary safety outcome: Major bleeding, minor bleeding, no bleeding, adverse events (AEs) and serious adverse events (SAEs) were monitored from day 0 up to day 37. |
|
| Notes | Use of adjunctive anticoagulative methods: no mention of use of any adjunctive anticoagulative method | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized, open label" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multicentre, randomised, open‐label study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "open‐label" study Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "evaluator‐blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 85.7% and 95.7% of participants in the 2 study groups completed medication. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
Li 2015.
| Methods | Randomised controlled study | |
| Participants |
Total number of participants: 36 Number of participants allocated to each group: fondaparinux group: 18, low molecular weight heparin (LMWH) group: 18 Number of participants excluded and/or lost to follow‐up: 0 Inclusion criteria: confirmed infection in trauma patients; hypercoagulopathy: prothrombin time (PT) < 3 seconds or longer than normal, abnormal international normalised ratio (INR) or activated partial prothrombin time (APTT) < 10 seconds or longer than normal; > 18 and < 70 years old; without haematological disorders; an informed consent Exclusion criteria: anticoagulation therapy before enrolment; haematological or bleeding disorders; active or recent‐stroked peptic ulcer; malignant disease; diluting coagulopathy and low platelets counts; hepatic and renal failure |
|
| Interventions |
Fondaparinux (FX): Participants in group F were given fondaparinux sodium (2.5 mg, 1/d for 11 d). Low molecular weight heparin (LMWH): Participants in group L were given the standard LMWH (4100 U, 1/12 hours for 11 days) recipe and served as controls. |
|
| Outcomes | Endpoints of clinical observation were discharge and death. All participants were followed up for 3 months. Clinical parameters included deep vein thrombosis (DVT), bleeding events, occurrence of multiple organ dysfunction syndrome (MODS) and mortality. Laboratory parameters included serum fibrinogen, D‐dimer and antithrombin III. Observations were made on days 1, 3, 5, 7 and 11 after admission. Major or minor bleeding events were monitored and recorded. Major bleeding events were defined as lethal or life‐threatening bleeding and intracranial or abdominal bleeding; minor ones consisted of occasional small bleeding that did not require further treatment. | |
| Notes | Use of adjunctive prophylaxis methods: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators used the randomisation sequence list to generate the random sequence. This information was obtained by contacting the study authors. Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Investigators used the randomisation sequence list to generate the random sequence. This information was obtained by contacting the study authors. Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinded to participants but not to healthcare staff This information was obtained by contacting the study authors. Comment: may affect participants' treatment and outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to outcome evaluator This information was obtained by contacting the study authors. Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% of enrolled participants received responsive treatment, and data were analysed. |
| Selective reporting (reporting bias) | Low risk | All endpoints listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Low risk | No risk of other bias was identified. |
PEGASUS.
| Methods | Multi‐centre, multi‐national, randomised, double‐blind study | |
| Participants |
Total number of participants: 2927 Number of participants allocated to each group: fondaparinux (FX) group: 1465, dalteparin (DA) group: 1462 Number of participants excluded and/or lost to follow‐up: FX group: 127 (62 adverse events, 9 lack of efficacy, 56 other reasons), DA group: 119 (57 adverse events, 2 lack of efficacy, 60 other reasons) Inclusion: Patients undergoing abdominal surgery under general anaesthesia, planned to last longer than 45 minutes (from incision to incision closure), and > 60 years of age with or without any other risk factor for VTE, or > 40 years of age and at risk for thromboembolic complications, were eligible. Patients at risk included those who were obese (body mass index (BMI) > 30 kg/m2 for men and 28.6 kg/m2 for women), undergoing cancer surgery, with a history of DVT or PE, with congestive heart failure (CHF) (grade III or IV of the New York Heart Association (NYHA) classification), with chronic obstructive pulmonary disease or with inflammatory bowel disease Exclusion: Exclusion criteria were based on the labelling of LMWH in force at the time study was conducted (e.g. active clinically significant bleeding, presence or history of low platelet count (< 100 x 109/L), medical condition associated with a bleeding risk, hypersensitivity to heparin or LMWH), or criteria related to contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 μmol/L) or hypersensitivity to contrast media) or criteria related to trial methods (e.g. use of anticoagulant or fibrinolytic therapy within 2 days before first drug administration). |
|
| Interventions | FX: 2.5 mg once daily given by sc injection up to day 7 ± 2, with the first injection between 6 and 7 hours after incision closure, provided haemostasis had been established DA: 2500 IU sc 2 hours preoperatively and 12 hours after the preoperative injection (and at least 6 hours after incision closure), then DA 5000 IU (once daily sc) up to day 7 ± 2 | |
| Outcomes | Primary efficacy outcome: VTE (asymptomatic and/or symptomatic DVT or PE or both) recorded until the time of screening venography or day 10, whichever occurred first. Secondary efficacy outcomes included individual events of total DVT, proximal DVT, distal DVT, symptomatic VTE up to day 10 and symptomatic VTE up to day 30 ± 2. Venography was considered positive if an intraluminal filling defect was seen on 2 different views, or after repeated injection of contrast medium; thrombi in the popliteal vein or above were considered proximal. A venogram was considered adequate if the entire deep venous system was visualised from the calf veins to the common iliac vein in both legs. Primary safety outcome: major bleeding detected between first injection of study drug (dalteparin or placebo) and 2 calendar days after last injection. Major bleeding was defined as fatal bleeding; bleeding that was retroperitoneal, intracranial or intraspinal or involved any other critical organ; bleeding leading to reoperation or intervention; and a bleeding index ≥ 2.0. The bleeding index was derived by adding the number of transfused units of packed red blood cells or whole blood to the difference in Hb level measured in grams per decilitre before and after a bleeding event. Secondary safety outcomes were death, other reported bleeding, thrombocytopenia and any other adverse events. | |
| Notes |
Use of adjunctive anticoagulative methods: The use of graded‐pressure elastic stockings was permitted. Eleven (0.4 %) of 2858 participants were given a diagnosis of CHF at baseline. Results were not stratified by baseline illness, but owing to the small numbers, we decided to include this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A multicenter, multinational, randomised, double‐blind study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "A multicenter, multinational, randomised, double‐blind study" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients given fondaparinux received a placebo injection 2 h before surgery ……received a placebo injection 6 h after surgery to correspond with the fondaparinux injection schedule" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"VTE outcomes evaluated (by an independent adjudicating committee)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 91.1% and 91.6% of patients in 2 study groups finished treatment. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
PENTAMAKS.
| Methods | Multi‐national, multi‐centre, randomised, double‐blind, parallel‐group study | |
| Participants |
Total number of participants: 1049 Number of participants allocated to each group: fondaparinux (FX) group: 526, enoxaparin (EN) group: 523 Number of participants excluded and/or lost to follow‐up: FX group: 36 (20 adverse events, 2 lack of efficacy, 7 withdrawn consent, 7 other reasons ), EN group: 36 (13 adverse events, 6 lack of efficacy, 11 withdrawn consent, 6 other reasons). Inclusion: Study population had to conform to the following criteria: men or women (of non‐childbearing potential, i.e. postmenopausal or with hysterectomy or bilateral tubal ligation), or women of childbearing potential using highly effective birth control and having a negative pregnancy test within 48 hours before randomisation; aged ≥ 18 years; undergoing elective major knee surgery or revision of at least 1 component (enrolment of participants with surgery limited to an osteotomy was not permitted); and haemostasis established on the calendar day of surgery, no later than 8 hours after closure of the incision. Exclusion: Exclusion criteria were based on the labelling of LMWH in force at the time study was conducted (e.g. active clinically significant bleeding, presence or history of low platelet count (< 100 x 109/L), medical condition associated with a bleeding risk), or criteria related to contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 µmol/L) or hypersensitivity to contrast media). |
|
| Interventions |
FX: Administration of FX (2.5 mg once daily as sc injection) started 6 ± 2 hours after surgical closure on day 1 (day of surgery). EN: EN (30 mg twice daily as sc injection) at least 12 hours but less than 24 hours after surgical closure To protect blinding (double‐dummy method), all participants received placebo to the active treatment they were not receiving. Study treatment was given up to 7 ± 2 days after surgical closure, or until the final venogram (positive unscheduled or mandatory) was obtained, whichever came first. Mandatory venography had to be performed between day 5 and day 11, but not more than 2 calendar days after the last study treatment administration. |
|
| Outcomes |
Primary efficacy outcome: cluster of the following VTE outcomes recorded up to day 11: adjudicated venogram positive for DVT or adjudicated symptomatic or asymptomatic DVT; adjudicated non‐fatal PE or fatal PE. All venography procedures, scheduled and unscheduled, were adjudicated by a blinded Central Independent Adjudication Committee (CIAC). Primary safety outcome: incidence of major bleeding, which included fatal bleeding; bleeding that was retroperitoneal, intracranial or intraspinal or that involved any other critical organ; bleeding leading to reoperation; and overt bleeding with a bleeding index ≥ 2. The bleeding index was calculated as the number of units of packed red cells or whole blood transfused plus Hb values before the bleeding episode minus Hb values after the episode (in grams per decilitre). Secondary safety outcomes were death, other bleeding, need for transfusion, thrombocytopenia and any other adverse event. Efficacy and safety outcomes were adjudicated by a central independent committee, whose members were unaware of treatment assignments, and included reviews of all venograms and reports of bleeding and death. |
|
| Notes | Use of adjunctive anticoagulative methods: In the FX group, 4 participants received prohibited treatment (anticoagulant or antiplatelet agents other than aspirin or thrombolytic therapy), 44 received discouraged treatment (non‐steroidal anti‐inflammatory agents or aspirin) and 298 wore graduated compression stockings; in the EN group, 11 participants received prohibited treatment (anticoagulant or antiplatelet agents other than aspirin or thrombolytic therapy), 60 received discouraged treatment (non‐steroidal anti‐inflammatory agents or aspirin) and 294 wore graduated compression stockings. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Immediately after surgery, patients were randomly assigned (in a ratio of 1:1 in blocks of four, stratified according to centre), through a central computer‐derived randomisation scheme" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Immediately after surgery, patients were randomly assigned (in a ratio of 1:1 in blocks of four, stratified according to centre), through a central computer‐derived randomisation scheme" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To protect the blind (double‐dummy method) all subjects received placebo (PBO) to the active treatment they were not receiving" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "were adjudicated by a blinded Central Independent Adjudication Committee (CIAC)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 93% participants completed the study. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
PENTATHLON.
| Methods | Multi‐centre, randomised, parallel, dose‐ranging study of FX with an assessor‐blind, comparative control group of EN | |
| Participants |
Total number of participants: 950 Number of participants allocated to each group: fondaparinux (FX) 0.75 mg group: 185, FX 1.5 mg group: 190, FX 3.0 mg group: 181, FX 6.0 mg group: 73, FX 8.0 mg group: 52; enoxaparin (EN) 60 mg group: 269 Number of participants excluded and/or lost to follow‐up: FX 0.75 mg group: 14 (8 adverse events, 0 lack of efficacy, 6 other reasons), FX 1.5 mg group: 15 (8 adverse events, 0 lack of efficacy, 7 other reasons), FX 3.0 mg group: 13 (7 adverse events, 0 lack of efficacy, 6 other reasons), FX 6.0 mg group: 12 (10 adverse events, 0 lack of efficacy, 2 other reasons), FX 8.0 mg group: 13 (9 adverse events, 0 lack of efficacy, 4 for other reasons); EN group: 27 (18 adverse events, 1 lack of efficacy, 8 other reasons). Inclusion: males and females of non‐childbearing potential ≥ 18 years of age undergoing elective primary hip replacement or revision of a primary procedure Exclusion: Exclusion criteria were based on the labelling of LMWH in force at the time study was conducted (such as active clinically significant bleeding, presence or history of low platelet count (< 100 x 109/L) or known bleeding disorder), criteria related to venogram (such as creatinine clearance > 1.6 mg/dL; or hypersensitivity to contrast media) or use of anticoagulant or thrombolytic therapy 1 week before the start of the study. |
|
| Interventions |
FX: Participants received a once daily sc injection of FX 0.75, 1.5, 3.0, 6.0 or 8.0 mg, starting 6 ± 2 hours postoperatively on day 1 (day of surgery). EN: twice daily sc injection of EN 30 mg that started within 12 to 24 hours postoperatively (day 1 or day 2) Participants were treated for a minimum of 5 days from day 1 until the final venogram was obtained, up to a maximum of 10 days. |
|
| Outcomes |
Primary efficacy outcome: incidence of participants with adjudicated mandatory venogram positive for DVT and/or symptomatic adjudicated PE. Independent experts of the Central Independent Adjudication Committee (CIAC) evaluated blindly all venograms and lung scans performed during the study. Primary safety outcome: major bleeding; bleeding was defined as major if it was clinically overt and fatal, intracranial or retroperitoneal; involved a critical organ; or led to reoperation for bleeding or hematoma at the operative site. Overt bleeding was also defined as major if Hb levels declined by more than 2 grams per decilitre, if more than 2 units of packed red cells or whole blood was transfused or if the number of units transfused plus the decline in Hb level in grams per decilitre was greater than 2. |
|
| Notes | Use of adjunctive anticoagulative methods: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multicentre, randomised, parallel, dose ranging study of FX with an assessor‐blind, comparative control group of EN" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Quote: "Multicentre, randomised, parallel, dose ranging study of FX with an assessor‐blind, comparative control group of EN" Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Without clear description Comment: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Independent experts of the Central Independent Adjudication Committee (CIAC) evaluated blindly all venograms and lung scans performed during the study" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 92.4%, 92%, 92.7%, 83.3%, 75% and 89.6% of participants in the 6 different treatment groups finished their treatment. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
PENTATHLON 2000.
| Methods | Multi‐centre, randomised, double‐blind study | |
| Participants |
Total number of participants: 2275 Number of participants allocated to each group: fondaparinux (FX) group: 1138, enoxaparin (EN) group: 1137 Number of participants excluded and/or lost to follow‐up: FX group: 61 (33 adverse events, 4 lack of efficacy, 19 withdrawn consent, 5 other reasons ), EN group: 66 (35 adverse events, 2 lack of efficacy, 15 withdrawn consent, 14 other reasons) Inclusion: Patients were eligible if they were undergoing an elective, primary, THR surgery or a revision of at least 1 component of a THR; > 18 years of age; men and women of non‐childbearing potential or women of childbearing potential using effective birth control with a negative pregnancy test within 48 hours before randomisation; haemostasis established on the calendar day of surgery, no later than 8 hours after incision closure; written informed consent Exclusion: Exclusion criteria were based on the labelling of LMWH in force at the time study was conducted (e.g. active clinically significant bleeding, presence or history of low platelet count (< 100 x109/L), medical condition associated with a bleeding risk) or criteria related to contrast dyes during venography (e.g. serum creatinine > 2 mg/dL (180 µmol/L) or hypersensitivity to contrast media). |
|
| Interventions |
FX: administration of FX (2.5 mg once daily as sc injection) started postoperatively at 6 ± 2 hours after surgical closure EN: administration of EN (30 mg twice daily as sc injection) started postoperatively at least 12 hours but no more than 24 hours post surgical closure Respective placebo to each drug was administered to protect the double‐blind (double‐dummy) method. Study treatment was given up to day 7 ± 2 (day 1 was the day of surgery) or until the final venogram (positive unscheduled or mandatory) was obtained, whichever came first. Mandatory venography had to be performed between day 5 and day 11, but not more than 2 calendar days after the last study treatment administration. |
|
| Outcomes |
Primary efficacy outcome: cluster of the following VTE outcome results recorded up to day 11: adjudicated venogram positive for DVT or adjudicated symptomatic/asymptomatic DVT; and adjudicated PE. All venograms, scheduled and unscheduled, and other diagnostic tests (ultrasonography, ventilation/perfusion, lung scan, pulmonary angiography, spiral computed tomography scan, autopsy report, etc.) were adjudicated blindly by independent experts of the Central Independent Adjudication Committee (CIAC). Primary safety outcome: incidence of major bleeding (any Investigator‐reported unusual bleeding adjudicated as major or minor bleeding by the CIAC) recorded between first injection of study drug (active drug or placebo) and day 11. Major bleeding was defined as fatal bleeding; clinically overt bleeding including retroperitoneal or intracranial bleeding, or bleeding into a critical organ (eye, adrenal gland, pericardium, spine); reoperation due to bleeding/haematoma at the operative site; and clinically overt bleeding leading to a fall in Hb > 2 g/dL (1.6 mmol/L) and/or transfusion ≥ 2 units of packed red blood cells or whole blood AND for which the combined calculated index was > 2. |
|
| Notes | Use of adjunctive anticoagulative methods: use of graduated compression stockings and physiotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a central computer‐derived randomisation scheme" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "a central computer‐derived randomisation scheme" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Respective placebo to each drug was administered to protect the double‐blind (double‐dummy method)" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "were adjudicated blindly by independent experts of the Central Independent Adjudication Committee (CIAC)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 94.6% and 94.2% of participants in the 2 study groups finished their relative treatments. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
PENTHIFRA.
| Methods | Multi‐national, multi‐centre, randomised, double‐blind, double‐dummy, parallel‐group study | |
| Participants |
Total number of participants: 1711 Number of participants allocated to each group: fondaparinux (FX) group: 849, enoxaparin (EN) group: 862 Number of participants excluded and/or lost to follow‐up: FX group: 55 (29 adverse events, 1 lack of efficacy, 25 other reasons), EN group: 67 (32 adverse events, 2 lack of efficacy, 33 other reasons) Inclusion: patients undergoing standard surgery for fracture of the upper third of the femur, including femoral head and neck, not more than 48 hours after admission; ≥ 18 years of age; men and women of non‐childbearing potential or women with a negative pregnancy test Exclusion: Exclusion criteria were based on the labelling of LMWH in force at the time study was conducted (such as active clinically significant bleeding, presence or history of low platelet count (< 100 x 109/L) or known bleeding disorder), criteria related to venogram (such as creatinine clearance > 2.0 mg/dL; or hypersensitivity to contrast media) or use of anticoagulant or thrombolytic therapy during the screening period. |
|
| Interventions |
FX: administration of FX (2.5 mg once daily as sc injection) started postoperatively (6 ± 2 hours after end of surgery) EN: EN (40 mg once daily as sc injection) preoperatively (12 ± 2 hours before surgery) when surgery was planned within 24 hours after hospital admission If surgery was delayed to 24 to 48 hours after admission, both study treatments were administered 12 ± 2 hours before the start of surgery. Study treatment was given up to day 7 ± 2 (day 1 was the day of surgery) or until the mandatory venogram was obtained, whichever came first. Mandatory venography had to be performed between days 5 and 11, but not more than 2 calendar days after the last study treatment administration. |
|
| Outcomes |
Primary efficacy outcome: cluster of the following VTE outcome results recorded up to day 11: adjudicated venogram positive for DVT or adjudicated symptomatic/asymptomatic DVT; adjudicated non‐fatal and fatal PE. All venograms, scheduled and unscheduled, and other available diagnostic tests (ultrasonography, ventilation/perfusion lung scan, pulmonary angiography, spiral computed tomography scan, autopsy report, etc) were adjudicated blindly by independent experts of the CIAC. Primary safety outcome: incidence of major bleeding, which included fatal bleeding; bleeding that was retroperitoneal, intracranial or intraspinal or that involved any other critical organ; bleeding leading to reoperation; and overt bleeding with a bleeding index ≥ 2. The bleeding index was calculated as the number of units of packed red cells or whole blood transfused plus Hb values before the bleeding episode minus Hb values after the episode (in g/dL). |
|
| Notes | Use of adjunctive anticoagulative methods: Use of graduated compression stockings and physiotherapy was recommended. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned to treatment groups in blocks of four, with stratification according to centre, with the use of a computer‐generated randomisation list" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Multi‐national, multi‐centre, randomised, double‐blind, double‐dummy, parallel‐group study Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study medications were packaged in boxes of identical appearance" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "were adjudicated blindly by independent experts of the CIAC" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 93.4% and 92% of participants in the 2 study groups finished the study. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
PENTHIFRA PLUS.
| Methods | Multi‐national, multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants |
Total number of participants: 656 Number of participants allocated to each group: fondaparinux (FX) group: 326, placebo group: 330 Number of participants excluded and/or lost to follow‐up: FX group: 40 (20 adverse events, 2 lack of efficacy, 18 other reasons), placebo group: 46 (14 adverse events, 12 lack of efficacy, 20 for other reasons) Inclusion: patients undergoing standard surgery for fracture of the upper third of the femur, including femoral head and neck, not more than 48 hours after admission; ≥ 18 years of age; men and women of non‐childbearing potential or women with a negative pregnancy test Exclusion: Exclusion criteria included active clinically significant bleeding, or known bleeding disorder, criteria related to venography (such as creatinine clearance > 2.0 mg/dL; or hypersensitivity to contrast media) or use of anticoagulant or thrombolytic therapy during the screening period. |
|
| Interventions | Before randomisation, participants received open‐label fondaparinux 2.5 mg once daily, sc postoperatively for 7 ± 1 days (day 1 = day of surgery). They then were randomised to receive double‐blind fondaparinux 2.5 mg once daily sc for 21 ± 2 days or placebo. | |
| Outcomes |
Primary efficacy outcome: cluster of the following adjudicated VTE outcomes, evaluated from day 1 (first day of double‐blind treatment) to day 24 of postrandomisation period: mandatory venogram positive for DVT; symptomatic DVTs and/or adjudicated non‐fatal and fatal PE. During the study, the investigator had to perform appropriate evaluations in case of clinical suspicion of VTE. In the absence of previous confirmation of VTE, a mandatory venogram had to be performed between day 19 and day 24, but not more than 1 calendar day after the last study treatment administration. A Central Independent Adjudication Committee (CIAC) adjudicated any diagnostic tests performed during the double‐blind period, and all reported bleedings and deaths. Primary safety outcome: incidence of major bleeding rate |
|
| Notes | Use of adjunctive anticoagulative methods: The use of graduated elastic stockings was permitted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Multinational, multicenter, randomised, double‐blind, placebo‐controlled, parallel‐group study" Comment: probably done, as earlier reports from the same company clearly describe use of random sequences |
| Allocation concealment (selection bias) | Low risk | Double‐blind study with effective method to confirm blindness Comment: probably done, as most earlier multi‐centre RCT reports clearly mentioned that studies of the same medicine organised by the same company were centrally randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "For the double blind period, study medications were packaged in the boxes" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A Central Independent Adjudication Committee (CIAC) adjudicated any diagnostic tests performed during the double‐blind period, and all reported bleedings and deaths" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 87.7% and 86.1% of participants in 2 study groups finished treatment. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Unclear risk | Company sponsored |
Shen 2014.
| Methods | Randomised controlled study | |
| Participants |
Total number of participants enrolled: 121 Number of participants allocated to each group: fondaparinux (FX) group: 59, nadroparin calcium (NA) group: 57 Number of participants excluded and/or lost to follow‐up: not mentioned Inclusion: clinical diagnosis of oesophageal carcinoma and planned for oesophagectomy; clinical diagnosis of lung carcinoma and planned for lung resection; general anaesthesia combined with epidural anaesthesia Exclusion: blood clotting dysfunction before surgery; anticoagulating or antiplatelet history before surgery; low blood platelet count; haemorrhagic disease; cerebral haemorrhage; cerebral, spinal or ophthalmologic operation history; peptic ulcer; bleeding > 400 mL during operation; bleeding > 100 mL/h after operation; blood transfusion during or after operation; severe renal or liver dysfunction; severe hypertension |
|
| Interventions |
FX: 2.5 mg IH once daily after operation NA: nadroparin calcium 4100 AxaIU IH once daily after operation |
|
| Outcomes | Primary efficacy outcomes: VTE events and drainage volume | |
| Notes | Use of adjunctive anticoagulative methods: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors confirmed that they used the computer‐derived randomisation table. Comment: low risk of bias |
| Allocation concealment (selection bias) | Low risk | Study authors confirmed that they used the computer‐derived randomisation table. Comment: low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study publication did not clearly describe methods of blinding, and communication with study authors did not provide further information. Comment: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study authors confirmed that outcome assessors did not know the group information. Comment: low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 121 patients were enrolled in this study, and 116 eligible patients were randomly assigned (Group H: 57 patients; Group F: 59 patients)" Comment: low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | Results of VTE rates were reported, but the number of events was not clearly reported. Drainage volumes of study participants were reported. |
| Other bias | Low risk | No risk of other bias was identified. |
Yokote 2011.
| Methods | Randomised controlled trial | |
| Participants |
Total number of participants: 250 Number of participants allocated to each group: fondaparinux (FX) group: 85, enoxaparin (EN): 86, placebo group: 85 Number of participants excluded and/or lost to follow‐up: FX group: 1, EN group: 2, placebo group: 2 Inclusion: patients older than 20 years of age undergoing elective primary unilateral THR Exclusion: patients who had undergone bilateral and revision THR. Other exclusion criteria included long‐term anticoagulation treatment such as unfractionated heparin, LMWH, vitamin K antagonists and antiplatelet agents for pre‐existing cardiac or cerebrovascular disease; history of VTE; coagulation disorder including antiphospholipid syndrome; presence of a solid malignant tumour or a peptic ulcer; and major surgery in the preceding 3 months. The study also excluded Caucasian patients. |
|
| Interventions |
FX: postoperative sc injections of fondaparinux (2.5 mg once daily) for 10 consecutive days EN: postoperative sc injections of enoxaparin (40 mg or 20 mg twice daily) for 10 consecutive days Placebo: placebo (0.5 mL of isotonic saline) for 10 consecutive days The first postoperative injections of fondaparinux, enoxaparin and saline took place at an average of 18 hours (SD 2), 17 hours (SD 2) and 18 hours (SD 2), respectively, after the operation. |
|
| Outcomes |
Primary efficacy outcome: incidence of DVT or VTE assessed by bilateral ultrasonographic studies from the external iliac vein to proximal portions of the calf veins at postoperative day 11. All scans were performed by experienced vascular technicians and were read by experienced radiologists who were blinded to participants' randomisation. Those with a negative scan were followed clinically for 12 weeks (until postoperative day 84) for signs or symptoms of DVT, pulmonary emboli or readmission to hospital because of a complication related to the chemical prophylaxis, a bleeding complication, a wound problem or any other clinical event. Participants who were found to have a distal (calf) DVT did not receive any chemical treatment. Those with proximal DVT received anticoagulant therapy, with initial administration of unfractionated heparin along with initiation of warfarin therapy. Primary safety outcome: incidence of any bleeding, major or minor. This was assessed daily during the treatment period of 10 days and within 24 hours after completion or discontinuation of treatment. An episode of bleeding was classified as major if it was retroperitoneal, intracranial or intraocular, or if it was associated with death, transfusion of more than 2 units of packed red blood cells or whole blood (except autologous), a reduction in level of Hb > 2 g/dL or a serious or life‐threatening clinical event requiring medical intervention. Suspected intra‐abdominal or intracranial bleeding was confirmed by ultrasonography, CT or MRI. Minor episodes of bleeding were defined as those with at least 1 of the following features: epistaxis lasting longer than 5 minutes or requiring intervention, ecchymosis or haematoma with maximum size of > 5 cm, haematuria not associated with trauma from the urinary catheter, gastrointestinal haemorrhage not related to intubation or to passage of a nasogastric tube, wound haematoma or haemorrhagic wound complications not associated with major haemorrhage or subconjunctival haemorrhage, requiring cessation of medication. |
|
| Notes |
Use of adjunctive anticoagulative methods: All participants received the same routine mechanical prophylaxis (intermittent pneumatic compression device and a thigh‐high elastic compression bandage) during and after operation. Used for sensitivity analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not clearly describe what randomisation method was used. Comment: unclear |
| Allocation concealment (selection bias) | Unclear risk | The study did not clearly describe what randomisation method was used and whether a strategy was used to confirm allocation concealment. Comment: unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study did not clearly describe blinding Comment: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All the scans were performed by experienced vascular technicians and were read by experienced radiologists who were blinded to the patient’s randomisation" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 250 of 267 participants finished the study. Comment: low risk of bias |
| Selective reporting (reporting bias) | Low risk | All primary efficacy and safety outcomes listed in the Methods section were reported. Comment: low risk of bias |
| Other bias | Low risk | No risk of other bias was identified. |
AE: adverse event. APTT: activated partial thromboplastin time. BI: bleeding index. BMI: body mass index. CG: control group. CUS: compression ultrasonography. DVT: deep vein thrombosis. EBL: estimated blood loss. IPC: intermittent pneumatic compression. EN: enoxaparin. FX: fondaparinux. HES: hydroxy ethyl starch. INR: international normalised ratio. LMWH: low molecular weight heparin. MRV: magnetic resonance venography. NYHA: New York Heart Association. PE: pulmonary embolism. PT: prothrombin time. PTT: partial thromboplastin time. SAE: serious adverse event. sc: subcutaneous. SFJ: sapheno‐femoral junction. SVT: superficial venous thrombosis. THR: total hip replacement. TKA: total knee arthroplasty. TKR: total knee replacement. VTE: venous thromboembolism.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACT1840 | Dose studied in the trial markedly extended the normal dose. |
| Amadeus 2008 | Study investigated idraparinux for prevention of embolism in patients with atrial fibrillation. Most thrombosis events in the study were arterial embolism, not venous thrombosis, and study results did not distinguish arterial embolism and venous thrombosis events. |
| AR3106206 | This study focused on initial treatment of VTE, not on the prophylaxis of VTE. |
| AR3106333 | Both groups in the study received fondaparinux therapy. |
| AR3106335 | Both groups in the study received fondaparinux therapy. |
| Argun 2013 | No reliable primary efficacy and safety results were reported. |
| Bonneux 2006 | The study did not report on VTE evaluation methods. In addition, it did not report the outcome major bleeding and did not indicate whether the evaluator was blinded to group allocation. |
| Buller 2014 | The study investigated idrabiotaparinux for prevention of thromboembolism in patients with atrial fibrillation. Study results did not distinguish between systemic embolism and venous thromboembolism. |
| Cassiopea | This study focused on initial treatment of VTE. |
| Cohen 2007 | Both groups in the study received fondaparinux therapy. |
| EQUINOX | Both groups in the study received pentasaccharide therapy; 1 group received idraparinux, and the other received idrabiotaparinux. |
| extended van Gogh | This study focused on treatment of VTE. |
| FLEXTRA | Both groups in the study received fondaparinux therapy. |
| Kawaji 2012 | This was not a randomised controlled study. |
| Li 2013 | This study focused on fondaparinux for non‐ST elevation acute coronary syndromes, not for prevention of venous thrombosis. |
| MATISSE‐DVT | This study examined initial treatment of VTE, not prophylaxis of VTE. |
| MATISSE‐PE | This study examined initial treatment of VTE, not prophylaxis of VTE. |
| NCT00521885 | This study was stopped owing to lack of accrual without outcomes reported. |
| NCT00539942 | This study was terminated because of problems with accrual. |
| PENTATAK | Participants in all 5 study groups received fondaparinux therapy. |
| PERSIST | This study focused on initial treatment of VTE. |
| Rembrandt | This study examined initial treatment of VTE, not prophylaxis of VTE. |
| SAFE‐AF | This study focused on fondaparinux for anticoagulation treatment of electric cardioversion of atrial fibrillation that was mainly done to prevent arterial embolism. |
| Sasaki 2009 | This was a quasi‐randomised study. |
| Sasaki 2011 | This was a quasi‐randomised study. |
| Savi 2005 | The outcome of the study was different from the outcomes that we studied. |
| Tsutsumi 2012 | This was not a randomised controlled study. |
| van Gogh‐DVT | This study focused on initial treatment of VTE. |
| van Gogh‐PE | This study focused on initial treatment of VTE. |
| Xin 2013 | This study focused on fondaparinux for non‐ST elevation acute coronary syndromes, not for prevention of venous thrombosis. |
| Yamaoka 2014 | This was not a randomised controlled study. |
| Zhao 2013 | This study focused on fondaparinux for non‐ST elevation acute coronary syndromes, not for prevention of venous thrombosis. |
| Zhao 2015 | This study focused on fondaparinux for non‐ST elevation acute coronary syndromes, not for prevention of venous thrombosis. |
VTE: venous thromboembolism.
Characteristics of ongoing studies [ordered by study ID]
EUCTR2007‐003746‐15‐DE.
| Trial name or title | Prospective randomised open study on the comparison of fondaparinux with the low‐molecular‐weight heparin enoxaparin in patients undergoing femoro‐distal venous bypass operation |
| Methods | Randomised controlled trial |
| Participants | Periperal arterial occlusive disease Fontaine IIb to IV Possibility of venous bypass reconstruction with intended oral anticoagulation Women with childbearing potential were included only when they were using correctly and consistently a highly effective method of birth control (i.e. Pearl‐Index < 1) during the study. Regarded as highly effective were sterilised women, vasectomised partner, combined oral contraceptives and hormone‐eluting IUDs. |
| Interventions | Trade name: Arixtra Product name: Arixtra Pharmaceutical form: anticoagulant and preservative solution for blood INN or proposed INN: fondaparinux‐sodium Concentration unit: mg/mL Concentration number: 2.5/0.5 Trade name: Clexane Product name: Clexane Pharmaceutical form: anticoagulant and preservative solution for blood INN or proposed INN: enoxaparin‐sodium Concentration unit: mg/mL Concentration number: 100 |
| Outcomes | Primary endpoint(s):
|
| Starting date | 27 March 2008 |
| Contact information | |
| Notes |
EUCTR2008‐001779‐31‐IT.
| Trial name or title | Markers of hypercoagulability and risk of death and rehospitalization in heart failure patients: a pilot study on the effects of fondaparinux ‐ fondaparinux and heart failure |
| Methods | Randomised placebo‐controlled trial |
| Participants | Diagnosis of heart failure according to the Framingham criteria (III to IV NYHA) for patients aged ≥ 60 years. Patients had a planned hospital stay ≥ 4 days. |
| Interventions | Trade name: ARIXTRA Pharmaceutical form: solution for injection INN or proposed INN: fondaparinux Concentration unit: mg/mL milligram(s)/millilitre Concentration type: equal Concentration number: 5 |
| Outcomes | Main objective: to evaluate the effects of fondaparinux on biochemical parameters and clinical events according to different duration of treatment in heart failure patients (III to IV NYHA). Primary endpoint: circulating D‐dimer plasma levels 2 months after enrolment Primary endpoint(s): circulating D‐dimer plasma levels 2 months after enrolment Secondary objective: secondary endpoints: circulating thrombin‐antithrombin complexes (TAT), prothrombin fragment 1+2 (F1+2), interleukin‐6 (IL‐6), C‐reactive protein (CRP), tumour necrosis factor‐alpha (TNF‐alpha) at discharge and 1, 2 and 12 months after enrolment. Circulating D‐dimer plasma levels at discharge and 1 and 12 months after enrolment. Total mortality, cardiovascular mortality and need for rehospitalisation 12 months after enrolment. Pulmonary emboly and/or deep vein thrombosis. Minor and major bleeding |
| Starting date | 9 May 2008 |
| Contact information | |
| Notes |
JPRN‐UMIN000002444.
| Trial name or title | Randomised study of anticoagulant therapy to prevent postoperative DVT/PE |
| Methods | Randomised double‐blind parallel trial |
| Participants | Patients older than 20 years of age after digestive surgery classified as at high risk of DVT/PE |
| Interventions | Fondaparinux 2.5 mg 1/d for 7 days Enoxaparin 2000 IU 2/d for 7 days Heparin sodium 5000 IU 2/d for 7 days |
| Outcomes | Primary efficacy outcome: frequency of VTE Primary safety outcome: frequency of adverse events, including haemorrhage and abnormal serological findings |
| Starting date | 1 September 2009 |
| Contact information | kurita@clin.med.tokushima‐u.ac.jp |
| Notes |
JPRN‐UMIN000007005.
| Trial name or title | The efficacy and safety of anticoagulant therapy Arixtra injection for the prevention of the vein thromboembolism in laparoscopic colorectal surgery |
| Methods | Randomised parallel open‐label study |
| Participants | Patients underwent laparoscopic surgery for colorectal cancer. |
| Interventions | Fondaparinux and no intervention |
| Outcomes | Efficacy of the incidence of venous thromboembolism. Safety of the incidence of major bleeding |
| Starting date | 10 January 2012 |
| Contact information | Osaka Medical College |
| Notes |
JPRN‐UMIN000008435.
| Trial name or title | Phase III study of efficacy of fondaparinux on the prevention of post‐operative venous thromboembolism in patients undergoing with laparoscopic colorectal cancer surgery |
| Methods | Randomised controlled trial |
| Participants | Patients undergoing laparoscopic colorectal surgery with additional risk factor for VTE |
| Interventions | Once daily fondaparinux (2.5 mg or 1.5 mg) for 4 to 8 days after 24 hours of surgery Intermittent pneumatic compression according to institutional guidelines |
| Outcomes | Incidence of venous thromboembolism (VTE) Incidence of major bleeding |
| Starting date | 1 September 2012 |
| Contact information | Multicenter Clinical Study Group of Osaka, Colorectal Cancer Treatment Group, 2‐2‐E2 Yamadaoka, Suita, Osaka 565‐0871, Japan Telephone number: 06‐6879‐3251 |
| Notes |
PROTECT.
| Trial name or title | Prophylaxis of thromboembolic complications trial: thromboprophylaxis needed in below knee plaster cast immobilisation for ankle and foot fractures (PROTECT) |
| Methods | Prospective, randomised, controlled, single‐blinded, multi‐centre trial |
| Participants | Patients 18 years of age or older with a non‐surgical fracture of the lower extremity requiring immobilisation in a below‐knee plaster cast for a minimum of 4 weeks |
| Interventions | One group receiving nadroparin (2850 IU anti‐Xa = 0.3 mL once daily), 1 group receiving fondaparinux (2.5 mg = 0.5 mL once daily) and 1 group receiving no prophylaxis |
| Outcomes | Primary outcome measure: deep vein trombosis as detected by venous duplex. Secondary outcome measure: bleeding complications |
| Starting date | 13 April 2009 |
| Contact information | rjderksen@hotmail.com |
| Notes |
CABG: coronary artery bypass graft. DVT: deep vein thrombosis. INN: international non‐proprietary name. IU: international unit. IUD: intrauterine device. PE: pulmonary embolism. VTE: venous thromboembolism.
Differences between protocol and review
We note here several differences between the review and its protocol (Song 2011).
First, in the protocol, we planned to perform analyses on an intention‐to‐treat (ITT) basis, but we considered that this would cause serious bias. Therefore, we chose to include for analysis all venous thromboembolism (VTE) events at the studies' time endpoints. The reasons were as follows: Most participants had VTE and deep venous thrombosis (DVT) diagnosed by venography; this could not be done for every participant, and it would be very difficult to arbitrate all participants without such an efficacy evaluation result. Therefore, if we arbitrated all of the missing data (about 10% of all randomised), the result would be at high risk of bias. We used per‐protocol rather than preplanned ITT data.
Second, Peto's method (Yusuf 1985) can be used only to pool odds ratios. This works well when intervention effects are small (i.e. odds ratios are close to one), events are not particularly common and studies include similar numbers in experimental and control groups. For some outcomes of our systematic review, events were not rare, and it was more appropriate to use the risk ratio (RR). We used the Mantel‐Haenszel RR, which can be used to analyse both rare and frequent events.
Third, because most major bleeding was reported as bleeding index greater than 2, not life‐threatening bleeding, we added the outcome 'fatal bleeding' to clarify the true life‐threatening bleeding rate.
Fourth, because events included in the outcome 'haemorrhagic complications' were the same as major bleeding events, and in fact, most of the included studies reported only major bleeding events, we did not analyse the 'haemorrhagic complications' outcome, as specified in our protocol.
Fifth, given that clinical heterogeneity was caused by different clinical settings, we performed subgroup analyses for different clinical settings; this was not prespecified in the protocol. In the protocol, we planned to perform subgroup analyses based on the half‐life time of the pentasaccharides. However, as we found no RCTs investigating long‐term use of pentasaccharides for VTE prevention, we studied only short‐term use of the pentasaccharide fondaparinux.
Finally, we added to the review proximal DVT, symptomatic VTE, total pulmonary embolism (PE) and fatal bleeding as secondary outcomes; this was not mentioned in the protocol. We added these for inclusion in additional intensive analyses.
Contributions of authors
Kezhou Dong (KD) searched and selected trials for inclusion, assessed methodological quality of included trials, extracted data, performed the statistical analysis and revised the review. Yanzhi Song (YS) searched and selected trials for inclusion, assessed methodological quality of included trials, extracted data, performed the statistical analysis and wrote the review. Xiaodong Li (XL) searched trials, selected trials for inclusion, assessed methodological quality of included trials and extracted data. Jie Ding (JD) arbitrated disagreements that could not be resolved through discussion regarding selection of trials and extraction of data processes. Zhiyong Gao (ZG): gave important advice and revised the review. Daopei Lu (DL): gave important advice and revised the review. Yimin Zhu revised the review on the basis of editorial comments.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office
Declarations of interest
KD: none known. YS: none known. XL: none known. JD: none known. ZG: none known. DL:none known. YZ: none known.
New
References
References to studies included in this review
ACT2545 {published data only}
- Anon. A multicentre dose finding study of once daily injection of natural pentasaccharide for the prevention of deep vein thrombosis after total hip replacement. GlaxoSmithKline Clinical Trial Register 2008. [URL : www.gsk‐clinicalstudyregister.com/study/ACT2545#rs (accessed 22 July 2014)]
- Turpie AG. Pentasaccharide (org31540/sr90107a) clinical trials update: lessons for practice. American Heart Journal 2001;142(2 Suppl):S9‐15. [DOI] [PubMed] [Google Scholar]
APOLLO {published data only}
- Anon. Safety study of fondaparinux sodium to prevent venous thromboembolic events (APOLLO). A multicenter, randomized, double‐blind, parallel group trial to demonstrate the efficacy of fondaparinux sodium in association with intermittent pneumatic compression versus intermittent pneumatic compression used alone for the prevention of venous thromboembolic events in subjects at increased risk undergoing major abdominal surgery. GlaxoSmithKline Clinical Trial Register 2011; Vol. Study No: AR3103414. [URL: www.gsk‐clinicalstudyregister.com/study/103414 (accessed 22 July 2014)]
- Priore G, Herzog TJ, Bauer KA, Caprini JA, Comp P, Gent M, et al. Fondaparinux combined with intermittent pneumatic compression (IPC) versus IPC alone in the prevention of venous thromboembolism after major abdominal surgery: results of the APOLLO study. Gynecologic Oncology 2006; Vol. 101:S21.
- Turpie AG, Bauer K, Caprini J, Comp P, Gent M, Muntz J. Fondaparinux combined with intermittent pneumatic compression (IPC) versus IPC alone in the prevention of VTE after major abdominal surgery: results of the APOLLO study. Journal of Thrombosis and Haemostasis 2005;3(Suppl 1):Abstract number: P1046. [DOI] [PubMed] [Google Scholar]
- Turpie AG, Bauer KA, Caprini JA, Comp PC, Gent M, Muntz JE, et al. Fondaparinux combined with intermittent pneumatic compression vs. intermittent pneumatic compression alone for prevention of venous thromboembolism after abdominal surgery: a randomized, double‐blind comparison. Journal of Thrombosis and Haemostasis 2007;5(9):1854‐61. [DOI] [PubMed] [Google Scholar]
- Turpie AGG, Bauer KA, Caprini JA, Comp PP, Gent M, Muntz JE. Fondaparinux combined with intermittent pneumatic compression (IPC) versus IPC alone in the prevention of venous thromboembolism after major abdominal surgery: the randomized APOLLO study. Blood 2005;106(11):Abstract 279. [Google Scholar]
AR3106116 {published data only}
- Anon. Clinical evaluation of GSK576428 (fondaparinux sodium) in prevention of venous thromboembolism (VTE) after abdominal surgery. GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/AR3106116 (accessed 22 July 2014)]
- Sakon M, Tsukamoto T, Kobayashi T, Fujita S, Kawashima T, Morito M. Clinical evaluation of fondaparinux for prevention of venous thromboembolism after abdominal surgery. A randomized open‐label study of fondaparinux and intermittent pneumatic compression as a benchmark. Rinsho Iyaku ‐ Journal of Clinical Therapeutics & Medicines 2008;24(7):679‐89. [Google Scholar]
ARTEMIS {published data only}
- Anon. A multinational, randomized, double‐blind comparison of once daily subcutaneous fondaparinux sodium with placebo for the prevention of venous thromboembolic events in acutely ill medical patients (ARTEMIS). GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/104619 (accessed 22 July 2014)]
- Cohen AT. Thromboprophylaxis with fondaparinux in acutely ill medical patients aged 75 years or more. The Hematology Journal 2004;5(Suppl 2):105. [Google Scholar]
- Cohen AT. Thromboprophylaxis with fondaparinux in acutely ill medical patients with moderate renal impairment. The Hematology Journal 2004;5(Suppl 2):54. [Google Scholar]
- Cohen AT, Davidson BL, Gallus AS. Fondaparinux for the prevention of VTE in acutely ill medical patients. Blood 2003;102(11):15a‐Abstract 42. [Google Scholar]
- Cohen AT, Davidson BL, Gallus AS, Lassen MR, Prins MH, Tomkowski W, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ 2006;332(7537):325‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AT, Davidson BL, Gallus AS, Lassen MR, Prins MH, Tomkowski W, et al. Thromboprophylaxis with fondaparinux in acutely Ill medical patients aged 75 years or more, or with moderate renal impairment. Journal of Thrombosis and Haemostasis 2005;3(1):Abstract number: P2303. [Google Scholar]
- Cohen AT, Gallus AS, Lassen MR, Tomkowski W, Turpie AGG, Davidson BL, et al. Fondaparinux vs. placebo for the prevention of venous thromboembolism in patients (artemis). Journal of Thrombosis and Haemostasis 2003;1(Suppl 1 July):Abstract P2046. [Google Scholar]
- Wilmott R, Cohen AT. Benefit of fondaparinux in medical patients: a subgroup analysis. 16th European Chapter Congress of the International Union of Angiology, 2005 Oct; Glasgow, UK.
- Wilmott R, Cohen AT. Thromboprophylaxis with fondaparinux in acutely ill medical patients aged 75 years or more, or with moderate renal impairment. 16th European Chapter Congress of the International Union of Angiology, 2005 Oct; Glasgow, UK.
Bern 2015 {published data only}
- Bern MM, Hazel D, Deeran E, Richmond JR, Ward DM, Spitz DJ, et al. Low dose compared to variable dose warfarin and to fondaparinux as prophylaxis for thromboembolism after elective hip or knee replacement surgery; a randomized, prospective study. Thrombosis Journal 2015;13(32):672‐83. [DOI: 10.1186/s12959-015-0062-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
CALISTO {published data only}
- Anonymous. Evaluation of fondaparinux (also called ARIXTRA) 2.5 mg subcutaneously once daily for the treatment of superficial thrombophlebitis (also known as superficial vein thrombosis). GlaxoSmithKline Clinical Trial Register 2013. [URL: www.gsk‐clinicalstudyregister.com/study/ART108053 (accessed 22 July 2014)]
- Decousus H, Prandoni P, Mismetti P, Bauersachs RM, Boda Z, Brenner B, et al. Fondaparinux for the treatment of superficial‐vein thrombosis in the legs. New England Journal of Medicine 2010;363(13):1222‐32. [DOI] [PubMed] [Google Scholar]
- Leizorovicz A, Becker F, Buchmuller A, Quere I, Prandoni P, Decousus H, et al. Clinical relevance of symptomatic superficial‐vein thrombosis extension: lessons from the CALISTO study. Blood 2013;122:1724‐9. [DOI] [PubMed] [Google Scholar]
Cho 2013 {published data only}
- Cho KY, Kim KI, Khurana S, Bae DK, Jin W. Is routine chemoprophylaxis necessary for prevention of venous thromboembolism following knee arthroplasty in a low incidence population?. Archives of Orthopaedic and Trauma Surgery 2013;133(4):551‐9. [DOI] [PubMed] [Google Scholar]
DRI4090 {published data only}
- Anon. A multicenter, randomized, double‐blind, placebo controlled, parallel group, dose response study of subcutaneous (Org31540/SR90107A) in the prevention of venous thromboembolism after elective total hip replacement surgery. GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/DRI4090 (accessed 22 July 2014)]
- Fuji T, Fujita S, Ochi T. Fondaparinux prevents venous thromboembolism after joint replacement surgery in Japanese patients. International Orthopaedics 2008;32(4):443‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
DRI4757 {published data only}
- Anonymous. A multicenter, randomized, double‐blind, placebo‐controlled, parallel group, dose response study of subcutaneous (Org31540/SR90107A) in the prevention of venous thromboembolism after elective total knee replacement surgery. GlaxoSmithKline Clinical Trial Register 2008; Vol. Study No.: DRI4757. [URL: http://www.gsk‐clinicalstudyregister.com/study/DRI4757?study_ids=DRI4757#rs (accessed 22 July 2014)]
- Fuji T, Fujita S, Ochi T. Fondaparinux prevents venous thromboembolism after joint replacement surgery in Japanese patients. International Orthopaedics 2008;32(4):443‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
EFFORT {published data only}
- Steele KE, Canner J, Prokopowicz G, Verde F, Beselman A, Wyse R, et al. The EFFORT trial. Preoperative enoxaparin versus postoperative fondaparinux for thromboprophylaxis in bariatric surgical patients: a randomized double‐blind pilot trial. Surgery for Obesity and Related Diseases 2015;11(3):672‐83. [DOI] [PubMed] [Google Scholar]
EPHESUS {published data only}
- Anon. European Pentasaccharide Hip Elective Surgery Study (EPHESUS). A multicenter, randomized, double‐blind comparison of once daily subcutaneous (Org31540/SR90107A) with enoxaparin for the prevention of deep vein thrombosis or symptomatic pulmonary embolism in patients undergoing elective hip replacement surgery. GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/63118 (accessed 22 July 2014)]
- Borja J, Olivella P, Curto J. Fondaparinux versus enoxaparin for prevention of venous thromboembolism. Lancet 2002;360(9345):1603‐4. [DOI] [PubMed] [Google Scholar]
- Lassen MR. Bauer K, Eriksson BI, Turpie AG, European Pentasaccharide Elective Surgery Study (EPHESUS) Steering Committee. Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip‐replacement surgery: a randomised double‐blind comparison. Lancet 2002;359(9319):1715‐20. [DOI] [PubMed] [Google Scholar]
FONDACAST {published data only}
- Anon. FONDAparinux in patients with a plaster CAST (FONDACAST). GlaxoSmithKline Clinical Trial Register 2011. [URL: www.gsk‐clinicalstudyregister.com/study/109350 (accessed 22 July 2014)]
- Samama CM, Lecoules N, Kierzek G, Claessens YE, Riou B, Rosencher N, et al. Comparison of fondaparinux with low molecular weight heparin for venous thromboembolism prevention in patients requiring rigid or semi‐rigid immobilization for isolated non‐surgical below‐knee injury. Journal of Thrombosis and Haemostasis 2013;11(10):1833‐43. [DOI] [PubMed] [Google Scholar]
- Samama CM, Lecoules N, Kierzek G, Claessens YE, Riou B, Rosencher N, et al. Comparison of fondaparinux with low‐molecular‐weight heparin for venous thromboembolism prevention in patients requiring rigid or semi‐rigid immobilization for isolated non‐surgical below‐knee injury. Annales Francaises de Medecine d'Urgence 2014;4(3):153‐66. [DOI] [PubMed] [Google Scholar]
- Samama CM, Riou B, Roy P‐M, Sautet A, Mismetti P, FONDACAST Study Group. Prevention of venous thromboembolism after an isolated, non‐surgical below‐knee injury. Benefit/risk of fondaparinux vs. low molecular weight heparin: the Fondacast study. Journal of Thrombosis and Haemostasis 2013;11(Suppl S3):5. [DOI] [PubMed] [Google Scholar]
- Samama CMR. Subgroup analysis of the FONDACAST study comparing fondaparinux to low‐molecular‐weight heparin for the prevention of venous thromboembolism after an isolated, non‐surgical below‐knee injury. Journal of Thrombosis and Haemostasis 2013;11 (Suppl S2):88. [DOI] [PubMed] [Google Scholar]
Fuji 2015 {published and unpublished data}
- Fuji T, Fujita S, Kawai Y, Abe Y, Kimura T, Fukuzawa M, et al. A randomized, open‐label trial of edoxaban in Japanese patients with severe renal impairment undergoing lower‐limb orthopedic surgery. Thrombosis Journal 2015;13:6. [DOI: 10.1186/s12959-014-0034-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kolluri 2016 {published data only}
- Kolluri R, Plessa AL, Sanders MC, Singh NK, Lucore C. A randomized study of the safety and efficacy of fondaparinux versus placebo in the prevention of venous thromboembolism after coronary artery bypass graft surgery. American Heart Journal 2016;171(1):1‐6. [DOI] [PubMed] [Google Scholar]
L‐8541 {published data only}
- Anon. Arixtra VTE Prevention Study ‐ Fondaparinux compared with enoxaparin in the prevention of venous thromboembolism (VTE) in Chinese patients undergoing elective knee replacement, hip surgery or a revision of components. GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/L‐8541 (accessed 22 July 2014)]
L‐8635 {published data only}
- Anon. Fondaparinux compared with enoxaparin in the prevention of venous thromboembolism in Taiwanese patients undergoing elective knee replacement. GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/L‐8635 (accessed 22 July 2014)]
Li 2015 {published and unpublished data}
- Li B, Wang K, Zhao X, Lin C, Sun H. Comparison of fondaparinux sodium and low molecular weight heparin in the treatment of hypercoagulability secondary to traumatic infection. Chinese Journal of Traumatology 2015;18:147‐9. [PubMed] [Google Scholar]
PEGASUS {published data only}
- Agnelli G, Bergqvist D, Cohen A, Gallus A, Gent M. A randomized double‐blind study to compare the efficacy and safety of fondaparinux with dalteparin in the prevention of venous thromboembolism after high‐risk abdominal surgery: the Pegasus study. Journal of Thrombosis and Haemostasis 2003; Vol. 1, issue Suppl 1:Abstract OC006.
- Agnelli G, Bergqvist D, Cohen A, Gallus AS, Gent M. A randomized double‐blind study to compare the efficacy and safety of postoperative fondaparinux (Arixtra) and preoperative dalteparin in the prevention of venous thromboembolism after high‐risk abdominal surgery: the PEGASUS study. Blood 2003;102(11 Pt 1):Abstract 40. [Google Scholar]
- Agnelli G, Bergqvist D, Cohen AT, Gallus AS, Gent M, PEGASUS Investigators. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high‐risk abdominal surgery. British Journal of Surgery 2005;92(10):1212‐20. [DOI] [PubMed] [Google Scholar]
- Anon. A multicenter, multinational, randomized, double‐blind study to compare the efficacy and safety of fondaparinux sodium (Org31540/SR90107A) with dalteparin (Fragmin) in the prevention of venous thromboembolic events in high‐risk abdominal surgery (PEGASUS). GlaxoSmithKline Clinical Trial Register 2005. [URL: www.gsk‐clinicalstudyregister.com/study/EFC3557 (accessed 22 July 2014)]
PENTAMAKS {published data only}
- Anon. A multicenter, multinational, randomized, double‐blind comparison of subcutaneous (Org31540/SR90107A) with enoxaparin in the prevention of deep vein thrombosis and symptomatic pulmonary embolism after elective major knee surgery or a revision (PENTAMAKS). GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/095‐002 (accessed 22 July 2014)]
- Bauer K. The PENTAMAKS Study. Comparison of the first synthetic factor Xa inhibitor with low molecular weight heparin in the prevention of venous thromboembolism (VTE) after elective major knee surgery. Blood 2000;96:490a. [Google Scholar]
- Bauer KA, Eriksson BI, Lassen MR, Turpie AGG, for the Steering Committee of the Pentasaccharide in Major Knee Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. New England Journal of Medicine 2001;345(18):1305‐10. [DOI] [PubMed] [Google Scholar]
- Warkentin TE, Cook RJ, Marder VJ, Sheppard JA, Moore JC, Eriksson BI, et al. Anti‐platelet factor 4/heparin antibodies in orthopedic surgery patients receiving antithrombotic prophylaxis with fondaparinux or enoxaparin. Blood 2005;106(12):3791‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
PENTATHLON {published data only}
- Turpie AG. Pentasaccharide (Org31540/SR90107A) clinical trials update: lessons for practice. American Heart Journal 2001;142(2 Suppl):S9‐15. [DOI] [PubMed] [Google Scholar]
- Turpie AG, Gallus AS, Hoek JA, Pentasaccharide Investigators. A synthetic pentasaccharide for the prevention of deep‐vein thrombosis after total hip replacement. New England Journal of Medicine 2001;344(9):619‐25. [DOI] [PubMed] [Google Scholar]
- Warkentin TE, Cook RJ, Marder VJ, Sheppard JI, Moore JC, Eriksson BI, et al. Anti‐platelet factor 4/heparin antibodies in orthopedic surgery patients receiving antithrombotic prophylaxis with fondaparinux or enoxaparin. Blood 2005;106(12):3791‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
PENTATHLON 2000 {published data only}
- Anon. A multicenter, multinational, randomized, double‐blind comparison of subcutaneous (Org31540/SR90107A) with enoxaparin in the prevention of deep vein thrombosis and symptomatic pulmonary embolism after elective hip replacement or a revision. PENTATHLON 2000. GlaxoSmithKline Clinical Trial 2008. [URL: www.gsk‐clinicalstudyregister.com/study/EFC2442 (accessed 22 July 2014)]
- Turpie AG. The PENTATHALON 2000 Study: comparison of the first synthetic factor Xa inhibitor with low molecular weight heparin in the prevention of venous thromboembolism. Blood 2000;96(491A):Abstract A2112. [Google Scholar]
- Turpie AG, Bauer KA, Eriksson BI, Lassen MR, PENTATHALON 2000 Study Steering Committee. Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip‐replacement surgery: a randomised double‐blind trial. Lancet 2002;359(9319):1721‐6. [DOI] [PubMed] [Google Scholar]
PENTHIFRA {published data only}
- Anon. A multicenter, multinational, randomized double‐blind comparison study of subcutaneous (Org31540/SR90107A) versus enoxaparin 40 mg o.d. in the prevention of deep vein thrombosis and symptomatic pulmonary embolism in hip fracture surgery (PENTHIFRA). GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/EFC2698 (accessed 22 July 2014)]
- Eriksson B. The PENTHIFRA Study. Comparison of the first synthetic Factor Xa inhibitor with low molecular weight heparin (LMWH) for the prevention of venous thromboembolism (VTE) after hip fracture surgery. Blood 2000;96(490A):Abstract 2110. [Google Scholar]
- Eriksson BI, Bauer KA, Lassen MR, Turpie AGG, for the Steering Committee of the Pentasaccharide in Hip‐Fracture Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip‐fracture surgery. The New England Journal of Medicine 2001;345(18):1298‐304. [DOI] [PubMed] [Google Scholar]
PENTHIFRA PLUS {published data only}
- Anon. A multicenter, multinational, randomized, double‐blind study of fondaparinux sodium (Org31540/SR90107A) versus placebo for the prolonged prevention of VTE in hip fracture surgery (PENTIHFRA PLUS). GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/EFC4582?study_ids=EFC4582#rs (accessed 22 July 2014)]
- Bauer KA, Eriksson BI, Lassen MR, Turpie AGG. No episode of thrombocytopenia after four‐week administration of fondaparinux, a new synthetic and selective inhibitor of factor Xa, in the PENTHIFRA‐PLUS study. Journal of Thrombosis and Haemostasis 2003;1(Suppl 1):P2050. [Google Scholar]
- Dobesh PP. Novel concepts: emerging data and the role of extended prophylaxis following hip fracture surgery. American Journal of Health‐System Pharmacy 2003;60(22 Suppl 7):S15‐9. [DOI] [PubMed] [Google Scholar]
- Eriksson BI. A multicenter, randomized, placebo‐controlled, double‐blind study of fondaparinux for the prolonged prevention of venous thromboembolism in hip fracture surgery. Abstracts of the Sicot/Sirot XXII World Congress 2002:318.
- Eriksson BI, Lassen MR. Consistency of efficacy of extended thromboprophylaxis with fondaparinux (Arixtra) in prevention of venous thromboembolism (VTE) after hip fracture surgery according to different composite efficacy endpoints: the PENTHIFRA‐PLUS study. Journal of Thrombosis and Haemostasis 2003;1(Suppl 1):Abstract P2064. [Google Scholar]
- Eriksson BI, Lassen MR, Colwell CW Jr. Efficacy of fondaparinux for thromboprophylaxis in hip fracture patients. Journal of Arthroplasty 2004;19(7 Suppl 2):78‐81. [DOI] [PubMed] [Google Scholar]
- Eriksson BI, Lassen MR, PENTasaccharide in HIp‐FRActure Surgery Plus (PENTHIFRA Plus) Investigators. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo‐controlled, double‐blind study. Archives of Internal Medicine 2003;163(11):1337‐42. [DOI] [PubMed] [Google Scholar]
- Lassen M, Bauer KA, Eriksson BI, Turpie AGG. Absence of transaminase increase after 4‐week administration of fondaparinux (Arixtra) a new synthetic and selective inhibitor of factor Xa in the PENTHIFRA‐PLUS study. Journal of Thrombosis and Haemostasis 2003;1(Suppl 1):Abstract number P2052. [Google Scholar]
- Lassen MR, Eriksson BI. Efficacy of fondaparinux (Arixtra) in extended thromboprophylaxis in hip fracture surgery is irrespective of patient and surgical characteristics: subgroup analyses of the Penthifra‐Plus study. Journal of Thrombosis and Haemostasis 2003;1(Suppl 1):Abstract P2062. [Google Scholar]
Shen 2014 {published and unpublished data}
- Shen Y, Zhong M, Tan L. Fondaparinux versus low molecular weight heparin following esophagectomy: results from a randomized and controlled trial. Chest 2014;145(3_Meeting Abstracts):535D. [Google Scholar]
Yokote 2011 {published data only}
- Yokote R, Matsubara M, Hirasawa N, Hagio S, Ishii K, Takata C. Is routine chemical thromboprophylaxis after total hip replacement really necessary in a Japanese population?. Journal of Bone and Joint Surgery British Volume 2011;93(2):251‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACT1840 {published data only}
- Anon. A multicentre pilot study of natural pentasaccharide (SR90107A /Org31540) for the prevention of deep venous thrombosis after total hip replacement. GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/ACT1840 (accessed 22 July 2014)]
Amadeus 2008 {published data only}
- Amadeus Investigators, Bousser MG, Bouthier J, Büller HR, Cohen AT, Crijns H, Davidson BL, et al. Comparison of idraparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: a randomised, open‐label, non‐inferiority trial. Lancet 2008;371(9609):315‐21. [DOI: 10.1016/S0140-6736(08)60168-3] [DOI] [PubMed] [Google Scholar]
AR3106206 {published data only}
- Anon. Clinical evaluation of GSK576428 (fondaparinux sodium) in the treatment of acute pulmonary thromboembolism (PE). GlaxoSmithKline Clinical Trial Register 2009. [NCT00981409; URL: www.gsk‐clinicalstudyregister.com/study/106206?study_ids=AR3106206#ps (accessed 22 July 2014)]
AR3106333 {published data only}
- Anon. Clinical evaluation of GSK576428 (fondaparinux sodium) in prevention of venous thromboembolism after elective total hip replacement surgery. GlaxoSmithKline Clinical Trial Register 2008. [URL: http://www.gsk‐clinicalstudyregister.com/study/AR3106333?study_ids=AR3106333#ps (accessed 22 July 2014)]
AR3106335 {published data only}
- Anon. Clinical evaluation of GSK576428 (fondaparinux sodium) in prevention of venous thromboembolism after hip fracture surgery. GlaxoSmithKline Clinical Trial Register 2008. [NCT00320424; URL: http://www.gsk‐clinicalstudyregister.com/study/AR3106335?study_ids=AR3106335#ps (accessed 22 July 2014)]
Argun 2013 {published data only}
- Argun M, Oner M, Saglamoglu M, Karaman I, Guney A, Halici M, et al. Fondaparınux versus nadroparin for prevention of venous thromboembolism after elective hip and knee arthroplasty. Current Therapeutic Research 2013;74:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonneux 2006 {published data only}
- Bonneux IM, Bellemans J, Fabry G. Evaluation of wound healing after total knee arthroplasty in a randomized prospective trial comparing fondaparinux with enoxaparin. Knee 2006;13(2):118‐21. [DOI] [PubMed] [Google Scholar]
Buller 2014 {published data only}
- Buller HR, Halperin J, Hankey GJ, Pillion G, Prins MH, Raskob GE. Comparison of idrabiotaparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: the Borealis‐Atrial Fibrillation Study. Journal of Thrombosis and Haemostasis 2014;12(6):824‐30. [DOI] [PubMed] [Google Scholar]
Cassiopea {published data only}
- Büller HR, Gallus AS, Pillion G, Prins MH, Raskob GE, on behalf of the Cassiopea Investigators. Enoxaparin followed by once‐weekly idrabiotaparinux versus enoxaparin plus warfarin for patients with acute symptomatic pulmonary embolism: a randomised, double‐blind, double‐dummy, non‐inferiority trial. Lancet 2012;379(9811):123‐9. [DOI] [PubMed] [Google Scholar]
Cohen 2007 {published data only}
- Cohen A, Brenkel I, Skinner J, Warwick D. Graduated compression stockings do not add to the efficacy of fondaparinux for thromboprophylaxis in hip replacement surgery. A multi‐centre, multi‐national, randomised, open‐label study. Journal of Bone and Joint Surgery British Volume 2009;91‐B(Suppl 1):73‐c. [Google Scholar]
- Cohen AT, Skinner JA, Warwick D, Brenkel I. The use of graduated compression stockings in association with fondaparinux in surgery of the hip. A multicentre, multinational, randomised, open‐label, parallel‐group comparative study. Journal of Bone and Joint Surgery British Volume 2007;89(7):887‐92. [DOI] [PubMed] [Google Scholar]
EQUINOX {published data only}
- The EQUINOX Invesstigators. Efficacy and safety of once weekly subcutaneous idrabiotaparinux in the treatment of patients with symptomatic deep venous thrombosis. Journal of Thrombosis and Haemostasis 2011;9(1):92‐9. [DOI] [PubMed] [Google Scholar]
extended van Gogh {published data only}
- van Gogh Investigators, Buller HR, Cohen AT, Davidson B, Decousus H, Gallus AS, et al. Extended prophylaxis of venous thromboembolism with idraparinux. New England Journal of Medicine 2007;357(11):1105‐12. [DOI] [PubMed] [Google Scholar]
FLEXTRA {published data only}
- Anon. Flexibility in administration of Arixtra for prevention of symptomatic venous thromboembolism in orthopedic surgery. GlaxoSmithKline Clinical Trial Register 2008. [URL: http://www.gsk‐clinicalstudyregister.com/study/L8518?study_ids=L851#rs (accessed 22 July 2014)]
- Colwell CW Jr, Kwong LM, Turpie AGG, Davidson BL. Flexibility in administration of fondaparinux for prevention of symptomatic venous thromboembolism in orthopaedic surgery. Journal of Arthroplasty 2006;21(1):36‐45. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Turpie AGG, Kwong LM, Colwell CW. FLEXTRA: early vs delayed initiation of postoperative fondaparinux prophylaxis after joint replacement: a clinical outcome study. Journal of Thrombosis and Haemostasis 2005;3(Suppl 1):Abstract OR061. [Google Scholar]
Kawaji 2012 {published data only}
- Kawaji H, Ishii M, Tamaki Y, Hamasaki M, Ishikawa H, Sasaki K, et al. Postoperative prophylactic effect of fondaparinux for prevention of deep venous thrombosis after cemented total hip replacement:a comparative study. Modern Rheumatology / The Japan Rheumatism Association 2012;22(2):216–22. [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li MW, Zhao XM, Rao LX. The clinical efficacy and safety of fondaparinux combined with tirofiban hydrochloride in patients with acute coronary syndrome undergoing complex percutaneous coronary intervention. Zhonghua Nei Ke Za Zhi 2013;52:1037‐40. [PubMed] [Google Scholar]
MATISSE‐DVT {published data only}
- Buller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, et al. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Annals of Internal Medicine 2004;140(11):867‐73. [DOI] [PubMed] [Google Scholar]
MATISSE‐PE {published data only}
- The Matisse Investigators. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. New England Journal of Medicine 2003;349(18):1695‐702. [DOI] [PubMed] [Google Scholar]
NCT00521885 {published data only}
- NCT00521885. Comparison of arixtra vs. lovenox to prevent blood clots in medically ill patients (BRiEF). ClinicalTrials.gov 2007. [URL: http://clinicaltrials.gov/show/NCT00521885 (accessed 8 March 2016)]
NCT00539942 {published data only}
- NCT00539942. Study of arixtra to prevent blood clots in women undergoing abdominopelvic surgery for likely gynecologic malignancy. ClinicalTrials.gov. [URL: http://clinicaltrials.gov/ct2/show/NCT00539942 (accessed 8 March 2016)]
PENTATAK {published data only}
- Anon. A multicenter, concurrent control, randomized, open‐label, assessor‐blind, dose‐ranging study (of Org 31540/SR90107A) in the prophylaxis of deep vein thrombosis in subjects undergoing total knee replacement surgery (PENTATAK). GlaxoSmithKline Clinical Trial Register 2008. [URL: www.gsk‐clinicalstudyregister.com/study/95001 (accessed 23 July 2014)]
PERSIST {published data only}
- PERSIST Investigators. A novel long‐acting synthetic factor Xa inhibitor (SanOrg34006) to replace warfarin for secondary prevention in deep vein thrombosis. A phase II evaluation. Journal of Thrombosis and Haemostasis 2004;2(1):47‐53. [DOI] [PubMed] [Google Scholar]
Rembrandt {published data only}
- The Rembrandt Investigators. Treatment of proximal deep vein thrombosis with a novel synthetic compound (SR90107A/ Org31540) with pure anti‐factor Xa activity. A phase II evaluation. Circulation 2000;102:2726‐31. [DOI] [PubMed] [Google Scholar]
SAFE‐AF {published data only}
- Cohen A, Stellbrink C, Heuzey JY, Faber T, Aliot E, Banik N, et al. Safety of fondaparinux in transoesophageal echocardiography‐guided electric cardioversion of atrial fibrillation (SAFE‐AF) study: a pilot study. Archives of Cardiovascular Diseases 2014;108:122‐31. [DOI] [PubMed] [Google Scholar]
Sasaki 2009 {published data only}
- Sasaki S, Miyakoshi N, Matsuura H, Saitoh H, Kudoh D, Shimada Y. Prospective randomized controlled trial on the effect of fondaparinux sodium for prevention of venous thromboembolism after hip fracture surgery. Journal of Orthopaedic Science 2009;14(5):491‐6. [DOI] [PubMed] [Google Scholar]
Sasaki 2011 {published data only}
- Sasaki S, Miyakoshi N, Matsuura H, Saito H, Nakanishi T, Kudo Y, et al. Prospective study on the efficacies of fondaparinux and enoxaparin in preventing venous thromboembolism after hip fracture surgery. Journal of Orthopaedic Science 2011;16(1):64‐70. [DOI] [PubMed] [Google Scholar]
Savi 2005 {published data only}
- Savi P, Chong BH, Greinacher A, Gruel Y, Kelton JG, Warkentin TE, et al. Effect of fondaparinux on platelet activation in the presence of heparin‐dependent antibodies: a blinded comparative multicenter study with unfractionated heparin. Blood 2005;105(1):139‐44. [DOI] [PubMed] [Google Scholar]
Tsutsumi 2012 {published data only}
- Tsutsumi S, Yajima R, Tabe Y, Takaaki T, Fujii F, Morita H, et al. The efficacy of fondaparinux for the prophylaxis of venous thromboembolism after resection for colorectal cancer. Hepatogastroenterology 2012;59:2477‐9. [DOI] [PubMed] [Google Scholar]
van Gogh‐DVT {published data only}
- van Gogh Investigators, Buller HR, Cohen AT, Davidson B, Decousus H, Gallus AS, et al. Idraparinux versus standard therapy for venous thromboembolic disease. New England Journal of Medicine 2007;357(11):1094‐104. [DOI] [PubMed] [Google Scholar]
van Gogh‐PE {published data only}
- van Gogh Investigators. Idraparinux versus standard therapy for venous thromboembolic disease. New England Journal of Medicine 2007;357(11):1094‐104. [DOI] [PubMed] [Google Scholar]
Xin 2013 {published data only}
- Xin Z, Ya‐Ling H, Xiao‐Zeng W, Bai‐Ge X. Efficacy and safety of fondaparinux during thrombolytic therapy in acute ST elevation myocardial infarction patients. Heart 2013;99:A54‐A55. [Google Scholar]
Yamaoka 2014 {published data only}
- Yamaoka YI, Nishikawa T. The impact of fondaparinux on the prophylaxis of venous thromboembolism: the efficacy, safety and prognosis after resection for colorectal cancer. Annals of Surgical Oncology. 2014; Vol. 21, issue 1 Suppl 1.
Zhao 2013 {published data only}
- Zhao X, Han Y, Wang X, Xu B. Efficacy and safety of fondaparinux during thrombolytic therapy in acute ST elevation myocardial infarction patients. Cardiology (Switzerland) 2013;126:138. [Google Scholar]
Zhao 2015 {published data only}
- Zhao XM, Gao CY, Chu YJ. Fondaparinux vs. enoxaparin in patients with non‐ST elevation acute coronary syndromes (NSTE‐ACS) treated with percutaneous coronary intervention and tirofiban: an exploratory study in China. Journal of Clinical Pharmacy and Therapeutics 2015;40:584‐9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
EUCTR2007‐003746‐15‐DE {published data only}
- EUCTR2007‐003746‐15‐DE. Prospective randomized open study on the comparison of fondaparinux with the low‐molecular‐weight heparin enoxaparin in patients undergoing femoro‐distal venous bypass operation. WHO International Clinical Trials Registry 2008. [URL: http://apps.who.int/trialsearch/Trial.aspx?TrialID=EUCTR2007‐003746‐15‐DE (accessed 19 September 2016)]
EUCTR2008‐001779‐31‐IT {published data only}
- Markers of hypercoagulability and risk of death and rehospitalization in heart failure patients: a pilot study on the effects of Fondaparinux ‐ Fondaparinux and heart failure. WHO International Clinical Trials Registry. [URL : http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2008‐001779‐31‐IT (accessed 19 September 2016)]
JPRN‐UMIN000002444 {published data only}
- JPRN‐UMIN000002444. Randomized study of anti‐coagulant therapy to prevent postoperative deep venous thrombosis/pulmonary embolism. WHO International Clinical Trials Registry 2009. [URL: http://apps.who.int/trialsearch/trial.aspx?trialid=JPRN‐UMIN000002444 (accessed 19 September 2016)]
JPRN‐UMIN000007005 {published data only}
- JPRN‐UMIN000007005. The efficacy and safety of anticoagulant therapy Arixtra Injection for the prevention of the vein thromboembolism in laparoscopic colorectal surgery. WHO International Clinical Trials Registry 2011. [URL: http://apps.who.int/trialsearch/Trial.aspx?TrialID=JPRN‐UMIN000007005 (accessed 19 September 2016)]
JPRN‐UMIN000008435 {published data only}
- Phase III study of efficacy of fondaparinux on the prevention of post‐operative venous thromboembolism in patients undergoing with laparoscopic colorectal cancer surgery. WHO International Clinical Trials Registry. [URL: http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000008435 (accessed 19 September 2016)]
PROTECT {published data only}
- NCT00881088. Prophylaxis of thromboembolic complications trial: thromboprophylaxis needed in below knee plaster cast immobilization for ankle and foot fractures (PROTECT). ClinicalTrials.gov 2009. [URL: http://clinicaltrials.gov/ct2/show/NCT00881088?term=NCT00881088&rank=1 (accessed 8 March 2016)]
Additional references
Ageno 2012
- Ageno W, Riva N, Noris P, Nisio M, Regina M, Arioli D, et al. The FONDAIR Study Group. Safety and efficacy of low dose fondaparinux (1.5 mg) for the prevention of venous thromboembolism in acutely ill medical patients with renal impairment: the FONDAIR study. Journal of Thrombosis and Haemostasis 2012;10(11):2291‐7. [DOI] [PubMed] [Google Scholar]
Agnelli 2000
- Agnelli G, Sonaglia F. Prevention of venous thromboembolism. Thrombosis Research 2000;97(1):V49‐62. [DOI] [PubMed] [Google Scholar]
Amiral 1997
- Amiral J, Lormeau JC, Marfaing‐Koka A, Vissac AM, Wolf M, Boyer‐Neumann C, et al. Absence of cross‐reactivity of SR90107A/ORG31540 pentasaccharide with antibodies to heparin‐PF4 complexes developed in heparin‐induced thrombocytopenia. Blood Coagulation and Fibrinolysis 1997;8(2):114‐7. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bauer 2003
- Bauer KA. New pentasaccharides for prophylaxis of deep vein thrombosis: pharmacology. Chest 2003;124(6 Suppl):364s‐70s. [DOI] [PubMed] [Google Scholar]
Bauersachs 2005
- Bauersachs RM. Fondaparinux: an update on new study results. European Journal of Clinical Investigation 2005;35(Suppl 1):27‐32. [DOI] [PubMed] [Google Scholar]
Bergqvist 2006
- Bergqvist D. Review of fondaparinux sodium injection for the prevention of venous thromboembolism in patients undergoing surgery. Vascular Health and Risk Management 2006;2(4):365‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bounameaux 2002
- Bounameaux H, Perneger T. Fondaparinux: a new synthetic pentasaccharide for thrombosis prevention. Lancet 2002;359(9319):1710‐1. [DOI] [PubMed] [Google Scholar]
Brito 2011
- Brito V, Ciapponi A, Kwong J. Factor Xa inhibitors for acute coronary syndromes. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD007038.pub2] [DOI] [PubMed] [Google Scholar]
Delavenne 2010
- Delavenne X, Zufferey P, Baylot D, Nguyen P, Borg JY, Fontenay M, et al. Population pharmacokinetics of fondaparinux administered at prophylactic doses after major orthopaedic surgery in everyday practice. Thrombosis and Haemostasis 2010;104(2):252‐60. [DOI] [PubMed] [Google Scholar]
Delavenne 2012
- Delavenne X, Zufferey P, Nguyen P, Rosencher N, Samama CM, Bazzoli C, et al. Pharmacokinetics of fondaparinux 1.5 mg once daily in a real‐world cohort of patients with renal impairment undergoing major orthopaedic surgery. European Journal of Clinical Pharmacology 2012;68(10):1403‐10. [DOI] [PubMed] [Google Scholar]
Erkens 2010
- Erkens PM, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD001100.pub3] [DOI] [PubMed] [Google Scholar]
Falck‐Ytter 2012
- Falck‐Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e278S‐325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fowkes 2003
- Fowkes FJ, Price JF, Fowkes FG. Incidence of diagnosed deep vein thrombosis in the general population: systematic review. European Journal of Vascular and Endovascular Surgery 2003;25(1):1‐5. [DOI] [PubMed] [Google Scholar]
Geerts 2001
- Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA, et al. Prevention of venous thromboembolism. Chest 2001;119(1 Suppl):132S‐75S. [DOI] [PubMed] [Google Scholar]
Geerts 2004
- Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126(3 Suppl):338S‐400S. [DOI] [PubMed] [Google Scholar]
Geerts 2008
- Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practive Guidelines (8th Edition). Chest 2008;133(6 Suppl):381S–453S. [DOI] [PubMed] [Google Scholar]
GradePro [Computer program]
- Brozek J, Oxman A, Schünemann H. [Computer program on www.gradepro.org]. GRADEpro. Version December 2014. McMaster University, 2014.
Hansson 2000
- Hansson PO, Sorbo J, Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Archives of Internal Medicine 2000;160(6):769‐74. [DOI] [PubMed] [Google Scholar]
Heit 2001
- Heit JA, Melton LJ 3rd, Lohse CM, Petterson TM, Silverstein MD, Mohr DN, et al. Incidence of venous thromboembolism in hospitalised patients vs community residents. Mayo Clinic Proceedings 2001;76(11):1102‐10. [DOI] [PubMed] [Google Scholar]
Heit 2005
- Heit JA, Cohen AT, Anderson FA Jr. Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism (VTE) events in the US. Blood 2005;106(11):Abstract 910. [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011], The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hills 2009
- Hills RK, Gray R, Wheatley K. Balancing treatment allocations by clinician or center in randomized trials allows unacceptable levels of treatment prediction. Journal of Evidence‐based Medicine 2009;2:196–204. [DOI] [PubMed] [Google Scholar]
Koopman 2003
- Koopman MM, Buller HR. Short‐ and long‐acting synthetic pentasaccharides. Journal of Internal Medicine 2003;254(4):335‐42. [DOI] [PubMed] [Google Scholar]
Metzger 2015
- Metzger A, Nagaraj T. New oral anticoagulants: clinical parameters and uses in practice. Consultant Pharmacist 2015;30(6):329‐45. [DOI: 10.4140/TCP.n.2015.329] [DOI] [PubMed] [Google Scholar]
Mismetti 2012
- Mismetti P, Samama CM, Rosencher N, Vielpeau C, Nguyen P, Deygas B, et al. Venous thromboembolism prevention with fondaparinux 1.5 mg in renally impaired patients undergoing major orthopaedic surgery. A real‐world, prospective, multicentre, cohort study. Thrombosis and Haemostasis 2012;107(6):1151‐60. [DOI] [PubMed] [Google Scholar]
Nijkeuter 2004
- Nijkeuter M, Huisman MV. Pentasaccharides in the prophylaxis and treatment of venous thromboembolism: a systematic review. Current Opinion in Pulmonary Medicine 2004;10:338‐44. [DOI] [PubMed] [Google Scholar]
Olson 1992
- Olson ST, Bjork I, Sheffer R, Craig PA, Shore JD, Choay J. Role of the antithrombin‐binding pentasaccharide in heparin acceleration of antithrombin‐proteinase reactions. Resolution of the antithrombin conformational change contribution to heparin rate enhancement. Journal of Biological Chemistry 1992;267(18):12528‐38. [PubMed] [Google Scholar]
Palareti 2012
- Palareti G, Schellong S. Isolated distal deep vein thrombosis: what we know and what we are doing. Journal of Thrombosis and Haemostasis 2012;10(1):11‐9. [DOI] [PubMed] [Google Scholar]
Paty 2007
- Paty I, Trellu M, Paquet N, Sibille M, Perez Y, Ortiz J. Neutralization by avidin of anticoagulant activity of biotinylated idraparinux, the first and unique reversible long‐acting anticoagulant. Journal of Thrombosis and Haemostasis. 2007; Vol. 5 (Suppl 2):O‐T‐050.
Paty 2010
- Paty I, Trellu M, Destors JM, Cortez P, Boelle E, Sanderink G. Reversibility of the anti‐FXa activity of idrabiotaparinux (biotinylated idraparinux) by intravenous avidin infusion. Journal of Thrombosis and Haemostasis 2010;8(4):722‐9. [DOI] [PubMed] [Google Scholar]
Pinede 2001
- Pinede L, Ninet J, Duhaut P, Chabaud S, Demolombe‐Rague S, Durieu I, et al. Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation 2001;103(20):2453‐60. [DOI] [PubMed] [Google Scholar]
Prandoni 2008
- Prandoni P, Tormene D, Perlati M, Brandolin B, Spiezia L. Idraparinux: review of its clinical efficacy and safety for prevention and treatment of thromboembolic disorders. Expert Opinion on Investigational Drugs 2008;17(5):773‐7. [DOI] [PubMed] [Google Scholar]
Salazar 2010
- Salazar CA, Malaga G, Malasquez G. Direct thrombin inhibitors versus vitamin K antagonists or low molecular weight heparins for prevention of venous thromboembolism following total hip or knee replacement. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD005981.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Savi 2008
- Savi P, Herault JP, Duchaussoy P, Millet L, Schaeffer P, Petitou M, et al. Reversible biotinylated oligosaccharides: a new approach for a better management of anticoagulant therapy. Journal of Thrombosis and Haemostasis 2008;6(10):1697‐706. [DOI] [PubMed] [Google Scholar]
Shoda 2015
- Shoda N, Yasunaga H, Horiguchi H, Fushimi K, Matsuda S, Kadono Y, et al. Prophylactic effect of fondaparinux and enoxaparin for preventing pulmonary embolism after total hip or knee arthroplasty: a retrospective observational study using the Japanese Diagnosis Procedure Combination database. Modern Rheumatology / The Japan Rheumatism Association 2015;25(4):625‐9. [DOI: 10.3109/14397595.2014.997424] [DOI] [PubMed] [Google Scholar]
Southworth 2013
- Southworth MR, Reichman ME, Unger EF. Dabigatran and post marketing reports of bleeding. New England Journal of Medicine 2013;368:1272‐4. [DOI] [PubMed] [Google Scholar]
Stoldt 1997
- Stoldt HS, Aftab F, Chinol M, Paganelli G, Luca F, Testori A, et al. Pretargeting strategies for radio‐immuno guided tumour localisation and therapy. European Journal of Cancer 1997;33(2):186‐92. [DOI] [PubMed] [Google Scholar]
Turpie 2003
- Turpie AG, Eriksson BI, Bauer KA, Lassen MR. New pentasaccharides for the prophylaxis of venous thromboembolism: clinical studies. Chest 2003;124(6 Suppl):371S‐8S. [PubMed] [Google Scholar]
Turpie 2003a
- Turpie AG. Future therapeutic directions for factor Xa inhibition in the prophylaxis and treatment of thrombotic disorders. American Journal of Health‐System Pharmacy 2003;60(22 Suppl 7):S20‐4. [DOI] [PubMed] [Google Scholar]
Uchino 2012
- Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta‐analysis of non inferiority randomized controlled trials. Archives of Internal Medicine 2012;172:397–402. [DOI] [PubMed] [Google Scholar]
Veyrat‐Follet 2009
- Veyrat‐Follet C, Vivier N, Trellu M, Dubruc C, Sanderink GJ. The pharmacokinetics of idraparinux, a long‐acting indirect factor Xa inhibitor: population pharmacokinetic analysis from Phase III clinical trials. Journal of Thrombosis and Haemostasis 2009;7(4):559‐65. [DOI] [PubMed] [Google Scholar]
Yusuf 1985
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomised trials. Progress in Cardiovascular Diseases 1985;27(5):335‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Nichol 2005
- Nichol IIE, Stansby GP, Kesteven P. Pentasaccharides for the prevention of venous thromboembolism. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD005134] [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2011
- Song Y, Liu L, Li X, Ding J. Pentasaccharides for the prevention of venous thromboembolism. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD005134.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


