Abstract
Background
Pulmonary embolism is a potentially life‐threatening condition in which a clot can travel from the deep veins, most commonly in the leg, up to the lungs. Previously, a pulmonary embolism was treated with the anticoagulants heparin and vitamin K antagonists. Recently, however, two forms of direct oral anticoagulants (DOACs) have been developed: oral direct thrombin inhibitors (DTI) and oral factor Xa inhibitors. The new drugs have characteristics that may be favourable over conventional treatment, including oral administration, a predictable effect, lack of frequent monitoring or re‐dosing and few known drug interactions. To date, no Cochrane review has measured the effectiveness and safety of these drugs in the long‐term treatment (minimum duration of three months) of pulmonary embolism.
Objectives
To assess the effectiveness of oral DTIs and oral factor Xa inhibitors for the long‐term treatment of pulmonary embolism.
Search methods
The Cochrane Vascular Trials Search Co‐ordinator searched the Specialised Register (last searched January 2015) and the Cochrane Register of Studies (last searched January 2015). Clinical trials databases were also searched for details of ongoing or unpublished studies. We searched the reference lists of relevant articles retrieved by electronic searches for additional citations.
Selection criteria
We included randomised controlled trials in which patients with a pulmonary embolism confirmed by standard imaging techniques were allocated to receive an oral DTI or an oral factor Xa inhibitor for the long‐term (minimum duration three months) treatment of pulmonary embolism.
Data collection and analysis
Two review authors (LR, JM) independently extracted the data and assessed the risk of bias in the trials. Any disagreements were resolved by discussion with the third author (PK). We used meta‐analyses when we considered heterogeneity low. The two primary outcomes were recurrent venous thromboembolism and pulmonary embolism. Other outcomes included all‐cause mortality and major bleeding. We calculated all outcomes using an odds ratio (OR) with a 95% confidence interval (CI).
Main results
We included five randomised controlled trials with a total of 7897 participants. Two studies tested oral DTIs (dabigatran) and three studies tested oral factor Xa inhibitors (one rivaroxaban, one edoxaban and one apixaban).
Analysis showed no difference in the effectiveness of oral DTIs and standard anticoagulation in preventing recurrent pulmonary embolism (OR 1.02, 95% CI 0.50 to 2.04; two studies; 1602 participants; high quality evidence), recurrent venous thromboembolism (OR 0.93, 95% CI 0.52 to 1.66; two studies; 1602 participants; high quality evidence), deep vein thrombosis (DVT) (OR 0.79, 95% CI 0.29 to 2.13; two studies; 1602 participants; high quality evidence) and major bleeding (OR 0.50, 95% CI 0.15 to 1.68; two studies; 1527 participants; high quality evidence).
For oral factor Xa inhibitors, when we combined the three included studies together in meta‐analyses, there was significant heterogeneity for recurrent pulmonary embolism (OR 1.08, 95% CI 0.46 to 2.56; two studies; 4509 participants; I2 = 58%; moderate quality evidence). The oral factor Xa inhibitors were no more or less effective in the prevention of recurrent venous thromboembolism (OR 0.85, 95% CI 0.63 to 1.15; three studies; 6295 participants; high quality evidence), DVT (OR 0.72, 95% CI 0.39 to 1.32; two studies; 4509 participants; high quality evidence), all‐cause mortality (OR 1.16, 95% CI 0.79 to 1.70; one study; 4817 participants; moderate quality evidence) or major bleeding (OR 0.97, 95% CI 0.59 to 1.62; two studies; 4507 participants; high quality evidence). None of the studies measured quality of life.
Authors' conclusions
There is no evidence of a difference between oral DTIs and standard anticoagulation in the prevention of recurrent pulmonary embolism. Using the GRADE criteria, the quality of evidence was high. The evidence of the effectiveness of oral factor Xa inhibitors for the prevention of recurrent pulmonary embolism was too heterogenous to combine in a pooled analysis. For the outcomes recurrent venous thromboembolism, DVT, all‐cause mortality and major bleeding there is no evidence of a difference between DOACs and standard anticoagulation. According to GRADE criteria, the quality of evidence was moderate to high.
Plain language summary
Novel oral anticoagulants (DOACs) for the treatment of pulmonary embolism
Background
Venous thromboembolism is a condition where a blood clot forms in the deep veins (DVT) (most commonly of the leg) and can travel up to block the arteries in the lungs (pulmonary embolism). Pulmonary embolism is life‐threatening and occurs in approximately 3 to 4 per 10,000 people. The chances of getting a pulmonary embolism can increase with risk factors, including previous clots, prolonged periods of immobility (such as travelling on aeroplanes or bed rest), cancer, exposure to oestrogens (pregnancy, oral contraceptives or hormone replacement therapy), blood disorders (thrombophilia) and trauma. A pulmonary embolism is diagnosed by determining the risk factors and scanning the lungs to check for a clot. If a pulmonary embolism is confirmed, patients are treated with an anticoagulant. This prevents further clots from forming. Until recently, the drugs of choice were heparin, fondaparinux and vitamin K antagonists. However, these drugs can cause side effects and have limitations. Recently two classes of direct oral anticoagulants (DOACs) have been developed: direct thrombin inhibitors (DTI) and factor Xa inhibitors. There are particular reasons why oral DTIs and factor Xa inhibitors might be better medicines to use. They can be given orally, they have a predictable effect, they do not require frequent monitoring or re‐dosing and they have few known drug interactions. This review measures the effectiveness and safety of these new drugs compared with conventional treatment.
Key results
After searching for relevant studies up to January 2015, we found five studies with a combined total of 7897 participants. Studies compared oral direct thrombin inhibitors and factor Xa inhibitors with conventional treatment. We looked at whether treatment for three months prevented further blood clots and pulmonary embolism. The main safety outcomes included mortality and adverse events such as bleeding. This review showed that there was no evidence of a difference between direct thrombin inhibitors and standard treatment in preventing recurrent clots in the lungs or legs. For oral factor Xa inihibitors and the prevention of recurrent clots in the lungs, the studies were too different to form any meaningful conclusion. Furthermore, there was no evidence of a difference in recurrent venous thromboembolism, DVT, mortality or bleeding. No study measured health‐related quality of life.
Quality of the evidence
For the outcomes recurrent pulmonary embolism and all‐cause mortality when comparing oral factor Xa inhibitors and standard anticoagulation we downgraded the quality of the evidence from high to moderate due to the differences in results between the studies and the small number of studies included in this review. The quality of the evidence for all other outcomes was high.
Summary of findings
Background
Description of the condition
Pulmonary embolism is a potentially life‐threatening condition in which a blood clot blocks the supply to the lungs. Pulmonary embolism is often a consequence of a thrombus in the deep veins of the legs (deep vein thrombosis (DVT)) that dislodges and travels in the blood to the pulmonary arteries. The prevalence of pulmonary embolism has been estimated as 3 to 4 per 10,000 people, although the true prevalence is hard to measure due to underestimation by diagnostic imaging and overestimation by postmortem data. DVT is present in approximately 70% to 80% of people with a pulmonary embolism, yet only 15% of pulmonary embolism cases have symptoms of DVT (Huerta 2007). One complication of pulmonary embolism is chronic thromboembolic pulmonary hypertension (CTPH). CTPH occurs when the clot obstructs the pulmonary arteries causing excessive pressure in the pulmonary artery and stress to the right ventricle. CTPH is less common but it can result in heart failure (NICE 2012a).
Risk factors for pulmonary embolism are similar to those for DVT and are classified as provoked or unprovoked (Kearon 2012). Provoked pulmonary embolism occurs following surgery or pregnancy, or by a non‐surgical transient risk factor such as a history of venous thromboembolism, venous insufficiency, chronic heart failure, thrombophilia, obesity, immobility (such as prolonged travel, acute medical illness or hospitalisation), cancer, oestrogens (pregnancy, use of oral contraceptives or hormone replacement therapy) and trauma (SIGN 2010).
Diagnosis of pulmonary embolism is made by general assessment of the patient's medical history, physical examination and clinical pre‐test probability. However, it can be particularly challenging as the symptoms (dyspnoea, pleuritic chest pain, retrosternal chest pain, cough and haemoptysis) are not specific (NICE 2012a). In severe cases, the right ventricle fails leading to dizziness, syncope, tachypnoea, tachycardia, hypoxia, elevated jugular venous pressure, systemic hypotension and cardiogenic shock (NICE 2012a). The UK National Institute for Health and Care Excellence recommend that people presenting with a suspected pulmonary embolism should be assessed using a two‐level pulmonary embolism Wells score (NICE 2012a; Wells 2000). Points are awarded for clinical features present including clinical signs of DVT, heart rate greater than 100 beats per minute, recent immobilisation or surgery, previous DVT, haemoptysis and malignancy (Wells 2000). For patients with a low pre‐test probability, the use of a D‐dimer assay combined with a clinical prediction rule has a high negative predictive value and avoids the need for unnecessary imaging (Qaseem 2007). However, for patients who have intermediate or high pre‐test probability of pulmonary embolism, imaging is essential. Patients with a score of greater than 4 are judged to be likely to have had a pulmonary embolism and should undergo immediate diagnostic imaging. If this cannot be performed immediately, patients should be given immediate interim parenteral anticoagulant therapy until the imaging test is done. Patients with a negative diagnosis in whom a DVT is likely should be given a proximal leg vein ultrasound scan (NICE 2012a).
There are two types of imaging technique used to diagnose pulmonary embolism: computed tomography pulmonary angiogram (CTPA) and ventilation perfusion (V/Q) scan.
1. Computed tomography pulmonary angiogram
CTPA involves injecting a contrast agent intravenously and performing a computed tomography (CT) scan of the chest to visualise the pulmonary arteries and detect any thrombi in the pulmonary arteries down to the subsegmental branches. The procedure has over 90% specificity and sensitivity in diagnosing pulmonary embolism in the main, lobar and segmental pulmonary arteries (Riedel 2004). However, the radiation dose administered to the patient is much larger than that of a V/Q scan, and thus patients who have a CTPA may be at an increased life‐time risk of cancer (Anderson 2009). CTPA is contraindicated in patients who have an allergy to contrast media, renal impairment or in whom the risk of radiation is too high. In these patients, a V/Q scan is performed instead (NICE 2013).
2. Ventilation perfusion scan
A V/Q scan comprises of two parts: the ventilation part where the patient breathes in a radioisotope (in the form of a gas or an aerosol) and the perfusion part where the patient is given an intravenous injection of the isotope. A gamma camera is used to detect where the isotopes are in the lungs and the images show which areas of the lungs are ventilated but not perfused (NICE 2012a). Another version of this test, the V/Q single photon emission computed tomography (V/Q SPECT) has been developed. The camera is rotated around the patient thus generating three‐dimensional images and leading to a more accurate diagnosis (Laurence 2012).
Description of the intervention
Until recently, standard treatment of a pulmonary embolism was with an indirect thrombin inhibitor, namely unfractionated heparin (UFH), low molecular weight heparin (LMWH) or vitamin K antagonists (VKAs). These drugs block the action of thrombin either by "activating naturally occurring thrombin inhibitors or by inhibiting specific factors in the coagulation system that subsequently impact on thrombin generation or activity" (Weitz 2003). Present guidelines recommend initial therapy for pulmonary embolism with a parenteral anticoagulant (UFH or LMWH or fondaparinux) and initial VKA initiation (Kearon 2012). Recommendations include the use of LMWH or fondaparinux over UFH for initial therapy of pulmonary embolism. Although heparin and VKAs are effective anticoagulants, there are limitations associated with each. LMWH must be administered parenterally and may be associated with an increased risk of bleeding and haemodynamic instability (Kearon 2012). Meanwhile VKAs have a narrow therapeutic window, require frequent monitoring and dosage adjustments, and can have multiple interactions with other drugs (Ageno 2012).
Two further classes of oral anticoagulants have been developed: direct thrombin inhibitors (DTI) and factor Xa inhibitors. DTIs and factor Xa inhibitors have characteristics that may be favourable over heparin and VKAs, including ease of oral administration, a predictable effect, lack of frequent monitoring or re‐dosing and fewer known drug interactions (compared with VKA) (Fox 2012).
How the intervention might work
Oral direct thrombin inhibitors
DTIs work by binding directly to the enzyme thrombin without the need for a co‐factor such as antithrombin. Unlike heparins and VKAs, DTIs can inhibit both soluble thrombin and fibrin‐bound thrombin (Kam 2005). Other advantages include a more predictable anticoagulant effect because of their lack of binding to other proteins, lack of an antiplatelet effect and no suspected concern of heparin‐induced thrombocytopenia (HIT) (Lee 2011). There are several types of DTIs.
1. Dabigatran
Dabigatran etexilate is a reversible oral DTI that is metabolised to its active ingredient, dabigatran, in the gastrointestinal tract (Ageno 2012). It does not require anticoagulation monitoring, is excreted by the kidneys and has a half‐life of 12 to 17 hours. As well as a treatment for venous thrombosis, this drug has been involved in many large randomised studies of atrial fibrillation (Connolly 2009), acute coronary syndromes (Oldgren 2011), and prevention of thrombosis following orthopaedic surgery (Eriksson 2007), and in patients with mechanical heart valves (Van de Werf 2012). In common with the other novel oral anticoagulants, dabigatran is associated with a lower incidence of intracranial haemorrhage (compared with VKA). However, again compared with VKA, dabigatran showed a higher incidence of indigestion and heartburn and a higher incidence of gastrointestinal bleeding. Dabigatran, in the atrial fibrillation studies, showed a tendency (although ultimately not statistically significant) to increased incidence of myocardial infarction (Baetz 2008).
2. Ximelagatran
Ximelagatran is a prodrug that is metabolised to melagatran as it is better absorbed from the gastrointestinal tract (Kam 2005). It has a plasma half‐life of three hours, has a predictable response after oral administration and does not require coagulation monitoring. Ximelagatran was found to be effective in the treatment of venous thromboembolism but caused unacceptable liver toxicity (Boudes 2006), and was, therefore, never licensed.
Oral factor Xa inhibitors
Factor Xa inhibitors bind directly to the active site of factor Xa, thus blocking the activity of the clotting factor. Unlike indirect factor Xa inhibitors such as fondaparinux, direct factor Xa inhibitors "inactivate free FXa and FXa incorporated with the prothrombinase complex equally well" and do not require interaction with the inhibitor antithrombin (Eriksson 2009). They have been shown to be non‐inferior to VKA but without the need for regular blood test monitoring. They appear to have fewer drug interactions (compared with VKA) and no food or alcohol interactions
1. Rivaroxaban
Rivaroxaban is a reversible direct factor Xa inhibitor. For the initial treatment of acute pulmonary embolism, the recommended dosage of rivaroxaban is 15 mg twice daily for the first 21 days followed by 20 mg once daily for continued treatment and prevention of recurrence (NICE 2012b). The plasma half‐life, if renal function is normal, is estimated to be 8 to 10 hours (Spyropoulos 2012).
2. Apixaban
Apixaban is an oral, small molecule, reversible inhibitor of factor Xa with a plasma half‐life of 8 to 15 hours, taken twice daily (Eriksson 2009).
3. Betrixaban
Betrixaban is an orally administered direct factor Xa inhibitor. It also has a half‐life of 15 hours, offers the convenience of once daily dosing and may exhibit fewer drug interactions than warfarin (Palladino 2013).
4. Edoxaban
Edoxaban is an oral direct inhibitor of activated factor X that is rapidly absorbed with a half‐life of 9 to 11 hours. Edoxaban has a dual mechanism of elimination with one‐third eliminated via the kidneys and the remainder excreted in the faeces. It also offers the convenience of once‐daily dosing (Eikelboom 2010), and is used in conjunction with LMWH for five days.
Why it is important to do this review
The effectiveness of oral DTIs and oral factor Xa inhibitors for the treatment of venous thromboembolism has been studied in several randomised controlled trials (EINSTEIN‐DVT Study (EINSTEIN Investigators), ODIXa‐DVT Study (Agnelli 2007), Botticelli Study (Botticelli Investigators), AMPLIFY Study (Agnelli 2013), RE‐COVER II Study (Schulman 2011), THRIVE Studies (Eriksson 2003)). One non‐Cochrane systematic review has examined the effectiveness of DTIs and factor Xa inhibitors versus VKAs in the treatment of acute venous thromboembolism (Fox 2012). The primary outcome was venous thromboembolism and results were not presented for DVT and pulmonary embolism separately. To date, no systematic review has been conducted examining the effectiveness of oral inhibitors in the treatment of pulmonary embolism alone.
A separate Cochrane systematic review assessing the effectiveness of oral DTIs and oral factor Xa inhibitors for the treatment of DVT was published recently (Robertson 2015).
Objectives
To assess the effectiveness of oral DTIs and oral factor Xa inhibitors for the long‐term treatment of pulmonary embolism.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials in which patients with a confirmed pulmonary embolism were allocated to receive an oral DTI or an oral factor Xa inhibitor for the treatment of pulmonary embolism. We included published studies and studies in progress if preliminary results were available. We placed no restrictions on publication status and non‐English studies were eligible for inclusion in the review. We exclude DTIs and factor Xa inhibitors that were not given by the oral route.
Types of participants
Patients with a pulmonary embolism, confirmed by standard imaging techniques (CTPA, V/Q scan).
Types of interventions
Oral DTIs (e.g. dabigatran, ximelagatran) (although ximelagatran was withdrawn from the market in 2006 due to safety issues, we have included it in the review to make the results as comprehensive as possible).
Oral factor Xa inhibitors (e.g. rivaroxaban, apixaban, betrixaban, edoxaban).
Other anticoagulants (e.g. LMWH, UFH, VKAs).
Comparisons included:
One oral DTI versus another oral DTI.
One oral factor Xa inhibitor versus another oral factor Xa inhibitor.
Oral DTI versus oral factor Xa inhibitor.
Oral DTI or oral factor Xa inhibitor versus another anticoagulant.
Treatment had to be for a minimum duration of three months as this is standard anticoagulation practice for a pulmonary embolism.
Types of outcome measures
Primary outcomes
Recurrent pulmonary embolism, confirmed by standard imaging techniques (CTPA, V/Q scan).
Recurrent venous thromboembolism (clinically overt DVT, confirmed by standard imaging techniques including proximal leg vein ultrasound scan or D‐dimer test, or both; or clinically overt pulmonary embolism, confirmed by CTPA or V/Q scan, or both).
Clinically overt DVT confirmed by standard imaging techniques (proximal leg vein ultrasound scan, venography) or D‐dimer test, or both.
Secondary outcomes
All‐cause mortality.
Adverse effects of treatment including major bleeding (as defined by the International Society on Thrombosis and Haemostasis (ISTH); Schulman 2005).
Fatal bleeding.
Symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome.
Bleeding causing a fall in haemoglobin level of 20 g/L (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells.
Any combination of points 1 to 3.
Health‐related quality of life, as reported in included studies.
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched January 2015) and the Cochrane Register of Studies (CRS) (http://www.metaxis.com/CRSWeb/Index.asp) (last searched January 2015). See Appendix 1 for details of the search strategy used to search the CRS. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL and AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Vascular module in The Cochrane Library (www.cochranelibrary.com)
The TSC also searched the following trial databases for details of ongoing and unpublished studies using the terms apixaban or betrixaban or dabigatran or edoxaban or rivaroxaban or ximelagatran.
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/).
ClinicalTrials.gov (clinicaltrials.gov/).
ISRCTN Register (http://www.isrctn.com/).
Searching other resources
We searched the reference lists of relevant articles retrieved by the electronic searches for additional citations.
Data collection and analysis
Selection of studies
One review author (LR) used the selection criteria to identify trials for inclusion and the second review author (JM) independently confirmed this selection. We resolved any disagreements by discussion.
Data extraction and management
Two review authors (LR, JM) independently extracted the data from the included studies. We recorded information about the trial design, diagnosis of pulmonary embolism, baseline characteristics of participants and type of prophylaxis. We recorded recurrent pulmonary embolism (fatal and non‐fatal) and DVT data as the primary outcome measures. We collected data on all‐cause mortality and adverse effects of treatment including clinically relevant bleeding and health‐related quality of life in accordance with the secondary outcome measures. We contacted authors of included studies if further information or clarification was required. We resolved any disagreements in data extraction and management by discussion and sought the opinion of the third author (PK) and an expert, if required.
Assessment of risk of bias in included studies
Two review authors (LR, JM) independently used the Cochrane 'Risk of bias' tool for assessing risk of bias for each of the included studies (Higgins 2011). The tool provides a protocol for judgements on sequence generation, allocation methods, blinding, incomplete outcome data, selective outcome reporting and any other relevant biases. We judged each of these domains as either high, low or unclear risk of bias according to Higgins 2011, and provided support for each judgement. We presented the conclusions in a 'Risk of bias' table. We resolved any disagreements by discussion with the third author (PK).
Measures of treatment effect
We based the analysis on intention‐to‐treat data from the individual clinical trials. As the primary and secondary outcomes were all binary measures, we computed odds ratios (ORs) using a fixed‐effect model and calculated the 95% confidence intervals (CI) for the effect sizes.
Unit of analysis issues
The unit of analysis in this review was the individual patient.
Dealing with missing data
We sought information about drop‐outs, withdrawals and other missing data and, if not reported, we contacted study authors for this information.
Assessment of heterogeneity
We assessed heterogeneity between the trials by visual examination of the forest plot to check for overlapping CIs, the Chi2 test for homogeneity with a 10% level of significance and using the I2 statistic to measure the degree of inconsistency between the studies. An I2 result of greater than 50% represented moderate to substantial heterogeneity (Deeks 2011).
Assessment of reporting biases
We planned to assess publication bias by funnel plots if a sufficient number of studies (10 or more) were available in the meta analyses. There are many reasons for funnel plot asymmetry, and we planned consult the Cochrane Handbook for Systematic Reviews of Interventions to aid the interpretation of the results (Sterne 2011).
Data synthesis
The review authors independently extracted the data. One review author (LR) input the data into Review Manager 5 (RevMan 2014), and the second review author (JM) cross‐checked data entry. We resolved any discrepancies by consulting the source publication.
We used a fixed‐effect model to meta‐analyse the data. If the I2 statistic indicated heterogeneity greater than 50%, we performed a random‐effects model analysis instead of a fixed‐effect model analysis.
Subgroup analysis and investigation of heterogeneity
History of venous thromboembolism.
Age.
Active cancer (treatment within last six months or palliative).
Pregnancy.
Major surgery requiring general or regional anaesthesia in the previous 12 weeks.
Recent period of immobility (bedridden three or more days in the previous 12 weeks).
Thrombophilia (genetic or acquired).
Sensitivity analysis
We planned to perform sensitivity analyses by excluding studies that we judged to be at high risk of bias. We also planned to perform sensitivity analyses with and without ximelagatran a priori given that this drug is no longer available. However, we found no studies that tested ximelagatran in patients with a pulmonary embolism.
'Summary of findings' table
We presented the main findings of the review results concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data for all outcomes of this review (Types of outcome measures) in a 'Summary of findings' table, according to the GRADE principles as described by Higgins 2011 and Atkins 2004. We used the GRADEprofiler (GRADEpro) software to assist in the preparation of the 'Summary of findings' table (www.guidelinedevelopment.org).
Results
Description of studies
Results of the search
See Figure 1.
1.
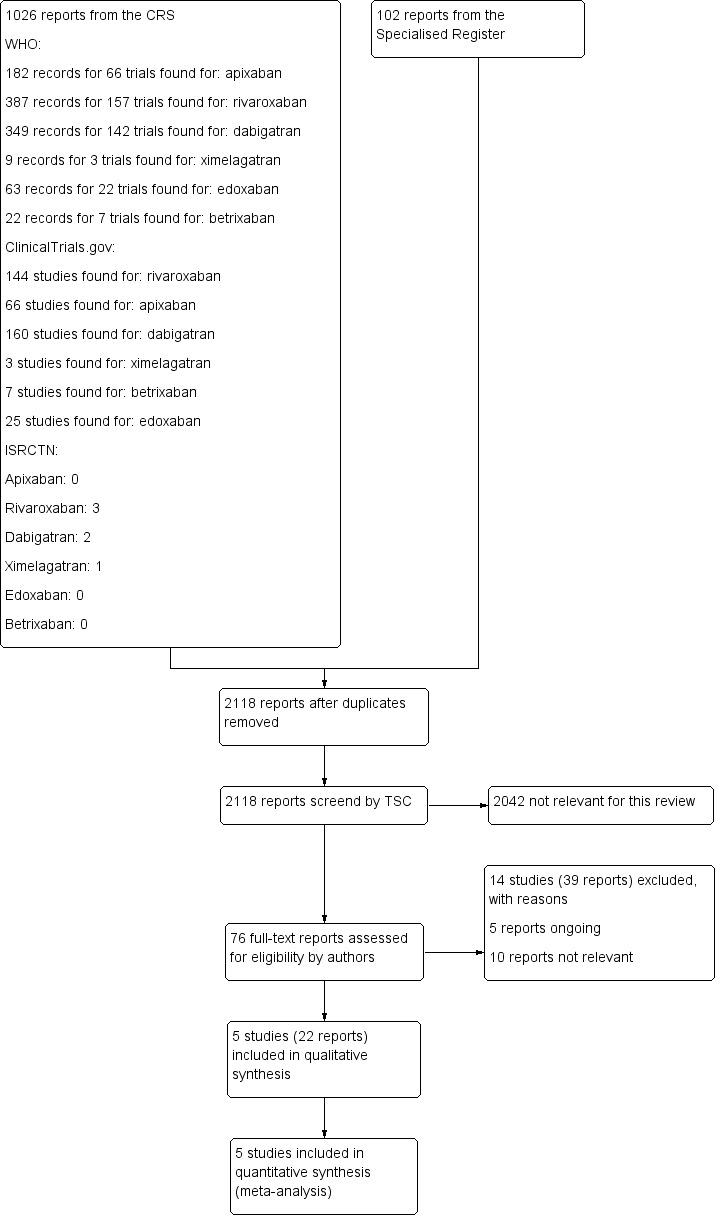
Study flow diagram.
Included studies
Five randomised controlled trials met the inclusion criteria for this review (AMPLIFY Study; EINSTEIN‐PE; Hokusai‐VTE Study; RE‐COVER; RE‐COVER II). See Characteristics of included studies.
The AMPLIFY Study was a double‐blind study in which 5395 patients with a DVT or pulmonary embolism were randomised to receive oral apixaban 10 mg twice daily for the first seven days, followed by 5 mg twice daily for six months or enoxaparin 1 mg/kg body weight every 12 hours for at least five days and warfarin concomitantly for six months. Patients were followed up for six months. Outcomes included a composite of recurrent symptomatic venous thromboembolism (fatal or non‐fatal pulmonary embolism and DVT), mortality related to venous thromboembolism, major bleeding and clinically relevant non‐major bleeding.
The EINSTEIN‐PE study was an open‐label study in which 4832 patients were randomised to receive oral rivaroxaban 15 mg twice daily for the first three weeks, followed by 20 mg once daily (n = 2419) or enoxaparin 1.0 mg per kg of body weight twice daily and either warfarin or acenocoumarol, started within 48 hours of randomisation (n = 2413). Participants were followed up at three, six and 12 months and outcomes included recurrent pulmonary embolism, recurrent DVT, major bleeding and all‐cause mortality.
The Hokusai‐VTE Study was a double‐blind study in which 4921 participants were randomised to receive 60 mg oral edoxaban once daily (n = 2468) or dose‐adjusted warfarin therapy and edoxaban‐like placebo (n = 2453). Outcomes were measured monthly for one year. Results were presented for all patients with a venous thromboembolism but specific outcome data for the subset of participants with a pulmonary embolism were obtained through communication with the author.
RE‐COVER was a phase III non‐inferiority, double‐blind, double‐dummy trial in which patients with a venous thromboembolism (n = 2539) were given 150 mg dabigatran twice daily or warfarin. In addition, initial treatment with an approved parenteral anticoagulant (unfractionated heparin administered intravenously or low molecular weight heparin administered subcutaneously) was started before patients were randomised. Treatment was for a period of six months and included sham monitoring of international normalised ratio (INR) and sham titration of warfarin in the control group. To gain regulatory approval, the study was repeated with an identical design (RE‐COVER II).
Excluded studies
See Characteristics of excluded studies.
We excluded 13 studies (Ageno 2014; AMPLIFY Extended Study; Botticelli DVT Study; Einstein‐DVT Dose Study; Einstein DVT Study; EINSTEIN Extension Study; ODIXa‐DVT Study; Piazza 2014; REMEDY; RE‐SONATE; THRIVE; THRIVE I; THRIVE III). We excluded five studies as patients had a DVT only (Botticelli DVT Study; Einstein‐DVT Dose Study; Einstein DVT Study; ODIXa‐DVT Study; Piazza 2014). We excluded one study as although all patients had a venous thromboembolism, specific data on the subgroup with a pulmonary embolism was not published (THRIVE I). We made attempts to contact the authors for these data but were unsuccessful. We excluded three studies as they were extended studies testing the effectiveness of DOACs as prophylaxis rather than the treatment of pulmonary embolism (AMPLIFY Extended Study; EINSTEIN Extension Study; REMEDY). We excluded the THRIVE study as treatment was for less than three months, while we excluded the THRIVE III study as the control arm was a placebo. We excluded one study as it was not a randomised controlled trial (Ageno 2014). Finally, we excluded the REMEDY study from this review as participants were already included in the RE‐COVER and RE‐COVER II studies.
Risk of bias in included studies
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
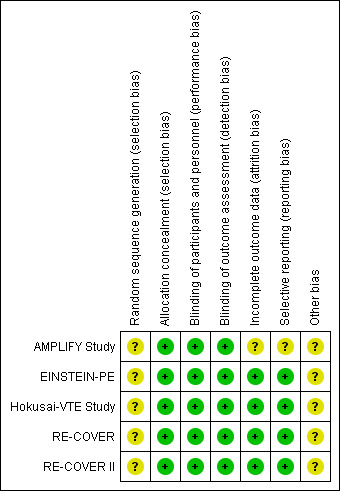
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All five included studies stated that they used a computerised system to conceal treatment allocation. However, the authors did not state the method by which the random sequence was generated and therefore we deemed the risk of selection bias relating to random sequence generation to be unclear. All five included studies reported that treatment allocation was concealed with the use of a computerised system and we therefore judged them at low risk of selection bias for allocation concealment.
Blinding
The EINSTEIN‐PE study was open‐label as the treatment arms comprised of rivaroxaban administered orally and subcutaneous enoxaparin. Therefore, blinding of participants and personnel was not possible. However, we judged that the lack of blinding in the control group was unlikely to have affected the outcome and therefore judged it to have a low risk of performance bias. The AMPLIFY Study, RE‐COVER, RE‐COVER II and Hokusai‐VTE Study were double‐blind and therefore we judged them to be at low risk of performance bias.
All studies used independent committees, whose members were unaware of the study group assignments, to adjudicate all suspected outcomes and the results of baseline imaging tests. Therefore we judged all included studies to be at low risk of detection bias.
Incomplete outcome data
Four studies accounted for all missing data and we judged them to be at low risk of attrition bias (EINSTEIN‐PE; Hokusai‐VTE Study; RE‐COVER; RE‐COVER II). The AMPLIFY Study inappropriately excluded a number of randomised patients from the intention‐to‐treat (ITT) analysis. Furthermore, a large number of patients within each treatment group were classified as discontinuing the study for "other reasons" with no given explanations and therefore we deemed the risk of attrition bias to be unclear
Selective reporting
Protocols were available for four studies (EINSTEIN‐PE; Hokusai‐VTE Study; RE‐COVER; RE‐COVER II). Furthermore, the study outcomes were clearly pre‐specified and data on the outcomes were presented. Therefore we judged these studies to be at low risk of reporting bias. The AMPLIFY Study pre‐defined minor bleeding as a secondary outcome but data were not reported in the paper and therefore we deemed the risk of reporting bias in this study to be unclear.
Other potential sources of bias
All five studies were funded by the pharmaceutical companies that manufacture dabigatran, rivaroxaban and edoxaban. This potentially could have influenced the time frame of reported safety outcomes and therefore we deemed the risk of other bias to be unclear. In addition, the AMPLIFY Study analysed non‐inferiority using an ITT analysis. When compared with the per‐protocol analysis, ITT favoured the finding of non‐inferior results. This may have skewed the result in favour of an increased efficacy of apixaban.
Effects of interventions
Summary of findings for the main comparison. Oral direct thrombin inhibitors (DTIs) versus standard anticoagulation for the treatment of pulmonary embolism.
| Oral direct thrombin inhibitors (DTIs) versus standard anticoagulation for the treatment of pulmonary embolism | ||||||
| Patient or population: patients with a pulmonary embolism, confirmed by standard imaging techniques Setting: hospital Intervention: oral direct thrombin inhibitors (DTIs) Comparison: standard anticoagulation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with standard anticoagulation | Risk with Oral DTI | |||||
| Recurrent pulmonary embolism1 | Study population | OR 1.02 (0.50 to 2.04) | 1602 (1 RCT) | ⊕⊕⊕⊕ HIGH2 3 | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 20 per 1000 | 20 per 1000 (10 to 40) | |||||
| Recurrent venous thromboembolism 4 | Study population | OR 0.93 (0.52 to 1.66) | 1602 (1 RCT) | ⊕⊕⊕⊕ HIGH2 3 | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 31 per 1000 | 29 per 1000 (16 to 50) | |||||
| Deep vein thrombosis5 | Study population | OR 0.79 (0.29 to 2.13) | 1602 (1 RCT) | ⊕⊕⊕⊕ HIGH2 3 | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 11 per 1000 | 9 per 1000 (3 to 23) | |||||
| All‐cause mortality | See comment | See comment | See comment | ‐ | The 2 RECOVER studies did not report on all‐cause mortality | |
| Major bleeding6 | Study population | OR 0.50 (0.15 to 1.68) | 1527 (1 RCT) | ⊕⊕⊕⊕ HIGH2 3 | The data from the 2 RECOVER studies were taken from 1 pooled analysis and are therefore shown as 1 study | |
| 10 per 1000 | 5 per 1000 (2 to 17) | |||||
| Health‐related quality of life | See comment | See comment | See comment | ‐ | The 2 RECOVER studies did not measure health‐related quality of life | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Confirmed by ventilation–perfusion lung scanning, angiography or spiral computed tomography of pulmonary arteries. 2Risk of bias was 'unclear' for random sequence generation but we did not consider it sufficient to downgrade the quality of evidence. 3The possibility of publication bias is not excluded but we did not consider it sufficient to downgrade the quality of evidence. 4Clinically overt DVT, confirmed by standard imaging techniques including proximal leg vein ultrasound scan or D‐dimer test, or both; or clinically overt pulmonary embolism, confirmed by ventilation–perfusion lung scanning, angiography or spiral computed tomography of pulmonary arteries. 5Clinically overt DVT confirmed by standard imaging techniques (proximal leg vein ultrasound scan, venography) or D‐dimer test, or both. 6As defined by the International Society on Thrombosis and Haemostasis (ISTH) (Schulman 2005). Fatal bleeding; symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome; bleeding causing a fall in haemoglobin level of 20 g/L (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells; any combination of points 1 to 3.
Summary of findings 2. Oral factor Xa inhibitors versus standard anticoagulation for the treatment of pulmonary embolism.
| Oral factor Xa inhibitors versus standard anticoagulation for the treatment of pulmonary embolism | ||||||
| Patient or population: patients with a pulmonary embolism, confirmed by standard imaging techniques Setting: hospital Intervention: oral factor Xa inhibitors Comparison: standard anticoagulation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with standard anticoagulation | Risk with oral factor Xa | |||||
| Recurrent pulmonary embolism1 | Study population | OR 1.08 (0.46 to 2.56) | 4509 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 2 3 4 | ‐ | |
| 22 per 1000 | 24 per 1000 (10 to 55) | |||||
| Recurrent venous thromboembolism5 | Study population | OR 0.85 (0.63 to 1.15) | 6295 (3 RCTs) | ⊕⊕⊕⊕ HIGH 2 4 | ‐ | |
| 24 per 1000 | 20 per 1000 (15 to 27) | |||||
| Deep vein thrombosis6 | Study population | OR 0.72 (0.39 to 1.32) | 4509 (2 RCTs) | ⊕⊕⊕⊕ HIGH 4 | ‐ | |
| 11 per 1000 | 8 per 1000 (4 to 15) | |||||
| All‐cause mortality | Study population | OR 1.16 (0.79 to 1.70) | 4817 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 4 7 | ‐ | |
| 16 per 1000 | 19 per 1000 (13 to 27) | |||||
| Major bleeding8 | Study population | OR 0.97 (0.59 to 1.62) | 4507 (2 RCTs) | ⊕⊕⊕⊕ HIGH 2 4 | ‐ | |
| 14 per 1000 | 13 per 1000 (8 to 22) | |||||
| Health‐related quality of life | See comment | See comment | See comment | ‐ | The studies did not measure health‐related quality of life | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; PE: pulmonary embolism; RCT: randomised controlled trial; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Confirmed by ventilation–perfusion lung scanning, angiography or spiral computed tomography of pulmonary arteries. 2Risk of bias was 'unclear' for random sequence generation but we did not consider it sufficient to downgrade the quality of evidence. 3Statistical heterogeneity was found for this outcome and could not be explained. 4The possibility of publication bias is not excluded but we did not consider it sufficient to downgrade the quality of evidence as only two studies were included in this comparison. 5Clinically overt DVT, confirmed by standard imaging techniques including proximal leg vein ultrasound scan or D‐dimer test, or both; or clinically overt pulmonary embolism, confirmed by ventilation–perfusion lung scanning, angiography or spiral computed tomography of pulmonary arteries. 6Clinically overt DVT confirmed by standard imaging techniques (proximal leg vein ultrasound scan, venography) or D‐dimer test, or both. 7Quality of evidence downgraded to moderate as only one study was included. 8As defined by the International Society on Thrombosis and Haemostasis (ISTH); Schulman 2005). Fatal bleeding; symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome; bleeding causing a fall in haemoglobin level of 20 g/L (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells; any combination of points 1 to 3.
We identified two studies that compared an oral direct thrombin inhibitor (DTI) versus standard anticoagulation with warfarin (RE‐COVER; RE‐COVER II), and two studies that compared an oral factor Xa inhibitor versus standard anticoagulation with warfarin (EINSTEIN‐PE; Hokusai‐VTE Study). We did not find any studies comparing one DTI with another DTI, one factor Xa inhibitor with another factor Xa inhibitor, or an oral DTI with a factor Xa inhibitor.
1. Oral direct thrombin inhibitor versus standard anticoagulation
In the meta‐analysis of oral DTIs versus standard anticoagulation, we used data from a paper, Schulman 2011, which combined the RE‐COVER and RE‐COVER II studies. This is reflected in the data analysis tables and 'Summary of findings' table by showing only one study for this comparison (Table 1).
Recurrent pulmonary embolism
Two studies on a combined total of 1602 patients measured recurrent pulmonary embolism (RE‐COVER; RE‐COVER II). The rate of recurrent pulmonary embolism was similar between patients treated with dabigatran (16 events/795 participants) and those treated with standard anticoagulation (16 events/807 participants) leading to an odds ratio (OR) of 1.02 (95% confidence interval (CI) 0.50 to 2.04) (Analysis 1.1).
1.1. Analysis.
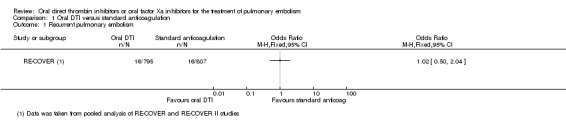
Comparison 1 Oral DTI versus standard anticoagulation, Outcome 1 Recurrent pulmonary embolism.
Recurrent venous thromboembolism
Two studies on a combined total of 1602 patients measured recurrent venous thromboembolism (RE‐COVER; RE‐COVER II). The rate of recurrent pulmonary embolism was similar between patients treated with dabigatran (23 events/795 participants) and those treated with standard anticoagulation (25 events/807 participants) leading to an OR of 0.93 (95% CI 0.52 to 1.66) (Analysis 1.2).
1.2. Analysis.

Comparison 1 Oral DTI versus standard anticoagulation, Outcome 2 Recurrent venous thromboembolism.
Deep vein thrombosis (DVT)
Two studies on a combined total of 1602 patients measured DVT (RE‐COVER; RE‐COVER II). The rate of DVT was similar between patients treated with dabigatran (seven events/795 participants) and those treated with standard anticoagulation (nine events/807 participants) leading to an OR of 0.79 (95% CI 0.29 to 2.13) (Analysis 1.3).
1.3. Analysis.

Comparison 1 Oral DTI versus standard anticoagulation, Outcome 3 Deep vein thrombosis.
All‐cause mortality
Neither study presented results on all‐cause mortality for the specific group of participants with pulmonary embolism.
Adverse effects of treatment
Both studies, RE‐COVER and RE‐COVER II, measured major bleeding (as defined by the International Society on Thrombosis and Haemostasis (ISTH); Schulman 2005). The rate of major bleeding was similar between patients treated with oral DTIs (four events/759 participants) and those treated with standard anticoagulation (eight events/768 participants) leading to an OR of 0.50 (95% CI 0.15 to 1.68) (Analysis 1.4).
1.4. Analysis.
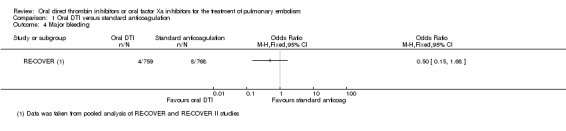
Comparison 1 Oral DTI versus standard anticoagulation, Outcome 4 Major bleeding.
Health‐related quality of life
Health‐related quality of life was not an outcome in any of the included studies.
2. Oral factor Xa inhibitor versus standard anticoagulation
See Table 2.
Recurrent pulmonary embolism
We included two studies on a combined total of 4509 patients in a meta‐analysis (EINSTEIN‐PE; Hokusai‐VTE Study). The rate of recurrent pulmonary embolism was similar between patients treated with oral factor Xa inhibitors (45 events/2253 participants) and those treated with standard anticoagulation (50 events/2256 participants), leading to an OR of 1.08 (95% CI 0.46 to 2.56). The I2 statistic was 58%, indicating significant heterogeneity. Therefore we used a random‐effects model in place of the planned fixed‐effect model (Analysis 2.1). The AMPLIFY Study did not present recurrent pulmonary embolism data for the subgroup of patients with a pulmonary embolism and therefore we did not include it in this meta‐analysis.
2.1. Analysis.
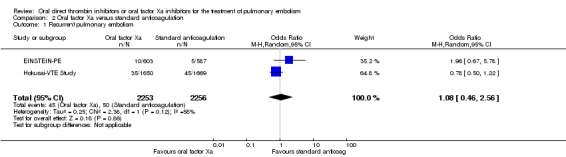
Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 1 Recurrent pulmonary embolism.
Recurrent venous thromboembolism
We included three studies on a combined total of 6295 patients in a meta‐analysis (AMPLIFY Study; EINSTEIN‐PE; Hokusai‐VTE Study). The rate of recurrent venous thromboembolism was similar between patients treated with oral factor Xa inhibitors (84 events/3153 participants) and those treated with standard anticoagulation (98 events/3142 participants), leading to an OR of 0.85 (95% CI 0.63 to 1.15) (Analysis 2.2).
2.2. Analysis.
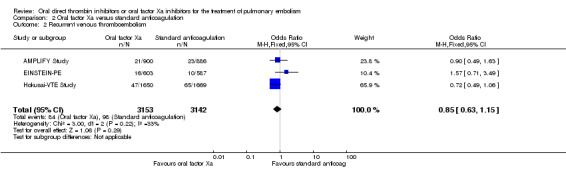
Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 2 Recurrent venous thromboembolism.
DVT
We included two studies on a combined total of 4509 patients in a meta‐analysis (EINSTEIN‐PE; Hokusai‐VTE Study). The rate of recurrent DVT was similar between patients treated with oral factor Xa inhibitors (18 events/2553 participants) and those treated with standard anticoagulation (25 events/2256 participants), leading to an OR of 0.72 (95% CI 0.39 to 1.32) (Analysis 2.3). The AMPLIFY Study did not present DVT data for the subgroup of patients with a pulmonary embolism and therefore we did not include it in this meta‐analysis.
2.3. Analysis.
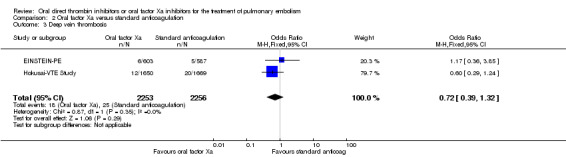
Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 3 Deep vein thrombosis.
All‐cause mortality
One study measured all‐cause mortality (EINSTEIN‐PE). The rate was similar between patients treated with the oral factor Xa inhibitor rivaroxaban (2.40%; 58 events/2412 participants) and those treated with standard anticoagulation (50 events/2405 participants), leading to an OR of 1.16 (95% CI 0.79 to 1.70) (Analysis 2.4). The AMPLIFY Study did not present all‐cause mortality data for the subgroup of patients with a pulmonary embolism and therefore we did not include it in this meta‐analysis
2.4. Analysis.
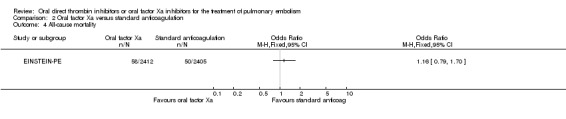
Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 4 All‐cause mortality.
Adverse effects of treatment
Both studies, EINSTEIN‐PE and Hokusai‐VTE Study, measured major bleeding (as defined by the International Society on Thrombosis and Haemostasis (ISTH); Schulman 2005). The rate of major bleeding was similar between patients treated with oral factor Xa inhibitors (30 events/2253 participants) and those treated with standard anticoagulation (31 events/2254 participants) leading to an OR of 0.97 (95% CI 0.59 to 1.61) (Analysis 2.5). The AMPLIFY Study did not present adverse effects of treatment data for the subgroup of patients with a pulmonary embolism and therefore we did not include it in this meta‐analysis
2.5. Analysis.
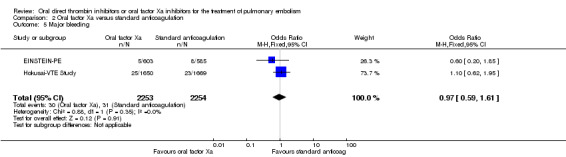
Comparison 2 Oral factor Xa versus standard anticoagulation, Outcome 5 Major bleeding.
Health‐related quality of life
Health‐related quality of life was not an outcome in any of the included studies.
Discussion
Summary of main results
Recurrent pulmonary embolism
Meta‐analyses showed that the rate of recurrent pulmonary embolism was similar between the oral direct thrombin inhibitor (DTI) dabigatran and standard anticoagulation, indicating that neither was more or less effective. For factor Xa inhibitors, there was substantial heterogeneity when we combined data from the two studies in a meta‐analysis. Therefore no meaningful conclusions can be drawn from this pooled analysis.
Recurrent venous thromboembolism
Meta‐analyses showed that the rate of recurrent venous thromboembolism was similar between the oral DTI dabigatran and standard anticoagulation, indicating that neither was more or less effective. Similarly, for oral factor Xa inhibitors, the rate of recurrent venous thromboembolism was similar to standard anticoagulation, indicating that neither was more or less effective.
Deep vein thrombosis (DVT)
Meta‐analyses showed that both oral DTIs and factor Xa inhibitors were no more effective than standard anticoagulation in preventing DVT.
All‐cause mortality
One study measured all‐cause mortality in patients treated with the oral factor Xa inhibitor rivaroxaban and found that it was no more effective in preventing deaths than standard therapy.
Major bleeding
Results of our meta‐analysis indicate that direct oral anticoagulants (DOACs) offer no reduction in major bleeding compared to standard anticoagulation. The included studies all used the strict definition of major bleeding provided by the International Society on Thrombosis and Haemostasis (ISTH) (Schulman 2005).
Health‐related quality of life
Health‐related quality of life was not reported in the included studies.
Overall completeness and applicability of evidence
This review assessed whether long‐term treatment with new oral anticoagulants, such as DTIs and factor Xa inhibitors, reduced the rate of recurrent venous thromboembolism, all‐cause mortality and major bleeding in patients with a pulmonary embolism. Two studies tested DTIs and three studies tested factor Xa inhibitors within similar study populations. With the exception of all‐cause mortality and health‐related quality of life, all of the addressed outcomes were analysed and reported by the trialists. Statistical heterogeneity was high for recurrent pulmonary embolism in the studies testing factor Xa inhibitors. This was unexpected as each individual study had strict inclusion criteria that resulted in the overall patient population of this review having almost identical conditions. Furthermore, for each particular drug, the concentrations used across studies were similar.
Subgroup analyses could not be performed because of the lack of patient level data. These analyses might be important to guide clinical management in patients with different risk factors for pulmonary embolism.
Although many consider DVT and pulmonary embolism to be manifestations of the same disorder, we elected to study these two conditions separately as there is evidence of clinically significant differences between them. The majority of recurrent events occur at the same site as the original thrombosis (in other words, in a patient presenting with a pulmonary embolism, a recurrent event after treatment is much more likely to be another pulmonary embolism); both oral contraceptive use and Factor V Leiden mutation are more likely to be associated with DVT than pulmonary embolism; on the other hand, lung disease is much more likely to be associated with pulmonary embolism. A review on the effectiveness of oral DTIs and factor Xa inhibitors for the long‐term treatment of DVT was recently published (Robertson 2015).
We did not find any studies comparing:
one oral DTI versus another anticoagulant;
one oral DTI versus another oral DTI;
one oral factor Xa inhibitor versus another oral factor Xa inhibitor;
oral DTI versus oral factor Xa inhibitor.
A recent cost‐effectiveness analysis conducted by the National Institute for Health Care and Excellence (NICE) used data from the RE‐COVER, RE‐SONATE and REMEDY trials to measure the cost‐effectiveness of DOACs versus standard anticoagulation for the treatment of DVT and pulmonary embolism (NICE 2014). While dabigatran and rivaroxaban were not compared directly, the report found no difference in efficacy between the two drugs and that the costs were also very similar.
Quality of the evidence
With the exception of selection and funding bias where the risk was unclear, the risk of bias was low in all included studies, reflecting good methodological quality. One of the five included studies was open‐label because of the complexity of monitoring international normalised ratio (INR) in the standard anticoagulation arm. However, all outcomes were assessed by observers who were blinded to the treatment and all safety outcomes were adjudicated by a central independent committee in each study. We could not investigate publication bias because we could not assess asymmetry in a funnel plot with the limited number of studies included in the meta‐analysis. All included studies were funded by the pharmaceutical company that formulated the particular drug being tested in the study. This could have led to funding bias. Currently there is no Cochrane tool to estimate the risk of this so we classified this as a potential other risk of bias. Funding by the pharmaceutical company could also have influenced the timeframe of reported safety outcomes and this has to be considered. All five included studies reported using a computerised system to generate the randomisation sequence. However, no further information was provided and for this reason we deemed that the risk of selection bias for random sequence generation was unclear.
For the comparison of oral DTIs versus standard anticoagulation, we graded the quality of the evidence as high. For oral factor Xa inhibitors versus standard anticoagulation, we downgraded the evidence for the outcome recurrent pulmonary embolism to moderate due to substantial heterogeneity that could not be explained. We also downgraded the evidence for all‐cause mortality to moderate as only one study was included for this outcome. However, for the outcomes recurrent venous thromboembolism, DVT and major bleeding, the evidence remained high as the outcomes were direct and effect estimates were consistent and precise, as reflected in the narrow confidence intervals around the ORs. See Table 1; Table 2.
One of the limitations of this review is the small number of included studies included. However, there were large numbers of participants within each study.
Potential biases in the review process
The search was as comprehensive as possible and we are confident that we have included all relevant studies. However, the possibility remains that some relevant trials, particularly in the 'grey' literature (for example conference proceedings), have been missed. Two review authors independently performed study selection and data extraction in order to minimise bias in the review process. We strictly adhered to the inclusion and exclusion criteria set out in the protocol in order to limit subjectivity. We performed data collection according to the process suggested by Cochrane. We also followed Cochrane processes as described by Higgins 2011 for assessing the risk of bias. For two of the included studies, RE‐COVER and RE‐COVER II, we took data from a pooled analysis published in one paper (Schulman 2011). This was the best available evidence. We tried to obtain data directly from the trialists but to no avail.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first review to measure the efficacy and safety of oral anticoagulants in patients with a pulmonary embolism. The same oral anticoagulants have been assessed in eight other systematic reviews (Antoniazzi 2103; Castellucci 2013; Fox 2012; Gomez‐Outes 2014; Hirschl 2014; Kang 2014; Sardar 2014; van der Huille 2014), but in patients with a venous thromboembolism. Five reviews found that novel oral anticoagulants are associated with less bleeding than conventional treatment (Antoniazzi 2103; Fox 2012; Gomez‐Outes 2014; Hirschl 2014; van der Huille 2014).
The review by Fox 2012 performed meta‐analysis by brand rather than class of drug and found no difference in recurrent venous thromboembolism between the two treatment groups. Rivaroxaban was the only drug found to be significantly associated with fewer major bleeding episodes (odds ratio (OR) 0.57, 95% confidence interval (CI) 0.39 to 0.84). All‐cause mortality did not differ between the two treatment groups.
The review by van der Huille 2014 showed no difference between the two treatment groups in terms of recurrent venous thromboembolism, fatal pulmonary embolism and all‐cause mortality. However, the novel oral anticoagulants were associated with a significant reduced risk of major bleeding (relative risk (RR) 0.60, 95% CI 0.41 to 0.88) and fatal bleeding (RR 0.36, 95% CI 0.15 to 0.87).
Hirschl 2014 found no differences between DOACs and standard treatment regarding recurrent venous thromboembolism and death. However, bleeding was reduced by rivaroxaban (RR 0.55, 95% CI 0.38 to 0.81), apixaban (RR 0.31, 95% CI 0.17 to 0.55) and edoxaban (RR 0.81, 95% CI 0.71 to 0.93).
The review by Gomez‐Outes 2014 found that the risk of recurrent venous thromboembolism was similar between the two treatment groups (RR 0.91, 95% CI 0.79 to 1.06) but the DOACs were associated with reduced major bleeding (absolute risk difference of ‐0.6%, 95% CI ‐1.0% to ‐0.3%).
The review by Kang 2014 found that DOACs did not differ in the risk of mortality or recurrent venous thromboembolism. However, dabigatran was associated with increased major bleeding compared to apixaban (RR 2.69, 95% CI 1.19 to 6.07) and edoxaban also had a higher bleeding rate compared to apixaban (RR 2.74, 95% CI 1.40 to 5.39).
The review by Antoniazzi 2103 included patients with venous thromboembolism and atrial fibrillation. Eight studies were included and results showed that the risk of major bleeding was lower in patients treated with dabigatran (RR 0.83, 95% CI 0.78 to 0.97).
The reviews by Castellucci 2013 and Sardar 2014 compared oral anticoagulants and antiplatelet drugs but the focus was on the secondary prevention of venous thromboembolism rather than treatment.
Authors' conclusions
Implications for practice.
There is no evidence of a difference between the oral DTIs and standard anticoagulation in the prevention of recurrent pulmonary embolism. Using the GRADE criteria, the quality of evidence was moderate to high. The evidence of the effectiveness of oral factor Xa inhibitors and the prevention of recurrent pulmonary embolism was too heterogenous to combine in a pooled analysis. For the outcomes recurrent venous thromboembolism, DVT, all‐cause mortality and major bleeding, evidence suggests that DOACs and standard anticoagulation are equivalent. According to GRADE criteria, the quality of evidence was moderate to high. DOACs such as direct thrombin inhibitors (DTIs) and factor Xa inhibitors may therefore be an alternative to conventional anticoagulation treatment for acute pulmonary embolism. The clear benefit of all DOACs is their ease of use due to fixed doses and no need for routine monitoring with blood tests.
Implications for research.
The lack of an antidote to DOACS is a potentially serious problem in patients with acute bleeding or who require emergency surgery. However, this is relatively rare as the DOACs have a short half‐life (if renal function is maintained). Antidotes to each of the DOACs are currently under trial and these are required urgently. There is also some evidence of wide inter‐individual variation in anticoagulant effect from the fixed doses of DOACs as currently prescribed. This may be of clinical importance, not only in emergencies or in patients requiring surgical or investigational interventions, but to answer the very basic question: is this patient both safely and adequately anticoagulated? Further research is also required to establish other factors associated with the use of DOACs, such as adherence, quality of life, cost‐effectiveness and tolerability. Future studies should also compare the DOACs directly with one another to see which one is most effective and safe. Finally, research is required in categories of venous thrombosis not specifically examined in the studies included here, such as those with malignancy, travel‐associated or patients carrying a thrombophilic abnormality such as the anti‐phospholipid syndrome.
Feedback
Feedback February 2016
Summary
Email received from Dr Lynda Ware.
1. The Hokusai‐VTE study. In both the narrative paragraph describing the study (page 11) and in the “characteristics of included studies” section (page 31) you describe a 'dabigatran‐like placebo'. This study was looking at edoxaban and the original paper speaks of an edoxaban placebo. I think therefore that there is a possible transcription error and I suggest that you actually meant “edoxaban‐like placebo” on pages 11 and 31.
Authors' response: Thank you for pointing this out. This is a transcription error and has now been changed.
2. Recurrent pulmonary embolism. In the summary of main results in the main body text you conclude that 'for Factor Xa inhibitors, there was substantial heterogeneity when we combined data from the two studies in a meta‐analysis. Therefore no meaningful conclusions can be drawn from this analysis'. The heterogeneity is measured as 58% using the I2 statistic and I can understand why you therefore decided not to combine the studies. When you say “no meaningful conclusions can be drawn from this analysis” do you mean the pooled analysis? Or the individual studies? Do you in fact draw some conclusions from those individual studies (see below)?
Authors' response: We meant to say "no meaningful conclusions can be drawn from the pooled analysis” and this has been been amended.
However, in the authors' conclusions in the abstract it states that 'moderate to high quality evidence suggests that there are no differences between DOACs and standard anticoagulation for the long‐term treatment of pulmonary embolism for the outcome(s) recurrent pulmonary embolism ....'. Your use of the term 'DOACs' implies both DTIs (represented in the data by dabigatran) and the Factor Xa inhibitors. In other words, you appear to be saying that (a) there is “moderate to high quality evidence that there is no difference between DTIs and standard anticoagulation for the long‐term treatment etc…”, and (b) there is “moderate to high quality evidence that there is no difference between the Factor Xa inhibitors and standard anticoagulation for the long‐term treatment etc…'
There are two problems I believe with this. A specific one is that for the Factor Xa inhibitors you have stated that “no meaningful conclusions can be drawn from this analysis”. How can you reconcile this with the above?
Authors' response: We agree with your point and have reworded the paragraph drawing conclusions for the separate drug classes rather than grouping them together.
Secondly, “the absence of evidence is not evidence of absence”. Can you really say that there is “moderate to high quality evidence that there is no difference between” the alternatives? Based on three trials? Or do you mean that “there is no evidence of a difference between the two treatments. Using the GRADE criteria, the quality of evidence supporting that statement is moderate to high” or “there is no evidence of a difference between the two treatments. The studies assessing this were at low risk of bias”.
Authors' response: Despite there being few studies for any of the comparisons, there is a relatively large number of patients. However, we can see your point and have amended accordingly.
Reply
The authors of the review have responded to this feedback giving a point by point response to the comments above.
Contributors
Feedback: Dr Lynda Ware, Cochrane UK Response: Dr Lindsay Robertson on behalf of the review authors
What's new
| Date | Event | Description |
|---|---|---|
| 16 December 2016 | Feedback has been incorporated | Review amended following comments received |
Acknowledgements
We would like to thank Dr Karen Welch for searching the Cochrane Vascular Specialised Register and the Cochrane Central Register of Controlled Trials. We would also like to thank Dr Marlene Stewart, Managing Editor of Cochrane Vascular, for her assistance and advice in completing this review
Appendices
Appendix 1. CRS search strategy
| Search run on Wed Jan 28 2015 | ||
| #1 | MESH DESCRIPTOR Antithrombins EXPLODE ALL TREES | 790 |
| #2 | MESH DESCRIPTOR Hirudin Therapy | 75 |
| #3 | (thrombin near3 inhib*):TI,AB,KY | 444 |
| #4 | hirudin*:TI,AB,KY | 327 |
| #5 | (dabigatran or Pradaxa or Rendix):TI,AB,KY | 199 |
| #6 | (BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048):TI,AB,KY | 9 |
| #7 | (ximelagatran or Exanta or Exarta or melagatran):TI,AB,KY | 147 |
| #8 | (AZD0837 or AZD‐0837):TI,AB,KY | 12 |
| #9 | (S35972 or S‐35972):TI,AB,KY | 0 |
| #10 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 | 1387 |
| #11 | MESH DESCRIPTOR Factor Xa Inhibitors | 1 |
| #12 | (Factor X* near4 (antag* or inhib* or block*)):TI,AB,KY | 415 |
| #13 | (FX* near4 (antag* or inhib* or block*)):TI,AB,KY | 33 |
| #14 | (10* near4 (antag* or inhib* or block*) ):TI,AB,KY | 842 |
| #15 | #11 OR #12 OR #13 OR #14 | 1237 |
| #16 | (rivaroxaban or Xarelto):TI,AB,KY | 251 |
| #17 | (Bay‐597939 or Bay597939):TI,AB,KY | 0 |
| #18 | (betrixaban or PRT054021):TI,AB,KY | 14 |
| #19 | apixaban:TI,AB,KY | 134 |
| #20 | (BMS‐562247 or BMS‐562247 or ELIQUIS):TI,AB,KY | 0 |
| #21 | (DU‐176b or DU176b):TI,AB,KY | 11 |
| #22 | (PRT‐054021 or PRT054021):TI,AB,KY | 1 |
| #23 | (YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176*):TI,AB,KY | 38 |
| #24 | (GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893):TI,AB,KY | 3 |
| #25 | edoxaban or lixiana | 51 |
| #26 | #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 | 456 |
| #27 | #10 OR #15 OR #26 | 2793 |
| #28 | MESH DESCRIPTOR Thrombosis | 1133 |
| #29 | MESH DESCRIPTOR Thromboembolism | 841 |
| #30 | MESH DESCRIPTOR Venous Thromboembolism | 159 |
| #31 | MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES | 1857 |
| #32 | (thrombus* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*):TI,AB,KY | 13382 |
| #33 | MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES | 676 |
| #34 | (PE or DVT or VTE):TI,AB,KY | 3057 |
| #35 | ((vein* or ven*) near thromb*):TI,AB,KY | 5003 |
| #36 | (blood near3 clot*):TI,AB,KY | 1305 |
| #37 | (pulmonary near3 clot*):TI,AB,KY | 5 |
| #38 | (lung near3 clot*):TI,AB,KY | 3 |
| #39 | #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 | 16505 |
| #40 | #27 AND #39 | 1026 |
Data and analyses
Comparison 1. Oral DTI versus standard anticoagulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent pulmonary embolism | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrent venous thromboembolism | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Deep vein thrombosis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Major bleeding | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Oral factor Xa versus standard anticoagulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent pulmonary embolism | 2 | 4509 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.46, 2.56] |
| 2 Recurrent venous thromboembolism | 3 | 6295 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.15] |
| 3 Deep vein thrombosis | 2 | 4509 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.39, 1.32] |
| 4 All‐cause mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Major bleeding | 2 | 4507 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.59, 1.61] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AMPLIFY Study.
| Methods |
Study design: randomised, double‐blind trial Duration of study: 6 months |
|
| Participants |
Setting: hospital Country: multinational No: 5395; apixaban 2691, enoxaparin + warfarin 2704 Age, mean (SD) years: apixaban 57.2 (16.0) years, enoxaparin + warfarin 56.7 (16.0) years Sex: apixaban 1569 M/1122 F; placebo 1598 M/1106 F Inclusion criteria: people ≥ 18 years of age with an objectively confirmed, symptomatic proximal DVT or PE (with or without DVT) Exclusion criteria: active bleeding, a high risk of bleeding, or other contraindications to treatment with enoxaparin and warfarin; if they had cancer and long‐term treatment with LMWH was planned; if their DVT or PE was provoked in the absence of a persistent risk factor for recurrence; if < 6 months of anticoagulant treatment was planned; or if they had another indication for long‐term anticoagulation therapy, dual antiplatelet therapy, treatment with aspirin at a dose > 165 mg daily, or treatment with potent inhibitors of cytochrome P‐450 3A4; if they had received > 2 doses of a once‐daily LMWH regimen, fondaparinux, or a VKA; > 3 doses of a twice daily LMWH regimen; or more than 36 hours of continuous intravenous heparin. Additional exclusion criteria were a haemoglobin level < 9 mg/dL, a platelet count < 100,000/mm3, a serum creatinine level > 2.5 mg/dL (220 μmol/L), or a calculated creatinine clearance < 25 mL/minute |
|
| Interventions |
Intervention 1: oral apixaban 10 mg twice daily for the first 7 days, followed by 5 mg twice daily for 6 months Intervention 2: enoxaparin 1 mg/kg body weight every 12 hours for at least 5 days and warfarin concomitantly for 6 months. Warfarin dose was adjusted to maintain the INR 2.0 to 3.0. Enoxaparin or placebo was discontinued when a blinded INR of ≥ 2.0 was achieved Follow‐up: weeks 2, 4, 8, 12, 16, 20 and 24 after randomisation and 30 days after the end of the intended treatment period |
|
| Outcomes |
Primary: composite of recurrent symptomatic VTE (fatal or non‐fatal PE and DVT), and mortality related to VTE; major bleeding; Secondary: recurrent symptomatic VTE, mortality related to VTE, mortality from cardiovascular causes, mortality from any cause and the composite of major bleeding and clinically relevant non‐major bleeding |
|
| Notes | Results were presented for all patients with a VTE but specific recurrent VTE data for the subset of participants with a PE was available in the supplementary material | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was performed with the use of an interactive voice‐response system" Comment: insufficient information to permit judgement of high or low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed with the use of an interactive voice‐response system" Comment: study judged at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind. Patients were assigned to receive apixaban tablets plus placebo enoxaparin injections and placebo warfarin tablets or conventional therapy with enoxaparin injections and warfarin tablets plus placebo apixaban tablets. The study used blinded INR monitoring with a point‐of‐care device that generated an encrypted code for INR results. Investigators reported the code to the interactive voice‐response system and received either an actual INR value (for patients assigned to warfarin) or a sham INR value (for patients receiving apixaban)" Comment: study judged at low risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent committee, whose members were unaware of the study‐group assignments, adjudicated the qualifying diagnosis, the anatomical extent of the initial deep vein thrombosis or pulmonary embolism, and all suspected outcomes." Comment: study judged at low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A number of randomised patients were inappropriately excluded from the intention‐to‐treat analysis. Additionally, 144/377 of apixaban patients and 142/413 patients given conventional treatment were classified as discontinuing for "other reasons", with no explanations given. Therefore we deemed the risk of attrition bias to be unclear |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was available. Minor bleeding was a pre‐defined secondary outcome but the data on this outcome were not reported in the paper. Therefore we deemed the risk of reporting bias to be unclear |
| Other bias | Unclear risk | The study was funded by Pfizer and Bristol‐Myers Squibb, the pharmaceutical companies that developed apixaban. In addition, the trial analysed non‐inferiority using an ITT analysis. When compared with the per‐protocol analysis, ITT favoured the finding of non‐inferior results. This may have skewed the result in favour of an increased efficacy of apixaban |
EINSTEIN‐PE.
| Methods |
Study design: randomised, open‐label, event‐driven, non‐inferiority trial Duration of study: 12 months |
|
| Participants |
Setting: hospital Country: 38 countries No: 4832; rivaroxaban 2419, warfarin 2413 Age, mean (SD) years: rivaroxaban 57.9 (7.3) years, warfarin 57.5 (7.2) years Sex: rivaroxaban 1309 M/1110 F, warfarin 1247 M/1166 F Inclusion criteria: patients aged 18 or older who had an acute, symptomatic pulmonary embolism with objective confirmation, with or without symptomatic deep vein thrombosis Exclusion criteria: contraindications to heparin or warfarin, had received treatment for more than 48 hours with therapeutic doses of heparin, had received more than one dose of a VKA, had cancer for which long‐term treatment with LMWH was anticipated, had another indication for warfarin therapy, continued to receive treatment with aspirin at a dose of more than 100 mg daily or dual antiplatelet therapy, or had a creatinine clearance of less than 30 mL per minute |
|
| Interventions |
Intervention 1: oral rivaroxaban 15 mg twice daily for the first 3 weeks, followed by 20 mg once daily Intervention 2: enoxaparin 1.0 mg per kg of body weight twice daily and either warfarin or acenocoumarol, started within 48 hours of randomisation. Enoxaparin was discontinued when the INR was 2.0 or more for 2 consecutive days and the patients had received at least 5 days of enoxaparin treatment. The dose of VKA was adjusted to maintain an INR of 2.0 to 3.0, determined at least once a month Follow‐up: 3, 6 and 12 months |
|
| Outcomes |
Primary: symptomatic recurrent VTE, defined as a composite of DVT or fatal or non‐fatal PE and clinically relevant bleeding, defined as a composite of major or clinically relevant non‐major bleeding. Death was classified as pulmonary embolism, bleeding or other established diagnoses. Pulmonary embolism was considered the cause of death if there was objective documentation of the condition or if death could not be attributed to a documented cause and pulmonary embolism could not be confidently ruled out. Bleeding was defined as major if it was clinically overt and associated with a decrease in the haemoglobin level if 2.0 g per decilitre or more, if bleeding led to the transfusion of 2 or more units of red blood cells, or if bleeding was intracranial or retroperitoneal, occurred in another critical site, or contributed to death. Clinically relevant non‐major bleeding was defined as overt bleeding that did not meet the criteria for major bleeding but was associated with medical intervention, unscheduled contact with a physician, interruption or discontinuation of a study drug, or discomfort or impairment of activities of daily life Secondary: major bleeding, death from any cause, vascular events (acute coronary syndrome, ischaemic stroke, transient ischaemic attack or systemic embolism) and net clinical benefit (defined as a composite of the primary efficacy outcome and major bleeding, as assessed in the intention‐to‐treat population) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was performed with the use of a computerised voice‐response system" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed with the use of a computerised voice‐response system" Comment: study judged to be at a low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Open‐label" Comment: only one dose of rivaroxaban was given and as the comparison was enoxaparin/VKA, blinding of participants and personnel was not possible. However, we judge that the lack of blinding in the control group was unlikely to have affected the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A central committee whose members were unaware of the study‐group assignments adjudicated the results of all baseline lung‐imaging tests and all suspected outcome events" Comment: study judged to be at low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's pre‐specified outcomes have been reported in the pre‐specified way |
| Other bias | Unclear risk | The study was funded by Bayer HealthCare, the pharmaceutical company that developed rivaroxaban. It is possible that this may have influenced the timeframe of reported safety outcomes |
Hokusai‐VTE Study.
| Methods |
Study design: randomised, double‐blind, non‐inferiority study Duration of study: 12 months |
|
| Participants |
Setting: multicentre Country: multinational No: 4921; edoxaban 2468, warfarin 2453 Age, mean (SD) years: edoxaban 55.7 (16.3) years, warfarin 55.9 (16.2) years Sex: edoxaban 2360 M/1758 F, warfarin 2356 M/1766 F Inclusion criteria: patients aged 18 or older who had objectively diagnosed, acute, symptomatic DVT involving the popliteal, femoral or iliac veins or acute, symptomatic PE (with or without DVT) Exclusion criteria: contraindications to heparin or warfarin, had received treatment for more than 48 hours with therapeutic doses of heparin, had received more than one dose of a VKA, had cancer for which long‐term treatment with LMWH was anticipated, had another indication for warfarin therapy, continued to receive treatment with aspirin at a dose of more than 100 mg daily or dual antiplatelet therapy, or had a creatinine clearance of less than 30 mL per minute |
|
| Interventions |
Intervention 1: oral edoxaban 60 mg once daily or 30 mg once daily in patients with a creatinine clearance of 30 to 50 mL per minute or a body weight of 60 kg or less or in patients who were receiving concomitant treatment with potent P‐glycoprotein inhibitors Intervention 2: dose‐adjusted warfarin therapy to achieve an INR of 2.0 to 3.0 and edoxaban‐like placebo Follow‐up: days 5, 12, 30 and 60 after randomisation, monthly while on study drug or every 3 months after discontinuing the study drug and finally at 12 months |
|
| Outcomes |
Primary: incidence of symptomatic recurrent VTE (DVT and fatal or non‐fatal PE), clinically relevant bleeding (major or clinically relevant non major) Secondary: none |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was performed with the use of an interactive Web‐base system" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed with the use of an interactive Web‐base system" Comment: study judged to be at a low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Edoxaban or warfarin was administered in a double‐blind fashion" Comment: study judged to be at a low risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent committee, whose members were unaware of the study‐group assignments, adjudicated all suspected outcome and the results of baseline imaging tests and assessed the anatomical extent of thrombosis" Comment: study judged to be at a low risk of performance bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's pre‐specified outcomes have been reported in the pre‐specified way |
| Other bias | Unclear risk | The study was funded by Daiichi‐Sankyo, the pharmaceutical company that developed edoxaban. It is possible that this may have influenced the timeframe of reported safety outcomes |
RE‐COVER.
| Methods |
Study design: randomised, double‐blind, double‐dummy non‐inferiority trial Duration of study: 6 months |
|
| Participants |
Setting: 228 clinical centres Country: 29 countries No: 2539; dabigatran 1273, warfarin 1266 Age, mean (range) years: dabigatran 56 (18 to 93) years, warfarin 55 (18 to 97) years Sex: dabigatran 738 M/535 F, warfarin 746 M/520 F Inclusion criteria: people aged ≥ 18 years who had acute, symptomatic, objectively verified proximal DVT of the legs or PE and for whom 6 months of anticoagulant therapy was considered an appropriate treatment Exclusion criteria: duration of symptoms > 14 days, PE with haemodynamic instability or requiring thrombolytic therapy, another indication for warfarin therapy, recent unstable cardiovascular disease, a high risk of bleeding, liver disease with an aminotransferase level that was 2 x ULN range, an estimated creatinine clearance < 20 mL/minute, a life expectancy < 6 months, contraindication to heparin or to radiographic contrast material, pregnancy or risk of becoming pregnant, requirement for long‐term anticoagulant therapy |
|
| Interventions |
Intervention 1: oral dabigatran 150 mg twice daily and warfarin‐like placebo Intervention 2: dose‐adjusted warfarin therapy to achieve an INR of 2.0 to 3.0 and dabigatran‐like placebo Follow‐up: 6 months |
|
| Outcomes |
Primary: recurrent VTE evaluated using the same diagnostic methods used for the initial diagnosis Secondary: bleeding that was defined as major if it was clinically overt and if it was associated with a fall in the haemoglobin level ≥ 20 g/L, resulted in the need for transfusion of ≥ 2 units of red cells, involved a critical site, or was fatal |
|
| Notes | 2539 participants were recruited into the trial but only 1602 had a PE and were included in the analysis of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Computer generated randomisation scheme" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Low risk | Quote: "Staff members at the clinical centres called an interactive voice‐response system that randomly assigned subjects to one of the supplied medication kits. The treatment‐group assignment was concealed from all the investigators and their staff at the coordinating centre and the clinical centres and from the clinical monitors" Comment: study judged to be at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind. The treatment‐group assignment was concealed from all the investigators and their staff at the coordinating centre and the clinical centres and from the clinical monitors. Warfarin or a placebo that looked identical to warfarin......Administration of dabigatran or a placebo that looked identical to dabigatran" Comment: study judged to be at low risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All suspected outcome events and deaths were classified by central adjudication committees, whose members were unaware of the treatment assignments" Comment: study judged to be at low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes have been reported in the pre‐specified way |
| Other bias | Unclear risk | The study was funded by Boehringer‐Ingelheim, the pharmaceutical company that developed dabigatran. It is possible that this may have influenced the timeframe of reported safety outcomes |
RE‐COVER II.
| Methods |
Study design: randomised, double‐blind, double‐dummy trial Duration of study: 6 months |
|
| Participants |
Setting: 208 study sites Country: 31 countries worldwide No: 2568: dabigatran 1280, warfarin 1288 Age, mean (SD) years: dabigatran 54.7 (16.2) years, warfarin 55.1 (16.3) years Sex: dabigatran 781 M/499 F, warfarin 776 M/512 F Inclusion criteria: patients aged 18 or older who had acute, symptomatic, objectively verified proximal deep vein thrombosis of the legs or pulmonary embolism and for whom 6 months of anticoagulant therapy was considered to be an appropriate treatment Exclusion criteria: duration of symptoms longer than 14 days; pulmonary embolism with haemodynamic instability or requiring thrombolytic therapy; another indication for warfarin therapy; recent unstable cardiovascular disease; a high risk of bleeding; liver disease with an aminotransferase level that was 3 times the upper limit of the normal range; an estimated creatinine clearance of less than 20 mL per minute; a life expectancy of less than 6 months; a contraindication to heparin or to radiographic contrast material; pregnancy or risk of becoming pregnant; requirement for long‐term anticoagulant therapy |
|
| Interventions |
Intervention 1: oral dabigatran 150 mg twice daily and warfarin‐like placebo for 6 months Intervention 2: active warfarin adjusted to achieve an INR of 2.0 to 3.0 and dabigatran‐like placebo for 6 months |
|
| Outcomes |
Primary: recurrent VTE objectively verified, preferably with the same method as for the index event Secondary: major bleeding defined according to the International Society on Thrombosis and Haemostasis criteria |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised by use of an interactive voice response system and a computer‐generated randomisation scheme in blocks of 4" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Low risk | Comment: no information given about how treatment allocation was concealed but study authors state that "the design of the trial was essentially identical to that of the first study with dabigatran for the treatment of acute VTE" (RE‐COVER), which we judged to be at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind" Comment: stated as double‐blind. No other information given about how blinding was maintained but study authors state that "the design of the trial was essentially identical to that of the first study with dabigatran for the treatment of acute VTE", which we judged to be at low risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A central adjudication committee, the members of which were unaware of the treatment assignments, classified all suspected outcome events, bleeding events, and deaths" Comment: study judged to be at low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes have been reported in the pre‐specified way |
| Other bias | Unclear risk | The study was funded by Boehringer‐Ingelheim, the pharmaceutical company that developed dabigatran. It is possible that this may have influenced the timeframe of reported safety outcomes. |
DVT: deep vein thrombosis F: female INR: international normalised ratio ITT: intention‐to‐treat LMWH: low molecular weight heparin M: male PE: pulmonary embolism SD: standard deviation ULN: upper limit of normal VKA: vitamin K antagonist VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ageno 2014 | Not a randomised controlled trial |
| AMPLIFY Extended Study | Extended study testing prophylaxis rather than treatment |
| Botticelli DVT Study | Patients with a pulmonary embolism were excluded from the study |
| Einstein DVT Study | Patients with a pulmonary embolism were excluded from the study |
| EINSTEIN Extension Study | Extended study testing prophylaxis rather than treatment |
| Einstein‐DVT Dose Study | Patients with a pulmonary embolism were excluded from the study |
| ODIXa‐DVT Study | Patients with a pulmonary embolism were excluded from the study |
| Piazza 2014 | Patients with a pulmonary embolism were excluded from the study |
| RE‐SONATE | Patients were already included in the RE‐COVER I and RE‐COVER II studies |
| REMEDY | Extended study testing prophylaxis rather than treatment |
| THRIVE | Treatment was for less than 3 months |
| THRIVE I | Unable to obtain specific outcome data for patients with a pulmonary embolism |
| THRIVE III | Control group were given a placebo |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐TRC‐14005223.
| Trial name or title | Efficacy and safety of rivaroxaban or warfarin on venous thromboembolic disease: a randomized controlled trial |
| Methods | Study design: randomised, parallel‐control trial |
| Participants |
Setting: hospitals Country: China Inclusion criteria: patients diagnosed with non‐high‐risk pulmonary thromboembolism with/without deep vein thrombosis Exclusion criteria : patients with active bleeding, high risk for bleeding complications or considered to be high‐risk for pulmonary thromboembolism. Aspartate aminotransferase (AST) and glutamic‐pyruvic transaminase (ALT) more than 3 times of the upper limit of normal in liver function test and ≤ 30 mL/min in kidney function test; systemic blood pressure < 90/50 mmHg, or those with uncontrolled, dangerous hypertension (B > 170/110 mmHg); patients who have to take azole antifungals, HIV protease inhibitors or strong CYP3A4 inducers during the period of treatment; pregnant, lactating women or who may be pregnant during the period of treatment |
| Interventions |
Intervention 1: rivaroxaban Intervention 2: warfarin |
| Outcomes |
Primary: thromboembolic events Secondary : bleeding events |
| Starting date | Not stated |
| Contact information | Chunli Liu, chunli@gird.cn |
| Notes | — |
NCT01780987.
| Trial name or title | A study to evaluate safety and efficacy of apixaban In Japanese acute deep vein thrombosis (DVT) and pulmonary embolism (PE) patients |
| Methods | Study design: randomised, multicentre, open‐label study |
| Participants |
Setting: 20 hospitals Country: Japan Inclusion criteria: men or women ≥ 20 years of age with acute symptomatic proximal DVT with evidence of proximal thrombosis or acute symptomatic PE with evidence of thrombosis in segmental or more proximal branches Exclusion criteria: active bleeding or high risk for bleeding contraindicating treatment with UFH and a VKA, uncontrolled hypertension: systolic blood pressure > 180 mmHg or diastolic blood pressure > 110 mmHg and participants requiring dual anti‐platelet therapy |
| Interventions |
Intervention 1: apixaban 10 mg twice a day for 7 days followed by 5 mg twice a day for 23 weeks Intervention 2: unfractionated heparin, dose adjustment based on activated partial thromboplastin time (APTT) = 1.5 to 2.5 times the control value, and until INR ≥ 1.5 for 5 days or more plus warfarin for 24 weeks at a dose to target INR range between 1.5 to 2.5 |
| Outcomes |
Primary: major bleeding and clinically relevant non‐major bleeding Secondary: symptomatic VTE or VTE‐related death, major bleeding and all bleeding |
| Starting date | January 2013 |
| Contact information | Pfizer CT.gov Call Centre |
| Notes | — |
NCT01895777 .
| Trial name or title | Open label study comparing efficacy and safety of dabigatran etexilate to standard of care in paediatric patients with venous thromboembolism (VTE) |
| Methods | Study design: randomised, open‐label study |
| Participants |
Setting: 61 hospitals Country: Argentina, Australia, Belgium, Brazil, Bulgaria, Canada, Colombia, Czech Republic, Finland, France, Greece, Israel, Italy, Lithuania, Mexico, Norway, Russia, Slovakia, Spain, Sweden, Switzerland, Taiwan, Thailand, Turkey, Ukraine Inclusion criteria: male or female participants < 18 years of age at the time of informed consent, body weight ≤ 40 kg, with a documented diagnosis of VTE per investigator judgment initially treated (generally 5 to 7 days) with an UFH or a LMWH and clinical indication for 3 months of treatment with anticoagulants for the VTE episode defined under the above inclusion criterion Exclusion criteria: conditions associated with an increased risk of bleeding, renal dysfunction or requirement for dialysis, active infective endocarditis, participants with a mechanical or a biological heart valve prosthesis, hepatic disease |
| Interventions |
Intervention 1: dabigatran at an age and weight appropriate dose given in capsules (50 mg, 75 mg and 110 mg) pellets or oral liquid formulation given twice a day in an open‐label fashion for 3 months Intervention 2: LMWH or VKA prescribed in an open‐label fashion for 3 months |
| Outcomes |
Primary: a combined efficacy endpoint of complete thrombus resolution plus freedom from recurrent VTE plus freedom from mortality related to VTE and freedom from major bleeding events Secondary: freedom from thrombus progression at baseline and at days 21 and 84 after randomisation, freedom from recurrence of VTE at 6, 9 and 12 months, freedom from occurrence of post‐thrombotic syndrome at 6, 9 and 12 months, all bleeding events and all‐cause mortality |
| Starting date | September 2013 |
| Contact information | clintriage.rdg@boehringer‐ingelheim.com |
| Notes | — |
NCT02234843.
| Trial name or title | EINSTEIN Junior phase III: oral rivaroxaban in children with venous thrombosis (EINSTEIN Jr) |
| Methods | Study design: randomised, open‐label study |
| Participants |
Setting: hospital Country: 20 countries Inclusion criteria: children aged 6 months to < 18 years with confirmed venous thromboembolism who receive initial treatment with therapeutic dosages of UFH (unfractionated heparin), LMWH (low molecular weight heparin) or fondaparinux and require anticoagulant therapy for at least 90 days Exclusion criteria: active bleeding or high risk for bleeding contraindicating anticoagulant therapy, estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2, hepatic disease that is associated with either: coagulopathy leading to a clinically relevant bleeding risk, or alanine transaminase (ALT) > 5x upper level of normal (ULN) or total bilirubin > 2x ULN with direct bilirubin > 20% of the total, platelet count < 50 x 109/L, hypertension defined as > 95th age percentile, life expectancy < 3 months, concomitant use of strong inhibitors of both cytochrome P450 isoenzyme 3A4 (CYP3A4) and P‐glycoprotein (P‐gp), concomitant use of strong inducers of CYP3A4, childbearing potential without proper contraceptive measures, pregnancy or breast feeding, hypersensitivity or any other contraindication listed in the local labelling for the comparator treatment or experimental treatment |
| Interventions |
Intervention 1: age and body weight‐adjusted dosing of rivaroxaban to achieve a similar exposure as that observed in adults treated for venous thromboembolism (VTE) with 20 mg rivaroxaban Intervention 2: subcutaneous low molecular weight heparin (LMWH), subcutaneous fondaparinux and/or oral vitamin K antagonist (VKA) |
| Outcomes |
Primary: composite number of all symptomatic recurrent venous thromboembolism and composite number of overt major and clinically relevant non‐major bleeding Secondary: composite number of all symptomatic recurrent venous thromboembolism and asymptomatic deterioration on repeat imaging |
| Starting date | November 2014 |
| Contact information | clinical‐trials‐contact@bayerhealthcare.com |
| Notes | — |
NCT02309411.
| Trial name or title | EINSTEIN Junior phase II: oral rivaroxaban in young children with venous thrombosis (EINSTEIN Jr) |
| Methods | Study design: randomised, open‐label study |
| Participants |
Setting: hospital Country: 20 countries Inclusion criteria: children aged 6 months to < 6 years who have been treated for at least 2 months or, in case of catheter‐related thrombosis, for at least 6 weeks with LMWH (low molecular weight heparin), fondaparinux and/or VKA (vitamin K antagonist) for documented symptomatic or asymptomatic venous thrombosis and who will enter their last month of intended anticoagulant treatment, haemoglobin, platelets, creatinine, alanine aminotransferase (ALT) and bilirubin evaluated within 10 days prior to randomisation and informed consent provided Exclusion criteria: active bleeding or high risk for bleeding contraindicating anticoagulant therapy, symptomatic progression of venous thrombosis during preceding anticoagulant treatment, planned invasive procedures, including lumbar puncture and removal of non‐peripherally placed central lines during study treatment, an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2, hepatic disease which is associated with either: coagulopathy leading to a clinically relevant bleeding risk, or ALT > 5x upper level of normal (ULN) or total bilirubin > 2x ULN with direct bilirubin > 20% of the total, platelet count < 100 x 109/L, hypertension defined as > 95th age percentile, life expectancy < 3 months, concomitant use of strong inhibitors of both cytochrome P450 isoenzyme 3A4 (CYP3A4) and P‐glycoprotein (P‐gp), concomitant use of strong inducers of CYP3A4, hypersensitivity or any other contraindication listed in the local labelling for the comparator treatment or experimental treatment, inability to co‐operate with the study procedures, previous randomisation to this study and participation in a study with an investigational drug or medical device within 30 days prior to randomisation |
| Interventions |
Intervention 1: age and body weight‐adjusted dosing of rivaroxaban to achieve a similar exposure as that observed in adults treated for venous thromboembolism (VTE) with 20 mg rivaroxaban Intervention 2: children randomised to the comparator group will continue with the anticoagulant treatment that was used prior to study randomisation (e.g. unfractionated heparin, low molecular weight heparin, fondaparinux, vitamin K antagonist therapy) |
| Outcomes |
Primary: composite number of all symptomatic recurrent venous thromboembolism and composite number of overt major and clinically relevant non‐major bleeding Secondary: composite number of all symptomatic recurrent venous thromboembolism and asymptomatic deterioration on repeat imaging |
| Starting date | January 2015 |
| Contact information | clinical‐trials‐contact@bayerhealthcare.com |
| Notes | — |
DVT: deep vein thrombosis INR: international normalised ratio LMWH: low molecular weight heparin PE: pulmonary embolism UFH: unfractionated heparin VKA: vitamin K antagonist VTE: venous thromboembolism
Differences between protocol and review
In a change from the protocol (Robertson 2014b), we excluded studies where treatment was for less than three months because a meta‐analysis of venous thromboembolism treatment strategies has demonstrated an increased rate of recurrence after less than three months anticoagulation but no significant difference with various longer periods of treatment (Boutitie 2011).
Contributions of authors
LR: drafted the protocol, selected studies for inclusion, extracted data, assessed the quality of studies, performed data analysis and wrote the review. PK: commented on the protocol, selected studies for inclusion, extracted data, assessed the quality of the studies and commented on the review. JM: selected studies for inclusion, extracted data, assessed the quality of the studies and commented on the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Programme Grant funding to Cochrane Vascular. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
LR: none known. PK: I have received consultancy fees for attendance at advisory boards of Boehringer‐Ingelheim, Bayer and Daiitchi‐Sankyo and payment from Bayer for lectures at the 2013 anticoagulation master class. My institution was paid travel/accommodation/meeting expenses by Boehringer‐Ingelheim for my attendance at the 2013 ISTH meeting and staff and NHS costs by Boehringer‐Ingelheim and Daiitchi‐Sankyo for involvement in phase III trials of novel anticoagulants in venous thrombosis. Since Summer 2014 I have declined all invitations to advisory boards, or lectures on behalf of the pharmaceutical industry. JM: I received travel, course fees, accommodation and meals from Medtronic as part of the Medtronic University program. This is an educational program, and includes registration and attendance at the European Vascular Course 2012. No financial remuneration was received by myself, other than costs of travel, accommodation, course fees and meals. I received sponsorship to attend the Vascular Society annual meeting 2012 and 2014 in the form of registration fees and accommodation/travel costs. I received sponsorship to attend a stenting master class, the Verve clinical meeting in 2013, and a technology forum in Phoenix, Arizona from Gore Medical. This was in the form of travel, accommodation and meals. No other financial remuneration was received. I received sponsorship to attend the LINC 2015 meeting in Leipzig, Germany from Abbott Medical in the form of registration, accommodation, travel and meals. I am a co‐founder of UKETS, a trainee initiative, which receives funding through sponsorship from endovascular technology and simulation companies. The majority of this is non‐financial (i.e. the companies supply trainers on the courses or allow use of their simulators), although some direct financial input is received from Vascutek and Mentice and is used to run events. No profit is derived from this initiative. Medtronic, Gore Medical, Abbott Medical, Vascutek and Mentice do not manufacture any pharmaceuticals, including anticoagulants.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
AMPLIFY Study {published data only}
- Agnelli G. Apixaban was noninferior to enoxaparin plus warfarin in patients with acute venous thromboembolism. Annals of Internal Medicine 2013;159(8):JC2. [DOI] [PubMed] [Google Scholar]
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. New England Journal of Medicine 2013;369(9):799‐808. [DOI] [PubMed] [Google Scholar]
- Agnelli GB, Masiukiewicz UP. Apixaban for the treatment of symptomatic deep‐vein thrombosis and pulmonary embolism: a randomized, double‐blind trial (AMPLIFY). Journal of Thrombosis and Haemostasis 2013;11(Suppl):18. [Google Scholar]
- NCT00633893. Efficacy and safety study of apixaban for the treatment of deep vein thrombosis or pulmonary embolism. http://clinicaltrials.gov/ct2/show/NCT00633893?term=ajax&rank=5 2009.
EINSTEIN‐PE {published data only}
- Buller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. New England Journal of Medicine 2012;366:1287‐97. [DOI] [PubMed] [Google Scholar]
- NCT00439777. Oral direct factor Xa inhibitor rivaroxaban In patients with acute symptomatic pulmonary embolism with or without symptomatic deep‐vein thrombosis: Einstein‐PE evaluation. https://clinicaltrials.gov/ct2/show/NCT00439777 (accessed June 2015) 2008.
- Prins M, Bamber L, Cano S, Wang M, Lensing AWA, Bauersachs R. Patient‐reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of acute symptomatic pulmonary embolism. Blood 2012;120(21):Abstract 1163. [Google Scholar]
- Prins MH, Lensing AW, Bauersachs R, Bellen B, Bounameaux H, Brighton TA, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN‐DVT and PE randomized studies. Thrombosis Journal 2013;11(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins MHE. Incidence of recurrent venous thromboembolism in patients following completion of the EINSTEIN DVT and EINSTEIN PE studies. Journal of Thrombosis and Haemostasis 2013;11(Suppl):257. [Google Scholar]
- Bellen B, Bamber L, Correa De Carvalho F, Prins M, Wang M, Lensing AWA. Reduction in the length of stay with rivaroxaban as a single‐drug regimen for the treatment of deep vein thrombosis and pulmonary embolism. Current Medical Research and Opinion 2014;30(5):829‐37. [DOI] [PubMed] [Google Scholar]
- Bellen B, Prins M, Bamber L, Wang M, Lensing AWA. Reduction in initial length of stay with rivaroxaban single‐drug regimen versus LMWH‐VKA standard of care: findings from the Einstein trial program. Blood 2012;120(21):Abstract 3419. [Google Scholar]
- Wang Y, Wang C. Rivaroxaban for the treatment of symptomatic deep vein thrombosis and/or pulmonary embolism in Chinese patients: a subgroup analysis of the EINSTEIN DVT and PE studies. Journal of Thrombosis and Haemostasis 2013;11(Suppl):694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hokusai‐VTE Study {published data only}
- Raskob G, Buller H, Prins M, Segers A, Shi M, Schwocho L, et al. Edoxaban for the long‐term treatment of venous thromboembolism: rationale and design of the Hokusai‐venous thromboembolism study ‐ methodological implications for clinical trials. Journal of Thrombosis and Haemostasis 2013;11(7):1287‐94. [DOI] [PubMed] [Google Scholar]
- Raskob GE, Buller H, Angchaisuksiri P, Oh D, Boda Z, Lyons RM, et al. Edoxaban for long‐term treatment of venous thromboembolism in cancer patients. Blood 2013;122(21):211. [Google Scholar]
- The Hokusai‐VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. New England Journal of Medicine 2013;369(15):1406‐15. [DOI] [PubMed] [Google Scholar]
RE‐COVER {published data only}
- NCT00291330. Efficacy and safety of dabigatran compared to warfarin for 6 month treatment of acute symptomatic venous thromboembolism (RE‐COVER I). http://clinicaltrials.gov/ct/show/NCT00291330 (accessed June 2015) 2007.
- Schulman S, Baanstra D, Eriksson H, Goldhaber S, Kakkar A, Kearon C. Dabigatran vs. placebo for extended maintenance therapy of venous thromboembolism. Journal of Thrombosis and Haemostasis 2011;9(Suppl 2):22. [Google Scholar]
- Schulman S, Baanstra D, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, et al. Benefit of extended maintenance therapy for venous thromboembolism with dabigatran etexilate is maintained over 1 year of post‐treatment follow‐up. Blood 2012;120(21):Abstract 332. [Google Scholar]
- Schulman S, Eriksson H, Feuring M, Hantel S. Efficacy of dabigatran versus warfarin in patients with acute venous thromboembolism and thrombophilia: a pooled analysis of RE‐COVER and RE‐COVER II. Circulation. 2014; Vol. 130:A18594.
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Mismetti P, et al. Safety of dabigatran vs. warfarin for acute venous thromboembolism: pooled analyses of RE‐COVER and RE‐COVER II. Journal of Thrombosis and Haemostasis 2013;11:225‐6. [Google Scholar]
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Mismetti P, et al. Treatment of acute pulmonary embolism with dabigatran or warfarin: a pooled analysis of efficacy data from RE‐COVER and RE‐COVER II. European Society of Cardiology Conference, Barcelona, Spain. Barcelona, 2014:Abstract: P5509.
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Schellong SM, et al. Influence of age on the efficacy and safety of dabigatran versus warfarin for the treatment of acute venous thromboembolism: a pooled analysis of RE‐cover and RE‐cover II. 55th Annual Meeting of the American Society of Hematology Abstracts. 2013.
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Schellong SM, et al. Influence of concomitant NSAID or ASA on the efficacy and safety of dabigatran versus warfarin for the treatment of acute venous thromboembolism: a pooled analysis from RE‐COVER and RE‐COVER II. Blood 2013;122(21):212. [Google Scholar]
- Schulman S, Kakkar AK, Schellong SM, Goldhaber SZ, Henry E, Mismetti P, et al. A randomized trial of dabigatran versus warfarin in the treatment of acute venous thromboembolism (RE‐COVER II). Blood 2011;118:Abstract 205. [Google Scholar]
- Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. New England Journal of Medicine 2009;361(24):2342‐52. [DOI] [PubMed] [Google Scholar]
RE‐COVER II {published data only}
- Schulman S. A randomized trial of dabigatran versus warfarin in the treatment of acute venous thromboembolism (RE‐COVER II). Blood 2011;118(21):95‐6. [Google Scholar]
- Schulman S, Eriksson H, Feuring M, Hantel S. Efficacy of dabigatran versus warfarin in patients with acute venous thromboembolism and thrombophilia: a pooled analysis of RE‐COVER and RE‐COVER II. Circulation. 2014; Vol. 130:A18594.
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Mismetti P, et al. Safety of dabigatran vs. warfarin for acute venous thromboembolism: pooled analyses of RE‐COVER and RE‐COVER II. Journal of Thrombosis and Haemostasis 2013;11:225‐6. [Google Scholar]
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Mismetti P, et al. Treatment of acute pulmonary embolism with dabigatran or warfarin: a pooled analysis of efficacy data from RE‐COVER and RE‐COVER II. European Society of Cardiology Conference, Barcelona, Spain. 2014:Abstract: P5509.
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Schellong SM, et al. Influence of age on the efficacy and safety of dabigatran versus warfarin for the treatment of acute venous thromboembolism: a pooled analysis of RE‐COVER and RE‐COVER II. Blood 2013;122:2375. [Google Scholar]
- Schulman S, Eriksson H, Goldhaber SZ, Kakkar A, Kearon C, Schellong SM, et al. Influence of concomitant NSAID or ASA on the efficacy and safety of dabigatran versus warfarin for the treatment of acute venous thromboembolism: a pooled analysis from RE‐COVER and RE‐COVER II. Blood 2013;122(21):212. [Google Scholar]
- Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014;129:764‐72. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ageno 2014 {published data only}
- Ageno W, Mantovani LG, Haas S, Kreutz R, Haupt V, et al. XALIA: Rationale and design of a non‐interventional study of rivaroxaban compared with standard therapy for initial and long‐term anticoagulation in deep vein thrombosis. Thrombosis Journal 2014;12(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
AMPLIFY Extended Study {published data only}
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Apixaban for extended treatment of venous thromboembolism. New England Journal of Medicine 2013;368(8):699‐708. [DOI] [PubMed] [Google Scholar]
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson MR, et al. Two doses of apixaban for the extended treatment of venous thromboembolism. Blood 2012;120(21):LBA‐1. [Google Scholar]
- Liu X, Thompson J, Phatak H, Mardekian J, Porcari AR, Johnson MR. Apixaban reduces hospitalization in patients with venous thromboembolism: an analysis of the AMPLIFY‐EXT trial. Blood 2013;122(21):Abstract 3638. [DOI] [PubMed] [Google Scholar]
Botticelli DVT Study {published data only}
- Barrett YC, Wang J, Knabb R, Mohan P. Apixaban decreases coagulation activity in patients with acute deep‐vein thrombosis. Thrombosis and Haemostasis 2011;105:181‐9. [DOI] [PubMed] [Google Scholar]
- Botticelli IWC, Buller H, Deitchman D, Prins M, Segers A. Efficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose‐ranging study. Journal of Thrombosis and Haemostasis 2008;6(8):1313‐8. [DOI] [PubMed] [Google Scholar]
- Buller HR. A dose finding study of the oral direct factor Xa inhibitor apixaban in the treatment of patients with acute symptomatic deep vein thrombosis ‐ The Botticelli Investigators. XXIst Congress of the International Society on Thrombosis and Haemostasis; 2007 Jul 6‐12; Geneva. 2007.
- NCT00252005. Oral direct factor Xa‐inhibitor apixaban in patients with acute symptomatic deep‐vein thrombosis ‐ the Botticelli DVT study. http://clinicaltrials.gov/ct/show/NCT00252005?order=1 2007.
Einstein‐DVT Dose Study {published data only}
- Buller H, Darius H. EINSTEIN DVT: Oral rivaroxaban versus standard therapy in the initial treatment of symptomatic deep vein thrombosis and long‐term prevention of recurrent venous thromboembolism. http://www.escardio.org/congresses/esc‐2010/congress‐reports/Pages/708‐4‐EINSTEIN‐DVT.aspx#.UvNXl03itMs 2010.
- Buller HR, Agnelli G. Once‐ or twice‐daily rivaroxaban for the treatment of proximal deep vein thrombosis: similar efficacy and safety to standard therapy in dose‐ranging studies. Blood 2006;108(11 Pt 1):172‐3. [Google Scholar]
- Buller HR, Lensing AW, Prins MH, Agnelli G, Cohen A, Gallus AS, et al. A dose‐ranging study evaluating once‐daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein‐DVT dose‐ranging study. Blood 2008;112(6):2242‐7. [DOI] [PubMed] [Google Scholar]
- NCT00395772. Once‐daily oral direct factor Xa inhibitor BAY 59‐7939 in patients with acute symptomatic deep‐vein thrombosis. The Einstein‐DVT dose‐finding study. http://clinicaltrials.gov/ct2/show/NCT00395772?term=einstein‐dvt&rank=2 2006.
Einstein DVT Study {published data only}
- Bamber L, Wang MY, Prins MH, Ciniglio C, et al. Patient‐reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of acute symptomatic deep‐vein thrombosis. Thrombosis and Haemostasis 2013;110(4):732‐41. [DOI] [PubMed] [Google Scholar]
- Buller HR. Oral rivaroxaban for the acute and continued treatment of symptomatic venous thromboembolism. The Einstein‐DVT and Einstein‐Extension study. Blood 2010;116(21):Abstract 187. [Google Scholar]
- Prandoni P. Treatment of patients with acute deep vein thrombosis and/or pulmonary embolism: efficacy and safety of non‐VKA oral anticoagulants in selected populations. Thrombosis Research 2014;134(2):227‐33. [DOI] [PubMed] [Google Scholar]
EINSTEIN Extension Study {published data only}
- NCT00439725. Once ‐ daily oral direct factor Xa inhibitor rivaroxaban In the long‐term prevention of recurrent symptomatic venous thromboembolism in patients with symptomatic deep‐vein thrombosis or pulmonary embolism. The Einstein‐Extension study. http://clinicaltrials.gov/ct2/show/NCT00439725?term=NCT00439725&rank=1 2008.
ODIXa‐DVT Study {published data only}
- Agnelli G, Gallus A, Goldhaber SZ, Haas S, Huisman MV, Hull RD, et al. Treatment of proximal deep‐vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59‐7939): the ODIXa‐DVT (oral direct factor Xa inhibitor BAY 59‐7939 in patients with acute symptomatic deep‐vein thrombosis) study. Circulation 2007;116(2):180‐7. [DOI] [PubMed] [Google Scholar]
- Anon. Oral direct factor Xa inhibitor BAY 59‐7939 in patients with acute symptomatic proximal deep vein thrombosis. ‐ ODIXa‐DVT study. http://trialfinder.bayerscheringpharma.de/html/pdf/11223_Study_Synopsis_CTP.pdf 2008. [DOI] [PubMed]
Piazza 2014 {published data only}
- NCT01662908. A randomized, open‐label, parallel‐group, multi‐center study for the evaluation of efficacy and safety of edoxaban monotherapy versus low molecular weight (LMW) heparin/warfarin in subjects with symptomatic deep‐vein thrombosis (eTRIS). http://www.clinicaltrials.gov/ct2/show/NCT01662908?term=edoxaban&rank=4 (accessed 3 February 2015).
- Piazza G, Mani V, Grosso M, Mercuri M, Lanz H, Schussler S, et al. A randomized, open‐label, multicenter study of the efficacy and safety of edoxaban monotherapy versus low‐molecular weight heparin/warfarin in patients with symptomatic deep vein thrombosis‐edoxaban thrombus reduction imaging study (eTRIS). Circulation 2014;130:A12074. [Google Scholar]
REMEDY {published data only}
- Liem TK, DeLoughery TG. Randomised controlled trial: extended‐duration dabigatran is non‐inferior to warfarin and more effective than placebo for symptomatic VTE. Evidence Based Medicine 2014;19(1):29. [DOI] [PubMed] [Google Scholar]
- Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. New England Journal of Medicine 2013;368(8):709‐18. [DOI] [PubMed] [Google Scholar]
RE‐SONATE {published data only}
- Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. New England Journal of Medicine 2013;368(8):709‐18. [DOI] [PubMed] [Google Scholar]
THRIVE {published data only}
- Fiessinger JN, Huisman MV, Davidson BL, Bounameaux H, Francis CW, Eriksson H, et al. Ximelagatran vs low‐molecular‐weight heparin and warfarin for the treatment of deep vein thrombosis: a randomized trial. JAMA 2005;293(6):681‐9. [DOI] [PubMed] [Google Scholar]
- Harenberg J, Ingrid J, Tivadar F. Treatment of venous thromboembolism with the oral thrombin inhibitor, ximelagatran. Israel Medical Association Journal 2002;4(11):1003‐5. [PubMed] [Google Scholar]
- Harenberg J, Joerg I, Weiss C. Incidence of recurrent venous thromboembolism of patients after termination of treatment with ximelagatran. European Journal of Clinical Pharmacology 2006;62(3):173‐7. [DOI] [PubMed] [Google Scholar]
THRIVE I {published data only}
- Eriksson H, Lundstrom T, Wahlander K, Clason SB, Schulman S. Prognostic factors for recurrence of venous thromboembolism (VTE) or bleeding during long‐term secondary prevention of VTE with ximelagatran. Thrombosis and Haemostasis 2005;94(3):522‐7. [PubMed] [Google Scholar]
- Eriksson H, Wahlander K, Gustafsson D, Welin LT, Frison L, Schulman S, et al. A randomized, controlled, dose‐guiding study of the oral direct thrombin inhibitor ximelagatran compared with standard therapy for the treatment of acute deep vein thrombosis: THRIVE I. Journal of Thrombosis and Haemostasis 2003;1(1):41‐7. [DOI] [PubMed] [Google Scholar]
- Eriksson H, Wahlander K, Lundstrom T, Billing Clason S, Schulman S. Extended secondary prevention with the oral direct thrombin inhibitor ximelagatran for 18 months after 6 months of anticoagulation in patients with venous thromboembolism: a randomized, placebo‐controlled trial. Blood 2002;100:81a. [Google Scholar]
- Francis CW, Ginsberg JS, Berkowitz SD, Bounameaux H, Davidson BL, Eriksson H, et al. Efficacy and safety of the oral direct thrombin inhibitor ximelagatran compared with current therapy for acute, symptomatic deep vein thrombosis, with or without pulmonary embolus: the THRIVE treatment study. Blood 2003;102(11):Abstract 7. [Google Scholar]
- Huisman MV, The THRIVETSI. Efficacy and safety of the oral direct thrombin inhibitor ximelagatran compared with current standard therapy for acute symptomatic deep vein thrombosis, with or without pulmonary embolism: a randomized, double‐blind, multinational study. Journal of Thrombosis & Haemostasis 2003;1(Suppl 1):Abstract OC003. [Google Scholar]
- Schulman S, Lundstrom T, Walander K, Billing Clason S, Eriksson H. Ximelagatran for the secondary prevention of venous thromboembolism: a complementary follow‐up analysis of the THRIVE III study 1828. Thrombosis and Haemostasis 2005;94(4):820‐4. [PubMed] [Google Scholar]
- Wimperis J, Fiessinger JN, Huisman MV, Davidson BL, Bounameaux H, Francis CW, et al. Ximelagatran, an oral direct thrombin inhibitor, compared with current standard therapy for acute, symptomatic deep vein thrombosis, with or without pulmonary embolism: the THRIVE Treatment Study. British Journal of Haematology 2004;125(Suppl 1):66. [Google Scholar]
THRIVE III {published data only}
- Harenberg J, Jorg I, Weiss C, Harenberg J, Jorg I, Weiss C. Observations of alanine aminotransferase and aspartate aminotransferase in THRIVE studies treated orally with ximelagatran. International Journal of Toxicology 2006;25(3):165‐9. [DOI] [PubMed] [Google Scholar]
- Schulman S, Wahlander K, Lundstrom T, Clason SB, Eriksson H, THRIVE III I. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. New England Journal of Medicine 2003;349(18):1713‐21. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR‐TRC‐14005223 {published data only}
- ChiCTR‐TRC‐14005223. Efficacy and safety of rivaroxaban or warfarin on venous thromboembolic disease: a randomized controlled trial. http://www.chictr.org/en/proj/show.aspx?proj=10248 (accessed 1 February 2015).
NCT01780987 {published data only}
- NCT01780987. A study to evaluate safety and efficacy of apixaban In Japanese acute deep vein thrombosis (DVT) and pulmonary embolism (PE) patients. http://clinicaltrials.gov/show/NCT01780987 (accessed 1 March 2014).
NCT01895777 {published data only}
- NCT01895777. Open label study comparing efficacy and safety of dabigatran etexilate to standard of care in paediatric patients with venous thromboembolism (VTE). http://clinicaltrials.gov/show/NCT01895777 (accessed 1 March 2014).
NCT02234843 {published data only}
- NCT02234843. EINSTEIN Junior phase III: oral rivaroxaban in children with venous thrombosis (EINSTEIN Jr). https://clinicaltrials.gov/ct2/show/NCT02234843 (accessed 1 February 2015).
NCT02309411 {published data only}
- NCT02309411. EINSTEIN Junior phase II: oral rivaroxaban in young children with venous thrombosis (EINSTEIN Jr). https://clinicaltrials.gov/ct2/show/NCT02309411 (accessed 1 February 2015).
Additional references
Ageno 2012
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G, American College of Chest Physicians. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141(Suppl 2):e44S‐88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agnelli 2007
- Agnelli G, Gallus A, Goldhaber SZ, Haas S, Huisman MV, Hull RD, et al. Treatment of proximal deep‐vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59‐7939): the ODIXa‐DVT (Oral direct factor Xa inhibitor BAY 59‐7939 in patients with acute symptomatic deep‐vein thrombosis) study. Circulation 2007;116(2):180‐7. [DOI] [PubMed] [Google Scholar]
Agnelli 2013
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. New England Journal of Medicine 2013;369(9):799‐808. [DOI] [PubMed] [Google Scholar]
Anderson 2009
- Anderson DR, Barnes DC. Computerized tomographic pulmonary angiography versus ventilation perfusion lung scanning for the diagnosis of pulmonary embolism. Current Opinion in Pulmonary Medicine 2009;15(5):425‐9. [DOI] [PubMed] [Google Scholar]
Antoniazzi 2103
- Antoniazzi S, Berdai D, Conti V, Robinson P, Radice S, Clementi E, et al. Risk of major bleeding with dabigatran versus active controls: a systematic review and meta‐analysis. Congres de Physiologie de Pharmacolgoie et de Therapeutique; 2013 April 22‐24. 2013.
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baetz 2008
- Baetz BE, Spinler SA. Dabigatran etexilate: an oral direct thrombin inhibitor for prophylaxis and treatment of thromboembolic diseases. Pharmacotherapy 2008;28(11):1354‐73. [DOI] [PubMed] [Google Scholar]
Botticelli Investigators
- Botticelli Investigators, Writing Committee, Buller H, Deitchman D, Prins M, Segers A. Efficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose‐ranging study. Journal of Thrombosis and Haemostasis 2008;6(8):1313‐8. [DOI] [PubMed] [Google Scholar]
Boudes 2006
- Boudes PF. The challenges of new drugs benefits and risks analysis: lessons from the ximelagatran FDA Cardiovascular Advisory Committee. Contemporary Clinical Trials 2006;27(5):432‐40. [DOI] [PubMed] [Google Scholar]
Boutitie 2011
- Boutitie F, Pinede L, Schulman S, Agnelli G, Raskob G, Julian J, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants' data from seven trials. BMJ 2011;342:d3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castellucci 2013
- Castellucci LA, Cameron C, Gal G, Rodger MA, Coyle D, Wells PS, et al. Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: systematic review and network meta‐analysis. BMJ 2013;347:f5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Connolly 2009
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. New England Journal of Medicine 2009;361(12):1139‐51. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Eikelboom 2010
- Eikelboom JW, Weitz JI. Update on antithrombotic therapy: new anticoagulants. Circulation 2010;121(13):1523‐32. [DOI] [PubMed] [Google Scholar]
EINSTEIN Investigators
- EINSTEIN Investigators, Bauersachs R, Berkowitzm SD, Brenner B, Buller HR, Decousus H, et al. Oral rivaroxaban for symptomatic venous thromboembolism. New England Journal of Medicine 2010;363(26):2499‐510. [DOI] [PubMed] [Google Scholar]
Eriksson 2003
- Eriksson H, Wåhlander K, Gustafsson D, Welin LT, Frison L, Schulman S, et al. A randomized, controlled, dose‐guiding study of the oral direct thrombin inhibitor ximelagatran compared with standard therapy for the treatment of acute deep vein thrombosis: THRIVE I. Journal of Thrombosis and Haemostasis 2003;1(1):41‐7. [DOI] [PubMed] [Google Scholar]
Eriksson 2007
- Eriksson BI, Dahl OE, Rosenecher N, Kurtha AA, Dijk CN, Frostick SP, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE‐MODEL randomized trial. Journal of Thrombosis and Haemostasis 2007;5(11):2178‐85. [DOI] [PubMed] [Google Scholar]
Eriksson 2009
- Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clinical Pharmacokinetics 2009;48(1):1‐22. [DOI] [PubMed] [Google Scholar]
Fox 2012
- Fox BD, Kahn SR, Langleben D, Eisenberg MJ, Shimony A. Efficacy and safety of novel oral anticoagulants for treatment of acute venous thromboembolism: direct and adjusted indirect meta‐analysis of randomised controlled trials. BMJ 2012;345:e7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomez‐Outes 2014
- Gomez‐Outes A, Terleira‐Fernandez AI, Lecumberri R, Suarez‐Gea ML, Vargas‐Castrillon E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta‐analysis. Thrombosis Research 2014;134(4):774‐82. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirschl 2014
- Hirschl M, Kundi M. New oral anticoagulants in the treatment of acute venous thromboembolism ‐ a systematic review with indirect comparisons. [Review]. Vasa 2014;43(5):353‐64. [DOI] [PubMed] [Google Scholar]
Huerta 2007
- Huerta C, Johansson S, Wallander MA, Garcia Rodriguez LA. Risk factors and short‐term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Archives of Internal Medicine 2007;167(9):935‐43. [DOI] [PubMed] [Google Scholar]
Kam 2005
- Kam PC, Kaur N, Thong CL. Direct thrombin inhibitors: pharmacology and clinical relevance. Anaesthesia 2005;60(6):565‐74. [DOI] [PubMed] [Google Scholar]
Kang 2014
- Kang N, Sobieraj DM. Indirect treatment comparison of new oral anticoagulants for the treatment of acute venous thromboembolism. Thrombosis Research 2014;133:1145‐51. [DOI] [PubMed] [Google Scholar]
Kearon 2012
- Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e419S‐94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laurence 2012
- Laurence IJ, Redman SL, Corrigan AJ, Graham RN. V/Q SPECT imaging of acute pulmonary embolus ‐ a practical perspective. Clinical Radiology 2012;67(10):941‐8. [DOI] [PubMed] [Google Scholar]
Lee 2011
- Lee CJ, Ansell JE. Direct thrombin inhibitors. British Journal of Clinical Pharmacology 2011;72(4):581‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2012a
- National Institute for Health and Care Excellence. Venous thromboembolic diseases: the management of thromboembolic diseases and the role of thrombophilia testing, 2012. guidance.nice.org.uk/CG144 (accessed 11 January 2014).
NICE 2012b
- National Institute of Health and Care Excellence. Rivaroxaban for the treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis and pulmonary embolism, 2012. guidance.nice.org.uk/TA261 (accessed 11 January 2014).
NICE 2013
- National Institute for Health and Care Excellence. Pulmonary embolism likely based on two‐level Wells score, 2013. http://pathways.nice.org.uk/pathways/venous‐thromboembolism/pulmonary‐embolism‐likely‐based‐on‐two‐level‐wells‐score (accessed 11 January 2013).
NICE 2014
- National Institute for Health Care and Excellence. Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism. NICE technology appraisal guidance [TA327] December 2014.
Oldgren 2011
- Oldgren J, Budaj A, Granger CB, Khder Y, Roberts J, Siegbahn A, et al. Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double‐blind, phase II trial. European Heart Journal 2011;32(22):2781‐9. [DOI] [PubMed] [Google Scholar]
Palladino 2013
- Palladino M, Merli G, Thomson L. Evaluation of the oral direct factor Xa inhibitor ‐ betrixaban. Expert Opinion on Investigational Drugs 2013;22(11):1465‐72. [DOI] [PubMed] [Google Scholar]
Qaseem 2007
- Qaseem A, Snow V, Barry PE, Hornbake R, Rodnick JE, Tobolic T, et al. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Annals of Internal Medicine 2007;146(6):454‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riedel 2004
- Riedel M. Diagnosing pulmonary embolism. Postgraduate Medicine Journal 2004;80(944):309‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 2015
- Robertson L, Kesteven P. Oral direct thrombin inhibitors or oral factor Xa inhibitors for the treatment of deep vein thrombosis. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD010956.pub2] [DOI] [PubMed] [Google Scholar]
Sardar 2014
- Sardar P, Chatterjee S, Mukherjee D. Efficacy and safety or new oral anticoagulants for extended treatment of venous thromboembolism: systematic review and meta‐analyses of randomised controlled trials. Drugs 2013;73:1171‐82. [DOI] [PubMed] [Google Scholar]
Schulman 2005
- Schulman S, Kearon C, and the Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. Journal of Thrombosis and Haemostasis 2005;3(4):692‐4. [DOI] [PubMed] [Google Scholar]
Schulman 2011
- Schulman S, Kakkar AK, Schellong SM, Goldhaber SZ, Henry E, Mismetti P, et al. A randomized trial of dabigatran versus warfarin in the treatment of acute venous thromboembolism (RE‐COVER II). Blood 2011;118:Abstract 205. [Google Scholar]
SIGN 2010
- Scottish Intercollegiate Guidelines Network. Prevention and management of venous thromboembolism: a national clinical guideline, 2010. www.sign.ac.uk/pdf/sign122.pdf (accessed 11 January 2014).
Spyropoulos 2012
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012;120(15):2954‐62. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Van de Werf 2012
- Werf F, Brueckmann M, Connolly SJ, Friedman J, Granger CB, Hartter S, et al. A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: the randomized, phase II study to evaluate the safety and pharmacokinetics of oral dabigatran etexilate in patients after heart valve replacement (RE‐ALIGN). American Heart Journal 2012;163(6):931‐7. [DOI] [PubMed] [Google Scholar]
van der Huille 2014
- Huille T, Exter PL, Dekkers OM, Klok FA. Effectiveness and safety of novel anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta‐analysis. Journal of Thrombosis and Haemostasis 2014;12:320‐8. [DOI] [PubMed] [Google Scholar]
Weitz 2003
- Weitz JI. A novel approach to thrombin inhibition. Thrombosis Research 2003;109(Suppl 1):S17‐22. [DOI] [PubMed] [Google Scholar]
Wells 2000
- Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D‐dimer. Thrombosis and Haemostasis 2000;83(3):416‐20. [PubMed] [Google Scholar]
References to other published versions of this review
Robertson 2014b
- Robertson L, Kesteven P. Oral direct thrombin inhibitors or oral factor Xa inhibitors for the treatment of pulmonary embolism. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD010957] [DOI] [PMC free article] [PubMed] [Google Scholar]


