Abstract
Background
Traumatic brain injury (TBI) is a leading cause of death and disability, and the identification of effective, inexpensive and widely practicable treatments for brain injury is of great public health importance worldwide. Progesterone is a naturally produced hormone that has well‐defined pharmacokinetics, is widely available, inexpensive, and has steroidal, neuroactive and neurosteroidal actions in the central nervous system. It is, therefore, a potential candidate for treating TBI patients. However, uncertainty exists regarding the efficacy of this treatment. This is an update of our previous review of the same title, published in 2012.
Objectives
To assess the effects of progesterone on neurologic outcome, mortality and disability in patients with acute TBI. To assess the safety of progesterone in patients with acute TBI.
Search methods
We updated our searches of the following databases: the Cochrane Injuries Group's Specialised Register (30 September 2016), the Cochrane Central Register of Controlled Trials (CENTRAL; Issue 9, 2016), MEDLINE (Ovid; 1950 to 30 September 2016), Embase (Ovid; 1980 to 30 September 2016), Web of Science Core Collection: Conference Proceedings Citation Index‐Science (CPCI‐S; 1990 to 30 September 2016); and trials registries: Clinicaltrials.gov (30 September 2016) and the World Health Organization (WHO) International Clinical Trials Registry Platform (30 September 2016).
Selection criteria
We included randomised controlled trials (RCTs) of progesterone versus no progesterone (or placebo) for the treatment of people with acute TBI.
Data collection and analysis
Two review authors screened search results independently to identify potentially relevant studies for inclusion. Independently, two review authors selected trials that met the inclusion criteria from the results of the screened searches, with no disagreement.
Main results
We included five RCTs in the review, with a total of 2392 participants. We assessed one trial to be at low risk of bias; two at unclear risk of bias (in one multicentred trial the possibility of centre effects was unclear, whilst the other trial was stopped early), and two at high risk of bias, due to issues with blinding and selective reporting of outcome data.
All included studies reported the effects of progesterone on mortality and disability. Low quality evidence revealed no evidence of a difference in overall mortality between the progesterone group and placebo group (RR 0.91, 95% CI 0.65 to 1.28, I² = 62%; 5 studies, 2392 participants, 2376 pooled for analysis). Using the GRADE criteria, we assessed the quality of the evidence as low, due to the substantial inconsistency across studies.
There was also no evidence of a difference in disability (unfavourable outcomes as assessed by the Glasgow Outcome Score) between the progesterone group and placebo group (RR 0.98, 95% CI 0.89 to 1.06, I² = 37%; 4 studies; 2336 participants, 2260 pooled for analysis). We assessed the quality of this evidence to be moderate, due to inconsistency across studies.
Data were not available for meta‐analysis for the outcomes of mean intracranial pressure, blood pressure, body temperature or adverse events. However, data from three studies showed no difference in mean intracranial pressure between the groups. Data from another study showed no evidence of a difference in blood pressure or body temperature between the progesterone and placebo groups, although there was evidence that intravenous progesterone infusion increased the frequency of phlebitis (882 participants). There was no evidence of a difference in the rate of other adverse events between progesterone treatment and placebo in the other three studies that reported on adverse events.
Authors' conclusions
This updated review did not find evidence that progesterone could reduce mortality or disability in patients with TBI. However, concerns regarding inconsistency (heterogeneity among participants and the intervention used) across included studies reduce our confidence in these results.
There is no evidence from the available data that progesterone therapy results in more adverse events than placebo, aside from evidence from a single study of an increase in phlebitis (in the case of intravascular progesterone).
There were not enough data on the effects of progesterone therapy for our other outcomes of interest (intracranial pressure, blood pressure, body temperature) for us to be able to draw firm conclusions.
Future trials would benefit from a more precise classification of TBI and attempts to optimise progesterone dosage and scheduling.
Plain language summary
Progesterone for traumatic brain injury
Review question
To find out whether using the hormone progesterone to treat people who have had an injury to the head that caused brain damage (traumatic brain injury (TBI)) is helpful and safe, if given within 24 hours of the injury.
Background
TBI is one of the main causes of death and disability in people with injuries. Damage to the brain can start at the time of the injury, but can continue for days after the injury too. Progesterone is a hormone that some doctors think could be used as a potential medicine for reducing brain damage if given shortly after TBI. However, as there is uncertainty about the effectiveness of this hormone, it is important that we assess the evidence.
Study characteristics
We searched the medical literature widely for randomised controlled trials that investigated the effects of progesterone in people with TBI up to 30 September 2016. Randomised controlled trials provide the most robust medical evidence. .
Key results We included five studies with a total of 2392 participants, and identified three ongoing studies. The studies all compared a group of participants who received progesterone within 24 hours of TBI against a group who received a pretend ‐ or dummy ‐ medicine (known as a placebo) that looked the same as the progesterone.
The results of our review did not find evidence that, when compared to placebo, progesterone could reduce death and disability in people with TBI. There were too few data available on the other outcomes that we were interested in (pressure inside the skull (intracranial pressure), blood pressure, body temperature and adverse events (harms)), for us to be able to analyse these in detail. However, although the information available shows no evidence of a difference in effect between the progesterone and control groups for intracranial pressure, blood pressure or body temperature, one study showed an increased level of an adverse event called phlebitis (inflammation in the vein) in the progesterone group, possibly because the progestreone was given into the vein through an intravascular infusion ('drip').
Quality of the evidence
We judged the quality of the evidence to be low for the data on risk of death, and moderate for the data on risk of disability. These judgements resulted from differences across studies, including different doses of progesterone and different time points for assessment of participants in the included studies. This means that we have limited confidence in the conclusions of this review.
Summary of findings
for the main comparison.
| Progesterone compared with no progesterone or placebo for traumatic brain injury | ||||||
|
Patient or population: people with acute TBI secondary to head injury Settings: hospitals, intensive care units Intervention: progesterone therapy Comparison: no progesterone or placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Progesterone | |||||
| Mortality at end of scheduled follow‐up | 192 per 1000 |
175 per 1000 (125 to 246) |
RR 0.91 (0.65 to 1.28) | 2376 (5 studies) | ⊕⊕⊝⊝ Low1,2,3 | There is no evidence of a reduction of mortality at the end of scheduled follow‐up as a result of progesterone therapy. Our confidence in this evidence is limited as we have assessed it as low quality. |
| Disability (unfavourable outcomes: death, vegetative state, severe disability; GOS 1‐3) at end of scheduled follow‐up | 533 per 1000 |
522 per 1000 (474 to 565) |
RR 0.98 (0.89 to 1.06) | 2260 (4 studies) |
⊕⊕⊕⊝ Moderate1,3 | There is no evidence of a difference in disability (unfavourable outcomes) at the end of scheduled follow‐up as a result of progesterone therapy. Our confidence in this evidence is somewhat limited as we have assessed it as moderate quality. |
| Intracranial pressure (ICP) within the treatment period | ‐ | ‐ | ‐ | 3 studies | ‐ | In Xiao 2008, ICP data were presented as mean values. In Wright 2006, ICP data were presented as the mean frequency of pressures exceeding threshold values. In Skolnick 2014, ICP data were presented as the population with increased ICP. We were therefore not able to perform meta‐analysis. There was no evidence that progesterone therapy has an effect on ICP. |
| Blood pressure | ‐ | ‐ | ‐ | 1 study | ‐ | "Throughout the three‐day infusion interval, there was no difference between the progesterone and placebo groups" (Wright 2006) |
| Body temperature | ‐ | ‐ | ‐ | 1 study | ‐ | "Progesterone group experienced a lower increase in mean temperature than the control group" (Wright 2006) |
| Adverse events | ‐ | ‐ | ‐ | 4 studies | ‐ | Wright 2014 reported that phlebitis or thrombophlebitis was significantly more frequent in the progesterone group than in the placebo group (882 cases, RR 3.03; 95% CI, 1.96 to 4.66). The rates of other serious and non‐serious adverse events were similar in the 4 studies. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;GOS: Glasgow Outcome Scale; ICP: intracranial pressure; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. We judged the overall risk of bias of two studies as high, and two studies as unclear. However, most data were from studies at low or unclear risk of bias, so we did not downgrade for risk of bias.
2. Downgraded once for inconsistency (substantial heterogeneity: I² = 62%, P value 0.03)
3. Downgraded once for inconsistency (the dosage, treatment routine and vehicles of progesterone varied across studies. Different time points were involved in the analysis of mortality and unfavourable outcomes).
Background
Description of the condition
Traumatic brain injury (TBI) is one of the main causes of death and disability in people with injuries (Ghajar 2000). Globally, at least 10 million people are killed or hospitalised every year because of TBI (Hyder 2007). The cost to society of TBI is considerable. In the USA, it is estimated that the cost of acute treatment and rehabilitation for patients with brain injury is around two billion dollars (USD) per year (McGarry 2002), not including indirect costs to families and society. The identification of effective, inexpensive and widely practicable treatments for brain injury is of great public health importance.
Although much of the neurological damage after TBI is caused at the time of the injury, secondary brain damage caused by mechanisms such as brain oedema, free radical formation or release of inflammatory mediators may exacerbate the primary injury. Severe injury sets in motion a cascade of events over several hours that can lead to secondary damage or death of brain tissue. To date, no pharmacologic agent has been shown to improve outcomes of TBI (Gultekin 2016). Methylprednisolone, once considered a mainstay of treatment, has been shown to be harmful (Alderson 2008), and there is no evidence to support the use of magnesium in patients with acute TBI (Arango 2008). It is important to search for safe and clinically effective neuroprotective drugs to prevent secondary brain damage after TBI, and progesterone has several features that make it an attractive candidate.
Description of the intervention
Progesterone, a hormone which is both widely available and inexpensive, has steroidal, neuroactive and neurosteroidal actions in the central nervous system. The pharmacokinetics of progesterone are well known, as the drug has been safely used for a long time (Allolio 1995; Goldfien 1989). Progesterone is present in the brains of men and women in small, roughly equal concentrations. Progesterone receptors are widely distributed throughout the central nervous system (Schumacher 1995). Although progesterone's non‐neurologic effects are well known, the steroid also has neuroprotective properties (Singh 2013). At the preclinical level, there is increasing evidence that progesterone could produce beneficial effects in brain and spinal cord injuries (Brotfain 2016), stroke (Yousuf 2016), brain haemorrhage (Hsieh 2016), and neurodegenerative diseases (De 2013).
How the intervention might work
A great number of preclinical studies have reported a therapeutic effect of progesterone in the central nervous system. Progesterone is thought to decrease brain oedema and help to maintain the integrity of the blood‐brain barrier (He 2014; Soltani 2016; Wang 2013), prevent apoptosis and necrosis (Li 2015; Yousuf 2016), reduce excitotoxicity by lessening the effect of neuroinflammation, reduce oxidative stress and alter glutamate receptor activity (Hong 2016; Luoma 2011; Webster 2015), and improve motor, sensory and cognitive recovery (Geddes 2016; Stein 2008; Wali 2016). However, so far, none of these encouraging preclinical results have led to evidence of any considerable improvement in clinical outcomes.
Why it is important to do this review
The limited evidence from the last version of our review published in 2012 revealed that progesterone might improve the neurologic outcome of acute TBI patients (Ma 2012). However, the previous systematic review has become outdated as the results of two recent phase III randomised controlled trials (RCTs) have now been published (Skolnick 2014; Wright 2014). An updated review was needed to provide a comprehensive assessment of the best available evidence of the safety and efficacy of progesterone treatment for acute TBI.
This updated systematic review of RCTs aims to quantify the evidence for the effects of progesterone administration on people with TBI. Because of the high incidence of TBI and its excessive cost each year, even a modest reduction in the risk of unfavourable outcomes could have major public health significance.
Objectives
To assess the effects of progesterone on neurologic outcome, mortality and disability in patients with acute TBI. To assess the safety of progesterone in patients with acute TBI.
Methods
Criteria for considering studies for this review
Types of studies
We included published randomised controlled trials (RCTs) of progesterone versus no progesterone (or placebo) for the treatment of acute TBI. In an attempt to improve the quality of our updated systematic review, we excluded non‐registered studies for which the study report was published after 2010 (Roberts 2015).
Types of participants
People, of any age, with clinically diagnosed with acute TBI secondary to head injury. All severities of head injury were included.
Types of interventions
Progesterone versus no progesterone or placebo, administered in any dose, by any route, for any duration and started within 24 hours of the head injury.
We only considered natural progesterone as the intervention. Synthetic progestin has different effects to natural progesterone in postinjury treatment. Consequently, we did not include the following compounds as interventions: medroxyprogesterone, megestrol acetate, chlormadinone, hydroxyprogesterone, norethindrone, norgestrel nor norethynodrel (Stein 2008).
Types of outcome measures
Primary outcomes
Mortality at end of scheduled follow‐up
Disability at end of scheduled follow‐up: assessed via the Glasgow Outcome Scale (GOS) and any other validated measures of neurological functioning and disability
Intracranial pressure within the treatment period
Secondary outcomes
Blood pressure
Body temperature
Complications and adverse events, including liver function abnormality, episodes of venous or arterial thromboembolism, allergic reactions, phlebitis
To determine the optimal information size we assumed a 20% control event rate (mortality) and a 25% relative risk reduction with 90% power and a 0.05 significance level. Our calculations indicated that the optimal information size needed to reliably detect a plausible treatment effect in mortality is 1212 patients in each group. For the disability outcome (GOS 1 to 3), we assumed a 50% control event and a 25% relative risk reduction with 90% power and a 0.05 significance level. The optimal information size needed to reliably detect a plausible treatment effect in disability is 329 patients in each group.
Search methods for identification of studies
The searches were not restricted by date, language or publication status.
Electronic searches
The Cochrane Injuries Group's Information Specialist updated searches of the following electronic databases and trials registries (2012 to date):
Cochrane Injuries Group's Specialised Register (30 September 2016);
Cochrane Central Register of Controlled Trials (CENTRAL; Issue 9, 2016, via Cochrane Register of Studies (CRSO));
MEDLINE (Ovid) (1950 to 30 September 2016);
Embase (Ovid) (1980 to 30 September 2016);
Web of Science Core Collection: Conference Proceedings Citation Index‐Science (CPCI‐S; 1990 to 30 September 2016);
Clinicaltrials.gov (30 September 2016);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (30 September 2016).
The full search strategies are presented in (Appendix 1).
Strategies for earlier searches (conducted in August 2012) are presented in (Appendix 2).
Data collection and analysis
Selection of studies
Two review authors (JM and YZ) independently screened the titles and abstracts of all citations found through the searches and decided whether or not the articles were potentially eligible for inclusion in the review. We obtained the full texts of all potentially relevant articles and the two review authors (JM and YZ) independently assessed whether each met the predefined inclusion criteria. We excluded any studies that did not fulfil the inclusion criteria, and the reasons for exclusion are noted in the 'Characteristics of excluded studies' table. We resolved any disagreement by discussion or arbitration with SH. We examined all duplicate study reports to verify that they presented unique sets of data.
Data extraction and management
Two review authors (JM and YZ) independently extracted data from the included studies on sequence generation, allocation concealment, loss to follow‐up, blinding of outcome assessment, types of participants, types of interventions, types of outcomes, methods of analysis (intention‐to‐treat (ITT) analysis or per protocol analysis, or both), comparability of groups at baseline, statistical methods used and source of study funding. Where necessary, we requested unpublished information from the study authors. We extracted data to allow an ITT method. For studies with a `modified ITT' method, if we had considered the reasons for exclusion of participants to be inappropriate and the data were available to the review author, we would have conducted analyses that include participants who were excluded by the study authors. We compared the data extracted by each author and resolved any disagreement by discussion or arbitration with SH. Data extracted are noted in the 'Characteristics of included studies' table.
Assessment of risk of bias in included studies
Two review authors (JM and YZ) assessed RCTs using the 'Risk of bias' assessment tool in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Each review author independently evaluated risk of bias through assessing: sequence generation, allocation concealment, blinding (assessments were made for each main outcome or class of outcomes), incomplete outcome data (assessments were made for each main outcome or class of outcomes), selective outcome reporting and other sources of bias. We resolved any disagreement through discussion or arbitration from SH. We came to a judgement relating to the risk of bias for each domain and we also categorised the overall risk of bias of individual studiesas being at: low, high or unclear risk of bias as follows (Higgins 2011):
low risk of bias (i.e. plausible bias unlikely to alter the results seriously) if all domains were at low risk of bias; ·
unclear risk of bias (i.e. plausible bias that raises some doubt about the results) if one or more domains had an unclear risk of bias;
high risk of bias (i.e. plausible bias that seriously weakens confidence in the results) if one or more domains were at high risk of bias.
Measures of treatment effect
We calculated the risk ratio (RR) and 95% confidence interval (CI) for mortality. We split GOS data into favourable (moderate disability, good recovery; GOS scores 4 and 5) and unfavourable outcomes (death, vegetative state, severe disability; GOS scores 1 to 3). We would have split other validated functional outcome data into favourable (modified Rankin Scale score<3, Barthel Index>60, etc) and unfavourable outcomes (modified Rankin Scale score graded 3 to 6 and Barthel Index 0 to 60,etc).These were also calculated by RR and 95% CI. For continuous outcomes such as intracranial pressure, body temperature, and blood pressure, we would have used arithmetic means and standard deviations for each group.
Dealing with missing data
We contacted study authors to obtain missing information. When the missing data were unavailable, we included data on only those particpants whose results were known to generate the outcome and considered the potential impact of the missing data during the interpretation of the results.
Assessment of heterogeneity
We assessed clinical heterogeneity between comparable trials by examining the participants, interventions and outcomes of the trials. In addition, we used visual inspections of graphs to assess heterogeneity. Statistical heterogeneity was examined with the I² statistic and Chi² test. Substantial heterogeneity was considered to exist when I² exceeded 60% and the Chi² test P value was less than 0.1.
Assessment of reporting biases
We planned to assess reporting bias by funnel plots and linear regression tests, however, there were too few included studies to enable meaningful analysis. We will assess reporting biases in future updates if there are 10 or more studies included in the meta‐analysis.
Data synthesis
We calculated the RRs and 95% CIs via a fixed‐effect model and conducted a meta‐analysis for dichotomous outcomes, if we judged that the included trials were clinically and statistically homogeneous. We employed the random‐effects model to pool studies when statistical heterogeneity occurred. For continuous data, we would have calculated mean differences (MD) or standardised mean differences (SMD) with 95% CI. We assessed possible sources of heterogeneity by subgroup and sensitivity analyses. We used Review Manager 2014 software, version 5.3 for all analyses.
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analysis by severity of brain injury:
severe TBI subgroup (Glasgow Coma Scale (GCS) ≤ 8) and moderate TBI subgroup (GCS 9 to 12).
Sensitivity analysis
We undertook sensitivity analyses to assess the robustness of the results, by excluding studies with an unclear or high risk of bias for allocation concealment.
Summarising findings and assessing the quality of the evidence
We developed a 'Summary of findings' table to present the results of this review. We included all outcomes: mortality, disability, intracranial pressure; blood pressure, body temperature, and complications and adverse events.
Where possible, we assessed the quality of the evidence for our effect estimates using the GRADE methods to account for the overall risk of bias of the included studies, inconsistency of the results, indirectness of the evidence, precision of the estimates and the risk of publication bias (GRADE 2004). This was done independently and in duplicate by JM and YZ. We rated the quality of the evidence as high, moderate, low or very low.
Results
Description of studies
We identified five completed studies that satisfied the inclusion criteria using the process described in Figure 1 (Skolnick 2014; Wright 2006; Wright 2014; Xiao 2007; Xiao 2008). All trials eligible for inclusion compared progesterone therapy with a control group. There were also three ongoing trials (IRCT2014042017356N1; CTRI/2009/091/000893, CTRI/2013/02/003396), which are detailed in Characteristics of ongoing studies.
1.
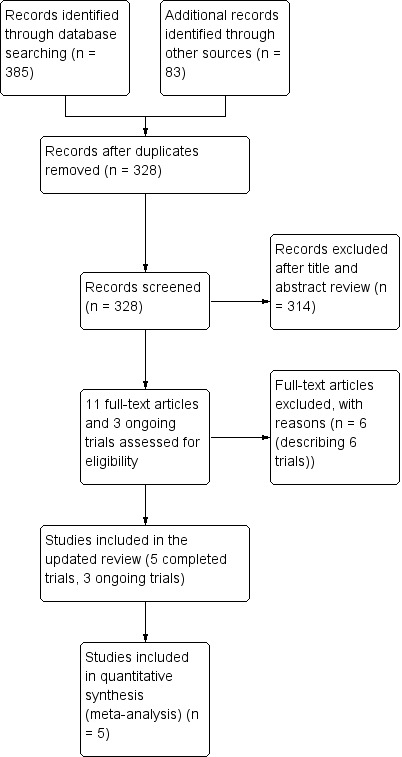
Study flow diagram.
Included studies
Full descriptions of all the included studies can be found in the Characteristics of included studies.
The five included studies had a total of 2392 participants; 1195 in Skolnick 2014;100 in Wright 2006; 882 in Wright 2014; 56 in Xiao 2007; and 159 in Xiao 2008.
Skolnick 2014: was a Mmulticenter phase III RCT that randomly assigned participants. Patients, (16 to 70 years of age, with severe TBI (GCS ≤ 8 and at least one reactive pupil)) were randomly assigned to receive progesterone or placebo. The modified ITT population excluded 16 participants (6 participants in the progesterone group and 10 in the placebo group) because they did not receive any study drug. Drug infusion (progesterone andor placebo) was started intravenously within 8eight hours afterof injury with a loading dose of 0.71 mg/kg/hour for one hour, followed by 0.50 mg/kg/hour for 119 hours.
Wright 2006 was a phase II RCT that recuited adults with acute severe TBI and a GCS score of 4 to 12 after resuscitation, and stabilisation within 11 hours of injury. Participants were assigned to eight clinical subgroups, then permuted block randomisation assigned four of every five consecutive patients to progesterone and the other to placebo. A 4:1 randomisation scheme was used to increase the number of patients receiving progesterone while maintaining blinding. The progesterone group received an infusion of 0.71 mg/kg of progesterone at 14 mL/h for the first hour and then an infusion of 0.5 mg/kg progesterone in 10 mL/h for the next 11 hours. Five additional 12‐hour maintenance infusions were delivered at the standard rate of 10 mL/hour, for a total of three days of treatment; the control group received placebo.
Wright 2014: was a Mmulticenter phase III RCT that recruited a. Adults patients with brain injury (GCS score of 4 to 12) were enrolled if the study treatment could be initiated within 4four hours afterof the injury. The study drug (progesterone or placebo) was infused continuously through a dedicated intravenous catheter at a dose of 14.3 mlL/h per hour for 1one hour and then at 10 mL/l per hour for 71 hours; the dose was then tapered by 2.5 mlL/ per hour every 8eight hours, for a total treatment duration of 96 hours.
Xiao 2007 recruited patients aged 15 to 65 years with severe TBI (GCS 5 to 8) after the time of injury, and randomised them according to a random number table. The progesterone group received 80 mg progesterone via intramuscular injection once every 12 hours for five consecutive days.
Xiao 2008 recruited adults with acute severe TBI and a GCS score ≤ 8 after resuscitation, and stabilisation within eight hours of injury. Participants were randomised according to a random number table. The progesterone group received a dose of progesterone of 1.0 mg/kg via intramuscular injection within eight hours of the time of injury, and then every 12 hours for five consecutive days. The control group received placebo.
Excluded studies
We excluded a total of six studies (Abokhabar 2012; Aminmansour 2012; Mofid 2016; Raheja 2016; Shakeri 2013; Wright 2005).
We excluded three studies because they were not prospectively registered and their reports were published after 2010. These included Aminmansour 2012 and Abokhabar 2012 for which we were unable to locate registration or protocols. Mofid 2016 claimed it was a prospective, single‐blind RCT performed from May 2013 to July 2015. However, we found the study protocol was approved by ethics committee of Kerman University of Medical Sciences in April 2014 and registered in the Iranian Registry of Clinical Trials in August 2014 (www.irct.ir, CT2014042017356N1). Because of this delay in trial registration, we judged that the study had not been prospectively registered.
We excluded two studies because they used biochemical outcome measures and did not address clinical outcomes. Wright 2005 evaluated the pharmacokinetics of progesterone given by intravenous infusion to people with TBI, while Raheja 2016 was part of a prospective blinded, randomized, placebo‐controlled phase II trial of progesterone with or without hypothermia (CTRI/2009/091/000893), which focused on the relationship between serum biomarkers and outcomes after TBI; the study report did not include the effect of progesterone on TBI.
We excluded Shakeri 2013 because medroxyprogesterone was used as the intervention rather than progesterone.
Risk of bias in included studies
Our assessments of the risk of bias in the included studies are recorded in the 'Risk of bias' tables, and are displayed in Figure 2 and Figure 3. The overall risk of bias of individual studies was judged as high for Xiao 2007 and Xiao 2008, unclear for Wright 2014 and Skolnick 2014, and low for Wright 2006.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Five studies are included in this review.
3.
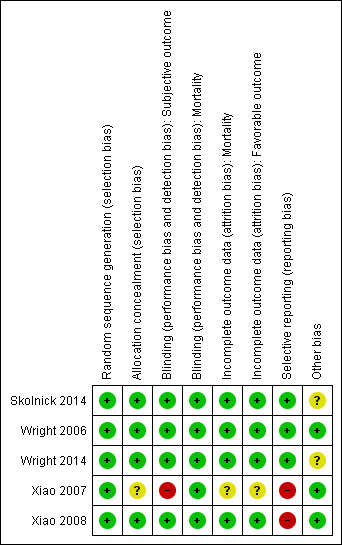
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Wright 2006 assigned four of every five consecutive participants to progesterone and the fifth to placebo via permuted block randomisation.
In Xiao 2007 and Xiao 2008, participants were allocated according to a random number table.
In Wright 2014, randomisation was performed with the use of a combination of minimisation and biased‐coin algorithms.
In Skolnick 2014, randomisation was implemented with the use of an interactive web‐based response system.
The risk of bias for this domain was judged to be low for all five studies.
Allocation concealment
In Wright 2006, Xiao 2008, Wright 2014 and Skolnick 2014, the appearance, packaging and administration of placebo and progesterone injections were the same for the two groups. Xiao 2007 did not describe the methods used for allocation concealment sufficiently for us to determine the risk of bias for this domain. We therefore assessed this study as being at unclear risk of bias.
The risk of bias for this domain was judged as low for Wright 2006, Xiao 2008, Wright 2014 and Skolnick 2014, and unclear for Xiao 2007.
Blinding
Blinding (GOS and other objective outcomes)
Wright 2006, Xiao 2008, Skolnick 2014 and Wright 2014 mentioned `double blinding', and achieved blinding by use of indistinguishable drug kits. We judged the risk of bias for this domain as low for these trials.
In the trial by Xiao 2007, there was no blinding and the outcome measurement was likely to be influenced by lack of blinding. We judged the risk of bias for this domain as high.
Blinding (mortality)
We decided that for the outcome of mortality, the outcome and its measurement were not likely to have been influenced by blinding. So we judged the risk of bias for this domain to be low for all included studies.
Incomplete outcome data
In Wright 2006, with the exception of three people who discontinued treatment (two died during infusion, one was taken into police custody), there were no withdrawals, dropouts, or losses to follow‐up by the end of the follow‐up period. In Xiao 2008, there were two withdrawals, three dropouts and 19 losses to follow‐up at the end of the follow‐up period. In Skolnick 2014, a total of 31 participants (17 in the progesterone group and 14 in the placebo group) were lost to follow‐up. Skolnick 2014 used a modified ITT population and excluded 16 participants (six in the progesterone group and 10 in the placebo group) because they did not receive any study drug. This method was described previously in its protocol, so we did not consider these exclusions were associated with industry funding or authors' conflicts of interest. In Wright 2014, 28 participants (6.3%) in progesterone group and 24 (5.5%) in placebo group were missing. Missing outcome data was balanced in numbers between progesterone group and placebo group in all of the above four studies. Attrition or exclusion of participants was not reported in Xiao 2007.
We judged the risk of bias for this domain as low for Wright 2006, Xiao 2008, Skolnick 2014 and Wright 2014, and as unclear for Xiao 2007.
Selective reporting
The protocols for Wright 2006, Wright 2014 and Skolnick 2014 were presented in Clinicaltrials.gov. It was clear that these published reports included all expected outcomes, so we judged the risk of bias for this domain as low for Wright 2006, Wright 2014 and Skolnick 2014.
Xiao 2007 and Xiao 2008 were registered in the Australian New Zealand Clinical Trials Registry (ACTRN12607000545460) and the Chinese Clinical Trial Register (ChiCTR‐TRC‐08000174), but this was done retrospectively. The dates of registration postdated the ends of the trials. We did not exclude these two trials because they were published before 2010 (Roberts 2015). We judged the risk of bias for selective reporting as high for these two trials.
Other potential sources of bias
Source of funding
Wright 2006 and Wright 2014 were supported by a grant from the National Institute for Neurological Disorders and Stroke, National Institutes of Health, USA.
Xiao 2007 and Xiao 2008 were supported by the Scientific Research Fund of Zhejiang Provincial Education Department, China.
Skolnick 2014 was funded by BHR Pharma, UK.
Stopping a trial early
The Wright 2014 trial intended to enrol 1140 participants, but the trial was abandoned after 882 people had been assessed. We did not consider reporting bias, because there was no selective revealing or suppression of the study information. After the second interim analysis, the trial was stopped because of futility (favourable outcomes were observed in 51% of participants who received progesterone after TBI, compared with 55.5% of controls. Stratification of the participants on the basis of injury severity did not reveal any effect of progesterone on recovery). We therefore assessed the risk of other bias for Wright 2014 as unclear.
Centre effects in multicenter RCTs
Skolnick 2014 was conducted in approximately 100 centres in 21 countries. The number of mortality or functional outcome events in each centre was quite low. We assessed potential bias from variation between‐centres as unclear because of factors such as different levels of expertise in treating TBI and outcome assessment.
Wright 2014 was conducted in 22 academic medical centres through the National Institute of Neurological Disorders and Stroke‐funded Neurological Emergencies Treatment Trials network in the USA. We considered the potential bias from centre effects in this trial to be low.
Effects of interventions
See: Table 1
Mortality
Overall mortality was evaluated in all five trials at the end of follow‐up (i.e. 30 days postinjury in Wright 2006, three months postinjury in Xiao 2007, and six months postinjury in Xiao 2008, Wright 2014 and Skolnick 2014).There was no evidence of a difference in mortality between the treatment and placebo groups. We assessed the quality of this evidence to be low (see Table 1).
The ITT analysis included 2376 participants pooled for meta‐analysis. The pooled RR of death at the end of follow‐up was 0.91 (95% CI 0.65 to 1.28, P value 0.60). We used a random‐effects model as there was substantial heterogeneity (Tau² = 0.08; Chi² = 10.40, df = 4 (P = 0.03); I² = 62%; Analysis 1.1).
1.1. Analysis.
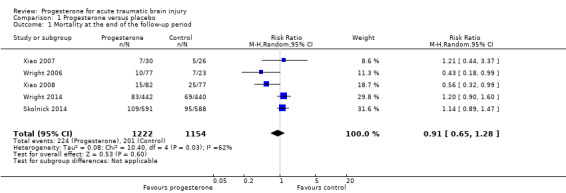
Comparison 1 Progesterone versus placebo, Outcome 1 Mortality at the end of the follow‐up period.
Disablity
All the included studies reported disability data assessed by GOS score. GOS data in Wright 2006 , Xiao 2008, Wright 2014 and Skolnick 2014 were sufficient to be dichotomised into favourable outcomes (moderate disability, good recovery; GOS 4 and 5) and unfavourable outcomes (sometimes referred to as unfavourable functional recovery: i.e. death, vegetative state, severe disability; GOS 1 to 3). Only Xiao 2007 reported the mean GOS at three months postinjury, and these data were insufficient to dichotomise into favourable and unfavourable outcomes.
We pooled unfavourable outcomes (death, vegetative state, severe disability; GOS 1 to 3) for four trials at the end of follow‐up (i.e. 30 days postinjury in Wright 2006, six months postinjury in Xiao 2008, Skolnick 2014 and Wright 2014). There was no evidence of a difference between the treatment and placebo groups and no substantial heterogeneity (RR = 0.98, 95% CI 0.89 to 1.06, P value 0.58; Chi² = 4.77, df = 3 (P = 0.19); I² = 37%; Analysis 1.2). We assessed the quality of this evidence to be moderate (see Table 1).
1.2. Analysis.
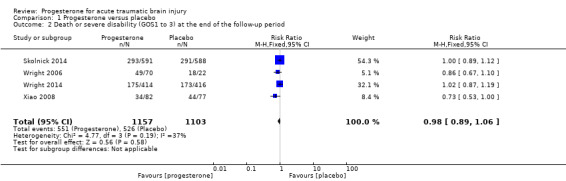
Comparison 1 Progesterone versus placebo, Outcome 2 Death or severe disability (GOS1 to 3) at the end of the follow‐up period.
In Wright 2006, disability in survivors was also assessed by the Disability Rating Score (DRS) at 30 days postinjury. Survivors with severe TBI in the placebo group were slightly less likely to be disabled than those in the progesterone group (DRS = 10.7, 95% CI 8.3 to ‐13.1 for progesterone‐treated participants versus DRS = 4.4, 95% CI 0.0 to 9.8 for placebo‐treated participants). The authors explained that a higher proportion of severely injured participants treated with progesterone survived, so survivors in the progesterone group may have been slightly more disabled. In the moderate TBI stratum, participants treated with progesterone were significantly less disabled than those who received placebo (DRS = 5.0, 95% CI 1.8 to 6.2 for progesterone‐treated participants versus DRS = 12.7, 95% CI 7.6 to 17.78 for placebo‐treated participants). The authors did not report a test for interaction between the high and moderate brain injury strata.
Xiao 2007 and Xiao 2008 presented data showing reduced disability in the progesterone therapy group relative to the control. However, these were the small studies and assessed to be at high overall risk of bias. We do not consider these data to outweigh the evidence from the much larger meta‐analysis. In Xiao 2007, disability data were assessed using GOS score, Karnofsky Performance Scale (KPS), verbal and motor function at three months postinjury. For GOS, a higher score equates to less disability, whilst for KPS, verbal and motor function scores, a lower score equates to lower disability. All of these data were presented as means and standard deviations in a table as follows:
the GOS score in the progesterone group was 5.0 ± 1.7 and in the control group was 4.0 ± 1.9, P < 0.05
the KPS score in the progesterone group was 4.9 ± 1.2 and in the control group was 4.0 ± 1.1, P < 0.05;
verbal function in the progesterone group was 3.1 ± 0.4 and in the control group was 2.3 ± 0.7, P < 0.05;
motor function in the progesterone group was 2.4 ± 0.7 and in the control group was 2.4 ± 0.4, P > 0.05.
No other details about these data were reported.
In Xiao 2008, disability was also assessed by Modified Functional Independence Measure (FIM) scores, where a higher score equates to less disability. At the three‐month follow‐up, the scores were 7.35 ± 1.89 for the placebo group and 8.02 ± 1.73 for the progesterone group (P < 0.05). At six months after injury, the scores were 8.95 ± 1.05 for the placebo group and 9.87 ± 1.17 (P < 0.01) for the progesterone group.
Intracranial pressure (ICP)
Wright 2006, Xiao 2008 and Skolnick 2014 reported intracranial pressure (ICP) data, but we were not able to perform meta‐analysis due to variations in the way the data were presented. Xiao 2008 presented ICP data as mean values, while Wright 2006 presented them as the mean frequency of pressures exceeding threshold values, and Skolnick 2014 presented them as the proportion with increased ICP.
In Wright 2006, the mean ICP level of the progesterone group remained stable, whereas that of the control group tended to increase during the first three days of treatment and for one day after treatment However, this trend was not significant. The mean ICP‐therapeutic intensity level scores presented did not differ between groups.
In Xiao 2008, there was no evidence of a difference between the two groups for mean ICP at 24 hours after trauma (progesterone group, 22.1 ± 4.3 mmHg versus placebo group, 23.2 ± 4.6 mmHg; P value 0.121). At 72 hours and at seven days after injury, there was still no evidence of a difference in the mean ICP of participants who were given progesterone and participants who received placebo (16.9 ± 3.8 mmHg and 14.8 ± 3.8 mmHg for progesterone treated participants versus 18.2 ± 5.1 mmHg and 15.9 ± 4.1 mmHg for placebo‐treated participants, respectively; P > 0.05).
In Skolnick 2014, the ICP of 130 participants in the progesterone group increased, as did the ICP of 137 participants in the placebo group. There was no evidence of a difference between the two groups.
Body temperature
No data for body temperature were available for meta‐analysis.
Wright 2006 collected detailed data on body temperature. Throughout the three‐day infusion period, the progesterone group experienced a lower increase in mean temperature than the control group; this was determined through analysis of a treatment‐by‐time interaction term for progesterone versus control participants, with had a slope of 0.0055 (95% CI ‐0.010 to ‐0.001).
Blood pressure
No data for blood pressure were available for meta‐analysis.
Wright 2006 collected detailed data on blood pressure. Throughout the three‐day infusion interval, there was no evidence of a difference between the progesterone and placebo groups.
Adverse events
A pooled analysis was not appropriate for adverse event data, so we have presented the data narratively.
Wright 2006 reported serious adverse events and adverse event rates for the progesterone‐treated group and the placebo‐treated group. There was no evidence of a difference in the rates of adverse and serious adverse events between groups. The only adverse event attributed to progesterone was a case of superficial phlebitis at the intravenous site.
Xiao 2008 did not report the specifics of any adverse events in either group, but did report that there were no additional adverse events after administration of progesterone and no further late toxicity up to six months.
Wright 2014 reported the rates of all reported adverse events and serious adverse events. Only phlebitis or thrombophlebitis was reported to be significantly more frequent in the progesterone group than in the placebo group (882 cases, RR 3.03; 95% CI, 1.96 to 4.66).
Skolnick 2014 recorded adverse events for the first 15 days, and serious adverse events were recorded throughout the duration of the study. There was no evidence of a difference in the rate of adverse events between the progesterone and placebo groups.
Subgroup analysis (severity of TBI)
The Wright 2006 and Wright 2014 trials involved 281 participants with moderate TBI and 701 participants with severe TBI. The Xiao 2007, Xiao 2008 and Skolnick 2014 studies enrolled a total of 1410 participants with severe TBI, but did not enrol people with moderate TBI. When we limited our analysis to the severe TBI subgroup or moderate TBI subgroup, there were minimal changes in the results.
In the severe TBI subgroup (GCS ≤ 8), the pooled RR for mortality at the end of follow‐up was 0.87 (95% CI 0.60 to 1.27; P value 0.48; 2111 participants, 2090 pooled for meta‐analysis). We used a random‐effects model as there was substantial heterogeneity (Tau² = 0.10; Chi² = 11.60, df = 4 (P value 0.02); I² = 66%). In the moderate TBI subgroup (GCS = 9 to 12) the pooled RR for mortality at the end of follow‐up was 1.30 (95% CI 0.70 to 2.41; P value 0.40; 281 participants, 279 pooled for meta‐analysis).There was no substantial heterogeneity (Chi² = 0.01, df = 1 (P = 0.91); I² = 0%). The test for subgroup differences showed no evidence that progesterone therapy has a differential effect on these two subgroups (P value 0.28) (Analysis 2.1).
2.1. Analysis.
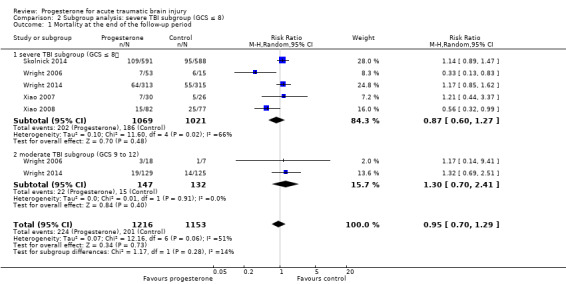
Comparison 2 Subgroup analysis: severe TBI subgroup (GCS ≤ 8), Outcome 1 Mortality at the end of the follow‐up period.
The pooled RR in the severe TBI subgroup for death or severe disability (GOS 1 to 3) at the end of follow‐up was 0.99 (95% CI 0.87 to 1.11; P value 0.80; 2055 participants, 2002 pooled for meta‐analysis). There was no substantial heterogeneity (Chi² = 4.25, df = 3 (P value 0.24); I² = 29%). The pooled RR in the moderate group for death or severe disability (GOS 1 to 3) at the end of follow‐up was 0.68 (95% CI 0.34 to 1.37 ; P value 0.28; 281 participants, 258 pooled for meta‐analysis); we used a random‐effects model as there was substantial heterogeneity (Tau² = 0.20; Chi² = 4.41, df = 1 (P = 0.04); I² = 77%). The test for subgroup differences showed no evidence that progesterone therapy had a differential effect on these two subgroups (P value 0.31). (Analysis 2.2).
2.2. Analysis.
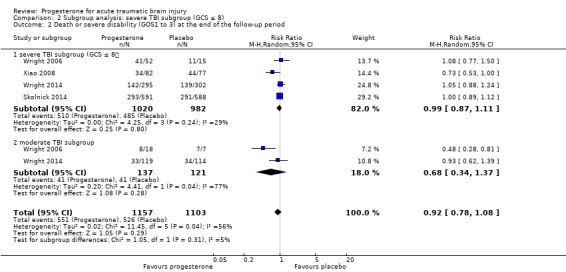
Comparison 2 Subgroup analysis: severe TBI subgroup (GCS ≤ 8), Outcome 2 Death or severe disability (GOS1 to 3) at the end of the follow‐up period.
Sensitivity analysis
When we removed studies that did not report allocation concealment procedures from the analysis, there was still no evidence of a difference in mortality (RR 0.88, 95% CI 0.60 to 1.28) or disability (unfavourable outcomes: death, vegetative state, severe disability; GOS 1 to 3) (RR 0.98, 95% CI 0.89 to 1.06) between the progesterone and placebo groups (Analysis 3.1; Analysis 3.2).
3.1. Analysis.
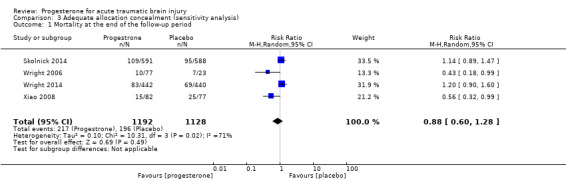
Comparison 3 Adequate allocation concealment (sensitivity analysis), Outcome 1 Mortality at the end of the follow‐up period.
3.2. Analysis.
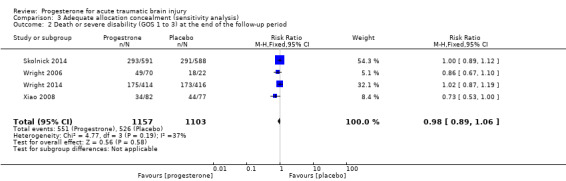
Comparison 3 Adequate allocation concealment (sensitivity analysis), Outcome 2 Death or severe disability (GOS 1 to 3) at the end of the follow‐up period.
Discussion
Summary of main results
We included five completed studies with 2392 participants. The main results are presented in the 'Summary of findings' table (Table 1). The results of the meta‐analyses did not find evidence that progesterone could improve overall mortality (RR 0.91, 95% CI 0.65 to 1.28; P = 0.60, I² = 62%; 2376 participants; low quality evidence) nor disability (unfavourable outcomes: death, vegetative state, severe disability; GOS 1 to 3) (RR 0.98, 95% CI 0.89 to 1.06; P = 0.58, I² = 37%; 2260 participants; moderate quality evidence) in participants with TBI. When we performed a subgroup analysis on participants with severe TBI versus moderate TBI, and tested for a difference between subgroups, there was no evidence of a difference between the two groups. When we performed sensitivity analysis and removed the study without adequate allocation concealment procedures from analysis, there were no significant changes to the results.
Data were not available for meta‐analysis for the outcomes of mean intracranial pressure, blood pressure, body temperature and adverse events. However, data from three studies showed no evidence of a difference in mean intracranial pressure among participants in either group. Data from one study showed no evidence of a difference in blood pressure and body temperature between the progesterone and placebo groups. Intravenous progesterone infusion increased the frequency of phlebitis in one trial. There was no evidence of a difference in the rate of other adverse events between progesterone treatment and placebo in the other three studies that looked at adverse events.
Overall completeness and applicability of evidence
Completeness
We were only able to perform meta‐analysis for two of our three primary outcomes (mortality and disability), as ICP was presented in three different formats in the three trials that looked at this outcome. For our secondary outcomes, blood pressure and body temperature were only recorded in one of our five included trials (Wright 2006), which limits the completeness of our results.
We were unable to obtain some information regarding the trial described in Xiao 2007. We judged the allocation concealment as unclear due to a lack of information in the study report. In addition, in this trial the data for GOS score were presented by mean difference and 95% CI in a table. These data were insufficient to calculate the pooled RR for disability. We tried to contact the study authors of Xiao 2007 to request details, but we did not receive a response.
Applicability
In terms of the applicability of our results, TBI patients are highly diverse in terms of aetiology, pathology, function and outcome, which leads to concerns that progesterone may affect the recovery of different TBI patients differentially. This is supported by the fact that although we performed subgroup analysis according to GCS grade, there was still high heterogeneity within these subgroups. This means that the results of the meta‐analyses should be interpreted with caution. We discuss the issue of heterogeneity among TBI patients in more detail under 'Quality of the evidence ‐ Categorisation of TBI'.
Quality of the evidence
According to the GRADE approach to assessing the quality of the evidence in the included studies (Table 1), we classified the quality of the evidence as low for mortality, and moderate for disability (unfavourable outcomes: death, vegetative state, severe disability; GOS 1 to 3)).
Although the overall risk of bias of two included studies was judged as high (Xiao 2007; Xiao 2008), and unclear for two studies (Skolnick 2014; Wright 2014), most of the data were from studies that were judged to be at low risk of bias across most domains, with no domains at high risk of bias, so we did not downgrade for risk of bias.
Although most of the trials included in this review were well conducted, several factors did influence our assessment of the quality of the overall body of evidence. Firstly, although subgroup analyses were performed according to the protocol, there was still substantial statistical heterogeneity. Secondly, there were three different time points involved in the analysis of mortality and two different time points involved in the analysis of unfavourable outcomes. Thirdly, the dosage, treatment routine and vehicles of progesterone varied across studies. Finally, we had concerns regarding the heterogeneity among TBI patients, and the fact that progesterone may affect TBI patients differentially.
Time at the end of follow‐up
For the mortality and disability outcome we pooled mortality and GOS scores at the end of the follow‐up in each trial; for Wright 2006 this was 30 days postinjury, for Xiao 2007 this was three months, and for Xiao 2008; Wright 2014; Skolnick 2014 this was six months. We should consider this as an inconsistency that has an impact on our confidence in our conclusions.
Dosage, mode of delivery and schedule of progesterone therapy
The dosage used in Wright 2006, Wright 2014 and Skolnick 2014 was six times that used in Xiao 2008 (approximately 12 mg/kg/day versus 2 mg/kg/day). The schedule and mode of delivery also varied between trials. This may explain the existence of significant heterogeneity. Examination of the pharmacokinetics of progesterone intravenous infusion in demonstrated that stable progesterone concentrations can be achieved rapidly via progesterone infusion following TBI. Unfortunately, none of the studies used allometric scaling to determine the most effective dose for humans based on the preclinical animal research (FDA 2005; Nair 2016), and there was no attempt to optimise either dose or schedule as part of any included study. It is possible that none of the doses in the included RCTs was optimal for improving motor, sensory and memory function according to preclinical dose‐response studies (Stein 2015; Wali 2014).
Categorisation of TBI
TBI is categorised according to GCS score on admission to hospital to estimate the severity of the underlying injury. As we know, TBI is regarded as a very heterogeneous and complex disease, with substantial heterogeneity in the aetiology, pathology, mechanisms, exposure time and outcome. There are obvious limitations to using the GCS for the classification of such a complicated disease (Maas 2012). The diverse mechanisms of TBI, as well as differences in age and gender, also lead to different potential for functional recovery and response to progesterone. Use of a 'one size fits all' approach for these different types of TBI is not appropriate (Meyfroidt 2016). Recently, an increasing number of articles suggest a more individualised classification and subgroup analysis of TBI based on biomarkers, age, gender, and clinical characteristics and imaging findings to identify the appropriate patients for progesterone therapy after acute TBI (Menon 2015). Unfortunately, in this review, we only performed subgroup analysis according to GCS grade due to the lack of inclusion of more detailed data in the included trials. These heterogeneities might explain the dissociation seen in the effect of progesterone between animal models and these human trials.
So far, all pharmacological interventions in TBI have failed, which led to discussion of the problematic neuroprotection end‐points in clinical trials (Gultekin 2016). It appears that the commonly used GOS and GOS‐E (Glasgow Outcome Scale‐extended), which measure global functioning after TBI, do not adequately reflect the patients' neurologic function, especially for specific deficits in behaviour, executive function, memory and emotion that may produce significant disabilities. This limitation can become even more evident when the scores are subjectively dichotomised into `unfavourable' and `favourable' outcomes (Poon 2015; Stein 2015).
Therefore, we consider that the different timescales of outcome measures, differing dosage of progesterone, and heterogeneity of participants, reduce our confidence in the conclusions of this review.
Potential biases in the review process
We have attempted to minimise bias in this review and to be as inclusive as possible. We included five studies, but we expect that there may have been other trials that have been conducted that we were unable to identify.
We excluded three studies that were not prospectively registered and published their reports after 2010 (Aminmansour 2012; Abokhabar 2012; Mofid 2016), in accordance with Roberts 2015. We consider that this will have reduced bias in the review process.
Agreements and disagreements with other studies or reviews
A series of preclinical studies and results of phase II RCTs indicated that progesterone might be an appropriate candidate for the treatment of people suffering from TBI (Stein 2008; Wright 2006; Xiao 2008). However, this updated systematic review did not find evidence that progesterone could provide a beneficial clinical outcome in people with TBI. It is possible that this negative result could be due to some of the problems with trial design that we have discussed under Quality of the evidence.
Authors' conclusions
Implications for practice.
The results of our updated systematic review did not find evidence that the use of progesterone therapy, started within 24 hours of injury, could reduce mortality or disability in people with traumatic brain injury (TBI). However, our confidence in these findings is limited, as the quality of the evidence for these outcomes is of low and moderate, respectively.
We are unable to draw firm conclusions about the effects of progesterone therapy on intracranial pressure, blood pressure or body temperature, as the results do not indicate any evidence of an effect.
At the moment there is no evidence that progesterone therapy is less safe than placebo for people with TBI, apart from a possible increase in phlebitis in the case of intravascular progesterone.
Implications for research.
Although some of the trials included in this review were well conducted in terms of sample size and minimising bias, their utility for determining the effects of progesterone therapy for acute TBI was limited by the heterogeneity of the populations, inconsistency of the interventions and limitations of the outcomes measured. Future trials would benefit from the following.
Population
TBI is a complex and heterogeneous condition, so precise characterisation ‐ based, for example, on biomarkers, clinical characteristics and imaging findings‐ is essential before further clinical trials are conducted. This way, more meaningful subgroup analyses can be conducted for TBI trials.
Intervention
Future trials should attempt to optimise progesterone dose and schedule. Allometric scaling could determine the most effective dose for humans based on the existing preclinical animal research.
Outcomes
There have been many criticisms of the subjectivity of the Glasgow Outcome Scale (GOS) and its extended version (GOS‐E) for measuring deficits or recovery from TBI. These scales rely on self‐assessment or assessment of a care‐giver, rather than quantifiable measurements of disability. It is possible that more quantitative outcome measures would be beneficial, although these may be more expensive and time‐consuming to implement.
In response to criticism of the lack of sensitivity of the GOS, use of multi‐faceted testing approaches to capture more of the relevant clinical information have been proposed.
At the very least, analysis methods for the GOS‐E that use more complex forms of statistical evaluation should be introduced to improve prediction of functional outcomes (Alali 2015).
What's new
| Date | Event | Description |
|---|---|---|
| 7 February 2017 | Amended | Minor copy‐editing amendment. |
History
Protocol first published: Issue 3, 2010 Review first published: Issue 1, 2011
| Date | Event | Description |
|---|---|---|
| 4 November 2016 | Amended | In an attempt to improve the quality of our systematic review, we only included prospectively registered studies for studies whose publication occurred after the publication of our 2012 review. |
| 4 November 2016 | New citation required and conclusions have changed | Two multicentre phase III trials (NCT00822900 and NCT01143064) have completed and been added into the review. The results and conclusions have changed. We have identified three ongoing studies. |
| 30 September 2016 | New search has been performed | The search has been updated to September 2016. |
| 18 August 2012 | New search has been performed | The search has been updated to July 2012. A new ongoing study was identified. The results and conclusions remain the same. |
| 16 August 2012 | New citation required but conclusions have not changed | The search has been updated to July 2012. The results and conclusions are unchanged. One new ongoing study has been identified. |
Notes
The results of the ongoing trials IRCT2014042017356N1, CTRI/2009/091/000893 and CTRI/2013/02/003396 were not considered to influence the results of the review because of their limited enrolments. We will update the review when other phase III trials are completed.
Acknowledgements
We thank the Cochrane Injuries Group editorial base staff for their support during the production of this review, and to the Information Specialist for developing and running the literature searches.
Appendices
Appendix 1. Search strategies 2016
Cochrane Register of Studies(including RCTs from the Injuries Group's Specialised Register) #1((progesteron* or progestagen* or progestin* or gestagen*) ):TI,AB,KY #2((TBI or ((trauma* or acute or severe* or acquired) and (brain injur* or brain trauma* or head injur* or head trauma*)))):TI,AB,KY #3(GLASGOW COMA SCALE):TI,AB,KY #4((acute NEAR (head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra cran* or inter cran* or intracran* or intercran* or multiple) and (injur* or trauma* or damag* or lesion* or wound* or destruction* or oedema* or edema* or contusion* or concus* or fracture*))):TI,AB,KY #5(Glasgow Outcome Scale or Glasgow Coma Scale):TI,AB,KY #6(#2 OR #3 OR #4 OR #5) #7(#1 AND #6)
Ovid MEDLINE (30/09/2016) (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present) 1. exp Craniocerebral Trauma/ 2. Glasgow Coma Scale/ or Glasgow Outcome Scale/ 3. (Glasgow adj (coma or outcome) adj (scale* or score*)).ab,ti,kf. 4. (injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus*).ti,ab,kf. 5. (acute adj5 (head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra cran* or inter cran* or intracran* or intercran* or multiple) adj3 (injur* or trauma* or damag* or lesion* or wound* or destruction* or oedema* or edema* or contusion* or concus* or fracture*)).ab,ti,kf. 6. (TBI or ((trauma* or acute or severe* or acquired) and (brain injur* or brain trauma* or head injur* or head trauma*))).ti,ab,kf. 7. 1 or ((2 or 3) and 4) or 5 or 6 8. exp Progesterone/ 9. Progestins/tu [Therapeutic Use] 10. (progesteron* or progestagen* or progestin* or gestagen*).ti,ab,kf,ot,rn. 11. or/ 8‐10 12. randomi#ed.ab,ti. 13. randomized controlled trial.pt. 14. controlled clinical trial.pt. 15. placebo.ab. 16. clinical trials as topic.sh. 17. randomly.ab. 18. trial.ti. 19. Comparative Study/ 20. or/12‐19 21. (animals not (humans and animals)).sh. 22. 20 not 21 23. (7 and 11 and 22) 24. (2012* or 2013* or 2014* or 2015* or 2016*).yr,ed. 25. 23 and 24
Ovid Embase (30/09/2016) (1974 to 2016 September 29) 1. exp Brain Injury/ 2. Head Injury/ 3. Brain Edema/ 4. Brain Perfusion/ 5. Glasgow Coma Scale/ or Glasgow Outcome Scale/ 6. (Glasgow adj (coma or outcome) adj (scale* or score*)).ab,ti,kw. 7. (injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus*).ti,ab,kw. 8. ((acute adj5 (head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra cran* or inter cran* or intracran* or intercran* or multiple)) and (injur* or trauma* or damag* or lesion* or wound* or destruction* or oedema* or edema* or contusion* or concus* or fracture*)).ab,ti,kw. 9. (TBI or ((trauma* or acute or severe* or acquired) and (brain injur* or brain trauma* or head injur* or head trauma*))).ti,ab,kw. 10. 1 or 2 or 3 or 4 or ((5 or 6) and 7) or 8 or 9 11. Progesterone/ or Progesterone Derivative/ 12. ((progesteron* or progestagen* or progestin* or gestagen*).ab,kw,ot,rn,ti. 13. Gestagen/ 14. or/11‐13 15. randomized controlled trial.de. 16. randomization.de. 17. randomly.ab. 18. randomi#ed.ab,ti,kw. 19. placebo.de,ti,ab. 20. trial.ti. 21. major clinical study/ 22. or/15‐21 23. ((animal or nonhuman) not (human and (animal or nonhuman))).de. 24. 22 not 23 25. 10 and 14 and 24 26. (2012* or 2013* or 2014* or 2015* or 2016*).yr,em. 27. 25 and 26
Web of Science (Core Collection): Conference Proceedings Citation Index‐ Science (CPCI‐S) ‐‐1990‐present Topic: (progesteron* and (TBI or ((trauma* or acute or severe* or acquired) and (brain injur* or brain trauma* or head injur* or head trauma*))) and (random* or placebo* or trial or study))
Clinicaltrials.gov Free‐text search: Progesterone AND TBI (30/09/2016) 2015 search: traumatic brain injury [DISEASE] AND ( Progestin OR gestagen OR progestagen OR progestogen OR progestation OR estrogen ) [TREATMENT] AND ( "01/01/2010" : "03/11/2015" ) [FIRST‐RECEIVED‐DATE
WHO International Clinical Trials Registry Platform (ICTRP) Free‐text search: Progesterone AND TBI OR Progesterone AND Traumatic Brain Injury (30/09/2016) 2015 search. Title: brain injury; Intervention: Progestin OR gestagen OR progestagen OR progestogen OR progestation OR estrogen; Recruitment: ALL; Registered: 01/01/2010 ‐ 11/03/2015
Controlled‐Trials.gov (ISRCTN) search: Condition: injury; Interventions: progestin OR gestagen OR progestagen OR progestogen OR progestation OR estrogen; Date applied: 01/01/2010 ‐ 11/03/2015 only
Appendix 2. Search strategies 2012
Cochrane Injuries Group's Specialised Register (head or brain or cranial or cerebral or brain* or intra‐cranial or inter‐cranial) and (injury or injur* or trauma* or damag* or wound* or fracture* or contusion* or polytrauma* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed*) and (progesterone or progestins or Gonadal Steroid Hormones or estrogens or Estrogens or Progestin* or gestagen* or progestagen* or progestogen* or progestation* or estrogen*)
MEDLINE (Ovid SP) 1950 to August Week 1 2012 and Cochrane Central Register of Controlled Trials (CENTRAL) 1.exp Progesterone/ 2.exp Progestins/ 3.exp Receptors, Progesterone/ 4.exp Gonadal Steroid Hormones/ 5.exp Estrogens/ 6.exp Receptors, Estrogen/ 7.(Progestin* or gestagen* or progestagen* or progestogen* or progestation* or estrogen*).ab,ti. 8.((gender* or gonad* or sex*) adj3 hormon*).ab,ti. 9.((gender or Sex* or hormon*) adj3 (differ* or effect* or influence* or function* or recover*)).ti,ab. 10.or/1‐9 11.exp Craniocerebral Trauma/ 12.exp Brain Edema/ 13.exp Glasgow Coma Scale/ 14.exp Glasgow Outcome Scale/ 15.exp Unconsciousness/ 16.exp Cerebrovascular Trauma/ 17.((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) adj5 (injur* or trauma* or damag* or wound* or fracture* or contusion*)).ab,ti. 18.((head or crani* or cerebr* or brain* or intra‐cran* or inter‐cran*) adj5 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure)).ti,ab. 19.(Glasgow adj (coma or outcome) adj (scale* or score*)).ab,ti. 20."Rancho Los Amigos Scale".ti,ab. 21.("diffuse axonal injury" or "diffuse axonal injuries").ti,ab. 22.((brain or cerebral or intracranial) adj3 (oedema or edema or swell*)).ab,ti. 23.((unconscious* or coma* or concuss* or 'persistent vegetative state') adj3 (injur* or trauma* or damag* or wound* or fracture*)).ti,ab. 24.exp coma/ 25.or/11‐24 26.randomi?ed.ab,ti. 27.randomized controlled trial.pt. 28.controlled clinical trial.pt. 29.placebo.ab. 30.clinical trials as topic.sh. 31.randomly.ab. 32.trial.ti. 33.or/26‐32 34.(animals not (humans and animals)).sh. 35.33 not 34 36.25 and 35 37.(rat* or rodent* or animal* or mice or murin* or dog* or canine* or cat* or feline* or rabbit* or guinea pig*).ti. 38.36 not 37 39.10 and 38
EMBASE(Ovid SP) 1. exp head injury/ 2. brain edema/ 3. exp Glasgow coma scale/ 4. exp Glasgow outcome scale/ 5. exp unconsciousness/ 6. exp cerebrovascular accident/ 7. ((head or crani$ or cerebr$ or capitis or brain$ or forebrain$ or skull$ or hemispher$ or intra‐cran$ or inter‐cran$) adj5 (injur$ or trauma$ or damag$ or wound$ or fracture$ or contusion$)).ab,ti. 8. ((head or crani$ or cerebr$ or brain$ or intra‐cran$ or inter‐cran$) adj5 (haematoma$ or hematoma$ or haemorrhag$ or hemorrhag$ or bleed$ or pressure)).ti,ab. 9. (Glasgow adj (coma or outcome) adj (scale$ or score$)).ab,ti. 10. "rancho los amigos scale".ti,ab. 11. ("diffuse axonal injury" or "diffuse axonal injuries").ti,ab. 12. ((brain or cerebral or intracranial) adj3 (oedema or edema or swell$)).ab,ti. 13. ((unconscious$ or coma$ or concuss$ or 'persistent vegetative state') adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$)).ti,ab. 14. exp coma/ 15. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 16. exp progesterone/ 17. exp progesterone receptor/ 18. exp sex hormone/ 19. exp estrogen/ 20. exp estrogen receptor/ 21. (Progestin* or gestagen* or progestagen* or progestogen* or progestation* or estrogen*).ab,ti. 22. ((gender* or gonad* or sex*) adj3 hormon*).ab,ti. 23. ((gender or Sex* or hormon*) adj3 (differ* or effect* or influence* or function* or recover*)).ti,ab. 24. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25. 15 and 24 26. exp Randomized Controlled Trial/ 27. exp controlled clinical trial/ 28. randomi?ed.ab,ti. 29. placebo.ab. 30. *Clinical Trial/ 31. randomly.ab. 32. trial.ti. 33. 26 or 27 or 28 or 29 or 30 or 31 or 32 34. exp animal/ not (exp human/ and exp animal/) 35. 33 not 34 36. 25 and 35 37. limit 36 to (exclude medline journals)
Zetoc 1.Progesterone trauma* brain 2.Progesterone trauma* head 3.Progesterone injur* brain 4.Progesterone injur* head 5.Progesterone trauma* crani* 6.Progesterone trauma* cerebr* 7.Or/1‐6
LILACs head OR brain OR cranial OR cerebral OR intra‐cranial OR inter‐cranial [Words] and haematoma OR hematoma OR hemorrhage OR bleedOR pressure OR injury OR injuriesOR traumaOR damageOR damagedOR wound OR fracture [Words] and progesterone or progestins or Gonadal Steroid Hormones or estrogens [Words]
Clinicaltrials.gov brain damage and (progesterone or progestins) brain injury and (progesterone or progestins) head injury (progesterone or progestins)
Controlled‐trials.com (ISRCTN) (progesterone or progestins) and (brain or head or cranial) and (trauma or injury or damage or wound)
Data and analyses
Comparison 1. Progesterone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at the end of the follow‐up period | 5 | 2376 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.28] |
| 2 Death or severe disability (GOS1 to 3) at the end of the follow‐up period | 4 | 2260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.06] |
Comparison 2. Subgroup analysis: severe TBI subgroup (GCS ≤ 8).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at the end of the follow‐up period | 5 | 2369 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.29] |
| 1.1 severe TBI subgroup (GCS ≤ 8) | 5 | 2090 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.60, 1.27] |
| 1.2 moderate TBI subgroup (GCS 9 to 12) | 2 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.70, 2.41] |
| 2 Death or severe disability (GOS1 to 3) at the end of the follow‐up period | 4 | 2260 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.78, 1.08] |
| 2.1 severe TBI subgroup (GCS ≤ 8) | 4 | 2002 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.11] |
| 2.2 moderate TBI subgroup | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.34, 1.37] |
Comparison 3. Adequate allocation concealment (sensitivity analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at the end of the follow‐up period | 4 | 2320 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.60, 1.28] |
| 2 Death or severe disability (GOS 1 to 3) at the end of the follow‐up period | 4 | 2260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.06] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Skolnick 2014.
| Methods | Multicentre randomised, double‐blind, placebo‐controlled phase III study | |
| Participants | Acute severe TBI patents, aged 16‐70 years (GCS score, ≤ 8 and at least 1 reactive pupil) | |
| Interventions | Both groups: The study drugs (progesterone and placebo) were provided in 250 ml bottles with identical appearance, containing a lipid emulsion consisting of 6% soybean oil and 1.2% egg lecithin phospholipids with the addition of 2.0 mg of progesterone per ml for the active treatment (BHR‐100, Fresenius Kabi). Drug infusion (progesterone or placebo) was started intravenously with 0.355 ml/kg/h for 1 h, followed by 0.25 ml/kg/h for 119 hours, through a dedicated peripheral intravenous catheter or dedicated lumen of a multilumen central catheter. | |
| Outcomes | Primary: GOS at 6 months Secondary: mortality and adverse events |
|
| Notes | Funded by BHR Pharma; Clinicaltrials.gov number, NCT01143064 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was implemented with the use of an interactive Web‐based response system, with a block design of four stratified according to geographic region (Asia, Europe, North America, and South America)." (p 2469) Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was implemented with the use of an interactive Web‐based response system, with a block design of four stratified according to geographic region (Asia, Europe, North America, and South America)." (p 2469) Quote: "The study drugs (progesterone and placebo) were provided in 250‐ml bottles with identical appearance, containing a lipid emulsion consisting of 6% soybean oil and 1.2% egg lecithin phospholipids, with the addition of 2.0 mg of progesterone per millilitre for the active treatment (BHR‐100, Fresenius Kabi)". (p 2469) Comment: probably done |
| Blinding (performance bias and detection bias) Subjective outcome | Low risk | Quote: "Double blind" "The study drugs (progesterone and placebo) were provided in 250‐ml bottles with identical appearance". (p 2469) Comment: probably done |
| Blinding (performance bias and detection bias) Mortality | Low risk | Obtained from medical records; review authors do not believe this introduced bias. |
| Incomplete outcome data (attrition bias) Mortality | Low risk | A total of 31 participants (17 in the progesterone group and 14 in the placebo group) were lost to follow‐up. Missing outcome data were balanced in numbers between the progesterone group and the placebo group. |
| Incomplete outcome data (attrition bias) Favorable outcome | Low risk | A total of 31 participants (17 in the progesterone group and 14 in the placebo group) were lost to follow‐up. Missing outcome data were balanced in numbers between the progesterone group and the placebo group. |
| Selective reporting (reporting bias) | Low risk | The protocol for this trial was presented in Clinicaltrials.gov. It was clear that the published report included all expected outcomes. |
| Other bias | Unclear risk | This multicentre RCT was conducted in approximately 100 centres in 21 countries. The number of outcome events in each centre was quite low. We assessed potential bias for variation between‐centres as unclear because of factors such as different levels of expertise in treating TBI and outcome assessment. |
Wright 2006.
| Methods | Block‐randomised, double‐blind, placebo‐controlled phase II trial | |
| Participants | Adults with acute severe TBI and a GCS score 4‐12 after resuscitation and stabilisation within 11 hours of injury Each participant was assigned to 1 of 8 clinical subgroups defined by sex, race (black versus others), and TBI severity (index GCS scores 4 to 8 were categorised as severe; 9 to 12 as moderate). Within each subgroup, permuted block randomisation assigned 4 of every 5 consecutive participants to progesterone and the other to placebo. A 4:1 randomisation scheme was used to increase the number of participants receiving progesterone while maintaining blinding. Exclusion criteria: indeterminate time of injury; pregnancy; a family reported history of active cancer, acute stroke or of older stroke with residual motor deficits; acute or chronic spinal cord injury with neurologic deficits; a blood alcohol concentration > 250 mg/dL; penetrating brain injury; < 18 years old |
|
| Interventions | Intervention group: progesterone was mixed in Intralipid 20% at a concentration designed to deliver a loading dose of 0.71 mg/kg at 14 mL/h for the first hour when a participant was enrolled. Then the infusion was reduced to 10 mL/h to deliver 0.5 mg/kg/h for the next 11 hours. Five additional 12‐hour maintenance infusions were delivered at the standard rate of 10 mL/h, for a total of 3 days of treatment. Control group: placebo |
|
| Outcomes | Mortality; dichotomised GOS; DRS; duration of coma; duration of post‐traumatic amnesia in 2 subgroups (index GCS scores 4‐8 severe; 9‐12 moderate) at 30 days postinjury, ICP, body temperature, blood pressure during the first 3 days of treatment and for 1 day after treatment, adverse events. | |
| Notes | Funding and support: "Supported by a grant from the National Institute for Neurological Disorders and Stroke, National Institutes of Health (1 R01 NS‐39097‐01A1 to AK) and the General Clinical Research Center at Emory University and Grady Memorial Hospital". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "permuted block randomisation assigned 4 of every 5 consecutive patients to progesterone and the other to placebo". (p 393) Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "permuted block randomisation" (p 393) Quote: "Drug kits were prepared and randomised off site by Emory's Investigational Drug Center. These kits were indistinguishable with respect to treatment assignment".(p 393) Comment: probably done |
| Blinding (performance bias and detection bias) Subjective outcome | Low risk | Quote: "Double blind" "Drug kits were prepared and randomised off site by Emory's Investigational Drug Center. These kits were indistinguishable with respect to treatment assignment". (p 393) Comment: probably done |
| Blinding (performance bias and detection bias) Mortality | Low risk | Obtained from medical records; review authors do not believe this introduced bias. |
| Incomplete outcome data (attrition bias) Mortality | Low risk | 100 participants were randomised; 1 participant who was randomised to progesterone died before the infusion could be started (this subject's data were retained in the analysis under the principle of ITT). Treatment for 3 participants was discontinued (1 was taken into police custody, 2 died during infusion). None of the participants was lost to follow‐up at 30 days. |
| Incomplete outcome data (attrition bias) Favorable outcome | Low risk | 92 participants were contacted to assess their functional status. All the data are presented. |
| Selective reporting (reporting bias) | Low risk | The protocol for this trial was presented in Clinicaltrials.gov and any remaining information was obtained from study authors. It was clear that the published report included all expected outcomes. |
| Other bias | Low risk | ‐‐ |
Wright 2014.
| Methods | Multicentre randomised, double‐blind, placebo‐controlled phase III study | |
| Participants | Adults who had severe, moderate‐to‐severe, or moderate TBI due to a blunt mechanism, with a GCS score of 4‐12. Participants were enrolled if the study treatment could be initiated within 4 h after injury. | |
| Interventions | Both groups: the study drug (progesterone or placebo) was infused continuously through a dedicated intravenous catheter at a dose of 14.3 mL/h for 1 hour and then at 10 mL/h for 71 hours; the dose was then tapered by 2.5 mL/h every 8 hours, for a total treatment duration of 96 hours. Site pharmacists prepared the coded kit assigned by the randomisation algorithm by mixing a weight‐based dose (progesterone 0.05 mg/kg/mLinfusate) from the provided vials and a 250‐mL bag of fat‐emulsion vehicle (Intralipid 20%, Fresenius Kabi) every 24 hours. | |
| Outcomes | Favourable outcome, as determined with the use of the stratified dichotomy of the GOS‐E score at 6 months after injury Mortality, DRS score and adverse events | |
| Notes | Funding and support: Supported by grants from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NS062778, 5U10NS059032, and U01NS056975) and the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000454) and by the Emory Emergency Neurosciences Laboratory in the Department of Emergency Medicine, Emory School of Medicine, and Grady Memorial Hospital. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed with the use of a combination of minimization and biased‐coin algorithms". (p 2459) Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed with the use of a combination of minimization and biased‐coin algorithms".(p 2459) Quote: "Study‐drug kits containing four vials of progesterone in ethanol (active treatment) or ethanol alone (placebo) were prepared by the Emory Investigational Drug Service. Drug kits and their contents were identical in appearance, and study assignments remained concealed from all site pharmacists and study teams".(p 2459) Comment: probably done |
| Blinding (performance bias and detection bias) Subjective outcome | Low risk | "Double blind." "Drug kits and their contents were identical in appearance, and study assignments remained concealed from all site pharmacists and study teams". (p 2459) Comment: probably done |
| Blinding (performance bias and detection bias) Mortality | Low risk | "Double blind" and review authors judged that the outcome and the outcome measurement were not likely to be influenced. |
| Incomplete outcome data (attrition bias) Mortality | Low risk | Missing outcome data balanced in numbers between progesterone group and placebo group. |
| Incomplete outcome data (attrition bias) Favorable outcome | Low risk | Data of 28 participants (6.3%) in progesterone group and 24 (5.5%) in placebo group were missing. |
| Selective reporting (reporting bias) | Low risk | The protocol for this trial was presented in Clinicaltrials.gov. It was clear that the published report included all expected outcomes. |
| Other bias | Unclear risk | The trial intended to enrol 1140 participants, but the trial was abandoned after 882 people had been assessed. The trial was because of futility: favourable outcomes were observed in 51% of participants who received progesterone after TBI, compared with 55.5% of controls. Stratification of the participants on the basis of injury severity did not reveal any effect of progesterone on recovery. We therefore assessed the risk of other bias to be unclear. |
Xiao 2007.
| Methods | RCT | |
| Participants | People with acute severe TBI and a GCS score of 5‐8 within 24 hours of the time of injury Exclusion criteria: pregnant or lactating women, taking other investigational drugs, have severe chronic disease | |
| Interventions | Intervention group: progesterone 80 mg via intramuscular injection repeated every 12 hours for 5 consecutive days Control group: No progesterone administration. No placebo |
|
| Outcomes | Mortality, ICP, GCS scores, complications during treatment GOS scores, verbal and motor function at 10 days and 3 months after injury |
|
| Notes | The study was approved by the Institutional Review Board and the ethical committees and supported by the Scientific Research Fund of Zhejiang Provincial Education Department, China. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients allocated according to random number table". Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | No information was reported. |
| Blinding (performance bias and detection bias) Subjective outcome | High risk | No blinding and the outcome measurement was likely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Mortality | Low risk | No blinding, but review authors judged that the outcome and the outcome measurement were not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Mortality | Unclear risk | No information was reported about withdrawals, dropouts, attrition between groups or losses to follow‐up. |
| Incomplete outcome data (attrition bias) Favorable outcome | Unclear risk | No information was reported about withdrawals, dropouts, attrition between groups or losses to follow‐up. |
| Selective reporting (reporting bias) | High risk | The trial was registered retrospectively. The registration date was after the end of the trial. |
| Other bias | Low risk | ‐‐ |
Xiao 2008.
| Methods | Randomised, double‐blind, placebo‐controlled trial; qualifying participants were randomly assigned in a 1:1 manner using random numbers | |
| Participants | People with acute severe TBI and a GCS score ≤ 8 after resuscitation and stabilisation within 8 hours of injury Exclusion criteria: pregnant or lactating women; people who had taken other investigational drugs within 30 days; who had severe anoxic intracerebral damage or brain death; whose clinical condition was unstable (partial pressure of oxygen < 60 mmHg or a systolic blood pressure < 90 mmHg, or both); those for whom there was doubt about whether the neurological status resulted from head trauma or acute or chronic spinal cord injury | |
| Interventions | Intervention group: progesterone 1.0 mg/kg via intramuscular injection within 8 hours of the documented time of injury and repeated every 12 hours for 5 consecutive days Control group: placebo | |
| Outcomes | Mortality, GOS scores and Modified Functional Independence Measure scores at 3 and 6 months after injury ICP, and average body temperature during treatment Complications and adverse events | |
| Notes | The study was approved by the Institutional Review Board and the ethical committees and supported by the Scientific Research Fund of Zhejiang Provincial Education Department, China. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Qualifying patients were randomly assigned in a 1:1 manner to receive the matching treatment with random numbers". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "The appearance, packaging and administration of placebo and progesterone injections were the same for the two groups". Comment: probably done |
| Blinding (performance bias and detection bias) Subjective outcome | Low risk | "Double blind" "The appearance, packaging and administration of placebo and progesterone injections were the same for the two groups. All patients, treating physicians, nursing staff, and pharmacists were blinded throughout the study period". Comment: probably done |
| Blinding (performance bias and detection bias) Mortality | Low risk | Double blinded, and review authors judged that the outcome and the outcome measurement were not likely to be influenced. |
| Incomplete outcome data (attrition bias) Mortality | Low risk | Data were available for 154 participants (96%) at the 3‐month follow‐up and for 135 (85%) at the 6‐month follow‐up. At 2 months, 19 participants (12%) were lost to follow‐up, 3 (2%) refused follow‐up, and 2 (1%) withdrew from the trial. Missing outcome data were balanced in numbers between the progesterone group and placebo group, with similar reasons for missing data across the groups. |
| Incomplete outcome data (attrition bias) Favorable outcome | Low risk | Missing outcome data were balanced in numbers between the progesterone group and placebo group, with similar reasons for missing data across the groups. |
| Selective reporting (reporting bias) | High risk | The trial was registered retrospectively. The registration date was after the end of the trial. |
| Other bias | Low risk | ‐‐ |
Abbreviations
DRS: Disability Rating Score GCS: Glasgow Coma Scale GOS: Glasgow Outcome Scale GOS‐E: extended Glasgow Outcome Scale ICP: intracranial pressure ITT: intention‐to‐treat analysis RCT: randomised controlled trial TBI: traumatic brain injury
Characteristics of excluded studies [ordered by year of study]
| Study | Reason for exclusion |
|---|---|
| Wright 2005 | This study evaluated the pharmacokinetics of progesterone given by intravenous infusion in TBI patients. No data on therapeutic effects were presented. |
| Abokhabar 2012 | Not a prospectively registered study. We were unable to locate the protocol. |
| Aminmansour 2012 | Not a prospectively registered study. We were unable to locate the protocol. |
| Shakeri 2013 | This study used medroxyprogesterone as the intervention rather than progesterone. Not a prospectively registered study. We were unable to locate the protocol. |
| Mofid 2016 | The authors claimed this was a prospective, single‐blind RCT, which was performed from May 2013‐July 2015. However, we found the study protocol was approved by ethics committee of Kerman University of Medical Sciences in April 2014 and the protocol was registered in the Iranian Registry of Clinical Trials in August 2014 (www.irct.ir, CT2014042017356N1). On the basis of this unreasonable schedule, we judged that the study was not prospective. |
| Raheja 2016 | This study was part of a prospective blinded, randomised, placebo‐controlled phase II trial of progesterone with or without hypothermia. It focused on the relationship between serum biomarkers and outcomes after TBI. The effect of progesterone on TBI was not published in this article. |
Abbreviations
RCT: randomised controlled trial TBI: traumatic brain injury
Characteristics of ongoing studies [ordered by study ID]
CTRI/2009/091/000893.
| Trial name or title | A randomized placebo controlled trial of progesterone with or without hypothermia in subjects with acute severe traumatic brain injury |
| Methods | RCT |
| Participants | Inclusion criteria: men or women aged 18‐65 years with suspected TBI; GCS score 4‐8 after resuscitation and stabilisation, who arrived within 8 hours of injury Exclusion criteria: people who have received any investigational drugs 30 days prior to enrolment, such as progesterone, oestrogen or any other investigational compound; people with severe anoxic intracerebral damage or brain death; those whose clinical condition is unstable (partial pressure of oxygen ≤60 mmHg or a systolic blood pressure ≤90 mmHg, or both); pregnant or lactating women; those for whom there is doubt about whether the altered neurological status resulted from head trauma or acute or chronic spinal cord injury |
| Interventions | Intervention group: progesterone 1.0 mg/kg via intramuscular injection, repeated every 12 hours for 5 consecutive days Control group: distilled water 1.0 mg/kg via intramuscular injection, repeated every 12 hours for 5 consecutive days |
| Outcomes | Primary outcome: GOS score at 1, 3 and 6 months Secondary outcomes: FIM score and mortality at 1,3 and 6 months |
| Starting date | January 2012 |
| Contact information | Dr Sumit Sinha, sumitaiims@yahoo.com Department of Neurosurgery, JPNA Trauma Center, All India Institute of Medical Sciences‐110029 New Delhi, DELHI |
| Sponsors and Collaborators | Department of Biotechnology, Government funding agency |
| Notes | Estimated enrolment: 250 participants |
CTRI/2013/02/003396.
| Trial name or title | A randomised, placebo‐controlled study to investigate the efficacy of progesterone in patients with severe traumatic brain injury |
| Methods | RCT |
| Participants | Inclusion criteria: men and women aged 16‐70 years (inclusive); weighing 45 kg‐135 kg (inclusive); who had sustained a closed head trauma ≤ 8 h before initiation of study drug infusion; TBI diagnosed by history and clinical examination; post‐resuscitation GCS score 4‐8 (inclusive); at least 1 reactive pupil; evidence of TBI confirmed by abnormalities consistent with trauma on CT scan upon admission (diffuse injury II‐IV, evacuated and non‐evacuated mass lesion, Marshall's CT Classification) Exclusion criteria: life expectancy < 24 hours as determined by the Investigator; prolonged and/or uncorrectable hypoxia or hypotension (systolic blood pressure 90 mmHg) upon admission; any spinal cord injury; pregnant; penetrating head injury; bilaterally fixed dilated pupils at the time of randomisation; coma suspected to be primarily due to other causes (e.g. alcohol); pure epidural haematoma; pre‐existing clinically significant disease or chronic condition that can be ascertained at the time of admission and could affect functional outcome; severe cardiac or haemodynamic instability after resuscitation; known treatment with other investigational drug therapy or procedure within 30 days of injury; a history of allergic reaction to progesterone and related drugs or any of the components of the infusion; any disease that, in the opinion of the investigator, is unstable or which could jeopardise the safety of the patient and his/her compliance in the study; those who, in the opinion of the investigator, would not be able or willing to comply with the protocol through the final visit (6 months postinjury) |
| Interventions | Intervention group: progesterone 1.0 mg/kg via intramuscular injection, repeated every 12 hours for 5 consecutive days Control group: distilled water 1.0 mg/kg via intramuscular injection, repeated every 12 hours for 5 consecutive days |
| Outcomes | Primary outcome: GOS at 6 months postinjury Secondary outcomes: mortality at 1 and 6 months postinjury; GOS at 3 months postinjury; GOS‐E at 3 and 6 months; quality of life using SF‐36 at 3 and 6 months; change in ICP and cerebral perfusion pressure; changes in intracranial pathology as assessed by admission and day 6 CT scans; changes in biochemical markers of severe TBI at 120 hours after the administration of the drug/ placebo |
| Starting date | July 2013 |
| Contact information | Dr Sumit Sinha, sumitaiims@yahoo.com Department of Neurosurgery, JPNA Trauma Center, All India Institute of Medical Sciences‐110029 New Delhi, DELHI |
| Sponsors and Collaborators | All India Institute of Medical Sciences |
| Notes | Estimated enrollment: 80 participants |
IRCT2014042017356N1.
| Trial name or title | Effect of female sex hormones on traumatic brain injury |
| Methods | RCT |
| Participants | Inclusion criteria: diffuse TBI; men aged 18‐60 years with moderate to severe brain injury (GCS grades 3‐8 = severe and 9‐12 = moderate). Exclusion criteria: people with blunt head trauma; damage at an unknown time; entering the hospital > 4 hours after the time of damage; severe hypothermia (temperature less than 28 °C); aged < 18 years and > 60 years; candidates for craniotomy; diseases related to the immune system, gastrointestinal, oncology, infectious diseases, and other associated trauma |
| Interventions | Intervention 1 (control group): routine and standard treatment only Intervention 2 (progesterone group): in addition to routine and standard treatment, within 4 hours of brain damage participants are given progesterone 1 mg/kg intramuscularly; dose is repeated every 12 hours for 5 consecutive days Intervention 3 (oestrogen group): in addition to routine and standard treatment, within 4 hours of brain damage participants are given a 40 µg oral oestrogen dose; dose is repeated daily for 5 consecutive days |
| Outcomes | CRS‐R , FIM, GOS‐E, complications and adverse effects, difference GCS between admission and discharge times, length of stay, mortality |
| Starting date | May 2014 |
| Contact information | Dr Mohammad Khaksari, physiolojy@kmu.ac.ir. Department of Physiology, Afzalipour Medicine College, 22 Bahman Blvd. Kerman, Islamic Republic of Iran. |
| Sponsors and Collaborators | Kerman Neuroscience Research Center |
| Notes | Estimated enrollment: 90 participants |
Abbreviations
CRS‐R: coma recovery scale ‐ revised CT: computed tomography FIM: Functional Independence Measure GCS: Glasgow Coma Scale GOS: Glasgow Outcome Scale GOS‐E: extended Glasgow Outcome Scale ICP: intracranial pressure ITT: intention‐to‐treat analysis RCT: randomised controlled trial SF‐36: Short Form (36) health survey TBI: traumatic brain injury
Differences between protocol and review
Yunhui Zeng and Chao You have been added as authors.
We have changed the inclusion criteria according to Cochrane Injuries Group policy (Roberts 2015). In order for a study to be eligible for inclusion, if the report of a study was published after 2010 then the study must have been prospectively registered.
We added a 'Summary of findings' table to the review, and described how we did this in the Methods.
For the outcome of disability at the end of follow‐up, measured using the Glasgow Outome Score (GOS), we split GOS data into favourable (moderate disability, good recovery; GOS 4 and 5) and unfavourable (death, vegetative state, severe disability; GOS 1 to 3) outcomes, as these groupings reflect two different disability levels.
Contributions of authors
JM and YZ undertook partial electronic database searches, screened the citations for eligibility, assessed the quality of papers, extracted data, entered data into RevMan and updated the review.
SH and CY conceived and designed the review, moderated disagreements during data collection, analysed and interpreted data, and helped to write the review.
Sources of support
Internal sources
The Sichuan University, China.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the UK National Institute for Health Research, through Cochrane Infrastructure funding to the Cochrane Injuries Group. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Junpeng Ma: none known
Siqing Huang: none known
Shu Qin: none known
Chao You: none known
Yunhui Zeng: none known
Edited (no change to conclusions)
References
References to studies included in this review
Skolnick 2014 {published data only}
- Skolnick BE, Maas AI, Narayan RK, Hoop RG, MacAllister T, Ward JD. A clinical trial of progesterone for severe traumatic brain injury. The New England Journal of Medicine 2014;371:2467‐76. [DOI] [PubMed] [Google Scholar]
Wright 2006 {published data only}
- Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Annals of Emergency Medicine 2007;49:391‐402. [DOI] [PubMed] [Google Scholar]
Wright 2014 {published data only}
- Wright DW, Yeatts SD, Silbergleit R, Palesch YY, Hertzberg VS, Frankel M. Very early administration of progesterone for acute traumatic brain injury. The New England Journal of Medicine 2014;371:2457‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiao 2007 {published data only}
- Xiao GM, Wei J, Wu ZH. Clinical study on the therapeutic effects and mechanism of progesterone in the treatment for acute severe head injury. Zhonghua Wai Ke Za Zhi 2007;45(2):106‐8. [PubMed] [Google Scholar]
Xiao 2008 {published data only}
- Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Critical Care 2008;12:R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abokhabar 2012 {published data only}
- Abokhabar H, Abouelela A, Mousa S. Impact of progesterone administration on outcome of patients with severe traumatic brain injury. Intensive Care Medicine. 2012.
Aminmansour 2012 {published data only}
- Aminmansour B, Nikbakht H, Ghorbani A, Rezvani M, Rahmani P, Torkashvand M. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: a randomized clinical trial with placebo group. Advanced Biomedical Research 2012;1:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mofid 2016 {published data only}
- Mofid B, Soltani Z, Khaksari M, Shahrokhi N, Nakhaee N, Karamouzian S. What are the progesterone‐induced changes of the outcome and the serum markers of injury, oxidant activity and inflammation in diffuse axonal injury patients?. International Immunopharmacology 2016;32:103‐10. [DOI] [PubMed] [Google Scholar]
Raheja 2016 {published data only}
- Raheja A, Sinha S, Samson N, Bhoi S, Subramanian A, Sharma P. Serum biomarkers as predictors of long‐term outcome in severe traumatic brain injury: analysis from a randomized placebo‐controlled Phase II clinical trial. Journal of Neurosurgery 2016;125:631‐41. [DOI] [PubMed] [Google Scholar]
Shakeri 2013 {published data only}
- Shakeri M, Boustani MR, Pak N, Panahi F, Salehpour F, Lotfinia I. Effect of progesterone administration on prognosis of patients with diffuse axonal injury due to severe head trauma. Clinical Neurology and Neurosurgery 2013;115:2019‐22. [DOI] [PubMed] [Google Scholar]
Wright 2005 {published data only}
- Wright DW, Ritchie JC, Mullins RE, Kellermann AL, Denson DD. Steady‐state serum concentrations of progesterone following continuous intravenous infusion in patients with acute moderate to severe traumatic brain injury. Journal of Clinical Pharmacology 2005;45:640‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
CTRI/2009/091/000893 {published data only}
- Sinha S. A clinical trial of progesterone with or without hypothermia in patients with severe head injury [A randomized placebo controlled trial (factorial design) of progesterone with or without hypothermia in subjects with acute severe traumatic brain injury]. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=1048 2011 (accessed 30 September 2016).
CTRI/2013/02/003396 {published data only}
- Sinha S. A clinical trial to Investigate the efficacy of progesterone in patients with severe traumatic brain injury [A randomized, placebo‐controlled study to investigate the efficacy of progesterone in patients with severe traumatic brain injury]. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=3871 2013 (accessed 30 September 2016).
IRCT2014042017356N1 {unpublished data only}
- Khaksari M. Effect of female sex hormones on traumatic brain injury [Evaluation of the efficacy and probable mechanism of estrogen and progesteron on the complications of moderate and severe diffuse traumatic brain injury in patients admitted in Kerman Shahid Bahonar Hospital: a clinical trial]. www.irct.ir/searchresult.php?id=17356&number=1 2014 (accessed 30 September 2016).
Additional references
Alali 2015
- Alali AS, Vavrek D, Barber J, Dikmen S, Nathens AB, Temkin NR. Comparative study of outcome measures and analysis methods for traumatic brain injury trials. Journal of Neurotrauma 2015;32:581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alderson 2008
- Alderson P, Roberts I. Corticosteroids for acute traumatic brain injury. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD000196.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allolio 1995
- Allolio B, Oremus M, Reincke M, Schaeffer HJ, Winkelmann W, Heck G, et al. High‐dose progesterone infusion in healthy males: evidence against antiglucocorticoid activity of progesterone. European Journal of Endocrinology 1995;133:696‐700. [DOI] [PubMed] [Google Scholar]
Arango 2008
- Arango MF, Bainbridge D. Magnesium for acute traumatic brain injury. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005400.pub3] [DOI] [PubMed] [Google Scholar]
Brotfain 2016
- Brotfain E, Gruenbaum SE, Boyko M, Kutz R, Zlotnik A, Klein M. Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord injury. Current Neuropharmacology 2016;14:641‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
De 2013
- Nicola AF, Gonzalez Deniselle MC, Garay L, Meyer M, Gargiulo‐Monachelli G, Guennoun R. Progesterone protective effects in neurodegeneration and neuroinflammation. Journal of Neuroendocrinology 2013;25:1095‐103. [DOI] [PubMed] [Google Scholar]
FDA 2005
- Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Available from www.fda.gov.
Geddes 2016
- Geddes RI, Peterson BL, Stein DG, Sayeed I. Progesterone treatment shows benefit in female rats in a pediatric model of controlled cortical impact injury. PLOS One 2016;11:e0146419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghajar 2000
- Ghajar J. Traumatic brain injury. Lancet 2000;356:923‐9. [DOI] [PubMed] [Google Scholar]
Goldfien 1989
- Goldfien A. The gonadal hormones and inhibitors. Basic and Clinical Pharmacology. 4th Edition. Norwalk, CT: Appleton and Lange: Katzung BG, 1989:493‐516. [Google Scholar]
GRADE 2004
- GRADE working group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gultekin 2016
- Gultekin R, Huang S, Clavisi O, Pattuwage L, König TC, Gruen R. Pharmacological interventions in traumatic brain injury: can we rely on systematic reviews for evidence?. Injury 2016;47:516‐24. [DOI] [PubMed] [Google Scholar]
He 2014
- He L, Zhang X, Wei X, Li Y. Progesterone attenuates aquaporin‐4 expression in an astrocyte model of ischemia/reperfusion. Neurochemical Research 2014;39:2251‐61. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hong 2016
- Hong Y, Wang X, Sun S, Xue G, Li J, Hou Y. Progesterone exerts neuroprotective effects against Aβ‐induced neuroinflammation by attenuating ER stress in astrocytes. International Immunopharmacology 2016;33:83‐9. [DOI] [PubMed] [Google Scholar]
Hsieh 2016
- Hsieh JT, Lei B, Sheng H, Venkatraman T, Lascola CD, Warner DS. Sex‐specific effects of progesterone on early outcome of intracerebral hemorrhage. Neuroendocrinology 2016;103:518‐30. [DOI] [PubMed] [Google Scholar]
Hyder 2007
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 2007;22:341‐53. [PubMed] [Google Scholar]
Li 2015
- Li X, Zhang J, Zhu X, Wang P, Wang X, Li D. Progesterone reduces inflammation and apoptosis in neonatal rats with hypoxic ischemic brain damage through the PI3K/Akt pathway. International Journal of Clinical and Experimental Medicine 2015;8:8197‐203. [PMC free article] [PubMed] [Google Scholar]
Luoma 2011
- Luoma JI, Kelley BG, Mermelstein PG. Progesterone inhibition of voltage‐gated calcium channels is a potential neuroprotective mechanism against excitotoxicity. Steroids 2011;76:845‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maas 2012
- Maas AI, Menon DK, Lingsma HF, Pineda JA, Sandel ME, Manley GT. Re‐orientation of clinical research in traumatic brain injury: report of an international workshop on comparative effectiveness research. Journal of Neurotrauma 2012;29:32‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGarry 2002
- McGarry LJ, Thompson D. Outcomes and costs of acute treatment of traumatic brain injury. Journal of Trauma 2002;53(6):1152‐9. [DOI] [PubMed] [Google Scholar]
Menon 2015
- Menon DK, Maas AI. Traumatic brain injury in 2014. Progress, failures and new approaches for TBI research. Nature Reviews Neurology 2015;11:71‐2. [DOI] [PubMed] [Google Scholar]
Meyfroidt 2016
- Meyfroidt G, Taccone FS. Another failed attempt of neuroprotection: progesterone for moderate and severe traumatic brain injury. Minerva Anestesiologica 2016;82:486‐91. [PubMed] [Google Scholar]
Nair 2016
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. Journal of Basic and Clinical Pharmacy 2016;7:27‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poon 2015
- Poon W, Vos P, Muresanu D, Vester J, Wild K, Homberg V. Cerebrolysin Asian Pacific trial in acute brain injury and neurorecovery: design and methods. Journal of Neurotrauma 2015;32:571–80. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2015
- Roberts I, Ker K, Edwards P, Beecher D, Manno D, Sydenham E. The knowledge system underpinning health care is not fit for purpose and must change. BMJ 2015;350:h2463. [DOI] [PubMed] [Google Scholar]
Schumacher 1995
- Schumacher M, Baulieu EE. Neurosteroids: synthesis and functions in the central and peripheral nervous systems. Ciba Foundation Symposium 1995;191:90‐106. [DOI] [PubMed] [Google Scholar]
Singh 2013
- Singh M, Su C. Progesterone and neuroprotection. Hormones and Behavior 2013;63:284‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soltani 2016
- Soltani Z, Khaksari M, Shahrokhi N, Mohammadi G, Mofid B, Vaziri A. Effect of estrogen and/or progesterone administration on traumatic brain injury‐caused brain edema: the changes of aquaporin‐4 and interleukin‐6. Journal of Physiology and Biochemistry 2016;72:33‐44. [DOI] [PubMed] [Google Scholar]
Stein 2008
- Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Research Reviews 2008;57(2):386‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2015
- Stein DG. Embracing failure: what the Phase III progesterone studies can teach about TBI clinical trials. Brain Injury 2015;29:1259‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wali 2014
- Wali B, Ishrat T, Won S, Stein DG, Sayeed I. Progesterone in experimental permanent stroke: a dose‐response and therapeutic time‐window study. Brain 2014;137:486‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wali 2016
- Wali B, Sayeed I, Guthrie DB, Natchus MG, Turan N, Liotta DC. Evaluating the neurotherapeutic potential of a water‐soluble progesterone analog after traumatic brain injury in rats. Neuropharmacology 2016;109:148‐58. [DOI] [PubMed] [Google Scholar]
Wang 2013
- Wang X, Zhang J, Yang Y, Dong W, Wang F, Wang L. Progesterone attenuates cerebral edema in neonatal rats with hypoxic‐ischemic brain damage by inhibiting the expression of matrix metalloproteinase‐9 and aquaporin‐4. Experimental and Therapeutic Medicine 2013;6:263‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Webster 2015
- Webster KM, Wright DK, Sun M, Semple BD, Ozturk E, Stein DG. Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long‐term outcomes in a rat model of repeated mild traumatic brain injury. Journal of Neuroinflammation 2015;12:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yousuf 2016
- Yousuf S, Atif F, Sayeed I, Wang J, Stein DG. Neuroprotection by progesterone after transient cerebral ischemia in stroke‐prone spontaneously hypertensive rats. Hormones and Behavior 2016;84:29‐40. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Junpeng 2011
- Junpeng M, Huang S, Qin S. Progesterone for acute traumatic brain injury. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008409.pub2] [DOI] [PubMed] [Google Scholar]
Ma 2012
- Ma J, Huang S, Qin S, You C. Progesterone for acute traumatic brain injury. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD008409.pub3] [DOI] [PubMed] [Google Scholar]


