Abstract
Background
Clavicle (collarbone) fractures account for around 4% of all fractures. Most (76%) clavicle fractures involve the middle‐third section of the clavicle. Treatment of these fractures is usually non‐surgical (conservative). Commonly used treatments are arm slings, strapping and figure‐of‐eight bandages.
This is an update of a Cochrane review first published in 2009 and updated in 2014.
Objectives
To evaluate the effects (benefits and harms) of different methods for conservative (non‐operative) treatment for acute (treated soon after injury) middle third clavicle fractures in adolescents and adults.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register, the Cochrane Central Register of Controlled Trials, MEDLINE (from 1966), Embase (from 1980), LILACS (from 1982), trial registers, orthopaedic proceedings and reference lists of articles. We applied no language or publication restrictions. The date of the last search was 5 January 2016.
Selection criteria
Randomised and quasi‐randomised controlled trials testing conservative interventions for treating adolescents and adults with acute middle third clavicle fractures. The primary outcomes were shoulder function or disability, pain and treatment failure.
Data collection and analysis
For this update, two review authors selected eligible trials, independently assessed risk of bias and cross‐checked data extraction. We calculated risk ratios and 95% confidence intervals for dichotomous variables, and mean differences and 95% confidence intervals for continuous variables. There was very limited pooling of data.
Main results
We included four trials in this review with 416 participants, who were aged 14 years or above. One new trial was included in this update.
Very low quality evidence was available from three trials (296 participants) that compared the figure‐of‐eight bandage with an arm sling for treating acute middle third clavicle fractures. The three trials were underpowered and compromised by poor methodology. Shoulder function was assessed in different ways in the three trials (data for 51, 61 and 152 participants); each trial provided very low quality evidence of similar shoulder function in the two groups. Pooled data from two trials (203 participants) showed no clinical difference between groups after two weeks in pain (visual analogue scale: 0 (no pain) to 10 (worst pain); mean difference (MD) 0.43, 95% confidence interval (CI) ‐0.35 to 1.21; I² = 74%; very low quality evidence). A third trial (61 participants) provided very low quality evidence based on a non‐validated scoring system of more pain and discomfort during the course of treatment in the figure‐of‐eight group. Treatment failure, measured in terms of subsequent surgery, was not reported in two trials; the third trial (152 participants) reported one participant in the arm sling group had surgery for secondary plexus nerve palsy. There was very low quality evidence from one trial (148 participants) of little difference in time to clinical fracture healing (MD 0.2 weeks, 95% CI ‐0.11 to 0.51); data from four non‐symptomatic non‐unions in the figure‐of‐eight group were not included. The very low evidence quality data for individual adverse outcomes (poor cosmetic appearance; change in allocated treatment due to pain and discomfort, worsened fracture position on healing; shortening > 15 mm; non‐symptomatic non‐union and permanent pain) did not confirm a difference between the two groups. There was no clear between group difference in the time to return to school or work activities (MD ‐0.12 weeks, 95% CI ‐0.69 to 0.45; 176 participants; very low quality evidence).
Moderate quality evidence was available from one trial (120 participants; reporting data for 101 participants), which evaluated therapeutic ultrasound. This trial was at low risk of bias but was underpowered and did not report on shoulder function or quality of life. The trial found no evidence of a difference between low‐intensity pulsed ultrasound and placebo in pain, treatment failure (subsequent surgery: 6/52 versus 5/49; RR 1.13, 95% CI 0.37 to 3.47), the time to clinical fracture healing (MD ‐0.32 days, 95% CI ‐5.85 to 5.21), adverse events (one case of skin irritation was reported in each group) or time to resume previous activities.
Authors' conclusions
The current evidence available from randomised controlled trials is insufficient to determine which methods of conservative treatment are the most appropriate for acute middle third clavicle fractures in adolescents and adults. Further research is warranted.
Plain language summary
Non‐surgical interventions for treating a broken collarbone in adolescents and adults
Background and aims
A broken collarbone (clavicle fracture) is a common injury, particularly in adolescents, and accounts for up to 4% of all fractures. Most collarbone fractures occur in the middle‐third section. These fractures are frequently treated with conservative treatments that do not involve surgery. Common conservative treatments are arm slings, strapping and figure‐of‐eight bandages.
This review aimed to evaluate the effects of different conservative treatments for treating collarbone fractures in adolescents and adults without surgery. The main outcomes we were interested in were long‐term function and pain.
Search results
We searched the scientific literature up to January 2016 and found four relevant studies with a total of 416 participants. The four small studies had methodological limitations that may affect the reliability of their findings. The types of conservative treatments evaluated were figure‐of‐bandage versus arm sling in three trials and therapeutic ultrasound versus sham treatment (placebo) in one trial.
Key results
The three studies (296 participants) comparing the figure‐of‐eight bandage versus an arm sling found similar shoulder function in the two groups at the end of follow‐up. Although data from two studies did not show a difference in pain at two weeks after injury, the third study reported more pain and discomfort in people in the figure‐of‐eight bandage group. One participant was recorded as having surgery for a complication. None of the three studies found differences in time for fracture healing, adverse outcomes or time to return to school or work activities.
The fourth study compared therapeutic ultrasound with sham treatment in 120 people with clavicle fractures. It found no difference in outcome, including the time for fracture healing, between the two groups.
Conclusions and quality of evidence
The evidence from the three studies that compared figure‐of‐eight bandage with arm sling was very low quality and so we cannot rely on it to draw conclusions about how collarbone fractures should be treated. We considered the evidence from one study that compared therapeutic ultrasound versus sham treatment to be moderate quality as the study was well conducted but it was not big enough to be conclusive.
Overall, there was not enough evidence to draw conclusions about the best methods of conservative treatment for these fractures.
Summary of findings
Background
The clavicle (or collarbone) has important functions which can be compromised by the occurrence of fractures and their complications. It acts as a prop to keep the shoulder and arm away from the sternum (breastbone) and thoracic (rib) cage. This helps to stabilise the shoulder girdle and to allow the arm a full range of movement. In addition to its role as a bony framework for muscle origins and insertions, the clavicle provides protection to vital neurovascular structures, supports the respiratory function and has a significant aesthetical role in the physical appearance of the person (Kotelnicki 2006; Lazarus 2001).
Description of the condition
Clavicle fractures are common, accounting for 2.6% to 4.0% of all fractures (Nordqvist 1994; Postacchini 2002). Epidemiological studies have reported an overall incidence of 64 per 100,000 population per year in Malmö, Sweden (Nordqvist 1994), 29 per 100,000 population per year in Edinburgh, Scotland (Robinson 1998) and 50 per 100,000 population per year in Uppsala, Sweden (Nowak 2000). The structure of the clavicle comprises medial and lateral flat expanses, linked by a thin, tubular middle. The medial and lateral segments are supported by muscular attachments and ligament structures, but the middle third is not fixed ‐ this area represents a weak link in the clavicular structure. Up to 80% of all clavicle fractures occur in the middle third (Neer 1984).
Clavicle fractures often occur after a fall onto an outstretched hand or after direct trauma to the shoulder. Deformity of the shoulder, as well as bruising, is generally obvious after a clavicle fracture, making diagnosis straightforward (Lazarus 2001; Stanley 1988).
In his study on clavicle fractures, Allman 1967 proposed the classification of clavicle fractures into three groups: group I (middle third fractures); group II (lateral third fractures) and group III (medial third fractures). In a large epidemiological study, Nordqvist 1994 classified 76% of all fractures as group I fractures, and found a median age of 13 years for participants in this group. Just over half (53%) of middle third fractures were undisplaced. Subsequently, due to the absence of a single system that has prognostic and therapeutic value, Robinson 1998 proposed a new classification, which includes prognostically important variables, such as degree of displacement and degree of comminution.
Description of the intervention
Conservative (non‐surgical) treatment is the norm for middle‐third clavicle fractures, and is recommended for these fractures (Robinson 2004); in particular, given the generally low incidence of non‐union after conservative treatment ‐ rates range from 0.03% to 5.9% (Nordqvist 1998; Robinson 2004; Zlowodzki 2005). Generally, conservative interventions to treat clavicle fractures can be grouped into arm‐supporting slings and bandages (simple sling, Velpeau bandage, or Sayre bandage) (Lester 1929) and bandages that aim to reduce the fracture (including a figure‐of‐eight dressing) (Quigley 1950). The most common treatments are the use of an arm sling or figure‐of‐eight bandage (also known as figure‐of‐eight splint, or backpack bandage), or a combination of these two methods (Andersen 1987b; Eiff 1997). There appears to be no consensus on the optimal duration of 'immobilisation'; some have recommended two to six weeks (Eiff 1997; Jeray 2007; Lazarus 2001). Other conservative treatment modalities include therapeutic ultrasound; there are three modalities: low‐intensity pulsed ultrasound, high intensity focused ultrasound and extracorporeal shock wave therapy (Griffin 2014). Often no subsequent therapy is suggested to the patient. Sometimes, however, a patient will require stretching exercises to regain motion.
Complications of clavicle fracture include non‐union, radiographic and symptomatic malunion, and shoulder deformity. Recent studies on displaced midshaft clavicular fractures have found non‐union rates of up to 15% (COTS 2007; Hill 1997; McKee 2006). These findings have prompted a recent increase in surgical interventions for displaced fractures. Fracture‐related risk factors for non‐union include open fracture, associated polytraumatic lesions, refracture, initial fracture displacement, comminution and shortening (Jupiter 1987; Marti 2003). Robinson 2004 observed that advanced age and female gender also predispose to non‐union.
How the intervention might work
Middle third clavicle fractures in adults have traditionally been treated conservatively, given the commonly low incidence of non‐union after conservative intervention. Using an arm sling is the simplest way to treat clavicle fractures and provides analgesia and support during the first four weeks (the period of more intense pain). Usually, the initial healing of a clavicle fracture occurs in approximately six to eight weeks when the soft tissue (callus) bridges the fracture, giving it initial stability. The arm sling provides support while the fracture heals but does not act to realign the fracture fragments, if displaced, and the positioning of the arm in the sling could act to shorten the clavicle by pushing the fracture fragments together in the wrong position. This, however, could lessen the risk of non‐union.
The rationale for treating clavicle fractures with a figure‐of‐eight bandage is that the shoulders are extended, which can facilitate the fracture reduction and lessen the risk of clavicle shortening and malunion while the fracture heals. This, however, could increase the risk of non‐union. Additionally, figure‐of‐eight bandages are more cumbersome and potentially inconvenient to patients than arm slings.
Low‐intensity pulsed ultrasonography (an acoustic energy) is used in the anticipation that it will accelerating fracture healing and thus recovery clinically. The acoustic energy can promote mechanical stimulation that induces a series of biochemical events at the cellular level (e.g. increased production of prostaglandins relating to the tissue repair process and increased congestion and local blood microcirculation) and may stimulate bone formation (Baker 2001).
Why it is important to do this review
This is an update of a Cochrane review first published in 2009 and updated in 2014. No randomised controlled trials (RCTs) have been published since the last published version of our review (between 2009 and 2014).
Middle third fracture of the clavicle is one of the most common fractures of the body. It frequently results in short‐term incapacity and pain, eventually causing longer‐term deformity and disability. As the majority of these fractures are treated conservatively, it is important to review the available evidence in order to inform management decisions for treating patients with these fractures.
Current literature highlights the controversy surrounding the best conservative treatment for middle third clavicle fractures. For example, most US surgeons (94% versus 6%) prefer to use a simple sling rather than the figure‐of‐eight bandage (Heuer 2014), while in Germany the converse holds, with the figure‐of‐eight bandage preferred in 88% of cases (Pieske 2008). This reinforces the importance of updating this review on conservative interventions for treating these fractures.
Objectives
To evaluate the effects (benefits and harms) of different methods for conservative (non‐operative) treatment for acute (treated soon after injury) middle third clavicle fractures in adolescents and adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised or quasi‐randomised (method of allocating participants to a treatment which is not strictly random e.g. by date of birth, hospital record number, alternation) controlled trials comparing conservative interventions for treating clavicle fractures.
Types of participants
We included trials with adolescent or adult participants diagnosed with an acute middle third clavicle fracture. We excluded trials exclusively including young children (aged less than 10 years) but included any trials that recruited young children as well as older people provided the proportion of young children was clearly under 10% or separate data were available. We did not include people with a diagnosis of any other disorders in the shoulder.
Types of interventions
We included trials evaluating the use of, or the optimal duration of use of, any conservative treatment (slings, strapping, figure‐of‐eight bandages and splints, and adjunct therapies such as therapeutic ultrasound).
Types of outcome measures
Primary outcomes
Shoulder function evaluated by upper limb functional outcome measures. Ideally, these should be patient‐reported measures of function validated for people with clavicle fractures (however, we are not aware of any outcome measures in this category). An example of a validated patient‐reported measure of upper limb function is the Disability of the Arm, Shoulder and Hand questionnaire (DASH) (Hudak 1996). A commonly‐used instrument for assessing shoulder function is the Constant score (Constant 1987), which is a composite score for shoulder function that includes subjectively rated pain and activities of daily living, as well as objectively rated range of movement and strength.
Pain. Preference was given to reports of pain measured using validated pain scales (visual analogue scale (VAS) or numerical rating scale (NRS)) and reported in terms of a clinically important change in pain score in the acute/short‐term phase (e.g. proportion with at least 30% improvement in pain) or patient‐reported long‐term pain (e.g. proportion above 30/100 mm VAS scale, i.e. worse than mild pain). Examples are drawn from recommendations in Eccleston 2010 and Moore 2010.
Treatment failure measured by the number of participants who have undergone or are being considered for a surgical intervention (e.g. symptomatic non‐union or malunion).
Timing of primary outcomes measurement
We extracted outcome data at the following time periods: short‐term follow‐up (up to six weeks following treatment); intermediate follow‐up (more than six weeks and up to six months after the end of treatment) and long‐term (longer than six months after the end of treatment).
Secondary outcomes
Clinical fracture healing: we treated this as a proxy for recovery of function in this review.
-
Adverse events, measured by:
cosmetic result: poor outcome such as deformity, asymmetrical result;
asymptomatic non‐union (i.e. the fracture has not healed radiographically) or symptomatic non‐union that is not being considered for surgery, radiographic malunion;
stiffness/restricted of range of shoulder movement;
other reported complication.
Health‐related quality of life, such as Short Form‐36 (Ware 1992).
Return to previous activities (work, sport, activities of daily living etc.), including time to return.
Patient dissatisfaction with method of treatment.
Patient preference and adherence to treatment.
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (7 January 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) (CRS Online; 2014 Issue 1 to 2016 Issue 1), MEDLINE (Ovid Online; 1946 to November Week 3 2015), Embase (Ovid Online; January 2014 to January 2016) and the Latin American and Caribbean Health Sciences Literature (LILACS) (BIREME; 1982 to 7 January 2016). We also searched the ISRCTN Registry, the WHO International Clinical Trials Registry Platform (WHO ICTRP) and ClinicalTrials.gov for ongoing and recently completed trials (11 January 2016).
In MEDLINE, a subject‐specific strategy was combined with the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011). The current search strategies for all databases can be found in Appendix 1. The previous search strategies are reported in Appendix 2.
We did not place any restrictions based on language or publication status.
Searching other resources
We contacted experts in the field and searched reference lists of relevant articles. We searched Orthopaedic Proceedings, a supplement to The Bone and Joint Journal, for abstracts of papers presented at scientific meetings (8 January 2016).
Data collection and analysis
Selection of studies
Two review authors (FF and ML) independently selected and assessed, using a pre‐piloted form, potentially eligible studies for inclusion in the review. We resolved any disagreements by discussion. The review authors were not blinded to the journal or to the authors.
Data extraction and management
Two review authors (FF and ML) extracted the following data using a pre‐piloted data extraction form: characteristics of the study methods including study design, duration of the study, whether the protocol was published before recruitment of participants, funding sources and details of trial registration; characteristics of the study participants including place of study, number of participants assigned, number of participants assessed, inclusion criteria, exclusion criteria, age and classification of injury; characteristics of the study interventions including timing of intervention, type of conservative interventions, rehabilitation and any co‐interventions; characteristics of the study outcomes including length of follow‐up, loss to follow‐up and outcome measures; as well as the methodological domains as outlined in Assessment of risk of bias in included studies.
We resolved any disagreements by discussion. Two review authors (FF and ML) entered data into Review Manager (RevMan 2014). We requested additional information or data from trial authors.
Assessment of risk of bias in included studies
Two independent review authors (FF and ML) assessed the risk of bias of included studies. As recommended by Cochrane's 'Risk of bias' tool (Higgins 2011), we assessed the following domains:
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective reporting.
Other bias (e.g. major baseline imbalance; inappropriate influence of funder; risk of bias associated with inexperience of care providers with the interventions, differences in rehabilitation).
We explicitly judged each of these criteria on the basis of low risk of bias, high risk of bias and unclear risk of bias (either lack of information or uncertainty over the potential for bias). We resolved disagreements between authors regarding the risk of bias for domains by consensus.
Measures of treatment effect
We calculated risk ratios (RRs) together with 95% confidence intervals (CIs) for dichotomous outcomes. We expressed continuous outcome data as mean differences (MDs) with 95% CIs.
When appropriate, we intended to report the number needed to treat to benefit (NNTB) with 95% CIs and the number needed to treat to harm (NNTH) with 95% CIs.
Unit of analysis issues
The unit of randomisation in the studies included in this review was the individual participant.
Dealing with missing data
We performed an intention‐to‐treat analysis with the aim of including all patients randomised to any intervention. When there was insufficient information relative to effect estimates, such as numbers of participants, means, measures of uncertainty (standard deviation or error), or numbers of events and participants, we contacted the lead authors of the included trials.
When it was impossible to acquire adequate data for the forest plot (e.g. means and standard deviations), we presented the data in the text.
We investigated the effects of dropouts and exclusions by conducting worst‐ and best‐case scenario analyses. For dichotomous outcomes, we analysed the worst‐case scenario using the number randomly assigned as the denominator, with the assumption that any participants missing at the end of treatment did not have positive outcomes (e.g. for the outcome 'number of participants experiencing treatment failure' we assumed that any missing participants had experienced an adverse event). We analysed the best‐case scenario using the number randomly assigned in the denominator, and ignored dropouts in our analyses of dichotomous outcomes (overall treatment failure).
Assessment of heterogeneity
We assessed the heterogeneity of effect estimates among the included studies by visual inspection of forest plots, and using the Chi² test and the I² statistic.
We quantified the magnitude of inconsistency (i.e. heterogeneity) through studies, using the I² statistic as follows: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% may represent considerable heterogeneity (Deeks 2008). In cases of considerable heterogeneity (defined as I² ≥ 75%), we planned to explore the data further by comparing the characteristics of individual studies and conducting subgroup analyses.
Assessment of reporting biases
We planned to create funnel plots of primary outcomes to assess the potential for publication bias (small study effects). However, the small number of included studies precluded this analysis.
Data synthesis
When considered appropriate, we planned to pool the results of comparable groups of trials using the fixed‐effect model and 95% confidence intervals (CIs). However, the results using the random‐effects model were also to be inspected where there was diversity in clinical or methodological characteristics.
Subgroup analysis and investigation of heterogeneity
Had sufficient data been available, we planned to carry out subgroup analyses by:
Age (adolescents, adults under 65 and people older than 65 years).
Fractures with two fragments versus more than two fragments.
Primarily undisplaced versus displaced fractures.
We planned to investigate whether the results of subgroups were significantly different by inspecting the overlap of CIs and by performing the test for subgroup differences available in the Review Manager software.
Sensitivity analysis
Where data become available for future review updates, we plan to develop sensitivity analyses to examine various aspects of trial and review methodology, including the effects of missing data and study quality (specifically allocation concealment and outcome assessor blinding).
'Summary of findings' tables and assessment of the quality of the evidence
We used the GRADE approach to assess the quality of evidence related to each of the key outcomes listed in the Types of outcome measures (see Section 12.2, Schünemann 2011).
We presented the main results of intramedullary fixation versus plate fixation for treating acute middle third clavicle fractures in a 'Summary of findings' table. The 'Summary of findings' table provides key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes.
Outcomes for 'Summary of findings' tables
We included the following outcomes in 'Summary of findings' tables: shoulder function, pain, treatment failure, clinical healing (of the fracture), adverse events, quality of life, and return to previous activities.
Results
Description of studies
Results of the search
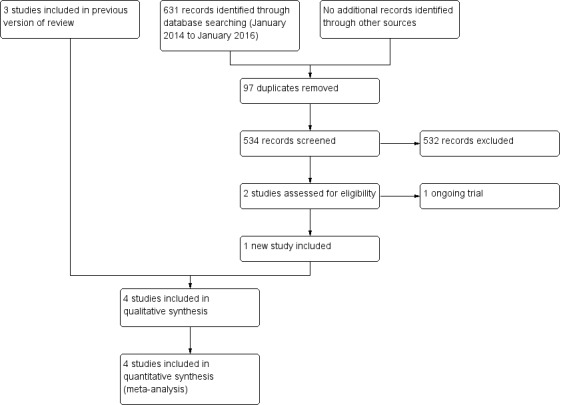
For this update we screened a total of 631 records from the following databases: the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (0), CENTRAL (50), MEDLINE (331), Embase (136), LILACS (20), Orthopaedic Proceedings (14), ISRCTN Registry (9), WHO ICTRP (40) and Clinical Trials.gov (31). We did not identify any potentially eligible studies from other sources.
The search update identified a total of two new studies for potential inclusion, for which full reports were obtained. Upon further analysis, one was included (Ersen 2015) and the other found to be an ongoing study (NCT02398006). A protocol for the ongoing study became available subsequently (Lenza 2016).
Overall, there are now four included studies, four excluded studies and one ongoing trial. We found no studies awaiting classification.
A flow diagram summarising the study selection process is shown in Figure 1. The results from the previous searches (up to January 2014) are reported in Appendix 3.
1.
Study flow diagram
Included studies
We included four randomised controlled trials (RCTs) in this review: Andersen 1987a (reported in English and Danish), Ersen 2015 (reported in English), Hoofwijk 1988 (reported in German) and Lubbert 2008 (reported in English). All trials were located in MEDLINE (PubMed). We also located two reports for both Andersen 1987a and Hoofwijk 1988 in the Cochrane Library (Wiley), and two reports of Andersen 1987a in Embase (OVID). See Characteristics of included studies.
Study design
Andersen 1987a, Ersen 2015 and Hoofwijk 1988 were single‐centre RCTs, conducted in hospitals in Denmark, Turkey and the Netherlands, respectively. All trials used a two‐group design comparing the same interventions (figure‐of‐eight bandage and arm sling).
Lubbert 2008 was a multicentre, double‐blind RCT, conducted in six hospitals in The Netherlands. This trial used a two‐group design comparing low‐intensity pulsed ultrasound (LIPUS) and placebo.
Participants
The four included trials enrolled a total of 416 participants; outcome data were available for a maximum of 365 participants (87.7%).
Age and gender
Andersen 1987a did not report the proportion of males and females. Ersen 2015 reported that 80.4% of trial participants were male, Hoofwijk 1988 reported that 72% were male and in Lubbert 2008, 84% were male.
Participants in Andersen 1987a were aged between 14 and 81 years; the median age of both groups was 19 years. Participants in Ersen 2015 were aged between 15 to 75 years; the mean age for the trial population was 31.6 years. All participants in Hoofwijk 1988 were older than 14 years; the mean age for the trial population was 24.9 years. Participants in Lubbert 2008 were aged between 19 and 74 years; the mean age for the trial population was 37.3 years.
Types of fractures
All trial participants had acute middle third clavicle fractures and were treated just after their diagnosis. Two trials did not use a specific classification for fractures (Andersen 1987a; Hoofwijk 1988). Andersen 1987a divided the fractures into types (two‐fragments, one intermediary fragment and two or more intermediary fragments) and dislocations (undisplaced, minor displacement, major displacement). Ersen 2015 divided the fractures in two types: displaced and not displaced. Hoofwijk 1988 divided the fractures according to displacement and shortening. Lubbert 2008 classified fractures using the Arbeitsgemeinschaft für Osteosynthesefragen (AO) system (Muller 1991).
Interventions
We grouped the included studies according to the comparisons studied.
Comparison 1: Immobilisation bandage (figure‐of‐eight and backpack‐bandage) versus sling
Three trials compared the figure‐of‐eight bandage versus sling immobilisation in 296 participants (Andersen 1987a; Ersen 2015; Hoofwijk 1988). Follow‐up data were available for 264 participants (89.2%).
Comparison 2: Therapeutic ultrasound versus placebo
Lubbert 2008 compared LIPUS versus placebo in 120 participants treated conservatively using a collar and cuff for passive support. Follow‐up data were available for 101 participants (84.2%).
Outcome measures
Primary outcomes
Shoulder function was evaluated in three trials (Andersen 1987a; Ersen 2015; Hoofwijk 1988). Andersen 1987a and Hoofwijk 1988 used non‐validated scores to assess function. Constant and the American Shoulder and Elbow Surgeons (ASES) scores were used for functional evaluation in Ersen 2015.
Pain was evaluated in all four studies: Andersen 1987a used a non‐validated score; Ersen 2015, Hoofwijk 1988 and Lubbert 2008 measured pain by applying a visual analogue scale (VAS: 0 (no pain) to 10 (worst pain)) and recording analgesic consumption.
Failure of treatment, in terms of surgery, was explicitly reported in Hoofwijk 1988 and Lubbert 2008.
Secondary outcomes
All four trials assessed fracture healing. Hoofwijk 1988 and Lubbert 2008 reported on time to clinical fracture consolidation.
Adverse events were reported in various ways in the four trials. Andersen 1987a reported cosmetic results and complications with treatment. Ersen 2015 reported on radiological fracture shortening. Data were available for subjectively‐reported cosmetic appearance and non‐union in Hoofwijk 1988; and for participants with skin irritation in Lubbert 2008.
None of the trials reported on health‐related quality of life.
Time to return to various previous activities was evaluated by Ersen 2015, Hoofwijk 1988 and Lubbert 2008.
Andersen 1987a and Ersen 2015 assessed patient dissatisfaction with the course of treatment.
Patient preference and adherence to treatment data were not specifically collected by the four trials. However, we noted cases where the allocated intervention had been discontinued or not fully taken up; these participants were typically excluded from follow‐up.
Excluded studies
We excluded four studies for the reasons stated in Characteristics of excluded studies (Bajuri 2011; Roberti 2008; Talbot 2008; Thompson 2005).
Ongoing studies
Our search for ongoing trials found one study (NCT02398006). This ongoing trial aims to recruit 110 participants comparing figure‐of‐eight bandage versus arm sling in adults with acute middle third clavicle fractures. See the Characteristics of ongoing studies.
Risk of bias in included studies
Lack of confirmation of allocation concealment, absence of blinding and inadequate treatment of withdrawals in Andersen 1987a, Ersen 2015 and Hoofwijk 1988 point to a high risk of bias in these trials. In contrast, particularly given the effective allocation concealment and blinding, Lubbert 2008 seemed to be at low risk of bias (Figure 2; Figure 3). A summary of the results and impressions of the likelihood of bias are presented below.
2.
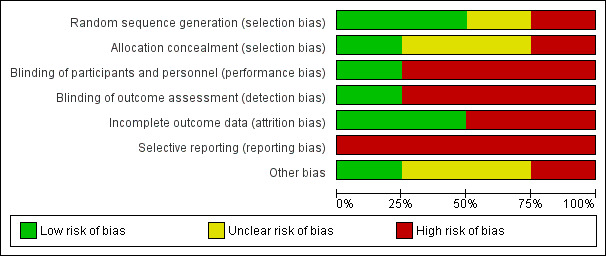
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
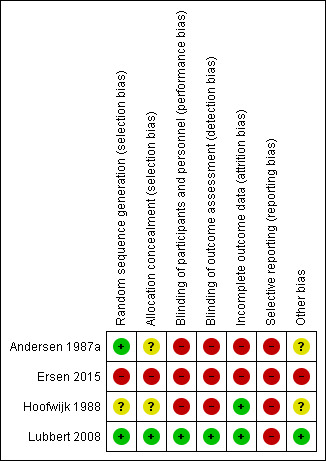
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
All four studies were randomised. We judged Andersen 1987a, which used a random numbers table, and Lubbert 2008, which used block randomisation, to be at low risk of bias relating to random sequence generation. We judged Hoofwijk 1988, which did not provide any information about the sequence generation process, to be at unclear risk of bias. We judged Ersen 2015 to be at high risk of bias because the authors employed non‐stratified randomisation in blocks of two using the sealed envelope method, so when one patient had chosen an envelope, the next patient would be allocated to a group according to the remaining envelope of the pair.
Neither Andersen 1987a, which used a random numbers table, nor Hoofwijk 1988, which used pre‐numbered envelopes, gave sufficient details to ascertain that allocation was concealed. We considered both to be at unclear risk of bias. Allocation was concealed in Lubbert 2008, which used a double‐blind, randomised method involving central randomisation by a third party (the manufacturer) and supply of identical packs containing either an active or placebo transducer. Hence, we judged Lubbert 2008 to be at low risk of selection bias. We considered Ersen 2015 to be at high risk of bias because, as described above, the allocation was not concealed for the second of pairs of patients.
Blinding
Only Lubbert 2008 blinded participants, care providers and the outcome assessors to treatment allocation. We considered this trial to be at low risk of bias for both blinding domains. Blinding of the assessment of most outcomes was impractical for the other three trials due to the type of intervention (Andersen 1987a; Ersen 2015; Hoofwijk 1988). Similarly, the type of intervention (bandage and sling) precluded participant and care provider blinding in these trials. We judged these three trials to be at high risk of bias.
Incomplete outcome data
We considered trials to be at low risk of attrition bias if more than 80% of participants completed the follow‐up, missing outcomes data were balanced in number across intervention groups and an intention‐to‐treat analysis was reported for the primary outcomes. As a result, two trials were at low risk of attrition bias (Hoofwijk 1988; Lubbert 2008); two were at high risk (Andersen 1987a; Ersen 2015).
We judged Hoofwijk 1988 at low risk of bias because more than 80% of participants completed the follow‐up, missing outcomes data were balanced in number across intervention groups and an intention‐to‐treat analysis was likely; however, outcome data for participants who had withdrawn from the trial or were lost to follow‐up were not presented. We classified Lubbert 2008 at low risk of bias because more than 80% of participants completed the follow‐up, missing outcome data were balanced in number across intervention groups, and an intention‐to‐treat analysis was reported for the primary outcomes; however, data for those patients who withdrew were not reported.
We judged Andersen 1987a to be at high risk of bias because only 61 (77%) of 79 participants completed follow‐up. We considered Ersen 2015 to be at high risk of bias because although 82% of participants completed the follow‐up, the missing outcome data were not balanced in numbers across intervention groups: more participants in the figure‐of‐eight group were lost to follow‐up at 12 months (2/30 (6.7%) in sling group vs. 7/30 (23.3%) in figure‐of‐eight group). This may have overestimated the benefits of sling.
Selective reporting
We classified all included trials at high risk of selective reporting bias because the study protocols were not available and in three trials function (primary outcomes) was not evaluated using a validated tool (Andersen 1987a; Hoofwijk 1988; Lubbert 2008). Function was measured by validated scores in Ersen 2015; however, this was only at the end of follow‐up that ranged from six to 12 months and the authors did not report functional outcomes at each time point.
Other potential sources of bias
We classified one trial at low risk of other bias (Lubbert 2008). We considered two trials at unclear of other bias, because they only provided baseline characteristics, split by treatment group at follow‐up rather than for the full study population at randomisation (Andersen 1987a; Hoofwijk 1988). Andersen 1987a only provided separate group data for fracture type and displacement; and Hoofwijk 1988, only for age and gender. In addition, neither Andersen 1987a nor Hoofwijk 1988 provided sufficient information to evaluate the possibility of confounding through differences between the intervention groups in other aspects of the care programmes. Andersen 1987a reported that all participants were encouraged to move the shoulder as soon as possible. However, participants allocated to figure‐of‐eight bandages were also advised to see their general practitioner for checks and adjustments to their bandages at two days, and one and two weeks after application. We judged Ersen 2015 to be at high risk of bias because the results were published in imprecise format and we found some data discrepancies between the results in the text and in the tables.
Effects of interventions
Summary of findings for the main comparison. Summary of findings: figure‐of‐eight bandage versus arm sling.
| Figure‐of‐eight bandage compared with arm sling for treating fractures of the middle third of the clavicle | ||||||
|
Patient or population: patients (mainly young male adults) with fractures of the middle third of the clavicle Settings: hospital (initially) Intervention: figure‐of‐eight bandage Comparison: arm sling | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Arm sling | Figure‐of‐eight bandage | |||||
|
Shoulder function Constant score (0 to 100 points: higher = better) Follow‐up: 6 to 12 months |
Mean (SD) population Constant score 89 (7)¹ | Mean function in the figure‐of‐eight bandage groups was 0.75 points lower (3.72 lower to 2.39 higher) | MD ‐0.75 points (‐3.72 to 2.39) | 51 (1 study) | ⊕⊝⊝⊝ very low² | The 95% CI does not include a clinically important difference.³ Shoulder function was measured using non‐validated measures in two other trials (61 and 152 participants). Both trials found evidence of similar shoulder function in the two groups |
|
Pain (early) Visual Analogue Scale ‐ VAS (0 (no pain) to 10 (worst pain)) Follow‐up: 2 weeks |
Mean pain in the arm sling groups ranged from 0.9 to 1.8 points | Mean pain in the figure‐of‐eight bandage groups was 0.43 points higher (0.35 lower to 1.21 higher) | MD 0.43 points (‐0.35 to 1.21) | 203 (2 studies) | ⊕⊝⊝⊝ very low⁴ | The 95% CI do not include a clinically important difference. A third trial (data for 61 participants) provided very low quality evidence based on a non‐validated scoring system of more pain and discomfort during the course of treatment in the figure‐of‐eight group |
|
Treatment failure (Number of participants who have undergone or are being considered for a surgical intervention) |
See comment | See comment | Not estimable | ‐ | See comment | Poorly reported outcome. One trial (152 participants) reported that one participant in the arm sling group had successful surgery for a secondary plexus nerve palsy |
| Clinical healing ‐ time to clinical fracture consolidation (weeks) | Mean clinical healing in the arm sling group was 3.6 weeks | Mean clinical healing in the figure‐of‐eight bandage group was 0.20 weeks longer (0.11 week shorter to 0.51 week longer) | MD 0.20 weeks (‐0.11 to 0.51) | 148 (1 study) | ⊕⊝⊝⊝ very low² | In addition, there were four non‐unions in the figure‐of‐eight group; none were problematic |
|
Adverse events ‐ total participants with adverse events |
See comment | See comment | Not estimable | ‐ | See comment | The very low evidence quality data for individual adverse outcomes (poor cosmetic appearance; change in allocated treatment due to pain and discomfort, worsened fracture position on healing; shortening > 15 mm; non‐symptomatic non‐union and permanent pain) did not confirm a difference between the two groups⁵ |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not measured in any trial |
| Return to previous activities ‐ Resumption of school/work (weeks) | Mean time to return to previous activities ranged across control groups from 3.5 to 4.6 weeks | Mean time to return to previous activities (weeks) ‐ resumption of school/work in the intervention groups was 0.12 weeks lower (0.69 lower to 0.45 higher) | MD ‐0.12 weeks (‐0.69 to 0.45) | 176 (2 studies) | ⊕⊝⊝⊝ very low⁶ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio SMD: standardised mean difference; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹ These are based on the Constant score in healthy people as reported in Yian 2005.
² We downgraded the evidence for this outcome two levels for high risk of bias reflecting serious study limitations, which included inadequately concealed treatment allocation and lack of blinding. We downgraded the evidence one further level for imprecision given the wide confidence interval and that the available data were from only one trial.
³ For the purposes of this review, the minimally clinical important difference was considered to be 10 points for the Constant score (Kukkonen 2013).
⁴ We downgraded the evidence for this outcome two levels for high risk of bias reflecting serious study limitations, which included inadequately concealed treatment allocation and lack of assessor blinding. We downgraded the evidence one further level for inconsistency given the considerable heterogeneity between the findings of the two groups (I² = 74%).
⁵ Data for individual adverse outcomes (poor cosmetic appearance; change in allocated treatment due to pain and discomfort, worsened fracture position on healing; shortening > 15 mm; non‐symptomatic non‐union and permanent pain) confirmed a difference between the two groups.
⁶ We downgraded the evidence for this outcome two levels for high risk of bias reflecting serious study limitations, which included inadequately concealed treatment allocation and lack of assessor blinding. We downgraded the evidence one further level for imprecision given the low numbers of participants contributing data to this outcome.
Summary of findings 2. Summary of findings: low‐intensity pulsed ultrasound.
| Low‐intensity pulsed ultrasound compared with placebo for treating fractures of the middle third of the clavicle | ||||||
|
Patient or population: patients with fractures of the middle third of the clavicle Settings: hospital Intervention: low‐intensity pulsed ultrasound Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Low‐intensity pulsed ultrasound | |||||
| Shoulder function | See comment | See comment | Not estimable | ‐ | See comment | Not measured in the trial |
|
Pain Visual analogue scale ‐ VAS (0 (no pain) to 10 (worst pain)) Follow‐up: in the 28‐day treatment period |
Mean pain in the control group was 3.55 points | Mean pain in the intervention group was 0.04 points lower (0.61 lower to 0.53 higher) | MD ‐0.04 (95% CI ‐0.61 to 0.53) | 101 (1 study) | ⊕⊕⊕⊝ moderate¹ | |
| Treatment failure ‐ Number who had surgical procedure | See comment | See comment | RR 1.13 (0.37 to 3.47) | 101 (1 study) | ⊕⊕⊕⊝ moderate¹ | Only one trial assessed this comparison |
| Clinical healing ‐ Time to clinical fracture consolidation (days) | Mean clinical healing in the control group was 27.09 days | Mean clinical healing: time to clinical/radiographic fracture consolidation (days) in the intervention group was 0.32 days lower (5.85 lower to 5.21 higher) | MD ‐0.32 weeks (‐5.85 to 5.21) | 101 (1 study) | ⊕⊕⊕⊝ moderate¹ | |
|
Adverse events ‐ total of adverse events (Skin irritation) Follow‐up: during the intervention |
See comment | See comment | RR 0.94 (0.06 to 14.65) | 101 (1 study) | ⊕⊕⊕⊝ moderate¹ | Only one trial assessed this comparison |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not measured in the trial |
| Return to previous activities ‐ Resumption of work (days) | Mean time to return to previous activities in the control group was 15.05 days | Mean time to return to previous activities (days) ‐ resumption of work in the intervention group was 1.95 weeks higher (2.18 lower to 6.08 higher) | MD 1.95 days (‐2.18 to 6.08) | 101 (1 study) | ⊕⊕⊕⊝ moderate¹ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹ We downgraded the evidence one level for imprecision given the wide confidence interval and that the available data were from only one trial.
Three studies (296 participants) compared the figure‐of‐eight bandage versus an arm sling. Follow‐up data were available for 264 participants (89.2%) (131 with figure‐of‐eight bandage and 133 with arm sling). One study (120 participants) compared low‐intensity pulsed ultrasound for fracture placebo. Follow‐up data were available for 101 participants (84.2%) (52 with LIPUS and 49 with control (placebo)).
The authors of Lubbert 2008 responded to our request for additional data to be used in the analyses and provided standard deviations (SDs) for outcomes. The authors of Hoofwijk 1988 were unable to provide the data we needed to calculate the radiographic outcomes. Ersen 2015clarified data discrepancies in their report by email but a promised erratum has yet to appear in the journal (last checked 14 December 2016).
Comparison 1: Immobilisation with figure‐of‐eight bandage versus arm sling
Bandage immobilisation was compared with sling immobilisation in three trials (Andersen 1987a; Ersen 2015; Hoofwijk 1988).
Shoulder function
Shoulder function was assessed in three trials. Andersen 1987a (61 participants at follow‐up) evaluated this outcome using a non‐validated score, but concluded that the functional results for both groups were identical. Ersen 2015 (51 participants at follow‐up) assessed shoulder function using Constant and ASES scores; they found no clinically‐important difference between the groups at the end of follow‐up (mean difference (MD) ‐0.75 points, 95% confidence interval (CI) ‐3.72 to 2.22 for Constant score; and MD ‐1.65 points, 95% CI ‐5.69 to 2.39 for ASES score; Analysis 1.1). Hoofwijk 1988, which measured function using subjective criteria, found there was no evidence of a difference in the number of participants with 'good function' (73/74 with figure‐of‐eight bandage versus 77/78 with arm sling; risk ratio (RR) 1.00, 95% CI 0.96 to 1.04; Analysis 1.2).
1.1. Analysis.
Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 1 Shoulder function.
1.2. Analysis.
Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 2 Shoulder function: number of participants with 'good function'.
Pain
Pain was assessed in all three trials. Andersen 1987a evaluated this outcome using a non‐validated score, but the results for pain from movement were identical at the final follow‐up examination. However, of the five trial participants excluded from the analysis because their allocated treatment was changed, four allocated to figure‐of‐eight bandage were switched to arm slings because they incurred pain and discomfort on application of the figure‐of‐eight bandage. Furthermore, based on a non‐validated scale, participants registered more pain and discomfort during the course of treatment. The pooled data from 203 participants for pain (visual analogue scale (VAS): 0 (no pain) to 10 (worst pain)) from Ersen 2015 and Hoofwijk 1988 showed no clear difference between groups at the first day of treatment (MD 0.63 favouring arm sling, 95% CI ‐0.57 to 1.83; I² = 77%); at the first week (MD 0.20, 95% CI ‐0.32 to 0.73; I² = 0%) or at the second week (MD 0.43, 95% CI ‐0.35 to 1.21; I² = 74%). The pooled results for pain on day one and at two weeks were highly heterogeneous (Analysis 1.3). Pain levels continued to drop at three weeks in Ersen 2015, with minimal differences between groups (Analysis 1.3). There was no clear difference between groups for duration of consumption of painkillers during the treatment (MD 0.60 days, 95% CI ‐0.82 to 2.02; 152 participants, 1 trial; Analysis 1.4).
1.3. Analysis.
Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 3 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)).
1.4. Analysis.
Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 4 Pain: duration of painkiller consumption (days).
Treatment failure
Only Hoofwijk 1988 (157 participants) referred to subsequent surgery for complications. They reported that surgery was not considered for any of the four non‐unions in the figure‐of‐eight group participants as these caused very little complaint but that one participant in the arm sling group had successful surgery for a secondary plexus nerve palsy. Given there was only one event, we did not present these data graphically.
Clinical fracture healing
Both Andersen 1987a and Ersen 2015 reported that all fractures had united. Hoofwijk 1988 reported four cases of non‐union (pseudo‐arthrosis). Hoofwijk 1988 found no clear difference between groups in the time to clinical fracture consolidation (MD 0.20 weeks, 95% CI ‐0.11 to 0.51; Analysis 1.5).
1.5. Analysis.
Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 5 Clinical healing: time to clinical fracture consolidation (weeks).
Adverse events
Hoofwijk 1988 (152 participants) found no clear between‐group difference in the number of participants with subjectively‐rated poor cosmetic appearance post healing of their fracture (10/74 versus 8/78; RR 1.32, 95% CI 0.55 to 3.16; Analysis 1.6). Andersen 1987a (61 participants at follow‐up) concluded that the cosmetic results of the two groups were identical. Andersen 1987a reported that more people in the figure‐of‐eight bandage group experienced greater discomfort and functional impairment; these and other outcome data were presented as part of a non‐validated score. Among the post randomisation exclusions in Andersen 1987a were five participants whose change of allocated treatment related to complications, all manifest in pain and discomfort, with the allocated treatment: the four figure‐of‐eight bandage group participants all had an arm sling and the one participant of the arm sling group was given a Velpeau's bandage (RR 3.02, 95% CI 0.35 to 25.83; 79 participants; Analysis 1.6).
1.6. Analysis.
Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 6 Adverse event.
Radiographic outcomes were evaluated in three trials; however, in Hoofwijk 1988, the exact numbers of participants assessed in the two groups were not available. All fractures healed in Andersen 1987a, who found no clear between‐group difference in the numbers of participants whose fracture position had worsened (3/34 versus 4/27; RR 0.60, 95% CI 0.15 to 2.44; Analysis 1.6). Ersen 2015 reported no clear between‐group difference in the numbers of participants with > 15 mm shortening (5/23 versus 6/28; RR 1.01, 95% CI 0.35 to 2.90); shortening was reported not to be associated with "lower functional results". As described above, cases of minimally symptomatic non‐union were reported only in Hoofwijk 1988 (4/131 versus 0/133; RR 9.48, 95% CI 0.52 to 173.09; 3 trials). Four participants in the figure‐of‐eight group reported 'permanent' pain at the end of clinical follow‐up (4/74 versus 0/78; RR 9.48, 95% CI 0.52 to 173.09) (Hoofwijk 1988).
Presenting data at three years from just eight of the 10 defaulters to clinical follow‐up at a median of three months, Andersen 1987a reported that of the five responders in the figure‐of‐eight group, three reported slight residual symptoms in the form of occasional aching at the former fracture site; one participant reported a skin problem caused by bandage and one participant complained about a lump at the fracture site. Of the three responders in the sling group, one reported a lump at the fracture site. These data are for illustration only given that they apply only to a small subset of the original trial population.
Health‐related quality of life
None of the included studies reported a validated health‐related quality of life measure.
Return to previous activities (work, sport, activities of daily living etc) including time to return
Pooled data from Ersen 2015 and Hoofwijk 1988 indicated no significant difference between groups in the time to return to school or work activities (MD ‐0.12 weeks, 95% CI ‐0.69 to 0.45; 176 participants). Hoofwijk 1988 also found no significant difference between groups in the time to return to sports activities (MD ‐0.60 weeks, 95% CI ‐1.48 to 0.28; 104 participants; Analysis 1.7).
1.7. Analysis.
Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 7 Time to return to previous activities (weeks).
Patient dissatisfaction with method of treatment
The difference in overall patient dissatisfaction with course of treatment was marginally in favour of the arm sling group (i.e. there was marginally less dissatisfaction in the sling group) (14/57 versus 5/55; RR 2.73, 95% CI 1.03 to 7.23; Analysis 1.8). The most common cause of patient dissatisfaction was symptoms related with bandage.
1.8. Analysis.
Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 8 Patient dissatisfaction with course of treatment.
Andersen 1987a found that 26.5% (9/34) of patients in the figure‐of‐eight group and 7.4% (2/27) of patients in the sling group reported dissatisfaction with treatment; the authors did not specify the causes of dissatisfaction.
Ersen 2015 reported that 18% (5/23) of the patients in the figure‐of‐eight group were dissatisfied with the treatment method; two had swelling of the injured extremity on day one; three had some abrasion of axillary skin by day seven because of the friction and compression from the bandage. In contrast, 12% (3/25) of arm sling group participants were dissatisfied because of mobility and crepitation of the fracture site. Also of note is that three participants lost to follow‐up in the figure‐of‐eight group and one in the arm sling group discontinued their allocated intervention; reasons were not provided, however.
Patient preference and adherence to treatment
These concepts were not specifically reported in any of the three trials. However, among the excluded participants in Andersen 1987a were five allocated figure‐of‐eight bandage who either discontinued treatment (one participant) or were treated with a simple sling (four participants) after incurring problems (pain, oedema and secondary fracture displacement upon initial application of the bandage). Of the three non‐adherers in the sling group, one opted for extended bed rest, one had Velpeau's bandage because of pain and one had an hemiplegic attack. Of four participants discontinuing their allocated intervention in Ersen 2015, three were in the figure‐of‐eight bandage group and one was in the sling group.
Comparison 2: Therapeutic ultrasound versus placebo
Therapeutic ultrasound (low‐intensity pulsed ultrasound, LIPUS) was compared with placebo in one study (Lubbert 2008) which reported results for 101 participants.
Shoulder function
Shoulder function was not assessed by Lubbert 2008.
Pain
Pain was assessed using a VAS and by measuring consumption of painkillers. There were no statistically significant differences between groups on the VAS (0 to 10; higher scores mean worse pain) in the 28‐day treatment period (MD ‐0.04, 95% CI ‐0.61 to 0.53; VAS 0 (no pain) to 10 (worst pain); Analysis 2.1) or in consumption of painkillers (MD 4.33 tablets/28 days, 95% CI ‐14.67 to 23.33; Analysis 2.2).
2.1. Analysis.
Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 1 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)).
2.2. Analysis.
Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 2 Pain: number of painkillers (tablets/28 days).
Treatment failure
There was no evidence of a difference between the two groups for subsequent surgery: five in each group had surgery because of lack of fracture healing; one other LIPUS group participant had surgery for the removal of a painful bone spike (Analysis 2.3).
2.3. Analysis.
Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 3 Treatment failure.
Clinical fracture healing
Lubbert 2008 reported no statistically significant difference between LIPUS and placebo in the number of days to clinical fracture consolidation (MD ‐0.32 days, 95% CI ‐5.85 to 5.21; Analysis 2.4).
2.4. Analysis.
Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 4 Clinical healing: time to clinical fracture consolidation (days).
Adverse events
Cosmetic results were not reported. Skin irritation was reported for one participant in each group (Analysis 2.5). The other "minor adverse side effects" were not enumerated. Notably, three participants in the placebo group, whose data were not included in the analyses, discontinued their treatment because of transducer failure or too much pain.
2.5. Analysis.
Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 5 Adverse events: skin irritation.
Health‐related quality of life
Health‐related quality of life was not evaluated in Lubbert 2008.
Return to previous activities (work, sport, activities of daily living etc.) including time to return
Lubbert 2008 reported the time to return to three types of activities (seeAnalysis 2.6). There were no statistically significant differences between the two groups in the number of days to return to household activities (MD ‐2.86 days, 95% CI ‐6.59 to 0.87) or professional work activities (MD 1.95 days, 95% CI ‐2.18 to 6.08). The difference in the time to return to sport activities was marginally in favour of LIPUS (MD ‐2.27 days, 95% CI ‐4.54 to 0.00).
2.6. Analysis.
Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 6 Time to return to previous activities (days).
Patient dissatisfaction with method of treatment
This was not reported in Lubbert 2008.
Patient preference and adherence to treatment
These concepts were not specifically reported in Lubbert 2008. However, among the excluded participants were nine in the LIPUS group and seven in the placebo group who had not completed their treatment diaries, as well as three participants in the placebo group who discontinued their treatment because of transducer failure or too much pain.
Subgroup analyses
We had planned to study the outcomes in different age groups and for different fracture types; however, this was not possible because of the lack of data.
Discussion
Summary of main results
Whilst there are several options for conservative treatment for middle third clavicle fractures, we found only four RCTs (416 participants) that met our inclusion criteria. Table 1 presents a summary of the evidence for figure‐of‐eight bandage compared with arm sling for people, mainly people (aged 14 years or over) with acute clavicle fractures. Very low quality evidence from three trials (Andersen 1987a; Ersen 2015; Hoofwijk 1988) comparing the figure‐of‐eight bandage with an arm sling found no evidence of between group differences in all the main outcomes. Overall, however, the available evidence from these three trials did not allow definitive conclusions about which intervention is better. Table 2 presents a summary of the evidence for low‐intensity pulsed ultrasound compared with placebo for adults with acute clavicle fractures. Moderate quality evidence from the fourth trial (Lubbert 2008) provided no evidence that application of therapeutic ultrasound influences recovery, including clinical fracture healing, or affects outcome after clavicle fractures.
Overall completeness and applicability of evidence
The search strategy for this review was designed, within reason, to locate all possible relevant trials. It included key electronic databases, including clinical trials registers, and contact with experts in the field. We included only RCTs or quasi‐RCTs in this review to restrict the possible selection bias.
We included four trials in this review. The included trials were not sufficient to evaluate the relative effectiveness of different conservative treatments for middle third clavicle fractures; this is attributable to the lack of available data, including 'missing' data that were irretrievable. Three trials compared figure‐of‐eight bandage versus arm sling (Andersen 1987a; Ersen 2015; Hoofwijk 1988). Two were conducted in the 1980s and missing data were not recorded (Andersen 1987a; Hoofwijk 1988). The evidence from the most recent trial that compared figure‐of‐eight bandage versus arm sling is not robust due to the risk of bias and small size (Ersen 2015). Historically, more than 200 different "devices", including bandages, have been reported for the conservative treatment of middle third clavicle fractures (Lester 1929). However, as shown in two surveys, the comparison of the figure‐of‐eight bandage versus the arm sling as tested in the three trials is relevant to current practice (Heuer 2014; Pieske 2008).
The trial that evaluated the effectiveness of therapeutic ultrasound treatment (LIPUS) found no differences between the experimental and control groups for the recorded outcomes (Lubbert 2008).
In accordance with the planning of this review, three included trials assessed adolescents and adults (Andersen 1987a; Ersen 2015; Hoofwijk 1988), and one trial assessed participants aged over 18 years (Lubbert 2008); however, with the data available we could not develop any subgroup analyses evaluating any association of age, skeletal maturity, fracture type or fracture displacement with outcome.
Quality of the evidence
All three trials comparing figure‐of‐eight bandage versus arm sling were at high risk of bias and individually underpowered. Additionally, two studies were conducted in the 1980s, when reporting and reporting standards were poorly developed and validated outcome measures of shoulder function were not available (Andersen 1987a; Hoofwijk 1988). We judged the quality of the evidence from this comparison to be very low; this reflects the downgrading by two levels because of the high risk of bias, and by one level because of imprecision reflecting the insufficiency of the data (often from one trial only) or inconsistency reflecting considerable heterogeneity for the pain at two weeks outcome. This means that we are very uncertain about the estimates of effect.
Lubbert 2008 was assessed at low risk of bias, where our judgement of the high risk of selective reporting bias reflected the absence of reporting of shoulder function. Despite being a multicentre study, it lacked power to determine if therapeutic ultrasound is a beneficial intervention after clavicle fracture. Thus, we downgraded our assessment of the quality of the evidence by one level for imprecision. The resulting judgement of moderate quality means that we think that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Potential biases in the review process
This review was conducted following the criteria and methods set out in a published protocol (Lenza 2008). It is possible but unlikely that we have missed potentially eligible trials. Our search strategy has been maintained and updated by the contact author (ML). The databases searched included LILACS, which captures studies from Latin America. Chinese studies reach the Cochrane Central Register of Controlled Trials through the Chinese Cochrane Centre.
We approached the authors of our included studies. We received unpublished data for two trials: numbers of participants, mean and standard deviations of all continuous endpoints and numbers of participants and number of events of all dichotomous endpoints for Ersen 2015; and standard deviations for Lubbert 2008. The missing data from Hoofwijk 1988 were no longer available. Authors of unpublished trials have been contacted with requests for information and trial reports.
Agreements and disagreements with other studies or reviews
We found one published review (Zlowodzki 2005) that assessed interventions to treat clavicle fractures and our results on effectiveness of conservative interventions are consistent with this review. However, our review adds consistent information for current clinical practice: we applied more rigorous methodology, restricting the included studies to RCTs or quasi‐RCTs, and performed a broader literature search that included non‐English literature. We also plan future updates in the light of new evidence.
Two recent systematic reviews concluded that the evidence of effectiveness of therapeutic ultrasound for treating acute fractures in adults is moderate to very low in quality and is insufficient to support the routine use of this intervention in current clinical practice (Busse 2009; Griffin 2014).
Authors' conclusions
Implications for practice.
Despite the high incidence of middle third clavicle, very few RCTs or quasi‐RCTs have examined their relative effectiveness of treatment. There is insufficient evidence from three trials to establish the relative effects on final functional outcome of the figure‐of‐eight bandage and an arm sling, although the bandage may be associated with more early pain and discomfort during use. Currently, based on the results of one underpowered trial, there is no evidence of enhanced recovery (specifically accelerated, clinically determined fracture healing) to support the use of therapeutic ultrasound treatment for these fractures. Health professionals involved in managing these injuries should continue to manage patients with midshaft clavicle fractures using established techniques, taking into consideration the nature of the fracture, their own experience and the circumstances of the patient.
Implications for research.
RCTs of conservative methods of treatment, including further trials comparing contemporary conservative interventions, such as an arm sling versus the figure‐of‐eight bandage for clavicle fractures, are warranted. These should meet current standards for the planning, conduct and reporting of RCTs, and be adequately powered. It would be useful if randomisation was stratified by skeletal maturity and the data from adult and adolescent subgroups were reported separately. Validated health‐related quality of life, pain and shoulder function tests should be used as outcome measures. Ideally, these should be patient‐reported measures of function validated for people with clavicle fracture.
What's new
| Date | Event | Description |
|---|---|---|
| 1 November 2016 | New search has been performed | In this update, published in 2016; the following changes were made: 1. The Background was updated, which included the addition of the new section on 'How the intervention might work'. 2. The search was updated to January 2016. 3. Two new studies were identified. Of these, one was included and one is an ongoing study. 4. Further modifications were made to 'Types of outcome measures'. 5. Summary of findings tables were generated. |
| 1 November 2016 | New citation required but conclusions have not changed | Changes were made to the authorship of the review. |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 29 May 2014 | New citation required but conclusions have not changed | No new included studies. Conclusion not changed. |
| 29 May 2014 | New search has been performed | This update included:
|
| 27 March 2008 | Amended | Converted to new review format. |
Acknowledgements
For this version of the review, we would like to thank Lindsey Elstub and Joanne Elliott for their support and Helen Handoll and Nigel Hanchard for their helpful feedback.
Thanks are also extended to the authors of the included trials who responded to requests for additional information/data: Dr Ali Ersen (Turkey), Dr Pieter Lubbert (The Netherlands), Professor Chris van der Werken (The Netherlands) and Dr Kjeld Andersen (Denmark).
We would like to recognise João Baptista Gomes dos Santos, João Carlos Belloti, and Régis B Andriolo who were authors on the previous version of this review.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies (Janurary 2014 to January 2016)
Cochrane Central Register of Studies (CRS Online)
#1 MESH DESCRIPTOR clavicle EXPLODE ALL TREES (66) #2 (clavic* or midclavic* or collarbone):TI,AB,KY (225) #3 #1 OR #2 (225) #4 MESH DESCRIPTOR Fracture Healing (333) #5 MESH DESCRIPTOR Fracture Fixation EXPLODE ALL TREES (1004) #6 MESH DESCRIPTOR Fractures, Bone EXPLODE ALL TREES (3120) #7 fracture*:TI,AB,KY (9958) #8 #4 OR #5 OR #6 OR #7 (9974) #9 #3 AND #8 (102) #10 31/01/2014 TO 31/01/2016:DL (181817) #11 #9 AND #10 (50)
MEDLINE (OvidSP)
1 Clavicle/ (4763) 2 (clavic* or midclavic* or collarbone).tw. (8153) 3 1 or 2 (9807) 4 Fracture Healing/ (10158) 5 exp Fracture Fixation/ (49809) 6 exp Fractures, Bone/ (149256) 7 fracture*.tw. (191373) 8 4 or 5 or 6 or 7 (237311) 9 3 and 8 (2997) 10 Randomized controlled trial.pt. (422103) 11 Controlled clinical trial.pt. (92600) 12 randomized.ab. (344099) 13 placebo.ab. (171790) 14 Drug therapy.fs. (1876199) 15 randomly.ab. (247903) 16 trial.ab. (359106) 17 groups.ab. (1540591) 18 or/10‐17 (3746261) 19 exp Animals/ not Humans/ (4176097) 20 18 not 19 (3223958) 21 9 and 20 (331)
Embase (Ovid Online)
1 Clavicle/ (5030) 2 (clavic* or midclavic* or collarbone).tw. (9473) 3 or/1‐2 (10957) 4 exp Fracture Healing/ or exp Fracture Treatment/ or exp Fracture/ (246703) 5 fracture*.tw. (219328) 6 or/4‐5 (306053) 7 and/3,6 (3289) 8 Randomized Controlled Trial/ (388340) 9 Controlled Clinical Study/ (391120) 10 random*.ti,ab. (1029619) 11 Randomization/ (68588) 12 Intermethod Comparison/ (203346) 13 placebo.ti,ab. (223165) 14 (compare or compared or comparison).ti. (387195) 15 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (1315917) 16 (open adj label).ti,ab. (47583) 17 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. (171854) 18 Double Blind Procedure/ (124856) 19 parallel group*1.ti,ab. (17424) 20 (crossover or cross over).ti,ab. (75833) 21 ((assign* or match or matched or allocation) adj5 (alternate or group*1 or intervention*1 or patient*1 or subject*1 or participant*1)).ti,ab. (221526) 22 (assigned or allocated).ti,ab. (263126) 23 (controlled adj7 (study or design or trial)).ti,ab. (228125) 24 (volunteer or volunteers).ti,ab. (187553) 25 Human Experiment/ (345374) 26 trial.ti. (189895) 27 or/8‐26 (3415088) 28 (exp Animal/ or Animal.hw. or Nonhuman/) not (exp Human/ or Human Cell/ or (human or humans).ti.) (5397275) 29 27 not 28 (2962221) 30 7 and 29 (461) 31 (2014* or 2015* or 2016*).em,dd. (3508884) 32 30 and 31 (136)
LILACS (BIREME)
Mh Clavicle OR Tw clavic$ OR Tw midclavic$ OR Tw collarbone [Words] and Mh Fracture healing OR Mh Fracture fixation OR Mh Fractures OR Tw fracture$ [Words] and ((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh Randomized controlled trials OR Mh Random allocation OR Mh Double‐blind method OR Mh Single‐blind method) AND NOT (Ct animals AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh Research design) AND NOT (Ct animals AND NOT (Ct human and Ct animals)) OR (Ct comparative study OR Ex E05.337$ OR Mh Follow‐up studies OR Mh Prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animals AND NOT (Ct human and Ct animals))) [Words] (20)
Orthopaedic Proceedings (The Bone and Joint Journal)
Title: clavic* or midclavic* or collarbone
Abstract or title: random*
Orthopaedic Proceedings = 14
ISRCTN Registry
fracture AND (clavicle OR clavicular OR midclavicle OR midclavicular) = 9
WHO International Clinical Trials Registry Platform
clavic* AND fracture* OR midclavic* AND fracture* OR mid‐clavic* AND fracture* = 40
ClinicalTrials.gov
fracture AND (clavicle OR clavicular OR midclavicle OR midclavicular) = 31
Appendix 2. Previous search strategies 2008 to 2014
The Cochrane Library (Wiley Online Library)
#1 MeSH descriptor: [Clavicle] this term only (75) #2 (clavic* or midclavic* or collarbone):ti,ab,kw (159) #3 (#1 or #2) (159) #4 MeSH descriptor: [Fracture Healing] this term only (364) #5 MeSH descriptor: [Fracture Fixation] explode all trees (1124) #6 MeSH descriptor: [Fractures, Bone] explode all trees (3803) #7 (fracture*):ti,ab,kw (8448) #8 (#4 or #5 or #6 or #7) (8464) #9 (#3 and #8) in Trials (54)
MEDLINE (PubMed)
(((Clavicle [mh] OR clavic* [tw] OR collarbone [tw]) AND (Fracture Healing [mh] OR Fracture Fixation [mh] OR Fractures, Bone [mh] OR fracture* [tw]) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp]) NOT (animals [mh] NOT humans [mh])))) AND 2008:2014 [edat] (89)
Embase (Ovid Online)
1 Clavicle/ (4726) 2 (clavic$ or midclavic$ or collarbone).tw. (9091) 3 or/1‐2 (10567) 4 exp Fracture Healing/ or exp Fracture Treatment/ or exp Fracture/ (232404) 5 fracture$.tw. (209206) 6 or/4‐5 (291855) 7 and/3,6 (3094) 8 Clinical trial/ (896067) 9 Randomized controlled trial/ (367792) 10 Randomization/ (64730) 11 Single blind procedure/ (18891) 12 Double blind procedure/ (122402) 13 Crossover procedure/ (39662) 14 Placebo/ (245995) 15 Randomi?ed controlled trial$.tw. (99640) 16 Rct.tw. (13563) 17 Random allocation.tw. (1368) 18 Randomly allocated.tw. (20584) 19 Allocated randomly.tw. (1974) 20 (allocated adj2 random).tw. (823) 21 Single blind$.tw. (14643) 22 Double blind$.tw. (150221) 23 ((treble or triple) adj blind$).tw. (377) 24 Placebo$.tw. (206316) 25 Prospective study/ (261999) 26 or/8‐25 (1429455) 27 Case study/ (23741) 28 Case report.tw. (270245) 29 Abstract report/ or Letter/ (920220) 30 or/27‐29 (1208582) 31 26 not 30 (1391203) 32 limit 31 to human (1265124) 33 and/7,32 (181) 34 (2008$ or 2009$ or 2010$ or 2011$ or 2012$ or 2013$ or 2014$).em. (7323623) 35 33 and 34 (108)
LILACS (BIREME)
Mh Clavicle OR Tw clavic$ OR Tw midclavic$ OR Tw collarbone [Words] and Mh Fracture healing OR Mh Fracture fixation OR Mh Fractures OR Tw fracture$ [Words] and ((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh Randomized controlled trials OR Mh Random allocation OR Mh Double‐blind method OR Mh Single‐blind method) AND NOT (Ct animals AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh Research design) AND NOT (Ct animals AND NOT (Ct human and Ct animals)) OR (Ct comparative study OR Ex E05.337$ OR Mh Follow‐up studies OR Mh Prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animals AND NOT (Ct human and Ct animals))) [Words] (18)
Orthopaedic Proceedings (The Bone and Joint Journal)
Title: clavic* or midclavic* or collarbone
Abstract or title: random*
Orthopaedic Proceedings = 13
Appendix 3. Results of the searches in previous versions of the review
We updated the search from December 2008 to January 2014. We screened a total of 282 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (0), the Cochrane Central Register of Controlled Trials (54), MEDLINE (89), Embase (108), LILACS (18) and Orthopaedic Proceedings (13). We did not identify any potentially eligible studies from trial registers or other sources.
The search update resulted in the identification of one potentially eligible study, for which we obtained a full report. We excluded this study (Bajuri 2011). We also excluded one study that was classified as ongoing in the last version of this review, after contact with the primary study author (Roberti 2008). A flow diagram summarising the study selection process is shown in Figure 1.
Overall, there are now a total of three included studies, four excluded studies, no ongoing trials and no studies awaiting classification.
The search of five main electronic databases for completed research yielded 159 references (Figure 1). JB and ML screened the titles and abstracts for references and firstly excluded 152 citations. Among these, 115 were duplicated citations or not relevant, 31 were excluded because they did not meet the inclusion criteria for participants or interventions, and six were not randomised or quasi‐randomised trials. The remaining seven potentially relevant studies were evaluated from full trial reports. Three trials were included (Andersen 1987a; Hoofwijk 1988; Lubbert 2008). A translation of one report (Jensen 1985) showed it that was a report of Andersen 1987a. One full report and two abstracts, one of which reported interim results (Hoofwijk 1986), were available for Hoofwijk 1988. Finally, one study (Thompson 2005) was excluded. Two trials were translated to English (see Acknowledgements). Of the two further trials identified from Trial Registers, one was excluded (Talbot 2008) and one is ongoing (Roberti 2008a).
Data and analyses
Comparison 1. Figure‐of‐eight bandage versus arm sling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Shoulder function | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Constant score (at end of follow‐up: 6 ‐ 12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 ASES score (at end of follow‐up: 6 ‐ 12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Shoulder function: number of participants with 'good function' | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Pain on 1st day | 2 | 203 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.57, 1.83] |
| 3.2 Pain on 1st week | 2 | 203 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.32, 0.73] |
| 3.3 Pain on 2nd week | 2 | 203 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.35, 1.21] |
| 3.4 Pain on 3rd week | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.21, 0.41] |
| 4 Pain: duration of painkiller consumption (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Clinical healing: time to clinical fracture consolidation (weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Adverse event | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Poor cosmetic appearance post fracture healing | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.55, 3.16] |
| 6.2 Change in allocated treatment due to pain and discomfort | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.02 [0.35, 25.83] |
| 6.3 Worsened fracture position on healing | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.15, 2.44] |
| 6.4 Shortening > 15 mm | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.35, 2.90] |
| 6.5 Non‐union | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.48 [0.52, 173.09] |
| 6.6 Permanent pain at mean 10 months | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.48 [0.52, 173.09] |
| 7 Time to return to previous activities (weeks) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Resumption of school/work | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.69, 0.45] |
| 7.2 Resumption of sports activities | 1 | 104 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.48, 0.28] |
| 8 Patient dissatisfaction with course of treatment | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.03, 7.23] |
Comparison 2. Low‐intensity pulsed ultrasound versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Pain: number of painkillers (tablets/28 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Treatment failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Number who had surgical procedure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Clinical healing: time to clinical fracture consolidation (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Adverse events: skin irritation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Time to return to previous activities (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Resumption of household activities | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Resumption of professional work | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Resumption of sport | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [author‐defined order]
Andersen 1987a.
| Methods |
Study design: RCT Duration of the study: June 1981 to September 1982 Protocol was published before recruitment of patients: not reported Details of trial registration: not reported Intention‐to‐treat analysis: Likely, but outcome data for participants who had withdrawn from the trial or were lost to follow‐up were not presented Funding sources: not reported |
|
| Participants |
Location: Denmark Number of participants assigned: 79 (49 figure‐of‐eight bandage group; 34 arm sling group) Number of participants assessed: 61 (34 figure‐of‐eight bandage group; 27 arm sling group) Inclusion criteria:
Exclusion criteria:
Age (median; range):
Gender: not reported Side of injury: not reported Classification of injury: not reported, just fracture types (2 fragments, 1 intermediary fragment and 2 or more intermediary fragments) and fracture dislocations (undisplaced, minor displacement, major displacement) |
|
| Interventions |
Timing of intervention: after diagnosis Intervention 1 (figure‐of‐eight bandage):
Intervention 2 (arm sling):
Rehabilitation: not reported Any co‐interventions: not reported |
|
| Outcomes |
Length of follow‐up: The figure‐of‐eight group lasted a median interval of 12 weeks (10 to 16) and the sling group lasted a median interval of 13 weeks (10 to 17); the figure‐of‐eight group also was assessed at 2 days, 1 and 2 weeks Loss of follow‐up: 18 participants lost to follow‐up: Figure‐of‐eight bandage group ‐ 11 participants lost to follow‐up:
Arm sling group ‐ two seven participants lost to follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes | The outcomes were evaluated by a non‐validated scoring system | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table was used |
| Allocation concealment (selection bias) | Unclear risk | Details to ascertain that allocation was concealed were not provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Less than 80% of participants completed the follow‐up (23% of withdrawals) |
| Selective reporting (reporting bias) | High risk | The authors used non‐validated scores to assess function and pain; treatment failure was not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Ersen 2015.
| Methods |
Study design: RCT Duration of the study: August 2012 and September 2013 Protocol was published before recruitment of patients: not reported Details of trial registration: not reported Intention‐to‐treat analysis: Likely, but outcome data for participants who had withdrawn from the trial or were lost to follow‐up were not presented Funding sources: the authors reported that no benefits in any form were received or will be received from a commercial party related directly or indirectly to the subject of this article |
|
| Participants |
Location: Istanbul, Turkey Number of participants assigned: 60 (30 figure‐of‐eight bandage group; 30 arm sling group) Number of participants assessed: 51 (23 figure‐of‐eight bandage group; 28 arm sling group) Inclusion criteria:
Exclusion criteria:
Age (mean; range):
Gender (male/female):
Side of injury (dominant/non‐dominant):
Classification of injury (displaced/not displaced):
|
|
| Interventions |
Timing of intervention: All participants were assessed on the day of injury Intervention 1 (figure‐of‐eight bandage):
Intervention 2 (arm sling):
Rehabilitation: not reported Any co‐interventions: not reported |
|
| Outcomes |
Length of follow‐up:
Loss of follow‐up: nine participants lost to follow‐up: Figure‐of‐eight bandage group ‐ seven participants lost to follow‐up:
Arm sling group ‐ two participants lost to follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes | We found some data discrepancies between text and tables of paper – all data were checked by authors’ via email. This confirmed the denominators at follow‐up were 23 figure‐of‐eight bandages and 28 arm slings | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The authors employed non‐stratified randomisation in blocks of two using the sealed envelope method, so when one patient had chosen an envelope, the next patient would be allocated to a group according to the remaining envelope of the pair |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 82% of participants completed the follow‐up. Missing outcome data were not balanced in numbers across intervention groups; more participants in the figure‐of‐eight group were lost to follow‐up at 12 months; (2/30 (6.7%) in sling group vs. 7/30 (23.3%) in figure‐of‐eight group). This may have overestimated the benefits of sling |
| Selective reporting (reporting bias) | High risk | The study protocol was not available. Function or disability was measured by validated scores, however, only at the end of follow‐up that raged by 6 to 12 months ‐ the authors did not report functional outcomes at each time point |
| Other bias | High risk | The results were published in imprecise format. We found some data discrepancies between text and tables of paper |
Hoofwijk 1988.
| Methods |
Study design: RCT Duration of the study: December 1983 to May 1987 Protocol was published before recruitment of patients: not reported Details of trial registration: not reported Intention‐to‐treat analysis: Likely, but outcome data for participants who had withdrawn from the trial or were lost to follow‐up were not presented Funding sources: not reported |
|
| Participants |
Location: Department of Surgery, Saint Elisabeth Hospital, Tilburg, The Netherlands Number of participants assigned: 157 (78 figure‐of‐eight bandage group; 79 arm sling group) Number of participants assessed: 152 (74 figure‐of‐eight bandage group; 78 arm sling group) Inclusion criteria:
Exclusion criteria:
Age (mean; SD):
Gender (male/female):
Side (left/right): 85/72 Classification of injury: not specified, just fracture displacement (undisplaced and displacement) and multiple fragment fractures (with or without shortening) |
|
| Interventions |
Timing of intervention: after diagnosis Intervention 1 (figure‐of‐eight bandage): details not reported Intervention 2 (arm sling): details not reported Rehabilitation: not reported Any co‐interventions: not reported |
|
| Outcomes |
Length of follow‐up:
Loss of follow‐up: five participants lost to follow‐up: Figure‐of‐eight bandage group ‐ four participants lost to follow‐up:
Arm sling group ‐ one participant lost to follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes | Nine participants ‐ all without complications ‐ refused x‐rays at final follow‐up. Participant numbers for each intervention were not known despite contacting the authors; thus we have used the numbers available at follow‐up for denominators | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomised to the 2 treatment groups by opening of pre‐numbered envelopes; however, details to ascertain that allocation was concealed were not provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | More than 80% of participants completed the follow‐up, missing outcomes data were balanced in number across intervention groups and an intention‐to‐treat analysis was likely, but outcome data for participants who had withdrawn from the trial or were lost to follow‐up were not presented |
| Selective reporting (reporting bias) | High risk | The authors used non‐validated scores to assess function and treatment failure was not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Lubbert 2008.
| Methods |
Study design: multicentre, double‐blind RCT Duration of the study: March 2001 and December 2003 Protocol was published before recruitment of patients: not reported Details of trial registration: not reported Intention‐to‐treat analysis: Likely, but data of those patients who withdrew could not be collected Funding sources: data collection and data analysis were supported by a financial grant from Smith and Nephew Inc, Memphis, USA. Transducers (placebo and active) were provided free of cost. No author had any financial or personal relationships with people or organisations that could inappropriately influence their work |
|
| Participants |
Location: 6 hospitals in the Netherlands participated in the study (Meander Medical Centre, Amersfoort; Onze Lieve Vrouwen Gasthuis Hospital, Amsterdam; Reinier de Graaf Hospital, Delft; Saint Antonius Hospital, Nieuwegein; Diakonessen Hospital, Utrecht; University Medical Centre Utrecht, Utrecht) Number of participants assigned: 120 (61 LIPUS; 59 control (placebo)) Number of participants assessed: 101 (52 LIPUS; 49 control (placebo)) Inclusion criteria:
Exclusion criteria:
*Age (mean/SD):
Gender (male/female):
Side (left/right):
Classification of injury: AO system (A1, A2, A3, B1, B2, B3, C1, C2, C3) |
|
| Interventions |
Timing of intervention: up to 5 days after the diagnosis Intervention 1 (LIPUS: low‐intensity pulsed ultrasound):
Intervention 2 (placebo):
Duration of treatment (mean): LIPUS = 25.38 days; control (placebo) = 24.43 days (mean difference 0.95, 95% CI ‐3.72 to +1.81, P = 0.49) Rehabilitation: it was not done All participants were treated with passive support for their own convenience. Free arm movements within pain range were allowed from day 1 Any co‐interventions: not reported |
|
| Outcomes |
Length of follow‐up:
Loss of follow‐up: 9 participants lost to follow‐up: LIPUS group ‐ nine participants lost to follow‐up:
Control (placebo) group ‐ 10 participant lost to follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes | *Data assessed by personal contact with the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was used |
| Allocation concealment (selection bias) | Low risk | Double‐blind, randomised, placebo‐controlled trial Each participating hospital was delivered consecutive numbered transducers in packs of 4 (2 LIPUS and 2 placebos) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | More than 80% of participants completed the follow‐up, missing outcomes data were balanced in number across intervention groups, and an intention‐to‐treat analysis was reported for the primary outcomes; however, data for those patients who withdrew were not reported |
| Selective reporting (reporting bias) | High risk | The study protocol is not available and function and/or disability were not evaluated using a validated score |
| Other bias | Low risk | The study appears to be free of other sources of bias |
<: less than >: more than ≥: more or equal to AO: Arbeitsgemeinschaft für Osteosynthesefragen CI: confidence interval DVT: deep‐venous thrombosis ITT: intention‐to‐treat LIPUS: low‐intensity pulsed ultrasound kHz: kilohertz MHz: megahertz mW/cm²: milliWatt per square centimetre µs: microsecond RCT: randomised controlled trial SATA: spatial average, temporal average VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bajuri 2011 | This was a prospective cohort study |
| Roberti 2008 | This study, which was registered in the Netherlands trial register, was listed as an ongoing trial in the first version of the review. It planned to compare Kinesio® tape plus sling versus sling alone, with a start date of October 2008 and end date October 2010. However, the contact author reported that for a variety of reasons the trial was ended and no data are available |
| Talbot 2008 | This study, logged in the National Research Register (UK), was intended to be a randomised trial of shoulder brace versus arm sling in 100 adults with isolated closed middle third clavicle fractures. It was planned to start in April 2002; however, the contact author indicated that for a variety of reasons this study never took place |
| Thompson 2005 | Not RCT or quasi‐RCT |
Characteristics of ongoing studies [ordered by study ID]
NCT02398006.
| Trial name or title | Figure‐of‐eight bandage versus arm sling for treating middle third clavicle fractures in adults: a randomised controlled trial |
| Methods |
Study design: parallel randomised controlled trial Random sequence generation: participants will be randomised according to computer generated randomisation Allocation concealment: by sealed opaque envelope Masking: open label |
| Participants |
Location: São Paulo, Brazil Target sample size: 110 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention 1: figure‐of‐eight bandage Intervention 2: arm sling |
| Outcomes |
Outcomes: function or disability measured by: DASH questionnaire and UCLA score; pain measured by VAS; failure of treatment; adverse events measured by: a) cosmetic results: perception of deformity or asymmetry (dichotomous data); b) asymptomatic nonunion (i.e. the fracture has not radiographically healed, although pain is absent); c) stiffness/restriction of the shoulder movement (compared with contralateral side); and numbers returning to previous activities Timing of outcomes measurement: 12 months |
| Starting date |
Main ID: NCT02398006 Date of registration: 12 March 2015 Last refreshed on: 19 March 2015 Date of first enrolment: January 2016 Status: recruiting |
| Contact information |
Name: Dr Mario Lenza Address: Hospital Israelita Albert Einstein – Avenida Albert Einstein, 627/701 – Jardim Leonor – CEP: 05652‐900 – São Paulo, SP, Brazil Telephone: 55 11 21511444 Email: mario.lenza@einstein.br Affiliation: Hospital Israelita Albert Einstein |
| Notes | A published protocol for this trial is available (Lenza 2016) |
DASH: Disability of the Arm, Shoulder and Hand questionnaire UCLA: University of California, Los Angeles VAS: visual analog score
Differences between protocol and review
In this update (2016), we made the following further changes from our published protocol.
We clarified that the first outcome was 'shoulder function' rather than a less specific 'function or disability'.
We modified the outcome 'patient satisfaction with method of treatment' to 'patient dissatisfaction with method of treatment', and added in 'patient preference and adherence to treatment'; see Types of outcome measures.
In the previous update (2014), we made the following changes from our published protocol.
We adjusted the outcomes to accord with the our most current review on these fractures (Lenza 2013) but modified these to a) add in 'clinical healing'; b) adjust the adverse events including removal of surgery‐related complications, and c) add in 'patient dissatisfaction with method of treatment'; see Types of outcome measures.
To search for ongoing and recently completed trials, we included the WHO International Clinical Trial Registry.
We assessed risk of bias and used the GRADE approach to assess the quality of evidence related to each of the key outcomes listed in the Types of outcome measures.
We listed three potential subgroups, should data become available for subgroup analysis in future.
Contributions of authors
For this version of this review, Mário Lenza contacted the authors of eligible trials for additional information and entered data into RevMan. Both authors performed trial selection, risk of bias assessment and data extraction. Both authors commented on and approved the final version of the review. Mário Lenza is the guarantor of the review.
Sources of support
Internal sources
Universidade Federal de São Paulo, Brazil.
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil.
The University of Manchester, UK.
Hospital Israelita Albert Einstein, Brazil.
External sources
No sources of support supplied
Declarations of interest
Mário Lenza: none known Flávio Faloppa: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andersen 1987a {published data only}
- Andersen K. Personal communication November 2008.
- Andersen K, Jensen PO, Lauritzen J. Treatment of clavicular fractures. Figure‐of‐eight bandage versus a simple sling. Acta Orthopaedica Scandinavica 1987;58(1):71‐4. [DOI] [PubMed] [Google Scholar]
- Jensen PO, Andersen K, Lauritzen J. Treatment of mid‐clavicular fractures. A prospective randomized trial comparing treatment with a figure‐eight dressing and a simple arm sling [Behandling af klavikelfrakturer. Sammenligning af behandling med 8‐talsbandage og mitella. En prospektiv, randomiseret undersøgelse]. Ugeskrift for Laeger 1985;147(25):1986‐8. [PubMed] [Google Scholar]
Ersen 2015 {published data only}
- Ersen A. Personal communication 28 February 2016.
- Ersen A, Atalar AC, Birisik F, Saglam Y, Demirhan M. Comparison of simple arm sling and figure of eight clavicular bandage for midshaft clavicular fractures: a randomised controlled study. Bone & Joint Journal 2015;97‐B(11):1562‐5. [DOI] [PubMed] [Google Scholar]
Hoofwijk 1988 {published data only}
- Hoofwijk AG, Werken C. Conservative treatment of clavicular fractures [Konservative behandlung der claviculafrakturen; eine prospektive Studie]. Zeitschrift für Unfallchirurgie, Versicherungsmedizin und Berufskrankheiten 1988;81(3):151‐6. [PubMed] [Google Scholar]
- Hoofwijk AGM, Werken C. Closed treatment of clavicular fractures: a prospective randomized trial. Acta Orthopaedica Scandinavica 1986;57(5):482‐3. [Google Scholar]
- Hoofwijk AGM, Werken C. Treatment of clavicula fractures; a prospective study [Behandlung der Claviculafrakturen; eine prospektive Studie]. Hefte zur Unfallheilkunde 1988;200:679. [Google Scholar]
- Werken C. Personal communication August 2008.
Lubbert 2008 {published and unpublished data}
- Lubbert PH. Personal communication August 2008.
- Lubbert PH, Rijt RH, Hoorntje LE, Werken C. Low‐intensity pulsed ultrasound (LIPUS) in fresh clavicle fractures: a multi‐centre double blind randomised controlled trial. Injury 2008;39(12):1444‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bajuri 2011 {published data only}
- Bajuri MY, Maidin S, Rauf A, Baharuddin M, Harjeet S. Functional outcomes of conservatively treated clavicle fractures. Clinics (São Paulo, Brazil) 2011;66(4):635‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberti 2008 {unpublished data only}
- Roberti BE. Personal communication February 2014.
- Roberti BE. Personal communication September 2008.
- Roberti BE. Conservative treatment of midclavicular fractures with Kinesio® clavicular tape and sling vs sling. WHO International Clinical Trial Registry at: http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=1374 (assessed 30 September 2008).
Talbot 2008 {unpublished data only}
- Talbot N. Personal communication 7 September 2008.
- Talbot N. The optimal conservative management of isolated midshaft clavicle fractures. National Research Register (NRR) Archive. https://portal.nihr.ac.uk (accessed 13 January 2009). [NRR Publication ID: N0185146336]
Thompson 2005 {published data only}
- Thompson DJ. Clavicle fracture and its complications. Trauma 2005;47(1):35‐61. [Google Scholar]
References to ongoing studies
NCT02398006 {unpublished data only}
- Lenza M, Taniguchi L, Ferretti M. Figure‐of‐eight bandage versus arm sling for treating middle‐third clavicle fractures in adults: study protocol for a randomised controlled trial. Trials 2016;17(229):DOI: 10.1186/s13063‐016‐1355‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenza M, Taniguchi LFP, Ferretti M. Conservative interventions for treating clavicle fractures in adults. clinicaltrials.gov/show/NCT02398006 (accessed 20 March 2016).
Additional references
Allman 1967
- Allman FL Jr. Fractures and ligamentous injuries of the clavicle and its articulation. Journal of Bone and Joint Surgery. American Volume 1967;49(4):774‐84. [PubMed] [Google Scholar]
Andersen 1987b
- Andersen K, Jensen PO, Lauritzen J. Treatment of clavicular fractures. Figure‐of‐eight bandage versus a simple sling. Acta Orthopaedica Scandinavica 1987;58(1):71‐4. [DOI] [PubMed] [Google Scholar]
Baker 2001
- Baker KG, Robertson VJ, Duck FA. A review of therapeutic ultrasound: biophysical effects. Physical Therapy 2001;81(7):1351‐8. [PubMed] [Google Scholar]
Busse 2009
- Busse JW, Kaur J, Mollon B, Bhandari M, Tornetta P 3rd, Schünemann HJ, et al. Low intensity pulsed ultrasonography for fractures: systematic review of randomised controlled trials. BMJ 2009;338:b351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Constant 1987
- Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clinical Orthopaedics and Related Research 1987;214:160‐4. [PubMed] [Google Scholar]
COTS 2007
- Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. Journal of Bone and Joint Surgery. American Volume 2007;89(1):1‐10. [DOI] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Eccleston 2010
- Eccleston C, Moore RA, Derry S, Bell RF, McQuay H. Improving the quality and reporting of systematic reviews. European Journal of Pain 2010;14(7):667‐9. [DOI] [PubMed] [Google Scholar]
Eiff 1997
- Eiff MP. Management of clavicle fractures. American Family Physician 1997;55(1):121‐8. [PubMed] [Google Scholar]
Griffin 2014
- Griffin XL, Parsons N, Costa ML, Metcalfe D. Ultrasound and shockwave therapy for acute fractures in adults. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD008579.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heuer 2014
- Heuer HJ, Boykin RE, Petit CJ, Hardt J, Millett PJ. Decision‐making in the treatment of diaphyseal clavicle fractures: is there agreement among surgeons? Results of a survey on surgeons' treatment preferences. Journal of Shoulder and Elbow Surgery 2014;23(2):e23‐33. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of InterventionsVersion 5.1.0 [updated March 2011].The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hill 1997
- Hill JM, McGuire MH, Crosby LA. Closed treatment of displaced middle‐third fractures of the clavicle gives poor results. Journal of Bone and Joint Surgery. British Volume 1997;79(4):537‐9. [DOI] [PubMed] [Google Scholar]
Hoofwijk 1986
- Hoofwijk AGM, Werken C. Closed treatment of clavicular fractures: a prospective randomized trial. Acta Orthopaedica Scandinavica 1986;57(5):482‐3. [Google Scholar]
Hudak 1996
- Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). American Journal of Industrial Medicine 1996;29(6):602‐8. [DOI] [PubMed] [Google Scholar]
Jensen 1985
- Jensen PO, Andersen K, Lauritzen J. Treatment of mid‐clavicular fractures. A prospective randomized trial comparing treatment with a figure‐eight dressing and a simple arm sling [Behandling af klavikelfrakturer. Sammenligning af behandling med 8‐talsbandage og mitella. En prospektiv, randomiseret undersøgelse]. Ugeskrift for Laeger 1985;147(25):1986‐8. [PubMed] [Google Scholar]
Jeray 2007
- Jeray KJ. Acute midshaft clavicular fracture. Journal of the American Academy of Orthopaedic Surgeons 2007;15(4):239‐48. [DOI] [PubMed] [Google Scholar]
Jupiter 1987
- Jupiter JB, Leffert RD. Non‐union of the clavicle. Associated complications and surgical management. Journal of Bone and Joint Surgery. American Volume 1987;69(5):753‐60. [PubMed] [Google Scholar]
Kotelnicki 2006
- Kotelnicki JJ, Bote HO, Mitts KG. The management of clavicle fractures. JAAPA 2006;19(9):50, 53‐4, 56. [DOI] [PubMed] [Google Scholar]
Kukkonen 2013
- Kukkonen J, Kauko T, Vahlberg T, Joukainen A, Aärimaa V. Investigating minimal clinically important difference for Constant score in patients undergoing rotator cuff surgery. Journal of Shoulder and Elbow Surgery 2013;22(12):1650‐5. [DOI] [PubMed] [Google Scholar]
Lazarus 2001
- Lazarus MD. Fractures of the clavicle. In: Bucholz RW, Heckman JD editor(s). Rockwood & Green's Fractures in Adults. 5th Edition. Philadelphia: Lippincott‐Wilkins, 2001:1041‐78. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lenza 2013
- Lenza M, Buchbinder R, Johnston RV, Belloti JC, Faloppa F. Surgical versus conservative interventions for treating fractures of the middle third of the clavicle. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD009363.pub2] [DOI] [PubMed] [Google Scholar]
Lenza 2016
- Lenza M, Taniguchi L, Ferretti M. Figure‐of‐eight bandage versus arm sling for treating middle‐third clavicle fractures in adults: study protocol for a randomised controlled trial. Trials 2016;17(229):DOI: 10.1186/s13063‐016‐1355‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lester 1929
- Lester CW. The treatment of fractures of the clavicle: a study of 422 cases observed in the out‐patient department of the Roosevelt Hospital of the city of New York. Annals of Surgery 1929;89(4):600‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marti 2003
- Marti RK, Nolte PA, Kerkhoffs GM, Besselaar PP, Schaap GR. Operative treatment of mid‐shaft clavicular non‐union. International Orthopaedics 2003;27(3):131‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKee 2006
- McKee MD, Pedersen EM, Jones C, Stephen DJ, Kreder HJ, Schemitsch EH, et al. Deficits following nonoperative treatment of displaced midshaft clavicular fractures. Journal of Bone and Joint Surgery. American Volume 2006;88(1):35‐40. [DOI] [PubMed] [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain‐‐establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI] [PubMed] [Google Scholar]
Muller 1991
- Muller M, Allgower M, Schneider R, Willenegger H. Manual of Internal Fixation: Techniques Recommended by the AO‐ASIF Group. 3rd Edition. Berlin: Springer‐Verlag, 1991. [Google Scholar]
Neer 1984
- Neer C. Fractures of the clavicle. In: Rockwood CA Jr, Green DP editor(s). Fractures in Adults. 2nd Edition. Philadelphia: Lippincott Williams & Wilkins, 1984:707‐13. [Google Scholar]
Nordqvist 1994
- Nordqvist A, Petersson C. The incidence of fractures of the clavicle. Clinical Orthopaedics and Related Research 1994;300:127‐32. [PubMed] [Google Scholar]
Nordqvist 1998
- Nordqvist A, Petersson CJ, Redlund‐Johnell I. Mid‐clavicle fractures in adults: end result study after conservative treatment. Journal of Orthopaedic Trauma 1998;12(8):572‐6. [DOI] [PubMed] [Google Scholar]
Nowak 2000
- Nowak J, Mallmin H, Larsson S. The aetiology and epidemiology of clavicular fractures. A prospective study during a two‐year period in Uppsala, Sweden. Injury 2000;31(5):353‐8. [DOI] [PubMed] [Google Scholar]
Pieske 2008
- Pieske O, Dang M, Zaspel J, Beyer B, Löffler T, Piltz S. Midshaft clavicle fractures ‐ classification and therapy. Results of a survey at German trauma departments [Die Klavikulaschaftfraktur – Klassifikation und Therapie]. Der Unfallchirurg 2008;111(6):387‐94. [DOI] [PubMed] [Google Scholar]
Postacchini 2002
- Postacchini F, Gumina S, Santis P, Albo F. Epidemiology of clavicle fractures. Journal of Shoulder and Elbow Surgery 2002;11(5):452‐6. [DOI] [PubMed] [Google Scholar]
Quigley 1950
- Quigley TB. The management of simple fracture of the clavicle in adults. New England Journal of Medicine 1950;243(8):286‐90. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 1998
- Robinson CM. Fractures of the clavicle in the adult. Epidemiology and classification. Journal of Bone and Joint Surgery. British Volume 1998;80(3):476‐84. [DOI] [PubMed] [Google Scholar]
Robinson 2004
- Robinson CM, Court‐Brown CM, McQueen MM, Wakefield AE. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. Journal of Bone and Joint Surgery. American Volume 2004;86(7):1359‐65. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glaziou P, et al. Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. London: The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org. [Google Scholar]
Stanley 1988
- Stanley D, Trowbridge EA, Norris SH. The mechanism of clavicular fracture. A clinical and biomechanical analysis. Journal of Bone and Joint Surgery. British Volume 1988;70(3):461‐4. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Yian 2005
- Yian EH, Ramappa AJ, Arneberg O, Gerber C. The Constant score in normal shoulders. Journal of Shoulder and Elbow Surgery 2005;14(2):128‐33. [DOI] [PubMed] [Google Scholar]
Zlowodzki 2005
- Zlowodzki M, Zelle BA, Cole PA, Jeray K, McKee MD. Treatment of acute midshaft clavicle fractures: systematic review of 2144 fractures: on behalf of the Evidence‐Based Orthopaedic Trauma Working Group. Journal of Orthopaedic Trauma 2005;19(7):504‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lenza 2008
- Lenza M, Belloti JC, Andriolo RB, Gomes dos Santos JB, Faloppa F. Conservative interventions for treating middle third clavicle fractures in adolescents and adults. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD007121] [DOI] [PubMed] [Google Scholar]
Lenza 2009a
- Lenza M, Belloti JC, Andriolo RB, Gomes dos Santos JB, Faloppa F. Conservative interventions for treating middle third clavicle fractures in adolescents and adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007121.pub2] [DOI] [PubMed] [Google Scholar]
Lenza 2014
- Lenza M, Belloti JC, Andriolo RB, Faloppa F. Conservative interventions for treating middle third clavicle fractures in adolescents and adults. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD007121.pub3] [DOI] [PubMed] [Google Scholar]


